Abstract
The signaling pathways that sense adverse stimuli and communicate with the nucleus to initiate appropriate changes in gene expression are central to the cellular stress response. Herein, we have characterized the role of the Sty1 (Spc1) stress-activated mitogen-activated protein kinase pathway, and the Pap1 and Atf1 transcription factors, in regulating the response to H2O2 in the fission yeast Schizosaccharomyces pombe. We find that H2O2 activates the Sty1 pathway in a dose-dependent manner via at least two sensing mechanisms. At relatively low levels of H2O2, a two component-signaling pathway, which feeds into either of the two stress-activated mitogen-activated protein kinase kinase kinases Wak1 or Win1, regulates Sty1 phosphorylation. In contrast, at high levels of H2O2, Sty1 activation is controlled predominantly by a two-component independent mechanism and requires the function of both Wak1 and Win1. Individual transcription factors were also found to function within a limited range of H2O2 concentrations. Pap1 activates target genes primarily in response to low levels of H2O2, whereas Atf1 primarily controls the transcriptional response to high concentrations of H2O2. Our results demonstrate that S. pombe uses a combination of stress-responsive regulatory proteins to gauge and effect the appropriate transcriptional response to increasing concentrations of H2O2.
INTRODUCTION
Reactive oxygen species (ROS), including superoxide anions, hydroxyl radicals, and hydrogen peroxide, are generated by the chemical reduction of oxygen by a variety of cellular enzymes, by exposure to UV or other environmental agents, and by incomplete reduction of oxygen to water in the mitochondrial respiratory chain. ROS are found in all aerobically growing cells and may have important functions in promoting cell growth, metabolism, and defense. However, when the levels of ROS increase beyond normal homeostatic concentrations oxidative stress occurs, causing damage to numerous cellular components and activating signaling pathways that may lead to cell death or disease (reviewed in Freeman and Crapo, 1982; Halliwell and Gutteridge, 1999). Under these conditions oxidative stress response mechanisms, which activate repair and antioxidant defense systems, are required for adaptation and survival.
Although a number of signaling pathways are likely to contribute to the response of cells to oxidative stress, studies performed over the past few years have highlighted the role of an evolutionarily conserved family of stress-activated mitogen-activated protein kinases (MAPKs). In the fission yeast Schizosaccharomyces pombe, the Sty1 (also known as Spc1 and Phh1) MAPK pathway is required for the cellular response to a wide range of adverse stimuli, including oxidative stress, osmotic stress, heat stress, and heavy metal toxicity and to DNA-damaging agents such as UV light (Millar et al., 1995; Shiozaki and Russell, 1995; Degols et al., 1996; Degols and Russell, 1997; Shieh et al., 1997). Sty1 is activated by phosphorylation by the mitogen-activated protein kinase kinase (MAPKK) Wis1, which in turn is activated through phosphorylation by two mitogen-activated protein kinase kinase kinases (MAPKKKs), Wak1 (also known as Wis4 and Wik1) (Samejima et al., 1997; Shieh et al., 1997; Shiozaki et al., 1997) and Win1 (Samejima et al., 1998; Shieh et al., 1998). Components of this MAPK cascade are homologous to components of the HOG1 osmosensing MAPK pathway in Saccharomyces cerevisiae and to the mammalian c-Jun NH2-terminal kinase (JNK) and p38 stress-activated protein kinase cascades (reviewed in Toone and Jones, 1998).
Recent studies indicate that oxidative stress activates the Sty1 pathway through a “two-component”-related response regulator protein, Mcs4 (Shieh et al., 1997; Shiozaki et al., 1997), which binds to Wak1 and controls activation of Sty1 in response to H2O2 (Buck et al., 2001). Mcs4 is controlled by two histidine kinases, Mak2 and Mak3, that apparently sense peroxide stress and initiate a multistep phosphorelay. This phosphorelay connects the kinase and receiver domains within Mak2 and Mak3 with the receiver domain of Mcs4 through the intermediary protein Mpr1 (Nguyen et al., 2000; Buck et al., 2001). The phosphorylation status of Mcs4 is predicted to regulate the activity of Wak1. This pathway is most similar to the two-component-like SLN1-YPD1-SSK1 pathway that lies upstream of the HOG1 osmosensing MAPK cascade in S. cerevisiae (Posas et al., 1996; Posas and Saito, 1998).
In fission yeast, two bZip transcription factors, Pap1 and Atf1, have been implicated in the oxidative stress response (Toda et al., 1991; Takeda et al., 1995; Kumada et al., 1996; Wilkinson et al., 1996; Toone et al., 1998; Yamada et al., 1999; Nguyen et al., 2000). Mammalian homologs of these factors, cJun and ATF2, are regulated by the JNK and p38 stress-activated protein kinases (reviewed in Tibbles and Woodgett, 1999). Studies have suggested that the Sty1 stress-activated protein kinase may also control the activity of Atf1 and Pap1. Atf1 is phosphorylated by Sty1 in response to stress and, although Pap1 does not appear to be a direct target of Sty1, H2O2-dependent changes in its subcellular localization are impaired in a sty1− mutant (Shiozaki and Russell, 1996; Wilkinson et al., 1996; Toone et al., 1998).
Stress responses are often studied by subjecting cells to a limited number of standardized stress conditions. However, in nature stresses vary in intensity and duration and consequently the response to stress must be appropriate for the particular condition. Herein, we have examined the specific roles of the Sty1 MAPK pathway, and the Pap1 and Atf1 transcription factors, in mediating gene expression in response to increasing intensities of H2O2 stress. Our data demonstrate that specific stress response genes are induced at different levels of H2O2 stress. Furthermore, we find that Pap1, Atf1, and components of the Sty1 pathway have distinct roles in controlling these transcriptional outputs. The Pap1 transcription factor is primarily required for the induction of target genes in response to low levels of H2O2 stress, whereas, Atf1 is primarily involved in the response to potentially lethal levels of H2O2. Upstream of the Sty1 kinase, we found that the two-component signaling pathway is required for Sty1 activation predominantly in response to low levels of H2O2 stress. Thus, to adapt to the different levels of oxidative stress, the cell uses a combination of stress-induced regulatory proteins to gauge and effect the appropriate response.
MATERIALS AND METHODS
Yeast Strains and Growth Conditions
S. pombe strains (Table 1) were grown in rich medium (YE5S) or in synthetic minimal medium (EMM2) as described previously (Moreno et al., 1991; Alfa et al., 1993).
Table 1.
S. pombe strains used in this study
| Strain | Genotype | Source or Reference |
|---|---|---|
| 972 | h− | Our stock |
| CHP429 | h− leu1 ura4 his7 ade6-216 | Gift from Charlie Hoffman |
| TP108-3C | h− leu1 his22 ura4 pap1::ura4+ | Our stock |
| NT224 | h− ura4 sty1-1 | Millar et al., 1995 |
| NT147 | h90 leu1 ura4 atf1::ura4+ | Our stock |
| MT100 | h− leu1 his2 ura4 pap1::ura4+ atf1::ura4+ | This study |
| JM1521 | h+ sty1(HA6xHis):ura4+ leu1 ura4 his7 | Shieh et al., 1998 |
| JM1828 | h+ sty1(HA6xHis): ura4+ mak1::LEU2 leu1 ura4 his7 | Buck et al., 2001 |
| JM1829 | h− sty1(HA6xHis): ura4+ mak2::LEU2 leu1 ura4 his7 | Buck et al., 2001 |
| JM1934 | h− sty1(HA6His): ura4+ mak3::kanR leu1 ura4 his7 | Buck et al., 2001 |
| KS1479 | h− atf1(HA6xHis): ura4+ leu1 ura4 | Shiozaki et al., 1996 |
| KS2086 | h− spc1(HA6xHis): ura4+ wis1AA(12myc): ura4+ leu1 ura4 | Shiozaki et al., 1998 |
| KS2096 | h− spc1(HA6xHis): ura4+ wis1(12myc): ura4+ leu1 ura4 | Shiozaki et al., 1998 |
| JM1436 | h+ wak1::ura4+ sty1(HA6xHis): ura4+ leu1 ura4 his7 | Shieh et al., 1998 |
| ED1293 | h− win1-1 wak1::ura4+ sty1(HA6xHis): ura4+ | Samejima et al., 1997 |
Note all strains are leu1-32, ura4-D18, or his7-366.
