Abstract
Pharmacovigilance, the science and practice of monitoring the effects of medicinals and their safety, is the responsibility of all stakeholders involved in the development, manufacture, regulation, distribution, prescription, and use of drugs and devices. The patient is the stakeholder most impacted by and the greatest source of information on safety issues. It is rare, however, for the patient to take a central role and exert leadership in the design and execution of pharmacovigilance. Patient organizations in the inherited bleeding disorders community are among the most established and empowered, particularly in the rare disorders. In this review, two of the largest bleeding disorders patient organizations, Hemophilia Federation of America (HFA) and National Hemophilia Foundation (NHF), offer insights into the priority actions required of all stakeholders to improve pharmacovigilance. The recent and ongoing increase in incidents raising safety concerns and a therapeutic landscape on the cusp of unprecedented expansion heighten the urgency of a recommitment to the primacy of patient safety and well-being in drug development and distribution.
Plain Language Summary
Patients at the center of product safety
Every medical device and therapeutic product has potential benefits and harms. The pharmaceutical and biomedical companies that develop them must demonstrate that they are effective, and the safety risks are limited or manageable, for regulators to approve them for use and sale. After the product has been approved and people are using it in their daily lives, it is important to continue to collect information about any negative side effects or adverse events; this is called pharmacovigilance. Regulators, like the United States (US) Food and Drug Administration, the companies that sell and distribute the products, and healthcare professionals who prescribe them are all required to participate in collecting, reporting, analyzing, and communicating this information. The people with the most firsthand knowledge of the benefits and harms of the drug or device are the patients who use them. They have an important responsibility to learn how to recognize adverse events, how to report them, and to stay informed of any news about the product from the other partners in the pharmacovigilance network. Those partners have a crucial responsibility to provide clear, easy-to-understand information to patients about any new safety concerns that come to light. The community of people with inherited bleeding disorders has recently encountered problems with poor communication of product safety issues, prompting two large US patient organizations, National Hemophilia Foundation and Hemophilia Federation of America, to hold a Safety Summit with all the pharmacovigilance network partners. Together they developed recommendations to improve the collection and communication of information about product safety so that patients can make well-informed, timely decisions about their use of drugs and devices. This article presents these recommendations in the context of how pharmacovigilance is supposed to work and some of the challenges encountered by the community.
Keywords: advocacy, community, drug safety, inherited bleeding disorders, patient safety, pharmacovigilance, postmarketing surveillance
Introduction
Hemophilia, the best known of the inherited bleeding disorders, is optimally managed by healthcare professionals (HCPs) functioning as a multidisciplinary comprehensive care team that collaborates with the patient who functions as the subject matter expert (SME) regarding disease manifestations and the safety and effectiveness of the interventions employed to manage it.1 This model is one in which decision-making is shared by HCPs, the SME, and their family.2 In this way, SMEs become advocates for their health and well-being, working with HCPs as true partners and not simply consumers of health care.3 The patient/SME is the stakeholder with the most intimate knowledge of and the greatest stake in product safety and should be at the center of pharmacovigilance.4 This review will focus on the role of the patient in pharmacovigilance, the importance of all other stakeholders committing to this patient-centric approach, and some practical directions for each stakeholder group to operationalize this commitment.
Unique history of people with hemophilia and pharmacovigilance
For decades the standard treatment of the clotting factor deficiencies, hemophilia A [factor (F) VIII deficiency] and hemophilia B (FIX deficiency), has been the prophylactic infusion of concentrates of the missing factor.5 Prior to their availability, people with hemophilia (PWH) experienced frequent debilitating bleeding, diminished quality of life, and significantly reduced life expectancy. In the 1920s, the life expectancy of a person with severe hemophilia was approximately 11 years6 with death often resulting from bleeding into vital organs.7 The development of plasma-derived clotting factor concentrates was heralded, by SMEs and HCPs alike, as a life-altering breakthrough; the treatment paradigm changed from treating bleeding events to preventing them. With the introduction of regular prophylactic infusions of factor concentrates bleeding, especially into joints, was greatly reduced resulting in better joint outcomes for children8,9 and adults.10 Today, prophylaxis combined with a patient-centered multidisciplinary team approach to care means that many PWH in high-income countries enjoy a life expectancy comparable with that of people without a bleeding disorder.1,11,12
Prior to the introduction of recombinant factor concentrates, many plasma-derived factor concentrates contained viruses responsible for hepatitis and acquired immunodeficiency syndrome (AIDS) which many PWH contracted and eventually succumbed to. During the 1981–1984 period, more than 50% of PWH in the United States (US) were infected with human immunodeficiency virus (HIV),13–15 and in 1988, over 99% of HIV-positive PWH in the US had also been infected with the hepatitis B, C, or D virus (HBV, HCV, HDV).15,16 Subsequent advances in viral inactivation techniques drastically reduced, if not eliminated, the risk of known blood-borne pathogens; in the currently well-regulated markets (e.g. North America and Europe), no infections from fractionated plasma products have been reported in over 25 years.17 The underappreciation of the consequences of non-A, non-B hepatitis transmission and the hesitancy of many leading hematologists, regulators, manufacturers, interest groups, and patient and governmental organizations to recognize that AIDS was transmitted by a blood-borne pathogen and require implementation of donor screening and heat-inactivation steps to eliminate it from factor products, however, prolonged the epidemic in the bleeding disorders community and cost lives.15,18,19
These events highly motivated the bleeding disorders community to pursue an agenda of accountability and transparency in blood product safety, but also engendered a hesitance to trust the system that initially failed them. Their extensive advocacy led to important advances in product safety regulations and patients’ rights legislation. In the US, the personal efforts and stories of many individuals drove the convening of an Institute of Medicine (IOM) committee to study the transmission of HIV through the blood supply.18 The committee conducted an in-depth investigation of the crisis and interviewed many of the people involved and affected, drawing important conclusions about what went wrong and making 14 detailed recommendations about what needed to be done differently going forward.18
Most of the 14 recommendations have been implemented including legislation such as the HIV Blood Supply and Ricky Ray Hemophilia Relief Acts, and significant changes to the way that blood products are regulated.18,19 The Assistant Secretary for Health (ASH) now also serves as the blood safety director with final responsibility and authority for decisions regarding blood safety and availability. The Blood, Organ and Tissue Safety Executive Council (BOTSEC), whose members include heads of Public Health Service and related agencies, provides regular updates and guidance regarding current and emergent issues to the ASH. An Advisory Committee on Blood and Tissue Safety and Availability (ACBTSA) was also created with the mandate to advise and make policy recommendations to the Secretary of Health and Human Services (HHS) regarding regulation of the collection, preparation, and distribution of blood, blood products, tissue and organs, and the potential transmission of communicable diseases. Both the ACBTSA and the US Food and Drug Administration (FDA) Blood Products Advisory Committee now include representatives of the communities most impacted by the transmission of viruses through the blood supply, as full voting members.19,20 This revised federal structure is designed to be more responsive in addressing blood safety and availability issues, and applies a risk management philosophy that remains data driven, but is more precautionary and patient-focused.19
In addition to effective HHS leadership to coordinate the response to any future threats to blood safety and availability, several other IOM conclusions and recommendations resonate particularly with the bleeding disorders community today.18 They highlighted the need for an agency to serve as the nation’s early warning system for threats to the health of the public, and recommended adequate funding and support (including from other federal agencies) for the US Centers for Disease Control and Prevention (CDC) to fulfill this responsibility. They recommended that the FDA periodically review important decisions made in a context of uncertainty surrounding some key decision variables, which may prove very relevant as first approvals are sought for novel bleeding disorders therapies such as gene therapy for hemophilia.21,22 The IOM emphasized the importance of HCPs/physicians and patients very intentionally discussing all the potential impacts of the full range of available options and utilizing a shared decision-making framework for these discussions.2,18 Importantly, they concluded that it is essential for voluntary health organizations, such as National Hemophilia Foundation (NHF) and Hemophilia Federation of America (HFA), who advise people with bleeding disorders about ongoing safety concerns, to avoid conflicts of interest, maintain independent judgment, and otherwise act to earn the confidence of the public and patients.18
Over many years, trustworthy relationships have been rebuilt between the bleeding disorders community and regulatory, industry, and healthcare establishment stakeholders. A heightened wariness and sense of responsibility for their own safety, however, continue to drive advocacy in the form of agency (e.g. timely access to information and education) and activism (e.g. awareness raising) efforts23 for ongoing vigilance. For many, honoring lost loved ones means preventing any potential future tragedy, and ensuring that lessons learned at such a high price were not in vain. Safety issues must be detected early, taken seriously, reported reliably, and acted upon. All stakeholders, including patients, must be held accountable, and every effort made to optimize prompt, effective communication of safety information.
Cause for concern: recent safety incidents
A number of drug safety incidents concerning bleeding disorder therapeutics occurred in 2019, 2020, and into 2021 (Table 1); recalling their tragic experience with HIV and hepatitis infections through tainted clotting factor products, the community response was rapid and robust. Issues continued to arise into 2021, and some will impact patients at least into 2024. In response, HFA and NHF initiated conversations with manufacturers and regulators to elucidate the nature of the issues, demand appropriate actions, and ensure transparent information exchange to and from patients and HCPs. Their efforts yielded only partial results as responses were often extremely limited. In addition to resurrecting past trauma, these incidents laid bare inadequacies in the current system that patients had only relatively recently come to trust again.
