Abstract
Introduction:
Post-operative radiation therapy (PORT) in resected NSCLC improves local-regional outcomes but recent randomized data do not support its unselected use. We assessed if tumor mutational burden (TMB) and mutations in genes associated with radiation sensitivity can select patients for PORT.
Methods:
Patients with resected NSCLC treated with and without PORT who underwent tumor genomic profiling were examined. The incidence of local-regional failures (LRF) in patients with deleterious mutations in DNA damage response and repair (DDR) genes and genes associated with radiation-resistance (KEAP1/NFE2L2/STK11/PIK3CA) were investigated. Cox modeling and receiver operating characteristic curve (ROC) analysis assessed the relationship between TMB and local-regional control (LRC).
Results:
Eighty-nine patients with NSCLC treated with PORT were analyzed with 2-year LRF rate of 19% (95% CI: 10–27%). Among PORT patients, those with mutations in radiation-resistance genes (n=16, 18%) had significantly more LRF than patient without (2-year LRF: 60% vs 11%, p<0.001). On multivariate analysis (MVA), radiation-resistance mutations associated with LRF after PORT (HR, 7.42, p<0.001). Patients with mutations identified in DDR genes (n=15, 17%) had significantly improved LRC (p=0.048) and no LRF events after PORT. On MVA, a higher TMB associated with improved LRC after PORT (HR, 0.86, p=0.01) and TMB was associated with PORT outcomes (area under ROC curve: 0.67–0.77). These genomic markers did not similarly associate with LRF in patients without PORT.
Conclusion:
Our data suggests that patients with radiation-resistance gene alterations may derive minimal benefit from PORT, whereas patients with high-TMB and/or alterations in DDR genes may benefit from PORT and be suited for future precision-RT strategies. Prospective studies are necessary to validate these findings.
Keywords: post-operative radiation therapy, non-small cell lung cancer, radiation resistance, DNA damage repair, tumor mutational burden
Introduction:
Improved local-regional disease control has been found to translate to superior survival outcomes in patients with non-small cell lung cancers (1–3). Post-operative thoracic radiation therapy (PORT) after surgical resection has therefore been a standard adjuvant treatment offering in patients with adverse pathological features, namely mediastinal node positivity. However, recent prospective data from the LungART and PORT-C trials suggest that PORT should not be universally recommended, and that strategies to better identify and select patients who would most benefit from PORT are needed (4, 5).
Despite the conflicting results on the impact of PORT on disease-free survival, both the LungART and PORT-C trials found PORT to significantly improve local-regional outcomes supporting its role in the management of resected NSCLC (4, 5). There is now a greater understanding of how tumor genetics contribute to radiation response and of the radiation-induced toxicities of PORT (5, 6). Therefore, tumor genomics could potentially be used to select patients who would most benefit from PORT and allow for precision radiation that can improve the therapeutic ratio.
In patients with NSCLC, tumor mutations in STK11, KEAP1, NFE2L2 and PIK3CA genes have been associated with radiation resistance, with treatment failures even after high-dose SBRT (7–9). On the other hand, mutations in DNA damage response and repair (DDR) genes have been associated with more favorable local-regional outcomes (10). Additionally, although there are limited data associating tumor mutational burden (TMB) with radiation response, multiple reports have found a higher TMB to associate with both DDR mutations and tumor immunogenicity, suggesting its potential utility as a novel radiation-response biomarker (11–14).
We, therefore, assessed patients with resected NSCLC treated with PORT who underwent tumor genomic profiling to determine if tumor genomics and TMB could be used to identify patient subgroups who may and may not benefit from PORT and guide PORT dosing. We also explored a cohort of resected NSCLC patients who although were candidates, did not receive PORT to assess if these genomic biomarkers are uniquely predictive of PORT outcomes. We hypothesized that patients with tumor mutations in genes associated with radiation-resistance would have limited benefit from PORT, whereas patients with tumor mutations in DDR genes and high-TMB would have favorable local-regional outcomes and be best suited for future precision-RT approaches.
Methods:
PORT Patients and Treatment:
We retrospectively examined consecutive patients with NSCLC treated with curative intent surgery who received PORT between January 2017 through September 2019. Evaluated patients gave informed consent, and underwent, targeted next generation sequencing (MSK-IMPACT; Integrated Mutation Profiling of Actionable Cancer Targets) (15, 16). Next generation sequencing was performed on available tissue from the primary tumor or regional nodal metastases. This research was conducted in accordance with the US Common Rule, and this study was Institutional Review Board approved.
Standard pre-treatment evaluation included a physical examination, computed tomography (CT) scan of the chest, abdomen and pelvis and/or whole-body fluorine-18 fluorodeoxyglucose positron emission tomography (PET), and magnetic resonance imaging (MRI) of the head when appropriate. Indications for PORT included mediastinal node positivity or positive surgical margin status. The standard PORT radiation dose was 54Gy in 1.8Gy fractions but ranged from 50 – 60Gy and treatment was standardly delivered using intensity-modulated radiation therapy. Tumor genomic profiling data were not used in PORT clinical decision-making. Radiation treatment planning included a 4-dimensional CT simulation and PORT target volumes included the involved nodal stations, bronchial stump an ipsilateral hilum (17). Adjuvant and neoadjuvant platinum-based systemic therapy was as per standard of care. PORT standardly followed after the completion of platinum-based systemic therapy. Additionally, a minority of patients received either EGFR directed tyrosine kinase inhibitor (TKI) or immune checkpoint inhibitor (ICI) on investigational protocols. Imaging with chest CT was performed every 6 months, or more frequently as clinically warranted. All patients suspected of disease progression underwent PET/CT imaging, and whenever feasible, biopsy.
Tumor Genomic Profiling and PORT Patient Cohorts:
Two cohorts of patients treated with PORT were defined through tumor genomic profiling based upon the presence of deleterious mutations (1) patients with mutations in genes associated with radiation resistance and (2) patients with mutations in DDR genes with no identified mutations in radiation resistance genes. Deleterious mutations included truncating, frame shift, splicing and fusion mutations predicted to impair protein function including as well as missense mutations predicted to be pathogenic based on OncoKB and/or ClinVar and literature review (18).
