Abstract
Binge eating (BE) is a maladaptive repetitive feeding behavior present across nearly all eating disorder diagnoses. Despite the substantial negative impact of BE on psychological and physiological health, its underlying neural mechanisms are largely unknown. Other repetitive behavior disorders (e.g., obsessive compulsive disorder) show dysfunction within corticostriatal circuitry. However, to date, no work has investigated the in vivo neural dynamics underlying corticostriatal activity during BE episodes. The aim of the current study was to longitudinally examine in vivo neural activity within corticostriatal regions – the infralimbic cortex (IL) and dorsolateral striatum (DLS)– in a robust pre-clinical model for BE. Female C57BL6/J mice (N=32) were randomized to receive: 1) intermittent (daily, 2-hour) binge-like access to palatable food (sweetened condensed milk) (BE), or 2) continuous, non-intermittent (24-hour) access to palatable food (control). In vivo calcium imaging was performed via fiber photometry at baseline and after chronic (4 weeks) engagement in the model for BE. Specific consummatory behaviors (feeding bout onset/offset) during recordings were captured using lickometers which generated TTL outputs for precise alignment of behavior to neural data. IL showed no specific changes in neural activity related to BE. However, BE animals showed decreased DLS activity at feeding onset and offset at the chronic timepoint when compared to activity at the baseline timepoint. Additionally, BE mice had significantly lower DLS activity at feeding onset and offset at the chronic timepoint compared to control mice. These results point to a role for DLS hypofunction in chronic BE, highlighting a potential target for future treatment intervention.
Keywords: Binge eating, Eating behavior, Palatable food, Neural activity, Corticostriatal
1. Introduction
Binge eating (BE) is a chronic and repetitive eating disorder behavior that is present across nearly all eating disorder diagnoses (i.e., anorexia nervosa binge-purge subtype, bulimia nervosa, binge eating disorder) (American Psychiatric Association, 2013) and is also widely prevalent in the general population. BE is strongly associated with elevated rates of obesity (Spitzer et al., 1993; Stice et al., 1999; Stice et al., 2002), poor psychosocial outcomes (e.g., suicidal ideation) (Conti et al., 2017; Telch & Stice, 1998), and significant medical consequences (e.g., type II diabetes) (Herpertz et al., 1998). Despite the substantial negative impact of BE, current psychological treatments for BE and binge-related eating disorders are often limited, as rates of relapse and persistence of eating disorder symptoms are high after intervention (Keel et al., 2005; Olmsted et al., 2015). Additionally, while lisdexamfetamine has been approved by the FDA for treatment of binge eating disorder and has demonstrated success in reduction of food intake and BE (McElroy et al., 2015; Schneider et al., 2021; Schneider et al., 2022), additional work is needed to fully understand the mechanisms underlying its effects. Together, these factors underscore a critical need for research aimed at identifying the neural mechanisms contributing to BE, which could lead to the development of biologically informed treatments.
Aberrant activity within corticostriatal circuitry has been found in psychiatric conditions associated with maladaptive repetitive behaviors such as obsessive compulsive disorder and substance use disorders (Burguiere et al., 2015; George & Koob, 2010; Graybiel & Rauch, 2000; Harrison et al., 2009; Kalivas, 2008). Despite evidence for corticostriatal dysfunction underlying other psychiatric conditions with prominent repetitive behaviors, there is little work directly investigating corticostriatal circuit activity during BE episodes. However, corticostriatal function contributes to inhibitory control (O’Hare et al., 2018), and clinical research has pointed to deficits in inhibitory control in individuals with BE (Mudan Wu et al., 2013). For example, impairments on the Stroop Task are found in BE compared to non-BE populations, suggesting that poor inhibitory control may be related to a history of BE (Manasse et al., 2015). Individuals with BE also have longer stop signal reaction times (M. Wu et al., 2013), and impaired response inhibition in Go/No-Go tasks using food-specific (Hege et al., 2015) and non-food related stimuli (Manasse et al., 2016), providing further evidence of impaired inhibitory control in BE. fMRI has been used to investigate neural correlates associated with inhibitory control in BE within corticostriatal circuitry, showing that women with BE have blunted prefrontal cortex activity on the Stroop task (Balodis et al., 2013), and lower activity within the sensorimotor cortex and dorsolateral striatum (DLS) during a food specific Go/No-Go task compared to women without BE (Skunde et al., 2016). Together, these findings highlight aberrant activity within corticostriatal circuits in BE populations.
While findings from previous work provide evidence that a history of BE behavior is associated with impairments in inhibitory control and corticostriatal circuitry function, to date, there have been no in vivo examinations of activity during episodes of BE in regions of corticostriatal circuitry that may be related to behavioral control. The aim of this study was to fill this gap by identifying corticostriatal dynamics associated with feeding behaviors during binge-like consumption of palatable food intake using in vivo fiber photometry to capture neural activity in the infralimbic cortex (IL) and DLS of BE and control mice. We hypothesized that after a chronic duration of binge-like consumption of palatable food, BE mice would show changes in neural activity within the IL and DLS during feeding bout onset and feeding bout offset compared to control mice, suggesting aberrant corticostriatal function after chronic BE.
2. Materials and Methods
2.1. Animals
Thirty-two adult female C57Bl6/J mice (from Jackson Laboratories or in house breeding which originated from Jackson Laboratory C57Bl6/J mice) were used for all experiments. We chose to focus our work on female mice given the well-established sex difference of greater incidence of BE in females vs. males in both humans (Klump et al., 2017) and rodents (Culbert et al., 2018; Klump et al., 2013). Mice were group housed with 3-5 mice per cage and had ad libitum access to food and water for entirety of study. Animals were maintained on a 12/12-hour light-dark cycle (lights off at 7:00 AM; on at 7:00PM). All experiments were approved by the Institutional Animal Use and Care Committee at the University of Pittsburgh in compliance with National Institutes of Health guidelines for the care and use of laboratory animals.
