Abstract
A growing body of evidence indicates that patients with cancer who receive cytotoxic treatments (such as chemotherapy or radiotherapy) have an increased risk of accelerated physical and cognitive ageing. Furthermore, accelerated biological ageing is a suspected driving force behind many of these observed effects. In this Review, we describe the mechanisms of biological ageing and how they apply to patients with cancer. We highlight the important role of specific behavioural factors, namely stress, sleep and lifestyle-related factors such as physical activity, weight management, diet and substance use, in the accelerated ageing of patients with cancer and cancer survivors. We also present a framework of how modifiable behaviours could operate to either increase the risk of accelerated ageing, provide protection, or promote resilience at both the biological level and in terms of patient-reported outcomes.
The physical and cognitive changes seen in a proportion of patients during and/or after receiving treatment for cancer have led to the hypothesis that certain malignancies and/or treatments might accelerate the ageing process1-9. Chemotherapy and radiotherapy are two examples of toxic exposures that could drive the increased risks of both physical and cognitive impairments (such as fatigue and memory complaints10-14), secondary morbidities and mortality1,2,9. Biological changes induced by cancer itself and by other treatment exposures (such as hormone therapy or immunotherapy) might also have a role15,16. Given the mounting evidence that cancer and cancer treatments might accelerate ageing in various ways, we consider what role behavioural factors could have in modifying these processes. We begin with a definition of ageing and a summary of the available evidence that cancer treatments accelerate this process, focusing on physical, cognitive and biological ageing. We then provide an overview of the evidence that biological ageing can be influenced by behaviour, with an emphasis on psychological stress and sleep disruptions, as well as a brief discussion of the roles of established protective health behaviours such as physical activity, weight management, healthy diet, moderate alcohol consumption and smoking cessation. Finally, we outline our model of biobehavioural modifiers of cancer and accelerated ageing and discuss possible avenues for future research and interventions.
Physical and cognitive ageing
Patterns of normative ageing vary across the population, with an increasing prevalence of physical and cognitive manifestations emerging as individuals reach a higher chronological age. Broadly, physical signs of ageing are characterized by common impairments that begin to interfere with day-to-day function, including increasing pain and fatigue, decreased mobility, declining strength, slowed gait speed and muscle loss17,18. Cognitive changes are also common in normative ageing, including poorer recall, short-term memory complaints, difficulties with maintaining concentration and working memory, slowed psychomotor speed, and impaired executive function19. These numerous manifestations of physical and cognitive ageing often co-occur and accumulate progressively with increasing chronological age, with the end stage of this process of deficit accumulation being described as a state of frailty1,20,21. Many of these same physical and cognitive effects are also commonly reported by both patients with cancer and cancer survivors as symptoms, including pain, fatigue and cognitive disturbances8,22,23. Importantly, these symptoms often occur at a higher frequency and/or at an earlier chronological age in those with ongoing or previous cancer compared to those without a cancer diagnosis, with substantial detrimental effects on quality of life2,13-15,24-30. In parallel with increased fatigue and declines in cognition, many patients with cancer and cancer survivors have declines in physical function after treatment as indicated by limitations in the ability to perform physical activities31,32, decreased mobility and impaired muscle strength3,6. Adult recipients of bone marrow transplants have an increased risk of early development of metabolic and cardiovascular disease, physical performance deficits, and premature death33,34. Similar to adults, childhood-cancer survivors can have an earlier onset of comorbidities several decades after completion of treatment, such as heart disease, lung disease, renal dysfunction, diabetes and secondary cancers, at incidences usually seen only among much older adults2,6,35,36. In cancer survivors, these changes are increasingly being conceptualized as a progressive loss of function or deficit accumulation and frailty37-39. Such changes in physical and cognitive function have been observed with several different types of cancer treatment, including radiotherapy, chemotherapy, targeted therapy and immunotherapies such as immune-checkpoint inhibitors40-45.
Biological ageing
Biological ageing is broadly defined as a gradual deterioration of tissue and cellular function as a direct result of damage accumulation over time. This damage and the failure of the related repair and/or clearance mechanisms are thought to be crucial drivers of ageing. Key hallmarks of biological ageing have been described, with general acceptance that this process begins with damage, commonly arising from inflammatory and/or oxidative metabolic sources, which affects several cellular structures, including DNA, lipids and proteins46,47. The consequences of such damage include genomic instability, telomere shortening, epigenetic alterations and a loss of proteostasis46,47. The functional effects of this damage include altered nutrient sensing and energy production, cellular replication arrest and senescence, and compromised mitochondrial performance46,47. Stem cells might replenish failing cells when supply permits, although depletion of or damage to stem cells from chemotherapy or radiation injury may lead to stem cell exhaustion46,47. As a cell accumulates damage, compromised performance can accelerate the phenotypic transition towards a state of senescence. This cellular senescence can be protective in that it prevents the replication of cells that harbour deleterious mutations that confer an increased cancer risk, yet the accumulation of these senescent cells is also thought to promote ageing48.
Senescent cells can act as a major source of inflammatory mediators, termed the senescence-associated secretory phenotype (SASP), and are thought to be a major source of chronic, low-grade inflammation often described as ‘inflammaging’49. SASP profiles probably vary across different cell types but often include common inflammatory mediators, such as damage-associated molecular patterns (DAMPs), that are known to be released from damaged and/or necrotic cells and the upregulation of an inflammatory intracellular signalling cascade (notably the transcription factor NF-κB50), resulting in the secretion of pro-inflammatory cytokines such as IL-6, IL-8, TNF and intracellular adhesion molecule 1. This inflammatory cascade has been implicated in several ageing-related diseases, including cancer, cardiovascular diseases, dementia, arthritis, osteoporosis, sarcopenia and immune compromise46,51-60. Reductions in chronic inflammation and the partial recovery of physical functions have been observed upon removal of senescent cells, suggesting that senescent cells are a major cause of the physical decline observed with ageing61-66. However, the removal of senescent cells might also have a cost and this possibility needs to be investigated further. Indeed, several lines of research indicate that senescent cells have roles in tissue remodelling and regeneration as well as in the maintenance of blood — tissue barriers and that their removal might impair these important cellular processes67-69. In summary, the gradual accumulation of damage arising from energy consumption and various exposures drives biological ageing, cellular senescence, inflammation, and the failure of organs and/or physiological systems (such as the cardiovascular system).
Cancer treatments and ageing
Cancer treatments are a source of cellular damage that can contribute to accelerated biological ageing. Chemotherapy and radiotherapy can both act on key mechanisms that are known to influence the signalling pathways that regulate biological ageing (reviewed in detail elsewhere9). In brief, the effectiveness of radiotherapy is dependent on causing DNA damage and the subsequent death of cancer cells during replication but might also involve the initiation of other downstream processes such as a localized inflammatory response that promotes the recruitment of immune cells to the tumour70. The non-malignant tissues surrounding a tumour are also likely to be altered by radiation exposure70. In preclinical models, whole-body irradiation leads to DNA damage71 and senescent cell accumulation66 as well as to secondary inflammation arising from the accumulation of senescent cells and tissue injury. Research involving animal models has also provided evidence that systemic chemotherapies (such as anthracyclines and taxanes) promote DNA damage, which induces systemic cellular senescence as indicated by the cellular expression of p16INK4a (REF.65). Mild exposure to DNA-damaging agents might promote stress resistance72, whereas excessive exposure is likely to contribute to the accumulation of damaged cells. Targeted therapies, including hormone receptor antagonists and immune-checkpoint inhibitors, can also activate key ageing-related pathways such as those involved in DNA damage and repair, cellular senescence, and maintenance of telomere length9.
Preliminary evidence from patients with cancer also suggests a role of cancer treatments in driving cellular senescence, with initial data suggesting greater levels of cellular ageing after exposure to cancer therapy, including increased expression of p16INK4a in T cells following chemotherapy in patients with breast cancer73,74. Similarly, we have observed increases in both epigenetic age and in cellular senescence immediately after chemotherapy and/or radiotherapy in patients with breast cancer75. We have also observed increased DNA damage and decreased telomerase activity in women with breast cancer 3–6 years after treatment with chemotherapy and/or radiotherapy, suggesting a lasting imprint on ageing biology76. Parallel findings have been observed in patients following autologous or allogeneic haematopoietic stem cell transplantation, with elevated cellular senescence observed after both 6 months and 2–9 years5. Similarly, data from patients with head and neck cancer demonstrate that treatment-related symptoms are correlated with greater epigenetic age and inferior overall survival outcomes77. Targeted therapies, including signal transduction inhibitors, immunotherapies and monoclonal antibodies78, can also have lasting complications and toxicities40-45. Although beyond the scope of this Review, further investigation of how these approaches might influence biological and physical ageing is warranted; this will require long-term follow-up data.
Taken together, the available evidence supports a putative role of cancer treatments in accelerating biological ageing, with estimates ranging from 3 to 14 years of age acceleration depending on the characteristics of the patients that were studied and the types of treatment exposure73-75. However, important variations exist in the extent of vulnerability to accelerated ageing, particularly in clinical research. The research described above73-77 reveals broad variations in the extent of biological ageing within cohorts, with some individuals having no notable alterations in biological age and others having more pronounced increases in ageing markers. These observations are consistent with symptom reports, in which a quarter to a third of patients will report persistent fatigue79 and a third to half of all patients report declines in health and physical function after treatment80, suggesting the manifestation of frailty among a subgroup of cancer survivors. Similar variability is evident for cognitive health, in that not all survivors report declines in perceived cognitive function81-84 or have declines in performance on neurocognitive function tests81-84. Host-specific factors associated with an increased risk of accelerated ageing need to be identified, especially targets that are potentially amenable to intervention.
Patient-specific factors
Patients receiving cancer treatments often present with pre-existing factors that can influence both their risk of cancer and responsiveness to cancer therapy. The life history of an individual before a cancer diagnosis might include external and internal environmental exposures, psychological stress and trauma, prior episodes of depression and anxiety disorders, and personal behaviours and habits that have the potential to affect cancer development as well as the patient’s ability to tolerate certain treatments (for example, tobacco and/or alcohol use, obesity, exogenous hormone use and viral infections). A holistic assessment of all possible patient-specific factors is rarely conducted by clinical oncologists, for whom the focus is almost entirely on the tumour and its specific pathological and genomic features. The interplay between cancer treatment and patient factors should not be overlooked and probably accounts for a proportion of the tremendous variability in outcomes seen among certain subgroups of patients, even in well-controlled clinical trials.
We posit that patients with psychosocial and/or behavioural risk factors that either accelerate tissue damage and/or limit repair processes might be particularly vulnerable to the harmful effects of cancer treatments. A number of psychosocial and behavioural risk factors have been linked to accelerated biological ageing outside of the context of cancer, including early-life adversity, social determinants of health (such as access to housing, food, adequate income, health insurance), psychological stress, sleep disturbance and lifestyle-related factors (such as obesity, a sedentary lifestyle or tobacco use)85-93. Notably, several of the same behavioural risk factors are associated with both cognitive and physical symptoms in cancer survivors79,94-96.
Our model proposes that these risk factors, some of which are pre-existing social, behavioural and psychological exposures as well as concurrent or newly developing behaviours, might act as accelerants of biological ageing from treatment, thereby increasing the risks of both physical and neurocognitive effects (FIG. 1). The model builds upon previous biobehavioural models focusing on inflammation as a contributor to cancer-related symptoms and morbidity outcomes94,95 by incorporating consideration of the accumulation of senescent cells as a potentially major source of inflammation. Our Review focuses on modifiable behaviours that could be targeted for intervention, although key patient characteristics (such as early-life stress, a history of depression or anxiety, or prior substance use) might also predispose patients to adverse effects of treatment and should be considered. In particular, accumulating evidence suggests that stress, insufficient sleep and other lifestyle-related factors can all directly interact with biological ageing pathways, and we outline a research agenda designed to test the role of behavioural factors that lead to accelerated ageing and interventions that might protect against cancer-related accelerated biological ageing. This view offers the potential for intervention and improvement of treatment outcomes.
