Abstract
The conclusions of the European Food Safety Authority (EFSA) following the peer review of the initial risk assessments carried out by the competent authorities of the rapporteur Member State Germany and co‐rapporteur Member State France for the pesticide active substance S‐metolachlor are reported. The context of the peer review was that required by Commission Implementing Regulation (EU) No 844/2012, as amended by Commission Implementing Regulation (EU) No 2018/1659. In September 2022, the European Commission asked EFSA to deliver its conclusion on the available outcomes of the assessments in all areas excluding the full assessment of endocrine disrupting properties as several critical areas of concern related to the protection of the environment were identified. The conclusions were reached on the basis of the evaluation of the representative uses of S‐metolachlor as a herbicide on maize and sunflower. The reliable end points, appropriate for use in regulatory risk assessment are presented. Missing information identified as being required by the regulatory framework is listed. The concerns identified are presented.
Keywords: S‐metolachlor, peer review, risk assessment, pesticide, herbicide
Summary
Commission Implementing Regulation (EU) No 844/2012, as amended by Commission Implementing Regulation (EU) No 2018/1659, lays down the procedure for the renewal of the approval of active substances submitted under Article 14 of Regulation (EC) No 1107/2009. The list of those substances is established in Commission Implementing Regulation (EU) No 686/2012. S‐metolachlor is one of the active substances listed in Regulation (EU) No 686/2012.
In accordance with Article 1 of Regulation (EU) No 844/2012, the rapporteur Member State (RMS), Germany, and co‐rapporteur Member State (co‐RMS), France, received an application from Syngenta Crop Protection AG for the renewal of approval of the active substance S‐metolachlor.
An initial evaluation of the dossier on S‐metolachlor was provided by the RMS in the renewal assessment report (RAR), and subsequently, a peer review of the pesticide risk assessment on the RMS evaluation was conducted by EFSA in accordance with Article 13 of Commission Implementing Regulation (EU) No 844/2012, as amended by Commission Implementing Regulation (EU) No 2018/1659.
In October 2020, expert meetings in the areas of mammalian toxicology, residues, environmental fate and ecotoxicology were held. EFSA requested the applicant to provide further information on the endocrine disrupting (ED) properties of S‐metolachlor in accordance with Article 13(3a) of Implementing Regulation (EU) No 844/2012, setting a deadline of 3 May 2023. In July 2022, EFSA informed the Commission that several critical areas of concern related to the protection of the environment have been identified, concerning contamination of groundwater and risks to mammals. On 27 September 2022, prior to completion of the peer review process, EFSA was mandated by the European Commission to deliver its conclusion on the available outcomes of the assessments in all areas except the assessment of the ED properties as several critical areas of concern related to the protection of the environment have been identified concerning contamination of groundwater and risks to mammals.
The following conclusions are derived.
The use of S‐metolachlor according to the representative uses, as proposed at EU level, as a herbicide, applied by foliar field spraying, on maize, and sunflower, results in a sufficient herbicidal efficacy against the target weeds.
The assessment of the data package revealed no issues that could not be finalised or that need to be included as critical areas of concern with respect to identity, physical and chemical properties and analytical methods. Two relevant impurities have been identified needing to be specified with a maximum content of 0.08 g/kg for each. It should be noted that the levels of these impurities in the representative batches and in the provided quality control data were above this level.
In the area of mammalian toxicology, an issue not finalised was identified for the risk assessment of two human metabolites (unique and disproportionate) for which further in vitro comparative metabolism data should be provided.
In the area of residues, the consumer dietary risk assessment could not be finalised as the residue definition for risk assessment for rotational crops proposed as ‘metolachlor including other mixtures of constituent isomers, including S‐metolachlor (sum of isomers)’ is provisional in view of the identified data gaps. In consequence, the livestock exposure assessment could also not be finalised as significant residues may occur in feed items derived from rotational crops.
The data available on environmental fate and behaviour are sufficient to carry out the required environmental exposure assessments at EU level for the representative uses, with the notable exception that information is missing to address the effect of water treatments processes on the nature of the residues that might be present in surface water when surface water is abstracted for the production of drinking water. This has led to the consumer risk assessment not being finalised regarding treatment of surface water. Critical areas of concern have been identified as the representative uses are shown to contaminate groundwater (by both the active substance and the groundwater relevant metabolites SYN547977, ESA (CGA354743), OXA (CGA51202), CGA50720, CGA368208, NOA436611, CGA357704, NOA413173, SYN542488, SYN542489, SYN542490, SYN542491, SYN542492, SYN542607, SYN545026, SYN545027 and SYN547969).
A critical area of concern was identified for mammals since high risk to earthworm‐eating mammals via secondary poisoning was concluded for all representative uses. In the absence of a study, the risk assessment for aquatic organisms could not be finalised. In the absence of toxicity data with sensitive species, the risk for non‐target terrestrial plants could not be finalised.
Background
Commission Implementing Regulation (EU) No 844/20121, as amended by Commission Implementing Regulation (EU) No 2018/16592, (hereinafter referred to as ‘the Regulation’), lays down the provisions for the procedure of the renewal of the approval of active substances, submitted under Article 14 of Regulation (EC) No 1107/20093. This regulates for the European Food Safety Authority (EFSA) the procedure for organising the consultation of Member States, the applicant(s) and the public on the initial evaluation provided by the rapporteur Member State (RMS) and co‐rapporteur Member State (co‐RMS) in the renewal assessment report (RAR), and the organisation of an expert consultation where appropriate.
In accordance with Article 13 of the Regulation, unless formally informed by the European Commission that a conclusion is not necessary, EFSA is required to adopt a conclusion on whether the active substance can be expected to meet the approval criteria provided for in Article 4 of Regulation (EC) No 1107/2009 within 5 months from the end of the period provided for the submission of written comments, subject to an extension of an additional 3 months where additional information is required to be submitted by the applicant(s) in accordance with Article 13(3). Furthermore, in accordance with Article 13(3a), where the information available in the dossier is not sufficient to conclude the assessment on whether the approval criteria for endocrine disruption (ED) are met, additional information can be requested to be submitted in a period of minimum 3 months, not exceeding 30 months, depending on the type of information requested.
In accordance with Article 1 of the Regulation, the RMS Germany and co‐RMS France received an application from Syngenta Crop Protection AG for the renewal of approval of the active substance S‐metolachlor. Complying with Article 8 of the Regulation, the RMS checked the completeness of the dossier and informed the applicant, the co‐RMS (France), the European Commission and EFSA about the admissibility.
The RMS provided its initial evaluation of the dossier on S‐metolachlor in the RAR, which was received by EFSA on 6 September 2018 (Germany, 2018).
In accordance with Article 12 of the Regulation, EFSA distributed the RAR to the Member States and the applicant, Syngenta Crop Protection AG, for consultation and comments on 29 November 2018. EFSA also provided comments. In addition, EFSA conducted a public consultation on the RAR. EFSA collated and forwarded all comments received to the European Commission on 29 January 2019. At the same time, the collated comments were forwarded to the RMS for compilation and evaluation in the format of a reporting table. The applicant was invited to respond to the comments in column 3 of the reporting table. The comments and the applicant's response were evaluated by the RMS in column 3.
The need for expert consultation and the necessity for additional information to be submitted by the applicant in accordance with Article 13(3) of the Regulation were considered in a telephone conference between EFSA, the RMS and the European Chemicals Agency (ECHA) on 10 July 2019. On the basis of the comments received, the applicant's response to the comments and the RMS's evaluation thereof, it was concluded that additional information should be requested from the applicant, and that EFSA should conduct an expert consultation in the areas of mammalian toxicology, residues, environmental fate and behaviour and ecotoxicology.
In addition, following a consultation with Member States in the Pesticides Peer Review Teleconferences 27 and 29 (October 2020), it was considered necessary to apply an additional clock stop of 30 months in accordance with Commission Implementing Regulation (EU) No 2018/1659, to be able to conclude whether the approval criteria for ED in line with the scientific criteria for the determination of endocrine disrupting (ED) properties, as laid down in Commission Regulation (EU) 2018/6054, are met. The deadline for the applicant to submit the additional ED data is on 3 May 2023.
The outcome of the telephone conference, together with EFSA's further consideration of the comments, is reflected in the conclusions set out in column 4 of the reporting table. All points that were identified as unresolved at the end of the comment evaluation phase and which required further consideration, including those issues to be considered in an expert consultation, were compiled by EFSA in the format of an evaluation table.
The conclusions arising from the consideration by EFSA, and as appropriate by the RMS, of the points identified in the evaluation table, together with the outcome of the expert consultation and the written consultation on the assessment of additional information, where these took place, were reported in the final column of the evaluation table.
In July 2022, EFSA informed the European Commission that several critical areas of concern related to the protection of the environment have been identified during the peer review, concerning contamination of groundwater and risks to mammals. In the light of these critical concerns already identified, it was considered necessary to act without delay should it be clear that one or more of the approval criteria laid down in Regulation (EC) No 1107/2009 are not met, so that further extension of approval, leading to continued presence of a potentially dangerous substance on the market for longer than strictly necessary is avoided. Given the critical concerns identified, it was clear that the outcome of the assessment of ED properties could not alter the overall outcome of the renewal procedure. In this context and although the peer review process is not yet fully completed, with the assessment of the ED properties according to point 3.6.5 and/or point 3.8.2 of Annex II to Regulation (EC) No 1107/2009 remaining pending, given that all other areas of the assessment were completed, the European Commission asked EFSA to deliver its conclusion excluding the full assessment of ED properties since several critical areas of concern related to the protection of the environment were identified concerning contamination of groundwater and risks to mammals. EFSA was requested to complete this mandate by 31 January 2023.
Based on that mandate, EFSA prepared a draft conclusion in November 2022 summarising the outcome of the peer review of the risk assessment of the active substance and the formulation for the representative uses of S‐metolachlor as a herbicide on maize and sunflower, as proposed by the applicant, excluding the assessment of the ED properties. In accordance with Article 12(2) of Regulation (EC) No 1107/2009, risk mitigation options identified in the RAR and considered during the peer review, if any, are presented in the conclusion.
A final consultation on the conclusions arising from the peer review of the risk assessment took place with Member States via a written procedure in December 2022–January 2023.
A list of the relevant end points for the active substance and the formulation is provided in Appendix B. In addition, the considerations as regards the cut‐off criteria for S‐metolachlor according to Annex II of Regulation (EC) No 1107/2009 are summarised in Appendix A.
A key supporting document to this conclusion is the peer review report (EFSA, 2023), which is a compilation of the documentation developed to evaluate and address all issues raised in the peer review, from the initial commenting phase to the conclusion. The peer review report comprises the following documents, in which all views expressed during the course of the peer review, including minority views, where applicable, can be found:
the comments received on the RAR;
the reporting table (18 July 2019);
the evaluation table (19 January 2023);
the reports of the scientific consultation with Member State experts (where relevant);
the comments received on the assessment of the additional information (where relevant);
the comments received on the draft EFSA conclusion.
Given the importance of the RAR, including its revisions (Germany, 2023), and the peer review report, both documents are considered as background documents to this conclusion and thus are made publicly available.
It is recommended that this conclusion and its background documents would not be accepted to support any registration outside the EU for which the applicant has not demonstrated that it has regulatory access to the information on which this conclusion report is based.
The active substance and the formulated product
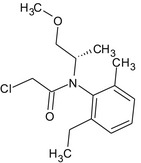
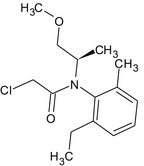
S‐metolachlor is the ISO common name for a reaction mixture of 80–100% 2‐chloro‐2′‐ethyl‐N‐[(1 S)‐2‐methoxy‐1‐methylethyl]‐6′‐methylacetanilide and 20–0% 2‐chloro‐2′‐ethyl‐N‐[(1R)‐2‐methoxy‐1‐methylethyl]‐6′‐methylacetanilide (IUPAC).
The formulated product for the representative uses in the context of the evaluation was ‘A9396G’, an emulsifiable concentrate (EC) containing 960 g/L S‐metolachlor.
The representative uses evaluated were hydraulic foliar spray application on maize and sunflower against annual grasses. Full details of the GAPs can be found in the list of end points in Appendix B.
Data were submitted to conclude that the use of S‐metolachlor according to the representative uses proposed at EU level results in a sufficient herbicidal efficacy against the target weeds, following the guidance document SANCO/2012/11251‐rev. 4 (European Commission, 2014b).
Conclusions of the evaluation
1. Identity, physical/chemical/technical properties and methods of analysis
The following guidance documents were followed in the production of this conclusion: European Commission, 2000a, 2000b, 2010.
The proposed specification for S‐metolachlor is based on batch data from industrial scale production and quality control data. The proposed minimum purity of the technical material is 960 g/kg (total content) with minimum content of S‐isomer 840 g/kg and maximum content of R‐isomer up to 130 g/kg, which complies with the ISO definition of the substance. 2‐chloro‐N‐(2‐ethyl‐6‐methylphenyl)acetamide (impurity 3, CGA13656) and 2,2‐dichloro‐N‐(2‐ethyl‐6‐methylphenyl)‐N‐[(2S)‐1‐methoxypropan‐2‐yl]acetamide (impurity 6, CGA50259) are considered relevant impurities with maximum content of 0.08 g/kg for each (see Section 2). It should be noted that the levels of these impurities in the representative batches and in the provided quality control data were above this level. The batches used in the (eco) toxicological assessment support the updated reference specification as proposed by the applicant but do not support the original reference specification (see Sections 2 and 5). Therefore, based on the data for renewal of the approval, it is proposed to update the reference specification. There is no FAO specification available for S‐metolachlor.
The main data regarding the identity of S‐metolachlor and its physical and chemical properties are given in Appendix B.
Adequate methods are available for the generation of data required for the risk assessment. Methods of analysis are available for the determination of the active substance in the technical material, in the formulation for representative uses and for the determination of the significant impurities in the technical material. Impurities 3 and 6 were concluded as relevant impurities as a consequence, data gaps for spectral data, methods for their determination in the formulation and data on their content before and after storage of the formulation were set (see Section 10). In addition, a data gap for more precise method(s) for their determination in the technical material with a LOQ appropriate for the specification levels was set (see Section 10).
The components of the residue definition in food and feed of plant origin (metolachlor including other mixtures of constituent isomers including S‐metolachlor (sum of isomers)) can be monitored by a quick, easy, cheap, effective and safe method (QuEChERS) using liquid chromatography with tandem mass spectrometry (LC–MS/MS) with a LOQ of 0.01 mg/kg in all commodity groups. The efficiency of the extraction procedures used was not verified but not required, since residues above LOQ in all matrix groups, as a result of the representative uses, were not found.
The components of the provisional residue definition (metolachlor including other mixtures of constituent isomers including S‐metolachlor (sum of isomers)) in food of animal origin (see Section 3) can be determined by QuEChERs using LC–MS/MS with a LOQ of 0.01 mg/kg in all animal matrices. The efficiency of the extraction procedures used was not verified. Whether the extraction efficiency needs to be addressed is pending upon the finalisation of the livestock exposure assessment and whether residues above the LOQ are expected in animal matrices (see Section 3).
Metolachlor (mixture of constituent isomers) in soil can be analysed by LC–MS/MS with LOQ of 0.01 mg/kg. Metolachlor (mixture of constituent isomers), OXA (CGA51202), ESA (CGA354743), SYN547977, SYN542492, CGA40172 and CGA41507 in water can be monitored by LC–MS/MS with LOQs of 0.01 μg/L for metolachlor and 0.05 μg/L for the metabolites. Metabolites CGA357704, CGA368208, CGA50720, NOA413173 and NOA436611 can be determined in water by LC–MS/MS with LOQs of 0.05 μg/L for each metabolite; however, an ILV of this method was missing (data gap, See Section 10). Methods were not available for the other relevant groundwater metabolites (data gap, see Section 10). Appropriate LC–MS/MS and gas chromatography with electron capture detection (GC‐ECD) methods exist for monitoring of metolachlor (mixture of constituent isomers) residue in air with LOQs of 4.5 and 5 μg/m3, respectively.
QuEChERs using the LC–MS/MS method can be used for monitoring of metolachlor (mixture of constituent isomers) residue in body fluids (blood) and tissues with LOQs of 0.01 mg/kg. However, the residue definition for monitoring in body fluids was concluded as metolachlor (mixture of constituent isomers) and the metabolite CGA46129 therefore a data gap for monitoring method for the metabolite CGA46129 in body fluids was set (see Section 10). In addition, it is noted that further assessment of a metabolite to be included in the residue definition for body fluids and tissues is needed (see Sections 2 and 10); therefore, a new monitoring method for biomonitoring might be required.
2. Mammalian toxicity
The toxicological profile of the active substance S‐metolachlor and its metabolites was discussed at the Pesticides Peer Review Teleconference 27 in October 2020. The assessment was based on the following guidance documents: European Commission, 2003, 2012; EFSA, 2014 and EFSA PPR Panel, 2012.
