Abstract
Eimeria tenella mainly invades and develops into cecal epithelial cells of chickens, resulting in cecal epithelial cell damage. Infectious intracellular pathogens possibly act by influencing the autophagy process after invading cells. The interaction between E. tenella and the autophagy of host cells was explored by infecting E. tenella with chick embryo cecal epithelial cells. Transmission electron microscopy, laser confocal microscopy, and Western blot analysis were used to demonstrate that E. tenella infection could induce autophagy in host cells. Results showed that infection with E. tenella induced the formation of autophagosomes in cells. The expression of ATG 5, Beclin-1, and LC3B-II proteins were significantly (P < 0.01) increased after E. tenella infected host cells. Expression of p62 protein levels were significantly (P < 0.01) decreased in host cells infected with E. tenella. Chloroquine (CQ) significantly (P < 0.01) increased the expression levels of LC3B-II and P62 in E. tenella-infected host cells. Rapamycin (RAPA) induced autophagy in host cells, thus reducing the intracellular infection of E. tenella. By contrast, the infection rate of E. tenella increased in cells treated with 3-Methyladenine (3-MA). Hence, E. tenella sporozoite infection could induce autophagy activation in chick embryo cecal epithelial cells, and enhanced autophagy could reduce the infection rate of E. tenella.
Key words: Eimeria tenella, chick embryo cecal epithelial cell, autophagy
INTRODUCTION
Chicken coccidiosis is a parasitic protozoan disease caused by one or several coccidiasis of the genus Eimeria apicellum parasitizing in the intestinal epithelial cells of chickens, causing blood dysentry and nutritional disorders. The annual global loss caused by coccidia infection in chickens can reach 10.4 billion pounds (Blake, et al., 2020). Eimeria tenella is one of the most pathogenic Eimeria in chicken, and it also causes the most serious economic loss. E. tenella usually parasitized only in chicken cecum and its adjacent intestinal segments (Matsubayashi et al., 2019). At present, the mechanism of coccidia damage to host cells is still not very clear. The molecular mechanism of interaction between E. tenella and host is the molecular basis of pathogenesis, vaccine and drug development. Therefore, it is of great significance to strengthen the research on the invasion mechanism of coccidia and the interaction mechanism between coccidia and host for the prevention and control of chicken coccidiosis.
Autophagy is an evolutionarily conserved cellular self-degradation process in all eukaryotes. Autophagy is regulated by autophagy-related genes under the conditions of nutrient deficiency or external stimulation, so that damaged organelles or denature-related macromolecules in the cytoplasm are wrapped by bilayer vesicles to form autophagosomes, and finally fuse with lysosomes to form autophagosomes (Lian et al., 2022). Then, the contents wrapped in lysosomes are decomposed by the action of a variety of enzymes to supply the necessary small molecule raw materials for cell repair, reconstruction and regeneration, so as to achieve cell homeostasis and organelle renewal (Rossman and Lamb, 2009; Messer, 2017; Li et al., 2021). In recent years, studies have shown that host cell autophagy interacts with intracellular pathogen infection in two ways. On the one hand, autophagy can assist in removing parasites that enter the cell. Studies have shown that the expression of autophagy-related gene Atg 5 was induced in macrophages activated by T. gondii infection, so that the GTPase of macrophages was activated and the vacuoles of nanworm were lysed. After the parasite's body membrane was stripped, the autophagic vacuoles wrapped the naked worms and transported to the lysosomes for degradation (Zhao et al., 2008). On the other hand, some pathogens can evade autophagy through specific mechanisms or use autophagy to provide nutrients for their own development. Studies have shown that Trypanosoma cruzi can use TcAtg4 and TcAtg8 homologues to induce autophagy in host cells and degrade cytoplasm and organelles in this way to provide nutrients for its own development (Alvarez et al., 2008a, b). Muniz-feliciano et al. proved that autophagy could be triggered after T. gondii infected cells, and T. gondii could not only regulate the signals of host cells through microwire proteins to escape the elimination of autophagy. It could also use the energy produced by autophagy for its own proliferation (Muniz-Feliciano et al., 2013b).
Qi Nanshan et al. demonstrated that the antiparasitic drug monensin induced autophagy and led to programmed death of E. tenella sporozoites (Qi et al., 2020). Qi Nanshan et al. also proved that starvation and the autophagy inducer rapamycin (RAPA) could induce autophagy in E. tenella sporozoites, while 3-Methyladenine (3-MA) could inhibit sporozoite autophagy. RAPA induced sporozoite autophagy could enhance sporozoite invasion into chick embryo fibroblasts, while 3-MA inhibited sporozoite autophagy and reduced sporozoite invasion (Qi et al., 2019). Zhang (2020b) found that the expression of Beclin-1 mRNA and LC3-II protein was significantly increased after E. tenella infection of chick embryo cecal epithelial cells. However, whether E. tenella infection could activate host cell autophagy flow, or whether autophagy plays a role in E. tenella infection have not been extensively investigated.
In the present study, an in vitro model of E. tenella sporozoite infection in chick embryo cecal epithelial cells was established. Moreover, the interaction between E. tenella and autophagy in chick embryo cecal epithelial cells was investigated by detecting the changes in the morphological characteristics of autophagy, the protein expression levels of autophagy-related factors in host cells and the infection rate.
