Abstract
Introduction: SARS-CoV-2, responsible for the coronavirus disease (COVID-19) pandemic, may impact other systems apart from the respiratory system, including the nervous system. In this systematic review, we aimed to establish the prevalence and determinants of neuropathic pain amongst COVID-19-infected individuals. Methodology: A literature search in the PubMed database was performed and 11 papers were eligible for inclusion in this systematic review and meta-analysis. Results: The pooled prevalence of COVID-19-related neuropathic pain was 6.7% (95% CI: 4.7–9.5%) for hospitalised patients during the acute phase and 34.3% (95% CI: 14.3–62%) for long COVID patients. The identified risk factors for COVID-19-related neuropathic pain development included depression, COVID-19 severity and azithromycin use. Conclusions: Neuropathic pain is a very common symptom in long COVID, indicating the urgency for further research in this direction.
Keywords: neuropathic pain, COVID-19, long COVID
1. Introduction
SARS-CoV-2, which was responsible for the coronavirus disease (COVID-19) pandemic, can lead to severe lung damage and has high mortality rates [1]. Apart from the respiratory system, a SARS-CoV-2 infection may impact multiple systems, including the cardiovascular [2] and the nervous systems. Long COVID syndrome is a term used to describe resisting symptoms months after the initial infection. A variety of symptoms have been described as part of long COVID syndrome. One under-researched complication is the development of neuropathic pain following a COVID-19 infection [3].
Neuropathic pain is caused by somatosensory nervous system diseases or lesions [4]. Many risk factors for the pathogenesis of neuropathic pain have been identified, including systemic diseases, autoimmune diseases, incisions, channelopathies, metabolic disorders, viral infections and chemotherapy drug-induced mechanisms [5,6,7,8]. Although the exact molecular mechanisms are still to be discovered [9], neuropathic pain is estimated to affect up to 10% of the general population [10].
Neuropathic pain has the potential to become severely debilitating, hugely impacting the quality of life of an individual [4]. Associated autonomic symptoms can also inflict long-term disabilities [11]. Mobility issues can limit socialisation and independence, leading to depression, anxiety and sleep disorders [12]. Additionally, neuropathic pain often presents with analgesic/opioid medication resistance [13], leaving treatment options limited and tailored to the individual. Subsequently, this highlights the necessity for neuropathic pain assessments across different cohorts, including COVID-19.
The exact prevalence of COVID-19-related neuropathic pain is unestablished to date [14]. COVID-19 is able to interact with the nervous system via multiple mechanisms, promoting nociceptor excitability and, therefore, neuropathic pain [15]. Furthermore, the evidence suggests that neuropathic pain is occasionally an immediate COVID-19 complication; in other cases, it is more delayed [3].
In this systematic review, we aimed to establish the prevalence and determinants of COVID-19-related neuropathic pain by systematically applying eligibility criteria and performing a quantitative analysis.
2. Methodology
2.1. Protocol and Registration
This review was registered in PROSPERO, an international database of prospectively registered systematic reviews in health and social care. The registration number for this review is CRD42022330381.
2.2. Literature Search Strategy
A systematic literature search in the PubMed database was performed on 16 May 2022 using two medical subject heading terms that had to be present in the title or the abstract. Term A was “Allodynia OR hyperalgesia OR hypoalgesia OR itchiness OR burning OR neuropathic OR ‘painful cold’ OR ‘electric shock’ OR tingling OR ‘pins and needles’ OR itchiness OR itching OR hypoesthesia” and term B was “COVID or SARS-CoV-2 or Coronavirus”. Human subjects, the English language and full-text filters were applied. The reference lists of eligible papers and relevant reviews were also meticulously searched to include further studies reporting on neuropathic pain related to COVID-19.
Decisions about the search term use involved the PICO (population, intervention, comparison and outcome) framework [16], highlighted in Table 1. The PICO framework aids the formulation/discovery of answers to healthcare research questions in an evidence-based manner [17]. In this review, how to best detect COVID-19-related neuropathic pain articles was deciphered from the PICO framework. This helped to answer the research aims regarding the prevalence.
Table 1.
