Abstract
The worldwide spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has urged scientists to present some novel vaccine platforms during this pandemic to provide a rather prolonged immunity against this respiratory viral infection. In spite of many campaigns formed against the administration of mRNA-based vaccines, those platforms were the most novel types, which helped us meet the global demand by developing protection against COVID-19 and reducing the development of severe forms of this respiratory viral infection. Some societies are worry about the COVID-19 mRNA vaccine administration and the potential risk of genetic integration of inoculated mRNA into the human genome. Although the efficacy and long-term safety of mRNA vaccines have not yet been fully clarified, obviously their application has switched the mortality and morbidity of the COVID-19 pandemic. This study describes the structural features and technologies used in producing of COVID-19 mRNA-based vaccines as the most influential factor in controlling this pandemic and a successful pattern for planning to produce other kind of genetic vaccines against infections or cancers.
Keywords: SARS-CoV-2, Vaccination, Immunization, Horizontal gene transfer (HGT), mRNA-based Vaccine
1. Introduction
Since end of 2019, there has been a global discussion on the coronavirus disease 2019 (COVID-19) pandemic and its consequences [1], [2]. After two historical pandemics with the coronavirus family, including the severe acute respiratory syndrome coronavirus (SARS-CoV) in 2002 and the Middle East Respiratory Syndrome coronavirus (MERS-CoV) in 2012, the world was poisoned with another dangerous type of coronavirus, SARS-CoV-2 [3], [4]. The evolutionary history of this causative viral infectious agent was previously clarified based on the phylogenetic studies on the emerging coronaviridae family. Comparing the nucleotide sequence of the recent virus with the well-known causative agents of SARS and MERS-CoV including, SARS-CoV BJ01 (GenBank: AY278488.2), MERS-CoV HCoV-EMC (GenBank: MH454272.1) and RaTG13 (GenBank: MN996532.1), confirmed their high similarities (75%, 55%, 96%, respectively).
SARS-CoV-2 infection can lead to a spectrum of various clinical symptoms, from asymptomatic, to partial flu, pneumonia and death due to acute respiratory distress syndrome (ARDS). However, all of those cases should be considered, because the asymptomatic carriers of the virus have led to a high prevalence of the disease worldwide. Several strategies including, isolating of the suspected and confirmed cases with moderate personal hygiene measures and strengthening immunity were used by the World Health Organization (WHO) to control the epidemic [3]. However, skiming the situation showed that almost all countries were suffering from the deadly SARS-CoV-2 attack [4].
According to the daily reports of theWHO website (https://covid19.who.int), since the beginning of the pandemic until today, the infected cases and deaths have been 670,000,000 and more than 6,700,000, respectively, with the largest share of infections in Europe and the largest share of deaths in the United States. Since December 2022, 1,900 people have been infected with COVID-19 every week throughout the world. Since the beginning of the year, the death rate of COVID-19 has been 12,000 per week. This report can bring some concerns. Since the beginning of 2023, statistics of deaths in has increased to 5,900 in the United States and 2,700 cases in Europe per week.
2. The COVID-19 vaccine development landscape
In the early days, no definitive treatment was proposed for the spread of COVID-19, but it was believed that the virus might be limited by social isolation of the patients, wearing masks[5]. Within two years to reach the peak of the pandemic, universal guidelines for everyone (health care workers and the general public) were issued by the WHO and the Centers for Disease Control and Prevention (CDC) to control the spread of COVID-19. The use of personal protective equipment such as masks, face shield/visors, goggles, gloves, and gowns was the first protective line for population. Due to the transmission of COVID-19 through airborne droplets, the transmission of SARS-CoV-2 is high in closed environments without ventilation even with social distancing. In general, according to past experiences, in the absence of medical measures (drug prescriptions and vaccines), to manage the spread of infection, wearing a mask with minimal cost and without disruption to social activities is the most effective.methods. The use of surgical and N95 masks, despite the different design and efficiency, have a high filtration capacity of bacterial and viral particles [6], [7]. According to the conducted studies, the level of personal health compliance in communities with COVID-19 was associated to themotivation and reasons of people in their decisions s [8]. The effectiveness of the using face masks in the prevention of respiratory infections is still controversial due to the insufficiency of precise scientific results and conclusions from them, especially in a society where there is insufficient evidence [9]. In a syudy has been performed by Li et al., through a meta-analysis, the effectiveness of the face masks in the prevention of respiratory infections and the performance of its application around the world were investigated. The information obtained from MEDLINE,PubMed, Cochrane, medRxiv, bioRxiv, and Web of Science databases since the beginning of August 2020about 21,341 records was found, which eight out of them were the gold standard research on mask prevention of infection and 78 out of them were studied on the intention and practice of using the face mask. According to the obtained results, 71% of them considered face masks appropriate to prevent infection and, 54% of people used face masks to prevent respiratory infections [9]. The wearing Face masks in public places was implemented as a law in more than 70 countries worldwide [10], [11]. Many people are considered as theasymptomatic carriers that cannot be detected without testing with high specificity. The quarantine people significantly prevent the transmission of the virus to other people. However, During the peak period of COVID-19, the quarantining ofpopulation has been resulted to high social and economic costs in the countries [12]. The negative psychological effects of quarantining people and especially vulnerable groups (chronic physical diseases, mental disorders, infected or suspected patients, people with low financial status, and frontline workers) have been studied a lot in the wake of the pandemic. In an online survey conducted in China in 2020 from February 28 to March 11, the results showed that house arresting or centralized quarantine had psychological consequences for those who had experienced this period. Accordingly, some pharmaceutical industries and scientific institutes started cooperation to develop new effective antiviral drugs [13], [14]. Antiviral drugs, Immunotherapy, were candidate strategies that applied against COVID-19 infection [14].
The administration of antiviral drugs is effective when they are used in the early stages of COVID-19. Once the diagnostic assay of SARS-CoV-2 would be positove, prompt action must be taken to prevent the spread of infection. In addition, pharmacotherapy methods are strongly influenced by the fair distribution of drugs among the population. As evidenced by the global approach to immunization by vaccines, a global approach to the equitable distribution of medicine is needed [15]. Referring to immunotherapy, many patients do not respond to dexamethasone and anti-IL-6 therapy and even after administration in some patients, the intensity of inflammation remains high, and no randomized controlled trials have been formally introduced. In many cases, the pathophysiological heterogeneity of COVID-19 was observed. In this way, the patients' response to SARS-CoV-2 was complex and the signaling pathways and immune response cascades can be both effective and harmful in the healing process [16].
To stop the pandemic in a limited time, researchers began developping novel effective vaccines against SARS-CoV-2, and the initial doses were administered in December 2020. During the pandemic, more than 100 vaccination products were developed and at least 24 products were authorized for emergency use [17]. Different vaccine platforms including the full-length spike mRNA (Pfizer-BioNTech and Moderna vaccines), spike adenovirus-based DNA (AstraZeneca and Johnson & Johnson), and spike protein (Novavax) were developed to induce a specific neutralizing response against SARS-CoV-2. Table S1 summaries the vaccines developed among COVID-19 pandemic.
To date, nearly five billion people worldwide (27% of the world's population) have been fully vaccinated and more than four billion people have received at least one shot. The possible number of lives saved by COVID-19 vaccines is unclear, however, based on the global statistics, more than 700,000 lives were saved [18]. Although these vaccines have effectively controlled the COVID-19 pandemic, but the crisis has not been resolved, and we are simply moving into a new phase of the epidemic [5].
Since the designinig and production of vaccines against COVID-19, many people were vaccinated and still many people have not vaccinated. In China, due to the resurgence of the SARS-CoV-2, people are still being quarantined. Although we have obtained a relative understanding of SARS-CoV-2, the changes of this virus into newer and more pathogenic and virulent variants can challenge the human immune system. Regarding to necessity of vaccination, people's immunity is a node in the path of infection transmission and they are able to control the infection transmission to a great extent [19]. Despite of vaccination, the COVID-19 pandemic continues to pose a threat to the public health of communities so the number of infected people is increasing. Two factors includingSARS-CoV-2 genome mutation and immunosuppression play a role in the long-term dynamics of COVID-19. In a study performed by Elisha et al., two world regions including South Africa and Canada were modeled based on the level of vaccination and health measurements. In this study, the relationship between endemicity and the level of immunity of people and the effectiveness of vaccines, transferability and the gradual reduction in incidence of COVID-19 were investigated. According to data analysis, gradual reduction in the restrictions, encouraging vaccination, careful planning and checking the evolution of SARS-CoV-2 can largely prevent the risks caused by pandemics. Regardless of these cases, BA.2.12.1, BA.4, and BA.5 at the mutant variants of Omicron, are currently worrisome variants circulating worldwide. Using the booster vaccination schedule (third and fourth dose inoculation), COVID-19 prevention was more controllable, although the immunity status lasts for a few months. The vaccination (before or after getting infected with COVID-19), which is called hybrid immunity, has also been applied to induce immunity against COVID-19. In a recent studyon Quebec healthcare workers during the Omicron BA.1 surge, showed that the use of a booster vaccine containing three-doses (BA.2.12.1, BA.4, and BA.5) of mRNA-based vaccine in people without previous infection increased immunity by 46%. Also, people infected with BA.1 variant reduced the risk of re-infection with BA.2 cariant by 72%, and its immunity level was 38% higher than the pre-Omicron SARS-CoV-2 strain. The combination of three doses (BA.2.12.1, BA.4, and BA.5) of vaccine and previous immunity with BA.1 variant was led to increase of immunity up to 95% for 5 months [20].
3. COVID-19 mRNA vaccines
3.1. mRNA vaccines
The first promissing in vitro expression of mRNA molecules in mouse skeletal muscle cells for sentesization purpose was conducted in 1990. Since then, studies have rapidly grown on mRNA production and related technologies. The initial experiments encountered with several limitations such as mRNA instability, low immunization potential, and in vivo delivery. To date, mRNA vaccines have been extensively studied in a variety of disorders, specially protection against viral infections caused by influenza virus H7N9, Zika virus, Ebola virus, dengue virus, respiratory syncytial virus, cytomegalovirus, rabies virus, and flaviviruses [21]. The development of SARS-CoV-2 mRNA vaccines began at an unprecedented rate, just 42 days after revealing its genetic information to the Moderna company. Despite the short time interval of the vaccine production efforts and its examination, an almost 90% efficiency was achieved at phase III clinical trial (Fig. S1) [22]. Pfizer-BioNTech COVID-19 vaccine was the first mRNA vaccine which was authorized by the food and drug administration (FDA) for emergency use and made available under the emergency use authorization (EUA) as a two-dose vaccine for primary protection. It is believed that such companies have already met the global demand by producing novel vaccines against COVID-19, even though, many campaigns developed against the administration of the COVID-19 mRNA vaccines. Both Pfizer-BioNTech (BNT162b2) and Moderna (mRNA-1273) vaccines have received widespread approval for human use, and the administration of these regimens started since December 2020 [23]. Although there is very limited information about other mRNA-based vaccine candidates, studies on this platform is ongoing. The approved COVID-19 mRNA vaccines and the relevant clinical trials have shown in Table 1 .
Table 1.
List of COVID-19 mRNA vaccines.
| COVID-19 mRNA vaccine | Target | mRNA dose (µg) | Ref. |
|---|---|---|---|
| mRNA-1273 | S-2P | 100 | [23] |
| CoV3 | S | 1 | [23] |
| Ptx-Covid19-B | N.A | 16–100 | [23] |
| HDT-301 | S | 1–25 | [23] |
| BNT1626b2 | S-2P | 30 | [24] |
| BNT162b1 | RBD | 1–100 | |
| BNT162a1 | RBD | – | |
| BNT162c2 | S-2P | – | |
| CVnCoV | S-2P | 12 | [25] |
| ARCoV | RBD | 15 | [26] |
| ARCT-021 | S | 5&7.5 | [27] |
| LNP-nCoVsaRNA-02 | S-2P | 0.1–10 | [23] |
| ChulaCov19 | S | 1–25 | [27] |
| DS5670a | N.A | 10–100 | [23] |
| MRT5500 | S-2P | 15–135 | [23] |
| EXG-5003 | RBD | – | [23] |
3.2. Advantage of mRNA vaccines
Protein-based and gene-based platforms were two main vaccine production approaches for targeting COVID-19 immunogens. In protein-based approach, direct administration of natural or recombinant immunogens results activation of adaptive immune responses, while, the gene-based approach is based on delivery of DNA or RNA molecules to host cells [28]. The mRNA vaccines have several advantages over other available platforms, including easy and high speed production, and rather feasible delivery to the cells [29]. The purpose of mRNA vaccination is production of the target immunogen through in vivo transcription. By identifying the coding sequence of the target immunogen, in vitro synthesis and formulation of the mRNA vaccine merely need minor changes regarding the ongoing platform.
