Abstract
Background
Both immune-mediated thrombotic thrombocytopenic purpura (iTTP) and septic disseminated intravascular coagulation (DIC) are life-threatening disorders developed by platelet-consuming microvascular thrombi and necessitate immediate therapeutic interventions. Although severe deficiencies of plasma haptoglobin in iTTP and factor XIII (FXIII) activity in septic DIC have been reported, few studies have focused on the possibility of using these markers to distinguish between iTTP and septic DIC.
Objectives
We investigated whether the plasma levels of haptoglobin and FXIII activity could be helpful for differential diagnosis.
Methods
Thirty-five patients with iTTP and 30 with septic DIC were enrolled in the study. Patient characteristics, coagulation, and fibrinolytic markers were collected from the clinical data. Plasma haptoglobin and FXIII activities were measured using chromogenic Enzyme-Linked Immuno Sorbent Assay and an automated instrument, respectively.
Results
The median plasma haptoglobin level was 0.39 mg/dL and 54.20 mg/dL in the iTTP and septic DIC groups, respectively. The median plasma FXIII activities were 91.3% and 36.3% in the iTTP and septic DIC groups, respectively. In the receiver operating characteristic curve analysis, the cutoff level of plasma haptoglobin was 2.868 mg/dL and the area under the curve was 0.832. The cutoff level for plasma FXIII activity and the area under the curve were 76.0% and 0.931, respectively. The thrombotic thrombocytopenic purpura (TTP)/DIC index was defined by FXIII activity (percentage) and haptoglobin (milligrams per decilitre). Laboratory TTP was defined as an index ≥60 and laboratory DIC <60. The sensitivity and specificity of the TTP/DIC index were 94.3% and 86.7%, respectively.
Conclusion
The TTP/DIC index, composed of plasma levels of haptoglobin and FXIII activity, is useful in differentiating iTTP from septic DIC.
Keywords: differential diagnosis, disseminated intravascular coagulation, factor XIII, haptoglobins, thrombotic thrombocytopenic purpura
Essentials
-
•
Plasma haptoglobin is decreased in immune-mediated thrombotic thrombocytopenic purpura (TTP).
-
•
Plasma factor XIII is decreased in disseminated intravascular coagulation (DIC).
-
•
Plasma levels of haptoglobin and factor XIII were almost normal in septic DIC and TTP, respectively.
-
•
Index of 2 laboratory tests is useful for differentiating immune-mediated TTP from septic DIC.
1. Introduction
Thrombotic thrombocytopenic purpura (TTP) is a well-known type of thrombotic microangiopathy (TMA), characterized by thrombocytopenia, microangiopathic hemolytic anemia, and systemic microvascular thrombi [1]. TTP is caused by a deficiency in the von Willebrand factor (VWF)–cleaving protease a disintegrin-like metalloproteinase with thrombospondin type 1 motifs 13 (ADAMTS-13) [2,3]. Severely diminished ADAMTS-13 activity results in unusually large VWF uncleaved multimers and form platelet-rich microvasculature thrombi [1]. There are 2 types of TTP: a hereditary form of TTP caused by ADAMTS-13 mutations, called congenital TTP, and another is the acquired form of TTP caused by the development of autoantibodies against ADAMTS-13, termed immune-mediated TTP (iTTP) [1]. More than 95% of patients with TTP are classified as iTTP. The mortality of patients with iTTP is >90% without plasma exchange but is <20% with plasma exchange [4]. Therefore, patients with iTTP should be promptly diagnosed and a treatment regimen of plasma exchange and corticosteroids should be initiated as soon as possible to improve prognosis [1].
Physicians often find it challenging to distinguish between iTTP and disseminated intravascular coagulation (DIC), especially in septic DIC, based only on clinical symptoms and laboratory findings, without ADAMTS-13 activity analysis [5]. A quick quantification of ADAMTS-13 activity is unavailable in most hospitals, necessitating the development of clinical prediction models to evaluate the probability of severe ADAMTS-13 deficiency. The French and PLASMIC scores are sometimes used for this purpose [6]. French score includes 2 criteria: platelet count and serum creatinine. The PLASMIC score has the following 7 components: active cancer, solid organ or stem cell transplant, platelet count, mean corpuscular volume, serum creatinine, prothrombin time (PT), and hemolytic value. Both scores were developed for adult populations with no complications such as pregnancy, cancer, or sepsis.
DIC is a serious condition in which systemic and persistent coagulation activation occurs because of the underlying diseases, such as sepsis, malignant tumor, aneurysm, and injury. Fibrin formation occurs in the microvasculature, leading to ischemic organ damage [[7], [8], [9]]. TTP and DIC differ based on the composition of microthrombi. Those in TTP contain VWF-rich thrombi, whereas those in DIC are characterized by fibrin formation. Our recent study indicated that platelet count, antithrombin activity, and fibrin/fibrinogen degradation products (FDPs) are useful markers for discriminating iTTP from septic DIC [5]. We explore specific makers for this purpose.
