Abstract
Background
There is a scarcity of validated rapid dietary screening tools for patient use in the clinical setting to improve health and reduce cardiovascular risk. The Healthy Eating Index (HEI) 2015 measures compliance with the 2015 to 2020 Dietary Guidelines for Americans but requires completion of an extensive diet assessment to compute, which is time consuming and impractical. The authors hypothesize that a 19‐item dietary survey assessing consumption of common food groups known to affect health will be correlated with the HEI‐2015 assessed by a validated food frequency questionnaire and can be further reduced without affecting validity.
Methods and Results
A 19‐item Eating Assessment Tool (EAT) of common food groups was created through literature review and expert consensus. A cross‐sectional survey was then conducted in adult participants from a preventive cardiology clinic or cardiac rehabilitation and in healthy volunteers (n=661, mean age, 36 years; 76% women). Participants completed an online 156‐item food frequency questionnaire, which was used to calculate the HEI score using standard methods. The association between each EAT question and HEI group was analyzed by Kruskal‐Wallis test. Linear regression models were subsequently used to identify univariable and multivariable predictors for HEI score for further reduction in the number of items. The final 9‐item model of Mini‐EAT was validated by 5‐fold cross validation. The 19‐item EAT had a strong correlation with the HEI score (r=0.73) and was subsequently reduced to the 9 items independently predictive of the HEI score: fruits, vegetables, whole grains, refined grains, fish or seafood, legumes/nuts/seeds, low‐fat dairy, high‐fat dairy, and sweets consumption, without affecting the predictive ability of the tool (r=0.71).
Conclusions
Mini‐EAT is a 9‐item validated brief dietary screener that correlates well with a comprehensive food frequency questionnaire. Future studies to test the Mini‐EAT's validity in diverse populations and for development of clinical decision support systems to capture changes over time are needed.
Keywords: cardiovascular disease prevention, dietary questionnaire, dietary survey, food frequency questionnaire, Healthy Eating Index 2015, preventive cardiology, rapid dietary screener
Subject Categories: Diet and Nutrition, Cardiovascular Disease, Lifestyle
Nonstandard Abbreviations and Acronyms
- AHEI‐10
Alternate Healthy Eating Index
- AHA
American Heart Association
- DASH
Dietary Approaches to Stop Hypertension
- EAT
Eating Assessment Tool
- FFQ
food frequency questionnaire
- HEI
Healthy Eating Index
- MEDAS
Mediterranean Diet Adherence Screener
- MDS
Mediterranean Diet Score
- REAP‐S
Modified, Shortened Rapid Eating Assessment for Participants
Clinical Perspective.
What Is New?
A 19‐item dietary assessment questionnaire was developed and validated against the Healthy Eating Index (HEI) 2015 score from a reference 156‐item food frequency questionnaire in adult volunteers from the community, a preventive cardiology clinic, and cardiac rehabilitation.
The dietary questionnaire was further reduced to 9 items and maintained good correlation with the HEI‐2015.
Mini‐Eating Assessment Tool (Mini‐EAT) is a 9‐item rapid dietary screener that includes fruits/vegetables, whole grains, refined grains, fish/seafood, legumes/nuts/seeds, low‐fat dairy, high‐fat dairy, and sweets consumption.
What Are the Clinical Implications?
The 9‐item Mini‐EAT could be integrated into clinical practice to rapidly screen diet quality in patients without substantially requiring more time, advanced education for patient providers, and resources.
Mini‐EAT provides an opportunity for discussion on small and actionable dietary interventions between patients and providers.
Mini‐EAT could be integrated in the electronic medical record and coupled with clinical decision support by an integrative multidisciplinary team with the main goal of improving patient dietary quality and prevention of cardiovascular disease.
T he quality of nutrition and diet are critical when managing patients with or at risk for cardiovascular disease. Improvement in dietary quality and cardiometabolic risk factors (body weight, total cholesterol, low‐density lipoprotein cholesterol, fasting glucose, and blood pressure) have been observed with both brief and intensive dietary counseling. 1 , 2 , 3 , 4 , 5 , 6 , 7 Standardized dietary assessments, including 24‐hour dietary recalls and validated food frequency questionnaires (FFQs), provide baseline data on dietary quality that can be used by dieticians and medical providers to initiate dietary plans and set goals for subsequent clinic visits. However, the American Heart Association (AHA) recently highlighted the barriers of implementation in an outpatient clinical setting attributable to time and workflow restraints, lack of nutrition education and provider confidence, and survey fatigue. 8 These limitations have led to the development of rapid dietary screener tools; however, only a handful of existing tools are validated and are comprised of 14 to 25 questions with poor to moderate correlations, respectively. 9 , 10 , 11 There continues to be an unmet clinical need for the development of a further abbreviated validated dietary assessment tool with comparable correlation to a standard FFQ to improve the frequency of nutrition assessment by clinical providers. Furthermore, by providing rapid and efficient dietary assessments, actionable management plans for patients to reduce cardiovascular disease risk and improve overall health during the same visit can be prioritized.
The aims of the current study were to stepwise: (1) compare and validate a short 19‐item dietary questionnaire we named EAT (Eating Assessment Tool) against a reference validated FFQ; and (2) reduce the number of EAT questions to a minimum without significantly affecting correlation or validity and develop a more simplified tool named Mini‐EAT. The main objective of this study was not to replace a full dietary assessment but rather identify the most important dietary components essential for healthy eating based on the Healthy Eating Index (HEI) that all patient providers can comfortably use. We also hypothesized that the reduction in number of items would be possible without losing significant correlation with the HEI score.
