Abstract
Background
The role of nutritional status and the risk of contracting and/or experiencing adverse outcomes from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection are unclear. Preliminary studies suggest that higher n-3 PUFA intakes are protective.
Objectives
This study aimed to compare the risk of 3 coronavirus disease 2019 (COVID-19) outcomes (testing positive for SARS-CoV-2, hospitalization, and death) as a function of the baseline plasma DHA levels.
Methods
The DHA levels (% of total fatty acids [FAs]) were measured by nuclear magnetic resonance. The 3 outcomes and relevant covariates were available for 110,584 subjects (hospitalization and death) and for 26,595 ever-tested subjects (positive for SARS-CoV-2) in the UK Biobank prospective cohort study. Outcome data between 1 January, 2020, and 23 March, 2021, were included. The Omega-3 Index (O3I) (RBC EPA + DHA%) values across DHA% quintiles were estimated. The multivariable Cox proportional hazards models were constructed, and linear (per 1 SD) relations with the risk of each outcome were computed as HRs.
Results
In the fully adjusted models, comparing the fifth to the first DHA% quintiles, the HRs (95% confidence intervals) for testing positive, being hospitalized, and dying with COVID-19 were 0.79 (0.71, 0.89, P < 0.001), 0.74 (0.58, 0.94, P < 0.05), and 1.04 (0.69–1.57, not significant), respectively. On a per 1-SD increase in DHA% basis, the HRs for testing positive, hospitalization, and death, were 0.92 (0.89, 0.96, P < 0.001), 0.89 (0.83, 0.97, P < 0.01), and 0.95 (0.83, 1.09), respectively. The estimated O3I values across DHA quintiles ranged from 3.5% (quintile 1) to 8% (quintile 5).
Conclusions
These findings suggest that nutritional strategies to increase the circulating n-3 PUFA levels, such as increased consumption of oily fish and/or use of n-3 FA supplements, may reduce the risk of adverse COVID-19 outcomes.
Keywords: EPA, DHA, Omega-3 Index, COVID-19, SARS-CoV-2, fish oil, epidemiology, observational, biomarker
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has arguably been the most disruptive worldwide event since World War II. Its impact was felt not only in the health sector (morbidity and mortality) but also in the economic, social, and political realms. A tremendous amount of research has been conducted on the causes of and treatments for the infection, including efforts to discover the factors that affect susceptibility to the disease. In addition to demographic and physiologic characteristics (e.g., obesity, age, underlying health conditions, and socioeconomic status [SES]), nutritional considerations have been explored. Nutrients, such as zinc and vitamins C and D, as well as dietary bioactives (e.g., probiotics) have been suggested as possible protective agents against severe acute respiratory syndrome coronavirus 2 SARS-CoV-2) infection and/or COVID-19 sequela [1]. In addition to these, the long-chain n-3 fatty acids (FAs) of marine origin— EPA and DHA—have been proposed to be protective against COVID-19 [[2], [3], [4], [5]]. DHA and EPA are the main precursors for inflammation-resolving mediators, including maresins, resolvins, and protectins [6]. These mediators downregulate the production of cytokines, improve macrophage-mediated removal of inflammatory debris and microbes and promote apoptosis of neutrophils [2]. Accordingly, higher levels of EPA and DHA in the tissues could reduce the severity of the inflammatory response to SARS-CoV-2 infection. Small-scale (<100 patients) studies have reported that higher RBC levels of EPA + DHA (i.e., the Omega-3 Index [O3I] [7]) at admission were associated with a lower risk of adverse COVID-19 outcomes [[8], [9], [10]], and pilot intervention studies have reported possible benefits from n-3 supplementation [[11], [12], [13]]. The availability of the plasma DHA levels in a subset of individuals in the UK Biobank (UKBB) cohort [14] affords the opportunity to explore the relationships between n-3 biostatus and the risk of COVID-19 outcomes. Two prior studies on this topic have been published. Julkunen et al. [15] and Sun et al. [16] both recently reported that higher DHA levels, among several other biomarkers, were associated with a lower risk of infection or hospitalization with COVID-19. In addition, the reported use of fish oil supplements was recently linked with a lower risk of COVID-19 in the UKBB cohort [17]. The present study aimed to expand upon the work of these investigators by examining the n-3 relations with additional COVID-19 outcomes (i.e., death), performing more granular analyses (i.e., quintiles), exploring the effects of several covariates on these relationships, and translating n-3 biostatus into the O3I, a more commonly used measure of n-3 status.
Methods
Participants
The UKBB is a prospective, population-based cohort of ∼500,000 individuals recruited between 2007 and 2010 at assessment centers across England, Wales, and Scotland. Baseline data derived from questionnaires, biological samples, and physical measurements were collected on all participating individuals, with longitudinal monitoring occurring via a mix of in-person and electronic medical record data [14, 18]. The participants completed a touchscreen questionnaire, which collected information on sociodemographic characteristics, diet, and lifestyle factors. Anthropometric measurements were taken using standardized procedures. The touchscreen questionnaire and other resources are shown on the UKBB website (http://www.ukbiobank.ac.uk/resources/). The UKBB has ethical approval (Ref. 11/NW/0382) from the North West Multicentre Research Ethics Committee as a Research Tissue Bank. This approval means that researchers do not require separate ethical clearance and can operate under the Tissue Bank approval. All participants gave electronic signed informed consent. The UKBB study was conducted according to the guidelines laid down in the Declaration of Helsinki. The UKBB protocol is available online (http://www.ukbiobank.ac.uk/wp-content/uploads/2011/11/UK-Biobank-Protocol.pdf).
A total of 117,946 individuals from the UKBB cohort had blood FA data (see Supplemental Figure 1). We first dropped individuals who died before 1 January, 2020 (i.e., before the pandemic began), leaving a sample of 111,240 individuals. After dropping a small number of individuals with missing covariates (listed under the Statistical methods section), our final analysis dataset consisted of 110,584 individuals.
Exposure
The primary exposure was plasma DHA (expressed as a percent of total plasma FAs) obtained from blood samples collected at the time of enrollment using nuclear magnetic resonance spectroscopy (NMR, Nightingale Health Plc [19]). To estimate the O3I values associated with these DHA levels, we used an equation described by Schuchardt et al. [20], which found a correlation between the observed O3I and estimated O3I (eO3I) of 0.83.
