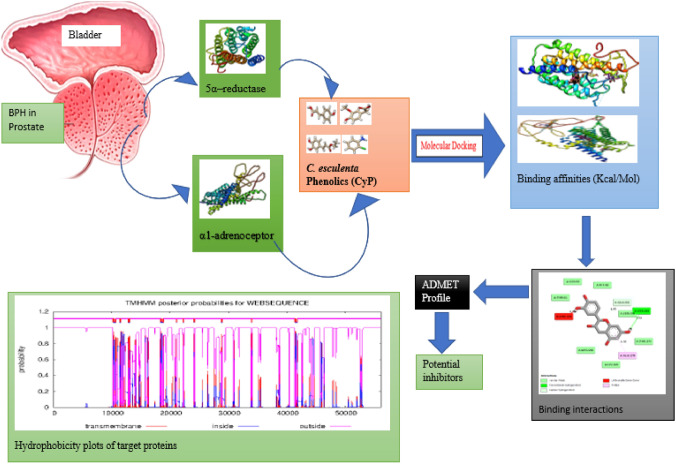
Abstract
Benign Prostatic Hyperplasia (BPH) is a major cause of lower urinary tract infections and erectile dysfunction thus a major contributor to lowering the quality of life among older men. In this study, we investigated the molecular mechanism of Colocasia esculenta (CE) as a novel agent for BPH chemotherapy. In vivo, we assigned 45 male Wistar albino rats about 6 weeks old into 9 experimental groups (n = 5). BPH was induced in groups 2–9 with 3 mg/kg of Testosterone Propionate (TP) subcutaneously. Group 2 (BPH) was not treated. Group 3 was treated with 5 mg/kg Finasteride (standard drug). Group 4–9 were treated each with 200 mg/kg body weight (b.w) of CE crude tuber extracts/fractions (ethanol, hexane, dichloromethane, ethyl acetate, butanol, aqueous). At the end of treatment, we sampled the rats’ serum to check the level of PSA. In silico, we conducted a molecular docking of the crude extract of CE phenolics (CyP) previously reported, targeting 5α-Reductase and α1-Adrenoceptor linked to the BPH progressions. We adopted the standard inhibitors/antagonists (5α-reductase: finasteride; α1-adrenoceptor: tamsulosin) of the target proteins as controls. Furthermore, the pharmacological properties of the lead molecules were studied in terms of ADMET using swissadme and pKCSM resources, respectively. Results showed that administration of TP in male Wistar albino rats significantly (p < 0.05) elevated serum PSA levels whereas CE crude extracts/fractions significantly (p < 0.05) lowered the serum PSA level. Also, fourteen of the CyPs bind to at least one or two of the target proteins with their binding affinity of between − 9.3 to − 5.6 kcal/mol and − 6.9 to − 4.2 kcal/mol, respectively. The CyPs possess better pharmacological properties compared to the standard drugs. Therefore, they have the potentials to be enlisted for clinical trials towards the management of BPH.
Graphical Abstract
Keywords: BPH, Colocasia esculenta phenolics, Molecular mechanism, 5α-reductase, α1-adrenoceptor, Lead molecules
Introduction
In the world, there are about 94 million cases of benign prostatic hyperplasia (BPH), which accounts for approximately 186 million (11.3–78) years of life lost to premature death, living in bad health, or being disabled (Miano et al. 2008; Ventura et al. 2011). While BPH may go unnoticed and asymptomatic in some men, the hyperplasic prostate, which is present in most older men, can clog the urethra and distort the base of the bladder, resulting in painful and incapacitating symptoms of the lower urinary tract (Rokosh and Simpson 2002; Ventura et al. 2011). Moreover, the symptoms caused by BPH can have an extremely detrimental effect on the quality of life of both the men suffering from the disease as well as their partners.
Pharmacological therapy is currently a significant therapeutic choice offered to the patient. Traditional pharmaceutical treatment aims to reduce either the prostate's physical enlargement or the BPH's increased smooth muscle tone. The proliferative effect of androgens is inhibited by medications that target the static component of BPH. These medications, which account for 23% of the global market for prostate pharmacotherapy (Eleazu et al. 2022), include finasteride and dutasteride. The use of 5α-reductase inhibitors is associated with important adverse effects including impotence, decreased libido, and abnormal ejaculation. In addition, drugs targeting the dynamic component (α1-adrenoceptor antagonists) (Miano et al. 2008) such as tamsulosin and alfuzosin (Ventura et al. 2011) produce both vasodilator side effects, and abnormal ejaculatory effects (Rokosh and Simpson 2002). Worse still, the use of surgery could be life-threatening, thus highlighting the need for safer alternatives.
Cocoyam (Colocasia esculenta L.) is a tropical starchy plant that is reportedly native to Asia, the Pacific, and the tropical regions (Eleazu 2016; Eleazu et al. 2021). Colocasia esculenta is major but sometimes neglected food (Ekwe et al. 2008) that contains a broad spectrum of functional foods (Niba 2003) as its nutritive components and phenolic constituents (Adegunwa et al. 2011; Olajide et al. 2011). Eleazu et al. (2021) have reported the phenolic constituents of cocoyam and demonstrated their potential in mitigating BPH in rat models. This suggests that the active component of C. esculenta tuber possesses 5α-reductase inhibitory properties, we hypothesized that C. esculenta tuber may have some relevance in the treatment of BPH. To further understand these active ingredients, and their mechanism of action, we used a computational approach based on targeting the key proteins.
Materials and methods
In vivo study
Plant materials sampling
Mature (6–8 months old) fresh tubers of C. esculenta were collected from Select local farmers. This was followed by authentication by a taxonomist, Professor S.O. Onyekwelu at the department of Applied Biology, Ebonyi state University. For reference purpose, a portion of the C. esculenta tuber was kept at the herbarium of Applied Biology Department, Ebonyi State University’s, Nigeria with voucher Reference number: EBSU-H-206. The C. esculent tubers were washed, boiled, peeled, sliced into chips, air-dried to a constant weight at room temperature, and processed into flour.
Extraction and purification of the plant sample
The powdered tuber 1280 g of C. esculenta were extracted with 8 L of 50% ethanol (Emsure®) overnight in a big stopper bottle with occasional stirring at room temperature. It was then sieved using a muslin cloth. The filtrates were air dried for 24 h to get the ethanol (crude) extracts. Slurry of the ethanol crude extract was made with distilled water. To purify the extract, a crude natural ethanol product was extracted with solvents of increasing polarity, first, hexane (Blulux), dichloro methane (UNILAB), and ethyl acetate (UNILAB) and butanol (AnalaR®) which depended on the chemical and physical nature of the target compounds. The fractions from hexane, dichloromethane, ethyl acetate and butanol were air dried to obtain solid fractions. The solid fractions were further dissolved in distilled water for the administration to the animals.
Animals handling
This study was done under the supervision and approval of the Office of Research, Innovation and Institutional Ethics Committee of Ebonyi state university, Nigeria (EBSU/BCH/ET/21/001). All procedures for animal studies were performed following guidelines and legislations consistent with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80‐23, revised in 1996) (National Institutes of Health 1985) as well as the National guidelines for the use of laboratory animals for research and teaching based on the principles of 3Rs, reduce refine or replace.