METHODS
Study Design
The data that support the findings of the current study are available from the corresponding author upon reasonable request. This was a cross‐sectional survey study at the Preventive Cardiology Clinic at the Mayo Clinic in Rochester, MN. Participants were enrolled online and were asked to complete both the EAT and a standard FFQ (Viocare) at least once. A random sample (N=74) completed EAT a second time within 24 hours to assess test–retest reliability. Participants received $15 per survey they completed. Data management included collection of EAT data using the RedCap database and VioScreen data were collected within their online platform. To control for incomplete surveys, a reminder was sent by email or phone call up to 3 times to request completion. The institutional review board of the Mayo Clinic approved the research protocol and all study participants provided written informed consent.
Study Population
Adults 18 years and older from the preventive cardiology clinic and cardiac rehabilitation at Mayo Clinic, Rochester, MN, and healthy volunteers were recruited for the study. All participants provided informed consent and the study protocol was approved by the institutional review board. Inclusion criteria included basic English literacy. Exclusion criteria included inability to provide consent.
Development of EAT
The investigational EAT is a shortened 19‐item FFQ that was developed by expert consensus via focus groups comprising dieticians and cardiologists. Established evidence‐based healthy dietary assessments were reviewed including the HEI, 12 Alternate Healthy Eating Index (AHEI‐10), 13 , 14 Mediterranean Diet Score (MDS), 9 and Dietary Approaches to Stop Hypertension (DASH) score. 15 , 16 Based on the 2015 to 2020 Dietary Guidelines for the United States, 17 we incorporated similar food items that were deemed important for the development of EAT. 18 Furthermore, compared with the AHA's more recent dietary recommendations for ideal cardiovascular health, 19 the EAT questionnaire encompasses most of the main guidelines including an abundance in fruits and vegetables, whole grain foods and products, plant proteins (legumes/nuts/seeds), fish/seafood, lean unprocessed meat and poultry, low‐fat and fat‐free dairy, and avoidance of processed meats, salty foods, saturated fats, sweet foods and beverages, ultraprocessed foods, and alcohol. 19 The main food groups important for distinguishing healthy and unhealthy dietary items and patterns were defined and discussed with registered dietician nutritionists and included clear examples of foods and portion sizes. The list of items was revised several times and internally piloted to ensure clarity and interpretation in people without formal training in diet and nutrition, and further revisions were made. EAT incorporates consumption of the main food groups (eg, fruits; vegetables; legumes, seeds, and nuts; grains; dairy; red meat and poultry; and fish and seafood), distinguishes saturated and unsaturated fat, whole and refined grains, cocoa products, processed food, food with high sugar, and salt content (Table S2). Fresh fruits included fresh or frozen fruits without added sugar and excluded preserved, dried, and fruit juices. Vegetables were defined in the screener as raw or cooked, nonfried, and nonstarchy with potatoes mentioned as the main example of starchy vegetables to exclude. Starchy vegetables (ie, potatoes, sweet potatoes, corn, and green peas), have 3 to 6 times more calories and carbohydrates than nonstarchy vegetables, with an association of lower mortality observed with increased consumption of fruits and vegetables but not potatoes, peas, and corn in cohort‐based studies. 20 Whole grains by definition require >51% of a food item to be composed of a whole grain by weight or have whole grain listed as the first ingredient. The example of “unsweetened ready‐to‐eat cereal” was confirmed by a nutritionist as an example of whole grains, which is an unsweetened version of a healthy option of a breakfast cereal, which predominantly includes different types of whole grains. For refined grains, we attempted to provide clear examples such as white bread, white rolls, bagels, English muffins, white rice or pasta, and wheat tortillas with a statement to not include whole grains consumption. Legumes included examples such as cooked or canned beans, lentils, chickpeas or peas, miso tofu, tempeh, and hummus. Regarding nut consumption, nut butters were initially discussed with the nutritionist as part of inclusion; however, evidence suggests that whole nuts rather than peanut butter consumption is associated with lower risk of all‐cause and disease‐specific mortality. 21 , 22 , 23 Seeds included examples such as sesame, sunflower, pumpkin, and flaxseeds. Fish or seafood included freshwater fish or sea water fish with examples including salmon, sardines, trout, Atlantic, and Pacific mackerel and seafood, with canned fish/seafood also included in this question. Oil consumption included olive oil, canola/rapeseed oil, sunflower oil, and avocados and also specifically mentions liquid oils used for cooking. Unprocessed red meat included pork, beef, and mutton meat. Poultry included unprocessed turkey, chicken, or rabbit meat. Processed meat included examples such as salami, sausage, ham, bacon, meat pies/pastries, meat snacks, deli (luncheon) meat, and canned meats (Spam Classic canned meat, Great Value corned beef, Keystone all‐natural beef). Low‐fat dairy included low‐fat milk (1%), fat‐free (skim) milk or soy milk, yogurt with reduced‐fat, low‐fat cheese, mozzarella, or cottage cheese. High‐fat dairy and saturated fats included 2% milk or whole milk, butter, cream, cream cheese, cheese with no reduced‐fat content, yogurt with 2% or higher milk fat, ice cream, butter, and coconut oil or shortening used for cooking. Sugar‐sweetened beverages included beverages with high sugar content such as soft drinks (eg, Coca Cola, Pepsi Cola, Mountain Dew, Dr Pepper, Sprite, Fanta, Mirinda), sugar‐sweetened iced teas (eg, Honest Tea, Snapple, Arizona, Lipton Pure Leaf, Gold Peak, and Nestea), sugar‐sweetened sport drinks (eg, Gatorade and Powerade), and fruit juices (both commercial brands and homemade). Sweets and sweet foods included commercial sweets, candies, cookies, cakes, pastries, and sweet snacks. Cocoa products included milk and dark chocolate, cocoa drinks, and other products with high cocoa content with the exception of white chocolate. Salty snacks included potato and vegetable chips, tortilla/tostada chips, ready‐to‐eat popcorn, pretzels, corn snacks, and pork rinds. Processed meals included canned, frozen, or dry mix soups, premade meals including frozen pizza or microwaveable frozen dinners, boxed meal mixes, and other canned ready‐to‐eat meals. Fast food restaurant meals included meals from fast food chains regardless of location of consumption (ie, restaurant/venue, home delivery, or takeout). Alcohol consumption examples included wine, beer, bourbon, rye whiskey, gin, rum, tequila, vodka, and vermouth.
