Abstract
After the onset of the AIDS pandemic, HIV-1 (genus Lentivirus) became the predominant model for studying retrovirus Env glycoproteins and their role in entry. However, HIV Env is an inadequate model for understanding entry of viruses in the Alpharetrovirus, Gammaretrovirus and Deltaretrovirus genera. For example, oncogenic model system viruses such as Rous sarcoma virus (RSV, Alpharetrovirus), murine leukemia virus (MLV, Gammaretrovirus) and human T-cell leukemia viruses (HTLV-I and HTLV-II, Deltaretrovirus) encode Envs that are structurally and functionally distinct from HIV Env. We refer to these as Gamma-type Envs. Gamma-type Envs are probably the most widespread retroviral Envs in nature. They are found in exogenous and endogenous retroviruses representing a broad spectrum of vertebrate hosts including amphibians, birds, reptiles, mammals and fish. In endogenous form, gamma-type Envs have been evolutionarily coopted numerous times, most notably as placental syncytins (e.g., human SYNC1 and SYNC2). Remarkably, gamma-type Envs are also found outside of the Retroviridae. Gp2 proteins of filoviruses (e.g., Ebolavirus) and snake arenaviruses in the genus Reptarenavirus are gamma-type Env homologs, products of ancient recombination events involving viruses of different Baltimore classes. Distinctive hallmarks of gamma-type Envs include a labile disulfide bond linking the surface and transmembrane subunits, a multi-stage attachment and fusion mechanism, a highly conserved (but poorly understood) “immunosuppressive domain”, and activation by the viral protease during virion maturation. Here, we synthesize work from diverse retrovirus model systems to illustrate these distinctive properties and to highlight avenues for further exploration of gamma-type Env structure and function.
Keywords: retrovirus, gammaretrovirus, alpharetrovirus, deltaretrovirus, filovirus, reptarenavirus, Env, Gp2, immunosuppressive domain, R-peptide, syncytin
1. Introduction
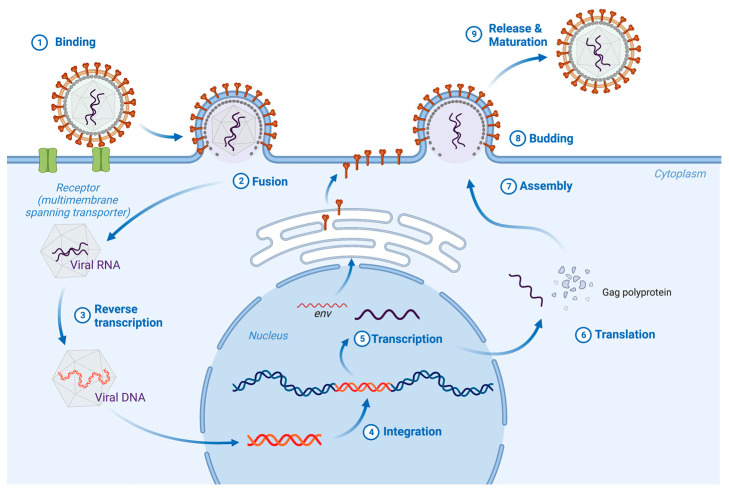
The family Retroviridae comprises two subfamilies, the Orthoretrovirinae and the Spumaretrovirinae [1]. These are further divided into six and five genera, respectively. For all retroviruses, viral attachment and entry is mediated by the Env glycoproteins, products of the viral env gene (Figure 1). Env is expressed as a precursor polyprotein in the secretory pathway. During transit through the secretory pathway, Env is glycosylated and cleaved into two subunits, SU (surface subunit) and TM (transmembrane subunit). Trimers of SU-TM heterodimers assemble into trimeric complexes that are incorporated into the membrane of budding virions. SU contains the primary binding determinants that confer the unique receptor-specificity associated with each virus, while TM mediates fusion of the virion and target cell membranes. For some retroviruses, Env expression also mediates receptor-dependent superinfection interference [2]. The Env proteins of HIV-1 and the related primate lentiviruses have received significant attention from virologists and structural biologists. However, HIV Env comes up short as a model for retroviruses of other genera. For example, viruses of the Alpharetrovirus, Gammaretrovirus and Deltaretrovirus genera share a similar Env protein that is both structurally and functionally distinct from the Env proteins of HIV and the other lentiviruses. Although considerable work has been done on the entry of viruses representing all three of these genera, gammaretrovirus Env proteins are convenient as prototypes both for illustrating the shared features and for highlighting differences between the genera. Therefore, when referring to these collectively we will use the term “gamma-type” Env [3,4].
Figure 1.
Gammaretroviral Replication Cycle. Schematic of a typical replication cycle for gammaretroviruses.
1.1. Retroviruses with Gamma-Type Env Proteins
Retroviruses that encode gamma-type Envs include, but are not limited to, the avian leukemia viruses (ALV; Alpharetrovirus genus), the murine, feline, and gibbon-ape leukemia viruses (MLV, FeLV, GaLV; Gammaretrovirus genus), and the human T-cell leukemia viruses (HTLV-I and HTLV-II; Deltaretrovirus genus). Additionally, among viruses in the Betaretrovirus genus, a subset known as the “type-D” betaretroviruses are natural recombinants that have acquired gammaretrovirus-related env genes, and therefore encode gamma-type Env proteins distinct from those of the canonical betaretroviruses [3,5,6]. Examples of type-D betaretroviruses include Mason-Pfizer monkey virus (MPMV, SRV-3) and squirrel monkey retrovirus (SMRV), among others.
The gamma-type Env can be distinguished from the Envs of lentiviruses, betaretroviruses, and retroviruses of the subfamily Spumaretrovirinae by a combination of several criteria, including the presence of an intersubunit disulfide bond between SU and TM, the presence of a C-terminal R-peptide in the immature trimer, two or three highly conserved protein sequence motifs and, in the case of the gammaretroviruses and deltaretroviruses, by a marked bias towards use of multi-pass membrane transporters of the solute carrier (SLC) superfamily as entry receptors [7,8]. Entry mediated by gamma-type Envs also differs from that of other retroviruses in requiring reduction of the intersubunit disulfide bond at a post-binding, but pre-fusion step [9,10,11]. Gammaretroviral entry also differs in multiple aspects from that of influenza A virus (IAV), the textbook example of a class I fusogen [12,13].
1.2. Variations on the Gamma-Type Env Are Also Found in Some Non-Retroviruses
The most notable examples of gamma-type Env outside of the Retroviridae are the Gp2 proteins of filoviruses (e.g., Ebolavirus, EBOV). Filovirus Gp2 is a distant homolog of the TM subunit of ALV Env [14,15,16]. Reptilian arenaviruses of boid snakes also encode an entry protein with striking sequence similarity to gamma-type Envs [17]. In addition to these RNA viruses, at least one double-stranded DNA virus, Chelonid herpesvirus 5 (ChHV-5), encodes a hypothetical protein (HP35) with significant similarity to a gamma-type Env [4,18]. Given these precedents, it seems likely that additional examples of horizontal gene transfer involving the gamma-type Env, and representatives of other viral taxa remain to be discovered.
1.3. Gamma-Type Env Encoded by Endogenous Retroviruses
Ancient endogenous retrovirus (ERV) loci often contain remnants of retroviral env genes, and in some cases, these may retain complete or nearly complete open reading frames with the potential to express functional gamma-type Env proteins [19,20,21,22,23,24,25,26,27]. Gammaretrovirus-related ERV loci are abundant in the genomes of a diverse array of vertebrate species, including mammals, birds/reptiles and amphibians [28,29,30,31,32]. Distantly related ERV loci encoding gamma-type Env can also be found in amphibian genomes [33] and in avian genomes, including recombinant forms [34] and loci closely related to those of exogenous avian leukemia virus (ALV) [29,30]. Mirroring the situation with exogenous betaretroviruses, ERV related to the betaretroviruses include recombinant forms that appear to have acquired a gamma-type env gene via recombination [6,34]. Endogenous deltaretrovirus sequences are rare, but a few have been identified in the genomes of a limited number of mammalian species [35,36]; at least one of these has a putative env sequence that once encoded a gamma-type Env distantly related to the Env of exogenous bovine leukemia virus (BLV) [37]. Therefore, not only is the gamma-type Env broadly distributed among extant viruses, it is also represented by a majority of the ERV loci found in vertebrate genomes. Collectively, the latter constitutes a “fossil” record of the natural history of the Retroviridae, attesting to the ancient origins and prehistoric host-distribution of the gamma-type Env over a period encompassing hundreds of millions of years of vertebrate evolution.
The exogenous epsilonretroviruses of fish include walleye dermal sarcoma virus and the walleye epidermal hyperplasia viruses (WDSV, WEHV; genus Epsilonretrovirus). These viruses encode a distinct Env lacking the hallmark features of a gamma-type Env, and gammaretrovirus-related ERV are largely absent from fish genomes [28,38]. However, classification of fish ERVs is based on similarity between the predicted reverse-transcriptase (RT) sequences of the ERV loci and the RT enzymes of exogenous epsilonretroviruses such as WDSV [39]. In contrast, when env sequences are used as the basis for phylogenetic comparison, it is evident that many fish ERV arose from viruses that encoded gamma-type Envs [4,40]. Notably, the predicted Env proteins of zebrafish ERV (ZfERV) loci include multiple motifs unique to gamma-type Envs [41]. The number and distribution of fish ERV loci with gamma-type env genes is evidence that related retroviruses once infected the ancestors of modern fish, and raises the possibility that retroviruses with gamma-type Env still circulate in natural fish populations. These observations also indicate that the common ancestor of the epsilonretroviruses employed a gamma-type Env, while the Env encoded by modern exogenous epsilonretroviruses represents a more recent evolutionary development.
1.4. Exaptation of ERV-Encoded Gamma-Type Env
ERV loci in vertebrate genomes are also substrates for natural selection, and there are now numerous reports of ERV-encoded proteins that have been coopted by host evolution to serve cellular/organismal functions [19,20,42]. For example, gammaretrovirus-related ERV also include the evolutionarily coopted placental syncytin genes of the different mammalian lineages [43]. Other endogenous retroviral genes have been co-opted during host evolution to confer resistance to infection by exogenous retroviruses [44]. In addition to the syncytin genes and the receptor interference examples, which are mostly on the order of tens-of-millions of years old, there are numerous examples of ERV loci with intact gamma-type Env ORFs of unknown function [23,27,45,46,47], including some that have remained intact for over 100 million years [22,24,48].
1.4.1. Syncytins
The discovery and characterization of the syncytins have been comprehensively reviewed elsewhere [21,43]. Briefly, the syncytins play an instrumental role in placental formation by facilitating cell-cell fusion and formation of the syncytiotrophoblast layer, which is key for the transfer of nutrients from mother to fetus. These include human Syncytin-1, which encodes a gamma-type Env derived from the HERV-W family of human ERVs (HERVs), and human Syncytin-2, which encodes a gamma-type Env derived from the HERV-FRD family [26,49,50]. Disruption of syncytin expression or function in mice disrupts cell-cell fusion and placental formation, and ultimately leads to termination of the fetus [21]. In addition to the syncytin genes of humans and related primates, syncytin genes have been identified in many other mammalian lineages as independent captures of retroviral env genes. Phylogenetically, most of the mammalian syncytins described thus far cluster with the Env proteins of gammaretroviruses [51,52,53,54].
1.4.2. Retrovirus Envs as Host-Encoded Resistance Genes
When expressed from ERV loci in the host genome, gamma-type Envs may act as barriers to exogenous infection of host tissues by viruses using the same receptor. The murine Fv4 gene (also known as Akvr-1) serves as a well understood example. Fv4 encodes a gamma-type Env closely related to the Env of ecotropic MLV, and Fv4 expression confers resistance to ecotropic MLV infection [55,56,57,58,59,60,61]. Fv4 was first identified in laboratory mice as a resistance locus for Friend MLV [56,57]. Later on, it was identified as an endogenous retrovirus closely related to MLV, containing an intact env gene [56,62]. Expression of the Fv4 env-like gene in cells has a similar effect to the expression of MLV Env in infected cells: in both cases, resistance to MLV infection occurs, supporting the conclusion that the Fv4 protein creates resistance through the mechanism of receptor interference. Receptor-interference is a common property of gamma-type Envs and is thought to provide a mechanism for superinfection resistance (discussed in a later section). Thus, it is possible that other ERV loci encoding gamma-type Envs evolved to become receptor-specific restriction factors as the result of selective pressure on the host imposed by a related exogenous virus. Indeed, other ERV loci for which there is compelling evidence for a resistance function include the Rmcf and Rmcf2 loci of mice and the Ev3, Ev6 and Ev9 loci of chickens [63,64,65,66].
Experimentally, at least, incorporation of Fv4 into MLV virions can also displace or negatively interfere with the function of the wild-type Env [67]. Similarly, Dewannieux et al. demonstrated that Env proteins from gammaretroviruses with different receptor specificities can heteromultimerize and have interfering effects on infectivity in cell culture, raising the possibility that dominant negative interference could be another mechanism by which endogenized Envs act as host restriction factors [68].
The Fv4 protein itself is processed like a normal envelope protein and retains the ability to bind to the mCAT1 receptor (the receptor used by ecotropic MLVs) but has lost its fusogenic potential due to a mutation in the fusion peptide [69]. The Fv4 Env can be incorporated into virions, and has been used in pseudotyping assays with MLV to demonstrate that, though binding occurs, no fusion or entry into the cell can occur [69]. Although difficult to prove, it is tempting to hypothesize that selection as an antiviral defense mechanism would favor ERV Envs that retain their receptor interference functions but lose the ability to mediate membrane fusion or viral entry.
As with many other vertebrates, the human genome contains a multitude of ERV loci, some of which retain some or all of the associated env ORF [25,27,70]. Proving that these have, or once had, antiviral functions is challenging. Among other things, the exogenous forms of the related viruses may have gone extinct millions of years ago. Experimentally, it has been shown that two human ERV-encoded gamma-type Envs (Suppressyn and Syncytin-1/HERV-W) that bind the ASCT2 receptor (SLC1A5) can also induce receptor-interference and render cells resistant to infection by extant retroviruses of other primates that also use ASCT2 as an entry receptor [25,71]. Another strategy is to use ERV sequence information to reconstruct the proteins of an extinct virus, allowing a direct test of the receptor-interference hypothesis. For example, HERV-T loci in the human genome are evidence for an ancient gammaretrovirus that would have infected the lineage leading to modern Old-World primates. Blanco-Melo et al. have identified the receptor that was once used by this virus as MCT1 (SLC16A1) [72]. Similar to Fv4 in mice, the intact env ORF of HERV-T in the human genome no longer encodes a fusogenic Env (in this case, due to a mutation in the furin cleavage site between SU and TM). It is, however, still capable of binding to MCT1 and blocking infection by virions carrying a reconstructed version of the ancient, functional HERV-T Env [72]. From this, it is thought likely that the HERV-T Env ORF may have been co-opted early in hominid evolution and preserved by natural selection because its expression conferred resistance to a closely related, exogenous gammaretrovirus.
1.5. Significance
The biological significance of gamma-type Envs is difficult to overestimate. They are associated with diverse viruses, including biomedically relevant viruses such as HTLV-I, HTLV-II and Ebolavirus, they include the mammalian syncytins, which are essential proteins in the development of the placental syncytiotrophoblasts, they are represented by both viral and host proteins, and they are likely to have had a significant impact on the coevolution of retroviruses and their vertebrate hosts. Despite their ubiquity in nature and ancient origins, work on gamma-type Envs for many years has been overshadowed by the focus on HIV-1 and the primate lentiviruses. However, lentiviral Env proteins are imperfect models for understanding gamma-type Envs and the viruses that encode them, and there are no known examples of endogenized, functional lentiviral Envs. In the following sections we attempt to summarize decades of work on retroviruses and ERVs with gamma-type Envs, emphasizing those unique features of gamma-type Envs that distinguish them from the Envs of other retroviruses and highlighting areas for further exploration.
2. Biogenesis
Complete maturation and fusion capability of the Env trimer is achieved through a combination of proteolytic events, including removal of the N-terminal signal peptide (SP) by signal peptidase, cleavage by host-cell furins to release the SU and TM subunits and expose the N-terminal fusion peptide of TM, and cleavage within the virion by the viral protease to remove a short C-terminal peptide (the so-called “R-peptide”) from TM.
The Env precursor protein is synthesized in the rough ER and co-translationally inserted across the membrane. After removal of the signal peptide by signal peptidase, Env travels from the rough ER to the Golgi and trans-Golgi network where it is processed into a trimer with subunits SU and TM. From there the envelope glycoprotein travels to the ER and then finally to the relevant membrane (typically the plasma membrane) [73,74]. Trafficking of gammaretroviral envelope proteins is dictated by specific sequences within the cytoplasmic tail [74,75].
Post-translational processing of gamma-type envelope glycoproteins is similar to other retroviral Envs, including glycosylation and furin-mediated cleavage in the secretory pathway. The number of possible glycosylation sites varies across gamma-type Envs with a range of 4–13 attachment sites; a majority of these are located in SU. While glycosylation varies considerably, furin cleavage sites are far more conserved. Cellular furin proteases recognize a specific motif within the envelope protein, typically RRKR, or a basic residue with a dibasic pair (K/R-X-K/R-R) [73,76]. Cleavage by furins leads to separation of the SU and TM subunits and dramatic reorganization of the structure of the envelope protein [77]. This restructuring into the final Env trimer occurs as the glycoprotein precursor travels from the trans-Golgi network to the endoplasmic reticulum, and correct processing by the furin protease has been linked to proper trafficking [73]. Conversely, it has also been reported that furin cleavage mutants result in only a small reduction in infectivity, as long as there are a small number of processed Env proteins available for incorporation [78]. Mutations to the furin cleavage site also lead to a 100-fold reduction of MLV Env’s ability to produce resistance to superinfection [79].