2.2. Stereotaxic Surgeries
Animals acclimated to the light-dark cycle for approximately 10 days prior to surgery. On day of surgery, mice were anesthetized using 5% isoflurane combined with oxygen for duration of surgery. For each animal, burr holes were drilled unilaterally in left hemisphere over target regions for virus injections and fiberoptic implants. A unilateral injection of 350 nL AAV1-Synapsin.NES-jRCaMP1b.WPRE.SV40 (titer 2.0x1012; Addgene, Watertown, Massachusetts, USA) and 500 nL AAV9-Synapsin-GCaMP6m-WPRE-SV40 (titer 2.7x1012; Addgene) were injected into IL (AP: 2.28, ML: 0.3, DV: −2.15) and DLS (AP: 0.9, ML: 2.6, DV: −2.3), respectively. Following viral injection, optical fibers (NA = .37, Neurophotometrics, San Diego, California, USA) were implanted into IL and DLS using the same coordinates noted above. This surgical protocol allowed for simultaneous in vivo recording from both the IL and DLS in each animal during the study- i.e., a total of 2 photometry fibers per animal. After the completion of surgery, mice were monitored until fully recovered from anesthesia, and then remained in home cage for three weeks to allow for recovery and viral incubation.
2.3. Binge Eating Paradigm
The BE paradigm is based on previous work using intermittent access to palatable food (PF) to elicit binge-like eating in rodents (see Hildebrandt & Ahmari, 2021 for summary). For the current study, mice were randomized to one of two feeding groups: 1) BE, in which animals received binge-like intermittent access to PF daily for 2-hours; or 2) control, in which animals received continuous (24-hour), non-intermittent access to PF. The BE condition selected for this paradigm recapitulates key clinical diagnostic characteristics of BE including intermittent/episodic consumption of a large amount of food which is consumed in a short period of time (American Psychiatric Association, 2013). While not a diagnostic requirement, food consumed during BE is typically palatable in nature–i.e., high in sweetness and fat, but low in nutritional value (Drewnowski et al., 1987; Gendall et al., 1999; Kales, 1990; Yanovski et al., 1992). In order to mimic the content of PF consumed during BE episodes, the PF used in the paradigm was sweetened condensed milk (Nestle; 3.25 kcal/g; 22% fat, 67% sugar, 10% protein; diluted in a 3:1 ratio with water; Furlong et al., 2014). All mice had ad libitum access to standard chow (Prolab Isopro 5P76; 4.1 kcal/g; 14% fat, 60% carbohydrates, 26% protein) throughout the paradigm and were never placed on food restriction.
Mice completed four weeks of daily PF consumption tests starting at approximately 2-3 hours after dark (active phase) onset. Prior to each test, mice were removed from group-housed conditions and placed in individual test cages for the duration of each 2-hour test. PF (delivered via 50mL conical tube with sipper attachment) and standard chow were weighed at the beginning and end of the 2-hour test, and body weight was measured daily. At completion of each test, mice were removed from test cages and returned to group housed home cages. Animals in the control group had a new tube of PF placed in the home cage daily for continuous access to PF. This bottle was weighed daily prior to test.
2.4. Fiber Photometry Recordings and Processing
All in vivo fiber photometry recordings took place at the same time of day as PF consumption tests; however, mice were placed in operant chambers (Med Associates) equipped with contact lickometers mounted to the chamber wall rather than test cages. Contact lickometers (Med Associates) registering TTL outputs for each individual lick were used to identify feeding onset (first lick of a PF consumption bout) and feeding offset (last lick of a PF consumption bout). Bouts were defined as ≥ 2 licks with breaks in between licks of no greater than 3 seconds. Prior to the start of the study, all animals were habituated over multiple days to the operant chamber and lickometer (containing only water). Animals were also habituated for at least two sessions to scruffing and cable attachment. There were two photometry recordings during the study– one at a baseline timepoint (prior to initiation of BE paradigm) and one at a chronic timepoint (after four weeks of engagement in BE paradigm). Animals were water restricted (and home cage PF restricted for control group before the chronic recording) overnight prior to recording days, while maintaining ad libitum access to standard chow. The first recording at the baseline timepoint occurred prior to initiation of BE paradigm, and animals were therefore naïve to PF. This design permits monitoring activity from first exposure/onset through chronic duration of behavior. Additionally, given that there has been no PF exposure at the start of the baseline recording, this provided a control timepoint equivalent to a chow-only access animal. The baseline recording was 40 minutes duration to account for neophobia to PF and ensure enough PF consumption bouts for meaningful interpretation of neural data. The second recording was 20 minutes and took place at the chronic timepoint after completion of BE paradigm (i.e., four weeks). After completion of the baseline and chronic recordings, mice were returned to their individual test cages to complete the daily PF consumption test.
Photometry recordings used a 3-color, 2-region system (Neurophotometrics). Animals were connected using a 4x branching cable (Doric, Quebec, Canada) to allow for simultaneous recording from 2 regions in each animal. The system pulsed at 40Hz and interleaved three LED channels (415nm, 470nm, 560nm) during recordings, resulting in detection of 1) isosbestic signal, 2) GCaMP6m activity (DLS), and 3) jRCaMP1b activity (IL). Bonsai was used to interface the photometry system with Med Associates TTL data and provide synchronization with photometry signal in real time. After recordings, traces were separated to examine activity within each channel independently. All photometry data were processed and analyzed using custom MATLAB scripts. Motion artifacts were first removed from 470nm (GCaMP6m) and 560nm (jRCaMP1b) channels using the 415nm channel (isosbestic) trace as a reference point. Next, traces were corrected for exponential decay and run through a low-pass filter (order 6, frequency 3). A moving minimum using a sliding minimum of 120 seconds was applied to remove large fluctuations from the traces. Finally, traces were z-scored for normalization. These traces were aligned with TTL outputs from lickometers for analysis of neural activity at onset and offset of PF consumption bouts. For each trace, data were aligned to behavior of interest (feeding onset, feeding offset) at timepoint “0”, and the trace analysis window included three seconds prior to the event and one second after the event.