Induction of BPH
We induced BPH in rats using testosterone propionate (TP) (Eleazu et al. 2022). The dose for induction was formulated as 3 mg/kg body weight and it was given by subcutaneous injection every day for 28 days. We prepared stock solution by dissolving 25 mg of TP in 8.33 ml olive oil. Three rats each from the groups were randomly selected after induction for confirmation of BPH before treatment.
Experimental design
The rats were grouped as follows with five rats in each group: Rats in group 1 (Normal control) received orally 1 ml of olive oil. BPH was induced in groups 2–9 with 3 mg/kg TP subcutaneously. Group 2 (BPH group) was not treated. Group 3 (Finasteride group) had rats that were treated with 5 mg/kg finasteride. The middle dose of 200 mg/kg was used for this study as reported by Eleazu et al. (2021). Group 4 had rats that were treated with 200 mg/kg body weight (b.w) of ethanol crude tuber extract of C. esculenta (ECTECE). Group 5 had rats that were treated with 200 mg/kg b.w of n-hexane fraction (HF). Group 6 had rats that were treated with 200 mg/kg b.w of dichloromethane fraction (DCMF). Group 7 had rats that were treated with 200 mg/kg b.w of ethyl acetate fraction (EAF). Group 8 had rats that were treated with 200 mg/kg b.w of butanol fraction (BF). And finally, group 9 had rats that were treated with 200 mg/kg b.w of aqueous fraction (AF). Oral administration of the extract or fractions or finasteride was done using oral gavage and all animal diets were provided ad libithum.
Blood sample collection and analysis
After 28 days, the rats were fasted overnight, sacrificed in anesthetized with halothane, and the blood was collected by cardiac puncture, using 5 ml syringes into EDTA vacutainers for determination of prostate-specific antigen (PSA) concentration. Enzyme-Linked Immunoassay kits (Biocheck, Incorporated) were used to determine the PSA concentration.
Statistical data analyses
Data were analyzed using Prism software (Graph-Pad Software; San Diego, CA) to determine statistical significance. The results are shown as mean ± SD of 5 rats per group. P < 0.05 was considered statistically significant.
In silico study
In this study, the molecular docking software used were UCSF Chimera (Pettersen et al. 2004), Autoduck Vina Plugin Pyrx (Valdés-Tresanco et al. 2020), and Discovery Studio (Studio 2008). Also, we used Web servers and databases like PubChem, Protein Databank (PDB), UniProt, Swissadme, pKCSM, and TMHMM.
Retrieval of ligands
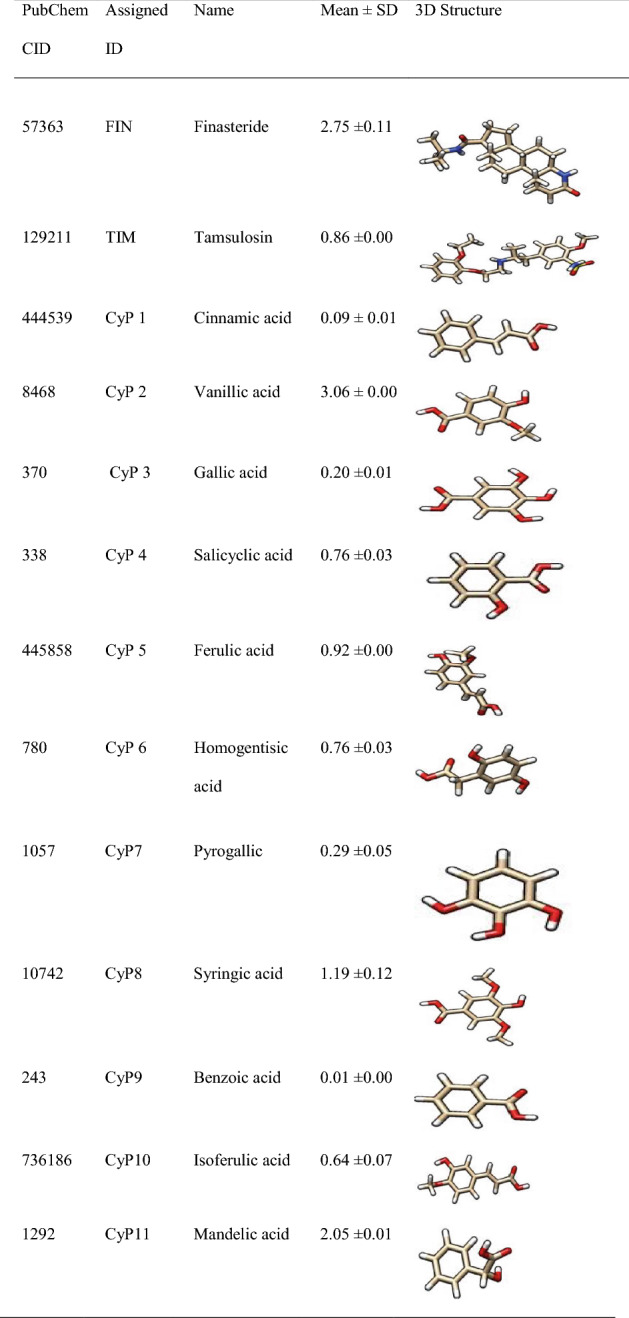
The 3D structures of the ligands in Table 1 which include standard inhibitors (finasteride and tamsulosin) as well as the 22 phenolics from boiled tubers of C. esculenta (Eleazu et al. 2022) were fetched by CID from the PubChem database into Chimera and saved in PDB format.
Table 1.
Phenolic profile of boiled C. esculenta tuber (mg/g) (Eleazu et al. 2021) and standard inhibitors
Retrieval of target proteins
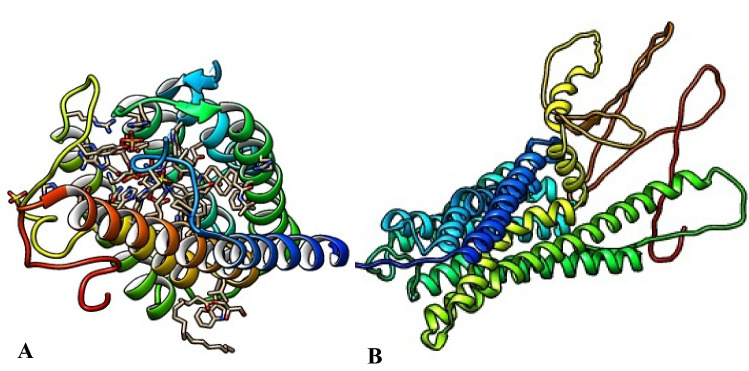
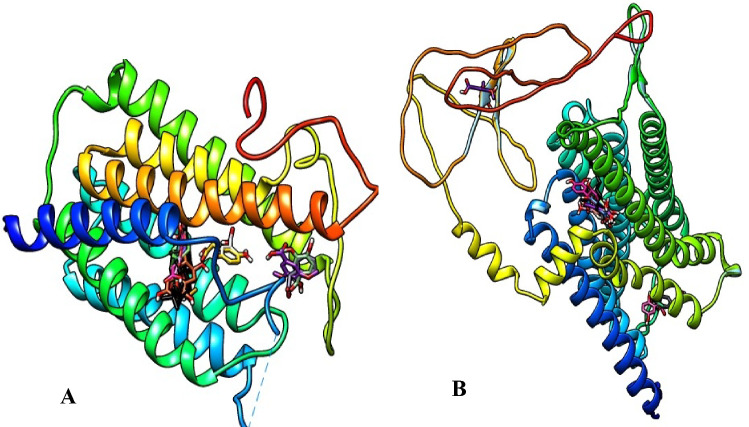
The 3D crystal structures of the target proteins, 5α-reductase (PDB ID: 7BW1) and α1-adrenoceptor (Uniprot KB-P35348), (Fig. 1) were retrieved from protein databank and Uniprot web servers respectively.
