Abstract
The female reproductive tract (FRT), similar to other mucosal sites, harbours a site-specific microbiome, which has an essential role in maintaining health and homeostasis. In the majority of women of reproductive age, the microbiota of the lower FRT (vagina and cervix) microenvironment is dominated by Lactobacillus species, which benefit the host through symbiotic relationships. By contrast, the upper FRT (uterus, Fallopian tubes and ovaries) might be sterile in healthy individuals or contain a low-biomass microbiome with a diverse mixture of microorganisms. When dysbiosis occurs, altered immune and metabolic signalling can affect hallmarks of cancer, including chronic inflammation, epithelial barrier breach, changes in cellular proliferation and apoptosis, genome instability, angiogenesis and metabolic dysregulation. These pathophysiological changes might lead to gynaecological cancer. Emerging evidence shows that genital dysbiosis and/or specific bacteria might have an active role in the development and/or progression and metastasis of gynaecological malignancies, such as cervical, endometrial and ovarian cancers, through direct and indirect mechanisms, including modulation of oestrogen metabolism. Cancer therapies might also alter microbiota at sites throughout the body. Reciprocally, microbiota composition can influence the efficacy and toxic effects of cancer therapies, as well as quality of life following cancer treatment. Modulation of the microbiome via probiotics or microbiota transplant might prove useful in improving responsiveness to cancer treatment and quality of life. Elucidating these complex host– microbiome interactions, including the crosstalk between distal and local sites, will translate into interventions for prevention, therapeutic efficacy and toxic effects to enhance health outcomes for women with gynaecological cancers.
The human body is colonized by a diverse community of commensal, symbiotic and pathogenic microorganisms, which include bacteria, archaea, fungi, protists and viruses1. When present in a particular environment, this community is referred to as microbiota, whereas the entire habitat — including microorganisms, their genomes and the surrounding environment — is referred to as the microbiome2. Technical limitations mean that human microbiome studies have previously been restricted to bacterial communities; however, over the past 5 years, studies characterizing viruses and fungi (the virome and mycobiome) residing on or in the human body have been emerging3–7. For the purpose of this Review, we focus on the bacterial communities. Notably, the number of bacteria in and on the human body is estimated to be of the same order as that of human cells8. Furthermore, the metagenome of bacterial communities in our body comprises at least 100 times more genes than the human genome9. Thus, the human microbiota and metagenome are broadly accepted to have a critical role in the maintenance of homeostasis and/or development of certain diseases, including cancer10,11.
The majority of the bacteria in our body reside in the gastrointestinal tract, particularly the colon; however, other body sites — including the urogenital tract — also harbour unique microbiota, which are distinct from the population of the gut1. In the past, the healthy urogenital tract was thought to contain bacteria only in the distal urethra and the vagina12. However, with advances in molecular and culture techniques, many tissues that have been traditionally considered sterile (including the bladder13–17, prostate18, uterus12,19–22, Fallopian tubes and ovaries23) have been shown to harbour low-abundant microbial communities, although these are present mostly in diseased states12. Studies also suggest the interconnection of the urogenital microbiota, as the same bacterial taxa are found in different organs (for example, the bladder and vagina in women or the urethra and prostate in men) and are shared between sexual partners (such as the penile skin, urethra and semen of male partners and the vagina of female partners)14,24,25. Furthermore, biological variables, including sex and age, influence changes in the urogenital microbiota that could be related to anatomy, hormones and hygiene practices13,17. The microbiota might have a role in the pathogenesis of urinary and male reproductive cancers13,17,18; this Review focuses specifically on the microbiota of the female reproductive tract (FRT) and gynaecological cancers. We highlight interactions between the host and the bacterial communities throughout the FRT and discuss how the genital microbiota might function as an important factor in the development and progression of gynaecological malignancies. We also consider the complex crosstalk between genital microbiota and distal mucosal sites, including the gut and bladder, and investigate the possible roles of microbiota in the aetiology of gynaecological cancer, prevention, therapeutic efficacy and toxic effects.
Microbiota of the FRT
Anatomically, the FRT can be divided into the lower (vagina and cervix) and upper (uterus, Fallopian tubes and ovaries) FRT and exhibits site-specific microenvironments. The majority of bacteria in the FRT reside in the vagina; in healthy women of reproductive age, the vaginal microbiota mostly exhibits low microbial diversity (defined as species richness and evenness) and consists of one or few Lactobacillus spp.26 (Fig. 1). This characteristic is in contrast to other mucosal sites (for instance, the colon), where high microbial diversity is considered to be a sign of health27. In contrast to the lower FRT, our knowledge about the normal microbiota that reside in the uterus, Fallopian tubes or ovaries (Fig. 1) is still rudimentary and challenging to assess12, and whether bacteria in the upper FRT are residents that maintain homeostasis, tourists that are readily eliminated or invaders that contribute to disease remains uncertain12.
Fig. 1. Microbial communities associated with health and gynaecological cancers.
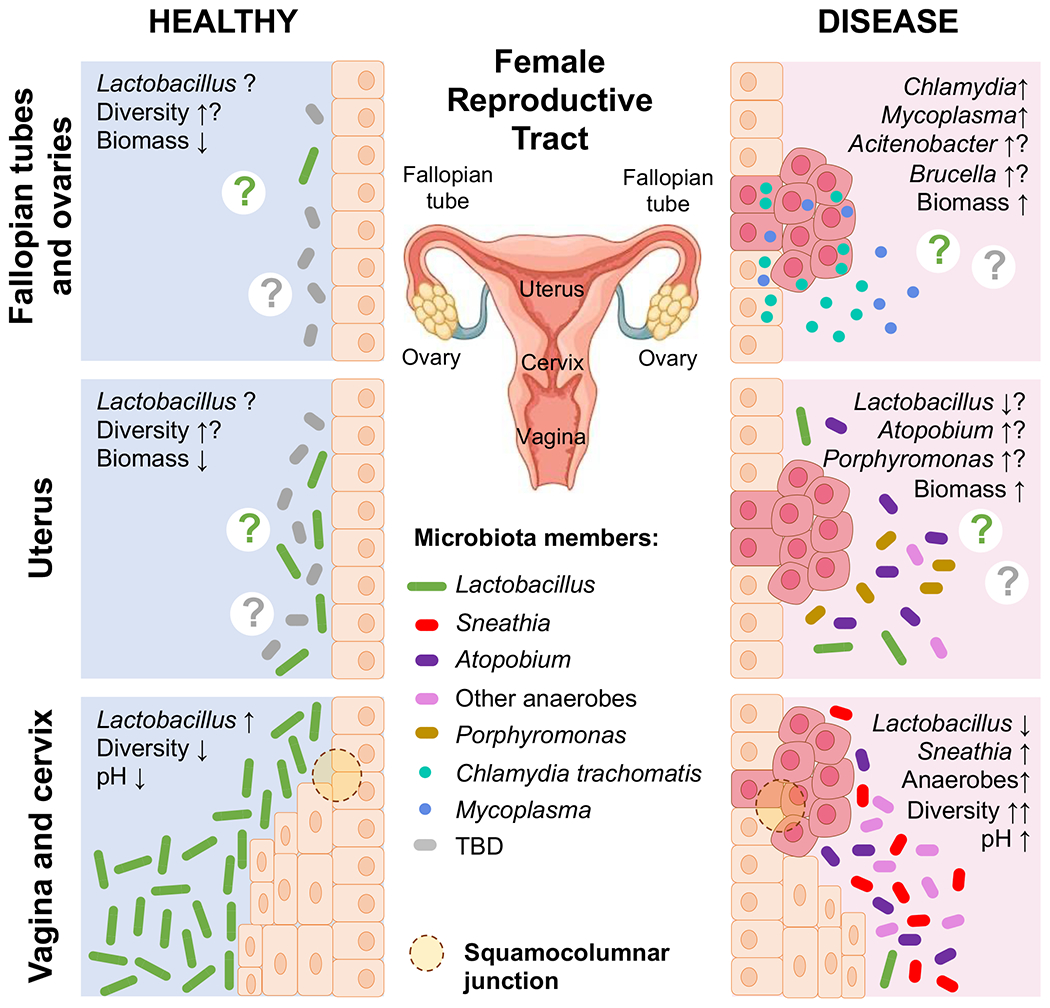
Microbiota composition and other features of the local microenvironment associated with health and gynaecological cancers across the female reproductive tract (FRT). In healthy reproductive-age women, the majority of bacteria reside in the lower FRT (vagina and cervix). The vaginal microbiota exhibits low microbial diversity and is dominated by Lactobacillus species, which acidify the local microenvironment through the production of lactic acid. By contrast, the upper FRT (endometrium, Fallopian tubes and ovaries) might contain no to low microbial biomass. The normal upper FRT microbiota is still not well characterized and is considered sterile by some groups. Available data suggest that the upper FRT microbiome exhibits higher microbial diversity than the lower FRT microbiome, especially in diseased states. The presence of Lactobacillus species and other microorganisms has been reported in the endometrium, Fallopian tubes and ovaries. In women with gynaecological malignancies (cervical, endometrial and ovarian cancer), the local microbiota composition and biomass loads change considerably. In women with cervical cancer, the vaginal microbiome exhibits high microbial diversity and is characterized by an overgrowth of diverse anaerobic bacteria, particularly Sneathia. Lactobacillus depletion results in elevated vaginal pH. In women with endometrial cancer, the simultaneous presence of Atopobium and Porphyromonas has been associated with disease. In women with ovarian cancer, potential intracellular pathogens, such as Brucella, Mycoplasma and Chlamydia, and pathobionts, such as Acinetobacter, were reported. Overall, these dysbiotic microbiomes might contribute to the aetiology, disease severity and/or treatment of gynaecological cancers. TBD, to be determined.
Microbiota of the lower FRT
In the lower FRT, Lactobacillus dominance is associated with vaginal health and depletion of these microorganisms can lead to numerous adverse conditions, such as increased risk of acquiring sexually transmitted infections (STIs), preterm birth, spontaneous miscarriage or pelvic inflammatory disease28. Interestingly, only one or few Lactobacillus species seem to be predominant in the vagina, such as Lactobacillus crispatus, Lactobacillus gasseri, Lactobacillus iners, Lactobacillus jensenii or Lactobacillus vaginalis (found mostly in women from sub-Saharan Africa), as demonstrated using both culture-dependent and molecular techniques26,28–33. These vaginal Lactobacillus species seem to be adapted for optimal colonization of the vaginal niche, as other Lactobacillus species, such as Lactobacillus acidophilus, do not colonize and dominate vaginal tissue33,34. Genetic and molecular mechanisms explaining this phenomenon still remain to be explored. Strikingly, Lactobacillus dominance seems to be unique to humans; distinct reproductive physiology, high risk of STI or a shift to diets rich in starch in agrarian societies (as opposed to hunter-gatherers) have been hypothesized to explain the evolutionary origin of the human vaginal microbiome35. Predominant vaginal Lactobacillus spp. exhibit a mutually beneficial relationship with their host, protecting the inhabited microenvironment against invading pathogens through various mechanisms, including production of anti-microbial products and by blocking adhesion of pathogens26,28,29,36. Production of lactic acid, which acidifies the vaginal microenvironment to pH <4.5, is a hallmark protective mechanism of vaginal Lactobacillus spp. in the lower FRT28,37. Numerous in vitro studies have demonstrated that protonated lactic acid kills or inactivates a broad range of sexually transmitted pathogens, including Neisseria gonorrhoeae38, Chlamydia trachomatis39, herpes simplex virus40 and human immunodeficiency virus41, as well as urinary tract pathogens, such as uropathogenic Escherichia coli42. The beneficial effect of Lactobacillus spp. has been also attributed to hydrogen peroxide; however, production of this antimicrobial compound seems unlikely in vivo owing to hypoxic conditions in the local microenvironment43,44. Furthermore, Lactobacillus spp. are able to block adhesion of invading pathogens to the vaginal epithelium through competitive exclusion and inhibit the growth of pathogens through the production of bacteriocins37,45. In return, Lactobacillus spp. thrive in the vaginal niche owing to the availability of glycogen by-products, which are used by these microorganisms as an energy source46.
Intriguingly, in some women, the vaginal microbiome seems to be devoid of a high proportion of Lactobacillus spp. and to consist of a diverse polymicrobial mixture of obligate and strict anaerobes (including Gardnerella vaginalis, Prevotella spp., Atopobium vaginae, Sneathia spp., Megasphaera spp. and others)26,47,48, many of which are commonly associated with bacterial vaginosis (BV). BV is the most common vaginal disorder among women of reproductive age, present in ~23–29% depending on the region49. However, the vaginal microbiome can also be dominated by G. vaginalis and exhibits a low diversity50. Whether the non-Lactobacillus-dominant vaginal microbiome exists as another vaginal microbial community type or actually reflects asymptomatic BV is still debatable30. Notably, these non-Lactobacillus-dominant microbial communities have been reported to be more prevalent in Hispanic and black women (30–40%) than in white and Asian women (10–20%)26,48,50,51. Furthermore, the data from the USA, Canada, Europe and Asia consistently demonstrate that groups representing minority populations within a country studied had higher rates of BV and lower prevalence of Lactobacillus dominance after adjustment for sexual practices and other confounders48,52–55. These racial and ethnic differences could relate to socioeconomic, behavioural and environmental factors, which can shape the vaginal microbiome, and require further investigation (Fig. 2). Finally, in some women, vaginal microbiome communities are characterized by the overgrowth of human pathobionts, such as Streptococcus spp., Staphylococcus spp. or Enterobacteriaceae50.
Fig. 2. Behavioural, socioeconomic, genetic and environmental factors contributing to genital dysbiosis and cancer.
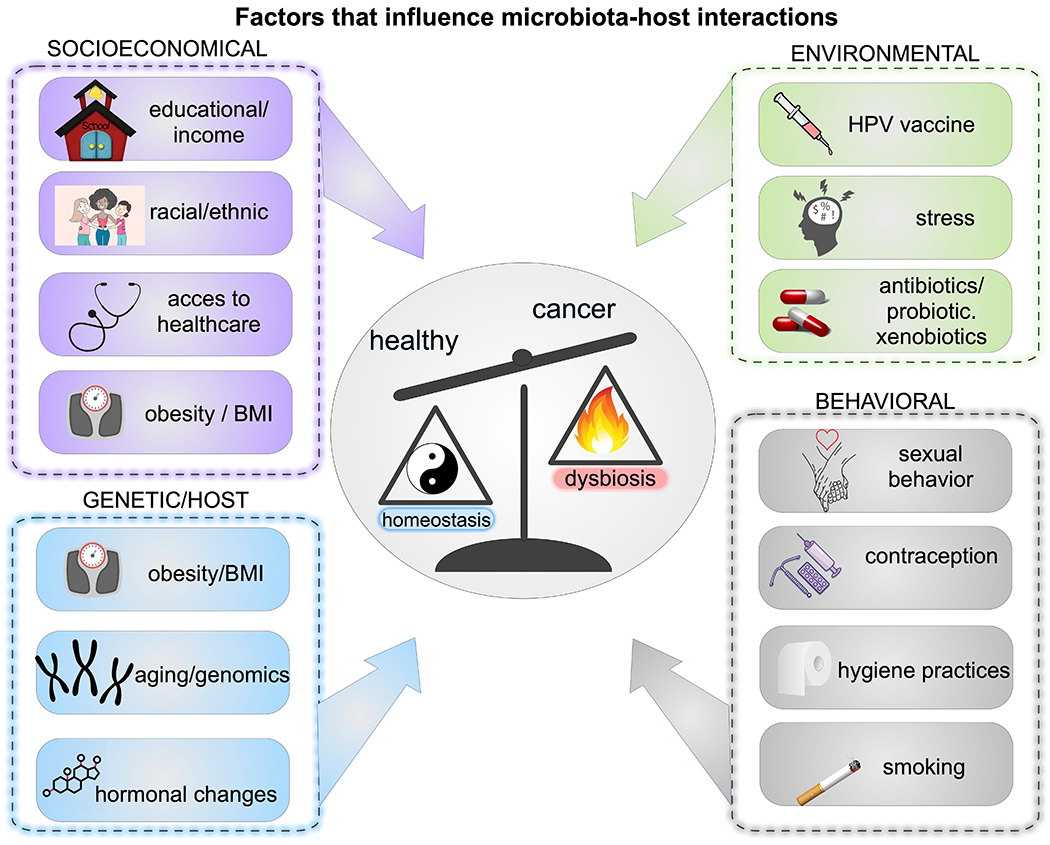
Complex interactions between the microbiome and host that increase the risk of gynaecological cancer can be influenced by behavioural factors (sexual orientation, sexual activity, number of sexual partners, use of lubricants and sex toys, contraception, feminine hygiene practices, smoking and vaping, alcohol consumption, diet and nutrition, obesity and physical activity), socioeconomic factors (education, income, structured racism or segregation, social policy and access to health care), genetic and host factors (age, genome and epigenome, hormonal status, pregnancy, altered immunity or other comorbidities (cardiometabolic, neuroendocrine and immuno-inflammatory)) and environmental factors (sexually transmitted infection (STI) status (including bacterial, viral, fungal and parasitic infections), human papilloma virus (HPV) vaccination, stress, antibiotics, probiotics, xenobiotics, toxins, carcinogens, geography and early life factors such as gestation, birth route and infancy).
