Abstract
The C–H bond activation of methylquinolines, quinoline, 3-methoxyquinoline, and 3-(trifluoromethyl)quinoline promoted by the square-planar rhodium(I) complex RhH{κ3-P,O,P-[xant(PiPr2)2]} [1; xant(PiPr2)2 = 9,9-dimethyl-4,5-bis(diisopropylphosphino)xanthene] has been systematically studied. Results reveal that the activation of the heteroring is preferred over the activation of the carbocycle, and the activated position depends upon the position of the substituent in the substrate. Thus, 3-, 4-, and 5-methylquinoline reacts with 1 to quantitatively form square-planar rhodium(I)-(2-quinolinyl) derivatives, whereas 2-, 6-, and 7-methylquinoline quantitatively leads to rhodium(I)-(4-quinolinyl) species. By contrast, quinoline and 8-methylquinoline afford mixtures of the respective rhodium(I)-(2-quinolinyl) and -(4-quinolinyl) complexes. 3-Methoxyquinoline displays the same behavior as that of 3-methylquinoline, while 3-(trifluoromethyl)quinoline yields a mixture of rhodium(I)-(2-quinolinyl), -(4-quinolinyl), -(6-quinolinyl), and -(7-quinolinyl) isomers.
Introduction
Direct C–H bond functionalization is a powerful and environmentally sustainable procedure, which converts simple compounds into valuable molecules, the C–H rupture being the key step in the processes of this class.1 The strength of the different C–H bonds of an organic substrate is in a narrow range, as proven by their dissociation energies; the range is particularly narrow when the entire molecule is aromatic. Transition metal complexes display ability to modify in a different manner the activation energy of the rupture of the diverse aromatic C–H bonds, as a function of their position at the aromatic ring and of the electronic and steric properties of the substituents of such a ring. Accordingly, to selectively perform the cleavage of a particular C–H bond, a metal species is usually needed.2 It is well established that the first step for a metal-promoted C–H bond rupture is the formation of a σ-intermediate LnM(η2-HC), which subsequently evolves by heterolytic or homolytic cleavage of the coordinated C–H bond. In this way, the activation energy of the rupture process is dependent on the stability of the σ-intermediate and the dissociation energy of the coordinated C–H bond. High chemo- and regioselectivities have been achieved by the kinetic governing of the rupture through the fine tuning of the electron density on the metal center and the design of the free space around the metal core, by choosing the ligands of the coordination sphere.3 From a thermodynamic point of view, strengths of the possible M–C bonds in the product dominate the selectivity of the cleavage.4
A quinoline core is present in many natural products and as a unit is a remarkable scaffold for the assembly of new pharmacological and agrochemical entities, given the diverse biological activity of this heterocycle and its derivatives.5 Transition-metal-catalyzed C–H functionalization of quinolines is currently receiving great attention as an environmentally sustainable alternative to the classical functionalization procedures, which generate more wastes.6 Functionalization of the C2 position is well established with a variety of transition-metal catalysts.7 The functionalization of C3,8 C4,9 and C810 positions has been also achieved, although it is always a real challenge. On the other hand, selective functionalization of C5–C7 positions requires the help of an assistant on some ligand of the metal coordination sphere of the catalyst and/or at an adjacent position of the substrate.11 The rationalization of these findings is certainly difficult, to a large extent due to the scarce number of systematic studies performed on the elemental reaction of metal-promoted C–H bond activation of quinolines. Such a stoichiometric process has been rarely observed at C2 and C8 positions. The activation of the C–H bond at the 2 position is facilitated by the proximity of the nitrogen atom, which many times acts as a coordination assistant,12 whereas the rupture of the C–H bond at the 8 position takes place thanks to the formation of five-membered metalacycles involving also the nitrogen.13
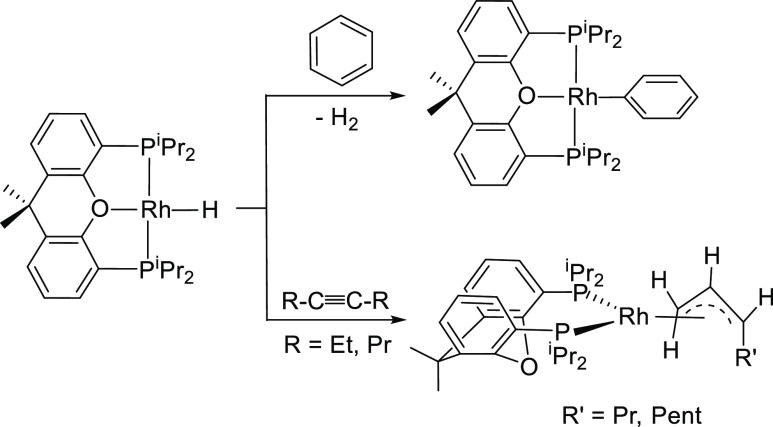
Rhodium catalysts are among the most utilized for the functionalization of quinolines, in particular, to build C–C, C–N, and C–halogen bonds.14 The square-planar monohydride complex RhH{κ3-P,O,P-[xant(PiPr2)2]} [xant(PiPr2)2 = 9,9-dimethyl-4,5-bis(diisopropylphosphino)xanthene] is a rare stable member of the family of late-transition metal unsaturated monohydride complexes, which was initially prepared in only moderate yield.15 In spite of this unfortunate handicap, its chemistry was studied in some recent years, proving to promote the activation of a wide range of σ-bonds,16 including C(sp2)–H bonds of arenes17 and C(sp3)–H bonds of bis(alkyl)alkynes18 to afford aryl and allyl derivatives (Scheme 1). In agreement with such ability, it was observed to be an efficient catalyst for C–H bond functionalization reactions such as the borylation of arenes17 and the dehydrogenative borylation of bis(alkyl)alkynes.18 Furthermore, it catalyzes the hydroboration of alkynes,19 the deuteration of boranes and hydrides of group 14 elements,20 the ammonia borane dehydrogenation,21 and the dehydropolymerization of amine-boranes.22 Two years ago, we improved significantly its synthesis, reducing the reaction time and increasing the yield, which now reaches 90%.20 The interest in the rhodium catalysts in the C–H functionalization of quinolines, the enhancement of yield in the preparation of the monohydride RhH{κ3-P,O,P-[xant(PiPr2)2]}, and its promising previous chemical behavior prompted us to employ this complex to perform a systematic study on the C–H bond activation of quinoline, its monomethyl-substituted counterparts, and related substituted quinolines.
Scheme 1. C(sp2)–H and C(sp3)–H Bond Activation of Arenes and Bis(alkyl)alkynes.
This paper reports the C–H bond activation of quinoline and substituted quinolines promoted by RhH{κ3-P,O,P-[xant(PiPr2)2]}, demonstrates that the activated position depends upon the position of the substituent and its nature, presents the selective formation of the first-metal-(4-quinolinyl) derivatives resulting from the direct C–H bond activation of a quinoline-type molecule, and reveals that the activation at 6 and 7 positions is also possible without the help of a coordination assistant.
Results and Discussion
C–H Bond Activation of Quinoline and Methylquinolines
In addition to quinoline, the substrates used were 2-, 3-, 4-, 5-, 6-, 7-, and 8-methylquinoline. The study was performed in n-octane, at 80 °C, using 1:1 molar ratios of rhodium/heterocycle. Reactions were followed by 31P{1H} NMR spectroscopy for 48–72 h, until quantitative transformation of rhodium(I)-hydride RhH{κ3-P,O,P-[xant(PiPr2)2]} (1) into the corresponding rhodium(I)-quinolinyl reaction products. Such species, which are the first square-planar transition metal complexes bearing a quinolinyl ligand, result from the direct C–H bond activation of the substrate (HQ) and the subsequent fast elimination of molecular hydrogen (Scheme 2). Although the trans-disposition of the hydride ligands appears to be favored in the rhodium(III)-dihydride intermediates similarly generated from 1 in related processes, the reductive elimination of molecular hydrogen rapidly occurs in all the cases because the hemilabile character of the oxygen atom of the ether-diphosphine allows the necessary three-center transition state.16a,17 The activated position depends on the position of the methyl substituent, with three different patterns of behavior being observed: selective activation of the C−H bond at position 2, selective activation of the C−H bond at position 4, and C−H bond activation competition between positions 2 and 4.
Scheme 2. C–H Bond Activation of Quinoline and Methylquinolines.
Selective Activation of the C–H Bond at Position 2
A methyl group at 3, 4, and 5 positions directs the C–H bond activation of the mono-substituted heterocycle to the 2 position. Thus, treatment of 1 with 3-, 4-, and 5-methylquinoline quantitatively leads to the rhodium(I)-(2-quinolinyl) derivatives 2–4 (Scheme 3), which were isolated as yellow solids in moderate yields, about 40%, due to their high solubility in alkanes including pentane.
Scheme 3. C–H Bond Activation of Position 2.
The three compounds were characterized by X-ray diffraction analysis. Figure 1a–c gives views of the structures, which demonstrate the activation of the C–H bond at the 2 position and the square-planar environment of the rhodium atom with the ether-diphosphine mer-coordinated and the quinolinyl group trans-disposed to the oxygen atom. The Rh–C bond lengths are similar, being in the range 1.957(14)–1.986(6) Å. It should be noted that unlike the previous cases involving activation of the C–H bond at position 2,12f coordination of the N atom is not observed despite the formally unsaturated character of the metal center. This is not entirely surprising given the ability of d8 ions to form square-planar species of 16 valence electrons. The NMR spectra are consistent with the structures (Figures S2–S10). Aromatic hydrogen atoms give rise to the patterns expected for the corresponding substitutions of the heterocycles, in the 1H spectra of the respective complexes (Figures S3, S6, and S9). The resonance due to the metalated carbon atom is observed as a doublet of triplets (1JC–Rh ≈ 43 Hz, 2JC–P ≈ 11 Hz), between 197 and 200 ppm, in the 13C{1H} spectra, whereas the 31P{1H} spectra display a doublet (1JP–Rh ≈ 185 Hz), close to 37 ppm, in agreement with the equivalence of the PiPr2 arms of the diphosphine.
Figure 1.
Molecular diagram of complexes 2–6, 8, and 11–13 [ellipsoids shown at 50% probability, except for 11 (30%)]. All hydrogen atoms are omitted for clarity.