Compared to traditional protein vaccines that mainly activate antibody production, the mRNA vaccines stimulate both humoral and cellular immune responses [30]. There is no other risk for application of mRNA vaccines compared to recombinant techniques [31]. In comparison with DNA vaccines, mRNA vaccines are just delivered to cytoplasmic region of the cell, eliminating the risk of genomic integration [31], [32]. Some studies show that DNA vaccines can be integrated into the host genome, leading to mutations that have been revolved in mRNA vaccines [31]. The mRNA vaccines can directly lead to high velocity immunogen production without necessity of crossing the nuclear membrane barrier, so, their expression is unrestricted in the packing step [32].The mRNA vaccines, with possible secondary and tertiary structures and negatively charged hydrophilic molecules, may show undesirable thermodynamic diffusion in the host cell membrane compared to DNA vaccines, posing a barrier to the chemical delivery approaches. Bacteria are often used to produce recombinant-protein-based vaccines, while the mRNA molecules should be translated by the host cells; therefore, post-translational changes occur in the in-situ produced proteins similar to what happens following virus infection [33].
3.3. Structure of mRNA vaccines
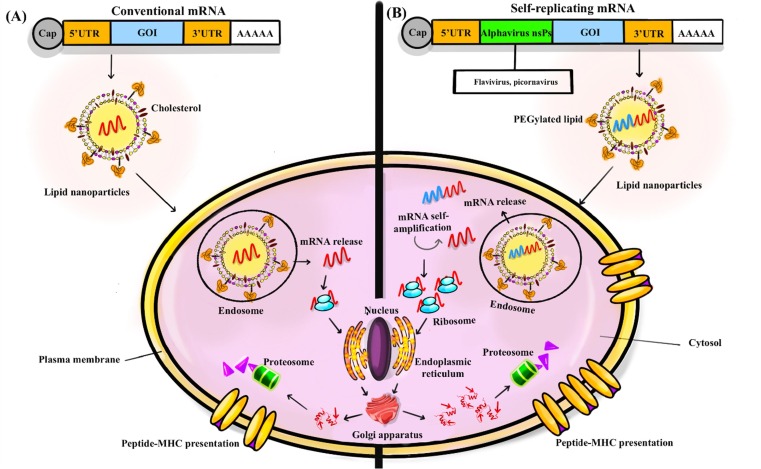
A typical mRNA vaccine consists of only sequences encoding the target antigen and the regulatory regions. Conventional mRNA vaccines are relatively small, limiting the stability and activity of the conventional mRNA in vivo. Due to the limited expression time of external mRNA molecules by cellular protein machinery, the expression and presentation of the target antigen can be optimized through vaccine formulation and modification of the RNA structural elements. For the stability of the mRNA molecule, access to ribosomes, and interaction with translation machines, using the 5′ cap UTRs, and 3′ poly-A tail are the commonly applied strategies (Fig. 1 ) [28]. UTRs affect the stability and translation rate of the mRNA encoding region. Poly-A tail also increase the mRNA stability, and its shortening and deletion destroys the mRNA structure. The 5′ cap plays role in the initiation of translation and the rate of protein production.
Fig. 1.
Target coding sequence for gene of interest (GOI), UTR5, and UTR3. (A) These sequences contain a terminal cap structure and a poly (A) tail in the conventional mRNA structure sequence. Once the mRNA enters the cell and is released from the endosome, the translation process begins. In the structure of self-amplifying mRNA sequences, the genomes of positive single-stranded RNA viruses, such as alphaviruses, are often used. The viral nonstructural proteins (nsPs) are required to amplify intracellular RNA; so, for high level expression of the target antigen nsPs are inserted into these vaccines. (B) High encoded antigens levels can be produced by generating RNA intermediates and many copies of antigen-encoded subgenomic mRNA by self-amplifying mRNA. The self-amplifying mRNA can control its selfamplification to produce RNA intermediates and many copies of antigen-encoding subgenomic mRNA, resulting in high amounts of the encoded antigen. The use of liposomal nanoparticles is the most common delivery method for cellular uptake in conventional mRNA vaccines and self-amplifying mRNA vaccines. Usually, the mRNA carrier enters the cytosol through endocytosis, which is followed by translation and protein processing to provide target peptides for major histocompatibility complex (MHC) clefts.
Furthermore, mRNA with higher guanine-cytosine content shows higher stability, and during the in silico vaccine designing steps, in combination with other strategies such as codon optimization, can lead to increased expression rate of the vaccine [34]. By selecting the “highly used coding sequences” secondary structures with longer functional half-lives and increased translation rate can be created. For example, using N1-Methylpseudouridine (m1Ψ) instead of uridine in the mRNA sequence, results increased protein expression. Recently, these modified uridines were considered, and application of m1Ψ has effectively increased the stability and melting point of Pfizer-BioNTech and Moderna mRNA vaccines [34].
Self-amplifying mRNA vaccines were designed based on replication characteristics of the single-strand and positive-sense RNA viruses such as flaviviruses, picornaviruses, and alphaviruses. The goal of engineering and producing these mRNAs was improving the expression rate and the immunogenicity of the encoded antigens. The genomes of alphaviruses, such as Sindbis virus, Venezuelan equine encephalitis viruses and Semliki forest virus were desirable models for production of self-amplifying mRNA vaccines [28]. Efficient delivery of mRNA vaccines in to the host cell is an important parameter in the efficacy of these vaccines. So, the mRNA vaccines were designed in different platforms such as RNA-conjugates, vectors-based mRNA vacccines, microparticle and nanoparticle embedded types [35].
4. Antigens of COVID-19 mRNA vaccines
Coronaviruses are enveloped, nonsegmented, single-stranded RNA viruses and with 26–32 kbp length, possess the longest genomic length among the RNA viruses. They have a spherical shape with diameter ranging 60–140 nm. Similar to SARS-CoV-1 and MERS-CoV, their outer surface is covered with distinct spikes of 9 to 12 nm length, giving an appearance of a rooster-like structure to the virus [36]. Coronaviruses encode structural antigenic proteins, including envelope (E), spike (S), nucleocapsid (N), and membrane (M) proteins, as well as nonstructural proteins. Virus surface antigens including S, E, and M proteins are accessible to antibodies. The viral RNA is surrounded by N protein. The genomic RNA consists of the noncoding 5′-UTR region, replicase encoding genes, accessory and structural proteins (N, S, E, and M), and the the noncoding 3′-UTR. About 15 non-structural proteins (nsp1-10 and nsp12-16) and two peptides including pp1a and pp1ab as well as replicase are encoded by the largest orf (orf1a/b) at the 5′ end of the RNA. Two cysteine proteases including a papain-like protease (PL2pro or nsp3) and a 3C-like protease (3CLpro or nsp5) are encoded by the viral genome and cleave the viral polypeptides. About 8 sub-genomic RNA-derived proteins (3a, 3b, 6, 7a, 7b, 8b, 9b, and orf14) are also encoded by the genomic structure [37] ( Fig. S2 ).
SARS-CoV-2 binds to host cells through the S glycoprotein with 1273 amino acids [38], consisting of signal peptide, extracellular, transmembrane, and intracellular domains (amino acids 1–14, 14–1211, 1214–1234, and 1234–1273) [39]. S protein has two functional subunits (S1 and S2). The C-terminus of the S1 subunit is a receptor-binding domain (RBD), which binds to human angiotensin-converting enzyme 2 (hACE2) and the S2 subunit mediates membrane fusion [39]. Four domains of the S1 fragments, including the N-terminal domain (NTD), RBD, C-terminal domain 1 (CTD1), and CTD2, wrap around the S2 fragment from the underneath. The S2 subunit is a symmetric trimer, which its first heptad bends back toward the viral membrane. The genome of SARS-CoV-2 has three unique features, setting it apart from other family members: (1) multiple mutations in the RBD, (2) a polybasic (furin-like) cleavage site (RRAR/S) at the boundary of S1/S2 subunits rather than the single arginine observed in SARS-CoV, (3) addition of three predicted O-linked glycans flanking the protease site [40], [41]. RBD has two structural components, including the hACE2 detection portion, which is located on the outer subdomain and characterized by a flexible ring fixed with a disulfide bond, and the conserved core subdomain with five antiparallel beta-plated strands [42]. Both the S-2P and RBD proteins are highly effective in inducing highly potent neutralizing antibodies and cellular immune responses [43]. Therefore, they are widely selected as antigens in the development of COVID-19 mRNA vaccines [27]. Pfizer-BioNTech mRNA vaccines (BNT162b1/BNT162b2) were designed accordingly with lipid nanoparticle formulations. BNT162b1 encodes a secreted RBD antigen that is trimerized to increase its immunogenicity through the multivalent display by the addition of a T4 fibritin (foldon) domain and BNT162b2 encodes S-2P full-length proteins [27].
5. Packaging COVID-19 mRNA vaccines in lipid nanoparticle
Extracellular mRNA delivery and dendritic cell targeting can be achived by encapsulating the mRNA in drug delivery systems such as polymers, lipids, cationic molecules, and nanoparticles [31]. RNA embeded lipid nanoparticles (LNPs) were mainly used for transfer of RNA molecules for gene therapy and immunological stimulations. The LNPsand associated formulation has been used as an effective delivery method for mRNA vaccines in the human body. These LNPs increase the efficacy of immunization modality to a large extent[10].
dendritic cells and other professional antigen-presenting cells are the main targets of mRNA vaccines. They induce specific immune response through processing and expression of the translated mRNA molecules [44].
mRNA-1273: LNPs help effective delivery of mRNA molecules and protect them against RNases [45]. They encapsulate the mRNA molecules within a stable lipid bilayer and help their entrance through endocytosis. The mRNA-1273 at least has two specific cationic LNPs, including WO2017070626 and WO2018115527. Also, SM-102, polyethylene glycol-2000-demiristoyl glycerol (PEG2000-DMG), cholesterol, and 1-distearoyl-sn-glycero-3-phosphocoline (DSPC) were used in its nanocarrier hydrophobic part [33].
BNT162b mRNA: An attempt has been made to improve the delivery efficiency of this vaccine (clinical trial # NCT04368728) [46]. Its nanocarrier is prepared from ionizable amino lipids, phospholipids, cholesterol and a PEGylated lipid with a ratio of 50/10/38.5/1.5 [46]. Both mRNA-1273 and BNT162b vaccines are administered intramuscularly.
5.1. Immunogenesity of LNPs
1,2-Dioleoyl-sn-Glycero-3 Phosphatidylethanolamine (DOPE) or 2-Distearoyl-sn-glycero-3-phosphocholine (DSPC), helper lipids such as ionizable amine-containing lipidoid, cholesterol, and polyethylene glycol (PEG) are lipid components of phospholipids. The presence of these lipids in mRNA delivery formulations avoids the mononuclear phagocytic system (MPS), low immunogenicity, endosomal trapping, facilitates cellular uptake, and protects against nucleases [47]. The effect of biophysical properties of LNPs in mRNA vaccines on their performance has not been investigated in detail yet [48]. Polydispersity index, charge and molar ratio of components, and surface chemistry are among the physicochemicalproperties of LNPs, whose impact on the immune system and biodistribution cannot be ignored. In the Kimberly's studies, the immunogenic effect of different lipid compounds used in mRNA vaccine was investigated in vivo. According to the obtained results, the immunogenicity level of the particles was directly associated to their size. LNPs with smaller size caused less immunogenicity in mice, whereas the larger size LNPs resulted to the greater immune response in non-human models. This is while all the particles caused an immune response after injection [48].
Stimulation of the immune system is also influenced by the surface charge of LNPs. Antibodies are more effective in neutralization under the influence of charged vesicles (positive–negative) than neutral lipids [49]. The formulation of mRNA vaccines with cationic lipids increases the response of the innate immune system, which elevates the effectiveness of the mRNA vaccines [50].
