Abstract
Background
Depression is a nontraditional risk factor for cardiovascular disease (CVD). Data on the association of depression and poor mental health with CVD and suboptimal cardiovascular health (CVH) among young adults are limited.
Methods and Results
We used data from 593 616 young adults (aged 18–49 years) from the 2017 to 2020 Behavioral Risk Factor Surveillance System, a nationally representative survey of noninstitutionalized US adults. Exposures were self‐reported depression and poor mental health days (PMHDs; categorized as 0, 1–13, and 14–30 days of poor mental health in the past 30 days). Outcomes were self‐reported CVD (composite of myocardial infarction, angina, or stroke) and suboptimal CVH (≥2 cardiovascular risk factors: hypertension, hypercholesterolemia, overweight/obesity, smoking, diabetes, physical inactivity, and inadequate fruit and vegetable intake). Using logistic regression, we investigated the association of depression and PMHDs with CVD and suboptimal CVH, adjusting for sociodemographic factors (and cardiovascular risk factors for the CVD outcome). Of the 593 616 participants (mean age, 34.7±9.0 years), the weighted prevalence of depression was 19.6% (95% CI, 19.4–19.8), and the weighted prevalence of CVD was 2.5% (95% CI, 2.4–2.6). People with depression had higher odds of CVD than those without depression (odds ratio [OR], 2.32 [95% CI, 2.13–2.51]). There was a graded association of PMHDs with CVD. Compared with individuals with 0 PMHDs, the odds of CVD in those with 1 to 13 PMHDs and 14 to 30 PHMDs were 1.48 (95% CI, 1.34–1.62) and 2.29 (95% CI, 2.08–2.51), respectively, after adjusting for sociodemographic and cardiovascular risk factors. The associations did not differ significantly by sex or urban/rural status. Individuals with depression had higher odds of suboptimal CVH (OR, 1.79 [95% CI, 1.65–1.95]) compared with those without depression, with a similar graded relationship between PMHDs and suboptimal CVH.
Conclusions
Depression and poor mental health are associated with premature CVD and suboptimal CVH among young adults. Although this association is likely bidirectional, prioritizing mental health may help reduce CVD risk and improve CVH in young adults.
Keywords: cardiovascular health, depression, mental health, young adults
Subject Categories: Cardiovascular Disease, Mental Health, Primary Prevention, Lifestyle
Nonstandard Abbreviations and Acronyms
- BRFSS
Behavioral Risk Factor Surveillance System
- CVH
cardiovascular health
- PMHD
poor mental health day
Clinical Perspective.
What Is New?
In this cross‐sectional study using a large nationally representative sample with less potential for selection bias, we investigated the association of depression and recent mental health (poor mental health days) with cardiovascular disease (CVD) in young adults, and we additionally excluded people with CVD and tested the association of depression and poor mental health days with suboptimal cardiovascular health.
Our findings showed depression and poor mental health days were associated with CVD, and the association did not vary by sex or urban/rural status.
In people without CVD, depression and poor mental health days were associated with suboptimal cardiovascular health.
What Are the Clinical Implications?
Although this association is likely bidirectional, our study implies that targeted interventions that improve mental health may be necessary in reducing CVD and improving overall cardiovascular health in young adults.
A multidisciplinary approach and collaborative and integrated care between health care professionals, such as mental health physicians, psychologists, psychiatrists, nutritionists and addiction specialists, primary care physicians, and cardiologists, may be needed to better improve mental health and reduce CVD risk.
Cardiovascular disease (CVD) remains the leading cause of death among adults in the United States and globally. 1 , 2 One of the targets of the global Sustainable Development Goals (target 3.4) is to reduce premature mortality from noncommunicable diseases, including CVD and cancer, by 30% and improve mental well‐being worldwide by 2030. 3 The concept of cardiovascular health (CVH) and metrics to monitor the goals were defined by the American Heart Association in 2010. 4 Until recently, the metrics to monitor CVH had been the “Life's Simple 7,” which included 4 modifiable behaviors (body mass index [BMI], physical activity, smoking status, and diet) and 3 biometric measures (blood pressure, total cholesterol, and fasting blood glucose). 4 CVD and poor CVH are increasingly prevalent in young adults, 5 , 6 underscoring the importance of early‐life risk factor modification, given the long time horizon over which young adults may accumulate risk burden. Improvement of CVH status would be expected to reduce the lifetime risk of CVD and CVD‐related mortality. 6 , 7 , 8 , 9
Depression is an emerging nontraditional risk factor for CVD, 10 and a leading cause of disability among young adults in the United States. 11 Prior literature has established high comorbidity of depression and CVD, suggesting a bidirectional relationship between the 2 factors. 12 , 13 , 14 However, data on the association of depression with CVD and suboptimal CVH among US young adults are limited. Another important question is whether these associations vary between urban and rural settings, given that rapid urbanization is associated with erosion of some of the protective factors for depression, such as traditional social and family support and healthy behaviors. 15 , 16
We sought to investigate the association of depression with CVD and suboptimal CVH in a nationally representative sample of young adults in the United States and whether the association differed by sex and urban/rural status. We also further explored whether there is a dose response to the association by studying the burden of mental health episodes during a 30‐day period.
METHODS
Data Source, Study Sample, and Study Design
In this cross‐sectional analysis, we used data from the 2017 to 2020 Behavioral Risk Factor Surveillance System (BRFSS), a Centers for Disease Control and Prevention–sponsored, nationally representative survey of noninstitutionalized adults (aged ≥18 years) in the United States performed at the state level. The BRFSS is an annual telephone survey that assesses health‐related risk behaviors, chronic health conditions, and the use of preventive services among US adults in all 50 US states, the District of Columbia, and participating US territories. 17 It uses iterative proportional fitting weighting method (raking), which incorporates demographic variables, such as age, sex, race and ethnicity, education level, and marital status, to make the data nationally representative and to address nonresponse. 18
In this current study, we used data from 593 616 young adults, aged 18 to 49 years, who had complete data on CVD and depression (Figure 1). The overall median survey response rate was 45.1% in 2017, 49.4% in 2018, 49.4% in 2019, and 47.9% in 2020. 17 All data are deidentified and publicly available at BRFSS (https://www.cdc.gov/brfss/annual_data/annual_data.htm); therefore, this study was deemed exempt from review by an institutional review board. We followed the Strengthening the Reporting of Observational Studies in Epidemiology guidelines in reporting our findings. 19
Figure 1. Flowchart of the analytical sample.
