Abstract
Background
Blockade of the lectin‐like oxidized low‐density lipoprotein receptor‐1 (LOX‐1) is a potentially attractive mechanism for lowering inflammatory and lipid risk in patients with atherosclerosis. This study aims to assess the safety, tolerability, and target engagement of MEDI6570, a high‐affinity monoclonal blocking antibody to LOX‐1.
Methods and Results
This phase 1, first‐in‐human, placebo‐controlled study (NCT03654313) randomized 88 patients with type 2 diabetes to receive single ascending doses (10, 30, 90, 250, or 500 mg) or multiple ascending doses (90, 150, or 250 mg once monthly for 3 months) of MEDI6570 or placebo. Primary end point was safety; secondary and exploratory end points included pharmacokinetics, immunogenicity, free soluble LOX‐1 levels, and change in coronary plaque volume. Mean age was 57.6/58.1 years in the single ascending doses/multiple ascending doses groups, 31.3%/62.5% were female, and mean type 2 diabetes duration was 9.7/8.7 years. Incidence of adverse events was similar among cohorts. MEDI6570 exhibited nonlinear pharmacokinetics, with terminal half‐life increasing from 4.6 days (30 mg) to 11.2 days (500 mg), consistent with target‐mediated drug disposition. Dose‐dependent reductions in mean soluble LOX‐1 levels from baseline were observed (>66% at 4 weeks and 71.61–82.96% at 10 weeks in the single ascending doses and multiple ascending doses groups, respectively). After 3 doses, MEDI6570 was associated with nonsignificant regression of noncalcified plaque volume versus placebo (−13.45 mm3 versus −8.25 mm3).
Conclusions
MEDI6570 was well tolerated and demonstrated dose‐dependent soluble LOX‐1 suppression and a pharmacokinetic profile consistent with once‐monthly dosing.
Registration
URL: https://clinicaltrials.gov/; Unique identifier: NCT03654313.
Keywords: atherosclerosis, cardiovascular disease, coronary CTA, diabetes, LOX‐1
Subject Categories: Clinical Studies; Acute Coronary Syndromes; Atherosclerosis; Inflammation; Diabetes, Type 2
Nonstandard Abbreviations and Acronyms
- ADA
anti‐drug antibody
- AE
adverse event
- AUC0–inf
area under the curve from time zero to infinity
- Cmax
peak plasma concentration
- CTA
computed tomography angiography
- FAI
fat attenuation index
- LLOQ
lower limit of quantification
- LOX‐1
low‐density lipoprotein receptor‐1
- MACE
major cardiovascular events
- MAD
multiple ascending dose
- SAD
single ascending dose
- sLOX‐1
soluble LOX‐1
Clinical Perspective.
What Is New?
In this phase 1 study in patients with type 2 diabetes, MEDI6570, a monoclonal antibody that blocks the pro‐atherogenic receptor low‐density lipoprotein receptor‐1, was well tolerated.
Soluble low‐density lipoprotein receptor‐1 was reduced by >66% at 4 weeks in the single ascending dose group, and by 71.61% to 82.96% at 10 weeks in the multiple ascending dose group.
What Are the Clinical Implications?
Blockade of low‐density lipoprotein receptor‐1 is a potentially attractive mechanism for lowering inflammatory and lipid risk in patients with atherosclerosis.
These findings warrant further clinical investigation of MEDI6570 as a potential additional therapy against atherosclerosis, alongside lipid‐lowering therapies and emerging anti‐inflammatory therapies.
Atherosclerosis is a chronic inflammatory disease that develops as a result of inflammation and lipid deposition in the coronary arteries and that can lead to ischemic heart disease and acute coronary syndrome. 1 Statins are first‐line therapy for primary and secondary prevention of cardiovascular disease because they lower both inflammatory and lipid‐associated risks in patients with atherosclerosis. 2 New lipid‐lowering therapies such as proprotein convertase subtilisin‐kexin type 9 inhibitors further lower low‐density lipoprotein (LDL) levels, resulting in a 15% relative risk reduction in major cardiovascular events (MACE), but without any robust anti‐inflammatory effects. 3 , 4 , 5 Independently, reduction of inflammation through anti‐interleukin 1β antibody administration in the Canakinumab Anti‐inflammatory Thrombosis Outcomes Study trial showed a 15% relative risk reduction in death, myocardial infarction, and stroke, but without any significant reduction in lipid levels. 6 Together these clinical studies show that residual inflammatory and lipid‐associated risks remain in patients treated with statins and can cause MACE. 4 , 7 Therapies that can lower both residual inflammatory and lipid‐associated risks are therefore highly attractive for reducing cardiovascular morbidity and mortality.
Blockade of the lectin‐like oxidized low‐density lipoprotein receptor‐1 (LOX‐1) could be an attractive mechanism for lowering inflammatory and lipid risk in patients with atherosclerosis. 8 LOX‐1 is found on many of the cells associated with proatherogenic processes, such as endothelial and smooth muscle cells, macrophages, neutrophils, and platelets. 9 , 10 As a scavenger receptor, LOX‐1 binds multiple ligands that are known to be atherogenic, including oxidized LDL, dysfunctional high‐density lipoprotein, C‐reactive protein, advanced glycosylation end products, activated platelets, and apoptotic cells. 11 , 12 , 13 LOX‐1 is upregulated in atherosclerotic plaques and has been shown to play an important role in cardiovascular disease initiation and progression. 14 , 15 , 16 Binding and internalization of ligands, such as oxidized LDL, by LOX‐1 on endothelial cells triggers increased expression of inflammatory cytokines and cellular adhesion molecules, production of vasoconstrictors and reactive oxygen species, and depletion of NO. 1 Therefore, there is a strong therapeutic potential in inhibiting the induction of the inflammatory response by blocking LOX‐1.
The level of LOX‐1 expression is reflected in the serum concentration of soluble LOX‐1 (sLOX‐1). LOX‐1 expression is minimal under healthy physiological conditions but is upregulated during chronic inflammatory conditions such as type 2 diabetes or cardiovascular disease. When LOX‐1 expression is upregulated, sLOX‐1 is released into the circulation via cleavage of the extracellular domain of the receptor by metalloproteinases. 1 Levels of circulating sLOX‐1 are markedly elevated in patients with acute coronary syndrome, previous myocardial infarction, and type 2 diabetes. 1 , 17 Levels of sLOX‐1 have been shown to predict long‐term all‐cause mortality and MACE. 18 Therefore, sLOX‐1 is a relevant biomarker of LOX‐1 expression and of the potential efficacy of LOX‐1 inhibition.
MEDI6570 is a high‐affinity human immunoglobulin G1 antibody to LOX‐1, designed to block the binding of multiple lipid and inflammatory ligands to LOX‐1. The aim of the present phase 1, first‐in‐human study in patients with type 2 diabetes was to evaluate the safety, tolerability, immunogenicity, and pharmacokinetics of single and multiple ascending doses of MEDI6570; and to establish the efficacy of MEDI6570 in reducing free sLOX‐1 levels. Patients with type 2 diabetes were selected because they are expected to have elevated baseline sLOX‐1 levels relative to healthy volunteers, allowing for target engagement and safety assessment, while avoiding potential associated risk to individuals with acute coronary syndrome. They are also broadly accessible and typically more clinically stable than those with atherosclerosis, making them a more suitable population for a phase 1 study investigating target engagement. The effects of MEDI6570 on atherosclerotic plaque volume and composition, and fat attenuation index (FAI), a marker of coronary inflammation, were also explored.
Methods
Overview
This was a phase 1, first‐in‐human, randomized, placebo‐controlled, dose‐escalation study of single (part A) and multiple (part B) ascending doses of MEDI6570 in patients with type 2 diabetes. The primary objective was to assess the safety and tolerability of MEDI6570. Secondary objectives were to evaluate the pharmacokinetics and immunogenicity of MEDI6570. Exploratory objectives included the characterization of target engagement in blood, the effect on inflammatory and disease pathogenesis biomarkers, and the effect on high‐risk coronary plaque volume and coronary artery inflammation.
