Abstract
Background and Objectives
Vascular theories of cognitive aging have focused on macrovascular changes and cognitive decline. However, according to the artery-size hypothesis, microvascular changes, such as those that underlie changes in erectile function, may also play an important role in contributing to cognitive decline. Thus, we examined associations between erectile function, sexual satisfaction, and cognition starting in middle age because this represents a transition period where declines in these areas emerge.
Research Design and Methods
We examined 818 men from the Vietnam Era Twin Study of Aging across three waves at mean ages 56, 61, and 68. Erectile function and sexual satisfaction were measured using the International Index of Erectile Function. Cognitive performance was measured using factor scores for episodic memory, executive function, and processing speed. We tested multilevel models hierarchically, adjusting for demographics, frequency of sexual activity, and physical and mental health confounders to examine how changes in erectile function and sexual satisfaction related to changes in cognitive performance.
Results
Lower erectile function at baseline was related to poorer performance in all cognitive domains at baseline and faster declines in processing speed over time. However, baseline sexual satisfaction was unrelated to cognitive performance. Decreases in erectile function and sexual satisfaction were both associated with memory decline.
Discussion and Implications
Decreasing sexual health may signal an increased risk for cognitive decline. We discuss potential mechanisms, including microvascular changes and psychological distress. Discussing and tracking sexual health in middle-aged men may help to identify those likely to face memory decline.
Keywords: Cognition, Erectile dysfunction, Men’s issues, Sexual function, Sexual health
Background and Objectives
By 2050, the older adult population will triple, increasing the cases of cognitive impairment and related disorders such as Alzheimer’s disease (AD; Matthews et al., 2019). Physical symptoms that capture early health changes might be important for early identification and intervention of those at risk for cognitive decline in later life. In men, erectile function is affected by changes in physical health, including microvascular changes, arterial blood flow, and endothelial function (Dong et al., 2011). Problems in erectile function increase 10-fold as men enter their sixties, coinciding with declines in cognitive performance (Stranne et al., 2019). In addition, changes in erectile function can exert secondary effects, such as changes in sexual satisfaction. Sexual satisfaction may be negatively related to cognitive performance in a similar manner to other measures of life satisfaction (Enkvist et al., 2013). However, few studies have examined how sexual health is related to cognition in middle-aged and older men.
The conceptual frameworks guiding the present study are vascular health models of cognitive aging (DeRight et al., 2015; Earles & Salthouse, 1995; Rockwood, 2002) and the artery-size hypothesis (Montorsi et al., 2005). There are multiple variables that have been causally linked to erectile dysfunction (ED); one of the leading causes is microvascular dysfunction (Montorsi et al., 2005). Vascular dysfunction has been linked to theories of cognitive aging, which show that vascular- and other health-related cognitive deficits are most evident in attention–executive–psychomotor speed domains (Earles & Salthouse, 1995; Rockwood, 2002). Two studies found associations between erectile function and cognitive performance. In a cross- sectional study of 651 men ages 51–60 from the Vietnam Era Twin Study of Aging (VETSA), having ED was associated with worse performance in several domains, such as processing speed, memory, and abilities requiring executive functions (i.e., inhibition, abstract reasoning, and verbal fluency; Moore et al., 2014). These associations survived adjustment for other standard risk factors, including high cholesterol, hypertension, obesity, and smoking. Additionally, ED predicted increased dementia risk in a population-based study of 4,153 Taiwanese men of all ages (Yang et al., 2015). Across 7 years, men with ED were 68% more likely to develop AD and non-AD dementia, even after adjusting for other cardiovascular risk factors, depression, and anxiety (Yang et al., 2015). Previous studies did not consider continuous measures of erectile function or longitudinal changes in cognitive performance.
Other studies examined associations between erectile function and sexual satisfaction in men. Clinical samples have shown associations between erectile function and sexual satisfaction, in which poor erectile function was associated with low sexual satisfaction (Bravi et al., 2020; Gomes et al., 2017; Nelson et al., 2007). Engagement in sexual activity can affect quality-of-life for some older adults (Hinchliff et al., 2018) and has been shown to be related to better cognitive performance (Wright et al., 2019). Poor erectile function may reduce sexual activity and sexual satisfaction, in turn reducing psychological well-being (Hinchliff et al., 2018). Sexual satisfaction has been associated with general life satisfaction measures (Fergusson et al., 2015; Lombardo et al., 2018), and a portion of 1,084 adults aged 50–90 stated that sexual difficulties were negatively affecting their psychological well-being (Hinchliff et al., 2018). Although ED is commonly vascular, a percentage of cases are considered psychogenic (Rosen, 2001). This source of dysfunction may have a greater influence on sexual satisfaction due to the subjective nature of satisfaction. Psychogenic ED is typically comorbid with major psychiatric disorders such as depression (Rosen, 2001). Thus, individuals with depressive symptoms and ED may be most susceptible to worsening sexual satisfaction and cognition.
Few studies have examined links between sexual satisfaction and cognitive performance, and the results are mixed. Hsu et al. (2018) found no association between baseline sexual satisfaction and changes in cognitive screening scores 5 years later in men aged 70 and older. Conversely, Smith et al. (2021) found a significant association between higher sexual satisfaction at baseline and a lower risk of cognitive impairment 10 years later in cognitively normal, married, adults aged 65 and older. More studies are thus needed to understand associations with cognitive performance, especially moving beyond baseline predictive associations and looking at changes in sexual satisfaction.
The purpose of the present study was to explore the relationship between erectile function, sexual satisfaction, and cognitive performance in men from midlife (average ages 56 and 62) to early older adulthood (average age 68). In line with the first cross-sectional study on erectile function and cognition (Moore et al., 2014), analyses were domain-specific and focused on changes in episodic memory, executive function, and processing speed. These domains are vulnerable to age-related cognitive decline (Gerstorf et al., 2011) and accelerated by vascular disease (DeRight et al., 2015). We first hypothesized that both erectile function and sexual satisfaction would decline as people aged, as shown by higher rates of ED starting as men enter their sixties (Stranne et al., 2019). Second, we hypothesized that declines in erectile function would be associated with declines in episodic memory, executive function, and processing speed. Associations between changes in sexual satisfaction and cognitive performance were not hypothesized as there are mixed findings on these associations between sexual satisfaction and cognitive performance. Like previous studies in this field, analyses simultaneously considered the role of other health factors. We further explored what health factors were associated with levels of erectile function and sexual satisfaction to identify potential confounders in any associations between these variables and cognitive performance.
Research Design and Methods
Participants
Participants were community-dwelling men who participated in up to three waves of the VETSA, a longitudinal study of risk and protective factors of cognitive and brain aging (Kremen et al., 2006, 2013). VETSA participants were randomly recruited from Vietnam Era Twin Registry (VETR) members who participated in the Harvard Drug Study; they were not selected based on substance use or any other diagnoses (Tsuang et al., 2001). All served in the United States military at some time between 1965 and 1975 (Goldberg et al., 2002). Approximately 80% of the VETSA sample did not report any combat exposure. VETSA participants are a nationally representative sample because participants are similar in lifestyle and health characteristics to similarly aged American men (Schoenborn & Heyman, 2009). To be eligible for the baseline assessment (VETSA 1; 2002–2008), participants had to be between 51 and 59 years old at recruitment, and both twins in each pair had to agree to participate (Kremen et al., 2006). At VETSA 1, participants were mean age 55.75 (standard deviation [SD] = 2.42, range = 51.10–60.69). The first follow-up (VETSA 2) was approximately 6 years later when participants were on average 61.36 years (SD = 2.41, range = 56.45–66.07). The second follow-up (VETSA 3) occurred approximately 6 years after VETSA 2 when participants were on average 67.20 years (SD = 2.54, range = 61.37–72.13).
VETSA participants traveled to either the University of California, San Diego (UCSD) or Boston University (BU) for testing, or, in rare circumstances, staff traveled to a participants’ city of residence. Written informed consent was obtained from all participants, and the research protocol was approved by the UCSD Human Research Protection Program and the BU Charles River Campus Institutional Review Board.
Here we included individuals who did not have mild cognitive impairment or ED (defined later) at VETSA 1 (n = 818). Descriptive statistics are provided in Table 1. Overall, 93% (n = 759) were non-Hispanic White with an average education of 13.98 years (SD = 2.16) and an average income of $64,800 (SD = 2.97). Most (93%, n = 758) were in a partnered relationship.
Table 1.
