Abstract
In this review, we summarize the data on the safety and side effect profile of coronavirus disease 2019 (COVID-19) vaccines during lactation to date, review what is known about mRNA vaccine components in breastmilk, and discuss the efficacy of COVID-19 vaccines in providing immune protection for the breastfeeding infant. The CDC and ACOG recommend lactating individuals receive COVID-19 mRNA vaccines, and stay up to date on booster doses, including the bivalent COVID-19 booster. The lack of serious side effects in mother or infant across numerous large studies and registries of COVID-19 vaccination in pregnancy and lactation is reassuring. While small quantities of mRNA may be transiently detectable in breastmilk after maternal vaccination, there are no data demonstrating that vaccine mRNA can survive the infant GI tract, and no evidence that breastmilk from lactating individuals who have received a COVID-19 mRNA vaccine can cause harm to breastfeeding infants. In contrast, numerous studies demonstrate that breastmilk of vaccinated individuals contains SARS-CoV-2-specific functional antibodies and T-cells, which benefit the breastfeeding infant’s developing immune system. Transfer of SARS-CoV-2-specific antibodies from mother to infant is highest when vaccination occurs during pregnancy compared with lactation, as the breastfeeding infant receives both long-lasting antibodies through the placenta and breastmilk antibodies through breastmilk. With clear data demonstrating efficacy and safety, and no data demonstrating harm to mother or infant following COVID-19 vaccine administration during lactation, any recommendations to avoid vaccination while breastfeeding, or withhold breastmilk from the infant for any period of time after vaccination, are not supported by available evidence.
Précis:
Data support the safety and efficacy of coronavirus disease 2019 (COVID-19) mRNA vaccination during lactation; there are no data demonstrating infant harm after ingesting breastmilk after maternal vaccination.
Introduction
Nearly two years have elapsed since COVID-19 mRNA vaccines initially became available to the public, during which time knowledge about the safety and efficacy of COVID-19 vaccination in pregnancy and lactation has grown substantially. It is well-established that COVID-19 mRNA vaccines are highly immunogenic in pregnant and lactating people,1-3 and provide excellent protection against severe COVID-19 in these high-risk groups, as they do in the general adult population.4-6 Receipt of a booster shot generates similar immune responses in pregnant and lactating individuals compared to nonpregnant controls, including against the Omicron variant, and is well-tolerated.7-9 The Centers for Disease Control and Prevention (CDC) and multiple professional organizations including the American College of Obstetricians and Gynecologists and the Society for Maternal Fetal Medicine have consistently recommended vaccination for people who are pregnant or breastfeeding with COVID-19 mRNA vaccines, and have recommended adherence to recommended booster dosing schedules, including with the bivalent COVID-19 vaccine booster.10-12
Although COVID-19 vaccination rates in pregnant populations initially lagged that of age-matched groups,13,14 with critical disparities in vaccine coverage noted in racial and ethnic minority groups,15 nearly 71% of currently pregnant individuals in the US have completed a primary COVID-19 vaccine series as of November 12, 2022,16 and many have received booster doses.17,18 Although serious illness and death from COVID-19 still occurs, with pregnancy being an important risk factor for severe disease,19 SARS-CoV-2 infection during the Omicron dominant period resulted in less severe disease compared with Delta and pre-Delta epochs in pregnant individuals.20 This reduced risk of severe-critical disease is attributable not only to differences in strain virulence, but also to higher vaccination rates in pregnant people.
Although the benefits of COVID-19 vaccination during pregnancy are clear, some individuals who are unvaccinated or due for a booster and are hesitant about receiving an mRNA vaccine during pregnancy may consider deferring vaccination to the postpartum period. A pressing question for these individuals has become: what is the optimal time to receive a COVID-19 vaccine that maximizes benefit and minimizes risk to both members of the mother-infant dyad? The recent publication from Hanna et al. in JAMA Pediatrics21 reporting transient detection of small levels of vaccine-derived mRNA in human breastmilk presents an opportunity to review the safety and efficacy of COVID-19 vaccination during lactation. In this review, we aim to: 1) briefly summarize the data on the safety and reactogenicity of COVID-19 vaccines during lactation to date; 2) contextualize the findings of the Hanna et al. study with what is known about mRNA vaccine components in breastmilk; and 3) discuss the efficacy of COVID-19 vaccines in providing immune protection for the breastfeeding infant. Key points are summarized in Box 1.
Box 1. Key Points.
COVID-19 mRNA vaccination is recommended for lactating individuals (CDC, ACOG).
Staying up to date on booster doses, including the bivalent COVID-19 booster, is recommended for lactating individuals (CDC, ACOG).
There is no evidence that breastmilk from lactating individuals who have received a COVID-19 mRNA vaccine can cause harm to breastfeeding infants.
The breastmilk of vaccinated individuals contains SARS-CoV-2-specific antibodies and T-cells that may benefit the breastfeeding infant’s developing immune system.
How much protection a vaccinated mother’s breastmilk affords the breastfeeding infant against COVID-19 and/or severe COVID-19 disease, and how long that protection lasts, is not known.
Transfer of SARS-CoV-2-specific antibodies from mother to infant is highest when vaccination occurs during pregnancy compared with lactation, as the breastfeeding infant receives both long-lasting antibodies through the placenta and breastmilk antibodies through breastmilk.