Fig. 1.
3D crystal structures of the target proteins (A 5α-reductase and B α1-adrenoceptor)
Preparation of target proteins
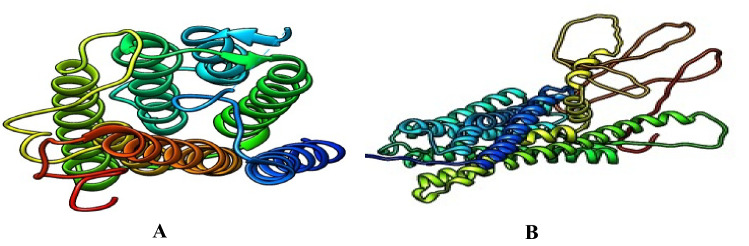
The 5α-reductase and α1-adrenoceptor were fetched in turns by ID into UCSF Chimera in complex with other chains. Those chains and nonstandard ligands were deleted leaving only chain A of each of the proteins (Fig. 2). Further, the protein was minimized in the default settings of the Chimera with the addition of hydrogen ions and charges Gastiger, and then saved in PDP format.
Fig. 2.
Prepared 3D crystal structure of the target proteins (A 5α-reductase and B α1-adrenoceptor)
Molecular docking
We used a method as previously reported (Aja et al. 2021). Briefly, the standard and the test ligands were imported into Pyrx, minimized with the addition of hydrogen ion and charges Gastiger, and then converted to pdbqt format. Further, in turn, the target proteins were loaded into the Pyrx and converted to Autodock ligand or macromolecules. The grid boxes were set to cover the proteins and the sizes recorded (Table 2).
Table 2.
Grid box orientation
| 5α-reductase | α1-adrenoceptor | |||||
|---|---|---|---|---|---|---|
| X | Y | Z | X | Y | Z | |
| Center | − 32.7331 | 14.3308 | 31.7573 | − 2.1865 | 15.1041 | − 2.9520 |
| Dimension | 45.9059 | 44.5969 | 63.7130 | 146.5743 | 86.5897 | 98.2684 |
Autodock vina was run in default settings with 8 exhaustiveness. For each of the screening, only the C. esculenta phenolic compounds which bound at the same site with the finasteride for 5α-reductase, or tamsulosin for α1-adrenoceptor were considered to be lead compounds (Aja et al. 2021) or potential inhibitors.
Post-docking analysis
The docking scores were recorded and the docking poses of the lead compounds with the target proteins were analyzed using the UCSF Chimera and Discovery Studio 2020 to decipher their molecular mechanisms of interacting with the proteins. Also, the hydrophobicity of the target proteins was plotted using the TMHMM resources to identify the region in which the lead compounds bind (Table 3).
Table 3.
Docking scores of the potential inhibitors
| 5α-reductase | α1-adrenoceptor | ||
|---|---|---|---|
| Compound PubChem CID |
Binding affinity (Kcal/mol) |
Compound PubChem CID |
Binding affinity (Kcal/mol) |
| FIN | − 11.6 | TAM | − 5.7 |
| CyP1 | − 6.3 | CyP1 | − 5.1 |
| CyP3 | − 6.4 | CyP3 | − 5.0 |
| CyP5 | − 7.0 | CyP5 | − 5.2 |
| CyP6 | − 6.0 | CyP7 | − 4.2 |
| CyP7 | − 5.7 | CyP9 | − 4.6 |
| CyP9 | − 5.8 | CyP10 | − 5.5 |
| CyP10 | − 6.8 | CyP11 | − 5.0 |
| CyP14 | − 6.2 | CyP15 | − 5.3 |
| CyP15 | − 6.7 | CyP20 | − 6.9 |
| CyP17 | − 5.8 | CyP21 | − 5.7 |
| CyP18 | − 6.9 | ||
| CyP20 | − 9.3 | ||
| CyP21 | − 7.1 | ||
| CyP22 | − 7.0 | ||
Pharmacological properties of the potential inhibitors
The pharmacological properties of the lead compounds were studied by predicting the drug-likeness in terms of the Lipinski Rule of five (Lipinski 2004) and pharmacokinetic properties like adsorption, distribution, metabolism, excretion, and toxicity (ADMET) using swissadme and pKCSM web servers respectively.
Results
In Vivo study
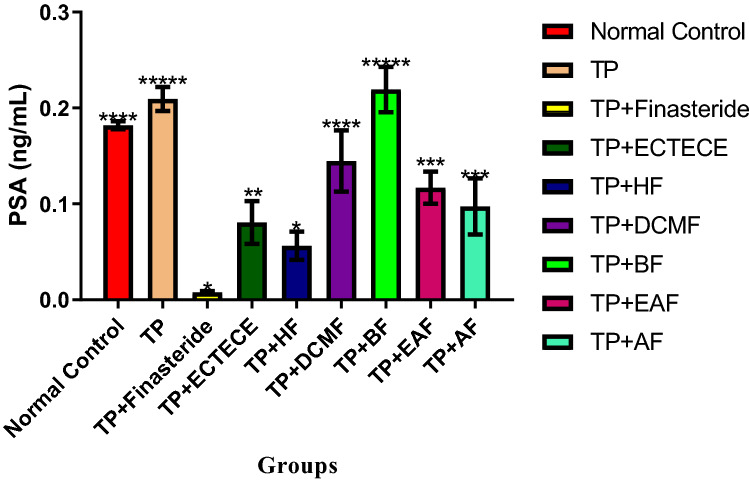
In this study, administration of TP in male Wistar albino rats significantly (p < 0.05) elevated serum PSA levels (Fig. 3). Co-administration of TP and ethanol crude Tuber extract of Colocasia esculenta (ECTECE), hexane fraction (HF), dichloromethane fraction (DCMF), butanol fraction (BF), Ethyl acetate fraction (EAF), and aqueous fraction (AF) in male rats significantly(p < 0.05) reduced the level of serum PSA. Interestingly, significant (p < 0.05) reductions in the level of serum PSA were observed in all the fractions except butanol fraction (Fig. 3).
Fig. 3.
Effect of ethanol crude Tuber extract of Colocasia esculenta and Fractions on Serum Prostate specific antigen in Testosterone propionate induced benign prostate hyperplasic Rats. Data are shown as mean ± S.D (n = 5). Mean values with the different signs are significantly different at P < 0.05. Testosterone propionate (TP), Ethanol crude Tuber extract of Colocasia esculenta (ECTECE), Hexane fraction (HF), Dichloromethane fraction (DCMF), Butanol fraction (BF), Ethyl acetate fraction (EAF) and Aqueous Fraction (AF)
In silico study
Binding target representation
We visualized the binding of standards and potential inhibitors/antagonists using UCSF Chimera (Fig. 4).
Fig. 4.
