Key Points
Question
What is the blood pressure–lowering effect of ultrasound renal denervation compared with a sham procedure in patients with hypertension without the confounding influence of antihypertensive medications?
Findings
In this sham-controlled, randomized (2:1) clinical trial including 224 patients withdrawn from antihypertensive medications, ultrasound renal denervation achieved clinically relevant reductions in daytime ambulatory systolic blood pressure (baseline-adjusted between-group difference, −6.3 mm Hg) as well as improvement for 6 of 7 secondary blood pressure outcomes compared with the sham procedure at 2 months. No major adverse events were reported in either group.
Meaning
Ultrasound renal denervation lowered daytime ambulatory systolic blood pressure among patients with hypertension.
Abstract
Importance
Two initial sham-controlled trials demonstrated that ultrasound renal denervation decreases blood pressure (BP) in patients with mild to moderate hypertension and hypertension that is resistant to treatment.
Objective
To study the efficacy and safety of ultrasound renal denervation without the confounding influence of antihypertensive medications in patients with hypertension.
Design, Setting, and Participants
Sham-controlled, randomized clinical trial with patients and outcome assessors blinded to treatment assignment that was conducted between January 14, 2019, and March 25, 2022, at 37 centers in the US and 24 centers in Europe, with randomization stratified by center. Patients aged 18 years to 75 years with hypertension (seated office systolic BP [SBP] ≥140 mm Hg and diastolic BP [DBP] ≥90 mm Hg despite taking up to 2 antihypertensive medications) were eligible if they had an ambulatory SBP/DBP of 135/85 mm Hg or greater and an SBP/DBP less than 170/105 mm Hg after a 4-week washout of their medications. Patients with an estimated glomerular filtration rate of 40 mL/min/1.73 m2 or greater and with suitable renal artery anatomy were randomized 2:1 to undergo ultrasound renal denervation or a sham procedure. Patients were to abstain from antihypertensive medications until the 2-month follow-up unless prespecified BP criteria were exceeded and were associated with clinical symptoms.
Interventions
Ultrasound renal denervation vs a sham procedure.
Main Outcomes and Measures
The primary efficacy outcome was the mean change in daytime ambulatory SBP at 2 months. The primary safety composite outcome of major adverse events included death, kidney failure, and major embolic, vascular, cardiovascular, cerebrovascular, and hypertensive events at 30 days and renal artery stenosis greater than 70% detected at 6 months. The secondary outcomes included mean change in 24-hour ambulatory SBP, home SBP, office SBP, and all DBP parameters at 2 months.
Results
Among 1038 eligible patients, 150 were randomized to ultrasound renal denervation and 74 to a sham procedure (mean age, 55 years [SD, 9.3 years]; 28.6% female; and 16.1% self-identified as Black or African American). The reduction in daytime ambulatory SBP was greater with ultrasound renal denervation (mean, −7.9 mm Hg [SD, 11.6 mm Hg]) vs the sham procedure (mean, −1.8 mm Hg [SD, 9.5 mm Hg]) (baseline-adjusted between-group difference, −6.3 mm Hg [95% CI, −9.3 to −3.2 mm Hg], P < .001), with a consistent effect of ultrasound renal denervation throughout the 24-hour circadian cycle. Among 7 secondary BP outcomes, 6 were significantly improved with ultrasound renal denervation vs the sham procedure. No major adverse events were reported in either group.
Conclusions and Relevance
In patients with hypertension, ultrasound renal denervation reduced daytime ambulatory SBP at 2 months in the absence of antihypertensive medications vs a sham procedure without postprocedural major adverse events.
Trial Registration
ClinicalTrials.gov Identifier: NCT03614260
This sham-controlled, randomized clinical trial compares the efficacy and safety of ultrasound renal denervation vs a sham procedure without the confounding influence of antihypertensive medications in adults with hypertension.
Introduction
Hypertension remains poorly controlled worldwide, and its prevalence is increasing.1,2 Lifestyle changes and pharmacotherapy are the mainstays of therapy for hypertension,3,4 but despite widespread availability of these approaches, many patients with hypertension are not adequately treated.5,6
Endovascular, catheter-based renal denervation has been studied as an adjunctive treatment to lower blood pressure (BP), but early trials reported inconsistent results.7,8 Four subsequent sham-controlled trials that had optimized designs to reduce or eliminate variability and variation of adjunctive medications, to improve procedural performance, and to further standardize outcome ascertainment provided evidence in support of the BP-lowering efficacy for both ultrasound renal denervation and radiofrequency-based renal denervation.9,10,11,12 Among these trials, the RADIANCE-HTN SOLO trial (A Study of the ReCor Medical Paradise System in Clinical Hypertension) and the RADIANCE-HTN TRIO trial were independently powered to detect a difference between ultrasound renal denervation and a sham procedure with respect to change in daytime ambulatory systolic BP (SBP) at 2 months (1) in the absence of antihypertensive medications in patients with mild to moderate hypertension,9 and (2) in the presence of antihypertensive medications in patients with hypertension that is resistant to treatment.10 Both trials used a circumferentially applied catheter-based system using ultrasound renal denervation to achieve consistent ablation of renal sympathetic nerves.
We designed a large pivotal trial, RADIANCE II, to further study the efficacy and safety of the ultrasound renal denervation procedure in the absence of the potentially confounding influence of antihypertensive medications.13
Methods
This international, multicenter, sham-controlled randomized clinical trial was conducted at 37 centers in the US and 24 centers in Europe (Belgium, France, Germany, the Netherlands, Poland, and the UK). The study was approved by local ethics committees or institutional review boards and conducted in accordance with the Declaration of Helsinki. All participants provided written informed consent. The trial protocol appears in Supplement 1 and the statistical analysis plan appears in Supplement 2.
Study Population
Eligible patients were men or women aged 18 years to 75 years with hypertension previously or currently treated with antihypertensive medications who had (1) uncontrolled hypertension while taking up to 2 antihypertensive medications from different medication classes (seated office SBP/diastolic BP [DBP] ≥140/90 mm Hg but <180/120 mm Hg), (2) no history of cardiovascular or cerebrovascular events, and (3) an estimated glomerular filtration rate (eGFR) of 40 mL/min/1.73 m2 or greater (using the formula from the Modification of Diet in Renal Disease Study14). All antihypertensive medications were discontinued for 4 weeks to establish a stable baseline BP without medications.
After the 4-week washout period, patients with daytime ambulatory SBP/DBP of 135/85 mm Hg or greater and SBP/DBP less than 170/105 mm Hg and suitable renal artery anatomy on preprocedural computed tomographic angiography or magnetic resonance angiography underwent renal angiography to confirm anatomical eligibility.
Race was identified by direct questioning of the patient with specific categories the patient could select or they could choose the category of “other/multiple” races. This information was included in the study per guidance from the US and Food and Drug Administration.15
Trial Procedures
Immediately after the qualifying renal angiogram, eligible patients were randomized 2:1 to undergo the ultrasound renal denervation procedure with the Paradise System (ReCor Medical Inc) or undergo a sham procedure (renal angiography) by proctored and trained interventionalists, as previously described.9 Ultrasound sonications were performed according to individual treatment plans guided by the prerandomization computed tomographic angiography or magnetic resonance angiography and then according to the renal angiogram. Sonications were sequentially delivered to the main right and left renal arteries proximal to the first bifurcation, as well as to any accessory renal arteries 3 to 8 mm in diameter.
