Abstract
A 1,067-bp cDNA, designated axeA, coding for an acetyl xylan esterase (AxeA) was cloned from the anaerobic rumen fungus Orpinomyces sp. strain PC-2. The gene had an open reading frame of 939 bp encoding a polypeptide of 313 amino acid residues with a calculated mass of 34,845 Da. An active esterase using the original start codon of the cDNA was synthesized in Escherichia coli. Two active forms of the esterase were purified from recombinant E. coli cultures. The size difference of 8 amino acids was a result of cleavages at two different sites within the signal peptide. The enzyme released acetate from several acetylated substrates, including acetylated xylan. The activity toward acetylated xylan was tripled in the presence of recombinant xylanase A from the same fungus. Using p-nitrophenyl acetate as a substrate, the enzyme had a Km of 0.9 mM and a Vmax of 785 μmol min−1 mg−1. It had temperature and pH optima of 30°C and 9.0, respectively. AxeA had 56% amino acid identity with BnaA, an acetyl xylan esterase of Neocallimastix patriciarum, but the Orpinomyces AxeA was devoid of a noncatalytic repeated peptide domain (NCRPD) found at the carboxy terminus of the Neocallimastix BnaA. The NCRPD found in many glycosyl hydrolases and esterases of anaerobic fungi has been postulated to function as a docking domain for cellulase-hemicellulase complexes, similar to the dockerin of the cellulosome of Clostridium thermocellum. The difference in domain structures indicated that the two highly similar esterases of Orpinomyces and Neocallimastix may be differently located, the former being a free enzyme and the latter being a component of a cellulase-hemicellulase complex. Sequence data indicate that AxeA and BnaA might represent a new family of hydrolases.
Plant cell walls are an important natural resource, composed mainly of cellulose, hemicellulose, and lignin. Hemicelluloses account for 20 to 30% of the dry weight of plant cell walls (10). Understanding how these complex polymers are degraded is important for applications in the pulp bleaching, food and feed processing, and fuel production industries. One form of hemicellulose, arabinoxylan, is prominent in grasses and consists of β-1,4-linked xylopyranosyl residues (27). The xylose residues are substituted with 4-O-methylglucuronic acid, arabinose, and acetyl residues. Some arabinose residues are substituted at the O-5 position with feruloyl or p-coumaroyl groups (4). Approximately 22 to 50% of the xylose residues are substituted with acetyl groups in the O-2 or O-3 position. The acetyl groups, along with the other previously mentioned substituents, hinder the complete breakdown of hemicellulose (8). The acetyl xylan esterases (EC 3.1.1.72) (AxeAs) cleave the ester bonds and thus remove the acetyl moieties from arabinoxylan. They are present in many bacteria, such as Fibrobacter succinogenes (23), Streptomyces lividans (29), Caldocellum saccharolyticum (21), and Thermoanaerobacterium sp. strain JW/SL YS485 (20, 28), as well as in fungi, such as Aspergillus niger (18), Schizophyllum commune (13), and Trichoderma reesei (25). Pectin acetyl esterase has been found in Erwinia chrysanthemi 3937 and Aspergillus aculeatus (15, 30). Although the AxeAs remove acetyl groups from complex carbohydrates, their sources are different, and very little homology has been found between the esterases so far sequenced (9, 12, 20, 22, 29, 32).
Anaerobic fungi produce hydrolytic enzymes which break down hemicellulose. These enzymes include xylanase, β-xylosidase, arabinofuranosidase, phenolic acid esterases, and AxeA (2). The presence of AxeA activity has been reported in both monocentric and polycentric fungi. Many of these enzymes occur in a hypothesized multienzyme complex similar to that of the cellulosome of Clostridium thermocellum (11, 16, 17). Thus many enzymes from anaerobic fungi have noncatalytic repeated peptide domains (NCRPDs) which have been postulated to bind to noncatalytic scaffolding-type proteins. The NCRPD is separated from its catalytic site by Ser-Thr-rich linkers. NCRPDs function as dockerins, but their sequences are completely different from the dockerin domains of C. thermocellum. Two AxeAs from the anaerobic fungus Neocallimastix patriciarum contain NCRPDs, and thus are believed to be involved in the cellulosomal complex of this fungus (9). In this study, we report the cloning, sequencing, purification, and characterization of an AxeA from Orpinomyces sp. strain PC-2. The enzyme lacks an NCRPD, which is in contrast to the AxeA from Neocallimastix (9).
MATERIALS AND METHODS
Fungal and bacterial strains, culture conditions, and vectors.