2.5. Histology
After completion of BE paradigm and photometry recordings, all mice were transcardially perfused using 4% paraformaldehyde (PFA). Brains were removed and remained in PFA for an additional 24 hours, cut into 35-micron sections, mounted onto slides, and cover slipped with DAPI mounting media. All sides were scanned using a slide scanning microscope (VS120 Virtual Slide Microscope, Olympus, Tokyo, Japan). Location of fiber implant was identified by examining damage tracks in tissue and the tip of the fibers. Placement of the fiber and spread of virus were used to include or exclude animals from the final sample, and all histology was independently reviewed by two blinded assessors. Only animals with the tip of the fiber falling directly above the virus were used for data analysis (Figure 1A; Supplementary Figure 1). These requirements for inclusion were used given that the area considered the “active zone” for photometry analysis is the area located immediately below the fiber tip and above the fluorescent expression of the virus (Resendez & Stuber, 2015). Notably, some fibers for the IL fall near the IL/prelimbic boundary (Supplementary Figure 1A) as defined by the atlas by Franklin and Paxinos (Franklin & Paxinos, 2007). However, given that the active zone for analysis is immediately beneath the fiber, data was gathered from the IL region. After histological examination, the final sample sizes included in the analysis by region were: IL = 12 BE, 11 control; DLS = 15 BE, 15 control (see Supplementary Figure 1A and 1B for fiber tip placements and representative histological images).
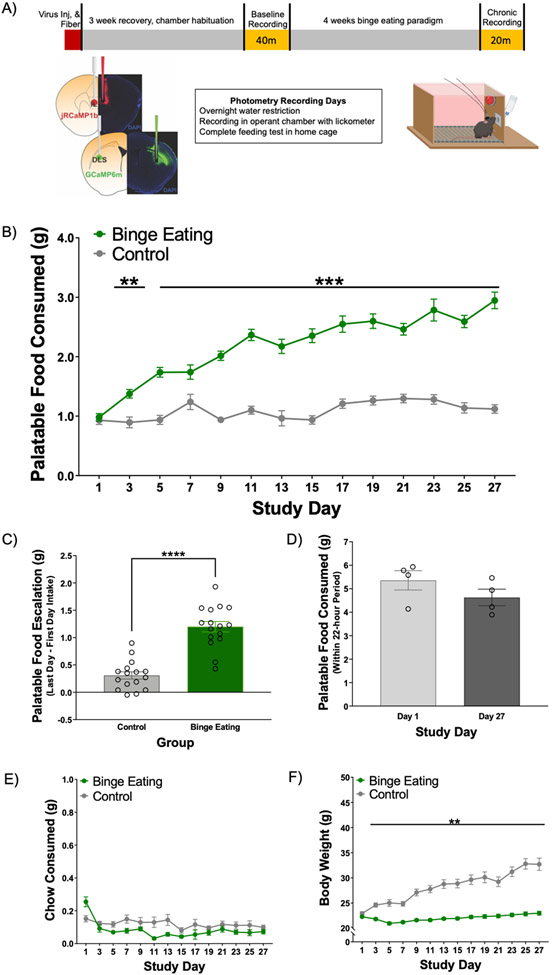
Figure 1. Study timeline and behavioral data.
N = 16 BE, 16 control. A) Study timeline. Mice were injected unilaterally in left hemisphere with AAV1-Synapsin.NED-jRCaMP1b.WPRE.SV40 into IL and AAV9-Synapsin-GCaMP6m-WPRE-SV40 into DLS. Optical fibers were implanted into IL and DLS after viral injection. After recovery, animals completed an in vivo recording with fiber photometry prior to engagement in the paradigm for binge eating (baseline), and after four weeks (chronic). For behavior data, palatable food (PF), chow, and body weight (BW) data are graphed showing every other day. B) BE animals consumed significantly more PF compared to control animals during feeding tests. C) Only BE animals escalated their PF intake across the study period. D) 24-hour home cage (N = 4 total cages) assessments of PF intake in control animals shows no increase or escalation of 24-hour PF intake over study period. E) There were no specific days identified where chow intake was different between BE and control mice. F) Body weight significantly increased in control group across the study period. **p < 0.01, ***p < .001, ****p < .0001.
2.6. Statistical Analyses
For all behavioral data including the BE paradigm and lick/bout data from photometry recording sessions, repeated measures ANOVAs (or mixed models in case of missing data) with Bonferroni post-hoc analyses or individual samples t-tests were used. Results are presented as mean ± SEM. A p-value of ≤ 0.05 was set to determine statistical significance. Statistical analyses were run using GraphPad Prism 9.
For fiber photometry data, multilevel spline regressions were run on normalized photometry data during PF consumption bouts (group analysis of IL and DLS data) or the residuals of the best fitting model (exploratory variance analysis of DLS data). The best fitting models were chosen via model comparisons that employed the Bayesian information criterion (BIC), which considers the degree of flexibility (i.e., number of change points) of the model and penalizes more flexible models (Kuha, 2004). All models were a full factorial analysis, and all categorical predictors were effect coded. For all analyses, the residual distributions were examined for normality and homoscedasticity. All group analysis models met assumptions, but the two variance analysis models were heteroscedastic. To satisfy the assumption of homoscedasticity, the residuals of the best fitting models were square root transformed prior to analysis. Predictors included the between-subjects categorical variable Group (BE, control), and the within-subjects categorical variable Stage (Baseline timepoint, Chronic timepoint) and continuous variable Time (−3 seconds prior to event of interest to 1 second after event of interest). The random effects structure included Intercept (subject) and two slopes (Stage and Time). Planned comparisons were run for significant interactions including Time, Stage, and Group using Type III Sums of Squares. All photometry data were analyzed using R (R 4.0.3). The highest order significant interactions (p < 0.05) including the variables Stage and Group were followed by planned comparisons using R’s emmeans package. Group × Stage × Time interactions were followed by planned comparisons at 500 ms intervals using emmeans. Results are presented as the model fit mean ± SEM. Raw values alongside model fits are presented in Supplementary Data Table 1 and 2.
