Abstract
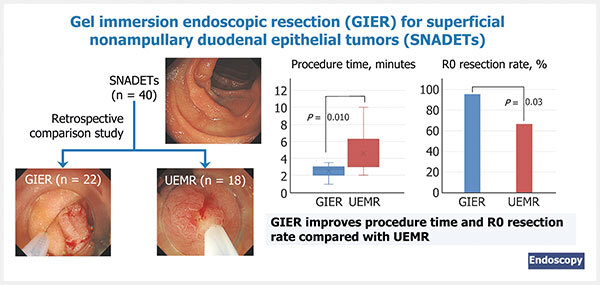
Background Although gel immersion endoscopic resection (GIER) is a potential alternative to underwater endoscopic mucosal resection (UEMR) for superficial nonampullary duodenal epithelial tumors (SNADETs), comparisons between the two are currently insufficient.
Methods 40 consecutive procedures performed in 35 patients were retrospectively reviewed; the primary outcome was procedure time, and the secondary outcomes were en bloc and R0 resection rates, tumor and specimen size, and adverse events.
Results Lesions were divided into GIER (n = 22) and UEMR groups (n = 18). The median (range) procedure time was significantly shorter in the GIER group than in the UEMR group (2.75 [1–3.5] minutes vs. 3 2 3 4 5 6 7 8 9 10 minutes; P = 0.01). The en bloc resection rate was 100 % in the GIER group, but only 83.3 % in the UEMR group. The R0 resection rate was significantly higher in the GIER group than in the UEMR group (95.5 % vs. 66.7 %; P = 0.03). The median specimen size was larger in the GIER group than in the UEMR group (14 mm vs. 7.5 mm; P < 0.001). The tumor size was not significantly different between the groups and no adverse events were observed.
Conclusions GIER is efficacious and safe to treat SNADETs, although additional studies are needed.
Introduction
Although superficial nonampullary duodenal epithelial tumors (SNADETs) were previously considered rare, their recognition has been increasing following an improvement in endoscopic accuracy 1 2 3 4 5 . The opportunities to perform endoscopic resection of SNADETs have therefore recently increased; however, it is considered extremely difficult because of the technical challenges posed by the complex anatomic features of the duodenum 2 4 5 . Recently, the safety and efficacy of underwater endoscopic mucosal resection (UEMR) have been increasingly reported 6 7 8 9 10 11 12 13 14 15 . UEMR is a water immersion method leading to easy snaring and a low perforation risk 2 7 11 12 13 14 . Therefore, UEMR is a favorable treatment and results in high resection and low adverse event (AE) rates 4 7 8 9 13 14 15 . However, continuously maintaining water in the lumen is difficult, leading to large-volume water injection and a long procedure time 6 13 .
Recently, a novel gelatinous liquid (Viscoclear; Otsuka Pharmaceuticals Factory, Inc., Tokushima, Japan) was developed to secure the visual field during endoscopy 16 17 . This gelatinous liquid comprises xanthan gum, locust bean gum, and glycerin 16 . The viscoelastic properties of the gelatinous liquid enable continuous filling of the lumen and prevent its mixing with fresh blood or feces, resulting in a favorable space for endoscopic visualization and treatment 16 17 . Yano et al. reported that use of an immersing gelatinous liquid during endoscopy resulted in a favorable visual field and named this method gel immersion endoscopy 16 17 18 . Applying these features, we devised gel immersion endoscopic resection (GIER), a gel-based EMR technique, as an alternative method to water-based UEMR, and reported its efficacy 19 .
Although GIER may effectively treat SNADETs, there have been no reports comparing GIER and UEMR. Therefore, we retrospectively investigated the short-term clinical outcomes of GIER as a treatment for SNADETs as compared with UEMR.
Methods
Study design and participants
We retrospectively reviewed the outcomes of 40 consecutive SNADET cases treated by GIER and UEMR in 35 patients between April 2019 and December 2021 at Asahi General Hospital, Japan. Because the gelatinous liquid was launched in the Japanese market in October 2020, the 18 consecutive cases before the launch (between April 2019 and September 2020) were treated with UEMR, whereas the 22 consecutive cases after the launch (between October 2020 and December 2021) were treated with GIER.
