Summary
Background
PARP inhibitors (PARPi) have revolutionized the management of advanced ovarian carcinoma, and were investigated as forefront treatment in recurrent disease. The objective was to explore if mathematical modeling of the early longitudinal CA-125 kinetics could be used as a pragmatic indicator of the subsequent rucaparib efficacy, like it is for platinum-based chemotherapy.
Methods
The datasets of ARIEL2 and Study 10 involving recurrent HGOC patients treated with rucaparib were retrospectively investigated. The same strategy as those successfully developed for platinum chemotherapy, based on CA-125 ELIMination rate constant K (KELIM™), was implemented. Individual values of rucaparib-adjusted KELIM (KELIM-PARP) were estimated based on the longitudinal CA-125 kinetics during the first 100 treatment days, and then scored as favorable (KELIM-PARP ≥1.0) or unfavorable (KELIM-PARP <1.0). The prognostic value of KELIM-PARP regarding treatment efficacy (radiological response, and progression-free survival (PFS)) was assessed using univariable/multivariable analyses, with respect to platinum-sensitivity and homologous recombination deficiency (HRD) status.
Findings
The data from 476 patients were assessed. The CA-125 longitudinal kinetics during the first 100-treatment days could be accurately assessed using the KELIM-PARP model. In patients with platinum-sensitive diseases, BRCA mutational status KELIM-PARP score and were associated with subsequent complete/partial radiological responses (KELIM-PARP: odds-ratio = 2.81, 95% CI 1.86–4.52), and PFS (KELIM-PARP: hazard-ratio = 0.67, 95% CI 0.50–0.91). The patients with BRCA-wild type cancer and favorable KELIM-PARP experienced long PFS with rucaparib regardless of HRD. In platinum-resistant disease patients, KELIM-PARP was associated with subsequent radiological response (odds-ratio = 2.80, 95% CI 1.82–4.72).
Interpretation
This proof-of-concept study confirms the early CA-125 longitudinal kinetics during rucaparib in recurrent HGOC patients are assessable by mathematical modeling, to generate individual a KELIM-PARP score associated with the subsequent efficacy. This pragmatic strategy might be useful for selecting the patients for PARPi-based combination regimens, when identifying efficacy biomarker is challenging. Further assessment of this hypothesis is warranted.
Funding
The present study was supported by Clovis Oncology with a grant to academic research association.
Keywords: Ovarian neoplasm, CA-125 antigen, Poly (ADP-ribose) polymerase inhibitors, Decision support techniques
Research in context.
Evidence before this study
In patients with recurrent high-grade ovarian carcinoma (HGOC), the standard systemic treatment includes chemotherapy with or without carboplatin, depending on the eligibility of the patients to platinum-based chemotherapy. The development of poly (ADP-ribose)-polymerase (PARP) inhibitors urged the investigation of chemotherapy-free regimens in these patients. Forefront treatment with PARP inhibitor in patients with recurrent platinum-sensitive or -resistant disease was found to be effective in several clinical trials, especially in patients with BRCA somatic or germline mutation, leading to the approvals of rucaparib and olaparib in patients previously treated with 2 or 3 more chemotherapy lines.
The combination of PARP inhibitors with other targeted agents, especially immune checkpoint inhibitors and anti-angiogenic drugs, is considered as a promising strategy to increase the number of recurrent HGOC patients who may benefit from PARP inhibitor-based forefront chemotherapy-free treatment beyond those with BRCA mutation, as recently reported in MEDIOLA (NCT02734004) and BOLD (NCT04015739) trials. However, identifying biomarkers of efficacy with these triplet regimens is challenging due to the concurrent blockade of three signaling pathways (DNA repair, immune tolerance, and angiogenesis).
In that context, investigating the potential prognostic and predictive value of the early longitudinal CA-125 kinetics during forefront treatment with a PARP inhibitor in patients with recurrent HGOC, as a potential pragmatic indicator of the treatment efficacy, is rationale since this strategy was found to be relevant for platinum-based chemotherapy.
We searched PubMed for articles published between January 1, 1990, and August 31, 2022, using the terms “ovarian cancer” AND “recurrent” AND « PARP inhibitor » (OR « rucaparib » OR « olaparib ») AND « CA-125 » to identify studies, which assessed CA-125 kinetic parameters associated with treatment efficacy with forefront PARP inhibitor. The main kinetic parameter retrieved in the litterature was based on the CA-125 percentage decline adopted by Gynecology Cancer Inter-group (GCIG) in 2004. The other most studied kinetic parameter is the modeled CA-125 ELIMination rate constant K (KELIM™), based on longitudinal CA-125 kinetics during the first 100 chemotherapy days, which was shown to be a reproducible prgamatic indicator of the tumor chemosensivity on the data of more than 13,000 patients treated with platinum-based chemotherapy in first-line or in platinum-sensitive recurrent setting.
Added value of this study
The outcomes of this exploratory analysis of the ARIEL2 and Study 10 trials confirm that the early CA-125 longitudinal kinetics during the first 100 treatment days with rucaparib given as a forefront therapy in recurrent HGOC can be accurately characterized using an adjusted version of the mathematical model of KELIM™ (rucaparib-adjusted KELIM, called KELIM-PARP). Moreover, the early CA-125 longitudinal kinetics exhibit independent prognostic value regarding the benefit from rucaparib in terms of subsequent radiological response and progression-free survival in univariable and multivariable analyses. Beyond patients with BRCA mutation, a favorable KELIM-PARP was associated with higher efficacy of rucaparib regardless of homologous recombination deficiency status.
Implications of all the available evidence
This proof-of-concept study suggests that the assessement of the early CA-125 longitudinal kinetics during the first 100 treatment days using mathematic modeling, known to be relevant for platinum-based chemotherapy, also provides an early indicator of the subsequent treatment efficacy in patients receiving a forefront PARP inhibitor-based chemotherapy-free regimen. This pramatic strategy may help identify the patients who will experience maximum benefit from combination treatments on development, and overcome the challenge of finding biomarkers of efficacy when several signaling pathways are targeted simultaneously (such as DNA repair, immune tolerance, and angiogenesis). Assessment of KELIM-PARP prognostic value in BOLD trial (NCT04015739) and KELIM-PARP predictive value in ARIEL4 trial (NCT02855944) is warranted.