For example, in a study performed by Lonez C et al., production of IL-6, IL-1β, IFN-γ, and TNF-α were induced by human or murine macrophage cell lines during the cascade activated by the cationic lipid RPR206252. NOD 3 (NLRP3) and Toll-like receptors 2 (TLR2) are the starting point in the production of inflammation during this inflammatory cascade [51]. In another example, the potency of self-amplifying mRNA (SAM) vaccines has been increased using LNP mannosylation. In the studies of Goswami et al, mannosylated LNPs were led to increased absorption of dendritic cells and increased immune system response [52]. Taken together, the immunogenicity and reactogenicity of COVID-19 mRNA vaccines are thought to be MDA5–IFNAR1 signaling-dependent by the vaccine-derived mRNA (60), adjuvant activity by vaccine-containing LNPs (30,44,62), and/or vaccine encoded S protein (66). Although, there are still some issues to be resolved, such as differences between human, and experimental animals and differences in LNP components, future detailed analyzes will facilitate the development of vaccines that balance efficacy and adverse events (AEs) [53]. The presence of PEG in the COVID-19 mRNA vaccine formulation produced by Moderna (mRNA-1273) was led to the production of anti-PEG antibodies and the activation of the associated immune response cascade. Therefore, their inoculation should be done with caution for people who are sensitive to allergic reactions [54].
6. Pharmacology and mechanism of COVID–19 mRNA vaccines
The idea behind the mRNA vaccination is straightforward. Once the target antigen is selected, the mRNA sequence can be optimally synthesized. Successful in vitro transcription of the mRNA vaccines could be followed by their in vivo administration. In the in vivo system, the mRNA vaccine mimics a viral infection by employing the host cell protein machinery to convert mRNA into a defiend antigen and to elicit a robust humoral and cellular immune response [55]. The mRNA activity is initiated following its enterance to the cytosol and translation to the target protein [56]. The engineered mRNA uses intracellular translation machine to synthesize antigenic protein, which eventually undergoes post-translational modifications, degradation by the proteasome system and presentation to immune system.
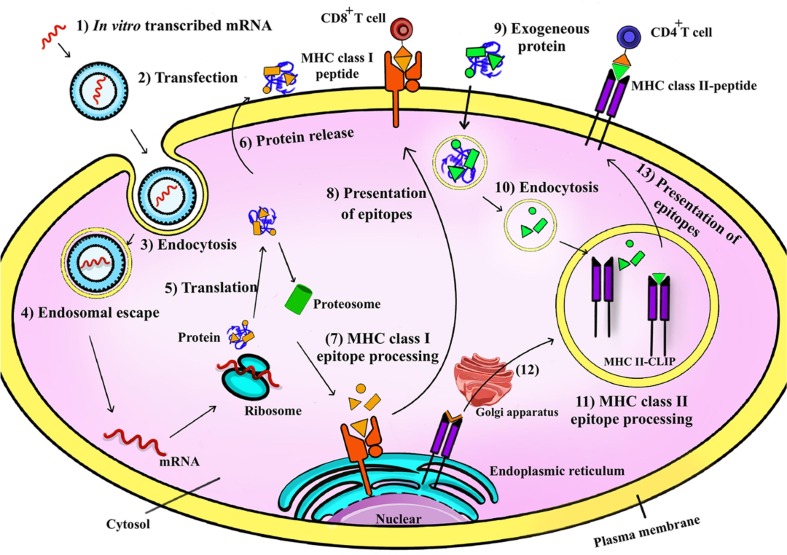
Two critical factors, including mRNA half-life and post-translational modifications of the final product play role in the pharmacokinetics of mRNA vaccines. Rapid RNase-based degradation and inactivation by plasma membranes due to high molecular weight and electrostatic repulsion between the negatively charged cell membranes and mRNA molecules are two major factors affecting the efficiency of foreign mRNA in the cytosol [57]. RNases rapidly target extracellular mRNA vaccines and reduce their effectiveness. Several mRNA delivery agents were designed to increase cellular uptake of the mRNA vaccines and simultaneously help their endosomal escape to the cytoplasm. Proteasome process the translated antigens and the antigenic determinants (epitopes) are actively transported to the endoplasmic reticulum to be expressed on major histocompatibility complex (MHC) class I molecules and to triger CD8+ cytotoxic T cells. Production and class switching of specific neutralizing antibodies are triggered by CD4+ helper T cells through MHC class II-bond peptides. In the endoplasmic reticulum, the invariant chain (Ii) protect the recently produced MHC-II molecules from binding to endogenous peptides. MHC II-Ii complex is exported via the Golgi to the late endosome compartments containing MHC II-containing vesicles, where Ii is replaced with antigenic peptides. Finally, the MHC II-peptide complex is exposed to CD4+ T cells [35] (Fig. 2 ).
Fig. 2.
Application of positively charged lipid nanoparticles in mRNA vaccines. Nanoparticles can enclose mRNA and protect against enzymatic degradation during endocytosis and passing through negatively charged cell membranes. Subsequently, mRNA escapes from the nanoparticles and is released in the cytosol. In this way, foreign proteins do not need to escape from the endosome to cytoplasm to be presented on MHC II. Endogenous proteins of pathogenic origin or intended purpose are initially displayed in the MHC I pathway. In the proteasome, proteins are broken down into smaller peptides, which are loaded onto MHC I molecules in the endoplasmic reticulum. The peptide-MHC I complex is subsecuently presented to CD8+ T cells at the cell surface.
Advances in the vaccine design increased our understanding on the complexity of innate immunity and Toll-like receptors (TLRs), which made it possible to recognizing patterns of mammalian immune system to a wide range of pathogens [58].
The structure of TLRs as the type I transmembrane proteins) consists of a TIR domain, a transmembrane region, and a leucine-rich repeat (LRR) module. TLRs recognize pathogen-associated molecular patterns with their LRR motif and generate intracellular signals with the TIR domain [59]. TLRs are divided into two general categories based on their location. TLR1, TLR2, TLR4, TLR5, TLR6, and TLR10 are located on the surface of the cell membrane, while TLR3, TLR7, TLR8, and TLR9 are located in intracellular vesicles. These TLRs are distributed in lysosomes, endoplasmic reticulum, and endosomes [59]. Since the mRNA molecules are considered as the strong activators of TLR7/8. The mRNA vaccines represent a series of endogenous adjuvants. Through designing mRNA vaccines, it is easy to change the activity of the immune system. For example, TLR7107 are activated by RNActive® vaccines, pseudouridine, or 1-methylpseudouridine (chemically modified nucleosides), which is led to production of reduced IFN type I. Regarding to design of vaccines against COVID-19, it is crucial to design more effective and safer vaccines in view of the successive changes of the virus. The application of adjuvants is useful for making the immune system more sensitive to antigens and increasing the duration and rate of the immune response. TLR agonists can be effective as adjuvants in stimulating the immune response against COVID-19 vaccines. The advantage of this type of adjuvants over the first generation adjuvants is to direct the dentritic cells to mature and subsequently produce stronger T cell responses. Using Pam3CSK4, poly (I:C), monophosphoryl lipid A (MPLA), rosiquimod (R848), and CpG oligonucleotide (ODN), as the candidates for TLR adjuvants, are being used in vaccine studies about COVID-19 prevention [59].
7. Comparison of COVID-19 mRNA vaccines against other vaccine platforms
7.1. Efficiency of COVID-19 mRNA vaccimes
Rapid development, high potency, low production costs, and safe administration have been led to interest in the new generation of mRNA vaccines. The advantages of using the mRNA vaccines compared to other platforms are: (1) mRNA is a non-infectious ribonucleic sequence that does not have any infectious or mutagenic part due to its non-integrated nature. The half-life of mRNA is controlled by normal cellular processes and is easily degraded. If there is a need to improve the structure stability, changes can be made in its sequence. (2) mRNA becomes more effective with the formulation in carrier molecules and the possibility of rapid adsorption and expression in the cell cytoplasm. Since mRNA is considered as a minimal genetic vector, it can be administered repeatedly. (3) due to the high efficiency of mRNA transcription in in vitro conditions, the mRNA vaccines have the better potentials within the large scale production and overally their costs are lower than other vaccine platforms [60], [61], [62].
The effectiveness of two trending vaccines including mRNA-1273 and BNT162b2 in reducing hospitalizations and death caused by the SARS-CoV-2 have been proven. It note that the effectiveness of these vaccines is somewhat different because of various factors. Both vaccines encode the SARS-CoV-2 spike protein, and the mRNA in these vaccines is modified nucleoside. The difference in the formulation of vaccines and the prescribed dose can be the root of this difference. The amount of injection per dose of BNT162b2 vaccine is 30 μg/0.3 ml (100 μg/mL), which is administered at 21-day intervals. And the immunogenic dose of Moderna is inoculated with amounts of 100 μg/0.5 ml (200 μg/mL) at 28-day intervals. Thus, even with the structure and formulation of these two vaccines being similar, each dose of BNT162b2 vaccine produces 1/3 of spike protein mRNA copy, which can ultimately be led to less effective priming for immune response. Comparing the level of antibodies produced by these two vaccines, significant results can be obtained on the effectiveness of these two vaccines. The two side effects of arthralgia and myalgia among the clinical trials of two vaccines were higher in the cases of vaccination with mRNA-1273 than BNT162b2, which had a direct relationship with the immunogenicity of this vaccine. The different lipid compositions through the formulation can be led to some differences in the effectiveness of them. ALC-0315, ALC-0159, distearolyphosphatidycholine (DSPC), and cholesterol are the lipid compounds formulated in BNT162b2, and SM-102, PEG-Colster, and DSPG-DMG constitute the lipid portion of the mRNA-1273 vaccine [63].
By optimizing the mRNA sequence (changes in codons, matching the frequency of host transfer RNA, (tRNA) or as a determinant for the introduction of secondary structures), the rate of protein synthesis and half-life of mRNA function can be altered. In improving mRNA translation, 1mΨ nucleotide changes can be used, which provide additional base pair stability and cause useful changes in the secondary structure. These changes make mRNA stable against the attacks of endonucleases and chemical degradation processes (hydrolysis) [64], [65], [66].
BNT162b2 and mRNA-1273 implement a combination of modified 1mΨ nucleotide substitution and removal of dsRNA fragments in the mRNA production process, which strongly attenuates innate immune signaling in response to mRNA through reduced activation of TLR signaling and cytosolic RNA sensors. Moderna claimed such this approach, both local and systemic innate immune effects of mRNA (vaccine) administration could be minimized in mice [36]. Unlike BNT162b2 and mRNA-1273, the CVnCoV vaccine candidate contains an “unmodified” mRNA that uses sequence engineering (eg reduced uridine content), selected UTRs, and a rigorous purification protocol to remove dsRNA fragments [67], [68].
7.2. Immunogenicity of COVID-19 mRNA vaccines
Out of about 270 COVID-19 vaccines in the market with different platforms, just for five vaccines an emergency license have been issued including (Moderna) and BNT162b2 mRNA vaccines (Pfizer/BioNTech) with mRNA platform and Gam-COVID-Vac vaccines (under the supervision of Gamale Research Institute), ChAdOx1 nCoV-19 (under the supervision of University of Oxford/AstraZeneca), and Johnson & Johnson or Ad26.COV2.S (Janssen) with an adenovirus platform. NVX-CoV2372; Novavax by protein subunit or BBV152; Bharat Biotech (based on an inactivated viral vaccine) has also reportedly been licensed in some countries for emergency use. Ad5-nCoV (CanSino Biologics) with an adenoviral vector and CoronaVac (Sinovac Biotech), BBIBP-CorV (Sinopharm), and WIBP-CorVS (WIBP-CorVS) are designed based on an inactivated virus platform and have been approved for emergency use in some countries. Peptide vaccine EpiVacCorona (VECTOR Center of Virology, Russia), inactivated vaccine CoviVac (Chumakov Center, Russia) and recombinant vaccine ZF2001 (Anhui Zhifei Longcom/Ch. Science) have been licensed in some countries without providing publicly available efficacy evidence are approved for emergency use in some countries.[22], [69], [70] Both mRNA-1273 and BNT162b2 vaccines, after a single dose, induce the production of anti-receptor binding domain (anti-RBD), antibodies that bind to the S protein receptor domain. This issue has been confirmed in clinical trials conducted on the serum of people inoculated with mRNA vaccine against positive COVID-19 patients who are recovering. In contrast, the level of CD8 + T responses at a low level appears after one or two doses of inoculation. These data would suggest the protection after one dose inoculation of these vaccines either requires extremely low levels of neutralizing antibodies leading to other effector mechanisms and/or is mediated by a relatively low frequency of antigen-specific T cells. Based on the collected information, adenoviral vector vaccines have similarities with mRNA vaccines in the way of activating the immune system, while they also have differences. In clinical trials, these vaccines have had variable efficacy. After one or two doses of ChAdOx-1 nCoV-19 administration, and after one administration of Ad26.COV2.S vaccine, their efficiency percentage was about 70%. With the administration of the second dose of the Gam-COVID-Vac vaccine, its effectiveness was reported to be 90%. The efficiency difference in this type of vaccine can be largely associated to the formulation of the vaccine with adenovirus 26 in the first dose and adenovirus 5 in the second dose [70], [71], [72]. Examining the rate of mortality, progressive infection, and hospitalization after administration of these vaccines, Ad26.COV2.S inoculated cases were farther than mRNA1273 [73]. Both ChAdOx1 nCoV-19 and BNT162b2 vaccines with two different platforms have been effective against hospitalization and severe disease above 80% [70], [74]. Both platforms (adenoviral- and mRNA-based) increased the level of neutralizing antibodies, which were higher than in patients who were recovered from the infection. Of course and in comparison, the level of induction of neutralizing antibodies was higher in mRNA vaccines. The ChAdOx1 nCov-19 vaccine with a single dose was led to the production of multifunctional antibodies, which introduced several effective mechanisms dependent on these antibodies, all of which were carried out with the aim of immunogenicity. These vaccines also facilitated the phagocytic activity of neutrophils and monocytes, which both were increased with the administration of the second dose [70], [75]. After the administeration the first dose, ChAdOx1 nCov-19 vaccine, antibodies were produced with antibody-dependent complement deposition mechanism. which was intensified by the second dose of inoculation. In addition, the studies on the production of TNF-α and IFN-γ from CD4 + T cells have shown that the immunogenic mechanism of the vaccines were associated with the induction of a strong T cell response [70]. Comparing these three popular vaccines in the United States and after collection of clinical trial data, it will be possible to consider a fair and rational vaccination policy in this country and other regions of the world [73].