3. Hallmark Features of Gamma-Type Envs
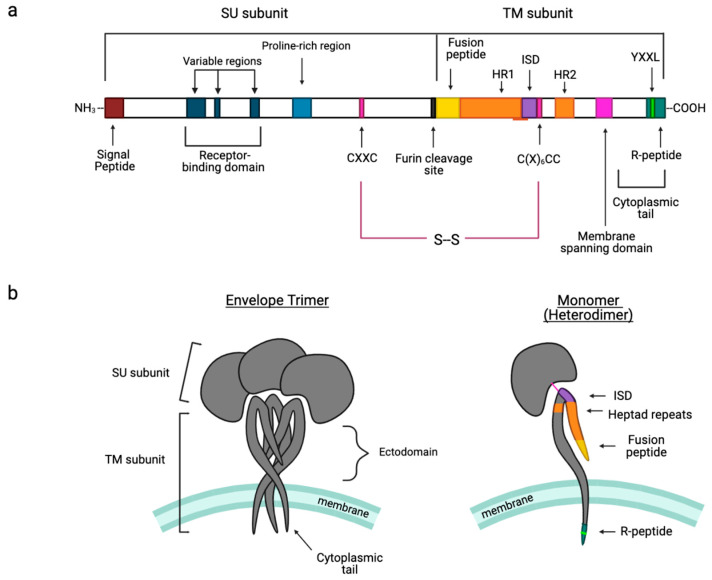
Many of the key insights into gamma-type Env structure and function comes from work on murine leukemia virus (MLV) and related gammaretroviruses, while studies of other gammaretroviruses as well as some viruses of other genera, allow for the identification of shared, conserved features and functions that distinguish gamma-type Envs from the Env proteins of HIV-1 and other retroviruses. Below, we highlight some of these, in order of their appearance in the linear protein sequence of MLV Env (Figure 2).
Figure 2.
Domains of the gammaretroviral envelope glycoprotein. (a) Linear depiction of a gammaretrovirus Env highlighting conserved features. S–S indicates a disulfide bond between the CXXC and C(X)6CC motifs. HR1 and HR2 are the two heptad repeat domains of the transmembrane subunit. HR1 overlaps slightly with the immunosuppressive domain (ISD). (b left) Schematic of an envelope glycoprotein trimer, composed of three heterodimers comprising SU and TM subunits. (b right) Depiction of an Env monomer, highlighting predicted locations of conserved regions including the ISD, HR1 and HR2, the fusion peptide and cytoplasmic tail domains. SU is linked to TM through a disulfide bond, depicted here by a pink line.
3.1. Signal Peptide
The amino terminus of retroviral Env precursors comprises a signal peptide (SP) responsible for targeting the protein to the secretory pathway. SPs also distinguish the gamma-type Env from those of other retroviruses. For example, the SPs of MLV, ALV and HTLV are short (~21–64 residues) and closely resemble canonical SPs of cellular proteins [80]. In contrast, the SPs of lentiviruses (e.g., HIV-1) often overlap with other ORFs, and the SPs of betaretroviruses (e.g., mouse mammary tumor virus (MMTV) and Jaagsiekte sheep retrovirus (JSRV) are longer (>90 residues), multifunctional, and bear little resemblance to cellular SPs [81,82,83,84,85,86].
The Env SP is cleaved off by cellular signal peptidases. Beyond functionality in trafficking and its role as an insertion signal, specific timing of cleavage of the SP may also act as a quality control for properly folded Envs [87]. The addition of cleavable signal peptides has also been shown to enhance packaging efficiency of pseudotyped viral particles [88]. Additionally, the composition of the signal peptide impacts glycosylation, and thus, antigenicity of the envelope glycoprotein [89]. This may indirectly subject the signal peptide sequence to immune related selective pressure. The signal peptide has also been shown to alter levels of incorporation of high mannose carbohydrates into particles which enhances infectivity through increased interactions with certain lectins [90].
3.2. Receptor Binding Domain (RBD)
The SU subunit of the envelope glycoprotein contains the receptor binding domain (RBD) that gives Env its specificity to a cellular receptor and permits binding of Env which in turn can trigger changes in TM which lead to fusion with the cell membrane. The RBDs of gamma-type Envs, including type-D betaretroviruses and representatives of the alpharetrovirus, gammaretrovirus and deltaretrovirus genera, are located in the amino-terminal half of SU [91,92,93,94,95,96,97,98].
The RBD of MLV minimally encompasses a region from amino acid 9 to 230 of the SU [94]. The MLV RBD is probably the best characterized gammaretroviral Env, and at least two variable regions (VRA and VRB) in SU contribute to receptor specificity of MLV Env [99]. Subsequent to coining of the terms VRA and VRB, a third variable region was named VRC. Consequently, in much of the relevant the literature, the N-to-C-terminal order of the variable domains in the linear SU sequence is not alphabetical—VRA, VRC, VRB. More distantly related gammaretroviruses may vary in both the number and sequence of variable regions, but in general these are found in the N-terminal half of SU. Similarly, the receptor binding determinants also map to multiple variable domains in the SU sequences of alpharetroviruses and deltaretroviruses [91,100,101,102,103,104,105,106,107]. Note, however, that while these serve analogous functions as receptor-specificity determinants, the number, location and sequence of variable regions can differ when comparing Env from different genera.
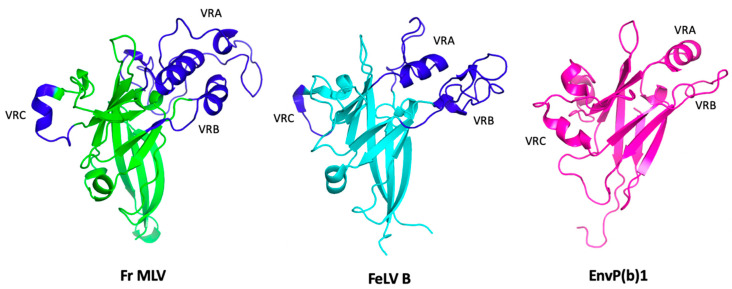
High-resolution crystal structures of the receptor-binding domains of two gammaretroviruses (MLV and FeLV) and one endogenous gamma-like ERV have been reported [23,108,109]. Together, the three RBDs represent tens-to-hundreds of millions of years of divergent evolution, yet comparison of the three structures reveals a highly similar core arrangement of beta strands, with the three variable domains arranged on the apical (receptor-facing) surface (Figure 3) [23]. This arrangement is consistent with a role for the variable domains in direct interactions with cell surface receptors.
Figure 3.
Crystal structures of gammaretrovirus receptor binding domains. Crystal structures of the receptor binding domain (RBD) of Friend MLV (PDB 1A0L), FeLV B (PDB 1LCS) and ENVp(b)1 (PDB 6W5Y). Defined variable regions A, B and C (VRA, VRB, VRC) are shown in blue for MLV and FeLV B. Approximate locations are indicated for EnvP(b)1 based on structurally similar locations (McCarthy et al., 2020). Variable regions are believed to provide receptor specificity among RBDs that recognize different receptors.
3.3. Proline-Rich Region (PRR)
In gamma-type Envs, the N-terminal domain of SU, including the RBD, is often separated from the C-terminal domain by a proline-rich region (PRR) [95,97,110]. The PRR is not well conserved across divergent gamma-type Envs, indicating that the proline-rich composition may be more important than the specific linear sequence. The PRR is a major antigenic determinant, although antibodies that bind the PRR are non-neutralizing [111]. Structural analysis of the FeLV Env PRR suggested it forms a beta-turn helix [112]. It is thought that the PRR connects the amino terminal half of SU (containing the RBD) to the C-terminal half of SU in a hinge-like way, and that this may be important for activation of TM-mediated fusion [113,114]. Notably, the PRR can tolerate large in-frame insertions, which has proven useful for structure/function studies [115,116,117].
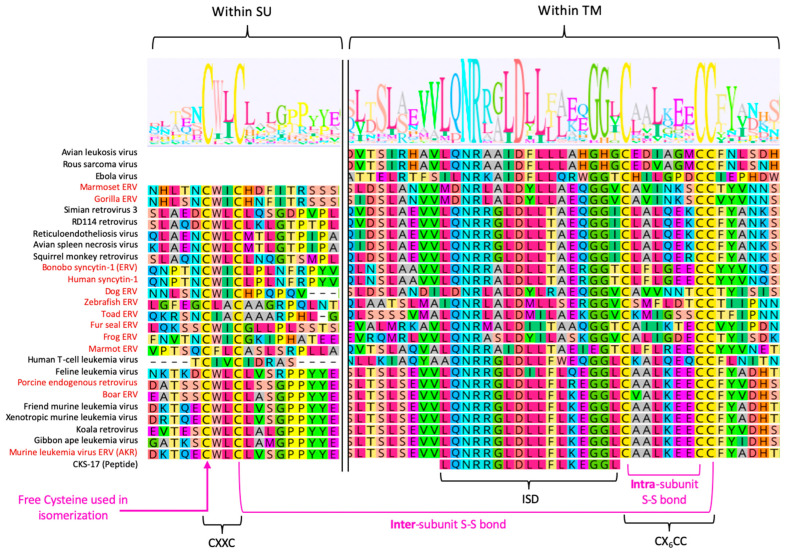
3.4. Intersubunit Disulfide Bond and the CXXC(SU) Motifs
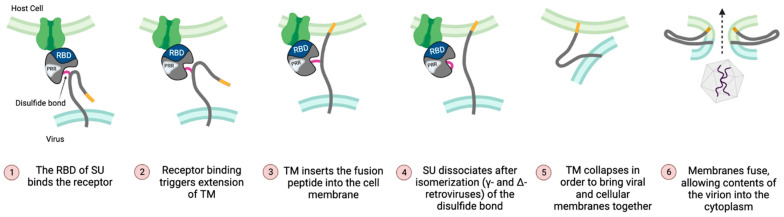
In contrast to the Env complexes of lentiviruses (e.g., HIV-1) and betaretroviruses (e.g., MMTV), the SU and TM subunits of gamma-type Envs are covalently linked via a labile disulfide bond that is crucial for proper Env stability and plays a role in the conversion of the receptor bound Env complex to a fusion-active form [10,11,118,119]. For gammaretroviruses, deltaretroviruses, and type-D betaretroviruses, the bond forms between a CXXC motif in SU and a highly conserved CXnCC motif in TM (typically, n = 6 residues) (Figure 2). Isomerization results in loss of the inter-subunit bond and formation of an intra-subunit bond between the cysteines of the CXXC motif, followed by dissociation of SU and refolding of TM into the hairpin, post-fusion conformation [120,121] (Figure 4).
Figure 4.
Binding and fusion of a Gamma-type Env protein with a host cell membrane. Env is shown as a single heterodimer rather than a trimer for simplicity. The receptor binding domain (RBD) is shown in blue, and the proline rich region (PRR) is shown in white. After binding of the SU subunit to a receptor, isomerization of the disulfide bond occurs, resulting in conversion to an intra-subunit bond within the CXXC motif of the SU subunit, releasing the SU subunit and ultimately resulting in fusion mediated by the TM subunit. The conformational change that TM undergoes is irreversible, and TM cannot revert to carry out fusion again.
The CXXC sequence is typically found within the C-terminal half of SU of gammaretroviruses, type-D betaretroviruses [122] and deltaretroviruses [107]. However, the location can vary, and in some gamma-type Env it may be located N-terminal to the RBD (e.g., SYNC2). The two internal positions display a bias towards hydrophobic residues, intriguingly similar to the active sites of disulfide exchange enzymes (Figure 5) [11]. The SU of alpharetrovirus Env proteins lack a CXXC motif, and instead the intersubunit bond forms between a cysteine in SU and the CX6CC motif in TM (see later subsection). ALV SU contain an unpaired cysteine, but surprisingly, this does not seem to be involved in isomerization or reduction of the intersubunit bond [123]. As with gammaretroviruses, binding of ALV SU to a receptor also triggers extension of TM and insertion of the fusion loop; however, in the case of ALV Envs, completion of fusion requires endocytosis and a concomitant drop in pH [100,124,125].
Figure 5.
Conservation of CXXC, ISD and CX6CC motifs of gamma-type Envs. Geneious amino acid alignment of 27 gammaretroviral and gamma-like Env sequences and the CKS-17 peptide. Endogenous sequences are shown in red while exogenous viruses are shown in black. On the left is a section from SU containing the CXXC motif (which is absent from alpharetroviral and filoviral entry proteins). The CXXC contributes to an intersubunit disulfide bond with the third cysteine in the CX6CC motif in TM. On the right is a section of sequence from TM that contains the highly conserved region of the immunosuppressive domain (ISD). The CKS-17 peptide was used to originally define the region of the ISD; also highlighted is the intra-subunit bond that forms within the CX6CC motif. RSV and ALV are classified as alpharetroviruses, SRV3 is a betaretrovirus with a gamma-like Env, and HTLV is a deltaretrovirus; also notable is the gamma-like glycoprotein of Ebola virus which is a member of Filoviridae. Accession #s in order of figure: AFV99542.1; BAD98245.1; Q05320.1; ACI62863.1; AGI61275.1; P07575.1; ABS71857.1; QXV86750.1; P31796.1; NP_041262.1; NP_001291475.1; Q9UQF0.1; XP_022270294.1; AAM34209.1; XP_040289417.1; XP_025743503.1; QXP50143.1; XP_015345157.1; AAAU04934.1; AYG96595.1; ACD35952.1; AAQ88184.1; P26804.1; AEI59727.1; ALX81658.1; ALV83307.1; AAB03092.1.
3.5. Fusion Peptide
Retrovirus Envs are class I fusion proteins, with fusion peptides (FP) located at or near the N-terminus of TM [126,127]. Gamma-type Env can have N-terminal FP, as is found in most gammaretroviruses and deltaretroviruses, or the FP can comprise an internal “loop” near the N-terminus of TM demarcated by disulfide bonded cysteines, as is the case for alpharetroviruses [100]. As with other class I fusion proteins, retroviral TM initiates fusion through insertion of the fusion peptide into the host cell membrane, after which refolding of TM brings the cell and viral membranes together to form a fusion pore and allow release of the contents of the virion into the host cell (Figure 4) [126]. The fusion peptide comprises primarily hydrophobic residues, although in the case of Moloney MLV (MoMLV) this hydrophobic region is followed by a glycine and threonine-rich region which may also be important for proper fusion [128]. Mutational analyses of varying retroviral Envs have shown that mutations within the fusion peptide often reduce fusogenicity and overall stability of the Env trimer [128,129]. When the fusion peptide corresponds to the N-terminus of TM it overlaps with the furin cleavage site, which is crucial for proper processing of the envelope protein into its two subunits. Mutations to this region have also been shown to result in incomplete processing, in addition to loss of fusion [128]. The general structure of the fusion peptide is conserved across Envs and commonly includes an ordered region, such as a 𝛽-sheet, followed by a turn or loop, followed by another ordered region such as an 𝛼-helix [130,131]. The peptide is capable of flipping between ⍺-helix and 𝛽-sheet conformations depending on the environment [130].
3.6. Heptad Repeats
In type I fusion proteins, including retroviral envelope glycoproteins, coiled-coil ⍺-helices formed from heptad repeats lie adjacent to regions directly involved in fusion [132]. In retroviral envelope proteins these comprise two heptad repeat regions (HR1 and HR2) in the ectodomain of the TM subunit. The first heptad repeat sequence, HR1, is just downstream of the fusion peptide, and is also referred to as the N-helix or N-heptad. Triggering of the fusion mechanism induces a series of conformational changes through which the three HR2 peptides wind up anti-parallel to the three internal HR1 regions in the form of a tightly packed six-helix bundle that represents the post-fusion conformation of TM [133,134]. The hairpin turn linking these is sometimes referred to as the “chain-reversal” region. Notably, the MLV HR2 (the C-helix) is significantly shorter than the corresponding C-helix of HIV [135]. Interestingly, peptides derived from the HR1 region (the N-helix) are capable of inhibiting fusion, possibly through inducing a post-fusion conformation and thus rendering the Env non-fusogenic [133,135].
Comparison of gamma-type Envs, including representatives of different genera and endogenous sequences found in a diverse range of vertebrate genomes, revealed that many non-mammalian gamma-type Envs share an N-glycan attachment site in or near HR1 [3,24]. Notably, the glycan attachment site is also found in Gp2 of filoviruses and certain snake arenaviruses [3,17,24,136,137,138]. The presence of a glycan attachment motif in this region of type 1 viral fusion proteins introduces a “heptad stutter” that disrupts the periodic 3-4-3-4 spacing of hydrophobic residues that define heptad repeats, and consequently has the potential to influence interhelical interactions, such as those that are involved in entry and fusion. Whether the presence or absence of the glycan (or the attachment motif) results in structural or functional differences between non-mammalian and mammalian gamma-type Envs remains to be determined.
3.7. Immunosuppressive Domain (ISD) and the CX6CC Motif
Gamma-type Env sequences are easily distinguished from those of other retroviruses by a stretch of ~26 highly conserved residues between HR1 and HR2 (Figure 5). This sequence comprises the most conserved, continuous stretch of sequence in gamma-type Envs, facilitating alignments and phylogenetic comparisons of highly divergent viruses [16]. The first ~17 residues comprise a motif commonly referred to as the immunosuppressive domain, or ISD. The next nine residues comprise the previously described CX6CC motif (see previous section). Two of the three cysteines form a conserved intrasubunit disulfide that is also found in TM subunits of non-gamma-type retroviruses, including betaretroviruses and lentiviruses. In contrast, the third cysteine is unique to the TM subunits of gamma-type Envs and is involved in forming the hallmark intersubunit disulfide bond with SU. Together, the combined ISD and CX6CC sequence comprises a telltale signature for reliable identification and classification of retroviruses and ERV that encode gamma-type Env proteins [3,119].
The ISD is so-named because a synthetic peptide corresponding to 17 residues immediately upstream of the CX6CC element of MLV was observed to have immunosuppressive properties [139,140]. The original peptide, CKS-17, was shown to have a variety of immunosuppressive effects on lymphocyte populations [139,140,141,142]. Later studies reported that the full envelope proteins of MLV and MPMV are capable of inhibiting tumor rejection in mice [143,144]. It has been proposed that the ISD of the placental syncytins also exerts immunosuppressive effects that contribute to maternal tolerance of foreign antigens expressed by the developing fetus, and the same tumor rejection model was used to test the immunosuppressive properties of the syncytin proteins. Intriguingly, it was found that Syncytin-1 showed no immunosuppression, which led to the isolation of two residues within the ISD that appear to switch immunosuppressive effects on and off [145]. While the immunotolerance hypothesis is intriguing, confirming that the results of cell-culture and murine models accurately reflect the in vivo function(s) of syncytins continues to be an open question, and a very difficult one to answer.
When similar amino acid mutations were introduced into the MPMV and MLV envelope proteins, inhibition of tumor rejection was lost, possibly reflecting a loss of immunosuppressive activity [145]. Wild type and ISD mutant MLV produced similar levels of infection in immunocompromised mice, while in immune competent mice the putative loss of immunosuppressive properties of Env correlated with reduced infectivity [146]. Together, these results suggest that the immunosuppressive functions of the ISD can be genetically separated from pleiotropic effects on viral entry. However, Eksmond et al. found that there was in fact a severe effect of the double mutant on MLV infectivity, but that the defect depended on expression of the cognate receptor in the producer cell [147]. In contrast, when the mutant virus was produced in human cells (lacking the receptor), there was little or no effect of the mutations on infectivity. Interestingly, when the receptor was overexpressed in human cells, infectivity of the wild-type virus was also affected. These observations highlight the difficulties in separating the proposed immunosuppressive effects from effects on the entry functions of Env. One intriguing possibility is that the conservation of the ISD belies a mechanism for stabilizing Env during biogenesis in receptor-expressing cells [147].
