Abstract
Airway mucus is a complex viscoelastic gel that provides a defensive physical barrier and shields the airway epithelium by trapping inhaled foreign pathogens and facilitating their removal via mucociliary clearance (MCC). In patients with respiratory diseases, such as chronic obstructive pulmonary disease (COPD), cystic fibrosis (CF), non-CF bronchiectasis, and asthma, an increase in crosslinking and physical entanglement of mucin polymers as well as mucus dehydration often alters and typically reduces mucus mesh network pore size, which reduces neutrophil migration, decreases pathogen capture, sustains bacterial infection, and accelerates lung function decline. Conventional aerosol particles containing hydrophobic drugs are rapidly captured and removed by MCC. Therefore, it is critical to design aerosol delivery systems with the appropriate size and surface chemistry that can improve drug retention and absorption with the goal of increased efficacy. Biodegradable muco-adhesive particles (MAPs) and muco-penetrating particles (MPPs) have been engineered to achieve effective pulmonary delivery and extend drug residence time in the lungs. MAPs can be used to target mucus as they get trapped in airway mucus by steric obstruction and/or adhesion. MPPs avoid muco-adhesion and are designed to have a particle size smaller than the mucus network, enhancing lung retention of particles as well as transport to the respiratory epithelial layer and drug absorption. In this review, we aim to provide insight into the composition of airway mucus, rheological characteristics of airway mucus in healthy and diseased subjects, the most recent techniques to study the flow dynamics and particle diffusion in airway mucus (in particular, multiple particle tracking, MPT), and the advancements in engineering MPPs that have contributed to improved airway mucus penetration, lung distribution, and retention.
Keywords: Rheology, microrheology, mucociliary clearance, multiple particle tracking, cystic fibrosis, aerosol, pulmonary drug delivery
Graphical Abstract
1. Introduction
Pulmonary drug delivery is a less invasive route for treatment of chronic respiratory airway diseases such as asthma, cystic fibrosis (CF), lung cancer, and chronic obstructive pulmonary disease (COPD) (Fröhlich and Salar-Behzadi, 2021; Mejías and Roy, 2019). This delivery route offers several advantages, including the ability to achieve local drug accumulation in the lungs, providing an alternative route to target systemic diseases, relatively low enzymatic activity, and good permeability due to an extensively vascularized membrane (Azarmi et al., 2008; Labiris and Dolovich, 2003; Yıldız-Peköz and Ehrhardt, 2020). Most inhaled drugs deposited in the conducting airways are subjected to clearance by three mechanisms, namely mucociliary clearance (MCC), macrophage uptake, and/or absorption into the epithelial layer (El-Sherbiny and Smyth, 2012; Matthews et al., 2020). The airway epithelium is the major physical barrier lined by ciliated and secretory cells that are physically connected with multiple layers of intercellular junctions (Bals and Hiemstra, 2004; Ganesan et al., 2013; Knowles and Boucher, 2002; Pohl et al., 2009).
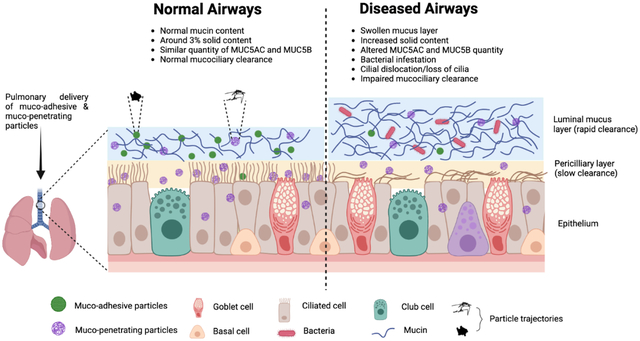
Airway mucus is a complex viscoelastic gel synthesized and secreted by specialized goblet and mucous glands in the surface epithelium (Birchenough et al., 2015; Rogers and Barnes, 2006). Native airway mucus protects the underlying epithelium by trapping and immobilizing inhaled foreign particles and pathogens, facilitating their removal via MCC and cough clearance, and prevents dehydrating the epithelial surface of the lung (Richard A Cone, 2009; Fahy and Dickey, 2010; Shah et al., 2019). Effective MCC mainly depends on the normal structure and function of cilia along with the optimum height, composition, and viscoelasticity of the mucus layer (Houtmeyers et al., 1999; Nawroth et al., 2020). In healthy individuals, mucus is produced continuously to protect the airway and humidify the air. In respiratory diseases, the composition and properties of airway secretions are altered and the airway epithelium often hyper-secretes mucus that disrupts MCC and airflow, resulting mucus retention (Daviskas and Rubin, 2013; Li and Tang, 2021; Livraghi and Randell, 2007; Shen et al., 2018). Defective cystic fibrosis transmembrane conductance regulator (CFTR) protein causes imbalance of sodium, chloride, and bicarbonate ions at the airway epithelial surface and dehydration of the airway surface liquid, increasing percent solids (mucins, salts, lipids, polypeptides, etc.) in the airway mucus (Boucher, 2007; Duncan et al., 2016b; Quinton, 2008). In most cases, mucus secreted by individuals with respiratory diseases has increased viscoelasticity compared to mucus from healthy individuals, causing mucus build-up in the airways leading to deterioration of lung function (Chisholm et al., 2019; Serisier et al., 2009). This is supported by studies that show increased solid mucus content in patients with lung diseases (Anderson et al., 2015; Hill et al., 2014).
Nanoparticles formulation is a promising approach for the treatment of respiratory diseases as it confers significant advantages in terms of sustained or controlled drug release, protection of encapsulated drug from premature degradation or loss-of-function by enzymatic/metabolic activities, and enhanced cellular uptake (D. Chen et al., 2021). However, success of this approach is highly dependent on the ability of nanoparticles to overcome various biological and physiological barriers (Alp and Aydogan, 2020; Porsio et al., 2018). In respiratory diseases, alteration in mucus properties can potentially impact the behavior of nanoparticles when used as a delivery vehicle, requiring careful consideration when designing and formulating nanoparticles (Chen et al., 2010a; Kan et al., 2020). It has been possible to improve the airway mucus penetration of nanoparticles in respiratory diseases using strategies such as modification of physicochemical properties of nanoparticles by coating with polymers, including polyethylene glycol (PEG); and modulation of barrier properties of airway mucus by mucolytics such as N-acetylcysteine (NAC) (Craparo et al., 2016; Kan et al., 2020; Nordgård and Draget, 2018). Nanoparticles densely surface coated with PEG5kDa demonstrated improved diffusion in normal, CF, and COPD airway mucus compared to uncoated nanoparticles (Forier et al., 2013; Schuster et al., 2014; Suk et al., 2009). Similarly, PEG-conjugated polymers (PEG-PEI and PEG-PBAE) based mucus penetrating particles (MPPs) confirmed widespread and uniform airway distribution, and prolonged retention in the mice lungs (Boylan et al., 2012; A. J. Kim et al., 2013).
In this review, we summarize the major functional components of mucus, highlighting the role of the gel-forming mucins as responsible for viscoelastic properties of airway mucus. Thereafter, the impact of mucus macrorheology and microrheology on the normal barrier and clearance function in the airways, and the altered viscoelastic properties of sputum as a biomarker of secondary infection and inflammation is discussed. We also provide an overview on the most recent techniques to study the flow dynamics of nanoparticles using a low volume of mucus. Moreover, we have discussed the contribution of mucus hypersecretion, microstructural alteration, and neutrophil extracellular traps (NETs) in respiratory diseases. Finally, we have introduced several studies focused on the design of muco-adhesive particles (MAPs) and MPPs and aerosols to overcome the airway mucus barrier for effective pulmonary drug delivery.
2. Healthy airway mucus: Composition, macrorheology and microrheology
The major functional component of viscoelastic mucus gel is mucin (1–5%), a family of high molecular weight, heavily glycosylated proteins containing hydrophobic polypeptide backbones connected to multiple hydrophilic oligosaccharide chains (Bansil and Turner, 2018). These mucin polypeptide backbones possess amino acid sequences with positive and negative charge that assist in trapping charged and hydrophobic particles (Lieleg et al., 2010; Suk et al., 2009). Besides mucin, numerous biomacromolecules - including proteins, antibodies, and lipids are present in the mucus and form a mesh-like structure through disulfide cross-linkages and/or physical entanglement, which is referred as the mucus microstructure (Duncan et al., 2016a). As per the HUGO Gene Nomenclature Committee, there are 24 human MUC genes (denoted with capital letters) of which 14 are located in the respiratory tract. Based on the ability to polymerize and if they are cell surface-bound or secreted, airway mucins are classified into three types: secreted monomeric mucins (MUC7, MUC8), secreted gel-forming mucins (MUC2, MUC5AC, MUC5B, MUC6, and MUC19), and non-secreted surface-bound mucins (MUC1, MUC4, MUC13, MUC16, MUC20, MUC21, and MUC22) (Atanasova and Reznikov, 2019; Ma et al., 2018). Each mucin is unique in size, sequence, and shares a common structural motif of tandem repeats that are rich in serine, threonine, and proline residues (Williams et al., 2006). Five of these secreted mucins possess cysteine domains at the terminal ends and can form disulfide bonds that impart a gel-like property. Among them, MUC5AC and MUC5B are the most abundant gel-forming mucins secreted by goblet cells in superficial epithelium and submucosal glands, respectively, which are key to the viscoelastic properties of airway mucus (Atanasova and Reznikov, 2019; Voynow and Rubin, 2009).
Mucus has complex and highly variable physical properties. The rheological behavior of mucus changes as a function of shear rate, shear stress, and length scale (Lai et al., 2009c). As the link between altered mucus rheology and difficulty in airway clearance has been established, findings have also correlated abnormal sputum viscoelastic properties as biomarkers of secondary infection (Dulfano and Adler, 1975; Patarin et al., 2020; Puchelle et al., 1985; Serisier et al., 2009). At the macroscopic level (bulk rheology), averaged physical properties of mucus such as flow and deformation can be measured. Macrorheological analysis of human mucus at low shear yields a viscosity as high as 104–106 folds to that of water. Depending on the anatomical region in the lungs, the rheological properties of respiratory mucus varies. In particular, small airway mucus has lower elasticity compared to mucus from the trachea (App et al., 1993). In conditions such as during coughing, the viscosity of mucus may be greatly reduced by increased shear stress (Ren et al., 2020).
Microrheology can be defined as the measure of rheology of a small volume of mucus probed by micro- or nanoparticles. Multiple particle tracking (MPT) has been widely used to study the flow dynamics of nanoparticles and gene nanocarriers within the network of mucus (Chai et al., 2020; Dawson et al., 2004; Schuster et al., 2014; Song et al., 2021; Suh et al., 2004). MPT involves studying the motion of individual particles or small groups of particles in real-time using fluorescence video microscopy. This reveals interactions of these particles with the mucus environment and the transport properties of these particles within the mucus network. In addition, post-acquisition analysis of time-resolved particle trajectories can be performed (Chai et al., 2020; Lai et al., 2007; Benjamin S. Schuster et al., 2013). MPT also provides quantitative information such as effective diffusivities, maximum velocity, and directionality along with qualitative information such as the mechanism of particle transport (Lai and Hanes, 2008; Selvaggi et al., 2010; Valentine et al., 2001). In brief, the x and y positions of particle centroids over time are extracted from the MPT movies and the trajectories of particles in the mucus are constructed. The time-averaged mean squared displacement (MSD) of each particle’s trajectory is calculated as:
| (1) |
Where, τ is the time scale, x(t) and y(t) represent the particles coordinates at a given time (Benjamin S Schuster et al., 2013). The particle tracking microrheology is performed by using the generalized Stokes-Einstein equation, which relates the thermal motion of particles to the viscoelastic moduli of the mucus probed. In 2D particle tracking microrheology complex shear modulus, G′ and G′′ are calculated as explained in detail by (Lai et al., 2009a). The phase angle (δ) determines whether the viscoelastic mucus is more solid (δ <45°, G′ > G′′) or more liquid like (δ > 45°, G′ < G′′). The following equation determines the phase angle of mucus (Ensign et al., 2012):
| (2) |
Any particles with sizes larger than the length scale of the mucus microstructure may experience steric obstruction and limited diffusion through the mucus. However, particles with lengths up to 55 nm demonstrate similar microviscosity in mucus and water (Lai et al., 2009c; Olmsted et al., 2001; Tan et al., 2020). A study by Lai et al. established that even larger PEG-coated particles up to 500 nm in diameter can diffuse through human mucus with effective diffusivities merely 4-times lower than water (Lai et al., 2007). Later, Schuster et al. studied the effect of particle size and surface chemistry on nanoparticle diffusion through airway mucus from healthy humans and showed that nanoparticles with particle sizes <500 nm in diameter and muco-inert properties can rapidly penetrate normal airway mucus (Benjamin S. Schuster et al., 2013). These studies highlight the importance that mucus mesh architecture might be more diverse than was recognized in previously reported diffusion experiments. To further understand the microscopic rheological properties of mucus using MPT, complex modulus (G* (ω)) and complex viscosity (ǀƞ* (ω)ǀ) can be calculated using the following formulae (Mason et al., 1997; Mason and Weitz, 1995; Benjamin S. Schuster et al., 2013):
| (3) |
| (4) |
Muco-inert particles (MIPs) of 100 and 200 nm diameters showed viscosities 16- and 38-fold greater compared to mucus interstitial fluid, respectively (Benjamin S. Schuster et al., 2013). Small-sized MIPs resist muco-adhesion and can diffuse through mucus mesh. However, particles larger than the mucus mesh are sensitive to the biopolymer network elasticity and its bulk rheology (Benjamin S. Schuster et al., 2013). Overall, a proper understanding of macrorheology and microrheology of airway mucus in healthy and diseased subjects might assist in the design of novel drug and gene delivery systems.
3. Airway mucus in respiratory disease and evaluation strategies
Mucin glycoproteins have a vital role in the airway’s innate immune system and actively take part in normal MCC (Rose and Voynow, 2006). Healthy airway secretions mainly contain MUC5AC which has a small mass to unit length ratio with shorter oligosaccharide chains, whereas MUC5B is predominant in chronic airway diseases and is present in both low-and high-charge glycoforms, suggesting both MUC5AC or MUC5B have distinct barrier functions in the mucus (Sheehan et al., 1991; Voynow and Rubin, 2009). However, analysis of mucin content in patients with acute and/or chronic airway inflammation, such as CF and COPD, demonstrated upregulation of mucin production, specifically MUC5AC and MUC5B (Kirkham et al., 2002). Esther Jr et al. analyzed mucus in the bronchoalveolar lavage fluid (BALF) from children with CF and control group without CF and found that the total airway mucin concentration was higher in CF compared to non-CF samples. Moreover, quantitative assessment of flake density and mucin content by immunohistochemistry (IHC) demonstrated that MUC5B and MUC5AC were mixed in the mucus flakes, and these particular mucins were more in CF samples compared to non-CF samples (Figure 1) (Esther Jr et al., 2019).
Figure 1.
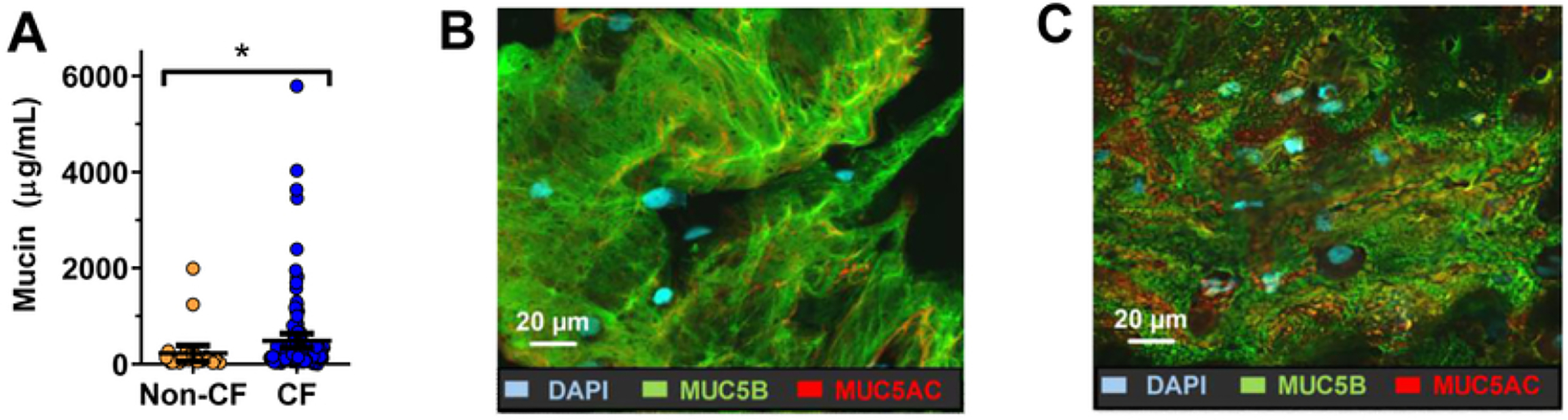
(A) BALF total mucin concentration from CF patients and non-CF controls; (B) & (C) represents IHC of MUC5B (green) and MUC5AC (red) in a cytospin from non-CF and CF BALF, respectively. *p<0.05 after multivariate analysis. Reproduced with permission from (Esther Jr et al., 2019).