Ethics
The study is registered on ClinicalTrials.gov (NCT03654313) and was conducted in accordance with the principles of the Declaration of Helsinki, the International Conference on Harmonization of Good Clinical Practice, and with any applicable laws and conditions required by relevant regulatory authorities. The study protocol and informed consent documents were reviewed and approved by the Institutional Review Board. All participants provided written informed consent before enrollment in the study. Data underlying the findings described in this article may be obtained in accordance with AstraZeneca's data sharing policy described at: https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Patients
Eligible patients in part A were men and women aged 18 to 65 years and in part B were men aged 18–65 years and women aged 40–65 years with type 2 diabetes and a body mass index of 18 to 45 kg/m2 (parts A and B). For part A cohort 6 (A6; see below), participants were ethnically Japanese, defined as having both parents and 4 grandparents who are Japanese. Patients were ineligible if they had a history of hepatic or renal disease, bleeding disorders, vascular abnormalities, or other clinically relevant conditions. Other key exclusion criteria were use of dual‐antiplatelet therapy, anticoagulation therapy, or thrombolytics in the previous month or planned use during the study, platelet count <100 000/μL, chronic anti‐inflammatory therapy, glycated hemoglobin >9.0% or insulin therapy, or history of ongoing infection or febrile illness within 30 days before randomization. Female participants were required to be of nonchildbearing potential. Full inclusion and exclusion criteria are listed in Data S1.
Trial Design and Interventions
In part A, participants were randomized into 6 cohorts (A1–A6; n=8 per cohort; Table 1; Figure 1). Cohort A6 comprised Japanese American participants. Each cohort was randomized 3:1 to receive single ascending doses (SAD) of MEDI6570 or placebo by subcutaneous injection. Safety was reviewed following dosing of each cohort before escalating to the next dose. Participants in cohorts A1–A4 received MEDI6570 10, 30, 90, or 250 mg, or placebo; participants in cohorts A5 and A6 received MEDI6570 500 mg or placebo. A sentinel dosing approach was used at each dose level.
Table 1.
Participant Demographics and Baseline Characteristics
| Part A, single ascending dose cohorts | Part B, multiple ascending dose cohorts | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo (n=12) | 10 mg (n=6) | 30 mg (n=6) | 90 mg (n=6) | 250 mg (n=6) | 500 mg (n=6) | 500 mg Japanese (n=6) | MEDI6570 total (n=36) | Placebo (n=10) | 90 mg (n=10) | 150 mg (n=10) | 250 mg (n=10) | MEDI6570 total (n=30) | |
| Age, mean (SD), y | 57.8 (6.9) | 52.8 (10.2) | 56.7 (5.3) | 59.7 (4.7) | 59.0 (5.2) | 60.0 (5.2) | 57.5 (7.2) | 57.6 (6.6) | 59.0 (5.5) | 57.3 (6.4) | 57.5 (9.3) | 58.4 (5.8) | 57.7 (7.1) |
| Female, n (%) | 3 (25.0) | 1 (16.7) | 1 (16.7) | 5 (83.3) | 1 (16.7) | 2 (33.3) | 2 (33.3) | 12 (33.3) | 5 (50.0) | 5 (50.0) | 7 (70.0) | 8 (80.0) | 20 (66.7) |
| Race, n (%) | |||||||||||||
| Asian | 2 (16.7) | 0 | 0 | 0 | 0 | 0 | 6 (100.0) | 6 (16.7) | 0 | 0 | 0 | 0 | 0 |
| Black | 2 (16.7) | 2 (33.3) | 1 (16.7) | 0 | 1 (16.7) | 2 (33.3) | 0 | 6 (16.7) | 1 (10.0) | 0 | 0 | 4 (40.0) | 4 (13.3) |
| White | 8 (66.7) | 4 (66.7) | 5 (83.3) | 6 (100.0) | 5 (83.3) | 4 (66.7) | 0 | 24 (66.7) | 9 (90.0) | 10 (100.0) | 9 (90.0) | 6 (60.0) | 25 (83.3) |
| Ethnicity, n (%) | |||||||||||||
| Hispanic | 3 (25.0) | 0 | 1 (16.7) | 4 (66.7) | 3 (50.0) | 0 | 0 | 8 (22.2) | 6 (60.0) | 7 (70.0) | 6 (60.0) | 2 (20.0) | 15 (50.0) |
| BMI, kg/m2, mean (SD) | 29.9 (3.8) | 30.8 (4.1) | 34.4 (5.0) | 30.5 (5.1) | 31.3 (5.9) | 31.9 (4.2) | 27.7 (5.2) | 31.1 (5.0) | 33.0 (4.4) | 34.6 (6.5) | 33.0 (5.2) | 34.2 (3.7) | 33.9 (5.1) |
| Prior CAD, n (%) | 1 (8.3) | 0 | 1 (16.7) | 1 (16.7) | 0 | 1 (16.7) | 0 | 3 (8.3) | 0 | 0 | 0 | 0 | 0 |
| Hypertension, n (%) | 10 (83.3) | 1 (16.7) | 5 (83.3) | 3 (50.0) | 4 (66.7) | 6 (100.0) | 5 (83.3) | 24 (66.7) | 6 (60.0) | 7 (70.0) | 5 (50.0) | 8 (80.0) | 20 (66.7) |
| Dyslipidemia, n (%) | 8 (66.7) | 4 (66.7) | 4 (66.7) | 4 (66.7) | 4 (66.7) | 4 (66.7) | 6 (100.0) | 26 (72.2) | 6 (60.0) | 7 (70.0) | 3 (30.0) | 6 (60.0) | 16 (53.3) |
| Current smoker, n (%) | 1 (8.3) | 0 | 0 | 0 | 1 (16.7) | 2 (33.3) | 0 | 3 (8.3) | 2 (20.0) | 1 (10.0) | 1 (10.0) | 2 (20.0) | 4 (13.3) |
| Prior PCI, n (%) | 1 (8.3) | 0 | 1 (16.7) | 1 (16.7) | 0 | 0 | 0 | 2 (5.6) | 0 | 0 | 0 | 0 | 0 |
| Prior MI, n (%) | 0 | 0 | 0 | 1 (16.7) | 0 | 0 | 0 | 1 (2.8) | 0 | 0 | 0 | 0 | 0 |
| Prior stroke or TIA, n (%) | 0 | 1 (16.7) | 1 (16.7) | 1 (16.7) | 0 | 2 (33.3) | 0 | 5 (13.9) | 0 | 0 | 0 | 1 (10.0) | 1 (3.3) |
| Duration of T2DM, mean (SD), y | 10.4 (4.7) | 6.2 (2.5) | 12.6 (6.8) | 11.1 (5.9) | 5.2 (6.2) | 7.4 (4.5) | 15.8 (13.1) | 9.7 (7.7) | 12.0 (7.1) | 6.3 (4.8) | 10.4 (4.9) | 9.5 (5.0) | 8.7 (5.1) |
| Baseline hs‐CRP, median (IQR), mg/L | 1.8 (0.7, 3.6) | 1.6 (0.7, 3.1) | 1.5 (1.2, 2.1) | 2.8 (2.3, 5.5) | 2.1 (1.8, 2.5) | 2.0 (1.0, 4.1) | 0.7 (0.2, 1.2) | 1.9 (1.0, 2.8) | 3.6 (1.3, 8.3) | 3.4 (2.1, 5.2) | 2.0 (0.5, 3.1) | 3.6 (2.6, 5.0) | 2.8 (1.8, 5.0) |
BMI indicates body mass index; CAD, coronary artery disease; hs‐CRP, high sensitivity C‐reactive protein; IQR, interquartile range; MI, myocardial infarction; PCI, percutaneous coronary intervention; T2DM, type 2 diabetes; and TIA, transient ischemic attack.
Figure 1. Study design.
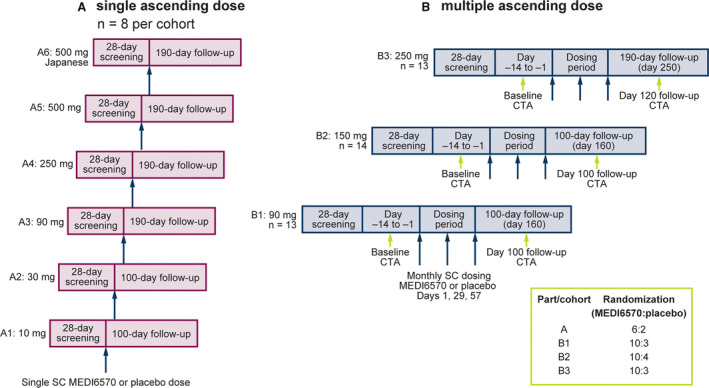
(A) Single ascending dose and (B) multiple ascending dose. CTA indicates computed tomography angiography; and SC, subcutaneous.