Orpinomyces sp. strain PC-2 used in this study was isolated and described by Borneman et al. (2). For enzyme production, the fungus was grown without shaking in 20-liter carboys at 39°C for 6 days in the basic medium described by Barichievich and Calza (1) with 0.2% Avicel as the growth substrate. Escherichia coli XL-1 Blue, λZAPII, and pBluescript were products of Stratagene Cloning Systems (La Jolla, Calif.) and were handled according to the manufacturer’s instructions.
Screening and sequencing of axeA.
Construction of the cDNA library of Orpinomyces was described previously (6). Briefly, Orpinomyces was grown for 3 days on 0.4% (wt/vol) Avicel cellulose. The harvested mycelium was immediately frozen in liquid nitrogen, ground in a mortar, and broken with a bead beater. RNA was extracted and isolated with a total RNA isolation kit (Promega, Madison, Wis.). Poly(A)+ RNA was purified on an oligo(dT)-cellulose column and used as a template for the construction of a cDNA library in phage λZAPII by a commercial kit (Stratagene). Screenings of clones were carried out according to procedures presented previously (6) with some modifications. Top agar containing isopropyl-β-d-thiogalactopyranoside (IPTG) (5 mM) was flooded with the substrate β-naphthyl acetate (βNA) prepared as previously described (26). Positive clones from an initial phage population of 50,000 PFU were identified by observing hydrolysis of βNA, producing a purple color around the plaque. A secondary screening resulted in isolation of pure clones. Positive clones were converted into pBluescript SK(−) by in vivo excision according to the manufacturer’s directions. The plasmid DNA was purified from an overnight culture of E. coli grown in Luria-Bertani broth containing 50 μg of ampicillin per ml by using the Qiaprep spin plasmid miniprep kit from Qiagen (Chatsworth, Calif.). The nucleotide sequence was determined with an automatic PCR sequencer (Applied Biosystems) using universal and specific primers to sequence both strands of the insert. The Genetics Computer Group version 8 software (University of Wisconsin Biotechnology Center, Madison, Wis.) on the VAX/VMS system of the Bioscience Computing Resource at the University of Georgia was used to analyze sequence data.
Enzyme assays.
Enzyme assays were carried out in 10 mM phosphate buffer, pH 6.7, at 37°C unless otherwise stated. For routine detection of acetyl esterase activity, p-nitrophenyl acetate (pNA) (Sigma, St. Louis, Mo.) was used as the substrate. In each well of a 96-well microtiter plate, a 200-μl aliquot of 100 μM pNA was added to the appropriate amount of enzyme solution (10 to 50 μl), and the change in absorbance at 405 nm was measured over time in an ATTC 960 microtiter plate reader (SLT-Labinstruments, Salzburg, Austria). p-Nitrophenol was used as a standard. In order to measure the effect of temperature on the enzyme, samples in 1 ml of 10 mM phosphate buffer, pH 6.7, were preincubated for 5 min at the appropriate temperature. The enzyme reaction was started by the addition of 10 μl of 100 μM pNA. The release of p-nitrophenol was measured with a Hewlett Packard diode array spectrophotometer. To measure the effect of pH on the enzyme, pNA was used as the substrate. It was incorporated into buffers over a range from pH 2 to 10.5 by using a universal phosphate buffer system. Activity was assayed by observing the release of p-nitrophenol in a microplate reader. Substrates for enzyme specificity studies included glucose pentaacetate, tri-O-acetyl-d-galactal, and xylose tetraacetate (Sigma) and O-{5-O-[(E)-feruloyl]-α-l-arabinofuranosyl}-(1→3)-O-β-d-xylopyranosyl-(1→4)-d-xylopyranose (FAXX) prepared from wheat bran (3). The amount of acetate released was determined with a Hewlett Packard 1100 series high-pressure liquid chromatograph equipped with an HP 1315 diode array detector. The column was an Aminex HPX 87H equilibrated with 5 mM H2SO4. Sodium acetate was used as a standard. The concentration of substrates was 10 mM. The assays were carried out at 40°C and pH 6.7. Feruloyl esterase activity was measured by determining the release of ferulic acid with a 5-μm Hypersil octyldecyl silane column (125 by 4 mm) with a mobile phase of 10 mM sodium formate and 30% methanol (3). Ferulic acid was used as a standard. The synergistic effect of xylanase on AxeA activity was measured by assaying released acetate as described above. The incubation mixture contained (per ml) 10 mg of chemically acetylated birchwood xylan (a gift from W. Lorenz, University of Georgia), 25 U of recombinant xylanase (XynA) from Orpinomyces sp. strain PC-2 (17), and 13 U of AxeA as assayed with pNA in 10 mM phosphate buffer, pH 6.7. The incubation was carried out at 40°C.
