Abstract
Introduction:
Breast cancer is a heterogeneous disease, consisting of multiple molecular subtypes. Obesity has been associated with an increased risk for postmenopausal breast cancer, but few studies have examined breast cancer subtypes separately. Obesity is often complicated by type 2 diabetes, but the possible association of diabetes with specific breast cancer subtypes remains poorly understood.
Methods:
In this retrospective case-control study, Louisiana Tumor Registry records of primary invasive breast cancer diagnosed in 2010–2015 were linked to electronic health records in the Louisiana Public Health Institute’s Research Action for Health Network. Controls were selected from Research Action for Health Network and matched to cases by age and race. Conditional logistic regression was used to identify metabolic risk factors. Data analysis was conducted in 2020–2021.
Results:
There was a significant association between diabetes and breast cancer for Luminal A, Triple-Negative Breast Cancer, and human epidermal growth factor 2–positive subtypes. In multiple logistic regression, including both obesity status and diabetes as independent risk factors, Luminal A breast cancer was also associated with overweight status. Diabetes was associated with increased risk for Luminal A and Triple-Negative Breast Cancer in subgroup analyses, including women aged ≥50 years, Black women, and White women.
Conclusions:
Although research has identified obesity and diabetes as risk factors for breast cancer, these results underscore that comorbid risk is complex and may differ by molecular subtype. There was a significant association between diabetes and the incidence of Luminal A, Triple-Negative Breast Cancer, and human epidermal growth factor 2–positive breast cancer in Louisiana.
INTRODUCTION
Breast cancer is the most common form of cancer and the second cause of cancer mortality in women of the U.S.1 Breast cancer is a group of molecularly and clinically distinct diseases and is classified on the basis of gene expression profiling.2 A commonly used surrogate for mRNA expression–based classification is based on immunohistochemistry (IHC) for ovarian hormone receptors (HRs) (estrogen receptor [ER] α and progesterone receptor [PR]), erythroblastosis oncogene B2/ human epidermal growth factor 2 (HER2), and proliferative marker Ki-67.3–5 On the basis of these IHC criteria, breast cancers are often classified into 4 main subtypes: Luminal A (HR+/ HER2−), Luminal B (HR+/HER2+), HER2 enriched (HR−/HER2+), and Triple-Negative Breast Cancer (TNBC) (HR−/HER2−).6–8 Luminal B breast cancer has a higher tumor grade by histology and high proliferative index as determined by Ki-67 compared with Luminal A breast cancer, resulting in a worse prognosis.7,9 TNBC does not express HR, although it can express androgen receptor, nor does it have genomic amplification of the erythroblastosis oncogene B2/HER2 gene. Clinically aggressive TNBCs that do not respond to neoadjuvant chemotherapy with a pathological complete remission have a high risk of early metastasis and a poor prognosis.10,11 The HER2-enriched subtype accounts for fewer breast cancer cases and is characterized by high-grade tumors with poor clinical outcomes.7,12
Obesity is considered an independent risk factor for several cancers, including breast cancer.13,14 Globally, the obese population (defined with a BMI ≥30 kg/m2) has grown rapidly in recent decades.15 The U.S. National Health and Nutrition Examination Survey reported that 40.4% of women were obese.16 Obesity produces a systemic inflammatory response that releases several cytokines and activates signaling pathways that promote tumor initiation, survival, and immune escape. In addition, estradiol and estrone produced by breast adipocytes can promote the growth of luminal breast cancers.17 Obesity is a multifactorial condition influenced by several factors, including diet, environment, sociodemographic factors, and physical activity.18,19 Picon-Ruiz et al. describe in detail the epidemiology and possible mechanistic links between obesity and breast cancer as well as possible risk mitigation interventions, including diet and exercise.20 The strongest link between obesity and breast cancer incidence has been found in postmenopausal ER-positive breast cancer (reviewed in Picon-Ruiz et al.20). By contrast, studies have reported that obesity was associated with lower premenopausal ER-positive breast cancer risk20–25 or no association at all.20,26,27 A number of studies indicate that obesity is associated with a higher risk of premenopausal ER-negative breast cancer22–27 and TNBC,20,27–33 but the risk is minimally or inversely linked after menopause.20 There are inconsistent results in describing a correlation between breast cancer development and obesity.34–38 A recent review described the paradoxical and controversial relationship between obesity and breast cancer, which is influenced by menopausal status.39 The correlation between obesity and breast cancer risk is also complicated by differences between racial/ethnic groups and sociodemographic factors.20,24,40–42
Type 2 diabetes (T2D) is a common comorbidity of obesity, and studies of the relationship between obesity and cancer risks or outcomes do not always distinguish between obesity with and without T2D. Numerous studies support multifaceted associations of diabetes with various cancers, including colorectal, prostate, and breast cancer.43–46 Diabetes has been reported to be linked with a 17% overall increased risk of breast cancer incidence in women.43,46 The association was significant among postmenopausal women and with ER+ breast cancer, and it remained significant after adjustment for multiple factors and was independent of age and obesity.43,46 T2D and obesity were linked with an increased risk for postmenopausal breast cancer.47–49 Women with diabetes were also reported to be at greater risk of developing TNBC than women without diabetes.44,50 Multiple factors linked to obesity, metabolic syndrome, and T2D may simultaneously or individually contribute to cancer progression. These include hyperinsulinemia, hyperglycemia, dyslipidemia, insulin-like growth factor, adipokines, and cytokines as well as the gut microbiome.43,44 Insulin resistance and hyperinsulinism are often associated with obesity but can occur independently of it.51 Insulin signaling can promote breast cancer growth.52 Therefore, examining T2D independently of obesity can inform researchers on the possible role of insulin resistance in various subtypes of breast cancer.
In summary, previous studies have produced inconsistent findings on the relationship between obesity and breast cancer development, particularly as it pertains to HR-negative tumors. Most previous studies did not include all breast cancer molecular subtypes and/or had inconclusive results on the association of obesity with breast cancer risk among Black women. Furthermore, limiting the definition of obesity to BMI does not capture the range of dysmetabolism among patients with obesity. T2D is a frequent metabolic complication of obesity and a diagnosis that can be readily established from electronic health records (EHRs). Louisiana has a high prevalence of obesity, T2D, and breast cancer as well as a diverse population that allows stratification by self-reported race. Therefore, we investigated the association between obesity, diabetes, and the most common breast cancer subtypes by race in Louisiana.
