Abstract
Vertebrate Tas2r taste receptors detect bitter compounds that are potentially poisonous. Previous studies found substantial variation in the number of Tas2r genes across vertebrates, with some frog species carrying the largest number. Peculiar among vertebrates, frogs undergo metamorphosis, often associated with a dietary shift between tadpoles and adults. A possible explanation for the large size of frog Tas2r families could be that distinct sets of Tas2r genes are required for tadpoles and adults, suggesting differential expression of Tas2r genes between tadpoles and adults. To test this hypothesis, we first examined 20 amphibian genomes and found that amphibians generally possess more Tas2r genes than do other vertebrate clades. We next focused on the American bullfrog (Lithobates catesbeianus) to examine the expression of its Tas2r genes in herbivorous tadpoles and insectivorous adult frogs. We report that close to one fifth of its 180 Tas2r genes are differentially expressed (22 genes enriched in adults and 11 in tadpoles). Tuning properties were determined for a subset of differentially expressed genes by a cell-based functional assay, with the adult-enriched Tas2r gene set covering a larger range of ligands compared to the tadpole-enriched subset. These results suggest a role of Tas2r genes in the ontogenetic dietary shift of frogs and potentially initiate a new avenue of ontogenetic analysis of diet-related genes in the animal kingdom.
Keywords: taste, diet, ontogeny, bitter, Tas2r
Diet is a key force driving the evolution of animal diversity. All basic sensory modalities of animals may be involved in food selection, including the sense of taste. Among the five basic taste modalities in vertebrates, the bitter taste is specialized to sense bitter-tasting compounds that are potentially poisonous in foods. Vertebrate bitter taste receptors are encoded by Tas2r genes, which vary substantially in number across vertebrate species but remain positively correlated with the amount of plant tissues in diets (1); however, see ref. 2. Among all vertebrates examined thus far, some frog species appear to possess the most Tas2r genes, which cannot be fully explained by their diets (1, 3, 4). However, these studies did not consider ontogenetic dietary shift—the change in diet during multiple life stages of an individual—which may impede a deeper understanding of the relationship between vertebrate bitter taste and diet.
Unlike other tetrapod vertebrates, most amphibians such as anurans undergo ontogenetic dietary shifts from herbivores to insectivores following metamorphosis, usually accompanied by habitat shifts from aquatic to terrestrial environments (5). These shifts may have resulted in exposure to distinct chemical environments, leading to the hypothesis that adult frogs inhabiting both aquatic and terrestrial environments encounter a greater diversity of bitter compounds in their diets than tadpoles existing exclusively in aquatic environments (4). To test whether bitter taste receptor (Tas2r) genes play a role in multiple life stages of amphibians associated with ontogenetic dietary shifts, we first examined 20 amphibian genomes to determine whether amphibians generally possess more Tas2r genes than other clades of vertebrates. Next, we focused on one frog species, the American bullfrog (Lithobates catesbeianus), to examine expression and functional differences of its Tas2r genes between tadpole and adult stages, aiming to test whether differences in Tas2r expression and function are associated with ontogenetic dietary shifts.
Results
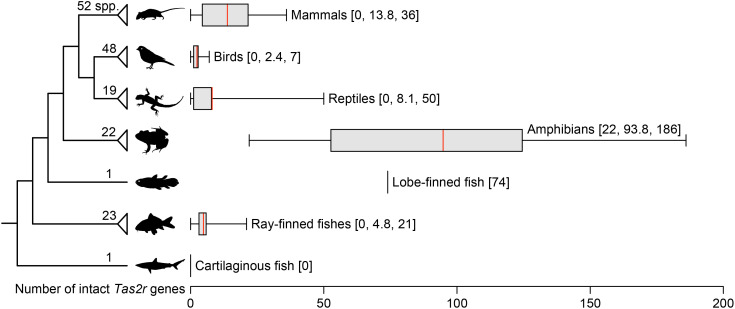
By examining publicly available high-quality genomes of 20 amphibian species, and published data from two additional species (6), we found that the number of intact Tas2r genes with an intact open reading frame and a full-length coding region ranges from 22 (Surinam toad) to 186 (Puerto Rican coqui) (mean 93.8; Fig. 1 and Datasets S1 and S2). A comparison with published data of other clades of vertebrates (cartilaginous fish, ray-finned fish, lobe-finned fish, reptiles, birds, and mammals) revealed that amphibians possess the most Tas2r genes (Fig. 1 and Dataset S1).
