Abstract
Dolutegravir (DTG) based dual therapies for treating PLWHIV are a standard of care nowadays. Switching to DTG and lamivudine (3TC) safety and efficacy were proven in TANGO randomized clinical trial. This multicenter retrospective study included 1032 HIV virologically suppressed patients switching to DTG+3TC from 13 Spanish hospitals. DTG+3TC provided high rates of undetectable viral load over 96%, corresponding to 96.6% (889/921) at 24 weeks, 97.5% (743/763) at 48 weeks, and 98.3% (417/425) at 96 weeks. No significant differences are evident when comparing the total population according to sex, presence of comorbidity, or presence of AIDS. The analysis for paired data showed an increase in CD4+ cell count. A statistically significant increase in CD4+ lymphocyte count was found in those without comorbidities in the three-time series analyzed [average increase at 24 weeks: 48.7 (SD: 215.3) vs. 25.8 (SD: 215.5), p-value = 0.050; a mean increase at 48 weeks: 75.1 (SD: 232.9) vs. 42.3 (SD: 255.6), p-value = 0.003; a mean increase at 96 weeks: 120.1 (SD: 205.0) vs. 63.8 (SD:275.3), p-value = 0.003]. In conclusion, our cohort demonstrates that DTG+3TC is an effective treatment strategy for virologically-suppressed PLWHIV independent of age, sex, and HIV stage, as well as a safe and durable strategy.
Keywords: DTG+3TC, switching, viral suppression, immune recovery, safety
1. Introduction
For the last 25 years, antiretroviral therapy (ART) for treating people living with HIV (PLWHIV) has stood on three-drug regimens (3-DR). They, most commonly, were designed as a backbone of two nucleos(t)ide reverse transcriptase inhibitors (NRTIs) with a third agent, either a retroviral protease inhibitor (PI), a non-nucleoside reverse transcriptase inhibitor (NNRTI), or more recently, an integrase strand transfer inhibitor (INSTI) [1]. Nevertheless, although current nucleos(t)ide reverse transcriptase inhibitors associated toxicity is remarkably inferior to that associated with the first drugs used for treating PLWHIV in the initial years of the pandemic, current NRTIs still represent a potential burden in terms of short and long-term side effects [2,3]. During the last decade, pursuing strategies with less toxicity led to exploring the possibility of dual therapy.
An initial breakthrough was made by several trials that combined lamivudine (3TC) with different PI [4]. Dolutegravir is a highly active INST against HIV, which remains to date as one of the cornerstones of current strategies for treating PLWHIV [5]. More recently, SWORD randomized clinical trial demonstrated the efficacy and safety of the combination of DTG plus rilpivirine for treatment-experienced PLWHIV with virological suppression [6]. In parallel, the combination of DTG plus 3TC has been approved for treatment-naïve and treatment-experienced PLWHIV after demonstrating its efficacy and safety in the TANGO and GEMINI randomized clinical trials [7,8].
Although DTG plus 3TC has proved its non-inferiority in terms of virological suppression and its durability as a treatment strategy, data regarding its impact on immune activation and inflammation are lacking. Therefore, this study aimed to collect real-life data in a multicenter cohort of PLWHIV treated with DTG plus 3TC as a switch strategy, not only in terms of virological suppression, safety, and durability, but also in terms of its immunological impact through the evolution of the CD4/CD8 ratio in these patients, a marker considered to be a surrogate marker of immune activation and systemic inflammation [9,10]. Data were collected from 1032 patients from 13 Spanish institutions (Spanish cohort of Dolutegravir and 3TC, SPADE-3).
2. Materials and Methods
2.1. Population and Study Design
From 1 November 2020 to 1 August 2021, a retrospective multicentre study was conducted at 13 hospitals in Spain. Data from 1032 virologically suppressed HIV patients over 18 years old who switched to DTG plus 3TC were collected. Included variables comprise demographics, HIV infection, comorbidities, efficacy in viral suppression, and immune recovery, safety, and tolerability. Since the experimental design requires the study to compare CD4+ and CD8+ lymphocyte counts in individuals at different stages, these data should be considered matched data. Undetectable HIV viral load was considered when <50 copies/mL.
REDcap was used as an online electronic database [11].
2.2. Outcomes
The primary outcome (efficacy analysis) was evaluated by determining the percentage of patients with undetectable viral load at weeks 24, 48, and 96 after switching. Virological failure was defined as the presence of an HIV viral load over 50 copies/mL during follow-up.
Secondary outcomes were: (a) patients’ immune recovery evolution through measuring total plasma CD4+/CD8+ cells count and CD4+/CD8+ ratio; (b) adverse events throughout the whole follow-up period; and (c) investigating reasons that led clinicians to switch to DTG plus 3TC.
2.3. Statistical Analysis
All statistical analyses were performed using R software (R Core Team, Vienna, Austria, 2021). Data for continuous quantitative variables are presented as medians and interquartile ranges (IQR). Qualitative variables are presented as percentages. Demographics, comorbidities, HIV infection, and diagnosis were compared by gender, the number of comorbidities, and the presence of AIDS. The contrast of medians between the different groups analyzed was compared with parametric (Student’s bilateral t-test) and non-parametric (U-Mann-Whitney) tests based on the result of the Kolmogorov-Smirnov normality test. In contrast, the comparison of proportions was carried out using the Chi-square and Fisher tests. In all cases, statistical significance was defined as p < 0.05.
