Highlights
-
•
The outcomes of zoledronic acid were assessed through a Bayesian meta-analysis.
-
•
Zoledronic acid reduces the incidence of skeletal-related events and pain.
-
•
No statistically significant treatment was found for overall survival.
Keywords: Zoledronic acid, Zoledronate, Bone metastasis, Clinical outcomes, Network meta-analysis, Adjuvant therapy, And neoadjuvant therapy
Abstract
Background
While considered the mainstay of treatment for specific bone metastases, ZA is used predominantly to treat osteolytic lesions. The purpose of this network meta-analysis is to compare ZA to other treatment options in its ability to improve specific clinical outcomes in patients with bone metastases secondary to any primary tumor.
Methods
PubMed, Embase and Web of Science were systematically searched from inception to May 5th, 2022. Keywords used were solid tumor, lung neoplasm, kidney neoplasm, breast neoplasm, prostate neoplasm, ZA and bone metastasis. Every randomized controlled trial and non-randomized quasi-experimental study of systemic ZA administration for patients with bone metastases and any comparator were included. A Bayesian network meta-analysis was done on the primary outcomes including number of SREs, time to developing a first on-study SRE, overall survival, and disease progression-free survival. Secondary outcome was pain at 3, 6 and 12 months after treatment.
Results
Our search yielded 3861 titles with 27 meeting inclusion criteria. For the number of SRE, ZA in combination with chemotherapy or hormone therapy was statistically superior to placebo (OR 0.079; 95 % CrI: 0.022–0.27). For the time to the first on study SRE, the relative effectiveness of ZA 4 mg was statistically superior to placebo (HR 0.58; 95 % CrI:0.48–0.77). At 3 and 6 months, ZA 4 mg was significantly superior to placebo for reducing pain with a SMD of −0.85 (95 % CrI:-1.6, −0.0025) and −2.6 (95 % CrI:-4.7, −0.52) respectively.
Conclusions
This systematic review shows the benefits of ZA in decreasing the incidence of SREs, increasing the time to the first on-study SRE, and reducing the pain level at 3 and 6 months.
1. Introduction
Over the past decades, oncology patients have seen their prognoses improve with new revolutionary therapies [1], [2]. Nevertheless, metastases are responsible for 90 % of cancer-related deaths [3], [4]. Along with liver and lung, bone is the most common site of metastases for many primary solid tumors such as prostate, lung, breast and kidney [5], [6], [7].
Bone metastases markedly disrupt normal bone remodelling [8]. This disturbance often results in skeletal pain, hypercalcemia and skeletal-related events (SREs) [9], [10]. SREs are a group of symptoms which comprise pathologic fractures, spinal cord compression, or events requiring radiation therapy or surgery to a bone. SREs are known for reducing physical capacity, prolonging hospital stays and shortening survival [11], [12]. Approved therapeutic agents for skeletal metastases include monoclonal antibodies such as denosumab, hormone therapy, radiotherapy, radioligand therapy, chemotherapy, immunotherapy and bisphosphonates (BPs) [13], [14].
Bisphosphonates are potent inhibitors of osteoclast-mediated bone resorption that are commonly prescribed for the treatment of osteoporosis, hypercalcemia of malignancy and bone metastases [15]. Zoledronic acid (ZA) is an intravenous, nitrogen-containing, third-generation bisphosphonate which was found to treat osteolytic lesions in vivo[13] in addition to its anti-tumor properties in vitro [16], [17], [18], [19], [20]. Moreover, ZA has been proven to be involved in inducing tumor cell apoptosis[17], modulating the immune system[20], inhibiting tumor invasion[18], decreasing tumor proliferation[19] and reducing tumor angiogenesis [16].
Therefore, ZA is considered a mainstay of treatment for bone metastases secondary to solid tumors such as breast cancer [21]. As an adjuvant therapy, ZA was also shown to prevent bone metastases secondary to the last-mentioned cancer type [22]. In addition, ZA has been used for other cancers such as lung, kidney and prostate [13]. Systematic reviews and network meta-analyses (NMAs) on bone metastases concluded that ZA was associated with a statistically significant decrease in SREs when compared to placebo [23], [24]. However, no NMA was performed regarding other clinical parameters such as overall survival, progression-free survival or level of pain [23], [24].
The purpose of this systematic review and Bayesian NMA is to compare ZA to other treatment options in its ability to improve overall survival, decrease the incidence of skeletal-related events and reduce pain in patients with bone metastases secondary to any primary tumors.
2. Materials and Methods
2.1. Search strategy
A systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [25]. The search was performed in the following databases: PubMed, Embase (via Ovid) and Web of Science. All databases were searched from inception to May 5th, 2022. The following keywords were used: solid tumor, lung neoplasm, kidney neoplasm, breast neoplasm, prostate neoplasm, ZA and bone metastasis. Synonyms of these terms were also used but not listed here (Supplementary Table 1–3). Each reference section was hand screened to identify additional studies. Endnote was used to remove the duplicates and tract removal or addition of studies.
2.2. Eligibility criteria
2.2.1. Population
Studies of human adults with bone metastases secondary to any solid tumors, regardless of the language, age or sex, were included.
2.2.2. Interventions and comparator
Randomized controlled trials and non-randomized quasi-experimental studies of systemic ZA administration for patients with bone metastases and any comparator, including placebo, were included. Subgroup analyses of broader studies that were already included in our study were excluded.
2.2.3. Outcomes
The primary outcomes are 1) the development of a new skeletal-related event, defined as new bone metastases, pathological fractures, spinal cord compression or disabling pain; 2) time to developing a first SRE during the study; 3) disease progression-free survival (PFS); and 4) overall survival (OS). The secondary outcome is pain at 3, 6 and 12 months after treatment as measured by the brief pain inventory (BPI) or a visual analogue score (VAS). Data from both scores were pooled since they share the same psychometric dimensions.
2.3. Screening and abstraction process
Titles and abstracts of articles identified were screened independently by two researchers (JPL and FGB). Any citation identified by either investigator as potentially relevant was then advanced to full-text review. Any discrepancies were resolved through a consensus discussion with a senior member of the research team (EA or JRG). Once full texts had been included in the study, data abstraction was performed in pairs (JPL, FGB, or JRG).
Treatments were categorized as ZA 4 or 8 mg every 3–4 weeks. When combined with any other drug (i.e. chemotherapeutics), regardless of ZA dosage, ZA was categorized as ZA in combination. Identified comparators included androgen blockade drugs, everolimus, denosumab, docetaxel, other bisphosphonates, and strontium-89 (Sr-89). Primary cancer type was categorized as breast, lung, prostate or other. The time of ZA treatment was categorized as before, during or after chemotherapy treatment.
For the relative effectiveness outcomes, the data extracted was the number of patients who presented with a SRE; its odds ratio (OR) and its 95 % credible intervals (CrI). Regarding the time to a first on-study SRE, OS, and PFS, hazard ratios (HR) and 95 % credible intervals (CrI) were calculated. Finally, for pain, the mean and standard error of the mean of the treatment effect were extracted and converted into standard mean differences (SMD). For HR calculation where data was not explicitly stated, Kaplan-Meier survival curves were used with the method described by Tierney et al. [26] using the Engauge Digitizer software, version 10.11 to estimate the values.
2.4. Risk of bias
Risk of bias in individual studies was assessed using the Risk of Bias v.2 tool [27]. Assessments were done in duplicate (JPL and FBG supervised by EA). If present, discrepancies were resolved between authors and if necessary, with other members of the research team (JRG and EA). Data visualization was done using the Robvis tool [28].
