Abstract
Background
Procaspase-3 (PC-3) is overexpressed in multiple tumour types and procaspase-activating compound 1 (PAC-1) directly activates PC-3 and induces apoptosis in cancer cells. This report describes the first-in-human, phase I study of PAC-1 assessing maximum tolerated dose, safety, and pharmacokinetics.
Methods
Modified-Fibonacci dose-escalation 3 + 3 design was used. PAC-1 was administered orally at 7 dose levels (DL) on days 1–21 of a 28-day cycle. Dose-limiting toxicity (DLT) was assessed during the first two cycles of therapy, and pharmacokinetics analysis was conducted on days 1 and 21 of the first cycle. Neurologic and neurocognitive function (NNCF) tests were performed throughout the study.
Results
Forty-eight patients were enrolled with 33 completing ≥2 cycles of therapy and evaluable for DLT. DL 7 (750 mg/day) was established as the recommended phase 2 dose, with grade 1 and 2 neurological adverse events noted, while NNCF testing showed stable neurologic and cognitive evaluations. PAC-1’s t1/2 was 28.5 h after multi-dosing, and systemic drug exposures achieved predicted therapeutic concentrations. PAC-1 clinical activity was observed in patients with neuroendocrine tumour (NET) with 2/5 patients achieving durable partial response.
Conclusions
PAC-1 dose at 750 mg/day was recommended for phase 2 studies. Activity of PAC-1 in treatment-refractory NET warrants further investigation.
Clinical trial registration
Clinical Trials.gov: NCT02355535.
Subject terms: Drug development, Oncology
Background
Members of the caspase family of cysteine proteases are key players in both the initiation and execution of apoptosis, a programmed form of cell death important in both the development and maintenance of higher organisms. Critical to apoptosis is the proteolytic conversion of procaspase-3 (PC-3) to caspase-3; as both the intrinsic and extrinsic apoptotic pathways converge to activate PC-3, and as caspase-3 has over 200 cellular substrates [1, 2], the activation of PC-3 to caspase-3 is a pivotal and committed event in the apoptotic cascade. Intriguingly, PC-3 levels are overexpressed in cancerous lesions relative to adjoining normal tissues, suggesting an oncogenic role of PC-3 in the processes of malignant transformation and progression [3]. PC-3 levels are elevated in a variety of hematopoietic and solid tumours including glioma, meningioma, pancreatic cancer, breast cancer, colon cancer, lung cancer, lymphoma, leukaemia, multiple myeloma, melanoma, liver cancer, and many additional tumour histologies as recently reviewed [3]. As a consequence of broad PC-3 overexpression in a variety of cancer types, there is considerable clinical interest in the preferential targeting of PC-3 in tumour cells as an emerging targeted anticancer treatment strategy.
Because of the differential overexpression of PC-3 in certain tumour types, a PC-3 activating compound would be predicted to exert preferential killing of cancer cells compared with normal cells. Through the screening of a collection of ~20,000 small molecules for their ability to induce activation of PC-3 in vitro and apoptosis of cancer cells in culture, PAC-1 (procaspase-activating compound 1) was discovered to have such characteristics [4]. Structure-activity relationship studies reveal that the activity of PAC-1 in vitro and in cell culture is dependent on the presence of the ortho-hydroxy N-acyl hydrazone moiety [5], a functional group known to participate in metal chelation. Indeed, zinc is a powerful inhibitor of PC-3 enzymatic activity [6], and the mechanism by which PAC-1 activates PC-3 in vitro is through chelation of labile inhibitory zinc from PC-3, which allows PC-3 to process itself to the active form [5, 6]. This feed-forward mechanism of PAC-1 leading to PC-3 activation is operational in cell culture as well, as zinc from cellular labile pools have been shown to co-localise with PC-3 [7], and the consequent chelation of intracellular labile zinc pools by PAC-1 enhances PC-3 activity, leading to apoptosis [8]. This direct activation of PC-3 by PAC-1 has also been confirmed in elegant experiments using caspase-specific inhibitors [9], in detailed studies using caspase-specific substrates [10], in studies using Bax/Bak double knockout cell lines [11, 12], and in studies (using PAC-1 derivatives) in caspase-3/caspase-7 knockout cell lines [13].
PAC-1 has been widely used as a research tool for studying programmed cell death [9, 11, 12, 14–27], and this large body of literature has confirmed key aspects about the therapeutic potential of PAC-1, including its negligible toxicity to non-cancerous cells (IC50 values over 100 μM) [10, 28–32]. In addition, PAC-1 has demonstrated translational promise as a novel anticancer strategy in a variety of preclinical and naturally occurring tumour models. In preclinical rodent studies, PAC-1 alone or in combination with conventional cytotoxins or small molecule inhibitors, exerts substantive growth inhibitory and cytoreductive activities in both solid (lung carcinoma, melanoma, osteosarcoma) and hematopoietic cancers (lymphoma) [33–35]. Furthermore, PAC-1 demonstrates favourable oral bioavailability and pharmacokinetics in large mammals [36], and uniquely biodistributes into the central nervous system, exerting anticancer activities in rodent glioma models [37, 38]. Provocatively, through the inclusion of pet dogs with naturally-occurring cancers serving as sophisticated models for recalcitrant cancers in people, PAC-1 combined with chemotherapy or chemoradiotherapy has proven to be safe and clinically active in clinically-challenging tumour histologies including metastatic sarcoma, peripheral T cell lymphoma, high-grade astrocytoma, and meningioma [35, 37, 39].
These detailed cell culture and preclinical model studies suggest that PAC-1 has several attributes that warrant further investigation as a potentially safe and efficacious anticancer drug for humans. The primary objective of this study was to determine the tolerability of PAC-1; given PAC-1’s blood–brain barrier penetration properties in mice [8], neurological and neurocognitive symptoms of CNS toxicity were specifically assessed throughout the trial. The secondary objectives were to characterise toxicity of PAC-1, assess pharmacokinetics, and document preliminary clinical activity in heavily pretreated patients with advanced cancers.
Methods
Patients
In this multicentre phase I study, patients 18 years or older with advanced solid tumours or lymphoma no longer responding to standard anticancer therapies were recruited (Clinical Trials.gov, NCT02355535). Patients were required to have an Eastern Cooperative Oncology Group performance status <3, measurable or evaluable disease per Response Evaluation Criteria in Solid Tumours (RECIST) version 1.1 [40], and adequate haematologic, hepatic, and renal function. Main exclusion criteria included: any history of primary brain tumours, brain metastases, seizures, or underlying brain injury; >grade 1 peripheral neuropathy, or active infection. The study was conducted according to International Conference on Harmonisation of Good Clinical Practice guidelines and the principles of the Declaration of Helsinki. The protocol was approved by the Institutional Review Board/Ethics Committee at each study location. All patients provided written informed consent before enrolment. The complete study protocol is available in the Supplementary Materials and Methods.
Treatment plan
PAC-1 was given at the assigned dose orally on days 1-21 of a 28-day cycle. Disease reassessment was performed every two cycles (8 weeks). Treatment continued until disease progression, unacceptable toxicity, or patient refusal. In order to ensure accuracy in pharmacokinetic analysis findings, PAC-1 administration was withheld on day 2 of cycle 1.