Safety and side effect profile of maternal COVID-19 mRNA vaccination in lactation
Although pregnant and lactating individuals were excluded from initial COVID-19 vaccine trials,13,22 at this point in the pandemic, numerous studies including thousands of lactating individuals receiving mRNA vaccines and their breastfed infants have been reported in the literature; a comprehensive summary of the literature is publicly available on the LactMed database.23 A search of the NIH/National Library of Medicine’s Drugs and Lactation Database (LactMed) conducted in November 2022 revealed no reports in the peer-reviewed literature of serious adverse events in either the breastfeeding recipient of COVID-19 mRNA vaccines, or to the breastfed infant. Side effects are similar in lactating individuals receiving a primary mRNA vaccine series compared with non-lactating individuals, with post-vaccination symptoms more common after the second dose.1,24-26 Rates of local or systemic post-vaccination symptoms ranged from 56% to 85% among lactating study participants, with pain at the injection site being the most commonly-reported post-vaccine symptom.24-27 In a large study of over 4400 lactating vaccine recipients, the mRNA-1273 vaccine was consistently more reactogenic than BNT162b2,24 and in a smaller study of only 86 patients, the Oxford/Astra-Zeneca vaccine (ChAdOx1 nCoV-19) was associated with more post-vaccine symptoms than either of the mRNA vaccines.28
No serious adverse events have been reported in breastfeeding infants whose mothers received a COVID-19mRNA vaccine. In an early prospective study of 84 lactating COVID-19 vaccine recipients, 4 subjects reported fever in their breastfeeding infant, although in all cases, fever occurred more than 7 days after maternal vaccination and all infants had symptoms of upper respiratory tract infections, which was thought to be the etiology of the fever.27 Most subsequent studies reporting outcomes of breastfed infants have been survey-based, which lack a non-vaccinated control group with which to compare and contextualize the responses and are subject to some recall bias. Regardless, these studies report low rates of observed effects in infants, with the most frequent events including changes in sleep or behavior (either increased sleepiness or irritability) and gastrointestinal symptoms, with a range of 1-31% of mothers reporting at least one symptom in these reports.9,24,25,29,30
Data are conflicting regarding effect on milk supply after receipt of the COVID-19 mRNA vaccines. A study including over 4400 lactating individuals receiving COVID-19 vaccines, 4% of recipients reported a transient increase in milk supply.24 However, the same study reported a transient decrease in milk supply in the days following receipt of a COVID-19 vaccine in 6% of recipients, and other studies have also reported a transient decrease in milk supply in 6-8% of vaccine recipients, with supply returning to normal within 3 days of the vaccine.24,25,31 Transient changes in milk color have also been reported.25,31 Available data suggest that lactational concerns may be less frequent following booster doses: in a follow-up survey study of over 10,000 lactating individuals, 96% of individuals reported no lactational concerns after vaccination, with 1.2% reporting any issue with their breastfed infant and 3.5% reporting decreased milk supply.9 In summary, although transient effects on milk supply and/or infant behavior have been reported following maternal vaccination, the lack of serious side effects in either mother or infant across numerous studies is reassuring.
Human milk extracellular vesicles and vaccine mRNA: detection is not evidence of harm
The COVID-19 mRNA vaccines deliver lipid nanoparticles that encapsulate mRNA encoding the SARS-CoV-2 spike protein to the vaccine recipient’s cells. Once taken up by the host cell, the mRNA is released and translated into the SARS-CoV-2 spike protein, which is then processed into peptides that get displayed on the cell surface for immune recognition.32 Vaccine mRNA has been detected in the plasma of vaccine recipients in low levels in the days following vaccination,33 so there exists a theoretical possibility that mRNA from the maternal circulation could be excreted intact into breastmilk.
To date, four studies have investigated levels of BNT162b2 or mRNA-1273 mRNA in breastmilk of vaccine recipients.21,34-36 In a small study of 14 lactating healthcare workers in Singapore receiving the BNT162b2 vaccine, 4 out of 40 breastmilk samples collected within a week of vaccination had detectable levels of vaccine mRNA at low levels (highest concentration of BNT162b2 mRNA was 2 ng/ml, which translates to 0.667% of the original vaccine dose per 100 mL of human milk given to the infant),35 and a second study of 35 lactating healthcare workers, also in Singapore, receiving a BNT162b2 vaccine detected mRNA (median 70 pg/mL) in 5 of 309 breastmilk samples collected within 1 week of vaccination; all positive samples were collected within 3 days.36 None of 5 breastfeeding infants recruited had detectable mRNA in their serum. In a small study of 7 individuals who received a COVID-19 mRNA vaccine during lactation in the US, breastmilk was collected 8-48 hours after vaccination, and milk supernatant, fat layer, and cells were tested for vaccine mRNA.34 Using highly sensitive assays with a lower limit of detection of 0.195 picograms/mL for BNT162b2 and 1.5 picograms/mL for the mRNA-1273 vaccine, none of the 13 samples had detectable vaccine mRNA.