METHODS
Study Population
Data were collected by the Louisiana Tumor Registry (LTR), a participant of the National Cancer Institute’s Surveillance, Epidemiology and End Results Program, and the Centers for Disease Control and Prevention’s National Program of Cancer Registries. The study included women aged ≥20 years with primary invasive breast cancer (International Classification of Diseases [ICD] for Oncology, third edition site codes C50.0-C50.9) diagnosed from 2010 to 2015. Breast cancers with ICD-O-3 histology codes 9050–9055, 9140, and 9590–9989 diagnosed by autopsy or death certificate were excluded. Eligible case records from LTR were linked to records from the Louisiana Public Health Institute’s Research Action for Health Network (REACHnet) to obtain BMI and diagnoses and chronic conditions recorded during patient encounters. REACHnet is an EHR-based clinical data repository that uses the National Patient-Centered Clinical Research Network Common Data Model.53 All research activities were approved by Louisiana State University Health Sciences Center, New Orleans IRB.
Measures
The molecular subtype was classified on the basis of joint HR status (estrogen/progesterone) and HER2 status. Joint HR status was considered negative if the tumor lacked both estrogen and progesterone reactivity. Borderline hormonal receptor was considered positive. Briefly, the 4 molecular breast cancer subtypes were defined as Luminal A (HR+/HER−), Luminal B (HR+/HER+), Triple Negative (HR−/HER2−), and HER2 (HR−/HER2+).
Patient age was categorized into age groups of 10 years. Self-reported race by patients was categorized as Black or African American, White or European American, or other. BMI was classified as lean (18.5≤BMI<25), overweight (25≤ BMI<30), mild obesity (30≤ BMI<35), and high obesity (BMI≥35). Records with extreme BMI values (>65) were excluded from data summarization to control for potential data entry errors. Diabetes and pregnancy were indicated by ICD, Ninth Revision and ICD-10 diagnoses codes (Appendix Table 2, available online).
Study Design
The study has a retrospective case-control design. Controls were matched to cases on the basis of age and race at a rate of up to 5:1. Matching was performed separately by molecular subtype. Patients who were missing BMI data, underweight (BMI<18.5), or pregnant during the study period were excluded. The stepwise schematic diagram of case and control eligibility for the study is presented in Appendix Figure 1 (available online).
For cases, date of diagnosis, age at diagnosis, race, and molecular subtype were obtained from the LTR database. Obesity status for each patient was determined as the mode or most prevalent BMI category obtained from REACHnet patient records spanning 12 months before diagnosis through 3 months (90 days) after diagnosis. Diabetes and pregnancy status were identified by condition and diagnosis codes from patient encounters in REACHnet that occurred no later than 3 months after the date of diagnosis. Patient information from the EHR was restricted to 3 months after the date of diagnosis to include patients who may have been referred into the health systems shortly after their breast cancer diagnosis while limiting cause-effect bias in the study results.
Controls were selected from a random sample of breast cancer–free patients in REACHnet with at least 1 encounter or visit during the study period. Age and self-reported race were obtained from the medical records, with age observed 12 months after the first encounter. Obesity status for each patient was determined as the mode or most prevalent BMI category across a period of 15 months after the first encounter. Diabetes and pregnancy status were identified by condition and diagnosis codes from patient encounters during the same period (15 months from the first encounter).
Statistical Analysis
Conditional logistic regression was performed to evaluate the risk factors associated with breast cancer in the case-control sample. This was conducted through effect stratification, with each case and its matched controls constituting 1 stratum.54 Case-control matching and all data analyses were performed in SAS (SAS Institute Inc, Cary, NC), version 9.4, during 2020–2021. Analyses were executed for each subtype. The primary analysis included 2 logistic regression models with obesity status and diabetes as a single risk factor and a multiple logistic regression with both obesity and diabetes as risk factors (multivariable model). Secondary analyses to confirm the consistency of results from the final model included subanalyses for women aged >50 years and among White/European women and Black/African American women.
RESULTS
Patient characteristics for the cases and controls for each molecular subtype are presented in Table 1. Most breast cancer cases in the study were Luminal A (n=1,584). TNBC was the next most common subtype with 364 cases. Luminal B and HER2+ were the least common subtypes with 232 and 115 cases, respectively. Among cases, obesity (mild and high) prevalence ranged from 46.3% (Luminal A) to 51.7% (TNBC). The prevalence of diabetes in cases ranged from 19.8% (Luminal B) to 28.9% (TNBC). Among age- and race-matched controls, the prevalence of obesity ranged from 44.1% to 48.9%, and the prevalence of diabetes ranged from 15.4% to 19%.
Table 1.
