Abstract
Background
Older females show greater cognitive gains from physical activity (PA) than males, which may be related to long-term consequences of female-specific reproductive events (eg, pregnancy) on cognitive health.
Methods
To determine whether previous parity could moderate the relationship between PA and cognitive decline in older women, we conducted secondary analyses of data from the Health, Aging, and Body Composition Study. We tested whether the association between average PA over 10 years and cognition (Modified Mini-Mental State Examination [3MS]) and executive functioning (digit symbol substitution test [DSST]) over 10 years varied by previous parity (nulliparity, low parity, medium parity, and grand multiparity). An analysis of covariance was performed with cognition (average and change over 10 years) as the dependent variables, parity as a categorical predictor, average PA as a continuous predictor, and a set of relevant covariates.
Results
Significant interactions were found between PA and parity group for all 4 comparisons: average 3MS (p = .014), average DSST (p = .032), change in 3MS (p = .016), and change in DSST (p = .017). Simple slope analyses indicated the positive relationship between PA and average 3MS and DSST was only significant in the nulliparity and grand multiparity groups, and the positive relationship between PA and change in 3MS and DSST was only significant in the grand multiparity group.
Conclusion
The findings suggest the relationship between self-reported walking and cognitive performance was strongest in the groups at risk for cognitive decline and dementia, the nulliparous and grand multiparous groups.
Keywords: Cognitive aging, Parity, Physical activity, Reproductive experience
Background
Physical activity (PA) is a promising strategy for improving brain and cognitive health during aging (1). Recent work suggests that older females may show greater cognitive benefits than older males (2–6); however, little is known as to why the female brain may respond differently to PA than the male brain. Potential sex differences in neuroplastic processes underlying PA effects may be involved. For example, engaging in 6 months of aerobic exercise increased levels of brain-derived neurotrophic factor, which is involved in neuroplasticity and neuronal health, to a greater extent in older females than in older males (3). Importantly, pregnancy and childbirth are sex-specific biological events that have long-term consequences for brain health and neuroplasticity (7,8), thus potentially influencing the ability of the female brain to respond to PA in older age.
Pregnancy has profound effects on female physiology, brain function, and disease risk in the long-term (9). Evidence suggests parity is related to Alzheimer’s disease (AD) risk and cognitive decline, with effects dependent on the specific number of births. Grand multiparity, defined as having 5 or more births, is associated with an increased risk of AD (10–12). Additionally, 4 or more pregnancies are associated with earlier age of onset of AD versus 3 or less pregnancies (13). After controlling for age at death and dementia severity, greater parity was correlated with more severe AD-pathology rating in the amygdala in the postmortem brains of females (14). Less studied is the potential role of nulliparity, having 0 births, on AD and cognition. Nulliparity has been associated with an increased risk of AD compared with having 1 birth but not having 2 or more births (15), although not all studies find this (10,16,17). Few studies have examined the effects of different amounts of parity on cognitive functioning in older age after controlling for potential confounding factors such as socioeconomic status, and the majority of these studies have been limited in sample size with a small range in parity, have not included nulliparous females, and have narrowly focused on global cognitive functioning as measured by the Mini-Mental State Examination (MMSE) at a single time point. Greater parity (1–6 children) has been associated with lower global cognition (18), and grand multiparity (≥5 births) has been linked to lower global cognition (10,11,19). In one of the only longitudinal studies, McLay et al. (20) found greater rates of decline in global cognition over years in parous females versus nulliparous females. Together, these results indicate that parity may alter cognitive function in the long-term.
We aimed to determine whether parity, defined as the number of live births, moderates the relationship between PA and cognition in older women by conducting a secondary analysis of existing data from the Health, Aging, and Body Composition (Health ABC) study―a 10-year longitudinal, cohort study of cognitively healthy older adults. We hypothesized a positive relationship between PA and cognition that increased in strength with increased parity, such that the strongest association will be observed in the grand multiparity group, the group most at risk for cognitive impairment and dementia.
Method
Participants
Participants were from the Health ABC study, a 10-year prospective, epidemiological, biracial cohort study of older adults 70–79 years of age at baseline that were recruited in 1997 from a random sample of community-dwelling White and Black older adults in Memphis, TN or Pittsburgh, PA. The Health ABC study enrolled a total of 3 075 older adults (50% female) that were well-functioning at baseline (21). Recruitment and study details can be found in previous Health ABC studies (22). Among the female participants in the Pittsburgh cohort, 596 females provided information about their pregnancy history in 2003 and 2004 as part of a substudy (23). The present study involves 442 of the 596 participants with complete data for the main variables of interest (ie, parity, cognition, and PA). This study was approved by the institutional review boards at the University of Tennessee Memphis, the University of Pittsburgh, and the University of California San Francisco. All eligible participants gave written informed consent.
Measures
Parity
Parity information was collected via questionnaire. Parity was defined as the number of births lasting at least 6 months gestation, and births were reported as live or stillborn. Number of preeclamptic and hypertensive pregnancies was determined as was the number of pregnancies with gestational diabetes. Parity was split into 4 distinct groups based on previous literature (10,11,23): nulliparous (0 births > 6 months), low parity (1–2 births > 6 months), medium parity (3–4 births > 6 months), and grand multiparous (≥ 5 births > 6 months).
Physical activity
Physical activity was ascertained from self-reported time spent walking (mins/week) that was measured annually from years 1 to 10 using a standardized questionnaire developed for the Health ABC study and modeled on a previous questionnaire (24). Walking per week was the only form of PA assessed in the full study sample. The questionnaire first determined whether participants had engaged in walking at least 10 times in the past 12 months and then whether participants had engaged in walking in the past 7 days. Participants answering yes were then queried about the total number of minutes of walking they had completed in the past 7 days. Average number of minutes spent walking over the last 7 days across the 10-year study period was computed. To help validate this self-report measure of PA, a subset of Health ABC participants (n = 114) completed an objective PA measurement using a SenseWear Armband (Body-Media Inc, Pittsburgh, PA) worn on the left upper arm over at least 3 days. As previously published, self-reported time spent walking was significantly correlated with objectively measured daily active energy expenditure (r = 0.27, p < .01), PA intensity (r = 0.29, p < .01), and objective step counts (r = 0.21, p < .02) (25).