Table 1.
Bleeding disorders product safety and recall incidents of approved therapeutics, 2019–2021.
| Product (date issue reported) | Manufacturer/distributor | Issue | Scope | Action taken | Communications, including initial notifications and correspondence with manufacturer |
|---|---|---|---|---|---|
| Kogenate® FS (19 Jun, 2019) | Bayer | Mislabeling resulted in distribution of wrong product, wrong dose, post-expiration | • Two lots 900+ vials • Distributed over 6 months |
Class 2 voluntary recall to end user | • Recall Notice • HFA-NHF Joint Statement 1 • HFA-NHF Joint Statement 2 • HFA-NHF Joint Statement 3 • HFA-NHF Joint Statement 4 • HFA-NHF Joint Statement 5 • Bayer Response, 9/27/19 • FDA Call Update to Community |
| Hemlibra® (21 Sept, 2019) | Genentech (through contract specialty pharmacy, MedVantx) | Shipment of incorrect length injection needles to patients who receive product through the Genentech Patient Foundation | • 124 families and 92 HCPs • Shipments involved took place over ~2 months |
No recall Genentech notified FDA via standard US Drug Safety reporting |
• Safety Announcement • HFA-NHF Joint Statement 1 • Genentech Patient Foundation Response |
| Hemlibra® (5 Oct, 2019) | Genentech | Particulate matter outside specifications found in vials of Hemlibra (issue is product deviation, not contamination) | • Genentech notified US, European, Canadian, and Japanese health authorities in Mar 2019 | No recall Regulators agreed there was no change in product benefit/risk profile |
• Genentech statement, MASAC response • HFA-NHF Joint Letter • Genentech Response, 10/17/19 |
| Humate-P® (15 Oct, 2019) | CSL Behring | Printing misalignment on product label could lead to confusion about dose/potency | • Lots of all fill sizes (600, 1200, 2400 IUs) distributed by CSL Behring since Feb 2019 | Filed ‘Important Drug Information Notice with FDA’ | • CSL Behring Announcement |
| VONVENDI® (25 Feb, 2020) | Takeda | Internal manufacturer audit revealed deviation in process, manufacturer self-reported to FDA. FDA provided improvement feedback to the manufacturer, who voluntarily recalled lots manufactured during this period, despite finding no impact on product safety or efficacy | • Two lots • 3425 vials • US only |
Voluntary pharmacy-level recall | • Pharmacy Level Recall Notification • HFA-NHF Joint Statement • Takeda response, 3/6/2020 |
| Stimate® nasal spray (21 Jul, 2020) | Ferring (manufacturer)/CSL Behring (distributor) | Product testing revealed low volume and therefore above-specification concentration of active ingredient | • All product world-wide | Voluntary recall (initiated as pharmacy-level recall;
subsequently extended to consumer level) Manufacturer does not anticipate resupply before 2023 |
• Initial Recall Notice • HFA-NHF Statement 1 • CSL Behring/Ferring pharmaceuticals response, 8/7/2020 • Recall extension notice • Product availability notice 1 • Product availability notice 2 |
| Tranexamic acid injection (1 Sept, 2020) | Mylan | Carton label mixup (some packages may have been mislabeled as an unrelated product, Amiodarone HCl Injection) | • Four lots of Tranexamic Acid Injection, USP 1000 mg/10 ml,
packaged in cartons of 10 single-dose 10 ml vials • Four lots of Amiodarone HCl Injection, USP |
Voluntary recall to the hospital/clinic level (product exclusively used in inpatient setting) | • Recall notice |
| Mononine®* (21 Jan, 2021) | CSL Behring | Unclear | • One lot | Voluntary pharmacy-level recall. Manufacturer stated that ‘patients can continue to use product they may have. Although the potential for safety risk to patients is considered low, it cannot be fully excluded’ |
• Recall notice |
FDA, US Food and Drug Administration; HCP, healthcare professional; HFA, Hemophilia Federation of America; NHF, National Hemophilia Foundation; US, United States.
Gray shading indicates an incident involving a voluntary FDA recall.
CSL Behring provided notice of discontinuation of this product 4 months previous.
Communications from manufacturers, distributors, and regulators were often minimal, vague or unclear, and inconsistent (readers are invited to review the communications available through the links in Table 1). Some issues were identified half a year before they were communicated to patients, and then only upon the insistence of patient organizations. Requests for information from the regulators sometimes garnered an uninformative ‘under investigation’ response. Recalls were communicated to certain stakeholders, such as pharmacies, but often not to patients, creating confusion and concern. Presenting issues not meeting the recall threshold as ‘quality control issues’ misrepresents the safety concern they constitute for patients and serves only to obfuscate the message and diminish trust in official statements. Communication tools, such as official statements, letters to stakeholders, activation of the patient notification system (PNS), and official regulatory channels have been employed inconsistently within and between incidents and companies. These challenges mean that patients are often left without clear, accessible, actionable information about whether to use a treatment product or not. These incidents have made clear that considerable work remains to achieve a system in which all incidents potentially related to product safety are handled promptly, transparently, consistently and with patient safety and well-being as the overarching guiding principles.
Changing therapeutic landscape heightens urgency
Decades of experience with clotting factor replacement therapeutics yielded a reasonably complete characterization of their benefit–risk profile. The development of inhibitors, alloantibodies that recognize replacement factor as foreign and inhibit its function in blood clotting, is the primary potential treatment complication, occurring in up to 25–30% of people with severe hemophilia A.24 Recent innovations brought a variety of nonfactor concentrate clotting factor deficiency treatment products to clinical trials.25 A humanized bispecific monoclonal antibody that mimics the function of activated factor VIII in the coagulation cascade has been approved for the treatment of hemophilia A, with or without inhibitors, in many countries.26 Multiple clinical trials of gene therapy for hemophilia A and B, phases I through III, are currently underway25 with one product approved by the European Commission (EC) to treat adults with severe hemophilia A, at the time of writing.21,22 Inhibition of anticoagulants such as antithrombin and tissue factor pathway inhibitor, through ribonucleic acid interference (RNAi) or a monoclonal antibody, respectively, are currently in phase III clinical trials.25 Other potential avenues to restoring hemostatic balance are also being explored. The bleeding disorders community must navigate from the familiar safety terrain of clotting factor concentrates into a more complex landscape of experimental therapies, clinical trials, novel mechanisms of action, and unknown unknowns.27,28 As recently reviewed in detail,27 novel therapies pharmacovigilance must now include lifelong surveillance for potential injection site reactions, thrombotic microangiopathies and thrombotic events, antidrug antibodies to monoclonal antibodies, liver transaminase elevations, liver fibrosis, oncogenicity, and insertional mutagenesis.27–29 This evolving context combined with the ongoing series of safety incidents heightens the urgency of optimizing all aspects of safety information communication between all stakeholders, to secure the safety and confidence of the bleeding disorders community.
Successful communication of safety information
Effective communication of safety information from and to all stakeholders requires familiarity with shared terms and definitions to avoid confusion on critical issues. Table 2 provides a glossary of useful terms often encountered in the communication of safety information.
Table 2.
Glossary of terms frequently used to communicate safety information.
| Term | Definition |
|---|---|
| Adverse (drug) event,30 A(D)E | Any untoward medical occurrence that may be present during treatment with a medicine but does not necessarily have a causal relationship with this treatment, that is, an adverse outcome that occurs while the patient is taking the medicine but is not, or not necessarily, attributable to it. |
| Adverse (drug) reaction,30 A(D)R | Any harm caused directly by a drug which is noxious and unintended, and which occurs at normal doses in normal use, such as for prophylaxis, diagnosis, or therapy of disease, or for the modification of physiological function. |
| Benefit31 | Helpful effects; help provided by a drug for the person who is taking it. |
| Causality30 | The probability that a particular medicine or substance is responsible for an isolated effect or adverse drug reaction. |
| Correlation32,33 | When two factors or variables (such as taking a drug and having an adverse reaction) tend to vary or occur together in a way that is not expected on the basis of chance alone, but one does not necessarily cause the other. |
| Effective34 | Providing services based on scientific knowledge to all who could benefit and refraining from providing services to those not likely to benefit (avoiding underuse and misuse, respectively). |
| Harm35 | Any physical or psychological injury or damage to the health of a person, including both temporary and permanent injury. |
| Hazard35 | A circumstance, agent, or action with the potential to cause harm. |
| Patient safety34 | The prevention of harm to patients. |
| Pharmacovigilance36 | The science and activities relating to the detection, assessment, understanding, and prevention of adverse effects or any other drug/device-related problem. |
| Risk35 | The combination of the probability of occurrence of harm and the severity of that harm. |
| Safe34 | Avoiding harm to patients from the care that is intended to help them. |
| Safety signal37 | A concern about an excess of adverse events compared with what would be expected to be associated with a product’s use. |
| Serious adverse effect,30 SAE | Any untoward medical occurrence at any dose that results in death, requires hospital admission or prolongation of existing hospital stay, results in persistent or significant disability or incapacity, or is life-threatening. |
| Side effect30 | Any unintended effect of a pharmaceutical product occurring at doses normally used in humans which is related to the pharmacological properties of the medicine. |
| Signal30 | Reported information on a possible causal relationship between an adverse event and a medicine, the relationship being previously unknown or incompletely documented. |
Patients as SMEs play a key role in drug and device safety. As the end users of treatments, they experience both the associated benefits and risks, and they must make timely and appropriate treatment decisions.38 Patients must be knowledgeable of the effects and the risks of medications and understand how to assess for the occurrence of a safety signal. All stakeholders performing a risk–benefit analysis require timely access to reliable information, but equally importantly, they must possess an ability to critically evaluate that information and contextualize it to their personal situation. They must be able to discern the appropriate degree of risk, causation from correlation, and anecdotal evidence from that obtained through scientific methodology. Patient safety has been defined by the IOM as ‘the prevention of harm to patients’39 that encompasses a goal of reducing the risk (i.e. the probability of encountering a danger) of adverse events (AEs). While the benefit–risk relationship is often summarized as a product being ‘safe and effective’ this can be misleading. ‘Safety’ and ‘safe’ are commonly used to mean an absence of harm, potentially leading to the public misinterpretation that once a drug reaches the market it is, or should be, risk-free. Clinical trials and ongoing pharmacovigilance monitor for the presence of harm. Patients and clinical trial participants have varied opinions about acceptable levels of harm or risk making these concepts relative to the individual.40 While a patient’s risk–benefit calculation may be heavily influenced by the current standard of care to which they have access, it is critically important that patient safety is upheld for all participants in clinical trials and all patients receiving an approved product.