Enzyme expression and purification.
E. coli, harboring the gene encoding AxeA, was grown in a 20-liter fermentor with Luria-Bertani broth containing 100 μg of ampicillin per ml at 37°C with an impeller speed of 250 rpm. Cultures were grown to an optical density at 600 nm of 0.5. Subsequently, IPTG was added to a final concentration of 1 mM, and the cells were grown for an additional 3 h. Cells were harvested by centrifugation at 10,000 × g. They were resuspended in 20 mM Tris, pH 7.5, in a ratio of 1 g of cells to 3 ml of buffer and were lysed using a French pressure cell. Residual cells and debris were removed by centrifugation at 100,000 × g for 15 min. Crude lysate (30 ml) was applied to an SP Sepharose high-performance column (Pharmacia, Piscataway, N.J.) equilibrated with 20 mM Tris, pH 7.5. Proteins were eluted with an 800-ml gradient from 0 to 1 M NaCl in the same buffer. Fractions with activity were pooled and concentrated with a Centricon 10 ultrafiltration device (Amicon, Beverly, Mass.) to a volume of 1 ml. This solution was loaded onto a TSK 3000SW (Tosohaas, Montgomeryville, Pa.) gel filtration column which was equilibrated with 20 mM Tris, pH 7.5, and 200 mM NaCl. Active fractions were combined and applied onto a MonoQ HR 5/5 (Pharmacia) column equilibrated with 20 mM Tris, pH 7.5. The purified forms of the enzyme were eluted with a 20-ml gradient of 1 M NaCl.
Analytical methods.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was carried out by the method of Laemmli (14). Zymogram analysis was performed by running PAGE gels of enzyme samples under native but reducing conditions according to the manufacturer’s instructions (Bio-Rad, Hercules, Calif.). After being run, the gels were equilibrated in 10 mM phosphate buffer, pH 6.7, for 15 min, after which substrate-coupler solution, as described by Rosenberg et al. (26), was added. PCR was carried out in a 480 thermal cycler (Perkin-Elmer) for 35 cycles of denaturation (1 min at 94°C), annealing (1.5 min at 40°C), and extension (1.5 min at 72°C). N-terminal amino acid sequencing was performed on an Applied Biosystems model 477A gas phase sequencer equipped with an automatic on-line phenylthiohydantoin analyzer. Localization of the enzyme in the periplasm was carried out as described before (7). Protein was measured by the method of Bradford (5).
Nucleotide sequence accession number.
The nucleotide sequence of axeA of Orpinomyces sp. strain PC-2 has been assigned the GenBank accession no. AF001178.
RESULTS
Isolation of axeA.
In previous publications we have described the screening of a cDNA library created in λZAPII from mRNA extracted from Orpinomyces sp. strain PC-2 (6). By similar methods, βNA was used as a substrate to screen the cDNA library. The substrate, when coupled to o-dianisidine, creates a purple color which can be readily visualized, and plaques can be subsequently isolated. One plaque was able to cleave the substrate which was obtained in the initial screening of 5 × 104 PFUs. This plaque was rescreened at a lower dilution to obtain plaques hydrolyzing βNA. Six plaques were picked and converted into pBluescript SK(−) by in vivo excision. The six plaques had the same EcoRI restriction pattern (results not shown). The insert had an approximate size of 1.2 kb. The plasmid harboring axeA, pAE, was sequenced from both ends by using universal primers. Internal primers were made from the original sequence and used to sequence both strands of the insert completely.
The cDNA library was screened a second time to isolate any additional gene(s) encoding AxeA. From a total phage population of 50,000 PFU, three plaques which hydrolyzed the substrate were isolated and converted into pBluescript SK(−) by previously described techniques (7). These plasmids were used as templates in a PCR with forward and reverse primers corresponding to the entire gene. The PCR products were of the same size as the first gene obtained, demonstrating that the same clones were isolated.
Nucleotide sequence and deduced amino acid sequence of axeA.