8. Future considerations on COVID-19 vaccines
Public confidence is one of the most critical factors in the successful and influential development and application of vaccines [1]. With the worldwide emergence of viral epidemics, conspiracy theories about COVID-19 and vaccination have been formed. One approach accused that COVID-19 and broad vaccination against it, was aimed for controlling the world's population and spying on microchips. Such theories about vaccination are not new, as similar theories were developed in the 1990 s about the association of MMR vaccine with autism and inflammatory bowel disease [76], [77], [78], [79], [80], [81], [82]. Conspiracy theories about the COVID-19 mRNA vaccines fall into four main areas. Firstly, there was doubts about the vaccine operating system and its safety. Indeed, the successful clinical trials vanished the main doubts about its safety and possible side effects. Secondly, there was doubt about the enough effectiveness of the mRNA vaccines during the pandemic. Thirdly, there was doubt whether COVID-19 is as dangerous as the medical authorities and experts explained! For example, given their history of the immoral treatment of minorities and the people color and race. In other words, can we accept that the system is trying to improve our standards of living? The vaccine and vaccine production combination was the fourth ethical skepticism that engaged public opinion [83].
The development and completion of COVID-19 vaccines are rapidly expanding, so, it is expected that the benefits of COVID-19 vaccines should outweigh the potential risks or their side effects [84]. Adenovirus-based vaccines may pose a risk of viral DNA aggregation at an unknown frequency with unpredictable epigenetic consequences, which may be observed several years after vaccination. Also, viral genes present in adenovirus DNA may be activated by intercellular agents, leading to different immune memory reactions experienced as transient symptoms after vaccination. Fortunately, the fatal consequences of severe immune responses have been very rare [85]. In dealing with such vaccines, people are concerned that “will the vaccine enter my genes”? There is no evidence that human adenoviruses are associated with tumorigenesis, while this possibility cannot be ruled out easily [86]. Concerns about the fate of foreign genetic material and the possibility of viral mRNA/DNA integration is a potential threat to human that needs further investigation. For the first time, Zhang et al. paid a scientific overview to recent clinical studies of SARS-CoV-2 mRNA vaccines to give a reasonable response to the worries of the society [87].
9. COVID-19 mRNA vaccine candidates and recommended dosage for different ages
According to the FDA's approval in the United States, to prevent further spread of COVID-19, vaccination of all ages above 6 months (infant) is recommended. According to the CDC recommendation, it is better for people to complete the primary series of vaccinations and complete them based on the recommendations for booster doses [88], [89].
The administration of monovalent or multivalent vaccines in booster doses is a very important issue that should be considered. MThe monovalent vaccines are prepared from one strain or one component of the virus, while bivalent vaccine contains the components of two strains or components of the virus..The primary vaccines produced to deal with the disease, while the main booster are considered to be monovalent, and the main strain of the virus has been used in the production of vaccines. The two-pots were the new productions of Pfizer and Moderna COVID-19 vaccines, which were used as booster doses, and the newer variant of Omicron including BA.4 and BA.5 were used together in the production of vaccine. These vaccines have induced protection and a longer period of immunity from disease/death against the Omicron variant than the primary produced vaccines [90]. The administration of bivalent mRNA vaccines in a booster dose is recommended to all people who have completed their primary series of vaccinations. However, 6 months to 4 years aged children were not allowed to use any monovalent or bivalent vaccines even after receiving the initial dose of Pfizer-BioNTech [88]. The vaccination of people over 18 years old with monovalent vaccines approved by the FDA in the cases where they have not received a booster dose, and are limited by vaccination with bivalent mRNA vaccines (unavailability, contraindications), they can use Novovax [88], [89].
9.1. Moderna COVID-19 mRNA vaccine
Booster dose of Moderna (bivalent) was recommended for 6 months to 4 year old children. The intervals between the first two doses are 4–8 weeks and for the booster dose two months after finishing the first series. The booster dose of Moderna COVID-19 mRNA vaccine is allowed for these ages when primary inoculation (two doses) has been done. In 5–11 year old children, the initial dose along with a booster dose (Moderna or Pfizer-BioNTech bivalent) was recommended [89], [91]. In 12 year and older vaccinated cases, two initial doses and one multivalent booster dose of Moderna or Pfizer-BioNTech were recommended. The time intervals of the first series of doses are 4–8 weeks and the booster dose is at least two months after the initial doses (either in people who did not receive a booster dose or those who received a monovalent booster dose) [88], [89].
9.2. Pfizer-BioNTech COVID-19 mRNA vaccine
In 4–6 year old children, three initial doses with Pfizer-BioNTech vaccine were recommended. The interval between the first two doses is 3–8 weeks. The administration of monovalent or bivalent vaccines werenot allowed for these children. In 5–11 year old children, two initial doses and one booster dose of bivalent Pfizer-BioNTech were used with an interval of two months. Indeed, the children of this age have not received a booster dose or at least two months have passed since their monovalent booster dose. Currently, bivalent Pfizer-BioNTech is authorized as a booster dose for children in the 5-year old, who have received two initial doses of Pfizer-BioNTech..In 12 year old and older cases, two initial doses and one multivalent booster dose of Moderna or PfizerBioNTech have been recommended. The time intervals of the first series of doses are 4–8 weeks and the booster dose is at least two months after the initial doses (either in people who did not receive a booster dose or those who received a monovalent booster dose) [88], [89], [91]. The types and dosages for each COVID-19 mRNA vaccines for different ages during primary and booster vaccination have been described in Table 2 .
Table 2.
COVID-19 mRNA vaccines and dosage administration in different ages.
|
Moderna vaccine | ||||||
|---|---|---|---|---|---|---|
|
Primary dosages |
Bosters dosage |
|||||
| Ages | Type of vaccine | Doses | Injection volume | Type of vaccine | Doses | Inoculation volume |
| 6 month-5 year old | monovalent | 25 µg | 0.25 ml | bivalent | 10 µg | 0.2 µg |
| 6–11 year old | monovalent | 50 µg | 0.5 ml | bivalent | 25 µg | 0.25 µg |
| 12 year old | monovalent | 100 µg | 0.5 ml | bivalent | 50 µg | 0.5 µg |
| Pfizer-BioNTech vaccine | ||||||
|
6 months-4 year old (1st&2nddosage) |
monovalent | 3 µg | 0.2 | – | – | – |
|
6 month-4 year old (3rd dosage) |
bivalent | 3 µg | 0.2 | – | – | – |
| 5–11 year old | monovalent | 10 µg | 0.3 | bivalent | 10 | 0.2 |
| 12 year old | monovalent | 30 µg | bivalent | 30 | 0.3 | |
10. Transportation of COVID-19 mRNA vaccines and its effect on properties
Since the designing and production of COVID-19 mRNA vaccines in the prevention against the pandemic has been dramatically demonstrated, however, there are challenges surrounding the maintenance and delivery of these type of vaccines to the end users [92]. The presence of the hydroxyl group in position 2 of the ribose sugar of mRNA makes the vaccine more sensitive to biological nucleases in the ambient condition. The mRNA phosphodiester bond is broken by intramolecular transesterification reaction. The proposed solution for this problem is freezing, freeze-drying, or lyophilization of the vaccines, which stabilizes mRNA. Cryo-protectants such as terehalose added to lyophilized mRNA vaccine formulations increased their stability up to 10 months at 4 °C. However, this method cannot be applied to mRNA vaccines formulated in liposomes and LNP. Because these nanoparticles are sensitive to freezmelting-thaw processes and lose their natural state. The COVID-19 mRNA vaccines produced by Pfizer-BioNTech are stable in liquid form at −80 °C. The storage of these vaccines for communities without the freeze-chain facilities limited the import of COVID-19 mRNA vaccines to the country. On the other hand, developed countries did not have any restrictions on keeping these vaccines. The application of other cryoprotectants such as sucrose or trehalose (5% w/v) in the lipid formulations of mRNA vaccines keeps the effectiveness of the vaccines stable for up to 3 months, whileupcoming challenge in association with the formulation is the storage of lipid-based mRNA vaccines in liquid nitrogen, which is challenging for the market delivery system [93].
11. Can the genetic content of COVID-19 mRNA vaccines integrates into the human genome?
Vaccines containing viral ORF sequences, due to cytoplasmic expression of chimeric mRNAs, have potential risk of enterance to the host chromosome [94]. The benefits of recently developed mRNA-based vaccines cover the delivery and stability issues, while more detailed studies are needed on RNA degradation and immunization concerns [95].
Several weeks after recovery from SARS-CoV-2 infection, some patients have positive diagnostic tests, which indicate presence of viral RNA without replication. To study the SARS-CoV-2 integration into the genome of infected cells, Zhangl et al. used three sequencing approaches, including long-term nanopore sequencing, whole-genome sequencing at the end of the ilumina pair, and Tn5-tagged DNA fusion enrichment sequencing. All three methods proved that SARS-CoV-2 sequences could be integrated into the host cell genome through reverse transcription methods. This may explain why some people are positive for SARS-CoV-2 test, several months after recovery from the initial infection, without re-infection and in the absence of the detectable virus. Meanwhile, there is no direct evidence of genome integration in the infected individuals [96].
Possible integration of exogenous genetic materials with human genome can be explained through horizontal gene transfer (HGT). Humans have been infected with many viruses during evolution, and the immune system has combated with viruses. However, certain genomic sections of viruses have been integrated into the human DNA. About 45% of the human genome is composed of transposable elements, including long and short interspersed elements (LINE and SINE), derived from 5 to 8 viral genetic sequences [97]. However, chromosomal integration of viral sequences in human might result in gene disruption, oncogenesis, premature cell death, and evolution of host species through hereditary genomic alteration [98].
RNA virus infections in insects appear to be systematically associated with endogenous events by reverse transcriptase-mediated process through host-encoded retrotransposons [99]. In viral infections, HGT usually occurs through host cell gene transfer to the attacking virus, while the reverse direction (HGT from virus to host cell) rarely occurs. Retroviruses and some circular single-stranded DNA viruses including Anelloviridae, Circoviridae, Geminiviridae and Nanoviridae are exceptions [100]. Extensive sequence similarities have been shown in general genome databases for different organisms [101], [102]. For example, the coding genes for RNA-dependent RNA polymerase and capsid protein of partiviruses and totiviruses have extensive homolog genes in the nuclear genomes of eukaryotic organisms, including fungi, protozoa, arthropods, nematodes, and plants [103]. Genome sequencing and other comparative evidence of linkages between the virus and host genome sequences show viral genome homologs in the genome of eukaryotic organisms, which were horizontally transferred [103]. Although the integration of the viral genetic segments into the host genome is required for some blood-borne viruses (Table S2) [98]. Following reverse transcription by LINE-1 or other transcription factors, parts of SARS-CoV-2 RNA can be reverse transcipted to DNA molecules and integrated into the genome at an unknown location and frequency. Therefore, the possibility of the integration of SARS-CoV-2 RNA transcripts into the infected cells, similar to vaccination with COVID-19 mRNA-based vaccine is not negligible [85].
