Abstract
The main aim of this study was to evaluate the oils from chamomile seeds as a new source of bioactive compounds suitable for human consumption. A green extraction technique with supercritical carbon dioxide (sc-CO2) at pressures up to 450 bar and temperatures up to 60 °C was employed for the production of a high amount of biologically active oil. Additionally, exhausted waste material was re-extracted using sc-CO2 with the addition of ethanol. By optimization in operating pressure, temperature, production cost, fraction of milled seeds, and co-solvent addition, the amount of separated chamomile oil increased from 2.4 to 18.6% and the content of unsaturated fatty acids up to 88.7%. Oils contained α-bisabolol oxide A and B in amounts up to 1.4%. Linoleic acid was detected in an amount up to 711.1 mg/g and α-linolenic acid up to 27.5 mg/g. The total phenolic content in separated oil reached 80.4 mg GAE/g while the total flavonoid content reached 11.6 mg QE/g. The obtained chamomile oils showed antioxidant activity with an IC50 of up to 3.9 mg/mL. Among the 23 tested microorganisms, the antimicrobial activity of oils was the most pronounced against Gram-positive bacteria. The cytotoxic activity of oils was tested on normal and cancer-derived cell lines. Results indicated a significant potential for oil from chamomile seeds, produced in an eco-friendly manner, as a functional food.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11947-023-03038-9.
Keywords: Antimicrobial activity, Antioxidant activity, Biological activity, Total phenolic content, Fatty acid composition, Linoleic acid
Introduction
Food trends have introduced the term “functional” to refer to certain types of foods that not only meet a nutritional function but also contain bioactive compounds that have the ability to provide health benefits. This highlights functional oils derived from plants that contain essential fatty acids, antioxidants, and phenolics (Fregapane et al., 2020; Lyashenko et al., 2019). The importance of functional oils in the diet and their health benefits gave consumers awareness of what they eat and how their diet can help reduce disease risks for better life quality (Fregapane et al., 2020). These oils are commercially obtained from seeds (e.g., milk thistle and linseed) and nuts (e.g., pistachio and walnut). In the light of mentioned food trend, the present study was aimed at exploring chamomile seeds as a new source of functional oils. Chamomile plant is an annual herb with thin, joint, and fibrous roots. The hairy stems are branched and erected (height up to 0.3 m) with the narrow and long leaves divided into thread-like segments (Lebanna, 2005). Flower heads (diameter of 10 to 30 mm) are pedunculate, heterogamous, and separately placed (Mihyaoui et al., 2022). The golden yellow tubular florets with 5 teeth are 1.5 to 2.5 mm long and always ending in a glandulous tube. The 11 to 27 white plant flowers are 6 to 11 mm long and 3.5 mm wide and arranged concentrically (Mihyaoui et al., 2022). The receptacle is 6 to 8 mm wide, flat in the beginning, and conical. The seeds are yellowish-brown cypsela (i.e., one-seeded dehiscent fruit derived from a bicarpellate ovary with a single chamber) with 3–5 ribs (Mihyaoui et al., 2022; Shil & Mukherjee, 2016; Skilbeck et al., 2019). Although the chamomile plant has been consumed worldwide in the form of various teas for centuries, to the best of our knowledge, its seeds have never been used before for the production of functional oils suitable for human consumption.
One of the most popular chamomile varieties is Roman chamomile (Chamaemelum nobile, Asteraceae), a traditional herb that can be used externally and internally for medicinal purposes due to its antibacterial, antifungal, anti-spasmodic, anti-allergic, antipyretic, analgesic, antiseptic, and carminative properties (Lebanna, 2005; Scalia et al., 1999; Srivastava et al., 2010). For local applications, chamomile extracts can be used as ointments and inhalations to treat skin rashes, nasal inflammation, ear and eye infections, etc. (Catani et al., 2021; Srivastava et al., 2010; Stanojevic et al., 2016). Internally, chamomile infusions can be taken to battle indigestion, poor appetite, flatulence, diarrhea, and nausea (Lebanna, 2005; Srivastava et al., 2010). It was reported that a mixture of chamomile essential oil and flour can treat the indurations of the spleen and liver (Lebanna, 2005). Chamomile can also decrease the pain associated with migraine, inflamed joints, arthritis, etc. (Catani et al., 2021; Lebanna, 2005; Srivastava et al., 2010). Furthermore, several studies have found that chamomile extracts inhibit normal cell growth while significantly reducing the viability of various human cancer cell lines (Al-Dabbagh et al., 2019; Sak et al., 2017; Srivastava et al., 2010). The mentioned notable activities are ascribed to a complex mixture of different compounds found in chamomile extracts, mainly flavonoids, terpenes, esters, phenolics, and fatty acids (Lebanna, 2005; Povh et al., 2001; Rahimi et al., 2011; Srivastava et al., 2010).
The interest in separating bioactive compounds from plants for new or improved applications has motivated researchers to find new renewable sources or develop new techniques such as supercritical fluid extraction (SFE). This technique, which has a “green” label, enables sustainable extraction with a decrease in solvent consumption and its recycling (Kulkarni et al., 2017). One of the most significant advantages of using supercritical CO2 (sc-CO2) as a solvent for SFE is its complete removal from the final extract (Marques et al., 2016). The SFE technique proved to be very efficient for the extraction of lipids and other non-polar compounds. For the extraction of polar compounds, the addition of a small amount of a polar co-solvent to sc-CO2 is required (Al-Suod et al., 2019; Ferrentino et al., 2019; Lebanna, 2005; Reverchon & Senatore, 1994; Scalia et al., 1999). Several reports in the available literature described the separation of bioactive extracts from chamomile flowers, flower heads, and herb material using SFE (Al-Suod et al., 2019; Čižmek et al., 2021; Kaiser et al., 2004; Kotnik et al., 2007; Lebanna, 2005; Omidbaigi et al., 2003; Pekić et al., 1995; Povh et al., 2001; Rahimi et al., 2011; Reverchon & Senatore, 1994; Scalia et al., 1999; Zekovic, 2000). However, the present study is the first one to explore the separation of high-quality oils suitable for human consumption from chamomile seeds using the SFE process. For the first time, the obtained chamomile oils were characterized in terms of composition (fatty acids, total phenolic, and total flavonoid content), antioxidant activity, antimicrobial, and cytotoxic potential. This manuscript is an attempt to unlock the new food potential of the chamomile plant, a commercially cultivated sustainable biomass.
Materials and Methods
Materials
Roman chamomile (Chamaemelum nobile, Asteraceae) seeds, produced in the year 2021, were purchased from HerbFarm Edwin Lewczuk (Poland). CO2 (purity 99.9%) produced by Grupa Azoty (Zaklady Azotowe “Pulawy” S.A., Poland) was used for SFE. Trimethylsulfonium hydroxide solution (TMSH ∼0.25 M in methanol, Sigma-Aldrich, USA) and methyl tert-butyl ether (MTBE, Avantor Performance Materials LLC, USA) were used for converting fatty acids into methyl esters. Folin and Ciocalteu (2 M with respect to acid), gallic acid (97.5–102.5%), quercetin (≥ 95%), and aluminum chloride (99.99%) purchased from Sigma-Aldrich (Germany) as well as isopropanol (min 99.8%, ChemSolute®, Germany) and sodium carbonate (99.8%, Poch, Poland) were used for the evaluation of phenolic and flavonoid content. 2,2-Diphenyl-1-picryl-hydrazyl (DPPH) and L-ascorbic acid (reagent grade) were obtained from Sigma-Aldrich (Germany) and used for evaluation of antioxidant activity.
Pretreatment of Chamomile Seeds
The chamomile seeds were stored in a dark place at a temperature of 22 °C before use. The moisture content in seeds was determined using a moisture analyzer (MAC 50/1/WH, RADWAG®, Poland). Prior to the extraction, seeds were milled using a basic mill (Ika® A11, Poland) and sieved using a set of sieves for the separation of fractions with an average particle size of 0.20 mm (Fr I) and 0.38 mm (Fr II).
Separation of Oils from Chamomile Seeds
The extraction process was performed in a high-pressure micronization unit (SITEC, Switzerland), described elsewhere in detail (Milovanovic et al., 2022c). The milled seeds (50.0 g of Fr I) were placed in the extractor and after achieving the desired temperature (40 or 60 °C) the pressure was elevated to 300 or 450 bar. During the process, CO2 flow was maintained at 11.0 kg/h and the oil was collected in a separator at 34 °C and 50 bar. The extraction yield (Y, %) was calculated as a ratio between the chamomile oil mass and the initial mass of milled seeds placed in the extractor.
The process parameters that enable the separation of the highest amount of chamomile oil from Fr I were employed for oil separation from Fr II. In addition, oil was separated from native and exhausted Fr I and Fr II with the addition of ethanol (40 w/w%) as a co-solvent.
Cost Estimation for Chamomile Oil Production
The estimation of cost for oil separation from chamomile seeds was based on the Turton methodology (Turton et al., 2003) as previously described (Milovanovic et al., 2022c). Namely, estimated costs were calculated using the equation:
where COM is the manufacturing cost, FCI is the fixed capital investment, COL is the operational labor cost, CUT is the utility cost, CWT is the waste treatment cost, and CRM is the raw material cost. Aside from the costs already mentioned, the specific cost (SC) is calculated as the manufacturing cost divided by the total mass of oil produced. The main parameters used in calculating the cost of chamomile seed oil production are (1) the price of a high-pressure unit consisting of three extractors, each with a volume of 1000 L (3,200,000 EUR (De Melo et al., 2014)), (2) 20 workers, i.e., 15 manual workers with minimal wages and 5 supervisors with 30% higher wages working in 5 teams (3 teams per day) with prices 3.49 EUR/h per worker in 2020, 3.76 EUR/h per worker in 2021, and 4.03 EUR/h per worker in 2022 (Statista, 2022) in Poland, (3) the price of wholesale electricity in Poland of 0.046 EUR/kWh in 2020 (Kasprzak, 2020), 0.140 EUR/kWh in 2021 (Poland electricity prices, 2022), and 0.182 EUR/kWh in 2022 (Sas, 2022), and (4) the price of chamomile seeds grown in Poland for 1 kg at 10.27 EUR (HerbShop, 2022).
GC/MS and GC/FID Analysis of Chamomile Oils
The derivatization of fatty acids in chamomile oils was performed prior to GC/MS and GC/FID analysis according to the previously described method (Mazurek et al., 2017).