Outcomes
We examined 3 primary endpoints: 1) death from COVID-19, 2) hospitalization with confirmed SARS-CoV-2 infection, and 3) having tested positive for SARS-CoV-2. Data were available on these 3 outcomes through 23 March, 2021, including the outcome itself (yes/no) and the date for which the outcome was recorded. Any individuals recorded as having died and/or been hospitalized with COVID-19 were imputed as having tested positive for COVID-19 if not already noted as such in the dataset (N = 174, 4% of the total). Because of inconsistencies in the reporting of testing data across regions of the United Kingdom (e.g., testing data were not available for Wales) and to provide a more precise outcome measure, the analysis sample for the “tested positive” outcome consisted of only individuals for whom a test result was available (e.g., ever tested, N = 26,597). Links to the UKBB outcomes, exposures, and covariates used here are shown in Supplemental Table 6. [I'm trying to leave a comment here but either I can't or don't know how! In any event, this Table is the first Suppl Table mentioned. I'd like to change it to Table 1 but I can't seem to edit it. I'm sure you can! I have updated the Suppl Materials table numbering to make this Table 1 instead of 6.]
Statistical methods
The Cox proportional hazards models were used to predict all 3 outcomes. For each outcome, 4 separate models were run predicting the outcome using DHA% while using different sets of covariates with prior evidence of association with confirmed infection with SARS-CoV-2 [21]: model 1, unadjusted; model 2, adjusted for age at start of pandemic, sex, and race (self-identified); model 3, which included model 2 covariates + waist circumference; and model 4, which included model 3 covariates + Townsend deprivation index (a measure of relative SES), time since enrollment, smoking status, education, self-reported health, blood pressure medications, slow walking pace, fresh fruit, dried fruit, salad/raw vegetables, cooked vegetables, and grain fiber (from cereals and breads [22]). For models with sets of covariates that substantially attenuated the hazard ratios (HRs) (particularly model 2 vs. 3), the relative contribution of each individual variable to the explained variation was evaluated by a drop-one analyses. The effect of dropping each individual covariate illuminates the relative impact of each on the model concordances. Other covariates identified in prior studies [21] for test positivity included cystatin C, hemoglobin A1c (HbA1c), forced expiratory volume, and high-density lipoprotein (HDL) cholesterol. However, these variables were missing for ∼17% of subjects; thus, we only included them in a sensitivity analysis (model 5, which included model 4 covariates + these 4 variables). As in prior studies, we focused primarily on modeling the relationships between DHA% and COVID-19 outcomes with quintiles and continuous linear relationships. In the secondary analyses, we further investigated potential nonlinearities using cubic splines. Cubic splines were compared with linear models using nested analysis of variance to test for significant model improvement. All analyses were conducted in R version 4.1.2 and used a statistical significance threshold of 0.05.
Results
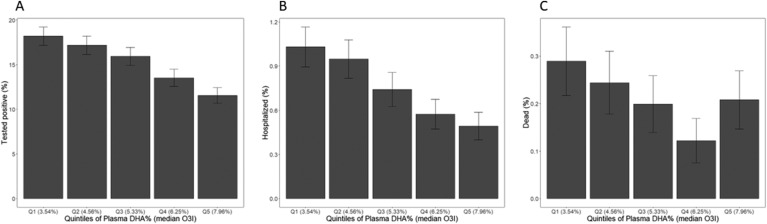
Descriptions of the analytic cohorts are presented in Table 1 . Among the 110,584 individuals included in this study, the mean age at the beginning of the pandemic was ∼68 y, the vast majority were White, and a little less than half were males. The mean plasma DHA level was 2%, and the eO3I value was 5.6%. Less than 1% of the full cohort were hospitalized with COVID-19, and ∼20% of those hospitalized died from it. Among the 26,597 individuals in the dataset who ever got tested for SARS-CoV-2 infection, ∼15% tested positive at some point between 1 January, 2020, and 23 March, 2021. In those who got tested, the plasma DHA% was similar to that of the whole cohort. When divided into quintiles, the plasma DHA% ranged from <1.49% in quintile 1 to >2.50% in quintile 5 (see TABLE 2, TABLE 3, TABLE 4 ). The median eO3I values corresponding to each plasma DHA% quintile ranged from 3.54% to 7.96% in quintiles 1 and 5, respectively (see Figure 1 ).
TABLE 1.
Distribution of analysis variables1
| All | Ever tested for COVID | |
|---|---|---|
| N | 110,584 | 26,597 |
| Sex, male | 49,638 (44.9%) | 12,368 (46.5%) |
| Race, White | 104,340 (94.4%) | 25,038 (94.1%) |
| Positive test results2 | 4081 (3.7%) | 4081 (15.3%) |
| Hospitalized with COVID-192 | 837 (0.8%) | 837 (3.1%) |
| Death with COVID-192 | 235 (0.2%) | 235 (0.9%) |
| Age (on 1 January, 2020) | 68.1 (8.1) | 68.6 (8.2) |
| Years since enrollment | 11.39 (0.88) | 11.39 (0.89) |
| Estimated3 Omega-3 Index (%) | 5.6 (1.89) | 5.56 (1.91) |
| Plasma DHA (% of total fatty acids) | 2.02 (0.68) | 2 (0.68) |
| Waist circumference (cm) | 90 (13.29) | 91.23 (13.74) |
| BMI (kg/m2) | 27.38 (4.73) | 27.77 (4.93) |
| Townsend deprivation index4 | −1.35 (3.07) | −1.17 (3.19) |
| Education, college/university | 42,106 (38.1%) | 9,657 (36.3%) |
| Smoking status, never | 61,236 (55.4%) | 13,822 (52%) |
| Self-report health, excellent | 18,606 (16.8%) | 3,946 (14.8%) |
| Long standing illness, none | 74,205 (67.1%) | 16,325 (61.4%) |
| Blood pressure medications, yes | 4844 (4.4%) | 1398 (5.3%) |
| Diabetes, yes | 5339 (4.8%) | 1629 (6.1%) |
| Walking pace, slow5 | 7943 (7.2%) | 2483 (9.3%) |
| Fresh fruit (pieces per day) | 2.23 (1.58) | 2.23 (1.65) |
| Dried fruit (pieces per day) | 1.89 (2.29) | 1.88 (2.37) |
| Fresh vegetables/salad (heaped tablespoon per day) | 2.16 (2.17) | 2.14 (2.15) |
| Cooked vegetables (heaped tablespoon per day) | 1.9 (2.51) | 1.91 (2.52) |
| Grain fiber (estimated g/week) | 21.76 (14.52) | 21.34 (14.32) |
COVID-19, coronavirus disease 2019.