To meaningfully participate in pharmacovigilance, patients must have a clear understanding of the process. It has been suggested that patient-reported AEs may result in earlier detection of safety signals.41,42 For this to be effective, the users of medications must be capable of discerning the presence of a potential AE and understand how to report that information. Data show that although patients and HCPs report about the same number of AEs, patients report AEs, in one study as much as 1 year, later than HCPs, resulting in a delay in identification of safety signals.43 This is likely due to inadequate patient knowledge and indicates the need for additional patient education regarding their prescribed medications, possible AEs, and how to report them. This study43 compared patient and HCP reports with the World Health Organization (WHO) global (>120 member countries) database of individual case safety reports, VigiBase;44 in most countries, patients can report AEs directly to their country’s national monitoring program themselves, or to manufacturers or HCPs who then report to those programs.45 Patients should be empowered and encouraged to report suspected AEs earlier. Telehealth and digital solutions such as online platforms and mobile phone apps facilitating spontaneous reporting or to follow specific patient groups over time may prove effective.45,46 Implementation of such tools, however, must not perpetuate or exacerbate access inequities; less than two in three US adults who identify as Hispanic or African American have broadband Internet at home, with similar patterns for those with lower income or education levels.47,48 HCPs in hospitals, clinics, and other healthcare delivery settings (e.g. nursing homes) must also have the necessary knowledge and tools to enable recognition and reporting of potential AEs.
Regulatory safety monitoring throughout the life cycle of a therapeutic
The complete safety information for any therapeutic can only be obtained by monitoring and reporting throughout the product’s life cycle, from preclinical studies to postmarketing surveillance and long-term data collection (Figure 1).
Figure 1.
Each phase in the life cycle of a therapeutic contributes important data to its complete safety profile, from preclinical studies through to postmarketing surveillance/pharmacovigilance.
Sources: https://www.who.int/teams/regulation-prequalification/pharmacovigilance,49 https://www.fda.gov/patients/drug-development-process/step-3-clinical-research#The_Investigational_New_Drug_Process,50 and https://www.fda.gov/science-research/clinical-trials-and-human-subject-protection/regulations-good-clinical-practice-and-clinical-trials.51
Clinical trials
In the US, drugs and biologics, among other things, are regulated by the FDA.52 The definitions and standards applied to safety reporting requirements by the FDA are intended to align with the guidelines developed by the Expert Working Group (Efficacy) of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) and recommended for adoption by the regulatory bodies of the European Union (EU), the US, and Japan.53
Throughout the clinical trials process, manufacturers have an obligation to respond to and share relevant safety information with the FDA and all participating investigators.54 They must promptly review any safety information they receive, from any source, and conduct periodic proactive reviews of potential sources, such as scientific literature55 and meetings.54 Clinical trial investigators also have obligations to report safety information to their Institutional Review Boards (IRBs) and to the FDA.54,56 Public availability of this information is not required by regulatory authorities but may help patients to make better informed decisions about participation in clinical trials and use of the therapeutic should it be approved.
Postmarketing surveillance
Many of the definitions (Table 2) and standards governing the preapproval phase of drug development also apply to the monitoring of safety signals postapproval. Safety information from investigational studies can now be bolstered by the experiences of patients using the therapeutic in the real world.27 Manufacturers must develop policies for the prompt, ongoing and continuous surveillance, receipt, evaluation, and reporting of this increased intelligence, and submit their plans as part of their application for market approval. Complete pharmacovigilance may be achieved through a combination of methods including passive surveillance, stimulated reporting, active surveillance, comparative observational studies, targeted clinical investigations, and descriptive studies. Patients and HCPs can report AEs concerning drugs or biologics to the manufacturer or directly to the FDA, through an online portal (MedWatch), regardless of the degree of causation certainty. Sponsors must submit postmarketing Safety Alerts to the FDA within 15 days of receiving a report of serious and unexpected AEs, from all sources. They must also submit Periodic Adverse Experience Reports of all other domestic spontaneous AEs quarterly for the first 3 years, and annually thereafter.57 International harmonization of these pharmacovigilance activities and requirements should minimize duplication of effort and benefit public health programs throughout the world as they consider potential new drugs.58
Communication of safety signal information to the public, particularly to patients impacted by a relevant therapeutic and their HCPs, is critical. Dear HCP letters, correspondence from the manufacturer or distributor of a therapeutic (or sometimes the FDA) intended to alert physicians and other HCPs about new or updated information, such as an important safety concern, are distributed via mass mailing, email, and posting to company websites, or through patient organizations. Industry is encouraged to consult the FDA on the content, targeting, and timing of the letter to ensure that the intended audience receives accurate information promptly.59 Drug Safety Communications (DSC) are developed and communicated directly to the public by the FDA independent of industry, whom they usually notify 24 h prior to posting to the FDA website. Highlighting new safety issues that pose potentially serious life-threatening risks or AEs they may contain information such as previously unknown drug interactions, a potential medication error like drug name confusion, or updated information about a known AE. DSCs are also disseminated through listservs, HCP newsletters, podcasts, social media, and MedWatch Safety Alerts via email.60 They are often picked up and amplified by the media, professional associations, and patient organizations.61
Product recalls
In the US, the FDA manages recalls of the products that it regulates, including all human drugs and blood products. Recalls are usually instigated by the manufacturing company, who alert the FDA that they have become aware that a product is defective or potentially harmful. The problem can also come to light as part of an FDA inspection of a manufacturing facility, through various health problem reporting systems, or via an alert from the CDC. Information about all recalls is posted on the FDA website in a weekly Enforcement Report, but the FDA only actively alerts the public if it feels there is a need to make them aware of a serious hazard. The Enforcement Report includes details of the product recalled, including lot or batch identifying information, reason for the recall, firm responsible, as well as initiation date, geographic scope, status (ongoing versus terminated), type, and class (Table 3).62 Individuals can subscribe to receive all notifications of listings or a subset (e.g. drugs or biologics) or to follow updates on a single recall. The FDA classifies the recall, works with the company concerned to identify and address the problem, evaluates the effectiveness of the recall and determines when it can be terminated (when it can be reasonably assumed that the product in question has been removed and disposed of or correction has been made, commensurate with the degree of hazard).52
Table 3.
Types and classes of FDA product recalls.
| Type | Details | Example |
|---|---|---|
| Voluntary | Manufacturer/distributor discovers a problem and initiates a
recall or FDA becomes aware of a problem and requests a recall |
All of the recent bleeding disorder product recalls (Table 1) |
| Mandatory | Various statutory provisions and regulations authorize the FDA to require recalls in particular circumstances of certain products | Products may include: infant formula medical devices tobacco products electronic products controlled substances biological products |
| Class | Details | Example |
| I | A dangerous or defective product could predictably cause a serious health problem or death | Superpotent drug |
| II | A product might cause a temporary health problem or poses only a slight threat of a serious nature | Vials mislabeled, contain similar but not identical product |
| III | A product is unlikely to cause any adverse health reaction but violates FDA labeling or manufacturing laws | External box label misaligned (vial labels correct) |
Source: U.S. Department of Health & Human Services. Product Recalls, Including Removals and Corrections: Guidance for Industry. Rockville, MD: U.S. Department of Health & Human Services; 2020.62
FDA, US Food and Drug Administration.
Safety information communication to patients and healthcare providers
Patients may receive notification of a product-related issue through multiple channels, some of which require action by the patient to establish contact with the relevant stakeholder, prior to any incident (Figure 2). Transparency and access to information is essential; however, the multiplicity of information available can be overwhelming. A whole host of communications on drug safety and safety signals are available on the FDA website (https://www.fda.gov/drugs/drug-safety-and-availability/postmarket-drug-safety-information-patients-and-providers), such as:
Figure 2.