For qualitative analysis using the 7000C Triple Quadrupole GC/MS (Agilent Technologies, USA), derivatized samples (100 μL) were diluted with a MTBE/CH3OH solution (9:1 v/v) at a 1:10 v/v as previously described (Milovanovic et al., 2022c).
For quantitative analysis using GC System 7820A (Agilent Technologies, USA) coupled with a flame ionizer detector (FID), derivatized samples (500 μL) were diluted with MTBE (500 μL) as previously described (Milovanovic et al., 2022a). For fatty acid quantification, palmitic, stearic, oleic, linoleic, and α-linolenic acid standards (purchased from Sigma-Aldrich, Germany) were used.
Total Phenolic Content in Chamomile Oils
Total phenolic content was estimated by the Folin–Ciocalteu (FC) method as previously described (Milovanovic et al., 2022a, b). In short, solutions of chamomile oils in isopropanol (20 mg/mL) were mixed with distilled water, FC reagent, and sodium carbonate solution. After 30 min, the absorbance of mixtures, filtered through a 0.45 μm syringe filter, was recorded at 765 nm using a spectrophotometer (Jasco V-650, Germany). Results were expressed as the mass of gallic acid equivalents per chamomile oil mass (mg GAE/g) using a calibration curve of gallic acid in isopropanol obtained for concentrations 0.01 − 1.00 mg/mL.
Total Flavonoid Content in Chamomile Oils
Total flavonoid content was estimated as previously described (Milovanovic et al., 2022a, b). For this purpose, chamomile oils were diluted with isopropanol to obtain solutions with a concentration of 2 − 20 mg/mL. After preparation of the calibration curve (quercetin in isopropanol for concentrations 3 − 60 μg/mL), results were expressed as the mass of quercetin equivalents per chamomile oil mass (mg QE/g).
Antioxidant Activity of Chamomile Oils
The DPPH radical scavenging capacity was analyzed as previously described (Milovanovic et al., 2022a, b). Chamomile oils were dissolved in isopropanol to obtain solutions with a concentration ranging from 2 to 20 mg/mL. The DPPH radical scavenging activity of oils was calculated using the equation:
where I is an inhibition percentage, Acontrol is the absorbance of the control, and Asample is the absorbance of oil solutions. Results were expressed as the concentration of a chamomile oil solution required for a 50% decrease in absorbance compared with the control (IC50). The method was validated by an analysis of the DPPH radical scavenging activity of vitamin C.
Antimicrobial Activity of Chamomile Oils
The minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC), or minimum fungicidal concentration (MFC) of the chamomile oils was determined for 17 reference strains (Gram-positive, Gram-negative bacteria, and fungi belonging to yeasts) of the American Type Culture Collection (ATCC) previously reported (Milovanovic et al., 2022a). In addition, the activity of chamomile oils was tested on Staphylococcus aureus ATCC 43300, Enterococcus faecalis ATCC 29212, Candida albicans ATCC 2091, Candida auris CDC B11903, Candida lusitaniae ATCC 34449, and Candida tropicalis ATCC 1369. Tests were performed using the microdilution broth method according to guidelines of the European Committee on Antimicrobial Susceptibility Testing (EUCAST, 2003) and our previously published method (Malm et al., 2021; Milovanovic et al., 2022a). Fluconazole (0.06–16 µg/mL), ciprofloxacin (0.015–16 µg/mL), and vancomycin (0.06–16 µg/mL) were used as standard antimicrobial substances active against yeasts, Gram-negative bacteria, and Gram-positive bacteria, respectively.
Cytotoxic Activity of Chamomile Oils
Cell lines used in experiments included normal VERO (ECACC, No. 84113001) cells and cancer-derived cell lines—FaDu (ATCC, HTB-43, human hypopharyngeal squamous cell carcinoma) and RKO (ATCC, Cat. No. CRL-2577, colon carcinoma). The VERO cells were cultured using Dulbecco Modified Eagle Medium (DMEM, Corning, USA), and cancer cells using Modified Eagle Medium (MEM, Corning). Media were supplemented with antibiotics (Penicillin–Streptomycin Solution, Corning) and fetal bovine serum (FBS, Corning)—10% (cell passaging) or 2% (cell maintenance and experiments). Phosphate-buffered saline (PBS) and trypsin were bought from Corning. The MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) and DMSO (dimethyl sulfoxide) were purchased from Sigma (Sigma-Aldrich, USA). Incubation was carried out in a 5% CO2 atmosphere at 37 °C (CO2 incubator, Panasonic Healthcare Co., Japan).
The cytotoxicity screening was performed using an MTT-based assay, as previously described (Zengin et al., 2020). The cells were seeded into 96-well plates (Falcon, TC-treated, Corning). Following overnight incubation, serial dilutions of oil stock solutions were applied and incubation was continued for 24 h. Afterwards, all media were removed, plates were washed with PBS, and 10% of MTT solution (5 mg/mL) in serum-free cell media was added and incubated for 3 h. Subsequently, 100 µL per well of SDS/DMF/PBS (14% SDS, 36% DMF, 50% PBS) solvent was added, and the plates were left at 37 °C until precipitated formazan crystals were dissolved. Lastly, the Synergy H1 Multi-Mode Microplate Reader (BioTek Instruments, Inc., USA) with Gen5 software (ver. 3.09.07; BioTek Instruments, Inc.) was used to measure the absorbance (540 and 620 nm) and results were exported to GraphPad Prism (version 7.04) to evaluate the CC50 values (50% cytotoxic concentration). The selectivity indexes (SI) were calculated in relation to normal VERO cells (SI = CC50VERO/CC50Cancer; SI > 1 is an indication of anticancer selectivity).
Statistical Analysis of Obtained Results
Quantitative data were reported as mean ± standard deviation. A one-way ANOVA with post-hoc Tukey HSD test was used to evaluate the significant difference between amount of separated oils, qualitative composition, content of fatty acids, total phenolic content, total flavonoid content, and concentrations of chamomile oils that scavenge 50% of DPPH radicals. Statistical analyses were performed using Astatsa online statistical calculator (Vasavada, n.d.).
Results
Oil Separation from Chamomile Seeds
Seeds were milled and sieved to obtain two fractions: Fr I with an average particle size of 0.20 mm (Fig. S1a) and Fr II with an average particle size of 0.38 mm (Fig. S1b). First, Fr I was used for oil production and the obtained results can be seen in Fig. 1a.
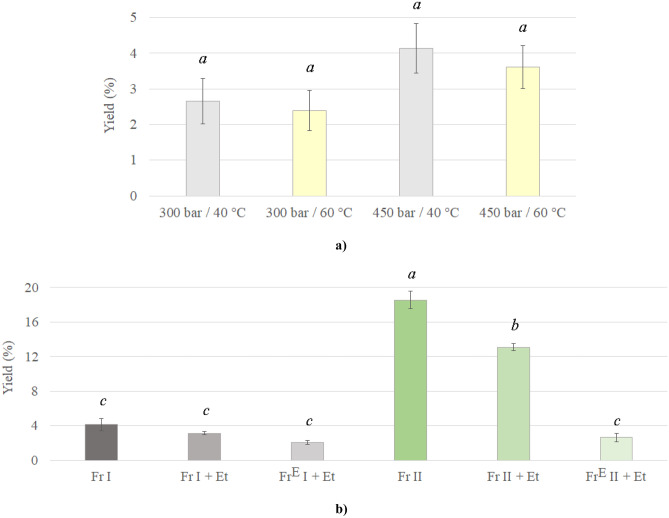
Fig. 1.
Changes in oil extraction yield for Fr I as a function of operating pressure and temperature (a) and Fr I and Fr II with material pretreatment at 450 bar and 40 °C (b). Different letters (a-c) strongly suggest that values are significantly different (p < 0.01)
To obtain a large amount of oil during the extraction process, the operating pressure was increased from 300 to 450 bars, and the temperature was raised from 40 to 60 °C. However, results show that an increase in temperature led to a slight decrease in extraction yield from 2.7 to 2.4% (at 300 bar) and from 4.1 to 3.6% (at 450 bar). The same observation was previously reported for chamomile flowers extracted at temperatures 36 − 64 °C and pressures 80 − 152 bar (Lebanna, 2005). In addition, it can be seen that an increase in pressure from 300 to 450 bar at 40 °C increased the extraction yield (Fig. 1a). These results confirm that oil separation from chamomile seeds follows the change in solvent density, i.e., sc-CO2 solvating power (Milovanovic et al., 2013; Pereira & Meireles, 2010). Specifically, an increase in sc-CO2 density from 830 to 975 kg/m3 increased the extraction yield from seeds. An amount of separated oils from Fr I of chamomile seeds is in agreement with previously reported values for chamomile flowers and herbs such as 1.9, 3.4, and 3.8% obtained from chamomile flowers/flowerheads at a temperature of 40 °C and pressures of 90, 202.6, and 250 bar, respectively (Kotnik et al., 2007; Lebanna, 2005; Reverchon & Senatore, 1994).
Besides the amount of separated oil from chamomile seeds, the costs of its production were an additional parameter that was estimated for the selection of the best pressure and temperature extraction conditions. The estimation of costs for the first year of industrial production of chamomile seed oils, based on parameters presented in the “Cost Estimation for Chamomile Oil Production” subsection and data presented in Fig. 1a, is shown in Table 1. It can be seen that the variation in yearly labor costs did not have a significant effect on the manufacturing cost (COM) as the yearly price of electricity had. The lowest price of chamomile oil would have been achieved in the year 2020, when the price of wholesale electricity was the lowest. Similar to our calculations, it was reported that slight variations in the price of labor costs do not have a significant share in the total COM for high-volume extraction vessels (Huang et al., 2020). In addition, a substantial contributor to the estimated price of chamomile oil production is the price of raw material, as previously reported (Huang et al., 2020). The price of the produced oil ranged from 534 to 1714 EUR/kg, due to the relatively high price of chamomile seeds of ca. 10 EUR/kg and relatively low extraction yield of up to 4%. Namely, previously reported estimated price for the milk thistle oil production ranged from 36 to 57 EUR/kg due to the seed price of ca. 2 EUR/kg and extraction yield of up to 26% (Milovanovic et al., 2022c). Also, it was reported that the estimated price for pistachio extract ranged from 713 to 876 EUR/kg due to leaves price of ca. 1 EUR/kg and an extraction yield of up to 0.3% (Aydi et al., 2020). By comparing chamomile oil manufacturing costs, it can be seen that the lowest estimated price can be achieved for the extraction process conducted at a pressure of 450 bar and temperature of 40 °C. Based on this result, the stated pressure and temperature conditions were selected for further investigation.