2.5. Calculations and statistical analysis
Following the guide by Harrer et al. [29], a NMA was performed under a Bayesian framework for each outcome using a random-effects model with the gemtc and rjags packages in the R v.4.1.0 [30]. For the binary outcome of SREs development, the model corresponded to a generalized linear model with a logit link. For time to event outcomes (time to develop a first SRE, OS and PFS), the model corresponded to a generalized linear model with a c-loglog link. Finally, for the continuous outcomes of pain, the model corresponded to a generalized linear model with an identity link. The between-study heterogeneity was assumed to be constant. Non-informative prior distributions was used for effectiveness model parameters given current uncertainty of the relative effectiveness of the treatments. Convergence of the 4 chains using the Gelman-Rubin statistic was used to assess convergence of the algorithm for the prior distributions, as well as visual inspection of trace plots. Convergence was deemed achieved if the potential scale reduction factor was lower than 1.05. Consistency of the network model was assessed through the nodesplit method. However, because the geometry of the networks had paucity of head-to-head trials, nodesplits were achieved only for some treatments.
A network plots was generated for each analysis. Summary results were presented as either OR, HR, or SMD with 95 % CrI. Treatment rankings were summarized by the surface under the cumulative ranking (SUCRA) that expressed the probability (0.00–1.00) of effectiveness. Each treatment has been compared with an ideal treatment ranked always first without uncertainty. Meta-regression analyses were done to assess whether the type of primary cancer or time of treatment with respect to chemotherapy administration influenced the primary outcomes.
3. Results
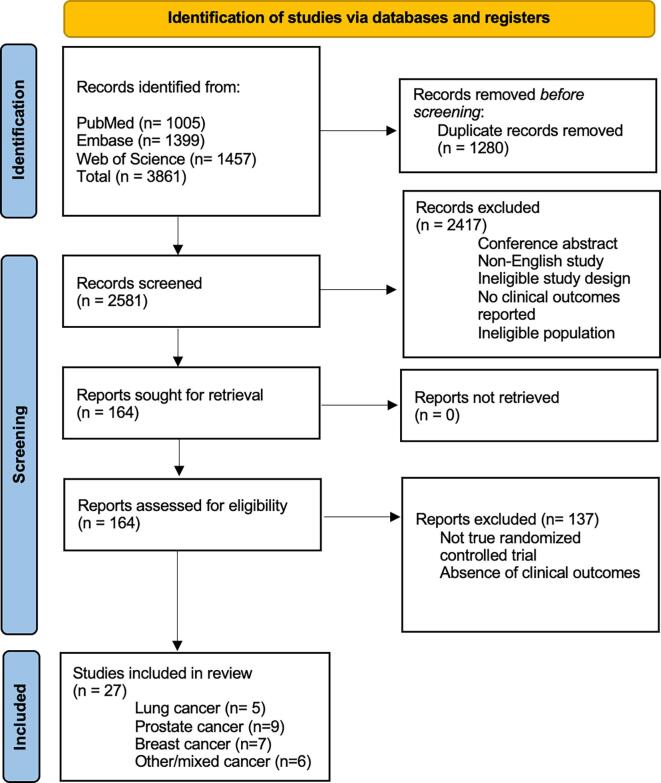
Our search yielded 3861 titles (Fig. 1). After removing duplicates, 2581 articles were screened based on titles and abstracts. After reviewing the bibliography of each paper, one more article was found to be eligible for the NMA. A total of 27 articles remained relevant based on the inclusion and exclusion criteria. The studies were divided based on the location of the primary tumors: lung (5), prostate (9), breast (7) and other/mixed (6). A total of 19,824 patients and 7 therapies were compared to ZA. The most frequent comparator was placebo. The mean number of participants in each group was 333 (range 15–1026) (Table 1). For each outcome, separation based on primary cancer or by time of administration was not associated with reduced heterogeneity of the data (Supplementary Table 4). The SUCRA was calculated for each outcome based on the different treatments. Cumulative rankings are represented in Supplementary Figs. 1–5. In addition, separation of prostate cancer studies based on castration resistant, castration sensitive or hormone naïve was performed. However, no differences were found in these three subcategories. This most likely represents underpower of the analysis as only 4, 2 and 2 studies were included for each category, respectively.
Fig. 1.
PRISMA figure illustrating the inclusion of the studies with the steps involved in the screening process. The separation highlighted in blue on the left shows the different sections of the process. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 1.
List of included studies. The data of each randomized controlled trial is represented in the columns. NSCLC = Non-small cell lung cancer, ZA = Zoledronic Acid, SR-89 = Strontium-89, SC = Subcutaneous, IV = Intravenous, MBq = Megabecquerel.
| Authors | Year | Primary cancer | Time of treatment compared to the main therapy | Treatment | Population size for treatment (n) | Comparator | Population size for comparator (n) |
|---|---|---|---|---|---|---|---|
| Barrett-Lee, P[33] | 2014 | Breast cancer | During | ZA- 4 mg/ 4 weeks | 699 | Ibandronic acid- 50 mg daily | 705 |
| Broom, R. J [34] | 2015 | Renal cell carcinoma | Before | Everolimus and ZA - 4 mg/ 4 weeks | 15 | Everolimus- 10 mg daily | 15 |
| Choudhury, K. B [31] | 2011 | NSCLC + others | During | ZA − 4 mg/ 4 weeks | 60 | 1. Ibandronate- 6 mg/ 4 weeks 2. Pamidronate- 90 mg/ 4 weeks |
1. 65 2. 62 |
| Cleeland, C. S [76] | 2013 | Breast cancer | Before | ZA − 4mg/ 4 weeks + SC placebo | 1020 | Denosumab- 120 mg/ 4 weeks + IV placebo | 1026 |
| Fizazi, K [35] | 2011 | Prostate cancer | After | ZA − 4 mg/ 4 weeks + SC placebo | 951 | Denosumab- 120 mg/ 4 weeks + IV placebo | 950 |
| Francini, F [36] | 2011 | NSCLC | During | ZA − 4mg/ 4 weeks | 28 | Ibandronic acid- 50 mg/day | 27 |
| Henry, D [37] | 2014 | NSCLC | Before | ZA - 4 mg/ 4 weeks + SC placebo | 797 | Denosumab SC- 120 mg/ 4 weeks with placebo IV | 800 |
| Hilton, J. F [38] | 2018 | Breast cancer | After | ZA − 4 mg/ 4 weeks | 38 | Pamidronate | 35 |
| Jacobs, C [39] | 2016 | Breast cancer | During | ZA − 4 mg/ 4 weeks | 35 | Pamidronate | 38 |
| James, N [40] | 2016 | Prostate cancer | Before | Docetaxel, ZA − 4 mg/ 4 weeks | 188 | 1. Docetaxel- 75 mg/m2/ 3 weeks, 2. Docetaxel and ZA 3. Docetaxel + Sr-89. |
1. 191 2. 188 3. 190 |
| Kamba, T [41] | 2016 | Prostate cancer | Before | ZA − 4 mg/ 4 weeks with combined androgen blockade | 115 | 80 mg of bicalutamide orally once a day and subcutaneous luteinizing hormone–releasing hormone agonist every 4 or 12 weeks | 112 |
| Kohno, N [42] | 2005 | Breast cancer | During | ZA − 4 mg/ 4 weeks | 114 | Placebo | 114 |
| Martin, M [43] | 2012 | Breast cancer | Before | ZA − 4 mg/ 4 weeks + SC placebo | 1020 | Denosumab- 120 mg/ 4 weeks + IV placebo | 1026 |