In the reference specification, the impurities 3 (CGA13656) and 6 (CGA50259) are currently considered toxicologically relevant based on genotoxic properties. For the impurity 3 tested alone, results were positive in an in vitro micronucleus test and equivocal in an in vivo micronucleus test. For the impurity 6 tested alone, results were positive in an Ames test and equivocal in an in vivo Comet assay. For both impurities 3 and 6, the applicability of the TTC approach should be considered, resulting in an acceptable value of 0.08 g/kg in the reference specification for an exposure at the ADI level.
The batches used in the toxicity studies were considered to be representative of the new technical specification as proposed by the applicant, and not of the reference specification from the first approval.
The analytical methods used in the toxicity studies were considered adequate (see Section 1).
The oral absorption of S‐metolachlor amounted to a value ≥ 85%. After absorption, the compound was strongly associated to red blood cells in the rat, with a wide distribution in well perfused organs without evidence of accumulation. Metabolic pathways of the racemic mixture (metolachlor) and the S‐enantiomer were similar, including mainly oxidative reactions and also glutathione conjugations. Excretion occurred mainly in faeces (via bile) and urine.
In the in vitro comparative metabolism study with rat and human microsomes, the metabolite M4 was evident in human microsomes only, while the metabolite M9 was shown as produced in amounts min 3 times higher in human microsomes than in rat microsomes. For these two metabolites, further investigations of in vitro comparative metabolism in other key species (e.g. mice, rabbits and dogs), chemical identification and toxicological assessment should be provided (data gap) in order to conclude on the risk assessment (issue not finalised).5
The proposed residue definition for body fluids is metolachlor and the metabolite CGA46129. Considering the literature findings on human biomonitoring, it should be further investigated if (S‐) metolachlor mercapturate is a major urinary metabolite of S‐metolachlor in humans (reaching levels higher than 10% of the administered dose) in order to conclude if it should also be included in the residue definition for body fluids (data gap, see Section 10).
Comparing acute toxicity studies, short‐term toxicity in rats and dogs, and developmental toxicity in rats and rabbits' studies, the results were comparable between metolachlor and S‐metolachlor. The experts agreed that there was sufficient evidence to bridge the missing data for S‐metolachlor from metolachlor studies (1‐year dog, long‐term, carcinogenicity and multigeneration studies).6
With regard to acute toxicity, S‐metolachlor showed a low toxicity profile in the available studies when administered orally, dermally or by inhalation, and no skin or eye irritating properties while it has a harmonised classification as Skin Sensitiser Cat. 1.7 Phototoxicity cannot be concluded on the basis of the available test since the compound is an UVB absorber and UVB radiations were not tested (data gap, see Section 10).
In the short‐term toxicity studies with S‐metolachlor, the target organs were the liver (rat and dog), the kidney (rat) and the blood (dog). For the rat, the overall no observed adverse effect level (NOAEL) for short‐term exposure is 20 mg/kg bw per day based on three 90‐day studies8 (2 with S‐metolachlor and 1 with metolachlor) where adverse effects included decreased body weight (gain), altered clinical chemistry parameters, increased liver and kidney weight and histopathological findings. For the dog, the overall short‐term NOAEL is 2.92 mg/kg bw per day based on the 6‐month study9 where adverse effects included decreased body weight (gain), altered red blood cell parameters and increased alkaline phosphatase. In a 21‐day dermal toxicity study with rabbits (with metolachlor), the NOAEL for systemic toxicity was 100 mg/kg bw per day based on increased liver weight.
The potential genotoxicity of S‐metolachlor was investigated in a test battery of both in vitro and in vivo studies addressing the different endpoints (mutagenicity, clastogenicity and aneugenicity). On the basis of the available results and weight‐of‐evidence considerations,10 S‐metolachlor was concluded unlikely to be genotoxic in humans. Testing for photogenotoxicity was not triggered for S‐metolachlor.
With regard to long term toxicity of metolachlor in rats, the identified NOAEL for systemic toxicity is 15 mg/kg bw per day based on decreased body weight (gain) and liver focal lesions; and the NOAEL for carcinogenicity is 15 mg/kg bw per day based on increased incidences of tumours in liver, thyroid, pituitary and nasal turbinates. For the liver tumours, the available mechanistic data were concluded as not sufficient to demonstrate the non‐relevance of rat liver tumours for humans.11 Additionally, some evidence of a link between exposure to metolachlor and increased incidences of liver tumours in humans was observed in epidemiological data. The classification proposed and agreed by the Committee for Risk Assessment (RAC) for S‐metolachlor is Carcinogen Category 2 (ECHA, 2022).
For the long‐term effects in mice, the available study with metolachlor presented many deviations and was concluded as not acceptable. It is noted that the RAC considered that the mouse study was inadequate for the assessment of carcinogenic potential of S‐metolachlor due to high mortality (data gap, see Section 10).
In the reproductive toxicity studies, adverse effects were not observed on the fertility and reproductive parameters in rats, while adverse developmental effects were observed in rats and rabbits. In the rat multigeneration study with metolachlor, both parental and offspring NOAELs were set at 17.7 mg/kg bw per day based on decreased body weight in pups; decreased food intake and increased relative thyroid and liver weights in parents. The NOAEL for reproductive toxicity was 54.9 mg/kg bw per day (top dose). From the rat developmental toxicity studies (one with S‐metolachlor and one with metolachlor), the relevant maternal NOAEL is 50 mg/kg bw per day based on clinical signs, decreased body weight (gain) and food consumption (observed after the initial doses); while the relevant developmental NOAEL is 300 mg/kg bw per day based on decreased foetal weight and increased incidence of skeletal variations and delayed ossification. For the rabbit developmental toxicity studies (one with S‐metolachlor and one with metolachlor), the relevant maternal NOAEL is 100 mg/kg bw per day based on clinical signs, decreased body weight (gain) and food consumption; while the relevant developmental NOAEL is 100 mg/kg bw per day based on decreased foetal weight and increased incidences of malformations and skeletal variations.
Based on signs of neurotoxicity observed in different toxicity studies and considering the weaknesses/limitations of the available database, the experts agreed that the neurotoxic potential of S‐metolachlor should be further investigated12 (data gap, see Section 10). A review of immune‐related parameters in the available toxicity studies and literature data showed some potential adverse effects on the immune system (e.g. increased globulins in rats, decreased leukocytes in rats and dogs, decreased spleen weight in offspring of the rat multigeneration study, decreased relative spleen weight and phagocytic index in mouse) (data gap, see Section 10).
All the toxicological reference values are derived applying a standard uncertainty factor of 100. The acceptable daily intake (ADI) is 0.03 mg/kg bw per day, based on decreased body weight (gain), altered red blood cell parameters and increased alkaline phosphatase in the 6‐month dog study with metolachlor at a dose level of 8.77 mg/kg bw per day. The acute reference dose (ARfD) is 0.5 mg/kg bw, based on clinical signs and decreased body weight (gain) after the initial doses in dams in the rat developmental toxicity study with S‐metolachlor at a dose level of 500 mg/kg bw per day. The acceptable operator exposure level (AOEL) is 0.03 mg/kg bw per day, on the same basis as the ADI and with no correction for oral absorption. The acute acceptable operator exposure level (AAOEL) is 0.5 mg/kg bw, on the same basis as the ARfD and with no correction for oral absorption.
Dermal absorption of S‐metolachlor in the product ‘A9396G’ has been assessed in an in vitro study with human skin. Based on the EFSA guidance of 2012, the dermal absorption values to be used for risk assessment are 0.4% for the concentrate, 12% for the 1:100 spray dilution, 8% for the 1:200 spray dilution and 13% for the 1:400 spray dilution. Values according to the EFSA guidance 2017 (EFSA, 2017) are also reported in Appendix B (and they might be further considered at MS level for national authorisations).
The non‐dietary exposure estimates for the operators are below the (A)AOEL with the use of gloves during mixing/loading and application (in addition to standard workwear), when considering the highest application rate of 1,440 g a.s./ha, based on the German model and EFSA calculator (EFSA, 2014). For the workers, the use of workwear is sufficiently protective during the inspection activities according to the EFSA calculator; while for residents and bystanders, a buffer strip of 10 m or drift‐reducing nozzles are required to reduce the exposure estimates below the (A)AOEL, together with a refined value for dislodgeable foliar residues (DFRs) of 2.43 μg/cm2 per kg a.s. per ha based on a field study.
Among the metabolites identified in groundwater, some could be identified as unlikely to be genotoxic but due to general limitations and deficiencies of the submitted read‐across approach, it was not possible to conclude on their general toxicity profile except for OXA (CGA51202) (see Table 1). Additionally, the available data did not allow to exclude the same toxicological properties as the parent, i.e. those triggering the proposed classification as Carcinogen category 2. Therefore, all groundwater metabolites are considered relevant (see Sections 4 and 7 and Table 6). For the plant metabolite CGA133275, its general toxicity and genotoxic properties could not be concluded in the absence of data (data gap, see also Sections 3 and 9.1). For the other metabolites that may be considered as quantitatively relevant in feed items (CGA49750, CGA380168, CGA41638, sugar conjugate of CGA118243) and in animal matrices (CGA41638, CGA217497 and CGA43826/CGA46576), data are not available to address the genotoxicity nor the general toxicity.
Table 1.
Summary of the toxicological profile of the metabolites
| Metabolite | Source | Genotoxicity | Reference values (RV) |
|---|---|---|---|
|
OXA (and OXA S‐enantiomer CGA351916) |
GW | Unlikely to be genotoxic | Reference values of S‐metolachlor apply |
|
ESA CGA354743 (a) (and ESA S‐enantiomer, CGA376944) |
GW | Equivocal results for mutagenicity and clastogenicity (data gap) | No conclusion, pending data gap in genotoxicity |
| NOA413173 (a) | GW | Equivocal results for clastogenicity in vitro, lack of investigation of the aneugenicity potential (data gap) | No conclusion, pending data gap in genotoxicity |
| CGA368208 | GW | Negative for mutagenicity and clastogenicity, aneugenicity not investigated (data gap) | No conclusion, pending data gap in genotoxicity |
| CGA50720 | GW | Unlikely to be genotoxic | No conclusion |
| CGA357704 | GW | Unlikely to be genotoxic | No conclusion |
| SYN548164 [ammonium salt of SYN542491] | [GW] | Unlikely to be genotoxic | No conclusion |
| NOA436611 | GW | Negative for mutagenicity and clastogenicity, aneugenicity not investigated (data gap) | No conclusion |
| SYN548163 [ammonium salt of SYN542489] | [GW] | Negative for mutagenicity and clastogenicity, aneugenicity not investigated (data gap) | No conclusion |
| SYN542492 | GW | Unlikely to be genotoxic | No conclusion |
| SYN542488 | GW | Equivocal results for mutagenicity in vitro (data gap) | No conclusion |
| SYN548165 [ammonium salt of SYN542607] | [GW] | Negative for mutagenicity and clastogenicity, aneugenicity not investigated (data gap) | No conclusion |
It was noted during the MS written procedure on the draft conclusion that new genotoxicity studies are available and have been submitted for national assessment in France. Based on a national assessment of these additional studies, it was concluded that both metabolites are unlikely to be genotoxic (see EFSA, 2023).
Table 6.
Groundwater (a)
| Compound (name and/or code) |
> 0.1 μg/L at 1 m depth for the representative uses (b) Step 2 |
Biological (pesticidal) activity/relevance Step 3a. |
Hazard identified Steps 3b. and 3c. |
Consumer RA triggered Steps 4 and 5 |
Human health relevance |
|---|---|---|---|---|---|
| S‐metolachlor | No | Yes | Carc Cat 2 | – | Yes |
| SYN547977 | Yes all scenarios except maize triennial applications 1,250 g/ha at Sevilla. All other scenarios and use patterns >0.135 μg/L with values up to 1.673 μg/L | Yes |
Yes Genotoxic potential not investigated Carcinogenic potential not excluded |
No, as hazard identified at step 3b,c |
Yes |
| ESA (CGA354743), (CGA376944 for S‐enantiomer, CGA380168 for its Sodium Salt) | Yes all scenarios 7.479–148.387 μg/L | No |
Yes Mutagenicity in vitro and clastogenicity in vivo equivocal Carcinogenic potential not excluded |
No, as hazard identified at step 3b,c |
Yes |
| OXA (CGA51202) | Yes all scenarios 5.511–90.127 μg/L | No |
Yes Unlikely to be genotoxic Carcinogenic potential not excluded |
No, as hazard identified at step 3c |
Yes |
| CGA40172 |
Yes 0.111 μg/L 1/8 maize scenarios (pre‐emergence, 1,440 g/ha, annual), 0.133 μg/L 1/2 sunflower scenarios (1,440 g/ha, annual) |
No |
Yes Genotoxic potential not investigated Carcinogenic potential not excluded |
No, as hazard identified at step 3b,c |
Yes |
| CGA50720 | Yes all scenarios 0.596–9.41 μg/L | No |
Yes Unlikely to be genotoxic Carcinogenic potential not excluded |
No, as hazard identified at step 3c |
Yes |
| CGA368208 | Yes all scenarios 0.461–10.427 μg/L | No |
Yes Aneugenic potential not investigated Carcinogenic potential not excluded |
No, as hazard identified at step 3b,c |
Yes |
| CGA37735 |
Yes 0.119 & 0.147 μg/L 2/8 maize scenarios (pre‐emergence, 1,440 g/ha, annual), 0.116 μg/L 1/2 sunflower scenarios (1,440 g/ha, annual) |
No |
Yes Genotoxic potential not investigated Carcinogenic potential not excluded |
No, as hazard identified at step 3b,c |
Yes |
| NOA436611 | Yes all scenarios 4.108–71.191 μg/L | No |
Yes Aneugenic potential not investigated Carcinogenic potential not excluded |
No, as hazard identified at step 3b,c |
Yes |
| CGA357704 | Yes all scenarios 7.819–137.912 μg/L | No |
Yes Unlikely to be genotoxic Carcinogenic potential not excluded |
No, as hazard identified at step 3c | Yes |
| NOA413173 | Yes up to 4.2 μg/L in a relevant lysimeter | No |
Yes Clastogenicity in vitro equivocal Aneugenic potential not investigated Carcinogenic potential not excluded |
No, as hazard identified at step 3b,c |
Yes |
| SYN542488 | Yes up to 1.7 μg/L in a relevant lysimeter | No |
Yes Mutagenicity in vitro equivocal Carcinogenic potential not excluded |
No, as hazard identified at step 3b,c |
Yes |
| SYN542489 | Yes up to 5.1 μg/L in a relevant lysimeter | No |
Yes Aneugenic potential not investigated Carcinogenic potential not excluded |
No, as hazard identified at step 3b,c |
Yes |
| SYN542490 | Yes up to 3.5 μg/L in a relevant lysimeter | No |
Yes Genotoxic potential not investigated Carcinogenic potential not excluded |
No, as hazard identified at step 3b,c |
Yes |
| SYN542491 | Yes up to 3.2 μg/L in a relevant lysimeter | No |
Yes Unlikely to be genotoxic Carcinogenic potential not excluded |
No, as hazard identified at step 3c |
Yes |
| SYN542492 | Yes up to 2.1 μg/L in a relevant lysimeter | No |
Yes Unlikely to be genotoxic Carcinogenic potential not excluded |
No, as hazard identified at step 3c |
Yes |
| SYN542607 | Yes up to 2.4 μg/L in a relevant lysimeter | No |
Yes Aneugenic potential not investigated Carcinogenic potential not excluded |
No, as hazard identified at step 3b,c |
Yes |
| SYN545026 | Yes up to 1.7 μg/L in a relevant lysimeter | Data gap |
Yes Genotoxic potential not investigated Carcinogenic potential not excluded |
No, as hazard identified at step 3b,c |
Yes |
| SYN545027 | Yes up to 2.4 μg/L in a relevant lysimeter | Data gap |
Yes Genotoxic potential not investigated Carcinogenic potential not excluded |
No, as hazard identified at step 3b,c |
Yes |
| SYN547969 | Yes up to 2.0 μg/L in a relevant lysimeter | No |
Yes Genotoxic potential not investigated Carcinogenic potential not excluded |
No, as hazard identified at step 3b,c |
Yes |
Assessment according to European Commission guidance of the relevance of groundwater metabolites (2003).
FOCUS scenarios or a relevant lysimeter.
3. Residues
The assessment in the residue section is based on the following guidance documents: OECD, 2009, 2011, European Commission, 2003, 2011 and JMPR, 2004, 2007.
S‐metolachlor was discussed at the Pesticides Peer Review Teleconference 30 in October 2020.