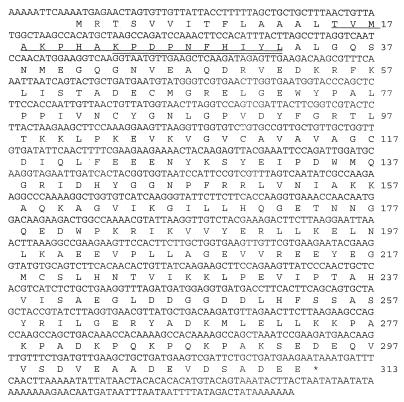
Figure 1 shows the complete nucleotide and deduced amino acid sequences of axeA. An insert of 1,067 nucleotides was obtained. A 939-bp open reading frame (ORF) containing axeA was detected. The protein was composed of 313 amino acids with a calculated molecular mass of 34,845 Da.
FIG. 1.
Nucleotide and deduced amino acid sequence of axeA from Orpinomyces sp. strain PC-2. Two forms of the enzyme were expressed in E. coli (see Fig. 2). The N-terminal sequences of the larger- and smaller-molecular-mass proteins are underlined and double underlined, respectively. The stop codon is indicated by an asterisk.
The determination of the translation start codon is based on the fact that this was the longest ORF observed, and there was a typical anaerobic fungal signal sequence comprised mainly of aliphatic amino acids at the N terminus of the predicted protein. N-terminal sequence data matched the deduced amino acid sequence. There is one stop codon in the ORF, with a heptamer poly(A) tail downstream.
Homology of AxeA with other esterases.
AxeA had 56% amino acid sequence identity with the acetyl xylan esterase BnaA from N. patriciarum. A search of the GenBank data base using the BLAST sequence analysis program showed no other protein with higher than 20% amino acid identity with AxeA. The data suggest that AxeA and BnaA belong to a new family of hydrolases.
Expression and purification of AxeA.
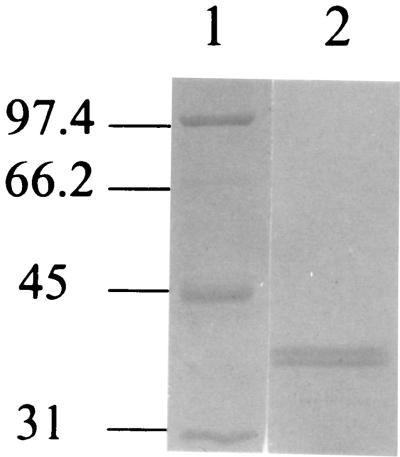
An E. coli strain harboring pAE was grown in a 20-liter fermentor in Luria-Bertani broth plus 50 μg of ampicillin per ml. The axeA was under the control of the lacZ promoter, and IPTG was used to induce the expression of the protein. AxeA activity was monitored during expression, and it peaked 3 h after the addition of IPTG. AxeA activity was associated with the cells, and no significant activity was detected in the supernatant. The enzyme was purified from the cell extract. The first step involving a SP Sepharose high-performance column achieved a 30-fold purification. Upon chromatography with a TSK 3000SW gel filtration column, esterase activity was found in two peaks. The activity detected in peak one had a higher molecular mass but lower specific activity than the activity detected in peak two. This may be explained by the possibility that the enzyme aggregates with other proteins. Peak one was not studied further. In the final step with the MonoQ HR 5/5 column, one peak of activity eluted that, when analyzed by SDS-PAGE, generated two bands (Fig. 2). The estimated molecular mass of the smaller and larger forms of AxeA were 39 and 40 kDa, respectively.
FIG. 2.
SDS-PAGE analysis of the purified recombinant AxeA. The recombinant protein was obtained in two active forms based on zymogram analysis (lane 2). The two forms were a result of differential signal peptide cleavage, which was confirmed by N-terminal sequencing (Fig. 1). Molecular mass markers are shown in lane 1, and molecular mass in kilodaltons is indicated on the left.
Zymogram analysis demonstrated that both of these bands had activity against βNA, indicating that there are two proteolytic forms of the enzyme produced by E. coli. N-terminal sequencing of these protein bands demonstrated that the higher-molecular-mass protein was eight amino acids longer and had the N-terminal sequence TVMAKPHAKP, and the lower-molecular-mass protein had the N-terminal sequence KPDPNFHIYL. Signal sequence cleavage occurred after the amino acid residues AAL and PHA, as seen in Fig. 1.
Localization of AxeA in E. coli.
To determine whether the signal peptide cleavage events resulted in secretion of the enzyme into the periplasm, an E. coli strain harboring axeA was spheroplasted as described by Chen et al. (7) with the method of Neu and Heppel (24). β-Galactosidase and alkaline phosphatase were used as intracellular and periplasmic markers, respectively. AxeA activity detected in the periplasm accounted for 13.3% of the total activity produced by the recombinant E. coli.
Characterization of AxeA.
