Abstract
Background:
The prevalence of liver disorders and metabolic syndrome has increased among youth. Glyphosate, the most widely used herbicide worldwide, could contribute to the development of these conditions.
Objective:
We aimed to assess whether lifetime exposure to glyphosate and its degradation product, aminomethylphosphonic acid (AMPA), is associated with elevated liver transaminases and metabolic syndrome among young adults.
Methods:
We conducted a prospective cohort study ( mother–child dyads) and a nested case–control study ( cases with elevated liver transaminases and 91 controls) using data from the Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS). We measured glyphosate and AMPA concentrations in urine samples collected during pregnancy and at child ages 5, 14, and 18 y from cases and controls. We calculated glyphosate residue concentrations: []. We estimated the amount of agricultural-use glyphosate applied within a radius of every residence from pregnancy to age 5 y for the full cohort using California Pesticide Use Reporting data. We assessed liver transaminases and metabolic syndrome at 18 y of age.
Results:
Urinary AMPA at age 5 y was associated with elevated transaminases [relative risk (RR) per , 95% confidence interval (CI): 1.06, 1.53] and metabolic syndrome (, 95% CI: 1.38, 3.11). Urinary AMPA and glyphosate residues at age 14 y were associated with metabolic syndrome [ (95% CI: 1.10, 2.93) and (95% CI: 1.03, 3.42), respectively]. Overall, a 2-fold increase in urinary AMPA during childhood was associated with a 14% and a 55% increased risk of elevated liver transaminases and metabolic syndrome, respectively. Living near agricultural glyphosate applications during early childhood (birth to 5 y of age) was also associated with metabolic syndrome at age 18 y in the case–control group (, 95% CI: 1.16, 2.02).
Discussion:
Childhood exposure to glyphosate and AMPA may increase risk of liver and cardiometabolic disorders in early adulthood, which could lead to more serious diseases later in life. https://doi.org/10.1289/EHP11721
Introduction
The prevalence of childhood obesity and metabolic syndrome has increased at an alarming rate in the United States,1,2 particularly among populations of color.1–3 Accompanying this, has been an increase in nonalcoholic fatty liver disease (NAFLD),4 a condition that can lead to cirrhosis and hepatocellular carcinoma later in life.5 Although diet and physical activity play an important role in cardiometabolic and liver disorders, some hypothesize that exposure to synthetic chemicals may also be involved.6,7
Use of the herbicide glyphosate has markedly increased in the United States in the last two decades and currently is the most commonly used broad-spectrum herbicide worldwide.8,9 It is used to control broadleaf grasses and weeds in agriculture, forestry, and right-of-way clearances, in parks, and in yards as a component in home weed killers (e.g., Round-up®). Exposure to glyphosate and its prime degradation product, aminomethylphosphonic acid (AMPA), can occur through consumption of contaminated food,10,11 air,12 dust,12 and water.13 In food, glyphosate has been detected primarily in grains14 and legumes, including soybeans,15 but it has also been detected in other fruits and vegetables10,16 and in baby formula.17 AMPA is also the degradation product of amino-polyphosphonates, which are extensively used in detergents, fire retardants, and other compounds.18
The potential impact of glyphosate on human health is controversial and widely debated.19–22 Like glyphosate, AMPA raises toxicologic concern.23 In 2015, the International Agency for Research on Cancer (IARC) classified glyphosate as probably carcinogenic to humans (Group 2A),21 but to date the U.S. Environmental Protection Agency (U.S. EPA) has found no evidence of risk to human health.20 Animal24–28 and human29–31 studies have suggested that exposure to glyphosate may be related to liver disease, and some researchers have hypothesized a potential relationship with metabolic disorders.32,33 In the current study, we investigated the association of prenatal and childhood exposure to glyphosate and AMPA—as indicated by urinary concentrations and registry data of nearby agricultural use of glyphosate—with markers of liver inflammation and metabolic syndrome in young adults.
Methods
Study Population
Participants are mother–child dyads enrolled in the Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS) longitudinal cohort of children born between 2000 and 2002 in California’s Salinas Valley.34 Briefly, pregnant women receiving prenatal care at community clinics primarily serving farmworker families were eligible for enrollment if they were at least 18 y old, wk gestation, spoke English or Spanish, met income requirements for public health insurance (Medi-Cal), and planned to deliver at the county hospital. Of 601 CHAMACOS women enrolled, 527 remained in the study at delivery ( live-born children, including twins) in the period 2000–2001 (CHAMACOS1 participants). In 2009–2010 we enrolled a second wave of 305 mother–child dyads of children born in 2000–2002 (CHAMACOS2 participants), using similar selection criteria. CHAMACOS1 mothers completed visits during pregnancy and delivery, and children were followed at approximately 1- to 2-y intervals. CHAMACOS2 mother–child dyads completed a baseline data collection visit at 9 y. Thereafter, CHAMACOS1 and CHAMACOS2 families completed the same study visits.
The present analyses included 480 CHAMACOS1 and CHAMACOS2 participants who completed the 18-y follow-up visit prior to our March 2020 COVID-19 closure and a subset of these who were selected for a nested case–control study of liver inflammation at age 18 y (Supplementary Figure 1). Cases () were defined by having an alanine transaminase (ALT) or an aspartate aminotransferase (AST) for males or for females (cutoffs are based on LabCorp reference ranges); controls () had normal ALT and AST levels and were randomly selected and frequency-matched to cases on sex. Cases and controls were required to have maternal pregnancy and/or child urine specimens collected and stored from earlier study waves ( were eligible for selection).
Mothers provided written informed consent; children provided verbal assent starting at age 7 y, written assent starting at age 12 y, and full written consent at age 18 y. All study activities were approved by the University of California, Berkeley Office for the Protection of Human Subjects.
Study Procedures
The specific data and sample collection procedures are described below:
Maternal and youth interviews.
Mothers were interviewed in English or Spanish by trained bilingual bicultural research assistants who used structured questionnaires at each study visit, including the prenatal visit at 26 wk gestation, and the child follow-up visits at 5, 14, and 18 y. We collected information on family occupation history, socioeconomic status, medical history, and lifestyle factors. Youth were interviewed in English at ages 14 and 18 y.
Dietary assessments.
Mothers were interviewed using a validated food frequency questionnaire (FFQ) of their diet at 26 wk gestation35–37 and their child’s diet at age 5 y.38,39 Youth completed a validated food screener at 14 y40 and basic diet questions at 18 y.41,42 In the FFQ administered during pregnancy, women were asked how often they ate various food items in the previous 3 months and how much they consumed each time. In the FFQ at 5 y, mothers were asked about the number of times their child ate various foods in the previous 4 wk. In the food screener at 14 y, the youth were asked the number of days in the previous week they ate or drank different food items and how much in 1 day. The validated FFQs and screener underwent proprietary data processing (Pregnancy35–37 and 14-y FFQL Nutritionquest40; 5-y FFQ: Harvard Nutrition Questionnaire Service Center)38,39,43 to convert reported dietary intake into summary variables and individual food items. We selected a priori those dietary variables that included foods commonly treated with glyphosate. We dichotomized continuous summary variables (i.e., total calories, total carbohydrates, whole grains, bran, fruits, and vegetables) into being above or below the median observed in our sample; we also dichotomized reported intake of individual foods (i.e., cold cereal, hot cereal, bread, tortillas, legumes) (e.g., time per day vs. time per day). In addition to the administration of the validated FFQs and screener, we asked mothers how frequently their 5-y-old children consumed fast food; we directly asked young adults this question at the 14-y and 18-y visits. We asked the 14- and 18-y-olds to report on their overall alcohol consumption; for 18-y-olds, we also asked about recent binge drinking ( drinks in a row for females, for males). At the 5-, 14-, and 18-y visits, we queried the mothers about family food security (U.S. Department of Agriculture Food Security Scale, Short Form).44
Body measurements.
At the 18-y visit, we recorded young adult participants’ height in triplicate using a wall-mounted stadiometer and their weight (a single measurement) using a Tanita bioimpedance scale (Tanita TBF-300A Body Composition Analyzer; Tanita Corporation). We calculated body mass index (BMI) based on the average recorded height and the single weight measurement. We measured waist circumference three times with a measuring tape wrapped around the abdomen, parallel to the floor, at the iliac crest. We present the average of these three measurements as the waist circumference. We measured blood pressure in triplicate using an automated oscillometric monitor (Dinamap Carescape V100). Participants sat and rested for two minutes prior to the first reading and had a 1-min rest between each subsequent reading. We present the average of the second two systolic and diastolic blood pressure readings, respectively, as the measured blood pressure.
Blood collection for clinical chemistries.
At the 18-y visit, a fasting blood sample was collected from the young adults via venipuncture and analyzed for ALT, AST, glucose, high-density lipoprotein (HDL) cholesterol, and serum triglycerides (LabCorp). For those with elevated ALT, we also measured bilirubin and Hepatitis B and C (LabCorp) as well as ceruloplasmin and actin smooth muscle antibodies (LabCorp) to rule out common causes of liver disease other than NAFLD.
Urinary measurements of glyphosate and AMPA.
For the case–control subgroup, we analyzed maternal urine collected at approximately 26 wk gestation and child urine collected at the 5-y, 14-y, and 18-y visits. All urine specimens were aliquoted into clean glass containers with Teflon caps and stored at at our Salinas research field office until shipment on dry ice to our University of California Berkeley–based biorepository, where they were stored at . Aliquots were kept in a frozen state until analysis with no intermediate freeze-thaw cycle. For prenatal, 5-y, and 14-y visits, spot urine samples were collected when participants had not fasted. However, at the 18-y visit, 40% ( out of 121) of participants provided a nonfirst morning void spot urine sample under fasting conditions to coincide with a fasting blood draw.