N (percent) or mean (SD).
Between 1 January, 2020, and 23 March, 2021.
Based on Schuchardt et al. (20) .
An index of 0 means “average deprivation” in the United Kingdom; a negative value means less deprived than average, and a positive means more deprived than average.
Less than 3 miles per hour (“steady” is 3–4, and “fast” is >4).
TABLE 2.
Hazard ratios (95% confidence interval) from the Cox proportional hazards models by quintile for testing positive for severe acute respiratory syndrome coronavirus 2 as a function of plasma DHA% among those who were ever tested (n = 26,597)
| Plasma DHA% quintile (Q; min–max) | Unadjusted | Model 21 | Model 31 | Model 41 |
|---|---|---|---|---|
| Q1 (<1.49, reference) | 1 | 1 | 1 | 1 |
| Q2 (1.49, 1.79) | 0.94 (0.86, 1.03) | 0.96 (0.88, 1.05) | 0.99 (0.9, 1.08) | 0.99 (0.91, 1.09) |
| Q3 (1.80, 2.09) | 0.86 (0.78, 0.94)∗∗ | 0.90 (0.82, 0.99)∗ | 0.95 (0.86,1.04) | 0.97 (0.89,1.07) |
| Q4 (2.10, 2.50) | 0.73 (0.66, 0.8)∗∗∗ | 0.79 (0.72, 0.87)∗∗∗ | 0.85 (0.76, 0.93)∗∗ | 0.89 (0.80, 0.98)∗ |
| Q5 (>2.50) | 0.60 (0.55, 0.67)∗∗∗ | 0.68 (0.61, 0.76)∗∗∗ | 0.74 (0.67, 0.82)∗∗∗ | 0.79 (0.71, 0.89)∗∗∗ |
| Linear across quintiles | 0.89 (0.87, 0.9)∗∗∗ | 0.91 (0.89, 0.93)∗∗∗ | 0.94 (0.91, 0.96)∗∗∗ | 0.95 (0.92, 0.97)∗∗∗ |
| Linear trend per SD | 0.84 (0.81, 0.86)∗∗∗ | 0.87 (0.84, 0.9)∗∗∗ | 0.90 (0.87, 0.93)∗∗∗ | 0.92 (0.89, 0.96)∗∗∗ |
Model 2, adjusted for age at start of pandemic, sex, and race; model 3, including model 2 covariates + waist circumference; and model 4, including model 3 covariates + Townsend deprivation index, time since enrollment, smoking status, education, self-reported health, blood pressure medications, slow walking pace, fresh fruit, dried fruit, fresh vegetables, cooked vegetables, and grain fiber. ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001.
TABLE 3.
Hazard ratios (95% confidence interval) from the Cox proportional hazards models for hospitalization with coronavirus disease 2019 as a function of plasma DHA% (n = 110,584)
| Plasma DHA% quintile (Q; min–max) | Unadjusted | Model 21 | Model 31 | Model 41 |
|---|---|---|---|---|
| Q1 (<1.49, reference) | 1 | 1 | 1 | 1 |
| Q2 (1.49, 1.79) | 0.92 (0.76,1.11) | 0.95 (0.79,1.15) | 1.07 (0.88,1.29) | 1.11 (0.92,1.34) |
| Q3 (1.80, 2.09) | 0.72 (0.59, 0.88)∗∗ | 0.74 (0.61, 0.91)∗∗ | 0.90 (0.74,1.11) | 0.97 (0.79,1.19) |
| Q4 (2.10, 2.50) | 0.56 (0.45, 0.69)∗∗∗ | 0.56 (0.45, 0.7)∗∗∗ | 0.73 (0.59, 0.92)∗∗ | 0.81 (0.65,1.02) |
| Q5 (>2.50) | 0.48 (0.38, 0.6)∗∗∗ | 0.46 (0.36, 0.58)∗∗∗ | 0.63 (0.5, 0.8)∗∗∗ | 0.74 (0.58, 0.94)∗ |
| Linear trend quintiles | 0.82 (0.78, 0.86)∗∗∗ | 0.82 (0.78, 0.86)∗∗∗ | 0.89 (0.84, 0.93)∗∗∗ | 0.92 (0.87, 0.97)∗∗ |
| Linear trend per SD | 0.75 (0.7, 0.81)∗∗∗ | 0.75 (0.69, 0.81)∗∗∗ | 0.84 (0.78, 0.91)∗∗∗ | 0.89 (0.83, 0.97)∗∗ |
Model 2, adjusted for age at start of pandemic, sex, and race; model 3, including model 2 covariates + waist circumference; model 4, including model 3 covariates + Townsend deprivation index, time since enrollment, smoking status, education, self-reported health, blood pressure, slow walking pace, fresh fruit, dried fruit, fresh vegetables, cooked vegetables, and grain fiber. ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001.
TABLE 4.
Hazard ratios (95% confidence interval) from the Cox proportional hazards models for death with coronavirus disease 2019 as a function of plasma DHA% (n = 110,584)
| Plasma DHA% quintile (Q; min–max) | Unadjusted | Model 21 | Model 31 | Model 41 |
|---|---|---|---|---|
| Q1 (<1.49, reference) | 1 | 1 | 1 | 1 |
| Q2 (1.49, 1.79) | 0.84 (0.59,1.21) | 0.86 (0.6,1.24) | 0.97 (0.67,1.39) | 1.03 (0.71,1.48) |
| Q3 (1.80, 2.09) | 0.69 (0.47,1.01) | 0.69 (0.47,1.01) | 0.84 (0.57,1.24) | 0.93 (0.63,1.38) |
| Q4 (2.10, 2.50) | 0.42 (0.27, 0.66)∗∗∗ | 0.40 (0.25, 0.63)∗∗∗ | 0.53 (0.33, 0.83)∗∗ | 0.61 (0.39, 0.98)∗ |
| Q5 (>2.50) | 0.72 (0.49,1.05) | 0.61 (0.41, 0.9)∗ | 0.85 (0.57,1.27) | 1.04 (0.69,1.57) |
| Linear trend quintiles | 0.87 (0.8, 0.96)∗∗ | 0.84 (0.77, 0.92)∗∗∗ | 0.92 (0.83,1.01) | 0.96 (0.87,1.06) |
| Linear trend per SD | 0.83 (0.72, 0.95)∗∗ | 0.78 (0.68, 0.9)∗∗∗ | 0.88 (0.77,1.01) | 0.95 (0.83,1.09) |
Model 2, adjusted for age at start of pandemic, sex, and race; model 3, including model 2 covariates + waist circumference; model 4, including model 3 covariates + Townsend deprivation index, time since enrollment, smoking status, education, self-reported health, blood pressure, slow walking pace, fresh fruit, dried fruit, fresh vegetables, cooked vegetables, and grain fiber. ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001.
