Abstract
Allergic disease represents one of the most prominent global public health crises of the 21st century. Although many different substances are known to produce hypersensitivity responses, metals constitute one of the major classes of allergens responsible for a disproportionately large segment of the total burden of disease associated with allergy. Some of the most prevalent forms of metal allergy – including allergic contact dermatitis – are well-recognized; however, to our knowledge, a comprehensive review of the many unique disease variants implicated in human cases of metal allergy is not available within the current scientific literature. Consequently, the main goal in composing this review was to (1) generate an up-to-date reference document containing this information to assist in the efforts of lab researchers, clinicians, regulatory toxicologists, industrial hygienists, and other scientists concerned with metal allergy and (2) identify knowledge gaps related to disease. Accordingly, an extensive review of the scientific literature was performed – from which, hundreds of publications describing cases of metal-specific allergic responses in human patients were identified, collected, and analyzed. The information obtained from these articles was then used to compile an exhaustive list of distinctive dermal/ocular, respiratory, gastrointestinal, and systemic hypersensitivity responses associated with metal allergy. Each of these disease variants is discussed briefly within this review, wherein specific metals implicated in each response type are identified, underlying immunological mechanisms are summarized, and major clinical presentations of each reaction are described.
Keywords: Metal allergy, respiratory hypersensitivity, dermatitis, immunotoxicity, occupational health
Introduction
Allergic disease constitutes an enormous global public health burden that has been described by many as the “epidemic of the twenty first century” (Pawankar et al. 2008). Currently, it is estimated that up to 30% of the world’s population is afflicted with some form of allergic disease – the most prevalent manifestations of which include contact allergy, asthma, rhinosinusitis, and food hypersensitivities (Sánchez-Borges et al. 2018). These and other allergic disorders have been continually rising in prevalence over the past several decades in most countries (Asher et al. 2006; Pawankar et al. 2013; Prescott et al. 2013). Concurrently, the average age of disease onset is declining, sensitized individuals are experiencing allergic symptoms with increasing frequency and severity, and the pathogenic mechanisms involved in prototypical hypersensitivity responses are becoming more complex (Heck et al. 2017; Lowe et al. 2017; Mahmoudi, Craig, and Ledford 2019; Simpson et al. 2008; Wu et al. 2011). These trends are believed to be reflective of a widespread shift in humans’ susceptibility to allergy that is increasing as a result of industrialization, modern lifestyle factors, and environmental changes – associations which suggest that the prevalence of allergic disease might continue to increase into the foreseeable future (De Souza, Araujo-Souza, and Leme 2022; Ray and Ming 2020).
Thousands of different substances are capable of inducing allergic disease in humans. Over 4,000 agents have been classified as potential contact allergens and approximately 600 agents to date were identified as potential respiratory allergens (Kurt and Basaran 2020; Martin, Rustemeyer, and Thyssen 2018). Among these substances, some of the most common classes of allergens include environmental proteins, food antigens, animal venoms, drugs, and reactive chemicals. Metals also constitute a major group of sensitizers and are widely-recognized as some of the most frequent inducers of global allergic disease (Lim et al. 2018). It is currently estimated that around 20% of the global population exhibits allergic sensitivity to at least one metal (Schultzel et al. 2020). Accordingly, metal-induced hypersensitivity responses are responsible for a disproportionately large segment of the global burden of allergic disease. Metal allergy also constitutes a major source of occupational illness around the world (Kurt and Basaran 2020).
Metal allergy is a collective term used to describe a subset of allergic conditions that are all similarly mediated by metal-specific adaptive immune responses. Based upon the information reported herein, this allergic conglomerate is comprised of more than 50 unique disease variants involving distinctive biological mechanisms, causative agents, anatomical sites of involvement, and clinical manifestations. Although a few of the most common presentations of these disorders are widely-recognized, the complete spectrum of disease associated with metal allergy is less so. As the prevalence of allergic sensitivity to metals continues to increase globally, failure to recognize the diversity of potential hypersensitivity responses associated with this disorder constitutes a major barrier impeding advances in the development and implementation of effective strategies to manage the disease (Forte, Petrucci, and Bocca 2008).
A comprehensive and up-to-date reference document describing the unique disease variants that have been implicated in human cases of metal allergy has yet to be published within the scientific literature. Consequently, the primary objective in composing this review was to generate a reference document containing this information. This review has potential utility in an extensive number of different applications and diverse settings in which metal-specific hypersensitivity responses present a notable health concern. It has the capacity to become a valuable resource for lab researchers, clinicians, regulatory toxicologists, industrial hygienists, and other scientists in their efforts to understand metal allergy and identify areas where more investigation is needed. The document provides information related to specific allergic hazards imposed by individual metals and identification of potential immunotoxic outcomes in relation to specific routes of exposure to sensitizing metals.
A general overview of metal allergy
The term ‘metal allergy’ refers to a subset of allergic conditions wherein the inciting agent, and thus, immunological memory generated by the adaptive immune system, may be any number of different metal species. According to the Royal Society of Chemistry, the periodic table contains 92 elements that are classified as metals (Yao et al. 2020). Nearly half of these elements have limited or no biological involvement in allergic disease. Approximately 45 total elemental species of metals pose a potential risk of inducing allergic responses and most have been implicated in allergic disease in some capacity (Thyssen and Menné 2010). The Contact Dermatitis Institute’s allergen database currently lists 35 different metal elements as constituents of compounds known to specifically cause contact allergy (Contact Dermatitis Institute). In addition, the World Allergy Organization maintains an updated list of agents capable of inducing respiratory allergy, among which, 11 different metals are listed (World Allergy Organization). The metals identified in these compendiums represent specific entities that have been repeatedly demonstrated to exhibit notable allergenic potential in some or all exposed humans, and accordingly, constitute significant hazards in the context of allergy. Several other metal species not included in these lists have also been implicated in hypersensitivity responses, albeit far less frequently, and similarly, pose a lower threat level than other sensitizing metals.
The most fundamental requirement to induce sensitization of naïve subjects or elicit allergic responses in existing disease states is allergen exposure. In this context, metal allergy represents a particularly unique subset of allergic disease since metals are routinely encountered by all major exposure routes. Dermal contact with metals and metal-containing objects is inevitable for most members of today’s society since these constitute a class of materials indispensable to modern life. The skin is continuously exposed to metals as a result of their incorporation into cosmetics, tools, and personal electronics, as well as their countless applications in the transportation, biomedical, housing and construction sectors (Garner 2004; Hostýnek et al. 1993; Kim et al. 2021). In addition, since metal particulates become suspended in the ambient air as a result of both natural and anthropomorphic processes, inhalation exposure to these substances is also common (Nemery 1990). Ingestion of metals also occurs frequently. Many metals are essential elements of the human diet, and as major constituents of the Earth’s crust, they are often present in both foods and drinking water (Kavcar, Sofuoglu, and Sofuoglu 2009). Finally, in some instances, systemic exposure to metals may occur as a result of their absorption following exposure and/or use in various parenteral biomedical applications (Bijukumar et al. 2018; De Brouwere et al. 2012). Due to the propensity for metals to be encountered by all routes of exposure, and subsequently, capable of absorption by all 4 major portals of entry into the body, metal-induced hyper-sensitivity responses might develop in many different anatomical compartments.
In accordance with the topic of this review, it is important to distinguish between hypersensitivity reactions and other types of immunological responses that may develop following exposure to metals. Metals constitute a group of toxicants with the capacity to elicit a wide variety of distinctive adverse health effects mediated by numerous unique biological mechanisms, which may manifest in any tissue of the body (Borowska and Brzoska 2015; Mamtani et al. 2011; Mizutani et al. 2016). Similarly, toxic responses implicating the immune system as the primary target tissue represent only one of many possible adverse outcomes following exposure to metals (Di Gioacchino et al. 2007). Further, of the many different immunotoxic responses that may emerge, allergic reactions represent only a single potential outcome. Localized inflammatory reactions often develop following exposure to metals, and while these responses may appear indiscernible from symptomology of allergic reactions, these usually involve nonspecific mechanisms of immune responsivity and are mediated exclusively by cells of the innate immune system. In contrast, true hypersensitivity responses involve inflammatory reactions that are antigen-specific, driven by previously-generated immunological memory, and are primarily mediated by cells of the adaptive immune system (Bircher 2018). In accordance with this distinction, this review is focused on the latter immune responses.
Like all allergic disorders, metal allergy involves two distinctive stages of disease pathogenesis (Nauta et al. 2008). The first phase – sensitization – entails a subclinical cascade of immunological events prompted by an initial exposure, during which, antigen-specific immunological memory is generated. Following the completion of this process, the elicitation phase ensues, wherein subsequent antigen encounters trigger activation of the adaptive immune system and pre-established effector mechanisms intended to neutralize the allergen. These mechanisms mediate the emergence of prototypical allergic signs and symptoms – often the first discernable indication of an individual’s new allergic disposition (Anderson, Siegel, and Meade 2011).
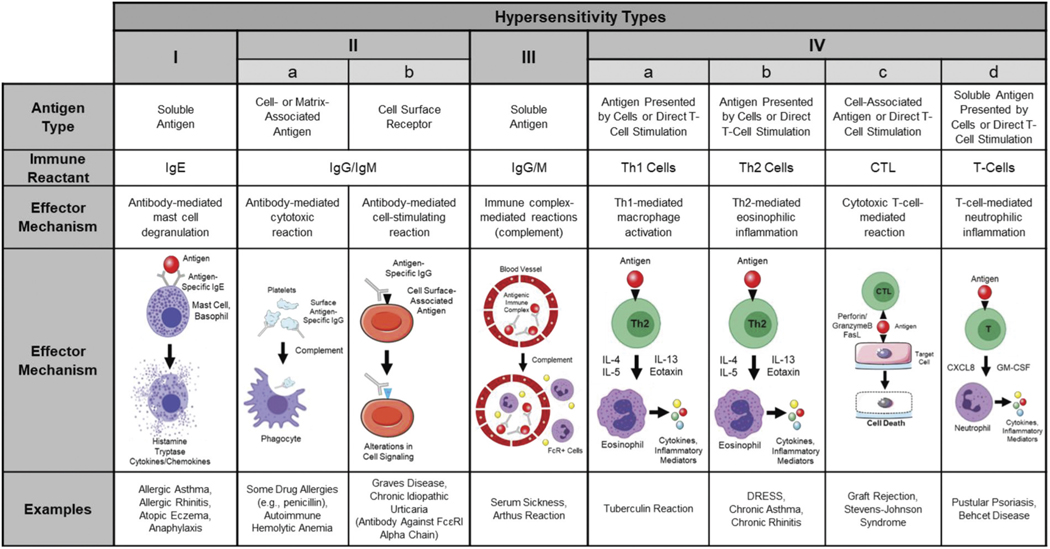
Exposure to allergenic metals and subsequent sensitization may lead to generation of many unique immunological mechanisms responsible for effector functions during the elicitation phase of metal hypersensitivity. Collectively, these different reactions might be broadly grouped based upon an existing paradigm utilized by immunologists to characterize allergic responses. The Gell and Coombs classification scheme was originally proposed in the 1960s but remains the most commonly-used approach for describing different classes of hypersensitivity responses to date (Coombs 1968; Gell 1963). Based upon this paradigm, 4 major types of hypersensitivity responses exist – some of which encompass additional response variants (Figure 1). Metals constitute a class of antigens with the capacity to produce all 4 types of hypersensitivity responses.
Figure 1.
The different types of hypersensitivity responses based on the Gell and Coombs classification scheme. Type I hypersensitivity reactions are mediated by antigen-specific IgE molecules that facilitate degranulation of mast cells following antigen exposure. Type II hypersensitivity reactions are mediated by antigen-specific IgG/M molecules that recognize cell-associated antigens, causing destruction of the target cell (type IIa) or alterations in target cell functionality (type IIb). Type III hypersensitivity responses involve soluble antigen recognition by IgG/M molecules. This leads to the formation of antigen/immune complexes that can deposit in various tissues of the body, activate complement, and cause local tissue damage. Type IV hypersensitivity reactions involve the effector functions of various subsets of T-lymphocytes. Type IVa responses are mediated by CD4+ Th1 cells and result in activation of macrophages. Type IVb reactions involve the actions of CD4+ Th2 cells, which promote eosinophilic inflammation. Type IVc responses implicate CD8+ CTLs, which exert direct cytotoxic effects on target cells. Finally, type IVd reactions are mediated by various subsets of T-cells that facilitate the development of neutrophilic inflammation.
Type I hypersensitivity responses are immediate-type allergic reactions mediated by antigen-specific immunoglobulin (Ig)E molecules. In sensitized individuals, B-cells produce these antibodies, which are then bound by FcεRI (high affinity IgE receptor) molecules expressed on granulocyte cell surfaces including mast cells and basophils. Antigen exposure facilitates IgE cross-linking, and subsequently, cellular degranulation (Murphy, Travers, and Walport 2012). Preformed molecular mediators, including histamine, tryptase, and various cytokines/chemokines, are released during this process and are responsible for the prototypical physiological alterations such as vasodilation or bronchoconstriction observed during this type of allergic response (Hausmann, Schnyder, and Pichler 2010; Moon, Befus, and Kulka 2014). Some examples of type I hypersensitivity responses include anaphylaxis, allergic asthma, allergic rhinitis, and contact urticaria.
Type II and III hypersensitivity responses are also considered immediate-type, antibody-mediated immune reactions; however, antigen-specific IgG (or IgM) molecules constitute the primary effectors in these reactions (Descotes and Choquet-Kastylevsky 2001). The major discerning feature of type II and III hypersensitivity reactions is the major antigen type, which tends to be cell- or matrix-associated proteins in type II responses and soluble antigens in type III reactions.
Two distinctive subsets of type II hypersensitivity reactions have been characterized. Both reaction types involve recognition of cell surface- or matrix-associated antigens by specific IgG/M molecules and subsequent destruction of the target cell or alterations in cellular functionality, which may or may not be accompanied by significant tissue damage (Murphy, Travers, and Walport 2012). Type IIa responses are often referred to as “cytotoxic” allergic responses. In these reactions, antigen-specific IgG/M molecules bind cell-associated proteins (antigen), triggering the activation of complement and subsequent destruction of the target cells (Uzzaman and Cho 2012). Examples of this response type include autoimmune hemolytic anemia and some drug allergies. Comparatively, type IIb responses involve antibody-mediated cell-stimulating reactions, wherein IgG/M molecules recognize cell surface- or matrix-associated antigens expressed by the target cell, following which, normal cell signaling processes may become augmented. This type of allergic response is associated with Grave’s disease and chronic idiopathic urticaria.
In type III hypersensitivity reactions (also called immune complex-mediated allergic reactions), host antibodies recognize and bind soluble antigen, forming a complex that may deposit within various tissues of the body, including blood vessel walls (Dispenza 2019). These complexes trigger complement activation, leading to local inflammation and tissue injury. Serum sickness and Arthus reactions constitute two of the most common manifestations of type III hypersensitivity reactions.
Finally, type IV hypersensitivity responses involve delayed-type allergic reactions mediated by antigen-specific T-lymphocytes. Four subtypes of type IV allergic responses have been described – each of which involves distinctive underlying immunological mechanisms orchestrated by different subsets of effector T-cells. Type IVa reactions involve the actions of CD4 + T helper 1 cells (Th1) and subsequent activation of macrophages (Phillips et al. 2019). Type IVb responses are mediated by T helper 2 cells (Th2) and facilitate eosinophilic inflammation. Type IVc hypersensitivity responses involve CD8+ cytotoxic T-lymphocytes (CTL) with direct cell-killing capabilities. The final subtype of delayed hypersensitivity reactions, type IVd responses, implicate T-cell-induced neutrophilic inflammation (Hausmann, Schnyder, and Pichler 2010). Common examples of type IV hypersensitivity responses include allergic contact dermatitis (ACD), tuberculin reactions, and Stevens-Johnson syndrome.
The earliest descriptions of metal allergy date back to the late 1800s (Thyssen et al. 2021). All preliminary reports of the disease selectively described cases of skin reactions in workers exposed to metals in their workplaces. Accordingly, allergic sensitivity to metals was initially recognized as a health concern preferentially associated with workers involved in activities such as electroplating, welding, smelting, and mining. This paradigm remained unchallenged for the first half of the 20th century; however, by the 1950s, cases of contact allergy to metals began emerging in the general population (Thyssen and Menné 2010). Industrialization and increased incorporation of metals into consumer goods led to increases in the frequency of cutaneous exposures to metals in the general public. For some metals – particularly those present in jewelry and stocking suspenders – the primary afflicted population shifted from male workers to females within the general population by the 1950s and 1960s. By the 1970s and 1980s, an elevation in prevalence of dermatitis had also become evident within the male segment of the general public as jeans began incorporating zippers and buttons capable of facilitating cutaneous exposures to sensitizing metals. Most countries experienced continuous increases in the prevalence of metal sensitivity in subsequent years, and by the 1990s, rates of allergic responsivity to some metals exceeded 30% in certain subsets of the global population (Thyssen and Menné 2010). Consequently, metal allergy became widely-recognized as a prominent public health concern in most nations by the 1990s.
Currently, in the 21st century, metal allergy remains a major health concern from both a public health standpoint and an occupational safety perspective. In the general public, dermal hypersensitivity responses constitute the most prevalent form of metal allergy (Thyssen and Menné 2010). Interestingly, contact sensitivity to metals has been identified as one of the most consistently problematic disorders around the globe and, unlike many diseases and other types of allergy, it is endemic to both industrialized and developing nations (Forte, Petrucci, and Bocca 2008). Studies originating from countries in North and South America, Europe, the Middle East, Africa, Asia, and the South Pacific all demonstrated that allergenic metals are some of the most common inducers of contact sensitivity amongst their respective citizen populations (Almutairi and Almutawa 2017; Belloni Fortina et al. 2015; Duarte et al. 2013; Goon 2018; Goon and Goh 2005; Mahler, Geier, and Schnuch 2014; Mirembe et al. 2016; Rui et al. 2013; Thyssen et al. 2010; Tiwari et al. 2018; Warshaw et al. 2013). Moreover, in all of these countries, the same 4 metals – nickel, cobalt, chromium, and gold – were identified to be responsible for nearly all cases of metal sensitivity (Cheng et al. 2008; Schuttelaar et al. 2018). In recent years, a few other general trends have been identified regarding the global public health burden imposed by metal allergy. For example, it is widely recognized that women are significantly more likely to develop metal allergy than men within the general population (Wöhrl et al. 2003). In addition, the risk of developing skin sensitivity to metal allergens has been positively correlated with the number of ear and/or body piercings by an individual (Ehrlich, Kucenic, and Belsito 2001; Markel et al. 2020). Finally, metal allergy is known to afflict all age groups within the general population, inducing disease in newborns and infants, toddlers and children, adolescents, adults, and the elderly (Cardona et al. 2011; Tuchman et al. 2015).
Sensitizing metals are also responsible for a large proportion of allergic responses reported specifically in worker populations (Warshaw et al. 2019). The profile of disease implicated in cases of metal allergy where occupational exposures are responsible for symptom emergence bears several unique features that differ from trends associated with the disease in the general population. For example, while most cases of metal allergy in the general public result from dermal contact with metals, occupationally-relevant cases might involve multiple routes of exposure (Bright et al. 1997; Fontenot and Amicosante 2008). Common industrial applications for metals not only facilitate skin contact, but also respiratory exposures in workers (Raulf et al. 2016). Consequently, both dermal and respiratory hypersensitivity responses to metals are frequently observed in occupational settings (Cristaudo et al. 2005; Fernandez-Nieto et al. 2006). In addition, while only a small number of metals are known to be responsible for most cases of metal allergy in the general public, workplace-associated cases tend to implicate a far greater number of metal allergens since the diversity of metals present in occupational settings is more extensive and in higher concentration than those likely to be encountered by the general public (Bircher 2018). Collectively, these discrepancies illustrate some of the distinctive hazards and challenges uniquely associated with occupationally-relevant cases of metal allergy.
A comprehensive list of disease variants associated with metal allergy
A comprehensive review of the scientific literature was performed and publications describing confirmed cases of metal allergy in human subjects were compiled. These reports were then analyzed and grouped according to similarities in the major route of exposure and primary anatomical site of allergic response manifestation described in each case. This categorical approach constitutes the organizational framework for the following sections, which describe the various dermal, respiratory, gastrointestinal (GI), and systemic hypersensitivity responses that have been implicated in metal allergy. For each of these categories of responses, the general prevalence of metal-induced allergic reactivity is noted, relevant sources of metal exposure are summarized, and subsets of the population at increased risk for disease are identified where applicable. An exhaustive compilation of distinctive metal-induced allergic responses associated with the corresponding biological compartment is then provided. Each of these unique disease manifestations is discussed briefly, wherein underlying immunological mechanisms, prototypical signs and symptoms, and specific metal entities implicated in each response type are highlighted. A summarized list of these conditions is provided in Table 1.
Table 1.
Summary of Disease Variants Implicated in Metal Allergy.
| Tissue | Primary Disease Presentation | Disease Variant(s) |
|---|---|---|
|
| ||
| Dermal | Allergic Contact Dermatitis (ACD): | |
| Hypersensitivity | Eczematous ACD | Nummular Dermatitis, Dyshidrotic Eczema, Recurrent Vesicular Hand Eczema Pruritic Papular, Lymphomatous, Lymphomatoid-Eosinophilic, Depigmented |
| Responses | Non-Eczematous ACD | Contact Dermatitis, Palmoplantar Pustulosis |
| Photo-Mediated ACD | ||
| Allergic Granulomatous Skin Reactions | ||
| IgE-Mediated Allergic Skin Responses: | ||
| Atopic Dermatitis/Contact Urticaria | ||
| Photo-Mediated Contact Urticaria | ||
| Oral Mucosal Allergy | Allergic Contact Stomatitis, Oral Lichen Planus, Orofacial Granulomatosis, Burning Mouth Syndrome, Geographic Tongue, Peri-Oral ACD, Allergic Contact Cheilitis | |
| Ocular Allergy | Allergic Conjunctivitis, Eyelid Contact Dermatitis, Atopic Keratoconjunctivitis, Contact Blepharoconjunctivitis | |
| Metal Hypersensitive Alopecia Areata Bullous Autoimmune Dermatoses: | ||
| Pemphigus | ||
| Pemphigoid | ||
| Respiratory | Allergic Asthma | Antibody-Mediated (IgE, IgG), Cell-Mediated; Th2/Eosinophilic, /Th1/17/Neutrophilic |
| Hypersensitivity | Allergic Rhinitis | |
| Responses | Immediate Hypersensitivity Laryngitis | |
| Hypersensitivity Pneumonitis: | ||
| Chronic Beryllium Disease | ||
| Gold Lung | ||
| Hard Metal Lung Disease | ||
| Pulmonary Eosinophilia | Eosinophilic bronchitis, Eosinophilic Pneumonia | |
| GI Hypersensitivity | Contact Allergic Mucositis/Gastritis | |
| Responses | Allergic Esophagitis: | |
| Eosinophilic Esophagitis | ||
| Lymphocytic Esophagitis | ||
| Systemic | Anaphylaxis | Type I, Mixed-Type Anaphylaxis |
| Hypersensitivity | Systemic ACD | Baboon Syndrome |
| Responses | Airborne ACD | |
| Chronic Urticaria Syndrome | ||
| Systemic Nickel Allergy Syndrome | ||
| Drug Reaction with Eosinophilia and Systemic Symptoms Syndrome | ||
| Kounis Syndrome | ||
| Systemic Sensitization | Implant Failure, Metallosis | |
Table 1: A comprehensive list of unique disease variants implicated in metal allergy. An extensive review of the scientific literature was performed and all reports describing metal-induced hypersensitivity responses in human subjects were compiled. All of the unique disease variants implicated in these publications are discussed in this manuscript and summarized in the table above. Responses are grouped according to primary tissue of involvement, as either dermal, respiratory, gastrointestinal, or systemic hypersensitivity reactions. Distinctive clinical manifestations and disease variants associated with specific conditions are listed where applicable.
In addition to the many direct manifestations of metal allergy, several other inflammatory conditions have also been identified within the scientific literature as disorders in which the concurrent existence of allergic sensitivity to metals has the capacity to play an indirect, but significant role in disease pathogenesis. These conditions are also highlighted and briefly discussed in the following sections, where applicable. A summarized list of these disease states is provided in Table 2.
Table 2.
Inflammatory Conditions in Which Metal Allergy May Indirectly Contribute to Disease Pathogenesis.
| Tissue | Disease |
|---|---|
|
| |
| Dermal | Rosacea |
| Responses | Psoriasis |
| Respiratory | Pulmonary Alveolar Proteinosis |
| Responses | Goodpasture’s Syndrome |
| GI | Celiac Disease |
| Responses | Gastro-Esophageal Reflux Disease Irritable Bowel Syndrome Ulcerative Colitis |
| Systemic Responses |
Systemic Lupus Erythematosus Rheumatoid Arthritis Chronic Fatigue Syndrome Sjogren’s Syndrome Goodpasture’s Syndrome Fibromyalgia Panniculitis Cutaneous T-Cell Lymphomas |
Table 2: A list of inflammatory conditions in which metal allergy may contribute to disease pathogenesis. In addition to the primary presentations of metal allergy listed in Table 1, allergic responsivity to sensitizing metals has also been indirectly associated with several other disease states. These conditions are listed in the table above, in accordance with the tissue/anatomical compartment of relevance. Although it remains largely unclear what role metal allergy plays in the pathogenesis of these disorders, ample evidence exists within the scientific literature to suggest that, in some cases, metal allergy can promote the development, progression, and severity of symptomology in these disease states.
Metals and topical hypersensitivity responses
Hypersensitivity responses of the skin constitute the most common form of metal allergy worldwide (Chen and Thyssen 2018). This is reflective of the ubiquitous nature of metal-containing items that regularly come into contact with the skin, which might facilitate absorption of immunogenic metalions through the epidermal barrier (Berardesca 2002; Lansdown 1995). Moreover, many items containing metals remain in contact with the skin for extended durations of time when used, increasing the magnitude of ion release and subsequent absorption. In the general population, jewelry, electronics, cosmetics, and clothing components (fasteners, buttons, snaps, zippers, etc.) constitute some of the major sources of dermal exposure to metals (Bocca and Forte 2009).
Skin contact with metals also occurs frequently in occupational settings, rendering dermal manifestations of metal allergy also a major concern in the workplace. Bankers, machine operators, cosmetologists, electroplaters, and healthcare workers are all known to be at increased risk for developing contact allergy to metals (Kanerva, Estlander, and Jolanki 1998; Kanerva et al. 1997; Thyssen et al. 2013; Warshaw et al. 2019). The risk of skin sensitization is also particularly high in workers that are required to perform their duties in environments with elevated temperatures (Sasseville 2008). In these individuals, elevations in core body temperature can result from excess heat that is generated either as a direct result of work activities – such welding or electroplating – or as a result of external working conditions, like those encountered by construction workers when working outdoors in the summer months. As body temperature rises and greater quantities of sweat are produced, many metal-containing items or compounds present on the skin undergo accelerated dissolution, enhancing the potential cumulative dose of the soluble metal form capable of penetrating the skin (Stefaniak et al. 2014). Consequently, these workers are especially vulnerable to skin sensitization by metals.
Many different types of metal-specific hypersensitivity responses might manifest in the skin and other topical surfaces of the body such as the eye, and will be discussed individually in the following sections. Some of the major defining features of these responses include the underlying immunological mechanism include type I, II, III, IV hypersensitivity and anatomical site(s) of involvement such as inflammation restricted to the site of contact or widespread skin eruptions. A summary of the most common topical hypersensitivity responses and corresponding metals associated with each condition is shown in Table 3.
Table 3.
Specific Metals Associated with Different Presentations of Dermal/Ocular Hypersensitivity.
Allergic Contact Dermatitis
Contact dermatitis is an inflammatory skin condition comprised of two major disease subsets that are mediated by distinctive immunological mechanisms. In cases of irritant contact dermatitis, dermal contact with skin irritants triggers the emergence of localized, nonspecific skin inflammation that becomes evident shortly after exposure (min to hr) (Tan, Rasool, and Johnston 2014). Cobalt is the metal most commonly implicated in this variant of dermatitis (Turčić, Marinović-Kulišić, and Lipozenčić 2013). Comparatively, ACD involves the elicitation of adaptive immune-mediated, antigen-specific skin inflammation at the site of exposure and represents the primary dermatitis subset of interest in the context of metal allergy.
ACD is a delayed-type hypersensitivity response of the skin that is exceptionally common, having been estimated to impact approximately 20–25% of the world’s population (Peiser et al. 2012). The disease is frequently observed in both the general public and working populations (Anderson, Long, and Dotson 2017). ACD is a disorder primarily produced by dermal contact with low molecular weight (LWM) sensitizers, which penetrate the uppermost layers of the epidermis and induce allergic sensitization via the skin (Fluhr et al. 2004). A population of antigen-specific T-cell clones is generated during ACD development, and upon future antigen encounters, these cells become activated, orchestrating inflammatory responses at the site of exposure in an attempt to destroy the antigen. Within 48–72 hr exposure, signs of the response become evident, presenting as eruptions of localized dermal inflammation (Bocca and Forte 2009).
Reactive chemicals represent one of the major classes of allergens most commonly implicated in ACD (Uter et al. 2018). Hair dyes, fragrances, preservatives, adhesives, and surfactants are all classes of chemicals with well-documented potential for dermal sensitization (Acer et al. 2020; Milam and Cohen 2019). Only one class of allergens is consistently implicated more frequently than reactive chemicals in cases of ACD – the sensitizing metals (Boonstra et al. 2015; Chen and Thyssen 2018). It has been estimated that approximately 20% of the general population exhibits delayed-type skin responses to at least one metal (Schuttelaar et al. 2018). Nickel (Ni), cobalt(Co), gold (Au), and chromium (Cr) account for the majority of these cases, and rates of sensitivity to these metals tend to be conserved between most countries (Ahlstrom et al. 2019; Davis et al. 2011).
Eczematous ACD: Several different clinical manifestations of ACD have been described to account for discrepancies in the nature of contact with the inciting antigen, pathophysiology of skin lesions, and anatomical sites of skin involvement (Pongpairoj et al. 2016). The most common clinical pattern of ACD reactions involves development of rashes described as ‘eczematous lesions,’ which present with visible erythema and itching and may result in blistering and chapping of the skin in exposed areas (Li and Li 2021). The specific metals most frequently implicated in prototypical eczematous forms of ACD include Ni, Co, and Cr (Turčić, Marinović-Kulišić, and Lipozenčić 2013). Similar cases of ACD initiated by palladium (Pd) and Au have also become increasingly recognized in recent years. Although reported far less frequently, this type of ACD was also associated with aluminum, beryllium, copper, iridium, rhodium, platinum, zirconium, and titanium in some individuals (Forte, Petrucci, and Bocca 2008; Mencia and Cawich 2021; Tous Romero et al. 2021).
A few specific clinical variants of eczematous ACD responses were observed and correlated with metal exposure. For example, nummular dermatitis describes the emergence of coin-shaped scaly patches of skin, primarily on the legs and buttocks, that do not itch (Kapur, Watson, and Carr 2018). Nickel, Co, and Cr are the three metals most commonly implicated in this variant of ACD (Bonamonte et al. 2012). The same three metals were also implicated in cases of dyshidrotic eczema (pompholyx) and recurrent vesicular hand eczema – chronic, intermittent forms of dermatitis that impact fingernails, hands, and feet (Boonstra et al. 2015; De Boer, Bruynzeel, and Van Ketel 1988; Nishizawa 2016; Stuckert and Nedorost 2008; Veien 2009; Veien et al. 1994; Vien and Kaaber 1979).
Non-Eczematous ACD: The other major clinical pattern of ACD involves the emergence of ‘non-eczematous’ responses (Pongpairoj et al. 2016). These types of reactions can include lichenoid, depigmented, bullous, and neutrophilic or eosinophilic ACD reactions (Li and Li 2021). Gold is a metal that has been associated with numerous different variants of this type of contact dermatitis. Similarly, soluble gold compounds and gold jewelry were reported to produce pruritic papular dermatitis, lymphomatous reactions, and lymphomatoid-eosinophilic responses (Conde-Taboada et al. 2007; Iwatsuki et al. 1982; Park et al. 1999; Sperber et al. 2003). Another clinical variant of non-eczematous ACD that selectively affects the palms and soles – palmoplantar pustulosis – might occur in subjects sensitive to Co, zinc (Zn), as well as other metals that have been associated with dentistry (Brunasso and Massone 2021; Song, Yin, and Ma 2011; Yanagi et al. 2005). Finally, depigmented contact dermatitis has also been associated with Ni sensitivity (Kim et al. 1991).
Photo-Mediated ACD: Another notable variant of ACD is known to emerge only under specific conditions that constitute its classification as a form of photoallergy. In this type of ACD, the formation of immunologically-active antigenic determinants is dependent upon a chemical transformation event. Most often, this involves deposition of low molecular weight (LMW) chemicals (referred to as prohaptens) onto the skin, where they remain biologically inert until exposed to ultraviolet (UV) or visible light (Kerr and Ferguson 2010). Subsequent chemical modifications result in the generation of haptenic entities, which then bind with proteins of the skin to form complete antigenic determinants. These allergens then trigger prototypical eczematous eruptions that are indistinguishable from non-photoallergy-mediated ACD responses (Li and Li 2021).
Only a few metals have been associated with photo-mediated forms of ACD and nearly all cases have been reported in workers that conduct their occupational duties in outdoor spaces. In one such report, a bricklayer with chronic and severe eczema exhibited a negative patch test result to Co, but a strong positive reaction to irradiated cobalt (Camarasa and Alomar 1981). In this instance, the elicitation of ACD reactions was uniquely specific for UV-transformed Co, which is often described as ‘photosensitization.’ Comparatively, several other cases of photoallergy to Co were described in which workers exhibit contact sensitivity to cobalt both in the presence and absence of sunlight (Romaguera et al. 1982). In many of these cases, metal exposure results in ACD eruptions, but concurrent exposure of the contact areas to sunlight might result in more severe reactions. This type of reaction is often referred to as ‘photoaggravation’ and was also found in workers with photosensitivity to Cr (Manciet et al. 2006).
Allergic granulomatous skin responses
Allergic granulomatous reactions are another form of contact allergy associated with metals. These responses involve the development of granulomas – which are defined as small, localized nodules that contain large cellular infiltrates comprised mostly of macrophages – in the dermis/hypodermis following contact with allergens (Beretta-Piccoli et al. 2018). Their development is known to involve delayed-type hypersensitivity mechanisms and Th1-related immune signaling pathways, similar to the mechanisms involved in ACD. Accordingly, allergic granuloma formation is sometimes reflective of a unique clinical manifestation of ACD; however, in other subjects with no history of ACD, granulomatous skin responses may represent the existence of a distinctive inflammatory skin condition, such as granuloma annulare or granulomatous dermatitis (Ţăranu et al. 2017; Tronnier and Mitteldorf 2015). Zirconium, aluminum (Al), titanium (Ti), Au, Co and Pd are all specific metals that were implicated in the development of hypersensitivity granulomas in the skin of sensitized subjects (Armstrong, Walsh, and Dawson 1997; Casper, Groth, and Hunzelmann 2004; Epstein and Allen 1964; Goossens et al. 2006; High et al. 2006; Lauren et al. 2016; Lopez et al. 1994; Martin et al. 1990; Mehta and Balachandran 2010; Montemarano et al. 1997; Skelton et al. 1993).
IgE-mediated allergic responses of the skin
Although the most common presentations of dermal hypersensitivity are mediated by delayed-type, cell-mediated mechanisms, immediate-type allergic skin responses involving antigen-specific IgE molecules also occur. Similar to ACD responses, these immediate-type skin reactions generally emerge following topical exposure to the agent and result in localized inflammatory reactions at the exposure site; however, the onset of clinical presentations and underlying mechanisms responsible for these reactions differ between the two response types. Antigen-specific IgE antibodies constitute the primary mediators of allergic response elicitation in these immediate-type allergic skin conditions, and accordingly, facilitate the emergence of visible eruptions within 30–60 min exposure (Li and Li 2021). By 24 hr, complete resolution of these reactions is generally observed.
Many metal species have been implicated in IgE-mediated allergic skin responses; however, these reactions were noted using inconsistent nomenclature within the scientific literature, complicating the interpretation of collective findings from these studies. In most of the existing publications, IgE-mediated dermal hypersensitivity responses elicited by metals are preferentially recognized as a variant of either atopic dermatitis or contact urticaria. Although these two allergic responses frequently entail similar clinical presentations of antigen-specific immunological responsivity and are often used interchangeably to describe immediate-type allergic skin responses orchestrated by IgE molecules, they constitute unique allergic entities.
Atopic dermatitis is a common allergic disorder associated with a lifetime prevalence of 15–30% (Pawankar et al. 2013). The primary clinical presentation implicated in this condition is the eruption of localized pruritic inflammatory reactions immediately following skin contact with antigen (Gaudinski and Milner 2017). The pathophysiological mechanisms responsible for atopic dermatitis are exceptionally complex; however, it is now well-accepted that both skin barrier dysfunction and immune dysregulation are two of the major contributing factors responsible for sustaining chronic skin inflammation in this condition (Kapur, Watson, and Carr 2018). Structural deficits in the epithelial barrier mediate polarization of local immune responses toward a Th2-dominant state and facilitate penetration of larger, high molecular weight (HMW) protein antigens through the skin. These effects ultimately promote dermal sensitization, but this process generally results in the production of antigen-specific IgE molecules, as opposed to antigen-specific T-cells like in ACD (Mocanu et al. 2021). Unlike other forms of contact allergy, atopic dermatitis as a condition is correlated with atopy – the genetic predisposition to generate IgE antibodies following exposure to common environmental proteins (e.g., pollens, dust mites, and food antigens) (Thomsen 2015). For many individuals, atopic dermatitis often constitutes one of the first indications of an atopic disposition since it tends to emerge early in life. Approximately 45% of cases are diagnosed in infants under 6 months of age and 85% of cases emerge by the age of 5 (Mocanu et al. 2021). Most subjects diagnosed with atopic dermatitis eventually develop additional allergic comorbidities associated with the atopic march such as asthma, rhinitis, and food allergy.