Dietary Assessment
All participants completed both a validated 156‐item FFQ (VioScreen) used as the reference standard and our investigational 19‐item nutritional assessment, EAT. VioScreen was performed online within 24 hours using an access link sent by email. Vioscreen calculates the updated HEI‐2015, which is a standardized measure for assessing compliance with the Dietary Guidelines for Americans (2015–2020). 24 The 13 components (total fruits, whole fruits, total vegetables, greens and beans, whole grains, dairy, total protein foods, seafood and plant proteins, fatty acids, refined grains, sodium, added sugars, and saturated fats) sum to a max score of 100, with a score >80 indicating a good diet, 51 to 80 reflecting a diet that needs improvement, and <51 indicating a poor diet. 25 Vioscreen uses standard exclusion criteria such as daily total intake <1000 kcal or >4500 kcal and/or reporting of <25 different foods. Vioscreen does not allow for any missing data and uses the Nutrition Data System for Research to prevent missing nutrient values.
Clinical Variables
All clinical data were collected via self‐reported surveys at the beginning of the 19‐item EAT questionnaire (Table S2). Demographic data including age, sex, ethnic group, education, marital status, and household income per year were obtained. Behavioral characteristics including smoking status, physical activity level, attention to nutrition levels, and current participation in a weight loss program or changes in diet were also gathered. Clinical characteristics obtained included height, weight, body mass index, and personal history data (hypertension, diabetes, impaired fasting glucose, dyslipidemia, heart attack, and stroke).
Statistical Analysis
Reliability analysis was performed by calculating the intraclass correlation between the 2 sets of the survey responses for participants who completed the EAT twice (N=74) (Table S1–S6). First and last responses were analyzed for participants who completed the EAT 3 times (N=2). The correlations between the answers to the same EAT questions ranged from 0.97 to 1. The correlation between the 2 sets of HEI scores was 0.86. (Figure S1–S6). Continuous variables were summarized as mean (SD) and median (range), while categorical variables were reported as frequency (percentage). Participants were divided into <51, 51 to 80, and >80 based on total HEI score. Continuous baseline variables were compared between the groups using Kruskal‐Wallis test and categorical baseline variables were compared with χ2 test. Variable reduction was done with the threshold of R 2=0.7. Linear regression model was used to find the best prediction model for the HEI score by removing the nonsignificant questions from the predictors (Tables S2 and S3). Ordinal food questions were converted to numeric variables, with the lowest level in the answer set to 1 and each higher‐level increases by 1. The coefficients in the final linear model were used to calculate the final Mini‐EAT score for each patient. Pearson correlation test was used to test the correlation between the Mini‐EAT score and HEI score. Receiver operating characteristic analysis was performed and area under the curve was calculated to reflect the predicting power of the Mini‐EAT score in predicting the HEI “healthy” group. Sensitivity and specificity of the Mini‐EAT score was calculated and the best cutoff point of the Mini‐EAT score in predicting the healthy class group defined by HEI score >80 was identified (Table S4), which achieved the highest true‐positive rate together with the lowest false‐positive rate. To test the model's performance, 5‐fold cross‐validation was performed, where the whole data were divided into 5 subgroups randomly (Table S5). In each fold, a subgroup was used as the test set and the rest of the groups were used to train the model. The calculated model performance metrics included residuals, correlation coefficient between observed and predicted HEI scores, and accuracy of assigning participants into HEI groups (<51, 51–80, >80). All tests were 2‐sided with an α level set at 0.05 for statistical significance. R3.6.2 was used for statistical analysis.
RESULTS
Participant Characteristics
A total of 742 survey records from 661 recruited participants were collected. The overall mean age was 36 years (SD, 13 years) with 75.5% women, 78.3% of White race, and 93.1% college graduates or higher degree (Table). Among the participants, a medical history of hypertension (13.1%), diabetes and impaired fasting glucose (4.6%), dyslipidemia (12.7%), heart attack (2.3%), and stroke (0.9%) were self‐reported.
Table .