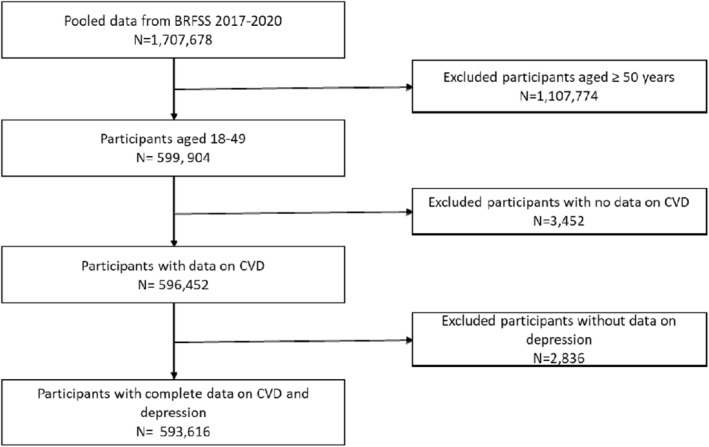
BRFSS indicates Behavioral Risk Factor Surveillance System; and CVD, cardiovascular disease.
Assessment of Depression and Poor Mental Health Days
Participants who responded “yes” to the question “Have you ever been told you have depressive disorder, including depression, major depression, dysthymia, or minor depression?” were considered as having depression, whereas those who responded “no” were considered as not having depression. Poor mental health days (PMHDs), which is the average number of self‐reported mentally unhealthy days in the past 30 days, is one of the Centers for Disease Control and Prevention's self‐reported measures of health‐related quality of life and a reliable estimate of a person's recent mental health. 20 , 21 PMHDs were assessed in response to the question “Now thinking about your mental health, which includes stress, depression, and problems with emotions, for how many days during the past 30 days was your mental health not good?” and categorized as 0, 1 to 13, and 14 to 30 days.
Assessment of CVD
The presence of CVD was assessed by the questions “Have you ever been told you had heart attack, also called myocardial infarction?”; “Have you ever been told you had angina or coronary heart disease?”; and “Have you ever been told you had a stroke?” Participants who responded “yes” to any of these questions were considered as having CVD, whereas those who responded “no” were not considered as having CVD.
Assessment of Suboptimal CVH
CVH was determined using 7 cardiovascular risk factors: hypertension, hypercholesterolemia, overweight/obesity, current smoking, diabetes, physical inactivity, and inadequate fruit and vegetable intake. Suboptimal CVH was defined as the presence of ≥2 of these 7 cardiovascular risk factors, whereas optimal CVH was 0 or 1 cardiovascular risk factor.
Although questions on smoking, diabetes, weight, and height (hence, BMI) were in all survey years, questions on hypertension, hypercholesterolemia, physical activity, and fruit/vegetable consumption were selectively included in surveys of odd‐numbered years (2017 and 2019). Hypertension, hypercholesterolemia, diabetes, and current smoking were systematically ascertained by participant self‐report. Overweight/obesity was defined as a BMI of ≥25 kg/m2 for non‐Asian respondents and ≥23 kg/m2 for Asian respondents, per World Health Organization guidelines. 22 Participants were considered physically inactive if they reported <150 minutes or the vigorous equivalent of physical activity per week, and consumption of <5 servings of fruits and vegetables was considered as inadequate fruit and vegetable intake.
Other Covariates
Other covariates included self‐reported sociodemographic characteristics, such as age, sex (men/women), race and ethnicity (non‐Hispanic White, non‐Hispanic Black, Hispanic, American Indian/Alaskan native, Asian, multiracial, and other), education level (<high school, high school/some college, or ≥college graduate), employment status (employed, unemployed, student, or retired), health care coverage (no health insurance plan or some health insurance plan), marital status (married, divorced, widowed, never married, or member of an unmarried couple), urban/rural status (urban/rural), and income level. Annual family income was adjusted using federal poverty guidelines for each state and categorized as follows: <100%, within 100% to 200%, or >200% of the federal poverty line. 23
Statistical Analysis
Sociodemographic characteristics and cardiovascular risk factors of survey participants were described by CVD status. Actual numbers of participants and weighted percentages were reported. The weighted prevalence rates of CVD, depression, and PMHDs were estimated. Using multivariable logistic regression, we investigated the association of depression and PMHDs with CVD using data pooled from 2017 to 2020. Model 1 was adjusted for sociodemographic factors: age, sex, race and ethnicity, education, employment, income, marital status, and health care coverage. Model 2 was additionally adjusted for cardiovascular risk factors, such as cigarette smoking, BMI, and diabetes. Using data from 2017 and 2019 only, we further adjusted for hypertension and hypercholesterolemia in a sensitivity analysis. We additionally tested the interaction of sex and urban/rural status with the exposure of interest (depression or PMHDs) and CVD.
In addition, among participants without CVD, we investigated the association of depression and PMHDs with suboptimal CVH using multivariable logistic regression and using data from 2017 and 2019 only (years with complete data on all 7 CVH metrics).
The survey command “svy” was used to account for the complex method of BRFSS. All analyses were conducted with Stata Version 16 (StataCorp, College Station, TX), and a 2‐sided α level of <0.05 was used to determine statistical significance.
RESULTS
Of the 593 616 participants (mean age, 34.7±9.0 years), the weighted proportion of women was 49.7%; non‐Hispanic White race and ethnicity, 54.1%; and college graduate, 28.4%. Most participants had some form of health insurance (82.5%) and annual income >200% of the federal poverty line (65.1%) (Table 1). Overall, the weighted prevalence of depression was 19.6% (95% CI, 19.4–19.8). A total of 55.7% of respondents (95% CI, 55.5–55.9) reported 0 PMHDs in the past 30‐days, whereas 29.2% (95% CI, 29.0–29.4) and 15.1% (95% CI,14.9–15.3) reported 1 to 13 and ≥14 days of poor mental health in the past 30 days, respectively.
Table 1.
