Abstract
The spatial heterogeneity of bacterial populations at a shallow-water hydrothermal vent in the Aegean Sea close to the island of Milos (Greece) was examined at two different times by using acridine orange staining for total cell counts, cultivation-based techniques, and denaturing gradient gel electrophoresis (DGGE) analysis of PCR-amplified 16S rRNA gene fragments. Concurrent with measurements of geochemical parameters, samples were taken along a transect from the center of the vent to the surrounding area. Most-probable-number (MPN) counts of metabolically defined subpopulations generally constituted a minor fraction of the total cell counts; both counting procedures revealed the highest cell numbers in a transition zone from the strongly hydrothermally influenced sediments to normal sedimentary conditions. Total cell counts ranged from 3.2 × 105 cells ml−1 in the water overlying the sediments to 6.4 × 108 cells g (wet weight) of sediment−1. MPN counts of chemolithoautotrophic sulfur-oxidizing bacteria varied between undetectable and 1.4 × 106 cells g−1. MPN counts for sulfate-reducing bacteria and dissimilatory iron-reducing bacteria ranged from 8 to 1.4 × 105 cells g−1 and from undetectable to 1.4 × 106 cells g−1, respectively. DGGE revealed a trend from a diverse range of bacterial populations which were present in approximately equal abundance in the transition zone to a community dominated by few populations close to the center of the vent. Temperature was found to be an important parameter in determining this trend. However, at one sampling time this trend was not discernible, possibly due to storm-induced disturbance of the upper sediment layers.
Submarine hydrothermal vents are well known for extremes in geochemical conditions, as well as for the exotic life they support. Cultivation-based studies and radiotracer experiments have revealed that at most marine hydrothermal vents, sulfur compounds seem to be important substrates for microbes (23, 26). On one hand, chemolithoautotrophic prokaryotes gain energy by the oxidation of reduced sulfur compounds. These organisms, which live either free at the interface between the anoxic hydrothermal fluid and the oxygenated seawater or in symbioses with animals, are thought to be the main primary producers at deep-sea vents (23, 25, 26, 53), where light is absent. However, at shallow-water vents, light is present and thus primary production by photosynthetic organisms can take place (see, e.g., references 10 and 41). On the other hand, oxidized sulfur compounds are used by many heterotrophic members of the Archaea and Bacteria as electron acceptors for the anaerobic degradation of organic matter, although some can also grow autotrophically. The habitat for these organisms are the anoxic parts of the hydrothermal system, and correspondingly many of them are thermophiles or hyperthermophiles (5, 26). However, due to sharp physical and chemical gradients, hydrothermal vents offer a variety of habitats and microniches, which can potentially be inhabited by metabolically diverse microorganisms (4, 5, 23, 26). Little is known, however, about the spatial distribution of the microbial populations thriving in these ecosystems (19, 22, 41) and the changes in community structure occurring along these gradients (20).
Cultivation-based methods are not well suited for investigations of the general composition of microbial communities, since only a small percentage of the microorganisms are cultivable (2) and cultivation may strongly bias our view of community structure (48). The advent of molecular tools in microbial ecology, e.g., the analysis of 16S rRNA sequences in natural samples, has made it possible to circumvent this limitation. Identification of the dominant populations in situ leads to a better understanding of how microbial communities are structured. However, molecular studies suffer from the drawback that physiology can only rarely be inferred from the 16S rRNA sequence data alone. Thus, cultivation and molecular methods may complement each other (19, 30, 39, 43). Nonetheless, the use of molecular tools for studying the microbial community structure at submarine hydrothermal vents to date has focused mainly on an assessment of the bacterial diversity and on an inventory of the taxa that are present, without making concomitant measurements of the geochemical parameters (19, 27, 28, 30, 34).
We have chosen a submarine shallow hydrothermal vent in the Aegean Sea near the island of Milos (Greece) to investigate the relationship between changes in physicochemical parameters and bacterial population distributions. By using denaturing gradient gel electrophoresis (DGGE) of PCR-amplified 16S rRNA gene fragments, we examined the spatial heterogeneity of bacterial populations on a vertical as well as horizontal scale along a transect from the center of the vent to the surrounding area. DGGE is a powerful tool to discern changes in microbial community structure in a variety of habitats (for a review, see reference 32). The DGGE analysis of the dominant phylotypes occurring at the vent system was accompanied by investigations of the vertical and horizontal distribution of specific physiological groups of bacteria, i.e., autotrophic sulfur-oxidizing bacteria (SOB), sulfate-reducing bacteria (SRB), and dissimilatory iron-reducing bacteria (DIRB), by using the most-probable-number (MPN) approach. The transect was concurrently physicochemically characterized by performing high-resolution in situ profiling of temperature, pH, redox potential, and O2 concentrations.
MATERIALS AND METHODS
Study site.
The study site was a solitary gaseous hydrothermal vent located 8 m deep in Palaeochori Bay (24°31.220′E, 36°40.391′N), a sandy bay on the southeastern coast of the island of Milos in the Aegean Sea (Greece). The mean composition of the discharged gases from different seeps was 80.5% CO2, 1.2% H2S, 0.8% CH4, and 0.4% H2 (11). The reduced hydrothermal fluid has an elevated salinity of up to 58‰ compared to 39‰ of the ambient seawater (44). Macrofauna which are dependent on endosymbiotic bacteria are absent at the Milos vents (10, 16, 44).
Sampling.
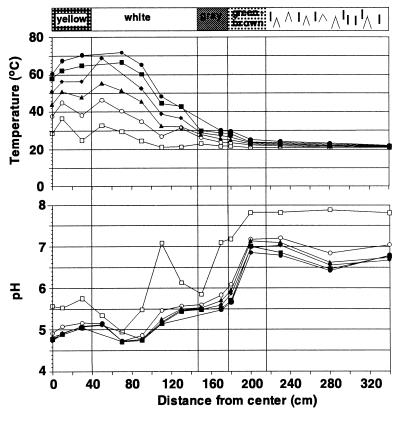
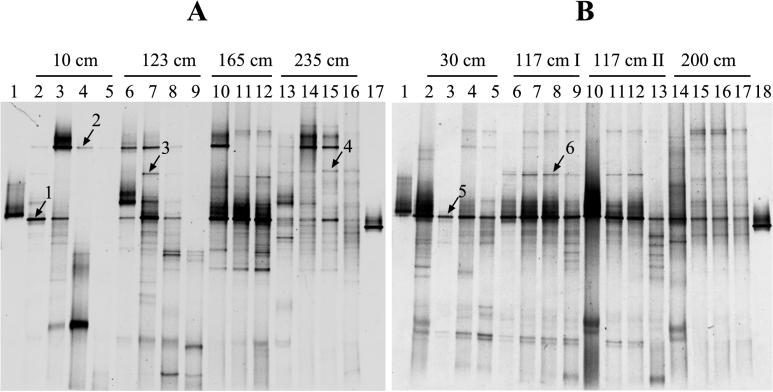
Sampling was carried out along a transect from the center of the almost circular vent to the surrounding area. At a specific area around the center of the vent site, a conspicuous white precipitate formed on the sediment surface, which increased in thickness under calm weather conditions (see Fig. 1 and 2). Sediment cores (polycarbonate tubes with an inner diameter of 37 mm) were taken by SCUBA divers at 10, 123, 165, and 235 cm from the vent center in June 1996 and at 30, 117, and 200 cm from the vent center in September 1996. Physicochemical measurements were carried out along the same transect.