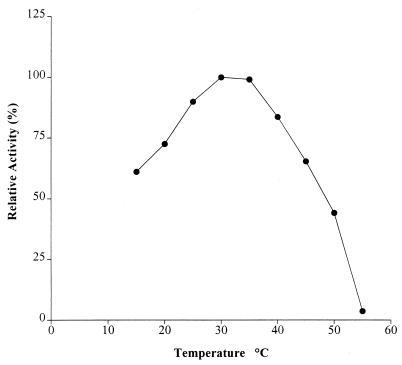
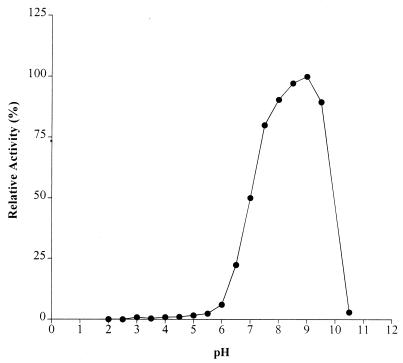
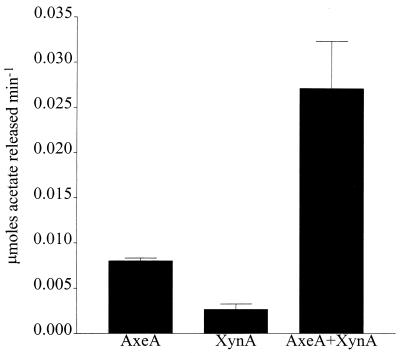
Temperature and pH optima for AxeA are 30°C and 9.0, respectively, as shown in Fig. 3 and 4. AxeA had activity against a variety of substrates. In a comparative assay the following activities (micromoles of acetate produced per minute) were obtained: pNA, 0.49; glucose pentaacetate, 0.31; xylose tetraacetate, 0.20; and tri-O-acetyl-d-galactal, 0.08. Neither FAXX, a substrate for phenolic acid esterase, nor cellulose acetate was hydrolyzed by the enzyme. The enzyme had Km and Vmax of 0.9 mM and 785 U/mg, respectively, when pNA was used as the substrate. Figure 5 shows that a xylanase from Orpinomyces, XynA, has a synergistic effect in the release of acetate by AxeA from acetylated xylan. When the esterase was incubated with XynA, there was a threefold increase in the amount of acetate released from acetylated birchwood xylan.
FIG. 3.
Plot of effect of temperature on AxeA with pNA as substrate. Experiments were carried out at pH 6.7.
FIG. 4.
Plot of pH optimum of AxeA with pNA as substrate. Experiments were carried out at 40°C.
FIG. 5.
Synergistic effect of xylanase on the release of acetate from acetylated xylan by AxeA. The reaction mixture contained (per milliliter of phosphate buffer, pH 6.7) 10 mg of acetylated xylan, 25 U of recombinant xylanase (XynA) from Orpinomyces sp. strain PC-2, and 13 U of AxeA. The incubation was carried out at 40°C.
DISCUSSION
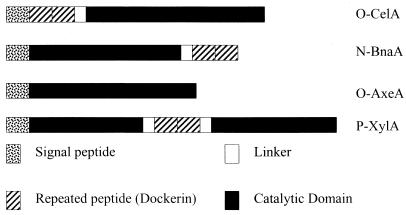
Anaerobic fungi are an important part of the microflora of the ruminal environment, producing hydrolytic enzymes which act synergistically to degrade plant cell walls. In particular, they are hypothesized to produce multienzyme complexes which aid in this degradation. The enzymes in these complexes have a typical domain structure (Fig. 6). The protein consists, at least, of a signal peptide, a catalytic domain, and a NCRPD (also known as a dockerin domain). The dockerin domains, separated by Ser-Thr-rich linkers, can be at the N terminus, the C terminus, or in the middle of the protein. Enzyme complexes have only been found in anaerobic microorganisms. Anaerobic fungi, like aerobic organisms, produce, in addition to enzyme complexes, free enzymes not associated with complexes. In this report, we show that Orpinomyces produces an AxeA which has homology with the catalytic domain of an esterase from N. patriciarum. In spite of this homology, the enzymes differ in that the Neocallimastix enzyme has a dockerin domain, while the esterase from Orpinomyces does not. It is possible that this is a result of gene transfer from one organism to the other and subsequent loss or addition of the dockerin sequences. Horizontal gene transfer has been postulated to occur in Orpinomyces due to evidence that several enzymes from other organisms are homologous with enzymes from Orpinomyces (6, 7, 19).