Self‐Reported Participant Characteristics by HEI Score
| Participant characteristics | HEI <51 (n=93) | HEI 51–80 (n=516) | HEI >80 (n=52) | Total (N=661) | P value |
|---|---|---|---|---|---|
| Age, y* | 0.970 | ||||
| Mean (SD) | 35.6 (11.3) | 36.4 (12.4) | 36.8 (13.8) | 36.3 (12.3) | |
| Median (range) | 33.0 (21.0–73.0) | 32.5 (19.0–79.0) | 32.0 (19.0–75.0) | 32.0 (19.0–79.0) | |
| Sex | 0.172 | ||||
| Women | 64 (12.3) | 398 (79.8) | 37 (7.4) | 499 (75.5) | |
| Men | 29 (17.9) | 118 (72.8) | 15 (9.3) | 162 (24.5) | |
| Racial or ethnic group | 0.014 | ||||
| Missing | 2 | 0 | 0 | 2 | |
| White | 62 (12.0) | 411 (79.7) | 43 (8.3) | 516 (78.3) | |
| Black | 1 (10.0) | 8 (80.0) | 1 (10.0) | 10 (1.5) | |
| Hispanic | 4 (12.9) | 26 (83.9) | 1 (3.2) | 31 (4.7) | |
| Native American | 0 (0.0) | 1 (100.0) | 0 (0.0) | 1 (0.2) | |
| Asian or Pacific Islander | 6 (13.0) | 38 (82.6) | 2 (4.3) | 46 (7.0) | |
| Indian | 10 (40.0) | 15 (60.0) | 0 (0.0) | 25 (3.8) | |
| Middle Eastern | 4 (26.7) | 8 (53.3) | 3 (20.0) | 15 (2.3) | |
| Other | 4 (26.7) | 9 (60.0) | 2 (13.3) | 15 (2.3) | |
| Marital status | 0.574 | ||||
| Missing | 0 | 2 | 0 | 2 | |
| Single | 40 (14.8) | 207 (76.4) | 24 (8.9) | 271 (41.1) | |
| Married | 45 (13.2) | 270 (79.2) | 26 (7.6) | 341 (51.7) | |
| Divorced | 8 (19.0) | 33 (78.6) | 1 (2.4) | 42 (6.4) | |
| Widow(er) | 0 (0.0) | 4 (80.0) | 1 (20.0) | 5 (0.8) | |
| Education | 0.108 | ||||
| Missing | 0 | 3 | 0 | 3 | |
| High school | 8 (17.8) | 36 (80.0) | 1 (2.2) | 45 (6.8) | |
| College | 55 (15.7) | 273 (77.8) | 23 (6.6) | 351 (53.3) | |
| Graduate school or higher | 30 (11.5) | 204 (77.9) | 28 (10.7) | 262 (39.8) | |
| Average household income | 0.146 | ||||
| Missing | 1 | 11 | 1 | 13 | |
| <$25 000 | 12 (23.1) | 36 (69.2) | 4 (7.7) | 52 (8.0) | |
| $25 000 to $34 999 | 12 (21.4) | 43 (76.8) | 1 (1.8) | 56 (8.6) | |
| $35 000 to $49 999 | 17 (18.5) | 71 (77.2) | 4 (4.3) | 92 (14.2) | |
| $50 000 to $74 999 | 20 (11.6) | 135 (78.0) | 18 (10.4) | 173 (26.7) | |
| $75 000 to $99 999 | 11 (11.0) | 82 (82.0) | 7 (7.0) | 100 (15.4) | |
| $100 000 to $149 999 | 16 (13.6) | 89 (75.4) | 13 (11.0) | 118 (18.2) | |
| $150 000 to $199 999 | 3 (7.0) | 36 (83.7) | 4 (9.3) | 43 (6.6) | |
| ≥$200 000 | 1 (7.1) | 13 (92.9) | 0 (0.0) | 14 (2.2) | |
| Hypertension | 0.272 | ||||
| Missing | 2 | 4 | 0 | 6 | |
| No | 73 (12.9) | 450 (79.4) | 44 (7.8) | 567 (86.6) | |
| Yes | 18 (20.9) | 60 (69.8) | 8 (9.3) | 86 (13.1) | |
| Unsure | 0 (0.0) | 2 (100.0) | 0 (0.0) | 2 (0.3) | |
| Diabetes/impaired fasting glucose | 0.406 | ||||
| Missing | 2 | 2 | 0 | 4 | |
| No | 84 (13.4) | 490 (78.4) | 51 (8.2) | 625 (95.1) | |
| Yes | 6 (20.0) | 23 (76.7) | 1 (3.3) | 30 (4.6) | |
| Unsure | 1 (50.0) | 1 (50.0) | 0 (0.0) | 2 (0.3) | |
| Dyslipidemia | 0.369 | ||||
| Missing | 1 | 0 | 0 | 1 | |
| No | 76 (13.3) | 452 (79.3) | 42 (7.4) | 570 (86.4) | |
| Yes | 15 (17.9) | 59 (70.2) | 10 (11.9) | 84 (12.7) | |
| Unsure | 1 (16.7) | 5 (83.3) | 0 (0.0) | 6 (0.9) | |
| Myocardial infarction | 0.825 | ||||
| Missing | 0 | 4 | 1 | 5 | |
| No | 92 (14.4) | 499 (78.0) | 49 (7.7) | 640 (97.6) | |
| Yes | 1 (6.7) | 12 (80.0) | 2 (13.3) | 15 (2.3) | |
| Unsure | 0 (0.0) | 1 (100.0) | 0 (0.0) | 1 (0.2) | |
| Stroke | 0.703 | ||||
| Missing | 0 | 6 | 0 | 6 | |
| No | 92 (14.2) | 506 (78.0) | 51 (7.9) | 649 (99.1) | |
| Yes | 1 (16.7) | 4 (66.7) | 1 (16.7) | 6 (0.9) | |
| Smoking history | 0.459 | ||||
| Missing | 1 | 4 | 0 | 5 | |
| Never smoker | 74 (14.0) | 414 (78.3) | 41 (7.8) | 529 (80.6) | |
| Former smoker, quit ≤12 mo ago | 4 (19.0) | 15 (71.4) | 2 (9.5) | 21 (3.2) | |
| Former smoker, quit >12 mo ago | 9 (10.3) | 69 (79.3) | 9 (10.3) | 87 (13.3) | |
| Current smoker | 5 (26.3) | 14 (73.7) | 0 (0.0) | 19 (2.9) | |
| Regular exercise | < 0.001 | ||||
| Missing | 0 | 3 | 0 | 3 | |
| I do not exercise regularly | 38 (32.8) | 74 (63.8) | 4 (3.4) | 116 (17.6) | |
| I exercise occasionally, but no more than twice a wk | 27 (12.9) | 171 (81.8) | 11 (5.3) | 209 (31.8) | |
| I exercise 3–5 times a wk | 27 (10.5) | 208 (80.6) | 23 (8.9) | 258 (39.2) | |
| I exercise most or all days of the wk | 1 (1.3) | 60 (80.0) | 14 (18.7) | 75 (11.4) |
Categorical variables are reported as number (row percentage) for subgroups and number (percentage) for overall. Abbreviations: HEI, Healthy Eating Index. Score <51=poor diet, 51 to 80=fair diet, >80=good diet.
