Abstract
Background and Objectives
Previous research has examined the association between cognition and flavonoids: bioactives found in foods, known to possess anti-inflammatory and antioxidant properties. We extend this research by investigating associations of dietary intakes of total flavonols and constituents (kaempferol, quercetin, myricetin, and isorhamnetin) on the change in cognitive performance in global cognition, episodic memory, semantic memory, visuospatial ability, perceptual speed, and working memory.
Methods
The study was conducted using 961 participants (aged 60–100 years) of the Rush Memory and Aging Project, a prospective cohort of community-dwelling Chicagoans who were followed for an average of 6.9 years. Diet was assessed using a validated semiquantitative food frequency questionnaire. Cognitive performance was assessed annually with a battery of 19 standardized tests. Flavonol intake was analyzed as a continuous variable using linear mixed-effects models. Cognitive domain scores were regressed on baseline calorie-adjusted flavonol variables.
Results
Higher dietary intakes of total flavonols and flavonol constituents were associated with a slower rate of decline in global cognition and multiple cognitive domains. In continuous models adjusted for age, sex, education, APOE ɛ4, late-life cognitive activity, physical activity, and smoking, total flavonol intake was associated with slower decline in global cognition β estimate = 0.004 (95% CI 0.001–0.006), episodic memory β = 0.004 (95% CI 0.002–0.006), semantic memory β = 0.003 (95% CI 0.001–0.007), perceptual speed β = 0.003 (95% CI 0.001–0.004), and working memory β = 0.003 (95% CI 0.001–0.005) and marginally associated with visuospatial ability β = 0.001 (95% CI −0.001 to 0.003). Analyses of individual flavonol constituents demonstrated that intakes of kaempferol and quercetin were associated with slower global cognitive decline (β = 0.01 [95% CI 0.006–0.02] and β = 0.004 [95% CI 0.0005–0.007]), respectively. Myricetin and isorhamnetin were not associated with global cognition.
Discussion
Results suggest that dietary intakes of total flavonols and several flavonol constituents may be associated with slower decline in global cognition and multiple cognitive abilities with older age.
Flavonoids are a large class of bioactive polyphenolic compounds found in plants, with a substantial amount found in fruits and vegetables, in particular. Many flavonoids are known to have antioxidant and anti-inflammatory properties. These inherent facets are recognized as having the capability to prevent or diminish cellular damage throughout the body, including the brain.1-3 Mechanistically, this is accomplished given their biochemical structure, which provides the capability to prevent oxidative stress from reactive oxygen species and free radicals and decrease harmful neuroinflammation.4-6
In previous studies, high total intake of flavonoids and some subclasses has been associated with a slower rate of age-related global cognitive decline and Alzheimer dementia.1,7-10 However, there has been limited investigation of flavonoid subclasses—and even further, individual constituents that make up those subclasses—and brain health.1,11 For example, much of the data on individual flavonoids originate from animal studies. Specifically, studies in mice have demonstrated that the constituents of the flavonoid subclass, flavonols—kaempferol, quercetin, myricetin, and isorhamnetin—are particularly important for learning and memory.12-14 Nevertheless, exploration of the relationship between the flavonol constituents and cognition in humans is limited. Therefore, in this study, we explored associations of total and individual flavonols, including kaempferol, quercetin, myricetin, and isorhamnetin, with cognitive decline in a community cohort of older persons. We also investigated the role of total and individual flavonols on specific cognitive domains including episodic memory, semantic memory, visuospatial ability, perceptual speed, and working memory.
Methods
Study Population
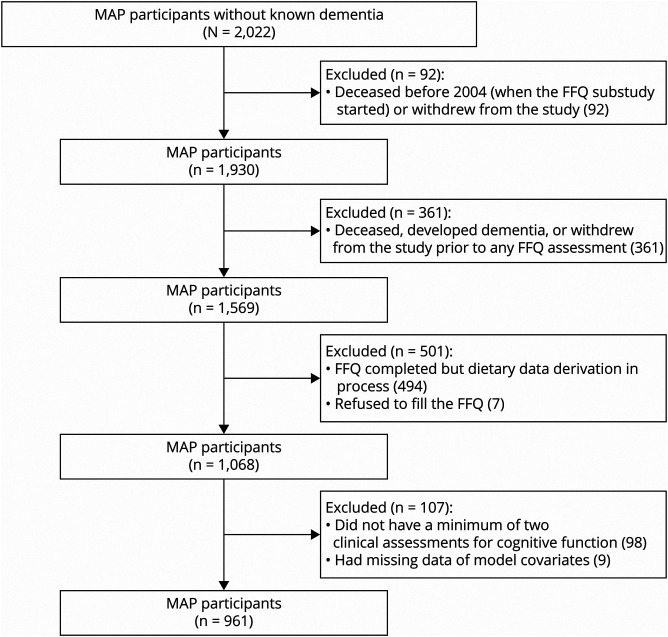
The study was completed in the Rush Memory and Aging Project (MAP), a 1997-present cohort study comprising Chicago residents of retirement communities and senior public housing with no known dementia at baseline. The participants undergo yearly, in person, clinical evaluations with detailed risk factor assessments and cognitive testing. Beginning in 2004, dietary data were collected using a validated self-administered food frequency questionnaire (FFQ). Starting August 2018, 2,022 participants without known dementia at baseline were enrolled in the MAP.15 Of those, 92 died or withdrew from the study before dietary data collection began in 2004. We further excluded those who were enrolled after 2004 but died or withdrew from the study prior to any FFQ assessment (n = 361), refused to fill out the FFQ (n = 7), or completed the FFQ, but the derivation of dietary data was in process (n = 494), leaving 1,068 participants with complete nutrient data. Of the 1,068 participants, 98 did not have a minimum of 2 clinical assessments to evaluate change in cognitive function, thus leaving 970 participants. We further excluded 9 individuals, from the analytical data set, who had missing data on model covariates. This provided 961 participants for whom we conducted analyses of dietary flavonol intake with cognitive change (Figure 1).