Particularly noteworthy is the behavior of 3-methylquinoline. Such a substrate undergoes C–H bond activation in the heteroring in a sterically hindered position, adjacent to the methyl group, which makes it difficult for the C–H bond to approach the metal center. At first glance, this is surprising because the steric congestion around the carbocycle positions is significantly lower. On the other hand, it suggests that the activation of the heteroring positions is strongly favored with regard to those of the carbocycle for electronic reasons. The exclusivity in selection between positions 2 and 4 is also a remarkable finding as both are equally hampered by the methyl substituent.
Selective Activation of the C–H Bond at Position 4
A methyl group at 2, 6, and 7 positions of the mono-substituted quinoline directs the C–H bond activation to the 4 position, in contrast to the activations shown in Scheme 3. Thus, treatment of 1 with 2-, 6-, and 7-methylquinoline quantitatively affords the rhodium(I)-(4-quinolinyl) derivatives 5–7 (Scheme 4), which were isolated as yellow solids with yields from moderate to high (33–64%).
Scheme 4. C–H Bond Activation of Position 4.
The formation of the first transition metal organometallic compounds resulting from the direct rupture of a C–H bond at the 4 position of a quinoline-type molecule was confirmed by the X-ray structures of 5 (Figure 1d) and 6 (Figure 1e). Both structures resemble those of 2–4 with Rh–C distances of 1.9692(14) (5) and 1.9720(11) (6) Å. The NMR spectra of 5–7 also support the activation of the aromatic C–H bond at the 4 position of the quinoline, mainly the 1H spectra (Figures S12, S15, and S18). Although the 13C{1H} and 31P{1H} spectra of these compounds and those of 2–4 are similar, there are between them enough differences to also convert these spectra into a characteristic feature of the activated position (Table 1). The 13C{1H} spectra of 5–7 display the doublet of triplets (1JC–Rh = 41–44 Hz, 2JC–P = 10–12 Hz) due to the metalated carbon atom of the heterocycle at about 187 ppm, shifted around 10 ppm toward a higher field with regard to the complexes 2–4. In contrast, in the 31P{1H} spectra, the doublet generated by the equivalent PiPr2 arms of the diphosphine appears at about 38 ppm, slightly shifted to a lower field, with values for the P–Rh coupling constant close to 171 Hz, which are about 14 Hz smaller than those observed in 2–4.
Table 1. Selected Spectroscopic Data of Complexes 2–11a.
|
31P{1H} NMR |
13C{1H} NMR |
||||
|---|---|---|---|---|---|
| compound | δ | 1JP–Rh | δRh–C | 1JC–Rh | 2JC–P |
| 2 | 36.9 (d) | 185.3 | 200.6 (dt) | 44.5 | 11.4 |
| 3 | 37.4 (d) | 185.3 | 197.9 (dt) | 43.1 | 10.0 |
| 4 | 37.6 (d) | 185.1 | 197.1 (dt) | 43.9 | 11.1 |
| 5 | 38.3 (d) | 171.7 | 187.0 (dt) | 43.1 | 10.5 |
| 6 | 38.4 (d) | 171.2 | 186.5 (dt) | 41.9 | 12.2 |
| 7 | 38.3 (d) | 171.2 | 187.5 (dt) | 42.7 | 12.0 |
| 8 | 38.5 (d) | 171.1 | 187.9 (dt) | 43.0 | 11.7 |
| 9 | 37.7 (d) | 184.7 | 196.0b | ||
| 10 | 38.1 (d) | 171.8 | 188.3 (dt) | 41.5 | 12.5 |
| 11 | 37.3 (d) | 184.8 | 197.4 (dt) | 43.1 | 10.9 |
NMR spectra registered in benzene-d6.
NMR spectrum registered in n-octane. Multiplicity not resolved.
Activation of the heteroring again was expected after observing the previous activations. However, the exclusive selection of the 4 position surprised us, given the activation of 3-methylquinoline at the 2 position and because the C–H bond at the 4 position is sterically more congested than those at 2 and 3 positions. One more time, steric reasons do not seem to be determinant in the C–H activation of these substrates. On the contrary, the substituent located in any position of both rings has a marked electronic repercussion in the activation of the C–H bonds of the heteroring; the activated C–H bond depends on the position of the methyl substituent, although the activation of the C–H bond in the 3 position seems hard to reach.
C–H Bond Activation Competition between Positions 2 and 4
In contrast to the previously mentioned heterocycles, quinoline and 8-methylquinoline undergo the rupture of the C–H bonds at 2 and 4 positions in a competitive manner, with the activation of the C–H bond at the 3 position being definitely elusive (Scheme 5). Treatment of 1 with quinoline under the standard conditions of the study gives rise to the formation of a yellow solid (51% yield) and an orange solution. The yellow solid was characterized as the rhodium(I)-(4-quinolinyl) derivative 8, by X-ray diffraction analysis. Figure 1f shows its structure [Rh–C = 1.980(2) Å]. The solution contains a mixture of 8 and its 2-quinolinyl isomer 9 in a 3:7 molar ratio. The presence of the latter is strongly supported by the NMR spectra of the mixture (Figures S23–S26). The 1H spectrum shows the expected pattern for a Rh substitution at the 2 position of the heterocycle (Figure S24), whereas the 13C{1H} spectrum contains a resonance at 196 ppm, and the 31P{1H} spectrum displays a doublet (1JP–Rh = 184.7 Hz) at 37.7 ppm, in agreement with 2–4. The reaction of 1 with 8-methylquinoline leads to a 1:1 mixture of the 4-quinolinyl derivative 10 and its 2-quinolinyl isomer 11. Orange single crystals of the latter, suitable for X-ray analysis, were isolated from the mixture. Figure 1g gives a view of the structure [Rh–C = 1.972(16) Å]. Complex 10 was fully characterized by NMR spectroscopy. The spectra show characteristic features of Rh(I)-(4-quinolinyl) complexes (Figures S27–S31). In accordance with 5–7, the 13C{1H} spectrum contains a doublet of triplets (1JC–Rh = 41.5 Hz, 2JC–P = 12.5 Hz) at 188.3 ppm, whereas the 31P{1H} spectrum displays a doublet (1JP–Rh = 171.8 Hz) at 38.1 ppm.
Scheme 5. Competitive C–H Bond Activations of Positions 4 and 2.
The formation of 8–11 confirms that the C–H bond activation of quinoline can be selectively directed to the 2 or 4 position by the introduction of a methyl substituent at the appropriate place on the heterocycle, with the exception of the 8 position.
C–H Bond Activation of Related Mono-Substituted Quinolines
Having established that a methyl group, located at any position of the quinoline rings, directs the C–H bond activation of the corresponding heterocycle to 2 or 4 positions and upon observing a scarce steric influence of the methyl group on the process, in contrast to the C–H bond activation of aromatic hydrocarbons,17,23 we decided to study the influence of markedly different substituents from an electronic point of view. We selected methoxide as a strongly electron-donating group, trifluoromethyl as a notable electron-withdrawing substituent, and the 3 position on being adjacent to both previously activated bonds.
The methoxide group does not change the rate or the selectivity of the activation with regard to methyl. Treatment of 1 with 3-methoxyquinoline quantitatively leads to a 2-quinolinyl derivative, 12 (Scheme 6), after 72 h as 3-methylquinoline. Complex 12 was isolated as an orange solid and characterized by X-ray diffraction analysis. Figure 1h shows a view of the structure. The Rh–C bond length of 1.9678(17) Å is statistically identical to that of the 3-methyl counterpart. The NMR spectra agree well with those of the 2-quinolinyl derivatives 2–4, 9, and 11 (Figures S32–S34). The 13C{1H} spectrum contains a doublet of triplets (1JC–Rh = 45.1 Hz, 2JC–P = 11.2 Hz) in the expected region, 192.9 ppm, whereas the 31P{1H} spectrum shows a doublet at 38.6 ppm with a P–Rh coupling constant displaying a value of 183.0 Hz, which lies in the same range as those of 2–4, 9, and 11.
Scheme 6. C–H Bond Activation of 3-Methoxyquinoline.
The trifluoromethyl group introduces very noticeable changes. It slows down the rate of the reaction and dramatically reduces the selectivity, promoting the activations of the C–H bonds at 2, 4, 6, and 7 positions (Scheme 7). Thus, the treatment of 1 with 3-(trifluoromethyl)quinoline gives a mixture of derivatives 13 (2-quinolinyl, 25%), 14 (4-quinolinyl, 14%), 15 (6-quinolinyl, 25%), and 16 (7-quinolinyl, 36%), after 5 days, according to the 31P{1H} NMR spectrum of the resulting solution in n-octane (Figure S35). The presence of 13 and 14 in the mixture is supported by the values of the P–Rh coupling constants obtained from the respective doublets at 35.3 and 37.5 ppm, of 183.9 and 174.6 Hz, which, respectively, lie in the range of the coupling constants observed in the previous 2-quinolinyl and 4-quinolinyl complexes (see Table 1). The evaporation of n-octane affords a reaction crude that when subsequently treated with pentane, yields a red solid only formed by 15 and 16 in a 1:2.5 molar ratio. Such a solid allowed us to obtain fine 1H, (1H,1H)–correlated spectroscopy (COSY), nuclear Overhauser effect spectroscopy (NOESY), (1H,13C)-heteronuclear single quantum coherence (HSQC), and (1H,13C)-heteronuclear multiple bond correlation (HMBC) NMR spectra to fully characterize both 15 and 16 (see Figures S36–S42). A noticeable feature of 15 is a doublet (1JP–Rh = 172.8 Hz) at 37.8 ppm in the 31P{1H} spectrum, whereas remarkable resonances of 16 are a doublet (1JP–Rh = 173.7 Hz) at 38.0 ppm in the 31P{1H} spectrum and a doublet of triplets (1JC–Rh = 40.4 Hz, 2JC–P = 12.5 Hz) at 179.7 ppm in the 13C{1H} spectrum. The formation of the 2-quinolinyl derivative 13, the 6-quinolinyl complex 15, and the 7-quinolinyl compound 16, as a result of the C–H bond activation of 3-(trifluoromethyl)quinoline, was furthermore confirmed by orange and red crystals obtained from the original solution in n-octane. X-ray diffraction of an orange single crystal provided the structure of 13 (Figure 1i), which showed a Rh–C distance of 1.977(4) Å. The X-ray diffraction analysis of a red crystal revealed that it was a pseudo-merohedral twin resulting from the co-crystallization of 15 and 16, in the same unit cell, in an approximately 1:2 proportion. Although the results of the analysis are not enough accurate to permit a deep discussion of the structural parameters, they confirm the bond sequence in both 15 and 16 and therefore their formation.
Scheme 7. C–H Bond Activation of 3-(Trifluoromethyl)quinoline.