FIGURE 1.
The percent of individuals experiencing coronavirus disease 2019 (COVID-19) outcomes between 1 January, 2020, and 23 March, 2021, by quintile of the plasma DHA%. The median estimated Omega-3 Index (O3I) of each quintile is also shown. Percent testing positive for severe acute respiratory syndrome coronavirus 2 (n = 26,597) (A), percent hospitalized with COVID-19 (n = 110,584) (B), and percent dying with COVID-19 (n = 110,584) (C).
There was a strong, inverse, and dose-related relationship between plasma DHA% and the risk of having a positive SARS-CoV-2 test result (see Figure 1A), which was partially attenuated with each level of multivariable adjustment (see Table 2). Nevertheless, in the fully adjusted model, individuals in quintile 5 were 21% less likely to test positive than those in quintile 1 (P < 0.001), and the risk of a positive test result was 8% lower for each 1- SD increase in the plasma DHA% (P < 0.001).
As regards hospitalization with COVID-19, again there was a significant inverse association with the plasma DHA%, which was partially attenuated with increasing adjustment (see Figure 1B). In the fully adjusted model (see Table 3), individuals in quintile 5 were at a 26% lower risk of hospitalization than those in quintile 1 (P < 0.05), and the risk was 11% lower per 1-SD higher in DHA% (P < 0.001).
Finally, for death from COVID-19, relations with the plasma DHA% were more complex (see Figure 1C, Table 4). In all models, the risk of death was significantly lower in quintile 4 than in quintile 1 (P < 0.05); however, in quintile 5, the risk reduction was partially attenuated and not significant in 3 of the 4 models (the exception being model 2 where the risk was 39% lower, P < 0.05). In the unadjusted model and model 2, the risks of death were 17% and 22%, respectively, lower (P < 0.05) per 1-SD increase in DHA%. However, in the fully adjusted model, it was attenuated to nonsignificance (see Table 4). Also in this model, the difference between the risk of death in quintiles 4 and 5 was significant (P = 0.04).
As mentioned for all 3 outcomes, there was some attenuation of the relationship between DHA% and risk with adjustment for covariates. Most of the attenuation in risk, particularly for hospitalization and death, was observed in model 3, which added waist circumference as a covariate in addition to age, sex, and race (from model 2). It was, therefore, of interest to examine each of these 4 covariates to determine the extent to which each was responsible for the observed attenuation in relationship. This was performed by computing the concordance between DHA% and each of the 3 outcomes and then systematically removing each of the variables from the model and calculating the resultant drop in concordance. The results of this exercise are shown in Table 5 . Regardless of the outcome, the removal of sex and then race from the covariate list had little effect on the concordance. Age and waist circumference were the more important covariates. For testing positive, the inclusion of age had the greatest impact on the observed association with DHA%, decreasing the concordance by 45%. Comparatively, the removal of waist circumference reduced the concordance by only 3%. For hospitalization, waist circumference was the more influential, reducing concordance by 19% compared with 10% for age. For death, age was again the major factor followed by waist circumference, reducing the concordances by 32% and 8%, respectively. Finally, there was some additional attenuation in model 4 (vs. model 3) when 12 more covariates were included in the analyses (see Table 4).
TABLE 5.
Effects of the removal of age, sex, race, and waist circumference from model 3 on model concordance for the coronavirus disease 2019 outcomes of interest
| Concordance | Absolute decrease |
Percent decrease1 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Age | Sex | Waist circumference | Race | Age | Sex | Waist circumference | Race | ||
| Positive test result | 0.6298 | 0.0584 | 0.0001 | 0.0038 | 0.0042 | 45.0% | 0.0% | 3.0% | 3.0% |
| Hospitalization | 0.7047 | 0.0213 | 0.0011 | 0.0379 | 0.0121 | 10.0% | 1.0% | 19.0% | 6.0% |
| Death | 0.8088 | 0.0998 | 0.0011 | 0.024 | 0.0181 | 32.0% | 0.0% | 8.0% | 6.0% |
Calculated by dividing the absolute decrease by the concordance—0.5.
As a sensitivity analysis, we included a fifth model with diminished sample sizes (owing to missing covariate data; see Supplemental Table 2) that additionally adjusted for cystatin C, HbA1c, forced expiratory volume, and HDL cholesterol. The inclusion of these variables (and the loss of 16.5% of the sample owing to missing data) resulted in further attenuation of the DHA% versus outcome relationships (see Supplemental Tables 2–4). Finally, the secondary analyses using spline models did not significantly improve upon the continuous models for any outcome and level of adjustment (data not shown).
As noted, there were monotonic inverse relations between DHA% and the risks of testing positive and hospitalization. This was also true for death through the fourth quintile; however, the trend reversed for the highest quintile. Thus, it was of interest to explore the differences in risk factor and demographic profiles across the quintiles of DHA% among the 235 individuals who died to determine if some characteristic of those in quintile 5 may help explain this reversal, especially versus quintile 4 (where the favorable trend was still evident; see Supplemental Table 5). The most obvious (and only statistically significant) difference between quintiles 5 and 4 was in reported fish oil supplement use, with 22% of the former reporting use and 61% of the latter (P = 0.002). This naturally explains why this group had the highest DHA levels. Quintile 5 subjects were also less likely to be White than quintile 4 subjects (74% vs. 93%, P = 0.1) and exercised less but also tended to be younger and less obese.
