Abstract
Background
Dyspnoea is a common persistent symptom after COVID-19. Whether it is associated with functional respiratory disorders remains unclear.
Methods
We assessed the proportion and characteristics of patients with “functional respiratory complaints” (FRCs) (as defined by Nijmegen Questionnaire >22) among 177 post-COVID-19 individuals who benefited from outclinic evaluation in the COMEBAC study (i.e., symptomatic and/or intensive care unit (ICU) survivors at 4 months). In a distinct explanatory cohort of 21 consecutive individuals with unexplained post-COVID-19 dyspnoea after routine tests, we also analysed the physiological responses to incremental cardiopulmonary exercise testing (CPET).
Findings
In the COMEBAC cohort, 37 patients had significant FRCs (20.9%, IC95: 14.9–26.9). The prevalence of FRCs ranged from 7.2% (ICU patients) to 37.5% (non-ICU patients). The presence of FRCs was significantly associated with more severe dyspnoea, lower 6-min walk distance, more frequent psychological and neurological symptoms (cognitive complaint, anxiety, depression, insomnia and post-traumatic stress disorders) and poorer quality of life (all p<0.01). In the explanatory cohort, seven out of 21 patients had significant FRCs. Based on CPET, dysfunctional breathing was identified in 12 out of 21 patients, five out of 21 had normal CPET, three out of 21 had deconditioning and one out of 21 had evidence of uncontrolled cardiovascular disease.
Interpretation
FRCs are common during post-COVID-19 follow-up, especially among patients with unexplained dyspnoea. Diagnosis of dysfunctional breathing should be considered in those cases.
Short abstract
Functional respiratory complaints are common during post-COVID-19 follow-up, especially among patients with unexplained dyspnoea. Diagnosis of dysfunctional breathing should be considered in those cases. https://bit.ly/3HXWaCq
Introduction
As the world faces the pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), there is increasing evidence of the long-term consequences of coronavirus disease 2019 (COVID-19) [1]. The various symptoms and organ-related injuries have been referred to as “post-acute COVID-19 syndrome” [2]. Carfì et al. [3] reported that 87.4% of COVID-19 patients discharged from hospital still had at least one symptom after a mean of 60 days, the most frequent being fatigue (53.1%) and dyspnoea (43.4%). Likewise, Garrigues et al. [4] reported that most patients requiring hospitalisation for COVID-19 still had persistent symptoms 110 days after being discharged, particularly fatigue (55%) and dyspnoea (42%). In the prospective COMEBAC (COnsultation Multi-Expertise de Bicêtre Après COVID-19) cohort (NCT04704388) evaluating COVID-19 survivors 4 months after hospitalisation in a university hospital in the Paris region (France), 51% of the patients declared at least one symptom that did not exist before COVID-19 [5]. The underlying mechanisms of post-COVID-19 dyspnoea remain unclear. In the present study we investigated post-COVID-19 “functional respiratory complaints” (FRCs) using the Nijmegen Questionnaire. As mentioned by van Dixhoorn and Folgering [6] who described this concept, the word “respiratory” refers to ventilation, dyspnoea and breathing movement; the word “functional” refers to the relationship with stress and anxiety. The presence of FRCs is associated with the diagnosis of dysfunctional breathing (DB), of which hyperventilation syndrome (HVS) is a well-known form [6, 7]. On the one hand, dyspnoea is a subjective symptom that poorly correlates with radiological findings among COVID-19 survivors [8], and HVS has been suggested as a cause of exercise intolerance among COVID-19 survivors [9]. On the other hand, there is evidence of long-term organic injuries that result in interstitial lung disease and impaired gas diffusion several months after the infection [10]. The objectives of this study were: 1) to investigate the proportion and characteristics of patients with FRCs after hospital discharge in the context of post-COVID-19 follow-up (COMEBAC study); and 2) to analyse the physiological responses to incremental cardiopulmonary exercise testing (CPET) in patients presenting with post-COVID-19 unexplained dyspnoea.
Material and methods
Patients and study design
The main cohort consisted of 177 patients from the COMEBAC study [5] who had been hospitalised in Bicêtre University Hospital (Université Paris-Saclay, AP-HP, France) during the first epidemic wave in France. They were evaluated at the outpatient facility 4 months after hospital discharge in the context of persistent symptoms and/or as a systematic follow-up after ICU management (supplementary figure S1). Psychological, cognitive and respiratory characteristics of patients with or without FRCs were compared. Details and thresholds of questionnaires and tests used for psychological, cognitive and respiratory assessment are presented in supplementary table 1.
The explanatory cohort consisted of 21 distinct, consecutive patients who had new or worsened dyspnoea 6 months after discharge from Bicêtre University Hospital for COVID-19 management during the second epidemic wave in France. They were offered CPET in the context of unexplained dyspnoea after completing routine tests at rest (i.e., detection for hypoxaemia and anaemia, computed tomography (CT) scan of the chest and pulmonary function tests, see details below).
All patients provided written informed consent to participate. This study was approved by The Ethics Committee of the French Intensive Care Society (CE20-56).
Respiratory assessment
We used the Nijmegen Questionnaire as a measure of FRCs, as has been suggested by the authors who initially elaborated this questionnaire [6]. A threshold >22 out of 64 defined patients with significant FRCs [11]. The functional impact of dyspnoea was evaluated using the modified Medical Research Council (mMRC) scale (supplementary table 2). A 6-min walk test was performed according to current recommendations [12].
Patients completed standard pulmonary function tests (PFTs) with spirometry, whole-body plethysmography and single-breath diffusing capacity of the lung for carbon monoxide (DLCO) according to the European Respiratory Society/American Thoracic Society (ERS/ATS) guidelines [13]. Forced vital capacity, forced expiratory volume in 1 s, total lung capacity and DLCO were expressed as percentages of predicted values using the Global Lung Function Initiative (GLI) 2012 [14] and European Community for Coal and Steel (ECCS) 1993 equations [15, 16]. A high-resolution lung CT scan was performed for all patients and blindly reviewed by two radiologists, who reached a consensus regarding any disagreements.
Hyperventilation provocation test
In the COMEBAC study, all patients with positive Nijmegen Questionnaire were offered a hyperventilation provocation test (HVPT). End-tidal carbon dioxide tension (PETCO2) was monitored with a single-use nasal cannula connected to a gas analyser through a sampling system (Perma Pure®; Perma Pure, Lakewood, NJ, USA), and tidal volume and respiratory rate (RR) were assessed breath-by-breath using a turbine flowmeter adapted with a silicon face mask (Cosmed Quark CPET; Cosmed, Rome, Italy). A 3-min baseline recording period of quiet breathing was followed by a 3-min voluntary hyperventilation period designed to reach both an RR >30·breaths·min−1 and a PETCO2 ≤20 mmHg. If the patient could not maintain these criteria because of clinical intolerance, the manoeuvre was interrupted before the end of the 3-min period. After the hyperventilation period, patients were instructed to breathe normally for 6 min. Patients were then asked to list the symptoms experienced during the test. The HVPT was considered positive if at least two daily symptoms were reproduced and/or in case of abnormal PETCO2 kinetic (PETCO2 <67% at 3 min and/or <91% at 5 min from baseline), as described elsewhere [17].