Additionally, differences in CD4+ and CD8+ lymphocyte count and CD4+/CD8+ ratio were calculated between the baseline and the three-time points analyzed (24, 48, and 96 weeks of dual DTG plus 3TC treatment) when data were available. Positive values or values greater than zero indicate an increase in the parameter under study. In this case, the average values of each difference are again contrasted according to the three defined population groups and represented graphically in the form of violin plots. Finally, we assessed whether the difference observed at the intra-group level was significant by applying paired means tests (Student’s t-test for paired variables; Wilcoxon signed-rank test).
2.4. Ethics
The study protocol was approved by the Ethics Committee of Hospital Universitario de Burgos in February 2021 with code 2454 and, subsequently, by the local committee of each institution involved in the study.
3. Results
The cohort evaluated 1032 patients from 13 centers in Spain. Baseline characteristics are summarized in Table 1. Men represented 78.6% of the study population with a median age of 48 years (38–57) compared to 53 (45–58) in women. AIDS was diagnosed in 11.4% of patients. At baseline, 44.5% of patients used FTC/TDF as the backbone, and INSTII was the most frequent third drug in 459 (44.5%) patients. Simplification was the leading reason for switching (56.9%), followed by toxicity (16.3%) and drug interaction (5.9%).
Table 1.
Main characteristics of HIV patients treated with DTG plus 3TC by sex, presence of comorbidities, and AIDS.
| Overall (n = 1032) |
Female (n = 221) |
Male (n = 811) |
p-Value | without Comorbidities (n = 617) |
with Any Comorbidity (n = 415) |
p-Value | No-AIDS Patient (n = 634) |
AIDS Patient (n = 118) |
p-Value | |
|---|---|---|---|---|---|---|---|---|---|---|
| DEMOGRAPHICS | ||||||||||
| Age, median [IQR] | 50.0 [40.0, 57.0] |
53.0 [45.0, 58.0] |
48.0 [38.0, 57.0] |
<0.001 | 45.0 [36.0, 54.0] |
54.0 [47.0, 59.5] |
<0.001 | 47.0 [37.2, 55.0] |
56.0 [53.0, 62.0] |
<0.001 |
| Age of HIV diagnosis, median [IQR] | 37.0 [27.0, 47.0] |
38.0 [29.0, 49.0] |
36.0 [26.0, 46.0] |
0.126 | 33.0 [24.0, 42.0] |
42.0 [30.0, 52.0] |
<0.001 | 34.0 [24.0, 45.0] |
45.5 [32.0, 53.8] |
<0.001 |
| Male, n (%) | 811 (78.6) | - | - | <0.001 | 491 (79.6) | 320 (77.1) | 0.384 | 512 (80.8) | 89 (75.4) | 0.229 |
|
Spanish nationality,
n (%) |
764 (76.9) | 156 (73.2) | 608 (77.8) | 0.186 | 460 (77.7) | 304 (75.6) | 0.492 | 405 (66.9) | 97 (82.2) | 0.001 |
| COMORBIDITIES, n (%) | ||||||||||
| Arterial hypertension | 119 (11.5) | 26 (11.8) | 93 (11.5) | 0.997 | - | - | - | 83 (13.1) | 34 (28.8) | <0.001 |
| Diabetes | 50 (4.8) | 9 (4.1) | 41 (5.1) | 0.67 | - | - | - | 35 (5.5) | 15 (12.7) | 0.007 |
| Dyslipidaemia | 211 (20.4) | 48 (21.7) | 163 (20.1) | 0.663 | - | - | - | 168 (26.5) | 41 (34.7) | 0.085 |
| Heart Disease | 29 (2.8) | 4 (1.8) | 25 (3.1) | 0.432 | - | - | - | 19 (3.0) | 9 (7.6) | 0.030 |
| Cerebrovascular disease | 9 (0.9) | 2 (0.9) | 7 (0.9) | 1.000 | - | - | - | 4 (0.6) | 5 (4.2) | 0.004 |
| Peripheral vascular disease | 11 (1.1) | 2 (0.9) | 9 (1.1) | 1.000 | - | - | - | 8 (1.3) | 3 (2.5) | 0.518 |
| Kidney failure | 40 (3.9) | 6 (2.7) | 34 (4.2) | 0.417 | - | - | - | 28 (4.4) | 12 (10.2) | 0.020 |
| Osteoporosis/Osteopenia | 31 (3.0) | 13 (5.9) | 18 (2.2) | 0.009 | - | - | - | 24 (3.8) | 7 (5.9) | 0.409 |