Discussion
This study aimed to examine the relationships between plasma DHA% at baseline in the UKBB and 3 COVID-19-related outcomes. We found that a 1-SD higher DHA% was associated with an 8% lower risk of testing positive for SARS-CoV-2 and an 11% lower risk of hospitalization in the multivariable adjusted models. The relationship with the risk of death with COVID-19 was also lower (5% lower per 1 SD of DHA%); however, it was not statistically significant. Thus, a higher n-3 level appeared to be protective against both getting infected with the virus and having a severe enough infection to require hospitalization. Regarding the risk of death with COVID-19, across the first 4 DHA% quintiles, the risk decreased in a strongly dose-related manner. However, in the highest DHA quintile, the trend reversed, and the difference (from quintile 1) was no longer significant. Beyond the quintile 5 enrichment in subjects reporting fish oil supplement use and nonWhite participants, the reasons for this partial reversal are unclear; however, caution should be used given the small number of deaths (n = 235). Finally, we were able translate DHA% quintiles into the more familiar O3I and found that the highest risk (i.e., quintile 1) eO3I value was ∼3.5%, whereas the lowest risk (quintile 5) value was ∼8%. These values comport well with the O3I risk cut points (originally proposed in 2004 for death from cardiovascular disease [7]) of <4% (high risk) and >8% (low risk) and imply that these target levels apply to COVID-19 outcomes as well.
In general, our findings support the previous work of Julkunen et al. [15] who reported on the relations between the risk of severe (i.e., hospitalization with) COVID-19 and multiple circulating biomarkers in the UKBB. They found that a 1-SD increase in DHA% was associated with a reduction of ∼23% in the risk of hospitalization in an age- and sex-adjusted model (compared with 25% here in model 2). They did not adjust for any other covariates, nor did they report on either test positivity or mortality. Sun et al. [16] also published on the relationships between multiple FA metrics and COVID-19 outcomes in the UKBB. Our report also differs from theirs in several ways. First, we were able to translate NMR plasma FA data to the eO3I, a metric that has been widely used clinically to risk stratify patients for a variety of conditions [[23], [24, [25], [26], [27], [28, [29]]. Second, although Sun et al. [16] adjusted for more variables that Julkunen et al. [15] did, their list included only age, sex, ethnicity, body mass index, Townsend deprivation index, and assessment center. As noted, we adjusted for additional variables, including those reported by Ho et al. [21] to be significant determinants of the risk of testing positive in hospital for SARS-CoV-2 and those reflecting better dietary habits. Third, similar to Julkunen et al. [15], Sun et al. [16] did not examine the risk of death. Finally, uniquely compared with others, we have reported quintile level relationships and explored nonlinear relationships between DHA% and COVID-19 outcomes. The latter could help define optimal or target blood O3I levels potentially useful in clinical practice.
Other reports from within the UKBB have described significant inverse relationships between the reported fish oil supplement use (which is associated with a higher O3I) and risks of death from all causes and incident cardiovascular events [30] as well as primary liver cancer [31], inflammatory bowel disease [32], dementia [33], and, as noted earlier, COVID-19 [17].
Outside of the UKBB, the associations between the O3I or other blood n-3 biomarkers and a variety of COVID-19-related outcomes in small-scale studies (<100 patients each) were reported. We [8] and a research group in Chile [9, 10] have both shown that higher O3I levels were associated with better clinical outcomes. A study on 26 children with multisystem inflammatory syndrome linked to COVID-19 infection found lower levels of EPA and DHA than those in historical controls [34]. Two double-blind, randomized controlled trials used n-3 PUFAs to treat COVID-19. Doaei et al. [13] compared an EPA + DHA-enriched enteral nutrition product (n = 28) with regular enteral nutrition (n = 73) in critically ill patients hospitalized with COVID-19 and reported a higher 1-mo survival rate, better renal function, improved lymphocyte counts, and better arterial blood gas parameters in the n-3 PUFA group. Sedighiyan et al. [35] compared hydroxychloroquine treatment alone with hydroxychloroquine plus n-3 FAs and found that patients randomized to the latter regimen had less myalgias and fatigue and lower erythrocyte sedimentation rates and C-reactive protein levels.
Mechanisms
There are several mechanisms that could, in theory, explain the relationships observed here. Beginning with the reduced risk of positive infection, SARS-COV-2 is known to infect the host cells via binding of the spike (S) glycoprotein with the surface angiotensin-converting enzyme 2 receptors, expressed in the pneumocytes and other cell types. An in silico study by Vivar-Sierra et al. [36] found that DHA and EPA had the potential to hold the S glycoprotein in a closed confirmation, reducing its ability to bind to surface receptors. These findings were confirmed by Goc et al. [37] who studied the effect of EPA on host cellular proteases, such as transmembrane protease serine protease-2 (TMPRSS2) and cathepsins, that cleave spike proteins and enable cellular entry. EPA effectively inhibited the enzymatic activity of cathepsin L and TMPRSS2. Finally, n-3 PUFAs may also play a role in the intracellular mechanisms of inhibiting viral replication. Middle East respiratory syndrome coronavirus is known to reprogram the sterol regulatory element binding protein (SREBP)-dependent lipogenesis pathway to ensure its replication [38]. N-3 FAs are intracellular inhibitors of SREBP transcription and maturation and may play a role in counteracting the viral activation of SREBP1/SREBP2, thus decreasing viral replication.
N-3 FAs are known to have anti-inflammatory actions via alterations in membrane physiochemistry, which alter the activity of toll-like receptor 4, diminishing signal transduction to nuclear factor kappa B, which, in turn, blunts the signaling cascade that produces inflammatory cytokines and adhesion molecules [39]. In addition, n-3 FAs serve as substrates for the production of a wide array of oxylipins (prostaglandins, leukotrienes, and inflammation-resolving mediators) that work in harmony to not only suppress inflammatory pathways but also actively promote the resolution of inflammation (for reviews, see the studies of Panigrahy et al. [2], Hathaway et al. [3], Regidor et al. [4], Kothapalli et al. [40], and Arnardottir et al. [41]). Taken together, there is a strong scientific rationale for a favorable effect of higher tissue n-3 levels and protection against both infection with SARS-CoV-2 and its downstream sequelae.