Contact urticaria is another common allergic response that is associated with a lifetime prevalence of over 20% (Pawankar et al. 2013). This reaction type also implicates development of immediate-type allergic skin inflammation at the site of antigen contact. Several different subtypes of urticaria were described, but the defining feature of these reactions is the emergence of angioedema and distinctive skin lesions referred to as ‘wheals and flares’ within approximately15 min of exposure, which tend to last for a few hr (Gaudinski and Milner 2017; Hon et al. 2019). These skin reactions result from dermal edema caused by vascular dilation and leakage of fluid into the skin following degranulation of mast cells, which might occur in response to antigen-induced dimerization of surface-bound IgE molecules, or via other mechanisms (Hennino et al. 2006).
Collectively, atopic dermatitis represents a well-established immunological disorder associated with the genetic predisposition for atopy, a distinctive timeline of disease emergence, concurrent existence of allergic comorbidities, and characteristic clinical symptoms that include immediate-type allergic responses of the skin following contact with antigen. Comparatively, the term ‘contact urticaria’ is generally used to refer to a specific clinical presentation of dermal hypersensitivity responses in which localized angioedema and eruption of wheal and flare-type lesions is observed immediately following dermal contact with antigen. Despite these subtle but fundamental discrepancies, the terms ‘atopic dermatitis’ and ‘contact urticaria’ are often used interchangeably to describe hypersensitivity responses involving localized, rapidly-emerging inflammatory skin reactions following allergen contact. Accordingly, case reports describing presentations of metal allergy in the context of either of these response types are discussed collectively in this review (Pongpairoj et al. 2016).
Many of the same metals associated with ACD responses have also been implicated in immediate-type allergic reactions of the skin – though far less frequently. Similar to ACD, the metal most commonly-associated with atopic and urticarial skin responses is Ni (Turčić, Marinović-Kulišić, and Lipozenčić 2013). Cobalt, copper (Cu), Cr, mercury (Hg), Al, Pd, and platinum (Pt) are also known to initiate immediate-type skin reactions in sensitized individuals (Chen and Thyssen 2018; Hostynek and Maibach 2004; Kal et al. 2008; Temesvari and Daroczy 1989). Interestingly, photo-aggravation of Co- and Cr-induced urticarial responses were also noted (Manciet et al. 2006).
Oral mucosal allergy
Oral mucosal allergy refers to a subset of contact hypersensitivity responses that selectively manifest in and around the tissues of the mouth (Bakula et al. 2011). Accordingly, these responses occur primarily in subjects undergoing various types of dental procedures that involve the use of materials with potentially-sensitizing constituents. Some chemical agents used by dentists, such as methacrylate and formaldehyde, might induce these hypersensitivity responses; however, metals constitute the main inciting agents in most of these reactions (Hosoki et al. 2009). Allergic responses of the oral mucosa were estimated to impact approximately 2% of the general population, most often occurring in middle-aged patients (aged 50–60 years old) and more frequently in females than males (Bakula et al. 2011; Gupta and Jawanda 2015). These reactions might involve different underlying immunological mechanisms, unique clinical signs, and selective anatomical sites of involvement – all of which are features that mat be employed in differential diagnosis of oral mucosal allergy.
Allergic contact stomatitis is a specific type of oral mucosal hypersensitivity characterized by the presence of allergic inflammation affecting the entire oral mucosa (Minciullo et al. 2016). Most commonly, metal ions released from implanted dental materials are responsible for these reactions, as the ions are readily absorbed by the oral mucosa. In this condition, delayed/type IV hypersensitivity mechanisms mediate development of outbreaks within 24–72 hr of antigen exposure, similar to responses observed in ACD (Bakula et al. 2011). Ulcerations and lesions present inside and around the mouth with noticeable erythema and edema. Palladium, Pt, and Au are all metals that were associated with allergic contact stomatitis; however, Hg is the metal most commonly implicated in the condition (Garau et al. 2005; Koch and Baum 1996; Laeijendecker and Van Joost 1994; Minciullo et al. 2016; Torgerson et al. 2007). In addition, two cases of Ni-induced oral mucosal hyperplasia were also described, wherein the condition was suggested to be a rare form of allergic contact stomatitis produced by Ni present in dental materials (Özkaya and Babuna 2011). Gold crowns were also implicated in cases of allergic contact gingivostomatitis – another specific clinical variant of contact stomatitis (Izumi 1982).
Oral lichen planus is another variant of oral mucosal allergy. Similar to allergic contact stomatitis, lichenoid reactions also emerge as a result of delayed-type hypersensitivity responses following local contact with antigen; however, this disease is associated with a distinctive pattern of clinical presentations. Oral lichen planus involves selective inflammation of the buccal mucosa, tongue, and gingiva and the corresponding development of plaque-like, papular, or erosive lesions (Lavanya et al. 2011). Specific metals associated with oral lichen planus include tin, silver, Cu,, manganese (Mn), Cr, and Pd; however, Au is the metal most often implicated in lichenoid reactions of the oral mucosa (Downey 1989; Finne, Göransson, and Winckler 1982; Gil et al. 2019; Gupta and Jawanda 2015; Laeijendecker and Van Joost 1994; Minciullo et al. 2016; Mizoguchi, Setoyama, and Kanzaki 1998; Ortiz-Ruiz, Ramírez-Espinosa, and López-Jornet 2006; Sockanathan, Setterfield, and Wakelin 2003; Vergara et al. 2004).
Orofacial granulomatosis is another unique presentation of oral mucosal allergy. This condition is characterized by antigen contact in or around the mouth that leads to persistent swelling of the face, lips, and oral tissues, concurrent with granuloma development in the surrounding areas (Lazarov et al. 2003). Similar to allergic granulomatous skin responses that occur at other anatomical locations, orofacial granuloma formation is also known to involve Th1-dominant mechanisms, and often, delayed-type/T-cell-mediated hypersensitivity reactions. Accordingly, orofacial granulomatosis may represent a specific clinical manifestation of other delayed-type allergic responses of the oral mucosa, or as a unique disease entity. Interestingly, Pryce and King (1990) reported an increased prevalence of atopy in patients with orofacial granulomatosis, suggesting that other allergic mechanisms – potentially involving IgE-mediated responses – may be involved in some cases of the disease. Allergenic metal ions released from dental materials are the most common inducers of orofacial granulomatosis. Accordingly, while case reports have implicated Au, Hg, Co, and indium as causative agents of the disease, Ni is the metal most commonly-associated with development of orofacial granulomatosis (Lazarov et al. 2003; Matsudate et al. 2019; Minciullo et al. 2016; Pryce and King 1990; Tomka et al. 2011).
Burning mouth syndrome is a complex disorder that remains poorly understood from a pathophysiological standpoint; however, the condition is known to emerge as a result of either hypersensitivity-mediated or non-allergic mechanisms (Jimson et al. 2015). Both variants of the disorder were proposed to involve enhancement in central and peripheral neuropathic pathway activation, as symptoms of burning mouth syndrome include a persistent warm, prickling, or burning sensation in the tip of the tongue, lateral tongue borders, lips, hard and soft palates (Minciullo et al. 2016). Interestingly, no visible signs of these symptoms are detectable in patients, which further complicates clinical assessment of the disease (Jimson et al. 2015). Cases of burning mouth syndrome involving allergic mechanisms most often occur in subjects with dental prostheses that contain contact allergens, such as metals. Some of the metals known to produce this disease include Zn, Ni, Co and Hg, but Au is the metal most commonly implicated in burning mouth syndrome (Koike 2005; Laeijendecker and Van Joost 1994; Shutty and Scheinman 2018). Although delayed-type hypersensitivity responses are presumed to be involved in most cases, it remains unclear why some subjects develop burning mouth syndrome over other forms of cell-mediated oral mucosal allergy to dental metals.
Geographic tongue is another particularly unique variant of oral mucosal allergy. In this condition, the tongue constitutes the primary target tissue of antigen-induced inflammatory responses. Antigen exposure triggers the eruption of depapillated and discolored erythematous lesions that appear selectively on the dorsal surface of the tongue, giving the appearance of a geographical map (Minciullo et al. 2016). A characteristic feature of this disorder is the spontaneous resolution of these lesions, following which, their rapid migration to different areas of the tongue occurs (Campana et al. 2019). Because of this unique clinical presentation, the disease is also frequently referred to as ‘benign migratory glossitis.’ Most subjects afflicted with geographic tongue experience recurrent cycling between periods of remission and active disease, but remain asymptomatic under most circumstances (Ogueta et al. 2019). As with the other forms of oral mucosal allergy, dental metals constitute the primary source of geographic tongue. Specifically, Ni, Co, silver (Ag), and Ti are known to induce this condition in sensitized patients with metal-containing dental implants (Evrard, Waroquier, and Parent 2010; Minciullo et al. 2016; Samuel, Soumya, and Koshy 2014; Waroquier et al. 2009).
Several other clinical manifestations of oral mucosal allergy have been associated with allergenic metals, although many exhibit considerable overlap with one or many of the previously-described presentations. For example, peri-oral ACD is a term commonly assigned in cases where allergic skin reactions are observed in the skin around the mouth, but no other discernable diagnostic criteria exist to implicate classification of the disease as another manifestation of oral mucosal allergy (Goh and Ng 1987). Cobalt, Au, Pd, and Ni are all metals that were identified as potential inducers of peri-oral ACD (Bakula et al. 2011; Khamaysi, Bergman, and Weltfriend 2006). In a similar regard, allergic contact cheilitis involves a superficial inflammation of the lip that often occurs simultaneously with stomatitis or peri-oral ACD. Gold, specifically, is commonly implicated in cases of contact allergic cheilitis (Bakula et al. 2011). Notably, both of these conditions have also been associated with eruptions in subjects following oral contact with musical instruments, topical medicines, and cosmetics containing metal allergens (Collet, Jeudy, and Dalac 2013).
Ocular allergy
Hypersensitivity responses that selectively manifest within the structures of the eye and the surrounding tissues are often referred to as variants of dermal allergy (and occasionally, as a subtype of respiratory/mucosal hypersensitivity responses) within the scientific literature. Although most ocular hypersensitivity responses emerge following similar exposure conditions as those responsible for dermal hypersensitivity reactions (topical antigen contact), and many implicate inflammation of the skin surrounding the eyes (e.g., palpebrae, eyelids), it is important to note that allergic responses of the eye are distinctive from true dermal hypersensitivity responses in many ways (Leonardi, Motterle, and Bortolotti 2008). As a sensory organ, the eye is comprised of many unique anatomical structures and cell types; the vascular networks and lymphatic channels associated with the ocular system also differ markedly from those found in the skin. Moreover, the eyes and skin are both populated with unique resident immune cell subsets, and many of the migratory inflammatory cells that readily infiltrate the skin in allergic responses lack similar access to certain structures of the eye (Chigbu 2009). Collectively, these and other anatomical and physiological discrepancies between the skin and eyes render allergic reactions in these tissues unique entities that are jointly referred to as “topical hypersensitivity reactions” for the purposes of this review.
It has been estimated that 40–60% of allergic subjects exhibit ocular symptoms concurrent with other clinical manifestations of hypersensitivity reactions; however, ocular allergy is also known to occur independently of other allergic conditions (Bucolo et al. 2015). Most allergic responses implicating the eyes involve exposed ocular surfaces like the eyelid, conjunctiva, limbus, and cornea (Chigbu 2009). Similarly, some of the most common hypersensitivity responses that remain localized to the eye area include allergic conjunctivitis, contact dermatitis of the eyelids, atopic keratoconjunctivitis, and contact blepharoconjunctivitis (Bielory 2008). These ocular hypersensitivity responses might emerge as a result of various underlying mechanisms that may be either IgE- or non-IgE-mediated. Major causative agents of ocular allergy include seasonal aeroallergens such as pollens and ragweed, animal proteins, reactive chemicals, and drugs (Bielory 2008; Soparkar et al. 1997). Occasional reports have also cited various metals as potential causative agents of ocular allergy. For example, occupational exposure to Au was found associated with the emergence of delayed-type blepharoconjunctivitis, while Cr and Ni have been implicated in cases of allergic conjunctivitis (Estlander et al. 1998; Gibb et al. 2000; Mancuso and Berdondini 2002). In addition, eyelid dermatitis is a common manifestation of contact hypersensitivity to Ni, Co, Au, iron (Fe), and Cr that merge following application of cosmetics and in response to dental metal exposure (Goossens 2004; Huang et al. 2021; McDaniel and Couch 2017; Oh et al. 2016; Poziomkowska-Gęsicka et al. 2018; Saxena, Warshaw, and Ahmed 2001).
Metal hypersensitive alopecia areata
Alopecia areata is an immune-mediated inflammatory condition that involves the selective destruction of hair follicles in afflicted subjects. The disorder manifests equally amongst male and female subjects, and has been associated with a lifetime prevalence of approximately 1.7% (Conde-Taboada et al. 2007). Several different subsets of the disease were identified, and while unique clinical characteristics are implicated in each disease variant, all forms of alopecia areata involve either autoimmune- or hypersensitivity-mediated inflammatory reactions.
Metal hypersensitive alopecia areata is one of the newest disease variants to be identified. The allergens responsible for this specific condition remained unknown until 2005, when the metal patch test series became readily available, and subsequently used to establish a causal association between allergenic metals and presentations of disease. As a result, it became apparent that the ingestion and systemic absorption of metal ions, including those released by dental materials, constitutes one of the primary antigenic sources responsible for the disease. Translocation of these ions to peripheral sites by way of the circulatory system facilitates their deposition within host hair follicles where the conjugation of haptenic metal ions with keratin proteins confers the formation of a complete antigen. Allergic sensitization then leads to the generation of antigen-specific CD4+ and CD8 + T-cell populations, which subsequently mediate the major effector functions responsible for allergic elicitation responses and development of metal hypersensitive alopecia areata.
It has been reported that amongst the collective population of patients diagnosed with alopecia areata (all clinical variants), 70% of subjects experiencing severe symptoms of disease are hypersensitive to metals (Juárez-Rendón et al. 2017). Accordingly, clinical presentations of metal hypersensitive alopecia areata are often evident in many of these individuals. An extensive number of metal species are capable of initiating the condition; however, a few specific metals are implicated far more frequently than others. A comprehensive study was executed in 2018 in order to better characterize these trends and generate quantitative information regarding the specific metal allergens involved in the disease. Accordingly, 104 subjects identified as having severe symptoms of metal hypersensitive alopecia areata were included in the study and patch tested with a panel of 18 different metals – some in varying concentrations (Nakayama and Chen 2018). It was subsequently determined that Hg, Ni, Co, and Cr were responsible for the greatest number of allergic responses, producing positive reactions in 33.7, 30.8, 26, and 23.1% of test subjects, respectively. The next most frequent metal allergens were Pt, Zn, tin (Sn), and Cu, which elicited positive reactions in 13.5, 11.5, 9.6, and 8.7% of subjects, respectively. Metals associated with positivity rates below 8% included Pd, cadmium (Cd), Au, Fe, indium, iridium, molybdenum, and Mn. Two metals – Ag and antimony – did not induce positive reactions in any test subjects.
Bullous autoimmune dermatoses
The term ‘bullous autoimmune dermatoses’ comprises several disease subtypes with shared but distinctive pathophysiological characteristics – the two most common of which are pemphigus and pemphigoid. In both of these diseases, autoantibodies are involved in blistering eruptions of the skin and oral mucosa. Pemphigus-type diseases involve the development of autoantibodies reactive toward desmogleins – proteins involved in cell-cell adhesion – which results in the loss of keratinocyte structural integrity within the epidermis and subsequent lesion formation (Hammers and Stanley 2016). Comparatively, pemphigoid-type conditions emerge in response to autoantibody formation wherein reactivity to hemidesmosomes – proteins that mediate cell adhesion to the basement membrane – results in fixation of complement and subsequent inflammation and lesion emergence (Hofmann, Juratli, and Eming 2018). Both diseases primarily implicate IgG isoforms of effector autoantibodies, however, IgA-mediated variants of both disease types also exist (Kasperkiewicz et al. 2017).
Although the causes of these disorders remain largely unclear, it has become recognized that some exposure conditions might promote the development of autoantibodies to epidermal proteins. For example, some drugs have been associated with the induction of structural changes in the epidermis that lead to sensitization. Although quite uncommon, dermal contact with metals was also implicated in similar mechanisms and subsequent development of pemphigus or pemphigoid in susceptible individuals. Accordingly, two reports described the emergence of pemphigus vulgaris in subjects with Ni-containing dental prostheses (Stransky 1998; Thongprasom et al. 2011). The extended duration of contact with the oral mucosa was suggested to result in the formation of novel antigens and subsequent sensitization, leading to pemphigus-like lesions in and around the mouth. Gold was also associated with the potential to initiate both pemphigus and pemphigoid in subjects receiving systemic Au therapy (Iveson et al. 1977; Lo Schiavo et al. 2008). Paradoxically, one of the indications for gold salt therapy is pemphigus (Faa et al. 2018). While some patients achieve relief from autoimmune symptoms following treatment, others subsequently develop autoreactive antibodies. This response was suggested to result from similar mechanisms known to occur in cases of drug-induced pemphigoid, wherein a drug triggers conformational changes in host proteins of the skin and subsequent sensitization of the patient to gold/host protein complexes (Van Der Voet 2010).
Other immune responses of the skin with potential implications in metal allergy
It is worth noting that several other autoimmune-mediated cutaneous responses have been correlated with dermal sensitivity to metals. For example, many patients with rosacea also exhibit symptoms of metal-induced ACD, particularly in response to Ni (Çifci 2019). Similarly, several investigators demonstrated that the prevalence of metal ACD tends to be elevated amongst subjects suffering from psoriasis (Heule et al. 1998; Kageyama et al. 2021; Weryńska-Kalemba et al. 2016). Allergic reactivity to Ni, Zn, and other dental metals is commonly observed in patients with psoriasis, as up to 70% of subjects exhibit skin sensitivity to one or more metal allergens (Nielsen and Menné 1997; Rasool et al. 2018). It remains unclear if these connections reflect the existence of a causative relationship between ACD and the two conditions or a simple association; however, Çifci (2019) proposed, based upon existing information that metal hypersensitivity may be a triggering factor for the development of rosacea. Comparatively, metal ACD was indicated by Krupashankar and Manivasagam (2012) to be a secondary effect of psoriatic responses. These two conditions are known to be mediated by opposing immunological mechanisms, though cutaneous eruptions might occur simultaneously (Quaranta et al. 2014). Overall, more information is needed in order to determine if autoimmune-associated skin conditions such as rosacea and psoriasis are directly associated with metal-specific ACD.
Metals & respiratory hypersensitivity responses
Aerosolized metal particulates become suspended in the ambient air as a result of both natural and anthropomorphic processes. Forest fires and volcanic eruptions, as well as traffic emissions and combustion reactions at industrial sites all facilitate the release of airborne metals into the environment where these substances subsequently are inhaled by humans; however, under normal circumstances, members of the general public are not likely to encounter these and other airborne sources of allergenic metals in concentrations high enough to induce sensitization (Aksu 2015). Consequently, unlike metal-induced dermal hypersensitivity responses, which are exceptionally prevalent within the general population, respiratory hypersensitivity responses initiated by metals are not commonly noted in members of the general public (Mayer and Hamzeh 2015). The vast majority of respiratory hypersensitivity responses induced by allergenic metals tend to occur selectively in working populations (Brooks 1981; Kastury, Smith, and Juhasz 2017).
The risk of developing metal-specific allergic airway responses tends to be significantly higher in workers than in non-workers since many common processes that generate large quantities of aerosolized metals are performed almost exclusively in occupational settings (Malo, Chan-Yeung, and Di 2013). Mining, refining, smelting, welding, electroplating, and many other industrial processes facilitate the generation of large quantities of airborne metal particulates, fumes, and vapors that are often released directly into the breathing spaces of workers. In susceptible workers that fail to utilize adequate personal protective equipment (PPE), inhalation of these substances can lead to respiratory sensitization, and subsequently, the development of metal-specific allergic airway responses (Wyman and Hines 2018).
Although direct sensitization of the airways following inhalation exposure to allergenic metals constitutes the most common and straightforward mechanism by which respiratory hypersensitivity responses develop, it is important to note that in some cases, other mechanisms may be involved (Jones 2008). As discussed in greater detail within the sections below, some metal-induced allergic lung responses emerge independently of inhalation exposures. A few allergenic metals (e.g., beryllium) are able to induce allergic lung responses following dermal sensitization and skin exposures (Tinkle et al. 2003). Similarly, some sensitizing metals (e.g., gold) may trigger the development of respiratory hypersensitivity reactions following systemic sensitization (Evans et al. 1987).
Metals have been associated with the development of many different forms of respiratory allergy – all of which are discussed in the sections below. Some of the major discriminating features of these responses include underlying immunological mechanisms (e.g., cell-mediated or IgE-mediated) and primary anatomical site of involvement within the respiratory tract (e.g., upper or lower airways). A summary of the most common respiratory hypersensitivity responses and corresponding metals associated with each condition is presented in Table 4.
Table 4.
Specific Metals Associated with Different Presentations of Respiratory Hypersensitivity.
Table 4: Metals implicated in different forms of respiratory allergy. Common presentations of metal allergy that selectively manifest in the respiratory tract are listed in the table above. Responses are grouped by primary mechanism of hypersensitivity and specific metals implicated in each disease variant are denoted by the parenthesized numbers within the assoicated column, which correspond to relevant citations.
Stainless steel is an alloy comprised of many metals (e.g., iron, nickel, chromium, titanium, etc.), and while the compound has been implicated in some cases of allergy, it is unclear which of the constituent metal(s) is responsible for the observed effects in many cases.
Allergic asthma
Similar to ACD, asthma is an inflammatory condition than may be mediated by one of two major overarching mechanisms. Non-immunologic, or irritant-induced asthma is a subset of the disease that describes non-specific, innate immune-orchestrated inflammation of the respiratory tract following inhalation exposure to respiratory irritants (e.g., reactive chemicals) (Maestrelli et al. 2009). Comparatively, allergic asthma constitutes the other disease subset, wherein true hypersensitivity reactions are responsible for development of airway inflammation following pulmonary exposure to antigen by a pre-sensitized individual. Although both conditions evoke similar clinical presentations and constitute major health concerns in the general public and the workplace, only allergic asthma is within the scope of this review.
Allergic asthma is one of the most common manifestations of respiratory allergy, afflicting an estimated 300 million individuals globally (Pawankar 2014). Asthma is a disease of the conducting airways characterized by increased responsivity to direct and indirect bronchoconstricting agents, as well as tightness in the chest, mucus hypersecretion, wheeze, and shortness of breath following allergen inhalation (Holgate et al. 2015). In cases of persistent asthma, chronic cycling between pathologic states of active allergic inflammation and inducible mechanisms of tissue repair in the resolution phase leads to airway remodeling – a collection of anatomical changes in the respiratory epithelium, airway smooth muscle layer, epithelial basement membrane, and pulmonary vasculature become evident over time, often leading to declines in lung function (Fehrenbach, Wagner, and Wegmann 2017; Warner and Knight 2008).
Most cases of asthma in the general population are induced by high molecular weight (HMW) environmental proteins, such as pollens and molds. While some HMW allergens, such as animal dander, are also responsible for cases of asthma in the workplace, LMW respiratory allergens are often selectively implicated in cases of occupational asthma (Bardana 2008). In addition to the various classes of reactive chemicals capable of inducing occupational asthma, metals constitute another group of potential asthmagens that are particularly concerning in the workplace.
Traditionally, the term ‘asthma’ has been used to describe a singular disease entity; however, it has recently become recognized that a notable degree of heterogeneity exists among asthmatic conditions (Erle and Sheppard 2014). As a result, a novel classification scheme was developed wherein multiple different subsets of the disorder identified. All of the asthma subtypes represented in this paradigm are derived from one of two general types of disease variants. Endotypic subtypes are distinguished according to differences in the underlying mechanisms responsible for presentations of disease. Specific endotypes implicated in allergic asthma include Th2, non-Th2, Th17, and neutrophilic subtypes (Guibas et al. 2017). Comparatively, phenotypic variants of disease are differentiated according to discrepancies in major clinical characteristics among disease subtypes. Common asthma phenotypes include adult-onset, obesity-related, smoking-associated, and virus-induced variants (Corren 2013).
Antibody-Mediated Asthma: The classic disease paradigm of allergic asthma involves immediate-type hypersensitivity responses mediated by antigen-specific IgE molecules (Nauta et al. 2008). After sensitization, antigen exposure triggers the degranulation of mast cells and subsequent release of preformed mediators that are responsible for the rapid onset of asthmatic symptoms (within 15 min of antigen exposure). Eosinophils and other Th2-associated immune effectors also play critical roles in the pathogenesis of this disease (Esteban-Gorgojo et al. 2018). Consistent with this asthmatic subtype, several metals are implicated in the development of IgE-mediated occupational asthma. Immediate onset of respiratory symptoms was observed in asthmatic workers following exposure to Cr, Mn, Hg, rhodium, tungsten, vanadium, Zn, Co, Ni, Fe, Pt, Pd, and Al (Daenen et al. 1999; De Raeve et al. 1998; Merget et al. 1994, 2010, 1988; Munoz et al. 2009; Thanasias et al. 2013; Vandenplas et al. 1998; Wittczak et al. 2008, 2012).
A few case reports also described cases of occupational asthma produced by metals wherein similar immunological mediators and clinical presentations of disease are observed, but specific IgG molecules, rather than IgE molecules, appear responsible for effector roles in the disease. Cobalt and Pt were both associated with inducing occupational asthma in workers were metal-specific IgG molecules were identified (Cirla 1994; Pepys et al. 1979).
Another common endotypic variant of asthma involves similar mechanisms as those associated with prototypical IgE-mediated, Th2-dominant reactions, as described above. The primary discriminating feature of the two asthmatic endotypes is the propensity for selective recruitment of different inflammatory cell subsets to the airways following antigen exposure. In the previously-described endotype of asthma, eosinophils represent the primary inflammatory cell type recruited to the lungs, while a characteristic influx of neutrophils is uniquely observed in this asthmatic endotype (Esteban-Gorgojo et al. 2018). Neutrophilic asthma represents a variation of the condition associated with more severe clinical symptoms including airway hyperreactivity (AHR), increased involvement of interleukin (IL)-17/Th17 signaling, and resistance to corticosteroid therapy (Gao, Ying, and Dai 2017; Ray and Kolls 2017). A few metals, including Fe, were found to initiate neutrophilic-dominant forms of occupational asthma (Munoz et al. 2009). Interestingly, both eosinophilic-and neutrophilic-dominant endotypes of asthma were observed in cases of Al -induced potroom asthma (Sjåheim et al. 2004). Moreover, both disease variants have been reported in workers employed by the same plant. The existence of similar exposure conditions between these two sets of workers suggests that other contributing factors are influential in determining the nature of allergic inflammation attributed to occupational metal exposures.
Cell-Mediated Asthma: In addition to eosinophilic and neutrophilic endotypes of IgE-mediated asthma, metals were also implicated in a form of occupational asthma associated with delayed-type, cell-mediated hypersensitivity mechanisms. In most of these cases, asthmatic responses are observed in workers with no detectable levels of circulating metal-specific antibodies. In the afflicted subjects, positive reactions are detected following specific inhalation challenge with the relevant metals, however, the onset of respiratory symptoms exhibits a characteristic temporal delay, consistent with cell-mediated responses. Chromium, Ni, and Co were all identified as metals implicated in these reactions (De Hauteclocque et al. 2002; Kusaka et al. 1991, 1989; Malo et al. 1985; Olaguibel and Basomba 1989). Interestingly, a few cases were described wherein metal-reactive subjects exhibit dual bronchial reactions in response to specific inhalation challenge, suggesting that both antibody- and cell-mediated mechanisms may be involved in some cases of metal-induced asthma. Both immediate and late asthmatic reactions were noted in sensitized subjects following inhalation of Pt, Cr, and Ni (Kazantzis 1978; Olaguibel and Basomba 1989; Sastre et al. 2001).
Allergic rhinitis
Allergic rhinitis is an allergic response of the nasal mucosa that occurs in 10–30% of the general population (Pawankar et al. 2013). The disease is characterized by the presence of immediate onset nasal congestion and itching, sneezing, and rhinorrhea following exposure to aeroallergens present in the air (Bousquet et al. 2020). Allergen-specific IgE molecules are responsible for the clinical manifestations of the disease, and similarly, allergic rhinitis often presents concurrently with asthma in many individuals; however, many individuals afflicted with rhinitis do not exhibit concomitant asthmatic responses. Other co-morbidities commonly implicated in cases of allergic rhinitis include allergic conjunctivitis, rhinosinusitis, and atopic dermatitis (Pawankar et al. 2013).
Although pollens, molds, and animal proteins tend to be the most common inducers of allergic rhinitis, several metals were also associated with the disorder. The majority of metal-induced rhinitis cases were reported to occur in workers with potential for exposure to airborne metals in their workplaces. Accordingly, Pd, Pt, rhodium, Ni, and mixed-metal alloys were all implicated in cases of occupational rhinitis (Estlander et al. 1993; Malo 2005; Merget et al. 2010; Niordson 1981; Pesonen et al. 2014).
Immediate hypersensitivity laryngitis
Immediate hypersensitivity laryngitis is an allergic response that manifests in the upper airways and selectively affects the larynx (Campagnolo and Benninger 2019). Although the larynx may be one of the tissues involved in other allergic responses of the respiratory tract (e.g., asthma and rhinitis), immediate hypersensitivity laryngitis is characterized by an isolated site of involvement following antigen challenge. The immediate onset of symptoms in this condition suggests involvement from antigen-specific IgE molecules, although the underlying mechanisms of immediate hypersensitivity laryngitis have yet to be specifically determined. Although similar immunological mechanisms may be involved in this condition and other immediate-type allergic responses of the respiratory tract, immediate hypersensitivity laryngitis tends to emerge independently of other allergic diseases and is rarely identified in conjunction with asthma.
A few metal species were reported to produce immediate hypersensitivity laryngitis in human subjects. Interestingly, existing accounts also described the manifestation of this allergic response following exposure to metals by different exposure routes. In Hannu, Piipari, and Toskala (2006) found development of immediate hypersensitivity laryngitis in a welder after lung exposure to stainless steel fumes. A specific challenge test was administered to the subject, and within 30 min, increased erythema and edema was detected selectively in the larynx. No indications of any other immediate-type respiratory hypersensitivity responses were evident. Lung function parameters typically altered during asthmatic responses remained unchanged and no symptoms of rhinitis were detected, indicating that the observed response was not associated with any other allergic condition. Buyukozturk et al. (2013) reported that Ni induced a similar set of symptoms in a different subject, although a different route of exposure was implicated in the response. In this case, a Ni-allergic individual began experiencing frequent laryngeal edema attacks requiring immediate treatment with epinephrine and corticosteroids (Buyukozturk et al. 2013). It was determined that a dental implant containing Ni was responsible for triggering laryngeal edema attacks in the patient. The local contact between the oral mucosa and the dental material was abrogated following removal of the device, and as a result, symptoms of hypersensitivity laryngitis disappeared in the patient.
Hypersensitivity pneumonitis
While the pathogenic effects of allergic asthma and rhinitis preferentially manifest in the upper airways and nasal region, hypersensitivity pneumonitis is an allergic response of the lungs that develops in the lower airways and lung interstitium (Moldoveanu et al. 2009). Hypersensitivity pneumonitis is less common than asthma and rhinitis, with an annual incidence of 1.28–1.94 cases per 100,000 individuals in the United States (Costabel et al. 2020; Fernández Pérez et al. 2018). In this disease, sensitization results in the development of antigen-specific CD4+ and CD8 + T-cells and Th1-polarized immune responsivity (Bogaert et al. 2009). Subsequent antigen exposures lead to an influx of effector T-cells to the lungs, alveolar macrophage activation, and lymphocytic inflammation of the alveoli and terminal bronchioles. Persistent alveolitis and granuloma formation might eventually lead to fibrosis and respiratory failure in subjects with hypersensitivity pneumonitis.
The most common antigens associated with hypersensitivity pneumonitis include various fungal species and proteins present in bird feces and feathers, which are associated with specific variants of the disease termed ‘Farmer’s lung’ and ‘Bird fancier’s lung,’ respectively (Woda 2008). A few metals were also implicated in cases of hypersensitivity pneumonitis. Two of these – beryllium (Be) and Au – are implicated in distinctive variants of the disease and are discussed separately below. Other metals known to initiate hypersensitivity pneumonitis include Zn, Co, Al, and zirconium, and tend to specifically afflict workers (Ameille et al. 1992; Chen, R.J. Monnat, and Mottet 1978; Liippo et al. 1993; Van Cutsem et al. 1987).
Chronic Beryllium Disease: Chronic Beryllium Disease (CBD) is a distinctive allergic condition of the lungs attributed to exposure to Be, which most frequently occurs in occupational settings. In 2009, it was estimated that over 800,000 workers are exposed to Be in the United States alone, and 2–5% of beryllium-exposed workers subsequently develop disease (Sood 2009). CBD is most common in workers employed in the aeronautics and transportation industry, Be manufacturing sector, and electronics and communications markets (Day et al. 2006; Forte, Petrucci, and Bocca 2008). CBD has been described as a form of granulomatous hypersensitivity pneumonitis that emerges following sensitization of susceptible individuals to Be. One of the unique aspects of this disease is that sensitization to the metal might occur following both inhalation exposure and dermal contact with Be (Tinkle et al. 2003). Irrespective of the exposure route involved, sensitization to Be involves the generation of metal-specific Th1-polarized CD4+ effector T-cells (McKee et al. 2015; Wade et al. 2018). Beryllium-specific T-cells are subsequently recruited to the airways, where their activity leads to inflammation of the alveolar spaces and formation of granulomas (Samuel and Maier 2008). Over time, CBD patients often develop decreases in lung volume and diffusing capacity, pulmonary fibrosis, and respiratory failure as a result of the disease (Sood 2009).
Gold Lung: Gold is another metal that has been associated with a distinctive variant of hypersensitivity pneumonitis. This condition is referred to as ‘gold lung’ and exclusively observed in chrysotherapy patients receiving monovalent gold salts for the treatment of autoimmune conditions such as rheumatoid arthritis (Evans et al. 1987). Notably, the disease appears to develop completely independently of respiratory exposures to the metal. Instead, gold lung was suggested to originate from a dose of intramuscularly- or intravenously-administrated gold salts that triggers systemic sensitization in a susceptible individual. A pool of gold-reactive T-cell clones is then generated by the subject’s immune system. As subsequent doses of gold salts are administered, the metal deposits and accumulates in various tissues of the body, including the lungs (Tomioka and King 1997). In some subjects, this leads to recruitment of gold-reactive T-cells to the lungs. Interestingly, CD8 + gold-reactive T-cells have been identified as the primary effector cell type responsible for the delayed-type allergic inflammation that results in the development of gold lung symptoms (Scherak et al. 1993; Slingerland et al. 1987). In accordance with these mechanisms, cases of gold lung are often diagnosed by confirming reactivity of circulating and the bronchoalveolar lavage (BAL)-associated lymphocytes to gold. The cellular profile of the BAL is also used in clinical evaluations, as gold lung patients tend to exhibit an overall lymphocytic predominance within the BAL and a decrease in the CD4:CD8 BAL T-cell ratio. Early clinical manifestations of the disease include dyspnea, fever, skin rash, and cough. Over time, a restrictive pattern of lung disease is often observed, consistent with the propensity for pulmonary fibrosis to develop in many gold lung patients. Failure to effectively treat and manage the condition might also lead to pleural effusion, hypoxia, and respiratory failure.
Hard Metal Lung Disease: Hard metal is a substance that is formed by compacting powdered tungsten carbide and Co into a polycrystalline material (Mizutani et al. 2016). The end result is a material that is comprised of approximately 90% tungsten carbide and approximately 10% Co, along with trace amounts of Ti, Ni, and chrome (Sergio et al. 2017). Hard metal is significantly stronger than hardened steel and exhibits strength almost equivalent to that of diamonds (Nemery and Abraham 2007). As a result, the material is frequently used to fabricate tools, machines, and drilling devices – objects whose use lead to liberation of airborne hard metal particulates that may be subsequently inhaled.
The term hard metal lung disease (HMLD) was first introduced in 1941 to describe an emerging inflammatory lung condition observed exclusively in workers exposed to hard metal dusts in the workplace (Nemery, Verbeken, and Demedts 2001). HMLD was initially characterized as a novel variant of pneumoconiosis – a group of occupational lung diseases caused by the deposition of various organic dusts within the respiratory tract; however, in the ensuing years it became evident that inhalation of hard metal dust lead to development of several unique disease variants with distinctive pathologies (Nemery and Abraham 2007). It also became apparent that different components of hard metal may be responsible for the major clinical presentations of HMLD in afflicted individuals. In accordance with this knowledge, the terminology used to describe hard metal-induced lung pathologies has expanded greatly but remains inconsistently reported within the scientific literature at present. Discrepancies in nomenclature used to describe HMLD include hard metal disease, hard metal pneumoconiosis, tungsten carbide pneumoconiosis, hard metal lung, giant cell interstitial pneumonia (GIP), and cobalt lung (Enriquez et al. 2007; Lison et al. 1996).