Descriptive Characteristics of the Analytical Sample from Waves 1, 2, and 3
| Variable | Wave 1 | Wave 2 | Wave 3 | Change over timea | ||||
|---|---|---|---|---|---|---|---|---|
| n = 818 | n = 648 | n = 589 | Dist.b | β | OR | t | p | |
| Age, M (SD) | 55.74 (2.41) | 61.36 (2.42) | 67.20 (2.54) | — | — | — | — | — |
| Education (years), M (SD) | 13.98 (2.16) | 14.93 (2.16) | 14.17 (2.14) | — | — | — | — | — |
| Race (Ref. = non-Hispanic White), n (%) | 759 (92.8) | 595 (91.8) | 542 (92.0) | — | — | — | — | — |
| Income,cM (SD) | 6.48 (2.97) | 6.14 (3.08) | 6.14 (3.16) | G | −0.06 | — | 3.58 | <.001 |
| Relationship status (Ref. = partnered), n (%) | 758 (92.7) | 571 (88.1) | 499 (84.7) | L | — | 1.07 | 4.37 | <.001 |
| Erectile function, M (SD) | 17.91 (2.65) | 11.02 (6.99) | 11.18 (6.69) | G | −0.44 | — | 22.91 | <.001 |
| Change in erectile function | — | −6.84 (6.83) | −6.73 (6.36) | — | — | — | — | — |
| Sexual satisfaction, M (SD) | 12.70 (2.35) | 9.11 (3.95) | 8.61 (4.15) | G | −0.44 | — | 23.35 | <.001 |
| Change in sexual satisfaction | — | −3.59 (3.91) | −4.11 (4.20) | — | — | — | — | — |
| Frequency of sexual activity,d median (IQR) | 2 (1) | 2 (2) | 1 (2) | M | −0.39 | — | 23.61 | <.001 |
| Episodic memory, M (SD) | 0.0 (1.00) | −0.28 (1.06) | −0.55 (1.15) | G | −0.24 | — | 19.71 | <.001 |
| Executive function, M (SD) | 0.0 (1.00) | −0.55 (0.94) | −0.86 (.95) | G | −0.37 | — | 30.57 | <.001 |
| Processing speed, M (SD) | 0.0 (1.00) | −0.76 (1.05) | −1.16 (1.15) | G | −0.44 | — | 39.38 | <.001 |
| Number of CVDs, n (%) | — | — | — | P | — | 1.16 | 13.42 | <.001 |
| 0 | 299 (36.6) | 137 (21.1) | 89 (15.1) | — | — | — | — | — |
| 1 | 182 (22.2) | 125 (19.3) | 87 (14.8) | — | — | — | — | — |
| 2+ | 336 (41.1) | 386 (59.6) | 413 (70.1) | — | — | — | — | — |
| Number of non-CVD medical conditions, n (%) | — | — | — | P | — | 1.15 | 13.56 | <.001 |
| 0 | 426 (52.1) | 313 (48.3) | 221 (37.5) | — | — | — | — | — |
| 1 | 221 (27.0) | 244 (37.7) | 221 (37.5) | — | — | — | — | — |
| 2+ | 120 (14.7) | 91 (14.0) | 147 (25.0) | — | — | — | — | — |
| Depressive symptoms (CESD), M (SD) | 7.33 (7.24) | 6.55 (7.48) | 6.11 (6.86) | G | −0.07 | — | 4.28 | <.001 |
| Physical function (SF-36), M (SD) | 27.34 (3.30) | 26.38 (4.04) | 25.76 (4.33) | G | −0.74 | — | 11.53 | <.001 |
| Current smoking status (Ref. = yes), n (%) | 186 (22.7) | 8 (1.2) | 76 (12.9) | L | — | 1.12 | 7.61 | <.001 |
| Alcohol consumption,en (%) | — | — | — | M | — | — | 0.002 | <.001 |
| 0 (zero) | 245 (30.0) | 226 (34.9) | 214 (36.3) | — | — | 0.97 | 3.04 | .002 |
| 1 (1–28 drinks) | 462 (56.5) | 342 (52.8) | 304 (51.6) | — | — | — | — | — |
| 2 (>28 drinks) | 111 (13.6) | 80 (12.3) | 71 (12.1) | — | — | — | — | — |
| Erectile dysfunction medications, n (%) | — | — | — | P | — | 2.51 | 3.27 | .001 |
| 0 | 801 (97.9) | 546 (84.3) | 459 (77.9) | — | — | — | — | — |
| ≥1 | 17 (2.1) | 102 (15.7) | 130 (14.3) | — | — | — | — | — |
Notes: Each major header the second to fourth column represents the outcome of an age-based multilevel model predicting variables listed in the first column. OR = odds ratio; SD = standard deviation; CESD = Center for Epidemiological Studies; CVD = cardiovascular disease; Ref. = reference group; SF-36 = Short Form Quality-of-Life Questionnaire—36 Questions; G = Gaussian; IQR = Interquartile range; L = Logistic; M = multinomial; P = Poisson.
aResults from an age-based multilevel model predicting variable to evaluate if change over time is significant (time refers to age in years).
bDistribution of the multilevel model predicting variable (G = Gaussian for continuous outcomes; B = binomial for models with dichotomous outcomes; M = multinomial for ordinal outcomes; P = Poisson for count outcomes).
cMedian of an ordinal variable where 1 = <10,000; 2 = 10,000 to 19,999; 3 = 20,000 to 29,999; 4 = 30,000 to 39,999; 5 = 40,000 to 49,999; 6 = 50,000 to 59,999; 7 = 60,000 to 69,999, 8 = 70,000 to 79,999; 9 = 80,000 to 89,999; 10 = 90,000 to 99,999; 11 = 100,000 to 109,999; 12 = 110,000 to 119,999, 13 = 120,000 or more.
dAverage of an ordinal variable with responses of 1 (1–2 attempts), 2 (3–4 attempts), 3 (5–6 attempts), 4 (7–10 attempts), or 5 (11+ attempts).
eThe number of drinks consumed in the past 2 weeks.
Measures
Erectile function
Erectile function was measured using four items from the International Index of Erectile Function (IIEF; Rosen et al., 1997), shown in Supplementary Table S1. Ratings were summed into a total erectile function composite score (α = 0.96), prorated by the number of completed items (multiply sum by [number of missing + total possible]/total possible). Classification of ED, used to remove people at baseline, was determined by prorating the original cutoff for moderate to severe ED on the IIEF five-item (≤11; Rhoden et al., 2002) to account for the use of only four items, leading to a cutoff of ≤8 on the total score (11 × 4/5 = ~8).
Sexual satisfaction
Sexual satisfaction was measured using other items from the IIEF (Rosen et al., 1997), shown in Supplementary Table S1. Three items measured overall sexual satisfaction with their relationship and intercourse over the past 4 weeks. Participants who answered at least two of the three items were included. Ratings were summed into a total sexual satisfaction composite score (α = .72), prorated by the number of completed items (multiply sum by [number of missing + total possible]/total possible).
Cognitive performance
We focused on episodic memory (Kremen et al., 2014), executive function (Gustavson et al., 2019), and processing speed (Sanderson-Cimino et al., 2019), which are cognitive domains vulnerable to aging and significantly worsened in trajectories toward dementia. We assessed episodic memory via a factor score based on recall scores across the California Verbal Learning Test-II (Delis et al., 2000), and the Wechsler Memory Scale-III (WMS-III) Logical Memory and Visual Reproductions tests (Wechsler, 1997). We captured executive function as a factor score based on measures of inhibition, shifting, and working memory span from the Stroop test (Golden & Freshwater, 2002), Delis-Kaplan Executive Function System Trail Making Test (Delis et al., 2001), and WMS-III Letter-Number Sequencing and Digit Span tasks (Wechsler, 1997). We captured processing speed as a factor score based on simple and choice reaction time tests developed by Teng (1990) and the number of correctly named colors and words on congruent conditions of the Stroop (Golden & Freshwater, 2002). More details on these measures and factor score creation are available in prior work (Gustavson et al., 2019; Kremen et al., 2014; Sanderson-Cimino et al., 2019). Cognitive factor scores at follow-ups were adjusted for practice effects via methods previously described (Elman et al., 2018).
Covariates
For demographics, we measured two time-invariant variables of race (non-Hispanic White vs. non-White) and lifetime education (years completed) reported at baseline. Time-varying demographic covariates, that is, covariates measured at each wave, included age, relationship status (married/partnered vs. single/widowed), and frequency of sexual activity. The frequency of sexual activity was measured using one question from the IIEF, “Over the past four weeks, how many times have you attempted sexual intercourse?” Participants rated frequency of sexual activity, coded as: (0) zero attempts, (1) 1–2 attempts, (2) 3–4 attempts, (3) 5–6 attempts, (4) 7–10 attempts, or (5) 11+ attempts.
