ABSTRACT
Background
Hypomagnesaemia with secondary hypocal-caemia (HSH) is a rare autosomal recessive disorder caused by pathogenic variants in TRPM6, encoding the channel-kinase transient receptor potential melastatin type 6. Patients have very low serum magnesium (Mg2+) levels and suffer from muscle cramps and seizures. Despite genetic testing, a subgroup of HSH patients remains without a diagnosis.
Methods
In this study, two families with an HSH phenotype but negative for TRPM6 pathogenic variants were subjected to whole exome sequencing. Using a complementary combination of biochemical and functional analyses in overexpression systems and patient-derived fibroblasts, the effect of the TRPM7-identified variants on Mg2+ transport was examined.
Results
For the first time, variants in TRPM7 were identified in two families as a potential cause for hereditary HSH. Patients suffer from seizures and muscle cramps due to magnesium deficiency and episodes of hypocalcaemia. In the first family, a splice site variant caused the incorporation of intron 1 sequences into the TRPM7 messenger RNA and generated a premature stop codon. As a consequence, patient-derived fibroblasts exhibit decreased cell growth. In the second family, a heterozygous missense variant in the pore domain resulted in decreased TRPM7 channel activity.
Conclusions
We establish TRPM7 as a prime candidate gene for autosomal dominant hypomagnesaemia and secondary hypocalcaemia. Screening of unresolved patients with hypocalcaemia and secondary hypocalcaemia may further establish TRPM7 pathogenic variants as a novel Mendelian disorder.
Keywords: genetics, HSH, magnesium deficiency, TRPM6, TRPM7
Graphical Abstract
Graphical Abstract.
KEY LEARNING POINTS.
What is already known about this subject?
Hypomagnesaemia with secondary hypocalcaemia (HSH) is a rare autosomal recessive disorder characterized by a very low serum magnesium concentration, muscle cramps and seizures.
Biallelic pathogenic variants in TRPM6 have been identified as the cause for HSH, yet several families remain without a genetic diagnosis.
What this study adds?
We report an autosomal dominant inheritance pattern of HSH.
We describe for the first time the presence of rare variants in the TRPM7 gene in patients with HSH.
The identified variants result in defective splicing or impair TRPM7 channel activity.
What impact this may have on practice or policy?
We recommend screening patients with HSH negative for variants in TRPM6 for rare variants in TRPM7.
Finding more TRPM7 variants will improve diagnostics of patients with HSH, allowing genetic counseling.
INTRODUCTION
Hypomagnesaemia with secondary hypocalcaemia [HSH, (Mendelian Inheritance in Man #602014)] is a rare autosomal recessive disorder characterized by a very low serum magnesium (Mg2+) concentration (<0.3 mmol/L) [1, 2]. Hypocalcaemia is a secondary effect of hypomagnesaemia as a consequence of parathyroid failure or parathyroid hormone (PTH) resistance [3]. Patients often present during the newborn period with severe seizures, which can result in severe neurological damage if left untreated.
In 2002, variants in TRPM6 were identified to be causative for HSH [1, 2]. TRPM6 encodes a non-selective divalent cation channel, transient receptor potential melastatin type 6 (TRPM6), with high permeability for Mg2+ [4, 5]. A recent study shows that TRPM6 functions in tetramers with its close homologue TRPM7 and that this interaction is essential for TRPM6 activity [6]. Both channels are sensitive to intracellular Mg2+ and Mg-ATP levels and have differential concentration-dependent channel inhibition [7]. The activity of TRPM6/TRPM7 tetramers is, therefore, regulated by the relative expression of TRPM6 and TRPM7 subunits. TRPM6 decreases the Mg-adenosine triphosphate (ATP)-induced inhibition of TRPM7 and thereby increases Mg2+ transport activity [6].
Whereas TRPM7 is ubiquitously expressed throughout the body, TRPM6 is exclusively located in epithelia, with the highest expression in the colon and kidney [8]. Within the kidney, TRPM6/TRPM7 tetramers locate in the distal convoluted tubule (DCT) segment of the nephron, where they are believed to function as tetramers on the apical membrane [8–10]. Urinary Mg2+ excretion is ultimately determined in the DCT since no Mg2+ reabsorption takes place beyond this segment [11, 12]. As a consequence, impaired Mg2+ transport via TRPM6/TRPM7 in the DCT inevitably results in renal Mg2+ wasting and hypomagnesaemia [1–3, 13].
The pathophysiology of HSH comprises a primary defect in intestinal Mg2+ uptake and additional renal Mg2+ wasting. During phases of severe hypomagnesaemia, the renal wasting is not detectable. It can, however, be evidenced after normalization of plasmatic levels (Mg2+ loading test). Here we report HSH in two families: one with an autosomal inheritance pattern and one de novo. Using whole exome sequencing (WES), we identified rare variants in TRPM7 that were functionally evaluated using patch clamp electrophysiology and biochemical analyses.
MATERIALS AND METHODS
Patient analysis
Electrolytes in the blood and 24-h urine measurements of the patients were performed according to standard procedures. An intravenous Mg2+-loading test was executed in individuals F1-II.2, F1-II.3 and F1-III.3. The probands of both families were subjected to WES, which, in the case of F1-II.2, was performed at BGI-Europe (Copenhagen, Denmark), employing a HiSeq 2000 machine (Illumina, San Diego, CA, USA). In the case of family 2, DNA samples were sent to Macrogen (Seoul, South Korea) and WES analysis was carried out using an Illumina platform. Candidate genes were selected and analysed in the other family members by Sanger sequencing. Full methods are available as Supplementary material.
Messenger RNA (mRNA) analysis
Primary fibroblast cultures were established from a skin biopsy. mRNA isolation of patients’ blood was performed using the PAXgene Blood RNA kit (Qiagen, Manchester, UK). Regions of interest were amplified using reverse transcription–polymerase chain reaction (PCR) and directly Sanger sequenced according to standard methods. To identify the sequences of the alternatively spliced TRPM7, a 5′ rapid amplification of complementary DNA ends PCR was performed according to the manufacturer's protocol (Invitrogen, Carlsbad, CA, USA). Full methods are available as Supplementary material.
Functional experiments
HEK293 cells were transfected with wild-type and mutant TRPM7 constructs for 48 h. At the start of the 25Mg2+ uptake experiments, cells were transferred to Mg2+-free medium supplemented with 1 mmol/L 25Mg2+ (purity ± 98%; Cortecnet, Voisins le Bretonneux, France). After 15 min, the buffer was removed and the cells were washed and lysed. Lysates were subjected to inductively coupled plasma mass spectrometry analysis. Full methods are available as Supplementary material.