Cognition
Global cognitive functioning and executive functioning were measured in Health ABC. Specifically, global cognitive performance was measured with the Modified Mini-Mental State Examination (3MS) in years 0, 2, 4, 7, and 9 after baseline, with higher scores indicating better performance. Digit symbol substitution test (DSST) measured the executive functions of sustained attention and working memory in addition to processing speed (26–29) in years 0, 4, 7, and 9 after baseline, with higher scores indicating better performance. Average cognitive performance over 10 years and the annual rate of change in performance was computed for each test.
Demographics and covariates
Demographics, including age, race (White or Black), educational attainment (< or ≥ high school), and family income (<$10 000, ≥$10 000 to <$25 000, ≥$25 000 to <$50 000, and ≥$50 000), and health-related characteristics were collected and assessed at baseline. Depressive symptoms were assessed with the 20-item Center for Epidemiologic Studies-Depression Scale (CES-D) with scores above 16 indicative of depression (30). Baseline comorbidities were determined from self-report, medication use, physician diagnoses, and laboratory data, including cardiovascular disease, cerebrovascular disease, and hypertension. Blood pressure was calculated as the average of 2 measurements by mercury sphygmomanometer, taken in the seated position after 5 minutes of quiet rest. Alcohol use and smoking status (current/former and never) were collected via self-report. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Gait speed (m/s), a valid marker of physical performance in older adults (31), was measured at the usual pace over 6 meters. Apolipoprotein E (APOE) genotype was determined by standard single-nucleotide polymorphism techniques for rs429358 and rs7412 (carriers and noncarriers).
Statistical Analyses
The data for total minutes spent walking per week were positively skewed, thus this variable was log-transformed prior to analysis to achieve normality. Analyses were carried out using SPSS (version 23). Demographic and baseline characteristics were evaluated according to parity group using either ANOVA or chi-square tests. To determine whether parity groups differed in average and annual rate of change in 3MS and DSST, as well as in average PA, ANOVAs were run controlling for depression, education, race, age, diabetes, APOE4, smoking, alcohol, cardiovascular disease, cerebrovascular disease, hypertension, high blood pressure, and family income.
ANCOVAs were conducted with cognition (average and change) as the dependent variable, parity group (nulliparity, low parity, medium parity, and grand multiparity) as a categorical predictor, average PA (average time spent walking per week over 10 years) as a continuous predictor, and a set of covariates (depression, education, race, age, diabetes, APOE4, smoking, alcohol, cardiovascular disease, cerebrovascular disease, hypertension, high blood pressure, and family income). The interaction between parity group and average PA was included to test whether the nature of the association (ie, strength and sign) between average PA and cognition depended on parity. Additionally, because the primary effect of interest was an interaction between PA and parity group, the interactions between each covariate and parity group were also included to better adjust for confounding (32). In the presence of a significant interaction between parity group and average PA, simple slopes between average PA and cognition for each parity group were extracted from the model. Separate analyses were performed for average and annual rate of change for 3MS and for DSST. To examine whether parity moderated the association between average PA and cognitive function, as measured by the 3MS and DSST, we conducted 3 planned comparisons, comparing the nulliparity, medium parity, and grand multiparity groups with the low parity group (the reference group).
Results
Demographics and Baseline Characteristics
Table 1 shows the baseline characteristics of the sample of 442 women separated by parity group (nulliparity, low parity, medium parity, and grand multiparity). Between-group differences were found for baseline age, depression, 3MS, DSST, education, smoking, and diabetes. Briefly, the nulliparous group was slightly older and less depressed. The low parity group had slightly lower baseline 3MS and DSST scores. The medium parity group had higher education, both the nulliparous and low parity groups had more smokers at baseline, and the grand multiparity group had more women with diabetes. Parity groups did not differ in BMI, gait speed, pregnancy complications, income, race, drinking, peripheral arterial disease, cerebrovascular disease, high blood pressure, and APOE4 status.
Table 1.
Baseline (Year 1) Participant Characteristics for Each Parity Group, Where Appropriate, One-way ANOVA or Chi-square Tests Were Conducted to Compare Parity Groups
| Mean (SD) or N (%) | Nulliparous (0 Births) | Low Parity (1–2 Births) | Medium Parity (3–4 Births) | Grand Multiparity (≥5 births) | p Value |
|---|---|---|---|---|---|
| N | 70 (16%) | 150 (34%) | 165 (37%) | 57 (13%) | |
| Age in years | 74.4 (2.7) | 73.4 (2.9) | 72.6 (2.6) | 73.1 (3.0) | <.001* |
| BMI in kg/m2 | 28.7 (5.8) | 27.7 (4.7) | 27.8 (5.5) | 29.0 (5.2) | .279 |
| CES-D | 3.8 (4.3) | 6.6 (7.0) | 5.1 (5.3) | 5.4 (6.3) | .012* |
| 3MS | 92.0 (6.9) | 90.9 (6.7) | 93.1 (5.3) | 90.1 (7.5) | .003* |
| DSST | 42.0 (14.9) | 38.3 (11.7) | 43.6 (11.8) | 41.5 (12.7) | .002* |
| Gait speed in m/s | 1.1 (0.2) | 1.2 (0.2) | 1.2 (0.2) | 1.1 (0.2) | .415 |
| Pregnancy complications | 5 (3%) | 8 (5%) | 5 (9%) | .265 | |
| Education | .043* | ||||
| > HS | 12 (17%) | 26 (17%) | 14 (8.5%) | 12 (21.1%) | |
| ≤ HS | 58 (83%) | 124 (83%) | 151 (92%) | 45 (79%) | |
| Income | .066 | ||||
| <25 000 | 40 (57%) | 80 (53%) | 94 (57%) | 42 (73%) | |
| ≥25 000 | 30 (43%) | 70 (47%) | 71 (43%) | 15 (26%) | |
| White | 35 (50%) | 76 (51%) | 98 (59%) | 26 (46%) | .209 |
| Smoking | 37 (53%) | 74 (49%) | 59 (36%) | 20 (35%) | .017* |
| Drinking | 50 (71%) | 113 (75%) | 116 (70%) | 42 (74%) | .781 |
| Heart disease | 6 (9%) | 15 (10%) | 18 (11%) | 10 (18%) | .385 |
| Diabetes | 9 (13%) | 12 (8%) | 13 (8%) | 15 (26%) | .001* |
| Peripheral arterial disease | 3 (4%) | 2 (1%) | 3 (2%) | 3 (5%) | .276 |
| Cerebrovascular disease | 3 (4%) | 13 (9%) | 8 (5%) | 3 (5%) | .450 |
| High blood pressure | 37 (53%) | 68 (45%) | 72 (44%) | 27 (47%) | .623 |
| APOEε4 | 21 (30%) | 41 (27%) | 44 (27%) | 18 (32%) | .878 |
Notes: * = statistically significant; BMI = body mass index; CES-D = Center for Epidemiologic Studies-Depression Scale (scores above 16 indicative of depression); 3MS = Modified Mini-Mental State Examination (higher scores indicating better performance); DSST = Digit symbol substitution test (higher scores indicating better performance); APOE ε4: Apolipoprotein E epsilon 4 carriers; SD = standard deviation.