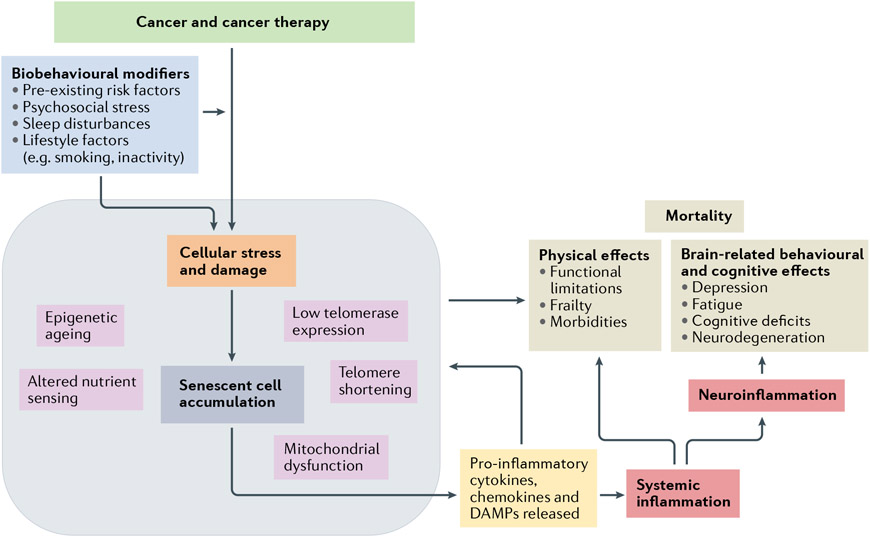
Fig. 1 ∣. Model of biobehavioural modifiers of cancer-related accelerated ageing.
In this model, several biobehavioural factors, including pre-existing risks and current psychological stress, sleep disturbances and lifestyle-related factors, along with exposure to cancer treatments, particularly chemotherapy and radiotherapy, result in cellular stress and damage276. This then leads to epigenetic ageing, telomere shortening and, if sufficient damage accumulation accrues, initiation of cellular senescence46,49. The senescent cells, which have an inflammation-biased secretome48,277, increase the degradation of nearby tissues and promote the release of inflammatory cytokines, chemokines and damage-associated molecular patterns (DAMPs), which collectively induce the secondary recruitment of inflammatory cells51,53,54,57 and lead to the further propagation of circulating inflammation and trafficking of immune cells into various tissue compartments. This gradual increase in inflammation impairs the function of several bodily systems, leading to alterations in both physical function and the ability to perform activities of daily living, such as slowing gait speed, declining muscle strength, increasing risks of frailty and an increased risk of comorbidities (such as cardiovascular disease, diabetes or osteoporosis). Inflammation can also signal across the blood–brain interface resulting in neuroinflammation, which can have both behavioural and cognitive consequences, including depression, fatigue and cognitive decline92,278,279.
Psychosocial stress and biological ageing
Psychosocial stress can be defined as any environmental demands that tax or exceed the adaptive capacity of an organism97,98. Sources of psychosocial stress include interpersonal relationships, work-related pressures, social and cultural factors such as structural racism and discrimination, exposure to violence, poverty and/or economic hardship, encountering existential threats, and numerous other negative daily life experiences. In addition to daily stress exposure, a history of early-life adversity, including stress exposure in utero, increases the risk of accelerated biological ageing as well as creating a heightened sensitivity and responsiveness to subsequent stressors99-104. Stress leads to both psychological and physiological responses, including activation of the body’s two key stress response systems, the sympathetic nervous system and the hypothalamic–pituitary–adrenal (HPA) axis, which have effects on several downstream processes mediated via the release of catecholamines and cortisol, respectively. These stress hormones might increase the risk of biological ageing through three main mechanisms: increased metabolic activity, cellular damage and activation of inflammation (FIG. 2). Catecholamine signalling results in increased release of stored energy (such as glucose and lipids) and in direct stimulation of cellular metabolic activity, which is known to produce reactive oxygen species, resulting in damage accumulation105-108. Glucocorticoids probably also have a role in the ageing process as regulators of metabolism85,109 and by decreasing telomerase activity110, thereby driving vulnerable cells towards senescence. Evidence also suggests that glucocorticoids impair DNA damage and repair pathways106. Damage within cells and in the nearby tissue microenvironment can initiate a cascade of inflammatory responses via the release of DAMPs, which are known to be elevated in ageing tissues and released from senescent cells111-113.
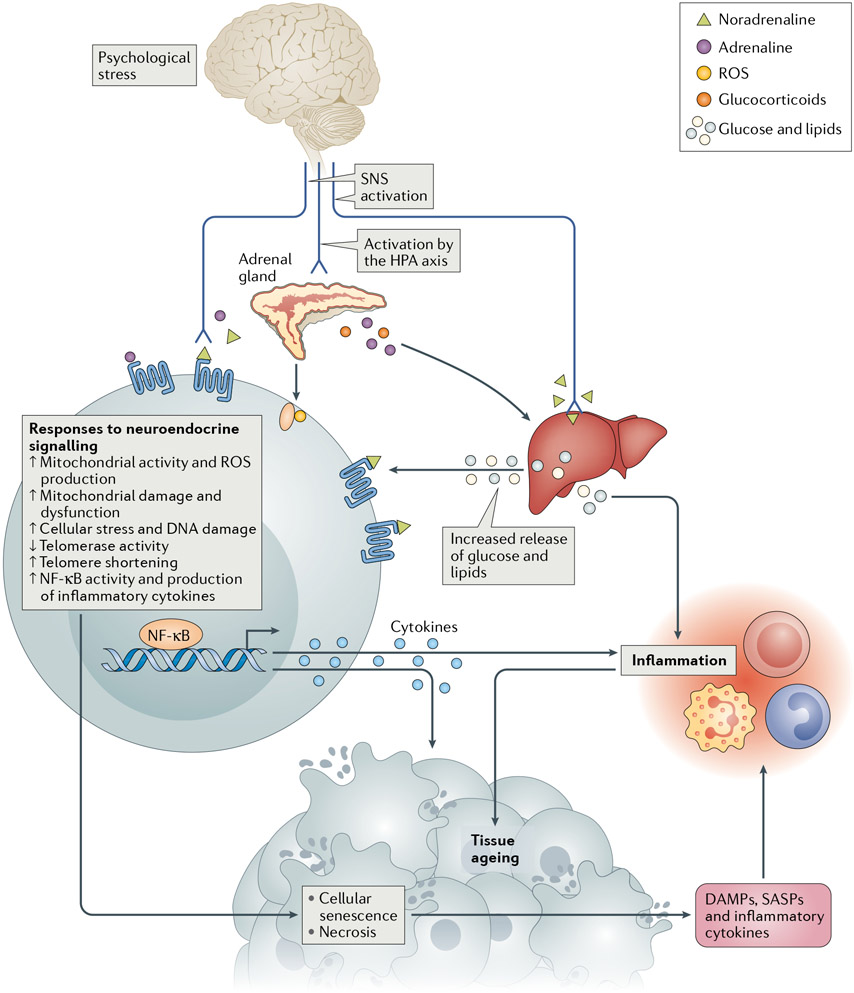
Fig. 2 ∣. Neuroendocrine-mediated pathways driving biological ageing following activation of the stress response.
The sympathetic nervous system (SNS) releases the catecholamines noradrenaline and adrenaline from nerve fibres and the adrenal gland, thus promoting the release of glucose and lipids from storage. These catecholamines also upregulate cellular metabolic activity, which produces reactive oxygen species (ROS), a source of tissue damage105-108. Damage to DNA and telomeres can lead to cellular senescence48,290. The hypothalamic–pituitary–adrenal (HPA) axis is activated during a stress response with release of glucocorticoids from the adrenal gland, thus further promoting metabolic activity85,109, leading to increased levels of glucose and lipids as well as of oxidative stress and inflammation291-293. Activation of the HPA axis also promotes decreased telomerase activity110 and impairs the function of DNA damage and repair pathways106. Damaged, necrotic and senescent cells release inflammatory signals, including damage-associated molecular patterns (DAMPs) and cytokines and chemokines that are characteristic of the senescence-associated secretory phenotype (SASP)111-113.
Stress hormones are also known to directly influence the production of pro-inflammatory cytokines by immune cells. These neuroendocrine–immune communication networks involve a distinct inflammatory pathway that can be activated by noradrenaline via catecholamine receptors expressed on immune cells, resulting in the activation of several inflammation-promoting transcription factors (such as NF-κB, AP1 and CREB)114. This pathway is reciprocally regulated by glucocorticoids, which typically have anti-inflammatory effects, although resistance to these effects can develop after prolonged exposure as is thought to occur under chronic stress114. Indeed, several studies have demonstrated increased inflammation as well as alterations in glucocorticoid resistance following both acute and chronic stress in individuals without cancer115-117. This excess inflammatory activity can then have direct effects on tissues, resulting in damage accumulation and accelerated ageing.
Animal models have also provided insights into the links between stress and biological ageing. For example, the upregulation of genes associated with the DNA damage response has been demonstrated in mice exposed to restraint stress118 and research demonstrates the ability of social defeat stress to shorten the lifespan of mice by increasing the numbers of senescent cells119. Parallel research in humans has begun to disentangle the role of stress in biological ageing, with a growing body of evidence implicating shortening of telomere length among individuals exposed to various chronic stress conditions86,120,121. Chronic stress has also been associated with other markers of biological ageing; for example, parents with high levels of perceived psychological stress have increased expression of p16INK4a in peripheral blood cells122 and data from several studies indicate associations of psychological adversity, including both early-life and adult trauma, with greater epigenetic age123-129.
We propose that individuals experiencing substantial psychological stress during and after cancer treatments might be more vulnerable to prolonged toxicities owing to the accumulation of stress-related damage and impairment of important repair mechanisms. The role of stress in the context of cancer is a crucially salient psychosocial factor given that both a cancer diagnosis and undergoing treatment pose considerable challenges to both psychological and physical wellbeing. Patients commonly experience concerns about their prognosis, the possibility of premature death, the adverse effects of adjuvant therapies, impacts on work and family life, and disruption of social ties and activities130. For some individuals, these concerns are more severe and persistent, with the potential for negative effects on both mental and physical health130. These issues are often exacerbated in individuals from socially and economically disadvantaged backgrounds and might be even more consequential among such patients131. A number of structural and institutional factors might also have important roles in the accessibility, adherence to and quality of cancer treatments received, thus limiting the potentially life-saving benefits of effective treatments132,133.
These multiple sources of stress and individual stress response patterns might directly influence biological responses to treatment and the function of recovery pathways, creating a biological environment that makes cells more vulnerable to damage accumulation and ageing. In addition, individuals diagnosed with cancer who have higher levels of childhood or lifetime stress exposure, economic or educational disadvantages, or prior episodes of depression or anxiety disorders might already be on a path towards accelerated ageing that further increases their risk of inflammation and poor outcomes after cancer therapy79,103,121,134-137. Indeed, a robust association exists between childhood adversity and inflammation and fatigue before, during and after treatment in women with breast cancer136,138-141. Similarly, greater levels of lifetime stress exposure, low socioeconomic status and a history of depression are all associated with cancer-related fatigue79 and declines in cognitive function8,142-144. Further research is warranted to examine other relevant pathways that might be altered during psychological stress in patients with cancer and in cancer survivors, including alterations in nutrient sensing and mitochondrial dysfunction108. Regardless, stress and the subsequent activation of neuroendocrine signalling pathways might be important targets both for interventions designed to improve psychological wellbeing (by reducing the severity of symptoms such as depression and anxiety) and in buffering the effects of treatment on physical and cellular ageing.
Sleep and biological ageing
Sleep is a state of rest, during which the body enters a period of relaxation and brain function is altered, with reduced levels of alertness and consciousness. This state is reached typically during a circadian 24-hour cycle. In humans, precise sleep requirements vary across individuals, although the optimal quantity of sleep for most adults is thought to be 7–8 hours of nocturnal sleep145. Sleep is thought to provide the body sufficient time to restore energy after daytime activity. This long-held concept in sleep science can be extended to include restoration at the biological level. In waking hours, the body is responding to repeated demands that require energy consumption, often with minimal time to rest and restore. Biologically speaking, these demands require metabolic activity that incurs damage and the accumulation of waste products that require repair and/or removal146. Theoretically, therefore, sleep provides the body with time to repair and restore the system to a healthy state. Inadequate sleep might lead to damage accumulation and a gradual deterioration in cellular function as seen in biological ageing.