RESULTS
Hypomagnesaemia with secondary hypocalcaemia
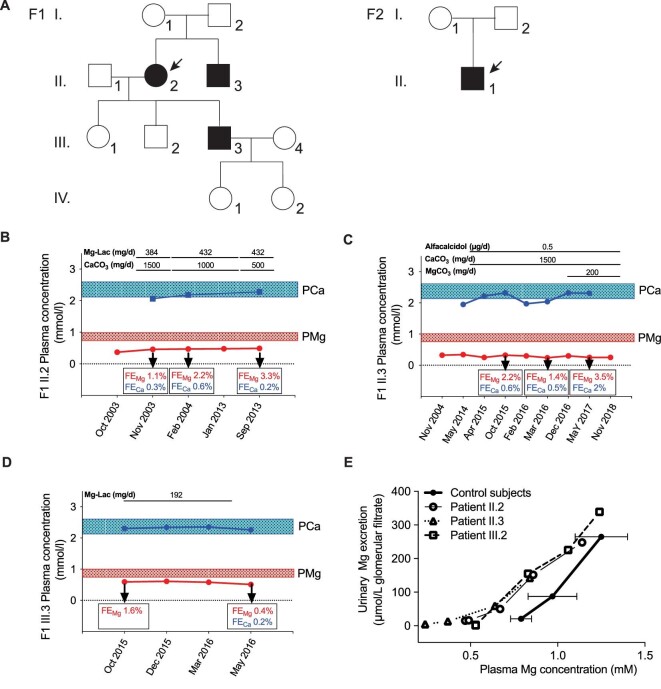
Two families were submitted for genetic testing under the suspicion of HSH (Fig. 1A). The first family, of French origin, presented with hypomagnesaemia (range 0.25–0.51 mmol/L) and episodes of hypocalcaemia in two affected family members (Fig. 1A, F1). All other serum electrolytes were in the normal range (Table 1). The proband (patient II.2, Fig. 1B) is a 78-year-old female with hypercholesterolaemia, hypertension and Hashimoto thyroiditis. Hypomagnesaemia and hypocalcaemia were discovered at the age of 68, when she was admitted for acute abdominal pain (plasma Mg2+ 0.37 mmol/L); fasting urinary Mg2+ excretion was low as well (2.8 µmol/L glomerular filtrate). Hypocalcaemia was corrected by oral calcium carbonate (1500 mg/d), but oral Mg2+ failed to correct hypomagnesaemia. On anamnesis, the patient disclosed she had episodes of muscle cramps throughout her life. The brother of the proband (patient II.3) had a medical history of arterial hypertension, dyslipidaemia, chronic kidney disease (CKD) stage 4 and Grave's disease that required a full thyroidectomy. He had an episode of tetany at the age of 63, after thyroidectomy, initially ascribed to post-surgery hypoparathyroidism. Then hypocalcaemia [serum ionized calcium (Ca2+) 1.10 mmol/L] and an inappropriately low PTH concentration (14 pg/mL) were confirmed (Fig. 1C, May 2014); hypomagnesaemia (0.25 mmol/L) was present with low urinary Mg2+ excretion (0.29 mmol/day). Of note, Mg2+ infusion (see below) normalized both PTH and blood Ca2+ concentrations, showing that hypoparathyroidism was not a result of thyroid surgery but a consequence of hypomagnesaemia. An episode of generalized seizures occurred when he was 77; no other cause than hypomagnesaemia could be retrieved. The son of the proband (patient III.3, Fig. 1D) had hypomagnesaemia (0.53 mmol/L) diagnosed at the age of 47, during family screening. His medical history showed asthma and occasional episodes of paresthesia. The affected individuals presented with low urinary Mg2+ excretion at the basal state (0.72, 0.29, 0.29 mmol/day in patients II.2, II.3 and III.3, respectively). Patients III.1, III.2, IV.1 and IV.2 had normal plasma Mg2+ concentrations (Table 1). A Mg2+ loading test in patients II.2, II.3 and III.3 demonstrated both intestinal and renal Mg2+ (re)absorption defects in all patients, similar to what is observed in patients with loss-of-function of TRPM6 [2] (Fig. 1E).
FIGURE 1:
HSH phenotype in two families with a dominant inheritance pattern. (A) Pedigree of the families with autosomal dominant hypomagnesaemia and secondary hypocalcaemia. Black symbols denote affected and genetically confirmed family members. (B–D) Clinical course of patients F1-II.2, II.3 and III.3. (E) Mg2+ loading test showing combined intestinal and renal Mg2+ wasting in individuals F1-II.2, F1-II.3 and F1-III.3. In patients on baseline (fasting) conditions, plasma Mg concentration was low and urinary Mg excretion was low as well (7.4 ± 6.6 µmol/L of glomerular filtrate), as expected under fasting conditions and in the same range as in control subjects under control conditions (19.2 ± 1.9 µmol/L of glomerular filtrate), showing that no urinary loss of Mg is seen when plasma Mg concentration is low. Then plasma Mg increased during Mg infusion in patients and controls and urinary Mg increased as well. However, for any plasma Mg concentration, urinary Mg excretion was higher in patients than in controls, showing that renal tubular reabsorption is impaired in patients; this can be clearly seen for any plasma Mg concentration ≥0.8 mmol/L. Therefore we conclude that the defect in renal tubular Mg reabsorption in patients manifests when plasma Mg reabsorption is normal or high.
Table 1.
Clinical data for families 1 and 2
| Family 1 | Family 2 | |||||
|---|---|---|---|---|---|---|
| Patient II-2 | Patient II-3 | Patient III-3 | Unaffected subjects (n = 4) | Patient II-1 | Normal values | |
| Age (years) | 78 | 76 | 48 | 3 | ||
| Height (cm) | 155 | 167 | 170 | 98 | ||
| Weight (kg) | 85.6 | 80.2 | 69.3 | 15.5 | ||
| Na+ (mmol/L) | 140 | 138 | 141 | 139 | 137–145 | |
| K+ (mmol/L) | 4.4 | 4.1 | 3.7 | 3.9 | 3.5–4.5 | |
| Cl− (mmol/L) | 105 | 101 | 104 | 102 | 99–106 | |
| Total CO2 (mmol/L) | 22 | 24 | 25 | 29 | 23–28 | |
| Ca2+ (mmol/L) | 2.28 | 2.31 | 2.26 | 2.35 ± 0.04 | 2.59 | 2.1–2.6 |
| Mg2+ (mmol/L) | 0.49 | 0.25 | 0.51 | 0.82 ± 0.04 | 0.61 | 0.7–1.0 |
| Pi (mmol/L) | 0.80 | 1 .05 | 0.70 | 1.30 | 0.82–1.39 | |
| Creatinine (µmol/L) | 101 | 226 | 75 | 44 | a53–97.2, b61.9–114.9 and c46–61 | |
| eGFR (mL/min/1.73 m2) | 46 | 26 | 96 | 130 | >90 | |
| Renin (pg/mL) | 16.5 | 4.8 | 12.7 | – | 9–30 | |
| PTH (pg/mL) | 72* | 17* | 49* | 58.3** | *11–57, **14.5–87.1 | |
| Aldosterone (pmol/L) | 168 | 270 | 216 | – | 80–1000 | |
| 24-hr urine volume (mL) | 1100 | 1185 | 1811 | – | ||
| Na+ (mmol/day) | 83 | 125 | 60 | |||
| FENa (%) | 0.13 | |||||
| K+ (mmol/day) | 39 | 39 | 54 | |||
| FEK (%) | 5.78 | |||||
| Cl− (mmol/day) | 90 | 120 | 69 | |||
| FECl (%) | 0.16 | |||||
| Ca2+ (mmol/day) | 0.34 | 0.56 | 0.62 | |||
| Ca:Cr | 0.02 | |||||
| Mg2+ (mmol/day) | 0.89 | 0.19 | 0.29 | |||
| FEMg (%) | 7.5 | |||||
| Pi (mmol/day) | 10.8 | 13.5 | 16.7 | |||
| TRP | 84.58 | |||||
Estimated glomerular filtration rate (eGFR) was calculated by the Modification of Diet in Renal Disease formula (family 1) and by the Schwartz 2009 formula (family 2). FENa: fractional excretion of sodium; FEK: fractional excretion of potassium; FECl: fractional excretion of chloride; FEMg: fractional excretion of magnesium; Ca:Cr: calcium:creatinine ratio; TRP: tubular reabsorption of phosphate. Conversion factors: Ca2+ mmol/L = mg/dL × 0.25; Mg2+ mmol/L = mg/dL × 0.48; Pi mmol/L = mg/dL × 0.32; creatinine μmol/L = mg/dL × 88.5; renin mU/L = pg/mL × 1.65; aldosterone pmol/L = ng/dL × 27.75. Reference values for the fractional excretions are not usually included since they depend on the levels of electrolytes in the serum and the volaemia. They are interpreted in a clinical context. The asterisks indicate the reference values in the respective hospitals/countries.
