Abstract
Background and purpose:
Obesity is a public health problem and the existence of beige adipocytes has got interested as a potential therapeutic involvement for obesity and obesity-associated diseases. Adipose tissue M1 macrophage inhibition, also, has a vital role in obesity via down-regulating adipose tissue inflammation and the use of natural compounds such as oleic acid with exercise has been proposed. The present study aimed to evaluate the possible effects of oleic acid and exercise on diet-induced thermogenesis and obesity in rats.
Experimental approach:
Wister albino rats were categorized into six groups. Group I: normal control, group II: oleic acid group (9.8 mg/kg; orally), group III: high-fat diet (HFD), group IV: HFD plus oleic acid, group V: HFD plus exercise training, group VI: HFD plus exercise training and oleic acid.
Findings/Results:
Oleic acid administration and/or exercise significantly decreased body weight, TG, and cholesterol, as well as elevated HDL levels. Furthermore, oleic acid administration and/or exercise reduced serum MDA, TNF-α, and IL-6 levels, elevated the levels of GSH and irisin, increased the expression of UCP1, CD137, and CD206, and reduced CD11c expression.
Conclusion and implications:
Oleic acid supplementation and/or exercise could be used as therapeutic agents for treating obesity via its antioxidant and anti-inflammatory activities, stimulation of beige adipocyte differentiation, and macrophage M1 inhibition.
Keywords: Beige adipocyte, CD137, Macrophage M1, Oleic acid, TNF-α
INTRODUCTION
Obesity, one of the major medical problems of the 21st century, is an important risk factor for the origin of cardiovascular diseases, insulin resistance, and type 2 diabetes. High-fat diet (HFD) is a hallmark of obesity and metabolic diseases that have been casually associated with elevated circulating lipids such as non-esterified fatty acids and triacylglycerol(1).
White adipose tissue (WAT) is an endocrine organ secreting several types of adipocytokines, such as adiponectin, tumor necrosis factor-α (TNF-α), leptin, and plasminogen activator inhibitor-1(2). WAT in obese subjects induced secretion of undesirable adipocytokines provoking obesity and metabolic syndrome(3). In addition, obese adipose tissue is famed for the infiltration of macrophages, which stimulates the formation of inflammatory cytokines(4). like TNF-α, interleukin (IL)-1, and IL-6 which have been related to mediate fatty acid and cholesterol synthesis via regulation of fatty acid synthetase and hepatic beta-hydroxy-beta-methylglutaryl coenzyme A (HMG-CoA reductase)(5). Wu et al.(6) reported that thermogenic adipocytes located in mouse WAT and named them beige adipocytes that were characterized by high mitochondrial content, these beige adipocytes elevated the expression of uncoupling proteins (UCPs) that are involved in weight loss(7).
Physical inactivity provokes visceral fat accumulation causing systemic inflammation. The skeletal muscle is considered an endocrine organ activated by exercise(8) releasing peroxisome proliferator-activated receptor gamma coactivator 1 alpha, modulating lipid, and inducing the fibronectin type III domain containing 5 gene expression which is cleaved and stimulates irisin hormone secretion(9). Irisin promotes browning of WAT and stimulates thermogenesis(10) resulting in an improvement of obesity.
A high-fat or high-carbohydrate consumption leads to the accumulation of excess body fat, while a low incidence of atherosclerosis and improvement of impaired pancreatic β-cell secretory function have been associated with the intake of diets rich in monounsaturated fatty acids, especially oleic acid, 18:1(n-9)(11). Oleic acid is not found only in olive oil, but also in many vegetable oils (e.g. high-oleic varieties of soybean and canola), nuts, fruits, and animal products (e.g. beef, pork, and eggs). Diets supplemented with monounsaturated oleic acid can be correlated with valuable outcomes on body structure, thereby adding to the controlling and inhibition of obesity. Also, a product of oleic acid, oleoylethanolamide, has been shown to decrease hunger and consequent food intake(12). Consequently, the purpose of this study was to investigate the effects of oleic acid and/or exercise on obesity induced by HFD and their effects on beige adipocytes and macrophages (M1 macrophages) inducing metabolic disorder and inflammation in obese rats.
MATERIAL AND METHODS
Animals
Sixty adult male Wister albino rats (7 to 8 weeks old, weighing 120-180 g) were purchased from National Research Centre, Egypt. They were housed in standard cages (10 rats each; 25 × 30 × 30 cm cage), under specific pathogen-free conditions in facilities maintained at controlled room temperature (21-24 °C) with a 40-60% relative humidity, and under normal dark/light cycles. All animals had access to a rat chow diet and water ad libitum and were acclimated for two weeks prior to the initiation of the experiment. The animal experiments were performed according to recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (NIH No. 85:23 revised 1985) in accordance with the guidelines provided by National Research Centre, Egypt.
Diets
The commercial rat chow diet (balanced diet), containing 67% carbohydrates, 10% fat, and 23% protein as the energy sources (overall calories: 3.6 kcal/g), was purchased from El Gomhorya Company, Cairo, Egypt. HFD was composed of the following energy sources: 36.2% carbohydrates, 44.8% fat, and 19% protein (overall calories: 4.6 kcal/g)(13,14).
Chemicals
Oleic acid was obtained from Merck, Germany.
Experimental design
The rats were divided into 6 equal groups (10 each). Group I: rats were given normal balanced chow, received saline (10 mg/kg) orally, for 12 weeks and served as the normal control group. Group II: rats were given normal balanced chow and received oleic acid orally (9.8 mg/kg) according to Gonçalves-de-Albuquerque et al. who used 0.28 mg of oleic acid for mice weighing 20 g equivalent to 1.96 mg to rat 200 mg using Pagetś scale(15) for 12 weeks. Group III: HFD group, rats were given HFD and received saline orally, for 12 weeks to induce obesity(14). Group IV: HFD-oleic acid group, rats were kept on HFD for 12 weeks and then received oleic acid for 6 weeks. Group V: HFD-exercise group, rats were kept on HFD for 12 weeks then underwent exercise training for 6 weeks. Group VI: HFD-oleic acid-exercise group, rats were received HFD for 6 weeks and then rats were received oleic acid orally and exercised for 6 weeks with HFD to th e end of 12th week.