The institutional review board of Asahi General Hospital approved this retrospective study (16 March 2021). Written informed consent for the procedure was obtained from all the patients. Endoscopic resection was indicated for SNADETs ≤ 20 mm in size.
Procedures
Video 1 Demonstration of the gel immersion endoscopic resection technique for a superficial nonampullary duodenal epithelial tumor.
Two board-certified fellows of the Japan Gastroenterological Endoscopy Society and one trainee performed all of the procedures. The patients were admitted 1 day prior to endoscopic resection or on the day of the procedure, and remained in the hospital for approximately 5 days. A gastroscope (GIF-Q260J; Olympus Medical Systems, Tokyo, Japan) was used with carbon dioxide insufflation.
For GIER, the gelatinous liquid was injected gently using a syringe, continuously filling the lumen; for UEMR, water was injected. Carbon dioxide insufflation was discontinued during immersion and resection. The lesion was captured using a 10-mm (AG-5076–241023; Hangzhou AGS MedTech Co., Ltd., Zhejiang, China), 13-mm (M00562451; Boston Scientific, Massachusetts, United States), 15-mm (AG-5076–241523; Hangzhou AGS MedTech Co., Ltd.), and 20-mm electrocautery snare (M00561831; Boston Scientific) as appropriate under liquid immersion. The current was passed using a high frequency generator (VIO300D; Erbe Elektromedizin, Tübingen, Germany). The high frequency generator was used with dry-cut mode (effect 4, 40 W) for mucosal resection. Prophylactic clip closure was performed for all cases using endoclips (HX-610–135S; Olympus).
A second-look endoscopy was routinely performed in all cases. All patients received 20 mg intravenous omeprazole for the first 2 days, beginning on the day of the procedure. Thereafter, 20 mg rabeprazole daily was prescribed for approximately 30 days. Treatment of a patient using GIER is shown in Fig. 1 and Video 1 .
Fig. 1.
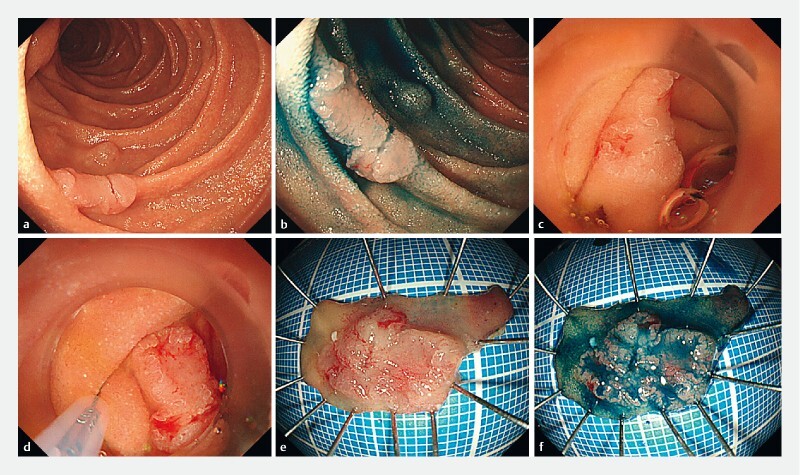
Example of gel immersion endoscopic resection of a superficial nonampullary duodenal epithelial tumor. a–d Endoscopic views showing: a,b a superficial 11-mm elevated and depressed lesion in the second part of the duodenum; c the favorable visual field after the lesion had been immersed in a gelatinous liquid; d a sufficient horizontal margin being easily confirmed with the lesion under the gelatinous liquid. e,f Macroscopic appearance of the en bloc resected lesion after it had been easily captured using an electrocautery snare, resulting in a specimen of 25 × 10 mm, containing a tumor of 11 × 8 mm that was pathologically diagnosed as a well-differentiated adenocarcinoma with negative margins.
The resected specimens were embedded in paraffin and subjected to staining with hematoxylin and eosin for histopathological diagnosis. R0 resection was defined as an en bloc resection where both the horizontal and vertical margins were negative. For en bloc resection specimens, we considered adenomas with unclear lateral margins and expected lateral burning effect as negative margins.