We also included time-varying health covariates. Using items from the Charlson index (Charlson et al., 1994), we created a total medical condition score for cardiovascular disease (CVD) conditions (stroke, heart attack, heart failure, peripheral vascular disease, thrombolysis, hypertension, and angina) and non-CVD conditions (diabetes, bronchitis, asthma, cancer, osteoarthritis, rheumatoid arthritis, and cirrhosis). We also adjusted for depressive symptoms, using the Centers for Epidemiological Studies Depression Scale (CESD; Radloff, 1977) and physical function, using the Short Form-36 (SF-36; Ware & Sherbourne, 1992) Physical Function subscale (i.e., ratings of limitations in 10 everyday activities). Finally, we measured alcohol consumption over the past 2 weeks (three-level ordinal variable: nondrinker; 1–28 drinks; and >28 drinks), current smoker status (currently smoking or not currently smoking), and the number of ED medications used. Medical conditions and alcohol consumption were based on a medical history interview. Coding of covariates is provided in the notes of Supplementary Table S2.
Statistical Analyses
We conducted analyses using SPSS Version 26 software (IBM Corp, 2020). We calculated means and standard deviations for continuous and ordinal data followed by frequencies and percentages for categorical group membership. Descriptive statistics for measures across waves are presented in Table 1. Changes in these variables over time were determined using multilevel models predicting outcomes using time calculated as age after 55 and baseline age, later referred to as age-based multilevel models (Mendes de Leon, 2007; Sliwinski et al., 2010). Gaussian, multinomial, and logistic multilevel models were used as required for continuous, ordinal, and binary outcome variables, respectively. For additional descriptive purposes, we examined how planned covariates predicted erectile function (Model 1) and sexual satisfaction (Model 2) over time using age-based multilevel models with random intercept and random effect of time-varying age using MIXED and GENLINMIXED syntax in SPSS. Observations of erectile function and sexual satisfaction were nested within individuals (i) and twin pairs (t). Equations for Models 1 and 2 are shown in Supplementary Table S2.
For main analyses, age-based multilevel models fitted with Gaussian distributions examined how erectile function and sexual satisfaction related to episodic memory, executive function, and processing speed in different models. As shown in Table 3, models were examined separately for erectile function (Model 3) and sexual satisfaction (Model 4) due to multicollinearity (r = 0.88) preventing model convergence. In these models, observations of cognitive performance over time (j) were nested within individuals (i) and twin pairs (t), that is, Yijt. Random effects were modeled for the intercept and time-varying age. As shown in Table 2, there were significant interindividual differences in baseline episodic memory, executive function, and processing speed (intraclass coefficients [σ 2/total variance] >60%). Meanwhile, there was little interindividual variability in the slope of cognitive change over time (random effect of time accounted for less than <1% of the total variance).
Table 3.
Multilevel Models of Erectile Function and Sexual Satisfaction Predicting Cognitive Performance at Baseline and Across Time
| Variable | Episodic memory | Executive function | Processing speed | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β (95% CI) | p | σ 2 | SE | β (95% CI) | p | σ 2 | SE | β (95% CI) | p | σ 2 | SE | |
| Fixed effects | ||||||||||||
| Time-varying Age (centered at 56) | −0.19 (−0.17, −0.55) | <.001 | −0.35 (−0.39, −0.32) | <.001 | −0.42 (−0.44, −0.39) | <.001 | ||||||
| Model 3: erectile function | ||||||||||||
| 3a Baseline erectile function (γ01) | 0.11 (0.05, 0.16) | <.001 | 0.08 (0.03, 0.14) | .003 | 0.11 (0.06, 0.17) | <.001 | ||||||
| 3a Change in erectile function (β2) | 0.08 (0.04, 0.13) | <.001 | 0.03 (−0.01, 0.08) | .164 | 0.04 (−0.01, 0.08) | .088 | ||||||
| 3b Baseline erectile function (γ01) | 0.11 (0.05, 0.16) | <.001 | 0.07 (0.02, 0.13) | .014 | 0.10 (0.04, 0.15) | .003 | ||||||
| 3b Change in erectile function (β2) | 0.09 (0.04, 0.13) | <.001 | 0.02 (−0.03, 0.06) | .435 | 0.03 (−0.02, 0.07) | .248 | ||||||
| Model 4: sexual satisfaction | ||||||||||||
| 4a Baseline sexual satisfaction (γ01) | 0.06 (−0.0003, 0.11) | .051 | 0.03 (−0.02, 0.09) | .224 | 0.04 (−0.01, 0.09) | .185 | ||||||
| 4a Change in sexual satisfaction (β2) | 0.07 (0.02, 0.11) | .005 | 0.03 (−0.02, 0.07) | .228 | 0.04 (.001, 0.08) | .049 | ||||||
| 4b Baseline sexual satisfaction (γ01) | 0.04 (−0.01, 0.10) | .127 | 0.01 (−0.04, 0.07) | .640 | 0.03 (−0.03, 0.08) | .360 | ||||||
| 4b Change in sexual satisfaction (β2) | 0.06 (0.02, 0.11) | .004 | 0.01 (−0.04, 0.05) | .717 | 0.03 (−0.01, 0.07) | .182 | ||||||
| Random effects | ||||||||||||
| Residual | 0.19 | 0.01 | 0.21 | 0.01 | 0.16 | 0.01 | ||||||
| Intercept | 0.45 | 0.03 | 0.44 | 0.03 | 0.48 | 0.03 | ||||||
| Time-varying age | 0.03 | 0.009 | 0.01 | 0.01 | 0.004 | 0.007 |
Notes: Each model represents an age-based multilevel model predicting the outcome shown in each column header. Model 3a/Model 4a was adjusted for age centered at age 56, race, income, education in years, committed status, and sexual activity. Model 3b/Model 4b was adjusted for depressive symptoms, physical health, number of cardiovascular diseases (CVDs), number of non-CVD chronic health conditions, alcohol consumption, current smoking status, and erectile dysfunction medication use. Model 3 only included erectile function predictors and Model 4 only included sexual satisfaction predictors. Changes in erectile function and sexual satisfaction were calculated by subtracting follow-up values from an individual’s baseline values. Baseline erectile function/sexual satisfaction predict baseline intercept (β0it). Change in those measures predicted change in cognitive performance (Yijt). CI = confidence interval; SE = standard error.
Table 2.