Investigated genes associated with radiation resistance included STK11, KEAP1, NFE2L2 and PIC3KA, as these genes all have both pre-clinical and clinical data demonstrating them to associate with radiation resistance in patients with NSCLC (7–9, 19). A panel of 43 genes involving major DNA damage and response pathways were selected based upon prior investigation (10, 20–22). (Supplemental Table 1).
Exploratory No-PORT Cohort:
To determine whether associations that were observed within the PORT cohort were radiation-specific and distinct from those patients receiving surgery and systemic therapy alone, a cohort of consecutive patients with AJCC 8th edition stage III NSCLC with pathological involved mediastinal nodes also treated between January 2017 through September 2019 with curative surgery but who did not receive PORT were also reviewed. Patients gave informed consent, and underwent, targeted next generation sequencing as aforementioned. No-PORT patients were similarly examined based on presented of tumor mutations associated with radiation resistance and mutations in DDR genes.
Statistical Analysis:
Data on patient age, sex, stage, smoking history, histology, Eastern Cooperative Oncology Group (ECOG) performance status, surgery type, surgical margin status, involved mediastinal nodal stations, TMB in units of mutations per megabase (mt/Mb), systemic therapy, and radiation dose and technique were collected. Baseline characteristics between PORT patients with and without tumor mutations in genes associated with radiation resistance and in patients with and without tumor mutations identified only in DDR genes were compared using the chi-square test, Fisher’s exact or the Wilcoxon test. We assessed for association between patient and tumor characteristics and local-regional failure using univariate and multivariate Cox proportional hazards modeling. Variables with p < 0.05 on univariable analysis were analyzed in multivariate analysis. TMB was evaluated as a continuous variable, and the number of involved mediastinal stations was assessed categorically as < 2 stations vs ≥ 2 stations.
Among PORT patients, overall survival was defined from the start of radiotherapy to disease-progression or death. Local-regional failure (LRF) and distant-metastasis free survival (DMFS) were defined from the start of radiotherapy to disease progression, with distant failure defined as metastatic disease progression per AJCC 8th edition staging. Among patients not treated with PORT, outcomes were defined from the date of surgery. Investigators were blinded to tumor mutation results when determining disease status. Patients were censored from analysis at time of their first progression event. Kaplan-Meier analysis was used to determine overall survival, cumulative incidence of LRF and DMFS. The log-rank test was used to compare overall survival, LRF and DMFS between patients with and without deleterious mutations in genes-associated with radiation resistance and with and without deleterious mutations identified only in DDR genes. Additionally, receiver operator characteristic curve analysis was performed to assess the relationship between TMB and LRF among patients with PORT. Differences were described as statistically significant for p-values < 0.05. All statistical computations were performed using SPSS software Version 27 (IBM, Armonk, NY).
Results:
Characteristics of Patients Treated with PORT:
We identified 89 consecutive patients who received PORT and had tumor genomic profiling completed on their primary or regional disease. Most patients had stage IIIA or IIIB disease (n=81, 91%), adenocarcinoma histology (n=78, 88%) and underwent a lobectomy (n=79, 89%) with a negative-margin resection (n=78, 88%). In total, 89% (n=79) received platinum-based chemotherapy either as neoadjuvant (n=32) or adjuvant (n=46) therapy. Additionally, 14% (n=12) and 11% (n=10) of patients received ICI or TKI therapy, respectively (Table 1). The median TMB was 7 mt/Mb. The PORT prescription most prescribed was 54Gy in 1.8Gy fractions (n=76, 85%) and 80% (n=71) received IMRT. Median follow-up after PORT was 36 months (IQR: 27 – 43 months).
Table 1.
Patient and Treatment Characteristics
| Characteristic | All Patients (n = 89) No. of Patients (%) |
|---|---|
|
| |
| Median age, range | 68 (46 – 83) |
|
| |
| Smoking History | |
| Never | 19 (21) |
| Former | 66 (74) |
| Current | 4 (5) |
|
| |
| Sex at Birth | |
| Female | 57 (64) |
| Male | 32 (36) |
|
| |
| Performance Status | |
| ECOG 0 | 54 (61) |
| ECOG 1 | 35 (39) |
|
| |
| Histology | |
| Adenocarcinoma | 78 (88) |
| Squamous Cell | 6 (7) |
| Other | 5 (5) |
|
| |
| Tumor Mutational Burden | |
| Median, IQR (mt/Mb) | 7 (3.5 – 11.63) |
|
| |
| AJCC 8th Overall Stage | |
| I | 1 (1) |
| IIB | 7 (8) |
| IIIA | 67 (75) |
| IIIB | 14 (16) |
|
| |
| Surgery Type | |
| Wedge / Segmentectomy | 8 (9) |
| Lobectomy | 79 (89) |
| Pneumonectomy | 2 (2) |
|
| |
| Margin Status | |
| Negative | 78 (88) |
| Positive | 11 (12) |
|
| |
| Involved Mediastinal Nodal Stations | |
| 0 | 10 (11) |
| 1 | 53 (60) |
| 2 | 23 (26) |
| 3 | 3 (3) |
|
| |
| Chemotherapy | |
| Yes | 79 (89) |
| Neoadjuvant | 32 (46) |
| Adjuvant | 46 (52) |
|
| |
| Neo/Adjuvant ICI | |
| Yes | 12 (14) |
|
| |
| Adjuvant TKI | |
| Yes | 10 (11) |
|
| |
| Radiation Dose | |
| Median, range (Gy) | 54 (50 – 60) |
|
| |
| Radiation Technique | |
| 3D-CRT | 18 (20) |
| IMRT | 71 (80) |
In total, 16 (18%) PORT patients had a deleterious mutation in a gene associated with radiation-resistance: STK11 (n=11), KEAP1 (n=4), NFE2L2 (n=1) and PIK3CA (n=2). Patients with and without these radiation-resistance tumor mutations were similar in stage, surgical margins status, number of involved mediastinal stations and TMB, but patients without mutations were older in age (p=0.006). Table 2A.
Table 2A.