Patient Characteristics for Breast Cancer Cases and Matched Controls
| Luminal A | Luminal B | TNBC | HER2 | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| Characteristics | Cases (n=1,584) | Controls (n=5,436) | Cases (n=232) | Controls (n=1,095) | Cases (n=364) | Controls (n=1,686) | Cases (n=115) | Controls (n=562) |
|
| ||||||||
| Age, years | ||||||||
| 20–29 | 0.3 (5) | 0.5 (25) | 0.4 (1) | 0.5 (5) | 0.6 (2) | 0.6 (10) | 0.9 (1) | 0.9 (5) |
| 30–39 | 1.9 (30) | 2.7 (149) | 7.8 (18) | 8.1 (89) | 6 (22) | 6.5 (110) | 6.1 (7) | 6.2 (35) |
| 40–49 | 10.9 (172) | 13.8 (748) | 19.4 (45) | 19.8 (217) | 14.6 (53) | 14.8 (250) | 7.8 (9) | 8 (45) |
| 50–59 | 24.4 (387) | 27.1 (1,471) | 23.7 (55) | 23.8 (261) | 26.7 (97) | 26.3 (444) | 31.3 (36) | 31.7 (178) |
| 60–69 | 31.3 (495) | 29.3 (1,591) | 26.7 (62) | 26.7 (292) | 28 (102) | 28.1 (473) | 31.3 (36) | 31.1 (175) |
| ≥70 | 31.3 (495) | 26.7 (1,452) | 22 (51) | 21.1 (231) | 24.2 (88) | 23.7 (399) | 22.6 (26) | 22.1 (124) |
| Race | ||||||||
| White | 69.6 (1,103) | 66.6 (3,620) | 62.1 (144) | 61.4 (672) | 50 (182) | 51.1 (861) | 54.8 (63) | 54.6 (307) |
| Black | 28.1 (445) | 30.6 (1,662) | 35.8 (83) | 36.4 (399) | 48.9 (178) | 47.8 (805) | 41.7 (48) | 41.8 (235) |
| Other | 2.3 (36) | 2.8 (154) | 2.2 (5) | 2.2 (24) | 1.1 (4) | 1.2 (20) | 3.5 (4) | 3.6 (20) |
| Obesity | ||||||||
| Lean | 22.4 (354) | 25.8 (1,400) | 22.4 (52) | 23.1 (253) | 21.2 (77) | 21.8 (368) | 23.5 (27) | 21.4 (120) |
| Overweight | 31.4 (497) | 30.2 (1,641) | 29.7 (69) | 29.5 (323) | 27.2 (99) | 30.5 (514) | 27 (31) | 29.7 (167) |
| Mild obesity | 23.4 (371) | 21.8 (1,183) | 24.6 (57) | 22.3 (244) | 24.5 (89) | 22.4 (377) | 19.1 (22) | 22.2 (125) |
| High obesity | 22.9 (362) | 22.3 (1,212) | 23.3 (54) | 25.1 (275) | 27.2 (99) | 25.3 (427) | 30.4 (35) | 26.7 (150) |
| Type II diabetes | ||||||||
| No | 76.8 (1,217) | 82.3 (4,476) | 80.2 (186) | 84.6 (926) | 71.2 (259) | 81 (1,366) | 73 (84) | 81.9 (460) |
| Yes | 23.2 (367) | 17.7 (960) | 19.8 (46) | 15.4 (169) | 28.9 (105) | 19 (320) | 27 (31) | 18.2 (102) |
HER2, human epidermal growth factor 2; TNBC, Triple-Negative Breast Cancer.
The association of obesity and diabetes with breast cancer was assessed with conditional logistic regression models, controlling for age and race matching (Table 2). In the first model, there was an increased risk of obesity among cases compared with that among the controls for Luminal A breast cancer, where the odds of overweight, mild obesity, and high obesity among cases were 17%–24% greater than those among the controls. There were no significant associations between elevated BMI groups and Luminal B, TNBC, or HER2+ breast cancer subtypes. The second model indicated a significant association between diabetes and breast cancer for Luminal A (OR [95% CI]=1.37 [1.19, 1.58]), TNBC (OR [95% CI]=1.82 [1.38, 2.39]), and HER2+ (OR [95% CI]=1.68 [1.05, 2.71]) subtypes. In multiple logistic regression, including both obesity status and diabetes as independent risk factors, the association between Luminal A breast cancer and overweight (OR [95% CI]=1.20 [1.01, 1.42]) and diabetes (OR [95% CI]=1.35 [1.16, 1.56]) remained significant. Similarly, there were significant associations between diabetes and TNBC (OR [95% CI] =1.8 [1.36, 2.37]) and HER2+ (OR [95% CI]=1.69 [1.04, 2.76]) (Table 2 and Figure 1).
Table 2.
Results From Case-Control Analyses of Metabolic Risk for Breast Cancer, by Molecular Subtype
| Risk factors | Obesity model, OR (95% CI) | Diabetes model, OR (95% CI) | Multivariable model, OR (95% CI) |
|---|---|---|---|
|
| |||
| Luminal A (n=1,584) | |||
| Overweight | 1.17 (1.00, 1.38) | 1.16 (0.99, 1.36) | |
| Mild obesity | 1.24 (1.05, 1.47) | 1.20 (1.01, 1.42) | |
| High obesity | 1.22 (1.02, 1.45) | 1.13 (0.95, 1.35) | |
| Diabetes | 1.37 (1.19, 1.58) | 1.35 (1.16, 1.56) | |
| Luminal B (n=232) | |||
| Overweight | 1.04 (0.70, 1.54) | 1.01 (0.68, 1.50) | |
| Mild obesity | 1.14 (0.74, 1.74) | 1.10 (0.71, 1.69) | |
| High obesity | 0.96 (0.62, 1.48) | 0.89 (0.57, 1.38) | |
| Diabetes | 1.39 (0.95, 2.04) | 1.43 (0.97, 2.11) | |
| TNBC (n=364) | |||
| Overweight | 0.92 (0.67, 1.28) | 0.90 (0.65, 1.25) | |
| Mild obesity | 1.13 (0.81, 1.60) | 1.05 (0.74, 1.49) | |
| High obesity | 1.12 (0.79, 1.57) | 0.99 (0.70, 1.40) | |
| Diabetes | 1.82 (1.38, 2.39) | 1.80 (1.36, 2.37) | |
| HER2 (n=115) | |||
| Overweight | 0.82 (0.46, 1.46) | 0.78 (0.43, 1.39) | |
| Mild obesity | 0.77 (0.40, 1.48) | 0.74 (0.38, 1.43) | |
| High obesity | 1.03 (0.58, 1.85) | 0.92 (0.51, 1.68) | |
| Diabetes | 1.68 (1.05, 2.71) | 1.69 (1.04, 2.76) | |
Note: Boldface indicates statistical significance (p<0.05).
Results are provided as ORs and 95% CIs from conditional logistic regression models. Obesity and diabetes models include a single fixed effect for the risk factor of interest. The multivariable model includes both obesity status and diabetes as fixed effects. All models were conditioned on case-control matching (age group 10 years and race).
HER2, human epidermal growth factor 2; TNBC, Triple-Negative Breast Cancer.
Figure 1.