Competitive binding of standard and potential inhibitors/antagonists visualized in UCSF Chimera (A ligands vs 5αreductase and B ligands vs α1-adrenoceptor)
Binding affinities of the potential inhibitors
Molecular interactions visualization
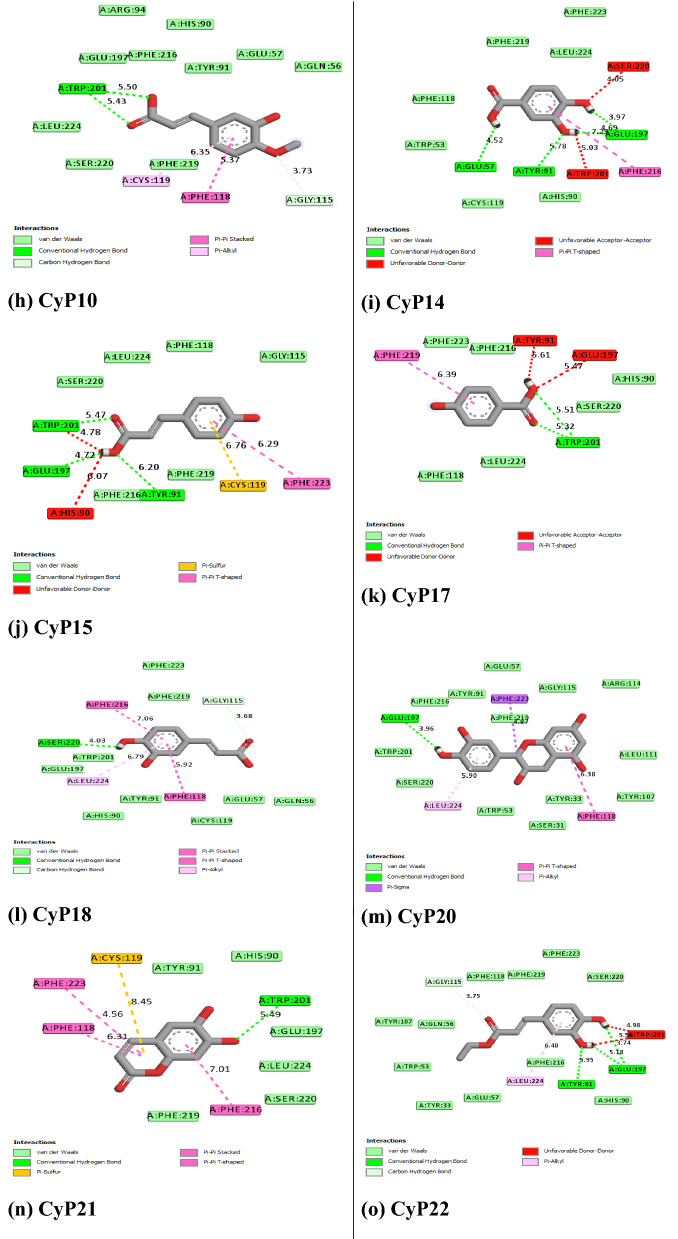
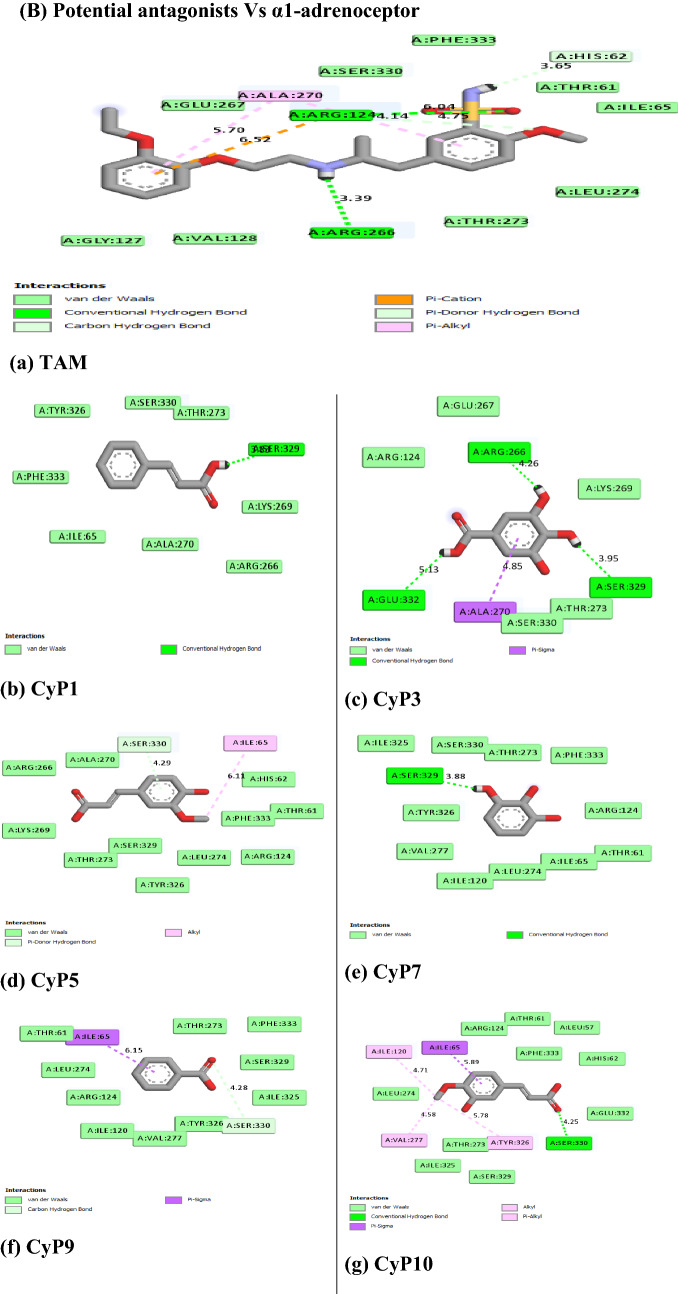
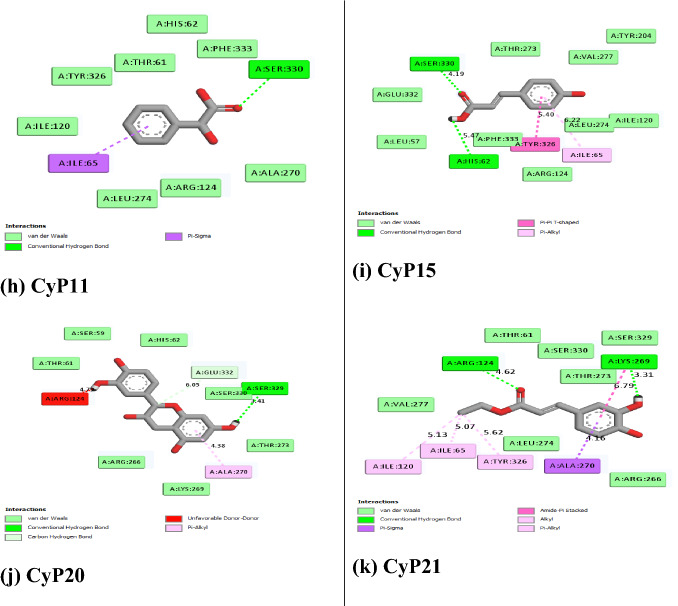
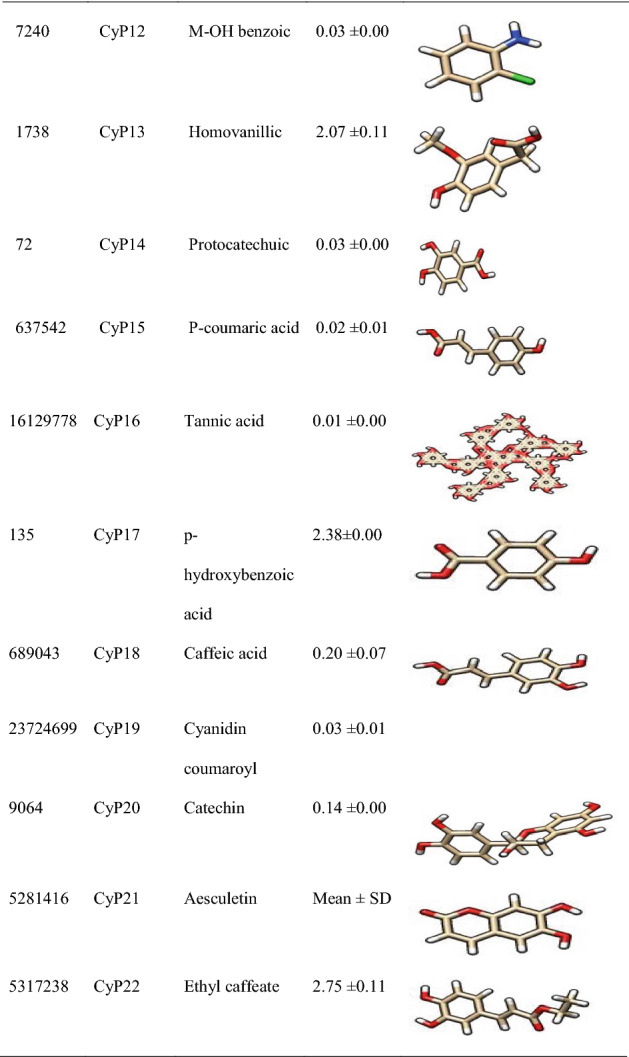
Figure 5 revealed the molecular interactions of the potential inhibitors and their comparison with the standard inhibitors. It showed the kinds of bonds, interacting amino acid residues within the pockets, and the bond lengths.
Fig. 5.