MATERIALS AND METHODS
Ethics Statement
All experiments involving animals were carried out in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC). This study was approved by the Animal Protection and Utilization Committee of Shanxi Agricultural University, China.
Experimental Animals
Fifteen-day-old SPF chicken embryos were obtained from Beijing Meri Avigon Laboratory Animal Technology Co., Ltd. (Beijing, China).
Parasites
E. tenella Shanxi virulent strain was provided by the Laboratory of Veterinary Pathology in the College of Veterinary Medicine, Shanxi Agricultural University.
Primary Culture of Chicken Embryo Cecal Epithelial Cells
Chick embryo cecal epithelial cells were isolated and cultured according to the reported method (Zhang et al., 2019, Zhang et al., 2020a). Cecum of 15-day-old SPF chick embryos was removed, the mesentery was carefully removed, washed with PBS (pH 7.4) containing penicillin streptomycin mixture (Solarbio, Beijing, China), cut into 1 mm3 size tissue blocks and washed. The tissue blocks were placed in 50 mg/L thermophilic protease (Sigma, CA) and digested at 37°C for 95 to 110 min at 80 r/min. The digestion was terminated with PBS and centrifuged at 1,000 r/min for 5 min. Cell precipitates were resuspended with 10% fetal bovine serum (FBS) (Silkgreen, Hangzhou, China) high-glucose DMEM cell medium, inoculated into cell culture bottles, and cultured in 41°C 8% CO2 incubator for 70 min. Unadherent cells in culture flasks were collected and centrifuged to remove cell culture medium. Viable cell masses were diluted to a concentration of 15 × 104/mL using cell inoculation medium (1% glutamine, 1% sodium heparin, 1% sodium pyruvate, 1% dual antibody, 0.02% hyperinsulin, and 2.5% fetal bovine serum). The cells were seeded at 100 μL, 500 μL, and 2 mL per well on 96-well, 24-well, and 6-well cell culture plates, respectively. At the same time, the cell slide was placed in a part of the 6-well plate and the next test was carried out when the cell adhesion rate reached about 90%.
Preparation of E. tenella Sporozoites
E. tenella sporozoites were prepared according to the method reported by Zhang et al., (2019). The oocysts were ground with a glass grinding rod to release the sporangia from the oocysts. Sporozoites were digested by bile trypsin to form sporozoites. The sporozoites were filtered through an 800-mesh sieve (10 μm) and diluted to 2.0 × 105 sporozoites /mL with MEM 199 (Gibco, CA).
Cell Viability Assay
Cell viability was assessed with the Cell Counting Kit-8 (CCK-8) assay (Bioss, Beijing, China) according to manufacturer's instructions. When the cell adhesion rate of 96-well plates reached about 90%, the cells were treated with RAPA (Selleck, TX) (30, 50, 100, 200, or 250 nM), Chloroquine (CQ) (MCE, Shanghai, China) (5, 10, 15, 25, or 35 µM), 3-MA (Selleck, TX) (0.5, 1, 5, 10, or 15 mM) or DMEM/F12 medium (Gibco, CA). After 4 h of culture, culture media were replaced and 10 μL CCK-8 solution were added to each well and incubated in 41°C for 1 h. The absorbance was measured at 450 nm with a microplate reader.
Experimental Protocol in Vitro
The chicken embryo cecal epithelial cells in the 6-well cell culture plates (some of chamber slides) were randomly divided into control group, E. tenella group, CQ group, CQ + E. tenella group, RAPA group, RAPA + E. tenella group, 3-MA group and 3-MA + E. tenella group. After inoculation of sporozoites (4.0 × 105 sporozoites/per well) for 4 h, the culture medium in each group was changed. The medium was then changed every 48 h. The cell culture plates in each group were cultured at 41°C and 8% CO2. Samples were taken at 4, 24, 72, and 120 h after inoculation of sporozoites.
Transmission Electron Microscopy (TEM)
When the cell adhesion rate reached about 90%, cells in E. tenella group were inoculated into 6-well plates at a dose of 4 × 105 sporozoites/well, while cells in Control group were not inoculated. Cells were incubated at 41°C in an 8% carbon dioxide incubator. The cells were removed after 72 h of culture, washed with PBS for 3 times, and then digested with trypsin for 2 min. After digestion was terminated by medium, the supernatant was centrifuged at 1,000 r/min for 5 min and discarded. The cell precipitate was resuspended in PBS buffer and washed twice. The last time, the cells were transferred to a 1.5 mL pointed centrifuge tube, centrifuged at 1,000 r/min for 5 min, and the supernatant was discarded. Cells were fixed for 2 h by gently adding 1 mL of freshly prepared 2.5% glutaraldehyde (Solarbio, Beijing, China). It was fixed with 1% osmotic acid for 3 h. Gradient dehydration with 30%, 50%, 70%, 85%, 95%, 100% I, 100% II alcohol; Soak in isoamyl acetate for 20 min; Embedding polymerized sections and staining. The ultrastructure of cells was observed and images were taken by transmission electron microscope.