a, b and c Creatinine normal values for adult women, men and children, respectively.
An unrelated child of Spanish origin was hospitalized with convulsions at the age of 7 months (Fig. 1A, F2). Nystagmus had been observed since birth. Blood analysis demonstrated the presence of severe hypomagnesaemia (0.44 mmol/L) (Table 1). Serum ionized Ca2+ levels were low during episodes of convulsions (0.94 mmol/L), but were restored to normal values. Mg2+ supplementation was unable to restore Mg2+ levels (0.51 mmol/L) and urinary Mg2+ excretion was increased (fractional excretion of magnesium 22%, normal value <2.2%) and was associated with low urinary calcium excretion. He did not present proteinuria (protein:creatinine ratio <22 mg/mmol) and PTH levels were in the normal range. Ultrasound evaluation showed normal kidneys. Since the first diagnosis, the patient has suffered repeatedly from seizures (epilepsy associated with psychomotor retardation). Other complementary examinations aimed at studying psychomotor retardation and epilepsy were negative. Both parents were healthy and had normal serum Mg2+ and Ca2+ levels (mother: Mg2+ 0.77 mmol/L, total Ca 2.32 mmol/L; father: Mg2+ 0.82 mmol/L, Ca2+ 2.35 mmol/L).
Identification of TRPM7 variants
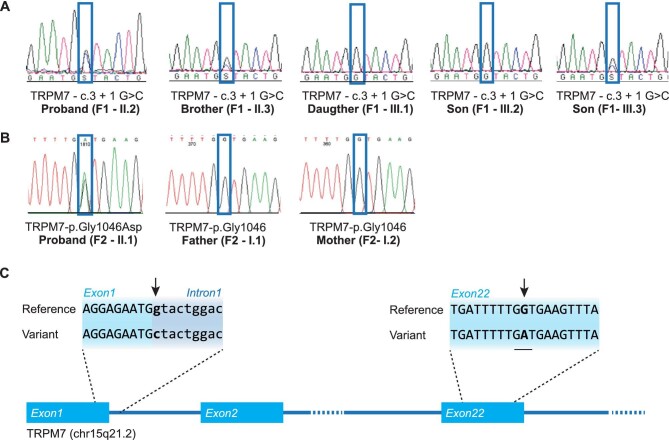
WES identified rare TRPM7 variants in both families. A heterozygous chr15:50978727G>C (c.3+1 G>C) variant was found in family 1, which is located at the first position of intron 1 of the TRPM7 gene. This substitution in the canonical splice donor site is predicted to abolish the splicing score (Alamut splicing prediction module) (Fig. 2A–C). Subsequent Sanger sequencing of the patients and two non-affected family members indicated that the variant cosegregated with the hypomagnesaemia phenotype (Fig. 2A, Supplementary data, Figure S1). In family 2, a heterozygous chr15:50891345C>T (c.3137G>A) variant was identified in exon 22, resulting in a p.Gly1046Asp change (Fig. 2B). The Combined Annotation-Dependent Depletion (CADD)-Phred scaled CADD score for this variant was 27.8, which ranked it in the top of damaging variants, and was predicted pathogenic with very high scores by the variant pathogenicity prediction tools PolyPhen-2 HVAR and SIFT. Parental testing by Sanger sequencing did not show the variant, indicating a de novo origin. Both identified variants were absent in our in-house database, dbSNP, the 1000-Genomes Project and the Genome Aggregation Database (gnomAD). Moreover, there were no rare variants detected in known hypomagnesaemia-causing genes [14–21]. Nor did we detect other rare variants that could explain the phenotype (Supplementary data, Tables S1–S4). The new TRPM7 variants were submitted to ClinVar and were included with accession numbers VCV000974783 and SCV001482422.
FIGURE 2:
Identification of TRPM7 mutations. (A) Mutation analysis chromatograms of F1, demonstrating the presence of the mutation in the proband (II.2), the brother of the proband (II.3) and one child of the proband (III.3). (B) Mutation analysis chromatograms of F2, demonstrating the presence of the mutation in the proband (II.1). (C) Affected family members from F1 carry a TRPM7 splice site mutation at the first nucleotide of the first intron (c.3+1 G>C). The de novo mutation in F2 is located in exon 22 and results in a p.Gly1046Asp missense mutation. Mutations are indicated by the black arrows.