Metabolism in primary crops was investigated in root crops (potatoes), pulses and oilseeds (soya bean) and cereals (maize). Pre‐ and early post‐emergence treatment was studied in soya bean with S‐metolachlor, and in potatoes with metolachlor. Comparative metabolism studies with S‐metolachlor and metolachlor were performed on maize and demonstrated a similar metabolic profile for both isomeric mixtures. S‐metolachlor and metolachlor were extensively metabolised in the immature parts of the plants. In soya bean, numerous minor metabolites, characterised mainly as polar aqueous compounds, accounted individually for very low proportions and concentrations in soya bean seeds (< 0.01 mg eq./kg and < 10% TRR). However, significant residue levels of CGA380168 were found in soya bean whole tops, dried hay and stalks (up to 15% TRR; 1.124 mg eq./kg). In maize immature plant parts, the major part of the radioactive residues was characterised as polar conjugated metabolites (32–72% TRR); metabolites CGA40172 and CGA41507 occurred at a level below 0.05 mg eq./kg in shoots (silage stage) and in stalks while identification of metabolites was not attempted in maize grain because of the very low residue levels. In potato tubers and tops/leaves, metolachlor was not detected. Only minor metabolites were identified in potato tubers (< 10% TRR; < 0.01 mg/kg), while in tops and leaves metabolites CGA41638 and sugar conjugates of CGA118243 were found at a level > 0.05 mg eq./kg.
A guideline‐compliant rotational crops metabolism study covering the intended maximal seasonal dose rate confirmed the metabolic picture observed in primary crops. For some soil metabolites (see PECsoil in the LOEP) accumulation in soil may be expected. If the tested application rate in the rotational crop metabolism study covers also the maximum PECaccu for the persistent soil metabolites is unclear and should have been assessed. S‐metolachlor was extensively degraded and found only in lettuce leaves (1% TRR; 0.001 mg eq./kg) at 30‐day plant‐back interval (PBI). In lettuce, the metabolite with the highest proportions and concentrations was OXA (likely the OXA S‐isomer CGA351916 in a study with S‐metolachlor but enantiomer specific analysis was not used) at PBI 120 days (11% TRR; 0.01 mg eq./kg). Metabolite CGA133275, free and its glucose and malonyl‐glucose conjugated forms were the predominant compounds of the total residues in radish tops (up to 19.3% TRR; 0.04 mg eq./kg at PBI 120 days), in spring wheat forage (up to 40% TRR; 0.06 mg eq./kg at PBI 120 days), in spring wheat fodder at maturity (straw and husks; up to 32.7% TRR; 0.26 eq./kg at PBI 120 days). In two residue field trials in rotational crops, conducted with S‐metolachlor, the residues of CGA133275 were below the LOQ of 0.01 mg/kg in all plant parts and at all PBIs. However, the analytical method used in these field trials did not include a hydrolysis step in the extraction procedure to release the conjugates of CGA133275, representing the major fraction of the residues of this compound as indicated by the metabolism study. Therefore, sufficient field trials should have been provided with rotational crops in NEU and SEU, analysing food and feed commodities for free and conjugated residues of CGA133275, using a validated analytical method with a hydrolysis step to release also conjugated CGA133275. In the absence of further evidence persistent soil metabolites are addressed by the rotational crops metabolism study, metabolites OXA [CGA51202], ESA [CGA354743], CGA40172, CGA368208, NOA436611 and CGA357704 will need also to be quantified in the rotational crops field trials (data gap in Section 9.1). This request is also supported by the fact that the genotoxicity potential of CGA133275 ESA [CGA354743], CGA368208, NOA436611 have not been fully addressed and that carcinogenic potential cannot be excluded for any of these metabolites (see Sections 2 and 9.1). Further assessment of residues in rotational crops is necessary.
For primary crops, and in the absence of measurable residue levels of the parent compound, a default residue definition is proposed for monitoring and risk assessment purposes as ‘metolachlor including other mixtures of constituent isomers, including S‐metolachlor (sum of isomers)’.
The default residue definition, as derived for primary crops, could only be provisionally applied for rotational crops (see Section 9.1). A final confirmation is pending upon the outcome of the identified data gaps for rotational to finalise the assessment of the significance of CGA133275, free and conjugated, in feed items of rotational crops, and further clarification if additional metabolites that are persistent in soil could also lead to measurable residues in rotational crops if they were present at the maximum PECaccu in the soil.
A sufficient number of residue field trials compliant with the critical GAP for maize and sunflower, covered by acceptable storage stability data and a validated analytical method demonstrated that the residues of S‐metolachlor were below the LOQ of 0.01 mg/kg.
Guideline‐compliant metabolism studies with laying hens and lactating goat were submitted. In all poultry and ruminant matrices, the parent compound S‐metolachlor was extensively metabolised. In poultry matrices most of the identified metabolites were very minor (< 10% TRR; < 0.001 mg eq./kg), except in liver where metabolites 1EX (β‐glucuronic acid conjugate of CGA41638), 2EX (dicarboxylic acid derivative of CGA41638) and 3EX (hydroxy metabolite of CGA41638) were found each at a level of ca. 0.1 mg eq./kg but below 10% of the TRR. In ruminants, metabolites CGA217497 and the cysteine conjugate of CGA43826 were predominant in milk (up to 35.4% TRR and 46.3% TRR, respectively) and in tissues (22% TRR in kidney and 30% TRR in liver, respectively) while the β‐glucuronide acid conjugate of CGA41638 was also a major compound of the total residues in kidney, muscle and fat (27% TRR, 17.2% TRR and 14.8% TRR, respectively).
Considering that the dietary burden was calculated only based on S‐metolachlor residue levels in maize and sunflower feed items and by‐products, the submitted poultry and ruminant metabolism studies were highly overdosed (> 1,000 N rate). (Geno)toxicity data are currently not required as the compounds identified as the pertinent metabolites in animal commodities based on their proportions (%) are expected to be found at trace concentrations at the calculated dietary burden. However, it is stressed that the potential carry‐over of the residues of CGA133275, free and conjugated from the rotational crops feed items to the animal commodities may need to be considered in the light of the outcome of the requested field trials analysing the magnitude of this compound in rotational crops (see data gap here above). The livestock exposure assessment cannot therefore be finalised (see Section 9.1).
Meanwhile, the residue definition for monitoring and risk assessment of products of animal origin is provisionally proposed as ‘metolachlor including other mixtures of constituent isomers, including S‐metolachlor (sum of isomers)’.
EFSA also emphasises that the submitted livestock metabolism studies conducted with S‐metolachlor may not properly reflect the realistic exposure situation for animals if forage/fodder uses were authorised in the Member States. Indeed, from the plant metabolism data, metabolites CGA49750, CGA380168, CGA41638, CGA357704, sugar conjugates of CGA118243 and CGA133275 (free and glucose/malonyl‐glucose conjugated) may occur in significant concentrations (> 0.05 mg/kg) in feed items (mainly tops and leaves of root crops and in soya bean forage, stalks, hay) while most of these metabolites were not observed in the metabolic pathway depicted in livestock when the animals are dosed with S‐metolachlor. It is recommended that if such specific feed uses representative of pulses and oilseeds crops group and tops/leaves of root crops are requested/authorised in the Member States, the actual occurrence of these metabolites in feed items, a revisited dietary burden calculation and the toxicological relevance of these metabolites may need to be assessed to address the potential carry‐over of these pertinent metabolites to the animal commodities.
A dairy ruminant feeding study with S‐metolachlor was considered acceptable despite some shortcomings of the analytical method used for the analysis of S‐metolachlor residues in animal matrices. The residue concentrations of the parent compound were below the LOQ (0.01 mg/kg) in milk and tissues for all the dosing levels. However, pending the outcome of the data gap to determine the magnitude of residues of CGA133275 (free and glucose/malonyl‐glucose conjugated) in the rotational crops and potential carry‐over of the residues of this compound into animal matrices, it cannot be concluded whether this feeding study can be representative of the actual exposure of the animals.
Considering the representative uses in maize and sunflower, a fish metabolism study is not triggered as the estimated intake by fish is below 0.1 mg/kg dry matter.
If data to determine the residues in pollen and bee products for human consumption resulting from residues taken up by honeybees from crops at blossom will be necessary is pending upon the magnitude of residues of CGA133275 and its conjugates in rotational crops.
The consumer dietary intake calculation is regarded as provisional in view of the data gaps identified to finalise the residue definition for risk assessment for rotational crops. Using the EFSA PRIMo rev. 3.1 model and considering the representative uses on maize and sunflower, the chronic dietary intake accounted for 0.2% of the ADI (NL toddler) while the highest acute intake was < 1% of the ARfD. In the context of this process for the renewal of the approval of S‐metolachlor, as the ADI value was lowered and an ARfD has been set, a screening assessment considering the maximum residue levels (MRLs) derived for the authorised uses under the Art 12 MRL review (EFSA, 2012) was carried out, using, where appropriate, the provisional residue definitions from the current peer review. The chronic dietary intake increased to 3% of the ADI (NL toddler) and the acute dietary intake accounted for 1% of the ARfD for pineapples.
The PECgw values calculated for numerous metabolites exceeded 0.75 μg/L (see Section 4 and Table 6). However, as these compounds were considered toxicologically relevant groundwater metabolites (see Section 2), the consumer risk assessment through drinking water was not carried out according to the current guidance document (European Commission, 2003). Finally, the consumer risk assessment is also not finalised regarding the nature of residues that may result from the treatment of surface water when it is abstracted for the production of drinking water (see Section 4 and Section 9.1).
4. Environmental fate and behaviour
S‐metolachlor was discussed at the Pesticides Peer Review Teleconference 28 in October 2020.
The rates of dissipation and degradation in the environmental matrices investigated were estimated using FOCUS (2006) kinetics guidance. In soil laboratory incubations under aerobic conditions in the dark, S‐metolachlor exhibited moderate to high persistence, forming the following eight metabolites which reached levels triggering assessment (2 × > 5% applied radioactivity (AR)): ESA (CGA354743, CGA376944 for S‐enantiomer, CGA380168 (S‐enantiomer Sodium Salt), max. 21.3%), OXA (CGA51202/CGA351916, max. 21.1% AR), CGA40172 (max. 6.5% AR), CGA50720 (max. 8.2% AR), CGA368208 (max. 7.6% AR), CGA37735 (max. 7.1% AR), NOA436611 (max. 9.1% AR) and CGA357704 (max. 21.9% AR), which respectively exhibited moderate to very high persistence (ESA), moderate to very high persistence (OXA), high persistence (CGA40172), low to moderate persistence (CGA50720), moderate to high persistence (CGA368208), very low to low persistence (CGA37735), moderate to very high persistence (NOA436611) and moderate to very high persistence (CGA357704). Mineralisation of the 14C‐phenyl radiolabelled ring to carbon dioxide accounted for 0.3–29% AR after 90 days. The formation of unextractable residues (not extracted by acetonitrile/water (sometimes acidified) then Soxhlet methanol/water or acidified acetone) for this radiolabel accounted for 5–45% AR after 90 days. Aerobic soil incubations in 7 soils dosed with metolachlor (racemate) where chiral analysis was carried out at the beginning of the experiments and a time interval at around the DT50 determined for the sum of isomers in each soil, indicated that preferential degradation of any of the four atropisomers had not taken place. In anaerobic soil incubations, S‐metolachlor formed the major metabolite CGA41507, the dechlorinated parent compound, with a max. of 44.2% AR. Taking into consideration the representative uses under evaluation in this conclusion, the formation of this metabolite in soil is considered not relevant for these uses. S‐metolachlor exhibited medium to high mobility in soil. The exhibited mobility of the metabolites was: very high for ESA, high to very high for OXA, medium to high for CGA40172, very high for CGA37735 and NOA436611 and high for CGA41507. In the absence of experimental data on the adsorption properties, for metabolites CGA357704, CGA50720 and CGA368208, a default worst‐case KFoc value of 1 mL/g was used in the exposure assessment. For S‐metolachlor and all major metabolites in soil, a pH dependency of degradation rate and of adsorption were not found nor expected. In satisfactory field dissipation studies carried out at nine sites in Germany, three in France, one in Italy and four in Switzerland (spray application to the soil surface on bare soil or maize covered plots) S‐metolachlor dissipated exhibiting low to moderate persistence. Sample analyses were only carried out for the parent S‐metolachlor (sum of isomers). For metabolites ESA and OXA, a justification for not providing the field dissipation studies due to their mobility was provided. Field dissipation studies under conditions representative of European agricultural conditions where metabolites CGA357704, CGA368208, CGA40172 and NOA436611 had been analysed for, at three different study locations with varying soils, were not available. This is identified as a data gap (see Section 10). Field study DegT50 values for S‐metolachlor were not available, only dissipation endpoints could be derived from the available field dissipation studies.
The leaching behaviour of S‐metolachlor and its metabolites was investigated in two outdoor lysimeter studies, both with duration of 3 years, and in two field leaching studies having the durations of 10 and 4 years, respectively.
In the lysimeter studies, several new metabolites were observed in lysimeter leachate, compared to the degradation products found in the soil laboratory incubations under aerobic conditions. In the first lysimeter study (Germany, 1,250 g a.s./ha/year), S‐metolachlor was detected at a max. annual average concentration of 0.08 μg/L during the first year, while for the other 2 years concentrations were < 0.01 μg/L. The max. annual average concentrations, expressed as mass of a.s. equivalents/L, were observed for the following metabolites: 28.0 μg a.s./L (ESA), 16.3 μg a.s./L (OXA), 7.8 μg a.s./L (CGA368208), 4.7 μg a.s./L (CGA50720), 5.1 μg a.s./L (CGA357704) and 0.98 μg a.s./L (CGA37735). In the second lysimeter (Switzerland, 1,500 g a.s./ha/year), S‐metolachlor was never detected. The metabolites identified, including their individual max. annual average concentrations expressed as mass equivalent of the a.s./L in the leachates were: 32.5 μg a.s./L (ESA), 26.5 μg a.s./L (OXA), 5.0 μg a.s./L (CGA368208), 1.1 μg a.s./L (CGA50720), 6.1 μg a.s./L (CGA357704), 2.8 μg a.s./L (NOA436611), 4.2 μg a.s./L (NOA413173), 4.2 μg a.s./L (SYN542489), 1.7 μg a.s./L (SYN542488), 3.5 μg a.s./L (SYN542490), 3.2 μg a.s./L (SYN542491), 2.1 μg a.s./L (SYN542492), 1.2 μg a.s./L (SYN542607), 1.7 μg a.s./L (SYN545026) and 2.4 μg a.s./L (SYN545027). The overall metabolites in the leachate of the lysimeter 2 with highest annual mean 14C concentration (year 2) after HR‐MS identification, showed the following concentrations expressed as mass equivalent of the a.s./L: 29.3 μg a.s./L (ESA), 24.1 μg a.s./L (OXA), 2.4 μg a.s./L (CGA368208), 5.3 μg a.s./L (CGA357704), 1.8 μg a.s./L (NOA436611), 3.0 μg a.s./L (NOA413173), 5.1 μg a.s./L (SYN542489), 0.8 μg a.s./L (SYN542488), 3.1 μg a.s./L (SYN542490), 1.2 μg a.s./L (SYN542491), 1.7 μg a.s./L (SYN542492), 2.4 μg a.s./L (SYN542607), 2.0 μg a.s./L (SYN547969) and 0.1 μg a.s./L (SYN547977). A data gap was identified because degradation and adsorption endpoints for the metabolites found only in the lysimeters (except for SYN547977), i.e. NOA413173, SYN542489, SYN542488, SYN542490, SYN542491, SYN542492, SYN542607 (only for degradation), SYN545026, SYN545027 and SYN547969, were not available (see Section 10). In aerobic laboratory soil incubations SYN547977 exhibited moderate to medium persistence and high to very high mobility in batch adsorption experiments.
A German field leaching study (province of Hessen) investigated the mobility of metolachlor/S‐metolachlor and the metabolites ESA and OXA with applications to maize at 1,500 g a.s./ha (metolachlor) or 1,250 g a.s./ha (S‐metolachlor), following seven applications between 1995 and 2003. It was stated that applications had also been made in some of the preceding years (1989, 1990, 1992 and 1994). The monitoring was performed in 14 groundwater wells, eight located downgradient and six upgradient, all outside the treated area (screen depths 1.37–3.86 m). S‐metolachlor was observed once at 0.11 μg/L in one well. ESA residues were always present in samples at 0.19–44.2 μg/L. OXA residues were always present in samples at 0.1–19 μg/L. A second field leaching study was conducted in a USA vulnerable area (Sherburne county Minnesota) cultivated with maize, applying S‐metolachlor at 3,000 g a.s./ha from 1996 to 1999. The monitoring was performed in nine sampling points including the control plot, formed by well/lysimeter clusters for a total of 34 lysimeters (ceramic suction cup samplers in the unsaturated zone) and 17 monitoring wells installed (where sampling was from the saturated zone 6–7.1 m below the soil surface). The annual average soil pore water concentration of S‐metolachlor, corrected for the lower dose rate of the proposed EU GAP, did not exceeded the 0.1 μg/L trigger. The annual average soil pore water concentration of ESA corrected for the lower dose rate of the EU GAP was 12.4 μg/L in the first year after the application in the 1.8 m depth lysimeter and relatively high (i.e.: 2.8–7.0 μg/L) during the first 2 years in the 2.7 m and 4 m depth lysimeters. These OXA residues corrected for the lower dose rate of the EU GAP never exceeded 10 μg/L as annual average concentration but was generally high in the first year of application with a max. annual average concentration of 6.1 μg/L in the 1.8 m depth lysimeter. In the 6 to 7.1 m depth well samples, concentrations calculated to the EU dose rates were < 0.1 μg/L for S‐metolachlor, 1.2–7.5 μg/L for ESA and < 0.1–2.5 μg/L for OXA.