As per a report comparing the pathophysiology of TMA and DIC, TMA forms microvascular thrombosis on the arterial side, whereas DIC forms microvascular thrombosis on the venous side [10]. Thrombus of iTTP formed on the arterial side, where the bloodstream is fast, causes hemolytic anemia, elevated total bilirubin (T-Bil) (especially indirect bilirubin [I-Bil]), elevated lactate dehydrogenase (LDH), and a marked decrease in haptoglobin [11]. Haptoglobin is produced mainly in the liver. It binds rapidly and with high affinity to free hemoglobin released into the blood so that it may be degraded by the reticuloendothelial system [12]. In hemolytic diseases, haptoglobin is markedly reduced owing to the release of large amounts of free hemoglobin in the blood [13]. In DIC, anemia and elevated bilirubin and LDH levels are seen less frequently than in iTTP [10], but there are no reports discussing haptoglobin levels in DIC.
Overactive coagulation in DIC leads to factor XIII (FXIII) and secondary FXIII deficiency. Factor XIII, also known as fibrin stabilizing factor, contributes to fibrin clot stabilization and wound healing after hemostasis [14] by incorporating α2 plasmin inhibitor [15,16] and fibronectin within the fibrin clot. In congenital FXIII deficiency [17] and acquired FXIII inhibitors, FXIII factor activity is reduced, resulting in bleeding symptoms. DIC also reportedly shows a secondary FXIII deficiency [18,19]. However, there are no reports focusing on the levels of FXIII in iTTP.
In this study, the plasma levels of haptoglobin and FXIII were compared between patients with iTTP and septic DIC. We also investigated whether these 2 markers could help in differentiating iTTP from septic DIC.
2. Methods
2.1. Patients
Our laboratory at Nara Medical University has been managing the TMA registry and functioning as a TMA referral center in Japan by analyzing ADAMTS-13 activity, as requested by physicians across Japan [20]. Citrated plasma of patients in the acute phase was sent from the referral hospitals to our laboratory along with a registration form including the patient background and clinical laboratory data performed in referral hospitals. Without ADAMTS-13 activity, it is difficult to determine whether these plasmas are iTTP, TMAs, or DIC from routine laboratory data.
Patients with iTTP were enrolled from the TMA registry between 2014 and 2017. iTTP was diagnosed by hemolytic anemia and thrombocytopenia with ADAMTS-13 activity verging at <10% of normal and the presence of ADAMTS-13 inhibitor (0.5 Bethesda units/mL or more). Patients with apparent underlying diseases such as infections, autoimmune diseases, or malignancies were excluded from this study. Thirty-five patients with iTTP were included in the study.
Patients with DIC were also referred to our laboratory by physicians across Japan to analyze ADAMTS-13 activity. Among patients without severely diminished ADAMTS-13 activity, DIC was diagnosed according to the diagnostic criteria of the International Society on Thrombosis and Haemostasis (ISTH) [21] with some modifications [22]. Sepsis is defined as a life-threatening organ dysfunction resulting from dysregulated host responses to infection by the Third International Consensus Definition for Sepsis and Septic Shock (Sepsis-3) [23]. Sepsis can be defined as the presence of an infection combined with an acute change in the sequential organ failure assessment score of 2 points or more [24]. A diagnosis of septic DIC was made in patients who met the definition of sepsis and diagnostic criteria for DIC. Thirty patients with septic DIC were included in TMA Registry between 2008 and 2017 and enrolled in this study. The difference in duration between the iTTP and septic DIC groups is to maintain the number of patients in the analysis approximately the same in both groups.
2.2. Measurements
In this study, the following coagulation and fibrinolytic markers were obtained from the registration form: PT–international normalized ratio (INR), activated partial thromboplastin time (aPTT), fibrinogen, FDP, D-dimer, antithrombin activity, thrombin-antithrombin complex, and plasmin-α2 plasmin inhibitor complex.
Plasma ADAMTS-13 activity, ADAMTS-13 inhibitor, haptoglobin, and FXIII activities were measured in our laboratory. ADAMTS-13 activity was measured using the chromogenic ADAMTS-13 act Enzyme-Linked Immuno Sorbent Assay (ELISA) [25]. Normal plasma was prepared using a mixture of 20 healthy plasma samples (10 male and 10 female volunteers) aged 20 to 40 years. ADAMTS-13 inhibitor titer was evaluated by measuring ADAMTS-13 activity in a mixture of heat-inactivated patient plasma at 56 °C for 30 minutes and normal plasma by chromogenic ADAMTS-13 act ELISA. One Bethesda unit/mL was defined as the amount that reduced ADAMTS-13 activity to 50% of the control activity [26,27]. The plasma levels of haptoglobin were analyzed using Human Quantikine ELISA Kits (DPHAGO, R&D systems, Inc), according to the manufacturer’s instructions. Factor XIII activity was measured using an automated instrument (CN-3000, Sysmex) with Berichrom FXIII (Sysmex).
2.3. Clinical prediction models for severe ADAMTS-13 activity
Seven components of the PLASMIC score were not included in our registry data. Therefore, only French scores were analyzed in this study. The French score is based on 2 parameters: platelet count <30 × 109/L +1 and serum creatine level <2.26 mg/dL +1. The probability of severe deficiency of ADAMTS-13 activity (<10%) using the French score was 2% in score 0, 70% in score 1, and 94% in score 2 [6].
2.4. Statistical analysis
Each data point was described as the median (first quartile to third quartile). In the statistical analysis, the data between the iTTP and septic DIC groups were compared using the Mann–Whitney U-test or Pearson’s chi-squared test. Receiver operating characteristic curve analysis was used to calculate the area under the curve (AUC), specificity, and sensitivity of the test values for differentiating between iTTP and septic DIC. All statistical analyses were performed using EZR software [28].
