Abstract
Objective:
Some cationic anti-microbial peptides show a wide range of cytotoxic action versus malignant cells, which may lead to developing a novel group of antitumor medications. In the present study, the anticancer activity of pleurocidin-like peptide WF3 isoform X2 (AMP-WF3), from the Poecilia Mexicana fish, against leukemic cell line Jurkat was evaluated, and the cytotoxicity compared with the effects on normal cells, including peripheral blood mononuclear cells (PBMCs) and human dermal fibroblast (HDF) cells.
Materials and Methods:
In this experimental study, cells were treated with various dosages of AMP-WF3 for 24 hours. Using methyl thiazole tetrazolium salt reduction (MTT test), the effects of the AMP-WF3 on cell viability and toxicity were evaluated. The impact of this peptide on apoptotic pathways was examined using flow cytometry and Annexin V-PI stains. Additionally, the relative expression of the P53, P21, and BCL-2 genes was evaluated using a real-time polymerase chain reaction.
Results:
The Jurkat cell line was more susceptible to AMP-WF3 cytotoxicity [half-maximal inhibitory concentration (IC50)=50 μM], while normal cells (PBMCs and HDF) were less susceptible. Flow cytometry verified that the apoptotic activity of AMP-WF3 on Jurkat cells was significantly higher than that of HDF and PBMCs. Peptide-treated Jurkat cells were associated with increased expression of P21, and P53 genes. In contrast, the changes in P21, P53, and BCL-2 genes differed in PBMCs and HDF cells. In HDF cells, simultaneous increase of P21, P53, and BCL-2, and in PBMCs, only the increase of BCL-2 was observed.
Conclusion:
Our research showed that AMP-WF3 could be developed as a novel treatment agent with minimum side effects for ALL patients.
Keywords: Acute Leukemia, Apoptosis, Cationic Peptide
Introduction
Despite considerable advances in cancer research and treatment, the illness continues to be one of the major causes of death worldwide. In 2018, approximately 18,000,000 instances of cancer were reported, with 9,000,000 dead as a result (1). Leukemia, caused by the uncontrolled growth of the hematopoietic cell lineage, is one of the most prevalent kinds of cancer. Multiple causes, including genetics, infections, toxins, and radio waves, can result in leukemia (2). In recent decades, there have been advancements in treating childhood leukemia with stem cell transplantation. Still, chemo drug continues to be the dominant therapeutic option for adults, with less treatment response, particularly in those aged over 50 years (3).
Chemotherapeutic medicines used to treat leukemia are nonspecific, affecting both malignant and healthy cells and organs (4). Some medications, such as Doxorubicin and Arsenic trioxide, have serious side effects (5). Doxorubicin causes oxidative stress, which harms vital organs like the heart, kidneys, and brain (6). Arsenic, one of the components used in the chemotherapy regimen for refractory leukemia treatment, can induce mandibular bone necrosis (7). Due to the challenges connected with current therapies, such as many mental and physical problems in patients and the inefficacy of these treatments in reducing cancer cells, new safe and effective medications for leukemia treatment must be developed as early as possible (8).
Cationic anti-microbial peptides (CAPs) are polypeptides that typically contain between 5 and 100 amino acids (9). These peptides, which are made up of alkaline amino acids, play a significant role in the host immune response (10). CAPs are classified as a-helical, b-sheet, loop, or extended peptides based on the secondary structure they develop while interacting with biological membranes (11). These peptides can stimulate the secretion of cytokines, decrease inflammation, and destroy cancer cells and bacteria (12). CAPs were shown to kill malignant cells in experimental and clinical investigations (13). Some of the targets these peptides interact with to kill cancer cells include phosphatidylserine, heparan sulfate proteoglycans, chondroitin sulfate, and O-sialoglycoproteins (14). Pleurocidins are a novel family of CAPs resembling cathelicidins in structure and function (15). This peptide was first discovered in the winter flounder’s skin-secreted mucus (pleuronectes americanus) (16). In 2003, 20 types of NRCs of pleurocidin-like peptides were discovered in various species of Flanders (17). These peptides, like magainin 2, create pores in lipid membranes. In addition, Pleurocidins have weak hemolytic and moderate antibacterial activity (18). When these peptides interact with biological membranes, they form an alpha-helix (19). These peptides’ apoptosis mechanism is twofold: i. Membranolytic targets: they cause cell death by reacting with extracellular receptors or creating instability in the plasma membrane, ii. Non-membranolytic targets: they cause cell death by reacting with intracellular proteins and inducing apoptosis (12). The amount of negatively charged on the outer layer of different malignant cell types determines the mode of action of these peptides. In investigations on pleurocidin’s anti-cancer potential, the peptides NRC-07 and NRC-03 from the pleurocidin family were found to be effective in killing multiple myeloma and breast cancer cells (20). Additionally, it was demonstrated in the recent study that NRC-03 may trigger apoptosis in oral squamous cell carcinoma by activating the CypD-mPTP axis, which is brought on by mitochondrial oxidative stress (21).
In the present studies, we evaluated the cytotoxicity of a pleurocidin-like peptide WF3 (AMP-WF3) isoform X2 from Poecilia Mexicana fish against Jurkat cells, as well as its impact on P21, P53, and BCL-2 expression.