Upon identification of a product-related issue, notifications are sent from the manufacturer/drug sponsor to multiple stakeholders who, in turn, have a responsibility to transmit the information rapidly and accurately to patients. In order to receive notifications from several of these stakeholders (indicated by blue dashed arrows), the patient must first establish contact with them, whereas other stakeholders (indicated by solid purple arrows) have direct contact with patients.
FDA, US Food and Drug Administration; HCP, healthcare professional; PNS, patient notification system.
DSCs.
Notifications of potential safety signals found in the FDA Adverse Event Reporting System (FAERS) database.
MedWatch Safety Alerts.
A database of drug safety labeling changes (SLCs).
Another of Risk Evaluation and Mitigation Strategies (REMS).
How the US bleeding disorders community operates within this context may serve as a useful example for adoption or adaptation by other chronic disorder communities. The community benefits from an additional communication channel established in 1998 by the Plasma Protein Therapeutics Association (PPTA), in collaboration with patient organizations and in consultation with the FDA: the PNS. Part of the legacy of the AIDS epidemic, this free, confidential service provides direct information to registrants of voluntary and mandated recalls of plasma protein therapies and their recombinant analogs. It includes brands of immune globulins, blood clotting factors, alpha-1 proteinase inhibitors, and other plasma protein products. In recent years, it expanded to include recombinant analog therapies. Registrants are directly notified via email, telephone, or fax as well as by post, with information also available via a website and a 24-h toll-free telephone number. The service provides logbooks to facilitate tracking of identifying information of the products patients use.63
Administered by the PPTA and funded by its member manufacturers, PNS is operated by an independent organization that specializes in public notification of pharmaceutical withdrawals and recalls, and all registrant information is kept strictly confidential.63,64 Voluntary paid membership of therapeutics manufacturers was originally limited to plasma products, eventually expanding to include recombinant analogs. To maintain its relevance as a trusted source of information on recalls of bleeding disorders therapeutics, it must evolve apace with the treatment landscape to encompass, for example, all recombinant factor products, nonfactor replacement products, and gene therapy. Manufacturers considering entering this marketing space must engage with this opportunity/responsibility before they ever need to avail themselves of its capabilities. Patients must also proactively register with the system, leaving them at risk of exclusion from notification should they, or their HCPs, be unaware of the system and how it works. Patients and HCPs who did not experience the contaminated blood products crisis personally may not recognize the need to proactively engage with a notification system, requiring further education.
Recommendations for improvements
Poor communication and multiple safety incidents in 2019–2021 (Table 1), despite the detailed and multifaceted pharmacovigilance formalized by the FDA, combined with the rapidly evolving treatment landscape, spurred the bleeding disorders community to closely examine the effectiveness of existing systems, and seek avenues for improvement. The two largest US patient organizations – NHF and HFA – co-hosted a Safety Summit in Washington, DC, over 3 days at the end of January 2020. The Safety Summit’s mission was to articulate expectations for monitoring, informing, educating, and communicating issues related to product safety, with attention to the role of each stakeholder in the process.
Eighty-five stakeholders participated in the summit, including patients, patient organizations (national, international, and state level), clinicians, manufacturers, specialty pharmacy, federal health agencies, and more. The event featured presentations, panel discussions, and workshops addressing safety notification standards and processes (what they are now, where gaps exist, and how safety communication practices could improve); rights, roles, and responsibilities of stakeholder groups with respect to product safety and safety reporting; and new considerations that come into play as novel and gene therapies come onto the market. This collaborative engagement resulted in concrete recommendations for improvements across the safety surveillance system targeting all stakeholders. Articulated in the context of the bleeding disorders community, they are applicable to all therapeutic areas. Active affirmation of the primacy of patient well-being and the optimization of prompt bidirectional communication prior to the urgent need to transmit important safety information emerged as overarching central themes.
All stakeholders
All segments of the bleeding disorders community have a role to play in the prompt and transparent bidirectional dissemination of information about the safety of drugs and biologics. To ensure that these communications are clear, patient-centered and timely, all stakeholders must:
Reaffirm commitment to patient-centered communication.
Recognize that regulatory requirements are the floor (the minimum acceptable), not the ceiling (the maximum expected).
Educate/self-educate on applicable standards, regulations, rights, roles, and responsibilities regarding product safety.
Improve communication channels within and outside the specific disorder community (in this case, inherited bleeding disorders).
Identify and define expectations for monitoring, informing, educating, and communicating issues related to product safety.
Base actions on scientific data.
Leverage social media as appropriate/challenge social media dissemination of incorrect or misleading information.
Be mindful of language used to describe novel and gene therapies: ensure that it is accurate, does not create unrealistic expectations, and is patient-centered and accessible.
Engage in international collaborations in safety communication and reporting to enhance consistency of messaging and completeness of reported data.
Develop checklists for reporting safety concerns and communicate processes to other stakeholder groups.
Each stakeholder plays an important role in the safety information communication network with unique responsibilities and opportunities to action the priorities outlined above. Some of these are described in the following.
Patients
Patients are the stakeholders most impacted by product safety information, and they are the primary source of safety information, the SMEs on living with the disorder. All other stakeholders have an obligation to recognize that patients are situated at the center of safety (Figure 3). An important trend in today’s society is the increasing determination of healthcare consumers to better manage their own health, gathering information (including from online sources), and self-organizing through social media networking. They hold the potential to provide massive amounts of data that may power advances in systems medicine, to influence important health outcomes, and to maximize the positive impact of pharmacovigilance on the safe and effective use of medicine.65,66
Figure 3.
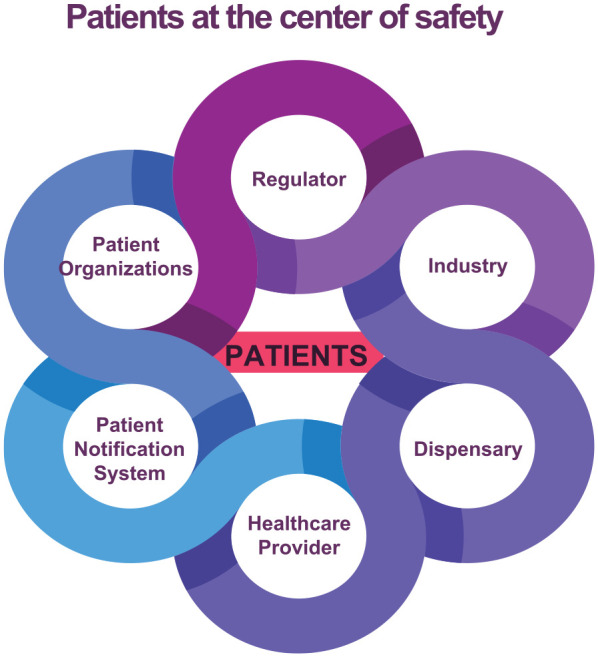
Patients are the stakeholders most impacted by product safety information, and they are the primary source of safety information. All other stakeholders have an obligation to recognize that patients are situated at the center of safety.
With such a central role to play, and regulators increasingly mandating initiatives to incorporate patient perspectives into pharmacovigilance activities,67 patients also have important responsibilities to:
- Educate themselves about:
- how to identify an AE; what concerns to report to their provider;
- basics of clinical trials, informed consent, drug safety reporting, and novel therapies in the pipeline;
- reliable sources of information.
Understand that they play a vital role as a primary source of safety information and in reporting issues to their medical providers.
Track identifying information of the products they take (e.g. lot numbers).
Enroll in a PNS (for the bleeding disorders community, this is the PNS).
Maintain strong ongoing communications with their HCP.
Understand the importance of participation in broad-based registries to allow for information gathering and safety signal monitoring around existing and novel therapies.
