Abstract
Background & Aims
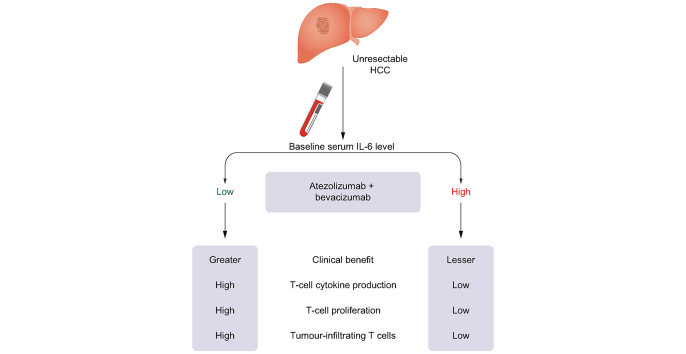
We elucidated the clinical and immunologic implications of serum IL-6 levels in patients with unresectable hepatocellular carcinoma (HCC) treated with atezolizumab and bevacizumab (Ate/Bev).
Methods
We prospectively enrolled 165 patients with unresectable HCC (discovery cohort: 84 patients from three centres; validation cohort: 81 patients from one centre). Baseline blood samples were analysed using a flow cytometric bead array. The tumour immune microenvironment was analysed using RNA sequencing.
Results
In the discovery cohort, clinical benefit 6 months (CB6m) was defined as complete or partial response, or stable disease for ≥6 months. Among various blood-based biomarkers, serum IL-6 levels were significantly higher in participants without CB6m than in those with CB6m (mean 11.56 vs. 5.05 pg/ml, p = 0.02). Using maximally selected rank statistics, the optimal cut-off value for high IL-6 was determined as 18.49 pg/ml, and 15.2% of participants were found to have high IL-6 levels at baseline. In both the discovery and validation cohorts, participants with high baseline IL-6 levels had a reduced response rate and worse progression-free and overall survival after Ate/Bev treatment compared with those with low baseline IL-6 levels. In multivariable Cox regression analysis, the clinical implications of high IL-6 levels persisted, even after adjusting for various confounding factors. Participants with high IL-6 levels showed reduced interferon-γ and tumour necrosis factor-α secretion from CD8+ T cells. Moreover, excess IL-6 suppressed cytokine production and proliferation of CD8+ T cells. Finally, participants with high IL-6 levels exhibited a non-T-cell-inflamed immunosuppressive tumour microenvironment.
Conclusions
High baseline IL-6 levels can be associated with poor clinical outcomes and impaired T-cell function in patients with unresectable HCC after Ate/Bev treatment.
Impact and implications
Although patients with hepatocellular carcinoma who respond to treatment with atezolizumab and bevacizumab exhibit favourable clinical outcomes, a fraction of these still experience primary resistance. We found that high baseline serum levels of IL-6 correlate with poor clinical outcomes and impaired T-cell response in patients with hepatocellular carcinoma treated with atezolizumab and bevacizumab.
Keywords: Hepatocellular carcinoma, IL-6, Atezolizumab, Bevacizumab, Immunotherapy
Abbreviations: AFP, alpha-foetoprotein; Ate/Bev, atezolizumab and bevacizumab; BCLC, Barcelona Clinic Liver Cancer; CB6m, clinical benefit 6 months; CONSORT, Consolidated Standards of Reporting Trials; CR, complete response; CRAFITY, C-reactive protein and AFP in immunotherapy; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; DC, dendritic cell; ECOG, Eastern Cooperative Oncology Group; FFPE, formalin-fixed paraffin-embedded; HCC, hepatocellular carcinoma; HR, hazard ratio; IFN-γ, interferon-γ; MDSC, myeloid-derived suppressor cell; MSI, microsatellite instability; MVI, macrovascular invasion; ORR, objective response rate; OS, overall survival; PBMC, peripheral blood mononuclear cell; PD, progressive disease; PD-1, programmed-death-1; PD-L1, programmed-death ligand-1; PFS, progression-free survival; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumours; SD, stable disease; TME, tumour microenvironment; TNF-α, tumour necrosis factor-α; VEGF, vascular endothelial growth factor
Graphical abstract
Highlights
-
•
Patients with high baseline IL-6 had worse survival outcomes following Ate/Bev.
-
•
Excess IL-6 impaired cytokine production and proliferation of CD8+ T cells.
-
•
High serum IL-6 levels were associated with non-T-cell-inflamed tumours.
Introduction
Recently, combined treatment with atezolizumab, an anti-programmed-death ligand-1 (PD-L1) antibody, and bevacizumab, an anti-vascular endothelial growth factor (VEGF) neutralising antibody, has demonstrated superior survival outcomes compared with those associated with sorafenib in the IMbrave150 trial, and became the standard of care in the new first-line systemic therapy for unresectable hepatocellular carcinoma (HCC).[1], [2], [3] Although people who respond to this combination therapy have favourable clinical outcomes, a fraction of these still exhibit intrinsic resistance.[4], [5], [6], [7] This limitation could be overcome by optimising atezolizumab and bevacizumab (Ate/Bev) therapy using an optimal biomarker that can precisely predict clinical responses or intrinsic resistance before treatment initiation. However, the development of predictive biomarkers through tumour tissue analysis is limited in HCC relative to other solid cancers, because tissue biopsy is not mandatory for diagnosing HCC and can be limited by tumour inaccessibility and the person’s medical condition.[8], [9], [10] Considering these hurdles, circulating biomarkers can be clinically more feasible alternatives to tissue-based biomarkers for predicting the efficacy of systemic therapy in unresectable HCC.
Cytokines are immunological messengers that act not only locally in an autocrine and paracrine manner but also systemically on distant target organs.11 IL-6 is a multifunctional cytokine produced by a variety of cell types, including immune cells, fibroblasts, endothelial cells, and tumour cells.[12], [13], [14] IL-6 is upregulated in various malignancies and plays a key role in tumorigenesis by affecting the survival, proliferation, angiogenesis, invasiveness, and metastasis of tumour cells.13,[15], [16], [17] In the liver, IL-6 levels increase in a stepwise manner from a healthy status to hepatitis, cirrhosis, and HCC.18 Furthermore, several recent studies have reported mechanisms of resistance to immunotherapy through IL-6 signalling.[19], [20], [21], [22] Excessive activation of IL-6 signalling may attenuate Th1 responses and impair T-cell recruitment in lymph nodes and the tumour microenvironment (TME), thus dampening antitumour T-cell immunity.[23], [24], [25]
Although the Ate/Bev combination therapy has emerged as a new standard of care for HCC, the role of serum cytokines in people treated with Ate/Bev has not been comprehensively addressed. Here, we elucidated the clinical and immunological implications of serum IL-6 levels in patients with unresectable HCC treated with Ate/Bev.