In laboratory incubations in dark aerobic natural sediment water systems, S‐metolachlor exhibited moderate persistence, forming the major metabolites OXA (max. 17% AR in water, max. 5% in sediment and max. 21% in the whole system, with estimated very high persistence), ESA (max. 7% AR in water, max. 3% in sediment and max. 9% in the whole system, with estimated very high persistence) and CGA41507 (max 8% AR in water, 12% in sediment and 18% in the whole system with estimated very high persistence). The unextractable sediment fraction (not extracted by acetonitrile/water then Soxhlet with acidified acetone) was the major sink for the 14C‐phenyl ring radiolabel, accounting for max 57% AR and max 61% AR at the end of the two studies (respectively 180 and 362 days). Mineralisation of this radiolabel accounted for only max 3.1% and max 4.5% AR at the end of the two studies. The rates of decline of S‐metolachlor and metolachlor in laboratory sterile aqueous photolysis experiments were slow relative to that occurred in the aerobic sediment water incubations. No chromatographically resolved components (excluding metolachlor) accounted for > 5.5% AR. Chiral analysis in the sterile natural water test system dosed with metolachlor indicated that preferential degradation of any of the four atropisomers had not taken place.
The necessary surface water and sediment exposure assessments (predicted environmental concentrations (PEC) calculations) were carried out for the metabolites ESA, OXA, CGA40172, CGA50720, CGA368208, CGA37735, NOA436611, CGA41507 and CGA357704 using the FOCUS (FOCUS, 2001) step 1 and step 2 approach (version 3.2 of the Steps 1–2 in FOCUS calculator) for just pre‐emergence uses for the dose rate of 1,440 g a.s./ha as a worst case covering all the intended uses. For the active substance S‐metolachlor, appropriate step 3 (FOCUS, 2001) and step 4 calculations were available for all the representative uses. The step 4 calculations appropriately followed the FOCUS (2007, 2008) guidance, with no‐spray drift buffer zones of up to 20 m being implemented for the drainage scenarios (representing a 91–93% spray drift reduction), and combined no‐spray buffer zones with vegetative buffer strips of up to 20 m (reducing solute flux in run‐off by 80% and erosion runoff of mass adsorbed to soil by 95%) being implemented for the run‐off scenarios. The SWAN tool (version 4.0.1) and EVA 3 rev2e were appropriately used to implement these mitigation measures in the simulations. However, risk managers and others may wish to note that while run‐off mitigation is included in the step 4 calculations available, the FOCUS (2007) report acknowledges that for substances with KFoc < 2,000 mL/g (i.e. S‐metolachlor), the general applicability and effectiveness of run‐off mitigation measures had been less clearly demonstrated in the available scientific literature, than for more strongly adsorbed compounds.
The necessary groundwater exposure assessments were appropriately carried out using FOCUS (European Commission, 2014a) scenarios and the models PEARL 4.4.4 and PELMO 5.5.3 13 for the active substance and the metabolites OXA (CGA51202), ESA (CGA354743),14 CGA40172, CGA50720, CGA368208, CGA37735, NOA436611, CGA357704 and for metabolite SYN547977 found in the lysimeter studies. Single applications at rates of 1,250 and 1,440 g a.s./ha were considered, with applications every year, every second year and every third year (pre‐ and post‐emergent applications to maize and pre‐emergent applications to sunflower). The potential for groundwater exposure from the representative uses by S‐metolachlor above the parametric drinking water limit of 0.1 μg/L was concluded to be low in geoclimatic situations that are represented by all 8 pertinent FOCUS groundwater scenarios. For the groundwater relevant (see Sections 2 and 7) metabolites ESA, OXA, CGA368208, NOA436611, CGA357704, CGA50720 and SYN547977, 80th percentile annual average recharge concentrations moving below 1 m were calculated to be > 0.1 μg/L at all the FOCUS scenarios. SYN547977 is both human health groundwater relevant and herbicidally active (see Sections 2 and 7). This metabolite was only predicted to be < 0.1 μg/L when applied triennially at 1,250 g/ha post emergence on maize at just the Sevilla scenario. CGA37735 was only predicted > 0.1 μg/L at the Hamburg, Thiva and Piacenza scenarios. CGA40172 was only predicted > 0.1 μg/L at the Okehampton and Sevilla scenarios. These two metabolites were assessed as groundwater relevant (see Sections 2 and 7).
Following European Commission (2014a) guidance a targeted monitoring study was provided as the highest tier of groundwater risk assessment in order to clarify the leaching of S‐metolachlor and its metabolites ESA, OXA and herbicidally active SYN547977 to groundwater under realistic EU conditions. The monitoring wells from 121 reliable vulnerable sites were considered representative of a wide range of locations across Europe (wells were located in: Austria, Belgium, France, Germany, Hungary, Italy, the Netherlands, Poland, Romania, Slovakia and Spain) corresponding to a variety of environmental and agricultural conditions. The data set covered a period of 6 years, from Q1 2013 to Q3 2018, and more than 2,000 groundwater analyses for each substance were assessed. A quarterly sampling frequency was enacted at each well. The sampling well set up in the study design, was considered by the experts at the Pesticides Peer Review Teleconference 2815 to be in line with the spatial aspects of the exposure assessment goal/protection goal option 2, as described in Appendix 1 of the reference Gimsing et al. (2019). It should be noted that experts considered that as the sampling frequency at each site (quarterly) was quite low, that annual average concentrations for a monitored site should not be considered when using the results from this targeted monitoring exercise. Because sampling was from the saturated zone, samples taken 3 months apart were considered to already represent some temporal averaging.16 Farmer records of S‐metolachlor applications to their maize crops in designated fields up catchment of sampling wells were available. The summary of analytical results from the 121 monitoring sites (2013–2018) are reported in the following table (Table 2):
Table 2.
Analytical results from the 121 monitoring sites (2013–2018)
| S‐metolachlor | SYN547977 | ESA | OXA | ||
|---|---|---|---|---|---|
| Samples | Total number | 2215 | 2064 | 2299 | 2189 |
| Samples < LOQ (a) | 1,949 (88.0%) | 1,800 (87.2%) | 319 (13.9%) | 887 (40.5%) | |
| Samples ≥ 0.1 μg/L | 64 (2.9%) | 123 (6.0%) | 1,786 (77.7%) | 1,136 (51.9%) | |
| Samples ≥ 10 μg/L | Not calculated | Not calculated | 129 (5.6%) | 60 (2.7%) | |
| Wells | Total number | 119 | 121 | 119 | 117 |
| Wells < LOQ (a) | 84 (70.6%) | 80 (66.1%) | 2 (1.7%) | 10 (8.5%) | |
| Wells ≥ 0.1 μg/L | 24 (20.2%) | 25 (20.7%) | 113 (95.0%) | 97 (82.9%) | |
| Wells ≥ 10 μg/L | Not calculated | Not calculated | 25 (21.8%) | 14 (12.0%) |
LOQ: 0.01 μg/L for S‐metolachlor and 0.05 μg/L for ESA, OXA and SYN547977.
The study represents a detailed approach of a combined retrospective and prospective monitoring programme for groundwater exposure assessment, but as it is monitoring it represents the farmer practice (dose rate and frequency of application) at each location monitored. Therefore, an evaluation of the data with regards to the application rates (i.e. 1,250 or 1,440 g/ha) or to the application patterns (i.e.: annual, biennial or triennial) according to the representative uses defined in the GAP table was carried out. The approach for this was discussed and agreed at the Pesticides Peer Review Teleconference 28. This evaluation highlighted that the required minimum number of 20 sites (recommended in European Commission, 2014a guidance on the use of monitoring data) representing a significant area in Europe (applicant proposal agreed by the peer review monitored sites were attributed to FOCUS groundwater scenario climate zones, which represent a significant area) was satisfied only for the Hamburg FOCUS zone with 56 sites. The number of sites allocated to each of the other FOCUS zones was below 20. When combining the separate evaluation of the application rates and the application pattern, even for the FOCUS Hamburg scenario not enough sites were identified as available to draw a regulatory conclusion for the representative uses as defined in the GAP (for triennial applications only 13 sites remained for the 1,250 g/ha dose and 10 sites for the 1,440 g/ha dose). The summary of the statistical evaluation of the monitoring data related to the different FOCUS climate zones is reported in the Table 3 (conclusions on Okehampton, Porto and Sevilla scenarios were not possible as only one site was associated to the respective zones):
Table 3.
Summary of the statistical evaluation of the monitoring data related to the different FOCUS climate zones
| FOCUS zone | Châteudun | Hamburg | Kremsmünster | Piacenza | Thiva | |
|---|---|---|---|---|---|---|
| S‐metolachlor | ||||||
| Samples | Total number: | 274 | 1,070 | 247 | 259 | 315 |
| Samples < LOQ (a) | 229 (83.6%) | 988 (92.3%) | 205 (83.0%) | 229 (88.4%) | 270 (85.7%) | |
| Samples ≥ 0.1 μg/L | 5 (1.8%) | 27 (2.5%) | 17 (6.9%) | 5 (1.9%) | 10 (3.2%) | |
| Max value (μg/L) | 0.39 | 1.6 | 110.6 | 0.31 | 1.1 | |
| Wells | Total number: | 16 | 56 | 14 | 12 | 18 |
| Wells < LOQ (a) | 9 (56.3%) | 42 (75.0%) | 7 (50%) | 5 (41.7%) | 8 (44.4%) | |
| Wells ≥ 0.1 μg/L | 2 (12.5%) | 9 (16.1%) | 4 (28.6%) | 4 (33.3%) | 5 (27.8%) | |
| SYN547977 | ||||||
| Samples | Total number: | 247 | 952 | 261 | 260 | 294 |
| Samples < LOQ (a) | 229 (92.7%) | 796 (83.6%) | 195 (74.7%) | 242 (93.1%) | 288 (98.0%) | |
| Samples ≥ 0.1 μg/L | 18 (7.3%) | 156 (16.4%) | 66 (25.3%) | 18 (6.9%) | 6 (2.0%) | |
| Max value (μg/L) | 0.2 | 1.1 | 3.7 | 0.3 | 0.14 | |
| Wells | Total number: | 16 | 56 | 15 | 13 | 18 |
| Wells < LOQ (a) | 12 (75.0%) | 32 (57.1%) | 9 (60.0%) | 9 (69.2%) | 15 (83.3%) | |
| Wells ≥ 0.1 μg/L | 3 (18.8%) | 15 (26.8.%) | 5 (33.3%) | 1 (7.7%) | 1 (5.6%) | |
| ESA | ||||||
| Samples | Total number: | 294 | 1041 | 290 | 278 | 346 |
| Samples < LOQ (a) | 34 (11.6%) | 148 (14.2%) | 19 (6.6%) | 49 (17.6%) | 58 (16.8%) | |
| Samples ≥ 0.1 μg/L | 240 (81.6%) | 808 (77.6%) | 251 (86.6%) | 211 (75.9%) | 238 (68.8%) | |
| Samples ≥ 10 μg/L | 3 (1%) | 79(7.6%) | 34 (11.7%) | 0 (0%) | 13 (3.8%) | |
| Max value (μg/L) | 12 | 42.8 | 40.4 | 6.9 | 17.1 | |
| Wells | Total number: | 16 | 54 | 15 | 12 | 18 |
| Wells < LOQ (a) | 0 (0%) | 1 (1.9%) | 0 (0%) | 1 (7.7%) | 0 (0%) | |
| Wells ≥ 0.1 μg/L | 16 (100%) | 49 (90.7%) | 15 (100%) | 12 (92.3%) | 18 (100%) | |
| Wells ≥ 10 μg/L | 1 (6.3%) | 17 (31.5%) | 4 (26.7%) | 0 (0%) | 3 (16.7%) | |
| OXA | ||||||
| Samples | Total number: | 280 | 1015 | 262 | 276 | 310 |
| Samples < LOQ (a) | 132 (47.1%) | 313 (30.8%) | 82 (31.3%) | 165 (59.8%) | 159 (51.3%) | |
| Samples ≥ 0.1 μg/L | 127 (45.4%) | 641 (63.2%) | 165 (63.0%) | 80 (29.0%) | 116 (37.4%) | |
| Samples ≥ 10 μg/L | 0 (0%) | 34 (3.3%) | 13 (5.0%) | 0 (0%) | 13 (4.2%) | |
| Max value (μg/L) | 9.8 | 51.5 | 81.1 | 6.4 | 17.6 | |
| Wells | Total number: | 16 | 54 | 14 | 13 | 17 |
| Wells < LOQ (a) | 2 (12.5%) | 8 (14.8%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Wells ≥ 0.1 μg/L | 12 (75.0%) | 44 (81.5%) | 13 (92.9%) | 13 (100%) | 13 (76.5%) | |
| Wells ≥ 10 μg/L | 0 (0%) | 10 (18.5%) | 3 (21.4%) | 0 (0%) | 1 (5.9%) | |
LOQ: 0.01 μg/L for S‐metolachlor and 0.05 μg/L for ESA, OXA and SYN547977.
The following provides an overview of all the available information regarding the potential for groundwater exposure. FOCUS groundwater modelling indicates that S‐metolachlor when used according to the representative use assessed should not be present in groundwater above the parametric drinking water limit of 0.1 μg/L. However, at 24 of the 119 wells monitored (20%) for sites specifically selected to represent vulnerable situations, it was present in groundwater above the parametric limit. In general, public monitoring data from Belgium (up to 1.3% of samples), Slovenia (1.8% of samples) Switzerland (3% of samples), Italy (5% of samples) and Spain (6% of samples) metolachlor has been found above the parametric limit. (As this is general monitoring it will be the case that crops treated with S‐metolachlor will not necessarily have been present or had limited presence in some of the groundwater catchments where samples originated.) FOCUS groundwater modelling indicates herbicidally active and human health groundwater relevant SYN547977 has a high potential to be present in groundwater considering a kinetic formation fraction in soil (0.1) consistent with it having been present at less than 5% in laboratory soil incubations. This may be overestimating its actual soil formation, considering it was not determined as being present in these incubations. However, at 25 of the 121 wells monitored (21%) for sites specifically selected to represent vulnerable situations, it was present in groundwater at above the drinking water limit. SYN547977 has not been reported as having been analysed for in public monitoring data. Taken together, this information may be considered to indicate that the approval conditions relating to the protection of groundwater are not being respected. EFSA has therefore indicated this as a critical area of concern (see Section 9.2).
The FOCUS groundwater modelling, lysimeter study results, field leaching study results targeted monitoring data and general public monitoring data all indicate that groundwater relevant metabolites ESA and OXA will be in groundwater above the parametric drinking water limit of 0.1 μg/L from the representative uses. Lysimeter study results indicate that groundwater relevant metabolites NOA413173, SYN542488, SYN542489, SYN542490, SYN542491, SYN542492, SYN542607, SYN545026, SYN545027 and SYN547969 have the potential to be present in groundwater above this parametric limit.17 The FOCUS groundwater modelling indicates that the groundwater relevant metabolites CGA368208, NOA436611, CGA357704 and CGA50720 have the potential to be present in groundwater above this parametric limit at all pertinent FOCUS groundwater scenarios. EFSA has therefore indicated this as a critical area of concern regarding all the metabolites listed in this paragraph (see Section 9.2).
The applicant did not provide appropriate information to address the effect of water treatments processes on the nature of the residues that might be present in surface water and groundwater, when surface water or groundwater are abstracted for drinking water. This has led to the identification of a data gap and results in the consumer risk assessment not being finalised regarding treatment of surface water (see Section 9.1). Note as groundwater metabolites are relevant, uses would not be possible when groundwater exposure would result.
The PEC in soil, surface water, sediment and groundwater covering the representative uses assessed can be found in Appendix B. These PEC were all carried out on a sum of isomers basis. The available evidence from aerobic soil incubations and a sterile aqueous natural water photolysis experiment was that changes in the atropisomer composition of S‐metolachlor would not be expected. Information in this regard for metabolites: ESA, OXA, CGA40172, NOA436611, CGA357704, NOA413173, SYN542488, SYN542489, SYN542490, SYN542491, SYN542492, SYN542607, SYN545026, SYN545027, SYN547969 and CGA41507 was not available. Additional margins of safety in risk assessments for these metabolites should therefore be used. A key to the persistence and mobility class wording used, relating these words to numerical DT and Koc endpoint values can be found in Appendix C.
