Abstract
Accurate treatment adjustment to physical activity (PA) remains a challenging problem in type 1 diabetes (T1D) management. Exercise-driven effects on glucose metabolism depend strongly on duration and intensity of the activity, and are highly variable between patients. In-silico evaluation can support the development of improved treatment strategies, and can facilitate personalized treatment optimization. This requires models of the glucose-insulin system that capture relevant exercise-related processes. We developed a model of glucose-insulin regulation that describes changes in glucose metabolism for aerobic moderate- to high-intensity PA of short and prolonged duration. In particular, we incorporated the insulin-independent increase in glucose uptake and production, including glycogen depletion, and the prolonged rise in insulin sensitivity. The model further includes meal absorption and insulin kinetics, allowing simulation of everyday scenarios. The model accurately predicts glucose dynamics for varying PA scenarios in a range of independent validation data sets, and full-day simulations with PA of different timing, duration and intensity agree with clinical observations. We personalized the model on data from a multi-day free-living study of children with T1D by adjusting a small number of model parameters to each child. To assess the use of the personalized models for individual treatment evaluation, we compared subject-specific treatment options for PA management in replay simulations of the recorded data with altered meal, insulin and PA inputs.
Author summary
Exercise represents a cornerstone of diabetes management. Yet, many people with type 1 diabetes refrain from exercising, since it increases the risk for hypoglycemia and requires adjusted insulin treatment. The effect of exercise on blood glucose levels depends on exercise duration and intensity, but also varies strongly between individuals, making accurate adjustment a challenge. Mathematical models can help to better understand exercise physiology and to devise new treatment strategies. Here, we propose a model of glucose-insulin regulation that captures the effects of exercise on glucose metabolism and personalize it to individual children with type 1 diabetes, allowing subject-specific treatment assessment.
Introduction
Blood glucose (BG) homeostasis maintains glucose levels within a tight range in healthy individuals, where the two main hormones involved are insulin and glucagon to lower and raise glucose levels, respectively. In type 1 diabetes (T1D), BG regulation is impeded by the autoimmune destruction of insulin-secreting β-cells of the pancreas [1]. The resulting lack of insulin leads to elevated glucose levels if untreated. People with T1D therefore rely on exogenous insulin either from multiple daily injections or an insulin pump together with BG monitoring to keep glucose levels stable within a target range of usually 70–180 mg/dl, with insulin requirements varying strongly between individuals. Tight glucose control is essential to avoid long-term complications such as cardiovascular disease and retinopathy from persistent hyperglycemia, or acute complications such as loss of consciousness and seizures from severe hypoglycemia.
Mathematical models of glucose-insulin regulation are a valuable tool for the in-silico evaluation of treatment strategies in T1D and play a critical role in the development of decision support and closed-loop insulin delivery systems (artificial pancreas) [2–4]. One prominent example is the UVa/Padova type 1 diabetes simulator [5] that has been approved by the FDA for preclinical testing of control algorithms for insulin treatment. While such models are typically used with hypothetical in-silico patients, a recent approach uses a personalized model to replay recorded data of individuals with T1D with altered carbohydrate (CHO) and insulin inputs, allowing subject-specific treatment assessment for improved BG control [6].
T1D treatment also needs to be adjusted to physical activity (PA), but complex PA-driven changes in glucose metabolism pose major challenges for accurate PA management. Changes occur on different time scales and strongly depend on duration and intensity of PA. Glucose demand increases drastically during the activity and insulin sensitivity remains elevated for several hours following exercise [7], leading to an increased risk for both acute and late-onset hypoglycemia. Current guidelines for treatment adjustment consider only coarse categories of glycemia, PA duration and intensity, and need further tailoring to the individual person [8, 9]. Tailoring largely relies on trial-and-error, and while PA has numerous benefits and represents a cornerstone in diabetes management [10, 11], fear of hypoglycemia restrains many people with T1D from exercising [12].
Extended models that capture exercise metabolism can help evaluate PA guidelines and treatment strategies [13] and the need for such models has long been recognized [14–16]. Roy et al. [17] proposed a PA extension of the Bergman minimal model [18], considering acute, insulin-independent effects of moderate-intensity PA on glucose uptake and production. They also included effects of liver glycogen depletion for prolonged PA. In an alternative proposal, Breton [19] studied increased glucose effectiveness and prolonged PA-driven changes in insulin sensitivity during an euglycemic hyperinsulemic clamp protocol in people with T1D. However, the effects of exercise intensity and duration on insulin action were not incorporated. Dalla Man et al. [20] integrated the model into their simulation model of the glucose-insulin system [21] in an in-silico study and added intensity- and duration-dependence, while Alkhateeb et al. [22] evaluated different variations of the Bergman minimal model and selected a model that features an increase in glucose effectiveness and insulin sensitivity. Other models have been proposed [23–26] and a virtual patient population has been generated [27] that incorporates PA [24].
However, these models have been developed under very controlled conditions, e.g. in clamp studies, have not been tailored to a T1D population, do not permit varying PA intensities or prolonged duration, or cover only a subset of the relevant processes. In addition, they often do not consider insulin and carbohydrate inputs. Hence, they are not suited for (personalized) treatment evaluation under everyday-life conditions.
Recently, Romeres et al. [28–30] and Nguyen et al. [31] conducted two elegant studies in which they evaluated exercise-induced changes in glucose utilization and endogenous glucose production, and separated and quantified insulin-dependent and –independent contributions. Incorporating their findings into models of exercise metabolism could alleviate some of the persistent problems and is useful for several reasons. A more accurate representation of exercise physiology by considering insulin-dependent and –independent effects separately facilitates prediction of exercise-driven changes in glucose levels and hypoglycemic events. In turn, this could be used to develop and evaluate improved insulin treatment strategies for PA in T1D. Furthermore, the quantification of overall glucose uptake and production rates allows to develop separate model components for each process. Previously, insulin-independent changes in glucose metabolism were often summarized in an exercise-induced increase in glucose effectiveness in PA models for T1D. As discussed by Alkhateeb et al. [22], this allows for decreasing glucose levels for moderate-intensity PA, but high-intensity PA can not be described by such models due to rising BG levels. In addition, it is difficult to incorporate liver glycogen depletion that affects the rate of glucose production for prolonged PA.
Here, we utilize these newly available data and develop a glucose-insulin regulation model for exercise that explicitly considers insulin-dependent and -independent effects on glucose uptake and production, and allows realistic full-day simulations and personalized replay simulations. The model captures the acute and prolonged changes in glucose metabolism during PA and subsequent recovery for moderate- to high-intensity exercise, and considers CHO intake and insulin injections. We first calibrate the model for a healthy population, before adjusting relevant parameters to people with T1D. We validate the model on independent data from increasingly complex scenarios including PA, insulin and CHO intake.
We show how exercise duration, intensity and time of day alter BG dynamics in full-day simulations. As a main result, we demonstrate that our model can describe real-world data of individual patients. We personalize the model on data from children with T1D recorded in a free-living observational study, using only data from sensors readily available during everyday-life. We then perform replay simulations of the original scenarios with altered meal, insulin and PA inputs to evaluate different treatment strategies and PA effects on the individual subject level.
Methods
Ethics statement
We used data from the ‘DiaActive’ study conducted in children with T1D under free-living conditions [32] with ethics approval no. 341/12 provided by the Ethics Commission Cantons of Basel on February 14, 2013. Written formal consent was obtained from the parent or guardian for each study participant.