Outcome definitions
The primary outcome was procedure time, defined as the time from liquid immersion into the lumen to resection. The secondary outcomes were en bloc resection rate, R0 resection rate, tumor size, specimen size, and AEs, such as delayed bleeding and perforation. Delayed bleeding was defined as hematemesis or melena requiring endoscopic hemostasis or blood transfusion, or leading to a reduction ≥ 2 g/dL in hemoglobin level.
Statistical analyses
Continuous variables were reported as the median and range, and compared using the Mann–Whitney–Wilcoxon test. Differences in the categorical variables were analyzed using the likelihood ratio test and Fisher’s exact test. These analyses were conducted at the Clinical Research Support Center of Asahi General Hospital. A two-sided P value of < 0.05 was considered statistically significant. All statistical analyses were performed using the JMP version 16.1.0 software (SAS Institute Japan, Tokyo, Japan).
Results
Patient and lesion characteristics in the GIER and UEMR groups
Overall, 35 patients were treated with 40 consecutive endoscopic resection procedures. UEMR was performed for the first 18 cases, while GIER was performed for the latter 22 cases. The comparison of patient and lesion characteristics between GIER and UEMR groups is presented in Table 1 . No significant differences were observed in the patient and lesion characteristics.
Table 1. Patient and lesion characteristics in the gel immersion endoscopic resection (GIER) and underwater endoscopic mucosal resection (UEMR) groups.
| GIER | UEMR | P value | |
| Patients, n | 19 | 16 | |
| Age, median (range), years | 67 (45–81) | 63 (35–80) | 0.25 1 |
| Sex, n (%) | 0.72 2 | ||
|
13 (68.4) | 12 (75.0) | |
|
6 (31.6) | 4 (25.0) | |
| Lesions, n | 22 | 18 | |
| Location, n (%) | 0.43 3 | ||
|
3 (13.6) | 1 (5.6) | |
|
16 (72.7) | 16 (88.9) | |
|
3 (13.6) | 1 (5.6) | |
| Macroscopic type, n (%) | 0.19 3 | ||
|
6 (27.3) | 1 (5.6) | |
|
9 (40.9) | 10 (55.6) | |
|
3 (13.6) | 5 (27.8) | |
|
4 (18.2) | 2 (11.1) | |
0-I, protruding; 0-IIa, superficial elevated; 0-IIc, superficial depressed.
Mann–Whitney–Wilcoxon test.
Fisher’s exact test.
Likelihood ratio test.
Treatment outcomes in the GIER and UEMR groups
The median (range) procedure time was significantly shorter in the GIER group than in the UEMR group (2.75 [1–3.5] minutes vs. 3 2 3 4 5 6 7 8 9 10 minutes; P = 0.01) ( Table 2 ). The en bloc resection rate was 100 % (95 %CI 85.1 %–100 %) in the GIER group, but only 83.3 % (95 %CI 60.8 %–94.2 %) in the UEMR group ( P = 0.08). The R0 resection rate was significantly higher in the GIER group than in the UEMR group (95.5 % [95 %CI 78.2 %–99.2 %] vs. 66.7 % [95 %CI 43.7 %–83.7 %]; P = 0.03). The median (range) specimen size was larger in the GIER group than in the UEMR group (14 [7–27] mm vs. 7.5 [4–11] mm; P < 0.001). Tumor size and histological type were not significantly different between the groups. No AEs, such as perforation and delayed bleeding, were observed in the groups. The median (range) amount of gelatinous liquid used during a procedure was 180 (60–300) mL in the GIER group.
