Summary
Background
Onset of effect of advanced therapies is an important parameter due to symptom load and risk of disease complications in moderate-to-severe ulcerative colitis (UC), but comparative data are lacking. Therefore, we aimed to assess the comparative onset of efficacy of biological therapies and small molecules for this patient population.
Methods
In this systematic review and network meta-analysis, we searched MEDLINE, Embase, and Cochrane Central Register of Controlled Trials from inception to 24 August 2022, for randomised controlled trials or open-label studies assessing the efficacy of biologics or small molecule drugs within the first six weeks of treatment in adults with UC. The co-primary outcomes were the induction of clinical response and clinical remission at week 2. Network meta-analyses was conducted under the Bayesian framework. This study is registered with PROSPERO: CRD42021250236.
Findings
The systematic literature search identified 20,406 citations, of which 25 studies comprising 11,074 patients fulfilled the eligibility criteria. Upadacitinib ranked highest for induction of clinical response and clinical remission at week 2 and was significantly superior to all agents but tofacitinib, which ranked second highest. Although the rankings remained consistent, no differences between upadacitinib and biological therapies were demonstrated in the sensitivity analyses of partial Mayo clinic score response or resolution of rectal bleeding at week 2. Tumor necrosis factor-α (TNF) inhibitors were significantly superior to vedolizumab and ustekinumab for patient-reported outcome-2 (PRO-2) remission at week 2 in bio-naïve patients. Filgotinib 100 mg, ustekinumab, and ozanimod ranked lowest across all endpoints.
Interpretation
In this network meta-analysis, we found upadacitinib to be significantly superior to all agents but tofacitinib for the induction of clinical response and clinical remission two weeks after treatment initiation. In contrast, ustekinumab and ozanimod ranked lowest. Our findings help to establish the evidence regarding the onset of efficacy of advanced therapies.
Funding
None.
Keywords: Ulcerative colitis, Biological therapies, Small molecules, Network meta-analysis, Rapidity, Speed of onset
Research in context.
Evidence before this study
Moderate to severe ulcerative colitis is associated with substantial symptom burden and disability and carries a high risk of progression with the need for hospitalization and colectomy, requiring rapid resolution. We systematically searched PubMed, Embase, the Cochrane Central Register of Controlled Trials, and conference proceedings from European Crohn's and Colitis Organization, Digestive Disease Week, and United European Gastroenterology Week from inception to 24 August 2022 and identified no systematic reviews, meta-analyses or network meta-analyses focusing on the rapidity of onset of efficacy. We subsequently identified original studies with the following search terms: “ulcerative colitis”, “inflammatory bowel disease”, or “colitis” in combination with the terms “biologics”, “anti-TNF”, “infliximab”, “Remicade”, “adalimumab”, “Humira”, “golimumab”, “CNTO-148”, “certolizumab”, “Simponi”, “CDP870”, “Cimzia”, “anti-integrin”, “vedolizumab”, “MLN-0002”, “Entyvio”, “ustekinumab”, “CNTO-1275”, “small molecule”, “JAK inhibitor”, “tofacitinib”, “CP-690550”, “Xeljanz”, “filgotinib”, “GLPG0634”, “upadacitinib”, “ABT-494”, “sphingosine-1-phosphate receptor modulator”, “ozanimod”, or “RPC1063”.
Added value of this study
Based on 25 original studies, we found upadacitinib and tofacitinib to rank highest across all analyses, while TNF inhibitors were significantly superior to vedolizumab and ustekinumab for the induction of patient-reported outcome-2 (PRO-2) remission at week 2 in bio-naïve patients. However, in subgroup analyses, upadacitinib and tofacitinib were not significantly superior to biological therapies for the induction of a partial Mayo clinical score response or resolution of rectal bleeding at week 2. Filgotinib 100 mg, ustekinumab and ozanimod ranked lowest in the analyses.
Implications of all the available evidence
This first systematic review and network meta-analysis on the comparative onset of efficacy sheds light on differences in the speed of onset of efficacy. The outcome might have implications in informed decision making and in guidelines when considering the choice of biological or small molecule agents. Further, the study is important when framing the patient's expectations in the clinical setting.
Introduction
Ulcerative colitis (UC) is the most prevalent entity of inflammatory bowel disease and is associated with an increasing worldwide incidence and prevalence.1 UC is a chronic, idiopathic, potentially disabling disease characterised by continuous mucosal and submucosal inflammation that results in bloody diarrhea and tenesmus and has a deleterious impact on the quality of life.1
First-line therapeutic options for moderate-to-severe UC include mesalamine as well as glucocorticoids followed by thiopurines.2,3 However, due to lack of efficacy and the risk of systemic adverse events especially with glucocorticoids, biological therapies, which are more specific and potent agents, are increasingly forming the backbone of the medical management of moderate-to-severe.4,5 The efficacy of biological therapies and small molecules is usually assessed after the induction period, which can be up to three to four months after treatment initiation.6,7 However, in light of the increasing diversity of mechanisms of action, the speed of onset of efficacy becomes more important in order to choose treatments and matters to clinicians and patients.8 As such, rapid symptomatic relief is associated with an improved quality of life and might also predict long-term outcomes and affect the disease course.9, 10, 11 Further, a recent machine learning model found that the Partial Mayo score reduction from baseline to week 2 was the most important predictor of an overall clinical response to tofacitinib at week 8.12 Accordingly, early clinical response is highlighted as an important short-term treatment goal in UC in the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE)-II recommendations.13 The comparative onset of effect of biological therapies and small molecule agents has not been investigated in any available systematic reviews or meta-analyses.6,7 The aim of the current study was, therefore, to provide evidence via a systematic review, meta-analysis, and network meta-analysis on the rapidity of onset of efficacy within the induction period of all biological therapies and small molecules that are approved for patients with moderate-to-severe UC.
Methods
This systematic review and network meta-analysis was conducted according to the Cochrane recommendations,14 and is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension statement for network meta-analyses.15 The study protocol was defined and registered prior to study initiation at the PROSPERO database (Registration Number CRD42021250236).
Search strategy and selection criteria
Two authors (M.A. and E.K.D.) independently searched MEDLINE (1946–24 August 2022), Embase and Embase Classic (1947–24 August 2022), the Cochrane Central Register of Controlled Trials (CENTRAL), the latter including clinicaltrials.gov as a bibliographical source. Second, the three largest congress databases from European Crohn's and Colitis Organization, Digestive Disease Week, and United European Gastroenterology Week were reviewed from 1 January 2019 to 24 August 2022 to obtain data published in abstract form. Third, bibliographies of all eligible studies and pre-existing systematic reviews were hand-searched. The corresponding or senior authors of studies with insufficient data were contacted to provide additional information before being excluded.
The search algorithm, which is listed in the protocol and was without any language restriction, included the following terms: “ulcerative colitis” OR “inflammatory bowel disease” OR “colitis” (both as medical subject headings and as free text terms). These terms were combined with the operator ‘AND’ to identify studies that included [“biologics” OR “anti-TNF” OR (“infliximab” OR “Remicade” OR “adalimumab” OR “Humira” OR (“golimumab” OR “CNTO-148” OR “certolizumab” OR “Simponi” OR “CDP870)” OR “Cimzia”) OR “anti-integrin” OR (“vedolizumab” OR “MLN-0002” OR “Entyvio”) OR (“ustekinumab” OR “CNTO-1275”) OR “small molecule” OR “JAK inhibitor” OR (“tofacitinib” OR “CP-690550” OR “Xeljanz”) OR (“filgotinib” OR “GLPG0634”) OR (“upadacitinib” OR “ABT-494”) OR “sphingosine-1-phosphate receptor modulator” OR (“ozanimod” OR “RPC1063”)].