In chronic respiratory diseases such as COPD, CF, respiratory syncytial virus (RSV) infection, and non-CF bronchiectasis, a network of copolymerized extracellular DNA and filamentous (F-) actin are released due to NETosis (Kater et al., 2007; McGuckin et al., 2015; Rubin, 2007; Thiam et al., 2020b). NETosis is a program for formation of neutrophil extracellular traps (NETs), characterized by the release of decondensed chromatin and granular contents to the extracellular space, leading to increased viscoelasticity of airway mucus (Thiam et al., 2020a; Vorobjeva and Chernyak, 2020). However, due to a lack of suitable in vivo and in situ measurement techniques, most of the rheological measurements of airway sputum are from in vitro studies that are subjected to changes in handling, which may not provide a true in vivo representation (Chen et al., 2019; Dulfano et al., 1971). Cone and plate rheometers have been used for bulk rheological analysis of the mucus, in which the mucus sample is suspended between a flat plate and an inverted cone. Under oscillation, the plate is rotated with a known amplitude and shear rate which results in a certain torque and input strain. This obtained data is used to calculate shear stress, phase angle, apparent viscosity, viscous modulus (G′′), and elastic modulus (G′) (Lai et al., 2009c; Benjamin S. Schuster et al., 2013). Recently, Abrami et al. proposed the combined use of bulk rheology and low field NMR to study the mesh size distribution in mucus. The study demonstrated that this combination approach can provide a more detailed picture of the mucus three-dimensional architecture along with mesh size distribution of the mucus network (Abrami et al., 2022, 2021).
Scanning electron microscope (SEM) imaging of airway mucus has confirmed a mesh-like architecture with the pore size ranging up to hundreds of nanometers in diameter (Figure 2). Both, normal airway mucus and CF sputum demonstrated similar overall appearances when observed using SEM; however, preparation of SEM samples likely contracts the mucus microstructure (Richard A. Cone, 2009; Benjamin S. Schuster et al., 2013; Suk et al., 2011b). Confocal Raman microscopy also has been used as a label-free and chemically selective technique to visualize the composition and structure of human airway mucus (Vukosavljevic et al., 2017). Despite these advantages, it is a time-consuming technique that uses a laser beam to create heat that dries the mucus sample, resulting in analytical challenges (Alinovi et al., 2020).
Figure 2.

SEM of airway mucus that shows a mesh-like architecture with the pore size ranging up to hundreds of nanometers in diameter (scale bar 500 nm). Reproduced with permission from (Benjamin S. Schuster et al., 2013).
In respiratory diseases, increase in mucin disulfide bond density (both inter- and intra-) or impaired secretion of bicarbonates, leads to the formation of mucus with a tighter mesh network (Chen et al., 2010b; Quinton, 2008; van der Vliet et al., 2018). These alterations contribute to the change in permeability and microstructural properties such as decreases in mucus mesh size, which reduces neutrophil migration and decreases bacterial capture, thereby sustaining infection (Duncan et al., 2016b; Linssen et al., 2020; Matsui et al., 2005). Microstructural analysis of mucus may serve as marker of lung disease severity; however, microstructural analysis in its native state is often challenging and necessitates preservation of the hydration state of hydrogels to prevent collapse of the mesh (Duncan et al., 2016b; Vukosavljevic et al., 2017).
Previous studies have shown that the microrheology and mucosal microstructure in respiratory diseases can be quantitatively determined by monitoring transport or diffusion of muco-inert particle (MIP) probes in the mucus via MPT, which has been discussed in detail in the previous section (Lai et al., 2011, 2007; Benjamin S. Schuster et al., 2013). Polyethylene glycol (PEG) coated MIPs as large as 500 nm can diffuse through normal airway mucus from healthy subjects, whereas the same size uncoated conventional polymeric particles are restricted by the mucus microstructure due to adhesive interaction between particles and mucus components (Richard A. Cone, 2009; Lai et al., 2007; Benjamin S Schuster et al., 2013). PEG-coated MIPs up to 200 nm in diameter diffused about 90-times faster through sputum from CF patients, compared to uncoated particles of similar diameter (Lai et al., 2011; Suk et al., 2009). However, there is a large variation in particle diffusion for sputum samples from different CF patients, even while using the same size particle probes, suggesting patient-specific inconsistencies in microstructural properties (Schuster et al., 2014). Studying the effect of key biochemical components of CF sputum on mucus microstructure reveals that the increase in mucin, DNA, and/or solid content is heavily linked with the decrease in mucus mesh size (Duncan et al., 2016b). These real-time dynamics studies using various sized particles have probed the 3D mesh sizes and structures for mucus from healthy, CF, and other respiratory conditions (Lai et al., 2007; Suk et al., 2009). MPT has also been used to study the viscoelasticity of human CF sputum, which demonstrated a lower magnitude for microviscosity compared to macroviscosity, suggesting increased microheterogeneity in particle tracking (Dawson et al., 2003).
4. Characteristics of airway mucus in respiratory diseases and alteration of mucus microstructure
Airway mucus secretions represent the first-line defense of the respiratory tract. In addition, particle permeability through airway mucus depends on the microstructural organization of the mucus, size, and surface chemistry of the particulate matter (Krupa et al., 2020). However, mucus hypersecretion, abnormal secretions, and decreased clearance can cause obstruction of airways, limit airflow, and accelerate lung function decline, leading to obstructive pulmonary diseases. Even though chronic bronchial diseases share distinct origins and mechanisms, the common pathophysiological and clinical manifestations of these diseases are mucus hypersecretion, airway inflammation, and impaired MCC (Fahy and Dickey, 2010; Kirkham et al., 2002). Interestingly, patients with COPD, CF, non-CF bronchiectasis, and asthma have altered mucins and water content at the airway epithelial surface, higher solid percentage, altered viscosity due to the release of DNA and F-actin filaments from stimulated neutrophils and damaged epithelial cells (Daviskas and Rubin, 2013; Thiam et al., 2020a; Williams et al., 2006). Recently, Patarin et al. assessed both quasi-static (linear storage and loss moduli) and dynamic rheological (flow point) properties of sputum samples collected from several chronic bronchial diseases and healthy subjects. Their study demonstrated that respiratory pathologies substantially influence the linear and flow properties of sputum and highlighted the relevance of determining quasi-static and dynamic rheological properties of sputum as a possible marker of chronic respiratory diseases (Patarin et al., 2020). Fahy and Dickey discussed that the conversion of mucus from healthy to pathologic condition occurs through several mechanisms via alteration in hydration state, biochemical constituents, and physical properties of mucus, including abnormal secretion of water and electrolytes, neutrophilic inflammation, and bronchovascular permeability (shown in Table 1 (Fahy and Dickey, 2010; Meldrum and Chotirmall, 2021)). The above findings provide broad implications that the characteristics of mucus in airway diseases may be a potential determinant of respiratory diseases.
Table 1.
Characteristics of mucus in airway mucosal diseases
| Airway mucus | Mucus characteristics | References |
|---|---|---|
| Healthy airway mucus |
|
(Fahy and Dickey, 2010; McGuckin et al., 2015; Rubin, 2007) |
| Asthma |
|
(Bonser et al., 2016; Bonser and Erle, 2017; Kuyper et al., 2003; Lachowicz-Scroggins et al., 2016) |
| Chronic obstructive pulmonary disease (COPD) |
|
(Anderson et al., 2015; Lin et al., 2020; Radicioni et al., 2021) |
| Cystic fibrosis (CF) |
|
(Button et al., 2016; Livraghi-Butrico et al., 2017; Martens et al., 2011) |
4.1. COPD and mucus microstructure
Airways from COPD patients have surface epithelial mucous metaplasia and hyperplasia along with increased submucosal glands and sputum with increased mucin polymerization and elasticity (Yuan et al., 2015). A key risk factor for COPD patients is cigarette smoke which can lead to the activation of pulmonary epidermal growth factor receptor (EGFR) cascades that enhance mucin production and goblet cell hyperplasia and epithelial barrier dysfunction (Mishra et al., 2016; Mohammad et al., 2013). Mucus obstruction is thought to be due to hypersecretion, airway dehydration, and rheological changes (Chisholm et al., 2019). Radicioni et al. highlighted the role of MUC5AC hypersecretion in the initiation, progression, exacerbation, and pathogenesis of COPD. Published data also suggests a change in MUC5AC concentration as a function of COPD severity (Radicioni et al., 2021). Sputum expectoration in COPD patients assists in the removal of phlegm from the large airways, but small airway mucus obstruction is considered a characteristic abnormality in COPD. Bulk rheological properties of airway mucus in patients with COPD have been widely explored and revealed to have higher viscoelasticity compared to samples from healthy subjects and subjects with CF. In contrast, watery mucus produced from asymptomatic tobacco smokers were cleared faster by cilia than normal mucus (Rubin et al., 1992). However, only limited studies have investigated the correlation between COPD sputum mesh size to sputum composition and lung function.
Chisholm et al. studied sputum microstructure in smokers with and without airway obstruction by using MPT with MAPs and MIPs (Chisholm et al., 2019). In sputum samples from smokers without airway obstruction, 100 nm MIPs highly diffused through sputum, whereas MAPs were largely hindered regardless of their particle diameter. Furthermore, same-sized MIPs and MAPs displayed unhindered diffusion and highly confined trajectories, respectively, in sputum samples from COPD patients with MSD values significantly higher for the MIPs compared to the MAPs. This study demonstrated that MIPs prevented sputum adhesion and the diffusion rates of MIPs can be explored to distinguish the biophysical properties of sputum. However, MIPs and MAPs with particle diameters greater than 300 nm showed confined trajectories in both non-COPD and COPD sputum. This indicated that the MSD values were significantly greater for the MIPS compared to that of the MAPs, suggesting tighter sputum mesh (smaller mesh size) in COPD. The authors also reported significantly higher solid content and mucin concentration in COPD sputum samples compared to non-COPD samples, whereas DNA content was not statistically significant between these groups.
Lin et al. use MPT to study mucus derived from primary human bronchial epithelial (HBE) cells with or without exogenous cigarette smoke exposure or cholinergic stimulation (Figure 3A) (Lin et al., 2020). As shown in Figure 3B, estimation of the MSD demonstrated decreased transport of MIP (1 μm particles) in mucus from COPD patient’s than to the mucus from normal and healthy smoker donors. The microviscosity as well as the solid content of mucus from COPD patients was significantly higher than that of healthy smokers and non-smoking donors (Figure 3C). Sputum collected from COPD donors had higher viscosity at 0.6 Hz and a higher percentage of solid content (both positively correlated) compared to mucus from healthy individuals and smokers (Figures 3D and E). Above data shows that change in mucin structure, mucus mesh size, and mucociliary transport occur in response to chronic exposure to cigarette smoke.
Figure 3.
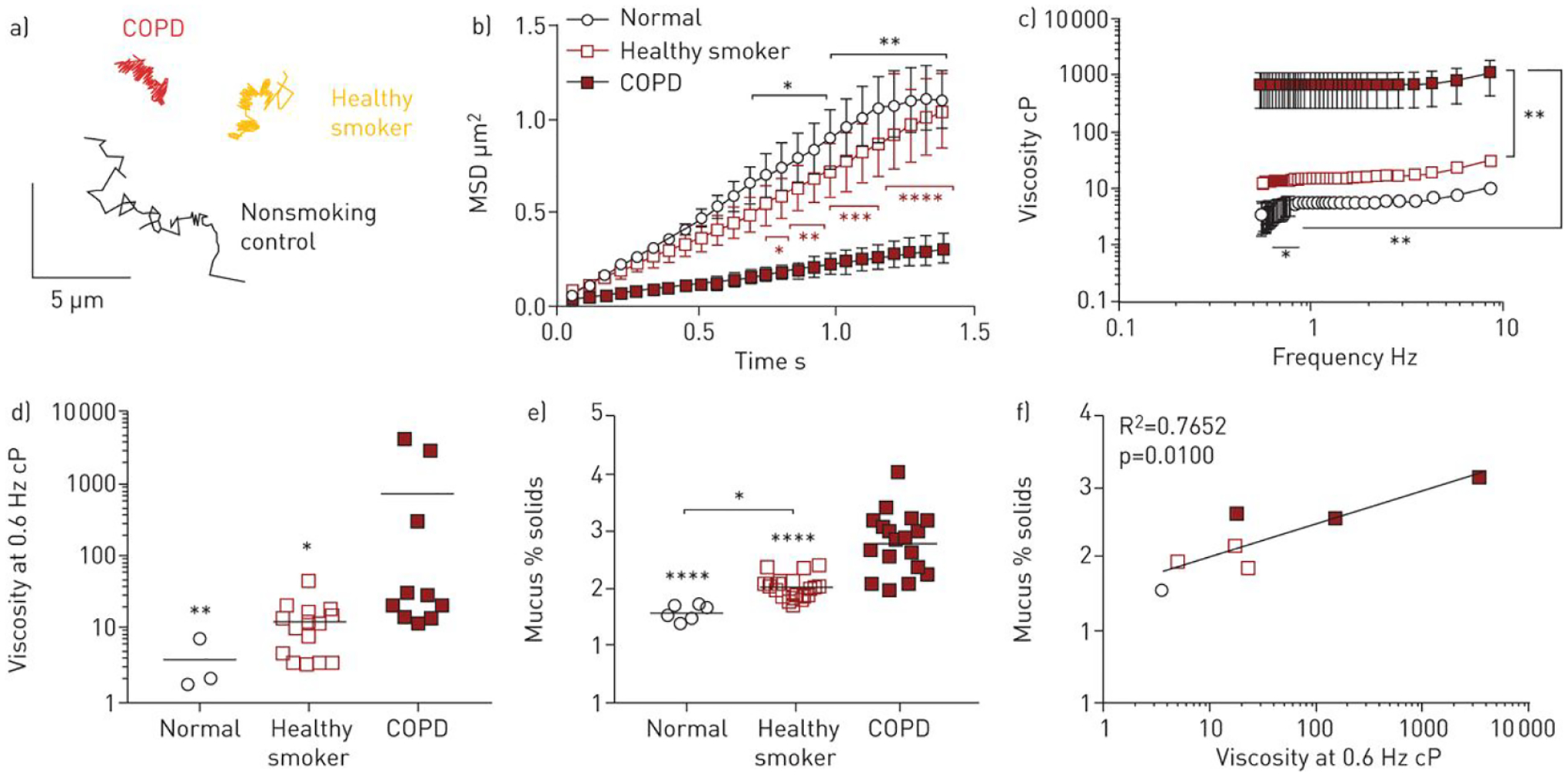
Diffusion of MIPs (1 μm) in mucus from primary HBE cells. (A) Representative particle trajectories of 1 μm MIPs in mucus from normal control, healthy smoker, and COPD patients. Scale bar, 5 μm. (B) Ensemble-averaged geometric mean square displacements (<MSD>) as a function of time scale. (C) Effective viscosity of mucus from normal control, healthy smoker, and COPD patients. (D) Comparative effective viscosity of each mucus samples at 0.6 Hz. (E) Mucus percent solids content by weight in each mucus samples. (F) Plot between mucus solid content and effective viscosity for mucus from each group. Reproduced with permission from (Lin et al., 2020)
4.2. Microstructural alterations in cystic fibrosis sputum
It is thought that increased mucin concentration in CF sputum is due, in part, to defects in CFTR which leads to an imbalance of water and electrolyte secretion in the CF airway(Chen et al., 2010b; Quinton, 2008). CFTR dysfunction causes decreased airway surface liquid (ASL) leading to increased osmotic pressure in the mucus layer (Button et al., 2012). Another hypothesis suggests that mucus dehydration causes an increase in mucin concentration along with a reduction in mucin pH, increase in cellular debris, reduction in glutathione, and elevation in myeloperoxidase levels, resulting in additional inter-chain cross-links (Perez-Vilar and Boucher, 2004). In contrast to the hydrated normal airway mucus that has a mesh size of around 0.2 – 1 μm, the mesh size in CF sputum is <100 nm (Clunes and Boucher, 2007). The decrease in the mesh size of CF sputum leads to the inability of neutrophils to penetrate through the mucus to capture bacteria (Matsui et al., 2005). Increase in the viscoelasticity of sputum from CF patient’s greatly reduced particles diffusion, thereby reducing the efficiency of drug delivery systems to lung epithelial cells (Dawson et al., 2003; Prasher et al., 2022).
Suk et al. estimated that fresh CF sputum has the mesh spacing of approximately 140 ± 50 nm using a fitted transport rate of various sized MIP (100, 200, and 500 nm in diameter) to an obstruction-scaling model (Suk et al., 2009). They also analyzed the extent of impediments to particle penetration in native CF sputum compared to N-acetyl L-cysteine (NAC) treated CF sputum. NAC is a mucolytic drug that cleaves disulfide bonds and so enhance MIP penetration in CF sputum. The diffusion exponent, α (for pure unhindered Brownian diffusion, α = 1; as the particle diffusion is obstructed, α approaches to 0) was 0.91 for the 200 nm MIP particles in CF sputum from NAC-treated group but in native sputum the value of α was 0.70 (Suk et al., 2011b). While experimentation of MIP diffusion through sputum can be used to evaluate microrheological and microstructural properties of mucus, there exists an interpatient variability roughly 50-times higher than intra-patient sputum samples. This might be due to differences in disease severity (Duncan et al., 2016b). The same study reported that the decrease in sputum mesh size (log10 [MSD1s]) was linked to the increase in mucin, DNA, and total solid concentration in sputum. Previous studies made several efforts to improve particle diffusion through the CF sputum by decreasing the barrier properties of the sputum or surface coating the particles with non-muco-adhesive polymers such as PEG (Dawson et al., 2003; Duncan et al., 2016b; Forier et al., 2013; Suk et al., 2011b, 2009).