Age-Based Multilevel Models Examining Variables Related to Erectile Function and Sexual Satisfaction
| Variable | Model 1: erectile function | Model 2: sexual satisfaction | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β (95% CI) | t | p | σ 2 | SE | β (95% CI) | t | p | σ 2 | SE | |
| Fixed effects | ||||||||||
| Time variable | ||||||||||
| Time-varying age at each wave (centered at 56) | −0.24 (−0.29, −0.19) | 10.33 | <.001 | −0.06 (−0.08, −0.03) | 3.96 | <.001 | ||||
| Time-invariant covariates predicting baseline level (β0it) | ||||||||||
| Age at baseline | 0.11 (0.07, 0.16) | 5.30 | <.001 | 0.02 (0.01, 0.03) | 3.62 | <.001 | ||||
| Race (Ref. = non-Hispanic White) | 0.13 (−0.01, 0.28) | 1.80 | .073 | 0.06 (−0.03, 0.15) | 1.40 | .162 | ||||
| Baseline erectile function | — | — | — | 0.09 (0.06, 0.11) | 6.40 | <.001 | ||||
| General cognitive ability at age 20 | −0.01 (−0.05, 0.03) | 0.29 | .775 | 0.01 (−0.02, 0.03) | 1.03 | .303 | ||||
| Time-variant covariates predicting outcomes over time (Yijt) | ||||||||||
| Incomea | −0.01 (−0.04, 0.03) | 0.13 | .900 | −0.02 (−0.05, −0.001) | 2.04 | .041 | ||||
| Committed status(Ref. = Partnered) | 0.16 (0.04, 0.28) | 2.58 | .010 | 0.14 (0.05, 0.23) | 3.04 | .002 | ||||
| Individual change in erectile function | — | — | — | 0.69 (0.65, 0.73) | 33.97 | <.001 | ||||
| Frequency of sexual activityb | 0.27 (0.25, 0.30) | 19.43 | <.001 | 0.18 (0.15, 0.22) | 10.14 | <.001 | ||||
| Depressive symptoms (CESD) | −0.05 (−0.09, −0.01) | 2.26 | .024 | −0.09 (−0.13, −0.06) | 5.64 | <.001 | ||||
| Physical function (SF-36) | 0.09 (0.04, 0.14) | 3.66 | <.001 | 0.02 (−0.02, 0.04) | 0.94 | .346 | ||||
| Number of CVDs | −0.03 (−0.07, 0.01) | 1.46 | .143 | 0.01 (−0.02, 0.04) | 0.56 | .573 | ||||
| Number of non-CVD medical conditions | −0.01 (−0.04, −0.03) | 0.50 | .616 | 0.01 (−0.01, 0.04) | 1.04 | .299 | ||||
| Alcohol consumptionc | 0.04 (−0.01, 0.08) | 1.92 | .055 | −0.02 (−0.05, 0.01) | 1.07 | .283 | ||||
| Current smoking status (Ref. = yes) | 0.20 (0.09, 0.31) | 3.57 | <.001 | 0.06 (−0.02, 0.14) | 1.42 | .155 | ||||
| Erectile dysfunction medications (Ref. = yes) | −0.31 (−0.38, −0.24) | 8.28 | <.001 | −0.04 (−0.09, 0.01) | 1.45 | .147 | ||||
| Random effects | ||||||||||
| Residual | 0.54 | .02 | 0.21 | .01 | ||||||
| Intercept | 0.05 | .02 | 0.08 | .01 | ||||||
| Time-varying age | <0.001 | <.001 | 0.002 | .01 |
Notes: Results in the first column represent a single age-based multilevel model predicting changes in erectile function over time from all row-listed variables. Results in the second column represent a single age-based multilevel model predicting changes in sexual satisfaction from all row-listed variables. Change in erectile function was calculated as erectile function at follow-up subtracted by an individual’s baseline value. CI = confidence interval; SE = standard error; CESD = Center for Epidemiological Studies; CVD = cardiovascular disease; Ref. = reference group; SF-36 = Short Form Quality-of-Life Questionnaire—36 Questions.
aAverage of an ordinal variable where 1 = <10,000; 2 = 10,000 to 19,999; 3 = 20,000 to 29,999; 4 = 30,000 to 39,999; 5 = 40,000 to 49,999; 6 = 50,000 to 59,999; 7 = 60,000 to 69,999, 8 = 70,000 to 79,999; 9 = 80,000 to 89,999; 10 = 90,000 to 99,999; 11 = 100,000 to 109,999; 12 = 110,000 to 119,999, 13 = 120,000 or more.
bAverage of an ordinal variable with responses of 1 (1–2 attempts), 2 (3–4 attempts), 3 (5–6 attempts), 4 (7–10 attempts), or 5 (11+ attempts).
cThe number of drinks consumed in the past 2 weeks.
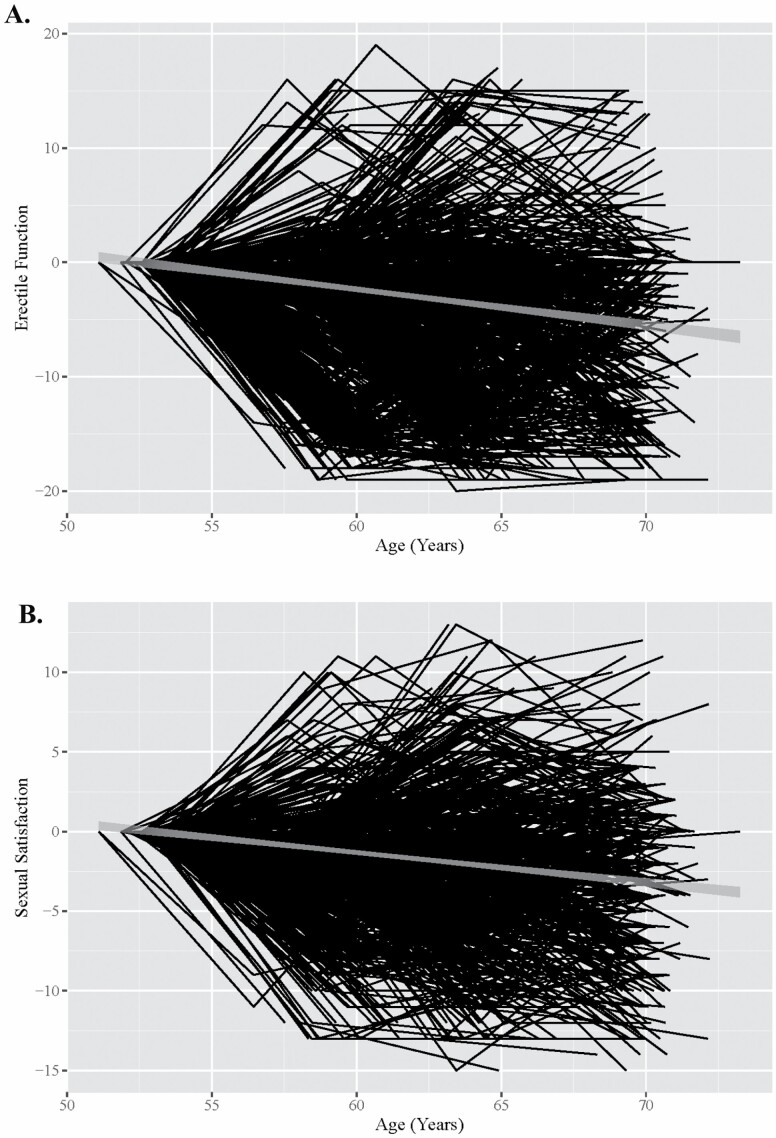
The models included three main components: First, the models estimated the association between interindividual (between-person) differences in erectile function and interindividual differences in cognitive function (intercept, β0it, effect). This was assessed by entering grand-mean centered baseline values of erectile function (Erectile Functionij=1,t grand mean centered) and sexual satisfaction (Sexual Satisfactionij=1,t grand mean centered) from wave 1 into the models. Second, models used interactions between time-varying age and baseline measures to examine interindividual differences in the rate of cognitive change (slope effect). Third, we used established procedures to examine intraindividual (within-person) associations of erectile function and cognitive change (Sliwinski & Buschke, 1999, 2004). This involved subtracting baseline values from time-varying measures of erectile function (Erectile Functionijt–Erectile Functionij=1,t) and sexual satisfaction (Sexual Satisfactionijt–Sexual Satisfactionij=1,t). Intraindividual changes in erectile function and sexual satisfaction are shown in Figure 1.
Figure 1.
Change in erectile function (A) and sexual satisfaction (B) from midlife to older adulthood.
Notes: Variables are centered by person-specific baseline values and unstandardized. A = change in erectile function; B = change in sexual satisfaction. Gray line in the middle of the black lines represents the 95% confidence interval of the trend.
Each main model was adjusted for covariates hierarchically. In the first step, models were adjusted for age (our time variable), race, education, and the rated frequency of sexual activity (Model 3a and Model 4a). In the second step, models were adjusted for health confounders (Model 3b and Model 4b), including the number of CVDs, number of non-CVD medical conditions, depressive symptoms, current smoking status, alcohol consumption, self-rated physical function, and erectile function medication use. Ordinal variables of sexual activity and alcohol consumption were examined using dummy-coded categories. Additional analyses examined sexual activity, a six-level ordinal variable, as a continuous measure after optimal scaling (Casacci & Pareto, 2015). All equations are shown in Supplementary Table S2. All code can be viewed online (https://github.com/trbellucsd/sexualsatisfaction). Significance was determined using an alpha level of .05.
Results
Descriptive Statistics
As shown in Table 1, average erectile function and sexual satisfaction decreased across waves. Age-based multilevel models showed this decline was significant (erectile function: β = −0.44, 95% confidence interval [CI; −0.42, −0.37]; sexual satisfaction: β = −0.44, 95% CI [−0.40, −0.33]; ps < .001). Changes over time are shown visually in Figure 1. Decline across time was significant for episodic memory (β = −0.24, 95% CI [−0.26, −0.21]), executive function (β = −0.37, 95% CI [−0.39, −0.34]), and processing speed (β = −0.44, 95% CI [−0.46, −0.42], ps < .001).