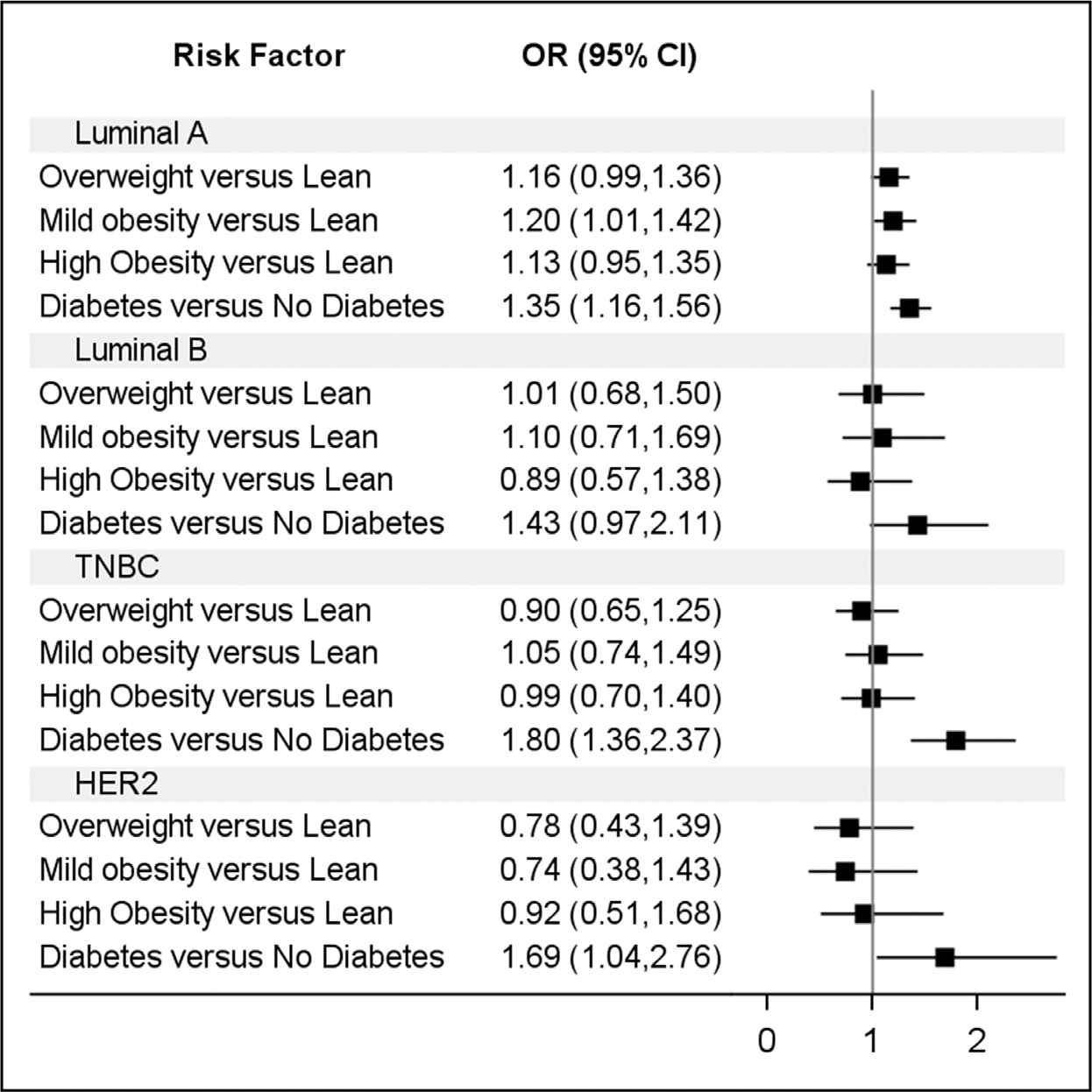
AORs and 95% CIs from multivariable conditional logistic regression model.
HER2, human epidermal growth factor 2; TNBC, Triple-Negative Breast Cancer.
Table 3 contains the conditional OR estimates and 95% CIs from the full case-control sample and subgroup analyses. For Luminal A breast cancer, the association between overweight, mild obesity, and high obesity and breast cancer incidence was most pronounced for women aged ≥50 years, a common proxy for menopausal status, with OR (95% CI) ranging from 1.21 (1.01, 1.43) to 1.33 (1.10, 1.60), and among Black women, with OR (95% CI) ranging from 1.39 (0.96, 2.02) to 1.52 (1.05, 2.20). The association between diabetes and breast cancer was greatest among Black women for Luminal A (OR [95% CI]=1.48 [1.17, 1.87]) and TNBC (OR [95% CI]=1.96 [1.35, 2.85]).
Table 3.
Results From Subgroup Case-Control Analyses of Metabolic Risk for Breast Cancer, by Molecular Subtype
| Risk factors | All, OR (95% CI) | Age >50 years, OR (95% CI) | White, OR (95% CI) | Black, OR (95% CI) |
|---|---|---|---|---|
|
| ||||
| Luminal A (n=1,584) | ||||
| Overweight | 1.16 (0.99, 1.36) | 1.21 (1.01, 1.43) | 1.10 (0.92, 1.32) | 1.52 (1.05, 2.20) |
| Mild obesity | 1.20 (1.01, 1.42) | 1.33 (1.10, 1.60) | 1.17 (0.96, 1.43) | 1.39 (0.96, 2.02) |
| High obesity | 1.13 (0.95, 1.35) | 1.25 (1.03, 1.52) | 1.05 (0.85, 1.30) | 1.47 (1.02, 2.13) |
| Diabetes | 1.35 (1.16, 1.56) | 1.31 (1.13, 1.53) | 1.33 (1.10, 1.61) | 1.48 (1.17, 1.87) |
| Luminal B (n=232) | ||||
| Overweight | 1.01 (0.68, 1.50) | 0.92 (0.57, 1.50) | 1.15 (0.73, 1.81) | 0.60 (0.26, 1.38) |
| Mild obesity | 1.10 (0.71, 1.69) | 1.24 (0.75, 2.05) | 1.01 (0.59, 1.72) | 0.94 (0.42, 2.11) |
| High obesity | 0.89 (0.57, 1.38) | 0.95 (0.56, 1.61) | 0.90 (0.52, 1.57) | 0.68 (0.30, 1.53) |
| Diabetes | 1.43 (0.97, 2.11) | 1.39 (0.91, 2.12) | 1.33 (0.76, 2.34) | 1.53 (0.88, 2.66) |
| TNBC (n=364) | ||||
| Overweight | 0.90 (0.65, 1.25) | 0.91 (0.63, 1.31) | 0.95 (0.63, 1.43) | 0.80 (0.45, 1.43) |
| Mild obesity | 1.05 (0.74, 1.49) | 1.09 (0.74, 1.61) | 1.06 (0.67, 1.69) | 1.06 (0.61, 1.86) |
| High obesity | 0.99 (0.70, 1.40) | 1.03 (0.69, 1.53) | 0.78 (0.47, 1.28) | 1.15 (0.67, 1.98) |
| Diabetes | 1.80 (1.36, 2.37) | 1.87 (1.39, 2.51) | 1.68 (1.10, 2.57) | 1.96 (1.35, 2.85) |
| HER2 (n=115) | ||||
| Overweight | 0.78 (0.43, 1.39) | 0.77 (0.40, 1.50) | 0.60 (0.29, 1.24) | 1.03 (0.33, 3.23) |
| Mild obesity | 0.74 (0.38, 1.43) | 0.90 (0.44, 1.82) | 1.01 (0.46, 2.21) | 0.45 (0.12, 1.64) |
| High obesity | 0.92 (0.51, 1.68) | 1.17 (0.60, 2.28) | 0.62 (0.27, 1.42) | 1.17 (0.40, 3.47) |
| Diabetes | 1.69 (1.04, 2.76) | 1.66 (1.00, 2.75) | 1.93 (0.95, 3.92) | 1.39 (0.68, 2.85) |
Note: Boldface indicates statistical significance (p<0.05).