3. Results
3.1. Binge-like feeding leads to escalated palatable food intake
All animals completed four weeks of the BE paradigm (Figure 1A). On PF consumption test days, total intake of PF and chow during each feeding test was measured. Body weight was also measured daily. For PF intake, there was a significant Time x Group interaction (F(8.32, 237.4) = 31.60, p < .0001) such that BE animals consumed significantly more PF compared to control mice during feeding tests starting on Day 2 (Figure 1B). This pattern persisted for the duration of the study (all ps < .001; except for Day 2, 3: p < 01; Day 7: p = .21). BE animals also significantly escalated their PF intake (average PF intake week 4 – average PF intake week 1) during feeding tests compared to control animals across the four week BE paradigm (Figure 1C; t(30) = 7.63, p < .0001). PF intake from the home cages of control animals with continuous PF access was also assessed daily and showed no escalation in total PF consumed across the study period (p > .99, Figure 1D), providing further support that escalation of PF intake was specific to BE mice with intermittent access to PF. There was a significant Time x Group interaction for chow intake during PF consumption tests (Figure 1E, F(26, 764) = 2.67, p < .0001); however, post-hoc analysis identified no significant differences across days between BE and control mice. There was also a significant Time x Group interaction for body weight (Figure 1F, F(26, 780) = 50.65, p < .0001), with control mice weighing significantly more than BE animals; this difference emerged on study Day 2 and persisted across study (all p’s < .01). Note, this is similar to observations in other work examining binge-like eating in rodents, with the BE group maintaining a normal weight range across study duration in contrast to progressively increasing weight within the control group (Boggiano et al., 2007; Czyzyk et al., 2010).
3.2. Binqe-like consumption of palatable food leads to an increase in feeding bouts during in vivo recordings
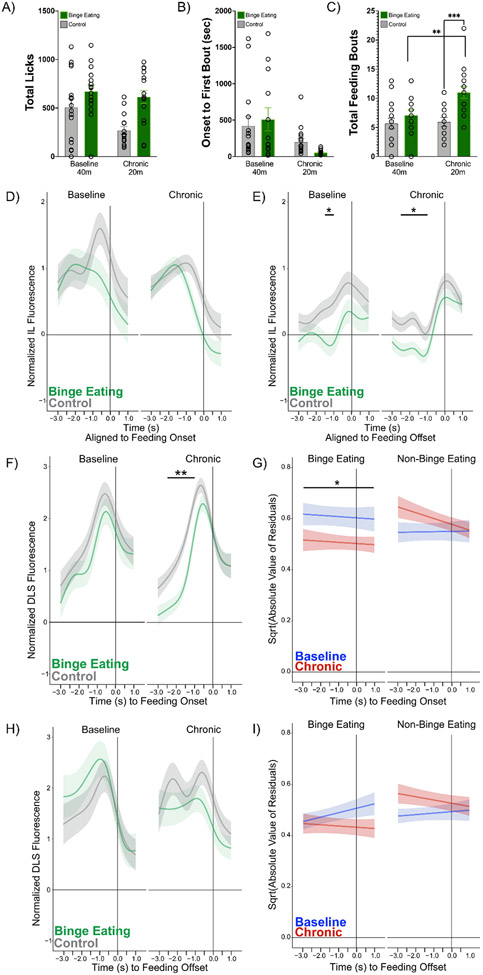
During in vivo fiber photometry recordings, consumption behaviors (licks; onset to first feeding bout; total feeding bouts) were assessed using data collected from contact lickometers inside the operant chamber. There were no significant interactions for total licks (Figure 2A, F(1, 28) = 2.72, p = .11) or onset to first bout (Figure 2B, F(1, 26) = 1.44, p = .24). However, there was a significant Timepoint x Group interaction for total feeding bouts (Figure 2C, F(1, 28) = 6.37, p = .02), such that the BE mice had significantly more bouts at the chronic timepoint compared to controls (p = .0003). Additionally, BE mice increased their total bouts from between baseline and chronic recording timepoints (p = .002), while the control mice showed no change in total bouts over time.
Figure 2. In vivo fiber photometry in IL and DLS at feeding onset and offset.
Placement of virus and fiber were verified using histology to determine final sample size (IL = 12 BE, 11 control; DLS = 15 BE, 15 control). A) Total licks during baseline and chronic recordings. B) Time in seconds of onset to first bout during recordings. C) Total bouts during baseline and chronic recordings. BE had significantly more bouts at the chronic duration (p = .0003) and increased their bouts over time (p = .002). All in vivo photometry data are aligned to feeding onset (D, F, G) or feeding offset (E, H, I) at Time = 0. D) There were no differences between BE and control mice in feeding onset-associated IL activity. E) At baseline, BE animals had significantly lower activity vs. control animals −1.5 and 1.0 seconds before feeding offset at baseline. At the chronic timepoint, BE animals had significantly lower activity −2.5, −2.0, −1.5, and −1.0 seconds before feeding offset vs. control animals. F) BE animals had significantly lower activity vs. control animals at the chronic timepoint −2.5 to −1.0 seconds prior to feeding onset. G) Variance in activity associated with feeding onset significantly decreased from the acute to chronic timepoint in BE, but not in control mice. Variance at chronic timepoint was lower in BE vs. control mice. Group × Stage interaction (χ2 (1) = 4.25, p = 0.04). H) Trend toward significant difference at chronic timepoint with lower activity at −0.5 and 0.0 seconds before feeding offset in BE vs. control mice. I) No differences in variance related to feeding offset in BE and control mice. *p < 0.05, **p < 0.01, ***p < .001.