4.3. Mucus hypersecretion in asthma and mucus microstructure
In patients with asthma, the relative proportion of MUC5AC and MUC5B mucins in sputum is altered with disease severity (Bonser and Erle, 2017). Asthmatic subjects had higher levels of MUC5AC compared to MUC5B, while in healthy individuals, MUC5B was more prevalent in respiratory mucus (Bonser and Erle, 2017; Kirkham et al., 2002). Goblet cell hyperplasia, secretory cell hypertrophy, and upregulated expression of MUC5AC might contribute to mucus accumulation and plugging, ultimately causing small airway obstruction (Evans et al., 2015; Ha and Rogers, 2016; Shen et al., 2018). Measurements of bulk rheology of respiratory secretions from asthmatic patients during acute exacerbations showed increased mucin cross-linking rather than mucin concentrations, which was demonstrated by the marked increase in elastic modulus (Innes et al., 2009; Yuan et al., 2015).
Morgan et al. examined the biophysical properties of airway mucus from asthma patients using the MPT technique (Morgan et al., 2021). To study whether mucus gel structure, MCC, and airway obstruction in asthma can be improved by disulfides disruption of mucus, mucus samples collected from asthma patients were treated with the reducing agent tris(2-carboxyethyl) phosphine (TCEP, 10 mM) and examined by MPT using PEG5k-conjugated 100 nm polystyrene beads. TCEP treatment increased MSD values, decreased the viscoelasticity of mucus, caused rapid depolarization of mucins, normalized homogeneity and level of MSD to that similar to controls. Another study examined the effect of MUC5B:MUC5AC mucin ratios on the macro- and microrheology of synthetic mucus and compared the elastic moduli of mucin-based hydrogels (Song et al., 2021). Higher MUC5AC content resulted in greater elastic and viscous moduli. MPT analysis using 100 nm MIP probes showed that increased MUC5AC content was associated with decreased particle diffusion, suggesting increased viscoelastic and a decrease in the mesh network size. A diffusion study using larger-sized MIP probes of 500 nm demonstrated a further reduction in log[MSD1s], which suggests an increase in the viscoelasticity with increasing MUC5AC. The authors also evaluated the gel network mesh size via MPT analysis and reported that MUC5B-rich hydrogels had larger mesh sizes (200–400 nm), whereas MUC5AC-rich gels had reduced network mesh sizes (100 nm).
4.4. Hyperconcentration of airway mucus in Non-CF bronchiectasis
Non-CF bronchiectasis is characterized by chronic airway obstruction and infection leading to dilation of the bronchi. These airways become filled with phlegm (Ramos et al., 2015; Rubin, 2009; Van der Schans, 2007). The relationship between secreted mucin levels and the presence of bacterial infection in the airways of stable bronchiectasis patients demonstrated that the highest levels of airway MUC2 was in patients’ airways infected with P. aeruginosa. Overall, airway mucus from non-CF bronchiectasis patients exhibited increased solid percentage as well as increased major secreted airway mucins compared with sputum from healthy individuals (Kesimer et al., 2017; Ramsey et al., 2020).
5. Contribution of Neutrophil Extracellular Traps (NETs) in respiratory diseases
Neutrophils are the first immune cells recruited to the site of inflammation and infection in pulmonary diseases. Neutrophil defense mechanisms include phagocytosis, degranulation, producing reactive oxygen species (ROS) and pro-inflammatory cytokines, and extruding neutrophil extracellular traps (NETs) (Brinkmann et al., 2004; Tucker et al., 2021). NETs are web-like structures composed of DNA in association with histones, antimicrobial granular, and cytoplasmic proteins such as neutrophil elastase, myeloperoxidase, and α-defensins (Keir and Chalmers, 2022; Porto and Stein, 2016; Tucker et al., 2021). The primary role of NETs is to trap and kill extracellular bacteria and other pathogens during infections (Brinkmann et al., 2004; Lefrançais et al., 2018). NET scaffold consists of chromatin fibers, DNA, and histones as the major structural constituents (Brinkmann et al., 2004). In patients with lung diseases, production of NETs decorated with histones causes cell cytotoxicity leading to inflammation (Cheng and Palaniyar, 2013; Saffarzadeh et al., 2012). A review by Block and Zarbock discussed the essential steps of NET formation (Block and Zarbock, 2021). As illustrated in Figure 4, different pathogens are capable of inducing NET formation and receptors such as toll-like-, G-protein coupled-, and Fc-receptors communicate with the cells to activate NADPH-oxidase complex, subsequently catalyzing ROS generation. Neutrophil elastase is released from the membranes in a ROS-dependent manner and results in the decondensation and release of chromatins decorated with antimicrobial agents.
Figure 4.
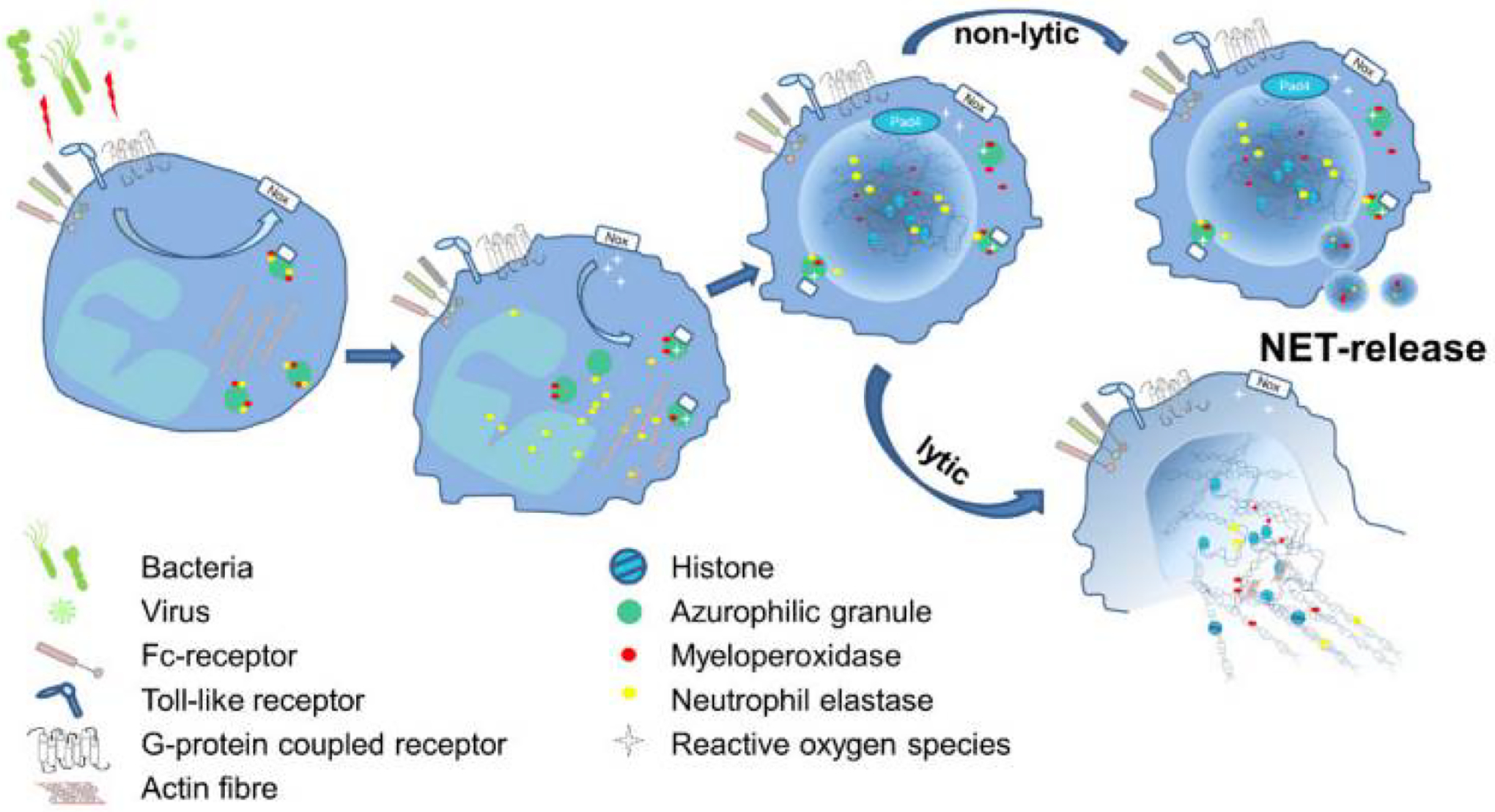
Schematic illustration of the essential steps of NET formation. Reproduced with permission (Block and Zarbock, 2021).
NETs have been identified in the sputum of pulmonary disease patients and the ultrastructural characterization of these sputum demonstrated NETs contribute to increased viscosity (Lethem et al., 1990; Saffarzadeh et al., 2012). The contribution of NETs on airway mucus viscoelasticity was recently explored by our team (Linssen et al., 2020). Human airway mucus was collected from endotracheal tubes of healthy adult patients admitted for elective surgery and were coincubated with NETs from phorbol 12-myristate 13-acetate-stimulated neutrophils. The study demonstrated that mucus coincubated with NETs resulted in significant increase in mucus viscoelasticity and decrease in mesh size of the mucus, and decreased movement of MIPs (100 nm and 500 nm) through the mucus as measured by MPT. However, the underlying mechanisms behind the development of NETs and their role in airway inflammation and increased mucus production still remain unclear (X. Chen et al., 2021; Zou et al., 2018). Studies suggest that production of NETs and delayed NET clearance are a contributor to chronic inflammation and lung tissue damage (Grabcanovic-Musija et al., 2015; Keir and Chalmers, 2022; Liu et al., 2017; Uddin et al., 2019). The bactericidal activity of neutrophils and NETosis is affected with pH of airway surface liquid and airway infections (Khan et al., 2019; Moraes et al., 2006; Pezzulo et al., 2012). Increased secretion of pro-inflammatory cytokines in CF lungs might induce NET formation and its release (Dwyer et al., 2014; Marcos et al., 2010). NET formation is also induced by the resistance of P. aeruginosa to NET-mediated killing, bacterial mutations in chronic infections, and the release of eicosanoid hepoxilin A3 by lung epithelial cells (Douda et al., 2015; Hurley et al., 2004; Limoli et al., 2014; Oliver et al., 2000; Rahman and Gadjeva, 2014). Dornase alfa can cleave polymerized DNA and reduce the viscosity of CF sputum is a widely accepted therapy for CF. In addition, use of dornase alfa in patients with COPD and non-CF bronchiectasis has shown potentially harmful effects in clinical trials (Aaron, 2017; McShane et al., 2013; Pasteur et al., 2010). Sputum from COPD patients also contains NETs and NET-forming neutrophils (Dicker et al., 2018; Grabcanovic-Musija et al., 2015; Obermayer et al., 2014). COVID-19 patients also have NETs in their airways (Middleton et al., 2020; Radermecker et al., 2020).
6. Muco-adhesive and muco-penetrating particles designed to overcome the respiratory mucus barrier
Particles with sizes larger than the airway mucus meshwork are trapped by steric obstruction while smaller particles can diffuse through the mucus pores (Huckaby and Lai, 2018). Negatively charged mucins can capture particles with cationic domains. This strategy has been applied to develop muco-adhesive particles (Figure 5) (Huck et al., 2022). The high density of glycans in the mucin fibers can also form hydrogen bonds with carboxyl and hydroxyl groups on the particle surfaces (Lele and Hoffman, 2000) and the non-glycosylated domain of the mucin forms a hydrophobic backbone thereby providing hydrophobic interactions with particles (Richard A. Cone, 2009; Murgia et al., 2018). Therapeutic aerosol delivery has been a primary approach to treat lung conditions such as asthma, CF, and COPD, however, it is important to design nanoparticles with appropriate sizes and surface chemistry for improved drug delivery to the lungs.
Figure 5.
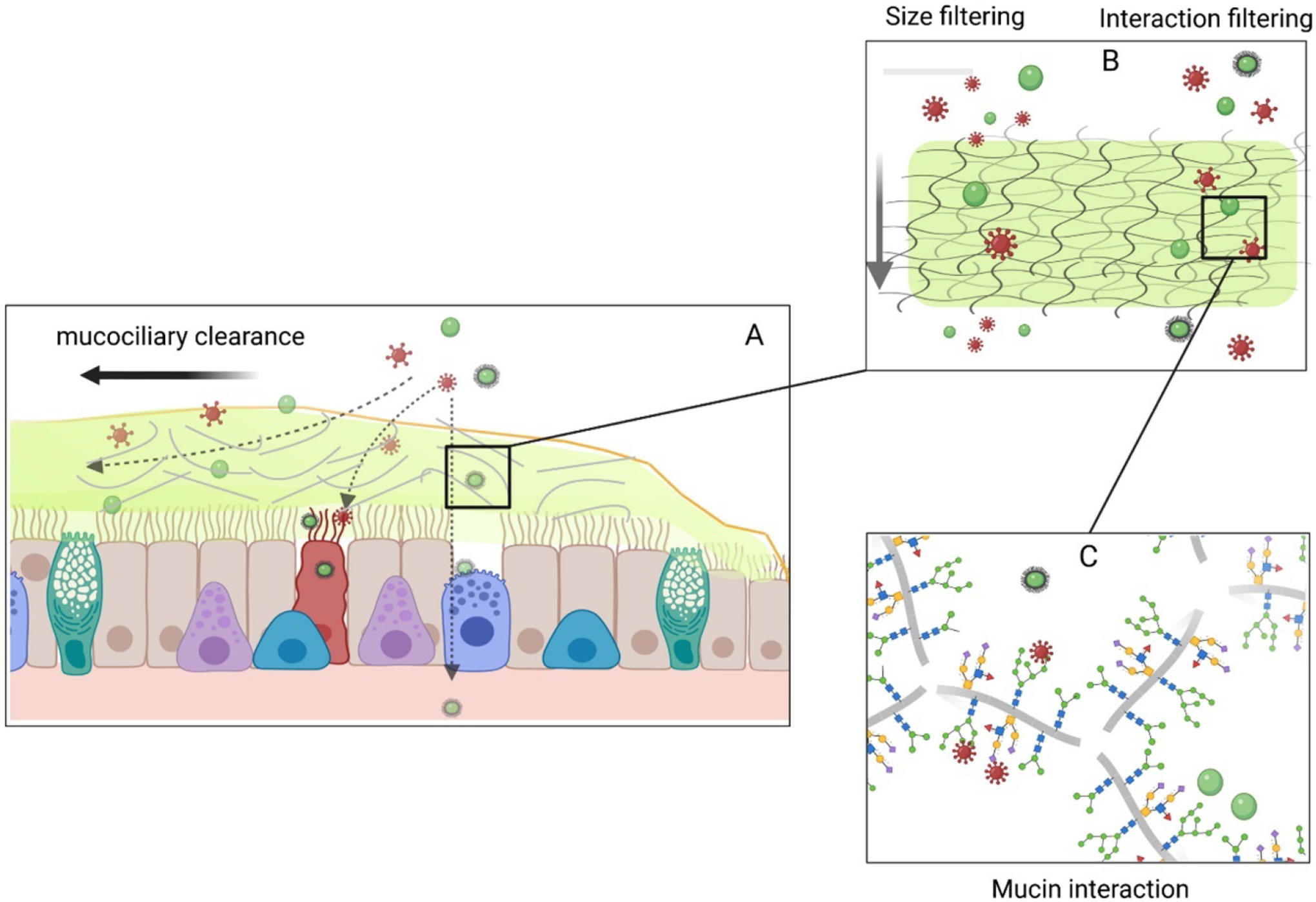
Schematic on the role of mucus barrier. (A) As shown in figure, airway mucus entraps hazardous (red spiked structures) and therapeutic (green structures) inhaled particles. (B) Depending on the size and surface charge, the inhaled particles either penetrate the mucus barrier or (C) interacts with mucin backbone. Reproduced with permission from (Huck et al., 2022).
As discussed above, pulmonary drug delivery is an effective approach to treating lung diseases, however, the mucus layer in the airways has evolved in protecting the lungs by rapidly trapping and removing foreign particles through the MCC (Da Silva et al., 2023; Ji et al., 2020; Osman et al., 2018). Several formulation-based approaches have been developed to extend the drug residence time in the lungs of poorly water-soluble drugs (Table 2). Among them, surface-modified biodegradable nanoparticles that can adhere to the mucus, namely MAPs (muco-adhesive particles), have been developed. Since mucus has highly glycosylated proteins, MAPs have been engineered to possess a positive surface charge to enhance electrostatic interactions with mucin fibers (Linden et al., 2008). Chitosan is one of the most commonly used polymers for developing MAPs through chemical conjugation or physical absorption on particle surfaces (M Ways et al., 2018; Rawal et al., 2018; Vieira et al., 2018). Another approach to enhancing the muco-adhesive properties of nanoparticles is to engineer nanoparticles using thiol-derived chitosan that can form disulfide bonds with mucin strands via thiol groups (Makhlof et al., 2010). It has been demonstrated that the chitosan-based MAPs can provide higher permeability and a better pharmacokinetic profile in healthy rat lungs than uncoated particles; however, it is still controversial whether MAPs can support the clinical application in some chronic inflammatory airway diseases with secretion retention (Bustamante-Marin and Ostrowski, 2017; Morrison et al., 2019). These cationic and thiol-coated nanoparticles are usually formulated with polymeric cores indicating that the muco-adhesive properties of the nanoparticles may also be due to the hydrophobic interactions of the nanoparticles and the mucus (Benjamin S Schuster et al., 2013; Suk et al., 2009). An alternative approach to achieving muco-adhesion is to use polymers that can physically entangle with mucin fibers, such as polyacrylic acid, alginates, and hyaluronic acid-coated nanoparticles (Cui et al., 2006; H. Kim et al., 2013; Müller et al., 2012). Despite this, MAPs mainly stick to the superficial layer of the airway mucus and are quickly cleared due to the rapid MCC and steady airway mucus turnover (~10–20 min). Therefore, it is expected that fewer MAPs will reach the underlying periciliary layer (PCL), potentially decreasing their bioavailability (Ali and Pearson, 2007).