Aliquots of maternal prenatal and child urine samples for our selected cases and controls were shipped on dry ice to the Center de Toxicologie du Québec, Institut National de Santé Publique du Québec (INSPQ). Glyphosate and AMPA were measured in a single extraction by ultraperformance liquid chromatography (UPLC)–mass spectroscopy/mass spectroscopy (MSMS) at INSPQ. This method was performed as previously published.45 External quality control for glyphosate and/or AMPA was ensured by INSPQ’s successful participation in the Quebec External Quality Assessment Scheme for Organic Substances in Urine (OSEQAS), German External Quality Assessment Scheme (G-EQUAS), and Human Biomonitoring for Europe (HBM4EU, reference laboratory) program (see certificates of participation in the Supplementary Material). Limits of detection (LOD) were for glyphosate and for AMPA. Specific gravity was measured by a refractometer (Atago Company Ltd.).
California Pesticide Use Reporting (PUR) data.
For the full cohort, we recorded families’ residential addresses each time they attended a study visit. In addition, at the 16-y visit, mothers completed a detailed residential history interview in which all residences from the start of their pregnancy through their child’s 16-y visit were obtained.
To characterize potential exposure, we estimated agricultural glyphosate use near each participant’s residence during the prenatal and postnatal (birth to 5-y visit) time periods using PUR data from 1999 to 2007.46–48 PUR data include the amount (kilograms) of active ingredient applied, application date, and location to a 1-square-mile section () defined by the U.S. Public Land Survey System (PLSS).47–49 We weighted the amount of glyphosate applied in each section by the proportion of land area that was included in a radius and accounted for the potential downwind transport of glyphosate from the application site using wind direction from the closest meteorological station50 based on the daily proportion of time the wind blew from each of eight directions. We summed all glyphosate agricultural applications to determine estimates of the wind-weighted amount of glyphosate (kg) applied around all residences for each participant during pregnancy and from birth to the 5-y visit.
Data Analysis
We used chi-square tests to compare the detection frequencies of glyphosate and AMPA concentrations measured in urine samples collected from the mother during pregnancy and from the child at 5, 14, and 18 y. We fitted crude and multivariable Poisson regression models using robust standard errors for specific-gravity adjusted urinary glyphosate and AMPA concentrations, as well as total glyphosate residue concentrations, in relationship to a) case–control status defined by liver transaminases, b) metabolic syndrome, and c) other clinical chemistry and anthropometry measures measured at 18 y of age (dichotomized as within or outside normal clinical limits; see more details below). We estimated exposure to total glyphosate residues using the formula [].29,51,52 This formula, proposed by the Joint Meeting on Pesticide Residues,53 is derived from the ratio of the AMPA molecular weight to the glyphosate molecular weight () and assumes that AMPA and glyphosate have similar human toxicity. We used glyphosate, AMPA, and total glyphosate residue concentrations to reduce the influence of outliers. Values below the LOD were randomly imputed based on a log-normal distribution using maximum likelihood estimation.54 Models were run for time points when at least half of participants had concentrations above the LOD, which included 14- and 18-y glyphosate and glyphosate residue concentrations, and 5-, 14-, and 18-y AMPA concentrations. We fitted models for 18-y nonfasting urinary concentrations and for all 18-y urinary concentrations.
In the models for metabolic syndrome and other clinical chemistry and anthropometry measures collected at 18 y, we corrected for oversampling of individuals with elevated markers of liver inflammation (and by extension, males) using stratum-specific weights for elevated ALT/AST and sex, based on the ratio of the proportions of each group in the case–control subset and full study population (i.e., male controls: 0.887; male cases: 0.373; female controls: 2.668; and female cases: 0.364).55,56 The following clinical cutoffs for adults were used for these models: high-density lipoprotein (HDL) cholesterol for males or for females, serum triglycerides , fasting glucose , BMI , waist circumference inches for males or inches for females, and systolic blood pressure Hg and diastolic Hg.57–59 The presence of metabolic syndrome was indicated by having at least three of the following five factors: a) high systolic blood pressure or diastolic blood pressure; b) large waist circumference; c) elevated fasting serum glucose; d) elevated serum triglycerides; and e) low HDL cholesterol.59
To approximate lifetime glyphosate exposure, we fitted multiple informant models with repeated urinary concentrations at the 5-y, 14-y, and 18-y visits, using mixed-effects Poisson models with a random intercept for each participant60 (we did not include urinary concentrations during pregnancy because the detection frequency was low). For participants who provided a fasting urine sample at 18 y, their 18-y measurement was excluded, but their 5-y and 14-y measurements were retained in the models. To determine whether exposure–outcome associations differed across the visits at which samples were collected and thus the appropriateness of multiple informant models, we also ran models that included exposure × visit interaction terms.
We examined whether BMI (continuous) at 14 y mediated the observed associations of urinary AMPA and glyphosate residue concentrations with the outcomes of interest using Structural Equation Models (SEMs).61 In the case–control study group, we conducted sensitivity analyses excluding eight cases who had high actin ( U) or low ceruloplasmin ( male, female) levels (based on LabCorp adult reference ranges) and/or who reported recent binge drinking in the past 30 d. We also used t-tests to examine the associations of dietary factors (dichotomous summary variables and individual food items) with glyphosate, AMPA, and glyphosate residues, measured concurrently.
We examined the correlation of maternal and child urinary concentrations of glyphosate and AMPA and PUR data from birth to 5 y. We constructed models of prenatal and postnatal PUR data in relationship to dichotomized clinical chemistry measures, anthropometric measures, and metabolic syndrome for the case–control subset () and the entire 18-y sample ( for clinical chemistries, for anthropometry). Because only 50.1% of the women lived within of an agricultural glyphosate application during pregnancy, we modeled prenatal exposure to glyphosate as a binary measure (zero vs. nonzero use within ). Because 95.2% of children lived near agricultural glyphosate between birth and age 5 y, we modeled postnatal exposure using the sum () of all agricultural glyphosate used during this period.
Covariates for multivariable models using urinary concentrations and PUR data were selected using a directed acyclic graph (DAG) (Supplemental Figure 2) and included youths’ sex and any alcohol consumption at 18 y (yes vs. no), maternal prepregnancy BMI (continuous), parental work in agriculture during the prenatal period (yes vs. no), as well as household poverty (above the poverty line vs. below)62 and food security (high/marginal food security vs. low/very low food security)44 at the time of sample collection (for models of urinary concentrations) or at the 18-y visit (for models of PUR data).
All statistical analyses were conducted using Stata 15.0 (StataCorp) and ArcMap 10.6.1 (Esri Corp.).
Results
In Table 1, we present demographic information of the study participants. Most mothers were overweight or obese before pregnancy. Seventy percent of the young adult cases were male in comparison with 47.5% in the full 18-y cohort. At the 18-y visit, 42.5% of the families were living at or below the federal poverty line, and 28.2% were at low or very low food security. Among the 18-y young adults, 10.6% fulfilled the criteria for metabolic syndrome, and 57.2% were overweight or obese (Table 2). In the case–control subset, 28.8% of cases vs. 4.4% of controls fulfilled the criteria for metabolic syndrome, and 85.0% of cases were overweight or obese vs. 57.2% of controls.
Table 1.
Demographic characteristics of participants in the liver disease nested case–control study and all 18-y-old participants, CHAMACOS study, 1999–2020 [n (%)].
| All 18-y-old participantsa () |
Casesb () |
Controlsb () |
|
|---|---|---|---|
| Maternal age at delivery (y) | |||
| 18–24 | 200 (41.7) | 30 (50.0) | 32 (35.2) |
| 25–29 | 148 (30.8) | 15 (25.0) | 35 (38.5) |
| 30–34 | 84 (17.5) | 7 (11.7) | 11 (12.1) |
| 35–45 | 48 (10.0) | 8 (13.3) | 13 (14.3) |
| Maternal education | |||
| grade | 207 (43.1) | 25 (41.7) | 47 (51.6) |
| 7th–12th grade | 162 (33.8) | 24 (40.0) | 31 (34.1) |
| High school graduate | 111 (23.1) | 11 (18.3) | 13 (14.3) |
| Marital status at pregnancy | |||
| Not married/living as married | 70 (14.6) | 8 (13.6) | 16 (17.6) |
| Married/living as married | 408 (85.4) | 51 (86.4) | 75 (82.4) |
| Missing | 2 | 1 | 0 |
| Years in U.S. prior to delivery | |||
| y | 81 (16.9) | 7 (11.7) | 22 (24.2) |
| 2–5 y | 139 (29.0) | 17 (28.3) | 22 (24.2) |
| 6–10 y | 121 (25.2) | 22 (36.7) | 26 (28.6) |
| y, nonnative | 96 (20.0) | 12 (20.0) | 16 (17.6) |
| Entire life | 43 (9.0) | 2 (3.3) | 5 (5.5) |
| Language spoken at home (during pregnancy) | |||
| Spanish primarily | 438 (91.6) | 57 (96.6) | 84 (92.3) |
| Spanish and English equally | 17 (3.6) | 1 (1.7) | 4 (4.4) |
| English primarily | 19 (4.0) | 1 (1.7) | 2 (2.2) |
| Other | 4 (0.8) | 0 (0.0) | 1 (1.1) |
| Missing | 2 | 1 | 0 |
| Maternal prepregnancy BMI | |||
| Normal or underweight () | 160 (33.4) | 14 (23.3) | 34 (37.4) |
| Overweight () | 201 (42.0) | 22 (36.7) | 38 (41.8) |
| Obese () | 118 (24.6) | 24 (40.0) | 19 (20.9) |
| Missing | 1 | 0 | 0 |
| Parental work in agriculture during pregnancy | |||
| Yes | 356 (74.5) | 41 (69.5) | 70 (76.9) |
| No | 122 (25.5) | 18 (30.5) | 21 (23.1) |
| Missing | 2 | 1 | 0 |
| Participant sex | |||
| Male | 228 (47.5) | 42 (70.0) | 64 (70.3) |
| Female | 252 (52.5) | 18 (30.0) | 27 (29.7) |
| Any alcohol consumption at 18 y | |||
| Yes | 249 (52.1) | 27 (45.8) | 48 (52.7) |
| No | 229 (47.9) | 32 (54.2) | 43 (47.3) |
| Missing | 2 | 1 | 0 |
| Household poverty at 18 y | |||
| At or below poverty line | 196 (42.5) | 31 (52.5) | 37 (41.1) |
| Above the poverty line | 265 (57.5) | 28 (47.5) | 53 (58.9) |
| Missing | 19 | 1 | 1 |
| Food security at 18 y | |||
| High or marginal | 338 (71.8) | 42 (70.0) | 61 (67.0) |
| Low | 97 (20.6) | 13 (21.7) | 21 (23.1) |
| Very low | 36 (7.6) | 5 (8.3) | 9 (9.9) |
| Missing | 9 | 0 | 0 |
Note: BMI, body mass index; CHAMACOS, Center for the Health Assessment of Mothers and Children of Salinas; U.S., United States: .