The behavior of 3-(trifluoromethyl)quinoline points out that the introduction of an electron-withdrawing substituent at the heteroring allows the direct activation of the C–H bond at the elusive positions 6 and 7 of the heterocycle, although the selectivity of the activation is significantly reduced.
Concluding Remarks
This study reveals that complex RhH{κ3-P,O,P-[xant(PiPr2)2]} promotes the C–H bond activation of quinoline and mono-substituted quinolines, to form square-planar rhodium(I)-quinolinyl derivatives. Activation of the heteroring is preferred over activation of the carbocycle. Such selectivity could be related to some contribution of an anionic carbene resonance form to the rhodium–quinolinyl bond. The chemical shifts of the resonance corresponding to the metalated carbon, in a relatively low field, in the 13C{1H} NMR spectra seem to point out in this direction. Within the heteroring, the 3 position is always elusive, while it is possible to select between activation of 2 and 4 positions by the introduction of a methyl substituent at the appropriate place on the heterocycle, with the exception of the 8 position. The presence of an electron-withdrawing substituent such as trifluoromethyl at the 3 position of the heteroring allows us to activate the carbocycle at 6 and 7 positions, although the selectivity of the activation suffers a significant decrease.
In summary, the first systematic study on the transition metal-promoted C–H bond activation of substituted quinolines yielded clear patterns of behavior, which allow us to control the selectivity of the activation and to observe direct C–H bond activations of unprecedented positions.
Experimental Section
General Information
All reactions were carried out with exclusion of air using Schlenk-tube techniques or in a drybox. Instrumental methods and X-ray details are given in the Supporting Information. In the NMR spectra (Figures S2–S42), the chemical shifts (in ppm) are referenced to residual solvent peaks (1H, 13C{1H}) or external 85% H3PO4 (31P{1H}), while J and N (N = JP–H + JP′–H for 1H and N = JP–C + JP′–C for 13C{1H}) are given in hertz. RhH{κ3-P,O,P-[xant(PiPr2)2]} (1)20 was prepared according to the reported procedure.
Reaction of 1 with 3-Methylquinoline: Preparation of Rh(κ1-C2-Quinolinyl-3-Me){κ3-P,O,P-[xant(PiPr2)2]} (2)
A solution of 1 (200 mg, 0.37 mmol) in n-octane (3 mL) was treated with 3-methylquinoline (49 μL, 0.37 mmol), and the resulting mixture was stirred at 80 °C for 48 h. After this time, the solution was evaporated to dryness to afford a brown residue. Addition of pentane (4 mL) afforded an orange solid that was washed with pentane (2 × 2 mL) and dried in vacuo. Yield: 67 mg (26%). The reaction is quantitative, but the isolated yield is low due to its high solubility in pentane. The solid is extremely sensitive to air, preventing us from getting its elemental analysis. HRMS (electrospray, m/z): calcd for C37H49NOP2Rh [M + H]+, 688.2339; found, 688.2325. IR (cm–1): ν(C=N) 1585 (m), ν(C–O–C) 1150 (m). 1H NMR (300.13 MHz, benzene-d6, 298 K): δ 8.25 (d, 3JH–H = 8.3, 1H, CH C-ring qn), 7.63 (d, 3JH–H = 8.0, 1H, CH C-ring qn), 7.49 (m, 1H, CH C-ring qn), 7.33 (s, 1H, CH N-ring qn), 7.30–7.19 (m, 3H, 2H CH-arom POP + 1H CH C-ring qn), 7.07 (dd, 3JH–H = 7.7, 4JH–H = 1.5, 2H, CH-arom POP), 6.87 (t, 3JH–H = 7.5, 2H, CH-arom POP), 3.32 (s, 3H, CH3 qn), 2.52 (m, 2H, PCH(CH3)2), 2.20 (m, 2H, PCH(CH3)2), 1.13 (s, 6H, CH3), 1.36–0.91 (m, 24H, PCH(CH3)2). 13C{1H}-apt NMR (75.48 MHz, benzene-d6, 298 K): δ 200.6 (dt, 1JC–Rh = 44.5, 2JC–P = 11.4, Rh–C qn), 157.0 (vt, N = 16.0, C-arom POP), 147.1 (d, 3JC–Rh = 3.6, C qn), 138.0 (s, C–CH3 qn), 131.8 (s, C-arom POP), 131.0 (s, CH-arom POP), 128.7 (s, CH C-ring qn), 128.1 (s, CH C-ring qn, inferred from the HSQC spectrum), 127.4 (s, CH C-ring qn), 126.9 (s, CH-arom POP), 126.5 (s, CH C-ring qn), 125.7 (vt, N = 16.5, C-arom POP), 125.1 (s, C qn), 124.7 (d, 2JC–Rh = 8.9, CH N-ring qn), 124.2 (s, CH-arom POP), 121.4 (s, CH C-ring qn), 34.5 (s, C(CH3)2), 31.5 (s, C(CH3)2), 25.8 (m, PCH(CH3)2), 26.0 (s, CH3 qn), 25.1 (m, PCH(CH3)2), 19.0 (vt, N = 9.7, PCH(CH3)2), 18.6, 18.3, 18.1 (all s, PCH(CH3)2). 31P{1H} NMR (121.49 MHz, benzene-d6, 298 K): δ 36.9 (d, 1JRh–P = 185.3).
Reaction of 1 with 4-Methylquinoline: Preparation of Rh(κ1-C2-Quinolinyl-4-Me){κ3-P,O,P-[xant(PiPr2)2]} (3)
A solution of 1 (200 mg, 0.37 mmol) in n-octane (3 mL) was treated with 4-methylquinoline (49 μL, 0.37 mmol), and the resulting mixture was stirred at 80 °C for 48 h. After this time, the solution was evaporated to dryness to afford a brown residue. Addition of pentane (4 mL) afforded an orange solid that was washed with pentane (2 × 2 mL) and dried in vacuo. Yield: 96 mg (38%). The reaction is quantitative, but the isolated yield is low due to its high solubility in pentane. Anal. Calcd. for C37H48NOP2Rh: C, 64.63; H, 7.04; N, 2.04. Found; 64.84; H, 6.98; N, 2.03. HRMS (electrospray, m/z): calcd for C37H49NOP2Rh [M + H]+, 688.2339; found, 688.2343. IR (cm–1): ν(C=N) 1576 (m), ν(C–O–C) 1194 (m). 1H NMR (300.13 MHz, benzene-d6, 298 K): δ 8.35 (d, 3JH–H = 8.3, 1H, CH C-ring qn), 8.01 (s, 1H, CH N-ring qn), 7.80 (d, 3JH–H = 9.1, 1H, CH C-ring qn), 7.5 (m, 1H, CH C-ring qn), 7.3 (m, 2H, CH-arom POP), 7.20 (m, 1H, CH C-ring qn), 7.05 (dd, 3JH–H = 7.7, 4JH–H = 1.5, 2H, CH-arom POP), 6.86 (t, 3JH–H = 7.7, 2H, CH-arom POP), 2.45 (m, 4H, PCH(CH3)2), 2.42 (s, 3H, CH3 qn), 1.24 (s, 6H, CH3), 1.22 (dvt, 3JH–H = 7.4, N = 14.5, 24H, PCH(CH3)2). 13C{1H}-apt NMR (75.48 MHz, benzene-d6, 298 K): δ 197.9 (dt, 1JC–Rh = 43.1, 2JC–P = 10.0, Rh–C qn), 156.2 (vt, N = 15.8, C-arom POP), 148.6 (d, 3JC–Rh = 3.8, C qn), 135.6 (s, CH N-ring qn), 131.3 (s, CH-arom POP), 131.1 (vt, N = 5.2, C-arom POP), 130.4 (s, C qn), 128.7 (s, CH C-ring qn), 127.5 (s, CH-arom POP), 127.3 (s, CH C-ring qn), 126.0 (vt, N = 16.2, C-arom POP), 125.8 (s, C–CH3 qn), 124.3 (s, CH C-ring qn), 124.1 (s, CH-arom POP), 121.3 (s, CH C-ring qn), 34.2 (s, C(CH3)2), 32.4 (s, C(CH3)2), 25.8 (dvt, 2JC–Rh = 2.5, N = 18.1, PCH(CH3)2), 19.5 (vt, N = 9.0, PCH(CH3)2), 18.9 (s, PCH(CH3)2), 18.3 (s, CH3 qn). 31P{1H} NMR (121.49 MHz, benzene-d6, 298 K): δ 37.4 (d, 1JRh–P = 185.3).
Reaction of 1 with 5-Methylquinoline: Preparation of Rh(κ1-C2-Quinolinyl-5-Me){κ3-P,O,P-[xant(PiPr2)2]} (4)
A solution of 1 (200 mg, 0.37 mmol) in n-octane (3 mL) was treated with 5-methylquinoline (49 μL, 0.37 mmol), and the resulting mixture was stirred at 80 °C for 48 h. After this time, the solution was evaporated to dryness to afford a brown residue. Addition of pentane (4 mL) afforded an orange solid that was washed with pentane (2 × 2 mL) and dried in vacuo. Yield: 95 mg (38%). The reaction is quantitative, but the isolated yield is low due to its high solubility in pentane. Anal. Calcd. for C37H48NOP2Rh: C, 64.63; H, 7.04; N, 2.04. Found; C, 64.89; H, 7.32; N, 2.18. HRMS (electrospray, m/z): calcd for C37H49NOP2Rh [M + H]+, 688.2339; found, 688.2321. IR (cm–1): ν(C=N) 1587 (m), ν(C–O–C) 1188 (m). 1H NMR (300.13 MHz, benzene-d6, 298 K): δ 8.19 (d, 3JH–H = 8.3, 1H, CH C-ring qn), 8.10 (d, 3JH–H = 8.7, 1H, CH N-ring qn), 7.49–7.37 (m, 2H, CH C- and N-rings qn), 7.27 (m, 2H, CH-arom POP), 7.06 (dd, 3JH–H = 7.0, 4JH–H = 1.3, 2H, CH-arom POP), 7.00 (d, 2JH–H = 6.9, 1H, CH N-ring qn), 6.87 (t, 3JH–H = 7.6, 2H, CH-arom POP), 2.47 (s, 3H, CH3 qn), 2.45 (m, 4H, PCH(CH3)2), 1.25 (s, 6H, CH3), 1.23 (dvt, 3JH–H = 7.4, N = 14.4, 24H, PCH(CH3)2). 13C{1H}-apt NMR (75.48 MHz, benzene-d6, 298 K): δ 197.1 (dt, 1JC–Rh = 43.9, 2JC–P = 11.1, Rh–C qn), 156.2 (vt, N = 16.0, C-arom POP), 149.2 (d, 3JC–Rh = 3.5, C qn), 134.5 (dt, 2JC–Rh = 3.4, 3JC–P = 3.4, CH N-ring qn), 134.3 (s, C–CH3 qn), 131.4 (s, CH-arom POP), 131.1 (vt, N = 5.1, C-arom POP), 127.5 (s, CH-arom POP), 127.1 (s, CH C-ring qn), 126.0 (vt, N = 15.5, C-arom POP), 124.4 (s, C qn), 124.1 (s, CH-arom POP), 122.6 (s, CH C-ring qn), 121.0 (s, CH N-ring qn), 34.2 (s, C(CH3)2), 32.4 (s, C(CH3)2), 25.8 (dvt, 2JC–Rh = 2.6, N = 18.1, PCH(CH3)2), 19.6 (vt, N = 8.7, PCH(CH3)2), 18.9 (s, PCH(CH3)2), 18.7 (s, CH3 qn). 31P{1H} NMR (121.49 MHz, benzene-d6, 298 K): δ 37.6 (d, 1JRh–P = 185.1).