Materials and Methods
Database search
The AMP-WF3 signal sequences MKCATLFLVLSMVVLMAEPGDA were predicted as a query for searching against fish in NCBI (https://www.ncbi.nlm.nih.gov) using the parameters of the BLOSUM62 matrix method, Gap Costs, existence 11, and extension 1.
Sequence analysis
The coding DNA sequence was identified using the ORF finder (https://www.ncbi.nlm.nih.gov/orffinder). The amino acid sequence of pleurocidin-like WF3 isoform X2 (XP 014834597.1) was provided in P 4.0 (http://www.cbs. dtu.dk/services/SignalP/) and (https:/www.genome.jp/ tools/motif/) to find the peptide signals and motifs. CAMP (http://www.camp.bicnirrh.res.in/predict) predicted the position of mature anti-microbial peptide (AMP) on its precursor. Anti-microbial characteristics were predicted using an anti-microbial peptide calculator and predictor (APD3) (http://aps.unmc.edu/AP/prediction/prediction main.php), and most AMPs that were comparable to prospective AMPs were discovered. The protein was provided in the helix rotation scheme (http://rzlab.ucr. edu/scripts/wheel/wheel.cgi) to forecast the helix and the hydrophilic interaction and hydrophobicity on the secondary structure of the peptide, the peptide sequence, and the amino acid sequence. The peptide’s 3D structure was predicted using I-TASSER (http://zhanglab.ccmb. med.umich.edu/ I-TASSER /), an online protein structure prediction site.
Peptide synthesis
AMP-WF3 (amino acid sequence: FLKSLWRGVKAIFNGARQGYKEHKN) from Poecilia Mexicana fish (Pepmic, Suzhou, China) was produced using a solid phase technique based on Fmoc (9-fluorenyl-methoxycarbonyl) chemistry. This peptide was isolated using a SHIMADZU Inertsil ODS-SP (4.6 250 mm 5 m) column in RP-HPLC. For 30 minutes, the peptide was eluted with a 0-100% H2O/acetonitrile gradient containing 0.1 percent trifluoroacetic acid (TFA). The peptide was homogeneous in an analytical high-performance homogeneity experiment utilizing an Inertsil ODSSP column, indicating a purity of 95%. The atomic mass of isolated peptides was successfully determined using mass spectrometry (MS).
Cell culture
The Pasteur Institute provided us with Jurkat and human dermal fibroblast (HDF) cell lines (Tehran, Iran). Jurkat cell lines were maintained in RPMMI1640 medium with L-glutamine (Caisson, USA), and HDF cell lines were maintained in DMEM.F12 medium (Caisson, USA) and 1% non-essential amino acid solution (Sigma, USA), supplemented with 10% heat-inactivated fetal bovine serum (FBS, Gibco, USA), 100 U/ml penicillin, and 100 µg/ml streptomycin (Caisson, USA) in 5% CO2 environment at 37˚C. Cells were passaged for optimum development and proven free of Mycoplasma and Endotoxin contamination. The cells were only employed if trypan blue exclusion revealed that more than 95% of them were viable. Healthy human peripheral blood mononuclear cells (PBMCs) were extracted from heparinized blood using Ficoll and utilized immediately.
Isolation of PBMCs via Ficoll
Whole blood was obtained in 10 mL EDTA tubes to isolate PBMCs. Phosphate Buffer Saline was added to fresh blood in equal amounts. In a sterile tube, phosphate buffer saline was mixed with an equal amount of fresh blood. Whole blood with PBS was added to a 50 ml canolical tube in a 2: 1 ratio, and centrifuged in a swinging bucket rotor without brake for 30 minutes at 20˚C at 800 g. The middle layer was slowly pouredinto a new 50 ml falcon, then washed twice with PBS at 300 g for 10 minutes with the break. After washing twice, the formed pellet was dissolved in 1 ml of RPMI1640 medium, and the cells were counted.
MTT assay
Different concentrations of AMP-WF3 were incubated with Jurkat cells, PBMCs, and HDF cells, and cytotoxic activity was measured using the MTT test (ROTH, USA). In brief, 2×104 Jurkat cells and PBMCs were seeded in 96-well plates in medium with different concentrations of AMP-WF3 (6.25, 12.5, 25, 50 μM), and 1×104 HDF cells were sown in 96-well plates in media with varying concentrations of AMP-WF3 (6.25, 12.5, 25, 50 μM). After 24 hours, 10 μL MTT was added to each well, and the plates were incubated at 37˚C for 4 hours, and then the formazan crystals were subsequently solubilized in dimethyl sulfoxide (DMSO, Bioscience, USA, 100 μl/ well). The optical absorbance was measured at 570 nm after 30 minutes. The final findings were calculated using an average of at least three repeated testings, and control cell survival was assumed to be 100%. The halfmaximal inhibitory concentration (IC50) value for Jurkat cells was the peptide dosage that resulted in a 50% drop in absorbance compared to the control. The viable cell percentage was estimated using the formula: absorbance sample - absorbance blank/absorbance control - absorbance blank ×100.