Introduction
In patients with recurrent high-grade ovarian carcinoma (HGOC), the standard treatment relies on chemotherapy, without/with carboplatin, depending on the expected platinum-sensitivity of the relapse, and/or the eligibility of the patients to platinum-based chemotherapy.1, 2, 3 The development of poly (ADP-ribose)-polymerase (PARP) inhibitors gave the opportunity to consider chemotherapy-free regimens in patients with platinum-sensitive or -resistant disease relapse. The favorable outcomes of trials investigating PARP inhibitors as forefront single agent therapeutics in patients with recurrent HGOC (ARIEL2 & Study 10 for rucaparib4, 5, 6; NCT00753545 trial for olaparib)7 led to the approvals of these drugs in adult patients with HGOC who were previouly treated with ≥2 chemotherapies for rucaparib,8 and ≥3 chemotherapies for olaparib.9 Subsequent studies showed that the main biomarkers of efficacy of rucaparib were the platinum-sensitivity of the relapse (platinum-sensitive recurrence if platinum-free interval (PFI) >6 months, versus platinum-resistant recurrence if PFI <6 months) and the homologous recombination status (BRCA mutation; homologous recombination deficiency (HRD) status, characterized by the level of loss-of-heterozygosity (LOH-high, or LOH-low)).4,10 On a practical point of view, these HRD assays are technologically complicated and costly to implement.11, 12, 13
PARP inhibitors are now being investigated as forefront treatment in combination with immunotherapy without/with anti-angiogenic drugs, as a way of enlarging the population of patients, who may benefit from them beyond BRCA mutation, as reported in MEDIOLA, or BOLD trials.14,15 Several assumptions support this strategy: higher neo-antigen load in HRD cancer leading to more effective immune response; STING-dependent innate immune response, by inducing type I interferon and pro-inflammatory cytokine production; glycogen synthase kinase-3 (GSK-3) inactivation and upregulation of PDL1 leading to increased cancer cell apoptosis.16 The promising preliminary outcomes of these trials imply the development of companion tests able to select the patients deriving the maximum benefit of these regimens. However, the high number of signaling pathways involved by these combinations (DNA repair, immune tolerance, and angiogenesis) will make the identification of several biomarkers of efficacy complicated and expensive. In the future, technologies of proteomics might help monitor cancer cell response, and uncover drug resistance emergence.17
Another option is to assess the early CA-125 longitudinal kinetics, as a pragmatic indicator of the treatment efficacy, as it was developed with success for platinum-based chemotherapy.18 The modeled CA-125 ELIMination rate constant K (KELIM™) is calculated with the mathematical equation driving the CA-125 longitudinal kinetics (≥3 values) during the first 100 days of treatment.19 KELIM™ can be understood as the rate of CA-125 decline during systemic treatment. The higher KELIM™, the faster the CA-125 elimination with systemic treatment, and the higher the treatment efficacy. The reliability of KELIM™ as a pragmatic independent indicator of tumor platinum-based chemosensitivity has been reproducibly shown on the data of more than 13,000 patients enrolled in 13 randomized trials, the Netherlands Cancer Registry, and the Gynecology Cancer InterGroup (GCIG) meta-analysis database.20, 21, 22, 23, 24, 25 In all studies performed so far, KELIM™ was found to exhibit better prognostic value than the official CA-125 response defined by the GCIG as a 50% reduction in CA-125 levels maintained for at least 28 days, in patients treated for recurrent disease.20,26, 27, 28, 29, 30 More recently, KELIM™ was reported to be a predictor of the benefit from maintenance treatments with PARP inhibitor in VELIA trial, and with bevacizumab in ICON-7 and GOG-0218 trials.25, 31 These favorable outcomes will lead to the adoption of the modeled CA-125 KELIM™ (easily assessable online by any clinician for their patients on https://www.biomarker-kinetics.org/presentation) as a useful numeric medical tool in the future European disease management algorithms.
The same pragmatic approach could be relevant for PARP inhibitor-based chemotherapy-free regimens. We hypothesized that the early longitudinal kinetics of CA-125 observed during treatment with rucaparib, and assessed using KELIM™ adjusted to rucaparib (called KELIM-PARP), may be helpful for identifying the patients who will benefit from rucaparib. The objective of the present post-hoc study of ARIEL2 and Study 10 was to assess the prognostic value of KELIM-PARP regarding the benefit from rucaparib, with respect to the other reported prognostic factors, especially platinum-sensitivity and HRD biomarkers.
Methods
Patients and data retrieved
ARIEL2 (NCT01891344) and Study 10 (NCT01482715) were international multicenter, two-part, phase 2 open-label studies assessing oral rucaparib given at 600 mg twice daily until disease progression, unacceptable toxicity, or death in adult women with platinum-sensitive or platinum-resistant recurrent HGOC. The methodology of these trials was previously reported.6 Eligibility criteria are detailed in Supplementary Material.
ARIEL2 study was done in Australia, Canada, France, Spain, the UK, and the USA; whilst Study 10 was conducted in the USA, the UK and Canada. ARIEL2 and Study 10 were approved by the institutional review board at each study site and was done in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines of the International Conference on Harmonisation (Supplementary Material). Patients provided written informed consent before participation.
CA-125 was measured every 3 (Study 10) or 4 weeks (ARIEL2). The patients analyzed in the present retrospective study should have ≥3 available CA-125 values during the first 100 days of treatment. HRD status was based on the presence of a deleterious BRCA1/2 mutation, or loss-of-heterozygosity (LOH)-high (≥16%) in tumor tissues using Foundation Medicine's next-generation sequencing assay.10 In the current study, platinum-sensitivity was defined as: platinum-refractory disease with PFI <1 month; platinum-resistant disease with PFI 1–6 months; and platinum-sensitive disease with PFI >6 months.
Modeling of CA-125 kinetics
To normalize the distribution of CA-125 concentrations and to eliminate right-skewness in the distribution, CA-125 levels were log-transformed. The mathematical modeling of early CA-125 kinetics with a non-linear mixed-effect model was previously described.20,21 Basic details about the semi-mechanistic kinetic-pharmacodynamic (K-PD) model adjustment and qualification are presented in the Supplementary Material.32
Consistently with previous analyses of ARIEL2 and Study 10 reporting the platinum-sensitivity of the relapse as a major prognostic factor of efficacy, the different kinetics of CA-125 among patients with platinum-sensitive or –resistant relapse led us to use the same model for both cohorts, and to estimate different population parameters for the baseline CA-125 and for KELIM-PARP. KELIM-PARP was standardized by a cutoff, as a way of providing an easy reading of patient KELIM-PARP outcome, with the following equation: Standardized (std) KELIM-PARP = KELIM-PARP estimated by the model/cutoff. Based on our experience for identifying the optimal cutoff for KELIM™ in patients treated with chemotherapy concluding that the best KELIM™ thresholds were similar to the median values,21, 22, 23,33 the cutoffs in each platinum-sensitive and –resistant cohort were selected as the respective median values of KELIM-PARP. As a consequence, std KELIM-PARP was a continuous covariate centered by 1.0. To help the interpretation of KELIM-PARP for prognostic analyses, std KELIM-PARP was dichotomized with a KELIM-PARP score: std KELIM <1.0 was considered as unfavorable, whilst std KELIM ≥1.0 was considered as favorable.