FIG. 1.
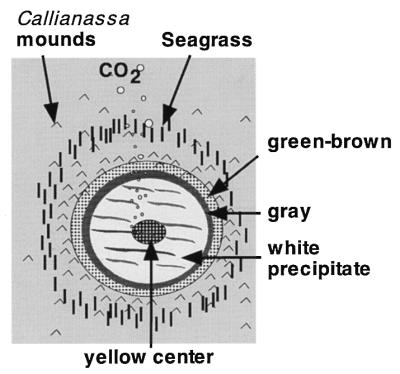
Schematic diagram of the solitary hydrothermal vent illustrating the zonation pattern observable on the sediment surface. See the text for further explanations.
FIG. 2.
Vertical profiles of temperature and pH along a transect from the surrounding area to the vent center for June 1996. The profiles from September 1996 were nearly identical. Sediment depth is indicated by the following symbols: □, 0 cm; ○, 1 cm; ▴, 2 cm; ⧫, 3 cm; ■, 4 cm; ●, 5 cm.
Slicing of sediment cores.
Sediment cores were immediately subsampled by extruding the sediment from the polycarbonate tubes and slicing each core. In June 1996, the first 30 mm of every sediment core was subsampled at 10-mm intervals. In September 1996, the layers from 0 to 5, 8 to 13, and 16 to 26 mm were subsampled. At both sampling times, the upper sample of each core consisted of water from just above the sediment surface, which was sampled with a sterile syringe. For the cores from 123 cm (June) and 117 cm (September), the upper sample consisted of the white precipitate, which was 2 to 5 mm thick. In September, a second core was taken at 117 cm from the center of the vent 1 week later, and no precipitate was present.
Physicochemical measurements.
Vertical profiles of pH, temperature, redox potential, and O2 concentration were determined in situ along a transect radiating out from the center of the vent site. The sensors were attached to a micromanipulator mounted on a tripod. The instruments were self-contained and held in watertight housings. The signals were transferred to and stored by a 12-bit data logger. The sensors for temperature and pH were combined; thus both parameters were measured at the same location and the pH measurements were temperature compensated. Vertical profiles of redox potential were measured with a platinum electrode. The dissolved-oxygen concentration was measured by using Clark-type microelectrodes with a built-in reference and a guard cathode (35). Measurements were done at horizontal distances of 20 to 30 cm along the transect up to a distance of 3.5 m from the center and in vertical increments of 1 cm for pH, temperature, and redox potential and 250 μm for dissolved oxygen.
Total cell counts.
A sediment sample (1 cm3) was fixed by the addition of 9 ml of borate-buffered formaldehyde (4% [wt/vol]) in 3.5% (wt/vol) NaCl. Water samples were fixed by the addition of borate-buffered formaldehyde (4% [wt/vol] final concentration). The borate-buffered formaldehyde was prepared by adding 4% (wt/vol) borax (Sigma, St. Louis, Mo.) to 37% (wt/vol) formaldehyde. After 16 h, the solution was filtered through Nuclepore polycarbonate filters (pore size, 0.2 μm; Costar, Cambridge, Mass.) to obtain a particle-free solution. Samples were stored at 4°C in the dark. For further processing, samples were put on ice and sonicated (three times for 15 s at 30-s intervals) with a microtip (Sonopuls HD 200; Bandelin, Berlin, Germany) to dislodge the cells from the sediment particles. After the particles were allowed to settle for 30 s, the supernatant was collected. The remaining sediment was washed eight times with 5 ml of a filter-sterilized 3.5% (wt/vol) NaCl solution. The supernatants were combined, the sample was diluted, and a minimum of 2 ml was filtered through black Nuclepore polycarbonate filters (pore size, 0.2 μm). Two filters per sample were stained with acridine orange (final concentration, 0.01% [wt/vol]) and mounted on glass slides with low-fluorescence immersion oil (type A; Cargille, Cedar Grove, N.J.), and cells were counted with a Axiolab epifluorescence microscope (Zeiss, Oberkochen, Germany) at a magnification of 1,000×. In addition to fixed samples, we examined fresh material by phase-contrast microscopy with a Zeiss Standard 20 microscope to get a general impression of the appearance of the material.
MPN counts.
The MPN technique was used to estimate the abundance of SRB and DIRB in June and of SOB in September. Subsamples were serially diluted (1:10) with artificial seawater medium without substrate. Between every dilution step, the samples were vigorously shaken on a vortex mixer to disaggregate cell clumps and to dislodge cells from sediment particles. Sonication was not used in this case, since we believed that it would have adverse effects on the viability of the cells. From each dilution, three replicate tubes containing growth medium were inoculated and incubated at their approximate in situ temperature. For anaerobic bacteria, the first dilution step was carried out in a glove box flushed with N2. All further dilution steps and the inoculation were done by transferring an aliquot of fluid with syringes from one anaerobic tube to the next by injection through butyl rubber stoppers. The tubes of the anaerobic MPN series were filled with medium under anaerobic conditions (a mixture of 90% N2 and 10% CO2 in the headspace) and closed with butyl rubber stoppers (51). The numbers of cultivable bacteria were determined as described previously (3).
(i) MPN counts of SRB.
Artificial seawater medium defined for SRB (51) contained 10 mM acetate as the carbon source and nonchelated trace element mixture no. 1. In marine sediments, acetate-oxidizing SRB dominate (see, e.g., reference 24), and hence acetate is the main electron donor for sulfate reduction (see, e.g., reference 38). The presence of SRB in the MPN tubes was determined by a semiquantitative detection of sulfide (8) and microscopically verified.
(ii) MPN counts of DIRB.
Artificial, sulfate-free seawater (51) was supplemented with washed (three times with distilled water) and autoclaved maghemite (γ-Fe2O3, 40 mM) with a surface area of 130 m2 g−1 (Bayer, Krefeld, Germany) as the electron donor, acetate (5 mM) as the electron acceptor, and trace element solution with EDTA (51). In marine sediments, maghemite yields cell numbers of DIRB comparable to those obtained with ferrihydrite (12). Tubes were counted positive if the color changed from red (maghemite) to black (magnetite). Positive tubes of DIRB were also checked microscopically.
(iii) MPN counts of SOB.
Mineral medium with 20 mM thiosulfate (added by sterile filtration to the autoclaved medium) as the sole electron donor was used. The composition of the mineral medium (in grams per liter) was: NaCl, 29; (NH4)2SO4, 1; MgSO4 · 7H2O, 1.5; CaCl2 · 2H2O, 0.42; K2HPO4, 0.5; KCl, 0.7; vitamin B12, 0.05; and trace element solution with EDTA, 1 ml liter (51). Bromthymol blue was added as pH indicator at a concentration of 4 mg liter−1. K2HPO4 was autoclaved separately and added to the medium after autoclaving. To test for the presence of SOB able to grow under anaerobic conditions, MPN series were set up with the same medium as for aerobic SOB but supplemented with 10 mM nitrate as the electron acceptor and buffered with 15 mM NaHCO3. This MPN series was performed under strict anaerobic conditions. The MPN series for SOB (aerobic and anaerobic) for the second core (117 cm from the vent center) was performed with the same medium as described above, but the salinity was increased to 55‰ by adding NaCl. This salinity was similar to that of the outflowing brine (44). In all cases, growth was determined by pH changes upon acid formation due to the oxidation of thiosulfate and was microscopically verified. The MPN cultures were incubated in the dark to avoid the growth of phototrophic organisms.
DNA extraction.