Figure 1. Study Population Flowchart.
Standard Protocol Approvals, Registrations, and Patient Consents
The Institutional Review Board of the Rush University Medical Center approved the study, and all participants provided written informed consent.
Data Availability
The anonymized data, used for the study, are available through the Rush Alzheimer's Disease Center (RADC). Qualified investigators can find information regarding the formal requirements for access to data on the RADC research resource-sharing hub (radc.rush.edu).
Cognitive Assessment
The annual structured clinical evaluations include a battery of 19 cognitive tests administered by trained and certified technicians. Composite scores of global cognition (all 19 tests) and 5 cognitive domains were calculated using the average of standardized scores16 of the individual tests, including for episodic memory (7 tests, including word list, word list recall, word list recognition, East Boston immediate recall, East Boston delayed recall, logical memory immediate recall, and logical memory delayed recall), semantic memory (3 tests, including Boston naming 15 items, category fluency, and reading test 10 items), visuospatial ability (2 tests, including line orientation and progressive matrices 16 items), perceptual speed (4 tests, including symbol digits modality test, number comparison, Stroop color naming, and Stroop word reading), and working memory (3 tests, including digits forward, digits backward, and digits ordering). The composite measure of each aforementioned domain was formed by converting raw scores on each cognitive test, making up the domain, to z scores, using the mean and SD of the cohort, and then averaging the z scores to yield the composite.
Dietary Assessment
Diet was assessed using a modified version of the Harvard FFQ that was validated for use in an older Chicago population.17 For these analyses, the baseline FFQ was used to establish the dietary intake of flavonols. The MAP FFQ ascertains usual frequency of intake of 144 food items consumed over the previous 12 months. Total calorie intake and nutrient levels for each of the food items were based on natural proportion sizes, (e.g., 1 egg) or according to age- and sex-specified serving sizes reported in national diet surveys. The various flavonol contents of foods are based on the Nutrition Coordinating Center Flavonoid and Proanthocyanidin Provisional Table from the University of Minnesota,18 which draws heavily on the United States Department of Agriculture (USDA) Database for the Flavonoid Content of Selected Foods, Release 3.3 (March 2018) and The USDA Database for the Proanthocyanidin Content of Selected Foods, Release 2.1 (March 2018), with additional data from other study publications.19
Using the aforementioned database, we derived the flavonoid content for each food item within our FFQ as milligrams per 100 g. We then multiplied this by the serving size, grams per serving, to obtain the flavonoid amounts (milligrams per serving) for each food item. The flavonoid contents were multiplied by the frequency of intake (servings per day) and summed across all food items to obtain the total daily intake of each flavonoid in mg/day.
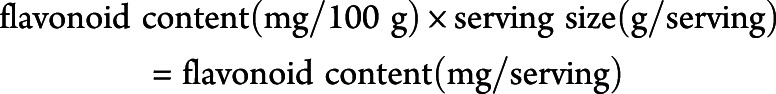 |
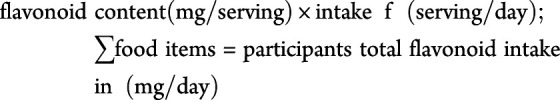 |
The top food item contributors to the individual flavonoids (subgroup flavonols) presented in this current study, shown as the mean (SD) of flavonol intake (in milligrams/day), were kale 0.07 (0.15), beans 0.19 (0.22), tea 0.22 (0.34), spinach 0.12 (0.15), and broccoli 0.20 (0.18) for kaempferol; tomatoes 0.10 (0.09), kale 0.07 (0.15), apples 0.29 (0.29), and tea 0.22 (0.34) for quercetin; tea 0.21 (0.34), wine 0.18 (0.41), kale 0.06 (0.15), oranges 0.22 (0.29), and tomatoes 0.28 (0.28) for myricetin, and pears 0.10 (0.17), olive oil 0.15 (0.24), wine 0.18 (0.41), and tomato sauce 0.06 (0.06) for isorhamnetin. Nutrients and polyphenols were calorie adjusted using the regression residual method, a method of analyses by which the dietary intakes of nutrients, and/or bioactives are evaluated in relation to total dietary calories.17 For analyses, the first completed valid FFQ established the baseline for each participant. Total flavonol intake was moderately stable (Spearman r = 0.60; p < 0.0001), between the first and last acquired FFQ, which were on average, 3 years apart with an intraclass correlation between r = 0.37 and r = 0.60.
