Abstract
Food additives are used to enhance freshness, safety, appearance, flavour, and texture of food. Depending on the absorbed dose, exposure method, and length of exposure, heavy metals in diet may have a negative impact on human health. The X-Ray Fluorescence (XRF) Analyzer from Niton Thermo Scientific (Mobile Test S, NDTr-XL3t-86956, com 24) was used in this work to measure the heavy metal content in saltpetre, a food additive that mostly contains potassium nitrate. The average essential metal concentrations in the samples were determined to be 27044.27 ± 10905.18 mg kg−1, 24521.10 ± 6564.28 mg kg−1, 2418.33 ± 461.50 mg kg−1, and 4.615 ± 3.59 mg kg−1 for Ca, K, Fe and Zn respectively. Toxic metals (As, Pb) were present in the saltpetre samples at 4.13 ± 2.47 mg kg−1 and 2.11 ± 1.87 mg kg−1 average concentrations. No traces of mercury or cadmium were detected. Studies on exposure, health risks, and bio-accessibility identified arsenic as a significant risk factor for potential illnesses. The need to monitor heavy metal content of saltpetre and any potential health effects on consumers is brought to light by this study.
Keywords: Saltpetre, Heavy metals, Exposure, Health risk assessment, Bio-accessibility
1. Introduction
For centuries, several food additives have been developed and patronized by consumers due to their nutritional benefits, as well as, enhancing the stability of the food. According to the World Health Organization, any substance that is added to food to preserve the nutritional value and maintain the taste, appearance and texture of the food is referred to as a food additive [1]. The safety of food additive is evaluated by Joint FAO/WHO Expert Committee on Food Additives (JEFCA) and should meet the maximum permitted levels that has been established by the Codex Alimentarius Commission [2]. The safe levels of Potassium nitrite (E 249), potassium nitrate (E 252), sodium nitrite (E 250) and sodium nitrate (E 251) was confirmed by the European Food Safety Authority as authorized food additives in European union (EU) [3]. The addition of nitrates and nitrites of potassium and sodium to meat and other food products serve as a preservative against clostridium botulinium that produce harmful neurotoxin which cause food poisoning [4]. The reduction of nitrates to nitrites during the preservation and storage of curing meat [5] help in colour retention of the meat, hinder fat rancidity and enhance the flavour of the meat products [6]. Saltpetre is a natural mineral source obtained from complex geochemical reactions as a result of the conversion of nitrogeneous biological waste into nitrates (NO3) and nitrites (NO2) by nitrifying bacteria and usually found in water, soil and plant materials. Saltpetre is mainly known as potassium nitrate (KNO3); however, calcium nitrate (CaCO3) and sodium nitrate (NaNO3) may be present in minute quantities when freshly extracted. Despite the beneficial use of saltpetre such as providing essential macro elements (K, Ca, Na) to the human body, saltpetre as a food additive may contain toxic substances which may have adverse effects on human health. Heavy metals may be present in saltpetre as a result of the complex processes involved in its formation. For example, after the nitrification process, the nitrates may penetrate the soil or may dissolve in rainwater which results in the evaporation of the deposits onto the surface to form the crude saltpetre. Heavy metals are ubiquitous in the environment, non-biodegradable and most of them are toxic or carcinogenic even at low concentration [[7], [8], [9]]. Human exposure to heavy metals occurs in the environment through different pathways such as ingestion of contaminated soil, water, food as well as through inhalation of air and dust. However, the greatest source of exposure to heavy metals is through diet [8]. Several researchers have determined the levels of heavy metals food sources such as fishes [10,11], rice grains [[12], [13], [14], [15]], yam [16,17], canned fruits, vegetables [[18], [19], [20]] and meat products [[21], [22], [23], [24]]. According to Makay et al. [6], heavy metals are highly stable and cannot be broken down during the processing of meat, and eventually bio-accumulates in the human tissues after consumption of the product. Heavy metals toxicity depends on the route of exposure, duration of exposure and absorbed dose. The toxicity of the heavy metals comes with several health risks which can interfere with metabolic processes and cause many disorders within the human body [25]. These metals have the potential to cause multiple organ damage even at low concentrations of exposure [8]. The high toxicity as well as its persistence in the environment make them high priority pollutants. To the best of our knowledge, this is the first research that uniquely investigated the levels and % bio-accessibility of heavy metals in saltpetre (a food addictive) despite extensive research on heavy metals in water bodies, sediment and soils in Ghana. This research aims to examine the essential and toxic metal content of saltpetre samples from a number of Kumasi Metropolitan markets using different analytical methods and assessing the potential human health risk associated with the consumption of saltpetre. The main objective for this research was to determine the potential health risk associated with the consumption of saltpetre within the Kumasi metropolis. This was achieved by.
-
(i)
Analysing the amounts of essential and toxic metals in saltpetre samples, (ii) Determination of human health risk factors by assessing targeted hazard quotient and cancer risk through the ingestion of saltpetre as a food addictive. (iii) Determination of bio-accessibility of the toxic heavy metals from saltpetre matrix through ingestion.
2. Materials and methods
2.1. Study area
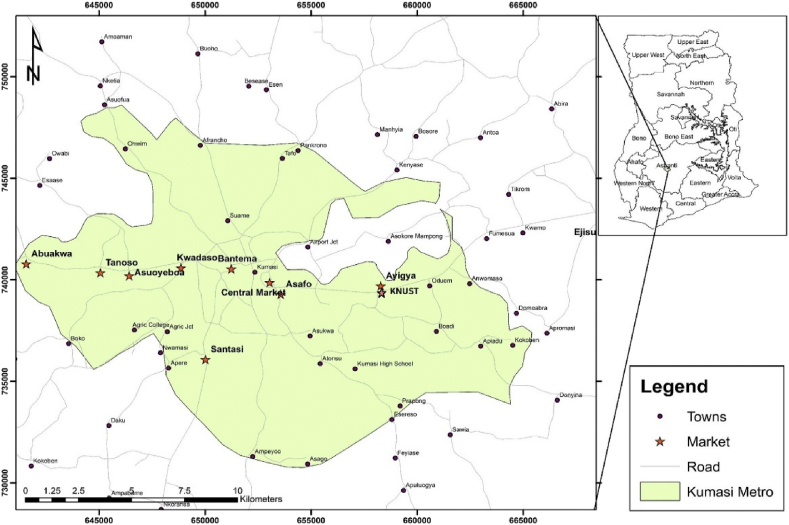
The Kumasi metropolis has a total land with 299 km2 area and a 71 elevation which extends between 250 and 300 m above sea level. It lies between longitude 1.30°-1.37° and latitude 6.35°-6.40°. The metropolis has a tropical climate which is characterized by an average temperature of 26.3 °C throughout the year. The metropolis can boast of the largest market in West Africa, which is at the centre of distribution for most products to other markets in the metropolis and Ashanti Region as a whole [26]. A map of the study area is presented in Fig. X.
Fig. X.
Customised map of the study area.
2.2. Experimental procedures
2.2.1. Sampling
The sampling criteria for saltpetre samples within the Kumasi Metropolis were based on the availability of the saltpetre samples as well as the location of the various satellite markets within the metropolis. The samples were randomly procured by random walk and based on the availability of the saltpetre at those random locations within the market. This helps to simulate how a random person will likely purchase saltpetre from these markets.
The sampling locations (the various market) are located at different positions on the map of the Kumasi Metropolis (ie North, East, South and West) which gives a good representation of the saltpetre samples in the Metropolis, especially with the levels of heavy metals obtained from this study, it will give an indication of how people are at risk after consuming the saltpetre samples that are sold on the various satellite market. Prior to analysis, samples were transported to the lab in labelled zip lock bags. Three composite saltpetre samples were procured at random from ten (10) large markets within the Kumasi metropolis constituting a sample size of thirty (30). The samples were obtained from the following markerts; Asafo, Abuakwa, Asuoyeoboah, Ayigya, Bantama, Kumasi Central Market, Kwadaso, Kwame Nkrumah University of Science and Technology (KNUST), Santasi and Tanoso.