To date, there is no unifying molecular mechanism that satisfactorily explains all of the various experimental phenotypes attributed to the ISD. It is particularly challenging to conceive of a hypothetical immunosuppressive mechanism that would be operational in hosts as divergent as mammals, fish, birds/reptiles and amphibians, let alone one that would involve viruses representing a wide range of tissue tropism and disease manifestations and mediate immune tolerance of fetal antigens during pregnancy in mammals. One possibility is that the immunosuppressive effects are unique to a subset of mammalian retroviruses; however, the broad conservation of the ISD sequence among non-mammalian viruses still needs to be explained. Alternatively, the high degree of conservation of the ISD sequence could be an indication that it forms a critical structure or provides an essential function related to viral entry. Indeed, mutations of the ISD that result in defects in infectivity or replication in cell culture are consistent with the possibility that the ISD has a fundamental role in viral replication [147,148]. Such a structure or function could be independent of, or in addition to, a secondary immunosuppressive effect. What those structures/functions might be remains to be discovered. The invariant co-occurrence of the adjacent ISD and the CX6CC motifs in all gamma-type Envs hints at one intriguing possibility: perhaps the ISD motif contributes to formation or stabilization of the hallmark disulfide bond between TM and SU. This would not preclude an additional immunosuppressive function, but would necessarily complicate experiments aimed at disentangling putative immunosuppressive functions from the well-established contributions of Env to virion assembly, attachment, fusion, entry and receptor-interference.
3.8. Cytoplasmic Tail
The cytosolic C-terminal domain of TM, or cytoplasmic tail (CT), begins just after the hydrophobic membrane-spanning segment. TM retains this topology in the virion, with the ectodomain on the outside of the viral membrane and the CT oriented towards the interior of the virion where it can interact with the viral structural proteins and the viral protease. The CT varies in length considerably between retroviruses; lentiviruses, such as HIV, have CTs over 100 residues long. Gammaretroviral envelope proteins, by contrast, typically have short cytoplasmic tails of around 25–35 residues [74]. CT sequences are highly variable and difficult to align, but often contain similar motifs that are thought to function in proper trafficking, cellular localization, and viral assembly [149]. For both the MLV and MPMV Envs, the cytoplasmic tails contain two conserved motifs, a dileucine motif and a tyrosine motif. The dileucine motif directs trafficking of Env from the TGN to endosomal compartments. These Envs then appear to traffic back to the TGN, which is controlled by the tyrosine motif. Mutations in both of motifs cause mis-localization of the envelope protein [150]. Additionally, both motifs may be involved in recruiting clathrin adapter protein complexes although the exact mechanisms and cellular factors involved in correct trafficking are still unknown [151]. Gamma-type Env CTs also often contain at least one YXXΦ motif that is thought to be essential for Env incorporation in virions [152].
Beyond trafficking, there is evidence that the cytoplasmic tail is involved in direct interactions with matrix (MA) and is crucial for incorporation of Env into particles [153]. Consistent with this observation, it has been shown that the CT determines pseudotyping compatibility and viral core preference by envelope proteins [154]. When Envs were expressed in cells in the absence of the other viral structural proteins, they were found to be initially randomly distributed across the plasma membrane. However, once viral core proteins were also expressed, Env trimers were found non-randomly clustered at viral budding sites. This demonstrated that the envelope proteins were actively recruited to sites of assembly, and additionally showed preference for certain viral cores over others that was dictated by the cytoplasmic tail [154]. Similarly, work in HIV has also shown that sites of virion release at the cell surface are determined by the envelope glycoprotein.
3.9. R-Peptide
Another distinguishing feature of gamma-type Envs is the presence of a so-called “R-peptide”, comprising the C-terminal most residues of the CT. The R-peptide must be cleaved off during virion maturation to activate the fusogenic potential of the Env complex [155,156,157,158,159,160]. The addition of the R-peptide of MLV to heterologous entry proteins, including divergent ones such as influenza HA, is sufficient to inhibit fusion [161]. However, incompatibility between a viral protease and the R-peptide cleavage site can reduce infectivity of virions pseudotyped with heterologous Env proteins [162]. It is interesting to speculate that such an incompatibility could limit the acquisition of new env genes by recombination between divergent retroviruses. For an interesting perspective on the origins of the term “R-peptide” see Rein, 2021 [163].
The MLV Env R-peptide comprises the C-terminal 16 amino acids of the cytoplasmic tail [156,164,165]. The viral protease cleaves the R-peptide during assembly and maturation of the viral particle. Cleavage of the R-peptide primes the transmembrane subunit for fusion, as MLV particles with an Env that contain an R-peptide, or are incapable of cleaving the R-peptide, do not induce cell-cell fusion [165]. Experimental cleavage or truncation of the R-peptide is sufficient to restore fusogenicity and infectivity to the MLV Env [165]. The domain containing the R-peptide forms a coiled coil prior to cleavage and fusion, and it is thought that this conformation blocks isomerization of the disulfide bond between SU and TM, or keeps Env in a non-fusogenic conformation [166,167]. However, the R-peptide does not appear to be a completely independent inhibitory mechanism, as mutations upstream in the cytoplasmic tail can reduce the inhibitory effects [168].
Interestingly, in addition to control of fusion, MLV envelope proteins with an R-peptide bind to the mCAT1 receptor less efficiently than envelope proteins without an R-peptide, and truncation of the R-peptide results in reduced incorporation of envelope protein into virions [169]. When the R-peptide was removed from a gammaretroviral Env it spread poorly in cell culture, but given time it acquired a compensatory extension of the C-terminus. This also restored the colocalization between the envelope protein and Gag, implying an additional role for the R-peptide in assembly of viral particles [170]. The R-peptide of MLV, and the entire cytoplasmic tail, is dispensable for assembly with HIV particles, consistent with the fact that HIV glycoproteins do not contain an R-peptide. However, the presence of the MLV R-peptide provides specificity for MLV Gag particles over HIV Gag [162]. This role in assembly is still poorly understood, as are the precise mechanisms through which the R-peptide is able to inhibit fusion.
4. Receptor Interactions of Gamma-Type Envs
The gamma-type Env complex engages with a cognate receptor at two distinct stages in the viral life cycle. First, as with all retroviruses, receptor-binding by mature gamma-type Env present on the virion initiates the cascade of events leading to fusion and viral entry into a target cell. Second, interactions between the immature gamma-type Env and the viral receptor can occur within the infected, virus-producing cell, resulting in reduced susceptibility of the cell to re-infection—a phenomenon known as receptor interference or superinfection resistance. As described in a previous section, the immature, cellular form of Env is distinguished from the mature, virion-associated form by the presence of a C-terminal R-peptide. One likely function of the R-peptide is to prevent these intracellular interactions (i.e., the interactions that result in receptor-interference) from premature triggering of fusion. Similarly, the R-peptide can suppress premature fusion between the producer cell and adjacent target cells. Only those Env molecules that successfully traffic to sites of assembly and are incorporated into nascent virions are activated by the viral protease, ensuring that Env-receptor interactions involving the mature virion and a target cell initiate the fusion and entry process.
4.1. The RBD Regulates Both Initiation and Completion of Fusion
For both ALV and MLV, receptor binding results in extension of TM and insertion of the fusion peptide into the target cell membrane [121,133,171]. However, a second trigger is required before TM can refold into the hairpin structure typically associated with completion of fusion [124,125]. For ALV Env proteins, this occurs when low pH is encountered during viral entry via the endosome [124,172]. By contrast, for MLV (and other gammaretroviruses), isomerization of the intersubunit disulfide bond between SU and TM is necessary for refolding of TM and completion of fusion [120,121].
The pH-dependence for ALV Env is well established [124,172]. By comparison, entry by amphotropic and xenotropic MLV have repeatedly been shown to be pH-independent, demonstrating no defect in fusion or infection when treated in ways that raise pH and prevent acidification of endosomes [173,174]. Interestingly, lower pH within endosomes has been suggested to play a role in fusion for ecotropic MLV, with pH dependence relying on c-terminal residues of SU [173]. However, it has also been shown that once the R-peptide of ecotropic MLV Env is cleaved off, low pH is no longer required for fusion [159]. This suggests that pH-dependence for ecotropic MLV may not be connected to fusion, and may be important after membrane fusion has occurred [159]. Whether or not pH plays a role in entry, and the underlying molecular basis for pH-dependence when it is observed, may differ even among related viruses. Given the natural host distribution and extreme diversity of gamma-type Envs, the effects of pH cannot be assumed based on taxonomic relationships, but rather must be determined experimentally for any given virus.
Multiple, independent lines of evidence point towards involvement of gammaretrovirus RBDs in a second, post-binding step that leads to isomerization of the intersubunit disulfide bond and refolding of TM into the hairpin structure associated with completion of fusion. For example, receptor binding and fusion can be uncoupled by mutation of a key histidine at the N-terminus of the MLV RBD. Virions containing the ΔHis mutant Env bind to the cognate receptor, but do not complete the fusion process [94]. The mutant can be rescued by providing a soluble RBD (sRBD) in-trans, and it is thought that rescue is evidence for a second interaction between the RBD and the C-terminal domain of SU [175,176,177]. Similar observations have been reported for gibbon ape leukemia virus (GaLV) [178]. The phenomenon also extends to the T strain of feline leukemia virus (FeLV-T). FeLV-T virions can bind to target cells, but entry depends on transactivation by a naturally occurring, secreted RBD encoded by a defective FeLV-B related ERV locus [108,179,180,181]. In some cases, rescue in-trans can be achieved using combinations of Envs with sRBDs derived from related viruses with different receptor-specificities; however, the target cells must express the cognate receptor for the transacting sRBD [12,176].
These observations led to a model comprising two sequential interactions involving an RBD [12]. First, an RBD binds to a cognate receptor, triggering the release of the TM fusion peptide (FP) and its insertion into the target cell membrane. A receptor-induced rearrangement in SU then promotes a second interaction between an RBD and the C-terminal domain of SU, promoting isomerization of the intersubunit disulfide bond, dissociation of SU and TM, and transition of TM from its extended form to the six-helix bundle associated with completion of fusion [120,182]. Isomerization is followed by fusion of the viral and cellular membranes. The experimental observations that sRBDs can rescue fusion in trans, together with direct and indirect evidence for cross-talk, raises the possibility that these steps could involve interactions between protomers of the same trimer or between adjacent trimers [177,183,184] Interestingly, an Env chimera comprising the N-terminal domain and PRR from HTLV-1 (a deltaretrovirus) and the C-terminal domain of SU and all of TM from MLV can mediate superinfection-interference specific for HTLV-1 and is functional for entry [107]. If post-binding interactions between the N- and C-termini are required to complete fusion, this result suggests that the mechanism may also extend to deltaretroviruses.
4.2. Gamma-Type Env Preferentially Use SLC Superfamily Transporters as Entry Receptors
A striking property of gamma-type Envs is a significant bias towards use of multi-membrane spanning transporters as entry receptors [7,8,12]. Indeed, a majority of the receptors that have been identified for gammaretroviruses and deltaretroviruses fall into the solute carrier (SLC) superfamily of cellular transporters (Table 1). This is in contrast to viruses in the betaretrovirus, epsilonretrovirus and lentivirus genera, for which no discernible, genus-specific patterns have emerged with respect to receptor preferences [8].
Table 1.
Known receptors used by viruses with gamma-type Envs for cell entry.
| Receptor | Transporter of | Fold | # of Membrane-Spanning Domains | Virus | Classification | Source |
|---|---|---|---|---|---|---|
| Slc1a4 (ASCT1) | Neutral AA | Glt | 8 | REV | Gammaretrovirus | [185] |
| Slc1a4 (ASCT1) | Neutral AA | Glt | 8 | RD114 | Gammaretrovirus | [186] |
| Slc1a4 (ASCT1) | Neutral AA | Glt | 8 | SNV | Gammaretrovirus | [187] |
| Slc1a4/Slc1a5 (ASCT1/ASCT2) | Neutral AA | Glt | 8 | HERV-W/SYNCYTIN-1 | Gamma-like ERV | [50] |
| Slc1a5 (ASCT2) | Neutral AA | Glt | 8 | SUPYN | Gamma-like ERV | [188] |
| Slc1a5 (ASCT2) | Neutral AA | Glt | 8 | SRV1, SRV2, SRV3 (MPMV), SRV4, SRV5 |
Betaretrovirus * | [189] |
| Slc1a5 (ASCT2) | Neutral AA | Glt | 8 | BaERV | Gammaretrovirus | [190] |
| Slc2A1 (Glut-1) | Glucose | MFS | 12 | HTLV | Deltaretrovirus | [191] |
| Slc5a3 (Smit1) | Sodium/myo-inositol | LeuT | 12 | M813 | Gammaretrovirus | [192] |
| Slc5a3 (Smit1) | Sodium/myo-inositol | LeuT | 12 | HEMV | Gammaretrovirus | [193] |
| Slc7a1 (mCAT1) | Cationic AA | LeuT | 12 | MoMLV | Gammaretrovirus | [194] |
| Slc7a1 (CAT1) | Cationic AA | LeuT | 12 | BLV | Deltaretrovirus | [195] |
| Slc9a1 (NHE-1) | Sodium/Hydrogen | NHE1 | 13 | ALV-J | Alpharetrovirus | [196] |
| Slc16a1 (MCT1) | monocarboxylate | MFS | 12 | HERV-T | Gammaretrovirus | [72] |
| Slc19a1 | Folate | MFS | 12 | GLN-MLV | Gammaretrovirus | [197] |
| Slc19a2 (THTR1) | Thiamine | MFS | 12 | KoRV-B | Gammaretrovirus | [198] |
| Slc19a2 (THTR1) | Thiamine | MFS | 12 | KoRV-J | Gammaretrovirus | [199] |
| Slc19a2 (THTR1) | Thiamine | MFS | 12 | FeLV-A | Gammaretrovirus | [200] |
| Slc20a1 (PiT1) | Phosphate | PiT | 10, 12 | KoRV-A | Gammaretrovirus | [201] |
| Slc20a1 (PiT1) | Phosphate | PiT | 10, 12 | FeLV-B | Gammaretrovirus | [202] |
| Slc20a1/Slc20a2 (PiT1/PiT2) | Phosphate | PiT | 10, 12 | GALV/WMV | Gammaretrovirus | [203] |
| Slc20a1 (PiT1) | Phosphate | PiT | 10, 12 | AMLV (4070A, 229A) | Gammaretrovirus | [204] |
| Slc20a1 (PiT1) | Phosphate | PiT | 10, 12 | FeLV-T | Gammaretrovirus | [179] |
| Slc20a1 (PiT1) | Phosphate | PiT | 10, 12 | 10A1-MLV | Gammaretrovirus | [205] |
| Slc31a1 (CTR1) | Copper ion | Ctr | 3 | cERV-1/2 | Gamma-like ERV | [206] |
| Slc31a1 (CTR1) | Copper ion | Ctr | 3 | FeLV-D | Gammaretrovirus | [207] |
| Slc35f2 | Nucleotide sugar | - | 10 | FeLV-A5 * | Gammaretrovirus | [208] |
| Slc49a1/Slc49a2 | Heme | MFS | 12 | FeLV-C | Gammaretrovirus | [209] |
| Slc52a2 (GHB/RFVT2/ PAR-2) |
Riboflavin | MFS | 12 | PERV-A, FeLV-CP * | Gammaretrovirus | [210,211] |
| Slc53a1 (Xpr1) | Phosphate | Slc53 | 8 | XMLV | Gammaretrovirus | [212,213] |
| Slc53a1 (Xpr1) | Phosphate | Slc53 | 8 | PMLV | Gammaretrovirus | [212,213] |
| Slc59a1 (MFSD2A) | Lysophosphatidylcholine | MFS | 12 | HERV FRD/SYNCYTIN-2 | Gamma-like ERV | [214] |
| Slc65a1 (NPC1) | Cholesterol | NPC1 | 13 | Ebola virus | Non-retrovirus (filovirus) | [215] |
| TVA | Cobalamin (?) | LDLR | 1 | ALV-A/RSV-A | Alpharetrovirus | [216,217] |
| CAR1 (TVB) | Tumor necrosis factor | TNFR | 1 | ALV-B/D/E | Alpharetrovirus | [218] |
| TVC | Immunoglobulin | Ig | 1 | ALV-C | Alpharetrovirus | [219] |
Receptors are listed with their SLC classification and commonly used name, if applicable. Fold refers to classification used in [220]. The “*” indicates artificial isolate selected from a library screen. Slc = designates member of the solute carrier superfamily. ASCT1/2 = Alanine Serine Cysteine Transporter type 1/2; Glut-1 = Glucose transporter protein type 1; Smit-1 = Sodium myo-inositol transporter type 1; mCAT1 = murine cationic amino acid transporter type 1; CAT1 = cationic amino acid transporter type 1; NHE-1 = sodium hydrogen exchanger type 1; MCT1 = Monocarboxylate transporter type 1; THTR1 = Thiamine transporter 1; PiT1 = Phosphate transporter; CTR1 = Copper uptake protein 1; GHB = gamma-hydroxybutyrate receptor; RFVT2 = Riboflavin uptake transporter; PAR2 = Protease activated receptor; Xpr1 = xenotropic and polytropic retrovirus receptor 1; MFSD2 = Major facilitator superfamily domain-containing protein 2; NPC1 = Niemann-Pick type C1 cholesterol transporter; TVA/B/C = tumor virus A/B/C.
While alpharetroviruses encode a gamma-type Env, a majority do not use SLC receptors; a notable exception is ALV-J, which uses avian Slc9a1 [196]. This distinction between alpharetroviruses and the gammaretroviruses and deltaretroviruses is intriguing. One possibility is that SLC preference is an ancestral property of gamma-type Env, and that this bias was lost during the early evolution of the alpharetrovirus lineage, allowing access to a broader range of potential receptor-specificities. Experimentally, ALV Envs could provide a useful comparator for exploring possible explanations for the SLC preference exhibit by the gammaretroviruses and deltaretroviruses.