Reaction of 1 with 2-Methylquinoline: Preparation of Rh(κ1-C4-Quinolinyl-2-Me){κ3-P,O,P-[xant(PiPr2)2]} (5)
A solution of 1 (200 mg, 0.37 mmol) in n-octane (3 mL) was treated with 2-methylquinoline (50 μL, 0.37 mmol), and the resulting mixture was stirred at 80 °C for 72 h. After this time, the solution was evaporated to dryness to afford a brown residue. Addition of pentane (4 mL) afforded a yellow solid that was washed with pentane (2 × 2 mL) and dried in vacuo. Yield: 161.7 mg (64%). Anal. Calcd. for C37H48NOP2Rh: C, 64.63; H, 7.04; N, 2.04. Found; C, 64.24; H, 7.32; N, 1.87. HRMS (electrospray, m/z): calcd for C37H49NOP2Rh [M + H]+, 688.2339; found, 688.2326. IR (cm–1): ν(C=N) 1546 (m), ν(C–O–C) 1197 (m). 1H NMR (300.13 MHz, benzene-d6, 298 K): δ 9.48 (m, 1H, CH C-ring qn), 8.40 (m, 1H, CH C-ring qn), 8.09 (d, 3JH–Rh = 2.4, 1H, CH N-ring qn), 7.5 (m, 2H, CH C-ring qn), 7.17 (m, 2H, CH-arom POP), 7.05 (dd, 3JH–H = 7.7, 4JH–H = 1.5, 2H, CH-arom POP), 6.84 (t, 3JH–H = 7.6, 2H, CH-arom POP), 2.88 (s, 3H, CH3 qn), 2.38 (m, 2H, PCH(CH3)2), 2.14 (m, 2H, PCH(CH3)2), 1.30 (s, 3H, CH3), 1.19 (s, 3H, CH3), 1.02 (dvt, 3JH–H = 6.8, N = 15.4, 12H, PCH(CH3)2), 1.02 (dvt, 3JH–H = 7.5, N = 14.4, 6H, PCH(CH3)2), 0.81 (dvt, 3JH–H = 9.2, N = 16.3, 6H, PCH(CH3)2). 13C{1H}-apt NMR (75.48 MHz, benzene-d6, 298 K): δ 187.0 (dt, 1JC–Rh = 43.1, 2JC–P = 10.5, Rh–C qn), 156.1 (vt, N = 15.6, C-arom POP), 152.6 (s, C–CH3 qn), 147.5 (s, C qn), 138.7 (s, CH C-ring qn), 138.4 (s, C qn), 132.7 (s, CH N-ring qn), 131.1 (s, CH-arom POP), 131.0 (vt, N = 5.0, C-arom POP), 130.0 (s, CH C-ring qn), 128.0 (s, CH-arom POP, inferred from the HSQC spectrum), 127.3 (s, CH C-ring qn), 125.0 (vt, N = 16.2, C-arom POP), 124.3 (s, CH-arom POP), 120.9 (s, CH C-ring qn), 34.6 (s, C(CH3)2), 34.1 (s, C(CH3)2), 31.0 (s, C(CH3)2), 25.7 (vt, N = 18.4, PCH(CH3)2), 25.6 (s, CH3 qn), 24.9 (vt, N = 15.1, PCH(CH3)2), 19.2 (vt, N = 6.8, PCH(CH3)2), 18.5 (vt, N = 8.3, PCH(CH3)2), 18.3, 18.1 (both s, PCH(CH3)2). 31P{1H} NMR (121.49 MHz, benzene-d6, 298 K): δ 38.3 (d, 1JRh–P = 171.7).
Reaction of 1 with 6-Methylquinoline: Preparation of Rh(κ1-C4-Quinolinyl-6-Me){κ3-P,O,P-[xant(PiPr2)2]} (6)
A solution of 1 (200 mg, 0.37 mmol) in n-octane (3 mL) was treated with 6-methylquinoline (49 μL, 0.37 mmol), and the resulting mixture was stirred at 80 °C for 48 h. After this time, the solution was evaporated to dryness to afford a brown residue. Addition of pentane (4 mL) afforded a yellow solid that was washed with pentane (2 × 2 mL) and dried in vacuo. Yield: 156 mg (62%). Anal. Calcd. for C37H48NOP2Rh: C, 64.63; H, 7.04; N, 2.04. Found: C, 64.31; H, 6.88; N, 2.30. HRMS (electrospray, m/z): calcd for C37H49NOP2Rh [M + H]+, 688.2339; found, 688.2324. IR (cm–1): ν(C=N) 1547 (m), ν(C–O–C) 1198 (m). 1H NMR (300.13 MHz, benzene-d6, 298 K): δ 9.36 (s, 1H, CH C-ring qn), 8.62 (d, 3JH–H = 4.6, 1H, CH N-ring qn), 8.42 (d, 3JH–H = 8.5, 1H, CH C-ring qn), 8.07 (m, 1H, CH N-ring qn), 7.35 (dd, 3JH–H = 8.5, 4JH–H = 2.1, H, CH C-ring qn), 7.18 (m, 2H, CH-arom POP), 7.04 (d, 3JH–H = 7.6, 4JH–H = 1.5, 2H, CH-arom POP), 6.84 (t, 3JH–H = 5.6, 2H, CH-arom POP), 2.55 (s, 3H, CH3 qn), 2.34 (m, 2H, PCH(CH3)2), 2.16 (m, 2H, PCH(CH3)2), 1.29 (s, 3H, CH3), 1.19 (s, 3H, CH3), 1.19–1.02 (m, 18H, PCH(CH3)2), 0.82 (dvt, 3JH–H = 8.8, N = 16.2, 6H, PCH(CH3)2). 13C{1H}-apt NMR (100.62 MHz, benzene-d6, 298 K): δ 186.5 (dt, 1JC–Rh = 41.9, 2JC–P = 12.2, Rh–C qn), 156.0 (vt, N = 15.6, C-arom POP), 146.3 (s, C qn), 145.0 (s, CH N-ring qn), 140.5 (s, C qn), 138.0 (s, CH C-ring qn), 132.6 (s, CH N-ring qn), 131.2 (s, CH-arom POP), 130.9 (vt, N = 4.9, C-arom POP), 130.5 (s, CH C-ring qn), 130.1 (s, C–CH3 qn), 129.3 (s, CH C-ring qn), 128.4 (s, CH-arom POP), 125.0 (vt, N = 16.3, C-arom POP), 124.3 (s, CH-arom POP), 34.5 (s, C(CH3)2), 34.1 (s, C(CH3)2), 31.4 (s, C(CH3)2), 25.6 (dvt, 2JC–Rh = 2.0, N = 18.7, PCH(CH3)2), 24.8 (dvt, 2JC–Rh = 2.7, N = 19.0, PCH(CH3)2), 21.8 (s, CH3 qn), 19.3 (vt, N = 7.1, PCH(CH3)2), 18.4 (vt, N = 8.7, PCH(CH3)2), 18.3, 18.0 (both s, PCH(CH3)2). 31P{1H} NMR (161.98 MHz, benzene-d6, 298 K): δ 38.4 (d, 1JRh–P = 171.2).
Reaction of 1 with 7-Methylquinoline: Preparation of Rh(κ1-C4-Quinolinyl-7-Me){κ3-P,O,P-[xant(PiPr2)2]} (7)
A solution of 1 (200 mg, 0.37 mmol) in n-octane (3 mL) was treated with 7-methylquinoline (49 μL, 0.37 mmol), and the resulting mixture was stirred at 80 °C for 48 h. After this time, the solution was evaporated to dryness to afford a brown residue. Addition of pentane (4 mL) afforded a yellow solid that was washed with pentane (2 × 2 mL) and dried in vacuo. Yield: 84 mg (33%). The reaction is quantitative, but the isolated yield is low due to its high solubility in pentane. Anal. Calcd. for C37H48NOP2Rh: C, 64.63; H, 7.04; N, 2.04. Found; 64.93; H, 6.78; N, 2.23. HRMS (electrospray, m/z): calcd for C37H49NOP2Rh [M + H]+, 688.2339; found, 688.2342. IR (cm–1): ν(C=N) 1547 (m), ν(C–O–C) 1196 (m). 1H NMR (400.13 MHz, benzene-d6, 298 K): δ 9.44 (d, 3JH–H = 7.8, 1H, CH C-ring qn), 8.60 (d, 3JH–H = 4.5, 1H, CH N-ring qn), 8.27 (s, 1H, CH C-ring qn), 8.05 (m, 1H, CH N-ring qn), 7.36 (d, 3JH–H = 7.8, 1H, CH C-ring qn), 7.19 (m, 2H, CH-arom POP), 7.07 (d, 3JH–H = 7.0, 2H, CH-arom POP), 6.85 (t, 3JH–H = 7.5, 2H, CH-arom POP), 2.38 (s, 3H, CH3 qn), 2.36 (m, 2H, PCH(CH3)2), 2.17 (m, 2H, PCH(CH3)2), 1.31 (s, 3H, CH3), 1.21 (s, 3H, CH3), 1.11 (m, 12H, PCH(CH3)2), 1.05 (dvt, 3JH–H = 7.4, N = 14.4, 6H, PCH(CH3)2), 0.82 (dvt, 3JH–H = 7.4, N = 15.7, 6H, PCH(CH3)2). 13C{1H}-apt NMR (100.62 MHz, benzene-d6, 298 K): δ 187.5 (dt, 1JC–Rh = 42.7, 2JC–P = 12.0, Rh–C qn), 156.0 (vt, N = 15.3, C-arom POP), 148.1 (s, C qn), 145.5 (s, CH N-ring qn), 138.9 (s, C qn), 138.7 (s, CH C-ring qn), 136.5 (s, C–CH3 qn), 132.0 (s, CH N-ring qn), 131.2 (s, CH-arom POP), 130.9 (vt, N = 4.8, C-arom POP), 129.9 (s, CH C-ring qn), 128.0 (s, CH-arom POP, inferred from the HSQC spectrum), 125.0 (vt, N = 14.5, C-arom POP), 124.3 (s, CH-arom POP), 123.7 (s, CH C-ring qn), 34.5 (s, C(CH3)2), 34.1 (s, C(CH3)2), 31.3 (s, C(CH3)2), 25.6 (dvt, 2JC–Rh = 1.6, N = 18.9, PCH(CH3)2), 24.8 (dvt, 2JC–Rh = 2.5, N = 18.9, PCH(CH3)2), 21.8 (s, CH3 qn), 19.3 (vt, N = 6.0, PCH(CH3)2), 18.4, 18.0 (both s, PCH(CH3)2). 31P{1H} NMR (161.98 MHz, benzene-d6, 298 K): δ 38.3 (d, 1JRh–P = 171.2).