H2O2 Sensitivity Tests
Acute and adaptive responses to H2O2 were performed in liquid culture. Overnight cultures in midlog were diluted in prewarmed medium and incubated for 4–5 h until OD595 = 0.025–0.05. Cultures were then split into two flasks and one was pretreated with 0.15 mM H2O2 for 1 h to initiate an adaptive response. An acute dose of H2O2 (final concentration of 25 mM, used fresh; Sigma, Poole, Dorset, United Kingdom) was then added to both flasks. Cells were taken at various time points, diluted, and then plated on YE5S agar to determine surviving cell numbers. Plates were incubated for 3–4 d and survival was expressed as a percentage of the time 0 sample.
RNA Analysis
Overnight cultures were grown to midlog, diluted in fresh prewarmed medium, and grown for 4–5 h until they had reached OD595 = 0.2. Cells were treated as indicated and collected by centrifugation. RNA was prepared for each time point from 25 ml of cells by a hot phenol method essentially as described in White et al. (1987). A 5-μg sample of total RNA was denatured with glyoxal, separated on a 1.2% agarose gel, and transferred to a GeneScreen hybridization membrane (PerkinElmer Life Sciences, Boston, MA). Gene-specific probes were prepared from polymerase chain reaction-generated fragments by labeling with 32P by using a DNA Megaprime labeling kit (Amersham, Little Chalfont, Buckinghamshire, United Kingdom). Hybridization conditions were as described in the GeneScreen protocol. Probes for his3+ or hmg1+ were used as loading controls.
Fluorescence Microscopy
Immunolocalization of Pap1 was carried out essentially as described by Hagan and Ayscough (2000). Samples (10 ml) of exponentially growing cells (OD595 = 0.2), untreated or treated with indicated concentrations of H2O2, were collected and fixed in 3.7% formaldehyde [freshly prepared in PEM; 100 mM piperazine-N,N′-bis(2-ethanesulfonic acid), 1 mM EGTA, 1 mM MgSO4, pH 6.9] for 10 min. Cells were washed in PEM, resuspended in PEMS (PEM + 1.2 M sorbitol) containing 0.25 mg/ml zymolase (100T; ICN Biomedicals; Costa Mesa, CA), and incubated at 37°C for 70 min. Cells were then washed in PEM, resuspended in PEMBAL (PEM + 1% globulin-free bovine serum albumin [Sigma], 0.1% NaN3, 100 M lysine hydrochloride) for at least 30 min. Cells were pelleted and resuspended in 10% Pap1 antisera at 4°C overnight. Cells were washed with PEM and resuspended in a 1/10,000 dilution of Alexa 546 goat anti-rabbit (Molecular Probes, Eugene, OR) secondary antibody, and placed at 4°C overnight. Cells were then washed with PEMBAL and then with phosphate-buffered saline and finally resuspended in 50 μl of phosphate-buffered saline + 1% NaN3. Cells were spread onto poly-l-lysine–coated coverslips, dried, and mounted onto slides with ProLong mounting medium (Molecular Probes). Cells were observed using an Olympus BX51 upright microscope, with a Plan-Apochromat 100× objective. Using a 100-W Ushio mercury bulb and a Chroma wide-band fluorescence cube (exciter 520–550 nm, detection 570–580) the Alexa label was examined via a cooled Colorview 12 camera and the analySIS imaging acquisition and processing system (SiS; Munster, Germany). Images were captured at 1300 × 1030, 24-bit resolution with an exposure time of 5 s. Images were then imported into Adobe PhotoShop 6.0 (Adobe Systems, Mountain View, CA).
For green fluorescent protein (GFP)-Pap1 analysis samples of cells, untreated or treated with indicated concentrations of H2O2, were collected by centrifugation and fixed by resuspending in −20°C methanol for at least 10 min. For nuclear staining fixed cells were stained with 4′,6-diamino-2-phenyl-indole. The cells were washed with water and mounted onto poly-l-lysine–coated coverslips. Cells were observed with a fluorescein isothiocyanate filter block (exciter 475 nm, detection 510–550 nm) as described above.
Sty1 Phosphorylation Assays
Strains bearing an integrated six-histidine- (6His) and hemagglutinin (HA)-tagged version of Sty1 (Millar et al., 1995) were grown in YE5S medium at 30°C and incubated for the times indicated in the same medium containing various concentrations of H2O2. Approximately 2 × 108 cells were harvested at each time point, lysed under native conditions, and the Sty1 protein precipitated using Ni2+-nitrilotriacetic acid (NTA) (QIAGEN; GmbH, Germany) agarose. Precipitated proteins were resolved by SDS-PAGE and Western blots probed for the presence of phosphorylated Sty1 by using an anti-phospho p38 antibody (New England Biolabs, Beverly, MA) (Millar et al., 1995). Blots were stripped and reprobed with an HA antibody (Sigma) as a loading control.
Atf1 Phosphorylation Assay
A strain bearing an integrated 6His- and HA-tagged version of Atf1 was grown in YE5S medium at 30°C and incubated for 10 min in the same medium containing the indicated concentrations of H2O2. Approximately 4 × 108 cells were harvested at each concentration, lysed under denaturing conditions, and the Atf1 protein precipitated using Ni2+-NTA (QIAGEN) agarose (Shiozaki and Russell, 1996). Differentially phosphorylated forms of Atf1 were resolved by SDS-PAGE and detected by Western blot with an HA-antibody (Sigma).
RESULTS
Transcription Factors Atf1 and Pap1 Have Complementary Roles in the Oxidative Stress Response
Deletion of the genes encoding the Sty1 MAPK, or the transcription factors Pap1 or Atf1, results in hypersensitivity to oxidants and an inability to induce oxidative stress response genes. However, the sensitivity to oxidative stress displayed by these mutants varies depending on the nature and intensity of the stress imposed. For example, an atf1− mutant is insensitive to H2O2 as measured by the ability to grow on solid media containing low concentrations of H2O2 (0.2 mM H2O2; our unpublished data) but is hypersensitive to a high dose of H2O2 in liquid culture (Nguyen et al., 2000). In contrast, a pap1− mutant is extremely sensitive to low concentrations of H2O2 on solid media (0.2 mM H2O2; our unpublished data) but is less sensitive than an atf1− mutant to high-dose H2O2 treatment in liquid culture (see below).
Exposure to low levels of stress often induces an adaptive response resulting in a transient resistance to subsequent higher levels of exposure to the same stress or to other types of stress (Collinson and Dawes, 1992; Jamieson, 1992; Lee et al., 1995; reviewed in Moradas-Ferreira and Costa, 2000). The results discussed above suggest that Pap1 may play a role in the low-level H2O2 response, whereas Atf1 might be more important for the response to acute stress. Therefore, we examined the relative ability of wild-type atf1− and pap1− mutants, as well as a double atf1− pap1− mutant strain, to mount either an adaptive or acute response to H2O2. Less than 10% of wild-type cells survive an acute stress of 25 mM H2O2 for 2 h (Figure 1A). However, pretreatment of wild-type cells with 0.15 mM H2O2 for 1 h induces an adaptive response resulting in 85–95% of the cells surviving a subsequent acute stress (Figure 1B). An atf1− mutant is hypersensitive to acute H2O2 stress, with <0.01% survival after a 2-h exposure. However, after pretreatment with 0.15 mM H2O2, 41% of the atf1− cells survived a 2-h exposure to 25 mM H2O2. Thus, atf1− cells are able to mount a significant adaptive response that can protect them from high-level H2O2 exposure. Similar survival curves were also obtained for cells deficient in Pcr1, the heterodimeric partner of Atf1, and for cells lacking both Atf1 and Pcr1, confirming that these proteins function together in the same pathway (our unpublished data).
