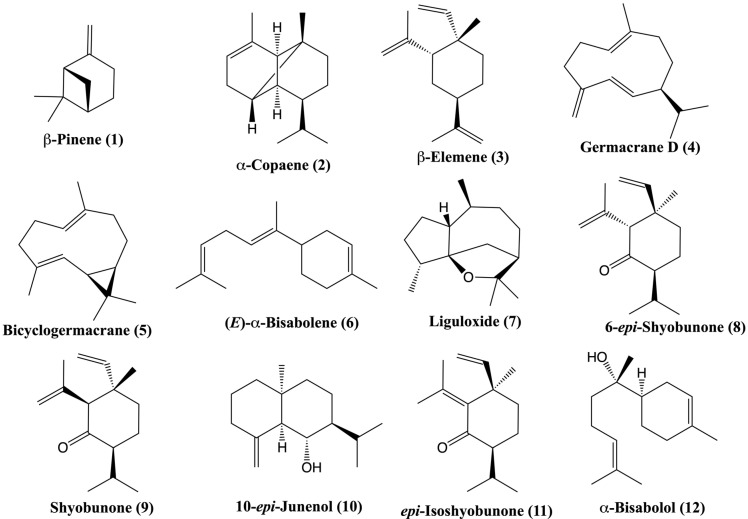
Abstract
In the present study, preliminary phytochemical investigations were performed on the fruit essential oil and antioxidant-rich methanolic extracts of the fruits and roots of Ferula drudeana, the putative Anatolian ecotype of the Silphion plant, to corroborate its medicinal plant potential and identify its unique characteristics amongst other Ferula species. The essential oil from the fruits of the endemic species Ferula drudeana collected from Aksaray was analyzed by GC and GC/MS. The main components of the oil were determined as shyobunone (44.2%) and 6-epishyobunone (12.6%). The essential oil of the fruits and various solvent extracts of the fruits and roots of F. drudeana were evaluated for their antibacterial and anticandidal activity using microbroth dilution methods. The essential oil of the fruits, methanol, and methylene chloride extracts of the fruits and roots showed weak to moderate inhibitory activity against all tested microorganisms with MIC values of 78–2000 µg/mL. However, the petroleum ether extract of the roots showed remarkable inhibitory activity against Candida krusei and Candida utilis with MIC values of 19.5 and 9.75 µg/mL, respectively. Furthermore, all the samples were tested for their antioxidant activities using DPPH• TLC spot testing, online HPLC–ABTS screening, and DPPH/ABTS radical scavenging activity assessment assays. Methanolic extracts of the fruits and roots showed strong antioxidant activity in both systems.
Keywords: Ferula drudeana, GC/MS, HPLC–ABTS•+, essential oil, antibacterial, anticandidal, antioxidant
1. Introduction
The genus Ferula (Apiaceae) comprises more than 220 species [1] and is widespread throughout the Mediterranean and Central Asia. It represents 26 species and 15 endemics in the flora of Turkey [2,3,4]. Ferula species are traditionally used in folk medicine for the treatment of many disorders, such as gastrointestinal problems, diarrhea, intestinal parasites, ulcer, hypotension, neurological disorders, epilepsy, rheumatism, and diabetes, and also used as a sedative, antispasmodic, expectorant, anticonvulsant, and tonic aphrodisiac [5]. About two thousand years ago, Pedanius Dioscorides described five drugs obtained from Ferula species in the third book of De Materia Medica [6], namely, Narthex (Ferula communis L.), Sagapenon (F. persica Wild.), Chalbane (Galbanum, F. gummosa Boiss.), Ammoniakon (F. tingitana L. or F. marmarica Asch. & Taub.), and Silphion, which clearly illustrates the span of medicinal use of Ferula species in ancient times.
Ferula drudeana Korovin (Figure 1) is a rare endemic species growing in the Central Anatolia region of Turkey. This species is the only member of the subgenus Narthex (Falc.) Drude of Ferula genus in Turkey [7]. With its unique morphological features and extremely limited local distributions near former Greek villages in Central Anatolia, F. drudeana has been proposed as an Anatolian ecotype of the silphion plant [8]. The silphion plant was used for many medicinal and culinary purposes during ancient times in Mediterranean countries. Pliny the Elder declared that “it would be an endless task to enumerate all the uses to which laser (i.e., the resin of silphion plant) is put” [9]. Theophrastus of Eresus calls the fruits of the silphion plant phyllon (i.e., leaf-like) [10]. Unfortunately, the use of the phyllon name inadvertently created an ambiguous terminology in the literature. The fruits of Ferula drudeana (Figure 2), like the fruits of other Ferula species, is a schizocharpic fruit that splits into two mericarps during their maturity. The fruits of Apiaceae plants are widely accepted sources of medicinal drugs due to their high essential oil/resin content. In contrast, their leaves are rarely used as a source of medicinal drugs [11]. Probably due to the use of ambiguous phyllon names for the fruits of the silphion plant by Theophrastus, instead of the biologically active metabolite-rich fruits, some authors referred to the leaves of the silphion plant as a medicinally used part [12]. In contrast, the medicinal use of the fruits of the silphion plant was not mentioned in the literature. As part of our study investigating the biological activities of the secondary metabolites of Ferula drudeana, we herein report on the analyses of the terpenoid content of the essential oil of the fruits as well as the major phenolic compounds of the methanolic extracts of the fruits and roots.
Figure 1.
(A) The general view of Ferula drudeana is similar to the numismatic silphion plant figures on the early period Cyrenaic coins (upper coin figure). (B) The numismatic figures on the later period Cyrenaic coins (lower coin figure) resemble the developing premature flowering stem of F. drudeana. Copyrights of coin figures: Trustees of the British Museum.
Figure 2.
Fruits of Ferula drudeana during their development stages: (A) immature fruits, (B) young fruits, (C) maturing young fruits become yellow due to the loss of chlorophyll, (D) golden brown mature fruits.
2. Results and Discussion
2.1. Volatile Composition of the Fruit Essential Oil of Ferula drudeana
The fruits of F. drudeana were collected in July and air-dried, and the coarsely crushed fruits were subjected to hydrodistillation to obtain its essential oil. The essential oil yield of the fruits of F. drudeana was 3.8%. The essential oil of the fruits was analyzed by GC-GC/MS systems, and 28 compounds representing 89.1% of the essential oil were characterized. The results of the analysis are shown in Table 1 and Figure 3.
Table 1.
Volatile composition of the fruit essential oil of Ferula drudeana.
| No. | RRI a | RRI b | Molecular Formula/MW | Compound Name | Peak Area (%) | IM |
|---|---|---|---|---|---|---|
| 1 | 1032 | 1032 c, 1008–1039 d | C10H16/136 | α-Pinene | 0.2 | tR, MS |
| 2 | 1118 | 1118 c, 1085–1130 d | C10H16/136 | β-Pinene (1) | 5.8 | tR, MS |
| 3 | 1132 | 1132 c | C10H16/136 | Sabinene | tr | tR, MS |
| 4 | 1174 | 1174 c | C10H16/136 | Myrcene | tr | tR, MS |
| 5 | 1299 | 1299 e | C10H20O2/172 | 2-Methylbutyl isovalerate | tr | MS |
| 6 | 1491 | 1495 c | C15H24/204 | Bicycloelemene | 0.1 | MS |
| 7 | 1492 | 1445–1549 d | C15H24/204 | Cyclosativene | 0.4 | MS |
| 8 | 1497 | 1497 c | C15H24/204 | α-Copaene (2) | 0.5 | MS |
| 9 | 1550 | 1559 d, 1534–1580 d | C15H24/204 | cis-α-Bergamotene | 0.2 | MS |
| 10 | 1597 | 1583–1668 d | C15H24/204 | α-Guaiene | 0.3 | MS |
| 11 | 1600 | 1565–1608 d | C15H24/204 | β-Elemene (3) | 0.6 | MS |
| 12 | 1612 | 1612 c | C15H24/204 | β-Caryophyllene | 0.2 | tR, MS |
| 13 | 1621 | - | -/204 | Unknown 1 | 1.8 | MS |
| 14 | 1668 | 1669 e | C15H24/204 | (Z)-β-Farnesene | 0.2 | MS |
| 15 | 1669 | 1668 c, 1627–1668 d | C15H24/204 | Sesquisabinene | 0.3 | tR, MS |
| 16 | 1671 | 1643–1684 d | C15H24/204 | (E)-β-Farnesene | 0.1 | MS |
| 17 | 1687 | 1687 c | C15H24/204 | α-Humulene | 0.1 | tR, MS |
| 18 | 1719 | 1718 f | C15H24/204 | γ-Guaiene | 0.2 | MS |
| 19 | 1726 | 1726 c | C15H24/204 | Germacrene D (4) | 1.5 | MS |
| 20 | 1755 | 1755 c | C15H24/204 | Bicyclogermacrene (5) | 2.7 | tR, MS |
| 21 | 1772 | 1773 c | C15H24/204 | δ-Cadinene | 0.2 | tR, MS |
| 22 | 1784 | 1773–1786 d | C15H24/204 | (E)-α-Bisabolene (6) | 1.0 | MS |
| 23 | 1804 | 1812 g, 1808 h | C15H26O/222 | Liguloxide (7) | 1.6 | MS |
| 24 | 1868 | 1861 c | C15H24O/220 | 6-epi-Shyobunone (8) | 12.6 | MS |
| 25 | 1900 | 1893 c | C15H24O/220 | Isoshyobunone | tr | MS |
| 26 | 1916 | 1903 c | C15H24O/220 | Shyobunone (9) | 44.2 | MS |
| 27 | 1977 | 2028 k, 2052 k | C15H26O/222 | 10-epi-Junenol (10) | 5.8 | MS |
| 28 | 2053 | 2044 c | C15H24O/220 | epi-Isoshyobunone (11) | 9.8 | MS |
| 29 | 2084 | - | -/236 | Unknown 2 | 2.2 | MS |
| 30 | 2092 | - | -/220 | Unknown 3 | 1.1 | MS |
| 31 | 2232 | 2178–2234 d | C15H26O/222 | α-Bisabolol (12) | 0.5 | tR, MS |
| Monoterpene Hydrocarbons | 6.0 | |||||
| Sesquiterpene Hydrocarbons | 8.6 | |||||
| Oxygenated Sesquiterpenes | 74.5 | |||||
| Others | 5.1 | |||||
| Total % | 94.2 |
Compounds listed in order of their elution in HP Innowax FSC GC column. RRI a: relative retention indices experimentally calculated against n-alkanes; RRI b: RRI from literature (c [13], d [14], e [15], f [16], g [17], h [18], and k [19]) for polar column values, with % calculated from FID data; tr: trace (<0.1%); IM: identification method; tR: identification based on comparison with coinjected standards on an HP Innowax column; MS: identification based on computer matching of the mass spectra libraries.
Figure 3.
Main terpenoid compounds of the fruit essential oil of Ferula drudeana.
The main components of the essential oil were identified as shyobunone (9) (44.2%), 6-epi-shyobunone (8) (12.6%), epi-isoshyobunone (11) (9.8%), and β-pinene (1) (5.8%). Oxygenated sesquiterpenes (74.5%), sesquiterpene hydrocarbons (8.6%), and monoterpene hydrocarbons (6.0%) were the main groups present in the oil. Oxygenated sesquiterpenes were the most abundant among these groups representing 74.5%. Previously, the fruit essential oil of another population of Ferula drudeana was analyzed, and its major components were identified as epi-isoshyobunone (38%), shyobunone (25%), and 6-epi-shyobunone (6%) [20]. Recently, the presence of high level of shyobunone derivatives, namely, isoshyobunone (23.9%), epi-shyobunone (18.9%), and shyobunone (2.7%), were discovered in the essential oil of fresh leaves of Siparuna guianensis Aubl. (Siparunaceae), a well-known Amazonian medicinal plant. The essential oil of S. guianensis was shown to have strong cholinesterase inhibitory, anti-Alzheimer, and neuroprotective activities due to its content of shyobunone derivatives [21]. The presence of shyobunone isomers in the essential oil of the aerial parts of Daucus carota L. var. carota (Apiaceae) was also reported in small quantities, namely, shyobunone (1.3%) and 6-epi-shyobunone (0.5%) [22]. Shyobunone and its isomers epi-shyobunone and isoshyobunone were originally isolated from the essential oil of the rhizomes of Acorus calamus L. (Acoraceae) [23]. The essential oil of the rhizomes of Acorus calamus, also known as sweet flag oil, was found to contain shyobunone (1.5–13.3%), 6-epi-shyobunone (0.4–3.1%), isoshyobunone (0.1–0.5%), and epi-isoshyobunone (3.3–7.3%) [13,24].
The essential oil composition of Ferula drudeana is unique amongst the Ferula species. Unlike other investigated Ferula species growing in the Mediterranean, Middle East, and North African countries, it has a very high percentage of oxygenated sesquiterpene compounds [8]. The proportion of rare elemane sesquiterpene ketone compounds in the essential oil is 66.6%.