FIG. 6.
Domain organization of enzymes cloned from anaerobic fungi. O-CelA, cellulase A from Orpinomyces sp. strain PC-2 (16); N-BnaA, AxeA from N. patriciarum (9); O-AxeA, AxeA from Orpinomyces sp. strain PC-2; P-XylA, xylanase from Piromyces communis (11).
The amino acid sequence of AxeA is not similar to any other protein in the GenBank data base except for BnaA. A new family of hydrolases including BnaA was first postulated by Dalrymple et al. (9). This family would also include acetyl esterases and BnaB, and BnaC of Neocallimastix. BnaC also has an NCRPD like BnaA and is postulated to be a part of the multienzyme complex produced by anaerobic fungi, while BnaB does not have an NCRPD. BnaB has 40% amino acid identity to a domain of unknown function in the CelE cellulase from C. thermocellum, while BnaC has 52% amino acid identity to a domain of unknown function in the XynB xylanase from Ruminococcus flavefaciens. We suggest that these domains may encode acetyl esterases, which would suggest bifunctional activities for these enzymes. Furthermore, Dalrymple et al. (9) showed that the sequences of these esterases, plus other esterases and some proteins of unknown function, have conserved residues, including glycine, asparagine, and histidine (Fig. 7), which are thought to be part of the active site of these enzymes (9). However, outside of these sequences, no similarity exists between AxeA and other esterases, and thus we suggest that AxeA and BnaA are in a family separate from the other enzymes mentioned. At the position of the linker sequence found in BnaA, AxeA has a sequence with repeated amino acids KPAKPADKPQKPQKPA (Fig. 7), the function of which is not known.
FIG. 7.
AxeA has 56% amino acid identity with BnaA from N. patriciarum (accession no. U66251). The sequence for AxeA from Orpinomyces sp. strain PC-2 is shown on top. The linker sequence and the dockerin sequences present in BnaA are underlined and double underlined, respectively. These sequences are not present in AxeA. Residues in boldface indicate residues conserved among esterases.
Zymogram analysis with concentrated culture supernatant from Orpinomyces revealed only one band of activity in the native PAGE gel. This differs from Neocallimastix, in which there were four bands of activity using βNA (9). These data suggest that the axeA cloned from Orpinomyces is the only copy of that gene. This is supported by the fact that no activity against pNA was found in a purified, cellulosomal-type enzyme complex from Orpinomyces sp. strain PC-2. Thus, the AxeA from Orpinomyces sp. strain PC-2 may not be part of the multienzyme complex of this fungus. This is in contrast to esterases of Neocallimastix. Chen et al. (7) have shown that a lichenase is present in the culture supernatant of Orpinomyces sp. strain PC-2. This enzyme was not found in Neocallimastix sp. strain MC-2. These observations suggest that monocentric and polycentric fungi differ by their enzyme types and not just by morphological differences.
AxeA had affected a variety of acetylated substrates, including acetylated xylan, proving that this is indeed an AxeA. When the enzyme was incubated with the xylanase from Orpinomyces, XynA, there was a threefold increase in activity. This indicates that there is a synergism between AxeA and XynA from Orpinomyces. This is possibly due to the fact that the xylanase cleaves the xylan chain into many shorter chains, making them more readily available to the AxeA. Another study showed that Bacillus stearothermophilus AxeA worked synergistically with a xylanase from the same organism (31).
Zymogram analysis and Coomassie-blue-stained gels of the purified enzyme indicate that two forms of the enzyme are produced by E. coli. We hypothesize that there is differential processing of the signal peptide, but both enzymes are secreted into the periplasmic space. The cleavage site in each enzyme isoform is preceded by an aliphatic amino acid similar to a lichenase from Orpinomyces which is secreted into the periplasm (7). The amino acids in the predicted proteolytic site are similar to the amino acids predicted to occur in other periplasmic proteins. The reason for the existence of two forms of the recombinant enzyme is not known, but this could be explained by the likelihood that cleavage events occur at these sites. The two isozymes, as visualized by SDS-PAGE, occur in almost equal amounts.
ACKNOWLEDGMENTS
The work presented in this publication was funded by a grant from the Department of Energy (DE-FG02-93ER0127). Support by a Georgia Power Distinguished Professorship in Biotechnology (to L.G.L.) is also gratefully acknowledged.