Moreover, the CA-125 response according to the GCIG was assessed in the same patients who had baseline CA-125 > 70 IU/mL, as per Rustin et al. rules.27,34 A GCIG CA-125 response was confirmed when a decline of CA-125 by minimum 50% was observed and maintained on a 28 day period.
Relationships between std KELIM-PARP and homologous recombination deficiency (HRD) status
The distributions of std KELIM-PARP in patients carrying a BRCA mutation, BRCA wild-type (WT) LOH-high, BRCA-WT LOH-low were assessed using box plots. The statistical significances of differences were assessed using Kruskal–Wallis test.
Relationships between std KELIM-PARP and radiological response to rucaparib
The distributions of std KELIM-PARP among patients experiencing complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD) as the best responses according to RECIST 1.1 criteria, observed after the 100th day of treatment, were assessed using box plots and waterfall plots. The ORR was defined as the percentage of patients who experienced CR or PR, as the best responses, while disease control rate (DCR) was defined as the percentage of patients who experienced best response of CR, PR, or SD. The statistical significances of differences were assessed using Wilcoxon rank sum test with continuity correction.
Univariable logistic regressions were used to assess the covariates significantly associated with the probability of CR/PR (compared to SD/PD) among KELIM-PARP (considered as a continuous covariate; or a categorical covariate (favorable, vs. unfavorable)); histology (clear cell, vs. others); HRD status (BRCA mutation, vs. BRCA-WT LOH-high, vs. BRCA-WT LOH-low); and GCIG CA-125 response.10 Those found significant in univariable analyses were then tested with a multivariable logistic regression model with backward selection procedure. The diagnostic accuracy was assessed using Area Under the ROC curve (ROC AUC). Moreover, C-index analyses were used to assess the prediction improvement related to the incorporation of KELIM-PARP and the other covariates in the logistic regression models. Accuracy of the final logistic model was evaluated using a repeated 10-fold cross-validation method.
Prognostic value of KELIM-PARP score regarding progression-free survival (PFS)
The prognostic value of KELIM-PARP score regarding PFS, categorized as unfavorable or favorable, was assessed using Log-rank test, Kaplan–Meier method, and multivariable hazard-ratio Cox models. The other prognostic factors tested in univariable analyses were the same as those described above. Those found significant in univariable analyses (P < 0.10), were included in the multivariable Cox model, and assessed using backward selections.
All survival analyses were implemented with a landmark time point set at 100 days after the start of rucaparib. As already done in other KELIM studies, CA-125 was modeled from day 0–100, and exclusion of the early progressions observed during the first 100 days avoided the biases related to the links between early progressions and CA-125 kinetics, or radiological tumor responses.35 Progression-free survival was calculated as the time elapsed between inclusion and disease progression or death, whichever occurred first. Missing data were automatically excluded from analyses.
Statistics and computing process
All tests were implemented using a two-sided 0.05 alpha risk. NONMEM 7.5 (ICON Development Solutions, Ellicott City, MD, USA) software was used to fit the semi-mechanistic model to CA-125 kinetic data.36 The XPOSE4 program was used for graphical evaluation of model fits.37 Logistic analyses, cross-validation, survival analyses and concordance probability (C-index) were obtained in R software version 4·1·0. The cross validation was performed under R (4.1.0) software using the function cv.glm: Cross-validation for Generalized Linear Models (boot package). Additonal details are presented in Supplementary Material.
Role of funders
The present study was supported by Clovis Oncology with a grant to the academic research association of Lyon University laboratory EA3738 CICLY. Clovis Oncology provided the data of ARIEL2 and Study 10 trials. The statistical analyses, and the manuscript writing, were independently performed by Lyon University team.
Results
Patients
Out of 545 enrolled patients (ARIEL2 n = 491; Study 10, n = 54), the data from 476 patients (87.3%) could be assessed for KELIM-PARP (Table 1; Supplementary Fig. S1). 63% of them had platinum-sensitive disease, whilst 29% had platinum-resistant disease, and 8% had refractory disease. 37% of patients carried a BRCA mutation (BRCA1, 25%; BRCA2, 12%), and 63% of them had BRCA-WT tumors. The patients with BRCA-WT tumor were classified as LOH-high (126 patients; 26%), LOH-low (152 patients; 32%), and LOH-unknown (24 patients; 5%).
Table 1.
Characteristics of assessed patients.
| Variable | N = 476 |
|---|---|
| Cancer type | |
| Epithelial ovarian cancer | 386 (81%) |
| Fallopian tube cancer | 43 (9%) |
| Primary peritoneal cancer | 47 (10%) |
| Histological classification | |
| Serous | 449 (94%) |
| Others | 27 (6%) |
| Platinum-sensitivity of the recurrent disease | |
| Sensitive | 299 (63%) |
| Resistant | 138 (29%) |
| Refractory | 39 (8%) |
| BRCA mutational status | |
| BRCA1 | 117 (25%) |
| BRCA2 | 57 (12%) |
| BRCA wild-type | 302 (63%) |
| Homologous recombination deficiency (HRD) status | |
| BRCA1 mutation | 117 (25%) |
| BRCA2 mutation | 57 (12%) |
| BRCA, wild type LOH-high | 126 (26%) |
| BRCA, wild type LOH-low | 152 (32%) |
| BRCA, wild type LOH(unknown) | 24 (5%) |
| Best radiological response after 100 days according to RECIST criteria | |
| Complete response | 23 (5%) |
| Partial response | 125 (26%) |
| Stable disease | 141 (30%) |
| Progressive disease | 63 (13%) |
| Not evaluable | 124 (26%) |
| CA-125 response according to the GCIG | |
| Unfavorable | 175 (37%) |
| Favorable | 107 (22%) |
| Not evaluablea | 194 (41%) |
| Key outcome measures | |
| Platinum-sensitive cohort (n = 299) | |
| Best subsequent radiological response | |
| Complete response | 21 (7%) |
| Partial response | 96 (32%) |
| Stable disease | 92 (31%) |
| Progressive disease | 37 (12%) |
| Not evaluable | 53 (18%) |
| Progression-free survival (PFS) with a 100 day landmark | 5.3 [4.1–6.00] |
| Platinum-resistant cohort (n = 177) | |
| Best subsequent radiological response | |
| Complete response | 2 (1%) |
| Partial response | 29 (16%) |
| Stable disease | 49 (28%) |
| Progressive disease | 26 (15%) |
| Not evaluable | 71 (40%) |
| Progression-free survival (PFS) with a 100 day landmark | 4.1 [4.0–5.5] |
LOH: loss-of-heterozygosity.