Exercise program
Rats were habituated to the swimming exercise during the first week in a tank filled with water maintained at 35 °C. Initially, rats swam for 15 min; this swimming time was then increased in 15 min increments daily until a swimming period of 1 h was achieved. Subsequently, a daily swimming period of 1 h five times per week was maintained for 6 weeks. At the end of each exercise session, animals were dried and kept in a warm environment(16).
Serum and tissue preparation
At the end of the experiment, after overnight fasting, rats were anesthetized in the morning, and blood samples were collected from retro-orbital venous plexus by capillary tubes under light anesthesia using pentobarbital sodium(17). The blood was then centrifuged at 3000 rpm for 15 min for serum collection. Serum was separated in aliquots in Eppendorf tubes and stored frozen at-80 °C until analysis. The separated serum was analyzed for estimation of the levels of lipid profile and oxidative stress markers. The visceral adipose tissues (VATs) were excised and dissected out and rinsed with PBS to remove excess blood. Parts from both kidneys were homogenized (MPW-120 homogenizer, Med instruments, Poland) to obtain 20% homogenate that was stored overnight at-20 °C. The homogenates were centrifuged for 5 min at 5000 g using a cooling centrifuge (Sigma and Laborzentrifugen, 2k15, Germany). The supernatant was collected and stored at-80 °C(18) and then used for the measurement of inflammatory markers, irisin hormone, and gene expression of adipose tissue UCP1, and CD137.
Biochemical analysis
Estimation of lipid profile and oxidative stress biomarkers
Serum triglycerides (TGs), total cholesterol (TC), and high-density lipoprotein (HDL) were determined according to Burstein et al.(1970), Fossati & Prencipe (1982), and Richmond W. (1973), respectively(19,20,21).
Reduced glutathione (GSH) and malondialdehyde (MDA) were measured using kits Biodiagnostic Co., Egypt. The GSH estimation is based on the reduction of 5,5 dithiobis (2-nitrobenzoic acid) (DTNB) to produce a yellow compound. The reduced chromogen is directly proportional to GSH concentration and its absorbance can be measured at 405 nm(22). The MDA estimation method depends on the formation of the MDA end product of lipid peroxidation which reacts with thiobarbituric acid producing a thiobarbituric acid reactive substance, a pink chromogen, which can be measured spectrophotometrically at 532 nm(23).
Estimation of adipose contents of IL-6, TNF-α, and irisin
IL-6 and TNF-α were measured by ELISA kits (Ray Biotech, Inc. Norcross, GA, USA) and irisin was measured by ELISA kits (MyBiosource, Inc.).
Adipose contents of IL-6, TNF-α, and irisin were determined using an ELISA kit. Standards and samples were pipetted into wells with immobilized antibodies specific for rat IL-6, TNF-α, and irisin and then were incubated for 30 min at 37 °C. After incubation and washing, horseradish peroxidase-conjugated streptavidin was pipetted into the wells and incubated for 30 min at 37 °C, which were washed once again. Tetramethylbenzidine (TMB) substrate solution was added to the wells and incubated for 15 min at 37 °C; a color was developed proportionally to the amount of IL-6, TNF-α, and irisin bound. Color development was discontinued (stop solution) and after 10 min color intensity was measured at 450 nm(24).
Quantitative real-time reverse transcription polymerase chain reaction analysis of UCP-1 and CD137 in VAT
RNA was extracted, reversely transcribed into cDNA and amplified by quantitative real-time polymerase chain reaction qRT-PCR and then detected using agarose gel electrophoresis(25). Total RNA was extracted from the tissue using TRIzol reagent (Invitrogen, San Diego, California, USA). The concentration of total RNA was measured by absorbance at 260/280 nm. The reverse transcription reaction for the first-strand cDNA synthesis was carried out with reverse transcriptase (Bio-Rad, Hercules, California, USA) using 2 μg of total RNA. RT-PCR was initiated on Step One Plus Real Time. PCR system (ABI Applied Biosystems, San Francisco, USA) using Power SYBR green PCR master mix. The cycling conditions were as follows: 12 min at 95 °C, followed by 40 cycles of 15 s at 95 °C and 1 min at 60 °C. The expression of β-actin served as the internal control(26). The primers’ sequences used are indicated in Table 1.
Table 1.
List of primers used in a quantitative real-time polymerase chain reaction.
| Genes | Forward (5' to 3') | Reverse (5' to 3') |
|---|---|---|
| β-actin | GCTTCTTTGCAGCTCCTTCGT | ATATCGTCATCCATGGCGAAC |
| UCP1 | CGACTCAGTCCAAGAGTACTTCTCTTC | GCCGGCTGAGATCTTGTTTC |
| CD137 | AACATCTGCAGAGTGTGTGC | AGACCTTCCGTCTAGAGAGC |
Histopathological examination
A midline incision about 4 cm in length was made in the abdomen, and the intra-viceral bad of fat (VAT) was dissected out from the base of the skull to the distal tibia, then fixed in 10% neutral formalin and routinely processed. Tissue sections of 5 μm thickness were cut and stained by hematoxylin and eosin (H&E). Ten sections per group were examined by light microscopy for evaluation of the histopathological alterations. The mean adipocyte diameter was estimated, according to the method of Wentworth et al.(27) with some modifications, in ten random high microscopic fields (40×).