Table 2.
Formulation strategies for the design of muco-adhesive and muco-penetrating particles to overcome airway mucus barrier
| Type of Formulation | Drug/Formulation details | Formulation characterization | Findings | References |
|---|---|---|---|---|
| Muco-adhesive nanoparticles |
|
|
|
(Rawal et al., 2018) |
|
|
|
(Vieira et al., 2018) | |
|
|
|
(Makhlof et al., 2010) | |
|
|
|
(H. Kim et al., 2013) | |
| Muco-penetrating nanoparticles |
|
|
|
(Benjamin S. Schuster et al., 2013) |
|
|
|
(Suk et al., 2009) | |
|
|
|
(Schneider et al., 2017) | |
|
|
|
(Boylan et al., 2012) | |
|
|
|
(Suk et al., 2014) |
Mucins are condensed and/or bundled into a thick cable through hydrophobic interaction, so with MAP treatment the structure of the mucus might be altered via interaction with MAPs (Lai et al., 2010). Wang et al. engineered MIP by dense coating of carboxylated polystyrene particles (1 μm in diameter) with PEG2k (surface charge −6.1 ± 0.9 mV), whose diffusion in the mucus is hindered only by steric obstruction. These MIP were used to determine the microstructural changes of mucus caused by MAP (200 nm PS-NH2, surface charge 13 ± 1.1 mV at pH 4). The study demonstrated that a high concentration (0.24% w/v) of MAP induced increased average effective diffusivity of the probe particles (MIP). In addition, use of obstruction-scaling model showed that the higher MAP concentration increased the average mucus mesh size from 380 nm to 470 nm, which was not observed at low concentration of MAP (0.0006% w/v) (Wang et al., 2011). Although the MAPs did not alter the rheology of the mucus, the MAPs may have cross-linked with the mucus fibers and caused them to bundle together, enlarging mucus meshwork and compromising its ability to trap foreign particles and pathogens. Since larger nanoparticles can provide higher drug loading and longer drug release profiles, the authors proposed to use “Sacrificial MAPs” to transiently enlarge the mucus pores facilitating the later administration of larger drug-loaded nanoparticles. Unfortunately, this may not be applicable for pulmonary drug delivery considering there is much faster airway mucus clearance (10~20 min) than cervicovaginal mucus (up to hours) (Ali and Pearson, 2007; Lai et al., 2009b).
Mucus penetration particles (MPP) or muco-inert particles (MIP) engineered by dense PEG-coating can minimize the interaction with airway mucus (Craparo et al., 2016; Schneider et al., 2017). Study conducted by Forier et al. to characterize the mobility of 100 nm and 200 nm PS-PEG MPP and PS-COOH MAP in CF sputum and biofilm suggested that PEGylation increased diffusion of nanoparticles in CF sputum and a better accumulation in the biofilm (Forier et al., 2013). Later studies further demonstrated that nanoparticles that are densely coated with high molecular weight PEG (e.g. 10 kDa and 40 kDa) can also prevent the mucus interactions, as long as the PEG coating is a dense brush conformation (Γ/ Γ*>2) (Maisel et al., 2016; Xu et al., 2013). These results highlighted the importance of engineering MPPs with suitable physiochemical properties for characterizing the microstructure of airway mucus and effective drug delivery to the lungs.
Schneider et al. compared the behavior of PS-PEG5k (MPPs) and PS-COOH (MAPs) particles in mouse lungs after intranasal administration (Schneider et al., 2017). MPPs as large as 300 nm were uniformly distributed and retained in the airway while MAPs, regardless of particle sizes, formed large aggregates that were poorly distributed through the airway. In order to facilitate future clinical translation, biodegradable MPPs and MAPs were further engineered using poly(lactic-co-glycolic acid) (PLGA) polymers, first using a PLGA-PEG5k polymer. Uncoated PLGA nanoparticles (biodegradable MAPs control) and MPPs showed a particle size around 130–140 nm. However, the average zeta potential of uncoated and PEG5k coated nanoparticles were −73 mV and −6 mV, respectively. A previous study demonstrated that dense F127 coated PLGA/F127 nanoparticles can rapidly penetrate through human cervicovaginal mucus (CVM), while F68 with insufficient PEG coating limited the trajectories of nanoparticles in the CVM (Yang et al., 2011). Based on these findings, the authors then engineered b-MPPs and b-MAPs with comparable particle sizes around 180 – 200 nm using PLGA/F127 (densely PEG coating) and PLGA/F68 (non-densely PEG coating), respectively. Similar to the PS-PEG MPP, the biodegradable PLGA/F127 MPPs diffused rapidly in CF sputum ex vivo and had more uniform distribution and enhanced retention time in mouse lungs than biodegradable PLGA/F68 MAPs. Moreover, the dexamethasone sodium phosphate-loaded PLGA/F127 nanoparticles significantly reduced LPS-induced murine lung inflammation when compared to the drug-loaded PLGA/F68 nanoparticles.
Uniform distribution and expression of genes through the airway epithelial layer is important for gene therapy of lung diseases, however there are barriers that limit the effective penetration of gene carriers to the lungs (Suk et al., 2016). Bolyan et al. engineered highly compacted DNA-nanoparticles using CK30 polymer (poly-L-lysine) conjugated with various PEG MW (2kDa, 5kDa, and 10 kDa) forming CK30PEG2k, CK30PEG5k, and CK30PEG10k nanoparticles with particle sizes around 220, 300, and 350 nm, respectively and nearly neutral surface charges (Boylan et al., 2012). CK30PEG10k nanoparticles presented a flexible-rod shape structure (Figure 6A). Although the hydrodynamic diameters were 4-fold less than that of the CK30PEG2k nanoparticles, all the DNA-nanoparticles formulations were immobilized and the diffusion in CF sputum was 40-fold slower than muco-inert PS-PEG2k nanoparticles (Figure 6B). To improve the diffusive rate of CK30PEG DNA-nanoparticles, mucolytic agents such as dornase alfa, NAC or a combination of NAC, and dornase were added to CF sputum before the CK30PEG10k DNA-nanoparticles’ incubation (Suk et al., 2011a). Treatment alone with dornase did not enhance gene carrier diffusion; however, NAC and NAC plus dornase increased the average diffusive rates by 6-fold and 13-fold, respectively. While a mucolytic can improve the sputum penetration of CK30PEG10k DNA nanoparticles, many DNA nanoparticles were restrained in the CF sputum. This suggest that adhesive interactions rather than steric interactions are the primary factor that limits the mobility of the CK30PEG10k DNA nanoparticles in the sputum. The PEG surface density on all the CK30PEG DNA nanoparticles ranges from 4 to 10 molecules/100 nm2, which is 12–30-fold less than that of the muco-inert PS-PEG2k nanoparticles. A previous study reported that 40% reduction in the surface PEG density might cause 700-fold decrease in the average mobility of the PS-PEG in the CVM, suggesting insufficient PEG coating on the surface of the CK30PEG DNA-nanoparticles is responsible for the DNA-nanoparticles trapped in the CF sputum (Wang et al., 2008). However, to effectively minimize nanoparticles binding with mucin, a dense brush conformation is required ([Γ]/[Γ*]>2), which reflects how densely packed and constrained the PEG molecules are on the nanoparticle surface. Where, [Γ] is the number of PEG chains (molecules) on the nanoparticle surface per 100 nm2 surface area and [Γ*] represents the number of unconstrained (mushroom conformation) PEG molecules that occupy a surface area of 100 nm2 on the nanoparticle surface (Shi et al., 2021; Xu et al., 2015).
Figure 6.
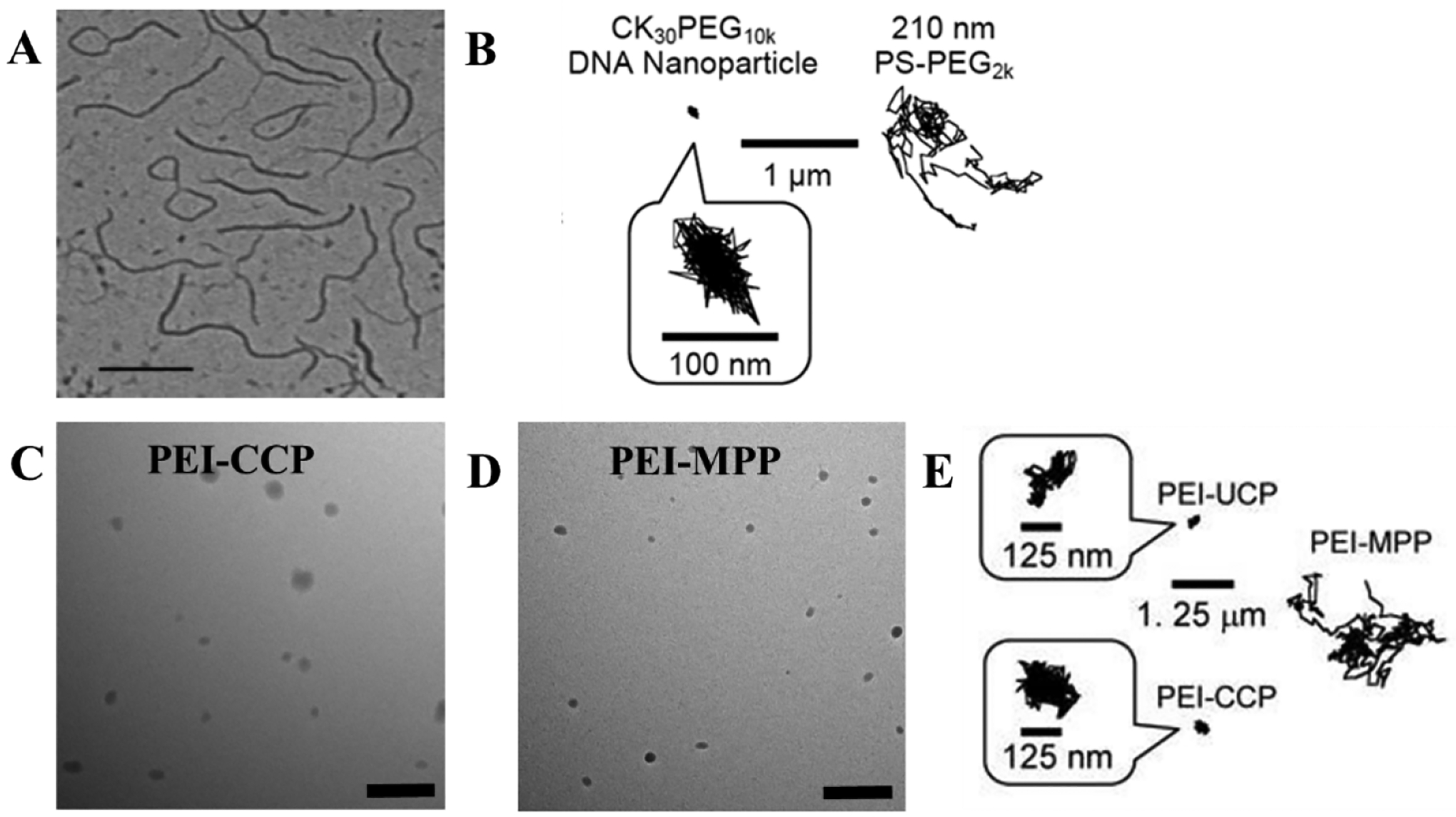
Diffusion of gene carriers in CF sputum. (A) TEM image of CK30PEG10k DNA-nanoparticles with hydrodynamic diameter of 60 nm which presented a rod-shape structure. (B) Representative trajectories of CK30PEG10k DNA-nanoparticles which are immobilized in CF sputum, and muco-inert PS-PEG2K nanoparticles with larger particle size. (C) TEM image of conventionally coated PEI/DNA NP (PEI-CCP) which had a loose spherical shape. (D) TEM image of PEI-MPP presented a more compacted spherical structure. (E) Representative trajectories of PEI-CCP nanoparticles, un-PEGylated PEI-nanoparticles (PEI-UCP) and PEI-MPP. PEI-CCP and PEI-UCP nanoparticles were restrained in CF sputum, while PEI-MPP can penetrate through CF sputum. Reproduced with permission from (Boylan et al., 2012) and (Suk et al., 2014).
A dense PEG surface coating is important for minimizing the muco-adhesion of gene carriers (Craparo et al., 2020). Nonetheless, a high degree of PEG-grafting on the cationic polymer impedes the DNA compaction and leads to larger particle sizes, due to the fewer positive charges available for DNA compaction and the higher degree of steric interferences from PEG. Loss of DNA compaction and large nanoparticle sizes can further compromise the surface PEG density causing poor sputum penetration. To overcome this dilemma, Suk et al. developed highly compacted small DNA-nanoparticles with a dense PEG-coating by using the mixed polymers of PEI and PEG5K-PEI at a 1:3 ratio (PEI-MPP) (Suk et al., 2014). A similar MPP formulation was also developed by using poly-l-lysine (PLL-MPP) polymers. Both PEI-MPPs and PLL-MPPs have particle sizes around 50 nm with a near neutral surface charge. Although both the conventionally coated particles (PEI-CCPs) and the PEI-MPPs have spherical-shaped structures, the PEI-MPPs have a more compact structure compared with the PEI-CCPs (Figures 6C and 6D). The PEI-MPPs can penetrate through human CF sputum, whereas the PEI-CCPs and un-PEGylated PEI-nanoparticles (PEI-UCP) with similar particle sizes are immobilized (Figure 6E). PEI-MPPs had more uniform distribution and more than 70% retention in mouse airways 6 hours after intranasal administration, in contrast with a restrained distribution and faster clearance of conventional PEGylated nanoparticles with 20% remaining at 2 hours after administration.
Unlike the conventional PEG conjugation method, Kim et al. designed PEG-dendrons through polyamidoamine (PAMAM) dendrimers which have a high density of primary amines on their surface and a cleavable disulfide core for preparing densely coated gene carriers that can penetrate through CF sputum (A. J. Kim et al., 2013). The PEG-dendrons were first synthesized by conjugating PEG5k to the terminal amine groups on the PAAM dendrimer surface, followed by PAMAM S-S disulfide bond reduction to two single-site sulfhydryl functional PEG-dendrons (-SH). Then the cationic polymers, either PEI or the G4 PAMAM polymer was connected to PEG-dendrons(-SH) through cross-linking, forming PEG-dendron conjugated cationic polymers. The plasmid DNA vector was encapsulated into nanoparticles using the synthesized PEG-dendron conjugated cationic polymers, namely dPEG5k-PAMAM/DNA or dPEG5k-PEI/DNA. Both nanoparticles have a spherical shape with particle sizes less than 80 nm and nearly neutral surface charges. Compared with conventional PEGylated PAMAM/DNA or PEI/DNA, the PEG-dendron systems provided more compact DNA nanoparticles with smaller particle sizes. Additionally, dPEG5k-PAMAM/DNA and dPEG5k-PEI/DNA have high surface PEG densities around 33 PEG/100 nm2 and 28 PEG/100 nm2, respectively. The surface PEG density was comparable to the muco-inert nanoparticles and was 6–8 fold higher than that of CK30PEG10k, which was unable to penetrate through CF sputum. The high surface PEG density on dPEG5k-PAMA/DNA and dPEG5k-PEI/DNA contributes to their uniform distribution and rapid penetration in CF sputum. In human bronchial epithelial cells (BEAS-2B), dPEG5k-PAMA/DNA and dPEG5k-PEI/DNA showed higher luciferase activity compared to plasmid DNA control, however, gene transfection efficiencies were lower compared to uncoated counterparts, which might be due to decreased cellular uptake. PEG-dendron/DNA can stably induce the expression of CFTR genes in CF bronchial epithelial cells. This might be due to sterically shielding of particle core by hydrophilic PEG as well as reducing the particles uptake by surrounding immune cells.