Controlled studies and open-label interventional studies examining the efficacy of the aforementioned agents within six weeks after treatment initiation in adult patients with moderate-to-severe UC were eligible for inclusion.
Outcome assessment
The co-primary outcomes were the overall clinical response and clinical remission of biological therapies or small molecules for UC at week 2 after treatment initiation, compared with placebo or each other. These outcomes were stratified according to resolution of Mayo rectal bleeding Subscore, partial Mayo score response, partial Mayo score remission and 2-item patient-reported outcome (PRO-2) remission. Secondary outcomes included clinical response, clinical remission, biochemical response, biochemical remission, and endoscopic remission at weeks 2 and 6.
Data extraction
Data from all eligible studies were extracted independently by at least two investigators (M.A, J.B.S, J.B., and J.G.) onto a Microsoft Excel spreadsheet (XP professional edition; Microsoft Corp, Redmond, WA, USA). The data extraction of the two authors was compared, and disagreements were resolved by consensus. Data were extracted as intention-to-treat analyses assuming all dropouts to be treatment failures (i.e., no response to biological therapy, small molecule, or placebo).
Quality assessment and risk of bias
Version 2 of the Cochrane Risk of Bias tool was used by at least two authors (M.A., J.B.S, and O.H.N) to independently rate the quality of all eligible studies and estimate the risk of bias herein.16 Discrepancies were solved by discussion. Egger's test for funnel plot asymmetry was performed.
Data synthesis and statistical analysis
Data from controlled and uncontrolled studies and studies on moderate-to-severe UC were analysed separately. In addition, different formulations of the same drugs were investigated separately due to potential pharmacokinetic differences that might affect the timing of onset of efficacy. Analyses were conducted as intention-to-treat (ITT) instead of per protocol analyses (PP), as the former approach is more conservative and reduces the risk of type I error. Second, there appears to be a discrepancy between findings in controlled settings (e.g. RCTs) and real-life observations, which might be attributed to differences in baseline predictive factors that are neutralised during randomization processes in the ITT analysis.17 We did not perform PP analyses as sensitivity analyses due to the fact that most trials were superiority trials and not non-inferiority trials. This strategy is in line with previous network meta-analyses within the field.6,7
The network meta-analyses were conducted within a Bayesian framework using Markov chain Monte Carlo methods with the statistical packages ‘BUGSnet’ and ‘GeMTC’ in R (V.4.0.2),18,19 assuming consistency of treatment efficacies across all eligible trials and following the recommendations by National Institute for Health and Care Excellence Decision Support Unit (NICE-DSU).20, 21, 22 Hence, both fixed-effects and random effects network meta-analysis were conducted and these were compared with respect to leverage, which is a measure of complexity and parsimony, the posterior mean of the residual deviance, and the deviance information criterion. Importantly, only RCTs were able to enter the network model as open-label studies lacked a comparator arm. The final model was used to calculate the probability of the ranking of each treatment within a surface under the cumulative ranking curve (SUCRA), which represents the percentage of efficacy achieved by an agent compared with an imaginary agent that is always best. Findings were reported as relative risks (RR) and 95% credible intervals (CrIs), the Bayesian equivalent to 95% confidence intervals (95% CI). Pre-planned subgroup analyses included prior tumor necrosis factor-α (TNF) antagonist exposure and endpoint definitions. Pre-planned network meta-regression comprised timing of follow-up and concomitant immunomodulators or corticosteroids as co-variates.
Second, direct comparison of each agent with its comparator was conducted using inverse-variance weighted fixed-effects and random effects models and was reported as a relative risk using RevMan software version 5.4 (Cochrane Collaboration, Copenhagen, Denmark). Third, meta-analyses on proportions were conducted using the ‘metafor’ R package. Proportion data were transformed using the logit transformation and the DerSimonian and Laird method to estimate Tau.2 Heterogeneity was assessed using Cochrane Q and I2 statistics according to the Cochrane Handbook for Systematic Reviews, where I2>50% indicated substantial heterogeneity.14
The confidence in estimates of outcomes derived from the network meta-analyses were evaluated following the Confidence in Network Meta-Analysis (CINeMA) approach,23 which is broadly based on Grading of Recommendations Assessment, Development, and Evaluation (GRADE).24
Ethical statement
Relevant data were retrieved from public databases, including MEDLINE, Embase and Embase Classic, and the Cochrane Central Register of Controlled Trials (CENTRAL). Ethical approval and informed consent was covered in the original studies and was not applicable for this study.
Role of the funding source
There was no funding source for this study. All authors had full access to all the data in the study. J.B.S. had the final decision to submit for publication.
Results
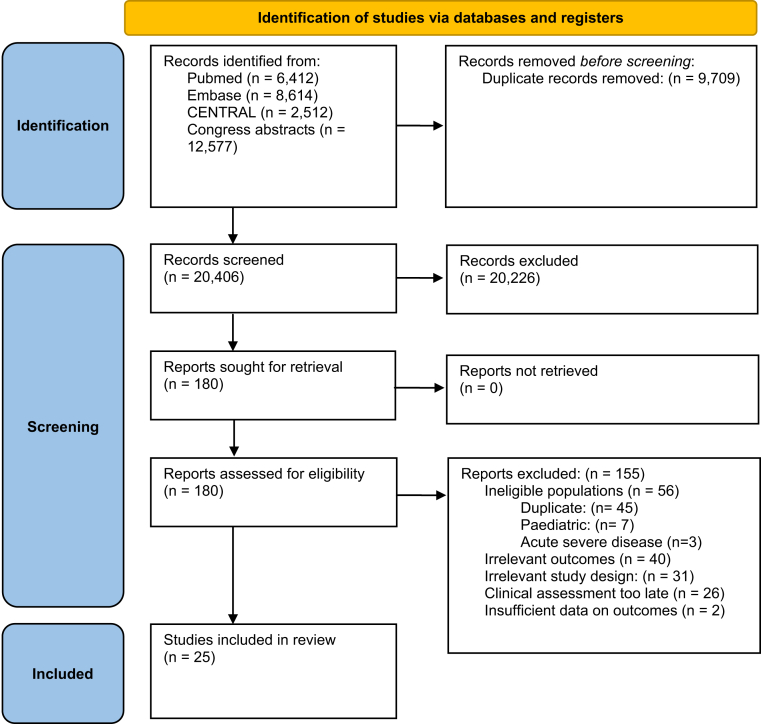
The bibliographical search generated 20,406 citations, of which 180 studies were identified as potentially eligible based on abstract screening and were retrieved for full-text assessment. Of these, 25 studies comprising 11,074 patients fulfilled the eligibility criteria and were included (Fig. 1). The agreement in the assessment of study eligibility was excellent between the reviewers (Kappa statistics = 0.86). There were six studies on vedolizumab (four randomised clinical trials (RCT),25, 26, 27, 28 and two post hoc analyses on the GEMINI trials and Japanese RCT10,29), three studies on infliximab (two RCTs,30,31 and one post hoc analysis of ACT-1 and 2 studies32), five studies on adalimumab (two RCTs,28,33 one post hoc analysis on ULTRA-1 and 2 studies,34 and two open-label studies35,36), five studies on golimumab (three RCTs,37, 38, 39 one post hoc analysis on PURSUIT-SC trial,32 and one open-label study40), three studies on upadacitinib (two RCTs41,42 and one post hoc analysis on the U-ACHIEVE trial43), two studies on tofacitinib (both post hoc studies on the OCTAVE trials11,12), one study on ustekinumab (post hoc study on the UNIFI trial44), one study on filgotinib (post hoc study on the SELECTION RCT45), and one study on ozanimod (post hoc study on the True North RCT46).