Cardiopulmonary exercise testing
All patients from the explanatory cohort underwent a maximal symptom-limited incremental exercise on a cycle ergometer (Quark CPET, Cosmed, Italy). The following data were recorded: 1 min of rest period, followed by 3 min of warmup with minimal workload and incrementally increased load until the patient reached maximum exhaustion, or until the physician stopped the test due to safety concerns. The work rate increment was estimated to attain maximal exertion after 8–12 min of loaded exercise (range from 10 to 30 W·min−1). Spiroergometric variables were measured using breath-by-breath analysis and included oxygen consumption (V′O2), output of carbon dioxide (V′CO2), PETCO2, tidal volume (VT), breathing frequency and minute ventilation (V′E) from which was derived the V′E/V′CO2 ratio. As previously suggested by other authors [18], the CPET pattern was suggestive of DB in the absence of cardiac, ventilatory, gas exchange or metabolic abnormality associated with one or more of the following features: high V′E/V′CO2 (>35 at 40 W), low PETCO2 (<30 mmHg) both at rest and during work; erratic VT and/or RR response to workload. Deconditioning was defined as reduced oxygen uptake at peak exercise (peak V′O2 <80%), without cardiocirculatory impairment or ventilatory limitation.
Psychiatric, cognitive and general assessment
Global cognitive assessment was performed through the Montreal Cognitive Assessment (MoCA) adapted to age and education level, and attention was assessed through the d2-R test. Memory complaints were assessed through the McNair self-questionnaire and personal interview with a neuropsychologist. A cognitive complaint was defined by a low McNair score, reports of cognitive symptoms or both. Cognitive impairment was defined by an impairment of either the MoCA or the d2-R score.
Anxiety symptoms were evaluated through the anxiety subscale of the Hospital Anxiety and Depression Scale (HADS-A); depression symptoms through the 13-item Beck Depressive Inventory (BDI-13) score; and post-traumatic symptoms through the Post-traumatic Stress Disorder (PTSD) Checklist for DSM-5 (PCL-5). Insomnia was evaluated through the Insomnia Severity Index (ISI). Psychiatric symptoms were defined as HADS-A >7 or BDI-13 >7 or PCL-5 >30 [5]. Quality of life was assessed through the 36-item short-form health survey (SF-36).
Statistical analysis
Study data were collected and managed with Research Electronic Data Capture tools hosted at Assistance Publique – Hôpitaux de Paris. Raw data were extracted with Omnia software (Cosmed, Italy). For the RR and PETCO2, the mean values obtained every 10 s were plotted against time. For tidal volumes, instantaneous values were used to detect deep sighing. Out-of-ranges values were all displayed and analysed, and automatic curve smoothing was applied. No assumption was made for missing values. Quantitative data are expressed as the mean±SD or median (interquartile range (IQR): first quartile to third quartile), according to the normality of the distribution. Qualitative data are expressed as the number of occurrences, i.e., n (%). To compare continuous variables between two groups, the t-test or Mann–Whitney U-test (if the variables were not normally distributed) was used. Pearson's chi-squared test or Fisher's exact test, as appropriate, was used to compare discrete variables between two groups. The most relevant variables associated with DB with a p-value <0.20 in the bivariate analysis were entered in a multivariable logistic regression model. The adjusted odds ratio (OR) of DB and the 95% confidence interval (95% CI) were calculated for all independent factors associated with DB. Statistical analyses were performed with R (version 4.01, http://cran.rproject.org). All p-values were two-sided, and values <0.05 were deemed statistically significant.
Results
Main cohort (COMEBAC)
General characteristics
Among the 177 patients (97 ICU patients and 80 non-ICU patients) evaluated in the outpatient clinic, 37 (20.9%, IC95: 14.9–26.9) had significant FRCs (7.2% in ICU patients and 37.5% in non-ICU patients). Compared with the rest of the population evaluated in the outpatient clinic (n=140), these patients were more often female (59.5% versus 32.9%, p<0.01) but had a similar age, body mass index and degree of tobacco exposure. COVID-19-related comorbidities did not differ significantly between the two groups (table 1).
TABLE 1.
Baseline and hospitalisation characteristics according to Nijmegen score
| Nijmegen score ≤22 | Nijmegen score >22 | p-value | |
| Patients n | 140 | 37 | |
| Age years | 57.3±13.7 | 55.2±11.1 | 0.39 |
| Female | 46 (32.9) | 22 (59.5) | <0.01 |
| Body mass index kg·m−2 | 28.9±5.1 | 30.5±6.4 | 0.28 |
| Obesity (>30 kg·m−2) | 53 (37.9) | 14 (41.2) | 0.99 |
| Hypertension | 63 (45.0) | 12 (32.4) | 0.17 |
| Diabetes | 43 (30.7) | 9 (24.3) | 0.45 |
| Chronic kidney disease | 17 (3.9) | 0 | - |
| COPD | 4 (2.9) | 1 (2.7) | 0.61 |
| Asthma | 13 (9.3) | 7 (18.9) | 0.17 |
| Smoking (n=169) | 0.64 | ||
| Active | 13 (9.6) | 2 (5.9) | |
| Former (>5 pack-years) | 21 (15.6) | 4 (11.8) | |
| No (<5 pack-years) | 101 (74.8) | 28 (82.4) | |
| Management of COVID-19 | |||
| Duration of hospitalisation days | 13 (6–23) | 7.5 (3–15) | <0.01 |
| Pulmonary embolism | 25 (17.9) | 4 (10.8) | 0.30 |
| Corticosteroids | 7 (5.0) | 0 | - |
| COVID-19-related ICU admission | 90 (64.3) | 7 (18.9) | <0.001 |
| Intubation | 48 (34.3) | 3 (8.1) | <0.01 |
| HFNC | 7 | 1 | 0.88 |
| ECMO | 7 | 1 | 0.88 |
Data are presented as n (%), mean±sd or median (IQR) unless otherwise stated. ICU: intensive care unit; HFNC: high-flow nasal cannula; ECMO: extracorporeal membrane oxygenation.