2.5. Ethics statement
This study was approved by the ethics committee of Nara Medical University (#2397) and was conducted according to the principles of the Declaration of Helsinki. Written informed consent was obtained from all the patients.
3. Results
3.1. Patient characteristics
The patient characteristics for both iTTP and septic DIC are shown in Table 1. Patients with iTTP were significantly younger than those with septic DIC. There was no significant difference in the sex ratio between the 2 groups. In the iTTP group, ADAMTS-13 activity was markedly decreased and ADAMTS-13 inhibitor was positive, whereas ADAMTS-13 activity was mildly decreased and ADAMTS-13 inhibitor was not detected in the septic DIC group. The iTTP group showed severe anemia and the septic DIC group showed mild anemia, with a significant difference between the 2 groups. White blood cell counts and T-Bil were not significantly different between the 2 groups. Severe thrombocytopenia was observed in both groups; however, the iTTP group had significantly lower platelet counts. Elevated LDH levels were observed in both groups but were significantly higher in the iTTP group. Creatinine levels were within the normal range in the iTTP group and significantly elevated in the septic DIC group. To summarize, significant differences between the 2 groups were found in age, ADAMTS-13 activity, ADAMTS-13 inhibitor, hemoglobin, platelet count, LDH, and creatinine.
Table 1.
Patient characteristics.
| Patient characteristics | Reference | iTTPa | Septic DICa | P value |
|---|---|---|---|---|
| Numberb | - | 35 | 30 | - |
| Age | - | 55.0 (42.0 to 68.5) | 70.5 (64.5 to 77.0) | <.01 |
| Sex (M/F) | - | 15/20 | 16/14 | .11 |
| ADAMTS-13 activity (%) | 50 to 150 | <0.5 (<0.5 to <0.5) | 31.8 (20.0 to 38.9) | <.01 |
| ADAMTS-13 inhibitor (BU/mL) | <0.5 | 3.0 (1.8 to 4.2) | <0.5 (<0.5 to <0.5) | <.01 |
| Hemoglobin (g/dL) | M:13.7 to 16.8 | 8.4 (7.1 to 9.4) | 10.5 (9.5 to 12.6) | <.01 |
| F:11.6 to 14.8 | ||||
| White blood cell (×103/μL) | 3.3 to 8.6 | 7.1 (4.7 to 10.0) | 9.5 (5.1 to 12.9) | .31 |
| Platelet (×109/L) | 158 to 348 | 12.0 (7.3 to 17.8) | 39.0 (18.5 to 48.8) | <.01 |
| LDH (U/L) | 120 to 220 | 888 (641 to 1438) | 462 (309 to 777) | <.01 |
| Creatinine (mg/dL) | M:0.65 to 1.05 | 0.77 (0.88 to 1.17) | 2.13 (1.27 to 3.50) | <.01 |
| F:0.45 to 0.80 | ||||
| T-Bil (mg/dL) | 0.2 to 1.1 | 2.7 (2.2 to 4.5) | 1.9 (0.8 to 4.7) | .13 |
ADAMTS-13, a disintegrin-like metalloproteinase with thrombospondin type 1 motifs 13; BU, Bethesda unit; DIC, disseminated intravascular coagulation; iTTP, immune-mediated thrombotic thrombocytopenic purpura; LDH, lactate dehydrogenase; T-Bil, total bilirubin.
Each data are described as median (first quartile to third quartile).
All patients were Japanese.
3.2. DIC scores of each group
The profile of DIC scores by ISTH diagnostic criteria, which defines DIC as ≥5 points, is shown in Supplementary Figure. In the iTTP group, 25 of the 35 patients (71.4%) had 4 points and 10 of the 35 patients (28.6%) had 5 points for DIC diagnosis. Patients in the TTP group who met the diagnostic criteria for DIC had severe thrombocytopenia (<50 × 109/L, 2 points) and markedly increased D-dimer levels (>8.0 μg/mL, 3 points). All patients in the septic DIC group met the DIC diagnostic criteria, with 5 or 6 points accounting for 77.1% (27/35) of all cases.
3.3. Coagulation and fibrinolytic markers
A comparison of coagulation and fibrinolytic markers in iTTP and septic DIC is shown in Table 2. In the iTTP group, PT-INR and aPTT were within the normal range, whereas the septic DIC group showed higher PT-INR and prolonged aPTT. These markers showed significant differences between the 2 groups. The fibrinogen levels were within the normal range in both groups and did not differ significantly between the groups. FDP and D-dimer levels increased slightly in the iTTP group but markedly in the septic DIC group, showing a significant difference between the 2 groups. The antithrombin activity was within the normal range in the iTTP group but decreased in the septic DIC group, with significant differences between the 2 groups. thrombin-antithrombin complex, a marker of coagulation activation, and plasmin-α2 plasmin inhibitor complex, a marker of fibrinolysis activation, were slightly increased in both groups, with no significant difference between the 2 groups. In short, significant differences between the 2 groups were found in PT-INR, aPTT, FDP, D-dimer level, and antithrombin activity.
Table 2.