The computer-generated randomization sequence was stratified by center using randomized block sizes of 4 or 6. Patients were sedated and wore headphones and eye covers. Treatment assignment was masked for patients and staff performing follow-up assessments for 12 months after randomization. The effectiveness of patient masking was assessed at hospital discharge and at 2-month follow-up. Patients completed a questionnaire asking if they believed they were treated, were not treated, or did not know whether they were or were not treated. This response was then compared with the actual treatment assignment to calculate a blinding index per the methods of Bang et al16 and James et al.17
All patients did not take antihypertensive medications until 2 months after randomization unless their office SBP/DBP exceeded 180/110 mm Hg and their home SBP/DBP exceeded 170/105 mm Hg as described in the trial protocol (Supplement 1), in which case patients received an escape antihypertensive treatment. All patients had monthly visits to (1) undergo seated office BP, heart rate, and laboratory assessments as well as spot urine collection for subsequent chemical adherence testing18 after the 2-month outcome assessment (all of these were assessed by a core laboratory masked to treatment assignment), (2) assess their 7-day home BP measured prior to each onsite visit, and (3) record medications and adverse events.
Seated office BP and home BP were measured with the same validated electronic device (Omron M10-IT) as previously described.9 Ambulatory BP measurements (Microlife WatchBP) were performed at baseline and at 2 months, and were sent to a core laboratory (dabl Ltd) with treatment assignment masked as previously described.9 Urine chemical adherence testing results were not made available to site investigators per the trial protocol. A follow-up renal computed tomographic angiography or magnetic resonance angiography was to be performed for all patients at 6 months.
Trial Outcomes
The primary efficacy outcome was the mean change in daytime ambulatory SBP at 2 months. The secondary outcomes specified for hierarchical analysis included testing for change in 24-hour ambulatory SBP, home SBP, office SBP, daytime ambulatory DBP, 24-hour ambulatory DBP, home DBP, and office DBP at 2 months.
Tertiary outcomes included change at 2 months in nighttime ambulatory measurements and ambulatory, home, and office heart rate measurements as the proportion of patients (1) with at least a decrease by 5 mm Hg, 10 mm Hg, or 15 mm Hg in daytime and 24-hour ambulatory SBP, (2) with controlled daytime BP (SBP/DBP <135/85 mm Hg) and 24-hour BP (SBP/DBP <130/80 mm Hg), and (3) treated with antihypertensive medications at 2 months.
The primary safety composite outcome included the following prespecified major adverse events: all-cause mortality, kidney failure, an embolic event, renal artery or vascular complications requiring intervention, and hospitalization for hypertensive or hypotensive crisis or for major cardiovascular or cerebrovascular events within 30 days as well as renal artery stenosis greater than 70% detected by noninvasive imaging at 6 months. We also assessed change in eGFR from baseline to 2 months. Additional prespecified safety outcomes appear in the statistical analysis plan in Supplement 2. An independent data and safety monitoring board reviewed the trial data quarterly for all enrolled patients.
Statistical Analysis
Assuming a 6-mm Hg difference in change in daytime ambulatory SBP at 2 months between the ultrasound renal denervation group and the sham procedure group7 with a common SD of 12 mm Hg and a 2-sided type I error rate of 5%, a sample size of 192 evaluable patients (128 in the ultrasound renal denervation group and 64 in the sham procedure group) yielded 90% power. To account for missing data up to 15% for the primary efficacy outcome, we planned to randomize 225 patients in the study. Enrollment of 128 patients to undergo ultrasound renal denervation provided 95% power for the primary safety composite outcome, assuming an observed major adverse event rate of 3% compared with a safety performance goal of 9.8%.8
The primary statistical analysis of the primary efficacy outcome was performed on the intention-to-treat population, which was defined as all randomized patients analyzed according to their original randomization assignment (N = 224) (other population definitions appear in the eAppendix in Supplement 3). In the intention-to-treat population, 10 of 224 patients who received escape antihypertensive treatment because of a high office or home BP before the 2-month outcome assessment had their baseline daytime ambulatory SBP (last observed BP prior to medication being added) imputed as their 2-month daytime ambulatory SBP value. Furthermore, for 12 additional patients who had added antihypertensive medications before the 2-month outcome assessment based on patient or physician decision without meeting the trial protocol–defined escape criteria, we used the observed value at 2 months in the intention-to-treat analysis. In addition, for 6 other patients with missing 2-month ambulatory BP measurements, multiple imputations were performed.
Similar methods were used for the analysis of the secondary outcomes. For the secondary outcomes specified for hierarchical analysis, the tests were performed in order until the first nonsignificant test result (P > .05), such that subsequent secondary outcomes would not be used to make claims. These results and corresponding significance tests are provided for descriptive purposes only. The statistical analysis of the secondary outcomes followed the methods of the primary efficacy outcome, including use of multiple imputation for missing data. Evaluable data were used for all other analyses. In addition, a tipping point analysis was performed on the primary efficacy outcome to evaluate the effect of missing observations. Missing observations (ie, changes in daytime ambulatory SBP from baseline to 2 months) were imputed over a range of possible scenarios for the treatment effect (best-case scenario was the greatest reduction in BP in the ultrasound renal denervation group and the greatest increase in BP in the sham procedure group; and the worst-case scenario was the greatest increase in BP in the ultrasound renal denervation group and the greatest reduction in BP in the sham procedure group; and assessment of the quartiles among these values) to identify the scenario or tipping point where the treatment effect in patients with missing data overturns the significant treatment effect observed in the primary outcome analysis.
We also used a modified intention-to-treat population, a complete ambulatory BP population, a per-protocol population, and an as-treated population for the supportive analyses. The modified intention-to-treat population excluded patients that received escape antihypertensive treatment. The complete ambulatory BP population included only patients who had ambulatory BP measurements at both baseline and at 2 months. The per-protocol population excluded patients who (1) did not meet the baseline daytime ambulatory BP criteria or the renal artery anatomical inclusion criteria, (2) were in the ultrasound renal denervation group but did not receive at least 2 emissions bilaterally, (3) restarted antihypertensive medications for any reason before the 2-month ambulatory BP measurement (according to trial protocol criteria or according to physician or patient decision), or (4) did not complete the 2-month ambulatory BP assessment. The as-treated population excluded patients randomized to ultrasound renal denervation who did not receive at least 2 emissions bilaterally.
Continuous variables are expressed as mean (SD) and categorical variables as frequency (%). Between-group differences are expressed as means and 2-sided 95% CIs or medians and 95% CIs estimated using the Hodges-Lehmann method when appropriate. Between-group treatment differences from baseline to 2 months were assessed using analysis of covariance, including the baseline value as a covariate. When the change in a parameter from baseline was not normally distributed, a baseline-adjusted analysis of covariance based on ranks was performed.19
The analyses for the prespecified subgroups (ethnicity, age, sex, geography, baseline daytime ambulatory SBP, office SBP, home SBP, abdominal obesity, baseline 24-hour ambulatory heart rate, eGFR, and enrollment before or after COVID-19) were performed using linear regression analyses with change in daytime ambulatory SBP at 2 months as the dependent variable. Baseline daytime SBP, treatment group, and a subgroup × treatment group interaction term were included as independent variables in the models (1 model per subgroup).