| Murakami, H [44] | 2014 | NSCLC | After | ZA − 4 mg/ 4 weeks with Docetaxel | 50 | Docetaxel- 60 mg/m2 | 50 |
| Pan, Y [45] | 2014 | Prostate cancer | After | Docetaxel-based chemotherapy + ZA - 4 mg/ 3 weeks | 53 | Docetaxel-based chemotherapy- 75 mg/m2 of docetaxel for 21 days and placebo | 52 |
| Pandya, K. J [56] | 2010 | NSCLC | After | Docetaxel, Carboplatin and ZA − 4 mg/ 4 weeks | 64 | Docetaxel- 75 mg/m2 for 1 h and carboplatin- IV for 15 min | 64 |
| Price, N [32] | 1999 | NSCLC + others | Before | ZA - A. 4 mg / 3 weeks B. 8 mg/ 3 weeks; reduced to 4 mg/ 3 weeks |
A. 254 B. 265 |
Placebo | 247 |
| Rosen, L. S [46] | 2003 | Multiple myeloma and breast cancer | During | ZA - A. 4 mg/ 4 weeks B. 8 mg/ 4 weeks; later switched to 4 mg/ 4 weeks |
A. 564 B. 526 |
Pamidronate- 90 mg/ 3 or 4 weeks | 558 |
| Rosen, L. S [47] | 2004 | NSCLC + others | During | ZA - A. 4 mg/ 4 weeks B. 8 mg/ 4 weeks; later switched to 4 mg/ 4 weeks |
A. 257 B. 266 |
Placebo | 250 |
| Saad, F [48] | 2002 | Prostate cancer | During | ZA - A. 4 mg / 4 weeks B. 8 mg/ 4 weeks;later switched to 4 mg/ 4 weeks |
A. 214 B. 221 |
Placebo | 208 |
| Smith, M. R [50] | 2014 | Prostate cancer | Before | ZA − 4 mg/ 4 weeks | 323 | Placebo | 322 |
| Smith, M. R. [49] | 2015 | Prostate cancer | After | ZA − 4 mg/ 4 weeks | 951 | Denosumab- 120 mg/ 4 weeks | 950 |
| Stopeck, A.T [51] | 2010 | Breast cancer | During | ZA − 4 mg/ 4 weeks + SC placebo | 1020 | Denosumab- 120 mg/ 4 weeks + IV placebo | 1026 |
| Ueno, S [52] | 2013 | Prostate cancer | Before | ZA − 4 mg/ 4 weeks with combined androgen blockade | 29 | Combined androgen blockade − 80 mg/ day | 31 |
| Wang, F [53] | 2013 | Prostate cancer | Before | ZA − 4 mg/ 4 weeks | 69 | Clodronate- 1600 mg / day | 68 |
| Wang, Y [55] | 2013 | NSCLC | Before | Sr-89, 0.9 % sodium chloride and ZA − 4 mg/ 4 weeks | 45 | 1. ZA − 4 mg every 3/4 weeks 2. Sr-89–150 MBq of Sr-89 every 6 months 3. Chemotherapy |
1. 45 2. 45 3. 45 |
| Zaghloul, MS [54] | 2010 | Bladder cancer | After | ZA − 4 mg/ 4 weeks | 20 | Placebo | 20 |
3.1. Study quality
Among the included studies, 14 had an overall risk of bias considered “low risk”, 11 studies had “some concerns” and 2 were classified “high risk”. These two studies, one from Choudhoury et al. [31] and another one from Price et al. [32] had biases due to deviation from intended intervention. Furthermore, Price et al. [32] had high risk bias due to missing outcome data (Supplementary Fig. 6).
3.2. Skeletal-related events
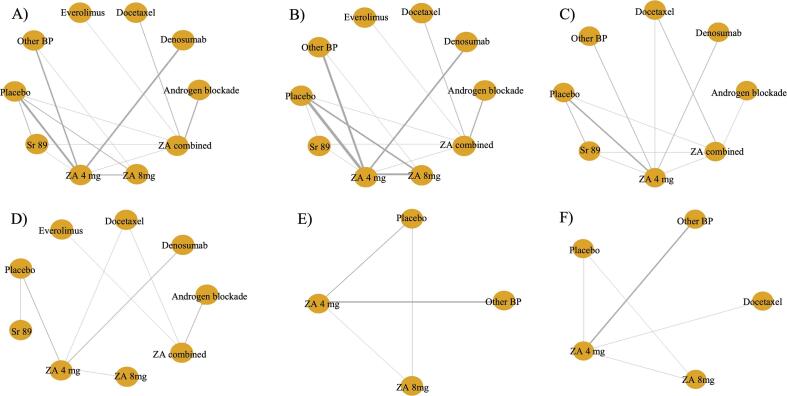
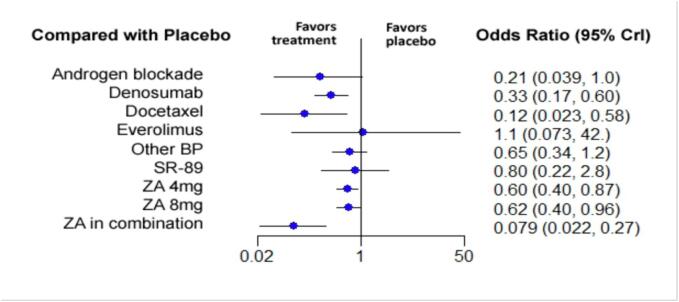
A total of 24 studies were included in the analysis of the SREs [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55]. Nine therapies were compared together and with placebo. These include ZA 4 mg, ZA 8 mg, ZA combined with another treatment, androgen blockade, denosumab, docetaxel, everolimus, Sr-89 and all the other bisphosphonates pooled in one group (Fig. 2). The NMA showed that three therapies were associated with a clinically significant decrease in the number of SREs when compared to placebo. Among them, ZA in combination with chemotherapy or hormone therapy was superior to placebo for preventing SREs with an OR of 0.079 (95 % CrI:0.022–0.27), followed by docetaxel and denosumab with ORs of 0.12 (95 % CrI:0.023–0.58) and 0.33 (95 % CrI:0.17–0.60) respectively. The relative impact of the different treatment groups when compared to placebo is depicted in Fig. 3. Pairwise comparison between the different treatments is depicted in the league table (Supplementary Table 5).
Fig. 2.
Network graphs for each different outcome analyzed in the study. The orange nodes correspond to each different study. The thickness of the grey lines is proportional to the number of trials between each pair of treatment nodes. More precisely, there is A) Time to first skeletal-related event, B) number of skeletal-related events, C) Overall survival, D) progression-free survival, E) pain at 3 months and F) Pain at 6- and 12-month. ZA = Zoledronic Acid, BP = Bisphosphonate, SR-89 = Strontium-89. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 3.
Forest plot of the impact of the different therapies in preventing the development of a SRE when compared to placebo. On the left, the different therapies are labelled. On the right, the odds ration corresponding to each therapy when compared to placebo are represented. In parenthesis, the 95 % credible intervals are shown. BP = Bisphosphonate, ZA = Zoledronic Acid, SR-89 = Strontium-89, CrI = Credible Intervals.
3.3. Time to a first on-study skeletal-related event
Twenty studies with 10 different treatments were included in the analysis of the time to develop a first on-study SRE [33], [34], [35], [37], [38], [40], [41], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55]. Included therapies were placebo, ZA 4 mg, ZA 8 mg, ZA combined with another treatment, androgen blockade, denosumab, docetaxel, everolimus, Sr-89 and all other bisphosphonates grouped (Fig. 2). When compared to placebo, the treatment with the greatest relative effectiveness for protecting against the development of a first on-study SRE was denosumab (HR 0.51 with 95 % CrI:0.35–0.78), followed by ZA 4 mg (HR 0.58 with 95 % CrI:0.48–0.77). No other treatment had a statistically significant increase in the time to treat a first SRE. The relative effectiveness of the different therapies when compared to placebo is depicted in Fig. 4. Pairwise comparison between the different treatments is depicted in the league table (Supplementary Table 6).