5. Ecotoxicology
The risk assessment was based on the following documents: European Commission (2002), SETAC (2001), EFSA (2009, 2013) and EFSA PPR Panel (2013).
Several aspects related to the risk assessment of S‐metolachlor were discussed at the Pesticide Peer Review Teleconference 29 (October 2020).
The batches used in the (eco)toxicity studies were considered to be representative of the new technical specification as proposed by the applicant, and not of the reference specification from the first approval. The results of comparable ecotoxicity studies conducted with metolachlor and S‐metolachlor showed similar toxicity.
Suitable acute and reproductive studies with S‐metolachlor where available for assessing the risk to birds. In addition, an acute study with a formulation equivalent to A9396G and several reproductive studies with metolachlor18 were also submitted. The endpoint for the long‐term risk assessment was agreed at the experts' meeting.19 A low acute and reproductive risk was concluded for all representative uses at the screening step and at Tier‐1, respectively.
For wild mammals, acute and reproductive studies with S‐metolachlor and metolachlor, respectively, were available. The reproductive endpoint was agreed at the experts' meeting.20 A low acute risk was concluded at the screening step for all representative uses whereas a high reproductive risk was indicated at Tier‐1 for all uses.21 The refined reproductive risk assessment was discussed at the experts' meeting.22 The experts agreed to:
Consider the wood mouse and the European brown hare and rabbit as key focal species for omnivorous and herbivorous mammals, respectively, based on the results of three monitoring studies in maize fields at early stages (BBCH 00–16);
Investigate whether the common vole should also be included as a key focal species for herbivorous mammals. After a further analysis,23 the common vole was proposed as a key focal species until additional evidence is available to exclude the occurrence of voles in maize at BBCH stages 17–18;
Refine the default value for the proportion of time spent in the treated field‐ (PT) for the omnivorous wood mouse;24
Accept the worst‐case foliar dissipation half‐time (DT50) from four studies performed in the central zone. Although the experts agreed that the refined DT50 would also cover the southern zone as the major route of dissipation was volatilisation, it could not be demonstrated that the temperature in the available residue decline studies was sufficiently representative of the temperature in the northern zone. Therefore, the revised DT50 was used to further refine the risk to herbivorous focal species for the post‐emergence uses in maize in the central and southern zones.
The refined assessment indicated low reproductive risk for omnivorous mammals (relevant for all representative uses of S‐metolachlor) and for herbivorous mammals in the central and southern zones (only relevant for the post‐emergence uses in maize), and high risk for herbivorous mammals in the northern zone. On this basis, a low risk could be concluded for all pre‐emergence uses in maize (BBCH 00–10) and the uses in sunflower (BBCH 00–09) and a high risk remained for the post‐emergence uses in maize (BBCH 11–18).
A qualitative assessment of the relevant plant metabolites of S‐metolachlor present in plants to which birds and wild mammals can be exposed to (CGA46576/CGA43826 and CGA380168) was available and low risk was concluded for all uses. A low risk from the consumption of S‐metolachlor contaminated water was indicated at the screening step for both birds and mammals. The risk to fish and earthworm‐eating birds due to secondary poisoning was concluded to be low for all representative uses. However, a high risk for earthworm‐eating mammals was indicated at Tier 1 for all representative uses. In the absence of sufficient evidence, 23 the risk could not be refined, leading to a critical area of concern (see Section 9.2).
Several valid studies with S‐metolachlor, metolachlor,25 A9396G and an equivalent formulation were available covering the relevant aquatic taxa (i.e. fish, aquatic invertebrates, algae, aquatic macrophytes and sediment dwelling organisms). A mesocosm study was also available as supporting information.
At the meeting, the acute endpoints for fish and aquatic invertebrates were agreed and the toxicity data for aquatic macrophytes was discussed.26 Considering the differences in methodology between the available studies and the limitations identified in the study with the most sensitive macrophyte species, Elodea canadensis,27 the experts agreed that it was not appropriate calculating a geomean and that a study with E. canadensis with a 14‐day exposure period is needed. In the absence of such data, the risk assessment to aquatic macrophytes could not be finalised (data gap and issue not finalised; see Section 9.1).
The outcome of the quantitative risk assessment for S‐metolachlor is summarised in Table 4. The acute risk to fish and aquatic invertebrates and the risk to sediment dwelling organisms was low for all representative uses at FOCUS Step 3 PECsw values. High chronic risk to fish and aquatic invertebrates and high risk to algae and aquatic plants was concluded for all uses of S‐metolachlor at FOCUS Step 3. By applying risk mitigation measures, low risk to algae, which was the most sensitive group in the absence of a valid endpoint for macrophytes, was indicated for all the representative FOCUS surface water scenarios except for R1/, R2/, R3/ and R4/stream (10 m buffer, resulting in 60% runoff reduction) and for R3/ and R4/stream (20 m vegetative buffer, resulting in 80% solute run‐off reduction) for the pre‐ and post‐emergence uses in maize and for R1/, R3/ and R4/stream (10 m buffer, resulting in 60% runoff reduction) and for R3/ and R4/stream (20 m vegetative buffer, resulting in 80% run‐off reduction) for the uses in sunflower, for which high risk was still indicated (although not for a majority of the scenarios).
Table 4.
Outcome of the aquatic risk assessment for the representative uses of S‐metolachlor at FOCUS Step 3
| Use | Fish | Aquatic invertebrates | Algae (a) | Aquatic macrophytes (b) | Sediment‐dwelling | |||
|---|---|---|---|---|---|---|---|---|
| Acute | Chronic | Acute | Chronic | |||||
| Maize |
1 × 1,440 g a.s./ha (BBCH 00–10 pre‐emergence) |
Low |
High (2/8 scenarios: R3/ and R4/ stream) |
Low |
High (3/8 scenarios: R1/,R3/ and R4/stream) |
High (8/8 scenarios: D3/ and D6/ditch; D4/, D5/, R1/, R2/, R3/ and R4/stream) |
High (8/8 scenarios: D3/ and D6/ditch; D4/, D5/, R1/, R2/, R3/ and R4/stream) |
Low |
|
1 × 1,250 g a.s./ha (BBCH 00–10 pre‐emergence) |
Low |
High (2/8 scenarios: R3/ and R4/ stream) |
Low |
High (3/8 scenarios: R1/, R3/ and R4/stream) |
High (7/8 scenarios: D3/ and D6/ditch; D5/, R1/, R2/, R3/ and R4/stream) |
High (6/8 scenarios: D3/ and D6/ditch; R1/, R2/, R3/ and R4/stream) |
Low | |
|
1 × 1,440 g a.s./ha (BBCH 11–18 post‐emergence) |
Low |
High (3/8 scenarios: R1/, R3/ and R4/stream) |
Low |
High (4/8 scenarios: R1/, R2/, R3/ and R4/stream) |
High (8/8 scenarios: D3/ and D6/ditch; D4/, D5/, R1/, R2/, R3/ and R4/stream) |
High (8/8 scenarios: D3/ and D6/ditch; D4/, D5/, R1/, R2/, R3/ and R4/stream) |
Low | |
|
1 × 1,250 g a.s./ha (BBCH 11–18 post‐emergence) |
Low |
High (2/8 scenarios: R3/ and R4/ stream) |
Low |
High (3/8 scenarios: R1/, R3/ and R4/stream) |
High (8/8 scenarios: D3/ and D6/ditch; D4/, D5/, R1/, R2/, R3/ and R4/stream) |
High (6/8 scenarios: D3/ and D6/ditch; R1/, R2/, R3/ and R4/stream) |
Low | |
| Sunflower |
1 × 1,440 g a.s./ha (BBCH 00–09 pre‐emergence) |
Low |
High (2/4 scenarios: R3/ and R4/stream) |
Low |
High (3/4 scenarios: R1/, R3/ and R4/stream) |
High (4/4 scenarios: D5/, R1/ R3/ and R4/stream) |
High (4/4 scenarios: D5/, R1/ R3/ and R4/stream) |
Low |
|
1 × 1,250 g a.s./ha (BBCH 00–09 pre‐emergence) |
Low |
High (2/4 scenarios: R3/ and R4/stream) |
Low |
High (2/4 scenarios: R3/ and R4/stream) |
High (4/4 scenarios: D5/, R1/ R3/ and R4/stream) |
High (3/4 scenarios: R1/ R3/ and R4/stream) |
Low | |
Most sensitive group among those for which a reliable endpoint was available. By applying risk mitigation measures, low risk was indicated for all the representative FOCUS surface water scenarios except for R1/, R2/, R3/ and R4/stream (10 m buffer, resulting in 60% run off reduction) and for R3/ and R4/stream (20 m vegetative buffer, resulting in 80% solute run‐off reduction) for the pre‐ and post‐emergence uses in maize and for R1/, R3/ and R4/stream (10 m buffer, resulting in 60% run off reduction) and for R3/ and R4/stream (20 m vegetative buffer, resulting in 80% solute run‐off reduction) for the uses in sunflower.
The risk assessment for aquatic macrophytes could not be finalised in the absence of a study covering the full life‐cycle of the sensitive species Elodea canadensis.
Acute studies with pertinent metabolites were available. For metabolites in surface water (Table 7), low risk was indicated for all representative uses considering the available FOCUS Step 2 PECsw. Low risk was also concluded for metabolites when groundwater becomes surface water considering the available groundwater PEC and annual average lysimeter leachate concentrations (Table 7).
Table 7.
Surface water and sediment
| Compound (name and/or code) | Ecotoxicology |
|---|---|
| S‐metolachlor | Risk assessment not finalised |
| ESA (CGA354743), (CGA376944 for S‐enantiomer, CGA380168 for its sodium salt) | Low risk |
| OXA (CGA51202) | Low risk |
| CGA40172 | Low risk |
| CGA50720 | Low risk |
| CGA368208 | Low risk |
| CGA37735 | Low risk |
| NOA436611 | Low risk |
| CGA41507 | Low risk |
| CGA357704 | Low risk |
| Following metabolites when groundwater becomes surface water | |
| SYN547977 | Low risk |
| NOA413173 | Low risk |
| SYN542488 | Low risk |
| SYN542489 | Low risk |
| SYN542490 | Low risk |
| SYN542491 | Low risk |
| SYN542492 | Low risk |
| SYN542607 | Low risk |
| SYN547969 | Low risk |
From the available literature data, the acute toxicity of S‐metolachlor to aquatic life stages of amphibians seemed to be comparable with respect to the acute toxicity to fish. While recognising the absence of a risk assessment scheme for amphibians and noting the shortcomings in the study from which the amphibian acute endpoint was derived, the risk for aquatic life‐stages of amphibians was assumed to be covered by the risk assessment for fish.
Acute (oral and contact) studies with honey bees were available with S‐metolachlor and the representative formulation. Chronic toxicity data with the active substance was only available for larvae (22‐day study with repeated exposure), whereas chronic studies with A9396G were submitted for both larvae (8‐day study) and adults. The risk assessment performed in line with the EFSA bee guidance document (EFSA, 2013) indicated a low acute risk to honey bees from contact and oral exposure for all representative uses at the screening step. The same conclusion would be reached by applying the SANCO guidance on terrestrial ecotoxicology (European Commission, 2002). A low chronic risk to larvae and adults was concluded at the screening step and at Tier‐1, respectively, for all uses. No relevant plant metabolites occurring in pollen and nectar were identified; therefore, the risk to plant metabolites was not assessed further. A suitable assessment of accumulative and sublethal effects (e.g. hypopharyngeal glands) was not available (data gap for sublethal effects, see Section 10). Furthermore, no risk assessment was performed to address the oral exposure via contaminated surface water and guttation (data gap, see Section 10). Finally, toxicity data and risk assessments were not available for bumble bees and solitary bees.
For non‐target arthropods other than bees, extended laboratory studies with a formulation equivalent to A9396G were conducted with the standard species Aphidius rhopalosiphi and Typhlodromus pyri and with two ground‐dwelling species, i.e. Aleochara bilineata and Poecilus cupreus. A low in‐ and off‐field risk was concluded for the representative uses of S‐metolachlor.
The risk to earthworms and other soil macroorganisms (i.e. the Collembola Folsomia candida and the soil mite Hypoaspis aculeifer) was evaluated using chronic toxicity studies with the representative formulation and the relevant soil metabolites CGA354743 (ESA), CGA51202 (OXA), CGA368208, CGA40172, CGA50720, CGA37735 and NOA436611. Toxicity data with the metabolite CGA357704 were not submitted for any soil taxa (data gap, see Section 10). A reliable field study showing no significant effects on earthworm abundance and biomass up to one year after application of 1,250 and 1,900 g S‐metolachlor/ha was submitted. Based on this body of evidence and the Tier‐1 risk assessment, a low long‐term risk was concluded for S‐metolachlor and the soil metabolites (for which data was available) for all representative uses.
Nitrogen transformation studies with S‐metolachlor, the representative formulation and all relevant soil metabolites were available to address the risk to soil microorganisms except the metabolite CGA357704 (data gap, see Section 10). A low risk was indicated for all representative uses.
Several aspects related with the hazard and risk assessment to non‐target terrestrial plants were discussed at the experts' meeting.28 The experts agreed that the vegetative vigour and seedling emergence studies available with the representative formulation are reliable. However, the experts concluded that none of the regulatory studies could be used for the hazard characterisation since most non‐target plant species tested belonged to labelled crops tolerant to S‐metolachlor. Furthermore, a peer‐reviewed publication from the systematic literature search indicated a higher sensitivity of non‐crop plants.
Owing to the lack of suitable endpoints covering non‐tolerant species, the risk to non‐target terrestrial plants could not be assessed for any of the representative uses (data gap and issue not finalised, see Section 9).
A low risk to organisms involved in biological methods for sewage treatment could be concluded for all representative uses.
6. Endocrine disruption properties
The assessment of the ED potential of S‐metolachlor according to the ECHA/EFSA guidance (2018) has not been finalised as the ED data submission from the applicant is due by 3 May 2023. According to the mandate received on 27 September 2022 by the European Commission, EFSA should provide its completed conclusion on all areas of the assessment, except for ED properties.
7. Overview of the risk assessment of compounds listed in residue definitions triggering assessment of effects data for the environmental compartments (Tables 5, 6, 7, 8–5, 6, 7, 8)
Table 5.
Soil
Table 8.
Air
| Compound (name and/or code) | Toxicology |
|---|---|
| S‐metolachlor | Rat LC50 by inhalation > 2.91 mg/L air/4 h (nose only) |
8. Particular conditions proposed to be taken into account by risk managers
Risk mitigation measures (RMMs) identified following consideration of Member State (MS) and/or applicant's proposal(s) during the peer review, if any, are presented in this section. These measures applicable for human health and/or the environment leading to a reduction of exposure levels of operators, workers, bystanders/residents, environmental compartments and/or non‐target organisms for the representative uses are listed below. The list may also cover any RMMs as appropriate, leading to an acceptable level of risks for the respective non‐target organisms.
It is noted that final decisions on the need of RMMs to ensure the safe use of the plant protection product containing the concerned active substance will be taken by risk managers during the decision‐making phase. Consideration of the validity and appropriateness of the RMMs remains the responsibility of MSs at product authorisation, taking into account their specific agricultural, plant health and environmental conditions at national level (Table 9).
Table 9.
Risk mitigation measures proposed for the representative uses assessed
| Representative use | Maize 1,440 g/ha | Maize 1,248 g/ha | Maize 1,440 g/ha | Maize 1,248 g/ha | Sunflower 1,440 g/ha | Sunflower 1,248 g/ha |
|---|---|---|---|---|---|---|
| foliar spray BBCH 00–10 | foliar spray BBCH 00–10 | foliar spray BBCH 11–18 | foliar spray BBCH 11–18 | foliar spray BBCH 00–09 | foliar spray BBCH 00–09 | |
| Operator risk | Use of PPE is required (a) | – | Use of PPE is required (a) | – | Use of PPE is required (a) | – |
| Worker exposure | – | – | – | – | – | – |
| Bystander/resident exposure | Buffer strip 10 m or drift reducing nozzles | Buffer strip 5 m or drift reducing nozzles | Buffer strip 10 m or drift reducing nozzles | Buffer strip 5 m or drift reducing nozzles | Buffer strip 10 m or drift reducing nozzles | Buffer strip 5 m or drift reducing nozzles |
| Risk to aquatic organisms | RMM equivalent to 10 m (b) or 20 m no‐spray buffer zone (c) | RMM equivalent to 10 m (b) or 20 m no‐spray buffer zone (c) | RMM equivalent to 10 m (b) or 20 m no‐spray buffer zone (c) | RMM equivalent to 10 m (b) or 20 m no‐spray buffer zone (c) | RMM equivalent to 10 m (d) or 20 m no‐spray buffer zone (e) | RMM equivalent to 10 m (d) or 20 m no‐spray buffer zone (e) y |
For tractor‐mounted applications: gloves during ML (mixing/loading) and A (application) (EFSA, 2014; and German model).