The current digital age presents both opportunities and challenges to engaging patients in accurate safety information communication. In a 2013 study, 35% of Americans reported that they had searched the Internet for help with health-related concerns and 16% had used it to try and connect with others with similar concerns.68 A 2021 survey of UK users of an online platform offering connections with others in disorder-specific groups, which receives approximately 4.5 million visits per month, found that a third of respondents reported no knowledge about methods of monitoring medication safety, although 89% had personally experienced side effects from their medications. Two-thirds supported the idea of providing links to regulatory reporting sites from the platform.69 As increasing numbers of patients turn to social media to share their experiences, automated text mining methods to detect AE mentions from social media posts are being explored.70–74 Artificial intelligence (AI) techniques, such as natural language processing and deep learning with neural networks, may prove determinant in the extraction of useful information from the very large volume of heterogeneous data generated by digital sources.73,75,76 The appropriate role for these data in pharmacovigilance is being studied, with particular attention to determining the accuracy and reliability of information shared on social media (e.g. Twitter).70,71,75,77 Even as AI techniques continue to be optimized to improve the accuracy and sensitivity with which they detect and classify AE mentions, limitations such as the degree to which social media users are representative of populations (e.g. the population of an entire country or the target population for a specific medication) and post authenticity issues, suggest that linked analysis with external trustworthy data sources may be required.72,78 The integration of AI into any healthcare process must be done with explicit awareness of, and efforts to eliminate, algorithmic bias that may result from the design of the algorithm, previous data collection, coding, and selection.79,80 When queried about their comfort level with researchers or the Medicines and Healthcare products Regulatory Agency (MHRA), which monitors and regulates postmarketing drug safety in the UK, using content posted to the aforementioned online platform to help monitor side effects, over 90% of respondents were favorable to the idea.69 They did, however, express concerns about data privacy, ethics, and the ability of such techniques to truly understand the nuanced and personal content being shared. Similarly, while promising progress has been made, the application of AI to the rapid and effective detection of existing and new AEs in large data sets of patient information such as electronic health records, health information technologies, and pharmacovigilance database systems, challenges remain in the ability to detect undocumented or unknown AEs, privacy concerns, and technical difficulties.76
Social media applications allow individuals to rapidly publish and re-publish highly personal, compelling, and emotional experiences.81 These peer-to-peer sharings can provide important support and validation to people living with similar concerns, propagate helpful information, and combat isolation, especially within rare disorders communities.82 The openness, user participation, rapid proliferation, and network effects that facilitate such impactful personal sharing, however, also empower the dissemination of false information.81 Some antivaccination groups, for example, intentionally harness social media to spread misinformation,83 while many well-intentioned individuals also share information they do not know to be false or poorly sourced. Closed social media groups exacerbate the challenge of ascertaining what erroneous information may be circulating. Reliable sources, such as patient organizations, public health offices, and HCP associations must capitalize upon the features and ubiquity of social media to proactively disseminate sound, well-sourced, transparent information, thus minimizing the opportunity for misinformation to proliferate.84
Patient organizations
Pharmacovigilance and the entire therapeutic safety ecosystem is complex and can be overwhelming for individual patients to navigate alone, often resulting in communication failure.85 Patient organizations are uniquely positioned to advocate for and educate patients while also facilitating relationships and transmission of information between patients, industry, and regulators. In the current context of the bleeding disorders community, the Safety Summit concluded that patient organizations must:
Earn and preserve their roles as trusted sources of information.
Identify designated contact persons among their staff for industry to notify in event of product recall.
Implement strategies to allow for prompt and accurate dissemination of product safety information via channels accessible to community members, in patient-friendly language, understanding that some patients may be off the grid and unconnected to specialized treatment centers.
Provide patient education about information sources and protocols, including where patients should go for accurate news and information.
Align messaging with one another (i.e. between different patient organizations).
Promote strong ties and robust communications between national and state/local organizations.
Promote enrollment in PNSs (in this case: PNS).
Offer foundational education to community members about clinical trials, safety reporting, and so on.
For concrete examples of patient organization action steps and communications following notification of different types of product issues, see the NHF/HFA protocol: Best practices for patient organization response upon notification of product issue (Supplementary Material 1).
Recent studies suggest that most patient organizations do not actively engage with pharmacovigilance. A survey of European patient organizations reported that while just over 40% would like to increase awareness among their members of specific adverse drug reactions related to their therapeutics, just under 40% have no stated pharmacovigilance goals and nearly 35% indicated no involvement in pharmacovigilance. The principal barriers were identified as a lack of sufficient funding and resources, and a lack of support from national competent authorities, who are primarily responsible for the authorization of medicines that do not pass through the centralized European Medicines Agency (EMA) process.86 Probing further revealed that a tendency not to prioritize pharmacovigilance by patient organizations may be tied to beliefs that this is not their responsibility, that patients do not expect it from them, and that their focus should be elsewhere.85
Bleeding disorders patient organizations appear to be exceptional in their advanced pharmacovigilance engagement, likely a legacy of the AIDS epidemic. Suggestions to enhance patient organization engagement tend to hinge on outreach, education, and support from national competent authorities and industry to patient organizations.85,86 While these are necessary and welcome, the bleeding disorders community has demonstrated the important strides that can be made when patient organizations take the lead, driving dialogue and accountability. Providing resources to enable best practice sharing and capacity building between patient organizations is a promising avenue whose potential has yet to be fully explored.
Many patient organizations receive funding from industry partners who also market products for their community. This can lead to issues of, or perceptions of, conflict of interest. The organization may be simultaneously stewarding a relationship with a funder and attempting to hold the same entity accountable on product safety issues and communications. Patient organizations must establish clear boundaries between funding and policy and advocacy initiatives. These parameters should be clearly and publicly declared to protect the integrity of the organization, secure the faith of its members, and forewarn funders of the impossibility of influence. An example, linked to more detailed policy language, follows:
While HFA does accept pharmaceutical funding, we have developed and stringently maintain clear boundaries and policies and procedures1 that preserve our independence and prevent undue influence on how we operate as an organization. A pharmaceutical company’s support, at any level, has absolutely zero impact on how policy and advocacy decisions are made at HFA. To do so would go against HFA’s very mission, and would dishonor the foundation the organization was built upon. Our duty is always patient-focused and patient-centered; whether it be lawsuits amongst manufacturers, a product safety issue, or threat to access, it is HFA’s intention is to achieve policy outcomes that ensure access to safe therapies and quality, affordable healthcare by mobilizing the bleeding disorders community as leaders in rare disease.
NHF and HFA are two large patient organizations with extensive experience in this arena and for whom pharmacovigilance is an important ongoing commitment. Discussions at the Safety Summit prompted the prioritization of a number of specific steps for near-term implementation by HFA/NHF:
Create (additional) patient-centered educational resources about safety issues.
Work with industry partners to ensure that all communications regarding product safety or availability are provided for public dissemination on letterhead or via a web link that readily identifies the product manufacturer and distributor.
Revise and align the two organizations’ respective Patient Bill of Rights and Responsibilities2,3 to reflect the critical role that patients play in safety monitoring.
Co-create a specific bleeding disorders educational hub (Blood Safety Academy) to train the next generation of safety advocates.
Provide specific and comprehensive contact information for each of their organizations to pharmaceutical companies.
Establish a means for all stakeholders to endorse or show their commitment to, and to hold stakeholders accountable to, the principles outlined herein.
Pharmaceutical companies (including sponsors of investigational therapeutics)
A number of recent regulatory guidances and related initiatives aim to emphasize the importance of patients’ perspectives in drug safety,67 yet most patient organizations surveyed in a recent European study reported no impact on their positions as pharmacovigilance stakeholders.85 Regulators increasingly require manufacturers to implement risk minimization strategies but only a handful has been shown to be effective. Most are designed by industry and regulators; however, their success depends largely on the target population, the context in which they interact with the therapeutic and effective communication with them.87 Pharmaceutical companies will benefit from developing a more patient-centered culture, employing a framework-driven approach to patient engagement, and becoming proficient in a range of patient-centered competencies.67 A constructive, respectful engagement between companies and patients promises to be mutually beneficial.
The HFA NHF Safety Summit discussions identified several key priority actions for and with companies that develop, manufacture, and distribute drugs and biologics to the bleeding disorders community:
Explicitly affirm and orient themselves around the end goal of optimizing patient well-being (Figure 3).
Recognize that regulatory requirements are the minimum expectations of the community, to be surpassed, not just met.
- Provide accurate, timely, and transparent guidance and information about safety issues to all stakeholder groups. To this end, safety information communications must be:
- in patient-friendly language;
- identifiable to the manufacturer (e.g. on letterhead);
- clear actionable guidance such as where and how recipients should report AEs, return product, and so on;
- published promptly, with the understanding that multiple communications may be required as further information develops.
Make the information contained in Dear HCP letters available to the public.
Establish and maintain direct communication with identified staff at national patient organizations.
- Participate in and make effective use of the PNS (in this case: PNS):
- companies should activate PNS to communicate recalls;
- company personnel should receive regular training about PNS, including how to access and activate prior to any need to activate.
Provide long-term follow-up and support for recipients of novel therapies.
Consider including patient and patient advocacy organization representatives on safety advisory boards.
Specialty pharmacy providers (therapeutic dispensaries)
The entity that dispenses therapeutics to patients constitutes a bridge between end users and specific product lots. For noninvestigational bleeding disorder therapeutics in the US, this role falls to specialty pharmacy providers [these may be separate entities or hemophilia treatment center (HTC)–affiliated pharmacies].88 Patients carry a responsibility to track the lot numbers of the products they use. Dispensing pharmacies, however, are the ones in direct and regular communication with the distributor positioning them uniquely to facilitate bidirectional exchange of essential information with end users. The Safety Summit articulated that they have an obligation to:
Recognize their critical role in receiving and disseminating safety information bidirectionally in the stakeholder chain.
- Proactively ensure that they receive correct information:
- from manufacturers or distributors, regarding any recall or safety event;
- from patients, regarding any suspected or reported AE.
Reach out to patients, via individually optimized channels such as text messaging, email, phone calls, postal mail, and other preferred communications technology.
In the case of a recall, notify not only patients, but also patients’ HCPs, as HCPs do not have the data linking patients to individual lot numbers.
Ensure delivery of replacement product to patients where applicable.
Healthcare providers
HCPs enjoy a privileged relationship with patients, especially those with lifelong conditions such as bleeding disorders. As partners in the management of their health they must:
Take patient safety concerns seriously and listen with empathy.