2.2.2. Sample preparation
Samples of saltpetre procured from the markets were dried until a consistent weight was attained at room temperature. This was achieved by determining the weight after every 24 h of drying process. This was repeated until the same weight was obtained 3 consecutive times. The crucible was used to crush the dried samples in turns and sieved through a 250 μm sieve. To avoid contamination, the crucible and sieve were completely cleaned after preparing each sample. Prior to analysis, the prepared samples were put into tidy polythene bags, sealed, and labelled for identification.
2.2.3. Determination of metals in saltpetre samples by X-ray fluorescence
The Thermo scientific Niton XRF Analyser (Mobile Test S, NDTrXL3t-86956, com 24) was used to determine the concentration of heavy metals in the saltpetre samples. X-ray Fluorescence is a non-destructive analytical technique which is excellent for quantitative and qualitative analysis of the composition of samples [27]. The analytical method measures the fluorescence x-ray emitted from a sample when it is excited by a primary source of x-ray and the individual elements present in the sample give rise to a set of characteristic fluorescence x-ray which is specific for a particular element. The analysis used X-Ray Fluorescence Spectrometry (XRF) in accordance with USEPA Method 6200, for Field Portable X-Ray Fluorescence Spectrometry, in analysing the Concentrations of Elements in Soils and Sediments [28]. Reference material (OC USGS SAR-M 180–673) was used to calibrate the instrument. The sieved material was placed halfway (∼3.0 g) into the polythene sample holder. A Mylar film was placed over and cupped the sample holder. To get the desired result, the sample which was cupped was then put in the XRF shroud and scanned for 180 s. The treatment of each sample was the same.
2.2.4. Wet acid digestion of representative saltpetre samples for bio-accessibility studies
Wet Digestion, an analytical technique was used to evaluate the elemental composition of the saltpetre samples at its aqueous state. In order to increase the solubility of the metals, this technique involves the chemical degradation of sample matrices in solution, typically with a mixture of acids. After obtaining the liquified sample solutions. After modified aqua regia digestion, a total of six (6) of the sieved saltpetre samples were chosen for this investigation and evaluated for total metals. A 3 mL combination of 1:1:1 HCl, HNO3, and water was prepared and an aliquot of 0.5 g of the sample was added. The mixture was digested in a heating block at 95 °C for 1 h. ICP-MS analysis was performed after the sample was made up to volume with diluted HCl.
2.2.5. Extraction of saltpetre samples
The EPA Method 9200.2-86, Standard Operating Procedure for an in-vitro bio-accessibility assay for Lead in soil served as the basis for the extraction process [29]. A method blank and a laboratory control sample were used for QA/QC. The sieved sample was weighed into a 125 mL acid cleaned HPDE container by difference (1.00 ± 0.05 g). The extraction fluid was measured out and added to the bottle in an aliquot of 100 ± 0.5 mL. A mass-to-fluid ratio of 1:100 for the sample was produced as a result. Measurements were made of the pH of the soil/extraction fluid mixture. The extraction solution was composed of concentrated HCl (Fisher Scientific, trace metal grade) and 30 g/L glycine (Calbiochem) that had been pH-adjusted to 1.5. In batches of eight, the sealed bottles were then put into the extractor and spun end over end in a 37 ± 2 °C water bath for 1 h. The bottles were taken out after the extraction was finished. Each extract was directly drawn with a luer slip into a disposable 20 ml plastic syringe (National Scientific). The extract was filtered into a clean 20 mL polyethylene scintillation vial using a 0.45 m cellulose acetate filter (25 mm diameter, Cole Palmer) (Wheaton). The filtered extract was then kept at 4 °C and subjected to an ICP-MS metal analysis.
2.2.6. Determination of metals in extracts
The United States Environmental Protection Agency (USEPA) SW846 Method 6020A, Inductively Coupled Plasma Mass Spectrometry, was used to conduct the metal analysis with an Agilent Model 7500ce Collision Cell ICP-MS [28].
2.2.7. Health risk assessment
Exposure to toxicants such as heavy metals can have adverse effects on human health. As a result, it is important to evaluate the possibility of occurrence of any probable extent of adverse effects on human health over a specific period of time. In Ghana, there is no established limit for maximum non-carcinogenic and carcinogenic risks at present; therefore, to assess the potential health risks to consumers, USEPA threshold values were used. In this study, different health risk indicators such as non-carcinogenic risks (Targeted Hazard Quotient, Hazard Index) and the carcinogenic risks of the toxic metals (Pb and As) in the saltpetre samples were assessed. The potential acute or long-term hazards from exposure to Pb and As were examined via ingestion of saltpetre as food addictive.
The estimated daily intake (EDI) of the toxic metals (Pb, As) was calculated using the method of Zeinali et al. [29] and Maky [6] and the results were presented in Table 3;
MC: Mean concentration of metal in food (ug g−1), FDC: The daily intake of a specified food (for example meat) which is estimated to be 4 g/person/day [6,30], BW: The average body weight (60 kg).
Table 3.
Estimated daily intake of toxic metals (ug/day) in saltpetre samples.
| Sampling site | Lead ± SD | Arsenic ± SD |
|---|---|---|
| ABU | 0.202 ± 0.027 | 0.243 ± 0.077 |
| ASA | 0.199 ± 0.035 | 0.288 ± 0.064 |
| ASY | 0.250 ± 0.025 | 0.273 ± 0.077 |
| AYI | 0.172 ± 0.000 | 0.246 ± 0.019 |
| BAN | 0.246 ± 0.000 | 0.191 ± 0.034 |
| CEN | 0.229 ± 0.029 | 0.397 ± 0.147 |
| KNU | 0.282 ± 0.061 | 0.432 ± 0.136 |
| KWA | 0.252 ± 0.088 | 0.496 ± 0.119 |
| SAN | – | 0.280 ± 0.128 |
| TAN | 0.189 ± 0.000 | 0.1567 ± 0.039 |
* Cadmium and mercury were below detection limit in almost all the saltpetre samples and are therefore not indicated in this table.
The probability of an individual experiencing health risks due to exposure to a carcinogen was estimated using the equation; Cancer risk = CDI*SF.
3. Results and discussion
3.1. Concentration of essential metals and toxic metals in saltpetre samples analysed using XRF
X-ray fluorescence analysis of the saltpetre samples from separate markets have high concentrations of essential metals (Ca, Fe, K, Zn) as presented in Table 1a. According to the joint FAO/WHO expert consultation on human vitamins and minerals held in Bangkok (Thailand) in 2001 established the recommended daily intake of calcium for adolescents and adults to be 1000 mg/day and 750–800 mg/day respectively. Also, the Food and Drug Administration and World Health Organization have also suggested the maximum values intake of essential metals such as iron, potassium, and zinc to be 15 mg/day, 3500 mg/day and 15 mg/day respectively. The estimated daily intake for both calcium and iron obtained from the two different analytical techniques was higher than the recommended daily intake established by WHO/FAO and FDA as indicated in Table 1b and 6b. In spite of the critical biological functions, essential metals play in the human body such as acting as catalyst for enzymatic activity, promoting growth, enabling muscles to contract etc, high concentrations of essential metals can also be toxic and may have detrimental effects on human health [31]. For example, iron is an important element for human health which plays critical roles in cell division and differentiation, oxygen and electron transport as well as regulation of gene expression in the human body. However, the levels of iron in the human body tissues should be closely regulated due to its potential to form free radicals which can lead to tissue damage. Also, calcium is the most abundant essential metal in the body and is involved in broad functions such as muscle contraction, immune response, enzyme activation, neuronal activity and programmed cell death. High consumption of calcium could increase the risk of ischemic stroke, kidney stones and myocardial infarction.
Table 1a.