Development of a glucoregulatory model including physical activity
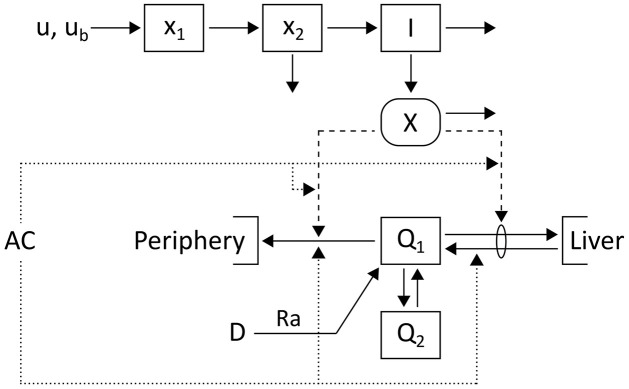
Our proposed model is outlined in Fig 1 and comprises a simple core model extended by meal intake and insulin injections. Accelerometer counts AC quantify the input for PA processes affecting glucose regulation.
Fig 1. Schematic of the glucose-insulin model.
Glucose, Q1, and insulin, I, dynamics are described using a two-compartment model [33]. Extensions capture plasma insulin kinetics after subcutaneous injection u with basal insulin infusion rate ub [34] and glucose appearance with rate Ra after a meal D [35]. PA is measured via accelerometer counts AC and leads to changes in glucose metabolism indicated by dotted lines.
We use the Cobelli two-compartment minimal model [33] to describe glucose-insulin regulation at rest and extend it to incorporate PA-driven changes in glucose metabolism:
| (1) |
The two glucose compartments Q1 and Q2 [mg/kg] represent glucose mass in plasma and in a remote compartment, respectively. Plasma insulin I [μU/ml] promotes the disappearance of plasma glucose into liver and tissue, and suppresses hepatic glucose production via the dynamic state X [1/min]. The constants Q1b [mg/kg] and Xb = p3/p2 ⋅ Ib [1/min] provide the basal levels of plasma glucose and state X, respectively, with the basal plasma insulin level Ib [μU/ml]. The ratio p3/p2 represents insulin sensitivity and p1 describes glucose effectiveness. The rate parameters p4 and p5 quantify the exchange between the two glucose compartments. Glucose appearance from meals is described by Ra [mg/min] and scaled with bodyweight, BW [kg]. The rates rGU [1/min] and (rGP − rdepl) [1/min] provide the exercise-induced insulin-independent increase in glucose uptake (GU) and production (GP), respectively, while (1 + Z) captures a PA-driven rise in insulin sensitivity. These processes depend on PA intensity (see below) and introduce additional nonlinearities to the model as they are further modulated by changing glucose levels. Finally, G [mg/dl] is the plasma glucose concentration and Vg [dl/kg] the glucose distribution volume.
Measure of exercise intensity and duration
We consider accelerometer (AC) counts to capture movement and link them to PA intensity Y [counts/min] following previous approaches [17, 19, 23]:
| (2) |
The delay τAC [min] allows initial adaptation to PA.
We also track PA duration tPA [min], integrated AC count PAint [counts] and time spent at high intensity th [min]:
| (3) |
where transfer functions f(AC; aAC, n2) and f(AC; ah, n2), defined as
| (4) |
capture the transition in AC count from rest to exercise, respectively from moderate to high intensity with corresponding AC thresholds aAC [counts/min] and ah [counts/min]. The exponent n2 defines the steepness of the transition and q5 delays the switch back from the high- to moderate-intensity mode during recovery. The use of transfer functions to introduce exercise-related changes was previously proposed by Breton [19].
Insulin sensitivity
Insulin sensitivity increases during exercise and stays elevated afterwards for up to 48 hours to replete liver glycogen stores [36]. Previous studies have further established that the increase depends linearly on PA intensity and duration [20, 22], and we consequently describe this rise Z by
| (5) |
where f(Y; aY, n1) defines the minimal intensity Y considered as PA with intensity threshold aY [counts/min] and exponent n1, parameter b [1/count] specifies the proportional rise, and τZ [min] the time for insulin sensitivity to return to its baseline level.
Insulin-independent glucose uptake and production
Glucose demand by active muscles increases acutely during PA and glucose uptake from plasma is upregulated. Simultaneously, hepatic glucose production by gluconeogenesis and glycogenolysis increases to maintain plasma glucose levels [37]. These processes are linear in PA intensity [17]. We therefore define the insulin-independent rise in GU (rGU [1/min]) and GP (rGP [1/min]) rates as
| (6) |
where qi are rate parameters.
Glycogen depletion
Liver glycogen stores may deplete during prolonged PA and GP cannot be maintained by gluconeogenesis alone, causing an accelerated drop in glucose levels [7, 38]. We follow Roy et al. [17] and assume that glycogen stores deplete in proportion to exercise intensity and duration. The time tdepl [min] to depletion determined from the integrated AC count and PA duration is then given by:
| (7) |
After depletion sets in, we allow a drop in GP rate, rdepl [1/min], defined by
| (8) |
where the transfer function f(tPA; tdepl, n1) indicates whether exercise time exceeds tdepl and q6 is a rate parameter. The maximum decrease rm [1/min] in GP is the sum of the basal resting GP rate, rGPb, and the PA-driven GP rate at steady state, q3/q4 ⋅ Y(t), scaled by the proportion of net hepatic glucose production attributed to glycogenolysis, β.
High-intensity exercise
During high-intensity PA (> 80% ), GP may (initially) exceed GU and result in rising plasma glucose levels due to an increase in catecholamines and cortisol [39]. We mimic the drastic rise in GP by modulating parameters q3 and q4 between low- (subscript l) and high-intensity (subscript h) values
| (9) |
where we use the transfer function f(th; tp, n2) to smoothly transition between the two exercise regimes when time spent at a high PA intensity th exceeds tp [min].
Model extensions for full-day simulations
To enable full-day simulations, we further include existing models to provide plasma insulin concentration after insulin injections and rate of glucose appearance after meals, and use these as inputs to the exercise model.
Insulin kinetics
We use a model with two subcutaneous compartments of insulin masses x1 and x2 [μU] and a plasma insulin compartment I [μU/ml] to model plasma insulin after a subcutaneous injection [34]:
| (10) |
Insulin is injected into x1, with u [μU/min] and ub [μU/min] defining the rates of correction and basal insulin infusion, respectively. VI [ml/kg] is the insulin distribution volume and ki are rate parameters. We estimated the model parameters from insulin measurements obtained after a subcutaneous injection of 0.3 U/kg insulin aspart [40] (Section 2 in S1 File).
Carbohydrate absorption
We describe the glucose appearance rate Ra [mg/min] after a meal with carbohydrate content D [mg] with an established model [35]:
| (11) |
A fraction f of glucose is absorbed into plasma and the time constant τm [min] characterizes the time-of-maximum appearance rate. We determine f and τm individually for each meal (see below).
Model calibration
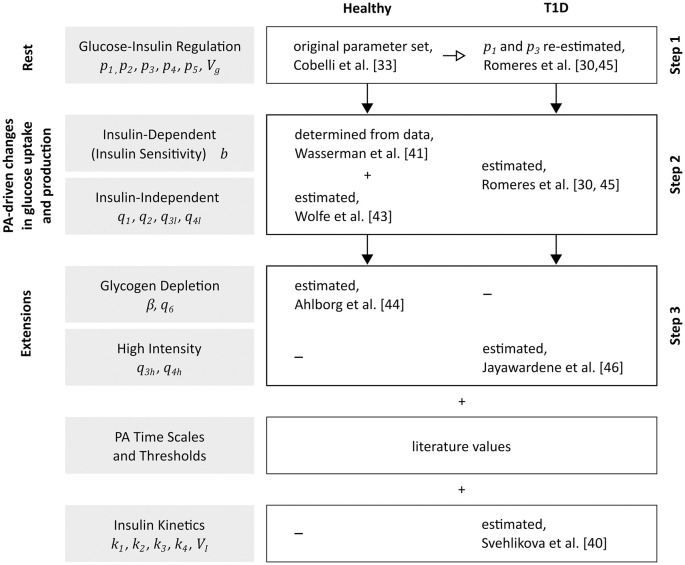
We obtained parameter values from literature or physiological knowledge when feasible and estimated the remaining parameters from published data. We followed a stepwise approach for parameter estimation and calibrated a population-average model on data of healthy subjects acquired during exercise, before adjusting parameters to describe glucose metabolism and PA effects in people with T1D (Fig 2 and Table A in S1 File).