A majority of the mammalian syncytins described are gamma-type Env proteins and with one exception, the known syncytin receptors belong to the SLC superfamily [50,214,221,222]. The exception is mouse Syncytin-A, which uses a glycosylphosphatidylinositol (GPI)-anchored membrane protein, lymphocyte antigen 6E (Ly6e), as a receptor [221]. Thus, murine Syncytin-A provides another opportunity to employ comparative approaches to explore the underlying basis for the observed bias of gammaretroviral Envs for SLC receptors.
The gamma-type Env receptors identified thus far include 10 divergent SLC families, including examples with distinct structural folds and different numbers of membrane spanning segments (Table 1). Several gammaretroviruses, and even some retroviruses of divergent genera, have been found to share the same SLC receptors [7]. Additionally, it is common for these viruses to bind to closely related receptors as well, of which there are many within the SLC superfamily. Some viruses are able to use multiple receptors within the same subfamily. For example, gibbon ape leukemia virus (GALV) and feline leukemia virus B (FeLV-B) can both utilize slc20a1 (PiT1) and slc20a2 (PiT2), though slc20a1 is the primary receptor. FeLV-B can also bind to both the feline and human versions of these receptors [7,12]. Thus, receptor-switching may not necessarily be limited to related receptors of the same SLC family. Experimental screening of FeLV based libraries containing randomized sequences in the RBD on a nonpermissive cell line (i.e., receptor-null) identified an Env mutant that could use a unique SLC receptor, SLC35F2, and another mutant that could use human SLC52A2 [208,211,223,224]. SLC52A2 is also used by porcine endogenous retrovirus PERV-A, whereas SLC35F2 is unrelated to any of the previously identified FeLV receptors, and in fact, belongs to a different SLC class and has a different number of membrane-spanning domains (Table 1). Together, these observations suggest that the gammaretroviral Env structure is optimized for use of SLC receptors in a way that is independent of the sequence-specific binding interactions that define the unique host/tissue tropism characteristics of individual viruses.
Given the diversity of SLCs used by viruses with gamma-type Envs, it is highly improbable that this bias reflects a common binding site sequence or receptor motif, and there are no obvious sequence similarities between the mapped viral binding sites recognized by different gammaretroviruses using different SLC receptors. Instead, the receptor preferences of gamma-type Envs may involve two levels of specificity. One level is represented by sequence-specific binding unique to each virus-receptor pair, while another level reflects dependence on one or more sequence-independent features shared by otherwise highly divergent SLC transporter proteins. The former reflects each virus’s association with a specific SLC, while the latter suggests a general dependence on the distinctive architecture of SLC proteins for optimal viral entry. How SLC architecture is involved (and at which step in the entry process) is unclear but represents an intriguing avenue for future exploration.
4.3. Recent Structural Insights into the SU-Receptor Interaction
High resolution structural insights into the gamma-type Env include crystal structures of at least three unbound RBDs (Figure 4), as well as papers describing structures of a PRR and the post-fusion conformation of TM [23,108,109,112,225,226,227]. There are also at least two cryo-EM reconstructions of gammaretroviral Envs bound to their cognate receptors [117,228]. Riedel et al. reported low-resolution reconstructions of the MLV trimer, including a receptor-bound form [117]. The trimer roughly resembles a three-sided pyramid, with a base comprising three widely spaced SU C-terminal domains, “steps” consisting of the three PRRs, and an apex consisting of the three RBDs. The authors speculate that the receptor-bound structure represents an intermediate step in entry, with the RBD bound to receptor and TM in its extended conformation linking the viral and cell membranes.
A high-resolution Cryo-EM-derived structure of a syncytin in complex with its cognate receptor has also been described [228]. The Env was derived from a consensus of homologs of Syncytin-2 (SYNC2), one of the two major, placental syncytins of humans and related primates [26]. The receptor for SYNC2 is MFSD2A (SLC59A1) [214]. Micrograph image data included evidence that a single SYNC2 trimer can interact with up to three receptor molecules, with the caveat that this could be a consequence of the experimental procedure which relies on presentation of SYNC2 and MFSD2A on individual micelles [228]. While prior high-resolution structures were limited to the N-terminal RBD of gammaretroviral SU domains, the SYNC2-MFSD2A complex includes high-resolution detail of the RBD as well as most of the C-terminal half of SU. To prevent isomerization and dissociation of SU and TM, the first C in the CXXC motif of the SYNC2 consensus was substituted with an S. Thus, the SYNC2-MFSD2A complex structure may also represent an intermediate receptor-bound step in which TM is still in its extended conformation (and still covalently linked to SU). Unfortunately, the first ~45 residues of SU and all of TM were not modeled in the structure and, in contrast to MLV, the CXXC motif in SYNC2 SU is N-terminal to the RBD at positions 43–46. Thus, the location of the intersubunit disulfide bond during this stage of entry was not revealed by the SYNC2-MFSD2A structure.
One face of the reported SYNC2 SU structure is largely devoid of N-glycans, and the authors suggest that this represents the SU-TM interacting surface [228]. If so, this places the proline-rich region (PRR), which acts as a hinge connecting the N- and C-terminal domains of SU, on the opposite side of the protomer from the extended TM. This placement is also consistent with the MLV structure reported by Riedel et al., with the PRR oriented towards the outside of the trimer [117].
The structure of SYNC2 SU reveals two domains: a beta-barrel C-terminal core, and an N-terminal RBD consisting of a five-stranded beta sheet and an alpha-helix, connected by the PRR [228]. The RBD includes the three variable regions (referred to by the authors as receptor-binding loops or RBLs 1–3). The interface between SYNC2 and MFSD2A involves extensive contacts between the variable loops and interstices formed by multiple extracellular loops (ECLs) of the receptor. This places the tips of the variable loops very close to the surface of the membrane, including one that penetrates into the plane of the target membrane and puts a phenylalanine (F275) in possible contact with lipid. Importantly, several features of the structure are consistent with mutagenesis and functional assays, including those conducted by the authors and those reported in the literature [118,228,229]. It is tempting to speculate that the penetration of the SYNC2 RBLs close to or within the plane of the target membrane could reflect a common feature of gamma-type Envs that use SLC receptors, and that this may help to explain the observed bias towards SLC superfamily members as entry receptors.
Human Synctyin-1 (SYNC-1) is homologous to the ENV proteins of retroviruses in the RDR receptor interference group, including the simian type-D betaretroviruses. All RDR Env tested to date, including SYNC1, use homologs of ASCT2/ASCT1 (SLC1a5, SLC1a4) as receptors [6]. In addition to extant exogenous retroviruses, the RDR Env is found in a diverse array of ERV families found in the genomes of various mammalian species, including human HERV-W loci (and SYNC1 is encoded by a HERV-W env gene). RDR represents a group of retroviruses that share a homologous Env protein, very likely as a consequence of numerous recombination events between different retroviral lineages taking place over a span of tens of millions of years [3,6]. Recently published Alphafold predictions suggest that the RDR RBDs may adopt a fold distinct from those of MLV, FeLV and EnvP(b)1 [230], such that current structures may not be suitable for gaining insights into this important class of gamma-type Env, including human SYNC1.
4.4. Receptor Interference
Actively infected cells often exhibit resistance to further infection from retroviruses that utilize the same receptor, a phenomenon commonly referred to as receptor interference or superinfection resistance (Figure 6). This property of retroviruses can be used to group viruses based on pairwise comparisons of interference patterns even when the cognate receptors are unknown [2,100,185,231,232,233,234,235]. For HIV, superinfection resistance involves multiple proteins, including Env and the accessory proteins Vpu and Nef. Vpu prevents trafficking of new CD4 receptors to the cell surface, while Nef causes endocytosis of cell surface CD4 [236]. Retroviruses with gamma-type Env typically have simple genomes with few if any apparent accessory genes (except the deltaretroviruses). Of the few that have been studied (e.g., MLV glycogen, HTLV HBZ) none have yet been shown to mediate receptor interference. Instead, receptor-interference is governed predominantly or exclusively by Env.
Figure 6.
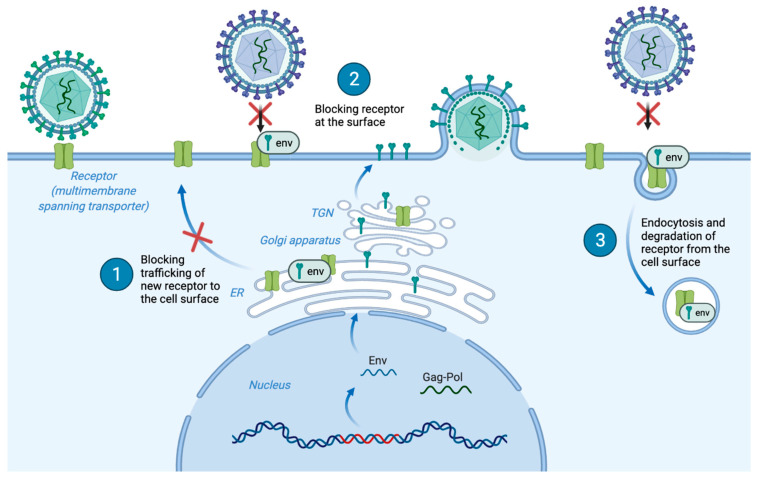
Receptor Interference (Superinfection Resistance). Env expression in infected, virus-producing cells induces receptor interference. Proposed mechanisms include: (1) blocking trafficking of the receptor to the cell surface; (2) binding of the receptor at the cell surface, thereby blocking new viral particles from binding, and (3) inducing endocytosis and subsequent degradation of the receptor.
Previous studies have shown that expression of the murine leukemia virus (MLV) Env is correlated with an internalization of the receptor such that it is distributed throughout the cytoplasm, ER, Golgi and lysosomes, and no longer found on the cell surface [237,238]. Internalization and subsequent degradation have also been demonstrated for a human endogenous retrovirus (HERV) Env [72]. Expression of these Envs, while they are not associated with any active exogenous virus, causes internalization and overall reduction of the receptor, as shown through immunofluorescent imaging experiments and Western blot, respectively [72]. Another study altered Env through addition of ligand sequences into the RBD and showed that this chimeric Env was able to induce strong resistance to superinfection and downregulate its new receptor from the cell surface [239]. Interestingly, previous studies using reticuloendotheliosis virus (REV) have shown that the envelope protein of this virus can induce resistance without being properly trafficked to the cell surface and does not need to be fusogenic to induce resistance, indicating that the regions of the TM subunit of Env involved in fusion are likely not required for inducing resistance to superinfection [96,240].
Additionally, multiple endogenous Envs that are no longer capable of fusion are still capable of inducing resistance to further infection, and likely have been co-opted for this purpose, as in the case of murine Fv4 [55]. Multiple point mutations to the RBD of the SNV Env abolished the envelope protein’s ability to induce resistance to superinfection in the cell [96]. On the other side of the Env-receptor coin, mutations to the receptor that reduce binding affinity of the envelope protein also reduce or abolish superinfection resistance [241]. Gibbon ape leukemia virus (GALV) can induce superinfection resistance in a cell without causing any change in density of the receptor at the cell surface; however, it is possible GALV uses two receptors, which may account for the lack of change in the receptor measured [242,243].
The commonality of the receptor-interference phenomenon, and the variety of mechanisms employed, implies that inducing cellular resistance to superinfection reflects a benefit to viral fitness. In multiple retroviruses it has been shown that a loss of superinfection resistance is strongly correlated with higher cytotoxicity and abnormal pathogenesis [104,244,245,246,247,248]. As retroviruses rely on their cellular host to produce copies of their genomes and proteins, they depend on the cell’s continued survival, at least long enough to produce progeny. Another possible benefit for the virus may be involved in clearing a path for new envelope proteins trafficking to the cell surface or sites of assembly. Downregulation of receptors would prevent these Envs from prematurely binding and could prevent them from losing fusogenicity before they have been incorporated into virions.
Based on these and other studies, proposed mechanisms for receptor-interference by gamma-type Env typically fall into one of three general categories (Figure 6). Briefly, these are: (1) Env prevents trafficking of new receptors to the surface, resulting in overall reduction of cell-surface receptor levels; (2) Env binds to the receptor at the cell surface, blocking a subsequent viral particle from binding to that receptor, referred to as receptor masking, and (3) Env promotes downregulation of the receptor from the cell surface through induction of endocytosis. Regardless of mechanism, it is clear that the receptor-recognition function of SU is necessary for receptor interference. It is likely that retroviruses with gamma-type Env differ in which of these mechanisms (or combinations of these mechanisms) are employed to induce superinfection resistance. Further work is needed to uncover the molecular interactions underlying each mechanism, including identification of other regions of Env that contribute and the cellular co-factors and trafficking pathways that are involved.
5. Summary and Outlook
The biological and temporal distribution of the gamma-type Envs in nature is remarkable. They are represented by at least four of the seven genera comprising the Orthoretrovirinae subfamily (five, if one includes the type-D betaretroviruses), and they have been acquired by RNA viruses in two other families and at least one large DNA virus [3,14,15,16,17,18,137,230]. The origins of the gamma-type Env are obscure, but likely date back hundreds of millions of years and span most of vertebrate evolution. Gamma-type env genes are also present in thousands of copies in most vertebrate genomes, including many instances in which coding capacity has been preserved and exapted for important host functions (e.g., human SYNCYTIN-1 and SYNCYTIN-2) [4,28,38,43]. Thanks to a combination of distinctive features, the gamma-type Env is easily identified, and readily distinguished from the Env proteins of other retroviruses and from other class I fusogens [3,4]. Comparative studies of the gamma-type Env can illuminate additional aspects of viral entry, provide insights into intraspecies and interspecies transmission and emergence of viruses, improve our understanding of Syncytin function (and by extension mammalian evolution), and serve as models for understanding how functional constraints influence evolution of protein structure and protein-protein interactions over deep evolutionary timescales. Among the many possibilities for further exploration, the following represent some of the most significant gaps in current knowledge.
5.1. Structural Insights into the Distinctive Entry and Fusion Mechanisms of Gamma-Type Env
While HIV Env has emerged as a model for retroviral entry, the gamma-type Env has several distinctive features that are absent from HIV Env, including regulation by a C-terminal R-peptide, a labile intersubunit disulfide bond, and involvement of the RBD at two steps in the entry process. Understanding these features and how they interact to perform the various functions of the gamma-type Env will necessarily require additional structural insights drawn directly from comparative work involving viruses with gamma-type Env, such as ALV, MLV and HTLV. Work on the structural biology of these viruses lags far behind HIV-1, and in particular, structural details of the immature precursor (with an R-peptide), the mature trimer, and the intermediate stages of fusion are still lacking. Moreover, given the very broad range of viruses that encode gamma-type Env (and the correspondingly broad host distribution), it is likely that novel folds have evolved, particularly in the N-terminal domain of SU. Some SU proteins have an N-terminal CXXC, for example, which may reflect differences in the arrangement or structure of SU and its interface with TM. One of these is SYNC2, and it is noteworthy that the RBD portion of the published SU structure differs significantly from the reported structures of the MLV, FeLV and Envp(b)1 RBDs, all of which represent Env with a CXXC motif in the C-terminal half of SU [23,108,109,228]. The ALVs lack a CXXC, which could also be reflected in differences in conformation of SU and the ALV trimer. Finally, based on alphafold predictions, Hötzel has proposed that distinctive structures may exist for viruses of the RDR group and human Syncytin-1 [230].
5.2. Conservation of the ISD Motif
The function(s) of the ISD motif need to be further defined, and a satisfying explanation for the extraordinary conservation of the ISD sequence, across multiple genera and hundreds of millions of years of viral evolution, is lacking. Whether the proposed immunosuppressive function alone is sufficient to explain this conservation will depend on whether immunosuppression is shared across divergent retroviruses representing the alpharetrovirus, gammaretrovirus and deltaretrovirus genera, and a correspondingly wide range of vertebrate hosts, and on identifying a plausible molecular mechanism that unifies the various published observations. Alternatively, or possibly in addition to immunosuppression, it is possible that conservation of the ISD sequence belies a critical contribution to the essential entry-related functions shared by all gamma-type Envs.
5.3. Receptor Bias
There is as yet no molecular explanation for the observed bias of gammaretroviruses and deltaretroviruses towards use of SLC transporters as receptors. Solving this puzzle may depend on obtaining additional high-resolution structures or robust structural models of Env trimers in various stages of the entry process. The recent high-resolution structure of SYNC2 in complex with MSFD2A revealed the insertion of variable domains deep into the receptor, close to or within the plane of the target membrane. If this tight junction between the RBD and the cell is shared by other Env-receptor pairs and is optimal for a subsequent step in the entry process, this may begin to explain the bias towards the low-profile receptors typical of the SLC superfamily. The technical challenges of experimenting with complex, multi-functional viral glycoproteins and multi-membrane spanning receptors are formidable. In addition, confirming fundamental hypotheses for SLC receptor preference will require expanding on traditional single-virus model systems to include broader, comparative approaches involving parallel analysis of divergent gammaretroviruses with different receptor specificities in order to identify common themes. Some clues may also come from a closer examination of the “exceptions to the rule”—these include avian leukemia virus subgroups A-E, which do not use SLCs as receptors (except for ALV subtype J [196]), and murine Syncytin-A, which uses the GPI-anchored Ly6e protein as a receptor [221].
5.4. Virus-Host “Arms Races” and the Evolution of Individual SLC Receptors
There are a few studies that have examined the potential impact of gammretroviruses on the evolution of the genes that encode their cognate receptors [249,250,251]. There are now several examples of SLC receptors that are used by multiple retroviruses (Table 1), as well as cases where the receptors used in the deep past by ancient ERV Env have been identified [50,72,188,206,214,221,222]. Testing the Env proteins of these viruses against an expanded array of receptor homologs, coupled with molecular evolutionary analysis of the corresponding host genes, could be used to gauge the impact of viruses on vertebrate evolution generally and receptor evolution specifically.
5.5. The Link between Entry and Post-Entry
The ubiquity of gamma-type ENVs and the diversity of receptors involved is a unique opportunity to understand how Env-receptor interactions dictate both entry pathways and subsequent trafficking of the capsid core (e.g., [252,253]).
5.6. Env Trafficking and Receptor-Interference
While it is well-established that gammaretrovirus Env proteins achieve superinfection resistance through receptor-interference, detailed mechanisms remain to be identified, and alternative hypotheses have been proposed (and it is possible that different viruses have evolved distinct mechanisms to achieve receptor-interference). Recently, Eksmond et al. revisited an MLV Env ISD mutant and uncovered a receptor-dependent, producer cell effect on infectivity [147]. Given that superinfection resistance is also a receptor-dependent, producer-cell effect mediated by Env, their observations could be related to the underlying mechanism of receptor interference. More detailed examination of Env biogenesis and trafficking in the presence and absence of cognate receptors, and the identification of any cellular cofactors involved, may also shed light on the superinfection process and its relevance to viral replicative fitness, virion release and cell-cell spread [242].