Coagulation and fibrinolytic markers of immune-mediated thrombotic thrombocytopenic purpura and septic disseminated intravascular coagulation.
| Coagulation/fibrinolytic marker | Reference | iTTPa | Septic DICa | P value |
|---|---|---|---|---|
| PT-INR | 0.90-1.15 | 1.08 (1.01-1.11) | 1.33 (1.11-1.60) | <.01 |
| aPTT (sec) | 24.0-37.7 | 31.0 (28.0-34.0) | 47.0 (32.3-82.5) | <.01 |
| Fibrinogen (mg/dL) | 200-400 | 310 (235-374) | 326 (213-553) | .73 |
| FDP (μg/mL) | <5.0 | 8.5 (4.3-16.9) | 43.4 (24.1-74.4) | <.01 |
| D-dimer (μg/mL) | <1.0 | 4.3 (1.5-7.7) | 17.9 (9.4-28.0) | <.01 |
| Antithrombin activity (%) | 80-100 | 100 (90-111) | 62 (48-81) | <.01 |
| TAT (ng/mL) | <3.9 | 9.6 (7.1-14.2) | 15.0 (7.5-28.3) | .11 |
| PIC (μg/mL) | <0.8 | 2.0 (1.2-2.6) | 2.5 (0.9-4.6) | .38 |
aPTT, activated partial thromboplastin time; DIC, disseminated intravascular coagulation; FDP, fibrin/fibrinogen degradation products; iTTP, immune thrombotic thrombocytopenic purpura; PIC, plasmin-α2 plasmin inhibitor complex; PT-INR, prothrombin time–international ratio; TAT, thrombin-antithrombin complex.
Each data point is described as median (first quartile to third quartile).
3.4. Haptoglobin and FXIII activity
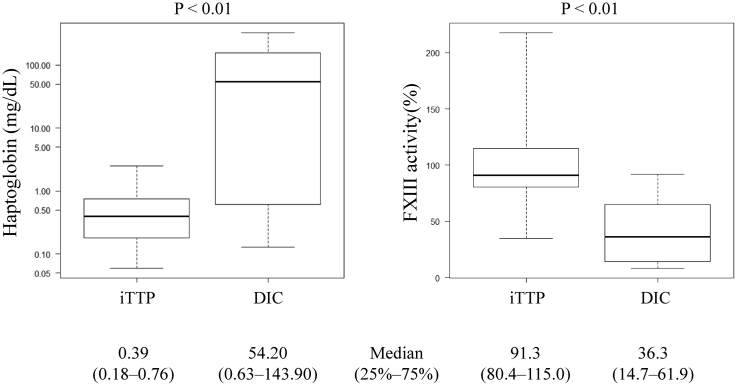
A comparison of the plasma haptoglobin and FXIII activity levels in the iTTP and septic DIC groups is shown in Figure 1. The reference levels of haptoglobin are 19 to 170 mg/dL and FXIII activity 70% to 140%. Haptoglobin was 0.39 mg/dL (0.18-0.76 mg/dL) in the iTTP group and 54.20 mg/dL (0.63-143.90 mg/dL) in the septic DIC group. A significant difference was observed between the 2 groups (P < .01). Plasma FXIII activity was 91.3% (80.4%-115.0%) in the iTTP group and 36.3% (14.7%-61.9%) in the septic DIC group. There was also a significant difference between the 2 groups.
Figure 1.
Comparison of haptoglobin and factor XIII (FXIII) activity in immune-mediated thrombotic thrombocytopenic purpura (iTTP) and septic disseminated intravascular coagulation (DIC) groups. There were significant differences in the haptoglobin levels and FXIII activity between the iTTP and septic DIC groups. The reference value for haptoglobin is 19 to 170 mg/dL, and FXIII activity is 70% to 140%.
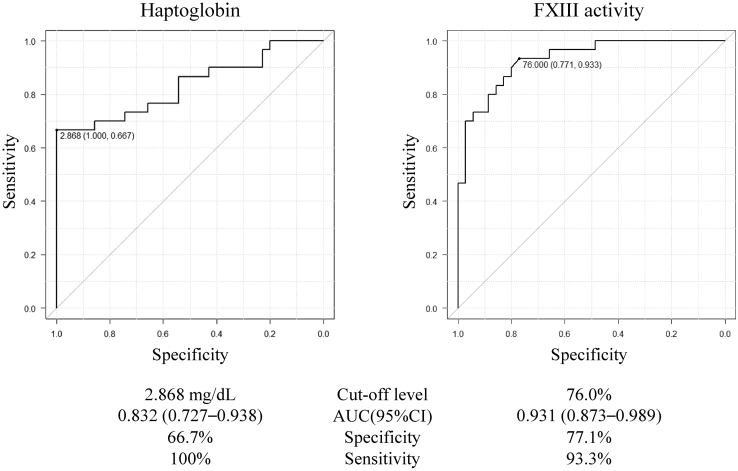
The receiver operating characteristic curves for haptoglobin and FXIII activity in the differential diagnosis of iTTP and septic DIC are shown in Figure 2. The cutoff level of haptoglobin was 2.868 mg/dL, and the AUC was 0.832 (95% CI: 0.727-0.938). All patients with iTTP had haptoglobin levels less than 2.868 mg/dL. In patients with septic DIC, 20 of the 30 patients (66.7%) had haptoglobin levels greater than 2.868 mg/dL, and 10 had levels less than 2.868 mg/dL. The cutoff level for FXIII activity was 76.0%, and the AUC was 0.931 (95% CI: 0.873-0.989). In patients with iTTP, 27 of the 35 patients (77.1%) had FXIII activity levels greater than 76.0%. In patients with DIC, 28 (93.3%) had FXIII activity levels of less than 76.0%.