12. The impact of mRNA vaccines on the next generation of humans
Although the main goal of using mRNA vaccine is reaching high efficiency, it should be noted that the life code manipulation can have completely unpredictable, long-term, or even permanent and irreversible adverse effects [104]. A potential risk is the worrying status of mRNA vaccination in women who becomes pregnant soon after receiving mRNA vaccines. The mRNA embedded in the liposome can enter the sperm and convert to DNA by LINE-1. In this way circulating plasmids containing the coding gene for S protein may be produced, which eventually fertilizes the ovum. In this case, the baby cannot respond to S protein and the immune system recognizes it as a self molecule, predisposing the baby to SARS-CoV-2 infections over the time. Although this is just a hypothesis, such a scenario cannot be easily ruled out. Therefore, evidence of a link between retrotransposons, sperm, fertilization, the immune system, and viruses are not imposible [104].
There have been several rumors of damage by COVID-19 vaccines to the reproductive system, and in this regard, Pfizer-BioNTech vaccines were highly blamed [105]. It has been claimed that antibodies that detect the SARS-CoV-2 spike protein, may interact with the maternal syncytine 1 protein and damage placenta. According this hypothesis, all vaccine platforms and natural infections can damage placenta. However, women infected with SARS-CoV-2 shortly before or early in pregnancy were less likely to have an abortion than their non-infected peers. Although this observation refuted this hypothesis, immunologists provided more reasonable responses. They found no significant serological cross-reactivity between SARS-CoV-2 antigens and syncytin-1. Because, the convalescent COVID-19 patients sera did not reacted with syncytine 1 [105].
The administration of commercially available vaccines for young adults (especially students) and children is a topic subject in many countries that continues to be debated. Since end of August 2021, different countries have not reached the same decision about prescribing vaccines to children [106].
In a study performed by Walter et al., the subject of the study was the safe vaccination of COVID-19 in children under 12 year old. The clinical trial phase 1for BNT162b2 vaccine was investigated in 6 month to 11 year old children with interval of 21 days. The doses were studied in the clinical trial phase 1 were 10, 20 and 30 μg, each dose was administered to 16 children. A dose level of 10 μg was used to investigate immunological reactions. In the continuation of the research, 2268 studied children were selected for the clinical trial phase 2, of which 1517 children received vaccine and 751 children received placebo. These studies were conducted during 2–3 months. The obtained results indicated the immunogenicity of the vaccine prescribed in the vaccination regimen (two doses of 10 μg; 21 days apart) [107].
Contrary to concerns about vaccination during pregnancy and breastfeeding, adverse effects of the COVID-19 vaccines have not been observed so far. Investigating the effects of vaccines on pregnant and lactating women, usually the vaccines have not been used because of the threat to the health of the mother, baby and fetus [108]. Due to limitations, there are limited data on the effects of the vaccine on the health and immunity of the lactating and pregnant mothers. According to results, the abortion rate among vaccinated and unvaccinated pregnant women was not different, and it seems that the immunogenicity of vaccines in the population of the pregnant and lactating women is not much different from the general population [109].
Analyzing the results of studies from Janssen, Pfizer/BioNTech and Moderna on animal models revealed no adverse effects on reproduction, embryogenesis, and fetal growth. Even fetuses born after birth were not aborted. Previous studies of mRNA vaccines against Zika, influenza, and rabies viruses have also shown positive effects with high immunogenicity during pregnancy of women before the pandemic [110], [111].
In a study performed by McLaurin-Jiang et al. the aim was to investigate the effect of vaccination on the breastfeeding of the studied mothers. In this study, children's symptoms were also monitored. According to the obtained data, the symptoms including fatigue, headache, muscle pain, painful injection site, chills, fever, or allergic reactions were more common in 4455 nursing mothers in the second dose of vaccination, while the adverse effect on their breastfeeding and emerging symptoms in no children were observed generally [112].
13. COVID-19 booster doses
13.1. SARS-CoV-2 mRNA boster vaccines for primary vaccination or after COVID-19 infection
It seems that the administration of booster doses of COVID-19 vaccines is very important in controlling the disease in populations and reducing the economic and health standards of this disease. Since the examination of the consequences of administering vaccines in the initial dose has been a necessity of vaccination, the time and type of booster vaccine is an important factor that should be taken into consideration in subsequent inoculations [113].
In the initial studies, homologous vaccines were used to enhance immunogenicity (using the same vaccine as the initial dose) and their immunogenicity and side effects were investigated. These types of homologous boosters have led to an increase in antibody titers against delta variant of SARS-CoV-2. Mixing & matching involve a special vaccination program for the booster dose, which consists of vaccines with different platforms (such as Ad26.COV2.S and mRNA-1273) or different types of vaccines from the same platforms such as (such as Ad26. COV2.S and mRNA-1273) to be used as the heterologous variant containing booster dose in the vaccination program. The success rate in this strategy not only can provide similar immunogenicity with homologous vaccination, but also increase the scope and longevity of the immunogenicity of vaccines based on their specific platforms and increase the possibility of immunogenicity against SARS-CoV-2 new variants [113].
Many countries started their national vaccination program as soon as the COVID-19 vaccines were approved by the WHO under the emergency use listing. This approval includes COVID-19 vaccines produced by Pfizer-BioNTech, Moderna, AstraZeneca, Janssen, Sinovac, and Sinopharm. Pfizer-BioNTech, Moderna, and Janssen are vaccines that are used as the booster doses in the United States. According to the CDC approval, people can choose any of these types of vaccines as a booster dose, regardless of what type or platform they have used before. However, with mixing these vaccines for a series of two primary doses, therefore no additional doses should be used. The administration of COVID-19 mRNA vaccines platform was approved by the FDA on August 12, 2021 as a booster dose for immunocompromised patients. The booster dose prescription for Pfizer-BioNTech and Moderna has been extended to people over 65 year old whose jobs are associated with the high risk of COVID-19 contacting. In November 2021, these two types of vaccines were approved by the FDA for those with 18 year old and older who used this platform in the initial series of vaccinations [89], [114].
The administration of Pfizer-BioNTech and Moderna as a booster dose should be done at least 6 months after the initial vaccination and at least 2 months for other platforms [114]. The extensive vaccination in Israel was done when some countries were trying to get the first dose in other countries. As the result, more than 50% of the Israeli population had been vaccinated with two doses of the Pfizer-BioNTech COVID-19 vaccine by the end of March 2021. Despite of this vaccination, the fourth wave of the epidemic was encountered in Israel, which necessitated the administration of the third dose of the Pfizer-BioNTech vaccine in immunocompromised people by the Ministry of Health of this country. Through this program, people over 60 year old were vaccinated. ByAugust 30, 2021, all people over 12 year old received a booster dose [89], [114].
According to Joint Committee on Vaccination and Immunisation (JCVI) of the UK, the administration of booster doses (third dose) using Pfizer-BioNTech is preferred regardless of the type of platform used in the primary vaccination. Although both Pfizer-BioNTech and Moderna vaccines provide an high immunity rate through the booster dose, for those who cannot use COVID-19 mRNA vaccines, they can use the AstraZeneca vaccine as the third dose [114].
13.2. SARS-CoV-2 mRNA boster vaccines against different variants
In the spring of 2020, the shape of the SARS-CoV-2 changed with four mutations in the genome (point mutations in the spike protein). Next, emerging SARS-CoV-2 variants were replaced by previous ones that had a higher ability to transmit and escape from the immune system. Alpha variant in the UK (B.1.1.7), Beta variant in the South Africa (B.1.351), Gamma variant in Brazil (P.1), Delta variant in the India, and Omicron variant (B.1.1.529) were a common variant that all emerged during the pandemic. Now, the Omicron has remained the most common variant. Among all these variants, Omicron with more than 50 mutations in its structure (at least 30 mutations occurred in the spike protein) has located in the first place [41], [115]. These mutations increased the SARS-CoV-2 ability to escape from the attack of antibodies produced during vaccination or previous infection with a previous variant. The subvariants of Omicron are BA.1, BA.1.1, BA.2, BA.2.12.1, BA.4, and BA.5, which the titer of neutralizing antibody for the last subvariant (BA.5) has increased three times more than its previous subvariant (BA.4) [115].
The first mutant strain of acute coronavirus syndrome (B.1.1.529 [omicron]) appeared in south Africa. Then this variety spreaded rapidly around the world, to the point where, according to the WHO declaration, it became a concern about re-emergence. [116]. One of the challenges in developing vaccines is their effectiveness and safety against new strains. The efficacy of Moderna and Pfizer-BioNTech vaccines was evaluated in two studies in Mayo Clinic in 2021, when alpha or delta strains were prevalent. Both vaccines showed good efficacy against hospitalization. The efficacy of mRNA-1273 was higher than that of BNT162b2, but the effectiveness of both vaccines against infection with recent variants was reduced. The effectiveness of BNT162b2 was lower than that of mRNA-1273. The results of the similar studies on the reduction of the infection rate among identical people who were fully vaccinated showed that mRNA-1273 could reduce the risk of new infection slightly more than BNT162b2 [63]. After inoculation of two doses of most vaccines, the efficacy rate increases. So, efforts should be made to maximize the effectiveness of the vaccine with two doses among the vulnerable population [117]. In a study performed in the Malaysia on the effectiveness of AZD1222, BNT162b2, and CoronaVac vaccines against and the of Omicron variant, showed that the administration of the booster doses, inhibits the infection 95.4%, inhibits the symptomatic infection 97.4%, and the mortality rate was calculated 91.7%. The effectiveness of AZD1222, BNT162b2, and CoronaVac booster doses on the mortality rate was 95.2%, 91.8%, and 88.8%, respectively. According to the obtained results, booster doses had been more effective against the sysmtoms caused by infection than non-boosted vaccination [118].
13.3. Hypothesis of association between myocarditis and COVID-19 mRNA vaccines
In addition to the satisfactory results of mRNA vaccination, myocarditis as a rare phenomenon, can be considered a possible threat to COVID-19 mRNA vaccination. This immune inflammatory reaction was mostly observed in adult men and could be due to dsRNA impurities produced during mRNA preparation steps. While, there is no exact explanation, the source of the inflammation could be linked to possible side effects of vaccination [119]. Myocarditis could be a side effect of mRNA vaccine in people receiving BNT162b2 with an incidence of 2.13 per 100,000 with a rather higher incidence in males (16 out of 29) [120]. To provide a reliable answer to this question, it is necessary to evaluate the genetic potential, sex differences, clinical incidence and long-term impact of myocarditis after COVID-19 vaccination [120].
Association between immunosuppressants and COVID-19 mRNA vaccines One of the important notes that the vaccine producing companies against COVID-19 are trying to get approval, is to improve the effectiveness of their vaccines in the elderly. Contrary to the fact that the vaccination program is a necessity for the elderly, these people are usually excluded in the clinical trials. For the clinical trial phase 3 of the Pfizer-BioNTech and Moderna vaccines, information on their effectiveness in the elderly people has been published. However, elderly people with underlying diseases and disability have not been studied to a large extent. With increasing the age, the strength of the immune system decreases. Also, the production of T cells responding to the vaccine is reduced. As the result, the CD8 T cells count decreases and consequently, the normal ratio of cells (CD4:CD8) is disturbed. The variety of receptors of these cells also decreases that all of which are effective in reducing the survival of T cells. With increasing age, the antibody titer decreases due to the reduction of selected proteins, which can justify the less effectivness of the vaccines in the elderly [121]. In relation to patients with autoimmune diseases or taking immunosuppressive drugs, limited information on the effectiveness of the vaccines has been reported, which is due to the exclusion of these specific groups from the clinical trials. Also, most of the deaths in this population were due to the COVID-19 infection. However, according to the rheumatology data, it has not been shown about these people's infection with COVID-19 and infectious side effects, and it has been recorded just in the cases used to the high doses of corticosteroids [122]. Methotrexate and rituximab are two humoral immunosuppressive drugs that play a role in neutralizing the antibody response against neoantigens. Rituximab targets CD20 + B cells and methotrexate attacks B-cell activating factor (BAFF) and suppresses immune system B cells by increasing immunosuppressive adenosine. Previous studies on the role of these drugs on the level of immunogenicity and the experience on the seasonal influenza vaccine have demonstrated that after administeration of the COVID-19 vaccine and stopping the drug, the immunogenicity of the vaccine increased for a maximum of two weeks without side effects or the exacerbation of rheumatoid arthritis. The effect of the COVID-19 vaccines in relation to these patients is not yet known precisely due to the limitations and need further evaluations [122]. In the studies of Luca et al., the immunogenicity of BNT162b2 vaccine was investigated on 260 people with cystic fibrosis and 18 lung transplant recipients. Anti-SARS-CoV-2 IgG and IgA levels were checked after inoculation of two doses. According to the study, the level of IgG and IgA produced after vaccination was significantly comparable with the healthy population. The efficacy of the BNT162b2 vaccine was impaired in transplant recipients, especially in those taking mycophenolate mofetil. Also, it is very important to check the effectiveness of the COVID-19 vaccines and measure the level of immunogenicity in the sensitive segment of the society [123].