| Chronic pulmonary disease | 48 (4.7) | 14 (6.3) | 34 (4.2) | 0.246 | - | - | - | 36 (5.7) | 11 (9.3) | 0.196 |
| Psychiatric disorders | 78 (7.6) | 23 (10.4) | 55 (6.8) | 0.096 | - | - | - | 60 (9.5) | 17 (14.4) | 0.144 |
| Cancer | 14 (1.4) | 5 (2.3) | 9 (1.1) | 0.325 | - | - | - | 10 (1.6) | 4 (3.4) | 0.334 |
| Chronic liver disease | 106 (10.3) | 30 (13.6) | 76 (9.4) | 0.089 | - | - | - | 71 (11.2) | 35 (29.7) | <0.001 |
| Number of comorbidities, n (%) | ||||||||||
| One | 617 (59.8) | 126 (57.0) | 491 (60.5) | 0.439 | - | - | - | 315 (49.7) | 26 (22.0) | <0.001 |
| Two | 220 (21.3) | 45 (20.4) | 175 (21.6) | - | - | 181 (28.5) | 37 (31.4) | |||
| Three | 105 (10.2) | 24 (10.9) | 81 (10.0) | - | - | 79 (12.5) | 25 (21.2) | |||
| Four | 54 (5.2) | 17 (7.7) | 37 (4.6) | - | - | 35 (5.5) | 18 (15.3) | |||
| Five | 29 (2.8) | 8 (3.6) | 21 (2.6) | - | - | 19 (3.0) | 10 (8.5) | |||
| Six | 4 (0.4) | 0 (0.0) | 4 (0.5) | - | - | 4 (0.6) | 0 (0.0) | |||
| HIV INFECTION | ||||||||||
| Transmission pathways, n (%) | ||||||||||
| Sexual intercourse | 684 (67.8) | 118 (53.6) | 566 (71.7) | <0.001 | 455 (75.5) | 229 (56.4) | <0.001 | 426 (69.4) | 64 (54.7) | <0.001 |
| Intravenous drug injectors | 191 (18.9) | 61 (27.7) | 130 (16.5) | 81 (13.4) | 110 (27.1) | 84 (13.7) | 35 (29.9) | |||
| Immune status, median [IQR] | ||||||||||
| Baseline CD4+ (cells/mm3) | 753.0 [549.0, 977.0] |
763.0 [590.5, 985.0] |
744.0 [543.0, 975.8] |
0.358 | 752.5 [551.5, 980.0] |
759.0 [531.0, 975.0] |
0.944 | 786.5 [596.5, 1005.8] |
604.0 [404.5, 933.0] |
<0.001 |
| 24 weeks CD4+ (cells/mm3) | 770.5 [592.8, 980.0] |
785.0 [601.0, 986.5] |
766.0 [592.0, 970.8] |
0.482 | 766.0 [596.5, 976.5] |
773.0 [590.0, 982.0] |
0.933 | 808.0 [630.5, 1000.5] |
644.0 [449.0, 875.5] |
<0.001 |
| 48 weeks CD4+ (cells/mm3) | 782.0 [574.0, 1004.0] |
779.0 [606.0, 978.0] |
784.5 [567.0, 1012.5] |
0.996 | 789.0 [589.0, 1034.0] |
776.0 [559.5, 948.8] |
0.179 | 801.0 [591.5, 1029.5] |
628.0 [424.2, 866.0] |
<0.001 |
| 96 weeks CD4+ (cells/mm3) | 823.0 [613.2, 1048.0] |
802.0 [652.0, 1037.0] |
839.0 [604.5, 1051.0] |
0.854 | 831.0 [633.5, 1058.5] |
803.0 [560.2, 1036.5] |
0.306 | 851.0 [678.0, 1125.0] |
649.0 [440.5, 856.2] |
<0.001 |
| Baseline CD8+ (cells/mm3) | 867.5 [630.0, 1179.5] |
805.0 [589.5, 1084.5] |
878.0 [653.0, 1196.5] |
0.048 | 880.0 [632.0, 1156.0] |
861.0 [627.0, 1198.0] |
0.752 | 875.0 [637.5, 1199.5] |
827.0 [609.0, 1100.0] |
0.141 |
| 24 weeks CD8+ (cells/mm3) | 897.0 [656.0, 1220.0] |
792.0 [603.0, 1188.5] |
913.0 [674.0, 1247.0] |
0.020 | 895.0 [677.5, 1244.5] |
898.0 [636.0, 1211.2] |
0.793 | 899.5 [656.8, 1241.0] |
871.0 [635.0, 1134.0] |
0.517 |
| 48 weeks CD8+ (cells/mm3) | 908.0 [638.5, 1229.8] |
817.0 [533.0, 1119.0] |
922.0 [661.5, 1248.5] |
0.020 | 910.0 [639.5, 1257.5] |
907.0 [640.0, 1208.0] |
0.897 | 900.0 [635.5, 1220.5] |
959.5 [603.2, 1224.8] |
0.651 |
| 96 weeks CD8+ (cells/mm3) | 906.0 [628.5, 1241.5] |
922.0 [625.0, 1222.5] |
906.0 [634.5, 1268.5] |
0.903 | 980.0 [673.2, 1280.0] |
893.0 [617.0, 1227.0] |
0.491 | 956.0 [672.0, 1246.0] |
862.0 [484.5, 1152.5] |
0.094 |
| Baseline CD4+/CD8+ (cells/mm3) | 0.9 [0.6, 1.2] |
0.9 [0.7, 1.4] |
0.9 [0.6, 1.2] |
0.033 | 0.9 [0.6, 1.2] |
0.9 [0.6, 1.3] |
0.601 | 0.9 [0.7, 1.3] |
0.8 [0.5, 1.1] |