Western Blot Analysis
At the indicated time when the sporozoite infected the cells, the medium in the cell plate was discarded. The cells were lysed in RIPA buffer containing protease phosphatase inhibitors (Beyotime, Shanghai, China) for 30 min on ice under shaking. The cells were centrifuged at 4°C and 12,000 r/min for 10 min, and the supernatant was collected. Protein concentration was determined using a bicinchoninic acid (BCA) protein assay kit (Thermo Scientific, MA). The protein sample was mixed with 5 × protein loading buffer (Boster biological, Wuhan, China) at a ratio of 4:1 and denatured in a boiling water bath for 10 min. Protein samples (20 µg/ lane) were separated on 10% or 12% polyacrylamide gels. After electrophoresis, proteins were transferred to a 0.22 or 0.45 μm nitrocellulose membrane (NC membrane) using a wet transfer system (Junyi, Beijing, China). The NC membranes was blocked in 5% BSA blocking solution for 1 h at 37°C. NC membranes were incubated overnight at 4°C with indicated primary antibodies, including ATG5 (Abmart, Shanghai, China), Beclin-1 (Abmart, Shanghai, China), LC3B (Abmart, Shanghai, China), and P62 (Proteintech, Wuhan, China). After washing off the primary antibody, the NC membrane was incubated in Goat Anti-Rabbit Ig-G-HRP (Abmart, Shanghai, China) secondary antibody for 2 h at 28°C. The secondary antibody was washed, and the ECL chemiluminescence reaction was carried out. Imaging was conducted with a high-sensitivity chemiluminescence imager (BIO RAD, ChemiDOC XRS + Imaging System, CA). We used β-Actin (CST, MA) as an internal reference protein.
Fluorescence Stain
The chick embryo cecal epithelial cells were seeded into a 24-well plate which had been preplaced with the treated cell crawl sheet, and the culture was continued at 41°C. The experiment was started when the cell growth reached about 90% adherent rate. The E. tenella group and the Control group were set respectively. The E. tenella group was co-cultured with sporozoites of E. tenella (2 × 105 sporozoites/well), while the Control group did not add any material. The cells were removed after 72 h of sporozoite infection and washed 3 times with preheated PBS buffer. After fixing the cell slides with 4% paraformaldehyde at room temperature for 20 min, permeate the cells with 0.2% TritonX-100 for 5 min. After blocking with 5% goat serum for 1 h at 37°C, cells were incubated with anti-LC3B Antibody (Abmart, Shanghai, China) overnight at 4°C. After washing with PBS 3 times, Goat Anti-rabbit IgG H&L/FITC secondary antibody (Bioss, Beijing, China) was incubated with PBS at 37°C for 1 h. Hoechst 33342 Stain (Beyotime, Shanghai, China) was used to stain the nuclei. After washing the cells three times with PBS, the coverslips were mounted onto glass with antifade solution before visualization on a confocal microscope. All images were acquired randomly using a laser scanning confocal fluorescence microscope.
Hematoxylin and Eosin Staining
The chamber slides in E. tenella group, RAPA + E. tenella group and 3-MA + E. tenella group were taken out at indicated times of infection, respectively. The slides were stained with H&E according to the method of Zhang et al. (2019). Sample E. tenella infections were observed by light microscopy in 200 randomly selected cells. Infection rate (%) was calculated using the following equation: number of infected parasite cells/200 × 100.
Image and Statistical Analyses