Reaction of 1 with Quinoline
A solution of 1 (200 mg, 0.37 mmol) in n-octane (3 mL) was treated with quinoline (43 μL, 0.37 mmol), and the resulting mixture was stirred at 80 °C for 48 h. After this time, the obtained suspension was evaporated to dryness to afford a brown residue. Addition of pentane (4 mL) afforded a yellow solid that was washed with pentane (2 × 2 mL) and dried in vacuo. Yield: 126 mg (51%). The 31P{1H} NMR spectra of the mother liquors show the presence of complexes Rh(κ1-C4-quinolinyl){κ3-P,O,P-[xant(PiPr2)2]} (8) and Rh(κ1-C2-quinolinyl){κ3-P,O,P-[xant(PiPr2)2]} (9) in a ratio 37:63, while the NMR spectra of the solid only show the presence of 8, that was obtained in a pure form due to the different solubility of 8 and 9 in pentane.
Data for 8
Anal. Calcd. for C36H46NOP2Rh: C, 64.19; H, 6.88; N, 2.08. Found; 63.82; H, 7.00; N, 2.23. HRMS (electrospray, m/z): calcd for C36H47NOP2Rh [M + H]+, 674.2182; found, 674.2198. IR (cm–1): ν(C=N) 1548 (m), ν(C–O–C) 1196 (m). 1H NMR (300.13 MHz, benzene-d6, 298 K): δ 9.54 (m, 1H, CH C-ring qn), 8.60 (d, 3JH–H = 4.4, 1H, CH N-ring qn), 8.50 (m, 1H, CH C-ring qn), 8.09 (m, 2H, CH N-ring qn), 7.51 (m, 2H, CH C-ring qn), 7.17 (m, 2H, CH-arom POP), 7.05 (dd, 3JH–H = 7.2, 4JH–H = 1.5, 2H, CH-arom POP), 6.84 (t, 3JH–H = 7.5, 2H, CH-arom POP), 2.34 (m, 2H, PCH(CH3)2), 2.14 (m, 2H, PCH(CH3)2), 1.30 (s, 3H, CH3), 1.19 (s, 3H, CH3), 1.10 (dvt, 3JH–H = 6.5, N = 13.6, 12H, PCH(CH3)2), 1.02 (dvt, 3JH–H = 7.5, N = 14.7, 6H, PCH(CH3)2), 0.78 (dvt, 3JH–H = 7.9, N = 15.7, 6H, PCH(CH3)2). 13C{1H}-apt NMR (75.48 MHz, benzene-d6, 298 K): δ 187.9 (dt, 1JC–Rh = 43.0, 2JC–P = 11.7, Rh–C qn), 156.0 (vt, N = 15.5, C-arom POP), 148.1 (s, C qn), 145.4 (s, CH N-ring qn), 140.8 (s, C qn), 138.9 (s, CH C-ring qn), 132.6 (s, CH N-ring qn), 131.2 (s, CH-arom POP), 131.0 (vt, N = 5.0, C-arom POP), 130.8 (s, CH C-ring qn), 127.9 (s, CH-arom POP, inferred from the HSQC spectrum), 127.3 (s, CH C-ring qn), 125.0 (vt, N = 16.4, C-arom POP), 124.3 (s, CH-arom POP), 121.5 (s, CH C-ring qn), 34.5 (s, C(CH3)2), 34.1 (s, C(CH3)2), 31.2 (s, C(CH3)2), 25.7 (dvt, 2JC–Rh = 2.1, N = 18.4, PCH(CH3)2), 24.8 (dvt, 2JC–Rh = 2.8, N = 19.3, PCH(CH3)2), 19.3 (vt, N = 6.9, PCH(CH3)2), 18.3 (m, PCH(CH3)2), 18.0 (s, PCH(CH3)2). 31P{1H} NMR (121.49 MHz, benzene-d6, 298 K): δ 38.5 (d, 1JRh–P = 171.1).
Spectroscopic Data for 9 Obtained from Figures S23–S25
1H NMR (300.13 MHz, benzene-d6, 298 K): δ 8.38 (d, 3JH–H = 8.3, 1H, CH C-ring qn), 8.19 (d, 3JH–H = 8.4, 1H, CH N-ring qn), 7.69 (d, 3JH–H = 8.6, 1H, CH C-ring qn), 7.58 (m, 1H, CH C-ring qn), 7.44–7.32 (m, 3H, CH N-ring qn + 2 CH-arom POP), 7.27 (m, 1H, CH C-ring qn), 7.16 (d, 3JH–H = 7.7, 2H, CH-arom POP), 6.97 (t, 3JH–H = 7.6, 2H, CH-arom POP), 2.54 (m, 4H, PCH(CH3)2), 1.35 (s, 6H, CH3), 1.39–1.25 (m, 24H, PCH(CH3)2). 31P{1H} NMR (121.49 MHz, benzene-d6, 298 K): δ 37.7 (d, 1JRh–P = 184.7).
Reaction of 1 with 8-Methylquinoline
A solution of 1 (200 mg, 0.37 mmol) in n-octane (3 mL) was treated with 8-methylquinoline (50 μL, 0.37 mmol), and the resulting mixture was stirred at 80 °C for 48 h. After this time, the solution was evaporated to dryness to afford a brown residue. Addition of pentane (4 mL) afforded an orange solid that was washed with pentane (2 × 2 mL) and dried in vacuo. Yield: 91 mg (36%). The reaction is quantitative, but the isolated yield is low due to the high solubility in pentane. The 31P{1H} NMR spectra of the crude solution show that the ratio of Rh(κ1-C4-quinolinyl-8-Me){κ3-P,O,P-[xant(PiPr2)2]} (10): Rh(κ1-C2-quinolinyl-8-Me){κ3-P,O,P-[xant(PiPr2)2]} (11) is 1:1. Upon isolation and due to their different solubility in pentane, the ratio of 10:11 changes to 35:65. Anal. Calcd. for C37H48NOP2Rh: C, 64.63; H, 7.04; N, 2.04. Found; 64.78; H, 7.00; N, 2.27. HRMS (electrospray, m/z): calcd for C37H49NOP2Rh [M + H]+, 688.2339; found, 688.2325. IR (cm–1): ν(C=N) 1580 (m), ν(C–O–C) 1193 (m).
Spectroscopic Data for 10
1H NMR (500.13 MHz, benzene-d6, 298 K): δ 9.56 (m, 1H, CH C-ring qn), 8.72 (d, 3JH–H = 4.4, 1H, CH N-ring qn), 8.21 (broad s, 1H, CH N-ring qn), 7.65–7.54 (m, 1H, CH C-ring qn), 7.31–7.22 (m, 3H, 2H CH-arom POP + 1H CH C-ring qn), 7.20–7.13 (m, 2H, CH-arom POP), 7.00–6.92 (m, 2H, CH-arom POP), 3.27 (s, 3H, CH3 qn), 2.45 (m, 2H, PCH(CH3)2), 2.26 (m, 2H, PCH(CH3)2), 1.46–1.12 (m, 24H, 6H CH3 + 18H PCH(CH3)2), 0.90 (dvt, 3JH–H = 8.3, N = 15.6, 6H, PCH(CH3)2). 13C{1H}-apt (100.62 MHz, benzene-d6, 298 K): δ 188.3 (dt, 1JC–Rh = 41.5, 2JC–P = 12.5, Rh–C qn), 156.0 (vt, N = 15.2, C-arom POP), 146.8 (s, C qn), 144.2 (s, CH N-ring qn), 140.5 (s, C qn), 137.5 (s, C qn), 137.3 (s, CH C-ring qn), 132.6 (s, CH N-ring qn), 131.1 (s, CH-arom POP), 130.9 (s, C-arom POP), 127.8 (s, CH-arom POP, inferred from the HSQC spectrum), 127.5 (s, CH C-ring qn, inferred from the HSQC spectrum), 125.0 (vt, N = 15.6, C-arom POP), 124.3 (s, CH-arom POP), 121.3 (s, CH C-ring qn), 34.5 (s, C(CH3)2), 34.1 (s, C(CH3)2), 31.2 (s, C(CH3)2), 26.0–25.4 (m, PCH(CH3)2), 24.8 (vt, N = 18.7, PCH(CH3)2), 19.4 (s, CH3 qn), 19.3, 18.5, 18.4, 18.0 (all s, PCH(CH3)2). 31P{1H} NMR (161.98 MHz, benzene-d6, 298 K): δ 38.1 (d, 1JRh–P = 171.8).
