Abstract
Introduction
Erectile dysfunction (ED) is a common disease among elderly men, and novel therapy methods are needed for drug-refractory ED. As an extracellular vesicle, stem cell–derived exosomes displayed erectile function improvement in rat ED models in some preclinical studies. However, the therapeutic efficacy has not been comprehensively evaluated.
Aim
To study the therapeutic effects of stem cell–derived exosomes on ED in preclinical studies and to investigate the potential mechanisms responsible for the efficacy.
Methods
The systematic literature search was conducted in Web of Science, PubMed, and Embase to retrieve studies utilizing stem cell–derived exosomes for ED treatment. We extracted data of intracavernous pressure/mean artery pressure (ICP/MAP), and cavernosum structural changes in rat ED models before and after stem cell-derived exosome therapy. RevMan 5.3 was used to perform meta-analyses of ICP/MAP and cavernosum microstructural changes. Publication bias was assessed with the Egger test and funnel plot by Stata 15.0 (StataCorp).
Main Outcome Measures
Outcomes included ICP/MAP, smooth muscle, and endothelial markers—such as the ratio of smooth muscle to collagen and the expression of α-SMA (alpha smooth muscle actin), CD31 (cluster of differentiation 31), nNOS and eNOS (neuronal and endothelial nitric oxide synthase), TGF-β1 (transforming growth factor β1), and caspase 3 protein-to evaluate erectile function and microstructural changes. Forest plots of effect sizes were performed.
Results
Of 146 studies retrieved, 11 studies were eligible. Pooled analysis showed that stem cell–derived exosomes ameliorated damaged ICP/MAP (standardized mean difference, 3.68; 95% CI, 2.64-4.72; P < .001) and structural changes, including the ratio of smooth muscle to collagen and the expression of α-SMA, CD31, nNOS, eNOS, TGF-β1, and caspase 3 protein. Subgroup analysis indicated that exosome type and ED model type made no difference to curative effects.
Conclusion
This meta-analysis suggests the therapeutic efficacy of stem cell–derived exosomes for ED. Exosomes may restore erectile function by optimizing cavernosum microstructures.
Keywords: exosome, erectile dysfunction, intracavernous pressure/mean artery pressure, stem cell, structural changes, meta-analysis
Introduction
Erectile dysfunction (ED) refers to the impotence to obtain or maintain an erection enough to permit satisfactory sexual intercourse.1 The incidence grows with age, especially in men aged >40 years, and it affects quality of life, causing physiologic and psychological problems.2 ED is an important complication in men with diabetes mellitus for its multifactorial pathophysiology, and more attention has been focused on postradical prostatectomy ED due to the growing incidence of prostate cancer in line with an increasing male life expectancy.3 Many other factors are reported to be involved with ED, such as cardiovascular diseases, metabolic syndrome, neuropathic damage, lower urinary tract symptoms, Peyronie disease (PD), obstructive sleep apnea, and psychiatric disorders.4–7 In animal models of ED, intracavernous pressure measurement for penile erection induced by electrical stimulation of the cavernous nerve has been widely adopted by researchers for evaluation of erectile function.8,9 It has been reported that ED was associated with decreased expression of endothelial markers (VEGF, endothelial nitric oxide synthase [eNOS], cluster of differentiation 31 [CD31], etc), smooth muscle markers (α-actin, smoothelin, etc), and pericyte markers (CD146 and NG2).10,11
In terms of therapies and management, oral phosphodiesterase type 5 inhibitors, such as sildenafil and tadalafil,12,13 were regarded as the first-line treatment of ED.4 Other treatment modalities include intracavernous injection therapy, testosterone therapy, vacuum constrictive devices, and penile prostheses. In addition, some researchers utilized low-intensity extracorporeal shock wave therapy and low-intensity pulsed ultrasound therapy to improve erectile function and penile hemodynamic by inducing neovascularization and promoting tissue regeneration.14,15 However, most of them are far from flawless. A certain proportion of patients with ED do not respond to phosphodiesterase type 5 inhibitors.16 Vacuum constrictive devices are expensive and may induce unnatural erections, which cannot meet the satisfaction of patients. Low-intensity extracorporeal shock wave therapy costs too much, and the actual physiologic changes of the penile tissue and the long-term risk of shock waves are not fully elucidated. Therefore, there is still a great need for more effective treatments that can provide long-lasting improvement for ED.