Model Covariates
Nondietary covariates were obtained from measurements and structured interview questions at the participants' baseline clinical evaluation. Education (years) is self-reported years of regular schooling. APOE genotyping was performed using high-throughput sequencing as previously described.20 Age (in years) at the analytical baseline visit was computed from self-reported birth date and the date of the baseline cognitive assessment and is used in these analyses as a continuous variable. Age is an established risk factor for cognitive decline. Physical activity (hours per week) was computed from self-reported minutes spent within the previous 2 weeks on 5 activities: walking for exercise, yard work, calisthenics, biking, and water exercise.21 Participation in cognitively stimulating activities was computed as the average frequency rating, based on a 5-point scale, of different activities (e.g., reading, playing games, writing letters, and visiting the library).22 Using the FFQ responses, calorie measures are calculated by Harvard University. The total energy of each food item, calories, is based on age-specific portion sizes from national dietary surveys or natural portion sizes. Smoking status was self-reported at baseline using 2 questions, “Do you smoke cigarettes now?” and “Did you ever smoke cigarettes?” The responses to these questions determined smoking history, defined as never smokers vs former smokers vs current smokers.23 Body mass index (BMI) was computed from objectively measured weight (kg) and height (m2) and modeled as 2 indicator variables, BMI ≤ 20 kg/m2 and BMI ≥30 kg/m2, with the referent group 20 < BMI <30 kg/m2. The number of depressive symptoms was evaluated by a modified 10-item version of the Center for Epidemiological Studies–Depression scale.24 Myocardial infarction history was based on self-reported medical diagnosis or use of cardiac glycosides (e.g., lanoxin and digitoxin). Diabetes history was determined by self-reported medical diagnosis or current use of diabetic medications. Clinical diagnosis of stroke was based on the clinician review of self-reported history and neurologic examination.25 Hypertension history was determined by self-reported medical diagnosis, measured blood pressure (average of 2 measurements ≥160 mm Hg systolic or ≥90 mm Hg diastolic), or current use of hypertensive medications.15 Dietary intakes of nutrients that have been associated with cognition, vitamin E26, saturated fat,27 folate,28 lutein,29 and omega-3 fatty acids30,31 are measured via the aforementioned food frequency questionnaire. The individual nutrient measures were calculated from the FFQ responses by determining the nutrient content (grams per serving) for each food item and multiplying by the frequency of intake. In the same fashion as was done with flavonoids, the intake was summed across all food items to obtain total daily intake of each nutrient in milligrams per day.
Statistical Analyses
To investigate the association between dietary intakes of total flavonols and flavonol constituents to global cognition and 5 cognitive domains, we used linear mixed models in SAS 9.4. Basic-adjusted models included covariates with the most established scientific evidence for association with cognitive decline,32 including age, sex, education, participation in cognitively stimulating activities, physical activity, smoking, and APOE ε4 status. In an effort to investigate and address potential confounding due to disease processes with established associations to cognitive decline, further models included additional covariates (e.g., depressive symptoms and BMI and individual cardiovascular diseases) to the basic-adjusted model. We analyzed flavonol intake as a continuous variable. All model estimates are presented as β-coefficients and 95% CIs. Interactions were investigated between flavonol associations and various covariates by including the products of the flavonol variables and potential effect modifiers (e.g., age, sex, education, and APOE ε4 status) in the basic-adjusted model, with a significance level at p ≤ 0.05. Statistically significant interactions were further investigated by stratifying on the effect modifier and reanalyzing the basic-adjusted models.