3.3. Binge eating paradigm does not lead to significant differences in IL activity measured using fiber photometry
Comparing Bayesian Information Criteria (BICs), the best fitting model for IL feeding onset included five change points. There was no significant Group × Stage (χ2(1) = 0.05, p = 0.81) or Group × Stage × Time (χ2(5) = 6.42, p = 0.27) interaction. There were no differences in activity between or within BE and control groups (Figure 2D). Other lower order effects are shown in Supplementary Data Table 1. Comparing BICs, the best fitting model for IL feeding offset included six change points. There was a significant Group × Stage × Time interaction (χ2(6) = 145.84, p < 0.01). The interaction revealed that the control group had reduced activity compared to the baseline timepoint at −1.0 seconds (z = 2.51, p = 0.02) prior to feeding offset. There were no differences within the BE group (all p > 0.10). At baseline, BE animals had lower activity than the control group at −1.5 (z = −2.41, p = 0.03) and −1.0 (z = −2.74, p = 0.01) seconds prior to feeding offset (Figure 2E). At the chronic timepoint, BE animals had lower activity at −2.5 (z = −2.48, p = 0.02), −2.0 (z = −3.03, p < 0.01), −1.5 (z = −2.96, p = 0.01), and −1.0 (z = 2.07, p = 0.05) seconds compared to the control group (Figure 2E). However, these results at the chronic timepoint are inconclusive given the significant differences in activity at the baseline timepoint between groups (Figure 2E, left panel). Other lower order effects are shown in Supplementary Data Table 1.
3.4. Binge-like consumption of palatable food leads to decreased DLS activity at feeding onset
Comparing BICs, the best fitting model for DLS feeding onset included five change points. There was a significant Group × Stage × Time interaction (χ2(5) = 41.17, p < 0.01). The BE group had reduced DLS activity at the chronic timepoint compared to the baseline timepoint at −2.5 (z = 2.13, p = 0.03) and −2.0 (z = 2.16, p = 0.03) seconds prior to feeding onset. There were no significant differences between baseline and chronic timepoints in control animals. Between group analysis showed that, compared to the control group, BE animals had reduced activity from −2.5 to −1.0 seconds (zs = −4.46 to −2.72, all p < 0.01) prior to feeding bout onset at the chronic timepoint (Figure 2F). Other lower order effects can be found in Supplementary Data Table 2. Exploratory analyses examining variance across groups found a significant Group × Stage interaction (χ2(1) = 4.32, p = 0.04). The BE group had reduced variability in their neural activity related to feeding onset at the chronic timepoint compared to the baseline timepoint (z = 2.29, p = 0.02) (Figure 2G), while there were no changes in variance in the control group from the baseline to chronic timepoints (z = −1.27, p = 0.21) (Figure 2G). The interaction also showed that at the chronic timepoint, the BE group had lower variability than the control group (z = −2.34, p = 0.02); this difference was not observed during the baseline timepoint (z = 1.17, p = 0.24) (Figure 2G).
3.5. Trend towards decreased DLS activity at feeding offset after binge-like consumption of palatable food
Comparing BICs, the best fitting model for DLS feeding offset included five change points. There was a significant Group × Stage × Time interaction (χ2(5) = 30.51, p < 0.01). The BE group had reduced activity in DLS at the chronic compared to the baseline timepoint at −1.5 (z = 1.97, p = 0.048) and −1.0 (z = 2.33, p = 0.02) seconds prior to feeding offset. The control group had increased activity at the chronic timepoint compared to the baseline timepoint at −2.5 (z = −2.13, p = 0.03) seconds. Compared to the control group, BE animals trended towards having reduced activity at −0.5 (z = −1.80, p = 0.07) and 0 (z = −1.76, p = 0.08) seconds prior to feeding bout offset at the chronic, but not baseline, timepoint (Figure 2H). Other lower order effects are shown in Supplementary Data Table 2. Exploratory analyses examining variance in DLS activity associated with feeding offset found no significant interactions including both Group and Stage (all ps > 0.05) (Figure 2I).
4. Discussion
This study used in vivo fiber photometry to investigate longitudinal changes in neural activity within corticostriatal circuitry in BE and control mice. We examined activity during naturalistic binge-like feeding episodes aligned to specific consumption behaviors (feeding onset, feeding offset) that can also be quantitatively measured in humans. While IL activity showed no specific changes after undergoing the BE paradigm (Figure 2 D-E; observed differences in BE group are present at both baseline and chronic timepoints), we found differential activity patterns in the DLS between BE and control mice after a chronic duration of PF intake (Figure 2 F-I). Additionally, findings highlighted that a history of binge-like feeding leads to a stronger impact on feeding onset versus offset in DLS (Figures 2F and H). Together, these findings suggest that reduced recruitment of DLS, particularly during feeding onset, is specific to animals with a history of binge-like consumption of PF (i.e., intermittent/episodic) and not general (i.e., continuous, non-intermittent/non-episodic) PF intake. These results point to a neural mechanism in the DLS that may underlie chronic BE, highlighting a potential target for future treatment intervention.