In a recently published study by Hanna et al., breastmilk from 11 lactating vaccine recipients was collected up to 5 days after vaccination and analyzed for the presence of vaccine mRNA in both whole breastmilk and in extracellular vesicles (EVs) isolated from breastmilk supernatant.21 EVs are particles released from cells that have a phospholipid bilayer and can carry biologically important molecules, including nucleic acids and proteins, in body fluids.37 The authors report that 5 of 11 samples had detectable vaccine mRNA in breastmilk at levels ranging from 1.3 to 16.8 pg/mL, with mRNA being detectable at timepoints ranging from 1 hour to 45 hours post vaccination. No breastmilk samples had detectable mRNA after 45 hours. Two of 5 positive samples had detectable vaccine mRNA in EVs only.21 To put these amounts in perspective, even at the highest detected concentration of vaccine mRNA in EVs (16 pg/mL), a 100 mL breastmilk sample would contain at most 0.002% of the amount of mRNA in the mRNA-1273 vaccine.
The study of milk-derived EVs is still relatively nascent. Because human milk EVs have been found to contain various types of RNA,37,38 it is not surprising that vaccine mRNA would be detected in a greater proportion of breastmilk samples when isolated breastmilk EVs are examined. Evidence is limited, however, as to whether EVs can survive the infant’s highly acidic gastric environment or enzymatic digestion of the small intestine. Although a small in vitro study suggests such survival is possible, and perhaps key to the potential biological relevance of breastmilk EVs to the infant,39 there is no direct in vivo evidence that breastmilk EVs can traverse the mucous layer of the intestine and enter the infant blood stream intact.37 Naked mRNA is highly unstable, subject to rapid degradation by RNases, and poorly taken up by cells in the absence of encapsulation.40-43 While small quantities of vaccine mRNA in breastmilk may minutely augment the substantial natural EV-RNA cargo, they are unlikely to have biological effects if released. While small quantities of mRNA may be transiently detectable in breastmilk after maternal vaccination, there are no data to suggest harm to the breastfeeding infant.
Benefits of maternal COVID-19 mRNA vaccination to the breastfeeding infant
Pregnant and lactating individuals mount immunologic responses to the COVID-19 mRNA vaccines that are comparable to those of nonpregnant reproductive-aged females.1,44 Receiving a primary COVID-19 mRNA vaccination series during pregnancy protects the vaccinated mother from serious illness and protects the fetus/neonate by lowering the risk of COVID-19-associated preterm birth.14,45 Vaccination during pregnancy also provides the infant with protection against COVID-19 hospitalization in the first 6 months of life via transplacental transfer of durable vaccine-derived anti-SARS-CoV-2 IgG, in addition to breastmilk transfer of vaccine-derived IgA, IgM and IgG if the infant is breastfed (Figure 1A).3,46-48 Receiving a COVID-19 vaccine during pregnancy does not increase the risk of side effects, miscarriage, preterm birth, or fetal growth restriction,49-51 and may in fact protect against stillbirth.6 Vaccination during pregnancy has clear benefits for the vaccinated individual and the infant, regardless of breastfeeding status. Despite these benefits, some pregnant individuals remain hesitant about vaccination during pregnancy and choose to pursue vaccination in the postpartum/lactational period, which still is beneficial as it offers protection for the postpartum individual as well as protection for the infant if breastfed. In addition, some individuals who were vaccinated prior to, during, or after pregnancy have become eligible for a booster dose while lactating. To aid with COVID-19 vaccine decision-making in lactating individuals, a conversation of any risks associated with vaccination should be balanced with discussion of the potential benefits breastfeeding provides for SARS-CoV-2-specific and overall newborn immunity.
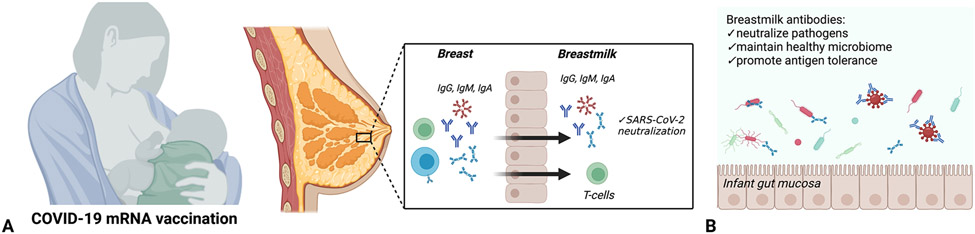
Figure 1:
Coronavirus disease 2019 (COVID-19) vaccine-induced breastmilk antibodies. A. Maternal COVID-19 vaccination has been demonstrated to be associated with generation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)–specific immune globulin (Ig)G, IgM and IgA, with neutralizing capabilities. In addition, coronavirus disease 2019 (COVID-19) vaccination has been demonstrated to generate SARS-CoV-2–specific T-cells that are detectable in human breastmilk. B. Breastmilk antibodies serve diverse functions in the neonatal gut, including neutralizing pathogens, promoting antigen tolerance, and maintaining a healthy gut microbiome through selection of favorable commensal bacteria. Created with BioRender.com.