Figure 1.
Comparison of the degrees of sensitivity of mutant S. pombe strains to H2O2. (A) Sensitivity of different midlog cultures to treatment with 25 mM H2O2 (acute stress). (B) Sensitivity of different midlog cultures to treatment with 25 mM H2O2 after pretreatment with 0.15 mM H2O2 for 1 h (adaptive response). After incubation of the cultures with H2O2 for the indicated times cells were diluted and plated on YE5S agar plates and survival measured as a percentage of colony number at time 0. The experiments were each repeated at least three times and the data from one representative experiment are shown.
The sensitivity of pap1− cells to acute stress is intermediate between that of wild-type and atf1− cells. After pretreatment with 0.15 mM H2O2 for 1 h, only 5% of pap1− cells were able to survive a subsequent treatment with 25 mM H2O2 for 2 h (Figure 1, A and B). Thus, the ability of a pap1− strain to mount an adaptive response is severely impaired. These results indicate that Atf1 is more important for cell survival after exposure to high levels of H2O2, whereas Pap1 is more important for the response to low levels of H2O2. An atf1− pap1− double mutant was extremely hypersensitive to acute stress (Figure 1A) and cell survival was not improved by pretreatment with 0.15 mM H2O2 (Figure 1B), indicating that, although there may be overlap in the functions of Pap1 and Atf1, both the adaptive and acute response are absent in an atf1− pap1− double mutant. Furthermore, a sty1− mutant behaved almost identically to the atf1− pap1− double mutant, being unable to mount either an adaptive or acute response (Figure 1, A and B). The same results were obtained with the MAPKK wis1− mutant (our unpublished data).
Role of Sty1, Pap1, and Atf1 in Controlling H2O2-induced Gene Expression
The H2O2-sensitive phenotypes presented above suggest that specific factors differentially control gene expression, depending on the level of stress. Three proteins that are critical for the enzymatic degradation of H2O2 are catalase, encoded by ctt1+; glutathione peroxidase, encoded by gpx1+; and thioredoxin peroxidase, encoded by tpx1+. To investigate whether Sty1, Pap1, and Atf1 regulate the expression of these genes differently, depending on the level of H2O2, Northern blots were performed to examine the transcription profiles of these genes in wild-type and mutant strains after exposure to increasing concentrations of H2O2.
ctt1+ was induced in wild-type cells over a wide range of H2O2 concentrations from 0.07 to 6.0 mM (Figure 2). At 0.25–6.0 mM H2O2 induction of ctt1+ expression is severely reduced, or absent, in the sty1− strain; however, at very low levels of H2O2 (0.07–0.25 mM) ctt1+ induction is less dependent on Sty1 (Figure 2; see below). Pap1 was found to be more important for ctt1+ expression at 0.07 and 0.25 mM, whereas Atf1 was more important for expression at high concentrations of H2O2 (6 mM). In the absence of both Pap1 and Atf1 there was no induction of ctt1+ at any concentration of H2O2. Hence, the roles played by Atf1 and Pap1 in ctt1+ expression at different concentrations of H2O2 are consistent with the sensitivity phenotypes associated with the corresponding mutant strains.
Figure 2.
H2O2 dose-dependent induction of peroxidase gene expression. Northern blot analyses of RNA isolated from midlog cultures of wild-type (wt) and different mutant strains treated with 0, 0.07, 0.25, 1.0, and 6.0 mM H2O2 for the times indicated with probes specific for ctt1+, gpx1+, tpx1+, and his3+.
gpx1+ expression has been reported previously to be regulated by Atf1 and is induced under a number of stress conditions known to activate Atf1 (Yamada et al., 1999). We found that in a wild-type strain, gpx1+ was maximally induced only after exposure to high concentrations of H2O2 (1.0–6.0 mM) and showed relatively little induction at lower H2O2 concentrations. In agreement with previous studies, this induced expression of gpx1+ required Atf1 (Figure 2). Sty1 is required for the induction of gpx1+ but loss of Sty1 does not have as profound an effect on basal level expression as loss of Atf1, suggesting that Atf1 has a role in maintaining basal level expression independent of Sty1 (Figure 2). Inactivation of pap1+ results in hyperactivation of gpx1+; in pap1− cells H2O2-dependent induction of gpx1+ expression occurs at considerably lower H2O2 levels (Figure 2). However, this induction remains dependent on Atf1 because gpx1+ is not expressed at any level of H2O2 stress in an atf1− pap1− double mutant. The superinduction of gpx1+ in a pap1− mutant has been observed previously (Nakagawa et al., 2000) and may result from an accumulation in the level of reactive oxygen species in a pap1− background such that less exogenous H2O2 is needed to obtain maximal induction. Alternatively, Pap1 may interfere with the activity of Atf1 and loss of Pap1 may result in increased activation of Atf1 on some promoters.
tpx1+ expression was induced by relatively low concentrations of H2O2 (0.07–1.0 mM H2O2) and this induction was controlled primarily by Pap1 and Sty1 (Figure 2). Inactivation of Atf1 had little affect on tpx1+ induction at any level of H2O2 (Figure 2). After exposure to 0.07 mM H2O2, some induction of tpx1+ occurred in the absence of Sty1, similar to that seen with ctt1+ expression. Thus, Sty1 appears to be more important for Pap1-dependent gene expression at 0.25–1.0 mM H2O2 than at lower concentrations. The switch from Sty1 independence to Sty1 dependence of Pap1-controlled genes occurs over a relatively small range of H2O2 concentrations (0.07–0.2 mM). To illustrate this further we compared the Sty1-dependent expression of ctt1+ to that of a known Sty1 and Atf1 target gene, pyp2+. ctt1+ expression is inducible in the absence of Sty1 at 0.15 mM H2O2 but is completely dependent on Sty1 at 1.0 mM H2O2 (Figure 3). pyp2+, which encodes a tyrosine-specific phosphatase involved in dephosphorylating the Sty1 kinase, is induced by H2O2 treatment in a Sty1-dependent manner (Wilkinson et al., 1996), but, as predicted from the above-mentioned data, maximal induction occurs only after exposure to >1 mM H2O2.
Figure 3.
Role of Sty1 in stress-induced gene expression after exposure to increasing concentrations of H2O2. Northern blot analyses of RNA isolated from midlog cultures of the wild-type (wt) and a sty1− mutant strain, treated with 0, 0.07, 0.15, and 1 mM H2O2 for 15 min, with probes specific for ctt1+, pyp2+, and hmg1+.