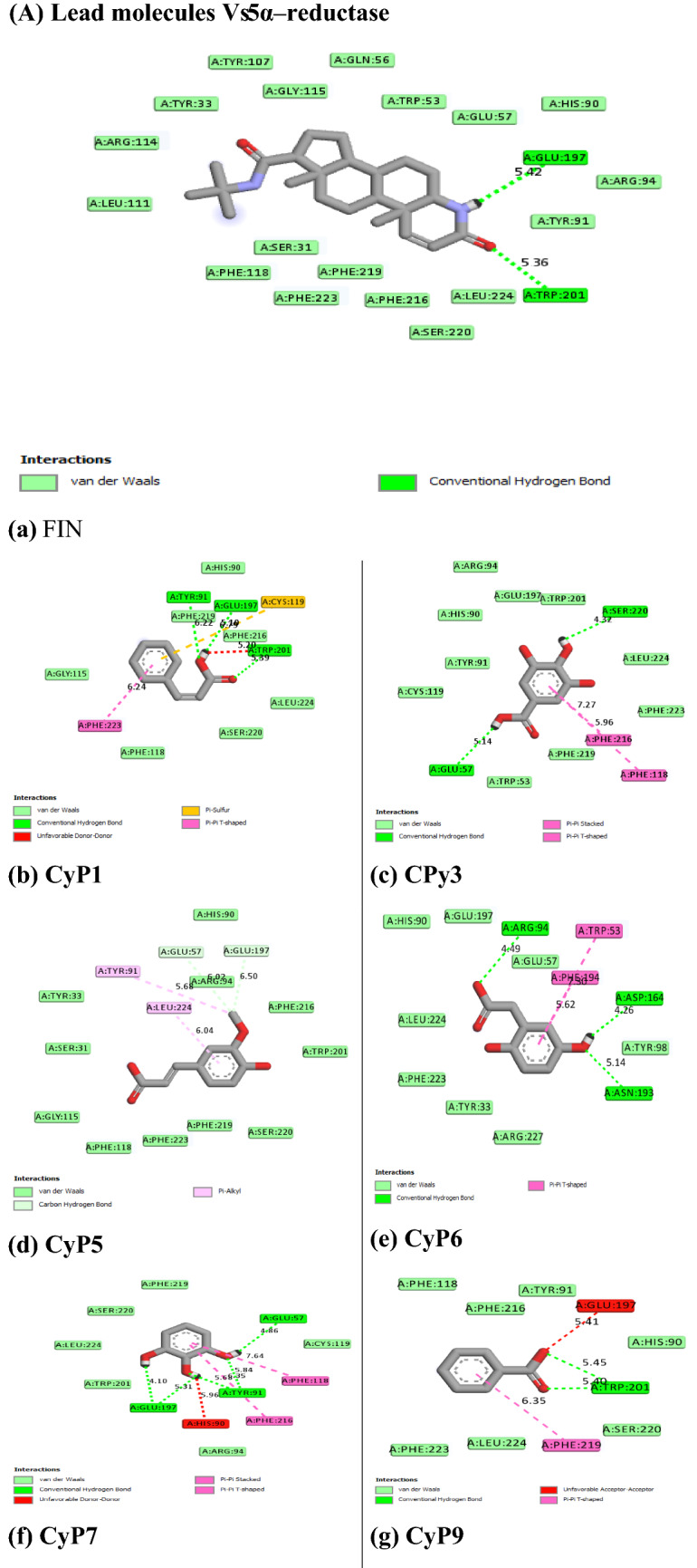
A Molecular interactions between 5α-reductase and the potential inhibitors (a standard inhibitor; b–o potential inhibitors). B Molecular interactions between α1-adrenoceptor and the potential inhibitors (a standard inhibitor; b–o: potential inhibitors
Hydrophobicity plots of the target proteins
Figures 6 and 7 showed the hydrophobicity of the target proteins studied using the TMHMM webserver. The target proteins have transmembrane regions for hydrophobic interactions with the potential inhibitor molecules.
Fig. 6.
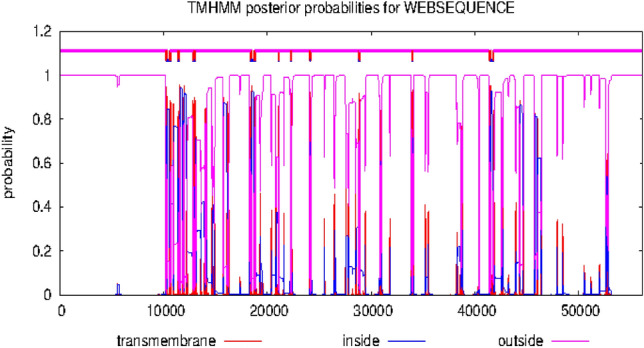
Output of hydrophobicity plot of 5α-reductase in TMHMM webserver
Fig. 7.