Human breastmilk plays an important, multifaceted role in providing immune protection to the breastfeeding infant.52 Although multiple biologically active components of human breastmilk provide non-specific immune defenses, maternal immunoglobulins (Igs) transferred in breastmilk to the lactating infant are key to supporting antigen-specific immunity.53 Secretory IgA is the most abundant Ig isotype in human breastmilk, although secretory IgM and IgG are also present in breastmilk and play important roles in both immune tolerance and defense against pathogens.52 By populating and coating the infant’s mucosal surfaces, secretory IgA provides barrier immunity by neutralizing pathogens (Figure 1A) 54 and supports the development and maintenance of a healthy gut microbiome through selection of useful commensal bacteria (Figure 1B).55 Although the roles of breastmilk-derived secretory IgM and IgG are less well understood, IgG has been shown to protect against pathogenic bacteria in the gut as well as respiratory viruses such as Respiratory Syncytial Virus (RSV),56,57 particularly in preterm infants who have altered gut permeability.58
Multiple studies have demonstrated the presence of SARS-CoV-2 specific IgA and IgG in human breastmilk in the weeks following primary maternal COVID-19 mRNA vaccination during pregnancy and lactation.59 In one large study of 98 breastfeeding mRNA vaccine recipients, SARS-CoV-2-specific IgA was detected in 89% of samples and IgG in all samples collected 14 days after the second vaccine dose.60 Studies across multiple cohorts and vaccine platforms consistently demonstrate the presence of SARS-CoV-2 specific IgA and IgG in breastmilk, with breastmilk antibody levels correlating with levels in maternal blood.59,61,62 The capability of breastmilk from vaccinated, lactating individuals to neutralize the SARS-CoV-2 virus, including variants of concern, has been demonstrated in multiple studies.62-64 Although SARS-CoV-2-specific IgA and IgG levels decrease over time in breastmilk, levels of both IgA and IgG remain elevated up to 6 months after vaccination.65,66 In a small study of 10 lactating participants a mRNA booster shot, boosting significantly improves antibody levels and breastmilk neutralizing capability in vitro, from 12% to 66% inhibition of the SARS-CoV-2 virus.67 Although the neutralizing SARS-CoV-2-specific antibodies in breastmilk likely confer some level of protection to the breastfeeding infant, the degree and durability of infant protection that maternal breastmilk antibodies provide is not known. However, these data support the ability of maternal mRNA COVID-19 vaccination to generate significant levels of SARS-CoV-2-specific functional antibodies in breastmilk, which may be boosted by vaccination during lactation.
SARS-CoV-2 specific antibodies detected in the breastmilk of breastfeeding vaccine recipients are transferred to the mucosal surfaces of the breastfeeding infant, and limited evidence suggests they may be capable of transiting beyond the infant’s mouth and upper respiratory tract into the lower GI tract. Breastmilk-derived SARS-CoV-2-specific antibodies have been detected in significant amounts in the breastfeeding infants’ saliva and stool,64,65,68 and studies performed in vitro support the capability of breastmilk-derived anti-SARS-CoV-2 IgG and secretory IgA to resist degradation in the infant gut.69,70 Importantly, however, anti-SARS-CoV-2 antibodies have not been detected in the blood of infants whose mothers were vaccinated during lactation only,29,68 suggesting that maternally-derived antibodies from breastmilk likely do not cross the gut mucosal barrier in detectable quantities. This stands in contrast to antibodies that are transplacentally transferred from mothers vaccinated during pregnancy, which are detectable in the infant’s circulation for up to 6 months in the majority of cases.46 Primary vaccination during pregnancy likely affords more long-lasting infant protection than breastfeeding alone, given that IgG can only be transferred to the newborn circulation via transplacental transfer and not via breastmilk.
Although breastmilk of vaccinated mothers likely confers antibody-mediated neutralization of SARS-CoV-2 that lasts only hours to days after cessation of breastfeeding, there is increasing evidence that breastmilk antibodies play a more complex role in neonatal protection than simple neutralization of pathogens at the mucosal surface. Breastmilk antibodies may serve a more durable immune function in the neonate and infant by helping to establish and maintain the gut microbiome, and training the immune system to “tolerate” antigens at the mucosal surface (Figure 1B).52 In addition, whether intact immune cells, which could provide longer-lasting immunity against SARS-CoV-2, can transfer to the breastfed infant is an open question. The presence of SARS-CoV-2-reactive CD4+ T-cells in breastmilk has been demonstrated after maternal vaccination (Figure 1A),71 with expansion of Spike-specific T-cell receptors in breastmilk observed following a COVID-19 mRNA booster.72 High levels of mucosal-homing markers in breastmilk T cells suggest that these cells may be derived from a T-cell population residing in the breast tissue itself that are modulated by maternal vaccination.72 In addition, recent evidence suggests breastmilk-derived maternal cells may be able to traffic across the infant gut mucosa and take up residence in infant tissues.73 Breastmilk immunity is likely far more complex than simple antibody persistence on infant mucosal surfaces, and the durability of breastmilk-transferred cellular immunity to the infant, as well as breastmilk education of the infant gut microbiome are key areas for future study.52,74,75 Longitudinal studies of breastfed infants born to vaccinated mothers are needed to better understand the potential short-and long-term protective benefits conferred by breastfeeding.