For the purposes of this review, the many variants of HMLD may be broadly categorized into one of two groups based upon the extent of immunological involvement in disease pathogenesis (Zheng, Marron, and Sehgal 2020). The most common presentations of HMLD bear many similarities to prototypical cases of pneumoconiosis and other conditions associated with a restrictive pattern of lung disease (Nemery, Verbeken, and Demedts 2001). In subjects experiencing this disease type, inhalation of hard metals induces inflammation of the lung parenchyma, a response that is primarily mediated by oxidant injury mechanisms and is orchestrated exclusively by cells of the innate immune system (Adams et al. 2017). The lung interstitium and alveolar walls become heavily infiltrated with mononuclear cells in these variants of HMLD, often resulting in noncaseating granuloma formation and development of fibrosis (Sergio et al. 2017). Prominent symptoms include cough, dyspnea, and weight loss (Kelleher, Pacheco, and Newman 2000).
The second group of disease variants associated with hard metal inhalation implicates involvement of the adaptive immune system, and thus, is representative of true allergic conditions. Two major disease states have been described in this context. The first is hard metal asthma (Chiba et al. 2019). Most cases of hard metal asthma implicate allergic sensitivity to Co as previously discussed. Tungsten is only associated with modest allergenic potential, as the metal has only been implicated in a few cases of immediate-type asthma in the literature (Bruckner 1967; Miyamoto, Inoue, and Watanabe 2005). The second variant of HMLD involving hypersensitivity-mediated mechanisms resembles a form of hypersensitivity pneumonitis and is most commonly referred to as GIP (Nemery, Verbeken, and Demedts 2001). GIP, like other forms of hypersensitivity pneumonitis, is mediated by antigen-specific lymphocytic inflammation within the alveolar region of the respiratory tract (Seaman, Meyer, and Kanne 2015). One of the distinctive characteristics of this HMLD variant is the presence of multinucleated giant cells in the airway lumen and lung interstitium of affected individuals (Mayer and Hamzeh 2015). These cells develop from macrophage precursors under specific physiological conditions, and often emerge as a result of fusion of multiple macrophages (McNally and Anderson 2011). The cells, which are often observed engulfing other immune cells in the lungs, are a hallmark of GIP, but their role in the pathogenesis of the condition remains largely unknown (Lison et al. 1996). Similar to non-allergic forms of HMLD, GIP might also result in granuloma formation within the lungs; however, this disease variant is not associated with development of fibrosis (Fontenot and Amicosante 2008).
A correlation between tungsten-specific allergic responses and GIP development following hard metal exposure has yet to be established in any published reports (Okuno et al. 2010). Correspondingly, nearly all cases of GIP associated with hard metal exposure were attributed to allergenic effects of Co (Lison et al. 1996; Sakai et al. 2010; Sakamoto, Kosai, and Kohrogi 2008). In subjects afflicted with the condition, cobalt-specific CD8 + T-cells are readily identified within the inflamed lung tissue and circulating lymphocytes exhibit reactivity to Co in vitro (Fontenot and Amicosante 2008). In some cases, inhalation of hard metal dust appears to be responsible for the initial sensitizing event that leads to generation of cobalt-specific lymphocytes and subsequent development of GIP (Davison et al. 1983). For other individuals, preexisting allergic responsivity to Co seemingly primes the respiratory tract for GIP development in response to hard metal exposure (Nemery, Verbeken, and Demedts 2001). In most of these cases, subjects that develop GIP report a history of contact sensitivity to the metal, suggesting that the existence of a previously-established pool of cobalt-reactive T-cells can predispose for development of hard metal-induced GIP (Nakamura et al. 2014). Interestingly, a few published reports also described cases of cobalt-associated GIP wherein the afflicted workers reported a history of Co-induced asthma of the immediate type (Davison et al. 1983; Satoh-Kamachi et al. 1998).
Pulmonary eosinophilia
Another inflammatory response of the airways that has been associated with inhalation of allergenic metals is pulmonary eosinophilia. Pulmonary eosinophilia encompasses several distinctive disorders, wherein the primary presentation of disease is increased influx of eosinophils to the respiratory tract and subsequent development of localized inflammation that can result from both hypersensitivity-mediated mechanisms, as well as non-allergic processes (Scott and Wardlaw 2006). Although eosinophilic inflammation is a cardinal sign of allergic asthma, rhinitis, and other chronic lung conditions, pulmonary eosinophilia generally refers to eosinophil-driven airway inflammation that occurs independently of these diseases. Accordingly, the diagnostic criteria used to identify cases of pulmonary eosinophilia generally include a BAL/sputum eosinophil count of > 2.5% of total cells, in addition to the absence of prototypical symptoms associated with other lung conditions like asthma (e.g., AHR, bronchoconstriction, mucus hypersecretion) (Gibson, Fujimura, and Niimi 2002). Two of the most common forms of pulmonary eosinophilia are eosinophilic bronchitis and eosinophilic pneumonia, which produce symptoms ranging from cough, dyspnea, fever, and blood eosinophilia (Akuthota and Weller 2012; Brightling 2006; Pala, Pignatti, and Moscato 2012; Yıldız and Dülger 2018).
At present, the only metals that have been consistently identified as potential causative agents of pulmonary eosinophilia are Al and Pt. Several reports have been published describing workers exposed to aerosolized forms of Al that subsequently develop elevated sputum eosinophil levels in the absence of other prototypical asthma symptoms (Schwarz et al. 1994; Sorgdrager et al. 1995). There exist fewer reports pertaining to Pt-induced pulmonary eosinophilia; however, Merget et al. (2015) in particular elucidates an interesting correlation between the disorder and metal allergy that is worth highlighting. In this case report, a precious metals refinery worker who had been employed in her position for 12 years began to develop a runny nose and cough when working with aerosolized forms of Pt (Merget et al. 2015). The subject also reported a history of recurrent skin outbreaks following dermal contact with the metal. Consequently, a specific inhalation challenge, prick tests, and patch tests were performed on the worker using different test formulations of Pt. The inhalation challenge yielded results inconsistent with an asthmatic response, though increased lung eosinophil burden was observed in the days following the test. The worker was prick test negative, but patch test positive for the metal. Collectively, these results indicate that while the subject had previously developed delayed-type, cell-mediated allergic responsivity to Pt (consistent with ACD), the subsequent emergence of pulmonary eosinophilia occurred independently of platinum-specific IgE-mediated mechanisms. This observation constitutes an interesting finding since eosinophilic lung responses are traditionally associated with Th2-dominated immune reactivity and Pt is one of the metals most commonly implicated in IgE-mediated asthmatic responses (Scott and Wardlaw 2006).
While only two individual metal species have been acknowledged as potential causative agents of pulmonary eosinophilia to date, there are numerous other reports describing development of this condition following exposure to mixed metal particulates. For example, lung eosinophilia was found in workers exposed to airborne sources of stainless steel, hard metal, metalworking fluids, and fly ash (Ghio et al. 2002; Schwarz et al. 1994; Wiggans and Barber 2017; Yacoub et al. 2005). These substances are often comprised of multiple metal elements. Stainless steel is an alloy comprised of Fe, Cr, Ni, and many other metals in varying concentrations, while hard metal is a term used to describe a metallic carbide comprised of tungsten, vanadium, and/or titanium that is mixed with Ni or Co (Antonini et al. 2004). Metalworking fluids are water- or oil-based lubricants used to reduce heat and friction during industrial machining operations, and although these substances are inherently metal-free when produced, they often become a vehicle for metal particulates generated during their use and might be subsequently inhaled (Wiggans and Barber 2017). ‘Fly ash’ refers to the inorganic residue that is generated following the combustion of carbonaceous materials such as coal, and is generally comprised of metals including Ni, Fe, and vanadium (Ghio et al. 2002). Existing publications describing the development of pulmonary eosinophilia following exposure to these substances often fail to elucidate the specific constituent metals responsible for the observed reactions, and thus, several additional metals capable of inducing lung eosinophilia likely exist but have yet to be identified.
Other immune responses of the respiratory tract with potential implications in metal allergy
Several other inflammatory conditions of the airways have been correlated with allergic responsivity to metals. Although metal-specific hypersensitivity reactions do not constitute the primary mechanism of pathogenesis in these disorders, evidence suggests that metal allergy may play a critical role in disease development, progression, and symptom severity.
Pulmonary alveolar proteinosis (PAP) is a relatively uncommon lung disease characterized by the accumulation of surfactant lipids and proteins within the alveolar space (Wang et al. 2012). Impaired clearance mechanisms are responsible for the buildup of these acellular components, which impair gas exchange, and eventually produce respiratory failure and death. Several different sources of PAP have been identified, and three corresponding variants of the disease were described to reflect these different origins of disease. Congenital PAP results from genetic mutations in innate immune cell receptors, autoimmune PAP involves adaptive immune-mediated interference with normal pulmonary clearance mechanisms, and secondary PAP emerges as a complication of infections, malignancies, or toxic exposures (Ben-Dov and Segel 2014; Santos et al. 2020). Accordingly, PAP is known to occur in both worker populations and the general public. The cytokine granulocyte macrophage-colony stimulating factor (GM-CSF) plays a central role in the pathogenesis of all three variants of PAP, as it mediates the terminal differentiation of alveolar macrophages, which are responsible for catabolizing the offending molecules and clearing them from the lower airways (Trapnell et al. 2019). Accordingly, disease onset corresponds with the introduction of disruptions in GM-CSF signaling and alveolar macrophage functionality.
Several metals have been implicated in PAP. Occupational exposures to indium, silica, Sn, Ti and Al have all been shown to produce disruptions in normal alveolar macrophage clearance mechanisms leading to development of secondary PAP (Bomhard 2017; Huaux et al. 2018a, 2018b; Igbokwe, Igwenagu, and Igbokwe 2019; Keller et al. 1995; Miller et al. 1984; Sauni et al. 2007; Yorozuya et al. 2019). Although these responses do not constitute a form of metal hypersensitivity, several studies have correlated the induction of secondary PAP and metal inhalation exposures to the subsequent development of autoimmune PAP (Chew, Nigam, and Sivakumaran 2016; Inoue et al. 2008). The mechanisms by which inhalation of metal particulates may facilitate the generation of autoantibodies remain largely unclear; however, Costabel and Nakata (2010) suggested that metal-induced structural alterations in proteins associated with GM-CSF signaling and alveolar macrophage functionality may be involved. Accordingly, if novel antigenic determinants that implicate metal/host protein complexes are formed following exposure, the subsequent development of autoimmune PAP may be regarded as a form of metal-specific hypersensitivity occurring in the lower airways.
In addition to PAP, inhalation exposure to allergenic metals was also proposed to play a role in some cases of Goodpasture’s syndrome. Goodpasture’s syndrome is an autoimmune condition that develops in subjects who produce autoantibodies specific for type IV collagen (Borza, Neilson, and Hudson 2003). In afflicted individuals, these circulating IgG autoantibodies recognize and bind antigens present in the basement membrane of the lungs and kidneys, producing localized inflammation. In some cases, these reactions trigger widespread immune activation and vasculitis, which lead to respiratory and/or renal failure and death (Greco et al. 2015).
Several case reports have been published describing development of Goodpasture’s syndrome in workers following inhalation exposures to hard metal dust, welding fumes, and silica (Bal et al. 2014; Dahlgren, Wardenburg, and Peckham 2010; Pedchenko, Vanacore, and Hudson 2011). Lechleitner et al. (1993) suggested that these types of exposures mediate the development of autoantibodies by inducing significant tissue damage within the lungs, and thus, exposing alveolar basement membrane proteins for recognition by the immune system. Moreover, localized inflammatory reactions initiated by the metals likely act as an adjuvant, further promoting the recruitment of the adaptive immune system. Although the existence of metal allergy was proposed as a potential mechanism that may prime for the development of Goodpasture’s syndrome in the lungs, this connection has yet to be definitively established.
Metals & gastrointestinal hypersensitivity responses
In addition to the skin and lungs, the GI tract is another organ system involved in a diverse assortment of hypersensitivity reactions. GI allergy emerges following exposure to antigen via the oral route and subsequent ingestion (Biermé, Nowak-Wegrzyn, and Caubet 2017). The ensuing hypersensitivity reactions can manifest as local responses that remain isolated within the intestinal mucosa and surrounding tissues; contrarily, antigen ingestion can also facilitate its systemic absorption and the emergence of allergic symptoms in other anatomical compartments of the body. The various manifestations of GI allergy are often broadly grouped according to similar underlying immunological mechanisms. The three general mechanisms responsible for these reactions include IgE-mediated, non-IgE-mediated, and mixed IgE/non-IgE type responses (Azouz and Rothenberg 2019). Irrespective of these discrepancies, nearly all forms of GI allergy implicate a conserved set of risk factors and key immunological alterations known to promote the development of disease. Increased susceptibility to GI allergy was associated with diet, previous infections, microbiome composition and diversity, prior ingestion exposures (e.g., antibiotics and chemicals), as well as various genetic and epigenetic factors (Wang et al. 2021). Concurrently, the inherent resistance to allergic responsivity observed in the gut under normal circumstances is frequently disrupted prior to allergy development by a consistent pattern of alterations including compromised GI mucosal barrier efficacy, a breakdown in immunological tolerance, and polarization of local immune networks toward Th2 directionality (Meyer et al. 2019).
Collectively, Pawankar et al. (2013) estimated that allergic responses of the digestive tract affect over 550 million individuals worldwide. Children tend to be disproportionately impacted by GI allergy since many conditions involve atopic mechanisms that emerge early in life (Scurlock et al. 2010). Allergic responses of the GI tract are often collectively referred to as ‘food allergy.’ Although this terminology adequately conveys the selective association of the disorders with the ingestion of antigen, this nomenclature can also be misleading, given that oral exposure to many substances other than foods might also result in exposure to allergens capable of inducing GI allergy. Protein allergens derived from various food sources – such as milk, eggs, soy, nuts, and fish – are undoubtedly the most frequent inducers of GI allergy globally; however, metals are another class of allergens that are commonly ingested and subsequently mediate hypersensitivity responses of the GI tract (Tomar and Hogan 2020).
Many metals are essential trace elements required by the body for execution of various routine physiological functions. Since many of these metal elements are naturally found in the Earth’s crust such as Ni, Al or Fe, they are often present in considerable concentrations in fruits, vegetables, legumes, and other foods that facilitate their ingestion (Zirwas and Molenda 2009). Drinking water might also be a major source of metal ingestion, particular for metals including Ni, Cu, and Zn (Organization” 2011). Notably, food and water may also serve as vehicles for the ingestion of non-essential, toxic metals – including lead (Pb), Cd, Hg and arsenic (As) – present as contaminants in these sources (Donald, Wissel, and Anas 2015; Khan et al. 2010; Onakpa, Njan, and Kalu 2018). Ingestion exposures to metals might also result from their accidental transfer into food items during various handling processes. For example, the use of metal cookware and utensils may facilitate the transfer of metals like Ni, Cr and Fe into consumables while preparing food (Kuligowski and Halperin 1992). An assortment of different metals might also be accidentally or unintentionally ingested independently of their association with food, water, and other consumables. For example, metal particulates are often unwittingly transferred from the hands to the mouth during daily activities and subsequently ingested.
In spite of the numerous unique presentations of GI allergy and the high frequency of metal ingestion, a limited number of metal-associated hypersensitivity responses were reported to occur in the GI tract; however, it should be noted that metal allergens were only recently identified as potential causative agents of the few GI hypersensitivity reactions described below. Correspondingly, it is likely that additional presentations of metal-induced GI allergy might be identified in the near future as advances in our understanding of the digestive tract’s unique immunological functions are established.
Contact allergic gastritis/mucositis
Contact allergic gastritis (sometimes referred to as contact allergic mucositis in the context of the GI tract) is one of the more recently-identified variants of GI allergy. This condition is characterized by eruptions of localized inflammation within the epithelial lining of the digestive tract following antigen ingestion (Mahdi, Israel, and Hassall 1996; Pföhler, Vogt, and Müller 2016). Interestingly, contact allergic gastritis has been almost exclusively associated with metal antigens. Accordingly, metals capable of triggering contact allergic gastritis are most often ingested as a result of their natural occurrence in various food items or following the release of ions from dental materials and subsequent transport to the GI tract by saliva. Clinical symptoms of contact allergic gastritis tend to remain isolated to the GI tract and include stomach upset, cramping, and bloating. Cell-mediated hypersensitivity reactions orchestrated within the intestinal mucosa are responsible for these symptoms, and often reflect a secondary manifestation of an established allergic condition (Nakajima 1977). For example, patients experiencing symptoms of contact allergic gastritis often report a history of metal-induced ACD or allergic contact stomatitis (Pföhler et al. 2012). It has been suggested that the same metal-reactive T-cell populations involved in these allergic disorders are responsible for development and pathogenesis of contact allergic gastritis, explaining the apparent correlation between the two conditions; however, more research is needed to confirm the validity of this suspected causal relationship.
Contact allergic gastritis is commonly associated with Ni sensitivity and was examined in this context by several investigators (Borghini et al. 2016). Notably, Di Gioacchino et al. (2000) showed that ingestion of Ni by sensitized individuals led to an influx of immune cells to the lamina propria and epithelium of the GI tract. Memory T-cells, specifically, were found to accumulate in the intestinal mucosa, consistent with the cell-mediated hypersensitivity mechanisms responsible for the disease. Collectively, these observations support the existence of a causal link between ACD and subsequent emergence of allergic contact gastritis. Pföhler, Vogt, and Müller (2016) noted a case of contact allergic gastritis in a patient experiencing concurrent GI pain and mucosal lesions shortly after the implantation of a dental bridge and crown comprised of Au, Pd, and zirconium. Subsequent patch tests revealed the prior existence of delayed-type allergic reactivity to Au, Mn, Ni, Pd, vanadium, and zirconium in the individual, and the dental implants were subsequently removed. The subject experienced immediate resolution of both dermal and GI symptoms upon removal of the devices.
Allergic esophagitis
Allergic esophagitis is one of the most common presentations of GI hypersensitivity that manifests within the upper segments of the digestive tract (Gómez-Aldana et al. 2019). The condition develops in sensitized individuals who, following antigen ingestion, develop localized allergic inflammation of the esophagus (Kuźmiński et al. 2020). This presentation of GI allergy may be either acute or chronic in nature. Although different immunological mechanisms might lead to emergence of allergic esophagitis, prototypical signs and symptoms are largely conserved between disease endotypes, and include difficulty swallowing, reflux-like sensations, localized pain, and esophageal lesions (Hill and Spergel 2016).
Eosinophilic Esophagitis: The most common variant of allergic esophagitis is eosinophilic esophagitis, which is characterized by significant influx and accumulation of eosinophils within the esophageal mucosa following antigen ingestion (D’alessandro et al. 2015). In patients with suspected disease, histological analysis might be performed, wherein the diagnostic criteria for the condition is the existence of ≥ 15 eosinophils at 400x magnification on a 0.3 mm2 surface of tissue (Kuźmiński et al. 2020). Prototypical Th2 immune responses are responsible for the pathogenesis of eosinophilic esophagitis, and similarly, the disease tends to be more common in atopic individuals (Vinit et al. 2019). The major allergens associated with this disease include protein epitopes of milk, wheat, soy, eggs, peanuts/tree nuts, and fish/seafood; however, eosinophilic esophagitis may also emerge following the ingestion of metal allergens in some subjects. Nickel was implicated in the majority of these cases and shown to initiate eosinophilic esophagitis alone or in combination with other clinical manifestations of GI allergy (Nucera et al. 2019).
Lymphocytic Esophagitis: Lymphocytic esophagitis is another subtype of esophagitis mediated by allergic mechanisms (Rubio, Sjödahl, and Lagergren 2006). This disease variant is far less common than eosinophilic esophagitis and characterized by intraepithelial lymphocytosis (no established numbers for diagnostic criteria) and minimal granulocyte presence within the esophagus (Avila et al. 2021; Purdy et al. 2008). In most patients suffering from lymphocytic esophagitis, CD8 + T-cells constitute the predominant lymphocytic subtype detected in tissue biopsies (Moiseff et al. 2021; Muller et al. 2021). Biopsies also frequently reveal the existence of spongiosis – lesions that closely resemble those seen in cases of ACD (Purdy et al. 2008). Consistent with these clinical patterns, some of the same antigens known to cause ACD were also implicated in lymphocytic esophagitis. Only a few studies correlated metal allergens to this condition at present. In one such report, a woman presenting with lymphocytic esophagitis and concurrent presentations of allergic reactivity to various antigens was examined (Wojas et al. 2021). Although the subject was found to exhibit immediate-type allergic responsivity to many common food and aeroallergens (e.g., birch pollen, hazelnuts, grasses, rye), it was also determined that she had developed delayed-type hypersensitivity responses to Ni, which were responsible for a history of chronic ACD outbreaks. Consequently, ingestion of Ni by this patient was identified as a potential cause of lymphocytic esophagitis.
Other immune responses of the gi tract with potential implications in metal allergy
By some estimates, GI allergy has become the most prevalent form of allergic disease worldwide (Tomar and Hogan 2020). Despite the frequency of these disorders, only a small fraction of GI hypersensitivity responses was reported to involve metal allergens. Accordingly, the digestive tract represents one of the tissues least commonly implicated in metal allergy. Although ingestion of metals is common, this route of exposure more frequently results in the systemic absorption of metals (or their excretion), as opposed to their local retention within tissues of the digestive tract (Mamtani et al. 2011). As a result, the general lack of causative associations observed between ingested metal allergens and local GI hypersensitivity reactions is likely to be at least partially explained by the physicochemical properties of metal ions, which facilitate their rapid absorption through the intestinal mucosa and into the circulation.
Although sensitization to food allergens is known to occur following their ingestion, it remains unclear if sensitization to metals might occur by similar mechanisms. Borghini et al. (2020) suggested that metal sensitivity may emerge, specifically, in susceptible individuals with Celiac disease following Ni ingestion. This association reflects the propensity for celiac patients to consume greater amounts of dietary Ni (e.g., from corn) than healthy individuals due to their avoidance of gluten-containing foods. As a result, it seems that these subjects may be at increased risk for the development of Ni hypersensitivity via the GI tract, although no definitive evidence for such effects in humans has been published. Similarly, few studies examined the potential for metal sensitization following ingestion in animal models. In one of the few existing reports pertaining to this concept, Al was shown to effectively induce colorectal hypersensitivity in rats and mice following ingestion (Esquerre et al. 2019).
Several chronic inflammatory conditions of the digestive tract have been specifically correlated with metal hypersensitivity in human subjects. These findings suggest that allergic inflammation induced by metal allergens may be directly or indirectly involved in the pathogenesis of these disorders. For example, the prevalence of Ni-induced ACD is known to be significantly elevated amongst patients with non-celiac wheat sensitivity (D’alcamo et al. 2017).
Gastro-esophageal reflux disease (GERD) is one of the most frequently-diagnosed diseases of the digestive tract in Western countries and estimated to affect up to 28% of the general population in North America (Clarrett and Hachem 2018). Ineffective control of GERD was associated with profound physical discomfort, decreased quality of life, and significant morbidity (Castell et al. 2004). Although some cases of GERD emerge as a result of non-immunological mechanisms, allergic-type mechanisms are known to be involved in many instances. Hypersensitivity-associated forms of GERD may be related to food allergens derived from shrimp, milk, and barley, among others (Pomiecinski et al. 2010). Metal allergens have not been implicated in GERD, although several investigators demonstrated a significant correlation between the disease and allergic sensitivity to Ni (Aslan, Sezikli, and Erdal 2017; Stanghellini et al. 2016). Moreover, the adoption of a low-nickel diet was found in many instances to significantly improve GERD symptoms (Yousaf et al. 2020). Although these observations suggest that Ni sensitivity may be involved in some cases of GERD, a definitive causal relationship has yet to be established.
Several studies also established a connection between metal allergy and irritable bowel syndrome (IBS). When compared to healthy controls, patients with IBS are significantly more likely to exhibit allergic reactivity to metals including Ni and Zn (Kageyama et al. 2019). In one study, 56.5% of the 147 subjects experiencing symptoms of IBS were noted to be hypersensitive to at least one metal. It has been proposed that dental metals, specifically, induce delayed-type hypersensitivity responses within the digestive tract of sensitized individuals, which contributes to disease pathogenesis in a subset of IBS patients. The potential involvement of metal hypersensitivity reactions in IBS is further supported by observations that adoption of a low Ni diet markedly improves symptoms of the disease (Rizzi et al. 2017).
A similar association between dental metals and ulcerative colitis (UC) was demonstrated in a recent study. As described in a 2020 report, 65 patients with UC and 22 healthy controls – all with metallic dental implants or prosthetics – were included in the study and tested for allergic reactivity to various metal allergens (Kageyama et al. 2020). It was determined that 60% of the UC patients in the study were allergic to at least one metal species, whereas only 32% of the healthy controls exhibited metal reactivity. Nickel and Pd were identified as the two metals most commonly implicated in these responses. Further, a greater degree of lymphocyte responsivity was seen in UC patients compared to healthy controls upon metal allergen exposure. Similar to the apparent involvement of metal allergy in IBS, the pathogenic mechanisms of UC have now also been suggested to involve metal- induced hypersensitivity responses in some subsets of the disease.
Metals and systemic hypersensitivity responses
Dermal contact, inhalation, and ingestion are all common means of exposure to metals in the general population that might lead to allergic responses. Although metals are absorbed, metabolized, and distributed following these exposures, which result in systemic responses, direct systemic exposures to metals might also induce hypersensitivity responses, although this type of exposure is only relevant in a small subset of the population. Most of the scenarios in which systemic exposures to immunogenic metals occur originate from within the biomedical sector (Chen and Thyssen 2018). The use of metal-containing objects and metal-based reagents in various medical and dental applications are often implcaited, and similarly, healthcare patients constitue the majority of individuals at risk for systemic exposure to metals. Specifically, surgical implantation of orthopedic and intracoronary devices, placement of orthodontic appliances and utilization of other dental materials, and administration of therapeutic substances containing metals constitute the most common sources of systemic metal exposures (Hallab and Jacobs 2009; Pigatto et al. 2014).
From 2000 to 2010, approximately 5.2 million total knee replacements were performed in the United State alone (Teo and Schalock 2016). In 2008 and 2010, respectively, an estimated 27,000 total shoulder arthroplasties and 311,000 total hip replacement surgeries were also performed. In these and other similar orthopedic procedures, the joints are reconstructed using artificial structures that frequently contain several different metal constituents. The metal elements most commonly found in these devices include Ni, Co, Cr, molybdenum, zirconium, and Ti alloys, along with stainless steel (Teo and Schalock 2016). Over time, ions of these metal species are released from the implant and absorbed into the circulation, which facilitate development of various immunologial reactions within the body.
Cardiovascular and endovascular implants also frequently contain metal constituents and might mediate systemic exposures to the respective ions. Coronary stents tend to be associated with the release of Ni, Au, Co, and Ti ions directly into the circulation (Honari et al. 2008; Honari, Taylor, and Ellis 2005). Comparatively, the implantation of pacemakers has been selectively implicated in the release of Ti ions (Honari et al. 2008; Peters et al. 1984). Intrauterine contraceptive devices represent another potential source of systemic metal ion exposure; however, since these implants are comprised solely of Cu, their use is associated with selective exposure to Cu ions, which may be continually released following implantation (Hostynek and Maibach 2004). As a result, patients implanted with these devices represent another subset of individuals at risk for systemic metal exposures and potential immune responses that may ensue.
Systemic exposures to metals might also result from the use of metal-based dental materials (Přikrylová, Procházková, and Podzimek 2019). Orthodontic appliances, fillings, bridges, and restorations are all likely to contain various metal constituents capable of releasing ions over time. As previously discussed, dental materials are a potential source of contact exposure to metals, which lead to localized allergic responses in the oral mucosa; however, several anatomical and physiological characteristics of the oral mucosa might interfere with the elicitation of local dermal hypersensitivity responses to metals in the oral mucosa (Hosoki et al. 2009; Kim et al. 2015). For example, the protein content within the oral mucosa is significantly lower than that of keratinized skin, and thus, may present a greater challenge for haptenization of small molecules (Lesueur and Yiannias 2003). In addition, the effective dose of irritants and allergens that come into contact with the oral mucosa may become significantly reduced as a result of their dilution with saliva (Minciullo et al. 2016). Saliva might also mediate the solubilization and degradation of many allergens, compromising their biological activity in the skin around the mouth. Comparatively, the high level of vascularization in the oral mucosa promotes the systemic absorption of antigens, such as metal ions, released from dental materials. Saliva might also mediate ingestion of these antigens, which then are absorbed into the circulation via the GI tract (Chen and Thyssen 2018). Accordingly, the potential for systemic responses to metal allergens released from dental materials is often a greater concern than dermal exposures. Some of the major metals of concern in this context include Hg, Ag, Ni, Co, Cr, Au, Al, Pd, and Cu (Hosoki et al. 2009; Lee et al. 2001).
Finally, pharmaceutical agents constitute another potential source of systemic exposure to metals that may result in allergic reactions. Gadolinium-based contrast agents, Fe supplements, Pt anticancer agents, and Au salts (used in chrysotherapy) are all administered intravenously or intramuscularly to patients in certain biomedical settings (Faa et al. 2018; Fok and Smith 2017; M.f.h and Barbosa 2016; Makrilia et al. 2010). As one of the most common immunological adjuvants used in commercial vaccine formulations, systemic exposure to Al salts following intramuscular injections also occurs frequently (Mbow, De Gregorio, and Ulmer 2011). The parenteral administration of these substances results in exposure to a significantly higher dose of metals compared to the other previously-mentioned sources of systemic exposure. As a result, these patients may represent a population that may be more susceptible to subsequent metal-induced immune reactions.
Although systemic exposures constitute the least common mechanism by which humans are likely to encounter allergenic metals, an extensive number of unique metal-induced systemic hypersensitivity reactions have been described in the literature. Some of the major discriminating features of these responses include underlying immunological mechanisms (e.g., delayed-type, immediate) and primary site of elicitation signs/symptoms (e.g., widespread, concentrated within the skin). Distinctive features of the elicitation response might also be employed to categorize, compare, and differentiate between the various systemic allergic responses initiated by metals. Unlike the majority of dermal and respiratory allergic responses that were described in previous sections, many of the systemic hypersensitivity reactions associated with metals manifest pro-foundly unique symptom profiles. For example, some of the unique clinical manifestations of metal-induced systemic hypersenstivity responses include the formation of foreign body granulomas and tissue necrosis, neurological impairments and chronic fatigue, and myocardial infarction with coronary spasm (Fernandes et al. 2019; Teo and Schalock 2016).
All of the different presentations of metal-induced systemic hypersensitivity that were reported in the literature are discussed in the sections below. The defining features of each allergic response are higlighted, and specific metals that have been associated with the reactions are listed. A summary of the most common systemic hypersensitivity responses and corresponding metals associated with each condition is presented in Table 5.
Table 5.
Specific Metals Associated with Different Presentations of Systemic Hypersensitivity.
Table 5: Metals implicated in different forms of systemic allergy. Common systemic presentations of metal allergy are listed in the table above. Responses are grouped by primary mechanism of hypersensitivity and specific metals implicated in each disease variant are denoted by the parenthesized numbers within the assoicated column, which correspond to relevant citations.
Anaphylaxis
Anaphylaxis is an acute, potentially life-threatening, systemic response that develops immediately following the sudden release of molecular mediators by mast cells and basophils (Loverde et al. 2018). Activation of these cells and their subsequent degranulation might result from both allergic and non-allergic mechanisms. Similarly, while all presentations of anaphylaxis are immunologically-mediated, only some of these reactions implicate true allergic processes.
Anaphylaxis has become increasingly recognized as a heterogeneous group of immune responses in recent years (Tomar and Hogan 2020). Several phenotypic and endotypic variants of the syndrome were identified and characterized within the scientific literature. Phenotypes of anaphylaxis include type I, cytokine-release, complement, and mixed reactions (Jimenez-Rodriguez et al. 2018). Corresponding endotypes associated with anaphylactic responses include IgE- and non-IgE-mediated mechanisms, cytokine-mediated responses, mixed processes, and complement/bradykinin-induced direct activation reactions. Further, several distinctive anaphylaxis response patterns were also recently identified and based upon parameters including the absence/recurrence of symptom cycling, as well as symptom onset, peak response, and reaction resolution times (Loverde et al. 2018). Accordingly, anaphylaxis can occur in a uniphasic, biphasic, or protracted response pattern.
The most common form of anaphylaxis is mediated by type I hypersensitivity mechanisms. In sensitized individuals, systemic antigen exposure triggers IgE-dependent activation of mast cells and basophils, leading to the release of many unique preformed mediators such as tryptase, histamine, and chemokines with various physiological functions (Loverde et al. 2018). The actions of these molecules are responsible for the subsequent emergence prototypical anaphylactic symptoms, which range from eruption of widespread urticarial lesions and angioedema in the skin, to profound bronchoconstriction with potential for respiratory insufficiency, and severe hypotension that lead to dizziness and syncope (Pawankar et al. 2013). In some cases, symptoms may be mild and readily managed with minimal intervention; comparatively, catastrophic reactions may also ensue, requiring immediate medical attention to monitor and treat life-threatening symptoms of anaphylaxis (Tomar and Hogan 2020). Pawankar et al. (2013) estimated that the lifetime occurrence of anaphylaxis ranges from 0.05–2% in the general population. Some of the major causative agents of anaphylaxis include pharmaceutical agents, insect venom, and food allergens (Muñoz-Cano et al. 2016). A small number of case reports also described the induction of anaphylactic responses in metal-sensitive subjects following various exposure conditions.
One of the major mechanisms by which metal antigens induce anaphylactic responses involves systemic administration of various metal-containing pharmaceutical agents. Accordingly, gadolinium-based contrast agents were associated with potential to induce anaphylactic responses in some subjects (Rodriguez-Nava et al. 2019) Systemic administration of platinum-containing antineoplastic agents were also found to produce similar responses in susceptible individuals (Makrilia et al. 2010). Anaphylactic responses were also reported following the administration of magnesium sulfate during preterm labor, barium enemas for diagnostic imaging, and intravenous iron supplements in anemic patients (Janower 1986; Rampton et al. 2014; Thorp et al. 1989).
Anaphylactic responses to metals might also occur following exposure by other routes. For example, ingestion of Ni was found to induce anaphylactic responses in some sensitized individuals (Antico and Soana 1999). Several instances of dermal metal exposure leading to anaphylaxis were also reported. Dermal exposure to Co was shown by Krecisz et al. (2009) to be sufficiently capable of triggering anaphylactic responses in a worker with established dermal reactivity to the metal. In addition, in one worker with existing symptoms of respiratory allergy triggered by occupational exposures to iridium salts, routine clinical evaluations were performed to elucidate the involvement of different immunological mechanisms associated with his responses (Bergman, Svedberg, and Nilsson 1995). Subsequent scratch testing with iridium salts led to the immediate onset of anaphylactic symptoms – a type I allergic response determined to be consistent with the mechanisms responsible for the worker’s respiratory symptoms.’
Although the underlying immunological mechanisms responsible for allergic anaphylaxis have been largely attributed to effector molecules including IgE, IgG, and immune complexes, there have also been occasional reports of metal-associated anaphylaxis that appear to be at least partially mediated by T-lymphocytes. In one such case, a specific inhalation challenge was used to evaluate the time course of allergic reactivity in a Cr-sensitized welder (Moller et al. 1986). Although prototypical indices of anaphylaxis were subsequently observed in the subject, the response did not become evident until several hr post-exposure, leading to the conclusion that cell-mediated mechanisms may be involved in some cases of anaphylaxis, wherein a delayed onset of symptoms may be observed.
Systemic allergic contact dermatitis
As previously described, ACD responses typically emerge as a result of skin contact with allergens; however, in some cases, allergens capable of entering the circulation might accumulate in the skin, leading to ACD-like eruptions. This response is termed ‘systemic ACD’ and has been associated with various metals including Ni, Co, Au, Zn, Al, and Cr (Wicks et al. 1988; Yoshihisa and Shimizu 2012). Systemic ACD is known to emerge following the release of ions from cardiovascular implants, orthopedic devices, and other surgical implantations containing Ni, Co and Cr directly into the bloodstream (Giménez-Arnau et al. 2000; Nosbaum et al. 2008; Zhubrak and Bar-David 2014). Aluminum-based vaccine adjuvants also facilitate development of systemic ACD reactions following intramuscular administration (Mistry and Dekoven 2021). In addition, dental materials also release metal ions that may be absorbed into the systemic circulation and subsequently trigger widespread ACD eruptions (Aquino and Rosner 2019). Zinc, Hg, Ni, Co, and Cr are all metals used in dental materials known to induce systemic ACD (Nedorost 2009; Pigatto et al. 2014). Gold-induced systemic ACD responses also occur and most commonly reported in patients undergoing chrysotherapy following intravenous or intramuscular injection of Au salts for treatment of various immune disorders (Wicks et al. 1988). Oral formulations of Au salts were also shown to produce systemic ACD eruptions, along with the ingestion of Ni present in food items (Malinauskiene, Isaksson, and Bruze 2013; Zirwas and Molenda 2009).