In part B, participants were randomized 10:3 (cohorts B1 and B3) or 10:4 (cohort B2) to receive multiple ascending doses (MAD; days 1, 29, and 57) of MEDI6570 90, 150, or 250 mg, or placebo by subcutaneous injection.
In part A, participants in cohorts A1–A5 were observed in the clinical research facility for 48 hours post dose. In part B, participants were observed for 24 hours after the first dose and at least 8 hours after the second and third doses of MEDI6570. Participants were followed up for 100 days (cohorts A1, A2, B1, and B2) or 190 days (cohorts A3–A6 and B3) after the last dose of MEDI6570 to take account of the expected longer target suppression by MEDI6570 at higher doses.
Safety was assessed throughout the trial and included monitoring of adverse events (AEs), which were evaluated for seriousness, severity, and relationship to study drug. Clinical chemistry and hematology, urinalysis, physical examinations (including assessment of injection site), vital signs, and electrocardiography were also assessed throughout the trial.
Blood samples for evaluation of the pharmacokinetics, immunogenicity, and pharmacodynamics of MEDI6570 were collected at prespecified timepoints through the study. Validated immunoassays were used for the quantification of MEDI6570 and relative‐quantification of free sLOX‐1 in serum. For immunogenicity assessment, a validated immunoassay was used for tiered analyses, which included screening, confirmatory, and titer assays.
Participants in part B underwent coronary computed tomography angiography (CTA) up to 14 days before randomization and at day 100 (cohorts B1 and B2) or day 120 (cohort B3) after the first dose (Figure 1). If necessary, participants received heart rate–lowering medication with oral and/or intravenous β‐blockers at least 60 minutes before CTA. Contrast‐enhanced CTA was performed using a 16 to 18‐gauge intravenous injection of 50 to 100 mL of iodinated contrast agent. Imaging was performed with prospective‐ECG triggering or, when necessary, tube‐current dose modulation to limit radiation exposure. CTA images were transferred to a blinded core laboratory (Caristo Diagnostics, Oxford, UK) for central interpretation. Plaque characteristics were analyzed using AutoPlaque v2.0 and v2.5 (Cedars Sinai, Los Angeles, CA). 19 iNtuition v4.4.13 (TeraRecon, Durham, NC) 20 was used for perivascular segmentations, and FAI was calculated using the CaRi‐Heart algorithm (CaRi‐CLOUD v1.0; Caristo Diagnostics, Oxford, UK).
End Points
The primary end points for assessment of the safety and tolerability of MEDI6570 were incidence of AEs, serious adverse events, and treatment‐emergent AEs and serious adverse events, and incidence of clinically meaningful changes in 12‐lead ECG, vital signs, or safety laboratory analyses. Secondary end points for evaluation of the pharmacokinetics of MEDI6570 included area under the curve from time zero to infinity (AUC0–inf), peak plasma concentration (Cmax), time to Cmax, and terminal half‐life. Additional secondary end points for evaluation of immunogenicity were anti‐drug antibody (ADA) positivity and titer. Exploratory pharmacodynamic end points included changes from baseline in sLOX‐1 concentration, hs‐CRP (high‐sensitivity C‐reactive protein) concentration, coronary plaque characterization, and quantification by CTA, including noncalcified, low‐attenuation (lipid core), calcified, total plaque volume, and FAI.
Statistical Analysis
The sample sizes for Part A and Part B of the study were empirically determined to provide adequate safety, tolerability, pharmacokinetics, and pharmacodynamics data to achieve study objectives while exposing as few subjects as possible to the investigational product and study procedures. Analyses were conducted on an as‐treated basis. The pharmacokinetic population included participants who received MEDI6570 and had detectable postdosing serum concentrations of MEDI6570. Data from participants receiving placebo were pooled across all cohorts within each study part. Analyses were performed using SAS v9.4 or later (SAS Institute Inc., Cary, NC).
Descriptive statistics were used to summarize results. The pharmacokinetic parameters were estimated by noncompartmental analysis using Phoenix WinNonlin v8.1 (Pharsight, Inc., Mountain View, CA). Pharmacodynamic parameters (sLOX‐1 and hs‐CRP levels) were calculated as absolute change from baseline and percent change from baseline. Fisher exact tests were used to compare the proportion of participants achieving free sLOX‐1 suppression (defined as a 90% reduction from baseline in free sLOX‐1 level or a free sLOX‐1 level below the lower limit of quantification [LLOQ]) in the MEDI6570 groups versus the pooled placebo group. An analysis of covariance model adjusting for baseline was used to compare percent change from baseline between the MEDI6570 groups and the pooled placebo group on hs‐CRP and FAI levels. No adjustment for multiplicity was applied.
Results
Participants
Of 253 patients screened across 9 sites in the United States from September 28, 2018 to July 21, 2020, 88 were randomly assigned to part A (n=48) or part B (n=40). The main reasons for screen failures were patients not meeting the inclusion criteria (n=47) and the cohort being full (n=18). In part A, all participants completed treatment; 1 participant in the 30 mg cohort (16.7%) withdrew from the study after completion of treatment but before study end because of personal obligations. In part B, 1 participant each in the 90 mg cohort (10%; because of commitments outside the study) and the 150 mg cohort (10%; no longer wished to take part) withdrew from the study after 2 doses of MEDI6570, and 1 participant (10%) in the 250 mg cohort withdrew after completing treatment but before study end because of concerns about COVID‐19.
In part A, the mean age of participants was 57.6 years, 31.3% were female, and the mean body mass index was 30.8 kg/m2, with the Japanese participants in cohort A6 having the lowest mean body mass index at 27.7 kg/m2 (Table 1). In part B, the mean age was 58.1 years, 62.5% were female, and mean body mass index was 33.7 kg/m2. The majority of participants had a history of hypertension (of those receiving MEDI6570/placebo: part A, 66.7%/83.3%; part B, 66.7%/60.0%) and dyslipidemia (of those receiving MEDI6570/placebo: part A, 72.2%/66.7%; part B, 53.3%/60.0%). The mean duration of type 2 diabetes for those receiving MEDI6570/placebo was 9.7 years/10.4 years for part A participants, and 8.7 years/12.0 years for part B participants (Table 1).
The overall median CTA radiation exposure at baseline was 4.97mSv (interquartile range, 3.63–6.66) and at follow‐up was 4.06mSv (2.67–6.90). The median cumulative exposure across baseline and follow‐up CTA scans was 9.03mSv (7.08–13.17).
Safety, Tolerability, and Immunogenicity
There were no deaths, life‐threatening AEs, or AEs leading to study withdrawal. The incidence of AEs was similar in the MEDI6570 and placebo SAD (part A) and MAD cohorts (part B; Table 2). There were 4 serious AEs (2 in the SAD cohorts and 2 in the MAD cohorts); none were considered by the study investigator to be related to MEDI6570. No bleeding, coagulation, or platelet‐related AEs were reported. There were no clinically relevant changes in vital signs, ECG, or standard safety laboratory parameters. Investigator‐reported injection site reactions were rare and mild in severity; injection site reactions occurred in 2 participants who received MEDI6570 (1 in the SAD cohorts and 1 in the MAD cohorts) and in 1 participant who received placebo (in the MAD cohorts). Treatment‐emergent AEs are detailed in Tables S1 and S2.
Table 2.