TRPM7 splice donor variant results in alternative splicing and decreased cell growth
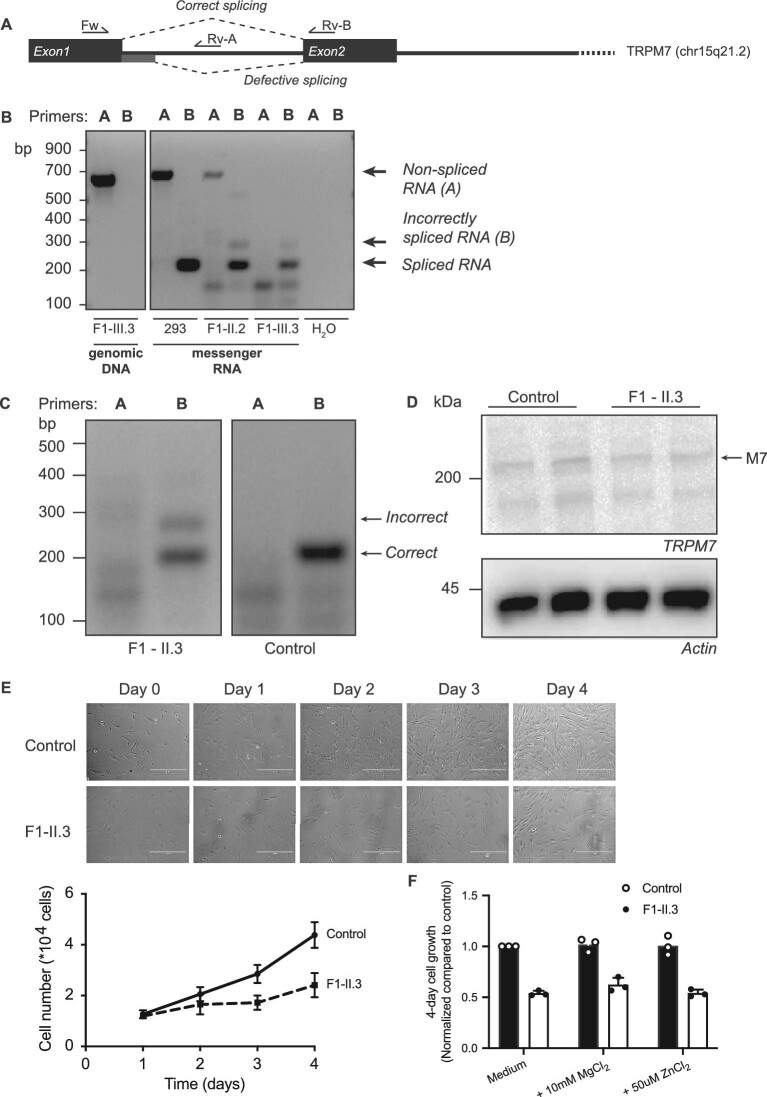
To examine the effects of the intronic TRPM7 variant on splicing of the TRPM7 mRNA, mRNA was isolated from the whole blood of patients II.2 and III.3. The PCR amplification of the spliced mRNA using primers in exon 1 and 2 demonstrated the presence of the normal spliced mRNA and also a larger band in minor quantities (Fig. 3A and B). Sanger sequencing of this band indicated that this transcript consists of several defective spliced mRNAs that all contain parts of intron 1. The most common transcript (∼80% of all defective transcripts) includes the first part of intron 1 and splices from position +71 to exon 2 (Supplementary data, Figure S2). The defective splicing caused a frame shift and a premature stop codon close to the beginning of exon 2.
FIGURE 3:
mRNA analysis demonstrates defective splicing of TRPM7 in family 1. (A) Schematic overview of the TRPM7 gene, indicating the primer locations and the correct and defective splice sites. (B) mRNA analysis of the non-spliced (primer set A) and spliced (primer set B) first intron of TRPM7 using mRNA isolated from blood of the proband (F1-II.2) and individual F1-III.3. As controls, water, genomic DNA and mRNA from HEK293 cells were included. The analysis shows the presence of alternatively spliced mRNA in the patient samples, indicated with an arrow. (C) mRNA splicing analysis using mRNA from fibroblasts shows the presence of alternatively spliced mRNA in individual F1-II.3, indicated with an arrow. (D) Representative immunoblot demonstrating the protein expression of TRPM7 in control fibroblasts and fibroblasts from individual F1-II.3. Actin was used as a loading control. (E) Cell proliferation was determined by cell counting during 4 days of culture and shows decreased cell growth in fibroblasts from individual F1-II.3. Representative images of control and F1-II.3 fibroblasts are shown in the top panel. (F) Cell proliferation could not be rescued by the addition of MgCl2 or ZnCl2. Graphs show the mean of three independent experiments, which each contained three replicates ± standard error of the mean.
To determine the functional consequences of the defective splicing, fibroblasts were isolated from patient II.3. Fibroblasts express TRPM7 and are dependent on its function for cell growth and survival [22, 23]. Indeed, fibroblasts from patient II.3 show the presence of the incorrectly spliced mRNA transcript (Fig. 3C), but low-normal TRPM7 protein expression (Fig. 3D). Proliferation assays showed that patient-derived fibroblasts demonstrated decreased cell growth (Fig. 3E). The decreased cell growth could not be rescued by culturing the cells in medium supplemented with 10 mM MgCl2 or 50 µM zinc chloride (Fig. 3F).
TRPM7 missense variant decreases the activity of the channel
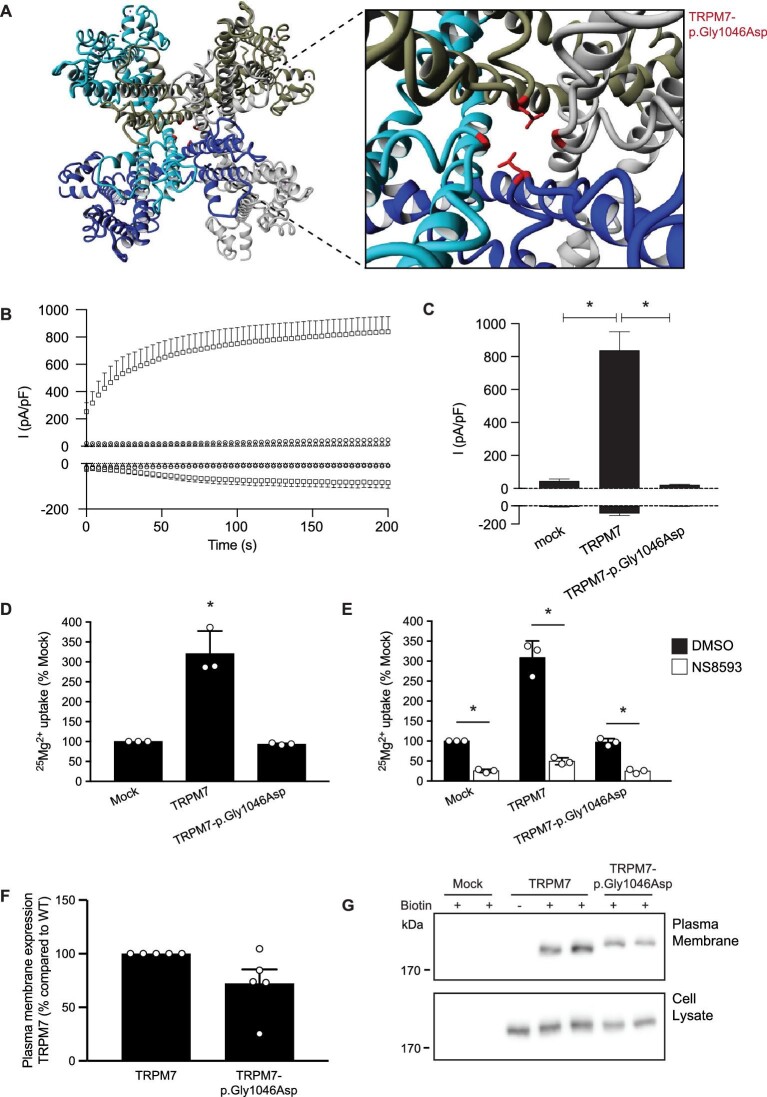
The Gly1046 residue is located in the TRPM7 channel pore (Fig. 4A). The change of a hydrophobic glycine for the large side chain of aspartic acid and its negative charge is predicted to disturb the pore structure and interfere with the channel function. To determine the functional implications of the variant on TRPM7 channel activity, we performed electrophysiological recordings. Whole-cell patch clamp of mock-transfected cells displayed small endogenous outward currents (46 ± 11 pA/pF), while the averaged current density for wild-type TRPM7 was 838 ± 112 pA/pF (Fig. 4B and C). A significant decrease in current amplitude was observed for the p.Gly1046Asp mutant, which showed outward currents comparable to the mock-transfected cells (22 ± 4 pA/pF; P > .99). 25Mg2+ uptake experiments demonstrated that HEK293 cells overexpressing TRPM7-p.Gly1046Asp show comparable uptake to mock-transfected cells (Fig. 4D). The TRPM7-mediated 25Mg2+ uptake was significantly inhibited by the specific TRPM7 inhibitor NS8593 (Fig. 4E) [24]. Interestingly, 25Mg2+ uptake in both mock- and TRPM7-p.Gly1046Asp-expressing cells was decreased by NS8593, suggesting that the mutant has no dominant negative effect on endogenous TRPM7 activity (Fig. 4E). Cell-surface biotinylation showed that wild-type TRPM7 and TRPM7-p.Gly1046Asp are both expressed at the plasma membrane (Fig. 4F and G). Of note, the expression of TRPM7-p.Gly1046Asp in cell lysate and plasma membrane fractions was marginally lower than wild-type TRPM7.
