Abstract
Globally, depression is a leading cause of disability and has remained so for decades. Antidepressant medications have suboptimal outcomes and are too frequently associated with side effects, highlighting the need for alternative treatment options. Although primarily known for its robust physical health benefits, exercise is increasingly recognized for its mental health and antidepressant benefits. Empirical evidence indicates that exercise is effective in treating individuals with depression; however, the mechanisms by which exercise exerts anti-depressant effects are not fully understood. Acute bouts of exercise have been shown to transiently modulate circulating levels of serotonin and norepinephrine, brain-derived neurotrophic factor, and a variety of immuno-inflammatory mechanisms in clinical cohorts with depression. However, exercise training has not been demonstrated to consistently modulate such mechanisms, and evidence linking these putative mechanisms and reductions in depression is lacking. The complexity of the biological underpinnings of depression coupled with the intricate molecular cascade induced by exercise are significant obstacles in the attempt to disentangle exercise’s effects on depression. Notwithstanding our limited understanding of these effects, clinical evidence uniformly argues for the use of exercise to treat depression. Regrettably, exercise remains underutilized despite being an accessible, low-cost alternative/adjunctive intervention that can simultaneously reduce depression and improve overall health. To address the gaps in our understanding of the clinical and molecular effects of exercise on depression, we propose a model that leverages systems biology and multidisciplinary team science with a large-scale public health investment. Until the science matches the scale of complexity and burden posed by depression, our ability to advance knowledge and treatment will continue to be plagued by fragmented, irreproducible mechanistic findings and no guidelines for standards of care.
Introduction
While depression is a highly variable psychiatric condition, both within and between individuals, it does have core clinical features. These consist of persistent sadness or low mood, loss of interest or pleasure in activities once enjoyed, loss of energy, changes in eating and sleeping patterns, feelings of worthlessness and guilt, difficulty concentrating or making decisions, and thoughts of death or suicide — all of which markedly impair an individual’s physical, psychological, social, and occupational function.1 Depressive disorders affect over 320 million people worldwide (WHO).2 At its most severe, depression can lead to suicide, which accounts for 1.5% of deaths worldwide.2 Depression has remained a leading cause of years lived with disability for the last 30 years and consequently is a major global health burden.3 The absolute burden of disability and suicide caused by depression is predominately experienced by low- and middle-income countries although after controlling for population size, disability and suicide are comparable across countries of all income levels.2 The persistent and ubiquitous nature of depression coupled with its penchant to drive disability, morbidity, and mortality indicate the pressing need to develop innovative and broadly effective interventions.
Depression is one of the most common mental health diagnoses in primary health care4 and is associated with cardiovascular5 and metabolic disease,6 multimorbidity,7 and mortality.8 While first line pharmacotherapy for depression has demonstrated benefits, approximately half of individuals receiving initial selective serotonin reuptake inhibitor (SSRI) treatment do not respond (≥50% reduction in depressive symptoms from baseline) to treatment and only a third remit (post-intervention depressive symptoms are mild or absent).9 Following failure to respond to initial SSRI treatment, a second course of either SSRI, SNRI (serotonin and norepinephrine reuptake inhibitor), or atypical antidepressant (bupropion) treatment yields reduced response (SSRI: 27%; SNRI: 28%; bupropion: 26%) and remission rates (SSRI: 27%; SNRI: 25%; bupropion: 26%).10 The suboptimal outcomes achieved with these medications are further complicated by the fact that an equal proportion (SSRI: 21%; SNRI: 21%; bupropion: 27%) of patients discontinue pharmacological treatment due to intolerable side effects (e.g., impaired sexual functioning, weight gain, etc.).10 Suboptimal treatment outcomes, poor treatment compliance, and low treatment uptake11, 12 all conspire to highlight a clear need for alternative and/or adjunctive treatments. Particularly attractive are treatments that can enhance response and remission rates while simultaneously reducing cardiovascular/metabolic risk factors, improving brain function and mitigating medication side-effects. The focus of this review is on just such an intervention, namely exercise.
While definitions of what constitutes exercise abound, here we use the term to mean physical activity that is planned, structured, repetitive, and performed to improve one or more components of health-related fitness (i.e., cardiovascular endurance, muscular strength, muscular endurance, flexibility, body composition).13 There are few ideas less controversial than the notion that exercise improves fitness, global physical function and overall health independent of age and morbidity status.14–17 Although not traditionally thought of as a component of health-related fitness, brain (or mental) health is inextricably bound to physical health18 and exercise has a potent ability to stimulate communication between skeletal muscle and the brain.19 Two of the most commonly employed modes of exercise interventions are aerobic and resistance exercise. Aerobic exercise (AEx) involves activities performed in a continuous or interval nature with the intention of improving the efficiency of the cardiovascular and pulmonary systems and increasing aerobic capacity (i.e., rate of oxygen uptake []).16 Resistance exercise (REx) involves activities that require sustained or intermittent exertion of forces against resistance with the intention of improving musculoskeletal function and enhancing muscular strength or endurance.16 Both, AEx training and REx training have demonstrated antidepressant effects20–23 and improve cardiometabolic24, 25 and brain health26, 27 yet remain underutilized in the clinical management of depression.
Exercise as a treatment to maintain or regain health is not a new concept; Hippocrates (460 – c. 370 BC) and Galen (129 – c. 216 AD) were among the first to extoll the virtues of exercise (or motion).28, 29 Many centuries later, Robert Burton specifically referenced exercise as one of several ‘cures’ for melancholy in the Anatomy of Melancholy (c.1621);30 and yet, the benefit of exercise for neuropsychiatric conditions was not more formally recognized until the middle of the 20th century.31 The systematic study of exercise as a potential therapeutic modality for depression began with pioneering work by William P. Morgan in the late 1960’s,32–35 which was followed by a handful of small investigations into the effects of exercise on the clinical outcomes of depression.36–38 Since then, interest in developing exercise as a treatment for depression has grown exponentially. Similarly, interest in the underlying molecular mechanisms by which exercise may exert antidepressant effects and improve overall brain health is an area of emerging study. As the line of inquiry examining exercise as a treatment for depression has evolved so have inconsistencies in the existing literature. Specifically, exercise dosing parameters are variably reported and inclusion (e.g., depression diagnosis criteria) and exclusion (e.g., comorbid psychiatric or other conditions) criteria are unevenly applied throughout the literature. Consequently, this heterogeneity has led to substantial difficulty ascertaining the molecular mechanisms impacted by exercise in depressed cohorts, and the relationship between exercise-mediated regulation of molecular mechanisms and clinical outcomes. In this review we seek to describe the state of the art of exercise for depression as it relates to: 1) what is known about antidepressant effects of exercise and dosing parameters in randomized controlled trials of unipolar depression; 2) the shared molecular mechanisms by which exercise and pharmacotherapy may exert anti-depressant effects in preclinical models and humans; and 3) the relationship between exercise-induced anti-depressant effects and changes in candidate molecular mechanisms in humans. By examining these areas of the literature, we aim to identify strengths and liabilities in our current approach to studying exercise and depression and propose a way forward that will advance this body of literature and ultimately lead to novel and improved treatments for depression.
Antidepressant effects of exercise - clinical outcomes
Based on systematic reviews and meta-analyses findings, exercise has an anti-depressant effect and even potential protective benefits.39–41 Chekroud et al. analyzed the responses of 1.2 million adults from the 2011, 2013, and 2015 Centers for Disease Control and Prevention Behavioral Risk Factors Surveillance System Survey to explore the relationship between self-reported physical activity (PA) and days of poor mental health over the past 30 days.39 Their findings support the theory that exercise positively impacts mental health. When looking specifically at those with a reported depression diagnosis at any time in the past, individuals who exercised experienced 3.75 (34.5%) fewer days of poor mental health (W=1.61x109, p<2.2x10−16) compared to those who did not exercise in the past month.39 The authors noted a U-shaped relationship between poor mental health days and exercise; the greatest associations between exercise parameters and fewer poorer mental health days were exercising 30 to 60 minutes, 3 to 5 days/week.39 Additionally, associations were larger among exercise variables (frequency, time, and type) than social or demographic variables like income and education, highlighting the importance of exercise parameters.39
Pearce et al. assessed the dose-response between PA and depression in their meta-analysis.40 They found an inverse curvilinear dose-response association between PA and depression among 15 studies, which included 191,130 participants.40 Those who exercised the recommended amount (equivalent to 2.5 hrs of brisk walking) had a 25% (95%CI, 18%–32%) lower risk of depression, and those who exercised at half of the recommended PA levels had an 18% (95%CI,13%-23%) lower risk of depression.40 Interestingly, the authors reported great uncertainty and potentially less benefit when exercising at higher levels.40 Lastly, in their meta-analysis of 111 prospective studies, Dishman et al. identified a 22% reduction in adjusted odds of incident cases of depression or an increase in subclinical depressive symptoms with PA (0.79, 0.75 to 0.82; I2=87.6).41 They reported that moderate to vigorous PA that met public health recommendations was associated with lower odds (OR=0.73 (95% CI: 0.68 to 0.78)) of depression, as did an increase in PA during the study compared to single exposure at baseline (OR=0.69 (95% CI: 0.61 to 0.79), k=50).41 For reference, the public health guidelines for PA include activity 5 days/week for a minimum of 150 minutes/week of moderate-intensity or 3 days/week for a minimum of 75 minutes/week at vigorous intensity and REx at least two days/week.42–44
Additionally, exercise has been found to have a moderate to large anti-depressant effect when compared to no treatment or control groups.45–48 While exercise is no more or less effective when compared to pharmacological or psychological therapies,46, 47 it has a significant moderate effect ([g= −0.48 95% CI= −0.80 to −0.16, p≤0.001]46 ; [SMD = −0.62, p<0.00001, I2 = 70%]48) compared to treatment as usual (TAU) or usual care from providers.46, 48 Kvam et al. reported a moderate, but non-significant anti-depressant effect (g= −0.50, 95% CI= −1.10 to 0.11, p=0.11) of exercise combined with antidepressants when compared to antidepressants alone.46 The antidepressants identified in this meta-analysis included SSRI, SNRI, norepinephrine reuptake inhibitors, norepinephrine reuptake inhibitors, and tricyclic antidepressants at varying dosages.46 As Stubbs et al. determined from their analysis of control group responses in exercise randomized controlled trials, proving efficacy is challenging, “as the control group response in exercise studies is large and almost double what is observed for antidepressant randomized controlled trials.” 49
One challenge in analyzing the exercise and depression literature is the substantial heterogeneity that exists. Studies vary widely regarding the approach used to identify depression and evaluate depressive symptoms, inclusion and/or exclusion of comorbid conditions, whether psychological, neurological, cardiovascular, or musculoskeletal, and application and dosing of exercise. While often overlooked, exercise dosing is vital as a threshold level is needed to achieve therapeutic benefit, just as a therapeutic dose of medication is essential, as demonstrated by the findings of Chekroud et al., Pearce et al., and Dishman et al.39–41 However, exercise dosing variables, or FITT (i.e., frequency, intensity, time, and type) parameters, are inconsistently reported throughout the literature, thereby leading to reproducibility problems, difficulties accounting for FITT parameters in meta-analyses, and challenges translating findings to clinical practice.
Although this is not a systematic review, we wanted to demonstrate the limitations within the existing literature regarding the heterogeneity of exercise parameters among studies focusing on clinical outcomes. PubMed, OvidSP MEDLINE, and PyscINFO databases were searched using the following parameters: (1) randomized controlled trials published in peer-reviewed journals; (2) unipolar depression diagnosed by established criteria, such as the Diagnostic and Statistical Manual of Mental Disorders, International Classification of Diseases, 10th Revision diagnosis codes, or the Mini International Neuropsychiatric Interview; (3) no additional comorbid diagnoses; (4) AEx or REx intervention(s) employed as a standalone treatment or an adjunct to traditional therapies; (5) control group received no intervention, TAU, education, or a stretching or low dose exercise program; and (6) depression outcomes were, at minimum, assessed pre- and post-intervention. Reference lists of previous systematic reviews and meta-analyses were used to identify potential studies meeting our inclusion criteria. Studies published before March 2022 were considered for inclusion.
Our search yielded a cohort of 34 studies in which exercise was employed in a myriad of strategies. For clarity, we subdivided the cohort based on the exercise intervention(s) application: exercise as a monotherapy20–23, 38, 50, 51 (Table 1), exercise as an adjunct to psychotherapy52–54 (Table 2), exercise as an adjunct to pharmacotherapy50, 55–64 (Table 3), and exercise as an adjunct to standard care65–77 (Table 4). The publication year of the included studies ranged from 198565 to 2021.77 Among the studies that utilized exercise as a monotherapy for depression20–23, 38, 50, 51 (Table 1), the most recent publication was in 2012,51 while the majority of the studies that employed exercise as an adjunctive therapy are more recent (2014–2021)52–54, 57–64, 69–77 (Tables 2–4). This could suggest a movement towards employing exercise as an augmentative treatment to existing anti-depressant therapies rather than as a monotherapy.
Table 1.
Randomized controlled trials of exercise as a monotherapy for major depressive disorder
| Trial | Depression diagnosis criteria and type | Sample | Interventions | Study Duration | Treatment arms (n) | Outcomes |
||
|---|---|---|---|---|---|---|---|---|
| Adherence | Drop-outs n(%) | Remission Rate % | ||||||
| Doyne et al., 198738 | RDC Major (78%) Minor (22%) |
Females only, 18–35y/o, met depression criteria, agreed not to participate in any other depression treatment options or exercise programs Exclusion: manic-depressive disorder, imminent suicide threat, physical contraindications to ex, or hospitalized for depression in preceding yr n=40 28.5±4.4y/o; 0% males |
Outpatient setting AEx: 5–10min stretching warm-up, running at 80% APMHR for NR duration, 5–10min cool-down REx: 5-10min warmup, Self-paced, 10-station program on universal machines at or below 50–60% APMHR, 5–10min cool-down CON: Waitlist (no treatment) |
8 weeks AEx and REx: 3–4d/wk/8wks (32 supervised individual sessions) CON: No treatment |
AEx (n=NR) | NR Mean 2.64 sessions/wk |
NR(40%) | 67% |
| REx (n=NR) | NR Mean 2.95 sessions/wk | NR(29%) | 80% | |||||
| CON (n=NR) | NA | NR(13%) | 17% | |||||
| Singh et al., 199720 | DSM-IV Unipolar major or minor, dysthymia |
Males and females, ≥60y/o, BDI >12 Exclusion: cognitive impairment, have unstable disease(s), bipolar disorder, active psychosis, suicidal plans, receiving psych, antidepressant use within past 3mos, exercising >2xs/wk in the past month (REx or AEx) n=32 57±6.5y/o; 37.5% males |
Outpatient setting PREx: 5 exercises (chest press, latissimus dorsi pulldown, leg press, knee extension, and knee flexion), 3x8 reps at 80% 1-RM with progression (~45mins), 5min stretching cool-down CON: 1hr health education session (lectures, videos, and discussions) |
10 weeks PREx: 3d/wk/10wks (30 sessions) CON: 2d/wk/10wks (20 sessions) PREx and CON sessions: supervised, but combination of individual and group |
PREx (n=17) | 93% | 0 | Remission: NR Response: 59% |
| CON (n=15) | 95% | 0 | Remission: NR Response: 26% |
|||||
| Blumenthal et al., 199950 | DSM-IV DIS Major |
Males and females, ≥50y/o, HAM-D17 ≥13 Exclusion: current antidepressant use, use of other medications that would preclude random assignment of exercise (e.g., quinidine, metoprolol), alcohol or substance abuse, medical contraindications to ex (e.g., orthopedic or cardiopulmonary disease), primary diagnois other than depression (e.g., bipolar, psychosis) acute suicidal risk, psych initiated in past yr, established exercise program n=156 57±6.5y/o; 27.6% males |
Outpatient setting AEx: 10min warm-up, 30mins walking or jogging at 70–85% HRR, 5min cool-down Pharm: Sertraline (50–200mg/d) titrated as needed after meeting with study psychiatrist(s) |
16 weeks AEx: 3d/wk/16wks (48 supervised group sessions) Pharm: 6 sessions (Study onset and Week 2, 6, 10, 14, 16) |
AEx (n=55) | 89.6% | 14(26.4%) | 60.4% |
| AEx + Pharm (n=55) | AEx: 91.7% Pharm: 95% |
11(20.0%) | 65.5% | |||||
| Pharm (n=48) | ~95% | 7(14.6%) | 68.8% | |||||
| Dunn et al., 200521 | DSM-IV-SCID Major |
Males and females, 20–45 y/o, HAM-D17=12–25, sedentary, live within 15 miles from center, not receiving any other treatment for depression Exclusion: ≥160% over ideal weight; consuming >21 alcoholic drinks/wk, substance abuse or recreational drug use, suicide attempt in past 2yrs, acute suicidal risk, hospitalization in last 5yrs for psychiatric disorder, participating in other clinical trials, inability to ex due to a medical condition, exercising ≥3d/wk for ≥20mins, planned or current pregnancy n=80 35.9±6.4y/o; 25% males |
Outpatient setting AEx: Treadmill or stationary cycling PHD: 17.5 kcal/kg/wk LD: 7 kcal/kg/wk CON: 15–20mins of stretching |
12 weeks AEx-3d: 3d/wk/12wks (36 sessions) AEx-5d: 5d/wk/12wks (60 sessions) CON: 3d/wk/12wks (36 sessions) AEx and CON sessions: Supervised and individual |
AEx PHD-3d (n=17) | 71% | 6(35.3%) | Efficacy: 31% ITT: 41% |
| AEx PHD-5d (n=16) | 71% | 4(25.0%) | Efficacy: 55% ITT: 31% | |||||
| AEx LD-3d (n=16) | 72% | 2(12.5%) | Efficacy: 31% ITT: 25% |
|||||
| AEx LD-5d (n=18) | 72% | 7(38.9%) | Efficacy: 19% ITT: 11% |
|||||
| CON (n=13) | 42% | 8(61.5%) | Efficacy: 11% ITT: 15% |
|||||
| Singh et al., 200522 | DSM-IV, SCID Unipolar major or minor, dysthymia |
Males and females, >60y/o, GDS≥14 Exclusion: demented, unstable medical condition that would prevent participation in REx, bipolar disorder, active psychosis, actively suicidal, receiving psych, antidepressant use within past 3mos, exercising >2xs/wk n=60 Range: 60–85y/o 45.0% males |
Outpatient setting HI-PREx: 3x8reps of 6 exercises (chest press, upright row, shoulder press, leg press, knee extension, and knee flexion) at 80% 1-RM with progression (~60mins) and Borg RPE of 15–18, 5mins stretching LO-REx: 3×8reps of 6 exercises (chest press, upright row, shoulder press, leg press, knee extension, and knee flexion) at 20% 1-RM without progression (~60mins), 5mins stretching CON: TAU initiated by GP Formal treatment: 52% Pharm: 42% Counseling only: 10% Psychiatrist referral: 5% |
8 weeks HI-PREx and LO-PREx: 3d/wk/8wks (24 supervised sessions; combination of individual and group) CON: Unrestricted with average of 5 health care profession visits |
HI-PREx (n=20) | 95%-100% | 2(10%) | Remission: NR Response: 61% |
| LO-REx (n=20) | ~99% | 3(15%) | Remission: NR Response: 29% |
|||||
| CON (n=20) | NR | 1(5%) | Remission: NR Response: 21% |
|||||
| Blumenthal et al., 200723 | DSM-IV-SCID Major |
Males and females, ≥40y/o, BDI ≥12, sedentary, Exclusion: receiving psych, comorbid primary psychiatric diagnosis, medical comorbidities that preclude participation (musculoskeletal difficulties), taking antidepressants or other psychotropic medication, alcohol or drug abuse or dependency, acute suicidal intent, established exercise program, failed medical screening (physical exam, blood work, blood pressure, pregnancy) n=202 52±8y/o; 24.3% males |
Outpatient setting AEx: 10min warm-up, 30mins walking or jogging at 70–85% HRR, 5min cool-down Pharm: Sertraline (50–200mg/d), titrated as needed after meeting with study psychiatrist(s) Placebo: 50–200mg/day, titrated as needed after meeting with study psychiatrist(s) |
16 weeks All AEx: 3d/wk/16wks (48 sessions) AEx-Home: unsupervised individual sessions AEx-Group: supervised group sessions Pharm and Placebo: 6 sessions (Study onset and Week 2, 6, 10, 14, 16) |
AEx-Home (n=53) | 93.9% | 3(5.7%) | 40% |
| AEx-Group (n=51) | 82.9% | 10(19.6%) | 45% | |||||
| Pharm (n=49) | 83% attended all sessions | 7(14.3%) | 47% | |||||
| Placebo (n=49) | 72% attended all sessions | 14(28.6%) | 31% | |||||
| Krogh et al., 201251 | DSM-IV-MINI Major |
Males and females, 18-60 y/o, HAM-D ≥12 Exclusion: drug use, antidepressant use in past 2mos, receiving psych, contraindications to physical exercise, >1hr of PA/wk, suicidal behavior, current/previous psychotic or manic symptoms, pregnancy n=115 41.6y/o (19–59 y/o); 33.0% males |
Outpatient setting AEx: 10min warm-up, 30mins on cycle ergometer (at least 65% of maximal capacity with progression to 80%), 5min cool-down CON: 10min warm-up at low intensity on stationary bike, 20min stretching program, 15mins of low intensity exercise |
12 weeks AEx: 3x/wk/12wks (36 supervised sessions) CON: 3x/wk/12wks (36 supervised sessions) |
AEx (n=56) | 39.3% attended >18 sessions Mean 13.5 |
11(19.6%) | ITT: 28.6% |
| CON (n=59) | 35.6% attended >18 sessions Mean 12.5 |
18(30.5%) | ITT: 30.5% | |||||
AEx = aerobic exercise; APMHR = age-predicted maximum heart rate; BDI = Beck Depression Inventory; CON = control; d = day; DIS = Diagnostic Interview Schedule; DSM-IV= Diagnostic and Statistical Manual of Mental Disorders, fourth edition; ex = exercise; GDS = Geriatric Depression Scale; GP = general practitioner; HAM-D = Hamilton Rating Scale of Depression; HI = high intensity; hr(s) = hour(s); HRR = heart rate reserve; ITT = intent to treat; LD = low dose; LO = low intensity; min(s) = minute(s); MINI – Mini International Neuropsychiatric Interview; mo(s) = month(s); NR = not reported; PA = physical activity, Pharm = pharmacotherapy; PHD = public health dose; PHD = public health dose; PREx = progressive resistance exercise; Psych = psychotherapy; RDC = research diagnostic criteria; REx = resistance exercise; RPE = rate of perceived exertion; SCID = Structured Clinical Interview of DSM Disorders; TAU = treatment as usual; wk(s) = week(s); y/o = years old; yr(s) = year(s); 1-RM = one repetition maximum
Table 2.