Figure 2.
Receiver operating characteristic curve analysis for haptoglobin and factor XIII (FXIII) activity. The cutoff level, specificity, and sensitivity of both markers are shown in the figure. AUC, area under the curve.
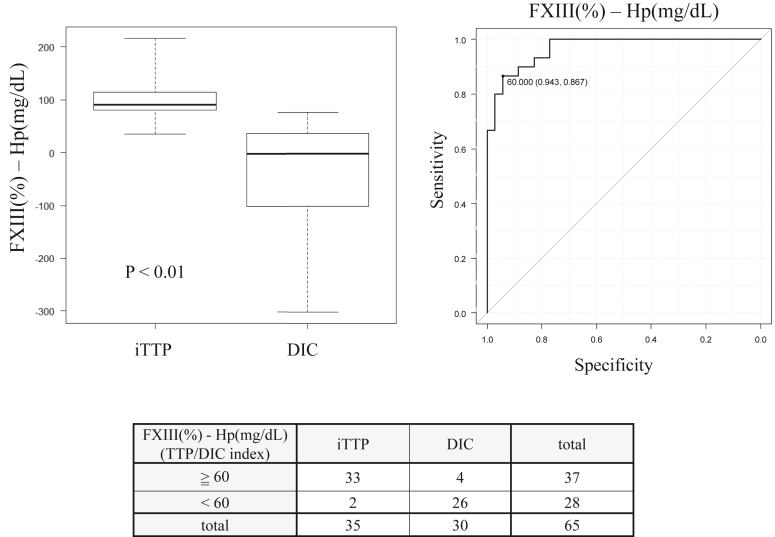
3.5. TTP/DIC index: FXIII activity (%)–haptoglobin (mg/dL)
The combination of haptoglobin and FXIII activity for the differential diagnosis of iTTP and septic DIC is shown in Figure 3. We developed a scoring system to differentiate iTTP from septic DIC using a combination of plasma FXIII activity and haptoglobin levels:
Figure 3.
Differentiation of immune-mediated thrombotic thrombocytopenic purpura (iTTP) and septic disseminated intravascular coagulation (DIC) by thrombotic thrombocytopenic purpura (TTP)/DIC index. The TTP/DIC index was defined as factor XIII (FXIII) activity (%)–haptoglobin (Hp) (mg/dL). The reference value for haptoglobin is 19 to 170 mg/dL, and plasma FXIII activity is 70% to 140%. The median index of iTTP was 90.8 (80.2-114.0) and the median index of septic DIC was −1.834 (−94.9 to 36.7). The area under the curve for the TTP/DIC index was 0.968 (95% CI: 0.935-1) and the cutoff level of TTP/DIC index was 60. Laboratory TTP was defined as an index ≥60, and laboratory DIC was defined as an index <60. Each data point was described as the median (first quartile to third quartile).
[TTP/DIC index = FXIII activity (%) − haptoglobin (mg/dL)]
The AUC for the TTP/DIC index was 0.968 (95% CI: 0.935-1) and the cutoff level of TTP/DIC index was 60. Laboratory TTP was defined as an index ≥60, and laboratory DIC was defined as an index <60. The accuracy of laboratory TTP in the iTTP group was 94.3% (33/35) and the accuracy of laboratory DIC in the septic DIC group was 86.7% (26/30).
3.6. Comparison between French scores and our index
The sensitivity and specificity of 1 or 2 points in French scores for the diagnosis of iTTP were 100% and 30.0%, respectively, whereas those of the 2 points were 77.1% and 86.7%, respectively (Supplementary Table 1). In contrast, the sensitivity and specificity of the TTP/DIC index were 94.3% and 86.7%, respectively.
4. Discussion
In this study, we analyzed the levels of plasma haptoglobin and FXIII activity in iTTP and DIC. We found that plasma haptoglobin was severely decreased in iTTP and normal to mildly decreased in DIC and that FXIII was decreased in DIC and normal in iTTP. We also mentioned the possibility of clear differentiation between iTTP and DIC by TTP/DIC index using these 2 markers.
For coagulation and fibrinolytic markers, there were significant differences between the iTTP group and the DIC group for PT-INR, aPTT, FDP, D-dimer, and antithrombin activity, similar to our previous report [5]. In septic DIC, the increase in PT-INR, prolonged aPTT, and decreased antithrombin activity were due to decreased coagulation factor attributable to hyperconsumption associated with overactive coagulation. FDP and D-dimer levels were increased because of fibrin thrombolysis. In particular, FDP, D-dimer, and antithrombin activity had high AUC, which is also similar to our previous report (Supplementary Table 2) [5]. These tests are routinely used in clinical practice and are easy to perform. Therefore, these coagulation markers can be used to easily distinguish iTTP from septic DIC. However, in practice, when using the ISTH criteria for DIC incorporating these coagulation markers, some patients with iTTP (10/35, 28.6%) had 5 points suggestive of DIC (Supplementary Figure). In addition, these markers can be easily varied. For example, vitamin K deficiency causes prolonged PT and aPTT; liver failure and undernutrition cause prolonged PT and aPTT and decreased antithrombin activity; extravasation due to inflammation causes decreased antithrombin activity; and pleural effusion, ascites, and hematoma cause an increase in FDP and D-dimer. Therefore, differentiating between iTTP and septic DIC using these markers alone may be unreliable. Although these markers may be highly useful for classifying DIC types [8,29,30], they were found to be insufficient for differentiating iTTP from septic DIC.