Participants with blood draw and clinical chemistry at 18-y visit prior to onset of COVID-19 shelter in place (March 2020).
Participants with urinary glyphosate measurements.
Table 2.
Liver clinical chemistry measures and metabolic outcomes of all 18-y-old CHAMACOS participants and those in the nested case–control subset {GM [GSD] or n (%)}.
| Outcome | All 18-y-old participants () |
Cases () |
Controls () |
|---|---|---|---|
| Elevated liver transaminases | 61 (14.7) | 60 (100.0) | 0 (0.0) |
| ALT (IU/L)a | 18.9 [1.9] | 57.8 [1.6] | 17.1 [1.5] |
| AST (IU/L)a | 19.0 [1.5] | 35.6 [1.5] | 17.8 [1.3] |
| BMI category | — | — | — |
| Normal () | 203 (42.8) | 9 (15.0) | 39 (42.9) |
| Overweight () | 130 (27.4) | 13 (21.7) | 30 (33.0) |
| Obese () | 141 (29.8) | 38 (63.3) | 22 (24.2) |
| Metabolic syndrome | 43 (10.6) | 17 (28.8) | 4 (4.4) |
| Blood pressure (systolic or diastolic Hg) | 52 (11.0) | 15 (25.4) | 8 (8.8) |
| Waist circumference ( in for male, in for female) | 185 (39.2) | 42 (70.0) | 24 (26.4) |
| Fasting glucose () | 20 (4.8) | 8 (13.3) | 4 (4.4) |
| Triglycerides () | 50 (12.1) | 18 (30.0) | 6 (6.6) |
| HDL cholesterol ( male, female) | 158 (38.1) | 29 (48.3) | 29 (31.9) |
Note: —, no data; ALT, alanine transaminase; AST, aspartate aminotransferase; BMI, body mass index; CHAMACOS, Center for the Health Assessment of Mothers and Children of Salinas; GM, geometric mean; GSM, geometric standard deviation; HDL, high-density lipoprotein; in, inches: .
Geometric mean (geometric standard deviation).
Urinary Glyphosate and AMPA Concentrations
Few prenatal samples had detectable concentrations of glyphosate (4.2% ) or AMPA (14.1% ), and these percentages did not differ between cases and controls (Supplemental Tables 1 and 2). Overall, detection frequencies of glyphosate and AMPA were higher for children than for pregnant mothers (Supplemental Table 1).
The detection frequency of glyphosate was low at age 5 y (35.2% ) and differed somewhat between cases and controls (46.9% vs. 28.6%, ) (Supplemental Table 2), whereas the detection frequency of AMPA was higher than for glyphosate at age 5 y (76.9% ) with a specific gravity-corrected geometric mean () of and a geometric standard deviation () of 2.53 (Supplemental Table 1) but did not differ between cases and controls (Supplemental Table 2).
At 14 and 18 y, cases and controls did not differ in the proportion of urine samples with detectable levels of glyphosate or AMPA (Supplemental Table 2). Both glyphosate and AMPA had higher detection frequencies and geometric means at age 14 y than at all other ages [78.9% ; (2.37); and 93.3% ; (2.27); respectively] (Supplemental Table 1). Specifically, 18-y-olds overall had lower detection frequencies and geometric means of glyphosate [54.6% ; (2.77)] and AMPA [66.9% ; (2.46)] than at 14 y, even among those who had not fasted [glyphosate: 65.8% ; (2.80) and AMPA: 75.3% ; (2.76)]. In comparison with 18-y-olds who had fasted, those who had not fasted had a greater proportion of samples above the detection limit for glyphosate (65.8% vs. 37.5%) and AMPA (75.3% vs. 54.2%).
Urinary concentrations of glyphosate and AMPA were correlated within each study wave () (Supplemental Table 3). Dietary factors were only modestly associated with urinary glyphosate and AMPA concentrations (Supplemental Table 4): Higher consumption of cold cereal was associated with somewhat higher AMPA and glyphosate residue concentrations at 5 y; higher total caloric and carbohydrate intake and higher consumption of hot cereal, bread, and fruits and vegetables were associated with higher concentrations of glyphosate at 14 y.
Residential Proximity to Glyphosate Use
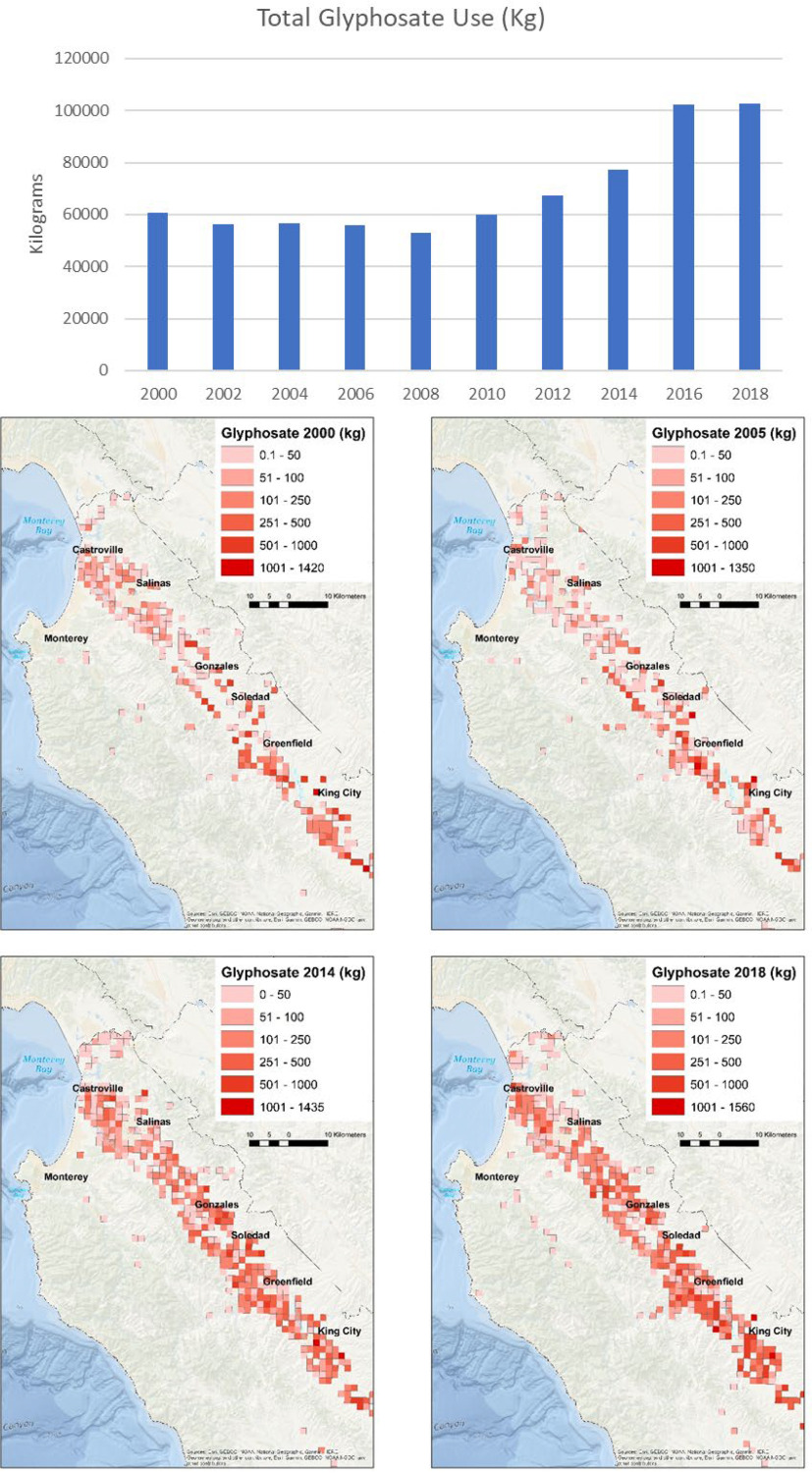
Agricultural applications of glyphosate were low during the time of pregnancy () and age 5 y visits () but higher at ages 14 () and 18 () (Figure 1; Supplemental Table 5). Glyphosate use near the child’s residence during early childhood (birth to 5 y) was not correlated with urinary glyphosate concentrations at age 5 () and weakly correlated with urinary AMPA concentrations at this same age () (Supplemental Table 3).