0.003 |
| 24 weeks CD4+/CD8+ (cells/mm3) | 0.9 [0.6, 1.2] |
1.0 [0.7, 1.3] |
0.8 [0.6, 1.2] |
0.011 | 0.9 [0.7, 1.2] |
0.9 [0.6, 1.2] |
0.855 | 0.9 [0.7, 1.2] |
0.7 [0.5, 1.1] |
0.001 |
| 48 weeks CD4+/CD8+ (cells/mm3) | 0.9 [0.6, 1.2] |
1.0 [0.7, 1.4] |
0.9 [0.6, 1.2] |
0.034 | 0.9 [0.6, 1.3] |
0.9 [0.6, 1.2] |
0.635 | 0.9 [0.7, 1.3] |
0.7 [0.5, 1.0] |
<0.001 |
| 96 weeks CD4+/CD8+ (cells/mm3) | 0.9 [0.7, 1.3] |
0.9 [0.7, 1.3] |
0.9 [0.7, 1.3] |
0.770 | 0.9 [0.7, 1.3] |
0.9 [0.7, 1.3] |
0.981 | 0.9 [0.7, 1.4] |
0.8 [0.7, 1.2] |
0.198 |
| HIV DIAGNOSIS n (%) | ||||||||||
| Previous treatments, n (%) | ||||||||||
| ABC/3TC | 384 (37.2) | 77 (34.8) | 307 (37.9) | 0.458 | 156 (25.3) | 228 (54.9) | <0.001 | 319 (50.3) | 64 (54.2) | 0.495 |
| FTC/TDF | 459 (44.5) | 96 (43.4) | 363 (44.8) | 0.784 | 193 (31.3) | 266 (64.1) | <0.001 | 367 (57.9) | 90 (76.3) | <0.001 |
| FTC/TAF | 149 (14.4) | 22 (10.0) | 127 (15.7) | 0.042 | 70 (11.3) | 79 (19.0) | <0.001 | 131 (20.7) | 18 (15.3) | 0.220 |
| PI ^ | 271 (26.3) | 79 (35.7) | 192 (23.7) | <0.001 | 77 (12.5) | 194 (46.7) | <0.001 | 202 (31.9) | 66 (55.9) | <0.001 |
| INSTI | 475 (46.0) | 82 (37.1) | 393 (48.5) | 0.003 | 229 (37.1) | 246 (59.3) | <0.001 | 407 (64.2) | 67 (56.8) | 0.153 |
| NNRTI | 340 (32.9) | 82 (37.1) | 258 (31.8) | 0.161 | 113 (18.3) | 227 (54.7) | <0.001 | 270 (42.6) | 67 (56.8) | 0.006 |
| Reasons for switching, n (%) | ||||||||||
| Toxicity | 168 (16.3) | 45 (20.4) | 123 (15.2) | 0.080 | 40 (6.5) | 128 (30.8) | <0.001 | 119 (18.8) | 48 (40.7) | <0.001 |
| Drug interaction | 61 (5.9) | 22 (10.0) | 39 (4.8) | 0.007 | 15 (2.4) | 46 (11.1) | <0.001 | 48 (7.6) | 13 (11.0) | 0.282 |
| Simplification | 587 (56.9) | 105 (47.5) | 482 (59.4) | 0.002 | 269 (43.6) | 318 (76.6) | <0.001 | 493 (77.8) | 93 (78.8) | 0.895 |
| Transition therapy to injectable drugs | 9 (0.9) | 1 (0.5) | 8 (1.0) | 0.727 | 5 (0.8) | 4 (1.0) | 1.000 | 9 (1.4) | 0 (0.0) | 0.400 |
| Simplicity | 33 (3.2) | 5 (2.3) | 28 (3.5) | 0.499 | 16 (2.6) | 17 (4.1) | 0.244 | 24 (3.8) | 8 (6.8) | 0.218 |
| Cost | 26 (2.5) | 5 (2.3) | 21 (2.6) | 0.974 | 6 (1.0) | 20 (4.8) | <0.001 | 13 (2.1) | 13 (11.0) | <0.001 |
| COINFECTIONS, n (%) | ||||||||||
| HBV diagnosis | 192 (27.9) | 36 (27.1) | 156 (28.1) | 0.904 | 56 (18.6) | 136 (35.1) | <0.001 | 139 (24.3) | 51 (44.0) | <0.001 |
| HBsAg positive | 10 (5.3) | 1 (2.8) | 9 (5.9) | 0.738 | 3 (5.7) | 7 (5.1) | 1 | 4 (2.9) | 6 (11.8) | 0.043 |
| HCV positive ELISA | 160 (23.0) | 41 (30.4) | 119 (21.2) | 0.032 | 19 (6.3) | 141 (35.7) | <0.001 | 111 (19.2) | 49 (42.2) | <0.001 |
| HCV positive PCR | 52 (34.7) | 16 (42.1) | 36 (32.1) | 0.359 | 4 (23.5) | 48 (36.1) | 0.451 | 26 (25.5) | 26 (54.2) | 0.001 |
| VIRAL LOAD < 50 copies/mL, n (%) | ||||||||||
| Baseline | 943 (96.0) | 206 (95.4) | 737 (96.2) | 0.716 | 565 (95.4) | 378 (96.9) | 0.318 | 576 (95.8) | 107 (96.4) | 0.991 |
| 24 weeks | 889 (96.6) | 196 (97.5) | 693 (96.4) | 0.574 | 539 (96.6) | 350 (96.7) | 1.000 | 532 (96.7) | 100 (95.2) | 0.638 |
| 48 weeks | 743 (97.5) | 162 (96.4) | 581 (97.8) | 0.463 | 467 (98.1) | 276 (96.5) | 0.256 | 393 (97.3) | 81 (94.2) | 0.258 |
| 96 weeks | 417 (98.3) | 98 (97.0) | 319 (98.8) | 0.456 | 304 (99.0) | 113 (96.6) | 0.181 | 126 (97.7) | 38 (95.0) | 0.735 |
^ In some patients previous treatments included PI in combination con INSTI.