FIGURE 4:
The TRPM7-p.Gly1046Asp mutant of family 2 results in decreased Mg2+ uptake. (A) Molecular modelling using the structure of human TRPM7 (pdb: 5ZX5). The outtake shows the heterozygous p.Gly1046Asp mutation present in two out of the four subunits of the channel. (B) Averaged time course of outward (+80 mV) and inward (–80 mV) from HEK293 cells transfected with TRPM7 wild-type (squares, n = 9) or TRPM7-p.Gly1046Asp (triangles, n = 9) and non-transfected HEK293 cells as control (circles, n = 6). (C) Bar graph presenting the current densities at +80 mV and –80 mV of indicated conditions at 200 s after establishing whole-cell configuration. Mean + standard error of the mean (SEM) is shown. An asterisk indicates significance compared with TRPM7 wild-type. (D and E) 25Mg2+ uptake assay of HEK293 cells expressing mock, wild-type TRPM7 and mutant TRPM7-p.Gly1046Asp. Cells were incubated for 15 min in a buffer containing 1 mM 25Mg2+ (98% purity) in the presence or absence of 0.4 (v/v)% DMSO (E, black bars) or 30 µM NS8593 (E, white bars). Intracellular 25Mg2+ content was measured by inductive-coupled plasma mass spectrometry. Graphs show the mean of three independent experiments, which each contained three replicates ± standard deviation; *P < .05 compared with mock (D) or DMSO (E). (F and G) Immunoblots showing similar membrane expression between TRPM7 proteins (upper blot) and a TRPM7 expression control (lower blot). The quantifications show the mean ± SEM of five independent experiments.
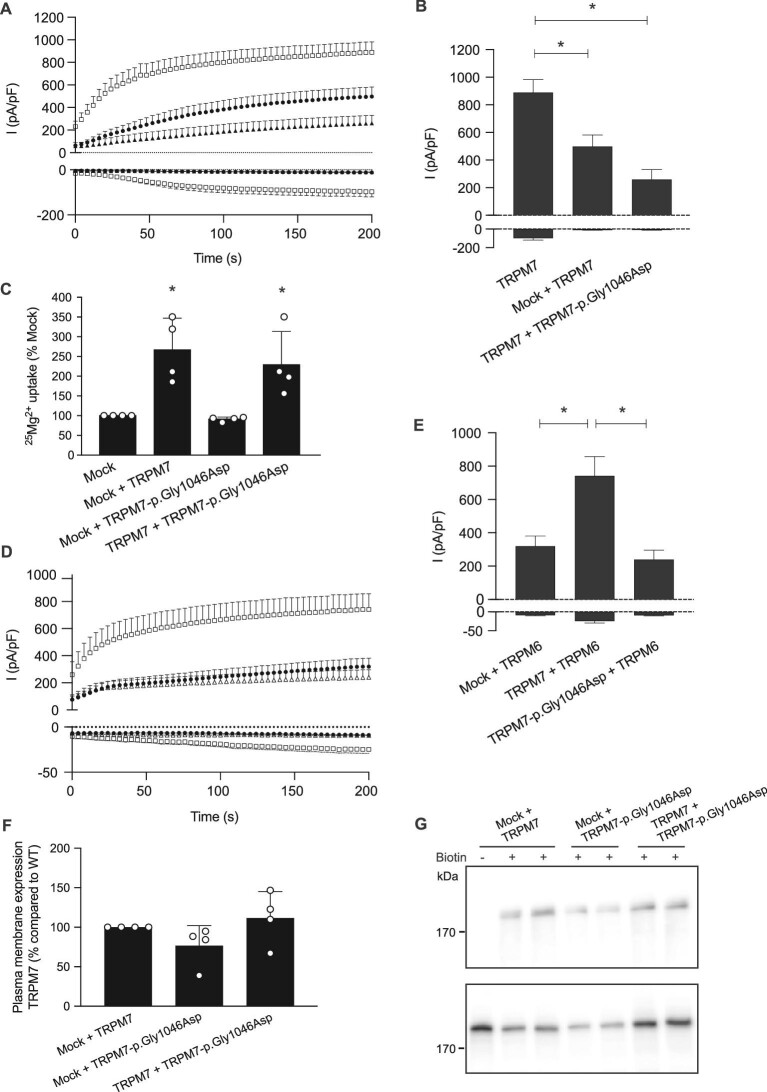
In the kidney, TRPM7 functions in homotetrameric complexes or in heterotetrameric complexes with TRPM6 to facilitate Mg2+ reabsorption. To further examine whether TRPM7-p.Gly1046Asp has a dominant negative effect on wild-type TRPM7 activity, TRPM7 wild-type and TRPM7-p.Gly1046Asp plasmids were cotransfected in HEK293 cells. Cells co-expressing TRPM7 and TRPM7-p.Gly1046Asp showed a comparable current density to TRPM7- and mock-expressing cells (260 ± 70 versus 497 ± 84 pA/pF; P = .34; Fig. 5A and B). Of note, cells cotransfected with TRPM7 and mock plasmids showed a decreased current density compared with TRPM7-expressing cells, in line with 50% lower expression levels (Fig. 5B). Indeed, co-expression of wild-type TRPM7 and TRPM7-p.Gly1046Asp resulted in similar 25Mg2+ uptake as cells transfected with TRPM7 and mock (P = .79; Fig. 5C), demonstrating that wild-type TRPM7 activity is not decreased by the presence of the mutant. Similar results were obtained when co-expressing TRPM6 and TRPM7. In line with previous studies [6], co-expression of TRPM7 significantly increases the TRPM6 current density (742 ± 116 pA/pF) (Fig. 5D and E). In contrast, cells expressing TRPM7-p.Gly1046Asp and TRPM6 displayed a current density similar to cells with TRPM6 and mock (240 ± 56 versus 320 ± 61 pA/pF; P = .93), indicating that TRPM7-p.Gly1046Asp did not affect TRPM6 current density (Fig. 5D and E). Cell surface biotinylation of TRPM7 and TRPM7-p.Gly1046Asp demonstrated equal TRPM7 expression among all conditions (Fig. 5F and G).