PICO criteria applied to literature/systematic search strategy.
| Population | Adults Only (≥18 Years of Age) |
|---|---|
| Intervention | Neuropathic pain following COVID-19 infection. |
| Comparison |
|
| Outcome | Prevalence (COVID-19-related neuropathic pain). |
2.3. Inclusion/Exclusion Criteria
Articles eligible to be included in this review had to meet the following criteria: (i) papers describing patients with neuropathic pain as defined by the International Association for the Study of Pain [18] that were clearly linked to COVID-19; (ii) human adult subjects involved; and (iii) the article was written in English.
The exclusion criteria for this review comprised: (i) a description of neuropathic pain that was not within the primary aims of the study; (ii) case series/cohorts with fewer than 10 patients; (iii) non-original articles (reviews, medical hypotheses, letters to the editor, etc.); and (iv) articles describing non-neuropathic pain.
In descending order, title screening, abstract screening, full-text screening and reference screening were implemented to filter out non-relevant papers. This left the relevant articles suitable for inclusion. Decisions regarding which papers were eligible for inclusion were made by two researchers. Reference screening was implemented using Google Scholar, purely to identify any papers that may have been missed during the original search. However, reference screening yielded no additional literature.
2.4. Data Extraction
Following the identification of eligible articles, information covering the phenotypes, incidence, natural history, treatments and narrative summary for COVID-19-related neuropathic pain was collated. Colour-coding assigning the content of each paper was utilised to achieve a narrative summary. Other factors such as the COVID-19 severity, patient demographics and comorbidities were extracted and taken into consideration. Neuropathy types and the reported time between an initial COVID-19 infection and neuropathic pain manifestation were extracted. A meta-analysis was conducted, in which the article details regarding long COVID were included. A full-text scrutiny of each included article aided the risk factor identification, contributing to the overall field of COVID-19-related neuropathic pain.
2.5. Synthesis of Results
The prevalence, descriptives, frequencies and other determinants were investigated via the extracted data. The means, 95% confidence intervals and meta-analysis (p < 0.05) were calculated, in line with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines.
2.6. Statistical Analysis
Aggregated data were used where possible in this review. Statistically pooled proportion calculations were conducted in R language using the default settings of the “meta” package and the “metaprop” function with a random fixed effects model [19]. The heterogeneity was evaluated using the I2 statistic and corresponding forest plot [20]. The I2 statistic suggested whether the study heterogeneity or chance were responsible for the variation. Negative I2 values were set as equal to 0 and the values ranged between 0% and 100% [20]. The heterogeneity could be quantified as low, moderate and high, with upper limits of 25%, 50% and 75% for I2, respectively [20]. Occasionally, a meta-analysis was not suitable for the data. Resultantly, a narrative approach was taken.
2.7. Assessment of Bias
Bias assessments adapted from [21] assessed the methodological quality of the included articles via a checklist. The response rate, sample representativeness, data collection method, measures used, sampling techniques and statistical methods were covered by nine questions. Each question was labelled as “0” or “1” (0 = low risk of bias; 1 = high risk of bias). The total scores ranged from 0–9 and were categorised as follows [22]: 7–9: high risk of bias; 4–6: moderate risk of bias; 0–3: low risk of bias.
Supplementary Table S1 details this bias assessment.