Table 2. Treatment outcomes of the gel immersion endoscopic resection (GIER) and underwater endoscopic mucosal resection (UEMR) groups.
| GIER | UEMR | P value | |
| Tumor size, median (range), mm | 7 (2–18) | 5 (3–10) | 0.48 1 |
| Specimen size, median (range), mm | 14 (7–27) | 7.5 (4–11) | < 0.001 1 |
| Pathology, n (%) | 0.18 3 | ||
|
1 (4.6) | 4 (22.2) | |
|
18 (81.8) | 13 (72.2) | |
|
3 (13.6) | 1 (5.6) | |
| Procedure time, median (range), minutes | 2.75 (1–3.5) | 3 (2–10) | 0.01 1 |
| En bloc resection, n (% [95 %CI]) | 22 (100 [85.1 %–100 %]) | 15 (83.3 [60.8 %–94.2 %]) | 0.08 2 |
| R0 resection, n (% [95 %CI]) | 21 (95.5 [78.2 %–99.2 %]) | 12 (66.7 [43.7 %–83.7 %]) | 0.03 2 |
| Adverse events, n | 0 (0) | 0 (0) | |
| Liquid consumption, median (range), mL | 180 (60–300) | No data | |
Discussion
This study observed that GIER, a form of gel immersion endoscopy, resulted in a short procedure time. Moreover, GIER enabled the resection of a larger specimen size and led to an increased R0 resection rate.
We observed that the procedure time was significantly shorter in the GIER group than in the UEMR group. A Japanese multicenter prospective study reported that the procedure time for treatment with UEMR was 5.4 (SD 4.3) minutes 14 . Moreover, the previous reports comparing UEMR and conventional EMR revealed that the procedure times were 4–7 minutes and 9.5–12 minutes, respectively 12 13 20 . We believe that our procedure time in patients treated with GIER is markedly shorter than previously reported for treatment with UEMR and conventional EMR. The gelatinous liquid used in GIER allows for easier maintenance of continuous lumen filling compared with water, and an additional infusion is unnecessary, leading to a short infusion time 19 . Furthermore, the viscoelastic properties of the gelatinous liquid enable a favorable transparent visual field to be maintained, resulting in easy snaring 19 . These factors might have led to a short procedure time.
The en bloc resection rate tended to be higher in the GIER group, while the R0 resection rate was significantly higher in the GIER group than in the UEMR group. Moreover, the specimen size was larger in the GIER group than in the UEMR group. The previous reports comparing UEMR and conventional EMR revealed that the en bloc resection rates were 75.4 %–96.4 % and 60.9 %–96.4 %, respectively 11 12 13 15 20 . Moreover, R0 resection rates were 50.8 %–76.1 % and 34.8 %–79.6 %, respectively 11 12 13 15 20 . In comparison with these reports, we believe that the en bloc and R0 resection rates for treatment with GIER are favorable. As mentioned previously, the favorable visual field under the gelatinous liquid might assist with snaring by allowing a sufficient horizontal margin to be determined, leading to a high resection rate and a large specimen.
A Japanese multicenter retrospective study reported that the en bloc resection rate was significantly lower in patients undergoing UEMR compared with conventional EMR, especially for large lesions 20 . Furthermore, a single-center study revealed that UEMR had lower en bloc and R0 resection rates compared with conventional EMR, although the rate of conversion to endoscopic submucosal dissection was reduced 11 . We believe that GIER leads to a large specimen with en bloc resection and can eliminate the drawbacks of UEMR. Moreover, as these advantages may be applicable to the colon, validation through further research is warranted.
When performing GIER, it is necessary to consider the electrical conduction properties of the gelatinous liquid, as well as the risks of current transmission to the wall and delayed transmural thermal injury. As the gelatinous liquid does not contain electrolytes, it has the advantage of not discharging when monopolar devices are used 16 17 . Yano et al. established appropriate electrical conductivity using rat liver and developed the dedicated gel 16 . They also verified the efficacy of electrocoagulation under the gelatinous liquid with monopolar hemostatic forceps in a porcine model and revealed that severe coagulative necrosis was not observed in the muscularis but was limited to the mucosa 16 . Therefore, we believe that the efficacy of electrocoagulation is favorable and that the risks of current transmission to the wall and delayed transmural thermal injury are low.