Pap1 Localizes to the Nucleus in Response to Low but not High Levels of H2O2
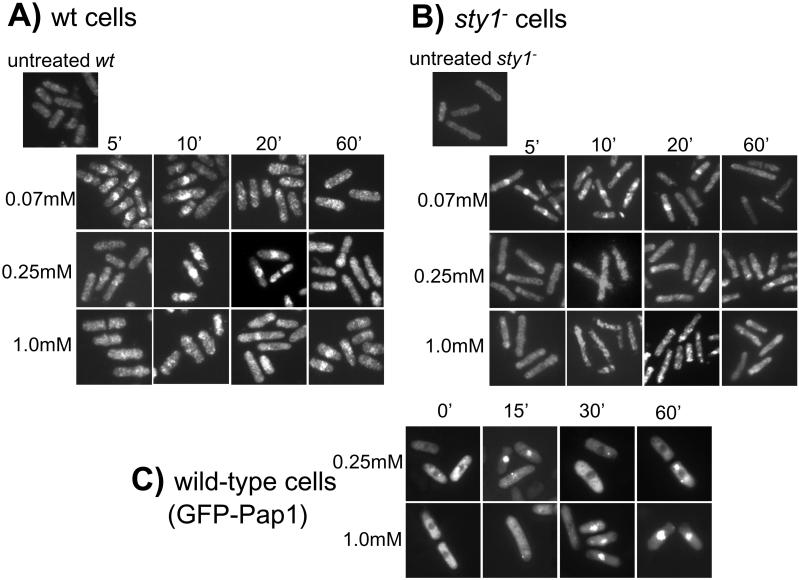
The data suggest that Pap1 functions to induce gene expression primarily at low concentrations of H2O2 (<1.0 mM H2O2), whereas Atf1 is more important at high concentrations of H2O2 (6.0 mM H2O2). Because previous studies have shown that Pap1 is regulated by changes in its subcellular localization (Toone et al., 1998), we examined Pap1 localization over a range of H2O2 concentrations. Immunolocalization experiments with an antibody raised against the Pap1 protein show that it accumulates in the nucleus within 0–5 min of exposure to 0.07 mM H2O2 and is effectively gone by 20 min (Figure 4A). Moreover, as the concentration of H2O2 increases, the time required for Pap1 to accumulate in the nucleus also increases. Thus, maximal nuclear accumulation of Pap1 took ∼15 min at 0.25 mM H2O2 and ∼60 min at 1.0 mM H2O2. Furthermore, the nuclear accumulation of Pap1 that was observed at 1.0 mM H2O2 was not as intense or as prevalent as at lower concentrations, with many cells showing little or no accumulation. No nuclear accumulation of Pap1 was seen at 6 mM H2O2 (at least over the 1-h duration of the experiment; our unpublished data) (Figure 4A). These results were confirmed using a GFP-Pap1 fusion protein described previously (Toone et al., 1998). As with wild-type Pap1, the time taken for nuclear accumulation of the GFP-Pap1 fusion protein increased with increasing H2O2 concentration, displaying approximately the same kinetics at 0.25 and 1.0 mM H2O2 as the wild-type protein (Figure 4C).
Figure 4.
Nuclear accumulation of Pap1 after treatment with increasing concentrations of H2O2. The cellular localization of Pap1 was examined by fluorescence microscopy in cells treated with the indicated concentration of H2O2 over a 1-h time period. (A) Immunolocalized Pap1 in wild-type cells. (B) Immunolocalized Pap1 in sty1− cells. (C) GFP-Pap1 in wild-type cells.
Previously, we demonstrated that a GFP-Pap1 fusion protein failed to accumulate in the nucleus of sty1− cells at 0.2 mM H2O2 (Toone et al., 1998). However, considering the expression data described above, we tested whether the status of Sty1 affected the nuclear localization of Pap1 at different concentrations of H2O2. Interestingly, Pap1 accumulated in the nucleus of sty1− cells at 0.07 mM H2O2 but failed to accumulate, or accumulated very poorly (<2% of cells) at higher concentrations of oxidant (Figure 4B). This correlates well with the switch from Sty1 independence to Sty1 dependence of Pap1-controlled gene expression shown previously (Figure 2).
Phosphorylation of Sty1 and Atf1 Increases with Rising Concentrations of H2O2
The results show that sty1− and atf1− strains are particularly sensitive to exposure to acute doses of H2O2 and that both factors are mainly required for the induction of target genes at high concentrations of H2O2. Previous studies have demonstrated that the Sty1 MAPK is cytoplasmic under nonstressed conditions, and translocates to the nucleus in response to stress, where it binds to and phosphorylates the Atf1 transcription factor (Shiozaki and Russell, 1996; Wilkinson et al., 1996; Gaits et al., 1998; Gaits and Russell, 1999). Hence, the effect of increasing concentrations of H2O2 on the phosphorylation status of both Sty1 MAPK and Atf1 was examined.
A wild-type strain bearing a 6His- and HA-tagged Sty1 was subjected to increasing concentrations of H2O2. Phosphorylation of Sty1 was monitored by Western blotting by using an antibody that recognizes only the phosphorylated, and by inference, activated form of Sty1 (Gaits et al., 1998). The cellular levels of phosphorylated Sty1 were found to increase with increasing concentrations of H2O2 (Figure 5A).
Figure 5.
Sty1 MAP kinase is activated in a dose-dependent manner by H2O2. (A) Western blot of Ni2+-NTA (QIAGEN) agarose-purified Sty1, tagged with 6His residues and HA, probed with the anti-p38 antibody (α-pp38) or the anti-HA antibody (α-HA). Sty1 was purified after treatment of a wild-type strain carrying tagged Sty1 with 0, 0.07, 0.15, 0.25, 0.5, 1.0, 2.0, or 6.0 mM H2O2 for 10 min. (B) Western blot of Ni2+-NTA agarose-purified Atf1, tagged with 6His residues and HA (ATF1–6His-HA), probed with the anti-HA antibody. Atf1 was purified after treatment of a wild-type strain carrying tagged Atf1 with 0, 0.07, 0.15, 0.25, 0.5, 1.0, 2.0, or 6.0 mM H2O2 for 10 min. A nonspecific band that reacts with the anti-HA antibody and runs slightly above Atf1 is shown to emphasize the change in mobility of Atf1.
To examine phosphorylation of Atf1 in response to increasing H2O2 concentrations, a strain carrying a 6His- and HA-tagged Atf1 was subjected to the same H2O2 concentrations used to assay Sty1 phosphorylation (Figure 5B). Differentially phosphorylated forms of Atf1 were resolved by SDS-PAGE and detected by Western blotting with an HA antibody (Shiozaki and Russell, 1996). Increasing the concentration of H2O2 was found to cause a gradual decrease in the mobility of Atf1, suggesting that the Atf1 protein is increasingly phosphorylated as H2O2 levels increase (Figure 5B). The increase in phosphorylation of Atf1 is most likely due to an increase in Sty1 activation because phosphorylation of Atf1 has previously been shown to be Sty1 dependent (Shiozaki and Russell, 1996). This gradual increase in phosphorylation may be important for Atf1 function (see DISCUSSION).
Role of MAPKKKs Wak1 and Win1 in Response to Low and High Levels of Peroxide Stress
As shown above, the Sty1 MAPK and the transcription factors Atf1 and Pap1 are regulated differently, depending on the level of stress imposed. Hence, we next investigated the role of the upstream components of the Sty1 pathway, the Wis1 MAPKK and the Wak1/Win1 MAPKKKs, in regulating Sty1 activation in response to increasing levels of H2O2. Wild-type cells and win1-1 and wak1− mutants, all carrying a 6His- and HA-tagged Sty1, were treated with a range of H2O2 concentrations and Sty1 phosphorylation examined. Interestingly, a high basal level of Sty1 activation is consistently observed in the win1-1 mutant but not in wak1− cells. However, at both 0.2 and 1 mM concentrations of H2O2, wild-type levels of Sty1 activation are observed in both MAPKKK mutant strains, although the kinetics of Sty1 activation is slightly delayed in the wak1− mutant at 0.2 mM H2O2 (Figure 6A). These results concur with previous observations (Samejima et al., 1997; Shiozaki et al., 1998) and demonstrate that Wak1 and Win1 may have overlapping functions in the regulation of the Sty1 pathway at low levels of H2O2. However, upon treating cells with 6 mM H2O2 we reproducibly observed a large decrease in Sty1 activation in both the wak1− and win1-1 strains (Figure 6A). Quantification of the immunoblot revealed that the fold induction of Sty1 was equally impaired in both MAPKKK mutant strains. This result demonstrates that both Wak1 and Win1 are necessary for maximal Sty1activation in response to high levels of H2O2.
Figure 6.