Characteristics of Study Participants, Stratified by CVD Status, BRFSS (2017–2020)
| Characteristic | Overall (n=593 616)* | No CVD (n=577 343)* | CVD (n=16 273)* | P value |
|---|---|---|---|---|
| Age, y | ||||
| 18–24 | 101 696 (23.3) | 100 695 (23.7) | 1001 (9.3) | <0.001† |
| 25–29 | 85 458 (15.3) | 84 355 (15.5) | 1103 (8.8) | <0.001† |
| 30–34 | 94 589 (17.7) | 92 799 (17.8) | 1790 (13.1) | <0.001† |
| 35–39 | 101 617 (15.1) | 99 007 (15.1) | 2610 (15.4) | <0.001† |
| 40–44 | 99 917 (15.3) | 96 119 (15.0) | 3798 (24.4) | <0.001† |
| 45–49 | 110 339 (13.3) | 104 368 (12.9) | 5971 (29.0) | <0.001† |
| Sex | 0.57† | |||
| Men | 288 809 (50.3) | 280 898 (50.3) | 7911 (50.7) | |
| Women | 304 471 (49.7) | 296 123 (49.7) | 8348 (49.3) | |
| Race and ethnicity | ||||
| Non‐Hispanic White | 383 093 (54.1) | 373 564 (54.2) | 9529 (50.0) | <0.001† |
| Non‐Hispanic Black | 52 586 (12.7) | 50 650 (12.6) | 1936 (16.9) | <0.001† |
| American Indian/Alaskan native | 16 561 (1.3) | 15 746 (1.2) | 815 (2.6) | <0.001† |
| Asian | 22 951 (7.1) | 22 638 (7.2) | 313 (3.2) | <0.001† |
| Hispanic | 88 063 (22.7) | 85 518 (22.7) | 2545 (24.5) | 0.003† |
| Other | 4209 (0.5) | 4036 (0.5) | 173 (0.7) | <0.001† |
| Multiracial | 17 006 (1.6) | 16 340 (1.6) | 666 (2.1) | <0.001† |
| Education level | ||||
| <High school | 41 637 (12.3) | 39 269 (11.9) | 2368 (23.9) | <0.001† |
| High school/some college | 321 895 (59.3) | 311 817 (59.3) | 10 078 (60.1) | <0.001† |
| College graduate | 228 560 (28.4) | 224 781 (28.8) | 3779 (16.0) | <0.001† |
| Employment status | ||||
| Employed | 436 408 (71.0) | 428 036 (71.4) | 8372 (52.7) | <0.001† |
| Unemployed | 103 018 (18.3) | 96 047 (17.7) | 6971 (42.1) | <0.001† |
| Student | 43 049 (10.3) | 42 650 (10.5) | 399 (3.3) | <0.001† |
| Retired | 2445 (0.4) | 2155 (0.4) | 290 (1.9) | <0.001† |
| Marital status | ||||
| Married | 274 882 (42.7) | 268 353 (42.7) | 6529 (40.3) | <0.001† |
| Divorced | 67 629 (9.6) | 63 607 (9.3) | 4022 (21.3) | <0.001† |
| Widowed | 4519 (0.6) | 4136 (0.6) | 383 (2.2) | <0.001† |
| Never married | 201 610 (39.5) | 197 413 (39.8) | 4197 (29.0) | <0.001† |
| Member of an unmarried couple | 41 433 (7.6) | 40 383 (7.6) | 1050 (7.2) | 0.007† |
| Health care coverage | <0.001 | |||
| No health insurance plan | 86 228 (17.5) | 83 392 (17.4) | 2836 (20.3) | |
| Some health insurance plan | 503 613 (82.5) | 490 249 (82.6) | 13 364 (79.8) | |
| Annual family income | ||||
| Below federal poverty line | 82 109 (16.5) | 77 491 (16.1) | 4618 (30.6) | <0.001† |
| Within 100%–200% of poverty line | 104 506 (18.4) | 100 871 (18.4) | 3635 (22.4) | <0.001† |
| At >200% of the poverty line | 374 229 (65.1) | 367 119 (65.5) | 7110 (47.0) | <0.001† |
| Urban/rural status | <0.001† | |||
| Urban | 377 262 (94.4) | 367 425 (94.5) | 9837 (92.3) | |
| Rural | 52 677 (5.6) | 50 844 (5.5) | 1833 (7.7) | |
| Diabetes | <0.001† | |||
| No | 566 810 (96.1) | 553 798 (96.4) | 13 012 (81.6) | |
| Yes | 26 083 (3.9) | 22 871 (3.6) | 3212 (18.4) | |
| Smoking status | ||||
| Never smoked | 364 060 (66.7) | 357 411 (67.2) | 6649 (45.2) | <0.001† |
| Former smoker | 101 438 (16.5) | 98 002 (16.3) | 3436 (21.5) | <0.001† |
| Current smoker | 99 998 (16.9) | 94 557 (16.4) | 5441 (33.3) | <0.001† |
| Overweight/obese | <0.001† | |||
| No | 192 840 (37.8) | 188 983 (38.1) | 3857 (26.7) | |
| Yes | 345 372 (62.2) | 334 412 (61.9) | 10 960 (73.3) | |
| Depression | <0.001† | |||
| No | 465 934 (80.4) | 457 169 (81.0) | 8765 (56.4) | |
| Yes | 127 682 (19.6) | 120 174 (19.0) | 7508 (43.6) | |
| Hypercholesterolemia‡ | <0.001† | |||
| No | 210 711 (83.3) | 206 681 (84.0) | 4030 (56.3) | |
| Yes | 44 652 (16.7) | 41 337 (16.0) | 3315 (43.7) | |
| Hypertension‡ | <0.001† | |||
| No | 242 540 (83.9) | 238 815 (84.7) | 3725 (49.0) | |
| Yes | 52 704 (16.1) | 48 430 (15.3) | 4274 (51.0) | |
Data are given as number (weighted percentage). BRFSS indicates Behavioral Risk Factor Surveillance System; and CVD, cardiovascular disease.
The numbers represent actual number of participants. All percentages are weighted to reflect final survey weights.
P<0.05, which is significant.
Data available for only 2017 and 2019.
Characteristics of Participants by CVD Status
The overall weighted prevalence of CVD was 2.5% (95% CI, 2.4–2.6). Individuals with CVD, compared with those without CVD, were more likely to be older (aged>44 years: 29.0% versus 12.9%; P<0.001), non‐Hispanic Black race and ethnicity (16.9% versus 12.6%; P<0.001), and uninsured (20.3% versus 17.4%; P<0.001) (Table 1). Those with CVD had a greater proportion of current smokers (33.3% versus 16.4%), overweight/obesity (73.3% versus 61.9%), diabetes (18.4% versus 3.6%), depression (43.6% versus 19.0%), hypertension (51.0% versus 15.3%), and hypercholesterolemia (43.7% versus 16.0%) (all P<0.001), compared with people without CVD. The description of the study population by CVH is shown in Table S1.
State‐Level Prevalence of CVD and Depression
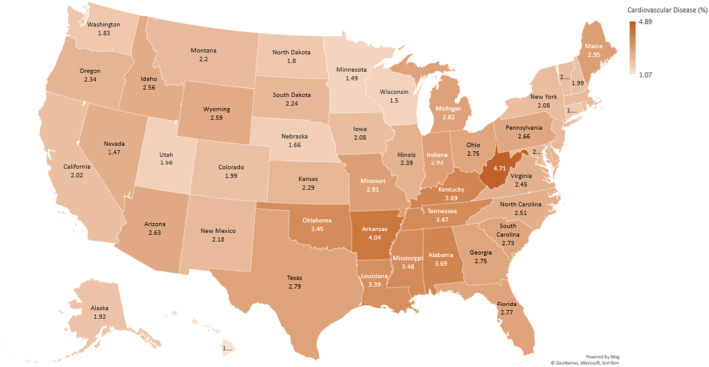
State‐level prevalence rates of depression and CVD are presented in Figures 2 and 3, respectively. There was high prevalence of both depression and CVD in West Virginia (depression: 29.74%; CVD: 4.71%). Hawaii had the lowest prevalence of depression (13.07%) and CVD (1.07%). There was a positive correlation between prevalent depression and CVD (correlation coefficient r=0.22).