3. Results
3.1. COVID-19-Related Neuropathic Pain
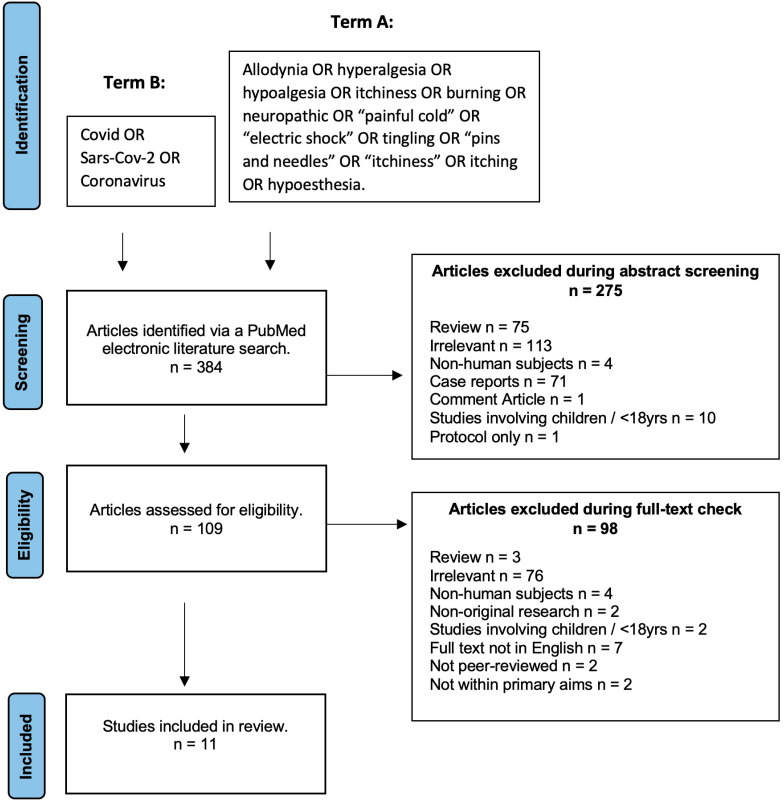
Following the systematic literature search strategy described above, 384 papers were initially identified. However, 275 were excluded during the abstract screening and 98 were excluded during the full-text eligibility screening. Therefore, 11 articles met the eligibility and were finally included in this review. The screening and inclusion process is highlighted in Figure 1 (PRISMA chart). The characteristics of the included papers are summarised in Supplementary Table S2.
Figure 1.
PRISMA chart displaying the screening and inclusion process for the COVID-19-related neuropathic pain systematic review and meta-analysis.
3.2. Epidemiology
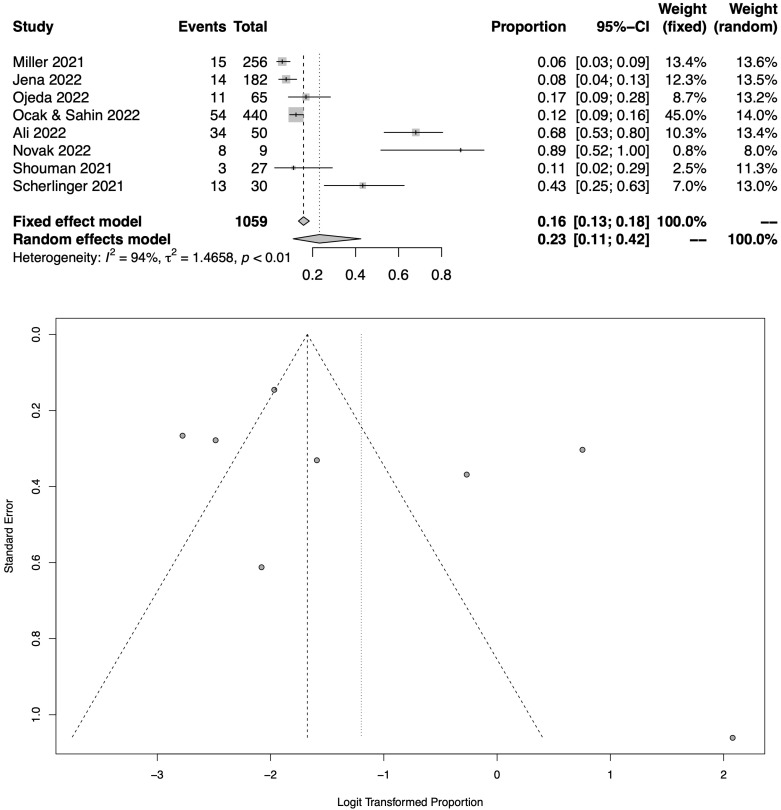
Figure 2 shows the total pooled prevalence of COVID-19-related neuropathic pain in COVID-19 sufferers following the meta-analysis of 8 eligible studies [23,24,25,26,27,28,29,30], which assessed a total of 1059 patients. The pooled prevalence of neuropathic pain was 23.2% (95% CI: 11.0–42.4%). As also shown in the respective funnel plot (Figure 2), the heterogeneity was high amongst these studies (I2 = 94%).