The presence of comorbidity was diagnosed in 617 patients (59.8%), and 192 (18.6%) were with three or more comorbidities. Dyslipidaemia was the most frequent one (20.4%), followed by hypertension (11.5%) and chronic liver disease (10.3%). Patients with associated comorbidities were older than those without, p < 0.001. AIDS patients had more comorbidities, including hypertension, diabetes, and chronic liver disease.
In addition, 27.9% (192) of overall patients had been diagnosed with hepatitis B virus infection, but only 10/192 (5.3%) presented a positive HBs antigen during the switch to DTG+3TC. On the other hand, 3/56 patients (5.7%) in the comorbidity group showed positive HBs antigen and were on treatment with entecavir. No issues were found within this aspect.
During follow-up, available data provided high rates of undetectable viral load over 96%, corresponding to 96.6% (889/921) at 24 weeks, 97.5% (743/763) at 48 weeks, and 98.3% (417/425) at 96 weeks. No significant differences are evident when comparing the total population according to sex, presence of comorbidity, or presence of AIDS. Nevertheless, there is a tendency related to more virological failures in the groups without comorbidities or AIDS. In those patients with virological failure, no resistance mutations were found when drug resistance tests were performed.
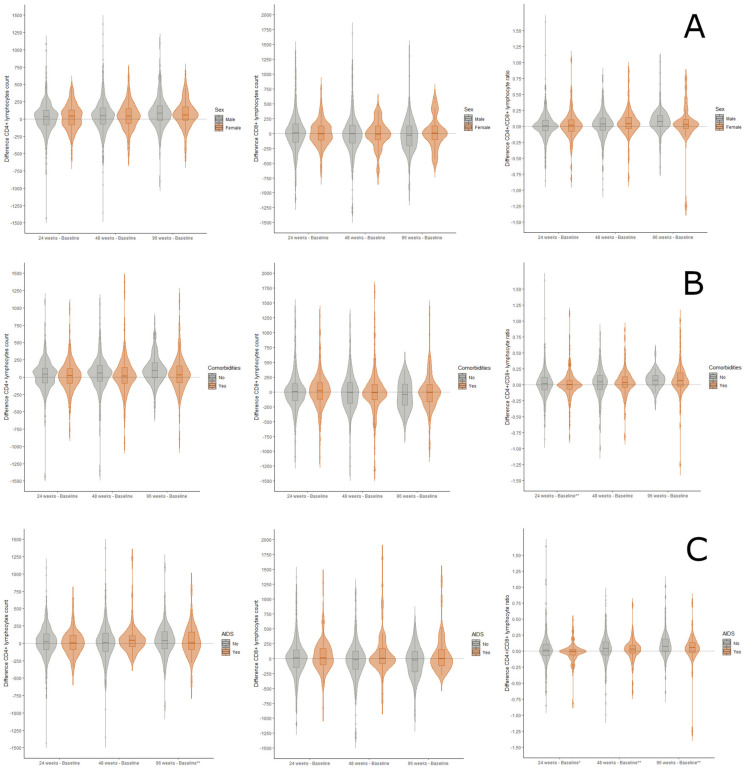
Immune status was assessed through the median baseline CD4+ and CD8+ lymphocyte count and CD4+/CD8+ ratio (Figure 1). No significant differences were found in the CD4+/CD8+ ratio (0.9) during follow-up from baseline to weeks 24 and 96. No statistically significant differences were observed in the study of paired samples based on gender. On the other side, a statistically significant increase in CD4+ lymphocyte count was found in those without comorbidities in the three-time series analyzed [average increase at 24 weeks: 48.7 (SD: 215.3) vs. 25.8 (SD: 215.5), p-value = 0.050; a mean increase at 48 weeks: 75.1 (SD: 232.9) vs. 42.3 (SD: 255.6), p-value = 0.003; a mean increase at 96 weeks: 120.1 (SD: 205.0) vs. 63.8 (SD:275.3), p-value = 0.003]. When this analysis was performed comparing patients with and without AIDS, a significant reduction (p < 0.05) was observed in the number of CD8+ lymphocytes in the subgroup without AIDS at 48 and 96 weeks [−27.8 (SD: 318.3); −54.0 (SD: 266.1)], as well as an increase in CD8+ lymphocyte count in the group with AIDS [91.3 (SD: 358.2); 131.4 (SD: 454.4)].
Figure 1.
Difference between baseline absolute CD4+ lymphocytes (cells/mm3), baseline absolute CD8+ lymphocytes (cells/mm3), and baseline CD4/CD8 ratio of HIV-positive patients at 24, 48, and 96 weeks of DTG plus 3TC treatment. Panel (A): Gender differences; Panel (B): Differences between patients with and without comorbidities; Panel (C): Differences in patients with and without AIDS. Significant values: p-value < 0.05 = *; p-value < 0.01 = **.
Regarding immune status, patients with AIDS presented, as expected, a lower lymphocyte count (around 150–200 cells/mm3 less than non-AIDS patients) without paired data analysis. Nevertheless, the absolute value of CD8+ lymphocytes remained constant in both groups. This trend translates into a lower CD4+/CD8+ ratio in the AIDS group up to week 48 of dual DTG plus 3TC therapy.
Adverse events (AEs) were scarce and only reported in 14 patients (1.4%). They included renal toxicity in 5 (0.5%), central nervous system toxicity in 8 (0.8%), and gastrointestinal issues in 1 (0.1%). Only three (0.29%) patients discontinued treatment because of AEs.