Exosomes refer to a class of extracellular vesicles with a diameter of 50 to 100 nm, which are secreted by almost all cells.17 They usually encapsulate a complex payload containing lipids, signaling proteins, and nucleic acids, thus enabling cells to exchange information for multiple physiologic and pathologic functions.18 Accordingly, the beneficial effects of exosomes on ED in rat models have been found in recent experiments.19,20 Among these studies, exosomes are mostly derived from stem cells, including bone marrow–derived mesenchymal stem cells (BMSCs), adipose-derived mesenchymal stem cells (ADSCs), and human urine–derived stem cells. However, the value of stem cell–derived exosomes in ED treatment has not been comprehensively interpreted. We tried to explore whether exosomes derived from stem cells have therapeutic effects on ED in rat models. Additionally, we attempted to address the following problems: (1) Among exosomes derived from different stem cells—ADSCs, BMSCs, and human urine–derived stem cells, which have better therapeutic efficacy? (2) Among different ED models—diabetic mellitus, cavernous nerve injury, PD, artery injury, and chronic intermittent hypoxia, which can be ameliorated better by exosomes therapy?
Methods
Literature search strategy and selection criteria
This meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses.21 Literature retrieval was conducted in PubMed, Web of Science, and Embase for pertinent studies. We also utilized preprint databases, including bioRxiv and medRxiv, to find potential articles without peer review to avoid publication bias.
The keywords were as follows: (“stem cell” or “SC”) and (“exosomes” or “extracellular vesicles”) and (“erectile dysfunction” or “ED”). Additionally, we hand-searched the references of all relevant articles if necessary. We did not apply any language restrictions. Reviews, duplicates, conference abstracts, and clinical trials were excluded. Abstracts were screened for relevance, and the full texts were read when it was unclear from the abstracts.
The inclusion criteria were as follows: randomized/nonrandomized controlled animal experiment, rat/mouse model, and the utilization of exosomes to treat ED.
Quality assessment
Two investigators were assigned to separately assess the methodological quality of included studies. The ARRIVE criteria22 and ESSM guidelines23 for reporting intracavernous pressure/mean artery pressure (ICP/MAP) were applied in assessment standards. There are 27 criteria, 1 point for each (not mentioned or unclear, 0; yes, 1). Studies with a score ≥18 were considered high quality, and studies with a score <18 were considered moderate quality.
Data extraction
Data were extracted independently by 2 authors of our team. The third author was involved when the 2 independent authors disagreed and failed to reach consensus after referring to the original articles. The following information from each study was extracted: first author, year of publication, source of exosomes, exosome indicators, ED model, species, follow-up time, injection frequency, injection methods, dose of exosomes injected, and molecular changes after exosome therapy. ICP/MAP was the primary outcome. Structural changes were also collected: the ratio of smooth muscle to collagen (SM/collagen), CD31, alpha smooth muscle antibody (α-SMA), eNOS, neural nitric oxide synthase (nNOS), the apoptotic protein cleaved caspase 3, and transforming growth factor β1 (TGF-β1).
The mean and SEM or SD were extracted from the included article texts. The software Web Plot Digitizer (https://automeris.io/WebPlotDigitizer/) was used to extract numeric values from charts if final results were displayed only as graphs and we failed to receive a reply from the corresponding authors of articles.
Statistical analyses
We used RevMan 5.3 software (The Nordic Cochrane Center) to analyze extracted data. To show the difference of ICP/MAP between the exosome therapy groups and the ED control groups, we used standardized mean difference with 95% CIs, which was also applied to structural changes in the corpus cavernosum, including SM/collagen and the expression of CD31, α-SMA, eNOS, nNOS, TGF-β1, and caspase 3 protein. Heterogeneity was evaluated with the I2 statistic test. A random effects model was adopted if I2 ≥ 50%, and a fixed effects model would be applicable if I2 < 50%. Stata (version 15.0; StataCorp) was used to examine publication bias with the Egger test24,25 and funnel plot. In addition, a P value <.05 in the Egger test was considered statistically significant for publication bias.
Meanwhile, subgroup analysis was used to investigate the possible source of heterogeneity among these studies. Subgroup analysis of ICP/MAP was based on 2 factors:
Exosome source cell: ADSCs vs BMSCs vs human urine–derived stem cells
ED model type: diabetic mellitus vs cavernous nerve injury vs PD vs artery injury vs chronic intermittent hypoxia resembling obstructive sleep apnea
Results
Study selection and characteristics
As shown in Figure 1, 146 publications were identified after the search. We eventually enrolled 11 studies as a result of full-text review. The characteristics of eligible studies are described in Table 1.
Figure 1.