Another approach to develop MPP nanoparticles is using zwitterionic polymers, such as poly(phosphobetaine), poly(sulfobetaine), and poly(carboxybetaine) (Cao and Jiang, 2012). Zwitterionic polymers are polyelectrolytes that have both positively and negatively charged groups but have an overall neutral surface charge (Zhang et al., 2019). Poly(zwitterionic) molecules display strong binding with water molecules through electrostatic interactions, forming a super hydrophilic surface that contributes to their non-fouling characteristic against protein and biofilms (Jiang and Cao, 2010; Zhang et al., 2019). Studies have shown that zwitterionic poly(carboxybetaine) (PCB) and poly(sulfobetaine) (PSB) coated nanoparticles can resist nonspecific protein absorption in plasma (Li et al., 2015; van Andel et al., 2017). Hu et al. evaluated the ability of PLGA-based nanoparticles with different surface modifications such as PEG, polyvinyl alcohol (PVA), F127, and zwitterionic polydopamine (PDA) to penetrate mucus (Hu et al., 2022). Bare PLGA-NPs were first prepared using the nanoprecipitation method, followed by dropwise addition of 20 mL of 1% PVA, PEG, or F127 solution. For PDA nanoparticles, PLGA nanoparticles were resuspended in dopamine solution modified with Tris buffer (pH 10) and allowed to react for 4 h under magnetic stirring. All the nanoparticles had particle sizes ranging from 190 to 270 nm, whereas surface zeta charges for PLGA/F127, PLGA/PDA, and PLGA nanoparticles were −26.9 ± 0.9, −26.3 ± 2.8, and −13.0 ± 2.7 mV, respectively, except for PLGA-PEG nanoparticles which had near-neutral surface charges (−6.2 ± 1.7 mV). Particle tracking studies revealed that PDA-coated PLGA-nanoparticles can penetrate through porcine mucin, showing similar trajectories to muco-inert particles including PLGA/F127 and PLGA-PEG nanoparticles, whereas PLGA nanoparticles were immobilized. However, the particle tracking was conducted using commercialized porcine mucus mixed with agarose gel and thus the study cannot mimic airway mucus in chronic pulmonary diseases that generate compacted mucus (Bustamante-Marin and Ostrowski, 2017; Morrison et al., 2019).
Although, MPPs penetrate mucus more than MAPs and the residence time is less affected by MCC, MPPs may have back-diffusion due to the particle’s concentration gradient (Netsomboon and Bernkop-Schnürch, 2016). Another issue with MPPs may be the limited endocytosis by the epithelial cells due to neutral charges and hydrophilic surfaces. Therefore, design of nanoparticles should be such that they penetrate the mucus layer and then get endocytosed by epithelial cells. This can be achieved by varying the PEG coating percentage on nanoparticle surface along with coating of polymers such as poly(2-ethyl-2-oxazoline) (POZ), polydopamine (PDA), or N-(2-hydroxypropyl) methacrylamide copolymer (PHPMA) (Liu et al., 2016; Mansfield et al., 2015; Poinard et al., 2019). Fasquelle et al. designed lipidated maltodextrin nanoparticles by incorporating anionic phospholipid core (NPL) in the nanoparticles and studied the interaction of cationic maltodextrin based nanoparticles, PEG-coated MPPs and chitosan-coated MAPs to the airway mucins and epithelial cells (Fasquelle et al., 2020). There was increased mobility of NPLs in airway mucin as well as unhindered uptake and delivery of proteins into the airway epithelial cells. In another study, the diffusion behavior of PDA-coated polystyrene nanoparticles and PEGylated polystyrene nanoparticles through reconstituted mucin solution was similar (Poinard et al., 2019). However, the cellular uptake of PDA-coated nanoparticles in T24 mucosal cells was three folds higher compared to that of PEGylated nanoparticles, suggesting enhanced muco-penetration by minimizing interaction with the negatively charged mucins and increased cellular uptake by interaction with positively charged choline groups on the lipid membrane.
PCB and its derivatives can also delay biofilm formation and inhibit biofilm adhesion (Cheng et al., 2009, 2008). Cheng et al. constructed zwitterionic poly (sulfobetaine methacrylate) (pSBMA) coated surfaces through atom transfer radical polymerization and evaluated the adhesion of S. epidermidis and P. aeruginosa using a laminar flow chamber (Cheng et al., 2007). Compared with bare glass and the methyl-terminated (CH3) coated glass surfaces which provided strong biofilm adhesion, pSBMA reduced the short-term (3h) S. epidermidis and P. aeruginosa adhesion by 92% and 96%, respectively. In long-term biofilm resistant studies, pSBMA coating can inhibit biofilm attachment up to 48 h for S. epidermidis and 24 h for P. aeruginosa. Zwitterionic polymers mainly conjugate to proteins or polymers, forming polymer-protein complexes or copolymers that can be formulated into muco-inert nanoparticles or liposomes (Tsao et al., 2020). Currently, zwitterionic polymers are mainly applied for oral drug delivery. Considering that they can resist non-specific protein binding and biofilm formation, the zwitterionic polymers may also have a broad application in developing muco-inert nanoparticles for pulmonary delivery.
7. Aerosolized muco-adhesive and muco-penetrating particles for pulmonary delivery
Inhalable systems employing a carrier excipient offer numerous advantages, for example, reduced particle size, increased surface area, and altered drug release in the lung tissues (Chenthamara et al., 2019; Maurya et al., 2020; Pontes and Grenha, 2020; Youngren-Ortiz et al., 2017). In particular, biodegradable MAPs and MIPs can be delivered into the lungs via nebulization of colloidal dispersions or pressurized metered dose inhalation (pMDI) or dry powder inhalation (DPI) (Kumar et al., 2022; Mangal et al., 2017; Wiedmann et al., 1997; Yang et al., 2008). Polymer-based carriers, liposomes and lipid-based carriers, emulsions, and dendrimers have been used as carrier systems; however, colloidal dispersion formulations have limitations such as strong interparticle interactions within a nanosystem, agglomeration and settling of particles, and chemical instability leading to carrier hydrolysis and drug degradation (Ahmad et al., 2005; Bivas-Benita et al., 2004; Cryan et al., 2006; Joshi and Misra, 2001; Plumley et al., 2009). To address these issues, dry powder-based carriers containing nanoparticles or microparticles with better particle size, surface morphology, aerodynamic diameter, and interparticulate interactions were designed (Xu et al., 2011). DPI are an attractive pulmonary delivery platform for the treatment of lung diseases (Son et al., 2013). However, commercial DPIs often deliver particles in the size range of 2 – 5 μm which produce extra-thoracic losses of 40~70% of the dose, in part due to incomplete particle deaggregation during inhalation (Hindle and Longest, 2012). The excipient enhanced growth (EEG) technology has been used to deliver particles with an initial size of <2 μm to reduce mouth-throat loss. The particles increase in size to 2–5 μm by hygroscopic growth after inhalation to achieve lung deposition and more uniform delivery throughout the airways (Boc et al., 2021; Hassan et al., 2020). Nevertheless, sputum and mucus are still critical barriers that limit effective drug penetration into the lung.
Muco-adhesive powder microspheres loaded with a poorly soluble model drug were developed using hydroxypropylcellulose polymers (HPC) (Sakagami et al., 2001). The crystalline HPC microspheres showed sustained absorption, whereas amorphous HPC microspheres demonstrated rapid absorption. Moreover, microspheres prepared using HPC-H (high viscosity) and a drug-HPC ratio of greater than 1:4 successfully enhanced absorption and reduced MCC compared to microspheres containing HPC-SL (the least viscous) and the lowest drug-HPC ratio of 1:1. Dong et al. compared muco-adhesive and muco-penetrating characteristics of baicalein-phospholipid complex loaded polymeric nanoparticles that were surface coated with either chitosan (CS-BA nanoparticles) and Pluronic F127 (F127-BA nanoparticles) (Dong et al., 2020). Due to the cationic amino group of chitosan, CS-BA nanoparticles had muco-adhesive properties, whereas the Pluronic F127 coating enhanced muco-penetration in the F127-BA nanoparticles, thereby generating a higher diffusion rate across porcine airway mucus ex-vivo. Findings from this study indicated that the F127-BA nanoparticles-based formulation could deliver baicalein with a higher diffusion rate to allow deeper penetration in healthy airway mucus in mouse model compared to the muco-adhesive formulation.
Our group conducted a proof-of-concept study using densely PEG coated muco-inert nanoparticles and uncoated polystyrene nanoparticles (PS-PEG nanoparticles and PS nanoparticles) with a particle size of 100 nm, which were further formulated as a powder using an EEG strategy (Chai et al., 2020). EEG is a recently introduced strategy that uses the powder formulation to produce aerosol size growth and minimizes extra thoracic losses by delivering micrometer-sized particle formulation (Walenga et al., 2017). PS-nanoparticles, both colloidal suspension or powdered EEG aerosols were highly hindered in sputum from CF patients, whereas PEG-coated nanoparticles had greater diffusion as shown in Figure 7A. Representative single particle trajectory showed greater movement for PEG-coated nanoparticles in the CF sputum compared with PS nanoparticles (Figure 7B). Moreover, the diffusion rate distribution study showed similar movement for the PS-PEG nanoparticle colloidal suspension and muco-inert nanoparticles loaded into the EEG dry powder aerosol (Figure 7C). Although, the diffusion of the aerosol was slightly slower compared to the suspension, this study showed that the muco-inert nanoparticles are released from the aerosol and maintain rapid mucus diffusion, which are still 22-fold faster than uncoated PS nanoparticles loaded EEG powder aerosols. Besides, hygroscopic growth of the EEG aerosols in the lungs will create a suspension before deposition and supply additional water, which could enhance mucus diffusion. In addition, densely PEG-coated nanoparticles of particle size smaller than mucus or sputum meshwork diffused efficiently. Overall, muco-adhesive and muco-penetrating aerosols can be successfully designed to achieve reduced drug loading for inhalation, superior aerosol performance, and deep lung deposition.
Figure 7.
Diffusion experiment using MIP suspension or MIP-containing EEG dry powder in CF sputum. (A) Ensemble-averaged geometric mean square displacements (<MSD>) as a function of time scale for PS suspension, PS-PEG suspension, PS aerosol, and PS-PEG aerosol in the CF mucus. (B) Representative particles trajectories of PS suspension, PS-PEG suspension, PS aerosol, and PS-PEG aerosol in the CF mucus in CF mucus. Scale bar, 1 μm. (C) The diffusion rate distribution of PS suspension, PS-PEG suspension, PS aerosol, and PS-PEG aerosol in the CF mucus. Reproduced with permission from (Chai et al., 2020).
8. Conclusions and perspectives
Pulmonary drug delivery is made more challenging, in part due to airway barrier functions that either trap and remove inhaled drugs or inactivate them when they are deposited. Over the last decades, the role of airway mucus in health and disease has been extensively investigated and it has been found that airway mucus hypersecretion, decreased mucus clearance, upregulation of mucin production (particularly MUC5AC and MUC5B), pulmonary infection and inflammation, and the formation of NETs are critical factors in airway obstruction and lung function decline. In addition, an increase in the disulfide bond density and the total solid contents of mucus leads to the formation of a tighter mesh network in subjects with respiratory disease, which directly contributes to the alteration in microstructural properties of mucus such as mesh size and viscosity. SEM and magnetic microrheometry measurements have their unique advantages in studying the rheology of airway mucus. More recently, MPT has been widely accepted for investigating the interaction of nanoparticles and their mucus environment along with quantitative determination of the effective diffusivities, maximum velocity, and time-resolved particle trajectories. Substantial progress has been made in terms of formulations and delivery devices to improve lung deposition of aerosol drugs. Therefore, it is important to understand the fate of aerosol drugs or carrier systems after deposition in the lungs as well as the potential barriers to pulmonary delivery.
In this review, we discussed some approaches for engineering nanoparticles that can overcome the heterogenous airway mucus barrier. These nanoparticles can be modified for particle size, surface morphology, surface charge, and targeting moieties as each of these characteristics are critical for distinguishing muco-adhesive or muco-penetrating properties of nanoparticles. MAPs coated with chitosan, polyacrylic acid, hyaluronic acid, and surfactant phospholipids provide higher permeability and better pharmacokinetic profiles compared to non-coated nanoparticles and extend the drug residence time in the lungs. However, the clinical efficacy in the case of chronic pulmonary diseases with mucus over production is still poor. Unlike MAPs that adhere to mucus polymers, nanoparticles densely coated with muco-inert chemicals with particle size below the mesh network size can easily diffuse through the mucus and achieve a higher absorption of drug into the lungs, which is an effective alternative to conventional therapy. This has encouraged researchers to explore biodegradable polymers to design MAPs and MPPs and the use nanotechnology to engineer nanoparticles with higher efficacy to treat pulmonary disease.
Acknowledgements
This work was supported by the National Institutes of Health (R01HD087339), and the George and Lavinia Blick Research Fund.
Abbreviations
- AMP
Antimicrobial peptide
- ASL
airway surface liquid
- BALF
bronchoalveolar lavage fluid
- CF
cystic fibrosis
- CFTR
cystic fibrosis transmembrane conductance regulator
- COPD
chronic obstructive pulmonary disease
- CVM
cervicovaginal mucus
- DPI
dry powder inhaler
- EEG
excipient enhanced growth
- EGFR
epidermal growth factor receptor
- HBE
human bronchial epithelial
- IHC
immunohistochemistry
- MAP
muco-adhesive particles
- MCC
mucociliary clearance
- MIP
muco-inert particles
- MPP
mucus penetrating particles
- MPT
multiple particle tracking
- MSD
mean squared displacement
- NAC
N-acetyl L-cysteine
- PAMAM
polyamidoamine
- PCB
poly(carboxybetaine)
- PCL
periciliary layer
- PDA
polydopamine
- PEG
polyethylene glycol
- PEI
polyethylenimine
- PLGA
poly(lactic-co-glycolic acid)
- PS
polystyrene
- PSB
poly(sulfobetaine)
- PSBMA
poly (sulfobetaine methacrylate)
- PTM
particle tracking microrheology
- PVA
poly vinyl alcohol
- rhDNase
recombinant human deoxyribonuclease
- RSV
respiratory syncytial virus
- SEM
scanning electron microscopy
- TCEP
tris(2-carboxyethyl) phosphine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest
The EEG aerosol technologies described in this publication have been filed as patent applications through Virginia Commonwealth University (VCU). Michael Hindle and Worth Longest are the co-inventors, which are subject to certain rules and restrictions under VCU policy. The terms of this arrangement are being managed by VCU in accordance with its conflict of interest policies.
CRediT authorship contribution statement
Rudra Pangeni: Methodology, Writing – Original draft. Tuo Meng: Methodology, Writing – Original draft. Sagun Poudel: Writing- review and editing. Divya Sharma: Writing- review and editing. Hallie Hutsell: Writing- review and editing. Jonathan Ma: Writing- review and editing. Bruce Rubin: Supervision, Writing- review and editing. Worth Longest: Supervision, Writing- review and editing. Michael Hindle: Supervision, Writing- review and editing. Qingguo Xu: Supervision, Writing- review and editing.