Fig. 1.
Flow diagram of assessment of studies identified in the systematic review.
Detailed trial and patient characteristics of all included studies are summarised in Supplementary Table S1. The endpoint definitions used in the studies are listed in Supplementary Table S2, and the risk of bias are reported in Supplementary Table S3, indicating that only a few studies were of high risk of bias. Funnel plots are presented in Supplementary Fig. S2 indicating no funnel plot asymmetry. The fixed-effects models had the best fit for modelling the induction of clinical response and clinical remission as summarised in Supplementary Table S4, and the network map is shown in Supplementary Figs. S1a and S1b. All analyses are therefore presented under the fixed-effects framework. However, random effects models were not different in any of the findings.
Clinical response
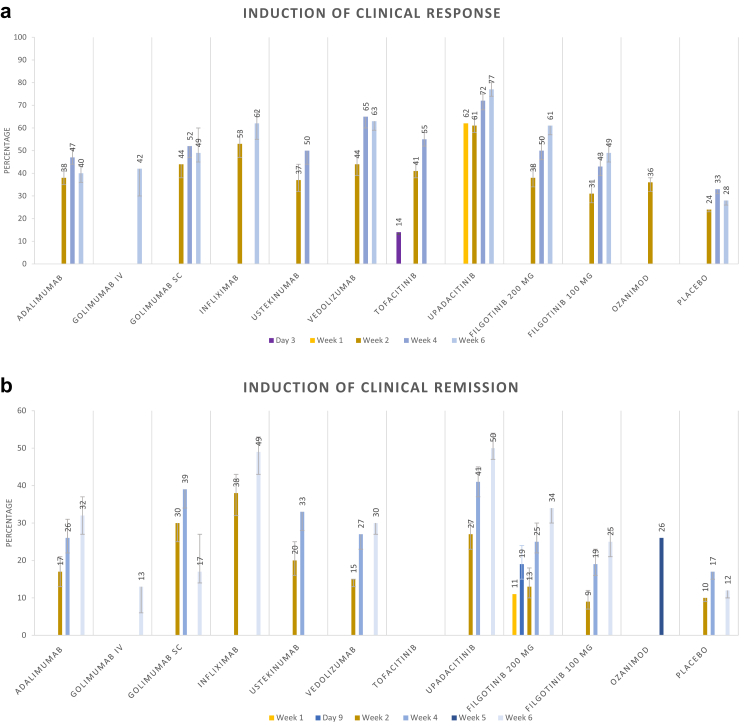
Results from the pooled-proportion meta-analyses of induction of clinical response over time are presented in Fig. 2a.
Fig. 2.
Meta-analysis on proportions of induction of a) clinical response and b) clinical remission.
Clinical response within two weeks of treatment
Clinical response within two weeks of treatment has only been reported in three studies on moderate-to-severe UC, which precluded statistical analysis. The post hoc analysis on the OCTAVE trials demonstrated separation of tofacitinib and placebo within three days in terms of achieving Mayo rectal bleeding Subscore = 0 (130/905 (14.4%) vs. 19/234 (8.2%), p < 0.05) and seven days in terms of achieving Mayo stool score = 0 (83/905 (9.2%) vs. 5/234 (2.3%), p < 0.01).11 A post hoc analysis on the U-ACHIEVE trial demonstrated significantly higher clinical response at day 8 among patients treated with upadacitinib as compared to placebo in terms of Mayo stool score = 0 (21/53 (39.6%) vs. 6/43 (14.0%), p = 0.01) and Mayo rectal bleeding Subscore = 0 (33/53 (62.3%) vs. 9/43 (20.9%), p < 0.01).43 A post hoc analysis on the SELECTION trial demonstrated that filgotinib 200 mg induced significantly more often rectal bleeding subscore of 0 than placebo by day 6 in biologic-naive patients (20.8% vs 12.4%, p = 0.04), and day 5 in biologic-experienced patients (17.2% vs 9.2%, p = 0.02).45
Clinical response at week 2
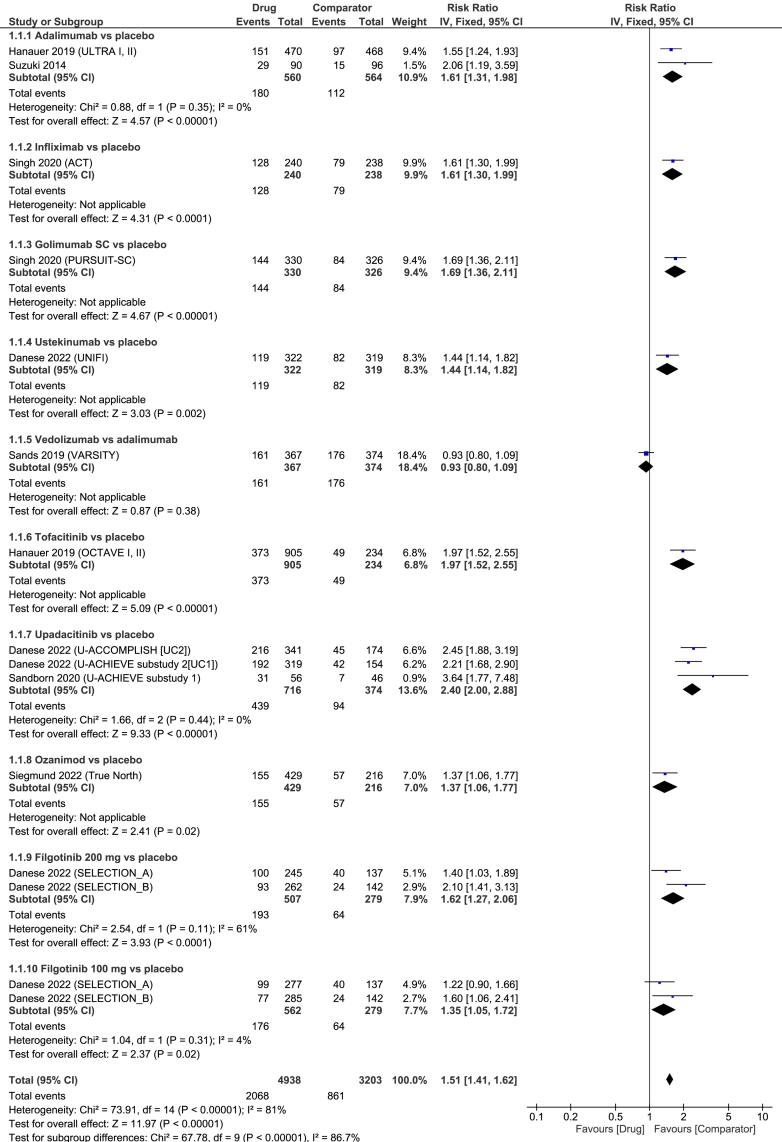
The first systematic assessment of clinical response in patients with moderate-to-severe UC across the majority of biological therapies and small molecules was found to be two weeks after treatment initiation with no sign of funnel asymmetry (Egger's p = 0.37). All agents were significantly superior to placebo for the induction of clinical response at week 2 in the direct, pair-wise meta-analysis (Fig. 3). No comparison between vedolizumab and placebo at week 2 was identified; however, data from the VARSITY trial indicated no difference between vedolizumab and adalimumab (RR = 0.93 (95% CI 0.80–1.09), Fig. 3).28 The overall heterogeneity was high (I2 = 78%).