FIGURE 5:
The TRPM7-p.Gly1046Asp mutant does not affect the wild-type TRPM7 activity. (A and B) Whole-cell patch clamp recordings with (A) averaged time course of outward (+80 mV) and inward (–80 mV) current densities from HEK293 cells transfected with either TRPM7 wild-type (open squares, n = 17) alone or cotransfected with either TRPM7 wild-type and mock (closed circles, n = 7) or TRPM7 wild-type and TRPM7-p.Gly1046Asp (closed triangles, n = 9). Bar graph (B) presenting the current densities at +80 mV and –80 mV of indicated conditions at 200 s after establishing whole-cell configuration. Mean + standard error of the mean (SEM) is shown. An asterisk indicates significance (P < .05). (C) 25Mg2+ uptake assay of HEK293 cells co-expressing mock, wild-type TRPM7 and mutant TRPM7-p.Gly1046Asp. Cells were incubated for 15 min in a buffer containing 1 mM 25Mg2+ (98% purity). Intracellular 25Mg2+ content was measured by inductive-coupled plasma mass spectrometry. Graphs show the mean of three independent experiments, which each contained three replicates ± standard deviation; *P < .05 compared with mock. (D and E) Whole-cell patch clamp recordings with (D) averaged time course of outward (+80 mV) and inward (–80 mV) current densities from HEK293 cells cotransfected with TRPM6 wild-type and TRPM7 wild-type (open squares, n = 13), TRPM6 wild-type and mock (closed circles, n = 15) and TRPM6 wild-type and TRPM7-p.Gly1046Asp (open triangles, n = 12). Bar graph (E) presenting the current densities at +80 mV and –80 mV of indicated conditions at 200 s after establishing whole-cell configuration. Mean + SEM is shown. An asterisk indicates significance (P < .05). (F and G) Immunoblots showing comparable membrane expression between TRPM7 proteins (upper blot) and a TRPM7 expression control (lower blot). The semiquantifications show the mean ± SEM of five independent experiments.
DISCUSSION
Here we report two families with hypomagnesaemia and secondary hypocalcaemia. WES identified a pathogenic de novo missense variant that alters the TRPM7 channel pore and a splice site variant that results in defective splicing of TRPM7 transcripts. Our results establish the TRPM7 gene as a prime candidate for de novo and autosomal dominant inheritance of HSH.
Our results show for the first time the autosomal dominant inheritance of HSH. The cardinal symptom in our patients is hypomagnesaemia, which was associated with renal Mg2+ wasting. Additionally, intestinal Mg2+ malabsorption was demonstrated by an Mg2+ loading test in patients F1-II.2, II.3 and III.3. In line with HSH patients with TRPM6 pathogenic variants, hypocalcaemia was not detected in all measurements but generally only during episodes of hypoparathyroidism. The presence of hypocalcaemia secondary to hypomagnesaemia is commonly explained by decreased PTH secretion [3]. Although the mechanism for decreased PTH secretion in chronic hypomagnesaemia is still not completely clear, it has been postulated that the inhibition of PTH secretion is caused by increased activity of the alpha subunit of the G protein downstream of the calcium-sensing receptor [25, 26]. Indeed, hypocalcaemia accompanied by a PTH level in the low-normal range was detected in patient F1-II.3. Hypocalcaemia was relieved by Mg2+ supplementation in all subjects.
The identification of two rare variants in TRPM7 in two independent families suggests that TRPM7 variants may be causative for autosomal dominant HSH. Nevertheless, it should be noted that the gnomAD lists a large number of observed missense variants in TRPM7 compared with the missense expected: 756 versus 950 (Z score = 2.23). Although this suggests that TRPM7 is relatively tolerant to missense variants, it should be noted that this score does not exclude pathogenicity of individual variants. Importantly, gnomAD does not list any nonsense or missense variants in residues that form the cation-specific pore domain: E1047, G1046 and F1045. In line with this observation, missense variants are also absent for the lower gate residue N1097 and the cysteine residues forming the pore disulfide bond: C1056 and C1066. Indeed, we demonstrate that the identified variants have functional consequences: the splice site variant impaired splicing of the first exon resulting in a premature stop codon and the p.Gly1046Asp variant was demonstrated to induce loss-of-function in patch clamp analysis. Moreover, TRPM7 has a low loss-of-function observed/expected upper bound fraction (LOEUF) score of 0.56 in gnomAD [27], which is comparable to genes that are essential for human cell viability (mean LOEUF = 0.63). Although this may be indicative of pathogenicity, we feel that the identification of more families with TRPM7 pathogenic variants is essential to provide a definitive answer.
To date, TRPM6 has been considered as the main determinant of intestinal and renal Mg2+ (re)absorption [12, 28]. TRPM6 channel activity is highly regulated by Mg2+, ATP and hormonal factors [5, 29–31]. Pathogenic variants in TRPM6 cause HSH and result in very low serum Mg2+ levels [1–3]. Our findings indicate that the presence of TRPM7 may be equally important for renal and intestinal Mg2+ uptake. Indeed, both zebrafish and intestine-specific knockout mouse models of TRPM7 demonstrate decreased serum Ca2+ and Mg2+ levels [9, 32].
Recently, using TRPM7-deficient trophoblast cells, Chubanov et al. [6] showed that TRPM6 activity requires the presence of TRPM7. TRPM6/TRPM6 homotetramers are not functional, whereas TRPM6/TRPM7 heterotetramers have increased Mg2+ transport activity compared with TRPM7/TRPM7 homotetramers [6, 7]. TRPM7/TRPM7 homotetramers are more susceptible to concentration-dependent inhibition by cytosolic Mg-ATP than TRPM6/TRPM7 heterotetramers [6]. The heterozygous TRPM7 variants cause decreased expression of TRPM7, which may result in more non-functional TRPM6/TRPM6 homotetramers and fewer TRPM6/TRPM7 heterotetramers. Thus the total Mg2+ (re)absorption capacity will be decreased, as shown by the Mg2+ loading test in all affected individuals of family 1.