Fig. 4.
Forest plot of the relative effectiveness of the different therapies to modify the time to develop a first SRE when compared to placebo. On the left, the different therapies are labelled. On the right, the hazard ratios corresponding to each therapy when compared to placebo are represented. In parenthesis, the 95 % credible intervals are shown. BP = Bisphosphonate, ZA = Zoledronic Acid, SR-89 = Strontium-89, CrI = Credible Intervals.
3.4. Progression-free survival
A total of 10 studies were included in the analysis of the progression-free survival [34], [35], [37], [40], [41], [44], [46], [50], [52], [56]. Nine different therapies were compared including placebo, ZA 4 mg, ZA 8 mg, ZA combined with another treatment, androgen blockade, denosumab, docetaxel, everolimus and strontium-89 (Fig. 2). The treatment with the highest relative effectiveness was Sr-89 (HR 0.88 with 95 % CrI:0.61–1.3), followed by denosumab (HR 0.92 with 95 % CrI:0.64–1.2) and ZA 8 mg (HR 0.95 with 95 % CrI:0.62–1.4). However, no treatment was statistically significant for increasing the PFS. The relative effectiveness of the different therapies is depicted in Fig. 5. Pairwise comparison between the different treatments is depicted in the league table (Supplementary Table 7).
Fig. 5.
Forest plot of the relative effectiveness of the different therapies for progression-free survival when compared to placebo. On the left, the different therapies are labelled. On the right, the hazard ratios corresponding to each therapy when compared to placebo are represented. In parenthesis, the 95 % credible intervals are shown. ZA = Zoledronic Acid, SR-89 = Strontium-89, CrI = Credible Intervals.
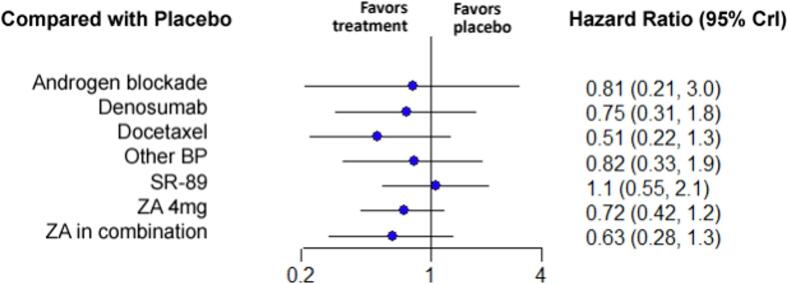
3.5. Overall survival
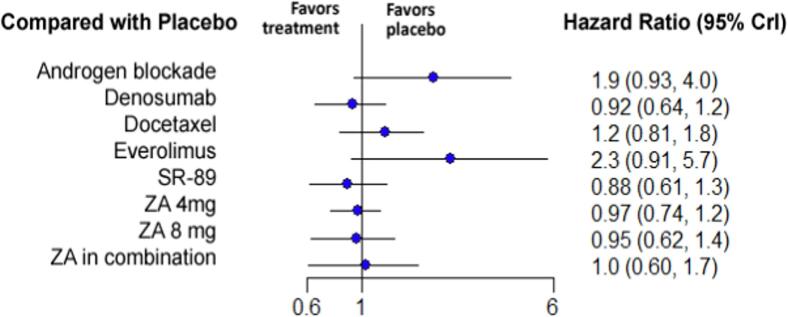
For the overall survival, 12 studies with 8 treatments were included in the analysis [33], [35], [40], [41], [44], [45], [50], [51], [53], [54], [55], [56]. Included therapies were placebo, ZA 4 mg, ZA combined with another treatment, androgen blockade, denosumab, docetaxel, Sr-89 and all other bisphosphonates pooled (Fig. 2). The treatment associated with the highest survival was docetaxel (HR 0.51 with 95 % CrI:0.22–1.3), followed by ZA in combination with chemotherapy or hormone therapy (HR 0.63 with 95 % CrI:0.28–1.3) and ZA 4 mg (HR 0.72 with 95 % CrI:0.42–1.2). However, no treatment was associated with a statistically significant decrease in overall survival when compared with placebo. The relative effectiveness of the different therapies when compared to placebo is illustrated in Fig. 6. Pairwise comparison between the different treatments is depicted in the league table (Supplementary Table 8).
Fig. 6.
Forest plot of the relative effectiveness of the different therapies for overall survival when compared to placebo. On the left, the different therapies are labelled. On the right, the hazard ratios corresponding to each therapy when compared to placebo are represented. In parenthesis, the 95 % credible intervals are shown. BP = Bisphosphonate, ZA = Zoledronic Acid, SR-89 = Strontium-89, CrI = Credible Intervals .
3.6. Pain
A total of 6 studies were included in the analysis of the pain at the 3- and 6-month follow-ups, with four and five different treatments, respectively [31], [33], [45], [48], [53], [54]. Five studies with 5 treatments were selected for the 12-month comparison [31], [33], [45], [48], [53]. These treatments include ZA 4 mg, ZA 8 mg, docetaxel and all the other bisphosphonates in one group (Fig. 2). At 3 and 6 months, ZA 4 mg was significantly superior to placebo for reducing pain with a SMD of −0.85 (95 % CrI:-1.6, −0.0025) and −2.6 (95 % CrI:-4.7, −0.52) respectively. No other treatment was statistically superior to placebo at 3 and 6 months. At 12 months, no treatment was statistically superior to placebo (Fig. 7). Pairwise comparison between the different treatments is depicted in the league table (Supplementary Table 9).
Fig. 7.
Forest plot of the relative effectiveness of the different therapies to modify pain BPI/VAS scores when compared to placebo at A) 3 months, B) 6 months and C) 12 months. On the left, the different therapies are labelled. On the right, the standardized mean difference corresponding to each therapy when compared to placebo is represented. In parenthesis, the 95 % credible intervals are shown. BP = Bisphosphonate, ZA = Zoledronic Acid, CrI = Credible Intervals.
4. Discussion
Zoledronic acid has been shown to improve the quality of life of patients with bone metastases while maintaining an acceptable safety profile for long-term use [57], [58]. Our Bayesian NMA showed that ZA decreases the number of SREs, increases the time to a first on-study SRE and reduces short-term pain level in patients with metastatic bone tumors.
Only few systematic reviews and meta-analyses were published on the use of ZA in patients with bone metastases. When compared to ZA, denosumab was significantly better at delaying the time to the first skeletal-event and reducing pain. Overall survival and disease progression were similar in both groups [59], [60]. These results are in accordance with our study regarding the time to the first skeletal-related event. However, our study demonstrated superiority of ZA 4 mg in reducing pain at the 3- and 12-month follow ups given the paucity of data for the denosumab group. A NMA comparing the different BPs with denosumab concluded that denosumab was superior to ZA in reducing the number of SREs [23]. This is coherent with our results which showed more benefits with denosumab than ZA 4 mg alone. However, the current meta-analysis demonstrates that ZA, when combined with chemotherapy or hormone therapy, is superior to denosumab alone.
While clinical trials include data that is highly curated, the use of ZA for breast cancer and bone metastases has been proven to be efficacious in real-world evidence [61]. Futhermore, a cost-effectiveness analysis has shown that bisphosphonates, compared to no therapy, are either cost-saving or highly cost effective [62]. In the same study, ZA was shown to be the most effective and less expensive of all options for reducing SREs and improving the quality of life of patients with bone metastases [62]. Another cost-analysis study for the same type of tumors demonstrated that the cost of generic ZA treatment every 3 months was approximately ninefold lower than monthly denosumab therapy in addition to being more cost-effective in reducing the number of SREs [63]. Altogether, this supports our findings that ZA is the best option for preventing SREs secondary to bone metastases.