Future directions and conclusions
A key area for future study is the extent to which enhanced protection of the neonate is afforded by the combination of transplacentally-transferred maternal IgG and breastmilk-acquired IgA, IgM and IgG. As more and more individuals are entering pregnancy vaccinated, it is increasingly important to understand how receipt of a COVID-19 vaccine during lactation complements the transplacental transfer of SARS-CoV-2-specific immunity from prior vaccination. Several studies have demonstrated population of the breastfeeding newborn’s gut with protective and functional (e.g. capable of activation of neutrophil phagocytosis) vaccine-induced SARS-CoV-2-specific antibodies,64,65,68,76 but the extent to which this protection can be augmented by a booster dose during lactation remains unknown. In addition, whether intranasal COVID-19 vaccines, which target induction of mucosal immunity against SARS-CoV-2 and are currently in development,77 might enhance breastmilk immunity will be important to assess, as some evidence has shown enhanced breastmilk immunity following intranasal influenza vaccination compared with the intramuscular vaccine.78
Without any evidence of harm to mother or infant following COVID-19 vaccine administration during lactation, recommendations to avoid vaccination while breastfeeding, or to withhold breastmilk from the infant for any period of time after vaccination, are inappropriate. Although misinformation about the safety of COVID-19 mRNA vaccines in reproduction continues to impact the public’s perception of vaccine safety,79,80 the benefits of COVID-19 vaccination for the mother-infant dyad are clear, whether during pregnancy or lactation, and far outweigh any potential theoretical risks. Pregnant individuals desiring to optimize infant protection against COVID-19 should be encouraged to vaccinate during pregnancy, rather than deferring vaccination until after delivery. As COVID-19 variants become increasingly transmissible over time, pregnant and lactating individuals should be encouraged to stay on schedule with mRNA booster doses, as this remains an important strategy for protecting both members of the breastfeeding pair from COVID-19 disease.
Supplementary Material
Funding sources:
Eunice Kennedy Shriver National Institute of Child Health and Human Development Grants 1R01HD100022-01 and 3R01HD100022-02S2 (to A.G.E.), 5K12HD103096-03 (to L.L.S)
National Institute of Allergy and Infectious Diseases Grant 1U19AI167899-01 (to A.G.E.)
Simons Foundation SFARI award, Grant 870754 (to A.G.E.)
Footnotes
Financial Disclosure: Andrea G. Edlow serves as a consultant for Mirvie, Inc, and receives research funding from Merck Pharmaceuticals to study maternal vaccination in pregnancy. Lydia L. Shook did not report any potential conflicts of interest.
Each author has confirmed compliance with the journal’s requirements for authorship.
References
- 1.Gray KJ, Bordt EA, Atyeo C, et al. COVID-19 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol. 2021. Sep;225(3):303.e1–303.e17. doi: 10.1016/j.ajog.2021.03.023. Epub 2021 Mar 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beharier O, Plitman Mayo R, Raz T, et al. Efficient maternal to neonatal transfer of antibodies against SARS-CoV-2 and BNT162b2 mRNA COVID-19 vaccine. J Clin Invest. Published online May 20, 2021. doi: 10.1172/JCI150319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prabhu M, Murphy EA, Sukhu AC, et al. Antibody Response to Coronavirus Disease 2019 (COVID-19) Messenger RNA Vaccination in Pregnant Women and Transplacental Passage Into Cord Blood. Obstet Gynecol. Published online April 28, 2021. doi: 10.1097/AOG.0000000000004438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldshtein I, Nevo D, Steinberg DM, et al. Association Between BNT162b2 Vaccination and Incidence of SARS-CoV-2 Infection in Pregnant Women. JAMA. 2021;326(8):728–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dagan N, Barda N, Biron-Shental T, et al. Effectiveness of the BNT162b2 mRNA COVID-19 vaccine in pregnancy. Nat Med. 2021;27(10):1693–1695. [DOI] [PubMed] [Google Scholar]
- 6.Prasad S, Kalafat E, Blakeway H, et al. Systematic review and meta-analysis of the effectiveness and perinatal outcomes of COVID-19 vaccination in pregnancy. Nat Commun. 2022;13(1):2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atyeo C, Shook LL, Nziza N, et al. COVID-19 booster dose induces robust antibody response in pregnant, lactating, and nonpregnant women. Am J Obstet Gynecol. Published online July 19, 2022. doi: 10.1016/j.ajog.2022.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang YJ, Murphy EA, Singh S, et al. Association of Gestational Age at Coronavirus Disease 2019 (COVID-19) Vaccination, History of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection, and a Vaccine Booster Dose With Maternal and Umbilical Cord Antibody Levels at Delivery. Obstet Gynecol. Published online December 28, 2021. doi: 10.1097/AOG.0000000000004693 [DOI] [PubMed] [Google Scholar]
- 9.Kachikis A, Englund JA, Covelli I, et al. Analysis of Vaccine Reactions After COVID-19 Vaccine Booster Doses Among Pregnant and Lactating Individuals. JAMA Netw Open. 2022;5(9):e2230495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.CDC. COVID-19 Vaccines While Pregnant or Breastfeeding. Centers for Disease Control and Prevention. Published February 18, 2022. Accessed February 28, 2022. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/pregnancy.html [Google Scholar]