14. Increased risk of autoimmune diseases related to COVID-19
Although the vaccination program successfully was led to the control of COVID-19 pandemic, the side effects and their effectiveness are still important points that need to be further investigated. Recently, there have been reports about the emergence of autoimmune diseases including immune thrombocytopenia, autoimmune liver diseases, Guillain-Barre syndrome, IgA nephropathy, rheumatoid arthritis and systemic lupus erythematosus after COVID-19 vaccination. Regarding to the phenomena of autoimmunity after vaccination, it must be noted that the production of autoantibodies, molecular mimicry, and vaccine adjuvants can be screened [124]. Many cases have been observed in association with the development of thrombotic thrombocytopenia after vaccination [125]. In addition, the association of immune thrombocytopenic purpura with COVID-19 vaccination has been challenged since a 28 year old woman was diagnosed with headache, purpura on the trunk, fever, and hemorrhagic lesions in the oral cavity after administration of AZD1222 vaccine [126]. The second related case to the Moderna vaccine was a 72 year old woman, which showed symptoms such as a decrease in platelets, skin rash, spontaneous oral bleeding, headache, widespread petechiae in the arms, legs, and abdomen, and self-bleeding blisters a day after receiving the vaccine [127].
In addition, a 35 year old Caucasian woman was infected with SARS-CoV-2 andabout 13 days after the injection of the vaccine, the symptoms such as general itching, cholera, and jaundice with positive antinuclear antibody and double-stranded DNA antibodies arose, the doubt arose were observed [128]. Also, a 36 year old Iraqi physician was seen with symptoms such as mild febrile reaction and abnormal liver function tests 26 days after vaccination [129]. These two and other cases are still under investigation. The results revealed thatwe must be careful on the long term complications appeared after the COVID-19 vaccination [124]. However, the studies performed on the autoimmune patients showed that these vaccines are almost safe for these patients.
Another autoimmune disease that is related to the peripheral nervous system is Guillain-Barré syndrome (GBS). This disease is caused by viral infections such as Epstein-Barr virus, cytomegalovirus, and Zika virus. Also, its relationship with hepatitis A and B, rabies, and flu vaccines has been determined. There is evidence of incidence of GBS after vaccination with COVID-19 [124]. An 82 year old woman with signs of general discomfort and body pain after a week of receiving the vaccine was the first case that showed the association between the GBS and vaccination [130]. In addition, clinical signs related to the GBS and the vaccines from Janssen and ChAdOx1-S/NCoV-19 were observed in India and England [130], [131].
In Iran, 3.3% of people were vaccinated with Sputnik V developed arthralgia. Studies on 724 rheumatic and musculoskeletal patients showed that four out of them got this disease after being vaccinated with BBV152 and ChAdOx1. The first case of arthritis was appeared in a person without a history of chronic disease or infected with SARS-CoV-2, who showed symptoms after receiving vaccination with Sputnik V. The CoronaVac vaccination in a 23 year old woman resulted in swelling of her left knee joint, which improved with treatment with betamethasone. The association of rheumatoid arthritis with the COVID-19 mRNA vaccine (mRNA-1273) has been approved by the FDA.
15. Discussion
In the context of worldwide vaccination programs, paying attention to the biological side effects of vaccine components is necessary and they could be investigated through meta-analysis. There is currently no available alternative procedure for conventional vaccination; therefore, following worldwide vaccination and pandemic subsiding, emergence of any unexpected disease or any increament in the frequency of the possible human diseases, especially in the vaccinated individuals should be stated [85]. Despite using novel technologies, vaccine development and scale-up is a labour and time consuming procedure, which requires extensive testing for quality and safety issues [132]. Fortunately, mRNA vaccines could be scaled up rapidly and sound promising candidate in emerging conditions. During the COVID-19 pandemic, various vaccine platforms were mass-produced in a short period and by controling the problem, build a public trust. Meanwhile, due to large-scale production, maintaining their inherent quality was a priority. Given that most communities have also been vaccinated with the third dose of mRNA vaccine, the population of vaccinated individuals is dramastically increasing and we may encounter with some unexpected safety findings such as myocarditis, infertility, inefficiency against upcomming variants, and integration of the virus nucleic components into the genome of mRNA vaccines. It is clear that trust in mRNA vaccines must be obtained with deeper familiarity and consideration on all aspects to align public opinion with the goals of vaccination [18]. Although the integration of exogenous mRNA into the host chromosomes is not a common phenomenon [133], we should be careful about this possibility and rule it out [134]. In the human body, numerus types of retroviruses and retrotransposons such as LINE retrotransposons could be found, which can excrete parts of the mobile RNA in the cytoplasm and transcribe the mRNA from vaccine into a cDNA in stress conditions, inflammation, and unbalanced immune response stimulations. Therefore, retrotransposons may facilitate this integration whenever they are present in the cytoplasm or when the retrovirus is replicating and capable to lead the migration of cDNA into the nucleus and its integration into the human genome. Such a phenomenon during some viral infections (such as HIV) can increase the possibility of integration. Although there is still no clear understanding of the specificity of retroviral integration within the target site, viral integrases can affect the integration efficiency. There are several control and repair systems in cellular disorders. The regulation of many processes are epigenetic-mediated phenomenon in the cell. However, such structures are usually condemned to post-transcriptional processes such as methylation in the event of integration, or they are removed by the immune system. Although there are few investigations showing the danger of integration of reversely transcribed RNA from SARS-CoV-2 can integrate into the genome of cultured human cells and can be consequently expressed in COVID-19 patient-derived tissues [134], the fact is that we still have very little information about the side effects of such vaccines, their immunogenicity, and efficacy, and recent comments based on scientific evidence are often unclear and sometimes exaggerated. Still, nobody knows about the long-term side effects of mRNA vaccines; we have not even summarized the short-term and medium-term side effects. At last and due to the high morbidity and mortality rate of the COVID-19 pandemic, COVID-19 mRNA vaccines were approved by the FDA only for emergency use. Luckily, the vaccination programs are under the strict control of regulators from the European Medicines Agency (EMA), FDA, and WHO. The adverse events described in association with COVID-19 mRNA vaccines are not too many cases and the benefits of them outweighs the risks and side effects [135].Vaccination has been a cornerstone of the response to the COVID-19 pandemic, while it still does not have the full support of communities. In this study, an attempt has been done to provide information, first to reduce the doubts of many communities about widespread vaccination and then as a small window to increase people's awareness of new generation of vaccines such as COVID-19 mRNA vaccines.
16. Conclusions
The prevalence of the development of new coronaviruses in recent decades suggests that another new virus from this family or other emerging viruses may develop in the future, so rapid development of safe, reliable, and robust vaccines is essential. It is noteworthy that in addition to the common challenges with emerging vaccine substrates, which include the unavailability of an appropriate preclinical model, existence of natural immunity against the target pathogen, along with several challenges such as vaccine bioprocessing scale up, enthusiasm or optimism in the area of vaccinology is extremely important to address these issues. Furthermore, we must present clear and scientific documents to anti-vaccine populations and satisfy them with benefits of these high-technology vaccines and explain the urge of public vaccination with any accessible vaccine to prevent circulation and development of mutant variants of SARS-CoV-2. The envision of living with COVID-19 in the future and looking to a high morbidity and mortality rate of this infection is too painful. We must alter the preference of anti-vaccine populations to a safe vaccination idea. This review article tried to consider all aspects of vaccines without any prejudice to improve the human race after the universal vaccination program. Efforts to better raise awareness and comprehensive understanding of COVID-19 vaccines should not distrust the manufacturers. However, it must be considered that mRNA-based COVID-19 vaccine has the potential to be a successful scientific pattern for designing and production of other kind genetic vaccines against infections and non-infectious diseases like cancers.
Author contributions
Mona Sadat Mirtaleb prepared the first draft of the manuscript. All authors participated in reviewing the manuscript. Reza Zolfaghari Emameh supervised the study and reviewed the final version of the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We thank the National Institute of Genetic Engineering and Biotechnology (NIGEB), Tehran, Iran for preparing the conditions to perform this study.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.intimp.2023.109934.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Data availability
No data was used for the research described in the article.
References
- 1.Lappeman J., Munyai K., Kagina B.M. Negative sentiment towards COVID-19 vaccines: A comparative study of USA and UK social media posts before vaccination rollout. F1000Research. 2021;10(472):472. [Google Scholar]
- 2.Zolfaghari Emameh R., Nosrati H., Taheri R.A. Combination of biodata mining and computational modelling in identification and characterization of ORF1ab Polyprotein of SARS-CoV-2 Isolated from Oronasopharynx of an Iranian Patient. Biol. Proced. Online. 2020;22:8. doi: 10.1186/s12575-020-00121-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pecetta S., et al. Immunology and technology of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines. Pharmacol. Rev. 2022;74(1):313–339. doi: 10.1124/pharmrev.120.000285. [DOI] [PubMed] [Google Scholar]
- 4.Zolfaghari Emameh R., et al. Expansion of Single Cell Transcriptomics Data of SARS-CoV Infection in Human Bronchial Epithelial Cells to COVID-19. Biol. Proced. Online. 2020;22:16. doi: 10.1186/s12575-020-00127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma O., et al. A Review of the Progress and Challenges of Developing a Vaccine for COVID-19. Front. Immunol. 2020;11:2413. doi: 10.3389/fimmu.2020.585354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li T., et al. Mask or no mask for COVID-19: A public health and market study. PLoS One. 2020;15(8):e0237691. doi: 10.1371/journal.pone.0237691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y., Deng Z., Shi D. How effective is a mask in preventing COVID-19 infection? Medical devices & sensors. 2021;4(1):e10163. doi: 10.1002/mds3.10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan D.K., Zhang C.-Q., Weman-Josefsson K. Why people failed to adhere to COVID-19 preventive behaviors? Perspectives from an integrated behavior change model. Infect. Control Hosp. Epidemiol. 2021;42(3):375–376. doi: 10.1017/ice.2020.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li H., et al. Efficacy and practice of facemask use in general population: a systematic review and meta-analysis. Transl. Psychiatry. 2022;12(1):1–15. doi: 10.1038/s41398-022-01814-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cirrincione L., et al. COVID-19 Pandemic: New Prevention and Protection Measures. Sustainability. 2022;14(8):4766. [Google Scholar]
- 11.Zhang M., et al. Clinical features of 2019 novel coronavirus pneumonia in the early stage from a fever clinic in Beijing. Zhonghua jie he he hu xi za zhi= Zhonghua jiehe he huxi zazhi= Chinese journal of tuberculosis and respiratory diseases. 2020;43:E013–E. doi: 10.3760/cma.j.issn.1001-0939.2020.0013. [DOI] [PubMed] [Google Scholar]