Randomized controlled trials of exercise as an adjunct to psychotherapy for major depressive disorder
| Trial | Depression diagnosis criteria and type | Sample | Interventions | Study Duration | Treatment arms (n) | Outcomes |
||
|---|---|---|---|---|---|---|---|---|
| Adherence | Drop-outs n(%) | Remission Rate % | ||||||
| Jacquart et al., 201452 | DSM-IV Major |
Males and females, ≥50 y/o, admitted to inpatient unit, met depression criteria, Exclusion: cognitive impairment, Fall Risk Assessment ≥25 n=88 (analysis: n=78) 59.7±8.5y/o 38.5% males |
Inpatient setting TAU: GT, OT, pharm Psych: Validation therapy techniques Psych_Walk: 5min warm-up, 20mins walking (intensity NR), 5min cool-down while receiving psych Psych_Sit: 30min psych session in seated position CON: TAU only |
Duration dependent on length of stay (mean 6.3±3.9d) Psych_Walk: mean of 3.5±1.7 supervised, individual sessions Psych_Sit: mean of 3.1±2.1 supervised, individual sessions TAU and CON: NR |
Psych_Walk + TAU (n=29) | NR | 3(10) | Remission: NR Change in GDS: 80.0% reduction |
| Psych_Sit + TAU (n=29) | NR | 3(10) | Remission: NR Change in GDS: 46.6% reduction |
|||||
| CON (n=30) | NA | 4(13) | Remission: NR Change in GDS: 32.1% reduction |
|||||
| Oertel-Knochel et al., 201453 | DSM-IV-SCID Major Study included individuals with schizophrenia disorder, but those participants are excluded from this review. |
Males and females, admitted to inpatient facility, met depression criteria, disease duration ≥5yrs, pharm permitted if stable dosage at least ≥1mo prior to pre-testing and for study duration Exclusion: Comorbid Axis- I or II disorders n=22 40.0±14.1y/o 50.0% males |
Inpatient setting Permitted to take steady dose of medication(s) Cog: 30mins of group and individual tasks AEx: 10min warm-up, 25mins of boxing and circuit training at 60–70% of MHR, 10min cool-down Relax: 45mins of breathing, imagery, relaxation, and awareness training CON: Waitlist |
4 weeks Cog: 3d/wk/4wks (12 supervised group sessions) AEx: 3d/wk/4wks (12 supervised sessions) Relax: 3d/wk/4wks (12 supervised sessions) CON: NA |
Cog + AEx (n=8) | NR | NR | Est change in BDI:~31% reduction |
| Cog + Relax (n=6) | NR | NR | Est change in BDI:~19% reduction | |||||
| CON (n=8) | NA | NR | Est change in BDI:~8% reduction | |||||
| Kerling et al., 201554 | DSM-IV-SCID Major |
Males and females, ≥18y/o, admitted to inpatient facility, met depression criteria Exclusion: Acute or chronic infectious disease, immunological disorders, diabetes mellitus, cardiovascular disorders, taking beta-blockers or other cardiologic treatment, cognitive impairment, schizophrenia, bipolar disorder, substance abuse/dependency pregnancy n=40 Age: NR 60.0% males |
Inpatient setting CBT: NR AEx: 25mins on bicycle ergometer at 60–70 RPM, 20mins on choice of cross-trainer stepper, arm ergometer, treadmill, recumbent bike, or rower at moderate intensity of 50% of max workload from exercise tolerance testing and progressed by 10% of workload. TAU: Optional participation in daily 20min activity program (walking, ball games and stretching) Pham: administer per treating physicians’ orders CBT + AEx: 77% CBT + TAU: 75% |
6 weeks CBT: NR AEx: 3d/wk/6wks (18 supervised sessions) TAU: NR |
CBT + AEx + Pharm (n=22) | CBT: NR AEx: >90% |
0(0) | BDI Remission: 64% Response: 64% MADRS Remission: 42% Response: 64% |
| CBT + TAU + Pharm (n=20) |
NR | 0(0) | BDI Remission: 40% Response: 45 MADRS Remission: 25% Response: 30% |
|||||
AEx = aerobic exercise; BDI = Beck Depression Inventory; CBT = cognitive-behavioral group therapy; Cog = cognitive training; CON = control; d = days; DSM-IV= Diagnostic and Statistical Manual of Mental Disorders, fourth edition; Est = estimated; GDS = Geriatric Depression Scale; GT = group therapy; MADRS = Montgomery- Depression Scale; MHR = maximum heart rate; min(s) = minute(s); NA = not applicable; NR = not reported; OT = occupational therapy; Pharm = pharmacotherapy; Psych = psychotherapy; Relax = relaxation training; RPM = revolutions per minute; SCID = Structured Clinical Interview of DSM Disorders; TAU = treatment as usual; wk(s) = weeks; y/o = years old; yr(s) = years
Table 3.
Randomized controlled trials of exercise as an adjunct to pharmacotherapy for major depressive disorder (exercise combined with pharmacotherapy as a treatment arm or all subjects on stable dose of pharmacotherapy)
| Trial | Depression diagnosis criteria and type | Sample | Interventions | Study Duration | Treatment arms (n) | Outcomes |
||
|---|---|---|---|---|---|---|---|---|
| Adherence | Drop-outs n(%) | Remission Rate % | ||||||
| Blumenthal et al., 199950 | DSM-IV DIS Major |
Males and females, ≥50y/o, HAM-D17 ≥13 Exclusion: current antidepressant use, use of other medications that would preclude random assignment of exercise (e.g., quinidine, metoprolol), alcohol or substance abuse, medical contraindications to ex (e.g., orthopedic or cardiopulmonary disease), primary diagnosis other than depression (e.g., bipolar, psychosis), acute suicidal risk, psych initiated in past yr, established exercise program n=156 57±6.5y/o 27.6% males |
Outpatient setting AEx: 10min warm-up, 30mins walking or jogging at 70–85% HRR, and 5min cool-down Pharm: Sertraline (50–200mg/d) titrated as needed after meeting with study psychiatrist(s) |
16 weeks AEx: 3d/wk/16wks (48 supervised group sessions) Pharm: 6 sessions (study onset and Week 2, 6, 10, 14, 16) |
AEx (n=55) | 89.6% | 14(26.4%) | 60.4% |
| AEx + Pharm (n=55) | AEx: 91.7% Pharm: 95% |
11(20.0%) | 65.5% | |||||
| Pharm (n=48) | ~95% | 7(14.6%) | 68.8% | |||||
| Pilu et al., 200755 | DSM-IV-SCID Major; comorbid GAD, SP, PD included |
Females, 40–60y/o; non-responders (HAM-D >13) to ≥1 antidepressant after 2mos Exclusion: psychotic disorders, comorbid psychiatric disorders (excluding, GAD, SP, and PD), contraindications to PA; diagnosis of neurological or orthopedic disorders n=30 Age: NR 0(0%) males |
Outpatient setting PA: 5min warm-up, 50mins physiological strengthening with multiple machines available for arms, leg and postural muscles (intensity NR), 5min cool-down Pharm: Varied medication types, dosages, and number taken |
8 months PA: 2d/wk/8mos (64 supervised group sessions) Pharm: Session information NR |
PA + Pharm (n=10) | NR | NR | Remission: NR Change in HAM-D: 60.5% reduction |
| Pharm (n=20) | NR | NR | Remission: NR Change in HAM-D: 13.4% reduction |
|||||
| Mota-Pereira al., 201156 | DSM-IV Major; treatment resistant |
Males and females, 18–60y/o, met depression criteria, non-responder to pharm for >9 and <15mos treatment, physical fitness confirmed by physician, normal ECG Exclusion: psychiatric and relevant clinical co-morbidities, psychotic symptoms, acute suicidal risk, receiving psych, change in pharm <6wks prior to study, participating in regular AEx n=33 Range: 26–60y/o 34.5% males |
Outpatient setting AEx: 30–45mins walking at >3METs on treadmill and overground Pharm: Varied medication types and dosages at therapeutic level, but did not change during study period |
12 weeks AEx: 5d/wk/12wks (60 sessions; supervised 1d/wk and unsupervised 4d/wk) Pharm: 1d/wk/12wks meeting with study staff for 30–45mins |
AEx + Pharm (n=22) | 91.0% | 3(13.6%) | Remission: 26% Response: 21% |
| Pharm (n=11) | NR | 1(9.1%) | Remission: 0% Response: 0% |
|||||
| Danielsson et al., 201457 | DSM-IV-MINI Major |
Males and females, 18–65y/o, met depression criteria, taking 1–2 antidepressants and followed by physician Exclusion: psychotic disorder, substance abuse, previous manic episode, acute suicide risk, untreated heart condition, current exercise program of moderate to high intensity or mind-body activities, pregnancy n=62 Age: NR 22.6% males |
Outpatient setting PA: 5min warm-up, 45min interval training program at 13–14 (low) and 16–17 (high) on Borg RPE with multiple machines (cross-trainer, stationary bikes, step-up boards, rowing machine, treadmill, jumping ropes, balls, free weights, and cable machine), 5min cool-down BBAT: 50mins of body awareness activities, 10mins of verbal reflection Pharm: Taking 1–2 antidepressants. Type, dosage, and titration NR. Advice: 1 session for advice and support for low to moderate PA |
10 weeks PA and BBAT: Week 1–2: 1d/wk/2wks Week 3–10: 2d/wk/8wks (18 supervised sessions; First two sessions were individual; remainder of sessions were group) Advice: 1 individual meeting |
PA + Pharm (n=22) |
85% | 4(18.2%) | Remission: 32% Response: 9% |
| BBAT + Pharm (n=20) |
75% | 6(30.0%) | Remission: 25% Response: 0% |
|||||
| Advice + Pharm (n=20) | 90% | 6(30.0%) | Remission: 15% Response: 10% |
|||||
| Belvederi Murri et al., 201558 | MINI by psychiatrist Major |
Males and females, 65–85y/o, HAM-D17 ≥18, sedentary but health status compatible with exercise Exclusion: other Axis I disorder(s), substance or alcohol misuse, cognitive impairment, physical illness that would prevent exercise (orthopedic, cardiovascular, and neurologic) n=121 75±6y/o 28.9% males |
Outpatient setting P-AEx: 10min warm-up, 40mins of cycling at 60–85% PHR, 5–10min cool-down NP-AEx: 60mins of activity at ≤70% PHR Pharm: Sertraline (≥50mg/d) prescribed and titrated by psychiatrist |
24 weeks P- and NP-AEx: 3d/wk/24wks (72 supervised group sessions) Pharm: Session information NR |
P-AEx + Pharm (n=42) |
P-AEx: ~70% Pharm: 93% |
4(9.5%) | 81.0% |
| NP-AEx + Pharm (n=37) |
NP-AEx: ~70% Pharm: 84% |
5(13.5%) | 73.0% | |||||
| Pharm (n=42) | Pharm: 74% | 6(14.3%) | 45.0% | |||||
| Carneiro et al., 201559 | ICD-10; psychiatrist confirmed ICD-10 codes: F32.1, F33.1, F43.1 Major, dysthymia |
Females, 18–65y/o, sedentary, met depression criteria, have physical fitness to participate in exercise, normal ECG Exclusion: psychotic comorbidities, participation in other clinical trials, medical history indicating medical constraints, taking beta-blockers, planned or current pregnancy, alcohol/drug abuse/dependence, receiving additional complementary therapies (psych), pharm changes in past 6wks or change during study, attend <60% of study sessions n=26 50.2±12.1y/o 0% males |
Outpatient setting AEx: 5min warm-up, 30mins of various activities (games, circuit workouts with resistance bands, jump ropes, fitness balls, brisk walking, and dancing) at 65–80 % MHR, 5min stretching cool-down Pharm: SSRIs and anxiolytics/hypnotics, if needed, at constant individualized dosages |
16 weeks AEx: 3d/wk/16wks (48 supervised group sessions) Pharm: Session information with psychiatrist over 16wks NR |
AEx + Pharm (n=13) | 82% | 4(31.0%) | Remission: NR Change in BDI: 23.4% reduction |
| Pharm (n=13) | NR | 3(23.0%) | Remission: NR Change in BDI: 7.1% increase |
|||||
| Legrand & Neff, 201660 | DSM-IV Major |
Males and females, admitted to inpatient facility, started medication <2wks prior to enrollment, BDI-II ≥29, ability to run or walk briskly, Exclusion: medical contraindication for exercise, psychotic features, receiving beta-blocking drugs or other therapy (sleep deprivation, ECT) n=35 45.3±13.2y/o 31.4% males |
Inpatient setting AEx: 30mins of daily walking/jogging at 65–75% of APMHR Stretch: 30mins of stretching (60s holds and 60s rests) Pharm: starting medication (SSRIs, SNRIs, and/or dopamine antagonist) <2wks prior to enrollment. Dosage and titration NR. |
10 days AEx: 1x/d/10d (10 supervised outdoor sessions with 92.2% of sessions individual) Stretch: 1x/d/10d (10 supervised indoor sessions with 95.3% of sessions individual) |
AEx + Pharm (n=14) |
AEx: 92.9% attended ≥8 sessions Pharm: NR |
1(17.1%) | Remission: NR Response: 57.1% Change in BDI-II: 47.5% reduction |
| Stretch + Pharm (n=11) | Stretch: 81.8% attended ≥8 sessions Pharm: NR |
2(18.2%) | Remission: NR Response: 9.1% Change in BDI-II: 24.8% reduction |
|||||
| Pharm (n=10) | Pharm: NR | 1(10.0%) | Remission: NR Response: 10.0% Change in BDI-II: 18.0% reduction |
|||||
| Salehi et al., 201661 | DSM-IV Major |
Males and females, 25–40y/o, admitted to inpatient care, BDI ≥30, HAM-D ≥25, no comorbid psychiatric disorders Exclusion: history of epilepsy, physical illness, refused ECT n=60 29.7±5.8y/o 70% males |
Inpatient setting Initial 2wk washout period with pharm Pharm: 40mg/d of citalopram AEx: 40–45mins of cycling at 60–75% of VO2max ECT: 1.5 times seizure threshold dose at 1.0ms pulse width |
4 weeks AEx: 3d/wk/4wks (12 supervised individual sessions) ECT: 3d/wk/4wks (12 supervised individual sessions) |
AEx + ECT + Pharm (n=20) | AEx:100% ECT: 100% Pharm:100% |
NR | 76.5% |
| AEx + Pharm (n=20) | AEx:100% Pharm:100% |
NR | 11.8% | |||||
| ECT + Pharm (n=20) | ECT: 100% Pharm:100% |
NR | 11.8% | |||||
| Siqueira et al., 201662 | DSM-IV Major |
Males and females, met depression criteria, drug free ≥5wks prior to enrollment Exclusion: any contraindication to exercise (disabling medical condition), cardiovascular disease, infection, neurological disorder, drug or alcohol abuse, medical comorbidities, active suicidal ideations, history of any Axis I disorder n=57 38.8±10.7y/o 28.1% males |
Outpatient setting AEx: Duration and activity NR, but progression of intensity from 60–85% of VO2max Pharm: Sertraline (50–100mg/d) titrated as needed |
4 weeks AEx: 4d/wk/4wks (16 supervised individual sessions) Pharm: NR |
AEx + Pharm (n=29) | NR | 9(31.0%) | Remission: NR Change in: HAM-D: 40.7% reduction BDI: 37.7% reduction |
| Pharm (n=28) | NR | 8(28.5%) | Remission: NR Change in: HAM-D: 38.0% reduction BDI: 40.9% reduction |
|||||
| Gujral et al., 201963 | DSM-V, PRIME-MD Major |
Males and females, 20–39y/o and 60–79y/o, sedentary, met criteria for major depressive episode Exclusion: self-reported active lifestyle (3d/wk for >20mins/d), gait or balance impairments, unsafe to participate in moderate AEx, disproval from participant’s physician, uncontrolled hypertension, cardiovascular event in past 12mos, substance use problems in past 3mos, lifetime diagnosis of bipolar disorder or any psychotic disorder, clinically significant cognitive impairment, contraindications to MRI n=15 (10 younger, 5 older) Age: NR Sex: NR |
Outpatient setting Initial 2wk taper off antidepressants AEx: warm-up, 45mins stationary cycling or treadmill at 60–75% APMHR or 13–15 Borg RPE (if taking beta-blocker), cool down (~60mins) Pharm: Venlafaxine XR (dosage NR) |
12 weeks Ex: 3d/wk/12wks (36 supervised sessions) Pharm: biweekly medication management by study clinicians |
AEx + Pharm (n=7) | 91% | 1(14%) | Remission: NR Response: NR Change in MADRS: 74% reduction (unclear if analysis was ITT or per protocol) |
| Pharm (n=8) | NR (States completers were adherent) | 3(38%) | Remission: NR Response: NR Change in MADRS: 64% reduction (unclear if analysis was ITT or per protocol) |
|||||
| Moraes et al., 202064 | DSM-IV confirmation by psychiatrist Major |
Males and females, >60y/o; met criteria for depression, sedentary, HAM-D ≤18, taking antidepressants and anxiolytics (if needed) for ≥ 4wks at therapeutic dosage prior to study Exclusion: low functional capacity, poor mobility, balance impairment, severe visual and/or auditory impairment, cognitive impairment, cerebro- vascular infarction; neurodegenerative disease; cardiovascular problems, clinical and psychiatric comorbidities n=27 Range: 60–81y/o 16.0% males |
Outpatient setting PREx: 3×8–12 reps of 4 exercises (chest press, low rows, leg press, knee extension, and knee flexion) at 70% of 1-RM (~30mins). Progressed when completed max number of reps for each set NP-AEx: 5min warm-up, 20mins of walking or stationary cycling at 60% of VO2max or 70% of MHR, 5min cool-down LO-PA: 5mins of low intensity walking (2.5 km/hr) or stationary cycling (<40 RPM), 1×8 reps of 4 exercises (chest press, low rows, leg press, knee extension, and knee flexion) with minimal load (“one plate”), series of stretching with 10sec holds (30mins total) Pharm: Varying therapeutic dosages of antidepressants (fluoxetine or sertraline) and anxiolytics as needed (diazepam or clonazepam) |
12 weeks PREx, NP-AEx, and LO-PA: 2d/wk/12wks (24 supervised sessions) Pharm: Session information NR |
PREx + Pharm (n=9) |
PREx: ≥75% Pharm: NR |
0(0%) | HAM-D Remission: 44.4% Response: 22.2% BDI Remission: 22.2% Response: 55.6% |
| NP-AEx + Pharm (n=9) |
NP-AEx: ≥75% Pharm: NR |
0(0%) | HAM-D Remission:55.6% Response: 55.6% BDI Remission: 33.3% Response: 22.2% |
|||||
| LO-PA + Pharm (n=9) |
LO-PA: ≥75% Pharm: NR |
2(2.2%) | HAM-D Remission: 0% Response: 0% BDI Remission: 0% Response: 0% |
|||||
AEx = aerobic exercise; min(s) = minute(s); APMHR = age-predicted maximum heart rate; BBAT = basic body awareness therapy; BDI = Beck Depression Inventory; d = day(s); DIS = Diagnostic Interview Schedule; DSM-IV= Diagnostic and Statistical Manual of Mental Disorders, fourth edition; ECG = electrocardiogram; ECT = electroconvulsive therapy; GAD = generalized anxiety disorder; HAM-D = Hamilton Rating Scale of Depression; HRR = heart rate reserve; ICD-10 = International Classification of Diseases, 10th Revision; LO-PA = low intensity physical activity; MADRS = Montgomery Depression Rating Scale; MET = metabolic equivalent; MHR = maximum heart rate; MINI = Mini International Neuropsychiatric Interview; mo(s) = month(s); MRI = magnetic resonance imaging; NP-AEx = non-progressive aerobic exercise; NR = not reported; PA = physical activity; P-AEx = progressive aerobic exercise; PD = panic disorder; Pharm = pharmacotherapy; PHR = peak heart rate; PREx = progressive resistance exercise; PRIME-MD = Primary Care Evaluation of Mental Disorders; Psych = psychotherapy; RPE = rating of perceived exertion; RPM = revolutions per minute; SCID = Structured Clinical Interview of DSM Disorders; SNRIs = serotonin and norepinephrine reuptake inhibitors; SP = social phobia; SSRIs = selective serotonin reuptake inhibitors; VO2max = maximal oxygen uptake; wk(s) = weeks; y/o = years old; 1-RM = one-repetition maximum
Table 4.