Fig. 1.
Number of intact Tas2r genes in vertebrates. Numbers on each branch are previously reported species numbers, including 20 amphibian species that were newly examined in this study. The box indicates the interquartile range of the number of intact Tas2rs; whiskers indicate minimum and maximum numbers; the red line indicates the mean. Minimum, mean, and maximum values are also shown in brackets on the right. Only one species of lobe-finned fish (Latimeria chalumnae) and one species of cartilaginous fish (Callorhinchus milii) have a reported Tas2r number. Silhouettes of vertebrates were taken from PhyloPic (phylopic.org).
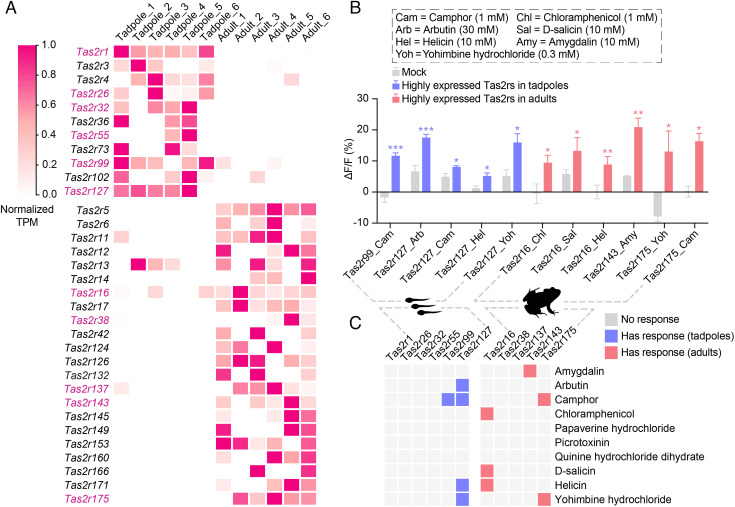
We next focused on the American bullfrog as an exemplar of amphibians to examine expression changes of its Tas2r genes between tadpole and adult stages because it switches its diet upon metamorphosis (from herbivorous tadpoles to insectivorous adults) (7, 8), and it may have one of the largest Tas2r repertoires (1, 4). Using RNA sequencing technology, we estimated expression levels of Tas2r genes by characterizing the transcriptomes of taste tissues from six tadpoles and six adult frogs. Of note, we detected sequencing reads for taste signaling elements such as genes encoding α subunit of gustducin (α-gustducin), Transient receptor potential cation channel subfamily M member 5 (Trpm5), Phospholipase C beta 2 (PLCβ2), and G protein γ subunit 13 (Gγ13), suggesting that the taste tissues collected from both tadpoles and adults contain taste papillae and taste discs (9). The American bullfrog genome contains 180 intact Tas2r genes (Dataset S1), of which 140 were expressed in at least one individual examined (Dataset S3). Of these, 33 were significantly differentially expressed between tadpoles and adults (Fig. 2A), which is significantly greater than that expected by chance (P < 0.0001, unpaired t test); Among them, 11 were preferentially expressed in tadpoles, while 22 were preferentially expressed in adults (Fig. 2A).
Fig. 2.
Expression and functional differences of Tas2r genes between tadpole and adult stages of the American bullfrog. (A) Thirty-three Tas2rs differentially expressed in tadpoles and adults. Tas2rs examined in functional assays are highlighted in purple. (B) Responses of bitter taste receptors to natural bitter compounds. (C) Response profiles of all examined receptors. Tadpole and adult receptors are indicated by blue and red, respectively.