Subsamples from sliced sediment cores were immediately deep-frozen in liquid N2. For long-term storage, the samples were kept at −80°C. DNA was extracted by the method of Zhou et al. (54). We modified the method by using 2 g of sample and including five cycles of thawing at 30°C and freezing in liquid nitrogen before starting the extraction. The lysis efficiency was monitored by epifluorescence microscopy after staining subsamples after the extraction step with acridine orange. No intact cells could be observed. The extracted DNA was stored at −20°C until further analysis.
PCR.
PCR amplifications were performed by a touchdown PCR with the bacterial primer pair GM5F-GC clamp and 907R (30). The annealing temperature was lowered from 65 to 55°C over 20 cycles, and after the final annealing temperature of 55°C was reached, 16 more cycles were performed. In all cases, bovine serum albumin (Sigma) was added to the PCR solution (final concentration, 3 mg ml−1) to prevent inhibition of enzymatic amplification by humic substances. Amplification products were analyzed by electrophoresis in 2% (wt/vol) SeaKem LE agarose (FMC Bioproducts, Rockland, Maine) gels stained with ethidium bromide (0.5 μg ml−1) before being subjected to further analysis.
DGGE.
DGGE analysis of PCR-amplified 16S rRNA gene fragments was performed as described by Muyzer et al. (31) with the D-Gene system (Bio-Rad, Hercules, Calif.). In this analysis, 1-mm-thick, 6% (wt/vol) polyacrylamide gels with a 20 to 70% denaturing gradient were run for 20 h in 1× TAE buffer (40 mM Tris, 20 mM acetic acid, 1 mM EDTA [pH 8.3]) at a constant voltage of 100 V. After electrophoresis, the gels were stained with ethidium bromide (0.5 μg ml−1) and photographed on a UV transillumination table (302 nm) with a Polaroid camera. The photograph of the gel was scanned with Fotolook version 2.05 (Agfa) software and edited with Photoshop 4.0 (Adobe) software. A linear regression analysis between the numbers of DGGE bands in each lane and various physicochemical parameters was performed with the statistical StatView version 4.02 (Abacus) software.
RESULTS
Physicochemical characterization of the vent site.
The solitary vent site at a water depth of 8 m had a characteristic concentric zonation of colored surface deposits surrounding the gas outlet (Fig. 1 and 2). The sediment at the center was covered with a bright yellow sulfur deposit. This was surrounded by a zone covered with white flocculent material on top of black sediment. This zone was approximately 110 cm wide and extended 150 cm from the vent center. The white zone was followed by a ring of gray sediment (ca. 30 cm wide) and a brown or green outer zone (ca. 40 cm wide). The transitions between the different zones were very distinct. In situ measurements of pH and temperature demonstrated that the shallow vent site constitutes an extreme environment (Fig. 2). At about 300 cm from the vent center, there was a distinct increase in temperature with depth. This gradient became more pronounced within the gray zone, with an increase of 10°C between the sediment surface and 5 cm deep in the sediment. The sediment temperature reached a maximum of 103°C at a depth of 10 cm at the vent center (data not shown). At this location, the temperature increased by 50°C between the sediment surface and 5 cm deep (Fig. 2). The pH values decreased horizontally toward the center, but in contrast to temperature, there was no change in pH with increasing sediment depth (Fig. 2). At a distance of 200 cm, the pH values decreased below 6. The redox potential decreased from positive values to low negative values at the same location at 200 cm from the center (data not shown). Sediments outside the venting region showed dissolved-oxygen profiles with the characteristic shape of diffusive transport from the overlying water to the sediment (data not shown). The penetration depth of 3 mm was typical of coastal sediments (35, 55). In the zone between 200 and 230 cm from the vent center, oxygen penetrated two to three times deeper into the sediment, indicating that transport processes other than diffusion are responsible for the high concentrations (data not shown). The outflow of gas and hot fluid probably induces a convective flow of pore water entrainment to the sediment surface and into the water column. This upward transport of reduced solutes is compensated by an inflow of oxic overlying water within a distinct zone. Oxygen was not detected toward the vent center or beneath the white flocculent material in the reduced black sediment (data not shown).
Total cell counts.
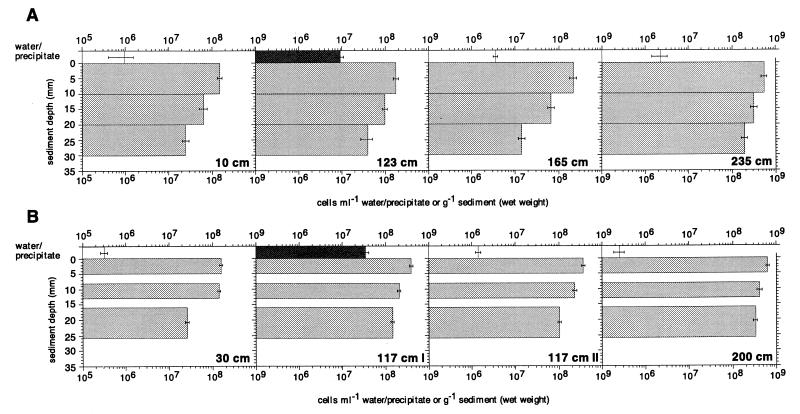
Total cell numbers varied between 9.5 × 105 cells ml−1 (in overlying water 10 cm from the vent center) and 5.5 × 108 cells g (wet weight) of sediment−1 (uppermost sediment layer at 235 cm from the vent center) in June (Fig. 3A) and between 3.2 × 105 cells ml−1 (overlying water at 30 cm from the vent center) and 6.4 × 108 cells g−1 (uppermost sediment layer at 200 cm from the vent center) in September (Fig. 3B). The largest numbers were observed at both sampling times in the upper sediment layers, and the numbers decreased with increasing sediment depth. On a horizontal scale, two regions could be detected. The largest numbers were found at 200 and 235 cm from the vent center in a transition zone between obviously hydrothermally influenced sediments (higher temperature, low pH, and redox potential) and ambient sediment conditions (mesophilic temperature, neutral pH, and positive redox potential). In the region which was more significantly affected by the hydrothermal fluid, i.e., between the center and 200 cm, the cell numbers were significantly smaller at a given depth compared to the outer region. Differences among the inner three cores or between the outer two cores were not apparent, however. In the water just above the sediment surface, the situation was quite different. The largest numbers were found in the white precipitate at 123 cm from the vent center in June and at 117 cm from the vent center in September (Fig. 3). When the precipitate was absent, no enrichment of bacteria relative to the ambient seawater was observed (117 cm II; Fig. 3B).
FIG. 3.
Total cell counts at specified locations along a transect from the vent center to the surrounding area for June 1996 (A) and September 1996 (B). The error bars indicate the 95% confidence interval. The cores at 117 cm from the vent center in September 1996 were obtained 1 week apart. During the first sampling (117 cm I), the white precipitate on the sediment surface was present, whereas during the second sampling (117 cm II), it was absent. The open bars indicate samples from the water above the sediment surface, the medium-shaded bars indicate sediment samples, and the dark-shaded bars indicate samples of the white precipitate.
Microscopic observations of unfixed, fresh material revealed that the white precipitate had a filamentous structure. The filaments had a diameter of 0.5 to 2 μm and were not stainable with the nuclear stain acridine orange. A highly motile, vibroid prokaryote was a dominant morphotype in the white precipitate. Gliding diatoms were also entangled in the fluffy material.
MPN counts of SRB.