- Constitute a trusted source of information, providing patient-friendly information and encouraging patients to ask questions:
- work with patients toward a shared understanding of what constitutes an AE, educating around the definition of an AE;
- provide patients advanced education (e.g. about products in the pipeline).
Stringently adhere to AE reporting requirements.
Respond to manufacturer follow-up inquiries and make use of manufacturer Medical Science Liaison (MSL) resources.
Promote patient enrollment in the PNS (in this case: PNS).
Federal partners
Pharmacovigilance and communication of drug safety information occur within the legal context of each country. Federal partners, such as regulators, national competent authorities, ministries of health, and others must ensure that stakeholders uphold their legal requirements. The Safety Summit determined that there are important opportunities for federal partners to influence more than these minimal requirements. They should:
Ensure industry compliance with postmarketing surveillance and reporting obligations.
Require regulated entities to provide communications in patient-friendly language, and apply the same standards in their own public statements.
Ensure that they, and industry, provide good education regarding patient participation in clinical trials (before, during, and after the trials) especially regarding how to recognize and report potential AEs or other safety concerns.
Encourage and support community surveillance initiatives.
PNS
Regulatory authorities and federal partners usually host a variety of platforms that the public can search for diverse drug safety information, but those platforms are often neither well-known to nor easily navigated by laypeople. A system that can proactively and promptly notify patients of important safety information relevant to their specific therapeutics can serve as one of the most important tools to achieve successful communication. PNS was designed to offer the US bleeding disorders community timely information about voluntary and mandated recalls of plasma protein therapies, and now also recombinant analogs. The system occupies a critical and trusted position within the community and to meet the expectations that it has established it must:
- Allow participation by manufacturers/sponsors of novel and gene therapies:
- initially created for plasma-derived products, it must evolve with the treatment landscape.
Provide timely notifications in a range of formats and via a range of media that are self-tailored to individual recipients, understanding that some patients may be off the grid and not connected to specialized treatment centers.
Conduct regular tests to ensure that all stakeholders are engaged appropriately (both those initiating and receiving notifications).
Conclusion
The value of patient participation in the design and implementation of pharmacovigilance practices has been recognized27,28,45,67,89 and even mandated;67 however, most approaches to inclusion tend to be top-down and prescriptive. Patient organizations are uniquely positioned to foster constructive relationships and play a leadership role in driving improvements. Patients have the most at stake, as end users of the therapeutic. Patients are also the SMEs on living with a disorder, with much to contribute to risk minimization87 and safety communication initiatives that are effective in the real world.38
Two large bleeding disorders patient organizations (NHF and HFA) recently convened a summit of all stakeholders to identify opportunities for improvement in the context of an alarming number of sometimes poorly communicated safety incidents and a rapidly evolving treatment landscape. Their priority conclusions centered upon the following:
Patient perspective: all stakeholders must exercise constant respect for the vulnerability of patients.
Patient role: patients must take an active, supported, role in identifying and reporting potential AEs.
Regulatory awareness and roles: stakeholders must have a practical understanding of and engagement with safety information communication, from preclinical to postmarketing.
Clinical trials: education of patients regarding potential safety events and reporting must be improved.
Data: collaboration between all stakeholders is required to strengthen the scientific and statistical validity of detection/analysis of potential risks and hazards.
Pharmacovigilance and recalls: renewed commitment is required from manufacturers, HCPs, and regulators to improve fulfillment of responsibilities.
Trusted sources (including patient organizations): the highest standards of transparency, timeliness, alignment, and accessibility must be upheld.
Patient-friendly: patient-friendly safety information must be available for all therapies.
Communication lines, protocols, and infrastructure: these must be reviewed and improved to optimize transmission of information, before an urgent situation arises.
Communication strategies and outreach: social media and related opportunities must be capitalized upon to maximize the reach of accurate information.
Committing to the primacy of patient well-being and establishing, and stewarding reliable, collaborative channels of communication before a crisis arises in which they are urgently needed, emerged as the two overarching principles from the bleeding disorder stakeholder discussions. The application of these two principles to pharmacovigilance in all therapeutic areas will benefit patients, industry, and regulators alike.
“Our hearts bleed from love, pain and suffering, fear and isolation. We battled on the fringe, warriors fighting stigma, ignorance, and inaction. We came together as families, communities, and fierce friends, to build community, and protect one another. We felt betrayal. We learned resilience. We promise this will never happen again.”
Hemophilia Circle, AIDS Memorial Grove, San Francisco
Supplemental Material
Supplemental material, sj-docx-1-taw-10.1177_20420986221146418 for Patient-centered pharmacovigilance: priority actions from the inherited bleeding disorders community by Fiona Robinson, Sonji Wilkes, Nathan Schaefer, Miriam Goldstein, Michelle Rice, Johanna Gray, Sharon Meyers and Leonard A. Valentino in Therapeutic Advances in Drug Safety
Acknowledgments
The authors gratefully acknowledge Clifford Goodman for his role in moderating the HFA NHF Safety Summit, and Dr Goodman and Evelyn Nkooyooyo, both of The Lewin Group, for compiling notes and summaries of the Summit meeting content. The authors also thank all of the staff at NHF and HFA whose contributions helped make the Safety Summit a reality.
Footnotes
ORCID iD: Fiona Robinson  https://orcid.org/0000-0003-4286-271X
https://orcid.org/0000-0003-4286-271X
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Fiona Robinson, Communications, Montréal, QC, Canada.
Sonji Wilkes, Hemophilia Federation of America, Washington, DC, USA.
Nathan Schaefer, National Hemophilia Foundation, New York, NY, USA.
Miriam Goldstein, Hemophilia Federation of America, Washington, DC, USA.
Michelle Rice, National Hemophilia Foundation, New York, NY, USA.
Johanna Gray, Artemis Policy Group, Washington, DC, USA.
Sharon Meyers, Hemophilia Federation of America, Washington, DC, USA.
Leonard A. Valentino, National Hemophilia Foundation, 7 Penn Plaza, Suite 102, New York, NY 1001, USA; Internal Medicine and Pediatrics, Rush University, Chicago, IL, USA.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Fiona Robinson: Investigation; Methodology; Validation; Visualization; Writing – original draft; Writing – review & editing.
Sonji Wilkes: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing.
Nathan Schaefer: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Supervision; Validation; Writing – original draft; Writing – review & editing.
Miriam Goldstein: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Validation; Writing – original draft; Writing – review & editing.
Michelle Rice: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Validation; Writing – original draft; Writing – review & editing.
Johanna Gray: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Validation; Writing – original draft; Writing – review & editing.
Sharon Meyers: Data curation; Formal analysis; Investigation; Methodology; Validation; Writing – original draft; Writing – review & editing.
Leonard A. Valentino: Data curation; Formal analysis; Investigation; Methodology; Validation; Writing – original draft; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The entire Safety Summit initiative and this manuscript were funded by NHF and HFA, with support from LFB Pharmaceuticals, HEMA Biologics, Pfizer, Bayer, Octapharma, CSL Behring, Genentech, Spark Therapeutics, BioMarin, Novo Nordisk, Sanofi, Kedrion, Grifols, Takeda, BPL, and Roche. Additional support from Takeda and Genentech was provided to support the preparation of this manuscript. F.R. provided professional consulting services to NHF/HFA for this article.
The authors declared the following potential conflict of interest with respect to the research, authorship, and/or publication of this article: HFA and the NHF, co-leaders of the 2020 Product Safety Summit, sought participation in and support of the Safety Summit from all manufacturers of bleeding disorders medications. HFA and NHF contributed equally to the development and deployment of the Safety Summit and subsequent work and materials; all funding supporting was split equally between the two nonprofit organizations. S.W. is employed by HFA as VP for Public Affairs. S.M. is employed by HFA as President & CEO. HFA receives honoraria for their participation on patient advocacy advisory boards for Takeda, BioMarin, and Sanofi. M.G. is employed by HFA as a director of policy. HFA is a national nonprofit organization that assists, educates, and advocates for the bleeding disorders community. HFA receives funding in the form of donations from individuals, philanthropic foundations, drug/biotech manufacturers, and specialty pharmacies; a cooperative grant from the US Centers for Disease Control; an engagement award from the Patient-Centered Outcomes Research Institute; sponsorship income from a symposium it holds annually. A list of corporate supporters can be found at https://www.hemophiliafed.org/home/our-role-and-programs/what-is-hfa/our-supporters/. F.R. is a consultant and was paid by NHF/HFA to contribute her expertise to this article. She has received consulting fees from F. Hoffmann-La Roche Ltd for speaking at a symposium, and for work with the World Federation of Hemophilia, American Thrombosis and Hemostasis Network, and European Haemophilia Consortium. The opinions expressed in this article by N.S. and L.A.V. are their own and not necessarily reflective of those of the NHF or its Board of Directors. The other authors have no conflicts of interest relevant to this article.
Availability of data and materials: Not applicable.