Feeding bouts involve a repetitive sequence of behaviors including feeding onset, consumption, and feeding offset, and previous work has shown that DLS plays a key role in initiating and completing sequences. Training on a 2-step lever pressing task showed that DLS lesions impaired acquisition of correct sequence completion in mice (Yin, 2010), and work using a viral strategy to specifically suppress activity of D1+ spiny projection neurons in DLS found impairments in sequence accuracy (Rothwell et al., 2015). In the current study, we measured DLS activity during distinct phases of the feeding sequence (feeding onset, feeding offset). At both feeding onset and offset, BE animals showed a reduction in DLS activity from their baseline to the chronic timepoint. At the chronic timepoint, BE animals also had lower activity compared to control animals at feeding onset (Figure 2F). At feeding offset, BE mice showed a blunting of activity from baseline to the chronic timepoint (Figure 2H) and trended towards lower DLS activity than control mice at the chronic timepoint (Figure 2H). Our findings thus suggest that decreased/blunted DLS activity in BE mice may contribute to difficulty in executing proper feeding sequences in BE mice.
Reduced activity in DLS of BE mice may result from decreased activity in upstream regions that project to the DLS and contribute to sequence performance, such as secondary motor cortex (M2), a primary input to DLS (Lee et al., 2019; Rothwell et al., 2015). Pharmacological inactivation of M2 in rats with a history of BE increased PF consumption (Corwin et al., 2016), suggesting that lower M2 activity may contribute to impairments in feeding offset/feeding sequence completion. M2→DLS projections are critical for correct execution and termination of behavioral sequences (Rothwell et al., 2015). Therefore, the blunted activity found at feeding onset in DLS of BE mice may represent less input from M2→DLS projections, thus promoting ongoing repetitive action. While these data underscore the importance of DLS and associated inputs in generating sequenced behavior, future work should use alternative sequence testing paradigms in chronic BE and control mice to determine if these findings are unique to binge-like consumption of PF or generalize to other sequenced behaviors. Additionally, manipulation of M2→DLS projections during distinct phases of the feeding sequence will help directly dissect the role of these projections in binge-like eating.
Previous work has suggested that BE leads to an imbalance in goal-directed and habitual behavior. Clinical research of BE populations indicates that individuals with BE show increased recruitment of brain regions involved in goal-directed behavior (e.g., ventromedial prefrontal cortex) when viewing images of PF (Neveu et al., 2018). However, on a decision making task unrelated to food, individuals with BE disorder engaged in significantly more habitual responding (Voon et al., 2015). Together, these findings suggest that feeding and food related tasks may differently recruit goal-directed/habitual circuitry compared to tasks assessing habitual decision-making (unrelated to feeding and food) in individuals with BE. Pre-clinical studies have attempted to further dissect the mechanisms underlying goal-directed vs. habitual behavior in BE. Work has shown that baseline deficits in goal-directed responding in rats naïve to BE were associated with higher levels of subsequent BE upon exposure to PF (LeMon et al., 2019), suggesting that impaired goal-directed responding may be a risk factor for BE development. Other work has shown that rats with a history of BE engaged in more lever pressing during outcome devaluation testing, suggesting BE led to more habitual responding compared to continuous access or control rats (Furlong et al., 2014). This pattern of habitual responding was associated with increased outcome-devaluation-test-related-c-Fos activity in areas associated with habit (i.e., DLS, IL) (Furlong et al., 2014). This contrasts with our findings of decreased DLS activity after chronic binge-like consumption of PF at feeding onset and offset, suggesting that in our paradigm, BE-related feeding behaviors are not driven by habit. However, the current study examined neural activity in real time rather than using an indirect marker of neural activity like c-Fos, which may explain the differences in results. Nonetheless, future work should investigate engagement of corticostriatal regions associated with goal-directed behavior (e.g., prelimbic cortex, dorsomedial striatum) during binge-like consumption of PF to help disentangle the relationship between goal-directed and habitual behavior in control of pathological BE feeding behavior.
Despite the strengths of this study, it is important to note the limitations. Prior to the chronic photometry recording, the PF tube was removed from the home cages of control animals overnight to maximize the potential for PF consumption during the recording. This manipulation was unable to be completed in the BE group as they did not have continuous PF access. Despite this difference in experimental manipulation, the control animals did not differ in their PF consumption after overnight PF restriction compared to the three days prior to the recording (all ps > .10, data not shown), thus suggesting that this manipulation did not impact feeding behavior. Animals were also water restricted overnight before the chronic recording. However, comparison of within-group PF and chow intake from the feeding test on the day prior to the recording versus the recording day, we found no within-group intake differences in the BE or control group (all ps > .10, data not shown). This suggests that feeding patterns were maintained despite water restriction, and that the isolated overnight water restriction performed in this study likely had minimal effects on intake, and thus, minimal effects on neural data. Nonetheless, future studies will examine neural activity in BE and control animals that are not water restricted or PF restricted (for the control animals). Additionally, there were significant body weight differences between the BE and control animals. While follow-up analyses showed that inclusion of body weight as a random effect variable in the models did not impact or change the findings reported above (Supplementary Data Table 3), it is worth noting that other factors associated with body weight differences (e.g., metabolic changes, adiposity) may differentially contribute to findings, and future studies should further examine potential group differences related to these factors. Finally, while results did not reach statistical significance, IL activity appears to increase prior to feeding onset in control mice at baseline (Figure 2D). Despite not reaching significance, these effects are characterized by more variability than findings in DLS. Therefore, it will be critical for future investigations to explore neural activity in IL with larger sample sizes to fully understand the potential relevance of these subtle but potentially biologically relevant effects to binge-like consumption of PF.
In conclusion, we capitalized on pre-clinical approaches to longitudinally examine in vivo neural activity associated with core feeding behaviors during binge-like feeding episodes, an approach currently unavailable in clinical research. Our results demonstrate decreased calcium activity in the DLS after chronic BE, pointing to a potential target for development of biologically informed treatment interventions. Future work should further investigate corticostriatal contributions to BE behavior and the interplay between goal-directed and habitual behavior in this context.
Supplementary Material
Funding:
This work was supported by the BRAIN Initiative (F32MH118687, BH) and the National Institute of Mental Health (T32MH016804).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest: The authors declare no competing financial interests.