4. Discussion
The efficacy and safety of DTG plus 3TC for treating virologically-suppressed PLWHIV without resistance mutations against DTG and 3TC were settled by the TANGO randomized clinical trial [12]. Similar results are found in our real-life cohort of more than 1000 patients with a suppression efficacy of over 96% and a rate of adverse events lower than 1.5%. However, in the TANGO trial, DTG plus 3TC branch (n = 369) included only 7% of women, and patients older than 50 accounted for 21%. On the contrary, in our cohort (n = 1032), women represented 21.4% of included patients, and individuals older than 50 accounted for 48.8% of the cohort. This fact provides additional helpful information regarding the safety and efficacy of DTG plus 3TC in this population, which are underrepresented in clinical trials.
Beyond this demographic data, in the intention-to-treat exposed population of the TANGO study, 344 patients (93.2%) had an HIV plasma viral load lower than 50 copies/mL at 48 weeks. In our cohort, in the intention-to-treat, 97.5% of patients achieved viral suppression of fewer than 50 copies/mL. Thus, our study confirms and reaffirms the results depicted in the pivotal clinical trial of DTG plus 3TC as a switch study, lining up with some other real-life data from smaller cohorts with similar results [13,14].
Another relevant issue on behalf of DTG plus 3TC treatment is its safety, as severe adverse effects are rarely reported, leading to durability and infrequent switch to other treatment strategies due to adverse effects. Instead, central nervous system events are the most frequently reported AE leading to treatment discontinuation. This data correlates with the TANGO trial [8] and other real-life studies [15,16,17].
A systematic review of the efficacy and safety of DTG plus 3TC from clinical trials and real-life cohorts [18] revealed that neuropsychiatric symptoms, including anxiety, depression, and insomnia, led to the discontinuation of DTG plus 3TC in 1 to 3% of patients [18,19,20]. In our cohort, only three patients (0.29%) had treatment discontinuation due to severe AEs, and only one (0.09%) was related to neuropsychiatric symptoms.
On behalf of CD4+ T cell count and CD4+/CD8+ ratio after switching, our study shows how CD4+ T cell count increased sequentially and significantly from baseline to 96 weeks. Remarkably, this increase was independent of sex, comorbidities, or pre-existing AIDS infection stage. In addition, a reduction in the absolute CD8+ value is also observed in the non-AIDS group.
Our study was designed and carried out during the most challenging days of the coronavirus disease 2019 (COVID-19) pandemic, requiring extra effort by bedside clinicians. Thus, a simple and user-friendly database was developed with a small number of variables to recruit centers. Unfortunately, it led to several limitations that may have clinical implications. They include the study’s retrospective nature; a lack of clinically interesting data, such as body weight gain; and data regarding archived M184V, commonly missing in medical records. Nevertheless, it has undoubted strengths that complement the clinical trials’ data. First, it represents real-life data in the most challenging time of last decades, the COVID-19 pandemic, in which proper adherence to the treatment was probably tackled by many circumstances, adding additional stress to this treatment strategy in terms of performance. Furthermore, this issue probably makes this cohort a completely differential one, as the circumstances in which this dual strategy has been evaluated, with patients having problems accessing their medication or physicians, were unprecedented. These facts have been well described previously [20], underlining how COVID-19 pushed many healthcare systems to their limits in many unpredictable ways, negatively impacting many aspects of patient’s life, including, of course, PLWHIV [21,22]. Thus, the impressive performance of DTG+3TC in very harsh times adds invaluable information to more regular, structured, and controlled data provided by randomized clinical trials and real-life cohorts. Second, it includes a remarkably higher percentage of women and patients over 50 years old than those included in randomized clinical trials, thus providing additional and necessary data in this commonly underrepresented PLWHIV. Third, these cohort results strengthen the results of the TANGO trial, and last but not least, it suggests a potential immunological benefit in increasing CD4+ lymphocyte count and CD4+/CD8+ ratio independently of age, sex, HIV stage, and pre-existing AIDS. Thus, it places DTG plus 3TC as a durable and safe treatment strategy, lined up with previously reported data.
5. Conclusions
In conclusion, our cohort confirms previous data from the TANGO study. Furthermore, it demonstrates that DTG+3TC is an effective treatment strategy for virologically-suppressed PLWHIV independent of age, sex, and HIV stage, as well as a safe and durable strategy, even under the stress of the COVID-19 pandemic. In addition, a potential immunological benefit in terms of CD4+ lymphocyte count and increased CD4+/CD8+ ratio is also suggested.
Acknowledgments
The SPADE-3 investigators want to acknowledge all those living with HIV. SPADE Study Group ** Hospital Burgos, Burgos, Spain (Luis Buzón, Carolina Navarro, María Fernández, Leticia Sánchez); Hospital de Valladolid, Valladolid, Spain (Carlos Dueñas, Laura Rodríguez, Sara Gutiérrez, Genoveva Zapico); Hospital Universitario Infanta Leonor, Madrid, Spain (Jesús Troya, Guillermo Cuevas), Complejo Hospitalario de Navarra, Pamplona, Spain (Estela Moreno-García); Hospital Álvaro Cunqueiro, Vigo, Spain (Guillermo Pousada); Hospital de Huesca, Huesca, Spain (Miguel Egido); Complejo asistencial de Zamora, Zamora, Spain (Cristina Martín); Hospital de Segovia, Segovia, Spain (Eva Ferreira); Hospital la Princesa, Madrid, Spain (Ignacio Santos, Marta del Rey); Hospital Puerta de Hierro, Madrid, Spain (Sara Fuente, Alberto Díaz); Hospital Río Hortega, Valladolid, Spain (Julia Gómez); Hospital Universitario de Áraba, Vitoria, Spain (Miguel Ángel Moran); Hospital Universitario de Donostia, Donostia, Spain (Josean Iribarren); Hospital Universitario de Salamanca, Salamanca, Spain (Alicia Iglesias); Hospital Marqués de Valdecialla, Santander, Spain (Claudia González), Hospital Virgen de la Salud Toledo, Toledo, Spain (María Antonia Sepúlveda). They all collaborated at their local institutions for data gathering. The SPADE-3 cohort was presented at IDWEEK 2022, hosted in Washington DC, poster number 1262.