- 11.ACOG Practice Advisory. COVID-19 Vaccination Considerations for Obstetric–Gynecologic Care. Published July 30, 2021. Accessed September 3, 2021. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/covid-19-vaccination-considerations-for-obstetric-gynecologic-care [Google Scholar]
- 12.ACOG and SMFM Recommend COVID-19 Vaccination for Pregnant Individuals. Published July 30, 2021. Accessed August 13, 2021. https://www.acog.org/news/news-releases/2021/07/acog-smfm-recommend-covid-19-vaccination-for-pregnant-individuals [Google Scholar]
- 13.Shook L, Kishkovich T, Edlow A. Countering COVID-19 vaccine hesitancy in pregnancy: the “4 Cs.” Am J Perinatol. Published online October 19, 2021. doi: 10.1055/a-1673-5546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stock SJ, Carruthers J, Calvert C, et al. SARS-CoV-2 infection and COVID-19 vaccination rates in pregnant women in Scotland. Nat Med. Published online January 13, 2022. doi: 10.1038/s41591-021-01666-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kriss JL, Hung MC, Srivastav A, et al. COVID-19 Vaccination Coverage, by Race and Ethnicity - National Immunization Survey Adult COVID Module, United States, December 2020-November 2021. MMWR Morb Mortal Wkly Rep. 2022;71(23):757–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.CDC. COVID-19 vaccination among pregnant people aged 18-49 years overall, by race/ethnicity, and date reported to CDC - Vaccine Safety Datalink,* United States. Accessed November 28, 2022. https://covid.cdc.gov/covid-data-tracker/#vaccinations-pregnant-women [Google Scholar]
- 17.CDC. COVID-19 Vaccine Booster Shots. Published October 29, 2021. Accessed November 2, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/booster-shot.html [Google Scholar]
- 18.Razzaghi H, Meghani M, Crane B, et al. Receipt of COVID-19 Booster Dose Among Fully Vaccinated Pregnant Individuals Aged 18 to 49 Years by Key Demographics. JAMA. Published online April 22, 2022. doi: 10.1001/jama.2022.6834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zambrano LD, Ellington S, Strid P, et al. Update: Characteristics of Symptomatic Women of Reproductive Age with Laboratory-Confirmed SARS-CoV-2 Infection by Pregnancy Status - United States, January 22-October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(44):1641–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adhikari EH, MacDonald L, SoRelle JA, Morse J, Pruszynski J, Spong CY. COVID-19 Cases and Disease Severity in Pregnancy and Neonatal Positivity Associated With Delta (B.1.617.2) and Omicron (B.1.1.529) Variant Predominance. JAMA. 2022;327(15):1500–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanna N, Heffes-Doon A, Lin X, et al. Detection of Messenger RNA COVID-19 Vaccines in Human Breast Milk. JAMA Pediatr. Published online September 26, 2022. doi: 10.1001/jamapediatrics.2022.3581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shook L, Fallah P, Silberman J, Edlow A. COVID-19 Vaccination in Pregnancy and Lactation: Current Research and Gaps in Understanding. Front Cell Infect Microbiol. 2021;11:899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.COVID-19 Vaccines. In: Drugs and Lactation Database (LactMed). National Library of Medicine (US); 2020. [Google Scholar]
- 24.McLaurin-Jiang S, Garner CD, Krutsch K, Hale TW. Maternal and Child Symptoms Following COVID-19 Vaccination Among Breastfeeding Mothers. Breastfeed Med. 2021;16(9):702–709. [DOI] [PubMed] [Google Scholar]
- 25.Bertrand K, Honerkamp-Smith G, Chambers CD. Maternal and Child Outcomes Reported by Breastfeeding Women Following Messenger RNA COVID-19 Vaccination. Breastfeed Med. 2021;16(9):697–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fu W, Sivajohan B, McClymont E, et al. Systematic review of the safety, immunogenicity, and effectiveness of COVID-19 vaccines in pregnant and lactating individuals and their infants. Int J Gynaecol Obstet. 2022;156(3):406–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perl SH, Uzan-Yulzari A, Klainer H, et al. SARS-CoV-2-Specific Antibodies in Breast Milk After COVID-19 Vaccination of Breastfeeding Women. JAMA. Published online April 12, 2021. doi: 10.1001/jama.2021.5782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Selma-Royo M, Bäuerl C, Mena-Tudela D, et al. Anti-SARS-CoV-2 IgA and IgG in human milk after vaccination is dependent on vaccine type and previous SARS-CoV-2 exposure: a longitudinal study. Genome Med. 2022;14(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Golan Y, Prahl M, Cassidy AG, et al. COVID-19 mRNA Vaccination in Lactation: Assessment of Adverse Events and Vaccine Related Antibodies in Mother-Infant Dyads. Front Immunol. 2021;12:777103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romero Ramírez DS, Suárez Hernández MI, Fernández Vilar AM, et al. Evaluation of Adverse Effects in Nursing Mothers and Their Infants After COVID-19 mRNA Vaccination. Breastfeed Med. 2022;17(5):412–421. [DOI] [PubMed] [Google Scholar]