Summary of Adverse Events
| Adverse events, number of participants (%)* | Part A, single ascending dose cohorts | Part B, multiple ascending dose cohorts | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo (n=12) | 10 mg (n=6) | 30 mg (n=6) | 90 mg (n=6) | 250 mg (n=6) | 500 mg (n=6) | 500 mg Japanese (n=6) | Total (n=48) | Placebo (n=10) | 90 mg (n=10) | 150 mg (n=10) | 250 mg (n=10) | Total (n=40) | |
| Any | 6 (50.0) | 2 (33.3) | 5 (83.3) | 3 (50.0) | 3 (50.0) | 4 (66.7) | 5 (83.3) | 28 (58.3) | 7 (70.0) | 7 (70.0) | 6 (60.0) | 9 (90.0) | 29 (72.5) |
| Any treatment‐related | 2 (16.7) | 0 | 0 | 0 | 1 (16.7) | 0 | 1 (16.7) | 4 (8.3) | 2 (20.0) | 2 (20.0) | 0 | 0 | 4 (10.0) |
| ≥Grade 3 severity | 0 | 0 | 0 | 0 | 1 (16.7) | 1 (16.7) | 0 | 2 (12.5) | 1 (10.0) | 0 | 0 | 1 (10.0) | 2 (5.0) |
| Leading to death | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Serious | 0 | 0 | 0 | 0 | 1 (16.7) | 1 (16.7) | 0 | 2 (12.5) | 1 (10.0) | 0 | 1 (10.0) | 1 (10.0) | 3 (7.5) |
| Serious and ≥ grade 3 severity | 0 | 0 | 0 | 0 | 1 (16.7) | 1 (16.7) | 0 | 2 (12.5) | 1 (10.0) | 0 | 1 (10.0) | 1 (10.0) | 3 (7.5) |
| Serious and related to MEDI6570 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Leading to study discontinuation | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Patients are counted once per category regardless of the number of events.
In the SAD cohorts, 6 participants (16.7%) had ADA‐positive results; 1 of these was a preexisting positive response that was not boosted by administration of MEDI6570 and the remaining 5 participants (13.9%) were treatment‐emergent and classed as persistent. ADA titers from these 5 participants ranged from 20 to 640. In the MAD cohorts, 5 participants (16.7%) had ADA‐positive results; all were classed as treatment‐emergent and persistent (ADA titers, range 20–320). ADA‐positive results were not associated with treatment‐emergent AEs or with any impact on serum drug concentrations.
Pharmacokinetics
MEDI6570 exhibited nonlinear pharmacokinetics consistent with target‐mediated drug disposition after subcutaneous administration in patients with type 2 diabetes. In part A, the concentration–time profiles showed absorption of MEDI6570 with a peak in serum concentration at ≈7 days after a single dose, followed by a nonlinear elimination phase (Figure 2A). Cmax and AUC0–inf increased more than dose‐proportionally after administration of a single dose of MEDI6570 10 to 250 mg. For MEDI6570 250 to 500 mg, Cmax and AUC0–inf increased in proportion with dose (Table 3), indicating that target‐mediated drug disposition was overcome and linear elimination was reached at doses of 250 mg or higher. Terminal half life tended to increase with ascending dose, from 4.6 days with MEDI6570 30 mg to 11.2 days with MEDI6570 500 mg. The interparticipant variation in Cmax, AUC0–inf, and terminal half‐life decreased with increasing dose. In the Japanese cohort (A6), mean Cmax and AUC0–inf were 1.4 and 1.8 times higher, respectively, than for the non‐Japanese 500 mg cohort; however, individual serum concentration–time profiles of MEDI6570 overlapped between these 2 cohorts. In part B, the serum concentration of MEDI6570 accumulated with a ratio of 1.5 to 1.6 for third to first dose at 14 days post dose (day 69 versus day 14; Figure 2B).
Figure 2. Free serum concentration of MEDI6570 after single dose (A) or multiple doses (B) in patients with T2DM.
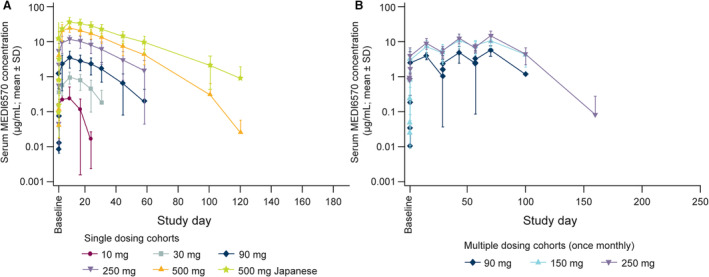
T2DM indicates type 2 diabetes.
Table 3.
Summary of the Pharmacokinetic Parameters of MEDI6570 After Single Subcutaneous Doses
| Pharmacokinetic parameter* | Part A, single ascending dose cohorts | |||||
|---|---|---|---|---|---|---|
| 10 mg (n=6) | 30 mg (n=6) | 90 mg (n=6) | 250 mg (n=6) | 500 mg (n=6) | 500 mg Japanese (n=6) | |
| Cmax, μg/mL | 0.157 (257.25) | 0.966 (53.61) | 3.356 (46.32) | 12.525 (30.09) | 24.761 (28.43) | 34.331 (33.61) |
| Tmax, d | 7.012 (1.000–7.054) | 6.973 (5.927–7.024) | 6.985 (5.958–8.030) | 7.028 (2.000–14.029) | 6.004 (1.973–7.108) | 6.963 (5.960–15.269) |
| AUC0–inf, μg.d/mL | NC† | 18.834 (78.62)‡ | 83.306 (66.67) | 350.376 (42.19) | 783.149 (36.48) | 1379.739 (39.02) |
| T1/2λz, d | NC† | 4.624 (38.35)‡ | 6.892 (71.30) | 9.209 (50.01) | 11.159 (20.04) | 16.188 (26.65) |
| CL/F, L/d | NC† | 1.593 (78.62)‡ | 1.080 (66.67) | 0.714 (42.19) | 0.638 (36.48) | 0.362 (39.02) |
| Vz/F, L | NC† | 10.627 (62.78)‡ | 10.741 (34.27) | 9.480 (53.14) | 10.278 (24.03) | 8.463 (48.20) |
AUC0–inf indicates area under the concentration–time curve from time zero to infinity; CL/F, apparent systemic clearance; Cmax, peak plasma concentration; NC, not calculated; T1/2λz, terminal elimination half‐life; Tmax, time to peak plasma concentration; and Vz/F, apparent volume of distribution.
Values are geometric mean (geometric percent coefficient of variation) except Tmax, which is median (range).
n=1.
n=5.
Pharmacodynamics
sLOX‐1 levels were reduced with MEDI6570 in a dose‐dependent manner. In part A, up to day 29 after single doses of MEDI6570 90 to 500 mg, mean serum sLOX‐1 levels were reduced by >66% relative to baseline or to below the LLOQ (32.8 pg/mL; number of participants below LLOQ=13) suggesting target engagement by MEDI6570 (Figure 3A). In part B, the mean percent change from baseline in sLOX‐1 levels at day 70 (after 3 doses) was −80.57%, −71.61%, and −82.96% with 90 mg, 150 mg, and 250 mg MEDI6570, respectively. Furthermore, sLOX‐1 suppression of 90% relative to baseline or to below LLOQ was achieved in 66.7% (P=0.003), 83.3% (P=0.001), and 90.0% (P<0.001) of participants at day 70 following 3 doses of 90 mg, 150 mg, and 250 mg, respectively. Following 2 doses of MEDI6570 90 mg or 150 mg, mean serum sLOX‐1 levels were reduced by >64% relative to baseline or to below LLOQ (n=6) up to day 57 after first dose. Following 2 doses of MEDI6570 250 mg, mean serum sLOX‐1 levels were reduced by >81% relative to baseline or to below LLOQ (n=6) up to day 57 after first dose (Figure 3B).
Figure 3. Free serum sLOX‐1 levels in patients with T2DM receiving single (A) or multiple (B) doses of MEDI6570 or placebo.
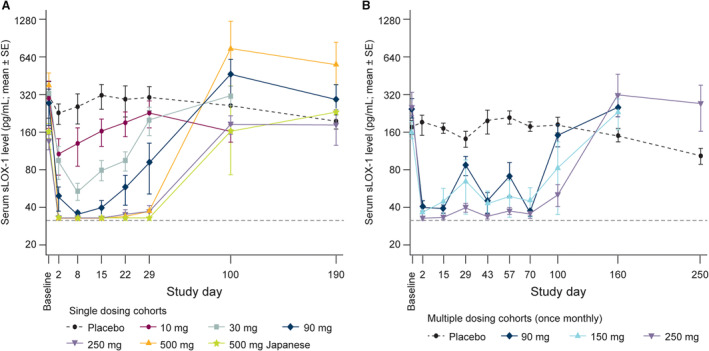
Dashed gray line indicates lower limit of quantification. In a categorical analysis, sLOX‐1 suppression of 90% relative to baseline or to below LLOQ was achieved in 66.7% (P=0.003), 83.3% (P=0.001), and 90.0% (P<0.001) of participants at day 70, respectively, following 3 doses of 90, 150, and 250 mg of MEDI6570. LLOQ indicates lower limit of quantification; sLOX‐1, soluble lectin‐like oxidized low‐density lipoprotein receptor 1; and T2DM, type 2 diabetes.