Although we propose GIER as a novel replacement method for UEMR, GIER has some drawbacks. First, the gelatinous liquid costs 2000 Japanese Yen per 200 mL, which is more expensive than water. Therefore, a validation of GIER cost-effectiveness is warranted. Second, the specific gravity of the gelatinous liquid is heavier than that of water, which may cause loss of buoyancy of the mucosal and submucosal layers, thereby reducing the advantage offered by the water immersion method. Furthermore, the viscosity of the gelatinous liquid may possibly affect the opening and closing of the snare. These issues might make snaring difficult and lead to perforation and a low resection rate; however, our previous reports revealed that the buoyancy of the mucosal and submucosal layers was favorable and similar to that in underwater endoscopy based on endoscopic ultrasonography findings 19 . Additionally, there was no difficulty in manipulating the snare. In fact, the resection rate was high in the present study and no cases of perforation were observed.
This study has several limitations. First, this was a single-center retrospective pilot study with a small sample size. Furthermore, some patients were included several times. As a result, we cannot exclude the possibility of statistical dependencies. It should be noted however that GIER is a novel treatment method, and there are no case–control studies or comparative studies with regard to the treatment of SNADET with GIER. Second, the impact of a learning curve and device improvement cannot be denied. In this study, UEMR was performed for the first 18 cases, while GIER was performed for the latter 22 cases. The improvement in technique owing to the learning curve and the use of a novel snare might have led to favorable outcomes in the GIER group. Third, the consumption of water was not recorded in the UEMR group. Therefore, it is unclear whether GIER really reduced the liquid consumption compared with UEMR. Finally, long-term clinical outcomes, such as local residual recurrence, were not validated. The evaluation of whether GIER is definitely an effective treatment requires the verification of long-term outcomes, and additional time is needed.
In conclusion, the gelatinous liquid enables continuous lumen filling and secures a favorable visual field. GIER is a method that applies these features and might result in a short and safe procedure. Therefore, GIER has the potential to be a novel treatment method for SNADETs. To confirm its efficacy, multicenter prospective studies on the short- and long-term clinical outcomes are needed.
Acknowledgments
We are grateful to Yasunori Sato (Department of Preventive Medicine and Public Health, Keio University School of Medicine, Tokyo, Japan) for his insightful comments. This study was supported by the Clinical Research Support Center at Asahi General Hospital, Chiba, Japan. We thank all support team members.
Footnotes
Competing interests The authors declare that they have no conflict of interest.
References
- 1.Goda K, Kikuchi D, Yamamoto Y et al. Endoscopic diagnosis of superficial non-ampullary duodenal epithelial tumors in Japan: multicenter case series. Dig Endosc. 2014;26 02:23–29. doi: 10.1111/den.12277. [DOI] [PubMed] [Google Scholar]
- 2.Yamasaki Y, Uedo N, Takeuchi Y et al. Current status of endoscopic resection for superficial nonampullary duodenal epithelial tumors. Digestion. 2018;97:45–51. doi: 10.1159/000484112. [DOI] [PubMed] [Google Scholar]
- 3.Yoshida M, Yabuuchi Y, Kakushima N et al. The incidence of non-ampullary duodenal cancer in Japan: the first analysis of a national cancer registry. J Gastroenterol Hepatol. 2021;36:1216–1221. doi: 10.1111/jgh.15285. [DOI] [PubMed] [Google Scholar]
- 4.Vanbiervliet G, Moss A, Arvanitakis M et al. Endoscopic management of superficial nonampullary duodenal tumors: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2021;53:522–534. doi: 10.1055/a-1442-2395. [DOI] [PubMed] [Google Scholar]