Results are presented as arithmetic mean ± standard error. Each experiment was repeated at least three times. Data were normally distributed. The SPSS 22.0 statistical software package (Chicago) was used to perform one-way analysis of variance (ANOVA) of all data. The Tukey post-hoc test was used for comparison between groups. Histograms were prepared with GraphPad Prism 6.0 software (San Diego, CA). Grayscale value was analyzed by ImageJ software (National Institutes of Health).
RESULTS
E. tenella Induces Autophagy in Chick Embryo Cecal Epithelial Cells
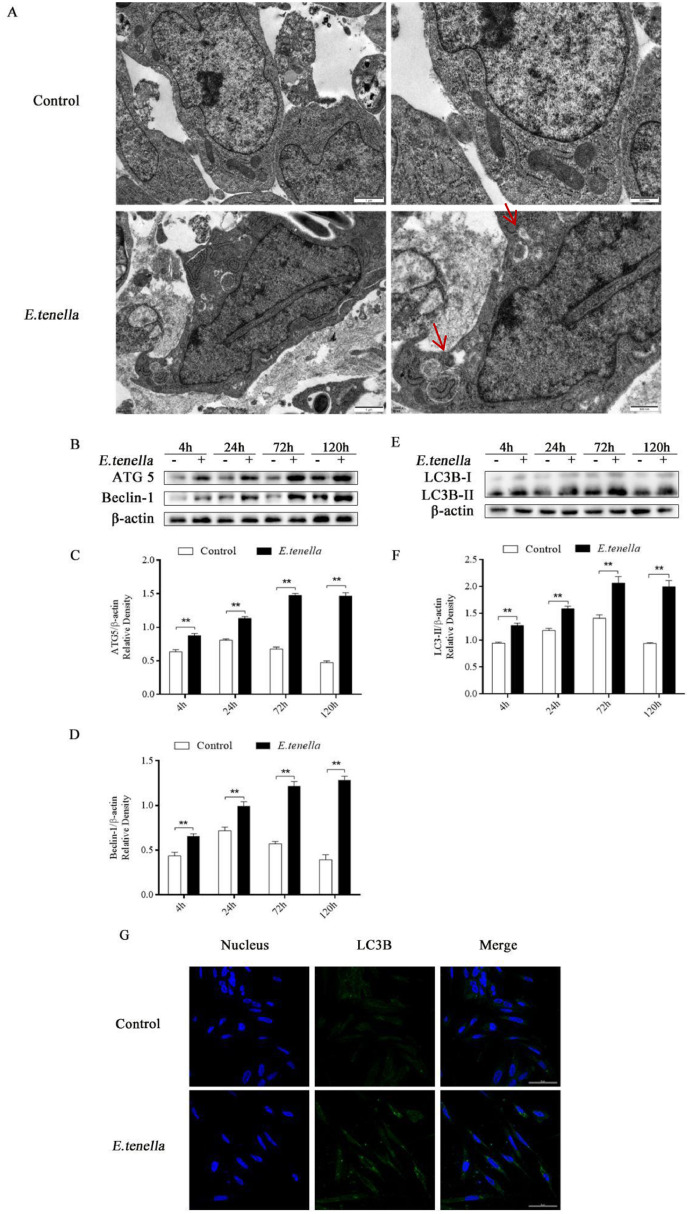
To investigate whether E. tenella induces autophagy in chick embryo cecal epithelial cells, TEM (considered the most intuitive detection method for autophagosomes) (Chakraborty, et al., 2020) was used to observe the ultrastructural autophagosomes of E. tenella infected cells for 72 h. It was found that the number of autophagosomes in the E. tenella group was significantly higher than that in the Control group (Figure 1 A). Western blot analysis was carried out to detect the protein expression levels of ATG 5, Beclin-1 and LC3B-II in E. tenella group and control group at 4, 24, 72, and 120 h. After the inoculation of sporozoites for 4 to 120 h, the protein levels expression of ATG 5, Beclin-1, and LC3B-II were significantly higher than that of the control group (P < 0.01) (Figure 1B–F). The changes and distribution of LC3B protein in cells were observed by laser confocal microscopy. In E. tenella group, a large number of LC3B green fluorescent spots appeared in cells, which were clearly visible. However, a small number of diffuse LC3B fluorescent spots appeared in the cells of the control group (Figure 1 G), indicated that E. tenella sporozoite infection could promote the secretion of LC3B protein in chick embryo cecal epithelial cells. These findings showed that E. tenella infection could induce autophagy in chick embryo cecal epithelial cells.
Figure 1.
E.tenella activates autophagy in chick embryo cecal epithelial cells. (A) Autophagic vacuoles in control and E. tenella-infected cells detected by using transmission electron microscopy (TEM) at 72 h. The arrow points to the autophagosome. The scale bar on the left is 1 µm. The scale bar on the right is 500 nm. (B–D) The expression levels of ATG 5 and Beclin-1 proteins in host cells infected with E. tenella sporozoites for 4 to 120 h. (E, F) The expression levels of LC3B-II protein in host cells infected with E. tenella sporozoites for 4 to 120 h. The protein levels of ATG 5, Beclin-1, and LC3B-II compared to β-actin protein levels were determined by densitometry. (G) LC3 dots (green) per cell captured under a confocal immunofluorescence microscopy at 72 h. Scale bar, 50 µm. "*" indicates P < 0.05; "**" indicates P < 0.01. The same as the figure below.
The Occurrence of Autophagy Flux in Chick Embryo Cecal Epithelial Cells During E. tenella Sporozoite Infection
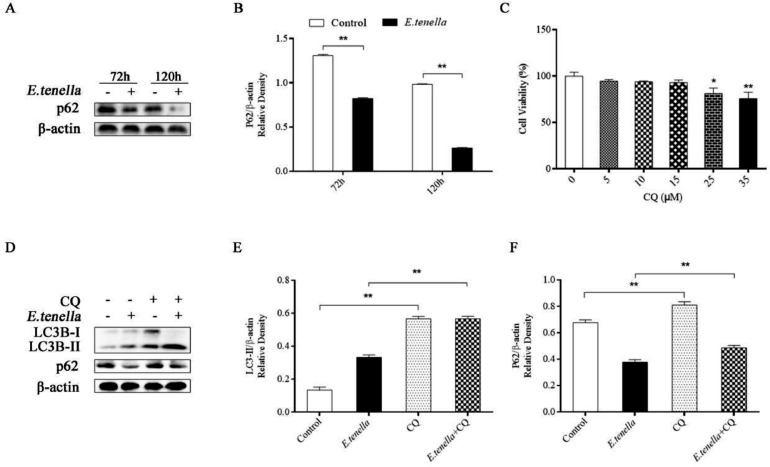
After inoculation with E. tenella for 72 to 120 h, the protein expression levels of P62 in host cells were significantly lower than that in the control group (P < 0.01) (Figure 2A, B).
Figure 2.
Autophagic flow in chick embryo cecal epithelial cells during E. tenella infection. (A, B) The expression levels of P62 protein in host cells infected with E. tenella sporozoites for 72 to 120 h. The protein levels of p62 compared to β-actin protein levels were determined by densitometry. (C) Effects of different concentrations of CQ on cell proliferation. (D, F) Changes in protein expression levels of LC3B-II and P62 in cells treated with CQ (15 µM).