5.7. Evolutionary Cooption of ERV-Encoded Gamma-Type Env Proteins
Because their origins and underlying virological functions are well understood, ERV-encoded Env glycoproteins can serve as model systems for understanding the phenomenon of evolutionary cooption and the events that occur after germline integration of an env gene, such as acquisition and fine-tuning of host-cell regulatory mechanisms, integration within the regulatory networks of other interacting host genes, and further adaptations of the encoded Env protein for optimal host function [25,27]. For example, decades of work on exogenous viruses such as MLV-guided identification of the fusion defects in the murine Fv4 and human envHERV-T viral resistance proteins [66,69,72]). Syncytins, by contrast, retain their fusogenic activity, and do so in the absence of activation by a viral protease. This suggests that Syncytins must either acquire alternative activation mechanisms, or evolve to function independently of R-peptide cleavage [254]. Aside from work performed in experimental mouse models [255,256], additional research is also needed to confirm and further define the presumed function(s) of many of the placental syncytins of other mammalian lineages. Finally, there are many ERV gamma-type Env “orphans” in the genomes of humans and other vertebrates [27,45,46], including the human HEMO and EnvP(b)1 proteins [22,23,48] and the Percomorf protein of ray-finned fish [24], for which host functions remain to be identified.
Acknowledgments
We thank Monica Roth, Alvaro Dafonte Imedio, Zachary Williams, Julia Harmon and members of the Johnson lab for helpful discussions and feedback. Figures were Created with BioRender.com.
Author Contributions
V.H. and W.E.J. drafted and edited the manuscript. V.H. generated all of the figures and Table 1. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created for this review article.
Conflicts of Interest
The authors have no conflict to declare.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Coffin J., Blomberg J., Fan H., Gifford R., Hatziioannou T., Lindemann D., Mayer J., Stoye J., Tristem M., Johnson W. ICTV Virus Taxonomy Profile: Retroviridae 2021. J. Gen. Virol. 2021;102:1712. doi: 10.1099/jgv.0.001712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sommerfelt M.A., Weiss R.A. Receptor interference groups of 20 retroviruses plating on human cells. Virology. 1990;176:58–69. doi: 10.1016/0042-6822(90)90230-O. [DOI] [PubMed] [Google Scholar]
- 3.Henzy J.E., Johnson W. Pushing the endogenous envelope. Philos Trans. R Soc. Lond B Biol. Sci. 2013;368:20120506. doi: 10.1098/rstb.2012.0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Y., Wang X., Liao M.-E., Song Y., Zhang Y.-Y., Cui J. Evolution and Genetic Diversity of the Retroviral Envelope in Anamniotes. J. Virol. 2022;96:e0207221. doi: 10.1128/jvi.02072-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sonigo P., Barker C., Hunter E., Wain-Hobson S. Nucleotide sequence of Mason-Pfizer monkey virus: An immunosuppressive D-type retrovirus. Cell. 1986;45:375–385. doi: 10.1016/0092-8674(86)90323-5. [DOI] [PubMed] [Google Scholar]
- 6.Sinha A., Johnson W. Retroviruses of the RDR superinfection interference group: Ancient origins and broad host distribution of a promiscuous Env gene. Curr. Opin. Virol. 2017;25:105–112. doi: 10.1016/j.coviro.2017.07.020. [DOI] [PubMed] [Google Scholar]
- 7.Greenwood A.D., Ishida Y., O’Brien S.P., Roca A., Eiden M.V. Transmission, Evolution, and Endogenization: Lessons Learned from Recent Retroviral Invasions. Microbiol. Mol. Biol. Rev. 2018;82:e00044-17. doi: 10.1128/MMBR.00044-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Overbaugh J., Miller A., Eiden M. Receptors and entry cofactors for retroviruses include single and multiple transmembrane-spanning proteins as well as newly described glycophosphatidylinositol-anchored and secreted proteins. Microbiol. Mol. Biol. Rev. 2001;65:371–389. doi: 10.1128/MMBR.65.3.371-389.2001. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li K., Zhang S., Kronqvist M., Wallin M., Ekström M., Derse D., Garoff H. Intersubunit disulfide isomerization controls membrane fusion of human T-cell leukemia virus Env. J. Virol. 2008;82:7135–7143. doi: 10.1128/JVI.00448-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Opstelten D.J., Wallin M., Garoff H. Moloney murine leukemia virus envelope protein subunits, gp70 and Pr15E, form a stable disulfide-linked complex. J. Virol. 1998;72:6537–6545. doi: 10.1128/JVI.72.8.6537-6545.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pinter A., Kopelman R., Li Z., Kayman S.C., A Sanders D. Localization of the labile disulfide bond between SU and TM of the murine leukemia virus envelope protein complex to a highly conserved CWLC motif in SU that resembles the active-site sequence of thiol-disulfide exchange enzymes. J. Virol. 1997;71:8073–8077. doi: 10.1128/jvi.71.10.8073-8077.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tailor C.S., Lavillette D., Marin M., Kabat D. Cell surface receptors for gammaretroviruses. Curr. Top. Microbiol. Immunol. 2003;281:29–106. doi: 10.1007/978-3-642-19012-4_2. [DOI] [PubMed] [Google Scholar]
- 13.Harrison S.C. Viral membrane fusion. Nat. Struct Mol. Biol. 2008;15:690–698. doi: 10.1038/nsmb.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallaher W.R. Similar structural models of the transmembrane proteins of Ebola and avian sarcoma viruses. Cell. 1996;85:477–478. doi: 10.1016/S0092-8674(00)81248-9. [DOI] [PubMed] [Google Scholar]
- 15.Volchkov V.E., Blinov V., Netesov S. The envelope glycoprotein of Ebola virus contains an immunosuppressive-like domain similar to oncogenic retroviruses. FEBS Lett. 1992;305:181–184. doi: 10.1016/0014-5793(92)80662-Z. [DOI] [PubMed] [Google Scholar]
- 16.Benit L., Dessen P., Heidmann T. Identification, phylogeny, and evolution of retroviral elements based on their envelope genes. J. Virol. 2001;75:11709–11719. doi: 10.1128/JVI.75.23.11709-11719.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stenglein M.D., Sanders C., Kistler A.L., Ruby J.G., Franco J.Y., Reavill D.R., Dunker F., Derisi J.L. Identification, characterization, and in vitro culture of highly divergent arenaviruses from boa constrictors and annulated tree boas: Candidate etiological agents for snake inclusion body disease. mBio. 2012;3:e00180-12. doi: 10.1128/mBio.00180-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrison C.L., Iwanowicz L., Work T.M., Fahsbender E., Breitbart M., Adams C., Iwanowicz D., Sanders L., Ackermann M., Cornman R.S., et al. Genomic evolution, recombination, and inter-strain diversity of chelonid alphaherpesvirus 5 from Florida and Hawaii green sea turtles with fibropapillomatosis. PeerJ. 2018;6:e4386. doi: 10.7717/peerj.4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malfavon-Borja R., Feschotte C. Fighting fire with fire: Endogenous retrovirus envelopes as restriction factors. J. Virol. 2015;89:4047–4050. doi: 10.1128/JVI.03653-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson W.E. Origins and evolutionary consequences of ancient endogenous retroviruses. Nat. Rev. Microbiol. 2019;17:355–370. doi: 10.1038/s41579-019-0189-2. [DOI] [PubMed] [Google Scholar]
- 21.Dupressoir A., Lavialle C., Heidmann T. From ancestral infectious retroviruses to bona fide cellular genes: Role of the captured syncytins in placentation. Placenta. 2012;33:663–671. doi: 10.1016/j.placenta.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Heidmann O., Béguin A., Paternina J., Berthier R., Deloger M., Bawa O., Heidmann T. HEMO, an ancestral endogenous retroviral envelope protein shed in the blood of pregnant women and expressed in pluripotent stem cells and tumors. Proc. Natl. Acad. Sci. USA. 2017;114:E6642–E6651. doi: 10.1073/pnas.1702204114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCarthy K.R., Timpona J.L., Jenni S., Bloyet L.-M., Brusic V., Johnson W.E., Whelan S.P.J., Robinson-McCarthy L.R. Structure of the Receptor Binding Domain of EnvP(b)1, an Endogenous Retroviral Envelope Protein Expressed in Human Tissues. mBio. 2020;11:e02772-20. doi: 10.1128/mBio.02772-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henzy J.E., Gifford R.J., Kenaley C.P., Johnson W.E. An Intact Retroviral Gene Conserved in Spiny-Rayed Fishes for over 100 My. Mol. Biol. Evol. 2017;34:634–639. doi: 10.1093/molbev/msw262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frank J.A., Singh M., Cullen H.B., Kirou R.A., Benkaddour-Boumzaouad M., Cortes J.L., Pérez J.G., Coyne C.B., Feschotte C. Evolution and antiviral activity of a human protein of retroviral origin. Science. 2022;378:422–428. doi: 10.1126/science.abq7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blaise S., de Parseval N., Bénit L., Heidmann T. Genomewide screening for fusogenic human endogenous retrovirus envelopes identifies syncytin 2, a gene conserved on primate evolution. Proc. Natl. Acad. Sci. USA. 2003;100:13013–13018. doi: 10.1073/pnas.2132646100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Parseval N., Lazar V., Casella J.F., Benit L., Heidmann T. Survey of human genes of retroviral origin: Identification and transcriptome of the genes with coding capacity for complete envelope proteins. J. Virol. 2003;77:10414–10422. doi: 10.1128/JVI.77.19.10414-10422.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayward A., Grabherr M., Jern P. Broad-scale phylogenomics provides insights into retrovirus-host evolution. Proc. Natl. Acad. Sci. USA. 2013;110:20146–20151. doi: 10.1073/pnas.1315419110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weiss R.A. The discovery of endogenous retroviruses. Retrovirology. 2006;3:67. doi: 10.1186/1742-4690-3-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gifford R., Kabat P., Martin J., Lynch C., Tristem M. Evolution and distribution of class II-related endogenous retroviruses. J. Virol. 2005;79:6478–6486. doi: 10.1128/JVI.79.10.6478-6486.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gifford R., Tristem M. The evolution, distribution and diversity of endogenous retroviruses. Virus Genes. 2003;26:291–315. doi: 10.1023/A:1024455415443. [DOI] [PubMed] [Google Scholar]
- 32.Gifford R.J., Blomberg J., Coffin J.M., Fan H., Heidmann T., Mayer J., Stoye J., Tristem M., Johnson W.E. Nomenclature for endogenous retrovirus (ERV) loci. Retrovirology. 2018;15:59. doi: 10.1186/s12977-018-0442-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yedavalli V.R.K., Patil A., Parrish J., Kozak C.A. A novel class III endogenous retrovirus with a class I envelope gene in African frogs with an intact genome and developmentally regulated transcripts in Xenopus tropicalis. Retrovirology. 2021;18:20. doi: 10.1186/s12977-021-00564-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henzy J.E., Gifford R.J., Johnson W.E., Coffin J.M. A novel recombinant retrovirus in the genomes of modern birds combines features of avian and mammalian retroviruses. J. Virol. 2014;88:2398–2405. doi: 10.1128/JVI.02863-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farkasova H., Hron T., Pačes J., Hulva P., Benda P., Gifford R.J., Elleder D. Discovery of an endogenous Deltaretrovirus in the genome of long-fingered bats (Chiroptera: Miniopteridae) Proc. Natl. Acad. Sci. USA. 2017;114:3145–3150. doi: 10.1073/pnas.1621224114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hron T., Farkašová H., Gifford R.J., Benda P., Hulva P., Görföl T., Pačes J., Elleder D. Remnants of an Ancient Deltaretrovirus in the Genomes of Horseshoe Bats (Rhinolophidae) Viruses. 2018;10:185. doi: 10.3390/v10040185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hron T., Elleder D., Gifford R. Deltaretroviruses have circulated since at least the Paleogene and infected a broad range of mammalian species. Retrovirology. 2019;16:33. doi: 10.1186/s12977-019-0495-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hayward A., Cornwallis C., Jern P. Pan-vertebrate comparative genomics unmasks retrovirus macroevolution. Proc. Natl. Acad. Sci. USA. 2015;112:464–469. doi: 10.1073/pnas.1414980112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Basta H.A., Cleveland S.B., Clinton R.A., Dimitrov A.G., McClure M.A. Evolution of teleost fish retroviruses: Characterization of new retroviruses with cellular genes. J. Virol. 2009;83:10152–10162. doi: 10.1128/JVI.02546-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bai J., Yang Z.-Z., Li H., Hong Y., Fan D.-D., Lin A.-F., Xiang L.-X., Shao J.-Z. Genome-Wide Characterization of Zebrafish Endogenous Retroviruses Reveals Unexpected Diversity in Genetic Organizations and Functional Potentials. Microbiol. Spectr. 2021;9:e0225421. doi: 10.1128/spectrum.02254-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen C.H., Steiner L. Genome structure and thymic expression of an endogenous retrovirus in zebrafish. J. Virol. 2004;78:899–911. doi: 10.1128/JVI.78.2.899-911.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frank J.A., Feschotte C. Co-option of endogenous viral sequences for host cell function. Curr. Opin. Virol. 2017;25:81–89. doi: 10.1016/j.coviro.2017.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lavialle C., Cornelis G., Dupressoir A., Esnault C., Heidmann O., Vernochet C., Heidmann T. Paleovirology of ‘syncytins’, retroviral env genes exapted for a role in placentation. Philos Trans. R Soc. Lond B Biol. Sci. 2013;368:20120507. doi: 10.1098/rstb.2012.0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Best S., Le Tissier P., Stoye J. Endogenous retroviruses and the evolution of resistance to retroviral infection. Trends Microbiol. 1997;5:313–318. doi: 10.1016/S0966-842X(97)01086-X. [DOI] [PubMed] [Google Scholar]
- 45.Simpson J., Kozak C., Boso G. Cross-species transmission of an ancient endogenous retrovirus and convergent co-option of its envelope gene in two mammalian orders. PLoS Genet. 2022;18:e1010458. doi: 10.1371/journal.pgen.1010458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diehl W.E., Patel N., Halm K., Johnson W.E. Tracking interspecies transmission and long-term evolution of an ancient retrovirus using the genomes of modern mammals. Elife. 2016;5:e12704. doi: 10.7554/eLife.12704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ueda M.T., Kryukov K., Mitsuhashi S., Mitsuhashi H., Imanishi T., Nakagawa S. Comprehensive genomic analysis reveals dynamic evolution of endogenous retroviruses that code for retroviral-like protein domains. Mob. DNA. 2020;11:29. doi: 10.1186/s13100-020-00224-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kasperek A., Béguin A., Bawa O., De Azevedo K., Job B., Massard C., Scoazec J., Heidmann T., Heidmann O. Therapeutic potential of the human endogenous retroviral envelope protein HEMO: A pan-cancer analysis. Mol. Oncol. 2022;16:1451–1473. doi: 10.1002/1878-0261.13069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mi S., Lee X., Li X.P., Veldman G.M., Finnerty H., Racie L., LaVallie E., Tang X.Y., Edouard P., Howes S., et al. Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature. 2000;403:785–789. doi: 10.1038/35001608. [DOI] [PubMed] [Google Scholar]
- 50.Blond J.L., Lavillette D., Cheynet V., Bouton O., Oriol G., Chapel-Fernandes S., Mandrand B., Mallet F., Cosset F.-L. An envelope glycoprotein of the human endogenous retrovirus HERV-W is expressed in the human placenta and fuses cells expressing the type D mammalian retrovirus receptor. J. Virol. 2000;74:3321–3329. doi: 10.1128/JVI.74.7.3321-3329.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cornelis G., Heidmann O., Bernard-Stoecklin S., Reynaud K., Véron G., Mulot B., Dupressoir A., Heidmann T. Ancestral capture of syncytin-Car1, a fusogenic endogenous retroviral envelope gene involved in placentation and conserved in Carnivora. Proc. Natl. Acad. Sci. USA. 2012;109:E432–E441. doi: 10.1073/pnas.1115346109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Imakawa K., Nakagawa S. The Phylogeny of Placental Evolution Through Dynamic Integrations of Retrotransposons. Prog. Mol. Biol. Transl. Sci. 2017;145:89–109. doi: 10.1016/bs.pmbts.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 53.Cornelis G., Funk M., Vernochet C., Leal F., Tarazona O.A., Meurice G., Heidmann O., Dupressoir A., Miralles A., Ramirez-Pinilla M.P., et al. An endogenous retroviral envelope syncytin and its cognate receptor identified in the viviparous placental Mabuya lizard. Proc. Natl. Acad. Sci. USA. 2017;114:E10991–E11000. doi: 10.1073/pnas.1714590114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Funk M., Cornelis G., Vernochet C., Heidmann O., Dupressoir A., Conley A., Glickman S., Heidmann T. Capture of a Hyena-Specific Retroviral Envelope Gene with Placental Expression Associated in Evolution with the Unique Emergence among Carnivorans of Hemochorial Placentation in Hyaenidae. J. Virol. 2019;93:e01811-18. doi: 10.1128/JVI.01811-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gardner M.B., Kozak C., O’Brien S.J. The Lake Casitas wild mouse: Evolving genetic resistance to retroviral disease. Trends Genet. 1991;7:22–27. doi: 10.1016/0168-9525(91)90017-K. [DOI] [PubMed] [Google Scholar]
- 56.Ikeda H., Sugimura H. Fv-4 resistance gene: A truncated endogenous murine leukemia virus with ecotropic interference properties. J. Virol. 1989;63:5405–5412. doi: 10.1128/jvi.63.12.5405-5412.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suzuki S. FV-4: A new gene affecting the splenomegaly induction by Friend leukemia virus. Jpn J. Exp. Med. 1975;45:473–478. [PubMed] [Google Scholar]