Wak1 and Win1 MAPKKKs and the Wis1 MAPKK are required for the dose-dependent activation of Sty1 by H2O2. (A) Strains carrying inactive alleles of wak1+ or win1+ display near wild-type levels of Sty1 phosphorylation in response to low (0.2 mM) and intermediate (1.0 mM) levels of H2O2. However, at 6 mM H2O2, a decrease in Sty1 phosphorylation is seen in both the wak1− and win1-1 strains. Strains were grown to exponential phase and treated with a range of H2O2 concentrations for 0, 5, and 10 min. Cells were harvested, lysed, and Sty1 was purified on Ni2+-NTA (QIAGEN) agarose. Purified Sty1 was Western blotted and probed with the anti-p38 antibody (α-pp38). Blots were stripped and reprobed with the anti-HA antibody to ensure even loading of Sty1 (our unpublished data). Note that the basal activities seen at 0.2 and 1 mM H2O2 are not seen at 6 mM H2O2 because shorter exposures are necessary due to the high level of Sty1 activation seen in the wild-type strain at this level of H2O2. (B) A strain carrying inactive alleles of both wak1+ and win1+ demonstrates reduced activation of Sty1 at low (0.2 mM), intermediate (1.0 mM), and high concentrations of H2O2 (6.0 mM). A strain carrying mutations in the conserved activating phosphorylation sites of Wis1 (Wis1AA) is unable to phosphorylate Sty1 in response to high or low levels of H2O2. Purified Sty1 was Western blotted and probed with the anti-p38 antibody (α-pp38).
To investigate the potential redundancy that exists between the two MAPKKKs, Sty1 phosphorylation was examined in a wak1− win1-1 double mutant and also in a strain carrying an unphosphorylatable Wis1 MAPKK: wis1AA. At all H2O2 concentrations, Sty1 phosphorylation was significantly inhibited in the wak1− win1-1 strain and barely detectable in the wis1AA mutant (Figure 6B). These data demonstrate the importance of Wis1 phosphorylation and confirm the overlapping functions of the two MAPKKKs at low concentrations of H2O2 in the oxidative stress response. At high levels of H2O2, the amount of Sty1 activation in the wak1− win1-1 double mutant was slightly less than in the single wak1− mutant, indicating that some redundancy still exists between the MAPKKKs in response to acute stress (our unpublished data).
Collectively, these results agree with previous work using the wis1AA allele (Shieh et al., 1998; Shiozaki et al., 1998) but contradict the work of Samejima et al. (1997) who report that Sty1 activation is unimpaired in the wak1− win1-1 strain in response to H2O2. The level of Sty1 phosphorylation that we observe in the wak1− win1-1 strain is higher than that observed in the wis1AA mutant, which may suggest the presence of a third kinase that can phosphorylate Wis1. However, the data indicate that the majority of the signal from peroxide stress is signaling through the MAPKKKs Wak1 and Win1.
Two-Component Signaling Is Required for Activation of Sty1 in Response to Low Levels of Peroxide Stress
A two-component-like phosphorelay system has recently been identified, which is specifically required for activation of Sty1 in response to peroxide stress (Nguyen et al., 2000; Buck et al., 2001). The phosphorelay system in S. pombe comprises three histidine kinases, Mak1, Mak2, and Mak3; the phosphorelay protein Mpr1; and the response regulator protein Mcs4 (Nguyen et al., 2000; Buck et al., 2001). Mcs4 has been shown to bind to Wak1, thus directly linking the two-component system and the Sty1 pathway (Buck et al., 2001). Deletion of either of the histidine kinase genes mak2+ or mak3+, but not mak1+, prevents phosphorylation of Sty1 in response to 1 mM H2O2 (Buck et al., 2001). This suggests that the peroxide signal in fission yeast is sensed by a heterodimeric complex, including Mak2 and Mak3, which is then signaled through Mpr1 and Mcs4 to Wak1 (and possibly Win1).
We next investigated the role of this two-component signaling system in regulating Sty1 activation over a range of peroxide concentrations. Wild-type cells, or cells individually deleted for the three histidine kinases mak1+, mak2+, or mak3+ (all carrying a 6His- and HA-tagged Sty1) were treated with a range of H2O2 concentrations and phosphorylation of Sty1 monitored (Figure 7). In agreement with previous findings, treatment with 1 mM H2O2 results in a rapid increase in Sty1 activation in both wild-type and mak1− cells, whereas the response is considerably diminished in mak2− and mak3− strains. Moreover, Mak2 and Mak3 appear to play a similar role in the response to low concentrations of peroxide because a significant decrease in Sty1 activation is seen in mak2− and mak3− strains after treatment with 0.07 and 0.2 mM H2O2. In contrast, deletion of mak1+ results in a stimulation of Sty1 activation compared with wild-type cells, at these concentrations of H2O2. This is particularly evident at very low concentrations of H2O2 (0.07 mM) in which phosphorylation of Sty1 is barely detectable in wild-type cells. These results suggest that the Mak1 histidine kinase is also a sensor of peroxide stress but, unlike Mak2 and Mak3, has an inhibitory effect on Sty1 activation at low H2O2 concentrations.
Figure 7.
Activation of Sty1 at low concentrations of H2O2 is regulated by a phophorelay initiated by the Mak2/3 histidine kinases. Mak2 and Mak3 are required for Sty1 phosphorylation in response to low but not high concentrations of H2O2. Exponentially growing wild-type cells or cells carrying inactive alleles of mak1+, mak2+, or mak3+ were treated with a range of H2O2 concentrations for 0, 5, and 10 min and then subjected to a Sty1 phosphorylation assay as described previously.
Surprisingly, significant Sty1 phosphorylation occurs rapidly in all the histidine kinase mutants after treatment with 6 mM H2O2. This suggests that activation of the Sty1 pathway in response to high peroxide concentrations can occur independently of, or in addition to, the two-component pathway. It was possible that under such acute conditions the Mak2 and Mak3 histidine kinases function independently to transmit the signal to the MAPK cascade. However, a a mak2− mak3− double mutant strain shows significant Sty1 phosphorylation after exposure to 6 mM H2O2 (our unpublished data). Furthermore, a strain carrying a mutant allele of the response regulator Mcs4, containing a nonphosphorylatable asparagine at position 412, also demonstrates considerable Sty1 activation at 6 mM H2O2 (our unpublished data). Thus, another pathway(s) independent of the two-component system is involved in sensing high levels of peroxide stress.
DISCUSSION
Studies on stress signaling pathways have historically concentrated on a few standardized stress conditions. This methodology has proved invaluable in providing the basic framework by which such systems operate. However, in reality, stresses imposed on the cell vary in intensity and, therefore, in the kind of damage they inflict. Hence, it is important to address how the cell senses the level of stress to generate an appropriate response. We have addressed this important biological question by examining the role of the Sty1 pathway, and the downstream transcription factors Pap1 and Atf1, in controlling the response to varying levels of H2O2 in the model eukaryote S. pombe.
Role of Sty1 in the H2O2 Response
In this study we found that S. pombe mounts two separate responses to H2O2 stress: an adaptive response to low-level H2O2 exposure, which protects the cell from subsequent exposures to higher concentrations of H2O2; and an acute or survival response, which allows the cell to survive a sudden and potentially lethal exposure to H2O2 (summarized in Figure 8). Inactivation of sty1+ prevents either a low-level or acute response to H2O2 stress. Indeed, we find that Sty1 is required for target gene expression over a wide range of H2O2 concentrations. Interestingly, this dependence on Sty1 diminishes at very low levels of H2O2 where the expression of genes, such as tpx1+ and ctt1+, becomes progressively Sty1 independent.
Figure 8.
Dose-dependent response to H2O2 is regulated by distinct signaling and transcription factors. A peroxide-sensing two-component pathway, which regulates the MAP kinase module, is required for the response to low but not high concentrations of the oxidant. This pathway interacts with the MAPK pathway through either of the two MAPKKKs. A distinct peroxide-sensing pathway responds to high levels of peroxide stress in which both MAPKKKs are needed to relay the signal to Sty1. Thus, different sensing pathways are being used to respond to different intensities of the same stress. With increasing levels of peroxide stress more of the cellular Sty1 MAPK is phosphorylated. At the level of target gene expression, two transcription factors, Pap1 and Atf1, were found to have distinct roles in controlling the response to H2O2, inducing specific target genes in response to either low or high concentrations of oxidant.