CA-125 response according to the GCIG: baseline CA-125 not available or baseline <2 N (70 kU/L).
The median follow-up was 6 months (95% CI 5.5–7.1). Taking into account the 100 days landmark analyses, the data from 352 to 353 patients could be assessed for the radiological response and PFS, respectively (Supplementary Fig. S1). In patients with platinum-sensitive recurrent disease, the ORR and DCR were 50% and 96%, respectively. In the platinum-resistant cohort, these numbers were 30% and 90%, respectively. The median PFS was 4.5 months (95% CI 4.1–5.8) for the whole population, including 5.3 months (95% CI 4.1–6.0) in the platinum-sensitive cohort, and 4.1 months (95% CI 4.1–5.5) in the platinum-resistant/refractory cohort.
Modeling of early longitudinal CA-125 kinetics
Median of 4 CA-125 values (range: 3–9) were available in each patient. Strong differences in CA-125 kinetics were observed between patients with platinum-sensitive disease and those with platinum-resistant/refractory disease (Supplementary Fig. S2). The qualification analyses from the final semi-mechanistic models, are presented in Supplementary Fig. S3. The median values of KELIM-PARP in patients with platinum-sensitive disease and platinum-resistant/refractory populations were 0.020 days−1 and 0.010 days−1, respectively (Wilcoxon rank sum test, P < 0.001). These values were used for standardizing (std) KELIM-PARP, and scoring them as unfavorable (<1.0) or favorable (≥1.0) for the rest of the study.
Relationships between std KELIM-PARP and homologous recombination biomarkers
As expected, KELIM-PARP tended to be higher in patients carrying HRD diseases. In the platinum-sensitive cohort, std KELIM-PARP was gradually higher among patients carrying BRCA mutations, followed by those with BRCA-WT LOH-high tumors, and then by those with BRCA-WT LOH-low tumors (median std KELIM-PARP, 1.53, vs. 1.05, vs. 0.68, respectively (Wilcoxon rank sum test, P < 0.001 for all pairwise comparisons) (Supplementary Fig. S4).
In the platinum-resistant/refractory cohort, std KELIM-PARP was higher among patients carrying BRCA mutation compared to those with BRCA-WT LOH-high tumors (median, 1.08 vs. 0.48, Wilcoxon rank sum test, P < 0.001). However, unlike the above platinum-sensitive population, std KELIM-PARP was not different between patients with BRCA-WT diseases associated with LOH-high or LOH-low (median, 0.48 vs. 0.52, P = 0.29, Wilcoxon rank sum test, P = 0.29) (Supplementary Fig. S4).
Prognostic value of std KELIM-PARP regarding subsequent radiological response and PFS
Platinum-sensitive recurrent cohort
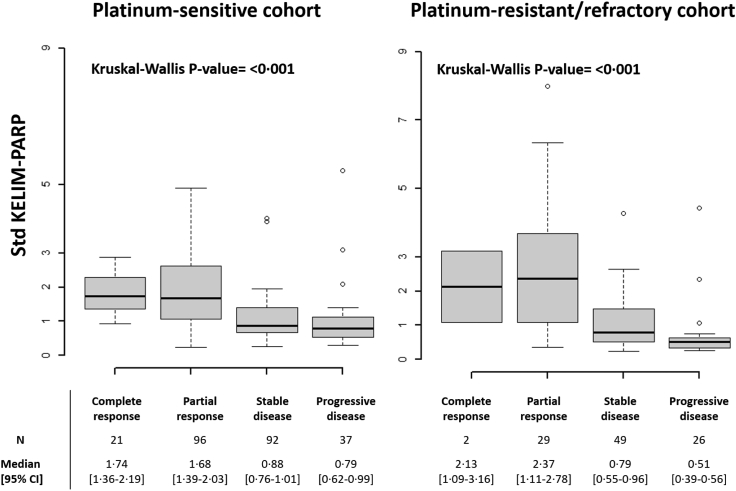
A strong correlation was found between std KELIM-PARP and subsequent radiological response assessed using RECIST criteria. Indeed, patients experiencing CR, or PR, had higher std KELIM-PARP than those experiencing SD, or PD (Fig. 1; Supplementary Table S1). The association between std KELIM-PARP and ORR was observed among patients regardless of the HRD status (Supplementary Fig. S5).
Fig. 1.
Best radiological responses as per RECIST criteria according to std KELIM-PARP in the platinum-sensitive and platinum-resistant/refractory cohort (Kruskal–Wallis test).
The univariable logistic regression models identified three significant prognostic factors associated with the probability of subsequent complete/partial response to rucaparib: HRD status; GCIG CA-125 response; and KELIM-PARP (Supplementary Table S2). The GCIG CA-125 response that was assessable in only 53% of patients was not kept for the multivariable analysis. In the final multivariable logistic analysis, std KELIM-PARP and HRD status were significantly associated with the likelihood of complete/partial response to rucaparib: std KELIM-PARP (odds-ratio (OR), 2.81, 95% CI 1.86–4.52); HRD status (BRCA mutation, reference, BRCA-WT LOH-high, OR 0.38, 95% CI 0.18–0.77, BRCA-WT LOH-low, OR 0.12, 95% CI 0.05–0.27) (Table 2; Supplementary Fig. S8).
Table 2.