Figure 2. Heat map showing prevalence of depression among young US adults: Behavioral Risk Factor Surveillance System 2017 to 2020.
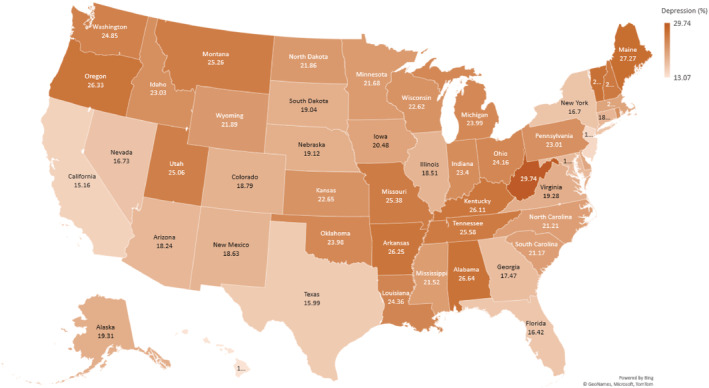
Figure 3. Heat map showing prevalence of cardiovascular disease among young US adults: Behavioral Risk Factor Surveillance System 2017 to 2020.
Association of Depression and PMHDs With CVD
Individuals with depression had higher odds of prevalent CVD than those without depression, after adjusting for sociodemographic characteristics (odds ratio [OR], 2.69 [95% CI, 2.49–2.90]) and after further adjusting for diabetes, smoking, and BMI (OR, 2.32 [95% CI, 2.13–2.51]) (Table 2). When additionally adjusted for hypertension and hypercholesterolemia, using data from only 2017 and 2019, the association only modestly attenuated (OR, 1.92 [95% CI, 1.71–2.16]) (Table S2). In addition, there was a positive graded association between PMHDs and CVD. Compared with participants with 0 PMHDs, participants with 1 to 13 days had 1.5‐fold odds of CVD (adjusted OR [aOR], 1.48 [95% CI, 1.34–1.62]), whereas participants with 14 to 30 days had even higher odds of CVD (aOR, 2.29 [95% CI, 2.08–2.51]) after adjustment for sociodemographic factors, diabetes, BMI, and smoking (Table 2), with similar associations observed after further adjustment for hypertension and hypercholesterolemia (Table S2).
Table 2.
Association of Depression and Poor Mental Health With CVD Outcomes, BRFSS (2017–2020)
| Variable | No. (%) of respondents | Weighted prevalence of CVD (95% CI) | Model 1 | Model 2 | Model 3 |
|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |||
| Depression | |||||
| No | 465 934 (80.4) | 1.8 (1.7–1.9) | Reference | Reference | Reference |
| Yes | 127 682 (19.6) | 5.6 (5.4–5.9) | 3.29 (3.09–3.51) | 2.69 (2.49–2.90) | 2.32 (2.13–2.51) |
| Poor mental health days | |||||
| 0 | 322 733 (55.7) | 1.8 (1.7–1.9) | Reference | Reference | Reference |
| 1–13 | 174 374 (29.2) | 2.3 (2.2–2.5) | 1.30 (1.20–1.42) | 1.59 (1.46–1.75) | 1.48 (1.34–1.62) |
| 14–30 | 88 491 (15.1) | 5.5 (5.2–5.8) | 3.19 (2.96–3.43) | 2.71 (2.49–2.96) | 2.29 (2.08–2.51) |
Model 1: crude. Model 2: adjusted for age, sex, race, education, employment, income, marital status, and health care coverage. Model 3: model 2+combustible cigarette smoking, body mass index, and diabetes. BRFSS indicates Behavioral Risk Factor Surveillance System; CVD, cardiovascular disease; and OR, odds ratio.
There was no significant interaction between depression and sex (P for interaction=0.13) or urban/rural status (P for interaction=0.26) on the association of depression with CVD. Similarly, there was no significant interaction between categories of PMHDs and sex (P for interaction 0–13 PMHDs: 0.35; 14–30 PMHDs: 0.88) or urban/rural status (P for interaction 0–13 PMHDs: 0.62; 14–30 PMHDs: 0.89). Estimates of the association of depression and PMHDs with CVD stratified by sex and urban/rural status are presented in Tables S3 and S4.
Association of Depression and PMHDs With Suboptimal CVH Among Participants Without CVD
Among those without prevalent CVD, 79.8% had suboptimal CVH. Depression was associated with higher odds of suboptimal CVH after adjusting for age, sex, race and ethnicity, education, employment, income, marital status, and health coverage (OR, 1.76 [95% CI, 1.65–1.87]) (Table 3). Similarly, PMHDs were associated with suboptimal CVH in a graded manner. Compared with participants with 0 days of poor mental health, participants with 1 to 13 days had 1.2 times higher odds of suboptimal CVH (aOR, 1.23 [95% CI, 1.17–1.29]), and those with 14 to 30 days had 1.8 times higher odds of suboptimal CVH (aOR, 1.79 [95% CI, 1.65–1.95]).
Table 3.
Association of Depression and Poor Mental Health With Suboptimal CVH, Among Participants With No CVD, BRFSS (2017 and 2019)
| Among participants with no CVD | ||||
|---|---|---|---|---|
| Variable | No. (weighted %) of respondents | Weighted prevalence of suboptimal CVH (95% CI) | Model 1 | Model 2 |
| OR (95% CI) | OR (95% CI) | |||
| Depression | ||||
| No | 150 682 (80.2) | 77.96 (77.56–78.36) | Reference | Reference |
| Yes | 41 537 (19.8) | 84.77 (84.15–85.38) | 1.57 (1.49–1.66) | 1.77 (1.67–1.88) |
| Poor mental health days | ||||
| 0 | 104 413 (54.8) | 78.59 (78.11–79.07) | Reference | Reference |
| 1–13 | 59 668 (31.0) | 77.92 (77.31–78.52) | 0.96 (0.92–1.01) | 1.23 (1.17–1.29) |
| 14–30 | 26 468 (14.2) | 85.01 (84.15–85.83) | 1.54 (1.43–1.66) | 1.82 (1.68–1.98) |
Model 1: crude. Model 2: adjusted for age, sex, race, education, employment, income, marital status, and health care coverage. Suboptimal CVH health: ≥2 cardiovascular risk factors. BRFSS indicates Behavioral Risk Factor Surveillance System; CVD, cardiovascular disease; CVH, cardiovascular health; and OR, odds ratio.