RNA extraction, cDNA synthesis, and real-time polymerase chain reaction
The TRIzol reagent was used to extract total RNA from cultivated cells in each group (Yekta Tajhiz Azma, Iran). Gel electrophoresis and the Nanodrop spectrometer were used to assess the RNA quality and quantity. Complementary DNA was produced according to the manufacturer’s instructions using a random hexamer primer and the reverse transcriptase M-MLV (Yekta Tajhiz Azma, Iran). Bonbiotech (Bonbiotech, Inc., Iran) synthesized primers for polymerase chain reaction (PCR) amplification, as shown in Table 1. Step One Plus Real-Time PCR devices were used to assess the mRNA expression in the cells (Applied Biosystems, USA). According to the manufacturer’s instructions, a 2X YTASYBR Green PCR Master Mix kit (Yekta Tajhiz Azma, Iran) was used to evaluate the expression of p53, p21, and BCL-2 mRNAs in Jurkat cells, PBMCs, and HDF cells. Briefly, 2 μl of cDNA product, 1 μl of each primer, 10 μl of 2X YTASYBR Green, and 7 μl of DEPC water were diluted to a final volume of 20 μl. (Sina Gene, Iran). An initial denaturation phase at 95˚C for 2 minutes was followed by 40 cycles of denaturation at 95˚C for 5 seconds, annealing, and extension at 60˚C for 30 seconds. HPRT was used as a reference gene to assess the mRNA expression levels of P21, P53, and BCL-2.
Table 1.
Real-time polymerase chain reaction primer sequences
|
| ||
|---|---|---|
| Target gene | Primer sequence (5´-3´) | Product size (bp) |
|
| ||
| HPRT | F: GGACAGGACTGAACGTCTTGC | 88 |
| R: ATAGCCCCCCTTGAGCACAC | ||
| P21 | F: GAGGCCGGGATGAGTTGGGAGGAG | 221 |
| R: CAGCCGGCGTTTGGAGTGGTAGAA | ||
| P53 | R: TAACAGTTCCTGCATGGGCGGC | 121 |
| R: AGGACAGGCACAAACACGCACC | ||
| BCL-2 | F: GGTGGGGTCATGTGTGTGG | 89 |
| R: CGGTTCAGGTACTCAGTCATCC | ||
|
| ||
Apoptosis assay
Apoptosis was determined using a bicolor flow cytometric technique with an Annexin V/PI testing kit. In summary, 2×104 cells were inoculated into 6-well plates with or without AMP-WF3 in the medium. Following overnight incubation, the cells were collected, washed in PBS, and re-suspended in 500 µL of binding buffer. The sample was combined with FITC-conjugated Annexin V and PI, and the combination was then incubated for 10 minutes on ice in the dark. Following flow cytometry examination of the labeled cells, the percentage of apoptotic cells was calculated using Cell Quest software.
Statistical analysis
All experiments were performed in triplicate, and results were reported as mean and standard deviation. The results were analyzed by the Wilcoxon rank test and ANOVA test by Graph Pad Prism version 9 (GraphPad Software Inc, USA) and SPSS v.22 software (IBM SPSS Statistics, USA). The significance level of statistical tests was considered to be 0.05 (P<0.05).
Ethical issue
This investigation has been approved by the Ethical Committee of Bushehr University of Medical Sciences (IR.BPUMS.REC.1398.013).
Results
Database search
When the pleurocidin peptide signal was used as a query in BLASTP against fish, a precursor to a new AMP, the pleurocidin-like peptide (XP_014834597.1) was found. The output list was checked for conserved peptide signal, and a score (81.82% detection score, 100% coverage score) was presented in the amino acid sequence as a potential, pleurocidin-like peptide (XP_014834597.1). After BLASTP, the results were as follows: The signaling region of pleurocidin-like peptide is 1 to 22 amino acids, and the cut-off region is between 22 and 23 amino acids (Fig .1). The desired sequence used in the research is as follows: FLKSLWRGVKAIFNGARQGYKEHKN, which is one of the amino acids from 23 to 44 (Table 2).
Fig 1.
Peptide signal and cleavage area were predicted using SignalP 5.0 server. Positions 22 and 23 are the protein sequences of the cleavage area.
Table 2.
The score of each sequence is ranked according to the sequence position with anti-microbial properties (AMP)
|
| ||||
|---|---|---|---|---|
| Seq ID | Position | Sequence | Class | AMP probability |
|
| ||||
| 1 | 1-25 | FLKSLWRGVKAIFNGARQGYKEHKN | AMP | 0.969 |
| 1 | 2-26 | LKSLWRGVKAIFNGARQGYKEHKNQ | AMP | 0.853 |
| 1 | 3-27 | KSLWRGVKAIFNGARQGYKEHKNQR | AMP | 0.761 |
| 1 | 4-28 | SLWRGVKAIFNGARQGYKEHKNQRR | AMP | 0.786 |
| 1 | 5-29 | LWRGVKAIFNGARQGYKEHKNQRRE | AMP | 0.661 |
| 1 | 6-30 | WRGVKAIFNGARQGYKEHKNQRREE | AMP | 0.536 |
| 1 | 7-31 | RGVKAIFNGARQGYKEHKNQRREEK | AMP | 0.595 |
| 1 | 8-32 | GVKAIFNGARQGYKEHKNQRREEKL | AMP | 0.575 |
| 1 | 9-33 | VKAIFNGARQGYKEHKNQRREEKLA | AMP | 0.566 |
| 1 | 10-34 | KAIFNGARQGYKEHKNQRREEKLAN | AMP | 0.533 |
| 1 | 11-35 | AIFNGARQGYKEHKNQRREEKLANA | AMP | 0.500 |
| 1 | 12-36 | IFNGARQGYKEHKNQRREEKLANAK | AMP | 0.515 |
| 1 | 13-37 | FNGARQGYKEHKNQRREEKLANAQ | AMP | 0.484 |
| 1 | 14-38 | NGARQGYKEHKNQRREEKLANAKD | AMP | 0.450 |
| 1 | 15-39 | GARQGYKEHKNQRREEKLANAKDM | AMP | 0.358 |
| 1 | 16-40 | ARQGYKEHKNQRREEKLANAKDMQ | AMP | 0.327 |
| 1 | 17-41 | RQGYKEHKNQRREEKLANAKDMQD | AMP | 0.350 |
| 1 | 18-42 | QGYKEHKNQRREEKLANAKDMQDQ | AMP | 0.366 |
| 1 | 19-43 | GYKEHKNQRREEKLANAKDMQDQQ | AMP | 0.404 |
|
| ||||
Jurkat cell line viability was inhibited by an AMPWF3
The MTT assay was used to test the peptide’s anticancer efficacy in Jurkat cells. After treatment with 6.25 µM pleurocidin-like peptide, more than 90% of Jurkat cells survived, and the viability of Jurkat cells decreased substantially as the peptide concentration increased. The IC50 of the pleurocidin-like peptide was 50 µM in Jurkat cells (Fig .2).