Study design
This study consisted of dose escalation of single agent PAC-1. Up to nine dose levels were planned (75, 150, 250, 375, 450, 625, 750, 875, 1000 mg), and a total of seven doses were tested. MTD of PAC-1 was determined using a modified-Fibonacci dose-escalation 3 + 3 design. Three patients were initially enrolled into each dose level cohort, but additional patients were enrolled, as needed, to ensure that three patients completed two full treatment cycles and were evaluable for dose-limiting toxicity (DLT) assessment. Escalation to the next dose continued unless a patient experienced a DLT, at which time a cohort was expanded to up to six patients evaluable for DLT assessment and able to complete two full cycles of treatment.
To allow longer evaluation of possible neurological toxicity, this study was designed to assess DLT in first two cycles (56 days) of therapy. Serious adverse events other than neurological toxicity have been also carefully recorded throughout the treatment. Per protocol, patients who had progression of disease within first two cycles of therapy had to be replaced in order to allow for dose escalation.
A DLT was defined as one of the following treatment related events occurring during the first cycle with exception of neurological toxicity: (a) grade 3 or greater treatment-related haematologic toxicity for >48 h during the first cycle (28 days) of therapy; (b) cerebrovascular ischaemia or haemorrhage of any duration or grade; (c) grade 3 or greater treatment-related clinical non-haematological toxicity (excluding ≥grade 3 nausea, vomiting, or diarrhoea without maximal medical intervention and/or prophylaxis) during the first cycle (28 days) of therapy; (d) delay of cycle 2 treatment start by more than two weeks due to incomplete haematologic recovery (ANC > 1.5 × 109/L or platelets >100 × 109/L) or unresolved treatment-related grade 3 or greater non-haematologic toxicity; (e) grade 2 or greater treatment-related neurological toxicity occurring during the first two cycles of therapy and lasting more than 72 h. The highest dose level was to be expanded initially to a total of nine patients to assure safety. Up to six additional patients with unresectable pancreatic or other gastrointestinal neuroendocrine tumours could have been included to assess for initial signals of efficacy.
Evaluation of toxicity and response
Toxicity and adverse side effects were classified according to NCI’s Common Terminology Criteria for Adverse Events Version 4.0 (CTCAE v 4) [41] and assessed on day 1 of each cycle. Computed tomography of the chest, abdomen and pelvis was performed at the time of study enrolment and every 8 weeks of treatment to assess disease status. Standard clinical measures were used to assess response using RECIST version 1.1 [40].
Pharmacokinetics
For all dose cohorts, pharmacokinetics (PK) of PAC-1 were assessed following doses administered on days 1 and 21 of the first cycle. The dose on day 2 was withheld in order to accurately define the absorption and metabolism of PAC-1 in patients at 24, 32, and 48 h post drug administration. Blood samples for analysis were collected on the mornings of days 1 and 21 of cycle 1 immediately prior to oral ingestion of PAC-1 and at the following time points after ingestion: 0.5, 1, 2, 3, 4, 6, 8,12, 24, 32, and 48 h. Non-compartmental analysis of the PAC-1 plasma concentration-time data following doses on days 1 and 21 for cycle 1 for each dose cohort were performed using WinNonlin® 8.1 (Certara, L.P., Princeton, NJ).
Neurologic and neurocognitive tests
A neuropsychologist conducted neurological exams and tested neurocognitive abilities in subjects at baseline (screening), on day 1 of each cycle (except the first cycle), and 30 days after the final dose of PAC-1. The neurological assessment included the use of the Mini-Mental State Examination [42]. Neurocognitive function (NCF) evaluation consisted of the Hopkins Verbal Learning Test-Revised (HVLT-R) [43–45], the Trail Making Test Part A (TMTA) and Part B (TMTB) [46, 47], and the Controlled Oral Word Association test (COWA) [48, 49]. These three tests were recommended to assess cognitive functioning in patients with cancer by an International Cognition and Cancer Task Force [50]. All tests at all time points were independently scored and standardised scores (i.e., z-scores) were calculated. Rules were in place to either decrease PAC-1 dose or terminate the subject from the study if a certain degree of neurological/neurocognitive changes was detected during the examination relative to baseline functioning (see protocol for further explanation) [51–53]. Change in NCF was also evaluated using both the Reliable Change Index (RCI)-defined Decline approach and a neurocognitive adverse events (NCAE) z-score decline approach allowing for differences in degree of neurocognitive deterioration to be appreciated. In the interest of mapping changes in neurocognitive test scores onto the framework of the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE), Version 4.0, an ad hoc definition of Grade 1–3 NCAE was defined based on changes in z-score units—−1.00 to −1.99 (Grade 1, mild), −2.00 to −2.99 (Grade 2, moderate), and >−3.00 or new inability to complete test that the patient was previously able to complete (Grade 3, severe).
Immunohistochemistry
Immunohistochemical (IHC) staining was performed on formalin-fixed, paraffin-embedded (FFPE) tumour tissues using an indirect immunoperoxidase technique with diaminobenzidine (DAB) as the chromogen for PC-3 and IHC staining was performed using an autostainer (intelliPATH FLX, Biocare, Concord, CA). Processed slides were deparaffinized in xylene and rehydrated in alcohol. Endogenous peroxidase activity was blocked with Biocare PX968 Peroxidazed 1 at RT for 5 min, rinsed with TBS wash buffer, and then incubated for 10 min at RT with Biocare BP974 Background Punisher. Slides were incubated with PC-3 antibody (Abcam, ab32150) for 30 min, washed, and then incubated with HRP-Polymer (Biocare, RC542) for 30 min. Slides were washed with TBS, then the reaction was developed using DAB substrate for 5 min. Slides were counterstained with Mayer’s haematoxylin. Human lymph node served as an internal positive control.
Scoring of immunoreactivity data
Twenty-six archival tumour tissues from 24 patients were available for additional histologic evaluation. Tumour histologies included 14 epithelial malignancies (carcinoma, adenocarcinoma, cystadenocarcinoma), six neuroendocrine tumours (from five patients), and one each for the following tumour histologies—adamantinoma, hemangioendothelioma, melanoma, basal cell carcinoma, leiomyosarcoma, and carcinosarcoma. All 26 tumours were evaluated for PC-3 immunohistochemical staining briefly described above [38]. The use of human tumour samples in the research conducted was approved by the Human Subjects Institutional Review Board at three participating institutes, the University of Illinois at Chicago (Chicago, IL, USA), Johns Hopkins Kimmel Cancer Center (Baltimore, MD, USA) and Regions Hospital (Saint Paul, MN, USA). Samples were de-identified and assigned a 5-digit numerical designation.
Five hundred cells—or as many as were available—from each sample were graded by one observer (TMF), and the percentage of negative, faintly staining, moderately staining, and strongly staining cells were recorded. Negatively staining samples contained <10% positive cells. Cells that had <50% cytoplasmic staining were graded as “faintly stained”, those with >50% cytoplasmic staining were graded as “moderately stained”, and those with >50% cytoplasmic staining and in which nuclear detail was obscured by staining intensity were categorised as “strongly stained”.