- 31.Jacob-Chow B, Vasundhara KL, Cheang HK, Lee LY, Low JM, Amin Z. Reactogenicity of mRNA- and Non-mRNA-Based COVID-19 Vaccines among Lactating Mother and Child Dyads. Vaccines (Basel). 2022;10(7). doi: 10.3390/vaccines10071094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le TK, Paris C, Khan KS, Robson F, Ng WL, Rocchi P. Nucleic Acid-Based Technologies Targeting Coronaviruses. Trends Biochem Sci. 2021;46(5):351–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fertig TE, Chitoiu L, Marta DS, et al. Vaccine mRNA Can Be Detected in Blood at 15 Days Post-Vaccination. Biomedicines. 2022;10(7). doi: 10.3390/biomedicines10071538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Golan Y, Prahl M, Cassidy A, et al. Evaluation of Messenger RNA From COVID-19 BTN162b2 and mRNA-1273 Vaccines in Human Milk. JAMA Pediatr. Published online July 6, 2021. doi: 10.1001/jamapediatrics.2021.1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Low JM, Gu Y, Ng MSF, et al. Codominant IgG and IgA expression with minimal vaccine mRNA in milk of BNT162b2 vaccinees. NPJ Vaccines. 2021;6(1):105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yeo KT, Chia WN, Tan CW, et al. Neutralizing Activity and SARS-CoV-2 Vaccine mRNA Persistence in Serum and Breastmilk After BNT162b2 Vaccination in Lactating Women. Front Immunol. 2021;12:783975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu Y, Thaler J, Nieuwland R. Extracellular Vesicles in Human Milk. Pharmaceuticals . 2021;14(10). doi: 10.3390/ph14101050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chutipongtanate S, Morrow AL, Newburg DS. Human Milk Extracellular Vesicles: A Biological System with Clinical Implications. Cells. 2022;11(15). doi: 10.3390/cells11152345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liao Y, Du X, Li J, Lönnerdal B. Human milk exosomes and their microRNAs survive digestion in vitro and are taken up by human intestinal cells. Mol Nutr Food Res. 2017;61(11). doi: 10.1002/mnfr.201700082 [DOI] [PubMed] [Google Scholar]
- 40.Kowalski PS, Rudra A, Miao L, Anderson DG. Delivering the Messenger: Advances in Technologies for Therapeutic mRNA Delivery. Mol Ther. 2019;27(4):710–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov. 2018;17(4):261–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsui NBY, Ng EKO, Lo YMD. Stability of endogenous and added RNA in blood specimens, serum, and plasma. Clin Chem. 2002;48(10):1647–1653. [PubMed] [Google Scholar]
- 43.Kauffman KJ, Webber MJ, Anderson DG. Materials for non-viral intracellular delivery of messenger RNA therapeutics. J Control Release. 2016;240:227–234. [DOI] [PubMed] [Google Scholar]
- 44.Atyeo CG, Shook LL, Brigida S, et al. Maternal immune response and placental antibody transfer after COVID-19 vaccination across trimester and platforms. Nat Commun. 2022;13(1):3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Theiler RN, Wick M, Mehta R, Weaver AL, Virk A, Swift M. Pregnancy and birth outcomes after SARS-CoV-2 vaccination in pregnancy. Am J Obstet Gynecol MFM. 2021;3(6):100467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shook LL, Atyeo CG, Yonker LM, et al. Durability of Anti-Spike Antibodies in Infants After Maternal COVID-19 Vaccination or Natural Infection. JAMA. Published online February 7, 2022. doi: 10.1001/jama.2022.1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burns MD, Muir C, Atyeo C, et al. Relationship between Anti-Spike Antibodies and Risk of SARS-CoV-2 Infection in Infants Born to COVID-19 Vaccinated Mothers. Vaccines (Basel). 2022;10(10). doi: 10.3390/vaccines10101696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Halasa NB, Olson SM, Staat MA, et al. Maternal Vaccination and Risk of Hospitalization for Covid-19 among Infants. N Engl J Med. 2022;387(2):109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shimabukuro TT, Kim SY, Myers TR, et al. Preliminary Findings of mRNA Covid-19 Vaccine Safety in Pregnant Persons. N Engl J Med. Published online April 21, 2021. doi: 10.1056/NEJMoa2104983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zauche LH, Wallace B, Smoots AN, et al. Receipt of mRNA Covid-19 Vaccines and Risk of Spontaneous Abortion. N Engl J Med. Published online September 8, 2021. doi: 10.1056/NEJMc2113891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lipkind HS, Vazquez-Benitez G, DeSilva M, et al. Receipt of COVID-19 Vaccine During Pregnancy and Preterm or Small-for-Gestational-Age at Birth - Eight Integrated Health Care Organizations, United States, December 15, 2020-July 22, 2021. MMWR Morb Mortal Wkly Rep. 2022;71(1):26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Atyeo C, Alter G. The multifaceted roles of breast milk antibodies. Cell. 2021;184(6):1486–1499. [DOI] [PubMed] [Google Scholar]
- 53.Andreas NJ, Kampmann B, Mehring Le-Doare K. Human breast milk: A review on its composition and bioactivity. Early Hum Dev. 2015;91(11):629–635. [DOI] [PubMed] [Google Scholar]
- 54.Brandtzaeg P Induction of secretory immunity and memory at mucosal surfaces. Vaccine. 2007;25(30):5467–5484. [DOI] [PubMed] [Google Scholar]
- 55.Rogier EW, Frantz AL, Bruno MEC, et al. Secretory antibodies in breast milk promote long-term intestinal homeostasis by regulating the gut microbiota and host gene expression. Proc Natl Acad Sci U S A. 2014;111(8):3074–3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zheng W, Zhao W, Wu M, et al. Microbiota-targeted maternal antibodies protect neonates from enteric infection. Nature. 2020;577(7791):543–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mazur NI, Horsley NM, Englund JA, et al. Breast Milk Prefusion F Immunoglobulin G as a Correlate of Protection Against Respiratory Syncytial Virus Acute Respiratory Illness. J Infect Dis. 2019;219(1):59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gregory KE, Walker WA. Immunologic Factors in Human Milk and Disease Prevention in the Preterm Infant. Curr Pediatr Rep. 2013;1(4). doi: 10.1007/s40124-013-0028-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Muyldermans J, De Weerdt L, De Brabandere L, Maertens K, Tommelein E. The Effects of COVID-19 Vaccination on Lactating Women: A Systematic Review of the Literature. Front Immunol. 2022;13:852928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Romero Ramírez DS, Lara Pérez MM, Carretero Pérez M, et al. SARS-CoV-2 Antibodies in Breast Milk After Vaccination. Pediatrics. 2021;148(5). doi: 10.1542/peds.2021-052286 [DOI] [PubMed] [Google Scholar]