Final multivariable logistic regression model regarding the probability of complete/partial response in the platinum-sensitive cohort.
| N = 237 (progressive or stable disease = 124, partial or complete response = 113) | |||||||
|---|---|---|---|---|---|---|---|
| n | Estimate | OR | 95% CI | P | C-index [95% CI] | Accuracy | |
| Intercept | −0.73 | 0.48 | 0.22–1.03 | 0.063 | 0.84 [0.78–0.89] | 77% | |
| Std KELIM-PARP | 237 | 1.03 | 2.81 | 1.86–4.52 | <0.001 | ||
| Homologous recombination deficiency (HRD) status | |||||||
| BRCA mutation | 105 | REF | |||||
| BRCA, wild type LOH-high | 64 | −0.96 | 0.38 | 0.18–0.77 | 0.007 | ||
| BRCA, wild type LOH-low | 68 | −2.11 | 0.12 | 0.05–0.27 | <0.001 | ||
OR: odds ratio; 95% CI: 95% confidence interval; REF: reference class; Accuracy: repeated 10-fold cross-validation accuracy; LOH: loss-of-heterozygosity.
The following covariates were associated with PFS in univariable analyses: HRD status; GCIG CA-125 response; and KELIM-PARP score (Supplementary Table S3). The GCIG CA-125 response that was assessable in 54.5% of patients only, was not kept in the multivariable analysis. In the final multivariable Cox hazard-ratio model, both KELIM-PARP score and HRD status were significant and independent prognostic factors of PFS: KELIM-PARP score (favorable vs. unfavorable, HR, 0.67, 95% CI 0.50–0.91); HRD status (BRCA mutation (reference) vs. BRCA-WT LOH-high, HR, 1.35 (95% 0.97–1.87); BRCA mutation (reference) vs. BRCA-WT LOH-low, HR, 1.98 (95% 1.39–2.81)) (Table 3).
Table 3.
Final multivariable cox model and C-index regarding PFS in the platinum-sensitive recurrent cohort.
| N = 237a | HR | 95% CI | P | Analysis of deviance | C-index [95% CI] |
|---|---|---|---|---|---|
| KELIM-PARP score | 0.63 [0.59–0.66] | ||||
| Unfavorable score | REF | 0.01 | |||
| Favorable score | 0.67 | 0.50–0.91 | 0.009 | ||
| Homologous recombination deficiency (HRD) status | |||||
| BRCA mutation | REF | <0.01 | |||
| BRCA, wild type LOH-high | 1.35 | 0.97–1.87 | 0.07 | ||
| BRCA, wild type LOH-low | 1.98 | 1.39–2.81 | <0.001 | ||
HR: hazard-ratio; 95% CI: 95% confidence interval; REF: reference class; Analysis of Deviance Table (Type II tests): wald; LOH: loss-of-heterozygosity.
9 Observations deleted due to missing data.
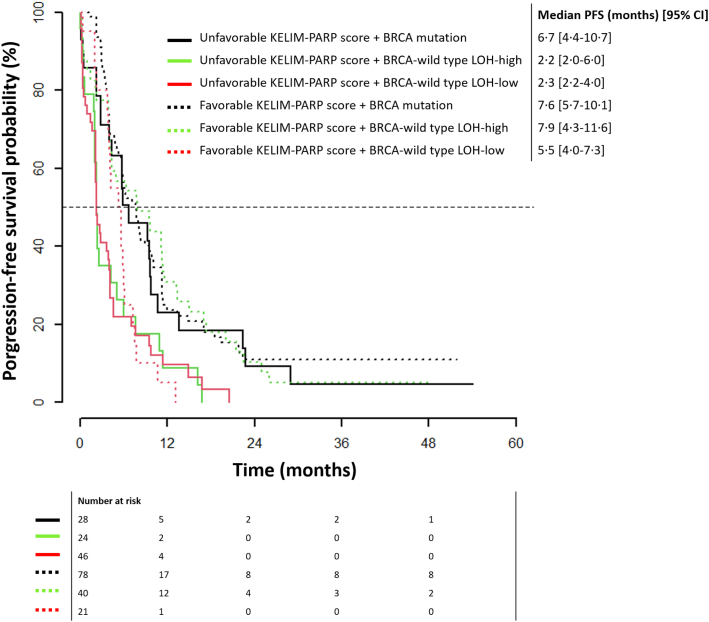
In line with these data, the Kaplan–Meier PFS curves according to KELIM-PARP score and HRD status suggest that patients with BRCA-mutated disease had long PFS regardless of KELIM-PARP score (median PFS 6.7–7.6 months) (Fig. 2). However, among patients with BRCA-WT tumor, only those with favorable KELIM-PARP score had long PFS regardless of LOH status (favorable KELIM-PARP score, median PFS 5.5–7.9 months; unfavorable KELIM-PARP score, median PFS 2.2–2.3 months). Of note, the PFS difference between patients with BRCA-WT LOH-high or BRCA-WT LOH-low tumors was not significant.
Fig. 2.
Kaplan–Meier PFS curves according to KELIM-PARP score and HRD status (BRCA mutation; BRCA wild-type LOH-high; BRCA wild-type LOH-low) in the platinum-sensitive cohort.
Platinum-resistant recurrent cohort
In patients with platinum-resistant/refractory recurrent disease, equivalent relationships between std KELIM-PARP and radiological response was found (Fig. 1, and Supplementary Table S4). The ORR was consistently higher among patients with favorable KELIM-PARP score, regardless of the HRD status (Supplementary Fig. S6).
In univariable logistic regression analysis, three significant prognostic factors were significantly associated with the probability of CR/PR to rucaparib: BRCA mutational status (mutated vs. not); GCIG CA-125 response (yes vs. no); and std KELIM-PARP (Supplementary Table S5). In the multivariable logistic analysis, only std KELIM-PARP was significant (OR, 2.80, 95% CI 1.82–4.72) (Supplementary Table S6; Supplementary Fig. S7).
No covariate was significantly associated with PFS in this cohort (Supplementary Fig. S9, Supplementary Table S7).
Discussion
This study is the first analysis of the prognostic value of the early modeled CA-125 kinetics during a forefront treatment with the PARP inhibitor rucaparib. The outcomes show that: 1) CA-125 kinetics during rucaparib treatment can be successfully described using the same model structure as developed for platinum-based chemotherapy; 2) the early decline of CA-125 during rucaparib monotherapy is less marked than those observed with chemotherapy, especially in patients with platinum-resistant disease; 3) there are strong relationships between the early CA-125 kinetics and: a) subsequent radiological tumor response to rucaparib in patients with platinum-sensitive or resistant/refractory disease; b) PFS in patients with platinum-sensitive relapse. In contrast with patients with platinum-sensitive relapse disease, the strong impact of rucaparib on tumor bulk observed in those with platinum-resistant relapse with a favorable std KELIM-PARP (ORR, 47%–63%, Supplementary Fig. S5) did not translate into a PFS advantage, probably as a result of the poor prognosis of these patients.