- 5.Kato M, Kanai T, Yahagi N et al. Endoscopic resection of superficial non-ampullary duodenal epithelial tumor. DEN Open. 2022;2:e54. doi: 10.1002/deo2.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Binmoeller K F, Shah J N, Bhat Y M et al. “Underwater” EMR of sporadic laterally spreading nonampullary duodenal adenomas (with video) Gastrointest Endosc. 2013;78:496–502. doi: 10.1016/j.gie.2013.03.1330. [DOI] [PubMed] [Google Scholar]
- 7.Yamasaki Y, Uedo N, Takeuchi Y et al. Underwater endoscopic mucosal resection for superficial nonampullary duodenal adenomas. Endoscopy. 2018;50:154–158. doi: 10.1055/s-0043-119214. [DOI] [PubMed] [Google Scholar]
- 8.Shibukawa G, Irisawa A, Sato A et al. Endoscopic mucosal resection performed underwater for nonampullary duodenal epithelial tumor: evaluation of feasibility and safety. Gastroenterol Res Pract. 2018;2018:7.490961E6. doi: 10.1155/2018/7490961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhogal N, Mohan B, Chandan S et al. Efficacy and safety of underwater endoscopic mucosal resection for superficial non-ampullary duodenal epithelial tumors: a systematic review and meta-analysis. Ann Gastroenterol. 2020;33:379–384. doi: 10.20524/aog.2020.0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iwagami H, Takeuchi Y, Yamasaki Y et al. Feasibility of underwater endoscopic mucosal resection and management of residues for superficial non-ampullary duodenal epithelial neoplasms. Dig Endosc. 2020;32:565–573. doi: 10.1111/den.13541. [DOI] [PubMed] [Google Scholar]
- 11.Kiguchi Y, Kato M, Nakayama A et al. Feasibility study comparing underwater endoscopic mucosal resection and conventional endoscopic mucosal resection for superficial non-ampullary duodenal epithelial tumor < 20 mm. Dig Endosc. 2020;32:753–760. doi: 10.1111/den.13524. [DOI] [PubMed] [Google Scholar]
- 12.Hirasawa K, Ozeki Y, Sawada A et al. Appropriate endoscopic treatment selection and surveillance for superficial non-ampullary duodenal epithelial tumors. Scand J Gastroenterol. 2021;56:342–350. doi: 10.1080/00365521.2020.1867896. [DOI] [PubMed] [Google Scholar]
- 13.Furukawa M, Mitoro A, Ozutumi T et al. Efficacy of underwater endoscopic mucosal resection for superficial non-ampullary duodenal epithelial tumor. Clin Endosc. 2021;54:371–378. doi: 10.5946/ce.2020.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamasaki Y, Uedo N, Akamatsu T et al. Nonrecurrence rate of underwater EMR for ≤20-mm nonampullary duodenal adenomas: a multicenter prospective study (D-UEMR study) Clin Gastroenterol Hepatol. 2022;20:1010–1018. doi: 10.1016/j.cgh.2021.06.043. [DOI] [PubMed] [Google Scholar]
- 15.Okimoto K, Maruoka D, Matsumura T et al. Utility of underwater EMR for nonpolypoid superficial nonampullary duodenal epithelial tumors ≤20 mm. Gastrointest Endosc. 2022;95:140–148. doi: 10.1016/j.gie.2021.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Yano T, Ohata A, Hiraki Y et al. Development of a gel dedicated to gel immersion endoscopy. Endosc Int Open. 2021;9:E918–E924. doi: 10.1055/a-1396-4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yano T, Takezawa T, Hashimoto K et al. Gel immersion endoscopy: innovation in securing the visual field–clinical experience with 265 consecutive procedures. Endosc Int Open. 2021;9:E1123–E1127. doi: 10.1055/a-1400-8289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yano T, Nemoto D, Ono K et al. Gel immersion endoscopy: a novel method to secure the visual field during endoscopy in bleeding patients (with videos) Gastrointest Endosc. 2016;83:809–811. doi: 10.1016/j.gie.2015.09.048. [DOI] [PubMed] [Google Scholar]
- 19.Miyakawa A, Kuwai T, Miyauchi T et al. Gel immersion endoscopy-facilitated endoscopic mucosal resection of a superficial nonampullary duodenal epithelial tumor: a novel approach. VideoGIE. 2021;6:422–426. doi: 10.1016/j.vgie.2021.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kato M, Takeuchi Y, Hoteya S et al. Outcomes of endoscopic resection for superficial duodenal tumors: 10 years’ experience in 18 Japanese high volume centers. Endoscopy. 2022;54:663–670. doi: 10.1055/a-1640-3236. [DOI] [PubMed] [Google Scholar]