Spectroscopic Data for 11
1H NMR (500.13 MHz, benzene-d6, 298 K): δ 8.16 (d, 3JH–H = 8.4, 1H, CH N-ring qn), 7.65–7.54 (m, 2H, CH C-ring qn), 7.40 (m, 2H, CH-arom POP), 7.36 (d, 3JH–H = 8.4, 1H, CH N-ring qn), 7.31–7.22 (m, 1H, CH qn), 7.20–7.13 (m, 2H, CH-arom POP), 7.00–6.92 (m, 2H, CH-arom POP), 3.25 (s, 3H, CH3 qn), 2.51 (m, 4H, PCH(CH3)2), 1.46–1.12 (m, 30H, 6 CH3, 24 PCH(CH3)2). 13C{1H}-apt NMR (100.62 MHz, benzene-d6, 298 K): δ 197.4 (dt, 1JC–Rh = 43.1, 2JC–P = 10.9, Rh–C qn), 156.4 (vt, N = 15.7, C-arom POP), 147.6 (s, C qn), 134.9 (s, CH N-ring qn), 134.7 (s, C qn), 131.3 (s, CH-arom POP), 131.2 (s, C-arom POP), 127.5 (s, CH qn, inferred from the HSQC spectrum), 127.4 (s, CH-arom POP), 126.5 (s, CH qn), 126.0 (vt, N = 15.6, C-arom POP), 125.2 (s, CH N-ring qn), 125.0 (s, C qn), 124.1 (s, CH-arom POP), 121.2 (s, CH qn), 34.3 (s, C(CH3)2), 32.2 (s, C(CH3)2), 26.0–25.4 (m, PCH(CH3)2), 19.6 (vt, N = 7.8, PCH(CH3)2), 19.2 (s, CH3 qn), 18.9 (s, PCH(CH3)2). 31P{1H} NMR (161.98 MHz, benzene-d6, 298 K): δ 37.3 (d, 1JRh–P = 184.8).
Reaction of 1 with 3-Methoxyquinoline: Preparation of Rh(κ1-C2-Quinolinyl-3-OMe){κ3-P,O,P-[xant(PiPr2)2]} (12)
A solution of 1 (200 mg, 0.37 mmol) in n-octane (3 mL) was treated with 3-methoxyquinoline (53 μL, 0.37 mmol), and the resulting mixture was stirred at 80 °C for 72 h. After this time, the solution was evaporated to dryness to afford a brown residue. Addition of pentane (4 mL) afforded an orange solid that was washed with pentane (2 × 2 mL) and dried in vacuo. Yield: 126 mg (49%). The reaction is quantitative, but the isolated yield is low due to its high solubility in pentane. Anal. Calcd. for C37H48NO2P2Rh: C, 63.16; H, 6.88; N, 2.04. Found; C, 63.38; H, 6.62; N, 2.21. HRMS (electrospray, m/z): calcd for C37H48NO2P2Rh [M]+, 703.2210; found, 703.2226. IR (cm–1): ν(C=N) 1580 (m), ν(C–O–C) 1090 (s), 1017(s). 1H NMR (400.13 MHz, benzene-d6, 343 K): δ 8.21 (d, 3JH–H = 8.1, 1H, CH C-ring qn), 7.63 (d, 3JH–H = 7.8, 1H, CH C-ring qn), 7.40 (m, 1H, CH C-ring qn), 7.34 (m, 2H, CH-arom POP), 7.21 (m, 1H, CH C-ring qn), 7.12 (d, 3JH–H = 7.7, 2H, CH-arom POP), 6.90 (t, 3JH–H = 7.5, 2H, CH-arom POP), 6.53 (s, 1H, CH N-ring qn), 3.70 (s, 3H, CH3 qn), 2.39 (m, 4H, PCH(CH3)2), 1.31 (s, 6H, CH3), 1.24 (dvt, 3JH–H = 7.1, N = 14.1, 12H, PCH(CH3)2), 1.15 (dvt, 3JH–H = 7.6, N = 15.9, 12H, PCH(CH3)2). 13C{1H}-apt NMR (100.62 MHz, benzene-d6, 343 K): δ 192.9 (dt, 1JC–Rh = 45.1, 2JC–P = 11.2, Rh–C qn), 157.7 (s, C–CH3 qn), 156.8 (vt, N = 16.0, C-arom POP), 145.2 (d, 3JC–Rh = 3.6, C qn), 131.5 (s, C-arom POP), 131.2 (s, CH-arom POP), 128.0 (s, CH C-ring qn, inferred from the HSQC spectrum), 127.1 (s, CH-arom POP), 126.8 (s, C qn), 126.7 (s, CH C-ring qn), 126.2 (s, C-arom POP), 124.9 (s, CH C-ring qn), 124.1 (s, CH-arom POP), 121.6 (s, CH C-ring qn), 101.8 (s, CH, qn N-ring), 54.1 (s, OCH3), 34.5 (s, C(CH3)2), 31.9 (s, C(CH3)2, inferred from the HSQC spectrum), 26.3 (vt, N = 14.5, PCH(CH3)2), 19.2 (vt, N = 8.9, PCH(CH3)2), 18.9 (s, PCH(CH3)2). 31P{1H} NMR (161.98 MHz, benzene-d6, 343 K): δ 38.6 (d, 1JRh–P = 183.0).
Reaction of 1 with 3-(Trifluoromethyl)quinoline
A solution of 1 (200 mg, 0.37 mmol) in n-octane (3 mL) was treated with 3-(trifluoromethyl)quinoline (72.1 mg, 0.37 mmol), and the resulting mixture was stirred at 80 °C for 5 days. After this time, the 31P{1H} NMR spectra of the crude solution show a mixture of derivatives Rh(κ1-C2-quinolinyl-3-CF3){κ3-P,O,P-[xant(PiPr2)2]} (13; δ 35.3, d, 1JRh–P = 183.9 Hz; 25%), Rh(κ1-C4-quinolinyl-3-CF3){κ3-P,O,P-[xant(PiPr2)2]} (14; δ 37.5, d, 1JRh–P = 174.6 Hz; 14%), Rh(κ1-C6-quinolinyl-3-CF3){κ3-P,O,P-[xant(PiPr2)2]} (15; δ 37.8, d, 1JRh–P = 172.8 Hz; 25%), and Rh(κ1-C7-quinolinyl-3-CF3){κ3-P,O,P-[xant(PiPr2)2]} (16; δ 38.0, d, 1JRh–P = 173.7 Hz; 36%). The solution was evaporated to dryness to afford a brown residue. Addition of pentane (4 mL) afforded a red solid that was washed with pentane (2 × 2 mL) and dried in vacuo. Yield: 53 mg (20%). 1H and 31P{1H} NMR spectra of the red solid show only complexes 15 and 16 in a ratio of 1:2.5. Anal. Calcd. for C37H45F3NOP2Rh: C, 59.92; H, 6.12; N, 1.89. Found: 60.31; H, 6.43; N, 2.08. HRMS (electrospray, m/z): calcd for C37H46F3NOP2Rh [M + H]+, 742.2056; found, 742.2058. IR (cm–1): ν(C=N) 1577 (m), ν(C–O–C) 1151 (m).
Spectroscopic Data of Complex 15
1H NMR (400.13 MHz, benzene-d6, 298 K): δ 9.05 (broad singlet, 1H, CH N-ring qn), 8.66 (d, 3JH–H = 8.4, 1H, CH C-ring qn), 8.28 (s, 1H, CH C-ring qn), 8.15 (s, 1H, CH C-ring qn), 8.12 (d, 3JH–H = 8.5, CH C-ring qn), 7.27–7.18 (m, 2H, 2 CH-arom POP), 7.10–7.01 (m, 2H, CH-arom POP), 6.91–6.80 (m, 2H, CH-arom POP), 2.31 (m, 4H, PCH(CH3)2), 1.24 (s, 6H, CH3), 1.17–0.99 (m, 24H, PCH(CH3)2). 13C{1H}-apt NMR (100.62 MHz, benzene-d6, 298 K): δ 156.0 (m, C-arom POP), 147.6 (s, C qn), 146.1 (s, CH C-ring qn), 140.9 (s, CH N-ring qn), 135.2 (s, CH C-ring qn), 131.3 (s, CH-arom POP), 130.9 (m, C-arom POP), 129.8 (s, CH N-ring qn), 128.2 (s, CH-arom POP, inferred from the HSQC spectrum), 125.7 (s, C qn), 125.0 (s, C-arom POP), 124.3 (s, CH-arom POP), 123.7 (s, CH C-ring qn), 123.1 (q, 1JC–F = 279.2, CF3 qn), 118.7 (q, 2JC–F = 31.8, C–CF3), 34.1 (s, C(CH3)2), 33.0 (s, C(CH3)2), 25.4 (m, PCH(CH3)2), 19.3 (s, PCH(CH3)2), 18.5 (s, PCH(CH3)2); the signal for the Rh–C atom was not observed. 31P{1H} NMR (161.98 MHz, benzene-d6, 298 K): δ 37.8 (d, 1JRh–P = 172.8).
Spectroscopic Data of Complex 16
1H NMR (400.13 MHz, benzene-d6, 298 K): δ 9.14 (broad singlet, 1H, CH N-ring qn), 8.96 (s, 1H, CH C-ring qn), 8.52 (d, 3JH–H = 8.1, 1H, CH C-ring qn), 8.05 (s, H, CH N-ring qn), 7.27–7.18 (m, 3H, 1H CH C-ring qn, 2H CH-arom POP), 7.10–7.01 (m, 2H, CH-arom POP), 6.91–6.80 (m, 2H, CH-arom POP), 2.31 (m, 4H, PCH(CH3)2), 1.23 (s, 6H, CH3), 1.17–0.99 (m, 24H, PCH(CH3)2). 13C{1H}-apt NMR (100.62 MHz, benzene-d6, 298 K): δ 179.7 (dt, 1JC–Rh = 40.4, 2JC–P = 12.5, Rh–C qn), 156.0 (m, C-arom POP), 147.9 (s, C qn), 144.8 (s, CH N-ring qn), 142.3 (s, CH C-ring qn), 137.7 (s, CH C-ring qn), 133.3 (s, CH N-ring qn), 131.3 (s, CH-arom POP), 130.9 (m, C-arom POP), 128.2 (s, CH-arom POP, inferred from the HSQC spectrum), 125.0 (m, C-arom POP), 124.6 (s, C qn), 124.3 (s, CH-arom POP), 123.1 (q, 1JC–F = 279.2, CF3 qn), 121.2 (s, CH C-ring qn), 118.7 (q, 2JC–F = 31.8, C–CF3), 34.1 (s, C(CH3)2), 33.0 (s, C(CH3)2), 25.4 (m, PCH(CH3)2), 19.3 (s, PCH(CH3)2), 18.5 (s, PCH(CH3)2). 31P{1H} NMR (161.98 MHz, benzene-d6, 298 K): δ 38.0 (d, 1JRh–P = 173.7).
Acknowledgments
Financial support from the MICIN/AEI/10.13039/501100011033 (PID2020-115286GB-I00 and RED2018-102387-T), Gobierno de Aragón (E06_20R and LMP23_21), FEDER, and the European Social Fund is acknowledged. L.A.d.l.H. thanks the MECD for her FPU contract (FPU17/04813, “ESF investing in your future”).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.organomet.2c00270.