Strengths and weakness
The greatest strength of this study was the ability to query the UKBB database with >110,000 individuals with baseline n-3 FA levels measured and then followed for >10 y. Second, we were able to link blood n-3 levels with 3 important COVID-19-related outcomes: positive test result, hospitalization, and death. We also considered several more relevant covariates in our modeling than previous authors have included, reducing the chances for—but by no means eliminating—unmeasured confounding. The HRs for each outcome were not materially affected by the inclusion of fruit, vegetable, and fiber intakes as covariates. This suggests that higher DHA levels are not simply a surrogate marker of a healthier diet. Finally, we translated NMR-derived n-3 metrics into the more familiar and clinically useful O3I metric.
As to the limitations, the first is one common to all studies linking circulating FA levels measured at baseline with disease outcomes occurring many years later: how stable are blood n-3 FA levels over time? How confident can we be that the levels measured at baseline were the same at the outbreak of the pandemic? In the Framingham Offspring cohort, we found that within persons, the RBC-based O3I was stable between 1999 and 2006 in subjects who did not initiate fish oil supplementation; naturally, it increased in those who did [42]. Other studies have examined plasma FA stability over time and reported precorrelations to postcorrelations of between 0.5 and 0.8 [[43], [44], [45], [46]]. Importantly, when the baseline and follow-up levels were each used to predict the risk of incident heart failure in one study [46], both metrics were equally predictive. In the UKBB cohort, the baseline (2006–2010) fish oil supplement use was reported by 31%, and the consumption of ≥2 meals per week of oily fish was reported by 18% [20]. At a random repeat visit between 2012 and 2013 in 20,334 subjects, the supplement use was reported by 32.7%, and >1 oily fish serving per week was reported by 20%. Hence, it is reasonable to assume that because the 2 major determinants of blood n-3 levels remained stable, the baseline n-3 biomarker levels generally reflected the levels later in life. Indeed, any major shifts in DHA% after baseline would likely have biased our results toward the null, suggesting that the relationships detected may, in fact, be stronger than they appear.
Another potential limitation is the impact of vaccination status on our findings. The study period covered by this report was from 1 January, 2020 (herein defined as the beginning of the pandemic), to 23 March, 2021. By the latter date, ∼38% of the UK population had received ≥1 dose of the vaccine; however, only 2.6% had received 2 doses [47]. Vaccination has been shown to not only reduce the severity of COVID-19 once contracted but also lower the risk of testing positive [48]. Unfortunately, data on vaccination status were not available for this analysis; however, in order for differential vaccination status to have biased our results, one would have to posit that individuals with higher n-3 levels were more likely to have been vaccinated than those with lower levels during the ∼3–4-mo period of this study for which vaccines were available (December 2020 to March 2021). Although this is plausible (i.e., higher n-3 levels are associated with higher SES and healthful behaviors and exercise and a greater consumption of oily fish and fish oil supplements), we are unable to confirm or deny this hypothesis.
Finally, by its very nature, the UKBB is not a random selection of all individuals in the United Kingdom; it is limited to those in a fixed age range who agreed to participate. Thus, the results should not be generalized beyond that context, and outcome data (all taken from electronic medical records) were not formally adjudicated, potentially introducing some bias.
In conclusion, in this study, we confirmed the findings of previous investigations that a low n-3 status was associated with an increased risk of hospitalization with COVID-19. We extended these findings by also showing a reduced risk of testing positive with the virus and providing evidence that the risk of death may also be reduced. Furthermore, we identified the eO3I levels associated with the least (<4%) and greatest (>8%) protection from COVID-19. Taken together, these results suggest that increased intakes of n-3 (from oily fish and/or taking n-3 supplements) should be encouraged to increase the O3I and possibly reduce the risk of COVID-19 and perhaps other future pulmonary viral infections.
Author Contribution
The authors’ responsibilities were as follows—WSH: designed the research; NLT: conducted the research, analyzed the data, and performed statistical analysis; JW: assisted in variable and dataset creation; WSH, NLT, and SPS: wrote the manuscript; WSH: had primary responsibility for the final content; and all authors: read and approved the final manuscript. WSH holds stock in OmegaQuant Analytics, LLC, a laboratory that offers blood fatty acid testing (including the Omega-3 Index) for researchers, clinicians, and consumers, and is a member of the Journal’s Editorial Board. All other authors report no conflicts of interest.
Data Availability
Codebook and analytic code related to data described in the manuscript will be made available upon reasonable request pending application to and approval by the Fatty Acid Research Institute. Raw data are available via standard application procedures directly from the UK Biobank.