Lactobacillus depletion and dominance of diverse anaerobes, which is characteristic of dysbiosis, has been associated with numerous gynaecological and obstetric sequelae, including increased risk of acquiring STIs, preterm birth, spontaneous miscarriages, pelvic inflammatory disease, endometritis and gynaecological cancer28 (Fig. 1). However, not all Lactobacillus-dominant communities benefit the host in the same manner. For example, one of the vaginal Lactobacillus species, L. iners, commonly occurs as a component of the diverse non-Lactobacillus-dominant communities56. Furthermore, L. iners-dominant vaginal microbiomes also frequently transition to the non-Lactobacillus-dominant communities, whereas L. crispatus-dominant microbiomes rarely undergo this transition56. Additionally, L. iners produces only the L-isoform of lactic acid, which is produced by vaginal epithelial cells, as well as by some BV-associated microorganisms57, whereas the other vaginal Lactobacillus spp. produce the D-isoform of lactic acid (L. jensenii) or both the D-isoform and L-isoform (L. crispatus and L. gasseri), which was confirmed in vitro57. Notably, the higher ratios of L-lactic acid to D-lactic acid in L. iners-dominant or diverse non-Lactobacillus-dominant communities have been shown to correlate with elevated levels of extracellular matrix metalloproteinase inducer and, consequently, matrix metalloproteinase 8 (MMP8) in vaginal secretions, which might alter the epithelial barrier integrity in these women57. Overall, these data suggest that dominance of the vaginal microbiome with L. crispatus might be optimal for vaginal health, whereas dominance with L. iners might be less beneficial.
Multiple factors have been shown to affect the vaginal microbiome. These can be behavioural (sexual orientation, sexual activity, number of sexual partners, use of lubricants and sex toys, contraception, feminine hygiene practices, smoking and vaping, alcohol consumption, diet and/or nutrition, obesity and physical activity), socioeconomic (education, income, structured racism and/or segregation, social policy and access to health care), genetic or host-related (age, genome and epigenome, hormonal status, pregnancy and altered immunity), other comorbidities (cardiometabolic, neuroendocrine and immuno-inflammatory) and environmental (STI status, human papilloma virus (HPV) vaccination, stress, antibiotics, probiotics, xenobiotics, toxins, carcinogens, geography and early life factors such as gestation, birth route and infancy)28,36,37,55,58 (Fig. 2). For example, cigarette smoking has been strongly associated with an increased prevalence of BV and vaginal microbiome alterations48,55,59,60. Women who douche are also at a higher risk of developing BV61,62. In addition, a diet rich in fat, with a high glycaemic load and nutritional density, and obesity have been linked to BV55,63,64. Additionally, in a human 3D in vitro model of vaginal epithelium, lubricants — particularly products with high osmolality — have been shown to alter the vaginal epithelial barrier65 and might also affect the vaginal microbiota.
Oestrogen levels, in particular, have a profound effect on the composition of the vaginal microbiome. For instance, before puberty or in postmenopausal women, when circulating oestrogen levels are low, the vaginal microbiome is devoid of Lactobacillus spp. and consists of a diverse mixture of anaerobic bacteria29,66. By contrast, the vaginal microbiomes of pregnant women, which are subject to elevated levels of oestrogen, are more stable and typically dominated by L. crispatus or L. iners67. This phenomenon is thought to occur via the oestrogen-mediated production and secretion of glycogen by the vaginal epithelium; the high levels of free glycogen promote the growth of Lactobacillus spp., which are able to use glycogen breakdown products as an energy source through fermentation46. As a consequence of this metabolic process, Lactobacillus spp. produce lactic acid, which protects the vaginal microenvironment. The changes in the oestrogen levels during the menstrual cycle might also affect vaginal microbiome structure, particularly Lactobacillus dominance. Indeed, longitudinal studies revealed that the vaginal microbiome is a dynamic ecosystem, which can fluctuate over short periods of time in some women or be relatively stable in others31,56,67. Two key longitudinal studies showed that the changes in the vaginal microbiome composition are affected by the phase of the menstrual cycle, by the type of vaginal microbiome community and, to a certain extent, by sexual activity31,56. Another longitudinal study also demonstrated that the stability of the vaginal microbiome was significantly higher in pregnant women than that of non-pregnant women (P < 0.001)67.
Microbiota of the upper FRT
The available data suggest that the microbiota of the upper FRT differ substantially from that of the vagina in both quantity and composition (Fig. 1). First, the upper FRT must be considered as a low-abundance site. For instance, the estimated number of bacteria in the uterus is ~10,000-fold lower than the bacterial load in the vagina23. Owing to this low bacterial biomass in the upper FRT microenvironment and the proximity to a microbial rich site, such as the vagina, sample contamination during collection and processing is a major hindrance in acquiring physiologically relevant data and, consequently, can lead to false biological conclusions12,68,69. In the majority of studies, samples for analysis of the uterine microbiome have been collected transcervically, which introduces a risk of cross-contamination with bacteria from the lower FRT12. An alternative would be to collect uterine samples directly from the bivalved uterus following hysterectomy, which would prevent contamination of specimens with bacteria from the cervix12. Other precautions, such as vaginal disinfection with povidone iodine, could further reduce cross-contamination68. Thus, careful sampling and rigorous controls during sample collection and processing are necessary in future studies to help identify possible contaminants12,68,69. Second, available data suggest that the upper FRT microbiota exhibit higher bacterial diversity than the vaginal microbiome23 (Fig. 1). However, the microbiota compositions identified vary greatly across studies, so whether these bacterial species are genuine members of the upper FRT microbiome or transient colonizers is unclear. Multiple studies have reported that Lactobacillus is found in the upper FRT20–22,70,71. Interestingly, the relative abundance of Lactobacillus gradually decreases throughout the upper FRT, with the highest levels in the vagina and cervix (100% and 97.6%, respectively), with notable, but no longer dominant, levels in the endometrium (30.6%) and with the lowest abundance in the Fallopian tubes (1.7%)23 (Fig. 1).
An important limitation of upper FRT microbiome studies is the fact that the samples have not been from healthy individuals. In most studies, participants underwent hysterectomy for benign conditions, such as fibroids, uterine bleeding or chronic pelvic pain. These conditions are likely to affect the physical and biological barriers in the cervix, consequently allowing bacteria residing in the lower FRT (such as Lactobacillus) to ascend and misleadingly be considered a normal component of the upper FRT microbiome. As the collection of endometrial biopsies from healthy women is outside clinical practice guidelines, women undergoing in vitro fertilization procedures could be a more suitable population for future studies aiming to define the microbiota in the upper FRT under non-disease conditions12. However, the presence of infertility in these women might itself affect the FRT microbiome72; thus, the restriction of inclusion criteria to couples presenting with male factor infertility might provide a better control group for these studies12. However, microbial communities present in semen could potentially affect the composition of the endometrial microbiomes25.
Genital and other microbiome interactions
Within individuals, the genital microbiota can interact with other body sites, both proximal (such as the urinary tract) and distal (such as the gut or oral cavity) (Fig. 3). Numerous studies have also shown that partners share and/or exchange members of the genital microbiota through sexual activity73–83. Thus, multiple sites can serve as potential reservoirs of genital microorganisms. Next-generation sequencing studies also demonstrated that common vaginal bacteria (such as Lactobacillus, Sneathia, Prevotella, Gardnerella, Atopobium, Peptoniphilus, Gemella and Finegoldia) are components of the urinary tract microbiota in both women and men14–17,84,85, suggesting the interconnectivity of microbiota (bladder–vagina axis) within the urogenital tract. Furthermore, the predominant vaginal Lactobacillus spp. and other common vaginal bacteria, such as G. vaginalis, are commonly found in catheterized urine samples and can be cultured using an expanded quantitative urine culture (EQUC) protocol85. In a study using EQUC and whole-genome sequencing techniques, phylogenetically similar strains of L. crispatus, L. iners, E. coli and Streptococcus anginosus were found to be present in the bladder and vagina of the same individual14. Furthermore, the same study showed that vaginal and bladder microbiota exhibit similar functional capacities, which are distinct from those of the gut microbiota14. These findings strongly suggest microbial translocation within the urogenital tract, which is not limited to uropathogens but also includes health-associated bacteria. Lactobacillus spp. found in both the bladder and the vagina have been hypothesized to protect the urinary tract against invading uropathogens14. However, mechanisms related to the protective effect of urinary tract microbiota need to be determined in future studies86. Multiple studies showed that vaginal microbiome members (including Lactobacillus spp. and BV-associated bacteria) can be detected in male penile skin, urethral, urine and semen specimens25,73–77, which suggests that sexual partners share and exchange microorganisms inhabiting their urogenital tracts. Furthermore, a study that examined vaginal, penile and male urethral microbiota among monogamous heterosexual couples with or without BV revealed high similarity between the penile skin and urethral microbiota of the male partner and the vaginal microbiota of the female partner with BV, which supports sexual transmission of BV24. Furthermore, previous culture-based studies showed that the BV-associated organism G. vaginalis can be cultivated from male urethra or penile skin78,79. By contrast, in BV-negative couples, neither the urethral nor penile skin microbiota was similar to the vaginal microbiota24, suggesting that the penile epithelial microenvironment might favour BV-associated bacteria colonization. In addition, multiple reports in women who have sex with women support the sexual transmission of BV-associated microorganisms80–83. Overall, this indicates that some vaginal bacteria can be shared between sexual partners and affect the species composition of each other’s urogenital microbiota. In addition, other sexual activities, such as oral and anal sex, can affect the microbial continuum across distal mucosal sites, such as the gut and the oral cavity55,87. However, more studies, in particular using microbial culturomics and metagenomics approaches, are needed to assess relationships and dynamics among the microbiomes of urogenital and other extravaginal mucosal sites of sexual partners. In addition, the most prevalent vaginal Lactobacillus species (L. crispatus, L. iners, L. jensenii and L. gasseri) have also been shown to colonize the rectum; co-colonization of the vagina and rectum correlated with the lowest prevalence of BV88,89. These studies support the concept that the rectum is a key reservoir for vaginal lactobacilli and that rectal colonization with these microorganisms contributes to maintenance of health-associated vaginal microbiota. Finally, haematogenous spread of bacteria emanating from the distal mucosal sites, such as the gut or oral cavity, should be considered as a potential route of interplay between the genital microbiota, particularly that of the upper FRT, and distal mucosal sites12.
Fig. 3. Female microbiome axes.
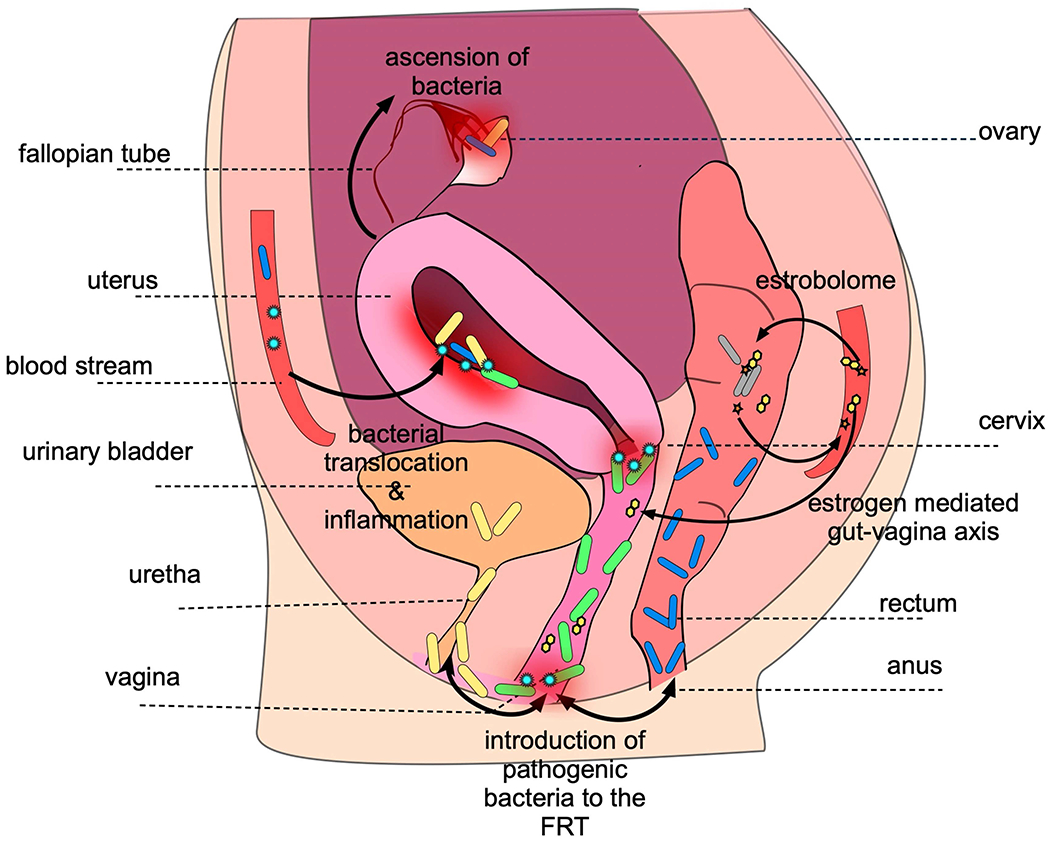
The female reproductive tract (FRT) microbiota interacts with the gut (vagina–gut axis) and the urinary tract microbiota (vagina–bladder axis) and possibly other distal mucosal sites (for example, the oral cavity) through direct or oestrogen-mediated mechanisms. The bacteria residing in the lower FRT, including Lactobacillus species and dysbiotic anaerobes, can ascend to the upper FRT. Common vaginal bacteria, such as Lactobacillus species, are components of the urinary tract microbiota. Vaginal Lactobacillus species can also colonize the rectum. Furthermore, gut microbiota can indirectly influence genital microbiota through the oestrobolome. Finally, haematogenous spread of bacteria (for example, from the oral cavity) might be a putative seeding route for the upper FRT microbiome. Potential extravaginal reservoirs of genital microorganisms are depicted with arrows.
Intriguingly, the gut microbiota can also indirectly influence the genital microbiota composition through oestrogen-mediated mechanisms (Fig. 3). In the lower FRT, oestrogen has a crucial role in homeostasis by facilitating growth of Lactobacillus spp. through induction of glycogen production29. However, studies have shown that circulating oestrogen levels in the human body are influenced by gut microbiota90,91, leading to the concept of an oestrogen-mediated gut–vagina axis92. The interplay between these two distal mucosal sites involves enteric bacteria that are able to metabolize oestrogens; the collection of these microorganisms and their genes is termed the ‘oestrobolome’90. These microorganisms secrete β-glucuronidase and β-glucosidase, which deconjugate hepatically conjugated oestrogens and promote their reabsorption to circulation91. After deconjugation, free oestrogen is transported to distal sites (including the FRT), where it binds to its receptors and triggers intracellular signalling, resulting in increased glycogen production and other physiological changes such as mucus production and thickening of the epithelium. Thus, a reduction in the gut microbiota diversity (resulting in a lack of oestrogen-metabolizing bacteria) could influence the vaginal microbiome composition (particularly Lactobacillus dominance) via the oestrobolome.