Statistical analysis
The primary endpoint of this study was to establish MTD of PAC-1 using a modified-Fibonacci dose-escalation 3 + 3 design. The Maximum Tolerated Dose (MTD) would be defined as that dose of PAC-1 with DLT of <33% in first cycle of therapy or the first two cycles of therapy (neurological toxicity). The influence of different doses and single and multiple dosing (e.g., day 1 area under the plasma concentration-time curve from zero to infinity [AUC0–∞] vs. day 21 AUC24) on the pharmacokinetics of PAC-1 were evaluated using a mixed-effects generalised linear models approach. Natural logarithm (ln) transformations were done to AUC0–∞, AUC0–24, and maximum plasma concentration (Cmax) to normalise the data. Repeated-measures analysis of variance models or mixed-effects regression models were employed to evaluate dose effects and associations between dose levels and the pharmacokinetic measures. For clinical data, descriptive statistics were used to describe the study sample. Three clinical endpoints of interests, response (stable disease (SD) or partial response (PR)), progression-free survival, and overall survival times, were estimated using 95% confidence interval estimation. Bi-variate associations between demographic, disease or treatment factors and the clinical endpoints were tested using Chi-squared or Log Rank tests. All statistical tests were 2-sided, controlling for a probability of Type I error of 0.05. Statistical analyses were performed using Tibco Spotfire S+ version 8.2 and SAS software version 9.4.
Results
Patient characteristics
Thirty-three out of 48 patients received at least two cycles of PAC-1 treatment between March 2015 and September 2019. Demographics, baseline characteristics, and tumour types for all 48 patients are summarised in Table 1. The median age was 59 years (range, 31–81 years).
Table 1.
Characteristics of patients (N = 48).
| Age, median (range) | 59 (31, 81) |
| Sex (N, %) | |
| Female | 24 (50) |
| Male | 24 (50) |
| ECOG performance status, (N, %) | |
| 0 | 11 (23) |
| 1 | 30 (62) |
| 2 | 7 (15) |
| Race (N, %) | |
| White | 36 (75) |
| Black or African American | 9 (19) |
| Asian | 3 (6) |
| Ethnicity | |
| Hispanic or Latino | 6 (12) |
| Non-Hispanic | 42 (88) |
| Primary cancer type (N, %) | |
| Neuroendocrine tumour | 5 (11) |
| Colon cancer | 6 (13) |
| Pancreatic cancer | 6 (13) |
| Ovarian cancer | 6 (13) |
| Epithelioid hemangioendothelioma | 2 (4) |
| Melanoma | 2 (4) |
| Breast cancer | 3 (6) |
| Leiomyosarcoma | 2 (4) |
| Adamantinoma | 1 (2) |
| Parotid cancer | 1 (2) |
| Nasopharyngeal carcinoma | 1 (2) |
| Squamous cell cancer of tongue | 1 (2) |
| Endometrial cancer | 1 (2) |
| Cholangiocarcinoma | 2 (4) |
| Hepatocellular carcinoma | 2 (4) |
| Basal cell carcinoma | 1 (2) |
| Soft tissue sarcoma | 3 (6) |
| Lung cancer | 2 (4) |
| Prostate cancer | 1 (2) |
| Prior chemotherapy regimens (N, %) | |
| >3 | 38 (79) |
| <3 | 10 (21) |
Patient drop-out due to disease progression before completion of DLT period
One patient in DL 1 was removed from study and replaced because of disease progression in the first cycle of therapy. Similarly, in DL 2 one patient had to be replaced because of disease progression before end of cycle 2. In DL 3, three patients had to be replaced because they could not complete the second cycle of therapy due to disease progression. In DL 5, one patient had to be replaced because of disease progression before completion of cycle 2. In DL 6, three patients had to be replaced, because of disease progression before completion of cycle 2. In addition, this cohort was expanded to a total of 10 patients due to one episode of intracranial bleed that occurred during cycle 4. This event was assessed initially as possibly related to study drug, but later attributed to new brain metastasis. There were 17 patients treated in DL 7; 5 had to be replaced because of disease progression in the first 2 cycles of therapy. Six episodes of grade 2 dizziness, 2 episodes of grade 2 ataxia, and 1 episode of grade 3 ataxia were noted throughout treatment period at this level (750 mg/day), and although the MTD was not reached, the decision was made to not further dose escalate due to concern for potential increased toxicity with higher dose. DL 7 was expanded to nine patients to assure safety, and furthermore, to this expanded DL 7 cohort, an additional three patients were added with neuroendocrine tumours to assess preliminary efficacy in this specific tumour type. All six episodes of dizziness and ataxia observed in DL7 were transient and resolved without intervention before next day dose was due. No dose interruption or delay were necessary.
Safety and adverse events
The safety population consisted of 48 patients, with the median number of treatment cycles being two (range, 1 to 12). Drug-related adverse events did not result in any dose delays; however, they accounted for 4% (2/48) of treatment discontinuations. The most common cause of treatment discontinuation was disease progression, occurring in 96% (46/48) of patients recruited for study. The incidence of grade 3 adverse events related to drug occurred in 6% (3/48) of patients; one event of anaemia, one of headache, and one of ataxia. Ataxia was transient and, lasting a few hours after oral administration of PAC-1 and resolved without intervention. There were no recorded grade 4 toxicities. GI toxicity such as constipation, diarrhoea, abdominal pain, nausea, and vomiting all grades were reported with notable increased incidence in DL6 (20 events) and DL7 (13 events). No intervention was required. No grade 3 or 4 GI toxicity was reported. Table 2 shows all treatment-related events reported in the study.
Table 2.
Treatment-related adverse events.