Figure 2.
Meta-analysis results (all RCTs with available data) as illustrated in the forest plot and the respective funnel plot regarding the pooled prevalence estimation for COVID-19-related neuropathic pain amongst COVID-19 sufferers [23,24,25,26,27,28,29,30].
As two of the studies provided data for the acute phase and six studies for the long COVID phase, a subgroup analysis was performed.
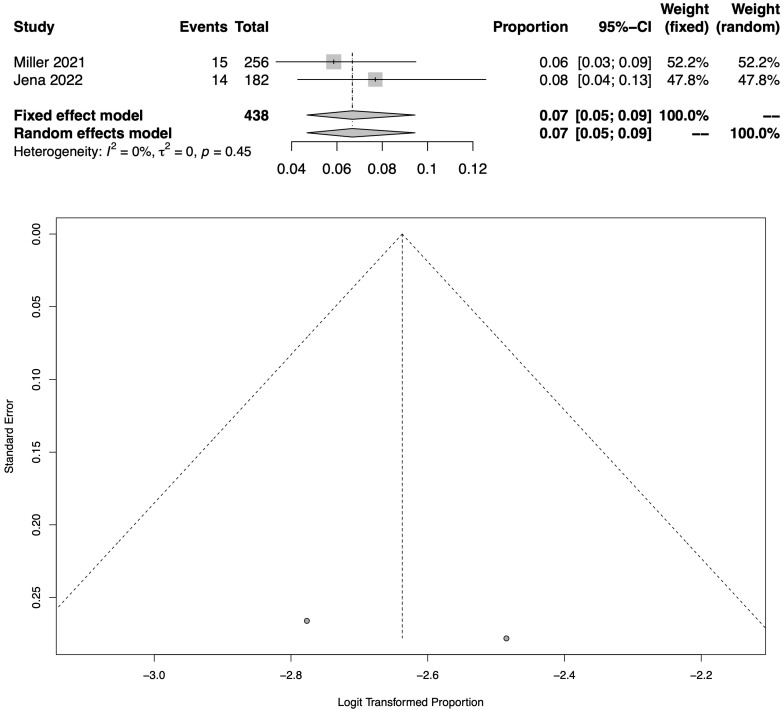
Figure 3 shows the total pooled prevalence of COVID-19-related neuropathic pain in hospitalised patients following the meta-analysis of 2 eligible studies [24,25], which assessed a total of 438 patients. The pooled prevalence during hospitalisation (acute phase of COVID-19) was 6.7% (95% CI: 4.7–9.5%). As also shown in the respective funnel plot (Figure 3), there was a high heterogeneity across these studies (I2 = 0.0%).
Figure 3.
Meta-analysis results (all RCTs with available data) as illustrated in the forest plot and the respective funnel plot regarding the pooled prevalence estimation for COVID-19-related neuropathic pain amongst hospitalised patients [24,25].