Microbial dysbiosis and carcinogenesis
Microorganisms, including viruses and bacteria, have been suspected to have a role in carcinogenesis for a long time. However, pinpointing specific bacterial species that can cause cancer has been challenging. The International Agency for Research on Cancer has classified only one bacterium, Helicobacter pylori, as a human carcinogen93 — this common bacterium colonizes the human gastric mucosa and induces chronic inflammation and, consequently, gastric ulcers, which can progress to stomach cancer94. Additional malignancies presumed to be caused by single bacterial species include gallbladder cancer (associated with chronic Salmonella enterica serovar Typhi or Paratyphi infections)95, immunoproliferative small intestine disease (associated with Campylobacter jejuni infections)96 or certain lymphomas (associated with Borrelia burgdorferi or Chlamydia psittaci infections)97,98. Several viruses, including Epstein–Barr virus, HPV and hepatitis C virus, are also known causative agents of cancer99. These oncogenic bacteria and viruses exhibit the capacity to directly modulate carcinogenesis through specific toxins that can damage host DNA or the integration of oncogenes into host genomes, respectively100. However, clinical studies, as well as those performed in germ-free, gnotobiotic and antibiotic-treated mice, suggest that microbially driven carcinogenesis is frequently related to global changes in the microbiome, rather than attributable to single pathogens101. In contrast to gastric cancer, which is caused by infection with a specific pathogen, other malignancies, such as colorectal and liver cancer, seem to be caused by dysbiosis. In this case, carcinogenesis seems to be driven indirectly by the altered host defence responses to dysbiotic microbiota, pathobionts and/or pathogens100. Studies in mouse models of colorectal and liver cancer have demonstrated that antibiotic treatment and germ-free status lead to a substantial reduction in the number of tumours101. Additional studies also revealed that the transmission of dysbiotic gut microbiota triggered colorectal cancer development102. For example, a 2019 study demonstrated that transplanting faecal material from patients with colorectal cancer into germ-free mice caused lesions and epigenetic changes characteristic of the development of malignancy103. These reports provide strong evidence of the tumour-promoting effects of dysbiotic gut microbiota in several malignancies, particularly colorectal and liver cancers. Similar to the gut, microbial dysbiosis might also promote tumorigenesis in other organs inhabited by microorganisms, such as the skin, oral cavity, lungs and the genital tract104.
Considerable efforts have been made to understand the pathophysiological mechanisms underlying microbially driven carcinogenesis. Specific bacteria or dysbiotic bacterial communities are well documented to cause epithelial barrier failure, immune dysregulation and/or genotoxicity and, consequently, create a tumour-permissive microenvironment100,101,105 (Fig. 4). In addition, chronic inflammation is a well-characterized mechanism modulating the hallmarks of cancer106 and cancer-associated bacteria (for example, Fusobacterium nucleatum in colorectal cancer) have been shown to activate nuclear factor-κB (NF-κB), a key regulator of cancer-associated inflammation, through engagement of Toll-like receptors and nucleotide-binding oligomerization domain-like receptors100. Furthermore, the carcinogenic potential of intestinal bacteria leads to increased production of IL-6 and tumour necrosis factor (TNF), activation of signal transducer and activator of transcription 3 (STAT3) and activation of IL-17–IL-23 pathways100,107. Collectively, these microbiota-induced innate and adaptive host immune responses can contribute to tumour development and progression by triggering cancer-promoting inflammation and promoting resistance to cell death100–102,105,106. Moreover, several bacteria, such as H. pylori (associated with gastric cancer), F. nucleatum and enterotoxigenic Bacteroides fragilis (both associated with colorectal cancer) produce proteins that can directly influence host Wnt–β-catenin signalling108,109, which regulates cell proliferation, survival and migration, and angiogenesis, all of which are hallmarks of cancer100,101 (Fig. 4). Interestingly, some bacteria, such as the sexually transmitted pathogen C. trachomatis, are able to induce epithelial-to-mesenchymal transition of infected cells, which might promote tumorigenesis through the loss of epithelial cell adhesion and downregulation of DNA damage responses110. Finally, bacteria can also promote carcinogenesis via a direct effect on cell transformation through production of DNA-damaging toxins. For example, colibactin (produced by E. coli) and cytolethal distending toxin (produced by Gram-negative bacteria such as E. coli, C. jejuni, Helicobacter spp. and S. Typhi) exert direct effects on DNA damage and genome instability, which are linked to the development of colorectal, gastric and gallbladder cancers100,101. In addition, toxins produced by other bacteria, such as B. fragilis toxin can indirectly damage DNA through induction of high levels of reactive oxygen species101. Members of the urogenital microbiota, particularly species associated with genital dysbiosis, are likely to use similar biological mechanisms, which could contribute to the development and progression of gynaecological cancers. Thus, mechanistic studies are urgently needed to investigate the functional effects of specific genital bacteria or their products on the hallmarks of cancer in the genital tract.
Fig. 4. Effect of microbiota on mucosal homeostasis and hallmarks of cancer.
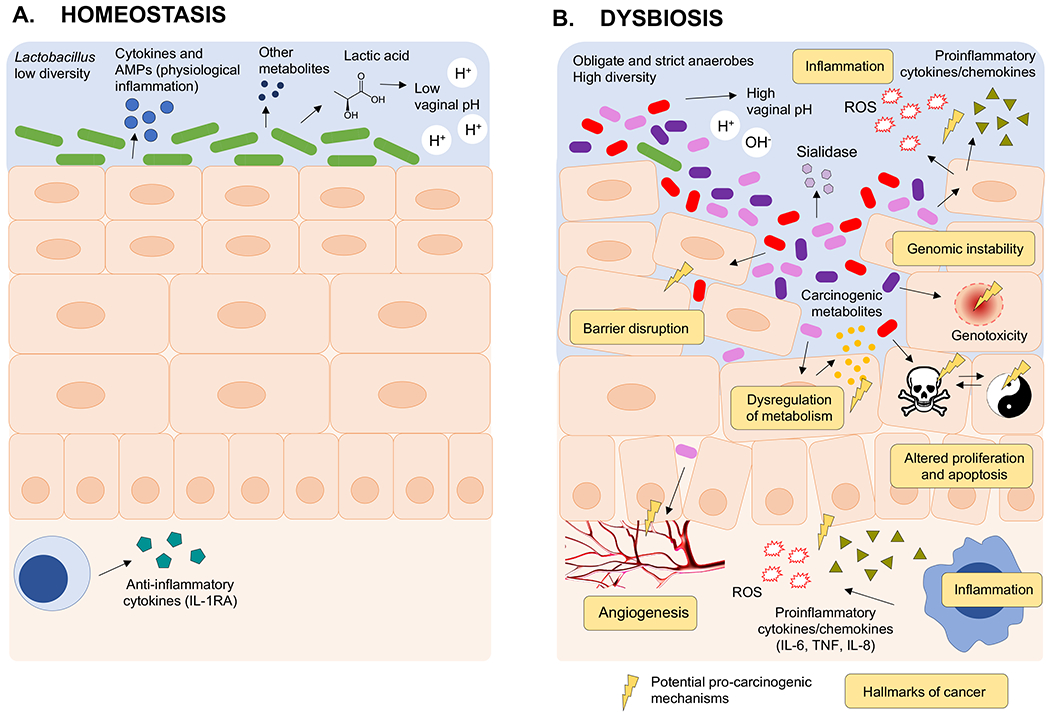
a) Health-associated Lactobacillus spp. have a number of roles in homeostasis of the cervicovaginal microenvironment, including anti-inflammatory properties and improving barrier function. Lactobacillus species produce lactic acid, which acidifies the local microenvironment to pH <4.5 and protects the host from invading pathogens with a physiological level of inflammation. In addition, metabolites produced by Lactobacillus species can stimulate the host to produce antimicrobial peptides and anti-inflammatory cytokines. b) Dysbiotic genital bacteria might affect hallmarks of cancer, including chronic inflammation, barrier disruption, genomic instability, altered proliferation and/or apoptosis and angiogenesis. When dysbiosis occurs, Lactobacillus species are replaced with a diverse mixture of anaerobic bacteria (such as Anaerococcus, Atopobium, Dialister, Fusobacterium, Gardnerella, Gemella, Prevotella, Megasphaera, Parvimonas, Peptoniphilus, Peptostreptococcus, Porphyromonas, Shuttleworthia and Sneathia). These microorganisms induce the production of proinflammatory immune mediators and reactive oxygen species (ROS). Oxidative damage by ROS can exhibit genotoxic effects on epithelial cells or alter the proliferation of epithelial cells, which can consequently lead to cell apoptosis. Putative microbial products or metabolites might also directly affect cell proliferation and cause barrier disruption. Bacterial enzymes (for example, sialidase) can degrade the protective mucous layer. Finally, the vaginal bacteria might affect angiogenesis, for example, via stimulation of the Janus kinase (JAK)– signal transducer and activator of transcription (STAT) pathway and the production of angiogenic factors such as vascular endothelial growth factor. TNF, tumour necrosis factor. AMPs, antimicrobial peptides.
The microbiome and gynaecological cancers
A growing body of literature supports the concept that bacterial communities within the FRT might contribute to the aetiology, disease severity and/or treatment of gynaecological malignancies111.
HPV, microbiota and cervical cancer
Cervical cancer is the most common HPV-related malignancy and the fourth most common cancer in women worldwide, with an estimated 570,000 new cases and 311,000 deaths in 2018112. Notably, the incidence of cervical cancer differs among racial and ethnic groups113,114. In the USA, Hispanic women are 60% more likely to be diagnosed with cervical cancer and 30% more likely to die from cervical cancer than non-Hispanic white women113. High-risk HPV genotypes, such as HPV16 or HPV18, are well-established oncogenic factors in cervical carcinogenesis. The squamocolumnar junction or transformation zone of the cervix is particularly susceptible to HPV infection and is the site at which cervical cancers arise115,116. In fact, 99.7% of cervical cancer biopsy samples contain the virus (confirmed by PCR)117. However, 85–90% of high-risk HPV infections are spontaneously cleared and only 10–15% persist, consequently leading to the development of precancerous cervical intraepithelial neoplasia (CIN) and subsequent progression to invasive cervical carcinoma (ICC)118. This discrepancy suggests that other factors in the local cervicovaginal microenvironment might promote carcinogenesis in conjunction with HPV. Several factors, including age of sexual debut, multiparity, contraceptives and hormone treatments, other STIs (chlamydia, gonorrhoea, syphilis and herpes) and smoking, have been shown to increase the risk of progression of cervical neoplasia among HPV-infected women119–124. Over the past decade, emerging evidence suggests that the vaginal microbiome also has a role in cervical carcinogenesis51,125–127. Epidemiological studies have revealed associations between the diverse non-Lactobacillus-dominant vaginal microbiome and HPV infection and persistence51,125,126,128–136. Additionally, BV, which is characterized by depletion of Lactobacillus spp. and overgrowth of diverse obligate and strict anaerobic bacteria, has been associated with an increased risk of HPV acquisition and decreased clearance of HPV128–130. Initial cross-sectional studies involving a Korean twin cohort (n = 68 selected from 912 women participating in the Healthy Twin Study, part of the Korean Genome Epidemiology Study, which excluded postmenopausal women from the analysis owing to the effect of menopause on vaginal microbiome composition) or a Chinese cohort (n = 70) revealed that women infected with HPV without cervical dysplasia have a more diverse vaginal microbiome and had substantially higher abundance of L. gasseri and several BV-associated bacteria, such as Gardnerella, Sneathia, Megasphaera or Dialister, than HPV-negative women66,126,131,132. Furthermore, two small longitudinal 16S rRNA sequencing studies (n = 32 and n = 72) have identified that the abundance of vaginal species L. gasseri and Atopobium spp. is associated with HPV clearance and HPV persistence, respectively133,134. Only three microbiome studies have included women with cervical dysplasia and cancer and they are cross-sectional studies with modest sample sizes51,125,126. However, all of these reports consistently show depletion of Lactobacillus spp. and a substantial increase in vaginal microbiome diversity in women with CIN and ICC compared with healthy individuals (Fig. 1). These studies were also aimed at identifying particular bacterial taxa associated with cervical disease. The first (n = 169) identified that three BV-associated microorganisms — Sneathia sanguinegens (P < 0.01), Anaerococcus tetradius (P < 0.05) and Peptostreptococcus anaerobius (P < 0.05) — were considerably more abundant in the vaginal microbiome of patients with high-grade dysplasia than in that of patients with low-grade dysplasia125. A separate study (n = 32) also identified that Sneathia and Fusobacterium spp. were only present in women with cervical dysplasia or cancer, but not in women without neoplasia126. Furthermore, in a study of 100 women, decreased Lactobacillus dominance and increased rates of diverse vaginal microbiome were observed in patients with precancerous lesions and cervical cancer (P < 0.05)51. Notably, a significant increase in vaginal pH (P = 0.01) was also observed, which was related to the severity of cervical neoplasia and strongly correlated with the depletion of Lactobacillus spp.51. With regard to specific taxa, the majority of bacteria that were enriched in HPV-infected women or women with dysplasia or cancer are not only limited to microorganisms associated with BV (such as Gardnerella, Atopobium, Prevotella, Megasphaera, Parvimonas, Peptostreptococcus, Anaerococcus, Sneathia, Shuttleworthia and Gemella) but also include those that cause other forms of dysbiosis (including Streptococcus agalactiae and Clostridium). Intriguingly, Sneathia, a member of the phylum Fusobacteria, was the only microorganism that was enriched in women throughout the continuum of cervical carcinogenesis51. As these data are in accordance with previous reports that identified this microorganism to be associated with cervical dysplasia, the presence of Sneathia in the vaginal microbiome might be a metagenomic marker for HPV persistence and progression of cervical neoplasm. Interestingly, Sneathia was enriched in women of Hispanic ethnicity in this study51. Taken together with the observation that Hispanic women exhibit decreased Lactobacillus dominance and elevated vaginal pH51, these observations might begin to explain increased rates of cervical cancer incidence and cancer disparity in this ethnic group. Finally, a 2018 systematic review and meta-analysis of longitudinal studies supported a causal link between a diverse non-Lactobacillus-dominant vaginal microbiome and cervical carcinogenesis via the effect of the microbiome on HPV acquisition (overall RR 1.33; RR among young women 1.4; 95% confidence interval (CI); statistical heterogeneity (I2) 0%) and persistence (RR 1.14; I2 44.2%), as well as on the development of precancerous dysplasia (RR 2.01; I2 0%)137. Another network meta-analysis of cross-sectional and longitudinal studies also revealed that women with a non-Lactobacillus-dominant vaginal microbiome or a vaginal microbiome dominated by L. iners had 2–3 times higher odds of high-risk HPV prevalence and cervical neoplasia and 3–5 times higher odds of any prevalent HPV (95% CI) than women with a vaginal microbiome dominated by L. crispatus138. Notably, a vaginal microbiome dominated by L. gasseri had the highest odds for high-risk HPV (odds ratio (OR), 3.3; 95% CI) compared with L. crispatus138. In addition, an independent meta-analysis validated these findings, showing that L. crispatus, but not L. iners, is associated with decreased detection of high-risk HPV (OR 0.49; 95% CI; I2 10%) and dysplasia (OR 0.50; 95% CI; I2 0%)139.
Mechanisms of HPV and microbiome interaction.
To date, and to our knowledge, no published reports have investigated the mechanism of interaction between HPV and vaginal bacteria, besides those reported in clinical studies. HPV is difficult to cultivate in vitro, making these studies challenging to perform. In addition, mouse models for HPV-mediated cervical dysplasia and cancer140–143 or BV using polymicrobial cocktails are limited144,145; however, future use of these mouse models might yield important insights into the role of vaginal bacteria in cervical carcinogenesis. In addition, human in vitro cell lines and 3D organotypic models can be used to study host-vaginal microbiota interactions144,146. Studies are ongoing in our laboratory to evaluate the role of individual, polymicrobial cocktails and clinical communities of vaginal bacteria using human 3D vaginal, cervical and endometrial epithelial models147–153 on characteristic hallmarks of cancer (Fig. 4).
Effects on host response.