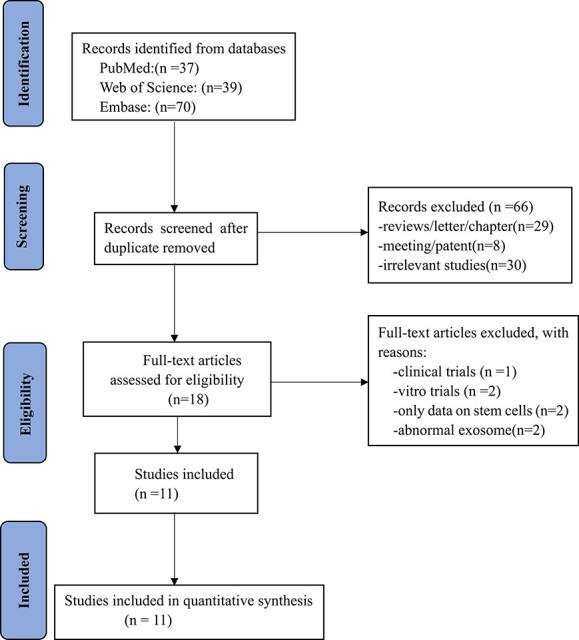
Flowchart of study selection.
Table 1.
Characteristics of included studies.
| Year | First author | Producer cell | Isolation method | Exosome labels | Exosome dose, μg a | ED model | Establishment method | |
|---|---|---|---|---|---|---|---|---|
| 2017 | Chen26 | ADSC | Multistep centrifugation | CD63, CD81, calnexin | 100 | T2DM | High-fat diet, intraperitoneal injection of low-dose STZ (30 mg/kg) | |
| 2021 | Liang27 | ADSC | ExoQuick-TC reagent | CD9, CD63, TSG101 | 400 | Hypoxia | Chronic intermittent hypoxia exposure | |
| 2020 | Song32 | ADSC, BMSC | Multistep centrifugation | CD9, CD63, TSG101, calnexin | 100 | T1DM | Intraperitoneal injection of STZ (60 mg/kg) | |
| 2018 | Li20 | BMSC, ADSC | PureExo exosome isolation kit | CD63, HSP70, CD81 | 100 | CNI | Bilateral cavernous nerve crush injury | |
| 2020 | Wang28 | ADSC | Ultracentrifugation, ultrafiltration | CD31, CD9, CD63, CD81 | 200 | T1DM | Intraperitoneal injection of STZ (60 mg/kg) | |
| 2019 | Liu31 | BMSC | Multistep centrifugation | CD9, TSG101 | 50 or 100 | AI b | Internal iliac artery ligation | |
| 2018 | Ouyang19 | BMSC | Multistep centrifugation | CD63, TSG101, flotillin-1 | 100 | CNI | Bilateral cavernous nerves crush injury | |
| 2022 | Liang29 | ADSC | Differential centrifugation | CD63, CD9 | 150 c | CNI | Bilateral cavernous nerves crush injury | |
| 2018 | Zhu30 | ADSC | Exosome precipitation solution, ExoQuick | CD63, CD9 | 10 or 100 | T1DM | Intraperitoneal injection of STZ (65 mg/kg) | |
| 2020 | Yang33 | HUSC | Ultracentrifugation, ultrafiltration | CD9, CD63, TSG101, alix | 100 | PD | Intratunical injection of TGF-β1 | |
| 2019 | Ouyang34 | HUSC | Ultracentrifugation | CD63, calnexin | 100 | T1DM | Intraperitoneal injection of STZ (50 mg/kg) | |
| Year | First author | Species | Animal age, wk | Injection method | Frequency | Follow-up, wk | Investigated parameters | Cargo |
| 2017 | Chen26 | SD rats | 6 | IC | 1 | 4 | ICP/MAP, CD31, Bcl-2, caspase 3 | Not mentioned |
| 2021 | Liang27 | SD rats | Not mentioned | IC | 8 | 8 | ICP/RT-AP, α-SMA, eNOS | miR-301a-3p |
| 2020 | Song32 | SD rats | 8 | IC | 1 | 4 | ICP/MAP, NO, cGMP | Not mentioned |
| 2018 | Li20 | SD rats | 12 | IC | 1 | 3 | nNOS, vWF,a-SMA, SM/collagen | Not mentioned |
| 2020 | Wang28 | SD rats | 8 | Intravenous | 1 | 2 | ICP/MAP, ANP, BNP, nNOS | Corin |
| 2019 | Liu31 | SD rats | 12 | IC | 1 | 4 | ICP/MAP, CD31, VEGFA, nNOS, eNOS, α-SMA, SM/collagen, collagen content | Not mentioned |
| 2018 | Ouyang19 | SD rats | 10 | IC | 1 | 4 | ICP/MAP, nNOS, SM/collagen, caspase 3 | Not mentioned |
| 2022 | Liang29 | SD rats | 6-8 | IC | 1 | 3 | MICP/MAP, α-SMA, eNOS, nNOS | Not mentioned |
| 2018 | Zhu30 | SD rats | 10 | Corpus cavernosum injection | 1 | 4 | ICP/MAP, SM/collagen, endothelial content | microRNAs |
| 2020 | Yang33 | SD rats | Not mentioned | Intratunical | 1 | 4 | ICP/MAP, elastin, TGF-β1, collagen III, SM/collagen, Smad2/3 protein | Not mentioned |
| 2019 | Ouyang34 | SD rats | Not mentioned | IC | 1 | 4 | CD31, eNOS, phospho-eNOS, nNOS, SM/collagen | miRNA |
Abbreviations: α-SMA, alpha smooth muscle actin; ADSC, adipose-derived mesenchymal stem cell; ANP, pro-atrial natriuretic peptide; BMSC, bone marrow–derived mesenchymal stem cell; BNP, pro-brain natriuretic peptide; CD31, cluster of differentiation 31; cGMP, cyclic guanosine monophosphate; CNI, cavernous nerve injury; ED, erectile dysfunction; eNOS, endothelial nitric oxide synthase; HUSC, human urine–derived stem cells; IC, intracavernous injection; ICP/MAP, intracavernous pressure/mean artery pressure; MICP, maximum intracavernous pressure; nNOS, neuronal nitric oxide synthase; NO, nitric oxide; PD, Peyronie disease; RT-AP, real-time carotid arterial pressure; SD, Sprague-Dawley; SM/collagen, ratio of smooth muscle to collagen; STZ, streptozotocin; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; TGF-β1, transforming growth factor β1; vWF, von willebrand factor.