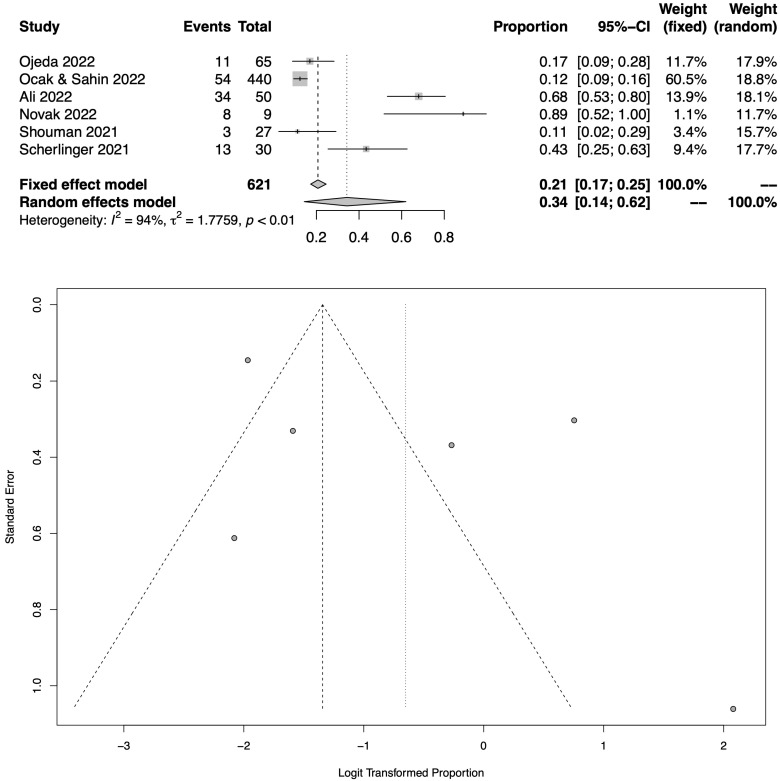
Figure 4 shows the total pooled prevalence of COVID-19-related neuropathic pain in long COVID patients following the meta-analysis of 6 eligible studies [23,26,27,28,29,30], which assessed a total of 621 patients. The pooled prevalence of COVID-19-related neuropathic pain in long COVID patients was 34.3% (95% CI: 14.3–62%). As also shown in the respective funnel plot (Figure 4), the heterogeneity was high amongst these studies (I2 = 94%).
Figure 4.
Meta-analysis results (all RCTs with available data) as illustrated in the forest plot and the respective funnel plot regarding the pooled prevalence estimation for COVID-19-related neuropathic pain amongst long COVID cohorts [23,26,27,28,29,30].
3.3. Risk Factors for Neuropathic Pain
3.3.1. Demographics
Neither age nor gender were associated with a neuropathic pain symptom presence overall [23,24,25,26,27,28,29,30,31,32]. However, the male gender was suggested to be a predictor of increased pain severity (odds ratio 4.3; 95% CI: 1.8–10.6) [24] and a younger age was proposed to contribute towards new-onset neuropathic pain following a severe COVID-19 infection. In their observational study, Ojeda et al. showed that patients with new-onset pain had a mean age of 60 years versus a mean age of 68 years for patients not developing new-onset pain (p = 0.005; univariate analysis) [28].
3.3.2. COVID-19 Severity
A neuropathic pain symptom presence correlated with the COVID-19 severity [24] for hospitalised COVID-19 patients. Moreover, those with an increased COVID-19 severity who had to be hospitalised in an intensive care unit were more likely to acquire peripheral nerve injuries due to a prone positioning for an extended amount of time [25].
Although the results on whether the COVID-19 severity was linked to neuropathic pain in long COVID were contradictory, the majority of the studies reported that severe COVID-19 increased the likelihood of COVID-19 neuropathic pain development up to 6.3 times [28,29,31,32,33].
3.3.3. Treatment with Azithromycin
Several studies suggested that a treatment with azithromycin increased the likelihood of developing neuropathic pain following a COVID-19 infection by up to 5.4 times [28,31]. Azithromycin may induce neuronal cell autophagy [31]. Concerningly, the odds of COVID-19-related neuropathic pain significantly increased following an azithromycin treatment [28].
3.3.4. Depression, Anxiety and Psychological Distress
A two-way relationship between pain and the mental state exists. Depression, anxiety and psychological distress may occur in those with neuropathic pain following a COVID-19 infection [29]; depression was also considered to be an independent predictor of COVID-19-related neuropathic pain in [31] as it may increase the risk of pain by 4.5 times.
3.4. Locations
Different areas of the body have been reportedly affected by neuropathic pain after a COVID-19 infection. The locations reported were the leg/calf, knees, hands, muscles, neck, shoulders, lower abdomen, head and feet [24,27]. Neuropathic pain presented diffusely across the whole body in a few cases [24]. Other cases displayed COVID-19-related neuropathic pain within the extremities [31,32]. An interesting neuropathic pain syndrome that was described was trichodynia, which occurred within 4 weeks of the initial COVID-19 infection [33].