Results are provided as ORs and 95% CIs from conditional logistic regression models. All models include both obesity status and diabetes as fixed effects and were conditioned on case-control matching (age group of 10 years and race).
HER2, human epidermal growth factor 2; TNBC, Triple-Negative Breast Cancer.
DISCUSSION
In this retrospective case-control study, metabolic risk factors were significantly associated with breast cancer. This study also highlights the heterogeneity of risk factors across breast cancer molecular subtypes. Results indicated that elevated BMI was a risk factor for Luminal A breast cancer, especially in postmenopausal women. In women aged ≥50 years, cases of Luminal A breast cancer had 21%–33% greater odds of presenting with BMI ≥25, which includes the categories of overweight, mild obesity, and high obesity, than controls. These results are in congruence with a body of literature that indicates a positive relationship between body mass and the risk of HR+ breast cancers in postmenopausal women, including several meta-analyses,31,42,55,56 and although not addressed in this study, literature further suggests that this particular relationship is strongest when there is significant weight gain in adulthood.31,57 In this study, the relationship between elevated BMI and HR− breast cancers was not significant. A consensus on the role of body mass in these breast cancer subtypes has yet to be reached, with contradictory findings in past studies.42 For instance, a meta-analysis of case-control TNBC studies suggested that body mass is a significant risk factor, but several studies included did not control for race or other covariates.58 Other meta-analyses of HR− breast cancers found no significant association.55,56 Conversely, a rigorous study by the African American Breast Cancer Epidemiology and Risk consortium, which aimed to specify the relationship between body type and breast cancer among Black women, found that recently elevated BMI correlated with decreased risk of TNBC.31 Additional studies have also suggested a protective effect of elevated BMI on HR− breast cancer tumors.59–62
Diabetes and prediabetes have been reported as significant risk factors for breast cancer in many populations, with excess risk in the range of 10%–30%.47–49,63–66 There was a significant association between diabetes and three molecular subtypes of breast cancer in Louisiana–Luminal A, TNBC, and HER2+−in this study. Diabetes exhibited the greatest risk in TNBC, where cases had 82% greater odds of diabetes than controls. After controlling for obesity status, the OR was nearly unchanged, at 80%. There were similar results for Luminal A and HER2+ subtypes, where the risk associated with diabetes was not attenuated after adjusting for BMI. These results support diabetes as a strong independent risk factor for breast cancer. This contrasts with studies that have reported that the associations between diabetes and breast cancer were attenuated after adjusting for BMI.47,48,66
Limitations
Limitations of this study include the retrospective observational study design, which only allows for the determination of association, not causation. The strength of the control population in the study is that it originates from large health systems in the greater New Orleans area, which not only maintain hospitals and emergency departments but also maintain walk-in clinics and urgent-care facilities. However, system-based controls do have the potential for selection bias because this population is more likely to include individuals who receive routine medical care. In the event that the selection bias favors individuals with poorer health status, those who require more frequent care, this would bias results toward the null. The linkage of the EHR records to LTR to identify breast cancer cases ensured that there was no loss to follow-up among controls. The external validity of the sample is fair because cancer cases were identified through the state cancer registry (LTR) and linked to a large regional EHR network (REACHnet). However, REACHnet does not have complete regional coverage; thus, the sample may not be fully representative.
A distinct objective and strength of this study were to assess metabolic risk factors by molecular subtype. However, low case counts for Luminal B and HER2 molecular subtypes in the study population likely lead to diminished statistical power for these models. The limited sample also precluded the ability to assess risk factors among premenopausal women or postmenopausal women by race. Molecular subtypes were assigned on the basis of IHC commonly used in clinical practice, as opposed to whole-transcriptome gene expression profiling. This prevented an investigation of the molecular subsubtypes of TNBC that are defined strictly through gene expression profiling.
Although the classification of BMI in this study was based on the WHO’s classification, it did not differentiate between Class II (35≤ BMI<39.9) and Class III (BMI≥40) obesity owing to low case counts. Instead, these categories were combined into a single category (high obesity), which is consistent with previous literature in the field.31,67 The diabetes exposure was defined by an indication of diabetes in the medical record. This did not include duration or control of disease, which may have the potential to modify risk. Finally, this analysis was limited to data in the National Patient-Centered Clinical Research Network Common Data Model for EHRs and thus did not control for other known risk factors regarding health behaviors (e.g., diet, physical activity), reproductive history, or social determinants of health (e.g., SES, environmental exposures) that were not available in the database.
There are known shortcomings of using BMI as an indicator of obesity in cancer research, with several reviews highlighting the inability of BMI to accurately characterize body composition across races.42,68,69 The Carolina Breast Cancer Study found that waist–hip ratio was associated with breast cancer incidence in both White and Black women, whereas there was no risk associated with BMI.70 Unfortunately, other measures of body composition are often not as readily available as BMI, especially in EHR-derived data. Results from this study support the notion that future studies should use multiple or composite measures of metabolic risk that include other comorbid conditions or laboratory profiles, such as Edmonton Obesity staging or metabolic syndrome criteria.71,72 For example, a meta-analysis found that 3 components of metabolic syndrome–obesity, diabetes, and hypertension–posed a significant breast cancer risk, and the presence of metabolic syndrome was associated with a twofold increase in breast cancer risk in postmenopausal women.72
CONCLUSIONS
There was a significant association between diabetes and the incidence of Luminal A, TNBC, and HER2+ breast cancer in Louisiana. These results suggest that the severity of metabolic sequelae of obesity may be better indicators of breast cancer risk than elevated BMI alone. Future breast cancer research should also consider additional factors that may modify risk in patients with diabetes, such as duration and control of disease, social determinants, and genomic risks.