Fig 2. Model calibration.
Schematic of the stepwise calibration process for healthy individuals and people with T1D.
Parameter determination for healthy subjects
We used the original parameter values of the two-compartment minimal model [33] and explicitly included the effect of basal insulin on glucose. To calibrate the exercise model, we separated parameters into process-specific sets and individually estimated these on data sets acquired during the corresponding exercise modes using least squares regression.
We set the delay parameter τAC to 5 min [19, 20] and chose a time constant τZ of 600 min [20] such that insulin sensitivity stays elevated for up to 48 hours in accordance with literature reports [36].
We obtained the increase in insulin sensitivity during PA (parameter b) from measurements of the insulin-dependent rate of glucose disappearance during rest and 100 min of cycling at 80% [41]. We converted to accelerometer count using
| (12) |
estimated from simultaneous AC count (Actigraph model 7164; Actigraph, LLC; Pensacola, Florida, USA) and measurements for different types and intensities of PA [42].
We estimated the insulin-independent GU and GP parameters q1, q2, q3l and q4l from total GU and GP rates measured during 60 min of PA at 40% [43] (Fig A (a) in S1 File). We distinguished between resting and exercise-driven contributions by separating the net rate of glucose change into endogenous glucose production and glucose uptake:
| (13) |
where we determined α from measurements at rest (Z = rGU = rGP = rdepl = 0). We assumed that the prolonged exercise-driven change in insulin sensitivity affects both GP and GU as found in Romeres et al. [29, 30] and subtracted its contribution to the total GU and GP rates based on the insulin sensitivity parameter defined above.
We determined the time until hepatic glycogenolysis decreases due to glycogen depletion from reported depletion times for different intensities [38] (parameters adepl and bdepl). We estimated glycogen depletion parameters β and q6 from plasma glucose measurements [44] recorded during 180 min of cycling at 58% , where we restricted q6 to 0.1 min−1 to avoid an overshoot in GP after PA and kept the remaining parameters fixed (Fig A (b) in S1 File).
Finally, moderate-intensity PA is defined by AC counts above 2296 counts/min [42] and we enforced the transition from rest to PA between 1000 and 2000 counts/min with parameters aY = 1500 counts/min and n1 = 20. Accordingly, we defined aAC = 1000 counts/min and n2 = 100 to track duration and AC count immediately from the start of PA. High-intensity PA commences at 80% (5800 counts/min) [39], and we set ah = 5600 counts/min and tp = 2 min for a transition between intensity regimes at 75%-80% .
Adjustment of model parameters to T1D
To re-calibrate the exercise model to persons with T1D, we relied on the study by Romeres et al. [30, 45], where people with T1D performed 60 min of exercise at 65% during a glucose clamp under three different glucose and insulin conditions (V1: euglycemia—low insulin, V2: euglycemia—high insulin, V3: hyperglycemia—low insulin). Plasma glucose and insulin concentrations were measured and glucose disappearance and production rates were determined from recorded data. We estimated parameters defining insulin-independent (p1, q1-q4l) and -dependent (p3, b) contributions to GU and GP at rest and during PA for all conditions, and defined the resulting parameters determined under condition V1 as our standard T1D model, since V1 represents physiologically ‘normal’ conditions. For further details on the estimation procedure, see Section 1.2.1 in S1 File. Additionally, we computed confidence intervals for all parameter estimates from profile likelihoods to determine practical identifiability (Section 1.2.2 in S1 File).
We estimated the high-intensity exercise parameters q3h and q4h from interstitial glucose measurements [46] of people with T1D performing 45 min of 4 min intervals at 82.5% using least squares regression (Fig D in S1 File). We introduced the parameter q5 = 0.03 min−1 to prevent a switch to low-intensity parameters during recovery. For the remaining parameters, we used the values determined for the hyperglycemia—low insulin condition (V3) of the previously discussed data, as the high-intensity activity was recorded under comparable conditions.
Model validation
We used independent data from six additional studies covering a range of exercise intensities and durations for validating our model. Importantly, several studies include pre-exercise meal intake and insulin bolus injections as well as different insulin reduction strategies. This allowed us to validate the individual model parts and their interplay in the full model. The data sets are the following:
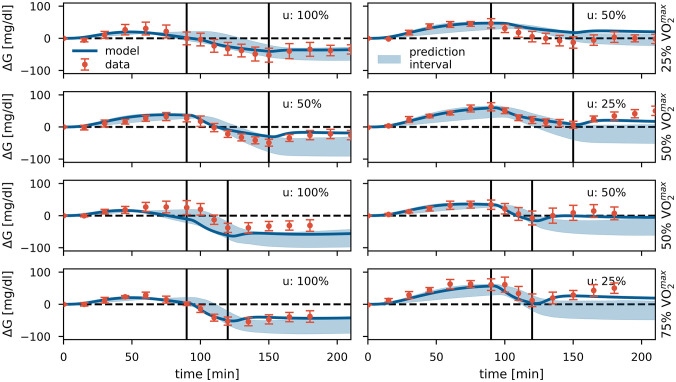
In a study by Rabasa-Lhoret et al. [47], participants with T1D performed exercise at three intensities (25%, 50% and 75% ) for different durations (30 and 60 min). Breakfast with 75g of CHO and varying insulin bolus sizes (25%, 50% and 100% of typical dose) was consumed 90 min prior to PA. Plasma glucose was measured.
In a second study conducted by Maran et al. [48], participants with T1D performed 30 min of exercise at 40% . The changes in plasma glucose and insulin concentrations were recorded.
Participants with T1D exercised for 45 min at 67.8% in a study presented by Iscoe and Riddell [49], where the change in interstitial glucose levels was measured.
The effect of basal insulin suspension during exercise was studied by Zaharieva et al. [50]. Exercise was performed for 40 min at 45% and the change in plasma glucose concentration was assessed.
In a study by Dubé et al. [51], exercise was performed for 60 min at 50% , 2h after lunch including a pre-meal insulin bolus. Plasma glucose levels were monitored and participants did or did not consume a drink containing 30g of glucose 15 min pre-exercise.
Healthy subjects cycled for 240 min at 30% in a study conducted by Ahlborg et al. [52] and plasma glucose was measured. For this PA duration, glycogen depletion affects GP and subsequently glucose levels.
We generated 95% prediction intervals for studies (1)–(5) based on the T1D model derived under condition V1. The predicted glucose ranges are shown in Fig 3 and Fig F (a)-(e) in S1 File (shaded areas). However, there is a high variation in the physiological response to PA between individuals, regarding both the increase in insulin sensitivity and in the insulin-independent processes [30]. Therefore, differences in study populations require tuning of the model parameters to accurately reflect the data, and we re-estimated parameters b, q1l and q2l. Note that we kept the ratio between q1l and q2l constant, enforcing a fixed steady-state level in the insulin-independent rise in GU to maintain the original ratio between PA-driven changes in GU and GP. The resulting glucose trajectories are shown as solid lines (Fig 3 and Fig F (a)-(e) in S1 File). For study (6), we used the parameter values of the healthy population and kept them unchanged (Fig F (f) in S1 File).