Fig. 3.
Forest plot for achievement of clinical response at week 2 among patients with moderate to severe ulcerative colitis.
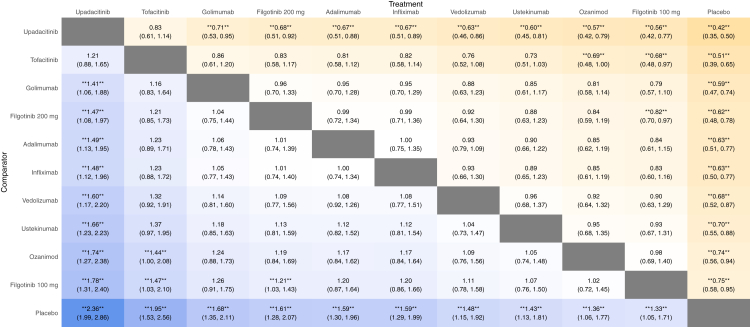
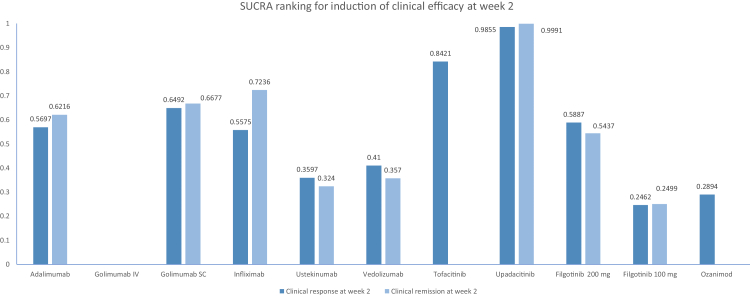
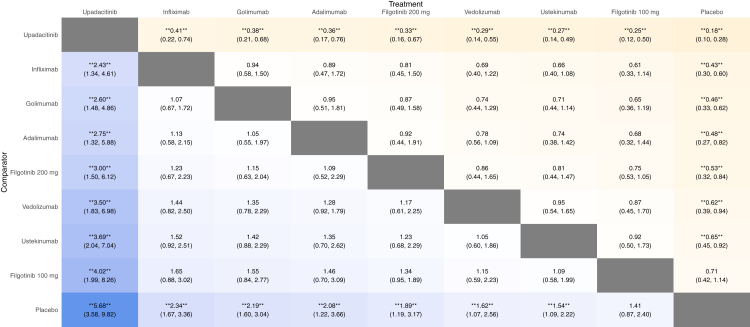
Results from the network meta-analysis are shown in Fig. 4. When comparing the active medications, upadacitinib was significantly superior to all agents (high confidence, Supplementary Table S6) but tofacitinib (moderate confidence) in the overall analysis. Further, tofacitinib was significantly superior to filgotinib 100 mg and ozanimod. Accordingly, upadacitinib and tofacitinib ranked highest for this endpoint, while filgotinib 100 mg, ozanimod, and ustekinumab ranked lowest (Fig. 5, Supplementary Table S5).
Fig. 4.
Indirect comparison of biologics and small molecule drugs for the induction of clinical response at week 2 in patients with moderate-to-severe ulcerative colitis. The values in each cell represent the relative treatment effect (relative risk and 95% credible intervals) of the treatment on the top compared to the treatment on the left. A double asterisk indicates statistical significance.
Fig. 5.
Relative efficacy of different interventions for induction of clinical response and clinical remission at week 2 in patients with moderate-to-severe ulcerative colitis.
When looking specifically on Mayo clinical response at week 2, upadacitinib ranked highest and was significantly superior to filgotinib 200 mg and 100 mg, but not adalimumab, nor vedolizumab (Supplementary Fig. S3). In terms of inducing a Mayo rectal bleeding Subscore of 0, tofacitinib (SUCRA 0.9153) ranked highest (in the absence of upadacitinib) but was not superior to golimumab (SUCRA 0.6745), infliximab (SUCRA 0.5673), adalimumab (0.4911), and ustekinumab (0.3517) (Supplementary Fig. S4). No difference was observed when comparing TNF antagonists to other biologics or infliximab versus other TNF antagonists (data not shown).
Subgroup-analysis demonstrated that upadacitinib and ustekinumab ranked highest for the induction of clinical response at week 2 among bio-naïve patients; however, statical significance was not achieved in any of the comparative analyses. Further, filgotinib 100 mg and ozanimod ranked lowest and were not superior to placebo (Supplementary Fig. S5–S7 and Supplementary Table S10). A post hoc analysis on the OCTAVE trials demonstrated no reduction in the efficacy of tofacitinib when stratified according to previous bio-exposure.11 Further, another post hoc analysis from the UNIFI trial demonstrated that the superiority of ustekinumab over placebo in terms of inducing clinical response at week 2 was more apparent in bio-naïve (41.5% vs. 19.9%) than bio-exposed patients (34.3% vs. 31.1%).44 Lastly, post hoc analyses on upadacitinib demonstrated a high rate of clinical response at week 2 when compared to placebo both in patients without (64.3% vs. 16.7%) and patients with (52.4% vs. 14.7%, p < 0.001) previous bio-exposure.42
Clinical response at week 6
In a direct, pair-wise meta-analysis, all agents apart from intravenous golimumab were significantly superior to placebo for the induction of clinical response at week 6 (Supplementary Fig. S8, I2 = 76%) (Egger's p = 0.84). In network meta-analysis, upadacitinib, which ranked highest, was significantly superior to infliximab, adalimumab, and filgotinib 100 mg but not vedolizumab or filgotinib 200 mg (Supplementary Fig. S9, Supplementary Table S5, high confidence). Vedolizumab was superior to adalimumab (moderate confidence) and TNF antagonists as a whole, but not infliximab. No difference was observed between the TNF antagonists (data not shown). The sensitivity analysis on Mayo clinical response was consistent with the findings for week 2; however, upadacitinib was not significantly superior to filgotinib 200 mg (Supplementary Fig. S10). Further, resolution of rectal bleeding at week 6 was only reported for infliximab and adalimumab, demonstrating similar efficacy (data not shown). Subgroup analysis according to prior bio-exposure is presented in Supplementary Fig. S11. We found the efficacy relative to placebo of all agents, apart from tofacitinib and vedolizumab, to slightly decrease over time (Supplementary Figs. S12 and S13).
Clinical remission
Results from the pooled-proportion meta-analyses regarding induction of clinical remission within the first six weeks of treatment are presented in Fig. 2b.