CCK8 was used to detect the toxicity of CQ on chick embryo cecal epithelial cells at the appropriate concentration. Fifteen micrometer CQ had no significant effect on the proliferation of chick embryo cecal epithelial cells (P > 0.05) (Figure 2 C). Therefore, in this study, cells were treated with 15 μM CQ.
The protein expressions levels of LC3B-II and P62 in the CQ group were significantly higher than that in the control group (P < 0.01). The protein expressions levels of LC3B-II and P62 in the CQ + E. tenella group were significantly higher than those in the E. tenella group at 72 h after inoculation (P < 0.01) (Figure 2 D–F). These results demonstrated that the increased expression of LC3B-II induced by E. tenella infection could be due to enhanced autophagosome synthesis, rather than blockade of autolysates.
Effect of Autophagy on Cecal Epithelial Cells of Chick Embryo Infected With E. tenella
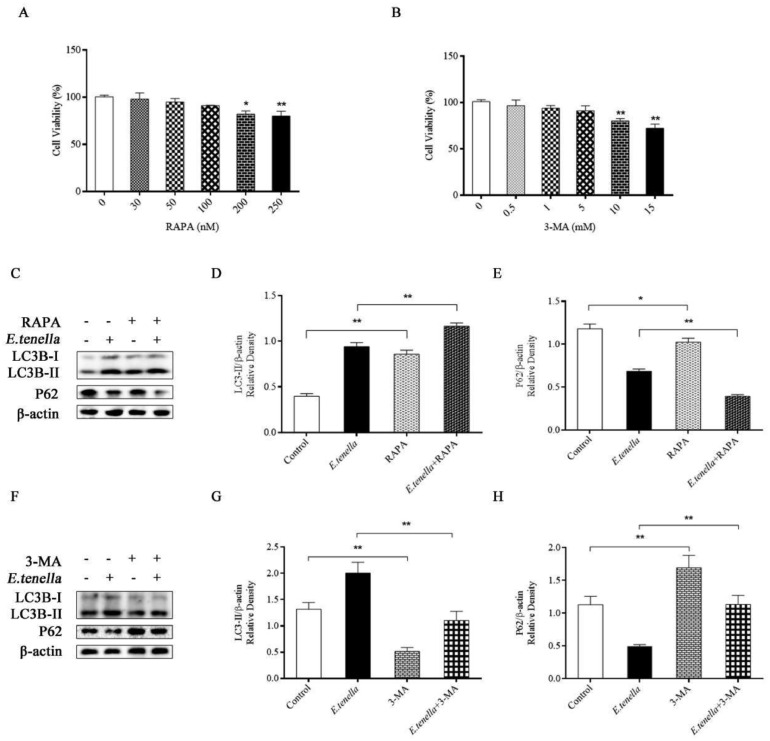
CCK8 was used to detect the proliferative activity of the two drugs at the appropriate concentration. 100 nM RAPA and 5 mM 3-MA had no significant effect on the proliferation of chick embryo cecal epithelial cells (P > 0.05) (Figure 3A, B). According to the CCK8 results, we selected the conditions of 100 nM RAPA and 5 mM 3-MA for 4 h of treatment for subsequent experiments.
Figure 3.
Effects of RAPA and 3-MA on autophagy. (A, B) Effects of different concentrations of RAPA and 3-MA on cell proliferation. (C–H) The expression levels of LC3B-II and P62 proteins in chick embryo cecal epithelial cells treated with RAPA (100 nM) (C–E) or 3-MA (5 mM) (F–H) during E. tenella infection.
The protein expression of LC3B-II in the RAPA group was significantly higher than that in the control group (P < 0.01), while the protein expression of P62 in the RAPA group was lower than that in the control group (P < 0.05). After 72 h of E. tenella infection, the expression of LC3B-II protein in RAPA + E. tenella group was significantly higher than that in E. tenella group (P < 0.01), while the expression of P62 protein was significantly lower than that in E. tenella group (P < 0.01) (Figure 3 C–E).
The protein expression of LC3B-II in the 3-MA group was significantly lower than that in the control group (P < 0.01), while the protein expression of P62 in the 3-MA group was significantly higher than that in the control group (P < 0.01). After 72 h of E. tenella infection, the expression of LC3B-II protein in 3-MA + E. tenella group was significantly lower than that in E. tenella group (P < 0.01), while the expression of P62 protein was significantly higher than that in E. tenella group (P < 0.01) (Figure 3 F–H). The results showed that RAPA could activate autophagy in chick embryo cecal epithelial cells, and 3-MA inhibited autophagy activation.