In multiple cell systems and animal models, TRPM7 has been shown to be essential for life [9, 22, 23, 33, 34]. TRPM7 knockout impairs cell proliferation and therefore the channel is considered as the main determinant of intracellular Mg2+ levels [33]. Of note, the importance of TRPM7 in Mg2+ homeostasis has also been questioned by some studies, since the deletion of TRPM7 in T lymphocytes did not affect cellular Mg2+ handling [34]. However, TRPM7-deficient mice demonstrated that TRPM7 is essential for the organismal balance of zinc (Zn2+), Mg2+ and Ca2+. Indeed, the channel pore of TRPM7 is permeable to Zn2+, Mg2+ and Ca2+ [9]. Interestingly, patient-derived fibroblasts show decreased growth rates. However, this growth could not be rescued by Mg2+ or Zn2+ supplementation.
Although TRPM7 is considered necessary for cell survival, it is important to note that the reported variants are present in a heterozygous state and will not completely impair TRPM7 function. The unique splice site variant reported here will not affect functional channels and will only result in haplotype insufficiency. Moreover, our 25Mg2+ uptake and patch clamp experiments demonstrated that the missense variant does not have a dominant negative effect on the functional wild-type protein. This may explain the relatively mild phenotype compared with knockout cells and mice [22, 23]. As heterozygous mice also have one functional allele, it is interesting to compare the patients with the TRPM7-deficient mice with the deletion of the alpha-kinase domain that were generated by Ryazanova et al. [23]. Heterozygous TRPM7Δkinase mice were viable and had a defect in intestinal Mg2+ absorption, which is also present in family 1. Cells taken from heterozygous TRPM7Δkinase mice demonstrated decreased TRPM7 activity.
A limitation of our study is the identification of only two families with pathogenic variants in TRPM7. One may speculate that variants in TRPM7 with more severe pathogenicity that entirely impair TRPM7 function are not compatible with life. Indeed, rare variants in TRPM7 have been associated with stillbirth [35]. Unfortunately, the Mg2+ status of the affected children is unknown. Our study suggests that the location of the variant may contribute to the severity of the disease. The fibroblasts from family 1 showed a significant TRPM7 expression despite the heterozygous splice site variant, which may also be reflected in the older age of presentation of the patients. The strength of our study is the extensive phenotypic characterization of the patient's phenotype, including the Mg2+ loading test, clearly demonstrating a mixed phenotype of intestinal and renal mal(re)absorption.
In conclusion, we describe for the first time heterozygous pathogenic variants in TRPM7 in patients with HSH. TRPM7 should be screened in patients with an autosomal dominant inheritance pattern or individual cases with low blood Mg2+ levels.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Willem Bosman and Gijs Franken for excellent technical support and Dr Hanka Venselaar for the visualization of the protein structure.
Contributor Information
Rosa Vargas-Poussou, Département de Génétique, Centre de référence des Maladies Rénales Héréditaires de l'Enfant et de l'Adulte, Hôpital Européen Georges Pompidou, Paris, France.
Felix Claverie-Martin, Unidad de Investigación, Renal Tube Group, Hospital Universitario Nuestra Señora de Candelaria, Santa Cruz de Tenerife, Spain.
Caroline Prot-Bertoye, Centre de Recherche des Cordeliers, Sorbonne Université, INSERM, Université de Paris, CNRS, Paris, France; Department of Physiology, Assistance Publique-Hôpitaux de Paris, Hôpital Européen Georges Pompidou, Paris, France; Centre de Référence des Maladies Rénales Héréditaires de l'Enfant et de l'Adulte, Paris, France.
Valentina Carotti, Department of Physiology, Radboud Institute for Molecular Life Sciences, Radboud University Medical Center, Nijmegen, The Netherlands.
Jenny van der Wijst, Department of Physiology, Radboud Institute for Molecular Life Sciences, Radboud University Medical Center, Nijmegen, The Netherlands.
Ana Perdomo-Ramirez, Unidad de Investigación, Renal Tube Group, Hospital Universitario Nuestra Señora de Candelaria, Santa Cruz de Tenerife, Spain.
Gloria M Fraga-Rodriguez, Sección de Nefrología Pediátrica, Hospital de la Santa Creu I Sant Pau, Barcelona, Spain.
Marguerite Hureaux, Département de Génétique, Centre de référence des Maladies Rénales Héréditaires de l'Enfant et de l'Adulte, Hôpital Européen Georges Pompidou, Paris, France.
Caro Bos, Department of Physiology, Radboud Institute for Molecular Life Sciences, Radboud University Medical Center, Nijmegen, The Netherlands.
Femke Latta, Department of Physiology, Radboud Institute for Molecular Life Sciences, Radboud University Medical Center, Nijmegen, The Netherlands.
Pascal Houillier, Centre de Recherche des Cordeliers, Sorbonne Université, INSERM, Université de Paris, CNRS, Paris, France; Department of Physiology, Assistance Publique-Hôpitaux de Paris, Hôpital Européen Georges Pompidou, Paris, France; Centre de Référence des Maladies Rénales Héréditaires de l'Enfant et de l'Adulte, Paris, France.
Joost G J Hoenderop, Department of Physiology, Radboud Institute for Molecular Life Sciences, Radboud University Medical Center, Nijmegen, The Netherlands.
Jeroen H F de Baaij, Department of Physiology, Radboud Institute for Molecular Life Sciences, Radboud University Medical Center, Nijmegen, The Netherlands.
FUNDING
This work was financially supported by ZonMW under the frame of the European Joint Programme on Rare Diseases (EJPRD2019-40) and by the IMAGEN project, which is cofunded by the PPP Allowance made available by Health∼Holland, Top Sector Life Sciences & Health, to stimulate public–private partnerships (IMplementation of Advancements in GENetic Kidney Disease, LSHM20009) and the Dutch Kidney Foundation (20OP+018). In addition, this project has received funding from the European Union's Horizon 2020 research and innovation programme under the EJP RD COFUND-EJP No 825575 and the Netherlands Organization for Scientific Research (NWO Veni 016.186.012. Vici 016.130.668). Work in Santa Cruz de Tenerife was financially supported by grant PI17/00153 (to F.C.M. and A.P.R.), integrated in the Plan Nacional de I+D+I 2013-2016 and co-financed by the ISCIII-Subdirección General de Evaluación y Fomento de la Investigación and the European Regional Development Fund ‘Another way to build Europe’.