Low risk indicated for 7/11 scenarios; high risk still indicated for R1/, R2/, R3/ and R4/stream.
Low risk indicated for 9/11 scenarios; high risk still indicated for R3/ and R4/stream.
Low risk indicated for 3/6 scenarios; high risk still indicated for R1/, R3/ and R4/stream.
Low risk indicated for 4/6 scenarios; high risk still indicated for R3/ and R4/stream.
9. Concerns and related data gaps
9.1. Issues that could not be finalised
An issue is listed as ‘could not be finalised’ if there is not enough information available to perform an assessment, even at the lowest tier level, for one or more of the representative uses in line with the uniform principles in accordance with Article 29(6) of Regulation (EC) No 1107/2009 and as set out in Commission Regulation (EU) No 546/201129 and if the issue is of such importance that it could, when finalised, become a concern (which would also be listed as a critical area of concern if it is of relevance to all representative uses).
An issue is also listed as ‘could not be finalised’ if the available information is considered insufficient to conclude on whether the active substance can be expected to meet the approval criteria provided for in Article 4 of Regulation (EC) No 1107/2009.
The following issues or assessments that could not be finalised have been identified, together with the reasons including the associated data gaps where relevant, which are reported directly under the specific issue to which they are related:
-
1
The risk assessment for two metabolites identified as unique for humans (M4) or in higher amounts in humans (M9) based on in vitro comparative metabolism data could not be finalised (see Section 2).
Further investigations of in vitro comparative metabolism for S‐metolachlor were not available, including identification of the metabolites M4 and M9, comparison with the metabolites measured in vivo, further toxicological assessment of these two metabolites, and further investigations of comparative metabolism in other key species (e.g. mice, rabbits and dogs).
-
2
The consumer dietary risk assessment could not be finalised since the residue definition for risk assessment for rotational crops proposed as ‘metolachlor including other mixtures of constituent isomers, including S‐metolachlor (sum of isomers)’ is provisional in view of the identified data gaps. Data addressing the actual levels of free and conjugated residues of CGA133275 in food and feed commodities derived from rotational crops are not available. In consequence, the livestock exposure assessment and the assessment of transfer of residues in animal commodities could also not be finalised (see Section 3).
Sufficient rotational crops field trials in NEU and SEU and analysing for CGA133275, free and glucose/malonyl glucose conjugated in food and feed edible parts of the rotational crops with a validated analytical method including a hydrolysis step to release the conjugates of CGA133275 were not available and, in the absence of further evidence persistent soil metabolites are addressed by the rotational crops metabolism study, metabolites OXA [CGA51202], ESA [CGA354743], CGA40172, CGA368208, NOA436611 and CGA357704 will need also to be quantified in rotational crops field trials (relevant for the representative uses). By ‘sufficient rotational crops field trials’ it is understood limited field studies (at two sites in major growing areas on three representative crops, including a root crop) or extended field trials, as appropriate, in case the limited set of studies show residues above 0.01 mg/kg.
The assessment of the toxicological relevance of CGA133275, free and glucose/malonyl glucose conjugated was not available (relevant for the representative uses, see Sections 2 and 3).
-
3
The consumer risk assessment is not finalised with regard to the unknown nature of residues that might be present in drinking water, consequent to water treatment following abstraction of surface water that might contain the active substance and its metabolites (see Sections 3 and 4).
Satisfactory information to address the effect of water treatment processes on the nature of residues present in surface water and groundwater, when surface water or groundwater are abstracted for drinking water was not available. In the first instance, a consideration of the processes of ozonation and chlorination would appear appropriate. If an argumentation is made that concentrations at the point of abstraction for drinking water purposes will be low, this argumentation should cover metabolites predicted to be in groundwater and surface water, as well as the active substance. Should this consideration indicate that novel compounds might be expected to be formed from water treatment, the risk to human or animal health through the consumption of drinking water containing them would be needed (relevant to comply with the conditions of approval, not dependent of any specific use, see Section 4).
-
4
The risk to aquatic organisms could not be finalised (see Section 5).
A specific study with the aquatic macrophyte E. canadensis covering the full life cycle (i.e., 14‐day study) for the active substance was not available (relevant for all representative uses).
-
5The risk to non‐target terrestrial plants could not be finalised (see Section 5).
- Toxicity data for terrestrial plants with sensitive species were not available (relevant for all representative uses).
9.2. Critical areas of concern
An issue is listed as a critical area of concern if there is enough information available to perform an assessment for the representative uses in line with the uniform principles in accordance with Article 29(6) of Regulation (EC) No 1107/2009 and as set out in Commission Regulation (EU) No 546/2011, and if this assessment does not permit the conclusion that, for at least one of the representative uses, it may be expected that a plant protection product containing the active substance will not have any harmful effect on human or animal health or on groundwater, or any unacceptable influence on the environment.
An issue is also listed as a critical area of concern if the assessment at a higher tier level could not be finalised due to lack of information, and if the assessment performed at the lower tier level does not permit the conclusion that, for at least one of the representative uses, it may be expected that a plant protection product containing the active substance will not have any harmful effect on human or animal health or on groundwater, or any unacceptable influence on the environment.
An issue is also listed as a critical area of concern if, in the light of current scientific and technical knowledge using guidance documents available at the time of application, the active substance is not expected to meet the approval criteria provided for in Article 4 of Regulation (EC) No 1107/2009.
The following critical areas of concern are identified, together with any associated data gaps, where relevant, which are reported directly under the specific critical area of concern to which they are related:
-
6
The available information indicates that the representative uses assessed result in a potential for S‐metolachlor and its relevant herbicidally active metabolite SYN547977 to be present in groundwater above the parametric drinking water limit of 0.1 μg/L. This happened at 24 of the 119 wells monitored (20%) and 25 of the 121 wells monitored (21%) respectively for sites specifically selected to represent vulnerable situations. This is not contradicted by the results of general public monitoring where metolachlor was present in groundwater above the parametric limit in Belgium (up to 1.3% of samples), Slovenia (1.8% of samples) Switzerland (3% of samples), Italy (5% of samples) and Spain (6% of samples), (relevant for all representative uses evaluated; see Sections 4 and 7).
-
7
High potential for groundwater exposure above the parametric drinking water limit of 0.1 μg/L by the human health relevant metabolites ESA (CGA354743), OXA (CGA51202), CGA50720, CGA368208, NOA436611, CGA357704, NOA413173, SYN542488, SYN542489, SYN542490, SYN542491, SYN542492, SYN542607, SYN545026, SYN545027, SYN547969 in situations represented by all FOCUS scenarios or a relevant lysimeter, with this being confirmed by an extensive targeted monitoring programme and public monitoring data for ESA (CGA354743) and OXA (CGA51202), (relevant for all representative uses evaluated; see Sections 2, 4 and 7).
-
8
High risk to earthworm‐eating mammals from secondary poisoning (see Section 5).
9.3. Overview of the concerns identified for each representative use considered (Table 10)
Table 10.
Overview of concerns reflecting the issues not finalised, critical areas of concerns and the risks identified that may be applicable for some but not for all uses or risk assessment scenarios
| Representative use | Maize 1,440 g/ha | Maize 1,248 g/ha | Maize 1,440 g/ha | Maize 1,248 g/ha | |
|---|---|---|---|---|---|
| foliar spray BBCH00‐10 | foliar spray BBCH00‐10 | foliar spray BBCH11‐18 | foliar spray BBCH11‐18 | ||
| Operator risk | Risk identified | ||||
| Assessment not finalised | |||||
| Worker risk | Risk identified | ||||
| Assessment not finalised | |||||
| Resident/bystander risk | Risk identified | ||||
| Assessment not finalised | |||||
| Consumer risk | Risk identified | ||||
| Assessment not finalised | X2,3 | X2,3 | X2,3 | X2,3 | |
| Risk to wild non‐target terrestrial vertebrates | Risk identified | X8 | X8 | X (c) ,8 | X (c) ,8 |
| Assessment not finalised | |||||
| Risk to wild non‐target terrestrial organisms other than vertebrates | Risk identified | ||||
| Assessment not finalised | X5 | X5 | X5 | X5 | |
| Risk to aquatic organisms | Risk identified | ||||
| Assessment not finalised | X4 | X4 | X4 | X4 | |
| Groundwater exposure to active substance | Legal parametric value breached | 24/119 wells6 | 24/119 wells6 | 24/119 wells6 | 24/119 wells6 |
| Assessment not finalised | |||||
| Groundwater exposure to metabolites | Legal parametric value breached (a) | X6,7 | X6,7 | X6,7 | X6,7 |
| Parametric value of 10 μg/L (b) breached | |||||
| Assessment not finalised | |||||
The superscript numbers relate to the numbered points indicated in Sections 9.1 and 9.2. Where there is no superscript number, see Sections 2 to 7 for further information.
When the consideration for classification proposed by RAC is confirmed under Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008.
Value for non‐relevant metabolites prescribed in SANCO/221/2000‐rev. 10 final, European Commission, 2003.
High risk for herbivorous mammals for the post‐emergence uses in maize (BBCH 11–18).
(If a particular condition proposed to be taken into account to manage an identified risk, as listed in Section 8, has been evaluated as being effective, then ‘risk identified’ is not indicated in Table 10).
| Representative use | Sunflower 1,440 g/ha | Sunflower 1,248 g/ha | |
|---|---|---|---|
| foliar spray BBCH00‐09 | foliar spray BBCH00‐09 | ||
| Operator risk | Risk identified | ||
| Assessment not finalised | |||
| Worker risk | Risk identified | ||
| Assessment not finalised | |||
| Resident/bystander risk | Risk identified | ||
| Assessment not finalised | |||
| Consumer risk | Risk identified | ||
| Assessment not finalised | X2,3 | X2,3 | |
| Risk to wild non‐target terrestrial vertebrates | Risk identified | X8 | X8 |
| Assessment not finalised | |||
| Risk to wild non‐target terrestrial organisms other than vertebrates | Risk identified | ||
| Assessment not finalised | X5 | X5 | |
| Risk to aquatic organisms | Risk identified | ||
| Assessment not finalised | X4 | X4 | |
| Groundwater exposure to active substance | Legal parametric value breached | 24/119 wells6 | 24/119 wells6 |
| Assessment not finalised | |||
| Groundwater exposure to metabolites | Legal parametric value breached (a) | X6,7 | X6,7 |
| Parametric value of 10 μg/L (b) breached | |||
| Assessment not finalised | |||
The superscript numbers relate to the numbered points indicated in Sections 9.1 and 9.2. Where there is no superscript number, see Sections 2 to 7 for further information.
When the consideration for classification proposed by RAC is confirmed under Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008.
Value for non‐relevant metabolites prescribed in SANCO/221/2000‐rev. 10 final, European Commission, 2003.
10. List of other outstanding issues
Remaining data gaps not leading to critical areas of concern or issues not finalised but considered necessary to comply with the data requirements, and which are relevant for some or all of the representative uses assessed at EU level. Although not critical, these data gaps may lead to uncertainties in the assessment and are considered relevant.
These data gaps refer only to the representative uses assessed and are listed in the order of the sections:
Spectral data for the relevant impurities (impurity 3 and impurity 6) (relevant for all representative uses evaluated; see Section 1).
Method for analysis of the relevant impurities (impurity 3 and impurity 6) in the formulation (relevant for all representative uses evaluated; see Section 1).
Content of the relevant impurities (impurity 3 and impurity 6) before and after the storage of the formulation (relevant for all representative uses evaluated; see Section 1).
Method (s) for analysis of the relevant impurities (impurity 3 and impurity 6) in the technical material with a LOQ at least 20% less than the specification limit of 0.08 g/kg (relevant for all representative uses evaluated; see Section 1).
An ILV of the monitoring method for ground/drinking water for CGA357704, CGA368208, CGA50720, NOA413173 and NOA436611 (relevant for all representative uses evaluated; see Section 1).
Monitoring methods for ground/drinking water for CGA37735, SYN542488, SYN542489, SYN542490, SYN542491, SYN542607, SYN545026, SYN545027, SYN547969 (relevant for all representative uses evaluated; see Section 1).
Monitoring method for metabolite CGA46129 in body fluids (relevant for all representative uses evaluated; see Section 1).
Further assessment of major metabolites in humans (e.g. (S‐)metolachlor mercapturate), for their possible inclusion in the residue definition for body fluids (relevant for all representative uses evaluated; see Section 2).
Further assessment of the phototoxicity of S‐metolachlor, covering exposure to UVB light (wavelengths below 320 nm) since S‐metolachlor is an UVB absorber. Based on new OECD guideline 432 (June 2019), UVB absorbers can also be properly assessed by the test (with appropriate UVB filters attenuating the cytotoxicity) (relevant for all representative uses evaluated; see Section 2).
Further investigations of the long‐term toxicity of S‐metolachlor in mice (relevant for all representative uses evaluated, though not needed to complete the risk assessment; see Section 2).
Further investigations of the neurotoxic potential of S‐metolachlor (relevant for all representative uses evaluated, though not needed to complete the risk assessment; see Section 2).
Further investigations of the potential immunotoxicity of S‐metolachlor (relevant for all representative uses evaluated, though not needed to complete the risk assessment; see Section 2).
Screening for the biological activity against the target weeds according to SANCO/221/2000‐rev.10‐final (European Commission, 2003) Step 3a Stage 1, for SYN545026 and SYN545027 (relevant for all representative uses evaluated; see Section 7, Table 2).
Field soil dissipation information from the analysis of CGA357704, CGA368208, CGA40172 and NOA436611 (SYN546829) at 3 different study locations with 3 varying soils (relevant for all representative uses evaluated; see Section 4).
Updated kinetic fitting for all laboratory soil incubations that can be considered as true replicates (relevant for all representative uses evaluated; see evaluation table Section 4 in the peer review report EFSA (2023)).
Degradation and adsorption endpoints for all the metabolites found only in the lysimeters (except for SYN547977), i.e. NOA413173, SYN542489, SYN542488, SYN542490, SYN542491, SYN542492, SYN542607 (only for degradation), SYN545026, SYN545027 & SYN547969 (relevant for all representative uses evaluated; see Section 4).
Study reports reporting the detail necessary for an evaluation and assessment of the results of the analysis of groundwater monitoring well samples for the metabolites CGA357704, SYN542489, CGA368208 and NOA436611 (relevant for all representative uses evaluated; see evaluation table Section 4 in the peer review report EFSA (2023)).
Further data to address the risk to honeybees from sublethal effects (e.g. effects on hypopharyngeal glands), via exposure to guttation and surface water (relevant for all representative uses; see Section 5).
Further data to address the effects of the metabolite CGA357704 to soil organisms (i.e. earthworms, soil macro‐organisms other than earthworms, soil micro‐organisms) (relevant for all representative uses; see Section 5).
Abbreviations
- a.s.
active substance
- AAOEL
acute acceptable operator exposure level
- ADI
acceptable daily intake
- AOEL
acceptable operator exposure level
- AR
applied radioactivity
- ARfD
acute reference dose
- bw
body weight
- DFR
dislodgeable foliar residues
- DT50
period required for 50% dissipation (define method of estimation)
- DT90
period required for 90% dissipation (define method of estimation)
- ECHA
European Chemicals Agency
- EEC
European Economic Community
- FAO
Food and Agriculture Organization of the United Nations
- FOCUS
Forum for the Co‐ordination of Pesticide Fate Models and their Use
- GAP
Good Agricultural Practice
- GC
gas chromatography
- ISO
International Organization for Standardization
- IUPAC
International Union of Pure and Applied Chemistry
- Kdoc
organic carbon linear adsorption coefficient
- KFoc
Freundlich organic carbon adsorption coefficient
- LC50
lethal concentration, median
- LC–MS‐MS
liquid chromatography with tandem mass spectrometry
- LOQ
limit of quantification
- MRL
maximum residue level
- NOAEL
no observed adverse effect level
- OECD
Organisation for Economic Co‐operation and Development
- PEC
predicted environmental concentration
- PECair
predicted environmental concentration in air
- PECgw
predicted environmental concentration in groundwater
- PECsed
predicted environmental concentration in sediment
- PECsoil
predicted environmental concentration in soil
- PECsw
predicted environmental concentration in surface water
- PPE
personal protective equipment
- RAC
regulatory acceptable concentration
- RAR
Renewal Assessment Report
- SMILES
simplified molecular‐input line‐entry system
- TRR
total radioactive residue
- UV
Ultraviolet
- WG
water‐dispersible granule
- WHO
World Health Organization
Appendix A – Consideration of cut‐off criteria for S‐metolachlor according to Annex II of Regulation (EC) No 1107/2009 of the European Parliament and of the Council
| Properties | Conclusion | |
|---|---|---|
| CMR | Carcinogenicity (C) |
S‐metolachlor is classified as Carcinogen category 2 (ECHA, RAC opinion adopted on 6 February 2022) |
| Mutagenicity (M) | – | |
| Toxic for Reproduction (R) | – | |
| Endocrine disrupting properties | Not finalised | |
| POP | Persistence | S‐metolachlor is not considered to be a persistent organic pollutant (POP) according to point 3.7.1 of Annex II of Regulation (EC) 1107/2009. |
| Bioaccumulation | ||
| Long‐range transport | ||
| PBT | Persistence | S‐metolachlor is not considered to be a persistent, bioaccumulative and toxic (PBT) substance according to point 3.7.2 of Annex II of Regulation (EC) 1107/2009. |
| Bioaccumulation | ||
| Toxicity | ||
| vPvB | Persistence | S‐metolachlor is not considered to be a very persistent, very bioaccumulative substance according to point 3.7.3 of Annex II of Regulation (EC) 1107/2009. |
| Bioaccumulation | ||
(a): origin of data to be included where applicable (e.g. EFSA, ECHA RAC, Regulation).