Randomized controlled trials of exercise as an adjunct to standard care for major depressive disorder
| Trial | Depression diagnosis criteria and type | Sample | Interventions | Study Duration | Treatment arms (n) | Outcomes |
||
|---|---|---|---|---|---|---|---|---|
| Adherence | Drop-outs n(%) | Remission Rate % | ||||||
| Martinsen et al., 198565 | DSM Major |
Males and females, 17–60y/o, admitted to the hospital, met criteria for depression Exclusion: psychosis, physical contraindications to exercise n=49 40±NR y/o NR% males |
Inpatient setting AEx: 1hr at 50–70% of VO2max TAU: Individual psych, OT, pharm Pharm n(%) AEx: 9(NR%) CON: 14(NR%) CON: 1hr of OT |
9 weeks AEx: 3d/wk/9wks (27 supervised group sessions) CON: 3d/wk/9wks (27 supervised group sessions) |
AEx + TAU: (n=28) | NR | 4(14.1%) | NR |
| CON + TAU (n=21) | NR | 2(9.5%) | NR | |||||
| Veale et al., 199266 Study 1 |
CIS Major |
Males and females, 18–60y/o, CIS ≥17 and depression severity ≥2, concurrent treatment (pharm, psych, etc.) permitted n=83 Study 1: Age and Sex: NR Study 1 & 2: 35.5±NR y/o 36% males |
Outpatient setting AEx: Stretching warm-up, running program. Duration and intensity NR TAU: Pharm and/or psych Pharm, Psych (%): AEx: 45%, NR CON: 34%, NR CON: TAU only |
12 weeks AEx: 3d/wk/12wk (36 supervised group sessions) TAU: NR CON: No sessions beyond assessments |
AEx + TAU (n=48) | AEx: NR TAU: NA |
12(25.0%) | Remission: NR Change in BDI: 30% reduction |
| CON (n=35) | NA | 6(17.2%) | Remission: NR Change in BDI: 32% reduction |
|||||
| Veale et al., 199266 Study 2 |
CIS Major |
Males and females, 18–60y/o, CIS ≥17 and depression severity ≥2, concurrent treatment (pharm, psych, etc.) permitted n=41 Study 2: Age and Sex: NR Study 1 & 2: 35.5±NR y/o 36% males |
Outpatient setting AEx: Stretching warm-up, running program. Duration and intensity NR LO-PA: Relaxation, stretching, and yoga. Duration and intensity NR TAU: Pharm and/or psych Pharm, Psych (%): AEx: 41%, NR LO-PA: 11%, NR |
12 weeks AEx: 3d/wk/12wk (36 supervised group sessions) LO-PA: 3d/wk/12wk (36 supervised group sessions) TAU: NR |
AEx + TAU (n=63) | AEx: NR TAU: NA |
17(26.9%) | Remission: NR Change in BDI: 35% reduction |
| LO-PA + TAU (n=26) | LO-PA: NR TAU: NA |
4(15.8%) | Remission: NR Change in BDI: 40% reduction |
|||||
| Knubben et al., 200767 | DSM-IV Major |
Males and females, 20–70y/o, BRMS >12, admitted to inpatient facility, ambulatory Exclusion: associated organic disease, schizophrenic symptoms, epilepsy, ECT referral n=41 Age: NR 44.7% males |
Inpatient setting AEx: 30mins of interval (1:1) walking (5 reps of 3mins at 80% APHRM and Borg RPE 13–14 and 3mins at half speed of high interval) TAU: Pham, sleep deprivation CON: 30mins light stretching of calves, thighs, back, shoulders, and pectoral muscles (20s hold, 40s rest) |
10 days AEx:1x/d/10d (10 supervised sessions) CON: 1x/d/10d (10 supervised sessions) |
AEx + TAU (n=20) | NR | 1(5.0%) | Remission: NR Response: 65% Change in BRMS: 36% reduction |
| CON + TAU (n=18) | NR | 2(11.1%) | Remission: NR Response: 22% Change in BRMS: 18% reduction |
|||||
| Krogh et al., 200968 | ICD-10 /DSM-IV-MDI Unipolar Major |
Males and females, 18–55y/o, referred by medical doctor or psychologist and met ICD-10 criteria, Exclusion: alcohol or substance abuse, acute suicidal risk, psychotic symptoms, medical conditions that contraindicated physical exercise, been on sick leave for >24mos, exercising >1hr/wk n=165 38.9±9.5y/o 26.1% males |
Outpatient setting P-AEx: 90mins of interval training; initially 2mins on: 2mins rest at 70% MHR and progress to 3mins on:1min rest at 89% MHR. AEx included cycling, running, stepping, abdominal exercises, rowing, trampoline, step bench, jump rope, and Ski Fitter PREx: 90mins for 2–3sets x12 reps at 50% 1-RM progressed to 2-3 sets x8-10 reps at 75% 1-RM. 6 exercises on machines (leg extension, leg press, total abdominal, lower back, chest press, vertical traction) and 3 with weights (calves, arm abductors, and triceps) Relax: 90mins of light activity at Borg RPE ≤12; 20–30mins exercise on mattresses or back massage, 10–20mins of light balance activities, and 20–30mins of supine relaxation. TAU: Pharm and/or psych Pharm, Psych (%): P-AEx: 64.6%, 47.9% PREx: 66.0%, 48.9% Relax: 52.4%, 47.6% |
16 weeks All groups: 2d/wk/16wks (32 supervised group sessions) TAU: NR |
P-AEx + TAU (n=55) | P-AEx: 56.2% TAU: NA |
8(14.6%) | 40.4% |
| PREx + TAU (n=55) | PREx: 50.6% TAU: NA |
7(12.7%) | 29.2% | |||||
| Relax + TAU (n=55) | Relax: 32.8% TAU: NA |
13(23.6%) | 31.7% | |||||
| Doose et al., 201569 | ICD-10 CIS ICD-10 codes: F32.0/1/2, F33.0/1/2 Major |
Males and females, 18–65y/o, HAM-D ≥25, admitted to inpatient facility, met ICD-10 criteria, not involved in any other PA during hospitalization Exclusion: severe depressive episode (ICD-10: F32.3 or F33.3), psychotic symptoms, psychiatric comorbidities (bipolar, schizophrenia, drug addiction), pregnancy, change in psych or pharm treatment, beginning psych, relevant orthopedic disease, relevant surgery in past 6mos, acute general disease (infectious disease, anemia, cancer), poorly controlled diabetes, severe cardiovascular disease, enrolling in other clinical trial n=46 48±NR y/o 37.0% males |
Inpatient setting AEx: 10–15min warm-up; 30–45mins walking or running at self-selected intensity (mean ~12 on Borg RPE); 10–15min cool-down TAU: Pharm and/or psych Pharm, Psych (%): AEx: 50%, 53.3% CON: 75%, 68.8% CON: TAU while waitlist |
8 weeks AEx: 3d/wk/8wks (24 supervised group sessions) TAU: NR CON: Only pre- and post-intervention assessment sessions |
AEx + TAU (n=30) | AEx: 58% TAU: NA |
7(23.3%) | 63.3% |
| CON (n=16) | NA | 4(25.0%) | 0% | |||||
| Hallgren et al., 201570 | DSM-IV-MINI Major, anxiety, comorbid anxiety |
Males and females, ≥18y/o, PHQ-9 >9, Exclusion: severe somatic illness, primary drug or alcohol use disorder, psychiatric diagnosis requiring specialist treatment (i.e., psychosis) n=946 43±12 y/o 27.2% males |
Outpatient setting Ex: Randomized to 60mins of light (yoga), moderate (intermediate-level aerobics class), or vigorous (higher intensity AEx, REx and balance) exercise ICBT: Online, self-help manual with weekly interaction with clinician; additional support provided as needed TAU: Managed by PCP and included pharm and/or CBT, or no treatment Antidepressants (%) Ex + TAU: 31% ICBT + TAU: 31% CON: 24% CON: TAU “Standard tx”: 33% Pharm: 24% CBT: NR No tx: 25% |
12 weeks Ex: 3d/wk/12wks (36 supervised group sessions) ICBT: Multiple (self-selected) visits/wk/12wks (mean of 4 logins/wk and accessed 7.8±5 of 13 modules) TAU and CON: NR |
Ex + TAU (n=317) | Ex: 33% TAU: NR |
68(21.5%) | Remission: NR Change in MADRS: 49.1% reduction |
| ICBT + TAU (n=317) | ICBT: 60% TAU: NR |
58(18.3%) | Remission: NR Change in MADRS: 47.9% reduction |
|||||
| CON (n=312) | NA | 80(25.6%) | Remission: NR Change in MADRS: 34.0% reduction |
|||||
| Schuch et al., 201571 | DSM-IV-MINI Major Inpatient |
Males and females, 18–60y/o, admitted to inpatient facility, HAM-D ≥25, not involved in any other PA during hospitalization Exclusion: taking beta-blockers, psychiatric diagnosis of bipolar, schizophrenia, anorexia, substance abuse or dependence, 3 or more cardiovascular risk factors on PAR-Q, any medical condition that limits or contraindicates exercise n=50 40.3±NR y/o 28.0% males |
Inpatient setting AEx: warm-up (stretching 4 lower limb muscle groups and 4min treadmill walk), exercise bout with choice of stationary bike, treadmill, or “transport” machine at 16.5 kcal/kg body mass/wk (~59% HRR), and cool-down TAU: Pharm and/or ECT and access to OT, but no psych CON: TAU only |
Duration dependent on length of stay AEx: 3d/wk (average ~23±9d hospitalized) (Supervised individual sessions) CON: NR with average ~21±8d hospitalized |
AEx + TAU (n=25) | AEx: ~91% TAU: NA |
2(8%) | Remission: 48% Response: 84% |
| CON (n=25) | NA | 1(4%) | Remission: 32% Response: 60% |
|||||
| Helgadóttir et al., 201672 | DSM-IV-MINI Major, anxiety, comorbid anxiety |
Males and females, 18–67 y/o, PHQ-9 ≥10, Exclusion: primary diagnosis or alcohol or drug dependency, serious somatic disorder, psychiatric diagnosis requiring specialist treatment (i.e., psychosis) n=620 42.6±12.0 y/o 26.3% males |
Outpatient setting AEx-Vig: 60mins of strenuous group aerobics class AEx-Mod: 60mins of intermediate-level group aerobics class AEx-Light: 60mins of yoga-based stretching and balance TAU: Managed by PCP and included pharm and/or CBT, or no treatment CON: TAU |
12 weeks AEx groups: 3d/wk/12wks (36 supervised group sessions and weekly individual session with study personnel) TAU and CON: NR |
AEx-Vig + TAU (n=99) |
AEx: NR TAU: NA |
19(19.2%) | Remission: NR Change in MADRS: 38.4% reduction |
| AEx-Mod + TAU (n=105) |
AEx: NR TAU: NA |
27(25.7%) | Remission: NR Change in MADRS: 32.9% reduction |
|||||
| AEX-Light + TAU (n=106) |
AEx: NR TAU: NA |
21(19.8%) | Remission: NR Change in MADRS: 44.1% reduction |
|||||
| CON (n=310) | NA | 79(25.5%) | Remission: NR Change in MADRS: 25.4% reduction |
|||||
| Olson et al., 201773 | DSM-IV-MINI Major |
Males and females, 18–30y/o, met non-psychotic depression criteria, no pharm or psych beyond >6wk stable dose of antidepressants or mood stabilizers, no regular exercise or pst mo (<35kcal/kg/day or <3d/wk for ≤20min/session), no physical limitations or contraindications to exercise, normal or corrected-to-normal vision Exclusion: severe psychopathology (substance dependence, bipolar, schizophrenia disorders), suicidal risk, planned or current pregnancy n=50 Age: NR 24.0% males |
Outpatient setting AEx: 45mins of steady-state exercise (treadmill or cycle ergometer) at 40–65% HRR TAU: Stable dose of antidepressants or mood stabilizers permitted, but no psych ~ 14% of sample reported using pharm CON: 30–45mins of light stretching in sitting and standing |
8 weeks AEx: 3d/wk/8wks (24 supervised sessions) TAU: NR CON: 3d/wk/8wks (24 supervised sessions) |
AEx + TAU (n=25) | AEx: 100% TAU: NR (only completers reported) |
10(40%) | 60% |
| CON + TAU (n=25) | NR | 10(40%) | 33% | |||||
| Buschert et al., 201974 | ICD-10 Unipolar depression Comorbid: ICD-10 chapters of F4, 5, 6 permitted |
Males and females, admitted to inpatient facility with unipolar depression Exclusion: psychotic symptoms, comorbid psychiatric disorders (except ICD-10 chapters F4, 5, and 6), cardiovascular or neurological disease, cognitive impairment n=38 41.2±9.1 y/o 36.7% males completed study |
Inpatient setting AEx: 30mins of endurance training (outdoor walking, Nordic walking, running, or stationary cycling) at 85% of APMHR TAU: Only pharm reported CON: Additional 30mins OT or AT session |
3 to 4 weeks AEx: 2–3d/wk/3–4wks (6 –12 supervised group sessions) TAU: NR CON: 2–3d/wk/3–4wks (6 –12 supervised group sessions) |
AEx + TAU (n=20) |
Mean sessions: 10.00±3.0 | 5(25.0%) | Remission: NR Change in BDI-II: 37.2% reduction HAM-D7: 34.5% reduction |
| CON + TAU (n=18) |
Mean sessions: 14.3±8.2 | 3(16.7%) | Remission: NR Change in BDI-II: 31.5% reduction HAM-D7: 33.0% reduction |
|||||
| Chau et al., 202075 | ICD-10 Major (F33.0 – F3.9 ICD-10 codes) |
Males and females, 18–64y/o, met ICD-10 diagnosis codes (F33.0–F33.9) Exclusion: unstable medical or psychological states (suicidal risk), severe cognitive, language or hearing deficits; and orthopedic conditions or other diseases that limit physical fitness assessment n= 84 47.4±10.6 y/o 20.2% males |
Outpatient setting Ex: 45mins for stretching warmup, circuit training (3 stations of AEx at 50–70% MHR and 3 stations of REx [3×10 reps of each major muscle group]), and 15mins stress management, mindful breathing, stretching, and body awareness (60min total) TAU: Only pharm noted Ex: 83% CON: 83% CON: TAU waitlist instructed not to initiate structured exercise program |
12 weeks Ex: 3ds/wk/12wks (36 supervised group sessions) CON: NA |
Ex + TAU (n=42) | NR | 6(15%) | Remission: NR Change in HAM-D17: 51.5% reduction |
| CON + TAU (n=42) | NR | 7(17.5%) | Remission: NR Change in HAM-D17: 15.6% reduction |
|||||
| Haussleiter et al., 202076 | DSM-IV-SCID Major |
Males and females, admitted for inpatient care, HAM-D ≥17, Exclusion: Acute suicidality, severe comorbid psychiatric disorders, medical contraindications to PA, cognitive impairment, unable to complete self-administered questionnaires n=111 per protocol analysis: n=76 45.05±12.19 y/o 33.3% male (full sample) ~68% male (per protocol) |
Inpatient setting GET: 50mins of group exercise of mixed modalities SOA: Encouraged to perform PA. Meetings to discuss physical conditions, depressive symptoms, and motivational troubles. TAU: antidepressant and psychoactive medications as prescribed (type and dosage NR) Mean number of antidepressants GET: 1.26±0.60 SOA: 1.02±0.73 Mean number of psychoactives GET: 1.72±1.00 SOA: 2.03±1.14 |
6 weeks GET: 3d/wk/6wks (18 supervised group sessions) SOA: 3d/wk/6wks (18 supervised group meetings) TAU: NR |
GET + TAU (n=36) |
NR | Full sample: Within 3wks: 35 (31.5%) Baseline to 6wks: NR |
HAM-D at 3wks: Response: 38.9% Remission: 27.8% HAM-D at 6wks: Response: 54.5% Remission: 40.9% |
| SOA + TAU (n=40) |
NR | HAM-D at 3wks: Response: 25.0% Remission: 10.0% HAM-D at 6wks: Response: 55.6% Remission: 25.9% |
||||||
| La Rocque et al., 202177 | DSM-V-SCID Unipolar depressive disorder |
Females, non-chronic unipolar depressive disorder Exclusion: psychotic, bipolar, or substance disorder, suicidal intent, medical condition interfering with PA, pregnancy, recent change (<3mos) in pharm or psych, participating in group or yoga exercise bi-weekly or more not obtaining physician clearance with moderate or high risk on PAR-Q n=53 Per protocol analysis: n=42 ~33 y/o 0% males |
Outpatient setting BY: 90mins of instructor-led group Bikram yoga class AEx: 50–60min group exercise class of choice (choreography-based cardio, aerobics, light muscular conditioning, and stretching; cardio, plyometric, and strength training; high intensity aerobic exercise with intermittent rest periods; circuit-based cardio and strength training; stepper-based exercises; and Latin-inspired dance/ fitness) CON: Waitlist with no interventions TAU: Continue pharm and/or psych with participant’s own healthcare provider BY: 8(44.4%) Pharm: 4(22.2%) Psych: 3(16.7%) Both: 1(5.6%) AEx: 9(45.0%) Pharm: 4(20.0%) Psych: 4(20.0%) Both: 1(5.0%) CON: 7(46.6%) Pharm: 7(46.6%) Psych: 0(0.0%) Both: 0(0.0%) |
8 weeks BY: 2d/wk/8wks (instructor led group classes) AEx: 2d/wk/8wks (instructor led group class of choice) CON: no access to classes TAU: as prescribed by healthcare provider |
BY + TAU (n=18) | ITT: 68.8% Per protocol analysis: 80% |
3(16.7%) | Remission (ITT): 61% Response (per protocol): 73.3% |
| AEx + TAU (n=20) | ITT: 66.9% Per protocol analysis: 86% |
5(25.0%) | Remission (ITT): 60% Response ITT: 60.0% Per protocol; 80.0% |
|||||
| CON + TAU (n=15) | N/A | 3(20.0%) | Remission: NR Response ITT: 6.7% Per protocol: 8.3% |
|||||
AEx = aerobic exercise; AEx-Light = exercise-light intensity; AEx-Mod = exercise-moderate intensity; AEx-Vig = exercise-vigorous intensity; APHRM = Age predicted maximum heart rate; AT = art therapy; BDI = Beck Depression Inventory; BMI = body mass index; BRMS = Bech-Rafaelsen Melancholy Scale; BY = bikram yoga; CBT = cognitive behavioral therapy; CIS = Clinical Interview Schedule; CON= control; d = day(s); DSM = Diagnostic and Statistical Manual of Mental Disorders, edition not reported; DSM-IV = Diagnostic and Statistical Manual of Mental Disorders, fourth edition; ECT = electroconvulsive therapy; Ex = exercise; GET = guided exercise therapy; HAM-D = Hamilton Depression Rating Scale; hr(s) = hours(s); HRR = heart rate reserve; ICBT = internet-based cognitive-behavioral therapy; ICD-10 = International Classification of Diseases, 10th Revision; ITT = intention to treat; LO-PA = low intensity physical activity; MADRS = Montgomery Depression Rating Scale; MDI = Major Depression Inventory; MHR = maximum heart rate; min(s) = minute(s); MINI = Mini International Neuropsychiatric Interview; NA= not applicable; NR = not reported; OT = occupational therapy; PA = physical activity; P-AEx = progressive aerobic exercise; PAR-Q = Physical Activity Readiness Questionnaire; PCP = primary care physician; Pharm = pharmacotherapy; PHQ-9: Patient Health Questionare-9; PREx = progressive resistance exercise; Psych = psychotherapy; Relax = relaxation; REx = resistance exercise; RPE = rate of perceived exertion; s = seconds; SCID = Structured Clinical Interview of DSM Disorders; SNRIs = serotonin and norepinephrine reuptake inhibitors; SOA = self-organized activity; SSRIs = selective serotonin reuptake inhibitors; TAU = treatment as usual; VO2max = maximal oxygen uptake; wk(s) = week(s); y/o = years old; 1-RM = one-repetition maximum
While Tables 1–4 note the inclusion/exclusion criteria, interventions, and results for each study, Table 5 illustrates if (1) the FITT variables were described sufficiently and (2) the intervention arm(s) met public health recommendations for PA.42–44 To classify the intensity of the exercise intervention(s) as moderate or vigorous, the reported percentages of intensity measures such as maximum heart rate (MHR), heart rate reserve, , rate of perceived exertion, and the metabolic equivalent of task were used.42, 78 Exercise dosing variables (FITT variables) were at least partially described in all identified studies. However, a complete description of dosing variables was often missing or difficult to discern (see Table 5).
Table 5.
Assessment of reported exercise variables of frequency, intensity, time, and type (FITT) in a manner that would permit intervention replication and determination if intervention met public health physical activity guidelines.44
| Trial | Exercise Study Arm(s) | Frequency | Intensity | Time | Type | Total FITT Criteria Reported | Meet Current PA Guidelines (AEx|REx) |
|---|---|---|---|---|---|---|---|
| Randomized controlled trials of exercise as a monotherapy (Table 1) | |||||||
| Doyne et al., 198738 | AEx | 3 | |||||
| REx | 2 | ||||||
| Singh et al., 199720 | PREx | 4 | |||||
| Blumenthal et al., 199950 | AEx | 4 | |||||
| Dunn et al., 200521 | AEx PHD-3d | 2 | |||||
| AEx PHD-5d | 2 | ||||||
| AEx LD-3d | 2 | ||||||
| AEx LD-5d | 2 | ||||||
| Singh et al., 200522 | HI-PREx | 4 | |||||
| LO-REx | 4 | ||||||
| Blumenthal et al., 200723 | AEx | 4 | |||||
| Krogh et al., 201251 | AEx | 4 | |||||
| Randomized controlled trials of exercise as an adjunct to psychotherapy for major depression (Table 2) | |||||||
| Jacquart et al., 201452 | Psych-Walk | 2 | |||||
| Oertel-Knochel et al., 201453 | AEx | 4 | |||||
| Kerling et al., 201554 | AEx | 4 | |||||
| Randomized controlled trials of exercise as an adjunct to pharmacotherapy for major depression (exercise combined with pharmacotherapy as a treatment arm or all subjects on stable dose of pharmacotherapy) (Table 3) | |||||||
| Blumenthal et al., 199950 | AEx | 4 | |||||
| Pilu et al., 200755 | PA | 2 | |||||
| Mota-Pereira al., 201156 | AEx | 4 | |||||
| Danielsson et al., 201457 | PA | 3 | |||||
| Belvederi Murri et al., 201558 | P-AEx | 4 | |||||
| NP-AEx | 4 | ||||||
| Carneiro et al., 201559 | AEx | 3 | |||||
| Legrand & Neff, 201660 | AEx | 4 | |||||
| Salehi et al., 201661 | AEx | 4 | |||||
| Siqueira et al., 201662 | AEx | 2 | |||||
| Gujral et al., 201963 | AEx | 4 | |||||
| Moraes et al., 202064 | PREx | 4 | |||||
| NP-AEx | 4 | ||||||
| Randomized controlled trials of exercise as an adjunct to standard care for major depression (Table 4) | |||||||
| Martinsen et al., 198565 | AEx | 3 | |||||
| Veale et al., 199266 Study 1 |
AEx | 2 | |||||
| Veale et al., 199266 Study 2 |
AEx | 2 | |||||
| Knubben et al., 200767 | AEx | 4 | |||||
| Krogh et al., 200968 | P-AEx | 4 | |||||
| PREx | 4 | ||||||
| Doose et al., 201569 | AEx | 4 | |||||
| Hallgren et al., 201570 | Ex | 2 | |||||
| Schuch et al., 201571 | AEx | 2 | |||||
| Helgadóttir et al., 201672 | Ex-Light | 3 | |||||
| Ex-Mod | 3 | ||||||
| Ex-Vig | 3 | ||||||
| Olson et al., 201773 | AEx | 4 | |||||
| Buschert et al., 201974 | AEx | 2 | |||||
| Chau et al., 202075 | Ex | AEx | 3-AEx 2-REx |
AEx | |||
| REx | REx | ||||||
| Haussleiter et al., 202076 | Ex | 2 | |||||
| La Rocque et al., 202177 | AEx | 2 | |||||
| Clinical trials examining the relationship of molecular mechanisms and clinical outcomes (Table 6) | |||||||
| Krogh et al., 2010161 | P-AEx | 4 | |||||
| PREx | 4 | ||||||
| Toups et al., 2011162 | AEx | 0 | |||||
| Krogh et al., 2012;51 | AEx | 4 | |||||
| Rethorst et al., 2013155 | AEx | 1 | |||||
| Krogh et al. 2014152 | AEx | 0 | |||||
| Krogh et al., 2014163 | AEx | 4 | |||||
| Schuch et al., 2014164 | AEx | 2 | |||||
| Salehi et al., 201661 | AEx | 4 | |||||
| Carniero et al., 2017117 | AEx | 3 | |||||
| Euteneuer et al., 2017153 | Ex | 2 | |||||
| Kerling et al., 2017165 | AEx | 3 | |||||
| Lavebratt et al., 2017154 | Ex-Vig | 3 | |||||
| Ex-Mod | 3 | ||||||
| Ex-Light | 3 | ||||||
| Rahman et al., 2017166 | Ex | 2 | |||||
| Gourgouvelis et al., 2018167 | Ex (AEx + REx) |
4 | AEx | ||||
| REx | |||||||
| Szuhany & Otto, 2020138 | Ex | 1 | |||||
| Gerber et al., 2020168 | Ex | 4 | |||||
PA = physical activity; AEx = aerobic exercise; REx = resistance exercise; PREx = progressive resistance exercise; PHD = public health dose; 3d = 3 days/week; 5d = 5days/week; HI = high intensity; LO = low intensity; Psych = psychotherapy; P-AEx = progressive aerobic exercise; NP-AEx = non-progressive aerobic exercise; Ex = exercise; Ex-Vig = exercise-vigorous intensity; Ex-Mod = exercise-moderate intensity; Ex-Light = exercise-light intensity
Assessment of FITT variables
 /
/  = sufficient/insufficient detail provided in manuscript to replicate the frequency (e.g. times per week), intensity (e.g., % heart rate reserve (AEx); % repetition maximum (REx), time (e.g., time spent exercising; time in target intensity zone), type (e.g. stationary cycling).
= sufficient/insufficient detail provided in manuscript to replicate the frequency (e.g. times per week), intensity (e.g., % heart rate reserve (AEx); % repetition maximum (REx), time (e.g., time spent exercising; time in target intensity zone), type (e.g. stationary cycling).
Assessment of intervention compliance with public health physical activity guidelines
 = intervention required at least 150 minutes of moderate intensity AEx, or 75 minutes of vigorous intensity AEx, per week; and/or intervention required at least two days of REx (utilizing most muscle groups) per week.
= intervention required at least 150 minutes of moderate intensity AEx, or 75 minutes of vigorous intensity AEx, per week; and/or intervention required at least two days of REx (utilizing most muscle groups) per week.
 = insufficient detail provided to determine total time and intensity; prescribed or reported time and intensity were below recommended thresholds.
= insufficient detail provided to determine total time and intensity; prescribed or reported time and intensity were below recommended thresholds.
Although the public health guidelines for PA recommend both AEx and REx,42–44 an overwhelming majority of studies employed AEx as the primary intervention.21, 23, 50–63, 65–67, 69–74, 77 Two studies employed REx-only (compared to control),20, 22 three studies employed AEx and REx arms,38, 64, 68 and one study developed a single intervention that included both AEx and REx.75 AEx interventions are generally more practical to implement within and outside of a research setting, as less equipment and experience is required for implementation and it allows for greater ease in controlling dosing variables, which may explain the overwhelming use of AEx compared to REx for depression.
Among the 27 studies that utilized an AEx-only intervention arm(s), thirteen (48%) sufficiently reported all FITT criteria that would enable replication of the intervention,23, 50, 51, 53, 54, 56, 58, 60, 61, 63, 67, 69, 73 and only eleven (40%) met public health recommendations for PA.21, 23, 50, 51, 56, 58, 60, 61, 65, 67, 72 Of these 27 studies, 22 (81.5%) reported a positive anti-depressant effect in the AEx arm.21, 23, 50, 52–54, 56–62, 65, 67, 69–73, 75–77 Interestingly, over half (55%) of the studies with positive anti-depressant effects are not repicable21, 52, 57, 59, 62, 65, 70–72, 76, 77 and/or did not meet PA guidelines52–54, 57, 59, 62, 69–71, 73, 76, 77 (Table 5).