Patient and Treatment Characteristics
| No. of Patients (%) | |||
|---|---|---|---|
|
| |||
| Characteristic | Radiation Resistance WT or VUS (n = 73) | Radiation Resistance mt (n = 16) | p – value |
|
| |||
| Age | 0.006 | ||
| Median, Range (years) | 68 (52 – 83) | 64 (46 – 79) | |
|
| |||
| Ever Smoker | 0.104 | ||
| Yes | 55 (75) | 15 (94) | |
|
| |||
| Sex | 0.665 | ||
| Female | 46 (63) | 11 (69) | |
| Male | 27 (37) | 5 (31) | |
|
| |||
| Performance Status | 0.689 | ||
| ECOG 0 | 45 (62) | 9 (56) | |
| ECOG 1 | 28 (38) | 7 (44) | |
|
| |||
| Histology | 0.391 | ||
| Adenocarcinoma | 65 (89) | 13 (81) | |
| Other | 8 (11) | 3 (19) | |
|
| |||
| Tumor Mutational Burden | 0.163 | ||
| Median, IQR (mt/Mb) | 6.1 (2.6–10.5) | 10.5 (6.1–15.8) | |
|
| |||
| AJCC 8th Overall Stage | 0.672 | ||
| < III | 7 (10) | 1 (6) | |
| IIIA or IIIB | 66 (90) | 15 (94) | |
|
| |||
| Margin Status | 0.985 | ||
| Negative | 64 (88) | 14 (88) | |
| Positive | 9 (12) | 2 (12) | |
|
| |||
| Involved Mediastinal Nodal Stations | 0.158 | ||
| <2 | 54 (74) | 9 (56) | |
| ≥2 | 19 (26) | 7 (44) | |
|
| |||
| Received Chemotherapy | 0.116 | ||
| Yes | 63 (86) | 16 (100) | |
|
| |||
| Received ICI | 0.350 | ||
| Yes | 11 (15) | 1 (6) | |
|
| |||
| Received TKI | 0.113 | ||
| Yes | 10 (14) | 0 | |
|
| |||
| Radiation Dose | 0.616 | ||
| Median, range (Gy) | 54 (50 – 60) | 54 (50.4 – 60) | |
|
| |||
| Radiation Technique | |||
| 3D-CRT | 15 (20) | 3 (19) | 0.871 |
| IMRT | 58 (80) | 13 (81) | |
In total, 15 (17%) PORT patients had a deleterious tumor mutation identified only in a DDR gene. Most common deleterious DDR mutations occurred in ARID1A (n=4), ATM (n=3), POLE (n=3), PMS2 (n=2) and TP53BP1 (n=2). Patients with and without these DDR mutations were mostly similar disease and treatment characteristics, but patients with mutations were found to have a significantly higher TMB (median 14 vs 6.1 mt/Mb, p=0.003). Table 2B. Among patients with deleterious tumor mutations in DDR genes, 6 received neoadjuvant chemotherapy, of whom all had >10% viable tumor remaining within the tumor bed after chemotherapy. Tumor genomic profiling results are shown in Supplemental Table 2.
Table 2B.
Patient and Treatment Characteristics
| No. of Patients (%) | |||
|---|---|---|---|
|
| |||
| Characteristic | DDR WT or VUS (n = 74) | DDR mt (n = 15) | p – value |
|
| |||
| Age | 0.951 | ||
| Median, Range (years) | 68 (46 – 83) | 69 (52 – 80) | |
|
| |||
| Ever Smoker | 0.406 | ||
| Yes | 57 (77) | 13 (87) | |
|
| |||
| Sex | 0.720 | ||
| Female | 48 (65) | 9 (60) | |
| Male | 26 (35) | 6 (40) | |
|
| |||
| Performance Status | 0.523 | ||
| ECOG 0 | 46 (62) | 8 (53) | |
| ECOG 1 | 28 (38) | 7 (46) | |
|
| |||
| Histology | 0.9 | ||
| Adenocarcinoma | 65 (88) | 13 (87) | |
| Other | 9 (12) | 2 (13) | |
|
| |||
| Tumor Mutational Burden | 0.003 | ||
| Median, IQR (mt/Mb) | 6.1 (3–10.5) | 14 (7.9–19.4) | |
|
| |||
| AJCC 8th Overall Stage | 0.182 | ||
| < III | 8 (11) | 0 | |
| IIIA or IIIB | 66 (89) | 15 (100) | |
|
| |||
| Margin Status | 0.9 | ||
| Negative | 65 (88) | 13 (87) | |
| Positive | 9 (12) | 2 (13) | |
|
| |||
| Involved Mediastinal Nodal Stations | 0.314 | ||
| <2 | 54 (73) | 9 (60) | |
| ≥2 | 20 (27) | 6 (40) | |
|
| |||
| Received Chemotherapy | 0.778 | ||
| Yes | 66 (89) | 13 (87) | |
|
| |||
| Received ICI | 0.397 | ||
| Yes | 11 (15) | 1 (7) | |
|
| |||
| Received TKI | 0.587 | ||
| Yes | 9 (12) | 1 (7) | |
|
| |||
| Radiation Dose | 0.616 | ||
| Median, range (Gy) | 54 (50 – 60) | 54 (50 – 60) | |
|
| |||
| Radiation Technique | |||
| 3D-CRT | 16 (22) | 2 (13) | 0.862 |
| IMRT | 58 (78) | 13 (87) | |
Overall Disease and Treatment Outcomes Among PORT Patients:
Across all patients treated with PORT, the 2 and 3-year incidence of LRF was 19% (95% CI: 10 – 27%) and 30% (13 – 42%), respectively. In total, 21 patients developed LRF and all but one patient had of component of an in-field failure seen on imaging. In total, 7 patients had local, in-field failure within the lung parenchyma and 14 patients had regional, in-field failure within the thoracic nodes. The 2- and 3-year DMFS estimates were 68% (58 – 78%) and 60% (49 – 71%). The median OS was not reached, the 2 and 3-year OS estimates were 78% (70 – 87%) and 71% (61 – 81%), respectively. In total, 43% (n=38) of patients had recurrent disease, and most patients (89% of recurrences, n=34) had a component of distant metastatic disease at first relapse.
Mutations in Radiosensitivity Genes Predict Local-Regional Outcomes with PORT.
Patients with deleterious tumor mutations in genes associated with radiation resistance had a significantly higher rate of LRF after PORT compared to patients without deleterious mutations in radiation resistance genes (p<0.001). The 2-year cumulative incidence of LRF in patients with vs without deleterious mutations was 60% (33 – 87) vs 11% (3 – 18%). (Figure 1A).