Fig 2.
Viability of Jurkat, PBMCs, and HDF cells after treatment with pleurocidin-like peptide (24 hours). PBMCs; Peripheral blood mononuclear cells and HDF; Human dermal fibroblast.
The AMP-WF3 was less cytotoxic against PBMCs and HDF cells compared with Jurkat cells
MTT test was used to determine the cytotoxicity of the pleurocidin-like peptide against normal cells (PBMCs and HDF cells). Following treatment with 6.25 µM of the pleurocidin-like peptide, more than 90% of the cells remained alive, and after treatment with 50 µM, more than 70% remained viable, demonstrating that this pleurocidin-like peptide had minimal cytotoxicity against PBMCs and HDF cells (Fig .2).
Examining necrosis and apoptosis in leukemia cells treated with peptide versus normal cells treated with peptide
A red fluorescent dye called PI was used to precisely mark necrotic cells. A dual marking with Annexin V-FITC/PI was used to examine apoptosis and necrosis. Treatment of Jurkat cells with 50 μM Pleurocidin resulted in 13% early apoptosis, 24% late apoptosis, and 14% necrosis (Fig .3A, B). While HDF cells also showed about 14% early apoptosis, about 16.5% late apoptosis, and 2% necrosis after Pleurocidin treatment (Fig .3C, D). Additionally, around 16.5 % early apoptosis, 9% late apoptosis, and 10% necrosis were observed in PBMCS treated with peptide (Fig .3E, F).
Fig 3.
Based on the annexin assay. A. Flow cytometry of Jurkat cells in the control group, B. Jurkat cells treated with Pleurocidin peptide, C. HDF cells in the control group, D. HDF cells treated with Pleurocidin peptide, E. PBMC cells in the control group, and F. PBMCs cells treated with Pleurocidin peptide. PBMCs; Peripheral blood mononuclear cells and HDF; Human dermal fibroblast.
Expression of genes P53, P21, and BCL-2 in JURKAT cells in response to the AMP-WF3
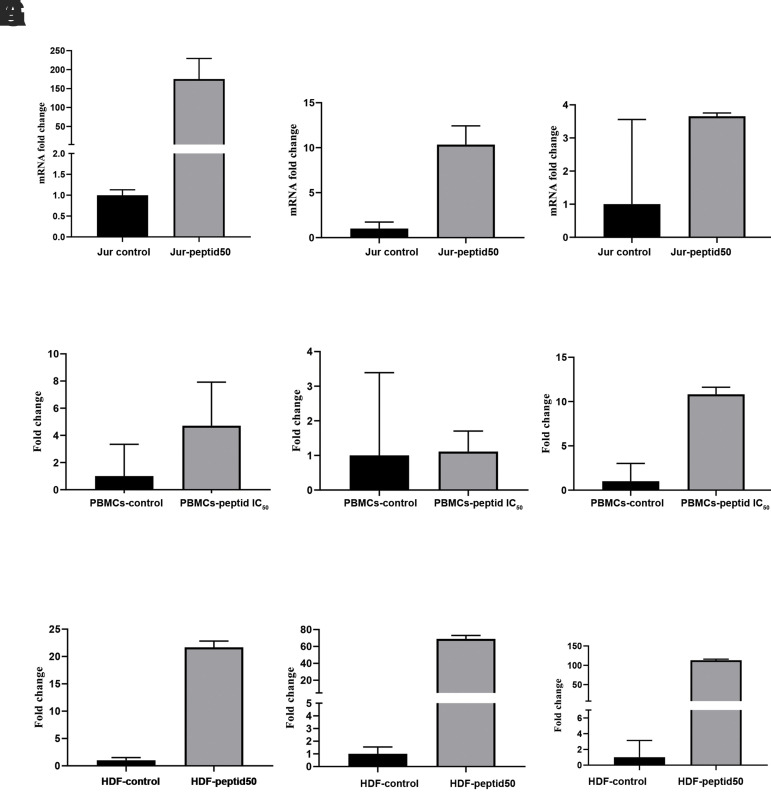
qRT PCR was used to evaluate the expression levels of P21, P53, and BCL-2 in Jurkat cells treated with the AMP-WF3 at a concentration of 50 μM versus untreated cells. P21 and P53 mRNA levels rose considerably after 24 hours in the pleurocidin-like peptide-treated group compared with the untreated group. P21 and P53 gene expression rose by 175.095 and 10.33 folds, respectively (Fig .4A, B).