Respiratory assessment
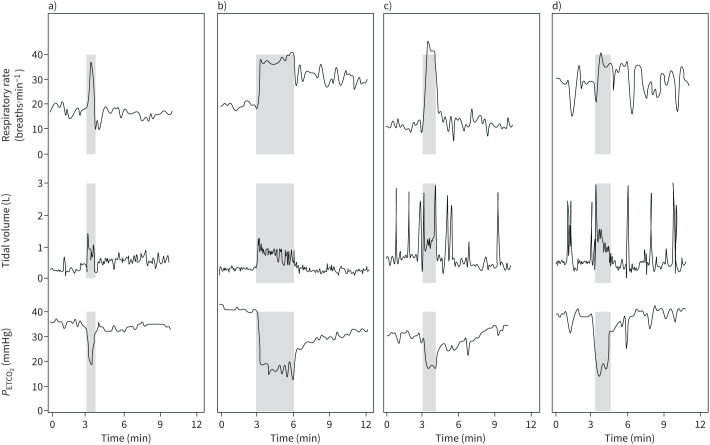
As shown in table 2, patients with FRCs reported a more significant functional impact of their dyspnoea, with 13.5%, 67.6% and 18.9% having an mMRC score of 0, 1–2 and 3–4, respectively, compared to 58.6%, 36.4% and 5% among the other patients (p<0.001). The distance covered during the 6-min walk test was shorter among patients with FRCs (404 versus 474 m, p<0.01). Cough was significantly more frequently observed in patients with FRCs (30.6% versus 8.8%, p<0.01). At revaluation, patients with FRCs were more likely to have normal CT scans of the chest (58.3% versus 31.1%, p<0.01). Persistent ground glass opacities and fibrotic lesions were observed in 37.1% and 5.7% of patients with FRCs, respectively, versus 45.9% (p<0.01) and 23.0% (p<0.01) of patients without FRCs. The results of the PFTs did not significantly differ between the two groups (table 2). Details regarding the distribution of each item of the Nijmegen Questionnaire in the 37 patients with FRCs are given in table 3. “Shortness of breath” was the most reported item, with 81% of patients describing this sensation as occurring “often” or “very often”; “anxiety” was the second most frequent finding, followed by “unable to breathe deeply” and “palpitations”. Abnormal responses to HVPT were found in 21 out of 25 (84%) patients, thus representing 12% of the patients evaluated at the outpatient clinic. Typical examples of abnormal breathing patterns are shown in figure 1.
TABLE 2.
Results of the in-person outpatient clinic visit according to Nijmegen score
| Nijmegen score ≤22 | Nijmegen score >22 | p-value | |
| Patients n | 140 | 37 | |
| mMRC (0–4) (n=177) | <0.001 | ||
| 0 | 82 (58.6) | 5 (13.5) | |
| 1–2 | 51 (36.4) | 25 (67.6) | |
| 3–4 | 7 (5) | 7 (18.9) | |
| Cough (n=172) | 12 (8.8) | 11 (30.6) | <0.01 |
| Lung CT scan (n=171) | <0.01 | ||
| Normal lung CT scan | 42 (31.1) | 21 (58.3) | |
| Persistent ground-glass opacities | 62 (45.9) | 13 (37.1) | |
| Lung fibrotic lesions | 31 (23) | 2 (5.7) | |
| Pulmonary function tests (n=157) | |||
| FEV1 % pred | 91.4±18.6 | 88.7±14.8 | 0.37 |
| FVC % pred | 89.7±16.4 | 87.0±16.5 | 0.40 |
| FEV1/FVC | 82.1±7.7 | 82.2±6.5 | 0.96 |
| TLC % pred | 82.4±15.7 | 84.2±13.5 | 0.51 |
| DLCO % pred | 86.7±22.7 | 88±20.5 | 0.70 |
| Obstructive pattern | 5 (4.1) | 1 (2.9) | 0.84 |
| Restrictive pattern | 55 (47.4) | 12 (36.4) | 0.26 |
| DLCO <70% pred | 27 (22.7) | 6 (18.2) | 0.93 |
| 6MWT distance m | 474 (395–516) | 404 (338–472) | <0.01 |
| Psychological and neurological assessment | |||
| Cognitive complaint (n=159) | 55 (43.7) | 24 (72.7) | <0.01 |
| Cognitive impairment (n=159) | 48 (38.1) | 13 (39.4) | 1.00 |
| Symptoms of anxiety (HADS-A) (n=169) | 32 (23.9) | 21 (61.8) | <0.001 |
| Symptoms of depression (BDI-13) (n=170) | 20 (14.7) | 14 (43.8) | <0.01 |
| Insomnia (ISI) (n=168) | 64 (47.8) | 26 (76.5) | <0.01 |
| Symptoms of PTSD (PCL-5) (n=169) | 12 (8.9) | 12 (35.3) | <0.01 |
| 36-item Short-form Health Survey (n=130) | |||
| Physical functioning | 80 (55–90) | 50 (35–65) | <0.001 |
| Role physical | 50 (25–100) | 25 (0–25) | <0.01 |
| Mental health | 66.7 (33.3–100) | 33.3 (0–66.7) | <0.01 |
| Vitality | 56.2 (37.5–75) | 31.2 (25–37.5) | <0.001 |
| Role emotional | 80 (65–90) | 55 (40–55) | <0.001 |
| Social functioning | 75 (50–100) | 50 (37.5–62.5) | <0.001 |
| Bodily pain | 83 (66.5–100) | 29 (16.5–58) | <0.001 |
| General health | 60 (45–80) | 35 (25–60) | <0.001 |
Data are presented as n (%), mean±sd or median (IQR) unless otherwise stated. Cognitive complaint was defined as an impaired McNair score, reported cognitive symptoms or both. Cognitive impairment was defined as an impairment of either the MoCA or d2-R score. Symptoms of anxiety are defined as a HADS-A score >7. Symptoms of depression were defined as a BDI-13 test score >7. Insomnia was defined as an ISI >7, and PTSD was defined as a PCL-5 score >30 (supplementary table 1). The European Community for Coal and Steel (ECCS) reference values were used for lung volume and TLC was expressed without adjustment for ethnicity. mMRC: modified Medical Research Council; CT: computed tomography; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; TLC: total lung capacity; DLCO: diffusing capacity of the lung for carbon monoxide; 6MWT: 6-min walk test; HADS-A: Hospital Anxiety and Depression Scale; BDI-13: Beck Depression Inventory-13 items; ISI: Insomnia Severity Index; PTSD: post-traumatic stress disorder; PCL-5: Post-traumatic Stress Disorder Checklist for DSM-5.
TABLE 3.