2.2. Antimicrobial Testing of the Fruit Essential Oil of Ferula drudeana
Hydrodistilled fruit essential oil of Ferula drudeana demonstrated weak antimicrobial effects against all tested pathogenic Gram (+) and Gram (−) bacterial strains and the Candida panel with MIC values of 500–2000 μg/mL using CLSI M7-A7 and M27-A2 reference microdilution broth methods. Methanol and methylene chloride extracts of the fruits and roots displayed weak to moderate inhibitory effects against all tested microorganisms at concentrations between 78 and 2500 μg/mL. However, the petroleum ether extract of the roots (R1) showed remarkable inhibitory effects on Candida krusei and Candida utilis with MIC values of 19.5 and 9.75 μg/mL, respectively (Table 2 and Table 3). Our results are similar to previous works [25,26,27,28,29,30,31,32,33,34] on different Ferula essential oils and extracts.
Table 2.
Antibacterial activity of the fruit essential oil, fruit, and root extracts of Ferula drudeana (MIC, μg/mL).
| Microorganisms | EOF | F1 | F2 | F3 | R1 | R2 | R3 | S1 | S2 |
|---|---|---|---|---|---|---|---|---|---|
| Escherichia coli | 2000 | 1250 | 1250 | 2500 | 1250 | 1800 | 1800 | 3.9 | 1 |
| Pseudomonas aeruginosa | 2000 | 625 | 1250 | 2500 | 1250 | 900 | 900 | 62.5 | 15.6 |
| Salmonella typhimurium | 500 | 1250 | 1250 | 2500 | 625 | 900 | 900 | 3.9 | 1 |
| Bacillus cereus | 1000 | 2500 | 625 | 1250 | 1250 | 3600 | 450 | 7.8 | 1 |
| Bacillus subtilis | 1000 | 1250 | 1250 | 2500 | 1250 | 450 | 900 | 1.9 | 1 |
| Serratia marcescens | 1000 | 625 | 625 | 1250 | 1250 | 450 | 900 | 15.6 | 15.6 |
| Staphylococcus epidermidis | 2000 | 2500 | 625 | 625 | 312 | 1800 | 900 | 3.9 | 1 |
| E. coli O157:H7 | 2000 | 1250 | 1250 | 2500 | 625 | 900 | 1800 | 3.9 | 1 |
EOF: essential oil of the fruits; F: fruit extracts; R: root extracts (1: petroleum ether, 2: methylene chloride, and 3: methanol); S1: chloramphenicol; S2: ampicillin.
Table 3.
Anticandidal activity of the fruit essential oil, fruit, and root extracts of Ferula drudeana (MIC, μg/mL).
| Microorganisms | EOF | F1 | F2 | F3 | R1 | R2 | R3 | S1 | S2 |
|---|---|---|---|---|---|---|---|---|---|
| Candida albicans * | 250 | 312 | 1250 | 1250 | 156 | 450 | 900 | 0.05 | 0.1 |
| Candida utilis | 500 | 39 | 78 | 312 | 19.5 | 112 | 225 | 1.6 | 0.05 |
| Candida tropicalis | 2000 | 625 | 312 | 1250 | 310 | 900 | 450 | 0.2 | 0.2 |
| Candida krusei | 500 | 39 | 312 | 625 | 9.75 | 450 | 900 | 1.6 | 0.2 |
| Candida albicans | 2000 | 312 | 1250 | 1250 | 156 | 450 | 450 | 0.1 | 0.2 |
| Candida glabrata | 2000 | 156 | 625 | 156 | 78 | 225 | 450 | 3.2 | 0.2 |
EOF: essential oil of the fruits; F: fruit extracts; R: root extracts (1: petroleum ether, 2: methylene chloride, and 3: methanol); S1: ketoconazole; S2: amphotericin-B; *: clinically isolated strain.
In contrast to the essential oil fraction, the presence of strong antifungal activity in the fruit and root petroleum ether extracts suggests that the antifungal activity must be due to nonvolatile component(s) of these extracts. Further studies are in progress to identify the most potent antifungal compound(s) of these extracts. C. albicans, C. tropicalis, and C. glabrata represent the most clinically isolated Candida species. In contrast, other species, such as C. krusei, C. parapsilosis, C. guilliermondii, and C. kefyr, have also been isolated and are thought to be less virulent. However, recent data indicate that >30% of nosocomial Candida infections are due to species other than C. albicans, and in recent years, there has been a significant increase in C. krusei, a human pathogen causing systemic and ocular infections [35]. Identifying potent anticandidal substances in the petroleum ether extracts of F. drudeana may provide a promising antifungal agent for the treatment of such infections.
2.3. Antioxidant Activity Determination
2.3.1. Qualitative TLC Spot Testing Evaluation of the Antioxidant Activities of the Fruit Essential Oil, Fruit, and Root Extracts of Ferula drudeana
The methanolic extracts of the fruits and roots of Ferula drudeana Korovin showed strong antioxidant activity by DPPH• reagent treatment on a TLC silica gel plate (Figure S1, Supplementary Material) [36,37]. Following the application of the fruit essential oil, fruit, and root extract solutions to a silica gel plate, the plate was sprayed with DPPH (2,2-diphenyl-1-picryl-hydrazylhydrate) solution. No antioxidant activity was observed in the petroleum ether and methylene chloride extracts of the fruits and roots. Only a slight discoloration was observed on the periphery of the spot where the essential oil was applied to the silica gel plate. Peripheral discoloration of essential oil is probably related to the microlevel distribution of the essential oil by blank dilution solvent on the silica gel plate at the application point. The strong discoloration of the methanolic extracts of the fruits and roots of F. drudeana indicates the presence of antioxidant compounds in these extracts.
2.3.2. Online HPLC–ABTS•+ Identification of Major Antioxidant Compounds of the Methanolic Extracts of Ferula drudeana
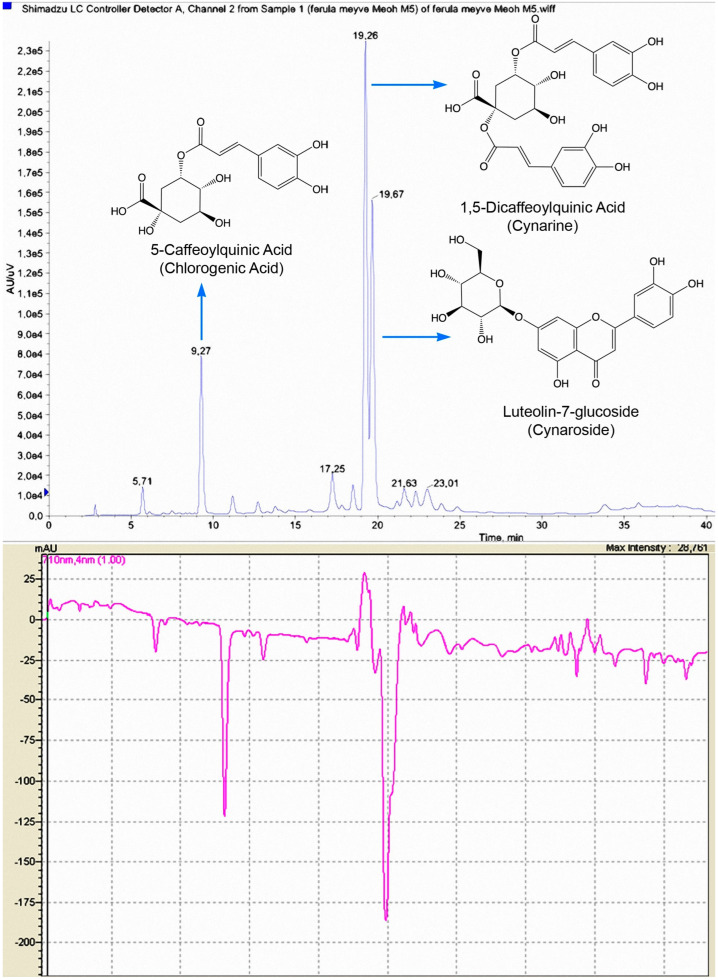
The methanolic extracts of the fruits and roots of F. drudeana were subjected to online high-performance liquid chromatography (HPLC)—2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) radical cation (ABTS•+)-based screening assay for the identification of phenolic antioxidants of the fruits and roots of F. drudeana [38]. HPLC elute of the methanolic extracts of the fruits and roots of F. drudeana was split into two lines. The elute of one line mixed with a stabilized solution of ABTS•+ reagent, so the formation of negative peaks, indicating the antioxidant activity of the corresponding compound peaks, were monitored by measuring the decrease in absorbance at 734 nm (Figure 4). The elute of the second line was subjected to electrospray ionization mass spectrometry (EIMS) to identify the phenolic compounds responsible for the antioxidant activity of the methanolic extracts of fruits and roots of F. drudeana.
Figure 4.
Online HPLC–ABTS•+ chromatogram (lower section) of the methanolic extract of fruits of Ferula drudeana display negative peaks, indicating the antioxidant activity of the corresponding compound peaks in the upper HPLC chromatogram.
2.3.3. HPLC–MS/MS Analysis of the Methanolic Extract of the Fruits of Ferula drudeana
The methanolic extract of the fruits of F. drudeana was subjected to high-performance liquid chromatography–MS/MS analysis to identify the phenolic compounds responsible for the antioxidant activity (Figure 5).
Figure 5.
Structures of the antioxidant phenolic compounds of the fruit and root methanolic extracts of Ferula drudeana.
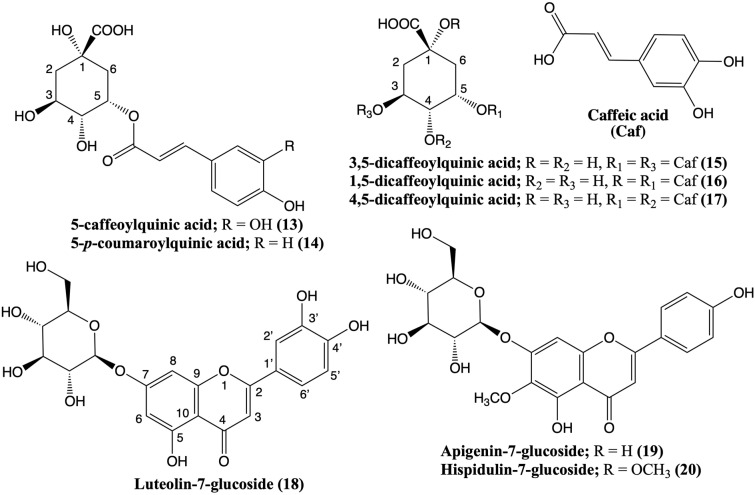
The mass spectrum of compound 13 (Figure S2, Supplementary Material; Table 4) (Rt 9.3 min) displayed a pseudo molecular [M-H]− ion at m/z 353 and a base peak ion at m/z 191 [quinic acid-H]− due to the loss of caffeoyl moiety (i.e., m/z 162), indicating a caffeic acid ester of quinic acid structure for 13. When the caffeoyl group residue esterified at the 3-OH position of quinic acid, the intensity of the caffeoyloxy moiety (i.e., [caffeic acid-H]−) at m/z 179 is more than the connection to the 5-OH position of quinic acid [39]. Thus, the structure of 13 was identified as 5-caffeoylquinic acid (i.e., chlorogenic acid). The mass spectrum of compound 14 (Rt 12.8 min) was similar to that of 13 except for a pseudo molecular [M-H]− at m/z 337, indicating the presence of a p-coumaric acid ester in 14 instead of a caffeic acid ester [40]. Consequently, the structure of 14 was confirmed as 5-p-coumaroylquinic acid.
Table 4.