Concentration (mg kg−1) of essential metals in saltpetre samples.
| Sample ID | Ca | K | Fe | Zn |
|---|---|---|---|---|
| LOD | 3.70 | 3.40 | 3.30 | 1.80 |
| ABU1 | 14437 ± 88.94 | 17455 ± 125.60 | 2679 ± 45.78 | 9.34 ± 3.23 |
| ABU2 | 32191 ± 148.85 | 19725 ± 155.70 | 2250 ± 41.20 | 4.45 ± 2.95 |
| ABU3 | 34934 ± 165.55 | 23776 ± 181.40 | 2021 ± 38.69 | <LOD |
| ASA1 | 24883 ± 138.11 | 31378 ± 198.30 | 2125 ± 39.87 | 4.75 ± 2.90 |
| ASA2 | 35082 ± 169.27 | 32325 ± 211.70 | 2227 ± 40.70 | <LOD |
| ASA3 | 27372 ± 147.5 | 11368 ± 131.2 | 2347 ± 39.62 | <LOD |
| ASY1 | 27517 ± 142.78 | 18759 ± 156.70 | 3094 ± 48.69 | 5.26 ± 3.09 |
| ASY2 | 19950 ± 124.65 | 22410 ± 170.60 | 2974 ± 45.83 | 8.90 ± 3.03 |
| ASY3 | 10632 ± 91.74 | 21328 ± 161.00 | 1546 ± 32.65 | <LOD |
| AYI1 | 18293 ± 119.51 | 27452 ± 185.18 | 1909 ± 36.85 | 5.57 ± 2.80 |
| AYI2 | 16236 ± 113.56 | 23590 ± 173.80 | 1920 ± 36.92 | 4.28 ± 2.75 |
| AYI3 | 30368 ± 153.02 | 26948 ± 188.40 | 2487 ± 42.36 | 4.41 ± 2.85 |
| BAN1 | 33360 ± 163.29 | 18801 ± 164.80 | 2614 ± 43.37 | <LOD |
| BAN2 | 18299 ± 116.47 | 25175 ± 173.80 | 2258 ± 40.90 | <LOD |
| BAN3 | 21155 ± 124.43 | 17922 ± 150.74 | 1913 ± 37.43 | <LOD |
| CEN1 | 16265 ± 115.53 | 36002 ± 210.20 | 2797 ± 44.20 | 6.26 ± 2.93 |
| CEN2 | 22863 ± 130.91 | 26156 ± 180.40 | 2371 ± 41.67 | 5.33 ± 2.92 |
| CEN3 | 21897 ± 131.48 | 22668 ± 173.60 | 2476 ± 41.80 | 7.38 ± 2.92 |
| KNU1 | 15870 ± 112.30 | 27913 ± 186.00 | 2327 ± 40.20 | 6.41 ± 2.84 |
| KNU2 | 29677 ± 153.12 | 20371 ± 168.50 | 3247 ± 50.41 | 8.57 ± 3.24 |
| KNU3 | 28539 ± 148.67 | 30658 ± 199.00 | 2647 ± 43.84 | 4.73 ± 2.92 |
| KWA1 | 26452 ± 145.11 | 26224 ± 187.70 | 2468 ± 41.85 | 4.91 ± 2.84 |
| KWA2 | 28837 ± 153.32 | 32382 ± 209.08 | 3522 ± 51.45 | 14.49 ± 3.35 |
| KWA3 | 22178 ± 132.93 | 40581 ± 223.40 | 2184 ± 41.25 | 8.44 ± 3.13 |
| SAN1 | 50701 ± 197.67 | 16135 ± 154.90 | 2047 ± 39.39 | <LOD |
| SAN2 | 19202 ± 122.63 | 27244 ± 185.50 | 2131 ± 38.66 | 7.16 ± 2.84 |
| SAN3 | 38487 ± 180.42 | 26552 ± 198.60 | 3384 ± 49.06 | 5.9 ± 2.92 |
| TAN1 | 27697 ± 146.17 | 31750 ± 201.30 | 2006 ± 39.03 | 5.82 ± 2.93 |
| TAN2 | 32558 ± 162.06 | 17273 ± 159.60 | 2043 ± 38.09 | <LOD |
| TAN3 | 65396 ± 234.84 | 15312 ± 161.10 | 2536 ± 44.28 | 6.09 ± 3.01 |
| AVERAGE | 27044.27 ± 10905.18 | 24521.10 ± 6564.28 | 2418.33 ± 461.50 | 4.615 ± 3.60 |
ABU = Abuakwa, ASA = Asafo, ASY = Asuoyeboah, AYI = Ayigya, BAN = Bantama, CEN = Central market, KNU =Knust campus, KWA=Kwadaso, SAN = Santasi, TAN = Tanoso.
Table 1b.
The concentration of essential metals in 4 g of processed food sample (meat) containing saltpetre as a food addictive analysed using XRF method compared to recommended daily intake.
| Sampling Site | Recommended Daily intake (mg/day) |
|||
|---|---|---|---|---|
| Ca (1000 mg) | K (3500 mg) | Zn (15 mg) | Fe (15 mg) | |
| ABU1 | 962 | 1164 | 0.6226 | 178.6 |
| ASA1 | 1658 | 2091 | 0.3166 | 141.7 |
| BAN1 | 2224 | 1253 | – | 174.3 |
| CEN1 | 1084 | 2400 | 0.4173 | 186.5 |
| KWA1 | 1763 | 1748 | 0.3273 | 164.5 |
| SAN1 | 3380 | 1076 | – | 136.5 |
Table 6b.
The concentration of essential metals in 4 g of processed food sample (meat) containing saltpetre as a food addictive analysed using ICP-MS method compared to recommended daily intake.
| Sampling Site | Recommended Daily intake (mg/day) |
|||
|---|---|---|---|---|
| Ca (1000 mg) | K (3500 mg) | Zn (15 mg) | Fe (15 mg) | |
| ABU1 | 1520 | 2113 | 0.6667 | 206.7 |
| ASA1 | 1620 | 2720 | 0.3600 | 153.3 |
| BAN1 | 2340 | 1187 | 0.3533 | 173.3 |
| CEN1 | 1180 | 3493 | 0.4467 | 193.3 |
| KWA1 | 15467 | 2060 | 0.4000 | 180.0 |
| SAN1 | 2713 | 1080 | 0.30667 | 153.3 |
According to Plum et al. [33] and the Adamo and Oteiza [34], both low and high concentrations of zinc can cause apoptosis and result in neuronal death following brain injury, ischemia or epileptic seizures. Our findings indicate high concentrations of As and Pb in the saltpetre samples ranging between (1.8–9.36) mg kg−1 and (2.46–5.41) mg kg−1 respectively as shown in Table 1c contrary to the proposed general limit of heavy metals in food additive set by Joint FAO/WHO Expert Committee on Food Additives (JECFA) as indicated in Table 2a. Zeinali [29] suggested that the ingestion of heavy metals especially Pb and Cd can retard foetal growth and cause immunological dysfunction due to depletion of some essential micronutrients. Epidemiological studies have established that the accumulation of arsenic in the human body can cause deficits in verbal intelligence quotient, neurobehavioral changes and may alter cognitive function such as memory, especially in children [35,36]. However, levels of Hg and Cd in the saltpetre samples were below the limit of detection (LOD) of 1.70 and 1.90 mg kg−1 respectively.
Table 1c.
Concentration (mg kg−1) of toxic metals in saltpetre samples.