The Effect of Previous Parity on Cognition
A significant main effect of parity group was found for average DSST score across time (F[3 425] = 2.575, p = .05, η p2 = 0.018). The low parity group had a significantly lower average DSST score than the other 3 parity groups (all p’s ≤ .04). A main effect of parity group was not found for average 3MS score (F[3 425] = 0.580, p > .05, η p2 = 0.004), change in 3MS over 10 years (F[3 425] = 0.036, p > .05, η p2 = 0.000), or change in DSST over 10 years (F[3 425] = 0.069, p > .05, η p2 = 0.000). Supplementary Table 1 provides adjusted means for each cognitive variable.
PA Levels Do Not Differ Between Parity Groups
A main effect of parity group was not found for average PA (ie, time spent walking) over 10 years (F[3 425] = 0.472, p > .05, η p2 = 0.003), suggesting that the 4 parity groups did not differ in average PA (Supplementary Table 1).
The Association Between Average PA and Average Global Cognition on the 3MS Differs by Previous Parity
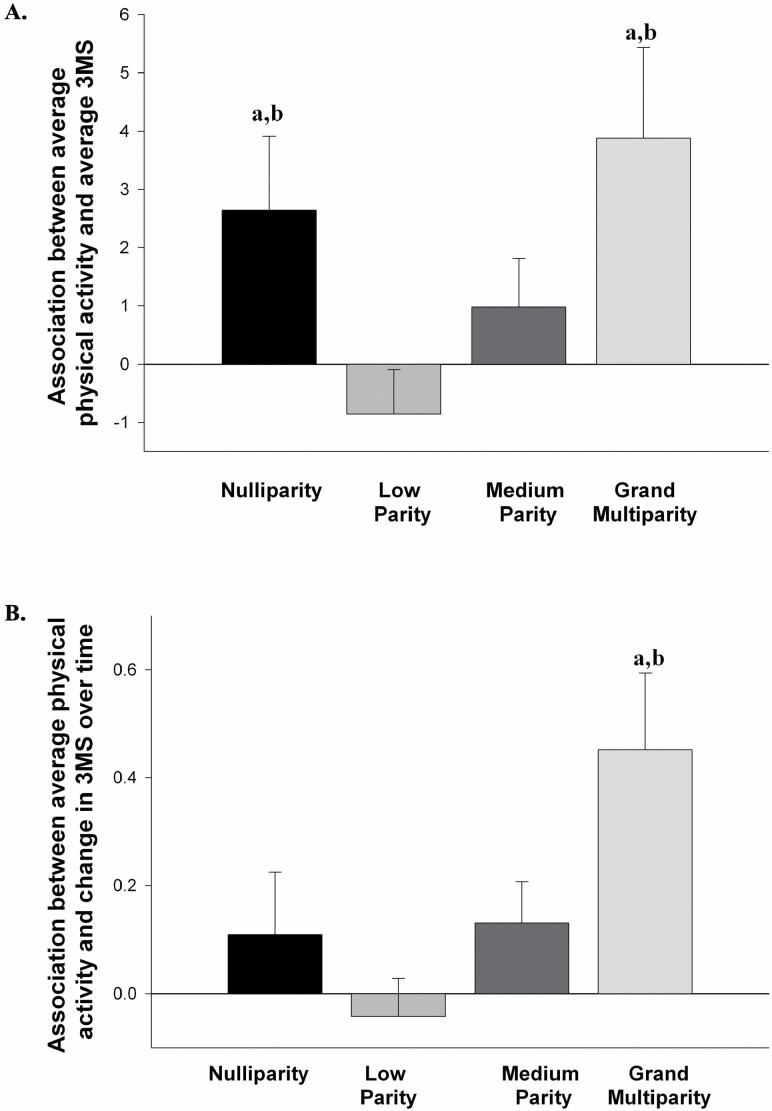
A significant interaction between the parity group and average PA was found for the average 3MS score (F[3 382) = 3.597, p = .014, η p2 = 0.027; see Figure 1A), indicating that the nature of the association between average PA and average global cognitive functioning varied by parity. The association between average PA and average 3MS was significant in both the nulliparity (B = 2.641, p = .038, η p2 = 0.011) and grand multiparity (B = 3.881, p = .013, η p2 = 0.016) groups, indicating greater average time spent walking was associated with better global cognition in these 2 groups. The association between average PA and average 3MS was not significant in the low parity group (B = −0.853, p > .05, η p2 = 0.003) or in the medium parity group (B = 0.979, p > .05, η p2 = 0.004). Compared with the low parity group, the associations between PA and average 3MS were significantly stronger in the nulliparity (t = 2.360, p = .019, η p2 = 0.014) and grand multiparity (t = 2.736, p = .007, η p2 = 0.019) groups, but not the medium parity group (t = 1.627, p = .105, η p2 = 0.007). There was also a significant main effect of average PA (F[1 382] = 8.348, p = .004, η p2 = 0.021), but not of parity group (F[3 382] = 2.413, p = .066, η p2 = 0.019).