Clinical remission within two weeks of treatment
The literature search identified only one study reporting the clinical remission within two weeks of treatment. The post hoc analysis from the SELECTION trial found that filgotinib induced significantly higher PRO-2 remission rates compared to placebo nine days after treatment initiation in both bio-naïve (day 9: 46/245 (18.8%) vs. 13/137 (9.5%), p = 0.01) and bio-exposed patients (day 7: 28/262 (10.7%) vs. 6/142 (4.2%), p = 0.02).45
Clinical remission at week 2
A network map of trials assessing clinical remission at week 2 in patients with moderate-to-severe UC is shown in Supplementary Fig. S1b with no evidence of funnel asymmetry (Egger's p = 0.09). In direct, pair-wise meta-analysis, all agents available for analysis apart from filgotinib 100 mg were significantly superior to placebo (I2 = 78%, Supplementary Fig. S14). The ULTRA 1 trial, which was deemed insufficient for inclusion, demonstrated no statistically significant difference between adalimumab and placebo in terms of this endpoint.47 Further, a post hoc analysis of the TRUE NORTH trial demonstrated no difference in clinical remission at week 2 between ozanimod and placebo (difference 1.9% (95% CI -3.4–7.1)).46 In network meta-analysis, upadacitinib ranked highest and was significantly superior to infliximab, adalimumab, golimumab, filgotinib 200 mg, vedolizumab, ustekinumab, and filgotinib 100 mg for the induction of clinical remission at week 2 (Fig. 6, high confidence). No data on tofacitinib were retrieved. TNF antagonists were significantly superior to other biological therapies in the overall analysis of clinical remission and numerically superior in the sensitivity analysis on PRO-2 remission ((RR = 1.43 (95% CI 1.00–2.05), Supplementary Figs. S15 and S16); in contrast, no difference between infliximab and other TNF antagonists was observed (data not shown). Sensitivity analysis on Mayo clinical remission confirmed the difference between upadacitinib, filgotinib 200 mg, and filgotinib 100 mg (Supplementary Fig. S17).
Fig. 6.
Indirect comparison of biologics and small molecule drugs for the induction of clinical remission at week 2 in patients with moderate-to-severe ulcerative colitis. The values in each cell represent the relative treatment effect (relative risk and 95% credible intervals) of the treatment on the top compared to the treatment on the left. A double asterisk indicates statistical significance.
Subgroup analysis showed that infliximab, golimumab, vedolizumab, and ustekinumab were significantly superior to placebo for the induction of PRO-2 remission at week 2 in bio-naïve patients (Supplementary Fig. S18, high confidence). Vedolizumab and ustekinumab achieved the highest and lowest rankings, respectively (Supplementary Table S7). Further, data on ustekinumab indicated that bio-exposed patients had a lower probability of achieving this endpoint than bio-naïve patients (16.3% vs. 25.2%). Similar data were retrieved for vedolizumab (14.7% vs. 22.3%).29 Unfortunately, sensitivity analysis was not possible for Mayo clinical remission.
Clinical remission at week 6
In the direct, pair-wise meta-analysis, Infliximab, SC golimumab, vedolizumab, upadacitinib, filgotinib 200 mg, and filgotinib 100 mg were significantly superior to placebo in terms of inducing clinical remission at week 6 (I2 = 76%, Supplementary Fig. S19), Egger's p = 0.09). The network meta-analysis showed that upadacitinib was significantly superior to all agents apart from SC golimumab (high confidence), while vedolizumab and SC golimumab remained significantly superior to adalimumab (moderate confidence) but not infliximab (moderate confidence, Supplementary Fig. S20, Supplementary Table S5). No difference was observed between TNF antagonists and biologics with other mechanisms of action or within the group of TNF inhibitors (data not shown). In the sensitivity analysis of Mayo clinical remission at week 6, all agents apart from intravenous golimumab were superior to placebo. Further, upadacitinib ranked highest and was significantly superior to filgotinib 200 mg and 100 mg (Supplementary Fig. S21). Unfortunately, sensitivity analysis was not possible for PRO-2 remission. Sensitivity analysis according to prior bio-exposure is presented in Supplementary Figs. S22 and S23, demonstrating no difference between biologics and filgotinib 200 mg, with SC golimumab ranking highest (Supplementary Table S7).
We found the relative efficacy compared to placebo to decrease for all agents apart from vedolizumab and ustekinumab (Supplementary Figs. S24 and S25).
Endoscopic, histological, and biochemical response and remission
The direct, pair-wise meta-analysis on endoscopic remission at week 6 is summarised in Supplementary Fig. S26. The network-meta-analysis did not add further information apart from ranking vedolizumab highest (Supplementary Fig. S27, Supplementary Table S5). No histological data were found. Early biochemical normalization was reported only for ustekinumab and upadacitinib. A higher proportion of patients treated with ustekinumab achieved normalization of C-reactive protein at week 2 (29.1% vs. 19.5%, p = 0.046) compared to placebo. This was consistent with regard to normalization of fecal calprotectin (week 2: 13.5% vs. 8.0%, p = 0.039).44 Finally, a higher proportion of patients achieved normalization of fecal calprotectin with upadacitinib compared to placebo (U-ACHIEVE: 29.4% vs. 4.8%, p < 0.001; U-ACCOMPLISH: 29.8% vs. 5.4%, p < 0.001).41
Discussion
To our knowledge, this is the first systematic review with a network meta-analysis to evaluate the comparative onset of efficacy of marketed biological therapies and small molecules for patients with UC. Several key observations have emerged from this network meta-analysis. First, upadacitinib was superior to all biological therapies and filgotinib 200 mg in terms of inducing a clinical remission at week 2, and upadacitinib and tofacitinib ranked highest for the induction of clinical response and clinical remission across all analyses. However, in sensitivity analysis, upadacitinib and tofacitinib were not superior to biological therapies for the induction of a Mayo clinical response or resolution of rectal bleeding at week 2. Second, all biological therapies induced significantly higher rates of PRO-2 remission than placebo at week 2, with TNF antagonists being superior to vedolizumab and ustekinumab in bio-naïve patients. Third, ozanimod, and ustekinumab ranked lowest in the majority of analyses.