REFERENCES
- 1.Barichievich E M, Calza R E. Supernatant protein and cellulase activities of the anaerobic ruminal fungus Neocallimastix frontalis EB188. Appl Environ Microbiol. 1990;56:43–48. doi: 10.1128/aem.56.1.43-48.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borneman W S, Akin D E, Ljungdahl L G. Fermentation products and plant cell wall-degrading enzymes produced by monocentric and polycentric anaerobic ruminal fungi. Appl Environ Microbiol. 1989;55:1066–1073. doi: 10.1128/aem.55.5.1066-1073.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borneman W S, Hartley R D, Himmelsbach D S, Ljungdahl L G. Assay for trans-p-coumaroyl esterase using a specific substrate from plant cell walls. Anal Biochem. 1990;190:129–133. doi: 10.1016/0003-2697(90)90145-y. [DOI] [PubMed] [Google Scholar]
- 4.Borneman W S, Ljungdahl L G, Hartley R D, Akin D E. Feruloyl and p-coumaroyl esterases from the anaerobic fungus Neocallimastix strain MC-2: properties and functions in plant cell wall degradation. In: Coughlan M P, Hazlewood G P, editors. Hemicellulose and hemicellulases. London, England: Portland Press; 1993. pp. 85–102. [Google Scholar]
- 5.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of proteins utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 6.Chen H, Li X L, Ljungdahl L G. A cyclophilin from the polycentric anaerobic rumen fungus Orpinomyces sp. strain PC-2 is highly homologous to vertebrate cyclophilin B. Proc Natl Acad Sci USA. 1995;92:2587–2591. doi: 10.1073/pnas.92.7.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen H, Li X L, Ljungdahl L G. Sequencing of a 1,3-1,4-beta-d-glucanase (lichenase) from the anaerobic fungus Orpinomyces strain PC-2: properties of the enzyme expressed in Escherichia coli and evidence that the gene has a bacterial origin. J Bacteriol. 1997;179:6028–6034. doi: 10.1128/jb.179.19.6028-6034.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chesson A, Forsberg C W. Polysaccharide degradation by rumen microorganisms. In: Hobson P N, editor. The rumen microbial ecosystem. New York, N.Y: Elsevier Applied Science; 1988. pp. 251–284. [Google Scholar]
- 9.Dalrymple B P, Cybinski D H, Layton I, McSweeney C S, Xue G P, Swadling Y J, Lowry J B. Three Neocallimastix patriciarum esterases associated with the degradation of complex polysaccharides are members of a new family of hydrolases. Microbiology. 1997;143:2605–2614. doi: 10.1099/00221287-143-8-2605. [DOI] [PubMed] [Google Scholar]
- 10.Eriksson K-E L, Blanchette R A, Ander P. Microbial and enzymatic degradation of wood and wood components. Berlin, Germany: Springer-Verlag; 1990. [Google Scholar]
- 11.Fanutti C, Ponyi T, Black G W, Hazlewood G P, Gilbert H J. The conserved noncatalytic 40-residue sequence in cellulases and hemicellulases from anaerobic fungi functions as a protein docking domain. J Biol Chem. 1995;270:29314–29322. doi: 10.1074/jbc.270.49.29314. [DOI] [PubMed] [Google Scholar]
- 12.Ghosh D, Duax W, Jornvall H, Eyzaguirre J. Acetyl xylan esterase II from Penicillium purpurogenum is similar to an esterase from Trichoderma reesei but lacks a cellulose binding domain. FEBS Lett. 1998;423:35–38. doi: 10.1016/s0014-5793(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 13.Halgasova N, Kutejova E, Timko J. Purification and some characteristics of the acetyl xylan esterase from Schizophyllum commune. Biochem J. 1994;298:751–755. doi: 10.1042/bj2980751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 15.Leeuwen M J F S-v, v. d. Broek L A M, Schols J A, Beldman G, Voragen A G J. Rhamnogalacturonan acetylesterase: a novel enzyme from Aspergillus aculeatus, specific for the deacetylation of hairy (ramified) regions of pectins. Appl Microbiol Biotechnol. 1992;38:347–349. [Google Scholar]