Levels of hs‐CRP were not affected by MEDI6570 administration. In part A, the Japanese cohort (A6) had a lower mean hs‐CRP level (0.968 mg/L) than all other cohorts (mean range 2.010–4.008 mg/L) and hs‐CRP levels over time were similar for MEDI6570‐ and placebo‐treated participants (Figure 4A). In part B, mean baseline hs‐CRP values ranged from 3.258 to 6.020 mg/L across cohorts. Multiple doses of MEDI6570 did not result in statistically significant changes in hs‐CRP levels compared with placebo (Figure 4B).
Figure 4. Mean hs‐CRP levels in patients with T2DM receiving single (A) or multiple (B) doses of MEDI6570 or placebo.
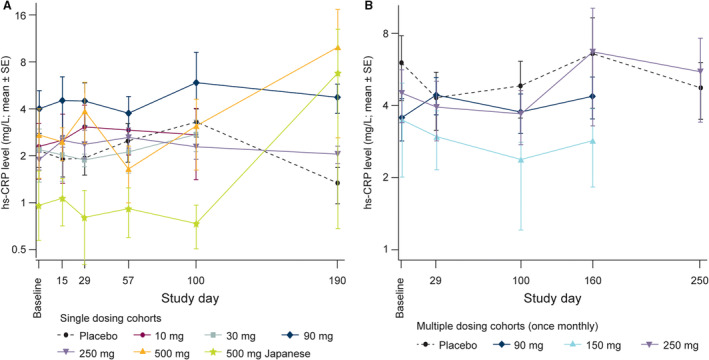
Extreme outliers have been removed. No comparisons were statistically significant. hs‐CRP indicates high‐sensitivity C‐reactive protein; and T2DM, type 2 diabetes.
Baseline and follow‐up CTA measurements were available for 37 participants, of whom 20 had plaque present at baseline. The least‐squares mean changes from baseline for the MEDI6570 versus placebo groups, respectively, were: noncalcified plaque volume, −13.45 mm3 (90% CI, −26.11, 0.79) versus −8.25 mm3 (−30.42, 13.92; P=0.732); low attenuation plaque volume, −5.29 mm3 (−8.72, −1.87) versus −2.97 mm3 (−8.97, 3.03; P=0.574); calcified plaque volume, −2.73 mm3 (−5.03, −0.43) versus −2.04 mm3 (−6.05, 1.97); total plaque volume −16.29 mm3 (−30.04, −2.55) versus −9.75 mm3 (−33.80, 14.29); and percent atheroma volume, −1.78% (−5.81, 2.26) versus 3.61% (−3.41, 10.63; P=0.268) (Figure 5A).
Figure 5. Change from baseline in plaque volumes (A) and FAI (B) in patients with T2DM receiving multiple doses of MEDI6570.
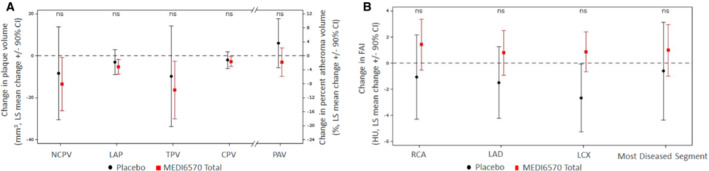
Dashed lines indicated baseline. CPV, calcified plaque volume; FAI, fat attenuation index; HU, Hounsfield unit; LAD, left anterior descending artery; LAP, low attenuation plaque; LCX, left circumflex artery; LS, least‐squares; NCPV, noncalcified plaque volume; ns, not significant; PAV, percent atheroma volume; RCA, right coronary artery; TPV, total plaque volume; and T2DM, type 2 diabetes.
FAI was measured in 37 participants with baseline and follow‐up CTA. The mean baseline values of FAI (Hounsfield units) in the right coronary artery, left anterior descending artery, left circumflex artery, and most diseased coronary segment of MEDI6570‐treated participants were normal at −80.37 (SD, 7.73), −81.57 (6.27), −77.57 (6.18), and −80.08 (7.86), respectively, compared with −84.20 (7.58), −81.53 (6.38), −79.04 (8.04), and −81.55 (4.06), respectively, in the placebo groups. The least‐squares mean changes from baseline in FAI for the MEDI6570 versus placebo groups, respectively, were: right coronary artery, 1.42 (90% CI, −0.52, 3.36) versus −1.06 (−4.30, 2.18); left anterior descending artery, 0.79 (−0.92, 2.49) versus −1.49 (−4.23, 1.26); left circumflex artery, 0.87 (−0.65, 2.39) versus −2.68 (−5.27, −0.08); and most diseased segment, 0.99 (−1.01, 2.98) versus −0.60 (−4.35, 3.14). There were no significant changes in FAI (Figure 5B).
Discussion
In the present phase 1, first‐in‐human study, MEDI6570, a high‐affinity blocking antibody to LOX‐1, was found to be well tolerated in both the SAD and MAD cohorts. There were no deaths, life‐threatening AEs, treatment‐related serious adverse events, or AEs that led to study withdrawal. Target engagement of MEDI6570 was demonstrated by reductions in free sLOX‐1 concentration in serum. There were associated changes in coronary plaque volume and composition that favored MEDI6570, but these were not statistically significant.
LOX‐1 plays a critical role in the initiation and progression of cardiovascular disease 14 , 15 , 16 and is found on endothelial cells, macrophages, neutrophils, smooth muscle cells, fibroblasts, and cardiomyocytes. 15 , 21 Increased LOX‐1 expression has been detected in human atherosclerotic plaques in vivo, 22 and elevated sLOX‐1 levels have been shown to be higher in patients with plaque rupture compared with controls. 23 In healthy individuals, the expression of LOX‐1 is low. 24 , 25 However, sLOX‐1 levels are elevated during chronic inflammation in patients with cardiovascular risk factors such as hypertension and type 2 diabetes. 26 , 27 , 28 , 29 Furthermore, elevated levels of sLOX‐1 identify those at risk of MACE. 24 , 25 , 30 , 31 For these reasons, patients with type 2 diabetes were chosen as the study population.
As a scavenger receptor, LOX‐1 binds atherogenic and proinflammatory ligands, including modified LDL (eg, oxidized LDL), dysfunctional high‐density lipoprotein, advanced glycosylation end products, apoptotic cells, and activated platelets. 27 , 28 Binding of these ligands activates downstream signaling, leading to reduced NO production associated with endothelial dysfunction, generation of reactive oxygen species, stimulation of the nuclear factor‐κB and NLRP3 pathways, and further stimulation of LOX‐1 expression and signaling. 32 , 33 , 34 , 35 , 36 At the level of the endothelial cell, evidence suggests that LOX‐1 is involved in the transcytosis of oxidized LDL into the cell and through the basement membrane. 8 , 37 Additionally, LOX‐1 facilitates monocyte adhesion to endothelial cells. 35 Monocytes migrate, differentiate into tissue macrophages, and form foam cells when exposed to accumulated oxidized LDL. Foam cell formation is recognized as an important step in the development of atherosclerotic plaques. 38 LOX‐1 has also been implicated in the retention of macrophages in atherosclerotic lesions. 39 , 40 At the level of the smooth muscle cell, evidence suggests that LOX‐1 can promote plaque instability by colocalizing with matrix metalloproteinase 9 and monocyte chemoattractant protein 1 (chemokine C‐C motif ligand 2) 41 and inducing smooth muscle cell apoptosis. 8 , 42
Several efforts to develop anti‐LOX‐1 therapies are ongoing. Naturally occurring LOX‐1 modulators have been identified, and research groups have focused on developing small‐molecule inhibitors. 15 , 43 , 44 A monoclonal antibody approach to therapy may offer more specific targeting and fewer off‐target effects than a small‐molecules approach, but few monoclonal antibodies against human LOX‐1 have been developed. 15 , 44 Potential immunogenicity and selectivity are limiting factors when developing human LOX‐1 antibodies because C‐type lectin domain of LOX‐1 is highly conserved among mammalian species. 1 High specificity for LOX‐1 is critical for the success of MEDI6570.