Results
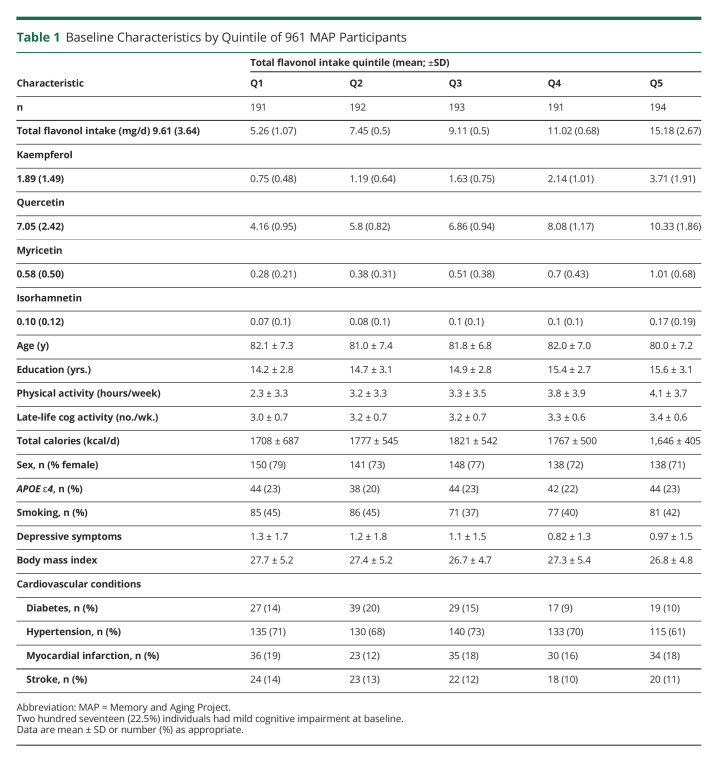
The study sample population, of 961 individuals, was followed for an average of 6.9 years (±3.6) and had a mean age at baseline of 81.4 years (SD = 7.2) with an age range of 58–100 years. The analytical sample had an average educational level of 15.0 years (±2.9) and was primarily female (75%) and non-Latino White (98%), with 22% having at least 1 APOE ɛ4 allele and 42% reporting a history of smoking. Participants with the highest levels of flavonol intake were on average younger, more educated, consumed fewer calories, and were more physically and cognitively active than those with the lowest flavonol intakes (Table 1). Dietary intake of the individual flavonols had low to moderate correlations with each other (Spearman r range 0.11–0.49; all p ≤ 0.05). Mean intake of total flavonols was 9.6 (SD = 3.6) mg/d, kaempferol 1.89 (1.49) mg/d, quercetin 7.05 (2.42) mg/d, myricetin 0.58 (0.50) mg/d, and isorhamnetin 0.10 (0.012) mg/d.
Table 1.
Baseline Characteristics by Quintile of 961 MAP Participants
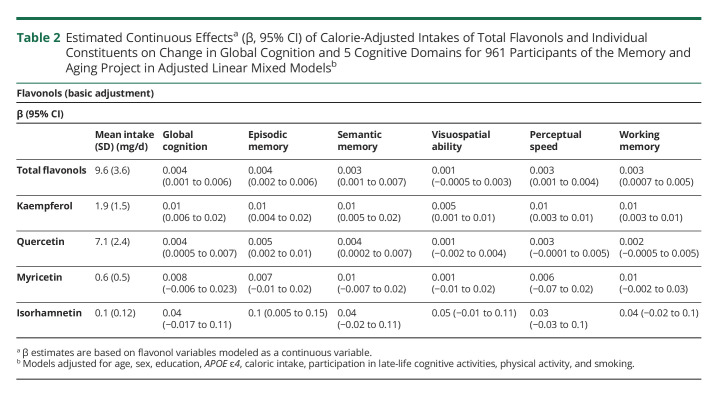
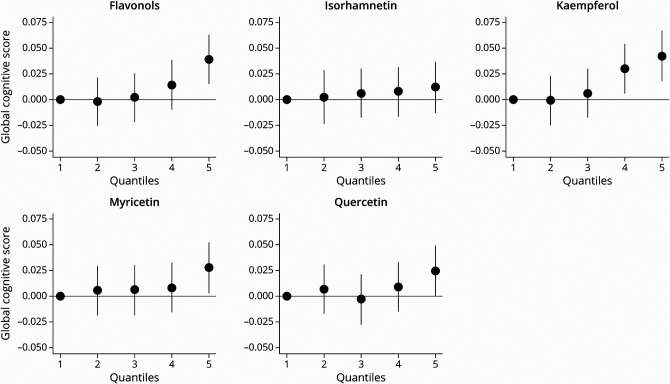
Higher dietary intakes of total flavonols and flavonol constituents were associated with a slower rate of decline in global cognition and several cognitive domains (Table 2). In the basic-adjusted continuous model, higher intake of total dietary flavonols was associated with a slower rate of decline in global cognition, episodic memory, semantic memory, perceptual speed, and working memory (Table 2). Total flavonol intake through diet was not associated with change in visuospatial ability (Table 2). This is redemonstrated when comparing quintile 5 vs quintile 1; dietary intake of total flavonols was associated with global cognition β = 0.04 (95% CI 0.02–0.06) (Figure 2), episodic memory β = 0.05 (95% CI 0.03–0.08), semantic memory β = 0.04 (95% CI 0.01–0.06), perceptual speed β = 0.02 (95% CI 0.0003–0.04), and working memory β = 0.03 (95% CI 0.002–0.05).
Table 2.