Ethical Statement
All experiments were approved by the Institutional Animal Use and Care Committee at the University of Pittsburgh (Protocol #22020586) in compliance with National Institutes of Health guidelines for the care and use of laboratory animals.
Conflict of Interest Statement
The authors declare no competing financial interests.
Data Availability:
Data will be made available on request.
References
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders (5th ed.). American Psychiatric Association. [Google Scholar]
- Balodis IM, Molina ND, Kober H, Worhunsky PD, White MA, Sinha R, … Potenza MN (2013). Divergent neural substrates of inhibitory control in binge eating disorder relative to other manifestations of obesity. Obesity, 21(2), 367–377. 10.1002/oby.20068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggiano MM, Artiga AI, Pritchett CE, Chandler-Laney PC, Smith ML, & Eldridge AJ (2007). High intake of palatable food predicts binge-eating independent of susceptibility to obesity: an animal model of lean vs obese binge-eating and obesity with and without binge-eating. International Journal of Obesity, 31(9), 1357–1367. 10.1038/sj.ijo.0803614 [DOI] [PubMed] [Google Scholar]
- Burguiere E, Monteiro P, Mallet L, Feng G, & Graybiel AM (2015). Striatal circuits, habits, and implications for obsessive–compulsive disorder. Current Opinion in Neurobiology, 30, 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti C, Lanzara R, Scipioni M, Iasenza M, Guagnano MT, & Fulcheri M (2017). The relationship between binge eating disorder and suicidality: A systematic review. Frontiers in Psychology, 8, 2125. 10.3389/fpsyg.2017.02125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin RL, Wojnicki FHE, Zimmer DJ, Babbs RK, McGrath LE, Olivos DR, … Hayes MR (2016). Binge-type eating disrupts dopaminergic and GABAergic signaling in the prefrontal cortex and ventral tegmental area. Obesity, 24(10), 2118–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbert KM, Sinclair EB, Hildebrandt BA, Sisk CL, & Klump KL (2018). Perinatal testosterone contributes to mid-to-post pubertal sex differentiated risk for binge eating in male and female rats. Journal of Abnormal Psychology, 127(2), 239–250. 10.1037/abn0000334 [DOI] [PubMed] [Google Scholar]
- Czyzyk TA, Sahr AE, & Statnick MA (2010). A model of binge-like eating behavior in mice that does not require food deprivation or stress. Obesity, 18(9), 1710–1717. 10.1038/oby.2010.46 [DOI] [PubMed] [Google Scholar]
- Drewnowski A, Bellisle F, Aimez P, & Remy B (1987). Taste and bulimia. Physiology & Behavior, 41(6), 621–626. 10.1016/0031-9384(87)90320-9 [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, & Paxinos G (2007). The Mouse Brain in Stereotaxic Coordinates, 3rd Edn San Diego. In: Elsevier Academic Press. [Google Scholar]
- Furlong TM, Jayaweera HK, Balleine BW, & Corbit LH (2014). Binge-like consumption of a palatable food accelerates habitual control of behavior and is dependent on activation of the dorsolateral striatum. Journal of Neuroscience, 34(14), 5012–5022. 10.1523/JNEUROSCI.3707-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendall KA, Joyce PR, & Abbott RM (1999). The effects of meal composition on subsequent craving and binge eating. Addictive Behaviors, 24(3), 305–315. 10.1016/S0306-4603(98)00046-X [DOI] [PubMed] [Google Scholar]
- George O, & Koob GF (2010). Individual differences in prefrontal cortex function and the transition from drug use to drug dependence. Neuroscience & Biobehavioral Reviews, 35(2), 232–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM, & Rauch SL (2000). Toward a neurobiology of obsessive-compulsive disorder. Neuron, 28(2), 343–347. [DOI] [PubMed] [Google Scholar]
- Harrison BJ, Soriano-Mas C, Pujol J, Ortiz H, López-Solà M, Hernández-Ribas R, … Pantelis C (2009). Altered corticostriatal functional connectivity in obsessive-compulsive disorder. Archives of General Psychiatry, 66(11), 1189–1200. [DOI] [PubMed] [Google Scholar]
- Hege MA, Stingl KT, Kullmann S, Schag K, Giel KE, Zipfel S, & Preissl H (2015). Attentional impulsivity in binge eating disorder modulates response inhibition performance and frontal brain networks. International Journal of Obesity, 39(2), 353–360. [DOI] [PubMed] [Google Scholar]
- Herpertz S, Albus C, Wagener R, Kocnar M, Wagner R, Henning A, … Thomas W (1998). Comorbidity of Diabetes and Eating Disorders: Does diabetes control reflect disturbed eating behavior? Diabetes Care, 21(7), 1110–1116. 10.2337/diacare.21.7.1110 [DOI] [PubMed] [Google Scholar]
- Hildebrandt BA, & Ahmari SE (2021). Breaking It Down: Investigation of Binge Eating Components in Animal Models to Enhance Translation. Frontiers in psychiatry, 1387. 10.3389/fpsyt.2021.728535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kales EF (1990). Macronutrient analysis of binge eating in bulimia. Physiology & Behavior, 48(6), 837–840. 10.1016/0031-9384(90)90236-W [DOI] [PubMed] [Google Scholar]
- Kalivas PW (2008). Addiction as a pathology in prefrontal cortical regulation of corticostriatal habit circuitry. Neurotoxicity Research, 14(2–3), 185–189. [DOI] [PubMed] [Google Scholar]
- Keel PK, Dorer DJ, Franko DL, Jackson SC, & Herzog DB (2005). Postremission predictors of relapse in women with eating disorders. American Journal of Psychiatry, 162(12), 2263–2268. 10.1176/appi.ajp.162.12.2263 [DOI] [PubMed] [Google Scholar]