Both TTP and DIC form microvascular thromboses, but the site of thrombus formation differs. When red blood cells (RBCs) flowing rapidly in the artery collide with the thrombus, the collision causes mechanical disintegration of the RBCs, resulting in hemolysis [1]. In DIC, the thrombus is formed on the venous side, and the RBCs, in a slower blood flow, collide with the thrombus [10]; consequently, hemolysis is milder than that in TTP. In addition, the production of haptoglobin may be increased due to inflammation in septic DIC [31,32].
The cause of FXIII deficiency in DIC is believed to be FXIII hyperconsumption due to overactive coagulation. There have been some reports of reduced FXIII activity in DIC and reports discussing the use of FXIII preparations as a treatment for bleeding caused by DIC [33,34]. Furthermore, there is a report that FXIII activity was significantly lower in overt DIC than in nonovert DIC, and DIC scores and FXIII activity levels were inversely correlated [35]. On the other hand, there are no reports discussing FXIII activity levels in iTTP. Pathologically, platelet-rich thrombi are seen in TTP, whereas fibrin-rich thrombi are seen in DIC [36]. This implies that platelet activation plays an important role in platelet-rich thrombus formation during TTP, whereas coagulation activation plays an important role in fibrin-rich thrombus formation during DIC. The difference in the factors involved in thrombus formation may have influenced the differences in plasma FXIII activity levels.
ADAMTS-13 activity is the most important marker for differentiating between iTTP and DIC. However, it takes several days to obtain ADAMTS-13 activity results in most countries including Japan. The French and PLASMIC scores are used to predict severe ADAMTS-13 activity in iTTP, other form of TMA, or DIC in routine clinical situations. External validation of the PLASMIC score showed that the sensitivity and specificity of 6 or 7 points were 90% and 92%, respectively [37]. However, although the PLASMIC score is highly reliable, it may be somewhat complicated because of the large number of constituent items. The present results suggest that the TTP/DIC index based on haptoglobin and FXIII activity is comparable or even superior to the French and PLASMIC scores in sensitivity and specificity. In addition, both scores were reported to have reduced sensitivity at ≥60 years of age [38]. As we reported previously, Japanese patients with iTTP were much older than those reported from Western countries [39]. Therefore, there is a need for a reliable method for predicting severe ADAMTS-13 activity. Although a small number, the sensitivity and specificity of the TTP/DIC index at ≥60 years of age were 100% (17/17) and 87.0% (20/23), respectively. TTP/DIC index has high sensitivity and specificity even at ≥60 years of age.
This study has several limitations. First, we did not include enhanced fibrinolytic-type DIC [8], which is often seen in acute promyelocytic leukemia [8,40], aortic aneurysm [8,29,30], or vascular malformations [30,41]. The trends of plasma haptoglobin and FXIII in enhanced fibrinolytic–type DIC may differ from the results in septic DIC. Second, examinations of haptoglobin and FXIII activity can be performed using commercially available kits, which can provide prompt results. However, most hospitals are unable to perform such analyses in house. Finally, the number of patients analyzed in this study was small. In the development of a prediction platform, a validation cohort is usually used to confirm if the prediction model actually works, but we cannot perform the validation cohort. Furthermore, we compared iTTP without an underlying disease to septic DIC, which is difficult to differentiate from iTTP based on routine clinical data alone. Further studies are required to determine whether the TTP/DIC index is also applicable to iTTP with underlying disease and definite septic DIC.
In conclusion, a comparison of iTTP and septic DIC showed significant differences in haptoglobin levels and FXIII activity. As for haptoglobin, iTTP showed a significant decrease, whereas septic DIC showed a normal-to-mild decrease. Regarding FXIII activity levels, iTTP showed normal levels, whereas septic DIC showed a marked decrease. In addition, the TTP/DIC index, composed of 2 laboratory tests, is useful in differentiating iTTP from septic DIC.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Funding
This study is financially supported by a research grant from the Ministry of Health, Labour, and Welfare of Japan (20FC1024).
Author contributions
S.Y. performed the data and sample collection, experiments, and statistical analysis and wrote the manuscript. H.A. and T.M. interpreted the results and wrote the manuscript. M.K. and K.S. performed the data and sample collection. M.M. designed the study, interpreted the results, and wrote the paper.
Relationship Disclosure
M.M., the inventor of the Enzyme-Linked Immuno Sorbent Assay used to assess ADAMTS-13 activity, received funding from Chugai Pharmaceutical, and Asahi Kasei Pharma received a consultant fee from Sanofi. No other potential conflicts of interest relevant to this article were reported.
Footnotes
Funding information Ministry of Health, Labour, and Welfare of Japan, research grant: 20FC1024.