Appendix B – List of end points for the active substance and the representative formulation
Appendix B can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2023.7852.
Appendix C – Wording EFSA used in Section 4 of this conclusion, in relation to DT and Koc ‘classes’ exhibited by each compound assessed
| Wording | DT50 normalised to 20°C for laboratory incubations 30 or not normalised DT50 for field studies (SFO equivalent, when biphasic, the DT90 was divided by 3.32 to estimate the DT50 when deciding on the wording to use) |
|---|---|
| very low persistence | < 1 day |
| low persistence | 1 to < 10 days |
| moderate persistence | 10 to < 60 days |
| medium persistence | 60 to < 100 days |
| high persistence | 100 days to < 1 year |
| very high persistence | A year or more |
Note these classes and descriptions are unrelated to any persistence class associated with the active substance cut‐off criteria in Annex II of Regulation (EC) No 1107/2009. For consideration made in relation to Annex II, see Appendix A.
| Wording | Koc (either KFoc or Kdoc) mL/g |
|---|---|
| very high mobility | 0 to 50 |
| high mobility | 51 to 150 |
| medium mobility | 151 to 500 |
| low mobility | 501 to 2,000 |
| slight mobility | 2,001 to 5,000 |
| Immobile | > 5,000 |
Based on McCall et al. (1980).
Appendix D – Used compound codes
| Code/trivial name (a) | IUPAC name/SMILES notation/InChiKey (b) | Structural formula (c) |
|---|---|---|
| S‐metolachlor |
reaction mixture of 80–100% 2‐chloro‐2′‐ethyl‐N‐[(1 S)‐2‐methoxy‐1‐methylethyl]‐6′‐methylacetanilide and 20–0% 2‐chloro‐2′‐ethyl‐N‐[(1R)‐2‐methoxy‐1‐methylethyl]‐6′‐methylacetanilide ClCC(=O)N([C@@H](C)COC)c1c(C)cccc1CC (S‐isomer) WVQBLGZPHOPPFO‐LBPRGKRZSA‐N (S‐isomer) ClCC(=O)N([C@H](C)COC)c1c(C)cccc1CC (R‐isomer) WVQBLGZPHOPPFO‐GFCCVEGCSA‐N (R‐isomer) |
S‐Isomer (major component)
R‐isomer |
| metolachlor |
2‐chloro‐2′‐ethyl‐N‐[(1RS)‐2‐methoxy‐1‐methylethyl]‐6′‐methylacetanilide ClCC(=O)N(C(C)COC)c1c(C)cccc1CC WVQBLGZPHOPPFO‐UHFFFAOYSA‐N |
|
| Impurities 3 (CGA13656) |
2‐chloro‐N‐(2‐ethyl‐6‐methylphenyl)acetamide O=C(Nc1c(C)cccc1CC)CCl SMINYPCTNJDYGK‐UHFFFAOYSA‐N |
|
| Impurities 6 (CGA50259) |
2,2‐dichloro‐N‐(2‐ethyl‐6‐methylphenyl)‐N‐[(2 S)‐1‐methoxypropan‐2‐yl]acetamide ClC(Cl)C(=O)N([C@@H](C)COC)c1c(C)cccc1CC AJMAPKQENYREOY‐NSHDSACASA‐N |
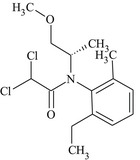
|
| SYN547977 |
N‐(2‐acetyl‐6‐methylphenyl)‐2‐chloro‐N‐(1‐methoxypropan‐2‐yl)acetamide ClCC(=O)N(C(C)COC)c1c(C)cccc1C(C) = O JQPYVRCWJNISTB‐UHFFFAOYSA‐N |
|
|
ESA (CGA354743) (though the material used in standards was prepared from sodium salt, in practice the acid is the moiety of interest and all quantifications were reported for the acid) |
2‐[(2‐ethyl‐6‐methylphenyl)(1‐methoxypropan‐2‐yl)amino]‐2‐oxoethane‐1‐sulfonic acid O=S(=O)(O)CC(=O)N(C(C)COC)c1c(C)cccc1CC CIGKZVUEZXGYSV‐UHFFFAOYSA‐N |
|
|
ESA S‐enantiomer (CGA376944) (CGA380168 is used for sodium salt) |
2‐{(2‐ethyl‐6‐methylphenyl)[(2 S)‐1‐methoxypropan‐2‐yl]amino}‐2‐oxoethane‐1‐sulfonic acid O=S(=O)(O)CC(=O)N([C@@H](C)COC)c1c(C)cccc1CC CIGKZVUEZXGYSV‐LBPRGKRZSA‐N |
|
| OXA (CGA51202) |
[(2‐ethyl‐6‐methylphenyl)(1‐methoxypropan‐2‐yl)amino](oxo)acetic acid O=C(O)C(=O)N(C(C)COC)c1c(C)cccc1CC LNOOSYCKMKZOJB‐UHFFFAOYSA‐N |
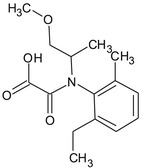
|
| CGA351916 (OXA S‐isomer) |
{(2‐ethyl‐6‐methylphenyl)[(2 S)‐1‐methoxypropan‐2‐yl]amino}(oxo)acetic acid O=C(O)C(=O)N([C@@H](C)COC)c1c(C)cccc1CC LNOOSYCKMKZOJB‐NSHDSACASA‐N |
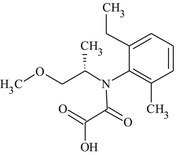
|
| CGA40172 |
N‐(2‐ethyl‐6‐methylphenyl)‐2‐hydroxy‐N‐(1‐methoxypropan‐2‐yl)acetamide OCC(=O)N(C(C)COC)c1c(C)cccc1CC YRHZCHBPHOEWCA‐UHFFFAOYSA‐N |
|
| CGA50720 |
(2‐ethyl‐6‐methylanilino)(oxo)acetic acid O=C(Nc1c(C)cccc1CC)C(=O)O SAWXESXDACFEPC‐UHFFFAOYSA‐N |
|
| CGA368208 |
2‐(2‐ethyl‐6‐methylanilino)‐2‐oxoethane‐1‐sulfonic acid O=C(Nc1c(C)cccc1CC)CS(=O)(=O)O QPVPJECIHVSBLZ‐UHFFFAOYSA‐N |
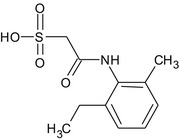
|
| CGA37735 |
N‐(2‐ethyl‐6‐methylphenyl)‐2‐hydroxyacetamide O=C(Nc1c(C)cccc1CC)CO MXMPHDJVYOMMTN‐UHFFFAOYSA‐N |
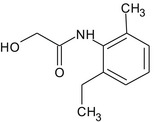
|
|
NOA436611 |
(2‐{(2‐ethyl‐6‐methylphenyl)[(2 S)‐1‐methoxypropan‐2‐yl]amino}‐2‐oxoethanesulfinyl)acetic acid O=C(O)CS(=O)CC(=O)N([C@@H](C)COC)c1c(C)cccc1CC FVHURMWINUOTIB‐LNHXHEARSA‐N |
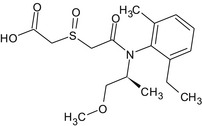
|
| SYN546829 |
{2‐[(2‐ethyl‐6‐methylphenyl)(1‐methoxypropan‐2‐yl)amino]‐2‐oxoethanesulfinyl}acetic acid O=C(O)CS(=O)CC(=O)N(C(C)COC)c1c(C)cccc1CC FVHURMWINUOTIB‐UHFFFAOYSA‐N |
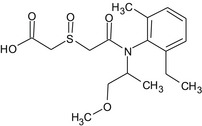
|
| CGA357704 |
N‐(2‐ethyl‐6‐methylphenyl)‐N‐oxaloalanine CC(N(C(=O)C(=O)O)c1c(C)cccc1CC)C(=O)O IMFSUYMDPTXKCC‐UHFFFAOYSA‐N |
|
|
NOA413173 |
disodium 2‐[(2‐ethyl‐6‐methylphenyl)(sulfonatoacetyl)amino]propanoate [Na+].[Na+].CC(N(C(=O)CS([O‐])(=O) = O)c1c(C)cccc1CC)C([O‐]) = O YYDFJGNRSFJICN‐UHFFFAOYSA‐L |
|
| SYN542488 |
{[2‐(carboxymethyl)‐6‐methylphenyl](1‐methoxypropan‐2‐yl)amino}(oxo)acetic acid O=C(O)C(=O)N(C(C)COC)c1c(C)cccc1CC(=O)O MQFPMRGMSDIXKQ‐UHFFFAOYSA‐N |
|
|
SYN542489 (SYN548163 is used for ammonium salt) |
{[2‐(1‐hydroxyethyl)‐6‐methylphenyl](1‐methoxypropan‐2‐yl)amino}(oxo)acetic acid O=C(O)C(=O)N(C(C)COC)c1c(C)cccc1C(C)O HZWHUPBAPXSVDP‐UHFFFAOYSA‐N |
|
| SYN542490 |
2‐[(2‐acetyl‐6‐methylphenyl)(1‐methoxypropan‐2‐yl)amino]‐2‐oxoethane‐1‐sulfonic acid O=S(=O)(O)CC(=O)N(C(C)COC)c1c(C)cccc1C(C) = O PJFBPRJNNJJYAR‐UHFFFAOYSA‐N |
|
|
SYN542491 (SYN548164 is used for ammonium salt) |
N‐[2‐(1‐hydroxyethyl)‐6‐methylphenyl]‐N‐oxaloalanine CC(N(C(=O)C(=O)O)c1c(C)cccc1C(C)O)C(=O)O ZKWNAPUMTOVAFX‐UHFFFAOYSA‐N |
|
| SYN542492 |
3‐ethyl‐2‐[(hydroxyacetyl)(1‐methoxypropan‐2‐yl)amino]benzoic acid OCC(=O)N(C(C)COC)c1c(cccc1CC)C(=O)O CGIPUXKKFSZMOD‐UHFFFAOYSA‐N |
|
|
SYN542607 (SYN548165 is used for ammonium salt) |
2‐{[2‐(1‐hydroxyethyl)‐6‐methylphenyl](1‐methoxypropan‐2‐yl)amino}‐2‐oxoethane‐1‐sulfonic acid O=S(=O)(O)CC(=O)N(C(C)COC)c1c(C)cccc1C(C)O YQSCHHLLFBCRTF‐UHFFFAOYSA‐N |
|
| SYN545026 |
N‐(2‐acetyl‐6‐methylphenyl)‐N‐(carboxycarbonyl)alanine CC(N(C(=O)C(=O)O)c1c(C)cccc1C(C) = O)C(=O)O NOAZNMSOODZCGC‐UHFFFAOYSA‐N |
|
| SYN545027 |
N‐[2‐(1‐hydroxyethyl)‐6‐methylphenyl]alanine CC(Nc1c(C)cccc1C(C)O)C(=O)O KEYSXCAXPBFABN‐UHFFFAOYSA‐N |
|
| SYN547969 |
3‐ethyl‐2‐[(1‐methoxypropan‐2‐yl)(oxalo)amino]benzoic acid O=C(O)C(=O)N(C(C)COC)c1c(cccc1CC)C(=O)O IZDYJGUMTAAPSM‐UHFFFAOYSA‐N |
|
| CGA41507 |
N‐(2‐ethyl‐6‐methylphenyl)‐N‐(1‐methoxypropan‐2‐yl)acetamide CC(=O)N(C(C)COC)c1c(C)cccc1CC AQQKRTUHCOLVTD‐UHFFFAOYSA‐N |
|
| CGA46129 |
N‐(2‐ethyl‐6‐methylphenyl)‐N‐(hydroxyacetyl)alanine CC(N(C(=O)CO)c1c(C)cccc1CC)C(=O)O GFKJCKIIVUVZFZ‐UHFFFAOYSA‐N |
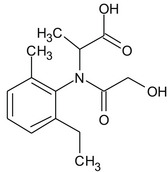
|
| CGA217498 |
N‐(2‐ethyl‐6‐methylphenyl)‐2‐(methanesulfonyl)‐N‐(1‐methoxypropan‐2‐yl)acetamide CS(=O)(=O)CC(=O)N(C(C)COC)c1c(C)cccc1CC BSRFFIFXMLDYOI‐UHFFFAOYSA‐N |
|
| CGA133275 |
N‐(2‐ethyl‐6‐methylphenyl)‐N‐(1‐hydroxypropan‐2‐yl)‐2‐(methanesulfonyl)acetamide CS(=O)(=O)CC(=O)N(C(C)CO)c1c(C)cccc1CC JXGGXQRJANRQKP‐UHFFFAOYSA‐N |
|
| CGA37913 |
2‐(2‐ethyl‐6‐methylanilino)propan‐1‐ol CC(CO)Nc1c(C)cccc1CC ALFXNLHLMQREPR‐UHFFFAOYSA‐N |
|
| CGA369873 |
2‐(2,6‐dimethylanilino)‐2‐oxoethane‐1‐sulfonic acid O=C(Nc1c(C)cccc1C)CS(=O)(=O)O ZNKNVJGSYJFDHT‐UHFFFAOYSA‐N |
|
| CGA50267 |
N‐(2‐ethyl‐6‐methylphenyl)alanine CC(Nc1c(C)cccc1CC)C(=O)O ACQCQKSFTBFYDO‐UHFFFAOYSA‐N |
|
| (S‐) metolachlor mercapturate |
N‐acetyl‐S‐{2‐[(2‐ethyl‐6‐methylphenyl)(1‐methoxypropan‐2‐yl)amino]‐2‐oxoethyl}‐L‐cysteine O=C(O)[C@@H](NC(C) = O)CSCC(=O)N(C(C)COC)c1c(C)cccc1CC HEFXMEPCHCUHDE‐JRZJBTRGSA‐N |
|
| CGA49750 |
4‐(2‐ethyl‐6‐methylphenyl)‐5‐methylmorpholine‐2,3‐dione CC1COC(=O)C(=O)N1c1c(CC)cccc1C IOUQBHFOFHGTFI‐UHFFFAOYSA‐N |
|
| CGA41638 |
2‐chloro‐N‐(2‐ethyl‐6‐methylphenyl)‐N‐(1‐hydroxypropan‐2‐yl)acetamide ClCC(=O)N(C(C)CO)c1c(C)cccc1CC RNRZTRIOAPZEME‐UHFFFAOYSA‐N |
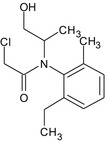
|
|
sugar conjugate of CGA118243 |
Structure undefined, a unique name/SMILES/InChiKey cannot be allocated |
|
| CGA217497 |
S‐{2‐[(2‐ethyl‐6‐methylphenyl)(1‐hydroxypropan‐2‐yl)amino]‐2‐oxoethyl}cysteine O=C(O)C(N)CSCC(=O)N(C(C)CO)c1c(C)cccc1CC GYISPTFVDMOMNA‐UHFFFAOYSA‐N |
|
| CGA43826/CGA46576 |
S‐{2‐[(2‐ethyl‐6‐methylphenyl)(1‐methoxypropan‐2‐yl)amino]‐2‐oxoethyl}cysteine O=C(O)C(N)CSCC(=O)N(C(C)COC)c1c(C)cccc1CC BCQUDRDUZNNSJM‐UHFFFAOYSA‐N |
|
|
1EX β‐glucuronic acid conjugate of CGA41638 |
2‐[(chloroacetyl)(2‐ethyl‐6‐methylphenyl)amino]propyl β‐D‐glucopyranosiduronic acid Cc1cccc(CC)c1N(C(C)CO[C@@H]1O[C@@H]([C@@H](O)[C@H](O)[C@H]1O)C(=O)O)C(=O)CCl NGLAXUTWCMXXAP‐XCPAYJINSA‐N |
|
| 2EX |
N‐(2‐ethyl‐6‐methylphenyl)‐N‐oxaloalanine CC(N(C(=O)C(=O)O)c1c(C)cccc1CC)C(=O)O IMFSUYMDPTXKCC‐UHFFFAOYSA‐N |
|
| 3EX |
2‐chloro‐N‐[2‐(1‐hydroxyethyl)‐6‐methylphenyl]‐N‐(1‐hydroxypropan‐2‐yl)acetamide ClCC(=O)N(C(C)CO)c1c(C)cccc1C(C)O CZABLDSDDUXPSQ‐UHFFFAOYSA‐N |
|
The name of compounds/metabolites in bold is the name used in the conclusion.