This theory of the utility of sleep is grounded in evidence from the past few years that sleep deprivation and sleep fragmentation accelerate ageing. In rats, chronic sleep fragmentation promotes the accumulation of senescent cells90 and sleep deprivation contributes to increased cellular stress and accumulation of unfolded protiens147. Paralleling these observations, the clearance of waste products, including amyloid plaques in the brain, is significantly enhanced during deep sleep148,149, suggesting that restrictions to sleep might compromise brain clearance of waste and contribute to brain ageing. In an experimental model of sleep restriction to 4 hours at night in older adults (61–86 years of age), we observed increased expression of genes associated with the DNA damage response and with a SASP profile, as well as greater expression of p16INK4a following a night of partial sleep deprivation89. Cross-sectional data have also linked sleep disturbances and insufficient sleep to shorter telomere length150-159 and older epigenetic age88. Similar to psychological stress, sleep influences inflammation160, with experimental and cross-sectional evidence documenting that insufficient sleep increases cellular and systemic inflammatory activity161.
Sleep difficulties are highly salient in the context of cancer and its treatments. Sleep quality is often compromised in patients with cancer, with estimates suggesting that 30–70% of patients have sleep disturbances prior to and/or during treatment for cancer162 and that those with sleep disturbances have an elevated risk of depression, fatigue, cognitive problems and earlier death163-167. Many cancer survivors also report sustained sleeping difficulties after completion of treatment, with as many as half having persistent reductions in sleep quality167-170. We propose that sleep disturbances occurring during and after treatment might lead to accelerated biological ageing and reduce the extent of damaged tissue repair, thus impairing recovery. By contrast, good quality sleep might reduce the risk of treatment-related damage and accelerated biological ageing. Thus, sleep problems during and after cancer treatments might be a particularly important modifiable factor in patients receiving treatment for cancer who seek to avoid treatment-related accelerated ageing.
Health behaviours
In addition to reducing stress and improving sleep, interventions targeting several other modifiable behaviours (such as physical inactivity, unhealthy diet and substance use) could protect the body from accelerated ageing in the context of cancer treatments and patient recovery. These include increased physical activity (adjusted according to performance status), maintenance of a healthy body weight, adequate nutrient intake and limited substance use. Extensive recommendations have been published outlining the importance of diet, physical activity, weight management and avoidance of substance use171. A large, established body of literature on these topics already exists; therefore, we focus here on highlighting the relevance of these behaviours in the context of accelerated ageing in patients with cancer.
Physical inactivity.
Physical inactivity is associated with an increased risk of the cognitive and physical declines seen with ageing. Accordingly, sedentary lifestyles are associated with accelerated biological ageing92,172,173 and increased physical activity seems to be protective93,174,175, although excessive and/or inappropriate exercise might be damaging176. The biological ageing pathways involved in this effect include inflammation, dysfunctional metabolism and poor energy utilization, greater cellular damage and accumulated waste, and more accumulation of senescent cells93,177. Therefore, engaging in regular physical activity is potentially an important behavioural modification that has shown improvements in strength and mental wellbeing178,179 as well as initial evidence of reductions in cellular senescence180; likewise, it could substantially improve ageing outcomes in patients with cancer. Inactivity has also been linked with inferior cancer-related outcomes, including an increased risk of disease recurrence and earlier mortality181-183, as well as increased behavioural symptoms among patients with cancer, including fatigue95, poor cognitive function184,185 and mood disturbances186-188.
Obesity.
Several lines of research demonstrate that individuals who are obese also have signs of accelerated ageing, including shortened telomere length and a greater degree of epigenetic ageing92,189-192. Whether obesity accelerates biological ageing or accelerated ageing increases the propensity to accumulate adipose tissue is not fully defined. However, research in animal models suggests that the induction of obesity using high-fat diets promotes the accumulation of senescent cells193, whereas the elimination of senescent cells in obese mice reversed the metabolic syndrome64. Reduced caloric intake has been shown to alter several metabolic parameters and slows biological ageing in preclinical models194,195, although evidence supporting the efficacy of caloric restriction in humans is growing yet currently inconclusive196,197. Components of the Mediterranean diet, such as high levels of fibre, healthier fats and antioxidants, are thought to prevent cellular damage198, thereby protecting from biological ageing. Further research, including human intervention trials, is needed to understand what dietary factors might lead to accelerated ageing. Thus, a combination of poor diet and obesity might be particularly detrimental to long-term health outcomes and lead to accelerated ageing.
Obesity is a risk factor for both inferior cancer outcomes and the development of secondary cancers199,200 and has also been shown to increase the risk of physical manifestations of ageing such as fatigue201. Weight management is therefore a behavioural target that could protect patients with cancer from accelerated ageing. Further research examining this pathway is needed and some studies that primarily focus on physical activity are currently under way (for example, NCT01635413)202. For example, the identification of weight management interventions that are most effective in protecting against accelerated ageing in patients with cancer might differ from those that are most effective in non-cancer populations.
Alcohol consumption and tobacco use.
Both of these exposures are associated with an increased risk of several cancers, primarily owing to genotoxic effects203-207. Excess blood alcohol levels lead to increased inflammation and tissue damage, including through the accumulation of metabolites (such as acetaldehyde) in the liver208. Evidence of accelerated ageing among heavy drinkers is mixed, with a number of studies linking high alcohol consumption with an older epigenetic age209 and shortened telomere length208,210, whereas such associations have not been observed in others211. Inhaling smoke from cigarettes and other related products can trigger damage in lung tissues, leading to inflammation and oxidative stress206,207. Indeed, a positive correlation exists between pack years of smoking and accelerated biological ageing as demonstrated by DNA methylation-based biomarkers of age92,209,212,213 and shortened telomere length211,214-216. These behaviours are also potential accelerants of biological ageing in patients with cancer and should continue to be important targets of both public health and clinician-led interventions.
Interventions
Intriguing evidence from preclinical models raises the possibility that biological ageing and, therefore, the related physical manifestations of ageing can be remedied using interventions that target the removal of senescent cells. Indeed, clinical trials testing the efficacy of senolytic agents in reversing cancer treatment-related ageing are currently under way217,218. Importantly, as outlined earlier in this Review, behavioural factors can also influence biological ageing and interventions targeting these processes offer considerable promise. Here, we outline and highlight several relationships between these aspects that could be considered targets for intervention (FIG. 3).
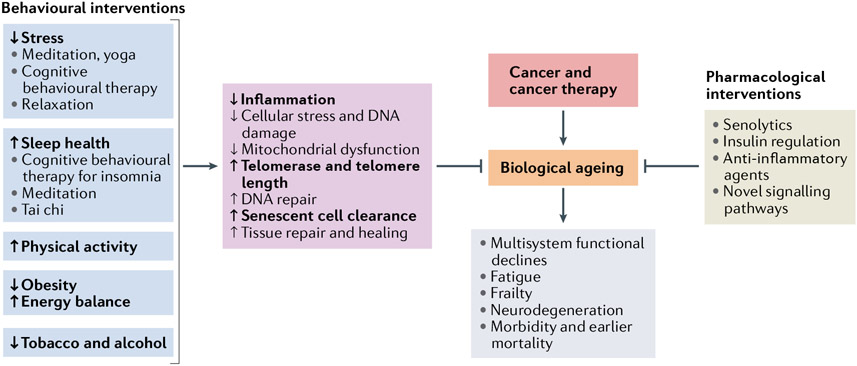
Fig. 3 ∣. Interventions proposed to inhibit the effects of cancer treatments on biological ageing and to modify long-term physical and cognitive health.
Patient-specific biobehavioral factors can influence biological ageing processes and are potential targets for interventions designed to slow or reverse the effects of cancer and its treatments on these outcomes. A variety of interventions targeting these host factors may act directly on biological ageing pathways such as inflammation, cellular stress, mitochondrial function, the telomere maintenance system, repair pathways and cellular senescence. As cancer and cancer therapies are thought to act as accelerators of biological ageing, these modifiable factors might also be potential targets for interventions designed to slow or reverse the effects of cancer and its treatments. Biobehavioural interventions targeting stress, sleep health, physical activity, obesity and substance use have been demonstrated to have beneficial effects on inflammation and some ageing biomarkers (highlighted with bold font), including empirical support for cognitive behavioural therapy, mind–body interventions, and exercise, dietary and substance use interventions. All of these interventions could then have implications for survivorship risk, including the risks of functional decline, fatigue, frailty, neurodegeneration, and early morbidities and mortality. Alternatively, pharmacological agents, such as senolytics or anti-inflammatory agents, might be able to directly reverse or modify the effects of biological ageing.
Targeting stress
Stress reduction might help to slow or reverse ageing. The first option for reducing stress is removal of the stressor, which is sometimes feasible but often not. A second option would be to block the neuroendocrine mediators driving the intracellular changes, for example, through administration of β2-adrenoceptor antagonists (β-blockers). Several clinical trials examining the efficacy of this approach, specifically for delaying disease progression and the recurrence of cancer, are currently under way, with promising preliminary results pointing to reductions in metastasis potential and risk of disease recurrence219-223. These trials might be well positioned to also investigate the effects of β-adrenergic blockade on patient-reported outcomes and on the hallmark features of biological ageing. A third option is to alter patients’ psychological and physiological responses to the stressors using behavioural interventions that target the cognitive, behavioural and/or biological processes associated with the stress response, with the goal of reducing the frequency and intensity of this response pattern. Importantly, several effective behavioural interventions have been demonstrated to reduce stress both in patients with cancer and in cancer survivors, including cognitive behavioural therapies and mind–body approaches224-226. These interventions can also have effects on ageing-related biological processes in patients with cancer. For example, cognitive behavioural stress management has been shown to reduce levels of anxiety and expression of pro-inflammatory genes in women with breast cancer227. Furthermore, intervention-related changes in inflammatory and antiviral gene expression predicted a lower risk of breast cancer recurrence in this study, although the sample size was small228. Mindfulness interventions have also been shown to reduce levels of stress and depression and to improve wellbeing in breast cancer survivors while also leading to reductions in inflammation-related gene expression229,230. Furthermore, regular meditation has been proposed to influence other aspects of biological ageing, including telomere length and the epigenetic clock109,231. Yoga interventions have also demonstrated beneficial effects on ageing-related symptoms (such as fatigue or cognitive complaints) as well as inflammatory processes in breast cancer survivors232-234. Thus, interventions designed to reduce levels of stress might be a method of promoting resilience to both biological and physical ageing.
Treating sleep disturbances
Sleep disturbances can be addressed using one of several established treatment modalities. The gold standard of cognitive behavioural therapy for insomnia (CBT-I) has been demonstrated to be effective in cancer survivors235,236. Several new methods of accessing this therapy have been developed, including online administration, with similar efficacy237. In addition, mind–body therapies, including tai chi238, mindfulness training229,239,240 and yoga241, have been shown to ameliorate sleep disturbances both in patients with cancer and in cancer survivors. Initial results suggest that acupuncture might also provide benefit for cancer survivors with insomnia, although the overall effectiveness of this intervention was lower than with CBT-I242. Notably, many of the interventions targeting insomnia also resulted in improvements in other symptoms, including fatigue and cognitive function83,235,236,241. These interventions have been efficacious in reducing levels of inflammation among those with insomnia243,244, thus further highlighting the benefits of addressing sleep disturbances in patients with cancer.
Pharmaceutical treatments for sleep disturbances, which are typically less effective than cognitive behavioural therapies, are often desired by patients seeking temporary relief from acute symptoms and/or during a vital window for optimal healing (such as after surgery, radiotherapy or chemotherapy). However, such treatments are associated with declining efficacy with long-term use and high secondary health costs (such as dependency and a risk of falls for older adults)162,166,245. Novel data are beginning to demonstrate the crucial role of deep sleep for maintaining brain health and might lead to the development of novel targeted strategies that assist in maintaining deep sleep, such as auditory closed loop stimulation246, which might in turn offer long-term amelioration of biological ageing in patients with cancer and cancer survivors. The availability of multiple effective treatments for sleep disturbances and the high prevalence of sleep difficulties both in patients with cancer and in cancer survivors, many of whom have ongoing sleep problems and are not offered remedies162,166, make sleep an important target for clinical cancer care247,248.