An important outcome of the present study is the independent and complementary prognostic values of both the BRCA mutational status and CA-125 KELIM-PARP in terms of PFS in patients with platinum-sensitive recurrent HGOC. This exploratory analysis confirms our assumption that the early CA-125 longitudinal kinetics could be a potential pragmatic indicator of the subsequent efficacy to expect with rucaparib, and other PARP inhibitors, when used as forefront therapeutics in patients with recurrent HGOC. Based on these outcomes, the assessment of the predictive value of KELIM-PARP regarding rucaparib activity is planned in the randomized clinical trial ARIEL4 (NCT02855944), which assessed the superiority of rucaparib over standard-of-care chemotherapy in patients with recurrent HGOC and BRCA mutation.38,39
The same success story as those seen with KELIM™ in patients treated with platinum-based chemotherapy can be expected. KELIM™ is being prospectively investigated as prognostic factor in on-going large phase III trials (such as NIRVANA trial, assessing niraparib ± bevacizumab in first-line setting (NCT04734665)), and will soon be recognized as a useful numeric tool by the European guidelines. Patient KELIM™ score calculation is easily available online to clinicians (https://www.biomarker-kinetics.org/presentation).
More largely, this positive exploratory study may have important consequences for the development of the future chemotherapy-free combination regimens based on PARP inhibitors being investigated in patients with recurrent HGOC. Indeed, it shows that the early CA-125 longitudinal kinetics assessed using mathematical modeling could provide relevant information about the benefit to expect in patients, and overcome the current challenge for finding efficacy biomarkers in the context of multiple signaling pathways blockade. The assessment of the prognostic value of KELIM-PARP is planned in recurrent HGOC patients treated with olaparib + durvalumab + bevacizumab in BOLD trial (NCT04015739), as an external validation dataset.
The results presented here should be analyzed with caution due to several limitations. This is the post-hoc retrospective pooled analysis of two single-arm open-label phase II trials. Moreover, KELIM-PARP was assessable in only 87% of study patients, because there were not enough available CA-125 values for the other ones. Of note, this percentage remains higher than those of patients assessable for the GCIG CA-125 response (∼59%), due to the complexity of the Rustin et al. algorithm. The reduced number of patients evaluable for the GCIG CA-125 response led us to eliminate it for multivariable tests in order to maintain the statistical power. Nevertheless, the final multivariable analysis with all the covariates found to be significant in univariable tests showed that this criterion was not significant after backward elimination procedure. Beyond HRD status, other biomarkers might have been relevant for prognostic analyses, such as the mismatch repair status.40 However, these covariates were not available in the datasets. Another limiting point relates to the 100 day time-window required for KELIM-PARP assessment, which obliges to apply a landmark analysis at 100 days, and excludes all patients who progressed within this time window, representing 26% of patients for PFS analysis. On the other hand, the objective of KELIM-PARP calculation is to identify patients who would experience long PFS while treated with rucaparib. If KELIM-PARP predictive value was confirmed, patient KELIM-PARP could be easily calculated online. In the future, technologies of proteomics may help.
In summary, this proof-of-concept study suggests that the early longitudinal kinetics of CA-125 during the first 100 days of treatment with rucaparib, are associated with subsequent radiological response and PFS in patients with recurrent ovarian cancer. The mathematical modeling-based approach, found to be useful for characterizing the tumor primary chemosensitivity in patients treated with platinum-based chemotherapy, may also be relevant for patients treated with PARP inhibitor-based chemotherapy-free regimens in recurrent setting. The modeled CA-125 kinetic parameter KELIM-PARP might represent a pragmatic numeric tool, complementary to platinum-sensitivity and HRD status, for identifying the patients who will derive the maximum benefit from forefront combination regimens based on PARP inhibitor, when the identification of efficacy biomarker is challenging. Additional studies on clinical trials and meta-analyses are warranted to confirm this assumption.
Contributors
Conceptualisation: BY.
Data curation: OC, BY.
Formal analysis: OC.
Funding acquisition: BY.
Investigation: All.
Methodology: BY.
Project administration: BY.
Resources: OC, BY.
Software: BY.
Supervision: OC, BY.
Validation: All.
Visualisation: OC, BY.
Writing – original draft: OC, BY.
Writing – review & editing: All.
Final validation: All.
All authors read and approved the final version of the manuscript.
Data sharing statement
Clovis Oncology (Clovis) is committed to ensuring that health care professionals (HCPs), researchers, trial participants, regulators, and other individuals or parties can access clinical trial data. Upon request, Clovis will provide access to clinical trial data from Clovis-sponsored trials in response to unsolicited requests for scientifically or clinically valid research proposals.
Clovis will consider requests from qualified researchers. To request data from Clovis, please contact medinfo@clovisoncology.com or a representative from Clovis's Medical Affairs team.
Once Clovis receives your request, a committee comprised of subject matter experts that may include nonclinical researchers, clinical scientists, and/or biostatisticians will evaluate your proposal. Requests for data will be evaluated using the following criteria.
-
•
Is your nonclinical or clinical research question clearly stated?
-
•
Does your question have a valid scientific and/or clinical rationale? Is it achievable?
-
•
Does the researcher have the expertise to answer the research question?
-
•
Does your proposal have a valid statistical analysis plan?
-
•
What do you intend to do with the data? Do you have a plan to present or publish the data at a scientific meeting and/or in a peer-reviewed journal?
-
•
Will the research or data be conducted and disclosed in good faith?
Although Clovis will make a reasonable effort to fulfill all requests for data, there may be instances in which Clovis may not be able to retrieve or deliver data. Reasons may include, but are not limited to, permission, patient privacy, local or regional laws or regulations, competitive reasons, and/or conflicts of interest.
If additional approval is required (e.g., permission or approval from a research institution, institutional review board, ethics committee, or other body), it is up to the researcher or health care professional to obtain this secondary approval.
Clovis Oncology adheres to the principles for responsible data sharing that are described in local and regional guidelines, including the European Federation of Pharmaceutical Industries and Associations (EFPIA) and the Pharmaceutical Research and Manufacturers of America (PhRMA). Clovis only provides de-identified data and takes every measure to ensure and safeguard patient and provider confidentiality.
Terms and conditions
-
1.
Recipient shall not use or disclose data in a manner that will violate local rules or regulations of the HCP's country.