Fig 4.
mRNA expression of apoptotic and anti-apoptotic genes. A. P21 in Jurkat cells, B. P53 in Jurkat cells, C. BCL-2 in Jurkat cells, D. P21 in PBMCs, E. P53 in PBMCs, F. BCL-2 in PBMCs, G. P21 in HDF cells, H. P53 in HDF cells, and I. BCL-2 in HDF cells. PBMCs; Peripheral blood mononuclear cells and HDF; Human dermal fibroblast. Error bars denote standard deviation.
The treated group’s BCL-2 gene expression was enhanced 3.655-fold compared to the untreated, but this was not statistically significant (Fig .4C).
Effect of the AMP-WF3 on P53, P21, and BCL-2 gene expression in PBMCs
The expression levels of P21, P53, and BCL-2 in PBMCs treated with peptide at a concentration of 50 μM versus control pBMCs were evaluated using qRT PCR. After 24 hours, the expression of P53 and P21 genes in the treated groups did not change compared to the control group, but the expression of BCL-2 in the treated group increased compared to the untreated group (Fig .4D-F).
Effect of the AMP-WF3 on P53, P21, and BCL-2 gene expression in HDF cell lines
qRT PCR was used to compare the expression levels of P53, P21, and BCL-2 in HDF cell lines treated with a 50 μM dose of AMP-WF3 to untreated cells. P21, P53, and BCL-2 expression were significantly higher in the peptide-treated groups than in the control group. P21, P53, and BCL-2 gene expression levels were elevated by 21.67, 68.97, and 112.98 times, respectively, as a result of this (Fig .4G-I).
The comparison of apoptotic and anti-apoptotic gene expression in peptide-treated leukemia and peptidetreated normal cells
P21 gene expression rose 37-fold in the peptide-treated Jurkat cells compared with the peptide-treated PBMCs and 8-fold in the peptide-treated fibroblast cells, both of which were statistically significant (P=0.032 and P=0.026, respectively). P53 gene expression increased 9-fold in the peptide-treated Jurkat cells compared with the peptide-treated PBMCs (P=0.057) and decreased 6.67-fold compared with the peptide-treated fibroblast cells, which was statistically significant (P=0.043). BCL-2 expression of genes raised 7.64-fold in the peptide-treated Jurkat cells compared with the peptide-treated PBMCs (P=0.110) and 1.36-fold in the peptide-treated fibroblast cells (P=0.842) and was not statistically significant.
Discussion
Current anti-cancer medications have many side effects and must be absorbed into the cell to work, so cancer cells can build resistance to them by pumping them out of the cell. Some AMPs, on the other hand, appear to have unique anti-cancer capabilities, making them promising new anti-cancer options. In this study, the effects of the pleurocidin-like peptide on Jurkat cells as a target group and HDF cells and PBMCs as control groups were investigated.
We found that changes in the dose of AMP-WF3 were associated with the lethality of Jurkat cells and that the peptide IC50 in Jurkat cells was 50 μM. Furthermore, this peptide exhibited decreased toxicity in normal PBMC and HDF cells.
The structure of the cell membrane is important in the function of peptides because AMP interaction with cancer cells can disrupt the cell membrane or penetrate the cell and influence intracellular proteins (22).
The cytotoxicity of pleurocidin family members such as NRC-03 and NRC-07 in multiple myeloma cells was studied, and it was shown that these cells were more vulnerable to NRC-03 than to NRC-07 (20). On the other hand, HL60 cell lines were similarly sensitive to different types of peptides NRC of this family (23). Furthermore, a previous study on the effect of pleurocidin peptide on breast cancer cells revealed that, in comparison to leukemia cells, a higher peptide concentration is required to achieve an effective rate of cell death in breast cancer cells, which is likely because breast cancer cells are larger than leukemia cells (20). According to recent research, NRC-03 suppresses OSCC cell proliferation at concentrations between 15 and 75 µg/ml throughout the course of the therapy. While NRC-03 at higher doses, 60 and 75 µg/ml, mainly causes cytotoxic in normal Human Oral Keratinocytes cells (21).
One of the leading causes for the differences in peptide effects on cancer cell types is the negative charge on the cell membrane surface. The outer lipid membrane of Jurkat cells contains high levels of cholesterol, phosphatidylserine, phosphatidylethanolamine, and phosphatidylinositol, all of which are associated with poor prognosis, while the outer surface of normal cell membranes of PBMCs and HDF is mainly made of Phosphatidylcholine and Sphingomyelin, which has zwitterionic properties (24).
The strong bonding and selective breakdown of cancer cell membranes are thought to be promoted by electrostatic adsorption between negatively charged cancer cell components and positively charged AMPs. The cationic anti-microbial peptide concentration is another factor involved in peptide’s action on target cells; at toxic concentrations, the peptide disrupts cancer cell membranes, but at lower concentrations, it enters the cell and activates apoptotic signaling pathways (13).
Although the permeability system of this peptide in the Jurkat cell membrane has not been studied, the behavior of the pleurocidin family in two planar lipid layers has shown that ion channels are formed based on the toroidal model (25).