Peak assignment for the HPLC–MS/MS analysis of the methanol extracts of Ferula drudeana.
| No | Rt (min) | [M-H]− (m/z) | Fragment Ion (m/z) | Identification | Reference |
|---|---|---|---|---|---|
| 1 | 9.3 | 353 | 353 (16), 191 (100), 179 (5) | 5-Caffeoylquinic acid (13) | [40,41] |
| 2 | 11.4 | 293 | 293 (100), 131 (20) | Unknown 4 | |
| 3 | 12.8 | 337 | 337 (16), 191 (100) | 5-p-Coumaroylquinic acid (14) | [40] |
| 4 | 17.4 | 515 | 191 (31), 179 (31), 173 (46), 135 (100) | Unknown 5 | |
| 5 | 18.7 | 515 | 515 (43), 353 (43) 191 (100) 179 (38) | 3,5-Dicaffeoylquinic acid (15) | [40] |
| 6 | 19.4 | 515 | 515 (23), 353 (35), 335 (5), 191 (100), 179 (12), 161 (9) | 1,5-Dicaffeoylquinic acid (16) | [41] |
| 7 | 19.7 | 447 | 447 (73), 285 (100) | Luteolin-7-glucoside (18) | [42] |
| 8 | 21.8 | 515 | 515 (17), 353 (17), 191 (39), 179 (44), 173 (100) | 4,5-Dicaffeoylquinic acid (17) | [41] |
| 9 | 23.1 | 431 | 431 (84), 268 (100) | Apigenin-7-glucoside (19) | [43] |
| 10 | 23.9 | 461 | 461 (100), 446 (32), 313 (11), 298 (42), 283 (37), 255 (68) | Hispidulin-7-glucoside (20) | [44] |
The mass spectra of compounds 15 (Rt 18.7 min), 16 (Rt 19.4 min) (Figure S3, Supplementary Material), and 17 (Rt 21.8 min) displayed the pseudo molecular ion [M-H]− at m/z 515 and characteristic ion at m/z 353 due to the loss of a caffeoyl moiety (i.e., m/z 162). All of these compounds showed the quinic acid fragment ion at m/z 191 (i.e., [quinic acid-H]−) following the loss of the second caffeoyl moiety. These data suggested that compounds 15, 16, and 17 were isomers of the dicaffeoylquinic acid derivatives. The presence of m/z 191 [quinic acid-H]− as the base peak and the absence of m/z 173 fragment in the mass spectra of compounds 15 and 16 (Figure S3, Supplementary Material) suggested that these compounds could be 1,3-, 1,5-, or 3,5-dicaffeoyl-quinic acid derivatives [40]. Furthermore, the presence of a strong m/z 179 ion (i.e., [caffeoyloxy-H]−) fragment vs. the lack of a weak m/z 335 ion fragment suggested that the structure of 15 should be 3,5-dicaffeoylquinic acid [40]. In contrast, the presence of m/z 179 ion and weak m/z 335 ion fragments in the mass spectrum of 16 (Figure S3, Supplementary Material) confirmed its structure as 1,5-dicaffeoylquinic acid (i.e., cynarine) [41].
The mass spectrum of compound 17 showed the main fragment ion at m/z 173, indicating the presence of a 4-OH substituted quinic acid. Consequently, this compound could be 3,4-dicaffeoylquinic acid or 4,5-dicaffeoylquinic acid. As the mass spectrum of 3,4-dicaffeoylquinic acid has an additional fragment at m/z 335 [41] and this fragment was absent in the mass spectrum of 17, its structure should be 4,5-dicaffeoylquinic acid.
So far, several caffeoylquinic acid derivatives have been reported from the polar extracts of other Ferula species [45,46,47,48,49].
The mass spectrum of compound 18 (Figure S4, Supplementary Material) showed m/z 447 [M-H]− pseudo molecular ion and m/z 285 (luteolin aglycone) base peak ion due to the loss of 162 (i.e., hexose) moiety, which confirmed the structure of 18 as luteolin-7-glucoside. Previously, luteolin-7-glucoside has also been detected in other Ferula species [42,50].
The mass spectrum of compound 19 showed m/z 431 [M-H]− pseudo molecular ion and m/z 268 (apigenin aglycone) base peak ion, which suggested the structure of 19 was apigenin glucoside. Apigenin-7-glucoside (19) has previously been identified in other Ferula species [43]. The mass spectroscopic data of compound 20 displayed m/z 461 [M-H]− pseudo molecular and m/z 299 (hispidulin aglycone) ions as well as m/z 446, 283, and 255 fragment ions, which are in agreement with previously reported data. Thus, the structure of compound 20 was identified as hispidulin-7-glucoside [44].
2.3.4. Determination of the Antioxidant Potential of the Methanolic Extracts of Ferula drudeana by DPPH and ABTS Free Radical Scavenging Activity Assessment
The antioxidant potential of the methanolic extracts of the fruits and roots of F. drudeana as well as two of their major antioxidant compounds, namely, chlorogenic acid and luteolin 7-glucoside, were determined by DPPH and ABTS radical scavenging activity tests. The results are shown in Table 5.
Table 5.
Antioxidant activities of Ferula drudeana extracts and standards.
| Extracts/Compounds | DPPH [IC50, mg/mL] 2 | TEAC 1 [mM] 1 mg/mL |
TEAC [mM] 0.1 mg/mL |
|---|---|---|---|
| Fruit Methanolic Extract of F. drudeana | 0.087 ± 0.011 | 0.41 ± 0.07 | na 3 |
| Root Methanolic Extract of F. drudeana | 0.189 ± 0.048 | 0.25 ± 0.09 | na |
| Chlorogenic acid 4 | 0.013 ± 0.001 | 2.94 ± 0.29 | 0.16 ± 0.07 |
| Luteolin-7-glucoside 4 | 0.0073 ± 0.0005 | 3.02 ± 0.05 | 1.03 ± 0.02 |
| Gallic acid 5 | 0.002 ± 0.0001 | 3.22 ± 0.02 | 3.24 ± 0.01 |
| BHT 5 | 0.042 ± 0.008 | 3.16 ± 0.04 | 0.37 ± 0.05 |
| Ascorbic acid 5 | 0.006 ± 0.001 | 3.24 ± 0.05 | 0.73 ± 0.07 |
1 TEAC: Trolox equivalent antioxidant capacity; 2 IC50: 50% inhibition concentration; 3 na: not active; 4 standard; 5 positive control standard.
The DPPH radical scavenging test results indicated that the methanolic fruit extract of F. drudeana was about 2.2 times more active than the root extract. Chlorogenic acid and luteolin-7-glucoside, two of the major antioxidant compounds of methanolic extracts, were more potent antioxidants than butylated hydroxytoluene (BHT) but were not as effective as gallic or ascorbic acids.
The results of the TEAC experiment (i.e., ABTS radical scavenging activity) suggested that the methanolic extract of the fruits of F. drudeana was approximately 1.6 times more effective than the root extract at 1 mg/mL concentration. However, at 0.1 mg/mL concentration, none of the methanolic extracts was active. The antioxidant activity of luteolin-7-glucoside was higher than both BHT and ascorbic acid at 0.1 mg/mL concentration but lower than gallic acid. In this assay, chlorogenic acid was found to be a more potent antioxidant than any of the standards used.
2.4. Biological Activities of the Ferula drudeana Metabolites
The biological activities of the main essential oil terpenoids and phenolic compounds of the methanolic extracts of Ferula drudeana are summarized in Table 6 below.
Table 6.
Biological activities of the compounds identified in the fruit essential oil, fruit, and root methanol extracts of Ferula drudeana.
| Secondary Metabolite | Biological Activities |
|---|---|
| β-Pinene (1) | Antibacterial, anticandidal [51,52], antibiofilm [53], phytotoxic [54], antidepressant-like activity [55], cytotoxic [56], gastroprotective [57], anticonvulsant [58] |
| α-Copaene (2) | Cytotoxic, antioxidant, antigenotoxic [59,60], insect attractant [61], analgesic and anti-inflammatory [62] |
| β-Elemene (3) | Antitumor, anticancer activity [63,64,65,66,67,68], antimigraine [69] |
| Germacrene D (4) | Cytotoxic, antioxidant, insecticidal [70], insect attractant [71], antibacterial [72] |
| Bicyclogermacrene (5) | Larvicidal [73], radical scavenger [74] |
| (E)-α-Bisabolene (6) | Antioxidant [75], cytotoxic [76], anti-inflammatory [77], antifungal [78] |
| Shyobunone (8) | Insecticidal, repellent activity [79], neuroprotective, cholinesterase inhibitor, anti-Alzheimer [21], antibacterial (against Helicobacter pylori) [80] |
| 6-epi-shyobunone (9) | Cholinesterase inhibitor, anti-Alzheimer, neuroprotective [21] |
| Epi-isoshyobunone (11) | Insecticidal, repellent activity [79], cholinesterase inhibitor, anti-Alzheimer, neuroprotective [21] |
| α-Bisabolol (12) | Anti-inflammatory, analgesic [81], antioxidant, anti-infective [82], cytotoxic [83], gastroprotective [84], nephroprotective [85], uterorelaxant [86], antileishmanial [87], antitumoral [88] |
| Cynarine (16) (1,5-Dicaffeoylquinic acid) | Antimicrobial [89], hepatoprotective [90], antihypertensive, vasodilator [91], choleretic [92], antioxidant [93], anti-inflammatory [94,95], antidiabetic [96,97], antidepressant [98], antivitiligo [99], anti-HIV-1 [100], Ebola virus inhibitor [101], Janus kinase (JAK) inhibitor [102] |
| Chlorogenic acid (13) (5-Caffeoylquinic acid) | Antimicrobial [89], hepatoprotective [90], antihypertensive, vasodilator [91], antitumor [103], anti-inflammatory [104], improves late diabetes [105], protects against cholestatic liver injury [106], neuroprotective [107], antiviral activity against influenza A (H1N1/H3N2) virus [108], antidiabetic and antilipidemic [109], inhibits hepatocellular carcinoma [110], anxiolytic and antioxidant [111], antihyperalgesic [112], cardioprotective [113], neuroprotective and cognitive improvement [114], improves hepatic steatosis and insulin resistance [115], alleviates obesity and modulates gut microbiota [116], ameliorates ulcerative colitis [117], inhibits glioblastoma growth [118], induces 4T1 breast cancer tumor’s apoptosis [119], strong matrix metalloproteinase-9 inhibitor [120] |
| 3,5-Dicaffeoylquinic acid (15) (Isochlorogenic acid A) | Promotes melanin synthesis [121], antirosacea [122], antioxidant [93,123] |
| 4,5-Dicaffeoylquinic acid (17) (Isochlorogenic acid C) | Antirosacea [122], antioxidant [93,123] |
| Cynaroside (18) (Luteolin-7-glucoside) | Choleretic and anticholestatic [124], antioxidant [125,126,127,128], anticholinesterase [125], antibacterial against multidrug-resistant clinical isolate strains [129], anti-inflammatory [128,130,131,132], antiallergic [132], inhibitor of monoamine oxidase B [133], inhibitor of low-density lipoprotein oxidation [134], antidiabetic [135,136,137], antidepressant [138], cytotoxic, anticancer [139,140,141,142,143,144], antimicrobial [89,145], antimutagenic [145], hepatoprotective [90], chondroprotective [146], CYP1A2 inhibitor [147], intestinal motility [148], retinal protective [149] |
| Apigenin-7-glucoside (19) | Antibacterial [150], antibacterial and antifungal [151], inhibition of α-amylase activity [152], anticandidal [153], cytotoxic, anticancer [142,153,154,155] |
| Homoplantaginin (20) (Hispidulin- 7-glucoside) | Antioxidant [156,157], antiproteasomal [158], collagenase, elastase and hyaluronidase enzyme inhibitory [159] |
3. Materials and Methods
3.1. General Experimental Procedures
The GC–FID analyses were carried out with capillary GC using an Agilent 6890N GC system (Agilent, Santa Clara, CA, USA), and the GC/MS analyses were performed on an Agilent 5975 GC–MSD system (Agilent, Santa Clara, CA, USA). An HP-Innowax FSC column (60 m × 0.25 mm, 0.25 μm film thickness, Agilent, Wilmington, DE, USA) was used for the analyses. The HPLC chromatographic separations were carried out using Shimadzu LC 20 System (Shimadzu, Tokyo, Japan). The mass spectra were recorded with AB Sciex 3200 Q TRAP mass spectrometer (AB Sciex, Toronto, Canada). GL Science Inertsil ODS 250 × 4.6 mm, 5 μm i.d. particle size, analytical column (GL Sciences, Tokyo, Japan) was used for the HPLC analyses. The turbidity of the standardized microbial sample solutions was measured using McFarland densitometer (Biosan McFarland Densitometer, Model Den-1B, Riga, Latvia). Antioxidant activity absorbances were recorded with a Biotek microplate reader (BioTek, Winooski, Vermont, USA). Chlorogenic acid, luteolin 7-glucoside, gallic acid, butylated hydroxytoluene (BHT), and L-ascorbic acid were purchased from Sigma-Aldrich (St. Louis, MO, USA).
3.2. Material
The plant material was collected (07 July 2012) near Mount Hasan in Aksaray, Turkey. A voucher specimen identified by Prof. Dr. H. Duman (Gazi University, Ankara) was deposited in the Herbarium of Gazi University (GAZI Nr. 9898000001568).
3.3. Extraction
Essential oil of the fruits of Ferula drudeana Korovin was obtained by hydrodistillation as described in Section 2.1 and subjected to GC–GC/MS analyses and antimicrobial testing. Air-dried and coarsely powdered fruits and roots (each 20 g) of F. drudeana were extracted in a Soxhlet extractor successively with petroleum ether (600 mL, 8 h), methylene chloride (600 mL, 8 h), and methanol (600 mL, 8 h). Each extract was concentrated in vacuo to remove the extraction solvent using a rotary evaporator and subjected to antimicrobial and antioxidant activities testing. The extract yields were as follows: fruit petroleum ether extract (F1) 1.916 g, fruit methylene chloride extract (F2) 0.265 g, fruit methanol extract (F3) 1.252 g, root petroleum ether extract (R1) 2.268 g, root methylene chloride extract (R2) 0.299 g, and root methanol extract (R3) 1.224 g.