| Treatment-related adverse events (all grades) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose level 1—75 mg | Dose level 2—150 mg | Dose level 3—250 mg | Dose level 4—375 mg | Dose level 5—450 mg | Dose level 6—625 mg | Dose level 7—750 mg | ||||||||
| N = 4 | N = 4 | N = 6 | N = 3 | N = 4 | N = 10 | N = 17 | ||||||||
| Toxicity | All grades | Grades 3/4 | All grades | Grades 3/4 | All grades | Grades 3/4 | All grades | Grades 3/4 | All grades | Grades 3/4 | All grades | Grades 3/4 | All grades | Grades 3/4 |
| Haematologic | ||||||||||||||
| Anaemia | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 1 | 0 |
| Non-haematologic | ||||||||||||||
| Hypokalaemia | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Constipation | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| Fatigue | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 7 | 0 |
| Chills | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Flushing | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Diarrhoea | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 |
| Atrial flutter | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Abdominal pain | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 1 | 0 |
| Nausea | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 0 | 5 | 0 |
| Vomiting | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 0 | 6 | 0 |
| Myalgia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Dizziness | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 0 | 6 | 0 |
| Somnolence | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Alopecia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Weight loss | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Anorexia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 |
| Ataxia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 1 |
| Headache | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 1 |
| Insomnia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Tremor | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Peripheral sensory neuropathy | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 |
| Generalised muscle weakness | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Nervous system disorder—other | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 0 |
Pharmacokinetics
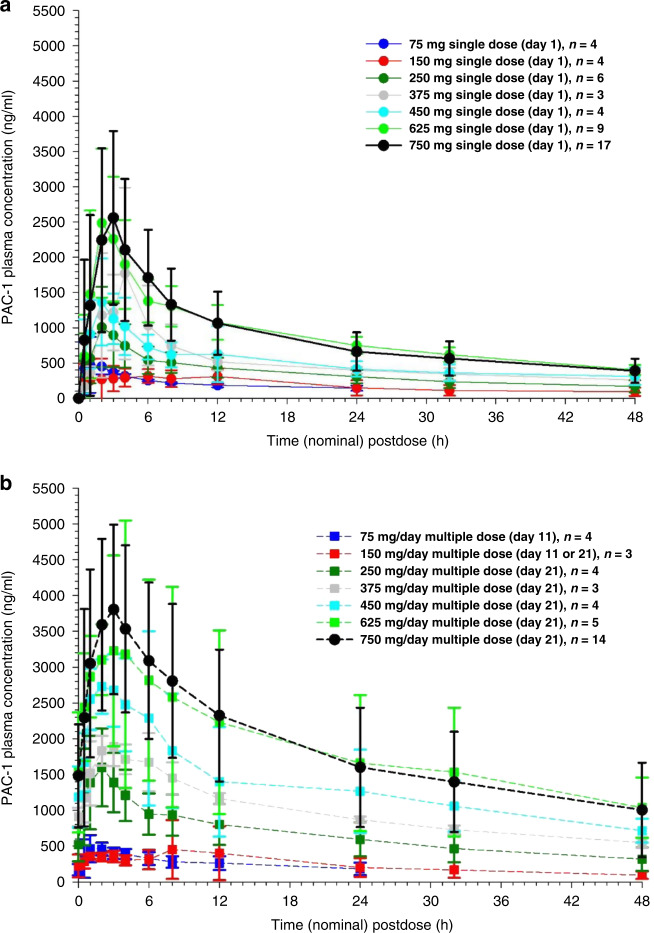
Following oral administration, PAC-1’s maximum concentration (Cmax) generally occurred between 1 and 4 h, and PAC-1 plasma concentrations declined in a bi-exponential fashion. Mean PAC-1 plasma concentrations showed minimal change between DL 1 (75 mg/day) and DL 2 (150 mg/day). In contrast, PAC-1 concentrations increased almost proportionally with dosage at subsequent dose levels. Figure 1a, b contrast the mean PAC-1 plasma concentration versus time curves among the seven dose levels following single- and multiple-dose administration.
Fig. 1. Pharmacokinetics of PAC-1.
a PAC-1 plasma concentration-time profiles following a single oral dose of 75, 150, 250, 375, 450, 625, or 750 mg (mean ± standard deviation). b PAC-1 plasma concentration-time profiles following a multiple oral dose of 75, 150, 250, 375, 450, 625, or 750 mg administered daily for 11 or 21 days (mean ± standard deviation).
The pharmacokinetics of PAC-1 appeared similar with single- and multiple-dose administration. Single- versus multiple-dose administration and dose had minimal influence on the elimination half-life (t1/2-λ). The mean t1/2-λz across dose groups was 25.6 h following single-dose administration and 28.5 h following multiple-dose administration. Among dose groups, the t1/2-λz ranged from 13.4 to 30.6 h at DL 1 (75 mg/day), from 9.5 to 28.0 h at DL 2 (150 mg/day), from 17.5 to 41.4 h at DL 3 (250 mg/day), from 17.9 to 42.0 h at DL 4 (375 mg/day), from 22.9 to 56.9 h at DL 5 (450 mg/day), from 17.4 to 41.4 h at DL 6 (625 mg/day) and from 11.9 to 60.3 h at DL7 (750 mg/day). Median Tmax was identical, 2.0 h, with single-dose and multiple-dose administration. Similarly, there was no significant difference in PAC-1 apparent clearance (CL/F) among dosage groups (p = 0.227) and single- versus multiple-dose administration (p = 0.613).
Treatment efficacy and duration
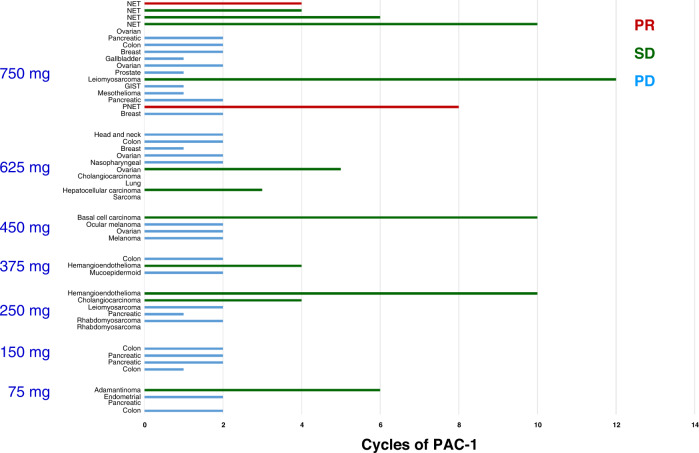
Disease response for all 48 patients evaluated by RECIST is summarised in Fig. 2 and includes SD across diverse tumour histologies including metastatic refractory ovarian cancer (5 months), two subjects with hemangioendothelioma (4 and 10 months), hepatocellular cancer (3 months), cholangiocarcinoma (4 months), adamantinoma (6 months), and metastatic to lung basal cell carcinoma (10 months). The longest SD of 12 months was achieved in a patient with chemotherapy-resistant leiomyosarcoma.
Fig. 2. Duration of therapy and response to treatment at different dose levels.
Responses were assessed in accordance with RECIST v1.1. PD progressive disease, SD stable disease, PR partial response, NET neuroendocrine tumour.
Out of five patients with NET enrolled in the trial, two had PR and three patients has SD. In one patient with pancreatic NET metastatic to the liver, which previously progressed on sunitinib and subsequently on an experimental immunotherapy agent prior to PAC-1 treatment, tumour burden was reduced by 64.3% (as assessed by RECIST 1.1). In a second patient with an ileal NET metastatic to the mesentery that previously progressed on sunitinib and octreotide, and subsequently on everolimus and octreotide, the tumour decreased in size by 36%. Furthermore, SD was achieved in three additional patients with NET (Fig. 2 and Table 3), with longest disease control of 10 months in a patient whose gastrointestinal NET that had metastasised to the peritoneal cavity and had progressed on cabozantinib, lanreotide, and single-agent everolimus.
Table 3.