Figure 1.
Agricultural use of glyphosate in Monterey County, California, 2000–2018. Note: Sources: Esri, General Bathymetric Chart of the Oceans (GEBCO), National Oceanic and Atmospheric Administration, National Geographic, Garmin, HERE, Geonames.org, and other contributors.
Associations of Urinary Glyphosate and AMPA Concentrations with Liver and Cardiometabolic Outcomes
We observed associations of urinary AMPA and glyphosate residues with markers of liver inflammation (Table 3; Supplemental Table 6). Higher AMPA concentrations at 5 y were associated with elevated liver transaminases at 18 y [relative risk ; 95% confidence interval (CI): 1.06, 1.53]. Higher AMPA and glyphosate residue concentrations at 18 y among nonfasting participants were marginally associated with elevated liver enzymes [ (95% CI: 0.98, 1.32) and (95% CI: 1.00, 1.35), respectively]; these associations were further attenuated when fasting 18-y-olds were included (Supplemental Table 7). RRs remained similar when we excluded the eight cases with high actin, with low ceruloplasmin, and/or who binge-drank alcohol (Supplemental Table 8).
Table 3.
Adjusteda RRs and 95% CI for 2-fold increases in child urinary glyphosate, AMPA, and glyphosate residue concentrations (specific gravity-corrected, ) and abnormal markers of liver inflammation and metabolic syndrome (and its components) in CHAMACOS young adults in case–control group.
| Outcome | Glyphosate | AMPA | Glyphosate residuesb | ||||
|---|---|---|---|---|---|---|---|
| 14 y () |
18 yc () |
5 y () |
14 y () |
18 yc () |
14 y () |
18 yc () |
|
| Elevated liver transaminases | 1.07 (0.89, 1.29) | 1.11 (0.97, 1.27) | 1.27 (1.06, 1.53) | 1.16 (0.96, 1.39) | 1.13 (0.98, 1.32) | 1.15 (0.93, 1.42) | 1.16 (1.00, 1.35) |
| Metabolic syndrome | 1.22 (0.76, 1.96) | 1.20 (0.71, 2.02) | 2.07 (1.38, 3.11) | 1.80 (1.10, 2.93) | 1.59 (0.99, 2.54) | 1.88 (1.03, 3.42) | 1.54 (0.94, 2.52) |
| High blood pressure | 1.30 (0.83, 2.04) | 0.98 (0.70, 1.37) | 1.29 (0.80, 2.10) | 1.50 (0.98, 2.30) | 1.28 (0.88, 1.86) | 1.55 (0.93, 3.60) | 1.23 (0.85, 1.79) |
| Large waist circumference | 0.88 (0.71, 1.10) | 1.19 (0.94, 1.50) | 1.11 (0.88, 1.40) | 1.15 (0.87, 1.53) | 1.26 (0.96, 1.65) | 1.06 (0.74, 1.52) | 1.33 (1.02, 1.73) |
| High glucose | 0.95 (0.57, 1.60) | 1.75 (0.20, 15.49) | 2.95 (1.70, 5.14) | 1.63 (0.92, 2.88) | 4.29 (0.31, 59.55) | 1.55 (0.75, 3.20) | 3.16 (0.30, 33.36) |
| High triglycerides | 0.95 (0.70, 1.31) | 1.18 (0.92, 1.52) | 1.39 (0.81, 2.37) | 1.51 (1.03, 2.22) | 1.45 (1.07, 1.96) | 1.40 (0.90, 2.18) | 1.39 (1.04, 1.88) |
| Low HDL cholesterol | 0.86 (0.70, 1.05) | 1.16 (0.94, 1.42) | 0.90 (0.72, 1.12) | 1.19 (0.96, 1.48) | 1.15 (0.89, 1.48) | 1.11 (0.84, 1.45) | 1.20 (0.93, 1.54) |
Note: AMPA, aminomethylphosphonic acid; BMI, body mass index; CHAMACOS, Center for the Health Assessment of Mothers and Children of Salinas; CI, confidence interval; HDL, high-density lipoprotein; RR, relative risk.
Models adjusted for sex, any alcohol consumption at 18 y (yes/no), maternal prepregnancy BMI, parental work in agriculture during pregnancy (yes/no), household poverty status at time of visit (above vs. below the poverty threshold), and food security at time of visit (high/marginal security vs. low and very low security).
Calculated using the formula: [].
Limited to participants with nonfasting urine samples.
We also found associations of urinary AMPA and total glyphosate residue concentrations with metabolic syndrome and related conditions. Two-fold increases in AMPA at 5 y (, 95% CI: 1.38, 3.11) and in AMPA and glyphosate residues at 14 y [ (95% CI: 1.10, 2.93) and (95% CI: 1.03, 3.42), respectively] and at 18 y (among nonfasters) [ (95% CI: 0.99, 2.54) and (95% CI: 0.94, 2.52), respectively] were associated with a 50% or greater increased risk of metabolic syndrome at 18 y (Table 3; Supplemental Table 6). In addition, higher AMPA concentrations at age 5 y were associated with elevated glucose levels (, 95% CI: 1.70, 5.14), and higher AMPA concentrations at ages 14 y and 18 y (among nonfasters) were associated with elevated triglycerides [ (95% CI: 1.03, 2.22) and (95% CI: 1.07, 1.96) respectively]. Higher urinary glyphosate residue concentrations at age 18 y (among nonfasters) were associated with elevated triglycerides (, 95% CI: 1.04, 1.88) and large waist circumference (, 95% CI: 1.02, 1.73).
In multiple informant models, we did not find evidence of interaction by visit in the associations of glyphosate, AMPA, or glyphosate residue concentrations with our outcomes (Supplemental Table 9). Therefore, we fitted models without interaction terms, using repeated measurements to approximate lifetime exposure (Table 4). In these models, a 2-fold increase in childhood urinary concentrations of AMPA was associated with a 14% increased risk of elevated liver transaminases (95% CI: 1.05, 1.23) and a 55% increased risk of metabolic syndrome (95% CI: 1.19, 2.02) at age 18 y. Higher childhood urinary concentrations of AMPA were also associated with elevated blood pressure, glucose, and triglycerides and with larger waist circumference. Higher childhood glyphosate residue concentrations were also associated with increased risks of elevated liver transaminases (RR per 2-fold increase in , 95% CI: 1.05, 1.22), metabolic syndrome (, 95% CI: 1.12, 2.06), and elevated triglycerides (, 95% CI: 1.01, 1.46) (Table 4).
Table 4.
Multiple informant models (RRs and 95% CI) for repeated child urinary glyphosate, AMPA, and glyphosate residue concentrations (specific gravity-corrected, ) at the 5-y, 14-y, and 18-y visits and abnormal markers of liver inflammation and metabolic syndrome (and its components), using mixed-effects Poisson models with a random intercept for each CHAMACOS participant.a,b
| Glyphosate () |
AMPA () |
Glyphosate residuesc () |
|
|---|---|---|---|
| Elevated liver transaminases | 1.05 (0.98, 1.13) | 1.14 (1.05, 1.23) | 1.13 (1.05, 1.22) |
| Metabolic syndrome | 1.14 (0.82, 1.59) | 1.55 (1.19, 2.02) | 1.52 (1.12, 2.06) |
| High blood pressure | 1.08 (0.85, 1.38) | 1.23 (1.01, 1.50) | 1.22 (0.99, 1.51) |
| Large waist circumference | 1.02 (0.94, 1.12) | 1.06 (0.99, 1.14) | 1.06 (0.99, 1.14) |
| High glucose | 1.11 (0.82, 1.49) | 1.35 (1.02, 1.77) | 1.30 (0.96, 1.77) |
| High triglycerides | 0.99 (0.86, 1.14) | 1.27 (1.07, 1.52) | 1.22 (1.01, 1.46) |
| Low HDL cholesterol | 0.94 (0.87, 1.02) | 1.01 (0.96, 1.07) | 0.99 (0.93, 1.06) |
Note: AMPA, aminomethylphosphonic acid; BMI, body mass index; CHAMACOS, Center for the Health Assessment of Mothers and Children of Salinas; CI, confidence interval; HDL, high-density lipoprotein; RR, relative risk.
Fasting urine samples taken at 18 y are not included in models, but 5-y and 14-y samples are still included for those participants.
Models adjusted for sex, any alcohol consumption at 18 years (yes/no), maternal prepregnancy BMI, parental work in agriculture during pregnancy (yes/no), household poverty status at 18 y (above vs. below the poverty threshold), and food security at 18 y (high/marginal security vs. low and very low security).
Calculated using the formula: [].
In the SEM models, we found no evidence that associations of urinary AMPA or glyphosate residues with elevated liver transaminases or metabolic syndrome were mediated by young adult BMI (Supplemental Table 10).
Association of Residential Proximity to Glyphosate Use with Liver and Cardiometabolic Outcomes
Any agricultural use of glyphosate near the home during pregnancy was associated with an increased risk of metabolic syndrome in the case–control subset (, 95% CI: 1.12, 10.42) but not in the full sample (, 95% CI: 0.66, 2.05) (Table 5; Supplemental Table 11). A 2-fold increase in nearby glyphosate use during early childhood was also associated with an increased risk of metabolic syndrome in the case–control group (, 95% CI: 1.16, 2.02), and with a somewhat elevated risk in the full sample (, 95% CI: 0.97, 1.35) (Table 5, Supplemental Table 11). In the case–control group, we also observed increased risk of elevated triglyceride levels with any nearby agricultural glyphosate use during pregnancy (RR per , 95% CI: 1.36, 8.33) as well as with all agricultural glyphosate use during early childhood (, 95% CI: 1.11, 1.88) (Table 5).