3.4. Gas Chromatography–Gas Chromatography/Mass Spectrometry Analyses of Ferula drudeana Essential Oil
The oil was analyzed by capillary GC and GC/MS using an Agilent GC–MSD system (Agilent Technologies Inc., Santa Clara, CA, USA).
The GC/MS analysis was carried out with an Agilent 5975 GC-MSD system. Innowax FSC column (60 m × 0.25 mm, 0.25 μm film thickness) was used with helium as carrier gas (0.8 mL/min). GC oven temperature was kept at 60 °C for 10 min and programmed to 220 °C at a rate of 4 °C/min and kept constant at 220 °C for 10 min and then programmed to 240 °C at a rate of 1 °C/min. The split ratio was adjusted to 40:1. The injector temperature was set at 250 °C. MS were taken at 70 eV. The mass range was from m/z 35 to 450.
The GC analysis was carried out with an Agilent 6890N GC system fitted with a FID detector set at a temperature of 300 °C. To obtain the same elution order with GC/MS, simultaneous autoinjection was carried out on a duplicate of the same column applying the same operational conditions. Relative percentages of the separated compounds were calculated from FID chromatograms.
The components of essential oils were identified by comparing their mass spectra with those in the Baser Library of Essential Oil Constituents, Wiley GC/MS Library, Adams Library, and Mass Finder Library and confirmed by comparison of their retention indices. Alkanes were used as reference points to calculate the relative retention indices (RRI). Relative percentages of the separated compounds were calculated from FID chromatograms. The results of the analysis are shown in Table 1.
3.5. Tested Microorganisms and Standard Antimicrobial Agents
Escherichia coli NRRL B-3008, Pseudomonas aeruginosa ATCC 27853, Salmonella typhimurium ATCC 13311, Bacillus cereus NRRL B-3711, B. subtilis NRRL B-4378, Serratia marcescens NRRL B-2544, Staphylococcus epidermidis ATCC 12228, E. coli O157:H7 RSSK 234 (RSSK; RSHM National Type Culture Collection Strains of Bacteria), two different strains of Candida albicans (clinically isolated, Osmangazi University, Faculty of Medicine, Department of Microbiology and ATCC 90028), C. utilis NRRL Y-12968, C. krusei NRRL Y-7179, and C. glabrata (clinically isolated, Osmangazi University, Faculty of Medicine, Department of Microbiology and ATCC 90028) were used as the test microorganisms. Chloramphenicol (Merck, Rahway, NJ, USA), ampicillin (Merck), amphotericin-B (Sigma-Aldrich), and ketoconazole (Sigma-Aldrich) were used as standard antimicrobial agents.
3.6. Antimicrobial Activity
Antibacterial and antifungal activities of the samples were evaluated using slightly modified CLSI (formerly NCCLS) microdilution broth methods M7-A7 and M27-A2, respectively [160,161].
3.7. Antioxidant Activity
The antioxidant activity of Ferula drudeana essential oil and extracts were evaluated by DPPH• TLC spot testing assay, the major antioxidant compounds of the active extracts were identified by online HPLC–ABTS•+ screening assay coupled with HPLC–UV–MS/MS system, and the antioxidant potential of methanolic extracts of F. drudeana was determined with DPPH and ABTS radical scavenging activity testing.
3.7.1. DPPH• TLC Spot Testing
The DPPH• TLC spot testing method was used to find the active antioxidant extracts. The extract and essential oil solutions (10 μL of 1 mg/mL) of Ferula drudeana were spotted on a silica gel TLC plate, then 2.54 mM DPPH–methanol solution was sprayed using a Camag TLC sprayer, and the results were evaluated after 30 min. Spots with the DPPH solution scavenging activity were observed as white-yellow spots on a purple background (Figure S1, Supplementary Material) [36,37].
3.7.2. HPLC–ABTS•+ Derivatization
Online HPLC–ABTS screening and HPLC–UV–MS/MS method were used, and the extract’s active compounds were identified using the method developed by Koleva and He [30,162]. HPLC coupled with ABTS assay was performed using a stock solution containing 3.5 mM potassium persulphate, and 2 mM ABTS was prepared and kept at room temperature in darkness for 16 h to stabilize the radical. The radical reagent was prepared by diluting the stock solution with pure water to an absorbance of 0.70 ± 0.02 at 734 nm. The extracts (at 10 mg/mL concentration, 10 µL) were injected into a Shimadzu HPLC system. HPLC separation was carried out as described in the previous section. HPLC analytes from the column arrived at a T-junction, where the ABTS reagent was added. A Shimadzu reagent pump delivered the ABTS reagent at a 0.7 mL/min flow rate. After the analytes were mixed with ABTS reagent in a reaction coil (15 m–0.25 mm i.d. PEEK tubing), DAD measured the negative peaks at 734 nm. Data were analyzed using LC Solution Software.
3.7.3. LC–MS/MS Analysis
AbSciex 3200 MS/MS detector was used for LC–MS/MS analysis. A negative ionization mode was preferred for ionization. Chromatographic separations were carried out with GL science Inertsil ODS 250 × 4.6 mm, i.d., 5 µm column using Shimadzu 20A HPLC. The column oven temperature was set to 40 °C, and the flow rate was adjusted to 1 mL/minute. Mobile phases were (A) methanol: water: formic acid (10:89:1, v/v/v) and (B) methanol: water: formic acid (89:10:1, v/v/v). The B concentration increased from 15% to 40% in 15 min, then increased to 45% within 3 min; it was at 45% B at 12 min, then increased to 75% within 5 min and 100% within 5 min. For mass scanning (EMS), a mass range of 100–1000 amu was chosen.
3.7.4. Determination of the Antioxidant Potential of Methanolic Extracts
DPPH Radical Scavenging Activity
Using a modified version of the Brand-Williams method [163,164], the DPPH radical scavenging capacity of the fruit and root methanolic extracts, standards, and positive control standards were assessed.
Solutions of the methanolic extracts of fruits and roots of F. drudeana (1.25 mg/mL), two standard compounds (0.1 mg/mL of each luteolin-7-glucoside and chlorogenic acid), and three positive control standards (0.1 mg/mL of each gallic acid and ascorbic acid and 1 mg/mL BHT) in methanol were prepared. In 96-well flat-bottom plates, 100 μL of the sample solutions (extracts, standards, and positive control standards) were serially diluted with 100 μL of methanol. Then, diluted samples were mixed with 100 μL of DPPH solution (0.08 mg/mL in methanol). As a control, 100 μL of methanol and 100 μL of DPPH solution were combined. The mixtures were kept in the dark for 30 min. Absorbance of each well was recorded at 517 nm. The percentage of inhibition was calculated using the following equation:
| % Inhibition = [(Acontrol − Asample)/Acontrol] × 100 |
where Acontrol is the absorbance of the solution that contains all reagents with the exception of extract or standard chemical. The 50% inhibition concentration (IC50) values of DPPH radical of each sample were calculated using SigmaPlot (Version 12.0).
ABTS Radical Scavenging Activity
ABTS radical scavenging test was performed following the method described by Re et al. [165] with slight modification. In order to produce the ABTS•+ free radical cation, 7 mM ABTS and 2.5 mM K2S2O8 were dissolved in 10 mL ultrapure water and incubated at room temperature for 16 h in the dark. The ABTS•+ solution was diluted with 100% ethanol prior to the experiment to obtain an absorbance of 0.7–0.8 at 734 nm. Solutions were prepared for extracts (0.1–1 mg/mL), standards (0.1–1 mg/mL), and Trolox (3.0, 2.0, 1.0, 0.5, 0.25, and 0.125 mM). During the experiment, 990 μL of ABTS•+ solution was combined with 10 μL of the sample (extract/standard) solution, and the combined mixtures were allowed to incubate for 30 min at room temperature in the dark. Trolox equivalent antioxidant capacity (TEAC) of the extracts and standards were calculated using the linear equation obtained for Trolox (y = 20.227x + 4.1663 R2_ = 0.9968).
4. Conclusions
This is the first report on the bioactivities of terpenoids present in the essential oil and major phenolic compounds of methanolic extracts of Ferula drudeana. The fruit essential oil of F. drudeana was analyzed by GC–MS, and 28 terpenoid compounds were identified. About 75% of the essential oil consisted of oxygenated sesquiterpene compounds. The presence of unusually high levels of shyobunone derivatives with reported anti-Alzheimer and neuroprotective activities in the essential oil of F. drudeana clearly highlights the medicinal value of this species. Although the antimicrobial activity of the fruit essential oil of F. drudeana was weak to moderate, the presence of strong anticandidal activity in the fruit and root petroleum ether extracts suggested the source of anticandidal activity was probably the nonvolatile component(s) of this extract. The results of DPPH•· TLC spot testing, online HPLC–ABTS•+ screening assay, and DPPH/ABTS radical scavenging tests indicated that F. drudeana was rich in polyphenolic compounds and exhibited good antioxidant activity. Three flavonoid glucosides and five hydroxycoumaric acid esters of quinic acid were determined in the methanol extracts of the fruits and roots of F. drudeana by HPLC–MS/MS analyses, and their antioxidant activities were detected simultaneously by online coupled HPLC–ABTS•+ based assay. Furthermore, based on the literature survey, known biological activities of the major essential oil terpenoids and methanolic extract phenolic compounds of F. drudeana were reviewed to confirm the medicinal values of F. drudeana secondary metabolites.
Acknowledgments
We thank H. Duman (Gazi University, Ankara, Turkey) for the identification of plant material and John A. Beutler for his invaluable comments on the manuscript.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants12040830/s1, Figure S1: The DPPH• reagent evaluation of the essential oil and extracts of Ferula drudeana; Figure S2: LC-MS/MS spectrum of 5-caffeoylquinic acid (13, chlorogenic acid); Figure S3: LC-MS/MS spectrum of 1,5-dicaffeoylquinic acid (16, cynarine); Figure S4: LC-MS/MS spectrum of Luteolin 7-glucoside (18, cynaroside).
Author Contributions
Conceptualization, F.T. and M.M.; methodology, M.M., M.K. and G.İ., F.G.; formal analysis, M.K., G.İ., F.G. and F.K.K.; investigation, F.T., M.M., M.K., G.İ., F.G. and F.K.K.; resources, F.T., M.M., M.K., G.İ. and F.G.; data curation, M.K., G.İ., F.G. and F.K.K.; writing—original draft preparation, F.T. and M.M.; writing—review and editing, F.T., M.M., M.K., G.İ. and F.G.; project administration, M.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The Supplementary Materials for the DPPH• TLC spot testing and LC-MS/MS analyses of chloro-genic acid, cynarine and cynaroside are available online.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.POWO Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Published on the Internet. [(accessed on 31 December 2022)]. Available online: https://powo.science.kew.org/taxon/30105171-2#publications.