In the ultrasound renal denervation group only, we assessed the effect of the number of ablations performed, the presence of untreated accessory arteries, and the balloon size. The blinding indices were calculated per the methods of Bang et al16 and James et al.17 The analyses were performed using SAS version 9.4 (SAS Institute Inc). A 2-sided P<.05 was considered significant.
Results
Patients
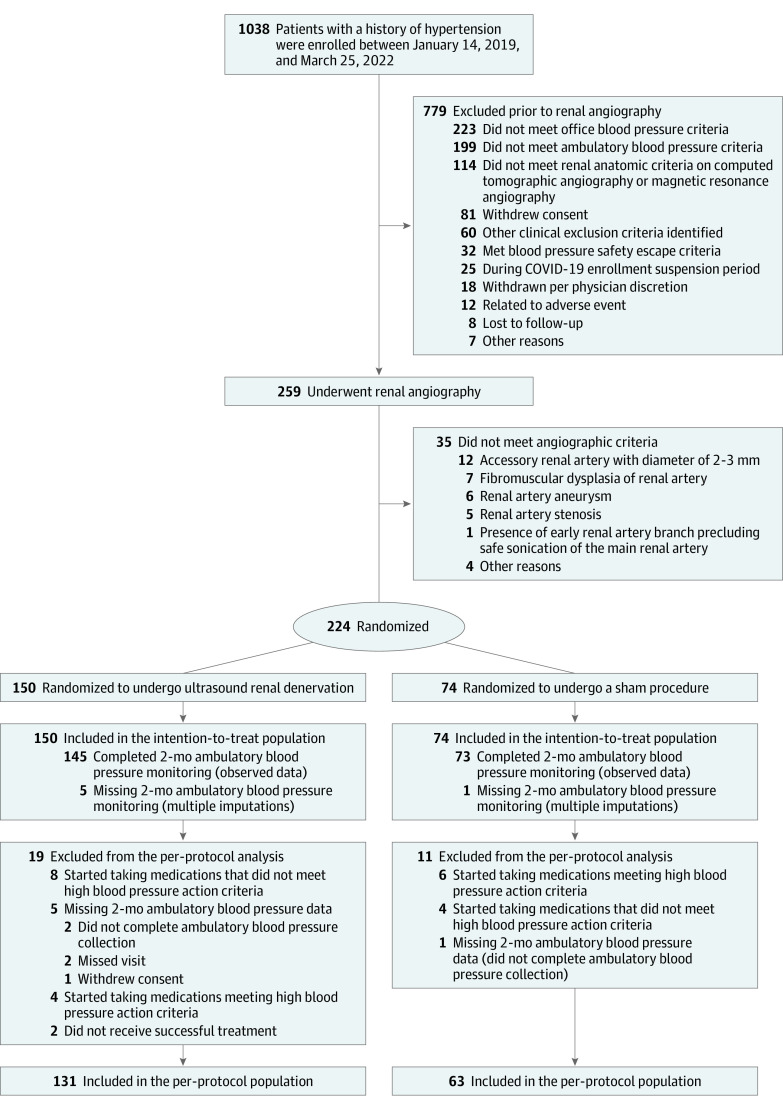
Between January 14, 2019, and March 25, 2022, 1038 patients were enrolled and 224 met all eligibility criteria for randomization (n = 150 in the ultrasound renal denervation group and n = 74 in the sham procedure group; Figure 1). Enrollment was paused due to the COVID-19 pandemic from March 13, 2020, to June 24, 2020, with 95 patients randomized prior to stopping for COVID-19. The baseline characteristics were similar across both study groups (Table 1). The mean age was 55 years (SD, 9.3 years). Female patients represented 28.6% of the trial population, and patients self-identifying as Black or African American represented 16.1%. A total of 147 of 224 patients (65.6%) were being treated with antihypertensive medications at the time of enrollment; the remainder had been previously treated with antihypertensive medications but were no longer receiving them at the time of enrollment.
Figure 1. Patient Flow Through the RADIANCE II Trial.
RADIANCE indicates A Study of the ReCor Medical Paradise System in Clinical Hypertension.
Table 1. Baseline Demographics and Clinical Characteristics of the Intention-to-Treat Population.
| No. (%)a | ||
|---|---|---|
| Ultrasound renal denervation (n = 150) | Sham procedure (n = 74) | |
| Age, mean (SD), y | 55.1 (9.9) | 54.9 (7.9) |
| Sex | ||
| Female | 47 (31.3) | 17 (23.0) |
| Male | 103 (68.7) | 57 (77.0) |
| Raceb | ||
| Black or African American | 21 (14.0) | 15 (20.3) |
| White | 114 (76.0) | 56 (75.7) |
| Other/multiple racesc | 15 (10.0) | 3 (4.1) |
| Body mass index, mean (SD)d | 30.1 (5.2) | 30.6 (5.2) |
| Abdominal obesitye | 90 (60.0) | 46 (62.2) |
| Estimated glomerular filtration rate | ||
| Mean (SD), mL/min/1.73 m2 | 81.4 (14.4) | 82.3 (14.9) |
| <60 mL/min/1.73 m2 | 7 (4.7) | 3 (4.1) |
| Type 2 diabetes | 9 (6.0) | 5 (6.8) |
| Sleep apnea syndrome | 21 (14.0) | 13 (17.6) |
| Prior hospitalization for hypertensive crisis | 9 (6.0) | 3 (4.1) |
| History of heart failure | 1 (0.7) | 0 |
| Blood pressure at office screening, mean (SD), mm Hg | ||
| Systolic | 155.8 (11.1) | 154.3 (10.6) |
| Diastolic | 101.3 (6.7) | 99.1 (5.6) |
| Heart rate at office screening, mean (SD), beats/min | 74.1 (12.0) | 73.6 (11.9) |
| Not taking antihypertensive medications at screening | 54 (36.0) | 23 (31.1) |
| Taking antihypertensive medications at screening | ||
| 1 Medication | 52 (34.7) | 25 (33.8) |
| 2 Medications | 44 (29.3) | 25 (33.8) |
| >2 Medications | 0 | 1 (1.4)f |
| Type of antihypertensive medication at screening in patients taking medications | (n = 96) | (n = 51) |
| Renin-angiotensin system blockers | 73 (76.0) | 35 (68.6) |
| Calcium channel blockers | 28 (29.2) | 22 (43.1) |
| Diuretics | 25 (26.0) | 13 (25.5) |
| β-Blockers | 10 (10.4) | 5 (9.8) |
| Aldosterone antagonists | 1 (1.0) | 1 (2.0) |
| Otherg | 3 (3.1) | 3 (5.9) |
Unless otherwise indicated.
Identified by direct questioning of the participant with specific categories the patient could select.