Detailed results of the Nijmegen Questionnaire item in the 37 patients with a score >22
| Item | Mean±sd | Median (IQR) | Sum |
| Shortness of breath | 3.13±0.85 | 3 (3–4) | 75 |
| Anxiety | 2.42±1.53 | 3 (1–4) | 58 |
| Unable to breathe deeply | 2.29±1.20 | 2 (2–3) | 55 |
| Palpitations | 2.29±1.20 | 2 (1.75–3) | 55 |
| Bloated abdominal sensation | 2.21±1.22 | 2 (2–3) | 53 |
| Constricted chest | 2.08±1.10 | 2 (2–3) | 50 |
| Chest pain | 2.08±1.02 | 2 (1.75–3) | 50 |
| Accelerated or deepened breathing | 2.04±0.91 | 2 (2–3) | 49 |
| Dizzy spells | 1.88±0.90 | 2 (1–3) | 45 |
| Cold hands or feet | 1.79±1.14 | 2 (1–3) | 43 |
| Feeling tense | 1.75±1.11 | 2 (1–3) | 42 |
| Tingling fingers | 1.71±1.16 | 2 (1–2.25) | 41 |
| Blurred vision | 1.58±1.02 | 2 (1–2) | 38 |
| Stiffness of fingers or arm | 1.58±1.32 | 1.5 (0.75–2) | 38 |
| To be confused, losing touch with environment | 1.21±1.02 | 1 (0–2) | 29 |
| Tightness around the mouth | 1.17±1.09 | 1 (0–3) | 28 |
| Total score | 31.21±5.05 | 29.5 (27–35) | 749 |
Each item was quantified as 0: never, 1: rarely, 2: sometimes, 3: often, 4: very often.
FIGURE 1.
Representative examples of positive hyperventilation provocation test (HVPT) from four patients with normal pulmonary function tests and lung computed tomography scans at evaluation in the outpatient clinic. The hyperventilation manoeuvre (grey area) began at the 3rd min and was interrupted at the 6th min or when clinical intolerance was reached. The first 3 min and the last 6 min characterise the breathing pattern at rest. a) Premature interruption of the hyperventilation manoeuvre. The HVPT provoked a rapid reproduction of daily symptoms with major discomfort that led to premature interruption of the hyperventilation (HV) manoeuvre. The patient's breathing pattern was considered normal, as the mean respiratory rate at rest was <20 breaths·min−1 (upper panel) and the tidal volume remained stable without hyperpnoea or deep sighing (middle panel), allowing quick recovery of the baseline end-tidal carbon dioxide tension (PETCO2) after HV (lower panel). b) Hyperventilation. After completion of the HV manoeuvre, an abnormal breathing pattern appeared with persistent tachypnoea that reached 30 breaths·min−1 even after 6 min of resting breathing (upper panel). Tidal volumes were normal (middle panel). The recovery of PETCO2 was delayed and it remained below its baseline value at the end of the test (lower panel). c) and d) Deep sighing. The HVPT provoked a rapid reproduction of daily symptoms with major discomfort that led to premature interruption of the HV manoeuvre. The patient's breathing pattern consisted of either normal (c) or increased (d) respiratory rate at rest (upper panel) with frequent deep sighs that resulted in several spikes on the volume–time curve (middle panel) which are mirrored by transient drops in the PETCO2 (lower panel).
Quality of life, psychiatric and cognitive assessment
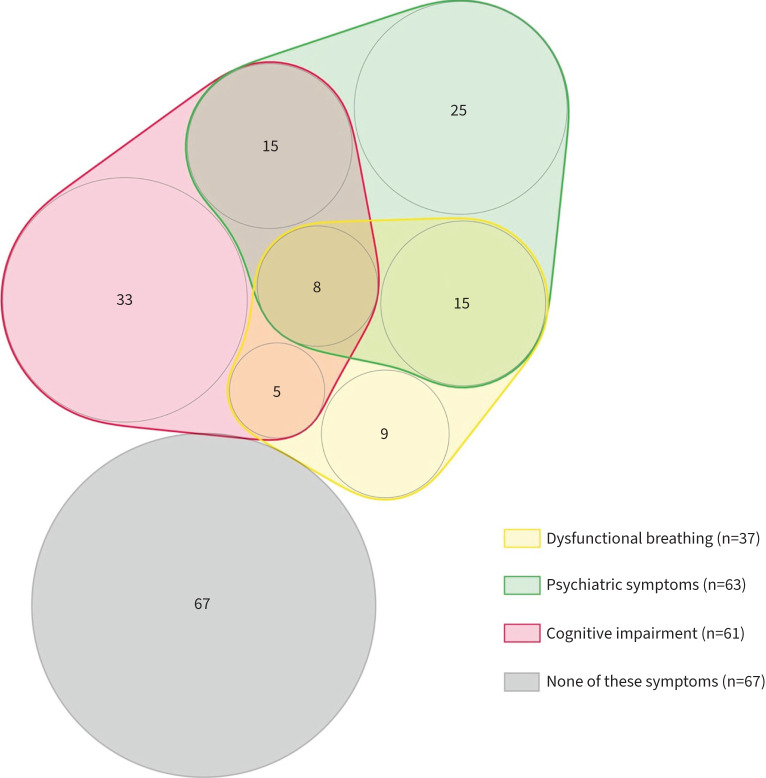
Patients with FRCs reported a poorer quality of life throughout the eight dimensions of the SF-36 score (physical functioning, role physical, mental health, vitality, role emotional, social functioning, bodily pain, general health) (all p<0.01). Having FRCs was associated with more cognitive complaints (61.8% versus 23.9%, p<0.001), but no difference was observed in cognitive impairment (39.4% and 38.1%, respectively). Symptoms of anxiety (HADS-A), depression (BDI-13), post-traumatic symptoms (PCL-5) and insomnia (ISI) were significantly increased in patients with FRCs (table 2). Figure 2 shows a visualisation of symptoms in the 37 patients with FRCs at the outpatient clinic 4 months after COVID-19 hospitalisation.
FIGURE 2.
Visualisation of symptoms in 37 patients with functional respiratory complaints (i.e. Nijmegen score >22) at the outpatient clinic 4 months after COVID-19 hospitalisation. Numbers represent patients with the symptoms or association of symptoms; 67 patients did not report these symptoms. Psychiatric symptoms were defined as Hospital Anxiety and Depression Scale >7, 13-item Beck Depressive Inventory >7 or Post-Traumatic Stress Disorder Checklist for DSM-V >30.
Multivariate analysis
In patients evaluated in the outpatient clinic, the following variables were considered clinically relevant and included in the multivariate analysis: sex, ICU admission, cognitive complaint, psychiatric symptoms and pathological CT scan of the chest at revaluation. The following factors were independently associated with higher risk of FRCs: having cognitive complaints (OR=3.41, IC95=1.32–9.58, p=0.014) and psychiatric symptoms (OR=3.19, IC95=1.23–8.68, p=0.019). ICU admission was not associated with higher risk of FRCs (OR=0.15, IC95=0.05–0.45, p=0.001) (table 4).
TABLE 4.
Multivariate analysis
| Variable | Odds ratio (95% CI) | p-value |
| Sex (male) | 0.85 (0.33–2.27) | 0.748 |
| ICU admission | 0.15 (0.05–0.45) | 0.001 |
| Cognitive complaints | 3.41 (1.32–9.58) | 0.014 |
| Psychiatric symptoms# | 3.19 (1.23–8.68) | 0.019 |
| Pathological CT scan of the chest | 0.78 (0.29–2.16) | 0.625 |
ICU: intensive care unit; CT: computed tomography;. #: psychiatric symptoms were defined as Hospital Anxiety and Depression Scale >7 or 13-item Beck Depression Inventory >7 or Post-Traumatic Stress Disorder Checklist for DSM-V >30.