- 2.Peşmen H. Ferula, Flora of Turkey and the East Aegean Islands. Volume 4. Edinburgh University Press; Edinburgh, UK: 1972. pp. 440–453. [Google Scholar]
- 3.Pimenov M., Leonov M. The Asian Umbelliferae biodiversity database (ASIUM) with particular reference to South-West Asian taxa. [(accessed on 7 February 2023)];Turk. J. Bot. 2004 28:139–145. Available online: https://journals.tubitak.gov.tr/botany/vol28/iss1/13. [Google Scholar]
- 4.Akalın E., Tuncay H.O., Olcay B., Miski M. A new Ferula (Apiaceae) species from Southwest Anatolia: Ferula pisidica Akalın & Miski. Plants. 2020;9:740. doi: 10.3390/plants9060740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohammadhosseini M., Venditti A., Sarker S.D., Nahar L., Akbarzadeh A. The genus Ferula: Ethnobotany, phytochemistry and bioactivities—A review. Ind. Crop. Prod. 2019;129:350–394. doi: 10.1016/j.indcrop.2018.12.012. [DOI] [Google Scholar]
- 6.Gunther R.T. The Greek Herbal of Dioscorides. Hafner Publishing Company; London, UK: New York, NY, USA: 1968. [Google Scholar]
- 7.Korovin E.P. Generis Ferula (Tourn.) L. monographia Illustrata. Academiae Scientiarum UzRSS; Tashkent, Uzbekistan: 1947. [Google Scholar]
- 8.Miski M. Next chapter in the legend of silphion: Preliminary morphological, chemical, biological and pharmacological evaluations, initial conservation studies, and reassessment of the regional extinction event. Plants. 2021;10:102. doi: 10.3390/plants10010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bostock J., Riley H.T. Pliny the Elder, Natural History; Collected Works of Pliny the Elder. Delphi Publishing Ltd.; East Sussex, UK: 2015. Book XXII, The Properties of Plants and Fruits, Chapter 49; Laser: Thirty-nine remedies. Delphi Classics, Hastings. [Google Scholar]
- 10.Hort A.F. Theophrastus, Enquiry into Plants; Collected Works of Theophrastus. Delphi Publishing Ltd.; East Sussex, UK: 2019. Book VI of Under-Shrubs [3.2–3.4] Delphi Classics, Hastings. [Google Scholar]
- 11.French D.H. The Biology and Chemistry of the Umbelliferae. Academic Press Inc.; London, UK: 1971. Ethnobotany of the Umbelliferae; pp. 385–412. [Google Scholar]
- 12.Totelin L.M.V. Hippocratic Recipes, Oral and Written Transmission of Pharmacological Knowledge in Fifth- and Fourth-Century Greece. Brill; Leiden, The Netherlands: 2009. pp. 158–161. [Google Scholar]
- 13.Süzgeç-Selçuk S., Özek G., Meriçli A.H., Baser K.H.C., Haliloglu Y., Özek T. Chemical and biological diversity of the leaf and rhizome volatiles of Acorus calamus L. from Turkey. J. Essent. Oil Bear. Plants. 2017;20:646–661. doi: 10.1080/0972060X.2017.1331142. [DOI] [Google Scholar]
- 14.Babushok V.I., Linstrom P.J., Zenkevich I.G. Retention indices for frequently reported compounds of plant essential oils. J. Phys. Chem. Ref. Data. 2011;40:043101-1–043101-47. doi: 10.1063/1.3653552. [DOI] [Google Scholar]
- 15.Demirci F., Demirci B., Gurbuz I., Yeşilada E., Başer K.H.C. Characterization and biological activity of Achillea teretifolia Willd. and A. nobilis L. subsp. neilreichii (Kerner) Formanek Essential Oils. Turk. J. Biol. 2009;33:129–136. doi: 10.3906/biy-0808-1. [DOI] [Google Scholar]
- 16.Demirci B., Koltuksuz Yasdikcıoglu G., Baser K.H.C. Sesquiterpene hydrocarbons of the essential oil of Actinolema macrolema Boiss. Turk. J. Chem. 2013;37:917–926. doi: 10.3906/kim-1103-24. [DOI] [Google Scholar]
- 17.Ozek G., Chidibayeva A., Ametov A., Nurmahanova A., Ozek T. Chemical composition of flower volatiles and seeds fatty acids of Rosa iliensis Chrshan, an endemic species from Kazakhstan. Rec. Nat. Prod. 2022;16:225–235. doi: 10.25135/rnp.271.2105.2083. [DOI] [Google Scholar]
- 18.Merle H., Verdeguer M., Blázquez M.A., Boira H. Chemical composition of the essential oils from Eriocephalus africanus L. var. africanus populations growing in Spain. Flav. Fragr. J. 2007;22:461–464. doi: 10.1002/ffj.1821. [DOI] [Google Scholar]
- 19.10-Epijunenol (Compound) [(accessed on 31 December 2022)]; Available online: https://pubchem.ncbi.nlm.nih.gov/compound/573015#section=Experimental-Properties.
- 20.Baser K.H.C., Demirci B., Sagiroglu M., Duman H. Essential oil of Ferula species of Turkey; Proceedings of the 38th International Symposium on Essential Oils; Graz, Austria. 9–12 September 2007. [Google Scholar]
- 21.Martins R.M., Xavier-Júnior F.H., Barros M.R., Menezes T.M., de Assis C.R., de Melo A.C.G., Veras B.O., Ferraz V.P., Filho A.A.M., Yogui G.T., et al. Impact on cholinesterase-inhibition and in silico investigations of sesquiterpenoids from Amazonian Siparuna guianensis Aubl. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021;252:119511. doi: 10.1016/j.saa.2021.119511. [DOI] [PubMed] [Google Scholar]
- 22.Rossi P.G., Bao L., Luciani A., Panighi J., Desjobert J.M., Costa J., Casanova J., Bolla J.-M., Berti L. (E)-Methylisoeugenol and elemicin: Antibacterial components of Daucus carota L. essential oil against Campylobacter jejuni. J. Agric. Food Chem. 2007;55:7332–7336. doi: 10.1021/jf070674u. [DOI] [PubMed] [Google Scholar]
- 23.Yamamura S., Iguchi A., Nishiyama A., Niwa M., Koyama H., Hirata Y. Sesquiterpenes from Acorus calamus L. Tetrahedron. 1971;27:5419–5431. doi: 10.1016/S0040-4020(01)91707-7. [DOI] [Google Scholar]
- 24.Garneau F.X., Collin G., Gagnon H., Bélanger A., Lavoie S., Savard N., Pichette A. Aromas from Quebec. I. Composition of the essential oil of the rhizomes of Acorus calamus L. J. Essent. Oil Res. 2008;20:250–254. doi: 10.1080/10412905.2008.9700004. [DOI] [Google Scholar]
- 25.Znati M., Jabrane A., Hajlaoui H., Harzallah-Skhiri F., Bouajila J., Casanova J., Jannet H.B. Chemical composition and in vitro evaluation of antimicrobial and anti-acetylcholinesterase properties of the flower oil of Ferula lutea. Nat. Prod. Comm. 2012;7:947–950. doi: 10.1177/1934578X1200700738. [DOI] [PubMed] [Google Scholar]
- 26.Zellagui A., Gherraf N., Hegazy M., Akkal S., Rhouati S., Dendougui H. Phytochemical investigation and antimicrobial activity of crude extract of the roots of Ferula vesceritensis. Chem. Nat. Compd. 2012;48:891–892. doi: 10.1007/s10600-012-0414-y. [DOI] [Google Scholar]
- 27.Pavlović I., Petrović S., Radenković M., Milenković M., Couladis M., Branković S., Drobac M.P., Niketić M. Composition, antimicrobial, antiradical and spasmolytic activity of Ferula heuffelii Griseb. ex Heuffel (Apiaceae) essential oil. Food Chem. 2012;130:310–315. doi: 10.1016/j.foodchem.2011.07.043. [DOI] [Google Scholar]
- 28.Özek G., Özek T., Işcan G., Başer K.H.C., Duran A., Hamzaoglu E. Composition and antimicrobial activity of the oils of Ferula szowitsiana DC. from Turkey. J. Essent. Oil Res. 2008;20:186–190. doi: 10.1080/10412905.2008.9699987. [DOI] [Google Scholar]
- 29.Maggi F., Cecchini C., Cresci A., Coman M.M., Tirillini B., Sagratini G., Papa F. Chemical composition and antimicrobial activity of the essential oil from Ferula glauca L. (F. communis L. subsp. glauca) growing in Marche (central Italy) Fitoterapia. 2009;80:68–72. doi: 10.1016/j.fitote.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Kavoosi G., Rowshan V. Chemical composition, antioxidant and antimicrobial activities of essential oil obtained from Ferula assa-foetida oleo-gum-resin: Effect of collection time. Food Chem. 2013;138:2180–2187. doi: 10.1016/j.foodchem.2012.11.131. [DOI] [PubMed] [Google Scholar]
- 31.Iranshahi M., Hassanzadeh-Khayat M., Bazzaz B.S.F., Sabeti Z., Enayati F. High content of polysulphides in the volatile oil of Ferula latisecta Rech. F. et Aell. fruits and antimicrobial activity of the oil. J. Essent. Oil Res. 2008;20:183–185. doi: 10.1080/10412905.2008.9699986. [DOI] [Google Scholar]
- 32.Ibraheim Z.Z., Abdel-Mageed W.M., Dai H., Guo H., Zhang L., Jaspars M. Antimicrobial antioxidant daucane sesquiterpenes from Ferula hermonis Boiss. Phytother. Res. 2012;26:579–586. doi: 10.1002/ptr.3609. [DOI] [PubMed] [Google Scholar]
- 33.Asili J., Sahebkar A., Bazzaz B.S.F., Sharifi S., Iranshahi M. Identification of essential oil components of Ferula badrakema fruits by GC-MS and 13C-NMR methods and evaluation of its antimicrobial activity. J. Essent. Oil-Bear. Plants. 2009;12:7–15. doi: 10.1080/0972060X.2009.10643685. [DOI] [Google Scholar]
- 34.Al-Yahya M.A., Muhammad I., Mirza H.H., El-Feraly F.S. Antibacterial constituents from the rhizomes of Ferula communis. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 1998;12:335–339. doi: 10.1002/(SICI)1099-157312:53.0.CO;2-H. [DOI] [Google Scholar]
- 35.Samaranayake Y.H., Samaranayake L. Candida krusei: Biology, epidemiology, pathogenicity and clinical manifestations of an emerging pathogen. J. Med. Microbiol. 1994;41:295–310. doi: 10.1099/00222615-41-5-295. [DOI] [PubMed] [Google Scholar]
- 36.Kumar S., Sandhir R., Ojha S. Evaluation of antioxidant activity and total phenol in different varieties of Lantana camara leaves. BMC Res. Notes. 2014;7:560. doi: 10.1186/1756-0500-7-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sathuvan M., Vignesh A., Thangam R., Palani P., Rengasamy R., Murugesan K. In vitro antioxidant and anticancer potential of bark of Costus pictus D. Don. Asian Pac. J. Trop. Biomed. 2012;2:S741–S749. doi: 10.1016/S2221-1691(12)60307-4. [DOI] [Google Scholar]
- 38.He W., Liu X., Xu H., Gong Y., Yuan F., Gao Y. On-line HPLC-ABTS screening and HPLC-DAD-MS/MS identification of free radical scavengers in Gardenia (Gardenia jasminoides Ellis) fruit extracts. Food Chem. 2010;123:521–528. doi: 10.1016/j.foodchem.2010.04.030. [DOI] [Google Scholar]
- 39.Gouveia S., Goncalves J., Castilho P.C. Characterization of phenolic compounds and antioxidant activity of ethanolic extracts from flowers of Andryala glandulosa ssp varia (Lowe ex DC.) R.Fern., an endemic species of Macaronesia region. Ind. Crop. Prod. 2013;42:573–582. doi: 10.1016/j.indcrop.2012.06.040. [DOI] [Google Scholar]
- 40.Clifford M.N., Johnston K.L., Knight S., Kuhnert N. Hierarchical scheme for LC-MS n identification of chlorogenic acids. J. Agric. Food Chem. 2003;51:2900–2911. doi: 10.1021/jf026187q. [DOI] [PubMed] [Google Scholar]
- 41.Clifford M.N., Knight S., Kuhnert N. Discriminating between the six isomers of dicaffeoylquinic acid by LC-MS. J. Agric. Food Chem. 2005;53:3821–3832. doi: 10.1021/jf050046h. [DOI] [PubMed] [Google Scholar]
- 42.Kontogianni V.G., Tomic G., Nikolic I., Nerantzaki A.A., Sayyad N., Stosic-Grujicic S., Stojanovic I., Gerothanassis I.P., Tzakos A.G. Phytochemical profile of Rosmarinus officinalis and Salvia officinalis extracts and correlation to their antioxidant and anti-proliferative activity. Food Chem. 2013;136:120–129. doi: 10.1016/j.foodchem.2012.07.091. [DOI] [PubMed] [Google Scholar]
- 43.Topdas E.F., Sengul M., Taghizadehghalehjoughi A., Hacimuftuoglu A. Neuroprotective potential and antioxidant activity of various solvent extracts and essential oil of Ferula orientalis L. J. Essent. Oil-Bear Plants. 2020;23:121–138. doi: 10.1080/0972060X.2020.1729247. [DOI] [Google Scholar]
- 44.Romo Vaquero M., Yáñez-Gascón M.J., Garcia Villalba R., Larrosa M., Fromentin E., Ibarra A., Roller M., Tomas-Barberan F., de Gea J.C.E., García-Conesa M.T. Inhibition of gastric lipase as a mechanism for body weight and plasma lipids reduction in Zucker rats fed a rosemary extract rich in carnosic acid. PLoS ONE. 2012;7:e39773. doi: 10.1371/journal.pone.0039773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kızıltaş H., Gören A., Bingol Z., Alwasel S., Gülçin İ. Anticholinergic, antidiabetic and antioxidant activities of Ferula orientalis L. determination of its polyphenol contents by LC-HRMS. Rec. Nat. Prod. 2021;15:513–528. doi: 10.25135/rnp.236.21.02.1983. [DOI] [Google Scholar]