Validation of EAT and Development of Mini‐EAT
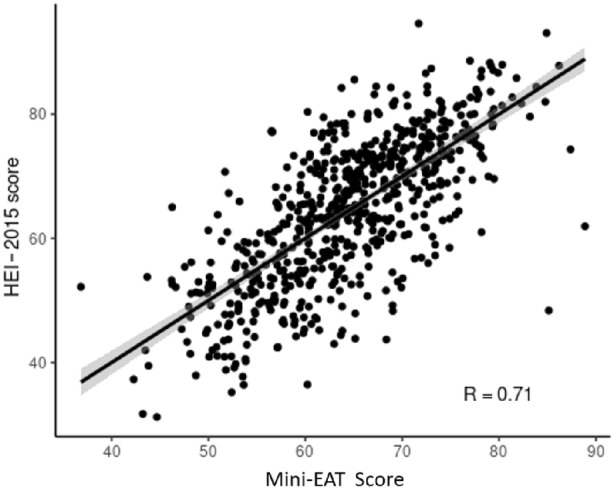
The 19‐item EAT answers by total HEI group (HEI score <51, HEI score 51–80, HEI score >80) are provided in Table S6. Among the 74 participants who completed the EAT survey twice, the intraclass correlation between the 2 sets of HEI score was 0.86 (95% CI, 0.79–0.91), indicating good test–retest reliability. Using a multivariable linear regression model for the 19 items, fruits (2.12 [95% CI, 1.67–2.56]; P<0.01), vegetables (0.85 [95% CI, 0.33–1.37]; P<0.01), whole grains (1.44 [95% CI, 1.05–1.84]; P<0.01), refined grains (−1.14 [95% CI, −1.52 to −0.76]; P<0.01), fish or seafood (0.90 [95% CI, 0.22–1.57]; P<0.01), legumes/nuts/seeds (0.72 [95% CI, 0.25–1.18]; P<0.01), low‐fat dairy (0.81 [95% CI, 0.48–1.15]; P<0.01), high‐fat dairy (−1.03 [95% CI, −1.42 to −0.63]; P<0.01), and sweets (−0.89 [95% CI, −1.33 to −0.46]; P<0.01) remained statistically significant with predicting the HEI score after adjusting for each other (Table S3). These 9 questions were subsequently used in the final model and were strongly correlated with the HEI score (R=0.71) (Figure 1). The remaining 10 questions of EAT were nonsignificant and therefore not included in the final model (Table S2). In the 5‐fold cross‐validation step, mean residual of the models ranged from −0.86 to 1.18, correlation coefficients between the HEI score and predicted HEI score ranged between 0.65 and 0.73, and the accuracy of predicting the correct HEI group ranged from 0.73 to 0.86.
Figure 1. Scatter plot of Mini‐Eating Assessment Tool (Mini‐EAT) score vs Healthy Eating Index 2015 (HEI‐2015) score.
Receiver operating characteristic analysis of Mini‐EAT as a continuous variable resulted in good predictive power of an HEI score >80 with a sensitivity of 0.73 and specificity of 0.85 (area under the curve, 0.87 [95% CI, 0.83–0.92]) (Figure 2). The Mini‐EAT cutoff score >69 for a healthy diet was determined by receiver operating characteristic analysis using Mini‐EAT as a binary variable (sensitivity 0.73, specificity 0.85). Utilizing the same analysis, the best cutoff point in a Mini‐EAT score for the unhealthy class was <60 (sensitivity 0.73, specificity 0.87).
Figure 2. Receiver operating characteristic curve for the predictive power of the Healthy Eating Index (HEI) (HEI score >80) by using Mini‐EAT score as a continuous variable.
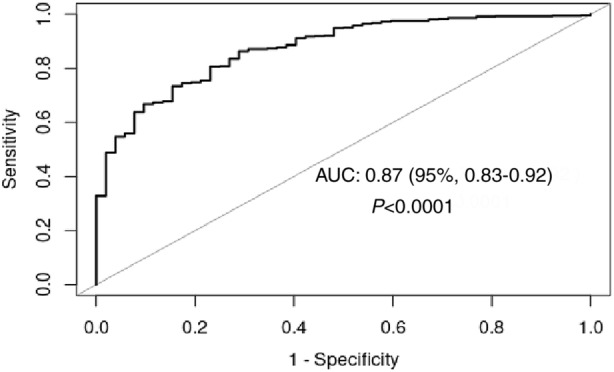
Mini‐Eat score as a continuous variable with sensitivity of 0.85 and specificity of 0.73. Best cutoff point for Mini‐EAT score: 69. AUC indicates area under curve.