Fig 3. Model validation.
Data (mean ± SEM, n = 6) [47] and model predictions for validation study (1). PA sessions are marked by vertical lines. PA was performed at different intensities (25%, 50% and 75% ) for durations of 30 or 60 min. A meal was consumed 90 min prior to PA, with a meal insulin bolus u of 100%, 50% or 25% of the full dose. The shaded areas show the 95% prediction intervals of the T1D model (V1), and solid BG trajectories display the tuned model (b = 1.83 ⋅ 10−6, q1 = 2.93 ⋅ 10−6, q2 = 2.93 ⋅ 10−6). Meal parameters f and τm = 105 min were determined from glucose levels at rest.
Overall, we observe good agreement between data and model predictions across all validation studies for both the prediction intervals based on the original calibration and the glucose trajectories using tuned parameters.
Results
Effect of physical activity in full-day simulations
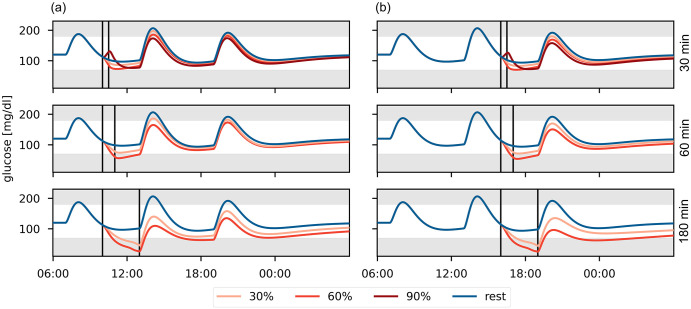
We evaluate our model’s performance in full-day simulations for a range of PA scenarios to confirm that it reproduces clinical knowledge. We define a standard day consisting of three meals and corresponding insulin bolus injections. We include a PA session in the morning or afternoon and consider different intensities (30%, 60% and 90% ) and durations (30, 60 and 180 min) (Fig 4).
Fig 4. Comparison of glucose trajectories for different PA scenarios in a full-day simulation.
PA is performed at 30%, 60% and 90% for 30, 60 and 180 minutes (a) in the morning, or (b) in the afternoon. The PA session is marked by vertical lines. Meals are eaten at 7:00, 13:00 and 19:00 containing 40g, 60g and 50g CHO, respectively.
During moderate-intensity PA, BG levels decrease with increasing intensity and duration [8] and drop even further once glycogen stores deplete [7]. In contrast, BG levels may rise [8] during high-intensity PA, which can provide protection against acute hypoglycemia [53], but the risk for late-onset hypoglycemia still increases with higher PA intensity and duration [48, 54]. Time of day also affects the risk for nocturnal hypoglycemia, which is higher for afternoon- compared to morning-PA [55].
Our model accurately reflects the duration- and intensity-dependence in glucose trends for moderate- and high-intensity PA. As expected, BG levels increase during high-intensity PA, but drop below those of moderate-intensity PA after the activity. We also find lower nocturnal BG levels following afternoon- compared to morning-PA. Our model thus reproduces clinical observations regarding hypoglycemia risk for a range of different PA scenarios.
Model personalization on data from children with T1D
To establish our model’s capability to describe individual subject data, we personalize the model on multi-day at-home data from five children aged 8–14 with T1D [32]. For each participant, interstitial glucose levels were measured by continuous glucose monitoring (CGM), exercise was monitored using an accelerometer, and CHO content and timing of meals as well as timing and dosing of insulin injections were self-reported in logbooks. We provide participant characteristics in Section 5.2.1 in S1 File and discuss data preparation in Section 5.2.2 in S1 File.
To personalize the model to each participant, we followed a strategy presented by Hughes et al. [6] and determined subject-specific parameter values for insulin sensitivity (p3) and meal parameters (f and τm) using least squares regression. We also estimated glucose effectiveness (p1) and the basal glucose concentration Gb. We computed the basal insulin level Ib based on the basal insulin infusion rate ub, and kept the remaining parameter values, including all exercise-related parameters, at their previously determined population-average level (Table A in S1 File, V1). We confirmed local structural identifiability of the personalized parameters using the STRIKE-GOLDD toolbox [56]. Furthermore, we established our model’s capacity for personalization and replay simulations with altered meal and insulin inputs–but no PA–using the UVa/Padova simulator (Python implementation [57]) (Section 5.1 in S1 File). Note that we forewent the deconvolution step originally proposed to address further model mismatch.
We consider two–not necessarily consecutive–24 hour periods for each of the five children. We estimate parameters p1 and p3 on data from the first day and keep these values for the second day to confirm that the personalized models generalize to new scenarios. We estimate basal glucose for each day, and estimate meal parameters independently for each meal to account for inaccuracies in the self-reported meal sizes and for different meal compositions.
The individual model fits show very good overall agreement with the recorded data and we only observe a small number of non-explainable glucose excursions, which we attribute to unrecorded meals or unknown residual dynamics carried over from the previous day (Fig 5, and Fig H and Tables D-F in S1 File). We quantified the model fits using the root mean square difference (RMSD) and the mean absolute relative difference (MARD) (Table 1). We reach commonly used targets of RMSD below 25 mg/dl [58] and MARD below 10% [6] in most cases.
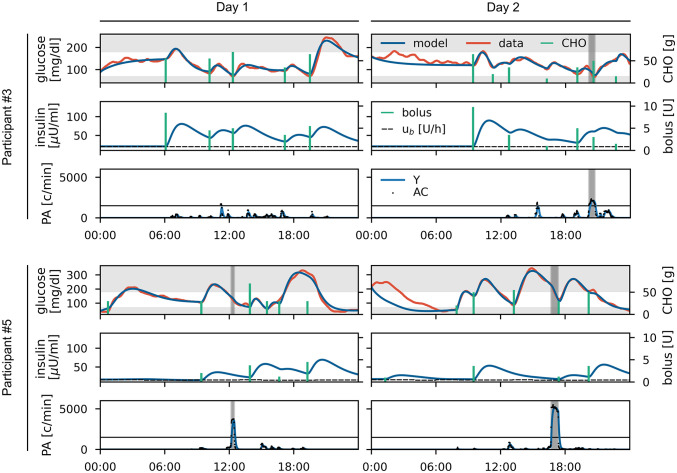
Fig 5. Data and personalized model for study participants #3 and #5 for two days each.
For each day, recorded (red) and fitted (blue) glucose data and carbohydrate inputs (green) are shown in the upper panel. Modelled insulin concentration (blue) and insulin inputs (green) including the basal insulin infusion rate (dashed) are shown in the middle panel. Accelerometer counts (dotted) and modelled PA intensity Y (blue) are shown in the lower panel with periods of physical activity highlighted in grey. The remaining participants are shown in Fig H in S1 File.
Table 1. Evaluation of personalized model fits.
Unexplained glucose excursions are excluded and results for the full 24h period are given in brackets in these cases.
| Participant | # 1 | # 2 | # 3 | # 4 | # 5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| day 1 | day 2 | day 1 | day 2 | day 1 | day 2 | day 1 | day 2 | day 1 | day 2 | |
| RMSD [mg/dl] | 11.4 | 12.1 | 3.7 | 6.6 | 9.3 | 15.8 | 11.0 | 16.8 | 12.8 | 9.0 |
| (84.6) | (34.2) | (36.6) | (43.1) | |||||||
| MARD [%] | 6.7 | 10.3 | 2.9 | 5.4 | 5.9 | 7.6 | 5.3 | 10.7 | 10.1 | 4.9 |
| (22.0) | (7.8) | (15.7) | (15.9) | |||||||
Replay simulations using personalized models
Next, we use the personalized models to demonstrate their potential in replay simulations, a promising approach for comparing and evaluating subject-specific treatment strategies in-silico, on two 24 hour episodes selected from our data.