Explanatory cohort
General characteristics
The 21 consecutive patients who reported new or worsened dyspnoea at 6 months had a mean age of 55±10 years; 12 out of 21 were women. 11 reported at least grade 2 on the mMRC scale. The mean±SD Nijmegen Questionnaire in the overall population was 22±11. 16 had no evidence of organ damage on routine tests; the five other patients had disproportionate dyspnoea with regard to their tests (three had mild ground-glass lesions on CT scan, one had chronic and stable anaemia due to β-thalassaemia and one had isolated mild hypoxaemia at rest). Overall, seven patients (33%) had a Nijmegen Questionnaire >22 indicating significant FRCs.
Results of CPET
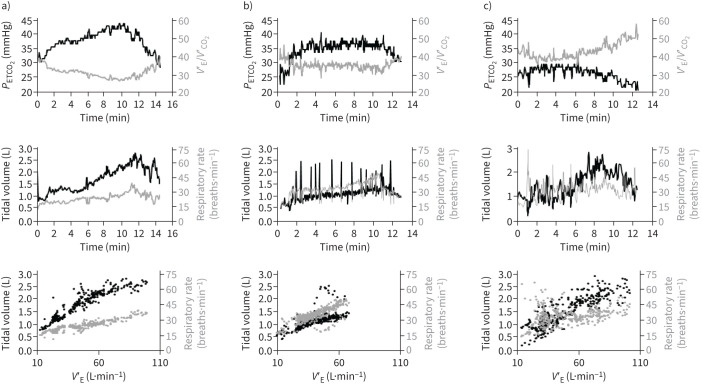
Among the 21 patients with unexplained dyspnoea, none had evidence of effort hypoxaemia; 12 had anomalies consistent with DB, five had normal CPET and three had evidence of deconditioning (associating low value of peak V′O2, decreased oxygen pulse and early anaerobic threshold). One patient had symptomatic systemic hypertension leading to premature interruption of exercise. Among patients with DB, 10 out of 12 had PETCO2 <30 mmHg at rest and during exercise; 10 out of 12 had increased V′E/V′CO2 (>35 at 40 W) and 11 out of 12 had evidence of erratic breathing pattern, including two patients (17%) with deep sighs. Representative examples are presented in figure 3.
FIGURE 3.
Representative examples of breathing patterns during cardiopulmonary exercise testing (CPET) in three patients with post-COVID-19 unexplained dyspnoea. a) Normal CPET. End-tidal carbon dioxide tension (PETCO2) >30 mmHg both at rest and during exercise; minute ventilation (V′E)/carbon dioxide production (V′CO2) <35 at 40 W (upper panel); predictable pattern of breathing frequency and tidal volume increases (middle and lower panels). b) Dysfunctional breathing with deep sighs. PETCO2 is broadly normal and V′E/V′CO2 is just above the limit of 35 at 40 W (upper panel); breathing pattern response is abnormal with typical deep sighing as reflected by spikes on the volume–time curve (middle and lower panels). c) Dysfunctional breathing with hyperventilation. PETCO2 <30 mmHg both at rest and during exercise; V′E/V′CO2 >35 regardless of power (upper panel); erratic breathing pattern (middle and lower panel).
Discussion
In the context of the COMEBAC study, patients underwent extensive workup in an outpatient clinic, including multidimensional dyspnoea assessments, PFTs, chest CT scans, HVPT, and psychiatric symptoms and cognitive evaluation. Implementing the Nijmegen Questionnaire, we found that 20.9% of post-COVID-19 patients had significant FRCs 4 months after hospital discharge. 12 of 21 patients with post-COVID-19 unexplained dyspnoea at 6 months showed evidence of DB on CPET. Taken together, these results support the idea that functional respiratory disorders should not be overlooked during COVID-19 follow-up.
The prevalence of FRCs is higher than the prevalence of 9.5% previously reported in a general primary care population [19] but lower than in other conditions such as difficult-to-treat asthma (47%) [20]. Consistent with the previously reported sex ratio imbalance [19], 59.5% of patients with FRCs were female. Despite the lower number of pathological CT scans and similar DLCO values, individuals with FRCs were more likely to report severe breathlessness. Using HVPT and breath-by-breath analysis we were able to identify abnormal breathing patterns in most cases. Notably, some patients displayed a pattern of isolated “deep sighing”, which is thought to be related to anxiety state [21]. The major strength of this study is to provide a detailed assessment of psychological and neurological symptoms, and quality of life and their relationships with FRCs. Our study demonstrates that FRCs are strongly associated with symptoms of anxiety and depression, post-traumatic stress disorders and cognitive complaints. However, whether FRCs are causative or secondary effects of psychiatric symptoms remains uncertain. Some authors have suggested that it could be the consequence of severe psychological trauma [22], while others have emphasised the role of underlying organic respiratory diseases such as asthma [23]. Our results do not support a major role of altered COVID-19-related lung properties in the pathophysiology of FRCs, since patients with DB had less severe disease at the acute phase and infrequent fibrotic sequelae. To note, mental disorders are risk factors of COVID-19 [24], and psychiatric symptoms are broadly reported in COVID-19 survivors [25, 26]. The overlap between FRCs and anxiety symptoms [27] could also explain, at least in part, the high rate of FRCs in COVID-19 survivors.
Since SARS-CoV-2 has neuro-invasive potential [28], other hypotheses can be proposed to explain post-COVID-19 FRCs. First, SARS-CoV-2-mediated neuronal inflammation might interfere with the respiratory drive since the viral receptor angiotensin-converting enzyme 2 is found in the brainstem nuclei involved in the regulation of ventilation [29]. Second, COVID-19 can trigger several neuropsychiatric manifestations, including anxiety [28], which was strongly correlated with FRCs in our study. We found indeed that psychiatric symptoms were independently associated with FRCs. Direct viral infiltration of the central nervous system and immune-based reactions are two potential underlying mechanisms [30]. Studies investigating the relationship between biomarkers and post-acute COVID-19 syndrome are required. We can also speculate that COVID-19 might worsen a pre-existing or latent functional respiratory disorder, favoured by the negative socioeconomics effects of the pandemic on mental health [31]. However, in our cohort, the majority of patients did not report any symptoms before their hospitalisation.
Finally, since FRCs are subjective symptoms, we can also hypothesise that FRCs are part of a larger post-COVID-19 somatoform disorder that includes other manifestations of unclear aetiology, such as headache, fatigue and cognitive complaints. Of note, we observed more cognitive complaints (either self-reported or after evaluation by a neuropsychologist) in patients with FRCs but similar cognitive impairment after objective evaluation (MoCA or D2R scores). This difference between subjective and objective symptoms might be related to fatigue, anxiety or depression [5]. As previously described with HVS [32], we highlight that FRCs severely impact the quality of life of post-COVID-19 patients, which may induce a significant burden for healthcare services.