The combined peptides and lipids generate well-defined pores in the toroidal model. In contrast, the hydrophilic portions of the head groups of peptides and phospholipids face the center of the pores and produce aqueous pores. Due to the tilt of lipid molecules, the membrane bends inward, generating a hole surrounded by lipid head groups and filled by peptides (26). The findings show that peptides combine and establish a channel-like hole throughout the membrane after 2.5 μs of simulations (27).
Resistance to apoptotic processes is a major strategic factor in pathogenesis of acute lymphoblastic lukemia (ALL). As a result, many medications aim to stimulate apoptosis to accomplish the desired outcomes (28). Understanding the molecular processes that generate leukemia might lead to the development of novel treatments that extend the lives of patients (29). Most alpha-helical AMPs primarily inhibit cancer by inducing necrosis and apoptosis (30). P53 protein levels rise in response to DNA-damaging anti-cancer stimulation, and rising p53 levels, in turn, lead to apoptosis. In cells undergoing either p53-mediated G1 arrest or apoptosis, the p21 protein has been observed to be induced (31). P21 is an essential regulator of cell growth arrest, especially when the genome is harmed by damaging agents. However, because P21 plays such a critical function in cell cycle regulation, mutations in this gene are extremely rare (32). Some cationic anti-microbial peptides, such as LL37, are structurally and functionally similar to pleurocidins (15). This peptide inhibits colon cancer by initiating a caspase-independent apoptotic cascade driven by p53 (33). Significantly, treatment of LL-37 with induction of caspase-independent apoptosis kills Jurkat T leukemia cells (34). LL-37 is paradoxically involved in spreading cancers of the breast, ovaries, lungs, prostate, and pancreas (35). Another cationic anti-microbial peptide is Buforin IIb alpha-helix, which leads to the death of prostate cancer cells by decreasing BCL2 and increasing P21 and P53 (36). Another peptide that has an anti-cancer role is temporin-1CE, which in high concentrations, disrupts the plasma membrane and causes the lysis of breast cancer cells. In contrast, its lower levels cause cell death by intracellular mechanisms (37). The pleurocidinlike peptide WF3 was linked to elevated levels of p53 and p21 gene expression in this study, indicating that Jurkat cell growth was reduced.
In earlier studies, p53 activation was shown as a result of DNA damage caused by the CAP reaction and a lack of DNA repair (38).
Bcl-2 is an inhibiting apoptosis protein that promotes cell survival and proliferation by inhibiting the function of pro-apoptotic proteins. In leukemia, the expression of inhibiting apoptosis proteins such as BCL2 is increased (39). The Bcl-2 family of proteins has long been thought to be essential regulators of drug-induced mitochondrial apoptosis. Furthermore, elevated BCL-2 levels have been linked to poor treatment response (40). Our data further showed that in Jurkat cells, AMP-WF3 did not affect the BCL2 gene.
This is the first research to look at the influence of the pleurocidin-like peptide WF3 isoform X2 on the expression of the p53, p21, and Bcl-2 genes, as well as apoptosis and cell growth inhibition in the Jurkat cell line.
Conclusion
In brief, the present investigation demonstrated that AMP-WF3 exhibited good cytotoxicity versus acute lymphoblastic cell line but had lower cytotoxicity toward normal cells. The apoptotic and necrotic activity of AMPWF3 on Jurkat cells was much higher than that of HDF and PBMCs, according to Annexi V-PI. In Jurkat cells, the IC50- AMP-WF3 activates signaling pathways P21 and P53, leading to cell cycle arrest and cell apoptosis, and also did not affect the BCL-2 gene. However, the effect of the peptides on HDF cells showed a high expression of BCL-2 anti-apoptotic protein along with an increase in P53 and P21 protein compared to control cells. The effect of peptides on PBMC cells in a healthy individual did not change the expression of P21 and P53, but the expression of BCL-2 in the treated group showed an increase compared to the untreated group.
Acknowledgements
This study was done with the financial support of the Research Council of Bushehr University of Medical Sciences. There is no conflict of interest to declare.