- 46.Rahali F.Z., Lamine M., Rebey I.B., Wannes W.A., Hammami M., Selmi S., Mliki A., Sellami I.H. Biochemical characterization of fennel (Ferula communis L.) different parts through their essential oils, fatty acids and phenolics. Acta Sci. Pol. Hortorum Cultus. 2021;20:3–14. doi: 10.24326/asphc.2021.1.1. [DOI] [Google Scholar]
- 47.Rahali F.Z., Kefi S., Bettaieb Rebey I., Hamdaoui G., Tabart J., Kevers C., Franck T., Mouithys-Mickalad A., Hamrouni Sellami I. Phytochemical composition and antioxidant activities of different aerial parts extracts of Ferula communis L. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2019;153:213–221. doi: 10.1080/11263504.2018.1461696. [DOI] [Google Scholar]
- 48.Pavlović I., Radenković M., Branković S., Milenković M.T., Niketić M., Ušjak L., Petrović S. Spasmolytic, gastroprotective and antioxidant activities of dry methanol extract of Ferula heuffelii underground parts. Chem. Biodivers. 2022;19:e202200047. doi: 10.1002/cbdv.202200047. [DOI] [PubMed] [Google Scholar]
- 49.Kahraman C., Baysal I., Çankaya I., Goger F., Kirimer N., Akdemir Z. Acetylcholinesterase inhibitory activities and LC-MS analysis of the antioxidant Ferula caspica M. Bieb. and F. halophila Pesmen extracts. J. Res. Pharm. 2019;23:543–551. doi: 10.12991/jrp.2019.161. [DOI] [Google Scholar]
- 50.Chen X., Liu Q. Luteolin glycosides as taxonomic markers in Ferula and related genera. Biochem. Syst. Ecol. 1989;17:309–310. doi: 10.1016/0305-1978(89)90008-2. [DOI] [Google Scholar]
- 51.Rivas da Silva A.C., Lopes P.M., Barros de Azevedo M.M., Costa D.C., Alviano C.S., Alviano D.S. Biological activities of α-pinene and β-pinene enantiomers. Molecules. 2012;17:6305–6316. doi: 10.3390/molecules17066305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.İşcan G. Antibacterial and anticandidal activities of common essential oil constituents. Rec. Nat. Prod. 2017;11:374–388. [Google Scholar]
- 53.de Macedo Andrade A.C., Rosalen P.L., Freires I.A., Scotti L., Scotti M.T., Aquino S.G., de Castro R.D. Antifungal activity, mode of action, docking prediction and anti-biofilm effects of (+)-beta-pinene enantiomers against Candida spp. Curr. Top. Med. Chem. 2018;18:2481–2490. doi: 10.2174/1568026618666181115103104. [DOI] [PubMed] [Google Scholar]
- 54.Chowhan N., Singh H.P., Batish D.R., Kaur S., Ahuja N., Kohli R.K. β-Pinene inhibited germination and early growth involves membrane peroxidation. Protoplasma. 2013;250:691–700. doi: 10.1007/s00709-012-0446-y. [DOI] [PubMed] [Google Scholar]
- 55.Guzmán-Gutiérrez S.L., Bonilla-Jaime H., Gómez-Cansino R., Reyes-Chilpa R. Linalool and β-pinene exert their antidepressant-like activity through the monoaminergic pathway. Life Sci. 2015;128:24–29. doi: 10.1016/j.lfs.2015.02.021. [DOI] [PubMed] [Google Scholar]
- 56.Machado T.Q., Felisberto J.R.S., Guimarães E.F., Queiroz G.A.D., Fonseca A.C.C.D., Ramos Y.J., Marques A.M., Moreira D.L., Robbs B.K. Apoptotic effect of β-pinene on oral squamous cell carcinoma as one of the major compounds from essential oil of medicinal plant Piper rivinoides Kunth. Nat. Prod. Res. 2015;36:1636–1640. doi: 10.1080/14786419.2021.1895148. [DOI] [PubMed] [Google Scholar]
- 57.Salehi B., Upadhyay S., Erdogan Orhan I., Jugran A.K., Jayaweera S.L.D., Dias D.A., Sharopov F., Taheri Y., Martins N., Baghalpour N., et al. Therapeutic potential of α-and β-pinene: A miracle gift of nature. Biomolecules. 2019;9:738. doi: 10.3390/biom9110738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Felipe C.F.B., Albuquerque A.M.S., de Pontes J.L.X., de Melo J.Í.V., Rodrigues T.C.M.L., de Sousa A.M.P., Monteiro A.B., da Silva Ribeiro A.E., Lopes J.P., de Menezes I.R.A., et al. Comparative study of alpha-and beta-pinene effect on PTZ-induced convulsions in mice. Fundam. Clin. Pharmacol. 2019;33:181–190. doi: 10.1111/fcp.12416. [DOI] [PubMed] [Google Scholar]
- 59.Türkez H., Çelik K., Toğar B. Effects of copaene, a tricyclic sesquiterpene, on human lymphocytes cells in vitro. Cytotechnology. 2014;66:597–603. doi: 10.1007/s10616-013-9611-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Turkez H., Togar B., Tatar A., Geyikoglu F., Hacımuftuoglu A. Cytotoxic and cytogenetic effects of α-copaene on rat neuron and N2a neuroblastoma cell lines. Biologia. 2014;69:936–942. doi: 10.2478/s11756-014-0393-5. [DOI] [Google Scholar]
- 61.Nishida R., Shelly T.E., Whittier T.S., Kaneshiro K.Y. α-Copaene, a potential rendezvous cue for the Mediterranean fruit fly, Ceratitis capitata. J. Chem. Ecol. 2000;26:87–100. doi: 10.1023/A:1005489411397. [DOI] [Google Scholar]
- 62.Chavan M.J., Wakte P.S., Shinde D.B. Analgesic and anti-inflammatory activities of the sesquiterpene fraction from Annona reticulata L. bark. Nat. Prod. Res. 2012;26:1515–1518. doi: 10.1080/14786419.2011.564583. [DOI] [PubMed] [Google Scholar]
- 63.Dong R., Chen X., Wu T., Liu G.J. Elemene for the treatment of lung cancer. Cochrane Database Syst. Rev. 2007:CD005054. doi: 10.1002/14651858.CD006054.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li Q.Q., Lee R.X., Liang H., Zhong Y. Anticancer activity of β-elemene and its synthetic analogs in human malignant brain tumor cells. Anticancer Res. 2013;33:65–76. [PMC free article] [PubMed] [Google Scholar]
- 65.Guan C., Liu W., Yue Y., Jin H., Wang X., Wang X.J. Inhibitory effect of β-elemene on human breast cancer cells. Int. J. Clin. Exp. Pathol. 2014;7:3948. [PMC free article] [PubMed] [Google Scholar]
- 66.Wang J., Xu C., Chen Y., Shao L., Li T., Fan X., Yu L., Zhang R., Chen B., Chen H., et al. β-elemene enhances the antitumor activity of erlotinib by inducing apoptosis through AMPK and MAPK pathways in TKI-resistant H1975 lung cancer cells. [(accessed on 7 February 2023)];J. Cancer. 2021 12:2285–2294. doi: 10.7150/jca.53382. Available online: https://www.jcancer.org/v12p2285.htm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen X., Huang C., Li K., Liu J., Zheng Y., Feng Y., Kai G.Y. Recent advances in biosynthesis and pharmacology of β-elemene. Phytochem. Rev. 2022:1–18. doi: 10.1007/s11101-022-09833-0. [DOI] [Google Scholar]
- 68.Zhai B., Zeng Y., Zeng Z., Zhang N., Li C., Zeng Y., Yu Y., Wang S., Chen X., Sui X., et al. Drug delivery systems for elemene, its main active ingredient β-elemene, and its derivatives in cancer therapy. Int. J. Nanomed. 2018;13:6279. doi: 10.2147/IJN.S174527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yuan R., Zhang D., Yang J., Wu Z., Luo C., Han L., Yang F., Lin J., Yang M. Review of aromatherapy essential oils and their mechanism of action against migraines. J. Ethnopharmacol. 2021;265:113326. doi: 10.1016/j.jep.2020.113326. [DOI] [PubMed] [Google Scholar]
- 70.Casiglia S., Bruno M., Bramucci M., Quassinti L., Lupidi G., Fiorini D., Maggi F. Kundmannia sicula (L.) DC: A rich source of germacrene D. J. Essent. Oil Res. 2017;29:437–442. doi: 10.1080/10412905.2017.1338625. [DOI] [Google Scholar]
- 71.Røstelien T., Borg-Karlson A.K., Fäldt J., Jacobsson U., Mustaparta H. The plant sesquiterpene germacrene D specifically activates a major type of antennal receptor neuron of the tobacco budworm moth Heliothis virescens. Chem. Senses. 2000;25:141–148. doi: 10.1093/chemse/25.2.141. [DOI] [PubMed] [Google Scholar]
- 72.Zamora C.M.P., Torres C.A., Nufiez M.B. Antimicrobial activity and chemical composition of essential oils from Verbenaceae species growing in South America. Molecules. 2018;23:544. doi: 10.3390/molecules23030544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Govindarajan M., Benelli G. Eco-friendly larvicides from Indian plants: Effectiveness of lavandulyl acetate and bicyclogermacrene on malaria, dengue and Japanese encephalitis mosquito vectors. Ecotoxicol. Environ. Saf. 2016;133:395–402. doi: 10.1016/j.ecoenv.2016.07.035. [DOI] [PubMed] [Google Scholar]
- 74.Xu Y., Wu B., Cao X., Zhang B., Chen K. Citrus CmTPS1 is associated with formation of sesquiterpene bicyclogermacrene. Sci. Hortic. 2017;226:133–140. doi: 10.1016/j.scienta.2017.08.032. [DOI] [Google Scholar]
- 75.Kazemi M., Rostami H. Chemical composition, antimicrobial and antioxidant activities of the essential oil of Psammogeton canescens. Nat. Prod. Res. 2015;29:277–280. doi: 10.1080/14786419.2014.951357. [DOI] [PubMed] [Google Scholar]
- 76.Yeo S.K., Ali A.Y., Hayward O.A., Turnham D., Jackson T., Bowen I.D., Clarkson R. β-Bisabolene, a sesquiterpene from the essential oil extract of opoponax (Commiphora guidottii), exhibits cytotoxicity in breast cancer cell lines. Phytother. Res. 2016;30:418–425. doi: 10.1002/ptr.5543. [DOI] [PubMed] [Google Scholar]
- 77.de Almeida Júnior J.S., da Silva É.B.S., Moraes T.M.P., Kasper A.A.M., Sartoratto A., Baratto L.C., de Oliveira E.C.P., Oliveira E., Barata L.E.S., Minervino A.H.H., et al. Anti-Inflammatory potential of the oleoresin from the Amazonian tree Copaifera reticulata with an unusual chemical composition in rats. Vet. Sci. 2021;8:320. doi: 10.3390/vetsci8120320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.He X., Zhang L., Chen J., Sui J., Yi G., Wu J., Ma Y. Correlation between chemical composition and antifungal activity of Clausena lansium essential oil against Candida spp. Molecules. 2019;24:1394. doi: 10.3390/molecules24071394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen H.P., Yang K., Zheng L.S., You C.X., Cai Q., Wang C.F. Repellant and insecticidal activities of shyobunone and isoshyobunone derived from the essential oil of Acorus calamus rhizomes. [(accessed on 7 February 2023)];Pharmacogn. Mag. 2015 11:675–681. doi: 10.4103/0973-1296.165543. Available online: https://www.phcog.com/text.asp?2015/11/44/675/165543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hindu M., Bhavana Harendra H., Jain A.S., Srinivasa C., Chandrappa K.G., Shreevatsa B., Pradeep S., Dharmashekara C., Kollur S.P., Deepak T.S., et al. Computational approach assessing the antibacterial activity of Acorus calamus against Helicobacter pylori. Lett. Appl. NanoBioSci. 2022;12:91. doi: 10.33263/LIANBS124.091. [DOI] [Google Scholar]
- 81.Ramazani E., Akaberi M., Emami S.A., Tayarani-Najaran Z. Pharmacological and biological effects of alpha-bisabolol: An updated review of the molecular mechanisms. Life Sci. 2022;21:120728. doi: 10.1016/j.lfs.2022.120728. [DOI] [PubMed] [Google Scholar]
- 82.Kamatou G.P., Viljoen A.M. A review of the application and pharmacological properties of α-bisabolol and α-bisabolol-rich oils. JAOCS. 2010;87:1–7. doi: 10.1007/s11746-009-1483-3. [DOI] [Google Scholar]