One of the distinctive clinical presentations of systemic ACD is referred to as ‘baboon syndrome,’ which reflects the characteristic distribution pattern of dermal eruptions following antigen exposure (Andersen, Hjorth, and Menné 1984). In these cases, symmetric diffuse erythema becomes present on the buttocks, upper inner surfaces of the thighs, and the armpits. Cases of baboon syndrome were detected following inhalation of Hg vapors, exposures to broken thermometers, and topical application of Hg-containing disinfectants (Fernandez et al. 1995; Le Coz et al. 1996; Tschanz and Prins 2000). Nickel was also associated with initiating baboon syndrome in some sensitized individuals following ingestion and systemic exposures (Antico and Soana 1999; Bibas et al. 2013; Kolodziej et al. 2003; Sánchez-Morillas and Ar 2004).
Airborne allergic contact dermatitis
Systemic immune responses are known to mediate another variant of ACD in which exposure to the inciting antigen occurs by inhalation. This condition is called airborne ACD and often involves the emergence of symmetrical rashes on the face, neck, and eyelids approximately 24 hr post antigen exposure (Pongpairoj et al. 2016). Airborne ACD involves similar delayed-type hypersensitivity mechanisms as those involved in other variants of ACD, and also implicates many of the same metal allergens. Most cases of airborne ACD produced by metals were noted to occur in workers (Kanerva et al. 1999). This is reflective of the greater propensity for metal aerosolization and subsequent inhalation to occur in certain occupational settings. Accordingly, specific metals that have been consistently implicated in cases of airborne ACD include Ni, Be, Cr, Co, and Au (Kimyon and Warshaw 2019; Watsky 2007). Less commonly, Hg, Sn, and Ag were also attributed to outbreaks of airborne ACD (Dooms-Goossens et al. 1986; Quenan et al. 2014).
Chronic urticaria syndrome
Chronic urticaria is a systemic allergic response with an estimated lifetime prevalence of approximately 1% in the general population (Hon et al. 2019). The disease might involve both acute allergic responses following antigen exposure, as well as episodic responses spanning over extended durations of time in the absence of any detectable encounters with antigen (Sachdeva et al. 2011). Acute inducible responses involved in the condition most often result from ingestion or systemic absorption of antigen, following which, widespread eruptions of skin rashes become evident within min or hr. In some individuals, clinical presentations might mirror many of the prototypical signs and symptoms of anaphylaxis, and may include the development of angioedema and bronchoconstriction (Gomułka and Panaszek 2014). Both IgE and IgG molecules were implicated in the immediate-type hypersensitivity mechanisms ultimately responsible for mast cell degranulation and subsequent physiological responses involved in acute presentations of the disease (Hon et al. 2019). By comparison, mechanisms responsible for the sporadic, recurrent manifestations of chronic urticaria in the absence of antigen exposure remain largely unclear. Asero et al. (2017) suggested that physical stimuli, autoimmune mechanisms, pseudoallergic reactions, or vasculitic triggers may be involved. Causative agents of chronic urticaria include food allergens, drugs, and metals. Nickel ingestion as part of the normal diet may trigger development of chronic urticaria in some sensitized subjects (Abeck et al. 1993; Antico and Soana 1999; Buyukozturk et al. 2015). Dental metals are also known to initiate chronic urticaria following systemic absorption or ingestion of Cu, Au, Hg, Cr, and Co ions (Barranco Sanz et al. 1989; Mikhailova et al. 2017; Moller et al. 1986).
Systemic nickel allergy syndrome
Systemic nickel allergy syndrome (SNAS) is a unique allergic condition that was reported to occur in approximately 20% of individuals afflicted with contact sensitivity to the metal (Nucera et al. 2019). Patients affected by this disease often experience symptoms following ingestion and subsequent systemic absorption of nickel. Local GI effects such as cramping, nausea, and vomiting, along with systemic manifestations including headache and fatigue and chronic dermatological symptoms are among the most common clinical presentations associated with SNAS elicitation responses (Di Gioacchino et al. 2014). In some cases, respiratory symptoms may also emerge.
These symptoms and the immunological mechanisms responsible for SNAS are believed to involve both cell-mediated effects, as well as prototypical Th2-type responses. The existence of nickel-reactive T-cell populations is a common feature of the disease, and subjects with SNAS often exhibit a significant increase in number of CD45RO+ memory cells present in the GI mucosa (Falagiani et al. 2008). Similarly, while established populations of nickel-specific regulatory T-cells may be detected in healthy individuals, this tolerogenic cell type is non-existent in SNAS patients. Although T-cells play a prominent role in the pathogenesis of SNAS, the simultaneous involvement of several critical Th2-associated effector functions led to the classification of this disease as a mixed-type hypersensitivity response initiated by Ni (Di Gioacchino et al. 2018). Accordingly, one of the major cytokines responsible for SNAS responses is IL-5. Consistent with this molecule’s role in immediate-type allergic responses, the enhanced production of IL-5 detected in SNAS patients leads to eosinophilic-dominant inflammation (Falagiani et al. 2008). As a result, many patients develop eosinophilic esophagitis and other eosinophil-mediated reactions in the GI tract.
Treatment of SNAS often requires elimination of foods that contain high levels of Ni from the diet. This modification leads to symptom improvement in many subjects and was also shown to attenuate dermal responsivity to Ni in some individuals (Antico and Soana 2015). Oral hyposensitization regiments were also employed to treat subjects suffering from SNAS, although relapses following treatment are commonly reported (Bonamonte et al. 2011).
Drug reaction with eosinophilia and systemic symptoms syndrome
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) syndrome is a severe inflammatory response traditionally associated with the administration of certain pharmaceutical agents (Choudhary et al. 2013). The condition is characterized by the existence of a long latency period (2–8 weeks), followed by the emergence of a variety of clinical symptoms ranging from fever and rash to systemic eosinophilia and liver enzyme abnormalities. In severe cases, respiratory symptoms may emerge, which include development of acute respiratory distress syndrome and hypoxic respiratory failure (Taweesedt et al. 2019).
Some metals are also known to induce DRESS in certain individuals. Most of these cases implicate the emergence of symptoms as a result of hypersensitivity-mediated immune mechanisms, and most subjects exhibit allergic sensitivity to the inciting metal prior to DRESS symptom development. Cases of DRESS were reported to occur following implantation of a titanium-based bioprosthesis, oral administration of strontium ranelate, and topical application of a mercury-based disinfectant (Cacoub et al. 2013; Di Meo et al. 2016; Nawaz and Wall 2007; Tschanz and Prins 2000).
Kounis syndrome
One of the most distinctive systemic hypersensitivity responses associated with metal exposure is Kounis coronary hypersensitivity syndrome. This condition is characterized by the emergence of concurrent acute coronary syndromes including coronary spasm, myocardial infarction, and stent thrombosis and physiological responses mediated by the degranulation of intracardiac and intracoronary mast cells (Almpanis et al. 2010). Subsequent clinical presentations of this response include EKG alterations, acute chest pain, dyspnea, and headache (Biteker 2010). Although several variants of the disease were described according to variations in underlying mechanisms, one type of Kounis syndrome is particularly relevant in the context of metal allergy.
In this disorder, individuals with existing metal sensitivity namely Ni allergy prior to the implantation of metal-containing endovascular devices, or subjects that become subsequently sensitized after device implantation, are likely to experience chronic allergic irritation to the coronary intima (Koniari, Kounis, and Hahalis 2016; Kounis 2016). As a result, stented areas tend to become populated by increasing numbers of mast cells and other inflammatory cells. Subsequent release of metal ions from the stent lead to activation of localized mast cells and the corresponding release of mediators including histamine, chemokines, arachidonic acid metabolites, platelet-activating factor, and neural proteases – many of which exert potent effects on the cardiovascular system that might trigger activation of the coronary component involved in Kounis syndrome (Kounis 2013).
Aside from Ni, the only other metal that implicated in Kounis syndrome is gadolinium. While metal endovascular devices constitute the primary source of Ni exposure in Kounis syndrome cases, gadolinium-associated reactions most commonly occur following the parenteral administration of biomedical contrast agents containing the metal (Abusnina et al. 2019; Kounis et al. 2020).
Systemic sensitization and implant failure
For some individuals, the first indication of metal-specific allergic sensitivity emerges following the implantation of metal-based devices within the body cavity. The release of metal ions from joint prostheses, intracoronary stents, surgical screws, electrical devices, and other various biomedical implants can trigger systemic sensitization, following which, an assortment of different biological responses may be experienced by the patient. In this context, one of the outcomes of greatest concern to clinicians is implant rejection.
Total joint arthroplasty is a common surgical procedure that involves the replacement of a patient’s arthritic or damaged joint with a prosthetic device, often containing metal subunits, in order to restore normal function and relieve chronic pain. Although these procedures tend to be exceptionally successful for most patients, implant failure does occur in approximately 10–20% of cases (Samelko et al. 2019). The most frequently-encountered complication responsible for total joint arthroplasty failure (approximately 75% of cases) is implant loosening due to aseptic osteolysis – a process that is often attributed to development of metal hypersensitivity in patient’s post-surgery (Hallab and Jacobs 2009). Cobalt-chromium-molybdenum alloys are one of the most common metal compounds used to construct metal-on-metal implants (Teo and Schalock 2016). Normal wear processes lead to the release of both ionic and particulate debris from the prosthetic device over time, which in some individuals, might result in immune activation (Van Der Merwe 2021). This heightened state of innate immune responsivity might trigger the transition from immunological tolerance to allergic sensitivity in some patients (Samelko et al. 2016). Several investigators demonstrated that this process is associated with the preferential polarization of immune reactivity toward a Th1/Th17-dominant state, which often occurs as a result of debris-induced inflammasome activation and pattern recognition receptor (PRR) ligation by metal ions (Hallab et al. 2008; Samelko et al. 2016). Subsequently, metal-specific T-lymphocytes are generated and recruited to the implant location, where chronic peri-implant inflammation lead to implant failure (Thomas et al. 2009). Zirconium, Pd, and Ti are other metals that are associated with similar sensitizing effects following debris release from orthopedic implants (Dawson-Amoah et al. 2020; Kręcisz, Kieć-Świerczyńska, and Chomiczewska-Skóra 2012; Teo and Schalock 2016; Towers and Kurtom 2020).
Similarly, development of allergic reactivity to metals was demonstrated in numerous studies to be correlated with increased potential for restenosis (reoccurrence of arterial narrowing following surgical intervention) following implantation of intracoronary stents. Many case reports have been published describing patients who, following gold stent placement, subsequently develop delayed-type allergic sensitivity to the metal, and then later experienced restenosis (Ekqvist et al. 2007; Svedman et al. 2009, 2005). Similar observations were noted in subjects implanted with stents comprised of Ni, Co, Cr, and molybdenum (Aliağaoğlu et al. 2012; Bui et al. 2022; Fujii et al. 2021; Köster et al. 2000; Nagura et al. 2022). Accordingly, restenosis constitutes another presentation of implant failure associated with development of allergic responsivity to biomaterials comprised of metal constituents.
In some cases, systemic sensitization following the implantation of metal-containing devices results in unique complications aside from implant rejection (Eliaz 2019). Several cases of impaired fracture healing were reported following osteosynthesis (surgical repair of a fractured bone), wherein the compromised capacity for bone repair was attributed to patients’ development of allergic sensitivity to metals such as Ni or Ti used in the repair process (Thomas et al. 2006a, 2006b). Nonspecific, widespread symptoms including chronic fever and abdominal pain were also reported to occur in patients following the implantation of metal-based devices (Luvsannyam et al. 2021; Wang et al. 2016). Stejskal et al. (2006) suggested that allergic sensitization initiated by biomedical implants results in cytokine-mediated systemic inflammation that might impact the hypothalamus-pituitary-adrenal axis, resulting in vague systemic symptoms that fundamentally emerge due to metal-induced hypersensitivity reactions.
Metallosis: Metallosis is another potential cause of implant failure. It is a condition characterized by deposition and accumulation of metal debris in the soft tissues associated with metal-on-metal implants (Vaz et al. 2019). The subsequent inflammatory reactions initiated by this process may lead to pain and swelling, pseudotumor formation, aseptic fibrosis, and osteolysis – symptoms that remain localized at the implant site (Oliveira et al. 2015). In some cases, however, metallosis might produce systemic effects. Systemic symptoms associated with metallosis generally involve nonspecific complaints including neurological impairments, memory loss, and chronic fatigue (Sahan and Anagnostakos 2020). It has been estimated that metallosis develops in approximately 5% of patients following implantation of metal-containing prosthetic devices. Vanadium, Co, Cr and Ti have all been associated with the development of metallosis (Breen and Stoker 1993; Czekaj et al. 2016; Pesce et al. 2013).
Metallosis is a condition that has only recently been identified as a unique syndrome involving distinctive characteristics that differentiate it from other metal-induced systemic inflammatory responses. Accordingly, the underlying mechanisms of pathogenesis responsible for metallosis have not yet been fully elucidated (Sahan and Anagnostakos 2020). Some investigators asserted that metal hypersensitivity plays a key role in the disease; comparatively, other accounts described the condition as a form of autoimmunity induced by metal implant debris (Oliveira et al. 2015). Notably, several publications classified metallosis as a form of autoimmune/autoinflammatory syndrome induced by adjuvants (ASIA) (Vaz et al. 2019). This collection of diseases encompasses many diverse autoimmune-mediated conditions that are known to emerge following adjuvant triggers (Schiff et al. 2021). In accordance with this classification scheme, it was proposed that metal implant debris facilitates the development of autoinflammatory responses in cases of metallosis; however, it remains unclear if these autoimmune reactions develop as a result of metal-induced novel antigen formation or in response to existing host proteins (Loyo et al. 2012). This proposed paradigm is consistent with the frequent occurrence of prototypical autoimmune responses, such as hemolytic anemia, observed in subjects with metallosis (Nakamura et al. 1997; Oliveira et al. 2015).
The predominant subset of immune cells populating peri-implant tissues was shown to vary amongst patients with metallosis. In some cases, a predominance of macrophages and T-cells is observed, consistent with the existence of granulomas at the implant site, and occasionally, the formation of granulomas in distal tissues like the lungs (Balbouzis, Georgiadis, and Grigoris 2016; Mahendra et al. 2009). Interestingly, multi-nucleated giant cells were also observed in a few of these patients (Sokół et al. 2020). By comparison, other analyses demonstrated a selective infiltration of B-cells in implant-associated pseudotumors and surrounding tissues (Hasegawa et al. 2012). Occasionally, eosinophilic-dominant inflammation was also described in patients with metallosis (Levy et al. 2016). These discrepancies suggest that the primary pathophysiological mechanisms implicated in metallosis may be patient-specific and reflective of differential involvement from the adaptive immune system (Sagoo et al. 2021). Several investigators suggested that T-cell-dominant influx to the implant site may indicate that hypersensitivity-based mechanisms of inflammation are responsible for cases of metallosis in subjects with existing metal sensitivity; however, it was noted by others that the frequency of lymphocyte reactivity to Ni, Co and Cr did not differ between groups of patients with and without implant-associated psuedotumors (Hasegawa, Iino, and Sudo 2016; Kwon et al. 2010).
Other systemic immune responses with potential implications in metal allergy
In addition to metallosis, other potential forms of systemic ASIA have been associated with allergenic metals (Kagan et al. 2020). In one patient, implantation of metal plates led to development of delayed-type hypersensitivity to molybdenum. In subsequent months, symptoms of systemic lupus erythematosus (SLE) began to emerge, suggesting that sensitization to the metal may have been a trigger for the development of autoimmunity in this patient (Federmann et al. 1994). This observation is consistent with knowledge that the occurrence of type IV hypersensitivity to metals is elevated in patients with SLE. A similar trend is also evident amongst patients afflicted with similar autoimmune conditions, such as rheumatoid arthritis, chronic fatigue syndrome, and Sjogren’s syndrome (Bjørklund, Dadar, and Aaseth 2018; Geier and Geier 2021; Stejskal, Reynolds, and Bjørklund 2015; Sterzl et al. 1999). Allergic reactivity to Ni, Au, and Hg are often implicated in these cases (Bjørklund, Dadar, and Aaseth 2018; Loyo et al. 2012).
Although many of the underlying mechanisms responsible for fibromyalgia remain unknown, it is well-accepted that inflammatory responses play a critical role in disease pathogenesis (Bellato et al. 2012). Interestingly, these inflammatory mechanisms appear closely associated with metal-induced allergic inflammation in many individuals (Patten, Schultz, and Berlau 2018; Sluka and Clauw 2016). In one study of 15 female patients, all subjects diagnosed with fibromyalgia also exhibited contact sensitization to one or more metals (Stejskal, Ockert, and Bjørklund 2013). Sensitivity to Ni was most common in these subjects, followed by reactivity to inorganic Hg, Cd, and Pb, respectively. Subsequent avoidance of these metals was associated with notable improvement of symptoms, suggesting that allergic inflammation caused by metals is closely intertwined with disease presentations in fibromyalgia. Although the onset of disease was not able to be discerned in this study, it has been proposed that metal-induced ACD may precede development of fibromyalgia, thus, representing another variant of ASIA with specific relevance to metal allergy. Metal-induced inflammation involving non-allergic mechanisms was also demonstrated to be a potential trigger of ASIA. Gadolinium, Al, Ni and silicon exposures were associated with subsequent development of fibromyalgia and chronic fatigue syndrome in a sensitization-independent manner (Colafrancesco et al. 2014; Exley et al. 2009; Kötter et al. 1995; Lattanzio 2019; Lattanzio and Imbesi 2020; Stejskal 2014).
Panniculitis is another type of ASIA that has been correlated with metal allergy. This condition is characterized by the development of lesions within the host’s adipose tissue – most frequently presenting as erythematous nodules within the subcutaneous fat layer (Wick 2017). A number of different immune cell subsets may be detected within these lesions, including lymphocytes, neutrophils, eosinophils, and macrophages, and their development is often accompanied by general symptoms of malaise including fever and fatigue (Requena and Yus 2001). Although panniculitis might develop following exposure to many different types of antigens, allergenic metals were cited as causative agents of the disorder in many instances. As described in one report, implantation of a metal-based orthopedic device was responsible for the development of panniculitis in one subject. Following surgery, the patient’s incision site failed to heal (no microbial infection), lesions of the adipose tissue were detected at the site of implantation, and general symptoms of ASIA were present (myalgia, low-grade fever, and arthralgia) (Radenska-Lopovok et al. 2021). Although it was concluded that the metal-containing implant was responsible for ASIA/panniculitis development, specific metals responsible for the condition were not identified. In another report, an accidental molten aluminum burn was identified as the cause of panniculitis that subsequently developed in an exposed worker (Chao, Lee, and Lee 2010). Directly following the accident, the individual developed localized panniculitis and eosinophilic cellulitis that resolved following a month of systemic corticosteroid therapy. Several months later, the patient had a relapse and reemergence of symptoms. It was determined that the subject had become sensitized to Al following the initial accident, and subsequent exposures to the metal caused similar outbreaks.
In addition to the many variants of ASIA that were correlated with metal hypersensitivity, allergic reactivity to sensitizing metals was also suggested to play a role in the promotion of a certain type of cancer – cutaneous T-cell lymphoma (CTCL). Many subtypes of CTCL were identified, but all variants of the disease are classified as extranodal non-Hodgkin’s lymphomas, wherein malignant monoclonal T-lymphocytes selectively infiltrate the skin (Bagherani and Smoller 2016). In the early stages of disease development, CTCL is often misdiagnosed as one of many common inflammatory skin conditions including ACD, psoriasis, lichen planus, folliculitis, or vitiligo (Hristov, Tejasvi, and R 2021). Interestingly, many subjects that develop CTCL have a history of these and other similar skin disorders. It is believed that these conditions often represent a precursor to cancerous transformation due to recurrent antigenic stimulation associated with chronic disease states. Accordingly, several cases of CTCL were correlated with chronic ACD induced by Cr, Ni, and Co (Khamaysi et al. 2011; Tilakaratne and Sidhu 2015).
Complex disease states and clinical presentations of metal allergy
The vast majority of subjects afflicted with metal allergy experience a single, primary presentation of the condition, consistent with one of the characteristic disease variants described in the previous sections; however, exposure to allergenic metals might trigger development of an increasingly complex state of immunological responsivity in some individuals, who subsequently experience unique allergic implications as a result. Several case reports describing such responses have been published over the past few decades. From these reports, at least three unique variants of complex allergic responses have been identified and associated with metal allergy and are discussed in the following sections. Overall, these types of responses are relatively uncommon (though likely underdiagnosed and underreported). As a result, they remain largely overlooked in clinical settings, the workplace, and research endeavors at present, and minimal information is currently available regarding the underlying mechanisms, susceptible populations, and general prevalence of these complex allergic responses to metals.
Concurrent allergic reactivity to multiple metals
One of the most common complex clinical presentations of metal allergy involves development of allergic sensitivity to more than one metal by an individual (Lidén et al. 2016). Polysensitization may emerge following simultaneous sensitization to multiple metals or sequential development of allergic reactivity to multiple individual metals. Sequential development of metal-specific reactivity appears to occur more frequently, as existing contact sensitivity to a single metal was shown to predispose for development of subsequent allergic reactivity to other metals (Carlsen et al. 2008; Kränke and Aberer 1996; Lammintausta et al. 1985).
Although concurrent allergic reactivity to multiple different metals is known to occur in the general population, the prevalence of co-sensitization is higher amongst workers (Hegewald et al. 2005; Rastogi et al. 2018; Zigante et al. 2020). As illustrated in one study, 25% of hard metal workers with existing contact sensitivity to Ni subsequently became sensitized to Co during the study, whereas a similar trend was only observed in 5% of the general population (Rystedt and Fischer 1983). Several unique exposure conditions associated with the workplace may be responsible for enhanced susceptibility of workers to metal polysensitization. For example, occupational settings often facilitate exposures to more hazardous formulations of metals, larger quantities of allergenic metals, and mixtures of substances such as irritants and adjuvants via multiple exposure routes and for extended durations of time (Anderson and Meade 2014; Dickel et al. 2001).
Nickel, Co, and Cr are the three metals most commonly investigated in the context of co-sensitization and occupational metal allergy (Rui et al. 2010). Concurrent sensitization to different combinations of these metals was associated with numerous different occupations. In construction workers, professional cleaners, and metal industry workers, common patterns of polysensitization to these metals include Ni+ Co and Co + Cr sensitivity (Román-Razo et al. 2019). Co-sensitization to all three metals was also noted in textile and leather workers, as well as bartenders (Rui et al. 2012).
Concurrent allergic sensitivity to multiple transition metals including Ni, Co, and Cr is more common than co-sensitization to other combinations of metals in the workplace; however, dental professionals are a subset of workers that are known to exhibit co-sensitivity to many different combinations of metals – some of which belong to the transition series of metals, and some of which do not (Santucci et al. 1996). Lyapina et al. (2018) demonstrated that the metal most commonly implicated in co-sensitization within a group of 128 dental professionals was Cr. Several pairs of metals associated with a significant elevation in the incidence of co-sensitization were also identified. Although some combinations of metals exhibited differing degrees of association within specific subsets of study participants, most of the correlations between metals were similarly evident amongst dental students, technicians, and dental professionals. Accordingly, Ni+ Co, Ni+ Pd, Cr+ Co, Cr +Cu, Cr +Au, and Cr+ Al were some of the most common metal co-sensitization patterns reported.
Mixed-type allergic responses with single metal specificity
A less commonly-reported presentation of metal allergy in which a heightened state of immunological complexity is implicated involves mixed-type allergic responsivity. Several case reports were published in which both populations of effector T-cells and IgE antibodies specific for the same metal antigen were identified in a single individual (Abeck et al. 1993; Spinelli et al. 2005; Walsh, Smith, and King 2010). Accordingly, these subjects often experience concurrent type I and type IV hypersensitivity responses following exposure to the offending metal. Nickel is one of the metals most frequently implicated in mixed-type hypersensitivity responses, but Co and Hg are also known to induce similar states of immunological reactivity in susceptible individuals (Barranco Sanz et al. 1989; Krecisz et al. 2009).
The immunological mechanisms responsible for development of mixed-type responses in metal allergy remain unclear; however, several general trends may be elucidated from the existing collection of published case reports. For example, most cases of mixed-type hypersensitivity to metals were noted to develop in workers, suggesting that unique exposure conditions in the workplace may selectively promote these complex responses (Redlich and Herrick 2008). Further the existence of both immediate- and delayed-type allergic responsivity to metals were correlated with an elevated risk of developing both systemic hypersensitivity responses and chronic disease states (Buyukozturk et al. 2015). Finally, in some of the published reports, a temporal association between the existence and emergence of different symptom profiles might be discerned. The majority of these cases depict a subject with established type IV responsivity and a history of prototypical metal-induced ACD symptoms who subsequently develops type I reactivity to the same metal over the course of ensuing months and years (Estlander et al. 1993; Krecisz et al. 2009; Kusaka 1983).
The development of concurrent delayed- and immediate-type allergic responsivity to metals most frequently results in the emergence of immunological responsivity within multiple biological compartments, and thus, allergic symptoms involving multiple tissues – a scenario that will be explored in additional detail in the following section (Mann et al. 2010; Tsui et al. 2020; Xue et al. 2019). Although reported far less commonly, mixed-type hypersensitivity to metals might also result in development of simultaneous, but mechanistically distinctive presentations of allergy within the same tissue. This type of response is most commonly observed in the skin. Accordingly, dermal contact with allergenic metals in previously-sensitized subjects might lead to the simultaneous elicitation of immediate-type urticarial reactions and delayed-type ACD responses. Nickel, Co and Hg have all been implicated in this variant of tissue-restricted, mixed-type metal hypersensitivity (Estlander et al. 1993; Krecisz et al. 2009; Temesvari and Daroczy 1989). Interestingly, concomitant presentations of delayed- and immediate-type photo-induced ACD responses were also observed occurring in sensitized workers exposed to both Cr and Co in the presence of UV radiation (Manciet et al. 2006). Only one report describing concurrent metal-specific mixed-type hypersensitivity responses isolated within the respiratory tract was published to date. In this case, a diamond polisher with a history of exposure to aerosolized Co in his place of employment developed concurrent symptoms of both Co-induced asthma and hypersensitivity pneumonitis (Van Cutsem et al. 1987).
Presentations of allergic sensitivity involving multiple anatomical compartments
Most individuals afflicted with metal allergy exhibit one primary presentation of disease that tends to remain isolated within a single anatomical compartment or immunologically-responsive tissue; however, some subjects have the capacity to develop metal-specific allergic responses that simultaneously manifest in multiple tissues of the body.
Mixed-type allergic responsivity is frequently responsible for the emergence of metal-specific allergic symptoms in multiple biological compartments. In accordance with this mechanism, elicitation of metal allergy might trigger immunological activation in multiple anatomical locations, wherein subsequent biological responses are mediated by different hypersensitivity mechanisms. ACD is the disease most commonly-implicated in this type of mixed-type, multi-tissue metal allergy presentation. ACD responses induced by Hg, Pt, and Cr have been associated with concurrent elicitation of anaphylaxis, eosinophilic airway reactions, and asthmatic responses, respectively (Hernández et al. 1994; Merget et al. 2015).
The underlying mechanisms responsible for development of compound disease presentations in metal allergy remain largely unclear. It does appear, however, based upon observations reported in existing publications describing these responses, that the emergence of allergic responsivity in multiple tissues tends to occur sequentially, and not simultaneously. Several investigators described temporal patterns implicated in development of primary symptoms in workers with metal allergy and subsequent emergence of secondary disease presentations. In this context, one of the most commonly observed disease patterns entails the concurrent existence of ACD and asthma. In most of these subjects, an established state of metal-specific ACD precedes the emergence of asthmatic responses, often by many years. Nickel, Cr, Co, and Pt were all implicated in this response type and corresponding pattern of temporal disease progression (De Raeve et al. 1998; Estlander et al. 1993; Krecisz et al. 2009; Marshall 1952; Onizuka et al. 2006). This example represents a logical scenario in which the first presentation of metal sensitivity involves delayed-type skin responses (consistent with the prevalence of ACD). Subsequent respiratory exposures to metals are then responsible for development of immediate-type asthmatic responses (consistent with the infrequent occurrence of metal inhalation and ACD-mediated increase in airway responsivity to allergen exposure).
There have also been reports, though far less common, wherein immediate-type allergic responsivity to metals is shown to precede the development of delayed-type ACD responses. For example, in one worker employed by the smoldering industry, rhinitis was the subject’s primary indication of metal allergy, following which, Ni-induced ACD responses developed after a year (Niordson 1981). In a similar report, a lab worker exposed to Hg via inhalation and dermal contact first developed asthmatic responses to the metal, then subsequently developed ACD reactions (Marshall 1952).
In other instances, manifestation of allergic responses in multiple different anatomical locations may involve a single, conserved metal-specific mechanism of hypersensitivity. Delayed-type hypersensitivity mechanisms are more commonly implicated in this type of complex metal allergy presentation. Several reports have been published detailing cases involving workers with established metal-specific ACD symptoms who subsequently develop delayed-type, cell-mediated asthmatic responses to the same metal. The major metals implicated in these responses include Ni, Cr and Co (De Hauteclocque et al. 2002; Kusaka et al. 1991; Olaguibel and Basomba 1989). Although the mechanisms responsible for these responses have yet to be directly investigated, it is generally accepted that the same populations of metal-reactive T-cells responsible for ACD responses are recruited to the lungs following respiratory exposures to the metal and subsequently mediate delayed-type asthmatic symptoms (Kusaka et al. 1989). In accordance with this mechanism, similar responses were observed in subjects with ACD initiated by Co, Cr, and Pd who subsequently developed HMLD, cell-mediated asthma, and contact allergic gastritis, respectively (Nakamura et al. 2014; Pföhler, Vogt, and Müller 2016).
Immediate-type hypersensitivity mechanisms were also implicated in a few cases of metal allergy involving multi-tissue responses. Concurrent emergence of contact urticaria and immediate-type systemic responses (anaphylaxis) were found in response to Ni and iridium exposure (Antico and Soana 1999; Bergman, Svedberg, and Nilsson 1995; Olaguibel and Basomba 1989). Similarly, simultaneous elicitation of asthmatic responses and anaphylactic reactions was observed in a worker sensitized to Co (Baik, Yoon, and Park 1995). Finally, immediate-type contact urticaria and asthmatic responses was shown to occur concurrently in Pt-sensitized workers (Santucci et al. 2000).
Conclusions
The primary objective of this review was to generate a comprehensive and up-to-date compendium of unique disease variants, clinical presentations, and related mechanisms implicated in metal allergy. Accordingly, the scientific literature was extensively reviewed and hundreds of publications describing metal-specific hypersensitivity responses in human subjects were compiled. From the information provided in these reports, over 50 unique clinical manifestations of metal allergy were identified and categorically grouped as either dermal, respiratory, GI, or systemic hypersensitivity responses. Each of these allergic conditions is discussed individually within this manuscript in accordance with this organizational framework. In addition to the direct manifestations of metal allergy, our review of the scientific literature also identified several inflammatory conditions in which metal hypersensitivity appears to play an indirect role in disease pathogenesis. These conditions are also discussed briefly within relevant sections of this document. Finally, a small assemblage of published articles describing increasingly complex and multifaceted allergic responses to metal allergens is discussed and collective findings are presented.
In addition to establishing a comprehensive reference document, another major goal of this review paper was to highlight knowledge gaps associated with metal allergy and identify specific areas where further investigations are needed. Overall, dermal hypersensitivity responses to allergenic metals constitute the most well-characterized and best-understood manifestations of the disease since ACD and other presentations of metal allergy involving the skin constitute the most prevalent form of disease within the general population. The various systemic hypersensitivity responses associated with metal allergy are also well-characterized due to their clinical significance and increased potential for profound morbidity. Although several metal-induced allergic responses of the airways have been identified, many of the underlying mechanisms responsible for these reactions remain unclear. This lack of information is at least partially reflective of the fact that metal-specific respiratory hypersensitivity responses are relatively uncommon and tend to emerge selectively in working populations. Accordingly, future scientific endeavors intended to improve our current understanding of metal allergy in the lungs are likely to facilitate considerable advancements in minimizing the burden of disease imposed by occupational metal allergy.
Collectively, the GI tract constitutes the anatomical compartment within which the effects of metal allergy are most poorly-characterized. To date, only a handful of hypersensitivity-mediated responses were identified as potential presentations of metal allergy within the digestive tract despite the frequent ingestion of metals by humans. Most of these responses were only recently identified, suggesting that the scientific niche concerned with metal-specific immune responses in the gut is in its infancy. Moreover, although several tissues comprising the GI tract (e.g., the intestinal mucosa and esophagus) were identified as potential sites of immunological responsivity and metal allergy symptomology, most of the associated mechanisms remain unclear. Future investigations need to be directed toward identifying unique presentations of metal allergy in the digestive tract and clarifying the underlying biological processes responsible for metal-induced GI allergy.
Despite the existence of numerous publications describing complex and mixed-type allergic reactions to metals, little information is currently available regarding these types of hypersensitivity responses. It remains entirely unclear why some individuals - specifically, workers - have the capacity to develop presentations of metal allergy that implicate concurrent, but distinctive immunological mechanisms, as well as responses that manifest in multiple anatomical compartments. Future research endeavors also need to be executed in order to identify the cellular mechanisms underlying these types of responses, as well as predisposing factors, individual metals of concern, and specific routes of exposure that may promote the development of complex and mixed-type allergic responses to metals.
Finally, although many reports have been published correlating the existence of metal hypersensitivity with other diverse inflammatory disorders including psoriasis, SLE, rheumatoid arthritis, and fibromyalgia, more information needs to be collected in order to better understand the nature of these relationships. It remains unknown exactly what role metal-induced adaptive immune responses play in the pathogenesis of these diseases, but it is believed that chronic inflammatory processes associated with metal allergy may promote the development of such disorders, accelerate disease progression, exacerbate symptom frequency and severity, and complicate disease management and treatment strategies (Bjørklund, Dadar, and Aaseth 2018; Drenovska, Shahid, and Vassileva 2020; Stejskal 2014; Stejskal et al. 2006). Accordingly, attempts to better understand the specific biological processes involved in disease overlap will be particularly beneficial for managing the growing number of individuals afflicted with metal allergy and various other comorbidities.
Funding
The author(s) reported there is no funding associated with the work featured in this article.
Disclosure statement
The authors received no specific funding for this work.