Figure 1.
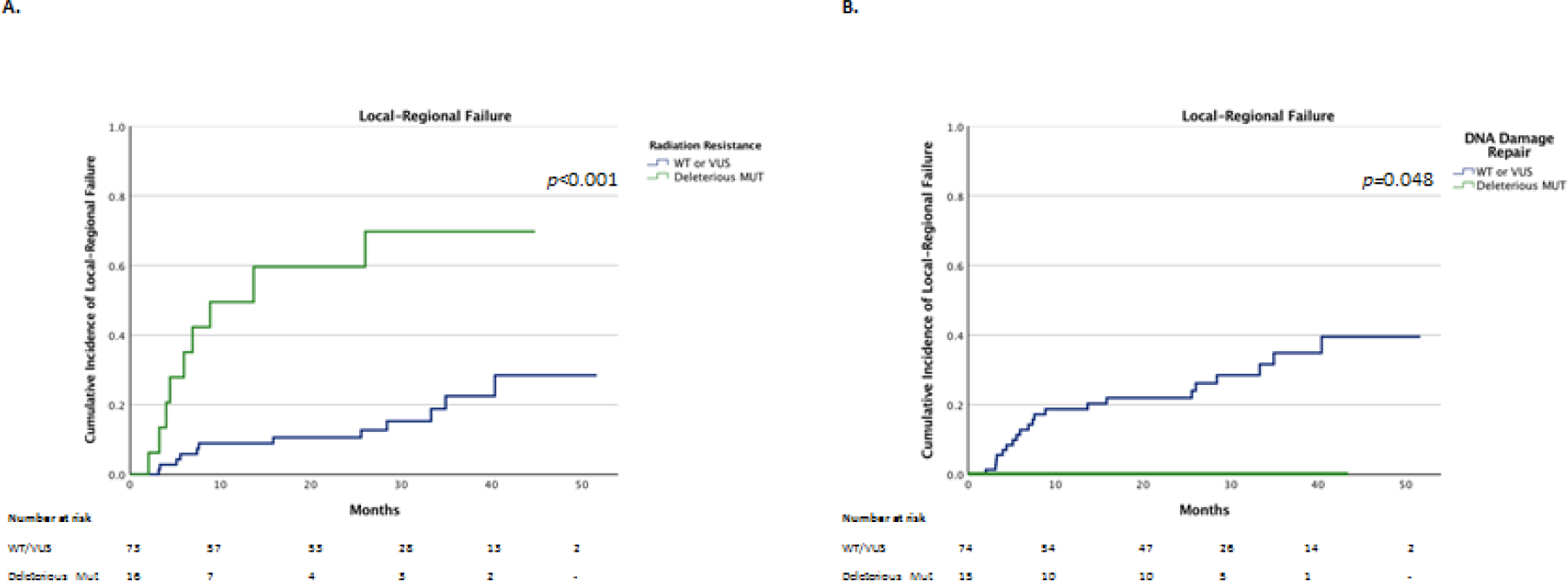
Comparison of local-regional failures between PORT patients with and without deleterious tumor mutations in genes associated with radiation resistance (A) and with and without identified deleterious tumor mutations only in DDR genes (B).
Patients with deleterious tumor mutations identified only in DDR genes had a significantly lower incidence of LRF (p=0.048) after PORT compared to patients without deleterious DDR mutations. There were no LRF events among these patients with mutations vs a 2-year incidence of LRF of 22% (12 – 32%) in patients without mutations identified in DDR genes. (Figure 1B).
TMB and Mutations in Radiation-Resistance Genes Predict Benefit of PORT
On univariate analysis neither age, sex, ECOG status, margin status, number of involved mediastinal stations nor receipt of ICI or TKI associated with LRF after PORT. On univariate analysis, a higher-TMB (p = 0.04) associated with improved LRC, and the presence of a deleterious radiation resistance mutation (p < 0.001) associated with increased LRF after PORT. On multivariate analysis, higher-TMB [hazards ratio (HR), 0.86, 95% CI, 0.77 – 0.97, p = 0.01] independently associated with improved LRC, and deleterious radiation resistance mutations (HR, 7.42, 95% CI, 2.83 – 19.44, p < 0.001) independently associated with increased LRF after PORT (Table 3).
Table 3:
Predictors for Local-Regional Failure After PORT
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | p - value | HR (95% CI) | p - value | |
| Age | 1.01 (0.95 – 1.06) | 0.84 | ||
| Sex | 1.67 (0.7 – 3.96) | 0.25 | ||
| ECOG 0 | 1.47 (0.62 – 3.46) | 0.38 | ||
| Margin Status | 1.27 (0.37 – 4.29) | 0.71 | ||
| Involved N2 Stations | 2.05 (0.84 – 4.97) | 0.11 | ||
| Receipt of ICI | 0.53 (0.12 – 2.29) | 0.39 | ||
| Receipt of TKI | 0.32 (0.04 – 2.39) | 0.27 | ||
| TMB | 0.91 (0.83–0.99) | 0.04 | 0.86 (0.77 – 0.97) | 0.01 |
| Radiation Resistance mut | 5.41 (2.26–12.94) | <0.001 | 7.42 (2.83 – 19.44) | <0.001 |
Age assessed as continuous variable. Sex (male vs female [ref]); ECOG 0 [ref] vs ECOG 1. Margin status (positive vs negative [ref]). Involved N2 stations (2 stations [ref] vs ≥2 stations). Receipt of ICI (yes [ref] vs no). Receipt of TKI (yes [ref] vs no). TMB assessed as continuous variable. Radiation Resistance mut (yes vs no [ref]).
Across all PORT patients, receiver operating characteristic curve analysis of TMB and local regional control found an area under the curve of 0.67 (0.54–0.8) (Figure 2A). When excluding patients with tumor mutations in radiation resistance genes, ROC analysis of TMB and LRF found an area under the curve of 0.77 (0.62–0.92) with a high-TMB (≥ 10 mt/Mb) having a 92% sensitivity for predicting LRC after PORT (Figure 2B).
Figure 2.