There were 15 patients in the ultrasound renal denervation group and 2 patients in the sham procedure group who selected the category “other/multiple races.” One patient selected another category but because there were fewer than 5 patients in that category, that patient is listed with “other.”
Calculated as weight in kilograms divided by height in meters squared.
Defined as a waist circumference greater than 102 cm for men and greater than 88 cm for women.
One patient was discovered to be taking 4 medications after randomization.
Included α1 receptor blockers, centrally acting drugs, and vasodilators.
Procedures
The average procedure length was 77 minutes for ultrasound renal denervation vs 44 minutes for the sham procedure. The total length of sonication for patients treated with ultrasound renal denervation was 38.9 seconds with an average of 5.6 sonications. Successful bilateral ablation was performed in 148 of 150 patients (98.7%); of whom, 30 (20.0%) had accessory renal artery ablations (eTable 1 in Supplement 3). The blinding indices calculated per the methods of Bang et al16 and James et al17 appear in eTable 2 in Supplement 3.
A total of 12 patients (8.0%) in the ultrasound renal denervation group and 10 patients (13.5%) in the sham procedure group received antihypertensive medications prior to 2 months by physicians blinded to treatment group; of these 22 patients, 4 (2.7%) and 6 (8.1%), respectively, were treated after meeting criteria for escape antihypertensive treatment (eTable 3 in Supplement 3). However, urine chemical adherence testing performed after the assessment of the 2-month primary efficacy outcome revealed hidden antihypertensive medication intake at baseline in 21 of 152 patients (13.8%) with urine samples available and in 13 of 147 patients (8.8%) at 2 months (9 of 97 patients [9.3%] in the ultrasound renal denervation group and 4 of 50 patients [8.0%] in the sham procedure group). In the 14 patients who were prescribed antihypertensive medications and had urine samples available at 2 months, the urine samples showed that these patients had been adherent to treatment.
Outcomes
Primary Efficacy Outcome
There was a greater reduction in daytime ambulatory SBP at 2 months in the ultrasound renal denervation group (mean, −7.9 mm Hg [SD, 11.6 mm Hg]) compared with the sham procedure group (mean, −1.8 mm Hg [SD, 9.5 mm Hg]) and a baseline-adjusted between-group difference of −6.3 mm Hg (95% CI, −9.3 to −3.2 mm Hg; P < .001) (Table 2 and Figure 2). In a sensitivity analysis to evaluate the effect of missing daytime ambulatory SBP data, a tipping point between the significant and nonsignificant primary outcome results did not occur. All 25 imputation scenarios resulted in the primary efficacy outcome as statistically significant, thus showing the robustness of the results (eTable 4 in Supplement 3).
Table 2. Change in Ambulatory, Home, and Office Blood Pressure Levels at 2 Months Compared With Baseline.
| Observed data | Baseline-adjusted for observed data | Baseline-adjusted with multiple imputations for missing data | Median between-group difference (95% CI)c | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ultrasound renal denervation, mean (SD) | Sham procedure, mean (SD) | ||||||||||
| At baseline | At 2 mo | Difference | At baseline | At 2 mo | Difference | Mean between-group difference (95% CI)a | P valueb | Mean between-group difference (95% CI)a | P valueb | ||
| Systolic blood pressure, mm Hg | |||||||||||
| Daytime ambulatoryd | 150.2 (8.6) | 142.3 (13.4) | −7.9 (11.6) | 151.3 (9.0) | 149.5 (11.1) | −1.8 (9.5) | −6.3 (−9.4 to −3.2) | <.001 | −6.3 (−9.3 to −3.2) | <.001 | −7.0 (−9.0 to −4.0)e |
| 24-h ambulatoryf | 143.2 (9.0) | 135.6 (13.0) | −7.7 (10.7) | 144.5 (9.7) | 142.9 (10.5) | −1.7 (9.3) | −6.3 (−9.2 to −3.4) | <.001 | −6.2 (−9.1 to −3.4) | <.001 | |
| Nighttime ambulatoryf | 132.1 (12.6) | 125.5 (15.0) | −6.6 (12.8) | 133.8 (13.3) | 132.4 (12.2) | −1.3 (11.3) | −5.9 (−9.1 to −2.6) | <.001 | −5.8 (−9.0 to −2.6) | <.001 | −6.0 (−9.0 to −3.0)e |
| Homeg | 152.4 (9.5) | 143.4 (12.3) | −9.0 (9.5) | 149.7 (10.3) | 148.8 (12.3) | −0.9 (7.9) | −7.8 (−10.4 to −5.1) | <.001 | −7.6 (−10.1 to −5.0) | <.001 | |
| Officeh | 156.8 (13.3) | 145.8 (15.9) | −11.0 (13.5) | 156.7 (12.9) | 151.2 (16.4) | −5.5 (12.9) | −5.5 (−9.2 to −1.8) | .004 | −5.4 (−9.0 to −1.8) | .004 | |
| Diastolic blood pressure, mm Hg | |||||||||||
| Daytime ambulatoryd | 93.8 (5.2) | 88.4 (7.4) | −5.4 (6.5) | 93.2 (5.6) | 91.8 (6.7) | −1.3 (5.7) | −3.9 (−5.6 to −2.2) | <.001 | −3.9 (−5.6 to −2.2) | <.001 | |
| 24-h ambulatoryf | 88.4 (5.8) | 83.1 (7.6) | −5.3 (6.4) | 88.2 (5.8) | 87.0 (6.3) | −1.2 (5.4) | −4.1 (−5.7 to −2.4) | <.001 | −4.1 (−5.7 to −2.4) | <.001 | |
| Nighttime ambulatoryf | 79.8 (8.3) | 75.1 (9.7) | −4.7 (8.2) | 80.2 (8.0) | 79.6 (7.5) | −0.5 (6.7) | −4.3 (−6.3 to −2.2) | <.001 | −4.2 (−6.3 to −2.2) | <.001 | −5.0 (−7.0 to −3.0)e |
| Homeg | 97.8 (6.3) | 92.7 (7.4) | −5.1 (6.0) | 95.7 (7.6) | 95.5 (8.1) | −0.3 (4.5) | −4.4 (−6.0 to −2.9) | <.001 | −4.3 (−5.9 to −2.8) | <.001 | |
| Officeh | 101.9 (7.8) | 96.0 (10.2) | −5.9 (9.4) | 101.4 (7.5) | 98.1 (11.2) | −3.3 (9.2) | −2.4 (−5.1 to 0.2) | .07 | −2.3 (−4.9 to 0.2) | .08 | |
Estimate of treatment difference used baseline-adjusted analysis of covariance.
Calculated using baseline-adjusted analysis of covariance.
Data provided because change from baseline in either cohort was not normal. These data were calculated using the Hodges-Lehmann estimator of location shift without adjustment for baseline data or multiple imputations.
There were 145 patients in the ultrasound renal denervation group and 73 patients in the sham procedure group with data.
The P value comparison for these data yielded a value of P<.001 and was calculated using baseline-adjusted analysis of covariance based on ranks and using observed data without multiple imputations.
There were 144 patients in the ultrasound renal denervation group and 72 patients in the sham procedure group with data.