Table 1.
Values of estimated costs for chamomile oil production from Fr I by SFE based on literature data (for the first year of production)
| Year | P (bar) / T (°C) | mOIL (kg/year) | FCI (EUR/year) | COL (EUR/year) | CUT (EUR/year) | CRM (EUR/year) | COM (EUR/year) | SC (EUR/kg) |
|---|---|---|---|---|---|---|---|---|
| 2020 | 300 / 40 | 13,242 | 3,248,286 | 219,102 | 2,548,020 | 5,197,029 | 11,112,038 | 839 |
| 300 / 60 | 11,993 | 3,246,000 | 219,102 | 2,569,853 | 5,197,029 | 11,138,198 | 929 | |
| 450 / 40 | 20,688 | 3,247,229 | 219,102 | 2,488,053 | 5,197,029 | 11,037,957 | 534 | |
| 450 / 60 | 18,040 | 3,247,229 | 219,102 | 2,626,739 | 5,197,029 | 11,208,541 | 621 | |
| 2021 | 300 / 40 | 13,242 | 3,248,286 | 236,053 | 7,754,845 | 5,197,029 | 17,562,707 | 1,326 |
| 300 / 60 | 11,993 | 3,246,000 | 236,053 | 7,821,292 | 5,197,029 | 17,643,743 | 1,471 | |
| 450 / 40 | 20,688 | 3,247,229 | 236,053 | 7,572,336 | 5,197,029 | 17,337,900 | 838 | |
| 450 / 60 | 18,040 | 3,247,229 | 236,053 | 7,994,424 | 5,197,029 | 17,857,068 | 990 | |
| 2022 | 300 / 40 | 13,242 | 3,248,286 | 253,003 | 10,064,127 | 5,197,029 | 20,449,399 | 1,544 |
| 300 / 60 | 11,993 | 3,246,000 | 253,003 | 10,150,362 | 5,197,029 | 20,554,773 | 1,714 | |
| 450 / 40 | 20,688 | 3,247,229 | 253,003 | 9,827,270 | 5,197,029 | 20,157,744 | 974 | |
| 450 / 60 | 18,040 | 3,247,229 | 253,003 | 10,375,049 | 5,197,029 | 20,831,513 | 1,155 |
mOIL mass of oil produced, FCI fixed capital investment, COL operational labor cost, CUT utility cost, CRM raw material cost, COM manufacturing cost, SC specific cost
In order to produce high amounts of high-quality oils, further extraction processes were performed by varying the seeds’ pretreatment at optimal pressure and temperature (450 bar and 40 °C). For this purpose, Fr II, Fr I and Fr II with the addition of ethanol (denoted as Fr I + Et and Fr II + Et), and exhausted Fr I and Fr II with the addition of ethanol (denoted as FrE I + Et and FrE II + Et) were subjected to extraction. The chamomile seed structure and steps for its pretreatment enabled the preparation of two different fractions (Fr I and Fr II). Namely, chamomile seeds are composed of the pericarp, endosperm, and embryo. While the pericarp has a thickness of only 65 − 72 μm, the embryo takes a major part of the chamomile seed (Shil & Mukherjee, 2016). During milling, seed walls crack releasing the seeds’ inner content. The seed’s kernel (endosperm and embryo) is easily crushed, while the pericarp gets only slightly damaged at the beginning of the milling (Milovanovic et al., 2022b). This occurrence, combined with sieving, enables the separation and collection of material with smaller particle size mostly composed from seeds’ kernel, i.e., Fr I (Fig. S1a). After separation of Fr I, most of the remaining milled material is composed of chamomile seeds’ pericarp, i.e., Fr II (Fig. S1b). The color of the tested plant material differs between fractions (beige for Fr I and dark brown for Fr II). These different ratios of pericarp to kernel in milled chamomile seeds could be one of the reasons for the different extraction results obtained for Fr I and Fr II presented in Fig. 1b. Oil secretory glands that are located in the pericarp of chamomile seeds (Skilbeck et al., 2019) can be responsible for the extraction yield of 18.6% obtained for Fr II (pericarp rich fraction) while the extraction yield for Fr I (kernel rich fraction) was 4.5-fold lower. A similar value of 19% extraction yield for chamomile seeds was reported for conventional extraction that employed hexane as a solvent (Pereira et al., 2005). An additional difference between fractions is their particle size. Although Fr I had a smaller particle size, which is advised for improving extraction efficacy, Fr II contained so significantly higher amount of extractible compounds that not even a larger particle size could impede SFE. Namely, Kaiser et al. (2004) stated that a decrease in chamomile flower particle size from 3.2 to 0.5 mm increases extractability due to the reduction in mass transfer resistance, which was not the case for the plant material tested in this study.
Besides variation in the milled seed fraction selected for oil production, the addition of co-solvent was tested for improvement in the extraction yield and oil quality. It was interesting to see that the addition of ethanol to sc-CO2 led to a decrease in extraction yields for both Fr I (from 4.1 to 3.2%) and Fr II (from 18.6 to 13.1%) because the addition of a co-solvent during SFE usually increases the amount of separated extract from plant material. For instance, Kaiser et al. (2004) reported that methanol as a co-solvent increased the extraction yield for chamomile flowers from 9.6 to 16.3% at 100 bar and 70 °C. However, this is not the case for oil-rich plant materials. Namely, it was reported that the addition of ethanol to sc-CO2 for separation of oil from milk thistle seeds at 300 bar and 40 °C decreased extraction yield from 21.4 to 18.2% (Lukic et al., 2022a). Sc-CO2 is a non-polar solvent and its ability to dissolve polar compounds is limited. The addition of a co-solvent to sc-CO2 ameliorates the extraction efficiency of polar compounds by increasing their solubility (Ferrentino et al., 2019). This would mean that the content of polar compounds like polyphenols in separated oil increased while the content of non-polar compounds like fatty acids decreased. This hypothesis was further tested by analysis of obtained oils.
After the plant material (Fr I and Fr II) was exhausted at 450 bar and 40 °C using neat sc-CO2, an attempt was made towards separating the remaining compounds using ethanol as a co-solvent. Exhausted plant materials are a valuable source of bioactive compounds that could be re-extracted using sc-CO2 with a co-solvent, which was previously demonstrated for milk thistle seeds primarily defatted using petroleum ether (Lukic et al., 2022a). The result from the current study shows that chamomile seed waste material could be re-extracted, achieving extraction yields of 2.1 and 2.6% for Fr I and Fr II, respectively. The reasons for the separation of the additional oil are numerous. For instance, the operating time of the first SFE was not sufficient to enable complete plant material exhaustion; the oil could not pass through cell walls since a part of cells containing oil remained intact; remaining extractible compounds were more tightly bonded to the cell structure matrix; more polar components with higher solubility in ethanol remained in seeds after exhaustion with sc-CO2; remaining extractible compounds had a significantly lower affinity towards sc-CO2 compared to compounds primarily extracted, etc. (Lukic et al., 2022a, b).
Chemical Composition of Chamomile Oils
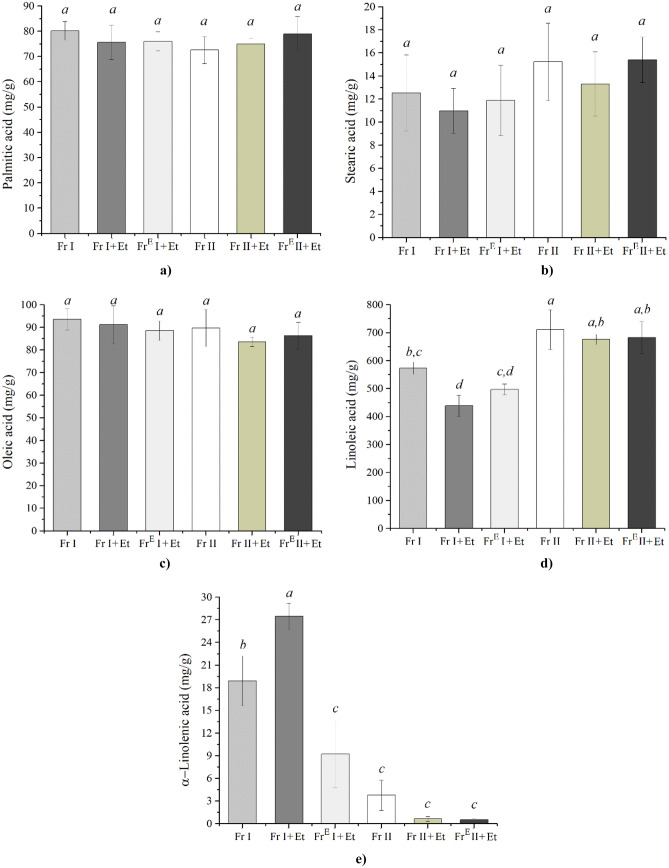
The results of GC/MS analysis for chamomile oils are presented in Table 2. It can be seen that oils contain about 10.4 − 13.1% of saturated fatty acids (SFA) and about 80.3 − 88.7% of unsaturated fatty acids (UFA). This UFA content in chamomile oils is higher compared with UFA content in mango seed kernel oils (52 − 56%) (Choudhary et al., 2022) and melon seeds (83.7%) (Bimakr et al., 2016). Chamomile seed oils also have higher content of poly unsaturated fatty acids (PUFA) compared with olive seed oils (which was around 17.0%) (Ranalli et al., 2009), fennel seed oil (12.8%) (Bettaieb Rebey et al., 2019), and anise seed oil (25.0%) (Bettaieb Rebey et al., 2019). It can be seen that the dominant compound is linoleic fatty acid, reaching values from 63.2 to 77.8%. Besides linoleic acid, the main constituents of oils were palmitic (from 9.2 to 11.6%) and oleic fatty acid (from 8.7 to 11.8%). Oil from chamomile seeds obtained by extraction with hexane contained 69.5% linoleic acid, 12.5% palmitic acid, and 13.1% oleic acid (Pereira et al., 2005). When compared to the previously reported fatty acid composition of chamomile essential oil (Hmamou et al., 2012), oils from chamomile seeds obtained in this study contained a similar amount of palmitic acid; a lower amount of stearic acid, α-linolenic acid, and oleic acid; and a higher amount of linoleic acid and palmitoleic acid. For instance, the content of linoleic acid in chamomile essential oil was 54.8% (Hmamou et al., 2012). Also, it was reported that supercritical extracts from chamomile flowers contained 2.8% of both palmitic and linoleic acid (Rahimi et al., 2011).