Handling Editor: Pantep Angchaisuksiri
The online version contains supplementary material available at https://doi.org/10.1016/j.rpth.2023.100076
Supporting Information
References
- 1.Sadler J.E. Pathophysiology of thrombotic thrombocytopenic purpura. Blood. 2017;130:1181–1188. doi: 10.1182/blood-2017-04-636431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furlan M., Robles R., Galbusera M., Remuzzi G., Kyrle P.A., Brenner B., et al. von Willebrand factor-cleaving protease in thrombotic thrombocytopenic purpura and the hemolytic-uremic syndrome. N Engl J Med. 1998;339:1578–1584. doi: 10.1056/NEJM199811263392202. [DOI] [PubMed] [Google Scholar]
- 3.Tsai H.M., Lian E.C. Antibodies to von Willebrand factor-cleaving protease in acute thrombotic thrombocytopenic purpura. N Engl J Med. 1998;339:1585–1594. doi: 10.1056/NEJM199811263392203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rock G.A., Shumak K.H., Buskard N.A., Blanchette V.S., Kelton J.G., Nair R.C., et al. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. Canadian Apheresis Study Group. N Engl J Med. 1991;325:393–397. doi: 10.1056/NEJM199108083250604. [DOI] [PubMed] [Google Scholar]
- 5.Sakai K., Wada H., Nakatsuka Y., Kubo M., Hayakawa M., Matsumoto M. Characteristics behaviors of coagulation and fibrinolysis markers in acquired thrombotic thrombocytopenic purpura. J Intensive Care Med. 2021;36:436–442. doi: 10.1177/0885066619899637. [DOI] [PubMed] [Google Scholar]
- 6.Zheng X.L., Vesely S.K., Cataland S.R., Coppo P., Geldziler B., Iorio A., et al. ISTH guidelines for the diagnosis of thrombotic thrombocytopenic purpura. J Thromb Haemost. 2020;18:2486–2495. doi: 10.1111/jth.15006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levi M., Ten Cate H. Disseminated intravascular coagulation. N Engl J Med. 1999;341:586–592. doi: 10.1056/NEJM199908193410807. [DOI] [PubMed] [Google Scholar]
- 8.Asakura H. Classifying types of disseminated intravascular coagulation: clinical and animal models. J Intensive Care. 2014;2:20. doi: 10.1186/2052-0492-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asakura H. Diversity of disseminated intravascular coagulation and selection of appropriate treatments. Int J Hematol. 2021;113:10–14. doi: 10.1007/s12185-020-03030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wada H., Matsumoto T., Suzuki K., Imai H., Katayama N., Iba T., et al. Differences and similarities between disseminated intravascular coagulation and thrombotic microangiopathy. Thromb J. 2018;16:14. doi: 10.1186/s12959-018-0168-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsumoto M., Fujimura Y., Wada H., Kokame K., Miyakawa Y., Ueda Y., et al. Diagnostic and treatment guidelines for thrombotic thrombocytopenic purpura (TTP) 2017 in Japan. Int J Hematol. 2017;106:3–15. doi: 10.1007/s12185-017-2264-7. [DOI] [PubMed] [Google Scholar]
- 12.Kristiansen M., Graversen J.H., Jacobsen C., Sonne O., Hoffman H.J., Law S.K., et al. Identification of the haemoglobin scavenger receptor. Nature. 2001;409:198–201. doi: 10.1038/35051594. [DOI] [PubMed] [Google Scholar]
- 13.Shih A.W.Y., McFarlane A., Verhovsek M. Haptoglobin testing in hemolysis: measurement and interpretation. Am J Hematol. 2014;89:443–447. doi: 10.1002/ajh.23623. [DOI] [PubMed] [Google Scholar]
- 14.Richardson V.R., Cordell P., Standeven K.F., Carter A.M. Substrates of Factor XIII-A: roles in thrombosis and wound healing. Clin Sci (Lond) 2013;124:123–137. doi: 10.1042/CS20120233. [DOI] [PubMed] [Google Scholar]
- 15.Muszbek L., Yee V.C., Hevessy Z. Blood coagulation factor XIII: structure and function. Thromb Res. 1999;94:271–305. doi: 10.1016/s0049-3848(99)00023-7. [DOI] [PubMed] [Google Scholar]
- 16.Ichinose A. Physiopathology and regulation of factor XIII. Thromb Haemost. 2001;86:57–65. [PubMed] [Google Scholar]
- 17.Menegatti M., Peyvandi F. Treatment of rare factor deficiencies other than hemophilia. Blood. 2019;133:415–424. doi: 10.1182/blood-2018-06-820738. [DOI] [PubMed] [Google Scholar]
- 18.Schroeder V., Kohler H.P. New developments in the area of factor XIII. J Thromb Haemost. 2013;11:234–244. doi: 10.1111/jth.12074. [DOI] [PubMed] [Google Scholar]
- 19.Johansson P.I., Sørensen A.M., Perner A., Welling K.L., Wanscher M., Larsen C.F., et al. Disseminated intravascular coagulation or acute coagulopathy of trauma shock early after trauma? An observational study. Crit Care. 2011;15:R272. doi: 10.1186/cc10553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujimura Y., Matsumoto M. Registry of 919 patients with thrombotic microangiopathies across Japan: database of Nara Medical University during 1998-2008. Intern Med. 2010;49:7–15. doi: 10.2169/internalmedicine.49.2706. [DOI] [PubMed] [Google Scholar]