Authors’ Contributions
Gh.R.Kh., M.M., N.O., M.E.; Participated in study design, contributed to all experimental work, data and statistical analysis, interpretation of data, and reviewed the literature for the manuscript. S.A.M; Contributed extensively to the interpretation of the data and the conclusion. Gh.R.Kh.; Performed editing and approved the final version of this manuscript for submission and approved the final draft. All authors read and approved the final manuscript.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Napier RJ, Norris BA, Swimm A, Giver CR, Harris WA, Laval J, et al. Low doses of imatinib induce myelopoiesis and enhance host anti-microbial immunity. PLoS Pathog. 2015;11(3):e1004770–e1004770. doi: 10.1371/journal.ppat.1004770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szebeni GJ, Balog JA, Demjén A, Alföldi R, Végi VL, Fehér LZ, et al. Imidazo[1,2-b]pyrazole-7-carboxamides induce apoptosis in human leukemia cells at nanomolar concentrations. Molecules. 2018;23(11):2845–2845. doi: 10.3390/molecules23112845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Papo N, Shai Y. Host defense peptides as new weapons in cancer treatment. Cell Mol Life Sci. 2005;62(7-8):784–790. doi: 10.1007/s00018-005-4560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Talati C, Pinilla-Ibarz J. Resistance in chronic myeloid leukemia: definitions and novel therapeutic agents. Curr Opin Hematol. 2018;25(2):154–161. doi: 10.1097/MOH.0000000000000403. [DOI] [PubMed] [Google Scholar]
- 6.Keeney JTR, Ren X, Warrier G, Noel T, Powell DK, Brelsfoard JM, et al. Doxorubicin-induced elevated oxidative stress and neurochemical alterations in brain and cognitive decline: protection by MESNA and insights into mechanisms of chemotherapy-induced cognitive impairment (“chemobrain”) Oncotarget. 2018;9(54):30324–30339. doi: 10.18632/oncotarget.25718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar M, Vineetha R, Kudva A. Medication related osteonecrosis of jaw in a leukemia patient undergoing systemic arsenic trioxide therapy: a rare case report. Oral Oncol. 2019;99:104343–104343. doi: 10.1016/j.oraloncology.2019.06.024. [DOI] [PubMed] [Google Scholar]
- 8.Huang YB, He LY, Jiang HY, Chen YX. Role of helicity on the anticancer mechanism of action of cationic-helical peptides. Int J Mol Sci. 2012;13(6):6849–6862. doi: 10.3390/ijms13066849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohammadi M, Hasan-Abad AM, Dehghani P, Nabipour I, Roozbehani M, Hemphill A, et al. Dicentracin-like from asian sea bass fish and moronecidine-like from hippocampus comes: two candidate antimicrobial peptides against leishmanina major infection.Int J Pept Res Ther. Int J Pept Res Ther; 2021. pp. 769–778. [Google Scholar]
- 10.Hoskin DW, Ramamoorthy A. Studies on anticancer activities of antimicrobial peptides. Biochim Biophys Acta. 2008;1778(2):357–375. doi: 10.1016/j.bbamem.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jenssen H, Hamill P, Hancock RE. Peptide antimicrobial agents. Clin Microbiol Rev. 2006;19(3):491–511. doi: 10.1128/CMR.00056-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leite ML, da Cunha NB, Costa FF. Antimicrobial peptides, nanotechnology, and natural metabolites as novel approaches for cancer treatment. Pharmacol Ther. 2018;183:160–176. doi: 10.1016/j.pharmthera.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 13.Deslouches B, Di YP. Antimicrobial peptides with selective antitumor mechanisms: prospect for anticancer applications. Oncotarget. 2017;8(28):46635–46651. doi: 10.18632/oncotarget.16743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riedl S, Leber R, Rinner B, Schaider H, Lohner K, Zweytick D. Human lactoferricin derived di-peptides deploying loop structures induce apoptosis specifically in cancer cells through targeting membranous phosphatidylserine. Biochim Biophys Acta. 2015;1848(11 Pt A):2918–2931. doi: 10.1016/j.bbamem.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 15.Pundir P, Catalli A, Leggiadro C, Douglas SE, Kulka M. Pleurocidin, a novel antimicrobial peptide, induces human mast cell activation through the FPRL1 receptor. Mucosal Immunol. 2014;7(1):177–187. doi: 10.1038/mi.2013.37. [DOI] [PubMed] [Google Scholar]
- 16.Tiwari BK, Valdramidis VP, O’Donnell CP, Muthukumarappan K, Bourke P, Cullen PJ. Application of natural antimicrobials for food preservation. J Agric Food Chem. 2009;57(14):5987–6000. doi: 10.1021/jf900668n. [DOI] [PubMed] [Google Scholar]
- 17.Patrzykat A, Gallant JW, Seo JK, Pytyck J, Douglas SE. Novel antimicrobial peptides derived from flatfish genes. Antimicrob Agents Chemother. 2003;47(8):2464–2470. doi: 10.1128/AAC.47.8.2464-2470.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshida K, Mukai Y, Niidome T, Takashi C, Tokunaga Y, Hatakeyama T, et al. Interaction of pleurocidin and its analogs with phospholipid membrane and their antibacterial activity. J Pept Res. 2001;57(2):119–126. doi: 10.1034/j.1399-3011.2001.00802.x. [DOI] [PubMed] [Google Scholar]
- 19.Hilchie AL, Doucette CD, Pinto DM, Patrzykat A, Douglas S, Hoskin DW. Pleurocidin-family cationic antimicrobial peptides are cytolytic for breast carcinoma cells and prevent growth of tumor xenografts. Breast Cancer Res. 2011;13(5):R102–R102. doi: 10.1186/bcr3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hilchie AL, Conrad DM, Coombs MR, Zemlak T, Doucette CD, Liwski RS, et al. Pleurocidin-family cationic antimicrobial peptides mediate lysis of multiple myeloma cells and impair the growth of multiple myeloma xenografts. Leuk Lymphoma. 2013;54(10):2255–2262. doi: 10.3109/10428194.2013.770847. [DOI] [PubMed] [Google Scholar]