Singh and colleagues (1997 & 2005) conducted two studies that examined the impact of REx monotherapy on depression (Table 1).20, 22 The first study compared a 10-week progressive REx program (80% of 1-repetition maximum [1-RM]) to a health education control group.20 The second study examined the impact of three interventions on depression: a progressive, high-intensity REx program (80% of 1-RM), a non-progressive, low-intensity REx program (20% of 1-RM), and TAU.22 Only 52% of participants in the TAU arm received some formal treatment, which included antidepressants (42%), only counseling (10%), and/or psychiatric referral (5%).22 In both studies, a greater response rate (≥50% improvement in Hamilton Rating Scale of Depression–17 [HAM-D17] score) was noted in the REx group compared to the control,20 TAU,22 and the low intensity, non-progressive REx group.22 In both studies, the authors sufficiently described the study arms, and the active and high-intensity interventions met the PA guidelines,20, 22 while, as expected, the low-intensity REx study arm did not.22
Three studies have directly compared the anti-depressant effect of AEx vs. REx,38, 64, 68 but only Krogh et al. (2009) and Moraes et al. sufficiently described both study arms (Table 5).64, 68 In Doyne et al.’s monotherapy study, the remission rate (Beck Depression Inventory score <9) was 67% for the AEx group (running at 80% of age-predicted MHR [APMHR]), 80% for the REx group (50–60% of APMHR) and 17% for the waitlist control, with no significant difference, noted between the two exercise groups (Table 1).38 The authors did not report the exercise duration (time) for both AEx and REx groups;38 thus, the study was identified as not meeting PA guidelines (Table 5). Moraes et al. examined AEx and REx as an adjunct to pharmacotherapy (Table 3).64 In this 12-week study, pharmacotherapy (fluoxetine or sertraline at varying therapeutic dosages) was paired with a progressive REx program (70% of 1-RM), a non-progressive AEx program (60% of or 70% of MHR), and a low-intensity PA control.64 Again, while no significant differences were noted between the remission (HAM-D17 score ≤7) and response (≥50% improvement in HAM-D17 score) rates in the REx and AEx groups, both intervention groups had significant improvements in both outcomes compared to the low-intensity control group.64 Based on the authors’ description, the REx arm met PA guidelines, while the AEx arm did not (Table 5). Alternatively, Krogh et al. (2009) completed a 16-week study that compared three exercise interventions while all participants were receiving TAU (pharmaco- and/or psycho-therapies): a progressive AEx program (90 minutes of interval training at 70–80% of MHR), a progressive REx program (75% 1-RM), and a relaxation control group.68 They found no significant difference in remission (HAM-D17 score <8) rates between all three groups (Table 4).68 Of note, the AEx and REx interventions met PA guidelines (Table 5). Although in its infancy, this growing body of evidence demonstrates the positive anti-depressant effects of high-intensity REx, whether as a monotherapy or as an adjunct to pharmacotherapy64 or TAU.68 Studies that directly compared AEx to REx,38, 64, 68 revealed no significant difference in their anti-depressant effects, but additional studies are needed to increase confidence in this conclusion. Additionally, there is the option of developing a combined AEx and REx exercise program, as done by Chau and colleagues (Table 4).75
Of the studies identified in this review, only four examined the dose-response of exercise.21, 22, 58, 72 Dunn et al.21 and Singh et al. (2005)22 used exercise as a monotherapy and compared different exercise dosing parameters to a control group (Table 1). The AEx dosing parameters utilized by Dunn et al. were based on public health exercise recommendations.21 They used a total weekly energy expenditure per kilogram of body weight (kcal/kg/wk) and an exercise frequency of three or five days per week.21 For the 12-week AEx intervention, the total energy expenditure for the public health dose was 17.5 kcal/kg/wk. In contrast, the low dose group was only 7 kcal/kg/wk, and the control group completed stretching exercises.21 While these exercise parameters fit within the PA guidelines, the translation of kcal/kg/wk could be challenging as it does not utilize the FITT principle. As noted in greater detail above, Singh et al. (2005) conducted an eight-week study comparing a progressive, high-intensity REx program and a non-progressive, low-intensity REx program to TAU.22 Dunn et al. and Singh et al. (2005), both reported greater remission (HAM-D17 score of ≤7)21 and response (≥50% improvement in HAM-D17 score)22 rates in the higher exercise dose arms compared to the lower dose and stretching control groups21 or TAU.22 While such findings indicate a potential dose-response relationship between exercise and reduction in depression, other studies have documented contrasting results.
Belvederi Murri and colleagues studied a 24-week intervention of either progressive or non-progressive AEx as an adjunct to pharmacotherapy (≥50mg/d of sertraline) (Table 3).58 Compared to pharmacotherapy alone, the progressive AEx intervention (40 minutes of cycling at 60–85% peak heart rate) plus pharmacotherapy and the non-progressive AEx program (60 minutes at ≤70% peak heart rate) plus pharmacotherapy both achieved greater remission (HAM-D17 score of ≤10) than the pharmacotherapy group.58 The authors noted that while the remission rates did not differ between the two exercise interventions, the progressive AEx group achieved remission earlier than the pharmacotherapy group.58 Similarly, Helgadóttir et al. employed light, moderate, or vigorous exercise as adjuncts to TAU (e.g., pharmacotherapy, psychotherapy, supportive counseling).72 Light, moderate, and vigorous exercise plus TAU all elicited significant reductions in the severity of depressive symptoms compared to TAU only.72 However, there was no significant effect of exercise intensity as all three exercise groups demonstrated comparable reductions in depressive symptoms.72
Such contrasting findings urge caution about the dose-response effects of exercise on depression, although subtle differences in exercise prescription may explain the heterogeneous results. Each study utilized a different method to differentiate the dose of exercise. Dunn et al. prescribed exercise dose by energy expenditure,21 Singh et al. (2005) manipulated REx dose based on a percentage of 1-RM,22 Belvederi Murri et al. dosed exercise based on a percentage of peak heart rate,58 and Helgadóttir et al. prescribed group exercise classes and differentiated groups by the expected intensity of the classes.72 Additionally, Singh et al. and Belvederi Murri et al. implemented progressed and non-progressed exercise groups to manipulate the exercise dose.22, 58 However, in all four studies, exercise doses that met current public health guidelines42–44 (Table 5) led to significant reductions in depressive symptoms. Taken together, these findings highlight: 1) the many methods that can be used to manipulate exercise dose; 2) the importance of reporting all exercise dosing variables; and 3) the significance of an exercise dose that meets current health recommendations.
It is also essential to acknowledge some factors outside exercise dosing that could impact study outcomes. While most of the studies identified in this review were completed in an outpatient setting, eleven studies were conducted in an inpatient mental health setting.52–54, 60, 61, 65, 67, 69, 71, 74, 76 Exercise for inpatients, and outpatients with depression has been shown to be well-tolerated, acceptable, and yields lower dropout rates in exercise arms compared to control arms in randomized controlled trials.79 Although exercise in an inpatient setting has been demonstrated to be feasible and beneficial to patients, it is rarely deployed. In the United States, this is a missed opportunity. In the clinical studies identified, the exercise interventions were supervised in-person one day a week at a minimum.20–23, 38, 50–77 Other published reviews also endorse supervised exercise by properly trained professionals to maximize compliance and adherence rates, reduce dropouts, and ensure safe and appropriate exercise intensity is achieved.80–82 By harnessing mobile technology, a future trend in this line of research could be remote intervention administration with video-recorded adherence and remote data collection to help ease any additional burden on the research participants, facilitate adherence, and allow for application outside of a research laboratory environment.
With institutional commitment, organized and strategic programming, and appropriately trained multidisciplinary teams, exercise intervention studies can be aptly designed and safely implemented in inpatient and outpatient mental health settings. These actions will aid in furthering the knowledge and understanding of how to best prescribe and integrate exercise into the treatment of depression. Going a step further and including transdisciplinary specialists, it will also be possible to examine the biological underpinnings to maximize the known anti-depressant benefits of exercise.
Mechanisms of the intervention-target interface for antidepressant treatment
The serotonergic and noradrenergic pathways serve an integral, yet complex, role in the pathophysiology of depression and are the primary targets of first line pharmacotherapeutic treatments (i.e., SSRI, SNRI).83 Pharmacological depletion of serotonin (5-HT) and norepinephrine (NE) in previously remitted depressed patients leads to a clinically significant recurrence of depressive symptoms.84, 85 This indicates the substantial role each may play in the development of depressive symptoms as well the potential for each to serve as treatment targets. Mechanistically, SSRIs and SNRIs reduce depressive symptoms through antagonist action on autoreceptor feedback and blockade of the reuptake of 5-HT and NE, ultimately leading to an increase in extracellular 5-HT and NE.86 Interestingly, depressive symptoms do not uniformly worsen (and in some cases unexpectedly improve) when 5-HT and NE are depleted in untreated depressed patients,87, 88 and healthy subjects without history of depression do not develop depressive symptoms following 5-HT depletion.89 This indicates that the causal relationship between 5-HT and NE and depressive symptoms is neither simple nor direct.90, 91 Disruptions in hypothalamo-pituitary-adrenal axis (HPA-axis) function,92 reductions in brain-derived neurotrophic factor (BDNF),93 and immuno-inflammation94 have all been implicated in depression. SSRI, and to a lesser extent SNRI, treatment may restore or ‘normalize’ HPA-axis function,95, 96 BDNF,97, 98 and immuno-inflammation99 although it is unclear how such changes relate to reductions in depression. This highlights the abundant complexity of mechanistic pathways in depression. Similarly, the interplay between putative mechanisms of action by which exercise exerts its antidepressant effects is not entirely clear. Although exercise’s antidepressant effects may occur through serotonergic and noradrenergic signaling, considerable evidence also indicates that it influences HPA-axis, BDNF, and immuno-inflammatory function. AEx- or REx-induced contractions of skeletal muscle release cytokines into circulation. Briefly, cytokines are proteins that are produced in response to infection, inflammation and stress (e.g. exercise) and act on tissues throughout the body, including the brain.100–102 Cytokines released by muscle fibers in response to exercise may be classified as myokines (as described by Pedersen and colleagues).103 Myokines, the ‘active ingredient’ of an exercise dose, can exert anti-inflammatory effects locally (e.g., muscle tissue) and distally (e.g., heart, liver, brain) and serve an integral role in exercise-induced health benefits.104 In this sense, skeletal muscle serves as a secretory organ, and AEx and REx are catalytic in the release of myokines that stimulate anti-inflammatory and antidepressant effects.105
Exercise effects on 5-HT and NE
Pre-clinical chronic stress models of depression suggest that depression is associated with reduced levels of 5-HT in the hippocampus (HPC)106, 107 pre-frontal cortex (PFC),107 and striatum,108 and reduced levels of NE in the PFC109 and HPC.110 Exercise can enhance brain 5-HT106, 111 and NE112 and can reduce depression-like behaviors.112–114 Exercise trials in humans utilize assessments of peripheral 5-HT and NE (as well as the remainder of the biological markers to be discussed) as surrogates of brain concentrations, although there are limitations to these assumptions.
Lechin and colleagues reported significant increases in both 5-HT and NE following a brief bout (5 minutes) of treadmill exercise in subjects with major depressive disorder (MDD), indicating that exercise has the potential to acutely influence serotonergic or noradrenergic activity in MDD.115 Similarly, data from non-depressed, healthy subjects support an acute, exercised-induced increase in 5-HT.116 The effects of exercise training on 5-HT and NE in clinical cohorts with depression are relatively limited and inconsistent with the results from acute exercise studies. Carneiro and colleagues reported that 16 weeks of AEx combined with SSRI treatment or SSRI treatment alone did not significantly enhance 5-HT in subjects with depression.117 NE was enhanced in both groups following the intervention, although these changes were also not significant. Reductions in 5-HT following AEx training have also been reported in an older breast cancer cohort with mild-to-no depressive symptoms118 and a younger healthy cohort with mild-to-no depressive symptoms.119 Interestingly, Melancon and colleagues reported that a 60-minute bout of moderate intensity AEx (~70% ) acutely increases free tryptophan (i.e., precursor to 5-HT) in older non-depressed men. However, following 16 weeks of AEx training, the same bout (duration and intensity) of AEx attenuated free tryptophan compared to baseline.120 Thus, it appears the cumulative effect of regular AEx training enhances tryptophan metabolism. Such an increase in peripheral tryptophan metabolism may occur through activation of the kynurenine pathway. The majority of peripheral tryptophan is converted to kynurenine, with only a small percentage of peripheral tryptophan converted to serotonin.121 Kynurenine is converted to quinolinic acid (neurotoxic) or kynurenic acid (neuroprotective) and exercise appears to stimulate the conversion of kynurenine to kynurenic acid via kynurenine aminotransferases (KAT). Agudelo and colleagues report that three weeks of intensive (2 training sessions per day/6 days per week) AEx training in healthy adults increases the expression of PGC-1α1, an activator of tryptophan-kynurenine metabolism, and subsequently KAT in human skeletal muscle.122 These findings were corroborated in their murine model which performed free wheel running and demonstrated significantly increased expression of PGC-1α1 and KAT in skeletal muscle as well as increases in plasma kynurenic acid compared to sedentary mice.122 Moreover, their transgenic murine model that overexpresses PGC-1α1 is resilient to developing depressive behaviors when subjected to chronic stress indicating the potential importance of this pathway in depression.122 Recent work by Contrepois and colleagues indicates that following an acute bout of AEx peripheral levels of kynurenic acid increase while tryptophan decreases suggesting that AEx stimulates tryptophan metabolism.123 Taken together, such findings provide novel evidence indicating that enhanced tryptophan-kynurenine metabolism is protective against depression and that exercise may elicit antidepressant effects via this pathway.
Data from depressed and non-depressed clinical cohorts are inconsistent in their findings on the acute and chronic effects of exercise on 5-HT and NE. Single sessions of AEx appear to increase tryptophan, 5-HT, and NE, yet AEx training has not been shown to reliably increase resting levels of any such mechanisms. Furthermore, assessing such mechanisms in the periphery will likely tell an incomplete story of the central effects given the complex pathways involved and whether peripheral changes in tryptophan, 5-HT, or NE mediate reductions in depression in clinical cohorts. A potential interesting avenue for further study is the effect of exercise on kynurenine metabolism and its subsequent effect on tryptophan, 5-HT, NE and ultimately depression.
Exercise effects on BDNF
Brain-derived neurotrophic factor is a protein that promotes neuronal growth and survival and enhances synaptic transmission and neurogenesis.124 In preclinical models of depression, hippocampal BDNF is reduced110, 125 and treatment with an SSRI or SNRI upregulates hippocampal BDNF expression and reduces depressive-like behavior.126, 127 Exercise also enhances BDNF and attenuates depressive-like behaviors 128 suggesting that BDNF may be a mediator of the antidepressant effects of exercise in clinical cohorts.
BDNF is produced by skeletal muscle cells in response to a bout of exercise129 and appears to be released into circulation by the brain during exercise.130 Clinical evidence indicates that AEx acutely enhances BDNF in cohorts with depression.131–133 The intensity of AEx appears to be an important variable in the acute enhancement of BDNF in depression, however a true dose-response relationship between AEx intensity and BDNF is not consistently reported.133, 134 It does appear that moderate-to-high intensity AEx generally elicits greater magnitude response of BDNF compared to low intensity or resting conditions.131, 135 In healthy, non-depressed cohorts, an acute bout of REx has been shown to enhance BDNF136 and it appears that acute BDNF enhancement occurs after both high intensity and high volume REx.137 The acute effects of REx on BDNF in depressed cohorts have not been studied. Conversely, chronic adaptations in BDNF response to exercise training interventions in depression are not well understood. A meta-analysis by Dinoff and colleagues identified six clinical trials (n=176) that examined the effect of AEx training on BDNF in MDD. There was no significant effect of AEx training on resting levels of BDNF, although there was heterogeneity in the duration, frequency, and intensity of the AEx interventions employed.138 Recently, Szuhany and Otto reported that 12 weeks of moderate intensity exercise combined with behavioral activation had no significant effect on resting BDNF.139 Interestingly, an acute maximal exercise test employed at 4 week intervals indicated that BDNF was acutely enhanced and the magnitude of this effect did not appear to change over the course of the intervention. Thus, consistent with much of the literature, their results highlight that AEx acutely enhances BDNF yet does not appear to substantially enhance resting levels. Regarding REx training, Pereira and colleagues demonstrated that either 10 weeks of REx or AEx reduces depressive symptoms in older, community dwelling females although only REx significantly increases resting BDNF.140 With the exception of these findings, it appears that the chronic effects of REx training on BDNF in MDD remains unexplored, highlighting an area in need of further investigation.
Exercise effects on immuno-inflammation
The response to stress and inflammation is complex. It involves the activation of a myriad of immune cells (i.e., leukocytes, granulocytes, lymphocytes, monocytes) and their associated cytokine (i.e., interleukins [IL], tumor necrosis factors [TNF], interferons [IFN]) production. Clinical evidence indicates increased central levels of pro-inflammatory markers IL-6 and TNF-α in MDD.101 Assessment of peripheral levels of IL-6 and TNF-α indicate that both are elevated in MDD, although when only high quality studies (Newcastle-Ottawa quality assessment scale score ≥ 6) were considered, the association between MDD and TNF-α was attenuated.141 Consequently, C-reactive protein (CRP), a marker of inflammation produced by the liver following rises in IL-6, has been shown to be elevated in individuals with depression.141, 142 Thus, inflammation may play an integral role in the pathophysiology of depression and may serve as a potential therapeutic target.
Preclinical evidence indicates that chronic administration of fluoxetine protected against the development of inflammation (IL-1β, IL-6, TNF-α) and depressive behaviors in rats exposed to chronic mild stress.143, 144 Similarly, swimming exercise reduced TNF-α in the PFC and attenuated stress induced depressive behavior.145 Wheel running reduced immobility time, promoted movement distance, and reduced circulating IL-6 in chronically stressed rats.128 This preclinical work suggests the possibility that antidepressant treatments concomitantly attenuate inflammation and depressive behavior.
Acute bouts of exercise stimulate the release of myokines into the circulation which may underlie its anti-inflammatory and anti-depressant effects.19, 104 In clinical cohorts, acute bouts of exercise appear to modulate several inflammatory molecules. Boetteger and colleagues examined the acute effects of a maximal bout of AEx on circulating inflammatory markers in patients with MDD and age-, sex-, and body mass index-matched non-depressed control subjects. Prior to exercise, patients with MDD had significantly higher levels of cellular inflammation compared to control subjects as indicated by elevated levels leukocytes, granulocytes, and monocytes although no significant differences in IL-6, IL-1β, or IL-10.146 Following a single bout of maximal AEx both groups demonstrated similar increases in cellular inflammation (leukocytes, granulocytes, lymphocytes, and monocytes) and IL-6 and IL-10. This suggests that although an acute bout of AEx can be a potent stimulator of the immune system, patients with MDD do not appear to demonstrate an exacerbated exercised-induced inflammatory response. Although IL-6 is already notably elevated in depression, the acute exercise-induced release of IL-6 into circulation may actually serve an anti-inflammatory role.147 IL-6 appears to acutely inhibit production of pro-inflammatory TNF-α148 and enhance the production of IL-10,149 which subsequently inhibits a wide range of pro-inflammatory cytokines. Conversely, Hallberg and colleagues reported acute increases in IL-6 and TNF-α, but not IL-10, following an acute bout of maximal AEx in unmedicated patients with MDD and non-depressed control subjects.150 Provided that the former investigation included mostly medicated subjects (93%), the findings of these studies may highlight the importance of combining both pharmacotherapy and exercise to maximize anti-inflammatory benefits.
Given that exercise can acutely stimulate an immuno-inflammatory response, it is unsurprising that the cumulative effects of exercise training also appear to regulate inflammation. Evidence in older adults indicates that higher levels of exercise and physical activity were associated with reduced levels of CRP, IL-6, and TNF-α,151 although the cross-sectional nature of the investigation does not permit identification of a true cause-and-effect of exercise. However, such findings are corroborated by Kohut and colleagues who reported significant reductions in CRP, IL-6, and TNF-α following 10 months of moderate to vigorous AEx training (progressing from 45-80% ) in older adults.152 Conversely, subjects receiving 10 months of flexibility training demonstrated a significant reduction in only TNF-α, perhaps indicating the importance of vigorous, repetitive muscle contraction (rather than low intensity muscle stretching) in the anti-inflammatory response of exercise. Yet, trials in cohorts with depression yield inconsistent effects of exercise on immuno-inflammation (see Table 6). Krogh and colleagues (2014) reported no significant effects of 12 weeks of AEx training on CRP or IL-6.153 Similarly, Euteneuer and colleagues reported no significant effects of 16 weeks of combined cognitive behavioral therapy (CBT) and exercise on CRP or IL-6, although IL-10 was significantly enhanced for the combined CBT and exercise group (compared to CBT and waitlist control groups).154 Lavebratt and colleagues investigated the effects of low, moderate, or vigorous intensity AEx on IL-6 and reported no significant difference between the exercise conditions, although low and moderate AEx elicited modest decreases in IL-6 whereas vigorous AEx elicited an increase in IL-6.155 Comparably, Rethorst and colleagues reported that neither 12 weeks of low dose nor high dose exercise were associated with significant changes in IL-6, TNF-α, IL-1β, or IFN-γ.156
Table 6.