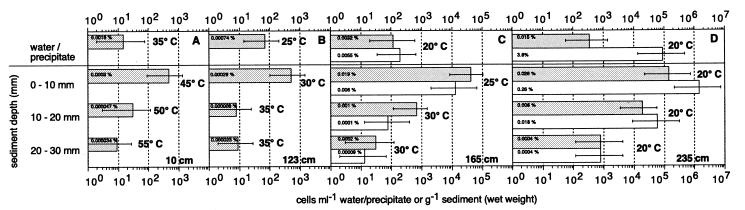
The numbers of acetate-oxidizing SRB varied between 8 and 1.43 × 105 cells g (wet weight) of sediment−1 (Fig. 4), with the highest numbers at 235 cm from the center (Fig. 4D). In general, numbers declined from the top sediment layer to the deeper layers. SRB were also detected in the oxygenated water above the sediment surface at all distances from the center, as well as in oxidized sediment layers at 235 cm from the center (Fig. 4D; 0 to 10 mm). At a maximum, SRB comprised 0.026% of the total counts (Fig. 4).
FIG. 4.
MPN counts of SRB (shaded bars) and DIRB (open bars) at 10 cm (A), 123 cm (B), 165 cm (C), and 235 cm (D) from the vent center in June 1996. The error bars denote the 95% confidence interval. The incubation temperature and the percentage of the total counts accounted for by the MPN counts are shown outside and inside the bars, respectively.
MPN counts of DIRB.
The numbers of DIRB capable of growing on acetate varied between undetectable and 1.4 × 106 cells g (wet weight) of sediment−1 (Fig. 4). As with the SRB, the largest numbers were found at 235 cm from the vent center (Fig. 4D), and they generally decreased from the surface to deeper layers. At 30 and 123 cm from the center, the numbers of DIRB were below the detection limit (Fig. 4A and B). At 235 cm from the center, DIRB were present in numbers equal to or greater than SRB (Fig. 4D). DIRB constituted up to 3.8% of the total cell numbers (Fig. 4).
MPN counts of SOB.
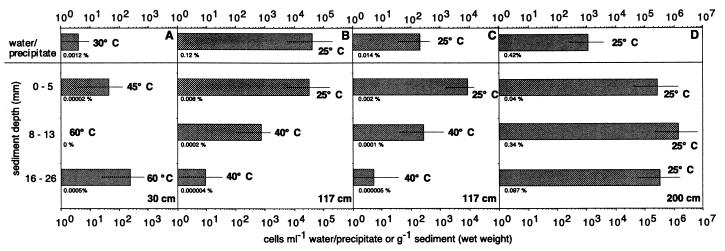
The numbers of aerobic SOB in September varied between undetectable and 1.42 × 106 cells g (wet weight) of sediment−1 and accounted at most for 0.41% of the total cell numbers (Fig. 5). The largest numbers were recorded at 200 cm from the vent center, which corresponds to the zone with the brownish to greenish surface (Fig. 5D). In the zone with the white precipitate on the surface, the largest numbers of SOB were found in the white precipitate and not in the sediment (Fig. 5B). The MPN counts with the higher-salinity medium were comparable to the MPN counts with the standard salt concentration. The single exception was for the samples collected from the water overlying the sediments (Fig. 5B and C). MPN counts of anaerobic SOB showed growth only in the first two dilutions of the samples collected 200 cm from the vent center (data not shown).
FIG. 5.
MPN counts of SOB at 30 cm (A), 117 cm (B and C), and 200 cm (D) from the vent center in September 1996. The bars indicate the 95% confidence interval. The incubation temperature and the percentage of the total cells accounted for by the MPN counts are given beside and below the bars, respectively. The two cores at 117 cm were obtained 1 week apart. During the first sampling, a white precipitate was present on the sediment surface (B), whereas during the second sampling, it was absent (C). The MPN series for the second core (C) was performed with medium with an increased salinity of 55‰.
DGGE analysis.
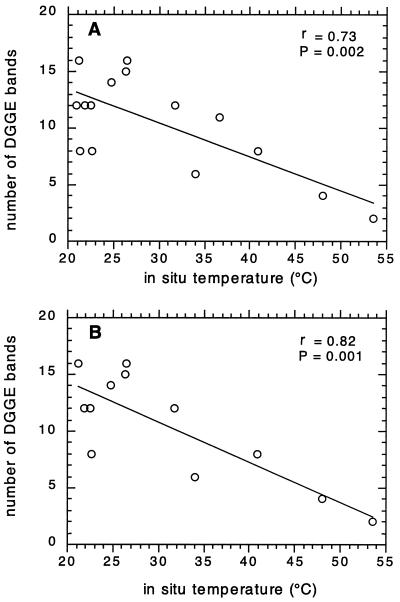
PCR-amplified 16S rRNA fragments obtained from environmental DNA were separated by DGGE to profile the bacterial communities at different locations along the transect in June and September (Fig. 6). For June, there was a trend of decreasing numbers of bands when approaching the vent center (Fig. 6A). In addition, there was a vertical decrease, which was most obvious at 10 and 123 cm from the center (Fig. 6A, lanes 2 to 5 and 6 to 9, respectively). The only physicochemical parameter that showed a significant correlation (P < 0.05) to the numbers of DGGE bands was temperature (Fig. 7A; r = 0.73; P = 0.002). The significance of this negative correlation increased when only sediment samples were included (Fig. 7B; r = 0.82; P = 0.001). The pH did not correlate significantly with the numbers of DGGE bands (r = 0.28; P = 0.079 [data not shown]). In September, a trend of decreasing numbers of bands when approaching the vent center was not discernible and the DGGE banding pattern appeared to be more homogeneous. In addition, there was no correlation between temperature and numbers of DGGE bands (r = 0.2; P = 0.45 [data not shown]).
FIG. 6.
DGGE analysis of 16S rDNA fragments obtained after PCR amplification with the bacterial primer pair GM5F-GC-clamp and 907R of genomic DNA from environmental samples and standards with a known melting behavior for June 1996 (A) and September 1996 (B). The environmental samples were obtained at specific locations along a transect from the vent center to the surrounding area. The two cores obtained at 117 cm from the vent center in September were taken 1 week apart. During the first sampling (117 cm I), the white precipitate on the sediment surface was present, whereas during the second sampling (117 cm II), it was absent. No PCR product could be obtained from the overlying water at 165 cm. The numbered bands are described in the text. (A) DGGE pattern of the samples taken in June 1996. Lanes: 1 and 17, standards; 2 to 5, samples taken at 10 cm from the vent center (lane 2, surface, lane 3, 0 to 10 mm; lane 4, 10 to 20 mm; lane 5, 20 to 30 mm); 6 to 9, samples taken at 123 cm (lane 6, surface; lane 7, 0 to 10 mm; lane 8, 10 to 20 mm; lane 9, 20 to 30 mm); 10 to 12, samples taken at 165 cm (lane 10, 0 to 10 mm; lane 11, 10 to 20 mm; lane 12, 20 to 30 mm); 13 to 16, samples taken at 235 cm (lane 13, surface; lane 14, 0 to 10 mm; lane 15, 10 to 20 mm; lane 16, 20 to 30 mm). (B) DGGE pattern of the samples taken in September 1996. Lanes: 1 and 18, standards; 2 to 5, samples taken at 30 cm from the vent center (lane 2, surface; lane 3, 0 to 5 mm; lane 4, 8 to 13 mm; lane 5, 16 to 26 mm); 6 to 9, samples taken at 117 cm (lane 6, surface; lane 7, 0 to 5 mm; lane 8, 8 to 13 mm; lane 9, 16 to 26 mm); 10 to 13, samples taken at 117 cm (lane 10, surface; lane 11, 0 to 5 mm; lane 12, 8 to 13 mm; lane 13, 16 to 26 mm); 14 to 17, samples taken at 200 cm (lane 14, surface; lane 15, 0 to 5 mm; lane 16, 8 to 13 mm; lane 17, 16 to 26 mm).