ACD/Name 2021.1.3 ACD/Labs 2021.1.3 (File Version N15E41, Build 123232, 7 July 2021).
ACD/ChemSketch 2021.1.3 ACD/Labs 2021.1.3 (File Version C25H41, Build 123835, 28 August 2021).
Supporting information
List of end points for the active substance and the representative formulation
Suggested citation: EFSA (European Food Safety Authority) , Alvarez F, Arena M, Auteri D, Binaglia M, Castoldi AF,Chiusolo A, Colagiorgi A, Colas M, Crivellente F, De Lentdecker C, De Magistris I, Egsmose M, Fait G, Ferilli F, Gouliarmou V, Nogareda LH, Ippolito A, Istace F, Jarrah S, Kardassi D, Kienzler A, Lanzoni A, Lava R, Leuschner R, Linguadoca A, Lythgo C, Magrans O, Mangas I, Miron I, Molnar T, Padovani L, Panzarea M, Parra Morte JM, Rizzuto S, Serafimova R, Sharp R, Szentes C, Szoradi A, Terron A, Theobald A, Tiramani M, Vianello G and Villamar‐Bouza L, 2023. Conclusion on the peer review of the pesticide risk assessment of the active substance S‐metolachlor excluding the assessment of the endocrine disrupting properties. EFSA Journal 2023;21(2):7852, 46 pp. 10.2903/j.efsa.2023.7852
Requestor: European Commission
Question number: EFSA‐Q‐2022‐00621
Declarations of interest: If you wish to access the declaration of interests of any expert contributing to an EFSA scientific assessment, please contact interestmanagement@efsa.europa.eu.
Acknowledgements: EFSA wishes to thank the rapporteur Member State Germany for the preparatory work on this scientific output.
EFSA may include images or other content for which it does not hold copyright. In such cases, EFSA indicates the copyright holder and users should seek permission to reproduce the content from the original source.
Approved: 27 January 2023
Notes
Commission Implementing Regulation (EU) No 844/2012 of 18 September 2012 setting out the provisions necessary for the implementation of the renewal procedure for active substances, as provided for in Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market. OJ L 252, 19.9.2012, pp. 26–32.
Commission Implementing Regulation (EU) No 2018/1659 of 7 November 2018 amending Implementing Regulation (EU) No 844/2012 in view of the scientific criteria for the determination of endocrine disrupting properties introduced by Regulation (EU) 2018/605.
Regulation (EC) No 1107/2009 of 21 October 2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. OJ L 309, 24.11.2009, pp. 1–50.
Commission Regulation (EU) 2018/605 of 19 April 2018 amending Annex II to Regulation (EC) No 1107/2009 by setting out scientific criteria for the determination of endocrine disrupting properties. OJ L 101, 20.4.2018, pp. 33–36.
See Experts' consultation 2.1 at the Pesticides Peer Review Teleconference 27 (EFSA, 2023).
See Experts' consultation 2.2 at the Pesticides Peer Review Teleconference 27 (EFSA, 2023).
Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. OJ L 353, 31.12.2008, pp. 1–1355.
See Experts' consultation 2.3 at the Pesticides Peer Review Teleconference 27 (EFSA, 2023).
See Experts' consultation 2.4 at the Pesticides Peer Review Teleconference 27 (EFSA, 2023).
See Experts' consultation 2.5 at the Pesticides Peer Review Teleconference 27 (EFSA, 2023).
See Experts' consultation 2.6 at the Pesticides Peer Review Teleconference 27 (EFSA, 2023).
See Experts' consultation 2.9 at the Pesticides Peer Review Teleconference 27 (EFSA, 2023).
Simulations utilised the agreed Q10 of 2.58 (following EFSA, 2008) and Walker equation coefficient of 0.7.
The kinetic endpoint approach used for the derivation of arithmetic mean formation fractions and geometric mean DegT50 of ESA and OXA (regarding which soil incubations might be considered as having the same soil properties) used in groundwater exposure modelling used by the RMS was different to that concluded correct in this conclusion and included in the list of agreed endpoints (Appendix A) of this conclusion. This has the particular context here, that all groundwater metabolites have been concluded as relevant and those simulated to have ESA and OXA as precursor (CGA368208, CGA37735, CGA50720 via ESA and CGA50720 directly from OXA) were simulated to be above 0.1 μg/L. Therefore, there was no practical consequence that the available simulation results that used the RMS approach for the derivation of arithmetic mean formation fractions and geometric mean DegT50 may be underestimating CGA368208, CGA37735, CGA50720 and CGA50720 concentrations. Note that there is a data gap associated with this issue in Section 10. The RMS disagreed with the selection of arithmetic mean formation fractions and geometric mean DegT50 of ESA and OXA that have been included in Appendix B.
See Experts' consultation 4.2 at the Pesticides Peer Review Teleconference 28 (EFSA, 2023).
This means that the results tabulated deviate from the temporal statistical population of concentrations described for option 2 in the Appendix 1 of Gimsing et al., 2019.
In one lysimeter SYN547977 was present in annual average recharge at the parametric drinking water limit, in addition to being detected above the limit in 21% of the sites included in the available targeted monitoring data.
S‐metolachlor and metolachlor showed similar toxicity to birds.
See Experts' consultation point 5.2 at the Pesticide Peer Review Teleconference 29 (EFSA, 2023).
See Experts' consultation point 5.1 at the Pesticide Peer Review Teleconference 29 (EFSA, 2023).
Toxicity‐exposure ratio values exceeded the trigger value for the following indicator species and crop group/scenarios: small omnivorous ‘mouse’ (bare soils and maize BBCH 10–29) and the small herbivorous ‘vole’ (maize BBCH 10–29).
See Experts' consultation point 5.4 at the Pesticide Peer Review Teleconference 29 (EFSA, 2023).
See S‐Metolachlor RAR Volume 3CP – B.9 Ecotoxicology data and assessment of risks for non‐target species (Germany, 2021).
The PT = 0.139 was the worst‐case value from a study with seven consumer individuals performed in Germany (EU central regulatory zone). There is some uncertainty on the transferability of the PT value for wood mouse to the northern and southern zones (https://ec.europa.eu/assets/sante/food/plants/pesticides/lop/index.html).
S‐metolachlor and metolachlor showed similar toxicity towards algae and Lemna sp. in Tier‐1 studies.
See Experts' consultation points 5.5 and 5.6 at the Pesticide Peer Review Teleconference 29 (EFSA, 2023).
The study did not cover the full life cycle of E. canadensis and it was shorter than the duration indicated in the OECD TG 239. Therefore, the study was not considered sufficiently reliable for the hazard characterisation for this species.
See Experts' consultation points 5.7 and 5.8 at the Pesticide Peer Review Teleconference 29 (October 2020) (EFSA, 2023).
Commission Regulation (EU) No 546/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorisation of plant protection products. OJ L 155, 11.6.2011, pp. 127–175.
For laboratory soil incubations normalisation was also to field capacity soil moisture (pF2/10 kPa). For laboratory sediment water system incubations, the whole system DT values were used.
References
- ECHA (European Chemicals Agency) , 2022. Committee for Risk Assessment (RAC) Opinion proposing harmonised classification and labelling at EU level of S‐metolachlor. CLH‐O‐0000007145‐77‐01/F. Adopted 2 June 2022. Available online: www.echa.europa.eu
- ECHA (European Chemicals Agency) , EFSA (European Food Safety Authority) with the technical support of the Joint Research Centre (JRC) , Andersson N, Arena M, Auteri D, Barmaz S, Grignard E, Kienzler A, Lepper P, Lostia AM, Munn S, Parra Morte JM, Pellizzato F, Tarazona J, Terron A and Van der Linden S, 2018. Guidance for the identification of endocrine disruptors in the context of Regulations (EU) No 528/2012 and (EC) No 1107/2009. EFSA Journal 2018;16(6):5311, 135 pp. 10.2903/j.efsa.2018.5311. ECHA‐18‐G‐01‐EN. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , 2008. Opinion on a request from EFSA related to the default Q10 value used to describe the temperature effect on transformation rates of pesticides in soil. EFSA Journal 2008;6(1):622, 32pp. 10.2903/j.efsa.2008.622 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2009. Guidance on Risk Assessment for Birds and Mammals on request from EFSA. EFSA Journal 2009;7(12):1438, 358 pp. 10.2903/j.efsa.2009.1438 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2012. Review of the existing maximum residue levels (MRLs) for S‐metolachlor according to Article 12 of Regulation (EC) No 396/2005. EFSA Journal 2012;10(2):2586, 42 pp. 10.2903/j.efsa.2012.2586 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2013. EFSA Guidance Document on the risk assessment of plant protection products on bees (Apis mellifera, Bombus spp. and solitary bees). EFSA Journal 2013;11(7):3295, 268 pp. 10.2903/j.efsa.2013.3295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , 2014. Guidance on the assessment of exposure of operators, workers, residents and bystanders in risk assessment for plant protection products. EFSA Journal 2014;12(10):3874, 55 pp. 10.2903/j.efsa.2014.3874 Available online: www.efsa.europa.eu/efsajournal [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , Buist H, Craig P, Dewhurst I, HougaardBennekou S, Kneuer C, Machera K, Pieper C, Court Marques D, Guillot G, Ruffo F and Chiusolo A, 2017. Guidance on dermal absorption. EFSA Journal 2017;15(6):4873, 60 pp. 10.2903/j.efsa.2017.4873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , 2023. Peer review report to the conclusion regarding the peer review of the pesticide risk assessment of the active substance S‐metolachlor. Available online: www.efsa.europa.eu
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues) , 2012. Guidance on dermal absorption. EFSA Journal 2012;10(4):2665, 30 pp. 10.2903/j.efsa.2012.2665 [DOI] [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues) , 2013. Guidance on tiered risk assessment for plant protection products for aquatic organisms in edge‐of‐field surface waters. EFSA Journal 2013;11(7):3290, 186 pp. 10.2903/j.efsa.2013.3290 [DOI] [Google Scholar]
- European Commission , 2000a. Residues: guidance for generating and reporting methods of analysis in support of pre‐registration data requirements for Annex II (Part A, Section 4) and Annex III (Part A, Section 5) of Directive 91/414. SANCO/3029/99‐rev. 4, 11 July 2000.
- European Commission , 2000b. Technical material and preparations: guidance for generating and reporting methods of analysis in support of pre‐ and post‐registration data requirements for Annex II (Part A, Section 4) and Annex III (Part A, Section 5) of Directive 91/414. SANCO/3030/99‐rev. 4, 11 July 2000.
- European Commission , 2002.Guidance Document on Terrestrial Ecotoxicology Under Council Directive 91/414/EEC. SANCO/10329/2002‐rev. 2 final, 17 October 2002.
- European Commission , 2003. Guidance Document on Assessment of the Relevance of Metabolites in Groundwater of Substances Regulated under Council Directive 91/414/EEC. SANCO/221/2000‐rev. 10 final, 25 February 2003.
- European Commission , 2004. Review report for the active substance S‐Metolachlor. Finalised in the Standing Committee on the Food Chain and Animal Health at its meeting on 8 October 2004 in view of the inclusion of S‐Metolachlor in Annex I of Directive 91/414/EEC. SANCO/1426/2001 – rev. 3, 4 October 2004, 25 pp.
- European Commission , 2010. Guidance Document on residue analytical methods. SANCO/825/00‐rev. 8.1, 16 November 2010.
- European Commission , 2011. Guidelines on comparability, extrapolation, group tolerances and data requirements for setting MRLs. SANCO 7525/VI/95‐rev. 9 March 2011. p. 1–46.
- European Commission , 2012. Guidance document on the assessment of the equivalence of technical materials of substances regulated under Regulation (EC) No 1107/2009. SANCO/10597/2003‐rev. 10.1, 13 July 2012.
- European Commission , 2014a. Assessing potential for movement of active substances and their metabolites to ground water in the EU. Report of the FOCUS Workgroup. EC Document Reference SANCO/13144/2010‐v. 3, 613 pp.
- European Commission , 2014b. Guidance document on the renewal of approval of active substances to be assessed in compliance with Regulation (EU) No 844/2012. SANCO/2012/11251‐rev. 4, 12 December 2014.
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use) , 2001. FOCUS surface water scenarios in the EU evaluation process under 91/414/EEC. Report of the FOCUS Working Group on Surface Water Scenarios. EC Document Reference SANCO/4802/2001‐rev. 2, 245 pp., as updated by Generic guidance for FOCUS surface water scenarios, v. 1.4 May 2015.
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use) , 2006. Guidance document on estimating persistence and degradation kinetics from environmental fate studies on pesticides in EU Registration Report of the FOCUS Work Group on Degradation Kinetics. EC Document Reference SANCO/10058/2005‐v. 2.0, 434 pp., as updated by the Generic guidance for Estimating Persistence and Degradation Kinetics from Environmental Fate Studies on Pesticides in EU Registration, v. 1.1 December 2014.
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use) , 2007. Landscape and mitigation factors in aquatic risk assessment. Volume 1. Extended summary and recommendations. Report of the FOCUS Working Group on Landscape and Mitigation Factors in Ecological Risk Assessment. EC Document Reference SANCO/10422/2005 v. 2.0, 169 pp.
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use) , 2008. Pesticides in air: considerations for exposure assessment. Report of the FOCUS Working Group on Pesticides in Air. EC Document Reference SANCO/10553/2006‐rev. 2 June 2008.
- Germany , 2018. Renewal Assessment Report (RAR) on the active substance S‐metolachlor prepared by the rapporteur Member State Germany, in the framework of Commission Implementing Regulation (EU) No 844/2012, September 2018. Available online: www.efsa.europa.eu
- Germany , 2023. Revised Renewal Assessment Report (RAR) on S‐metolachlor prepared by the rapporteur Member State Germany in the framework of Commission Implementing Regulation (EU) No 844/2012, January 2023. Available online: www.efsa.europa.eu
- Gimsing AL, Agert J, Baran N, Boivin A, Ferrari F, Gibson R, Hammond L, Hegler F, Jones R, Konig W, Kreuger J, van der Linden T, Liss D, Loiseau L, Massey A, Miles B, Monrozies L, Newcombe A, Poot A, Reeves GL, Reichenberger S, Rosenbom AE, Staudenmaier H, Sur R, Schwen A, Stemmer M, Tuting W and Ulrich U, 2019. Conducting groundwater monitoring studies in Europe for pesticide active substances and their metabolites in the context of Regulation (EC) 1107/2009. J Consum Prot Food Saf, 14, 1–93. 10.1007/s00003-019-01211-x [DOI] [Google Scholar]
- JMPR (Joint Meeting on Pesticide Residues) , 2004. Report of the Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group on Pesticide Residues, Rome, Italy, 20–29 September 2004, 383 pp.
- JMPR (Joint Meeting on Pesticide Residues) , 2007. Report of the Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group on Pesticide Residues, Geneva, Switzerland, 18–27 September 2007, 164 pp.
- McCall PJ, Laskowski DA, Swann RL and Dishburger HJ, 1980. Measurements of sorption coefficients of organic chemicals and their use in environmental fate analysis. Test Protocols for Environmental Fate and Movement of Toxicants. Proceedings of the 94th Annual Meeting of the American Association of Official Analytical Chemists (AOAC). Oct 21–22, Washington, DC. pp. 89–109. [Google Scholar]
- OECD (Organisation for Economic Co‐operation and Development) , 2009. Guidance document on overview of residue chemistry studies. ENV/JM/MONO(2009)31, 28 July 2009.
- OECD (Organisation for Economic Co‐operation and Development) , 2011. OECD MRL calculator: spreadsheet for single data set and spreadsheet for multiple data set, 2 March 2011. In: Pesticide Publications/Publications on Pesticide Residues. Available online: www.oecd.org
- SETAC (Society of Environmental Toxicology and Chemistry) , Candolfi MP, Barrett KL, Campbell PJ, Forster R, Grandy N, Huet MC, Lewis G, Oomen PA, Schmuck R and Vogt H (eds), 2001. Guidance document on regulatory testing and risk assessment procedures for plant protection products with non‐target arthropods. ESCORT 2 workshop.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of end points for the active substance and the representative formulation