The present study is the first to demonstrate the effect of a high‐affinity LOX‐1 blocking antibody reducing free sLOX‐1 levels. MEDI6570 demonstrated high selectivity and low immunogenicity, and no safety concerns related to immunogenicity were identified. Free sLOX‐1 levels were used to assess engagement of MEDI6570 with LOX‐1 because sLOX‐1 levels are thought to reflect membrane‐bound LOX‐1 expression levels. 8 MEDI6570 significantly reduced free sLOX‐1 levels in a dose‐dependent manner. Additionally, MEDI6570 exhibited nonlinear pharmacokinetics that became linear at higher doses, suggesting that target‐mediated drug disposition was overcome at doses of 250 mg or higher and confirming that target engagement of soluble and membrane LOX‐1 was achieved.
The present study also explored the effect of MEDI6570 on coronary atherosclerosis and inflammation. After 3 monthly doses of MEDI6570, regression of noncalcified plaque volume, low attenuation plaque volume, and total plaque volume was observed, but the changes were not statistically significant. Single and multiple doses of MEDI6570 did not affect levels of hs‐CRP, similar to previous findings with proprotein convertase subtilisin‐kexin type 9 inhibitors. 45 , 46 Although hs‐CRP and sLOX‐1 levels are significantly correlated, 47 hs‐CRP is a systemic marker of inflammation and the mechanism of action of LOX‐1 inhibition may not affect systemic levels. To explore whether inhibition of LOX‐1 with MEDI6570 reduced inflammation on the local vessel level, a novel measurement of vascular inflammation in the form of perivascular FAI from the CTA scans was used. FAI is thought to be a more specific marker of vascular inflammation and predictor of cardiovascular mortality than CTA‐derived plaque measurements. 48 However, in this phase 1 population of patients with type 2 diabetes, baseline measurements of FAI were within the normal range for nearly all patients, which did not allow evaluation of the effect of MEDI6570 on coronary vasculature–specific inflammation. Further studies are warranted to investigate whether the reduction in serum sLOX‐1 levels translates into downstream effects on coronary vascular inflammation and plaque volume.
Conventional pharmacological interventions for atherosclerosis focus on reducing plasma LDL cholesterol levels, and it is possible to drive LDL to low levels with treatment. Statins, the most widely prescribed class of lipid‐lowering medications, have been highly successful at reducing the burden of atherosclerosis; however, when used as monotherapy, statins are sometimes insufficient or poorly tolerated. 49 Proprotein convertase subtilisin‐kexin type 9 inhibitors are now available and are an effective option, but these agents do not affect inflammation 45 and are associated with a high cost and the burden of self‐injection. 50 As the role of inflammation in atherosclerosis has been revealed, anti‐inflammatory therapies have become a major focus of research, with mixed results to date in reducing the risk of MACE. 6 , 51 Anti‐LOX‐1 therapy may offer an additional line of defense against atherosclerosis, alongside lipid‐lowering therapies and emerging anti‐inflammatory therapies. 6
Limitations of this work include the fact that this was a small phase 1 study in patients with type 2 diabetes who were eligible for a first‐in‐human study. The study did not enroll patients with a history of acute coronary syndrome, who might derive the greatest benefit from an anti‐LOX‐1 therapy. Patients with type 2 diabetes were selected because sLOX‐1 is elevated in this population and because hemoglobin A1C and sLOX‐1 levels are significantly correlated. 17 The inclusion of patients with type 2 diabetes enabled assessment of the pharmacokinetics, pharmacodynamics, and target engagement of MEDI6570, which would not have been possible in healthy volunteers who have low LOX‐1 expression levels. Another limitation of the study was that there were no patient selection criteria for baseline hs‐CRP levels. There were also limitations in the assessment of coronary plaque and inflammation by CTA. Only 20 of the 37 patients in the multiple dose cohorts had identifiable plaque on their CTA scans, limiting the assessment of plaque volume and composition. In addition, the time between the baseline CTA and follow‐up scan was quite short, limiting the amount of plaque regression that may have been realized by MEDI6570. It is worth noting that plaque regression has been detected after as little as 3 months in the carotid arteries with 3‐dimensional ultrasound, and after as little as 9 months with coronary CTA. 52 , 53 Perivascular FAI was used to measure coronary inflammation in this study. This novel method was chosen over fluorodeoxyglucose‐positron emission tomography, the other established vascular inflammation imaging marker, because FAI can be conveniently measured using data already available from the CTA scans and is not known to be limited in patients with diabetes. Although all 37 scans were evaluable for FAI, nearly all participants had a normal baseline measurement, limiting the assessment of the effect of MEDI6570 on coronary vascular inflammation.
In summary, MEDI6570 was well tolerated, with no safety‐ or efficacy‐related immunogenicity issues identified. The pharmacokinetic and pharmacodynamic profiles of MEDI6570 support a once‐monthly dosing regimen for future clinical development. Target engagement of MEDI6570 with LOX‐1 was demonstrated, with dose‐dependent decreases in free sLOX‐1. Further studies in patients with coronary heart disease are required to establish whether these initial results translate into reductions in atherosclerosis and underlying inflammation, and benefits for longer term survival.
Sources of Funding
This work was supported by AstraZeneca.
Disclosures
The study was sponsored by MedImmune LLC, owned by AstraZeneca. The sponsor was responsible for the design and conduct of the study; the collection, analysis, and interpretation of the data; and the preparation, review, and approval of the manuscript. A.L.V., M.S., E.L.O., Y.Y., V.V., J.G., C.J., V.D., A.L.Q., and A.C. are employees of AstraZeneca. R.T.G. was an employee of AstraZeneca at the time of the study and is currently an employee of Regeneron. A.L.V., M.S., E.L.O., Y.Y., V.V., J.G., C.J., V.D., A.C., and R.T.G. have stock ownership and/or stock options in AstraZeneca. S.K.K. is an employee of Enveda Biosciences. L.A. had stock ownership/options in AstraZeneca at the time the research was performed, and is currently an employee of Horizon Therapeutics. M.J.K. served as a clinical investigator on this study and is an employee of a company that received research funding from AstraZeneca to enroll participants and conduct the clinical trial. R.G. is an employee of Gilead Sciences.
Supporting information
Data S1
Tables S1–S2
Acknowledgments
The authors thank the participants and staff involved in the study. Medical writing support was provided by Katie Willetts, PhD, from Oxford PharmaGenesis, Oxford, UK, which was funded by AstraZeneca.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.027540
For Sources of Funding and Disclosures, see page 11.