We first consider day 1 of participant #3 with no PA and replay these data with changes in lunch size or with an altered meal bolus (Fig 6(a)). As expected, a larger meal or lower bolus increase BG levels, while a smaller meal or larger bolus lead to a corresponding reduction. Differences between these treatments reduce over time and virtually vanish after dinner.
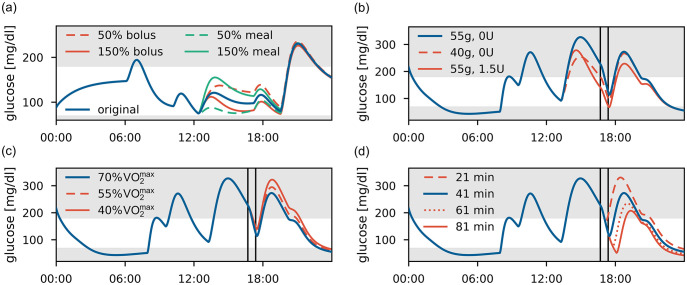
Fig 6. Replay simulations.
(a) Participant #3, day 1. Variations in meal size and insulin dose at lunch to 50% and 150% of their original size. (b)-(d) Participant #5, day 2, with a PA session marked by vertical lines. The original glucose trajectory is shown in blue. (b) Meal or bolus adjustment for pre-PA meal. (c) Alterations in PA intensity from 70% to 55% and 40% . (d) Alterations in PA duration from 41 min to 21, 61 and 81 min, with the post-exercise meal following directly after the session.
For our second scenario, we use the data for day 2 of participant #5, who exercises for 41 min at almost constant intensity of 70% in the afternoon. Likely in anticipation of the planned PA session, the participant used no insulin bolus for the preceding meal and commenced the session in hyperglycemia. We therefore ask if an alternative treatment decision might have led to a more favorable BG trajectory, and consider reducing the pre-PA meal size from 55g to 40g CHO or to administer an insulin bolus of 1.5U (Fig 6(b)). The replay simulation indicates that a smaller pre-PA meal could have been favorable, reducing hyperglycemia before PA without increasing the risk of hypoglycemia during the activity.
We also use this scenario to study the effect of exercise intensity and duration. First, we replay the scenario with lower PA intensities (Fig 6(c)). Notably, BG trajectories only start to diverge substantially after the post-PA meal as insulin sensitivity remains elevated for several hours during recovery, and further measures to avoid post-PA hyperglycemia might be required. Next, we consider varying PA duration (Fig 6(d)). The effect of elevated insulin sensitivity is clearly visible as the BG trajectories stay separated for the remaining simulation time, and results suggest that no additional adjustments are necessary to protect against hypoglycemia for exercise up to an hour.
Discussion
Mathematical models are a valuable tool to develop and evaluate treatment strategies for T1D in-silico. Finding accurate individual treatment adjustments for physical activity remains a complex process that could be facilitated by in-silico treatment evaluation, but comprehensive models including all relevant aspects of exercise metabolism suitable for this task are currently lacking.
PA-driven changes in glucose metabolism act on different time scales and require different treatment adjustments. In particular, insulin-independent processes affect BG levels mainly during PA, while insulin-dependent effects are the main cause for late-onset hypoglycemia and need to be considered for several hours post-PA. BG levels often fall during moderate-intensity PA when GU exceeds GP, while a drastic rise in GP during high-intensity PA can in contrast cause rising BG levels. It is therefore crucial to incorporate all relevant exercise processes in a PA model to study PA management in-silico.
In this work, we presented a model of glucose-insulin regulation in T1D that covers acute insulin-independent changes in GU and GP during PA, and the prolonged PA-induced rise in insulin sensitivity. We considered PA of moderate to high intensities, and accounted for depletion effects during prolonged exercise. We suggested the use of transfer functions to switch between these different exercise regimes, with the aim to keep the model compact without affecting the individual PA processes. The model includes modules for insulin bolus injections and meal intake as additional inputs to capture all aspects of daily life and diabetes management, allowing simulation of realistic scenarios.
We proposed a stepwise approach for model calibration, estimating parameters of the different model components separately on corresponding population-average data from healthy subjects. While the full model is not identifiable—a common problem for models of the glucose-insulin system—this allowed us to quantify individual contributions of the different PA-related processes accurately. Next, we adjusted the full model to a T1D population and computed profile likelihoods to determine practical parameter identifiability. We validated the model on independent data sets covering PA of different intensities and duration, and PA in conjunction with CHO intake and insulin injections. The resulting prediction intervals show the correct behavior in glucose trends, and the model accurately predicts the observed BG trajectories after tuning a small subset of parameters with high inter-patient variability to the examined patient population, demonstrating the feasibility of stepwise model identification and its potential for calibrating complex T1D models. Additionally, we evaluated the model’s prediction capabilities in full-day simulations with a range of PA scenarios against clinical knowledge.
The presented model structure is consistent with literature reports studying exercise-induced changes in glucose metabolism. Studies demonstrated that glucose utilization increases with exercise intensity for healthy subjects [59, 60] and for people with T1D [61]. It was shown by Romeres et al. [30] and Nguyen et al. [31] that this increase can be separated into insulin-dependent and –independent contributions. In particular, they confirmed that insulin-mediated GU increases gradually during the activity and remains elevated for several hours post-PA, while non-insulin-mediated GU increases rapidly at PA onset and drops to its baseline level immediately after. Nguyen et al. [31] did not find intensity-dependence for GU, but discuss that this might have been caused by PA intensities that were not sufficiently different or by varying levels of fitness between participants.
Similarly, it was shown that endogenous glucose production increases with exercise intensity to counteract the rise in GU [31, 59–62]. In contrast to its effect on GU, insulin suppresses GP. Romeres et al. [28] found that the PA-driven rise in GP is consequently inhibited in people with T1D with hyperinsulinemia, and identified a delayed effect of insulin on GP [29]. In contrast, Nguyen et al. [31] did not find insulin-mediated changes in GP. In this work, we followed the findings of Romeres et al. [29], since GU and GP rates were estimated in a model-independent way, and our model is able to describe their data well when including a PA-driven, elevated effect of insulin on GP.
Furthermore, the rate of hepatic glycogenolysis during PA increases linearly with intensity [38], supporting our assumption that the time until depletion occurs decreases in proportion to PA intensity. However, we estimated depletion parameters of the model only on data from healthy subjects, and data from individuals with T1D are required to validate this model part for a T1D population. Petersen et al. [62] observed that differences in GP between healthy and T1D subjects arise from varying contributions of gluconeogenesis, and that glycogenolysis at rest and for different PA intensities is similar for both populations. Hence, we believe that our model assumptions also hold for individuals with T1D and that predictions are therefore qualitatively correct for this population.
During high-intensity PA, counterregulatory hormones such as catecholamines and cortisol are upregulated. They are associated with increased hepatic GP that exceeds GU, and thus lead to (initially) rising BG levels during exercise [9, 39]. The rise in BG levels persists only while these hormone levels are elevated, and is followed by several hours with an increased risk for hypoglycemia [63]. We incorporated the drastic rise in GP in our model and were able to accurately reflect the resulting glucose dynamics. However, we were unable to perform an independent validation for this scenario due to lack of additional data. Moreover, we applied a fixed threshold to transition between moderate and high intensities, although we expect this transition to be different between individuals, especially when considering different age groups. In addition, the threshold might depend on the specific situation and type of PA the person is performing, where for example stress in competitive scenarios could lead to an earlier onset of high-intensity-like glucose dynamics.