Patients and methods
Study design and patients
This study prospectively enrolled patients with unresectable or metastatic HCC treated with Ate/Bev in multiple tertiary cancer centres in Korea. The study was conducted in two stages. In the first stage, we aimed to identify clinically relevant biomarkers, including various cytokines, in patients with HCC treated with Ate/Bev at three independent cancer centres in Korea, namely CHA Bundang Medical Center, Ulsan University Hospital, and Haeundae Paik Hospital (discovery cohort). In the second stage, we aimed to validate the clinical impact of biomarkers in an independent cohort of patients with HCC treated with Ate/Bev at the CHA Bundang Medical Center (validation cohort). The eligibility criteria were age ≥20 years, unresectable or metastatic HCC confirmed by histologic or radiologic diagnosis according to the American Association for the Study of Liver Diseases criteria,26 no prior systemic anticancer therapy, Child–Pugh class A or B7, and an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 1. The study adhered to the ethical guidelines of the Declaration of Helsinki and was approved by the participating hospitals’ institutional review boards (CHA Bundang Medical Center, CHA-2017-11-052 and CHA-2017-11-054; Ulsan University Hospital, 2020-12-006; and Haeundae Paik Hospital, 2020-12-019-001). Written informed consent was obtained from all participants.
Treatments and outcome evaluation
Participants were treated with atezolizumab (1,200 mg fixed dose) and bevacizumab (15 mg/kg) every 3 weeks as first-line systemic therapy. Dose interruptions or reductions were determined according to the IMbrave150 protocol.1 Treatment was continued until intolerable toxicity, progressive disease (PD), or withdrawal of consent was observed. Response evaluation was performed every 6 or 9 weeks using computed tomography or magnetic resonance imaging, according to RECIST (Response Evaluation Criteria in Solid Tumours) version 1.1. Overall survival (OS) was defined as the time from treatment initiation to the date of death. Progression-free survival (PFS) was defined as the time between treatment initiation and the date of PD or death. The objective response rate (ORR) was defined as the sum of complete response (CR) and partial response (PR).
Sample collection and measurement of serum cytokines
Intravenous blood samples were obtained immediately before the first administration of Ate/Bev. To obtain serum, blood was centrifuged at 1,000×g for 5 min; serum samples were subsequently stored at -80 °C. Serum cytokine concentrations were quantified using a cytometric bead array (560484, BD Biosciences) according to the manufacturer’s instructions. In brief, the capture beads were incubated with serum samples and the detection reagent for 3 h at room temperature. After several washes, cytokine-bead complexes were measured using a flow cytometer (Beckman Coulter), and the data were analysed using the FlowJo software (Tree Star Inc.).
Flow cytometry
Before antibody staining, cells were stained with Fixable Viability Dye eFluor™ 780 (eBioscience) on ice for 30 min to exclude dead cells, followed by treatment with a human Fc receptor-binding inhibitor (eBioscience) for 15 min at room temperature. Surface proteins were stained on ice for 30 min with the following fluorochrome-conjugated antibodies: antihuman CD3 (clone SK7, eBioscience), anti-human CD4 (clone RPA-T4, BioLegend), and antihuman CD8 (clone RPA-T8, eBioscience). For intracellular staining, cells were fixed and permeabilised with a FoxP3 staining buffer kit (Thermo Fisher Scientific) and stained with the following fluorochrome-conjugated antibodies: antihuman interferon-γ (IFN-γ) (clone B27, BD Biosciences), and antihuman tumour necrosis factor-α (TNF-α) (clone Mab11, BD Biosciences). Flow cytometry was performed using a CytoFLEX flow cytometer (Beckman Coulter), and the results were analysed using the FlowJo software (Tree Star Inc.).
Cytokine and proliferation assays
Peripheral blood mononuclear cells (PBMCs) were obtained by density gradient centrifugation with Ficoll-Paque PLUS (GE Healthcare). To assess cytokine secretion, PBMCs were activated with a plate-bound anti-CD3 antibody (1 μg/ml), with or without recombinant IL-6 (100 pg/ml). After 4 h, cells were treated with brefeldin A (3 μg/ml, eBioscience) and monensin (2 μM, eBioscience). After 20 h, the stimulated cells were harvested and cytokine production was evaluated by flow cytometry. To assess cellular proliferation, we stained the cells using a Cell Trace Violet cell proliferation kit (Thermo Fisher Scientific) according to the manufacturer’s instructions. Briefly, cells were incubated with Cell Trace Violet for 20 min at room temperature and then washed. T cells were stimulated using anti-CD3 antibody-coated plates. After 72 h, the cells were harvested and the dilution of Cell Trace Violet was evaluated by flow cytometry.
RNA isolation and gene expression analysis
A total of 20 tumour samples with formalin-fixed paraffin-embedded (FFPE) slides were retrieved. RNA was extracted using Trizol (Thermo Fisher Scientific) and isolated using the AllPrep FFPE tissue kit (Qiagen, Germany), following the manufacturer’s instructions. The quality of FFPE-derived RNA was measured by the proportion of fragments >200 b (DV200). FFPE-derived RNA from all 20 samples was processed on FFPE-compatible RNAseq library preparation using QuantSeq 3′ mRNA-Seq Library Prep Kit FWD (Lexogen, USA). RNA sequencing was performed using the QuantSeq 3′ mRNA-sequencing kit (Lexogen, Vienna, Austria) to obtain the gene expression profiles of samples from the IL-6-high and IL-6-low groups.
Estimation of immune cell infiltration into the TME
The absolute score and relative fraction of the infiltrated immune subsets in the samples were estimated using the CIBERSORT algorithm. The leucocyte signature matrix LM22 was used as a signature gene set to infer the abundance of immune subsets in the gene expression data. The permutations were performed 100 times for statistical analysis. Student’s t test was used to compare the IL-6-high and IL-6-low groups, and to assess the statistical significance of IL-6-related enrichment of the immune subsets. Signatures of T-cell dysfunction and exclusion were calculated based on the algorithm of the previous report.27 Immunohistochemical staining of tumour tissues was performed with anti-CD8 (Ventana) and anti-CD4 (Abcam) antibodies, as previously described.28 Densities of CD8+ and CD4+ T cells were quantified using the ImageJ software (https://imagej.nih.gov/ij/download.html) as previously described.29
Statistical analysis
Independent-sample t tests, ANOVA, and chi-square tests were used to compare variables. Survival analysis was performed using the Kaplan–Meier method, and the subgroups were compared using the log-rank test. Univariable and multivariable analyses of survival outcomes were performed using Cox proportional hazards regression analysis. The optimal cut-off value for IL-6 was determined using the maxstat (maximally selected rank statistics) package of the R software (R Foundation for Statistical Computing, Vienna, Austria; https://www.r-project.org/).30 All other statistical analyses were performed using the IBM SPSS version 18.0 software (IBM, Armonk, NY, USA). Statistical significance was set at p < 0.05.