DISCUSSION
In this cross‐sectional analysis of a nationally representative sample of young adults, depression and PMHDs were independently associated with CVD, with no significant interaction by sex or urban/rural status. In addition, among people with no CVD, depression and PMHDs were independently associated with suboptimal CVH, with a dose‐response relationship.
Self‐report of depression was highly prevalent in our study, with ≈1 in 5 young adults reporting depression, 2‐fold higher than the national prevalence of at least 1 major depressive disorder of 8.4% in all US adults in 2020 24 but similar to recent prevalence estimates in young US adults. 24 , 25 Furthermore, during the COVID‐19 pandemic, the percentage of US adults who experienced depression or anxiety jumped from 36.4% to 41.5%, with the highest spike among people aged 18 to 29 years, according to Centers for Disease Control and Prevention data, 26 highlighting the need to develop targeted interventions to address the burden of poor mental health in young adults to reduce the depression‐associated health risks. 27
Our finding of higher odds of CVD in participants with depression is consistent with prior studies in other geographic locations. In a pooled cohort from >30 countries, Harshfield et al found baseline depression symptoms to be associated with incidence of CVD among an older population (mean age, 63±9 years). 13 In a multicenter study focusing on low‐ and middle‐income countries, having ≥4 depressive symptoms in patients without a history of CVD was associated with 14% increased risk of incident CVD and all‐cause mortality. 14 A recent study among individuals of European ancestry showed depression frequency was associated with incident coronary artery disease, type 2 diabetes, and atrial fibrillation. 28 In the United States, a smaller single‐center study of 882 participants found moderate to severe depression to be associated with low (worse) CVH score, 29 whereas similar findings were reported in moderate sized population‐based studies. 30 , 31 Our study explores the association of depression and PMHDs, which represent recent and active mental health conditions, with CVD in a larger population of US young adults, with subanalyses by sex and urban/rural status; it also assesses the association of depression and PMHDs, with suboptimal CVH, in people without CVD.
The relationship between CVD and depression has been regarded as bidirectional. There is evidence that depression is an independent risk factor for CVD. 12 , 13 , 32 The increased odds of CVD in people with depression may stem from the unhealthy lifestyle that may be associated with depression, such as sedentary behavior, 33 , 34 unhealthy eating, 35 , 36 and smoking. 37 , 38 , 39 There are multiple physiological pathways, such as abnormalities in glucose and lipid homeostasis and coagulation cascade abnormalities related to chronic stress, by which psychological health and well‐being may influence CVH and CVD risk. 40 , 41 , 42 Other possible mechanisms that may explain the association of depression with CVD include the neurohormonal imbalances and overactivation of the sympathoadrenal and hypothalamic‐pituitary‐adrenal axis that have been demonstrated among people with depression. 12 , 43
It is important to recognize that CVD can also lead to depression. Indeed, several studies have reported higher rates of depression among people with CVD. 44 , 45 , 46 , 47 A rate of 15% of major depressive disorder has been reported in patients after myocardial infarction or coronary artery bypass grafting, 44 and this rate is >20% in patients with heart failure and is much higher in advanced heart failure. 45 In another study, poststroke depression was seen in 5% of patients within 3 years after ischemic stroke diagnosis, and a higher 3‐year mortality was observed in patients with poststroke depression. 48
Our findings showed no sex differences in the association of depression with CVD, contrary to the findings of some prior studies in which stronger associations between depression and incident CVD, CVD mortality, and all‐cause mortality were found in men. 49 , 50 Men tend to underreport poor mental health and are also less likely to seek treatment, 51 , 52 , 53 which may contribute to the apparent increase in risk. Other studies have also shown stronger association of depression and incident coronary artery disease among women, 28 demonstrating that sex differences in this association are highly heterogeneous across the literature.
Studies on urbanization and risk of depression have been inconsistent in different geographic locations. Although some studies suggest an increased risk of depression in individuals residing in urban areas, some have found a protective or a null association. 18 , 54 , 55 Evidence from a meta‐analysis showed higher odds of depression in urban areas compared with rural areas in developed countries, with a pooled OR of 1.44. 18 Factors, such as unhealthy diets, reduced physical activity, stress, unsafe neighborhoods and isolation, lack of social support, and lack of green spaces, in urban areas may be contributory. 19 , 56 In this study, however, the association of mental health and CVD did not vary by urban/rural status. Last, studies have found that depression, stress, and anxiety, attributable to disparities in social determinants of health, adverse childhood experiences, general trauma, and structural racism, could place certain populations and racial and ethnic underrepresented groups at a higher risk of CVD and poor mental health. 57 , 58 Future research should focus on addressing the role of social determinants of health and health disparities in improving the intersection between mental health and health outcomes.
Our findings have several important clinical and public health implications. Our study highlights the need for clinicians to recognize the risk of CVD in patients with depression and poor mental health. Given the increased odds of CVD among patients with depression, it may be necessary to enhance screening for depression and evaluation of mental health in general, as an additional screening tool in evaluating CVH and monitoring for CVD. Among people without a clinical diagnosis of depression, a unique group that is likely captured by our assessment of PMHDs, improvement in their mental health may likely be beneficial for CVD prevention. At least, Screening of individuals who have had a major cardiovascular event for mental health conditions and emotional and psychological well‐being, and communicating with close family members and friends, can prove to be beneficial. Mental health screening among those with established CVD may be important to optimize secondary prevention.
Targeted interventions that improve mental health and reduce mood disorders may be necessary in reducing CVD and improving overall CVH. Cardiac rehabilitation programs often incorporate stress reduction and mindfulness, but mental health could be more strongly integrated, particularly for those with mental health conditions. Interventions to address mood disorders in young adults should consider targeting CVH, such as physical activity, 59 weight management, and smoking cessation. Although lifestyle changes may be particularly challenging in patients with depression, a multidisciplinary approach and collaborative and integrated care between health care professionals, such as mental health physicians, psychologists, psychiatrists, nutritionists and addiction specialists, primary care physicians, and cardiologists, may be needed to better improve mental health and reduce CVD risk. Future research should focus on incorporating a validated screening tool in the Life's Essential 8 metrics for appropriate screening and interventions in a patient‐centered approach. Last, these data are reflective of the pre–COVID‐19 world, and future studies are encouraged to explore these associations in the post–COVID‐19 period.
Study Strengths
The BRFSS is a nationally representative sample with less potential for selection bias. Using data from 4 years provided a large sample size to assess not only depression but recent mental health (PMHDs) with CVD. We additionally excluded people with CVD and tested the association of depression and PMHDs with suboptimal CVH.