Table 5.
Adjusteda RRs and 95% CI for living within of agricultural glyphosate use during maternal pregnancy (any use) and from birth to age 5 y (all use, in kilograms, ) based on the California Pesticide Use Reporting (PUR) data and presence of elevated markers of liver inflammation or metabolic syndrome (and its components) among all CHAMACOS young adult participants and in case–control subset.
| Outcome | Any PUR use near home residence during pregnancy (yes/no) | Sum of PUR use near home residence from birth to age 5 y () | ||
|---|---|---|---|---|
| All 18-y-old participants () |
Case–control subset () |
All 18-y-old participants () |
Case–control subset () |
|
| Elevated liver transaminases | 0.83 (0.53, 1.30) | 1.14 (0.77, 1.71) | 0.93 (0.80, 1.08) | 0.98 (0.86, 1.11) |
| Metabolic syndrome | 1.16 (0.66, 2.05) | 3.42 (1.12, 10.42) | 1.15 (0.97, 1.35) | 1.53 (1.16, 2.02) |
| High blood pressure | 1.00 (0.60, 1.67) | 0.89 (0.40, 1.99) | 0.95 (0.78, 1.16) | 1.11 (0.85, 1.46) |
| Large waist circumference | 1.04 (0.84, 1.29) | 1.23 (0.74, 2.05) | 1.05 (0.97, 1.13) | 0.97 (0.82, 1.15) |
| High glucose | 0.78 (0.31, 1.95) | 0.39 (0.10, 1.54) | 0.87 (0.65, 1.15) | 0.79 (0.56, 1.11) |
| High triglycerides | 0.99 (0.58, 1.67) | 3.37 (1.36, 8.33) | 1.09 (0.90, 1.32) | 1.45 (1.11, 1.88) |
| Low HDL cholesterol | 1.04 (0.81, 1.32) | 1.07 (0.64, 1.78) | 1.00 (0.91, 1.09) | 0.91 (0.76, 1.07) |
Note: BMI, body mass index; CHAMACOS, Center for the Health Assessment of Mothers and Children of Salinas; CI, confidence interval; HDL, high-density lipoprotein; PUR, Pesticide Use Reporting; RR, relative risk.
Models adjusted for sex, alcohol use at 18 y (never/ever), maternal prepregnancy BMI, parental work in agriculture during pregnancy (yes/no), household poverty status at 18 y (above vs. below the poverty threshold), and food security at 18 y (high/marginal security vs. low and very low security).
Discussion
We observed associations of glyphosate or AMPA exposure during childhood with liver inflammation and metabolic syndrome at young adulthood. More specifically, after accounting for multiple potential confounders, we found that higher urinary concentrations of AMPA, a degradation product of glyphosate and amino-polyphosphonates, and glyphosate residues between ages 5 and 18 y were associated with both elevated liver transaminases and metabolic syndrome at age 18 y. This association could not be explained by mediation by body mass. In addition, we found that agricultural glyphosate use during the prenatal period and/or childhood (from birth to age 5 y) was associated with metabolic syndrome at 18 y.
Our findings are consistent with Mills et al.,29 who observed that urinary concentrations of AMPA and total glyphosate residues were elevated in 34 patients with nonalcoholic steatohepatitis (NASH) in comparison with 63 controls and more elevated in cases with more advanced fibrosis than those with less. These findings are also consistent with hepatotoxicity noted at much higher doses in glyphosate poisoning cases and with occupational exposures.30,31 In rodent studies, even low dosages of glyphosate or glyphosate-based herbicide formulations produced signs of NAFLD,24 as evidenced by fibrosis, steatosis, and necrosis of the liver.25 Glyphosate and glyphosate-based herbicide formulations have been found to alter the metabolome, proteome,24 transcriptome,26 epigenome,27 and DNA28 of the liver in rodent studies. Exposure to glyphosate, glyphosate-based herbicides, and AMPA has also induced epigenetic modifications in in vitro studies of human peripheral blood mononuclear cells.27
In a study of male rats, Prasad et al.33 found a dose-related increase in fasting blood glucose and serum insulin in glyphosate-exposed groups in comparison with controls. To our knowledge, the association of glyphosate with insulin resistance and other metabolic disorders has not been previously explored in human populations, although researchers have hypothesized that glyphosate has the potential to induce metabolic disease because of its ability to induce oxidative stress in preadipocytes and in other tissues.32,33,63–65 A second hypothesis for glyphosate’s etiologic role in metabolic disorders is its adverse effect on the gut microbiota, which have been shown in animal studies to be a source of oxidative stress.66 Recent investigations in rats have shown that glyphosate-containing herbicides inhibit the shikimate pathway in the gut microbiome.66 A third hypothesis for the association is through endocrine disruption,67 with evidence that glyphosate and glyphosate-containing herbicides can disrupt endocrine-signaling systems.33,68,69 Although the association of exposure to AMPA with metabolic disorders has been neither explored nor hypothesized, a recent in vitro study based on induced pluripotent stem cells (iPSCs) found changes in glucose metabolism following treatment to glyphosate or AMPA.70
Most of our prenatal urine samples, all collected around year 2000, had nondetectable levels of glyphosate and AMPA, consistent with the low use of glyphosate in agriculture around that time.8 With this exception, detection frequencies and concentrations in urine samples collected during childhood were within the range of those reported in other studies of children.71–74 Adolescents (12- to 19-y-olds) participating in the 2013–2014 National Health and Nutrition Examination Survey (NHANES) had a higher weighted detection frequency (87.2% ) and wet weight geometric mean () of urinary glyphosate concentrations than the 14-y-olds included in our study (who provided urine samples collected around the same time) (78.9% , , respectively)75; urinary AMPA concentrations were not measured in NHANES adolescents. However, our 14-y-old participants had higher urinary glyphosate and AMPA detection frequencies (78.9 and 93.3% , respectively) and wet weight geometric means (, respectively) than 14- to 17-y-old children participating in the 2015–2017 German Environmental Survey for Children and Adolescents (glyphosate: 46% , GM , respectively; AMPA: 42% , GM , respectively) ( for both glyphosate and AMPA).72 It is likely that diet was a major source of glyphosate and AMPA exposure among our study participants at age 14 y, as indicated by higher urinary glyphosate or AMPA concentrations among those who ate more cereal, fruits, vegetables, bread, and in general, carbohydrates. We observed lower urinary concentrations of glyphosate and AMPA at age 18 y, even among the participants who had not fasted, than at age 14 y, despite increases in agricultural glyphosate use. It is possible that differences in diet may explain the lower concentrations at age 18 y; unfortunately, our 18-y dietary questionnaire was too limited to test this hypothesis. Similar to our findings at age 18 y, the NHANES data revealed lower urinary glyphosate concentrations in those who had fasted more than 8 h in comparison with those who fasted less, supporting the importance of dietary intake in glyphosate exposure.75
A strength of our study was that we could characterize agricultural glyphosate use near homes using California’s unique PUR database. However, our estimates do not reflect the full extent of ambient glyphosate exposure; they do not account for agricultural use near participants’ schools, workplaces, or nonagricultural uses (e.g., homes, roadways, parks).46 We also did not consider use of specific formulations of glyphosate-based pesticides, which may differ in their toxicity.76 Despite these limitations, we observed associations between PUR-assessed agricultural glyphosate exposure and metabolic syndrome that are interesting and merit additional research.77
An important limitation of our study is that a single measure of glyphosate or AMPA concentrations in the urine, and even multiple measures at different developmental periods, might not accurately reflect exposure, given the short half-life of glyphosate and AMPA in humans of between 3.5 and 14.5 h.78 This possibility, along with inaccuracy in dietary recall, could explain the modest associations of urinary AMPA and glyphosate residue concentrations with dietary factors that we observed. The short half-life of glyphosate in the human body78 as well as in the environment79 (unlike AMPA, which has been classified as persistent in soil80 and groundwater81) may have contributed to the weak correlations we observed between glyphosate use near residences and urinary glyphosate or AMPA concentrations. It can also explain the lower detection and urinary concentrations among those 18-y-olds who had fasted (vs. the nonfasters), given that food was likely an important route of exposure and that the maximum concentration of glyphosate and AMPA in urine is 1–3 h and 5–6 h after exposure, respectively.82 Given these short half-lives, the urinary concentrations measured concurrently with the outcomes at 18 y might not accurately reflect the exposure to glyphosate and AMPA preceding the onset of disease, which would be important criterion to establish a causal relationship.
Although we observe some associations of nearby agricultural use of glyphosate during the prenatal period and childhood with metabolic syndrome, urinary glyphosate concentrations were not associated with any health outcomes in the present study. Nevertheless, we observed associations of urinary AMPA and glyphosate residue concentrations (with the latter largely driven by AMPA) with elevated liver transaminases and/or metabolic syndrome. It is likely that urinary AMPA was derived from degradation of glyphosate in the environment. For example, microbial degradation of glyphosate in soil results in the accumulation of AMPA in soil, plants, and animal products.18 AMPA is highly soluble in water, more persistent in the environment than glyphosate,83 and therefore frequently detected at higher concentrations than glyphosate in most hydrological settings, with groundwater and soil water samples having the highest values.81 Tracer studies in Canada have shown that AMPA in groundwater is mainly derived from glyphosate degradation rather than wastewater sources, such as those contaminated with phosphonates.84 Although few studies have measured urinary AMPA concentrations in human populations, it is known that AMPA is poorly metabolized in the human body. For example, in a study of volunteers who ingested glyphosate,82 total dose recovered as unchanged glyphosate was low (1%–6%) but extremely low for AMPA—0.01%–0.04% of the total dose of glyphosate. This low excretion of AMPA in urine after glyphosate exposure has also been demonstrated in other studies.31,85 Thus, urinary AMPA likely results from direct exposure to AMPA from food residues and water, with a lesser extent from the metabolism of glyphosate in vivo. However, additional research is needed to identify the major pathways of AMPA exposure.