Clinical trials examining the relationship of molecular mechanisms and clinical outcomes in major depressive disorder
| Trial | Depression diagnosis criteria and type | Sample | Interventions | Study Duration | Treatment arms (n) | Outcomes |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mechanism | Clinical | Relationship | ||||||||
| Krogh et al., 2010162 | ICD-10 /DSM-IV-MDI Unipolar Major |
Males and females, 18–55y/o, referred by medical doctor or psychologist and met ICD-10 criteria, Exclusion: alcohol or substance abuse, acute suicidal risk, psychotic symptoms, medical conditions that contraindicated physical exercise, been on sick leave for >24mos, exercising >1hr/wk n=88 (only ‘completers’ included in analysis) ~39y/o 27% males |
Outpatient setting P-AEx: 90mins of interval training; initially 2mins on: 2mins rest at 70% MHR and progress to 3mins on:1min rest at 89% MHR. AEx included cycling, running, stepping, abdominal exercises, rowing, trampoline, step bench, jump rope, and Ski Fitter PREx: 90mins for 2–3sets x12 reps at 50% 1-RM progressed to 2-3 sets x8-10 reps at 75% 1-RM. 6 exercises on machines (leg extension, leg press, total abdominal, lower back, chest press, vertical traction) and 3 with weights (calves, arm abductors, and triceps) Relax: 90mins of light activity at Borg RPE ≤12; 20–30mins exercise on mattresses or back massage, 10–20mins of light balance activities, and 20–30mins of supine relaxation. TAU: Pharm Pharm (%): P-AEx: 64.5% PREx: 62.1% Relax: 67.9% |
16 weeks All groups: 2d/wk/16wks (32 supervised group sessions) TAU: NR |
P-AEx + TAU (n=31) | pCORT ←→ |
HAM-D17 Δ NR |
NR | ||
| PREx + TAU (n=29) | pCORT ←→ |
HAM-D17 Δ NR |
NR | |||||||
| Relax + TAU (n=28) | pCORT ←→ |
HAM-D17 Δ NR |
NR | |||||||
| Note: No significant effect of intervention on resting cortisol or cortisol response to maximal exercise test. Changes in depression and relationship of cortisol with changes depression were not reported. Only completers (subjects completed intervention and provided pre and post blood samples) were included analysis. | ||||||||||
| Toups et al., 2011163 |
DSM-IV Major |
Males and females, 18–70y/o, all had been treated with SSRI for 2–6mos prior and were partial or non-responders (HAM-D ≥14), not engaged in regular exercise Exclusion: history, physical exam or laboratory results indicating significant medical condition, depression due to comorbid psychiatric disorder; additional pharm, or psych n= 104 (70 completers) 47.6±9.4 y/o ~20% male |
Outpatient setting AEx: either LD (4KKW) or HD (16KKW). Subjects self-selected intensity with the goal of meeting weekly energy expenditure through supervised and home-based exercise |
12 weeks All sessions: initial sessions were supervised and tapered to one supervised session per week in week 3. |
LD AEx (n=38) | sBDNF ←→ | IDS-C ↓ | τ = −.01 | ||
| HD AEx (n=32) | sBDNF ←→ | IDS-C ↓ | τ = −.01 | |||||||
| Note: Higher baseline levels of sBDNF predicted more rapid antidepressant response. High BMI at baseline amplified this response. No between group differences. | ||||||||||
| Krogh et al., 2012;51 Krogh et al. 2014153
|
DSM-IV-MINI Major |
Males and females, 18–60 y/o, HAM-D ≥12 Exclusion: drug use, antidepressant use in past 2mos, receiving psych, contraindications to physical exercise, >1hr of PA/wk, suicidal behavior, current/previous psychotic or manic symptoms, pregnancy n= 115 41.6y/o (19–59 y/o) 33% male |
Outpatient setting AEx: 10min warm-up, 30 mins on cycle ergometer (at least 65% of maximal capacity with progression to 80%), and 5min cool-down CON: 10min warm-up at low intensity on stationary bike, 20min program of stretching, 15min of low intensity exercise |
12 weeks AEx: 3d/wk/12wks (36 supervised sessions) CON: 3d/wk/12wks (36 supervised sessions) |
AEx (n=56) | hsCRP ←→ IL-6 ←→ |
HAM-D17 ↓ | NR (non- significant) | ||
| CON(n=59) | hsCRP ←→ IL-6 ←→ |
HAM-D17 ↓ | NR (non- significant) | |||||||
| Note: No effect of group on HAM-D17 or hsCRP. Does not appear there was a time effect on IL-6. HAMD17 was reduced by 41% and 44% in AEx and CON groups, respectively. hsCRP remained unchanged. Regression analyses revealed no significant relationship between inflammatory markers and change in depressive symptoms. | ||||||||||
| Rethorst et al., 2013156 | DSM-IV Major |
Males and females, 18–70y/o, all had been treated with SSRI for 2–6mos prior and were partial or non-responders, not engaged in regular exercise Exclusion: depression due to comorbid psychiatric disorder; additional pharma or psych, history, physical exam or laboratory results indicating significant medical condition, n= 105 (73 completers) 47.5±9.4 y/o ~20% male |
Outpatient setting AEx: either LD (4KKW) or HD (16KKW). Subjects self-selected intensity with the goal of meeting weekly energy expenditure through supervised and home-based exercise |
12 weeks All sessions: initial sessions were supervised and tapered to one supervised session per week in week 3. |
LD–AEx (n=40) | IFN-γ ←→ IL-1β ←→ IL-6 ←→ TNF-α ←→ | *IDS-C ↓ |
rs = −0.19 rs = 0.20 rs = 0.23 rs = −0.04 |
||
|
HD–AEx (n=33) |
IFN-γ ←→ IL-1β ←→ IL-6 ←→ TNF-α ←→ |
*IDS-C ↓ |
rs = 0.12 rs = 0.29 rs = 0.15 rs = 0.09 |
|||||||
| Note: Moderator analyses indicated that TNF- α above median at baseline predicted greater magnitude reduction in IDS-C. There was a significant, weak correlation between IL-1β and all depression measures for both groups combined. Larger reductions in IL-1β associated with reductions in depression. | ||||||||||
| Krogh et al., 2014164 |
DSM-IV-MINI Major |
Males and females, 18–60 y/o, HAM-D ≥12 Exclusion: drug use, antidepressant use in past 2mos, receiving psych, contraindications to physical exercise, >1hr of PA/wk, suicidal behavior, current/previous psychotic or manic symptoms, pregnancy n= 79 41.3±12.1 y/o 33%/male |
Outpatient setting AEx: 10min warm-up, 30 mins on cycle ergometer (at least 65% of maximal capacity with progression to 80%), and 5min cool-down CON: 10min warm-up at low intensity on stationary bike, 20min program of stretching, 15min of low intensity exercise |
12 weeks AEx: 3x/wk/12wks (36 supervised sessions) CON: 3x/wk/12wks (36 supervised sessions) |
AEx (n=41) | sBDNF ←→ sVEGF ←→ sIGF-1 ←→ R-HPCvol←→ L-HPC vol ←→ | HAM-D17 Δ NR | NR NR NR rs = 0.30 NR |
||
| CON(n=38) | sBDNF ←→ sVEGF ←→ sIGF-1 ←→ R-HPCvol←→ L-HPCvol ←→ | HAM-D17 Δ NR | NR NR NR rs = 0.30 NR |
|||||||
| Note: Sub-study of Krogh et al., 2012.51 Right hippocampal volume increased significantly associated with reduction in HAM-D17 independent of group. | ||||||||||
| Schuch et al., 2014165 |
DSM-IV-MINI Major |
Male and female,, 18–60 y/o, HAM-D ≥25, not involved in any other PA during hospitalization Exclusion: three or more cardiovascular risk factors on PAR-Q, not able to exercise due to medical condition, taking beta blocker medication, psychiatric diagnosis of bipolar, schizophrenia, anorexia, alcohol or drug abuse or dependence n= 50 40.3±NR y/o 24% male |
Inpatient setting AEx: warm-up (stretching 4 lower limb muscle groups and 4min treadmill walk), exercise bout included choice of stationary bike, treadmill, or “transport” machine to complete 16.5 kcal/kg body mass/wk (~59% HRR), and cool-down TAU: Pharm and/or ECT and access to OT, but no psych CON: TAU only |
Duration dependent of length of stay AEx: 3d/wk (average ~21±5d hospitalized) (Supervised individual sessions) CON: NR with average ~24±6d hospitalized |
AEx + TAU (n=15) | sTBARS↓ sBDNF↑ | HAM-D Δ NR | NR NR |
||
| CON (n=11) | sTBARS↑ sBDNF↑ | HAM-D Δ NR | NR NR |
|||||||
| Note: Significant group by time interaction at discharge for sTBARS. sTBARS was significant lower for AEx + TAU group. Significant time effect on sBDNF. sBDNF was significantly elevated in second week but did not differ by group. No regression analyses performed | ||||||||||
| Salehi et al., 201661 |
DSM-IV-MINI Major |
Males and females, 25–40 y/o, admitted to inpatient care, BDI ≥30, HAM-D ≥25, no comorbid psychiatric disorders, no systemic disorders such as diabetes, hypertension, hyper- or hypothyroidism Exclusion: history of epilepsy, physical illness, refused ECT n=60 29.7±5.8 y/o 70% male |
Inpatient setting Initial 2wk washout period with pharm Pharm: 40mg/d of citalopram AEx: 40–45mins of cycling at 60–75% of VO2max ECT: 1.5 times seizure threshold dose at 1.0ms pulse width |
4 weeks AEx: 3xs/wk/4wks (12 supervised individual sessions) ECT: 3xs/wk/4wks (12 supervised individual sessions) |
AEx + ECT + Pharm (n=20) | pBDNF↑ | HAM-D21↓ | r = −.02 | ||
| AEx + Pharm (n=20) | pBDNF←→ | HAM-D21↓ | r = −.02 | |||||||
| ECT + Pharm (n=20) | pBDNF↑ | HAM-D21↓ | r = −.02 | |||||||
| Note: pBDNF greater at post in ECT and AEx+ ECT compared to AEx. HDRS decreased significantly in all groups with AEx+ECT being significantly lower at post compared to other groups. AEx+ ECT was significantly associated with remission; 76.5% remitted. Correlations provided are associations between post assessments. Correlations of change scores not performed. | ||||||||||
| Carniero et al., 2017117 | ICD-10; psychiatrist confirmed Major, dysthymia |
Females, 18–65 y/o, sedentary, physical fitness to endure exercise confirmed by physician, normal ECG Exclusion: psychotic comorbidities, substance or alcohol abuse, receiving additional treatment, pharmacotherapy changes in past 6wks, significant medical constraints, taking beta-blocker medication, n=19 ~50 y/o 100% female |
Outpatient setting AEx: 5min warm-up, 30mins of various activities (games, circuit workouts with resistance bands, jump ropes, fitness balls, brisk walking, and dancing) at 65–76% MHR and 5min stretching cool-down Pharm: SSRIs and anxiolytics/hypnotics, if needed, at constant individualized dosages |
16 weeks AEx: 3d/wk/16wks (48 supervised group sessions) Pharm: Session information with psychiatrist over 16 wks NR |
AEx + Pharm (n=9) | pDA←→ pNA↑ pAD↑ p5-HT↓ pCORT↓ |
BDI-II↓ | NR all | ||
| Pharm (n=10) | pDA↓ pNA↑ pAD←→ p5-HT↑ pCORT↓ |
BDI-II↑ | NR all | |||||||
| Note: Sub-study of Carniero et al., 2015.59 Only group by time interaction was on dopamine. BDI-II results from previous publication indicate significant reduction in DEP in AEx group. No regression or correlation performed with mechanisms and clinical outcomes. Unclear how any measure mediates depression. | ||||||||||
| Euteneuer et al., 2017154 | DSM-IV-SCID Major |
Males and females, 18–65 y/o Exclusion: psychotic comorbidities, neurological illness, substance or alcohol abuse, unstable dose of pharm (change within previous 2wks or planning to change) n=98 37.3±12.2 y/o 51% male |
Outpatient setting CBT: 50min session of CBT with clinical psychologist. Ex: Patients provided a manual of exercise activities. Instructed to complete at least 40mins of ‘moderate’ intensity exercise 4xs/wk. Exercise habits reinforced during CBT sessions (CBT+Ex group only). Euthymic activity: Instructed to complete 40mins of pleasurable activities 4xs/wk. Included activities that were enjoyable but did not lead to substantial increase in physical activity. These activities were reinforced during CBT sessions. |
16 weeks CBT: 1x/wk/ beginning week 1 (16 individual supervised sessions) Ex: 4xs/wk/beginning week 5. (Encouraged 44 unsupervised sessions) Euthymic activity: 4xs/wk/beginning week 5 (Encouraged 44 unsupervised sessions) |
CBT + Ex (n=36) | CRP←→ IL-6↑ IL-10↑ |
BDI-II↓ | NR all | ||
| CBT + Euthymic activity (n=35) |
CRP←→ IL-6↑ IL-10←→ |
BDI-II↓ | NR all | |||||||
| CON (n=30) |
CRP←→ IL-6←→ IL-10←→ |
BDI-II←→ | NR all | |||||||
| Note: MDD patients had significantly higher CRP levels compared to healthy controls. No significant change in CRP amongst any group. Sub sample of subjects with CRP > 1 indicated that Ex significantly reduced CRP levels at 16 weeks. IL-10 significantly increased in CBT+Ex group at 8 and 16 weeks compared to CBT or CON. Both CBT+Ex and CBT had significant reductions in DEP compared to CON so the role of IL-10 in reduction of DEP is unclear. | ||||||||||
| Kerling et al., 2017166 | DSM-IV-SCID Major |
Males and females, 18–60 y/o, medical exam confirmed no evidence for previous coronary artery disease, myocardial infarction, or angioplasty, no use of beta-blocker or other cardiologic medication Exclusion: BMI ≥ 30, cardiovascular, metabolic, or immune disorder, current substance abuse, schizophrenia, cognitive impairment, bipolar disorder n= 42 ~42 y/o 62% male |
Inpatient setting AEx: 45mins at 50% maximal workload achieved during baseline exercise test. Mode consisted of combination of cycle ergometer, treadmill, rower, and crosstrainer TAU: pharm and psych. Light unstructured activities permitted for 20mins (walking, stretching, ball games) |
6 weeks AEx: 3d/wk/6wks (18 supervised sessions) TAU: pharm and psych as prescribed. Light activities daily |
AEx + TAU (n=22) | sBDNF↑ | BDI-II↓ MADRS↓ |
NR BDI-II n.s. |
||
| TAU (n=20) | sBDNF↓ |
BDI-II↓ MADRS↓ |
NR BDI-II n.s. |
|||||||
| Note: Both groups demonstrated significant reductions in DEP as reported in Kerling et al. 201554 (NR here). Group effect but not time effect on BDNF. No relationship reported between change in BDNF and change in DEP | ||||||||||
| Lavebratt et al., 2017155 | DSM-IV-MINI Major, anxiety, comorbid anxiety |
Males and females, 18–64 y/o, PHQ-9 >9, Exclusion: severe somatic illness, substance abuse, psychiatric diagnosis requiring specialist treatment (i.e., psychosis) n=116 43±12 y/o 34% male |
Outpatient setting Ex: 60mins of light (yoga), moderate (AEx), or vigorous (AEx+REx) TAU: Managed by PCP and included pharm and/or CBT, or no treatment Antidepressant use Light: 8% Moderate: 6% Vigorous: 9% |
12 weeks Ex: 3d/wk/12wks (36 supervised group sessions) Light: yoga, stretching, controlled breathing (~54% APMHR) Moderate: ‘intermediate’ level AEx class (~70% APMHR) Vigorous: higher intensity AEx, REx, and balance training class (~76% APMHR) |
Light (n=48) | IL-6↓ | MADRS↓ | β = 0.19 | ||
| Moderate (n=36) | IL-6↓ | MADRS↓ | β = 0.19 | |||||||
| Vigorous (n=32) | IL-6↑ | MADRS↓ | β = 0.19 | |||||||
| Note: Sub-study of Hallgren et al.70 Subjects appeared to also receive TAU. Changes in IL-6 did not significantly differ between exercise intensities. Regression analyses indicated that: 1) Higher baseline IL-6 level was associated with greater reductions in MADRS; 2) Higher baseline MADRS was associated with greater reductions in IL-6; and 3) there was a significant, positive relationship between change in IL-6 and change in MADRS. | ||||||||||
| Rahman et al., 2017167 | DSM-IV-MINI Major, anxiety, comorbid anxiety |
Males and females, ≥18 y/o, PHQ-9 >9, Exclusion: severe somatic illness, substance abuse, psychiatric diagnosis requiring specialist treatment (i.e., psychosis) n=547 (subjects providing sample for BDNF genotyping) 43±12 y/o 27.2% males n=117 (subjects in exercise group that provided pre- and post- samples for BDNF genotyping and assessment of circulating BDNF) age NR sex NR |
Outpatient setting Exercise: 60mins of light (yoga), moderate (AEx), or vigorous (AEx+REx) ICBT: Online, self-help manual with weekly interaction with clinician; additional support provided as needed TAU: Managed by PCP and included pharm and/or CBT, or no treatment Antidepressant use Exercise: 33% ICBT: 32% TAU: 26% |
12 weeks Exercise: 3d/wk/12wks (36 supervised group sessions) ICBT: Multiple (self-selected) TAU: NR |
Exercise (n=197, BDNF SNP) (n=120, circulating BDNF) |
BDNF rs6265 SNP (Val66Met) 71% ValVal 29% ValMet mBDNF←→ proBDNF←→ |
MADRS Δ NR |
NR (n.s.) NR (n.s) |
||
| ICBT (n=208, BDNF SNP) | BDNF rs6265 SNP (Val66Met) 69% ValVal 31% ValMet |
MADRS Δ NR |
NA |
|||||||
| TAU (n=142 BDNF SNP) | BDNF rs6265 SNP (Val66Met) 65% ValVal 35% ValMet |
MADRS Δ NR |
NA |
|||||||
| Note: Sub-study of Hallgren et al.70 Circulating BDNF only studied in a sub-sample of exercise group. Neither proBDNF nor mBDNF were associated with baseline depression severity or depressive symptom response to exercise. ValMet SNP carriers, without exposure to childhood adversity, were more likely to be responders to exercise. ValMet carriers had higher serum mBDNF at baseline but this was not augmented by exercise. | ||||||||||
| Gourgouvelis et al., 2018168 | DSM-IV- Major and anxiety |
Males and females, low-activity levels, comorbid anxiety, BDI-II ≥ 20, medication permitted if taking stable dosage at least 6wks prior to pre-testing and through study duration, experience depressive symptoms ≥ 6mos, no medical contraindications to exercise assessed by PAR-Q Exclusion: Axis I disorder other than anxiety, substance abuse n=16 39.3±7.0y/o 25% male |
Outpatient setting CBT: NR Ex: At least 150mins MVPA/wk with AEx and REx sessions. AEx: 1x/wk with progression up to 60mins at 60–80% APMHR REx: 8–12 reps at 95% 10-RM Pharm: Individual stable dose |
8 weeks CBT: Group sessions Ex: 3x/wk/8wks (24 individual, supervised sessions; 8 AEx and 16 REx sessions) |
CBT + Ex (AEx & REx) + Pharm (n=8) | pBDNF↑ CTHB←→ |
BDI-II↓ |
R2 = −.50 CTHB NR |
||
| CBT + Pharm (n=8) | pBDNF←→ CTHB←→ |
BDI-II↓ |
R2 = −.50 CTHB NR |
|||||||
| Note: Both groups demonstrated significant reductions in DEP although AEx group had significantly greater reduction in DEP at 8 weeks. Reductions in DEP were significantly correlated with increases in BDNF across groups. Sleep improvements were also significantly associated with increases BDNF (see Rethorst et al. 2015192). Inflammatory markers were measured but >50% were below detection level so not included in analyses. | ||||||||||
| Szuhany & Otto, 2020139 | DSM-V via ADIS-5 Major or persistent |
Males and females, 18–65 y/o, sedentary (<2d/wk of 30mins of moderate exercise for previous 3mos) Exclusion: past or current psychotic, schizoaffective, bipolar, anorexia, bulimia, or substance abuse disorders, high suicide risk, not medically stable to exercise as assessed by the PAR-Q, participating in psychosocial treatment or on unstable dose of pharm (no dose changes in the previous 8wks) n=29 ~34y/o 24% male |
Outpatient setting BA: 60mins of standard BA treatment (psychoeducation, activity monitoring scheduling activities, role of social support Ex: 150mins of moderate Ex/wk; Ex performed on own w/guidance from therapist. 30min sessions following first 6 BA sessions to enhance adherence Stretching: 150mins/wk. Stretching performed on own w/guidance from therapist. 30min sessions following first 6 BA sessions to enhance adherence |
12 weeks BA: 1x/wk for weeks 1–6; Biweekly booster sessions for weeks 7–12 (9 supervised sessions) Ex: Accumulate 150ms/wk/12wks Stretching: Accumulate 150mins/wk/12wks |
BA + Ex (n=14) | Resting sBDNF←→ Exercise induced Δ sBDNF↑ |
MADRS↓ BDI-II↓ MADRS↓ BDI-II↓ |
r = −0.19 r = 0.25 r = 0.35 r = −0.26 |
||
| BA + Stretching (n=15) | Resting sBDNF←→ Exercise induced Δ sBDNF↑ |
MADRS↓ BDI-II↓ MADRS↓ BDI-II↓ |
r = −0.19 r = 0.25 r = 0.35 r = −0.26 |
|||||||
| Note: Both groups demonstrated significant reductions in DEP at 16wks. Intervention was 12wks, but no testing completed at that point. No effect of group on resting BDNF or exercise induced change in BDNF. No significant relationship between change in resting BDNF or change in exercise induced BDNF and changes in DEP. Report from Szuhany & Otto, 2020193 indicated that patients increased MVPA over the course of the intervention independent of group assignment potentially explaining the lack of group difference on BDNF and/or DEP. | ||||||||||
| Gerber et al., 2020169 | ICD-10 Major, recurrent (1 bipolar patient included) |
Males and female, 19–60 y/o, admitted for inpatient care, met ICD-10 criteria (F32, F33, and F31) Exclusion: HAM-D17 <17, BMI >35, comorbid major psychiatric disorder, somatic condition preventing participation in exercise, participation in regular vigorous intensity exercise n=25 38.1±7.0y/o 48% male |
Inpatient setting Ex: 40–50mins of stationary cycling at 60–75% APMHR CON: coordination and stretching activities for all major muscle groups TAU: pharm (SSRI, SNRI, and lithium), psych (individual and group) |
6 weeks Ex: 3d/wk/6wks (18 sessions) CON: 3d/wk/6wks (18 sessions) Ex and CON sessions supervised and performed individually or with one other patient TAU: as prescribed |
Ex + TAU |
TSST induced Δ in salCORT←→ | BDI↓ | r = 0.02 | ||
| CO N+ TAU | TSST induced Δ in salCORT←→ | BDI↓ | r = 0.02 | |||||||
| Note: Significant effect of time on DEP. No group by time interaction. No significant changes in cortisol response to TSST and changes in cortisol response were not associated with changes in DEP. | ||||||||||
ADIS= Anxiety and Related Disorders Interview Schedule; AEx = aerobic exercise; APMHR = age predicted maximum heart rate; BA= behavioral activation; BDI = Beck Depression Inventory; BMI = body mass index; CBT = cognitive-behavioral therapy; CON = control; CRP= C-reactive protein; DEP = depression; DSM= Diagnostic and Statistical Manual of Mental Disorders; ECG = electrocardiogram; ECT= electroconvulsive therapy; Ex = exercise; HAM-D = Hamilton Rating Scale of Depression; HD = high dose (exercise); HPCvol = hippocampal volume; hr(s) = hour(s); HRR = heart rate reserve; hsCRP = high sensitive C-reactive protein; ICBT = internet-based cognitive-behavioral therapy; ICD-10 = International Classification of Diseases, 10th Revision; IDS-C= Inventory of Depression Symptomatology-clinician rated; IFN-γ= interferon γ; IL-10= interleukin 10; IL-1β= interleukin 1β; IL-6= interleukin 6; KKW= kilocalorie per kilogram body weight per week; L = left; LD = low dose (exercise); MADRS = Montgomery Depression Rating Scale; mBDNF = mature brain-derived neurotrophic factor; MDD = major depressive disorder; MDI = Medical Depression Inventory; Met = Methionine; MHR = maximum heart rate; MINI = Mini International Neuropsychiatric Interview; mo(s) = month(s); MVPA = moderate to vigorous physical activity; n.s. = nonsignificant; NR = not reported; OT = occupational therapy; p5-HT= plasma serotonin; PA = physical activity; P-AEx = progressive aerobic exercise; PAR-Q = Physical Activity Readiness Questionnaire; pBDNF= plasma BDNF; pCORT= plasma cortisol; PCP = primary care physician; pCTHB= plasma cysteine proteinases; pDA= plasma dopamine; pharm = Pharmacotherapy; PHQ- 9 = Patient Health Questionare-9; pNA= plasma noradrenaline; PREx = progressive resistance exercise; proBDNF = precursor brain-derived neurotrophic factor; Psych = psychotherapy; R = right; Relax = relaxation; REx = resistance exercise; RPE = rating of perceived exertion; salCORT= salivary cortisol; sBDNF= serum brain-derived neurotrophic factor; SCID = Structured Clinical Interview of DSM Disorders; sIGF-1= serum insulin-like growth factor; SNP = single nucleotide polymorphism; SSRI = serotonin reuptake inhibitors; sTBARS = serum thiobarbituric acid-reactive substances; sVEGF= vascular endothelial growth factor; TAU = treatment as usual;TNF-α= tumor necrosis factor α; TSST = Trier Social Stress Test; Val = Valine; VO2max = maximal oxygen uptake; wk(s) = week(s); y/o = years old; 1-RM = 1 repetition maximum; 10-RM = 10 repetition maximum; τ=Kendall’s tau rank correlation coefficient; rs= spearman’s rank correlation coefficient; Δ= change; β = multiple linear regression beta coefficient; R2 = coefficient of determination ; ←→↓↑= general direction of change in outcome measure, not an indication of statistical significance
Although exercise may acutely stimulate 5-HT/NE, BDNF, or immuno-inflammation, evidence supporting the ability of exercise training to chronically and reliably change such mechanisms in depressed cohorts is lacking. Consequently, as will be described below, the mechanisms by which exercise elicits anti-depressant effects remain hypothetical, despite its robust clinical effects. While the aforementioned mechanisms do not appear to singularly mediate the antidepressant benefits of exercise, they may serve complementary roles to other putative mechanisms (e.g., kynurenine). The heterogenous pathophysiology of depression coupled with the complex “molecular choreography” (as described by Contrepois and colleagues123) of exercise suggests the need to consider how exercise may influence multiple biological processes rather than the now common practice of characterizing the effects of exercise on one or a few such processes (see Figure 1).
Figure 1.
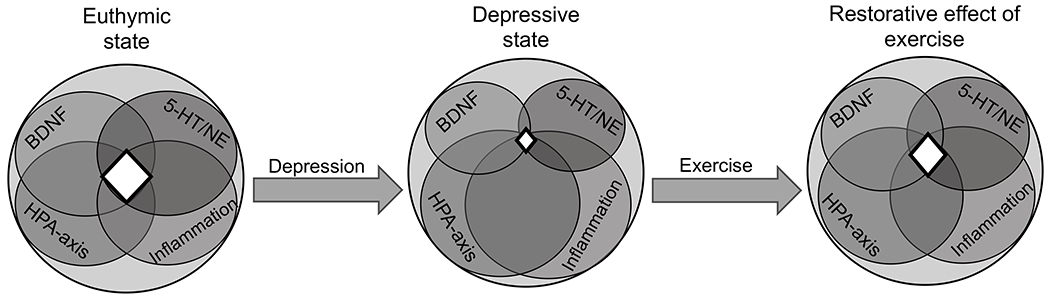
Early conceptual model of the effect of exercise on depression.
Circles represent the theoretical relative contribution of each mechanism and how each may change from euthymia to depression (and then restored by exercise). White shaded area represents a theoretical level of homeostasis amongst such mechanisms.
BDNF, 5-HT/NE, inflammation, and the HPA-axis have each been identified as possible candidate neurobiological mechanisms/pathways involved in depression. In a euthymic state, these implicated mechanisms function appropriately, resulting in homeostasis (white shaded area). While the interaction between these mechanisms and their scaled contributions remains unclear, there is a disruption of homeostasis in depression as abnormalities associated with these mechanisms have been identified: reduced BDNF and 5HT/NE signaling, high levels of inflammation, and HPA-axis dysfunction. Since targeting one specific mechanism or pathway has not been found to completely ameliorate depression, a proposed conceptual approach is to view these mechanisms as a whole working system. Exercise is a viable contender to address multiple aspects of the biological underpinnings of depression. Specifically, aerobic exercise may deliver antidepressant effects via its ability to potentially modulate BDNF, 5HT-NE, inflammation, and HPA-axis function and help restore homeostasis amongst such mechanisms. Resistance exercise may deliver similar benefits, however it remains a scantly studied and underutilized mode of exercise for the treatment of depression.
Antidepressant effects of exercise - do proposed mechanisms predict/mediate clinical outcomes?