There were 140 patients in the ultrasound renal denervation group and 69 patients in the sham procedure group with data.
There were 137 patients in the ultrasound renal denervation group and 71 patients in the sham procedure group with data.
Figure 2. Twenty-Four Hour Ambulatory Profiles of Systolic Blood Pressure in Patients With Complete Ambulatory Measurements at Baseline and at 2 Months.
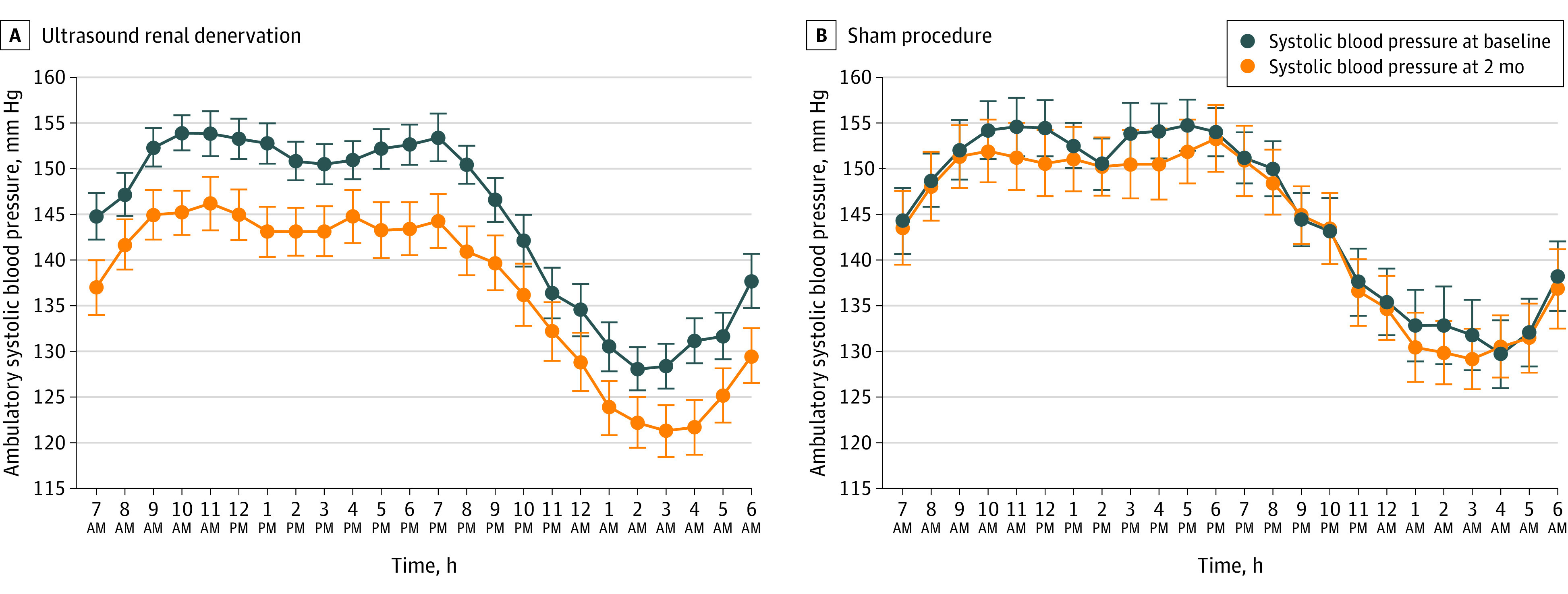
There were 145 patients in the ultrasound renal denervation group and 73 patients in the sham procedure group with complete data. Data are expressed as mean (95% CI).
Primary Safety Composite Outcome
The primary safety composite outcome was met and there were no major adverse events in either group (eTable 5 in Supplement 3). No renal artery stenosis greater than 70% was detected by computed tomographic angiography or magnetic resonance angiography at 6 months; data were available in 138 of 150 patients (92%) who underwent ultrasound renal denervation (eTable 5 in Supplement 3).
Prespecified Secondary Outcomes
Of the 7 prespecified secondary BP outcomes, 6 were significantly improved in the ultrasound renal denervation group vs the sham procedure group (Table 2). Examination of the ambulatory BP monitoring demonstrated lower BP throughout the 24-hour circadian cycle (Figure 2). The time course of changes in home and office SBP from baseline to 2 months appears in eFigure 1 in Supplement 3. In the modified intention-to-treat population, complete ambulatory BP population, per-protocol population, and the as-treated population, the between-group differences for changes in daytime ambulatory SBP and in other BP parameters were statistically significant (eTables 6-9 in Supplement 3).
Additional Analyses
Individual changes in daytime ambulatory SBP related to their respective individual BP values at baseline appear in Figure 3 and the frequency distribution of the BP response to ultrasound renal denervation vs the sham procedure appears in eFigure 2 in Supplement 3. A total of 93 of 145 patients (64.1%) had a change of at least −5 mm Hg in daytime ambulatory SBP in the ultrasound renal denervation group vs 25 of 73 patients (34.2%) in the sham procedure group (P < .001; eTable 10 in Supplement 3). In the ultrasound renal denervation group, 25 of 133 patients (18.8%) attained controlled daytime ambulatory BP (SBP/DBP <135/85 mm Hg) in the absence of added antihypertensive medications vs 3 of 63 patients (4.8%) in the sham procedure group (P = .009; eTable 11 in Supplement 3).
Figure 3. Individual Changes From Baseline to 2 Months in Daytime Ambulatory Systolic Blood Pressure in Patients With Complete Ambulatory Measurements.