FIG. 7.
Correlation of the in situ temperature with the number of DGGE bands in each lane for June 1996, including water samples (A) and excluding water samples (B). The Pearson correlation coefficient, r, and its significance, P, are shown.
One dominant band was present in every region and at almost every depth at both sampling times (Fig. 6; bands 1 and 5). However, its dominance was less pronounced at 200 and 235 cm from the vent center. The position of the band relative to the two standards was the same at both sampling times. Other bands seemed to be restricted to certain depths or zones, such as bands 2, 3 and 4 in June (Fig. 6A), and band 6 in September (Fig. 6B).
DISCUSSION
Total-cell counts and MPN counts.
There are only a few studies where the fraction of culturable subpopulations of natural communities growing autotrophically or with single carbon sources was related to the total cell counts (see, e.g., references 39, 43, and 53). The small numbers found in the present study fall within the range of values reported previously, although the fraction might be increased when complex media are used (19, 22). However, since typically less than 1% of the total prokaryotic populations in natural habitats may generally be cultivable (2), the present numbers might represent a substantial fraction of the culturable organisms in this habitat. In addition, since total-cell counts do not differentiate between active and inactive cells, a substantial fraction of the total-cell counts might consist of dead cells (57). Although we tried to maximize the viable-cell counts by incubating samples at the in situ temperature (48), we certainly underestimated the absolute numbers of the respective physiological groups by not providing the appropriate growth media. In addition, the association of cells in clumps or with particles cannot be ruled out, although we vigorously vortexed the samples to disaggregate and dislodge the cells. Clumping would also lead to an underestimation, since the MPN evaluation assumes that only one cell initiated the growth at the highest dilution (3). Cells may also have been present at higher dilutions but were not detected due to the insensitivity of the detection methods used (43, 45, 53).
The large total-cell numbers at 200 and 235 cm from the vent center as well as the relatively high abundance of DIRB and SRB at 235 cm were probably related to the moderate environmental conditions, e.g., neutral pH and temperatures of about 20°C, in these sediments and to the presence of diatoms and other primary producers such as SOB (see below). The organic matter produced by autotrophic organisms could be used by the anaerobic heterotrophs, a possibility supported by their confinement to the upper sediment layers. Similar observations have been made in a marine microbial mat (46) and in other marine sediments (24). In addition, organic matter (such as seagrass fragments) transported by bottom currents from the surrounding area to the vent site could form the basis of a phytodetrital food chain (44). The large numbers of SRB at 235 cm from the vent center correlated well with sulfate reduction rates, which were also highest in this zone and peaked in the upper 2 cm at the studied vent (56) and at similar vents in Palaeochori Bay (10). The small numbers of SRB in the cores obtained at 30 and 123 cm from the vent center were probably related to the low pH in the sediments, which is known to be inhibitory to sulfate reducers (50). The presence of thermophilic SRB in this zone (36) could be related to the presence of microniches (17, 50).
The relatively large numbers of DIRB compared to SRB in the brownish zone at 235 cm from the vent center indicate that organic matter degradation by dissimilatory iron reduction is likely to be an important process in the hydrothermally influenced sediments of Palaeochori Bay. This hypothesis is supported by the co-occurrence of the largest numbers of DIRB and high concentrations of Fe(III) (56). The absence of DIRB closer to the center of the vent could be explained by low concentrations of Fe(III) in these zones due to the reduced conditions at the sediment surface. Temperature was not likely to be an important parameter in restricting the occurrence of DIRB in these zones, since dissimilatory Fe(III)-reducing bacteria which are able to grow at temperatures up to 74°C were isolated (37).
In addition to phototrophic organisms, e.g., diatoms and cyanobacteria, the green-brownish transition zone also contained large numbers of aerobic SOB (Fig. 5B). These organisms are capable of autotrophic growth and therefore most probably contributed to primary production. The slight increase in temperature indicates that this zone was affected by the hydrothermal processes. The reduced sulfur compounds contained in the hydrothermal fluid, mainly H2S, would provide the necessary electron donor for the SOB. However, SOB could also use sulfide produced by SRB. The observation that numbers of SOB at 200 cm from the center were also large in deeper sediment layers could be related to the increased penetration depth of O2 at this site. At 200 cm from the vent center, O2 penetration reached up to 9 mm deep in the sediment, whereas O2 did not penetrate into the sediment at 30 and 117 cm from the vent center. The nature of the electron acceptor for SOB below the oxygen penetration depth remains unclear, since anaerobic SOB using nitrate as electron acceptor were present in much smaller numbers. One explanation might be that the oxygen penetration depth is temporally variable, e.g., influenced by tidal effects on hydrothermal fluid discharge (1, 16). In the German Wadden Sea (North Sea), although aerobic Thiomicrospira populations were homogeneously distributed in the upper 1 cm of the sediment, only the populations which were in the O2-penetrating zone seemed to be metabolically active (6). The lower abundance of SOB at 30 cm and 117 cm from the vent center is probably related to the elevated temperatures and the lack of O2 as electron acceptor in the sediments.
Since the outflowing hydrothermal fluid has an elevated salinity relative to ambient seawater (44), we were concerned whether we would miss additional cultivable SOB by using the standard medium. However, with the exception of the water above the sediment surface, both high- and normal-salinity media showed comparable numbers, although there may have been differences among the types of SOB present in the dilutions (7). The significantly larger numbers of SOB in the water above the sediment surface in the first core at 117 cm from the vent center compared to the second core at 117 cm are probably not related to the difference in salinity but to the absence of the white precipitate in the second core, since the same trend was found for the total cell numbers. This result indicates that the precipitate was a suitable habitat for certain bacteria, such as SOB and a vibrio, which was further identified to be an Arcobacter species by using an oligonucleotide probe specific for this genus (18).
Recently, Taylor and Wirsen (42) described the formation of filamentous sulfur by a highly motile vibroid chemoautotrophic strain of H2S-oxidizing bacterium in a continuous-flow, H2S-enriched seawater reactor. The filaments resembled the precipitate at deep-sea hydrothermal vents (42) and at the vent site investigated in the present study. Since this vibrio also belongs to the genus Arcobacter within the epsilon subdivision of the Proteobacteria (52), it is possible that the vibroid Arcobacter species detected in the present study is involved in the formation of the white precipitate. As argued by Taylor and Wirsen (42), precipitate formation could be a strategy for retainment in environments characterized by active fluid motion. The precipitate would also favor the growth of other SOB, since it stabilized the gradient between the sulfidic brine and the oxygenated seawater. In addition, the H2S is rapidly transformed into the more stable form of sulfur, which could still be used as an electron donor (42).
DGGE analysis.