Author Contributions
Conceptualization, methodology, formal analysis, investigation, writing-original draft preparation, supervision, review, and editing: L.B., C.D. and J.T. contributed equally to these issues. Methodology and software: R.P. did the whole statistical analysis. Investigation, data review, and editing: The rest of the authors reviewed their patient’s medical records and incorporated them into the database. They participated at the end in reviewing the manuscript before sending it. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study protocol was approved by the Ethics Committee of Hospital Universitario de Burgos in February 2021 with code 2454 and subsequently by the local committee of each institution involved in the study.
Informed consent statement
Informed consent was waived due to the impossibility of getting it during the COVID-19 pandemic.
Data Availability Statement
All data are kept by the investigators of the SPADE-3 project.
Conflicts of Interest
Luis Buzon, Jesús Troya, and Carlos Dueñas declare to have received advisory board and lecture fees from ViiV Healthcare, GILEAD, Jannsen, and MSD. They do not own stock options from any of those companies.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Kanters S., Vitoria M., Doherty M., Socias M.E., Ford N., Forrest J.I., Popoff E., Bansback N., Nsanzimana S., Thorlund K., et al. Comparative efficacy and safety of first-line antiretroviral therapy for the treatment of HIV infection: A systematic review and network meta-analysis. Lancet HIV. 2016;3:e510–e520. doi: 10.1016/S2352-3018(16)30091-1. [DOI] [PubMed] [Google Scholar]
- 2.Margolis A.M., Heverling H., Pham P.A., Stolbach A. A review of the toxicity of HIV medications. J. Med. Toxicol. 2014;10:26–39. doi: 10.1007/s13181-013-0325-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carr A., Miller J., Law M., Cooper D.A. A syndrome of lipoatrophy, lactic acidaemia and liver dysfunction associated with HIV nucleoside analogue therapy: Contribution to protease inhibitor-related lipodystrophy syndrome. AIDS. 2000;14:F25–F32. doi: 10.1097/00002030-200002180-00001. [DOI] [PubMed] [Google Scholar]
- 4.Arribas J.R., Girard P.M., Landman R., Pich J., Mallolas J., Martínez-Rebollar M., Zamora F.X., Estrada V., Crespo M., Podzamczer D., et al. Dual treatment with lopinavir-ritonavir plus lamivudine versus triple treatment with lopinavir-ritonavir plus lamivudine or emtricitabine and a second nucleos(t)ide reverse transcriptase inhibitor for maintenance of HIV-1 viral suppression (OLE): A randomized, open-label, non-inferiority trial. Lancet Infect. Dis. 2015;15:785–792. doi: 10.1016/S1473-3099(15)00096-1. [DOI] [PubMed] [Google Scholar]
- 5.Osterholzer D.A., Goldman M. Dolutegravir: A next-generation integrase inhibitor for the treatment of HIV infection. Clin. Infect. Dis. 2014;59:265–271. doi: 10.1093/cid/ciu221. [DOI] [PubMed] [Google Scholar]
- 6.Spinelli F., Prakash M., Slater J., van der Kolk M., Bassani N., Grove R., Wynne B., van Wyk J., Clark A. Dolutegravir-based regimens in treatment-naive and treatment-experienced aging populations: Analyses of 6 phase III clinical trials. HIV Res. Clin. Pract. 2021;22:46–54. doi: 10.1080/25787489.2021.1941672. [DOI] [PubMed] [Google Scholar]
- 7.Cahn P., Madero J.S., Arribas J.R., Antinori A., Ortiz R., Clarke A.E., Hung C.C., Rockstroh J.K., Girard P.M., Sievers J., et al. Durable Efficacy of Dolutegravir Plus Lamivudine in Antiretroviral Treatment-Naive Adults With HIV-1 Infection: 96-Week Results From the GEMINI-1 and GEMINI-2 Randomized Clinical Trials. J. Acquir. Immune Defic. Syndr. 2020;83:310–318. doi: 10.1097/QAI.0000000000002275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Wyk J., Ajana F., Bisshop F., De Wit S., Osiyemi O., Portilla Sogorb J., Routy J.P., Wyen C., Ait-Khaled M., Nascimento M.C., et al. Efficacy and Safety of Switching to Dolutegravir/Lamivudine Fixed-Dose 2-Drug Regimen vs. Continuing a Tenofovir Alafenamide-Based 3- or 4-Drug Regimen for Maintenance of Virologic Suppression in Adults Living With Human Immunodeficiency Virus Type 1: Phase 3, Randomized, Noninferiority TANGO Study. Clin. Infect. Dis. [Internet] 2020;71:1920–1929. doi: 10.1093/cid/ciz1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mussini C., Lorenzini P., Cozzi-Lepri A., Lapadula G., Marchetti G., Nicastri E., Cingolani A., Lichtner M., Antinori A., Gori A., et al. Icona Foundation Study Group. CD4/CD8 ratio normalization and non-AIDS-related events in individuals with HIV who achieve viral load suppression with antiretroviral therapy: An observational cohort study. Lancet HIV. 2015;2:e98–e106. doi: 10.1016/S2352-3018(15)00006-5. [DOI] [PubMed] [Google Scholar]