Results
Participant characteristics and treatment outcome
From June 2020 to October 2021, 165 participants with unresectable HCC treated with Ate/Bev were prospectively registered in the discovery (n = 84) and validation (n = 81) cohorts at three tertiary cancer centres in Korea. A CONSORT (Consolidated Standards of Reporting Trials) diagram is shown in Fig. S1. The baseline clinical and laboratory characteristics are shown in Table 1 and Fig. 1, respectively. The median participant age was 61 years, and 84.2% were male. Most participants were Child–Pugh class A (87.3%) and Barcelona Clinic Liver Cancer (BCLC) stage C (79.4%). Hepatitis B (67.3 %) was the most common cause of HCC. Most participants (66.1%) had received at least one prior local therapy for HCC. Participants in the validation cohort had worse ECOG performance status and more Child–Pugh B7 class than those in the discovery cohort. The discovery cohort showed an ORR of 33.3% and a 1-year OS rate of 90.0%, with a median follow-up duration of 11.4 months. The validation cohort showed an ORR of 24.7% and a 1-year OS rate of 82.4%, with a median follow-up of 6.6 months.
Table 1.
Baseline demographics of participants with HCC.
| Total HCC cohort (N = 165) | Discovery cohort (n = 84) | Validation cohort (n = 81) | |
|---|---|---|---|
| Age (years), median (IQR) | 61 (55–68) | 61 (55–69) | 61 (56–68) |
| Male sex | 139 (84.2%) | 70 (83.3%) | 69 (85.2%) |
| ECOG performance status | |||
| 0 | 68 (41.2%) | 48 (57.1%) | 20 (24.7%) |
| 1 | 97 (58.8%) | 36 (42.9%) | 61 (75.3%) |
| Child–Pugh classification | |||
| A5 | 99 (60.0%) | 59 (70.2%) | 40 (49.4%) |
| A6 | 45 (27.3%) | 20 (23.8%) | 25 (30.9%) |
| B7 | 21 (12.7%) | 5 (6.0%) | 16 (19.7%) |
| BCLC stage | |||
| B | 34 (20.6%) | 21 (25.0%) | 13 (16.0%) |
| C | 131 (79.4%) | 63 (75.0%) | 68 (84.0%) |
| AFP ≥400 ng/ml, n (%) | 56 (33.9%) | 26 (31.0%) | 30 (37.0%) |
| Presence of MVI | 61 (37.0%) | 24 (28.6%) | 37 (45.7%) |
| Presence of extrahepatic spread | 96 (58.2%) | 46 (54.8%) | 50 (61.7%) |
| Aetiology of HCC | |||
| Hepatitis B | 117 (67.3%) | 60 (71.4%) | 57 (70.4%) |
| Hepatitis C | 12 (6.7%) | 5 (6.0%) | 7 (8.6%) |
| Alcohol | 19 (11.5%) | 12 (14.3%) | 7 (8.6%) |
| Other or unknown | 17 (14.5%) | 7 (8.3%) | 10 (12.3%) |
| Prior local therapy for HCC | 109 (66.1%) | 58 (69.0%) | 51 (63.0%) |
| IL-6 at baseline, median (IQR) | 2.89 (0.8–8.9) | 3.29 (1.3–8.3) | 2.32 (0.0–9.4) |
| IL-6 at baseline | |||
| Negative or low (<18.49 pg/ml) | 140 (84.8%) | 74 (88.1%) | 66 (81.5%) |
| High (≥18.49 pg/ml) | 25 (15.2%) | 10 (11.9%) | 15 (18.5%) |
AFP, alpha-foetoprotein; BCLC, Barcelona Clinic Liver Cancer; ECOG, Eastern Cooperative Oncology Group; HCC, hepatocellular carcinoma; MVI, macrovascular invasion.
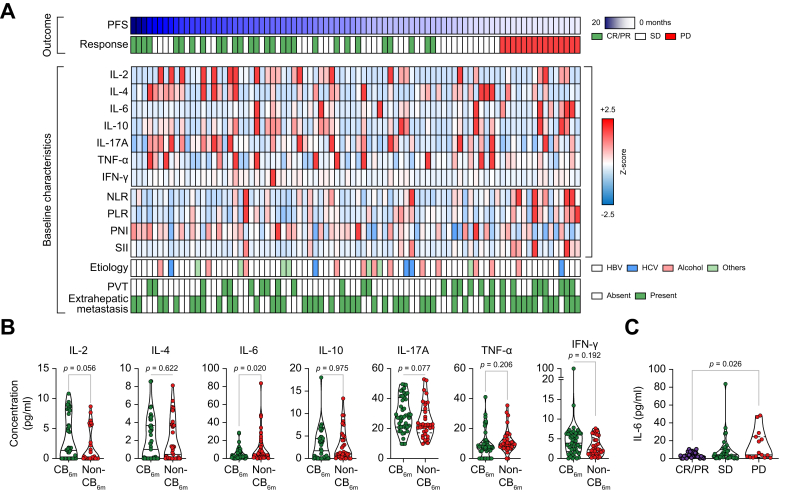
Fig. 1.
Baseline serum IL-6 levels are higher in patients without clinical benefit than in those with clinical benefit. (A) Comparisons of baseline serum cytokine levels, laboratory markers, and clinical characteristics. (B) Comparisons of baseline serum IL-2, IL-4, IL-6, IL-10, IL-17A, IFN-γ, and TNF-α levels according to CB6m (CB6m, n = 47; non-CB6m, n = 37). (C) Comparisons of serum IL-6 levels according to the best response. Values were compared using unpaired t tests (B) or ANOVA with Tukey’s post hoc test (C). CB6m, clinical benefit 6 months; CR, complete response; IFN-γ, interferon-γ; NLR, neutrophil-to-lymphocyte ratio; PD, progressive disease; PFS, progression-free survival; PLR, platelet-to-lymphocyte ratio; PNI, prognostic nutritional index; PR, partial response; PVT, portal vein thrombosis; SD, stable disease; SII, systemic immune-inflammation index; TNF-α, tumour necrosis factor-α.
Serum IL-6 levels were elevated in participants without clinical benefit
Next, we compared the baseline serum cytokine levels, laboratory markers, and clinical characteristics between participants with and without clinical benefit 6 months (CB6m) (Fig. 1A). CB6m was defined as CR, PR, or stable disease (SD) with ≥6 months PFS after Ate/Bev treatment. Among the various markers, serum IL-6 levels were significantly higher in participants without CB6m than in those with CB6m (mean 11.56 vs. 5.05, p = 0.02) (Fig. 1A and B). Moreover, participants who had PD at the first response evaluation had remarkably high baseline serum IL-6 levels compared with those with objective responses (CR + PR) (mean 14.15 vs. 3.62, p = 0.026).