Limitations
In an effort to improve the management of the most fragile individuals, we invited all ICU patients to join the COMEBAC cohort (whether or not they complained of persistent symptoms). This recruitment is from a real-life setting (i.e., symptomatic and/or ICU patients); however it may have contributed to reduce the proportion of patients with FRCs among ICU patients. Nevertheless, the presence of FRCs in patients with mild or moderate COVID-19 suggests that post-COVID-19 functional respiratory disorders should not be sought only in patients with severe pneumonia. In the explanatory cohort of patients with post-COVID-19 unexplained dyspnoea, we established the diagnosis of DB based on criteria available in the current literature [18]. Since there is no current consensus-determined gold standard for the diagnosis of DB, misdiagnoses cannot be excluded. However, we found evidence of abnormal breathing pattern during CPET in 12 out of 21 patients, including two patients with typical deep sighing, a feature that we also observed in other patients who underwent HVPT. Our results are consistent with those of Frésard et al. [33] who also described using CPET in post-COVID-19 patients, an erratic type of breathing mainly without hyperventilation corresponding to deep sighs. Other approaches might have been relevant to assess the ventilatory response of patients. It has been suggested that higher regional inhomogeneity (as assessed by Electrical Impedance Tomography) may have contributed to dyspnoea in post-COVID-19 patients [34]. Using CPET, other authors evaluated a method to classify the breathing pattern in terms of inter-rater agreement: among 20 patients, seven had an abnormal breathing pattern associated with lower exercise capacity, which could possibly explain exercise-related symptoms in some patients with post-acute COVID-19 syndrome [35]. The role of DB and deconditioning has been highlighted in larger cohorts [36, 37]. Deconditioning was uncommon in our explanatory cohort, which was characterised by long-term dyspnoeic patients with normal routine tests and mostly evidence of DB on CPET.
When indicated, patients were invited to perform breathing exercises with a physiotherapist. A systematic Cochrane review was unable to inform clinical practice based on the inclusion of only small and poorly reported randomised controlled trials [38]. In our experience, this strategy is effective when the patient is compliant and has access to a well-trained physiotherapist. Unfortunately, these conditions are difficult to meet in a pandemic situation. Promising new therapeutic approaches have emerged, such as the English programme “ENO Breathe”, which is based on singing techniques [39].
In conclusion, this study provides new data regarding the occurrence and mechanisms of COVID-19-related FRCs. and their relationships with psychological and neurological symptoms, and quality of life. Physicians should be aware of these symptoms and incorporate them into their decision-making algorithm when treating patients with post-acute COVID-19 syndrome.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00063-2023.SUPPLEMENT (392.2KB, pdf)
Figure S1 00063-2023.SUPPLEMENT (513.6KB, jpg)
Acknowledgements
The authors thank the patients who participated in this cohort, as well as the physicians, psychologists, nurses, care helpers, biologists, pharmacists, other allied health professionals and administrators of the Bicêtre Hospital.
Provenance: Submitted article, peer reviewed.
Comebac Investigators: Luc Morin (Université Paris-Saclay, AP-HP, Service de Réanimation Pédiatrique et Médecine Néonatale, Hôpital de Bicêtre, DMU 3 Santé de l'Enfant et de l'Adolescent, Le Kremlin-Bicêtre, France), Laurent Savale (Université Paris-Saclay, AP-HP, Service de Pneumologie et Soins Intensifs Respiratoires, Hôpital de Bicêtre, DMU 5 Thorinno, Inserm UMR_S999, Le Kremlin-Bicêtre, France), Tài Pham (Université Paris-Saclay, AP-HP, Service de Médecine Intensive-Réanimation, Hôpital de Bicêtre, DMU 4 CORREVE Maladies du Cœur et des Vaisseaux, Inserm UMR_S999, Le Kremlin-Bicêtre, France), Romain Colle (Université Paris-Saclay, AP-HP, Service de Psychiatrie, Hôpital de Bicêtre, DMU 11, Equipe MOODS, INSERM U1178, CESP, Le Kremlin-Bicêtre, France), Samy Figueiredo (Université Paris-Saclay, AP-HP, Service de Réanimation Chirurgicale, Hôpital de Bicêtre, DMU 12 Anesthésie, Réanimation, Douleur, Le Kremlin-Bicêtre, France), Anatole Harrois (Université Paris-Saclay, AP-HP, Service de Réanimation Chirurgicale, Hôpital de Bicêtre, DMU 12 Anesthésie, Réanimation, Douleur, Le Kremlin-Bicêtre, France), Matthieu Gasnier (Université Paris-Saclay, AP-HP, Service de psychiatrie, Hôpital de Bicêtre, DMU 11, Equipe MOODS, INSERM U1178, CESP, Le Kremlin-Bicêtre, France), Anne-Lise Lecoq (Université Paris-Saclay, AP-HP, Centre de Recherche Clinique Paris-Saclay, DMU 13 Santé Publique, Information Médicale, Appui à la Recherche Clinique, INSERM U1018, CESP, Le Kremlin-Bicêtre, France), Olivier Meyrignac (Université Paris-Saclay, AP-HP, Service de Radiologie Diagnostique et Interventionnelle, Hôpital de Bicêtre, DMU 14 Smart Imaging, BioMaps, Le Kremlin-Bicêtre, France), Nicolas Noel (Université Paris-Saclay, AP-HP, Service de Médecine Interne et Immunologie Clinique, Hôpital de Bicêtre, DMU 7 Endocrinologie-Immunités-Inflammations-Cancer-Urgences, Le Kremlin-Bicêtre, France), Elodie Baudry (Université Paris-Saclay, AP-HP, Service de Gériatrie Aiguë, Hôpital de Bicêtre, DMU 1 Médecine Territoire Gériatrie, Le Kremlin-Bicêtre, France), Marie-France Bellin (Université Paris-Saclay, AP-HP, Service de Radiologie Diagnostique et Interventionnelle, Hôpital de Bicêtre, DMU 14 Smart Imaging, BioMaps, Le Kremlin-Bicêtre, France), Antoine Beurnier (Université Paris-Saclay, AP-HP, Service de Réanimation Pédiatrique et Médecine Néonatale, Hôpital de Bicêtre, DMU 3 Santé de l'Enfant et de l'Adolescent, Le Kremlin-Bicêtre, France), Walid Choucha (Université Paris-Saclay, AP-HP, Service de Psychiatrie, Hôpital de Bicêtre, DMU 11, Equipe MOODS, INSERM U1178, CESP, Le Kremlin-Bicêtre, France), Emmanuelle Corruble (Université Paris-Saclay, AP-HP, Service de Psychiatrie, Hôpital de Bicêtre, DMU 11, Equipe MOODS, INSERM U1178, CESP, Le Kremlin-Bicêtre, France), Laurent Dortet (Université Paris-Saclay, AP-HP, Service de Réanimation Pédiatrique et Médecine Néonatale, Hôpital de Bicêtre, DMU 3 Santé de l'Enfant