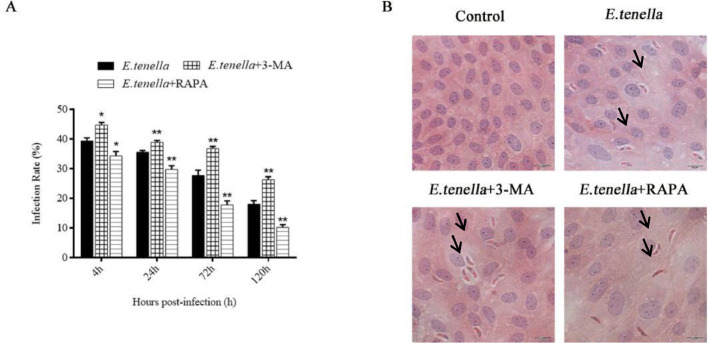
At 4 h after sporozoite inoculation, the infection rate of 3-MA+ E. tenella group was higher than that of E. tenella group (P < 0.05), and the infection rate of RAPA + E. tenella group was significantly lower than that of E. tenella group (P < 0.05). After sporozoite inoculation for 24 to 120 h, the infection rate of 3-MA + E. tenella group was significantly higher than that of E. tenella group (P < 0.01). On the contrary, the infection rate of RAPA + E. tenella group was significantly lower than that of E. tenella group (P <0.01) (Figure 4). Based on the results, RAPA activated autophagy and decreased the infection rate of E. tenella in host cells, while 3-MA inhibited autophagy and increased the infection rate.
Figure 4.
E. tenella infection of host cells. (A) Infection rate of cells at 4, 24, 72, and 120 h after inoculation with E. tenella. (B) H.E staining of cells. Scale bar, 10 µm. The arrow points to the sporozoite.
DISCUSSION
Chicken coccidiosis is a global parasitic disease with extremely serious damage. Coccidia can infect chickens of all ages, parasitizing in the epithelial cells of the digestive tract, mainly damaging the intestinal mucosa, leading to severe bleeding in the intestinal lumen, abdominal pain, diarrhea and bloody dysentery (Guo et al., 2013). In some cases, coccidia may lead to growth and development arrest and production performance decline of chickens, and in some cases, large numbers of deaths of chickens. At present, anti-coccidiosis drugs and vaccines are the main methods for prevention and control of chicken coccidiosis, but drug resistance and the pathogenicity of live vaccines limit their wide application. Therefore, it is of great significance to strengthen the research on the invasion mechanism of coccidia and the interaction mechanism between coccidia and host to provide theoretical basis for finding the targets of anti-coccidia drugs and to develop new vaccines. The results of this study showed that E. tenella infection activated autophagy in chick embryo cecal epithelial cells, and induced autophagy could reduce the infection rate of E. tenella in host cells.
Autophagy refers to the formation of autophagosomes by cells wrapping the substances to be degraded in monolayer or bilayer membranes, and the fusion of autophagosomes and lysosomes to form autophagosomes, followed by digestion and degradation of various enzymes, so as to realize the metabolic needs of cells themselves and the renewal of some organelles (Wang et al., 2019). Studies have shown that autophagy plays an important role in the process of parasite removal by host cells, and parasites have evolved mechanisms to avoid being removed, and can even use autophagy to provide energy (Lopez Corcino et al., 2019). Studies have shown that selective autophagy occurs in mouse hepatocytes infected with Plasmodium berghei. In the infrared phase of infection, highly propagated Plasmodium parasites are able to destroy autophagic vesicles and use them as a source of nutrients for growth, thus facilitating the growth of Plasmodium in the infrared phase (Wacker et al., 2017). Autophagy of host cells can promote the development of Trypanosoma brucei. The growth of Trypanosoma brucei requires the production of ATP by autophagy of host cells. The level of autophagy of host infected with Trypanosoma brucei is positively correlated with the level of cellular ATP (Li and He, 2017). When Leishmania SPP. infects host macrophages, it inhibits autophagy activation, resulting in reduced survival of intracellular parasites. However, host autophagy improved the survival rate of Leishmania SPP (Crauwels et al., 2015; Thomas et al., 2018). N. caninum infection induced autophagy in caprine EECs via inhibition of mTOR signaling. The enhancement of autophagy significantly promoted the intracellular proliferation of N. caninum in vitro (Zhao et al., 2022). CD40 in endothelial cells infected with T. gondii can induce increased expression of heat shock protein 70, trigger HSP70-dependent autophagy, and inhibit T. gondii activity, thereby limiting T. gondii invasion of neural tissues (Portillo et al., 2019). In addition, T. gondii infected cells, on the one hand, upregulated the phosphorylation level of EGFR through MIC 3 and MIC 6 (Muniz-Feliciano et al., 2013a), and on the other hand, it upregulated the self-phosphorylation level of EGFR and increased the phosphorylation level of ECFR downstream substrate Akt by activating PKCa/PKCB-Src signaling pathway in the late stage of infection. At the same time, the CD40-CD40L signaling pathway is regulated by activating EGFR-Akt, and the bilayer autophagic membrane structure expressing autophagosome marker protein LC3 is prevented from targeting T. gondii, so as to avoid the damage of PV and killing of T. gondii by lysosomes (Lopez Corcino et al., 2019). In this study, compared with the Control group, the protein expression levels of ATG 5, Beclin-1, and LC3B-II in cecal epithelial cells of chick embryo in E. tenella group were increased after 4 to 120 h. A large number of autophagosomes were observed by transmission electron microscopy and LC3B protein accumulation by confocal laser observation at 72 h after E. tenella infection in chick embryo cecal epithelial cells. These results suggest that E. tenella infection induces autophagy in chick embryo cecal epithelial cells. The result of Western blot showed that the expression of p62 protein was significantly decreased at 72 to 120 h after E. tenella infection. In addition, CQ treatment significantly increased the expression of P62 protein in E. tenella infected cells, which further confirmed that E. tenella infected chicken embryo cecal epithelial cells could promote the fusion of autophagosomes and lysosomes. These results indicated that E. tenella infection could induce autophagy activation in chick embryo cecal epithelial cells. To examine the effect of autophagy on E. tenella infected host cells, we treated chick embryo cecal epithelial cells with autophagy inducer (RAPA) and inhibitor (3-MA). RAPA induced autophagy in host cells significantly reduced the intracellular infection of E. tenella. In contrast, the infection rate of E. tenella increased in cells treated with 3-MA. These results proved that induction of autophagy could reduce E. tenella infection rate. This study revealed for the first time the mechanism of interaction between E. tenella infection and host cell autophagy, which is of great significance for the prevention and treatment of chicken coccidiosis.