- 83.Rigo A., Ferrarini I., Lorenzetto E., Darra E., Liparulo I., Bergamini C., Sissa C., Cavalieri E., Vinante F. BID and the α-bisabolol-triggered cell death program: Converging on mitochondria and lysosomes. Cell Death Dis. 2019;10:1–13. doi: 10.1038/s41419-019-2126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rocha N.F.M., de Oliveira G.V., de Araújo F.Y.R., Rios E.R.V., Carvalho A.M.R., Vasconcelos L.F., Macedo D.S., Soares P.M.G., de Sousa D.P., de Sousa F.C.F. (−)-α-Bisabolol-induced gastroprotection is associated with reduction in lipid peroxidation, superoxide dismutase activity and neutrophil migration. Eur. J. Pharm. Sci. 2011;44:455–461. doi: 10.1016/j.ejps.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 85.Sampaio T.L., de Menezes R.R.P.P.B., da Costa M.F.B., Meneses G.C., Arrieta M.C.V., Filho A.J.M.C., de Morais G.B., Libório A.B., Alves R.S., Evangelista J.S.A.M., et al. Nephroprotective effects of (−)-α-bisabolol against ischemic-reperfusion acute kidney injury. Phytomedicine. 2016;23:1843–1852. doi: 10.1016/j.phymed.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 86.Muñoz-Pérez V.M., Ortiz M.I., Ponce-Monter H.A., Monter-Pérez V., Barragán-Ramírez G. Anti-inflammatory and utero-relaxant effect of α-bisabolol on the pregnant human uterus. Korean J. Physiol. Pharmacol. 2018;22:391–398. doi: 10.4196/kjpp.2018.22.4.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hajaji S., Sifaoui I., López-Arencibia A., Reyes-Batlle M., Jiménez I.A., Bazzocchi I.L., Piñero J.E. Leishmanicidal activity of α-bisabolol from Tunisian chamomile essential oil. Parasitol. Res. 2018;117:2855–2867. doi: 10.1007/s00436-018-5975-7. [DOI] [PubMed] [Google Scholar]
- 88.Seki T., Kokuryo T., Yokoyama Y., Suzuki H., Itatsu K., Nakagawa A., Mizatuni T., Miyake T., Uno M., Yamauchi K., et al. Antitumor effects of α-bisabolol against pancreatic cancer. Cancer Sci. 2011;102:2199–2205. doi: 10.1111/j.1349-7006.2011.02082.x. [DOI] [PubMed] [Google Scholar]
- 89.Zhu X., Zhang H., Lo R. Phenolic compounds from the leaf extract of artichoke (Cynara scolymus L.) and their antimicrobial activities. J. Agric. Food Chem. 2004;52:7272–7278. doi: 10.1021/jf0490192. [DOI] [PubMed] [Google Scholar]
- 90.Adzet T., Camarasa J., Laguna J.C. Hepatoprotective activity of polyphenolic compounds from Cynara scolymus against CCl4 toxicity in isolated rat hepatocytes. J. Nat. Prod. 1987;50:612–617. doi: 10.1021/np50052a004. [DOI] [PubMed] [Google Scholar]
- 91.Hakkou Z., Maciuk A., Leblais V., Bounani N.E., Mekhfi H., Bnouham M., Aziz M., Ziyyat A., Rauf A., Hadda T.B., et al. Antihypertensive and vasodilator effects of methanolic extract of Inula viscosa: Biological evaluation and POM analysis of cynarin, chlorogenic acid as potential hypertensive. Biomed. Pharmacother. 2017;93:62–69. doi: 10.1016/j.biopha.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 92.Kirchhoff R., Beckers C., Kirchhoff G.M., Trinczek-Gärtner H., Petrowicz O., Reimann H.J. Increase in choleresis by means of artichoke extract. Phytomedicine. 1994;1:107–115. doi: 10.1016/S0944-7113(11)80027-9. [DOI] [PubMed] [Google Scholar]
- 93.Li X., Li K., Xie H., Xie Y., Li Y., Zhao X., Jiang X., Chen D. Antioxidant and cytoprotective effects of the Di-O-Caffeoylquinic acid family: The mechanism, structure–activity relationship, and conformational effect. Molecules. 2018;23:222. doi: 10.3390/molecules23010222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim D.B., Unenkhuu B., Kim G.J., Kim S.W., Kim H.S. Cynarin attenuates LPS-induced endothelial inflammation via upregulation of the negative regulator MKP-3. Animal Cells Syst. 2022;26:119–128. doi: 10.1080/19768354.2022.2077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wu C., Chen S., Liu Y., Kong B., Yan W., Jiang T., Tian H., Liu Z., Shi Q., Wang Y., et al. Cynarin suppresses gouty arthritis induced by monosodium urate crystals. Bioengineered. 2022;13:11782–11793. doi: 10.1080/21655979.2022.2072055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen Y., Geng S., Liu B. Three common caffeoylquinic acids as potential hypoglycemic nutraceuticals: Evaluation of α-glucosidase inhibitory activity and glucose consumption in HepG2 cells. J. Food Biochem. 2020;44:13361. doi: 10.1111/jfbc.13361. [DOI] [PubMed] [Google Scholar]
- 97.Etemadi-Tajbakhsh N., Faramarzi M.A., Delnavazi M.R. 1, 5-dicaffeoylquinic acid, an α-glucosidase inhibitor from the root of Dorema ammoniacum D. Don. Res. Pharm. Sci. 2020;15:429–436. doi: 10.4103/1735-5362.297845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Murlanova K., Cohen N., Pinkus A., Vinnikova L., Pletnikov M., Kirby M., Gorelick J., Drori E., Pinhasov A. Antidepressant-like effects of a chlorogenic acid- and cynarine-enriched fraction from Dittrichia viscosa root extract. Sci. Rep. 2022;12:3647. doi: 10.1038/s41598-022-04840-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mamat N., Lu X.Y., Kabas M., Aisa H.A. Potential anti-vitiligo properties of cynarine extracted from Vernonia anthelmintica (L.) Willd. Int. J. Mol. Med. 2018;42:2665–2675. doi: 10.3892/ijmm.2018.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Slanina J., Táborská E., Bochořáková H., Slaninova I., Humpa O., Robinson W.E., Schram K.H. New and facile method of preparation of the anti-HIV-1 agent, 1,3-dicaffeoylquinic acid. Tetrahedron Lett. 2001;42:3383–3385. doi: 10.1016/S0040-4039(01)00448-8. [DOI] [Google Scholar]
- 101.Corona A., Fanunza E., Salata C., Morwitzer M.J., Distinto S., Zinzula L., Sanna C., Frau A., Daino G.L., Quartu M., et al. Cynarin blocks Ebola virus replication by counteracting VP35 inhibition of interferon-beta production. Antiviral Res. 2022;198:105251. doi: 10.1016/j.antiviral.2022.105251. [DOI] [PubMed] [Google Scholar]
- 102.Liao H.J., Tzen J.T.C. The potential role of phenolic acids from Salvia miltiorrhiza and Cynara scolymus and their derivatives as JAK inhibitors: An in silico study. Int. J. Mol. Sci. 2022;23:4033. doi: 10.3390/ijms23074033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mileo A.M., Di Venere D., Linsalata V., Fraioli R., Miccadei S. Artichoke polyphenols induce apoptosis and decrease the invasive potential of the human breast cancer cell line MDA-MB231. J. Cell Physiol. 2012;227:3301–3309. doi: 10.1002/jcp.24029. [DOI] [PubMed] [Google Scholar]
- 104.Hwang S.J., Kim Y.-W., Park Y., Lee H.J., Kim K.-W. Anti-inflammatory effects of chlorogenic acid in lipopolysaccharide stimulated RAW 264.7 cells. Inflamm. Res. 2014;63:81–90. doi: 10.1007/s00011-013-0674-4. [DOI] [PubMed] [Google Scholar]
- 105.Jin S., Chang C., Zhang L., Liu Y., Huang X., Chen Z. Chlorogenic acid improves late diabetes through adiponectin receptor signaling pathways in db/db mice. PLoS ONE. 2015;10:e0120842. doi: 10.1371/journal.pone.0120842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wu D., Bao C., Li L., Fu M., Wang D., Xie J., Gong X. Chlorogenic acid protects against cholestatic liver injury in rats. J. Pharm. Sci. 2015;129:177–182. doi: 10.1016/j.jphs.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 107.Kwon S.-H., Lee H.-K., Kim J.-A., Hong S.-I., Kim H.-C., Jo T.-H., Park Y.-I., Lee C.-K., Kim Y.-B., Lee S.-Y., et al. Neuroprotective effects of chlorogenic acid on scopolamine-induced amnesia via anti-acetylcholinesterase and anti-oxidative activities in mice. Eur. J. Pharmacol. 2010;649:210–217. doi: 10.1016/j.ejphar.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 108.Ding Y., Cao Z., Cao L., Ding G., Wang Z., Xiao W. Antiviral activity of chlorogenic acid against influenza A (H1N1/H3N2) virus and its inhibition of neuraminidase. Sci. Rep. 2017;7:45723. doi: 10.1038/srep45723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ong K.W., Hsu A., Tan B.K.H. Anti-diabetic and anti-lipidemic effects of chlorogenic acid are mediated by ampk activation. Biochem. Pharmacol. 2013;85:1341–1351. doi: 10.1016/j.bcp.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 110.Yan Y., Liu N., Hou N., Dong L., Li J. Chlorogenic acid inhibits hepatocellular carcinoma in vitro and in vivo. J. Nutr. Biochem. 2017;46:68–73. doi: 10.1016/j.jnutbio.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 111.Bouayed J., Rammal H., Dicko A., Younos C., Soulimani R. Chlorogenic acid, a polyphenol from Prunus domestica (Mirabelle), with coupled anxiolytic and antioxidant effects. J. Neurol. Sci. 2007;262:77–84. doi: 10.1016/j.jns.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 112.Bagdas D., Cinkilic N., Ozboluk H.Y., Ozyigit M.O., Gurun M.S. Antihyperalgesic activity of chlorogenic acid in experimental neuropathic pain. J. Nat. Med. 2012;67:698–704. doi: 10.1007/s11418-012-0726-z. [DOI] [PubMed] [Google Scholar]
- 113.Mubarak A., Bondonno C.P., Liu A.H., Considine M.J., Rich L., Mas E., Croft K.D., Hodgson J.M. Acute effects of chlorogenic acid on nitric oxide status, endothelial function, and blood pressure in healthy volunteers: A randomized trial. J. Agric. Food Chem. 2012;60:9130–9136. doi: 10.1021/jf303440j. [DOI] [PubMed] [Google Scholar]
- 114.Heitman E., Ingram D.K. Cognitive and neuroprotective effects of chlorogenic acid. Nutr. Neurosci. 2017;20:32–39. doi: 10.1179/1476830514Y.0000000146. [DOI] [PubMed] [Google Scholar]
- 115.Ma Y., Gao M., Liu D. Chlorogenic acid improves high fat diet-induced hepatic steatosis and insulin resistance in mice. Pharm. Res. 2015;32:1200–1209. doi: 10.1007/s11095-014-1526-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang Z., Lam K.-L., Hu J., Ge S., Zhou A., Zheng B., Zeng S., Lin S. Chlorogenic acid alleviates obesity and modulates gut microbiota in high-fat-fed mice. Food Sci. Nutr. 2019;7:579–588. doi: 10.1002/fsn3.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zeng J., Zhang D., Wan X., Bai Y., Yuan C., Wang T., Yuan D., Zhang C., Liu C. Chlorogenic acid suppresses miR-155 and ameliorates ulcerative colitis through NF-kB/NLRP3 inflammasome pathway. Mol. Nutr. Food Res. 2020;64:2000452. doi: 10.1002/mnfr.202000452. [DOI] [PubMed] [Google Scholar]
- 118.Xue N., Zhou Q., Ji M., Jin J., Lai F., Chen J., Zhang M., Jia J., Yang H., Zhang J., et al. Chlorogenic acid inhibits glioblastoma growth through repolarizating macrophage from M2 to M1 phenotype. Sci. Rep. 2017;7:39011. doi: 10.1038/srep39011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Changizi Z., Moslehi A., Rohani A.H., Eidi A. Chlorogenic acid induces 4T1 breast cancer tumor’s apoptosis via p53, Bax, Bcl-2, and caspase-3 signaling pathways in BALB/c mice. J. Biochem. Mol. Toxicol. 2020;35:e22642. doi: 10.1002/jbt.22642. [DOI] [PubMed] [Google Scholar]
- 120.Jin U.-H., Lee J.-Y., Kang S.-K., Kim J.-K., Park W.-H., Kim J.-G., Moon S.-K., Kim C.-H. A phenolic compound, 5-caffeoylquinic acid (chlorogenic acid), is a new type and strong matrix metalloproteinase-9 inhibitor: Isolation and identification from methanol extract of Euonymus alatus. Life Sci. 2005;77:2760–2769. doi: 10.1016/j.lfs.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 121.Mamat N., Dou J., Lu X., Eblimit A., Akber A.H. Isochlorogenic acid A promotes melanin synthesis in B16 cell through the β-catenin signal pathway. Acta Biochim. Biophys. Sin. 2017;49:800–807. doi: 10.1093/abbs/gmx072. [DOI] [PubMed] [Google Scholar]
- 122.Roh K.-B., Jang Y., Cho E., Park D., Kweon D.-H., Jung E. Chlorogenic acid isomers isolated from Artemisia lavandulaefolia exhibit anti-Rosacea effects in vitro. Biomedicines. 2022;10:463. doi: 10.3390/biomedicines10020463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Moradi-Afrapoli F., Saremi G., Nasseri S., Emami S.A., Mojarrab M. Isolation of two isochlorogenic acid isomers from phenolic rich fraction of Artemisia turanica Krasch. Iranian J. Pharm. Res. 2020;19:59–66. doi: 10.22037/ijpr.2019.15182.12919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gebhardt R. Choleretic and anticholestatic activities of flavonoids of artichoke (Cynara scolymus L. subsp. scolymus (L.) Hayek) Acta Hort. 2005;681:429–435. doi: 10.17660/ActaHortic.2005.681.60. [DOI] [Google Scholar]