Our research suggests that lifetime exposure to glyphosate and AMPA may increase risk of liver and metabolic disease in early adulthood, which could lead to more serious diseases later in life, such as liver cancer,86 diabetes, and cardiovascular disease.87 Longitudinal lifelong studies in humans, such as CHAMACOS, are necessary to connect potential impact of glyphosate and AMPA on organ damage and other intermediate outcomes to chronic illness in adulthood. Future research should include frequent measurements of exposure biomarkers during fetal and child development to determine windows of susceptibility; examine the effects of glyphosate and AMPA in the context of exposure to pesticide mixtures88,89; and explore associations with other outcomes, such as reproductive and endocrine function.67,90 In addition, studies with sufficient sample size should examine differences in susceptibility by sex, as seen in animal studies.91,92
Conclusions
Metabolic and liver diseases are increasing among youth and young adults.93 Our study suggests that glyphosate, the most commonly used herbicide worldwide, and AMPA, a degradation product of glyphosate and amino-phosphonates, may increase risk of liver inflammation and/or cardiometabolic disease in young adulthood. Although previous research on glyphosate in humans has largely focused on its potential carcinogenicity, this study indicates the need for further investigation of its association with metabolic and liver outcomes.
Supplementary Material
Acknowledgments
The authors thank the Salinas research team for their dedicated work collecting these data and the UC Berkeley biorepository team for preserving and managing biological samples. The authors also thank the CHAMACOS families for their years of participation.
This work was funded by research grant numbers UH3 ES030631, R24 ES028529, R01 ES026994, P01 ES009605, R01 ES017054, and R01 ES021369 from the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH); R01 DA035300 from the National Institute on Drug Abuse (NIDA, NIH); and R82670901, RD83171001, and RD83451301 from the U.S. EPA. Additional support for this study was provided by The Solomon Dutka Fund in the New York Community Trust and The Westreich Foundation.
References
- 1.Sanyaolu A, Okorie C, Qi X, Locke J, Rehman S. 2019. Childhood and adolescent obesity in the United States: a public health concern. Glob Pediatr Health 6:2333794X19891305, PMID: , 10.1177/2333794X19891305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wittcopp C, Conroy R. 2016. Metabolic syndrome in children and adolescents. Pediatr Rev 37(5):193–202, PMID: , 10.1542/pir.2014-0095. [DOI] [PubMed] [Google Scholar]
- 3.Jeans MR, Ghaddar R, Vandyousefi S, Landry MJ, Gray MJ, Leidy HJ, et al. 2022. Distinct racial and ethnic metabolic syndrome characteristics: a comparative assessment in low-income children 7–10 years of age. Pediatr Obes 17(10):e12925, PMID: , 10.1111/ijpo.12925. [DOI] [PubMed] [Google Scholar]
- 4.Doycheva I, Watt KD, Alkhouri N. 2017. Nonalcoholic fatty liver disease in adolescents and young adults: the next frontier in the epidemic. Hepatology 65(6):2100–2109, PMID: , 10.1002/hep.29068. [DOI] [PubMed] [Google Scholar]
- 5.Goyal NP, Schwimmer JB. 2016. The progression and natural history of pediatric nonalcoholic fatty liver disease. Clin Liver Dis 20(2):325–338, PMID: , 10.1016/j.cld.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haverinen E, Fernandez MF, Mustieles V, Tolonen H. 2021. Metabolic syndrome and endocrine disrupting chemicals: an overview of exposure and health effects. Int J Environ Res Public Health 18(24):13047, PMID: , 10.3390/ijerph182413047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y, Peterson KE. 2015. Maternal exposure to synthetic chemicals and obesity in the offspring: recent findings. Curr Environ Health Rep 2(4):339–347, PMID: , 10.1007/s40572-015-0068-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benbrook CM. 2016. Trends in glyphosate herbicide use in the United States and globally. Environ Sci Eur 28(1):3, PMID: , 10.1186/s12302-016-0070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guyton KZ, Loomis D, Grosse Y, El Ghissassi F, Benbrahim-Tallaa L, Guha N, et al. 2015. Carcinogenicity of tetrachlorvinphos, parathion, malathion, diazinon, and glyphosate. Lancet Oncol 16(5):490–491, PMID: , 10.1016/S1470-2045(15)70134-8. [DOI] [PubMed] [Google Scholar]
- 10.Roy DN, Konar SK, Banerjee S, Charles DA, Thompson DG, Prasad R. 1989. Uptake and persistence of the herbicide glyphosate (Vision®) in fruit of wild blueberry and red raspberry. Can J for Res 19(7):842–847, 10.1139/x89-128. [DOI] [Google Scholar]
- 11.Wang YS, Jaw CG, Chen YL. 1994. Accumulation of 2,4-D and glyphosate in fish and water hyacinth. Water Air Soil Pollut 74(3–4):397–403, 10.1007/BF00479802. [DOI] [Google Scholar]
- 12.Bento CPM, Goossens D, Rezaei M, Riksen M, Mol HGJ, Ritsema CJ, et al. 2017. Glyphosate and AMPA distribution in wind-eroded sediment derived from loess soil. Environ Pollut 220(pt B):1079–1089, PMID: , 10.1016/j.envpol.2016.11.033. [DOI] [PubMed] [Google Scholar]
- 13.Alferness PL, Iwata Y. 1994. Determination of glyphosate and (aminomethyl)phosphonic acid in soil, plant and animal matrixes, and water by capillary gas chromatography with mass-selective detection. J Agric Food Chem 42(12):2751–2759, 10.1021/jf00048a020. [DOI] [Google Scholar]
- 14.Granby K, Johannesen S, Vahl M. 2003. Analysis of glyphosate residues in cereals using liquid chromatography-mass spectrometry (LC-MS/MS). Food Addit Contam 20(8):692–698, PMID: , 10.1080/0265203031000109477. [DOI] [PubMed] [Google Scholar]
- 15.Çetin E, Şahan S, Ülgen A, Şahin U. 2017. DLLME-spectrophotometric determination of glyphosate residue in legumes. Food Chem 230:567–571, PMID: , 10.1016/j.foodchem.2017.03.063. [DOI] [PubMed] [Google Scholar]
- 16.Chen MX, Cao ZY, Jiang Y, Zhu ZW. 2013. Direct determination of glyphosate and its major metabolite, aminomethylphosphonic acid, in fruits and vegetables by mixed-mode hydrophilic interaction/weak anion-exchange liquid chromatography coupled with electrospray tandem mass spectrometry. J Chromatogr A 1272:90–99, PMID: , 10.1016/j.chroma.2012.11.069. [DOI] [PubMed] [Google Scholar]
- 17.Rodrigues NR, Ferreira de Souza AP. 2018. Occurrence of glyphosate and AMPA residues in soy-based infant formula sold in Brazil. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 35(4):723–730, PMID: , 10.1080/19440049.2017.1419286. [DOI] [PubMed] [Google Scholar]
- 18.Grandcoin A, Piel S, Baurès E. 2017. AminoMethylPhosphonic acid (AMPA) in natural waters: its sources, behavior and environmental fate. Water Res 117:187–197, PMID: , 10.1016/j.watres.2017.03.055. [DOI] [PubMed] [Google Scholar]
- 19.Benbrook CM. 2019. How did the US EPA and IARC reach diametrically opposed conclusions on the genotoxicity of glyphosate-based herbicides? Environ Sci Eur 31(1):2, 10.1186/s12302-018-0184-7. [DOI] [Google Scholar]
- 20.U.S. EPA (U.S. Environmental Protection Agency). 2020. Glyphosate, Interim Registration Review Decision Case Number 0178. https://www.epa.gov/sites/default/files/2020-01/documents/glyphosate-interim-reg-review-decision-case-num-0178.pdf [accessed 8 June 2022].
- 21.IARC (International Agency for Research on Cancer). 2015. IARC Monographs Volume 112: evaluation of five organophosphate insecticides and herbicides. https://www.iarc.who.int/wp-content/uploads/2018/07/MonographVolume112-1.pdf [accessed 8 June 2022].
- 22.Van Bruggen AHC, He MM, Shin K, Mai V, Jeong KC, Finckh MR, et al. 2018. Environmental and health effects of the herbicide glyphosate. Sci Total Environ 616–617:255–268, PMID: , 10.1016/j.scitotenv.2017.10.309. [DOI] [PubMed] [Google Scholar]
- 23.FAO (Food and Agriculture Organization of the United Nations), WHO (World Health Organization). 1998. Pesticide Residues in Food 1997 - Joint FAO/WHO Meeting on Pesticide Residues Part I - Residues. https://www.fao.org/publications/card/en/c/CB2747EN/ [accessed 24 August 2022].