Here, we only considered aerobic exercise of moderate to high intensity. We did not incorporate anaerobic exercise that is encountered for example in high-intensity interval or strength training. Anaerobic exercise can cause different trends in glucose levels for people with T1D [15], and it would be useful to integrate this modality into a PA model. Additionally, exercise has been reported to induce changes in insulin absorption that might affect plasma insulin concentrations [8, 64]. Our model currently does not consider this effect, as exact mechanisms remain elusive and potential changes cannot be estimated from our available data sets.
Exercise was recorded with accelerometers in our patient data, and we therefore chose AC count as PA input for our model. Other models use heart rate or instead, which might be better suited to quantify PA as they measure direct physiological responses to exercise. However, it would be straightforward to convert these measures and adjust the model to different inputs, since AC count, and heart rate are all linearly dependent [42, 65].
We applied our model to evaluate subject-specific treatment strategies in-silico based on model personalization and replay simulations. First, we validated this approach for altered meal and insulin inputs–but without exercise–against the UVa/Padova simulator. We then personalized our model to several children with T1D by adjusting a small number of parameters to each child, and accurately reproduced their glucose data recorded under real-life conditions. We presented examples of replay simulations from these personalized models to study subject-specific treatment alternatives and PA effects. Our results provide a promising proof-of-principle for adjusting treatment strategies to the individual person to improve PA management. The approach only requires data easily available in everyday settings from CGM devices and activity trackers, and we therefore expect that it also applies to the challenging case of unplanned and unstructured PA typical for children.
We anticipate that our model could be used in practice to describe and simulate blood glucose levels and to predict hypoglycemia associated with PA. Model personalization allows replay of recorded data and simulation of alternative treatment strategies to improve individual patient care, which would provide entirely new possibilities for clinical assessment and treatment adjustment. In addition, more fine-grained solutions to different exercise scenarios can be provided compared to current clinical guidelines that rely on observations of glucose changes during PA. We also anticipate that our model might find application in decision support systems or meal bolus calculators to determine insulin requirements for improved glycemic control, and would in particular allow to consider PA-induced changes in insulin sensitivity that can lead to late-onset hypoglycemia. Further applications might include the development of control algorithms for insulin treatment adjusted to glucose metabolism during and after exercise.
Conclusion
We proposed a model of glucose-insulin regulation that captures the acute and prolonged effects of moderate- to high-intensity PA on glucose metabolism. The model accurately predicts BG during PA and subsequent recovery and is capable of describing data from individuals with T1D. We illustrated its use in replay simulations for personalized PA management in children, which could support clinicians in tailoring treatment strategies to individuals in the future. We also anticipate that it finds applications as an ‘exercise calculator’ [14] for clinical decision support, as well as for improving control algorithms for closed-loop insulin delivery.
We evaluated the model’s performance on several data sets, but further validation of the model and personalized replay are warranted before application in a clinical setting.
Supporting information
Definition of standard patient. Additional information on replay simulations.
(PDF)
Acknowledgments
The authors would like to thank Jörg Stelling for valuable feedback and discussions.
Data Availability
All data and Python source code to use the model and reproduce its calibration, validation, and all analyses in the manuscript are available from https://gitlab.com/csb.ethz/t1d-exercise-model. Our individual patient data as well as the Python source code are additionally available at the ETH Research Collection, a FAIR repository, under DOI https://doi.org/10.3929/ethz-b-000589840.
Funding Statement
This work was supported by the two Cantons of Basel through project grant PMB-01-17 granted by the ETH Zurich. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2014;37(Supplement 1):S81–S90. doi: 10.2337/dc14-S081 [DOI] [PubMed] [Google Scholar]
- 2. Cinar A, Turksoy K. Advances in Artificial Pancreas Systems—Adaptive and Multivariable Predictive Control. Springer; 2017. [Google Scholar]
- 3. Kovatchev B. Automated closed-loop control of diabetes: the artificial pancreas. Bioelectronic Medicine. 2018;4(1):1–12. doi: 10.1186/s42234-018-0015-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kovatchev B. A Century of Diabetes Technology: Signals, Models, and Artificial Pancreas Control. Trends in Endocrinology and Metabolism. 2019;30(7):432–444. doi: 10.1016/j.tem.2019.04.008 [DOI] [PubMed] [Google Scholar]
- 5. Dalla Man C, Micheletto F, Lv D, Breton M, Kovatchev B, Cobelli C. The UVA/PADOVA type 1 diabetes simulator: New features. Journal of Diabetes Science and Technology. 2014;8(1):26–34. doi: 10.1177/1932296813514502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hughes J, Gautier T, Colmegna P, Fabris C, Breton MD. Replay Simulations with Personalized Metabolic Model for Treatment Design and Evaluation in Type 1 Diabetes. Journal of Diabetes Science and Technology. 2021;15(6):1326–1336. doi: 10.1177/1932296820973193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Camacho RC, Galassetti P, Davis SN, Wasserman DH. Glucoregulation during and after exercise in health and insulin-dependent diabetes. Exercise and sport sciences reviews. 2005;33(1):17–23. [PubMed] [Google Scholar]
- 8. Riddell MC, Gallen IW, Smart CE, Taplin CE, Adolfsson P, Lumb AN, et al. Exercise management in type 1 diabetes: a consensus statement. The Lancet Diabetes and Endocrinology. 2017;5(5):377–390. doi: 10.1016/S2213-8587(17)30014-1 [DOI] [PubMed] [Google Scholar]
- 9. Adolfsson P, Riddell MC, Taplin CE, Davis EA, Fournier PA, Annan F, et al. ISPAD Clinical Practice Consensus Guidelines 2018: Exercise in children and adolescents with diabetes. Pediatric Diabetes. 2018;19(June):205–226. doi: 10.1111/pedi.12755 [DOI] [PubMed] [Google Scholar]
- 10. American Diabetes Association. 4. Foundations of care: Education, nutrition, physical activity, smoking cessation, psychosocial care, and immunization. Diabetes Care. 2015;38(January):S20–S30. [DOI] [PubMed] [Google Scholar]