How does Sty1 regulate two distinct responses to H2O2 stress? We show that as H2O2 levels increase there is a corresponding increase in the levels of active Sty1. The magnitude and duration of MAPK activation have been proposed as a mechanism by which signaling through a single pathway results in distinct responses. For example, activation of the ERK2 pathway in PC12 cells can lead either to proliferation or differentiation, depending on the level of MAPK activation, and, dose-dependent activation of the S. cerevisiae mating pathway predicates whether cells mate or differentiate into filamentous cells (Marshall, 1995; Sabbagh et al., 2001).
Transcriptional Control of H2O2-responsive Genes
In this study we have shown that Pap1 is required primarily for the response to low-level H2O2 stress, whereas Atf1 is more important for the response to acute levels of H2O2. Thus, tpx1+expression was induced in response to low levels of H2O2 in a Pap1-dependent manner, whereas gpx1+ expression was induced primarily at high concentrations of H2O2 and required Atf1. These data imply that different oxidative stress response genes are important in the low-level versus acute responses to peroxide stress. A similar strategy is used by S. cerevisiae where the ALO1 gene, involved in the synthesis of the antioxidant d-erythroascorbic acid, is required for resistance to acute levels of H2O2 but apparently plays no role in the adaptive response to H2O2 (Huh et al., 1998).
In S. pombe, ctt1+ was expressed over a wide range of H2O2 concentrations. Interestingly, the transcription factor requirements for ctt1+ expression changes depending on the concentration of H2O2; at low levels of H2O2, Pap1 is used predominantly, but as H2O2 levels increase Atf1 becomes more important than Pap1. Previous studies have shown that the ctt1+ promoter contains both Pap1 and Atf1 binding sites (Nakagawa et al., 1998, 2000). Taken together, our results imply that these promoter elements are differentially used in response to specific concentrations of H2O2.
The differences in the expression patterns of ctt1+, tpx1+, and gpx1+, over a range of H2O2 concentrations, and in different mutant backgrounds, highlight the complexity of the oxidative stress response. Thus, we find that although Pap1 plays a predominant role in the low-dose response, there is some induction of ctt1+ in a pap1− strain at low concentrations of H2O2, which is dependent on Atf1 (Figure 2). At intermediate doses of H2O2 either Pap1 or Atf1 can regulate the expression of ctt1+, and at high levels of stress (although Atf1 is the more important factor) there is some induction of ctt1+ that is dependent on Pap1. Clearly, the responses to low versus high doses of H2O2 overlap. Determining the extent of overlap is complicated by compensatory interactions that become apparent when we inactivate the specific regulatory factors (see below). On the whole, however, our data show that Pap1 functions primarily in the response to low levels of peroxide stress, whereas Atf1 primarily regulates the response to high levels of H2O2.
How do different levels of H2O2 stress regulate Pap1 and Atf1 activities? We have shown that as H2O2 levels increase a greater proportion of Sty1 is activated and that Atf1, a known target of Sty1, is increasingly phosphorylated. These results correlate with the observations that Atf1 and Sty1 are specifically required for survival and for the transcriptional response at high levels of H2O2. The role of phosphorylation in regulating Atf1 activity, however, remains unclear. ATF2 in mammalian cells is phosphorylated on Thr69 and Thr71 residues by both JNK and p38 kinases (Livingstone et al., 1995), and these modifications result in an increased ability to activate target gene expression. In fission yeast, there are 11 potential phosphorylation sites on Atf1 and a cumulative level of phosphorylation may be more critical than phosphorylation of specific sites. Indeed, studies with other proteins, such as the cyclin kinase inhibitor Sic1 in S. cerevisiae, suggest that phosphorylation of multiple residues allows proteins to be regulated in a switch-like manner (Nash et al., 2001).
Previously, it has been shown that Pap1 activity is regulated primarily by oxidative stress-dependent changes in subcellular localization (Toone et al., 1998; Toone et al., 2001). Herein, we show that in response to low levels of H2O2 Pap1 quickly accumulates in the nucleus, but as the dose of H2O2 increases, the time taken for accumulation also increases. This observation fits well with the gene expression and H2O2 sensitivity data, which indicate that Pap1 primarily controls the response to low-level H2O2 stress. Studies in S. cerevisiae have shown that a homolog of Pap1, Yap1, is also regulated at the level of nuclear localization (Kuge et al., 1997, 1998). In response to H2O2 intramolecular disulfide bonds are formed in Yap1, which mask the accessibility of the nuclear export machinery to a C-terminal nuclear export sequence, resulting in accumulation of Yap1 in the nucleus (Delaunay et al., 2000; Kuge et al., 2001). Similar mechanisms appear to be operating to control Pap1 localization (Kudo et al., 1999; E. Hidalgo, unpublished data). Interestingly, Kuge et al. (2001) have shown that different cysteine residues are required, and different disulfide linkages formed within Yap1, depending on the type of the oxidative stress, as well as on the duration of the stress. Moreover, Delaunay et al. (2000) have shown that Yap1 becomes increasingly oxidized as H2O2 levels increase. These observations provide the basis for a model for how nuclear accumulation of Pap1 might be delayed by increasing concentrations of H2O2. It is likely that only certain oxidation states, imposed by specific concentrations of H2O2, result in an active Pap1. At high concentrations of H2O2 Pap1 may assume an inactive conformation. At these and intermediate concentrations, degradation of H2O2 by antioxidants, present either at steady-state levels within the cell or induced by Atf1, would be required before Pap1 could reach and active oxidized form.
Nuclear accumulation of Pap1, and expression of Pap1-dependent genes in response to H2O2, are impaired in sty1− cells, suggesting a role for Sty1 in the regulation of Pap1. Interestingly, inactivation of Pap1 results in the superinduction of Sty1 and Atf1-dependent genes such as gpx1+. Thus, there appears to be cross-talk among the Sty1, Atf1, and Pap1 proteins. An explanation for the apparent role for Sty1 in Pap1-dependent gene expression has been presented by Nguyen et al. (2000) who showed that, in the absence of Sty1, unphosphorylated Atf1 (the predominant form of Atf1 in sty1− cells) is able to repress Pap1-dependent genes such as ctt1+. This repression is lost in the absence of Atf1 such that Pap1-dependent ctt1+ induction is recovered in a sty1− atf1− double mutant (Nguyen et al., 2000). Similarly, we have found that induction of other genes, such as apt1+ and trr1+, which requires Pap1 but not Atf1, is impaired in sty1− cells but recovered in a sty1− atf1− double mutant (our unpublished data). Atf1, therefore, may negatively regulate Pap1 target genes by binding directly to their promoters, or alternatively, the phosphorylation state of Atf1 may affect Pap1 localization/activity. Why this interplay between Pap1 and Sty1-Atf1 changes at low levels of H2O2, where Pap1-dependent transcription is less reliant on Sty1, is unclear and will require further investigation.