- 24.Mesnage R, Renney G, Séralini GE, Ward M, Antoniou MN. 2017. Multiomics reveal non-alcoholic fatty liver disease in rats following chronic exposure to an ultra-low dose of roundup herbicide. Sci Rep 7:39328, PMID: , 10.1038/srep39328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mesnage R, Arno M, Costanzo M, Malatesta M, Séralini GE, Antoniou MN. 2015. Transcriptome profile analysis reflects rat liver and kidney damage following chronic ultra-low dose Roundup exposure. Environ Health 14:70, PMID: , 10.1186/s12940-015-0056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mesnage R, Teixeira M, Mandrioli D, Falcioni L, Ibragim M, Ducarmon QR, et al. 2021. Multi-omics phenotyping of the gut–liver axis reveals metabolic perturbations from a low-dose pesticide mixture in rats. Commun Biol 4(1):471, PMID: , 10.1038/s42003-021-01990-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rossetti MF, Canesini G, Lorenz V, Milesi MM, Varayoud J, Ramos JG. 2021. Epigenetic changes associated with exposure to glyphosate-based herbicides in mammals. Front Endocrinol (Lausanne) 12:671991, PMID: , 10.3389/fendo.2021.671991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mesnage R, Ibragim M, Mandrioli D, Falcioni L, Tibaldi E, Belpoggi F, et al. 2022. Comparative toxicogenomics of glyphosate and roundup herbicides by mammalian stem cell-based genotoxicity assays and molecular profiling in Sprague-Dawley rats. Toxicol Sci 186(1):83–101, PMID: , 10.1093/toxsci/kfab143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mills PJ, Caussy C, Loomba R. 2020. Glyphosate excretion is associated with steatohepatitis and advanced liver fibrosis in patients with fatty liver disease. Clin Gastroenterol Hepatol 18(3):741–743, PMID: , 10.1016/j.cgh.2019.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kamijo Y, Takai M, Sakamoto T. 2016. A multicenter retrospective survey of poisoning after ingestion of herbicides containing glyphosate potassium salt or other glyphosate salts in Japan. Clin Toxicol (Phila) 54(2):147–151, PMID: , 10.3109/15563650.2015.1121271. [DOI] [PubMed] [Google Scholar]
- 31.Zouaoui K, Dulaurent S, Gaulier JM, Moesch C, Lachâtre G. 2013. Determination of glyphosate and AMPA in blood and urine from humans: about 13 cases of acute intoxication. Forensic Sci Int 226(1–3):e20–e25, PMID: , 10.1016/j.forsciint.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 32.De Long NE, Holloway AC. 2017. Early-life chemical exposures and risk of metabolic syndrome. Diabetes Metab Syndr Obes 10:101–109, PMID: , 10.2147/DMSO.S95296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prasad M, Gatasheh MK, Alshuniaber MA, Krishnamoorthy R, Rajagopal P, Krishnamoorthy K, et al. 2022. Impact of glyphosate on the development of insulin resistance in experimental diabetic rats: role of NFκB signalling pathways. Antioxidants (Basel) 11(12):2436, PMID: , 10.3390/antiox11122436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eskenazi B, Kogut K, Huen K, Harley KG, Bouchard M, Bradman A, et al. 2014. Organophosphate pesticide exposure, PON1, and neurodevelopment in school-age children from the CHAMACOS study. Environ Res 134:149–157, PMID: , 10.1016/j.envres.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Block G, Woods M, Potosky A, Clifford C. 1990. Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol 43(12):1327–1335, PMID: , 10.1016/0895-4356(90)90099-b. [DOI] [PubMed] [Google Scholar]
- 36.Block G, Thompson FE, Hartman AM, Larkin FA, Guire KE. 1992. Comparison of two dietary questionnaires validated against multiple dietary records collected during a 1-year period. J Am Diet Assoc 92(6):686–693, PMID: , 10.1016/S0002-8223(21)00707-0. [DOI] [PubMed] [Google Scholar]
- 37.Harley K, Eskenazi B, Block G. 2005. The association of time in the US and diet during pregnancy in low-income women of Mexican descent. Paediatr Perinat Epidemiol 19(2):125–134, PMID: , 10.1111/j.1365-3016.2005.00640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosas LG, Harley K, Fernald LCH, Guendelman S, Mejia F, Neufeld LM, et al. 2009. Dietary associations of household food insecurity among children of Mexican descent: results of a binational study. J Am Diet Assoc 109(12):2001–2009, PMID: , 10.1016/j.jada.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stein AD, Shea S, Basch CE, Contento IR, Zybert P. 1992. Consistency of the Willett semiquantitative food frequency questionnaire and 24-hour dietary recalls in estimating nutrient intakes of preschool children. Am J Epidemiol 135(6):667–677, PMID: , 10.1093/oxfordjournals.aje.a116346. [DOI] [PubMed] [Google Scholar]
- 40.Hunsberger M, O’Malley J, Block T, Norris JC. 2015. Relative validation of Block Kids Food Screener for dietary assessment in children and adolescents. Matern Child Nutr 11(2):260–270, PMID: , 10.1111/j.1740-8709.2012.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.CDC (U.S. Centers for Disease Control and Prevention). 2022. Behavioral Risk Factor Surveillance System (BRFSS). https://www.cdc.gov/brfss/index.html [accessed 7 June 2022].
- 42.UCLA Center for Health Policy Research. 2009. Dietary Screener in the 2009 California Health Interview Survey. https://healthpolicy.ucla.edu/chis/data/public-use-data-file/Documents/CHIS2009_Diet_Screener_Intro.pdf [accessed 8 June 2022].
- 43.Harvard T. H. Chan School of Public Health. 2022. General Documentation. Harvard Nutrition Questionnaire Service Center. https://www.hsph.harvard.edu/nutrition-questionnaire-service-center/general-documentation/ [accessed 24 August 2022].
- 44.USDA Economic Research Service. 2012. U.S. Household Food Security Survey Module: Six-Item Short Form Economic Research Service, United States Department of Agriculture (USDA). https://www.ers.usda.gov/media/8282/short2012.pdf [accessed 8 June 2022].
- 45.Bienvenu JF, Bélanger P, Gaudreau É, Provencher G, Fleury N. 2021. Determination of glyphosate, glufosinate and their major metabolites in urine by the UPLC-MS/MS method applicable to biomonitoring and epidemiological studies. Anal Bioanal Chem 413(8):2225–2234, PMID: , 10.1007/s00216-021-03194-x. [DOI] [PubMed] [Google Scholar]
- 46.CDPR (California Department of Pesticide Regulation). 2022. Pesticide Use Reporting (PUR). https://www.cdpr.ca.gov/docs/pur/purmain.htm [accessed 25 May 2022].
- 47.Gunier RB, Ward MH, Airola M, Bell EM, Colt J, Nishioka M, et al. 2011. Determinants of agricultural pesticide concentrations in carpet dust. Environ Health Perspect 119(7):970–976, PMID: , 10.1289/ehp.1002532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gunier RB, Jerrett M, Smith DR, Jursa T, Yousefi P, Camacho J, et al. 2014. Determinants of manganese levels in house dust samples from the CHAMACOS cohort. Sci Total Environ 497–498:360–368, PMID: , 10.1016/j.scitotenv.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Esri (Environmental Systems Research Institute). 2020. World Ocean Base. https://www.arcgis.com/home/item.html?id=1e126e7520f9466c9ca28b8f28b5e500%2F [accessed 4 January 2023].
- 50.CIMIS (California Irrigation Management Information System). 2014. California Department of Water Resources. https://cimis.water.ca.gov/Default.aspx [accessed 25 May 2022].
- 51.FAO and WHO. 2019. Pesticide residues in food 2019 - Report 2019 - Extra Joint FAO/WHO Meeting on Pesticide Residues. Rome. https://www.fao.org/3/ca5711en/ca5711en.pdf [accessed 24 August 2022].
- 52.FAO and WHO. 2005. Pesticide residues in food 2005 - Report 2005 - Joint FAO/WHO Meeting on Pesticide Residues. Series number 183. Rome. https://www.fao.org/3/a0209e/A0209E.pdf [accessed 24 August 2022].
- 53.van Apeldoorn E, van Hoeven P. 1997. Toxicological and Environmental Evaluations 1994. International Peer Reviewed Chemical Safety Information. https://inchem.org/documents/jmpr/jmpmono/v097pr04.htm [accessed 24 August 2022].
- 54.Lubin JH, Colt JS, Camann D, Davis S, Cerhan JR, Severson RK, et al. 2004. Epidemiologic evaluation of measurement data in the presence of detection limits. Environ Health Perspect 112(17):1691–1696, PMID: , 10.1289/ehp.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reilly M, Torrång A, Klint A. 2005. Re-use of case-control data for analysis of new outcome variables. Stat Med 24(24):4009–4019, PMID: , 10.1002/sim.2398. [DOI] [PubMed] [Google Scholar]
- 56.Richardson DB, Rzehak P, Klenk J, Weiland SK. 2007. Analyses of case-control data for additional outcomes. Epidemiology 18(4):441–445, PMID: , 10.1097/EDE.0b013e318060d25c. [DOI] [PubMed] [Google Scholar]
- 57.CDC. 2022. About Adult BMI. https://www.cdc.gov/healthyweight/assessing/bmi/adult_bmi/index.html [accessed 8 June 2022].
- 58.NHLBI (National Heart, Lung, and Blood Institute). 2022. Metabolic Syndrome Diagnosis. https://www.nhlbi.nih.gov/health/metabolic-syndrome/diagnosis [accessed 30 May 2022].
- 59.Alberti KGMM, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. 2009. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120(16):1640–1645, PMID: , 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 60.Sánchez BN, Hu H, Litman HJ, Téllez-Rojo MM. 2011. Statistical methods to study timing of vulnerability with sparsely sampled data on environmental toxicants. Environ Health Perspect 119(3):409–415, PMID: , 10.1289/ehp.1002453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baron RM, Kenny DA. 1986. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol 51(6):1173–1182, PMID: , 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 62.U.S. Census Bureau. n.d. Poverty Thresholds: Poverty Thresholds by Size of Family and Number of Children; 2000 & 2018. https://www.census.gov/data/tables/time-series/demo/income-poverty/historical-poverty-thresholds.html [accessed 25 May 2022].