General information for the Experimental Section, structural analysis, and NMR spectra (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Hartwig J. F. Evolution of C-H Bond Functionalization from Methane to Methodology. J. Am. Chem. Soc. 2016, 138, 2–24. 10.1021/jacs.5b08707. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Gunsalus N. J.; Koppaka A.; Park S. H.; Bischof S. M.; Hashiguchi B. G.; Periana R. A. Homogeneous Functionalization of Methane. Chem. Rev. 2017, 117, 8521–8573. 10.1021/acs.chemrev.6b00739. [DOI] [PubMed] [Google Scholar]; c Das R.; Kapur M. Transition-Metal-Catalyzed C-H Functionalization Reactions of π-Deficient Heterocycles. Asian J. Org. Chem. 2018, 7, 1217–1235. 10.1002/ajoc.201800204. [DOI] [Google Scholar]; d Dalton T.; Faber T.; Glorius F. C-H Activation: Toward Sustainability and Applications. ACS Cent. Sci. 2021, 7, 245–261. 10.1021/acscentsci.0c01413. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Pulcinella A.; Mazzarella D.; Noël T. Homogeneous catalytic C(sp3)-H functionalization of gaseous alkanes. Chem. Commun. 2021, 57, 9956–9967. 10.1039/d1cc04073a. [DOI] [PubMed] [Google Scholar]; f Jana R.; Begam H. M.; Dinda E. The emergence of the C-H functionalization strategy in medicinal chemistry and drug discovery. Chem. Commun. 2021, 57, 10842–10866. 10.1039/d1cc04083a. [DOI] [PubMed] [Google Scholar]; g Liu X.-L.; Jiang L.-B.; Luo M.-P.; Ren Z.; Wang S.-G. Recent advances in catalytic enantioselective direct C-H bond functionalization of electrondeficient N-containing heteroarenes. Org. Chem. Front. 2022, 9, 265–280. 10.1039/d1qo01223a. [DOI] [Google Scholar]
- a Shilov A. E.; Shul’pin G. B. Activation of C-H Bonds by Metal Complexes. Chem. Rev. 1997, 97, 2879–2932. 10.1021/cr9411886. [DOI] [PubMed] [Google Scholar]; b Eisenstein O.; Milani J.; Perutz R. N. Selectivity of C-H Activation and Competition between C-H and C-F Bond Activation at Fluorocarbons. Chem. Rev. 2017, 117, 8710–8753. 10.1021/acs.chemrev.7b00163. [DOI] [PubMed] [Google Scholar]; c Pabst T. P.; Chirik P. J. A Tutorial on Selectivity Determination in C(sp2)-H Oxidative Addition of Arenes by Transition Metal Complexes. Organometallics 2021, 40, 813–831. 10.1021/acs.organomet.1c00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcells D.; Clot E.; Eisenstein O. C-H Bond Activation in Transition Metal Species from a Computational Perspective. Chem. Rev. 2010, 110, 749–823. 10.1021/cr900315k. [DOI] [PubMed] [Google Scholar]
- Jones W. D.; Feher F. J. Comparative Reactivities of Hydrocarbon C-H Bonds with a Transition-Metal Complex. Acc. Chem. Res. 1989, 22, 91–100. 10.1021/ar00159a002. [DOI] [Google Scholar]
- a Shang X.-F.; Morris-Natschke S. L.; Liu Y.-Q.; Guo X.; Xu X.-S.; Goto M.; Li J.-C.; Yang G.-Z.; Lee K.-H. Biologically active quinoline and quinazoline alkaloids part I. Med. Res. Rev. 2018, 38, 775–828. 10.1002/med.21466. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Matada B. S.; Pattanashettar R.; Yernale N. G. A comprehensive review on the biological interest of quinoline and its derivatives. Bioorg. Med. Chem. 2021, 32, 115973. 10.1016/j.bmc.2020.115973. [DOI] [PubMed] [Google Scholar]
- a Iwai T.; Sawamura M. Transition-Metal-Catalyzed Site-Selective C-H Functionalization of Quinolines beyond C2 Selectivity. ACS Catal. 2015, 5, 5031–5040. 10.1021/acscatal.5b01143. [DOI] [Google Scholar]; b Stephens D. E.; Larionov O. V. Recent advances in the C-H-functionalization of the distal positions in pyridines and quinolines. Tetrahedron 2015, 71, 8683–8716. 10.1016/j.tet.2015.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Prabagar B.; Yang Y.; Shi Z. Site-selective C-H functionalization to access the arene backbone of indoles and quinolines. Chem. Soc. Rev. 2021, 50, 11249–11269. 10.1039/d0cs00334d. [DOI] [PubMed] [Google Scholar]; d Corio A.; Gravier-Pelletier C.; Busca P. Regioselective Functionalization of Quinolines through C-H Activation: A Comprehensive Review. Molecules 2021, 26, 5467. 10.3390/molecules26185467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See for example:; a Lewis J. C.; Bergman R. G.; Ellman J. A. Direct Functionalization of Nitrogen Heterocycles via Rh-Catalyzed C-H Bond Activation. Acc. Chem. Res. 2008, 41, 1013–1025. 10.1021/ar800042p. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Tobisu M.; Hyodo I.; Chatani N. Nickel-Catalyzed Reaction of Arylzinc Reagents with N-Aromatic Heterocycles: A Straightforward Approach to C-H Bond Arylation of Electron-Deficient Heteroaromatic Compounds. J. Am. Chem. Soc. 2009, 131, 12070–12071. 10.1021/ja9053509. [DOI] [PubMed] [Google Scholar]; c Colby D. A.; Bergman R. G.; Ellman J. A. Rhodium-Catalyzed C-C Bond Formation via Heteroatom-Directed C-H Bond Activation. Chem. Rev. 2010, 110, 624–655. 10.1021/cr900005n. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Ren X.; Wen P.; Shi X.; Wang Y.; Li J.; Yang S.; Yan H.; Huang G. Palladium-Catalyzed C-2 Selective Arylation of Quinolines. Org. Lett. 2013, 15, 5194–5197. 10.1021/ol402262c. [DOI] [PubMed] [Google Scholar]; e Sun K.; Wang X.; Liu L.; Sun J.; Liu X.; Li Z.; Zhang Z.; Zhang G. Copper-Catalyzed Cross-Dehydrogenative C-N Bond Formation of Azines with Azoles: Overcoming the Limitation of Oxidizing N-O Activation Strategy. ACS Catal. 2015, 5, 7194–7198. 10.1021/acscatal.5b02411. [DOI] [Google Scholar]
- a Li B.-J.; Shi Z.-J. Ir-catalyzed highly selective addition of pyridyl C-H bonds to aldehydes promoted by triethylsilane. Chem. Sci. 2011, 2, 488–493. 10.1039/c0sc00419g. [DOI] [Google Scholar]; b Cheng C.; Hartwig J. F. Iridium-Catalyzed Silylation of Aryl C-H Bonds. J. Am. Chem. Soc. 2015, 137, 592–595. 10.1021/ja511352u. [DOI] [PubMed] [Google Scholar]; c He Y.; Wu Z.; Ma C.; Zhou X.; Liu X.; Wang X.; Huang G. Palladium-Catalyzed Selective C-H Activation: A Simple Method to Synthesize C-3 Site Arylated Quinoline Derivatives. Adv. Synth. Catal. 2016, 358, 375–379. 10.1002/adsc.201500763. [DOI] [Google Scholar]
- a Yamamoto S.; Saga Y.; Andou T.; Matsunaga S.; Kanai M. Cobalt-Catalyzed C-4 Selective Alkylation of Quinolines. Adv. Synth. Catal. 2014, 356, 401–405. 10.1002/adsc.201300991. [DOI] [Google Scholar]; b Zhu L.; Sheng X.; Li Y.; Lu D.; Qiu R.; Kambe N. Nickel-Catalyzed Remote C4-H Arylation of 8-Aminoquinolines. Org. Lett. 2019, 21, 6785–6789. 10.1021/acs.orglett.9b02403. [DOI] [PubMed] [Google Scholar]
- a Kwak J.; Kim M.; Chang S. Rh(NHC)-Catalyzed Direct and Selective Arylation of Quinolines at the 8-Position. J. Am. Chem. Soc. 2011, 133, 3780–3783. 10.1021/ja111670s. [DOI] [PubMed] [Google Scholar]; b Konishi S.; Kawamorita S.; Iwai T.; Steel P. G.; Marder T. B.; Sawamura M. Site-Selective C-H Borylation of Quinolines at the C8 Position Catalyzed by a Silica-Supported Phosphane-Iridium System. Chem.—Asian J. 2014, 9, 434–438. 10.1002/asia.201301423. [DOI] [PubMed] [Google Scholar]; c Murai M.; Nishinaka N.; Takai K. Iridium-Catalyzed Sequential Silylation and Borylation of Heteroarenes Based on Regioselective C-H Bond Activation. Angew. Chem., Int. Ed. 2018, 57, 5843–5847. 10.1002/anie.201801229. [DOI] [PubMed] [Google Scholar]
- a Ramakrishna K.; Biswas J. P.; Jana S.; Achar T. K.; Porey S.; Maiti D. Coordination Assisted Distal C-H Alkylation of Fused Heterocycles. Angew. Chem., Int. Ed. 2019, 58, 13808–13812. 10.1002/anie.201907544. [DOI] [PubMed] [Google Scholar]; b Liu X.; Mao G.; Qiao J.; Xu C.; Liu H.; Ma J.; Sun Z.; Chu W. Nickel-catalyzed C-H bond trifluoromethylation of 8-aminoquinoline derivatives by acyl-directed functionalization. Org. Chem. Front. 2019, 6, 1189–1193. 10.1039/c9qo00173e. [DOI] [Google Scholar]; c Shi H.; Lu Y.; Weng J.; Bay K. L.; Chen X.; Tanaka K.; Verma P.; Houk K. N.; Yu J.-Q. Differentiation and functionalization of remote C-H bonds in adjacent positions. Nat. Chem. 2020, 12, 399–404. 10.1038/s41557-020-0424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Khot N. P.; Mahato P.; Sajeev T. K.; Mukherjee S.; Kapur M. Rh(III)-Catalyzed C(7)-H Alkylation of Quinolines in the Synthesis of Angular π-Extended Pyrroloquinolines for Single-Component White-Light Emission. Org. Lett. 2022, 24, 2186–2191. 10.1021/acs.orglett.2c00503. [DOI] [PubMed] [Google Scholar]