References
- 1. Valentino LA, Baker JR, Butler R, et al. Integrated hemophilia patient care via a national network of care centers in the United States: a model for rare coagulation disorders. J Blood Med 2021; 12: 897–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Valentino LA, Blanchette V, Negrier C, et al. Personalising haemophilia management with shared decision making. J Haemop Pract 2021; 8: 69–79. [Google Scholar]
- 3. Karazivan P, Dumez V, Flora L, et al. The patient-as-partner approach in health care: a conceptual framework for a necessary transition. Acad Med 2015; 90: 437–441. [DOI] [PubMed] [Google Scholar]
- 4. Hubinette M, Dobson S, Voyer S, et al. ‘We’ not ‘I’: health advocacy is a team sport. Med Educ 2014; 48: 895–901. [DOI] [PubMed] [Google Scholar]
- 5. Srivastava A, Santagostino E, Dougall A, et al. WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia 2020; 26: 1–158. [DOI] [PubMed] [Google Scholar]
- 6. Larsson SA. Life expectancy of Swedish haemophiliacs, 1831–1980. Br J Haematol 1985; 59: 593–602. [DOI] [PubMed] [Google Scholar]
- 7. Jones PK, Ratnoff OD. The changing prognosis of classic hemophilia (factor VIII ‘deficiency’). Ann Intern Med 1991; 114: 641–648. [DOI] [PubMed] [Google Scholar]
- 8. Manco-Johnson MJ, Abshire TC, Shapiro AD, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med 2007; 357: 535–544. [DOI] [PubMed] [Google Scholar]
- 9. Warren BB, Thornhill D, Stein J, et al. Young adult outcomes of childhood prophylaxis for severe hemophilia A: results of the Joint Outcome Continuation Study. Blood Adv 2020; 4: 2451–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Manco-Johnson MJ, Lundin B, Funk S, et al. Effect of late prophylaxis in hemophilia on joint status: a randomized trial. J Thromb Haemost 2017; 15: 2115–2124. [DOI] [PubMed] [Google Scholar]
- 11. Mejia-Carvajal C, Czapek EE, Valentino LA. Life expectancy in hemophilia outcome. J Thromb Haemost 2006; 4: 507–509. [DOI] [PubMed] [Google Scholar]
- 12. Plug I, Van Der Bom JG, Peters M, et al. Mortality and causes of death in patients with hemophilia, 1992–2001: a prospective cohort study. J Thromb Haemost 2006; 4: 510–516. [DOI] [PubMed] [Google Scholar]
- 13. Chorba TL, Holman RC, Clarke MJ, et al. Effects of HIV infection on age and cause of death for persons with hemophilia A in the United States. Am J Hematol 2001; 66: 229–240. [DOI] [PubMed] [Google Scholar]
- 14. Evatt BL, Gomperts ED, McDougal JS, et al. Coincidental appearance of LAV/HTLV-III antibodies in hemophiliacs and the onset of the AIDS epidemic. N Engl J Med 1985; 312: 483–486. [DOI] [PubMed] [Google Scholar]
- 15. Evatt BL. The tragic history of AIDS in the hemophilia population, 1982–1984. J Thromb Haemost 2006; 4: 2295–2301. [DOI] [PubMed] [Google Scholar]
- 16. Troisi CL, Hollinger FB, Hoots WK, et al. A multicenter study of viral hepatitis in a United States hemophilic population. Blood 1993; 81: 412–418. [PubMed] [Google Scholar]
- 17. Farrugia A, Liumbruno GM, Candura F, et al. Factors affecting the quality, safety and marketing approval of clotting factor concentrates for haemophilia. Blood Transfus 2018; 16: 525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leveton LB, Sox HC, Jr, Stoto MA. HIV and the blood supply: an analysis of crisis decision making – executive summary: the Institute of Medicine, National Academy of Sciences Committee to Study HIV Transmission through Blood and Blood Products. Transfusion 1996; 36: 919–927. [DOI] [PubMed] [Google Scholar]
- 19. Epstein JS, Jaffe HW, Alter HJ, et al. Blood system changes since recognition of transfusion-associated AIDS. Transfusion 2013; 53: 2365–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. U.S. Department of Health & Human Services. Charter, Advisory Committee on Blood and Tissue Safety and Availability, https://www.hhs.gov/oidp/advisory-committee/blood-tissue-safety-availability/charter/index.html (accessed 15 June 2021).
- 21. BioMarin. First gene therapy for adults with severe hemophilia A, BioMarin’s ROCTAVIAN™ (valoctocogene roxaparvovec), approved by European Commission (EC), https://investors.biomarin.com/2022-08-24-First-Gene-Therapy-for-Adults-with-Severe-Hemophilia-A,-BioMarins-ROCTAVIAN-TM-valoctocogene-roxaparvovec-,-Approved-by-European-Commission-EC (accessed 26 August 2022).
- 22. European Medicines Agency. First gene therapy to treat severe haemophilia A, https://www.ema.europa.eu/en/news/first-gene-therapy-treat-severe-haemophilia (accessed July 20 2022).
- 23. Hubinette M, Dobson S, Scott I, et al. Health advocacy. Medical Teacher 2017; 39: 128–135. [DOI] [PubMed] [Google Scholar]
- 24. Peyvandi F, Miri S, Garagiola I. Immune responses to plasma-derived versus recombinant FVIII products. Front Immunol 2020; 11: 591878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Croteau SE, Wang M, Wheeler AP. 2021 clinical trials update: innovations in hemophilia therapy. Am J Hematol 2021; 96: 128–144. [DOI] [PubMed] [Google Scholar]
- 26. Shima M. Bispecific antibodies and advances in non-gene therapy options in hemophilia. Res Pract Thromb Haemost 2020; 4: 446–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Peyvandi F, Garagiola I, Mannucci PM. Post-authorization pharmacovigilance for hemophilia in Europe and the USA: independence and transparency are keys. Blood Rev 2021; 49: 100828. [DOI] [PubMed] [Google Scholar]
- 28. Pierce GF. Uncertainty in an era of transformative therapy for haemophilia: addressing the unknowns. Haemophilia 2021; 27: 103–113. [DOI] [PubMed] [Google Scholar]
- 29. Konkle BA, Coffin D, Pierce GF, et al. World Federation of Hemophilia gene therapy registry. Haemophilia 2020; 26: 563–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Management Sciences for Health and World Health Organization. Drug and Therapeutics Committee training course, submitted to the U.S. Agency for International Development by the Rational Pharmaceutical Management plus program. Arlington, VA: Management Sciences for Health and World Health Organization. [Google Scholar]
- 31. U.S. Food and Drug Administration. Drug advertising: a glossary of terms, https://www.fda.gov/drugs/prescription-drug-advertising/drug-advertising-glossary-terms (accessed 25 January 2022).
- 32. Merriam-Webster.com. Dictionary: ‘correlation’, https://www.merriam-webster.com/dictionary/correlation (accessed 25 January 2022).
- 33. MRC/CSO Social and Public Health Sciences Unit University of Glasgow. Correlation and causation, https://www.understandinghealthresearch.org/useful-information/correlation-and-causation-15 (accessed 7 September 2022).
- 34. Institute of Medicine (IOM). Crossing the quality chasm: a new health system for the 21st century. Washington, DC: National Academy Press, 2001. [PubMed] [Google Scholar]
- 35. World Health Organization & WHO Patient Safety. Conceptual framework for the international classification for patient safety version 1.1: final technical report January 2009. Geneva: World Health Organization, 2010. [Google Scholar]
- 36. World Health Organization. The safety of medicines in public health programmes: pharmacovigilance an essential tool. Geneva: World Health Organization, 2006. [Google Scholar]
- 37. Ramya. Safety signal: introduction, https://allaboutpharmacovigilance.org/31-signal-introduction/ (accessed 25 January 2022).
- 38. Malikova MA. Practical applications of regulatory requirements for signal detection and communications in pharmacovigilance. Ther Adv Drug Saf 2020; 11: 909614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Institute of Medicine Committee on Data Standards for Patient Safety. Patient safety: achieving a new standard for care (eds P Aspden, JM Corrigan, J Wolcott, et al.). Washington, DC: National Academies Press, 2004. [PubMed] [Google Scholar]
- 40. CIOMS Working Group VI. Management of safety information from clinical trials: report of CIOMS Working Group VI. Geneva: CIOMS Working Group IV, 2005. [Google Scholar]
- 41. Mitchell AS, Henry DA, Sanson-Fisher R, et al. Patients as a direct source of information on adverse drug reactions. Br Med J 1988; 297: 891–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Egberts TC, Smulders M, de Koning FH, et al. Can adverse drug reactions be detected earlier? A comparison of reports by patients and professionals. BMJ 1996; 313: 530–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rolfes L, van Hunsel F, Caster O, et al. Does patient reporting lead to earlier detection of drug safety signals? A retrospective comparison of time to reporting between patients and healthcare professionals in a global database. Br J Clin Pharmacol 2018; 84: 1514–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Uppsala Monitoring Centre. VigiBase services, https://who-umc.org/vigibase/vigibase-services/ (accessed 8 September 2022).
- 45. Berrewaerts J, Delbecque L, Orban P, et al. Patient participation and the use of e-health tools for pharmacovigilance. Front Pharmacol 2016; 7: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Spanakis M, Sfakianakis S, Kallergis G, et al. PharmActa: personalized pharmaceutical care eHealth platform for patients and pharmacists. J Biomed Inform 2019; 100: 103336. [DOI] [PubMed] [Google Scholar]
- 47. Pew Research Center. Internet/broadband fact sheet, https://www.pewresearch.org/internet/fact-sheet/internet-broadband/?menuItem=3109350c-8dba-4b7f-ad52-a3e976ab8c8f (accessed 26 August 2022).