Immunohistochemical analysis
CD68 immunohistochemical staining was performed for assessment of the crown density. While CD11c and CD206 immunohistochemical staining were performed for quantification of inflammatory M1 subtype adipose tissue macrophages (ATMs) and anti-inflammatory M2 subtype ATMs, respectively. The tissue sections were deparaffinized in xylene and rehydrated in ethanol. Antigen retrieval was performed by heating in sodium citrate solutions for 30 min. For blocking the endogenous peroxidase activity, the sections were immersed in 0.3% H2O2 in methanol for 15 min. The sections were subsequently incubated with the primary antibodies, mouse anti-rat CD68 (Cat# ab53444), mouse anti-rat CD11c (Cat# ab11029), and Rabbit anti-rat CD206 (Cat# ab64693) at 4 °C overnight. The sections were then incubated with anti-mouse IgG3 (Dako) as the secondary antibody. The immune reaction was visualized using diaminobenzene. Finally, the sections were counterstained with hematoxylin. The crown density was assessed according to the method of Wentworth et al.(27). Three or more CD68-positive cells surrounding an adipocyte are considered a crown. Crown density is the number of crowns per high microscopic power field (40×). On the other hand, CD11c and CD206 immune-stained cells were counted in five random high microscopic fields (40×)(28).
Statistical analysis
All the values are presented as means ± SEM. Comparisons between different groups were carried out using a one-way analysis of variance (ANOVA) followed by Tukey's multiple comparisons test. GraphPad Prism software, version 5 (Inc., San Diego, USA) was used to carry out these statistical tests. The difference was considered significant when P-values were smaller than 0.05.
RESULTS
Effect of exercise and/or oleic acid on body weight and serum lipid profile
HFD for 12 weeks elevated body weight by 52% in comparison with normal control rats; exercise, oleic acid administration, and their combination normalized body weight, furthermore, HFD-induced dyslipidemia that evidenced by a significant elevation of TG by 46% and TC by 43%, and decrease in HDL-C by 49% as compared to normal control. On the other hand, exercise and/or oleic acid administration showed a significant reduction of TG by 22%, 13%, and 26%, respectively, and TC by 18%, 17%, and 25%, respectively, and an increase in HDL-C by 21%, 44%, and 57% as compared to HFD group. In addition, exercise with oleic acid administration showed a better significant reduction of TG and TC levels by 15% and 9%, respectively and elevation of HDL level by 9% than oleic acid alone (Table 2).
Table 2.
Effect of exercise and/or oleic acid on body weight and serum lipid profile. Data are expressed as mean ± SEM.
| Parameters | Normal control | Oleic acid (9.8 mg/kg) | HFD | HFD + exercise | HFD + oleic acid | HFD + exercise + oleic acid |
|---|---|---|---|---|---|---|
| Body weight (g) | 123.0 | 127.0 | 187.2 | 126.0 | 128.4 | 123.6 |
| ± 0.45 | ± 2.30b | ± 5.23a | ± 1.14b | ± 1.86b | ± 0.68b | |
| Total cholesterol (mg/dL) | 166.29 | 171.70 | 237.70 | 193.82 | 196.50 | 179.12 |
| ± 1.56 | ± 2.95b | ± 0.79a | ± 1.58ab | ± 1.63ab | ± 1.04abc | |
| Triglyceride (mg/dL) | 91.67 | 96.07 | 133.70 | 104.28 | 116.36 | 99.28 |
| ± 0.88 | ± 1.50b | ± 2.37a | ± 1.39ab | ± 3.28ab | ± 0.88abc | |
| High-density lipoprotein (mg/dL) | 73.21 | 72.81 | 37.70 | 45.48 | 54.24 | 59.32 |
| ± 1.25 | ± 0.91b | ± 0.79a | ± 1.65ab | ± 0.29ab | ± 0.50ab |
HFD, High-fat diet; aP < 0.05 Indicates significant differences compared to the normal control (saline); bP < 0.05 versus HFD control; cP < 0.05 against the oleic acid group.
Effect of exercise and/or oleic acid on oxidative stress
MDA serum level was elevated after HFD supplementation by 13.45 folds and serum GSH activity was reduced by 62% compared to the normal control. Notably, exercise and/or oleic acid administration successfully declined MDA serum levels by 57%, 54%, and 59%, respectively, and elevated serum GSH activities by 62%, 58%, and 75%, respectively, as compared with the HFD group. Moreover, exercise with oleic acid administration showed a better significant elevation of GSH level by 10% than oleic acid alone (Table 3).
Table 3.
Effect of exercise and/or oleic acid on oxidative stress. Data are expressed as mean ± SEM.
| Parameters | Normal control | Oleic acid (9.8 mg/kg) | HFD | HFD + exercise | HFD + oleic acid | HFD + exercise + oleic acid |
|---|---|---|---|---|---|---|
| Reduced glutathione (μΜ/mL) | 84.81 ± 1.72 | 83.42 ± 1.59b | 31.82 ± 0.01a | 51.68 ± 1.44ab | 50.44 ± 1.65ab | 55.56 ± 2.05abc |
| Malondialdehyde (nmol/mL) | 3.81 ± 0.05 | 3.82 ± 0.01b | 55.02 ± 1.96a | 23.52 ± 0.40ab | 25.28 ± 1.58ab | 22.36 ± 0.97ab |
HFD, High fat diet; aP < 0.05 Indicates significant differences compared to the normal control (saline); bP < 0.05 versus HFD control; cP < 0.05 against the oleic acid group.
Effect of exercise and/or oleic acid on inflammation
Adipose contents of IL-6 and TNF-α were elevated after HFD supplementation by 2.87 and 1.11 folds, respectively, compared to the normal control. Notably, exercise and/or oleic acid administration ameliorated adipose contents of IL-6 by 35%, 32%, and 50%, respectively, and TNF-α by 49%, 48%, and 52%, respectively, as compared with the HFD group. In addition, exercise with oleic acid administration showed a better significant reduction of IL-6 levels by 26% than oleic acid alone (Fig. 1).
Fig. 1.