Intervening in health behaviours
A large body of literature exists on the importance of intervening in negative health behaviours in the general population, with existing recommendations available for increasing physical activity249, reducing obesity250 and substance use251-253. Interventions targeting these aspects have demonstrated beneficial effects in cancer survivors254-257 and evidence from the past decade suggests that these interventions also directly modify biomarkers of ageing172,175,177,258,259.
Increasing exercise.
Exercise can potentially have a number of health benefits, including reducing stress, improving sleep quality, boosting mood, improving muscle strength and tone, and enhancing cardiovascular and lung health178,260. Therefore, physical activity is an important target for cancer prevention and control183,259 as well as for improving quality of life and ageing outcomes184,186,256,257 and should not be overlooked. Physical activity interventions not only have behavioural and health benefits but can also have positive effects on ageing-related biomarkers175,261, making this an excellent behavioural target for intervention. Maintaining an active lifestyle during and after cancer treatment can be challenging, although patients should be encouraged to maintain levels of activity in order to prevent and/or reduce the severity of fatigue and other adverse effects of cancer treatment and to avoid a loss of mobility262. Improving the level of physical activity is particularly important for patients with sedentary lifestyles, with several interventions having shown efficacy, including those with tailored goals for individual patients263, and increased accessibility owing to the use of remote instruction and/or smart devices264. Older cancer survivors might be more amenable to interventions such as gardening, which has the potential to increase both mobility and promote the consumption of fruits and vegetables265.
Targeting energy balance.
Improving metabolic function might have the added benefits of helping to heal treatment-related cellular damage and protecting against cancer-related accelerated ageing266. Indeed, interventions targeting weight management, a healthy diet and physical activity might be beneficial across the cancer care continuum254. Changes in the timing and/or frequency of caloric intake, such as caloric restriction and intermittent fasting, also seem to modify certain mechanisms of ageing267-269, including pathways involved in tumour growth (such as PI3K–AKT–mTOR signalling)270, which can now be inhibited with targeted therapies270. Thus, diets that are thought to be protective against cancer also seem to ameliorate certain aspects of ageing, although the effects might vary according to the age of the organism; therefore, further research is needed to understand whether the effects of dietary interventions also differ by age in humans271,272. Nonetheless, interventions that target obesity and energy balance might prove beneficial in preventing or slowing ageing after cancer treatments. Diet might also be crucial in maintaining a healthy gut microbiota and future research should consider the role that the microbiota might have in resistance and resilience during and after cancer treatments176. Further research is also needed to understand the relevance of specific diets on the effects of cancer treatments and to provide clear recommendations on the optimal energy balance and nutrient intake during both treatment and recovery.
Reducing alcohol consumption and tobacco use.
This remains a crucial target of intervention for cancer prevention and control that not only reduces the risk of primary cancer but could also potentially reduce the risk of poor treatment outcomes, secondary cancers and future morbidities252,253. The modification of these factors might also have major implications for efforts to ameliorate accelerated ageing in patients with cancer and future research should consider the direct effects of changes in alcohol consumption and tobacco use on markers of biological ageing. Existing guidelines on the use of several intervention strategies are available251,273-275 and other methods of reducing the severity of cravings or other withdrawal symptoms include cognitive behavioural therapy, pharmacological treatments and support groups, all of which have resulted in a certain level of success in the general population.
Pharmacological interventions.
A number of promising drugs that target biological ageing are currently in development and several have entered clinical trials (NCT04815902, NCT04063124, NCT04210986, NCT04733534, NCT02848131, NCT04770064 and NCT04313634). A few agents worth highlighting here include senolytic agents and anti-inflammatory drugs62,197,276. In brief, senolytic agents target and degrade senescent cells as a means of reducing a source of both inflammation and impaired tissue function. Animal models of senolytic agents suggest an improved overall healthy lifespan (referred to as healthspan) and lifespan, presenting an extremely promising approach to the treatment of accelerated biological ageing217. In addition to senolytic agents, anti-inflammatory agents that specifically target the regulatory pathways that promote the SASP might also provide benefit in terms of reducing both symptoms and ageing53. A detailed overview of the mechanisms of action of these agents is provided elsewhere62,276.
Future research priorities
Given the growing evidence linking behavioural factors with ageing, future research priorities should include a focus on characterizing the crucial role of these behaviours as a potential target to alter treatment-related accelerated biological ageing in patients with cancer and cancer survivors. Behavioural interventions might be beneficial at several points of the cancer trajectory; therefore, timing is likely to be an important consideration for the design of such interventions. The optimal timing of these targeted interventions will vary depending on a number of factors that clinicians and researchers will need to consider, including pre-existing patient-specific factors, interactions with treatment regimens and the time point at which these behavioural factors are most relevant (FIG. 3).
For interventions delivered at the time of diagnosis, clinicians will need to consider patient-specific factors and behaviours that might have put the patient at risk of cancer (such as tobacco and/or alcohol use or obesity) and directly address them as part of the cancer treatment plan (for example, encouraging tobacco use cessation). Certain interventions (such as exercise) might also increase the efficacy of treatments (such as chemotherapy277,278) and improve survival outcomes279. Screening for past and/or current symptoms of depression can be crucial for the management of patients in this setting as diagnosis and treatment might trigger a new episode that could interfere with treatment adherence279. Stress levels and sleep in particular might be affected at the time of diagnosis owing to considerable existential anxiety regarding the upcoming treatments and their probable outcomes280-284.
Owing to the known benefits of regular high-quality sleep, successful interventions for insomnia282 could, theoretically, be particularly beneficial as a means of clearing waste products (particularly in the brain)285,286, repairing damage caused by therapy, and/or aiding the body in healing tissue injury146. However, no research to date has tested this hypothesis. Likewise, in patients with circadian disruptions caused by surgery, light therapy could be delivered immediately after surgery to support the recovery of circadian alignment283,284. Several studies have also demonstrated substantial changes in sleep patterns in patients receiving chemotherapy287 and data demonstrate the feasibility and preliminary effectiveness of a brief CBT-I intervention administered during chemotherapy appointments288. Mindfulness, meditation-based interventions and other stress-reduction techniques have also shown benefit when delivered during treatment227,280,281,289.
After treatment completion, focused interventions designed to reduce excess fat stores by restoring a healthy energy balance through increasing levels of physical activity could assist in the prevention of a second primary cancer199. Intermittent fasting might also prove to be an important method of preventing secondary tumours and/or the growth or cancer cells not cleared by initial treatment given that this strategy alters the extent of glucose and/or insulin signalling270, which are key pathways involved in the promotion of ageing and/or tumour growth and, interestingly, are also inhibited by several novel targeted therapies (via suppression of PI3K–AKT–mTOR signalling)270. In addition, interventions targeting behavioural symptoms in cancer survivors (such as fatigue and insomnia) are crucial for preventing impairments in quality of life and further acceleration of ageing processes.
Future research priorities include the optimal timing of behavioural interventions, particularly in terms of their effectiveness in restoring treatment-related biological damage and providing protection from accelerated ageing, as well as considering the timing of delivery given the opportunity for a teachable moment and access to services. In particular, research tracking biobehavioural factors in relation to the physical and biological effects of cancer and the various available treatments is needed. Several important and commonly seen adverse ageing outcomes exist and might be amenable to behavioural intervention (FIG. 3). These modifiable outcomes include levels of biological ageing markers, physical functional measures, cognitive and mental health, new morbidities and secondary cancers, frailty, and early mortality. Cancer is primarily a disease of older individuals (those >65 years of age); therefore, addressing relevant behaviours primarily in this population will probably contribute to more immediate improved health and wellbeing. Moreover, adoption of these behaviours will probably also contribute to a reduction in disease burden or symptoms from other concomitant chronic diseases (such as diabetes and cardiovascular disease). By contrast, for children and young adults who have survived cancer, whose lifespan is considerably longer, focusing on biobehavioural interventions has the potential to influence accelerated ageing and is likely to yield considerable benefit in terms of reducing the risk of secondary primary cancers and other chronic health problems that might not emerge until 2–3 decades later266. For these individuals, the prevention pay-off might be substantial. By targeting these potential behavioural modifiers across the cancer continuum, the potential exists to substantially improve the healthy lifespan and longevity of cancer survivors of any age.
Conclusions
In summary, this Review has outlined the growing literature linking cancer and its treatments to accelerated physical, cognitive and biological ageing and extended this model to include several important behavioural modifiers that might ameliorate these patterns of ageing. Key behaviours that are known to affect ageing biology in individuals without cancer include perceived stress, sleep disturbances and insomnia, a sedentary lifestyle, adiposity, poor diet, and substance use. Herein, we propose that these factors might also act as modifiers in the context of cancer and lead to accelerated ageing. Intervening to reduce stress, improve sleep health, increase physical activity, manage weight, and/or reduce alcohol and tobacco use could all prove beneficial for the long-term healthspan and lifespan of patients and survivors by directly altering biological ageing patterns. We propose several directions for future research designed to carefully determine actionable targets for interventions.
Key points.
Cancer and its treatments are thought to promote accelerated biological ageing, leading to adverse cognitive, behavioural and functional outcomes in cancer survivors.
Modifiable host-specific factors are known to affect ageing biology in individuals without cancer, including psychosocial stress, poor sleep, physical inactivity, obesity, and tobacco and alcohol use.
Behavioural interventions and/or modifications targeting these host factors might directly alter biological ageing processes in patients with cancer and cancer survivors, thereby improving both healthspan and lifespan.
We propose that these host factors be considered in models of cancer-related age acceleration and that interventions designed to reduce stress, improve sleep health, increase physical activity, manage weight, and/or reduce alcohol and tobacco use be investigated as promising approaches to address accelerated ageing in this context.