- Klump KL, Culbert KM, & Sisk CL (2017). Sex differences in binge eating: Gonadal hormone effects across development. Annual Review of Clinical Psychology, 13, 183–207. http://www.annualreviews.org/doi/full/10.1146/annurev-clinpsy-032816-045309?url_ver=Z39.88-2003&rfrJd=ori%3Arid%3Acrossref.org&rfr_dat=cr_pub%3Dpubmed [DOI] [PubMed] [Google Scholar]
- Klump KL, Racine SE, Hildebrandt B, & Sisk CL (2013). Sex differences in binge eating patterns in male and female adult rats. International Journal of Eating Disorders, 46(7), 729–736. 10.1002/eat.22139 [DOI] [PubMed] [Google Scholar]
- Kuha J (2004). AIC and BIC: Comparisons of assumptions and performance. Sociological methods & research, 33(2), 188–229. [Google Scholar]
- Lee K, Bakhurin KI, Claar LD, Holley SM, Chong NC, Cepeda C, … Masmanidis SC (2019). Gain modulation by corticostriatal and thalamostriatal input signals during reward-conditioned behavior. Cell Reports, 29(8), 2438–2449. e2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMon JV, Sisk CL, Klump KL, & Johnson AW (2019). Reduced sensitivity to devaluation for instrumental but not consummatory behaviors in binge eating prone rats. Physiology & Behavior, 206, 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manasse SM, Forman EM, Ruocco AC, Butryn ML, Juarascio AS, & Fitzpatrick KK (2015). Do executive functioning deficits underpin binge eating disorder? A comparison of overweight women with and without binge eating pathology. International Journal of Eating Disorders, 48(6), 677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manasse SM, Goldstein SP, Wyckoff E, Forman EM, Juarascio AS, Butryn ML, … Nederkoorn C (2016). Slowing down and taking a second look: Inhibitory deficits associated with binge eating are not food-specific. Appetite, 96, 555–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neveu R, Neveu D, Carrier E, Gay A, Nicolas A, & Coricelli G (2018). Goal directed and self-control systems in bulimia nervosa: an fMRI study. EBioMedicine, 34, 214–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hare J, Calakos N, & Yin HH (2018). Recent insights into corticostriatal circuit mechanisms underlying habits. Current opinion in behavioral sciences, 20, 40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmsted MP, MacDonald DE, McFarlane T, Trottier K, & Colton P (2015). Predictors of rapid relapse in bulimia nervosa. International Journal of Eating Disorders, 48(3), 337–340. 10.1002/eat.22380 [DOI] [PubMed] [Google Scholar]
- Resendez SL, & Stuber GD (2015). In vivo calcium imaging to illuminate neurocircuit activity dynamics underlying naturalistic behavior. Neuropsychopharmacology, 40(1), 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell PE, Hayton SJ, Sun GL, Fuccillo MV, Lim BK, & Malenka RC (2015). Input-and output-specific regulation of serial order performance by corticostriatal circuits. Neuron, 88(2), 345–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skunde M, Walther S, Simon JJ, Wu M, Bendszus M, Herzog W, & Friederich HC (2016). Neural signature of behavioural inhibition in women with bulimia nervosa. Journal of Psychiatry & Neuroscience, 41(5), E69–E78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer RL, Yanovski S, Wadden T, Wing R, Marcus MD, Stunkard A, … Horne RL (1993). Binge eating disorder: its further validation in a multisite study. International Journal of Eating Disorders, 13(2), 137–153. 10.1002/1098-108X(199303)13:2<137::AID-EAT2260130202>3.0.CO;2-%23 [DOI] [PubMed] [Google Scholar]
- Stice E, Cameron RP, Killen JD, Hayward C, & Taylor CB (1999). Naturalistic weight-reduction efforts prospectively predict growth in relative weight and onset of obesity among female adolescents. Journal of Consulting and Clinical Psychology, 67(6), 967–974. 10.1037//0022-006x.67.6.967 [DOI] [PubMed] [Google Scholar]
- Stice E, Presnell K, & Spangler D (2002). Risk factors for binge eating onset in adolescent girls: a 2-year prospective investigation. Health Psychology, 21(2), 131–138. 10.1037/0278-6133.21.2.131 [DOI] [PubMed] [Google Scholar]
- Telch CF, & Stice E (1998). Psychiatric comorbidity in women with binge eating disorder: Prevalence rates from a non-treatment-seeking sample. Journal of Consulting and Clinical Psychology, 66(5), 768–776. 10.1037//0022-006x.66.5.768 [DOI] [PubMed] [Google Scholar]
- Voon V, Baek K, Enander J, Worbe Y, Morris LS, Harrison NA, … Daw N (2015). Motivation and value influences in the relative balance of goal-directed and habitual behaviours in obsessive-compulsive disorder. Translational Psychiatry, 5(11), e670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Giel KE, Skunde M, Schag K, Rudofsky G, de Zwaan M, … Friederich HC (2013). Inhibitory control and decision making under risk in bulimia nervosa and binge-eating disorder. International Journal of Eating Disorders, 46(7), 721–728. [DOI] [PubMed] [Google Scholar]
- Wu M, Hartmann M, Skunde M, Herzog W, & Friederich H-C (2013). Inhibitory control in bulimic-type eating disorders: a systematic review and meta-analysis. PloS One, 8(12), e83412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanovski SZ, Leet M, Yanovski JA, Flood M, Gold PW, Kissileff HR, & Walsh BT (1992). Food selection and intake of obese women with binge-eating disorder. The American Journal of Clinical Nutrition, 56(6), 975–980. 10.1093/ajcn/56.6.975 [DOI] [PubMed] [Google Scholar]
- Yin HH (2010). The sensorimotor striatum is necessary for serial order learning. Journal of Neuroscience, 30(44), 14719–14723. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.