Then, using the maximally selected rank statistics, we determined the cut-off value for high IL-6 that could optimally predict PFS as 18.49 pg/ml (Fig. S2). Using this cut-off value, 15.2% of the participants in this study (11.9% in the discovery cohort and 18.5% in the validation cohort) were defined as IL-6-high (Table 1).
Previous studies have shown that IL-6 levels are strongly associated with HCC development and serum IL-6 levels progressively increased from healthy liver status to hepatitis, liver cirrhosis, and HCC, according to disease stage.18 In this study, we confirmed that IL-6 levels increased proportionally with decreased liver function, and participants classified as Child–Pugh B7 had higher IL-6 levels than those who were Child–Pugh A. However, in terms of disease stage, BCLC stages B and C did not show a significant difference in IL-6 levels (Table 2). Recently, the C-reactive protein and alpha-foetoprotein (AFP) in immunotherapy (CRAFITY) score has been associated with clinical outcomes in patients with HCC treated with Programmed-death (ligand)-1 (PD-(L)1) immunotherapy.31 Intriguingly, there is a significant correlation between high IL-6 levels and high CRAFITY scores (p <0.001) (Table 2).
Table 2.
Clinical characteristics according to IL-6 status.
| IL-6-low (n = 140) | IL-6-high (n = 25) | p value | |
|---|---|---|---|
| Age (years), median (IQR) | 61 (55–68) | 64 (58–69) | 0.120 |
| Male sex | 119 (85.0%) | 20 (80.0%) | 0.527 |
| ECOG performance status | 0.058 | ||
| 0 | 62 (44.3%) | 6 (24.0%) | |
| 1 | 78 (55.7%) | 19 (76.0%) | |
| Child–Pugh classification | <0.001 | ||
| A5 | 92 (65.7%) | 7 (28.0%) | |
| A6 | 35 (25.0%) | 10 (40.0%) | |
| B7 | 13 (9.3%) | 8 (32.0%) | |
| BCLC stage | 0.287 | ||
| B | 31 (22.1%) | 3 (12.0%) | |
| C | 109 (77.9%) | 22 (88.0%) | |
| AFP ≥400 ng/ml, n (%) | 45 (32.1%) | 11 (44.0%) | 0.249 |
| Presence of MVI | 48 (34.3%) | 13 (52.0%) | 0.091 |
| Presence of extrahepatic spread | 79 (56.4%) | 17 (68.0%) | 0.280 |
| Aetiology of HCC | 0.720 | ||
| Hepatitis B | 100 (71.4%) | 17 (68.0%) | |
| Hepatitis C | 9 (6.4%) | 3 (12.0%) | |
| Alcohol | 17 (12.1%) | 2 (8.0%) | |
| Other or unknown | 14 (10.0%) | 3 (12.0%) | |
| CRAFITY score | <0.001 | ||
| 0 | 63 (45.0%) | 2 (8.0%) | |
| 1 | 64 (45.7%) | 10 (40.0%) | |
| 2 | 13 (9.3%) | 13 (52.0%) | |
| Prior local therapy for HCC | 98 (70.0%) | 11 (44.0%) | 0.011 |
AFP, alpha-foetoprotein; BCLC, Barcelona Clinic Liver Cancer; CRAFITY, C-reactive protein and AFP in immunotherapy; ECOG, Eastern Cooperative Oncology Group; HCC, hepatocellular carcinoma; MVI, macrovascular invasion.
High IL-6 levels were associated with unfavourable clinical outcomes in response to Ate/Bev in HCC
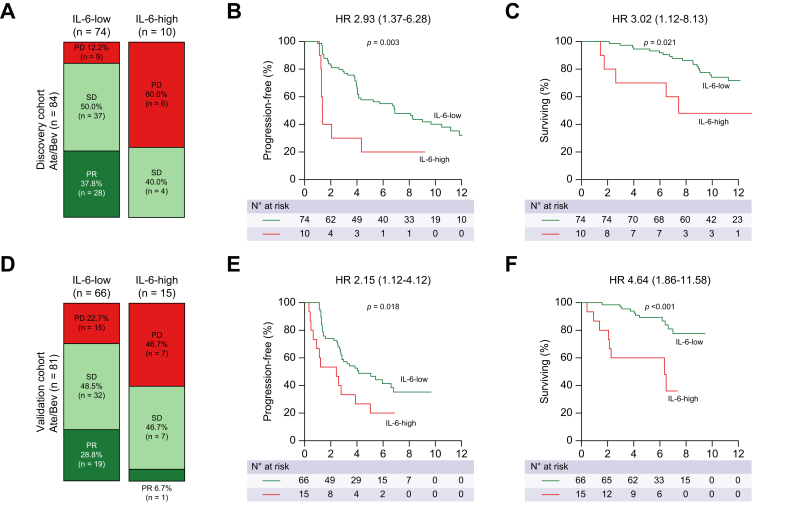
Next, we compared the clinical outcomes of Ate/Bev treatment according to baseline IL-6 levels in unresectable HCC. In the discovery cohort, participants with high IL-6 levels at baseline had a lower ORR than those with low IL-6 levels (0 vs. 37.8%; Fig. 2A). Moreover, PFS and OS were significantly worse in participants with high IL-6 levels than in those with low IL-6 levels (for PFS, hazard ratio [HR] 2.93, p = 0.003; for OS, HR 3.02, p = 0.021) (Fig. 2B and C). These findings were consistent with those of the validation cohort. Participants with high IL-6 levels showed a significantly lower ORR than those with low IL-6 levels (7 vs. 28.8%; Fig. 2D). Moreover, the IL-6-high group showed worse PFS and OS than the IL-6-low group (for PFS, HR 2.15, p = 0.018; for OS, HR 4.64, p <0.001) (Fig. 2E and F). Of note, almost half of the participants with high IL-6 levels at baseline (60.0% in the discovery cohort and 46.7% in the validation cohort) showed disease progression at their first radiologic response evaluation, compared with only 17.1% of participants with low IL-6 levels (12.2% in the discovery cohort and 22.7% in the validation cohort).
Fig. 2.