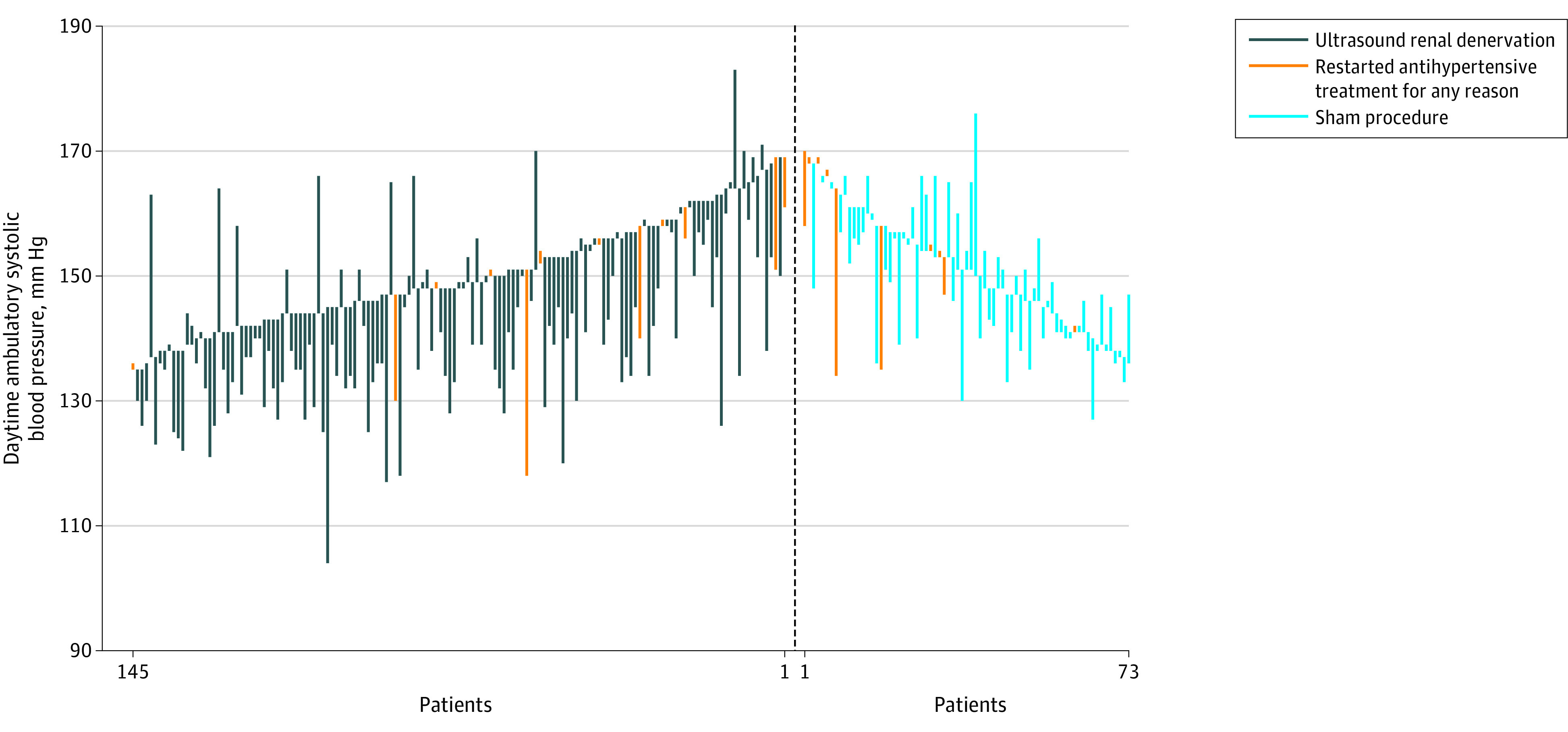
There were 145 patients in the ultrasound renal denervation group and 73 patients in the sham procedure group with complete data. There were 22 patients (total from both groups combined) who restarted antihypertensive treatment for any reason.
The between-group differences for changes in daytime SBP from baseline to 2 months were consistent across all prespecified subgroups with no effect modification (eFigure 3 in Supplement 3). The number of ultrasound emissions, the presence of nontreated accessory renal arteries, balloon size, and the number of ultrasound renal denervation procedures performed by treating interventionalists did not influence the BP response to ultrasound renal denervation (results not shown). In addition, there was no within-group or between-group difference in office or ambulatory heart rate at 2 months (eTable 12 in Supplement 3). Estimated GFR did not significantly change from baseline and was similar in both groups at 2 months (eTable 13 in Supplement 3).
Discussion
This randomized clinical trial that was conducted mainly among middle-aged men and women (mean age, 55 years) with hypertension demonstrated statistically significant reductions in daytime ambulatory SBP (primary efficacy outcome) and in 6 of 7 prespecified secondary BP outcomes with ultrasound renal denervation compared with the sham procedure and supports the efficacy of ultrasound renal denervation. The effect of ultrasound renal denervation on daytime ambulatory SBP was consistent across all prespecified subgroups and was achieved without major adverse events.
After the proof-of-concept demonstration of the BP-lowering efficacy of ultrasound renal denervation in the sham-controlled, off-medication, RADIANCE-HTN SOLO study,9 this larger sham-controlled randomized clinical trial was designed to further study the efficacy and safety. The RADIANCE-HTN SOLO study included patients with less severe hypertension (either naive to antihypertensive medications or had controlled or uncontrolled hypertension while taking 0-2 antihypertensive medications) than patients in the current trial. Similar to the RADIANCE-HTN SOLO trial,9 we limited the duration of patients being off antihypertensive medications in the current trial to 3 months after enrollment for safety, ethical, and regulatory reasons. Although the present trial was paused and then restarted due to the COVID-19 pandemic, we were able to ascertain that enrollment prior to or after the pandemic did not influence the BP response to ultrasound renal denervation.
In this randomized clinical trial, the ultrasound renal denervation group experienced an average 7.9-mm Hg reduction in daytime ambulatory SBP from baseline to 2 months, a 6.3-mm Hg greater reduction compared with the sham procedure group in the intention-to-treat analysis, despite proportionally more patients in the sham procedure group resuming antihypertensive medications according to the trial protocol–defined escape criteria. Moreover, consistent average reductions in 6 of 7 prespecified secondary BP outcomes at 2 months, including 24-hour SBP, home SBP, and office SBP, were achieved after ultrasound renal denervation. The homogeneity of the BP-lowering effect of ultrasound renal denervation, independent of the method of BP measurement, reinforces the strength of the results. Furthermore, monthly home and office BP measurements demonstrated that the SBP decrease in the ultrasound renal denervation group was already present as early as 1 month after undergoing the procedure (eFigure 1 in Supplement 3). The between-group difference in all DBP parameters also favored ultrasound renal denervation.
Moreover, the BP-lowering effect of ultrasound renal denervation was consistent over the 24-hour circadian cycle with similar BP decreases during daytime, nighttime, and early morning, which is a so-called always-on effect. Such consistent BP reductions during nighttime and the early morning phase may not be achieved with the once-daily use of short-acting antihypertensive medications. One further potential benefit of ultrasound renal denervation could be a reduction in the consequences of the variable timing of medication intake, forgetfulness of medication intake, and nonadherence to oral antihypertensive treatments, which are all frequently observed in patients with hypertension.20 This may be even more relevant given recent data that even the favorable effects of intensive BP control, as demonstrated within the Systolic Blood Pressure Intervention Trial (SPRINT) trial,21 wane over time.
The average decrease in daytime ambulatory SBP in the ultrasound renal denervation group at 2 months in this trial was of similar magnitude to that observed in the RADIANCE-HTN SOLO trial9 (reduction of −8.5 mm Hg from baseline, which is a 6.3-mm Hg greater reduction vs the sham procedure) following the same average number of sonications, using the same methods of ambulatory BP measurement, and with patients having similar daytime ambulatory SBP (approximately 150 mm Hg) and eGFR values at baseline. In the current trial, standardized follow-up on protocolized antihypertensive medication escalation in patients who have persistent elevation of BP from 2 months onward is planned for up to 6 months after randomization (without crossover of patients in the sham procedure group to ultrasound renal denervation until 12 months) with both patients and clinicians masked to initial treatment assignment.
In this trial, consistent with the RADIANCE-HTN SOLO trial and the RADIANCE-HTN TRIO trial, treatment with ultrasound renal denervation was safe with no major device-related or procedure-related adverse events. These findings are consistent with the overall safety profile of ultrasound renal denervation,9,10 and the safety profile of radiofrequency-based renal denervation.11,12 The long-term safety of ultrasound renal denervation will continue to be monitored for 60 months in the current trial with renal computed tomographic angiography or magnetic resonance angiography imaging at 12 months in the ultrasound renal denervation group.