Table 2.
Compounds present in oils obtained from chamomile seeds determined by GC/MS
| Compounds | Fr I (%) | Fr I + Et (%) | FrE I + Et (%) | Fr II (%) | Fr II + Et (%) | FrE II + Et (%) |
|---|---|---|---|---|---|---|
| α-Bisabolol oxide B | 0.35a ± 0.10 | 1.16a ± 0.01 | / | / | / | / |
| α-Bisabolol oxide A | 0.33b ± 0.09 | 1.35a ± 0.11 | 0.05b ± 0.02 | / | / | / |
| Palmitic acid (C16:0) | 10.80a,b,c ± 0.63 | 11.57a,b ± 0.13 | 11.60a ± 0.08 | 9.80b,c ± 0.32 | 9.25c ± 0.28 | 9.16c ± 0.11 |
| Palmitoleic (C16:1) | 0.10a ± 0.01 | 0.60a ± 0.06 | 0.23a ± 0.13 | 0.26a ± 0.16 | 0.52a ± 0.12 | 0.53a ± 0.14 |
| Stearic acid (C18:0) | 1.57a ± 0.17 | 1.47a ± 0.48 | 1.51a ± 0.10 | 1.56a ± 0.05 | 1.20a ± 0.06 | 1.19a ± 0.06 |
| Oleic acid (C18:1) | 10.72a,b ± 0.17 | 11.75a ± 0.42 | 10.80a,b ± 0.19 | 8.98c ± 0.21 | 8.58c ± 0.16 | 8.70c ± 0.14 |
| Elaidic acid (C18:1) | 1.67a ± 0.08 | 2.30a ± 0.20 | 1.95a ± 0.33 | 1.06b ± 0.02 | 1.79a ± 0.15 | 1.78a ± 0.13 |
| Linoleic acid (C18:2) | 72.23c,d ± 0.52 | 63.19e ± 1.18 | 69.48d ± 0.46 | 77.58a,b ± 0.05 | 77.83a ± 0.36 | 75.48a,b,c ± 0.29 |
| α-Linolenic acid (C18:3) | 0.65a ± 0.11 | 1.35a ± 0.32 | 0.48a ± 0.13 | 0.08b ± 0.03 | t | t |
| Arachidonic acid (C20:4) | 0.48a ± 0.20 | 1.10a ± 0.17 | 0.52a ± 0.06 | / | / | / |
| Total content | 98.88 ± 1.42 | 95.82 ± 0.33 | 96.60 ± 0.09 | 99.31 ± 0.28 | 99.17 ± 0.82 | 96.83 ± 0.59 |
| SFA | 12.37 ± 0.80 | 13.04 ± 0.36 | 13.11 ± 0.02 | 11.36 ± 0.27 | 10.45 ± 0.33 | 10.35 ± 0.17 |
| UFA | 85.84 ± 1.10 | 80.28 ± 0.13 | 83.44 ± 0.12 | 87.95 ± 0.01 | 88.72 ± 0.49 | 86.48 ± 0.42 |
| PUFA | 73.36 ± 1.42 | 65.63 ± 0.69 | 70.47 ± 0.53 | 77.65 ± 0.02 | 77.83 ± 0.36 | 75.48 ± 0.29 |
SFA saturated fatty acids, PUFA polyunsaturated fatty acid, UFA total unsaturated fatty acids, t found in traces
Different letters in the superscript represent significant differences (p < 0.05) (comparisons were made row-wise)
It was interesting to note that α-bisabolol oxide A and B were only present up to 1.4% in the oil obtained from Fr I. These values are comparable to previously reported values found in supercritical extracts from chamomile flowers obtained at 250 bar and 40 °C (Rahimi et al., 2011). However, Kotnik et al. (2007) reported bisabolol content to be 0.025% in chamomile flowerheads obtained at the same conditions. Pekic et al. (1995) also found up to 1% α-bisabolol oxide A and B summary content in the extract obtained at 80 bar and 40 °C. Results obtained in the current study are especially significant considering the anti-flogistic, anti-spasmodic, anti-allergic, anti-inflammatory, sedative, and vermifuge properties of α-bisabolol (Povh et al., 2001; Rahimi et al., 2011).
The use of ethanol as a co-solvent for extraction from Fr I and Fr II had a variable effect on the separation of fatty acids. While the addition of ethanol reduced the linoleic acid content of the oil from Fr I, it increased the α-linolenic acid content from 0.65 to 1.35%. For Fr II, the effect of ethanol addition on the content of mentioned unsaturated fatty acids was vice versa. Similarly, it was reported that ethanol as a co-solvent to sc-CO2 increased the content of linoleic acid while it decreased the content of oleic acid in oils from milk thistle seeds (Lukic et al., 2022a) and brewer’s spent grain (Ferrentino et al., 2019). It is interesting to notice that chamomile oils obtained from exhausted plant material (FrE I + Et and FrE II + Et) contained almost the same fatty acids as oils obtained from native plant material. A similar observation was reported for supercritical oil from hemp seeds primarily extracted by cold pressing (Lukic et al., 2022b).
The quantitative content of dominant fatty acids present in chamomile oils was determined using a GC/FID method, and the results are presented in Fig. 2. It can be seen that the content of palmitic and oleic acids is only slightly changing with a change in chamomile seeds’ pretreatment. However, the content of stearic and linoleic acids is changing with pretreatment achieving the highest values of 15.4 and 711.1 mg/g, respectively, in the oil obtained from Fr II. The detected content of linoleic acid present in oil from chamomile seeds is one of the highest ever reported. For instance, it was reported that the contents of linoleic acid in supercritical oil from milk thistle and dandelion seeds were 514.8 and 658.3 mg/g, respectively (Milovanovic et al., 2022a, b). In addition, it was reported that commercial oils obtained by conventional methods also contain smaller amounts of linoleic acid such as linseeds (150 mg/g) (Matthäus & Özcan, 2015), peanut seeds (283 mg/g) (Matthäus & Özcan, 2015), soybeans (340 mg/g) (Rymer et al., 2011), and cotton seeds (563 mg/g) (Matthäus & Özcan, 2015). To the best of our knowledge, only a higher value of linoleic acid content was reported for safflower oil (Orsavova et al., 2015).
Fig. 2.
Quantitative content of dominant fatty acids in oils obtained from chamomile seeds determined by GC/FID. Different letters (a–d) represent significant differences (p < 0.05) (comparisons were made separately for each fatty acid)
The content of α-linolenic acid in chamomile oils ranged from 0.5 to 27.5 mg/g. The highest amount was found in the oil obtained from Fr I with ethanol as a co-solvent (Fr I + Et) as shown in Fig. 2e. Obtained values of α-linolenic acid content are comparable to supercritical extracts from, e.g., silverweed (which was in the range from 0.32 to 0.78 mg/g) (Luan et al., 2022), wheat bran (37.3 mg/g) (Rebolleda et al., 2020), or grape seeds (5.2 mg/g) (Barriga-Sánchez et al., 2021).
Only two fatty acids are known to be essential for humans and both can be found in chamomile seed oils: α-linolenic acid (an omega-3 fatty acid) and linoleic acid (an omega-6 fatty acid). This information is especially important considering the numerous beneficial effects of essential fatty acids on human health such as antithrombotic, antiarrhythmic, anti-inflammatory, and hypolipidemic activity (Orsavova et al., 2015).
Total Phenolic Content in Chamomile Oils
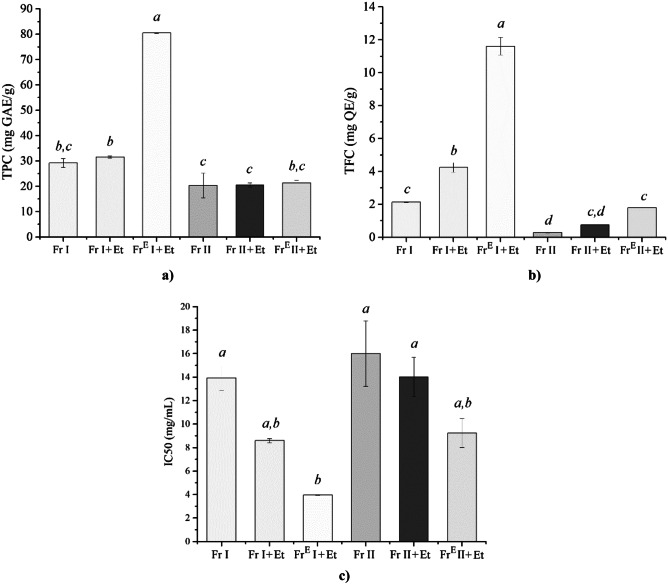
The distribution of phenolic compounds separated from chamomile seeds is depicted in Fig. 3a. Analysis showed that oils obtained from Fr I had total phenolic content (TPC) of 29.1 mg GAE/g and that oils from Fr II contained 20.3 mg GAE/g of phenolic compounds. These results were significantly higher compared with the recent report for TPC found in supercritical extracts from chamomile herbs obtained at 200 bar and 48 °C, which was 2.3 mg GAE/g (Čižmek et al., 2021). In addition, values recorded in the present study are higher compared with TPC found in functional oils from other plant materials like pistachio (up to 12.2 mg GAE/g) (Fregapane et al., 2020), walnut (up to 19.0 mg GAE/g) (Fregapane et al., 2020), canola seed (around 0.4 mg GAE/mL) (Fadairo et al., 2022), and brewer’s spent grain (4.6 − 8.4 mg GAE/g) (Ferrentino et al., 2019).
Fig. 3.