- 12.Ashcroft P., et al. Quantifying the impact of quarantine duration on COVID-19 transmission. Elife. 2021;10:e63704. doi: 10.7554/eLife.63704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tarighi P., et al. A review of potential suggested drugs for coronavirus disease (COVID-19) treatment. Eur. J. Pharmacol. 2021;895 doi: 10.1016/j.ejphar.2021.173890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mirtaleb M.S., et al. Potential therapeutic agents to COVID-19: An update review on antiviral therapy, immunotherapy, and cell therapy. Biomed Pharmacother. 2021;138 doi: 10.1016/j.biopha.2021.111518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gandhi R.T., Malani P.N., Del Rio C. COVID-19 Therapeutics for nonhospitalized patients. JAMA. 2022;327(7):617–618. doi: 10.1001/jama.2022.0335. [DOI] [PubMed] [Google Scholar]
- 16.van de Veerdonk F.L., et al. A guide to immunotherapy for COVID-19. Nat. Med. 2022;28(1):39–50. doi: 10.1038/s41591-021-01643-9. [DOI] [PubMed] [Google Scholar]
- 17.Gelfand, A.A. and G. Poland, Migraine treatment and COVID‐19 vaccines: No cause for concern. 2021, Wiley Online Library. [DOI] [PMC free article] [PubMed]
- 18.Altmann D.M., Boyton R.J. COVID-19 vaccination: The road ahead. Science. 2022;375(6585):1127–1132. doi: 10.1126/science.abn1755. [DOI] [PubMed] [Google Scholar]
- 19.Kominers S.D., Tabarrok A. Vaccines and the Covid-19 pandemic: lessons from failure and success. Oxf. Rev. Econ. Policy. 2022;38(4):719–741. [Google Scholar]
- 20.Hui D.S. Hybrid immunity and strategies for COVID-19 vaccination. Lancet Infect. Dis. 2023;23(1):2–3. doi: 10.1016/S1473-3099(22)00640-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khorramdelazad H., et al. Immunopathological similarities between COVID-19 and influenza: Investigating the consequences of Co-infection. Microb Pathog. 2021;152 doi: 10.1016/j.micpath.2020.104554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ndwandwe D., Wiysonge C.S. COVID-19 vaccines. Curr. Opin. Immunol. 2021;71:111–116. doi: 10.1016/j.coi.2021.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hogan M.J., Pardi N. mRNA Vaccines in the COVID-19 Pandemic and Beyond. Annu. Rev. Med. 2022;73:17–39. doi: 10.1146/annurev-med-042420-112725. [DOI] [PubMed] [Google Scholar]
- 24.Huang Q., Yan J. SARS-CoV-2 virus: vaccines in development. Fundamental Research. 2021;1(2):131–138. [Google Scholar]
- 25.Kremsner P.G., et al. Safety and immunogenicity of an mRNA-lipid nanoparticle vaccine candidate against SARS-CoV-2. Wien. Klin. Wochenschr. 2021;133(17):931–941. doi: 10.1007/s00508-021-01922-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bettini E., Locci M. SARS-CoV-2 mRNA vaccines: immunological mechanism and beyond. Vaccines. 2021;9(2):147. doi: 10.3390/vaccines9020147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang Q., Zeng J., Yan J. COVID-19 mRNA vaccines. J. Genet. Genomics. 2021;48(2):107–114. doi: 10.1016/j.jgg.2021.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maruggi G., et al. mRNA as a transformative technology for vaccine development to control infectious diseases. Mol. Ther. 2019;27(4):757–772. doi: 10.1016/j.ymthe.2019.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pardi N., et al. mRNA vaccines—a new era in vaccinology. Nat. Rev. Drug Discov. 2018;17(4):261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schlake T., et al. Developing mRNA-vaccine technologies. RNA Biol. 2012;9(11):1319–1330. doi: 10.4161/rna.22269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wadhwa A., et al. Opportunities and challenges in the delivery of mRNA-based vaccines. Pharmaceutics. 2020;12(2):102. doi: 10.3390/pharmaceutics12020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamamoto A., et al. Current prospects for mRNA gene delivery. Eur. J. Pharm. Biopharm. 2009;71(3):484–489. doi: 10.1016/j.ejpb.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 33.Park J.W., et al. mRNA vaccines for COVID-19: what, why and how. Int. J. Biol. Sci. 2021;17(6):1446. doi: 10.7150/ijbs.59233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schoenmaker L., et al. mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability. Int. J. Pharm. 2021;601 doi: 10.1016/j.ijpharm.2021.120586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reichmuth A.M., et al. mRNA vaccine delivery using lipid nanoparticles. Ther. Deliv. 2016;7(5):319–334. doi: 10.4155/tde-2016-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wrapp D., et al. Structural basis for potent neutralization of betacoronaviruses by single-domain camelid antibodies. Cell. 2020;181(5):1004–1015. doi: 10.1016/j.cell.2020.04.031. e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Santos, I.d.A., et al., Antivirals against coronaviruses: candidate drugs for SARS-CoV-2 treatment? Frontiers in microbiology, 2020: p. 1818. [DOI] [PMC free article] [PubMed]
- 38.Cao Y., et al. Potent neutralizing antibodies against SARS-CoV-2 identified by high-throughput single-cell sequencing of convalescent patients’ B cells. Cell. 2020;182(1):73–84. doi: 10.1016/j.cell.2020.05.025. e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cai Y., et al. Distinct conformational states of SARS-CoV-2 spike protein. Science. 2020;369(6511):1586–1592. doi: 10.1126/science.abd4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mittal A., et al. COVID-19 pandemic: Insights into structure, function, and hACE2 receptor recognition by SARS-CoV-2. PLoS Pathog. 2020;16(8):e1008762. doi: 10.1371/journal.ppat.1008762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zolfaghari Emameh R., et al. Identification and characterization of a silent mutation in RNA binding domain of N protein coding gene from SARS-CoV-2. BMC Res Notes. 2021;14(1):10. doi: 10.1186/s13104-020-05439-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang, Q., et al., Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell, 2020. 181(4): p. 894-904. e9. [DOI] [PMC free article] [PubMed]
- 43.Girum T., et al. Global strategies and effectiveness for COVID-19 prevention through contact tracing, screening, quarantine, and isolation: a systematic review. Tropical medicine and health. 2020;48(1):1–15. doi: 10.1186/s41182-020-00285-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cullis P.R., Hope M.J. Lipid nanoparticle systems for enabling gene therapies. Mol. Ther. 2017;25(7):1467–1475. doi: 10.1016/j.ymthe.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shin M.D., et al. COVID-19 vaccine development and a potential nanomaterial path forward. Nat. Nanotechnol. 2020;15(8):646–655. doi: 10.1038/s41565-020-0737-y. [DOI] [PubMed] [Google Scholar]
- 46.Vogel A.B., et al. BNT162b vaccines protect rhesus macaques from SARS-CoV-2. Nature. 2021;592(7853):283–289. doi: 10.1038/s41586-021-03275-y. [DOI] [PubMed] [Google Scholar]
- 47.Kiaie S.H., et al. Recent advances in mRNA-LNP therapeutics: immunological and pharmacological aspects. J. Nanobiotechnol. 2022;20(1):276. doi: 10.1186/s12951-022-01478-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hassett K.J., et al. Impact of lipid nanoparticle size on mRNA vaccine immunogenicity. J. Control. Release. 2021;335:237–246. doi: 10.1016/j.jconrel.2021.05.021. [DOI] [PubMed] [Google Scholar]
- 49.Nakanishi T., et al. Positively charged liposome functions as an efficient immunoadjuvant in inducing immune responses to soluble proteins. Biochem. Biophys. Res. Commun. 1997;240(3):793–797. doi: 10.1006/bbrc.1997.7749. [DOI] [PubMed] [Google Scholar]
- 50.Guevara M.L., Persano F., Persano S. Advances in lipid nanoparticles for mRNA-based cancer immunotherapy. Front. Chem. 2020;8 doi: 10.3389/fchem.2020.589959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lonez C., et al. Cationic lipid nanocarriers activate Toll-like receptor 2 and NLRP3 inflammasome pathways. Nanomed. Nanotechnol. Biol. Med. 2014;10(4):775–782. doi: 10.1016/j.nano.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 52.Goswami R., et al. Mannosylation of LNP results in improved potency for self-amplifying RNA (SAM) vaccines. ACS Infect. Dis. 2019;5(9):1546–1558. doi: 10.1021/acsinfecdis.9b00084. [DOI] [PubMed] [Google Scholar]
- 53.August A., et al. Clinical development of mRNA vaccines: challenges and opportunities. mRNA Vaccines. 2022:167–186. doi: 10.1007/82_2022_259. [DOI] [PubMed] [Google Scholar]
- 54.Nogueira S.S., et al. Polysarcosine-functionalized lipid nanoparticles for therapeutic mRNA delivery. ACS Applied Nano Materials. 2020;3(11):10634–10645. [Google Scholar]
- 55.Maruggi G., et al. Immunogenicity and protective efficacy induced by self-amplifying mRNA vaccines encoding bacterial antigens. Vaccine. 2017;35(2):361–368. doi: 10.1016/j.vaccine.2016.11.040. [DOI] [PubMed] [Google Scholar]
- 56.Miliotou A.N., Papadopoulou L.C. Chimeric Antigen Receptor T Cells. Springer; 2020. In Vitro-Transcribed (IVT)-mRNA CAR Therapy Development; pp. 87–117. [DOI] [PubMed] [Google Scholar]
- 57.Schlake T., et al. mRNA as novel technology for passive immunotherapy. Cell. Mol. Life Sci. 2019;76(2):301–328. doi: 10.1007/s00018-018-2935-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.O'neill, L.A., D. Golenbock, and A.G. Bowie, The history of Toll-like receptors—redefining innate immunity. Nature Reviews Immunology, 2013. 13(6): p. 453-460. [DOI] [PubMed]
- 59.Dai J., et al. Toll-like receptor signaling in severe acute respiratory syndrome coronavirus 2-induced innate immune responses and the potential application value of toll-like receptor Immunomodulators in patients with coronavirus disease 2019. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.948770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guan S., Rosenecker J. Nanotechnologies in delivery of mRNA therapeutics using nonviral vector-based delivery systems. Gene Ther. 2017;24(3):133–143. doi: 10.1038/gt.2017.5. [DOI] [PubMed] [Google Scholar]
- 61.Thess A., et al. Sequence-engineered mRNA without chemical nucleoside modifications enables an effective protein therapy in large animals. Mol. Ther. 2015;23(9):1456–1464. doi: 10.1038/mt.2015.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Verbeke R., et al. The dawn of mRNA vaccines: The COVID-19 case. J. Control. Release. 2021;333:511–520. doi: 10.1016/j.jconrel.2021.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Puranik A., et al. Comparison of two highly-effective mRNA vaccines for COVID-19 during periods of Alpha and Delta variant prevalence. MedRxiv. 2021 [Google Scholar]
- 64.Holtkamp S., et al. Modification of antigen-encoding RNA increases stability, translational efficacy, and T-cell stimulatory capacity of dendritic cells. Blood. 2006;108(13):4009–4017. doi: 10.1182/blood-2006-04-015024. [DOI] [PubMed] [Google Scholar]
- 65.Wayment-Steele H.K., et al. Theoretical basis for stabilizing messenger RNA through secondary structure design. Nucleic Acids Res. 2021;49(18):10604–10617. doi: 10.1093/nar/gkab764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mauger D.M., et al. mRNA structure regulates protein expression through changes in functional half-life. Proc. Natl. Acad. Sci. 2019;116(48):24075–24083. doi: 10.1073/pnas.1908052116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nelson, J., et al., Impact of mRNA chemistry and manufacturing process on innate immune activation. Science advances, 2020. 6(26): p. eaaz6893. [DOI] [PMC free article] [PubMed]
- 68.Lutz J., et al. Unmodified mRNA in LNPs constitutes a competitive technology for prophylactic vaccines. npj Vaccines. 2017;2(1):1–9. doi: 10.1038/s41541-017-0032-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abdool Karim S.S., de Oliveira T. New SARS-CoV-2 variants—clinical, public health, and vaccine implications. N. Engl. J. Med. 2021;384(19):1866–1868. doi: 10.1056/NEJMc2100362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sadarangani M., Marchant A., Kollmann T.R. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat. Rev. Immunol. 2021;21(8):475–484. doi: 10.1038/s41577-021-00578-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhu F.-C., et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396(10249):479–488. doi: 10.1016/S0140-6736(20)31605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhu F.-C., et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020;395(10240):1845–1854. doi: 10.1016/S0140-6736(20)31208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Naranbhai V., et al. Comparative immunogenicity and effectiveness of mRNA-1273, BNT162b2, and Ad26. COV2. S COVID-19 vaccines. J Infect Dis. 2022;225(7):1141–1150. doi: 10.1093/infdis/jiab593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.COVID, M., Vaccine. FDA Briefing Document. Vaccines and Related Biological Products Advisory Committee Meeting. December 17, 2020. 2020. 2021.