After building a phylogenetic tree of all 180 Tas2r genes identified from the bullfrog genome, we selected six and five differentially expressed genes, representing major phylogenetic positions, in tadpoles and adults, respectively (Datasets S4 and S5), for functional characterization. Using a cell-based functional assay (SI Appendix), we examined responsiveness of these 11 Tas2r genes in vitro toward 10 naturally occurring bitter compounds (Dataset S6) previously shown to activate human bitter receptors (10). We obtained functional responses for two of the six selected genes in tadpoles and three of the five selected genes in adults (Fig. 2 B and C). Tuning properties were different for each gene, whereas the width of tuning was similar between the genes preferentially expressed in tadpoles and adults (Fig. 2 B and C).
Discussion
Why do amphibians need so many Tas2r genes? One possibility is that the expansion of Tas2rs would increase their survival rate by preventing the ingestion of more bitter-tasting toxins in their diets, which could have facilitated their adaptations to diverse environments (11). It is well known that many insects sequester bitter-tasting secondary compounds from plants in their diets for use in their own defense systems (12); thus, insectivorous adult frogs, inhabiting both aquatic and terrestrial environments, would encounter many bitter-tasting compounds in their daily life. This wide environmental variation in amphibians may expose them to a larger number of bitter-tasting compounds in their diets compared to other vertebrates inhabiting solely aquatic or terrestrial environments. Additionally, it is plausible that they may use distinct sets of Tas2rs to evaluate potential foods in different life stages associated with ontogenetic dietary shifts. Indeed, our transcriptome analysis identified that tadpoles and adult bullfrogs express distinct sets of Tas2rs in taste tissues; our functional assays demonstrated that some of those distinctly expressed receptors have different tuning properties. These results are consistent with the hypothesis that tadpoles and adults of amphibians may require divergent functional profiles of Tas2rs to fulfill their needs in different life stages, potentially associated with ontogenetic dietary shifts. However, it is worth noting that the large majority of Tas2r genes do not show differential expression between tadpoles and adult frogs, suggesting that in addition to ontogenetic dietary shift, there must be other factors contributing to the expansion of Tas2r genes in frogs.
Previous studies on the evolution of diet-related genes typically attempted to link gene evolution and diets of adult forms; hence, the contribution of diet-related genes to ontogenetic dietary shifts has not been tested. Our study demonstrates a potential role of bitter receptor genes in ontogenetic dietary shifts of the bullfrog and suggests that ontogeny is more important than previously appreciated in studies of taste genes (1, 3, 4). Since ontogenetic dietary shifts are widespread in animals, as best seen in insects, amphibians, and fishes (13), our work potentially initiates a new avenue of evolutionary analysis of diet-related genes with consideration of ontogeny.
Materials and Methods
Identification of Tas2r genes and reconstruction of phylogenetic trees are described elsewhere (1). Expression differences of six tadpoles and six adult American bullfrogs were examined using RNA sequencing. Transcriptome analysis was performed as described previously (14). Cell-based functional assays of 11 representative Tas2rs were conducted following a previous study (11). See also SI Appendix for details.
Supplementary Material
Appendix 01 (PDF)
Dataset S01 (XLSX)
Dataset S02 (TXT)
Dataset S03 (XLSX)
Dataset S04 (TXT)
Dataset S05 (TXT)
Dataset S06 (XLSX)
Acknowledgments
This work was supported by the National Natural Science Foundation of China (32270436), National Key Research and Development Program of China (2021YFF0702004), and Fundamental Research Funds for the Central Universities (2042021kf0217).
Author contributions
H.Z. designed research; X.H., D.Z., and Q.L. performed research; H.Z. contributed new reagents/analytic tools; X.H., H.J., X.Y., W.L., P.J., and H.Z. analyzed data; and X.H., P.J., and H.Z. wrote the paper.
Competing interests
The authors declare no competing interest.