- 11. Colberg SR, Sigal RJ, Yardley JE, Riddell MC, Dunstan DW, Dempsey PC, et al. Physical activity/exercise and diabetes: A position statement of the American Diabetes Association. Diabetes Care. 2016;39(11):2065–2079. doi: 10.2337/dc16-1728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brazeau AS, Rabasa-Lhoret R, Strychar I, Mircescu H. Barriers to physical activity among patients with type 1 diabetes. Diabetes Care. 2008;31(11):2108–2109. doi: 10.2337/dc08-0720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Deichmann J, Bachmann S, Burckhardt MA, Szinnai G, Kaltenbach HM. Simulation-based evaluation of treatment adjustment to exercise in type 1 diabetes. Frontiers in Endocrinology. 2021. doi: 10.3389/fendo.2021.723812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Colberg SR, Laan R, Dassau E, Kerr D. Physical activity and type 1 diabetes: Time for a rewire? Journal of Diabetes Science and Technology. 2015;9(3):609–618. doi: 10.1177/1932296814566231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Riddell MC, Zaharieva DP, Yavelberg L, Cinar A, Jamnik VK. Exercise and the Development of the Artificial Pancreas: One of the More Difficult Series of Hurdles. Journal of diabetes science and technology. 2015;9(6):1217–1226. doi: 10.1177/1932296815609370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tagougui S, Taleb N, Molvau J, Nguyen É, Raffray M, Rabasa-Lhoret R. Artificial Pancreas Systems and Physical Activity in Patients with Type 1 Diabetes: Challenges, Adopted Approaches, and Future Perspectives. Journal of Diabetes Science and Technology. 2019;13(6):1077–1090. doi: 10.1177/1932296819869310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Roy A, Parker RS. Dynamic Modeling of Exercise Effects on Plasma Glucose and Insulin Levels. Journal of Diabetes Science and Technology. 2007;1(3):338–347. doi: 10.1177/193229680700100305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bergman RN, Ider YZ, Bowden CR, Cobelli C. Quantitative estimation of insulin sensitivity. American Journal of Physiology. 1979;236(6):E667–E677. [DOI] [PubMed] [Google Scholar]
- 19. Breton MD. Physical activity-the major unaccounted impediment to closed loop control. Journal of Diabetes Science and Technology. 2008;2(1):169–174. doi: 10.1177/193229680800200127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dalla Man C, Breton MD, Cobelli C. Physical Activity into the Meal Glucose–Insulin Model of Type 1 Diabetes: In Silico Studies. Journal of Diabetes Science and Technology. 2009;3(1):56–67. doi: 10.1177/193229680900300107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dalla Man C, Rizza RA, Cobelli C. Meal simulation model of the glucose- insulin system. IEEE Trans Biom Engin. 2007;54(10):1740–1749. doi: 10.1109/TBME.2007.893506 [DOI] [PubMed] [Google Scholar]
- 22. Alkhateeb H, El Fathi A, Ghanbari M, Haidar A. Modelling glucose dynamics during moderate exercise in individuals with type 1 diabetes. PLoS ONE. 2021;16(3 March):1–17. doi: 10.1371/journal.pone.0248280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lenart PJ, Parker RS. Modeling exercise effects in type I diabetic patients. IFAC Proceedings Volumes. 2002;35(1):247–252. doi: 10.3182/20020721-6-ES-1901.01350 [DOI] [Google Scholar]
- 24. Hernández-Ordoñez M, Campos-Delgado DU. An extension to the compartmental model of type 1 diabetic patients to reproduce exercise periods with glycogen depletion and replenishment. Journal of Biomechanics. 2008;41(4):744–752. doi: 10.1016/j.jbiomech.2007.11.028 [DOI] [PubMed] [Google Scholar]
- 25. Kim J, Saidel GM, Cabrera ME. Multi-scale computational model of fuel homeostasis during exercise: Effect of hormonal control. Annals of Biomedical Engineering. 2007;35(1):69–90. doi: 10.1007/s10439-006-9201-x [DOI] [PubMed] [Google Scholar]
- 26. Palumbo MC, Morettini M, Tieri P, Diele F, Sacchetti M, Castiglione F. Personalizing physical exercise in a computational model of fuel homeostasis. PLoS Computational Biology. 2018;14(4):1–23. doi: 10.1371/journal.pcbi.1006073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Resalat N, El Youssef J, Tyler N, Castle J, Jacobs PG. A statistical virtual patient population for the glucoregulatory system in type 1 diabetes with integrated exercise model. Plos One. 2019;14(7):e0217301. doi: 10.1371/journal.pone.0217301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Romeres D, Olson K, Carter R, Cobelli C, Dalla Man C, Basu A, et al. Hyperglycemia But Not Hyperinsulinemia Is Favorable for Exercise in Type 1 Diabetes: A Pilot Study. Diabetes Care. 2020; p. 6. doi: 10.2337/dc20-0611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Romeres D, Schiavon M, Visentin R, Basu A, Cobelli C, Basu R, et al. 691-P: Exercise Effect on Endogenous Glucose Production in Type 1 Diabetes: A Modeling Analysis. Diabetes. 2020;69(Supplement 1). [Google Scholar]
- 30. Romeres D, Schiavon M, Basu A, Cobelli C, Basu R, Dalla Man C. Exercise Effect on Insulin-Dependent and Insulin-Independent Glucose Utilization in Healthy and Type 1 Diabetes Individuals. A Modeling Study. American Journal of Physiology-Endocrinology and Metabolism. 2021. doi: 10.1152/ajpendo.00084.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nguyen TTP, Jacobs PG, Castle JR, Wilson LM, Kuehl K, Branigan D, et al. Separating insulin-mediated and non-insulin-mediated glucose uptake during and after aerobic exercise in type 1 diabetes. American Journal of Physiology—Endocrinology and Metabolism. 2021;320(3). doi: 10.1152/ajpendo.00534.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bachmann S, Hess M, Martin-Diener E, Denhaerynck K, Zumsteg U. Nocturnal hypoglycemia and physical activity in children with diabetes: New insights by continuous glucose monitoring and accelerometry. Diabetes Care. 2016;39(7):e95–e96. doi: 10.2337/dc16-0411 [DOI] [PubMed] [Google Scholar]
- 33. Cobelli C, Caumo A, Omenetto M. Minimal model SG overestimation and SI underestimation: improved accuracy by a Bayesian two-compartment model. The American journal of physiology. 1999;277(3 Pt 1):E481–E488. [DOI] [PubMed] [Google Scholar]
- 34. Nucci G, Cobelli C. Models of subcutaneous insulin kinetics. A critical review. Computer Methods and Programs in Biomedicine. 2000;62(3):249–257. doi: 10.1016/S0169-2607(00)00071-7 [DOI] [PubMed] [Google Scholar]
- 35. Hovorka R, Canonico V, Chassin LJ, Haueter U, Massi-Benedetti M, Federici MO, et al. Nonlinear model predictive control of glucose concentration in subjects with type 1 diabetes. Physiological Measurement. 2004;25(4):905–920. doi: 10.1088/0967-3334/25/4/010 [DOI] [PubMed] [Google Scholar]
- 36. Mul JD, Stanford KI, Hirshman MF, Goodyear LJ. Exercise and Regulation of Carbohydrate Metabolism. Progress in Molecular Biology and Translational Science. 2015;135:17–37. doi: 10.1016/bs.pmbts.2015.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wahren J, Felig P, Ahlborg G, Jorfeldt L. Glucose metabolism during leg exercise in man. The Journal of clinical investigation. 1971;50(12):2715–2725. doi: 10.1172/JCI106772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gonzalez JT, Fuchs CJ, Betts JA, van Loon LJC. Liver glycogen metabolism during and after prolonged endurance-type exercise. American Journal of Physiology—Endocrinology And Metabolism. 2016;311(3):E543–E553. doi: 10.1152/ajpendo.00232.2016 [DOI] [PubMed] [Google Scholar]
- 39. Marliss EB, Vranic M. Intense exercise has unique effects on both insulin release and its roles in glucoregulation: Implications for diabetes. Diabetes. 2002;51(SUPPL.):271–283. doi: 10.2337/diabetes.51.2007.S271 [DOI] [PubMed] [Google Scholar]
- 40. Svehlikova E, Mursic I, Augustin T, Magnes C, Gerring D, Jezek J, et al. Pharmacokinetics and pharmacodynamics of three different formulations of insulin aspart: A randomized, double-blind, crossover study in men with type 1 diabetes. Diabetes Care. 2021;44(2):448–455. doi: 10.2337/dc20-1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wasserman DH, Geer RJ, Rice DE, Bracy D, Flakoll PJ, Brown LL, et al. Interaction of exercise and insulin action in humans. American Journal of Physiology-Endocrinology and Metabolism. 1991;260(1):E37–E45. doi: 10.1152/ajpendo.1991.260.1.E37 [DOI] [PubMed] [Google Scholar]