| Sample ID | As | Cd | Hg | Pd |
|---|---|---|---|---|
| LOD | 1.8 | 1.9 | 1.7 | 1.7 |
| ABU1 | 5.23 ± 1.38 | <LOD | <LOD | 2.62 ± 1.67 |
| ABU2 | 2.49 ± 1.28 | <LOD | <LOD | 3.44 ± 1.66 |
| ABU3 | 3.2 ± 1.17 | <LOD | <LOD | <LOD |
| ASA1 | 3.36 ± 1.29 | <LOD | <LOD | 3.52 ± 1.64 |
| ASA2 | 5.29 ± 1.32 | <LOD | <LOD | 2.46 ± 1.60 |
| ASA3 | <LOD | <LOD | <LOD | <LOD |
| ASY1 | 5.26 ± 1.43 | <LOD | <LOD | 4.13 ± 1.79 |
| ASY2 | 2.94 ± 1.26 | <LOD | <LOD | 3.38 ± 1.62 |
| ASY3 | <LOD | <LOD | <LOD | <LOD |
| AYI1 | 4.09 ± 1.22 | <LOD | <LOD | <LOD |
| AYI2 | 3.37 ± 1.17 | <LOD | <LOD | <LOD |
| AYI3 | 3.61 ± 1.26 | <LOD | <LOD | 2.58 ± 1.59 |
| BAN1 | 2.35 ± 1.2 | <LOD | <LOD | <LOD |
| BAN2 | 3.38 ± 1.29 | <LOD | <LOD | 3.69 ± 1.64 |
| BAN3 | <LOD | <LOD | <LOD | <LOD |
| CEN1 | 8.68 ± 1.41 | <LOD | <LOD | 3.00 ± 1.60 |
| CEN2 | 5.95 ± 1.38 | <LOD | <LOD | 3.88 ± 1.67 |
| CEN3 | 3.27 ± 1.31 | <LOD | <LOD | 5.41 ± 1.69 |
| KNU1 | 4.9 ± 1.29 | <LOD | <LOD | 3.32 ± 1.58 |
| KNU2 | 5.18 ± 1.47 | <LOD | <LOD | 5.15 ± 1.86 |
| KNU3 | 9.36 ± 1.43 | <LOD | <LOD | 2.48 ± 1.59 |
| KWA1 | 5.07 ± 1.29 | <LOD | <LOD | 2.46 ± 1.80 |
| KWA2 | 7.87 ± 1.52 | <LOD | <LOD | 5.11 ± 1.80 |
| KWA3 | 9.37 ± 1.52 | <LOD | <LOD | 3.90 ± 1.75 |
| SAN1 | 2.65 ± 1.22 | <LOD | <LOD | <LOD |
| SAN2 | 3.05 ± 1.17 | <LOD | <LOD | <LOD |
| SAN3 | 6.92 ± 1.36 | <LOD | <LOD | <LOD |
| TAN1 | 3.17 ± 1.27 | <LOD | <LOD | 2.84 ± 1.61 |
| TAN2 | 1.8 ± 1.13 | <LOD | <LOD | <LOD |
| TAN3 | 2.08 ± 1.21 | <LOD | <LOD | <LOD |
| AVERAGE | 4.13 ± 2.47 | <LOD | <LOD | 2.11 ± 1.87 |
ABU = Abuakwa, ASA = Asafo, ASY = Asuoyeboah, AYI = Ayigya, BAN = Bantama, CEN = Central market, KNU =Knust campus, KWA=Kwadaso, SAN = Santasi, TAN = Tanoso.
Table 2a.
Joint FAO/WHO Expert Committee on Food Additives (JECFA); Limit test for heavy metals in food additive specifications [32].
| Heavy metal | Proposed general limit |
|---|---|
| Arsenic | None (except indicated by manufacturing method or source) |
| Lead | 2 mg kg−1 (1 mg kg−1 or lower in case of high consumption) |
| Cadmium | 1 mg kg−1 |
| Mercury | 1 mg kg−1 |
3.2. Importance of the calculated estimated daily intake (EDI) of saltpetre samples
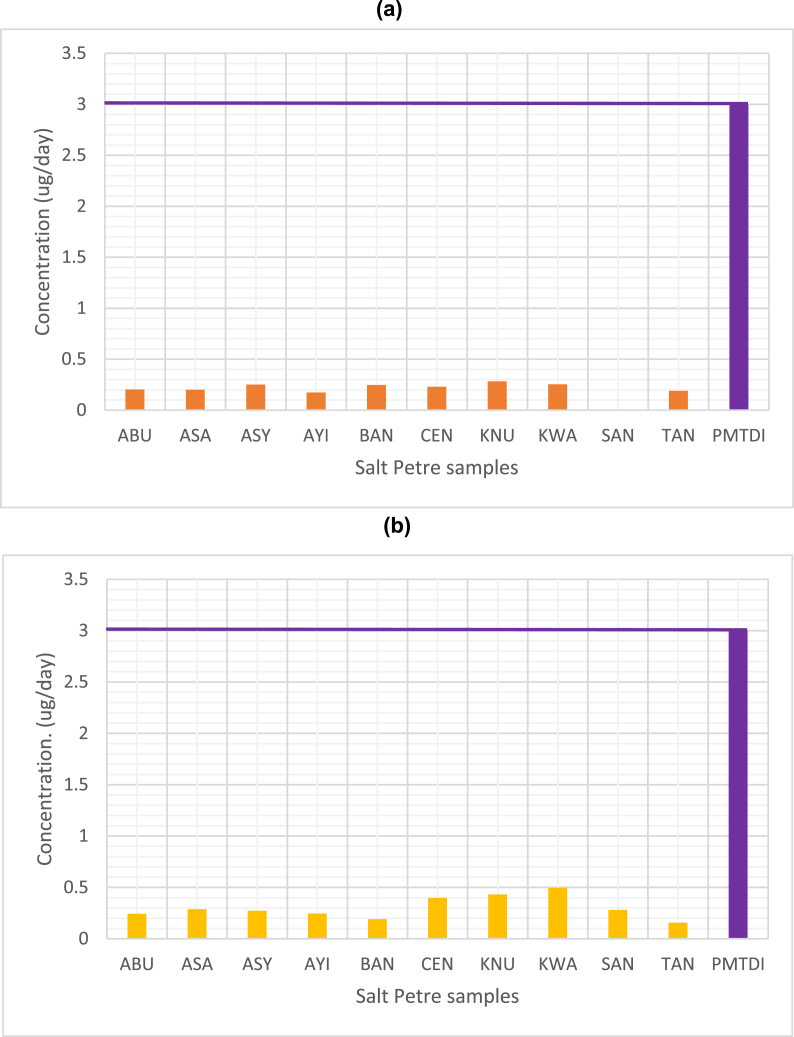
The EDI of Pb and As in the saltpetre samples is less than the permitted maximum tolerable daily intake (PMTDI) reported by Nkansah et al. [26] and Ternes et al. [37] as shown in Fig. 1a and b and Table 2b. However, according to the World Health Organization, chronic arsenic poisoning, skin cancer and skin lesions are some characteristic effects that may occur from long term exposure to arsenic especially through ingestion of food and drinking water. Exposure to arsenic have been linked to diabetes, cardiovascular diseases and may have negative effects on cognitive development in children and increased deaths in adults [38]. The World Health Organization has established that Pb is a cumulative toxicant and has the potential to affect several organs in the human body. In fact, no blood-lead concentration can be considered to be safe. Long term exposure to lead can cause kidney damage, high blood pressure and reproductive failure [39,40].
Fig. 1.
(a) The mean concentration of lead in 4 g of processed food sample (meat) containing saltpetre as a food addictive compared to permitted maximum tolerable daily intake (PMTDI).(b) The mean concentration of arsenic in 4 g of processed food sample (meat) containing saltpetre as a food addictive compared to permitted maximum tolerable daily intake (PMTDI).
Table 2b.
Permitted Maximum Tolerable Daily Intake (PMTDI) values for some heavy metals in food additives [26,33].