Funding
This study was funded in part by the William H. Donner Foundation, which played no role in any aspect of this investigation.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajcnut.2022.11.011.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Louca P., Murray B., Klaser K., Graham M.S., Mazidi M., Leeming E.R., et al. Modest effects of dietary supplements during the COVID-19 pandemic: insights from 445 850 users of the COVID-19 Symptom Study app. BMJ Nutr Prev Health. 2021;4(1):149–157. doi: 10.1136/bmjnph-2021-000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Panigrahy D., Gilligan M.M., Huang S., Gartung A., Cortés-Puch I., Sime P.J., et al. Inflammation resolution: a dual-pronged approach to averting cytokine storms in COVID-19? Cancer Metastasis Rev. 2020;39(2):337–340. doi: 10.1007/s10555-020-09889-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hathaway D., Pandav K., Patel M., Riva-Moscoso A., Singh B.M., Patel A., et al. Omega 3 fatty acids and COVID-19: a comprehensive review. Infect Chemother. 2020;52(4):478–495. doi: 10.3947/ic.2020.52.4.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Regidor P.A., Santos F.G., Rizo J.M., Egea F.M. Pro resolving inflammatory effects of the lipid mediators of omega 3 fatty acids and its implication in SARS COVID-19. Med Hypotheses. 2020;145 doi: 10.1016/j.mehy.2020.110340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weill P., Plissonneau C., Legrand P., Rioux V., Thibault R. May omega-3 fatty acid dietary supplementation help reduce severe complications in Covid-19 patients? Biochimie. 2020;179:275–280. doi: 10.1016/j.biochi.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiang N., Serhan C.N. Specialized pro-resolving mediator network: an update on production and actions. Essays Biochem. 2020;64(3):443–462. doi: 10.1042/EBC20200018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harris W.S., Von Schacky C. The Omega-3 Index: a new risk factor for death from coronary heart disease? Prev Med. 2004;39(1):212–220. doi: 10.1016/j.ypmed.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 8.Asher A., Tintle N.L., Myers M., Lockshon L., Bacareza H., Harris W.S. Blood omega-3 fatty acids and death from COVID-19: a pilot study. Prostaglandins Leukot Essent Fatty Acids. 2021;166 doi: 10.1016/j.plefa.2021.102250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramírez-Santana M., Zapata Barra R., Ñunque González M., Müller J.M., Vásquez J.E., Ravera F., et al. Inverse association between Omega-3 Index and severity of COVID-19: a case-control study. Int J Environ Res Public Health. 2022;19(11):6445. doi: 10.3390/ijerph19116445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zapata B.R., Müller J.M., Vásquez J.E., Ravera F., Lago G., Cañón E., et al. Omega-3 Index and clinical outcomes of severe COVID-19: preliminary results of a cross-sectional study. Int J Environ Res Public Health. 2021;18(15):7722. doi: 10.3390/ijerph18157722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suh W., Urits I., Viswanath O., Kaye A.D., Patel H., Hall W., et al. Three cases of COVID-19 pneumonia that responded to icosapent ethyl supportive treatment. Am J Case Rep. 2020;21 doi: 10.12659/AJCR.928422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berger A.A., Sherburne R., Urits I., Patel H., Eskander J. Icosapent ethyl - a successful treatment for symptomatic COVID-19 infection. Cureus. 2020;12(9) doi: 10.7759/cureus.10211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doaei S., Gholami S., Rastgoo S., Gholamalizadeh M., Bourbour F., Bagheri S.E., et al. The effect of omega-3 fatty acid supplementation on clinical and biochemical parameters of critically ill patients with COVID-19: a randomized clinical trial. J Transl Med. 2021;19(1):128. doi: 10.1186/s12967-021-02795-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sudlow C., Gallacher J., Allen N., Beral V., Burton P., Danesh J., et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3) doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Julkunen H., Cichońska A., Slagboom P.E., Würtz P., Nightingale Health UK Biobank Initiative Metabolic biomarker profiling for identification of susceptibility to severe pneumonia and COVID-19 in the general population. Elife. 2021;10 doi: 10.7554/eLife.63033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun Y., Chatterjee R., Ronanki A., Ye K. Circulating polyunsaturated fatty acids and COVID-19: a prospective cohort study and Mendelian randomization analysis. Front Med (Lausanne) 2022;9 doi: 10.3389/fmed.2022.923746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma Y., Zhang L., Zeng R., Luo D., Jiang R., Wu H., et al. Associations of habitual fish oil use with risk of SARS-CoV-2 infection and COVID-19-related outcomes in UK: national population based cohort study. medRxiv. 2022:2022. 09.14.22279933. [Google Scholar]
- 18.Collins R. What makes UK Biobank special? Lancet. 2012;379(9822):1173–1174. doi: 10.1016/S0140-6736(12)60404-8. [DOI] [PubMed] [Google Scholar]
- 19.Wurtz P., Raiko J.R., Magnussen C.G., Soininen P., Kangas A.J., Tynkkynen T., et al. High-throughput quantification of circulating metabolites improves prediction of subclinical atherosclerosis. Eur Heart J. 2012;33(18):2307–2316. doi: 10.1093/eurheartj/ehs020. [DOI] [PubMed] [Google Scholar]
- 20.Schuchardt J.P., Tintle N., Westra J., Harris W.S. Estimation and predictors of the Omega-3 Index in the UK Biobank. Br J Nutr. 2022:1–40. doi: 10.1017/S0007114522003282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho F.K., Celis-Morales C.A., Gray S.R., Katikireddi S.V., Niedzwiedz C.L., Hastie C., et al. Modifiable and non-modifiable risk factors for COVID-19, and comparison to risk factors for influenza and pneumonia: results from a UK Biobank prospective cohort study. BMJ Open. 2020;10(11) doi: 10.1136/bmjopen-2020-040402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bradbury K.E., Young H.J., Guo W., Key T.J. Dietary assessment in UK Biobank: an evaluation of the performance of the touchscreen dietary questionnaire. J Nutr Sci. 2018;7:e6. doi: 10.1017/jns.2017.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Block R.C., Harris W.S., Reid K.J., Sands S.A., Spertus J.A. EPA and DHA in blood cell membranes from acute coronary syndrome patients and controls. Atherosclerosis. 2008;197(2):821–828. doi: 10.1016/j.atherosclerosis.2007.07.042. [DOI] [PubMed] [Google Scholar]
- Ammann E.M., Pottala J.V., Robinson J.G., Espeland M.A., Harris W.S. Erythrocyte omega-3 fatty acids are inversely associated with incident dementia: secondary analyses of longitudinal data from the Women’s Health Initiative Memory Study (WHIMS) Prostaglandins Leukot Essent Fatty Acids. 2017;121:68–75. doi: 10.1016/j.plefa.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris W.S., Del Gobbo L., Tintle N.L. The Omega-3 Index and relative risk for coronary heart disease mortality: estimation from 10 cohort studies. Atherosclerosis. 2017;262:51–54. doi: 10.1016/j.atherosclerosis.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 26.McBurney M.I., Tintle N.L., Vasan R.S., Sala-Vila A., Harris W.S. Using an erythrocyte fatty acid fingerprint to predict risk of all-cause mortality: the Framingham Offspring Cohort. Am J Clin Nutr. 2021;114(4):1447–1454. doi: 10.1093/ajcn/nqab195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dai X.W., Zhang B., Wang P., Chen C.G., Chen Y.M., Su Y.X. Erythrocyte membrane n-3 fatty acid levels and carotid atherosclerosis in Chinese men and women. Atherosclerosis. 2014;232(1):79–85. doi: 10.1016/j.atherosclerosis.2013.10.028. [DOI] [PubMed] [Google Scholar]