-
2.
Recipient shall not use or disclose data in a fashion other than as permitted by Clovis or required by law.
-
3.
Recipient shall report any use or disclosure of the data to Clovis within five business days or when recipient becomes aware of such use or disclosure.
-
4.
All HCPs will adhere to local laws regarding patient privacy, data sharing, and confidentiality. Clovis will not provide information that can help identify a patient (e.g., name, address, date of birth, social security number, etc.).
-
5.
Recipient will not use data, either alone or in concert with any other information, to make any effort to identify or contact individuals who are or may be the sources of data without specific written approval from the HCP and/or other bodies (e.g., institutional review board).
-
6.
By signing this agreement, the recipient provides assurance that relevant institutional policies and applicable federal, state, or local laws and regulations are followed.
Declaration of interests
OC: None. Employee of Lyon University Hospital.
BY: Consulting for MSD, Astra-Zeneca, GSK-TESARO, BAYER, Roche-Genentech, ECS Progastrine, Novartis, LEK, Amgen, Clovis Oncology, Merck Serono, BMS, SEAGEN, Myriad.
SG, KKL, LM: Employees of Clovis Oncology.
Other coauthors: They disclose no conflict of interest.
Acknowledgements
The present study was supported by Clovis Oncology with a grant to academic research association.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2023.104477.
Appendix A. Supplementary data
References
- 1.Baert T., Ferrero A., Sehouli J., et al. The systemic treatment of recurrent ovarian cancer revisited. Ann Oncol. 2021;32(6):710–725. doi: 10.1016/j.annonc.2021.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Colombo N., Sessa C., Bois A.D., et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Int J Gynecol Cancer. 2019;30(5):672–705. doi: 10.1093/annonc/mdz062. [DOI] [PubMed] [Google Scholar]
- 3.Tew W.P., Lacchetti C., Ellis A., et al. PARP inhibitors in the management of ovarian cancer: ASCO guideline. J Clin Oncol. 2020;38(30):3468–3493. doi: 10.1200/JCO.20.01924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swisher E.M., Lin K.K., Oza A.M., et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): an international, multicentre, open-label, phase 2 trial. Lancet Oncol. 2017;18(1):75–87. doi: 10.1016/S1470-2045(16)30559-9. [DOI] [PubMed] [Google Scholar]
- 5.Domchek S.M., Aghajanian C., Shapira-Frommer R., et al. Efficacy and safety of olaparib monotherapy in germline BRCA 1/2 mutation carriers with advanced ovarian cancer and three or more lines of prior therapy. Gynecol Oncol. 2016;140(2):199–203. doi: 10.1016/j.ygyno.2015.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oza A.M., Tinker A.V., Oaknin A., et al. Antitumor activity and safety of the PARP inhibitor rucaparib in patients with high-grade ovarian carcinoma and a germline or somatic BRCA1 or BRCA2 mutation: integrated analysis of data from Study 10 and ARIEL2. Gynecol Oncol. 2017;147(2):267–275. doi: 10.1016/j.ygyno.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 7.Kaufman B., Shapira-Frommer R., Schmutzler R.K., et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol. 2015;33(3):244–250. doi: 10.1200/JCO.2014.56.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rucaparib FDA label. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/209115s004lbl.pdf
- 9.Olaparib FDA label. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208558s014lbl.pdf
- 10.Swisher E.M., Kwan T.T., Oza A.M., et al. Molecular and clinical determinants of response and resistance to rucaparib for recurrent ovarian cancer treatment in ARIEL2 (Parts 1 and 2) Nat Commun. 2021;12(1):2487. doi: 10.1038/s41467-021-22582-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gou R., Dong H., Lin B. Application and reflection of genomic scar assays in evaluating the efficacy of platinum salts and PARP inhibitors in cancer therapy. Life Sci. 2020;261 doi: 10.1016/j.lfs.2020.118434. [DOI] [PubMed] [Google Scholar]
- 12.Miller R.E., Leary A., Scott C.L., et al. ESMO recommendations on predictive biomarker testing for homologous recombination deficiency and PARP inhibitor benefit in ovarian cancer. Ann Oncol. 2020;31(12):1606–1622. doi: 10.1016/j.annonc.2020.08.2102. [DOI] [PubMed] [Google Scholar]
- 13.Ngoi N.Y.L., Tan D.S.P. The role of homologous recombination deficiency testing in ovarian cancer and its clinical implications: do we need it? ESMO Open. 2021;6(3) doi: 10.1016/j.esmoop.2021.100144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Domchek S.M., Postel-Vinay S., Im S.A., et al. Olaparib and durvalumab in patients with germline BRCA-mutated metastatic breast cancer (MEDIOLA): an open-label, multicentre, phase 1/2, basket study. Lancet Oncol. 2020;21(9):1155–1164. doi: 10.1016/S1470-2045(20)30324-7. [DOI] [PubMed] [Google Scholar]
- 15.Freyer G., Floquet A., Tredan O., et al. 733P Bevacizumab (Bev), olaparib (Ola) and durvalumab (Durva) in patients with recurrent advanced ovarian cancer (AOC): the GINECO BOLD study. Ann Oncol. 2021;32(Supplement 5):S734–S735. [Google Scholar]
- 16.Revythis A., Limbu A., Mikropoulos C., et al. Recent insights into PARP and immuno-checkpoint inhibitors in epithelial ovarian cancer. Int J Environ Res Public Health. 2022;19(14):8577. doi: 10.3390/ijerph19148577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghose A., Gullapalli S.V.N., Chohan N., et al. Applications of proteomics in ovarian cancer: dawn of a new era. Proteomes. 2022;10(2):16. doi: 10.3390/proteomes10020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lauby A., Colomban O., Corbaux P., et al. The increasing prognostic and predictive roles of the tumor primary chemosensitivity assessed by CA-125 elimination rate constant K (KELIM) in ovarian cancer: a narrative review. Cancers (Basel) 2021;14(1):98. doi: 10.3390/cancers14010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.You B., Freyer G., Gonzalez-Martin A., et al. The role of the tumor primary chemosensitivity relative to the success of the medical-surgical management in patients with advanced ovarian carcinomas. Cancer Treat Rev. 2021;100 doi: 10.1016/j.ctrv.2021.102294. [DOI] [PubMed] [Google Scholar]