Nevertheless, ZA treatment has some limitations such as osteonecrosis of the jaw. This complication was shown to be higher in smokers and in patients with poor dental hygiene [57], [64]. Patients also reported other less serious symptoms including flu-like symptoms, diarrhea, nausea and heartburn [57]. A more significant clinical limitation is the use of ZA in patients with pre-existing renal dysfunctions since ZA is predominantly excreted through the kidney and therefore is associated with a risk of nephrotoxicity [65], [66].
Based on the FDA recommendations [67], the ideal ZA dose is 4 mg infused over no less than 15 min every 3 to 4 weeks, which results in a peak serum concentration of 1–3 µM for a few hours following systemic administration [68]. Local continuous administration of drugs is an emerging approach [69], [70]. This would ensure high drug concentrations at the site and reduce the systemic absorption [70], [71]. Therefore, local delivery of ZA would reduce the risk of experiencing side effects [57], [72]. This can be achieved by using impregnated 3D-printed nanoporous scaffolds [73]. In vitro studies have shown that with a 3D-printer, it is possible to customize a model and insert it in a bone defect to allow bone marrow stem cells to infiltrate, adhere, proliferate, and form new bone [73]. Such approach showed promising results where approximately 3 µM of ZA impregnated into nanoscaffolds or beads was enough to achieve the same therapeutic concentration locally as the systemic dose of 4 mg upon intravenous administration [70], [71]. As shown in Table 1, every study involving a ZA infusion of 8 mg amended its protocol to decrease the infusion to 4 mg [32], [46], [47]. This is likely secondary to the detrimental effect of a higher systemic dosage of ZA. In fact, both studies by Rosen et al. reported an increase in creatinine level with 8 mg infusion [46], [47]. This effect might explain why ZA 4 mg performed better than ZA 8 mg.
Another area of research focuses on the combination of ZA with other treatments [74], [75]. A synergistic action would allow the patients to receive a lower dose from both medications, therefore reducing the risk of side effects. Investigations demonstrated that ZA combined with chemotherapy had a synergistic effect at the tumor site in vitro [74]. A similar synergistic effect was found in a study conducted with mice where immunotherapy and ZA had an increased anti-tumor efficacy [75].
An important strength of our study is its design. The network meta-analysis allows for a broader comparison of articles compared to a regular meta-analysis. We were able to analyze every treatment found in the included studies and compare them with each other. On the other hand, this study is limited by the reported data of the included studies. While primary cancer and time of administration were not associated with reduced heterogeneity, it was not possible to control for other factors given the lack of data. Future studies should attempt to control for other parameters such as Charlson comorbidity index and extent of disease.
5. Conclusion
Our Bayesian NMA shows the benefits of ZA 4 mg in reducing the incidence of new SREs, increasing the time to the development of a SRE, and decreasing pain in the short term. ZA in combination with chemotherapy or hormone therapy had the highest relative effectiveness at reducing the incidence of SREs. However, ZA 4 mg alone also showed a statistically significant improvement in the time to a first on-study SRE when compared to placebo. Unfortunately, for the overall survival and progression free survival, no treatment was associated with a statistically significant increase when compared with placebo.
CRediT authorship contribution statement
Justin-Pierre Lorange: Investigation, Data curation, Writing - original draft. Jose Ramirez Garcia Luna: Software, Formal analysis, Writing – review & editing. Frédéric Grou-Boileau: Investigation. Derek Rosenzweig: Writing – review & editing. Michael H. Weber: Writing – review & editing, Supervision. Elie Akoury: Conceptualization, Supervision, Project administration, Methodology, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
This research was funded by AO Start-Up grant number S-16-138W to M.H.W. and the Cancer Research Society (CRS) grant number 23014 to M.H.W. and D.H.R., by internal start-up funding from the Research Institute of the McGill University Health Centre (RI-MUHC) to D.H.R. and the Réseau de Recherche en Santé Buccodentaire et Osseuse (RSBO) to M.H.W. and D.H.R. E.A. was supported by postdoctoral fellowships from the RI-MUHC, RSBO and from McGill University-Faculty of Medicine.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jbo.2023.100470.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary figure 1.
Supplementary figure 2.
Supplementary figure 3.
Supplementary figure 4.
Supplementary figure 5.
Supplementary figure 6.
References
- 1.Kinch M.S. An analysis of FDA-approved drugs for oncology. Drug Discov. Today. 2014;19(12):1831–1835. doi: 10.1016/j.drudis.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2016. CA Cancer J. Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Seyfried T.N., Huysentruyt L.C. On the origin of cancer metastasis. Crit. Rev. Oncog. 2013;18(1–2):43–73. doi: 10.1615/critrevoncog.v18.i1-2.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dillekås H., Demicheli R., Ardoino I., Jensen S.A.H., Biganzoli E., Straume O. The recurrence pattern following delayed breast reconstruction after mastectomy for breast cancer suggests a systemic effect of surgery on occult dormant micrometastases. Breast Cancer Res. Treat. 2016;158(1):169–178. doi: 10.1007/s10549-016-3857-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Batista N., Tee J., Sciubba D., Sahgal A., Laufer I., Weber M., Gokaslan Z., Rhines L., Fehlings M., Patel S., Raja Rampersaud Y., Reynolds J., Chou D., Bettegowda C., Clarke M., Fisher C. Emerging and established clinical, histopathological and molecular parametric prognostic factors for metastatic spine disease secondary to lung cancer: helping surgeons make decisions. J. Clin. Neurosci. 2016;34:15–22. doi: 10.1016/j.jocn.2016.05.023. [DOI] [PubMed] [Google Scholar]
- 6.Clarke M.J., Molina C.A., Fourney D.R., Fisher C.G., Gokaslan Z.L., Schmidt M.H., Rhines L.D., Fehlings M.G., Laufer I., Patel S.R., Rampersaud Y.R., Reynolds J., Chou D., Bettegowda C., Mendel E., Weber M.H., Sciubba D.M. Systematic review of the outcomes of surgical treatment of prostate metastases to the spine. Global Spine J. 2017;7(5):460–468. doi: 10.1177/2192568217710911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coleman R.E., Croucher P.I., Padhani A.R., Clézardin P., Chow E., Fallon M., Guise T., Colangeli S., Capanna R., Costa L. Bone metastases. Nat Rev Dis Primers. 2020;6(1) doi: 10.1038/s41572-020-00216-3. [DOI] [PubMed] [Google Scholar]
- 8.Roodman G.D. Mechanisms of bone metastasis. N. Engl. J. Med. 2004;350(16):1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 9.Menshawy A., Mattar O., Abdulkarim A., Kasem S., Nasreldin N., Menshawy E., Mohammed S., Abdel-Maboud M., Gadelkarim M., El Ashal G.G., Elgebaly A.S. Denosumab versus bisphosphonates in patients with advanced cancers-related bone metastasis: systematic review and meta-analysis of randomized controlled trials. Support Care Cancer. 2018;26(4):1029–1038. doi: 10.1007/s00520-018-4060-1. [DOI] [PubMed] [Google Scholar]
- 10.Baek Y.H., Jeon H.L., Oh I.S., Yang H., Park J., Shin J.Y. Incidence of skeletal-related events in patients with breast or prostate cancer-induced bone metastasis or multiple myeloma: a 12-year longitudinal nationwide healthcare database study. Cancer Epidemiol. 2019;61:104–110. doi: 10.1016/j.canep.2019.05.013. [DOI] [PubMed] [Google Scholar]
- 11.Costa L., Badia X., Chow E., Lipton A., Wardley A. Impact of skeletal complications on patients' quality of life, mobility, and functional independence. Support Care Cancer. 2008;16(8):879–889. doi: 10.1007/s00520-008-0418-0. [DOI] [PubMed] [Google Scholar]
- 12.Pockett R.D., Castellano D., McEwan P., Oglesby A., Barber B.L., Chung K. The hospital burden of disease associated with bone metastases and skeletal-related events in patients with breast cancer, lung cancer, or prostate cancer in Spain. Eur. J. Cancer Care (Engl.) 2010;19(6):755–760. doi: 10.1111/j.1365-2354.2009.01135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gdowski A.S., Ranjan A., Vishwanatha J.K. Current concepts in bone metastasis, contemporary therapeutic strategies and ongoing clinical trials. J. Exp. Clin. Cancer Res. 2017;36(1):108. doi: 10.1186/s13046-017-0578-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez BD, Brownstein NC, Fan W, Dicker AP, Oswald LB, Dhillon HM, et al. Improvements in symptoms related to bone metastasis in recipients of Lutetium-177 PSMA-617 for prostate cancer. Journal of Clinical Oncology. 2022;40(6_suppl):96-.