- a Eisenstadt A.; Giandomenico C. M.; Frederick M. F.; Laine R. M. Potential Models of the Interactions between Nitrogen-Containing Heterocycles and the Active Catalyst Sites in Heterogeneous Hydrodenitrogenation Catalysts. Organometallics 1985, 4, 2033–2039. 10.1021/om00130a018. [DOI] [Google Scholar]; b Kabir S. E.; Siddiquee T. A.; Rosenberg E.; Smith R.; Hursthouse M. B.; Malik K. M. A.; Hardcastle K. I.; Visi M. Reactions of Triruthenium Clusters with Quinolines: X-Ray Structures of [Ru3(μ-CO)(CO)7{μ3-η2-P(C6H5)CH2P(C6H5)2)}{μ-η2-C9H5(CH3)N}] and [Ru3(CO)10(μ-H){μ-η2-C9H6N}]. J. Cluster Sci. 1998, 9, 185–199. 10.1023/a:1021994116953. [DOI] [Google Scholar]; c Hursthouse M. B.; Kabir S. E.; Malik K. M. A.; Tesmer M.; Vahrenkamp H. Phosphine derivatives of the 4-methylquinoline triosmium cluster [(μ-H)Os3(CO)10{μ-1,2-η2-C9H5(CH3)N}]: crystal structures of two isomeric compounds [(μ-H)Os3(CO)9{μ-1,2-η2-C9H5(CH3)N}(PPh3)] and [(μ-H)Os3(CO)9{μ-1,2-η2-C9H5(CH3)N}{P(OMe)3}]. J. Organomet. Chem. 1998, 568, 133–142. 10.1016/S0022-328X(98)00762-1. [DOI] [Google Scholar]; d Esteruelas M. A.; Fernández-Alvarez F. J.; Oñate E. Stabilization of NH Tautomers of Quinolines by Osmium and Ruthenium. J. Am. Chem. Soc. 2006, 128, 13044–13045. 10.1021/ja064979l. [DOI] [PubMed] [Google Scholar]; e Esteruelas M. A.; Fernández-Alvarez F. J.; Oliván M.; Oñate E. NH-Tautomerization of Quinolines and 2-Methylpyridine Promoted by a Hydride-Iridium(III) Complex: Importance of the Hydride Ligand. Organometallics 2009, 28, 2276–2284. 10.1021/om8011954. [DOI] [Google Scholar]; f Ren S.; Xie Z. Reaction of a Zirconocene-Carboryne Complex with Pyridines: Ligand C-H Activation. Organometallics 2011, 30, 5953–5959. 10.1021/om200772q. [DOI] [Google Scholar]; g Conejero S.; López-Serrano J.; Paneque M.; Petronilho A.; Poveda M. L.; Vattier F.; Álvarez E.; Carmona E. Tautomerisation of 2-Substituted Pyridines to N-Heterocyclic Carbene Ligands Induced by the 16 e- Unsaturated [TpMe2IrIII(C6H5)2] Moiety. Chem.—Eur. J. 2012, 18, 4644–4664. 10.1002/chem.201103507. [DOI] [PubMed] [Google Scholar]; h Cao Y.; Shih W.-C.; Bhuvanesh N.; Zhou J.; Ozerov O. V. Cooperative C-H activation of pyridine by PBP complexes of Rh and Ir can lead to bridging 2-pyridyls with different connectivity to the B-M unit. Chem. Sci. 2021, 12, 14167–14173. 10.1039/d1sc01850g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Kabir S. E.; Kolwaite D. S.; Rosenberg E.; Hardcastle K.; Cresswell W.; Grindstaff J. Synthesis, Structure, and Reactivity of Electron-Deficient Complexes of Quinolines with Triosmium Clusters. Organometallics 1995, 14, 3611–3613. [Google Scholar]; b Bergman B.; Holmquist R.; Smith R.; Rosenberg E.; Ciurash J.; Hardcastle K.; Visi M. Functionalizing Heterocycles by Electron-Deficient Bonding to a Triosmium Cluster. J. Am. Chem. Soc. 1998, 120, 12818–12828. 10.1021/ja981445e. [DOI] [Google Scholar]; c Din A. B.; Bergman B.; Rosenberg E.; Smith R.; Dastru W.; Gobetto R.; Milone L.; Viale A. The solution dynamics of adduct formation and electronic communication between ligand and metal core in electron deficient quinoline triosmium clusters. Polyhedron 1998, 17, 2975–2984. 10.1016/s0277-5387(98)00100-4. [DOI] [Google Scholar]; d Arcia E.; Kolwaite D. S.; Rosenberg E.; Hardcastle K.; Ciurash J.; Duque R.; Gobetto R.; Milone L.; Osella D.; Botta M.; Dastrú W.; Viale A.; Fiedler I. Mechanistic and Structural Studies of Electron-Deficient Quinoline Triosmium Clusters. Organometallics 1998, 17, 415–426. 10.1021/om970569h. [DOI] [Google Scholar]; e Esteruelas M. A.; Larramona C.; Oñate E. Osmium-Mediated Direct C-H Bond Activation at the 8-Position of Quinolines. Organometallics 2016, 35, 1597–1600. 10.1021/acs.organomet.6b00264. [DOI] [Google Scholar]
- Dhiman A. K.; Thakur A.; Kumar R.; Sharma U. Rhodium-Catalyzed Selective C-H Bond Functionalization of Quinolines. Asian J. Org. Chem. 2020, 9, 1502–1518. 10.1002/ajoc.202000341. [DOI] [Google Scholar]
- Esteruelas M. A.; Oliván M.; Vélez A. Xantphos-Type Complexes of Group 9: Rhodium versus Iridium. Inorg. Chem. 2013, 52, 5339–5349. 10.1021/ic4002658. [DOI] [PubMed] [Google Scholar]
- a Esteruelas M. A.; Oliván M.; Vélez A. POP-Pincer Silyl Complexes of Group 9: Rhodium versus Iridium. Inorg. Chem. 2013, 52, 12108–12119. 10.1021/ic401931y. [DOI] [PubMed] [Google Scholar]; b Curto S. G.; Esteruelas M. A.; Oliván M.; Oñate E.; Vélez A. Selective C-Cl Bond Oxidative Addition of Chloroarenes to a POP-Rhodium Complex. Organometallics 2017, 36, 114–128. 10.1021/acs.organomet.6b00615. [DOI] [Google Scholar]
- Esteruelas M. A.; Oliván M.; Vélez A. POP-Rhodium-Promoted C-H and B-H Bond Activation and C-B-Bond Formation. Organometallics 2015, 34, 1911–1924. 10.1021/acs.organomet.5b00176. [DOI] [Google Scholar]
- Curto S. G.; Esteruelas M. A.; Oliván M.; Oñate E. Rhodium-Mediated Dehydrogenative Borylation-Hydroborylation of Bis(alkyl)alkynes: Intermediates and Mechanism. Organometallics 2019, 38, 2062–2074. 10.1021/acs.organomet.9b00104. [DOI] [Google Scholar]
- Curto S. G.; Esteruelas M. A.; Oliván M.; Oñate E. Insertion of Diphenylacetylene into Rh-Hydride and Rh-Boryl Bonds: Influence of the Boryl on the Behavior of the β-Borylalkenyl Ligand. Organometallics 2019, 38, 4183–4192. 10.1021/acs.organomet.9b00513. [DOI] [Google Scholar]
- Esteruelas M. A.; Martínez A.; Oliván M.; Vélez A. A General Rhodium Catalyst for the Deuteration of Boranes and Hydrides of the Group 14 Elements. J. Org. Chem. 2020, 85, 15693–15698. 10.1021/acs.joc.0c01967. [DOI] [PubMed] [Google Scholar]
- Esteruelas M. A.; Nolis P.; Oliván M.; Oñate E.; Vallribera A.; Vélez A. Ammonia Borane Dehydrogenation Promoted by a Pincer-Square-Planar Rhodium(I) Monohydride: A Stepwise Hydrogen Transfer from the Substrate to the Catalyst. Inorg. Chem. 2016, 55, 7176–7181. 10.1021/acs.inorgchem.6b01216. [DOI] [PubMed] [Google Scholar]
- Adams G. M.; Colebatch A. L.; Skornia J. T.; McKay A. I.; Johnson H. C.; Lloyd–Jones G. C.; Macgregor S. A.; Beattie N. A.; Weller A. S. Dehydropolymerization of H3B·NMeH2 To Form Polyaminoboranes Using [Rh(Xantphos-alkyl)] Catalysts. J. Am. Chem. Soc. 2018, 140, 1481–1495. 10.1021/jacs.7b11975. [DOI] [PubMed] [Google Scholar]
- a Chotana G.; Rak M. A.; Smith M. R. Sterically Directed Functionalization of Aromatic C–H Bonds: Selective Borylation Ortho to Cyano Groups in Arenes and Heterocycles. J. Am. Chem. Soc. 2005, 127, 10539–10544. 10.1021/ja0428309. [DOI] [PubMed] [Google Scholar]; b Hartwig J. F. Regioselectivity of the borylation of alkanes and arenes. Chem. Soc. Rev. 2011, 40, 1992–2002. 10.1039/c0cs00156b. [DOI] [PubMed] [Google Scholar]; c Serratore N. A.; Anderson C. B.; Frost G. B.; Hoang T.-G.; Underwood S. J.; Gemmel P. M.; Hardy M. A.; Douglas C. J. Integrating Metal-Catalyzed C-H and C-O Functionalization To Achieve Sterically Controlled Regioselectivity in Arene Acylation. J. Am. Chem. Soc. 2018, 140, 10025–10033. 10.1021/jacs.8b06476. [DOI] [PubMed] [Google Scholar]; d Esteruelas M. A.; Martínez A.; Oliván M.; Oñate E. Direct C-H Borylation of Arenes Catalyzed by Saturated Hydride-Boryl-Iridium-POP Complexes: Kinetic Analysis of the Elemental Steps. Chem.—Eur. J. 2020, 26, 12632–12644. 10.1002/chem.202001838. [DOI] [PubMed] [Google Scholar]; e Esteruelas M. A.; Martínez A.; Oliván M.; Oñate E. Kinetic Analysis and Sequencing of Si-H and C-H Bond Activation Reactions: Direct Silylation of Arenes Catalyzed by an Iridium-Polyhydride. J. Am. Chem. Soc. 2020, 142, 19119–19131. 10.1021/jacs.0c07578. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.