- 20.You B., Colomban O., Heywood M., et al. The strong prognostic value of KELIM, a model-based parameter from CA 125 kinetics in ovarian cancer: data from CALYPSO trial (a GINECO-GCIG study) Gynecol Oncol. 2013;130(2):289–294. doi: 10.1016/j.ygyno.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 21.Colomban O., Tod M., Leary A., et al. Early modeled longitudinal CA-125 kinetics and survival of ovarian cancer patients: a gineco ago MRC CTU study. Clin Cancer Res. 2019;25(17):5342–5350. doi: 10.1158/1078-0432.CCR-18-3335. [DOI] [PubMed] [Google Scholar]
- 22.You B., Robelin P., Tod M., et al. CA-125 ELIMination rate constant K (KELIM) is a marker of chemosensitivity in patients with ovarian cancer: results from the phase II CHIVA trial. Clin Cancer Res. 2020;26(17):4625–4632. doi: 10.1158/1078-0432.CCR-20-0054. [DOI] [PubMed] [Google Scholar]
- 23.You B., Clamp A., Cook A., McNeish I., Colomban O. Differential benefit from fractionated dose-dense first-line chemotherapy for epithelial ovarian cancer (EOC) according to KELIM-evaluated tumor primary chemosensitivity: exploratory analyses of ICON-8 trial. J Clin Oncol. 2021;39(no 15_suppl):5530. [Google Scholar]
- 24.Corbaux P., You B., Glasspool R., et al. Survival prognostic and surrogate values of the early modeled CA-125 KELIM in newly diagnosed advanced ovarian cancer: data from the GCIG meta-analysis group. Ann Oncol. 2021;32(Supplement 5):S744. [Google Scholar]
- 25.You B., Sehgal V., Hosmane B., et al. CA-125 KELIM as a potential complementary tool for predicting veliparib benefit: an exploratory analysis from the VELIA/GOG-3005 study. J Clin Oncol. 2022;41(1):107–116. doi: 10.1200/JCO.22.00430. JCO2200430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rustin G.J., Nelstrop A.E., McClean P., et al. Defining response of ovarian carcinoma to initial chemotherapy according to serum CA 125. J Clin Oncol. 1996;14(5):1545–1551. doi: 10.1200/JCO.1996.14.5.1545. [DOI] [PubMed] [Google Scholar]
- 27.Rustin G.J., Vergote I., Eisenhauer E., et al. Definitions for response and progression in ovarian cancer clinical trials incorporating RECIST 1.1 and CA 125 agreed by the Gynecological Cancer Intergroup (GCIG) Int J Gynecol Cancer. 2011;21(2):419–423. doi: 10.1097/IGC.0b013e3182070f17. [DOI] [PubMed] [Google Scholar]
- 28.Lee C.K., Friedlander M., Brown C., et al. Early decline in cancer antigen 125 as a surrogate for progression-free survival in recurrent ovarian cancer. J Natl Cancer Inst. 2011;103(17):1338. doi: 10.1093/jnci/djr282. U7. [DOI] [PubMed] [Google Scholar]
- 29.Lee CK, Simes RJ, Brown C, et al. Prognostic nomogram to predict progression-free survival in patients with platinum-sensitive recurrent ovarian cancer. Br J Cancer. 2011;105(8):1144–1150. doi: 10.1038/bjc.2011.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee M., Kim E.J., Cho Y., et al. Predictive value of circulating tumor cells (CTCs) captured by microfluidic device in patients with epithelial ovarian cancer. Gynecol Oncol. 2017;145(2):361–365. doi: 10.1016/j.ygyno.2017.02.042. [DOI] [PubMed] [Google Scholar]
- 31.You B., Purdy C., Swisher E.M., et al. Identification of the ovarian cancer patients experiencing the highest benefit from bevacizumab in first-line setting based on their tumor intrinsic chemosensitivity (KELIM): GOG-0218 validation study. J Clin Oncol. 2022;40(34):3965–3974. doi: 10.1200/JCO.22.01207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dartois C., Brendel K., Comets E., et al. Overview of model-building strategies in population PK/PD analyses: 2002-2004 literature survey. Br J Clin Pharmacol. 2007;64(5):603–612. doi: 10.1111/j.1365-2125.2007.02975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Wagensveld L., Colomban O., Van der Aa M.A., Tod M., et al. Proceedings of ESMO 2020 virtual meeting abs 847P. 2020. The prognostic value of chemosensitivity, estimated by the modeled CA-125 KELIM, in ovarian cancer patients treated with neo-adjuvant chemotherapy in The Netherlands. [Google Scholar]
- 34.Rustin G.J.S., Quinn M., Thigpen T., et al. Re: new guidelines to evaluate the response to treatment in solid tumors (ovarian cancer) J Natl Cancer Inst. 2004;96(6):487–488. doi: 10.1093/jnci/djh081. [DOI] [PubMed] [Google Scholar]
- 35.Heller G., McCormack R., Kheoh T., et al. Circulating tumor cell number as a response measure of prolonged survival for metastatic castration-resistant prostate cancer: a comparison with prostate-specific antigen across five randomized phase III clinical trials. J Clin Oncol. 2018;36(6):572–580. doi: 10.1200/JCO.2017.75.2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beal S., Sheiner L.B., Boeckmann A., Bauer R.J. Icon; Ellicott City, MD, USA: 2009. NONMEM user’s guides (1989–2009) [Google Scholar]
- 37.Jonsson E.N., Karlsson M.O. Xpose - an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Methods Programs Biomed. 1999;58(1):51–64. doi: 10.1016/s0169-2607(98)00067-4. [DOI] [PubMed] [Google Scholar]
- 38.Kristeleit R.S., Lisyanskaya A., Fedenko A., et al. Proceedings of SGO 2021 Annual Meeting (Society of Gynecologic Oncology 2021 virtual annual meeting; March 19-25, 2021) Abs 11479. 2021. Rucaparib versus chemotherapy in patients with advanced, relapsed ovarian cancer and a deleterious BRCA mutation: efficacy and safety from ARIEL4, a randomized phase III study. [Google Scholar]
- 39.Kristeleit R. Proc SGO 2022, Abs 11479. 2022. Rucaparib versus chemotherapy in patients with advanced, relapsed ovarian cancer and a deleterious BRCA mutation: efficacy and safety from ARIEL4, a randomized phase III study. [Google Scholar]
- 40.Boussios S., Rassy E., Moschetta M., et al. BRCA mutations in ovarian and prostate cancer: bench to bedside. Cancers (Basel) 2022;14(16):3888. doi: 10.3390/cancers14163888. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.