Abbreviations:
- ACD
allergic contact dermatitis
- AHR
airway hyperreactivity
- ASIA
autoimmune/autoinflammatory syndrome induced by adjuvants
- BAL
bronchoalveolar lavage
- CBD
chronic beryllium disease
- CTCL
cutaneous T-cell lymphoma
- CTL
cytotoxic T-Lymphocyte
- DRESS
drug reaction with eosinophilia and systemic symptoms
- GERD
gastro-esophageal reflux disease
- GI
gastrointestinal
- GIP
giant cell interstitial pneumonia
- GM-CSF
granulocyte macrophage-colony stimulating factor
- HMLD
hard metal lung disease
- HMW
high molecular weight
- IBS
irritable bowel syndrome
- Ig
immunoglobulin
- IL
interleukin
- LMW
low molecular weight
- PAP
pulmonary alveolar proteinosis
- PPE
personal protective equipment
- PRR
pathogen recognition receptor
- SLE
systemic lupus erythematosus
- SNAS
systemic nickel allergy syndrome
- Th
helper T-cell
- UC
ulcerative colitis
- UV
ultraviolet
References
- Abeck D, Traenckner I, Steinkraus V, Vieluf D, and Ring J. 1993. Chronic urticaria due to nickel intake. Acta Derm. Venereol 73:438–39. doi: 10.2340/0001555573438439. [DOI] [PubMed] [Google Scholar]
- Abusnina W, Shehata M, Abouzid M, Price M, and Zeid F. 2019. Kounis syndrome secondary to gadolinium contrast agent. Proc. (Bayl. Univ. Med. Cent.) 32:253–55. doi: 10.1080/08998280.2019.1581319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acer E, Erdogan HK, Batan T, and Saracoglu ZN. 2020. European standard series patch test results in contact dermatitis patients in a tertiary care hospital. Şişli Etfal Hastan. Tıp Bul. 54:206–10. doi: 10.14744/SEMB.2020.02703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams TN, Butt YM, Batra K, and Glazer CS. 2017. Cobalt related interstitial lung disease. Respir. Med 129:91–97. doi: 10.1016/j.rmed.2017.06.008. [DOI] [PubMed] [Google Scholar]
- Agarwal S, and Gawkrodger DJ. 2002. Occupational allergic contact dermatitis to silver and colophonium in a jeweler. Am. J. Contact Dermatitis 13:74. [PubMed] [Google Scholar]
- Agarwal R, Sharma SK, and Malaviya AN. 1989. Gold-Induced hypersensitivity pneumonitis in a patient with rheumatoid arthritis. Clin. Exp. Rheumatol. 7:89–90. [PubMed] [Google Scholar]
- Aggarwal V, Jain A, and Kabi D. 2010. Oral lichenoid reaction associated with tin component of amalgam restorations: A case report. Am. J. Dermatopathol 32:46–48. doi: 10.1097/DAD.0b013e3181afcdab. [DOI] [PubMed] [Google Scholar]
- Ahlstrom MG, Thyssen JP, Wennervaldt M, Menne T, and Johansen JD. 2019. Nickel allergy and allergic contact dermatitis: A clinical review of immunology, epidemiology, exposure, and treatment. Contact Dermatitis. doi: 10.1111/cod.13327. [DOI] [PubMed] [Google Scholar]
- Aksu A 2015. Sources of metal pollution in the urban atmosphere (a case study: Tuzla, Istanbul). J. Environ. Health Sci 13:79–79. doi: 10.1186/s40201-015-0224-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akuthota P, and Weller PF. 2012. Eosinophilic pneumonias. Clin. Microbiol. Rev 25:649–60. doi: 10.1128/CMR.00025-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliağaoğlu C, Turan H, Erden I, Albayrak H, Ozhan H, Başar C, Gürlevik Z, and Alçelik A. 2012. Relation of nickel allergy with in-stent restenosis in patients treated with cobalt chromium stents. Ann Dermatol 24:426–29. doi: 10.5021/ad.2012.24.4.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almpanis GC, Tsigkas GG, Koutsojannis C, Mazarakis A, Kounis GN, and Kounis NG. 2010. Nickel allergy, kounis syndrome and intracardiac metal devices. Int. J. Cardiol 145:364–65. doi: 10.1016/j.ijcard.2010.02.038. [DOI] [PubMed] [Google Scholar]
- Almutairi N, and Almutawa F. 2017. Allergic contact dermatitis pattern in kuwait: nickel leads the pack. in-depth analysis of nickel allergy based on the results from a large prospective patch test series report. Postepy Dermatol. Alergol 34:207–15. doi: 10.5114/ada.2017.67843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameille J, Brechot JM, Brochard P, Capron F, and Dore MF. 1992. Occupational hypersensitivity pneumonitis in a smelter exposed to zinc fumes. Chest 101:862–63. doi: 10.1378/chest.101.3.862. [DOI] [PubMed] [Google Scholar]
- Andersen KE, Hjorth N, and Menné T. 1984. The baboon syndrome: systemically-induced allergic contact dermatitis. Contact Dermatitis. 10:97–100. doi: 10.1111/j.1600-0536. 1984.tb00343.x. [DOI] [PubMed] [Google Scholar]
- Anderson SE, Long C, and Dotson GS. 2017. Occupational allergy. Eur Med J (Chelmsf) 2:65–71. [PMC free article] [PubMed] [Google Scholar]
- Anderson SE, and Meade BJ. 2014. Potential health effects associated with dermal exposure to occupational chemicals. Environ. Health Insights 8:51–62. doi: 10.4137/EHI.S15258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SE, Siegel PD, and Meade BJ. 2011. The LLNA: A brief review of recent advances and limitations. J. Allergy 2011:1–10. doi: 10.1155/2011/424203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antico A, and Soana R. 1999. Chronic allergic-like dermatopathies in nickel-sensitive patients. Results of dietary restrictions and challenge with nickel salts. Allergy Asthma Proc 20:235–42. [DOI] [PubMed] [Google Scholar]
- Antico A, and Soana R. 2015. Nickel sensitization and dietary nickel are a substantial cause of symptoms provocation in patients with chronic allergic-like dermatitis syndromes. Allergy Rhinol (Providence) 6:56–63. doi: 10.2500/ar.2015.6. 0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonini JM, Taylor MD, Zimmer AT, and Roberts JR. 2004. Pulmonary responses to welding fumes: role of metal constituents. J. Toxicol. Environ. Health , A 67:233–49. [DOI] [PubMed] [Google Scholar]
- Aquino M, and Rosner G. 2019. Systemic contact dermatitis. Clin Rev Allergy Immunol 56:9–18. doi: 10.1007/s12016-018-8686-z. [DOI] [PubMed] [Google Scholar]
- Armstrong DK, Walsh MY, and Dawson JF. 1997. Granulomatous contact dermatitis due to gold earrings. Br. J. Dermatol 136:776–78. [PubMed] [Google Scholar]
- Asero R, Tedeschi A, Marzano AV, and Cugno M. 2017. Chronic urticaria: A focus on pathogenesis. F1000Res 6:1095. doi: 10.12688/f1000research.11546.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asher MI, Montefort S, Björkstén B, Lai CK, Strachan DP, Weiland SK, and Williams H. 2006. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: isaac phases one and three repeat multicountry cross-sectional surveys. Lancet 368:733–43. doi: 10.1016/s0140-6736(06)69283-0. [DOI] [PubMed] [Google Scholar]
- Aslan N, Sezikli M, and Erdal E. 2017. Nickel sensitivity in patients with gastroesophageal reflux disease. Cutan Ocul Toxicol 36:347–50. doi: 10.1080/15569527.2017.1295252. [DOI] [PubMed] [Google Scholar]
- Avila M, Ambelil M, Tong Y, Khurana S, Abraham F, Ertan A, Thosani NC, Dupont AW, Cash BD, and Younes M. 2021. Lymphocytic esophagitis is not similar to eosinophilic esophagitis except for seasonal variation. Ann. Clin. Lab. Sci 51:347–51. [PubMed] [Google Scholar]
- Azouz NP, and Rothenberg ME. 2019. Mechanisms of gastrointestinal allergic disorders. Clin. Invest. (Lond.) 129:1419–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagherani N, and Smoller BR. 2016. An overview of cutaneous T cell lymphomas. F1000Res 5:1882. doi: 10.12688/f1000research.8829.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baik JJ, Yoon YB, and Park HS. 1995. Cobalt-induced occupational asthma associated with systemic illness. J. Korean Med. Sci 10:200–04. doi: 10.3346/jkms.1995.10.3. 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakula A, Lugović-Mihić L, Situm M, Turcin J, and Sinković A. 2011. Contact allergy in the mouth: diversity of clinical presentations and diagnosis of common allergens relevant to dental practice. Acta Clin. Croat 50:553–61. [PubMed] [Google Scholar]
- Bal A, Das A, Gupta D, and Garg M. 2014. Goodpasture’s syndrome and p-ANCA associated vasculitis in a patient of silicosiderosis: an unusual association. Case Rep Pulmonol 2014:398238. doi: 10.1155/2014/398238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balbouzis T, Georgiadis T, and Grigoris P. 2016. Granulomatous lung disease: A novel complication following metallosis from hip arthroplasty. Hip Pelvis 28:249–53. doi: 10.5371/hp.2016.28.4.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardana EJ 2008. 10 occupational asthma. J. Allergy Clin. Immunol 121:S408–S411. doi: 10.1016/j.jaci.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Barranco Sanz P, Lopez Serrano MM, Esteban M, and Casas O. 1989. Hypersensitivity to mercuric fluorescein compounds. Allergol. Immunopathol. (Madr.) 17:219–22. [PubMed] [Google Scholar]
- Bellato E, Marini E, Castoldi F, Barbasetti N, Mattei L, Bonasia DE, and Blonna D. 2012. Fibromyalgia syndrome: Etiology, pathogenesis, diagnosis, and treatment. Pain Res. Treat 2012:426130–426130. doi: 10.1155/2012/426130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belloni Fortina A, Cooper SM, Spiewak R, Fontana E, Schnuch A, and Uter W. 2015. Patch test results in children and adolescents across Europe. analysis of the ESSCA network 2002–2010. Pediatr. Allergy Immunol 26:446–55. doi: 10.1111/pai.12397. [DOI] [PubMed] [Google Scholar]
- Ben-Dov I, and Segel MJ. 2014. Autoimmune pulmonary alveolar proteinosis: Clinical course and diagnostic criteria. Autoimmun. Rev 13:513–17. [DOI] [PubMed] [Google Scholar]
- Berardesca E 2002. Disorders of skin barriers: clinical implications. J. Eur. Acad. Dermatol. Venereol 16:559–61. doi: 10.1046/j.1468-3083.2002.00528.x. [DOI] [PubMed] [Google Scholar]
- Beretta-Piccoli BT, Mainetti C, Peeters M-A, and Laffitte E. 2018. Cutaneous granulomatosis: A comprehensive review. Clin. Rev. Allergy Immunol 54:131–46. [DOI] [PubMed] [Google Scholar]
- Bergman A, Svedberg U, and Nilsson E. 1995. Contact urticaria with anaphylactic reactions caused by occupational exposure to iridium salt. Contact Dermatitis 32:14–17. [DOI] [PubMed] [Google Scholar]
- Bibas N, Lassere J, Paul C, Aquilina C, and Giordano-Labadie F. 2013. Nickel-induced systemic contact dermatitis and intratubal implants: the baboon syndrome revisited. Dermatitis 24:35–36. [DOI] [PubMed] [Google Scholar]
- Bielory L 2008. Ocular allergy overview. Immunol. Allergy Clin. North Am 28:1–23. doi: 10.1016/j.iac.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Biermé P, Nowak-Wegrzyn A, and Caubet J-C. 2017. Non-IgE-mediated gastrointestinal food allergies. Curr. Opin. Pediatr 29:697–703. [DOI] [PubMed] [Google Scholar]
- Bijukumar DR, Segu A, Souza JCM, Li X, Barba M, Mercuri LG, J. J. J, and M. T. Mathew. 2018. Systemic and local toxicity of metal debris released from hip prostheses: A review of experimental approaches. Nanomedicine 14:951–63. doi: 10.1016/j.nano.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bircher AJ 2018. Metal allergy: other metals. In Metal allergy: from dermatitis to implant and device failure, ed. Chen JK, and Chen JK, 467–79. Cham: Springer International Publishing. [Google Scholar]
- Biteker M 2010. Current understanding of Kounis syndrome. Expert Rev. Clin. Immunol 6:777–88. doi: 10.1586/eci.10.47. [DOI] [PubMed] [Google Scholar]
- Bjørklund G, Dadar M, and Aaseth J. 2018. Delayed-type hypersensitivity to metals in connective tissue diseases and fibromyalgia. Environ. Res 161:573–79. doi: 10.1016/j.envres.2017.12.004. [DOI] [PubMed] [Google Scholar]
- Bocca B, and Forte G. 2009. The epidemiology of contact allergy to metals in the general population: prevalence and new evidences. Open Chem. Biomed. Meth 2:26–34. [Google Scholar]
- Bogaert P, Tournoy KG, Naessens T, and Grooten J. 2009. Where asthma and hypersensitivity pneumonitis meet and differ: non-eosinophilic severe asthma. Am. J. Pathol 174:3–13. doi: 10.2353/ajpath.2009.071151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhler-sommeregger K, and Lindemayr H. 1986. Contact sensitivity to aluminium. Contact Derm. 15:278–81. [DOI] [PubMed] [Google Scholar]
- Bomhard EM 2017. Particle-induced pulmonary alveolar proteinosis and subsequent inflammation and fibrosis: A toxicologic and pathologic review. Toxicol. Pathol 45:389–401. doi: 10.1177/0192623316688959. [DOI] [PubMed] [Google Scholar]
- Bonamonte D, Cristaudo A, Nasorri F, Carbone T, De Pità O, Angelini G, and Cavani A. 2011. Efficacy of oral hyposensitization in allergic contact dermatitis caused by nickel. Contact Dermatitis 65:293–301. doi: 10.1111/j.1600-0536.2011.01940.x. [DOI] [PubMed] [Google Scholar]
- Bonamonte D, Foti C, Vestita M, Ranieri LD, and Angelini G. 2012. Nummular eczema and contact allergy: A retrospective study. Dermatitis 23:153–57. doi: 10.1097/DER.0b013e318260d5a0. [DOI] [PubMed] [Google Scholar]
- Boonstra MB, Christoffers WA, Coenraads PJ, and Schuttelaar ML. 2015. Patch test results of hand eczema patients: relation to clinical types. J. Eur. Acad. Dermatol. Venereol 29:940–47. doi: 10.1111/jdv.12735. [DOI] [PubMed] [Google Scholar]
- Borghini R, De Amicis N, Bella A, Greco N, Donato G, and Picarelli A. 2020. Beneficial effects of a low-nickel diet on relapsing IBS-like and extraintestinal symptoms of celiac patients during a proper gluten-free diet: nickel allergic contact mucositis in suspected non-responsive celiac disease. Nutrients 12. doi: 10.3390/nu12082277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghini R, Puzzono M, Rosato E, Di Tola M, Marino M, Greco F, and Picarelli A. 2016. Nickel-related intestinal mucositis in IBS-like patients: Laser Doppler perfusion imaging and oral mucosa patch test in use. Biol. Trace Elem. Res 173:55–61. doi: 10.1007/s12011-016-0650-2. [DOI] [PubMed] [Google Scholar]
- Borowska S, and Brzoska MM. 2015. Metals in cosmetics: implications for human health. J. Appl. Toxicol 35:551–72. doi: 10.1002/jat.3129. [DOI] [PubMed] [Google Scholar]
- Borza DB, Neilson EG, and Hudson BG. 2003. Pathogenesis of goodpasture syndrome: A molecular perspective. Semin. Nephrol 23:522–31. doi: 10.1053/s0270-9295(03)00131-1. [DOI] [PubMed] [Google Scholar]
- Bousquet J, Anto JM, Bachert C, Baiardini I, Bosnic-Anticevich S, Walter Canonica G, Melén E, Palomares O, Scadding GK, and Togias A. 2020. Allergic rhinitis. Nat. Rev. Dis. Primers 6:95. doi: 10.1038/s41572-020-00227-0. [DOI] [PubMed] [Google Scholar]
- Breen DJ, and Stoker DJ. 1993. Titanium lines: A manifestation of metallosis and tissue response to titanium alloy megaprostheses at the knee. Clin. Radiol 47:274–77. doi: 10.1016/s0009-9260(05)81138-9. [DOI] [PubMed] [Google Scholar]
- Bregnbak D, Johansen JD, Jellesen MS, Zachariae C, Menné T, and Thyssen JP. 2015. Chromium allergy and dermatitis: Prevalence and main findings. Contact Derm. 73:261–80. doi: 10.1111/cod.12436. [DOI] [PubMed] [Google Scholar]
- Bright P, Burge PS, O’hickey SP, Gannon PF, Robertson AS, and Boran A. 1997. Occupational asthma due to chrome and nickel electroplating. Thorax 52:28–32. doi: 10.1136/thx.52.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brightling CE 2006. Chronic cough due to non-asthmatic eosinophilic bronchitis: ACCP evidence-based clinical practice guidelines. Chest 129:116S–121S. doi: 10.1378/chest.129.1_suppl.116S. [DOI] [PubMed] [Google Scholar]
- Brooks SM 1981. Lung disorders resulting from the inhalation of metals. Clin. Chest Med 2:235–54. doi: 10.1016/S0272-5231(21)00119-2. [DOI] [PubMed] [Google Scholar]
- Bruckner H 1967. Extrinsic asthma in a tungsten carbide worker. J. Occup. Med 9:518–19. [DOI] [PubMed] [Google Scholar]
- Brunasso G, and Massone C. 2021. Palmoplantar pustulosis: epidemiology, clinical features, and diagnosis. Fac Rev 10:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucolo C, Platania CBM, Drago F, and Salomone S. 2015. Ocular allergies. Ref. Module Biomed. Sci. Elsevier 1:1–5. [Google Scholar]
- Bui R, Boven L, Kaufman D, and Weinberger P. 2022. Metal allergy in tracheostomy tube placement resulting in complete subglottic stenosis: A case report. Ann. Otol. Rhinol. Laryngol 6:34894211070135. doi: 10.1177/00034894211070135. [DOI] [PubMed] [Google Scholar]
- Buyukozturk S, Gelincik A, Demirtürk M, Erdoğdu D, Pur L, Çolakoğlu B, Deniz G, and Erdem Kuruca S. 2013. Nickel dental alloys can induce laryngeal edema attacks: A case report. J. Dermatol 40:740–42. doi: 10.1111/1346-8138.12210. [DOI] [PubMed] [Google Scholar]
- Buyukozturk S, Gelincik A, Unal D, Demirturk M, Celik DD, Erden S, Colakoglu B, and Erdem Kuruca S. 2015. Oral nickel exposure may induce type I hypersensitivity reaction in nickel-sensitized subjects. Int. Immunopharmacol 26:92–96. doi: 10.1016/j.intimp.2015.03.012. [DOI] [PubMed] [Google Scholar]
- Cacoub P, Descamps V, Meyer O, Speirs C, Belissa-Mathiot P, and Musette P. 2013. Drug rash with eosinophilia and systemic symptoms (DRESS) in patients receiving strontium ranelate. Osteoporos. Int 24:1751–57. doi: 10.1007/s00198-013-2265-1. [DOI] [PubMed] [Google Scholar]
- Camarasa J, and Alomar A. 1981. Photosensitization to cobalt in a bricklayer. Contact Dermatitis 7:154–55. [DOI] [PubMed] [Google Scholar]
- Camarasa J, and Serra-Baldrich E. 1989. Allergic dermatitis caused by gold. Description of a new case. Med Cutan Ibero Lat Am 17:187–88. [PubMed] [Google Scholar]
- Campagnolo A, and Benninger MS. 2019. Allergic laryngitis: chronic laryngitis and allergic sensitization. Braz. J. Otorhinolaryngol 85:263–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campana F, Vigarios E, Fricain JC, and Sibaud V. 2019. Geographic stomatitis with palate involvement. An. Bras. Dermatol 94:449–51. doi: 10.1590/abd1806-4841.20197774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona V, Guilarte M, Luengo O, Labrador-Horrillo M, Sala-Cunill A, and Garriga T. 2011. Allergic diseases in the elderly. Clin. Transl. Allergy 1:11–11. doi: 10.1186/2045-7022-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen BC, Andersen KE, Menné T, and Johansen JD. 2008. Patients with multiple contact allergies: A review. Contact Dermatitis 58:1–8. doi: 10.1111/j.1600-0536.2007.01232.x. [DOI] [PubMed] [Google Scholar]
- Casper C, Groth W, and Hunzelmann N. 2004. Sarcoidal-type allergic contact granuloma: A rare complication of ear piercing. Am. J. Dermatopathol 26:59–62. [DOI] [PubMed] [Google Scholar]
- Castano R, and Suarthana E. 2014. Occupational rhinitis due to steel welding fumes. Am. J. Ind. Med 57:1299–302. doi: 10.1002/ajim.22365. [DOI] [PubMed] [Google Scholar]
- Castell DO, Murray JA, Tutuian R, Orlando RC, and Arnold R. 2004. Review article: the pathophysiology of gastro-oesophageal reflux disease -oesophageal manifestations. Aliment. Pharmacol. Ther 20 (Suppl 9):14–25. doi: 10.1111/j.1365-2036.2004.02238.x. [DOI] [PubMed] [Google Scholar]
- Chao SC, Lee YP, and Lee JY. 2010. Eosinophilic cellulitis and panniculitis with generalized vesicular pustular id reaction after a molten aluminum burn. Dermatitis 21:E11–15. [PubMed] [Google Scholar]
- Chen WJ, Monnat MCRJ Jr., and N. K. Mottet. 1978. Aluminum induced pulmonary granulomatosis. Human Pathol 9:705–11. doi: 10.1016/s0046-8177(78)80053-7. [DOI] [PubMed] [Google Scholar]
- Chen JK, and Thyssen JP. 2018. Metal allergy: from dermatitis to implant and device failure. Cham: Springer International Publishing. [Google Scholar]
- Cheng TY, Tseng YH, Sun CC, and Chu CY. 2008. Contact sensitization to metals in taiwan. Contact Dermatitis 59:353–60. [DOI] [PubMed] [Google Scholar]
- Chew R, Nigam S, and Sivakumaran P. 2016. Alveolar proteinosis associated with aluminium dust inhalation. Occup. Med 66:492–94. doi: 10.1093/occmed/kqw049. [DOI] [PubMed] [Google Scholar]
- Chiba Y, Kido T, Tahara M, Oda K, Noguchi S, Kawanami T, Yokoyama M, and Yatera K. 2019. Hard metal lung disease with favorable response to corticosteroid treatment: A case report and literature review. Tohoku J. Exp. Med 247:51–58. doi: 10.1620/tjem.247.51. [DOI] [PubMed] [Google Scholar]
- Chigbu DGI 2009. The pathophysiology of ocular allergy: A review. Contact Lens Anterior Eye 32:3–15. doi: 10.1016/j.clae.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Choudhary S, Mcleod M, Torchia D, and Romanelli P. 2013. Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome. J. Clin. Aesthet. Dermatol 6:31–37. [PMC free article] [PubMed] [Google Scholar]
- Çifci N 2019. Nickel sensitivity in rosacea patients: A prospective case control study. Endocr. Metab. Immune Disord. Drug Targets 19:367–72. doi: 10.2174/1871530319666190101120437. [DOI] [PubMed] [Google Scholar]
- Cirla AM 1994. Cobalt-related asthma: Clinical and immunological aspects. Sci. Total Environ 150:85–94. [DOI] [PubMed] [Google Scholar]
- Clarrett DM, and Hachem C. 2018. Gastroesophageal reflux disease (GERD). Mol. Med 115:214–18. [PMC free article] [PubMed] [Google Scholar]
- Colafrancesco S, Perricone C, Priori R, Valesini G, and Shoenfeld Y. 2014. Sjögren’s syndrome: Another facet of the autoimmune/inflammatory syndrome induced by adjuvants (Asia). J. Autoimmun 51:10–16. doi: 10.1016/j.jaut.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Collet E, Jeudy G, and Dalac S. 2013. Cheilitis, perioral dermatitis and contact allergy. Eur. J. Dermatol 23:303–07. doi: 10.1684/ejd.2013.1932. [DOI] [PubMed] [Google Scholar]
- Conde-Taboada A, Roson E, Fernandez-Redondo V, Garcia-Doval I, De La Torre C, and Cruces M. 2007. Lymphomatoid contact dermatitis induced by gold earrings. Contact Dermatitis 56:179–81. doi: 10.1111/j.1600-0536.2007.01022.x. [DOI] [PubMed] [Google Scholar]
- Contact Dermatitis Institute. “Allergen database.” Accessed 5/7/2021. https://www.contactdermatitisinstitute.com/database.php.
- Coombs R 1968. Classification of allergic reactions responsible for clinical hypersensitivity and disease. Clin. Aspects Immun 8:575–96. [Google Scholar]
- Corren J 2013. Asthma phenotypes and endotypes: an evolving paradigm for classification. Discov. Med 15:243–49. [PubMed] [Google Scholar]
- Costabel U, Miyazaki Y, Pardo A, Koschel D, Bonella F, Spagnolo P, Guzman J, Ryerson CJ, and Selman M. 2020. Hypersensitivity pneumonitis. Nat. Rev. Dis. Primers 6:65. doi: 10.1038/s41572-020-0191-z. [DOI] [PubMed] [Google Scholar]
- Costabel U, and Nakata K. 2010. Pulmonary alveolar proteinosis associated with dust inhalation: not secondary but autoimmune? Am. J. Respir. Crit. Care Med 181:427–28. [DOI] [PubMed] [Google Scholar]
- Cristaudo A, Sera F, Severino V, De Rocco M, Di Lella E, and Picardo M. 2005. Occupational hypersensitivity to metal salts, including platinum, in the secondary industry. Allergy 60:159–64. doi: 10.1111/j.1398-9995.2004.00521.x. [DOI] [PubMed] [Google Scholar]
- Czarnecki N, and Fritsch P. 1978. Contact sensitivity to lead. Hautarzt 29:445–47. [PubMed] [Google Scholar]
- Czekaj J, Ehlinger M, Rahme M, and Bonnomet F. 2016. Metallosis and cobalt–chrome intoxication after hip resurfacing arthroplasty. J. Orthop. Sci 21:389–94. [DOI] [PubMed] [Google Scholar]
- D’alcamo A, Mansueto P, Soresi M, Iacobucci R, Blasca FL, Geraci G, Cavataio F, Fayer F, Arini A, and Di Stefano L. 2017. Contact dermatitis due to nickel allergy in patients suffering from non-celiac wheat sensitivity. J Nutrients 9:103. [Google Scholar]
- D’alessandro A, Esposito D, Pesce M, Cuomo R, De Palma GD, and Sarnelli G. 2015. Eosinophilic esophagitis: From pathophysiology to treatment. World J. Gastrointest. Pathophysiol 6:150–58. doi: 10.4291/wjgp.v6.i4.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daenen M, Rogiers P, Van De Walle C, Rochette F, Demedts M, and Nemery B. 1999. Occupational asthma caused by palladium. Eur. Respir. J 13:213–16. [DOI] [PubMed] [Google Scholar]
- Dahlgren J, Wardenburg M, and Peckham T. 2010. Goodpasture’s syndrome and silica: A case report and literature review. Case Rep. Med 2010. doi: 10.1155/2010/426970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MD, Wang MZ, Yiannias JA, Keeling JH, Connolly SM, Richardson DM, and Farmer SA. 2011. Patch testing with a large series of metal allergens: findings from more than 1,000 patients in one decade at mayo clinic. Dermatitis 22:256–71. [DOI] [PubMed] [Google Scholar]
- Davison AG, Haslam PL, Corrin B, Coutts I, Dewar A, Riding WD, Studdy PR, and Newman-Taylor AJ. 1983. Interstitial lung disease and asthma in hard-metal workers: bronchoalveolar lavage, ultrastructural, and analytical findings and results of bronchial provocation tests. Thorax 38:119–28. doi: 10.1136/thx.38.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson-Amoah KG, Waddell BS, Prakash R, and Alexiades MM. 2020. Adverse reaction to zirconia in a modern total hip arthroplasty with ceramic head. Arthroplast Today 6:612–616.e611. doi: 10.1016/j.artd.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day GA, Stefaniak AB, Weston A, and Tinkle SS. 2006. Beryllium exposure: dermal and immunological considerations. Int. Arch. Occup. Environ. Health 79:161–64. doi: 10.1007/s00420-005-0024-0. [DOI] [PubMed] [Google Scholar]
- De Boer EM, Bruynzeel DP, and Van Ketel WG. 1988. Dyshidrotic eczema as an occupational dermatitis in metal workers. Contact Dermatitis 19:184–88. doi: 10.1111/j.1600-0536.1988.tb02891.x. [DOI] [PubMed] [Google Scholar]
- De Brouwere K, Buekers J, Cornelis C, Schlekat CE, and Oller AR. 2012. Assessment of indirect human exposure to environmental sources of nickel: oral exposure and risk characterization for systemic effects. Sci. Total Environ 419:25–36. doi: 10.1016/j.scitotenv.2011.12.049. [DOI] [PubMed] [Google Scholar]
- De Hauteclocque C, Morisset M, Kanny G, Kohler C, Mouget B, and Moneret-Vautrin DA. 2002. occupational asthma due to hard metals hypersensitivity. Rev. Mal. Respir 19:363–65. [PubMed] [Google Scholar]
- De La Cuadra J, and Grau-massanés M. 1991. Occupational contact dermatitis from rhodium and cobalt. Contact Derm. 25:182–84. [DOI] [PubMed] [Google Scholar]
- De Raeve H, Vandecasteele C, Demedts M, and Nemery B. 1998. Dermal and respiratory sensitization to chromate in a cement floorer. Am. J. Ind. Med 34:169–76. [DOI] [PubMed] [Google Scholar]
- De Souza IR, Araujo-Souza PS, and Leme DM. 2022. Genetic variants affecting chemical mediated skin immunotoxicity. J Toxicol Environ Health B 25:43–95. [DOI] [PubMed] [Google Scholar]
- Descotes J, and Choquet-Kastylevsky G. 2001. Gell and coombs’s classification: is it still valid? Toxicology 158:43–49. doi: 10.1016/S0300-483X(00)00400-5. [DOI] [PubMed] [Google Scholar]
- Di Gioacchino M, Boscolo P, Cavallucci E, Verna N, Di Stefano F, Di Sciascio M, Masci S, Andreassi M, Sabbioni E, and Angelucci D. 2000. Lymphocyte subset changes in blood and gastrointestinal mucosa after oral nickel challenge in nickel-sensitized women. Contact Dermatitis 43:206–11. doi: 10.1034/j.1600-0536.2000.043004206.x. [DOI] [PubMed] [Google Scholar]
- Di Gioacchino M, Gatta A, Della Valle L, Farinelli A, Caruso R, Pini C, Malandra A, Mangifesta R, Cavallucci E, and Petrarca C. 2018. Systemic nickel allergy syndrome. In Metal allergy: from dermatitis to implant and device failure, ed. Chen JK, and Chen JK, 551–61. Cham, Switzerland: Springer International. [Google Scholar]
- Di Gioacchino M, Ricciardi L, De Pità O, Minelli M, Patella V, Voltolini S, Di Rienzo V, Braga M, Ballone E, and Mangifesta R. 2014. Nickel oral hyposensitization in patients with systemic nickel allergy syndrome. Ann. Med 46:31–37. doi: 10.3109/07853890.2013.861158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Gioacchino M, Verna N, Di Giampaolo L, Di Claudio F, Turi MC, Perrone A, Petrarca C, Mariani-Costantini R, Sabbioni E, and Boscolo P. 2007. Immunotoxicity and sensitizing capacity of metal compounds depend on speciation. Int. J. Immunopathol. Pharmacol 20:15–22. doi: 10.1177/03946320070200S204. [DOI] [PubMed] [Google Scholar]
- Di Meo N, Gubertini N, Crocè L, Tiribelli C, and Trevisan G. 2016. DRESS syndrome with autoimmune hepatitis from strontium ranelate. Cutis 97:E22–26. [PubMed] [Google Scholar]
- Dickel H, Radulescu M, Weyher I, and Diepgen TL. 2001. Occupationally-induced “isolated cobalt sensitization.” Contact Dermatitis 45:246–47. doi: 10.1034/j.1600-0536.2001.450418.x. [DOI] [PubMed] [Google Scholar]
- Dispenza MC 2019. Classification of hypersensitivity reactions. Allergy Asthma Proc 40:470–73. doi: 10.2500/aap.2019.40.4274. [DOI] [PubMed] [Google Scholar]
- Donald DB, Wissel B, and Anas MU. 2015. Species-specific mercury bioaccumulation in a diverse fish community. Environ. Toxicol. Chem 34:2846–55. doi: 10.1002/etc.3130. [DOI] [PubMed] [Google Scholar]
- Dooms-Goossens AE, Debusschere KM, Gevers DM, Dupre KM, Degreef HJ, Loncke JP, and Snauwaert JE. 1986. Contact dermatitis caused by airborne agents. A review and case reports. J. Am. Acad. Dermatol 15:1–10. doi: 10.1016/s0190-9622(86)70135-7. [DOI] [PubMed] [Google Scholar]
- Downey D 1989. Contact mucositis due to palladium. Contact Dermatitis 21:54. doi: 10.1111/j.1600-0536.1989.tb04688.x. [DOI] [PubMed] [Google Scholar]
- Drenovska K, Shahid M, and Vassileva S. 2020. Nickel and skin: From allergy to autoimmunity. Endocr. Metab. Immune Disord. Drug Targets 20:1032–40. doi: 10.2174/1871530320666191231115437. [DOI] [PubMed] [Google Scholar]
- Drouet M, Le Sellin J, Bonneau JC, and Sabbah A. 1990. Mercury–Is it a respiratory tract allergen?. Allerg Immunol (Paris) 22 (81):84–88. [PubMed] [Google Scholar]
- Duarte IAG, Tanaka GM, Suzuki NM, Lazzarini R, Lopes ASDA, Volpini BMF, and Castro PCD. 2013. Patch test standard series recommended by the brazilian contact dermatitis study group during the 2006–2011 period. An. Bras. Dermatol 88:1015–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich A, Kucenic M, and Belsito DV. 2001. Role of body piercing in the induction of metal allergies. Am. J. Contact Dermat 12:151–55. doi: 10.1053/ajcd.2001.22774. [DOI] [PubMed] [Google Scholar]
- Ekqvist S, Svedman C, Moller H, Kehler M, Pripp CM, Bjork J, Gruvberger B, Holmstrom E, Gustavsson CG, and Bruze M. 2007. High frequency of contact allergy to gold in patients with endovascular coronary stents. Br. J. Dermatol 157:730–38. doi: 10.1111/j.1365-2133.2007.08119.x. [DOI] [PubMed] [Google Scholar]
- Eliaz N 2019. Corrosion of metallic biomaterials: A review. Materials (Basel) 12:407. doi: 10.3390/ma12030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhart S, and Segal RJ. 2017. Allergic reaction to vanadium causes a diffuse eczematous eruption and titanium alloy orthopedic implant failure. Cutis 99:245–49. [PubMed] [Google Scholar]
- Enriquez LS, Mohammed T-LH, Johnson GL, Lefor MJ, and Beasley MB. 2007. Hard metal pneumoconiosis: A case of giant-cell interstitial pneumonitis in a machinist. J Respir Care 52:196–99. [PubMed] [Google Scholar]
- Epstein WL, and Allen JR. 1964. Granulomatous hypersensitivity after use of zirconium-containing poison oak lotions. J. Am. Med. Assoc 190:940–42. [DOI] [PubMed] [Google Scholar]
- Erle DJ, and Sheppard D. 2014. The cell biology of asthma. J. Cell Biol 205:621–31. doi: 10.1083/jcb.201401050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esquerre N, Basso L, Dubuquoy C, Djouina M, Chappard D, Blanpied C, Desreumaux P, Vergnolle N, Vignal C, and Body-Malapel M. 2019. Aluminum ingestion promotes colorectal hypersensitivity in rodents. Cell. Mol. Gastroenterol. Hepatol 7:185–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban-Gorgojo I, Antolin-Amerigo D, Dominguez-Ortega J, and Quirce S. 2018. Non-eosinophilic asthma: current perspectives. J. Asthma Allergy 11:267–81. doi: 10.2147/jaa.S153097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estlander T, Kanerva L, Tupasela O, Keskinen H, and Jolanki R. 1993. Immediate and delayed allergy to nickel with contact urticaria, rhinitis, asthma and contact dermatitis. Clin. Exp. Allergy 23:306–10. [DOI] [PubMed] [Google Scholar]
- Estlander T, Kari O, Jolanki R, and Kanerva L. 1998. Occupational allergic contact dermatitis and blepharoconjunctivitis caused by gold. Contact Dermatitis 38:40–41. [DOI] [PubMed] [Google Scholar]
- Evans RB, Ettensohn DB, Fawaz-Estrup F, Lally EV, and Kaplan SR. 1987. Gold lung: Recent developments in pathogenesis, diagnosis, and therapy. Semin. Arthritis Rheum 16:196–205. [DOI] [PubMed] [Google Scholar]
- Evrard L, Waroquier D, and Parent D. 2010. Allergies to dental metals. titanium: a new allergen. Rev. Med. Brux 31:44–49. [PubMed] [Google Scholar]
- Exley C, Swarbrick L, Gherardi RK, and Authier FJ. 2009. A role for the body burden of aluminium in vaccine-associated macrophagic myofasciitis and chronic fatigue syndrome. Med. Hypotheses 72:135–39. doi: 10.1016/j.mehy.2008.09.040. [DOI] [PubMed] [Google Scholar]
- Faa G, Gerosa C, Fanni D, Lachowicz JI, and Nurchi VM. 2018. Gold - old drug with new potentials. Curr. Med. Chem 25:75–84. doi: 10.2174/0929867324666170330091438. [DOI] [PubMed] [Google Scholar]
- Falagiani P, Di Gioacchino M, Ricciardi L, Minciullo PL, Saitta S, Carní A, Santoro G, Gangemi S, Minelli M, and Bozzetti MP. 2008. Systemic nickel allergy syndrome (SNAS): A review. J Rev Port Imunoalergologia 16:135–47. [Google Scholar]