Figure 1.
(A) The association (simple slope) between average time spent walking over 10 years and average performance on the Modified Mini-Mental State Examination (3MS) for each parity group. (B) The association (simple slope) between average time spent walking over 10 years and annual rate of change on the 3MS for each parity group. (a) Indicates the association was significant within that parity group. (b) Indicates the association was significantly different from the low parity group.
The Association Between Average PA and Average Executive Function on the DSST Differs by Previous Parity
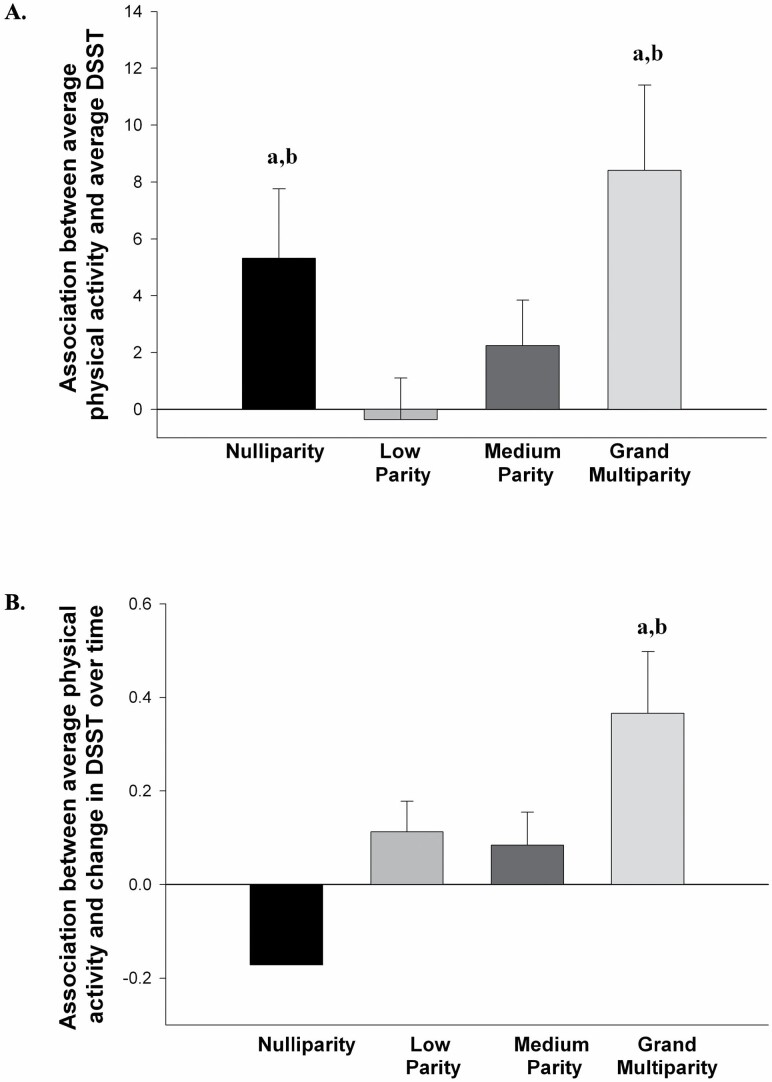
A significant interaction between the parity group and average PA was found for the average DSST score (F[3 382] = 2.969, p = .032, η p2 = 0.023; see Figure 2A), indicating that the nature of the association between average PA and average executive function varied by parity. The association between average PA and average DSST was significant in both the nulliparity (B = 5.310, p = .031, η p2 = 0.012) and grand multiparity (B = 8.407, p = .005, η p2 = 0.020) groups, indicating greater average time spent walking was associated with better executive function in these two groups. The association between average PA and average DSST was not significant in the low parity group (B = −0.357, p > .05, η p2 = 0.000) or in the medium parity group (B = 2.241, p = .163, η p2 = 0.005). Compared with the low parity group, the associations between PA and average DSST were significantly stronger in the nulliparity (t = 1.982, p = .048, η p2 = 0.010) and grand multiparity (t = 2.622, p = .009, η p2 = 0.018) groups, but not the medium parity group (t = 1.194, p = .233, η p2 = 0.004). There was also a significant main effect of average PA (F[1 382] = 12.319, p = .001, η p2 = 0.031), but not of parity group (F[3 382] = 1.182, p = .316, η p2 = 0.009).
Figure 2.
(A) The association (simple slope) between average time spent walking over 10 years and average performance on the digit symbol substitution test (DSST) for each parity group. (B) The association (simple slope) between average time spent walking over 10 years and annual rate of change on the DSST for each parity group. (a) Indicates the association was significant within that parity group. (b) Indicates the association was significantly different from the low parity group.
The Association Between Average PA and Change in Global Cognition Over 10 Years on the 3MS Differs by Previous Parity
A significant interaction between the parity group and average PA was found for change in 3MS over 10 years (F[3 382] = 3.466, p = .016, η p2 = 0.026; see Figure 1B). The association between average PA and change in 3MS was significant in the grand multiparity (B = 0.452, p = .002, η p2 = 0.026) group, indicating greater average time spent walking was associated with less decline in global cognition over time in this group only. The association between average PA and change in 3MS was not significant in the nulliparity group (B = 0.109, p = .348, η p2 = 0.002), the low parity group (B = −0.042, p > .05, η p2 = 0.001), or in the medium parity group (B = 0.131, p = .086, η p2 = 0.008). Compared with the low parity group, the association between PA and change in 3MS was significantly stronger in the grand multiparity group (t = 3.121, p = .002, η p2 = 0.025), but not the nulliparity (t = 1.120, p = .263, η p2 = 0.003) or medium (t = 1.680, p = .094, η p2 = 0.007) parity groups. There was also a significant main effect of average PA (F[1 382] = 9.504, p = .002, η p2 = 0.024), but not of parity group (F[3 382] = 1.133, p = .335, η p2 = 0.009).