Predictors of Erectile Function and Sexual Satisfaction
We examined factors associated with erectile function and sexual satisfaction across waves (Table 2). In Model 1, lower erectile function was related to older age (β = −0.17, 95% CI [−0.20, −0.14], p < .001), being single (β = 0.16, 95% CI [0.04, 0.28], p = .010), higher depressive symptoms (β = −0.05, 95% CI [−0.09, −0.01], p = .024), less frequent sexual activity (β = 0.27, 95% CI [0.25, 0.30], p <. 001), poorer physical functioning (β = 0.09, 95% CI [0.04, 0.14], p < .001), and more reported use of ED medications (β = −0.31, 95% CI [−0.38, −0.24], p < .001). In Model 2, lower sexual satisfaction over time was related to being single (β = 0.14, 95% CI [0.05, 0.23], p = .002), more depressive symptoms (β = −0.09, 95% CI [−0.13, −0.06], p < .001), less frequent sexual activity (β = 0.18, 95% CI [0.15, 0.22], p < .001), and decreasing erectile function over time (β = 0.69, 95% CI [0.65, 0.73], p < .001).
Main Models
Baseline interindividual differences in erectile function and sexual satisfaction at mean age 56
Baseline difference in cognitive function (intercept)
Erectile function
All main models are shown fully in Supplementary Table S3 and S4 and summarized in Table 3. Adjusting for demographics and frequency of sexual activity in Model 3a, lower baseline levels of erectile function were related to lower baseline levels of episodic memory (β = 0.11, 95% CI [0.05, 0.16], p < .001), executive function (β = 0.08, 95% CI [0.03, 0.14], p = .003), and processing speed (β = 0.11, 95% CI [0.06, 0.17], p < .001). These remained significant after adjusting for health confounders in Model 3b (all p < .05).
Sexual satisfaction
Adjusting for demographics and frequency of sexual activity in Model 4a, baseline levels of sexual satisfaction were not related to baseline levels of episodic memory, executive function, or processing speed (ps > .05). There were also no significant interactions of baseline sexual satisfaction and time-varying age (ps > .05). This was also true with adjustment for health confounders in Model 4b (ps > .05).
Differences in the rate of cognitive change (slope)
Erectile function
There was a significant interaction between baseline erectile function and time-varying age in Model 3a. Men with lower baseline erectile function had faster rates of decline in processing speed, which remained significant after adjusting for health confounders in Model 3b (β = 0.03, 95% CI [0.01, 0.05], p = .010).
Sexual satisfaction
There was also no significant association between baseline sexual satisfaction and the rate of cognitive change (ps > .05).
Longitudinal intraindividual changes in erectile function and sexual satisfaction
Erectile function
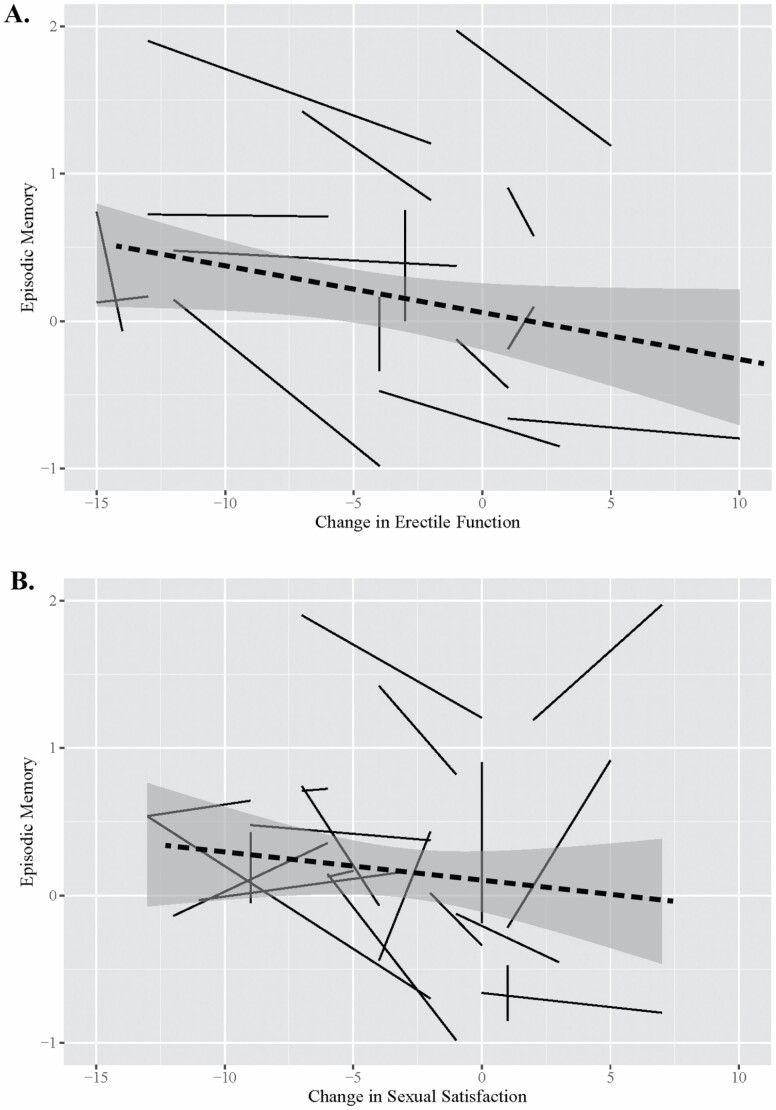
After adjusting for demographics and sexual activity in Model 3a, decreases in erectile function were related to declines in episodic memory, and increases were related to less steep decline in episodic memory (β = 0.08, 95% CI [0.04, 0.13], p < .001), which remained significant after adjusting for health confounders in Model 3b (β = 0.09, 95% CI [0.04, 0.13], p < .001). This association is visualized in Figure 2a. Intraindividual changes in erectile function were unrelated to changes in executive function and processing speed in any model (ps > .05).
Figure 2.
Association between change in erectile function and episodic memory (A) and change in sexual satisfaction and episodic memory (B).
Notes: Data are taken from a 20% random sample to visualize individual trajectories. Negative values of change in erectile function and sexual satisfaction represent a decline at any point in the study; positive values represent an increase at any point in the study. Perfectly vertical lines represent no change in erectile function or sexual satisfaction over time. Dashed line represents the general effect over time with a 95% confidence interval shown in gray. A = change in erectile function; B = change in sexual satisfaction.
Sexual satisfaction
After adjusting for demographics and sexual activity in Model 4a, decreases in sexual satisfaction were related to declines in episodic memory, and increases were related to less steep decline in episodic memory (β = 0.07, 95% CI [0.02, 0.11], p = .005). This association remained significant after added adjustment for health confounders in Model 4b (β = 0.06, 95% CI [0.02, 0.11], p = .004), and visualized in Figure 2b. Intraindividual changes in sexual satisfaction were unrelated to changes in executive function and processing speed in any model (ps > .05).
Covariate effects
As shown in Supplementary Table S3 and S4 in Model 3b, older age at baseline and lower general cognitive ability at age 20 were related to worse episodic memory, executive function, and processing speed at baseline (ps < .05). Time was related to worse episodic memory, executive function, and processing speed (ps < .05). Lower income was related to worse episodic memory at all timepoints (β = 0.05, 95% CI [0.01, 0.09], p = .016). Greater depressive symptoms were related to worse executive function at all time points (β = −0.08, 95% CI [−0.12, −0.04], p < .001). Worse physical function and lower alcohol consumption were related to worse processing speed (β = 0.06, 95% CI [0.02, 0.10], p = .003). Comparable associations were found in models examining sexual satisfaction as a predictor (Supplementary Table S3, Model 4b).
Discussion and Implications
We examined how changes in erectile function and sexual satisfaction after midlife affected cognitive decline. Overall, we found that decreases in erectile function and sexual satisfaction were related to declines in episodic memory, and increases were related to less decline in episodic memory. These associations survived adjustment for demographic and health factors. Our study showed that in addition to effects at midlife, continuously measured declines in erectile function thereafter correspond to declines in episodic memory. We found that erectile function was related to all cognitive domains at baseline and that men with lower erectile function at baseline had greater declines in memory over time. Even after accounting for baseline function, intraindividual changes in erectile function were related to intraindividual changes in episodic memory. That is, increases (or decreases) in erectile function from one wave to the next were associated with an increase (or decreases) in cognitive function.