DGGE allowed a general assessment of the spatial distribution of bacterial populations and the bacterial community structure at this particular submarine hydrothermal vent. The bacterial populations were neither vertically nor horizontally homogeneously distributed, as has been described for, e.g., soil bacterial communities (13). Inhomogeneous distribution is most probably due to changes in the physicochemical parameters that occurred on a relatively small scale. For June, a change in the bacterial community structure, i.e., in the number of populations and their relative abundance, was observed along the transect from the center of the vent to the surrounding area. This trend was not apparent in September, however (see below). If it is assumed that the intensity of the PCR product is proportional to the abundance of the template (see e.g., references 29 and 33), the changes observed in June support the hypothesis that extreme environmental conditions will lead to a community structure with a limited number of dominant populations, since fewer but stronger bands were found at the locations closer to the vent center. This finding is in accordance with results from other studies at different deep-sea hydrothermal vents in which molecular methods were used (27, 30, 34). An acidothermal soil from a thermal area in New Zealand also contained a limited microbial diversity compared to “normal” soil (49). However, previous studies have not investigated how the community structure might change with a decreasing influence of the hydrothermal fluid.
In June, we found the highest diversity of bacterial populations as indicated by DGGE bands at 165 and 235 cm from the vent center. However, the community structures at the two distances differed. At 165 cm, one population seemed to dominate, as indicated by the strong band, whereas at 235 cm, no such dominant population was found (Fig. 6A). This result could be explained by the more moderate environmental conditions at 235 cm, which probably allowed more populations to coexist rather than favoring a few populations. Interestingly, Thiermann et al. observed a general decrease in faunal diversity toward the hydrothermally active area at a similar vent system in Palaeochori Bay (44). In addition, the faunal community in the strongly hydrothermally influenced sediments was found to be uneven and dominated by tolerant, opportunistic species (44).
The significant correlation between temperature and the numbers of DGGE bands found in the June samples suggests that temperature was an important environmental parameter affecting the bacterial community structure at the vent site. Temperature is also a key factor in determining the distribution of bacterial populations in the outflow of a hot spring (14), and it has been demonstrated that the microbial diversity of hot spring mats decreased as the temperature of the environment increased (21). However, total cell numbers and numbers of culturable hyperthermophiles increased in the deeper layers (≥10 cm) in the hot sediments of a shallow submarine vent (22). Thus, the trend described above might change when the deeper, hyperthermophilic microbial community is analyzed. These populations are obviously present in Palaeochori Bay, albeit in unknown numbers (9).
Although PCR-DGGE has the advantage that it circumvents selective and potential ineffective cultivation, it also has limitations. First, only numerically dominant populations will be detected by DGGE (29, 39). This limitation precludes a determination of changes in species richness at this vent site, since numerically smaller populations, which would contribute to species richness, might have been present but were not detected. In addition, bacteria specific to this habitat may not contain the signature sites necessary for efficient amplification with the bacterial primers used and thus would not have been included in the analysis. An insufficient or preferential disruption of cells would also distort the view of the community composition (47). Although our microscopic observations indicated that complete lysis was achieved, the recovery might still have been reduced by degradation or adsorption of nucleic acids upon their release from cells to matrix material contained in the sediment. Such a process might have influenced the trend, since the sedimentary environment changed concurrently. Furthermore, PCR biases, e.g., a selective amplification of certain DNA fragments or differential amplification efficiencies, cannot be excluded (15, 40, 47). Since we did not know the amount of DNA attributable to bacteria and whether this proportion would change along the transect, we were concerned that the amount of template DNA would have an effect on the observed changes in the banding pattern. However, a PCR bias related to the amount of template DNA can be excluded, since the banding pattern of the DGGE and the relative intensities of the bands did not change when serial dilutions (up to 1,000-fold) of the template DNA were used (data not shown). An increased proportion of Archaea nearer the center of the vent might actually be responsible for the lack of variation in total cell counts along the transect. However, it is also important to note that two communities could contain the same quantity of cells but be structured differently.
The trend of a more uneven community structure with increasingly extreme environmental conditions was not discernible in the DGGE banding pattern from September, however. One reason might be the sampling interval, since the cores were sliced at a finer resolution in September than in June. This would suggest that the community changes are less obvious on a scale less then 1 cm. However, a storm occurred a few days prior to the sampling, and so another explanation could be the disturbance of the microbial community caused by resuspension of the upper sediment layers due to strong bottom currents caused by the wave action. Such a disturbance would also account for the more homogeneous DGGE banding pattern in September and for the lack of a negative correlation between temperature and numbers of DGGE bands. In addition, it would explain the presence of Thiomicrospira spp. in deeper sediment layers with unfavorable growth conditions close to the center of the vent as found by DGGE analysis (7). Thus, it could be speculated that longer periods of calm conditions are necessary for the establishment of distinct bacterial populations along the naturally occurring physicochemical gradients at this vent site.
The presence of bands 1 and 5 at the two sampling dates might indicate a certain degree of stability of the bacterial community over time, despite the highly dynamic changes that occur in this environment. That these bands were present in almost every zone and depth irrespective of the different physicochemical conditions further suggests that they belong to a population with a broad tolerance to environmental conditions. Judged from the intensity of the bands, it could be inferred that the preferred habitat for this population was between the center of the vent and 165 cm, i.e., in the more strongly hydrothermally influenced sediments. However, since it has been demonstrated that different sequences can have the same melting properties in DGGE (14, 29), this band does not a priori belong to the same population in every case. Other bands, e.g., bands 2, 3, 4, and 6, appeared to be more confined to certain depth and zones, suggesting that they belong to populations that are more specialized.
Conclusions.
The data indicate that the changing physicochemical conditions at the vent site affected bacterial distribution and community structure. The largest cell numbers, the greatest diversity of dominant populations, and an even community structure were found in a transition zone from the strongly hydrothermally influenced sediments to normal sedimentary conditions. Closer to the vent center, cell numbers were significantly smaller and the community structure was dominated by few populations, probably due to the harsh environmental conditions in these regions. This trend is likely to apply also to deep-sea hydrothermal vents, where sharp physicochemical gradients are known to occur (4, 5, 23, 26). DGGE seems to be an appropriate tool to address this question in future studies. The trend of a more uneven community structure with increasingly extreme environmental conditions, however, was not observed at the second sampling time. Resuspension of the upper sediment layers due to a storm prior to the sampling most probably was responsible for this observation. In future studies, the influence of such disturbances on the dynamics of the microbial community should be studied in greater detail. The relatively large numbers of DIRB in the mesophilic zone indicated that in addition to sulfate reduction, ferric iron reduction was an important pathway for the anaerobic degradation of organic matter at the vent site. The results of the MPN series for SOB indicate that chemoautotrophy based on reduced sulfur compounds adds to primary production at the vent site, most notably in the transition zone and possibly in the white precipitate. The detection of sulfur-oxidizing Thiomicrospira spp. among the predominant populations, as identified by DGGE (7), and the indication that the vibroid Arcobacter sp. is a SOB further support this assumption.
ACKNOWLEDGMENTS
We are grateful to S. Menger and G. Lützenkirchen for SCUBA diving, sampling, and assistance with the fieldwork; to the mechanical workshop of the institute for building the sampling devices; and to the technicians of the microsensor group for their help in constructing microsensors. We also thank R. Amann, J. Detmers, B. M. Fuchs, U. Nübel, K. Sahm, and H. Schäfer for helpful discussions and advice; C. O. Wirsen for permission to cite unpublished data; and C. Arnosti for linguistic improvements to the manuscript. Special thanks go to the participants of the EU-funded project Hydrothermal Fluxes and Biological Production in the Aegean for a variety of types of support and help. We also acknowledge the Greek authorities for permission to undertake SCUBA diving and fieldwork. Two anonymous reviewers provided valuable comments that improved the manuscript.