Acknowledgements
The work of J.E.C. was supported in part by the American Cancer Society Research Scholars grant 128660-RSG-15-187-01-PCsM and the National Cancer Institute at the National Institutes of Health grant R01CA237535 and R35CA197289. The work of J.E.B. was supported in part by the National Cancer Institute at the National Institutes of Health grant R01CA237535 and the Breast Cancer Research Foundation. The work of P.A.G. was supported by the Breast Cancer Research Foundation and the author notes that she serves on the Scientific Advisory Board of the Breast Cancer Research Foundation.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Guida JL et al. Measuring aging and identifying aging phenotypes in cancer survivors. J. Natl Cancer Inst 111, 1245–1254 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henderson TO, Ness KK & Cohen HJ Accelerated aging among cancer survivors: from pediatrics to geriatrics. Am. Soc. Clin. Oncol. Educ. B 34, e423–e430 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Maccormick RE Possible acceleration of aging by adjuvant chemotherapy: a cause of early onset frailty? Med. Hypotheses 67, 212–215 (2006). [DOI] [PubMed] [Google Scholar]
- 4.Bluethmann SM, Mariotto AB & Rowland JH Anticipating the “Silver Tsunami”: prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol. Biomark. Prev 25, 1029–1036 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wood WA et al. Chemotherapy and stem cell transplantation increase p16INK4a expression, a biomarker of T-cell aging. EBioMedicine 11,227–238 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ness KK et al. Frailty in childhood cancer survivors. Cancer 121, 1540–1547 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ness KK et al. Physiologic frailty as a sign of accelerated aging among adult survivors of childhood cancer: a report from the St Jude lifetime cohort study. J. Clin. Oncol 31,4496–4503 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mandelblatt JS et al. Cognitive effects of cancer and its treatments at the intersection of aging: what do we know; what do we need to know? Semin. Oncol 40, 709–725 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cupit-Link MC et al. Biology of premature ageing in survivors of cancer. ESMO Open 2, e000250 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goedendorp MM et al. Prolonged impact of chemotherapy on fatigue in breast cancer survivors: a longitudinal comparison with radiotherapy-treated breast cancer survivors and noncancer controls. Cancer 118, 3833–3841 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janelsins MC et al. Cognitive complaints in survivors of breast cancer after chemotherapy compared with age-matched controls: an analysis from a nationwide, multicenter, prospective longitudinal study. J. Clin. Oncol 35, 506–514 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bower JE et al. Fatigue in long-term breast carcinoma survivors. Cancer 106, 751–758 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Ganz PA et al. Quality of life in long-term, disease-free survivors of breast cancer: a follow-up study. J. Natl Cancer Inst 94, 39–49 (2002). [DOI] [PubMed] [Google Scholar]
- 14.Ganz PA, Rowland JH, Meyerowitz BE & Desmond KA Impact of different adjuvant therapy strategies on quality of life in breast cancer survivors. Recent Results Cancer Res. 152, 396–411 (1998). [DOI] [PubMed] [Google Scholar]
- 15.Ahles TA, Root JC & Ryan EL Cancer- and cancer treatment-associated cognitive change: an update on the state of the science. J. Clin. Oncol 30, 3675–3686 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sehl ME & Ganz PA Potential mechanisms of age acceleration caused by estrogen deprivation: do endocrine therapies carry the same risks? JNCI Cancer Spectr. 2, pky035 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan SS, Singer BD & Vaughan DE Molecular and physiological manifestations and measurement of aging in humans. Aging Cell 16, 624–633 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taffett GE in Geriatric Medicine: An Evidence-Based Approach, 4th ed. (ed. Cassel CK) 27–35 (Springer Science & Business Media, 2003). [Google Scholar]
- 19.Cabeza R, Nyberg L, Park DC Cognitive Neuroscience of Aging: Linking Cognitive and Cerebral Aging (Oxford Scholarship Online, 2009). 10.1093/acprof:oso/9780195156744.001.0001 [DOI] [Google Scholar]
- 20.Searle SD, Mitnitski A, Gahbauer EA, Gill TM & Rockwood K A standard procedure for creating a frailty index. BMC Geriatr. 8, 24 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fried LP et al. Frailty in older adults: evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci 56, M146–M156 (2001). [DOI] [PubMed] [Google Scholar]
- 22.Ganz PA et al. Cognitive complaints after breast cancer treatments: examining the relationship with neuropsychological test performance. J. Natl Cancer Inst 105, 791–801 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bower JE & Ganz PA Symptoms: fatigue and cognitive dysfunction. Adv. Exp. Med. Biol 862, 53–75 (2015). [DOI] [PubMed] [Google Scholar]
- 24.Stanton AL, Rowland JH & Ganz PA Life after diagnosis and treatment of cancer in adulthood: contributions from psychosocial oncology research. Am. Psychol 70, 159–174 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Chopra I & Kamal KM A systematic review of quality of life instruments in long-term breast cancer survivors. Health Qual. Life Outcomes 10, 14 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Howard-Anderson J, Ganz PA, Bower JE & Stanton AL Quality of life, fertility concerns, and behavioral health outcomes in younger breast cancer survivors: a systematic review. J. Natl Cancer Inst 104, 386–405 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Ahles TA & Root JC Cognitive effects of cancer and cancer treatments. Annu. Rev. Clin. Psychol 14, 425–451 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bernstein LJ, McCreath GA, Komeylian Z & Rich JB Cognitive impairment in breast cancer survivors treated with chemotherapy depends on control group type and cognitive domains assessed: a multilevel meta-analysis. Neurosci. Biobehav. Rev 83, 417–428 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Janelsins MC et al. An update on cancer- and chemotherapy-related cognitive dysfunction: current status. Semin. Oncol 38, 431–438 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lim ASP et al. Modification of the relationship of the apolipoprotein E ε4 allele to the risk of Alzheimer disease and neurofibrillary tangle density by sleep. JAMA Neurol. 70, 1544 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petrick JL et al. Functional status declines among cancer survivors: trajectory and contributing factors. J. Geriatr. Oncol 5, 359–367 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sehl M, Lu X, Silliman R & Ganz PA Decline in physical functioning in first 2 years after breast cancer diagnosis predicts 10-year survival in older women. J. CancerSurviv 7, 20–31 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arora M et al. Longitudinal trajectory of frailty in blood or marrow transplant survivors: report from the blood or marrow transplant survivor study. Cancer 127, 794–800 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baker KS, Armenian S & Bhatia S Long-term consequences of hematopoietic stem cell transplantation: current state of the science. Biol. Blood Marrow Transpl 16 (Suppl. 1), S90–S96 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oeffinger KC et al. Chronic health conditions in adult survivors of childhood cancer. N. Engl. J. Med 355, 1572–1582 (2006). [DOI] [PubMed] [Google Scholar]
- 36.Armenian SH et al. Long-term health-related outcomes in survivors of childhood cancer treated with HSCT versus conventional therapy: a report from the Bone Marrow Transplant Survivor Study (BMTSS) and Childhood Cancer Survivor Study (CCSS). Blood 118, 1413–1420 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ness KK et al. Progression of frailty in young adult survivors of childhood cancer: St. Jude Lifetime Cohort. J. Clin. Oncol 37, 10057–10057 (2019). [Google Scholar]
- 38.Bennett JA, Winters-Stone KM, Dobek J & Nail LM Frailty in older breast cancer survivors: age, prevalence, and associated factors. Oncol. Nurs. Forum 40, E126–E134 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arora M et al. Physiologic frailty in nonelderly hematopoietic cell transplantation patients. JAMA Oncol. 2, 1277 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu S & Kurzrock R Toxicity of targeted therapy: implications for response and impact of genetic polymorphisms. Cancer Treat. Rev 40, 883–891 (2014). [DOI] [PubMed] [Google Scholar]
- 41.Totzeck M, Schuler M, Stuschke M, Heusch G & Rassaf T Cardio-oncology - strategies for management of cancer-therapy related cardiovascular disease. Int. J. Cardiol 280, 163–175 (2019). [DOI] [PubMed] [Google Scholar]
- 42.Escalante CP et al. Meta-analysis of cardiovascular toxicity risks in cancer patients on selected targeted agents. Support. Care Cancer 24, 4057–4074 (2016). [DOI] [PubMed] [Google Scholar]
- 43.Joly F, Castel H, Tron L, Lange M & Vardy J Potential effect of immunotherapy agents on cognitive function in cancer patients. J. Natl Cancer Inst 112, 123–127 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cuzzubbo S et al. Neurological adverse events associated with immune checkpoint inhibitors: review of the literature. Eur. J. Cancer 73, 1–8 (2017). [DOI] [PubMed] [Google Scholar]
- 45.Weber JS, Yang JC, Atkins MB & Disis ML Toxicities of immunotherapy for the practitioner. J. Clin. Oncol 33, 2092–2099 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lopez-Otin C, Blasco MA, Partridge L, Serrano M & Kroemer G The hallmarks of aging. Cell 153, 1194–1217 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kennedy BK et al. Geroscience: linking aging to chronic disease. Cell 159, 709–713 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Campisi J Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell 120, 513–522 (2005). [DOI] [PubMed] [Google Scholar]
- 49.Franceschi C & Campisi J Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci 69 (Suppl. 1), S4–S9 (2014). [DOI] [PubMed] [Google Scholar]
- 50.Salminen A, Kauppinen A & Kaarniranta K Emerging role of NF-kB signaling in the induction of senescence-associated secretory phenotype (SASP). Cell Signal. 24, 835–845 (2012). [DOI] [PubMed] [Google Scholar]
- 51.Campisi J & d’Adda di Fagagna F Cellular senescence: when bad things happen to good cells. Nat. Rev. Mol. Cell Biol 8, 729–740 (2007). [DOI] [PubMed] [Google Scholar]
- 52.Coppé J-P et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 6, 2853–2868 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Freund A, Orjalo AV, Desprez P-Y & Campisi J Inflammatory networks during cellular senescence: causes and consequences. Trends Mol. Med 16, 238–246 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Erusalimsky JD Vascular endothelial senescence: from mechanisms to pathophysiology. J. Appl. Physiol 106, 326–332 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Effros RB The role of CD8 T cell replicative senescence in human aging. Discov. Med 5, 293–297 (2005). [PubMed] [Google Scholar]
- 56.Coppe J-P, Desprez P-Y, Krtolica A & Campisi J The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu. Rev. Pathol 5, 99–118 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu EPK & Bennett MR Mitochondrial DNA damage and atherosclerosis. Trends Endocrinol. Metab 25, 481–487 (2014). [DOI] [PubMed] [Google Scholar]
- 58.Maassen JA et al. Mitochondrial diabetes: molecular mechanisms and clinical presentation. Diabetes 53 (Suppl. 1), S103–S109 (2004). [DOI] [PubMed] [Google Scholar]
- 59.Sahin E & Depinho RA Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature 464, 520–528 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vasto S et al. Inflammation, ageing and cancer. Mech Ageing Dev. 130, 40–45 (2009). [DOI] [PubMed] [Google Scholar]
- 61.Xu M et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med 24, 1246–1256 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kirkland JL, Tchkonia T, Zhu Y, Niedernhofer LJ & Robbins PD The clinical potential of senolytic drugs. J. Am. Geriatr. Soc 65, 2297–2301 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baker DJ et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479, 232–236 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Palmer AK et al. Targeting senescent cells alleviates obesity-induced metabolic dysfunction. Aging Cell 25, e12950 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Demaria M et al. Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. Cancer Discov. 7, 165–176 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chang J et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat. Med 22, 78–83 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Elder SS & Emmerson E Senescent cells and macrophages: key players for regeneration? Open Biol. 10, 200309 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Da Silva-Álvarez S et al. Cell senescence contributes to tissue regeneration in zebrafish. Aging Cell 19, e13052 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grosse L et al. Defined p16High senescent cell types are indispensable for mouse healthspan. Cell Metab. 32, 87–99.e6 (2020). [DOI] [PubMed] [Google Scholar]
- 70.Kim JH, Jenrow KA & Brown SL Mechanisms of radiation-induced normal tissue toxicity and implications for future clinical trials. Radiat. Oncol. J 32, 103–115 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Conroy SK et al. Alterations in brain structure and function in breast cancer survivors: effect of post-chemotherapy interval and relation to oxidative DNA damage. Breast Cancer Res. Treat 137, 493–502 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ermolaeva MA et al. DNA damage in germ cells induces an innate immune response that triggers systemic stress resistance. Nature 501,416–420 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sanoff HK et al. Effect of cytotoxic chemotherapy on markers of molecular age in patients with breast cancer. J. Natl Cancer Inst 106, dju057 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shachar SS et al. Effects of breast cancer adjuvant chemotherapy regimens on expression of the aging biomarker, p16INK4a. JNCI Cancer Spectr. 4, pkaa082 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sehl ME, Carroll JE, Horvath S & Bower JE The acute effects of adjuvant radiation and chemotherapy on peripheral blood epigenetic age in early stage breast cancer patients. NPJ Breast Cancer 6, 23 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Scuric Z et al. Biomarkers of aging associated with past treatments in breast cancer survivors. NPJ Breast Cancer 3, 50 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xiao C et al. Association of epigenetic age acceleration with risk factors, survival, and quality of life in patients with head and neck cancer. Int. J. Radiat. Oncol. Biol. Phys 111, 157–167 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.National Cancer Institute. Targeted Cancer Therapies Fact Sheet - National Cancer Institute https://www.cancer.gov/about-cancer/treatment/types/targeted-therapies/targeted-therapies-fact-sheet (2020).