Supplementary Material
ACKNOWLEDGMENTS
The authors also thank the Louisiana Tumor Registry for data collections. The authors acknowledge the participation of REACHnet partner health systems Ochsner Health, Tulane University, and Louisiana State University Health Sciences Center in this project. FMH and DMD are cofirst authors.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies or data collection authorities.
This research was supported in part by U54 GM104940 from the National Institute of General Medical Sciences of the NIH, which funds the Louisiana Clinical and Translational Science Center; P20 CA233374 from the National Cancer Institute; and Obesity Health Disparities Research Center pilot grant supported by National Institute on Minority Health and Health Disparities U54MD000502. The research reported in this article was conducted in partnership with Research Action for Health Network (REACHnet), funded by the Patient-Centered Outcomes Research Institute Award RI-CRN-2020–008. REACHnet is a partner network in the National Patient-Centered Clinical Research Network, which was developed with funding from the Patient-Centered Outcomes Research Institute.
No financial disclosures were reported by the authors of this paper.
This article is part of a supplement entitled Obesity-Related Health Disparities: Addressing the Complex Contributors, which is sponsored by the National Institute on Minority Health and Health Disparities (NIMHD), National Institutes of Health (NIH), U.S. Department of Health and Human Services (HHS). The findings and conclusions in this article are those of the author (s) and do not necessarily represent the official position of NIMHD, NIH, or HHS.
Footnotes
CREDIT AUTHOR STATEMENT
Fokhrul M. Hossain: Conceptualization, Writing - original Draft. Denise M. Danos: Formal analysis, Methodology, Writing - original draft. Qiufan Fu: Formal analysis, Writing - review and editing. Xinnan Wang: Formal analysis. Richard A. Scribner: Conceptualization, Methodology. San T. Chu: Data curation, Writing - review and editing. Ronald L. Horswell: Data curation. Eboni G. Price-Haywood: Supervision. Bridgette M. Collins-Burow: Supervision. Xiao-Cheng Wu: Data curation, Supervision, Writing - review and editing. Augusto C. Ochoa: Funding acquisition, Supervision. Lucio Miele: Conceptualization, Supervision, Writing - review and editing.
SUPPLEMENTAL MATERIAL
Supplemental materials associated with this article can be found in the online version at https://doi.org/10.1016/j.amepre.2022.02.017.
REFERENCES
- 1.Cancer facts & figures 2022. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2022.html/. American Cancer Society. Updated January 12, 2022. Accessed March 8, 2022. [Google Scholar]
- 2.Sotiriou C, Neo SY, McShane LM, et al. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc Natl Acad Sci U S A. 2003;100(18):10393–10398. 10.1073/pnas.1732912100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joensuu K, Leidenius M, Kero M, Andersson LC, Horwitz KB, Heikkila P. ER, PR, HER2, Ki-67 and CK5 in early and late relapsing breast cancer-reduced CK5 expression in metastases. Breast Cancer (Auckl). 2013;7:23–34. 10.4137/BCBCR.S10701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Onitilo AA, Engel JM, Greenlee RT, Mukesh BN. Breast cancer subtypes based on ER/PR and Her2 expression: comparison of clinicopathologic features and survival. Clin Med Res. 2009;7(1–2):4–13. 10.3121/cmr.2009.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vasconcelos I, Hussainzada A, Berger S, et al. The St. Gallen surrogate classification for breast cancer subtypes successfully predicts tumor presenting features, nodal involvement, recurrence patterns and disease free survival. Breast. 2016;29:181–185. 10.1016/j.breast.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 6.Cheang MC, Martin M, Nielsen TO, et al. Defining breast cancer intrinsic subtypes by quantitative receptor expression. Oncologist.2015;20(5):474–482. 10.1634/theoncologist.2014-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haque R, Ahmed SA, Inzhakova G, et al. Impact of breast cancer subtypes and treatment on survival: an analysis spanning two decades. Cancer Epidemiol Biomarkers Prev. 2012;21(10):1848–1855. 10.1158/1055-9965.EPI-12-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–752. 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 9.Tran B, Bedard PL. Luminal-B breast cancer and novel therapeutic targets. Breast Cancer Res. 2011;13(6):221. 10.1186/bcr2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carey LA, Dees EC, Sawyer L, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13(8):2329–2334. 10.1158/1078-0432.CCR-06-1109. [DOI] [PubMed] [Google Scholar]
- 11.Millikan RC, Newman B, Tse CK, et al. Epidemiology of basal-like breast cancer [published correction appears in Breast Cancer Res Treat. 2008;109(1):141]. Breast Cancer Res Treat. 2008;109(1):123–139. 10.1007/s10549-007-9632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blows FM, Driver KE, Schmidt MK, et al. Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: a collaborative analysis of data for 10,159 cases from 12 studies. PLoS Med. 2010;7(5):e1000279. 10.1371/journal.pmed.1000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhaskaran K, Douglas I, Forbes H, dos-Santos-Silva I, Leon DA, Smeeth L. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5•24 million UK adults. Lancet. 2014;384(9945):755–765. 10.1016/S0140-6736(14)60892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–578. 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 15.GBD 2015 Obesity Collaborators, Afshin A, Forouzanfar MH, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377(1):13–27. 10.1056/NEJ-Moa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315(21):2284–2291. 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qureshi R, Picon-Ruiz M, Aurrekoetxea-Rodriguez I, et al. The major pre- and postmenopausal estrogens play opposing roles in obesity-driven mammary inflammation and breast cancer development. Cell Metab. 2020;31(6):1154–1172 e9. 10.1016/j.cmet.2020.05.008. [DOI] [PubMed] [Google Scholar]
- 18.Ng SW, Popkin BM. Time use and physical activity: a shift away from movement across the globe. Obes Rev. 2012;13(8):659–680. 10.1111/j.1467-789X.2011.00982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swinburn BA, Sacks G, Hall KD, et al. The global obesity pandemic: shaped by global drivers and local environments. Lancet. 2011;378 (9793):804–814. 10.1016/S0140-6736(11)60813-1. [DOI] [PubMed] [Google Scholar]