Data, Materials, and Software Availability
The raw RNA-sequencing (RNA-seq) data were deposited in the National Center for Biotechnology Information (NCBI) Sequence Read Archive under the accession number PRJNA863339 at https://www.ncbi.nlm.nih.gov/bioproject/PRJNA863339 (15); other data in this study are included in the article and/or SI Appendix.
Supporting Information
References
- 1.Li D., Zhang J., Diet shapes the evolution of the vertebrate bitter taste receptor gene repertoire. Mol. Biol. Evol. 31, 303–309 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Syed A. S., Korsching S. I., Positive Darwinian selection in the singularly large taste receptor gene family of an “ancient” fish, Latimeria chalumnae. BMC Genomics 15, 1–15 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi P., Zhang J., Contrasting modes of evolution between vertebrate sweet/umami receptor genes and bitter receptor genes. Mol. Biol. Evol. 23, 292–300 (2006). [DOI] [PubMed] [Google Scholar]
- 4.Zhong H., Huang J., Shang S., Yuan B., Evolutionary insights into umami, sweet, and bitter taste receptors in amphibians. Ecol. Evol. 11, 18011–18025 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trakimas G., et al. , Ontogenetic dietary shifts in European common frog (Rana temporaria) revealed by stable isotopes. Hydrobiologia 675, 87–95 (2011). [Google Scholar]
- 6.Behrens M., Di Pizio A., Redel U., Meyerhof W., Korsching S. I., At the root of T2R gene evolution: Recognition profiles of coelacanth and zebrafish bitter receptors. Genome Biol. Evol. 13, evaa264 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Da Silva E. T., Dos Reis E. P., Feio R. N., Ribeiro Filho O. P., Diet of the invasive frog Lithobates catesbeianus (Shaw, 1802) (Anura: Ranidae) in Viçosa, Minas Gerais State, Brazil. South Am. J. Herpetol. 4, 286–294 (2009). [Google Scholar]
- 8.Ruibal M., Laufer G., Bullfrog Lithobates catesbeianus (Amphibia: Ranidae) tadpole diet: Description and analysis for three invasive populations in Uruguay. Amphib. Reptil. 33, 355–363 (2012). [Google Scholar]
- 9.Behrens M., Meyerhof W., “Mammalian bitter taste perception” in Chemosensory Systems in Mammals, Fishes, and Insects, Korsching S., Meyerhof W., Eds. (Springer, 2009), pp. 77–96. [Google Scholar]
- 10.Lossow K., et al. , Comprehensive analysis of mouse bitter taste receptors reveals different molecular receptive ranges for orthologous receptors in mice and humans. J. Biol. Chem. 291, 15358–15377 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiao H., Wang Y., Zhang L., Jiang P., Zhao H., Lineage-specific duplication and adaptive evolution of bitter taste receptor genes in bats. Mol. Ecol. 27, 4475–4488 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duffey S. S., Sequestration of plant natural products by insects. Annu. Rev. Entomol. 25, 447–477 (1980). [Google Scholar]
- 13.Sánchez-Hernández J., Nunn A. D., Adams C. E., Amundsen P. A., Causes and consequences of ontogenetic dietary shifts: A global synthesis using fish models. Biol. Rev. 94, 539–554 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Pertea M., Kim D., Pertea G. M., Leek J. T., Salzberg S. L., Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 11, 1650–1667 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hao X., Zhao H., Transcriptomedata of taste tissues from tadpoles and adults of the American bullfrog. NCBI. https://www.ncbi.nlm.nih.gov/bioproject/PRJNA863339. Deposited 28 July 2022. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Dataset S01 (XLSX)
Dataset S02 (TXT)
Dataset S03 (XLSX)
Dataset S04 (TXT)
Dataset S05 (TXT)
Dataset S06 (XLSX)
Data Availability Statement
The raw RNA-sequencing (RNA-seq) data were deposited in the National Center for Biotechnology Information (NCBI) Sequence Read Archive under the accession number PRJNA863339 at https://www.ncbi.nlm.nih.gov/bioproject/PRJNA863339 (15); other data in this study are included in the article and/or SI Appendix.