- 58.Inaguma Y., Miyashita N., Moriwaki K., Huai W.C., Jin M.L., He X.Q., Ikeda H. Acquisition of two endogenous ecotropic murine leukemia viruses in distinct Asian wild mouse populations. J. Virol. 1991;65:1796–1802. doi: 10.1128/jvi.65.4.1796-1802.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Limjoco T.I., Dickie P., Ikeda H., Silver J. Transgenic Fv-4 mice resistant to Friend virus. J. Virol. 1993;67:4163–4168. doi: 10.1128/jvi.67.7.4163-4168.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gardner M.B., Rasheed S., Pal B.K., Estes J.D., O’Brien S.J. Akvr-1, a dominant murine leukemia virus restriction gene, is polymorphic in leukemia-prone wild mice. Proc. Natl. Acad. Sci. USA. 1980;77:531–535. doi: 10.1073/pnas.77.1.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kozak C.A., Gromet N.J., Ikeda H., E Buckler C. A unique sequence related to the ecotropic murine leukemia virus is associated with the Fv-4 resistance gene. Proc. Natl. Acad. Sci. USA. 1984;81:834–837. doi: 10.1073/pnas.81.3.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dandekar S., Rossitto P., Pickett S., Mockli G., Bradshaw H., Cardiff R., Gardner M. Molecular characterization of the Akvr-1 restriction gene: A defective endogenous retrovirus-borne gene identical to Fv-4r. J. Virol. 1987;61:308–314. doi: 10.1128/jvi.61.2.308-314.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Buller R.S., Ahmed A., Portis J. Identification of two forms of an endogenous murine retroviral env gene linked to the Rmcf locus. J. Virol. 1987;61:29–34. doi: 10.1128/jvi.61.1.29-34.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Buller R.S., Sitbon M., Portis J. The endogenous mink cell focus-forming (MCF) gp70 linked to the Rmcf gene restricts MCF virus replication in vivo and provides partial resistance to erythroleukemia induced by Friend murine leukemia virus. J. Exp. Med. 1988;167:1535–1546. doi: 10.1084/jem.167.5.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jung Y.T., Lyu M.S., Buckler-White A., Kozak C.A. Characterization of a polytropic murine leukemia virus proviral sequence associated with the virus resistance gene Rmcf of DBA/2 mice. J. Virol. 2002;76:8218–8224. doi: 10.1128/JVI.76.16.8218-8224.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu T., Yan Y., Kozak C. Rmcf2, a xenotropic provirus in the Asian mouse species Mus castaneus, blocks infection by polytropic mouse gammaretroviruses. J. Virol. 2005;79:9677–9684. doi: 10.1128/JVI.79.15.9677-9684.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takeda A., Matano T. Inhibition of infectious murine leukemia virus production by Fv-4 env gene products exerting dominant negative effect on viral envelope glycoprotein. Microbes Infect. 2007;9:1590–1596. doi: 10.1016/j.micinf.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 68.Dewannieux M., Collins M.K. Spontaneous heteromerization of gammaretrovirus envelope proteins: A possible novel mechanism of retrovirus restriction. J. Virol. 2008;82:9789–9794. doi: 10.1128/JVI.02696-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Taylor G.M., Gao Y., Sanders D. Fv-4: Identification of the defect in Env and the mechanism of resistance to ecotropic murine leukemia virus. J. Virol. 2001;75:11244–11248. doi: 10.1128/JVI.75.22.11244-11248.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nakagawa S., Takahashi M.U. gEVE: A genome-based endogenous viral element database provides comprehensive viral protein-coding sequences in mammalian genomes. Database (Oxford) 2016;2016:baw087. doi: 10.1093/database/baw087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ponferrada V.G., Mauck B., Wooley D. The envelope glycoprotein of human endogenous retrovirus HERV-W induces cellular resistance to spleen necrosis virus. Arch. Virol. 2003;148:659–675. doi: 10.1007/s00705-002-0960-x. [DOI] [PubMed] [Google Scholar]
- 72.Blanco-Melo D., Gifford R., Bieniasz P. Co-option of an endogenous retrovirus envelope for host defense in hominid ancestors. Elife. 2017;6:e22519. doi: 10.7554/eLife.22519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Murakami T. Retroviral env glycoprotein trafficking and incorporation into virions. Mol. Biol. Int. 2012;2012:682850. doi: 10.1155/2012/682850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tedbury P.R., Freed E.O. The cytoplasmic tail of retroviral envelope glycoproteins. Prog. Mol. Biol. Transl. Sci. 2015;129:253–284. doi: 10.1016/bs.pmbts.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sandrin V., Muriaux D., Darlix J.-L., Cosset F.-L. Intracellular trafficking of Gag and Env proteins and their interactions modulate pseudotyping of retroviruses. J. Virol. 2004;78:7153–7164. doi: 10.1128/JVI.78.13.7153-7164.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dong J.Y., Dubay J.W., Perez L.G., Hunter E. Mutations within the proteolytic cleavage site of the Rous sarcoma virus glycoprotein define a requirement for dibasic residues for intracellular cleavage. J. Virol. 1992;66:865–874. doi: 10.1128/jvi.66.2.865-874.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sjoberg M., Wu S.-R., Löving R., Rantalainen K., Lindqvist B., Garoff H., Shaltiel I.A., Aprelia M., Saurin A.T., Chowdhury D., et al. Furin cleavage of the Moloney murine leukemia virus Env precursor reorganizes the spike structure. Proc. Natl. Acad. Sci. USA. 2014;111:6034–6039. doi: 10.1073/pnas.1317972111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zavorotinskaya T., Albritton L.M. Failure To cleave murine leukemia virus envelope protein does not preclude its incorporation in virions and productive virus-receptor interaction. J. Virol. 1999;73:5621–5629. doi: 10.1128/JVI.73.7.5621-5629.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Freed E.O., Risser R. The role of envelope glycoprotein processing in murine leukemia virus infection. J. Virol. 1987;61:2852–2856. doi: 10.1128/jvi.61.9.2852-2856.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Owji H., Nezafat N., Negahdaripour M., Hajiebrahimi A., Ghasemi Y. A comprehensive review of signal peptides: Structure, roles, and applications. Eur. J. Cell Biol. 2018;97:422–441. doi: 10.1016/j.ejcb.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 81.Hoch-Marchaim H., Weiss A.M., Bar-Sinai A., Fromer M., Adermann K., Hochman J. The leader peptide of MMTV Env precursor localizes to the nucleoli in MMTV-derived T cell lymphomas and interacts with nucleolar protein B23. Virology. 2003;313:22–32. doi: 10.1016/S0042-6822(03)00236-8. [DOI] [PubMed] [Google Scholar]
- 82.Indik S., Günzburg W.H., Salmons B., Rouault F. A novel, mouse mammary tumor virus encoded protein with Rev-like properties. Virology. 2005;337:1–6. doi: 10.1016/j.virol.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 83.Mertz J.A., Simper M.S., Lozano M.M., Payne S.M., Dudley J.P. Mouse mammary tumor virus encodes a self-regulatory RNA export protein and is a complex retrovirus. J. Virol. 2005;79:14737–14747. doi: 10.1128/JVI.79.23.14737-14747.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Caporale M., Arnaud F., Mura M., Golder M., Murgia C., Palmarini M. The signal peptide of a simple retrovirus envelope functions as a posttranscriptional regulator of viral gene expression. J. Virol. 2009;83:4591–4604. doi: 10.1128/JVI.01833-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hofacre A., Nitta T., Fan H. Jaagsiekte sheep retrovirus encodes a regulatory factor, Rej, required for synthesis of Gag protein. J. Virol. 2009;83:12483–12498. doi: 10.1128/JVI.01747-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Byun H., Halani N., Gou Y., Nash A.K., Lozano M.M., Dudley J.P. Requirements for mouse mammary tumor virus Rem signal peptide processing and function. J. Virol. 2012;86:214–225. doi: 10.1128/JVI.06197-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McCaul N., Quandte M., Bontjer I., van Zadelhoff G., Land A., Crooks E.T., Binley J.M., Sanders R.W., Braakman I. Intramolecular quality control: HIV-1 envelope gp160 signal-peptide cleavage as a functional folding checkpoint. Cell Rep. 2021;36:109646. doi: 10.1016/j.celrep.2021.109646. [DOI] [PubMed] [Google Scholar]
- 88.Liu H., Wu R., Yuan L., Tian G., Huang X., Wen Y., Ma X., Huang Y., Yan Q., Zhao Q., et al. Introducing a cleavable signal peptide enhances the packaging efficiency of lentiviral vectors pseudotyped with Japanese encephalitis virus envelope proteins. Virus Res. 2017;229:9–16. doi: 10.1016/j.virusres.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 89.Yolitz J., Schwing C., Chang J., Van Ryk D., Nawaz F., Wei D., Cicala C., Arthos J., Fauci A.S. Signal peptide of HIV envelope protein impacts glycosylation and antigenicity of gp120. Proc. Natl. Acad. Sci. USA. 2018;115:2443–2448. doi: 10.1073/pnas.1722627115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Marzi A., Akhavan A., Simmons G., Gramberg T., Hofmann H., Bates P., Lingappa V.R., Pöhlmann S. The signal peptide of the ebolavirus glycoprotein influences interaction with the cellular lectins DC-SIGN and DC-SIGNR. J. Virol. 2006;80:6305–6317. doi: 10.1128/JVI.02545-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim F.J., Manel N., Garrido E.N., Valle C., Sitbon M., Battini J.-L. HTLV-1 and -2 envelope SU subdomains and critical determinants in receptor binding. Retrovirology. 2004;1:41. doi: 10.1186/1742-4690-1-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Davey R.A., A Hamson C., Healey J.J., Cunningham J.M. In vitro binding of purified murine ecotropic retrovirus envelope surface protein to its receptor, MCAT-1. J. Virol. 1997;71:8096–8102. doi: 10.1128/jvi.71.11.8096-8102.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Davey R.A., Zuo Y., Cunningham J. Identification of a receptor-binding pocket on the envelope protein of friend murine leukemia virus. J. Virol. 1999;73:3758–3763. doi: 10.1128/JVI.73.5.3758-3763.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bae Y., Kingsman S., Kingsman A. Functional dissection of the Moloney murine leukemia virus envelope protein gp70. J. Virol. 1997;71:2092–2099. doi: 10.1128/jvi.71.3.2092-2099.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Battini J.L., Danos O., Heard J. Receptor-binding domain of murine leukemia virus envelope glycoproteins. J. Virol. 1995;69:713–719. doi: 10.1128/jvi.69.2.713-719.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Martinez I., Dornburg R. Mutational analysis of the envelope protein of spleen necrosis virus. J. Virol. 1996;70:6036–6043. doi: 10.1128/jvi.70.9.6036-6043.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ott D., Friedrich R., Rein A. Sequence analysis of amphotropic and 10A1 murine leukemia viruses: Close relationship to mink cell focus-inducing viruses. J. Virol. 1990;64:757–766. doi: 10.1128/jvi.64.2.757-766.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ott D., Rein A. Basis for receptor specificity of nonecotropic murine leukemia virus surface glycoprotein gp70SU. J. Virol. 1992;66:4632–4638. doi: 10.1128/jvi.66.8.4632-4638.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Battini J.L., Heard J., Danos O. Receptor choice determinants in the envelope glycoproteins of amphotropic, xenotropic, and polytropic murine leukemia viruses. J. Virol. 1992;66:1468–1475. doi: 10.1128/jvi.66.3.1468-1475.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Federspiel M.J. Reverse Engineering Provides Insights on the Evolution of Subgroups A to E Avian Sarcoma and Leukosis Virus Receptor Specificity. Viruses. 2019;11:497. doi: 10.3390/v11060497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dorner A.J., Stoye J., Coffin J. Molecular basis of host range variation in avian retroviruses. J. Virol. 1985;53:32–39. doi: 10.1128/jvi.53.1.32-39.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kan N.C., Baluda M., Papas T. Sites of recombination between the transforming gene of avian myeloblastosis virus and its helper virus. Virology. 1985;145:323–329. doi: 10.1016/0042-6822(85)90166-7. [DOI] [PubMed] [Google Scholar]
- 103.Bova C.A., Manfredi J., Swanstrom R. Env genes of avian retroviruses: Nucleotide sequence and molecular recombinants define host range determinants. Virology. 1986;152:343–354. doi: 10.1016/0042-6822(86)90137-6. [DOI] [PubMed] [Google Scholar]
- 104.Dorner A.J., Coffin J.M. Determinants for receptor interaction and cell killing on the avian retrovirus glycoprotein gp85. Cell. 1986;45:365–374. doi: 10.1016/0092-8674(86)90322-3. [DOI] [PubMed] [Google Scholar]
- 105.Bova C.A., Olsen J., Swanstrom R. The avian retrovirus env gene family: Molecular analysis of host range and antigenic variants. J. Virol. 1988;62:75–83. doi: 10.1128/jvi.62.1.75-83.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Johnston E.R., Albritton L., Radke K. Envelope proteins containing single amino acid substitutions support a structural model of the receptor-binding domain of bovine leukemia virus surface protein. J. Virol. 2002;76:10861–10872. doi: 10.1128/JVI.76.21.10861-10872.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kim F.J., Seiliez I., Denesvre C., Lavillette D., Cosset F.-L., Sitbon M. Definition of an amino-terminal domain of the human T-cell leukemia virus type 1 envelope surface unit that extends the fusogenic range of an ecotropic murine leukemia virus. J. Biol. Chem. 2000;275:23417–23420. doi: 10.1074/jbc.C901002199. [DOI] [PubMed] [Google Scholar]
- 108.Barnett A.L., Wensel D.L., Li W., Fass D., Cunningham J.M. Structure and mechanism of a coreceptor for infection by a pathogenic feline retrovirus. J. Virol. 2003;77:2717–2729. doi: 10.1128/JVI.77.4.2717-2729.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fass D., Davey R.A., Hamson C.A., Kim P.S., Cunningham J.M., Berger J.M. Structure of a murine leukemia virus receptor-binding glycoprotein at 2.0 angstrom resolution. Science. 1997;277:1662–1666. doi: 10.1126/science.277.5332.1662. [DOI] [PubMed] [Google Scholar]
- 110.Koch W., Hunsmann G., Friedrich R. Nucleotide sequence of the envelope gene of Friend murine leukemia virus. J. Virol. 1983;45:1–9. doi: 10.1128/jvi.45.1.1-9.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Burkhart M.D., Kayman S.C., He Y., Pinter A. Distinct mechanisms of neutralization by monoclonal antibodies specific for sites in the N-terminal or C-terminal domain of murine leukemia virus SU. J. Virol. 2003;77:3993–4003. doi: 10.1128/JVI.77.7.3993-4003.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fontenot J.D., Tjandra N., Ho C., Andrews P.C., Montelaro R.C. Structure and self assembly of a retrovirus (FeLV) proline rich neutralization domain. J. Biomol. Struct Dyn. 1994;11:821–836. doi: 10.1080/07391102.1994.10508035. [DOI] [PubMed] [Google Scholar]
- 113.Lavillette D., Maurice M., Roche C., Russell S.J., Sitbon M., Cosset F.-L. A proline-rich motif downstream of the receptor binding domain modulates conformation and fusogenicity of murine retroviral envelopes. J. Virol. 1998;72:9955–9965. doi: 10.1128/JVI.72.12.9955-9965.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Machida C.A., Bestwick R.K., Boswell B.A., Kabat D. Role of a membrane glycoprotein in Friend virus-induced erythroleukemia: Studies of mutant and revertant viruses. Virology. 1985;144:158–172. doi: 10.1016/0042-6822(85)90314-9. [DOI] [PubMed] [Google Scholar]
- 115.Wu B.W., Lu J., Gallaher T.K., Anderson W., Cannon P.M. Identification of regions in the Moloney murine leukemia virus SU protein that tolerate the insertion of an integrin-binding peptide. Virology. 2000;269:7–17. doi: 10.1006/viro.2000.0201. [DOI] [PubMed] [Google Scholar]
- 116.Kayman S.C., Park H., Saxon M., Pinter A. The hypervariable domain of the murine leukemia virus surface protein tolerates large insertions and deletions, enabling development of a retroviral particle display system. J. Virol. 1999;73:1802–1808. doi: 10.1128/JVI.73.3.1802-1808.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Riedel C., Vasishtan D., Siebert C.A., Whittle C., Lehmann M.J., Mothes W., Grünewald K. Native structure of a retroviral envelope protein and its conformational change upon interaction with the target cell. J. Struct Biol. 2017;197:172–180. doi: 10.1016/j.jsb.2016.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cui L., Wang H., Lu X., Wang R., Zheng R., Li Y., Yang X., Jia W.-T., Zhao Y., Wang Y., et al. Effects of individually silenced N-glycosylation sites and non-synonymous single-nucleotide polymorphisms on the fusogenic function of human syncytin-2. Cell Adh. Migr. 2016;10:39–55. doi: 10.1080/19336918.2015.1093720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Henzy J.E., Coffin J.M. Betaretroviral envelope subunits are noncovalently associated and restricted to the mammalian class. J. Virol. 2013;87:1937–1946. doi: 10.1128/JVI.01442-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wallin M., Ekstrom M., Garoff H. Isomerization of the intersubunit disulphide-bond in Env controls retrovirus fusion. EMBO J. 2004;23:54–65. doi: 10.1038/sj.emboj.7600012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wallin M., Ekstrom M., Garoff H. Receptor-triggered but alkylation-arrested env of murine leukemia virus reveals the transmembrane subunit in a prehairpin conformation. J. Virol. 2006;80:9921–9925. doi: 10.1128/JVI.00380-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sitbon M., D’Auriol L., Ellerbrok H., André C., Nishio J., Perryman S., Pozo F., Hayes S.F., Wehrly K., Tambourin P. Substitution of leucine for isoleucine in a sequence highly conserved among retroviral envelope surface glycoproteins attenuates the lytic effect of the Friend murine leukemia virus. Proc. Natl. Acad. Sci. USA. 1991;88:5932–5936. doi: 10.1073/pnas.88.13.5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Smith J.G., Cunningham J.M. Receptor-induced thiolate couples Env activation to retrovirus fusion and infection. PLoS Pathog. 2007;3:e198. doi: 10.1371/journal.ppat.0030198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mothes W., Boerger A.L., Narayan S., Cunningham J.M., Young J.A. Retroviral entry mediated by receptor priming and low pH triggering of an envelope glycoprotein. Cell. 2000;103:679–689. doi: 10.1016/S0092-8674(00)00170-7. [DOI] [PubMed] [Google Scholar]