References
- Aaron SD, 2017. Mucolytics for COPD: negotiating a slippery slope towards proof of efficacy. Eur. Respir. J 50, 1701465. [DOI] [PubMed] [Google Scholar]
- Abrami M, Maschio M, Conese M, Confalonieri M, Gerin F, Dapas B, Farra R, Adrover A, Torelli L, Ruaro B, Grassi G, Grassi M, 2021. Combined use of rheology and portable low-field NMR in cystic fibrosis patients. Respir. Med 189, 106623. [DOI] [PubMed] [Google Scholar]
- Abrami M, Maschio M, Conese M, Confalonieri M, Salton F, Gerin F, Dapas B, Farra R, Adrover A, Milcovich G, Fornasier C, Biasin A, Grassi M, Grassi G, 2022. Effect of chest physiotherapy on cystic fibrosis sputum nanostructure: an experimental and theoretical approach. Drug Deliv. Transl. Res 12, 1943–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad Z, Sharma S, Khuller GK, 2005. Inhalable alginate nanoparticles as antitubercular drug carriers against experimental tuberculosis. Int. J. Antimicrob. Agents 26, 298–303. [DOI] [PubMed] [Google Scholar]
- Ali MS, Pearson JP, 2007. Upper airway mucin gene expression: a review. Laryngoscope 117, 932–938. [DOI] [PubMed] [Google Scholar]
- Alinovi M, Mucchetti G, Andersen U, Rovers TAM, Mikkelsen B, Wiking L, Corredig M, 2020. Applicability of confocal Raman microscopy to observe microstructural modifications of cream cheeses as influenced by freezing. Foods (Basel, Switzerland) 9, 679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alp G, Aydogan N, 2020. Lipid-based mucus penetrating nanoparticles and their biophysical interactions with pulmonary mucus layer. Eur. J. Pharm. Biopharm 149, 45–57. [DOI] [PubMed] [Google Scholar]
- Anderson WH, Coakley RD, Button B, Henderson AG, Zeman KL, Alexis NE, Peden DB, Lazarowski ER, Davis CW, Bailey S, Fuller F, Almond M, Qaqish B, Bordonali E, Rubinstein M, Bennett WD, Kesimer M, Boucher RC, 2015. The relationship of mucus concentration (hydration) to mucus osmotic pressure and transport in chronic bronchitis. Am. J. Respir. Crit. Care Med 192, 182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- App EM, Zayas JG, King M, 1993. Rheology of mucus and transepithelial potential difference: small airways versus trachea. Eur. Respir. J 6, 67–75. [PubMed] [Google Scholar]
- Atanasova KR, Reznikov LR, 2019. Strategies for measuring airway mucus and mucins. Respir. Res 20, 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azarmi S, Roa WH, Löbenberg R, 2008. Targeted delivery of nanoparticles for the treatment of lung diseases. Adv. Drug Deliv. Rev 60, 863–875. [DOI] [PubMed] [Google Scholar]
- Bals R, Hiemstra PS, 2004. Innate immunity in the lung: how epithelial cells fight against respiratory pathogens. Eur. Respir. J 23, 327–333. [DOI] [PubMed] [Google Scholar]
- Bansil R, Turner BS, 2018. The biology of mucus: Composition, synthesis and organization. Adv. Drug Deliv. Rev 124, 3–15. [DOI] [PubMed] [Google Scholar]
- Birchenough GMH, Johansson MEV, Gustafsson JK, Bergström JH, Hansson GC, 2015. New developments in goblet cell mucus secretion and function. Mucosal Immunol 8, 712–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bivas-Benita M, Oudshoorn M, Romeijn S, van Meijgaarden K, Koerten H, van der Meulen H, Lambert G, Ottenhoff T, Benita S, Junginger H, Borchard G, 2004. Cationic submicron emulsions for pulmonary DNA immunization. J. Control. release Off. J. Control. Release Soc 100, 145–155. [DOI] [PubMed] [Google Scholar]
- Block H, Zarbock A, 2021. A fragile balance: Does neutrophil extracellular trap formation drive pulmonary disease progression? Cells 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boc S, Momin MAM, Farkas DR, Longest W, Hindle M, 2021. Development and characterization of excipient enhanced growth (EEG) surfactant powder formulations for treating neonatal respiratory distress syndrome. AAPS PharmSciTech 22, 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonser LR, Erle DJ, 2017. Airway mucus and asthma: The role of MUC5AC and MUC5B. J. Clin. Med 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonser LR, Zlock L, Finkbeiner W, Erle DJ, 2016. Epithelial tethering of MUC5AC-rich mucus impairs mucociliary transport in asthma. J. Clin. Invest 126, 2367–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher RC, 2007. Airway surface dehydration in cystic fibrosis: pathogenesis and therapy. Annu. Rev. Med 58, 157–170. [DOI] [PubMed] [Google Scholar]
- Boylan NJ, Suk JS, Lai SK, Jelinek R, Boyle MP, Cooper MJ, Hanes J, 2012. Highly compacted DNA nanoparticles with low MW PEG coatings: in vitro, ex vivo and in vivo evaluation. J. Control. release Off. J. Control. Release Soc 157, 72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A, 2004. Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535. [DOI] [PubMed] [Google Scholar]
- Bustamante-Marin XM, Ostrowski LE, 2017. Cilia and mucociliary clearance. Cold Spring Harb. Perspect. Biol 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button B, Anderson WH, Boucher RC, 2016. Mucus hyperconcentration as a unifying aspect of the chronic bronchitic phenotype. Ann. Am. Thorac. Soc 13, S156–S162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button B, Cai L-H, Ehre C, Kesimer M, Hill DB, Sheehan JK, Boucher RC, Rubinstein M, 2012. A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science 337, 937–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Jiang S, 2012. Super-hydrophilic zwitterionic poly(carboxybetaine) and amphiphilic non-ionic poly(ethylene glycol) for stealth nanoparticles. Nano Today 7, 404–413. [Google Scholar]
- Chai G, Hassan A, Meng T, Lou L, Ma J, Simmers R, Zhou L, Rubin BK, Zhou Q. (Tony), Longest PW, Hindle M, Xu Q, 2020. Dry powder aerosol containing muco-inert particles for excipient enhanced growth pulmonary drug delivery. Nanomedicine Nanotechnology, Biol. Med 29, 102262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Liu J, Wu J, Suk JS, 2021. Enhancing nanoparticle penetration through airway mucus to improve drug delivery efficacy in the lung. Expert Opin. Drug Deliv 18, 595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen EYT, Wang Y-C, Chen C-S, Chin W-C, 2010a. Functionalized positive nanoparticles reduce mucin swelling and dispersion. PLoS One 5, e15434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen EYT, Yang N, Quinton PM, Chin W-C, 2010b. A new role for bicarbonate in mucus formation. Am. J. Physiol. Lung Cell. Mol. Physiol 299, L542–L549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Li Y, Qin L, He R, Hu C, 2021. Neutrophil extracellular trapping network promotes the pathogenesis of neutrophil-associated asthma through macrophages. Immunol. Invest 50, 544–561. [DOI] [PubMed] [Google Scholar]
- Chen Z, Zhong M, Luo Y, Deng L, Hu Z, Song Y, 2019. Determination of rheology and surface tension of airway surface liquid: a review of clinical relevance and measurement techniques. Respir. Res 20, 274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G, Li G, Xue H, Chen S, Bryers JD, Jiang S, 2009. Zwitterionic carboxybetaine polymer surfaces and their resistance to long-term biofilm formation. Biomaterials 30, 5234–5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G, Xue H, Zhang Z, Chen S, Jiang S, 2008. A switchable biocompatible polymer surface with self-sterilizing and nonfouling capabilities. Angew. Chem. Int. Ed. Engl 47, 8831–8834. [DOI] [PubMed] [Google Scholar]
- Cheng G, Zhang Z, Chen S, Bryers JD, Jiang S, 2007. Inhibition of bacterial adhesion and biofilm formation on zwitterionic surfaces. Biomaterials 28, 4192–4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng OZ, Palaniyar N, 2013. NET balancing: a problem in inflammatory lung diseases. Front. Immunol 4, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenthamara D, Subramaniam S, Ramakrishnan SG, Krishnaswamy S, Essa MM, Lin F-H, Qoronfleh MW, 2019. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res 23, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm JF, Shenoy SK, Shade JK, Kim V, Putcha N, Carson KA, Wise R, Hansel NN, Hanes JS, Suk JS, Neptune E, 2019. Nanoparticle diffusion in spontaneously expectorated sputum as a biophysical tool to probe disease severity in COPD. Eur. Respir. J 54, 1900088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clunes MT, Boucher RC, 2007. Cystic fibrosis: The mechanisms of pathogenesis of an inherited lung disorder. Drug Discov. Today. Dis. Mech 4, 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone Richard A, 2009. Barrier properties of mucus. Adv. Drug Deliv. Rev 61, 75–85. [DOI] [PubMed] [Google Scholar]
- Craparo EF, Drago SE, Mauro N, Giammona G, Cavallaro G, 2020. Design of new polyaspartamide copolymers for siRNA delivery in antiasthmatic therapy. Pharmaceutics [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craparo EF, Porsio B, Sardo C, Giammona G, Cavallaro G, 2016. Pegylated polyaspartamide–polylactide-based nanoparticles penetrating cystic fibrosis artificial mucus. Biomacromolecules 17, 767–777. [DOI] [PubMed] [Google Scholar]
- Cryan S-A, Devocelle M, Moran PJ, Hickey AJ, Kelly JG, 2006. Increased intracellular targeting to airway cells using octaarginine-coated liposomes: In vitro assessment of their suitability for inhalation. Mol. Pharm 3, 104–112. [DOI] [PubMed] [Google Scholar]
- Cui F, Qian F, Yin C, 2006. Preparation and characterization of mucoadhesive polymer-coated nanoparticles. Int. J. Pharm 316, 154–161. [DOI] [PubMed] [Google Scholar]
- Da Silva AL, De Oliveira GP, Kim N, Cruz FF, Kitoko JZ, Blanco NG, Martini SV, Justin H, Rocco PRM, Suk JS, Morales MM, 2023. Nanoparticle-based thymulin gene therapy therapeutically reverses key pathology of experimental allergic asthma. Sci. Adv 6, eaay7973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daviskas E, Rubin BK, 2013. Effect of inhaled dry powder mannitol on mucus and its clearance. Expert Rev. Respir. Med 7, 65–75. [DOI] [PubMed] [Google Scholar]
- Dawson M, Krauland E, Wirtz D, Hanes J, 2004. Transport of polymeric nanoparticle gene carriers in gastric mucus. Biotechnol. Prog 20, 851–857. [DOI] [PubMed] [Google Scholar]
- Dawson M, Wirtz D, Hanes J, 2003. Enhanced viscoelasticity of human cystic fibrotic sputum correlates with increasing microheterogeneity in particle transport. J. Biol. Chem 278, 50393–50401. [DOI] [PubMed] [Google Scholar]
- Dicker AJ, Crichton ML, Pumphrey EG, Cassidy AJ, Suarez-Cuartin G, Sibila O, Furrie E, Fong CJ, Ibrahim W, Brady G, Einarsson GG, Elborn JS, Schembri S, Marshall SE, Palmer CNA, Chalmers JD, 2018. Neutrophil extracellular traps are associated with disease severity and microbiota diversity in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol 141, 117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong W, Ye J, Zhou J, Wang W, Wang H, Zheng X, Yang Y, Xia X, Liu Y, 2020. Comparative study of mucoadhesive and mucus-penetrative nanoparticles based on phospholipid complex to overcome the mucus barrier for inhaled delivery of baicalein. Acta Pharm. Sin. B 10, 1576–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douda DN, Grasemann H, Pace-Asciak C, Palaniyar N, 2015. A lipid mediator hepoxilin A3 is a natural inducer of neutrophil extracellular traps in human neutrophils. Mediators Inflamm 2015, 520871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulfano MJ, Adler K, Philippoff W, 1971. Sputum viscoelasticity in chronic bronchitis. Am. Rev. Respir. Dis 104, 88–98. [DOI] [PubMed] [Google Scholar]
- Dulfano MJ, Adler KB, 1975. Physical properties of sputum. Am. Rev. Respir. Dis 112, 341–347. [DOI] [PubMed] [Google Scholar]
- Duncan GA, Jung J, Hanes J, Suk JS, 2016a. The mucus barrier to inhaled gene therapy. Mol. Ther 24, 2043–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan GA, Jung J, Joseph A, Thaxton AL, West NE, Boyle MP, Hanes J, Suk JS, 2016b. Microstructural alterations of sputum in cystic fibrosis lung disease. JCI insight 1, e88198–e88198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer M, Shan Q, D’Ortona S, Maurer R, Mitchell R, Olesen H, Thiel S, Huebner J, Gadjeva M, 2014. Cystic fibrosis sputum DNA has NETosis characteristics and neutrophil extracellular trap release is regulated by macrophage migration-inhibitory factor. J. Innate Immun 6, 765–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sherbiny IM, Smyth HDC, 2012. Controlled release pulmonary administration of curcumin using swellable biocompatible microparticles. Mol. Pharm 9, 269–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensign LM, Schneider C, Suk JS, Cone R, Hanes J, 2012. Mucus penetrating nanoparticles: Biophysical tool and method of drug and gene delivery. Adv. Mater 24, 3887–3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esther CR Jr, Muhlebach MS, Ehre C, Hill DB, Wolfgang MC, Kesimer M, Ramsey KA, Markovetz MR, Garbarine IC, Forest MG, Seim I, Zorn B, Morrison CB, Delion MF, Thelin WR, Villalon D, Sabater JR, Turkovic L, Ranganathan S, Stick SM, Boucher RC, 2019. Mucus accumulation in the lungs precedes structural changes and infection in children with cystic fibrosis. Sci. Transl. Med 11, eaav3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CM, Raclawska DS, Ttofali F, Liptzin DR, Fletcher AA, Harper DN, McGing MA, McElwee MM, Williams OW, Sanchez E, Roy MG, Kindrachuk KN, Wynn TA, Eltzschig HK, Blackburn MR, Tuvim MJ, Janssen WJ, Schwartz DA, Dickey BF, 2015. The polymeric mucin Muc5ac is required for allergic airway hyperreactivity. Nat. Commun 6, 6281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahy JV, Dickey BF, 2010. Airway mucus function and dysfunction. N. Engl. J. Med 363, 2233–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasquelle F, Carpentier R, Demouveaux B, Desseyn J-L, Betbeder D, 2020. Importance of the phospholipid core for mucin hydrogel penetration and mucosal cell uptake of maltodextrin nanoparticles. ACS Appl. Bio Mater 3, 5741–5749. [DOI] [PubMed] [Google Scholar]
- Forier K, Messiaen A-S, Raemdonck K, Deschout H, Rejman J, De Baets F, Nelis H, De Smedt SC, Demeester J, Coenye T, Braeckmans K, 2013. Transport of nanoparticles in cystic fibrosis sputum and bacterial biofilms by single-particle tracking microscopy. Nanomedicine (Lond) 8, 935–949. [DOI] [PubMed] [Google Scholar]
- Fröhlich E, Salar-Behzadi S, 2021. Oral inhalation for delivery of proteins and peptides to the lungs. Eur. J. Pharm. Biopharm 163, 198–211. [DOI] [PubMed] [Google Scholar]
- Ganesan S, Comstock AT, Sajjan US, 2013. Barrier function of airway tract epithelium. Tissue barriers 1, e24997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabcanovic-Musija F, Obermayer A, Stoiber W, Krautgartner W-D, Steinbacher P, Winterberg N, Bathke AC, Klappacher M, Studnicka M, 2015. Neutrophil extracellular trap (NET) formation characterises stable and exacerbated COPD and correlates with airflow limitation. Respir. Res 16, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha EVS, Rogers DF, 2016. Novel therapies to inhibit mucus synthesis and secretion in airway hypersecretory diseases. Pharmacology 97, 84–100. [DOI] [PubMed] [Google Scholar]
- Hassan A, Farkas D, Longest W, Hindle M, 2020. Characterization of excipient enhanced growth (EEG) tobramycin dry powder aerosol formulations. Int. J. Pharm 591, 120027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill DB, Vasquez PA, Mellnik J, McKinley SA, Vose A, Mu F, Henderson AG, Donaldson SH, Alexis NE, Boucher RC, Forest MG, 2014. A biophysical basis for mucus solids concentration as a candidate biomarker for airways disease. PLoS One 9, e87681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindle M, Longest PW, 2012. Condensational growth of combination drug-excipient submicrometer particles for targeted high-efficiency pulmonary delivery: evaluation of formulation and delivery device. J. Pharm. Pharmacol 64, 1254–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtmeyers E, Gosselink R, Gayan-Ramirez G, Decramer M, 1999. Regulation of mucociliary clearance in health and disease. Eur. Respir. J 13, 1177–1188. [DOI] [PubMed] [Google Scholar]
- Hu S, Yang Z, Wang S, Wang L, He Q, Tang H, Ji P, Chen T, 2022. Zwitterionic polydopamine modified nanoparticles as an efficient nanoplatform to overcome both the mucus and epithelial barriers. Chem. Eng. J 428, 132107. [Google Scholar]
- Huck BC, Murgia X, Frisch S, Hittinger M, Hidalgo A, Loretz B, Lehr C-M, 2022. Models using native tracheobronchial mucus in the context of pulmonary drug delivery research: Composition, structure and barrier properties. Adv. Drug Deliv. Rev 183, 114141. [DOI] [PubMed] [Google Scholar]
- Huckaby JT, Lai SK, 2018. PEGylation for enhancing nanoparticle diffusion in mucus. Adv. Drug Deliv. Rev 124, 125–139. [DOI] [PubMed] [Google Scholar]
- Hurley BP, Siccardi D, Mrsny RJ, McCormick BA, 2004. Polymorphonuclear cell transmigration induced by Pseudomonas aeruginosa requires the eicosanoid hepoxilin A3. J. Immunol 173, 5712–5720. [DOI] [PubMed] [Google Scholar]