Vascular theories of aging posit that changes in vascular function may explain cognitive decline as we age, with the emphasis on attention–executive–psychomotor speed domains (Earles & Salthouse, 1995; Rockwood, 2002). The artery-size hypothesis states that this could involve not only macrovascular changes, but microvascular changes that may be captured by changes in erectile function (Montorsi et al., 2005). Intraindividual changes in erectile function were associated with episodic memory. We found no associations between health and cognitive outcomes, and the effect did not change in models including health conditions. This may suggest that erectile function captured health changes related to cognitive function missed by a reliance on self-rated physical health or diagnoses of major cardiovascular conditions. Consistent with the artery-size hypothesis (Montorsi et al., 2005), changes in erectile function may correspond to the presence of early microvascular changes long before reports of major cardiovascular disease (Montorsi et al., 2005). This is supported by the fact that 74% of patients with ED ages 41–73 show cardiovascular risk factors (Dumbrăveanu et al., 2018). One such type of microvascular change preceding macrovascular events is arteriosclerosis, which can worsen verbal episodic memory (Hugenschmidt et al., 2013) and cause hippocampal atrophy (Jagust et al., 2008).
As we did not measure actual vasculature, there may be other mechanisms explaining the association between erectile function and memory decline. One, decreases in erectile function may correspond to declining levels of testosterone, which is linked to lower hippocampal volume and poorer memory performance in older men (Panizzon et al., 2018). A meta-analysis by Allen and Walter (2018) has linked erectile function to a host of lifestyle variables, which may also drive associations with cognition, such as diet and physical activity. In this analysis, physical functioning was associated with both sexual health and cognition, and alcohol consumption was associated with cognition, providing some support for the role of lifestyle influences (Allen & Walter, 2018). The lack of association between cardiovascular conditions and cognition is inconsistent with vascular theories of cognitive aging (DeRight et al., 2015; Earles & Salthouse, 1995; Rockwood, 2002). As proposed by the artery-size hypothesis, microvascular changes may be contributing to the association between erectile function and memory, as well as other factors, such as testosterone and lifestyle. Future studies clarifying etiologies and other contextual and psychosocial factors will contribute to our understanding of mechanisms.
We found that declines in sexual satisfaction were related to decline in episodic memory as well. This builds on the limited studies in this area. In fact, two studies examined sexual satisfaction and cognition, with mixed findings. Hsu et al. (2018) found no association between sexual satisfaction at baseline and performance on a cognitive screener 5 years later in men 70 years and older. Smith et al. (2021) found that people aged 65 and older with higher sexual satisfaction at baseline were less likely to have cognitive impairment over 10 years. Our research builds upon prior research in multiple ways. We used continuous factor scores reflecting neuropsychological tests measuring episodic memory, executive function, and processing speed, rather than screening instruments used in prior studies. We used a middle-aged sample and a longitudinal design incorporating intraindividual change that allowed us to capture possible changes in cognition related to early age-related changes in sexual satisfaction. We examined cognitive changes over a 12-year span (across three waves). Finally, by examining sexual satisfaction, our findings connect to the small literature linking life satisfaction and cognitive performance (Enkvist et al., 2013). It may be that sexual satisfaction captures psychological distress like general life satisfaction measures (Fergusson et al., 2015; Lombardo et al., 2018).
The association between sexual satisfaction and memory parallels research on psychological distress, which has been associated with worse episodic memory (Turner et al., 2017) and greater cognitive decline over time (Brailean et al., 2017). As such, they may share similar biological mechanisms. Psychological distress involves activation of the limbic system, which uses neural structures such as the hippocampus and frontal lobes to communicate distress, structures that also are needed for learning and memory recall. One negative side effect of chronic limbic system activation is the release of neurotoxic glucocorticoid/cortisol for which the hippocampus and the frontal lobes have many receptors, increasing their risk of atrophy therefrom (Dedovic et al., 2009; Jameison & Dinan, 2001). Poor sexual satisfaction may increase these levels of distress, which worsens episodic memory. More research will need to confirm that decreasing sexual satisfaction is a form of distress with physiological and inflammatory properties. Such findings may explain associations with worsening episodic memory over time. However, it is also possible that due to strong associations between erectile function and sexual satisfaction, biological mechanisms of erectile function may mostly mediate the association between sexual satisfaction and episodic memory.
The present study is not without its limitations. We cannot generalize about sexual satisfaction for women. In addition, the sample is largely non-Hispanic White, limiting generalizability to people from other races or ethnicities. Health and social disparities among ethnic/racial groups could alter the associations between erectile function, sexual satisfaction, and cognitive functioning. Participants were also all twins and former members of the U.S. military, which may limit generalization to other cohorts. However, nearly 80% reported no combat experience, and based on Centers for Disease Control and Prevention data, the sample is representative of American men in their age range in various health, education, and lifestyle factors (Schoenborn & Heyman, 2009). Second, we were unable to distinguish the sexual identities of the VETSA participants. Third, our measures of erectile function and sexual satisfaction were based on self-report, which is susceptible to interpretation bias when involving sensitive topics.
Our study also had several strengths. For one, data were collected during and after midlife, when changes in health and cognition begin to accelerate (Earles & Salthouse, 1995). Midlife is a pivotal period for several domains of life, and one’s health and well-being in midlife can affect one’s development into older adulthood (Infurna et al., 2020). Previous studies have largely focused on people in their seventies and eighties (Hsu et al., 2018; Smith et al., 2021; Yang et al., 2015), missing this key window. Second, our statistical approach focused on modeling within-person associations of erectile function and sexual satisfaction with cognition, which is critical for understanding how these variables change together as individuals age. Our measures of cognitive performance were also rigorous, including factor scores from validated neuropsychological tests, and were adjusted for general cognitive ability at average age 20.
As the older adult population is expected to double across the next three decades (Matthews et al., 2019), so too will the number of people who enter their sixties with declines in erectile function and sexual satisfaction. As shown here, such individuals may be more likely to decline in memory, which may increase their risk for dementia, as demonstrated in non-U.S. samples (Yang et al., 2015). Increasing the assessment and monitoring of erectile function as a vital sign of health may help identify those at risk of cognitive decline before their seventies.
Supplementary Material
Acknowledgments
The content of this article is the responsibility of the authors and does not necessarily represent official views of the National Institute on Aging, the National Institutes of Health, or The US Department of Veterans Affairs (VA). The VA, Department of Defense; National Personnel Records Center, National Archives and Records Administration; National Opinion Research Center; National Research Council, National Academy of Sciences; and the Institute for Survey Research, Temple University provided invaluable assistance in the creation of the Vietnam Era Twin (VET) Registry. The Cooperative Studies Program of the VA provided financial support for development and maintenance of the VET Registry. We would also like to acknowledge the continued cooperation and participation of the members of the VET Registry and their families. Data are available upon request and with a data authorization agreement. This study was not preregistered. Portions of this work were presented at the 2021 annual scientific meeting of The Gerontological Society of America.
Contributor Information
Riki E Slayday, Department of Psychology, San Diego State University, San Diego, California, USA; Department of Human Development and Family Studies, The Pennsylvania State University, University Park, Pennsylvania, USA.
Tyler R Bell, Department of Psychiatry, University of California San Diego, La Jolla, California, USA; Center for Behavior Genetics of Aging, University of California San Diego, La Jolla, California, USA.
Michael J Lyons, Department of Psychological and Brain Sciences, Boston University, Boston, Massachusetts, USA.
Teresa S Warren , BA, Department of Psychology, San Diego State University, San Diego, California, USA.
Rosemary Toomey, Department of Psychological and Brain Sciences, Boston University, Boston, Massachusetts, USA.
Richard Vandiver, Department of Psychological and Brain Sciences, Boston University, Boston, Massachusetts, USA.
Martin J Sliwinski, Department of Human Development and Family Studies, The Pennsylvania State University, University Park, Pennsylvania, USA.
William S Kremen, Department of Psychiatry, University of California San Diego, La Jolla, California, USA; Center for Behavior Genetics of Aging, University of California San Diego, La Jolla, California, USA.
Carol E Franz, Department of Psychiatry, University of California San Diego, La Jolla, California, USA; Center for Behavior Genetics of Aging, University of California San Diego, La Jolla, California, USA.
Funding
This work was supported the National Institute on Aging at the National Institutes of Health (R01AG050595 to W. S. Kremen, M. J. Lyons, and C. E. Franz; R01AG022381 and R01AG076838 to W. S. Kremen; P01AG055367 and R01AG059329 to C. E. Franz; and R25AG043364).
Conflict of Interest
None declared.