- 79.Bower JE The role of neuro-immune interactions in cancer-related fatigue: biobehavioral risk factors and mechanisms. Cancer 125, 353–364 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.de Moor JS et al. Cancer survivors in the United States: prevalence across the survivorship trajectory and implications for care. Cancer Epidemiol. Biomark. Prev 22, 561–570 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mandelblatt JS et al. Cancer-related cognitive outcomes among older breast cancer survivors in the thinking and living with cancer study. J. Clin. Oncol 3, JCO1800140 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bevans M et al. National Institutes of Health hematopoietic cell transplantation late effects initiative: the patient-centered outcomes working group report. Biol. Blood Marrow Transpl 23, 538–551 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jim HSL et al. Meta-analysis of cognitive functioning in breast cancer survivors previously treated with standard-dose chemotherapy. J. Clin. Oncol 30, 3578–3587 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McDougall GJ, Oliver JS & Scogin F Memory and cancer: a review of the literature. Arch. Psychiatr. Nurs 28, 180–186 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Epel ES & Lithgow GJ Stress biology and aging mechanisms: toward understanding the deep connection between adaptation to stress and longevity. J. Gerontol. A Biol. Sci. Med. Sci 69 (Suppl. 1), S10–S16 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rentscher KE, Carroll JE & Mitchell C Psychosocial stressors and telomere length: a current review of the science. Annu. Rev. Public Health 41, 223–245 (2020). [DOI] [PubMed] [Google Scholar]
- 87.Entringer S, de Punder K, Buss C & Wadhwa PD The fetal programming of telomere biology hypothesis: an update. Philos. Trans. R. Soc. B Biol. Sci 373, 20170151 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Carroll JE et al. Epigenetic aging and immune senescence in women with insomnia symptoms: findings from the Women’s Health Initiative study. Biol. Psychiatry 81, 136–144 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Carroll JE et al. Partial sleep deprivation activates the DNA damage response (DDR) and the senescence-associated secretory phenotype (SASP) in aged adult humans. Brain Behav. Immun 51, 223–229 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Carreras A et al. Chronic sleep fragmentation induces endothelial dysfunction and structural vascular changes in mice. Sleep 37, 1817–1824 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schafer MJ et al. Exercise prevents diet-induced cellular senescence in adipose tissue. Diabetes 65, 1606–1615 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Quach A et al. Epigenetic clock analysis of diet, exercise, education, and lifestyle factors. Aging 9, 419–446 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rebelo-Marques A et al. Aging hallmarks: the benefits of physical exercise. Front. Endocrinol 9, 258 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Miller AH, Ancoli-Israel S, Bower JE, Capuron L & Irwin MR Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J. Clin. Oncol 26, 971–982 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bower JE Cancer-related fatigue–mechanisms, risk factors, and treatments. Nat. Rev. Clin. Oncol 11, 597–609 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bower JE et al. Fatigue after breast cancer treatment: biobehavioral predictors of fatigue trajectories. Health Psychol. 37, 1025–1034 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lazarus RS & Folkman S Stress, Appraisal, and Coping (Springer, 1984). [Google Scholar]
- 98.Cohen S, Kessler RC & Gordon LU (eds) Measuring Stress: A Guide for Health and Social Scientists (Oxford Univ. Press, 1997). [Google Scholar]
- 99.Carroll JE et al. Childhood abuse, parental warmth, and adult multisystem biological risk in the coronary artery risk development in young adults study. Proc. Natl Acad. Sci. USA 110, 17149–17153 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shalev I et al. Exposure to violence during childhood is associated with telomere erosion from 5 to 10 years of age: a longitudinal study. Mol. Psychiatry 18, 576–581 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Marini S et al. Adversity exposure during sensitive periods predicts accelerated epigenetic aging in children. Psychoneuroendocrinology 113, 104484 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Belsky DW et al. Impact of early personal-history characteristics on the pace of aging: implications for clinical trials of therapies to slow aging and extend healthspan. Aging Cell 16, 644–651 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Miller GE, Chen E & Parker KJ Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychol. Bull 137, 959–997 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Carroll JE, Mahrer NE, Shalowitz M, Ramey S & Dunkel Schetter C Prenatal maternal stress prospectively relates to shorter child buccal cell telomere length. Psychoneuroendocrinology 121, 104841 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Antoni MH et al. The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat. Rev. Cancer 6, 240–248 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Flint MS, Baum A, Chambers WH & Jenkins FJ Induction of DNA damage, alteration of DNA repair and transcriptional activation by stress hormones. Psychoneuroendocrinology 32, 470–479 (2007). [DOI] [PubMed] [Google Scholar]
- 107.Hara MR et al. A stress response pathway regulates DNA damage through β2-adrenoreceptors and β-arrestin-1. Nature 477, 349–353 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Picard M, Juster R-P & McEwen BS Mitochondrial allostatic load puts the “gluc” back in glucocorticoids. Nat. Rev. Endocrinol 10, 303–310 (2014). [DOI] [PubMed] [Google Scholar]
- 109.Epel ES Psychological and metabolic stress: a recipe for accelerated cellular aging? Hormones 8, 7–22 (2009). [DOI] [PubMed] [Google Scholar]
- 110.Choi J, Fauce SR & Effros RB Reduced telomerase activity in human T lymphocytes exposed to cortisol. Brain Behav. Immun 22, 600–605 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Picca A et al. Fueling inflamm-aging through mitochondrial dysfunction: mechanisms and molecular targets. Int. J. Mol. Sci 18, 933 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Franceschi C, Garagnani P, Parini P, Giuliani C & Santoro A Inflammaging: a new immune–metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol 14, 576–590 (2018). [DOI] [PubMed] [Google Scholar]
- 113.Chen GY & Nuñez G Sterile inflammation: sensing and reacting to damage. Nat. Rev. Immunol 10, 826–837 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Irwin MR & Cole SW Reciprocal regulation of the neural and innate immune systems. Nat. Rev. Immunol 11, 625–632 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Marsland AL, Walsh C, Lockwood K & John-Henderson NA The effects of acute psychological stress on circulating and stimulated inflammatory markers: a systematic review and meta-analysis. Brain Behav. Immun 64, 208–219 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cohen S et al. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc. Natl Acad. Sci 109, 5995–5999 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kiecolt-Glaser JK et al. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc. Natl Acad. Sci. USA 100, 9090–9095 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Flint MS et al. Genomic profiling of restraint stress-induced alterations in mouse T lymphocytes. J. Neuroimmunol 167, 34–44 (2005). [DOI] [PubMed] [Google Scholar]
- 119.Razzoli M et al. Social stress shortens lifespan in mice. Aging Cell 17, e12778 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mathur MB et al. Perceived stress and telomere length: a systematic review, meta-analysis, and methodologic considerations for advancing the field. Brain Behav. Immun 54, 158–169 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shalev I et al. Stress and telomere biology: a lifespan perspective. Psychoneuroendocrinology 38, 1835–1842 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rentscher KE et al. Chronic stress exposure and daily stress appraisals relate to biological aging marker p16INK4a. Psychoneuroendocrinology 102, 139–148 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Boks MP et al. Longitudinal changes of telomere length and epigenetic age related to traumatic stress and post-traumatic stress disorder. Psychoneuroendocrinology 51,506–512 (2015). [DOI] [PubMed] [Google Scholar]
- 124.Simpkin AJ et al. Prenatal and early life influences on epigenetic age in children: a study of mother-offspring pairs from two cohort studies. Hum. Mol. Genet 25, 191–201 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Roberts AL et al. Posttraumatic stress disorder and accelerated aging: PTSD and leukocyte telomere length in a sample of civilian women. Depress Anxiety 34, 391–400 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sumner JA, Colich NL, Uddin M, Armstrong D & McLaughlin KA Early experiences of threat, but not deprivation, are associated with accelerated biological aging in children and adolescents. Biol. Psychiatry 85, 268–278 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zannas AS et al. Lifetime stress accelerates epigenetic aging in an urban, African American cohort: relevance of glucocorticoid signaling. Genome Biol. 16, 266 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jovanovic T et al. Exposure to violence accelerates epigenetic aging in children. Sci. Rep 7, 8962 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Marini S et al. Adversity exposure during sensitive periods predicts accelerated epigenetic aging in children. Psychoneuroendocrinology 113, 104484 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Stanton AL Psychosocial concerns and interventions for cancer survivors. J. Clin. Oncol 24, 5132–5137 (2016). [DOI] [PubMed] [Google Scholar]
- 131.O’Keefe EB, Meltzer JP & Bethea TN Health disparities and cancer: racial disparities in cancer mortality in the United States, 2000–2010. Front. Public Health 3, 51 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kumachev A, Trudeau ME & Chan KKW Associations among socioeconomic status, patterns of care and outcomes in breast cancer patients in a universal health care system: Ontario’s experience. Cancer 122, 893–898 (2016). [DOI] [PubMed] [Google Scholar]
- 133.Dignam JJ Disparities in breast cancer: narrowing the gap. J. Natl Cancer Inst 113, 349–350 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.de Punder K, Heim C, Wadhwa PD & Entringer S Stress and immunosenescence: the role of telomerase. Psychoneuroendocrinology 101,87–100 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cho HJ, Bower JE, Kiefe CI, Seeman TE & Irwin MR Early life stress and inflammatory mechanisms of fatigue in the coronary artery risk development in young adults (CARDIA) study. Brain Behav. Immun 26, 859–865 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Crosswell AD, Bower JE & Ganz PA Childhood adversity and inflammation in breast cancer survivors. Psychosom. Med 76, 208–214 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Epel ES & Prather AA Stress, telomeres, and psychopathology: toward a deeper understanding of a triad of early aging. Annu. Rev. Clin. Psychol 14, 371–397 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Han TJ et al. Association of childhood trauma with fatigue, depression, stress, and inflammation in breast cancer patients undergoing radiotherapy. Psychooncology 25, 187–193 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bower JE, Crosswell AD & Slavich GM Childhood adversity and cumulative life stress: risk factors for cancer-related fatigue. Clin. Psychol. Sci 2, 108–115 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bower JE et al. Childhood maltreatment and monocyte gene expression among women with breast cancer. Brain Behav. Immun 88, 396–402 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bower JE et al. Testing a biobehavioral model of fatigue before adjuvant therapy in women with breast cancer. Cancer 125, 633–641 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Papanastasiou A et al. Role of stress, age and adjuvant therapy in the cognitive function of patients with breast cancer. Oncol. Lett 18, 507–517 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Huehnchen P, van Kampen A, Boehmerle W & Endres M Cognitive impairment after cytotoxic chemotherapy. Neurooncol. Pract 7, 11–21 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kuring JK, Mathias JL & Ward L Risk of dementia in persons who have previously experienced clinically-significant depression, anxiety, or PTSD: a systematic review and meta-analysis. J. Affect. Disord 274, 247–261 (2020). [DOI] [PubMed] [Google Scholar]
- 145.Watson NF et al. Joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society on the recommended amount of sleep for a healthy adult: methodology and discussion. Sleep 38, 1161–1183 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Carroll JE & Prather AA Sleep and biological aging: a short review. Curr. Opin. Endocr. Metab. Res 18, 159–164 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Brown MK & Naidoo N The UPR and the anti-oxidant response: relevance to sleep and sleep loss. Mol. Neurobiol 42, 103–113 (2010). [DOI] [PubMed] [Google Scholar]
- 148.Jessen NA, Munk ASF, Lundgaard I & Nedergaard M The glymphatic system: a beginner’s guide. Neurochem. Res 40, 2583–2599 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Xie L et al. Sleep drives metabolite clearance from the adult brain. Science 342, 373–377 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tempaku PF, Mazzotti DR & Tufik S Telomere length as a marker of sleep loss and sleep disturbances: a potential link between sleep and cellular senescence. Sleep Med. 16, 559–563 (2015). [DOI] [PubMed] [Google Scholar]
- 151.Prather AA et al. Tired telomeres: poor global sleep quality, perceived stress, and telomere length in immune cell subsets in obese men and women. Brain Behav. Immun 47, 155–162 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cribbet MR et al. Cellular aging and restorative processes: subjective sleep quality and duration moderate the association between age and telomere length in a sample of middle-aged and older adults. Sleep 37, 65–70 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jackowska M et al. Short sleep duration is associated with shorter telomere length in healthy men: findings from the Whitehall II Cohort Study. PLoS ONE 7, e47292 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Huang P et al. The association between obstructive sleep apnea and shortened telomere length: a systematic review and meta-analysis. Sleep Med. 48, 107–112 (2018). [DOI] [PubMed] [Google Scholar]