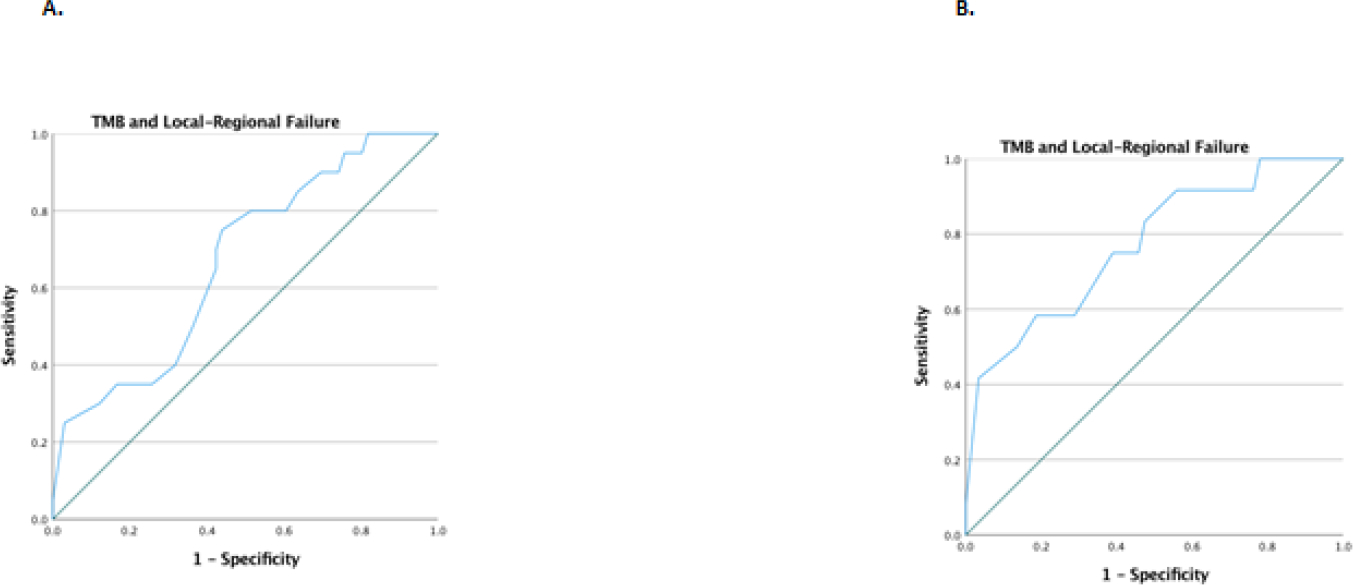
Receiver operating characteristic curve analysis of TMB and local regional control among all PORT patients (A) and PORT patients without deleterious tumor mutations in genes associated with radiation resistance (B)
Distant Control and Overall Survival After PORT in Radiation Resistant and Sensitive Cohorts:
Patients with radiation resistance mutations had significantly lower DMFS and overall survival versus patients without: two-year DMFS and OS rates of 25% (5 – 45%) vs 77% (68 – 87%) and 53% (27 – 79%) vs 83% (75 – 92%) (p<0.001, for both comparisons). (Supplemental Figure 1). Among patients with mutations only in DDR genes, DMFS and overall survival were not significantly different between patients with and without mutations (p=0.944 and p=0.25, respectively) (Supplemental Figure 2). However, compared to patients with radiation resistance mutations, patient with DDR mutations had significantly higher DMFS, with 2-year DMFS rates of 67% (33 – 91%) vs 25% (5 – 45%) (p=0.019) (Supplemental Figure 3).
Genomic Predictors for PORT Outcomes Do Not Associate with LRC in No-PORT Cohort
In total, 19 patients with stage III NSCLC with mediastinal nodal involvement who did not receive PORT were examined (Supplemental Table 3). Among these patients, 4 (21%) had a deleterious mutation in genes associated with radiation-resistance and 4 (21%) had a deleterious mutation identified only in a DDR Gene (Supplemental Table 4). The 2-year incidence of LRF was 57% (95% CI: 30–86%) with no significant difference in local-regional failure among patients with and without mutations in genes associated with radiation-resistance (p = 0.99) or between patients with and without mutations identified only in DDR genes (p = 0.322) (Supplemental Figure 4). On univariate variate analysis, radiation-resistance or DDR gene mutational status did not predict for local-regional failure, but a higher-TMB was associated with increased LRF (HR, 1.28, 95% CI, 1.05 – 1.55, p = 0.01) (Supplemental Table 5).
Discussion:
Recently published randomized trials evaluating PORT in patients with resected NSCLC have called out the need to better identify patients who may most benefit from PORT (4, 5). Although adjuvant therapy in resected NSCLC is evolving, local-regional failures are the predominant site of relapse even in patients treated with adjuvant ICI on recent trials (23). In this study, we assessed if tumor genomic profiling can be used to select patients for PORT. We found patients with mutations in tumor genes associated with radiation resistance to have high-rates of local-regional failure after PORT, suggesting a minimal benefit from PORT. However, in patients with mutations in DDR genes, local-regional failure was exceedingly low, suggesting that these patients not only benefit from PORT, but that a lower-dose precision-RT based approach may be warranted. Additionally, to our knowledge, this report is among the first to associate TMB with radiotherapy outcomes. A higher TMB has been associated with tumor DDR mutations and tumor immunogenicity (11, 12), and our data suggest that TMB may also be a novel biomarker for radiation-response. Given that we did not find these same genomic markers to associate with local-regional outcomes in patients without PORT, these markers potentially could be uniquely predictive of PORT outcomes and prospective studies are warranted to validate these findings.