Estimated Continuous Effectsa (β, 95% CI) of Calorie-Adjusted Intakes of Total Flavonols and Individual Constituents on Change in Global Cognition and 5 Cognitive Domains for 961 Participants of the Memory and Aging Project in Adjusted Linear Mixed Modelsb
Figure 2. Estimated Effects (β, 95% CI) of Energy-Adjusted Intakes of Total Flavonols and Flavonol Constituents by Quantile on Change in Global Cognition for 961 Participants of the Memory and Aging Project in Basic‐Adjusted* Linear Mixed Models.
*Basic adjustment—age, sex, education, APOE ɛ4, late-life cognitive activity, physical activity, and smoking.
In analyses of individual flavonol constituents as continuous variables, higher kaempferol intake was associated with slower decline in the global cognitive score and all 5 cognitive domains (Table 2). Quercetin intake was associated with slower decline in global cognition, episodic memory, and semantic memory and suggestive for both perceptual speed and working memory (Table 2). Isorhamnetin was associated with a slower rate of episodic memory and suggestive for visuospatial memory. Myricetin was not associated with change in any cognitive abilities but was suggestive for working memory (Table 2). The highest quintile vs lowest quintile intake of the flavonol constituents kaempferol β = 0.04 (95% CI 0.02–0.07), quercetin β = 0.02 (95% CI 0.0002–0.05), and myricetin β = 0.03 (95% CI 0.003–0.05) was associated with a decrease in the rate of global cognitive decline (Figure 2). Q5 vs Q1 intake of isorhamnetin was not associated with global cognition (Figure 2).
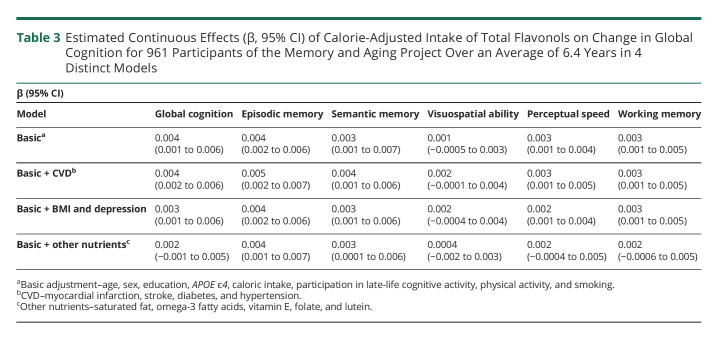
We investigated possible confounding due to cardiovascular conditions on the observed protective relations of flavonol intake to cognitive decline by adding these covariates (myocardial infarction, stroke, diabetes, and hypertension) to the basic-adjusted model. However, there were no material changes in the effect estimates or the findings (Table 3).
Table 3.
Estimated Continuous Effects (β, 95% CI) of Calorie-Adjusted Intake of Total Flavonols on Change in Global Cognition for 961 Participants of the Memory and Aging Project Over an Average of 6.4 Years in 4 Distinct Models
In further analyses, we reanalyzed the data with the addition of BMI and depressive symptoms to the basic-adjusted model, covariates that have the potential to be both confounders and clinical manifestations of neurodegenerative conditions. The effect estimates and findings for flavonols were virtually unchanged with these adjustments (Table 3).
We also explored possible confounding by other dietary components by adding to the basic model, dietary intakes of vitamin E, saturated fat, folate, lutein, and omega-3 fatty acids. After adjustment for these dietary components, neither quercetin nor myricetin was associated with global cognitive score (p-trend = 0.09) (Table 3).
We considered the possibility that the observed protective relations may be due to the latent effects of neurodegenerative conditions on dietary behaviors. The analytic sample excluded those with dementia at baseline; however, we conducted sensitivity analyses in an effort to account for reverse causation and further excluded 217 participants with mild cognitive impairment at their baseline of enrollment. We then reanalyzed the basic models with the continuous variable for total intake of dietary flavonols. The results were similar for intakes of total flavonols (and individual constituents) β = 0.04 (95% CI 0.02–0.07) for global cognition; β = 0.05 (95% CI 0.02–0.08) for episodic memory; β = 0.04 (95% CI 0.01–0.07) for semantic memory; β = 0.02 (95% CI 0.000–0.05) for visuospatial ability; β = 0.03 (95% CI 0.008–0.05) for perceptual speed; and β = 0.04 (95% CI 0.01–0.06) for working memory. There were no significant interactions observed by age (≤80, >80), sex, education (≤12 years, >12 years), or APOE ɛ4 status.
Discussion
In this prospective community-based cohort study of older people, we found evidence that higher dietary intakes of total flavonols and several individual flavonol constituents are associated with slower rates of decline in global cognition and multiple cognitive domains. The observed associations were independent of cardiovascular conditions and lifestyle factors. Furthermore, after adjusting for dietary intake of vitamin E, saturated fat, folate, lutein, and omega-3 fatty acids, flavonol associations with episodic memory, semantic memory, and visuospatial ability were unchanged.