The Association Between Average PA and Change in Executive Function Over 10 Years on the DSST Differs by Previous Parity
A significant interaction between the parity group and average PA was found for change in DSST over 10 years (F[3 382] = 3.439, p = .017, η p2 = 0.026; see Figure 2B). The association between average PA and change in DSST was significant in the grand multiparity (B = 0.366, p = .006, η p2 = 0.020) group, indicating greater average time spent walking was associated with less decline in executive function over time in this group only. The association between average PA and change in DSST was not significant in the nulliparity group (B = −0.171, p = .114, η p2 = 0.007), the low parity group (B = 0.113, p = .083, η p2 = 0.008), or in the medium parity group (B = 0.084, p = .236, η p2 = 0.004). Compared with the low parity group, the association between PA and change in DSST was significantly stronger in the nulliparity group (t = 2.255, p = .025, η p2 = 0.013), but not the grand multiparity (t = 1.718, p = .087, η p2 = 0.008) or medium (t = 0.301, p = .763, η p2 = 0.000) parity groups. There was also a significant main effect of average PA (F[1 382] = 3.987, p = .047, η p2 = 0.010), but not of parity group (F[3 382] = 2.513, p = .058, η p2 = 0.019).
Discussion
Our results provide preliminary evidence that parity moderates the relationship between self-reported time spent walking and cognition in older females. Specifically, we found that greater levels of average PA in the form of self-reported time spent walking were associated with better average executive function, processing speed, and global cognition in nulliparous and grand multiparous (≥5 births) older females, and with less declines over 10 years in executive function, processing speed and global cognition in grand multiparous older females. Physical activity was not associated with cognition and processing speed in low and medium parity groups. Together these findings suggest a nonlinear relationship between parity, PA, and cognitive aging.
We found a stronger relationship between PA and cognition in the parity groups most at risk of AD and cognitive impairment, the grand multiparity and nulliparous groups (10,15). Grand multiparity is associated with 1.7 fold higher risk of AD compared with having 1–4 births (10), 1.6 fold higher risk of cognitive impairment compared with 1–2 births (11), and 1.3 fold higher risk of cognitive impairment compared to 1–4 births (12). Nulliparity is associated with an increased risk of AD compared with having 1 birth. Interestingly a recent analysis of 4 European cohorts and 2 Asian cohorts found that only grand multiparity was associated with increased risk of non-Alzheimer’s dementia but not Alzheimer’s dementia (33) and an analysis of 3 Asian cohorts found that only nulliparity was associated with an increased risk of all-cause dementia (34). Grand multiparity is also associated with lower global cognition (10,11,19). Ning et al. (35) also showed that nulliparous females had slower response time and poorer visual memory compared to females with 1, 2, or 3 births but not compared to females with 4 or more births. Engaging in PA in older age is a promising lifestyle strategy for promoting healthy cognitive aging and preventing AD (1); thus, taken together, our findings suggest that promoting PA in grand multiparous and nulliparous women is of great importance.
Long-term consequences of parity also extend beyond cognition that may make nulliparous and grand multiparous females more responsive to PA. Nulliparity is associated with an increased risk of several autoimmune conditions, including scleroderma and rheumatoid arthritis (36,37) and earlier age of menopause (38) which may help explain why this group showed a greater relationship between higher PA and better cognitive profile as PA may act as an anti-inflammatory agent. Nulliparity and grand multiparity are both associated with an increased risk of cardiovascular disease, with grand multiparity associated with the greatest risk (39). Grand multiparity is further associated with several risk factors for cardiovascular diseases, including diabetes, adiposity, and hypertension (40–42) which are also risk factors that PA helps mitigate. Importantly, we did not see parity group differences in the level of PA.
We did not find a relationship between moderate levels of parity (ie, having 1–4 births) and PA levels. Moderate levels of parity have previously been shown to be beneficial for brain aging. For example, de Lange et al. (43), in their supplementary analyses, found that having 1–3 children was associated with a younger-looking brain, as defined by structural brain characteristics using machine learning to construct an estimated brain age, compared with nulliparity. Ning et al. (35) also found that 2–3 children were associated with better reaction time and fewer visual memory errors compared with nulliparity. Furthermore, moderate parity seems to be associated with the lowest risk of CVD (39). Thus it may be the case that moderate levels of parity protect the brain from the negative effects of aging, and PA does not affect cognition above and beyond this.
Greater responsivity to PA is likely related to long-term alterations in brain plasticity. The specific underlying mechanisms through which parity influences brain plasticity in later life are currently unknown. However, in parous females, this may be related to endocrinological modulation. Sex steroid hormones such as estradiol, progesterone, prolactin, oxytocin, and cortisol regulate neuroplasticity (44), and these hormones are altered during pregnancy, and the postpartum and have important implications for the brain in the long-term (7,9). Additionally, parity may exert a long-lasting effect on neuroplasticity through alterations in the immune system (45). Pregnancy is considered to be a state of low-level inflammation and is accompanied by immune adaptations such as an increase in regulatory T cells during pregnancy (46). This alteration in inflammatory processes during pregnancy has long-term effects on the immune system (47) and research suggests these immune processes affect brain health (45). In the nulliparous state, the lack of these immune adaptations due to pregnancy may lead an overly activated immune system after menopause, which has been proposed by the pregnancy-compensation hypothesis (48) and may negatively affect brain health in older age. Additionally, other factors may be involved, including differences in socioeconomic status, chronic stress, education level, and genetics. Furthermore, more targeted research is required to determine how different amounts of parity influence brain plasticity and how this in turn affects the relationship between PA and cognition in older age.