The autophagy induced by E. tenella infection was studied for the first time. The enhanced autophagy of host cells significantly inhibited E. tenella infection of host cells. These results suggest that E. tenella plays an important role in parasite intracellular infection by inducing autophagy in host cells.
ACKNOWLEDGMENTS
This study was funded by a grant from the National Natural Science Foundation of China (Grant No. 31602029), the Natural Science Research Project of Shanxi Province (No. 202103021224165) and the Key Research and Development Program of Jinzhong City (No. Y212010).
DISCLOSURES
Yu Zhang carried out most of the experiments, wrote the manuscript, and should be considered as primary author. Rui Bai critically revised the manuscript and the experiment design. Bu-ting Duan, Yong-juan Zhao, Kai-ling Cui, Tong Xu, Xue-song Zhang, Xiao-ling Lv, Lu-Lu Guo, and Ming-xue Zheng helped with the experiment. All the authors read and approved the final version of the manuscript.
References
- Alvarez V.E., Kosec G., Sant'Anna C., Turk V., Cazzulo J.J., Turk B. Autophagy is involved in nutritional stress response and differentiation in Trypanosoma cruzi. J. Biol. Chem. 2008;283:3454–3464. doi: 10.1074/jbc.M708474200. [DOI] [PubMed] [Google Scholar]
- Alvarez V.E., Kosec G., Sant Anna C., Turk V., Cazzulo J.J., Turk B. Blocking autophagy to prevent parasite differentiation: a possible new strategy for fighting parasitic infections? Autophagy. 2008;4:361–363. doi: 10.4161/auto.5592. [DOI] [PubMed] [Google Scholar]
- Blake D.P., Knox J., Dehaeck B., Huntington B., Rathinam T., Ravipati V., Ayoade S., Gilbert W., Adebambo A.O., Jatau I.D., Raman M., Parker D., Rushton J., Tomley F.M. Re-calculating the cost of coccidiosis in chickens. Vet. Res. 2020;51:115. doi: 10.1186/s13567-020-00837-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty J., Caicci F., Roy M., Ziviani E. Investigating mitochondrial autophagy by routine transmission electron microscopy: seeing is believing? Pharmacol. Res. 2020;160 doi: 10.1016/j.phrs.2020.105097. [DOI] [PubMed] [Google Scholar]
- Crauwels P., Bohn R., Thomas M., Gottwalt S., Jackel F., Kramer S., Bank E., Tenzer S., Walther P., Bastian M., van Zandbergen G. Apoptotic-like Leishmania exploit the host's autophagy machinery to reduce T-cell-mediated parasite elimination. Autophagy. 2015;11:285–297. doi: 10.1080/15548627.2014.998904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo A., Cai J., Gong W., Yan H., Luo X., Tian G., Zhang S., Zhang H., Zhu G., Cai X. Transcriptome analysis in chicken cecal epithelia upon infection by Eimeria tenella in vivo. PLoS One. 2013;8:e64236. doi: 10.1371/journal.pone.0064236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F.-J., He C.Y. Autophagy in protozoan parasites: trypanosoma brucei as a model. Future Microbiol. 2017:1337–1340. doi: 10.2217/fmb-2017-0158. [DOI] [PubMed] [Google Scholar]
- Li W., He P., Huang Y., Li Y.F., Lu J., Li M., Kurihara H., Luo Z., Meng T., Onishi M., Ma C., Jiang L., Hu Y., Gong Q., Zhu D., Xu Y., Liu R., Liu L., Yi C., Zhu Y., Ma N., Okamoto K., Xie Z., Liu J., He R.R., Feng D. Selective autophagy of intracellular organelles: recent research advances. Theranostics. 2021;11:222–256. doi: 10.7150/thno.49860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian C.-Y., Chu B.-X., Xia W.-H., Wang Z.-Y., Fan R.-F. and Wang L., Persistent activation of Nrf2 in a p62-dependent non-canonical manner aggravates lead-induced kidney injury by promoting apoptosis and inhibiting autophagy, J. Adv. Res, 2022. In press. [DOI] [PMC free article] [PubMed]
- Lopez Corcino Y., Ferrer S.Gonzalez, Mantilla L.E., Trikeriotis S., Yu J.S., Kim S., Hansen S., Portillo J.C., Subauste C.S. Toxoplasma gondii induces prolonged host epidermal growth factor receptor signalling to prevent parasite elimination by autophagy: perspectives for in vivo control of the parasite. Cell Microbiol. 