- 125.Olennikov D.N., Chirikova N.K., Vennos C. Chemical composition, antioxidant and anticholinesterase activities of Gentianella azurea from Russian Federation. Nat. Prod. Commun. 2017;12:55–56. doi: 10.1177/1934578X1701200115. [DOI] [PubMed] [Google Scholar]
- 126.Dong W., Chen D., Chen Z., Sun H., Xu Z. Antioxidant capacity differences between the major flavonoids in cherry (Prunus pseudocerasus) in vitro and in vivo models. LWT. 2021;141:110938. doi: 10.1016/j.lwt.2021.110938. [DOI] [Google Scholar]
- 127.Yu H., Li J., Hu X., Feng J., Wang H., Xiong F. Protective effects of cynaroside on oxidative stress in retinal pigment epithelial cells. J. Biochem. Mol. Toxicol. 2019;33:e22352. doi: 10.1002/jbt.22352. [DOI] [PubMed] [Google Scholar]
- 128.Zou Y., Zhang M., Zhang T., Wu J., Wang J., Liu K., Zhan N. Antioxidant and anti-inflammatory activities of cynaroside from Elsholtiza bodinieri. Nat. Prod. Commun. 2018;13:1501–1504. doi: 10.1177/1934578X1801301122. [DOI] [Google Scholar]
- 129.Mogana R., Adhikari A., Tzar M.N., Ramliza R., Viart C. Antibacterial activities of the extracts, fractions and isolated compounds from Canarium patentinervium Miq. against bacterial clinical isolates. BMC Complement. Med. Ther. 2020;20:55. doi: 10.1186/s12906-020-2837-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Odontoya G., Hoult J.R.S., Houghton P.J. Structure-activity relationship for anti-inflammatory effect of luteolin and its derived glycosides. Phytother. Res. 2005;19:782–786. doi: 10.1002/ptr.1723. [DOI] [PubMed] [Google Scholar]
- 131.Lee S.A., Park B.R., Moon S.M., Kim J.-S., Kim D.K., Kim C.S. Cynaroside protects human periodontal ligament cells from lipopolysaccharide-induced damage and inflammation through suppression of NF-κB activation. Arch. Oral Biol. 2020;120:104944. doi: 10.1016/j.archoralbio.2020.104944. [DOI] [PubMed] [Google Scholar]
- 132.Szekalska M., Sosnowska K., Tomczykowa M., Winnicka K., Kasacka I., Tomczyk M. In vivo anti-inflammatory and anti-allergic activities of cynaroside evaluated by using hydrogel formulations. Biomed. Pharmacother. 2020;121:109681. doi: 10.1016/j.biopha.2019.109681. [DOI] [PubMed] [Google Scholar]
- 133.Chung H.S. Inhibition of monoamine oxidase by a flavone and its glycoside from Ixeris dentata Nakai. Nutraceuticals Food. 2003;8:141–144. doi: 10.3746/jfn.2003.8.2.141. [DOI] [Google Scholar]
- 134.Brown J.E., Rice-Evans C.A. Luteolin-rich artichoke extract protects low density lipoprotein from oxidation in vitro. Free Rad. Res. 1998;29:247–255. doi: 10.1080/10715769800300281. [DOI] [PubMed] [Google Scholar]
- 135.Van L.V., Pham E.C., Nguyen C.V., Duong N.T.N., Thi V.L.T., Truong T.N. In vitro and in vivo antidiabetic activity, isolation of flavonoids, and in silico molecular docking of stem extract of Merremia tridentata (L.) Biomed. Pharmacother. 2022;146:112611. doi: 10.1016/j.biopha.2021.112611. [DOI] [PubMed] [Google Scholar]
- 136.Borges P.H.O., Pedreiro S., Baptista S.J., Geraldes C.F.G.C., Batista M.T., Maria M.C., Silva M.M.C., Figueirinha A. Inhibition of α-glucosidase by flavonoids of Cymbopogon citratus (DC) Stapf. J. Ethnopharmacol. 2021;280:114470. doi: 10.1016/j.jep.2021.114470. [DOI] [PubMed] [Google Scholar]
- 137.Zang Y., Igarashi K., Li Y.L. Anti-diabetic effects of luteolin and luteolin-7-O-glucoside on KK-Ay mice. Biosci. Biotechnol. Biochem. 2016;80:1580–1586. doi: 10.1080/09168451.2015.1116928. [DOI] [PubMed] [Google Scholar]
- 138.Kim J.H., Son Y.K., Kim G.H., Hwang K.H. Xanthoangelol and 4-hydroxyderricin are the major active principles of the inhibitory activities against monoamine oxidases on Angelica keiskei K. Biomol. Ther. 2013;21:234–240. doi: 10.4062/biomolther.2012.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Goodarzi S., Tabatabaei M.J., Jafari R.M., Shemirani F., Tavakolib S., Mansur M., Tofighi Z. Cuminum cyminum fruits as source of luteolin-7-O-glucoside, potent cytotoxic flavonoid against breast cancer cell lines. Nat. Prod. Res. 2020;34:1602–1606. doi: 10.1080/14786419.2018.1519824. [DOI] [PubMed] [Google Scholar]
- 140.Seelinger G., Merfort I., Wolfle U., Schempp C.M. Anti-carcinogenic effects of the flavonoid luteolin. Molecules. 2008;13:2628–2651. doi: 10.3390/molecules13102628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Velmurugan B.K., Lin J.T., Mahalakshmi B., Chuang Y.C., Lin C.C., Lo Y.S., Hsieh M.J., Chen M.K. Luteolin-7-O-glucoside inhibits oral cancer cell migration and invasion by regulating matrix metalloproteinase-2 expression and extracellular signal-regulated kinase pathway. Biomolecules. 2020;10:502–518. doi: 10.3390/biom10040502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Damasceno L.M.O., Silva A.L.N., dos Santos R.F., Feitosa T.A., Viana L.G.F., de Oliveira-Júnior R.G., Silva M.G., Larissa A., Rolim L.A., Camila S., et al. Cytotoxic activity of chemical constituents and essential oil from the leaves of Leonotis nepetifolia (Lamiaceae) Rev. Virtual Quim. 2019;11:517–528. doi: 10.21577/1984-6835.20190039. [DOI] [Google Scholar]
- 143.Yang R., Han S., Clayton J., Haghighatian M., Tsai C.-C., Yao Y., Li P., Shen J., Zhou Q. A proof-of concept inhibitor of endothelial lipase suppresses triple-negative breast cancer cells by hijacking the mitochondrial function. Cancers. 2022;14:3763. doi: 10.3390/cancers14153763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ji J., Wang Z., Sun W., Li Z., Cai H., Zhao E., Cui H. Effects of cynaroside on cell proliferation, apoptosis, migration and invasion though the MET/AKT/mTOR axis in gastric cancer. Int. J. Mol. Sci. 2021;22:12125. doi: 10.3390/ijms222212125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Žemlička L., Fodran P., Lukeš V., Vagánek A., Slováková M., Staško A., Dubaj T., Liptaj T., Karabín M., Birošová L. Physicochemical and biological properties of luteolin-7-O-β-d-glucoside (cynaroside) isolated from Anthriscus sylvestris (L.) Hoffm. Monatsh. Chem. 2014;145:1307–1318. doi: 10.1007/s00706-014-1228-3. [DOI] [Google Scholar]
- 146.Lee S.A., Park B.R., Moon S.M., Hong J.H., Kim D.K., Kim C.S. Chondroprotective effect of cynaroside in IL-1 β-induced primary rat chondrocytes and organ explants via NF-κB and MAPK signaling inhibition. Oxid. Med. Cell Longev. 2020;2020:9358080. doi: 10.1155/2020/9358080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang L., Ma X., Wang J., Li C. In vitro inhibitory effects of cynaroside on human liver cytochrome P450 enzymes. Pharmacology. 2019;104:296–302. doi: 10.1159/000502172. [DOI] [PubMed] [Google Scholar]
- 148.Mendel M., Chłopecka M., Latek U., Karlik W., Tomczykowa M., Straw J., Tomczyk M. Evaluation of the effects of Bidens tripartita extracts and their main constituents on intestinal motility—An ex vivo study. J. Ethnopharmacol. 2020;259:112982. doi: 10.1016/j.jep.2020.112982. [DOI] [PubMed] [Google Scholar]
- 149.Feng J.H., Dong X.W., Yu H.L., Shen W., Lv X.-Y., Wang R., Cheng X.-X., Xiong F., Hu X.-L., Wang H. Cynaroside protects the blue light-induced retinal degeneration through alleviating apoptosis and inducing autophagy in vitro and in vivo. Phytomedicine. 2021;88:153604. doi: 10.1016/j.phymed.2021.153604. [DOI] [PubMed] [Google Scholar]
- 150.Rigano D., Formisano C., Basile A., Lavitola A., Senatore F., Rosselli S., Bruno M. Antibacterial activity of flavonoids and phenylpropanoids from Marrubium globosum ssp. libanoticum. Phytother. Res. 2007;21:395–397. doi: 10.1002/ptr.2061. [DOI] [PubMed] [Google Scholar]
- 151.Moussaoui F., Zellagui A., Segueni N., Touil A., Rhouati S. Flavonoid Constituents from Algerian Launaea resedifolia (O.K.) and their antimicrobial activity. Rec. Nat. Prod. 2010;4:91–95. [Google Scholar]
- 152.Villa-Rodriguez J.A., Kerimi A., Abranko L., Tumova S., Ford L., Blackburn R.S., Rayner C., Williamson G. Acute metabolic actions of the major polyphenols in chamomile: An in vitro mechanistic study on their potential to attenuate postprandial hyperglycaemia. Sci. Rep. 2018;8:5471. doi: 10.1038/s41598-018-23736-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Smiljkovic M., Stanisavljevic D., Stojkovic D., Petrovic I., Vicentic J.M., Popovic J., Grdadolnik S.G., Markovic D., Sankovic-Babice S., Glamoclija J. Apigenin-7-O-glucoside versus apigenin: Insight into the modes of anticandidal and cytotoxic actions. EXCLI J. 2017;16:795–807. doi: 10.17179/excli2017-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Minda D., Avram S., Pavel I.Z., Kis B., Ghitu A., Zupkó I., Dehelean C., Buda V., Diaconeasa Z., Scurtu A., et al. An in vitro evaluation of apigenin and apigenin-7-O-glucoside against hela human cervical cancer cell line. Rev. Chim. 2020;71:140–144. doi: 10.37358/RC.20.2.7906. [DOI] [Google Scholar]
- 155.Liu M.M., Ma R.H., Ni Z.J., Thakur K., Cespedes-Acuña C.L., Jiang L., Wei Z.J. Apigenin 7-O-glucoside promotes cell apoptosis through the PTEN/PI3K/AKT pathway and inhibits cell migration in cervical cancer Hela cells. Food Chem. Toxicol. 2020;146:111843. doi: 10.1016/j.fct.2020.111843. [DOI] [PubMed] [Google Scholar]
- 156.Weng X.C., Wang W. Antioxidant activity of compounds isolated from Salvia plebeia. Food Chem. 2000;71:489–493. doi: 10.1016/S0308-8146(00)00191-6. [DOI] [Google Scholar]
- 157.Ai-li J., Chang-hai W. Antioxidant properties of natural components from Salvia plebeia on oxidative stability of ascidian oil. Process Biochem. 2006;41:1111–1116. doi: 10.1016/j.procbio.2005.12.001. [DOI] [Google Scholar]
- 158.Gülcemal D., Alankus-Çalıskan Ö., Karaalp C., Örs A.U., Ballar P., Bedir E. Phenolic Glycosides with antiproteasomal activity from Centaurea urvillei DC. subsp. urvillei. Carbohydr. Res. 2010;345:2529–2533. doi: 10.1016/j.carres.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 159.Genc Y., Guragac Dereli F.T., Saracoglu I., Kupeli Akkol E. The inhibitory effects of isolated constituents from Plantago major subsp. major L. on collagenase, elastase and hyaluronidase enzymes: Potential wound healer. Saudi Pharm. J. 2020;28:101–106. doi: 10.1016/j.jsps.2019.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Clinical and Laboratory Standards Institute . Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard. 2nd ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2006. CLSI Document M7-A7. [Google Scholar]
- 161.NCCLS . Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard. 2nd ed. NCCLS; Wayne, PA, USA: 2002. NCCLS Document M27-A2. [Google Scholar]
- 162.Koleva I.I., Niederländer H.A., van Beek T.A. Application of ABTS radical cation for selective on-line detection of radical scavengers in HPLC eluates. Anal. Chem. 2001;73:3373–3381. doi: 10.1021/ac0013610. [DOI] [PubMed] [Google Scholar]
- 163.Brand-Williams W., Cuvelier M., Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- 164.Bader A., Martini F., Schinella G.R., Rios J.L., Prieto J.M. Modulation of Cox-1, 5-, 12-and 15-Lox by Popular Herbal Remedies Used in Southern Italy Against Psoriasis and Other Skin Diseases. Phytother Res. 2015;29:108–113. doi: 10.1002/ptr.5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The Supplementary Materials for the DPPH• TLC spot testing and LC-MS/MS analyses of chloro-genic acid, cynarine and cynaroside are available online.