et de l'Adolescent, Le Kremlin-Bicêtre, France), Isabelle Hardy-Leger (Université Paris-Saclay, AP-HP, Service de Médecine Interne et Immunologie Clinique, Hôpital de Bicêtre, DMU 7 Endocrinologie-Immunités-Inflammations-Cancer-Urgences, Le Kremlin-Bicêtre, France), François Radiguer (Université Paris-Saclay, AP-HP, Service de Réanimation Chirurgicale, Hôpital de Bicêtre, DMU 12 Anesthésie, Réanimation, Douleur, Le Kremlin-Bicêtre, France), Sabine Sportouch (Université Paris-Saclay, AP-HP, Service de Médecine Intensive-Réanimation, Hôpital de Bicêtre, DMU 4 CORREVE Maladies du Cœur et des Vaisseaux, Inserm UMR_S999, Le Kremlin-Bicêtre, France), Christiane Verny (Université Paris-Saclay, AP-HP, Service de Gériatrie Aiguë, Hôpital de Bicêtre, DMU 1 Médecine Territoire Gériatrie, Le Kremlin-Bicêtre, France), Benjamin Wyplosz (Université Paris-Saclay, AP-HP, Service des Maladies Infectieuses et Tropicales, Hôpital de Bicêtre, DMU 7 Endocrinologie-Immunités-Inflammations-Cancer-Urgences, INSERM U1018, CESP, Le Kremlin-Bicêtre, France), Mohamad Zaidan (Université Paris-Saclay, AP-HP, Service de Néphrologie Transplantation, Hôpital de Bicêtre, DMU 4 CORREVE Maladies du Cœur et des Vaisseaux, Le Kremlin-Bicêtre, France), Laurent Becquemont (Université Paris-Saclay, AP-HP, Centre de Recherche Clinique Paris-Saclay, DMU 13 Santé Publique, Information Médicale, Appui à la Recherche Clinique, INSERM U1018, CESP, Le Kremlin-Bicêtre, France), David Montani (Université Paris-Saclay, AP-HP, Service de Pneumologie et Soins Intensifs Respiratoires, Hôpital de Bicêtre, DMU 5 Thorinno, Inserm UMR_S999, Le Kremlin-Bicêtre, France) and Xavier Monnet (Université Paris-Saclay, AP-HP, Service de Médecine Intensive-Réanimation, Hôpital de Bicêtre, DMU 4 CORREVE Maladies du Cœur et des Vaisseaux, Inserm UMR_S999, Le Kremlin-Bicêtre, France).
Support statement: This study was supported by Assistance Publique-Hôpitaux de Paris. Funding information for this article has been deposited with the Crossref Funder Registry.
Conflict of interest: A. Beurnier reports personal fees from Sanofi and AstraZeneca, outside the submitted work.
Conflict of interest: L. Savale reports personal fees and nonfinancial support from Janssen and MSD, and grants, personal fees and nonfinancial support from GSK, outside the submitted work.
Conflict of interest: X. Jaïs reports grants and personal fees from Janssen, grants and personal fees from MSD, and grants from Bayer and GSK, outside the submitted work.
Conflict of interest: T. Gille reports personal fees from Roche SAS, and other support from Oxyvie (oxygen provider), Vivisol France (oxygen provider) and Menarini France, outside the submitted work.
Conflict of interest: O. Sitbon reports grants from Acceleron, AOP Orphan, Janssen, GSK and MSD; consulting fees from Altavant, Gossamer Bio, Janssen and MSD; lecture honoraria from AOP Orphan, Janssen, Ferrer and MSD; and participation on advisory boards for Acceleron, Altavant, Gossamer Bio, Janssen, MSD and Ferrer, all outside the submitted work.
Conflict of interest: Marc Humbert reports grants from Acceleron, AOP Orphan, Janssen, Merck and Shou Ti; consulting fees from Acceleron, Aerovate, Altavant, AOP Orphan, Bayer, Chiesi, Ferrer, Janssen, Merck, MorphogenIX, Shou Ti and United Therapeutics; lecture honoraria from Janssen and Merck; and advisory board participation for Acceleron, Altavant, Janssen, Merck and United Therapeutics, all outside the submitted work.
Conflict of interest: D. Montani reports grants from Acceleron, Janssen and Merck; consulting fees from Acceleron; and lecture honoraria from Bayer, Janssen and Merck, all outside the submitted work.
Conflict of interest: All other authors have nothing to disclose.
Contributor Information
the Comebac Investigators:
Luc Morin, Laurent Savale, Tài Pham, Romain Colle, Samy Figueiredo, Anatole Harrois, Matthieu Gasnier, Anne-Lise Lecoq, Olivier Meyrignac, Nicolas Noel, Elodie Baudry, Marie-France Bellin, Antoine Beurnier, Walid Choucha, Emmanuelle Corruble, Laurent Dortet, Isabelle Hardy-Leger, François Radiguer, Sabine Sportouch, Christiane Verny, Benjamin Wyplosz, Mohamad Zaidan, Laurent Becquemont, David Montani, and Xavier Monnet
References
- 1.Yelin D, Wirtheim E, Vetter P, et al. Long-term consequences of COVID-19: research needs. Lancet Infect Dis 2020; 20: 1115–1117. doi: 10.1016/S1473-3099(20)30701-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med 2021; 27: 601–615. doi: 10.1038/s41591-021-01283-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carfì A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID-19. JAMA 2020; 324: 603–605. doi: 10.1001/jama.2020.12603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garrigues E, Janvier P, Kherabi Y, et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect 2020; 81: e4–e6. 10.1016/j.jinf.2020.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Writing Committee for the COMEBAC Study Group , Morin L, Savale L, et al. Four-month clinical status of a cohort of patients after hospitalization for COVID-19. JAMA 2021; 325: 1525–1534. doi: 10.1001/jama.2021.3331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Dixhoorn J, Folgering H. The Nijmegen Questionnaire and dysfunctional breathing. ERJ Open Res 2015; 1: 00001-2015. doi: 10.1183/23120541.00001-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vidotto LS, de Carvalho CRF, Harvey A, et al. Dysfunctional breathing: what do we know? J Bras Pneumol 2019; 45: e20170347. doi: 10.1590/1806-3713/e20170347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lerum TV, Aaløkken TM, Brønstad E, et al. Dyspnoea, lung function and CT findings three months after hospital admission for COVID-19. Eur Respir J 2021; 57: 2003448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Motiejunaite J, Balagny P, Arnoult F, et al. Hyperventilation: a possible explanation for long-lasting exercise intolerance in mild COVID-19 survivors? Front Physiol 2020; 11: 614590. doi: 10.3389/fphys.2020.614590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah AS, Wong AW, Hague CJ, et al. A prospective study of 12-week respiratory outcomes in COVID-19-related hospitalisations. Thorax 2021; 76: 402–404. doi: 10.1136/thoraxjnl-2020-216308 [DOI] [PubMed] [Google Scholar]