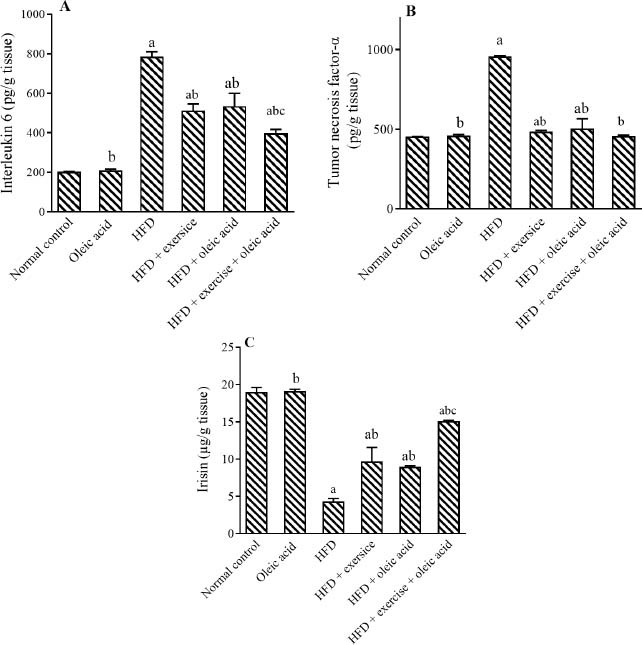
Effect of exercise and/or oleic acid on adipose contents of (A) interleukin 6, (B) tumor necrosis factor-α, and (C) Irisin. HFD, High-fat diet; aP < 0.05 Indicates significant diffrences compared to the normal control (saline); bP < 0.05 versus HFD control; c P < 0.05 against the oleic acid group.
Effect of exercise and/or oleic acid on adipose content of irisin
HFD showed a marked reduction in adipose contents of irisin level by 77% compared to its respective control. Conversely, exercise and/or oleic acid administration increased adipose contents of irisin level by 2.49, 2.07, and 2.25 folds, respectively, as compared with the HFD group. Moreover, exercise with oleic acid administration increased irisin more by 69% than oleic acid alone (Fig. 1).
Effect of exercise and/or oleic acid on adipose tissue UCP1 and CD137 gene expression
HFD showed a significant reduction in adipose tissue UCP1 gene expression by 74% and beige fat-specific marker CD137 gene expression by 97% compared to their respective controls. Conversely, exercise and/or oleic acid administration increased CD137 expression by 20.51, 17.72, and 21.33 folds, respectively, as compared with HFD group. Exercise and its combination with oleic acid administration increased UCP1 expression by 1.26 and 1.29 folds, respectively, while oleic acid administrated with HFD did not change UCP1 gene expression as compared with HFD group. In addition, exercise with oleic acid administration showed a better significant elevation of UCP1 and CD 137 by 18% and 19%, respectively, than oleic acid alone (Fig. 2).
Fig. 2.
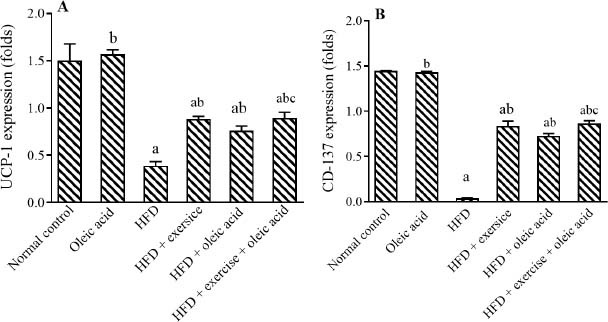
Effect of exercise and/or oleic acid on adipose tissue (A) UCP1 and (B) CD137 gene expression. HFD, High-fat diet; aP < 0.05 Indicates significant differences compared to the normal control (saline); bP < 0.05 versus HFD control; cP < 0.05 against the oleic acid group.
Histopathology
VAT of both control and oleic acid-supplemented rats showed normal WAT with large round adipocytes, with a mean diameter of 66.86 ± 3.16 and 67.34 ± 2.95, respectively, surrounded by a thin cytoplasmic rim (Fig. 3A and B, respectively). Whereas VAT of the HFD group revealed marked expansion and hypertrophy of adipocytes with a mean diameter of 97.78 ± 1.76 which is significantly different from the normal control group. One of the most characteristic lesions demonstrated in this group was the presence of numerous necrotic adipocytes which are surrounded by macrophages forming a distinctive crown-like structure (Fig. 3C) associated with intense macrophage and lymphocytic cell infiltration. On the contrary, a marked decrease in adipocytes size with a mean diameter of 59.52 ± 2.29, associated with mild leukocytic cell infiltration was recorded in the HFD-exercise group (Fig. 3D). Little improvement was recorded in the HFD-oleic acid group in which the mean diameter of adipocytes was 91.40 ± 3.11 (Fig. 3E). Pronounced amelioration with, mean adipocyte diameter of 58.63 ± 2.67, minimal macrophages and lymphocytic cell infiltration and sparse necrotic adipocytes were observed in HFD-exercise-oleic acid group (Fig. 3F).
Fig. 3.
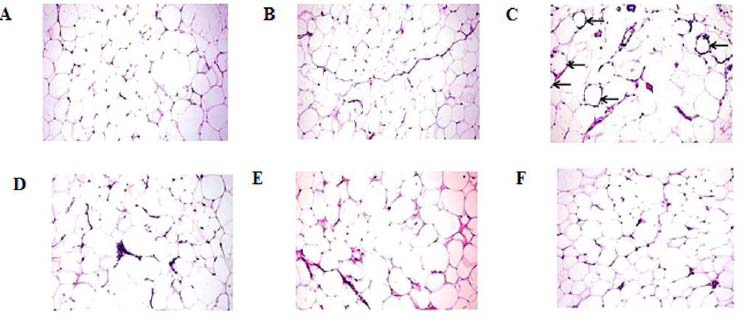
Photomicrograph of the visceral adipose tissues stained with hematoxylin and eosin of (A) control rats shows normal white adipose tissue with large round adipocytes, (B) oleic acid group showing normal adipose tissue, (C) HFD group showing numerous necrotic adipocytes surrounded by macrophages forming distinctive crown-like structure (arrows), (D) HFD-exercise group showing mild leukocytic cell infiltration, (E) HFD-oleic acid group showing large adipocytes, and (F) HFD-exercise-oleic acid group showing minimal macrophages and lymphocytic cell infiltration; magnification 20×. HFD, High-fat diet.