Total phenolic content in chamomile oils (a), total flavonoid content in chamomile oils (b), and concentrations of chamomile oils that scavenge 50% of DPPH radicals (c). Different letters (a–d) represent significant differences (p < 0.05)
The addition of ethanol as a co-solvent had a slight influence on TPC. The TPC value increased from 29.1 to 31.5 mg GAE/g for samples obtained from Fr I and from 20.3 to 20.5 mg GAE/g for samples obtained from Fr II, respectively. The obtained results are comparable to the content of phenolic compounds found in chamomile flower ethanolic extracts (Al-Dabbagh et al., 2019; Catani et al., 2021). The highest value of TPC (80.4 mg GAE/g) was recorded for the sample FrE I + Et that was re-extracted using co-solvent ethanol. It can be assumed that, after the removal of non-polar compounds in the first extraction process, the remaining more polar phenolic compounds could be re-extracted in the second SFE with polar co-solvent.
Values of TPC found in oils from chamomile seeds in the present study, which range from 20.3 to 80.4 mg GAE/g, are higher compared with previously reported TPC in oils obtained by SFE from various seeds. Namely, oil from milk thistle seeds contained 9.2 − 14.2 mg GAE/g (Milovanovic, 2022b), dandelion seeds contained 5.5 − 12.1 mg GAE/g (Milovanovic et al., 2022a), grape seeds contained 0.1 mg GAE/g (Barriga-Sánchez et al., 2021), purslane seeds contained 1.7 mg GAE/g (Sodeifian et al., 2018), burdock seeds contained 1.2 mg GAE/g (Stefanov et al., 2022), melon seeds contained 42.8 mg GAE/g (Bimakr et al., 2016), fennel seeds contained 0.5 mg GAE/g (Bettaieb Rebey et al., 2019), and anise seeds contained 0.9 GAE/g (Bettaieb Rebey et al., 2019).
Total Flavonoid Content in Chamomile Oils
Analysis showed that total flavonoid content (TFC) in oils, presented in Fig. 3b, has a similar trend to total phenolic content presented in Fig. 3a. Namely, TFC in Fr I of 2.1 mg QE/g was higher compared with that in Fr II (0.3 mg QE/g). Addition of ethanol as a co-solvent increased TFC in chamomile oil from 2.1 to 4.2 mg QE/g and from 0.3 to 0.8 mg QE/g for Fr I and Fr II, respectively. The highest value of TFC (11.6 mg QE/g) was recorded for the sample FrE I + Et that was re-extracted using co-solvent ethanol. This finding is in agreement with the results of Kaiser et al. (2004), who stated that the addition of a polar solvent to sc-CO2 increases the extraction of flavonoids from chamomile flowers due to their polar nature.
Values of TFC found in oils from chamomile seeds range from 0.3 to 11.6 mg QE/g. These values are slightly lower compared to previously reported TFC found in ethanolic extracts from chamomile flowers, which ranged from 15.3 to 27.3 mg/g (Catani et al., 2021). However, values obtained in the present study are similar or higher compared with previously reported TFC for oils obtained from various seeds using SFE. Namely, TFC found in oil from milk thistle seeds was 0.1 − 0.2 mg QE/g (Milovanovic, 2022b), dandelion seeds was 0.2 − 0.6 mg QE/g (Milovanovic et al. 2022a), mango seeds was 13.6 mg QE/g (Buelvas-Puello et al., 2021), and melon seeds was up to 0.4 mg QE/g (Bimakr et al., 2016).
DPPH Radical Scavenging Activity of Chamomile Oils
The DPPH radical scavenging activity test is the most commonly used for determination of antioxidant activity of a variety of plant extracts. Selected parameters for the method used in this study were validated by the analysis of the DPPH radical scavenging activity of vitamin C. This analysis showed that the required vitamin C concentration to scavenge 50% of DPPH radicals (IC50) was 10.2 μg/mL, which is in the range of literature reports. This test was used to assess the antioxidant activity of obtained oils. The results presented in Fig. 3c as IC50 value show that the antioxidant activity of oils follows the trend of TFC (Fig. 3b). The addition of ethanol as a co-solvent led to the separation of compounds that show stronger antioxidant activity. In addition, oil obtained from exhausted plant material (sample FrE I + Et) showed the best DPPH radical scavenging activity with IC50 of 3.9 mg/mL.
Values of IC50 found for oils from chamomile seeds ranged from 3.9 to 16.0 mg/mL. These results are in agreement with previously reported IC50 of 2.1 − 3.3 mg/mL for chamomile flowers essential oil (Stanojevic et al., 2016). In addition, it was previously reported that a solution of an extract from chamomile herbs obtained at 200 bar and 48 °C in a concentration of 25 mg/mL inhibits DPPH radicals around 90% (Čižmek et al., 2021). Results obtained in this study are better compared with previously reported ones for oils from various seeds separated by SFE. Namely, it was reported that IC50 value for oils from milk thistle seeds was in the range 12.7 − 24.4 mg/mL (Milovanovic, 2022b), hemp seeds was 9.2 mg/mL (Hong et al., 2015), and burdock seed was 54.5 mg/mL (Stefanov et al., 2022).
Antimicrobial Activity of Chamomile Oils
The results of an antimicrobial analysis performed using oils obtained from chamomile seeds are presented in Table 3. Chamomile oils showed differential activity against the tested reference bacteria (MIC values from 0.02 to ≥ 10 mg/mL) and yeasts (MIC = 1.25–10 mg/mL), which depended on the seeds’ pretreatment. Oils demonstrated relatively low activity against Gram-negative bacteria with MIC values in the range from 5 to ≥ 10 mg/mL. All oils showed the highest activity against Gram-positive bacteria Micrococcus luteus, Bacillus subtilis, and Bacillus cereus with MIC values ranging from 0.3 to 0.6 mg/mL, 0.02 to 0.3 mg/mL, and 0.16 to 1.25 mg/mL, respectively. The same stronger activity against Gram-positive bacteria compared to Gram-negative bacteria was previously reported for oils obtained from dandelion seeds (Milovanovic et al., 2022a) and mango seeds (Choudhary et al., 2022). It was also previously reported that essential oil from chamomile flowers has stronger activity against Gram-positive Staphylococcus aureus compared with Gram-negative Escherichia coli and Pseudomonas aeruginosa (Stanojevic et al., 2016). The antifungal activity of tested chamomile oils was almost unified against Candida strains. Oils showed good activity against all tested yeasts with MIC ranging from 1.25 to 10 mg/mL.
Table 3.
Activity of chamomile oils against reference microorganism strains expressed as MIC (mg/mL), MBC (mg/mL), and MFC (mg/mL)
| Microbial strains | Fr I | Fr I + Et | FrE I + Et | Fr II | Fr II + Et | FrE II + Et | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gram-positive bacteria | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC |
| Staphylococcus aureus ATCC 25923 | 5 | 5 | 2.5 | 10 | 2.5 | 5 | 2.5 | 5 | 1.25 | 2.5 | 1.25 | 2.5 |
| Staphylococcus aureus ATCC 29213 | 2.5 | 5 | 5 | 5 | 5 | 5 | 1.25 | 2.5 | 2.5 | 5 | 1.25 | 5 |
| Staphylococcus aureus ATCC 43300 | 5 | 5 | 2.5 | 5 | 2.5 | 5 | 1.25 | 5 | 2.5 | 5 | 2.5 | 5 |
| Staphylococcus aureus ATCC BAA1707 | 5 | 5 | 5 | 5 | 5 | 5 | 1.25 | 5 | 2.5 | 5 | 2.5 | 5 |
| Staphylococcus epidermidis ATCC 12228 | 5 | 10 | 5 | 10 | 10 | 10 | 5 | 10 | 5 | 10 | 5 | 10 |
| Enterococcus faecalis ATCC 29212 | 5 | 10 | 5 | 10 | 10 | 10 | 5 | 10 | 5 | 10 | 5 | 10 |
| Micrococcus luteus ATCC 10240 | 0.6 | 5 | 0.3 | 5 | 0.3 | 2.5 | 0.3 | 5 | 0.6 | 2.5 | 0.3 | 2.5 |
| Bacillus subtilis ATCC 6633 | 0.3 | 2.5 | 0.16 | 2.5 | 0.04 | 2.5 | 0.04 | 1.25 | 0.02 | 2.5 | 0.02 | 5 |
| Bacillus cereus ATCC 10876 | 0.3 | 2.5 | 1.25 | 5 | 0.16 | 1.25 | 0.6 | 2.5 | 0.3 | 2.5 | 0.3 | 2.5 |
| Gram-negative bacteria | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC |
| Salmonella Typhimurium ATCC 14028 | 10 | 10 | > 10 | > 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Proteus mirabilis ATCC 12453 | 10 | 10 | 5 | 5 | 5 | 5 | 10 | 10 | 10 | 10 | 5 | 5 |
| Bordetella bronchiseptica ATCC 4617 | 10 | > 10 | > 10 | > 10 | 10 | > 10 | 10 | 10 | > 10 | > 10 | 10 | > 10 |
| Escherichia coli ATCC 25922 | 10 | > 10 | 10 | > 10 | 10 | 10 | 10 | > 10 | 10 | > 10 | 10 | > 10 |
| Klebsiella pneumoniae ATCC 13883 | > 10 | > 10 | > 10 | > 10 | > 10 | > 10 | > 10 | > 10 | > 10 | > 10 | > 10 | > 10 |
| Pseudomonas aeruginosa ATCC 27853 | 10 | > 10 | 10 | > 10 | 10 | > 10 | 10 | > 10 | 10 | > 10 | 10 | > 10 |
| Yeasts | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC |
| Candida albicans ATCC 2091 | 5 | 5 | 2.5 | 5 | 2.5 | 5 | 5 | 5 | 5 | 5 | 2.5 | 5 |
| Candida albicans ATCC 10231 | 2.5 | 5 | 2.5 | 5 | 2.5 | 5 | 5 | 5 | 2.5 | 5 | 2.5 | 5 |
| Candida auris CDC B11903 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Candida glabrata ATCC 90030 | 5 | 5 | 5 | 10 | 5 | 10 | 5 | 5 | 5 | 5 | 5 | 5 |
| Candida parapsilosis ATCC 22019 | 1.25 | 5 | 2.5 | 5 | 1.25 | 5 | 1.25 | 5 | 1.25 | 5 | 1.25 | 5 |
| Candida krusei ATCC 14243 | 2.5 | 5 | 2.5 | 5 | 2.5 | 5 | 5 | 5 | 2.5 | 5 | 2.5 | 5 |
| Candida lusitaniae ATCC 34449 | 2.5 | 5 | 5 | 5 | 2.5 | 10 | 2.5 | 5 | 2.5 | 5 | 2.5 | 5 |
| Candida tropicalis ATCC 1369 | 5 | 5 | 5 | 10 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
MIC minimum inhibitory concentration, MBC minimum bactericidal concentration, MFC minimum fungicidal concentration
MIC values for the reference antimicrobial substances were 1 μg/mL of fluconazole for Candida albicans ATCC 10231, 1 μg/mL of vancomycin for Staphylococcus aureus ATCC 29213, and 0.015 μg/mL of ciprofloxacin for Escherichia coli ATCC 25922
It is interesting to notice that oils from Fr II, Fr II + Et, and FrE II + Et showed stronger antibacterial and antifungal activity even though they contained lower amounts of TPC, TFC, and DPPH radicals scavenging compounds compared with oils from Fr I, Fr I + Et, and FrE I + Et. One of the reasons for this occurrence could be the higher amount of linoleic acid present in oils from Fr II, Fr II + Et, and FrE II + Et. Namely, linoleic acid has been found to have antibacterial activity, particularly in inhibiting the growth of Gram-positive bacteria (Dilika et al., 2000; Kusumah et al., 2020). It was reported that the MIC value of linoleic acid against Bacillus subtilis is 0.02 mg/mL (Kusumah et al., 2020), Bacillus cereus is 0.01 mg/mL (Dilika et al., 2000), and Staphylococcus aureus is 1.0 mg/mL (Dilika et al., 2000). Similar MIC values were obtained in the present study for these bacteria. Nonetheless, additional research is required to determine which compounds have a dominant effect on the antimicrobial activity of chamomile oils.