- 42. Evenson KR, Catellier DJ, Gill K, Ondrak KS, McMurray RG. Calibration of two objective measures of physical activity for children. Journal of Sports Sciences. 2008;26(14):1557–1565. doi: 10.1080/02640410802334196 [DOI] [PubMed] [Google Scholar]
- 43. Wolfe RR, Nadel ER, Shaw JHF, Stephenson LA. Role of changes in insulin and glucagon in glucose homeostasis in exercise. Journal of Clinical Investigation. 1986;77(3):900–907. doi: 10.1172/JCI112388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ahlborg G, Felig P. Lactate and glucose exchange across the forearm, legs, and splanchnic bed during and after prolonged leg exercise. Journal of Clinical Investigation. 1982;69(1):45–54. doi: 10.1172/JCI110440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Romeres D, Basu A, Schiavon M, Cobelli C, Man CD, Basu R. Effects of Hyperglycemia and Hyperinsulinemia on Glucose Turnover during Exercise in Type 1 Diabetes. Diabetes. 2018;67(Supplement 1). [Google Scholar]
- 46. Jayawardene DC, McAuley SA, Horsburgh JC, Gerche AL, Jenkins AJ, Ward GM, et al. Closed-loop insulin delivery for adults with type 1 diabetes undertaking high-intensity interval exercise versus moderate-intensity exercise: A randomized, crossover study. Diabetes Technology and Therapeutics. 2017;19(6):340–348. doi: 10.1089/dia.2016.0461 [DOI] [PubMed] [Google Scholar]
- 47. Rabasa-Lhoret R, Bourque J, Ducros F, Chiasson JL. Guidelines for premeal insulin dose reduction for postprandial exercise of different intensities and durations in type 1 diabetic subjects treated intensively with a basal-bolus insulin regimen (ultralente-lispro). Diabetes Care. 2001;24(4):625–630. doi: 10.2337/diacare.24.4.625 [DOI] [PubMed] [Google Scholar]
- 48. Maran A, Pavan P, Bonsembiante B, Brugin E, Ermolao A, Avogaro A, et al. Continuous glucose monitoring reveals delayed nocturnal hypoglycemia after intermittent high-intensity exercise in nontrained patients with type 1 diabetes. Diabetes Technology and Therapeutics. 2010;12(10):763–768. doi: 10.1089/dia.2010.0038 [DOI] [PubMed] [Google Scholar]
- 49. Iscoe KE, Riddell MC. Continuous moderate-intensity exercise with or without intermittent high-intensity work: Effects on acute and late glycaemia in athletes with Type1 diabetes mellitus. Diabetic Medicine. 2011;28(7):824–832. doi: 10.1111/j.1464-5491.2011.03274.x [DOI] [PubMed] [Google Scholar]
- 50. Zaharieva D, Yavelberg L, Jamnik V, Cinar A, Turksoy K, Riddell MC. The effects of basal insulin suspension at the start of exercise on blood glucose levels during continuous versus circuit-based exercise in individuals with type 1 diabetes on continuous subcutaneous insulin infusion. Diabetes Technology and Therapeutics. 2017;19(6):370–378. doi: 10.1089/dia.2017.0010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dubé MC, Lavoie C, John Weisnagel S. Glucose or intermittent high-intensity exercise in glargine/glulisine users with T1DM. Medicine and Science in Sports and Exercise. 2013;45(1):3–7. doi: 10.1249/MSS.0b013e31826c6ad3 [DOI] [PubMed] [Google Scholar]
- 52. Ahlborg G, Felig P, Hagenfeldt L, Hendler R, Wahren J. Substrate turnover during prolonged exercise in man. Splanchnic and leg metabolism of glucose, free fatty acids, and amino acids. Journal of Clinical Investigation. 1974;53(4):1080–1090. doi: 10.1172/JCI107645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Guelfi K, Jones T, Fournier P. Intermittent high-intensity exercise does not increase the risk of early postexercise hypoglycemia in individuals with type 1 diabetes. Diabetes Care. 2005;28(2):416–418 3p. doi: 10.2337/diacare.28.2.416 [DOI] [PubMed] [Google Scholar]
- 54. Jaggers JR, King KM, Watson SE, Wintergerst KA. Predicting Nocturnal Hypoglycemia with Measures of Physical Activity Intensity in Adolescent Athletes with Type 1 Diabetes. Diabetes Technology and Therapeutics. 2019;21(7):406–408. doi: 10.1089/dia.2019.0048 [DOI] [PubMed] [Google Scholar]
- 55. Gomez AM, Gomez C, Aschner P, Veloza A, Munoz O, Rubio C, et al. Effects of performing morning versus afternoon exercise on glycemic control and hypoglycemia frequency in type 1 diabetes patients on sensor-augmented insulin pump therapy. Journal of Diabetes Science and Technology. 2015;9(3):619–624. doi: 10.1177/1932296814566233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Villaverde AF, Barreiro A, Papachristodoulou A. Structural Identifiability of Dynamic Systems Biology Models. PLoS Computational Biology. 2016;12(10):1–22. doi: 10.1371/journal.pcbi.1005153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xie J. Simglucose v0.2.1; 2018. Available from: https://github.com/jxx123/simglucose.
- 58. Kanderian SS, Weinzimer S, Voskanyan G, Steil GM. Identification of intraday metabolic profiles during closed-loop glucose control in individuals with type 1 diabetes. Journal of Diabetes Science and Technology. 2009;3(5):1047–1057. doi: 10.1177/193229680900300508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Romijn JA, Coyle EF, Sidossis LS, Gastaldelli A, Horowitz JF, Endert E, et al. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. The American Journal of Physiology. 1993;265(3p1):380–391. [DOI] [PubMed] [Google Scholar]
- 60. Van Loon LJC, Greenhaff PL, Constantin-Teodosiu D, Saris WHM, Wagenmakers AJM. The effects of increasing exercise intensity on muscle fuel utilisation in humans. Journal of Physiology. 2001;536(1):295–304. doi: 10.1111/j.1469-7793.2001.00295.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Shetty VB, Fournier PA, Davey RJ, Retterath AJ, Paramalingam N, Roby HC, et al. Effect of exercise intensity on glucose requirements to maintain euglycemia during exercise in type 1 diabetes. Journal of Clinical Endocrinology and Metabolism. 2016;101(3):972–980. doi: 10.1210/jc.2015-4026 [DOI] [PubMed] [Google Scholar]
- 62. Petersen KF, Price TB, Bergeron R. Regulation of net hepatic glycogenolysis and gluconeogenesis during exercise: Impact of type 1 diabetes. Journal of Clinical Endocrinology and Metabolism. 2004;89(9):4656–4664. doi: 10.1210/jc.2004-0408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Riddell MC, Perkins BA. Type 1 diabetes and vigorous exercise: Applications of exercise physiology to patient management. Canadian Journal of Diabetes. 2006;30(1):63–71. doi: 10.1016/S1499-2671(06)01010-0 [DOI] [Google Scholar]
- 64. Mallad A, Hinshaw L, Schiavon M, Man CD, Dadlani V, Basu R, et al. Exercise effects on postprandial glucose metabolism in type 1 diabetes: A triple-tracer approach. American Journal of Physiology—Endocrinology and Metabolism. 2015;308(12):E1106–E1115. doi: 10.1152/ajpendo.00014.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Howley ET. Type of activity: resistance, aerobic and leisure versus occupational physical activity. Medicine & Science in Sports & Exercise. 2001;33(6):364–369. doi: 10.1097/00005768-200106001-00005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Definition of standard patient. Additional information on replay simulations.
(PDF)
Data Availability Statement
All data and Python source code to use the model and reproduce its calibration, validation, and all analyses in the manuscript are available from https://gitlab.com/csb.ethz/t1d-exercise-model. Our individual patient data as well as the Python source code are additionally available at the ETH Research Collection, a FAIR repository, under DOI https://doi.org/10.3929/ethz-b-000589840.