High IL-6 levels are associated with reduced clinical outcomes following treatment with Ate/Bev in HCC. Treatment outcomes of the (A–C) discovery cohort (n = 84) and (D–F) validation cohort (n = 81). (A and D) Bar charts showing the best response to Ate/Bev as determined by IL-6 levels. (B, C, E, and F) Kaplan–Meier curves showing PFS and OS according to IL-6 levels. Values of p were calculated using the log-rank test. HRs and 95% CIs are shown in the survival curves. Ate/Bev, atezolizumab and bevacizumab; HCC, hepatocellular carcinoma; HR, hazard ratio; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PR, partial response; SD, stable disease.
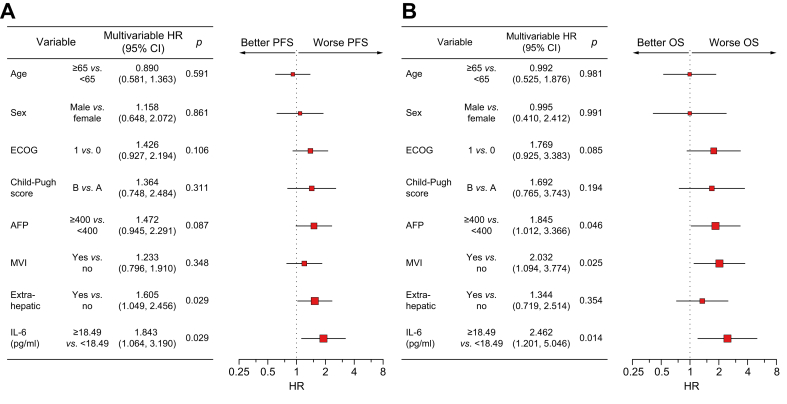
Because other variables such as Child–Pugh class may serve as confounding factors of survival outcome, we further validated the clinical impact of baseline IL-6 levels using a multivariable Cox proportional hazards model. On multivariable analysis, high baseline IL-6 levels were still the most significant factor associated with poor PFS and OS compared with other confounding variables, such as age, sex, ECOG performance status, Child–Pugh class, AFP levels, macrovascular invasion (MVI), and extrahepatic metastasis (for PFS, HR 1.843, p = 0.029; for OS, HR 2.462, p = 0.014) (Fig. 3A and B).
Fig. 3.
Multivariable survival analysis using the discovery and validation cohorts. Forest plots showing multivariable analyses of (A) PFS and (B) OS by age, sex, ECOG performance status, Child–Pugh score, AFP, MVI, extrahepatic spread, and serum IL-6 levels; N = 165, pooled data from the discovery and validation cohorts. Values of p were calculated using Cox proportional hazards regression analysis. AFP, alpha-foetoprotein; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; MVI, macrovascular invasion; OS, overall survival; PFS, progression-free survival.
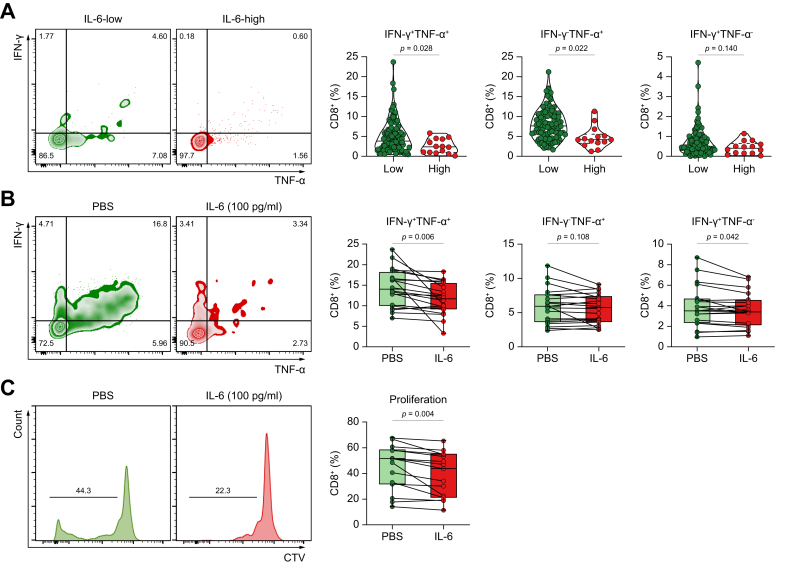
Highly elevated serum IL-6 levels correlated with reduced cytokine production and CD8+ T-cell proliferation
To further unveil how high levels of serum IL-6 influence T-cell immunity, we compared effector cytokine production by peripheral blood CD8+ T cells from IL-6-high and IL-6-low participants. CD8+ T cells from participants with high IL-6 levels produced less IFN-γ and TNF-α than those from participants with low IL-6 levels (Fig. 4A and B). To confirm that excess IL-6 affects CD8+ T-cell function, we examined whether the function of CD8+ T cells from participants with low IL-6 levels can be impaired by exogenous IL-6 treatment. Indeed, IL-6-treated CD8+ T cells produced less IFN-γ and TNF-α than the control CD8+ T cells (Fig. 4C and D). Moreover, excess IL-6 suppressed CD8+ T-cell proliferation (Fig. 4E and F). Therefore, high IL-6 levels may be associated with decreased effector function and proliferative capacity of cytotoxic T cells in patients with unresectable HCC.
Fig. 4.
Highly elevated serum IL-6 levels correlate with reduced cytokine production and CD8+ T-cell proliferation. (A) IFN-γ and TNF-α production in CD8+ T cells according to serum IL-6 levels (n = 102 for IL-6-low; n = 14 for IL-6-high); pooled data from the discovery and validation cohorts. (B) IFN-γ and TNF-α production by CD8+ T cells in the absence/presence of excess IL-6 (100 pg/ml); n = 20 for each group. (C) CD8+ T cell-proliferation in the absence/presence of excess IL-6 (100 pg/ml); n = 20 for each group. Values were compared using unpaired (A) or paired t tests (B and C). CTV, CellTrace Violet; IFN-γ, interferon-γ; TNF-α, tumour necrosis factor-α.