DISCUSSION
The aim of the current study was to evaluate the validity of our 19‐item abbreviated dietary questionnaire, EAT, against the HEI score assessed by an FFQ and identify healthy eating behaviors in adults presenting to a preventive cardiology clinic or cardiac rehabilitation and from the community. From our knowledge, our analysis of further reducing the 19‐item questionnaire to the 9‐item Mini‐EAT is novel and maintained very good predictive power when compared with the reference standard FFQ, which is extensive and time consuming. Mini‐EAT also meets most of the AHA's theoretical and practice‐based validity criteria including validity against a complete dietary assessment, brevity, and ability to be completed and scored at administration without special knowledge or software. 8 Additionally, it describes food consumption of the main components included in the AHA‐recommended dietary patterns and allows practitioners to identify room for improvement regarding specific food groups. The 9‐item Mini‐EAT further distinguishes itself by maintaining validity with substantial correlation (r=0.71) and may have the potential to be adopted into health clinics' electronic medical record.
Validation studies of rapid diet screener tools that assess total diet, reflect the most up‐to‐date dietary guidance, and meet most of the AHA's validity criteria include the Mediterranean Diet Adherence Screener (MEDAS), the modified, shortened Rapid Eating Assessment for Participants (REAP‐S), and the PrimeScreen tool. The original MEDAS tool was composed of 14 items and showed only moderate correlation (r=0.52, P<0.001) compared with a full‐length FFQ. 9 The REAP‐S was studied in 81 healthy adults and was composed of 16 questions and had poor correlation with the older 2010 HEI score (r=0.23, P=0.047). 10 The PrimeScreen dietary screening tool was validated with 160 adults and was composed of 25 items (including 7 questions on nutrients) and achieved a good correlation (r=0.70). 11 While these existing dietary screeners are similar to our 19‐item questionnaire, our analysis executes an additional step to remove items that do not contribute to the correlation to the reference standard, making it more efficient and able to be distributed in a busy clinical practice. Our Mini‐EAT tool was also validated with a significantly larger number of participants compared with prior studies.
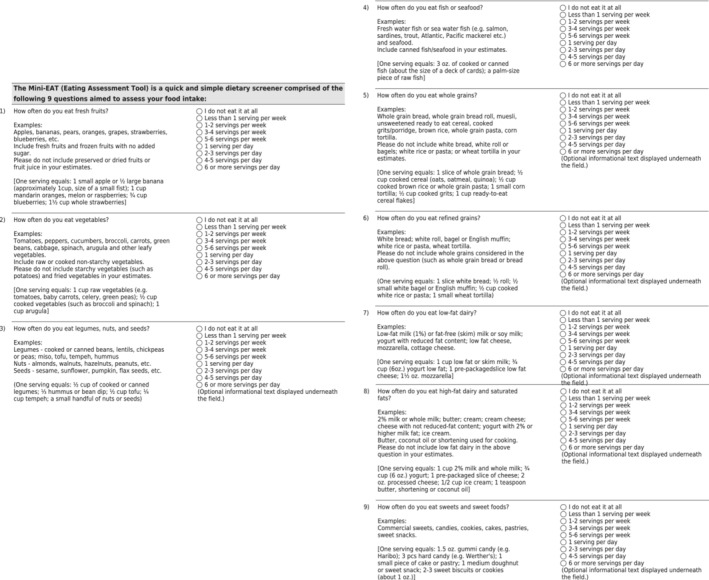
Mini‐EAT can be adopted by most health clinics because of its simplicity, convenience, and minimal expertise required in nutrition (Figure 3). It can be easily integrated in the electronic medical record and coupled with clinical decision support by an integrative multidisciplinary team including the clinical provider (physician, nurse practitioner, physician assistant), registered nurses, behavioral therapists, and registered dieticians. For example, one pathway to consider might include providing patients with a smart tablet that administers the Mini‐EAT in the waiting room after checking in. A low score would result in an automatic referral to a dietician for a more formal and comprehensive evaluation. The dietician would provide practical strategies for making healthier decisions based on childhood learned experiences, cultural barriers, psychosocial influences, and socioeconomic status implications. If a referral to dietetics is not feasible, online or physical educational materials on how to improve their diet can be disseminated at the end of the visit. Rather than a formal assessment, for an intermediate score, which likely reflects a balance of good and bad dietary habits, a conversation between the provider and patient with a goal of identifying 2 or 3 actionable predefined algorithmic modifications to improve diet with a plan for close follow‐up by phone would provide a quick yet efficient pathway. For example, a pop‐up screen after taking the Mini‐EAT would involve the patient selecting 2 goals from 5 or 6 options and may include any of the following examples: drinking at least 8 glasses of water per day, cutting the portion of refined carbohydrates by 50% and replacing with whole grain products, avoiding sweet foods 5 days of the week, and replacing at least 3 dinners with fish/seafood or plant proteins. Present comorbidities including obesity, prediabetes, diabetes, coronary heart disease, hypertension, and heart failure should prompt long‐term dietary follow‐up with the patient based on the Mini‐EAT score. Further ideas for development of the Mini‐EAT include rapid “click bait” of graphics or videos and “eat this not that” recommendations based on where their scores were specifically suboptimal would provide meaningful nutrition education for practical use. A high score would validate the patient with positive feedback and continued motivation to maintain a healthy diet.