PAC-1 activity in pancreatic and other gastrointestinal NETs.
| NET type | Metastases | Grade | Ki-67 (%) | Prior therapy #1 | Duration #1 | Reason to stop therapy | BR to #1 therapy | Prior therapy #2 | Duration #2 | Reason to stop therapy | BR to #2 therapy | Duration of PAC-1 | Reason to stop therapy | BR on PAC-1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pancreatic | Liver | 1 | 1 | Sunitinib | 4.5 | PD (283%) | 16% | Immunotherapy | 2 | Toxicity | −9% | 8 | PD | −64.3% |
| Gastrointestinal | Peritoneal | 2 | 7 | Cabozantinib/lanreotide | 5.5 | Anorexia, nausea | 6% | Everolimus | 3 | PD | 12% | 10 | PD | –2.5% |
| Colon | Liver | 1 | 1 | Everolimus | 5.5 | Nausea, dizziness | 17% | 6 | PD | 6% | ||||
| Pancreatic | Liver | 2 | 10 | Everolimus | 6 | PD | 24% | Sunitinib | 5 | PD | 15% | 4 | PD | 3.9% |
| Ileal | Mesenteric | 2 | 5 | Sunitinib/octreotide | 1.5 | PD | 28% | Everolimus/octreotide | 2 | Renal toxicity | 14% | 4 | Intestinal obstruction | −36% |
BR best response, NET neuroendocrine tumour, PAC-1 first procaspase-activating compound, PD progressive disease.
Procaspase-3 expression
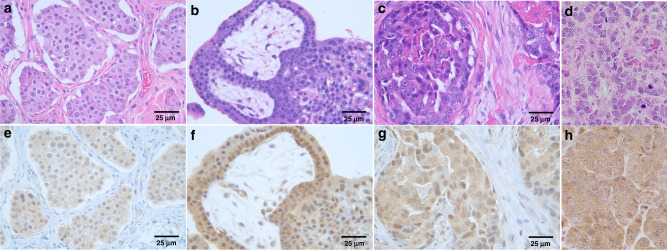
Twenty-six unique tumour samples were characterised for PC-3 expressions by immunohistochemistry. Positive immunoreactivity for PC-3 was identified in the majority of tumours, with 25/26 (96%) demonstrating either faint (4/26), moderate (18/26), or strong (3/26) immunostaining intensities. Only 1 tumour sample, a non-small cell lung carcinoma, was categorised as negative, based upon <10% cell positivity for PC-3. Moderate PC-3 immunoreactivity was identified in all NET samples and aggregate PC-3 immunoreactivity across different tumour histologies are represented in Fig. 3.
Fig. 3. Procaspase-3 (PC-3) expressions in diverse tumour histologies categorised as moderate-to-strong immunostaining intensities.
Paired H&E and PC-3 from a, e metastatic neuroendocrine tumour, b, f metastatic hemangioendothelioma, c, g ductal breast carcinoma, and d, h carcinosarcoma. Magnification ×400.
Neuro-cognitive studies
Thirty-eight patients were evaluable by longitudinal neurological and NCF test results with 32 patients completing at least two cycles of treatment. One patient in DL1 performed the tests in Korean and they were removed from the final analysis to avoid misinterpretation. On average, patients entered the study with NCF impairments on TMTB (i.e., executive function). The degree and distribution of NCF impairment across NCF tests/domains varied between the dose levels with the most consistent areas of cognitive impairment observed on TMTB. The mean for standardised scores for each test did not suggest global neurocognitive dysfunction over time. Between patient variability was observed, yet this was not clearly associated with either dose or time on study drug. NCAEs of any grade occurred at all dose levels with no clear relationship to dose. Grade 3 NCAEs were uncommon and ranged from 0-3% across study visits for all DLs except DL 5 where the proportion was 5%. NCAEs of any grade were most common on the HVLT-R DR and RECOG (i.e., memory) and TMTB. Grade 3 NCAEs were most common on the TMTB. Of the 9 grade 3 NCAEs during treatment, there was no further follow-up testing to evaluate persistence or resolution of the NCAE in 4 cases. In the other five cases, three improved in the severity on follow-up testing and two exhibited persistent grade 3 NCAE. No dose reduction or treatment discontinuation was made due to change in neurocognitive function as it was not attributed to the study drug, but to symptoms of cancer burden. Attributions of these events were also performed in a multidisciplinary way with input from neuro-oncologists on the team, based on timing and overall patient clinical condition.
Discussion
Programmed cell death is essential for normal tissue development and maintenance of multicellular organisms and is orchestrated through the complex interplay among proteins with counter-regulatory activities, i.e., pro-apoptotic vs. anti-apoptotic. Despite its universality in health, dysfunction of the apoptotic cascade is a well-recognised feature of tumour cells and contributes to cancer recurrence, relapse, and metastasis despite treatment with cytotoxic therapies. An alternative and direct activation of cancer cell apoptosis strategy was evaluated in this clinical trial whereby the first-in-human phase 1 trial with PAC-1, a small molecule activator of PC-3, was investigated in heavily pretreated cancer patients.
Given the pan-expression of PC-3 by all nucleated eukaryote cells, the druggability of PC-3 with PAC-1 as investigated in this clinical trial was essential to assess toxicity in human subjects with terminal pathologies. Derived from protein expression studies in normal tissues, PC-3 is basally present in higher amounts in epithelial tissues of gastrointestinal, respiratory, reproductive, and urinary tract origin, as well as in bone marrow hematopoietic precursors and primary/secondary lymphoid tissues (Human Protein Atlas). Based upon these basal expression patterns of PC-3 in normal tissues, indiscriminate and wide scale PC-3 activation could result in toxicities. However, given the role of PC-3 in oncogenesis and the consequent paradoxical overexpression of PC-3 by most cancers studied to date [3], the possible preferential activation of PC-3 in cancer cells was envisioned, with minimal on-target, off-tumour toxicity. Indeed, data from this study demonstrate that oral PAC-1 was safe in patients with advanced clinical stage diseases, even when drug levels in serum were substantial as judged by the pharmacokinetic analysis. Other than one episode of grade 3 anaemia at dose 150 mg/day, one episode of grade 3 headache at dose of 750 mg/day, and one episode of grade 3 ataxia at 750 mg/day dose, PAC-1 was safe when given daily for multiple cycles of therapy. This study was designed to assess dose limiting toxicities during first two cycles of therapy. This strategy permitted longer observation of patients for neurological toxicity before PAC-1 dose escalation in next cohort was allowed.
Based upon its chemical structure and lipophilicity, in silico modelling predicts that PAC-1 can rapidly traverse the blood-brain barrier with entry into the central nervous system (CNS) compartment, with experimental data generated in mice supporting the penetration of PAC-1 into the brain parenchyma [8]. While the CNS penetrant properties of PAC-1 have favoured the positive management of CNS malignancies in murine and spontaneous large animal models [37–39], as well as remain the focus of a current clinical trial for recurrent GBM (NCT03332355), unintended neurotoxicity as a consequence of PC-3 activation remains a possibility in patients receiving PAC-1. In the current phase 1 study, five episodes of grade 2 and one episode of grade 3 ataxia were experienced by patients in the highest evaluated DL (750 mg/day); however, these neurologic perturbations were transient and completely reversible. Furthermore, onset and severity of symptoms did not appear to be cumulative, and serial cognitive and neurologic function evaluations performed throughout treatment did not demonstrate any impact of PAC-1 on these functions. Grade 1 and 2 neurological adverse events of dizziness and hallucinations were also noted at 750 mg/day dose; however, none of the effects observed at this dose constituted a MTD. The dose-dependent nature of these effects, and the very high drug serum levels of PAC-1 at 750 mg, suggested that maximal patient benefit and safety ratio would be achieved at this dose. Therefore, further dose escalation was stopped, and 750 mg/day was recommended dose for phase 2 study.