This study extends the limited number of investigations into the association of dietary flavonols to objectively measure cognitive decline. A previous study by our group reported that kaempferol, a flavonol that is abundant in green leafy vegetables, was associated with a reduced rate of global cognitive decline.11 A limited number of cohort studies have reported significant associations between the dietary intake of flavonoids and improved subjective and objective cognition,1,7,11,33 the latter of which relied on participants' self-reported cognitive abilities (i.e., subjective cognitive decline), whereas our investigation assessed cognitive function using a battery of 19 cognitive tests (objective cognitive decline) by a trained study staff member. In addition, we conducted annual assessments of these standardized cognitive battery tests to study the potential change in cognition and the various cognitive domains. Furthermore, our study also investigates the association between the individual constituents found within the subclass of flavonols and individual cognitive domains. In addition, 2 studies have reported a positive association between total flavonol intake and cognition in middle-aged samples.34,35 As such, in the current study, we focused on flavonols, a subclass of flavonoids, as their biochemical structures suggest particularly effective antioxidant capabilities compared with other flavonoid subclasses.2,3 Although the mechanisms are not fully understood, the inherent antioxidant and anti-inflammatory properties of flavonols may have the ability to diminish or even prevent systemic cellular damage, extending to the brain.1-3 By having anti-inflammatory features, flavonols can decrease the amplitude or duration of neuroinflammation. In addition, containing antioxidant characteristics allows dietary flavonols to potentially prevent or reduce oxidative stress from reactive oxygen species and free radicals.4-6 Lending novelty to the present research, we investigated individual flavonol constituents and their associations with objective global cognition and the association of total flavonols and individual constituents to specific objective cognitive domains.
Several animal models demonstrate protective roles for individual flavonol constituents on cognition. Quercetin supplementation improved memory and learning in triple transgenic Alzheimer disease mice,13 whereas in other mouse models, kaempferol and myricetin treatments improved learning and memory and reduced oxidative stress.12 In mice with Aβ25–35-injected Alzheimer disease, oral quercetin supplementation improved cognitive function and memory.36 In transgenic mice with an induced Alzheimer disease–like pathology, 3 months of treatment with a myricetin derivative, dihydromyricetin, improved cognition as measured by object familiarization and subsequent novel object recognition.37
A number of strengths provide support for the findings of this study. The prospective design provides temporality, albeit causality cannot be established due to the nature of observational studies. However, the long follow-up time (up to 14 years) lends confidence to the results of change over time. Furthermore, tests of cognitive abilities were administered annually using a battery of 19 cognitive tests, thus providing a robust measure of cognitive function. The dietary intake data were based on a comprehensive food frequency questionnaire that was validated among older Chicago community-dwelling residents17 and found to be unbiased by age, sex, educational level, and cognitive impairment. In addition, lifestyle and other factors that are associated with one's cognitive status were assessed annually. These factors were adjusted to help minimize residual confounding, considering that higher dietary intake of flavonols may be a proxy for a healthy lifestyle. Even still, some residual confounding may persist. The current study also has limitations. Self-reports of dietary intake are prone to recall bias. Furthermore, based on their age, MAP participants are at risk of mild cognitive impairment or subclinical disease. This could lead to unreliable reports of consumption on the FFQ or changes in dietary habits, which may not have been captured, when using the baseline FFQ to establish a participant's dietary intake of flavonols. Both aspects could bias the results leading to an over- or under-estimation of reported associations. An additional limitation, reverse causality, remains due to the length of the prodromal phase revolving around the cognitive decline of Alzheimer disease and related dementias. However, the findings did not change in sensitivity analyses that reanalyzed the data after eliminating an additional 217 participants with mild cognitive impairment, beyond those with dementia, who were excluded in the baseline analysis. Another limitation rests in the ever-evolving science for estimating flavonol intake in foods. The derived estimates may be imprecise given that the flavonoid contents of food are inherently variable.38 Flavonoid content is dependent on several conditions, for example, sun exposure, soil quality, geographic location, and time of harvest, to name a few. Also, given there are two predominate flavonoid databases that exist—the Nutrition Data System for Research provisional flavonoid tables (based on USDA databases) and the Phenol-Explorer (a European-based polyphenol database)—objective food analyses via PCR and potential imputation of flavonoid content data can lead to variability in the observed flavonoid concentrations. Even then, the error, in nature, should be more random and bias toward the null. Although we had a mean follow-up time of 6.9 years (±3.6), some participants may not have consumed the specific flavonoids for an adequate amount of time to demonstrate benefit. Finally, the study has limited generalizability as it is based on a sample that is largely non-Latino White (98%), highly educated, and from the Midwest.
The current study's findings suggest that dietary intake of total flavonols and several constituents may slow the rate of decline in global cognition and several cognitive domains including episodic memory, semantic memory, visuospatial ability, perceptual speed, and working memory. There is great value in investigating additional cohorts and groups to aid in future validation. Further studies are required to confirm the observed associations in other populations and help elucidate and understand the underlying biologic mechanisms involved.