Limitations
There are limitations to this trial. First, the duration of follow-up was limited. However, extended follow-up is planned.
Second, the enrollment of patients was limited to those at low cardiovascular risk with an eGFR of 40 mL/min/1.73 m2 or greater and without significant comorbidities. This was by design because the trial mandated withdrawal of BP medications, potentially exposing patients to 3 months of uncontrolled BP, especially patients in the sham procedure group. As a result, the generalizability of these findings to patients with more severe hypertension is limited; however, the RADIANCE TRIO trial demonstrated BP decreases with ultrasound renal denervation in patients with hypertension that is resistant to treatment.
Third, the BP-lowering effect for a single individual patient is difficult to predict, partly because of the variability in the prevailing state of sympathetic hyperactivity22 or variable renal nerve ablation. At present, no simple and reproducible way exists to assess sympathetically mediated hypertension prior to consideration of ultrasound renal denervation and there is still no reliable periprocedural marker of successful ultrasound renal denervation, though preclinical models suggest complete ablation is likely with multiple circumferential sonications bilaterally in the main renal arteries and major accessory vessels (if present).23,24
Conclusions
In patients with hypertension, ultrasound renal denervation reduced daytime ambulatory SBP at 2 months in the absence of antihypertensive medications vs a sham procedure without postprocedural major adverse events.
Trial protocol
Statistical analysis plan
eAppendix. Population definitions
eTable 1. Angiographic and procedural characteristics in the ultrasound renal denervation group (uRDN) and in the sham group
eTable 2. Bang and James blinding indices at hospital discharge and 2 months
eTable 3. Patients with change in antihypertensive medications prior to the 2-month visit in the ultrasound renal denervation group (uRDN) and in the sham group (intention-to-treat population)
eTable 4. Tipping point analysis for primary endpoint of daytime ambulatory systolic blood pressure
eTable 5. Incidence of major adverse events adjudicated by the clinical events committee (primary safety endpoint)
eTable 6. Change in ambulatory, office, and home blood pressure at 2 months in the ultrasound renal denervation group (uRDN) and in the sham group in the modified-intention-to-treat population (n = 146 uRDN, n = 68 sham)
eTable 7. Change in ambulatory blood pressure at 2 months in the ultrasound renal denervation group (uRDN) and in the sham group in patients with complete ambulatory blood pressure data both at baseline and 2 months (n = 145 uRDN, n= 73 sham)
eTable 8. Change in ambulatory, office, and home blood pressure at 2 months in the ultrasound renal denervation group (uRDN) and in the sham group in the per-protocol population (n = 131 uRDN, n = 63 sham)
eTable 9. Change in ambulatory blood pressure at 2 months in the ultrasound renal denervation group (uRDN) and in the sham group in the as-treated population (n = 149 uRDN, n = 74 sham)
eTable 10. Number and percentage of patients with a decrease in daytime ambulatory blood pressure at 2 months ≥ 5 mmHg, ≥ 10 mmHg, and ≥ 15 mmHg in the ultrasound renal denervation group (uRDN) and in the sham group among patients with ambulatory blood pressure measurements at 2 months (n = 145 uRDN, n = 73 sham)
eTable 11. Number and percentage of patients with controlled blood pressure according to ambulatory and office blood pressure measurements in the ultrasound renal denervation group (uRDN) and in the sham group
eTable 12. Changes in ambulatory, office, and home heart rate at 2 months in the ultrasound renal denervation group (uRDN) and in the sham group in the intention-to-treat population
eTable 13. Estimated glomerular filtration rate (eGFR) and serum creatinine at baseline and 2 months in the ultrasound renal denervation group (uRDN) and in the sham group for subjects with data at both timepoints (n = 136, uRDN; n = 72, sham)
eFigure 1. Time course evolution of home (top) and office (bottom) systolic blood pressure (SBP) changes between baseline, 1, and 2 months in the ultrasound renal denervation group (uRDN, blue line) and in the sham (grey line) group (intention-to-treat population)
eFigure 2. Frequency distribution plots of daytime (A), nighttime (B), 24h (C), home (D) and office (E) systolic blood pressure (SBP) changes from baseline to 2 months in the ultrasound renal denervation group (uRDN, blue line) and in the sham (grey line) group
eFigure 3. Forest plot of between-group differences (95%CI) in daytime ambulatory systolic blood pressure changes across pre-specified sub-groups in favor of ultrasound renal denervation group (uRDN) vs sham
Nonauthor collaborators
Data sharing statement
References
- 1.Zhou B, Carrillo-Larco RM, Danaei G, et al. ; NCD Risk Factor Collaboration (NCD-RisC) . Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. 2021;398(10304):957-980. doi: 10.1016/S0140-6736(21)01330-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muntner P, Hardy ST, Fine LJ, et al. Trends in blood pressure control among US adults with hypertension, 1999-2000 to 2017-2018. JAMA. 2020;324(12):1190-1200. doi: 10.1001/jama.2020.14545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2018;138(17):e426-e483. doi: 10.1161/CIR.0000000000000597 [DOI] [PubMed] [Google Scholar]
- 4.Williams B, Mancia G, Spiering W, et al. ; ESC Scientific Document Group . 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021-3104. doi: 10.1093/eurheartj/ehy339 [DOI] [PubMed] [Google Scholar]
- 5.Choudhry NK, Kronish IM, Vongpatanasin W, et al. ; American Heart Association Council on Hypertension; Council on Cardiovascular and Stroke Nursing; and Council on Clinical Cardiology . Medication adherence and blood pressure control: a scientific statement from the American Heart Association. Hypertension. 2022;79(1):e1-e14. doi: 10.1161/HYP.0000000000000203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Egan BM, Yang J, Rakotz MK, et al. Self-reported antihypertensive medication class and temporal relationship to treatment guidelines. Hypertension. 2022;79(2):338-348. doi: 10.1161/HYPERTENSIONAHA.121.17102 [DOI] [PubMed] [Google Scholar]
- 7.Azizi M, Sapoval M, Gosse P, et al. ; Renal Denervation for Hypertension (DENERHTN) investigators . Optimum and stepped care standardised antihypertensive treatment with or without Renal Denervation for Resistant Hypertension (DENERHTN): a multicentre, open-label, randomised controlled trial. Lancet. 2015;385(9981):1957-1965. doi: 10.1016/S0140-6736(14)61942-5 [DOI] [PubMed] [Google Scholar]
- 8.Bhatt DL, Kandzari DE, O’Neill WW, et al. ; SYMPLICITY HTN-3 Investigators . A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370(15):1393-1401. doi: 10.1056/NEJMoa1402670 [DOI] [PubMed] [Google Scholar]
- 9.Azizi M, Schmieder RE, Mahfoud F, et al. ; RADIANCE-HTN Investigators . Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): a multicentre, international, single-blind, randomised, sham-controlled trial. Lancet. 2018;391(10137):2335-2345. doi: 10.1016/S0140-6736(18)31082-1 [DOI] [PubMed] [Google Scholar]