- 20.Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, Friedman ER, Slingerland JM. Obesity and adverse breast cancer risk and outcome: mechanistic insights and strategies for intervention. CA Cancer J Clin. 2017;67(5):378–397. 10.3322/caac.21405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.White AJ, Nichols HB, Bradshaw PT, Sandler DP. Overall and central adiposity and breast cancer risk in the Sister Study. Cancer. 2015;121(20):3700–3708. 10.1002/cncr.29552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cotterchio M, Kreiger N, Theis B, Sloan M, Bahl S. Hormonal factors and the risk of breast cancer according to estrogen- and progesterone-receptor subgroup. Cancer Epidemiol Biomarkers Prev. 2003;12(10):1053–1060. [PubMed] [Google Scholar]
- 23.Ma H, Bernstein L, Ross RK, Ursin G. Hormone-related risk factors for breast cancer in women under age 50 years by estrogen and progesterone receptor status: results from a case-control and a case-case comparison. Breast Cancer Res. 2006;8(4):R39. 10.1186/bcr1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.John EM, Sangaramoorthy M, Hines LM, et al. Overall and abdominal adiposity and premenopausal breast cancer risk among Hispanic women: the Breast Cancer Health Disparities study. Cancer Epidemiol Biomarkers Prev. 2015;24(1):138–147. 10.1158/1055-9965.EPI-13-1007-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagrani R, Mhatre S, Rajaraman P, et al. Central obesity increases risk of breast cancer irrespective of menopausal and hormonal receptor status in women of South Asian Ethnicity. Eur J Cancer. 2016;66:153–161. 10.1016/j.ejca.2016.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Enger SM, Ross RK, Paganini-Hill A, Carpenter CL, Bernstein L. Body size, physical activity, and breast cancer hormone receptor status: results from two case-control studies. Cancer Epidemiol Biomarkers Prev. 2000;9(7):681–687. [PubMed] [Google Scholar]
- 27.Kawai M, Malone KE, Tang MT, Li CI. Height, body mass index (BMI), BMI change, and the risk of estrogen receptor-positive, HER2-positive, and triple-negative breast cancer among women ages 20 to 44 years. Cancer. 2014;120(10):1548–1556. 10.1002/cncr.28601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kwan ML, Kushi LH, Weltzien E, et al. Epidemiology of breast cancer subtypes in two prospective cohort studies of breast cancer survivors. Breast Cancer Res. 2009;11(3):R31. 10.1186/bcr2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dolle JM, Daling JR, White E, et al. Risk factors for triple-negative breast cancer in women under the age of 45 years. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1157–1166. 10.1158/1055-9965.EPI-08-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen L, Cook LS, Tang MT, et al. Body mass index and risk of luminal, HER2-overexpressing, and triple negative breast cancer. Breast Cancer Res Treat. 2016;157(3):545–554. 10.1007/s10549-016-3825-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bandera EV, Chandran U, Hong CC, et al. Obesity, body fat distribution, and risk of breast cancer subtypes in African American women participating in the AMBER Consortium. Breast Cancer Res Treat. 2015;150(3):655–666. 10.1007/s10549-015-3353-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen FY, Ou HY, Wang SM, Wu YH, Yan GJ, Tang LL. Associations between body mass index and molecular subtypes as well as other clinical characteristics of breast cancer in Chinese women. Ther Clin Risk Manag. 2013;9:131–137. 10.2147/TCRM.S41203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gaudet MM, Press MF, Haile RW, et al. Risk factors by molecular subtypes of breast cancer across a population-based study of women 56 years or younger. Breast Cancer Res Treat. 2011;130(2):587–597. 10.1007/s10549-011-1616-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kolb R, Sutterwala FS, Zhang W. Obesity and cancer: inflammation bridges the two. Curr Opin Pharmacol. 2016;29:77–89. 10.1016/j.coph.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bulló M, Casas-Agustench P, Amigó-Correig P, Aranceta J, Salas-Salvadó J. Inflammation, obesity and comorbidities: the role of diet. Public Health Nutr. 2007;10(10A):1164–1172. 10.1017/S1368980007000663. [DOI] [PubMed] [Google Scholar]
- 36.Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 2014;105(2):141–150. 10.1016/j.diabres.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 37.Wang Z, Nakayama T. Inflammation, a link between obesity and cardiovascular disease. Mediators Inflammm. 2010;2010:535918. 10.1155/2010/535918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun B, Karin M. Obesity, inflammation, and liver cancer. J Hepatol.2012;56(3):704–713. 10.1016/j.jhep.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.García-Estévez L, Cortés J, Pérez S, Calvo I, Gallegos I, Moreno-Bueno G. Obesity and breast cancer: a paradoxical and controversial relationship influenced by menopausal status. Front Oncol. 2021;11:705911. 10.3389/fonc.2021.705911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robinson WR, Tse CK, Olshan AF, Troester MA. Body size across the life course and risk of premenopausal and postmenopausal breast cancer in Black women, the Carolina Breast Cancer Study, 1993–2001. Cancer Causes Control. 2014;25(9):1101–1117. 10.1007/s10552-014-0411-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prakash O, Hossain F, Danos D, Lassak A, Scribner R, Miele L. Racial disparities in triple negative breast cancer: a review of the role of biologic and non-biologic factors. Front Public Health. 2020;8:576964. 10.3389/fpubh.2020.576964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dietze EC, Chavez TA, Seewaldt VL. Obesity and triple-negative breast cancer: disparities, controversies, and biology. Am J Pathol. 2018;188(2):280–290. 10.1016/j.ajpath.2017.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gallagher EJ, LeRoith D. Obesity and diabetes: the increased risk of cancer and cancer-related mortality. Physiol Rev. 2015;95(3):727–748. 10.1152/physrev.00030.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kang C, LeRoith D, Gallagher EJ. Diabetes, obesity, and breast cancer. Endocrinology. 2018;159(11):3801–3812. 10.1210/en.2018-00574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steenland K, Nowlin S, Palu S. Cancer incidence in the National Health and Nutrition Survey I. Follow-up data: diabetes, cholesterol, pulse and physical activity. Cancer Epidemiol Biomarkers Prev. 1995;4(8):807–811. [PubMed] [Google Scholar]