The pre-clinical and clinical evidence discussed above suggests that exercise may have both acute and chronic effects on a range of markers that index putative neurobiological mechanisms that influence brain health and function. Similarly, a substantial number of randomized controlled trials demonstrate the antidepressant benefits of exercise as both a monotherapy21, 23, 50 and adjunctive therapy.58, 71, 157 Identification of neurobiological mechanisms that predict or mediate the response to antidepressant treatment remains an area of high interest yet elusive due to the complex nature of depression pathophysiology and lack of evidence supporting the effects of exercise on candidate mechanisms in depressed cohorts. Biomarker profiles representing several aspects of the underlying mechanisms of depression (i.e., neuroendocrine, neurotrophin, immuno-inflammation) have been examined in response to first-line pharmacotherapy treatment (SSRIs and SNRIs), providing insight into the mechanisms by which pharmacotherapy may reduce depression. Meta-analytic evidence indicates that pharmacotherapy reduces pro-inflammatory cytokines IL-6, IL-1β, TNF-α, and anti-inflammatory cytokine IL-10, and enhances BDNF.99, 158–160 However, reliable treatment efficacy biomarkers for pharmacotherapy have yet to be identified. Similarly, exercise may reduce depression via anti-inflammatory effects and enhancement of BDNF yet the link between these putative etiologic factors and clinical outcomes (e.g., remission) remains suggestive/equivocal.161 Although a limited number of clinical trials51, 117, 139, 153–156, 162–169 (see Table 6) examining the potential coupling of exercise-induced changes in neurobiological mechanisms and clinical outcomes have not yielded a reliable treatment biomarker, the results can be used to generate new hypotheses and inform future clinical trial design.
Identifying neurobiological phenotypes (e.g., single or combination of mechanisms – patient with low BDNF, reduced 5-HT/NE, hyperactive HPA-axis, elevated inflammation) that are most responsive to exercise will be useful in guiding the future development of exercise as a therapeutic option for depression. This is an emerging area of investigation with relatively few clinical trials examining both clinical outcomes and molecular mechanisms. Therefore, we expanded our search to include intervention trials that were uncontrolled and/or non-randomized with all other search criteria utilized in the ‘Clinical Outcomes’ section remaining. The effects of exercise on several molecular mechanisms have been examined in clinically depressed cohorts, including monoamines, HPA-axis function, BDNF, and a series of inflammatory molecules. Carneiro and colleagues reported that 16 weeks of an AEx intervention for MDD reduced depressive symptoms but elicited no significant effect on 5-HT, NE, or cortisol, and there did not appear to be a relationship between reduction in depression and monoamine or cortisol response.59, 117 Krogh et al. (2010) found no significant effect of four months AEx or REx, on resting cortisol and cortisol response to a maximal exercise test. However, the relationship between changes in cortisol and depression was not reported.162 Similarly, Gerber and colleagues reported that 6 weeks of AEx training for inpatients with MDD reduced depressive symptoms but had no significant impact on cortisol response to a stress reactivity test and there was no relationship between changes in depression and cortisol reactivity.169 These studies appear to be the extent of the literature exploring the effect of exercise training on monoamines or HPA-axis function and clinical outcomes in MDD. The commonly examined mechanisms appear to be BDNF and inflammatory processes, thus the focus here will be on such mechanisms and their relationship to clinical response to pharmacotherapy and exercise.
Although pharmacotherapy targets 5-HT and NE, it also appears to enhance resting peripheral BDNF.98 Higher levels of pre-treatment serum BDNF have been associated with greater reductions in depressive symptoms following SNRI or SSRI treatment in medication-free subjects with MDD, although changes in BDNF and depressive symptoms at post-treatment assessment were not associated.170, 171 Similarly, the predictive value of resting BDNF on post-treatment depression has also been reported in the exercise literature. Toups and colleagues examined the effects of 12 weeks of adjunctive low dose (4 kcal/kg/wk.) or high dose (16 kcal/kg/wk.) exercise on serum BDNF and depressive symptoms in SSRI-treated subjects with MDD.163 There was no significant increase in BDNF in either group, and no association between changes in BDNF and changes in depression. However, subjects with higher baseline serum BDNF demonstrated more rapid decline in depressive symptoms compared to those with lower serum BDNF irrespective of exercise dose. This may suggest that pre-emptively enhancing BDNF, in this case via pharmacotherapy, prior to beginning exercise training may moderate the antidepressant response to exercise.163 This study highlights the role of exercise as an adjunctive treatment that can serve alongside first line medications to manage depression. Other clinical trials examining exercise as a monotherapeutic164 or adjunctive treatment61, 139, 165–168 for MDD have yielded inconsistent results with respect to BDNF. Several investigations in MDD indicate that exercise training does not significantly enhance resting BDNF61, 139, 164, 165 compared to a non-exercise condition. In contrast, others report an enhancement in BDNF following exercise training,166, 168 but changes in depression do not appear to be strongly, if at all, correlated with changes in BDNF (see Table 6).61, 139, 163, 166, 167, 172 Therefore, the coupling of enhanced BDNF and reduction in depression in response to either exercise, or pharmacotherapy, is not well supported. While BDNF does not appear to be a reliable biomarker of treatment efficacy, basal levels appear to have at least some predictive value in pharmacotherapy170, 171 and exercise163, 173 treatments for MDD. Future trials may consider this in design or analysis methods or consider the potential for ‘priming’ BDNF with pharmacotherapy prior to exercise treatment to enhance response.
Immuno-inflammatory pathways appear to be a shared target of pharmacotherapeutic and exercise treatments for MDD. C-reactive protein (CRP), a marker of inflammation, is elevated in medication-naïve MDD subjects compared to non-depressed control subjects, indicating that system-level inflammation is involved in MDD pathophysiology.142 Although it does not appear that CRP is reduced with pharmacotherapy,174 CRP appears to inform pharmacotherapy treatment selection rather than serving as a biomarker of treatment efficacy.175, 176 Specifically, higher levels of CRP (≥ 1mg/L) indicate better response to tricyclic antidepressants175 or combined SSRI and bupropion176 treatment compared to SSRI monotherapy. Conversely, lower levels of pre-treatment CRP (< 1mg/L) indicate better response to SSRI monotherapy.175 Other inflammatory markers have been shown to respond differently to SSRI or SNRI therapies. Carboni et al. examined immuno-inflammatory pathways as potential prognostic or efficacy biomarkers of treatment with paroxetine (SSRI) or venlafaxine (SNRI).177 For paroxetine treatment, higher baseline TNF-α and IL-10 levels were associated with better response to treatment whereas for venlafaxine treatment lower levels of baseline CRP were associated with greater reductions in depressive symptoms. Increases in TNF-α (r = −0.22), IL-6 (r = −0.23), IL-10 (r = −0.23), and CRP (r = −0.30) over the paroxetine treatment period were associated with reductions in depression whereas there were no significant relationships between changes in immuno-inflammatory markers and depressive symptoms for those treated with venlafaxine. These findings are somewhat contradictory to evidence indicating that SSRIs reduce pro-inflammatory markers, specifically TNF-α, IL-6, IL-1β, and anti-inflammatory marker IL-10.99, 174 Consequently, whether inflammatory markers can serve as predictors of treatment outcome remains an important area of further investigation.
Evidence examining the effects of exercise on CRP in MDD is limited. Krogh and colleagues (2012) reported no significant differences in post-treatment CRP following 12 weeks of either AEx or control stretching51 and reported no significant relationship of change in CRP and change in depressive symptoms.153 Similarly, Euteneuer and colleagues reported that 16 weeks of combined exercise and cognitive behavioral therapy (CBT) significantly reduced depressive symptoms, although there was no significant effect on CRP.154 When all study subjects (Exercise [Ex] + CBT, low energy activity + CBT, waitlist; see Table 6 for descriptions) were stratified by CRP levels (≥ 1μg/ml or < 1 μg/ml), subjects with higher CRP levels ≥ 1μg/ml that received Ex + CBT had significantly reduced CRP at 16 weeks compared to those receiving other interventions. Ex + CBT significantly enhanced anti-inflammatory IL-10 at 8 and 16 weeks compared to the non-exercise treatment arms, however the Ex + CBT and low energy activity + CBT arms both demonstrated comparable reductions in depressive symptoms. The combined results on CRP and IL-10 may suggest that although exercise elicits anti-inflammatory and antidepressant effects, the two may occur independently of one another. These markers may be useful in identifying subgroups that may be most responsive to exercise treatment rather than biomarkers of exercise treatment efficacy. Rethorst and colleagues reported higher levels of TNF-α predicted more rapid reductions in MDD symptoms following either a low or high dose exercise intervention.156 Furthermore, reductions in IL-1β were significantly associated with reductions in MDD symptoms and although this relationship appeared to be driven by the high dose exercise group, a true dose-response in IL-1β was not observed. Baseline levels of IL-6 did not predict response to exercise treatment, nor were changes in IL-6 associated with changes in MDD symptoms.156 Conversely, Lavebratt and colleagues reported higher baseline levels of IL-6 were associated (β = −0.19) with greater reductions in depressive symptoms following 12 weeks of low (~54% maximum heart rate [MHR]), moderate (~70% MHR), or vigorous (~76% MHR) intensity AEx. Furthermore, from pre- to post-intervention, changes in IL-6 were positively associated (β = 0.19) with changes in depressive symptoms indicating that greater reductions in IL-6 were associated greater reductions in depression.155
Taken together, CRP, IL-6, IL-10, TNF-α, and IL-1β may provide insight into the anti-inflammatory effects of exercise although the paucity of evidence, specifically in MDD, does not allow for firm conclusions.154–156 The results from these trials, and others in non-MDD, suggest that exercise can attenuate inflammation104, 178 and enhance anti-inflammatory molecules179 although the role that such effects serve in reducing depression remains unclear. Pharmacotherapy has the potential to influence 5-HT/NE, BDNF, HPA-axis, and immuno-inflammation but was utilized inconsistently across all studies including several that excluded subjects receiving such treatment.51, 153, 164 Furthermore, conditions such as diabetes or cardiovascular disease, often comorbid to depression, were only explicitly excluded from some studies.61, 166 Others assessed the presence of risk factors for such conditions via Physical Activity Readiness Questionnaire,139, 165, 168 however the majority of studies in this section either did not formally exclude or assess cardiovascular or metabolic disease. Reduced levels of circulating BDNF,180 elevated levels of inflammatory markers,181 and HPA-axis dysfunction182 have been implicated in diabetes and serotonin signaling appears to play a role in glucose metabolism.183 Cardiovascular disease is positively associated with increased inflammation (IL-6 related pathways),184 and inversely associated with circulating BDNF.185 Attenuated HPA-axis reactivity and increased systemic inflammation (i.e. elevated CRP) have been demonstrated in response to an acute bout of exercise in patients with coronary artery disease.186, 187 It is possible that discrepant findings between studies may be attributable to the heterogeneity of depression and confounding variables such as medical comorbidities and pharmacotherapy status. Controlling for such potential confounders is of paramount importance in future mechanistic studies seeking to explore the exercise-depression phenomenon.
Identifying the mechanisms by which exercise attenuates depressive symptoms will be vital in further establishing exercise as an adjunct to traditional antidepressant therapies and most importantly, naturally provide valuable insight into the pathophysiology of depression. Exercise may target mechanisms that are not enhanced via pharmacotherapy (e.g., IL-10), perhaps leading to a more robust antidepressant response when the treatments are combined. Furthermore, this work will also facilitate the identification of subgroups that may be most responsive to exercise (treatment matching), optimal dosing of exercise, and may generate novel, non-traditional combination treatments for depression such as exercise as an adjunct to repetitive transcranial magnetic stimulation (rTMS),188 ECT,189 or ketamine-induced NMDAR antagonism.190
Conclusions
Current evidence indicates that exercise is as effective as pharmacotherapy in reducing depressive symptoms in individuals with depression.46, 47 Aerobic exercise is the most commonly deployed mode of exercise treatment, likely due to the ease of access (little to no equipment required) and ease of intensity prescription (Karvonen heart rate method). Resistance exercise can reduce depressive symptoms, although compared to AEx, it remains infrequently studied. Limited evidence indicates that both AEx and REx elicit comparable antidepressant effects and ideally a combination of both AEx and REx should be prescribed to ensure improvements in cardiovascular and muscular fitness. Exercise should be employed regularly, 3 to 5 sessions per week, for 45 to 60 minutes per session, and at a moderate to vigorous intensity although this may be adjusted based on a given individual’s initial fitness level. This recommended frequency, intensity, and time is supported directly by previous reviews and meta-analyses,40, 41, 45, 80, 81 public health guidelines,44 and a cross sectional analysis of exercise habits and mental health burden of 1.2 million people.39 As it currently stands, exercise interventions deployed in randomized controlled trials are not regularly meeting the recommended public health thresholds of 150 minutes of moderate intensity AEx or 75 minutes of vigorous intensity AEx and two days of REx (Table 5.) Additionally, exercise prescription parameters (i.e., frequency, intensity, time, type) are not consistently reported across studies making precise study replication or clinical application challenging.
Regarding the neurobiological effects of exercise, there are a multitude of mechanisms or pathways by which exercise may attenuate depression. Aerobic exercise can acutely modulate 5-HT/NE, BDNF, TNF-α, IL-6, and IL-10 yet regular AEx training has not been found to reliably alter resting levels of any such mechanisms in depressed cohorts. The acute and chronic effects of REx on 5-HT/NE, BDNF, HPA-axis function, or immuno-inflammation in clinical cohorts with depression has not been examined. Clinical evidence does not indicate a single mechanism or marker that changes reliably with depression (IL-6 and IL-1β had mild associations with reduction in depressive symptoms in two studies155, 156), and BDNF, IL-6, IL-10, and CRP may be better predictors of responders to treatment than biomarkers of treatment efficacy. It may be the case that traditional methods for studying the pathophysiology of exercise and depression, separately or conjointly, are simply too insensitive to properly characterize the shared and independent causal matrices involved in both. A new approach that harnesses some of the most recent developments in systems biology may be what is needed. Case in point, Contrepois and colleagues published the most extensive molecular study of the acute effects of exercise.123 This study examined system-wide (metabolic, cardiovascular, immune) molecular response to a cardio-pulmonary exercise test in individuals with varying levels of insulin resistance/sensitivity. Amongst a host of novel findings, they report a “fitness inflammatory signature” at fifteen minutes post-exercise in which individuals with greater demonstrated higher levels of inflammation. This appeared to be driven by an acute increase in IL-5 at two minutes post-exercise, which correlated with a marked increase in fourteen inflammatory molecules, “centered on IL-1β,” at 15 minutes post-exercise.123 This characterization of the post-exercise inflammatory response may be a sign of adaptation to exercise training and a marker of physical fitness (indicated by higher ) and IL-5 and IL-1β may be key mediators/regulators of the chronic anti-inflammatory benefits of exercise. Interestingly, individuals classified as insulin resistant demonstrated a prolonged post-exercise inflammatory response in which TNF-α and IL-6 returned to baseline levels 1 hour following exercise, whereas in insulin sensitive individuals both TNF-α and IL-6 returned to baseline levels 15 minutes following exercise. This perhaps indicates a dysregulated inflammatory response that occurs during metabolic disease processes. Given the association of depression with insulin resistance,191 this may be a shared mechanism in the pathogenesis of both disorders and could serve as a target for exercise-based interventions for insulin resistance and depression. While we have summarized only a few key aspects and outcomes of this investigation, the broader findings attest to the complexity of the overall molecular response to exercise. Such methods would be valuable in disentangling the complex relationship of 5-HT/NE, BDNF, HPA-axis function, immuno-inflammation and the antidepressant benefits of exercise and provide valuable information regarding the underlying pathophysiology of depression.
Despite the development of pharmacotherapeutic, psychotherapeutic, and non-invasive brain stimulation (i.e., rTMS) anti-depressant treatments, depression has remained a leading cause of disability and global health burden for decades. Thus, there is a dire need to re-evaluate current depression treatment paradigms. Pharmacotherapy is too often ineffective and can produce substantial side effects, whereas psychotherapy is costly, time intensive, and requires highly trained providers. Similarly, rTMS treatments, which are currently only approved for treatment-resistant depression, are costly and require access to specialized clinics and highly trained providers. Conversely, exercise treatment for depression is universally accessible, can be implemented at a fraction of the cost of other anti-depressant treatments, and produces a host of health benefits. Exercise applications for mobile devices are extremely comprehensive with respect to information content and further increase access to both AEx and REx. However, despite the clinical evidence indicating that exercise is as effective as pharmacotherapy and its viability as a first-line adjunct to pharmacotherapy and psychotherapy, exercise remains an afterthought in the treatment of depression. Although the underlying molecular mechanisms by which exercise produces anti-depressant benefits are not well understood, it is no less understood than other treatment approaches. The molecular elements (i.e., myokines) of the robust response to exercise likely hold the key to the anti-depressant and overall health benefits of exercise and warrant further study.
Exercise is best conceptualized as an infrequently deployed but potent weapon for depression and it is essential that its use becomes a more common feature of the treatment landscape; its continued underutilization represents a serious missed opportunity to treat the ‘depresso-genic’ complex that drives depression. This is not a call for exercise to replace existing therapeutic strategies but for exercise to be consistently integrated as an additional first-line treatment for depression. The potent biological effects of exercise can serve as a catalyst to enhance outcomes in those treated with pharmacotherapy or psychotherapy. Conversely, we recognize that lack of motivation, low energy, or fatigue may be common reasons to avoid incorporating exercise into a treatment plan. In this case, pharmacotherapy or psychotherapy can serve as a catalyst to improve depression and subsequently exercise can be introduced as a lifestyle change to combat relapse to depression. Additionally, exercise will also aid in attenuating cardiovascular or metabolic disease risk factors, which are often comorbid with depression. Incorporating exercise into the continuum of care for depression will require a multidisciplinary approach (psychiatry, psychology, physical therapy, and exercise physiology) where integrated care/coaching will provide the synergy needed to more successfully counter the burden of suffering meted out by depression.
Future Directions
We believe that the state of the art with respect to exercise’s effects on depression points to very broad conclusions: One, both resistance and aerobic exercise have favorable effects on depression and two, that the molecular/cellular underpinnings of these effects are not at all well understood. The latter epistemic shortfall will constrain efforts to optimize exercise’s effects on depression. Building a knowledge base about the molecular mechanisms of exercise’s broadly antidepressant effects (and most other aspects of human flourishing) can be achieved, at least in part, by overcoming two major problems with the current approach to the science of exercise: 1) a lack of integrated multidisciplinary team science and 2) a limit in the scale of public funding mechanisms. The former problem could be overcome by engaging a national community of basic and clinical scientists to build knowledge about the biological mechanisms (omics) of exercise (cf., Molecular Transducers of Physical Activity Consortium; motrpac.org) and its effects on important mental health liabilities (starting with depression but not restricted to it) while the latter problem could be resolved through a trans-Institutional NIH funding initiative to support a nationwide “scaling up” of science (the NIH Common Fund). While these two obstacles plague the landscape of all branches of basic and applied science, there are large-scale public health initiatives in existence that could serve as a model to advance the understanding and treatment of depression with exercise, thereby significantly reducing the influence of these obstacles.
One such model began in 1999/2000 when the National Institute on Drug Abuse (NIDA/NIH) initiated the National Drug Abuse Treatment Clinical Trials Network or CTN. This national research consortium was commissioned in response to the Institute of Medicine (1998) recommendations to bridge the gap between the research and practice of drug/alcohol abuse treatment. It now consists of 16 academic research centers or nodes across the US (e.g., Johns Hopkins, UCLA, University of Miami, etc.), each of which was connected to multiple community-based treatment centers. The goal was to efficiently test substance abuse treatments on a large scale and across geographically diverse regions of the country. Applying this template to the exercise treatment of depression would involve the establishment of national consortium of multidisciplinary research centers whose primary function is to characterize the neurobiological footprint of exercise while systematically/parametrically evaluating exercises effects on a spectrum of mood-related disorders (Figure 2). The involvement of a national network of academic centers in these efforts would ensure that the necessary systems biology approach (i.e., genomics, proteomics, metabolomics, etc.) would be applied to studying causal factors. Synergistically building on this mechanism focused research platform, the same national network of academic centers would perform efficacy and effectiveness focused clinical trials of (a) individual exercise treatments (e.g., short- and long-term effects of AEx or REx on depression), (b) combination exercise treatments (separate and interactive effects of AEx and REx on depression), and (c) mixed treatment types (exercise, diet, psychotherapy, pharmacotherapy, non-invasive brain stimulation). Until this scale of effort is made, the quality of conclusions that can be derived from research on exercise’s role in combating depression will remain obscured by the often irreconcilable, silo-produced patchwork of research findings that define the current state of the art.
Figure 2.
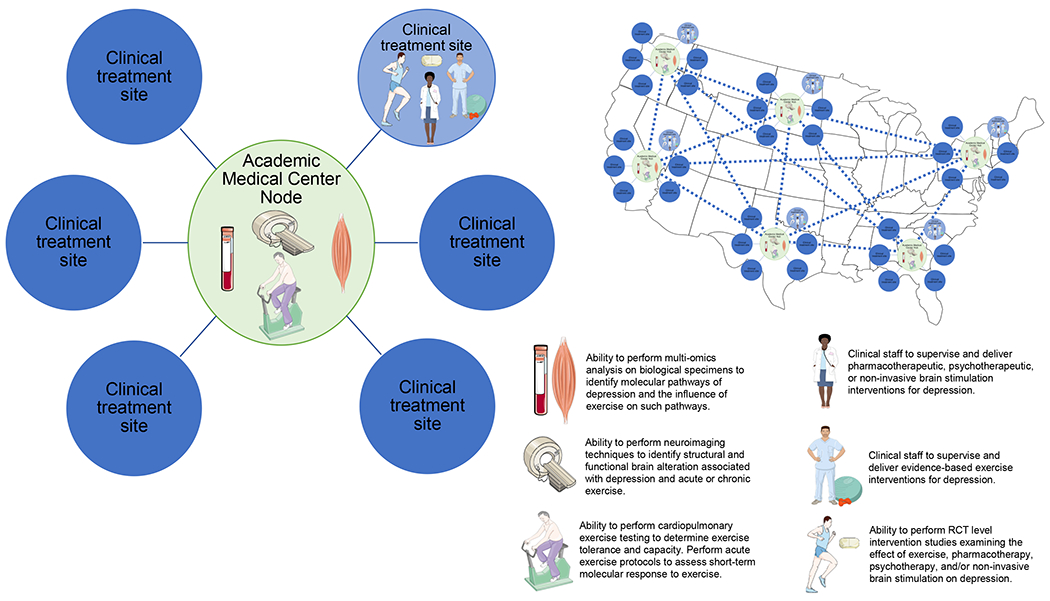
Proposed solution to advance the state of the art of the mechanistic understanding of exercise treatment for depression.
This proposed next generation research network would utilize the National Institute of Drug Abuse Clinical Trials Network (CTN) and the Molecular Transducers of Physical Activity Consortium (MoTrPAC) as models to advance the science of depression and the interface of exercise and depression. Each node will be an academic medical center with the capability of providing support to obtain, store and analyze biospecimens (i.e., ‘omics analyses). Outlying each node will be clinical treatment sites which will deliver singular exercise treatments (AEx, REx), combination exercise treatments (AEx and REx), or mixed treatments (exercise with nutritional counseling, psychotherapy, pharmacotherapy, or non-invasive brain stimulation). This model provides the infrastructure needed to perform large scale clinical trials while obtaining the biological data needed to elucidate the molecular mechanisms underlying depression, exercise, and the treatment response to exercise-based interventions. Ultimately this model can be leveraged to explore other mental health liabilities, develop novel treatments for such liabilities, and reduce the overall burden of mental health liabilities. Portion of illustration created with Servier Medical Art (smart.servier.com).
Funding
This work was supported by grants from the United States Department of Veterans Affairs ([RER] grant number RR&D IK1 RX002962), Craig H. Neilsen Foundation (CJV), and National Institutes of Health ([CMG] grant number R01HD095137). The views in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or United States government.
Footnotes
Competing Interests
The authors have no conflicts of interest to declare.