REFERENCES
- 1. Kattoor AJ, Goel A, Mehta JL. LOX‐1: regulation, signaling and its role in atherosclerosis. Antioxidants (Basel). 2019;8:218. doi: 10.3390/antiox8070218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, et al. Rosuvastatin to prevent vascular events in men and women with elevated c‐reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646 [DOI] [PubMed] [Google Scholar]
- 3. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–1722. doi: 10.1056/NEJMoa1615664 [DOI] [PubMed] [Google Scholar]
- 4. Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397. doi: 10.1056/NEJMoa1410489 [DOI] [PubMed] [Google Scholar]
- 5. Bittner VA, Szarek M, Aylward PE, Bhatt DL, Diaz R, Edelberg JM, Fras Z, Goodman SG, Halvorsen S, Hanotin C, et al. Effect of alirocumab on lipoprotein(a) and cardiovascular risk after acute coronary syndrome. J Am Coll Cardiol. 2020;75:133–144. doi: 10.1016/j.jacc.2019.10.057 [DOI] [PubMed] [Google Scholar]
- 6. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914 [DOI] [PubMed] [Google Scholar]
- 7. Bohula EA, Giugliano RP, Leiter LA, Verma S, Park JG, Sever PS, Lira Pineda A, Honarpour N, Wang H, Murphy SA, et al. Inflammatory and cholesterol risk in the FOURIER trial. Circulation. 2018;138:131–140. doi: 10.1161/CIRCULATIONAHA.118.034032 [DOI] [PubMed] [Google Scholar]
- 8. Barreto J, Karathanasis SK, Remaley A, Sposito AC. Role of LOX‐1 (lectin‐like oxidized low‐density lipoprotein receptor 1) as a cardiovascular risk predictor: mechanistic insight and potential clinical use. Arterioscler Thromb Vasc Biol. 2021;41:153–166. doi: 10.1161/ATVBAHA.120.315421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. He K, Yue LH, Zhao GQ, Li C, Lin J, Jiang N, Wang Q, Xu Q, Peng XD, Hu LT, et al. The role of LOX‐1 on innate immunity against aspergillus keratitis in mice. Int J Ophthalmol. 2016;9:1245–1250. doi: 10.18240/ijo.2016.09.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Condamine T, Dominguez GA, Youn JI, Kossenkov AV, Mony S, Alicea‐Torres K, Tcyganov E, Hashimoto A, Nefedova Y, Lin C, et al. Lectin‐type oxidized LDL receptor‐1 distinguishes population of human polymorphonuclear myeloid‐derived suppressor cells in cancer patients. Sci Immunol. 2016;1:aaf8943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Holvoet P, Vanhaecke J, Janssens S, Van de Werf F, Collen D. Oxidized LDL and malondialdehyde‐modified LDL in patients with acute coronary syndromes and stable coronary artery disease. Circulation. 1998;98:1487–1494. doi: 10.1161/01.CIR.98.15.1487 [DOI] [PubMed] [Google Scholar]
- 12. Ehara S, Ueda M, Naruko T, Haze K, Itoh A, Otsuka M, Komatsu R, Matsuo T, Itabe H, Takano T, et al. Elevated levels of oxidized low density lipoprotein show a positive relationship with the severity of acute coronary syndromes. Circulation. 2001;103:1955–1960. doi: 10.1161/01.CIR.103.15.1955 [DOI] [PubMed] [Google Scholar]
- 13. Lu J, Yang JH, Burns AR, Chen HH, Tang D, Walterscheid JP, Suzuki S, Yang CY, Sawamura T, Chen CH. Mediation of electronegative low‐density lipoprotein signaling by LOX‐1: a possible mechanism of endothelial apoptosis. Circ Res. 2009;104:619–627. doi: 10.1161/CIRCRESAHA.108.190116 [DOI] [PubMed] [Google Scholar]
- 14. Murphy JE, Vohra RS, Dunn S, Holloway ZG, Monaco AP, Homer‐Vanniasinkam S, Walker JH, Ponnambalam S. Oxidised LDL internalisation by the LOX‐1 scavenger receptor is dependent on a novel cytoplasmic motif and is regulated by dynamin‐2. J Cell Sci. 2008;121:2136–2147. doi: 10.1242/jcs.020917 [DOI] [PubMed] [Google Scholar]
- 15. Pothineni NVK, Karathanasis SK, Ding Z, Arulandu A, Varughese KI, Mehta JL. LOX‐1 in atherosclerosis and myocardial ischemia: biology, genetics, and modulation. J Am Coll Cardiol. 2017;69:2759–2768. doi: 10.1016/j.jacc.2017.04.010 [DOI] [PubMed] [Google Scholar]
- 16. Li D, Williams V, Liu L, Chen H, Sawamura T, Romeo F, Mehta JL. Expression of lectin‐like oxidized low‐density lipoprotein receptors during ischemia‐reperfusion and its role in determination of apoptosis and left ventricular dysfunction. J Am Coll Cardiol. 2003;41:1048–1055. doi: 10.1016/S0735-1097(02)02966-2 [DOI] [PubMed] [Google Scholar]
- 17. Tan KC, Shiu SW, Wong Y, Leng L, Bucala R. Soluble lectin‐like oxidized low density lipoprotein receptor‐1 in type 2 diabetes mellitus. J Lipid Res. 2008;49:1438–1444. doi: 10.1194/jlr.M700551-JLR200 [DOI] [PubMed] [Google Scholar]
- 18. Higuma T, Abe N, Tateyama S, Endo T, Shibutani S, Yokoyama H, Hanada K, Yamada M, Tomita H, Hanada H, et al. Plasma soluble lectin‐like oxidized low‐density lipoprotein receptor‐1 as a novel prognostic biomarker in patients with ST‐segment elevation acute myocardial infarction. Circ J. 2015;79:641–648. doi: 10.1253/circj.CJ-14-0904 [DOI] [PubMed] [Google Scholar]
- 19. Dey D, Cheng VY, Slomka PJ, Nakazato R, Ramesh A, Gurudevan S, Germano G, Berman DS. Automated 3‐dimensional quantification of noncalcified and calcified coronary plaque from coronary CT angiography. J Cardiovasc Comput Tomogr. 2009;3:372–382. doi: 10.1016/j.jcct.2009.09.004 [DOI] [PubMed] [Google Scholar]
- 20. Antonopoulos AS, Sanna F, Sabharwal N, Thomas S, Oikonomou EK, Herdman L, Margaritis M, Shirodaria C, Kampoli AM, Akoumianakis I, et al. Detecting human coronary inflammation by imaging perivascular fat. Sci Transl Med. 2017;9:eaal2658. doi: 10.1126/scitranslmed.aal2658 [DOI] [PubMed] [Google Scholar]
- 21. Mehta JL, Chen J, Hermonat PL, Romeo F, Novelli G. Lectin‐like, oxidized low‐density lipoprotein receptor‐1 (LOX‐1): a critical player in the development of atherosclerosis and related disorders. Cardiovasc Res. 2006;69:36–45. doi: 10.1016/j.cardiores.2005.09.006 [DOI] [PubMed] [Google Scholar]
- 22. West NEJ, Corrigan JP, Owen RHG, Hoole SP, Brown AJ, Blatcher S, Newby AC. Percutaneous sampling of local biomolecule gradients across coronary artery atherosclerotic plaques. JACC Basic Transl Sci. 2017;2:646–654. doi: 10.1016/j.jacbts.2017.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kobayashi N, Takano M, Hata N, Kume N, Yamamoto M, Yokoyama S, Shinada T, Tomita K, Shirakabe A, Otsuka T, et al. Soluble lectin‐like oxidized LDL receptor‐1 (sLOX‐1) as a valuable diagnostic marker for rupture of thin‐cap fibroatheroma: verification by optical coherence tomography. Int J Cardiol. 2013;168:3217–3223. doi: 10.1016/j.ijcard.2013.04.110 [DOI] [PubMed] [Google Scholar]
- 24. Skarpengland T, Skjelland M, Kong XY, Skagen K, Holm S, Otterdal K, Dahl CP, Krohg‐Sørensen K, Sagen EL, Bjerkeli V, et al. Increased levels of lectin‐like oxidized low‐density lipoprotein receptor‐1 in ischemic stroke and transient ischemic attack. J Am Heart Assoc. 2018;7:e006479. doi: 10.1161/JAHA.117.006479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yokota C, Sawamura T, Watanabe M, Kokubo Y, Fujita Y, Kakino A, Nakai M, Toyoda K, Miyamoto Y, Minematsu K. High levels of soluble lectin‐like oxidized low‐density lipoprotein receptor‐1 in acute stroke: an age‐ and sex‐matched cross‐sectional study. J Atheroscler Thromb. 2016;23:1222–1226. doi: 10.5551/jat.32466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhao ZW, Xu YW, Li SM, Guo JJ, Yi T, Chen LL. Higher serum lectin‐like oxidized low‐density lipoprotein receptor‐1 in patients with stable coronary artery disease is associated with major adverse cardiovascular events: a multicentre pilot study. Biochem Med (Zagreb). 2019;29:010705. doi: 10.11613/BM.2019.010705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pirillo A, Norata GD, Catapano AL. LOX‐1, OxLDL, and atherosclerosis. Mediators Inflamm. 