3.5. Natural History
The time between a COVID-19 initial infection and neuropathic pain development ranged from 1 month to 15 months for long COVID patients [23,28]. Upon discharge, half of the long COVID patients went on to report new-onset pain 4 weeks later [28]. Conclusions are difficult, as the papers did not investigate similar time lengths [23,26,29]. However, all these papers showed that COVID-19-related neuropathic pain could be acute (4 weeks) or delayed (4 months+). Therefore, COVID-19-related neuropathic pain should still be considered to be plausible in delayed circumstances.
4. Discussion
This review aimed to establish the prevalence of neuropathic pain following a COVID-19 infection. Although neuropathic pain can be a symptom in the acute phase of COVID-19 (the pooled prevalence for hospitalised patients was 6.7%), it is more common in the context of long COVID syndrome as it affects more than 1 in 3 sufferers (the pooled prevalence was 34.3%). The current literature suggests that long COVID syndrome affects one in three of those infected with COVID-19 [34]. Therefore, one in nine of those infected with COVID-19 will develop neuropathic pain. This is a significant number of people, considering the millions who have been infected with COVID-19 (Mercer and Salit, 2021).
The COVID-19-related neuropathic pain predictors that proved to be significant in the literature included the COVID-19 severity (hospitalised cohorts) [24], prolonged ICU prone positioning [25], azithromycin use and depression [31]. Therefore, psychological interventions to treat depression might prove useful in reducing the risk of COVID-19-related neuropathic pain. This is quite significant, considering 61% of COVID-19 patients were seeking neuropathic pain treatments at the follow-up [23].
Our findings should be interpreted with caution, given a few limitations. First, a gender bias impacted certain samples [24,25], possibly preventing detectable differences from being uncovered. Future replications require equally balanced samples. Second, most studies lacked control groups. Third, our search was restricted to a single database (PubMed). Despite the fact that PubMed is the largest medical database where the majority of (if not all) high-quality studies appear, there was a small risk that we might have missed a few relevant papers. Finally, publication bias may occur in systematic reviews and could have undermined the validity of the results. This was reflected in the significant heterogeneity among the studies of long COVID patients.
In conclusion, in this review we highlighted the prevalence and predictors of COVID-19-related neuropathic pain. More research is needed in the future to explore these findings in more detail, considering that several patients have been left unable to function due to neuropathic pain following COVID-19 [27].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm12041672/s1, Table S1: Quality assessment of the included papers; Table S2: Characteristics of the included papers.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Baud D., Qi X., Nielsen-Saines K., Musso D., Pomar L., Favre G. Real estimates of mortality following COVID-19 infection. Lancet Infect. Dis. 2020;20:773. doi: 10.1016/S1473-3099(20)30195-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Long B., Brady W.J., Koyfman A., Gottlieb M. Cardiovascular complications in COVID-19. Am. J. Emerg. Med. 2020;38:1504–1507. doi: 10.1016/j.ajem.2020.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joshi D., Gyanpuri V., Pathak A., Chaurasia R.N., Mishra V.N., Kumar A., Singh V.K., Dhiman N.R. Neuropathic Pain Associated with COVID-19: A Systematic Review of Case Reports. Curr. Pain Headache Rep. 2022;26:595–603. doi: 10.1007/s11916-022-01065-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finnerup N.B., Kuner R., Jensen T.S. Neuropathic pain: From mechanisms to treatment. Physiol. Rev. 2021;101:259–301. doi: 10.1152/physrev.00045.2019. [DOI] [PubMed] [Google Scholar]