- 15.Drake M.T., Clarke B.L., Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin. Proc. 2008;83(9):1032–1045. doi: 10.4065/83.9.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Salvatore M., Orlandi A., Bagalà C., Quirino M., Cassano A., Astone A., et al. Anti-tumour and anti-angiogenetic effects of zoledronic acid on human non-small-cell lung cancer cell line. Cell Prolif. 2011;44(2):139–146. doi: 10.1111/j.1365-2184.2011.00745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kiper H.D., Tezcanli Kaymaz B., Gokbulut A.A., Selvi N., Avci C.B., Kosova B., Iskender G., Yandim M.K., Gunduz C., Sahin F., Baran Y., Saydam G. STAT pathway in the regulation of zoledronic acid-induced apoptosis in chronic myeloid leukemia cells. Biomed. Pharmacother. 2013;67(6):527–532. doi: 10.1016/j.biopha.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Lin C., Xin S., Qin X., Li H., Lin L., You Y. Zoledronic acid suppresses metastasis of esophageal squamous cell carcinoma cells through upregulating the tight junction protein occludin. Cytotechnology. 2016;68(4):1233–1241. doi: 10.1007/s10616-015-9884-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsumoto S., Kimura S., Segawa H., Kuroda J., Yuasa T., Sato K., Nogawa M., Tanaka F., Maekawa T., Wada H. Efficacy of the third-generation bisphosphonate, zoledronic acid alone and combined with anti-cancer agents against small cell lung cancer cell lines. Lung Cancer. 2005;47(1):31–39. doi: 10.1016/j.lungcan.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Reuben J.S., Dinh L., Lee J., Stateson J., Kamara H., Xiang L., Opperman L.A. Bisphosphonates inhibit phosphorylation of signal transducer and activator of transcription 3 and expression of suppressor of cytokine signaling 3: implications for their effects on innate immune function and osteoclastogenesis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011;111(2):196–204. doi: 10.1016/j.tripleo.2010.09.068. [DOI] [PubMed] [Google Scholar]
- 21.Van Poznak C.H., Temin S., Yee G.C., Janjan N.A., Barlow W.E., Biermann J.S., Bosserman L.D., Geoghegan C., Hillner B.E., Theriault R.L., Zuckerman D.S., Von Roenn J.H. American Society of Clinical Oncology executive summary of the clinical practice guideline update on the role of bone-modifying agents in metastatic breast cancer. J. Clin. Oncol. 2011;29(9):1221–1227. doi: 10.1200/JCO.2010.32.5209. [DOI] [PubMed] [Google Scholar]
- 22.Gnant M., Dubsky P., Hadji P. Bisphosphonates: prevention of bone metastases in breast cancer. Recent Results Cancer Res. 2012;192:65–91. doi: 10.1007/978-3-642-21892-7_3. [DOI] [PubMed] [Google Scholar]
- 23.Wang Z, Qiao D, Lu Y, Curtis D, Wen X, Yao Y, et al. Systematic literature review and network meta-analysis comparing bone-targeted agents for the prevention of skeletal-related events in cancer patients with bone metastasis. Oncologist. 2015;20(4):440-9. [DOI] [PMC free article] [PubMed]
- 24.Ford J.A., Jones R., Elders A., Mulatero C., Royle P., Sharma P., Stewart F., Todd R., Mowatt G. Denosumab for treatment of bone metastases secondary to solid tumours: systematic review and network meta-analysis. Eur. J. Cancer. 2013;49(2):416–430. doi: 10.1016/j.ejca.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 25.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed]
- 26.Tierney J.F., Stewart L.A., Ghersi D., Burdett S., Sydes M.R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8(1):16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. Bmj. 2019;366:l4898. [DOI] [PubMed]
- 28.McGuinness L.A., Higgins J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods. 2021;12(1):55–61. doi: 10.1002/jrsm.1411. [DOI] [PubMed] [Google Scholar]
- 29.Harrer M, Cuijpers P, Furukawa TA, Ebert DD. Doing meta-analysis in R: A hands-on guide. PROTECT Lab Erlangen. 2019.
- 30.Foundation TR. The R Project for Statistical Computing 2020 [cited 2021 March 6th]. Available from: https://www.r-project.org.
- 31.Choudhury K.B., Mallik C., Sharma S., Choudhury D.B., Maiti S., Roy C. A randomized controlled trial to compare the efficacy of bisphosphonates in the management of painful bone metastasis. Indian J. Palliat. Care. 2011;17(3):210–218. doi: 10.4103/0973-1075.92338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Price N., Belani C.P., Jain V.K. Bisphosphonates to prevent skeletal morbidity in patients with lung cancer with bone metastases. Clin. Lung Cancer. 2004;5(5):267–269. doi: 10.1016/S1525-7304(11)70347-3. [DOI] [PubMed] [Google Scholar]
- 33.Barrett-Lee P., Casbard A., Abraham J., Hood K., Coleman R., Simmonds P., Timmins H., Wheatley D., Grieve R., Griffiths G., Murray N. Oral ibandronic acid versus intravenous zoledronic acid in treatment of bone metastases from breast cancer: a randomised, open label, non-inferiority phase 3 trial. Lancet Oncol. 2014;15(1):114–122. doi: 10.1016/S1470-2045(13)70539-4. [DOI] [PubMed] [Google Scholar]
- 34.Broom R.J., Hinder V., Sharples K., Proctor J., Duffey S., Pollard S., et al. Everolimus and zoledronic acid in patients with renal cell carcinoma with bone metastases: a randomized first-line phase II trial. Clin. Genitourin. Cancer. 2015;13(1):50–58. doi: 10.1016/j.clgc.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Fizazi K., Carducci M., Smith M., Damião R., Brown J., Karsh L., Milecki P., Shore N., Rader M., Wang H., Jiang Q.i., Tadros S., Dansey R., Goessl C. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377(9768):813–822. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Francini F., Pascucci A., Bargagli G., Francini E., Conca R., Miano S.T., Martellucci I., Migali C., Gotti G., Fiaschi A.I., Cozzolino A., Petrioli R. Effects of intravenous zoledronic acid and oral ibandronate on early changes in markers of bone turnover in patients with bone metastases from non-small cell lung cancer. Int. J. Clin. Oncol. 2011;16(3):264–269. doi: 10.1007/s10147-010-0179-x. [DOI] [PubMed] [Google Scholar]
- 37.Henry D., Vadhan-Raj S., Hirsh V., von Moos R., Hungria V., Costa L., Woll P.J., Scagliotti G., Smith G., Feng A., Jun S., Dansey R., Yeh H. Delaying skeletal-related events in a randomized phase 3 study of denosumab versus zoledronic acid in patients with advanced cancer: an analysis of data from patients with solid tumors. Support Care Cancer. 2014;22(3):679–687. doi: 10.1007/s00520-013-2022-1. [DOI] [PubMed] [Google Scholar]