This work was funded by the EU under MAST CT-95-0021 and the Max-Planck Society, Munich (Germany).
REFERENCES
- 1.Aliani S, Amici L, Dando P R, Meloni R. Time series and bottom temperature in a marine shallow water hydrothermal vent off Milos Island (Aegean Volcanic Arc) Rapp P-V Comm Reun Int Explor Sci Mer Mediterr. 1998;35:46–47. [Google Scholar]
- 2.Amann R I, Ludwig W, Schleifer K-H. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol Rev. 1995;59:143–169. doi: 10.1128/mr.59.1.143-169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Public Health Association. Standard methods for the examination of water and wastewater, including bottom sediments and sludge. Washington, D.C: American Public Health Association; 1969. pp. 604–609. [Google Scholar]
- 4.Baross J A, Hoffman S E. Submarine hydrothermal vents and associated gradient environments as sites for the origin of life. Origins Life. 1985;15:327–345. [Google Scholar]
- 5.Baross J A, Deming J W. Growth at high temperatures: isolation and taxonomy, physiology, and ecology. In: Karl D M, editor. Microbiology of deep-sea hydrothermal vents. Boca Raton, Fla: CRC Press, Inc.; 1995. [Google Scholar]
- 6.Brinkhoff T, Santegoeds C M, Sahm K, Kuever J, Muyzer G. A polyphasic approach to study the diversity and vertical distribution of sulfur-oxidizing Thiomicrospira species in coastal sediments of the German Wadden Sea. Appl Environ Microbiol. 1998;64:4650–4657. doi: 10.1128/aem.64.12.4650-4657.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brinkhoff T, Sievert S M, Kuever J, Muyzer G. Distribution and diversity of sulfur-oxidizing Thiomicrospira spp. at a shallow-water hydrothermal vent in the Aegean Sea (Milos, Greece) Appl Environ Microbiol. 1999;65:3843–3849. doi: 10.1128/aem.65.9.3843-3849.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cord-Ruwisch R. A quick method for the determination of dissolved and precipitated sulfides in cultures of sulfate-reducing bacteria. J Microbiol Methods. 1985;4:33–36. [Google Scholar]
- 9.Dando P R, Thomm M, Arab H, Brehmer M, Hooper L, Jochimsen B, Schlesner H, Stöhr R, Miquel J C, Fowler S. Microbiology of shallow hydrothermal sites off Palaeochori Bay, Milos (Hellenic Volcanic Arc) Cah Biol Mar. 1998;39:369–372. [Google Scholar]
- 10.Dando P R, Hughes J A, Thiermann F. Preliminary observations on biological communities at shallow hydrothermal vents in the Aegean Sea. In: Parson L M, Walker C L, Dixon D R, editors. Hydrothermal vents and processes. Special publication 87. London, United Kingdom: Geological Society; 1995. pp. 303–317. [Google Scholar]
- 11.Dando P R, Hughes J A, Leahy Y, Niven S J, Taylor L J, Smith C. Gas venting rates from submarine hydrothermal areas around the island of Milos, Hellenic Volcanic Arc. Continental Shelf Res. 1995;15:913–929. [Google Scholar]
- 12.Detmers J. M.Sc. thesis. Germany: University of Bremen; 1997. [Google Scholar]
- 13.Felske A, Akkermans A D L. Spatial homogeneity of abundant bacterial 16S rRNA molecules in grassland soils. Microb Ecol. 1998;36:31–36. doi: 10.1007/s002489900090. [DOI] [PubMed] [Google Scholar]
- 14.Ferris M J, Muyzer G, Ward D M. Denaturing gradient gel electrophoresis profiles of 16S rRNA-defined populations inhabiting a hot spring microbial mat community. Appl Environ Microbiol. 1996;62:340–346. doi: 10.1128/aem.62.2.340-346.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferris M J, Ward D M. Seasonal distributions of dominant 16S rRNA-defined populations in a hot spring microbial mat examined by denaturing gradient gel electrophoresis. Appl Environ Microbiol. 1997;63:1375–1381. doi: 10.1128/aem.63.4.1375-1381.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fitzimons M F, Dando P R, Hughes J A, Thiermann F, Akoumianaki I, Pratt S M. Submarine hydrothermal brine seeps off Milos, Greece: observations and geochemistry. Mar Chem. 1997;57:325–340. [Google Scholar]
- 17.Fortin D, Davis B, Beveridge T J. Role of Thiobacillus and sulfate-reducing bacteria in iron biocycling in oxic and acidic mine tailings. FEMS Microbiol Ecol. 1996;21:11–24. [Google Scholar]
- 18.Fuchs, B. M., S. M. Sievert, J. Kuever, and R. Amann. Unpublished data.
- 19.Harmsen H J M, Prieur D, Jeanthon C. Distribution of microorganisms in deep-sea hydrothermal vent chimneys investigated by whole-cell hybridization and enrichment culture of thermophilic subpopulations. Appl Environ Microbiol. 1997;63:2876–2883. doi: 10.1128/aem.63.7.2876-2883.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hedrick D B, Pledger R D, White D C, Baross J A. In situ microbial ecology of hydrothermal vent sediments. FEMS Microbiol Ecol. 1992;101:1–10. [Google Scholar]
- 21.Hiraishi A, Umezawa T, Yamamoto H, Kato K, Maki Y. Changes in quinone profiles of hot spring microbial mats with a thermal gradient. Appl Environ Microbiol. 1999;65:198–205. doi: 10.1128/aem.65.1.198-205.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoaki T, Nishijima M, Miyashita H, Maruyama T. Dense community of hyperthermophilic sulfur-dependent heterotrophs in a geothermally heated shallow submarine biotope near Kadakara-Jima island, Kagoshima, Japan. Appl Environ Microbiol. 1995;61:1931–1937. doi: 10.1128/aem.61.5.1931-1937.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jannasch H W, Mottl M J. Geomicrobiology of deep-sea hydrothermal vents. Science. 1985;229:717–725. doi: 10.1126/science.229.4715.717. [DOI] [PubMed] [Google Scholar]
- 24.Jørgensen B B, Bak F. Pathways and microbiology of thiosulfate and sulfate reduction in a marine sediment (Kattegat, Denmark) Appl Environ Microbiol. 1991;57:847–856. doi: 10.1128/aem.57.3.847-856.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karl D M, Wirsen C O, Jannasch H W. Deep-sea primary production at the Galápagos hydrothermal vents. Science. 1980;207:1345–1347. [Google Scholar]
- 26.Karl D M. Ecology of free-living, hydrothermal vent microbial communities. In: Karl D M, editor. Microbiology of deep-sea hydrothermal vents. Boca Raton, Fla: CRC Press, Inc.; 1995. [Google Scholar]
- 27.Moyer C L, Dobbs F C, Karl D M. Estimation of diversity and community structure through restriction fragment length polymorphism distribution analysis of bacterial 16S rRNA genes from a microbial mat at an active, hydrothermal vent system, Loihi Seamount, Hawaii. Appl Environ Microbiol. 1994;60:871–879. doi: 10.1128/aem.60.3.871-879.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moyer C L, Dobbs F C, Karl D M. Phylogenetic diversity of the bacterial community from a microbial mat at an active, hydrothermal vent system, Loihi Seamount, Hawaii. Appl Environ Microbiol. 1995;61:1555–1562. doi: 10.1128/aem.61.4.1555-1562.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muyzer G, de Waal E C, Uitterlinden A G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol. 1993;59:695–700. doi: 10.1128/aem.59.3.695-700.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muyzer G, Teske A, Wirsen C O, Jannasch H W. Phylogenetic relationships of Thiomicrospira species and their identification in deep-sea hydrothermal vent samples by denaturing gradient gel electrophoresis of 16S rDNA fragments. Arch Microbiol. 1995;164:165–172. doi: 10.1007/BF02529967. [DOI] [PubMed] [Google Scholar]
- 31.Muyzer G, Brinkhoff T, Nübel U, Santegoeds C, Schäfer H, Wawer C. Denaturing gradient gel electrophoresis (DGGE) in microbial ecology. In: Akkermans A D L, van Elsas J D, de Bruijn F J, editors. Molecular microbial ecology manual. 3rd ed. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1998. pp. 1–27. [Google Scholar]
- 32.Muyzer G, Smalla K. Application of denaturing gradient gel electrophoresis (DGGE) and temperature gradient gel electrophoresis (TGGE) in microbial ecology. Antonie Leeuwenhoek. 1998;73:127–141. doi: 10.1023/a:1000669317571. [DOI] [PubMed] [Google Scholar]
- 33.Nübel U, Garcia-Pichel F, Kühl M, Muyzer G. Quantifying microbial diversity: morphotypes, 16S rRNA genes, and carotenoids of oxygenic phototrophs in microbial mats. Appl Environ Microbiol. 1999;65:422–430. doi: 10.1128/aem.65.2.422-430.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Polz M F, Cavanaugh C M. Dominance of one bacterial phylotype at a Mid-Atlantic Ridge hydrothermal vent site. Proc Natl Acad Sci. 1995;92:7232–7236. doi: 10.1073/pnas.92.16.7232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Revsbech N P. An oxygen microelectrode with a guard cathode. Limnol Oceanogr. 1989;34:157–175. [Google Scholar]
- 36.Sievert, S. M., and J. Kuever. Unpublished data.