References
- Allen, M. S., & Walter, E. E. (2018). Health-related lifestyle factors and sexual dysfunction: A meta-analysis of population-based research. Journal of Sexual Medicine, 15(4), 458–475. doi: 10.1016/j.jsxm.2018.02.008 [DOI] [PubMed] [Google Scholar]
- Brailean, A., Aartsen, M. J., Muniz-Terrera, G., Prince, M., Prina, A. M., Comijs, H. C., Huisman, M., & Beekman, A. (2017). Longitudinal associations between late-life depression dimensions and cognitive functioning: A cross-domain latent growth curve analysis. Psychological Medicine, 47(4), 690–702. doi: 10.1017/S003329171600297X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravi, C. A., Tin, A., Montorsi, F., Mulhall, J. P., Eastham, J. A., & Vickers, A. J. (2020). Erectile function and sexual satisfaction: The importance of asking about sexual desire. Journal of Sexual Medicine, 17(2), 349–352. doi: 10.1016/j.jsxm.2019.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casacci, S., & Pareto, A. (2015). Methods for quantifying ordinal variables: A comparative study. Quality and Quantity, 49(5), 1859–1872. doi: 10.1007/s11135-014-0063-2 [DOI] [Google Scholar]
- Charlson, M., Szatrowski, T. P., Peterson, J., & Gold, J. (1994). Validation of a combined comorbidity index. Journal of Clinical Epidemiology, 47(11), 1245–1251. doi: 10.1016/0895-4356(94)90129-5 [DOI] [PubMed] [Google Scholar]
- Dedovic, K., Duchesne, A., Andrews, J., Engert, V., & Pruessner, J. C. (2009). The brain and the stress axis: The neural correlates of cortisol regulation in response to stress. Neuroimage, 47(3), 864–871. doi: 10.1016/j.neuroimage.2009.05.074 [DOI] [PubMed] [Google Scholar]
- Delis, D. C., Kaplan, E., & Kramer, J. H. (2001). Delis-Kaplan Executive Function System (D-KEFS). The Psychological Corporation. doi: 10.1037/t15082-000 [DOI] [Google Scholar]
- Delis, D. C., Kramer, J. H., Kaplan, E., & Ober, B. A. (2000). California verbal learning test–adult version. Manual (2nd ed.). The Psychological Corporation. [Google Scholar]
- DeRight, J., Jorgensen, R. S., & Cabral, M. J. (2015). Composite cardiovascular risk scores and neuropsychological functioning: A meta-analytic review. Annals of Behavioral Medicine, 49(3), 344–357. doi: 10.1007/s12160-014-9681-0 [DOI] [PubMed] [Google Scholar]
- Dong, J. Y., Zhang, Y. H., & Qin, L. Q. (2011). Erectile dysfunction and risk of cardiovascular disease: Meta-analysis of prospective cohort studies. Journal of the American College of Cardiology, 58(13), 1378–1385. doi: 10.1016/j.jacc.2011.06.024 [DOI] [PubMed] [Google Scholar]
- Dumbrăveanu, I., Branishte, T., Banov, P., Arian, I., Balutel, B., & Ceban, E. (2018). Incidence of primary cardiovascular risk factors in patients with erectile dysfunction. Medical-Surgical Journal, 122(1), 33–38. http://hdl.handle.net/20.500.12710/19608 [Google Scholar]
- Earles, J. L., & Salthouse, T. A. (1995). Interrelations of age, health, and speed. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 50(1), P33–P41. doi: 10.1093/geronb/50b.1.p33 [DOI] [PubMed] [Google Scholar]
- Elman, J. A., Jak, A. J., Panizzon, M. S., Tu, X. M., Chen, T., Reynolds, C. A., Gustavson, D. E., Franz, C. E., Hatton, S. N., Jacobson, K. C., Toomey, R., McKenzie, R., Xian, H., Lyons, M. J., & Kremen, W. S. (2018). Underdiagnosis of mild cognitive impairment: A consequence of ignoring practice effects. Alzheimer’s and Dementia, 10, 372–381. doi: 10.1016/j.dadm.2018.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enkvist, A., Ekström, H., & Elmståhl, S. (2013). Associations between cognitive abilities and life satisfaction in the oldest–old. Results from the longitudinal population study Good Aging in Skåne. Clinical Interventions in Aging, 8, 845. doi: 10.2147/CIA.S45382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson, D., McLeod, G., Horwood, L. J., Swain, N., Chapple, S., & Poulton, R. (2015). Life satisfaction and mental health problems (18 to 35 years). Psychological Medicine, 45(11), 2427–2436. doi: 10.1017/S0033291715000422 [DOI] [PubMed] [Google Scholar]
- Gerstorf, D., Ram, N., Hoppmann, C., Willis, S. L., & Schaie, K. W. (2011). Cohort differences in cognitive aging and terminal decline in the Seattle Longitudinal Study. Developmental Psychology, 47(4), 1026. doi: 10.1037/a0023426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg, J., Curran, B., Vitek, M., Henderson, W., & Boyko, E. (2002). The Vietnam Era Twin Registry. Twin Research, 5(5), 476–481. doi: 10.1375/twin.5.5.476 [DOI] [PubMed] [Google Scholar]
- Golden, C. J., & Freshwater, S. M. (2002). Stroop color and word test kit for adults. In A manual for clinical and experimental uses (2nd ed.). Stoelting. [Google Scholar]
- Gomes, C. M., Miranda, E. P., de BessaBellucci, J.C. H. S., Jr., Battistella, L. R., Abdo, C. H. N., Bruschini, H., Srougi, M., & Mulhall, J. P. (2017). Erectile function predicts sexual satisfaction in men with spinal cord injury. Journal of Sexual Medicine, 5(3), e148–e155. doi: 10.1016/j.esxm.2017.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavson, D. E., Panizzon, M. S., Franz, C. E., Reynolds, C. A., Corley, R. P., Hewitt, J. K., Lyons, M. J., Kremen, W. S., & Friedman, N. P. (2019). Integrating verbal fluency with executive functions: Evidence from twin studies in adolescence and middle age. Journal of Experimental Psychology General, 148(12), 2104–2119. doi: 10.1037/xge0000589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinchliff, S., Tetley, J., Lee, D., & Nazroo, J. (2018). Older adults’ experiences of sexual difficulties: Qualitative findings from the English Longitudinal Study on Ageing (ELSA). Journal of Sex Research, 55(2), 152–163. doi: 10.1080/00224499.2016.1269308 [DOI] [PubMed] [Google Scholar]
- Hsu, B., Hirani, V., Waite, L. M., Naganathan, V., Blyth, F. M., Le Couteur, D. G., Seibel, M. J., Cumming, R. G., & Handelsman, D. J. (2018). Temporal associations between sexual function and cognitive function in community-dwelling older men: The Concord Health and Ageing in Men Project. Age and Ageing, 47(6), 900–904. doi: 10.1093/ageing/afy088 [DOI] [PubMed] [Google Scholar]
- Hugenschmidt, C. E., Hsu, F. C., Hayasaka, S., Carr, J. J., Freedman, B. I., Nyenhuis, D. L., Williams, J. D., & Bowden, D. W. (2013). The influence of subclinical cardiovascular disease and related risk factors on cognition in type 2 diabetes mellitus: The DHS-Mind study. Journal of Diabetes and Its Complications, 27(5), 422–428. doi: 10.1016/j.jdiacomp.2013.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM Corp. Released 2020. IBM SPSS Statistics for Windows, Version 27.0. IBM Corp. [Google Scholar]
- Infurna, F. J., Gerstorf, D., & Lachman, M. E. (2020). Midlife in the 2020s: Opportunities and challenges. American Psychologist, 75(4), 470. doi: 10.1037/amp0000591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagust, W. J., Zheng, L., Harvey, D. J., Mack, W. J., Vinters, H. V., Weiner, M. W., Ellis, W. G., Zarow, C., Mungas, D., Reed, B. R., Kramer, J. H., Schuff, N., DeCarli, C., & Chui, H. C. (2008). Neuropathological basis of magnetic resonance images in aging and dementia. Annals of Neurology, 63(1), 72–80. doi: 10.1002/ana.21296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameison, K., & Dinan, T. G. (2001). Glucocorticoids and cognitive function: From physiology to pathophysiology. Human Psychopharmacology, 16(4), 293–302. doi: 10.1002/hup.304 [DOI] [PubMed] [Google Scholar]