- 38.Hilton J.F., Clemons M., Pond G., Zhao H., Mazzarello S., Vandermeer L., Addison C.L. Effects on bone resorption markers of continuing pamidronate or switching to zoledronic acid in patients with high risk bone metastases from breast cancer. J. Bone Oncol. 2018;10:6–13. doi: 10.1016/j.jbo.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jacobs C., Kuchuk I., Bouganim N., Smith S., Mazzarello S., Vandermeer L., Dranitsaris G., Dent S., Gertler S., Verma S., Song X., Simos S., Cella D., Clemons M. A randomized, double-blind, phase II, exploratory trial evaluating the palliative benefit of either continuing pamidronate or switching to zoledronic acid in patients with high-risk bone metastases from breast cancer. Breast Cancer Res. Treat. 2016;155(1):77–84. doi: 10.1007/s10549-015-3646-2. [DOI] [PubMed] [Google Scholar]
- 40.James N., Pirrie S., Pope A., Barton D., Andronis L., Goranitis I., Collins S., McLaren D., O’Sullivan J., Parker C., Porfiri E., Staffurth J., Stanley A., Wylie J., Beesley S., Birtle A., Brown J., Chakraborti P., Russell M., Billingham L. TRAPEZE: a randomised controlled trial of the clinical effectiveness and cost-effectiveness of chemotherapy with zoledronic acid, strontium-89, or both, in men with bony metastatic castration-refractory prostate cancer. Health Technol. Assess. 2016;20(53):1–288. doi: 10.3310/hta20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kamba T., Kamoto T., Maruo S., Kikuchi T., Shimizu Y., Namiki S., Fujimoto K., Kawanishi H., Sato F., Narita S., Satoh T., Saito H., Sugimoto M., Teishima J., Masumori N., Egawa S., Sakai H., Okada Y., Terachi T., Ogawa O. A phase III multicenter, randomized, controlled study of combined androgen blockade with versus without zoledronic acid in prostate cancer patients with metastatic bone disease: results of the ZAPCA trial. Int. J. Clin. Oncol. 2017;22(1):166–173. doi: 10.1007/s10147-016-1037-2. [DOI] [PubMed] [Google Scholar]
- 42.Kohno N., Aogi K., Minami H., Nakamura S., Asaga T., Iino Y., Watanabe T., Goessl C., Ohashi Y., Takashima S. Zoledronic acid significantly reduces skeletal complications compared with placebo in Japanese women with bone metastases from breast cancer: a randomized, placebo-controlled trial. J. Clin. Oncol. 2005;23(15):3314–3321. doi: 10.1200/JCO.2005.05.116. [DOI] [PubMed] [Google Scholar]
- 43.Martin M, Bell R, Bourgeois H, Brufsky A, Diel I, Eniu A, et al. Bone-related complications and quality of life in advanced breast cancer: results from a randomized phase III trial of denosumab versus zoledronic acid. Clin Cancer Res. 2012;18(17):4841-9. [DOI] [PubMed]
- 44.Murakami H., Yamanaka T., Seto T., Sugio K., Okamoto I., Sawa T., et al. Phase II study of zoledronic acid combined with docetaxel for non-small-cell lung cancer: West Japan Oncology Group. Cancer Sci. 2014;105(8):989–995. doi: 10.1111/cas.12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pan Y., Jin H., Chen W., Yu Z., Ye T., Zheng Y., Weng Z., Wang F. Docetaxel with or without zoledronic acid for castration-resistant prostate cancer. Int. Urol. Nephrol. 2014;46(12):2319–2326. doi: 10.1007/s11255-014-0824-9. [DOI] [PubMed] [Google Scholar]
- 46.Rosen L.S., Gordon D., Kaminski M., Howell A., Belch A., Mackey J., Apffelstaedt J., Hussein M.A., Coleman R.E., Reitsma D.J., Chen B.-L., Seaman J.J. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: a randomized, double-blind, multicenter, comparative trial. Cancer. 2003;98(8):1735–1744. doi: 10.1002/cncr.11701. [DOI] [PubMed] [Google Scholar]
- 47.Rosen L.S., Gordon D., Tchekmedyian N.S., Yanagihara R., Hirsh V., Krzakowski M., Pawlicki M., de Souza P., Zheng M., Urbanowitz G., Reitsma D., Seaman J. Long-term efficacy and safety of zoledronic acid in the treatment of skeletal metastases in patients with nonsmall cell lung carcinoma and other solid tumors: a randomized, Phase III, double-blind, placebo-controlled trial. Cancer. 2004;100(12):2613–2621. doi: 10.1002/cncr.20308. [DOI] [PubMed] [Google Scholar]
- 48.Saad F., Gleason D.M., Murray R., Tchekmedyian S., Venner P., Lacombe L., Chin J.L., Vinholes J.J., Goas J.A., Chen B. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J. Natl. Cancer Inst. 2002;94(19):1458–1468. doi: 10.1093/jnci/94.19.1458. [DOI] [PubMed] [Google Scholar]
- 49.Smith M.R., Coleman R.E., Klotz L., Pittman K., Milecki P., Ng S., Chi K.N., Balakumaran A., Wei R., Wang H., Braun A., Fizazi K. Denosumab for the prevention of skeletal complications in metastatic castration-resistant prostate cancer: comparison of skeletal-related events and symptomatic skeletal events. Ann. Oncol. 2015;26(2):368–374. doi: 10.1093/annonc/mdu519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith M.R., Halabi S., Ryan C.J., Hussain A., Vogelzang N., Stadler W., Hauke R.J., Monk J.P., Saylor P., Bhoopalam N., Saad F., Sanford B., Kelly W.K., Morris M., Small E.J. Randomized controlled trial of early zoledronic acid in men with castration-sensitive prostate cancer and bone metastases: results of CALGB 90202 (alliance) J. Clin. Oncol. 2014;32(11):1143–1150. doi: 10.1200/JCO.2013.51.6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stopeck A.T., Lipton A., Body J.-J., Steger G.G., Tonkin K., de Boer R.H., Lichinitser M., Fujiwara Y., Yardley D.A., Viniegra M., Fan M., Jiang Q.i., Dansey R., Jun S., Braun A. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J. Clin. Oncol. 2010;28(35):5132–5139. doi: 10.1200/JCO.2010.29.7101. [DOI] [PubMed] [Google Scholar]
- 52.Ueno S., Mizokami A., Fukagai T., Fujimoto N., Oh-Oka H., Kondo Y., et al. Efficacy of combined androgen blockade with zoledronic acid treatment in prostate cancer with bone metastasis: the ZABTON-PC (zoledronic acid/androgen blockade trial on prostate cancer) study. Anticancer Res. 2013;33(9):3837–3844. [PubMed] [Google Scholar]
- 53.Wang F., Chen W., Chen H., Mo L., Jin H., Yu Z., Li C., Liu Q., Duan F., Weng Z. Comparison between zoledronic acid and clodronate in the treatment of prostate cancer patients with bone metastases. Med. Oncol. 2013;30(3) doi: 10.1007/s12032-013-0657-x. [DOI] [PubMed] [Google Scholar]
- 54.Zaghloul M.S., Boutrus R., El-Hossieny H., Kader Y.A., El-Attar I., Nazmy M. A prospective, randomized, placebo-controlled trial of zoledronic acid in bony metastatic bladder cancer. Int. J. Clin. Oncol. 2010;15(4):382–389. doi: 10.1007/s10147-010-0074-5. [DOI] [PubMed] [Google Scholar]