Study Limitations
Our study has several limitations. First, the BRFSS is based on self‐reported data, and there is a potential for recall bias and misclassification. Standard questionnaires, such as Patient Health Questionnaire‐8 and the General Anxiety Disorder Scales, are not used herein. Second, CVD and cardiovascular risk factors may be underreported in people with low literacy and because of social desirability and underdiagnosed in people who do not have access to health care. Also, arrhythmias, heart failure, and peripheral vascular disease were not included in our definition of CVD because of unavailability of data and, thus, the prevalence of CVD may be underestimated. Although the recently revised components of CVH (Life's Essential 8) include sleep, we were unable to include sleep in our definition of CVH because the BRFSS did not have data available on sleep in the survey years in which the other 7 CVH metrics were assessed. Nevertheless, Life's Simple 7 and Life's Essential 8 are highly correlated (>0.8). In addition, information on antidepressants was not available and so could not be adjusted for in the analysis. Furthermore, information on hypertension and hypercholesterolemia is only asked biennially and, therefore, could only be assessed over odd‐numbered years. Last, because our study is observational and cross‐sectional, we cannot infer causality or determine the directionality of the association, although our findings are biologically plausible. We, therefore, encourage future longitudinal studies to explore these associations using standard questionnaires for assessment of depression and ascertainment of CVD.
CONCLUSIONS
In this large, nationally representative sample of young adults in the United States, we found that depression and PMHDs were independently associated with premature CVD and suboptimal CVH. Although this association is likely bidirectional, prioritizing mental health may help reduce CVD risk and improve CVH in young adults. Achieving the CVH‐related Sustainable Development Goals may necessitate the awareness of the physical health risks associated with depression and the prioritization of an integrated and comprehensive approach to tackling CVD and mental health disorders. In addition, broader public policies should promote mental well‐being and healthy behaviors as part of a comprehensive strategy to reduce CVD burden among young adults.
Sources of Funding
Dr Honigberg has funding support from the American Heart Association grants 940166 and 979465. Dr Sharma has funding support from American Heart Association grant 979462.
Disclosures
Dr Honigberg receives consulting fees from CRISPR Therapeutics and provides advisory board service for Miga Health, which is unrelated to this study. Dr Natarajan reports investigator‐initiated grants from Amgen, Apple, AstraZeneca, Boston Scientific, and Novartis; reports personal fees from Apple, AstraZeneca, Blackstone Life Sciences, Foresite Labs, Novartis, and Roche/Genentech; is a cofounder of TenSixteen Bio; is a scientific advisory board member of Esperion Therapeutics, geneXwell, and TenSixteen Bio; and reports spousal employment at Vertex, all unrelated to the present work.
Supporting information
Appendix S1
Presented in part at the Johns Hopkins University Department of Medicine and Whiting School of Engineering Poster Session in Baltimore, MD, on September 20, 2022.
Presented in part at the American Heart Association Scientific Sessions 2022 in Chicago, IL, November 5 to 7, 2022, and published in abstract form (Circulation. 2022;146:A13611 or https://www.ahajournals.org/doi/abs/10.1161/circ.146.suppl_1.13611).
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.028332
For Sources of Funding and Disclosures, see page 10.
References
- 1. Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, et al. Heart disease and stroke statistics—2021 update. Circulation. 2021;143:E254–E743. doi: 10.1161/CIR.0000000000000950 [DOI] [PubMed] [Google Scholar]
- 2. Cardiovascular diseases (CVDs) . World Health Organization Published in June 2021. Available at: https://www.who.int/news‐room/fact‐sheets/detail/cardiovascular‐diseases‐(cvds). Accessed June 20, 2022.
- 3. SDG Target 3.4|Noncommunicable diseases and mental health: by 2030, reduce by one third premature mortality from non‐communicable diseases through prevention and treatment and promote mental health and well‐being. World Health Organization. https://www.who.int/data/gho/data/themes/topics/indicator‐groups/indicator‐group‐details/GHO/sdg‐target‐3.4‐noncommunicable‐diseases‐and‐mental‐health. Accessed July 19, 2022.
- 4. Lloyd‐Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Horn LV, Geenlund K, Daniels S, Nichol G, Tormaselli GF, et al. Defining and setting National Goals for cardiovascular health promotion and disease reduction. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703 [DOI] [PubMed] [Google Scholar]
- 5. Andersson C, Vasan RS. Epidemiology of cardiovascular disease in young individuals. Nat Rev Cardiol. 2018;15:230–240. doi: 10.1038/NRCARDIO.2017.154 [DOI] [PubMed] [Google Scholar]
- 6. Lloyd‐Jones DM, Ning H, Labarthe D, Brewer L, Sharma G, Rosamond W, Foraker RE, Black T, Grander MA, Allen NB, et al. Status of cardiovascular health in US adults and children using the American Heart Association's new “Life's essential 8” metrics: prevalence estimates from the National Health and nutrition examination survey (NHANES), 2013–2018. Circulation. 2022;146:822–835. doi: 10.1161/CIRCULATIONAHA.122.060911 [DOI] [PubMed] [Google Scholar]
- 7. Wang L, Song L, Li D, Zhou Z, Chen S, Yang Y, Hu Y, Wang Y, Wu S, Tian Y. Ideal cardiovascular health metric and its change with lifetime risk of cardiovascular diseases: a prospective cohort study. J Am Heart Assoc. 2021;10:22502. doi: 10.1161/JAHA.121.022502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guo L, Zhang S. Association between ideal cardiovascular health metrics and risk of cardiovascular events or mortality: a meta‐analysis of prospective studies. Clin Cardiol. 2017;40:1339–1346. doi: 10.1002/CLC.22836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gaye B, Canonico M, Perier MC, Samieri C, Berr C, Dartigues JF, Tzourio C, Elbaz A, Empana JP. Ideal cardiovascular health, mortality, and vascular events in elderly subjects: the Three‐City study. J Am Coll Cardiol. 2017;69:3015–3026. doi: 10.1016/J.JACC.2017.05.011 [DOI] [PubMed] [Google Scholar]
- 10. Lichtman JH, Froelicher ES, Blumenthal JA, Carney RM, Doering LV, Frasure‐Smith N, Freedland KE, Jaffe AS, Leifheit‐Limson EC, Sheps DS, et al. Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: systematic review and recommendations: a scientific statement from the American Heart Association. Circulation. 2014;129:1350–1369. doi: 10.1161/CIR.0000000000000019 [DOI] [PubMed] [Google Scholar]
- 11. Mental Health Awareness, Genomics and Precision Health, CDC. https://www.cdc.gov/genomics/resources/diseases/mental.htm. Accessed June 26, 2022.