- Kremen, W. S., Fennema-Notestine, C., Eyler, L. T., Panizzon, M. S., Chen, C. H., Franz, C. E., Lyons, M. J., Thompson, W. K., & Dale, A. M. (2013). Genetics of brain structure: Contributions from the Vietnam Era Twin Study of Aging. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics, 162B(7), 751–761. doi: 10.1002/ajmg.b.32162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen, W. S., Panizzon, M. S., Franz, C. E., Spoon, K. M., Vuoksimaa, E., Jacobson, K. C., Vasilopoulos, T., McCaffery, J. M., Panizzon, M. S., Franz, C. E., Vuoksimaa, E., Xian, H., Rana, B. K., Toomey, R., McKenzie, R., & Lyons, M. J. (2014). Genetic complexity of episodic memory: A twin approach to studies of aging. Psychology and Aging, 29(2), 404. doi: 10.1037/a0035962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen, W. S., Thompson-Brenner, H., Leung, Y. M., Grant, M. D., Franz, C. E., Eisen, S. A., Jacobson, K. C., Boake, C., & Lyons, M. J. (2006). Genes, environment, and time: The Vietnam Era Twin Study of Aging (VETSA). Twin Research and Human Genetics, 9(6), 1009–1022. doi: 10.1375/183242706779462750 [DOI] [PubMed] [Google Scholar]
- Lombardo, P., Jones, W., Wang, L., Shen, X., & Goldner, E. M. (2018). The fundamental association between mental health and life satisfaction: Results from successive waves of a Canadian national survey. BMC Public Health, 18(1), 1–9. doi: 10.1186/s12889-018-5235-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews, K. A., Xu, W., Gaglioti, A. H., Holt, J. B., Croft, J. B., Mack, D., & McGuire, L. C. (2019). Racial and ethnic estimates of Alzheimer’s disease and related dementias in the United States (2015–2060) in adults aged ≥65 years. Alzheimer’s and Dementia, 15(1), 17–24. doi: 10.1016/j.jalz.2018.06.3063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes de Leon, C. F. (2007). Aging and the elapse of time: A comment on the analysis of change. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 62(3), S198–S202. doi: 10.1093/geronb/62.3.S198 [DOI] [PubMed] [Google Scholar]
- Montorsi, P., Ravagnani, P. M., Galli, S., Rotatori, F., Briganti, A., Salonia, A., Rigatti, P., & Montorsi, F. (2005). The artery size hypothesis: A macrovascular link between erectile dysfunction and coronary artery disease. The American Journal of Cardiology, 96(12), 19–23. doi: 10.1016/j.amjcard.2005.07.006 [DOI] [PubMed] [Google Scholar]
- Moore, C. S., Grant, M. D., Zink, T. A., Panizzon, M. S., Franz, C. E., Logue, M. W., Hauger, R. L., Kremen, W. K., & Lyons, M. J. (2014). Erectile dysfunction, vascular risk, and cognitive performance in late middle age. Psychology and Aging, 29(1), 163–172. doi: 10.1037/a0035463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson, C. J., Choi, J. M., Mulhall, J. P., & Roth, A. J. (2007). Erectile dysfunction: Determinants of sexual satisfaction in men with prostate cancer. Journal of Sexual Medicine, 4(5), 1422–1427. doi: 10.1111/j.1743-6109.2007.00547.x [DOI] [PubMed] [Google Scholar]
- Panizzon, M. S., Hauger, R. L., Xian, H., Jacobson, K., Lyons, M. J., Franz, C. E., & Kremen, W. S. (2018). Interactive effects of testosterone and cortisol on hippocampal volume and episodic memory in middle-aged men. Psychoneuroendocrinology, 91, 115–122. doi: 10.1016/j.psyneuen.2018.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff, L. S. (1977). The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1(3), 385–401. doi: 10.1177/014662167700100306 [DOI] [Google Scholar]
- Rhoden, E. L., Teloken, C., Sogari, P. R., & Souto, C. A. V. (2002). The use of the simplified International Index of Erectile Function (IIEF-5) as a diagnostic tool to study the prevalence of erectile dysfunction. International Journal of Impotence Research, 14(4), 245–250. doi: 10.1038/sj.ijir.3900859 [DOI] [PubMed] [Google Scholar]
- Rockwood, K. (2002). Vascular cognitive impairment and vascular dementia. Journal of the Neurological Sciences, 203, 23–27. doi: 10.1016/S0022-510X(02)00255-1 [DOI] [PubMed] [Google Scholar]
- Rosen, R. C. (2001). Psychogenic erectile dysfunction. Classification and management. Urologic Clinics of North America, 28(2), 269–278. doi: 10.1016/s0094-0143(05)70137-3 [DOI] [PubMed] [Google Scholar]
- Rosen, R. C., Riley, A., Wagner, G., Osterloh, I. H., Kirkpatrick, J., & Mishra, A. (1997). The international index of erectile function (IIEF): A multidimensional scale for assessment of erectile dysfunction. Urology, 49(6), 822–830. doi: 10.1016/s0090-4295(97)00238-0 [DOI] [PubMed] [Google Scholar]
- Sanderson-Cimino, M., Panizzon, M. S., Elman, J. A., Gustavson, D. E., Franz, C. E., Reynolds, C. A., Toomey, R., Lyons, M. J., & Kremen, W. S. (2019). Genetic and environmental architecture of processing speed across midlife. Neuropsychology, 33(6), 862–871. doi: 10.1037/neu0000551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenborn, C. A., & Heyman, K. M. (2009). Health characteristics of adults aged 55 years and over: United States, 2004–2007. National Health Statistics Reports, 16, 1–31. doi: 10.1037/e623972009-001 [DOI] [PubMed] [Google Scholar]
- Sliwinski, M., & Buschke, H. (1999). Cross-sectional and longitudinal relationships among age, cognition, and processing speed. Psychology and Aging, 14(1), 18–33. doi: 10.1037//0882-7974.14.1.18 [DOI] [PubMed] [Google Scholar]
- Sliwinski, M., & Buschke, H. (2004). Modeling intraindividual cognitive change in aging adults: Results from the Einstein aging studies. Aging, Neuropsychology and Cognition, 11(2–3), 196–211. doi: 10.1080/13825580490511080 [DOI] [Google Scholar]
- Sliwinski, M., Hoffman, L., & Hofer, S. M. (2010). Evaluating convergence of within-person change and between-person age differences in age-heterogeneous longitudinal studies. Research in Human Development, 7(1), 45–60. doi: 10.1080/15427600903578169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, A. G., Bardach, S. H., Barber, J. M., Williams, A., Rhodus, E. K., Parsons, K. K., & Jicha, G. A. (2021). Associations of future cognitive decline with sexual satisfaction among married older adults. Clinical Gerontologist, 44(3), 345–353. doi: 10.1080/07317115.2021.1887420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranne, J., Malmsten, U. G. H., Areskoug, B., Milsom, I., Molander, U., & Peeker, R. (2019). The rate of deterioration of erectile function increases with age: Results from a longitudinal population based survey. Scandinavian Journal of Urology, 53(2–3), 161–165. doi: 10.1080/21681805.2019.1596154 [DOI] [PubMed] [Google Scholar]
- Teng, E. L. (1990). The 3RT Test: Three reaction time tasks for IBM PC computers. Behavior Research Methods, Instruments, and Computers, 22(4), 389–392. doi: 10.3758/BF03203180 [DOI] [Google Scholar]
- Tsuang, M. T., Bar, J. L., Harley, R. M., & Lyons, M. J. (2001). The Harvard Twin Study of Substance Abuse: What we have learned. Harvard Review of Psychiatry, 9, 267–279. doi: 10.1080/10673220127912 [DOI] [PubMed] [Google Scholar]
- Turner, A. D., James, B. D., Capuano, A. W., Aggarwal, N. T., & Barnes, L. L. (2017). Perceived stress and cognitive decline in different cognitive domains in a cohort of older African Americans. The American Journal of Geriatric Psychiatry, 25(1), 25–34. doi: 10.1016/j.jagp.2016.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware, J. E., Jr., & Sherbourne, C. D. (1992). The MOS 36-item short-form health survey (SF-36). Conceptual framework and item selection. Medical Care, 30(6), 473–483. doi: 10.1097/00005650-199206000 [DOI] [PubMed] [Google Scholar]
- Wechsler, D. (1997). WMS-III: Wechsler memory scale administration and scoring manual. The Psychological Corporation. [Google Scholar]
- Wright, H., Jenks, R. A., & Demeyere, N. (2019). Frequent sexual activity predicts specific cognitive abilities in older adults. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 74(1), 47–51. doi: 10.1093/geronb/gbx065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, C. M., Shen, Y. C., Weng, S. F., Wang, J. J., & Tien, K. J. (2015). Increased risk of dementia in patients with erectile dysfunction: A population-based, propensity score-matched, longitudinal follow-up study. Medicine (Baltimore), 94(24), e990. doi: 10.1097/MD.0000000000000990 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.