Distinct Signaling Pathways Are Used to Detect H2O2 Stress
Distinct signaling pathways were found to be activated depending on the level of the stress. A two-component signaling system, regulated by the histidine kinases Mak2 and Mak3, is required for activation of Sty1 at low concentrations of H2O2 but upon exposure to high concentrations of H2O2, Sty1 is strongly activated independently of Mak2 and Mak3. It is possible that the two-component pathway still responds to high levels of H2O2, but this must be in addition to an unknown mechanism(s) that induces Sty1 activation. Interestingly, at very low concentrations of H2O2, a third histidine kinase, Mak1, exhibits an inhibitory effect on Sty1 activation because deletion of mak1+ results in increased phosphorylation of Sty1. Thus, all three histidine kinases in S. pombe have a function in controlling the response to H2O2. The inhibitory role of Mak1 at low concentrations of H2O2 may be to “dampen” the signal coming from Mak2 and Mak3, resulting in low levels of Sty1 activation and Atf1 phosphorylation, thus maintaining Pap1 as the critical transcription factor. At higher levels of peroxide stress, where Atf1 becomes increasingly important, the inhibitory effect of Mak1 on Sty1 activation is lost, resulting in increased Sty1 activation with a concomitant increase in Atf1 phosphorylation. The mechanisms controlling the regulation of these histidine kinases are unknown. However, the different arrangement of potential redox sensing domains in Mak1 compared with those in Mak2 and Mak3 may function to regulate the adjacent histidine kinase domain differently in response to H2O2 (Buck et al., 2001).
The MAPKKKs Wak1 and Win1, which function downstream of the two-component system, behave redundantly in response to low levels of H2O2. This implies that either of these MAPKKKs can interact with Mcs4 and respond to stimuli from upstream histidine kinases. Indeed, both Wak1 and Win1 share a sequence motif with the S. cerevisiae MAPKKK, Ssk2, which has been found to be critical for binding the Ssk1 response regulator (Posas and Saito, 1998; B. Morgan, unpublished observation). Interestingly, at high concentrations of H2O2, in which a two-component–independent signaling system is activated, Wak1 and Win1 are both required for maximal Sty1 activation.
In summary, we have uncovered distinct signal transduction pathways that control the graded transcriptional response to increasing H2O2 levels in the fission yeast S. pombe. These results provide insight into how the cell distinguishes between and responds to different levels of oxidative stress. In higher eukaryotes stress-activated protein kinase pathways transmit signals from a large range of agonists and instigate a variety of outcomes, including adaptive responses, repair, differentiation, transformation, and apoptosis. The mechanisms by which these pathways differentially respond to a range and intensity of stimuli are, in most cases, unclear. Because stress responses involve evolutionarily conserved signaling pathways, S. pombe presents a useful model for the way in which cells sense and respond to stress in other systems.
ACKNOWLEDGMENTS
We thank Hiromi Maekawa, Panagiota Malakasi, Deborah Smith, Elizabeth Veal, Simon Whitehall, Shusuke Kuge, and Ann Flenniken for discussions and comments on the manuscript; Janni Peterson and Steve Bagley for help with immunofluorescence; Vicky Buck for technical assistance; and E. Hidalgo, P. Fantes, and K. Shiozaki for the kind gift of strains and reagents. This work was funded by Cancer Research UK, the BBSRC, Medical Research Council (M.R.C.), and the Wellcome Trust. J.Q. is funded by an MRC Career Development Award.
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.01–06–0288. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.01–06–0288.
REFERENCES
- Alfa CE, Gallagher IM, Hyams JS. Antigen localization in fission yeast. Methods Cell Biol. 1993;37:201–222. doi: 10.1016/s0091-679x(08)60251-4. [DOI] [PubMed] [Google Scholar]
- Buck V, Quinn J, Soto Pino T, Martin H, Saldanha J, Makino K, Morgan BA, Millar JBA. Peroxide sensors for the fission yeast stress-activated mitogen-activated protein kinase pathway. Mol Biol Cell. 2001;12:407–419. doi: 10.1091/mbc.12.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinson LP, Dawes IW. Inducibility of the response of yeast cells to peroxide stress. J Gen Microbiol. 1992;138:329–335. doi: 10.1099/00221287-138-2-329. [DOI] [PubMed] [Google Scholar]
- Degols G, Shiozaki K, Russell P. Activation and regulation of the Spc1 stress-activated protein kinase in Schizosaccharomyces pombe. Mol Cell Biol. 1996;16:2870–2877. doi: 10.1128/mcb.16.6.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degols G, Russell P. Discrete roles of the Spc1 kinase and the Atf1 transcription factor in the UV response of Schizosaccharomyces pombe. Mol Cell Biol. 1997;17:3356–3363. doi: 10.1128/mcb.17.6.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaunay A, Isnard AD, Toledano MB. H2O2 sensing through oxidation of the Yap1 transcription factor. EMBO J. 2000;19:5157–5166. doi: 10.1093/emboj/19.19.5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman BA, Crapo JD. Biology of disease: free radicals and tissue injury. Lab Invest. 1982;47:412–426. [PubMed] [Google Scholar]
- Gaits F, Degols G, Shiozaki K, Russell P. Phosphorylation and association with the transcription factor Atf1 regulate localization of Spc1/Sty1 stress-activated kinase in fission yeast. Genes Dev. 1998;12:1464–1473. doi: 10.1101/gad.12.10.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaits F, Russell P. Active nucleocytoplasmic shuttling required for function and regulation of stress-activated kinase Spc1/Sty1 in fission yeast. Mol Biol Cell. 1999;10:1395–1407. doi: 10.1091/mbc.10.5.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan I, Ayscough KR. Fluorescence microscopy in yeast. In: Allan VJ, editor. Protein Localization by Fluorescence Microscopy. New York: Oxford University Press; 2000. pp. 179–206. [Google Scholar]
- Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. Oxford, UK: Oxford University Press; 1999. [Google Scholar]
- Huh WK, Lee BH, Kim ST, Kim YR, Rhie GE, Baek YW, Hwang CS, Lee JS, Kang SO. d-Erythroascorbic acid is an important antioxidant molecule in Saccharomyces cerevisiae. Mol Microbiol. 1998;30:895–903. doi: 10.1046/j.1365-2958.1998.01133.x. [DOI] [PubMed] [Google Scholar]
- Jamieson DJ. Saccharomyces cerevisiae has distinct adaptive responses to both hydrogen peroxide and menadione. J Bacteriol. 1992;174:6678–6681. doi: 10.1128/jb.174.20.6678-6681.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo N, Taoka H, Toda T, Yoshida M, Horinouchi S. A novel nuclear export signal sensitive to oxidative stress in the fission yeast transcription factor Pap1. J Biol Chem. 1999;274:15151–15158. doi: 10.1074/jbc.274.21.15151. [DOI] [PubMed] [Google Scholar]
- Kuge S, Jones N, Nomoto A. Regulation of yAP-1 nuclear localization in response to oxidative stress. EMBO J. 1997;16:1710–1720. doi: 10.1093/emboj/16.7.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuge S, Arita M, Murayama A, Maeta K, Izawa S, Inoue Y, Nomoto A. Regulation of the yeast Yap1p nuclear export signal is mediated by redox signal-induced reversible disulfide bond formation. Mol Cell Biol. 2001;15:6139–6150. doi: 10.1128/MCB.21.18.6139-6150.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuge S, Toda T, Iizuka N, Nomoto A. Crm1 (XpoI) dependent nuclear export of the budding yeast transcription factor yAP-1 is sensitive to oxidative stress. Genes Cells. 1998;3:521–532. doi: 10.1046/j.1365-2443.1998.00209.x. [DOI] [PubMed] [Google Scholar]