- 46.Michels KB, Solomon CG, Hu FB, et al. Type 2 diabetes and subsequent incidence of breast cancer in the Nurses’ Health Study. Diabetes Care. 2003;26(6):1752–1758. 10.2337/diacare.26.6.1752. [DOI] [PubMed] [Google Scholar]
- 47.Maskarinec G, Jacobs S, Park SY, et al. Type II diabetes, obesity, and breast cancer risk: the multiethnic cohort. Cancer Epidemiol Biomarkers Prev. 2017;26(6):854–861. 10.1158/1055-9965.EPI-16-0789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boyle P, Boniol M, Koechlin A, et al. Diabetes and breast cancer risk: a meta-analysis. Br J Cancer. 2012;107(9):1608–1617. 10.1038/bjc.2012.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Larsson SC, Mantzoros CS, Wolk A. Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J Cancer. 2007;121(4):856–862. 10.1002/ijc.22717. [DOI] [PubMed] [Google Scholar]
- 50.Bronsveld HK, Jensen V, Vahl P, et al. Diabetes and breast cancer subtypes. PLoS One. 2017;12(1):e0170084. 10.1371/journal.pone.0170084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106(4):473–481. 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yee LD, Mortimer JE, Natarajan R, Dietze EC, Seewaldt VL. Metabolic health, insulin, and breast cancer: why oncologists should care about insulin. Front Endocrinol (Lausanne). 2020;11:58. 10.3389/fendo.2020.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weeks J, Pardee R. Learning to share health care data: a brief timeline of influential common data models and distributed health data networks in U.S. health care research. EGEMs (Wash DC). 2019;7(1):4. 10.5334/egems.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.SAS/STAT(R) 9.2 user’s guide, second edition. SAS. https://support.sas.com/documentation/cdl/en/statug/63033/HTML/default/viewer.htm#statug_logistic_sect062.htm. Accessed December 13, 2021. [Google Scholar]
- 55.Suzuki R, Orsini N, Saji S, Key TJ, Wolk A. Body weight and incidence of breast cancer defined by estrogen and progesterone receptor status–a meta-analysis. Int J Cancer. 2009;124(3):698–712. 10.1002/ijc.23943. [DOI] [PubMed] [Google Scholar]
- 56.Munsell MF, Sprague BL, Berry DA, Chisholm G, Trentham-Dietz A. Body mass index and breast cancer risk according to postmenopausal estrogen-progestin use and hormone receptor status. Epidemiol Rev. 2014;36:114–136. 10.1093/epirev/mxt010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hao Y, Jiang M, Miao Y, et al. Effect of long-term weight gain on the risk of breast cancer across women’s whole adulthood as well as hormone-changed menopause stages: a systematic review and dose–response meta-analysis. Obes Res Clin Pract. 2021;15(5):439–448. 10.1016/j.orcp.2021.08.004. [DOI] [PubMed] [Google Scholar]
- 58.Pierobon M, Frankenfeld CL. Obesity as a risk factor for triple-negative breast cancers: a systematic review and meta-analysis. Breast Cancer Res Treat. 2013;137(1):307–314. 10.1007/s10549-012-2339-3. [DOI] [PubMed] [Google Scholar]
- 59.Berstad P, Coates RJ, Bernstein L, et al. A case-control study of body mass index and breast cancer risk in white and African-American women. Cancer Epidemiol Biomarkers Prev. 2010;19(6):1532–1544. 10.1158/1055-9965.EPI-10-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bandera EV, Chandran U, Zirpoli G, et al. Body fatness and breast cancer risk in women of African ancestry. BMC Cancer. 2013;13 (1):475. 10.1186/1471-2407-13-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Siddharth S, Sharma D. Racial disparity and triple-negative breast cancer in African-American women: a multifaceted affair between obesity, biology, and socioeconomic determinants. Cancers (Basel). 2018;10(12):514. 10.3390/cancers10120514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Agurs-Collins T, Ross SA, Dunn BK. The many faces of obesity and its influence on breast cancer risk. Front Oncol. 2019;9:765. 10.3389/fonc.2019.00765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hardefeldt PJ, Edirimanne S, Eslick GD. Diabetes increases the risk of breast cancer: a meta-analysis. Endocr Relat Cancer. 2012;19(6):793–803. 10.1530/ERC-12-0242. [DOI] [PubMed] [Google Scholar]
- 64.Hu Y, Zhang X, Ma Y, et al. Incident type 2 diabetes duration and cancer risk: a prospective study in two U.S. cohorts. J Natl Cancer Inst. 2021;113(4):381–389. 10.1093/jnci/djaa141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JP. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ. 2015;350:g7607. 10.1136/bmj.g7607. [DOI] [PubMed] [Google Scholar]
- 66.Redaniel MT, Jeffreys M, May MT, Ben-Shlomo Y, Martin RM. Associations of type 2 diabetes and diabetes treatment with breast cancer risk and mortality: a population-based cohort study among British women. Cancer Causes Control. 2012;23(11):1785–1795. 10.1007/s10552-012-0057-0. [DOI] [PubMed] [Google Scholar]
- 67.Kwan ML, Kroenke CH, Sweeney C, et al. Association of high obesity with PAM50 breast cancer intrinsic subtypes and gene expression. BMC Cancer. 2015;15(1):278. 10.1186/s12885-015-1263-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Caan BJ, Cespedes Feliciano EM, Kroenke CH. The importance of body composition in explaining the overweight paradox in cancer-counterpoint. Cancer Res. 2018;78(8):1906–1912. 10.1158/0008-5472.CAN-17-3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brown JC, Carson TL, Thompson HJ, Agurs-Collins T. The triple health threat of diabetes, obesity, and cancer-epidemiology, disparities, mechanisms, and interventions. Obesity (Silver Spring). 2021;29(6):954–959. 10.1002/oby.23161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hall IJ, Newman B, Millikan RC, Moorman PG. Body size and breast cancer risk in Black women and white women: the Carolina Breast Cancer Study. Am J Epidemiol. 2000;151(8):754–764. 10.1093/oxfordjournals.aje.a010275. [DOI] [PubMed] [Google Scholar]
- 71.Sharma AM, Kushner RF. A proposed clinical staging system for obesity. Int J Obes (Lond). 2009;33(3):289–295. 10.1038/ijo.2009.2. [DOI] [PubMed] [Google Scholar]
- 72.Zhao P, Xia N, Zhang H, Deng T. The metabolic syndrome is a risk factor for breast cancer: a systematic review and meta-analysis. Obes Facts. 2020;13(4):384–396. 10.1159/000507554. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