We found patients with identified deleterious mutations in either KEAP1, NFE2L, STK11 or PIK3CA to have a two-year LRF rate of approximately 60%. This high LRF rate suggests a limited benefit of PORT in this radiation-resistant subgroup as the 3-year LRF rate in the no-radiation arms of the PORT-C and LungART trials was approximately 45% (4, 5). The KEAP1/NFE2L2 pathway plays a role in regulating cellular stress, and mutations in these genes can lead to NFE2L2 overexpression thereby protecting cancer cells from the effects of radiation (8, 24, 25). Prior studies have not found mutations in the KEAP1/NFE2L2 pathway to predict for increased LRF in surgically treated patients without radiation or chemotherapy (8), suggesting the utility of KEAP1/NFE2L2 in predicting radiotherapy local-regional outcomes. Mutations in STK11 have also been found to promote resistance to radiation potentially through engaging the KEAP1/NFE2L2 pathway (7). Additionally, mutations in PIK3CA have been associated with radiation resistance both in pre-clinical models and in patients with NSCLC treated with radiation (19, 26). Approximately 20% of our patient population had an identified radiation-resistance mutation. This suggests that this sizeable cohort of patients with inherent radiation-resistance could have blunted the benefit of PORT across the unselected patient populations in the PORT-C and LungART trials. While our data requires further validation, standard PORT among this patient subgroup may expose patients to RT-associated toxicity without significant clinical benefit.
Patients with tumor mutations identified only in DDR genes were found to be at very-low risk for LRF, with no LRF events in this patient subgroup. This finding cannot be fully explained by a favorable response to chemotherapy as all patients in the DDR mutant cohort who received neoadjuvant chemotherapy had viable tumor at time of surgical resection. Therefore, suggesting that PORT did indeed contribute to their favorable local-regional outcomes. Across the large panel of DDR genes selected, deleterious mutations in ATM, POLE, PMS2, TP53BP1 or ARID1A were most identified. Importantly, while mutations in ATM and TP53BP1 are central mediators in double-strand DNA break repair and are associated with clinical radiation sensitivity (27, 28), the role POLE, ARID1A and PMS2 play in repair from DNA damage from radiotherapy is less clear (21, 29–31). Supporting our findings however is a similar analysis that assessed a panel of DDR genes from multiple DDR pathways and found NSCLC tumors with deleterious DDR mutations to have significantly improved local-regional control when treated with definitive chemoradiation (10). Given their exceedingly low rate of LRF with standard PORT, our data supports investigating a lower dose precision-PORT approach among these patients to improve the therapeutic ratio.
Most intriguing, we found a higher-TMB to predict for improved local-regional control after PORT on multivariate analysis. Additionally, TMB was identified as an acceptable-to-good tool on ROC analysis with high-sensitivity for identifying the benefit of PORT. A high-TMB has been found to predict for response to immunotherapy and pembrolizumab is FDA approved for the treatment of solid tumors based on high-TMB (32–34). However, TMB is not a prognostic biomarker as it has not been found to predict for outcomes in patients who have not received immunotherapy (33). Given that only 14% of patients in our study were treated with adjuvant ICI, our data imply that the association between TMB and local-regional outcomes is a reflection on radiation therapy. Furthermore, among our cohort of patients not treated with PORT, a higher-TMB was associated with higher local-regional failure. This finding is supported by a recent study that found higher-TMB to associate with aggressive clinicopathologic features that predict for local-regional recurrence (35, 36). All together, these data suggest that TMB is a radiation sensitivity biomarker that warrants investigation.
Multiple lines of evidence provide rationale to support our finding that a high-TMB can predict for radiation sensitivity. First, studies have found a higher TMB to correlate with DDR mutations and for the majority of NSCLC patients harboring DDR mutations to have a high-TMB (12–14). Given that DDR genes play a role in radiation repair, mutations in these genes, as our data also suggests, can lead to radiation sensitivity. Second, a higher TMB has been associated with tumor immunogenicity, as high-TMB tumors have more neoantigens that could be involved in antitumor immunity (11). Data have found radiation sensitivity to also be partly dependent on the anti-tumor immune response, therefore providing further biological rationale to support our findings (37, 38). Although validation of our work is necessary in other NSCLC patient populations with limited ICI exposure, TMB could represent a tool to select patients for precision-RT PORT approaches.
This work is limited by its retrospective nature and of its inclusion of a single cancer center. However, patients in this cohort had substantial follow-up and represent a modern cohort during which time tumor genomic profiling was routinely performed on primary tumor specimens. Given that the determination of the pathogenicity of a mutation is dependent on available data, our work is further constrained by incomplete data on certain tumor mutations. Additionally, given the rare frequency of mutations in individual DDR genes, we used a previously established panel of genes for analysis, however this work is limited by its sample size and larger cohorts will be necessary to identify outcomes from mutations in individual repair genes. Another limitation of this analysis is our inability to simultaneously control for TMB and DDR mutations on MVA, due to the lack of events in the DDR mutant group. Additionally, although our no-PORT cohort was limited in size given that these patients deviated from an institutional standard of care, the findings from this cohort are consistent with the published literature. There were imbalances in characteristics between PORT patients with and without pathogenic radiation resistance mutations, with patients with mutations being younger in age, that could have introduced bias. Additionally, multiple hypotheses were tested which could have inflated type I error, however our statistical methods are consistent with the exiting literature in this space (7, 24, 39, 40).
Local-regional failures in resected NSCLC patients represent a predominant site of relapse. PORT has been found to significantly improve local-regional outcomes but strategies to select patients for PORT have been limited. We found that tumor genomic profiling can potentially identify patients with inherent radiation -resistance for whom standard PORT may have limited clinical benefit and that there may be a cohort of patients with DDR mutations and high TMB for whom a precision-RT based PORT treatment warrants further prospective investigation. Although further work validating these findings are required, strategies that involve tumor genomic may allow for optimal patient selection for PORT.
Supplementary Material
Acknowledgements:
We are thankful to the Molecular Diagnostics Service in the Department of Pathology, and the Marie-Josee and Henry R. Kravis Center for Molecular Oncology.