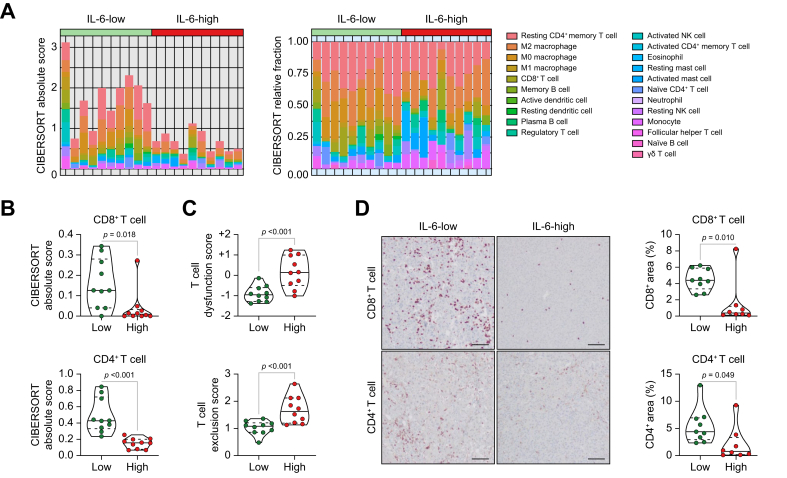
Highly elevated serum IL-6 levels may be associated with a non-T-cell-inflamed TME in unresectable HCC
To compare tumour immune phenotypes between the IL-6-high and IL-6-low groups, we examined intratumoural immune cell subsets from RNA sequencing data using CIBERSORT algorithms. Absolute scores from CIBERSORT algorithms estimated that the immune environment of tumours from the IL-6-high group consisted of a relatively lower quantity of immune subsets (Fig. 5A). To evaluate the difference in immune subsets between IL-6-high and IL-6-low tumours, hierarchical clustering was performed (Fig. S3A). In tumours with high IL-6 levels, CD8+ and CD4+ T-cell CIBERSORT scores were remarkably lower than in those with low IL-6 levels (Fig. 5B and Fig. S3B). Moreover, T-cell dysfunction and exclusion scores were remarkably higher in IL-6-high tumours than in IL-6-low tumours (Fig. 5C). Consistent with the findings of the CIBERSORT analysis, histological analysis showed that intratumoural CD8+ and CD4+ T cells were lower in IL-6-high tumours than in IL-6-low tumours (Fig. 5D). Therefore, patients with high serum IL-6 levels had non-T-cell-inflamed TME in their tumour tissues.
Fig. 5.
Highly elevated serum IL-6 levels are associated with a non-T-cell-inflamed TME in unresectable HCC. (A) Proportion of intratumoural immune cell subsets by CIBERSORT analysis according to serum IL-6 levels. (B) Comparison of CD8+ and CD4+ T cell CIBERSORT scores according to serum IL-6 levels. (C) Comparison of T-cell dysfunction and exclusion scores according to serum IL-6 levels. (D) Images and comparisons of CD8+ and CD4+ T cells in tumour tissues according to serum IL-6 levels. Values were compared using unpaired t tests. (A–C) n = 10 for each group. (D) n = 9 for IL-6-low and n = 8 for IL-6-high. HCC, hepatocellular carcinoma; TME, tumour microenvironment.
Discussion
Ate/Bev treatment has become the standard of care for unresectable HCC; however, a fraction of patients still exhibit primary resistance to this combination therapy. Pretreatment identification of these non-responders has been a crucial topic for both patients and clinicians. Herein, we found that a fraction (15%) of participants with unresectable HCC had high baseline IL-6 levels, and this was associated with reduced clinical benefits from Ate/Bev treatment. These findings were consistent even after adjusting for various confounding factors, including age, sex, ECOG, Child–Pugh class, AFP, MVI, and extrahepatic spread.
Although PD-L1 expression, microsatellite instability (MSI)-high status, and tumour mutational burden in tumour tissues are well-known predictive markers of response to immunotherapy in other solid tumours, their clinical use is less established in HCC.[32], [33], [34] Especially in HCC, given that tissue biopsy is not mandatory for diagnosis, the discovery of tumour tissue predictive biomarkers is very limited. In this regard, the discovery of circulating biomarkers that can predict immunotherapy efficacy is clinically meaningful in HCC. Thus, serum IL-6 is a promising candidate as an easily accessible circulating predictive biomarker for Ate/Bev treatment in HCC.
In this multicentre study, we verified the clinical implications of IL-6 using a multitude of evidence. First, we found that patients with high baseline IL-6 levels had unfavourable survival outcomes following Ate/Bev treatment. These findings were verified using a validation cohort and multivariable analysis. Second, we elucidated the immunological impact of high IL-6 levels on CD8+ T-cell function using serological and flow cytometric analyses. We demonstrated that patients with high IL-6 levels had reduced activation of peripheral CD8+ T cells compared with patients with low IL-6 levels. Moreover, excess IL-6 impaired cytokine production and proliferation of CD8+ T cells. These findings suggest that high baseline IL-6 levels may attenuate T-cell immunity in unresectable HCC. Third, we evaluated immune cell infiltrates within the HCC tissue by comparing RNA sequencing data between the high and low IL-6 groups. Notably, CD8+ and CD4+ T cells were markedly reduced in IL-6-high tumours compared with IL-6-low tumours. Therefore, high serum IL-6 levels be associated with a non-T-cell-inflamed TME in unresectable HCC.
In this study, we defined clinical benefit 6 months (CB6m) as CR, PR, or SD with ≥6 months PFS. The disease control rate is generally defined as the sum of CR, PR, and SD, regardless of the PFS.35 When we compared CB6m and disease control rate according to various cytokine levels, the overall trends were comparable, but CB6m better correlated with the differences in cytokine levels than disease control rate (Fig. S4). This may be because, in patients with SD, those with shorter PFS (<6 months) have higher levels of IL-6 than those with longer PFS (≥6 months).
Although we defined the IL-6 cut-off value for determining the clinical outcomes of first-line Ate/Bev therapy in unresectable HCC as 18.49 pg/ml, another recent study examining a small number of people treated with Ate/Bev as first- or second-line therapy suggested that a relatively low IL-6 level (3.2 pg/ml) could be used as a prognostic factor.36 However, this lower IL-6 cut-off value did not demonstrate significant differences in survival outcomes in our discovery and validation cohorts and T-cell functional differences, which suggests that sufficiently high IL-6 levels need to be applied to reproducibly predict the therapeutic efficacy of Ate/Bev.
The IL-6 signalling pathway is abnormally regulated in various malignancies,[15], [16], [17] and several recent studies have reported that IL-6 may be involved in mechanisms of resistance to immunotherapy.[19], [20], [21] IL-6 activates STAT3 in dendritic cells (DCs), leading to the downregulation of major histocompatibility complex class II expression on the surface of DCs.37 IL-6 is also capable of upregulating Agr-1 expression and chemokine receptor CCR5 in myeloid-derived suppressor cells (MDSCs), leading to MDSC recruitment and activation.38 Other recent studies have also revealed an association between high baseline IL-6 levels and reduced benefits from immunotherapy in patients with melanoma, renal cell carcinoma, and lung cancer.22,39,40 These findings suggest that high IL-6 levels may attenuate the efficacy of immunotherapy and that IL-6 blockade could augment antitumour immunity. A recent preclinical study revealed that IL-6 blockade could suppress tumour growth by enhancing CD4+/CD8+ effector T cells while suppressing Th17 and macrophages.21 Based on these previous findings, an ongoing phase Ib/II clinical trial (NCT04524871) is currently evaluating the efficacy and safety of anti-IL-6 add-on therapy in patients with unresectable HCC treated with Ate/Bev.