- 10.Trickey A., May M.T., Schommers P., Tate J., Ingle S.M., Guest J.L., Gill M.J., Zangerle R., Saag M., Reiss P., et al. Antiretroviral Therapy Cohort Collaboration (ART-CC). CD4:CD8 Ratio and CD8 Count as Prognostic Markers for Mortality in Human Immunodeficiency Virus-Infected Patients on Antiretroviral Therapy: The Antiretroviral Therapy Cohort Collaboration (ART-CC) Clin. Infect. Dis. 2017;65:959–966. doi: 10.1093/cid/cix466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scott L.J. Dolutegravir/Lamivudine Single-Tablet Regimen: A Review in HIV-1 Infection. Drugs. 2020;80:61–72. doi: 10.1007/s40265-019-01247-1. [DOI] [PubMed] [Google Scholar]
- 13.Hidalgo-Tenorio C., Cortés L.L., Gutiérrez A., Santos J., Omar M., Gálvez C., Sequera S., Jesús S.E., Téllez F., Fernández E., et al. DOLAMA study: Effectiveness, safety and pharmacoeconomic analysis of dual therapy with dolutegravir and lamivudine in virologically suppressed HIV-1 patients. Medicine (Baltimore) 2019;98:e16813. doi: 10.1097/MD.0000000000016813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maggiolo F., Gulminetti R., Pagnucco L., Digaetano M., Benatti S., Valenti D., Callegaro A., Ripamonti D., Mussini C. Lamivudine/dolutegravir dual therapy in HIV-infected, virologically suppressed patients. BMC Infect. Dis. 2017;17:215. doi: 10.1186/s12879-017-2311-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cento V., Perno C.F. Two-drug regimens with dolutegravir plus rilpivirine or lamivudine in HIV-1 treatment-naïve, virologically-suppressed patients: Latest evidence from the literature on their efficacy and safety. J. Glob. Antimicrob. Resist. 2020;20:228–237. doi: 10.1016/j.jgar.2019.08.010. [DOI] [PubMed] [Google Scholar]
- 16.Mendoza I., Lázaro A., Torralba M. Effectiveness, Durability, and Safety of Dolutegravir and Lamivudine Versus Dolutegravir, Lamivudine, and Abacavir in a Real-Life Cohort of HIV-Infected Adults. Ann. Pharmacother. 2022;56:412–421. doi: 10.1177/10600280211034176. [DOI] [PubMed] [Google Scholar]
- 17.Fabbiani M., Rossetti B., Ciccullo A., Oreni L., Lagi F., Celani L., Colafigli M., De Vito A., Mazzitelli M., Dusina A., et al. ODOACRE Study Group. Efficacy and durability of two- vs. three-drug integrase inhibitor-based regimens in virologically suppressed HIV-infected patients: Data from real-life ODOACRE cohort. HIV Med. 2021;22:843–853. doi: 10.1111/hiv.13146. [DOI] [PubMed] [Google Scholar]
- 18.Patel R., Evitt L., Mariolis I., Di Giambenedetto S., d’Arminio Monforte A., Casado J., Cabello Úbeda A., Hocqueloux L., Allavena C., Barber T., et al. HIV Treatment with the Two-Drug Regimen Dolutegravir Plus Lamivudine in Real-world Clinical Practice: A Systematic Literature Review. Infect. Dis. Ther. 2021;10:2051–2070. doi: 10.1007/s40121-021-00522-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baldin G., Ciccullo A., Capetti A., Rusconi S., Sterrantino G., Cossu M.V., Giacomelli A., Lagi F., Latini A., Bagella P., et al. Efficacy and safety of switching to dolutegravir plus emtricitabine/tenofovir disoproxil fumarate (TDF) or elvitegravir/cobicistat/emtricitabine/TDF in virologically suppressed HIV-infected patients in clinical practice: Results from a multicentre, observational study. HIV Med. 2019;20:164–168. doi: 10.1111/hiv.12688. [DOI] [PubMed] [Google Scholar]
- 20.Horton R. Offline: COVID-19 is not a pandemic. Lancet. 2020;396:874. doi: 10.1016/S0140-6736(20)32000-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bogart L.M., Ojikutu B.O., Tyagi K., Klein D.J., Mutchler M.G., Dong L., Lawrence S.J., Thomas D.R. COVID-19 Related Medical Mistrust, Health Impacts, and Potential Vaccine Hesitancy Among Black Americans Living With HIV. J. Acquir. Immune Defic. Syndr. 2021;86:200–207. doi: 10.1097/QAI.0000000000002570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meyer D., Slone S.E., Ogungbe O., Duroseau B., Farley J.E. Impact of the COVID-19 Pandemic on HIV Healthcare Service Engagement, Treatment Adherence, and Viral Suppression in the United States: A Systematic Literature Review. AIDS Behav. 2022;2:1–14. doi: 10.1007/s10461-022-03771-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are kept by the investigators of the SPADE-3 project.