Figure 3. Mini‐Eating Assessment Tool (Mini‐EAT) 9‐item survey.
Interestingly, fats, healthy oils, and meat consumption were not found to be statistically significant nor associated with the HEI score in the multivariable model and did not add to the correlation coefficient and were therefore not included in Mini‐EAT. This is likely explained by the food groups strongly correlating with other food items associated to HEI score. However, the exclusion of fat and meat intake in the Mini‐EAT should not be interpreted as noncontributors in the quality of diet but rather they do not provide incremental correlation with the HEI score. These findings contrast with prior studies, which utilize the above food groups as part of determining dietary patterns in their screening tools.
Limitations of the present study include selection bias, random and systematic errors in self‐reported dietary assessment, misclassification of food group reporting, and a nondiverse study population of mostly highly educated women. We acknowledge the potential challenge in accurately designating food items to the correct food groups, such as the vast majority of items such as cereal products that may market themselves as unrefined whole grain products but more accurately reflect refined grains. Future research is needed to develop specific definitions survey responders can understand with the goal of accurately categorizing food groupings. The inaccuracies of validated FFQs to measure dietary intake is also a known limitation in nutrition research. Although Mini‐EAT lacks Viocare's graphics of pictures and portions of food groups to assist in more accurate responses, Mini‐EAT was still able to achieve good correlation. The 2015 HEI is also commonly used in nutrition research; however, there are multiple ways to arrive at the same HEI score, resulting in more confidence in extreme scores due consistency in diet and less confidence in intermediate scores. Important information concerning macronutrients such as total protein intake may also not be captured because of the truncated HEI components and total scores. The lack of diversity in our sample population is a significant limitation of the current study and more extensive testing of the Mini‐EAT within different sample populations is essential for Mini‐EAT's generalizability. Furthermore, input from healthcare providers regarding the utility and effectiveness of Mini‐EAT will need to be further investigated.
Mini‐EAT is a validated rapid dietary screening tool that correlates well with the HEI‐2015 assessed by an FFQ and is feasible for a busy clinical practice to incorporate into the electronic medical record. Opportunities for the development of Mini‐EAT into a digital health tool or app would lend convenience to both providers and patients, which is consistent with the AHA's call to improve the integration of diet screener tools into clinical practice. 8 Furthermore, development into a clinical decision support tool will allow Mini‐EAT the ability to capture dietary changes over time and become incorporated into chronic disease management. Future studies validating Mini‐EAT in diverse populations along with predictive validity relating to cardiometabolic risk factors are warranted.
Sources of Funding
None.
Disclosures
Dr Lerman reported consultative services for Itamar Medical, Philips, and Shahal Telemedicine. Dr Kopecky reported consultative services for Prime Therapeutics, Amgen, and Merck; research support for True Health, DSMB Member for Applied Clinical Intelligence, Chair of Mayo Clinic Support Services in Texas, and board member for Mayo Clinic CV & Pharmacy and Therapeutics Task force; member for American Society for Men's Health; and author of “Live Younger Longer.” All other coauthors have no disclosures.
Supporting information
Table S1–S6
Figure S1
Presented in part at the American Heart Association Scientific Sessions 2021, held virtually from November 13–15, 2021, and published in abstract form [Circulation. 2021;144:A9748 or https://doi.org/10.1161/circ.144.suppl_1.9748].
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.025064
For Sources of Funding and Disclosures, see page 9.
See Editorial by Vadiveloo et al.
References
- 1. Barnard ND, Alwarith J, Rembert E, Brandon L, Nguyen M, Goergen A, Horne T, do Nascimento GF, Lakkadi K, Tura A, et al. A mediterranean diet and low‐fat vegan diet to improve body weight and cardiometabolic risk factors: a randomized, cross‐over trial. J Am Coll Nutr. 2022;41:127–139. [DOI] [PubMed] [Google Scholar]
- 2. Siervo M, Lara J, Chowdhury S, Ashor A, Oggioni C, Mathers JC. Effects of the Dietary Approach to Stop Hypertension (DASH) diet on cardiovascular risk factors: a systematic review and meta‐analysis. Br J Nutr. 2015;113:1–15. [DOI] [PubMed] [Google Scholar]
- 3. Djekic D, Shi L, Brolin H, Carlsson F, Särnqvist C, Savolainen O, Cao Y, Bäckhed F, Tremaroli V, Landberg R, et al. Effects of a vegetarian diet on cardiometabolic risk factors, gut microbiota, and plasma metabolome in subjects with ischemic heart disease: a randomized, crossover study. J Am Heart Assoc. 2020;9:e016518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Micha R, Peñalvo JL, Cudhea F, Imamura F, Rehm CD, Mozaffarian D. Association between dietary factors and mortality from heart disease, stroke, and type 2 diabetes in the United States. JAMA. 2017;317:912–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grossman DC, Bibbins‐Domingo K, Curry SJ, Barry MJ, Davidson KW, Doubeni CA, Epling JW Jr, Kemper AR, Krist AH, Kurth AE, et al. Behavioral counseling to promote a healthful diet and physical activity for cardiovascular disease prevention in adults without cardiovascular risk factors: US Preventive Services Task Force Recommendation Statement. JAMA. 2017;318:167–174. [DOI] [PubMed] [Google Scholar]