The symptom burden of moderate to severe active UC is associated a high degree of disability and risk of morbidity in terms of hospitalization and colectomy and early symptomatic relief is thus considered an important short-term treatment goal. Further, medicines with rapid response may in addition provide beneficial disease modifying capabilities.48 However, this topic is not addressed in any currently available network meta-analyses.6,7,49,50 Physicians must carefully balance the risks and benefits of continuing or discontinuing any therapy. On the other hand, premature switching of therapies increases the risk of exhausting treatment options sooner. Further, switching back to previous biological therapies may not be possible due to the risk of immunogenicity.51
The finding that upadacitinib and tofacitinib ranked highest for most endpoints is in line with results from network meta-analyses of post-induction and maintenance endpoints of patients with UC.6,7 The current study found upadacitinib to be significantly superior to filgotinib 200 mg in all analyses. An important finding is that upadacitinib and tofacitinib did not achieve statistical superiority in the sensitivity analyses of Mayo clinical response and resolution of rectal bleeding. This needs, however, to be interpreted with caution as week 2 endpoints, which represent the first clinical assessment across the trials, do not truly represent onset of efficacy but rather early efficacy. Accordingly, studies have demonstrated an onset of effectiveness of small molecules within days in UC and refractory rheumatoid arthritis.11,43,45,52 Further, a recently published post hoc analysis of the U-ACHIEVE and U-ACCOMPLISH trials found upadacitinib to induce a significant symptomatic improvement within a day in patients with UC.53 Second, the network model is anchored by the placebo-group, which experienced relatively higher clinical response than clinical remission, and therefore might conceal the clinical benefit of the agents of interest. Consequently, measuring clinical remission increases the threshold of effect and, thereby, reduces the risk of type I errors. This disparity does, however, highlight the increasing diversity of efficacy measures and, consequently, the need for rigorous standardization of measures that should always be reported within trials. Whether TNF inhibitors have an earlier onset of efficacy than other classes of biological therapies has long been postulated.32 We found that TNF antagonists were significantly superior to vedolizumab and ustekinumab for induction of clinical remission at week 2, but not for clinical response. Further, TNF inhibitors were superior to other ustekinumab and vedolizumab in terms of inducing PRO-2 remission at week 2 in bio-naïve patients. Representing an important finding in this study, ustekinumab and ozanimod ranked lowest in most endpoints. However, caution is advised when considering these findings as ustekinumab ranked second highest for the induction of clinical response at week 2 among bio-naïve patients and ranked second highest for induction of clinical remission at later time points in biologically experienced patients.6 In the current study, we found no statistically significant differences between infliximab and other TNF inhibitors. However, adalimumab ranked consistently lowest among the TNF inhibitors, which is in line with a previous network meta-analysis.49 Importantly, a recent post-hoc analysis of ACT-1, ACT-2, and PURSUIT-SC trials adjusting for patient-level differences found infliximab to resolve symptoms more rapidly, and to have greater efficacy for inducing remission, than golimumab in patients with moderate-to-severe UC.32 Further, previous real-world studies and network meta-analysis have given preference to infliximab over other TNF inhibitors for induction of clinical response in UC.50,54,55
With increasing emphasis on the rapidity of onset of efficacy, it is of interest to elucidate whether an early response to biologics and small molecule agents predicts long-term efficacy or results in any long-term benefit in moderate-to-severe UC. While emerging studies are pointing towards this notion for small molecules,10,11 our findings highlight a clinical rationale for continuing treatment throughout the induction period to reap the full benefits of agents, such as ustekinumab and ozanimod.44 A post hoc analysis of the VARSITY trial demonstrated that the proportion of delayed remitters to adalimumab and vedolizumab at week 14 were 11% and 13%, respectively, and a strategy of waiting for delayed remission only benefitted 6–7% at week 52.56 Nonetheless, to our knowledge, the current literature has not addressed this topic, which restricts the interpretations of the current study.
The current literature does not allow an analysis based on the timing of occurrence of adverse events; however, recent post-induction assessments have found upadacitinib to be associated with the highest risk of adverse events.6,7 This highlights that the time to onset of efficacy is not the only factor to consider when choosing the right therapy.
Our study had several strengths. First, all analyses were conducted at standardised time points based on a large number of patients and trials. Currently, available network meta-analyses combine data obtained 6–14 weeks after treatment initiation, which precludes interpretations regarding the speed of onset of effect. Second, we followed rigid recommendations regarding the methodology of the systematic review, network meta-analysis, and interpretations hereof.
However, we acknowledge several limitations in addition to those inherent in the trials. Most important is the inability of network-meta-analyses to adjust analyses for patient-level variables such as concomitant medications (e.g., corticosteroids) that may induce a rapid response which is indistinguishable from that of the agents of interest. Second, the network meta-analysis model does not consider variations in the design of RCTs. Therefore, the findings should be interpreted as hypothesis-generating in the absence of head-to-head trials.57 However, network models with indirect comparisons remain helpful,57 and the systematic review methodology comprises the highest level of evidence. Second, a high between-study heterogeneity was observed, which might be attributed to differing endpoint definitions. These were explored thoroughly in sensitivity analyses. Finally, bio-exposure was considered and explored in any possible sensitivity analysis; however, data on the bio-exposed subpopulation remained scarce and will require more attention in future studies.
In summary, we found that all available active agents induced significantly higher clinical response than placebo at week 2, with upadacitinib and tofacitinib ranking highest, while ustekinumab and ozanimod ranking lowest across all endpoints. This might have implications in informed decision-making when considering the choice of biological or small molecule agents. The decision should, however, also include an individualised risk-benefit assessment, including risks of pulmonary emboli, venous thromboembolism, or even death with use of JAK inhibitors.58 Head-to-head trials adding the time to onset of efficacy as an endpoint are warranted to differentiate further the currently available agents and their position in the clinical setting.
Contributors
Guarantor: JBS is guarantor. J.B.S. conceived the study idea. M.A., J.B.S., and O.H.N. planned and designed the study. M.A. drafted the study protocol. M.A. and E.K.D. conducted a systematic review of the literature. M.A., J.B.S, J.B., and J.G. extracted data from all eligible studies. M.A., J.B.S, and O.H.N performed an analysis of the risk of bias in all eligible studies. M.A. performed the statistical analyses. JG accessed and verified the data and validated the statistical analyses. M.A. drafted the manuscript. All authors interpreted the results and contributed with a critical revision of the manuscript for important intellectual content. All authors had full access to all the data in the study. J.B.S. had the final decision to submit for publication. All authors have approved the final draft of the manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Data sharing statement
Data are presented in the current manuscript, its supplementary materials, or within the manuscripts or appendices of the included studies.
Declaration of interests
M.A.: None. E.K.D.: reports research grants from Takeda, paid speaker by MSD, Tillotts. J.B.: reports grants and personal fees from AbbVie, grants and personal fees from Janssen-Cilag, personal fees from Celgene, grants and personal fees from MSD, personal fees from Pfizer, grants and personal fees from Takeda, grants and personal fees from Tillots Pharma, personal fees from Samsung Bioepis, grants and personal fees from Bristol Myers Squibb, grants from Novo Nordisk, personal fees from Pharmacosmos, personal fees from Ferring, personal fees from Galapagos, outside the submitted work. J.G.: None. O.H.N.: None. J.B.S.: has received research grants from Takeda, Janssen, the Danish Research Council, and the Capital Region Denmark, and is national coordinator of studies from AbbVie, Arena Pharmaceuticals, Ely Lilly, and Boehringer Ingelheim. None of these pertain to the research submitted here.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.101866.