| Heavy metal | WHO/FAO PMTDI (ug/kg BW/day) |
|---|---|
| Arsenic | 3.0 |
| Lead | 3.0 |
| Cadmium | 0.6 |
| Mercury | 0.3 |
3.3. Targeted hazard quotient (THQ) and hazard index (HI)
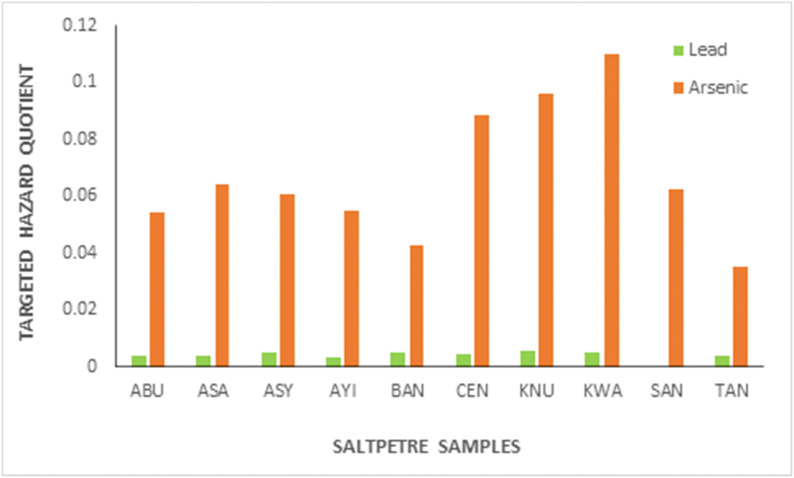
The non-carcinogenic risk which is expressed as the targeted hazard quotient of the various metals in the saltpetre samples was estimated. The THQ is the ratio of exposure to harmful compounds to the chronic reference dose (RFD) of the toxin (mg kg −1 d−1). A THQ value less than 1 (THQ <1) indicates that it is unlikely that the exposed population will incur negative consequences, whereas a THQ value above 1 (HQ > 1) non-carcinogenic effect is likely, and as the value increases the likelihood increase [33,41]. The THQ values estimated for Pb and As from the different markets were ranged from 0.0033 to 0.0054 and 0.0424–0.1202 as indicated in Fig. 2. The THQ values of the estimated metals were less than 1 indicating that the probability of adverse effects through the ingestion of saltpetre as a food addictive is negligible. Exposure to different toxicants may result in similar adverse effects. The hazard index (HI) of the toxic metals (Pb, As) was estimated by adding the individual hazard quotients of the metals. The HI for the studied metals via ingestion of saltpetre as food addictive was found to be 0.7058. Although non-carcinogenic risks of Pb and As was less than unity, continuous ingestion as well as long term exposure to saltpetre as a food additive are of great concern [42].
Fig. 2.
The targeted hazard quotient of arsenic and lead in the saltpetre samples.
3.4. Cancer risk
Over the years, several researchers have investigated the risks associated with heavy metals exposure. Based on their findings, it was suggested that skin, kidney, lung and liver cancers can be linked to heavy metals exposure. The International Agency for Cancer research has classified As, Pb, Cd, Hg and Cr as a probable group 1 carcinogen [43,44].
Where the chronic daily intake of carcinogens (mg kg−1 d−1) is denoted by CDI and the slope factor of hazardous substances (mg kg−1 d−1) is denoted by SF. The cancer risk values for As and Pb in the saltpetre samples were found to be 4.470E-3 and 1.718E-5 respectively as shown in Table 4. The cancer risk for As exceeded the level of 1E-06 to 1E-04 established by USEPA [45]. Based on the health risk assessment results, arsenic is the main contributor to heavy metal related risk among the four toxic metals.
Table 4.
Cancer risks of lead and arsenic in saltpetre samples.
| Metal | Estimated Daily intake (EDI) | Cancer Slope Factor (CSF) | Cancer Risk |
|---|---|---|---|
| Arsenic | 3.003E-3 | 1.490 | 4.470E-3 |
| Lead | 2.021E-3 | 0.0085 | 1.718E-5 |
3.5. Inter-elemental correlation of essential and toxic metals in saltpetre samples
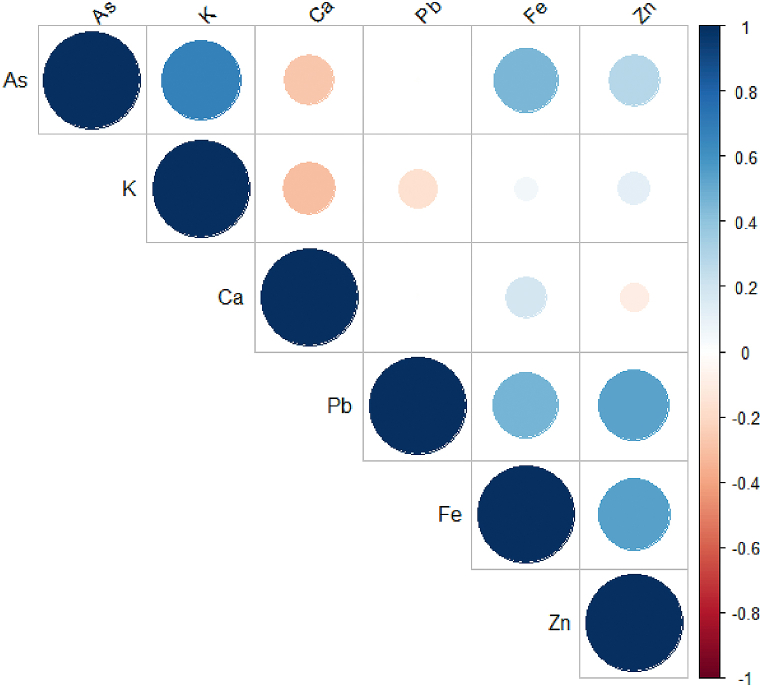
Saltpetre is formed from the decomposition of organic matter by the action of bacterial enzymes [46]. The deposits are usually dissolved in rainwater and evaporated on the surface as crude saltpetre. Most of these heavy metals have low solubility and are usually immobilized in soils [47]. For example Zn can form complexes with the organic matter easily which can affect the activity of Zn2+ in the soil [48]. Also, high concentration in groundwater can precipitate arsenic and reduce its bioavailability. Inter-elemental relationships of the heavy metals in the saltpetre samples were computed using Pearson Correlation Analysis and the results have been presented in Table 5 and Fig. 3. A significant positive correlation for K–As (r = 0.6753), Zn–Pb (r = 0.5309) and Zn–Fe (r = 0.548) at the level of p = 0.05 was obtained. Based on the correlation results, it can be suggested that the association between these metals likely indicate their shared or related sources. which may be affected by the soil forming materials. Also, the low solubility and immobilization nature of Zn, Fe, Pb and As may have contributed to their inter-elemental relationships.
Table 5.
Metal to metal correlation matrix for saltpetre samples (p = 0.05).
| As | Pb | Ca | K | Fe | Zn | |
|---|---|---|---|---|---|---|
| As | 1.0000 | |||||
| Pb | −0.0070 | 1.000 | ||||
| Ca | −0.2718 | −0.005 | 1.000 | |||
| K | 0.6753 | −0.1664 | −0.3047 | 1.000 | ||
| Fe | 0.4501 | 0.4622 | 0.1808 | 0.0597 | 1.000 | |
| Zn | 0.2896 | 0.5309 | −0.09299 | 0.1183 | 0.548 | 1.000 |
Fig. 3.
The inter-elemental relationship between the essential and toxic metals in the saltpetre samples.
3.6. Validation of analytical data for saltpetre samples from selected markets
The heavy metals in saltpetre samples were analysed using the ICP-MS method and the results have been presented in Table 6a. To ensure reliable and accurate results, the ICP-MS method was validated using a certified reference material (OREAS45EA Standard Reference Material).
Table 6a.
Concentration of essential and toxic metals in saltpetre samples analysed using ICP-MS.
| Sample ID | Ca | K | Fe | Zn | As | Cd | Hg | Pb |
|---|---|---|---|---|---|---|---|---|
| LOD | 100 | 100 | 100 | 0.1 | 0.1 | 0.01 | 0.005 | 0.01 |
| ASA1 | 24300 | 40800 | 2300 | 5.4 | 6.2 | 0.02 | 0.008 | 1.25 |
| ABU1 | 22800 | 31700 | 3100 | 10 | 8.1 | 0.01 | 0.008 | 2.36 |
| BAN1 | 35100 | 17800 | 2600 | 5.3 | 2.7 | 0.02 | 1.30 | |
| CEN1 | 17700 | 52400 | 2900 | 6.7 | 11.1 | 0.02 | 1.34 | |
| KWA1 | 23200 | 30900 | 2700 | 6.0 | 7.2 | 0.02 | 1.50 | |
| SAN1 | 40700 | 16200 | 2300 | 4.6 | 3.5 | 0.02 | 0.85 |
The concentration of the heavy metals in the blank samples were below the detection limit which suggests a void contamination effect on the ICP-MS method. The concentrations of the essential metals (Ca, K, Fe, Zn) exceeded the expected concentrations of the Certified Reference Materials Table 6b. In the case of the toxic metals, the values obtained correlated well with the certified values as indicated in Table 6c.
Table 6c.