While the findings from the present study are compelling and suggest previous parity has long-term consequences for the cognitive response to PA in the form of self-reported walking, several limitations to the study design should be considered. The 2 groups that show the positive relationship between PA and cognitive performance also have the smallest sample size as would be expected. This may possibly lead to a small sample bias and could inflate the effects we are seeing. However, it is reassuring that we are seeing the same relationships across different cognitive tests in this study. While the covariates included in our analysis are quite comprehensive, it is possible we have not been able to account for all possible confounding factors between previous parity and cognition. For example, while we do have information regarding possible complications during each pregnancy derived from participant recall, a more detailed assessment of medical documentation for each pregnancy would provide greater accuracy in the role of pregnancy complications on long-term cognitive health. The PA variable was derived from self-report time spent walking using a previously validated questionnaire adapted from Taylor (24). The participants in the current study spent on average between 76 and 98 minutes walking per week. To put this in a broader context, the World Health Organization recommendation for healthy adults over 65 years of age is to engage in at least 150 minutes of moderate-intensity aerobic PA throughout the week, which includes walking. Thus, on average, the participants in the study were engaged in less walking than is currently recommended, although it is feasible participants were also engaged in other forms of aerobic PA. Therefore, a more objective measure of PA that assesses several aspects of PA would increase accuracy. It is important to note, however, that walking is the most common form of PA (49) and its use as a proxy for PA levels has been validated and successfully used in previous studies (see (1) for a review of this literature). Furthermore, previous work using the Health ABC study showed a moderate strength relationship between the self-reported time spent walking measure of PA and 3 quantitative measures of PA. Additionally, the cognitive test utilized in this study, the DSST, is a well-known measure of processing speed and in more recent years has been shown to also measure aspects of executive functions (29). However, future studies should examine executive functions using a more comprehensive battery that includes more traditional tests such as the Trail Making Test and the Stroop test (50). And finally, the effect sizes associated with the effects seen in this study are relatively small. Thus, the findings of the present study should be taken with caution and considered preliminary as we await replication in larger studies with quantitively measured PA levels.
Conclusion
The positive relationship between PA and cognitive health in older age is stronger in females than males; however, little is known as to why the female brain may respond differently to PA than the male brain. The current study provides preliminary evidence that reproductive history is involved in the greater cognitive response to PA in older females. Specifically, we saw a greater positive relationship between cognition and PA in the form of self-reported walking in older age in nulliparous females and grand multiparous females, 2 groups that are at greater risk of AD and cognitive impairment. These results were not related to differences in self-reported walking levels between parity groups. Taken together, these preliminary findings may have profound implications for the treatment of cognitive decline with aging in parous versus nonparous females. Future studies should determine whether previous parity alters the effectiveness of interventions aimed at increasing PA to ameliorate cognitive decline in older women.
Supplementary Material
Contributor Information
Cindy K Barha, Djavad Mowafaghian Centre for Brain Health, Vancouver Coastal Health Research Institute, Vancouver, Canada; Department of Physical Therapy, University of British Columbia, Vancouver, Canada.
John R Best, Djavad Mowafaghian Centre for Brain Health, Vancouver Coastal Health Research Institute, Vancouver, Canada; Department of Physical Therapy, University of British Columbia, Vancouver, Canada.
Caterina Rosano, Department of Epidemiology, University of Pittsburgh , Pittsburgh, Pennsylvania, USA.
Kristine Yaffe, Department of Epidemiology and Biostatistics, University of California , California, San Francisco, USA; Departments of Psychiatry and Neurology, University of California, , San Francisco, California, USA.
Janet M Catov, Department of Epidemiology, University of Pittsburgh , Pittsburgh, Pennsylvania, USA.
Teresa Liu-Ambrose, Djavad Mowafaghian Centre for Brain Health, Vancouver Coastal Health Research Institute, Vancouver, Canada; Department of Physical Therapy, University of British Columbia, Vancouver, Canada.
Funding
Teresa Liu-Ambrose is a Canada Research Chair (Tier 2) in Physical Activity, Mobility and Cognitive Neuroscience. Cindy Barha is an Alzheimer’s Association and Brain Canada Research Fellow. This research was supported by National Institute on Aging (NIA) Contracts N01-AG-6-2101; N01-AG-6-2103; N01-AG-6-2106; NIA grant R01-AG028050, and NINR grant R01-NR012459. This research was funded in part by the Intramural Research Program of the NIH, National Institute on Aging.
Conflict of Interest
None declared.