Appendix A. Supplementary data
References
- 1.Kobayashi T., Siegmund B., Le Berre C., et al. Ulcerative colitis. Nat Rev Dis Prim. 2020;6(1):74. doi: 10.1038/s41572-020-0205-x. [DOI] [PubMed] [Google Scholar]
- 2.Ford A.C., Bernstein C.N., Khan K.J., et al. Glucocorticosteroid therapy in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 2011;106(4):590–599. doi: 10.1038/ajg.2011.70. quiz 600. [DOI] [PubMed] [Google Scholar]
- 3.Lamb C.A., Kennedy N.A., Raine T., et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68(Suppl 3):s1–s106. doi: 10.1136/gutjnl-2019-318484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nielsen O.H., Ainsworth M.A. Tumor necrosis factor inhibitors for inflammatory bowel disease. N Engl J Med. 2013;369(8):754–762. doi: 10.1056/NEJMct1209614. [DOI] [PubMed] [Google Scholar]
- 5.Zhao M., Sall Jensen M., Knudsen T., et al. Trends in the use of biologicals and their treatment outcomes among patients with inflammatory bowel diseases - a Danish nationwide cohort study. Aliment Pharmacol Ther. 2022;55(5):541–557. doi: 10.1111/apt.16723. [DOI] [PubMed] [Google Scholar]
- 6.Lasa J.S., Olivera P.A., Danese S., Peyrin-Biroulet L. Efficacy and safety of biologics and small molecule drugs for patients with moderate-to-severe ulcerative colitis: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol. 2022;7(2):161–170. doi: 10.1016/S2468-1253(21)00377-0. [DOI] [PubMed] [Google Scholar]
- 7.Burr N.E., Gracie D.J., Black C.J., Ford A.C. Efficacy of biological therapies and small molecules in moderate to severe ulcerative colitis: systematic review and network meta-analysis. Gut. 2021 doi: 10.1136/gutjnl-2021-326390. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 8.Gray J.R., Leung E., Scales J. Treatment of ulcerative colitis from the patient's perspective: a survey of preferences and satisfaction with therapy. Aliment Pharmacol Ther. 2009;29(10):1114–1120. doi: 10.1111/j.1365-2036.2009.03972.x. [DOI] [PubMed] [Google Scholar]
- 9.Feagan B.G., Sandborn W.J., Lazar A., et al. Adalimumab therapy is associated with reduced risk of hospitalization in patients with ulcerative colitis. Gastroenterology. 2014;146(1):110–118.e3. doi: 10.1053/j.gastro.2013.09.032. [DOI] [PubMed] [Google Scholar]
- 10.Nagahori M., Watanabe K., Motoya S., et al. Week 2 symptomatic response with vedolizumab as a predictive factor in Japanese anti-TNFα-naive patients with ulcerative colitis: a post hoc analysis of a randomized, placebo-controlled phase 3 trial. Digestion. 2021;102(5):742–752. doi: 10.1159/000512235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanauer S., Panaccione R., Danese S., et al. Tofacitinib induction therapy reduces symptoms within 3 Days for patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2019;17(1):139–147. doi: 10.1016/j.cgh.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Lees C.W., Deuring J.J., Chiorean M., et al. Prediction of early clinical response in patients receiving tofacitinib in the OCTAVE Induction 1 and 2 studies. Therap Adv Gastroenterol. 2021;14 doi: 10.1177/17562848211054710. 17562848211054710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turner D., Ricciuto A., Lewis A., et al. STRIDE-II: an update on the selecting therapeutic Targets in inflammatory bowel disease (STRIDE) initiative of the International Organization for the study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021;160(5):1570–1583. doi: 10.1053/j.gastro.2020.12.031. [DOI] [PubMed] [Google Scholar]
- 14.Higgins J., Thomas J., Chandler J., et al. Cochrane; 2022. Cochrane Handbook for Systematic Reviews of Interventions version 6.3.www.training.cochrane.org/handbook [Google Scholar]
- 15.Hutton B., Salanti G., Caldwell D.M., et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 16.Sterne J.A.C., Savović J., Page M.J., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 17.Ha C., Ullman T.A., Siegel C.A., Kornbluth A. Patients enrolled in randomized controlled trials do not represent the inflammatory bowel disease patient population. Clin Gastroenterol Hepatol. 2012;10(9):1002–1007. doi: 10.1016/j.cgh.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 18.van Valkenhoef G., Lu G., de Brock B., Hillege H., Ades A.E., Welton N.J. Automating network meta-analysis. Res Synth Methods. 2012;3(4):285–299. doi: 10.1002/jrsm.1054. [DOI] [PubMed] [Google Scholar]
- 19.Béliveau A., Boyne D.J., Slater J., Brenner D., Arora P. BUGSnet: an R package to facilitate the conduct and reporting of Bayesian network Meta-analyses. BMC Med Res Methodol. 2019;19(1):196. doi: 10.1186/s12874-019-0829-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ades A.E., Caldwell D.M., Reken S., Welton N.J., Sutton A.J., Dias S. London: National Institute for Health and Care Excellence (NICE); London:: 2012. NICE DSU Technical Support Document 7: Evidence Synthesis of Treatment Efficacy in Decision Making: A Reviewer's Checklist.https://pubmed.ncbi.nlm.nih.gov/27905719/ [PubMed] [Google Scholar]
- 21.Dias S., Welton N.J., Sutton A.J., Caldwell D.M., Lu G., Ades A.E. 2014. NICE DSU technical Support document 4: inconsistency in networks of evidence based on randomised controlled trials.https://pubmed.ncbi.nlm.nih.gov/27466656/ [internet]. London. [PubMed] [Google Scholar]
- 22.Dias S., Welton N.J., Sutton A.J., Ades A.E. 2014. NICE DSU Technical Support Document 2: A Generalised Linear Modelling Framework for Pairwise and Network Meta-Analysis of Randomised Controlled Trials [Internet]https://www.ncbi.nlm.nih.gov/books/NBK310366/ [PubMed] [Google Scholar]
- 23.Nikolakopoulou A., Higgins J.P.T., Papakonstantinou T., et al. CINeMA: an approach for assessing confidence in the results of a network meta-analysis. PLoS Med. 2020;17(4) doi: 10.1371/journal.pmed.1003082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guyatt G.H., Oxman A.D., Vist G.E., et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feagan B.G., Rutgeerts P., Bruce S.E., et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369(8):699–710. doi: 10.1056/NEJMoa1215734. [DOI] [PubMed] [Google Scholar]
- 26.Sandborn W.J., Baert F., Danese S., et al. Efficacy and safety of vedolizumab subcutaneous formulation in a randomized trial of patients with ulcerative colitis. Gastroenterology. 2020;158(3):562–572.e12. doi: 10.1053/j.gastro.2019.08.027. [DOI] [PubMed] [Google Scholar]
- 27.Feagan B.G., Greenberg G.R., Wild G., et al. Treatment of ulcerative colitis with a humanized antibody to the alpha4beta7 integrin. N Engl J Med. 2005;352(24):2499–2507. doi: 10.1056/nejmoa042982. [DOI] [PubMed] [Google Scholar]
- 28.Sands B.E., Peyrin-Biroulet L., Loftus E.V., et al. Vedolizumab versus Adalimumab for moderate-to-severe ulcerative colitis. N Engl J Med. 2019;381(13):1215–1226. doi: 10.1056/NEJMoa1905725. [DOI] [PubMed] [Google Scholar]