- 125.Smith J.G., Mothes W., Blacklow S.C., Cunningham J.M. The mature avian leukosis virus subgroup A envelope glycoprotein is metastable, and refolding induced by the synergistic effects of receptor binding and low pH is coupled to infection. J. Virol. 2004;78:1403–1410. doi: 10.1128/JVI.78.3.1403-1410.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Harrison S.C. Viral membrane fusion. Virology. 2015;479–480:498–507. doi: 10.1016/j.virol.2015.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lozada C., Barlow T.M.A., Gonzalez S., Lubin-Germain N., Ballet S. Identification and Characteristics of Fusion Peptides Derived From Enveloped Viruses. Front. Chem. 2021;9:689006. doi: 10.3389/fchem.2021.689006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhu N.L., Cannon P.M., Chen D., Anderson W.F. Mutational analysis of the fusion peptide of Moloney murine leukemia virus transmembrane protein p15E. J. Virol. 1998;72:1632–1639. doi: 10.1128/JVI.72.2.1632-1639.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hernandez L.D., White J.M. Mutational analysis of the candidate internal fusion peptide of the avian leukosis and sarcoma virus subgroup A envelope glycoprotein. J. Virol. 1998;72:3259–3267. doi: 10.1128/JVI.72.4.3259-3267.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Davies S.M., Kelly S.M., Price N.C., Bradshaw J.P. Structural plasticity of the feline leukaemia virus fusion peptide: A circular dichroism study. FEBS Lett. 1998;425:415–418. doi: 10.1016/S0014-5793(98)00274-9. [DOI] [PubMed] [Google Scholar]
- 131.Delos S.E., Gilbert J., White J. The central proline of an internal viral fusion peptide serves two important roles. J. Virol. 2000;74:1686–1693. doi: 10.1128/JVI.74.4.1686-1693.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chambers P., Pringle C., Easton A. Heptad repeat sequences are located adjacent to hydrophobic regions in several types of virus fusion glycoproteins. (Pt 12)J. Gen. Virol. 1990;71:3075–3080. doi: 10.1099/0022-1317-71-12-3075. [DOI] [PubMed] [Google Scholar]
- 133.Netter R.C., Amberg S.M., Balliet J.W., Biscone M.J., Vermeulen A., Earp L.J., White J.M., Bates P. Heptad repeat 2-based peptides inhibit avian sarcoma and leukosis virus subgroup a infection and identify a fusion intermediate. J. Virol. 2004;78:13430–13439. doi: 10.1128/JVI.78.24.13430-13439.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fass D., Harrison S., Kim P. Retrovirus envelope domain at 1.7 angstrom resolution. Nat. Struct. Biol. 1996;3:465–469. doi: 10.1038/nsb0596-465. [DOI] [PubMed] [Google Scholar]
- 135.Ou W., Silver J. Inhibition of murine leukemia virus envelope protein (env) processing by intracellular expression of the env N-terminal heptad repeat region. J. Virol. 2005;79:4782–4792. doi: 10.1128/JVI.79.8.4782-4792.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Harrison J.S., Koellhoffer J.F., Chandran K., Lai J.R. Marburg virus glycoprotein GP2: pH-dependent stability of the ectodomain alpha-helical bundle. Biochemistry. 2012;51:2515–2525. doi: 10.1021/bi3000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hetzel U., Sironen T., Laurinmäki P., Liljeroos L., Patjas A., Henttonen H., Vaheri A., Artelt A., Kipar A., Butcher S., et al. Isolation, identification, and characterization of novel arenaviruses, the etiological agents of boid inclusion body disease. J. Virol. 2013;87:10918–10935. doi: 10.1128/JVI.01123-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Koellhoffer J.F., Malashkevich V.N., Harrison J.S., Toro R., Bhosle R.C., Chandran K., Almo S.C., Lai J.R. Crystal structure of the Marburg virus GP2 core domain in its postfusion conformation. Biochemistry. 2012;51:7665–7675. doi: 10.1021/bi300976m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cianciolo G.J., Bogerd H.P., Kipnis R.J., Copeland T.D., Oroszlan S., Snyderman R. Inhibition of lymphocyte proliferation by a synthetic peptide homologous to envelope proteins of human and animal retroviruses. Trans. Assoc. Am. Physicians. 1985;98:30–41. [PubMed] [Google Scholar]
- 140.Cianciolo G.J., Copeland T.D., Oroszlan S., Snyderman R. Inhibition of lymphocyte proliferation by a synthetic peptide homologous to retroviral envelope proteins. Science. 1985;230:453–455. doi: 10.1126/science.2996136. [DOI] [PubMed] [Google Scholar]
- 141.Kadota J., Cianciolo G., Snyderman R. A synthetic peptide homologous to retroviral transmembrane envelope proteins depresses protein kinase C mediated lymphocyte proliferation and directly inactivated protein kinase C: A potential mechanism for immunosuppression. Microbiol. Immunol. 1991;35:443–459. doi: 10.1111/j.1348-0421.1991.tb01575.x. [DOI] [PubMed] [Google Scholar]
- 142.Kleinerman E.S., Lachman L.B., Knowles R.D., Snyderman R., Cianciolo G.J. A synthetic peptide homologous to the envelope proteins of retroviruses inhibits monocyte-mediated killing by inactivating interleukin 1. J. Immunol. 1987;139:2329–2337. doi: 10.4049/jimmunol.139.7.2329. [DOI] [PubMed] [Google Scholar]
- 143.Blaise S., Mangeney M., Heidmann T. The envelope of Mason-Pfizer monkey virus has immunosuppressive properties. (Pt 7)J. Gen. Virol. 2001;82:1597–1600. doi: 10.1099/0022-1317-82-7-1597. [DOI] [PubMed] [Google Scholar]
- 144.Mangeney M., Heidmann T. Tumor cells expressing a retroviral envelope escape immune rejection in vivo. Proc. Natl. Acad. Sci. USA. 1998;95:14920–14925. doi: 10.1073/pnas.95.25.14920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mangeney M., Renard M., Schlecht-Louf G., Bouallaga I., Heidmann O., Letzelter C., Richaud A., Ducos B., Heidmann T. Placental syncytins: Genetic disjunction between the fusogenic and immunosuppressive activity of retroviral envelope proteins. Proc. Natl. Acad. Sci. USA. 2007;104:20534–20539. doi: 10.1073/pnas.0707873105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Schlecht-Louf G., Renard M., Mangeney M., Letzelter C., Richaud A., Ducos B., Bouallaga I., Heidmann T. Retroviral infection in vivo requires an immune escape virulence factor encrypted in the envelope protein of oncoretroviruses. Proc. Natl. Acad. Sci. USA. 2010;107:3782–3787. doi: 10.1073/pnas.0913122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Eksmond U., Jenkins B., Merkenschlager J., Mothes W., Stoye J.P., Kassiotis G. Mutation of the Putative Immunosuppressive Domain of the Retroviral Envelope Glycoprotein Compromises Infectivity. J. Virol. 2017;91:e01152-17. doi: 10.1128/JVI.01152-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Brody B.A., Hunter E. Mutations within the env gene of Mason-Pfizer monkey virus: Effects on protein transport and SU-TM association. J. Virol. 1992;66:3466–3475. doi: 10.1128/jvi.66.6.3466-3475.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Song C., Micoli K., Hunter E. Activity of the Mason-Pfizer monkey virus fusion protein is modulated by single amino acids in the cytoplasmic tail. J. Virol. 2005;79:11569–11579. doi: 10.1128/JVI.79.18.11569-11579.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Blot V., Lopez-Vergès S., Breton M., Pique C., Berlioz-Torrent C., Grange M.-P. The conserved dileucine- and tyrosine-based motifs in MLV and MPMV envelope glycoproteins are both important to regulate a common Env intracellular trafficking. Retrovirology. 2006;3:62. doi: 10.1186/1742-4690-3-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bouard D., Sandrin V., Boson B., Nègre D., Thomas G., Granier C., Cosset F.-L. An acidic cluster of the cytoplasmic tail of the RD114 virus glycoprotein controls assembly of retroviral envelopes. Traffic. 2007;8:835–847. doi: 10.1111/j.1600-0854.2007.00581.x. [DOI] [PubMed] [Google Scholar]
- 152.Song C., Micoli K., Bauerova H., Pichova I., Hunter E. Amino acid residues in the cytoplasmic domain of the Mason-Pfizer monkey virus glycoprotein critical for its incorporation into virions. J. Virol. 2005;79:11559–11568. doi: 10.1128/JVI.79.18.11559-11568.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Johnson M.C. Mechanisms for Env glycoprotein acquisition by retroviruses. AIDS Res. Hum. Retrovir. 2011;27:239–247. doi: 10.1089/aid.2010.0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Jorgenson R.L., Vogt V., Johnson M. Foreign glycoproteins can be actively recruited to virus assembly sites during pseudotyping. J. Virol. 2009;83:4060–4067. doi: 10.1128/JVI.02425-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rein A., Mirro J., Haynes J.G., Ernst S.M., Nagashima K. Function of the cytoplasmic domain of a retroviral transmembrane protein: p15E-p2E cleavage activates the membrane fusion capability of the murine leukemia virus Env protein. J. Virol. 1994;68:1773–1781. doi: 10.1128/jvi.68.3.1773-1781.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Green N., Shinnick T.M., Witte O., Ponticelli A., Sutcliffe J.G., A Lerner R. Sequence-specific antibodies show that maturation of Moloney leukemia virus envelope polyprotein involves removal of a COOH-terminal peptide. Proc. Natl. Acad. Sci. USA. 1981;78:6023–6027. doi: 10.1073/pnas.78.10.6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sutcliffe J.G., Shinnick T.M., Green N., Liu F.-T., Niman H.L., Lerner R.A. Chemical synthesis of a polypeptide predicted from nucleotide sequence allows detection of a new retroviral gene product. Nature. 1980;287:801–805. doi: 10.1038/287801a0. [DOI] [PubMed] [Google Scholar]
- 158.Bobkova M., Stitz J., Engelstädter M., Cichutek K., Buchholz C.J. Identification of R-peptides in envelope proteins of C-type retroviruses. (Pt 9)J. Gen. Virol. 2002;83:2241–2246. doi: 10.1099/0022-1317-83-9-2241. [DOI] [PubMed] [Google Scholar]
- 159.Ragheb J.A., Anderson W.F. pH-independent murine leukemia virus ecotropic envelope-mediated cell fusion: Implications for the role of the R peptide and p12E TM in viral entry. J. Virol. 1994;68:3220–3231. doi: 10.1128/jvi.68.5.3220-3231.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kim F.J., Manel N., Boublik Y., Battini J.-L., Sitbon M. Human T-cell leukemia virus type 1 envelope-mediated syncytium formation can be activated in resistant Mammalian cell lines by a carboxy-terminal truncation of the envelope cytoplasmic domain. J. Virol. 2003;77:963–969. doi: 10.1128/JVI.77.2.963-969.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Li M., Li Z.-N., Yao Q., Yang C., Steinhauer D.A., Compans R.W. Murine leukemia virus R Peptide inhibits influenza virus hemagglutinin-induced membrane fusion. J. Virol. 2006;80:6106–6114. doi: 10.1128/JVI.02665-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lucas T.M., Lyddon T.D., Grosse S.A., Johnson M.C. Two distinct mechanisms regulate recruitment of murine leukemia virus envelope protein to retroviral assembly sites. Virology. 2010;405:548–555. doi: 10.1016/j.virol.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rein A. Across the Hall from Pioneers. Viruses. 2021;13:491. doi: 10.3390/v13030491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Henderson L.E., Sowder R., Copeland T.D., Smythers G., Oroszlan S. Quantitative separation of murine leukemia virus proteins by reversed-phase high-pressure liquid chromatography reveals newly described gag and env cleavage products. J. Virol. 1984;52:492–500. doi: 10.1128/jvi.52.2.492-500.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kubo Y., Amanuma H. Mutational analysis of the R peptide cleavage site of Moloney murine leukaemia virus envelope protein. (Pt 8)J. Gen. Virol. 2003;84:2253–2257. doi: 10.1099/vir.0.19126-0. [DOI] [PubMed] [Google Scholar]
- 166.Loving R., Li K., Wallin M., Sjöberg M., Garoff H. R-Peptide cleavage potentiates fusion-controlling isomerization of the intersubunit disulfide in Moloney murine leukemia virus Env. J. Virol. 2008;82:2594–2597. doi: 10.1128/JVI.02039-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Loving R., Wu S.-R., Sjöberg M., Lindqvist B., Garoff H. Maturation cleavage of the murine leukemia virus Env precursor separates the transmembrane subunits to prime it for receptor triggering. Proc. Natl. Acad. Sci. USA. 2012;109:7735–7740. doi: 10.1073/pnas.1118125109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Li M., Yang C., Compans R. Mutations in the cytoplasmic tail of murine leukemia virus envelope protein suppress fusion inhibition by R peptide. J. Virol. 2001;75:2337–2344. doi: 10.1128/JVI.75.5.2337-2344.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kubo Y., Izumida M., Togawa K., Zhang F., Hayashi H. Cytoplasmic R-peptide of murine leukemia virus envelope protein negatively regulates its interaction with the cell surface receptor. Virology. 2019;532:82–87. doi: 10.1016/j.virol.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 170.Schneider I.C., Eckhardt M., Brynza J., Collins M.K., Cichutek K., Buchholz C.J. Escape from R-peptide deletion in a gamma-retrovirus. Virology. 2011;418:85–92. doi: 10.1016/j.virol.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 171.Cardone G., Brecher M., Fontana J., Winkler D.C., Butan C., White J.M., Steven A.C. Visualization of the two-step fusion process of the retrovirus avian sarcoma/leukosis virus by cryo-electron tomography. J. Virol. 2012;86:12129–12137. doi: 10.1128/JVI.01880-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Melikyan G.B., Barnard R.J.O., Markosyan R.M., Young J.A.T., Cohen F.S. Low pH is required for avian sarcoma and leukosis virus Env-induced hemifusion and fusion pore formation but not for pore growth. J. Virol. 2004;78:3753–3762. doi: 10.1128/JVI.78.7.3753-3762.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Nussbaum O., Roop A., Anderson W. Sequences determining the pH dependence of viral entry are distinct from the host range-determining region of the murine ecotropic and amphotropic retrovirus envelope proteins. J. Virol. 1993;67:7402–7405. doi: 10.1128/jvi.67.12.7402-7405.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Rasmussen I., Vilhardt F. Macropinocytosis is the entry mechanism of amphotropic murine leukemia virus. J. Virol. 2015;89:1851–1866. doi: 10.1128/JVI.02343-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lavillette D., Ruggieri A., Russell S.J., Cosset F.-L. Activation of a cell entry pathway common to type C mammalian retroviruses by soluble envelope fragments. J. Virol. 2000;74:295–304. doi: 10.1128/JVI.74.1.295-304.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Barnett A.L., Davey R., Cunningham J. Modular organization of the Friend murine leukemia virus envelope protein underlies the mechanism of infection. Proc. Natl. Acad. Sci. USA. 2001;98:4113–4118. doi: 10.1073/pnas.071432398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.O’Reilly L., Roth M.J. Second-site changes affect viability of amphotropic/ecotropic chimeric enveloped murine leukemia viruses. J. Virol. 2000;74:899–913. doi: 10.1128/JVI.74.2.899-913.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Farrell K.B., Ting Y., Eiden M. Fusion-defective gibbon ape leukemia virus vectors can be rescued by homologous but not heterologous soluble envelope proteins. J. Virol. 2002;76:4267–4274. doi: 10.1128/JVI.76.9.4267-4274.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Anderson M.M., Lauring A.S., Burns C.C., Overbaugh J. Identification of a cellular cofactor required for infection by feline leukemia virus. Science. 2000;287:1828–1830. doi: 10.1126/science.287.5459.1828. [DOI] [PubMed] [Google Scholar]
- 180.Lauring A.S., Anderson M., Overbaugh J. Specificity in receptor usage by T-cell-tropic feline leukemia viruses: Implications for the in vivo tropism of immunodeficiency-inducing variants. J. Virol. 2001;75:8888–8898. doi: 10.1128/JVI.75.19.8888-8898.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lauring A.S., Cheng H.H., Eiden M.V., Overbaugh J. Genetic and biochemical analyses of receptor and cofactor determinants for T-cell-tropic feline leukemia virus infection. J. Virol. 2002;76:8069–8078. doi: 10.1128/JVI.76.16.8069-8078.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lavillette D., Boson B., Russell S.J., Cosset F.-L. Activation of membrane fusion by murine leukemia viruses is controlled in cis or in trans by interactions between the receptor-binding domain and a conserved disulfide loop of the carboxy terminus of the surface glycoprotein. J. Virol. 2001;75:3685–3695. doi: 10.1128/JVI.75.8.3685-3695.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zhao Y., Lee S., Anderson W. Functional interactions between monomers of the retroviral envelope protein complex. J. Virol. 1997;71:6967–6972. doi: 10.1128/jvi.71.9.6967-6972.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Rein A., Yang C., Haynes J.A., Mirro J., Compans R.W. Evidence for cooperation between murine leukemia virus Env molecules in mixed oligomers. J. Virol. 1998;72:3432–3435. doi: 10.1128/JVI.72.4.3432-3435.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Koo H.M., Gu J., Varela-Echavarria A., Ron Y., Dougherty J.P. Reticuloendotheliosis type C and primate type D oncoretroviruses are members of the same receptor interference group. J. Virol. 1992;66:3448–3454. doi: 10.1128/jvi.66.6.3448-3454.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Rasko J.E., Battini J.L., Gottschalk R.J., Mazo I., Miller A.D. The RD114/simian type D retrovirus receptor is a neutral amino acid transporter. Proc. Natl. Acad. Sci. USA. 1999;96:2129–2134. doi: 10.1073/pnas.96.5.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kewalramani V.N., Panganiban A., Emerman M. Spleen necrosis virus, an avian immunosuppressive retrovirus, shares a receptor with the type D simian retroviruses. J. Virol. 1992;66:3026–3031. doi: 10.1128/jvi.66.5.3026-3031.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Sugimoto J., Sugimoto M., Bernstein H., Jinno Y., Schust D. A novel human endogenous retroviral protein inhibits cell-cell fusion. Sci Rep. 2013;3:1462. doi: 10.1038/srep01462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Tailor C.S., Nouri A., Zhao Y., Takeuchi Y., Kabat D. A sodium-dependent neutral-amino-acid transporter mediates infections of feline and baboon endogenous retroviruses and simian type D retroviruses. J. Virol. 1999;73:4470–4474. doi: 10.1128/JVI.73.5.4470-4474.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Marin M., Tailor C.S., Nouri A., Kabat D. Sodium-dependent neutral amino acid transporter type 1 is an auxiliary receptor for baboon endogenous retrovirus. J. Virol. 2000;74:8085–8093. doi: 10.1128/JVI.74.17.8085-8093.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Manel N., Kinet S., Battini J.-L., Kim F.J., Taylor N., Sitbon M. The HTLV receptor is an early T-cell activation marker whose expression requires de novo protein synthesis. Blood. 2003;101:1913–1918. doi: 10.1182/blood-2002-09-2681. [DOI] [PubMed] [Google Scholar]