- 37.Slobodkin A I, Reysenbach A L, Strutz N, Dreier M, Wiegel J. Thermoterrabacterium ferrireducens gen. nov., sp. nov., a thermophilic anaerobic dissimilatory Fe(III)-reducing bacterium from a continental hot spring. Int J Syst Bacteriol. 1997;47:541–547. doi: 10.1099/00207713-47-2-541. [DOI] [PubMed] [Google Scholar]
- 38.Sørensen J, Christensen D, Jørgensen B B. Volatile fatty acids and hydrogen as substrates for sulfate-reducing bacteria in anaerobic marine sediment. Appl Environ Microbiol. 1981;42:5–11. doi: 10.1128/aem.42.1.5-11.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Straub K L, Buchholz-Cleven B E E. Enumeration and detection of anaerobic ferrous iron-oxidizing, nitrate-reducing bacteria from diverse European sediments. Appl Environ Microbiol. 1998;64:4846–4856. doi: 10.1128/aem.64.12.4846-4856.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suzuki M T, Giovannoni S J. Bias caused by template annealing in the amplification of mixtures of 16S rRNA genes by PCR. Appl Environ Microbiol. 1996;62:625–630. doi: 10.1128/aem.62.2.625-630.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tarasov V G, Propp M V, Propp L N, Zhirmunsky A V, Namsaraev B B, Gorlenko V M, Starynin D A. Shallow-water gasohydrothermal vents of Ushishir Volcano and the ecosystem of Kraternaya Bight (the Kurile Islands) Mar Ecol. 1990;11:1–23. [Google Scholar]
- 42.Taylor C D, Wirsen C O. Microbiology and ecology of filamentous sulfur formation. Science. 1997;277:1483–1485. [Google Scholar]
- 43.Teske A, Wawer C, Muyzer G, Ramsing N B. Distribution of sulfate-reducing bacteria in a stratified fjord (Mariager Fjord, Denmark) as evaluated by most-probable-number counts and denaturing gradient gel electrophoresis of PCR-amplified ribosomal DNA fragments. Appl Environ Microbiol. 1996;62:1405–1415. doi: 10.1128/aem.62.4.1405-1415.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thiermann F, Akoumianaki I, Hughes J A, Giere O. Benthic fauna of a shallow-water gaseohydrothermal vent area in the Aegean Sea (Milos, Greece) Mar Biol. 1997;128:149–159. [Google Scholar]
- 45.Vester F, Ingvorsen K. Improved most-probable-number method to detect sulfate-reducing bacteria with natural media and a radiotracer. Appl Environ Microbiol. 1998;64:1700–1707. doi: 10.1128/aem.64.5.1700-1707.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Visscher P T, Prins R A, van Gemerden H. Rates of sulfate reduction and thiosulfate consumption in a marine microbial mat. FEMS Microbiol Ecol. 1992;86:283–294. [Google Scholar]
- 47.Von Wintzingerode F, Göbel U B, Stackebrandt E. Determination of microbial diversity in environmental samples: pitfalls of PCR-based rRNA analysis. FEMS Microbiol Rev. 1997;21:213–229. doi: 10.1111/j.1574-6976.1997.tb00351.x. [DOI] [PubMed] [Google Scholar]
- 48.Ward D M, Santegoeds C M, Nold S C, Ramsing N B, Ferris M J, Bateson M M. Biodiversity within hot spring microbial mat communities: molecular monitoring of enrichment cultures. Antonie Leeuwenhoek. 1997;71:143–150. doi: 10.1023/a:1000131426164. [DOI] [PubMed] [Google Scholar]
- 49.Ward N, Raney F A, Goebel B, Stackebrandt E. Identifying and culturing ‘unculturables’: a challenge for microbiologists. In: Allsopp D, Colwell R R, Hawksworth D L, editors. Microbial diversity and ecosystem function. Wallingford, United Kingdom: CAB International; 1995. pp. 89–109. [Google Scholar]
- 50.Widdel F. Sulphate-reducing bacteria and their ecological niches. In: Barnes E M, Mead G C, editors. Anaerobic bacteria in habitats other than man. Oxford, United Kingdom: Blackwell Scientific Publications; 1986. pp. 157–184. [Google Scholar]
- 51.Widdel F, Bak F. Gram-negative mesophilic sulfate-reducing bacteria. In: Balows A, Trüper H G, Dworkin M, Harder W, Schleifer K H, editors. The prokaryotes. 2nd ed. Berlin, Germany: Springer-Verlag KG; 1992. pp. 3352–3378. [Google Scholar]
- 52.Wirsen, C. O. Personal communication.
- 53.Wirsen C O, Tuttle J H, Jannasch H W. Activities of sulfur-oxidizing bacteria at the 21°N East Pacific Rise vent site. Mar Biol. 1986;92:449–456. [Google Scholar]
- 54.Zhou J, Bruns M A, Tiedje J M. DNA recovery from soils of diverse composition. Appl Environ Microbiol. 1996;62:316–322. doi: 10.1128/aem.62.2.316-322.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ziebis W, Hüttel M, Forster S. Impact of bionic sediment topography on oxygen fluxes in permeable seabeds. Mar Ecol Prog Ser. 1996;140:227–237. [Google Scholar]
- 56.Ziebis, W., V. Brüchert, S. Forster, and B. B. Jørgensen. 1999. Unpublished data.
- 57.Zweifel U L, Hagström Å. Total counts of marine bacteria include a large fraction of non-nucleoid-containing bacteria (ghosts) Appl Environ Microbiol. 1995;61:2180–2185. doi: 10.1128/aem.61.6.2180-2185.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]