- 21.Taylor F.B., Toh C.H., Hoots W.K., Wada H., Levi M. Scientific Subcommittee on Disseminated Intravascular Coagulation (DIC) of the International Society on Thrombosis and Haemostasis (ISTH). Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86:1327–1330. [PubMed] [Google Scholar]
- 22.Larsen J.B., Aggerbeck M.A., Granfeldt A., Schmidt M., Hvas A.M., Adelborg K. Disseminated intravascular coagulation diagnosis: positive predictive value of the ISTH score in a Danish population. Res Pract Thromb Haemost. 2021;5 doi: 10.1002/rth2.12636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cecconi M., Evans L., Levy M., Rhodes A. Sepsis and septic shock. Lancet. 2018;392:75–87. doi: 10.1016/S0140-6736(18)30696-2. [DOI] [PubMed] [Google Scholar]
- 25.Kato S., Matsumoto M., Matsuyama T., Isonishi A., Hiura H., Fujimura Y. Novel monoclonal antibody-based enzyme immunoassay for determining plasma levels of ADAMTS13 activity. Transfusion. 2006;46:1444–1452. doi: 10.1111/j.1537-2995.2006.00914.x. [DOI] [PubMed] [Google Scholar]
- 26.Fujimura Y., Matsumoto M., Yagi H., Yoshioka A., Matsui T., Titani K. Von Willebrand factor-cleaving protease and Upshaw-Schulman syndrome. Int J Hematol. 2002;75:25–34. doi: 10.1007/BF02981975. [DOI] [PubMed] [Google Scholar]
- 27.Kasper C.K., Aledort L., Aronson D., Counts R., Edson J.R., van Eys J., et al. Proceedings: a more uniform measurement of factor VIII inhibitors. Thromb Diath Haemorrh. 1975;34:612. [PubMed] [Google Scholar]
- 28.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamada S., Asakura H. Therapeutic strategies for disseminated intravascular coagulation associated with aortic aneurysm. Int J Mol Sci. 2022;23:1296. doi: 10.3390/ijms23031296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamada S., Asakura H. Management of disseminated intravascular coagulation associated with aortic aneurysm and vascular malformations. Int J Hematol. 2021;113:15–23. doi: 10.1007/s12185-020-03028-z. [DOI] [PubMed] [Google Scholar]
- 31.di Masi A., De Simone G., Ciaccio C., D’Orso S., Coletta M., Ascenzi P. Haptoglobin: from hemoglobin scavenging to human health. Mol Aspects Med. 2020;73 doi: 10.1016/j.mam.2020.100851. [DOI] [PubMed] [Google Scholar]
- 32.Lan P., Yu P., Ni J., Zhou J. Higher serum haptoglobin levels were associated with improved outcomes of patients with septic shock. Crit Care. 2022;26:131. doi: 10.1186/s13054-022-04007-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamada S., Okumura H., Morishita E., Asakura H. Complete hemostasis achieved by factor XIII concentrate administration in a patient with bleeding after teeth extraction as a complication of aplastic anemia and chronic disseminated intravascular coagulation. Blood Coagul Fibrinolysis. 2020;31:274–278. doi: 10.1097/MBC.0000000000000902. [DOI] [PubMed] [Google Scholar]
- 34.Kawano H., Yamamoto D., Uchihashi Y., Wakahashi K., Kawano Y., Sada A., et al. Severe inhibitor-negative acquired factor XIII/13 deficiency with aggressive subdural haemorrhage. Blood Coagul Fibrinolysis. 2013;24:638–641. doi: 10.1097/MBC.0b013e32835facef. [DOI] [PubMed] [Google Scholar]
- 35.Song J.W., Choi J.R., Song K.S., Rhee J.H. Plasma factor XIII activity in patients with disseminated intravascular coagulation. Yonsei Med J. 2006;47:196–200. doi: 10.3349/ymj.2006.47.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawasaki H., Hayashi K., Awai M. Disseminated intravascular coagulation (DIC). Immunohistochemical study of fibrin-related materials (FRMs) in renal tissues. Acta Pathol Jpn. 1987;37:77–84. [PubMed] [Google Scholar]
- 37.Li A., Khalighi P.R., Wu Q., Garcia D.A. External validation of the PLASMIC score: a clinical prediction tool for thrombotic thrombocytopenic purpura diagnosis and treatment. J Thromb Haemost. 2018;16:164–169. doi: 10.1111/jth.13882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu A., Dhaliwal N., Upreti H., Kasmani J., Dane K., Moliterno A., et al. Reduced sensitivity of PLASMIC and French scores for the diagnosis of thrombotic thrombocytopenic purpura in older individuals. Transfusion. 2021;61:266–273. doi: 10.1111/trf.16188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsumoto M., Bennett C.L., Isonishi A., Qureshi Z., Hori Y., Hayakawa M., et al. Acquired idiopathic ADAMTS13 activity deficient thrombotic thrombocytopenic purpura in a population from Japan. PLoS One. 2012;7 doi: 10.1371/journal.pone.0033029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ikezoe T. Advances in the diagnosis and treatment of disseminated intravascular coagulation in haematological malignancies. Int J Hematol. 2021;113:34–44. doi: 10.1007/s12185-020-02992-w. [DOI] [PubMed] [Google Scholar]
- 41.Yamada S., Arahata M., Morishita E., Asakura H. Blue rubber bleb nevus syndrome complicated by enhanced-fibrinolytic-type DIC: a case report. Ann Vasc Dis. 2021;14:252–255. doi: 10.3400/avd.cr.20-00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.