- 63.Roberts CK, Sindhu KK. 2009. Oxidative stress and metabolic syndrome. Life Sci 84(21–22):705–712, PMID: , 10.1016/j.lfs.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 64.Martini CN, Gabrielli M, Vila MDC. 2012. A commercial formulation of glyphosate inhibits proliferation and differentiation to adipocytes and induces apoptosis in 3T3-L1 fibroblasts. Toxicol In Vitro 26(6):1007–1013, PMID: , 10.1016/j.tiv.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 65.Martini CN, Gabrielli M, Brandani JN, Vila MDC. 2016. Glyphosate inhibits PPAR gamma induction and differentiation of preadipocytes and is able to induce oxidative stress. J Biochem Mol Toxicol 30(8):404–413, PMID: , 10.1002/jbt.21804. [DOI] [PubMed] [Google Scholar]
- 66.Mesnage R, Teixeira M, Mandrioli D, Falcioni L, Ducarmon QR, Zwittink RD, et al. 2021. Use of shotgun metagenomics and metabolomics to evaluate the impact of glyphosate or roundup MON 52276 on the gut microbiota and serum metabolome of Sprague-Dawley rats. Environ Health Perspect 129(1):17005, PMID: , 10.1289/EHP6990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Myers JP, Antoniou MN, Blumberg B, Carroll L, Colborn T, Everett LG, et al. 2016. Concerns over use of glyphosate-based herbicides and risks associated with exposures: a consensus statement. Environ Health 15:19, PMID: , 10.1186/s12940-016-0117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guerrero Schimpf M, Milesi MM, Zanardi MV, Varayoud J. 2022. Disruption of developmental programming with long-term consequences after exposure to a glyphosate-based herbicide in a rat model. Food Chem Toxicol 159:112695, PMID: , 10.1016/j.fct.2021.112695. [DOI] [PubMed] [Google Scholar]
- 69.Romano MA, Romano RM, Santos LD, Wisniewski P, Campos DA, de Souza PB, et al. 2012. Glyphosate impairs male offspring reproductive development by disrupting gonadotropin expression. Arch Toxicol 86(4):663–673, PMID: , 10.1007/s00204-011-0788-9. [DOI] [PubMed] [Google Scholar]
- 70.Martinez A, Al-Ahmad AJ. 2019. Effects of glyphosate and aminomethylphosphonic acid on an isogeneic model of the human blood-brain barrier. Toxicol Lett 304:39–49, PMID: , 10.1016/j.toxlet.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 71.Dereumeaux C, Mercier F, Soulard P, Hulin M, Oleko A, Pecheux M, et al. 2022. Identification of pesticides exposure biomarkers for residents living close to vineyards in France. Environ Int 159:107013, PMID: , 10.1016/j.envint.2021.107013. [DOI] [PubMed] [Google Scholar]
- 72.Lemke N, Murawski A, Schmied-Tobies MIH, Rucic E, Hoppe H-W, Conrad A, et al. 2021. Glyphosate and aminomethylphosphonic acid (AMPA) in urine of children and adolescents in Germany – human biomonitoring results of the German Environmental Survey 2014–2017 (GerES V). Environ Int 156:106769, PMID: , 10.1016/j.envint.2021.106769. [DOI] [PubMed] [Google Scholar]
- 73.Stajnko A, Snoj Tratnik J, Kosjek T, Mazej D, Jagodic M, Eržen I, et al. 2020. Seasonal glyphosate and AMPA levels in urine of children and adolescents living in rural regions of northeastern Slovenia. Environ Int 143:105985, PMID: , 10.1016/j.envint.2020.105985. [DOI] [PubMed] [Google Scholar]
- 74.Sierra-Diaz E, Celis-de la Rosa A, Lozano-Kasten F, Trasande L, Peregrina-Lucano A, Sandoval-Pinto E, et al. 2019. Urinary pesticide levels in children and adolescents residing in two agricultural communities in Mexico. Int J Environ Res Public Health 16(4):562, PMID: , 10.3390/ijerph16040562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ospina M, Schütze A, Morales-Agudelo P, Vidal M, Wong LY, Calafat AM. 2022. Exposure to glyphosate in the United States: data from the 2013–2014 National Health and Nutrition Examination Survey. Environ Int 170:107620, PMID: , 10.1016/j.envint.2022.107620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mesnage R, Defarge N, Spiroux de Vendômois J, Séralini GE. 2015. Potential toxic effects of glyphosate and its commercial formulations below regulatory limits. Food Chem Toxicol 84:133–153, PMID: , 10.1016/j.fct.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 77.Rani V, Deep G, Singh RK, Palle K, Yadav UCS. 2016. Oxidative stress and metabolic disorders: pathogenesis and therapeutic strategies. Life Sci 148:183–193, PMID: , 10.1016/j.lfs.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 78.Connolly A, Jones K, Basinas I, Galea KS, Kenny L, McGowan P, et al. 2019. Exploring the half-life of glyphosate in human urine samples. Int J Hyg Environ Health 222(2):205–210, PMID: , 10.1016/j.ijheh.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 79.Jenkins M, Locke M, Reddy K, McChesney DS, Steinriede R. 2018. Glyphosate applications, glyphosate resistant corn, and tillage on nitrification rates and distribution of nitrifying microbial communities. Soil Sci Soc Am J 81:1371–1380, 10.2136/sssaj2017.02.0063. [DOI] [Google Scholar]
- 80.Bento CPM, Yang X, Gort G, Xue S, van Dam R, Zomer P, et al. 2016. Persistence of glyphosate and aminomethylphosphonic acid in loess soil under different combinations of temperature, soil moisture and light/darkness. Sci Tot Environ 572:301–311, PMID: , 10.1016/j.scitotenv.2016.07.215. [DOI] [PubMed] [Google Scholar]
- 81.Battaglin WA, Meyer MT, Kuivila KM, Dietze JE. 2014. Glyphosate and its degradation product AMPA occur frequently and widely in U.S. soils, surface water, groundwater, and precipitation. J Am Water Resour Assoc 50(2):275–290, 10.1111/jawr.12159. [DOI] [Google Scholar]
- 82.Faniband MH, Norén E, Littorin M, Lindh CH. 2021. Human experimental exposure to glyphosate and biomonitoring of young Swedish adults. Int J Hyg Environ Health 231:113657, PMID: , 10.1016/j.ijheh.2020.113657. [DOI] [PubMed] [Google Scholar]
- 83.Carretta L, Masin R, Zanin G. 2022. Review of studies analysing glyphosate and aminomethylphosphonic acid (AMPA) occurrence in groundwater. Environ Rev 30(1):88–109, 10.1139/er-2020-0106. [DOI] [Google Scholar]
- 84.Struger J, Van Stempvoort DR, Brown SJ. 2015. Sources of aminomethylphosphonic acid (AMPA) in urban and rural catchments in Ontario, Canada: glyphosate or phosphonates in wastewater? Environ Pollut 204:289–297, PMID: , 10.1016/j.envpol.2015.03.038. [DOI] [PubMed] [Google Scholar]
- 85.Zoller O, Rhyn P, Zarn JA, Dudler V. 2020. Urine glyphosate level as a quantitative biomarker of oral exposure. Int J Hyg Environ Health 228:113526, PMID: , 10.1016/j.ijheh.2020.113526. [DOI] [PubMed] [Google Scholar]
- 86.Massoud O, Charlton M. 2018. Nonalcoholic fatty liver disease/nonalcoholic steatohepatitis and hepatocellular carcinoma. Clin Liver Dis 22(1):201–211, PMID: , 10.1016/j.cld.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 87.Kahn R, Buse J, Ferrannini E, Stern M, American Diabetes Association. 2005. The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 28(9):2289–2304, PMID: , 10.2337/diacare.28.9.2289. [DOI] [PubMed] [Google Scholar]
- 88.Bonvallot N, Canlet C, Blas-Y-Estrada F, Gautier R, Tremblay-Franco M, Chevolleau S, et al. 2018. Metabolome disruption of pregnant rats and their offspring resulting from repeated exposure to a pesticide mixture representative of environmental contamination in Brittany. PLoS One 13(6):e0198448, PMID: , 10.1371/journal.pone.0198448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mesnage R, Mahmud N, Mein CA, Antoniou MN. 2021. Alterations in small RNA profiles in liver following a subchronic exposure to a low-dose pesticide mixture in Sprague-Dawley rats. Toxicol Lett 353:20–26, PMID: , 10.1016/j.toxlet.2021.10.001. [DOI] [PubMed] [Google Scholar]
- 90.Manservisi F, Lesseur C, Panzacchi S, Mandrioli D, Falcioni L, Bua L, et al. 2019. The Ramazzini Institute 13-week pilot study glyphosate-based herbicides administered at human-equivalent dose to Sprague Dawley rats: effects on development and endocrine system. Environ Health 18(1):15, PMID: , 10.1186/s12940-019-0453-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Giommi C, Ladisa C, Carnevali O, Maradonna F, Habibi HR. 2022. Metabolomic and transcript analysis revealed a sex-specific effect of glyphosate in zebrafish liver. IJMS 23(5):2724, PMID: , 10.3390/ijms23052724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lozano VL, Defarge N, Rocque L-M, Mesnage R, Hennequin D, Cassier R, et al. 2018. Sex-dependent impact of roundup on the rat gut microbiome. Toxicol Rep 5:96–107, PMID: , 10.1016/j.toxrep.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Haffner SM. 2006. The metabolic syndrome: inflammation, diabetes mellitus, and cardiovascular disease. Am J Cardiol 97(2A):3A–11A, PMID: , 10.1016/j.amjcard.2005.11.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.