- 55.Wang Y., Tao H., Yu X., Wang Z., Wang M. Clinical significance of zoledronic acid and strontium-89 in patients with asymptomatic bone metastases from non-small-cell lung cancer. Clin. Lung Cancer. 2013;14(3):254–260. doi: 10.1016/j.cllc.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 56.Pandya K.J., Gajra A., Warsi G.M., Argonza-Aviles E., Ericson S.G., Wozniak A.J. Multicenter, randomized, phase 2 study of zoledronic acid in combination with docetaxel and carboplatin in patients with unresectable stage IIIB or stage IV non-small cell lung cancer. Lung Cancer. 2010;67(3):330–338. doi: 10.1016/j.lungcan.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 57.Polascik T.J., Mouraviev V. Zoledronic acid in the management of metastatic bone disease. Ther. Clin. Risk Manag. 2008;4(1):261–268. doi: 10.2147/tcrm.s2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weinfurt K.P., Li Y., Castel L.D., Saad F., Timbie J.W., Glendenning G.A., Schulman K.A. The significance of skeletal-related events for the health-related quality of life of patients with metastatic prostate cancer. Ann. Oncol. 2005;16(4):579–584. doi: 10.1093/annonc/mdi122. [DOI] [PubMed] [Google Scholar]
- 59.Chen C., Li R., Yang T., Ma L., Zhou S., Li M., Zhou Y., Cui Y. Denosumab versus zoledronic acid in the prevention of skeletal-related events in vulnerable cancer patients: a meta-analysis of randomized. Controlled Trials. Clinical Therapeutics. 2020;42(8):1494–1507.e1. doi: 10.1016/j.clinthera.2020.05.019. [DOI] [PubMed] [Google Scholar]
- 60.Jiang L., Cui X., Ma H., Tang X. Comparison of denosumab and zoledronic acid for the treatment of solid tumors and multiple myeloma with bone metastasis: a systematic review and meta-analysis based on randomized controlled trials. J. Orthop. Surg. Res. 2021;16(1):400. doi: 10.1186/s13018-021-02554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mazziotti G., Pedersini R., Vena W., Cosentini D., Carrone F., Pigni S., Simoncini E.L., Torrisi R., Zambelli A., Farina D., Balzarini L., Lania A.G., Berruti A. Real-world effectiveness of denosumab and bisphosphonates on risk of vertebral fractures in women with breast cancer undergoing treatment with aromatase inhibitors. Calcif. Tissue Int. 2022;111(5):466–474. doi: 10.1007/s00223-022-01011-w. [DOI] [PubMed] [Google Scholar]
- 62.Botteman M., Barghout V., Stephens J., Hay J., Brandman J., Aapro M. Cost effectiveness of bisphosphonates in the management of breast cancer patients with bone metastases. Ann. Oncol. 2006;17(7):1072–1082. doi: 10.1093/annonc/mdl093. [DOI] [PubMed] [Google Scholar]
- 63.Shapiro C.L., Moriarty J.P., Dusetzina S., Himelstein A.L., Foster J.C., Grubbs S.S., Novotny P.J., Borah B.J. Cost-effectiveness analysis of monthly zoledronic acid, zoledronic acid every 3 months, and monthly denosumab in women with breast cancer and skeletal metastases: CALGB 70604 (alliance) J. Clin. Oncol. 2017;35(35):3949–3955. doi: 10.1200/JCO.2017.73.7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Van Poznak C.H., Unger J.M., Darke A.K., Moinpour C., Bagramian R.A., Schubert M.M., Hansen L.K., Floyd J.D., Dakhil S.R., Lew D.L., Wade J.L., Fisch M.J., Henry N.L., Hershman D.L., Gralow J. Association of osteonecrosis of the jaw with zoledronic acid treatment for bone metastases in patients with cancer. JAMA Oncol. 2021;7(2):246. doi: 10.1001/jamaoncol.2020.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Markowitz G.S., Fine P.L., Stack J.I., Kunis C.L., Radhakrishnan J., Palecki W., Park J., Nasr S.H., Hoh S., Siegel D.S., D'Agati V.D. Toxic acute tubular necrosis following treatment with zoledronate (Zometa) Kidney Int. 2003;64(1):281–289. doi: 10.1046/j.1523-1755.2003.00071.x. [DOI] [PubMed] [Google Scholar]
- 66.Chang J.T., Green L., Beitz J. Renal failure with the use of zoledronic acid. N. Engl. J. Med. 2003;349(17):1676–1679. doi: 10.1056/NEJM200310233491721. [DOI] [PubMed] [Google Scholar]
- 67.HIGHLIGHTS OF PRESCRIBING INFORMATION- ZOMETA New Jersey: Novartis Pharmaceuticals Corporation; 2016 [Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/021223s034lbl.pdf.
- 68.Berenson J.R.R.C., Ma P., Deckert F., Sasaki Y., Saeki T., Takashima S., LoRusso P., Goodin S., Seaman J., Schran H., Zhou H. Population Pharmacokinetics (PK) of Zometa. Proc ASCO. 2000 [Google Scholar]
- 69.Ahangar P., Akoury E., Ramirez Garcia Luna A., Nour A., Weber M., Rosenzweig D. Nanoporous 3D-printed scaffolds for local doxorubicin delivery in bone metastases secondary to prostate cancer. Materials (Basel) 2018;11(9):1485. doi: 10.3390/ma11091485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nooh A., Zhang Y.L., Sato D., Rosenzweig D.H., Tabariès S., Siegel P., Barralet J.E., Weber M.H. Intra-tumor delivery of zoledronate mitigates metastasis-induced osteolysis superior to systemic administration. J. Bone Oncol. 2017;6:8–15. doi: 10.1016/j.jbo.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Akoury E., Weber M.H., Rosenzweig D.H. 3D-printed nanoporous scaffolds impregnated with zoledronate for the treatment of spinal bone metastases. MRS Adv. 2019;4(21):1245–1251. [Google Scholar]
- 72.De Jong W.H., Borm P.J.A. Drug delivery and nanoparticles:applications and hazards. Int. J. Nanomed. 2008;3(2):133–149. doi: 10.2147/ijn.s596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fairag R., Li L.i., Ramirez-GarciaLuna J.L., Taylor M.S., Gaerke B., Weber M.H., Rosenzweig D.H., Haglund L. A composite lactide-mineral 3D-printed scaffold for bone repair and regeneration. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.654518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ibrahim T., Liverani C., Mercatali L., Sacanna E., Zanoni M., Fabbri F., et al. Cisplatin in combination with zoledronic acid: a synergistic effect in triple-negative breast cancer cell lines Corrigendum in/10.3892/ijo.2016.3613. Int. J. Oncol. 2013;42(4):1263–1270. doi: 10.3892/ijo.2013.1809. [DOI] [PubMed] [Google Scholar]
- 75.Li Y., Du Y., Sun T., Xue H., Jin Z., Tian J. PD-1 blockade in combination with zoledronic acid to enhance the antitumor efficacy in the breast cancer mouse model. BMC Cancer. 2018;18(1):669. doi: 10.1186/s12885-018-4412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cleeland C.S., Body J.-J., Stopeck A., von Moos R., Fallowfield L., Mathias S.D., Patrick D.L., Clemons M., Tonkin K., Masuda N., Lipton A., de Boer R., Salvagni S., Oliveira C.T., Qian Y.i., Jiang Q.i., Dansey R., Braun A., Chung K. Pain outcomes in patients with advanced breast cancer and bone metastases: results from a randomized, double-blind study of denosumab and zoledronic acid. Cancer. 2013;119(4):832–838. doi: 10.1002/cncr.27789. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.