Output of hydrophobicity plot of α1-adrenoceptor in TMHMM webserver
Pharmacological properties of the potential inhibitors
Discussion
Investigations aimed at the discovery of novel chemotherapeutic agents to tackle the upsurge of BPH have become imminent. The existing drugs have been reported with some degrees of toxicity amidst the global incidence of the disease. In vivo study has demonstrated the potential of C. esculenta against BPH and identified 22 phenolics to be present (Eleazu et al. 2021). In this study, testosterone propionate dramatically increased the PSA level in rats, according to the in vitro assay results. But when compared to the untreated group, the administration of C. esculenta crude extracts and fractions dramatically reduced PSA levels. This result is consistent with other research on BPH using different animal model send (Steiner et al. 1999; Tiwari et al. 2005; Li et al. 2018; Eleazu et al. 2021). Like the finasteride, the crude extract or fractions of CE were able to inhibit the progression of the testosterone-stimulated androgen-dependent processes. This could be due to inhibition of the growth factors regulating the expression of the disease proteins. Understanding this molecular mechanism inspired the use of in-silico approach to predict which of the phenolics competitive inhibitors of standard drugs were.
In this study, therefore, we targeted two proteins (5α-reductase and α1-adrenoceptor) linked to the disease proliferation (Ventura et al. 2011). The standard inhibitor/antagonist of the proteins was used for the precise and validity of the molecular target (Aja et al. 2021). The results showed that 14 out of the 22 compounds bound the same site with the standard inhibitor (Finasteride) of 5α-reductase whereas 10 out of the 22 compounds bound the same site with the standard antagonists (tamsulosin) of α1-adrenoceptor (Fig. 5), hence considered as the lead compounds.
For activity, ligand molecules should have an affinity for the target proteins (Elekofehinti et al. 2021). The Molecular docking scores (Table 4) showed that the lead compounds have affinities for the target proteins. Standard inhibitor/antagonists, finasteride and tamsulosin, have binding affinities of − 11.6 kcal/mol and − 5.7 kcal/mol, respectively for their target proteins. Within the target binding pocket of 5α-reductase CyP1, CyP3, CyP5, CyP6, CyP7, CyP9, CyP10, CyP14, CyP15, CyP17, CyP18, CyP20, CyP21, and CyP22 have affinities of − 9.3 to − 5.6 kcal/mole. Also, in the target binding pocket of α1-adrenoceptor CyP1, CyP3, CyP5, CyP7, CyP9, CyP10, CyP11, CyP15, CyP20, and CyP22 have affinities of − 6.9 to − 4.2 kcal/mol. Compared to the standards, CyP20 has the strongest affinities (− 9.3 kcal/mole for 5α-reductase and − 6.9 kcal/mol for α1-adrenoceptor) for each of the target proteins. Therefore, CyP20 together with these compounds are potential chemotherapeutic agents via inhibition or antagonism of the target proteins.
Table 4.
Predicted drug-likeness and pharmacokinetics of the lead compounds
| MW | #Rotatable bonds | #H-bond acceptors | #H-bond donors | MR | TPSA | iLOGP | |
|---|---|---|---|---|---|---|---|
| CyP1 | 148.16 | 2 | 2 | 1 | 43.11 | 37.3 | 1.55 |
| CyP3 | 170.12 | 1 | 5 | 4 | 39.47 | 97.99 | 0.21 |
| CyP5 | 194.18 | 3 | 4 | 2 | 51.63 | 66.76 | 1.62 |
| CyP6 | 168.15 | 2 | 4 | 3 | 42.03 | 77.76 | 0.6 |
| CyP7 | 126.11 | 0 | 3 | 3 | 32.51 | 60.69 | 0.97 |
| CyP9 | 122.12 | 1 | 2 | 1 | 33.4 | 37.3 | 1.11 |
| CyP10 | 194.18 | 3 | 4 | 2 | 51.63 | 66.76 | 1.79 |
| CyP11 | 152.15 | 2 | 3 | 2 | 39.15 | 57.53 | 0.99 |
| CyP14 | 154.12 | 1 | 4 | 3 | 37.45 | 77.76 | 0.66 |
| CyP15 | 164.16 | 2 | 3 | 2 | 45.13 | 57.53 | 0.95 |
| CyP17 | 138.12 | 1 | 3 | 2 | 35.42 | 57.53 | 0.85 |
| CyP18 | 180.16 | 2 | 4 | 3 | 47.16 | 77.76 | 0.97 |
| CyP20 | 290.27 | 1 | 6 | 5 | 74.33 | 110.38 | 1.47 |
| CyP21 | 178.14 | 0 | 4 | 2 | 46.53 | 70.67 | 1.25 |
| CyP22 | 208.21 | 4 | 4 | 2 | 56.29 | 66.76 | 2.04 |
| FIN | 372.54 | 3 | 2 | 2 | 113.18 | 58.2 | 3.32 |
| TAM | 408.51 | 11 | 7 | 2 | 108.24 | 108.26 | 3.36 |
| GI absorption | BBB permeant | Pgp substrate | CYP1A2 inhibitor | CYP2C19 inhibitor | CYP2C9 inhibitor | CYP2D6 inhibitor | |
|---|---|---|---|---|---|---|---|
| CyP1 | High | Yes | No | No | No | No | No |
| CyP3 | High | No | No | No | No | No | No |
| CyP5 | High | Yes | No | No | No | No | No |
| CyP6 | High | No | No | No | No | No | No |
| CyP7 | High | Yes | No | No | No | No | No |
| CyP9 | High | Yes | No | No | No | No | No |
| CyP10 | High | Yes | No | No | No | No | No |
| CyP11 | High | No | No | No | No | No | No |
| CyP14 | High | No | No | No | No | No | No |
| CyP15 | High | Yes | No | No | No | No | No |
| CyP17 | High | Yes | No | No | No | No | No |
| CyP18 | High | No | No | No | No | No | No |
| CyP20 | High | No | Yes | No | No | No | No |
| CyP21 | High | No | No | Yes | No | No | No |
| CyP22 | High | Yes | No | No | No | No | No |
| FIN | High | Yes | Yes | No | No | No | No |
| TAM | High | No | Yes | No | Yes | Yes | Yes |
Molecular mechanisms of action of chemotherapeutic agents can be explained by the interactions in terms of the kinds of bonds it establishes with the amino acids in the binding pockets (Powers 2009; Aja et al. 2021). Figures 4 and 5 showed the individual interactions between the potential inhibitors and their target receptors. The standard, as well as the potential inhibitor/antagonist, interacts with different amino acids in the binding sites although similar bonds are involved: Hydrogen bonding, van der Waals, various kinds of pi-bonds, unfavorable donor, and salt bridges. Of these binding interactions, hydrogen bonds are the strongest (Aja et al. 2021). Overall, all the potential inhibitors showed better interactions than the finasteride within the binding pockets of 5α-reductase as their interactions were stronger and involved multiple bonds (Umamaheswari et al. 2013). The interaction of cocoyam phenolic potential antagonists to alpha 1-adrenoceptors was as effective as the standard drug (tamsulosin). The hydrophobicity plot of the target proteins revealed each has a transmembrane region which further gives insight into the molecular interactions of the lead molecules. Molecules interact with α-helices of the hydrophobic domains to gain access to the inside and outside regions of the cells. Possibly, this allowed the novel identified potential chemotherapeutic agents to permeate membranes to interact with receptors. The phenolic compounds identified in cocoyam including CyP1, 3, 5, 6, 7, 9, 10, 14, 15, 17, 18, 20, 21, and 22, use a similar mechanism of action to standard drugs which include targeting either the static (size) or dynamic (muscular contraction) component of BPH (Ventura et al. 2011; Akanshka et al. 2018). The finasteride drugs target the static component of BPH by inhibiting the proliferative action of androgens (Carson III and Rittmaster 2003). Therefore, they have the potential to inhibit the action of the 5a-reductase enzyme which catalyzes the conversion of testosterone to the more potent androgen dihydrotestosterone (Ventura et al. 2011). This will perhaps potentiate relief of urethral obstruction as the physical size of the prostate is decreased (Carson III and Rittmaster 2003; Tarter and Vaughan Jr 2006). Unlike the finasteride, tamsulosin drugs act in the treatments for BPH targeting the dynamic component (Miano et al. 2008) as α1-adrenoceptor antagonists (Rigatti et al. 2003; Hasan et al. 2007; Lepor 2007). On the other hand, the cocoyam phenolics including CyP1, 3, 5, 7, 9, 10, 11, 15, 20, and 22 will perhaps decrease the stromal smooth muscle tone by blocking prostatic α1-adrenoceptors like the tamsulosin, thus relieving urethral obstruction and the associated troublesome voiding symptoms (Lepor 2007). There is a direct correlation between urethral obstruction and the amount of prostatic smooth muscle. Therefore, regulating the prostate’s size and muscular constriction could be an effective target of tackling the severity of BPH. The potential synergy that results from targeting multiple receptors by cocoyam phenolics give it tremendous advantage over conventional drugs for BPH treatment in older men who are usually struggling with polypharmacy (Hilmer and Gnjidic 2009).