- 155.Prather AA et al. Shorter leukocyte telomere length in midlife women with poor sleep quality. J. Aging Res 2011, 1–6 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lee KA et al. Telomere length is associated with sleep duration but not sleep quality in adults with human immunodeficiency virus. Sleep 37, 157–166 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zgheib NK et al. Short telomere length is associated with aging, central obesity, poor sleep and hypertension in lebanese individuals. Aging Dis. 9, 77–89 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Carroll JE et al. Obstructive sleep apnea, nighttime arousals, and leukocyte telomere length: the multi-ethnic study of atherosclerosis. Sleep 42, zsz089 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Carroll JE et al. Insomnia and telomere length in older adults. Sleep 39, 559–564 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Irwin MR & Opp MR Sleep health: reciprocal regulation of sleep and innate immunity. Neuropsychopharmacology 42, 129–155 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Irwin MR, Olmstead R & Carroll JE Sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol. Psychiatry 80, 40–52 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Garland SN, Mahon K & Irwin MR Integrative approaches for sleep health in cancer survivors. Cancer J. 25, 337–342 (2019). [DOI] [PubMed] [Google Scholar]
- 163.Palesh O et al. Actigraphy-measured sleep disruption as a predictor of survival among women with advanced breast cancer. Sleep 37, 837–842 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Trudel-Fitzgerald C et al. Sleep and survival among women with breast cancer: 30 years of follow-up within the Nurses’ Health Study. Br. J. Cancer 116, 1239–1246 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Irwin MR, Olmstead RE, Ganz PA & Haque R Sleep disturbance, inflammation and depression risk in cancer survivors. Brain Behav. Immun 30 (Suppl.), S58–S67 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Savard J & Morin CM Insomnia in the context of cancer: a review of a neglected problem. J. Clin. Oncol 19, 895–908 (2001). [DOI] [PubMed] [Google Scholar]
- 167.Carroll JE et al. Sleep disturbance and neurocognitive outcomes in older patients with breast cancer: interaction with genotype. Cancer 125, 4516–4524 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ancoli-Israel S et al. Sleep, fatigue, depression, and circadian activity rhythms in women with breast cancer before and after treatment: a 1-year longitudinal study. Support. Care Cancer 22, 2535–2545 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Savard J et al. Breast cancer patients have progressively impaired sleep-wake activity rhythms during chemotherapy. Sleep 32, 1155–1160 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Santoso AMM et al. Prevalence of sleep disturbances among head and neck cancer patients: a systematic review and meta-analysis. Sleep Med. Rev 47, 62–73 (2019). [DOI] [PubMed] [Google Scholar]
- 171.Basen-Engquist K et al. Agenda for translating physical activity, nutrition, and weight management interventions for cancer survivors into clinical and community practice. Obesity 25 (Suppl. 2), S9–S22 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Tucker LA Physical activity and telomere length in U.S. men and women: an NHANES investigation. Prev. Med 100, 145–151 (2017). [DOI] [PubMed] [Google Scholar]
- 173.Cherkas LF et al. The association between physical activity in leisure time and leukocyte telomere length. Arch. Intern. Med 168, 154–158 (2008). [DOI] [PubMed] [Google Scholar]
- 174.Soares-Miranda L et al. Physical activity, physical fitness, and leukocyte telomere length. Med. Sci. Sports Exerc 47, 2525–2534 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Puterman E & et al. Aerobic exercise lengthens telomeres and reduces stress in family caregivers: a randomized controlled trial - Curt Richter Award Paper 2018. Psychoneuroendocrinology 98, 245–252 (2018). [DOI] [PubMed] [Google Scholar]
- 176.Figueiredo N et al. Anthracyclines induce DNA damage response-mediated protection against severe sepsis. Immunity 39, 874–884 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Garatachea N et al. Exercise attenuates the major hallmarks of aging. Rejuvenation Res. 18, 57–89 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ramírez-Vélez R et al. Evidence-based exercise recommendations to improve mental wellbeing in women with breast cancer during active treatment: a systematic review and meta-analysis. Cancers 13, 264 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Speck RM, Courneya KS, Masse LC, Duval S & Schmitz KH An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J. Cancer Surviv 4, 87–100 (2010). [DOI] [PubMed] [Google Scholar]
- 180.Englund DA et al. Exercise reduces circulating biomarkers of cellular senescence in humans. Aging Cell 20, e13415 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kerr J, Anderson C & Lippman SM Physical activity, sedentary behaviour, diet, and cancer: an update and emerging new evidence. Lancet Oncol. 18, e457–e471 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Arem H et al. Pre- and postdiagnosis physical activity, television viewing, and mortality among patients with colorectal cancer in the national institutes of health-AARP diet and health study. J. Clin. Oncol 33, 180–188 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ligibel JA, Basen-Engquist K & Bea JW Weight management and physical activity for breast cancer prevention and control. Am. Soc. Clin. Oncol. Educ. B 39, e22–e33 (2019). [DOI] [PubMed] [Google Scholar]
- 184.Wagner MA, Erickson KI, Bender CM & Conley YP The influence of physical activity and epigenomics on cognitive function and brain health in breast cancer. Front. Aging Neurosci 12, 123 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Bender CM et al. Physical activity, cardiorespiratory fitness, and cognitive function in postmenopausal women with breast cancer. Support. Care Cancer 29, 3743–3752 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ho M et al. Effects of dietary and physical activity interventions on generic and cancer-specific health-related quality of life, anxiety, and depression in colorectal cancer survivors: a randomized controlled trial. J. Cancer Surviv 14, 424–433 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Thorsen L et al. The association between self-reported physical activity and prevalence of depression and anxiety disorder in long-term survivors of testicular cancer and men in a general population sample. Support. Care Cancer 13, 637–646 (2005). [DOI] [PubMed] [Google Scholar]
- 188.Ribeiro FE et al. Relationship of anxiety and depression symptoms with the different domains of physical activity in breast cancer survivors. J. Affect. Disord 273, 210–214 (2020). [DOI] [PubMed] [Google Scholar]
- 189.An R & Yan H Body weight status and telomere length in U.S. middle-aged and older adults. Obes. Res. Clin. Pract 11, 51–62 (2017). [DOI] [PubMed] [Google Scholar]
- 190.Hang D et al. Longitudinal associations of lifetime adiposity with leukocyte telomere length and mitochondrial DNA copy number. Eur. J. Epidemiol 33, 485–495 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Horvath S et al. Obesity accelerates epigenetic aging of human liver. Proc. Natl Acad. Sci. USA 111, 15538–15543 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Nevalainen T et al. Obesity accelerates epigenetic aging in middle-aged but not in elderly individuals. Clin. Epigenetics 9, 20 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ogrodnik M et al. Obesity-induced cellular senescence drives anxiety and impairs neurogenesis. Cell Metab. 29, 1061–1077.e8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Almendáriz-Palacios C, Mousseau DD, Eskiw CH & Gillespie ZE Still living better through chemistry: an update on caloric restriction and caloric restriction mimetics as tools to promote health and lifespan. Int. J. Mol. Sci 21, 9220 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Hoshino S, Kobayashi M & Higami Y Mechanisms of the anti-aging and prolongevity effects of caloric restriction: evidence from studies of genetically modified animals. Aging 10, 2243 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Dorling JL et al. Effects of caloric restriction on human physiological, psychological, and behavioral outcomes: highlights from CALERIE phase 2. Nutr. Rev 79, 98–113 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Gonzalez-Freire M et al. The road ahead for health and lifespan interventions. Ageing Res. Rev 59, 101037 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Del Bo’ CD et al. Overview of human intervention studies evaluating the impact of the mediterranean diet on markers of DNA damage. Nutrients 11, 391 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sung H, Hyun N, Leach CR, Yabroff KR & Jemal A Association of first primary cancer with risk of subsequent primary cancer among survivors of adult-onset cancers in the United States. J. Am. Med. Assoc 324, 2521–2535 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ganz PA & Casillas JN Incorporating the risk for subsequent primary cancers into the care of adult cancer survivors: moving beyond 5-year survival. J. Am. Med. Assoc 324, 2493–2495 (2020). [DOI] [PubMed] [Google Scholar]
- 201.Ruiz-Casado A, Álvarez-Bustos A, de Pedro CG, Méndez-Otero M & Romero-Elías M Cancer-related fatigue in breast cancer survivors: a review. Clin. Breast Cancer 21, 10–25 (2021). [DOI] [PubMed] [Google Scholar]
- 202.Lee JT et al. Impact of community-based exercise program participation on aerobic capacity in women with and without breast cancer. World J. Clin. Oncol 12, 468–481 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Hamajima N et al. Alcohol, tobacco and breast cancer - collaborative reanalysis of individual data from 53 epidemiological studies, including 58,515 women with breast cancer and 95,067 women without the disease. Br. J. Cancer 87, 1234–1245 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Znaor A et al. Independent and combined effects of tobacco smoking, chewing and alcohol drinking on the risk of oral, pharyngeal and esophageal cancers in Indian men. Int. J. Cancer 105, 681–686 (2003). [DOI] [PubMed] [Google Scholar]
- 205.Baan R et al. Carcinogenicity of alcoholic beverages. Lancet Oncol. 8, 292–293 (2007). [DOI] [PubMed] [Google Scholar]
- 206.National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. Advances in Knowledge of the Health Consequences of Smoking: From 1964–2014, https://www.ncbi.nlm.nih.gov/books/NBK294317/ (2014).
- 207.Centers for Disease Control and Prevention (US); National Center for Chronic Disease Prevention and Health Promotion (US); Office on Smoking and Health (US). How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General https://pubmed.ncbi.nlm.nih.gov/21452462/ (2020). [PubMed]
- 208.Harpaz T et al. The effect of ethanol on telomere dynamics and regulation in human cells. Cells 7, 169 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Beach SRH et al. Methylomic aging as a window onto the influence of lifestyle: tobacco and alcohol use alter the rate of biological aging. J. Am. Geriatr. Soc 63, 2519–2525 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Dixit S et al. Alcohol consumption and leukocyte telomere length. Sci. Rep 9, 1404 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Latifovic L, Peacock SD, Massey TE & King WD The influence of alcohol consumption, cigarette smoking, and physical activity on leukocyte telomere length. Cancer Epidemiol. Biomark. Prev 25, 374–380 (2016). [DOI] [PubMed] [Google Scholar]
- 212.Lu AT et al. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging 11, 303–327 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Lei M-K, Gibbons FX, Simons RL, Philibert RA & Beach SRH The effect of tobacco smoking differs across indices of DNA methylation-based aging in an African American sample: DNA methylation-based indices of smoking capture these effects. Genes 11, 311 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Verde Z et al. Effects of cigarette smoking and nicotine metabolite ratio on leukocyte telomere length. Environ. Res 140, 488–494 (2015). [DOI] [PubMed] [Google Scholar]
- 215.Needham BL et al. Socioeconomic status, health behavior, and leukocyte telomere length in the National Health and Nutrition Examination Survey, 1999–2002. Soc. Sci. Med 85, 1–8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Valdes A et al. Obesity, cigarette smoking, and telomere length in women. Lancet 366, 662–664 (2005). [DOI] [PubMed] [Google Scholar]
- 217.Collins F Connecting senescent cells to obesity and anxiety. NIH Director’s Blog, https://directorsblog.nih.gov/2019/01/08/connecting-senescent-cells-to-obesity-and-anxiety/ (2019). [Google Scholar]
- 218.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02652052 (2019). [DOI] [PubMed]
- 219.Hiller JG, Perry NJ, Poulogiannis G, Riedel B & Sloan EK Perioperative events influence cancer recurrence risk after surgery. Nat. Rev. Clin. Oncol 15, 205–218 (2017). [DOI] [PubMed] [Google Scholar]
- 220.Hiller JG et al. Preoperative β-blockade with propranolol reduces biomarkers of metastasis in breast cancer: a phase II randomized trial. Clin. Cancer Res 26, 1803–1811 (2020). [DOI] [PubMed] [Google Scholar]
- 221.Knight JM et al. Propranolol inhibits molecular risk markers in HCT recipients: a phase 2 randomized controlled biomarker trial. Blood Adv. 4, 467–476 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Haldar R et al. Perioperative COX2 and β-adrenergic blockade improves biomarkers of tumor metastasis, immunity, and inflammation in colorectal cancer: a randomized controlled trial. Cancer 126, 3991–4001 (2020). [DOI] [PubMed] [Google Scholar]
- 223.Cole SW & Sood AK Molecular pathways: beta-adrenergic signaling in cancer. Clin. Cancer Res 18, 1201–1206 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Carlson LE, Toivonen K & Subnis U Integrative approaches to stress management. Cancer J. 25, 329–336 (2019). [DOI] [PubMed] [Google Scholar]