Extensive neurologic and neurocognitive tests including MMSE, HVLT, TMTA and TMTB, and COWA were performed in this phase 1 trial with a unique approach of ad hoc definition of neurocognitive toxicity based on changes in z score units. While no neurocognitive toxicity was identified in this study related to PAC-1 administration, this novel approach could be applied to other phase 1 trials of drugs with potential neurocognitive toxicities.
The dose schedule of PAC-1 utilised in this study produced favourable pharmacokinetics. PAC-1 reached maximum plasma concentrations within 1 to 4 h following oral administration with achievement of steady-state with multiple dosing. The half-life of PAC-1 ranged from 9.5 to 60.3 h, depending upon DL, and these findings support the selection of once-a-day oral dosing. Importantly, at the recommended phase 2 dose of 750 mg/day, trough levels of PAC-1 were sustained at low μg/mL concentrations, which are predicted to activate apoptosis based upon expansive cell culture data of PAC-1 alone or in combination with other cytotoxins [34, 35, 37]. Collectively, the clinical use of PAC-1 was favourably supported by observed dose-proportionality with multiple-dose administration, predictable drug accumulation with multiple dosing, and acceptable absorption and elimination profiles.
Single-agent activity resulting in partial responses as per RECIST 1.1 [40] was seen in a patient with grade 1 pancreatic NET (64.3% reduction in target lesion) and a patient with grade 2 ileal NET (36% reduction in target lesion). Furthermore, three additional NET patients enrolled in the DL7 cohort (750 mg PAC-1) achieved disease stabilisation as best response lasting 4, 6, and 10 months in duration. All five NET patients had failed prior therapies preceding enrolment into the phase 1 trial with PAC-1, and these intriguing data clinically may justify further testing of PAC-1 alone or in combination (i.e., sunitinib) [54] in the setting of treatment-refractory NETs. In addition to provocative findings for NET patients, prolonged SD of 12 months was achieved in one patient with treatment-refractory leiomyosarcoma with 750 mg/day PAC-1, and in two patients with epithelial hemangioendotheliomas treated with 250 mg/day and 375 mg/day of PAC-1 for 10 and 4 months, respectively. Based upon these three patients, further inquiry of PAC-1’s activity in sarcomas is warranted, and further clinical justification for this exploration is supported by demonstrated activity of PAC-1 in combination with doxorubicin in pet dogs diagnosed with metastatic sarcoma [35].
A reliable biomarker that can accurately predict response to any targeted therapy would allow for a personalised medicine approach whereby cancer patients could be stratified for treatment and receive the most effective drug or drug combination. For PAC-1, existent data would indicate that relative PC-3 expressions can contribute to drug sensitivity and could aid in response predictions. However, as it is known that inhibitors of apoptotic proteins can inhibit proteolytically active caspase-3, sole reliance upon PC-3 expressions for predicting activity of PAC-1 is likely incomplete. In this study, PC-3 was expressed in the majority of available tumour tissues (25/26), and qualitatively enriched in NETs and gastrointestinal solid tumours. However, correlation of PC-3 expression levels with observed clinical responses was not performed in the context of this phase 1 trial, given the intent of the study and the number of confounding variables including limited sample population, different DLs evaluated and cumulative drug exposures, and diverse tumour histologies.
In summary, phase 2 recommended dose of PAC-1 was defined, and PAC-1 was found to be safe in heavily pretreated patients with advanced cancers and on-target/off-tumour toxicity did not cause any unexpected or irreversible adverse effects. While preliminary clinical activity of single-agent PAC-1 was identified in this phase 1 trial, existent preclinical data suggests that PAC-1 can be rationally and synergistically combined with cytotoxic [35, 37, 39] or targeted agents [34, 55]. Coupled with the provocative responses in NET and sarcoma histologies observed in this phase 1 trial, future phase 2 clinical studies could evaluate rationally designed combinatorial strategies inclusive of PAC-1 for the management of neuroendocrine and sarcoma tumour histologies.
Supplementary information
Acknowledgements
We thank James P. Zacny, PhD for his editorial support with the clinical protocol and manuscript. LC-MS/MS was performed at the Toxicology Research Laboratory, College of Medicine at the University of Illinois at Chicago. We thank the patients who participated and the Clinical Trials Office of the University of Illinois Cancer Center for providing the necessary clinical research infrastructure to execute this study.
Author contributions
AZD and OCD designed the study. OCD, MH, RAP, NKV, and MJR acquired the data. OCD, MH, RAP, and NKV provided RECIST assessments. JHF did the pharmacokinetic testing. LCL did the statistical analysis. AZD, PJH, TMF, TMT, and OCD provided data analysis. AZD and OCD prepared the initial draft of the manuscript. Revisions were prepared by AZD, PJH, TMF, TMT, and OCD. All authors contributed to subsequent manuscript drafts and agreed with the submission of the manuscript for publication.
Funding
Financial support was provided by Vanquish Oncology. Inc., the University of Illinois Cancer Center, the University of Illinois, the NIH (R01CA120439), and Engdahl Family Foundation.
Data availability
The data generated in this study are available upon request from the corresponding author.
Competing interests
PJH serves as Chief Scientific Officer and has equity in Vanquish Oncology, Inc. TMT serves as Chief Executive Officer and has equity in Vanquish Oncology, Inc. TMF serves as VP Preclinical Development and has equity in Vanquish Oncology, Inc. AZD reports research funding from Eli Lilly. AZD served as Chief Medical Officer for Vanquish Oncology, Inc. AZD serves as Chief Executive Officer for TTC Oncology, LLC, and Chief Medical Officer for IGF Oncology. All other authors declare no competing interests.
Ethics approval and consent to participate
The study was conducted according to International Conference on Harmonisation of Good Clinical Practice guidelines and the principles of the Declaration of Helsinki. The protocol was approved by the Institutional Review Board/Ethics Committee at each study location. All patients provided written informed consent before enrolment.
Consent for publication
Not applicable.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-022-02089-7.