Our study was limited in that it enrolled only East Asian participants from a hepatitis B virus-endemic region, therefore necessitating external validation in other ethnic groups. Furthermore, we did not evaluate the source of peripheral blood IL-6 levels. In HCC, IL-6 levels may be affected by various conditions, such as deterioration of liver function, progression of the HCC stage, and the immune phenotype of HCC, as shown by the RNA sequencing results of our study. Thus, further investigation is needed to clarify the source of elevated IL-6 levels. Moreover, in this study, we activated T cells in vitro for a short time; thus, some T cells may have remained in a naïve state, which could have affected the degree of cytokine production. Therefore, further mechanistic studies on the effect of IL-6 on T-cell function are needed to validate the findings of this study.
In conclusion, high baseline serum IL-6 level is associated with worse clinical outcomes in patients with unresectable HCC treated with Ate/Bev. Clinicians need to carefully monitor and perform early response evaluations when treating patients with high IL-6 levels; however, these individuals should not be excluded from receiving a potentially effective standard-of-care therapy. Further validation and standardisation of IL-6 assays are warranted to optimise the monitoring and management of the Ate/Bev combination therapy. In addition, future investigations are needed to explore whether the association between reduced clinical benefit in response to Ate/Bev treatment and high IL-6 levels observed in the present study can also be applied to other combination immunotherapies, such as anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and anti-PD-1/PD-L1.
Financial support
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2020R1C1C1010722 to HJC, NRF-2020R1A2C2004530 to CK, and NRF-2021R1F1A106196311 to BK).
Authors’ contributions
Supervision: CK, HJC. Conceptualisation: HY, JC, BK, SYR, CK, HJC. Funding acquisition: BK, CK, HJC. Participant recruitment: JC, IK, HK, SJ, BK, CK, HJC. Laboratory analysis: HY, YH, SHL, WSL. Data analysis and visualisation: HY, YH, GK, SHL, CK, HJC. Writing: HY, SHL, VEG, CK, HJC.
Data availability statement
Data are available upon request.
Conflicts of interest
HJC has a consulting or advisory role at Eisai, Roche, Bayer, ONO, MSD, BMS, Celgene, Sanofi, Servier, AstraZeneca, Sillajen, Menarini, and GreenCross Cell, and has received research grants from Roche, Dong-A ST, and Boryung Pharmaceuticals. CK has a consulting or advisory role at Roche, ONO, MSD, BMS, Oncocross, Virocure, Sillajen, and Panolos Biosciences, and has received research grants from Boryung Pharmaceuticals, Oncocross, Sillajen, and Virocure. JC has a consulting or advisory role at Roche, MSD China, Eisai, and Servier, and has received research grants from Bayer. VEG is an employee of F. Hoffmann-La Roche Ltd. and has stock options from F. Hoffmann-La Roche Ltd. The other authors have no potential conflicts of interest to disclose.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2023.100672.
Contributor Information
Jaekyung Cheon, Email: cheonjk0526@chamc.co.kr.
Chan Kim, Email: chan@cha.ac.kr.
Hong Jae Chon, Email: minidoctor@cha.ac.kr.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Finn R.S., Qin S., Ikeda M., Galle P.R., Ducreux M., Kim T.-Y., et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 2.Cheng A.-L., Qin S., Ikeda M., Galle P.R., Ducreux M., Kim T.-Y., et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76:862–873. doi: 10.1016/j.jhep.2021.11.030. [DOI] [PubMed] [Google Scholar]
- 3.Lee W.S., Yang H., Chon H.J., Kim C. Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity. Exp Mol Med. 2020;52:1475–1485. doi: 10.1038/s12276-020-00500-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheon J., Yoo C., Hong J.Y., Kim H.S., Lee D.-W., Lee M.A., et al. Efficacy and safety of atezolizumab plus bevacizumab in Korean patients with advanced hepatocellular carcinoma. Liver Int. 2022;42:674–681. doi: 10.1111/liv.15102. [DOI] [PubMed] [Google Scholar]
- 5.Maesaka K., Sakamori R., Yamada R., Tahata Y., Imai Y., Ohkawa K., et al. Hyperprogressive disease in patients with unresectable hepatocellular carcinoma receiving atezolizumab plus bevacizumab therapy. Hepatol Res. 2022;52:298–307. doi: 10.1111/hepr.13741. [DOI] [PubMed] [Google Scholar]
- 6.Yumita S., Ogasawara S., Nakagawa M., Maruta S., Okubo T., Itokawa N., et al. Analysis of hyperprogressive disease due to atezolizumab combined with bevacizumab treatment in patients with advanced hepatocellular carcinoma. Res Square. 2022 doi: 10.21203/rs.3.rs-1761220/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim C., Yang H., Kim I., Kang B., Kim H., Kim H., et al. Association of high levels of antidrug antibodies against atezolizumab with clinical outcomes and T-cell responses in patients with hepatocellular carcinoma. JAMA Oncol. 2022;8 doi: 10.1001/jamaoncol.2022.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.An H.J., Chon H.J., Kim C. Peripheral blood-based biomarkers for immune checkpoint inhibitors. Int J Mol Sci. 2021;22:9414. doi: 10.3390/ijms22179414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruix J., Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Tommaso L., Spadaccini M., Donadon M., Personeni N., Elamin A., Aghemo A., et al. Role of liver biopsy in hepatocellular carcinoma. World J Gastroenterol. 2019;25:6041–6052. doi: 10.3748/wjg.v25.i40.6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stenken J.A., Poschenrieder A.J. Bioanalytical chemistry of cytokines – a review. Anal Chim Acta. 2015;853:95–115. doi: 10.1016/j.aca.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirano T., Ishihara K., Hibi M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene. 2000;19:2548–2556. doi: 10.1038/sj.onc.1203551. [DOI] [PubMed] [Google Scholar]
- 13.Kumari N., Dwarakanath B., Das A., Bhatt A.N. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumor Biol. 2016;37:11553–11572. doi: 10.1007/s13277-016-5098-7. [DOI] [PubMed] [Google Scholar]
- 14.Fisher D.T., Appenheimer M.M., Evans S.S. The two faces of IL-6 in the tumor microenvironment. Semin Immunol. 2014;26:38–47. doi: 10.1016/j.smim.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoejberg L., Bastholt L., Schmidt H. Interleukin-6 and melanoma. Melanoma Res. 2012;22:327–333. doi: 10.1097/CMR.0b013e3283543d72. [DOI] [PubMed] [Google Scholar]