- 5.Bannister K., Sachau J., Baron R., Dickenson A.H. Neuropathic pain: Mechanism-based therapeutics. Annu. Rev. Pharmacol. Toxicol. 2020;60:257–274. doi: 10.1146/annurev-pharmtox-010818-021524. [DOI] [PubMed] [Google Scholar]
- 6.Cavalli E., Mammana S., Nicoletti F., Bramanti P., Mazzon E. The neuropathic pain: An overview of the current treatment and future therapeutic approaches. Int. J. Immunopathol. Pharmacol. 2019;33:1–10. doi: 10.1177/2058738419838383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaggi A.S., Singh N. Mechanisms in cancer-chemotherapeutic drugs-induced peripheral neuropathy. Toxicology. 2012;291:1–9. doi: 10.1016/j.tox.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 8.Mearns E.S., Taylor A., Thomas Craig K.J., Puglielli S., Cichewicz A.B., Leffler D.A., Sanders D.S., Lebwohl B., Hadjivassiliou M. Neurological manifestations of neuropathy and ataxia in celiac disease: A systematic review. Nutrients. 2019;11:380. doi: 10.3390/nu11020380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen H., Hu Y., Xie K., Chen Y., Wang H., Bian Y., Wang Y., Dong A., Yu Y. Effect of autophagy on allodynia, hyperalgesia and astrocyte activation in a rat model of neuropathic pain. Int. J. Mol. Med. 2018;42:2009–2019. doi: 10.3892/ijmm.2018.3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu S., Bono J., Tao Y.X. Long noncoding RNA (lncRNA): A target in neuropathic pain. Expert Opin. Ther. Targets. 2019;23:15–20. doi: 10.1080/14728222.2019.1550075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bakkers M., Faber C.G., Hoeijmakers J.G., Lauria G., Merkies I.S. Small fibers, large impact: Quality of life in small-fiber neuropathy. Muscle Nerve. 2014;49:329–336. doi: 10.1002/mus.23910. [DOI] [PubMed] [Google Scholar]
- 12.Smart K.M., Blake C., Staines A., Doody C. Self-reported pain severity, quality of life, disability, anxiety and depression in patients classified with ‘nociceptive’, ‘peripheral neuropathic’ and ‘central sensitisation’ pain. The discriminant validity of mechanisms-based classifications of low back (±leg) pain. Man. Ther. 2012;17:119–125. doi: 10.1016/j.math.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Clark A.K., Old E.A., Malcangio M. Neuropathic pain and cytokines: Current perspectives. J. Pain Res. 2013;6:803. doi: 10.2147/JPR.S53660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Attal N., Martinez V., Bouhassira D. Potential for increased prevalence of neuropathic pain after the COVID-19 pandemic. Pain Rep. 2021;6:e884. doi: 10.1097/PR9.0000000000000884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McFarland A.J., Yousuf M.S., Shiers S., Price T.J. Neurobiology of SARS-CoV-2 interactions with the peripheral nervous system: Implications for COVID-19 and pain. Pain Rep. 2021;6:e885. doi: 10.1097/PR9.0000000000000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richardson W.S., Wilson M.C., Nishikawa J., Hayward R.S. The well-built clinical question: A key to evidence-based decisions. ACP J. Club. 1995;123:A12–A13. doi: 10.7326/ACPJC-1995-123-3-A12. [DOI] [PubMed] [Google Scholar]
- 17.Davies K.S. Formulating the evidence-based practice question: A review of the frameworks. Evid. Based Libr. Inf. Pract. 2011;6:75–80. doi: 10.18438/B8WS5N. [DOI] [Google Scholar]
- 18.Raja S.N., Carr D.B., Cohen M., Finnerup N.B., Flor H., Gibson S., Keefe F., Mogil J.S., Ringkamp M., Sluka K.A., et al. The revised IASP definition of pain: Concepts, challenges, and compromises. Pain. 2020;161:1976. doi: 10.1097/j.pain.0000000000001939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Langfelder P., Horvath S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008;9:559. doi: 10.1186/1471-2105-9-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 21.Hoy D., Brooks P., Woolf A., Blyth F., March L., Bain C., Baker P., Smith E., Buchbinder R. Assessing risk of bias in prevalence studies: Modification of an existing tool and evidence of interrater agreement. J. Clin. Epidemiol. 2012;65:934–939. doi: 10.1016/j.jclinepi.