2019;21:e13084. doi: 10.1111/cmi.13084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubayashi M., Inaoka D.K., Komatsuya K., Hatta T., Kawahara F., Sakamoto K., Hikosaka K., Yamagishi J., Sasai K., Shiba T., Harada S., Tsuji N., Kita K. Novel characteristics of mitochondrial electron transport chain from Eimeria tenella. Genes (Basel) 2019;10:29. doi: 10.3390/genes10010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messer J.S. The cellular autophagy/apoptosis checkpoint during inflammation. Cell Mol. Life Sci. 2017;74:1281–1296. doi: 10.1007/s00018-016-2403-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muniz-Feliciano L., Van Grol J., Portillo J., Liew L., Liu B., Carlin C., Carruthers V., Matthews S., Subauste C. Toxoplasma gondii-induced activation of EGFR prevents autophagy protein-mediated killing of the parasite. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muniz-Feliciano L., Van Grol J., Portillo J.A., Liew L., Liu B., Carlin C.R., Carruthers V.B., Matthews S., Subauste C.S. Toxoplasma gondii-induced activation of EGFR prevents autophagy protein-mediated killing of the parasite. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portillo J., Van Grol J., Saffo S., Lopez Corcino Y., Rodriguez M., Fox B., Bzik D., Ward N., Dubyak G., Rojas R., Toosi Z., Subauste C. CD40 in endothelial cells restricts neural tissue invasion by Toxoplasma gondii. Infect. Immun. 2019;87:e00868–18. doi: 10.1128/IAI.00868-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi N., Liao S., Abuzeid A.M.I., Li J., Wu C., Lv M., Lin X., Hu J., Yu L., Xiao W., Sun M., Li G. The effect of autophagy on the survival and invasive activity of Eimeria tenella sporozoites. Sci. Rep. 2019;9:5835. doi: 10.1038/s41598-019-41947-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi N., Liao S., Mohiuddin M., Abuzeid A.M.I., Li J., Wu C., Lv M., Lin X., Hu J., Cai H., Yu L., Xiao W., Sun M., Li G. Autophagy induced by monensin serves as a mechanism for programmed death in Eimeria tenella. Vet. Parasitol. 2020;287 doi: 10.1016/j.vetpar.2020.109181. [DOI] [PubMed] [Google Scholar]
- Rossman J.S., Lamb R.A. Autophagy, apoptosis, and the influenza virus M2 protein. Cell Host Microbe. 2009;6:299–300. doi: 10.1016/j.chom.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Thomas S.A., Nandan D., Kass J., Reiner N.E. Countervailing, time-dependent effects on host autophagy promote intracellular survival of Leishmania. J. Biol. Chem. 2018;293:2617–2630. doi: 10.1074/jbc.M117.808675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker R., Eickel N., Schmuckli-Maurer J., Annoura T., Niklaus L., Khan S.M., Guan J.L., Heussler V.T. LC3-association with the parasitophorous vacuole membrane of Plasmodium berghei liver stages follows a noncanonical autophagy pathway. Cell Microbiol. 2017,;19:e12754. doi: 10.1111/cmi.12754. [DOI] [PubMed] [Google Scholar]
- Wang L., Fan R., Yang D., Zhang D., Wang L. Puerarin reverses cadmium-induced lysosomal dysfunction in primary rat proximal tubular cells via inhibiting Nrf2 pathway. Biochem. Pharmacol. 2019;162:132–141. doi: 10.1016/j.bcp.2018.10.016. [DOI] [PubMed] [Google Scholar]
- Zhang L. Study on pathogenicity differences and pathogenic genes of E. tenella precocious strains and virulent strains. Diss. Univ. Shanxi, Jinzhong; 2020. [Google Scholar]
- Zhao S.S., Tao D.L., Chen J.M., Chen X., Geng X.L., Wang J.W., Yang X., Song J.K., Liu Q., Zhao G.H. Neospora caninum infection activated autophagy of caprine endometrial epithelial cells via mTOR signaling. Vet. Parasitol. 2022;304 doi: 10.1016/j.vetpar.2022.109685. [DOI] [PubMed] [Google Scholar]
- Zhang X., Li S., Zheng M., Zhang L., Bai R., Li R., Hao S., Bai B., Kang H. Effects of the PI3K/Akt signaling pathway on the apoptosis of early host cells infected with Eimeria tenella. Parasitol. Res. 2020;119:2549–2561. doi: 10.1007/s00436-020-06738-9. [DOI] [PubMed] [Google Scholar]
- Zhang L., Zheng M.X., Xi R., Xu Z.Y., Zhang X.S., Zheng L.L., Bai R., Mi C.L., Hao F.F., Feng Y.P. Comparison of the host cells apoptosis induced by precocious strains and virulent strains of Eimeria tenella. Poult. Sci. 2019;98:4384–4390. doi: 10.3382/ps/pez218. [DOI] [PubMed] [Google Scholar]
- Zhao Z., Fux B., Goodwin M., Dunay I.R., Strong D., Miller B.C., Cadwell K., Delgado M.A., Ponpuak M., Green K.G., Schmidt R.E., Mizushima N., Deretic V., Sibley L.D., Virgin H.W. Autophagosome-independent essential function for the autophagy protein Atg5 in cellular immunity to intracellular pathogens. Cell Host Microbe. 2008;4:458–469. doi: 10.1016/j.chom.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]