The functional effect of the vaginal microbiome on the host response during cervical carcinogenesis has not been comprehensively studied. One small cross-sectional study (n = 32) showed that elevated cervical expression of anti-inflammatory IL-4 and transforming growth factor β1 (TGFβ1) were associated with the presence of Fusobacterium spp., which is involved in the development of the immunosuppressive microenvironment126. In a cross-sectional study (n = 100), systematic examination of multiple immune mediators in the local cervicovaginal microenvironment in women with cervical cancer or dysplasia and women without neoplasia (infected or not with HPV) revealed that several proinflammatory (IL-36γ) and chemotactic (IFNγ-induced protein 10 (IP10), macrophage-inflammatory protein 1β (MIP1β) and RANTES), haematopoietic (FLT3 ligand) and adaptive immune response cytokines (IL-2, IL-4 and soluble CD40 ligand) were associated with non-Lactobacillus dominance (P < 0.05)51. Moreover, known circulating cancer biomarkers, including growth and angiogenic factors (such as basic fibroblast growth factor (FGF2), stem cell factor (SCF), apoptosis-related proteins (soluble Fas ligand, TNF-related apoptosis-inducing ligand (TRAIL)), hormones (prolactin), proinflammatory cytokines and chemokines (macrophage migration inhibitory factor (MIF) and TNF) and osteopontin were present in the local cervicovaginal microenvironment and correlated positively not only with genital inflammation but also with a dysbiotic vaginal microbiome (that is, non-Lactobacillus dominance) (P < 0.05)154. Additionally, integration of multiple datasets, including those of cervicovaginal immune mediators and cancer biomarkers, the vaginal microbiome and vaginal pH, revealed potentially ‘high-risk’ features of the local cervicovaginal microenvironment, such as a substantial increase in levels of proinflammatory cytokines and specific cancer biomarkers, abnormal vaginal pH and depletion of lactobacilli, that might directly or indirectly contribute to cervical carcinogenesis154.
Previous metabolomic studies of the cervicovaginal microenvironment in women with BV suggested that the vaginal microbiome might drastically alter local metabolic profiles (mostly in amino acid, dipeptide, polyamine and ketone body pathways)59,155, which could thereby affect cervical carcinogenesis. Indeed, a cross-sectional study (n = 78) from our own group, which to our knowledge was the first report on cervicovaginal metabolomes in HPV-mediated cervical neoplasia, demonstrated the functional interplay between HPV, neoplastic disease and the vaginal microbiome127. In this study, cervicovaginal metabolic profiles were strongly driven by cancer, followed by genital inflammation, HPV infection and vaginal microbiome composition127. Perturbations in lipids, an emerging hallmark of cancer156, were observed in cancer metabolomes, whereas depletion in amino acid and nucleotide metabolites connected vaginal dysbiosis to HPV infection and precancerous cervical dysplasia. Specific metabolites, such as anti-inflammatory nucleotides, correlated with Lactobacillus dominance independently of the severity of the cervical neoplasm127. The multi-omics approach enabled the identification of specific sets of metabolites associated with Lactobacillus dominance involved mostly in peptide, amino acid and nucleotide pathways and genital inflammation involved in multiple lipid pathways127.
We and others hypothesize that microenvironmental factors, including microbial51,125,126,137,138, immune51,125–127 and metabolic signatures154, might favour HPV persistence in the local microenvironment and consequently increase the risk of neoplastic disease. Alternatively, the collective action of these conditions, including infection with high-risk HPV, might directly affect hallmarks of cancer (such as cell proliferation, evasion of apoptosis, senescence, DNA mutation and methylation, and angiogenesis) contributing to the development of precancerous lesions and progression to cancer (Fig. 4). Taken together, this increasing body of evidence suggests a complex relationship between the host, local microbiome and HPV during cervical carcinogenesis.
Microbiota axes and endometrial cancer
Endometrial cancer is the most common gynaecological cancer in developed countries and the fourth most common cancer affecting women in the USA111. Socioeconomic status, race and/or ethnicity might place some women at an increased risk of developing endometrial cancer157,158, as genetic alterations and hereditary factors underlie only 10–20% of cases159. Notably, in the USA, black and non-Hispanic white women have the highest incidence of endometrial cancer160; however, the mortality is twice as high in African American women compared with all other racial and ethnic groups161. Environmental factors, including obesity, inflammation, imbalances in oestrogen metabolism and oestrogen therapy after menopause, are major risk factors for the development of type I endometrial cancer162,163. Interestingly, these environmental factors are also associated with changes in the gut164,165 and vaginal51,166 microbiomes. A strong connection between the gut microbiota, oestrogen metabolism and obesity suggests a potential role of the microbiome in the aetiology of endometrial cancer92,111,167.
Advances in sequencing technologies have shown that oestrogen compounds can shape vaginal and distal microbial communities167–169 and that the oestrobolome can alter circulating oestrogen levels90. We and others postulate that the oestrobolome can influence the development and treatment of endometrial hyperplasia and cancer via its oestrogen modulatory effects92,170. Moreover, as oestrogen levels directly affect health and homeostasis in the vaginal microenvironment, oestrogen can also affect the gut–vaginal microbiome axis. Thus, the gut microbiome might indirectly promote endometrial carcinogenesis by altering genital microbial communities.
A 2016 study associated differences in the FRT microbiota with endometrial hyperplasia and cancer19. In this small (n = 31), cross-sectional study, several microbial taxa, including Atopobium, Porphyromonas, Dialister, Peptoniphilus, Ruminococcus, Anaerotruncus, Anaerostipes, Treponema, Bacteroides and Arthrospira, were reported to be enriched in women with hyperplasia and cancer along the reproductive tract, including the uterus, compared with benign uterine samples19 (Fig. 1). Furthermore, the simultaneous presence of Atopobium and Porphyromonas combined with abnormal vaginal pH (>4.5) was strongly associated with disease status19. These bacteria or other disturbances in the microbiome could alter hallmarks of cancer, including the promotion of damaging local inflammation that contributes to carcinogenesis in the endometrium92. A study from our group that used a 3D human vaginal epithelium model showed that A. vaginae induces proinflammatory cytokines and antimicrobial peptides148. The association between proinflammatory A. vaginae and Porphyromonas spp. in this endometrial cancer cohort19 supports a link between ascending BV-associated bacteria and other genital bacteria, inflammation and endometrial cancer. However, future studies with larger cohorts are needed to validate these initial findings. Mechanistic studies, employing in vitro151 and animal models171, will be also required to improve understanding of the functional role of genital microbiota and the oestrobolome in the aetiology of endometrial cancer.
Microbiota in tubal and ovarian cancer
Ovarian cancer is one of the deadliest malignancies in women — in 2018, an estimated 295,414 new cases of ovarian cancer were diagnosed and 184,799 women died of the disease worldwide112 — owing to its asymptomatic clinical presentation. Thus, prevention strategies and early diagnosis are priorities172. In contrast to cervical cancer, risk of developing ovarian cancer is 30% higher in non-Hispanic white women than in women of other races or ethnic groups172. However, the connection of this cancer disparity with the FRT microbiome is unknown. Additionally, multiparity, tubal ligation, use of oral contraceptives and oophorectomy are known to reduce the risk of developing ovarian cancer172. Conversely, dysbiosis in the genital microbiota has been associated with the development of ovarian cancer and proposed as a potential biomarker for the disease173,174.
Similar to endometrial cancer, chronic infections with sexually transmitted pathogens and inflammation in the genital tract have been associated with the development of ovarian tumours175. Two cross-sectional studies with modest sample sizes (n = 119 and n = 127) have compared ovarian tissues in women with ovarian cancer and healthy participants and found a distinct ovarian microbiome in women with cancer173,174. Moreover, the microbiome of the malignant ovarian tissue had distinct microbial signatures compared with the healthy surrounding ovarian tissues within the same individuals174. In particular, the presence of potentially pathogenic intracellular microorganisms, such as Brucella, Mycoplasma and Chlamydia spp., was found in 60–76% of ovarian tumours174,176,177 (Fig. 1). Additionally, an increase in Proteobacteria, especially in Acinetobacter spp., in the ovarian tumours was linked to inflammation-associated gene profiles in participants173. Finally, several pathogenic viruses and intracellular bacteria present in ovarian tissues, including HPV, cytomegalovirus and C. trachomatis, were identified as biosignatures of ovarian cancer175.
Although these pilot studies have identified the presence of various bacteria in ovarian cancer tissues and suggested a link with inflammation, the causational link between microbiota and ovarian cancer remains unclear. These microorganisms might induce carcinogenesis through direct or indirect mechanisms; however, the highly anoxic tumour microenvironment might also favour the recruitment and growth of anaerobic microorganisms, such as Chlamydia spp.174. Similar to other upper FRT sites, the Fallopian tubes and ovaries are low-abundant sites, which makes sample collection without cross-contamination and interpretation of the results challenging68. Site-specific differences related to the ovarian tumour microbiome173 warrant future comparative studies to improve understanding of the connection between microbiomes throughout the FRT and establish the role of the microbiome in this malignancy. Furthermore, the connection between the presence of pathogenic microorganisms, chronic inflammation and ovarian cancer needs further validation with epidemiological studies in large patient cohorts and/or longitudinal study designs.
Microbiome and therapy efficacy
Women with gynaecological cancers are typically treated with surgery (for example, hysterectomy or bilateral salpingo-oophorectomy), radiation, chemotherapy or a combination of chemoradiation and surgery depending on the site and stage of the cancer178. Immunotherapy has become an exciting new option for gynaecological cancer treatment, but it is currently in its early stages178. To date, the FDA has approved only a few immunotherapy options, including the immune checkpoint inhibitor targeting anti-programmed cell death 1 (PD1) and anti-angiogenic factor targeting VEGFR, for the treatment of advanced gynaecological cancers178. These immunotherapeutics have been specifically used for the treatment of recurrent endometrial cancer with either mismatch repair protein-deficient or microsatellite stability index-high (MSI-H), ovarian cancer, and advanced cervical cancer with disease progression during or after chemotherapy178. However, other immunotherapy agents are being evaluated in multiple clinical trials as well as combinations of these therapies178. The microbiome has been demonstrated to affect the efficacy of cancer therapies, including chemotherapy and immunotherapy, for various tumour types (including melanoma, lung and kidney cancers)179–182 (Fig. 5). A 2018 study reported a correlation between decreased efficacy of immunotherapy and survival in patients with melanoma who had received antibiotic treatment before immunotherapy, revealing that alteration of the gut microbiota via antibiotic treatment directly affects therapeutic responses179. These data have been reflected in several other studies in patients with cancer and in preclinical mouse models, which have demonstrated that the gut microbiome diversity and composition affect antitumour immunity and the efficacy of PD-1 immunotherapy for melanoma, lung and kidney cancers179–181. Notably, faecal microbiota transplantation (FMT) from human responders to antibiotic-treated or germ-free mice with xenograft tumours promoted the efficacy of PD-1 blockade against melanoma, lung and kidney cancers, whereas FMT from non-responders did not179–181. Particular bacterial taxa, for example, Akkermansia muciniphila, Bifidobacterium longum and Ruminococcaceae, were also found to be more abundant in faecal samples collected from PD-1-responding patients. Notably, the responsiveness to PD-1 or programmed cell death 1 ligand 1 (PD-L1) immunotherapy in mouse melanoma and lung cancer models could be induced through oral supplementation with A. muciniphila179 and in a mouse melanoma model with Bifidobacterium183. These findings strongly suggest a mechanistic role for the gut microbiota in facilitating antitumour immunity179,183.
Fig. 5. An overview of microbiota–cancer therapy interactions.
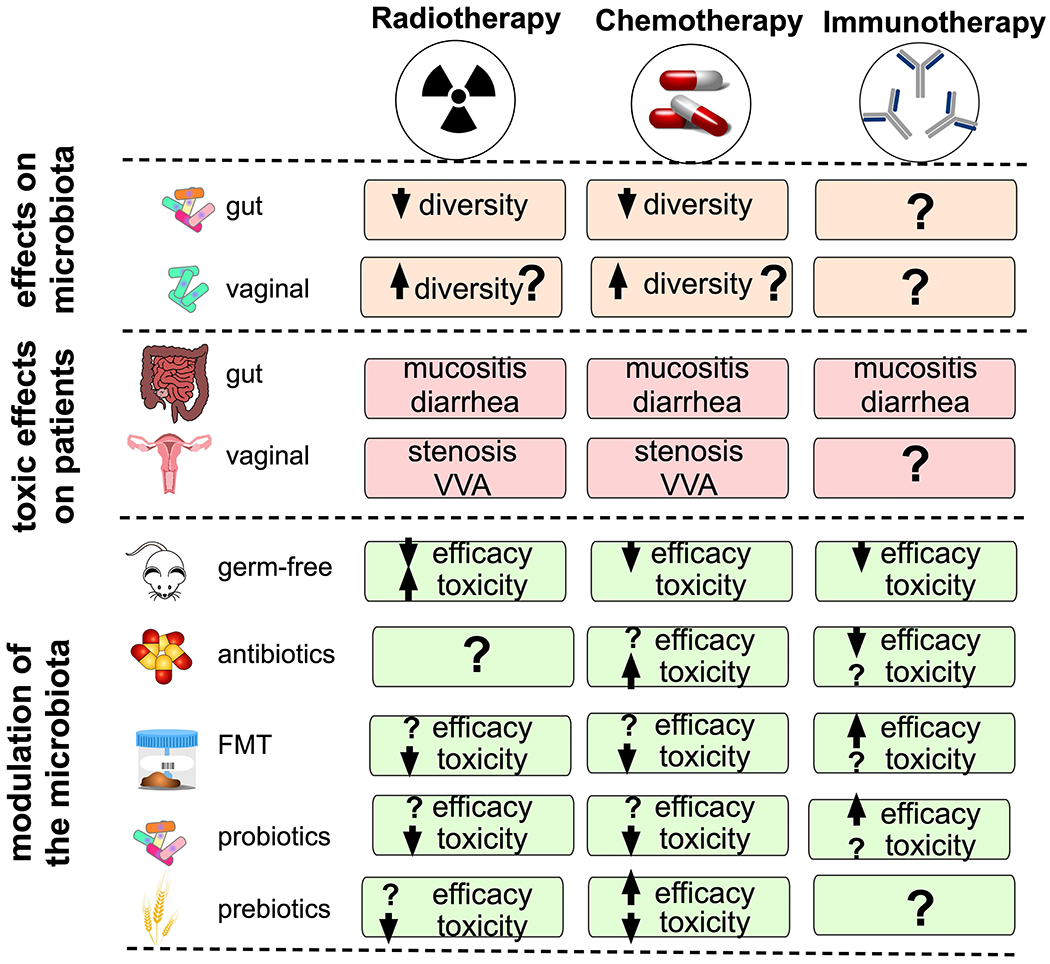
a) Gynaecological cancer therapies (surgery, radiotherapy, chemotherapy and immunotherapy) can affect microbial diversity and composition. The first line of treatment for gynaecological cancers is surgery (for example, hysterectomy); however, data are lacking regarding hysterectomy and modulation of the gut microbiome. The adverse effects of radiation and chemotherapies on gut microbiota are well characterized, whereas the effects of immunotherapy need further characterization. Toxic effects of chemotherapy and radiotherapy on the gut and vaginal ecosystems include diarrhoea or constipation, nausea, vomiting, abdominal pain, gastrointestinal bleeding, mucositis, vaginal stenosis and vulvovaginal atrophy (VVA). b) Modulation of the gut microbiota has been shown to alter the toxic effects and efficacy of cancer treatments. Diminishing the gut microbiota via antibiotics or other inhibitory agents, replenishing the microbiota via faecal microbiota transplantation (FMT) or vaginal microbiota transplantation (VMT) and supplementing the microbiota with prebiotics or probiotics might drive response to cancer therapy.