- 16.Li X-L, Chen H, Ljungdahl L G. Two cellulases, CelA and CelC, from the polycentric anaerobic fungus Orpinomyces strain PC-2 contain N-terminal docking domains for a cellulase-hemicellulase complex. Appl Environ Microbiol. 1997;63:4721–4728. doi: 10.1128/aem.63.12.4721-4728.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X-L, Chen H, Ljungdahl L G. Monocentric and polycentric anaerobic fungi produce structurally related cellulases and xylanases. Appl Environ Microbiol. 1997;63:628–635. doi: 10.1128/aem.63.2.628-635.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Linden J, Samara M, Decker S, Johnson E, Boyer M, Pecs M, Adney W, Himmel M. Purification and characterization of an acetyl esterase from Aspergillus niger. Appl Biochem Biotechnol. 1994;45-46:383–393. doi: 10.1007/BF02941813. [DOI] [PubMed] [Google Scholar]
- 19.Ljungdahl L G, Li X-L, Chen H Z. Evidence in anaerobic fungi of transfer of genes between them and from aerobic fungi, bacteria and animal hosts. In: Wiegel J, Adams M W W, editors. Thermophiles: the keys to molecular evolution and the origin of life? Philadelphia, Pa: Taylor and Francis, Inc.; 1998. pp. 187–197. [Google Scholar]
- 20.Lorenz W W, Wiegel J. Isolation, analysis, and expression of two genes from Thermoanaerobacterium sp. strain JW/SL YS485: a β-xylosidase and a novel acetyl xylan esterase with cephalosporin C deacetylase activity. J Bacteriol. 1997;179:5436–5441. doi: 10.1128/jb.179.17.5436-5441.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luthi E, Jasmat N B, Bergquist P L. Overproduction of an acetylxylan esterase from the extreme thermophile “Caldocellum saccharolyticum” in Escherichia coli. Appl Microbiol Biotechnol. 1990;34:214–219. doi: 10.1007/BF00166783. [DOI] [PubMed] [Google Scholar]
- 22.Margolles-Clark E, Tenkanen M, Penttila M. Acetyl xylan esterase from Trichoderma reesei contains an active-site serine residue and a cellulose-binding domain. Eur J Biochem. 1996;237:553–560. doi: 10.1111/j.1432-1033.1996.0553p.x. [DOI] [PubMed] [Google Scholar]
- 23.McDermid K P, Forsberg C W, MacKenzie C R. Purification and properties of an acetyl xylan esterase from Fibrobacter succinogenes. Appl Environ Microbiol. 1990;56:3805–3810. doi: 10.1128/aem.56.12.3805-3810.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neu H C, Heppel L A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965;240:3685–3692. [PubMed] [Google Scholar]
- 25.Poutanen K, Sundberg M, Korte H, Puls J. Deacetylation of xylans by acetyl esterases of Trichoderma reesei. Appl Microbiol Biotechnol. 1990;33:506–510. [Google Scholar]
- 26.Rosenberg M, Roegner V, Becker F F. The quantitation of rat serum esterases by densitometry of acrylamide gels stained for enzyme activity. Anal Biochem. 1975;66:206–212. doi: 10.1016/0003-2697(75)90738-1. [DOI] [PubMed] [Google Scholar]
- 27.Salinger L B, Forsberg C W, Cheng K-J. The rumen: a unique source of enzymes for enhancing livestock production. Anaerobe. 1996;2:263–284. doi: 10.1006/anae.1996.0036. [DOI] [PubMed] [Google Scholar]
- 28.Shao W, Wiegel J. Purification and characterization of two thermostable acetyl xylan esterases from Thermoanerobacterium sp. strain JW/SL YS485. Appl Environ Microbiol. 1995;61:729–733. doi: 10.1128/aem.61.2.729-733.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shareck F, Biely P, Morosoli R, Kluepfel D. Analysis of DNA flanking the xlnB locus of Streptomyces lividans reveals genes encoding acetyl xylan esterase and the RNA component of ribonuclease P. Gene. 1995;153:105–109. doi: 10.1016/0378-1119(94)00763-i. [DOI] [PubMed] [Google Scholar]
- 30.Shevchik V E, Hugouvieux-Cotte-Pattat N. Identification of a bacterial pectin acetyl esterase in Erwinia chrysanthemi 3937. Mol Microbiol. 1997;24:1285–1301. doi: 10.1046/j.1365-2958.1997.4331800.x. [DOI] [PubMed] [Google Scholar]
- 31.Suh J-H, Yong-Jin C. Synergism among endo-xylanase, β-xylosidase, and acetyl xylan esterase from Bacillus stearothermophilus. J Microbiol Biotechnol. 1996;6:173–178. [Google Scholar]
- 32.Tsujibo H, Ohtsuki T, Iio T, Yamazaki I, Miyamoto K, Sugiyama M, Inamori Y. Cloning and sequence analysis of genes encoding xylanases and acetyl xylan esterase from Streptomyces thermoviolaceus OPC-520. Appl Environ Microbiol. 1997;63:661–664. doi: 10.1128/aem.63.2.661-664.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]