- 10.Azizi M, Sanghvi K, Saxena M, et al. ; RADIANCE-HTN investigators . Ultrasound renal denervation for hypertension resistant to a triple medication pill (RADIANCE-HTN TRIO): a randomised, multicentre, single-blind, sham-controlled trial. Lancet. 2021;397(10293):2476-2486. doi: 10.1016/S0140-6736(21)00788-1 [DOI] [PubMed] [Google Scholar]
- 11.Böhm M, Kario K, Kandzari DE, et al. ; SPYRAL HTN-OFF MED Pivotal Investigators . Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED Pivotal): a multicentre, randomised, sham-controlled trial. Lancet. 2020;395(10234):1444-1451. doi: 10.1016/S0140-6736(20)30554-7 [DOI] [PubMed] [Google Scholar]
- 12.Kandzari DE, Böhm M, Mahfoud F, et al. ; SPYRAL HTN-ON MED Trial Investigators . Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet. 2018;391(10137):2346-2355. doi: 10.1016/S0140-6736(18)30951-6 [DOI] [PubMed] [Google Scholar]
- 13.Kandzari DE, Mahfoud F, Bhatt DL, et al. Confounding factors in renal denervation trials: revisiting old and identifying new challenges in trial design of device therapies for hypertension. Hypertension. 2020;76:1410-1417. doi: 10.1161/HYPERTENSIONAHA.120.15745 [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247-254. Published correction appears in Ann Intern Med. 2021;174(4):584. [DOI] [PubMed] [Google Scholar]
- 15.US Food and Drug Administration . Collection of race and ethnicity data in clinical trials. Accessed October 15, 2022. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/collection-race-and-ethnicity-data-clinical-trials
- 16.Bang H, Ni L, Davis CE. Assessment of blinding in clinical trials. Control Clin Trials. 2004;25(2):143-156. doi: 10.1016/j.cct.2003.10.016 [DOI] [PubMed] [Google Scholar]
- 17.James KE, Bloch DA, Lee KK, Kraemer HC, Fuller RK. An index for assessing blindness in a multi-centre clinical trial: disulfiram for alcohol cessation–a VA cooperative study. Stat Med. 1996;15(13):1421-1434. doi: [DOI] [PubMed] [Google Scholar]
- 18.Lane D, Lawson A, Burns A, et al. ; endorsed by the European Society of Hypertension (ESH) Working Group on Cardiovascular Pharmacotherapy and Adherence . Nonadherence in hypertension: how to develop and implement chemical adherence testing. Hypertension. 2022;79(1):12-23. doi: 10.1161/HYPERTENSIONAHA.121.17596 [DOI] [PubMed] [Google Scholar]
- 19.Conover WJ, Iman RL. Analysis of covariance using the rank transformation. Biometrics. 1982;38(3):715-724. doi: 10.2307/2530051 [DOI] [PubMed] [Google Scholar]
- 20.Burnier M, Egan BM. Adherence in hypertension. Circ Res. 2019;124(7):1124-1140. doi: 10.1161/CIRCRESAHA.118.313220 [DOI] [PubMed] [Google Scholar]
- 21.Jaeger BC, Bress AP, Bundy JD, et al. Longer-term all-cause and cardiovascular mortality with intensive blood pressure control: a secondary analysis of a randomized clinical trial. JAMA Cardiol. 2022;7(11):1138-1146. doi: 10.1001/jamacardio.2022.3345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grassi G, Mark A, Esler M. The sympathetic nervous system alterations in human hypertension. Circ Res. 2015;116(6):976-990. doi: 10.1161/CIRCRESAHA.116.303604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kandzari DE, Mahfoud F, Weber MA, et al. Clinical trial design principles and outcomes definitions for device-based therapies for hypertension: a consensus document from the Hypertension Academic Research Consortium. Circulation. 2022;145(11):847-863. doi: 10.1161/CIRCULATIONAHA.121.057687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahfoud F, Azizi M, Ewen S, et al. Proceedings from the 3rd European Clinical Consensus Conference for clinical trials in device-based hypertension therapies. Eur Heart J. 2020;41(16):1588-1599. doi: 10.1093/eurheartj/ehaa121 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
Statistical analysis plan
eAppendix. Population definitions
eTable 1. Angiographic and procedural characteristics in the ultrasound renal denervation group (uRDN) and in the sham group
eTable 2. Bang and James blinding indices at hospital discharge and 2 months
eTable 3. Patients with change in antihypertensive medications prior to the 2-month visit in the ultrasound renal denervation group (uRDN) and in the sham group (intention-to-treat population)
eTable 4. Tipping point analysis for primary endpoint of daytime ambulatory systolic blood pressure
eTable 5. Incidence of major adverse events adjudicated by the clinical events committee (primary safety endpoint)
eTable 6. Change in ambulatory, office, and home blood pressure at 2 months in the ultrasound renal denervation group (uRDN) and in the sham group in the modified-intention-to-treat population (n = 146 uRDN, n = 68 sham)
eTable 7. Change in ambulatory blood pressure at 2 months in the ultrasound renal denervation group (uRDN) and in the sham group in patients with complete ambulatory blood pressure data both at baseline and 2 months (n = 145 uRDN, n= 73 sham)
eTable 8. Change in ambulatory, office, and home blood pressure at 2 months in the ultrasound renal denervation group (uRDN) and in the sham group in the per-protocol population (n = 131 uRDN, n = 63 sham)
eTable 9. Change in ambulatory blood pressure at 2 months in the ultrasound renal denervation group (uRDN) and in the sham group in the as-treated population (n = 149 uRDN, n = 74 sham)
eTable 10. Number and percentage of patients with a decrease in daytime ambulatory blood pressure at 2 months ≥ 5 mmHg, ≥ 10 mmHg, and ≥ 15 mmHg in the ultrasound renal denervation group (uRDN) and in the sham group among patients with ambulatory blood pressure measurements at 2 months (n = 145 uRDN, n = 73 sham)
eTable 11. Number and percentage of patients with controlled blood pressure according to ambulatory and office blood pressure measurements in the ultrasound renal denervation group (uRDN) and in the sham group
eTable 12. Changes in ambulatory, office, and home heart rate at 2 months in the ultrasound renal denervation group (uRDN) and in the sham group in the intention-to-treat population
eTable 13. Estimated glomerular filtration rate (eGFR) and serum creatinine at baseline and 2 months in the ultrasound renal denervation group (uRDN) and in the sham group for subjects with data at both timepoints (n = 136, uRDN; n = 72, sham)
eFigure 1. Time course evolution of home (top) and office (bottom) systolic blood pressure (SBP) changes between baseline, 1, and 2 months in the ultrasound renal denervation group (uRDN, blue line) and in the sham (grey line) group (intention-to-treat population)
eFigure 2. Frequency distribution plots of daytime (A), nighttime (B), 24h (C), home (D) and office (E) systolic blood pressure (SBP) changes from baseline to 2 months in the ultrasound renal denervation group (uRDN, blue line) and in the sham (grey line) group
eFigure 3. Forest plot of between-group differences (95%CI) in daytime ambulatory systolic blood pressure changes across pre-specified sub-groups in favor of ultrasound renal denervation group (uRDN) vs sham
Nonauthor collaborators
Data sharing statement