- Federmann M, Morell B, Graetz G, Wyss M, Elsner P, Von Thiessen R, Wüthrich B, and Grob D. 1994. Hypersensitivity to molybdenum as a possible trigger of ana-negative systemic lupus erythematosus. Ann. Rheum. Dis 53:403–05. doi: 10.1136/ard.53.6.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehrenbach H, Wagner C, and Wegmann M. 2017. Airway remodeling in asthma: what really matters. Cell Tissue Res. 367:551–69. doi: 10.1007/s00441-016-2566-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes P, Sharma SR, Magee A, Michielon G, and Fraisse A. 2019. Severe migraine associated with nickel allergy requiring surgical removal of atrial septal device. Ann. Thorac. Surg 108:e183–e184. doi: 10.1016/j.athoracsur.2019.01.034. [DOI] [PubMed] [Google Scholar]
- Fernández Pérez ER, Kong AM, Raimundo K, Koelsch TL, Kulkarni R, and Cole AL. 2018. Epidemiology of hypersensitivity pneumonitis among an insured population in the United States: A claims-based cohort analysis. Ann. Am. Thorac. Soc 15:460–69. doi: 10.1513/AnnalsATS.201704-288OC. [DOI] [PubMed] [Google Scholar]
- Fernandez-Nieto M, Quirce S, Carnes J, and Sastre J. 2006. Occupational asthma due to chromium and nickel salts. Int Arch Occup Environ Health 79:483–86. doi: 10.1007/s00420-005-0078-z. [DOI] [PubMed] [Google Scholar]
- Fernandez L, Maquiera E, Garci-Abujeta J, Yanez S, Rodriguez E, Martin-Gil D, and Jerez J. 1995. Baboon syndrome due to mercury sensitivity. Contact Dermatitis 33:56–57. [DOI] [PubMed] [Google Scholar]
- Finne K, Göransson K, and Winckler L. 1982. Oral lichen planus and contact allergy to mercury. Int. J. Oral Surg 11:236–39. doi: 10.1016/S0300-9785(82)80073-2. [DOI] [PubMed] [Google Scholar]
- Fluhr JW, Elsner P, Berardesca E, and Maibach HI. 2004. Bioengineering of the skin: water and the. Stratum Corneum: CRC press. [Google Scholar]
- Fok JS, and Smith WB. 2017. Hypersensitivity reactions to gadolinium-based contrast agents. Curr. Opin. Allergy Clin. Immunol 17:241–46. doi: 10.1097/aci.0000000000000371. [DOI] [PubMed] [Google Scholar]
- Fontenot AP, and Amicosante M. 2008. Metal-induced diffuse lung disease. Semin. Respir. Crit. Care Med 29:662–69. doi: 10.1055/s-0028-1101276. [DOI] [PubMed] [Google Scholar]
- Forte G, Petrucci F, and Bocca B. 2008. Metal allergens of growing significance: Epidemiology, immunotoxicology, strategies for testing and prevention. Inflamm. Allergy Drug Targets 7:145–62. doi: 10.2174/187152808785748146. [DOI] [PubMed] [Google Scholar]
- Franzen DP, Lang C, Agorastos N, Freitag L, Kohler M, and Schmid-Grendelmeier P. 2017. Evaluation of nickel release from endobronchial valves as a possible cause of hypersensitivity pneumonitis in a patient treated with bronchoscopic lung volume reduction. Int. Arch. Allergy Immunol 174:144–50. doi: 10.1159/000481986. [DOI] [PubMed] [Google Scholar]
- Fregert S 1982. Rhinorrhea due to chromic acid etching in a chromium sensitive person. Contact Derm. 8:219. doi: 10.1111/j.1600-0536.1982.tb04199.x. [DOI] [PubMed] [Google Scholar]
- Fregert S, Kollander M, and Poulsen J. 1979. Allergic contact stomatitis from gold dentures. Contact Derm 5:63–64. doi: 10.1111/j.1600-0536.1979.tb05550.x. [DOI] [PubMed] [Google Scholar]
- Fujii S, Fujita K, Yamaoka H, Miki K, Hirai S, Nemoto S, and Sumita K. 2021. Refractory in-stent stenosis after flow diverter stenting associated with delayed cobalt allergic reaction. J. Neurointerv. Surg doi: 10.1136/neurintsurg-2021-017948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Ying S, and Dai Y. 2017. Pathological roles of neutrophil-mediated inflammation in asthma and its potential for therapy as a target. J. Immunol. Res 2017:3743048–−3743048. doi: 10.1155/2017/3743048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garau V, Masala MG, Cortis MC, and Pittau R. 2005. Contact stomatitis due to palladium in dental alloys: A clinical report. J. Prosthet. Dent 93:318–20. doi: 10.1016/j.prosdent.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Garcia-Nunez I, Algaba-Marmol MA, Suarez-Vergara M, Fuentes-Soltero J, Fernandez-Barrera C, Bartolome B, Grau-Bonete A, and Ignacio-Garcia JM. 2019. Vanadium contact dermatitis: Case report and studies performed. Contact Derm. 80:127–28. doi: 10.1111/cod.13138. [DOI] [PubMed] [Google Scholar]
- García AA, Rodríguez Martín AM, Serra Baldrich E, Manubens Mercade E, and Puig Sanz L. 2016. Allergic contact dermatitis to silver in a patient treated with silver sulphadiazine after a burn. J Eur Acad Dermatol Venereol 30:365–66. doi: 10.1111/jdv.12785. [DOI] [PubMed] [Google Scholar]
- Garg S, Loghdey S, and Gawkrodger DJ. 2010. Allergic contract dermatitis from aluminium in deodorants. Contact Derm. 62:57–58. [DOI] [PubMed] [Google Scholar]
- Garner LA 2004. Contact dermatitis to metals. Dermatol. Ther 17:321–27. doi: 10.1111/j.1396-0296.2004.04034.x. [DOI] [PubMed] [Google Scholar]
- Gaudinski MR, and Milner JD. 2017. Atopic dermatitis and allergic urticaria: cutaneous manifestations of immunodeficiency. Immunol. Allergy Clin. North Am 37:1–10. doi: 10.1016/j.iac.2016.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier DA, and Geier MR. 2021. Dental amalgams and the incidence rate of arthritis among American adults. Clin. Med. Insights Arthritis Musculoskelet. Disord 14:11795441211016261. doi: 10.1177/11795441211016261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gell P 1963. The classification of allergic reactions underlying disease. Clin. Aspects Immun 1963:317–37. [Google Scholar]
- Ghio AJ, Silbajoris R, Carson JL, and Samet JM. 2002. Biologic effects of oil fly ash. Environ. Health Perspect 110:89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb HJ, Lees PS, Pinsky PF, and Rooney BC. 2000. Clinical findings of irritation among chromium chemical production workers. Am. J. Ind. Med 38:127–31. doi: 10.1002/1097-0274(200008). [DOI] [PubMed] [Google Scholar]
- Gibson PG, Fujimura M, and Niimi A. 2002. Eosinophilic bronchitis: Clinical manifestations and implications for treatment. Thorax 57:178–82. doi: 10.1136/thorax.57.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil F, Rato M, Monteiro A, Parente J, and Aranha J. 2019. Occupational lichenoid allergic contact dermatitis caused by tin. Contact Dermatitis 81:71–73. [DOI] [PubMed] [Google Scholar]
- Giménez-Arnau A, Riambau V, Serra-Baldrich E, and Camarasa JG. 2000. Metal-induced generalized pruriginous dermatitis and endovascular surgery. Contact Dermatitis 43:35–40. doi: 10.1034/j.1600-0536.2000. 5.x. [DOI] [PubMed] [Google Scholar]
- Goh CL, and Ng SK. 1987. Nickel dermatitis mimicking sycosis barbae. Contact Dermatitis 16:42. doi: 10.1111/j.1600-0536.1987.tb02619.x. [DOI] [PubMed] [Google Scholar]
- Gómez-Aldana A, Jaramillo-Santos M, Delgado A, Jaramillo C, and Lúquez-Mindiola A. 2019. Eosinophilic esophagitis: Current concepts in diagnosis and treatment. World J. Gastroenterol 25:4598–613. doi: 10.3748/wjg.v25.i32.4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomułka K, and Panaszek B. 2014. Contact urticaria syndrome caused by haptens. Postepy. Dermatol. Alergol 31:108–12. doi: 10.5114/pdia.2014.40915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Ruiz L, Vergara De Caso E, Peña-Sánchez R, and Silvestre-Salvador JF. 2019. Delayed hypersensitivity to palladium dichloride: 15-year retrospective study in a skin allergy unit. Contact Derm. 81:249–53. doi: 10.1111/cod.13343. [DOI] [PubMed] [Google Scholar]
- Goon A 2018. Metal allergy in Asia. In Metal allergy: from dermatitis to implant and device failure, ed. Chen JK, and Chen JK, 515–20. Cham: Springer International Publishing. [Google Scholar]
- Goon AT, and Goh C. 2005. Metal allergy in Singapore. Contact Dermatitis 52:130–32. [DOI] [PubMed] [Google Scholar]
- Goossens A 2004. Contact allergic reactions on the eyes and eyelids. Bull. Soc. Belge Ophtalmol 292:11–17. [PubMed] [Google Scholar]
- Goossens A, De Swerdt A, De Coninck K, Snauwaert JE, Dedeurwaerder M, and De Bonte M. 2006. Allergic contact granuloma due to palladium following ear piercing. Contact Dermatitis 55:338–41. doi: 10.1111/j.1600-0536.2006.00952.x. [DOI] [PubMed] [Google Scholar]
- Greco A, Rizzo MI, De Virgilio A, Gallo A, Fusconi M, Pagliuca G, Martellucci S, Turchetta R, Longo L, and De Vincentiis M. 2015. Goodpasture’s syndrome: A clinical update. Autoimmun. Rev 14:246–53. doi: 10.1016/j.autrev.2014.11.006. [DOI] [PubMed] [Google Scholar]
- Guibas GV, Mathioudakis AG, Tsoumani M, and Tsabouri S. 2017. Relationship of allergy with asthma: there are more than the allergy “eggs” in the asthma “basket.” Front. Pediatr 5:92–92. doi: 10.3389/fped.2017.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, and Jawanda MK. 2015. Oral lichen planus: an update on etiology, pathogenesis, clinical presentation, diagnosis and management. Indian J. Dermatol 60:222–29. doi: 10.4103/0019-5154.156315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley PJ 1991. Mechanisms of granulomatous lung disease from inhaled beryllium: The role of antigenicity in granuloma formation. Toxicol Pathol 19:514–25. doi: 10.1177/019262339101900417. [DOI] [PubMed] [Google Scholar]
- Hallab NJ, Caicedo M, Finnegan A, and Jacobs JJ. 2008. Th1 type lymphocyte reactivity to metals in patients with total hip arthroplasty. J. Orthop. Surg. Res 3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallab NJ, and Jacobs JJ. 2009. Biologic effects of implant debris. Bull. NYU Hosp. Jt. Dis 67:1 82–188. [PubMed] [Google Scholar]
- Hammers CM, and Stanley JR. 2016. Mechanisms of disease: pemphigus and bullous pemphigoid. Annu. Rev. Pathol 11:175–97. doi: 10.1146/annurev-pathol-012615-044313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa T, Yoshioka E, Azukizawa H, Itoi S, Tani M, Kira M, and Katayama I. 2012. Systemic allergic contact dermatitis to palladium inlay manifesting as annular erythema. Eur J of Derm 22:697–98. [DOI] [PubMed] [Google Scholar]
- Hannu T, Piipari R, and Toskala E. 2006. Immediate hypersensitivity type of occupational laryngitis in a welder exposed to welding fumes of stainless steel. Am. J. Ind. Med 49:402–05. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Iino T, and Sudo A. 2016. Immune response in adverse reactions to metal debris following metal-on-metal total hip arthroplasty. BMC Musculoskel. Disord 17:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa M, Yoshida K, Wakabayashi H, and Sudo A. 2012. Pseudotumor with dominant b-lymphocyte infiltration after metal-on-metal total Hip arthroplasty with a modular cup. J. Arthroplasty 27:493.e495–493.e497. doi: 10.1016/j.arth.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Hassmanová V, Vanĕcková J, and Bousová K. 2000. Occupational diseases caused by chromium and its compounds. Acta Medica (Hradec Kralove) Suppl. (43):33–36. [PubMed] [Google Scholar]
- Hausmann O, Schnyder B, and Pichler WJ. 2010. Drug hypersensitivity reactions involving skin. In Adverse drug reactions, ed. Uetrecht J, 29–55. Berlin Heidelberg: Springer. [DOI] [PubMed] [Google Scholar]
- Heck S, Al-Shobash S, Rapp D, Le DD, Omlor A, Bekhit A, Flaig M, Al-Kadah B, Herian W, and Bals R. 2017. High probability of comorbidities in bronchial asthma in germany. NPJ Prim Care Respir Med 27:28. doi: 10.1038/s41533-017-0026-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedmer M, Karlsson JE, Andersson U, Jacobsson H, Nielsen J, and Tinnerberg H. 2014. Exposure to respirable dust and manganese and prevalence of airways symptoms, among swedish mild steel welders in the manufacturing industry. Int Arch Occup Environ Health 87:623–34. doi: 10.1007/s00420-013-0896-3. [DOI] [PubMed] [Google Scholar]
- Hegewald J, Uter W, Pfahlberg A, Geier J, and Schnuch A. 2005. A multifactorial analysis of concurrent patch-test reactions to nickel, cobalt, and chromate. Allergy 60:372–78. doi: 10.1111/j.1398-9995.2005.00693.x. [DOI] [PubMed] [Google Scholar]
- Helgesen AL, and Austad J. 1997. Contact urticaria from aluminium and nickel in the same patient. Contact Derm. 37:303–04. doi: 10.1111/j.1600-0536.1997.tb02475.x. [DOI] [PubMed] [Google Scholar]
- Hennino A, Berard F, Guillot I, Saad N, Rozieres A, and Nicolas JF. 2006. Pathophysiology of urticaria. Clin Rev Allergy Immunol 30:3–11. doi: 10.1385/criai:30:1:003. [DOI] [PubMed] [Google Scholar]
- Hernández AP, Aznar JVB, Martinez GJ, Puchades A, and Baixauli EB. 1994. Mercurochrome allergy: Concurrence of 2 hypersensitivity mechanisms in the same patient. Contact Dermatitis 30:48–49. [DOI] [PubMed] [Google Scholar]
- Heule F, Tahapary GJ, Bello CR, and Van Joost T. 1998. Delayed-type hypersensitivity to contact allergens in psoriasis. A clinical evaluation. Contact Dermatitis 38:78–82. doi: 10.1111/j.1600-0536.1998.tb05657.x. [DOI] [PubMed] [Google Scholar]
- High WA, Ayers RA, Adams JR, Chang A, and Fitzpatrick JE. 2006. Granulomatous reaction to titanium alloy: an unusual reaction to ear piercing. J. Am. Acad. Dermatol 55:716–20. doi: 10.1016/j.jaad.2006.02.028. [DOI] [PubMed] [Google Scholar]
- Hill DA, and Spergel JM. 2016. The immunologic mechanisms of eosinophilic esophagitis. Curr. Allergy Asthma Rep 16:9–9. doi: 10.1007/s11882-015-0592-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SC, Juratli HA, and Eming R. 2018. Bullous autoimmune dermatoses. J Dtsch Dermatol Ges 16:1339–58. doi: 10.1111/ddg.13688. [DOI] [PubMed] [Google Scholar]
- Holgate ST, Wenzel S, Postma DS, Weiss ST, Renz H, and Sly PD. 2015. Asthma. Nat. Rev. Dis. Primers 1:15025. doi: 10.1038/nrdp.2015.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hon KL, Leung AKC, Ng WGG, and Loo SK. 2019. Chronic urticaria: an overview of treatment and recent patents. Recent Pat. Inflamm. Allergy Drug Discov 13:27–37. doi: 10.2174/1872213X13666190328164931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honari G, Ellis SG, Wilkoff BL, Aronica MA, Svensson LG, and Taylor JS. 2008. Hypersensitivity reactions associated with endovascular devices. Contact Dermatitis 59:7–22. [DOI] [PubMed] [Google Scholar]
- Honari G, Taylor JS, and Ellis S. 2005. Contact allergy to gold in patients with gold-plated intracoronary stents. Contact Dermatitis 53:359. doi: 10.1111/j.0105-1873.2005.00719.x. [DOI] [PubMed] [Google Scholar]
- Hosoki M, Bando E, Asaoka K, Takeuchi H, and Nishigawa K. 2009. Assessment of allergic hypersensitivity to dental materials. Biomed. Mater. Eng 19:53–61. doi: 10.3233/bme-2009-0563. [DOI] [PubMed] [Google Scholar]
- Hostýnek JJ, Hinz RS, Lorence CR, Price M, and Guy RH. 1993. Metals and the skin. Crit. Rev. Toxicol 23:171–235. [DOI] [PubMed] [Google Scholar]
- Hostynek JJ, and Maibach HI. 2004. Copper hypersensitivity: dermatologic aspects. Dermatol. Ther 17:328–33. doi: 10.1111/j.1396-0296.2004.04035.x. [DOI] [PubMed] [Google Scholar]
- Hristov AC, Tejasvi T, and A. W. R. 2021. Cutaneous T-cell lymphomas: 2021 update on diagnosis, risk-stratification, and management. Am. J. Hematol 96:1313–28. doi: 10.1002/ajh.26299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CX, Yiannias JA, Killian JM, and Shen JF. 2021. Seven common allergen groups causing eyelid dermatitis: education and avoidance strategies. Clin. Ophthalmol 15:1477–90. doi: 10.2147/opth.S297754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huaux F, De Gussem V, Lebrun A, Yakoub Y, Palmai-Pallag M, Ibouraadaten S, Uwambayinema F, and Lison D. 2018a. New interplay between interstitial and alveolar macrophages explains pulmonary alveolar proteinosis (PAP) induced by indium tin oxide particles. Arch. Toxicol 92:1349–61. doi: 10.1007/s00204-018-2168-1. [DOI] [PubMed] [Google Scholar]
- Huaux F, De Gussem V, Lebrun A, Yakoub Y, Palmai-Pallag M, Ibouraadaten S, Uwambayinema F, and Lison D. 2018b. New interplay between interstitial and alveolar macrophages explains pulmonary alveolar proteinosis (PAP) induced by indium tin oxide particles. Arch. Toxicol 92:1349–61. [DOI] [PubMed] [Google Scholar]
- Huber J, Hawkes JE, and Powell DL. 2016. Cobalt-Induced contact urticaria presenting as chronic urticaria due to intramuscular vitamin b12 injections. Dermatitis 27 (5):313–14. [DOI] [PubMed] [Google Scholar]
- Ido T, Kumakiri M, Kiyohara T, Sawai T, and Hasegawa Y. 2002. Oral lichen planus due to zinc in dental restorations. Contact Derm. 47:51. doi: 10.1034/j.1600-0536.2002.470113. x. [DOI] [PubMed] [Google Scholar]
- Igbokwe IO, Igwenagu E, and Igbokwe NA. 2019. Aluminium toxicosis: A review of toxic actions and effects. Interdiscip. Toxicol 12:45–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue Y, Trapnell BC, Tazawa R, Arai T, Takada T, Hizawa N, Kasahara Y, Tatsumi K, Hojo M, and Ichiwata T. 2008. Characteristics of a large cohort of patients with autoimmune pulmonary alveolar proteinosis in Japan. Am. J. Respir. Crit. Care Med 177:752–62. doi: 10.1164/rccm.200708-1271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irsigler GB, Visser PJ, and Spangenberg PA. 1999. Asthma and chemical bronchitis in vanadium plant workers. Am. J. Ind. Med 35:366–74. [DOI] [PubMed] [Google Scholar]
- Iveson JM, Scott DG, Perera WD, Cunliffe WJ, and Wright V. 1977. Immunofluorescence of the skin in gold rashes - with particular reference to ige. Ann. Rheum. Dis 36:520–23. doi: 10.1136/ard.36.6.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatsuki K, Tagami H, Moriguchi T, and Yamada M. 1982. Lymphadenoid structure induced by gold hypersensitivity. Arch. Dermatol 118:608–11. [PubMed] [Google Scholar]
- Izumi AK 1982. Allergic contact gingivostomatitis due to gold. Arch. Dermatol. Res 272:387–91. [DOI] [PubMed] [Google Scholar]
- Janower ML 1986. Hypersensitivity reactions after barium studies of the upper and lower gastrointestinal tract. Radiology 161:139–40. doi: 10.1148/radiology.161.1.3763856. [DOI] [PubMed] [Google Scholar]
- Jimenez-Rodriguez TW, Garcia-Neuer M, Alenazy LA, and Castells M. 2018. Anaphylaxis in the 21st century: phenotypes, endotypes, and biomarkers. J. Asthma Allergy 11:121–42. doi: 10.2147/jaa.S159411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimson S, Rajesh E, Krupaa RJ, and Kasthuri M. 2015. Burning mouth syndrome. J. Pharm. Bioallied Sci 7:S194– 196. doi: 10.4103/0975-7406.155899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MG 2008. Exposure-response in occupational allergy. Curr. Opin. Allergy Clin. Immunol 8:110–14. doi: 10.1097/ACI.0b013e3282f4b5f1. [DOI] [PubMed] [Google Scholar]
- Juárez-Rendón K, Sánchez G, Reyes-López M, García-Ortiz J, Bocanegra-García V, Guardiola-Avila I, and Altamirano-García M. 2017. Alopecia areata: current situation and perspectives. Arch. Argent Pediatr 115:e404–e411. [DOI] [PubMed] [Google Scholar]
- Jung J-W, Kang H-R, Kim M-H, Lee W, Min K-U, Han M-H, and Cho S-H. 2012. Immediate hypersensitivity reaction to gadolinium-based mr contrast media. Radiology 264:414–22. doi: 10.1148/radiol.12112025. [DOI] [PubMed] [Google Scholar]
- Kagan P, Halpert G, Amital H, Shapira R, and Shoenfeld Y. 2020. Autoimmune/inflammatory syndrome induced by adjuvant associated with a metal implant in the mouth; explantation was followed by recovery. Israel. Med. Assoc. J 22:582–83. [PubMed] [Google Scholar]
- Kageyama Y, Aida K, Kawauchi K, Morimoto M, Akiyama T, and Nakamura T. 2019. Higher incidence of zinc and nickel hypersensitivity in patients with irritable bowel syndrome. Immun Inflamm Dis 7:304–07. doi: 10.1002/iid3.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama Y, Aida K, Kawauchi K, Morimoto M, Akiyama T, and N T 2021. Dental metal hypersensitivity in patients with psoriasis vulgaris: A case-control study using an in vitro quantitative sensitivity test. Health Sci Rep 4:e223. doi: 10.1002/hsr2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama Y, Shimokawa Y, Kawauchi K, Morimoto M, Aida K, Akiyama T, and Nakamura T. 2020. Higher prevalence of nickel and palladium hypersensitivity in patients with ulcerative colitis. Int. Arch. Allergy Immunol 181:456–61. doi: 10.1159/000506633. [DOI] [PubMed] [Google Scholar]
- Kal BI, Evcin O, Dundar N, Tezel H, and Unal I. 2008. An unusual case of immediate hypersensitivity reaction associated with an amalgam restoration. Br. Dent. J 205:547–50. doi: 10.1038/sj.bdj.2008.981. [DOI] [PubMed] [Google Scholar]
- Kanerva L, Alanko K, Jolanki R, and Estlander T. 1999. Laboratory assistant’s occupational allergic airborne contact dermatitis from nickel presenting as rosacea. Eur. J. Dermatol 9:397–98. [PubMed] [Google Scholar]
- Kanerva L, Estlander T, and Jolanki R. 1998. Bank clerk’s occupational allergic nickel and cobalt contact dermatitis from coins. Contact Dermatitis 38:217–18. doi: 10.1111/j.1600-0536.1998.tb05715.x. [DOI] [PubMed] [Google Scholar]
- Kanerva L, Kiilunen M, Jolanki R, Estlander T, and Aitio A. 1997. Hand dermatitis and allergic patch test reactions caused by nickel in electroplaters. Contact Dermatitis 36:137–40. doi: 10.1111/j.1600-0536.1997.tb00395.x. [DOI] [PubMed] [Google Scholar]
- Kapur S, Watson W, and Carr S. 2018. Atopic dermatitis. Allergy Asthma Clin. Immunol 14:52–52. doi: 10.1186/s13223-018-0281-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasperkiewicz M, Ellebrecht CT, Takahashi H, Yamagami J, Zillikens D, Payne AS, and Amagai M. 2017. Pemphigus. Nat. Rev. Dis. Primers 3:17026–17026. doi: 10.1038/nrdp.2017.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastury F, Smith E, and Juhasz AL. 2017. A critical review of approaches and limitations of inhalation bioavailability and bioaccessibility of metal(loid)s from ambient particulate matter or dust. Sci. Total Environ 574:1054–74. doi: 10.1016/j.scitotenv.2016.09.056. [DOI] [PubMed] [Google Scholar]
- Kavcar P, Sofuoglu A, and Sofuoglu SC. 2009. A health risk assessment for exposure to trace metals via drinking water ingestion pathway. Int. J. Hyg. Environ. Health 212:216–27. [DOI] [PubMed] [Google Scholar]
- Kawai A, Zhang XM, Nakagawa M, Kawai J, Okada T, and Kawai K. 1994. Allergic contact dermatitis due to mercury in a wedding ring and a cosmetic. Contact Derm. 31:330–31. [DOI] [PubMed] [Google Scholar]
- Kazantzis G 1978. The role of hypersensitivity and the immune response in influencing susceptibility to metal toxicity. Environ. Health Perspect 25:111–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher P, Pacheco K, and Newman LS. 2000. Inorganic dust pneumonias: The metal-related parenchymal disorders. Environ. Health Perspect 108:685–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller CA, Frost A, Cagle PT, and Abraham JL. 1995. Pulmonary alveolar proteinosis in a painter with elevated pulmonary concentrations of titanium. Chest 108:277–80. doi: 10.1378/chest.108.1.277. [DOI] [PubMed] [Google Scholar]
- Kerr A, and Ferguson JJP. 2010. Photoimmunology, and photomedicine. Photoallergic.Contact Derm 26:56–65. [DOI] [PubMed] [Google Scholar]
- Keskinen H, Kalliomaki PL, and Alanko K. 1980. Occupational asthma due to stainless steel welding fumes. Clin. Allergy 10:151–59. [DOI] [PubMed] [Google Scholar]
- Khamaysi Z, Bergman R, and Weltfriend S. 2006. Positive patch test reactions to allergens of the dental series and the relation to the clinical presentations. Contact Dermatitis 55:216–18. [DOI] [PubMed] [Google Scholar]
- Khamaysi Z, Weltfriend S, Khamaysi K, and Bergman R. 2011. Contact hypersensitivity in patients with primary cutaneous lymphoproliferative disorders. Int. J. Dermatol 50:423–27. doi: 10.1111/j.1365-4632.2010.04763.x. [DOI] [PubMed] [Google Scholar]
- Khan SI, Ahmed AKM, Yunus M, Rahman M, Hore SK, Vahter M, and Wahed MA. 2010. Arsenic and cadmium in food-chain in Bangladesh–an exploratory study. J Health Popul. Nutr 28:578–84. doi: 10.3329/jhpn.v28i6.6606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpikari I 1997. Contact allergy to gold with pharyngeal and laryngeal disorders. Contact Derm. 37:130–31. doi: 10.1111/j.1600-0536.1997.tb00321.x. [DOI] [PubMed] [Google Scholar]
- Kim T-W, Kim W-I, Mun J-H, Song M, Kim H-S, Kim B-S, Kim M-B, and Ko H-C. 2015. Patch testing with dental screening series in oral disease. Ann. Dermatol 27:389–93. doi: 10.5021/ad.2015.27.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HI, Kim DH, Yoon MS, Kim HJ, and Lee S. 1991. Two cases of nickel dermatitis showing vitiligo-like depigmentations. Yonsei Med. J 32:79–81.\. [DOI] [PubMed] [Google Scholar]
- Kim K-B, Kwack SJ, Lee JY, Kacew S, and Lee B-M. 2021. Current opinion on risk assessment of cosmetics. J Toxicol Environ Health B 24:137–61. [DOI] [PubMed] [Google Scholar]
- Kimyon RS, and Warshaw EM. 2019. Airborne allergic contact dermatitis: management and responsible allergens on the american contact dermatitis society core series. Dermatitis 30:106–15. [DOI] [PubMed] [Google Scholar]
- Koch P, and Baum HP. 1996. Contact stomatitis due to palladium and platinum in dental alloys. Contact Dermatitis 34:253–57. doi: 10.1111/j.1600-0536.1996.tb02195.x. [DOI] [PubMed] [Google Scholar]
- Koike M 2005. A case of burning mouth associated with dental metal allergy. J Nihon Hotetsu Shika Gakkai Zasshi 49:498–501. [DOI] [PubMed] [Google Scholar]
- Kolodziej T, Szepietowski JC, Sikora J, and Bialynick-Birula R. 2003. The baboon syndrome due to nickel. Acta Derm. Venereol 11:29–31. [PubMed] [Google Scholar]
- Koniari I, Kounis NG, and Hahalis G. 2016. In-stent restenosis and thrombosis due to metal hypersensitivity: implications for kounis syndrome. J. Thorac. Dis 8:3056–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köster R, Vieluf D, Kiehn M, Sommerauer M, Kähler J, Baldus S, Meinertz T, and Hamm CW. 2000. Nickel and molybdenum contact allergies in patients with coronary in-stent restenosis. Lancet 356:1895–97. doi: 10.1016/s0140-6736(00)03262-1. [DOI] [PubMed] [Google Scholar]
- Kötter I, Dürk H, Saal JG, Kroiher A, and Schweinsberg F. 1995. Mercury exposure from dental amalgam fillings in the etiology of primary fibromyalgia: A pilot study. J. Rheumatol 22:2194–95. [PubMed] [Google Scholar]
- Kounis NG 2013. Coronary hypersensitivity disorder: the kounis syndrome. Clin. Ther 35:563–71. doi: 10.1016/j.clinthera.2013.02.022. [DOI] [PubMed] [Google Scholar]
- Kounis NG 2016. Kounis syndrome: An update on epidemiology, pathogenesis, diagnosis and therapeutic management. Clin. Chem. Lab. Med 54:1545–59. doi: 10.1515/cclm-2016-0010. [DOI] [PubMed] [Google Scholar]
- Kounis NG, Koniari I, Tsigkas G, Soufras GD, Plotas P, Davlouros P, and Hahalis G. 2020. Gadolinium-induced Kounis syndrome including electrocardiographic considerations. Proc (Bayl Univ Med Cent) 33:474–76. doi: 10.1080/08998280.2020.1765665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kränke B, and Aberer W. 1996. Multiple sensitivities to metals. Contact Dermatitis 34:225. [DOI] [PubMed] [Google Scholar]
- Kręcisz B, Kieć-Świerczyńska M, and Chomiczewska-Skóra D. 2012. Allergy to orthopedic metal implants - a prospective study. Int J Occup Med Environ Health 25:463–69. doi: 10.2478/s13382-012-0029-3. [DOI] [PubMed] [Google Scholar]
- Krecisz B, Kiec-Swierczynska M, Krawczyk P, Chomiczewska D, and Palczynski C. 2009. Cobalt-induced anaphylaxis, contact urticaria, and delayed allergy in a ceramics decorator. Contact Dermatitis 60:173–74. doi: 10.1111/j.1600-0536.2008.01465.x. [DOI] [PubMed] [Google Scholar]
- Krupashankar DS, and Manivasagam SR. 2012. Prevalence and relevance of secondary contact sensitizers in subjects with psoriasis. Indian Dermatol Online J 3:177–81. doi: 10.4103/2229-5178.101813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuligowski J, and Halperin KM. 1992. Stainless steel cookware as a significant source of nickel, chromium, and iron. Arch. Environ. Contam. Toxicol 23:211–15. doi: 10.1007/bf00212277. [DOI] [PubMed] [Google Scholar]
- Kurt OK, and Basaran N. 2020. Occupational exposure to metals and solvents: Allergy and airway diseases. Curr. Allergy Asthma Rep 20:38–38. [DOI] [PubMed] [Google Scholar]
- Kusaka Y 1983. Asthma due to hard alloy dusts–a case of allergic bronchial asthma and contact dermatitis due to metallic cobalt. Nihon Kyobu Shikkan Gakkai Zasshi 21:582–86. [PubMed] [Google Scholar]
- Kusaka Y, Nakano Y, Shirakawa T, Fujimura N, Kato M, and Heki S. 1991. Lymphocyte transformation test with nickel in hard metal asthma: Another sensitizing component of hard metal. Indust. Health 29:153–60. doi: 10.2486/indhealth.29.153. [DOI] [PubMed] [Google Scholar]
- Kusaka Y, Nakano Y, Shirakawa T, and Morimoto K. 1989. Lymphocyte transformation with cobalt in hard metal asthma. J Indust Health 27:155–63. [DOI] [PubMed] [Google Scholar]
- Kuźmiński A, Przybyszewski M, Graczyk M, Żbikowska-Gotz M, Sokołowska-Ukleja N, Tomaszewska A, and Bartuzi Z. 2020. Selected allergic diseases of the gastrointestinal tract. Prz. Gastroenterol 15:194–99. doi: 10.5114/pg.2019.87681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon YM, Thomas P, Summer B, Pandit H, Taylor A, Beard D, Murray DW, and Gill HS. 2010. Lymphocyte proliferation responses in patients with pseudotumors following metal-on-metal hip resurfacing arthroplasty. J. Orthop. Res 28:444–50. doi: 10.1002/jor.21015. [DOI] [PubMed] [Google Scholar]
- Laeijendecker R, and Van Joost T. 1994. Oral manifestations of gold allergy. J. Am. Acad. Dermatol 30:205–09. doi: 10.1016/s0190-9622(94)70018-4. [DOI] [PubMed] [Google Scholar]
- Laeijendecker R, and Van Joost T. 1994. Oral manifestations of gold allergy. J. Am. Acad. Dermatol 30:205–09. doi: 10.1016/s0190-9622(94)70018-4. [DOI] [PubMed] [Google Scholar]
- Lammintausta K, Pitkänen O-P, Kalimo K, and Jansen CT. 1985. Interrelationship of nickel and cobalt contact sensitization. Contact Dermatitis 13:148–52. doi: 10.1111/j.1600-0536.1985.tb02527.x. [DOI] [PubMed] [Google Scholar]
- Lansdown ABG 1995. Physiological and toxicological changes in the skin resulting from the action and interaction of metal ions. Crit. Rev. Toxicol 25:397–462. doi: 10.3109/10408449509049339. [DOI] [PubMed] [Google Scholar]
- Lastovkova A, Klusackova P, Fenclova Z, Bonneterre V, and Pelclova D. 2015. Asthma caused by potassium aluminium tetrafluoride: A case series. Ind Health 53:562–68. doi: 10.2486/indhealth.2014-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattanzio SM 2019. The gadolinium hypothesis for fibromyalgia and unexplained widespread chronic pain. Med. Hypotheses 129:109240. doi: 10.1016/j.mehy.2019.109240. [DOI] [PubMed] [Google Scholar]
- Lattanzio SM, and Imbesi F. 2020. Fibromyalgia associated with repeated gadolinium contrast-enhanced MRI examinations. Radiol Case Rep 15:534–41. doi: 10.1016/j.radcr.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauren CT, Belsito DV, Morel KD, and Larussa P. 2016. Case report of subcutaneous nodules and sterile abscesses due to delayed type hypersensitivity to aluminum-containing vaccines. Pediatrics 138. doi: 10.1542/peds.2014-1690. [DOI] [PubMed] [Google Scholar]
- Lavanya N, Jayanthi P, Rao UK, and Ranganathan K. 2011. Oral lichen planus: an update on pathogenesis and treatment. J. Oral Maxillofac. Pathol 15:127–32. doi: 10.4103/0973-029X.84474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarov A, Kidron D, Tulchinsky Z, and Minkow B. 2003. Contact orofacial granulomatosis caused by delayed hypersensitivity to gold and mercury. J. Am. Acad. Dermatol 49:1117–20. doi: 10.1016/s0190-9622(03)02185-6. [DOI] [PubMed] [Google Scholar]
- Le Coz C, Boos V, Cribier BJ, Grosshans EM, and Heid E. 1996. An unusual case of mercurial baboon syndrome. Contact Dermatitis 35:112. [DOI] [PubMed] [Google Scholar]
- Lechleitner P, Defregger M, Lhotta K, Tötsch M, and Fend F. 1993. Goodpasture’s syndrome. unusual presentation after exposure to hard metal dust. Chest 103:956–57. doi: 10.1378/chest.103.3.956. [DOI] [PubMed] [Google Scholar]
- Lee JY, Yoo JM, Cho BK, and Kim HO. 2001. Contact dermatitis in korean dental technicians. Contact Dermatitis 45:13–16. doi: 10.1034/j.1600-0536.2001.045001013.x. [DOI] [PubMed] [Google Scholar]
- Leis Dosil VM, Cabeza Martinez R, Suarez Fernandez RM, and Lazaro Ochaita P. 2006. Allergic contact dermatitis due to manganese in an aluminium alloy. Contact Derm. 54:67– 68. doi: 10.1111/j.0105-1873.2006.0729i.x. [DOI] [PubMed] [Google Scholar]
- Leonardi A, Motterle L, and Bortolotti M. 2008. Allergy and the eye. Clin. Exp. Immunol 153 Suppl 1:17–21. doi: 10.1111/j.1365-2249.2008.03716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesueur BW, and Yiannias JA. 2003. Contact stomatitis. Dermatol. Clin 21:105–14. doi: 10.1016/S0733-8635(02)00070-0. [DOI] [PubMed] [Google Scholar]
- Levy B, Hanflik A, Bryk E, and Vigorita VJ. 2016. Eosinophilic metallosis: A newly described entity in failed metal-on-metal arthroplasty. J. Long. Term Eff. Med. Implants 26:341–46. doi: 10.1615/JLongTermEffMedImplants.2017020104. [DOI] [PubMed] [Google Scholar]
- Li Y, and Li L. 2021. Contact dermatitis: classifications and management. Clin Rev Allergy Immunol. doi: 10.1007/s12016-021-08875-0. [DOI] [PubMed] [Google Scholar]
- Lidén C, Andersson N, Julander A, and Matura M. 2016. Cobalt allergy: suitable test concentration, and concomitant reactivity to nickel and chromium. Contact Dermatitis 74:360–67. [DOI] [PubMed] [Google Scholar]