References
- 1. Liu-Ambrose T, Barha CK, Best JR. Physical activity for brain health in older adults. Appl Physiol Nutr Metab. 2018;43(11):1105–1112. doi: 10.1139/apnm-2018-0260 [DOI] [PubMed] [Google Scholar]
- 2. Barha CK, Liu-Ambrose T. Exercise and the aging brain: considerations for sex differences. Brain Plast. 2018;4(1):53–63. doi: 10.3233/BPL-180067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barha CK, Hsiung GR, Best JR, et al. Sex difference in aerobic exercise efficacy to improve cognition in older adults with vascular cognitive impairment: secondary analysis of a randomized controlled trial. J Alzheimers Dis. 2017;60(4):1397–1410. doi: 10.3233/JAD-170221 [DOI] [PubMed] [Google Scholar]
- 4. Barha CK, Davis JC, Falck RS, Nagamatsu LS, Liu-Ambrose T. Sex differences in exercise efficacy to improve cognition: a systematic review and meta-analysis of randomized controlled trials in older humans. Front Neuroendocrinol. 2017;46:71–85. doi: 10.1016/j.yfrne.2017.04.002 [DOI] [PubMed] [Google Scholar]
- 5. Barha CK, Best JR, Rosano C, et al. Sex-specific relationship between long-term maintenance of physical activity and cognition in the Health ABC Study: potential role of hippocampal and dorsolateral prefrontal cortex volume. J Gerontol A Biol Sci Med Sci. 2020;75(4):764–770. doi: 10.1093/gerona/glz093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14(2):125–130. doi: 10.1111/1467-9280.t01-1-01430 [DOI] [PubMed] [Google Scholar]
- 7. Duarte-Guterman P, Leuner B, Galea LAM. The long and short term effects of motherhood on the brain. Front Neuroendocrinol. 2019;53:100740. doi: 10.1016/j.yfrne.2019.02.004 [DOI] [PubMed] [Google Scholar]
- 8. Hillerer KM, Jacobs VR, Fischer T, Aigner L. The maternal brain: an organ with peripartal plasticity. Neural Plast. 2014;2014:574159. doi: 10.1155/2014/574159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Galea LA, Qui W, Duarte-Guterman P. Beyond sex differences: short and long-term implications of motherhood on women’s health. Curr Opin Physiol. 2018;6:82–88. doi: 10.1016/j.cophys.2018.06.003 [DOI] [Google Scholar]
- 10. Jang H, Bae JB, Dardiotis E, et al. Differential effects of completed and incomplete pregnancies on the risk of Alzheimer disease. Neurology. 2018;91(7):e643–e651. doi: 10.1212/WNL.0000000000006000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rasgon NL, Magnusson C, Johansson AL, Pedersen NL, Elman S, Gatz M. Endogenous and exogenous hormone exposure and risk of cognitive impairment in Swedish twins: a preliminary study. Psychoneuroendocrinology. 2005;30(6):558–567. doi: 10.1016/j.psyneuen.2005.01.004 [DOI] [PubMed] [Google Scholar]
- 12. Li FD, He F, Chen TR, et al. Reproductive history and risk of cognitive impairment in elderly women: a cross-sectional study in eastern China.. J Alzheimer’s Dis: JAD. 2016;49(1):139–147. doi: 10.3233/JAD-150444 [DOI] [PubMed] [Google Scholar]
- 13. Colucci M, Cammarata S, Assini A, et al. The number of pregnancies is a risk factor for Alzheimer’s disease. Eur J Neurol. 2006;13(12):1374–1377. doi: 10.1111/j.1468-1331.2006.01520.x [DOI] [PubMed] [Google Scholar]
- 14. Beeri MS, Rapp M, Schmeidler J, et al. Number of children is associated with neuropathology of Alzheimer’s disease in women. Neurobiol Aging. 2009;30(8):1184–1191. doi: 10.1016/j.neurobiolaging.2007.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yoo JE, Shin DW, Han K, et al. Female reproductive factors and the risk of dementia: a nationwide cohort study. Eur J Neurol. 2020;27(8):1448–1458. doi: 10.1111/ene.14315 [DOI] [PubMed] [Google Scholar]
- 16. Ptok U, Barkow K, Heun R. Fertility and number of children in patients with Alzheimer’s disease. Arch Womens Ment Health. 2002;5(2):83–86. doi: 10.1007/s00737-002-0142-6 [DOI] [PubMed] [Google Scholar]
- 17. Corbo RM, Gambina G, Ulizzi L, et al. Combined effect of apolipoprotein e genotype and past fertility on age at onset of Alzheimer’s disease in women. Dement Geriatr Cogn Disord. 2007;24(2):82–85. doi: 10.1159/000103866 [DOI] [PubMed] [Google Scholar]
- 18. Heys M, Jiang C, Cheng KK, et al. Life long endogenous estrogen exposure and later adulthood cognitive function in a population of naturally postmenopausal women from Southern China: the Guangzhou Biobank Cohort Study. Psychoneuroendocrinology. 2011;36(6):864–873. doi: 10.1016/j.psyneuen.2010.11.009 [DOI] [PubMed] [Google Scholar]
- 19. Jung JH, Lee GW, Lee JH, et al. Multiparity, brain atrophy, and cognitive decline. Front Aging Neurosci. 2020;12:159. doi: 10.3389/fnagi.2020.00159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McLay RN, Maki PM, Lyketsos CG. Nulliparity and late menopause are associated with decreased cognitive decline. J Neuropsychiatry Clin Neurosci. 2003;15(2):161–167. doi: 10.1176/jnp.15.2.161 [DOI] [PubMed] [Google Scholar]
- 21. Rooks RN, Simonsick EM, Miles T, et al. The association of race and socioeconomic status with cardiovascular disease indicators among older adults in the Health, Aging, and Body Composition study. J Gerontol B Psychol Sci Soc Sci. 2002;57(4):S247–S256. doi: 10.1093/geronb/57.4.s247 [DOI] [PubMed] [Google Scholar]
- 22. Visser M, Kritchevsky SB, Goodpaster BH, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the Health, Aging, and Body Composition study. J Am Geriatr Soc. 2002;50(5):897–904. doi: 10.1046/j.1532-5415.2002.50217.x [DOI] [PubMed] [Google Scholar]
- 23. Catov JM, Newman AB, Sutton-Tyrrell K, et al. Parity and cardiovascular disease risk among older women: how do pregnancy complications mediate the association? Ann Epidemiol. 2008;18(12):873–879. doi: 10.1016/j.annepidem.2008.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Taylor HL, Jacobs DR Jr, Schucker B, Knudsen J, Leon AS, Debacker G. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis. 1978;31(12):741–755. doi: 10.1016/0021-9681(78)90058-9 [DOI] [PubMed] [Google Scholar]