References
- 1.American Psychiatric Association., American Psychiatric Association. DSM-5 Task Force. Diagnostic and statistical manual of mental disorders : DSM-5. 5th edn. American Psychiatric Association: Washington, D.C., 2013, xliv, 947 ppp. [Google Scholar]
- 2.Depression and Other Common Mental Disorders: Global Health Estimates. vol. Licence:CC BY-NC-SA 3.0 IGO. World Health Organization: Geneva, 2017, pp Licence:CC BY-NC-SA 3.0 IGO. [Google Scholar]
- 3.Collaborators GDaIIaP. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016; 388(10053): 1545–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finley CR, Chan DS, Garrison S, Korownyk C, Kolber MR, Campbell S et al. What are the most common conditions in primary care? Systematic review. Can Fam Physician 2018; 64(11): 832–840. [PMC free article] [PubMed] [Google Scholar]
- 5.Rajan S, McKee M, Rangarajan S, Bangdiwala S, Rosengren A, Gupta R et al. Association of Symptoms of Depression With Cardiovascular Disease and Mortality in Low-, Middle-, and High-Income Countries. JAMA Psychiatry 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen PC, Chan YT, Chen HF, Ko MC, Li CY. Population-based cohort analyses of the bidirectional relationship between type 2 diabetes and depression. Diabetes Care 2013; 36(2): 376–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith DJ, Court H, McLean G, Martin D, Langan Martin J, Guthrie B et al. Depression and multimorbidity: a cross-sectional study of 1,751,841 patients in primary care. J Clin Psychiatry 2014; 75(11): 1202–1208; quiz 1208. [DOI] [PubMed] [Google Scholar]
- 8.Cuijpers P, Smit F. Excess mortality in depression: a meta-analysis of community studies. J Affect Disord 2002; 72(3): 227–236. [DOI] [PubMed] [Google Scholar]
- 9.Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. The American Journal of Psychiatry 2006; 163(1): 28–40. [DOI] [PubMed] [Google Scholar]
- 10.Rush AJ, Trivedi MH, Wisniewski SR, Stewart JW, Nierenberg AA, Thase ME et al. Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. The New England journal of medicine 2006; 354(12): 1231–1242. [DOI] [PubMed] [Google Scholar]
- 11.Kessler RC. The costs of depression. The Psychiatric clinics of North America 2012; 35(1): 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Birnbaum HG, Kessler RC, Kelley D, Ben-Hamadi R, Joish VN, Greenberg PE. Employer burden of mild, moderate, and severe major depressive disorder: mental health services utilization and costs, and work performance. Depress Anxiety 2010; 27(1): 78–89. [DOI] [PubMed] [Google Scholar]
- 13.Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep 1985; 100(2): 126–131. [PMC free article] [PubMed] [Google Scholar]
- 14.Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, Minson CT, Nigg CR, Salem GJ et al. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Medicine and science in sports and exercise 2009; 41(7): 1510–1530. [DOI] [PubMed] [Google Scholar]
- 15.Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR et al. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care 2010; 33(12): e147–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Medicine and science in sports and exercise 2011; 43(7): 1334–1359. [DOI] [PubMed] [Google Scholar]
- 17.Williams MA, Haskell WL, Ades PA, Amsterdam EA, Bittner V, Franklin BA et al. Resistance exercise in individuals with and without cardiovascular disease: 2007 update: a scientific statement from the American Heart Association Council on Clinical Cardiology and Council on Nutrition, Physical Activity, and Metabolism. Circulation 2007; 116(5): 572–584. [DOI] [PubMed] [Google Scholar]
- 18.Scott KM, Lim C, Al-Hamzawi A, Alonso J, Bruffaerts R, Caldas-de-Almeida JM et al. Association of Mental Disorders With Subsequent Chronic Physical Conditions: World Mental Health Surveys From 17 Countries. JAMA Psychiatry 2016; 73(2): 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pedersen BK. Physical activity and muscle-brain crosstalk. Nat Rev Endocrinol 2019; 15(7): 383–392. [DOI] [PubMed] [Google Scholar]
- 20.Singh NA, Clements KM, Fiatarone MA. A randomized controlled trial of progressive resistance training in depressed elders. J Gerontol A Biol Sci Med Sci 1997; 52(1): M27–M35. [DOI] [PubMed] [Google Scholar]
- 21.Dunn AL, Trivedi MH, Kampert JB, Clark CG, Chambliss HO. Exercise treatment for depression: efficacy and dose response. Am J Prev Med 2005; 28(1): 1–8. [DOI] [PubMed] [Google Scholar]
- 22.Singh NA, Stavrinos TM, Scarbek Y, Galambos G, Liber C, Fiatarone Singh MA. A randomized controlled trial of high versus low intensity weight training versus general practitioner care for clinical depression in older adults. J Gerontol A Biol Sci Med Sci 2005; 60(6): 768–776. [DOI] [PubMed] [Google Scholar]
- 23.Blumenthal JA, Babyak MA, Doraiswamy PM, Watkins L, Hoffman BM, Barbour KA et al. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosomatic medicine 2007; 69(7): 587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wewege MA, Thom JM, Rye KA, Parmenter BJ. Aerobic, resistance or combined training: A systematic review and meta-analysis of exercise to reduce cardiovascular risk in adults with metabolic syndrome. Atherosclerosis 2018; 274: 162–171. [DOI] [PubMed] [Google Scholar]
- 25.Ashton RE, Tew GA, Aning JJ, Gilbert SE, Lewis L, Saxton JM. Effects of short-term, medium-term and long-term resistance exercise training on cardiometabolic health outcomes in adults: systematic review with meta-analysis. Br J Sports Med 2020; 54(6): 341–348. [DOI] [PubMed] [Google Scholar]
- 26.Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci 2007; 30(9): 464–472. [DOI] [PubMed] [Google Scholar]
- 27.Herold F, Törpel A, Schega L, Müller NG. Functional and/or structural brain changes in response to resistance exercises and resistance training lead to cognitive improvements - a systematic review. Eur Rev Aging Phys Act 2019; 16: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tipton CM. The history of “Exercise Is Medicine” in ancient civilizations. Advances in Physiology Education 2014; 38(2): 109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berryman JW. Motion and rest: Galen on exercise and health. The Lancet 2012; 380(9838): 210–211. [DOI] [PubMed] [Google Scholar]
- 30.Bate J The Anatomy of Melancholy revisited. The Lancet 2017; 389(10081): 1790–1791. [Google Scholar]
- 31.Knudson AB, Davis JE. Medically prescribed exercises for neuropsychiatric patients; the Veterans Administration program. J Am Med Assoc 1949; 140(13): 1090–1095. [DOI] [PubMed] [Google Scholar]
- 32.Morgan WP. Selected physiological and psychomotor correlates of depression in psychiatric patients. Res Q 1968; 39(4): 1037–1043. [PubMed] [Google Scholar]
- 33.Morgan WP. A pilot investigation of physical working capacity in depressed and nondepressed psychiatric males. Res Q 1969; 40(4): 859–861. [PubMed] [Google Scholar]
- 34.Morgan WP. Physical working capacity in depressed and non-depressed psychiatric females: a preliminary study. Am Correct Ther J 1970; 24(1): 14–16. [PubMed] [Google Scholar]
- 35.Morgan WP, Roberts JA, Brand FR, Feinerman AD. Psychological effect of chronic physical activity. Med Sci Sports 1970; 2(4): 213–217. [PubMed] [Google Scholar]
- 36.Greist JH, Klein MH, Eischens RR, Faris J, Gurman AS, Morgan WP. Running as treatment for depression. Compr Psychiatry 1979; 20(1): 41–54. [DOI] [PubMed] [Google Scholar]
- 37.Sexton H, Maere A, Dahl NH. Exercise intensity and reduction in neurotic symptoms. A controlled follow-up study. Acta Psychiatr Scand 1989; 80(3): 231–235. [DOI] [PubMed] [Google Scholar]
- 38.Doyne EJ, Ossip-Klein DJ, Bowman ED, Osborn KM, McDougall-Wilson IB, Neimeyer RA. Running versus weight lifting in the treatment of depression. J Consult Clin Psychol 1987; 55(5): 748–754. [DOI] [PubMed] [Google Scholar]
- 39.Chekroud SR, Gueorguieva R, Zheutlin AB, Paulus M, Krumholz HM, Krystal JH et al. Association between physical exercise and mental health in 1·2 million individuals in the USA between 2011 and 2015: a cross-sectional study. Lancet Psychiatry 2018; 5(9): 739–746. [DOI] [PubMed] [Google Scholar]
- 40.Pearce M, Garcia L, Abbas A, Strain T, Schuch FB, Golubic R et al. Association between physical activity and risk of depression: A systematic review and meta-analysis. JAMA Psychiatry 2022; 79(6): 550–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dishman RK, McDowell CP, Herring MP. Customary physical activity and odds of depression: a systematic review and meta-analysis of 111 prospective cohort studies. Br J Sports Med 2021; 55(16): 926–934. [DOI] [PubMed] [Google Scholar]
- 42.American College of Sports Medicine, Riebe D, Ehrman JK, Liguori G, Magal M. ACSM’s Guidelines for Exercise Testing and Prescription. 10 edn. Wolters Kluwer: Philadelphia, 2018. [Google Scholar]
- 43.Bull FC, Al-Ansari SS, Biddle S, Borodulin K, Buman MA- O, Cardon G et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med 2020; 54(24): 1451–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA et al. The physical activity guidelines for Americans. JAMA 2018; 320(19): 2020–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schuch FB, Vancampfort D, Richards J, Rosenbaum S, Ward PB, Stubbs B. Exercise as a treatment for depression: A meta-analysis adjusting for publication bias. J Psychiatr Res 2016; 77: 42–51. [DOI] [PubMed] [Google Scholar]
- 46.Kvam S, Kleppe CL, Nordhus IH, Hovland A. Exercise as a treatment for depression: A meta-analysis. J Affect Disord 2016; 202: 67–86. [DOI] [PubMed] [Google Scholar]
- 47.Cooney GM, Dwan K, Greig CA, Lawlor DA, Rimer J, Waugh FR et al. Exercise for depression. The Cochrane database of systematic reviews 2013; 9: CD004366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee J, Gierc M, Vila-Rodriguez F, Puterman E, Faulkner G. Efficacy of exercise combined with standard treatment for depression compared to standard treatment alone: A systematic review and meta-analysis of randomized controlled trials. J Affect Disord 2021; 295: 1494–1511. [DOI] [PubMed] [Google Scholar]
- 49.Stubbs B, Vancampfort D, Rosenbaum S, Ward PB, Richards J, Ussher M et al. Challenges establishing the efficacy of exercise as an antidepressant treatment: A systematic review and meta-analysis of control group responses in exercise Rrandomised controlled trials. Sports Med 2016; 46(5): 699–713. [DOI] [PubMed] [Google Scholar]
- 50.Blumenthal JA, Babyak MA, Moore KA, Craighead WE, Herman S, Khatri P et al. Effects of exercise training on older patients with major depression. Arch Int Med 1999; 159(19): 2349–2356. [DOI] [PubMed] [Google Scholar]
- 51.Krogh J, Videbech P, Thomsen C, Gluud C, Nordentoft M. DEMO-II trial. Aerobic exercise versus stretching exercise in patients with major depression-a randomised clinical trial. PLoS One 2012; 7(10): e48316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jacquart SD, Marshak HH, Dos Santos H, Luu SM, Berk LS, McMahon PT et al. The effects of simultaneous exercise and psychotherapy on depressive symptoms in inpatient, psychiatric older adults. Adv Mind Body Med 2014; 28(4): 8–17. [PubMed] [Google Scholar]
- 53.Oertel-Knochel V, Mehler P, Thiel C, Steinbrecher K, Malchow B, Tesky V et al. Effects of aerobic exercise on cognitive performance and individual psychopathology in depressive and schizophrenia patients. Eur Arch Psychiatry Clin Neurosci 2014; 264(7): 589–604. [DOI] [PubMed] [Google Scholar]
- 54.Kerling A, Tegtbur U, Gutzlaff E, Kuck M, Borchert L, Ates Z et al. Effects of adjunctive exercise on physiological and psychological parameters in depression: a randomized pilot trial. J Affect Disord 2015; 177: 1–6. [DOI] [PubMed] [Google Scholar]
- 55.Pilu A, Sorba M, Hardoy MC, Floris AL, Mannu F, Seruis ML et al. Efficacy of physical activity in the adjunctive treatment of major depressive disorders: preliminary results. Clin Pract Epidemiol Ment Health 2007; 3(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mota-Pereira J, Silverio J, Carvalho S, Ribeiro JC, Fonte D, Ramos J. Moderate exercise improves depression parameters in treatment-resistant patients with major depressive disorder. J Psychiatr Res 2011; 45(8): 1005–1011. [DOI] [PubMed] [Google Scholar]
- 57.Danielsson L, Papoulias I, Petersson EL, Carlsson J, Waern M. Exercise or basic body awareness therapy as add-on treatment for major depression: a controlled study. J Affect Disord 2014; 168: 98–106. [DOI] [PubMed] [Google Scholar]
- 58.Belvederi Murri M, Amore M, Menchetti M, Toni G, Neviani F, Cerri M et al. Physical exercise for late-life major depression. Br J Psychiatry 2015; 207(3): 235–242. [DOI] [PubMed] [Google Scholar]
- 59.Carneiro LS, Fonseca AM, Vieira-Coelho MA, Mota MP, Vasconcelos-Raposo J. Effects of structured exercise and pharmacotherapy vs. pharmacotherapy for adults with depressive symptoms: A randomized clinical trial. J Psychiatr Res 2015; 71: 48–55. [DOI] [PubMed] [Google Scholar]
- 60.Legrand FD, Neff EM. Efficacy of exercise as an adjunct treatment for clinically depressed inpatients during the initial stages of antidepressant pharmacotherapy: An open randomized controlled trial. Journal of Affective Disorders 2016; 191: 139–144. [DOI] [PubMed] [Google Scholar]
- 61.Salehi I, Hosseini SM, Haghighi M, Jahangard L, Bajoghli H, Gerber M et al. Electroconvulsive therapy (ECT) and aerobic exercise training (AET) increased plasma BDNF and ameliorated depressive symptoms in patients suffering from major depressive disorder. J Psychiatr Res 2016; 76: 1–8. [DOI] [PubMed] [Google Scholar]
- 62.Siqueira CC, Valiengo LL, Carvalho AF, Santos-Silva PR, Missio G, de Sousa RT et al. Antidepressant efficacy of adjunctive aerobic activity and associated biomarkers in major depression: A 4-Week, randomized, single-blind, controlled clinical trial. PLoS One 2016; 11(5): e0154195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gujral S, Aizenstein H, Reynolds CF 3rd, Butters MA, Grove G, Karp JF et al. Exercise for depression: A feasibility trial exploring neural mechanisms. Am J Geriatr Psychiatry 2019; 27(6): 611–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moraes HS, Silveira HS, Oliveira NA, Matta Mello Portugal E, Araújo NB, Vasques PE et al. Is strength training as effective as aerobic training for depression in older adults? A randomized controlled trial. Neuropsychobiology 2020; 79(2): 141–149. [DOI] [PubMed] [Google Scholar]
- 65.Martinsen EW, Medhus A, Sandvik L. Effects of aerobic exercise on depression: a controlled study. Br Med J (Clin Res Ed) 1985; 291(6488): 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Veale D, Le Fevre K, Pantelis C, de Souza V, Mann A, Sargeant A. Aerobic exercise in the adjunctive treatment of depression: a randomized controlled trial. J R Soc Med 1992; 85(9): 541–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Knubben K, Reischies FM, Adli M, Schlattmann P, Bauer M, Dimeo F. A randomised, controlled study on the effects of a short-term endurance training programme in patients with major depression. Br J Sports Med 2007; 41(1): 29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Krogh J, Saltin B, Gluud C, Nordentoft M. The DEMO trial: a randomized, parallel-group, observer-blinded clinical trial of strength versus aerobic versus relaxation training for patients with mild to moderate depression. J Clin Psychiatry 2009; 70(6): 790–800. [DOI] [PubMed] [Google Scholar]
- 69.Doose M, Ziegenbein M, Hoos O, Reim D, Stengert W, Hoffer N et al. Self-selected intensity exercise in the treatment of major depression: A pragmatic RCT. Int J Psychiatry Clin Pract 2015; 19(4): 266–275. [DOI] [PubMed] [Google Scholar]
- 70.Hallgren M, Kraepelien M, Öjehagen A, Lindefors N, Zeebari Z, Kaldo V et al. Physical exercise and internet-based cognitive-behavioural therapy in the treatment of depression: randomised controlled trial. Br J Psychiatry 2015; 207(3): 227–234. [DOI] [PubMed] [Google Scholar]
- 71.Schuch FB, Vasconcelos-Moreno MP, Borowsky C, Zimmermann AB, Rocha NS, Fleck MP. Exercise and severe major depression: effect on symptom severity and quality of life at discharge in an inpatient cohort. J Psychiatr Res 2015; 61: 25–32. [DOI] [PubMed] [Google Scholar]
- 72.Helgadóttir B, Hallgren M, Ekblom Ö, Forsell Y. Training fast or slow? Exercise for depression: A randomized controlled trial. Prev Med 2016; 91: 123–131. [DOI] [PubMed] [Google Scholar]
- 73.Olson RL, Brush CJ, Ehmann PJ, Alderman BL. A randomized trial of aerobic exercise on cognitive control in major depression. Clin Neurophysiol 2017; 128(6): 903–913. [DOI] [PubMed] [Google Scholar]
- 74.Buschert V, Prochazka D, Bartl H, Diemer J, Malchow B, Zwanzger P et al. Effects of physical activity on cognitive performance: a controlled clinical study in depressive patients. Eur Arch Psychiatry Clin Neurosci 2019; 269(5): 555–563. [DOI] [PubMed] [Google Scholar]
- 75.Chau RMW, Tsui AYY, Wong EYW, Cheung EYY, Chan DYC, Lau PMY et al. Effectiveness of a structured physical rehabilitation program on the physical fitness, mental health and pain for Chinese patients with major depressive disorders in Hong Kong - a randomized controlled trial with 9-month follow-up outcomes. Disabil Rehabil 2020: 1–11. [DOI] [PubMed] [Google Scholar]
- 76.Haussleiter IS, Bolsinger B, Assion HJ, Juckel G. Adjuvant guided exercise therapy versus self-organized activity in patients with major depression. J Nerv Ment Dis 2020; 208(12): 982–988. [DOI] [PubMed] [Google Scholar]
- 77.La Rocque CL, Mazurka R, Stuckless TJR, Pyke K, Harkness KL. Randomized controlled trial of bikram yoga and aerobic exercise for depression in women: Efficacy and stress-based mechanisms. J Affect Disord 2021; 280(Pt A): 457–466. [DOI] [PubMed] [Google Scholar]
- 78.Schulz JM, Birmingham TB, Atkinson HF, Woehrle E, Primeau CA, Lukacs MJ et al. Are we missing the target? Are we aiming too low? What are the aerobic exercise prescriptions and their effects on markers of cardiovascular health and systemic inflammation in patients with knee osteoarthritis? A systematic review and meta-analysis. Br J Sports Med 2020; 54(13): 771–775. [DOI] [PubMed] [Google Scholar]
- 79.Stubbs B, Vancampfort D, Rosenbaum S, Ward PB, Richards J, Soundy A et al. Dropout from exercise randomized controlled trials among people with depression: A meta-analysis and meta regression. J Affect Disord 2016; 190: 457–466. [DOI] [PubMed] [Google Scholar]
- 80.Rethorst CD, Trivedi MH. Evidence-based recommendations for the prescription of exercise for major depressive disorder. J Psychiatr Pract 2013; 19(3): 204–212. [DOI] [PubMed] [Google Scholar]
- 81.Stubbs B, Vancampfort D, Hallgren M, Firth J, Veronese N, Solmi M et al. EPA guidance on physical activity as a treatment for severe mental illness: a meta-review of the evidence and Position Statement from the European Psychiatric Association (EPA), supported by the International Organization of Physical Therapists in Mental Health (IOPTMH). Eur Psychiatry 2018; 54: 124–144. [DOI] [PubMed] [Google Scholar]
- 82.Firth J, Stubbs B, Vancampfort D, Schuch F, Lagopoulos J, Rosenbaum S et al. Effect of aerobic exercise on hippocampal volume in humans: A systematic review and meta-analysis. NeuroImage 2018; 166: 230–238. [DOI] [PubMed] [Google Scholar]
- 83.Schatzberg AF. Pharmacological principles of antidepressant efficacy. Hum Psychopharmacol 2002; 17 Suppl 1: S17–22. [DOI] [PubMed] [Google Scholar]
- 84.Delgado PL, Charney DS, Price LH, Aghajanian GK, Landis H, Heninger GR. Serotonin function and the mechanism of antidepressant action. Reversal of antidepressant-induced remission by rapid depletion of plasma tryptophan. Arch Gen Psychiatry 1990; 47(5): 411–418. [DOI] [PubMed] [Google Scholar]
- 85.Miller HL, Delgado PL, Salomon RM, Berman R, Krystal JH, Heninger GR et al. Clinical and biochemical effects of catecholamine depletion on antidepressant-induced remission of depression. Arch Gen Psychiatry 1996; 53(2): 117–128. [DOI] [PubMed] [Google Scholar]
- 86.Nutt DJ. The neuropharmacology of serotonin and noradrenaline in depression. Int Clin Psychopharmacol 2002; 17 Suppl 1: S1–12. [DOI] [PubMed] [Google Scholar]
- 87.Delgado PL, Price LH, Miller HL, Salomon RM, Aghajanian GK, Heninger GR et al. Serotonin and the neurobiology of depression. Effects of tryptophan depletion in drug-free depressed patients. Arch Gen Psychiatry 1994; 51(11): 865–874. [DOI] [PubMed] [Google Scholar]
- 88.Berman RM, Sanacora G, Anand A, Roach LM, Fasula MK, Finkelstein CO et al. Monoamine depletion in unmedicated depressed subjects. Biol Psychiatry 2002; 51(6): 469–473. [DOI] [PubMed] [Google Scholar]
- 89.Praschak-Rieder N, Wilson AA, Hussey D, Carella A, Wei C, Ginovart N et al. Effects of tryptophan depletion on the serotonin transporter in healthy humans. Biol Psychiatry 2005; 58(10): 825–830. [DOI] [PubMed] [Google Scholar]
- 90.Ruhé HG, Mason NS, Schene AH. Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: a meta-analysis of monoamine depletion studies. Mol Psychiatry 2007; 12(4): 331–359. [DOI] [PubMed] [Google Scholar]
- 91.Moncrieff J, Cooper RE, Stockmann T, Amendola S, Hengartner MP, Horowitz MA. The serotonin theory of depression: a systematic umbrella review of the evidence. Mol Psychiatry 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vreeburg SA, Hoogendijk WJ, van Pelt J, Derijk RH, Verhagen JC, van Dyck R et al. Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: results from a large cohort study. Archives of General Psychiatry 2009; 66(6): 617–626. [DOI] [PubMed] [Google Scholar]
- 93.Bus BA, Molendijk ML, Tendolkar I, Penninx BW, Prickaerts J, Elzinga BM et al. Chronic depression is associated with a pronounced decrease in serum brain-derived neurotrophic factor over time. Mol Psychiatry 2015; 20(5): 602–608. [DOI] [PubMed] [Google Scholar]
- 94.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry 2009; 65(9): 732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ronaldson A, Carvalho LA, Kostich K, Lazzarino AI, Urbanova L, Steptoe A. The effects of six-day SSRI administration on diurnal cortisol secretion in healthy volunteers. Psychopharmacology (Berl) 2018; 235(12): 3415–3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hinkelmann K, Moritz S, Botzenhardt J, Muhtz C, Wiedemann K, Kellner M et al. Changes in cortisol secretion during antidepressive treatment and cognitive improvement in patients with major depression: a longitudinal study. Psychoneuroendocrinology 2012; 37(5): 685–692. [DOI] [PubMed] [Google Scholar]
- 97.Matrisciano F, Bonaccorso S, Ricciardi A, Scaccianoce S, Panaccione I, Wang L et al. Changes in BDNF serum levels in patients with major depression disorder (MDD) after 6 months treatment with sertraline, escitalopram, or venlafaxine. Journal of psychiatric research 2009; 43(3): 247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Molendijk ML, Bus BA, Spinhoven P, Penninx BW, Kenis G, Prickaerts J et al. Serum levels of brain-derived neurotrophic factor in major depressive disorder: state-trait issues, clinical features and pharmacological treatment. Mol Psychiatry 2011; 16(11): 1088–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang L, Wang R, Liu L, Qiao D, Baldwin DS, Hou R. Effects of SSRIs on peripheral inflammatory markers in patients with major depressive disorder: A systematic review and meta-analysis. Brain Behav Immun 2019; 79: 24–38. [DOI] [PubMed] [Google Scholar]