- 6. Patnode CD, Evans CV, Senger CA, Redmond N, Lin JS. Behavioral counseling to promote a healthful diet and physical activity for cardiovascular disease prevention in adults without known cardiovascular disease risk factors: updated evidence report and systematic review for the US preventive services task force. JAMA. 2017;318:175–193. [DOI] [PubMed] [Google Scholar]
- 7. Lv N, Azar KM, Rosas LG, Wulfovich S, Xiao L, Ma J. Behavioral lifestyle interventions for moderate and severe obesity: a systematic review. Prev Med. 2017;100:180–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vadiveloo M, Lichtenstein AH, Anderson C, Aspry K, Foraker R, Griggs S, Hayman LL, Johnston E, Stone NJ, Thorndike AN. Rapid diet assessment screening tools for cardiovascular disease risk reduction across healthcare settings: a scientific statement from the American Heart Association. Circ Cardiovasc Qual Outcomes. 2020;13:e000094. [DOI] [PubMed] [Google Scholar]
- 9. Schröder H, Fitó M, Estruch R, Martínez‐González MA, Corella D, Salas‐Salvadó J, Lamuela‐Raventós R, Ros E, Salaverría I, Fiol M, et al. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J Nutr. 2011;141:1140–1145. [DOI] [PubMed] [Google Scholar]
- 10. Johnston CS, Bliss C, Knurick JR, Scholtz C. Rapid Eating Assessment for Participants [shortened version] scores are associated with Healthy Eating Index‐2010 scores and other indices of diet quality in healthy adult omnivores and vegetarians. Nutr J. 2018;17:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rifas‐Shiman SL, Willett WC, Lobb R, Kotch J, Dart C, Gillman MW. PrimeScreen, a brief dietary screening tool: reproducibility and comparability with both a longer food frequency questionnaire and biomarkers. Public Health Nutr. 2001;4:249–254. [DOI] [PubMed] [Google Scholar]
- 12. Reedy J, Lerman JL, Krebs‐Smith SM, Kirkpatrick SI, Pannucci TE, Wilson MM, Subar AF, Kahle LL, Tooze JA. Evaluation of the Healthy Eating Index‐2015. J Acad Nutr Diet. 2018;118:1622–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McCullough ML, Willett WC. Evaluating adherence to recommended diets in adults: the Alternate Healthy Eating Index. Public Health Nutr. 2006;9:152–157. [DOI] [PubMed] [Google Scholar]
- 14. Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142:1009–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schwingshackl L, Hoffmann G. Diet quality as assessed by the Healthy Eating Index, the Alternate Healthy Eating Index, the Dietary Approaches to Stop Hypertension score, and health outcomes: a systematic review and meta‐analysis of cohort studies. J Acad Nutr Diet. 2015;115:780–800.e5. [DOI] [PubMed] [Google Scholar]
- 16. Apovian CM, Murphy MC, Cullum‐Dugan D, Lin PH, Gilbert KM, Coffman G, Jenkins M, Bakun P, Tucker KL, Moore TJ. Validation of a web‐based dietary questionnaire designed for the DASH (dietary approaches to stop hypertension) diet: the DASH online questionnaire. Public Health Nutr. 2010;13:615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Snetselaar LG, de Jesus JM, DeSilva DM, Stoody EE. Dietary Guidelines for Americans, 2020–2025: understanding the Scientific Process, Guidelines, and Key Recommendations. Nutrition Today. 2021;56:287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lloyd‐Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 19. Lichtenstein AH, Appel LJ, Vadiveloo M, Hu FB, Kris‐Etherton PM, Rebholz CM, Sacks FM, Thorndike AN, Van Horn L, Wylie‐Rosett J. 2021 dietary guidance to improve cardiovascular health: a scientific statement from the American Heart Association. Circulation. 2021;144:e472–e487. [DOI] [PubMed] [Google Scholar]
- 20. Wang DD, Li Y, Bhupathiraju SN, Rosner BA, Sun Q, Giovannucci EL, Rimm EB, Manson JE, Willett WC, Stampfer MJ, et al. Fruit and vegetable intake and mortality: results from 2 prospective cohort studies of US men and women and a meta‐analysis of 26 cohort studies. Circulation. 2021;143:1642–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Amba V, Murphy G, Etemadi A, Wang S, Abnet CC, Hashemian M. Nut and peanut butter consumption and mortality in the National Institutes of Health‐AARP diet and health study. Nutrients. 2019;11:1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ivey KL, Nguyen XT, Quaden RM, Ho YL, Cho K, Gaziano JM, Djoussé L. Association of nut consumption with risk of stroke and cardiovascular disease: the Million Veteran Program. Nutrients. 2021;13:3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van den Brandt PA, Schouten LJ. Relationship of tree nut, peanut and peanut butter intake with total and cause‐specific mortality: a cohort study and meta‐analysis. Int J Epidemiol. 2015;44:1038–1049. [DOI] [PubMed] [Google Scholar]
- 24. Hauk L. DGAC makes food‐based recommendations in the 2015–2020 dietary guidelines for Americans. Am Fam Physician. 2016;93:525. [PubMed] [Google Scholar]
- 25. Krebs‐Smith SM, Pannucci TE, Subar AF, Kirkpatrick SI, Lerman JL, Tooze JA, Wilson MM, Reedy J. Update of the Healthy Eating Index: HEI‐2015. J Acad Nutr Diet. 2018;118:1591–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1–S6
Figure S1