Validation method for quantitative analysis of essential and toxic metals in saltpetre samples.
| Sample | OREAS45EA Standard Reference Material | Measured value (mg kg−1) |
|---|---|---|
| Ca | 360 | 17700–40700 |
| K | 530 | 16200–52400 |
| Fe | 235100 | 2300–3100 |
| Zn | 31.40 | 4.6–10 |
| As | 3.70 | 2.7–11.1 |
| Cd | 0.03 | 0.01–0.02 |
| Hg | 0.010 | 0.008 |
| Pb | 14.30 | 0.85–1.25 |
3.7. Comparison of heavy metal concentrations of saltpetre samples using two different analytical methods (xrf and icp-ms)
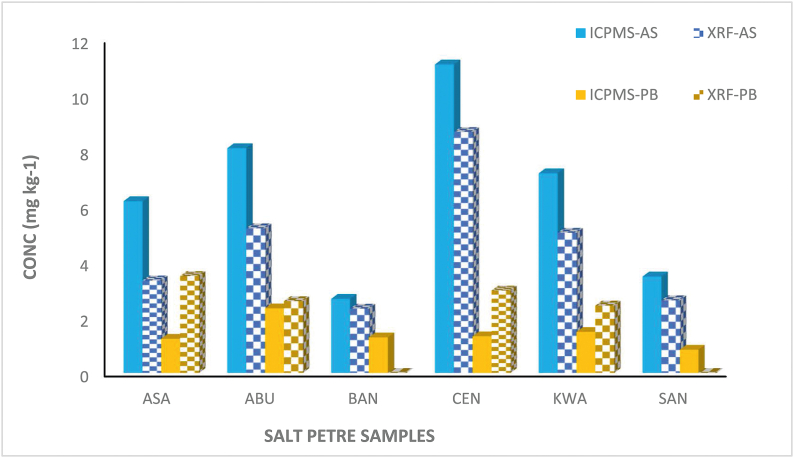
In this study, two different analytical techniques X-ray fluorescence spectroscopy (XRF) and Inductively Coupled Plasma-Mass Spectrometry (ICP-MS) were used to determine the concentrations of heavy metals in some selected saltpetre samples (ABU1, ASA1, BAN1, CEN1, KWA1, SAN1). Different concentrations of the same samples were obtained when quantified using XRF and ICP-MS. In the saltpetre sample analysis using ICP-MS, high concentrations of Arsenic (As) ranged from 2.7 to 11.1 mg kg−1 were obtained for all samples as presented in Table 6a contrary to the XRF analysis. However, in the case of Lead (Pb), low concentrations were obtained for all samples analysed using ICP-MS. This may be attributed to the ability of lead to bind tightly to the soil organic matter thereby decreasing its availability. During the acid digestion step which is an important requirement of ICP-MS analysis does not dissolve the sample entirety but decompose them to separate the soluble from the insoluble matrix. Hence the low concentration of Pb obtained for the selected saltpetre samples is due to the incomplete dissolution of the samples which lowers the detection limits of the analytes and the dissolved solid concentrations as shown in Fig. 4 [49].
Fig. 4.
The mean concentration (mg kg−1) of Pb and As in saltpetre samples from selected markets using ICP-MS and XRF analysis.
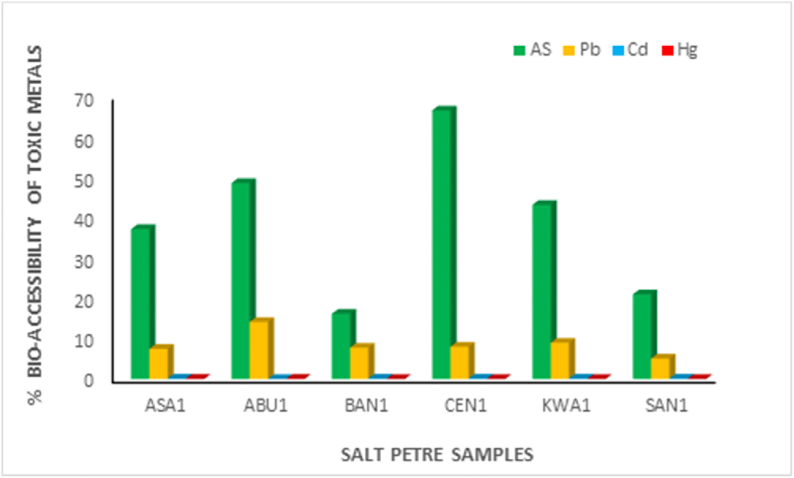
3.8. Significance of % bioaccessibility of metals
In addition to quantifying the total concentration of heavy metals in the saltpetre samples, the actual fraction of the heavy metals that will be mobilized from the solid matrix (saltpetre as a food addictive) in the human gastrointestinal tract that becomes absorbed by the intestine is essential for risk evaluation. Bio-accessibility analysis was done in this study for the toxic metals (AS, Pb, Cd, Hg) and the % bioaccessibility values have been shown in Fig. 5. High % As bioaccessibility values ranging from 21 to 66% were obtained for the selected saltpetre samples. According to Yager et al. [50], food contributes to about 70% of human arsenic (As) consumption based on As bioaccessibility studies in dietary composites and specific foods, which is in agreement to our findings. Gersztyn et al. [51] established that solubility of arsenic in soils increased substantially in strongly acidic conditions.The high % As bioaccessibility levels is due to the hydrolysis of As during the acid digestion of the saltpetre samples resulting in the high solubilization.The %Pb bio-accessibility values for the saltpetre samples varied between 5.10 and 14.16%, however the values obtained for Hg and Cd were less than 1%. The bio-accesssibility values obtained in this study suggests that although the total heavy metal concentrstions for As were low, it is highly soluble and available for intestinal absorption when ingested into our body. Therefore such findings can be important in application to human health risk assessments due to the fact that exposure to arsenic has been linked to a wide range of health issues, from short-term toxicity to long-term, chronic disorders.
Fig. 5.
The % bio-accessibility of toxic metals in saltpetre samples from selected markets.
3.9. Strengths and limitations of the study
The main strengths of our study have been outlined as follows: First, saltpetre (mainly potassium nitrate) has been established by the European Food and Safety Authority as an authorised food additive. There is very limited data on heavy metal profile of saltpetre. As a result, determining the levels of essential and heavy metals in saltpetre samples is critical for ensuring consumer safety.
Second, the levels of essential and toxic metals that are obtained from the analysis are important for assessing the potential health risks through its consumption, especially in the food industry where saltpetre is largely used for the preservation and storage of meat.
Third, some scientists in Ghana focus their research on determining heavy metals in vegetables, fish, and tea samples; therefore, the findings of this study will provide insight into the levels of heavy metals in saltpetre and the need to consider other food additives in their next research as well as a good source of information for the food industry.
Fourth, XRF, a non-destruction analytical technique as well as wet digestion-ICPMS an analytical technique which involves the chemical degradation of the sample matrix were used in this study. The levels of metals that will be obtained from the analysis will give good indication and information on the extent of the contamination of the saltpetre samples and how these methods differ in their detection.
Our study has some limitations: First, the estimated daily intake for the various metals was calculated by considering an average body weight of 60 kg which is specific for adults. Therefore, our study did not factor in the average body weights of children.
Second, the Gbolal Burden of Disease (GBD) Diet Collaborators established the optimal intake range of (0–4) g for daily consumption of meat preserved by curing, smoking, salting, or addition of chemical preservatives. Therefore, the highest value of 4 is used for the calculation of EDI. However, children and adults consume varying amounts of meat which may be lower or higher than the established optimal intake value by the GBD Diet Collaborators.
4. Conclusions
This study has offered insight into the heavy metal profile of saltpetre. Knowledge on heavy metal concentration in saltpetre which is used as a food addictive is limited, and the findings of this study have indicated the presence of essential and toxic metals in the saltpetre samples from selected markets within the Kumasi metropolis of Ghana. Although the estimated daily intake values of the toxic metals (As and Pb) were lower than the maximum tolerable daily intake, its cumulative effect is of concern. The bioaccessibility analysis and the health risk assessment data suggest arsenic (As) as the major contributor to potential health related problems among the two toxic metals present in the saltpetre samples. This research has provided insight into the levels of heavy metals in saltpetre samples a food addictive and an important source of information for the food processing industry where saltpetre is used for preservation of food especially meat products.The data obtained serves as baseline for future research. The findings have also informed consumer satefy and basis for policy response.