Antimicrobial substances can be regarded as bactericidal or fungicidal if the MBC/MIC or MFC/MIC ratios are lower than or equal to 4. If the MBC/MIC or MFC/MIC ratios are higher than 4, antimicrobial substances are usually regarded as bacteriostatic or fungistatic (EUCAST 2003). Based on the presented results, it can be concluded that chamomile oils showed bactericidal (MBC/MIC = 1–4) and fungicidal effects (MFC/MIC = 1–4) for most of the tested microorganisms. The bacteriostatic effect of oils was noted against Micrococcus luteus, Bacillus subtilis, and Bacillus cereus (MBC/MIC = 4–250).
Cytotoxic Activity of Chamomile Oils
The results of cytotoxicity testing for chamomile oils obtained by SFE are presented in Table 4. It can be seen that overall, the highest toxicity was observed for FrE I + Et, with CC50 of 439.91, 331.96, and 511.32 µg/mL against VERO, FaDu, and RKO, respectively. Interestingly, antineoplastic activity of FrE I + Et against hypopharyngeal cancer was observed with SI of 1.33. The sample Fr I + Et showed a similar anticancer selectivity towards FaDu. Other chamomile oils showed higher toxicity on normal cells than cancer ones.
Table 4.
Cytotoxicity and anticancer selectivity of oils from chamomile seeds
| Oil samples | VERO | FaDu | RKO | ||
|---|---|---|---|---|---|
| CC50 | CC50 | SI | CC50 | SI | |
| Fr I | 499.60 ± 51.48 | 807.06 ± 34.99 | 0.62 | 857.01 ± 62.93 | 0.58 |
| Fr I + Et | 571.45 ± 46.88 | 432.46 ± 66.81 | 1.32 | 537.39 ± 77.52 | 1.06 |
| FrE I + Et | 439.91 ± 31.52 | 331.96 ± 36.27 | 1.33 | 511.32 ± 49.33 | 0.86 |
| Fr II | 695.76 ± 75.86 | 795.17 ± 86.88 | 0.87 | 880.95 ± 37.97 | 0.79 |
| Fr II + Et | 696.46 ± 31.61 | 1002.78 ± 150.22 | 0.69 | 1018.60 ± 107.20 | 0.68 |
| FrE II + Et | 742.32 ± 62.78 | 795.17 ± 55.63 | 0.93 | 1122.91 ± 104.35 | 0.66 |
CC50 50% cytotoxic concentration (µg/mL), SI selectivity index (VERO CC50/cancer CC50)
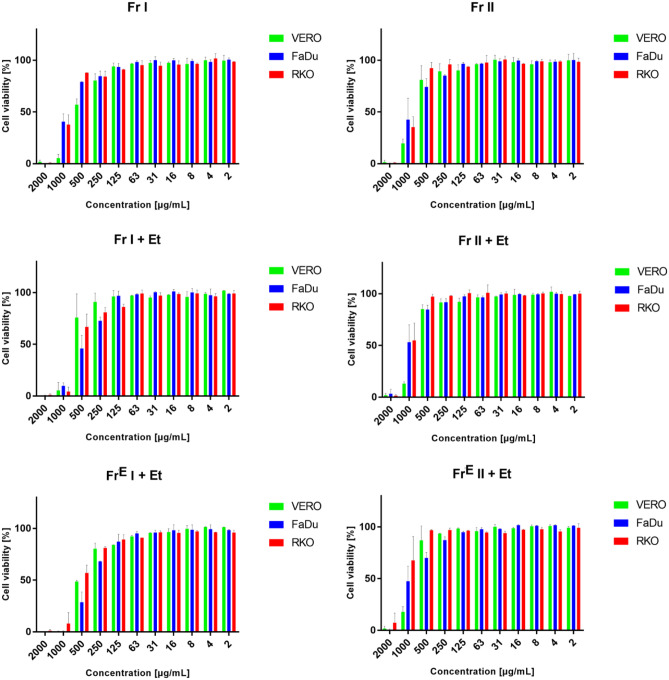
Analysis of the dose–response influence on cell lines, shown in Fig. 4, indicates nontoxic effect (viability of above 90%) of chamomile oils at a concentration of 125 µg/mL for Fr I, Fr I + Et, and FrE I + Et, whereas for Fr II, Fr II + Et, and FrE II + Et samples, the nontoxic effect was noticed at 250 µg/mL. Based on the criteria of cytotoxicity classification (Łaska et al., 2019), it can be concluded that chamomile oils were not cytotoxic to the tested cell lines (CC50 > 500 μg/mL). A slight deviation from this conclusion was noticed for the Fr I and FrE I + Et samples that showed weak toxicity on VERO cells due to CC50 values in the range 201 − 500 μg/mL (Łaska et al., 2019). Also, the samples FrE I + Et and Fr I + Et showed weak toxicity towards FaDu. Similarly, Catani et al. (2021) reported that ethanolic chamomile extracts did not exhibit an effect on viability of normal colon cell (Caco2 cells) at a low concentration (100 μg/mL), while cytotoxic effects were observed with the higher extract concentrations (i.e., 300 μg/mL led to 30% inhibition and 500 μg/mL reduced viability by about 70%). In addition, it was previously reported that extracts from chamomile flowers obtained using methanol show a potential to be used in anticancer treatments (Al-Dabbagh et al., 2019; Sak et al., 2017). For instance, these extracts significantly inhibited the level of prerequisite angiogenesis markers both in HepG2 cells and ex vivo (Al-Dabbagh et al., 2019) and showed the cytotoxic activity on melanoma cells (IC50 40.7 μg/mL) twofold higher than on epidermoid carcinoma cells (IC50 71.4 μg/mL) (Sak et al., 2017).
Fig. 4.
Dose–response influence of chamomile oils on normal and cancer cell lines
The results obtained in this study on composition and bioactivity of oils obtained from chamomile seeds suggest the possibility of its use as a functional food. According to Regulation (EU) 2015/2283 on novel foods, products that are considered “substantially equivalent” in composition, nutritional value, and levels of undesirable substances, to an existing food that is already marketed within the EU, have a simplified procedure for marketing (Lyashenko et al., 2019). This implies that chamomile seed oils could reach a rapid presence in the market.
Conclusions
The current study demonstrated a novel strategy for separating functional oils from chamomile seeds. Variation in the extraction process parameters (operating pressure, temperature, milled seed particle size, and addition of co-solvent) enabled an increase in the amount of separated oil (2.4 to 18.6%) and variation in the oil chemistry. Unsaturated fatty acid content ranged from 80.3 to 88.7%, with linoleic acid dominating in amounts ranging from 497.3 to 711.1 mg/g. The addition of ethanol as a co-solvent to sc-CO2 decreased extraction yield and, on the other hand, increased the TPC and TFC in oils. It was also shown that waste seeds material remained after the SFE process could be re-extracted using sc-CO2 with ethanol. These oils show higher TPC, TFC, and antioxidant activity compared with extracts from native seeds. TPC in separated oil reached 80.4 mg GAE/g, while TFC reached up to 11.6 mg QE/g. The obtained chamomile oils showed antioxidant activity with IC50 up to 3.9 mg/mL. The antimicrobial activity of oils was the most pronounced against Gram-positive bacteria with MIC in the range from 0.02 to 10 mg/mL. Chamomile oils were not cytotoxic on normal cells. Based on the presented results it can be concluded that oils obtained from chamomile seeds using an environmentally friendly technique are bioactive and have significant potential to be used as functional foods.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Stoja Milovanovic acknowledges the scholarship from the Polish National Agency for Academic Exchange (NAWA, Warsaw, Poland) (Ulam Programme 2020, the agreement number is PPN/ULM/2020/1/00023/U/00001). The Ministry of Science, Technological Development and Innovation of the Republic of Serbia supported this work (Contract No. 451-03-47/2023-01/200135).
Author Contribution
Stoja Milovanovic: conceptualization; Stoja Milovanovic, Agnieszka Grzegorczyk, Łukasz Świątek, Anita Grzęda, and Agnieszka Dębczak: methodology; Stoja Milovanovic, Agnieszka Grzegorczyk, Łukasz Świątek, and Anita Grzęda: investigation; Katarzyna Tyskiewicz and Marcin Konkol: resources; Stoja Milovanovic, Agnieszka Grzegorczyk, Łukasz Świątek, Anita Grzęda, and Agnieszka Dębczak: data curation; Stoja Milovanovic, Agnieszka Grzegorczyk, and Łukasz Świątek: writing, original draft preparation; Stoja Milovanovic, Agnieszka Grzegorczyk, Łukasz Świątek, Katarzyna Tyskiewicz, and Marcin Konkol: writing, review and editing.
Data Availability
The data presented in this study are available on request from the corresponding author.