AUTHORS’ CONTRIBUTIONS
R.V.P., R.C.M., P.H., J.H., F.C.M. and J.d.B. designed the research studies. R.V.P., F.C.M., C.P.B., G.M.F.R. and P.H. acquired clinical data. V.C., J.v.d.W., C.B., F.L., J.d.B., A.P.R and F.C.M. conducted experiments and/or analysed data. R.V.P., F.C.M., P.H. and J.d.B. wrote the manuscript. All authors corrected the manuscript and approved the final version.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Schlingmann KP, Weber S, Peters Met al. Hypomagnesaemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat Genet 2002; 31: 166–170 [DOI] [PubMed] [Google Scholar]
- 2. Walder RY, Landau D, Meyer Pet al. Mutation of TRPM6 causes familial hypomagnesaemia with secondary hypocalcemia. Nat Genet 2002; 31: 171–174 [DOI] [PubMed] [Google Scholar]
- 3. Schlingmann KP, Sassen MC, Weber Set al. Novel TRPM6 mutations in 21 families with primary hypomagnesaemia and secondary hypocalcemia. J Am Soc Nephrol 2005; 16: 3061–3069 [DOI] [PubMed] [Google Scholar]
- 4. Li M, Jiang J, Yue L. Functional characterization of homo- and heteromeric channel kinases TRPM6 and TRPM7. J Gen Physiol 2006; 127: 525–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li M, Du J, Jiang Jet al. Molecular determinants of Mg2+ and Ca2+ permeability and pH sensitivity in TRPM6 and TRPM7. J Biol Chem 2007; 282: 25817–25830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chubanov V, Ferioli S, Wisnowsky Aet al. Epithelial magnesium transport by TRPM6 is essential for prenatal development and adult survival. eLife 2016; 5: e20914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ferioli S, Zierler S, Zaisserer Jet al. TRPM6 and TRPM7 differentially contribute to the relief of heteromeric TRPM6/7 channels from inhibition by cytosolic Mg2+ and Mg. ATP. Sci Rep 2017; 7: 8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Voets T, Nilius B, Hoefs Set al. TRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorption. J Biol Chem 2004; 279: 19–25 [DOI] [PubMed] [Google Scholar]
- 9. Mittermeier L, Demirkhanyan L, Stadlbauer Bet al. TRPM7 is the central gatekeeper of intestinal mineral absorption essential for postnatal survival. Proc Natl Acad Sci USA 2019; 116: 4706–4715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Robak P, Ożgo M, Michałek Ket al. Identification of TRPM6 and TRPM7 expression changes in response to a diet supplemented with inulin in porcine kidney. Arch Anim Breed 2016; 59: 267–274 [Google Scholar]
- 11. de Baaij JH, Hoenderop JG, Bindels RJ. Regulation of magnesium balance: lessons learned from human genetic disease. Clin Kidney J 2012; 5: i15–i24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Baaij JH, Hoenderop JG, Bindels RJ. Magnesium in man: implications for health and disease. Physiol Rev 2015; 95: 1–46 [DOI] [PubMed] [Google Scholar]
- 13. Lainez S, Schlingmann KP, van der Wijst Jet al. New TRPM6 missense mutations linked to hypomagnesaemia with secondary hypocalcemia. Eur J Hum Genet 2014; 22: 497–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Arjona FJ, de Baaij JH, Schlingmann KPet al. CNNM2 mutations cause impaired brain development and seizures in patients with hypomagnesaemia. PLoS Genet 2014; 10: e1004267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bockenhauer D, Feather S, Stanescu HCet al. Epilepsy, ataxia, sensorineural deafness, tubulopathy, and KCNJ10 mutations. N Engl J Med 2009; 360: 1960–1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de Baaij JH, Dorresteijn EM, Hennekam EAet al. Recurrent FXYD2 p.Gly41Arg mutation in patients with isolated dominant hypomagnesaemia. Nephrol Dial Transplant 2015; 30: 952–957 [DOI] [PubMed] [Google Scholar]
- 17. Ferre S, de Baaij JH, Ferreira Pet al. Mutations in PCBD1 cause hypomagnesaemia and renal magnesium wasting. J Am Soc Nephrol 2014; 25: 574–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Groenestege WM, Thebault S, van der Wijst Jet al. Impaired basolateral sorting of pro-EGF causes isolated recessive renal hypomagnesaemia. J Clin Invest 2007; 117: 2260–2267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schlingmann KP, Bandulik S, Mammen Cet al. Germline de novo mutations in ATP1A1 cause renal hypomagnesaemia, refractory seizures, and intellectual disability. Am J Hum Genet 2018; 103: 808–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schlingmann KP, Renigunta A, Hoorn EJet al. Defects in KCNJ16 cause a novel tubulopathy with hypokalemia, salt wasting, disturbed acid-base homeostasis, and sensorineural deafness. J Am Soc Nephrol 2021; 32: 1498–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van der Wijst J, Konrad M, Verkaart SAJet al. A de novo KCNA1 mutation in a patient with tetany and hypomagnesaemia. Nephron 2018; 139: 359–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nadler MJ, Hermosura MC, Inabe Ket al. LTRPC7 is a Mg.ATP-regulated divalent cation channel required for cell viability. Nature 2001; 411: 590–595 [DOI] [PubMed] [Google Scholar]
- 23. Ryazanova LV, Rondon LJ, Zierler Set al. TRPM7 is essential for Mg2+ homeostasis in mammals. Nat Commun 2010; 1: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chubanov V, Ferioli S, Gudermann T. Assessment of TRPM7 functions by drug-like small molecules. Cell Calcium 2017; 67: 166–173 [DOI] [PubMed] [Google Scholar]
- 25. Quitterer U, Hoffmann M, Freichel Met al. Paradoxical block of parathormone secretion is mediated by increased activity of G alpha subunits. J Biol Chem 2001; 276: 6763–6769 [DOI] [PubMed] [Google Scholar]
- 26. Rodriguez-Ortiz ME, Canalejo A, Herencia Cet al. Magnesium modulates parathyroid hormone secretion and upregulates parathyroid receptor expression at moderately low calcium concentration. Nephrol Dial Transplant 2014; 29: 282–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Karczewski KJ, Francioli LC, Tiao Get al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 2020; 581: 434–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schaffers OJM, Hoenderop JGJ, Bindels RJMet al. The rise and fall of novel renal magnesium transporters. Am J Physiol Renal Physiol 2018; 314: F1027–F1033 [DOI] [PubMed] [Google Scholar]
- 29. Thebault S, Alexander RT, Tiel Groenestege WMet al. EGF increases TRPM6 activity and surface expression. J Am Soc Nephrol 2009; 20: 78–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nair AV, Hocher B, Verkaart Set al. Loss of insulin-induced activation of TRPM6 magnesium channels results in impaired glucose tolerance during pregnancy. Proc Natl Acad Sci U S A 2012; 109: 11324–11329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. de Baaij JH, Blanchard MG, Lavrijsen Met al. P2X4 receptor regulation of transient receptor potential melastatin type 6 (TRPM6) Mg2+ channels. Pflugers Arch 2014; 466: 1941–1952 [DOI] [PubMed] [Google Scholar]
- 32. Elizondo MR, Budi EH, Parichy DM. trpm7 Regulation of in vivo cation homeostasis and kidney function involves stanniocalcin 1 and fgf23. Endocrinology 2010; 151: 5700–5709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schmitz C, Perraud AL, Johnson COet al. Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell 2003; 114: 191–200 [DOI] [PubMed] [Google Scholar]
- 34. Jin J, Desai BN, Navarro Bet al. Deletion of TRPM7 disrupts embryonic development and thymopoiesis without altering Mg2+ homeostasis. Science 2008; 322: 756–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cartwright JH, Aziz Q, Harmer SCet al. Genetic variants in TRPM7 associated with unexplained stillbirth modify ion channel function. Hum Mol Genet 2020; 29: 1797–1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.