Immunohistochemistry
CD68 immunohistochemical staining of VAT of normal control and oleic acid-treated groups revealed few immune stained cells with the mean crown intensity of 0.6 ± 0.24 and 0.8 ± 0.37, respectively (Fig. 4A and B). The crown intensity was significantly increased in the HFD group (3.8 ± 0.37) in addition to abundant interstitial immune stained cells (Fig. 4C). On the other hand, a noticeable reduction of crown intensity was recorded in the HFD-exercise group (1.4 ± 0.24; Fig. 4D). HFD-oleic acid group showed a mild decrease of crown intensity (2.0 ± 0.32; Fig. 4E). In contrast, a significant decrease of CD68 positive cells with reduction of crown intensity was recorded in HFD-exercise-oleic acid group (0.8 ± 0.20; Fig. 4F) which was insignificant from the normal control group.
Fig. 4.
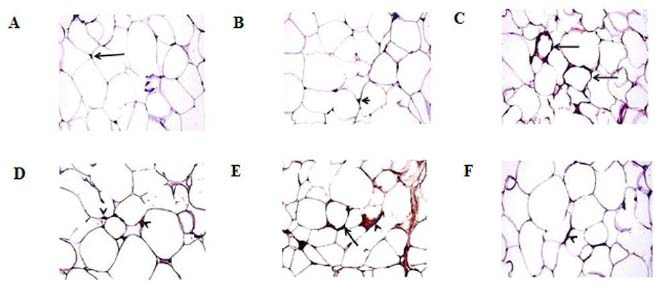
Photomicrograph of the visceral adipose tissues stained for CD68 shows crown (arrow) and resident adipose tissue macrophages (arrowhead) of (A) control group, (B) oleic acid group, (C) HFD group, (D) HFD-exercise group, (E) HFD-oleic acid group, and (F) HFD-exercise-oleic acid group. CD68 immunohistochemical staining, magnification 40×. HFD, High-fat diet.
CD11c and CD206 immunohistochemical staining of VAT revealed a significant increase of CD11c positive cells in the HFD group (19.6 ± 0.81), which surround adipocytes forming crown or aggregate in the form of syncytial giant cells (Fig. 5C), compared to normal control (3.0 ± 0.55; Fig. 5A) and oleic acid-treated group (4.4 ± 1.03; Fig. 5B) but CD206 positive cells were significantly decreased in HFD group (2.0 ± 0.32; Fig. 6C) compared to the normal control (6.2 ± 0.85; Fig. 6A) and oleic acid-treated group (4.0 ± 0.45; Fig. 5B). Significant reductions of CD11c positive cells were recorded in HFD-exercise group and HFD-oleic acid group (9.6 ± 0.40 and 11.6 ± 1.94, respectively; Fig. 5D and E) compared to HFD group. While a significant increase of CD206 positive cells was recorded in the HFD-exercise group and HFD-oleic acid group (3.6 ± 0.24 and 5.2 ± 0.58, respectively; Fig. 6D and E). HFD-exercise-oleic acid group revealed a remarkable decrease of CD11c positive cells (6.0 ± 1.26; Fig. 5F) which is significantly different from the other treated groups. Additionally, CD206 positive cells were markedly increased (5.6 ± 0.60; Fig. 6F) which was insignificantly different from the normal control group.
Fig. 5.
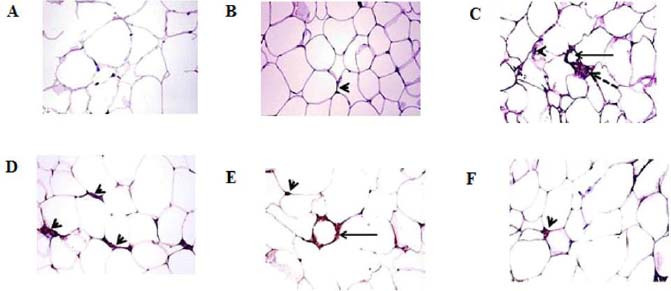
Photomicrograph of the visceral adipose tissues stained for CDllc shows crown (arrow), syncytial giant cell (dashed arrow), and resident adipose tissue macrophages (arrowhead) of (A) control group, (B) oleic acid group, (C) HFD group, (D) HFD-exercise group, (E) HFD-oleic acid group, and (F) HFD-exercise-oleic acid group. CD11c immunohistochemical staining, magnification 40×. HFD, High-fat diet.
Fig. 6.
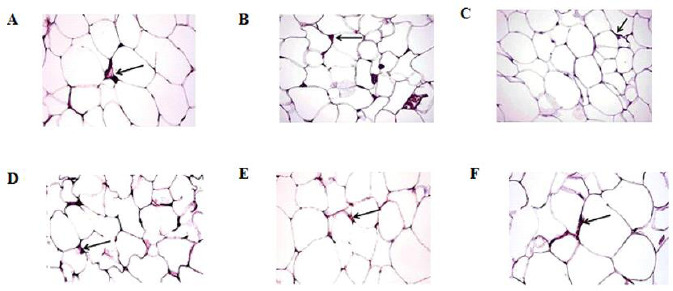
photomicrograph of the visceral adipose tissues stained for CD206 shows positive cells at the junction of two or more adipocytes (arrow) of (A) control group, (B) oleic acid group, (C) HFD group, (D) HFD-exercise group, (E) HFD-oleic acid group, and (F) HFD-exercise-oleic acid group. (CD206immunohistochemical staining, 40×. HFD, High fat diet.