Acknowledgment
The authors thank (the late) Martha Clare Morris, ScD, for her mentorship and guidance.
Glossary
- MAP
Memory and Aging Project
- FFQ
food frequency questionnaire
- RADC
Rush Alzheimer's Disease Center
Appendix. Authors
Study Funding
This work was supported by the NIH, National Institute on Aging grant RF1AG051641 (PIs: S.L.B. and J.A.S.), grant R01AG052583 (PIs: L.L.B. and F.M.S.), and grant R01AG017917 (PI: D.A.B.) and partially supported by the USDA Agricultural Research Service under Cooperative Agreement No. 58-1950-7-707. Any opinions, findings, or conclusions expressed in this publication are those of the authors and do not necessarily reflect the view of the US Department of Agriculture.
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Devore EE, Kang JH, Breteler MMB, Grodstein F. Dietary intakes of berries and flavonoids in relation to cognitive decline. Ann Neurol. 2012;72(1):135-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Youdim KA, Spencer JPE, Schroeter H, Rice-Evans C. Dietary flavonoids as potential neuroprotectants. Biol Chem. 2002;383(3-4):503-519. [DOI] [PubMed] [Google Scholar]
- 3.Pietta PG. Flavonoids as antioxidants. J Nat Prod. 2000;63(7):1035-1042. [DOI] [PubMed] [Google Scholar]
- 4.Sinyor B, Mineo J, Ochner C. Alzheimer's disease, inflammation, and the role of antioxidants. J Alzheimer's Dis Rep. 2020;4(1):175-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiecolt-Glaser JK. Stress, food, and inflammation: psychoneuroimmunology and nutrition at the cutting edge. Psychosomatic Med. 2010;72(4):365-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watzl B. Anti-inflammatory Effects of Plant-based Foods and of their ConstituentsInternationale Zeitschrift Fur Vitamin- und Ernahrungsforschung. Int J Vitamin Nutr Research. 2008;78(6):293-298. [DOI] [PubMed] [Google Scholar]
- 7.Letenneur L, Proust-Lima C, Le Gouge A, Dartigues JF, Barberger-Gateau P. Flavonoid intake and cognitive decline over a 10-year period. Am J Epidemiol. 2007;165(12):1364-1371. [DOI] [PubMed] [Google Scholar]
- 8.Shishtar E, Rogers GT, Blumberg JB, Au R, Jacques PF. Long-term dietary flavonoid intake and risk of Alzheimer disease and related dementias in the Framingham Offspring Cohort. Am J Clin Nutr. 2020;112(2):343-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agarwal P, Holland TM, Wang Y, Bennett DA, Morris MC. Association of strawberries and anthocyanidin intake with Alzheimer's dementia RisK. Nutrients. 2019;11(12):3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holland TM, Agarwal P, Wang Y, et al. Dietary flavonols and risk of Alzheimer dementia. Neurology. 2020;94(16):e1749–e1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morris MC, Wang Y, Barnes LL, Bennett DA, Dawson-Hughes B, Booth SL. Nutrients and bioactives in green leafy vegetables and cognitive decline: prospective study. Neurology. 2018;190(3):e214–e222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lei Y, Chen J, Zhang W, et al. In vivo investigation on the potential of galangin, kaempferol and myricetin for protection of D-galactose-induced cognitive impairment. Food Chem. 2012;135(4):2702-2707. [DOI] [PubMed] [Google Scholar]
- 13.Sabogal-Guáqueta AM, Muñoz-Manco JI, Ramírez-Pineda JR, Lamprea-Rodriguez M, Osorio E, Cardona-Gómez GP. The flavonoid quercetin ameliorates Alzheimer's disease pathology and protects cognitive and emotional function in aged triple transgenic Alzheimer's disease model mice. Neuropharmacology. 2015;93:134-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishola IO, Osele MO, Chijioke MC, Adeyemi OO. Isorhamnetin enhanced cortico-hippocampal learning and memory capability in mice with scopolamine-induced amnesia: role of antioxidant defense, cholinergic and BDNF signaling. Brain Res. 2019;1712:188-196. [DOI] [PubMed] [Google Scholar]
- 15.Bennett DA, Buchman AS, Boyle PA, Barnes LL, Wilson RS, Schneider JA. Religious orders study and Rush memory and aging Project. J Alzheimer's Dis. 2018;64(s1):S161–S189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson RS, Boyle PA, Yu L, et al. Temporal course and pathologic basis of unawareness of memory loss in dementia. Neurology. 2015;85(11):984-991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris MC, Tangney CC, Bienias JL, Evans DA, Wilson RS. Validity and reproducibility of a food frequency questionnaire by cognition in an older biracial sample. Am J Epidemiol. 2003;158(12):1213-1217. [DOI] [PubMed] [Google Scholar]