- 21.Hou D, Hu F, Mao Y, Yan L, Zhang Y, Zheng Z, et al. Cationic antimicrobial peptide NRC-03 induces oral squamous cell carcinoma cell apoptosis via CypD-mPTP axis-mediated mitochondrial oxidative stress. Redox Biol. 2022;54:102355–102355. doi: 10.1016/j.redox.2022.102355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang H, Han D, Lv T, Liu K, Yang Y, Xu X, et al. Novel peptide myristoly-CM4 induces selective cytotoxicity in leukemia K562/ MDR and Jurkat cells by necrosis and/or apoptosis pathway. Drug Des Devel Ther. 2019;13:2153–2167. doi: 10.2147/DDDT.S207224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morash MG, Douglas SE, Robotham A, Ridley CM, Gallant JW, Soanes KH. The zebrafish embryo as a tool for screening and characterizing pleurocidin host-defense peptides as anti-cancer agents. Dis Model Mech. 2011;4(5):622–633. doi: 10.1242/dmm.007310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szlasa W, Zendran I, Zalesińska A, Tarek M, Kulbacka J. Lipid composition of the cancer cell membrane. J Bioenerg Biomembr. 2020;52(5):321–342. doi: 10.1007/s10863-020-09846-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saint N, Cadiou H, Bessin Y, Molle G. Antibacterial peptide pleurocidin forms ion channels in planar lipid bilayers. Biochim Biophys Acta. 2002;1564(2):359–364. doi: 10.1016/s0005-2736(02)00470-4. [DOI] [PubMed] [Google Scholar]
- 26.Huang Y, Huang J, Chen Y. Alpha-helical cationic antimicrobial peptides: relationships of structure and function. Protein Cell. 2010;1(2):143–152. doi: 10.1007/s13238-010-0004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Talandashti R, Mehrnejad F, Rostamipour K, Doustdar F, Lavasanifar A. Molecular insights into pore formation mechanism, membrane perturbation, and water permeation by the antimicrobial peptide pleurocidin: a combined all-atom and coarse-grained molecular dynamics simulation study. J Phys Chem B. 2021;125(26):7163–7176. doi: 10.1021/acs.jpcb.1c01954. [DOI] [PubMed] [Google Scholar]
- 28.Azimi A, Hagh MF, Talebi M, Yousefi B, Hossein pour feizi AA, Baradaran B, et al. Time--and Concentration--dependent effects of resveratrol on mir 15a and mir16-1 expression and apoptosis in the CCRF-CEM acute lymphoblastic leukemia cell line. Asian Pac J Cancer Prev. 2015;16(15):6463–6468. doi: 10.7314/apjcp.2015.16.15.6463. [DOI] [PubMed] [Google Scholar]
- 29.Tzifi F, Economopoulou C, Gourgiotis D, Ardavanis A, Papageorgiou S, Scorilas A. The role of BCL2 family of apoptosis regulator proteins in acute and chronic leukemias. Adv Hematol. 2012;2012:524308–524308. doi: 10.1155/2012/524308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang Y, Feng Q, Yan Q, Hao X, Chen Y. Alpha-helical cationic anticancer peptides: a promising candidate for novel anticancer drugs. Mini Rev Med Chem. 2015;15(1):73–81. doi: 10.2174/1389557514666141107120954. [DOI] [PubMed] [Google Scholar]
- 31.el-Deiry WS, Harper JW, O’Connor PM, Velculescu VE, Canman CE, Jackman J, et al. WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res. 1994;54(5):1169–1174. [PubMed] [Google Scholar]
- 32.LaBaer J, Garrett MD, Stevenson LF, Slingerland JM, Sandhu C, Chou HS, et al. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 1997;11(7):847–862. doi: 10.1101/gad.11.7.847. [DOI] [PubMed] [Google Scholar]
- 33.Ren SX, Cheng AS, To KF, Tong JH, Li MS, Shen J, et al. Host immune defense peptide LL-37 activates caspase-independent apoptosis and suppresses colon cancer. Cancer Res. 2012;72(24):6512–6523. doi: 10.1158/0008-5472.CAN-12-2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mader JS, Mookherjee N, Hancock RE, Bleackley RC. The human host defense peptide LL-37 induces apoptosis in a calpain- and apoptosis-inducing factor-dependent manner involving Bax activity. Mol Cancer Res. 2009;7(5):689–702. doi: 10.1158/1541-7786.MCR-08-0274. [DOI] [PubMed] [Google Scholar]
- 35.Chen X, Zou X, Qi G, Tang Y, Guo Y, Si J, et al. Roles and mechanisms of human cathelicidin LL-37 in cancer. Cell Physiol Biochem. 2018;47(3):1060–1073. doi: 10.1159/000490183. [DOI] [PubMed] [Google Scholar]
- 36.Han Y, Lu M, Zhou J. Buforin IIb induces androgen-independent prostate cancer cells apoptosis though p53 pathway in vitro. Toxicon. 2019;168:16–21. doi: 10.1016/j.toxicon.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 37.Wang C, Zhou Y, Li S, Li H, Tian L, Wang H, et al. Anticancer mechanisms of temporin-1CEa, an amphipathic α-helical antimicrobial peptide, in Bcap-37 human breast cancer cells. Life Sci. 2013;92(20-21):1004–1014. doi: 10.1016/j.lfs.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 38.Cho J, Hwang IS, Choi H, Hwang JH, Hwang JS, Lee DG. The novel biological action of antimicrobial peptides via apoptosis induction. J Microbiol Biotechnol. 2012;22(11):1457–1466. doi: 10.4014/jmb.1205.05041. [DOI] [PubMed] [Google Scholar]
- 39.Marqus S, Pirogova E, Piva TJ. Evaluation of the use of therapeutic peptides for cancer treatment. J Biomed Sci. 2017;25(1):21–21. doi: 10.1186/s12929-017-0328-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sasaki M, Kumazaki T, Tanimoto K, Nishiyama M. Bcl-2 in cancer and normal tissue cells as a prediction marker of response to 5-fluorouracil. Int J Oncol. 2003;22(1):181–186. [PubMed] [Google Scholar]