- Filipovic M.G., Aeschbacher S., Reiner M.F., Stivala S., Gobbato S., Bonetti N., et al. Whole blood omega-3 fatty acid concentrations are inversely associated with blood pressure in young, healthy adults. J Hypertens. 2018;36(7):1548–1554. doi: 10.1097/HJH.0000000000001728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Markhus M.W., Skotheim S., Graff I.E., Froyland L., Braarud H.C., Stormark K.M., et al. Low omega-3 index in pregnancy is a possible biological risk factor for postpartum depression. PLoS One. 2013;8(7) doi: 10.1371/journal.pone.0067617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Z.H., Zhong W.F., Liu S., Kraus V.B., Zhang Y.J., Gao X., et al. Associations of habitual fish oil supplementation with cardiovascular outcomes and all cause mortality: evidence from a large population based cohort study. BMJ. 2020;368:m456. doi: 10.1136/bmj.m456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang W., Li F.R., Yang H.H., Chen G.C., Hua Y.F. Relationship between fish oil use and incidence of primary liver cancer: findings from a population-based prospective cohort study. Front Nutr. 2021;8 doi: 10.3389/fnut.2021.771984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang X., Li Y., Zhuang P., Liu X., Zhang Y., Zhang P., et al. Habitual fish oil supplementation and risk of incident inflammatory bowel diseases: a prospective population-based study. Front Nutr. 2022;9 doi: 10.3389/fnut.2022.905162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma H., Zhou T., Li X., Heianza Y., Qi L. Use of fish oil supplements is differently related to incidence of all-cause and vascular dementia among people with the distinct APOE ε4 dosage. Clin Nutr. 2022;41(3):731–736. doi: 10.1016/j.clnu.2022.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verduci E., Risé P., Di Profio E., Fiori L., Vizzuso S., Dilillo D., et al. Blood fatty acids profile in MIS-C children. Metabolites. 2021;11(11):721. doi: 10.3390/metabo11110721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sedighiyan M., Abdollahi H., Karimi E., Badeli M., Erfanian R., Raeesi S., et al. Omega-3 polyunsaturated fatty acids supplementation improve clinical symptoms in patients with Covid-19: a randomised clinical trial. Int J Clin Pract. 2021;75(12) doi: 10.1111/ijcp.14854. [DOI] [PubMed] [Google Scholar]
- 36.Vivar-Sierra A., Araiza-Macías M.J., Hernández-Contreras J.P., Vergara-Castañeda A., Ramírez-Vélez G., Pinto-Almazán R., et al. In silico study of polyunsaturated fatty acids as potential SARS-CoV-2 spike protein closed conformation stabilizers: epidemiological and computational approaches. Molecules. 2021;26(3):711. doi: 10.3390/molecules26030711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goc A., Niedzwiecki A., Rath M. Polyunsaturated ω-3 fatty acids inhibit ACE2-controlled SARS-CoV-2 binding and cellular entry. Sci Rep. 2021;11(1):5207. doi: 10.1038/s41598-021-84850-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yuan S., Chu H., Chan J.F., Ye Z.W., Wen L., Yan B., et al. SREBP-dependent lipidomic reprogramming as a broad-spectrum antiviral target. Nat Commun. 2019;10(1):120. doi: 10.1038/s41467-018-08015-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Calder P.C. Omega-3 fatty acids and inflammatory processes: from molecules to man. Biochem Soc Trans. 2017;45(5):1105–1115. doi: 10.1042/BST20160474. [DOI] [PubMed] [Google Scholar]
- 40.Kothapalli K.S.D., Park H.G., Brenna J.T. Polyunsaturated fatty acid biosynthesis pathway and genetics. implications for interindividual variability in prothrombotic, inflammatory conditions such as COVID-19✰,✰✰,★,★★. Prostaglandins Leukot Essent Fatty Acids. 2020;162 doi: 10.1016/j.plefa.2020.102183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arnardottir H., Pawelzik S.C., Öhlund Wistbacka U., Artiach G., Hofmann R., Reinholdsson I., et al. Stimulating the resolution of inflammation through omega-3 polyunsaturated fatty acids in COVID-19: rationale for the COVID-Omega-F Trial. Front Physiol. 2020;11 doi: 10.3389/fphys.2020.624657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harris W.S., Pottala J.V., Vasan R.S., Larson M.G., Robins S.J. Changes in erythrocyte membrane trans and marine fatty acids between 1999 and 2006 in older Americans. J Nutr. 2012;142(7):1297–1303. doi: 10.3945/jn.112.158295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lai H.T., de Oliveira Otto M.C., Lemaitre R.N., McKnight B., Song X., King I.B., et al. Serial circulating omega 3 polyunsaturated fatty acids and healthy ageing among older adults in the Cardiovascular Health Study: prospective cohort study. BMJ. 2018;363(363) doi: 10.1136/bmj.k4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma J., Folsom A.R., Eckfeldt J.H., Lewis L., Chambless L.E. Short- and long-term repeatability of fatty acid composition of human plasma phospholipids and cholesterol esters. The Atherosclerosis Risk in Communities (ARIC) Study Investigators. Am J Clin Nutr. 1995;62(3):572–578. doi: 10.1093/ajcn/62.3.572. [DOI] [PubMed] [Google Scholar]
- 45.Zheng J.S., Imamura F., Sharp S.J., Koulman A., Griffin J.L., Mulligan A.A., et al. Changes in plasma phospholipid fatty acid profiles over 13 years and correlates of change: European Prospective Investigation into Cancer and Nutrition-Norfolk Study. Am J Clin Nutr. 2019;109(6):1527–1534. doi: 10.1093/ajcn/nqz030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Djousse L., Petrone A.B., Weir N.L., Hanson N.Q., Glynn R.J., Tsai M.Y., et al. Repeated versus single measurement of plasma omega-3 fatty acids and risk of heart failure. Eur J Nutr. 2014;53(6):1403–1408. doi: 10.1007/s00394-013-0642-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richie H, Rodés-Guirao L, Appel C, Giattino C, Ortiz-Ospina E, Hasell J, et al. Causes of death [Internet]. https://ourworldindata.org/covid-vaccinations?country=GBR#citation. (20 August, 2022, Date accessed).
- 48.Zheng C., Shao W., Chen X., Zhang B., Wang G., Zhang W. Real-world effectiveness of COVID-19 vaccines: a literature review and meta-analysis. Int J Infect Dis. 2022;114:252–260. doi: 10.1016/j.ijid.2021.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Codebook and analytic code related to data described in the manuscript will be made available upon reasonable request pending application to and approval by the Fatty Acid Research Institute. Raw data are available via standard application procedures directly from the UK Biobank.