- 29.Feagan B.G., Lasch K., Lissoos T., et al. Rapid response to vedolizumab therapy in biologic-naive patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2019;17(1):130–138.e7. doi: 10.1016/j.cgh.2018.05.026. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi T., Suzuki Y., Motoya S., et al. First trough level of infliximab at week 2 predicts future outcomes of induction therapy in ulcerative colitis-results from a multicenter prospective randomized controlled trial and its post hoc analysis. J Gastroenterol. 2016;51(3):241–251. doi: 10.1007/s00535-015-1102-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Probert C.S.J., Hearing S.D., Schreiber S., et al. Infliximab in moderately severe glucocorticoid resistant ulcerative colitis: a randomised controlled trial. Gut. 2003;52(7):998–1002. doi: 10.1136/gut.52.7.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh S., Proudfoot J.A., Dulai P.S., et al. Comparative efficacy and speed of onset of action of infliximab vs golimumab in ulcerative colitis. Clin Gastroenterol Hepatol. 2020;18(2):424–431.e7. doi: 10.1016/j.cgh.2019.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suzuki Y., Motoya S., Hanai H., et al. Efficacy and safety of adalimumab in Japanese patients with moderately to severely active ulcerative colitis. J Gastroenterol. 2014;49(2):283–294. doi: 10.1007/s00535-013-0922-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanauer S., Sandborn W.J., Colombel J.-F., et al. Rapid changes in laboratory parameters and early response to adalimumab: a pooled analysis from patients with ulcerative colitis in two clinical trials. J Crohns Colitis. 2019;13(9):1227–1233. doi: 10.1093/ecco-jcc/jjz031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Afif W., Leighton J.A., Hanauer S.B., et al. Open-label study of adalimumab in patients with ulcerative colitis including those with prior loss of response or intolerance to infliximab. Inflamm Bowel Dis. 2009;15(9):1302–1307. doi: 10.1002/ibd.20924. [DOI] [PubMed] [Google Scholar]
- 36.Peyrin-Biroulet L., Laclotte C., Roblin X., Bigard M.-A. Adalimumab induction therapy for ulcerative colitis with intolerance or lost response to infliximab: an open-label study. World J Gastroenterol. 2007;13(16):2328–2332. doi: 10.3748/wjg.v13.i16.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sandborn W.J., Feagan B.G., Marano C., et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014;146(1):85. doi: 10.1053/j.gastro.2013.05.048. [DOI] [PubMed] [Google Scholar]
- 38.Rutgeerts P., Feagan B.G., Marano C.W., et al. Randomised clinical trial: a placebo-controlled study of intravenous golimumab induction therapy for ulcerative colitis. Aliment Pharmacol Ther. 2015;42(5):504–514. doi: 10.1111/apt.13291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hibi T., Imai Y., Senoo A., Ohta K., Ukyo Y. Efficacy and safety of golimumab 52-week maintenance therapy in Japanese patients with moderate to severely active ulcerative colitis: a phase 3, double-blind, randomized, placebo-controlled study-(PURSUIT-J study) J Gastroenterol. 2017;52(10):1101–1111. doi: 10.1007/s00535-017-1326-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Telesco S.E., Brodmerkel C., Zhang H., et al. Gene expression signature for prediction of golimumab response in a phase 2a open-label trial of patients with ulcerative colitis. Gastroenterology. 2018;155(4):1008–1011.e8. doi: 10.1053/j.gastro.2018.06.077. [DOI] [PubMed] [Google Scholar]
- 41.Danese S., Vermeire S., Zhou W., et al. Upadacitinib as induction and maintenance therapy for moderately to severely active ulcerative colitis: results from three phase 3, multicentre, double-blind, randomised trials. Lancet (London, England) 2022;399(10341):2113–2128. doi: 10.1016/S0140-6736(22)00581-5. [DOI] [PubMed] [Google Scholar]
- 42.Sandborn W.J., Ghosh S., Panes J., et al. Efficacy of upadacitinib in a randomized trial of patients with active ulcerative colitis. Gastroenterology. 2020;158(8):2139–2149.e14. doi: 10.1053/j.gastro.2020.02.030. [DOI] [PubMed] [Google Scholar]
- 43.D'Haens G., Loftus E.V., Jr., Higgins P.D.R., et al. P435 Rapidity of symptomatic and inflammatory biomarker improvements following upadacitinib induction treatment: data from the U-ACHIEVE study. J Crohn’s Colitis. 2019;13(Supplement_1):S326–S327. doi: 10.1093/ecco-jcc/jjy222.559. [DOI] [Google Scholar]
- 44.Danese S., Sands B.E., Abreu M.T., et al. Early symptomatic improvement after ustekinumab therapy in patients with ulcerative colitis: 16-week data from the UNIFI trial. Clin Gastroenterol Hepatol. 2022;1542-3565(22) doi: 10.1016/j.cgh.2022.02.050. 00207-5. [DOI] [PubMed] [Google Scholar]
- 45.Danese S., Ferrante M., Feagan B.G., et al. Rapid and sustained symptom relief in patients with ulcerative colitis treated with filgotinib: data from the phase 2b/3 SELECTION trial. Am J Gastroenterol. 2022 doi: 10.14309/ajg.0000000000001979. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siegmund B., Axelrad J., Pondel M., et al. DOP43 Rapidity of ozanimod-induced symptomatic response and remission in patients with moderately to severely active Ulcerative Colitis: results from the induction period of True North. J Crohn’s Colitis. 2022;16(Supplement_1):i092–i093. doi: 10.1093/ecco-jcc/jjab232.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reinisch W., Sandborn W.J., Hommes D.W., et al. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomised controlled trial. Gut. 2011;60(6):780–787. doi: 10.1136/gut.2010.221127. [DOI] [PubMed] [Google Scholar]
- 48.Haga K., Shibuya T., Osada T., et al. Early clinical remission is a predictor of long-term remission with the use of vedolizumab for ulcerative colitis. Biomedicines. 2022;10(10):2526. doi: 10.3390/biomedicines10102526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singh S., Murad M.H., Fumery M., Dulai P.S., Sandborn W.J. First- and second-line pharmacotherapies for patients with moderate to severely active ulcerative colitis: an updated network meta-analysis. Clin Gastroenterol Hepatol. 2020;18(10):2179–2191.e6. doi: 10.1016/j.cgh.2020.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Danese S., Fiorino G., Peyrin-Biroulet L., et al. Biological agents for moderately to severely active ulcerative colitis: a systematic review and network meta-analysis. Ann Intern Med. 2014;160(10):704–711. doi: 10.7326/M13-2403. [DOI] [PubMed] [Google Scholar]
- 51.Boschetti G., Nachury M., Laharie D., et al. Efficacy and safety of infliximab retreatment in Crohn's disease: a multicentre, prospective, observational cohort (REGAIN) study from the GETAID. Am J Gastroenterol. 2022;117(9):1482–1490. doi: 10.14309/ajg.0000000000001842. [DOI] [PubMed] [Google Scholar]
- 52.Strand V., Kremer J., Wallenstein G., et al. Effects of tofacitinib monotherapy on patient-reported outcomes in a randomized phase 3 study of patients with active rheumatoid arthritis and inadequate responses to DMARDs. Arthritis Res Ther. 2015;17:307. doi: 10.1186/s13075-015-0825-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Loftus E.V.J., Colombel J.-F., Takeuchi K., et al. Upadacitinib therapy reduces ulcerative colitis symptoms as early as day 1 of induction treatment. Clin Gastroenterol Hepatol. 2022 doi: 10.1016/j.cgh.2022.11.029. S1542-3565(22):1109-0. [DOI] [PubMed] [Google Scholar]
- 54.Thorlund K., Druyts E., Mills E.J., Fedorak R.N., Marshall J.K. Adalimumab versus infliximab for the treatment of moderate to severe ulcerative colitis in adult patients naïve to anti-TNF therapy: an indirect treatment comparison meta-analysis. J Crohns Colitis. 2014;8(7):571–581. doi: 10.1016/j.crohns.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 55.Trigo-Vicente C., Gimeno-Ballester V., García-López S., López-Del Val A. Systematic review and network meta-analysis of treatment for moderate-to-severe ulcerative colitis. Int J Clin Pharm. 2018;40(6):1411–1419. doi: 10.1007/s11096-018-0743-4. [DOI] [PubMed] [Google Scholar]
- 56.Narula N., Wong E.C.L., Marshall J.K., Jairath V., Dulai P.S., Reinisch W. Long-term outcomes of early versus delayed responders to vedolizumab and adalimumab: a post-hoc analysis of VARSITY. Am J Gastroenterol. 2022 doi: 10.14309/ajg.0000000000001987. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 57.Salanti G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Res Synth Methods. 2012;3(2):80–97. doi: 10.1002/jrsm.1037. [DOI] [PubMed] [Google Scholar]
- 58.Ytterberg S.R., Bhatt D.L., Mikuls T.R., et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386(4):316–326. doi: 10.1056/NEJMoa2109927. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.