- 75.Barrett J.R., et al. Phase 1/2 trial of SARS-CoV-2 vaccine ChAdOx1 nCoV-19 with a booster dose induces multifunctional antibody responses. Nat. Med. 2021;27(2):279–288. doi: 10.1038/s41591-020-01179-4. [DOI] [PubMed] [Google Scholar]
- 76.Beale A.J. Autism, inflammatory bowel disease, and MMR vaccine. Lancet. 1998;351(9106):906. doi: 10.1016/S0140-6736(05)70318-4. author reply 908–9. [DOI] [PubMed] [Google Scholar]
- 77.Bedford H., et al. Autism, inflammatory bowel disease, and MMR vaccine. Lancet. 1998;351(9106):907. doi: 10.1016/S0140-6736(05)70320-2. author reply 908–9. [DOI] [PubMed] [Google Scholar]
- 78.Black D., Prempeh H., Baxter T. Autism, inflammatory bowel disease, and MMR vaccine. Lancet. 1998;351(9106):905–906. doi: 10.1016/S0140-6736(05)70316-0. author reply 908–9. [DOI] [PubMed] [Google Scholar]
- 79.Lee J.W., et al. Autism, inflammatory bowel disease, and MMR vaccine. Lancet. 1998;351(9106):905. doi: 10.1016/S0140-6736(05)70316-0. author reply 908–9. [DOI] [PubMed] [Google Scholar]
- 80.Lindley K.J., Milla P.J. Autism, inflammatory bowel disease, and MMR vaccine. Lancet. 1998;351(9106):907–908. doi: 10.1016/S0140-6736(05)70321-4. author reply 908–9. [DOI] [PubMed] [Google Scholar]
- 81.O'Brien S.J., Jones I.G., Christie P. Autism, inflammatory bowel disease, and MMR vaccine. Lancet. 1998;351(9106):906–907. doi: 10.1016/S0140-6736(05)70318-4. author reply 908–9. [DOI] [PubMed] [Google Scholar]
- 82.Payne C., Mason B. Autism, inflammatory bowel disease, and MMR vaccine. Lancet. 1998;351(9106):907. doi: 10.1016/S0140-6736(05)70319-6. author reply 908–9. [DOI] [PubMed] [Google Scholar]
- 83.Mohammed D., Rossi M.G. The pandemic of argumentation. Springer; 2022. The argumentative potential of doubt: From legitimate concerns to conspiracy theories about COVID-19 vaccines; pp. 125–144. [Google Scholar]
- 84.Lai C.-C., et al. COVID-19 vaccines: concerns beyond protective efficacy and safety. Expert Rev. Vaccines. 2021;20(8):1013–1025. doi: 10.1080/14760584.2021.1949293. [DOI] [PubMed] [Google Scholar]
- 85.Doerfler, W., Adenoviral Vector DNA-and SARS-CoV-2 mRNA-Based Covid-19 Vaccines: Possible Integration into the Human Genome-Are Adenoviral Genes Expressed in Vector-based Vaccines? Virus Res, 2021. 302: p. 198466-198466. [DOI] [PMC free article] [PubMed]
- 86.Doerfler W. Viral Vector DNA-and RNA-Based SARS-CoV-2 Vaccines: Possible Integration into the Human Genome Are Adenoviral Genes Expressed in Vector-based Vaccines? Virus Res. 2021;302 doi: 10.1016/j.virusres.2021.198466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang, L., et al., SARS-CoV-2 RNA reverse-transcribed and integrated into the human genome. bioRxiv, 2020.
- 88.Rosenblum H.G., et al. Interim recommendations from the Advisory Committee on Immunization Practices for the use of bivalent booster doses of COVID-19 vaccines—United States, October 2022. Morb. Mortal. Wkly Rep. 2022;71(45):1436–1441. doi: 10.15585/mmwr.mm7145a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Control, C.f.D. and Prevention, Interim clinical considerations for use of COVID-19 vaccines currently authorized in the United States. 2021.
- 90.Besanko D., Gillis S., Shen S. Polio Eradication—Within Our Reach? Kellogg Sch. Manag. Cases. 2017 [Google Scholar]
- 91.Fleming-Dutra, K.E., et al., Interim recommendations of the Advisory Committee on Immunization Practices for use of Moderna and Pfizer-BioNTech COVID-19 vaccines in children aged 6 months–5 years—United States, June 2022. 2022. [DOI] [PubMed]
- 92.Crommelin D.J., et al. Addressing the cold reality of mRNA vaccine stability. J. Pharm. Sci. 2021;110(3):997–1001. doi: 10.1016/j.xphs.2020.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Karam M., Daoud G. mRNA vaccines: Past, present, future. Asian J. Pharm. Sci. 2022 doi: 10.1016/j.ajps.2022.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rauch S., et al. New vaccine technologies to combat outbreak situations. Front. Immunol. 2018;9:1963. doi: 10.3389/fimmu.2018.01963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang F., Kream R.M., Stefano G.B. An evidence based perspective on mRNA-SARS-CoV-2 vaccine development. Medical science monitor: international medical journal of experimental and clinical research. 2020;26:e924700–e924701. doi: 10.12659/MSM.924700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.The EMBO journalZhao, X., et al., Neutralisation of ZF2001-elicited antisera to SARS-CoV-2 variants. The Lancet Microbe, 2021. 2(10): p. E494. [DOI] [PMC free article] [PubMed]
- 97.Zhang, X.-O., H. Pratt, and Z. Weng, Investigating the potential roles of SINEs in the human genome. Annual Review of Genomics and Human Genetics, 2021. 22: p. pp 199-218. [DOI] [PubMed]
- 98.Desfarges, S. and A. Ciuffi, Viral integration and consequences on host gene expression, in Viruses: essential agents of life. 2012, Springer. p. 147-175.
- 99.Gilbert C., Cordaux R. Viruses as vectors of horizontal transfer of genetic material in eukaryotes. Curr. Opin. Virol. 2017;25:16–22. doi: 10.1016/j.coviro.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 100.Zerbini F.M., et al. ICTV virus taxonomy profile: Geminiviridae. J. Gen. Virol. 2017;98(2):131. doi: 10.1099/jgv.0.000738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Emameh R.Z., et al. Assessment of databases to determine the validity of β-and γ-carbonic anhydrase sequences from vertebrates. BMC Genomics. 2020;21(1):1–8. doi: 10.1186/s12864-020-6762-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Emameh, R.Z., S.N. Hosseini, and S. Parkkila, Application of beta and gamma carbonic anhydrase sequences as tools for identification of bacterial contamination in the whole genome sequence of inbred Wuzhishan minipig (Sus scrofa) annotated in databases. Database: the journal of biological databases and curation, 2021. 2021: p. baab029. [DOI] [PMC free article] [PubMed]
- 103.Liu H., et al. Widespread horizontal gene transfer from double-stranded RNA viruses to eukaryotic nuclear genomes. J. Virol. 2010;84(22):11876–11887. doi: 10.1128/JVI.00955-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Seneff S., Nigh G. Worse than the disease? Reviewing some possible unintended consequences of the mRNA vaccines against COVID-19. International Journal of Vaccine Theory, Practice, and Research. 2021;2(1):38–79. [Google Scholar]
- 105.Male V. Are COVID-19 vaccines safe in pregnancy? Nat. Rev. Immunol. 2021;21(4):200–201. doi: 10.1038/s41577-021-00525-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ioannidis J.P. COVID-19 vaccination in children and university students. Eur. J. Clin. Invest. 2021;51(11):e13678. doi: 10.1111/eci.13678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Walter E.B., et al. Evaluation of the BNT162b2 Covid-19 vaccine in children 5 to 11 years of age. N. Engl. J. Med. 2022;386(1):35–46. doi: 10.1056/NEJMoa2116298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Garg I., et al. COVID-19 vaccine in pregnant and lactating women: a review of existing evidence and practice guidelines. Infectious disease reports. 2021;13(3):685–699. doi: 10.3390/idr13030064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Falsaperla R., et al. COVID-19 vaccination in pregnant and lactating women: a systematic review. Expert Rev. Vaccines. 2021;20(12):1619–1628. doi: 10.1080/14760584.2021.1986390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Polack F.P., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Baden L.R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.McLaurin-Jiang S., et al. Maternal and child symptoms following COVID-19 vaccination among breastfeeding mothers. Breastfeed. Med. 2021;16(9):702–709. doi: 10.1089/bfm.2021.0079. [DOI] [PubMed] [Google Scholar]
- 113.Das R., et al. Emerging heterologous mRNA-based booster strategies within the COVID-19 vaccine landscape. Hum. Vaccin. Immunother. 2023:2153532. doi: 10.1080/21645515.2022.2153532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Thye A.-Y.-K., et al. COVID-19 Booster Vaccines Administration in Different Countries. Progress In Microbes & Molecular Biology. 2021;4(1) [Google Scholar]
- 115.Burki T.K. Omicron variant and booster COVID-19 vaccines. Lancet Respir. Med. 2022;10(2):e17. doi: 10.1016/S2213-2600(21)00559-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Takashita E., et al. Efficacy of Antibodies and Antiviral Drugs against Covid-19 Omicron Variant. N. Engl. J. Med. 2022 doi: 10.1056/NEJMc2119407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lopez Bernal, J., et al., Effectiveness of Covid-19 vaccines against the B. 1.617. 2 (Delta) variant. N. Engl. j. med, 2021. [DOI] [PubMed]
- 118.Wong M.T.J., et al. Effectiveness of Booster Vaccinations on the Control of COVID-19 during the Spread of Omicron Variant in Malaysia. Int. J. Environ. Res. Public Health. 2023;20(2):1647. doi: 10.3390/ijerph20021647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Milano G., et al. Myocarditis and COVID-19 mRNA vaccines: a mechanistic hypothesis involving dsRNA. Futur. Virol. 2022;17(3):191–196. doi: 10.2217/fvl-2021-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Witberg G., et al. Myocarditis after Covid-19 vaccination in a large health care organization. N. Engl. J. Med. 2021 doi: 10.1056/NEJMoa2110737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Soiza R.L., Scicluna C., Thomson E.C. Efficacy and safety of COVID-19 vaccines in older people. Age Ageing. 2021;50(2):279–283. doi: 10.1093/ageing/afaa274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sonani B., et al. COVID-19 vaccination in immunocompromised patients. Clin. Rheumatol. 2021;40(2):797–798. doi: 10.1007/s10067-020-05547-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lucca F., et al. Immunogenicity and Safety of the BNT162b2 COVID-19 Vaccine in Patients with Cystic Fibrosis with or without Lung Transplantation. Int. J. Mol. Sci. 2023;24(2):908. doi: 10.3390/ijms24020908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen Y., et al. New-onset autoimmune phenomena post-COVID-19 vaccination. Immunology. 2022;165(4):386–401. doi: 10.1111/imm.13443. [DOI] [PubMed] [Google Scholar]
- 125.Abed N., et al. Direct regulation of the pefI-srgC operon encoding the Rck invasin by the quorum-sensing regulator SdiA in S almonella T yphimurium. Mol. Microbiol. 2014;94(2):254–271. doi: 10.1111/mmi.12738. [DOI] [PubMed] [Google Scholar]
- 126.Candelli M., et al. Immune thrombocytopenic purpura after SARS-CoV-2 vaccine. Br. J. Haematol. 2021 doi: 10.1111/bjh.17508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Julian J.A., Mathern D.R., Fernando D. Idiopathic thrombocytopenic purpura and the moderna covid-19 vaccine. Ann. Emerg. Med. 2021;77(6):654–656. doi: 10.1016/j.annemergmed.2021.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bril F., et al. Autoimmune hepatitis developing after coronavirus disease 2019 (COVID-19) vaccine: causality or casualty? J. Hepatol. 2021;75(1):222–224. doi: 10.1016/j.jhep.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Clayton-Chubb D., et al. Autoimmune hepatitis developing after the ChAdOx1 nCoV-19 (Oxford-AstraZeneca) vaccine. J. Hepatol. 2021;75(5):1249–1250. doi: 10.1016/j.jhep.2021.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.McKean N., Chircop C. Guillain-Barré syndrome after COVID-19 vaccination. BMJ Case Reports CP. 2021;14(7):e244125. doi: 10.1136/bcr-2021-244125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sen P., et al. COVID-19 vaccination-related adverse events among autoimmune disease patients: results from the COVAD study. Rheumatology. 2023;62(1):65–76. doi: 10.1093/rheumatology/keac305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Amanat F., Krammer F. SARS-CoV-2 vaccines: status report. Immunity. 2020;52(4):583–589. doi: 10.1016/j.immuni.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Smits N., et al. No evidence of human genome integration of SARS-CoV-2 found by long-read DNA sequencing. Cell Rep. 2021;36(7) doi: 10.1016/j.celrep.2021.109530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang L., et al. Reverse-transcribed SARS-CoV-2 RNA can integrate into the genome of cultured human cells and can be expressed in patient-derived tissues. Proc. Natl. Acad. Sci. USA. 2021;118(21) doi: 10.1073/pnas.2105968118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bezbaruah R., et al. Developmental landscape of potential vaccine candidates based on viral vector for prophylaxis of COVID-19. Front. Mol. Biosci. 2021;8:96. doi: 10.3389/fmolb.2021.635337. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.