- 12. Halaris A. Inflammation‐associated Co‐morbidity between depression and cardiovascular disease. Curr Top Behav Neurosci. 2017;31:45–70. doi: 10.1007/7854_2016_28 [DOI] [PubMed] [Google Scholar]
- 13. Harshfield EL, Pennells L, Schwartz JE, Willeit P, Kaptoge S, Bell S, Shaffer JA, Bolton T, Spackman S, Wassertheil‐Smoller S, et al. Association between depressive symptoms and incident cardiovascular diseases. JAMA. 2020;324:2396–2405. doi: 10.1001/JAMA.2020.23068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rajan S, McKee M, Rangarajan S, Bangdiwala S, Rosengren A, Gupta R, Kutty VR, Wielgosz A, Lear S, Alhabib KF, et al. Association of symptoms of depression with cardiovascular disease and mortality in low‐, middle‐, and high‐income countries. JAMA Psychiatry. 2020;77:1052–1063. doi: 10.1001/JAMAPSYCHIATRY.2020.1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Purtle J, Nelson KL, Yang Y, Langellier B, Stankov I, Diez Roux AV. Urban–rural differences in older adult depression: a systematic review and meta‐analysis of comparative studies. Am J Prev Med. 2019;56:603–613. doi: 10.1016/J.AMEPRE.2018.11.008 [DOI] [PubMed] [Google Scholar]
- 16. Reddy KS. Cardiovascular diseases in the developing countries: dimensions, determinants, dynamics and directions for public health action. Public Health Nutr. 2002;5:231–237. doi: 10.1079/PHN2001298 [DOI] [PubMed] [Google Scholar]
- 17. CDC – Behavioral Risk Factor Surveillance System. https://www.cdc.gov/brfss/index.html. Accessed June 21, 2022.
- 18. Methodologic Changes in the Behavioral Risk Factor Surveillance System in 2011 and Potential Effects on Prevalence Estimates. Morbidity and Mortality Weekly Report, CDC, Published on June 8, 2012. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6122a3.htm. Accessed June 21, 2022. [PubMed]
- 19. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–349. doi: 10.1016/J.JCLINEPI.2007.11.008 [DOI] [PubMed] [Google Scholar]
- 20. Healthy Days Methods and Measures, Health Related Quality of Life, CDC. https://www.cdc.gov/hrqol/methods.htm. Accessed June 20, 2022.
- 21. Poor Mental Health Days, County Health Rankings & Roadmaps. https://www.countyhealthrankings.org/explore‐health‐rankings/measures‐data‐sources/county‐health‐rankings‐model/health‐outcomes/quality‐of‐life/poor‐mental‐health‐days Accessed June 20, 2022.
- 22. Nishida C, Barba C, Cavalli‐Sforza T, Cutter J, Deurenberg P, Darnton‐Hill I, Deurenberg‐Yap M, Gill T, James P, Ko G, et al. Appropriate body‐mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3 [DOI] [PubMed] [Google Scholar]
- 23. HHS Poverty Guidelines for 2022, Office of the Assistant Secretary for Planning and Evaluation ASPE. Published January 12, 2022. https://aspe.hhs.gov/topics/poverty‐economic‐mobility/poverty‐guidelines. Accessed July 19, 2022.
- 24. Major Depression, National Institute of Mental Health. https://www.nimh.nih.gov/health/statistics/major‐depression Accessed June 26, 2022.
- 25. Hasin DS, Sarvet AL, Meyers JL, Saha TD, Ruan WJ, Stohl M, Grant BF. Epidemiology of adult DSM‐5 major depressive disorder and its specifiers in the United States. JAMA Psychiatry. 2018;75:336–346. doi: 10.1001/JAMAPSYCHIATRY.2017.4602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vahratian A, Blumberg SJ, Terlizzi EP, Schiller JS. Symptoms of anxiety or depressive disorder and use of mental health care among adults during the COVID‐19 pandemic—United States, august 2020–February 2021. MMWR Morb Mortal Wkly Rep. 2021;70:490–494. doi: 10.15585/MMWR.MM7013E2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Greenberg PE, Fournier AA, Sisitsky T, Simes M, Berman R, Koenigsberg SH, Kessler RC. The economic burden of adults with major depressive disorder in the United States (2010 and 2018). Pharmacoeconomics. 2021;39:653–665. doi: 10.1007/S40273-021-01019-4/FIGURES/3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Honigberg MC, Ye Y, Dattilo L, Sarma AA, Scott NS, Smoller JW, Zhao H, Wood MJ, Natarajan P. Low depression frequency is associated with decreased risk of cardiometabolic disease. Nat Cardiovasc Res. 2022;1:125–131. doi: 10.1038/s44161-021-00011-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Patterson SL, Marcus M, Goetz M, Gooding HC. Depression and anxiety are associated with cardiovascular health in young adults. Circulation. 2021;143:AP192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang Z, Jackson S, Merritt R, Gillespie C, Yang Q. Association between cardiovascular health metrics and depression among U.S. adults: National Health and nutrition examination survey, 2007–2014. Ann Epidemiol. 2019;31:49–56.e2. doi: 10.1016/J.ANNEPIDEM.2018.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Medoff B, Baird A, Herbert B, Magnani JW. Depression worsens cardiovascular health: a population‐based cohort study (NHANES, 2015–2016). Circulation. 2020;142:A14193. [Google Scholar]
- 32. Seldenrijk A, Vogelzangs N, Batelaan NM, Wieman I, van Schaik DJF, Penninx BJWH. Depression, anxiety and 6‐year risk of cardiovascular disease. J Psychosom Res. 2015;78:123–129. doi: 10.1016/J.JPSYCHORES.2014.10.007 [DOI] [PubMed] [Google Scholar]
- 33. Huang Y, Li L, Gan Y, Wang C, Jiang H, Cao S, Lu Z. Sedentary behaviors and risk of depression: a meta‐analysis of prospective studies. Transl Psychiatry. 2020;10:1–10. doi: 10.1038/s41398-020-0715-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jiang L, Cao Y, Ni S, Chen X, Shen M, Lv H, Hu J. Association of sedentary behavior with anxiety, depression, and suicide ideation in college students. Front Psychiatry. 2020;11:1403. doi: 10.3389/FPSYT.2020.566098/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Paans NPG, Bot M, Brouwer IA, Visser M, Roca M, Kohls E, Watkins E, Penninx BWJH. The association between depression and eating styles in four European countries: the MooDFOOD prevention study. J Psychosom Res. 2018;108:85–92. doi: 10.1016/J.JPSYCHORES.2018.03.003 [DOI] [PubMed] [Google Scholar]
- 36. Paans NPG, Bot M, van Strien T, Brouwer IA, Visser M, Penninx BWJH. Eating styles in major depressive disorder: results from a large‐scale study. J Psychiatr Res. 2018;97:38–46. doi: 10.1016/J.JPSYCHIRES.2017.11.003 [DOI] [PubMed] [Google Scholar]