- 25. Best JR, Rosano C, Aizenstein HJ, et al. Long-term changes in time spent walking and subsequent cognitive and structural brain changes in older adults. Neurobiol Aging. 2017;57:153–161. doi: 10.1016/j.neurobiolaging.2017.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Murstein BI, Leipold WD. The role of learning and motor abilities in the Wechsler-Bellevue Digit Symbol Subtest. Educ Psychol Meas. 1961;21(1):103–112. doi: 10.1177/001316446102100109 [DOI] [Google Scholar]
- 27. Thornton KE, Carmody DP. Symbol digit and the quantitative EEG. J Neurother. 2012;16(3):210–222. doi: 10.1080/10874208.2012.705762 [DOI] [Google Scholar]
- 28. Nakahachi T, Ishii R, Iwase M, et al. Frontal activity during the digit symbol substitution test determined by multichannel near-infrared spectroscopy. Neuropsychobiology. 2008;57(4):151–158. doi: 10.1159/000147467 [DOI] [PubMed] [Google Scholar]
- 29. Jaeger J. Digit symbol substitution test: the case for sensitivity over specificity in neuropsychological testing. J Clin Psychopharmacol. 2018;38(5):513–519. doi: 10.1097/JCP.0000000000000941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Radloff L. The CES-D Scale: a self-reported depression scale for research in the general population. Appl Psychol Meas. 1977;1:385. doi: 10.1177/014662167700100306 [DOI] [Google Scholar]
- 31. Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332(9):556–561. doi: 10.1056/NEJM199503023320902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yzerbyt VY, Muller D, Judd CM. Adjusting researchers’ approach to adjustment: on the use of covariates when testing interactions. J Exp Soc Psychol. 2004;40(3):424–431. doi: 10.1016/j.jesp.2003.10.001 [DOI] [Google Scholar]
- 33. Bae JB, Lipnicki DM, Han JW, et al. Parity and the risk of incident dementia: a COSMIC study. Epidemiol Psychiatr Sci. 2020;29:e176. doi: 10.1017/S2045796020000876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bae JB, Lipnicki DM, Han JW, et al. Does parity matter in women’s risk of dementia? A COSMIC collaboration cohort study. BMC Med. 2020;18(1):210. doi: 10.1186/s12916-020-01671-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ning K, Zhao L, Franklin M, et al. Parity is associated with cognitive function and brain age in both females and males. Sci Rep. 2020;10(1):6100. doi: 10.1038/s41598-020-63014-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Spector TD, Roman E, Silman AJ. The pill, parity, and rheumatoid arthritis. Arthritis Rheum. 1990;33(6):782–789. doi: 10.1002/art.1780330604 [DOI] [PubMed] [Google Scholar]
- 37. Pisa FE, Bovenzi M, Romeo L, et al. Reproductive factors and the risk of scleroderma: an Italian case-control study. Arthritis Rheum. 2002;46(2):451–456. doi: 10.1002/art.10178 [DOI] [PubMed] [Google Scholar]
- 38. Mishra GD, Pandeya N, Dobson AJ, et al. Early menarche, nulliparity and the risk for premature and early natural menopause. Hum Reprod. 2017;32(3):679–686. doi: 10.1093/humrep/dew350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Parikh NI, Cnattingius S, Dickman PW, Mittleman MA, Ludvigsson JF, Ingelsson E. Parity and risk of later-life maternal cardiovascular disease. Am Heart J. 2010;159(2):215–221 e216. doi: 10.1016/j.ahj.2009.11.017 [DOI] [PubMed] [Google Scholar]
- 40. Klingberg S, Brekke HK, Winkvist A, Engstrom G, Hedblad B, Drake I. Parity, weight change, and maternal risk of cardiovascular events. Am J Obstet Gynecol. 2017;216(2):172 e171–172 e115. doi: 10.1016/j.ajog.2016.09.105 [DOI] [PubMed] [Google Scholar]
- 41. Nicholson WK, Asao K, Brancati F, Coresh J, Pankow JS, Powe NR. Parity and risk of type 2 diabetes: the Atherosclerosis Risk in Communities Study. Diabetes Care. 2006;29(11):2349–2354. doi: 10.2337/dc06-0825 [DOI] [PubMed] [Google Scholar]
- 42. Lawlor DA, Emberson JR, Ebrahim S, et al. Is the association between parity and coronary heart disease due to biological effects of pregnancy or adverse lifestyle risk factors associated with child-rearing? Findings from the British Women’s Heart and Health Study and the British Regional Heart Study. Circulation. 2003;107(9):1260–1264. doi: 10.1161/01.cir.0000053441.43495.1a [DOI] [PubMed] [Google Scholar]
- 43. de Lange AG, Kaufmann T, van der Meer D, et al. Population-based neuroimaging reveals traces of childbirth in the maternal brain. Proc Natl Acad Sci USA. 2019;116(44):22341–22346. doi: 10.1073/pnas.1910666116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Barha CK, Galea LA. Influence of different estrogens on neuroplasticity and cognition in the hippocampus. Biochim Biophys Acta. 2010;1800(10):1056–1067. doi: 10.1016/j.bbagen.2010.01.006 [DOI] [PubMed] [Google Scholar]
- 45. Barth C, de Lange AG. Towards an understanding of women’s brain aging: the immunology of pregnancy and menopause. Front Neuroendocrinol. 2020;58:100850. doi: 10.1016/j.yfrne.2020.100850 [DOI] [PubMed] [Google Scholar]
- 46. Wegienka G, Havstad S, Bobbitt KR, et al. Within-woman change in regulatory T cells from pregnancy to the postpartum period. J Reprod Immunol. 2011;88(1):58–65. doi: 10.1016/j.jri.2010.06.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fox M, Berzuini C, Knapp LA, Glynn LM. Women’s pregnancy life history and Alzheimer’s risk: can immunoregulation explain the link? Am J Alzheimers Dis Other Demen. 2018;33(8):516–526. doi: 10.1177/1533317518786447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Natri H, Garcia AR, Buetow KH, Trumble BC, Wilson MA. The pregnancy pickle: evolved immune compensation due to pregnancy underlies sex differences in human diseases. Trends Genet. 2019;35(7):478–488. doi: 10.1016/j.tig.2019.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Siegel PZ, Brackbill RM, Heath GW. The epidemiology of walking for exercise: implications for promoting activity among sedentary groups. Am J Public Health. 1995;85(5):706–710. doi: 10.2105/ajph.85.5.706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rosano C, Studenski SA, Aizenstein HJ, Boudreau RM, Longstreth WT Jr, Newman AB. Slower gait, slower information processing and smaller prefrontal area in older adults. Age Ageing. 2012;41(1):58–64. doi: 10.1093/ageing/afr113 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.