References
- 1.Dix MM, Simon GM, Cravatt BF. Global mapping of the topography and magnitude of proteolytic events in apoptosis. Cell. 2008;134:679–91. doi: 10.1016/j.cell.2008.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahrus S, Trinidad JC, Barkan DT, Sali A, Burlingame AL, Wells JA. Global sequencing of proteolytic cleavage sites in apoptosis by specific labeling of protein N termini. Cell. 2008;134:866–76. doi: 10.1016/j.cell.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boudreau MW, Peh J, Hergenrother PJ. Procaspase-3 overexpression in cancer: a paradoxical observation with therapeutic potential. ACS Chem Biol. 2019;14:2335–48. doi: 10.1021/acschembio.9b00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Putt KS, Chen GW, Pearson JM, Sandhorst JS, Hoagland MS, Kwon JT, et al. Small-molecule activation of procaspase-3 to caspase-3 as a personalized anticancer strategy. Nat Chem Biol. 2006;2:543–50. doi: 10.1038/nchembio814. [DOI] [PubMed] [Google Scholar]
- 5.Peterson QP, Hsu DC, Goode DR, Novotny CJ, Totten RK, Hergenrother PJ. Procaspase-3 activation as an anti-cancer strategy: structure-activity relationship of procaspase-activating compound 1 (PAC-1) and its cellular co-localization with caspase-3. J Med Chem. 2009;52:5721–31. doi: 10.1021/jm900722z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peterson QP, Goode DR, West DC, Ramsey KN, Lee JJY, Hergenrother PJ. PAC-1 activates procaspase-3 in vitro through relief of zinc-mediated inhibition. J Mol Biol. 2009;388:144–58. doi: 10.1016/j.jmb.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Truong-Tran AQ, Grosser D, Ruffin RE, Murgia C, Zalewski PD. Apoptosis in the normal and inflamed airway epithelium: role of zinc in epithelial protection and procaspase-3 regulation. Biochem Pharm. 2003;66:1459–68. doi: 10.1016/S0006-2952(03)00498-2. [DOI] [PubMed] [Google Scholar]
- 8.West DC, Qin Y, Peterson QP, Thomas DL, Palchaudhuri R, Morrison KC, et al. Differential effects of procaspase-3 activating compounds in the induction of cancer cell death. Mol Pharm. 2012;9:1425–34. doi: 10.1021/mp200673n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Putinski C, Abdul-Ghani M, Stiles R, Brunette S, Dick SA, Fernando P, et al. Intrinsic-mediated caspase activation is essential for cardiomyocyte hypertrophy. Proc Natl Acad Sci USA. 2013;110:E4079–87. doi: 10.1073/pnas.1315587110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang F, Wang L, Zhao Y, Li Y, Ping G, Xiao S, et al. A novel small-molecule activator of procaspase-3 induces apoptosis in cancer cells and reduces tumor growth in human breast, liver and gallbladder cancer xenografts. Mol Oncol. 2014;8:1640–52. doi: 10.1016/j.molonc.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seervi M, Sobhan PK, Mathew KA, Joseph J, Pillai PR, Santhoshkumar TR. A high-throughput image-based screen for the identification of Bax/Bak-independent caspase activators against drug-resistant cancer cells. Apoptosis. 2014;19:269–84. doi: 10.1007/s10495-013-0921-8. [DOI] [PubMed] [Google Scholar]
- 12.Monaco G, Decrock E, Akl H, Ponsaerts R, Vervliet T, Luyten T, et al. Selective regulation of IP3-receptor-mediated Ca2+ signaling and apoptosis by the BH4 domain of Bcl-2 versus Bcl-Xl. Cell Death Differ. 2012;19:295–309. doi: 10.1038/cdd.2011.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel V, Balakrishnan K, Keating MJ, Wierda WG, Gandhi V. Expression of executioner procaspases and their activation by a procaspase-activating compound in chronic lymphocytic leukemia cells. Blood. 2015;125:1126–36. doi: 10.1182/blood-2014-01-546796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao HJ, Liu T, Mao X, Han S, Liang R, Hui L, et al. Fructus phyllanthi tannin fraction induces apoptosis and inhibits migration and invasion of human lung squamous carcinoma cells in vitro via MAPK/MMP pathways. Acta Pharmacol Sin. 2015;36:758–68. doi: 10.1038/aps.2014.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma J, Bao G, Wang L, Li W, Xu B, Du B, et al. Design, synthesis, biological evaluation and preliminary mechanism study of novel benzothiazole derivatives bearing indole-based moiety as potent antitumor agents. Eur J Med Chem. 2015;96:173–86. doi: 10.1016/j.ejmech.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 16.Chakkath T, Lavergne SN, Fan TM, Bunick D, Dirikolu L. Preliminary metabolism of lomustine in dogs and comparative cytotoxicity of lomustine and its major metabolites in canine cells. Vet Sci. 2014;1:159–73. doi: 10.3390/vetsci1030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo WT, Wang XW, Yan YL, Li YP, Yin X, Zhang Q, et al. Suppression of epithelial-mesenchymal transition and apoptotic pathways by miR-294/302 family synergistically blocks let-7-induced silencing of self-renewal in embryonic stem cells. Cell Death Differ. 2015;22:1158–69. doi: 10.1038/cdd.2014.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu J, Li Z, Wang J, Chen H, Fang JY. Combined PTEN mutation and protein expression associate with overall and disease-free survival of glioblastoma patients. Transl Oncol. 2014;7:196–205. doi: 10.1016/j.tranon.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campbell DS, Okamoto H. Local caspase activation interacts with Slit-Robo signaling to restrict axonal arborization. J Cell Biol. 2013;203:657–72. doi: 10.1083/jcb.201303072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu H, Sun G, Dong J, Fei L. The role of PRRX1 in the apoptosis of A549 cells induced by cisplatin. Am J Transl Res. 2017;9:396–402. [PMC free article] [PubMed] [Google Scholar]
- 21.Dong T, Zhang Q, Hamblin MR, Wu MX. Low-level light in combination with metabolic modulators for effective therapy of injured brain. J Cereb Blood Flow Metab. 2015;35:1435–44. doi: 10.1038/jcbfm.2015.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dick SA, Chang NC, Dumont NA, Bell RAV, Putinski C, Kawabe Y, et al. Caspase 3 cleavage of Pax7 inhibits self-renewal of satellite cells. Proc Natl Acad Sci USA. 2015;112:E5246–52. doi: 10.1073/pnas.1512869112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu L, Zeng Z, Zhang W, Ren G, Ling X, Huang F, et al. RXRα ligand Z-10 induces PML-RARα cleavage and APL cell apoptosis through disrupting PML-RARα/RXRα complex in a cAMP-independent manner. Oncotarget. 2017;8:12311–22. doi: 10.18632/oncotarget.14812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Namjoshi P, Showalter L, Czerniecki BJ, Koski GK. T-helper 1-type cytokines induce apoptosis and loss of HER-family oncodriver expression in murine and human breast cancer cells. Oncotarget. 2019;10:6006–20. doi: 10.18632/oncotarget.10298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Auner HW, Beham-Schmid C, Dillon N, Sabbattini P. The life span of short-lived plasma cells is partly determined by a block on activation of apoptotic caspases acting in combination with endoplasmic reticulum stress. Blood. 2010;116:3445–55. doi: 10.1182/blood-2009-10-250423. [DOI] [PubMed] [Google Scholar]