- 18.Flavonoid Proanthocynidin Provisional Table. University of Minnesota Nutrition Coordinating Center, University of Minnesota. [Google Scholar]
- 19.Schakel SF, Buzzard I, Gebhardt SE. Procedures for estimating nutrient values for food composition databases. J Food Compost Anal. 1997;10(2):102-114. [Google Scholar]
- 20.Buchman AS, Boyle PA, Wilson RS, Beck TL, Kelly JF, Bennett DA. Apolipoprotein E e4 allele is associated with more rapid motor decline in older persons. Alzheimer Dis Associated Disord. 2009;23(1):63-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buchman AS, Boyle PA, Wilson RS, Bienias JL, Bennett D. Physical activity and motor decline in older persons. Muscle Nerve. 2007;35(3):354-362. [DOI] [PubMed] [Google Scholar]
- 22.Wilson RS, Barnes LL, Krueger KR, Hoganson G, Bienias JL, Bennett DA. Early and late life cognitive activity and cognitive systems in old age. J Int Neuropsychol Soc. 2005;11(4):400-407. [PubMed] [Google Scholar]
- 23.Aggarwal NT, Bienias JL, Bennett DA, et al. The relation of cigarette smoking to incident Alzheimer's disease in a biracial urban community population. Neuroepidemiology. 2006;26(3):140-146. [DOI] [PubMed] [Google Scholar]
- 24.Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D depression symptoms index. J Aging Health. 1993;5(2):179-193. [DOI] [PubMed] [Google Scholar]
- 25.Bennett DA. Secular trends in stroke incidence and survival, and the occurrence of dementia. Stroke. 2006;37(5):1144-1145. [DOI] [PubMed] [Google Scholar]
- 26.Morris MC, Evans DA, Bienias JL, Tangney CC, Wilson RS. Vitamin E and cognitive decline in older persons. Arch Neurol. 2002;59(7):1125-1132. [DOI] [PubMed] [Google Scholar]
- 27.Barnard ND, Bunner AE, Agarwal U. Saturated and trans fats and dementia: a systematic review. Neurobiol Aging. 2014;35:S65–S73. [DOI] [PubMed] [Google Scholar]
- 28.Morris MC, Evans DA, Bienias JL, et al. Dietary folate and vitamin B12 intake and cognitive decline among community-dwelling older persons. Arch Neurol. 2005;62(4):641-645. [DOI] [PubMed] [Google Scholar]
- 29.Yuan C, Fondell E, Ascherio A, et al. Long-Term intake of dietary carotenoids is positively associated with late-life subjective cognitive function in a prospective study in US women. J Nutr. 2020;150(7):1871-1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robinson JG, Ijioma N, Harris W. Omega-3 fatty acids and cognitive function in women. Womens Health. 2010;6(1):119-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morris MC, Evans DA, Bienias JL, et al. Consumption of fish and n-3 fatty acids and risk of incident alzheimer disease. Arch Neurol. 2003;60(7):940-946. [DOI] [PubMed] [Google Scholar]
- 32.A Bennett D, A Schneider J, S Buchman A, L Barnes L, A Boyle P, S Wilson R. Overview and findings from the Rush memory and aging Project. Curr Alzheimer Res. 2012;9(6):646-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yeh TS, Yuan C, Ascherio A, Rosner BA, Willett WC, Blacker D. Long-term dietary flavonoid intake and subjective cognitive decline in US men and women. Neurology. 2021;97(10):e1041–e1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Root M, Ravine E, Harper A. Flavonol intake and cognitive decline in middle-aged adults. J Med Food. 2015/12/01 2015;18(12):1327-1332. [DOI] [PubMed] [Google Scholar]
- 35.Kesse-Guyot E, Fezeu L, Andreeva VA, et al. Total and specific polyphenol intakes in midlife are associated with cognitive function measured 13 Years later. J Nutr. 2011;142(1):76-83. [DOI] [PubMed] [Google Scholar]
- 36.Kim JH, Lee J, Lee S, Cho EJ. Quercetin and quercetin-3-β-d-glucoside improve cognitive and memory function in Alzheimer's disease mouse. Appl Biol Chem. 2016;59(5):721-728. [Google Scholar]
- 37.Liang J, Kerstin Lindemeyer A, Shen Y, et al. Dihydromyricetin ameliorates behavioral deficits and reverses neuropathology of transgenic mouse models of Alzheimer's disease. Neurochem Res. 2014;39(6):1171-1181. [DOI] [PubMed] [Google Scholar]
- 38.Peterson JJ, Dwyer JT, Jacques PF, McCullough ML. Improving the estimation of flavonoid intake for study of health outcomes. Nutr Rev. 2015;73(8):553-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The anonymized data, used for the study, are available through the Rush Alzheimer's Disease Center (RADC). Qualified investigators can find information regarding the formal requirements for access to data on the RADC research resource-sharing hub (radc.rush.edu).