Declarations
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Al-Dabbagh B, Elhaty IA, Elhaw M, Murali C, Al Mansoori A, Awad B, Amin A. Antioxidant and anticancer activities of chamomile (Matricaria recutita L.) BMC Research Notes. 2019;12:1–8. doi: 10.1186/s13104-018-3960-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Suod H, Ratiu IA, Krakowska-Sieprawska A, Lahuta L, Górecki R, Buszewski B. Supercritical fluid extraction in isolation of cyclitols and sugars from chamomile flowers. Journal of Separation Science. 2019;42:3243–3252. doi: 10.1002/jssc.201900539. [DOI] [PubMed] [Google Scholar]
- Aydi, A., Zibetti, A. W., Al-Khazaal, A. Z., Eladeb, A., Adberraba, M., & Barth, D. (2020). Supercritical CO2 extraction of extracted oil from Pistacia lentiscus L.: Mathematical modeling, economic evaluation and scale-up. Molecules, 25, 199. 10.3390/molecules25010199 [DOI] [PMC free article] [PubMed]
- Barriga-Sánchez, M., Castro-Rumiche, C. F., Sanchez-Gonzales, G., & Rosales-Hartshorn, M. U. (2021). Functional and chemical qualities of Vitis labrusca grape seed oil extracted by supercritical CO2. Revista Colombiana de Quimica, 50, 3–9. 10.15446/rev.colomb.quim.v50n3.95469
- Bettaieb Rebey I, Bourgou S, Detry P, Wannes WA, Kenny T, Ksouri R, et al. Green extraction of fennel and anise edible oils using bio-based solvent and supercritical fluid: Assessment of chemical composition, antioxidant property, and oxidative stability. Food and Bioprocess Technology. 2019;12:1798–1807. doi: 10.1007/s11947-019-02341-8. [DOI] [Google Scholar]
- Bimakr M, Rahman RA, Ganjloo A, Taip FS, Adzahan NM, Sarker MZI. Characterization of valuable compounds from winter melon (Benincasa hispida (Thunb.) Cogn.) seeds using supercritical carbon dioxide extraction combined with pressure swing technique. Food and Bioprocess Technology. 2016;9:396–406. doi: 10.1007/s11947-015-1636-3. [DOI] [Google Scholar]
- Buelvas-Puello, L. M., Franco-Arnedo, G., Martínez-Correa, H. A., Ballesteros-Vivas, D., Sánchez-Camargo, A. D. P., Miranda-Lasprilla, D., et al. (2021). Supercritical fluid extraction of phenolic compounds from mango (Mangifera indica l.) seed kernels and their application as an antioxidant in an edible oil. Molecules, 26, 7516. 10.3390/molecules26247516 [DOI] [PMC free article] [PubMed]
- Catani, M. V., Rinaldi, F., Tullio, V., Gasperi, V., & Savini, I. (2021). Comparative analysis of phenolic composition of six commercially available chamomile (Matricaria chamomilla l.) extracts: Potential biological implications. International Journal of Molecular Sciences, 22, 10601. 10.3390/ijms221910601 [DOI] [PMC free article] [PubMed]
- Choudhary P, Devi TB, Tushir S, Kasana RC, Popatrao DS, K, N. Mango seed kernel: A bountiful source of nutritional and bioactive compounds. Food and Bioprocess Technology. 2022 doi: 10.1007/s11947-022-02889-y. [DOI] [Google Scholar]
- Čižmek L, Kralj MB, Čož-rakovac R, Mazur D, Ul’yanovskii, N., Likon, M., & Trebše, P. Supercritical carbon dioxide extraction of four medicinal mediterranean plants: Investigation of chemical composition and antioxidant activity. Molecules. 2021;26:5697. doi: 10.3390/molecules26185697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Melo MMR, Barbosa HMA, Passos CP, Silva CM. Supercritical fluid extraction of spent coffee grounds: Measurement of extraction curves, oil characterization and economic analysis. Journal of Supercritical Fluids. 2014;86:150–159. doi: 10.1016/j.supflu.2013.12.016. [DOI] [Google Scholar]
- Dilika F, Bremner PD, Meyer JJM. Antibacterial activity of linoleic and oleic acids isolated from Helichrysum pedunculatum: A plant used during circumcision rites. Fitoterapia. 2000;71:450–452. doi: 10.1016/S0367-326X(00)00150-7. [DOI] [PubMed] [Google Scholar]
- EUCAST, E. C. for A. S. T. of the E. S. of C. M. and I. D Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. Clinical Microbiology and Infection. 2003;9(8):1–7. doi: 10.1046/j.1469-0691.2003.00790.x. [DOI] [PubMed] [Google Scholar]
- Fadairo OS, Nandasiri R, Eskin NAM, Aluko RE, Scanlon MG. Air frying as a heat pre-treatment method for improving the extraction and yield of canolol from canola seed oil. Food and Bioprocess Technology. 2022 doi: 10.1007/s11947-022-02961-7. [DOI] [Google Scholar]
- Ferrentino G, Ndayishimiye J, Haman N, Scampicchio M. Functional activity of oils from brewer’s spent grain extracted by supercritical carbon dioxide. Food and Bioprocess Technology. 2019;12:789–798. doi: 10.1007/s11947-019-02249-3. [DOI] [Google Scholar]
- Fregapane, G., Guisantes-Batan, E., Ojeda-Amador, R. M., & Salvador, M. D. (2020). Development of functional edible oils enriched with pistachio and walnut phenolic extracts. Food Chemistry, 310, 125917. 10.1016/j.foodchem.2019.125917 [DOI] [PubMed]
- HerbShop. (2022). Common chamomile - seeds. https://herbshop.pl/pl/p/Rumianek-pospolity-zwyczajny-nasiona/73. Accessed 28 September 2022
- Hmamou, D. Ben, Salghi, R., Zarrouk, A., Hammouti, B., Al-Deyab, S. S., Bazzi, L., et al. (2012). Corrosion inhibition of steel in 1 M hydrochloric acid medium by chamomile essential oils. International Journal of Electrochemical Science, 7, 2361–2373. 10.48422/IMIST.PRSM/ajees-v3i2.8997
- Hong S, Sowndhararajan K, Joo T, Lim C, Cho H, Kim S, et al. Ethanol and supercritical fluid extracts of hemp seed (Cannabis sativa L.) increase gene expression of antioxidant enzymes in HepG2 cells. Asian Pacific Journal of Reproduction. 2015;4:147–152. doi: 10.1016/S2305-0500(15)30012-9. [DOI] [Google Scholar]
- Huang, Z., Ma, Q., Liu, S. F., & Guo, G. M. (2020). Benign recovery of carotenoids from Physalis alkekengi L.var. francheti through supercritical CO2 extraction: Yield, antioxidant activity and economic evaluation. Journal of CO2 Utilization, 36, 9–17. 10.1016/j.jcou.2019.10.015
- Kaiser CS, Römpp H, Schmidt PC. Supercritical carbon dioxide extraction of chamomile flowers: Extraction efficiency, stability, and in-line inclusion of chamomile-carbon dioxide extract in β-cyclodextrin. Phytochemical Analysis. 2004;15:249–256. doi: 10.1002/pca.775. [DOI] [PubMed] [Google Scholar]
- Kasprzak, M. (2020). Poland’s wholesale electricity prices rise to the highest in Europe. EMBER. https://ember-climate.org/insights/research/polands-wholesale-electricity-prices-rise-to-the-highest-in-europe/. Accessed 29 September 2022
- Kotnik P, Škerget M, Knez Ž. Supercritical fluid extraction of chamomile flower heads: Comparison with conventional extraction, kinetics and scale-up. Journal of Supercritical Fluids. 2007;43:192–198. doi: 10.1016/j.supflu.2007.02.005. [DOI] [Google Scholar]
- Kulkarni NG, Kar JR, Singhal RS. Extraction of flaxseed oil: A comparative study of three-phase partitioning and supercritical carbon dioxide using response surface methodology. Food and Bioprocess Technology. 2017;10:940–948. doi: 10.1007/s11947-017-1877-4. [DOI] [Google Scholar]
- Kusumah D, Wakui M, Murakami M, Xie X, Yukihito K, Maeda I. Linoleic acid, α-linolenic acid, and monolinolenins as antibacterial substances in the heat-processed soybean fermented with Rhizopus oligosporus. Bioscience, Biotechnology and Biochemistry. 2020;84:1285–1290. doi: 10.1080/09168451.2020.1731299. [DOI] [PubMed] [Google Scholar]
- Łaska G, Sieniawska E, Świątek Ł, Zjawiony J, Khan S, Boguszewska A, et al. Phytochemistry and biological activities of Polemonium caeruleum L. Phytochemistry Letters. 2019;30:314–323. doi: 10.1016/j.phytol.2019.02.017. [DOI] [Google Scholar]
- Lebanna, J. (2005). Composition of supercritical carbon dioxide derived extracts of Chamaemelum nobile. North-West University. Retrieved from https://repository.nwu.ac.za/handle/10394/588
- Luan, G., Yang, M., Nan, X., Lv, H., Liu, Q., Wang, Y., & Li, Y. (2022). Optimization and comparative study of different extraction methods of sixteen fatty acids of Potentilla anserina L. from twelve different producing areas of the Qinghai-Tibetan plateau. Molecules, 27, 5443. 10.3390/molecules27175443 [DOI] [PMC free article] [PubMed]
- Lukic, I., Milovanovic, S., Pantic, M., Srbljak, I., Djuric, A., Tadic, V., & Tyśkiewicz, K. (2022a). Separation of high-value extracts from Silybum marianum seeds: Influence of extraction technique and storage on composition and bioactivity. Lwt, 160, 113319. 10.1016/j.lwt.2022.113319
- Lukic, I., Pajnik, J., Tadic, V., & Milovanovic, S. (2022b). Supercritical CO2-assisted processes for development of added-value materials: Optimization of starch aerogels preparation and hemp seed extracts impregnation. Journal of CO2 Utilization, 61(May). 10.1016/j.jcou.2022.102036
- Lyashenko, S., González-Fernández, M. J., Gómez-Mercado, F., Yunusova, S., Denisenko, O., & Guil-Guerrero, J. L. (2019). Ribes taxa: A promising source of γ-linolenic acid-rich functional oils. Food Chemistry, 301, 125309. 10.1016/j.foodchem.2019.125309 [DOI] [PubMed]