- 225.Greenlee H et al. Clinical practice guidelines on the evidence-based use of integrative therapies during and after breast cancer treatment. CA Cancer J. Clin 67, 194–232 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Matis J, Svetlak M, Slezackova A, Svoboda M & Sumec R Mindfulness-based programs for patients with cancer via eHealth and mobile health: systematic review and synthesis of quantitative research. J. Med. Internet Res 22, e20709 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Antoni MH et al. Cognitive-behavioral stress management reverses anxiety-related leukocyte transcriptional dynamics. Biol. Psychiatry 71, 366–372 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Antoni MH et al. Stress management, leukocyte transcriptional changes and breast cancer recurrence in a randomized trial: an exploratory analysis. Psychoneuroendocrinology 74, 269–277 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Bower JE et al. Mindfulness meditation for younger breast cancer survivors: a randomized controlled trial. Cancer 121, 1231–1240 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Bower JE & Irwin MR Mind-body therapies and control of inflammatory biology: a descriptive review. Brain Behav. Immun 51, 1–11 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Chaix R et al. Epigenetic clock analysis in long-term meditators. Psychoneuroendocrinology 85, 210–214 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Bower JE et al. Yoga reduces inflammatory signaling in fatigued breast cancer survivors: a randomized controlled trial. Psychoneuroendocrinology 43, 20–29 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kiecolt-Glaser JK et al. Yoga’s impact on inflammation, mood, and fatigue in breast cancer survivors: a randomized controlled trial. J. Clin. Oncol 32, 1040–1049 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Danhauer SC et al. Yoga for symptom management in oncology: a review of the evidence base and future directions for research. Cancer 125, 1979–1989 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Garland SN et al. Sleeping well with cancer: a systematic review of cognitive behavioral therapy for insomnia in cancer patients. Neuropsychiatr. Dis. Treat 10, 1113–1124 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Johnson JA et al. A systematic review and meta-analysis of randomized controlled trials of cognitive behavior therapy for insomnia (CBT-I) in cancer survivors. Sleep Med. Rev 27, 20–28 (2016). [DOI] [PubMed] [Google Scholar]
- 237.Zachariae R et al. Internet-delivered cognitive-behavioral therapy for insomnia in breast cancer survivors: a randomized controlled trial. J. Natl Cancer Inst 110, 880–887 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Irwin MR et al. Tai Chi Chih compared with cognitive behavioral therapy for the treatment of insomnia in survivors of breast cancer: a randomized, partially blinded, noninferiority trial. J. Clin. Oncol 35, 2656–2665 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Garland SN et al. Mindfulness-based stress reduction compared with cognitive behavioral therapy for the treatment of insomnia comorbid with cancer: a randomized, partially blinded, noninferiority trial. J. Clin. Oncol 32, 449–457 (2014). [DOI] [PubMed] [Google Scholar]
- 240.Zhao Y et al. Effects of mindfulness-based cognitive therapy on breast cancer survivors with insomnia: a randomised controlled trial. Eur. J. Cancer Care 29, e13259 (2020). [DOI] [PubMed] [Google Scholar]
- 241.Mustian KM et al. Multicenter, randomized controlled trial of yoga for sleep quality among cancer survivors. J. Clin. Oncol 31,3233–3241 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Garland SN et al. Acupuncture versus cognitive behavioral therapy for insomnia in cancer survivors: a randomized clinical trial. J. Natl Cancer Inst 111, 1323–1331 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Irwin MR et al. Cognitive behavioral therapy vs. Tai Chi for late life insomnia and inflammatory risk: a randomized controlled comparative efficacy trial. Sleep 37, 1543–1552 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Irwin MR et al. Tai Chi, cellular Inflammation, and transcriptome dynamics in breast cancer survivors with insomnia: a randomized controlled trial. JNCI Monogr. 2014, 295–301 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Chen T-Y, Lee S & Buxton OM A greater extent of insomnia symptoms and physician-recommended sleep medication use predict fall risk in community-dwelling older adults. Sleep 10.1093/sleep/zsx142 (2017). [DOI] [PubMed] [Google Scholar]
- 246.Besedovsky L et al. Auditory closed-loop stimulation of EEG slow oscillations strengthens sleep and signs of its immune-supportive function. Nat. Commun 8, 1984 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Charalambous A et al. Cancer-related fatigue and sleep deficiency in cancer care continuum: concepts, assessment, clusters, and management. Support. Care Cancer 27, 2747–2753 (2019). [DOI] [PubMed] [Google Scholar]
- 248.Matthews E, Carter P, Page M, Dean G & Berger A Sleep-wake disturbance: a systematic review of evidence-based interventions for management in patients with cancer. Clin. J. Oncol. Nurs 22, 37–52 (2018). [DOI] [PubMed] [Google Scholar]
- 249.Piercy KL et al. The physical activity guidelines for Americans. J. Am. Med. Assoc 320, 2020–2028 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Jensen MD et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society. Circulation 129 (Suppl. 2), S102–S138 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Kaiser EG, Prochaska JJ & Kendra MS Tobacco cessation in oncology care. Oncology 95, 129–137 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Warren GW, Ostroff JS & Goffin JR Lung cancer screening, cancer treatment, and addressing the continuum of health risks caused by tobacco. Am. Soc. Clin. Oncol. Educ. B 35, 223–229 (2016). [DOI] [PubMed] [Google Scholar]
- 253.LoConte NK et al. Lifestyle modifications and policy implications for primary and secondary cancer prevention: diet, exercise, sun safety, and alcohol reduction. Am. Soc. Clin. Oncol. Educ. B 38, 88–100 (2018). [DOI] [PubMed] [Google Scholar]
- 254.Demark-Wahnefried W et al. Weight management and physical activity throughout the cancer care continuum. CA Cancer J. Clin 68, 64–89 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Ligibel JA et al. Impact of a pre-operative exercise intervention on breast cancer proliferation and gene expression: Results from the pre-operative health and body (PreHAB) study. Clin. Cancer Res 25, 5398–5406 (2019). [DOI] [PubMed] [Google Scholar]
- 256.Gerritsen JKW & Vincent AJPE Exercise improves quality of life in patients with cancer: A systematic review and meta-analysis of randomised controlled trials. Br. J. Sports Med 50, 796–803 (2016). [DOI] [PubMed] [Google Scholar]
- 257.Ballard-Barbash R et al. Physical activity, biomarkers, and disease outcomes in cancer survivors: a systematic review. J. Natl Cancer Inst 104, 815–840 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.García-Calzón S et al. Telomere length as a biomarker for adiposity changes after a multidisciplinary intervention in overweight/obese adolescents: the EVASYON study. PLoS ONE 9, e89828 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Mctiernan A et al. Physical activity in cancer prevention and survival: a systematic review. Med. Sci. Sports Exerc 51, 1252–1261 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Pinckard K, Baskin KK & Stanford KI Effects of exercise to improve cardiovascular health. Front. Cardiovasc. Med 6, 69 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Brown JC et al. Effect of exercise or metformin on biomarkers of inflammation in breast and colorectal cancer: a randomized trial. Cancer Prev. Res 13, 1055–1062 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Bower JE et al. Screening, assessment, and management of fatigue in adult survivors of cancer: an American Society of Clinical Oncology clinical practice guideline adaptation. J. Clin. Oncol 32, 1840–1850 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Pekmezi D et al. Physical activity maintenance following home-based, individually tailored print interventions for African American women. Health Promot. Pract 21, 268–276 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Mayer DK et al. SurvivorCHESS to increase physical activity in colon cancer survivors: can we get them moving? J. Cancer Surviv 12, 82–94 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Demark-Wahnefried W et al. Pilot randomized controlled trial of a home vegetable gardening intervention among older cancer survivors shows feasibility, satisfaction, and promise in improving vegetable and fruit consumption, reassurance of worth, and the trajectory of central adiposity. J. Acad. Nutr. Diet 118, 689–704 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Guida JL et al. Strategies to prevent or remediate cancer and treatment-related aging. J. Natl Cancer Inst 113, 112–122 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Brandhorst S et al. A periodic diet that mimics fasting promotes multi-system regeneration, enhanced cognitive performance, and healthspan. Cell Metab. 22, 86–99 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Longo VD & Mattson MP Fasting: molecular mechanisms and clinical applications. Cell Metab. 19, 181–192 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Mattson MP, Longo VD & Harvie M Impact of intermittent fasting on health and disease processes. Ageing Res. Rev 39, 46–58 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.De Cabo R & Mattson MP Effects of intermittent fasting on health, aging, and disease. N. Engl. J. Med 381, 2541–2551 (2019). [DOI] [PubMed] [Google Scholar]
- 271.Hahn O et al. A nutritional memory effect counteracts the benefits of dietary restriction in old mice. Nat. Metab 1, 1059–1073 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Espada L et al. Loss of metabolic plasticity underlies metformin toxicity in aged Caenorhabditis elegans. Nat. Metab 2, 1316–1331 (2020). [DOI] [PubMed] [Google Scholar]
- 273.Fucito LM et al. Pairing smoking-cessation services with lung cancer screening: a clinical guideline from the association for the treatment of tobacco use and dependence and the society for research on nicotine and tobacco. Cancer 122, 1150–1159 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Taylor KL et al. Preliminary evaluation of a telephone-based smoking cessation intervention in the lung cancer screening setting: a randomized clinical trial. Lung Cancer 108, 242–246 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Zbikowski SM, Hapgood J, Barnwell SS & McAfee T Phone and web-based tobacco cessation treatment: real-world utilization patterns and outcomes for 11,000 tobacco users. J. Med. Internet Res 10, e41 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.McHugh D & Gil J Senescence and aging: causes, consequences, and therapeutic avenues. J. Cell Biol 217, 65–77 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Van Waart H et al. Effect of low-intensity physical activity and moderate- to high-intensity physical exercise during adjuvant chemotherapy on physical fitness, fatigue, and chemotherapy completion rates: results of the PACES randomized clinical trial. J. Clin. Oncol 33, 1918–1927 (2015). [DOI] [PubMed] [Google Scholar]
- 278.Courneya KS et al. Effects of exercise during adjuvant chemotherapy on breast cancer outcomes. Med. Sci. Sports Exerc 46, 1744–1751 (2014). [DOI] [PubMed] [Google Scholar]
- 279.Cannioto RA et al. Physical activity before, during, and after chemotherapy for high-risk breast cancer: relationships with survival. J. Natl Cancer Inst 113, 54–63 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Antoni MH et al. Reduction of cancer-specific thought intrusions and anxiety symptoms with a stress management intervention among women undergoing treatment for breast cancer. Am. J. Psychiatry 163, 1791–1797 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Gudenkauf LM et al. Brief cognitive-behavioral and relaxation training interventions for breast cancer: a randomized controlled trial. J. Consult. Clin. Psychol 83, 677–688 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Palesh O et al. Feasibility and acceptability of brief behavioral therapy for cancer-related insomnia: effects on insomnia and circadian rhythm during chemotherapy: a phase II randomised multicentre controlled trial. Br. J. Cancer 119, 274–281 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Johnson JA et al. Bright light therapy improves cancer-related fatigue in cancer survivors: a randomized controlled trial. J. Cancer Surviv 12, 206–215 (2018). [DOI] [PubMed] [Google Scholar]
- 284.Redd WH et al. Systematic light exposure in the treatment of cancer-related fatigue: a preliminary study. Psychooncology 23, 1431–1434 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Fultz NE et al. Coupled electrophysiological, hemodynamic, and cerebrospinal fluid oscillations in human sleep. Science 366, 628–631 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Nedergaard M & Goldman SA Glymphatic failure as a final common pathway to dementia. Science 370, 50–56 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Savard J, Ivers H, Savard M-H & Morin CM Cancer treatments and their side effects are associated with aggravation of insomnia: results of a longitudinal study. Cancer 121, 1703–1711 (2015). [DOI] [PubMed] [Google Scholar]
- 288.Palesh O et al. Secondary outcomes of a behavioral sleep intervention: a randomized clinical trial. Health Psychol. 38, 196–205 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Carlson L, Rouleau CR & Garland SN The impact of mindfulness-based interventions on symptom burden, positive psychological outcomes, and biomarkers in cancer patients. Cancer Manag. Res 7, 121 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Blackburn EH Telomere states and cell fates. Nature 408, 53–56 (2000). [DOI] [PubMed] [Google Scholar]
- 291.Rodriguez–Mortera R, Bains Y & Gugliucci A Fructose at the crossroads of the metabolic syndrome and obesity epidemics. Front. Biosci 24, 186–211 (2019). [DOI] [PubMed] [Google Scholar]
- 292.Nowotny B et al. Effects of acute psychological stress on glucose metabolism and subclinical inflammation in patients with post-traumatic stress disorder. Horm. Metab. Res 42, 746–753 (2010). [DOI] [PubMed] [Google Scholar]
- 293.Fiorentino TV, Prioletta A, Zuo P & Folli F Hyperglycemia-induced oxidative stress and its role in diabetes mellitus related cardiovascular diseases. Curr. Pharm. Des 19, 5695–5703 (2013). [DOI] [PubMed] [Google Scholar]