- 61.Juncker HG, Mulleners SJ, Ruhé EJM, et al. Comparing the human milk antibody response after vaccination with four COVID-19 vaccines: A prospective, longitudinal cohort study in the Netherlands. EClinicalMedicine. 2022;47:101393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rosenberg-Friedman M, Kigel A, Bahar Y, et al. BNT162b2 mRNA vaccine elicited antibody response in blood and milk of breastfeeding women. Nat Commun. 2021;12(1):6222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Collier ARY, McMahan K, Yu J, et al. Immunogenicity of COVID-19 mRNA Vaccines in Pregnant and Lactating Women. JAMA. 2021;325(23):2370–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Narayanaswamy V, Pentecost BT, Schoen CN, et al. Neutralizing Antibodies and Cytokines in Breast Milk After Coronavirus Disease 2019 (COVID-19) mRNA Vaccination. Obstet Gynecol. 2022;139(2):181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stafford L, Valcarce V, Henry M, et al. Detection of SARS-CoV-2 IgA and IgG in human milk and breastfeeding infant stool 6 months after maternal COVID-19 vaccination. Res Sq. Published online August 19, 2022. doi: 10.21203/rs.3.rs-1950944/v1 [DOI] [PubMed] [Google Scholar]
- 66.Perez SE, Luna Centeno LD, Cheng WA, et al. Human Milk SARS-CoV-2 Antibodies up to 6 Months After Vaccination. Pediatrics. 2022;149(2). doi: 10.1542/peds.2021-054260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bender JM, Lee Y, Cheng WA, Marentes Ruiz CJ, Pannaraj PS. Coronavirus Disease 2019 Vaccine Booster Effects Are Seen in Human Milk Antibody Response. Front Nutr. 2022;9:898849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schwartz A, Nir O, Toussia-Cohen S, et al. Presence of SARS-CoV-2 antibodies in lactating women and their infants following BNT162b2 messenger RNA vaccine. Am J Obstet Gynecol. 2021;225(5):577–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pieri M, Maniori MA, Shahabian L, et al. Survival of Vaccine-Induced Human Milk SARS-CoV-2 IgG, IgA and SIgA Immunoglobulins across Simulated Human Infant Gastrointestinal Digestion. Nutrients. 2022;14(16). doi: 10.3390/nu14163368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Calvo-Lerma J, Bueno-Llamoga P, Bäuerl C, et al. Persistence of Anti SARS-CoV-2 Antibodies in Breast Milk from Infected and Vaccinated Women after In Vitro-Simulated Gastrointestinal Digestion. Nutrients. 2022;14(10). doi: 10.3390/nu14102117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gonçalves J, Juliano AM, Charepe N, et al. Secretory IgA and T cells targeting SARS-CoV-2 spike protein are transferred to the breastmilk upon mRNA vaccination. Cell Rep Med. 2021;2(12):100468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Armistead B, Jiang Y, Carlson M, et al. Spike-specific T cells are enriched in breastmilk following SARS-CoV-2 mRNA vaccination. medRxiv. Published online September 28, 2022. doi: 10.1101/2021.12.03.21267036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Molès JP, Tuaillon E, Kankasa C, et al. Breastfeeding-related maternal microchimerism. Nat Rev Immunol. 2017;17(11):729–721. [DOI] [PubMed] [Google Scholar]
- 74.Darby MG, Chetty A, Mrjden D, et al. Pre-conception maternal helminth infection transfers via nursing long-lasting cellular immunity against helminths to offspring. Sci Adv. 2019;5(5):eaav3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Camacho-Morales A, Caba M, García-Juárez M, Caba-Flores MD, Viveros-Contreras R, Martínez-Valenzuela C. Breastfeeding Contributes to Physiological Immune Programming in the Newborn. Front Pediatr. 2021;9:744104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Atyeo C, DeRiso EA, Davis C, et al. COVID-19 mRNA vaccines drive differential antibody Fc-functional profiles in pregnant, lactating, and non-pregnant women. Sci Transl Med. Published online October 19, 2021:eabi8631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Waltz E. How nasal-spray vaccines could change the pandemic. Nature Publishing Group UK. doi: 10.1038/d41586-022-02824-3 [DOI] [PubMed] [Google Scholar]
- 78.Pannaraj PS, da Costa-Martins AG, Cerini C, et al. Molecular alterations in human milk in simulated maternal nasal mucosal infection with live attenuated influenza vaccination. Mucosal Immunol. 2022;15(5):1040–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Abbasi J Widespread Misinformation About Infertility Continues to Create COVID-19 Vaccine Hesitancy. JAMA. 2022;327(11):1013–1015. [DOI] [PubMed] [Google Scholar]
- 80.Misinformation About COVID-19 Vaccines and Pregnancy is Widespread, Including Among Women Who are Pregnant or Planning to Get Pregnant. KFF. Published May 27, 2022. Accessed November 7, 2022. https://www.kff.org/coronavirus-covid-19/press-release/misinformation-about-covid-19-vaccines-and-pregnancy-is-widespread-including-among-women-who-are-pregnant-or-planning-to-get-pregnant/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.