- 192.Hein S., Prassolov V., Zhang Y., Ivanov D., Löhler J., Ross S.R., Stocking C. Sodium-dependent myo-inositol transporter 1 is a cellular receptor for Mus cervicolor M813 murine leukemia virus. J. Virol. 2003;77:5926–5932. doi: 10.1128/JVI.77.10.5926-5932.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Tipper C.H., Cingoz O., Coffin J. Mus spicilegus endogenous retrovirus HEMV uses murine sodium-dependent myo-inositol transporter 1 as a receptor. J. Virol. 2012;86:6341–6344. doi: 10.1128/JVI.00083-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Albritton L.M., Tseng L., Scadden D., Cunningham J.M. A putative murine ecotropic retrovirus receptor gene encodes a multiple membrane-spanning protein and confers susceptibility to virus infection. Cell. 1989;57:659–666. doi: 10.1016/0092-8674(89)90134-7. [DOI] [PubMed] [Google Scholar]
- 195.Bai L., Sato H., Kubo Y., Wada S., Aida Y. CAT1/SLC7A1 acts as a cellular receptor for bovine leukemia virus infection. FASEB J. 2019;33:14516–14527. doi: 10.1096/fj.201901528R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Chai N., Bates P. Na+/H+ exchanger type 1 is a receptor for pathogenic subgroup J avian leukosis virus. Proc. Natl. Acad. Sci. USA. 2006;103:5531–5536. doi: 10.1073/pnas.0509785103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Tsang J., Ribet D., Heidmann T., Dewannieux M. Identification of the Receptor Used by the Ecotropic Mouse GLN Endogenous Retrovirus. J. Virol. 2019;93:e01125-18. doi: 10.1128/JVI.01125-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Xu W., Stadler C.K., Gorman K., Jensen N., Kim D., Zheng H., Tang S., Switzer W.M., Pye G.W., Eiden M.V. An exogenous retrovirus isolated from koalas with malignant neoplasias in a US zoo. Proc. Natl. Acad. Sci. USA. 2013;110:11547–11552. doi: 10.1073/pnas.1304704110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Shojima T., Yoshikawa R., Hoshino S., Shimode S., Nakagawa S., Ohata T., Nakaoka R., Miyazawa T. Identification of a novel subgroup of Koala retrovirus from Koalas in Japanese zoos. J. Virol. 2013;87:9943–9948. doi: 10.1128/JVI.01385-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Mendoza R., Anderson M., Overbaugh J. A putative thiamine transport protein is a receptor for feline leukemia virus subgroup A. J. Virol. 2006;80:3378–3385. doi: 10.1128/JVI.80.7.3378-3385.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Oliveira N.M., Farrell K., Eiden M. In vitro characterization of a koala retrovirus. J. Virol. 2006;80:3104–3107. doi: 10.1128/JVI.80.6.3104-3107.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Takeuchi Y., Vile R.G., Simpson G., O’Hara B., Collins M.K., A Weiss R. Feline leukemia virus subgroup B uses the same cell surface receptor as gibbon ape leukemia virus. J. Virol. 1992;66:1219–1222. doi: 10.1128/jvi.66.2.1219-1222.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Olah Z., Lehel C., Anderson W.B., Eiden M.V., A Wilson C. The cellular receptor for gibbon ape leukemia virus is a novel high affinity sodium-dependent phosphate transporter. J. Biol. Chem. 1994;269:25426–25431. doi: 10.1016/S0021-9258(18)47267-5. [DOI] [PubMed] [Google Scholar]
- 204.Kavanaugh M.P., Miller D.G., Zhang W., Law W., Kozak S.L., Kabat D., Miller A.D. Cell-surface receptors for gibbon ape leukemia virus and amphotropic murine retrovirus are inducible sodium-dependent phosphate symporters. Proc. Natl. Acad. Sci. USA. 1994;91:7071–7075. doi: 10.1073/pnas.91.15.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wilson C.A., Eiden M.V., Anderson W.B., Lehel C., Olah Z. The dual-function hamster receptor for amphotropic murine leukemia virus (MuLV), 10A1 MuLV, and gibbon ape leukemia virus is a phosphate symporter. J. Virol. 1995;69:534–537. doi: 10.1128/jvi.69.1.534-537.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Soll S.J., Neil S., Bieniasz P. Identification of a receptor for an extinct virus. Proc. Natl. Acad. Sci. USA. 2010;107:19496–19501. doi: 10.1073/pnas.1012344107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Tury S., Giovannini D., Ivanova S., Touhami J., Courgnaud V., Battini J.-L. Identification of Copper Transporter 1 as a Receptor for Feline Endogenous Retrovirus ERV-DC14. J. Virol. 2022;96:e0022922. doi: 10.1128/jvi.00229-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Sarangi A., Bupp K., Roth M. Identification of a retroviral receptor used by an envelope protein derived by peptide library screening. Proc. Natl. Acad. Sci. USA. 2007;104:11032–11037. doi: 10.1073/pnas.0704182104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Quigley J.G., Burns C.C., Anderson M.M., Lynch E.D., Sabo K.M., Overbaugh J., Abkowitz J.L. Cloning of the cellular receptor for feline leukemia virus subgroup C (FeLV-C), a retrovirus that induces red cell aplasia. Blood. 2000;95:1093–1099. doi: 10.1182/blood.V95.3.1093.003k01_1093_1099. [DOI] [PubMed] [Google Scholar]
- 210.Ericsson T.A., Takeuchi Y., Templin C., Quinn G., Farhadian S.F., Wood J.C., Oldmixon B.A., Suling K.M., Ishii J.K., Kitagawa Y., et al. Identification of receptors for pig endogenous retrovirus. Proc. Natl. Acad. Sci. USA. 2003;100:6759–6764. doi: 10.1073/pnas.1138025100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Mazari P.M., Argaw T., Valdivieso L., Zhang X., Marcucci K.T., Salomon D.R., Wilson C.A., Roth M.J. Comparison of the convergent receptor utilization of a retargeted feline leukemia virus envelope with a naturally-occurring porcine endogenous retrovirus A. Virology. 2012;427:118–126. doi: 10.1016/j.virol.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Tailor C.S., Nouri A., Lee C.G., Kozak C., Kabat D. Cloning and characterization of a cell surface receptor for xenotropic and polytropic murine leukemia viruses. Proc. Natl. Acad. Sci. USA. 1999;96:927–932. doi: 10.1073/pnas.96.3.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Yang Y.L., Guo L., Xu S., Holland C.A., Kitamura T., Hunter K.W., Cunningham J.M. Receptors for polytropic and xenotropic mouse leukaemia viruses encoded by a single gene at Rmc1. Nat. Genet. 1999;21:216–219. doi: 10.1038/6005. [DOI] [PubMed] [Google Scholar]
- 214.Esnault C., Priet S., Ribet D., Vernochet C., Bruls T., Lavialle C., Weissenbach J., Heidmann T. A placenta-specific receptor for the fusogenic, endogenous retrovirus-derived, human syncytin-2. Proc. Natl. Acad. Sci. USA. 2008;105:17532–17537. doi: 10.1073/pnas.0807413105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Carette J.E., Raaben M., Wong A.C., Herbert A.S., Obernosterer G., Mulherkar N., Kuehne A.I., Kranzusch P.J., Griffin A.M., Ruthel G., et al. Ebola virus entry requires the cholesterol transporter Niemann-Pick C1. Nature. 2011;477:340–343. doi: 10.1038/nature10348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Bates P., Young J., Varmus H. A receptor for subgroup A Rous sarcoma virus is related to the low density lipoprotein receptor. Cell. 1993;74:1043–1051. doi: 10.1016/0092-8674(93)90726-7. [DOI] [PubMed] [Google Scholar]
- 217.Young J.A., Bates P., Varmus H. Isolation of a chicken gene that confers susceptibility to infection by subgroup A avian leukosis and sarcoma viruses. J. Virol. 1993;67:1811–1816. doi: 10.1128/jvi.67.4.1811-1816.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Brojatsch J., Naughton J., Rolls M.M., Zingler K., Young J.A. CAR1, a TNFR-related protein, is a cellular receptor for cytopathic avian leukosis-sarcoma viruses and mediates apoptosis. Cell. 1996;87:845–855. doi: 10.1016/S0092-8674(00)81992-3. [DOI] [PubMed] [Google Scholar]
- 219.Elleder D., Stepanets V., Melder D.C., Šenigl F., Geryk J., Pajer P., Plachý J., Hejnar J., Svoboda J., Federspiel M.J. The receptor for the subgroup C avian sarcoma and leukosis viruses, Tvc, is related to mammalian butyrophilins, members of the immunoglobulin superfamily. J. Virol. 2005;79:10408–10419. doi: 10.1128/JVI.79.16.10408-10419.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Ferrada E., Superti-Furga G. A structure and evolutionary-based classification of solute carriers. iScience. 2022;25:105096. doi: 10.1016/j.isci.2022.105096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Bacquin A., Bireau C., Tanguy M., Romanet C., Vernochet C., Dupressoir A., Heidmann T. A Cell Fusion-Based Screening Method Identifies Glycosylphosphatidylinositol-Anchored Protein Ly6e as the Receptor for Mouse Endogenous Retroviral Envelope Syncytin-A. J. Virol. 2017;91:e00832-17. doi: 10.1128/JVI.00832-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Heidmann O., Vernochet C., Dupressoir A., Heidmann T. Identification of an endogenous retroviral envelope gene with fusogenic activity and placenta-specific expression in the rabbit: A new "syncytin" in a third order of mammals. Retrovirology. 2009;6:107. doi: 10.1186/1742-4690-6-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Mazari P.M., Linder-Basso D., Sarangi A., Chang Y., Roth M.J. Single-round selection yields a unique retroviral envelope utilizing GPR172A as its host receptor. Proc. Natl. Acad. Sci. USA. 2009;106:5848–5853. doi: 10.1073/pnas.0809741106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Mazari P.M., Roth M.J. Library screening and receptor-directed targeting of gammaretroviral vectors. Future Microbiol. 2013;8:107–121. doi: 10.2217/fmb.12.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Aydin H., Cook J., Lee J. Crystal structures of beta- and gammaretrovirus fusion proteins reveal a role for electrostatic stapling in viral entry. J. Virol. 2014;88:143–153. doi: 10.1128/JVI.02023-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Lamb D., Schüttelkopf A.W., Van Aalten D.M.F., Brighty D.W. Charge-surrounded pockets and electrostatic interactions with small ions modulate the activity of retroviral fusion proteins. PLoS Pathog. 2011;7:e1001268. doi: 10.1371/journal.ppat.1001268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Dean T.T., Serrao V., Lee J. Structure of the Core Postfusion Porcine Endogenous Retrovirus Fusion Protein. mBio. 2022;13:e0292021. doi: 10.1128/mbio.02920-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Martinez-Molledo M., Nji E., Reyes N. Structural insights into the lysophospholipid brain uptake mechanism and its inhibition by syncytin-2. Nat. Struct Mol. Biol. 2022;29:604–612. doi: 10.1038/s41594-022-00786-8. [DOI] [PubMed] [Google Scholar]
- 229.Chen C.P., Chen L.F., Yang S.R., Chen C.Y., Ko C.C., Chang G.D., Chen H. Functional characterization of the human placental fusogenic membrane protein syncytin 2. Biol. Reprod. 2008;79:815–823. doi: 10.1095/biolreprod.108.069765. [DOI] [PubMed] [Google Scholar]
- 230.Hotzel I. Deep-Time Structural Evolution of Retroviral and Filoviral Surface Envelope Proteins. J. Virol. 2022;96:e0006322. doi: 10.1128/jvi.00063-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Vogt P.K., Ishizaki R. Patterns of viral interference in the avian leukosis and sarcoma complex. Virology. 1966;30:368–374. doi: 10.1016/0042-6822(66)90115-2. [DOI] [PubMed] [Google Scholar]
- 232.Rein A. Interference grouping of murine leukemia viruses: A distinct receptor for the MCF-recombinant viruses in mouse cells. Virology. 1982;120:251–257. doi: 10.1016/0042-6822(82)90024-1. [DOI] [PubMed] [Google Scholar]
- 233.Rein A., Schultz A. Different recombinant murine leukemia viruses use different cell surface receptors. Virology. 1984;136:144–152. doi: 10.1016/0042-6822(84)90255-1. [DOI] [PubMed] [Google Scholar]
- 234.Sommerfelt M.A., Williams B.P., Clapham P.R., Solomon E., Goodfellow P.N., Weiss R.A. Human T cell leukemia viruses use a receptor determined by human chromosome 17. Science. 1988;242:1557–1559. doi: 10.1126/science.3201246. [DOI] [PubMed] [Google Scholar]
- 235.Barnard R.J., Elleder D., Young J. Avian sarcoma and leukosis virus-receptor interactions: From classical genetics to novel insights into virus-cell membrane fusion. Virology. 2006;344:25–29. doi: 10.1016/j.virol.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 236.Wildum S., Schindler M., Münch J., Kirchhoff F. Contribution of Vpu, Env, and Nef to CD4 down-modulation and resistance of human immunodeficiency virus type 1-infected T cells to superinfection. J. Virol. 2006;80:8047–8059. doi: 10.1128/JVI.00252-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Jobbagy Z., Garfield S., Baptiste L., Eiden M.V., Anderson W.B. Subcellular redistribution of Pit-2 P(i) transporter/amphotropic leukemia virus (A-MuLV) receptor in A-MuLV-infected NIH 3T3 fibroblasts: Involvement in superinfection interference. J. Virol. 2000;74:2847–2854. doi: 10.1128/JVI.74.6.2847-2854.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Kim J.W., Cunningham J.M. N-linked glycosylation of the receptor for murine ecotropic retroviruses is altered in virus-infected cells. J. Biol. Chem. 1993;268:16316–16320. doi: 10.1016/S0021-9258(19)85423-6. [DOI] [PubMed] [Google Scholar]
- 239.Bahrami S., Pagh K., Ejegod D., Duch M., Tolstrup M., Pedersen F.S. Construction of a gammaretrovirus with a novel tropism and wild-type replication kinetics capable of using human APJ as entry receptor. J. Virol. 2012;86:10621–10627. doi: 10.1128/JVI.01028-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Delwart E.L., Panganiban A.T. Role of reticuloendotheliosis virus envelope glycoprotein in superinfection interference. J. Virol. 1989;63:273–280. doi: 10.1128/jvi.63.1.273-280.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Bahrami S., Ejegod D., Sørensen K.D., Pedersen F.S. Coupling of receptor interference and a host-dependent post-binding entry deficiency in a gammaretroviral envelope protein. Retrovirology. 2010;7:9. doi: 10.1186/1742-4690-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Liu M., Eiden M.V. A mutant retroviral receptor restricts virus superinfection interference and productive infection. Retrovirology. 2012;9:51. doi: 10.1186/1742-4690-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Liu M., Eiden M.V. The receptors for gibbon ape leukemia virus and amphotropic murine leukemia virus are not downregulated in productively infected cells. Retrovirology. 2011;8:53. doi: 10.1186/1742-4690-8-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Murphy S.L., Chung-Landers M., Honczarenko M., Gaulton G.N. Linkage of reduced receptor affinity and superinfection to pathogenesis of TR1.3 murine leukemia virus. J. Virol. 2006;80:4601–4609. doi: 10.1128/JVI.80.9.4601-4609.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Rainey G.J., Coffin J.M. Evolution of broad host range in retroviruses leads to cell death mediated by highly cytopathic variants. J. Virol. 2006;80:562–570. doi: 10.1128/JVI.80.2.562-570.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Reinhart T.A., Ghosh A.K., A Hoover E., I Mullins J. Distinct superinfection interference properties yet similar receptor utilization by cytopathic and noncytopathic feline leukemia viruses. J. Virol. 1993;67:5153–5162. doi: 10.1128/jvi.67.9.5153-5162.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Weller S.K., Joy A., Temin H. Correlation between cell killing and massive second-round superinfection by members of some subgroups of avian leukosis virus. J. Virol. 1980;33:494–506. doi: 10.1128/jvi.33.1.494-506.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Yoshimura F.K., Wang T., Nanua S. Mink cell focus-forming murine leukemia virus killing of mink cells involves apoptosis and superinfection. J. Virol. 2001;75:6007–6015. doi: 10.1128/JVI.75.13.6007-6015.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Yan Y., Liu Q., Wollenberg K., Martin C., Buckler-White A., Kozak C.A. Evolution of functional and sequence variants of the mammalian XPR1 receptor for mouse xenotropic gammaretroviruses and the human-derived retrovirus XMRV. J. Virol. 2010;84:11970–11980. doi: 10.1128/JVI.01549-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Boso G., Lam O., Bamunusinghe D., Oler A.J., Wollenberg K., Liu Q., Shaffer E., Kozak C.A. Patterns of Coevolutionary Adaptations across Time and Space in Mouse Gammaretroviruses and Three Restrictive Host Factors. Viruses. 2021;13:1864. doi: 10.3390/v13091864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Demogines A., Abraham J., Choe H., Farzan M., Sawyer S.L. Dual host-virus arms races shape an essential housekeeping protein. PLoS Biol. 2013;11:e1001571. doi: 10.1371/journal.pbio.1001571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Kizhatil K., Albritton L.M. Requirements for different components of the host cell cytoskeleton distinguish ecotropic murine leukemia virus entry via endocytosis from entry via surface fusion. J. Virol. 1997;71:7145–7156. doi: 10.1128/jvi.71.10.7145-7156.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Valle-Tenney R., Opazo T., Cancino J., Goff S.P., Arriagada G. Dynein Regulators Are Important for Ecotropic Murine Leukemia Virus Infection. J. Virol. 2016;90:6896–6905. doi: 10.1128/JVI.00863-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Cheynet V., Ruggieri A., Oriol G., Blond J.L., Boson B., Vachot L., Verrier B., Cosset F.-L., Mallet F. Synthesis, assembly, and processing of the Env ERVWE1/syncytin human endogenous retroviral envelope. J. Virol. 2005;79:5585–5593. doi: 10.1128/JVI.79.9.5585-5593.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Dupressoir A., Vernochet C., Bawa O., Harper F., Pierron G., Opolon P., Heidmann T. Syncytin-A knockout mice demonstrate the critical role in placentation of a fusogenic, endogenous retrovirus-derived, envelope gene. Proc. Natl. Acad. Sci. USA. 2009;106:12127–12132. doi: 10.1073/pnas.0902925106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Dupressoir A., Vernochet C., Harper F., Guegan J., Dessen P., Pierron G., Heidmann T. A pair of co-opted retroviral envelope syncytin genes is required for formation of the two-layered murine placental syncytiotrophoblast. Proc. Natl. Acad. Sci. USA. 2011;108:E1164–E1173. doi: 10.1073/pnas.1112304108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created for this review article.