- 48. Lin JL, Huber B, Amir O, et al. Barriers and facilitators to the implementation of family-centered technology in complex care: feasibility study. J Med Internet Res 2022; 24: e30902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. World Health Organization. Regulation and prequalification, https://www.who.int/teams/regulation-prequalification/pharmacovigilance (accessed 23 May 2021).
- 50. U.S. Food and Drug Administration. Step 3: clinical research, https://www.fda.gov/patients/drug-development-process/step-3-clinical-research#The_Investigational_New_Drug_Process (accessed 21 May 2021).
- 51. U.S. Food and Drug Administration. Regulations: good clinical practice and clinical trials. https://www.fda.gov/science-research/clinical-trials-and-human-subject-protection/regulations-good-clinical-practice-and-clinical-trials (accessed 20 May 2021).
- 52. U.S. Department of Health & Human Services. Applications for FDA approval to market a new drug. Food and Drug Administration HHS (ed.). Silver Spring, MD: Food and Drug Administration HHS, 2020. [Google Scholar]
- 53. Expert Working Group (Efficacy) of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). Clinical safety data management: definitions and standards for expedited reporting. New York: International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, 1995. [Google Scholar]
- 54. U.S. Department of Health & Human Services. Investigational new drug safety reporting requirements for human drug and biological products and safety reporting requirements for bioavailability and bioequivalence studies in humans. Silver Spring, MD: Food and Drug Administration HHS, 2010. [Google Scholar]
- 55. Ochyra B, Szewczyk M, Przybylkowski A. Impact of literature reports on drug safety signals. Wien Klin Wochenschr 2021; 133: 188–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. U.S. Department of Health & Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) and Center for Biologics Evaluation and Research (CBER). Guidance for industry and investigators: safety reporting requirements for INDs and BA/BE studies. Rockville, MD: U.S. Department of Health, 2012. [Google Scholar]
- 57. Tobenkin A. An introduction to drug safety surveillance and the FDA adverse event reporting system, https://www.fda.gov/about-fda/fda-pharmacy-student-experiential-program/introduction-drug-safety-surveillance-and-fda-adverse-event-reporting-system (2018, accessed 21 May 2021).
- 58. U.S. Department of Health & Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) and Center for Biologics Evaluation and Research (CBER). Guidance for industry: E2E pharmacovigilance planning. Rockville, MD: U.S. Department of Health, 2005. [Google Scholar]
- 59. U.S. Department of Health & Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) and Center for Biologics Evaluation and Research (CBER). Guidance for industry and FDA staff dear health care provider letters: improving communication of important safety information. Rockville, MD: U.S. Department of Health, 2014. [Google Scholar]
- 60. U.S. Food and Drug Administration. Subscribe to MedWatch safety alerts, https://www.fda.gov/safety/medwatch-fda-safety-information-and-adverse-event-reporting-program/subscribe-medwatch-safety-alerts (accessed 23 May 2021).
- 61. U.S. Food and Drug Administration. CDER’s drug safety communications: ensuring postmarket safety, https://www.fda.gov/drugs/news-events-human-drugs/cders-drug-safety-communications-ensuring-postmarket-safety (accessed 24 May 2021).
- 62. U.S. Department of Health & Human Services. Product recalls, including removals and corrections: guidance for industry. Rockville, MD: U.S. Department of Health & Human Services, 2020. [Google Scholar]
- 63. Plasma Protein Therapeutics Association. Patient notification system (PNS), https://www.pptaglobal.org/plasma/21-advocacy/75-patient-notification-system (accessed 24 May 2021).
- 64. Plasma Protein Therapeutics Association. About the patient notification system, https://www.patientnotificationsystem.org/about.asp (accessed 10 June 2021).
- 65. Arlett P, Straus S, Rasi G. Pharmacovigilance 2030: invited commentary for the January 2020 ‘futures’ edition of clinical pharmacology and therapeutics. Clin Pharmacol Ther 2020; 107: 89–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Flores M, Glusman G, Brogaard K, et al. P4 medicine: how systems medicine will transform the healthcare sector and society. Per Med 2013; 10: 565–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Smith MY, Benattia I. The patient’s voice in pharmacovigilance: pragmatic approaches to building a patient-centric drug safety organization. Drug Saf 2016; 39: 779–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Fox S, Duggan M. One in three American adults have gone online to figure out a medical condition. Health Online, 2013, https://www.pewresearch.org/internet/2013/01/15/health-online-2013/
- 69. Bulcock A, Hassan L, Giles S, et al. Public perspectives of using social media data to improve adverse drug reaction reporting: a mixed-methods study. Drug Saf 2021; 44: 553–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. De Rosa M, Fenza G, Gallo A, et al. Pharmacovigilance in the era of social media: discovering adverse drug events cross-relating Twitter and PubMed. Future Gener Comput Syst 2021; 114: 394–402. [Google Scholar]
- 71. Freifeld CC, Brownstein JS, Menone CM, et al. Digital drug safety surveillance: monitoring pharmaceutical products in twitter. Drug Saf 2014; 37: 343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hussain Z, Sheikh Z, Tahir A, et al. Artificial intelligence-enabled social media analysis for pharmacovigilance of COVID-19 vaccinations in the United Kingdom: observational study. JMIR Public Health Surveill 2022; 8: e32543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Jiang K, Zhang D, Bernard GR. Extraction of medication-effect relations in Twitter data with neural embedding and recurrent neural network. Stud Health Technol Inform 2022; 290: 767–771. [DOI] [PubMed] [Google Scholar]
- 74. Lyu T, Eidson A, Jun J, et al. Data veracity of patients and health consumers reported adverse drug reactions on Twitter: key linguistic features, Twitter variables, and association rules. Stud Health Technol Inform 2022; 290: 552–556. [DOI] [PubMed] [Google Scholar]
- 75. Aronson JK. Artificial intelligence in pharmacovigilance: an introduction to terms, concepts, applications, and limitations. Drug Saf 2022; 45: 407–418. [DOI] [PubMed] [Google Scholar]
- 76. Edrees H, Song W, Syrowatka A, et al. Intelligent telehealth in pharmacovigilance: a future perspective. Drug Saf 2022; 45: 449–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kim Y, Huang J, Emery S. Garbage in, garbage out: data collection, quality assessment and reporting standards for social media data use in health research, infodemiology and digital disease detection. J Med Internet Res 2016; 18: e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Pathak R, Catalan-Matamoros D. Can Twitter posts serve as early indicators for potential safety signals? A retrospective analysis. Int J Risk Saf Med. Epub ahead of print 26 April 2022. DOI: 10.3233/JRS-210024. [DOI] [PubMed] [Google Scholar]
- 79. Amann J, Blasimme A, Vayena E, et al. Explainability for artificial intelligence in healthcare: a multidisciplinary perspective. BMC Med Inform Decis Mak 2020; 20: 310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Delgado J, de Manuel A, Parra I, et al. Bias in algorithms of AI systems developed for COVID-19: a scoping review. J Bioeth Inq 2022; 19: 407–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Witteman HO, Zikmund-Fisher BJ. The defining characteristics of Web 2.0 and their potential influence in the online vaccination debate. Vaccine 2012; 30: 3734–3740. [DOI] [PubMed] [Google Scholar]
- 82. Charalambous A. Social media and health policy. Asia Pac J Oncol Nurs 2019; 6: 24–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wawrzuta D, Jaworski M, Gotlib J, et al. Characteristics of antivaccine messages on social media: systematic review. J Med Internet Res 2021; 23: e24564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Betsch C, Brewer NT, Brocard P, et al. Opportunities and challenges of Web 2.0 for vaccination decisions. Vaccine 2012; 30: 3727–3733. [DOI] [PubMed] [Google Scholar]
- 85. Chinchilla K, Matos C, Hall V, et al. Patient organizations’ barriers in pharmacovigilance and strategies to stimulate their participation. Drug Saf 2021; 44: 181–191. [DOI] [PubMed] [Google Scholar]
- 86. Matos C, Weits G, van Hunsel F. The role of European patient organizations in pharmacovigilance. Drug Saf 2019; 42: 547–557. [DOI] [PubMed] [Google Scholar]
- 87. Smith MY, Morrato E. Advancing the field of pharmaceutical risk minimization through application of implementation science best practices. Drug Saf 2014; 37: 569–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Malouin RA, Mckernan L, Forsberg A, et al. Impact of the 340B pharmacy program on services and supports for persons Served by hemophilia treatment centers in the United States. Matern Child Health J 2018; 22: 1240–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Mouchantaf R, Auth D, Moride Y, et al. Risk management for the 21st century: current status and future needs. Drug Saf 2021; 44: 409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-taw-10.1177_20420986221146418 for Patient-centered pharmacovigilance: priority actions from the inherited bleeding disorders community by Fiona Robinson, Sonji Wilkes, Nathan Schaefer, Miriam Goldstein, Michelle Rice, Johanna Gray, Sharon Meyers and Leonard A. Valentino in Therapeutic Advances in Drug Safety