Author contribution statement
Marian Asantewah Nkansah: Conceived and designed the experiments; Analysed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Mavis Korankyel: Performed the experiments; Analysed and interpreted the data; Wrote the paper.
Godfred Darko: Analysed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Matt Dodd: Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Francis Opoku: Analysed and interpreted the data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data associated with this study has been deposited at https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4147061.
Declaration of interest's statement
The authors declare no conflict of interest.
References
- 1.World Health Organization . 2018. Food Additives.https://www.who.int/news-room/fact-sheets/detail/food-additives [Google Scholar]
- 2.Subhan M.A., Chandra Saha P., Rahman M.M., Ahmed J., Asiri A.M., Al-Mamun M. Fabrication of a 2,4-dinitrophenol sensor based on Fe3O4@Ag@Ni nanomaterials and studies on their antibacterial properties. New J. Chem. 2018;42:872–881. [Google Scholar]
- 3.Ge T., Han J., Qi Y., Gu X., Ma L., Zhang C., Naeem S., Huang D. The toxic effects of chlorophenols and associated mechanisms in fish. Aquat. Toxicol. 2017;184:78–93. doi: 10.1016/j.aquatox.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Basak A.K., Chatterjee T., Ghosh S.K., Chakravarty A. Impacts of dietary exposure to sodium or potassium salts of nitrate and nitrite on the development of Drosophila melanogaster. Interdiscipl. Toxicol. 2017;10:70–78. doi: 10.1515/intox-2017-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen M.J., Tung V.C., Kaner R.B. Honeycomb carbon: a review of graphene. Chem. Rev. 2010;110:132–145. doi: 10.1021/cr900070d. [DOI] [PubMed] [Google Scholar]
- 6.Maky M.A., Abd-ElRasoul M.A.A., Salah M. Evaluation of some food additives and heavy metals in Egyptian meat products. Int. J. One Health. 2020;6:61–68. [Google Scholar]
- 7.Jia Z., Li S., Wang L. Assessment of soil heavy metals for eco-environment and human health in a rapidly urbanization area of the upper Yangtze Basin. Sci. Rep. 2018;8:3256. doi: 10.1038/s41598-018-21569-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ankar-Brewoo G.M., Darko G., Abaidoo R.C., Dalsgaard A., JohnsonW P.N., Ellis O., Brimer L. Health risks of toxic metals (Al, Fe and Pb) in two common street vended foods, fufu and fried-rice, in Kumasi, Ghana. Scientific African. 2020;7 [Google Scholar]
- 9.Wu X., Cobbina S.J., Mao G., Xu H., Zhang Z., Yang L. A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ. Sci. Pollut. Res. Int. 2016;23:8244–8259. doi: 10.1007/s11356-016-6333-x. [DOI] [PubMed] [Google Scholar]
- 10.Nyantakyi A.J., Wiafe S., Akoto O., Fei-Baffoe B. Heavy metal concentrations in fish from river tano in Ghana and the health risks posed to consumers. J. Environ Public Health. 2021 doi: 10.1155/2021/5834720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwaansa-Ansah E.E., Nti S.O., Opoku F. Heavy metals concentration and human health risk assessment in seven commercial fish species from Asafo market, Ghana. Food Sci. Biotechnol. 2018;28:569–579. doi: 10.1007/s10068-018-0485-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adam A.-A., Sackey L.N.A., Ofori L.A. Risk assessment of heavy metals concentration in cereals and legumes sold in the Tamale Aboabo market, Ghana. Heliyon. 2022;8 doi: 10.1016/j.heliyon.2022.e10162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Praveena S.M., Omar N.A. Heavy metal exposure from cooked rice grain ingestion and its potential health risks to humans from total and bioavailable forms analysis. Food Chem. 2017;235:203–211. doi: 10.1016/j.foodchem.2017.05.049. [DOI] [PubMed] [Google Scholar]
- 14.Satpathy D., Reddy M.V., Dhal S.P. Risk assessment of heavy metals contamination in paddy soil, plants, and grains (Oryza sativa L.) at the East Coast of India. BioMed Res. Int. 2014;2014 doi: 10.1155/2014/545473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ihedioha J.N., Ujam O.T., Nwuche C.O., Ekere N.R., Chime C.C. Assessment of heavy metal contamination of rice grains (Oryza sativa) and soil from Ada field, Enugu, Nigeria: estimating the human healtrisk. Hum. Ecol. Risk Assess. 2016;22:1665–1677. [Google Scholar]
- 16.Shin M.Y., Cho Y.E., Park C., Sohn H.Y., Lim J.H., Kwun I.S. The contents of heavy metals (cd, Cr, As, Pb, Ni, and Sn) in the selected commercial yam powder products in South Korea. Prev Nutr Food Sci. 2013;18:249–255. doi: 10.3746/pnf.2013.18.4.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raphael E.C., Eunice O.E., Frank E.O. Trace metals distribution in some common tuber crops and leafy vegetables grown in the Niger delta region of Nigeria. Pakistan J. Nutr. 2010;9:957–961. [Google Scholar]
- 18.Massadeh A.M., Al-Massaedh A.A.T. Determination of heavy metals in canned fruits and vegetables sold in Jordan market. Environ. Sci. Pollut. Res. Int. 2018;25:1914–1920. doi: 10.1007/s11356-017-0611-0. [DOI] [PubMed] [Google Scholar]
- 19.Taghipour H., Mosaferi M. Heavy metals in the vegetables collected from production sites. Health Promot. Perspect. 2013;3:185–193. doi: 10.5681/hpp.2013.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parvin R., Sultana A., Zahid M.A. Detection of heavy metals in vegetables cultivated in different locations in chittagong, Bangladesh. IOSR J. Environ. Sci. Toxicol. Food Technol. 2014;8:58–63. [Google Scholar]
- 21.Zaman M.M., Ahmed S., Ahsan A.A., Kazi A.I., Hossain S.M.A., Siddique Z.A., Chowdhury M.Z.A. Determination of essential and toxic metals in meats, meat products and eggs by spectrophotometric method. J. Bangladesh Chem. Soc. 1970;24:165–172. [Google Scholar]
- 22.Di Bella C., Traina A., Giosue C., Carpintieri D., Lo Dico G.M., Bellante A., Del Core M., Falco F., Gherardi S., Uccello M.M., Ferrantelli V. Heavy metals and PAHs in meat, milk, and seafood from augusta area (southern Italy): contamination levels, dietary intake, and human exposure assessment. Front. Public Health. 2020;8:273. doi: 10.3389/fpubh.2020.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korish M.A., Attia Y.A. Evaluation of heavy metal content in feed, litter, meat, meat products, liver, and table eggs of chickens. Animals (Basel) 2020;10:727. doi: 10.3390/ani10040727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barone G., Storelli A., Quaglia N.C., Garofalo R., Meleleo D., Busco A., Storelli M.M. Trace metals in pork meat products marketed in Italy: occurrence and health risk characterization. Biol. Trace Elem. Res. 2020;199:2826–2836. doi: 10.1007/s12011-020-02417-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaishankar M., Tseten T., Anbalagan N., Mathew B.B., Beeregowda K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscipl. Toxicol. 2014;7:60–72. doi: 10.2478/intox-2014-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nkansah M.A., Korankye M., Darko G., Dodd M. Heavy metal content and potential health risk of geophagic white clay from the Kumasi Metropolis in Ghana. Toxicol Rep. 2016;3:644–651. doi: 10.1016/j.toxrep.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melquiades F.L., Appoloni C.R. Application of XRF and field portable XRF for environmental analysis. J. Radioanal. Nucl. Chem. 2004;262(2):533–541. [Google Scholar]