The microbiota can alter therapeutic efficacy by a number of mechanisms, including translocation, immunomodulation, metabolism and enzymatic degradation, depending on the type of therapy184,185. For example, the composition of the gut microbiota has been shown to affect the level of efficacy for chemotherapeutics such as irinotecan via alterations in drug metabolism, including inhibited intestinal absorption and decreased activity of the irinotecan-activating enzyme carboxylesterase186. The gut microbiome has also been shown to affect immunotherapeutics including PD-1/PD-L1 blockade by modulating the host immune system, particularly by mediating T cell activation, increasing T cell priming and T cell accumulation at the tumour site179–181. Furthermore, early studies on microbiota in cancer demonstrated that interactions between the gut microbiota and the host immune system are bidirectional and that microbiota can also regulate the immune response to cancer cells179,187 (Fig. 5). Microorganism-associated molecular patterns, such as cellular wall polysaccharides and microbial metabolic products — including short-chain fatty acids — have been shown to interact with pattern recognition receptors of the innate immune system to modulate therapy responses188. Moreover, many structural and metabolic features of bacteria, including mobility, cell toxicity, immunogenicity and their preferential accumulation within the tumour microenvironment, suggest that bacteria are potential targets for mediating the efficacy of cancer therapy189. The targeted interactions between bacteria and the host immune system are key factors in patient responsiveness to immunotherapeutic agents179. Additionally, several reports have investigated the effect of the vaginal microbiome on the pharmacokinetics of topical antiretroviral drugs, including tenofovir gel and the dapivirine ring, with contradicting results190–192. One study demonstrated that vaginal microorganisms, such as L. crispatus and G. vaginalis, but not A. vaginae, decreased bioavailability of the drugs, resulting in decreased antiviral activity190. By contrast, another report showed no significant differences in tenofovir concentrations in cervicovaginal aspirates from women with Lactobacillus-dominant microbiome versus dysbiotic Lactobacillus-depleted microbiome (P > 0.07)191. However, the direct role of the vaginal microbiome in cancer therapies is unknown. Taking into account the connection between the gut and vaginal microbiomes111, the effect of cancer therapeutics on oestrogen metabolism92,111 and the known modulation of topical drug efficacies by the vaginal microbiome190,191,193, the vaginal microbiome could have a role in the efficacy of cancer therapeutics. An improved understanding of the microbiota–drug interactions185 and the vaginal–gut microbiota axis92 should be further studied and might provide unique insights into the biology of gynaecological cancer and therapeutic efficacy.
IrAEs and the microbiome
In addition to mediating therapeutic responses, the microbiome can be an important target for the management of therapeutic toxic effects or immune-related adverse events (irAEs). Radiotherapy and chemotherapy for gynaecological cancers can induce urogenital, vaginal, rectal and skin-related toxicities194–196. In general, the connection between the microbiome and therapeutic toxicity is poorly understood184; however, preliminary studies in animal models have demonstrated that gut microbiota can mediate the gastrointestinal toxicities related to chemotherapy197,198, which include diarrhoea, gastrointestinal pain and bleeding199,200 (Fig. 5). These events were also associated with alterations in the gut microbiome199 — a decrease in Bifidobacterium and Lactobacillus and an increase in E. coli and Staphylococcus in patients treated with chemotherapeutic agents such as cisplatin and carboplatin were associated with diarrhoea and increased levels of NF-κB, IL-1β and TNF200. Changes in the gut microbiome might also be related to adverse effects of poly(ADP-ribose) polymerase (PARP) inhibitors, which are standard maintenance therapeutics for the management of ovarian cancer201 and are known to induce nausea, vomiting, diarrhoea and constipation202. Indeed, differences in gut microbiota composition in Parp-knockout mice and wild-type mice were associated with inflammation, diarrhoea and reduced gut microbiota diversity in the knockout mice203. Additionally, gut microbial activity can induce the toxic effects of common chemotherapeutic agents. For example, gut commensals that produce β-glucuronidase (an oestrobolome enzyme) can induce diarrhoea by converting irinotecan to its toxic form, which damages the intestinal epithelia204. Future studies characterizing the effect of chemotherapeutics on microbiota in the gut and at other sites will enhance our understanding of these microbiome-related toxicities203 and could aid in the development of safe and effective therapies.
Radiotherapy and chemotherapies (for example, 5-fluorouracil) are also known to induce gastrointestinal mucositis, an inflammatory condition that affects the gastrointestinal tract caused by elimination of beneficial gut microorganisms and reduction of gut microbial diversity205,206. The damage to the proliferating epithelial barrier following administration of cancer drugs provides opportunities for pathobionts, such as Enterobacteriaceae and Corynebacterium, to translocate and induce mucositis206. In addition to radiation and chemotherapy, mucositis has been observed in patients treated with PD-1 inhibitors207, indicating that several commonly used cancer therapies have adverse effects on the gut microbiota. Animal studies in mice or rats treated with irinotecan have shown that the overgrowth of opportunistic microorganisms, such as Enterobacteriaceae, in the small intestine after chemotherapy is related to mucositis198,206 and that dietary fibre supplementation after chemotherapy reduced the mucositis by strengthening the gut epithelial barrier197. As dietary fibre enhances butyrate production in the colon and butyrate enhances the gut epithelial barrier197, prebiotic supplementation during cancer therapy might have the potential to reduce the severity of gastrointestinal symptoms of cancer therapeutics.
In addition to gastrointestinal toxicities, cancer treatment has adverse effects on the vagina and probably also the local microbiome111. Vaginal stenosis and vulvovaginal atrophy (VVA) are common adverse effects of pelvic radiotherapy or brachytherapy for the treatment of cervical and endometrial cancers196,208,209. Vaginal stenosis is defined as a shortening and narrowing of the vagina that causes dryness, itching and discomfort in patients and arises from cellular damage caused by radiotherapy, resulting in thinning of the vaginal walls111. Women with VVA have reduced levels of glycogen in their vaginal secretions, which can result in lower levels of health-associated Lactobacillus species66. Management of vaginal stenosis and VVA involves use of vaginal dilators, lubricants and moisturizers210, and patients are advised to have sexual intercourse211, all of which might also affect the vaginal microbiota composition and/or stability. Although the connection between vaginal stenosis and changes in the local cervicovaginal microenvironment and microbiome have not yet been investigated, one report has shown that radiation can adversely affect vaginal bacterial communities212. This pilot study (n = 19) characterizing vaginal microbiota profiles in women with gynaecological cancer before and after radiotherapy showed an increase in particular BV-associated bacteria, such as Mobiluncus, Atopobium and Prevotella, and a decrease in other BV-associated microorganisms, such as Gardnerella and Peptostreptococcus, as well as health-associated Lactobacillus spp. following treatment212. Acute and long-term toxic effects after gynaecological cancer therapy can profoundly affect the quality of life of survivors. Thus, investigation of microbiomes and their connection to therapeutic toxic effects is essential for the development of strategies to reduce these therapeutic toxicities or irAEs and enhance quality of life for these patients and survivors.
Modulating the microbiome
Gut microbiome modulation
Health benefits from the modulation of microbiomes have been successfully shown for many chronic and inflammatory diseases, including irritable bowel syndrome213 and recurring Clostridioides difficile infections214. The accumulating evidence connecting gynaecological cancer and dysbiosis identifies microbiota as a target for cancer prevention and therapy. Notably, the investigation of microbiome editing, sometimes referred to as ‘bugs as drugs’215, has been prompted by the observation that patients receiving antibiotics do not respond to immunotherapy179,216,217. Microbiome modulation includes the use of bacterial therapeutics, probiotics and/or prebiotics, antibiotics or other drugs, and microbiota transplantation.
FMT has been widely used to determine the functional effect of gut microbiota on a wide a range of health conditions218,219. FMT from patients responding and not responding to immune checkpoint inhibitors to cancer xenograft mouse models showed that the gut microbiome dictates responsiveness to immunotherapy179,181. Furthermore, FMT has been shown to reduce toxic effects associated with radiotherapy and chemotherapy220,221. These innovative studies have led to FMT being proposed as a promising adjunct to cancer therapies222. Additional safety and testing regulations are also needed for the investigational use of FMT in human patients, especially those who are immunocompromised. As immunotherapy for gynaecological cancers is still in its infancy, the use of FMT to improve therapeutic efficacy has not been evaluated for any gynaecological cancers.
Notably, FMT studies have led to the identification of specific gut microorganisms that modulate therapeutic response179,181. Oral administration of Bifidobacterium species to mice with melanoma was as effective as treatment with the PD-L1 inhibitor alone and also improved PD-L1 inhibitor efficacy when administered adjunctively by increasing the number of primed T cells and their accumulation at the tumour site183. In a similar melanoma mouse model, the presence of B. fragilis-mediated and Bacteroides thetaiotaomicron-mediated T cell activation and responsiveness towards monoclonal antibodies targeting cytotoxic T lymphocyte-associated protein 4 (CTLA-4), another immune checkpoint inhibitor187. Similar to the experiments with Bifidobacterium, oral supplementation of A. muciniphila, a gut commensal that has been identified as a predictive biomarker of responsiveness to PD-1 inhibitors in a mouse FMT model, restored the PD-1 inhibitor response in mice that received FMT from PD-1 non-responder patients179. Considering that Bifidobacterium and Bacteroides species have been associated with immune modulation, immunotherapy efficacy180,183 and oestrogen metabolism167,223, investigation of their therapeutic potential in oestrogen-driven cancers, and particularly endometrial and ovarian cancer, is compelling.
Based on the beneficial effects on gut homeostasis224, probiotics have also been shown to reduce therapeutic toxic effects. For example, in a wild-type mouse model, supplementation with Lactobacillus lactis engineered to secrete pancreatitis-associated protein, an antimicrobial peptide involved in gut homeostasis, following chemotherapy with 5-fluorouracil, reduced the abundance of pathobionts, such as Enterobacteriaceae, in the gut and alleviated mucositis severity206. Taken together, these data suggest that specific gut microbiota species induce antitumour responses and that associations between gut microbiota diversity and immunity indicate the exciting potential to develop microbiome-based or adjunct therapy regimens for various malignancies, including gynaecological cancers.
Vaginal microbiome modulation
Notably, vaginal probiotic lactobacilli, such as L. crispatus strain CTV-05 (as a vaginal suppository; LACTIN-V), have also been tested in clinical trials, mainly for the treatment of BV225,226 or urinary tract infection (UTI)227 (Fig. 6). A randomized placebo-controlled phase IIa clinical trial, involving women with BV after standard metronidazole treatment (n = 24), showed that vaginal colonization with LACTIN-V was achieved in 44% participants (at day 28 following LACTIN-V treatment) and inhibited the growth of particular BV-associated bacteria, especially Atopobium (P = 0.04), compared with women not colonized with LACTIN-V186. Additionally, the study revealed that vaginal intercourse during treatment (P = 0.003) or pre-colonization of the vagina with endogenous L. crispatus (P = 0.018) reduced odds of successful colonization with probiotic L. crispatus strain186. In 2019, a multicentre phase IIb clinical trial (NCT02766023; n = 228) assessing the long-term efficacy of repeated doses of LACTIN-V in preventing BV recurrence was completed; however, the results have not yet been published (ClinicalTrials.gov). Another phase II trial (n = 100) evaluated LACTIN-V supplementation for the prevention of recurrent UTI and demonstrated a reduction in recurrent UTI compared with placebo treatment (P < 0.01)227. Overall, these studies demonstrate the feasibility of the use of vaginal probiotics to modulate the vaginal microbiome.
Fig. 6. Novel approaches for modulating vaginal microbiota.
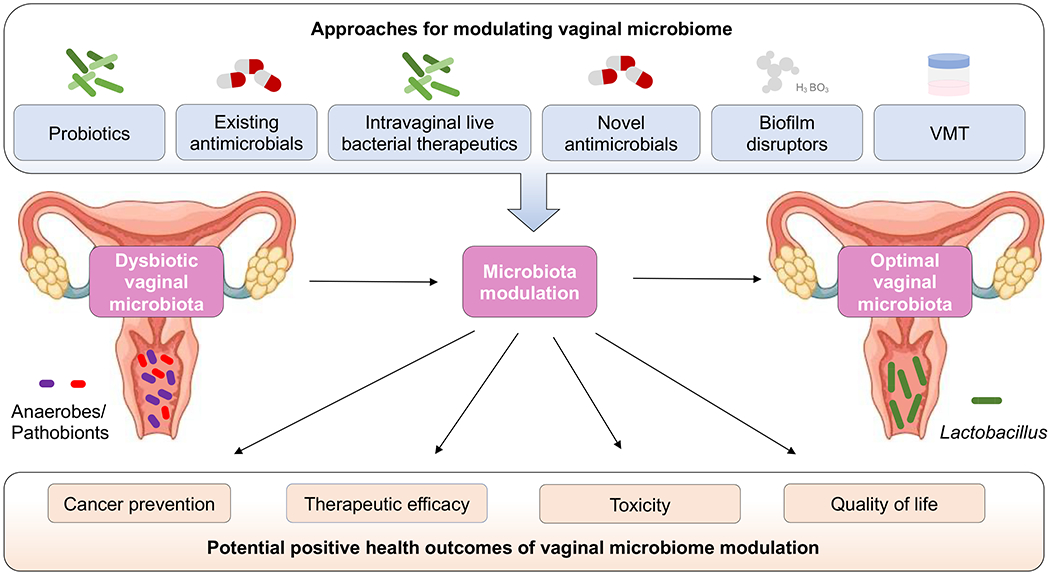
Combining current and experimental protocols for modulating the vaginal microbiota from dysbiotic to optimal Lactobacillus-dominant community state could affect carcinogenesis, therapeutic efficacy, toxicity and quality of life for women. Current and experimental protocols include existing antimicrobials, probiotics, intravaginally delivered vaginal lactobacilli formulations, novel antimicrobials, biofilm disruptors and vaginal microbiota transplantation (VMT).
Other promising novel therapies against BV or vaginal dysbiosis include a boric acid-based anti-infective with enhanced biofilm disruptive activity (TOL-463)228 and the nitroimidazole antibiotic secnidazole229 (Fig. 6). A phase II clinical trial (n = 106) demonstrated that TOL-463 treatment in either vaginal gel or insert forms is safe and well tolerated and led to a 59% and 50% clinical cure rate of BV for insert and gel, respectively, at days 9–12 (95% CI)228. A novel formulation of secnidazole (single-dose oral granule) has also been shown to be well tolerated with a low number of treatment-emergent adverse effects in a phase III clinical trial (n = 283)229. In this study, 72.5% of women were also assessed by investigators not to require additional BV treatment229.
Vaginal microbiota transplantation (VMT) from a donor with an optimal vaginal microbiome is a novel provocative treatment option under investigation for women with BV or vaginal disorders (Fig. 6). Currently, two phase I/II clinical trials in the USA (NCT03769688 and NCT04046900) and one clinical trial in Israel (NCT02236429) are recruiting participants (n = 10–126) to evaluate the safety and efficacy of this procedure in women with BV (ClinicalTrials.gov). A 2019 pilot study (n = 5) demonstrated the feasibility of using VMT from healthy donors as a therapeutic for women with intractable, antibiotic-unresponsive and recurrent BV230. Four of the five women treated with VMT had long-term remission, defined as marked improvement of symptoms and reconstitution of a Lactobacillus-dominant microbiome, at the follow-up 5–21 months after VMT230. To elicit a durable clinical response, repeat VMT was required in three of the five women in the exploratory study230. The authors did not observe adverse effects associated with VMT230; however, the long-term consequences of VMT remain unknown. The risks of this procedure, similar to other microbiome transplants, include transfer of antimicrobial-resistant microorganisms and undetected pathogens. Additionally, the transfer of sperm in vaginal fluid can result in unintended pregnancy. Thus, stringent inclusion/exclusion criteria and extensive testing of donor samples are imperative to minimize risks. A screening approach for universal VMT donors has been described and implemented in another 2019 pilot study (n = 20)231. Future studies with larger cohorts and randomized, placebo-controlled studies are required to determine the efficacy and durability of VMT.
In the future, vaginal probiotics, existing antimicrobials, live bacterial therapeutics, novel antimicrobials, biofilm disruptors and VMT have the potential to be used alone or in combination to modulate the vaginal microbiome by restoring homeostasis for prevention of gynaecological cancer and/or reduction of vaginal toxic effects related to gynaecological cancer therapies (Fig. 6).