- Innes AL, Carrington SD, Thornton DJ, Kirkham S, Rousseau K, Dougherty RH, Raymond WW, Caughey GH, Muller SJ, Fahy JV, 2009. Ex vivo sputum analysis reveals impairment of protease-dependent mucus degradation by plasma proteins in acute asthma. Am. J. Respir. Crit. Care Med 180, 203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji W, Peiyu L, Yang Y, Yuhong F, Hongye J, Min L, Zhiping S, Shibo J, Lu L, X. WM, 2020. Pulmonary surfactant–biomimetic nanoparticles potentiate heterosubtypic influenza immunity. Science (80-.) 367, eaau0810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S, Cao Z, 2010. Ultralow-fouling, functionalizable, and hydrolyzable zwitterionic materials and their derivatives for biological applications. Adv. Mater 22, 920–932. [DOI] [PubMed] [Google Scholar]
- Joshi M, Misra AN, 2001. Pulmonary disposition of budesonide from liposomal dry powder inhaler. Methods Find. Exp. Clin. Pharmacol 23, 531–536. [DOI] [PubMed] [Google Scholar]
- Kan S, Hariyadi DM, Grainge C, Knight DA, Bartlett NW, Liang M, 2020. Airway epithelial-targeted nanoparticles for asthma therapy. Am. J. Physiol. Lung Cell. Mol. Physiol 318, L500–L509. [DOI] [PubMed] [Google Scholar]
- Kater A, Henke MO, Rubin BK, 2007. The role of DNA and actin polymers on the polymer structure and rheology of cystic fibrosis sputum and depolymerization by gelsolin or thymosin beta 4. Ann. N. Y. Acad. Sci 1112, 140–153. [DOI] [PubMed] [Google Scholar]
- Keir HR, Chalmers JD, 2022. Neutrophil extracellular traps in chronic lung disease: implications for pathogenesis and therapy. Eur. Respir. Rev 31, 210241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesimer M, Ford AA, Ceppe A, Radicioni G, Cao R, Davis CW, Doerschuk CM, Alexis NE, Anderson WH, Henderson AG, Barr RG, Bleecker ER, Christenson SA, Cooper CB, Han MK, Hansel NN, Hastie AT, Hoffman EA, Kanner RE, Martinez F, Paine R 3rd, Woodruff PG, O’Neal WK, Boucher RC, 2017. Airway mucin concentration as a marker of chronic bronchitis. N. Engl. J. Med 377, 911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MA, Ali ZS, Sweezey N, Grasemann H, Palaniyar N, 2019. Progression of cystic fibrosis lung disease from childhood to adulthood: Neutrophils, neutrophil extracellular trap (NET) formation, and NET degradation. Genes (Basel) 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim AJ, Boylan NJ, Suk JS, Hwangbo M, Yu T, Schuster BS, Cebotaru L, Lesniak WG, Oh JS, Adstamongkonkul P, Choi AY, Kannan RM, Hanes J, 2013. Use of single-site-functionalized PEG dendrons to prepare gene vectors that penetrate human mucus barriers. Angew. Chem. Int. Ed. Engl 52, 3985–3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Park HT, Tae YM, Kong WH, Sung DK, Hwang BW, Kim KS, Kim YK, Hahn SK, 2013. Bioimaging and pulmonary applications of self-assembled Flt1 peptide-hyaluronic acid conjugate nanoparticles. Biomaterials 34, 8478–8490. [DOI] [PubMed] [Google Scholar]
- Kirkham S, Sheehan JK, Knight D, Richardson PS, Thornton DJ, 2002. Heterogeneity of airways mucus: variations in the amounts and glycoforms of the major oligomeric mucins MUC5AC and MUC5B. Biochem. J 361, 537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles MR, Boucher RC, 2002. Mucus clearance as a primary innate defense mechanism for mammalian airways. J. Clin. Invest 109, 571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupa L, Bajka B, Staroń R, Dupont D, Singh H, Gutkowski K, Macierzanka A, 2020. Comparing the permeability of human and porcine small intestinal mucus for particle transport studies. Sci. Rep 10, 20290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Mehta P, Shankar KR, Rajora MAK, Mishra YK, Mostafavi E, Kaushik A, 2022. Nanotechnology-assisted metered-dose inhalers (MDIs) for high-performance pulmonary drug delivery applications. Pharm. Res [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuyper LM, Paré PD, Hogg JC, Lambert RK, Ionescu D, Woods R, Bai TR, 2003. Characterization of airway plugging in fatal asthma. Am. J. Med 115, 6–11. [DOI] [PubMed] [Google Scholar]
- Labiris NR, Dolovich MB, 2003. Pulmonary drug delivery. Part I: physiological factors affecting therapeutic effectiveness of aerosolized medications. Br. J. Clin. Pharmacol 56, 588–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachowicz-Scroggins ME, Yuan S, Kerr SC, Dunican EM, Yu M, Carrington SD, Fahy JV, 2016. Abnormalities in MUC5AC and MUC5B protein in airway mucus in asthma. Am. J. Respir. Crit. Care Med 194, 1296–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai SK, Hanes J, 2008. Real-time multiple particle tracking of gene nanocarriers in complex biological environments BT - gene therapy protocols: Design and characterization of gene transfer vectors, in: Le Doux JM (Ed.),. Humana Press, Totowa, NJ, pp. 81–97. [DOI] [PubMed] [Google Scholar]
- Lai SK, Hanlon DE, Harrold S, Man ST, Wang Y-Y, Cone R, Hanes J, 2007. Rapid transport of large polymeric nanoparticles in fresh undiluted human mucus. Proc. Natl. Acad. Sci 104, 1482 LP – 1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai SK, Suk JS, Pace A, Wang YY, Yang M, Mert O, Chen J, Kim J, Hanes J, 2011. Drug carrier nanoparticles that penetrate human chronic rhinosinusitis mucus. Biomaterials 32, 6285–6290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai SK, Wang Y-Y, Cone R, Wirtz D, Hanes J, 2009a. Altering mucus rheology to “solidify” human mucus at the nanoscale. PLoS One 4, e4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai SK, Wang Y-Y, Hanes J, 2009b. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv. Drug Deliv. Rev 61, 158–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai SK, Wang Y-Y, Wirtz D, Hanes J, 2009c. Micro- and macrorheology of mucus. Adv. Drug Deliv. Rev 61, 86–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai SK, Ying-Ying W, Kaoru H, Richard C, Justin H, 2010. Nanoparticles reveal that human cervicovaginal mucus is riddled with pores larger than viruses. Proc. Natl. Acad. Sci 107, 598–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefrançais E, Mallavia B, Zhuo H, Calfee CS, Looney MR, 2018. Maladaptive role of neutrophil extracellular traps in pathogen-induced lung injury. JCI insight 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lele BS, Hoffman AS, 2000. Mucoadhesive drug carriers based on complexes of poly(acrylic acid) and PEGylated drugs having hydrolysable PEG-anhydride-drug linkages. J. Control. release Off. J. Control. Release Soc 69, 237–248. [DOI] [PubMed] [Google Scholar]
- Lethem MI, James SL, Marriott C, Burke JF, 1990. The origin of DNA associated with mucus glycoproteins in cystic fibrosis sputum. Eur. Respir. J 3, 19–23. [PubMed] [Google Scholar]
- Li Y, Liu R, Shi Y, Zhang Z, Zhang X, 2015. Zwitterionic poly(carboxybetaine)-based cationic liposomes for effective delivery of small interfering RNA therapeutics without accelerated blood clearance phenomenon. Theranostics 5, 583–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Tang XX, 2021. Abnormal airway mucus secretion induced by virus infection. Front. Immunol 12, 701443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieleg O, Vladescu I, Ribbeck K, 2010. Characterization of particle translocation through mucin hydrogels. Biophys. J 98, 1782–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limoli DH, Rockel AB, Host KM, Jha A, Kopp BT, Hollis T, Wozniak DJ, 2014. Cationic antimicrobial peptides promote microbial mutagenesis and pathoadaptation in chronic infections. PLoS Pathog 10, e1004083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin VY, Kaza N, Birket SE, Kim H, Edwards LJ, LaFontaine J, Liu L, Mazur M, Byzek SA, Hanes J, Tearney GJ, Raju SV, Rowe SM, 2020. Excess mucus viscosity and airway dehydration impact COPD airway clearance. Eur. Respir. J 55, 1900419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden SK, Sutton P, Karlsson NG, Korolik V, McGuckin MA, 2008. Mucins in the mucosal barrier to infection. Mucosal Immunol 1, 183–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linssen RS, Chai G, Ma J, Kummarapurugu AB, van Woensel JBM, Bem RA, Kaler L, Duncan GA, Zhou L, Rubin BK, Xu Q, 2020. Neutrophil extracellular traps increase airway mucus viscoelasticity and slow mucus particle transit. Am. J. Respir. Cell Mol. Biol 64, 69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Zhang J, Zhu X, Shan W, Li L, Zhong J, Zhang Z, Huang Y, 2016. Efficient mucus permeation and tight junction opening by dissociable “mucus-inert” agent coated trimethyl chitosan nanoparticles for oral insulin delivery. J. Control. release Off. J. Control. Release Soc 222, 67–77. [DOI] [PubMed] [Google Scholar]
- Liu T, Wang F-P, Wang G, Mao H, 2017. Role of neutrophil extracellular traps in asthma and chronic obstructive pulmonary disease. Chin. Med. J. (Engl) 130, 730–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livraghi-Butrico A, Grubb BR, Wilkinson KJ, Volmer AS, Burns KA, Evans CM, O’Neal WK, Boucher RC, 2017. Contribution of mucus concentration and secreted mucins Muc5ac and Muc5b to the pathogenesis of muco-obstructive lung disease. Mucosal Immunol 10, 395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livraghi A, Randell SH, 2007. Cystic fibrosis and other respiratory diseases of impaired mucus clearance. Toxicol. Pathol 35, 116–129. [DOI] [PubMed] [Google Scholar]
- M Ways TM, Lau WM, Khutoryanskiy VV, 2018. Chitosan and its derivatives for application in mucoadhesive drug delivery systems. Polymers (Basel) 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Rubin BK, Voynow JA, 2018. Mucins, mucus, and goblet cells. Chest 154, 169–176. [DOI] [PubMed] [Google Scholar]
- Maisel K, Reddy M, Xu Q, Chattopadhyay S, Cone R, Ensign LM, Hanes J, 2016. Nanoparticles coated with high molecular weight PEG penetrate mucus and provide uniform vaginal and colorectal distribution in vivo. Nanomedicine (Lond) 11, 1337–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makhlof A, Werle M, Tozuka Y, Takeuchi H, 2010. Nanoparticles of glycol chitosan and its thiolated derivative significantly improved the pulmonary delivery of calcitonin. Int. J. Pharm 397, 92–95. [DOI] [PubMed] [Google Scholar]
- Mangal S, Gao W, Li T, Zhou Q. (Tony), 2017. Pulmonary delivery of nanoparticle chemotherapy for the treatment of lung cancers: challenges and opportunities. Acta Pharmacol. Sin 38, 782–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield EDH, Sillence K, Hole P, Williams AC, Khutoryanskiy VV, 2015. POZylation: a new approach to enhance nanoparticle diffusion through mucosal barriers. Nanoscale 7, 13671–13679. [DOI] [PubMed] [Google Scholar]
- Marcos V, Zhou Z, Yildirim AO, Bohla A, Hector A, Vitkov L, Wiedenbauer E-M, Krautgartner WD, Stoiber W, Belohradsky BH, Rieber N, Kormann M, Koller B, Roscher A, Roos D, Griese M, Eickelberg O, Döring G, Mall MA, Hartl D, 2010. CXCR2 mediates NADPH oxidase-independent neutrophil extracellular trap formation in cystic fibrosis airway inflammation. Nat. Med 16, 1018–1023. [DOI] [PubMed] [Google Scholar]
- Martens CJ, Inglis SK, Valentine VG, Garrison J, Conner GE, Ballard ST, 2011. Mucous solids and liquid secretion by airways: studies with normal pig, cystic fibrosis human, and non-cystic fibrosis human bronchi. Am. J. Physiol. Lung Cell. Mol. Physiol 301, L236–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason TG, Ganesan K, van Zanten JH, Wirtz D, Kuo SC, 1997. Particle tracking microrheology of complex fluids. Phys. Rev. Lett 79, 3282–3285. [Google Scholar]
- Mason TG, Weitz DA, 1995. Optical measurements of frequency-dependent linear viscoelastic moduli of complex fluids. Phys. Rev. Lett 74, 1250–1253. [DOI] [PubMed] [Google Scholar]
- Matsui H, Verghese MW, Kesimer M, Schwab UE, Randell SH, Sheehan JK, Grubb BR, Boucher RC, 2005. Reduced three-dimensional motility in dehydrated airway mucus prevents neutrophil capture and killing bacteria on airway epithelial surfaces. J. Immunol 175, 1090 LP – 1099. [DOI] [PubMed] [Google Scholar]
- Matthews AA, Ee PLR, Ge R, 2020. Developing inhaled protein therapeutics for lung diseases. Mol. Biomed 1, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurya P, Singh S, Saraf SA, 2020. Inhalable hybrid nanocarriers for respiratory disorders. Target. Chronic Inflamm. Lung Dis. Using Adv. Drug Deliv. Syst [Google Scholar]
- McGuckin MA, Thornton DJ, Whitsett JA, 2015. Chapter 14 - Mucins and mucus, in: Mestecky J, Strober W, Russell MW, Kelsall BL, Cheroutre H, Lambrecht BNBT-MI (Fourth E. (Eds.),. Academic Press, Boston, pp. 231–250. [Google Scholar]
- McShane PJ, Naureckas ET, Tino G, Strek ME, 2013. Non–cystic fibrosis bronchiectasis. Am. J. Respir. Crit. Care Med 188, 647–656. [DOI] [PubMed] [Google Scholar]
- Mejías JC, Roy K, 2019. In-vitro and in-vivo characterization of a multi-stage enzyme-responsive nanoparticle-in-microgel pulmonary drug delivery system. J. Control. Release 316, 393–403. [DOI] [PubMed] [Google Scholar]
- Meldrum OW, Chotirmall SH, 2021. Mucus, microbiomes and pulmonary disease. Biomedicines 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton EA, He X-Y, Denorme F, Campbell RA, Ng D, Salvatore SP, Mostyka M, Baxter-Stoltzfus A, Borczuk AC, Loda M, Cody MJ, Manne BK, Portier I, Harris ES, Petrey AC, Beswick EJ, Caulin AF, Iovino A, Abegglen LM, Weyrich AS, Rondina MT, Egeblad M, Schiffman JD, Yost CC, 2020. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood 136, 1169–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra R, Foster D, Vasu VT, Thaikoottathil JV, Kosmider B, Chu HW, Bowler RP, Finigan JH, 2016. Cigarette smoke induces human epidermal receptor 2-dependent changes in epithelial permeability. Am. J. Respir. Cell Mol. Biol 54, 853–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad Y, Shaaban R, Al-Zahab BA, Khaltaev N, Bousquet J, Dubaybo B, 2013. Impact of active and passive smoking as risk factors for asthma and COPD in women presenting to primary care in Syria: first report by the WHO-GARD survey group. Int. J. Chron. Obstruct. Pulmon. Dis 8, 473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes TJ, Plumb J, Martin R, Vachon E, Cherepanov V, Koh A, Loeve C, Jongstra-Bilen J, Zurawska JH, Kus JV, Burrows LL, Grinstein S, Downey GP, 2006. Abnormalities in the pulmonary innate immune system in cystic fibrosis. Am. J. Respir. Cell Mol. Biol 34, 364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan LE, Jaramillo AM, Shenoy SK, Raclawska D, Emezienna NA, Richardson VL, Hara N, Harder AQ, NeeDell JC, Hennessy CE, El-Batal HM, Magin CM, Grove Villalon DE, Duncan G, Hanes JS, Suk JS, Thornton DJ, Holguin F, Janssen WJ, Thelin WR, Evans CM, 2021. Disulfide disruption reverses mucus dysfunction in allergic airway disease. Nat. Commun 12, 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison CB, Markovetz MR, Ehre C, 2019. Mucus, mucins, and cystic fibrosis. Pediatr. Pulmonol. 54 Suppl 3, S84–S96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller C, Leithner K, Hauptstein S, Hintzen F, Salvenmoser W, Bernkop-Schnürch A, 2012. Preparation and characterization of mucus-penetrating papain/poly(acrylic acid) nanoparticles for oral drug delivery applications. J. Nanoparticle Res 15, 1353. [Google Scholar]
- Murgia X, Loretz B, Hartwig O, Hittinger M, Lehr C-M, 2018. The role of mucus on drug transport and its potential to affect therapeutic outcomes. Adv. Drug Deliv. Rev 124, 82–97. [DOI] [PubMed] [Google Scholar]
- Nawroth JC, van der Does AM, Ryan (Firth) A, Kanso E, 2020. Multiscale mechanics of mucociliary clearance in the lung. Philos. Trans. R. Soc. B Biol. Sci 375, 20190160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netsomboon K, Bernkop-Schnürch A, 2016. Mucoadhesive vs. mucopenetrating particulate drug delivery. Eur. J. Pharm. Biopharm 98, 76–89. [DOI] [PubMed] [Google Scholar]
- Nordgård CT, Draget KI, 2018. Co association of mucus modulating agents and nanoparticles for mucosal drug delivery. Adv. Drug Deliv. Rev 124, 175–183. [DOI] [PubMed] [Google Scholar]
- Obermayer A, Stoiber W, Krautgartner W-D, Klappacher M, Kofler B, Steinbacher P, Vitkov L, Grabcanovic-Musija F, Studnicka M, 2014. New aspects on the structure of neutrophil extracellular traps from chronic obstructive pulmonary disease and in vitro generation. PLoS One 9, e97784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver A, Cantón R, Campo P, Baquero F, Blázquez J, 2000. High frequency of hypermutable Pseudomonas aeruginosa in cystic fibrosis lung infection. Science 288, 1251–1254. [DOI] [PubMed] [Google Scholar]
- Olmsted SS, Padgett JL, Yudin AI, Whaley KJ, Moench TR, Cone RA, 2001. Diffusion of macromolecules and virus-like particles in human cervical mucus. Biophys. J 81, 1930–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman G, Rodriguez J, Chan SY, Chisholm J, Duncan G, Kim N, Tatler AL, Shakesheff KM, Hanes J, Suk JS, Dixon JE, 2018. PEGylated enhanced cell penetrating peptide nanoparticles for lung gene therapy. J. Control. Release 285, 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasteur MC, Bilton D, Hill AT, 2010. British Thoracic Society guideline for non- CFbronchiectasis. Thorax 65, i1 LP–i58. [DOI] [PubMed] [Google Scholar]