DISCUSSION
Obesity is often associated with oxidative stress emergence(29). Oxidative stress is a candidate mediator of inflammatory and metabolic disease states, including obesity(30). Free radicals affect cell survival and damage cell membrane via the oxidative damage of lipids and protein and irreparable DNA alterations(31). This study demonstrated that HFD caused a significant increase in total weight gain, TG, and cholesterol, and decreased HDL-cholesterol, with induction of oxidative stress that was evidenced by increased serum level of MDA and GSH depletion. Administration of antioxidants neutralized cumulative damage induced by free radicals in the body, as in this study, oleic acid treatment and/or exercise reduced weight gain, TG, and cholesterol and increased HDL-cholesterol via reduction of fatty acid synthesis, triglyceride accumulation, and cholesterol biosynthesis(32,33). In addition, oleic acid treatment with exercise produced a significant decrease in the levels of MDA and an elevation in GSH activity when compared to obese rats. These results were confirmed previously as virgin olive oil is a source of antioxidants as monounsaturated fat (oleic acid) reducing the oxidative damage in cancer diseases provoked by diethylnitrosamine(34), moreover, physical exercise reduced oxidative stress ameliorating depression induced by reserpine through decreasing GSH levels(16). These findings suggested the beneficial effect of combining oleic acid treatment with exercise in this study in alleviating obesity induced by HFD.
Obesity stimulates chronic low-degree inflammation and intrinsic immune system activation(35). Obese adipose tissue includes further immune cells, involving M1-polarized pro-inflammatory macrophages which generate pro-inflammatory cytokines like IL-6, TNF-α, and IL-Ιβ(36). Our results exhibited that HFD elevated the production of TNF-α and IL-6 from macrophages. In adipose tissue, TNF-α has a high correlation with the body mass index(37), provokes liver lipogenesis through degrading triacyl-glycerol in the adipocytes, induces hepatic very-low-density lipoprotein production regulating body fat metabolism(38). TNF-α and IL-6 levels, in the current study, were suppressed after oleic acid treatment and/or exercise that regulates adipose tissue inflammation via the inhibition of M1 macrophage activity. Oleic acid, in another study, was able of reducing the inflammatory mediators in breast, ovarian, and stomach cancer by inhibiting intercellular adhesion molecule-1 expression and nuclear factor kappa B (NF-KB) phosphorylation(39).
Irisin hormone has a vital role in obesity and is described as a myokine that induces white fat “browning” and thermogenesis stimulating UCP1 expression(40). Swimming as an example of exercise elevated irisin levels and UCP1 expression provoking white adipocytes browning alleviating obesity(41). In the present study, HFD reduced thermogenesis as evidenced by decreased levels of irisin, UCP1, and CD137 expressions (beige adipocyte markers). These results are in accordance with the results of Tanaka-Yachi et al.(42). In this work, for the first time, oleic acid treatment with exercise elevated irisin level, UCP1, and CD137 expressions inducing browning and thermogenesis. These results suggest the use of oleic acid with exercise in obesity treatment.
Concerning histopathological alterations and immunohistochemical investigations, our results revealed the expansion of visceral adipocytes in HFD-fed animals which is in accordance with the findings of a study conducted by Huang et al.(43). The hypertrophied adipocytes enhance the secretion of free fatty acids which cause infiltration of macrophages and a switch in macrophage phenotypes into MI subtype which is confirmed in CD11c immunohistochemically stained sections. MI subtype ATMs produce pro-inflammatory cytokines such as IL-6(44). Adipocyte necrosis demonstrated in the HFD group is attributed to reactive oxygen species produced by M1 subtype ATMs(45). Additionally, increased adipocyte size with subsequent dysfunctional adipose tissue(46) is correlated with increased crown density and increased M1 subtype ATMs confirming the macrophages phenotypic shift. The crown is a dead adipocyte that is surrounded by a cluster of macrophages mostly CD11c subtype as confirmed in the present study and reported by another study(47). Moreover, CD11c subtype macrophage plays a vital role in glucose intolerance and metabolic disorder as confirmed by Shaul et al.(48). On the other hand, a significant decrease of CD206 positive cells in the HFD group denotes decreased M2 macrophages that produce anti-inflammatory cytokines and inhibit the inflammatory response(49). Shiomi and Watanabe showed that M1 macrophages treated with oleic acid were smaller than M1 macrophages activated by lipopolysaccharide(50). Our histopathological study indicated that oleic acid treatment and/or exercise significantly attenuate the pathological alterations in the HFD group and ameliorate obesity via a significant reduction of adipocyte size and crown-like structure relying on its potent anti-inflammatory and antioxidant properties.
CONCLUSION
Oleic acid treatment with exercise has proven to be efficient in the treatment of obesity through its inhibitory effect on macrophages M1 producing inflammatory cytokines, reducing oxidative stress, and inflammation as well as stimulating beige adipocyte differentiation that leads to overexpression of irisin, UCP2, and CD137 in obese rats.
Conflicts of interest statement
The authors declared no conflict of interest in this study.
Authors’ contributions
A. Salama contributed to the conceptualization, methodology, validation, formal analysis, investigation, resources, and writing, reviewing, and editing of the article; M.M. Amina provided the resources and edited the manuscript; A. Hassan contributed to the methodology, investigation, and writing of histopathology part. The finalized article was approved ay all authors.
Acknowledgments
The authors would like to thank the Pharmacology Department, National Research Centre, Egypt for supplying instrumental facilities to conduct the current study.