Gaps, challenges and future directions
The clinical relevance of the urogenital microbiota in gynaecological and reproductive diseases, including cancer, has just begun to be explored. The advances in microbiomics and metagenomics have enabled us to start identifying microbial communities and/or particular bacterial species that might promote pathological states in the FRT and consequently contribute to tumorigenesis. Nevertheless, in the future, clinical studies with larger cohorts are necessary to validate and extend these initial findings for potential clinical use, a fact illustrated by a statement from the International Cancer Microbiome Consortium in 2019, which concluded that data from longitudinal cohort studies are needed to confirm the role of the human microbiome as a key driver in the aetiopathogenesis of cancer232. Furthermore, future epidemiological studies must include women of different races, ethnicities and socioeconomic backgrounds, as considerable differences have been reported in the genital microbiota compositions among women of different ethnic origins26,48,50,51 and we hypothesize that these differences might contribute to disparities in cancer demographics, for example, the increased rate of cervical cancer in Hispanic, African American, and American Indian and Alaskan Native women. Other metadata collected from participants, such as behavioural, environmental, socioeconomic and genetic information (Fig. 2), might further help to identify possible covariates and confounders in association studies using cross-sectional study designs. In addition, longitudinal studies are required to determine the extent to which genital bacteria are causal factors, passengers or just a consequence of disease. Furthermore, rigorous and careful sample collection and standardized sample processing with appropriate controls must be employed in future clinical studies (particularly for studies evaluating low microbial biomass sites, such as the upper FRT) to be able to draw biologically meaningful conclusions. The 16S rRNA sequencing technique, which is commonly used for vaginal microbiome studies, has a number of limitations, particularly relating to the lack of absolute bacterial quantification, which is relevant not only for studies investigating low microbial biomass sites (upper FRT) but also those investigating vaginal dysbiosis in the lower FRT (characterized by overgrowth of anaerobic microorganisms). In future studies, other molecular techniques for bacterial quantification, for example, quantitative real-time PCR233 or flow cytometry234, could be used to determine absolute loads of specific bacteria associated with particular conditions. Integrated multi-omics approaches are also desirable in future studies to fully understand the complex relationships in the local microenvironment between the host, genital microbiota and the development and progression of neoplastic disease. These integrative analyses could lead to identification of new microbial and host signatures (such as bacterial communities and/or species, immune mediators and other proteins, and metabolites) to exploit as biomarkers for gynaecological cancers. Finally, mechanistic studies using in vitro and in vivo models are required to determine the role of the microbial communities and specific bacterial species in triggering and/or promoting tumorigenesis in the FRT. Physiologically relevant 3D cell cultures, including organoids and bioreactor-derived models, can be successfully used to study the interactions between the host and genital bacteria, and future studies using these models should investigate the effect of these bacteria (alone or in polymicrobial milieu) on hallmarks of cancer144,146,148–153. Moreover, mouse models of cervical143, endometrial171 and ovarian cancers235 could be infected with genital bacteria in order to further investigate the pathogenesis of clinically relevant bacteria and provide additional insights into microorganism-mediated mechanisms contributing to cancer development and/or progression.
Conclusions
Epidemiological studies suggest that the urogenital microbiota is linked to gynaecological cancers, particularly cervical cancer. Even so, the mechanisms of host defence to these microorganisms are still poorly understood. Further research is warranted to elucidate the functional effect of the microbial communities and/or particular bacterial species in the FRT on the local microenvironment related to tumorigenesis. Future studies using robust clinical datasets as well as in vitro human and animal models will help to define the influence of these microorganisms on homeostasis and disease and bring us closer to understanding how these microorganisms contribute to the development and progression of gynaecological malignancies. Finally, a clearer understanding of the complex host–microorganism interactions in the FRT might reveal new opportunities for cancer prevention, therapy and improving women’s quality of life and overall health.
Key points.
The majority of bacteria in the female reproductive tract (FRT) reside in the vagina and cervix; however, the upper FRT might have a distinct low-biomass microbiome and site-specific microenvironmental factors.
A vaginal microbiome dominated by Lactobacillus species benefits the host, whereas a dysbiotic vaginal microbiome consisting of anaerobic bacteria is linked to numerous gynaecological and obstetric conditions, including gynaecological cancer.
Multiple socioeconomic, behavioural, environmental, hormonal and genetic factors can affect the genital microbiome by disrupting homeostasis and promoting dysbiosis; the FRT microbiome is intimately interconnected with other mucosal sites.
Emerging evidence suggests that microbial communities within the FRT might contribute to aetiology, disease severity and/or treatment of gynaecological cancers; however, further well-designed, large-cohort and mechanistic studies are needed.
The gut microbiome can modulate oestrogen levels and thereby affect carcinogenesis of oestrogen-mediated cancers, might dictate therapeutic efficacy and toxicity for gynaecological cancer and, ultimately, influence quality of life.
Vaginal microbiome modulation via probiotics, novel antimicrobials and/or vaginal microbiota transplantation might be a novel approach to the prevention of gynaecological cancers and/or the reduction of vaginal toxicities related to cancer treatment.
Acknowledgements
We would like to acknowledge our clinical colleagues and past and present members of the Herbst-Kralovetz lab for thoughtful discussions on this topic. P.Ł., Z.E.I. and M.M.H.-K. have been supported by the Mary Kay Foundation Translational Research Grant (no. 017-48), the Valley Research Partnership Grant (no. VRP26), the Flinn Foundation Grant (no. 1974), the Alternatives Research and Development Foundation Grant, and the National Institutes of Health Grants from the National Institute of Allergy and Infectious Diseases (1R15AI113457-01A1) and the National Cancer Institute (NCI) and Office for Research on Women’s Health (P30CA023074 and 2U54CA143924-11).
Glossary
- Microbiota
A community of microorganisms in a particular environment.
- Microbiome
The entire habitat, which includes microorganisms, their genomes, and the surrounding environment.
- Metagenome
The collection of genomes and genes from the members of a microbial community.
- Pathobionts
Resident microorganisms with pathogenic potential, harmless to the host under normal conditions.
- Osmolality
A concentration of osmotic solution expressed as the number of solute particles in 1 kg of solvent.
- Microbial culturomics
An approach to identifying unknown bacteria that inhabit the human body utilizing bacterial culture techniques to provide unique insights into host–bacteria relationships.
- Metagenomics
An approach to characterizing microbial communities at genome and gene level without requiring culturing.
- Oestrobolome
The collection of microorganisms (and their genes) that are able to metabolize oestrogens.
- Metabolomic studies
Studies of small molecules (metabolites), which are substrates, intermediates and products of metabolism within microorganisms, cells, tissues or body fluids.
- Metabolomes
The collections of small molecules (metabolites) and interactions among these molecules within a biological system.
- Faecal microbiota transplantation (FMT)
A process of transplantation of faecal material from a healthy individual to a recipient for restoration of the gut microbiota.
- Probiotics
Live microorganisms that confer a health benefit on the host when taken as a dietary supplement in adequate amounts.
- Biofilm
An assemblage of microbial cells that form on and coat various surfaces.
- Vaginal microbiota transplantation (VMT)
A process of transplantation of vaginal secretions from a healthy individual to a recipient for restoration of the vaginal microbiota.
- Microbiomics
The study of microbial communities inhabiting a particular environment (for example, the human body).
Footnotes
Competing interests
M.M.H.-K. has been a consultant for Lupin Pharmaceuticals and Beckton Dickinson. P.Ł. and Z.E.I. declare no competing interests.
References:
- 1.Gilbert JA et al. Current understanding of the human microbiome. Nat. Med 24, 392–400 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marchesi JR & Ravel J The vocabulary of microbiome research: a proposal. Microbiome 3, 31 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santiago-Rodriguez TM, Ly M, Bonilla N & Pride DT The human urine virome in association with urinary tract infections. Front. Microbiol 6, 14 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mukhopadhya I, Segal JP, Carding SR, Hart AL & Hold GL The gut virome: the ‘missing link’ between gut bacteria and host immunity? Ther. Adv. Gastroenterol 12, 1756284819836620 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nash AK et al. The gut mycobiome of the Human Microbiome Project healthy cohort. Microbiome 5, 153 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradford LL & Ravel J The vaginal mycobiome: a contemporary perspective on fungi in women’s health and diseases. Virulence 8, 342–351 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garretto A, Miller-Ensminger T, Wolfe AJ & Putonti C Bacteriophages of the lower urinary tract. Nat. Rev. Urol 16, 422–432 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sender R, Fuchs S & Milo R Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 14, e1002533 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gill SR et al. Metagenomic analysis of the human distal gut microbiome. Science 312, 1355–1359 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raskov H, Burcharth J & Pommergaard HC Linking gut microbiota to colorectal cancer. J. Cancer 8, 3378–3395 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu HX et al. Difference of lower airway microbiome in bilateral protected specimen brush between lung cancer patients with unilateral lobar masses and control subjects. Int. J. Cancer 142, 769–778 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Baker JM, Chase DM & Herbst-Kralovetz MM Uterine microbiota: residents, tourists, or invaders? Front. Immunol 9, 208 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whiteside SA, Razvi H, Dave S, Reid G & Burton JP The microbiome of the urinary tract — a role beyond infection. Nat. Rev. Urol 12, 81–90 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Thomas-White K et al. Culturing of female bladder bacteria reveals an interconnected urogenital microbiota. Nat. Commun 9, 1557 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siddiqui H, Nederbragt AJ, Lagesen K, Jeansson SL & Jakobsen KS Assessing diversity of the female urine microbiota by high throughput sequencing of 16S rDNA amplicons. BMC Microbiol. 11, 244 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pearce MM et al. The female urinary microbiome: a comparison of women with and without urgency urinary incontinence. MBio 5, e01283–01214 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aragon IM et al. The urinary tract microbiome in health and disease. Eur. Urol. Focus. 4, 128–138 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Sfanos KS, Yegnasubramanian S, Nelson WG & De Marzo AM The inflammatory microenvironment and microbiome in prostate cancer development. Nat. Rev. Urol 15, 11–24 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Walther-Antonio MR et al. Potential contribution of the uterine microbiome in the development of endometrial cancer. Genome Med. 8, 122 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verstraelen H et al. Characterisation of the human uterine microbiome in non-pregnant women through deep sequencing of the V1–2 region of the 16S rRNA gene. PeerJ 4, e1602 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moreno I et al. Evidence that the endometrial microbiota has an effect on implantation success or failure. Am. J. Obstet. Gynecol 215, 684–703 (2016). [DOI] [PubMed] [Google Scholar]
- 22.Franasiak JM et al. Endometrial microbiome at the time of embryo transfer: next-generation sequencing of the 16S ribosomal subunit. J. Assist. Reprod. Genet 33, 129–136 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen C et al. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat. Commun 8, 875 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zozaya M et al. Bacterial communities in penile skin, male urethra, and vaginas of heterosexual couples with and without bacterial vaginosis. Microbiome 4, 16 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Altmae S, Franasiak JM & Mandar R The seminal microbiome in health and disease. Nat. Rev. Urol 16, 703–721 (2019). [DOI] [PubMed] [Google Scholar]
- 26.Ravel J et al. Vaginal microbiome of reproductive-age women. Proc. Natl Acad. Sci. USA 108, 4680–4687 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin DH & Marrazzo JM The vaginal microbiome: current understanding and future directions. J. Infect. Dis 214, S36–S41 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nunn KL & Forney LJ Unraveling the dynamics of the human vaginal microbiome. Yale J. Biol. Med 89, 331–337 (2016). [PMC free article] [PubMed] [Google Scholar]
- 30.Beamer MA et al. Bacterial species colonizing the vagina of healthy women are not associated with race. Anaerobe 45, 40–43 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jespers V et al. A longitudinal analysis of the vaginal microbiota and vaginal immune mediators in women from sub-Saharan Africa. Sci. Rep 7, 11974 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kyongo JK et al. Cross-sectional analysis of selected genital tract immunological markers and molecular vaginal microbiota in sub-Saharan African women, with relevance to HIV risk and prevention. Clin. Vaccine Immunol. 22, 526–538 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Antonio MA, Hawes SE & Hillier SL The identification of vaginal Lactobacillus species and the demographic and microbiologic characteristics of women colonized by these species. J. Infect. Dis 180, 1950–1956 (1999). [DOI] [PubMed] [Google Scholar]
- 34.Younes JA et al. Women and their microbes: the unexpected friendship. Trends Microbiol. 26, 16–32 (2018). [DOI] [PubMed] [Google Scholar]
- 35.Miller EA, Beasley DE, Dunn RR & Archie EA Lactobacilli dominance and vaginal pH: why is the human vaginal microbiome unique? Front. Microbiol 7, 1936 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hickey RJ, Zhou X, Pierson JD, Ravel J & Forney LJ Understanding vaginal microbiome complexity from an ecological perspective. Transl Res. 22, 267–282 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Łaniewski P & Herbst-Kralovetz M in Encyclopedia of Reproduction Vol. 2 (ed Skinner MK) 353–359 (Academic Press: Elsevier, 2018). [Google Scholar]
- 38.Graver MA & Wade JJ The role of acidification in the inhibition of Neisseria gonorrhoeae by vaginal lactobacilli during anaerobic growth. Ann. Clin. Microbiol. Antimicrob 10, 8 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gong Z, Luna Y, Yu P & Fan H Lactobacilli inactivate Chlamydia trachomatis through lactic acid but not H2O2. PLoS One 9, e107758 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Conti C, Malacrino C & Mastromarino P Inhibition of herpes simplex virus type 2 by vaginal lactobacilli. J. Physiol. Pharmacol 60, 19–26 (2009). [PubMed] [Google Scholar]
- 41.Tyssen D et al. Anti-HIV-1 activity of lactic acid in human cervicovaginal fluid. mSphere 3, e00055–18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cadieux PA, Burton J, Devillard E & Reid G Lactobacillus by-products inhibit the growth and virulence of uropathogenic Escherichia coli. J. Physiol. Pharmacol 60, 13–18 (2009). [PubMed] [Google Scholar]
- 43.O’Hanlon DE, Moench TR & Cone RA Vaginal pH and microbicidal lactic acid when lactobacilli dominate the microbiota. PLoS One 8, e80074 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tachedjian G, O’Hanlon DE & Ravel J The implausible “in vivo” role of hydrogen peroxide as an antimicrobial factor produced by vaginal microbiota. Microbiome 6, 29 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maldonado-Barragan A, Caballero-Guerrero B, Martin V, Ruiz-Barba JL & Rodriguez JM Purification and genetic characterization of gassericin E, a novel co-culture inducible bacteriocin from Lactobacillus gasseri EV1461 isolated from the vagina of a healthy woman. BMC Microbiol. 16, 37 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mirmonsef P et al. Glycogen levels in undiluted genital fluid and their relationship to vaginal pH, estrogen, and progesterone. PLoS One 11, e0153553 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou X et al. Differences in the composition of vaginal microbial communities found in healthy Caucasian and black women. ISME J. 1, 121–133 (2007). [DOI] [PubMed] [Google Scholar]
- 48.Fettweis JM et al. Differences in vaginal microbiome in African American women versus women of European ancestry. Microbiology 160, 2272–2282 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peebles K, Velloza J, Balkus JE, McClelland RS & Barnabas RV High global burden and costs of bacterial vaginosis: a systematic review and meta-analysis. Sex. Transm. Dis 46, 304–311 (2019). [DOI] [PubMed] [Google Scholar]