2013;2013:152786. doi: 10.1155/2013/152786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xu S, Ogura S, Chen J, Little PJ, Moss J, Liu P. LOX‐1 in atherosclerosis: biological functions and pharmacological modifiers. Cell Mol Life Sci. 2013;70:2859–2872. doi: 10.1007/s00018-012-1194-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dey AK, Gaddipati R, Elnabawi YA, Ongstad E, Goyal A, Chung JH, Teague HL, Rodante JA, Sajja AA, Sorokin AV, et al. Association between soluble lectin‐like oxidized low‐density lipoprotein receptor‐1 and coronary artery disease in psoriasis. JAMA Dermatol. 2020;156:151–157. doi: 10.1001/jamadermatol.2019.3595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhao ZW, Zhu XL, Luo YK, Lin CG, Chen LL. Circulating soluble lectin‐like oxidized low‐density lipoprotein receptor‐1 levels are associated with angiographic coronary lesion complexity in patients with coronary artery disease. Clin Cardiol. 2011;34:172–177. doi: 10.1002/clc.20847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhao ZW, Xu YW, Li SM, Guo JJ, Sun JM, Hong JC, Chen LL. Baseline serum sLOX‐1 concentrations are associated with 2‐year major adverse cardiovascular and cerebrovascular events in patients after percutaneous coronary intervention. Dis Markers. 2019;2019:4925767–4925768. doi: 10.1155/2019/4925767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tian K, Ogura S, Little PJ, Xu SW, Sawamura T. Targeting LOX‐1 in atherosclerosis and vasculopathy: current knowledge and future perspectives. Ann N Y Acad Sci. 2019;1443:34–53. doi: 10.1111/nyas.13984 [DOI] [PubMed] [Google Scholar]
- 33. Hein TW, Xu X, Ren Y, Xu W, Tsai SH, Thengchaisri N, Kuo L. Requisite roles of LOX‐1, JNK, and arginase in diabetes‐induced endothelial vasodilator dysfunction of porcine coronary arterioles. J Mol Cell Cardiol. 2019;131:82–90. doi: 10.1016/j.yjmcc.2019.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Akhmedov A, Rozenberg I, Paneni F, Camici GG, Shi Y, Doerries C, Sledzinska A, Mocharla P, Breitenstein A, Lohmann C, et al. Endothelial overexpression of LOX‐1 increases plaque formation and promotes atherosclerosis in vivo. Eur Heart J. 2014;35:2839–2848. doi: 10.1093/eurheartj/eht532 [DOI] [PubMed] [Google Scholar]
- 35. Li D, Mehta JL. Antisense to LOX‐1 inhibits oxidized LDL‐mediated upregulation of monocyte chemoattractant protein‐1 and monocyte adhesion to human coronary artery endothelial cells. Circulation. 2000;101:2889–2895. doi: 10.1161/01.CIR.101.25.2889 [DOI] [PubMed] [Google Scholar]
- 36. Ding Z, Liu S, Wang X, Dai Y, Khaidakov M, Deng X, Fan Y, Xiang D, Mehta JL. LOX‐1, mtDNA damage, and NLRP3 inflammasome activation in macrophages: implications in atherogenesis. Cardiovasc Res. 2014;103:619–628. doi: 10.1093/cvr/cvu114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sun SW, Zu XY, Tuo QH, Chen LX, Lei XY, Li K, Tang CK, Liao DF. Caveolae and caveolin‐1 mediate endocytosis and transcytosis of oxidized low density lipoprotein in endothelial cells. Acta Pharmacol Sin. 2010;31:1336–1342. doi: 10.1038/aps.2010.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Orekhov AN. LDL and foam cell formation as the basis of atherogenesis. Curr Opin Lipidol. 2018;29:279–284. doi: 10.1097/MOL.0000000000000525 [DOI] [PubMed] [Google Scholar]
- 39. Yang HY, Bian YF, Zhang HP, Gao F, Xiao CS, Liang B, Li J, Zhang NN, Yang ZM. LOX1 is implicated in oxidized low‐density lipoprotein‐induced oxidative stress of macrophages in atherosclerosis. Mol Med Rep. 2015;12:5335–5341. doi: 10.3892/mmr.2015.4066 [DOI] [PubMed] [Google Scholar]
- 40. Sun Y, Chen X. Ox‐LDL‐induced LOX‐1 expression in vascular smooth muscle cells: role of reactive oxygen species. Fundam Clin Pharmacol. 2011;25:572–579. doi: 10.1111/j.1472-8206.2010.00885.x [DOI] [PubMed] [Google Scholar]
- 41. Ishino S, Mukai T, Kume N, Asano D, Ogawa M, Kuge Y, Minami M, Kita T, Shiomi M, Saji H. Lectin‐like oxidized LDL receptor‐1 (LOX‐1) expression is associated with atherosclerotic plaque instability‐‐analysis in hypercholesterolemic rabbits. Atherosclerosis. 2007;195:48–56. doi: 10.1016/j.atherosclerosis.2006.11.031 [DOI] [PubMed] [Google Scholar]
- 42. Kataoka H, Kume N, Miyamoto S, Minami M, Morimoto M, Hayashida K, Hashimoto N, Kita T. Oxidized LDL modulates Bax/Bcl‐2 through the lectin‐like ox‐LDL receptor‐1 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2001;21:955–960. doi: 10.1161/01.ATV.21.6.955 [DOI] [PubMed] [Google Scholar]
- 43. Falconi M, Ciccone S, D'Arrigo P, Viani F, Sorge R, Novelli G, Patrizi P, Desideri A, Biocca S. Design of a novel LOX‐1 receptor antagonist mimicking the natural substrate. Biochem Biophys Res Commun. 2013;438:340–345. doi: 10.1016/j.bbrc.2013.07.073 [DOI] [PubMed] [Google Scholar]
- 44. Iwamoto S, Nishimichi N, Tateishi Y, Sato Y, Horiuchi H, Furusawa S, Sawamura T, Matsuda H. Generation and characterization of chicken monoclonal antibodies against human LOX‐1. MAbs. 2009;1:357–363. doi: 10.4161/mabs.1.4.8919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. O'Donoghue ML, Fazio S, Giugliano RP, Stroes ESG, Kanevsky E, Gouni‐Berthold I, Im K, Lira Pineda A, Wasserman SM, Češka R, et al. Lipoprotein(a), PCSK9 inhibition, and cardiovascular risk. Circulation. 2019;139:1483–1492. doi: 10.1161/CIRCULATIONAHA.118.037184 [DOI] [PubMed] [Google Scholar]
- 46. Cao YX, Li S, Liu HH, Li JJ. Impact of PCSK9 monoclonal antibodies on circulating hs‐CRP levels: a systematic review and meta‐analysis of randomised controlled trials. BMJ Open. 2018;8:e022348. doi: 10.1136/bmjopen-2018-022348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lubrano V, Del Turco S, Nicolini G, Di Cecco P, Basta G. Circulating levels of lectin‐like oxidized low‐density lipoprotein receptor‐1 are associated with inflammatory markers. Lipids. 2008;43:945–950. doi: 10.1007/s11745-008-3227-9 [DOI] [PubMed] [Google Scholar]
- 48. Oikonomou EK, Marwan M, Desai MY, Mancio J, Alashi A, Hutt Centeno E, Thomas S, Herdman L, Kotanidis CP, Thomas KE, et al. Non‐invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): a post‐hoc analysis of prospective outcome data. Lancet. 2018;392:929–939. doi: 10.1016/S0140-6736(18)31114-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Larsen LE, Stoekenbroek RM, Kastelein JJP, Holleboom AG. Moving targets: recent advances in lipid‐lowering therapies. Arterioscler Thromb Vasc Biol. 2019;39:349–359. doi: 10.1161/ATVBAHA.118.312028 [DOI] [PubMed] [Google Scholar]
- 50. Cho KH, Hong YJ. Proprotein convertase subtilisin/kexin type 9 inhibition in cardiovascular disease: current status and future perspectives. Korean J Intern Med. 2020;35:1045–1058. doi: 10.3904/kjim.2020.140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Libby P, Everett BM. Novel antiatherosclerotic therapies. Arterioscler Thromb Vasc Biol. 2019;39:538–545. doi: 10.1161/ATVBAHA.118.310958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ratchford EV, Gutierrez J, Lorenzo D, McClendon MS, Della‐Morte D, DeRosa JT, Elkind MS, Sacco RL, Rundek T. Short‐term effect of atorvastatin on carotid artery elasticity: a pilot study. Stroke. 2011;42:3460–3464. doi: 10.1161/STROKEAHA.111.625418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Budoff MJ, Muhlestein JB, Bhatt DL, Le Pa VT, May HT, Shaikh K, Shekar C, Kinninger A, Lakshmanan S, Roy SK, et al. Effect of icosapent ethyl on progression of coronary atherosclerosis in patients with elevated triglycerides on statin therapy: a prospective, placebo‐controlled randomized trial (EVAPORATE): interim results. Cardiovasc Res. 2021;117:1070–1077. doi: 10.1093/cvr/cvaa184 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S2