- Liippo KK, Anttila SL, Taikina-Aho O, Ruokonen EL, Toivonen ST, and Tuomi T. 1993. Hypersensitivity pneumonitis and exposure to zirconium silicate in a young ceramic tile worker. Am. Rev. Respir. Dis 148:1089–92. doi: 10.1164/ajrccm/148.4_Pt_1.1089. [DOI] [PubMed] [Google Scholar]
- Lim HP, Lee KM, Koh YI, and Park SW. 2012. Allergic contact stomatitis caused by a titanium nitride-coated implant abutment: A clinical report. J Prosthet Dent 108:209–13. doi: 10.1016/s0022-3913(12)60163-2. [DOI] [PubMed] [Google Scholar]
- Lim DS, Toh TH, Kim MK, Kwon YC, Choi SM, Kwack SJ, Kim KB, Yoon S, Kim HS, and Lee B-M. 2018. Non-cancer, cancer and dermal sensitization risk assessment of heavy metals in cosmetics. J. Toxicol. Environ. Health Part A 81:432–52. [DOI] [PubMed] [Google Scholar]
- Lison D, Lauwerys R, Demedts M, and Nemery B. 1996. Experimental research into the pathogenesis of cobalt/hard metal lung disease. Eur. Respir. J 9:1024–28. [DOI] [PubMed] [Google Scholar]
- Lo Schiavo A, Sangiuliano S, Puca RV, Brunetti G, Ruocco E, and Cozzi R. 2008. Pemphigus and chrysotherapy: all that glitters is not gold! Int. J. Dermatol 47:645–47. doi: 10.1111/j.1365-4632.2008.03530.x. [DOI] [PubMed] [Google Scholar]
- Lopez S, Peláez A, Navarro LA, Montesinos E, Morales C, and Carda C. 1994. Aluminium allergy in patients hyposensitized with aluminium-precipitated antigen extracts. Contact Dermatitis 31:37–40. doi: 10.1111/j.1600-0536.1994.tb01903.x. [DOI] [PubMed] [Google Scholar]
- Loverde D, Iweala OI, Eginli A, and Krishnaswamy G. 2018. Anaphylaxis. Chest 153:528–43. doi: 10.1016/j.chest.2017.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe AJ, Angelica B, Su J, Lodge CJ, Hill DJ, Erbas B, Bennett CM, Gurrin LC, Axelrad C, and Abramson MJ. 2017. Age at onset and persistence of eczema are related to subsequent risk of asthma and hay fever from birth to 18 years of age. Pediatr. Allergy Immunol 28:384–90. doi: 10.1111/pai.12714. [DOI] [PubMed] [Google Scholar]
- Loyo E, Jara LJ, López PD, and Puig AC. 2012. Autoimmunity in connection with a metal implant: A case of autoimmune/autoinflammatory syndrome induced by adjuvants. Autoimmun. Highlights 4:33–38. doi: 10.1007/s13317-012-0044-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luvsannyam E, Jayaraman A, Jain MS, Jagani RP, Velez V, Mirji AS, Tiesenga F, and Jorge J. 2021. Diffuse nickel hypersensitivity reaction post-cholecystectomy in a young female. Cureus 13:e17146. doi: 10.7759/cureus.17146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyapina M, Dencheva M, Cekova M, Deliverska M, and Kisselova-Yaneva A. 2018. Concurrent contact sensitization to metals in dental exposures. J IMAB 24:1941–52. [Google Scholar]
- M.f.h C, and Barbosa F Jr. 2016. Gold nanoparticles: A critical review of therapeutic applications and toxicological aspects. J Toxicol Environ Health B 19:129–48. [DOI] [PubMed] [Google Scholar]
- Maestrelli P, Boschetto P, Fabbri LM, and Mapp CE. 2009. Mechanisms of occupational asthma. J. Allergy Clin. Immunol 123:531–542; quiz 543–534. doi: 10.1016/j.jaci.2009.01.057. [DOI] [PubMed] [Google Scholar]
- Mahdi G, Israel DM, and Hassall E. 1996. Nickel dermatitis and associated gastritis after coin ingestion. J. Pediatr. Gastroenterol. Nutr 23:74–76. [DOI] [PubMed] [Google Scholar]
- Mahendra G, Pandit H, Kliskey K, Murray D, Gill HS, and Athanasou N. 2009. Necrotic and inflammatory changes in metal-on-metal resurfacing hip arthroplasties. Acta Orthop 80:653–59. doi: 10.3109/17453670903473016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler V, Geier J, and Schnuch A. 2014. Current trends in patch testing - new data from the German contact dermatitis research group (DKG) and the information network of departments of dermatology (IVDK). J Dtsch Dermatol Ges 12:583–92. doi: 10.1111/ddg.12371. [DOI] [PubMed] [Google Scholar]
- Mahmoudi M, Craig T, and Ledford DK. 2019. Allergy and asthma: the basics to best practices, edited by Mahmoudi M. Cham: Springer. [Google Scholar]
- Makrilia N, Syrigou E, Kaklamanos I, Manolopoulos L, and Saif MW. 2010. Hypersensitivity reactions associated with platinum antineoplastic agents: A systematic review. Met.-Based Drugs 2010:207084. doi: 10.1155/2010/207084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinauskiene L, Isaksson M, and Bruze M. 2013. Systemic contact dermatitis in a gold-allergic patient after treatment with an oral homeopathic drug. J. Am. Acad. Dermatol 68: e58. doi: 10.1016/j.jaad.2012.09.013. [DOI] [PubMed] [Google Scholar]
- Malo JL 2005. Occupational rhinitis and asthma due to metal salts. J Allergy 60:138–39. [DOI] [PubMed] [Google Scholar]
- Malo JL, and Cartier A. 1987. Occupational asthma due to fumes of galvanized metal. Chest 92:375–77. doi: 10.1378/chest.92.2.375. [DOI] [PubMed] [Google Scholar]
- Malo JL, Cartier A, and Dolovich J. 1993. Occupational asthma due to zinc. Eur. Respir. J 6:447–50. [PubMed] [Google Scholar]
- Malo J, Cartier A, Gagnon G, Evans S, and Dolovich J. 1985. Isolated late asthmatic reaction due to nickel sulphate without antibodies to nickel. Clin. Exp. Allergy 15:95–99. [DOI] [PubMed] [Google Scholar]
- Malo J, Chan-Yeung M, and Di B. 2013. Asthma in the workplace. Fourth Edition ed. New York: CRC Press. [Google Scholar]
- Mamtani R, Stern P, Dawood I, and Cheema S. 2011. Metals and disease: A global primary health care perspective. J. Toxicol 2011:319136–319136. doi: 10.1155/2011/319136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manciet J, Barrade A, Janssen F, and Morel P. 2006. Contact allergy with immediate and delayed photoaggravation to chromate and cobalt. Contact Dermatitis 33:282–84. doi: 10.1111/j.1600-0536.1995.tb00496.x. [DOI] [PubMed] [Google Scholar]
- Mancuso G, and Berdondini RM. 2002. Eyelid dermatitis and conjunctivitis as sole manifestations of allergy to nickel in an orthodontic appliance. Contact Dermatitis 46:245. doi: 10.1034/j.1600-0536.2002.460415.x. [DOI] [PubMed] [Google Scholar]
- Mann E, Ranft U, Eberwein G, Gladtke D, Sugiri D, Behrendt H, Ring J, Schäfer T, Begerow J, and Wittsiepe J. 2010. Does airborne nickel exposure induce nickel sensitization? Contact Dermatitis 62:355–62. [DOI] [PubMed] [Google Scholar]
- Marcusson JA, Cederbrant K, and Heilborn J. 1998. Indium and iridium allergy in patients exposed to dental alloys. Contact Derm. 38:297–98. doi: 10.1111/j.1600-0536.1998.tb05758.x. [DOI] [PubMed] [Google Scholar]
- Markel K, Silverberg N, Pelletier J, Watsky K, and Jacob S. 2020. Art of prevention: A piercing article about nickel. Int. J. Women Dermatol 6:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J 1952. Asthma and dermatitis caused by chloroplatinlc acid. S. Afr. Med. J 26:8–9. [PubMed] [Google Scholar]
- Martin N, Belinchón I, Fuente C, Vélez A, and Sánchez-Yus E. 1990. Granuloma annulare and gold therapy. Arch. Dermatol 126:1370–71. [PubMed] [Google Scholar]
- Martin SF, Rustemeyer T, and Thyssen JP. 2018. Recent advances in understanding and managing contact dermatitis. F1000Res 7:810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsudate Y, Yamashita M, Fujii Y, and Urano Y. 2019. Contact granulomatous hypersensitivity to indium in a patient with orofacial granulomatosis. Contact Dermatitis 81:293–94. doi: 10.1111/cod.13284. [DOI] [PubMed] [Google Scholar]
- Mayer A, and Hamzeh N. 2015. Beryllium and other metal-induced lung disease. Curr. Opin. Pulm. Med 21:178–84. doi: 10.1097/mcp.0000000000000140. [DOI] [PubMed] [Google Scholar]
- Mbow ML, De Gregorio E, and Ulmer JB. 2011. Alum’s adjuvant action: Grease is the word. Nat. Med 17:415–16. [DOI] [PubMed] [Google Scholar]
- McDaniel LM, and Couch SM. 2017. Allergic eyelid dermatitis as the sole manifestation of gold hypersensitivity. Ophthalmic Plast. Reconstr. Surg 33:e80–e82. doi: 10.1097/iop.0000000000000783. [DOI] [PubMed] [Google Scholar]
- McKee AS, Mack DG, Crawford F, and Fontenot AP. 2015. Myd88 dependence of beryllium-induced dendritic cell trafficking and cd4(+) t-cell priming. Mucosal Immunol 8:1237–47. doi: 10.1038/mi.2015.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally AK, and Anderson JM. 2011. Macrophage fusion and multinucleated giant cells of inflammation. Adv. Exp. Med. Biol 713:97–111. doi: 10.1007/978-94-007-0763-4_7. [DOI] [PubMed] [Google Scholar]
- Mcparland H, and Warnakulasuriya S. 2012. Oral lichenoid contact lesions to mercury and dental amalgam–a review. J. Biomed. Biotechnol 589569–589569. doi: 10.1155/2012/589569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta V, and Balachandran C. 2010. Persistent nodular contact dermatitis to gold: case report of two cases. Indian J. Dermatol. Venereol. Leprol 76:397–99. doi: 10.4103/0378-6323.66594. [DOI] [PubMed] [Google Scholar]
- Mencia MM, and Cawich SO. 2021. Allergic dermatitis following bilateral oxidized zirconium total knee replacements. J Orthop Case Rep 11:67–70. doi: 10.13107/jocr.2021.v11.i02.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merget R, Fartasch M, Sander I, Van Kampen V, Raulf M, and Bruning T. 2015. Eosinophilic airway disease in a patient with a negative skin prick test, but a positive patch test with platinum salts–implications for medical surveillance. Am. J. Ind. Med 58:1008–11. doi: 10.1002/ajim.22482. [DOI] [PubMed] [Google Scholar]
- Merget R, Reineke M, Rueckmann A, Bergmann E-M, and Schultze-Werninghaus G. 1994. Nonspecific and specific bronchial responsiveness in occupational asthma caused by platinum salts after allergen avoidance. Am. J. Respir. Crit. Care Med 150:1146–49. [DOI] [PubMed] [Google Scholar]
- Merget R, Sander I, Van Kampen V, Raulf-Heimsoth M, Ulmer HM, Kulzer R, and Bruening T. 2010. Occupational immediate-type asthma and rhinitis due to rhodium salts. Am. J. Ind. Med 53:42–46. doi: 10.1002/ajim.20786. [DOI] [PubMed] [Google Scholar]
- Merget R, Schultze-Werninghaus G, Muthorst T, Friedrich W, and Meier-Sydow J. 1988. Asthma due to the complex salts of platinum–a cross-sectional survey of workers in a platinum refinery. Clin. Allergy 18:569–80. [DOI] [PubMed] [Google Scholar]
- Meyer R, Fox AT, Chebar Lozinsky A, Michaelis LJ, and Shah N. 2019. Non-ige-mediated gastrointestinal allergies —do they have a place in a new model of the allergic march. Pediatr. Allergy Immunol 30:149–58. [DOI] [PubMed] [Google Scholar]
- Mikhailova ES, Кostyunichev VV, Ermolaeva LA, Socolovich NA, Ogrina NA, Sheveleva NA, Nikolaevich S, and Zhovtyy AAP. 2017. Clinical-diagnostic significance of recognition of specific antibodies in patients with allergies to metal alloys used in prosthetic dentistry. J. Biol 6:83–89. [Google Scholar]
- Milam EC, and Cohen DE. 2019. Contact dermatitis: Emerging trends. Dermatol. Clin 37:21–28. doi: 10.1016/j.det.2018.07.005. [DOI] [PubMed] [Google Scholar]
- Miller RR, Churg AM, Hutcheon M, and Lam S. 1984. Pulmonary alveolar proteinosis and aluminum dust exposure. Am. Rev. Respir. Dis 130:312–15. [DOI] [PubMed] [Google Scholar]
- Minciullo PL, Paolino G, Vacca M, Gangemi S, and Nettis E. 2016. Unmet diagnostic needs in contact oral mucosal allergies. Clin. Mol. Allergy 14:10–10. doi: 10.1186/s12948-016-0047-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirembe SK, Mulyowa GK, Jaeger G, Ofenloch R, Diepgen TL, and Weisshaar E. 2016. Hand eczema in Africa: clinical findings and experiences from south-west uganda. Acta Derm. Venereol 96:388–89. doi: 10.2340/00015555-2246. [DOI] [PubMed] [Google Scholar]
- Mistry BD, and Dekoven JG. 2021. Widespread cutaneous eruption after aluminum-containing vaccination: A case report and review of current literature. Pediatr. Dermatol 38:872–74. doi: 10.1111/pde.14613. [DOI] [PubMed] [Google Scholar]
- Miyamoto T, Inoue S, and Watanabe T. 2005. A case of immediate hypersensitivity reaction with tungsten. Allergy 60:415–16. [DOI] [PubMed] [Google Scholar]
- Mizoguchi S, Setoyama M, and Kanzaki T. 1998. Linear lichen planus in the region of the mandibular nerve caused by an allergy to palladium in dental metals. Dermatology 196:268–70. doi: 10.1159/000017891. [DOI] [PubMed] [Google Scholar]
- Mizutani RF, Terra-Filho M, Lima E, Freitas CSG, Chate RC, Kairalla RA, Carvalho-Oliveira R, and Santos UP. 2016. Hard metal lung disease: A case series. J. Bras. Pneumol 42:447–52. doi: 10.1590/S1806-37562016000000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocanu M, Vâță D, Alexa A-I, Trandafir L, Patrașcu A-I, Hâncu MF, and Gheucă-Solovăstru L. 2021. Atopic dermatitis-beyond the skin. Diagnostics 11:1553. Basel, Switzerland. doi: 10.3390/diagnostics11091553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moiseff R, Olson N, Suriawinata AA, Rothstein RI, and Lisovsky M. 2021. CD8 T-cell-predominant lymphocytic esophagitis is one of the major patterns of lymphocytic inflammation in gastroesophageal reflux disease. Arch. Pathol. Lab. Med 145:1138–43. doi: 10.5858/arpa.2020-0430-OA. [DOI] [PubMed] [Google Scholar]
- Moldoveanu B, Otmishi P, Jani P, Walker J, Sarmiento X, Guardiola J, Saad M, and Yu J. 2009. Inflammatory mechanisms in the lung. J. Inflamm. Res 2:1–11. [PMC free article] [PubMed] [Google Scholar]
- Moller DR, Brooks SM, Bernstein DI, Cassedy K, Enrione M, and Bernstein IL. 1986. Delayed anaphylactoid reaction in a worker exposed to chromium. J. Allergy Clin. Immunol 77:451–56. [DOI] [PubMed] [Google Scholar]
- Montemarano AD, Sau P, Johnson FB, and James WD. 1997. Cutaneous granulomas caused by an aluminum-zirconium complex: an ingredient of antiperspirants. J. Am. Acad. Dermatol 37:496–98. doi: 10.1016/s0190-9622(97)70159-2. [DOI] [PubMed] [Google Scholar]
- Moon TC, Befus AD, and Kulka M. 2014. Mast cell mediators: their differential release and the secretory pathways involved. Front. Immunol 5. doi: 10.3389/fimmu.2014.00569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller K, Xiao J, Putra J, Rothstein R, McCourt C, Konnikova L, and Lisovsky M. 2021. Lymphocytic esophagitis with predominance of CD4 T cells and expansion of th1 cells is associated with achalasia. Am. J. Clin. Pathol 156:278–87. doi: 10.1093/ajcp/aqaa239. [DOI] [PubMed] [Google Scholar]
- Muñoz-Cano R, Picado C, Valero A, and Bartra J. 2016. Mechanisms of anaphylaxis beyond ige j. invest. Allergol. Clin. Immunol 26:73–82. doi: 10.18176/jiaci.0046. [DOI] [PubMed] [Google Scholar]
- Munoz X, Cruz M, Freixa A, Guardino X, and Morell F. 2009. Occupational asthma caused by metal arc welding of iron. J Respir 78:455–59. [DOI] [PubMed] [Google Scholar]
- Murphy K, Travers P, and Walport M. 2012. Janeway’s Immunobiology, Vol. 8. New York: Garland Science. [Google Scholar]
- Musk AW, and Tees JG. 1982. Asthma caused by occupational exposure to vanadium compounds. Med. J. Aust 1:183–84. [DOI] [PubMed] [Google Scholar]
- Nagura S, Sakai M, Obi H, and Fukahara K. 2022. Aortic valve replacement in a patient with self-reported systemic multiple metal allergy. Gen. Thorac. Cardiovasc. Surg 70:79–82. doi: 10.1007/s11748-021-01712-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima H 1977. The induction of an allergic gastritis of delayed-type in guinea pigs sensitized to potassium bichromate–further studies on the gastritis related to contact allergy. Osaka City Med. J 23:109–15. [PubMed] [Google Scholar]
- Nakamura Y, Nishizaka Y, Ariyasu R, Okamoto N, Yoshida M, Taki M, Nagano H, Hanaoka K, Nakagawa K, and Yoshimura C. 2014. Hard metal lung disease diagnosed on a transbronchial lung biopsy following recurrent contact dermatitis. Intern. Med 53:139–43. doi: 10.2169/internalmedicine.53.1019. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Yasunaga Y, Ikuta Y, Shimogaki K, Hamada N, and Takata N. 1997. Autoantibodies to red cells associated with metallosis—a case report. J Acta Orthopaedica Scand 68:495–96. [DOI] [PubMed] [Google Scholar]
- Nakayama H, and Chen K-R. 2018. How to treat metal hypersensitive alopecia areata and atopic alopecia. Clin Dermatol Res J 3 (1):1. [Google Scholar]
- Nauta AJ, Engels F, Knippels LM, Garssen J, Nijkamp FP, and Redegeld FA. 2008. Mechanisms of allergy and asthma. Eur. J. Pharmacol 585:354–60. [DOI] [PubMed] [Google Scholar]
- Navarro-Triviño FJ, Cassini VA De Cádiz Gómez, and Ruiz-Villaverde R. 2021. Allergic connubial contact dermatitis caused by molybdenum from fiberglass. Int. J. Dermatol 60:e88–e90. doi: 10.1111/ijd.15117. [DOI] [PubMed] [Google Scholar]
- Nawaz F, and Wall BM. 2007. Drug rash with eosinophilia and systemic symptoms (DRESS) syndrome: suspected association with titanium bioprosthesis. Am. J. Med. Sci 334:215–18. doi: 10.1097/MAJ.0b013e318141f723. [DOI] [PubMed] [Google Scholar]
- Nedorost S 2009. Clinical patterns of hand and foot dermatitis: emphasis on rubber and chromate allergens. Dermatol. Clin 27:281–87. doi: 10.1016/j.det.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Nemery B 1990. Metal toxicity and the respiratory tract. Eur. Respir. J 3:202–19. [PubMed] [Google Scholar]
- Nemery B, and Abraham JL. 2007. Hard metal lung disease: still hard to understand. In: American Thoracic Society. [DOI] [PubMed] [Google Scholar]
- Nemery B, Verbeken EK, and Demedts M. 2001. Giant cell interstitial pneumonia (hard metal lung disease, cobalt lung). Semin. Respir. Crit. Care Med 22:435–48. doi: 10.1055/s-2001-17386. [DOI] [PubMed] [Google Scholar]
- Nielsen NH, and Menné T. 1997. Allergic contact dermatitis caused by zinc pyrithione associated with pustular psoriasis. Am. J. Contact Dermat 8:170–71. [PubMed] [Google Scholar]
- Niordson AM 1981. Nickel sensitivity as a cause of rhinitis. Contact Dermatitis 7:273–74. doi: 10.1111/j.1600-0536.1981.tb04067.x. [DOI] [PubMed] [Google Scholar]
- Nishizawa A 2016. Dyshidrotic eczema and its relationship to metal allergy. Curr. Probl. Dermatol 51:80–85. [DOI] [PubMed] [Google Scholar]
- Nosbaum A, Rival-Tringali AL, Barth X, Damon H, Vital-Durand D, Claudy A, and Faure M. 2008. Nickel-induced systemic allergic dermatitis from a sacral neurostimulator. Contact Dermatitis 59:319–20. doi: 10.1111/j.1600-0536.2008.01434.x. [DOI] [PubMed] [Google Scholar]
- Nucera E, Chini R, Rizzi A, Schiavino D, Buonomo A, Aruanno A, Ricci R, Mangiola F, Campanale M, and Gasbarrini A. 2019. Eosinophilic oesophagitis (in nickel-allergic patient) regressed after nickel oral desensitization: A case report. Int. J. Immunopathol. Pharmacol 33:2058738419827771. doi: 10.1177/2058738419827771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogueta CI, Ramírez PM, Jiménez OC, and Cifuentes MM. 2019. Geographic tongue: what a dermatologist should know. Actas Dermosifiliogr 110:341–46. (Engl Ed). doi: 10.1016/j.ad.2018.10.022. [DOI] [PubMed] [Google Scholar]
- Oh JE, Lee HJ, Choi YW, Choi HY, and Byun JY. 2016. Metal allergy in eyelid dermatitis and the evaluation of metal contents in eye shadows. J. Eur. Acad. Dermatol. Venereol 30:1518–21. doi: 10.1111/jdv.13646. [DOI] [PubMed] [Google Scholar]
- Okuno K, Kobayashi K, Kotani Y, Ohnishi H, Ohbayashi C, and Nishimura Y. 2010. A case of hard metal lung disease resembling a hypersensitive pneumonia in radiological images. J. Intern. Med 49:1185–89. [DOI] [PubMed] [Google Scholar]
- Olaguibel JM, and Basomba A. 1989. Occupational asthma induced by chromium salts. Allergol Immunopathol 17:133–36. [PubMed] [Google Scholar]
- Oliveira CA, Candelária IS, Oliveira PB, Figueiredo A, and Caseiro-Alves F. 2015. Metallosis: A diagnosis not only in patients with metal-on-metal prostheses. Eur. J. Radiol 2:3–6. doi: 10.1016/j.ejro.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onakpa MM, Njan AA, and Kalu OC. 2018. A review of heavy metal contamination of food crops in Nigeria. Ann. Glob. Health 84:488–94. doi: 10.29024/aogh.2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onizuka R, Tanabe K, Nakayama Y, Fukuchi T, Nakata K, and Hiki T. 2006. a case of chrome asthma induced by exposure to the stone cutter dust. Arerugi 55:1556–61. [PubMed] [Google Scholar]
- Ortiz-Ruiz AJ, Ramírez-Espinosa M, and López-Jornet P. 2006. Oral lichen planus and sensitization to manganese in a dental prosthesis. Contact Dermatitis 54:214–15. [DOI] [PubMed] [Google Scholar]
- Özkaya E, and Babuna G. 2011. Two cases with nickel-induced oral mucosal hyperplasia: A rare clinical form of allergic contact stomatitis? Dermatol. Online J 17:12. [PubMed] [Google Scholar]
- Pala G, Pignatti P, and Moscato G. 2012. Occupational nonasthmatic eosinophilic bronchitis: Current concepts. Med. Lav 103:17–25. [PubMed] [Google Scholar]
- Park YM, Kang H, Kim HO, and Cho BK. 1999. Lymphomatoid eosinophilic reaction to gold earrings. Contact Dermatitis 40:216–17. [DOI] [PubMed] [Google Scholar]
- Park HS, Uh ST, and Park CS. 1996. Increased neutrophil chemotactic activity is noted in alminum-induced occupational asthma. Korean J. Intern. Med 11:69–73. doi: 10.3904/kjim.1996.11.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten DK, Schultz BG, and Berlau DJ. 2018. The safety and efficacy of low-dose naltrexone in the management of chronic pain and inflammation in multiple sclerosis, fibromyalgia, Crohn’s disease, and other chronic pain disorders. Pharmacotherapy 38:382–89. [DOI] [PubMed] [Google Scholar]
- Pawankar R 2014. Allergic diseases and asthma: A global public health concern and a call to action. World Allergy Organ. J 7:12–13. doi: 10.1186/1939-4551-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawankar R, Baena-Cagnani CE, Bousquet J, Canonica GW, Cruz AA, Kaliner MA, Lanier BQ, and Henley K. 2008. State of world allergy report 2008: Allergy and chronic respiratory diseases. World Allergy Organ. J 1:S4–S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawankar R, Canonica GW, Holgate ST, and Lockey RF. 2013. World Allergy Organization. Milwaukee, WI: World Allergy Organization. [Google Scholar]
- Peat F, Coomber R, Rana A, and Vince A. 2018. Vanadium allergy following total knee arthroplasty. BMJ Case Rep bcr- 2017–222092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedchenko V, Vanacore R, and Hudson B. 2011. Goodpasture’s disease: Molecular architecture of the autoantigen provides clues to etiology and pathogenesis. Curr. Opin. Nephrol. Hypertens 20:290–96. doi: 10.1097/MNH.0b013e328344ff20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiser M, Tralau T, Heidler J, Api AM, Arts JHE, Basketter DA, English J, Diepgen TL, Fuhlbrigge RC, and Gaspari AA. 2012. Allergic contact dermatitis: epidemiology, molecular mechanisms, in vitro methods and regulatory aspects: current knowledge assembled at an international workshop at BFR, Germany. Cell. Mol. Life Sci 69:763–81. doi: 10.1007/s00018-011-0846-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelaez Hernandez A, Brasó Aznar J, Jorro Martinez G, Rochina Puchades A, and Burches Baixauli E. 1994. Mercurochrome allergy: Concurrence of 2 hypersensitivity mechanisms in the same patient. Contact Derm. 30:48–49. [DOI] [PubMed] [Google Scholar]
- Pepys J, Parish W, Cromwell O, and Hughes E. 1979. Specific IgE and IgG antibodies to platinum salts in sensitized workers. Monogr. Allergy 14:142. [PubMed] [Google Scholar]
- Pesce V, Maccagnano G, Vicenti G, Notarnicola A, Lovreglio P, Soleo L, Pantalone A, Salini V, and Moretti B. 2013. First case report of vanadium metallosis after ceramic-on-ceramic total hip arthroplasty. J. Biol. Regul. Homeost. Agents 27:1063–68. [PubMed] [Google Scholar]
- Pesonen M, Airaksinen L, Voutilainen R, Riekki R, Jungewelter S, and Suuronen K. 2014. Occupational contact urticaria and rhinitis caused by immediate allergy to palladium salts. Contact Dermatitis 71:176–77. doi: 10.1111/cod.12214. [DOI] [PubMed] [Google Scholar]
- Peters MS, Schroeter AL, Van Hale HM, and Broadbent JC. 1984. Pacemaker contact sensitivity. Contact Dermatitis 11:214–18. [DOI] [PubMed] [Google Scholar]
- Pföhler C, Körner R, Vogt T, and Müller CSL. 2012. Contact allergic gastritis: an underdiagnosed entity? BMJ Case Rep 2012:bcr2012006916. doi: 10.1136/bcr-2012-006916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pföhler C, Vogt T, and Müller CS. 2016. Contact allergic gastritis: rare manifestation of a metal allergy. Hautarzt 67:359–64. doi: 10.1007/s00105-016-3773-7. [DOI] [PubMed] [Google Scholar]
- Phillips EJ, Bigliardi P, Bircher AJ, Broyles A, Chang YS, Chung WH, Lehloenya R, Mockenhaupt M, Peter J, and Pirmohamed M. 2019. Controversies in drug allergy: testing for delayed reactions. J. Allergy Clin. Immunol 143:66–73. doi: 10.1016/j.jaci.2018.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigatto PD, Brambilla L, Ferrucci S, Zerboni R, Somalvico F, and Guzzi G. 2014. Systemic allergic contact dermatitis associated with allergy to intraoral metals. Dermatol. Online J 20:10. [PubMed] [Google Scholar]
- Pomiecinski F, Yang AC, Navarro-Rodrigues T, Kalil J, and Castro FFM. 2010. Sensitization to foods in gastroesophageal reflux disease and its relation to eosinophils in the esophagus: Is it of clinical importance? Ann. Allergy, Asthma Immunol 105:359–63. doi: 10.1016/j.anai.2010.08.021. [DOI] [PubMed] [Google Scholar]
- Pongpairoj K, Ale I, Andersen KE, Bruze M, Diepgen TL, Elsner PU, Goh CL, Goossens A, Jerajani H, and Lachapelle JM. 2016. Proposed ICDRG classification of the clinical presentation of contact allergy. J Dermatitis 27:248–58. [DOI] [PubMed] [Google Scholar]
- Poziomkowska-Gęsicka I, Summer B, Sokołowska M, Thomas P, and Kurek M. 2018. Allergic contact dermatitis caused by hypersensitivity to gold–description of a clinical case. Contact Dermatitis 78:363–65. [DOI] [PubMed] [Google Scholar]
- Prescott SL, Pawankar R, Allen KJ, Campbell DE, Sinn J, Fiocchi A, Ebisawa M, Sampson HA, Beyer K, and Lee BW. 2013. A global survey of changing patterns of food allergy burden in children. World Allergy Organ. J 6:21. doi: 10.1186/1939-4551-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Přikrylová J, Procházková J, and Podzimek Š. 2019. Side effects of dental metal implants: impact on human health (metal as a risk factor of implantologic treatment). Biomed. Res. Int 2019:2519205. doi: 10.1155/2019/2519205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryce DW, and King CM. 1990. Orofacial granulomatosis associated with delayed hypersensitivity to cobalt. Clin. Exp. Dermatol 15:384–86. doi: 10.1111/j.1365-2230.1990.tb02123.x. [DOI] [PubMed] [Google Scholar]
- Purdy JK, Appelman HD, Golembeski CP, and Mckenna BJ. 2008. Lymphocytic esophagitis: A chronic or recurring pattern of esophagitis resembling allergic contact dermatitis. Am. J. Clin. Pathol 130:508–13. doi: 10.1309/d3pcf6d6yymqrx9a. [DOI] [PubMed] [Google Scholar]
- Quaranta M, Eyerich S, Knapp B, Nasorri F, Scarponi C, Mattii M, Garzorz N, Harlfinger A, Jaeger T, and Grosber M. 2014. Allergic contact dermatitis in psoriasis patients: typical, delayed, and non-interacting. PLoS One 9:e101814. doi: 10.1371/journal.pone.0101814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quenan S, Huber C, Pasche-Koo F, and Piletta P. 2014. Airborne allergic contact dermatitis caused by tin. Contact Dermatitis 71:184–85. doi: 10.1111/cod.12237. [DOI] [PubMed] [Google Scholar]
- Radenska-Lopovok SG, Kolesnikova AO, Egorova ON, Severinova MV, and Musatov ID. 2021. Panniculitis as a manifestation of metal-associated autoimmune/inflammatory syndrome induced by adjuvants: A case-based review. Rheumatol. Int doi: 10.1007/s00296-021-04924-1. [DOI] [PubMed] [Google Scholar]
- Raith L, Schubert H, and Göring HD. 1982. Contact dermatitis from cadmium chloride?. Contact Derm. 8:267. doi: 10.1111/j.1600-0536.1982.tb04213.x. [DOI] [PubMed] [Google Scholar]
- Rampton D, Folkersen J, Fishbane S, Hedenus M, Howaldt S, Locatelli F, Patni S, Szebeni J, and Weiss G. 2014. Hypersensitivity reactions to intravenous iron: Guidance for risk minimization and management. Haematologica 99:1671–76. doi: 10.3324/haematol.2014.111492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasool F, Akhtar S, Hassan I, Zeerak S, Mubashir S, and Sheikh G. 2018. Common contact allergens in patients with palmoplantar and scalp psoriasis and impact of their avoidance on dermatology life quality index: A hospital-based study. Indian J. Dermatol 63:160–64. doi: 10.4103/ijd.IJD_760_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi S, Patel KR, Singam V, Lee HH, and Silverberg JI. 2018. Associations of nickel co-reactions and metal polysensitization in adults. Dermatitis 29:316–20. doi: 10.1097/der.0000000000000421. [DOI] [PubMed] [Google Scholar]
- Raulf M, Weiss T, Lotz A, Lehnert M, Hoffmeyer F, Liebers V, van Gelder R, Kafferlein HU, Hartwig A, Pesch B, et al. 2016. Analysis of inflammatory markers and metals in nasal lavage of welders. J. Toxicol. Environ. Health Part A 79:1144–57. [DOI] [PubMed] [Google Scholar]
- Ray A, and Kolls JK. 2017. Neutrophilic inflammation in asthma and association with disease severity. Trends Immunol. 38:942–54. doi: 10.1016/j.it.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray C, and Ming X. 2020. Climate change and human health: A review of allergies, autoimmunity and the microbiome. Int. J. Env. Res. Public Health 17:4814. doi: 10.3390/ijerph17134814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redlich CA, and Herrick CA. 2008. Lung/skin connections in occupational lung disease. Curr. Opin. Allergy Clin. Immunol 8:115–19. doi: 10.1097/ACI.0b013e3282f85a31. [DOI] [PubMed] [Google Scholar]
- Requena L, and Yus ES. 2001. Panniculitis. Part I. Mostly septal panniculitis. J. Am. Acad. Dermatol 45:163–86. doi: 10.1067/mjd.2001.114736. [DOI] [PubMed] [Google Scholar]
- Rizzi A, Nucera E, Laterza L, Gaetani E, Valenza V, Corbo GM, Inchingolo R, Buonomo A, Schiavino D, and Gasbarrini A. 2017. Irritable bowel syndrome and nickel allergy: what is the role of the low nickel diet? J. Neurogastroenterol. Motil 23:101–08. doi: 10.5056/jnm16027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robledo MA 2012. Chronic methyl mercury poisoning may trigger endemic pemphigus foliaceus ”fogo selvagem”. Med. Hypotheses 78:60–66. doi: 10.1016/j.mehy.2011.09.041. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Nava G, Kesler AM, Carrillo-Martin I, and Gonzalez-Estrada A. 2019. Gadolinium-induced anaphylaxis with positive skin test results. Ann. Allergy. Asthma. Immunol 122:654–55. doi: 10.1016/j.anai.2019.03.022. [DOI] [PubMed] [Google Scholar]
- Romaguera C, Lecha M, Grimalt F, Muniesa A, and Mascaro J. 1982. Photocontact dermatitis to cobalt salts. Contact Dermatitis 8:383–88. [DOI] [PubMed] [Google Scholar]
- Román-Razo EA, O’farrill PM, Cambray C, Herrera A, Mendoza-Revilla DA, and Aguirre D. 2019. allergic contact dermatitis to cobalt and nickel in a metal industry worker. case report and literature review. Rev. Alerg. Mex 66:371–74. doi: 10.29262/ram.v66i3.537. [DOI] [PubMed] [Google Scholar]
- Rossman MD, Kern JA, Elias JA, Cullen MR, Epstein PE, Preuss OP, Markham TN, and Daniele RP. 1988. Proliferative response of bronchoalveolar lymphocytes to beryllium: A test for chronic beryllium disease. J Annals of Internal Medicine 108:687–93. [DOI] [PubMed] [Google Scholar]
- Rubio CA, Sjödahl K, and Lagergren J. 2006. Lymphocytic esophagitis: A histologic subset of chronic esophagitis. Am. J. Clin. Pathol 125:432–37. [PubMed] [Google Scholar]
- Rui F, Bovenzi M, Prodi A, Belloni Fortina A, Romano I, Corradin MT, and Larese Filon F. 2013. Nickel, chromium and cobalt sensitization in a patch test population in north-eastern Italy (1996–2010). Contact Dermatitis 68:23–31. doi: 10.1111/j.1600-0536.2012.02133.x. [DOI] [PubMed] [Google Scholar]
- Rui F, Bovenzi M, Prodi A, Fortina AB, Romano I, Corradin MT, and Filon FL. 2012. Concurrent sensitization to metals and occupation. Contact Dermatitis 67:359–66. doi: 10.1111/j.1600-0536.2012.02100.x. [DOI] [PubMed] [Google Scholar]
- Rui F, Bovenzi M, Prodi A, Fortina AB, Romano I, Peserico A, Corradin MT, Carrabba E, and Filon FL. 2010. Nickel, cobalt and chromate sensitization and occupation. Contact Dermatitis 62:225–31. doi: 10.1111/j.1600-0536.2009.01650.x. [DOI] [PubMed] [Google Scholar]
- Rystedt I, and Fischer T. 1983. Relationship between nickel and cobalt sensitization in hard metal workers. Contact Dermatitis 9:195–200. doi: 10.1111/j.1600-0536.1983.tb04357.x. [DOI] [PubMed] [Google Scholar]
- Sachdeva S, Gupta V, Amin SS, and Tahseen M. 2011. Chronic urticaria. Indian J. Dermatol 56:622–28. doi: 10.4103/0019-5154.91817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagoo NS, Sharma R, Alaraj S, Sharma IK, Bruntz AJ, and Bajaj GS. 2021. Metal hypersensitivity and complex regional pain syndrome after bilateral total knee arthroplasty: A case report. JBJS Case Connect 11. doi: 10.2106/jbjs.Cc.21.00099. [DOI] [PubMed] [Google Scholar]
- Sahan I, and Anagnostakos K. 2020. Metallosis after knee replacement: A review. Arch. Orthop. Trauma Surg 140:1791–808. doi: 10.1007/s00402-020-03560-x. [DOI] [PubMed] [Google Scholar]
- Sakai T, Hatano Y, and Fujiwara S. 2013. Systemic contact dermatitis due to zinc successfully treated with a zinc-restricted diet: A case report. Allergol Int 62:265–67. doi: 10.2332/allergolint.12-LE-0518. [DOI] [PubMed] [Google Scholar]