- Kumada K, Yanagida M, Toda T. Caffeine-resistance in fission yeast is caused by mutations in a single essential gene, crm1+ Mol Gen Genet. 1996;250:59–68. doi: 10.1007/BF02191825. [DOI] [PubMed] [Google Scholar]
- Lee J, Dawes IW, Roe JH. Adaptive response of Schizosaccharomyces pombe to hydrogen peroxide and menadione. Microbiology. 1995;141:3127–3132. doi: 10.1099/13500872-141-12-3127. [DOI] [PubMed] [Google Scholar]
- Livingstone C, Patel G, Jones N. ATF-2 contains a phosphorylation-dependent transcriptional activation domain. EMBO J. 1995;14:1785–1797. doi: 10.1002/j.1460-2075.1995.tb07167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall CJ. Specificity of receptor tyrosine signaling: transient versus sustained extracellular signal regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- Millar JBA, Buck V, Wilkinson MG. Pyp1 and Pyp2 PTPases dephosphorylate an osmosensing MAP kinase controlling cell size at division in fission yeast. Genes Dev. 1995;9:2117–2130. doi: 10.1101/gad.9.17.2117. [DOI] [PubMed] [Google Scholar]
- Moradas-Ferreira P, Costa V. Adaptive response of the yeast Saccharomyces cerevisiae to reactive oxygen species. defenses, damage and death. Redox Rep. 2000;5:277–285. doi: 10.1179/135100000101535816. [DOI] [PubMed] [Google Scholar]
- Moreno S, Klar A, Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Nakagawa CW, Yamada K, Mutoh N. Two distinct upstream regions are involved in expression of the catalase gene in Schizosaccharomyces pombe in response to oxidative stress. J Biochem. 1998;123:1048–1054. doi: 10.1093/oxfordjournals.jbchem.a022042. [DOI] [PubMed] [Google Scholar]
- Nakagawa CW, Yamada K, Mutoh N. Role of Atf1, and Pap1 in the induction of the catalase gene of fission yeast Schizosaccharomyces pombe. J Biochem. 2000;127:233–238. doi: 10.1093/oxfordjournals.jbchem.a022599. [DOI] [PubMed] [Google Scholar]
- Nash P, Tang X, Orlicky S, Chen Q, Gertler FB, Mendenhall MD, Sicheri F, Pawson T, Tyers M. Multi-site phosphorylation of a CDK inhibitor sets a threshold for the onset of DNA replication. Nature. 2001;414:516–523. doi: 10.1038/35107009. [DOI] [PubMed] [Google Scholar]
- Nguyen AN, Lee A, Place W, Shiozaki K. Multistep phosphorelay proteins transmit oxidative stress signals to the fission yeast stress-activated protein kinase. Mol Biol Cell. 2000;14:1169–1181. doi: 10.1091/mbc.11.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posas F, Saito H. Activation of the yeast SSK2 MAP kinase kinase kinase by the SSK1 two-component response regulator. EMBO J. 1998;17:1385–1394. doi: 10.1093/emboj/17.5.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posas F, Wurgler-Murphy SM, Maeda T, Witten EA, Thai TC, Saito H. Yeast HOG1 MAP kinase cascade is regulated by a multi-step phospho-relay mechanism in the SLN1-YPD1-SSK1 ‘two-component’ system. Cell. 1996;86:865–875. doi: 10.1016/s0092-8674(00)80162-2. [DOI] [PubMed] [Google Scholar]
- Sabbagh W, Flatauer LJ, Bardwell AJ, Bardwell L. Specificity of MAP kinase signaling in yeast differentiation involves transient versus sustained MAPK activation. Mol Cell. 2001;8:683–691. doi: 10.1016/s1097-2765(01)00322-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samejima I, Mackie S, Fantes PA. Multiple modes of activation of the stress-responsive MAP kinase pathway in fission yeast. EMBO J. 1997;16:6162–6170. doi: 10.1093/emboj/16.20.6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samejima I, Mackie S, Warbrick E, Weisman R, Fantes PA. The fission yeast mitotic regulator win1+ encodes a MAP kinase kinase kinase that phosphorylates and activates Wis1 MAP kinase kinase in response to high osmolarity. Mol Biol Cell. 1998;9:2325–2335. doi: 10.1091/mbc.9.8.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh JC, Wilkinson MG, Buck V, Morgan BA, Makino K, Millar JB. The Mcs4 response regulator coordinately controls the stress-activated Wak1-Wis1-Sty1 MAP kinase pathway and fission yeast cell cycle. Genes Dev. 1997;11:1008–1022. doi: 10.1101/gad.11.8.1008. [DOI] [PubMed] [Google Scholar]
- Shieh JC, Wilkinson MG, Millar JB. The Win1 mitotic regulator is a component of the fission yeast stress-activated Sty1 MAPK pathway. Mol Biol Cell. 1998;9:311–322. doi: 10.1091/mbc.9.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozaki K, Russell P. Cell cycle control linked to extracellular environment by MAP kinase pathway in fission yeast. Nature. 1995;378:739–743. doi: 10.1038/378739a0. [DOI] [PubMed] [Google Scholar]
- Shiozaki K, Russell P. Conjugation, meiosis, and the osmotic stress response are regulated by Spc1 kinase through Atf1transcription factor in fission yeast. Genes Dev. 1996;10:2276–2288. doi: 10.1101/gad.10.18.2276. [DOI] [PubMed] [Google Scholar]
- Shiozaki K, Shiozaki M, Russell P. Mcs4 mitotic catastrophe suppressor regulates the fission yeast cell cycle through the Wik1-Wis1-Spc1 kinase cascade. Mol Biol Cell. 1997;8:409–419. doi: 10.1091/mbc.8.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozaki K, Shiozaki M, Russell P. Heat stress activates fission yeast Spc1/Sty1 MAPK by a MEKK-independent mechanism. Mol Biol Cell. 1998;9:1339–1349. doi: 10.1091/mbc.9.6.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda T, Toda T, Kominami K, Kohnosu A, Yanagida M, Jones N. Schizosaccharomyces pombe atf1+ encodes a transcription factor required for sexual development and entry into stationary phase. EMBO J. 1995;14:6193–6208. doi: 10.1002/j.1460-2075.1995.tb00310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbles LA, Woodgett JR. The stress-activated protein kinase pathways. Cell Mol Life Sci. 1999;55:1230–1254. doi: 10.1007/s000180050369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T, Shimanuki M, Yanagida M. Fission yeast genes that confer resistance to staurosporine encode an AP-1-like transcription factor and a protein kinase related to the mammalian ERK1/MAP2 and budding yeast FUS3 and KSS1 kinases. Genes Dev. 1991;5:60–73. doi: 10.1101/gad.5.1.60. [DOI] [PubMed] [Google Scholar]
- Toone WM, Jones N. Stress-activated signaling pathways in yeast. Genes Cells. 1998;3:485–498. doi: 10.1046/j.1365-2443.1998.00211.x. [DOI] [PubMed] [Google Scholar]
- Toone WM, Kuge S, Samuels M, Morgan BA, Toda T, Jones N. Regulation of the fission yeast transcription factor Pap1 by oxidative stress: requirement for the nuclear export factor Crm1 (Exportin) and the stress-activated MAP kinase Sty1/Spc1. Genes Dev. 1998;12:1453–1463. doi: 10.1101/gad.12.10.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toone WM, Morgan BA, Jones N. Redox control of AP-1-like factors in yeast, and beyond. Oncogene. 2001;20:2336–2346. doi: 10.1038/sj.onc.1204384. [DOI] [PubMed] [Google Scholar]
- White JH, Green SR, Barker DG, Dumas LB, Johnston LH. The CDC8 transcript is cell cycle regulated in yeast and is expressed coordinately with CDC9 and CDC21 at a point preceding histone transcription. Exp Cell Res. 1987;171:223–231. doi: 10.1016/0014-4827(87)90265-5. [DOI] [PubMed] [Google Scholar]
- Wilkinson MG, Samuels M, Takeda T, Toone WM, Shieh JC, Toda T, Millar JB, Jones N. The Atf1 transcription factor is a target for the Sty1 stress-activated MAP kinase pathway in fission yeast. Genes Dev. 1996;10:2289–2301. doi: 10.1101/gad.10.18.2289. [DOI] [PubMed] [Google Scholar]
- Yamada K, Nakagawa CW, Mutoh N. Schizosaccharomyces pombe homologue of glutathione peroxidase, which does not contain selenocysteine, is induced by several stresses and works as an antioxidant. Yeast. 1999;15:1125–1132. doi: 10.1002/(SICI)1097-0061(199908)15:11<1125::AID-YEA442>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]