- Patarin J, Ghiringhelli É, Darsy G, Obamba M, Bochu P, Camara B, Quétant S, Cracowski J-L, Cracowski C, Robert de Saint Vincent M, 2020. Rheological analysis of sputum from patients with chronic bronchial diseases. Sci. Rep 10, 15685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Vilar J, Boucher RC, 2004. Reevaluating gel-forming mucins’ roles in cystic fibrosis lung disease. Free Radic. Biol. Med 37, 1564–1577. [DOI] [PubMed] [Google Scholar]
- Pezzulo AA, Tang XX, Hoegger MJ, Abou Alaiwa MH, Ramachandran S, Moninger TO, Karp PH, Wohlford-Lenane CL, Haagsman HP, van Eijk M, Bánfi B, Horswill AR, Stoltz DA, McCray PBJ, Welsh MJ, Zabner J, 2012. Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature 487, 109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumley C, Gorman EM, El-Gendy N, Bybee CR, Munson EJ, Berkland C, 2009. Nifedipine nanoparticle agglomeration as a dry powder aerosol formulation strategy. Int. J. Pharm 369, 136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl C, Hermanns MI, Uboldi C, Bock M, Fuchs S, Dei-Anang J, Mayer E, Kehe K, Kummer W, Kirkpatrick CJ, 2009. Barrier functions and paracellular integrity in human cell culture models of the proximal respiratory unit. Eur. J. Pharm. Biopharm. Off. J. Arbeitsgemeinschaft fur Pharm. Verfahrenstechnik eV 72, 339–349. [DOI] [PubMed] [Google Scholar]
- Poinard B, Kamaluddin S, Tan AQQ, Neoh KG, Kah JCY, 2019. Polydopamine coating enhances mucopenetration and cell uptake of nanoparticles. ACS Appl. Mater. Interfaces 11, 4777–4789. [DOI] [PubMed] [Google Scholar]
- Pontes JF, Grenha A, 2020. Multifunctional nanocarriers for lung drug delivery. Nanomater. (Basel, Switzerland) 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsio B, Craparo EF, Mauro N, Giammona G, Cavallaro G, 2018. Mucus and cell-penetrating nanoparticles embedded in nano-into-micro formulations for pulmonary delivery of ivacaftor in patients with cystic fibrosis. ACS Appl. Mater. Interfaces 10, 165–181. [DOI] [PubMed] [Google Scholar]
- Porto BN, Stein RT, 2016. Neutrophil extracellular traps in pulmonary diseases: Too much of a good thing? Front. Immunol 7, 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasher P, Sharma M, Singh SK, Gulati M, Jha NK, Gupta PK, Gupta G, Chellappan DK, Zacconi F, de Jesus Andreoli Pinto T, Chan Y, Liu G, Paudel KR, Hansbro PM, George Oliver BG, Dua K, 2022. Targeting mucus barrier in respiratory diseases by chemically modified advanced delivery systems. Chem. Biol. Interact 365, 110048. [DOI] [PubMed] [Google Scholar]
- Puchelle E, Jacquot J, Beck G, Zahm JM, Galabert C, 1985. Rheological and transport properties of airway secretions in cystic fibrosis-relationships with the degree of infection and severity of the disease. Eur. J. Clin. Invest 15, 389–394. [DOI] [PubMed] [Google Scholar]
- Quinton PM, 2008. Cystic fibrosis: impaired bicarbonate secretion and mucoviscidosis. Lancet 372, 415–417. [DOI] [PubMed] [Google Scholar]
- Radermecker C, Detrembleur N, Guiot J, Cavalier E, Henket M, d’Emal C, Vanwinge C, Cataldo D, Oury C, Delvenne P, Marichal T, 2020. Neutrophil extracellular traps infiltrate the lung airway, interstitial, and vascular compartments in severe COVID-19. J. Exp. Med 217, e20201012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radicioni G, Ceppe A, Ford AA, Alexis NE, Barr RG, Bleecker ER, Christenson SA, Cooper CB, Han MK, Hansel NN, Hastie AT, Hoffman EA, Kanner RE, Martinez FJ, Ozkan E, Paine R, Woodruff PG, O’Neal WK, Boucher RC, Kesimer M, 2021. Airway mucin MUC5AC and MUC5B concentrations and the initiation and progression of chronic obstructive pulmonary disease: an analysis of the SPIROMICS cohort. Lancet Respir. Med 9, 1241–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman S, Gadjeva M, 2014. Does NETosis contribute to the bacterial pathoadaptation in cystic fibrosis? Front. Immunol 5, 378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos EMC, Ramos D, Moreira GL, Macchione M, Guimarães ET, Rodrigues FMM, de Souza AAL, Saldiva PHN, Jardim JR, 2015. Viscoelastic properties of bronchial mucus after respiratory physiotherapy in subjects with bronchiectasis. Respir. Care 60, 724 LP – 730. [DOI] [PubMed] [Google Scholar]
- Ramsey KA, Chen ACH, Radicioni G, Lourie R, Martin M, Broomfield A, Sheng YH, Hasnain SZ, Radford-Smith G, Simms LA, Burr L, Thornton DJ, Bowler SD, Livengood S, Ceppe A, Knowles MR, Noone PGS, Donaldson SH, Hill DB, Ehre C, Button B, Alexis NE, Kesimer M, Boucher RC, McGuckin MA, 2020. Airway mucus hyperconcentration in non-cystic fibrosis bronchiectasis. Am. J. Respir. Crit. Care Med 201, 661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawal T, Patel S, Butani S, 2018. Chitosan nanoparticles as a promising approach for pulmonary delivery of bedaquiline. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci 124, 273–287. [DOI] [PubMed] [Google Scholar]
- Ren S, Li W, Wang L, Shi Y, Cai M, Hao L, Luo Zihao, Niu J, Xu W, Luo Zujin, 2020. Numerical analysis of airway mucus clearance effectiveness using assisted coughing techniques. Sci. Rep 10, 2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers DF, Barnes PJ, 2006. Treatment of airway mucus hypersecretion. Ann. Med 38, 116–125. [DOI] [PubMed] [Google Scholar]
- Rose MC, Voynow JA, 2006. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol. Rev 86, 245–278. [DOI] [PubMed] [Google Scholar]
- Rubin BK, 2009. Mucus, phlegm, and sputum in cystic fibrosis. Respir. Care 54, 726 LP – 732. [DOI] [PubMed] [Google Scholar]
- Rubin BK, 2007. Mucus structure and properties in cystic fibrosis. Paediatr. Respir. Rev 8, 4–7. [DOI] [PubMed] [Google Scholar]
- Rubin BK, Ramirez O, Zayas JG, Finegan B, King M, 1992. Respiratory mucus from asymptomatic smokers is better hydrated and more easily cleared by mucociliary action. Am. Rev. Respir. Dis 145, 545–547. [DOI] [PubMed] [Google Scholar]
- Saffarzadeh M, Juenemann C, Queisser MA, Lochnit G, Barreto G, Galuska SP, Lohmeyer J, Preissner KT, 2012. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One 7, e32366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakagami M, Sakon K, Kinoshita W, Makino Y, 2001. Enhanced pulmonary absorption following aerosol administration of mucoadhesive powder microspheres. J. Control. Release 77, 117–129. [DOI] [PubMed] [Google Scholar]
- Schneider CS, Xu Q, Boylan NJ, Chisholm J, Tang BC, Schuster BS, Henning A, Ensign LM, Lee E, Adstamongkonkul P, Simons BW, Wang S-YS, Gong X, Yu T, Boyle MP, Suk JS, Hanes J, 2017. Nanoparticles that do not adhere to mucus provide uniform and long-lasting drug delivery to airways following inhalation. Sci. Adv 3, e1601556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster BS, Kim AJ, Kays JC, Kanzawa MM, Guggino WB, Boyle MP, Rowe SM, Muzyczka N, Suk JS, Hanes J, 2014. Overcoming the cystic fibrosis sputum barrier to leading adeno-associated virus gene therapy vectors. Mol. Ther 22, 1484–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster Benjamin S., Suk JS, Woodworth GF, Hanes J, 2013. Nanoparticle diffusion in respiratory mucus from humans without lung disease. Biomaterials 34, 3439–3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaggi L, Salemme M, Vaccaro C, Pesce G, Rusciano G, Sasso A, Campanella C, Carotenuto R, 2010. Multiple-Particle-Tracking to investigate viscoelastic properties in living cells. Methods 51, 20–26. [DOI] [PubMed] [Google Scholar]
- Serisier DJ, Carroll MP, Shute JK, Young SA, 2009. Macrorheology of cystic fibrosis, chronic obstructive pulmonary disease & normal sputum. Respir. Res 10, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah MR, Imran M, Ullah S, 2019. Chapter 6 - Nanocarrier-based targeted pulmonary delivery: novel approaches for effective lung cancer treatment, in: Shah MR, Imran M, Ullah, S.B.T.-N. for C.D. and T.C. (Eds.), Micro and Nano Technologies Elsevier, pp. 129–161. [Google Scholar]
- Sheehan JK, Thornton DJ, Somerville M, Carlstedt I, 1991. The structure and heterogeneity of respiratory mucus glycoproteins. Am. Rev. Respir. Dis 144, S4–9. [DOI] [PubMed] [Google Scholar]
- Shen Y, Huang S, Kang J, Lin J, Lai K, Sun Y, Xiao W, Yang L, Yao W, Cai S, Huang K, Wen F, 2018. Management of airway mucus hypersecretion in chronic airway inflammatory disease: Chinese expert consensus (English edition). Int. J. Chron. Obstruct. Pulmon. Dis 13, 399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Zhang J, Zhao M, Tang S, Cheng X, Zhang W, Li W, Liu X, Peng H, Wang Q, 2021. Effects of polyethylene glycol on the surface of nanoparticles for targeted drug delivery. Nanoscale 13, 10748–10764. [DOI] [PubMed] [Google Scholar]
- Son Y-J, Longest PW, Tian G, Hindle M, 2013. Evaluation and modification of commercial dry powder inhalers for the aerosolization of a submicrometer excipient enhanced growth (EEG) formulation. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci 49, 390–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song D, Iverson E, Kaler L, Bader S, Scull MA, Duncan GA, 2021. Modeling airway dysfunction in asthma using synthetic mucus biomaterials. ACS Biomater. Sci. Eng 7, 2723–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh J, Wirtz D, Hanes J, 2004. Real-time intracellular transport of gene nanocarriers studied by multiple particle tracking. Biotechnol. Prog 20, 598–602. [DOI] [PubMed] [Google Scholar]
- Suk JS, Boylan NJ, Trehan K, Tang BC, Schneider CS, Lin J-MG, Boyle MP, Zeitlin PL, Lai SK, Cooper MJ, Hanes J, 2011a. N-acetylcysteine enhances cystic fibrosis sputum penetration and airway gene transfer by highly compacted DNA nanoparticles. Mol. Ther 19, 1981–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suk JS, Kim AJ, Trehan K, Schneider CS, Cebotaru L, Woodward OM, Boylan NJ, Boyle MP, Lai SK, Guggino WB, Hanes J, 2014. Lung gene therapy with highly compacted DNA nanoparticles that overcome the mucus barrier. J. Control. Release 178, 8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suk JS, Lai SK, Boylan NJ, Dawson MR, Boyle MP, Hanes J, 2011b. Rapid transport of muco-inert nanoparticles in cystic fibrosis sputum treated with N-acetyl cysteine. Nanomedicine (Lond) 6, 365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suk JS, Lai SK, Wang Y-Y, Ensign LM, Zeitlin PL, Boyle MP, Hanes J, 2009. The penetration of fresh undiluted sputum expectorated by cystic fibrosis patients by non-adhesive polymer nanoparticles. Biomaterials 30, 2591–2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suk JS, Xu Q, Kim N, Hanes J, Ensign LM, 2016. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev 99, 28–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan M, Mao Y, Walker TW, 2020. Rheological enhancement of artificial sputum medium. Appl. Rheol 30, 27–38. [Google Scholar]
- Thiam HR, Wong SL, Qiu R, Kittisopikul M, Vahabikashi A, Goldman AE, Goldman RD, Wagner DD, Waterman CM, 2020a. NETosis proceeds by cytoskeleton and endomembrane disassembly and PAD4-mediated chromatin decondensation and nuclear envelope rupture. Proc. Natl. Acad. Sci. U. S. A 117, 7326–7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiam HR, Wong SL, Wagner DD, Waterman CM, 2020b. Cellular mechanisms of NETosis. Annu. Rev. Cell Dev. Biol 36, 191–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao C, Yuan Z, Zhang P, Liu E, McMullen P, Wu K, Hung H-C, Jiang S, 2020. Enhanced pulmonary systemic delivery of protein drugs via zwitterionic polymer conjugation. J. Control. release Off. J. Control. Release Soc 322, 170–176. [DOI] [PubMed] [Google Scholar]
- Tucker SL, Sarr D, Rada B, 2021. Neutrophil extracellular traps are present in the airways of ENaC-overexpressing mice with cystic fibrosis-like lung disease. BMC Immunol 22, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin M, Watz H, Malmgren A, Pedersen F, 2019. NETopathic inflammation in chronic obstructive pulmonary disease and severe asthma. Front. Immunol 10, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine MT, Kaplan PD, Thota D, Crocker JC, Gisler T, Prud’homme RK, Beck M, Weitz DA, 2001. Investigating the microenvironments of inhomogeneous soft materials with multiple particle tracking. Phys. Rev. E. Stat. Nonlin. Soft Matter Phys 64, 61506. [DOI] [PubMed] [Google Scholar]
- van Andel E, de Bus I, Tijhaar EJ, Smulders MMJ, Savelkoul HFJ, Zuilhof H, 2017. Highly specific binding on antifouling zwitterionic polymer-coated microbeads as measured by flow cytometry. ACS Appl. Mater. Interfaces 9, 38211–38221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Schans CP, 2007. Bronchial mucus transport. Respir. Care 52, 1150–1158. [PubMed] [Google Scholar]
- van der Vliet A, Janssen-Heininger YMW, Anathy V, 2018. Oxidative stress in chronic lung disease: From mitochondrial dysfunction to dysregulated redox signaling. Mol. Aspects Med 63, 59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira ACC, Chaves LL, Pinheiro S, Pinto S, Pinheiro M, Lima SC, Ferreira D, Sarmento B, Reis S, 2018. Mucoadhesive chitosan-coated solid lipid nanoparticles for better management of tuberculosis. Int. J. Pharm 536, 478–485. [DOI] [PubMed] [Google Scholar]
- Vorobjeva NV, Chernyak BV, 2020. NETosis: Molecular mechanisms, role in physiology and pathology. Biochemistry. (Mosc) 85, 1178–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voynow JA, Rubin BK, 2009. Mucins, mucus, and sputum. Chest 135, 505–512. [DOI] [PubMed] [Google Scholar]
- Vukosavljevic B, Murgia X, Schwarzkopf K, Schaefer UF, Lehr CM, Windbergs M, 2017. Tracing molecular and structural changes upon mucolysis with N-acetyl cysteine in human airway mucus. Int. J. Pharm 533, 373–376. [DOI] [PubMed] [Google Scholar]
- Walenga RL, Longest PW, Kaviratna A, Hindle M, 2017. Aerosol drug delivery during noninvasive positive pressure ventilation: Effects of intersubject variability and excipient enhanced growth. J. Aerosol Med. Pulm. Drug Deliv 30, 190–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y-Y, Lai SK, So C, Schneider C, Cone R, Hanes J, 2011. Mucoadhesive nanoparticles may disrupt the protective human mucus barrier by altering its microstructure. PLoS One 6, e21547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y-Y, Lai SK, Suk JS, Pace A, Cone R, Hanes J, 2008. Addressing the PEG mucoadhesivity paradox to engineer nanoparticles that “slip” through the human mucus barrier. Angew. Chem. Int. Ed. Engl 47, 9726–9729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedmann TS, DeCastro L, Wood RW, 1997. Nebulization of nanocrystals™: Production of a respirable solid-in-liquid-in-air colloidal dispersion. Pharm. Res 14, 112–116. [DOI] [PubMed] [Google Scholar]
- Williams OW, Sharafkhaneh A, Kim V, Dickey BF, Evans CM, 2006. Airway mucus: From production to secretion. Am. J. Respir. Cell Mol. Biol 34, 527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Boylan NJ, Cai S, Miao B, Patel H, Hanes J, 2013. Scalable method to produce biodegradable nanoparticles that rapidly penetrate human mucus. J. Control. release Off. J. Control. Release Soc 170, 279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Ensign LM, Boylan NJ, Schön A, Gong X, Yang J-C, Lamb NW, Cai S, Yu T, Freire E, Hanes J, 2015. Impact of surface polyethylene glycol (PEG) density on biodegradable nanoparticle transport in mucus ex vivo and distribution in vivo. ACS Nano 9, 9217–9227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Mansour HM, Hickey AJ, 2011. Particle interactions in dry powder inhaler unit processes: A review. J. Adhes. Sci. Technol 25, 451–482. [Google Scholar]
- Yang M, Lai SK, Wang Y-Y, Zhong W, Happe C, Zhang M, Fu J, Hanes J, 2011. Biodegradable nanoparticles composed entirely of safe materials that rapidly penetrate human mucus. Angew. Chem. Int. Ed. Engl 50, 2597–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Peters JI, Williams RO 3rd, 2008. Inhaled nanoparticles--a current review. Int. J. Pharm 356, 239–247. [DOI] [PubMed] [Google Scholar]
- Yıldız-Peköz A, Ehrhardt C, 2020. Advances in pulmonary drug delivery Pharmaceutics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngren-Ortiz SR, Hill DB, Hoffmann PR, Morris KR, Barrett EG, Forest MG, Chougule MB, 2017. Development of optimized, inhalable, gemcitabine-loaded gelatin nanocarriers for lung cancer. J. Aerosol Med. Pulm. Drug Deliv 30, 299–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan S, Hollinger M, Lachowicz-Scroggins ME, Kerr SC, Dunican EM, Daniel BM, Ghosh S, Erzurum SC, Willard B, Hazen SL, Huang X, Carrington SD, Oscarson S, Fahy JV, 2015. Oxidation increases mucin polymer cross-links to stiffen airway mucus gels. Sci. Transl. Med 7, 276ra27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu Y, Ren B, Zhang D, Xie S, Chang Y, Yang J, Wu J, Xu L, Zheng J, 2019. Fundamentals and applications of zwitterionic antifouling polymers. J. Phys. D. Appl. Phys 52, 403001. [Google Scholar]
- Zou Y, Chen X, Xiao J, Bo Zhou D, Xiao Lu X, Li W, Xie B, Kuang X, Chen Q, 2018. Neutrophil extracellular traps promote lipopolysaccharide-induced airway inflammation and mucus hypersecretion in mice. Oncotarget 9, 13276–13286. [DOI] [PMC free article] [PubMed] [Google Scholar]