- 11.Thomas M, McKinley RK, Freeman E, et al. Prevalence of dysfunctional breathing in patients treated for asthma in primary care: cross sectional survey. BMJ 2001; 322: 1098–1100. doi: 10.1136/bmj.322.7294.1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holland AE, Spruit MA, Troosters T, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J 2014; 44: 1428–1446. doi: 10.1183/09031936.00150314 [DOI] [PubMed] [Google Scholar]
- 13.Graham BL, Brusasco V, Burgos F, et al. 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur Respir J 2017; 49: 1600016. doi: 10.1183/13993003.00016-2016 [DOI] [PubMed] [Google Scholar]
- 14.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J 2012; 40: 1324–1343. doi: 10.1183/09031936.00080312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quanjer PH, Tammeling GJ, Cotes JE, et al. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official statement of the European Respiratory Society. Eur Respir J Suppl 1993; 16: 5–40. doi: 10.1183/09041950.005s1693 [DOI] [PubMed] [Google Scholar]
- 16.Cotes JE, Chinn DJ, Quanjer PH, et al. Standardization of the measurement of transfer factor (diffusing capacity). Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official statement of the European Respiratory Society. Eur Respir J Suppl 1993; 16: 41–52. doi: 10.1183/09041950.041s1693 [DOI] [PubMed] [Google Scholar]
- 17.Vansteenkiste J, Rochette F, Demedts M. Diagnostic tests of hyperventilation syndrome. Eur Respir J 1991; 4: 393–399. doi: 10.1183/09031936.93.04040393 [DOI] [PubMed] [Google Scholar]
- 18.Watson M, Ionescu MF, Sylvester K, et al. Minute ventilation/carbon dioxide production in patients with dysfunctional breathing. Eur Respir Rev 2021; 30: 200182. doi: 10.1183/16000617.0182-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas M, McKinley RK, Freeman E, et al. The prevalence of dysfunctional breathing in adults in the community with and without asthma. Prim Care Respir J 2005; 14: 78–82. doi: 10.1016/j.pcrj.2004.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Denton E, Bondarenko J, Tay T, et al. Factors associated with dysfunctional breathing in patients with difficult to treat asthma. J Allergy Clin Immunol Pract 2019; 7: 1471–1476. doi: 10.1016/j.jaip.2018.11.037 [DOI] [PubMed] [Google Scholar]
- 21.Tobin MJ, Chadha TS, Jenouri G, et al. Breathing patterns. 2. Diseased subjects. Chest 1983; 84: 286–294. doi: 10.1378/chest.84.3.286 [DOI] [PubMed] [Google Scholar]
- 22.Hancox RJ, Morgan J, Dickson N, et al. Rape, asthma, and dysfunctional breathing. Eur Respir J 2020; 55: 1902455. [DOI] [PubMed] [Google Scholar]
- 23.Veidal S, Jeppegaard M, Sverrild A, et al. The impact of dysfunctional breathing on the assessment of asthma control. Respir Med 2017; 123: 42–47. doi: 10.1016/j.rmed.2016.12.008 [DOI] [PubMed] [Google Scholar]
- 24.Wang Q, Xu R, Volkow ND. Increased risk of COVID-19 infection and mortality in people with mental disorders: analysis from electronic health records in the United States. World Psychiatry 2021; 20: 124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. The Lancet 2021; 397: 220–232. doi: 10.1016/S0140-6736(20)32656-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taquet M, Geddes JR, Husain M, et al. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry 2021; 8: 416–427. doi: 10.1016/S2215-0366(21)00084-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meuret AE, Ritz T. Hyperventilation in panic disorder and asthma: empirical evidence and clinical strategies. Int J Psychophysiol 2010; 78: 68–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guadarrama-Ortiz P, Choreño-Parra JA, Sánchez-Martínez CM, et al. Neurological aspects of SARS-CoV-2 infection: mechanisms and manifestations. Front Neurol 2020; 11: 1039. doi: 10.3389/fneur.2020.01039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baig AM, Khaleeq A, Ali U, et al. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci 2020; 11: 995–998. doi: 10.1021/acschemneuro.0c00122 [DOI] [PubMed] [Google Scholar]
- 30.Ferrando SJ, Klepacz L, Lynch S, et al. COVID-19 psychosis: a potential new neuropsychiatric condition triggered by novel coronavirus infection and the inflammatory response? Psychosomatics 2020; 61: 551–555. doi: 10.1016/j.psym.2020.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brenner MH, Bhugra D. Acceleration of anxiety, depression, and suicide: secondary effects of economic disruption related to COVID-19. Front Psychiatry 2020; 11: 592467.doi: 10.3389/fpsyt.2020.592467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chenivesse C, Similowski T, Bautin N, et al. Severely impaired health-related quality of life in chronic hyperventilation patients: exploratory data. Respir Med 2014; 108: 517–523. doi: 10.1016/j.rmed.2013.10.024 [DOI] [PubMed] [Google Scholar]
- 33.Frésard I, Genecand L, Altarelli M, et al. Dysfunctional breathing diagnosed by cardiopulmonary exercise testing in ‘long COVID’ patients with persistent dyspnoea. BMJ Open Respir Res 2022; 9: e001126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scaramuzzo G, Ronzoni L, Campo G, et al. Long-term dyspnea, regional ventilation distribution and peripheral lung function in COVID-19 survivors: a 1 year follow up study. BMC Pulm Med 2022; 22: 408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.von Gruenewaldt A, Nylander E, Hedman K. Classification and occurrence of an abnormal breathing pattern during cardiopulmonary exercise testing in subjects with persistent symptoms following COVID-19 disease. Physiol Rep 2022; 10: e15197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kersten J, Hoyo L, Wolf A, et al. Cardiopulmonary exercise testing distinguishes between post-COVID-19 as a dysfunctional syndrome and organ pathologies. Int J Environ Res Public Health 2022; 19: 11421. doi: 10.3390/ijerph191811421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ingul CB, Edvardsen A, Follestad T, et al. Changes in cardiopulmonary exercise capacity and limitations 3 to 12 months after COVID-19. Eur Respir J 2023: 61: 2200745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones M, Harvey A, Marston L, et al. Breathing exercises for dysfunctional breathing/hyperventilation syndrome in adults. Cochrane Database Syst Rev 2013; 5: CD009041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.English National Opera (ENO). About the ENO Breathe Programme. https://eno.org/eno-breathe/about-the-eno-breathe-programme/ Date last accessed: 14 March 2023.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00063-2023.SUPPLEMENT (392.2KB, pdf)
Figure S1 00063-2023.SUPPLEMENT (513.6KB, jpg)