The possible therapeutic compounds were shown to have good pharmacological characteristics. They obeyed the entire Lipinski rule of five (Lipinski 2004; Nisha et al. 2016). Their high gastrointestinal absorption gives good chances for oral administration as mode of delivery. Except for CyP3, CyP6, CyP11, CyP14, CyP20, and CyP21, others can permeate the blood–brain-barrier making them potent to target neuronal tissues. Similarly, others are neither inhibitors nor substrates of the p-glycoproteins and the metabolizing enzymes, respectively except for CyP20 which inhibits CYP1A2 (Faber et al. 2005). This suggests mild effects on central metabolism. Further studies might look into lead compounds from cocoyam as potential anti prostatic molecules.
In Table 5, we reported the prediction of excretion and potential toxicities of the drug-like molecules. All the drug-like molecules do not interfere with the organic cation transporter 2 hence can maintain good renal clearance. They have comparatively, high acute toxicity and require little dose. They are non-hERGI/hERGII inhibitors, and thus do not affect cardiac muscles (Aja et al. 2021). Only CyP6 is a potential carcinogen. The potential chemotherapeutic agents are non-hepatotoxic whereas the standards (both finasteride and tamsulosin) are hepatotoxic.
Table 5.
Predicted excretion and toxicity of the lead compounds
| ID | Excretion | Toxicity | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total clearance (log ml/min/kg) | Renal OCT2 substrate (Yes/No) | AMES toxicity (Yes/No) | Max. tolerated dose (human) log mg/kg/day) | hERG I inhibitor (Yes/No) |
hERG II inhibitor (Yes/No) |
Oral rat acute toxicity (LD50) (mol/kg) | Oral rat chronic toxicity (LOAEL) (Log mg/kg/bw/day) |
Hepatic toxicity (Yes/No) |
|
| CyP1 | 0.781 | No | No | 1.11 | No | No | 2.094 | 2.651 | No |
| CyP3 | 0.518 | No | No | 0.7 | No | No | 2.218 | 3.06 | No |
| CyP5 | 0.623 | No | No | 1.082 | No | No | 2.282 | 2.065 | No |
| CyP6 | 0.59 | No | Yes | 1.316 | No | No | 2.274 | 2.064 | No |
| CyP7 | 0.104 | No | No | -0.269 | No | No | 2.049 | 2.374 | No |
| CyP9 | 0.707 | No | No | 0.612 | No | No | 2.17 | 2.637 | No |
| CyP10 | 0.621 | No | No | 1.075 | No | No | 2.301 | 2.046 | No |
| CyP11 | 0.611 | No | No | 1.18 | No | No | 0.284 | 1.816 | No |
| CyP14 | 0.551 | No | No | 0.814 | No | No | 2.423 | 2.021 | No |
| CyP15 | 0.662 | No | No | 1.111 | No | No | 2.155 | 2.534 | No |
| CyP17 | 0.593 | No | No | 0.846 | No | No | 2.255 | 2.483 | No |
| CyP18 | 0.508 | No | No | 1.145 | No | No | 2.383 | 2.092 | No |
| CyP20 | 0.183 | No | No | 0.438 | No | No | 2.428 | 2.5 | No |
| CyP21 | 0.671 | No | No | − 0.262 | No | No | 2.337 | 1.504 | No |
| CyP22 | 0.564 | No | No | − 0.172 | No | No | 2.078 | 1.574 | No |
| FIN | 0.38 | Yes | No | − 1.355 | No | No | 2.424 | 1.453 | Yes |
| TAM | 0.691 | No | No | 0.113 | No | No | 1.99 | 1.109 | Yes |
Conclusion
The prospect of tackling the uprising cases of BPH which damages the quality of life is highly promising. Phenolic compounds present in C. esculenta are potential chemotherapeutic agents for the management of BPH. Their molecular mechanisms of action are either to halt the conversion of testosterone into dihydrotestosterone and stop the proliferation of prostatic cells or to induce vasodilation of the smooth muscle and control urethra obstruction, hence lowering the severity of the symptoms. Therefore, these novel chemotherapeutic agents have the potential to proceed for phase 1 clinical trial toward the discovery of a greener approach to the management of benign prostatic hyperplasia.