- 28.Tran Q.T., Hoa H.T.M., Yoo D.-H., Cuong T.V., Hur S.H., Chung J.S., Kim E.J., Kohl P.A. Reduced graphene oxide as an over-coating layer on silver nanostructures for detecting NH3 gas at room temperature. Sensor. Actuator. B Chem. 2014;194:45–50. [Google Scholar]
- 29.Zeinali T., Salmani F., Naseri K. Dietary intake of cadmium, chromium, copper, nickel, and lead through the consumption of meat, liver, and kidney and assessment of human health risk in birjand, southeast of Iran. Biol. Trace Elem. Res. 2019;191:338–347. doi: 10.1007/s12011-019-1637-6. [DOI] [PubMed] [Google Scholar]
- 30.Afshin A., Sur P.J., Fay K.A., Cornaby L., Ferrara G., Salama J.S., Mullany E.C., Abate K.H., Abbafati C., Abebe Z., Afarideh M., Aggarwal A., Agrawal S., Akinyemiju T., Alahdab F., Bacha U., Bachman V.F., Badali H., Badawi A., Bensenor I.M., Bernabe E., Biadgilign S.K.K., Biryukov S.H., Cahill L.E., Carrero J.J., Cercy K.M., Dandona L., Dandona R., Dang A.K., Degefa M.G., El Sayed Zaki M., Esteghamati A., Esteghamati S., Fanzo J., Farinha C.S.e.S., Farvid M.S., Farzadfar F., Feigin V.L., Fernandes J.C., Flor L.S., Foigt N.A., Forouzanfar M.H., Ganji M., Geleijnse J.M., Gillum R.F., Goulart A.C., Grosso G., Guessous I., Hamidi S., Hankey G.J., Harikrishnan S., Hassen H.Y., Hay S.I., Hoang C.L., Horino M., Islami F., Jackson M.D., James S.L., Johansson L., Jonas J.B., Kasaeian A., Khader Y.S., Khalil I.A., Khang Y.-H., Kimokoti R.W., Kokubo Y., Kumar G.A., Lallukka T., Lopez A.D., Lorkowski S., Lotufo P.A., Lozano R., Malekzadeh R., Marz W., Meier T., Melaku Y.A., Mendoza W., Mensink G.B.M., Micha R., Miller T.R., Mirarefin M., Mohan V., Mokdad A.H., Mozaffarian D., Nagel G., Naghavi M., Nguyen C.T., Nixon M.R., Ong K.L., Pereira D.M., Poustchi H., Qorbani M., Rai R.K., Razo-Garcia C., Rehm C.D., Rivera J.A., Rodriguez-Ramirez S., Roshandel G., Roth G.A., Sanabria J., Sanchez-Pimienta T.G., Sartorius B., Schmidhuber J., Schutte A.E., Sepanlou S.G., Shin M.-J., Sorensen R.J.D., Springmann M., Szponar L., Thorne-Lyman A.L., Thrift A.G., Touvier M., Tran B.X., Tyrovolas S., Ukwaja K.N., Ullah I., Uthman O.A., Vaezghasemi M., Vasankari T.J., Vollset S.E., Vos T., Vu G.T., Vu L.G., Weiderpass E., Werdecker A., Wijeratne T., Willett W.C., Wu J.H., Xu G., Yonemoto N., Yu C., Murray C.J.L. Health effects of dietary risks in 195 countries, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;393:1958–1972. doi: 10.1016/S0140-6736(19)30041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deng S., Tjoa V., Fan H.M., Tan H.R., Sayle D.C., Olivo M., Mhaisalkar S., Wei J., Sow C.H. Reduced graphene oxide conjugated Cu2O nanowire mesocrystals for high-performance NO2 gas sensor. J. Am. Chem. Soc. 2012;134:4905–4917. doi: 10.1021/ja211683m. [DOI] [PubMed] [Google Scholar]
- 32.P Gupta S. Roles of metals in human health. MOJ Bioorg. Org. Chemistry. 2018;2 [Google Scholar]
- 33.Plum L.M., Rink L., Haase H. The essential toxin: impact of zinc on human health. Int. J. Environ. Res. Publ. Health. 2010;7:1342–1365. doi: 10.3390/ijerph7041342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adamo A.M., Oteiza P.I. Zinc deficiency and neurodevelopment: the case of neurons. Biofactors. 2010;36:117–124. doi: 10.1002/biof.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hong Y.S., Song K.H., Chung J.Y. Health effects of chronic arsenic exposure. J. Prev. Med. Public Health. 2014;47:245–252. doi: 10.3961/jpmph.14.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tyler C.R., Allan A.M. The effects of arsenic exposure on neurological and cognitive dysfunction in human and rodent studies: a review. Curr Environ Health Rep. 2014;1:132–147. doi: 10.1007/s40572-014-0012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ternes T.A., Joss A., Siegrist H. Peer reviewed: scrutinizing pharmaceuticals and personal care products in wastewater treatment. Environ. Sci. Technol. 2004;38:392A–399A. doi: 10.1021/es040639t. [DOI] [PubMed] [Google Scholar]
- 38.Gurjar B.R., Molina L.T., Ojha C.S.P. CRC press; Boca Raton, US: 2010. Air Pollution: Health and Environmental Impacts. [Google Scholar]
- 39.Nagaraj V.J., Jacobs M., Vattipalli K.M., Annam V.P., Prasad S. Nanochannel-based electrochemical sensor for the detection of pharmaceutical contaminants in water. Environ. Sci.: Process. Impacts. 2014;16:135–140. doi: 10.1039/c3em00406f. [DOI] [PubMed] [Google Scholar]
- 40.Assi M.A., Hezmee M.N., Haron A.W., Sabri M.Y., Rajion M.A. The detrimental effects of lead on human and animal health. Vet. World. 2016;9:660–671. doi: 10.14202/vetworld.2016.660-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu X., Song Q., Tang Y., Li W., Xu J., Wu J., Wang F., Brookes P.C. Human health risk assessment of heavy metals in soil-vegetable system: a multi-medium analysis. Sci. Total Environ. 2013;463–464:530–540. doi: 10.1016/j.scitotenv.2013.06.064. [DOI] [PubMed] [Google Scholar]
- 42.Cairns T., Sherma J. CRC press; Boca Raton, Florida, USA: 1992. Emerging Strategies for Pesticide Analysis. [Google Scholar]
- 43.Yuan W., Yang N., Li X. Advances in understanding how heavy metal pollution triggers gastric cancer. BioMed Res. Int. 2016;2016 doi: 10.1155/2016/7825432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carver A., Gallicchio V.S. Cancer Causing Substances; 2018. Heavy Metals and Cancer. [Google Scholar]
- 45.Pye V.I., Patrick R. Ground water contamination in the United States. Science. 1983;221:713–718. doi: 10.1126/science.6879171. [DOI] [PubMed] [Google Scholar]
- 46.Butler A., Moffett J. Saltpetre in early and medieval Chinese medicine. Asian Med. 2009;5:173–185. [Google Scholar]
- 47.Xie X., Hu W., Fan X., Chen H., Tang M. Interactions between phosphorus, zinc, and iron homeostasis in nonmycorrhizal and mycorrhizal plants. Front. Plant Sci. 2019;10:1172. doi: 10.3389/fpls.2019.01172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rutkowska B., Szulc W., Bomze K., Gozdowski D., Spychaj-Fabisiak E. Soil factors affecting solubility and mobility of zinc in contaminated soils. Int. J. Environ. Sci. Technol. 2014;12:1687–1694. [Google Scholar]
- 49.Nyika J., Onyari E., Dinka M.O., Mishra S.B. A comparison of reproducibility of inductively coupled spectrometric techniques in soil metal analyses. Air Soil. Water Res. 2019;12 [Google Scholar]
- 50.Yager J.W., Greene T., Schoof R.A. Arsenic relative bioavailability from diet and airborne exposures: implications for risk assessment. Sci. Total Environ. 2015;536:368–381. doi: 10.1016/j.scitotenv.2015.05.141. [DOI] [PubMed] [Google Scholar]
- 51.Gersztyn L., Karczewska A., Galka B. Influence of pH on the solubility of arsenic in heavily contaminated soils. Environ. Prot. Nat. Resour. 2013;24:7–11. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data associated with this study has been deposited at https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4147061.