- Malm, A., Grzegorczyk, A., Biernasiuk, A., Baj, T., Rój, E., Tyśkiewicz, K., et al. (2021). Could supercritical extracts from the aerial parts of Helianthus salicifolius A. Dietr. and Helianthus tuberosus L. be regarded as potential raw materials for biocidal purposes? Agriculture (Switzerland), 11(1), 1–11. 10.3390/agriculture11010010
- Marques LLM, Panizzon GP, Aguiar BAA, Simionato AS, Cardozo-Filho L, Andrade G, et al. Guaraná (Paullinia cupana) seeds: Selective supercritical extraction of phenolic compounds. Food Chemistry. 2016;212:703–711. doi: 10.1016/j.foodchem.2016.06.028. [DOI] [PubMed] [Google Scholar]
- Matthäus B, Özcan MM. Oil content, fatty acid composition and distributions of vitamin-E-active compounds of some fruit seed oils. Antioxidants. 2015;4:124–133. doi: 10.3390/antiox4010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurek B, Chmiel M, Górecka B. Fatty acids analysis using gas chromatography-mass spectrometer detector (GC/MSD) - method validation based on berry seed extract samples. Food Analytical Methods. 2017;10(8):2868–2880. doi: 10.1007/s12161-017-0834-1. [DOI] [Google Scholar]
- Mihyaoui AE, Esteves Da Silva JCG, Charfi S, Castillo MEC, Lamarti A, Arnao MB. Chamomile (Matricaria chamomilla L.): A review of ethnomedicinal use, phytochemistry and pharmacological uses. Life. 2022;12:1–41. doi: 10.3390/life12040479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milovanovic S, Stamenic M, Markovic D, Radetic M, Zizovic I. Solubility of thymol in supercritical carbon dioxide and its impregnation on cotton gauze. Journal of Supercritical Fluids. 2013;84:173–181. doi: 10.1016/j.supflu.2013.10.003. [DOI] [Google Scholar]
- Milovanovic, Stoja, Grzegorczyk, A., Świątek, Ł., Dębczak, A., Tyskiewicz, K., & Konkol, M. (2022a). Dandelion seeds as a new and valuable source of bioactive extracts obtained using the supercritical fluid extraction technique. Sustainable Chemistry and Pharmacy, 29, 100796. 10.1016/j.scp.2022.100796
- Milovanovic, Stoja, Lukic, I., Kamiński, P., Dębczak, A., Klimkowska, K., Tyśkiewicz, K., & Konkol, M. (2022b). Green manufacturing of high-value extracts from milk thistle seeds: Parameters that affect the supercritical CO2 extraction process. Journal of CO2 Utilization, 63, 102134. 10.1016/j.jcou.2022.102134
- Milovanovic, Stoja, Lukic, I., Stamenic, M., Kamiński, P., Florkowski, G., Tyskiewicz, K., & Konkol, M. (2022c). The effect of equipment design and process scale-up on supercritical CO2 extraction : Case study for Silybum marianum seeds. Journal of Supercritical Fluids, 188, 105676. 10.1016/j.supflu.2022.105676
- Omidbaigi R, Sefldkon F, Kazemi F. Roman chamomile oil: Comparison between hydro-distillation and supercritical fluid extraction. Journal of Essential Oil-Bearing Plants. 2003;6:191–194. doi: 10.1080/0972-060X.2003.10643350. [DOI] [Google Scholar]
- Orsavova J, Misurcova L, Vavra Ambrozova J, Vicha R, Mlcek J. Fatty acids composition of vegetable oils and its contribution to dietary energy intake and dependence of cardiovascular mortality on dietary intake of fatty acids. International Journal of Molecular Sciences. 2015;16:12871–12890. doi: 10.3390/ijms160612871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekić B, Zeković Z, Petrović L, Tolić A. Behavior of (–)-α-bisabolol and (–)-α-bisabololoxides A and B in camomile flower extraction with supercritical carbon dioxide. Separation Science and Technology. 1995;30(18):3567–3576. doi: 10.1080/01496399508015137. [DOI] [Google Scholar]
- Pereira CG, Meireles MAA. Supercritical fluid extraction of bioactive compounds: Fundamentals, applications and economic perspectives. Food and Bioprocess Technology. 2010;3:340–372. doi: 10.1007/s11947-009-0263-2. [DOI] [Google Scholar]
- Pereira NP, Miguel OG, Miguel MD. Composição química do óleo fixo obtido dos frutos secos da [Chamomilla recutita (L.) Rauschert] produzida no município de Mandirituba. PR. Revista Brasileira De Farmacognosia. 2005;15:334–337. doi: 10.1590/s0102-695x2005000400014. [DOI] [Google Scholar]
- Poland electricity prices. (2022). GlobalPetrolPrices. https://www.globalpetrolprices.com/Poland/electricity_prices/. Accessed 29 September 2022
- Povh NP, Marques MOM, Meireles MAA. Supercritical CO2 extraction of essential oil and oleoresin from chamomile (Chamomilla recutita [L.] Rauschert) Journal of Supercritical Fluids. 2001;21:245–256. doi: 10.1016/S0896-8446(01)00096-1. [DOI] [Google Scholar]
- Rahimi E, Prado JM, Zahedi G, Meireles MAA. Chamomile extraction with supercritical carbon dioxide: Mathematical modeling and optimization. Journal of Supercritical Fluids. 2011;56:80–88. doi: 10.1016/j.supflu.2010.11.008. [DOI] [Google Scholar]
- Ranalli A, Marchegiani D, Pardi D, Contento S, Pardi D, Girardi F, Kotti F. Evaluation of functional phytochemicals in destoned virgin olive oil. Food and Bioprocess Technology. 2009;2:322–327. doi: 10.1007/s11947-008-0128-0. [DOI] [Google Scholar]
- Rebolleda S, José MLGS, Sanz MT, Beltrán S, Solaesa ÁG. Bioactive compounds of a wheat bran oily extract obtained with supercritical carbon dioxide. Foods. 2020;9:625. doi: 10.3390/foods9050625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reverchon E, Senatore F. Supercritical carbon dioxide extraction of chamomile essential oil and its analysis by gas chromatography-mass spectrometry. Journal of Agricultural and Food Chemistry. 1994;42:154–158. doi: 10.1021/jf00037a027. [DOI] [Google Scholar]
- Rymer C, Hartnell GF, Givens DI. The effect of feeding modified soyabean oil enriched with C18: 4n–3 to broilers on the deposition of n-3 fatty acids in chicken meat. British Journal of Nutrition. 2011;105:866–878. doi: 10.1017/S0007114510004502. [DOI] [PubMed] [Google Scholar]
- Sak K, Nguyen TH, Ho VD, Do TT, Raal A. Cytotoxic effect of chamomile (Matricaria recutita) and marigold (Calendula officinalis) extracts on human melanoma SK-MEL-2 and epidermoid carcinoma KB cells. Cogent Medicine. 2017;4:1333218. doi: 10.1080/2331205x.2017.1333218. [DOI] [Google Scholar]
- Sas, A. (2022). Wholesale prices of electricity in Poland from 2018 to 2022. Energy & Environment. https://www.statista.com/statistics/1066654/poland-wholesale-electricity-prices/#statisticContainer. Accessed 29 September 2022
- Scalia S, Giuffreda L, Pallado P. Analytical and preparative supercritical fluid extraction of chamomile flowers and its comparison with conventional methods. Journal of Pharmaceutical and Biomedical Analysis. 1999;21:549–558. doi: 10.1016/S0731-7085(99)00152-1. [DOI] [PubMed] [Google Scholar]
- Shil, A., & Mukherjee, S. K. (2016). Diversity of cypselar anatomy in nine species of the tribe Anthemideae (Asteraceae). Indian Journal of Plant Sciences, 5, 95–105. 10.13140/RG.2.2.16660.50561
- Skilbeck, C. A., Lynch, I., Ellenby, M., & Spencer, M. A. (2019). Achene morphology of British and Irish mayweeds and chamomiles: implications for taxonomy and identification. British & Irish Botany, 1, 128–166. 10.33928/bib.2019.01.128
- Sodeifian G, Ardestani NS, Sajadian SA, Moghadamian K. Properties of Portulaca oleracea seed oil via supercritical fluid extraction: Experimental and optimization. Journal of Supercritical Fluids. 2018;135:34–44. doi: 10.1016/j.supflu.2017.12.026. [DOI] [Google Scholar]
- Srivastava JK, Shankar E, Gupta S. Chamomile: A herbal medicine of the past with a bright future (review) Molecular Medicine Reports. 2010;3:895–901. doi: 10.3892/mmr.2010.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanojevic, L. P., Marjanovic-Balaban, Z. R., Kalaba, V. D., Stanojevic, J. S., & Cvetkovic, D. J. (2016). Chemical composition, antioxidant and antimicrobial activity of chamomile flowers essential oil (Matricaria chamomilla L.). Journal of Essential Oil Bearing Plants, 19(8), 2017–2028. 10.1080/0972060X.2016.1224689
- Statista. (2022). Minimum gross hourly wages and salaries in Poland from 2017 to 2023. https://www.statista.com/statistics/1085566/poland-minimum-gross-hourly-wages-and-salaries/. Accessed 29 September 2022
- Stefanov, S. M., Fetzer, D. E. L., de Souza, A. R. C., Corazza, M. L., Hamerski, F., Yankov, D. S., & Stateva, R. P. (2022). Valorization by compressed fluids of Arctium lappa seeds and roots as a sustainable source of valuable compounds. Journal of CO2 Utilization, 56, 101821. 10.1016/j.jcou.2021.101821
- Turton R, Bailie RC, Whiting WB, Shaeiwitz JA. Analysis, synthesis and design of chemical processes. 2. Prentice Hall; 2003. [Google Scholar]
- Vasavada, N. (n.d.). Online Web Statistical Calculators. https://astatsa.com/OneWay_Anova_with_TukeyHSD/. Accessed 12 February 2023
- Zekovic ZP. Chamomile ligulate flowers in supercritical CO2-extraction. Journal of Essential Oil Research. 2000;12:85–93. doi: 10.1080/10412905.2000.9712049. [DOI] [Google Scholar]
- Zengin G, Mahomoodally MF, Rocchetti G, Lucini L, Sieniawska E, Swiatek Ł, et al. Chemical characterization and bioactive properties of different extracts from Fibigia clypeata, an unexplored plant food. Foods. 2020;9:705. doi: 10.3390/foods9060705. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author.