- 37. Fluharty M, Taylor AE, Grabski M, Munafò MR. The Association of Cigarette Smoking with depression and anxiety: a systematic review. Nicotine Tob Res. 2017;19:3–13. doi: 10.1093/NTR/NTW140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chaiton MO, Cohen JE, O'Loughlin J, Rehm J. A systematic review of longitudinal studies on the association between depression and smoking in adolescents. BMC Public Health. 2009;9:1–11. doi: 10.1186/1471-2458-9-356/FIGURES/3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stubbs B, Vancampfort D, Firth J, Solmi M, Siddiqi N, Smith L, Carvalho AF, Koyanagi A. Association between depression and smoking: a global perspective from 48 low‐ and middle‐income countries. J Psychiatr Res. 2018;103:142–149. doi: 10.1016/J.JPSYCHIRES.2018.05.018 [DOI] [PubMed] [Google Scholar]
- 40. Levine GN, Cohen BE, Commodore‐Mensah Y, Fleury J, Huffman JC, Khalid U, Labarthe DR, Lavretsky H, Michos ED, Spatz ES, et al. Psychological health, well‐being, and the mind‐heart‐body connection: a scientific statement from the American Heart Association. Circulation. 2021;143:E763–E783. doi: 10.1161/CIR.0000000000000947 [DOI] [PubMed] [Google Scholar]
- 41. Slavich GM, Irwin MR. From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol Bull. 2014;140:774–815. doi: 10.1037/A0035302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Maia DB, Marmar CR, Mendlowicz MV, Metzler T, Nobrega A, Peres MC, Coutinho ES, Volchan E, Figuera I. Abnormal serum lipid profile in Brazilian police officers with post‐traumatic stress disorder. J Affect Disord. 2008;107:259–263. doi: 10.1016/J.JAD.2007.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Huffman JC, Celano CM, Beach SR, Motiwala SR, Januzzi JL. Depression and cardiac disease: epidemiology, mechanisms, and diagnosis. Cardiovasc Psychiatry Neurol. 2013;2013:14. doi: 10.1155/2013/695925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Colquhoun DM, Bunker SJ, Clarke DM, Glozier N, Hare DL, Hickie IB, Tatoulis J, Thompson DR, Toffler GH, Wilson A, et al. Screening, referral and treatment for depression in patients with coronary heart disease. Med J Austr. 2013;198:483–484. doi: 10.5694/MJA13.10153 [DOI] [PubMed] [Google Scholar]
- 45. Rutledge T, Reis VA, Linke SE, Greenberg BH, Mills PJ. Depression in heart failure: a meta‐analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. 2006;48:1527–1537. doi: 10.1016/J.JACC.2006.06.055 [DOI] [PubMed] [Google Scholar]
- 46. Kuhlmann SL, Arolt V, Haverkamp W, Martus P, Strohle A, Waltenberger J, Rieckmann N, Muller‐Nordhorn J. Prevalence, 12‐month prognosis, and clinical management need of depression in coronary heart disease patients: a prospective cohort study. Psychother Psychosom. 2019;88:300–311. doi: 10.1159/000501502 [DOI] [PubMed] [Google Scholar]
- 47. Thombs BD, Bass EB, Ford DE, Stewart KJ, Tsilidis KK, Patel U, Fauerbach JA, Bush DE, Ziegelsten RC. Prevalence of depression in survivors of acute myocardial infarction. J Gen Intern Med. 2006;21:30–38. doi: 10.1111/J.1525-1497.2005.00269.X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Williams LS, Ghose SS, Swindle RW. Depression and other mental health diagnoses increase mortality risk after ischemic stroke. Am J Psychiatry. 2004;161:1090–1095. doi: 10.1176/APPI.AJP.161.6.1090 [DOI] [PubMed] [Google Scholar]
- 49. Sun WJ, Xu L, Chan WM, Lam TH, Mary Schooling C. Are depressive symptoms associated with cardiovascular mortality among older Chinese: a cohort study of 64,000 people in Hong Kong? Am J Geriatr Psychiatry. 2013;21:1107–1115. doi: 10.1016/J.JAGP.2013.01.048 [DOI] [PubMed] [Google Scholar]
- 50. Penninx BWJH, Geerlings SW, Deeg DJH, van Eijk JTM, van Tilburg W, Beekman ATF. Minor and major depression and the risk of death in older persons. Arch Gen Psychiatry. 1999;56:889–895. doi: 10.1001/ARCHPSYC.56.10.889 [DOI] [PubMed] [Google Scholar]
- 51. Mackenzie CS, Gekoski WL, Knox VJ. Age, gender, and the underutilization of mental health services: the influence of help‐seeking attitudes. Aging Ment Health. 2006;10:574–582. doi: 10.1080/13607860600641200 [DOI] [PubMed] [Google Scholar]
- 52. Lynch L, Long M, Moorhead A. Young men, help‐seeking, and mental health services: exploring barriers and solutions. Am J Mens Health. 2018;12:138–149. doi: 10.1177/1557988315619469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Call JB, Shafer K. Gendered manifestations of depression and help seeking among men. Am J Mens Health. 2018;12:41–51. doi: 10.1177/1557988315623993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sampson L, Ettman CK, Galea S. Urbanization, urbanicity, and depression: a review of the recent global literature. Curr Opin Psychiatry. 2020;33:233–244. doi: 10.1097/YCO.0000000000000588 [DOI] [PubMed] [Google Scholar]
- 55. Adjaye‐Gbewonyo D, Rebok GW, Gallo JJ, Gross AL, Underwood CR. Urbanicity of residence and depression among adults 50 years and older in Ghana and South Africa: an analysis of the WHO study on global AGEing and adult health (SAGE). Aging Ment Health. 2019;23:660–669. doi: 10.1080/13607863.2018.1450839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kondo MC, Fluehr JM, McKeon T, Branas CC. Urban green space and its impact on human health. Int J Environ Res Public Health. 2018;15:15. doi: 10.3390/IJERPH15030445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Belgrave FZ, Abrams JA. Reducing disparities and achieving equity in African American women's health. Am Psychol. 2016;71:723–733. doi: 10.1037/AMP0000081 [DOI] [PubMed] [Google Scholar]
- 58. Paradies Y, Ben J, Denson N, Elias A, Priest N, Pieters A, Gupta A, Kelaher M, Gee G. Racism as a determinant of health: a systematic review and meta‐analysis. PLoS One. 2015;10:10. doi: 10.1371/JOURNAL.PONE.0138511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Schuch FB, Vancampfort D. Physical activity, exercise, and mental disorders: it is time to move on. Trends Psychiatry Psychother. 2021;43:177–184. doi: 10.47626/2237-6089-2021-0237 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