- 26.Wang H, Boussouar A, Mazelin L, Tauszig-Delamasure S, Sun Y, Goldschneider D, et al. The proto-oncogene c-Kit inhibits tumor growth by behaving as a dependence receptor. Mol Cell. 2018;72:413–25. doi: 10.1016/j.molcel.2018.08.040. [DOI] [PubMed] [Google Scholar]
- 27.Yosefzon Y, Soteriou D, Feldman A, Kostic L, Koren E, Brown S, et al. Caspase-3 regulates YAP-dependent cell proliferation and organ size. Mol Cell. 2018;70:573–87.e4. doi: 10.1016/j.molcel.2018.04.019. [DOI] [PubMed] [Google Scholar]
- 28.Chen Y, Sun M, Ding J, Zhu Q. SM-1, a novel PAC-1 derivative, activates procaspase-3 and causes cancer cell apoptosis. Cancer Chemother Pharm. 2016;78:643–54. doi: 10.1007/s00280-016-3115-6. [DOI] [PubMed] [Google Scholar]
- 29.Zhai X, Bao G, Wang L, Cheng M, Zhao M, Zhao S, et al. Design, synthesis and biological evaluation of novel 4-phenoxy-6,7-disubstituted quinolines possessing (thio)semicarbazones as c-Met kinase inhibitors. Bioorg Med Chem. 2016;24:1331–45. doi: 10.1016/j.bmc.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 30.Ma J, Chen D, Lu K, Wang L, Han X, Zhao Y, et al. Design, synthesis, and structure-activity relationships of novel benzothiazole derivatives bearing the ortho-hydroxy N-carbamoylhydrazone moiety as potent antitumor agents. Eur J Med Chem. 2014;86:257–69. doi: 10.1016/j.ejmech.2014.08.058. [DOI] [PubMed] [Google Scholar]
- 31.Ma J, Zhang G, Han X, Bao G, Wang L, Zhai X, et al. Synthesis and biological evaluation of benzothiazole derivatives bearing the ortho-hydroxy-N-acylhydrazone moiety as potent antitumor agents. Arch Pharm. 2014;347:936–49. doi: 10.1002/ardp.201400230. [DOI] [PubMed] [Google Scholar]
- 32.Wang F, Wang L, Li Y, Wang N, Wang Y, Cao Q, et al. PAC-1 and its derivative WF-210 inhibit angiogenesis by inhibiting VEGF/VEGFR pathway. Eur J Pharm. 2018;821:29–38. doi: 10.1016/j.ejphar.2017.12.035. [DOI] [PubMed] [Google Scholar]
- 33.Botham RC, Fan TM, Im I, Borst LB, Dirikolu L, Hergenrother PJ. Dual small-molecule targeting of procaspase-3 dramatically enhances zymogen activation and anticancer activity. J Am Chem Soc. 2014;136:1312–9. doi: 10.1021/ja4124303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peh J, Fan TM, Wycislo KL, Roth HS, Hergenrother PJ. The combination of vemurafenib and procaspase-3 activation is synergistic in mutant BRAF melanomas. Mol Cancer Ther. 2016;15:1859–69. doi: 10.1158/1535-7163.MCT-16-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Botham RC, Roth HS, Book AP, Roady PJ, Fan TM, Hergenrother PJ. Small-molecule procaspase-3 activation sensitizes cancer to treatment with diverse chemotherapeutics. ACS Cent Sci. 2016;2:545–59. doi: 10.1021/acscentsci.6b00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lucas PW, Schmit JM, Peterson QP, West DC, Hsu DC, Novotny CJ, et al. Pharmacokinetics and derivation of an anticancer dosing regimen for PAC-1, a preferential small molecule activator of procaspase-3, in healthy dogs. Invest N Drugs. 2011;29:901–11. doi: 10.1007/s10637-010-9445-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joshi AD, Botham RC, Schlein LJ, Roth HS, Mangraviti A, Borodovsky A, et al. Synergistic and targeted therapy with a procaspase-3 activator and temozolomide extends survival in glioma rodent models and is feasible for the treatment of canine malignant glioma patients. Oncotarget. 2017;8:80124–38. doi: 10.18632/oncotarget.19085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schlein LJ, Fadl-Alla B, Pondenis HC, Lezmi S, Eberhart CG, LeBlanc AK, et al. Immunohistochemical characterization of procaspase-3 overexpression as a druggable target with PAC-1, a procaspase-3 activator, in canine and human brain cancers. Front Oncol. 2019;9:96. doi: 10.3389/fonc.2019.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tonogai EJ, Huang S, Botham RC, Berry MR, Joslyn SK, Daniel GB, et al. Evaluation of a procaspase-3 activator with hydroxyurea or temozolomide against high-grade meningioma in cell culture and canine cancer patients. Neuro Oncol. 2021;23:1723–35. doi: 10.1093/neuonc/noab161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 41.USDHHS. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. 2010. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03.
- 42.Spencer MPF, Folstein MF. The Mini-Mental State Examination. In: Keller PAR, Ritt LG (eds). Innovations in clinical practice: a source book. Sarasota, FL: Professional Resource Exchange, Inc.; 1985) pp 307–8.
- 43.Shapiro AM, Benedict RH, Schretlen D, Brandt J. Construct and concurrent validity of the Hopkins Verbal Learning Test-revised. Clin Neuropsychol. 1999;13:348–58. doi: 10.1076/clin.13.3.348.1749. [DOI] [PubMed] [Google Scholar]
- 44.Brandt J, Benedict R. Hopkins Verbal Learning Test-revised. Lutz, FL: Psychological Assessment Resources, Inc.; 2001.
- 45.Benedict RHB, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test-Revised: Normative data and analysis of inter-form and test-retest reliability. Clin Neuropsychol. 1998;12:43–55. doi: 10.1076/clin.12.1.43.1726. [DOI] [Google Scholar]
- 46.Reitan R. Manual for administration of neuropsychological test batteries for adults and children. Tucson, AZ: Reitan Neuropsychology Laboratories; 1978.
- 47.Levine AJ, Miller EN, Becker JT, Selnes OA, Cohen BA. Normative data for determining significance of test-retest differences on eight common neuropsychological instruments. Clin Neuropsychol. 2004;18:373–84. doi: 10.1080/1385404049052420. [DOI] [PubMed] [Google Scholar]
- 48.Benton AL, Hamsher KD, Siva AB. Multilingual aphasia examination. Lutz, FL: Psychological Assessment Resources, Inc; 1994. [Google Scholar]
- 49.Ruff RM, Light RH, Parker SB, Levin HS. Benton Controlled Oral Word Association Test: reliability and updated norms. Arch Clin Neuropsychol. 1996;11:329–38. doi: 10.1093/arclin/11.4.329. [DOI] [PubMed] [Google Scholar]
- 50.Wefel JS, Vardy J, Ahles T, Schagen SB. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011;12:703–8. doi: 10.1016/S1470-2045(10)70294-1. [DOI] [PubMed] [Google Scholar]
- 51.Neurocognitive Test Summary provided by the Health Services and Research Outcomes (HSRO) Subcommittee of the Radiation Therapy Oncology Group (RTOG). 2011. www.rtog.org.
- 52.Jacobson NS, Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol. 1991;59:12–9. doi: 10.1037/0022-006X.59.1.12. [DOI] [PubMed] [Google Scholar]
- 53.Wefel JS, Cloughesy T, Zazzali JL, Zheng M, Prados M, Wen PY, et al. Neurocognitive function in patients with recurrent glioblastoma treated with bevacizumab. Neuro Oncol. 2011;13:660–8. doi: 10.1093/neuonc/nor024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501–13. doi: 10.1056/NEJMoa1003825. [DOI] [PubMed] [Google Scholar]
- 55.Peh J, Boudreau MW, Smith HM, Hergenrother PJ. Overcoming resistance to targeted anticancer therapies through small-molecule-mediated MEK degradation. Cell Chem Biol. 2018;25:996.e4–1005.e4. doi: 10.1016/j.chembiol.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in this study are available upon request from the corresponding author.