- 16.Ma Y., Ren Y., Dai Z.-J., Wu C.-J., Ji Y.-H., Xu J. IL-6, IL-8 and TNF-α levels correlate with disease stage in breast cancer patients. Adv Clin Exp Med. 2017;26:421–426. doi: 10.17219/acem/62120. [DOI] [PubMed] [Google Scholar]
- 17.Feng L., Qi Q., Wang P., Chen H., Chen Z., Meng Z., et al. Serum levels of IL-6, IL-8, and IL-10 are indicators of prognosis in pancreatic cancer. J Int Med Res. 2018;46:5228–5236. doi: 10.1177/0300060518800588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shakiba E., Ramezani M., Sadeghi M. Evaluation of serum interleukin-6 levels in hepatocellular carcinoma patients: a systematic review and meta-analysis. Clin Exp Hepatol. 2018;4:182–190. doi: 10.5114/ceh.2018.78122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalbasi A., Ribas A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat Rev Immunol. 2020;20:25–39. doi: 10.1038/s41577-019-0218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flint T.R., Janowitz T., Connell C.M., Roberts E.W., Denton A.E., Coll A.P., et al. Tumor-induced IL-6 reprograms host metabolism to suppress anti-tumor immunity. Cell Metab. 2016;24:672–684. doi: 10.1016/j.cmet.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hailemichael Y., Johnson D.H., Abdel-Wahab N., Foo W.C., Bentebibel S.-E., Daher M., et al. Interleukin-6 blockade abrogates immunotherapy toxicity and promotes tumor immunity. Cancer Cell. 2022;40:509–523.e506. doi: 10.1016/j.ccell.2022.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sang Y.B., Yang H., Lee W.S., Lee S.J., Kim S.-G., Cheon J., et al. High serum levels of IL-6 predict poor responses in patients treated with pembrolizumab plus axitinib for advanced renal cell carcinoma. Cancers (Basel) 2022;14:5985. doi: 10.3390/cancers14235985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsukamoto H., Senju S., Matsumura K., Swain S.L., Nishimura Y. IL-6-mediated environmental conditioning of defective Th1 differentiation dampens antitumour immune responses in old age. Nat Commun. 2015;6:1–15. doi: 10.1038/ncomms7702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakanishi Y., Lu B., Gerard C., Iwasaki A. CD8+ T lymphocyte mobilization to virus-infected tissue requires CD4+ T-cell help. Nature. 2009;462:510–513. doi: 10.1038/nature08511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsukamoto H., Fujieda K., Senju S., Ikeda T., Oshiumi H., Nishimura Y. Immune-suppressive effects of interleukin-6 on T-cell-mediated anti-tumor immunity. Cancer Sci. 2018;109:523–530. doi: 10.1111/cas.13433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heimbach J.K., Kulik L.M., Finn R.S., Sirlin C.B., Abecassis M.M., Roberts L.R., et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 27.Jiang P., Gu S., Pan D., Fu J., Sahu A., Hu X., et al. Signatures of T cell dysfunction and exclusion predict cancer immunotherapy response. Nat Med. 2018;24:1550–1558. doi: 10.1038/s41591-018-0136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park H.S., Kim Y.M., Kim S., Lee W.S., Kong S.J., Yang H., et al. High endothelial venule is a surrogate biomarker for T-cell inflamed tumor microenvironment and prognosis in gastric cancer. J Immunother Cancer. 2021;9 doi: 10.1136/jitc-2021-003353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang H., Lee W.S., Kong S.J., Kim C.G., Kim J.H., Chang S.K., et al. STING activation reprograms tumor vasculatures and synergizes with VEGFR2 blockade. J Clin Invest. 2019;129:4350–4364. doi: 10.1172/JCI125413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lausen B., Schumacher M. Maximally selected rank statistics. Biometrics. 1992;48:73–85. [Google Scholar]
- 31.Scheiner B., Pomej K., Kirstein M.M., Hucke F., Finkelmeier F., Waidmann O., et al. Prognosis of patients with hepatocellular carcinoma treated with immunotherapy – development and validation of the CRAFITY score. J Hepatol. 2022;76:353–363. doi: 10.1016/j.jhep.2021.09.035. [DOI] [PubMed] [Google Scholar]
- 32.Yavuz B.G., Hasanov E., Lee S.S., Mohamed Y.I., Curran M.A., Koay E.J., et al. Current landscape and future directions of biomarkers for immunotherapy in hepatocellular carcinoma. J Hepatocell Carcinoma. 2021;8:1195–1207. doi: 10.2147/JHC.S322289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hyung J., Cho E.J., Kim J., Kim J.H., Kim J.E., Hong Y.S., et al. Histopathologic and molecular biomarkers of PD-1/PD-L1 inhibitor treatment response among patients with microsatellite instability-high colon cancer. Cancer Res Treat. 2022;54:1175–1190. doi: 10.4143/crt.2021.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim C.W., Chon H.J., Kim C. Combination immunotherapies to overcome intrinsic resistance to checkpoint blockade in microsatellite stable colorectal cancer. Cancers (Basel) 2021;13:4906. doi: 10.3390/cancers13194906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Villaruz L.C., Socinski M.A. The clinical viewpoint: definitions, limitations of RECIST, practical considerations of measurement. Clin Cancer Res. 2013;19:2629–2636. doi: 10.1158/1078-0432.CCR-12-2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Myojin Y., Kodama T., Sakamori R., Maesaka K., Matsumae T., Sawai Y., et al. Interleukin-6 is a circulating prognostic biomarker for hepatocellular carcinoma patients Treated with Combined Immunotherapy. Cancers. 2022;14:883. doi: 10.3390/cancers14040883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kitamura H., Ohno Y., Toyoshima Y., Ohtake J., Homma S., Kawamura H., et al. Interleukin-6/STAT3 signaling as a promising target to improve the efficacy of cancer immunotherapy. Cancer Sci. 2017;108:1947–1952. doi: 10.1111/cas.13332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weber R., Groth C., Lasser S., Arkhypov I., Petrova V., Altevogt P., et al. IL-6 as a major regulator of MDSC activity and possible target for cancer immunotherapy. Cell Immunol. 2021;359 doi: 10.1016/j.cellimm.2020.104254. [DOI] [PubMed] [Google Scholar]
- 39.Hardy-Werbin M., Rocha P., Arpi O., Taus Á., Nonell L., Durán X., et al. Serum cytokine levels as predictive biomarkers of benefit from ipilimumab in small cell lung cancer. Oncoimmunology. 2019;8 doi: 10.1080/2162402X.2019.1593810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laino A.S., Woods D., Vassallo M., Qian X., Tang H., Wind-Rotolo M., et al. Serum interleukin-6 and C-reactive protein are associated with survival in melanoma patients receiving immune checkpoint inhibition. J Immunother Cancer. 2020;8 doi: 10.1136/jitc-2020-000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon request.