Acknowledgements
We are grateful to Emanuel Mutekanga, Victor Mukundane, and Bright Rujumba, for their valuable technical assistance. The authors declare no conflict of interest. I would also like to acknowledge the DAAD fellowship for funding the PMA staff exchange that established a collaboration between Mbarara University of science and technology and Ebonyi State University
Author contributions
PMA, APC, DT conceived the idea. All authors were involved in designing the experiments, analyzing data, writing and reviewing the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data Availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
Declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adegunwa M, Alamu E, Omitogun L. Effect of processing on the nutritional contents of yam and cocoyam tubers. J Appl Biosci. 2011;46:3086–3092. [Google Scholar]
- Aja P, Agu P, Ezeh E, Awoke J, Ogwoni H, Deusdedit T, Ekpono E, Igwenyi I, Alum E, Ugwuja E. Prospect into therapeutic potentials of Moringa oleifera phytocompounds against cancer upsurge: de novo synthesis of test compounds, molecular docking, and ADMET studies. Bull Natl Res Centre. 2021;45(1):1–18. doi: 10.1186/s42269-021-00554-6. [DOI] [Google Scholar]
- Akanshka M, Dhingra R, Dhingra N. In silico identification of potential 5α-reductase inhibitors for prostatic disease: QSAR modelling, molecular docking, and pre ADME prediction. MOJ DDD T. 2018;2(3):136–145. [Google Scholar]
- Carson C, III, Rittmaster R. The role of dihydrotestosterone in benign prostatic hyperplasia. Urology. 2003;61(4):2–7. doi: 10.1016/S0090-4295(03)00045-1. [DOI] [PubMed] [Google Scholar]
- Ekwe K, Nwosu K, Ekwe C, Nwachukwu I (2008) Examining the underexploited values of cocoyams (Colocasia and Xanthosoma species) for enhanced household food security, nutrition and economy in Nigeria. International Symposium on Underutilized Plants for Food Security, Nutrition, Income and Sustainable Development 806
- Eleazu C. Characterization of the natural products in cocoyam (Colocasia esculenta) using GC–MS. Pharm Biol. 2016;54(12):2880–2885. doi: 10.1080/13880209.2016.1190383. [DOI] [PubMed] [Google Scholar]
- Eleazu K, Maduabuchi Aja P, Eleazu CO. Cocoyam (Colocasia esculenta) modulates some parameters of testosterone propionate-induced rat model of benign prostatic hyperplasia. Drug Chem Toxicol. 2021 doi: 10.1080/01480545.2021.1892956. [DOI] [PubMed] [Google Scholar]
- Eleazu K, Maduabuchi Aja P, Eleazu CO. Cocoyam (Colocasia esculenta) modulates some parameters of testosterone propionate-induced rat model of benign prostatic hyperplasia. Drug Chem Toxicol. 2022;45(5):1923–1933. doi: 10.1080/01480545.2021.1892956. [DOI] [PubMed] [Google Scholar]
- Elekofehinti OO, Iwaloye O, Josiah SS, Lawal AO, Akinjiyan MO, Ariyo EO. Molecular docking studies, molecular dynamics and ADME/tox reveal therapeutic potentials of STOCK1N-69160 against papain-like protease of SARS-CoV-2. Mol Diversity. 2021;25(3):1761–1773. doi: 10.1007/s11030-020-10151-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber MS, Jetter A, Fuhr U. Assessment of CYP1A2 activity in clinical practice: why, how, and when? Basic Clin Pharmacol Toxicol. 2005;97(3):125–134. doi: 10.1111/j.1742-7843.2005.pto_973160.x. [DOI] [PubMed] [Google Scholar]
- Hasan M, Parveen F, Shamsuzzaman A, Kibria M. Comparison of efficacy between Tamsulosin and Finasteride on symptomatic Benign Prostatic Hyperplasia. Mymensingh Med j: MMJ. 2007;16(2):154–159. [PubMed] [Google Scholar]
- Hilmer S, Gnjidic D. The effects of polypharmacy in older adults. Clin Pharmacol Ther. 2009;85(1):86–88. doi: 10.1038/clpt.2008.224. [DOI] [PubMed] [Google Scholar]
- Lepor H. Alpha blockers for the treatment of benign prostatic hyperplasia. Rev Urol. 2007;9(4):181. [PMC free article] [PubMed] [Google Scholar]
- Li J, Tian Y, Guo S, Gu H, Yuan Q, Xie X. Testosterone-induced benign prostatic hyperplasia rat and dog as facile models to assess drugs targeting lower urinary tract symptoms. PLoS ONE. 2018;13(1):e0191469. doi: 10.1371/journal.pone.0191469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinski CA. Lead-and drug-like compounds: the rule-of-five revolution. Drug Discov Today Technol. 2004;1(4):337–341. doi: 10.1016/j.ddtec.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Miano R, De Nunzio C, Asimakopoulos AD, Germani S, Tubaro A. Treatment options for benign prostatic hyperplasia in older men. Med Sci Monit: Int Med J Exp Clini Res. 2008;14(7):94–102. [PubMed] [Google Scholar]
- National Institutes of Health (1985) Guide for the care and use of laboratory animals, National Academies
- Niba LL. Processing effects on susceptibility of starch to digestion in some dietary starch sources. Int J Food Sci Nutr. 2003;54(1):97–109. doi: 10.1080/0963748031000042038. [DOI] [PubMed] [Google Scholar]
- Nisha CM, Kumar A, Nair P, Gupta N, Silakari C, Tripathi T, Kumar A. Molecular docking and in silico ADMET study reveals acylguanidine 7a as a potential inhibitor of β-secretase. Adv Bioinform. 2016 doi: 10.1155/2016/9258578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olajide R, Akinsoyinu A, Babayemi O, Omojola A, Abu A, Afolabi K. Effect of processing on energy values, nutrient and anti-nutrient components of wild cocoyam (Colocasia esculenta (L.) Schott) corm. Pak J Nutr. 2011;10(1):29–34. doi: 10.3923/pjn.2011.29.34. [DOI] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem. 2004;25(13):1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Powers R. Advances in nuclear magnetic resonance for drug discovery. Expert Opin Drug Discov. 2009;4(10):1077–1098. doi: 10.1517/17460440903232623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigatti P, Brausi M, Scarpa R, Porru D, Schumacher H, Rizzi C. A comparison of the efficacy and tolerability of tamsulosin and finasteride in patients with lower urinary tract symptoms suggestive of benign prostatic hyperplasia. Prostate Cancer Prostatic Dis. 2003;6(4):315–323. doi: 10.1038/sj.pcan.4500680. [DOI] [PubMed] [Google Scholar]
- Rokosh DG, Simpson PC. Knockout of the α1A/C-adrenergic receptor subtype: the α1A/C is expressed in resistance arteries and is required to maintain arterial blood pressure. Proc Natl Acad Sci. 2002;99(14):9474–9479. doi: 10.1073/pnas.132552699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner MS, Couch RC, Raghow S, Stauffer D. The chimpanzee as a model of human benign prostatic hyperplasia. J Urol. 1999;162(4):1454–1461. doi: 10.1016/S0022-5347(05)68340-1. [DOI] [PubMed] [Google Scholar]
- Studio D (2008) Discovery Studio. Accelrys [2.1]
- Tarter T, Vaughan E., Jr Inhibitors of 5α-reductase in the treatment of benign prostatic hyperplasia. Curr Pharm Des. 2006;12(7):775–783. doi: 10.2174/138161206776056010. [DOI] [PubMed] [Google Scholar]
- Tiwari A, Krishna N, Nanda K, Chugh A. Benign prostatic hyperplasia: an insight into current investigational medical therapies. Expert Opin Investig Drugs. 2005;14(11):1359–1372. doi: 10.1517/13543784.14.11.1359. [DOI] [PubMed] [Google Scholar]
- Umamaheswari M, Madeswaran A, Asokkumar K. Virtual screening analysis and in-vitro xanthine oxidase inhibitory activity of some commercially available flavonoids. Iran J Pharmac Res: IJPR. 2013;12(3):317. [PMC free article] [PubMed] [Google Scholar]
- Valdés-Tresanco MS, Valdés-Tresanco ME, Valiente PA, Moreno E. AMDock: a versatile graphical tool for assisting molecular docking with Autodock Vina and Autodock4. Biol Direct. 2020;15(1):1–12. doi: 10.1186/s13062-020-00267-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura S, Oliver V, White C, Xie J, Haynes J, Exintaris B. Novel drug targets for the pharmacotherapy of benign prostatic hyperplasia (BPH) Br J Pharmacol. 2011;163(5):891–907. doi: 10.1111/j.1476-5381.2011.01332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.