REFERENCES
- 1.Timilsina S, Timilsina S, Mandal A, Paudel R, Gayam V. Triad of diabetic ketoacidosis, hypertriglyceridemia, and acute pancreatitis: severity of acute pancreatitis may correlate with the level of hypertriglyceridemia. Cureus. 2019;11(6):1–5. doi: 10.7759/cureus.4930. e4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumari R, Kumar S, Kant R. An update on metabolic syndrome: metabolic risk markers and adipokines in the development of metabolic syndrome. Diabetes Metab Syndr. 2019;13(4):2409–2417. doi: 10.1016/j.dsx.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Su R, Yan J, Yang H. Transgenerational glucose intolerance of tumor necrosis factor with epigenetic alteration in rat perirenal adipose tissue induced by intrauterine hyperglycemia. J Diabetes Res. 2016;2016:1–14. doi: 10.1155/2016/4952801. 4952801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peterson KR, Flaherty DK, Hasty AH. Obesity alters B cell and macrophage populations in brown adipose tissue. Obesity. 2017;25(11):1881–1884. doi: 10.1002/oby.21982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu SY, Liu Z, Chung S, Kim YC. Obesity-induced tumor necrosis factor alpha suppresses in vivo and in vitro retinoic acid synthesis and fatty acid oxidation in adipose tissue. Curr Dev Nutr. 2021;5(Suppl 2):955–955. doi: 10.1093/cdn/nzab050_022. [DOI] [Google Scholar]
- 6.Sidossis L, Kajimura S. Brown and beige fat in humans: thermogenic adipocytes that control energy and glucose homeostasis. J Clin Invest. 2015;125(2):478–486. doi: 10.1172/JCI78362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang J, Wu H, Ma S, Jing F, Yu C, Gao L, et al. Transcription regulators and hormones involved in the development of brown fat and white fat browning: transcriptional and hormonal control of brown/beige fat development. Physiol Res. 2018;67(3):347–362. doi: 10.33549/physiolres.933650. [DOI] [PubMed] [Google Scholar]
- 8.Keller P, Vollaard NB, Gustafsson T, Gallagher IJ, Sundberg CJ, Rankinen T, et al. A transcriptional map of the impact of endurance exercise training on skeletal muscle phenotype. J Appl Physiol (1985) 2011;110(1):46–59. doi: 10.1152/japplphysiol.00634.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jung S, Kim K. Exercise-induced PGC-1α transcriptional factors in skeletal muscle. Integr Med Res. 2014;3(4):155–160. doi: 10.1016/j.imr.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moreno-Navarrete JM, Ortega F, Serrano M, Guerra E, Pardo G, Tinahones F, et al. Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. J Clin Endocrinol Metab. 2013;98(4):E769–E778. doi: 10.1210/jc.2012-2749. [DOI] [PubMed] [Google Scholar]
- 11.Šrámek J, Němcová-Fürstová V, Polák J, Kovář J. Hypoxia modulates effects of fatty acids on NES2Y human pancreatic β-cells. Int J Mol Sci. 2019;20(14):1–12. doi: 10.3390/ijms20143441. 3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tutunchi H, Ostadrahimi A, Saghafi-Asl M. The effects of diets enriched in monounsaturated oleic acid on the management and prevention of obesity: a systematic review of human intervention studies. Adv Nutr. 2020;11(4):864–877. doi: 10.1093/advances/nmaa013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu ZJ, Fan JG, Ding XD, Qiao L, Wang GL. Characterization of high-fat, diet-induced, non-alcoholic steatohepatitis with fibrosis in rats. Dig Dis Sci. 2010;55(4):931–940. doi: 10.1007/s10620-009-0815-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hussain MA, Abogresha NM, Hassan R, Tamany DA, Lotfy M. Effect of feeding a high-fat diet independently of caloric intake on reproductive function in diet-induced obese female rats. Arch Med Sci. 2016;12(4):906–914. doi: 10.5114/aoms.2016.59790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonçalves-de-Albuquerque CF, Medeiros-de-Moraes IM, Oliveira FMdJ, Burth P, Bozza PT, Castro Faria MV, et al. Omega-9 oleic acid induces fatty acid oxidation and decreases organ dysfunction and mortality in experimental sepsis. PLoS One. 2016;11(4):1–18. doi: 10.1371/journal.pone.0153607. e0153607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teixeira AM, Trevizol F, Colpo G, Garcia SC, Charao M, Pereira RP, et al. Influence of chronic exercise on reserpine-induced oxidative stress in rats: behavioral and antioxidant evaluations. Pharmacol Biochem Behav. 2008;88(4):465–472. doi: 10.1016/j.pbb.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 17.El-Baz FK, Salama A, Salama RAA. Dunaliella salina attenuates diabetic neuropathy induced by STZ in rats: involvement of thioredoxin. Biomed Res Int. 2020;2020:1–12. doi: 10.1155/2020/1295492. 1295492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salama AAA, Mostafa RE, Elgohary R. Effect of L-carnitine on potassium dichromate-induced nephrotoxicity in rats: modulation of PI3K/AKT signaling pathway. Res Pharm Sci. 2022;17(2):153–163. doi: 10.4103/1735-5362.335174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burstein M, Scholnick HR, Morfin R. Rapid method for the isolation of lipoproteins from human serum by precipitation with polyanions. J Lipid Res. 1970;11(6):583–595. doi: 10.1016/S0022-2275(20)42943-8. [DOI] [PubMed] [Google Scholar]
- 20.Fossati P, Prencipe L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin Chem. 1982;28(10):2077–2080. doi: 10.1093/CLINCHEM/28.10.2077. [DOI] [PubMed] [Google Scholar]
- 21.Richmond W. Preparation and properties of a cholesterol oxidase from Nocardia sp. and its application to the enzymatic assay of total cholesterol in serum. Clin Chem. 1973;19(12):1350–1356. doi: 10.1093/clinchem/19.12.1350. [DOI] [PubMed] [Google Scholar]