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 22.Liampas A., Velidakis N., Georgiou T., Vadalouca A., Varrassi G., Hadjigeorgiou G.M., Tsivgoulis G., Zis P. Prevalence and management challenges in central post-stroke neuropathic pain: A systematic review and meta-analysis. Adv. Ther. 2020;37:3278–3291. doi: 10.1007/s12325-020-01388-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ali S.T., Kang A.K., Patel T.R., Clark J.R., Perez-Giraldo G.S., Orban Z.S., Lim P.H., Jimenez M., Graham E.L., Batra A., et al. Evolution of neurologic symptoms in non-hospitalized COVID-19 “long haulers”. Ann. Clin. Transl. Neurol. 2022;9:950–961. doi: 10.1002/acn3.51570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jena D., Sahoo J., Barman A., Gupta A., Patel V. Musculoskeletal and Neurological Pain Symptoms Among Hospitalized COVID-19 Patients. Am. J. Phys. Med. Rehabil. 2022;101:411–416. doi: 10.1097/PHM.0000000000001969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller C., O’Sullivan J., Jeffrey J., Power D. Brachial Plexus Neuropathies during the COVID-19 Pandemic: A Retrospective Case Series of 15 Patients in Critical Care. Phys. Ther. 2021;101:pzaa191. doi: 10.1093/ptj/pzaa191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Novak P., Mukerji S.S., Alabsi H.S., Systrom D., Marciano S.P., Felsenstein D., Mullally W.J., Pilgrim D.M. Multisystem Involvement in Post-Acute Sequelae of Coronavirus Disease 19. Ann. Neurol. 2022;91:367–379. doi: 10.1002/ana.26286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ocak O., Sahin E.M. Evaluation of Neuropathic Pain Features in COVID-19 Patients. Neurol. India. 2022;70:591. doi: 10.4103/0028-3886.344625. [DOI] [PubMed] [Google Scholar]
- 28.Ojeda A., Calvo A., Cuñat T., Mellado-Artigas R., Comino-Trinidad O., Aliaga J., Arias M., Ferrando C., Martinez-Pallí G., Dürsteler C. Characteristics and influence on quality of life of new-onset pain in critical COVID-19 survivors. Eur. J. Pain. 2022;26:680–694. doi: 10.1002/ejp.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scherlinger M., Felten R., Gallais F., Nazon C., Chatelus E., Pijnenburg L., Mengin A., Gras A., Vidailhet P., Arnould-Michel R., et al. Refining “Long-COVID” by a prospective multimodal evaluation of patients with long-term symptoms attributed to SARS-CoV-2 infection. Infect. Dis. Ther. 2021;10:1747–1763. doi: 10.1007/s40121-021-00484-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shouman K., Vanichkachorn G., Cheshire W.P., Suarez M.D., Shelly S., Lamotte G.J., Sandroni P., Benarroch E.E., Berini S.E., Cutsforth-Gregory J.K., et al. Autonomic dysfunction following COVID-19 infection: An early experience. Clin. Auton. Res. 2021;31:385–394. doi: 10.1007/s10286-021-00803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Magdy R., Eid R.A., Fathy W., Abdel-Aziz M.M., Ibrahim R.E., Yehia A., Sheemy M.S., Hussein M. Characteristics and risk factors of persistent neuropathic pain in recovered COVID-19 patients. Pain Med. 2022;23:774–781. doi: 10.1093/pm/pnab341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oguz-Akarsu E., Gullu G., Kilic E., Dinc Y., Ursavas A., Yilmaz E., Zarifoglu M., Karli N., Pandemic Study Team. Akalın H., et al. Insight into pain syndromes in acute phase of mild-to-moderate COVID-19: Frequency, clinical characteristics, and associated factors. Eur. J. Pain. 2022;26:492–504. doi: 10.1002/ejp.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Starace M., Iorizzo M., Sechi A., Alessandrini A.M., Carpanese M., Bruni F., Vara G., Apalla Z., Asz-Sigall D., Barruscotti S., et al. Trichodynia and telogen effluvium in COVID-19 patients: Results of an international expert opinion survey on diagnosis and management. JAAD Int. 2021;5:11–18. doi: 10.1016/j.jdin.2021.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Dowd A. COVID-19: Third of people infected have long term symptoms. BMJ Br. Med. J. 2021;373:n1626. doi: 10.1136/bmj.n1626. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author.