- Sakai M, Kato G, Fujioka T, Sugihara M, Koyanagi K, Kato O, Tominaga M, and Kawabata Y. 2010. A case of hard metal lung disease in a man who worked as an iron grinder. Nihon Kokyuki Gakkai Zasshi 48:282–87. [PubMed] [Google Scholar]
- Sakamoto O, Kosai S, and Kohrogi H. 2008. a case of hard metal lung disease presenting a type of chronic hypersensitivity pneumonitis. Nihon Kokyuki Gakkai Zasshi 46:535–41. [PubMed] [Google Scholar]
- Saltzer EI, and Wilson JW. 1968. Allergic contact dermatitis due to copper. Arch. Dermatol 98:375–376. [Google Scholar]
- Saluja SS, Davis CL, Chong TA, and Powell DL. 2016. Contact urticaria to nickel: A series of 11 patients who were prick test positive and patch test negative to nickel sulfate 2.5% and 5.0%. J Dermatitis 27:282–87. [DOI] [PubMed] [Google Scholar]
- Samelko L, Caicedo MS, Jacobs J, and Hallab NJ. 2019. Transition from metal-DTH resistance to susceptibility is facilitated by NLRP3 inflammasome signaling induced th17 reactivity: implications for orthopedic implants. PLoS One 14:e0210336. doi: 10.1371/journal.pone.0210336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samelko L, Landgraeber S, Mcallister K, Jacobs J, and Hallab NJ. 2016. Cobalt alloy implant debris induces inflammation and bone loss primarily through danger signaling, not TLR4 activation: implications for dampening implant related inflammation. PLoS One 11:e0160141. doi: 10.1371/journal.pone.0160141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel G, and Maier LA. 2008. Immunology of chronic beryllium disease. Curr. Opin. Allergy Clin. Immunol 8:126–34. doi: 10.1097/ACI.0b013e3282f824a4. [DOI] [PubMed] [Google Scholar]
- Samuel S, Soumya SV, and Koshy S. 2014. Delayed hypersensitivity or geographic tongue: A diagnostic dilemma. J Pierre Fauchard Acad (India Section) 28:122–24. doi: 10.1016/j.jpfa.2015.01.001. [DOI] [Google Scholar]
- Sánchez-Borges M, Martin BL, Muraro AM, Wood RA, Agache IO, Ansotegui IJ, Casale TB, Fleisher TA, Hellings PW, and Papadopoulos NG. 2018. The importance of allergic disease in public health: an ICALL statement. World Allergy Organ. J 11:8. doi: 10.1186/s40413-018-0187-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Morillas L, and Ar DL. 2004. Baboon syndrome. Allergol. Immunopathol. (Madr.) 32:43–45. [DOI] [PubMed] [Google Scholar]
- Santos GF, Portela J, Argyropoulou D, Varudo R, Pimenta I, Oliveira A, Lança S, and Fernandes A. 2020. Alveolar proteinosis due to toxic inhalation at workplace. Respir. Med. Case Rep 31:101199. doi: 10.1016/j.rmcr.2020.101199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santucci B, Cannistraci C, Cristaudo A, and Picardo M. 1996. Multiple sensitivities to transition metals: the nickel palladium reactions. Contact Dermatitis 35:283–86. [DOI] [PubMed] [Google Scholar]
- Santucci B, Valenzano C, De Rocco M, and Cristaudo A. 2000. Platinum in the environment: frequency of reactions to platinum-group elements in patients with dermatitis and urticaria. Contact Dermatitis 43:333–38. doi: 10.1034/j.1600-0536.2000.043006333.x. [DOI] [PubMed] [Google Scholar]
- Sasseville D 2008. Occupational contact dermatitis. Allergy Asthma Clin. Immunol 4:59–65. doi: 10.1186/1710-1492-4-2-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastre J, Fernández-Nieto M, Marañón F, Fernandez-Caldas E, Pelta R, and Quirce S. 2001. Allergenic cross-reactivity between nickel and chromium salts in electroplating-induced asthma. J. Allergy Clin. Immunol 108:650–51. doi: 10.1067/mai.2001.118294. [DOI] [PubMed] [Google Scholar]
- Satoh-Kamachi A, Munakata M, Kusaka Y, Amishima M, Furuya K, Takahashi T, and Kawakami Y. 1998. A case of sarcoidosis that developed three years after the onset of hard metal asthma. Am. J. Ind. Med 33:379–84. [DOI] [PubMed] [Google Scholar]
- Sauni R, Järvenpää R, Iivonen E, Nevalainen S, and Uitti J. 2007. Pulmonary alveolar proteinosis induced by silica dust? Occup. Med. (Lond.) 57:221–24. doi: 10.1093/occmed/kql162. [DOI] [PubMed] [Google Scholar]
- Saxena M, Warshaw E, and Ahmed DD. 2001. Eyelid allergic contact dermatitis to black iron oxide. Am. J. Contact Dermatol 12:38–39. doi: 10.1053/ajcd.2000.18398. [DOI] [PubMed] [Google Scholar]
- Schena D, Barba A, and Costa G. 1996. Occupational contact urticaria due to cisplatin. Contact Derm. 34:220–21. doi: 10.1111/j.1600-0536.1996.tb02181.x. [DOI] [PubMed] [Google Scholar]
- Scherak O, Popp W, Kolarz G, Wottawa A, Ritschka L, and Braun O. 1993. Bronchoalveolar lavage and lung biopsy in rheumatoid arthritis. In vivo effects of disease modifying antirheumatic drugs. J. Rheumatol 20:944–49. [PubMed] [Google Scholar]
- Schiff A, Jiries N, Goldsztein S, Schiff E, and Ginsberg S. 2021. A possible association of orthopedic metal implants and Asia syndrome. J. Rheumatol 60:e95–e96. [DOI] [PubMed] [Google Scholar]
- Schultzel M, Klein CM, Demirjian M, Blout C, and Itamura JM. 2020. Incidence of metal hypersensitivity in orthopedic surgical patients who self-report hypersensitivity history. Permanente J 24:19.091. doi: 10.7812/TPP/19.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuttelaar ML, Ofenloch RF, Bruze M, Cazzaniga S, Elsner P, Gonçalo M, Naldi L, Svensson Å, and Diepgen TL. 2018. Prevalence of contact allergy to metals in the european general population with a focus on nickel and piercings: the EDEN fragrance study. Contact Dermatitis 79:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz YA, Kivity S, Fischbein A, Ribak Y, Fireman E, Struhar D, Topilsky M, and Greif J. 1994. Eosinophilic lung reaction to aluminium and hard metal. Chest 105:1261–63. doi: 10.1378/chest.105.4.1261. [DOI] [PubMed] [Google Scholar]
- Scott KA, and Wardlaw AJ. 2006. Eosinophilic airway disorders. Semin. Respir. Crit. Care Med 27:128–33. doi: 10.1055/s-2006-939515. [DOI] [PubMed] [Google Scholar]
- Scurlock AM, Vickery BP, Hourihane JO, and Burks AW. 2010. Pediatric food allergy and mucosal tolerance. Mucosal Immunol 3:345–54. doi: 10.1038/mi.2010.21. [DOI] [PubMed] [Google Scholar]
- Seaman DM, Meyer CA, and Kanne JP. 2015. Occupational and environmental lung disease. Clin. Chest Med 36:249–268, viii–ix. doi: 10.1016/j.ccm.2015.02.008. [DOI] [PubMed] [Google Scholar]
- Sergio P, Ceruti M, Manotti L, Manuguerra R, Ceruti P, and Dell’osso A. 2017. Hard metal lung disease: unexpected CT findings. Indian J. Radiol. Imaging 27:256–59. doi: 10.4103/ijri.IJRI_404_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shutty BG, and Scheinman PL. 2018. Allergic contact mucositis caused by metal: A covertly located permanent dental retainer. Contact Dermatitis 78:88–90. doi: 10.1111/cod.12848. [DOI] [PubMed] [Google Scholar]
- Silverberg NB, Pelletier JL, Jacob SE, and Schneider LC. 2020. Nickel allergic contact dermatitis: Identification, treatment, and prevention. Pediatrics 145 (5):e20200682. doi: 10.1542/peds.2020-0628. [DOI] [PubMed] [Google Scholar]
- Simpson CR, Newton J, Hippisley-Cox J, and Sheikh A. 2008. Incidence and prevalence of multiple allergic disorders recorded in a national primary care database. J. Roy Soc. Med 101:558–63. doi: 10.1258/jrsm.2008.080196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjåheim T, Halstensen T, Lund M, Bjørtuft Ø, Drabløs P, Malterud D, and Kongerud J. 2004. Airway inflammation in aluminium potroom asthma. Occup. Environ. Med 61:779–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelton HG, Smith KJ, Johnson FB, Cooper CR, Tyler WF, and Lupton GP. 1993. Zirconium granuloma resulting from an aluminum zirconium complex: A previously unrecognized agent in the development of hypersensitivity granulomas. J. Am. Acad. Dermatol 28:874–76. doi: 10.1016/0190-9622(93)70122-a. [DOI] [PubMed] [Google Scholar]
- Slingerland R, Hoogsteden HC, Adriaansen HJ, Van Der Kwast TH, and Hilvering C. 1987. Gold-induced pneumonitis. Respiration 52:232–36. doi: 10.1159/000195330. [DOI] [PubMed] [Google Scholar]
- Sluka KA, and Clauw DJ. 2016. Neurobiology of fibromyalgia and chronic widespread pain. Neuroscience 338:114–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JD, Odom RB, and Maibach HI. 1975. Contact urticaria from cobalt chloride. Arch. Dermatol 111:1610–11. [PubMed] [Google Scholar]
- Sockanathan S, Setterfield J, and Wakelin S. 2003. Oral lichenoid reaction due to chromate/cobalt in dental prosthesis. Contact Dermatitis 48:342–43. doi: 10.1034/j.1600-0536.2003.00140.x. [DOI] [PubMed] [Google Scholar]
- Sokół K, Szostakowski B, Chraszczewska M, Goryń T, Wągrodzki M, Prochorec-Sobieszek M, and Szumera-Ciećkiewicz A. 2020. Histiocytic lymphadenopathy secondary to metallosis following endoprosthetic replacement in osteosarcoma patient–a potential diagnostic pitfall. J. Oncol 70:272–75. [Google Scholar]
- Song H, Yin W, and Ma Q. 2011. Allergic palmoplantar pustulosis caused by cobalt in cast dental crowns: A case report. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod 111:e8–10. doi: 10.1016/j.tripleo.2010.12.013. [DOI] [PubMed] [Google Scholar]
- Sood A 2009. Current treatment of chronic beryllium disease. J. Occup. Environ Hyg 6:762–65. doi: 10.1080/15459620903158698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soparkar CN, Wilhelmus KR, Koch DD, Wallace GW, and Jones DB. 1997. Acute and chronic conjunctivitis due to over-the-counter ophthalmic decongestants. Arch. Ophthalmol 115:34–38. [DOI] [PubMed] [Google Scholar]
- Sorgdrager B, Pal TM, De Looff AJ, Dubois AE, and De Monchy JG. 1995. Occupational asthma in aluminium potroom workers related to pre-employment eosinophil count. Eur. Respir. J 8:1520–24. [PubMed] [Google Scholar]
- Sperber BR, Allee J, Elenitsas R, and James WD. 2003. Papular dermatitis and a persistent patch test reaction to gold sodium thiosulfate. Contact Dermatitis 48:204–08. doi: 10.1034/j.1600-0536.2003.00081.x. [DOI] [PubMed] [Google Scholar]
- Spinelli V, Boniface S, Lehucher-Michel MP, Vervloet D, and Magnan A. 2005. L’asthme au nickel. Rev. Francaise D Allergol. Et D Immunol. Clin 45:103–07. doi: 10.1016/j.allerg.2004.12.003. [DOI] [Google Scholar]
- Stanghellini V, Tosetti C, Benedetto E, Condoluci M, De Bastiani R, Cogliandro R, Mastronuzzi T, De Polo M, Di Mita F, and Napoli L. 2016. Nickel sensitization in patients with gastro-esophageal reflux disease. United Eur. Gastroenterol. J 4:184–90. doi: 10.1177/2050640615595917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefaniak AB, Duling MG, Geer L, and Virji MA. 2014. Dissolution of the metal sensitizers ni, be, cr in artificial sweat to improve estimates of dermal bioaccessibility. Environ Sci Process Impacts 16:341–51. doi: 10.1039/c3em00570d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stejskal V 2014. Metals as a common trigger of inflammation resulting in non-specific symptoms: diagnosis and treatment. Israel Med. Assoc. J 16:753–58. [PubMed] [Google Scholar]
- Stejskal V, Hudecek R, Stejskal J, and Sterzl I. 2006. Diagnosis and treatment of metal-induced side-effects. Neuro Endocrinol. Lett 27 (Suppl 1):7–16. [PubMed] [Google Scholar]
- Stejskal V, Ockert K, and Bjørklund G. 2013. Metal-induced inflammation triggers fibromyalgia in metal-allergic patients. Neuro Endocrinol. Lett 34:559–65. [PubMed] [Google Scholar]
- Stejskal V, Reynolds T, and Bjørklund G. 2015. Increased frequency of delayed type hypersensitivity to metals in patients with connective tissue disease. J Trace Elem Med Biol 31:230–36. doi: 10.1016/j.jtemb.2015.01.001. [DOI] [PubMed] [Google Scholar]
- Sterzl I, Procházková J, Hrdá P, Bártová J, Matucha P, and Stejskal VD. 1999. Mercury and nickel allergy: risk factors in fatigue and autoimmunity. Neuro Endocrinol. Lett 20:221–28. [PubMed] [Google Scholar]
- Stransky L 1998. Contact pemphigus vulgaris? Contact Dermatitis 38:45–45. [DOI] [PubMed] [Google Scholar]
- Stuckert J, and Nedorost S. 2008. Low-cobalt diet for dyshidrotic eczema patients. Contact Dermatitis 59:361–65. [DOI] [PubMed] [Google Scholar]
- Suárez CP, Fernández-Redondo V, and Toribio J. 2002. Bingo-hall worker’s occupational copper contact dermatitis from coins. Contact Dermatitis 47:182–000. [DOI] [PubMed] [Google Scholar]
- Summer B, Fink U, Zeller R, Rueff F, Maier S, Roider G, and Thomas P. 2007. Patch test reactivity to a cobalt-chromium-molybdenum alloy and stainless steel in metal-allergic patients in correlation to the metal ion release. Contact Derm. 57:35–39. doi: 10.1111/j.1600-0536.2007.01139.x. [DOI] [PubMed] [Google Scholar]
- Svedman C, Ekqvist S, Möller H, Björk J, Pripp CM, Gruvberger B, Holmström E, Gustavsson CG, and Bruze M. 2009. A correlation found between contact allergy to stent material and restenosis of the coronary arteries. Contact Dermatitis 60:158–64. doi: 10.1111/j.1600-0536.2008.01502.x. [DOI] [PubMed] [Google Scholar]
- Svedman C, Tillman C, Gustavsson CG, Möller H, Frennby B, and Bruze M. 2005. Contact allergy to gold in patients with gold-plated intracoronary stents. Contact Dermatitis 52:192–96. doi: 10.1111/j.0105-1873.2005.00522.x. [DOI] [PubMed] [Google Scholar]
- Takeda Y, Suto Y, Ito K, Hashimoto W, Nishiya T, Ueda K, Narushima T, Takahashi T, and Ogasawara K. 2017. Trav7–2* 02 expressing cd8+ t cells are responsible for palladium allergy. J International Journal of Molecular Sciences 18:1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan CH, Rasool S, and Johnston GA. 2014. Contact dermatitis: allergic and irritant. Clin. Dermatol 32:116–24. doi: 10.1016/j.clindermatol.2013.05.033. [DOI] [PubMed] [Google Scholar]
- Ţăranu T, Grigorovici M, Constantin M, and Toader MP. 2017. Subcutaneous granuloma annulare. Acta Dermatovenerol Croat 25:292–94. [PubMed] [Google Scholar]
- Taweesedt PT, Nordstrom CW, Stoeckel J, and Dumic I. 2019. Pulmonary manifestations of drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome: A systematic review. Biomed. Res. Int 2019:7863815. doi: 10.1155/2019/7863815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temesvari E, and Daroczy J. 1989. Histological examination of immediate and delayed contact allergy provoked by mercuric chloride. Contact Dermatitis 21:271–72. doi: 10.1111/j.1600-0536.1989.tb03211.x. [DOI] [PubMed] [Google Scholar]
- Teo WZW, and Schalock PC. 2016. Metal hypersensitivity reactions to orthopedic implants. Dermatol. Ther 7:53–64. doi: 10.1007/s13555-016-0162-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrani I, Scherer Hofmeier K, and Bircher AJ. 2020. Indium and iridium: Two rare metals with a high rate of contact sensitization. Contact Derm. 83:94–98. doi: 10.1111/cod.13549. [DOI] [PubMed] [Google Scholar]
- Thanasias E, Polychronakis I, Van Kampen V, Brüning T, and Merget R. 2013. Occupational immediate-type allergic asthma due to potassium tetrachloroplatinate in production of cytotoxic drugs. Adv. Exp. Med. Biol 755:47–53. doi: 10.1007/978-94-007-4546-9_6. [DOI] [PubMed] [Google Scholar]
- Thomas P, Bandl WD, Maier S, Summer B, and Przybilla B. 2006a. Hypersensitivity to titanium osteosynthesis with impaired fracture healing, eczema, and T-cell hyperresponsiveness in vitro: Case report and review of the literature. Contact Dermatitis 55:199–202. doi: 10.1111/j.1600-0536.2006.00931.x. [DOI] [PubMed] [Google Scholar]
- Thomas P, Braathen L, Dörig M, Auböck J, Nestle F, Werfel T, and Willert H. 2009. Increased metal allergy in patients with failed metal-on-metal hip arthroplasty and peri-implant T-lymphocytic inflammation. Allergy 64:1157–65. [DOI] [PubMed] [Google Scholar]
- Thomas P, Gollwitzer H, Maier S, and Rueff F. 2006b. Osteosynthesis associated contact dermatitis with unusual perpetuation of hyperreactivity in a nickel allergic patient. Contact Dermatitis 54:222–25. doi: 10.1111/j.0105-1873.2006.0775j.x. [DOI] [PubMed] [Google Scholar]
- Thomsen SF 2015. Epidemiology and natural history of atopic diseases. Eur. Clin. Respir. J 2:10.3402/ecrj. v3402.24642. doi: 10.3402/ecrj.v2.24642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thongprasom K, Suvanpiyasiri C, Wongsa AS, Iamaroon A, Korkij W, Lohwongwatana B, Sinpitaksakul S, and Nakpipat P. 2011. Nickel-induced oral pemphigus vulgaris-like lesions. Acta Stomatol. Croat 45:3. [Google Scholar]
- Thorp JM Jr., Katz VL, Campbell D, and Cefalo RC. 1989. Hypersensitivity to magnesium sulfate. Am. J. Obstet. Gynecol 161:889–90. doi: 10.1016/0002-9378(89)90744-8. [DOI] [PubMed] [Google Scholar]
- Thyssen JP, Ahlström MG, Bruze M, Rustemeyer T, and Lidén C. 2021. “Chapter 40: Contact Allergy to Metals.“ In Contact Dermatitis. 16th ed., 757–802. [Google Scholar]
- Thyssen JP, Gawkrodger DJ, White IR, Julander A, Menné T, and Lidén C. 2013. Coin exposure may cause allergic nickel dermatitis: A review. Contact Dermatitis 68:3–14. doi: 10.1111/j.1600-0536.2012.02127.x. [DOI] [PubMed] [Google Scholar]
- Thyssen JP, and Menné T. 2010. Metal allergy–a review on exposures, penetration, genetics, prevalence, and clinical implications. Chem. Res. Toxicol 23:309–18. doi: 10.1021/tx9002726. [DOI] [PubMed] [Google Scholar]
- Thyssen JP, Ross-Hansen K, Menné T, and Johansen JD. 2010. Patch test reactivity to metal allergens following regulatory interventions: A 33-year retrospective study. Contact Dermatitis 63:102–06. doi: 10.1111/j.1600-0536.2010.01751.x. [DOI] [PubMed] [Google Scholar]
- Tilakaratne D, and Sidhu S. 2015. Heavy metal (monoclonal) bands: A link between cutaneous t-cell lymphoma and contact allergy to potassium dichromate, nickel and cobalt? Australas. J. Dermatol 56:59–63. doi: 10.1111/ajd.12182. [DOI] [PubMed] [Google Scholar]
- Tinkle SS, Antonini JM, Rich BA, Roberts JR, Salmen R, Depree K, and Adkins EJ. 2003. Skin as a route of exposure and sensitization in chronic beryllium disease. Environ. Health Perspect 111:1202–08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari SM, Gebauer K, Frydrych AM, and Burrows S. 2018. Dental patch testing in patients with undifferentiated oral lichen planus. Australas. J. Dermatol 59:188–93. [DOI] [PubMed] [Google Scholar]
- Toledo F, Silvestre JF, Cuesta L, Latorre N, and Monteagudo A. 2011. Contact allergy to beryllium chloride: Report of 12 cases. Contact Derm. 64:104–09. [DOI] [PubMed] [Google Scholar]
- Tomar S, and Hogan SP. 2020. Recent advances in mechanisms of food allergy and anaphylaxis. F1000Res 9. doi: 10.12688/f1000research.25638.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomioka R, and King TE Jr. 1997. Gold-induced pulmonary disease: clinical features, outcome, and differentiation from rheumatoid lung disease. Am. J. Respir. Crit. Care Med 155:1011–20. [DOI] [PubMed] [Google Scholar]
- Tomka M, Machovcová A, Pelclová D, Petanová J, Arenbergerová M, and Procházková J. 2011. Orofacial granulomatosis associated with hypersensitivity to dental amalgam. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod 112:335–41. doi: 10.1016/j.tripleo.2011.03.030. [DOI] [PubMed] [Google Scholar]
- Torgerson RR, Davis MDP, Bruce AJ, Farmer SA, and Rogers RS. 2007. Contact allergy in oral disease. J. Am. Acad. Dermatol 57:315–21. doi: 10.1016/j.jaad.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Tous Romero F, Palencia Perez SI, Rodriguez Peralto JL, and De Frutos FJO. 2021. Contact allergy to aluminum following vaccination: A report of 3 cases. Actas Dermosifiliogr (Engl Ed). doi: 10.1016/j.ad.2020.01.013. [DOI] [PubMed] [Google Scholar]
- Tous-Romero F, Velasco-Tamariz V, Prieto-Barrios M, and Ortiz De Frutos J. 2017. Active sensitization to cadmium, tin,and indium. Contact Derm. 77:332–33. doi: 10.1111/cod.12826. [DOI] [PubMed] [Google Scholar]
- Towers WS, and Kurtom K. 2020. Rare systemic response to titanium spinal fusion implant: case report. Cureus 12: e7109. doi: 10.7759/cureus.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell BC, Nakata K, Bonella F, Campo I, Griese M, Hamilton J, Wang T, Morgan C, Cottin V, and McCarthy C. 2019. Pulmonary alveolar proteinosis. Nat. Rev. Dis. Primers 5:16. doi: 10.1038/s41572-019-0066-3. [DOI] [PubMed] [Google Scholar]
- Tronnier M, and Mitteldorf C. 2015. Histologic features of granulomatous skin diseases. part 1: non-infectious granulomatous disorders. J Deutschen Dermatologischen Gesellschaft 13:211–16. [DOI] [PubMed] [Google Scholar]
- Tschanz C, and Prins C. 2000. Drug rash with eosinophilia and systemic symptoms caused by topical application of mercury. J. Dermatol 201:381–82. [DOI] [PubMed] [Google Scholar]
- Tsui HC, Ronsmans S, De Sadeleer LJ, Hoet PHM, Nemery B, and Vanoirbeek JAJ. 2020. Skin exposure contributes to chemical-induced asthma: what is the evidence? A systematic review of animal models. Allergy Asthma Immunol. Res 12:579–98. doi: 10.4168/aair.2020.12.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuchman M, Silverberg JI, Jacob SE, and Silverberg N. 2015. Nickel contact dermatitis in children. Clin. Dermatol 33:320–26. doi: 10.1016/j.clindermatol.2014.12.008. [DOI] [PubMed] [Google Scholar]
- Turčić P, Marinović-Kulišić S, and Lipozenčić J. 2013. Patch test reactions to metal salts in patients with different types of dermatitis. Acta Dermatovenerol.Croatica 21:180–180. [PubMed] [Google Scholar]
- Uter W, Werfel T, White IR, and Johansen JD. 2018. Contact allergy: A review of current problems from a clinical perspective. Int. J. Environ. Res. Public Health 15:1108. doi: 10.3390/ijerph15061108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzzaman A, and Cho SH. 2012. Chapter 28: classification of hypersensitivity reactions. Allergy Asthma Proc 33 (Suppl 1):96–99. [DOI] [PubMed] [Google Scholar]
- Vallejo Irastorza G, Lopez Cedrun JL, Marinez-Conde R, and Aguirre Urizar JM. 1990. Oral lichenoid reaction secondary to gold salt therapy. Av. Odontoestomatol 6:131–33. [PubMed] [Google Scholar]
- Van Cutsem EJ, Ceuppens JL, Lacquet LM, and Demedts M. 1987. Combined asthma and alveolitis induced by cobalt in a diamond polisher. Eur. J. Respir. Dis 70:54–61. [PubMed] [Google Scholar]
- Van Der Merwe J 2021. Trunnionosis and metal wear causing metal hypersensitivity: 2 sides of the same coin? Can. J. Surg 64:E534–e536. doi: 10.1503/cjs.013420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Voet G 2010. Metals. In Side effects of drugs annual: V22, ed. K J, 413–23. Amsterdam, The Netherlands: Aronson, Elsevier. [Google Scholar]
- Van Loon L, Van Elsas P, Van Joost T, and Davidson C. 1984. Contact stomatitis and dermatitis to nickel and palladium. J Contact Dermatitis 11:294–97. [DOI] [PubMed] [Google Scholar]
- Vandenplas O, Delwiche J, Vanbilsen M, Joly J, and Roosels D. 1998. Occupational asthma caused by aluminium welding. Eur. Respir. J 11:1182–84. [DOI] [PubMed] [Google Scholar]
- Vaz R, Xavier P, Brito S, Dantas J, Duque S, Consciência JG, and Campos L. 2019. Metallosis: A new form of autoimmune/autoinflammatory syndrome induced by adjuvants syndrome (Asia)? Eur. J. Case Rep. Intern. Med 6:001034–001034. doi: 10.12890/2019_001034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veien NK 2009. Acute and recurrent vesicular hand dermatitis. Dermatol. Clin 27:337–53. doi: 10.1016/j.det.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Veien NK, Borchorst E, Hattel T, and Laurberg G. 1994. Stomatitis or systemically-induced contact dermatitis from metal wire in orthodontic materials. Contact Dermatitis 30:210–13. doi: 10.1111/j.1600-0536.1994.tb00645.x. [DOI] [PubMed] [Google Scholar]
- Velásquez D, Majlis PZ, Fernández RS, and Ochaíta PL. 2010. Allergic contact dermatitis to manganese in a prosthodontist with orthodontics. Allergol Immunopath (Madr) 38:47–48. [DOI] [PubMed] [Google Scholar]
- Vergara G, Silvestre J, Botella R, Albares M, and Pascual J. 2004. Oral lichen planus and sensitization to copper sulfate. Contact Dermatitis 50:374–374. [DOI] [PubMed] [Google Scholar]
- Vien NK, and Kaaber K. 1979. Nickel cobalt and chromium sensitivity in patients with pompholyx (dyshidrotic eczema). Contact Dermatitis 5:371–74. [DOI] [PubMed] [Google Scholar]
- Vilaplans J, Romaguera C, and Grimalt F. 1992. Occupational and non-occupational allergic contact dermatitis from beryllium. Contact Derm. 26:295–98. [DOI] [PubMed] [Google Scholar]
- Vinit C, Dieme A, Courbage S, Dehaine C, Dufeu CM, Jacquemot S, Lajus M, Montigny L, Payen E, and Yang DD. 2019. Eosinophilic esophagitis: pathophysiology, diagnosis, and management. Arch. Pediatr 26:182–90. doi: 10.1016/j.arcped.2019.02.005. [DOI] [PubMed] [Google Scholar]
- Wade MF, Collins MK, Richards D, Mack DG, Martin AK, Dinarello CA, Fontenot AP, and McKee AS. 2018. TLR9 and Il-IRL promote mobilization of pulmonary dendritic cells during beryllium sensitization. J. Immunol 201:2232–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh ML, Smith VH, and King CM. 2010. Type 1 and type iv hypersensitivity to nickel. Australas. J. Dermatol 51:285–86. [DOI] [PubMed] [Google Scholar]
- Walters G, Moore V, Robertson A, Burge C, Vellore A-D, and Burge P. 2012. An outbreak of occupational asthma due to chromium and cobalt. J Occ Med 62:533–40. [DOI] [PubMed] [Google Scholar]
- Wang T, Lazar CA, Fishbein MC, and Lynch III JP. 2012. Pulmonary alveolar proteinosis. Semin. Respir. Crit. Care Med 33:498–508. [DOI] [PubMed] [Google Scholar]
- Wang LJ, Mu SC, Lin MI, Sung TC, Chiang BL, and Lin CH. 2021. Clinical manifestations of pediatric food allergy: A contemporary review. Clin Rev Allergy Immunol 62 (1):180–99. [DOI] [PubMed] [Google Scholar]
- Wang L-J, Mu S-C, Lin M-I, Sung T-C, Chiang B-L, and Lin C-H. 2022. Clinical manifestations of pediatric food allergy: A contemporary review. Clin. Rev. Allergy Immunol 62:180–99. [DOI] [PubMed] [Google Scholar]
- Wang LF, Wu J, Zheng C, Li SL, Huang RR, and Zhang JK. 2016. Long-term fever after hallux valgus surgery secondary to titanium allergy: A case report and review of the literature. J. Foot Ankle Surg 55:1282–86. doi: 10.1053/j.jfas.2015.06.021. [DOI] [PubMed] [Google Scholar]
- Warner SM, and Knight DA. 2008. Airway modeling and remodeling in the pathogenesis of asthma. Curr. Opin. Allergy Clin. Immunol 8:44–48. [DOI] [PubMed] [Google Scholar]
- Waroquier D, Evrard L, Nelis M, and Parent D. 2009. Allergic contact stomatitis presenting as geographical tongue with pruritus. Contact Dermatitis 60:106–106. doi: 10.1111/j.1600-0536.2008.01352.x. [DOI] [PubMed] [Google Scholar]
- Warshaw EM, Belsito DV, Taylor JS, Sasseville D, Dekoven JG, Zirwas MJ, Fransway AF, Mathias CGT, Zug KA, and DeLeo VA 2013. North American contact dermatitis group patch test results: 2009 to 2010. Dermatitis 24:50–59. doi: 10.1097/DER.0b013e3182819c51. [DOI] [PubMed] [Google Scholar]
- Warshaw EM, Schlarbaum JP, Dekoven JG, Silverberg JI, Zug KA, Marks JG, Belsito DV, Mathias T, and Reeder MJ. 2019. Occupationally related nickel reactions: A retrospective analysis of the North American contact dermatitis group data 1998–2016. Dermatitis 30:306–13. doi: 10.1097/der.0000000000000516. [DOI] [PubMed] [Google Scholar]
- Watchmaker J, Collins R, and Chaney K. 2015. Allergic contact dermatitis to manganese in metallic implant. Dermatitis 26:149–50. [DOI] [PubMed] [Google Scholar]
- Watsky KL 2007. Occupational allergic contact dermatitis to platinum, palladium, and gold. Contact Dermatitis 57:382–83. doi: 10.1111/j.1600-0536.2007.01144.x. [DOI] [PubMed] [Google Scholar]
- Weryńska-Kalemba M, Filipowska-Grońska A, Kalemba M, Krajewska A, Grzanka A, Bożek A, and Jarząb J. 2016. Analysis of selected allergic reactions among psoriatic patients. Postepy Dermatol. Alergol 33:18–22. doi: 10.5114/pdia.2014.44015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick MR 2017. Panniculitis: A summary. Semin. Diagn. Pathol 34:261–72. doi: 10.1053/j.semdp.2016.12.004. [DOI] [PubMed] [Google Scholar]
- Wicks IP, Wong D, McCullagh RB, and Fleming A. 1988. Contact allergy to gold after systemic administration of gold for rheumatoid arthritis. Ann. Rheum. Dis 47:421–22. doi: 10.1136/ard.47.5.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggans RE, and Barber CM. 2017. Metalworking fluids: A new cause of occupational non-asthmatic eosinophilic bronchitis. Thorax 72:579–80. doi: 10.1136/thoraxjnl-2016-208827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittczak T, Dudek W, Krakowiak A, Walusiak J, and Palczynski C. 2008. Occupational asthma due to manganese exposure: A case report. Int J Occup Med Environ Health 21:81–83. doi: 10.2478/v10001-007-0041-1. [DOI] [PubMed] [Google Scholar]
- Wittczak T, Dudek W, Walusiak-Skorupa J, Swierczynska-Machura D, Cader W, Kowalczyk M, and Palczynski C. 2012. Metal-induced asthma and chest x-ray changes in welders. Int J Occup Med Environ Health 25:242–50. doi: 10.2478/s13382-012-0031-9. [DOI] [PubMed] [Google Scholar]
- Woda BA 2008. Hypersensitivity pneumonitis: an immunopathology review. Arch. Pathol. Lab. Med 132:204–05. doi: 10.1043/1543-2165(2008)132[204:Hpair]2.0.Co;2. [DOI] [PubMed] [Google Scholar]
- Wöhrl S, Hemmer W, Focke M, Götz M, and Jarisch R. 2003. Patch testing in children, adults, and the elderly: Influence of age and sex on sensitization patterns. Pediatr. Dermatol 20:119–23. doi: 10.1046/j.1525-1470.2003.20204.x. [DOI] [PubMed] [Google Scholar]
- Wojas O, Żalikowska-Gardocka M, Krzych-Fałta E, Szczepankiewicz B, Samel-Kowalik P, Samoliński B, and Przybyłkowski A. 2021. A case of lymphocytic esophagitis in a woman with multiple allergies. Allergy Asthma Clin. Immunol 17:56. doi: 10.1186/s13223-021-00558-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Allergy Organization. “Sensitizing agents inducers of occupational asthma, hypersensitivity pneumonitis and eosinophilic bronchitis.” https://www.worldallergy.org/education-and-programs/education/allergic-disease-resource-center/professionals/sensitizing-agents-inducers-of-occupational-asthma .
- World Health Organization. 2011.Guidelines for Drinking Water Quality. Geneva: World Health Organization. [Google Scholar]
- Wu WF, Wan KS, Wang SJ, Yang W, and Liu WL. 2011. Prevalence, severity, and time trends of allergic conditions in 6-to-7-year-old schoolchildren in taipei. J. Invest. Allergol. Clin. Immunol 21:556–62. [PubMed] [Google Scholar]
- Wyman AE, and Hines SE. 2018. Update on metal-induced occupational lung disease. Curr. Opin. Allergy Clin. Immunol 18:73–79. doi: 10.1097/aci.0000000000000420. [DOI] [PubMed] [Google Scholar]
- Xue N, Zhao L, Luo Y, Liu J, Guo S, and Yan Y. 2019. Investigation of contact dermatitis caused by hard metal dust. Chin. J. Ind. Hyg 37:589–92. [DOI] [PubMed] [Google Scholar]
- Yacoub MR, Malo JL, Labrecque M, Cartier A, and Lemière C. 2005. Occupational eosinophilic bronchitis. Allergy 60:1542–44. doi: 10.1111/j.1398-9995.2005.00920.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Tsujino K, Urasaki K, Matsuki T, Fukushima K, and Kida H. 2020. Arc-Welders’ pneumoconiosis with atypical radiological and bronchoalveolar lavage fluid findings: A case report. Respir Med Case Rep 29:101023. doi: 10.1016/j.rmcr.2020.101023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagi T, Shimizu T, Abe R, and Shimizu H. 2005. Zinc dental fillings and palmoplantar pustulosis. Lancet 366:1050. [DOI] [PubMed] [Google Scholar]
- Yao B, Kuznetsov VL, Xiao T, Slocombe DR, Rao C, Hensel F, and Edwards PP. 2020. Metals and non-metals in the periodic table. Phil. Trans. Roy. Soc. London, Ser. A 378:20200213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yıldız T, and Dülger S. 2018. Non-astmatic eosinophilic bronchitis. Turkish Thoracic J. 19:41–45. doi: 10.5152/TurkThoracJ.2017.17017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorozuya T, Ikeda K, Chiba H, Saito A, Kuronuma K, Nishikiori H, Miyajima S, Takahashi M, Yoshikawa T, and Takahashi Y. 2019. Autoimmune pulmonary alveolar proteinosis diagnosed after exposure to a fire extinguisher containing silica powder. Intern. Med 58:2067–72. doi: 10.2169/internalmedicine.1557-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihisa Y, and Shimizu T. 2012. Metal allergy and systemic contact dermatitis: An overview. Dermatol. Res. Pract 2012:749561–749561. doi: 10.1155/2012/749561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousaf A, Hagen R, Mitchell M, Ghareeb E, Fang W, Correa R, Zinn Z, and Gayam S. 2020. The effect of a low-nickel diet and nickel sensitization on gastroesophageal reflux disease: A pilot study. Indian J. Gastroenterol doi: 10.1007/s12664-020-01090-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M, Marron RM, and Sehgal S. 2020. Hard metal lung disease: uspdate in diagnosis and management. Curr. Pulmonol. Rep 9:37–46. [Google Scholar]
- Zhubrak M, and Bar-David T. 2014. Systemic nickel allergy after internal fixation of a bunionectomy. J. Foot Ankle Surg 53:466–67. doi: 10.1053/j.jfas.2014.03. 006. [DOI] [PubMed] [Google Scholar]
- Zigante M, Mlinaric MR, Kastelan M, Perkovic V, Zrinski MT, and Spalj S. 2020. Symptoms of titanium and nickel allergic sensitization in orthodontic treatment. Prog. Orthod 21:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirwas MJ, and Molenda MA. 2009. Dietary nickel as a cause of systemic contact dermatitis. Jcad 2:39–43. [PMC free article] [PubMed] [Google Scholar]