Funding Statement:
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748
Footnotes
Conflict of Interest Statements:
N. Shaverdian: Reports research funding from Novartis. 0A. F. Shepard: Reports honoraria from ASCO0, M. Offin: Reports advisory role for PharMar, Novartis and Targeted Oncology. Reports honoraria from Bristol-Myers Squibb and Merck Sharp & Dohme. X. Li: No COI to report., H. B. Lengel: No COI to report., D. Y. Gelblum: No COI to report. 0 A. J. Wu: Reports research support from CivaTech Oncology, Inc., non-financial support from AlphaTau Medical, personal fees from MoreHealth, and personal fees from AstraZeneca., C. B. Simone II: Reports honoraria from Varian Medical Systems., A. Rimner: Reports grants from Varian Medical Systems, grants from Boehringer Ingelheim, grants from Pfizer, grants and personal fees from AstraZeneca, grants and personal fees from Merck, personal fees from Research to Practice, personal fees from Cybrexa, non-financial support from Philips/Elekta, personal fees from MoreHealth., D. R. Jones: Reports serving as senior medical advisor for Diffusion Pharmaceuticals, Inc, and consulting for Merck & Co and AstraZeneca, J. E. Chaft: Reports both research funding and consulting roles with Bristol-Myers Squibb, Merck, Genentech and AstraZeneca. ‘N. Riaz: Reports honoraria from PeerView, consulting role at Mirati Therapeutics, Repare Therapeutics, research funding from Bristol Myers Squibb, Pfizer, Repare Therapeutics and expenses from Varian Medical Systems, D. R. Gomez: Reports honoraria from Merck, BMS, AstraZeneca, Reflexion, Medscape, Vindico, US Oncology, and Varian. Reports research support from Merck, BMS, AstraZeneca, and Varian. Serves on advisory board for AstraZeneca.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Availability Statement:
Research data are not available at this time, deidentified data elements can be made available upon reasonable request from the corresponding author.
References:
- 1.Aupérin A, Le Péchoux C, Rolland E, Curran WJ, Furuse K, Fournel P, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(13):2181–90. [DOI] [PubMed] [Google Scholar]
- 2.Albain KS, Swann RS, Rusch VW, Turrisi AT, Shepherd FA, Smith C, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. The Lancet. 2009;374(9687):379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Machtay M, Paulus R, Moughan J, Komaki R, Bradley JE, Choy H, et al. Defining local-regional control and its importance in locally advanced non-small cell lung carcinoma. J Thorac Oncol. 2012;7(4):716–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hui Z, Men Y, Hu C, Kang J, Sun X, Bi N, et al. Effect of Postoperative Radiotherapy for Patients With pIIIA-N2 Non–Small Cell Lung Cancer After Complete Resection and Adjuvant Chemotherapy: The Phase 3 PORT-C Randomized Clinical Trial. JAMA oncology. 2021;7(8):1178–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le Pechoux C, Pourel N, Barlesi F, Faivre-Finn C, Lerouge D, Zalcman G, et al. LBA3_PR An international randomized trial, comparing post-operative conformal radiotherapy (PORT) to no PORT, in patients with completely resected non-small cell lung cancer (NSCLC) and mediastinal N2 involvement: Primary end-point analysis of LungART (IFCT-0503, UK NCRI, SAKK) NCT00410683. Annals of Oncology. 2020;31:S1178. [Google Scholar]
- 6.Shepherd AF, Yu AF, Iocolano M, Leeman JE, Wild AT, Imber BS, et al. Increasing Heart Dose Reduces Overall Survival in Patients Undergoing Postoperative Radiation Therapy for NSCLC. JTO Clinical and Research Reports. 2021;2(8):100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sitthideatphaiboon P, Galan-Cobo A, Negrao MV, Qu X, Poteete A, Zhang F, et al. <em>STK11</em>/LKB1 Mutations in NSCLC Are Associated with KEAP1/NRF2-Dependent Radiotherapy Resistance Targetable by Glutaminase Inhibition. Clinical Cancer Research. 2021;27(6):1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Binkley MS, Jeon Y-J, Nesselbush M, Moding EJ, Nabet BY, Almanza D, et al. KEAP1/NFE2L2 Mutations Predict Lung Cancer Radiation Resistance That Can Be Targeted by Glutaminase Inhibition. Cancer Discovery. 2020;10(12):1826–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lockney NA, Yang TJ, Barron D, Gelb E, Gelblum DY, Yorke E, et al. PIK3CA mutation is associated with increased local failure in lung stereotactic body radiation therapy (SBRT). Clin Transl Radiat Oncol. 2017;7:91–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo LY, Samstein RM, Dick-Godfrey R, Sidiqi B, Wang C, Oro F, et al. Genomic Analyses for Predictors of Response to Chemoradiation in Stage III Non-Small Cell Lung Cancer. Advances in Radiation Oncology. 2021;6(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGranahan N, Furness AJS, Rosenthal R, Ramskov S, Lyngaa R, Saini SK, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351(6280):1463–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mamdani H, Chen J, Kim S, Ibrahim Y, Asad MFB, Nieva JJ, et al. DNA damage response and repair (DDR) gene mutations and correlation with tumor mutation burden (TMB) in non-small cell lung cancer (NSCLC). Journal of Clinical Oncology. 2019;37(15_suppl):9100-. [Google Scholar]
- 13.Li Z, Niu Y, Ma T, Yuan H. MA13.03 DNA Damage Response Gene Alterations and their Association with Tumor Mutation Burden and Response to Immunotherapy in NSCLC and SCLC. Journal of Thoracic Oncology. 2021;16(3):S182. [Google Scholar]
- 14.Chae YK, Davis AA, Raparia K, Agte S, Pan A, Mohindra N, et al. Association of Tumor Mutational Burden With DNA Repair Mutations and Response to Anti-PD-1/PD-L1 Therapy in Non-Small-Cell Lung Cancer. Clinical lung cancer. 2019;20(2):88–96.e6. [DOI] [PubMed] [Google Scholar]