- 50.Borgdorff H et al. The association between ethnicity and vaginal microbiota composition in Amsterdam, the Netherlands. PLoS One 12, e0181135 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Łaniewski P et al. Linking cervicovaginal immune signatures, HPV and microbiota composition in cervical carcinogenesis in non-Hispanic and Hispanic women. Sci. Rep 8, 7593 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peipert JF et al. Bacterial vaginosis, race, and sexually transmitted infections: does race modify the association? Sex. Transm. Dis 35, 363–367 (2008). [DOI] [PubMed] [Google Scholar]
- 53.Cherpes TL, Hillier SL, Meyn LA, Busch JL & Krohn MA A delicate balance: risk factors for acquisition of bacterial vaginosis include sexual activity, absence of hydrogen peroxide-producing lactobacilli, black race, and positive herpes simplex virus type 2 serology. Sex. Transm. Dis 35, 78–83 (2008). [DOI] [PubMed] [Google Scholar]
- 54.Kenyon C, Colebunders R & Crucitti T The global epidemiology of bacterial vaginosis: a systematic review. Am. J. Obstet. Gynecol 209, 505–523 (2013). [DOI] [PubMed] [Google Scholar]
- 55.Lewis FM, Bernstein KT & Aral SO Vaginal microbiome and its relationship to behavior, sexual health, and sexually transmitted diseases. Obstet. Gynecol 129, 643–654 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gajer P et al. Temporal dynamics of the human vaginal microbiota. Sci. Transl Med 4, 132ra152 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Witkin SS et al. Influence of vaginal bacteria and D- and L-lactic acid isomers on vaginal extracellular matrix metalloproteinase inducer: implications for protection against upper genital tract infections. MBio 4, e00460–13 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Serrano MG et al. Racioethnic diversity in the dynamics of the vaginal microbiome during pregnancy. Nat. Med 25, 1001–1011 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nelson TM et al. Cigarette smoking is associated with an altered vaginal tract metabolomic profile. Sci. Rep 8, 852 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brotman RM et al. Association between cigarette smoking and the vaginal microbiota: a pilot study. BMC Infect. Dis 14, 471 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sabo MC et al. Association between vaginal washing and vaginal bacterial concentrations. PLoS One 14, e0210825 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brotman RM et al. A longitudinal study of vaginal douching and bacterial vaginosis — a marginal structural modeling analysis. Am. J. Epidemiol 168, 188–196 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thoma ME et al. Bacterial vaginosis is associated with variation in dietary indices. J. Nutr 141, 1698–1704 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Neggers YH et al. Dietary intake of selected nutrients affects bacterial vaginosis in women. J. Nutr 137, 2128–2133 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wilkinson EM, Herbst-Kralovetz MM & Brotman RM Clinical and personal lubricants alter cell viability, cytotoxicity and mucin production in human vaginal epithelial cell models. Am. J. Obstet. Gynecol 219, 638 (2018). [Google Scholar]
- 66.Muhleisen AL & Herbst-Kralovetz MM Menopause and the vaginal microbiome. Maturitas 91, 42–50 (2016). [DOI] [PubMed] [Google Scholar]
- 67.Romero R et al. The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome 2, 4 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Karstens L et al. Community profiling of the urinary microbiota: considerations for low-biomass samples. Nat. Rev. Urol 15, 735–749 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Perez-Munoz ME, Arrieta MC, Ramer-Tait AE & Walter J A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: implications for research on the pioneer infant microbiome. Microbiome 5, 48 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fang RL et al. Barcoded sequencing reveals diverse intrauterine microbiomes in patients suffering with endometrial polyps. Am. J. Transl Res 8, 1581–1592 (2016). [PMC free article] [PubMed] [Google Scholar]
- 71.Mitchell CM et al. Colonization of the upper genital tract by vaginal bacterial species in nonpregnant women. Am. J. Obstet. Gynecol. 212, 611.e1–611.e9 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Franasiak JM & Scott RT Jr. Reproductive tract microbiome in assisted reproductive technologies. Fertil. Steril 104, 1364–1371 (2015). [DOI] [PubMed] [Google Scholar]
- 73.Nelson DE et al. Characteristic male urine microbiomes associate with asymptomatic sexually transmitted infection. PLoS One 5, e14116 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nelson DE et al. Bacterial communities of the coronal sulcus and distal urethra of adolescent males. PLoS One 7, e36298 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dong Q et al. The microbial communities in male first catch urine are highly similar to those in paired urethral swab specimens. PLoS One 6, e19709 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Price LB et al. The effects of circumcision on the penis microbiome. PLoS One 5, e8422 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weng SL et al. Bacterial communities in semen from men of infertile couples: metagenomic sequencing reveals relationships of seminal microbiota to semen quality. PLoS One 9, e110152 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dawson SG, Ison CA, Csonka G & Easmon CS Male carriage of Gardnerella vaginalis. Br. J. Vener. Dis 58, 243–245 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kinghorn GR, Jones BM, Chowdhury FH & Geary I Balanoposthitis associated with Gardnerella vaginalis infection in men. Br. J. Vener. Dis 58, 127–129 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Olson KM, Boohaker LJ, Schwebke JR, Aslibekyan S & Muzny CA Comparisons of vaginal flora patterns among sexual behaviour groups of women: implications for the pathogenesis of bacterial vaginosis. Sex. Health 15, 61–67 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Muzny CA, Lensing SY, Aaron KJ & Schwebke JR Incubation period and risk factors support sexual transmission of bacterial vaginosis in women who have sex with women. Sex. Transm. Infect 95, 511–515 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vodstrcil LA et al. Incident bacterial vaginosis (BV) in women who have sex with women is associated with behaviors that suggest sexual transmission of BV. Clin. Infect. Dis 60, 1042–1053 (2015). [DOI] [PubMed] [Google Scholar]
- 83.Forcey DS et al. Factors associated with bacterial vaginosis among women who have sex with women: a systematic review. PLoS One 10, e0141905 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fouts DE et al. Integrated next-generation sequencing of 16S rDNA and metaproteomics differentiate the healthy urine microbiome from asymptomatic bacteriuria in neuropathic bladder associated with spinal cord injury. J. Transl Med. 10, 174 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hilt EE et al. Urine is not sterile: use of enhanced urine culture techniques to detect resident bacterial flora in the adult female bladder. J. Clin. Microbiol 52, 871–876 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brubaker L & Wolfe AJ The female urinary microbiota, urinary health and common urinary disorders. Ann. Transl Med 5, 34 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Carda-Dieguez M et al. Variations in vaginal, penile, and oral microbiota after sexual intercourse: a case report. Front. Med 6, 178 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Antonio MA, Rabe LK & Hillier SL Colonization of the rectum by Lactobacillus species and decreased risk of bacterial vaginosis. J. Infect. Dis 192, 394–398 (2005). [DOI] [PubMed] [Google Scholar]
- 89.El Aila NA et al. Identification and genotyping of bacteria from paired vaginal and rectal samples from pregnant women indicates similarity between vaginal and rectal microflora. BMC Infect. Dis 9, 167 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Plottel CS & Blaser MJ Microbiome and malignancy. Cell Host Microbe 10, 324–335 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Flores R et al. Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: a cross-sectional study. J. Transl Med 10, 253 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Baker JM, Al-Nakkash L & Herbst-Kralovetz MM Estrogen-gut microbiome axis: physiological and clinical implications. Maturitas 103, 45–53 (2017). [DOI] [PubMed] [Google Scholar]
- 93.Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7–14 June 1994. IARC Monogr. Eval. Carcinog. Risks Hum. 61, 1–241 (1994). [PMC free article] [PubMed] [Google Scholar]
- 94.Wang F, Meng W, Wang B & Qiao L Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. 345, 196–202 (2014). [DOI] [PubMed] [Google Scholar]
- 95.Welton JC, Marr JS & Friedman SM Association between hepatobiliary cancer and typhoid carrier state. Lancet 1, 791–794 (1979). [DOI] [PubMed] [Google Scholar]
- 96.Lecuit M et al. Immunoproliferative small intestinal disease associated with Campylobacter jejuni. N. Engl. J. Med. 350, 239–248 (2004). [DOI] [PubMed] [Google Scholar]
- 97.Cerroni L, Zochling N, Putz B & Kerl H Infection by Borrelia burgdorferi and cutaneous B-cell lymphoma. J. Cutan. Pathol 24, 457–461 (1997). [DOI] [PubMed] [Google Scholar]
- 98.Ferreri AJ et al. Chlamydophila psittaci eradication with doxycycline as first-line targeted therapy for ocular adnexae lymphoma: final results of an international phase II trial. J. Clin. Oncol 30, 2988–2994 (2012). [DOI] [PubMed] [Google Scholar]
- 99.Akram N et al. Oncogenic role of tumor viruses in humans. Viral Immunol. 30, 20–27 (2017). [DOI] [PubMed] [Google Scholar]
- 100.Garrett WS Cancer and the microbiota. Science 348, 80–86 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schwabe RF & Jobin C The microbiome and cancer. Nat. Rev. Cancer 13, 800–812 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kang M & Martin A Microbiome and colorectal cancer: unraveling host-microbiota interactions in colitis-associated colorectal cancer development. Semin. Immunol 32, 3–13 (2017). [DOI] [PubMed] [Google Scholar]
- 103.Sobhani I et al. Colorectal cancer-associated microbiota contributes to oncogenic epigenetic signatures. Proc. Natl Acad. Sci. USA 116, 24285–24295 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen J, Domingue JC & Sears CL Microbiota dysbiosis in select human cancers: evidence of association and causality. Semin. Immunol 32, 25–34 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rajagopala SV et al. The human microbiome and cancer. Cancer Prev. Res 10, 226–234 (2017). [DOI] [PubMed] [Google Scholar]
- 106.Fulbright LE, Ellermann M & Arthur JC The microbiome and the hallmarks of cancer. PLoS Pathog. 13, e1006480 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Moschen AR et al. Lipocalin 2 protects from inflammation and tumorigenesis associated with gut microbiota alterations. Cell Host Microbe 19, 455–469 (2016). [DOI] [PubMed] [Google Scholar]
- 108.Rubinstein MR et al. Fusobacterium nucleatum promotes colorectal cancer by inducing Wnt/betacatenin modulator Annexin A1. EMBO Rep. 20, e47638 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu N et al. Helicobacter pylori promotes angiogenesis depending on Wnt/beta-catenin-mediated vascular endothelial growth factor via the cyclooxygenase-2 pathway in gastric cancer. BMC Cancer 16, 321 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zadora PK et al. Integrated phosphoproteome and transcriptome analysis reveals Chlamydia-induced epithelial-to-mesenchymal transition in host cells. Cell Rep. 26, 1286–1302 e1288 (2019). [DOI] [PubMed] [Google Scholar]
- 111.Chase D, Goulder A, Zenhausern F, Monk B & Herbst-Kralovetz M The vaginal and gastrointestinal microbiomes in gynecologic cancers: a review of applications in etiology, symptoms and treatment. Gynecol. Oncol. 138, 190–200 (2015). [DOI] [PubMed] [Google Scholar]
- 112.Bray F et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin 68, 394–424 (2018). [DOI] [PubMed] [Google Scholar]
- 113.Siegel RL et al. Cancer statistics for Hispanics/Latinos, 2015. CA Cancer J. Clin 65, 457–480 (2015). [DOI] [PubMed] [Google Scholar]
- 114.Viens LJ et al. Human papillomavirus-associated cancers — United States, 2008–2012. Morb. Mortal. Wkly Rep 65, 661–666 (2016). [DOI] [PubMed] [Google Scholar]
- 115.Marsh M Original site of cervical carcinoma; topographical relationship of carcinoma of the cervix to the external os and to the squamocolumnar junction. Obstet. Gynecol 7, 444–452 (1956). [PubMed] [Google Scholar]
- 116.Herfs M et al. A discrete population of squamocolumnar junction cells implicated in the pathogenesis of cervical cancer. Proc. Natl Acad. Sci. USA 109, 10516–10521 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Walboomers JM et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol 189, 12–19 (1999). [DOI] [PubMed] [Google Scholar]
- 118.Shulzhenko N, Lyng H, Sanson GF & Morgun A Menage a trois: an evolutionary interplay between human papillomavirus, a tumor, and a woman. Trends Microbiol. 22, 345–353 (2014). [DOI] [PubMed] [Google Scholar]
- 119.Gravitt PE & Winer RL Natural history of HPV infection across the lifespan: role of viral latency. Viruses 9, E267 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ryser MD, Rositch A & Gravitt PE Modeling of US human papillomavirus (HPV) seroprevalence by age and sexual behavior indicates an increasing trend of HPV infection following the sexual revolution. J. Infect. Dis 216, 604–611 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Eldridge RC et al. Smoking and subsequent human papillomavirus infection: a mediation analysis. Ann. Epidemiol 27, 724–730.e721 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Castle PE et al. An association of cervical inflammation with high-grade cervical neoplasia in women infected with oncogenic human papillomavirus (HPV). Cancer Epidemiol. Biomarkers Prev. 10, 1021–1027 (2001). [PubMed] [Google Scholar]
- 123.Mhatre M et al. Cervical intraepithelial neoplasia is associated with genital tract mucosal inflammation. Sex. Transm. Dis 39, 591–597 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lehtinen M et al. Chlamydia trachomatis infection and risk of cervical intraepithelial neoplasia. Sex. Transm. Infect 87, 372–376 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mitra A et al. Cervical intraepithelial neoplasia disease progression is associated with increased vaginal microbiome diversity. Sci. Rep 5, 16865 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Audirac-Chalifour A et al. Cervical microbiome and cytokine profile at various stages of cervical cancer: a pilot study. PLoS One 11, e0153274 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ilhan ZE et al. Deciphering the complex interplay between microbiota, HPV, inflammation and cancer through cervicovaginal metabolic profiling. EBioMedicine 44, 675–690 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Watts DH et al. Effects of bacterial vaginosis and other genital infections on the natural history of human papillomavirus infection in HIV-1-infected and high-risk HIV-1-uninfected women. J. Infect. Dis 191, 1129–1139 (2005). [DOI] [PubMed] [Google Scholar]
- 129.Gillet E et al. Bacterial vaginosis is associated with uterine cervical human papillomavirus infection: a meta-analysis. BMC Infect. Dis 11, 10 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Guo YL, You K, Qiao J, Zhao YM & Geng L Bacterial vaginosis is conducive to the persistence of HPV infection. Int. J. STD AIDS 23, 581–584 (2012). [DOI] [PubMed] [Google Scholar]
- 131.Gao W, Weng J, Gao Y & Chen X Comparison of the vaginal microbiota diversity of women with and without human papillomavirus infection: a cross-sectional study. BMC Infect. Dis 13, 271 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lee JE et al. Association of the vaginal microbiota with human papillomavirus infection in a Korean twin cohort. PLoS One 8, e6351 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Brotman RM et al. Interplay between the temporal dynamics of the vaginal microbiota and human papillomavirus detection. J. Infect. Dis 210, 1723–1733 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Di Paola M et al. Characterization of cervicovaginal microbiota in women developing persistent high-risk Human Papillomavirus infection. Sci. Rep 7, 10200 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tuominen H, Rautava S, Syrjanen S, Collado MC & Rautava J HPV infection and bacterial microbiota in the placenta, uterine cervix and oral mucosa. Sci. Rep 8, 9787 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Oh HY et al. The association of uterine cervical microbiota with an increased risk for cervical intraepithelial neoplasia in Korea. Clin. Microbiol. Infect 21, 674.e1–674.e9 (2015). [DOI] [PubMed] [Google Scholar]
- 137.Brusselaers N, Shrestha S, Van De Wijgert J & Verstraelen H Vaginal dysbiosis, and the risk of human papillomavirus and cervical cancer: systematic review and meta-analysis. Am. J. Obstet. Gynecol. 21, 9–18.e8 (2018). [DOI] [PubMed] [Google Scholar]
- 138.Norenhag J et al. The vaginal microbiota, HPV and cervical dysplasia: a systematic review and network meta-analysis. BJOG 127, 171–180 (2020). [DOI] [PubMed] [Google Scholar]
- 139.Wang H et al. Associations of cervicovaginal lactobacilli with high-risk HPV infection, cervical intraepithelial neoplasia, and cancer: a systematic review and meta-analysis. J. Infect. Dis 220, 1243–1254 (2019). [DOI] [PubMed] [Google Scholar]
- 140.Mehta FF, Baik S & Chung SH Recurrence of cervical cancer and its resistance to progestin therapy in a mouse model. Oncotarget 8, 2372–2380 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Larmour LI et al. A patient derived xenograft model of cervical cancer and cervical dysplasia. PLoS One 13, e0206539 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Doorbar J Model systems of human papillomavirus-associated disease. J. Pathol 238, 166–179 (2016). [DOI] [PubMed] [Google Scholar]
- 143.Christensen ND, Budgeon LR, Cladel NM & Hu J Recent advances in preclinical model systems for papillomaviruses. Virus Res. 231, 108–118 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Herbst-Kralovetz MM, Pyles RB, Ratner AJ, Sycuro LK & Mitchell C New systems for studying intercellular interactions in bacterial vaginosis. J. Infect. Dis 214, S6–S13 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gilbert NM, Lewis WG & Lewis AL Clinical features of bacterial vaginosis in a murine model of vaginal infection with Gardnerella vaginalis. PLoS One 8, e59539 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Barrila J et al. Organotypic 3D cell culture models: using the rotating wall vessel to study host-pathogen interactions. Nat. Rev. Microbiol 8, 791–801 (2010). [DOI] [PubMed] [Google Scholar]