- 100.Zhang JM, An J. Cytokines, inflammation, and pain. Int Anesthesiol Clin 2007; 45(2): 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Enache D, Pariante CM, Mondelli V. Markers of central inflammation in major depressive disorder: A systematic review and meta-analysis of studies examining cerebrospinal fluid, positron emission tomography and post-mortem brain tissue. Brain Behav Immun 2019; 81: 24–40. [DOI] [PubMed] [Google Scholar]
- 102.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 2008; 9(1): 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pedersen BK, Akerström TC, Nielsen AR, Fischer CP. Role of myokines in exercise and metabolism. J Appl Physiol (1985) 2007; 103(3): 1093–1098. [DOI] [PubMed] [Google Scholar]
- 104.Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol (1985) 2005; 98(4): 1154–1162. [DOI] [PubMed] [Google Scholar]
- 105.Pedersen BK. Muscles and their myokines. J Exp Biol 2011; 214(Pt 2): 337–346. [DOI] [PubMed] [Google Scholar]
- 106.Wen L, Jin Y, Li L, Sun S, Cheng S, Zhang S et al. Exercise prevents raphe nucleus mitochondrial overactivity in a rat depression model. Physiol Behav 2014; 132: 57–65. [DOI] [PubMed] [Google Scholar]
- 107.Lu Q, Mouri A, Yang Y, Kunisawa K, Teshigawara T, Hirakawa M et al. Chronic unpredictable mild stress-induced behavioral changes are coupled with dopaminergic hyperfunction and serotonergic hypofunction in mouse models of depression. Behav Brain Res 2019; 372: 112053. [DOI] [PubMed] [Google Scholar]
- 108.Ahmad A, Rasheed N, Banu N, Palit G. Alterations in monoamine levels and oxidative systems in frontal cortex, striatum, and hippocampus of the rat brain during chronic unpredictable stress. Stress 2010; 13(4): 355–364. [DOI] [PubMed] [Google Scholar]
- 109.Shen M, Yang Y, Wu Y, Zhang B, Wu H, Wang L et al. L-theanine ameliorate depressive-like behavior in a chronic unpredictable mild stress rat model via modulating the monoamine levels in limbic-cortical-striatal-pallidal-thalamic-circuit related brain regions. Phytother Res 2019; 33(2): 412–421. [DOI] [PubMed] [Google Scholar]
- 110.Wu GF, Ren S, Tang RY, Xu C, Zhou JQ, Lin SM et al. Antidepressant effect of taurine in chronic unpredictable mild stress-induced depressive rats. Sci Rep 2017; 7(1): 4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Daniele TMDC, de Bruin PFC, Rios ERV, de Bruin VMS. Effects of exercise on depressive behavior and striatal levels of norepinephrine, serotonin and their metabolites in sleep-deprived mice. Behav Brain Res 2017; 332: 16–22. [DOI] [PubMed] [Google Scholar]
- 112.Lee H, Ohno M, Ohta S, Mikami T. Regular moderate or intense exercise prevents depression-like behavior without change of hippocampal tryptophan content in chronically tryptophan-deficient and stressed mice. PLoS One 2013; 8(7): e66996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kiuchi T, Lee H, Mikami T. Regular exercise cures depression-like behavior via VEGF-Flk-1 signaling in chronically stressed mice. Neuroscience 2012; 207: 208–217. [DOI] [PubMed] [Google Scholar]
- 114.Otsuka T, Nishii A, Amemiya S, Kubota N, Nishijima T, Kita I. Effects of acute treadmill running at different intensities on activities of serotonin and corticotropin-releasing factor neurons, and anxiety- and depressive-like behaviors in rats. Behav Brain Res 2016; 298(Pt B): 44–51. [DOI] [PubMed] [Google Scholar]
- 115.Lechin F, van der Dijs B, Orozco B, Lechin ME, Báez S, Lechin AE et al. Plasma neurotransmitters, blood pressure, and heart rate during supine-resting, orthostasis, and moderate exercise conditions in major depressed patients. Biol Psychiatry 1995; 38(3): 166–173. [DOI] [PubMed] [Google Scholar]
- 116.Zimmer P, Stritt C, Bloch W, Schmidt FP, Hübner ST, Binnebößel S et al. The effects of different aerobic exercise intensities on serum serotonin concentrations and their association with Stroop task performance: a randomized controlled trial. Eur J Appl Physiol 2016; 116(10): 2025–2034. [DOI] [PubMed] [Google Scholar]
- 117.Carneiro LS, Mota MP, Vieira-Coelho MA, Alves RC, Fonseca AM, Vasconcelos-Raposo J. Monoamines and cortisol as potential mediators of the relationship between exercise and depressive symptoms. Eur Arch Psychiatry Clin Neurosci, vol. 267: Germany, 2017, pp 117–121. [DOI] [PubMed] [Google Scholar]
- 118.Payne JK, Held J, Thorpe J, Shaw H. Effect of exercise on biomarkers, fatigue, sleep disturbances, and depressive symptoms in older women with breast cancer receiving hormonal therapy. Oncol Nurs Forum 2008; 35(4): 635–642. [DOI] [PubMed] [Google Scholar]
- 119.Wipfli B, Landers D, Nagoshi C, Ringenbach S. An examination of serotonin and psychological variables in the relationship between exercise and mental health. Scand J Med Sci Sports 2011; 21(3): 474–481. [DOI] [PubMed] [Google Scholar]
- 120.Melancon MO, Lorrain D, Dionne IJ. Changes in markers of brain serotonin activity in response to chronic exercise in senior men. Appl Physiol Nutr Metab 2014; 39(11): 1250–1256. [DOI] [PubMed] [Google Scholar]
- 121.Savitz J The kynurenine pathway: a finger in every pie. Mol Psychiatry 2020; 25(1): 131–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Agudelo LZ, Femenía T, Orhan F, Porsmyr-Palmertz M, Goiny M, Martinez-Redondo V et al. Skeletal muscle PGC-1α1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell 2014; 159(1): 33–45. [DOI] [PubMed] [Google Scholar]
- 123.Contrepois K, Wu S, Moneghetti KJ, Hornburg D, Ahadi S, Tsai MS et al. Molecular Choreography of Acute Exercise. Cell 2020; 181(5): 1112–1130.e1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Binder DK, Scharfman HE. Brain-derived neurotrophic factor. Growth factors (Chur, Switzerland) 2004; 22(3): 123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Qiao H, An SC, Xu C, Ma XM. Role of proBDNF and BDNF in dendritic spine plasticity and depressive-like behaviors induced by an animal model of depression. Brain Res 2017; 1663: 29–37. [DOI] [PubMed] [Google Scholar]
- 126.Lu Y, Ho CS, McIntyre RS, Wang W, Ho RC. Effects of vortioxetine and fluoxetine on the level of Brain Derived Neurotrophic Factors (BDNF) in the hippocampus of chronic unpredictable mild stress-induced depressive rats. Brain Res Bull 2018; 142: 1–7. [DOI] [PubMed] [Google Scholar]
- 127.Lapmanee S, Charoenphandhu J, Teerapornpuntakit J, Krishnamra N, Charoenphandhu N. Agomelatine, venlafaxine, and running exercise effectively prevent anxiety- and depression-like behaviors and memory impairment in restraint stressed rats. PLoS One 2017; 12(11): e0187671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Eldomiaty MA, Almasry SM, Desouky MK, Algaidi SA. Voluntary running improves depressive behaviours and the structure of the hippocampus in rats: A possible impact of myokines. Brain Res 2017; 1657: 29–42. [DOI] [PubMed] [Google Scholar]
- 129.Matthews VB, Astrom MB, Chan MH, Bruce CR, Krabbe KS, Prelovsek O et al. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia 2009; 52(7): 1409–1418. [DOI] [PubMed] [Google Scholar]
- 130.Rasmussen P, Brassard P, Adser H, Pedersen MV, Leick L, Hart E et al. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Experimental physiology 2009; 94(10): 1062–1069. [DOI] [PubMed] [Google Scholar]
- 131.Gustafsson G, Lira CM, Johansson J, Wisen A, Wohlfart B, Ekman R et al. The acute response of plasma brain-derived neurotrophic factor as a result of exercise in major depressive disorder. Psychiatry research 2009; 169(3): 244–248. [DOI] [PubMed] [Google Scholar]
- 132.Kallies G, Rapp MA, Fydrich T, Fehm L, Tschorn M, Teran C et al. Serum brain-derived neurotrophic factor (BDNF) at rest and after acute aerobic exercise in major depressive disorder. Psychoneuroendocrinology 2018; 102: 212–215. [DOI] [PubMed] [Google Scholar]
- 133.Ross RE, Saladin ME, George MS, Gregory CM. High-Intensity Aerobic Exercise Acutely Increases Brain-derived Neurotrophic Factor. Medicine and science in sports and exercise 2019; 51(8): 1698–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Meyer JD, Koltyn KF, Stegner AJ, Kim JS, Cook DB. Relationships between serum BDNF and the antidepressant effect of acute exercise in depressed women. Psychoneuroendocrinology 2016; 74: 286–294. [DOI] [PubMed] [Google Scholar]
- 135.Meyer JD, Ellingson LD, Koltyn KF, Stegner AJ, Kim JS, Cook DB. Psychobiological Responses to Preferred and Prescribed Intensity Exercise in Major Depressive Disorder. Medicine and science in sports and exercise 2016; 48(11): 2207–2215. [DOI] [PubMed] [Google Scholar]
- 136.Yarrow JF, White LJ, McCoy SC, Borst SE. Training augments resistance exercise induced elevation of circulating brain derived neurotrophic factor (BDNF). Neurosci Lett 2010; 479(2): 161–165. [DOI] [PubMed] [Google Scholar]
- 137.Church DD, Hoffman JR, Mangine GT, Jajtner AR, Townsend JR, Beyer KS et al. Comparison of high-intensity vs. high-volume resistance training on the BDNF response to exercise. J Appl Physiol (1985) 2016; 121(1): 123–128. [DOI] [PubMed] [Google Scholar]
- 138.Dinoff A, Herrmann N, Swardfager W, Gallagher D, Lanctot KL. The effect of exercise on resting concentrations of peripheral brain-derived neurotrophic factor (BDNF) in major depressive disorder: A meta-analysis. Journal of psychiatric research 2018; 105: 123–131. [DOI] [PubMed] [Google Scholar]
- 139.Szuhany KL, Otto MW. Assessing BDNF as a mediator of the effects of exercise on depression. J Psychiatr Res 2020; 123: 114–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pereira DS, de Queiroz BZ, Miranda AS, Rocha NP, Felício DC, Mateo EC et al. Effects of physical exercise on plasma levels of brain-derived neurotrophic factor and depressive symptoms in elderly women--a randomized clinical trial. Arch Phys Med Rehabil 2013; 94(8): 1443–1450. [DOI] [PubMed] [Google Scholar]
- 141.Haapakoski R, Mathieu J, Ebmeier KP, Alenius H, Kivimäki M. Cumulative meta-analysis of interleukins 6 and 1β, tumour necrosis factor α and C-reactive protein in patients with major depressive disorder. Brain Behav Immun 2015; 49: 206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shi Y, Song R, Wang L, Qi Y, Zhang H, Zhu J et al. Identifying Plasma Biomarkers with high specificity for major depressive disorder: A multi-level proteomics study. J Affect Disord 2020; 277: 620–630. [DOI] [PubMed] [Google Scholar]
- 143.Zavvari F, Nahavandi A. Fluoxetine increases hippocampal neural survival by improving axonal transport in stress-induced model of depression male rats. Physiol Behav 2020; 227: 113140. [DOI] [PubMed] [Google Scholar]
- 144.Lu Y, Xu X, Jiang T, Jin L, Zhao XD, Cheng JH et al. Sertraline ameliorates inflammation in CUMS mice and inhibits TNF-α-induced inflammation in microglia cells. Int Immunopharmacol 2019; 67: 119–128. [DOI] [PubMed] [Google Scholar]
- 145.Liu W, Sheng H, Xu Y, Liu Y, Lu J, Ni X. Swimming exercise ameliorates depression-like behavior in chronically stressed rats: relevant to proinflammatory cytokines and IDO activation. Behav Brain Res 2013; 242: 110–116. [DOI] [PubMed] [Google Scholar]
- 146.Boettger S, Müller HJ, Oswald K, Puta C, Donath L, Gabriel HH et al. Inflammatory changes upon a single maximal exercise test in depressed patients and healthy controls. Prog Neuropsychopharmacol Biol Psychiatry 2010; 34(3): 475–478. [DOI] [PubMed] [Google Scholar]
- 147.Pedersen BK, Febbraio M. Muscle-derived interleukin-6--a possible link between skeletal muscle, adipose tissue, liver, and brain. Brain Behav Immun 2005; 19(5): 371–376. [DOI] [PubMed] [Google Scholar]
- 148.Starkie R, Ostrowski SR, Jauffred S, Febbraio M, Pedersen BK. Exercise and IL-6 infusion inhibit endotoxin-induced TNF-alpha production in humans. FASEB J 2003; 17(8): 884–886. [DOI] [PubMed] [Google Scholar]
- 149.Steensberg A, Fischer CP, Keller C, Møller K, Pedersen BK. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am J Physiol Endocrinol Metab 2003; 285(2): E433–437. [DOI] [PubMed] [Google Scholar]
- 150.Hallberg L, Janelidze S, Engstrom G, Wisén AG, Westrin A, Brundin L. Exercise-induced release of cytokines in patients with major depressive disorder. J Affect Disord 2010; 126(1-2): 262–267. [DOI] [PubMed] [Google Scholar]
- 151.Colbert LH, Visser M, Simonsick EM, Tracy RP, Newman AB, Kritchevsky SB et al. Physical activity, exercise, and inflammatory markers in older adults: findings from the Health, Aging and Body Composition Study. J Am Geriatr Soc 2004; 52(7): 1098–1104. [DOI] [PubMed] [Google Scholar]
- 152.Kohut ML, McCann DA, Russell DW, Konopka DN, Cunnick JE, Franke WD et al. Aerobic exercise, but not flexibility/resistance exercise, reduces serum IL-18, CRP, and IL-6 independent of beta-blockers, BMI, and psychosocial factors in older adults. Brain Behav Immun 2006; 20(3): 201–209. [DOI] [PubMed] [Google Scholar]
- 153.Krogh J, Benros ME, Jørgensen MB, Vesterager L, Elfving B, Nordentoft M. The association between depressive symptoms, cognitive function, and inflammation in major depression. Brain Behav Immun 2014; 35: 70–76. [DOI] [PubMed] [Google Scholar]
- 154.Euteneuer F, Dannehl K, Del Rey A, Engler H, Schedlowski M, Rief W. Immunological effects of behavioral activation with exercise in major depression: an exploratory randomized controlled trial. Transl Psychiatry 2017; 7(5): e1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lavebratt C, Herring MP, Liu JJ, Wei YB, Bossoli D, Hallgren M et al. Interleukin-6 and depressive symptom severity in response to physical exercise. Psychiatry Res 2017; 252: 270–276. [DOI] [PubMed] [Google Scholar]
- 156.Rethorst CD, Toups MS, Greer TL, Nakonezny PA, Carmody TJ, Grannemann BD et al. Pro-inflammatory cytokines as predictors of antidepressant effects of exercise in major depressive disorder. Mol Psychiatry 2013; 18(10): 1119–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Trivedi MH, Greer TL, Church TS, Carmody TJ, Grannemann BD, Galper DI et al. Exercise as an augmentation treatment for nonremitted major depressive disorder: a randomized, parallel dose comparison. The Journal of clinical psychiatry 2011; 72(5): 677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Köhler CA, Freitas TH, Stubbs B, Maes M, Solmi M, Veronese N et al. Peripheral Alterations in Cytokine and Chemokine Levels After Antidepressant Drug Treatment for Major Depressive Disorder: Systematic Review and Meta-Analysis. Mol Neurobiol 2018; 55(5): 4195–4206. [DOI] [PubMed] [Google Scholar]
- 159.Molendijk ML, Spinhoven P, Polak M, Bus BA, Penninx BW, Elzinga BM. Serum BDNF concentrations as peripheral manifestations of depression: evidence from a systematic review and meta-analyses on 179 associations (N=9484). Molecular psychiatry 2014; 19(7): 791–800. [DOI] [PubMed] [Google Scholar]
- 160.Zhou C, Zhong J, Zou B, Fang L, Chen J, Deng X et al. Meta-analyses of comparative efficacy of antidepressant medications on peripheral BDNF concentration in patients with depression. PLoS One 2017; 12(2): e0172270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Schuch FB, Deslandes AC, Stubbs B, Gosmann NP, Silva CT, Fleck MP. Neurobiological effects of exercise on major depressive disorder: A systematic review. Neurosci Biobehav Rev 2016; 61: 1–11. [DOI] [PubMed] [Google Scholar]
- 162.Krogh J, Nordentoft M, Mohammad-Nezhad M, Westrin A. Growth hormone, prolactin and cortisol response to exercise in patients with depression. Journal of affective disorders 2010; 125(1-3): 189–197. [DOI] [PubMed] [Google Scholar]
- 163.Toups MS, Greer TL, Kurian BT, Grannemann BD, Carmody TJ, Huebinger R et al. Effects of serum Brain Derived Neurotrophic Factor on exercise augmentation treatment of depression. Journal of psychiatric research 2011; 45(10): 1301–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Krogh J, Rostrup E, Thomsen C, Elfving B, Videbech P, Nordentoft M. The effect of exercise on hippocampal volume and neurotrophines in patients with major depression--a randomized clinical trial. Journal of affective disorders 2014; 165: 24–30. [DOI] [PubMed] [Google Scholar]
- 165.Schuch FB, Vasconcelos-Moreno MP, Borowsky C, Zimmermann AB, Wollenhaupt-Aguiar B, Ferrari P et al. The effects of exercise on oxidative stress (TBARS) and BDNF in severely depressed inpatients. European archives of psychiatry and clinical neuroscience 2014; 264(7): 605–613. [DOI] [PubMed] [Google Scholar]
- 166.Kerling A, Kuck M, Tegtbur U, Grams L, Weber-Spickschen S, Hanke A et al. Exercise increases serum brain-derived neurotrophic factor in patients with major depressive disorder. Journal of affective disorders 2017; 215: 152–155. [DOI] [PubMed] [Google Scholar]
- 167.Rahman MS, Millischer V, Zeebari Z, Forsell Y, Lavebratt C. BDNF Val66Met and childhood adversity on response to physical exercise and internet-based cognitive behavioural therapy in depressed Swedish adults. J Psychiatr Res 2017; 93: 50–58. [DOI] [PubMed] [Google Scholar]
- 168.Gourgouvelis J, Yielder P, Clarke ST, Behbahani H, Murphy BA. Exercise Leads to Better Clinical Outcomes in Those Receiving Medication Plus Cognitive Behavioral Therapy for Major Depressive Disorder. Frontiers in psychiatry 2018; 9: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gerber M, Imboden C, Beck J, Brand S, Colledge F, Eckert A et al. Effects of Aerobic Exercise on Cortisol Stress Reactivity in Response to the Trier Social Stress Test in Inpatients with Major Depressive Disorders: A Randomized Controlled Trial. J Clin Med 2020; 9(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wolkowitz OM, Wolf J, Shelly W, Rosser R, Burke HM, Lerner GK et al. Serum BDNF levels before treatment predict SSRI response in depression. Progress in neuro-psychopharmacology & biological psychiatry 2011; 35(7): 1623–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mikoteit T, Beck J, Eckert A, Hemmeter U, Brand S, Bischof R et al. High baseline BDNF serum levels and early psychopathological improvement are predictive of treatment outcome in major depression. Psychopharmacology (Berl) 2014; 231(15): 2955–2965. [DOI] [PubMed] [Google Scholar]
- 172.Leong DP, Teo KK, Rangarajan S, Lopez-Jaramillo P, Avezum A, Orlandini A et al. Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet 2015; 386(9990): 266–273. [DOI] [PubMed] [Google Scholar]
- 173.Rethorst CD, South CC, Rush AJ, Greer TL, Trivedi MH. Prediction of treatment outcomes to exercise in patients with nonremitted major depressive disorder. Depress Anxiety 2017; 34: 1116–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Więdłocha M, Marcinowicz P, Krupa R, Janoska-Jaździk M, Janus M, Dębowska W et al. Effect of antidepressant treatment on peripheral inflammation markers - A meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry 2018; 80(Pt C): 217–226. [DOI] [PubMed] [Google Scholar]
- 175.Uher R, Tansey KE, Dew T, Maier W, Mors O, Hauser J et al. An inflammatory biomarker as a differential predictor of outcome of depression treatment with escitalopram and nortriptyline. Am J Psychiatry 2014; 171(12): 1278–1286. [DOI] [PubMed] [Google Scholar]
- 176.Jha MK, Minhajuddin A, Gadad BS, Greer T, Grannemann B, Soyombo A et al. Can C-reactive protein inform antidepressant medication selection in depressed outpatients? Findings from the CO-MED trial. Psychoneuroendocrinology 2017; 78: 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Carboni L, McCarthy DJ, Delafont B, Filosi M, Ivanchenko E, Ratti E et al. Biomarkers for response in major depression: comparing paroxetine and venlafaxine from two randomised placebo-controlled clinical studies. Transl Psychiatry 2019; 9(1): 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lavin KM, Perkins RK, Jemiolo B, Raue U, Trappe SW, Trappe TA. Effects of aging and lifelong aerobic exercise on basal and exercise-induced inflammation. J Appl Physiol (1985) 2020; 128(1): 87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Cabral-Santos C, de Lima Junior EA, Fernandes IMDC, Pinto RZ, Rosa-Neto JC, Bishop NC et al. Interleukin-10 responses from acute exercise in healthy subjects: A systematic review. J Cell Physiol 2019; 234(7): 9956–9965. [DOI] [PubMed] [Google Scholar]
- 180.Krabbe KS, Nielsen AR, Krogh-Madsen R, Plomgaard P, Rasmussen P, Erikstrup C et al. Brain-derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia 2007; 50(2): 431–438. [DOI] [PubMed] [Google Scholar]
- 181.Duncan BB, Schmidt MI, Pankow JS, Ballantyne CM, Couper D, Vigo A et al. Low-grade systemic inflammation and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes 2003; 52(7): 1799–1805. [DOI] [PubMed] [Google Scholar]
- 182.Dias JP, Joseph JJ, Kluwe B, Zhao S, Shardell M, Seeman T et al. The longitudinal association of changes in diurnal cortisol features with fasting glucose: MESA. Psychoneuroendocrinology 2020; 119: 104698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zhou L, Sutton GM, Rochford JJ, Semple RK, Lam DD, Oksanen LJ et al. Serotonin 2C receptor agonists improve type 2 diabetes via melanocortin-4 receptor signaling pathways. Cell Metab 2007; 6(5): 398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Sarwar N, Butterworth AS, Freitag DF, Gregson J, Willeit P, Gorman DN et al. Interleukin-6 receptor pathways in coronary heart disease: a collaborative meta-analysis of 82 studies. Lancet 2012; 379(9822): 1205–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kaess BM, Preis SR, Lieb W, Beiser AS, Yang Q, Chen TC et al. Circulating brain-derived neurotrophic factor concentrations and the risk of cardiovascular disease in the community. J Am Heart Assoc 2015; 4(3): e001544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Nijm J, Kristenson M, Olsson AG, Jonasson L. Impaired cortisol response to acute stressors in patients with coronary disease. Implications for inflammatory activity. J Intern Med 2007; 262(3): 375–384. [DOI] [PubMed] [Google Scholar]
- 187.Mahmood Z, Davidsson A, Olsson E, Leanderson P, Lundberg AK, Jonasson L. The effect of acute exercise on interleukin-6 and hypothalamic-pituitary-adrenal axis responses in patients with coronary artery disease. Sci Rep 2020; 10(1): 21390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.O’Reardon JP, Solvason HB, Janicak PG, Sampson S, Isenberg KE, Nahas Z et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biological psychiatry 2007; 62(11): 1208–1216. [DOI] [PubMed] [Google Scholar]
- 189.Husain MM, Rush AJ, Fink M, Knapp R, Petrides G, Rummans T et al. Speed of response and remission in major depressive disorder with acute electroconvulsive therapy (ECT): a Consortium for Research in ECT (CORE) report. J Clin Psychiatry 2004; 65(4): 485–491. [DOI] [PubMed] [Google Scholar]
- 190.Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 2011; 475(7354): 91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Pearson S, Schmidt M, Patton G, Dwyer T, Blizzard L, Otahal P et al. Depression and insulin resistance: cross-sectional associations in young adults. Diabetes Care 2010; 33(5): 1128–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Rethorst CD, Greer TL, Toups MS, Bernstein I, Carmody TJ, Trivedi MH. IL-1β and BDNF are associated with improvement in hypersomnia but not insomnia following exercise in major depressive disorder. Transl Psychiatry 2015; 5: e611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Szuhany KL, Otto MW. Efficacy evaluation of exercise as an augmentation strategy to brief behavioral activation treatment for depression: a randomized pilot trial. Cogn Behav Ther 2020; 49(3): 228–241. [DOI] [PMC free article] [PubMed] [Google Scholar]