- 15.Zehir A, Benayed R, Shah RH, Syed A, Middha S, Kim HR, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nature medicine. 2017;23(6):703–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng DT, Mitchell TN, Zehir A, Shah RH, Benayed R, Syed A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. The Journal of molecular diagnostics : JMD. 2015;17(3):251–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shepherd AF, Iocolano M, Leeman J, Imber BS, Wild AT, Offin M, et al. Clinical and Dosimetric Predictors of Radiation Pneumonitis in Patients With Non-Small Cell Lung Cancer Undergoing Postoperative Radiation Therapy. Practical Radiation Oncology. 2021;11(1):e52–e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horie Y, Suzuki T, Inoue J, Iso T, Wells G, Moore TW, et al. Molecular basis for the disruption of Keap1–Nrf2 interaction via Hinge & Latch mechanism. Communications Biology. 2021;4(1):576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta AK, Bakanauskas VJ, Cerniglia GJ, Cheng Y, Bernhard EJ, Muschel RJ, et al. The Ras radiation resistance pathway. Cancer research. 2001;61(10):4278–82. [PubMed] [Google Scholar]
- 20.Teo MY, Seier K, Ostrovnaya I, Regazzi AM, Kania BE, Moran MM, et al. Alterations in DNA Damage Response and Repair Genes as Potential Marker of Clinical Benefit From PD-1/PD-L1 Blockade in Advanced Urothelial Cancers. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2018;36(17):1685–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olivieri M, Cho T, Álvarez-Quilón A, Li K, Schellenberg MJ, Zimmermann M, et al. A Genetic Map of the Response to DNA Damage in Human Cells. Cell. 2020;182(2):481–96.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen J, Peng Y, Wei L, Zhang W, Yang L, Lan L, et al. ARID1A Deficiency Impairs the DNA Damage Checkpoint and Sensitizes Cells to PARP Inhibitors. Cancer Discovery. 2015;5(7):752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Felip EVCZ E, Wakelee H, Bondarenko I, Sakai H, Saito H, Ursol G, Kawaguchi K, Liu1 Y, Levchenko E, Kislov N, Reck M, Liersch R, McNally VA, Zhu Q, Ding B, Bennett E, Gitlitz B, Altorki NK. LBA9 - IMpower010: Sites of relapse and subsequent therapy from a phase III study of atezolizumab vs best supportive care after adjuvant chemotherapy in stage IB-IIIA NSCLC: ESMO; 2021. [Available from: https://oncologypro.esmo.org/meeting-resources/esmo-congress-2021/impower010-sites-of-relapse-and-subsequent-therapy-from-a-phase-iii-study-of-atezolizumab-vs-best-supportive-care-after-adjuvant-chemotherapy-in-s. [Google Scholar]
- 24.Jeong Y, Hoang NT, Lovejoy A, Stehr H, Newman AM, Gentles AJ, et al. Role of KEAP1/NRF2 and TP53 Mutations in Lung Squamous Cell Carcinoma Development and Radiation Resistance. Cancer Discov. 2017;7(1):86–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Best SA, Sutherland KD. “Keaping” a lid on lung cancer: the Keap1-Nrf2 pathway. Cell Cycle. 2018;17(14):1696–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lockney NA, Yang TJ, Barron D, Gelb E, Gelblum DY, Yorke E, et al. PIK3CA mutation is associated with increased local failure in lung stereotactic body radiation therapy (SBRT). Clin Transl Radiat Oncol. 2017;7:91–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pitter KL, Casey DL, Lu YC, Hannum M, Zhang Z, Song X, et al. Pathogenic ATM Mutations in Cancer and a Genetic Basis for Radiotherapeutic Efficacy. J Natl Cancer Inst. 2021;113(3):266–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwabuchi K, Hashimoto M, Matsui T, Kurihara T, Shimizu H, Adachi N, et al. 53BP1 contributes to survival of cells irradiated with X-ray during G1 without Ku70 or Artemis. Genes Cells. 2006;11(8):935–48. [DOI] [PubMed] [Google Scholar]
- 29.Gool I, Rayner E, Osse E, Nout R, Creutzberg C, Tomlinson I, et al. Adjuvant Treatment for POLE Proofreading Domain-Mutant Cancers: Sensitivity to Radiotherapy, Chemotherapy, and Nucleoside Analogues. Clinical Cancer Research. 2018;24:clincanres.0266.2018. [DOI] [PubMed] [Google Scholar]
- 30.Park Y, Chui MH, Suryo Rahmanto Y, Yu ZC, Shamanna RA, Bellani MA, et al. Loss of ARID1A in Tumor Cells Renders Selective Vulnerability to Combined Ionizing Radiation and PARP Inhibitor Therapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2019;25(18):5584–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu XS, Narayanan L, Dunklee B, Liskay RM, Glazer PM. Hypermutability to ionizing radiation in mismatch repair-deficient, Pms2 knockout mice. Cancer research. 2001;61(9):3775–80. [PubMed] [Google Scholar]
- 32.Galvano A, Gristina V, Malapelle U, Pisapia P, Pepe F, Barraco N, et al. The prognostic impact of tumor mutational burden (TMB) in the first-line management of advanced non-oncogene addicted non-small-cell lung cancer (NSCLC): a systematic review and meta-analysis of randomized controlled trials. ESMO Open. 2021;6(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samstein RM, Lee C-H, Shoushtari AN, Hellmann MD, Shen R, Janjigian YY, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nature Genetics. 2019;51(2):202–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones GD, Brandt WS, Shen R, Sanchez-Vega F, Tan KS, Martin A, et al. A Genomic-Pathologic Annotated Risk Model to Predict Recurrence in Early-Stage Lung Adenocarcinoma. JAMA Surg. 2021;156(2):e205601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kadota K, Nitadori J-I, Sima CS, Ujiie H, Rizk NP, Jones DR, et al. Tumor Spread through Air Spaces is an Important Pattern of Invasion and Impacts the Frequency and Location of Recurrences after Limited Resection for Small Stage I Lung Adenocarcinomas. J Thorac Oncol. 2015;10(5):806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen H-y, Xu L, Li L-f, Liu X-x, Gao J-x, Bai Y-r. Inhibiting the CD8+ T cell infiltration in the tumor microenvironment after radiotherapy is an important mechanism of radioresistance. Scientific Reports. 2018;8(1):11934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee Y, Auh SL, Wang Y, Burnette B, Wang Y, Meng Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114(3):589–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jeong Y, Hellyer JA, Stehr H, Hoang NT, Niu X, Das M, et al. Role of KEAP1/NFE2L2 Mutations in the Chemotherapeutic Response of Patients with Non–Small Cell Lung Cancer. Clinical Cancer Research. 2020;26(1):274–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shaverdian N, Offin M, Shepherd AF, Simone CB II, Gelblum DY, Wu AJ, et al. The Impact of Durvalumab on Local-Regional Control in Stage III NSCLCs Treated With Chemoradiation and on KEAP1-NFE2L2-Mutant Tumors. Journal of Thoracic Oncology. 2021;16(8):1392–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Research data are not available at this time, deidentified data elements can be made available upon reasonable request from the corresponding author.