- 22.Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- 23.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 24.Salama A, Fayed HM, Elgohary R. L-carnitine alleviated acute lung injuries induced by potassium dichromate in rats: involvement of Nrf2/HO-1 signaling pathway. Heliyon. 2021;7(6):1–9. doi: 10.1016/j.heliyon.2021.e07207. e07207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han L, Zi X, Garmire LX, Wu Y, Weissman SM, Pan X, et al. Co-detection and sequencing of genes and transcripts from the same single cells facilitated by a microfluidics platform. Sci Rep. 2014;4(1):1–9. doi: 10.1038/srep06485. 6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):2002–2007. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wentworth JM, Naselli G, Brown WA, Doyle L, Phipson B, Smyth GK, et al. Pro-inflammatory CD11c+CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes. 2010;59(7):1648–1656. doi: 10.2337/db09-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dinh CH, Szabo A, Yu Y, Camer D, Zhang Q, Wang H, et al. Bardoxolone methyl prevents fat deposition and inflammation in brown adipose tissue and enhances sympathetic activity in mice fed a high-fat diet. Nutrients. 2015;7(6):4705–4723. doi: 10.3390/nu7064705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu P, Zhang F, Dai Y, Han L, Chen S. Serum TNF-alpha, GTH and MDA of high-fat diet-induced obesity and obesity resistant rats. Saudi Pharm J. 2016;24(3):333–336. doi: 10.1016/jjsps.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brema I. The relationship between plasma Visfatin/Nampt and type 2 diabetes, obesity, insulin resistance and cardiovascular disease. Endocrinol Metab Int J. 2016;3(6):157–163. doi: 10.15406/emij.2016.03.00068. [DOI] [Google Scholar]
- 31.Pandey B, Kumar A, Rastogi L, Mishra K. The involvement of cellular oxidative damage in the apoptotic death induced in γ-irradiated mouse thymocytes. Int J Low Radiat. 2008;5(4):356–367. doi: 10.1504/IJLR.2008.020979. [DOI] [Google Scholar]
- 32.Sato K, Arai H, Mizuno A, Fukaya M, Sato T, Koganei M, et al. Dietary palatinose and oleic acid ameliorate disorders of glucose and lipid metabolism in Zucker fatty rats. J Nutr. 2007;137(8):1908–1915. doi: 10.1093/jn/137.8.1908. [DOI] [PubMed] [Google Scholar]
- 33.Aziz N, Si L, Budin S, Zainalabidin S. Establishment of high-fat diet-induced obesity with myocardial infarction rat model. Int J Cardiol. 2019;297:29. doi: 10.1016/j.ijcard.2019.11.079. [DOI] [Google Scholar]
- 34.Hassanen NH, Ahmed MH. Protective effect of fish oil and virgin olive oil on diethylnitrosamine toxicity in rats. Int J Food Sci Nutr. 2015;4(3):388–396. doi: 10.11648/j.ijnfs.20150403.27. [DOI] [Google Scholar]
- 35.Likitnukul S, Kalandakanond-Thongsong S, Thammacharoen S. Evidence of growth hormone effect on plasma leptin in diet-induced obesity and diet-resistant rats. Asian Biomed. 2018;12(5):219–228. doi: 10.1515/abm-2019-0023. [DOI] [Google Scholar]
- 36.Kang YE, Kim JM, Joung KH, Lee JH, You BR, Choi MJ, et al. The roles of adipokines, proinflammatory cytokines, and adipose tissue macrophages in obesity-associated insulin resistance in modest obesity and early metabolic dysfunction. PLoS One. 2016;11(4):1–14. doi: 10.1371/journal.pone.0154003. e0154003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kern PA, Saghizadeh M, Ong JM, Bosch RJ, Deem R, Simsolo RB. The expression of tumor necrosis factor in human adipose tissue. Regulation by obesity, weight loss, and relationship to lipoprotein lipase. J Clin Invest. 1995;95(5):2111–2119. doi: 10.1172/JCI117899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suo L, Wang W. Effects of liraglutide on omentin-1 and insulin resistance in high-fat diet obese rats. J China Med Univ. 2015;12:1129–1131. [Google Scholar]
- 39.Menendez JA, Papadimitropoulou A, Vellon L, Lupu R. A genomic explanation connecting “Mediterranean diet”, olive oil and cancer: oleic acid, the main monounsaturated fatty acid of olive oil, induces formation of inhibitory “PEA3 transcription factor-PEA3 DNA binding site” complexes at the Her-2/neu (erbB-2) oncogene promoter in breast, ovarian and stomach cancer cells. Eur J Cancer. 2006;42(15):2425–2432. doi: 10.1016/j.ejca.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 40.Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481(7382):463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Badawy E, El-laithy NA, Morsy SM, Ashour MN, Elias TR, Masoud MM, Aly O. Role of swimming on muscle PGC-1α FNDC5 mRNA, and assessment of serum omentin, adropin, and irisin in high carbohydrate high fat (HCHF) diet induced obesity in rats. Egypt J Med Hum Genet. 2020;21(1):1–8. doi: 10.1186/s43042-020-00080-6. [DOI] [Google Scholar]
- 42.Tanaka-Yachi R, Takahashi-Muto C, Adachi K, Tanimura Y, Aoki Y, Koike T, Kiyose C. Promoting effect of alpha-tocopherol on beige adipocyte differentiation in 3T3-L1 cells and rat white adipose tissue. J Oleo Sci. 2017;66(2):171–179. doi: 10.5650/jos.ess16137. [DOI] [PubMed] [Google Scholar]
- 43.Huang IC, Lee JL, Ketheeswaran P, Jones CM, Revicki DA, Wu AW. Does personality affect health-related quality of life? A systematic review. PloS One. 2017;12(3):1–31. doi: 10.1371/journal.pone.0173806. e0173806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.İzli G. Phenolic compounds change in table olives. Nutri Food Sci Int J. 2017;3(5):1–2. doi: 10.19080/NFSIJ.2017.03.555623. 555621. [DOI] [Google Scholar]
- 45.Boutens L, Stienstra R. Adipose tissue macrophages: going off track during obesity. Diabetologia. 2016;59(5):879–894. doi: 10.1007/s00125-016-3904-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zulkipli H, Din AM, Salim N, Froemming GA, Ismail AM, Nawawi H. Black tea polyphenols suppress adverse effects of TNFα-induced inflammation in osteoblast cells. Bone Abstracts. 2013;1:194. doi: 10.1530/boneabs.1.PP194. [DOI] [Google Scholar]
- 47.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46(11):2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 48.Shaul ME, Bennett G, Strissel KJ, Greenberg AS, Obin MS. Dynamic, M2-like remodeling phenotypes of CD11c+ adipose tissue macrophages during high-fat diet-induced obesity in mice. Diabetes. 2010;59(5):1171–1181. doi: 10.2337/db09-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nahrendorf M, Swirski FK. Monocyte and macrophage heterogeneity in the heart. Circ Res. 2013;112(12):1624–1633. doi: 10.1161/CIRCRESAHA.113.300890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shiomi N, Watanabe K. Effects of oleic acid on murine macrophage dysfunction. J Biomed Sci Eng. 2013;6(6):654–660. doi: 10.4236/jbise.2013.66080. [DOI] [Google Scholar]