- 147.Gardner J et al. IL-36gamma is elevated in cervicovaginal epithelial cells in women with bacterial vaginosis and in vitro after infection with microbes associated with bacterial vaginosis. J. Infect. Dis 10.1093/infdis/jiz514 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Doerflinger SY, Throop AL & Herbst-Kralovetz MM Bacteria in the vaginal microbiome alter the innate immune response and barrier properties of the human vaginal epithelia in a species-specific manner. J. Infect. Dis 209, 1989–1999 (2014). [DOI] [PubMed] [Google Scholar]
- 149.Radtke AL, Quayle AJ & Herbst-Kralovetz MM Microbial products alter the expression of membrane-associated mucin and antimicrobial peptides in a three-dimensional human endocervical epithelial cell model. Biol. Reprod 87, 132 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hjelm BE, Berta AN, Nickerson CA, Arntzen CJ & Herbst-Kralovetz MM Development and characterization of a three-dimensional organotypic human vaginal epithelial cell model. Biol. Reprod 82, 617–627 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Łaniewski P, Gomez A, Hire G, So M & Herbst-Kralovetz MM Human three-dimensional endometrial epithelial cell model to study host interactions with vaginal bacteria and Neisseria gonorrhoeae. Infect. Immun 85, e01049–16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Radtke AL & Herbst-Kralovetz MM Culturing and applications of rotating wall vessel bioreactor derived 3D epithelial cell models. J. Vis. Exp 62, 3868 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.McGowin CL, Radtke AL, Abraham K, Martin DH & Herbst-Kralovetz M Mycoplasma genitalium infection activates cellular host defense and inflammation pathways in a 3-dimensional human endocervical epithelial cell model. J. Infect. Dis 207, 1857–1868 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Łaniewski P et al. Features of the cervicovaginal microenvironment drive cancer biomarker signatures in patients across cervical carcinogenesis. Sci. Rep 9, 7333 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Srinivasan S et al. Metabolic signatures of bacterial vaginosis. MBio 6, e00204–15 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pavlova NN & Thompson CB The emerging hallmarks of cancer metabolism. Cell Metab. 23, 27–47 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rauh-Hain JA et al. Racial disparities in treatment of high-grade endometrial cancer in the Medicare population. Obstet. Gynecol 125, 843–851 (2015). [DOI] [PubMed] [Google Scholar]
- 158.Chatterjee S, Gupta D, Caputo TA & Holcomb K Disparities in gynecological malignancies. Front. Oncol 6, 36 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Doll A et al. Novel molecular profiles of endometrial cancer-new light through old windows. J. Steroid Biochem. Mol. Biol 108, 221–229 (2008). [DOI] [PubMed] [Google Scholar]
- 160.Doll KM & Winn AN Assessing endometrial cancer risk among US women: long-term trends using hysterectomy-adjusted analysis. Am. J. Obstet. Gynecol 221, 318.e1–318.e9 (2019). [DOI] [PubMed] [Google Scholar]
- 161.Doll KM, Snyder CR & Ford CL Endometrial cancer disparities: a race-conscious critique of the literature. Am. J. Obstet. Gynecol 218, 474–482 e472 (2018). [DOI] [PubMed] [Google Scholar]
- 162.Allen NE et al. Endogenous sex hormones and endometrial cancer risk in women in the European Prospective Investigation into Cancer and Nutrition (EPIC). Endocr. Relat. Cancer 15, 485–497 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dossus L et al. Obesity, inflammatory markers, and endometrial cancer risk: a prospective case-control study. Endocr. Relat. Cancer 17, 1007–1019 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tilg H, Moschen AR & Kaser A Obesity and the microbiota. Gastroenterology 136, 1476–1483 (2009). [DOI] [PubMed] [Google Scholar]
- 165.Candela M et al. Inflammation and colorectal cancer when microbiota-host mutualism breaks. World J. Gastroenterol 20, 908–922 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Si J, You HJ, Yu J, Sung J & Ko G Prevotella as a hub for vaginal microbiota under the influence of host genetics and their association with obesity. Cell Host Microbe 21, 97–105 (2017). [DOI] [PubMed] [Google Scholar]
- 167.Choi S, Hwang YJ, Shin MJ & Yi H Difference in the gut microbiome between ovariectomy-induced obesity and diet-induced obesity. J. Microbiol. Biotechnol 27, 2228–2236 (2017). [DOI] [PubMed] [Google Scholar]
- 168.Cox-York KA et al. Ovariectomy results in differential shifts in gut microbiota in low versus high aerobic capacity rats. Physiol. Rep 3, e12488 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Shen J et al. Effects of low dose estrogen therapy on the vaginal microbiomes of women with atrophic vaginitis. Sci. Rep 6, 24380 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kwa M, Plottel CS, Blaser MJ & Adams S The intestinal microbiome and estrogen receptor-positive female breast cancer. J. Natl Cancer Inst 108, djw029 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Joshi AR & Ellenson LH in Molecular Genetics of Endometrial Carcinoma (ed Ellenson LH) 261–273 (Springer, 2017). [Google Scholar]
- 172.Torre LA et al. Ovarian cancer statistics, 2018. CA Cancer J. Clin 68, 284–296 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhou B et al. The biodiversity composition of microbiome in ovarian carcinoma patients. Sci. Rep 9, 1691 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Banerjee S et al. The ovarian cancer oncobiome. Oncotarget 8, 36225–36245 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Shanmughapriya S et al. Viral and bacterial aetiologies of epithelial ovarian cancer. Eur. J. Clin. Microbiol. Infect. Dis 31, 2311–2317 (2012). [DOI] [PubMed] [Google Scholar]
- 176.Chan PJ, Seraj IM, Kalugdan TH & King A Prevalence of mycoplasma conserved DNA in malignant ovarian cancer detected using sensitive PCR-ELISA. Gynecol. Oncol 63, 258–260 (1996). [DOI] [PubMed] [Google Scholar]
- 177.Emara MM et al. Synchronous occurrence of brucellosis and ovarian cancer — a case report. Austral. Asian J. Cancer 6, 257–259 (2016). [Google Scholar]
- 178.Pakish JB & Jazaeri AA Immunotherapy in gynecologic cancers: are we there yet? Curr. Treat. Options Oncol 18, 59 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Routy B et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 359, 91–97 (2018). [DOI] [PubMed] [Google Scholar]
- 180.Matson V et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 359, 104–108 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gopalakrishnan V et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 359, 97–103 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Colbert LE et al. The gut and cervical microbiome promote immune activation and response to chemoradiation in cervical cancer. Cancer Cell 10.2139/ssrn.3199993 (2018). [DOI] [Google Scholar]
- 183.Sivan A et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 350, 1084–1089 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Alexander JL et al. Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat. Rev. Gastroenterol. Hepatol 14, 356–365 (2017). [DOI] [PubMed] [Google Scholar]
- 185.Wilkinson EM, Ilhan ZE & Herbst-Kralovetz MM Microbiota-drug interactions: impact on metabolism and efficacy of therapeutics. Maturitas 112, 53–63 (2018). [DOI] [PubMed] [Google Scholar]
- 186.Kurita A et al. Streptomycin alleviates irinotecan-induced delayed-onset diarrhea in rats by a mechanism other than inhibition of beta-glucuronidase activity in intestinal lumen. Cancer Chemother. Pharmacol 67, 201–213 (2011). [DOI] [PubMed] [Google Scholar]
- 187.Vetizou M et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 350, 1079–1084 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kamada N, Seo SU, Chen GY & Nunez G Role of the gut microbiota in immunity and inflammatory disease. Nat. Rev. Immunol 13, 321–335 (2013). [DOI] [PubMed] [Google Scholar]
- 189.Wong S & Slavcev RA Treating cancer with infection: a review on bacterial cancer therapy. Lett. Appl. Microbiol 61, 107–112 (2015). [DOI] [PubMed] [Google Scholar]
- 190.Taneva E et al. Vaginal microbiome modulates topical antiretroviral drug pharmacokinetics. JCI Insight 3, 99545 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Thurman AR et al. Vaginal microbiota and mucosal pharmacokinetics of tenofovir in healthy women using tenofovir and tenofovir/levonorgestrel vaginal rings. PLoS One 14, e0217229 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Donahue Carlson R et al. The female genital tract microbiome is associated with vaginal antiretroviral drug concentrations in human immunodeficiency virus-infected women on antiretroviral therapy. J. Infect. Dis. 216, 990–999 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Vitali B et al. Vaginal microbiome and metabolome highlight specific signatures of bacterial vaginosis. Eur. J. Clin. Microbiol. Infect. Dis 34, 2367–2376 (2015). [DOI] [PubMed] [Google Scholar]
- 194.Maduro JH, Pras E, Willemse PH & de Vries EG Acute and long-term toxicity following radiotherapy alone or in combination with chemotherapy for locally advanced cervical cancer. Cancer Treat. Rev 29, 471–488 (2003). [DOI] [PubMed] [Google Scholar]
- 195.Berkey FJ Managing the adverse effects of radiation therapy. Am. Fam. Physician 82, 381–388, 394 (2010). [PubMed] [Google Scholar]
- 196.Morris L, Do V, Chard J & Brand AH Radiation-induced vaginal stenosis: current perspectives. Int. J. Womens Health 9, 273–279 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Lin XB et al. The role of intestinal microbiota in development of irinotecan toxicity and in toxicity reduction through dietary fibres in rats. PLoS One 9, e83644 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Brandi G et al. Intestinal microflora and digestive toxicity of irinotecan in mice. Clin. Cancer Res 12, 1299–1307 (2006). [DOI] [PubMed] [Google Scholar]
- 199.Secombe KR, Coller JK, Gibson RJ, Wardill HR & Bowen JM The bidirectional interaction of the gut microbiome and the innae immune system: Implications for chemotherapy-induced gastrointestinal toxicity. Int. J. Cancer 144, 2365–2376 (2019). [DOI] [PubMed] [Google Scholar]
- 200.Stringer AM et al. Biomarkers of chemotherapyinduced diarrhoea: a clinical study of intestinal microbiome alterations, inflammation and circulating matrix metalloproteinases. Support. Care Cancer 21, 1843–1852 (2013). [DOI] [PubMed] [Google Scholar]
- 201.Audeh MW et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet 376, 245–251 (2010). [DOI] [PubMed] [Google Scholar]
- 202.Liu Y, Meng J & Wang G Risk of selected gastrointestinal toxicities associated with poly (ADP-ribose) polymerase (PARP) inhibitors in the treatment of ovarian cancer: a meta-analysis of published trials. Drug. Des. Devel. Ther 12, 3013–3019 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Vida A, Kardos G, Kovacs T, Bodrogi BL & Bai P Deletion of poly(ADPribose) polymerase-1 changes the composition of the microbiome in the gut. Mol. Med. Rep 18, 4335–4341 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wallace BD et al. Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science 330, 831–835 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Touchefeu Y et al. Systematic review: the role of the gut microbiota in chemotherapy- or radiation-induced gastrointestinal mucositis — current evidence and potential clinical applications. Aliment. Pharmacol. Ther 40, 409–421 (2014). [DOI] [PubMed] [Google Scholar]
- 206.Carvalho R et al. Gut microbiome modulation during treatment of mucositis with the dairy bacterium Lactococcus lactis and recombinant strain secreting human antimicrobial PAP. Sci. Rep 8, 15072 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Acero Brand FZ et al. Severe immune mucositis and esophagitis in metastatic squamous carcinoma of the larynx associated with pembrolizumab. J. Immunother. Cancer 6, 22 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Bruner DW et al. Vaginal stenosis and sexual function following intracavitary radiation for the treatment of cervical and endometrial carcinoma. Int. J. Radiat. Oncol. Biol. Phys 27, 825–830 (1993). [DOI] [PubMed] [Google Scholar]
- 209.Mac Bride MB, Rhodes DJ & Shuster LT Vulvovaginal atrophy. Mayo Clin. Proc 85, 87–94 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Stahl JM et al. Extended duration of dilator use beyond 1 year may reduce vaginal stenosis after intravaginal high-dose-rate brachytherapy. Support. Care Cancer 27, 1425–1433 (2019). [DOI] [PubMed] [Google Scholar]
- 211.Decruze SB, Guthrie D & Magnani R Prevention of vaginal stenosis in patients following vaginal brachytherapy. Clin. Oncol 11, 46–48 (1999). [DOI] [PubMed] [Google Scholar]
- 212.Bai J, Jhaney I, Daniel G & Watkins Bruner D Pilot study of vaginal microbiome using QIIME 2 in women with gynecologic cancer before and after radiation therapy. Oncol. Nurs. Forum 46, E48–E59 (2019). [DOI] [PubMed] [Google Scholar]
- 213.Colman RJ & Rubin DT Fecal microbiota transplantation as therapy for inflammatory bowel disease: a systematic review and meta-analysis. J. Crohns Colitis 8, 1569–1581 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Gough E, Shaikh H & Manges AR Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin. Infect. Dis 53, 994–1002 (2011). [DOI] [PubMed] [Google Scholar]
- 215.Weiman S Harnessing the power of microbes as therapeutics: bugs as drugs. Report on an American Academy of Microbiology Colloquium held in San Diego, CA, in April 2014 (ed. Fox J) (American Society for Microbiology, 2015). [PubMed] [Google Scholar]
- 216.Biancheri P, Divekar D & Watson AJM Could fecal transplantation become part of PD-1-based immunotherapy, due to effects of the intestinal microbiome? Gastroenterology 154, 1845–1847 (2018). [DOI] [PubMed] [Google Scholar]
- 217.Wang Y, Ma R, Liu F, Lee SA & Zhang L Modulation of gut microbiota: a novel paradigm of enhancing the efficacy of programmed death-1 and programmed death ligand-1 blockade therapy. Front. Immunol 9, 374 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Alang N & Kelly CR Weight gain after fecal microbiota transplantation. Open Forum Infect. Dis 2, ofv004 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Weingarden AR et al. Microbiota transplantation restores normal fecal bile acid composition in recurrent Clostridium difficile infection. Am. J. Physiol. Gastrointest. Liver Physiol 306, G310–G319 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Cui M et al. Faecal microbiota transplantation protects against radiation-induced toxicity. EMBO Mol. Med 9, 448–461 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Hefazi M et al. Safety and efficacy of fecal microbiota transplant for recurrent Clostridium difficile infection in patients with cancer treated with cytotoxic chemotherapy: a single-institution retrospective case series. Mayo Clin. Proc 92, 1617–1624 (2017). [DOI] [PubMed] [Google Scholar]
- 222.Wardill HR, Secombe KR, Bryant RV, Hazenberg MD & Costello SP Adjunctive fecal microbiota transplantation in supportive oncology: emerging indications and considerations in immunocompromised patients. EBioMedicine 44, 730–740 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Javurek AB et al. Effects of exposure to bisphenol A and ethinyl estradiol on the gut microbiota of parents and their offspring in a rodent model. Gut Microbes 7, 471–485 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.van Baarlen P, Wells JM & Kleerebezem M Regulation of intestinal homeostasis and immunity with probiotic lactobacilli. Trends Immunol. 34, 208–215 (2013). [DOI] [PubMed] [Google Scholar]
- 225.Ngugi BM et al. Effects of bacterial vaginosis-associated bacteria and sexual intercourse on vaginal colonization with the probiotic Lactobacillus crispatus CTV-05. Sex. Transm. Dis 38, 1020–1027 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Hemmerling A et al. Phase 2a study assessing colonization efficiency, safety, and acceptability of Lactobacillus crispatus CTV-05 in women with bacterial vaginosis. Sex. Transm. Dis 37, 745–750 (2010). [DOI] [PubMed] [Google Scholar]
- 227.Stapleton AE et al. Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin. Infect. Dis 52, 1212–1217 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Marrazzo JM et al. Safety and efficacy of a novel vaginal anti-infective, TOL-463, in the treatment of bacterial vaginosis and vulvovaginal candidiasis: a randomized, single-blind, phase 2, controlled trial. Clin. Infect. Dis 68, 803–809 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Chavoustie SE, Gersten JK, Samuel MJ & Schwebke JR A phase 3, multicenter, prospective, open-label study to evaluate the safety of a single dose of secnidazole 2 g for the treatment of women and postmenarchal adolescent girls with bacterial vaginosis. J. Womens Health 27, 492–497 (2018). [DOI] [PubMed] [Google Scholar]
- 230.Lev-Sagie A et al. Vaginal microbiome transplantation in women with intractable bacterial vaginosis. Nat. Med 25, 1500–1504 (2019). [DOI] [PubMed] [Google Scholar]
- 231.DeLong K et al. Conceptual design of a universal donor screening approach for vaginal microbiota transplant. Front. Cell Infect. Microbiol 9, 306 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Scott AJ et al. International Cancer Microbiome Consortium consensus statement on the role of the human microbiome in carcinogenesis. Gut 68, 1624–1632 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Lokken EM et al. Association between vaginal washing and detection of Lactobacillus by culture and quantitative PCR in HIV-seronegative Kenyan women: a cross-sectional analysis. Sex Transm. Infect (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Vandeputte D et al. Quantitative microbiome profiling links gut community variation to microbial load. Nature 551, 507–511 (2017). [DOI] [PubMed] [Google Scholar]
- 235.Lengyel E et al. Epithelial ovarian cancer experimental models. Oncogene 33, 3619–3633 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]