aThe amount of stem cell–derived exosomes dissolved in phosphate-buffered saline.
bArtery injury by bilateral internal iliac artery ligation.
cThis study utilized a polydopamine nanoparticle–incorporated poly(ethylene glycol)-poly(ε-caprolactone-co-lactide) gel as a solvent of exosomes.
Exosomes derived from ADSCs were applied in 5 studies,26–30 and exosomes derived from BMSCs were used in 2 studies.19,31 Both parental cells were utilized in another 2 studies.20,32 The remaining 2 studies exploited exosomes derived from human urine–derived stem cells.33,34
Regarding ED models, 5 studies26,28,30,32,34 injected streptozotocin into animals to establish diabetic mellitus model, in which 1 study constructed a type 2 diabetic mellitus model by high-fat diet. Three studies19,20,29 constructed a neurogenic ED model by damaging cavernous nerves surgically. Injection of TGF-β1 into rat tunica albuginea was utilized in 1 study to create a PD model,33 and 1 research utilized chronic intermittent hypoxia to mimic obstructive sleep apnea–induced ED.27 Another study focused on artery injury–indued ED.31
Administration of exosomes by intracavernous injection was used in 9 studies, while the other 2 studies used intratunical or intravenous injection.
Quality assessment
The quality score of 6 studies was ≥18, and the other 5 studies received <18 points. Details are shown in Table 2.
Table 2.
Quality assessment of included studies.
| Year | Study | Study design | Sample Size | Inclusion and exclusion | Randomization | Statistic | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Experiment unit | No. | Calculation | Criteria | Exclusion | No. | Randomization | Confounder | Blinding | Outcome measure | Methods, software | Assumption | ||||
| 2017 | Chen26 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | ||
| 2021 | Liang27 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | ||
| 2020 | Song32 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | ||
| 2018 | Li20 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | ||
| 2020 | Wang28 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | ||
| 2019 | Liu31 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | ||
| 2018 | Ouyang19 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | ||
| 2022 | Liang29 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | ||
| 2018 | Zhu30 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | ||
| 2020 | Yang33 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | ||
| 2019 | Ouyang34 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | ||
| Year | Study | Animals | Procedures | Results | ICP/MAP | Cell | Exosome | |||||||||
| Species | Further | What | When | Where | Why | Summary | Nommalization by MAP | Images of traces | APO test a | Phenotype b | Label | Morphology | Structural changes | Score | ||
| 2017 | Chen26 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 20 |
| 2021 | Liang27 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 20 |
| 2020 | Song32 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 20 |
| 2018 | Li20 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 18 |
| 2020 | Wang28 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 15 |
| 2019 | Liu31 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 18 |
| 2018 | Ouyang19 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 18 |
| 2021 | Liang29 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 13 |
| 2018 | Zhu30 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 17 |
| 2020 | Yang33 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 19 |
| 2019 | Ouyang34 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 21 |
Abbreviation: ICP/MAP, intracavernous pressure/mean artery pressure.
aApomorphine test to verify erectile dysfunction before administration of exosomes.
bStem cell phenotype identification.
Meta-analysis
Intracavernous pressure/mean artery pressure
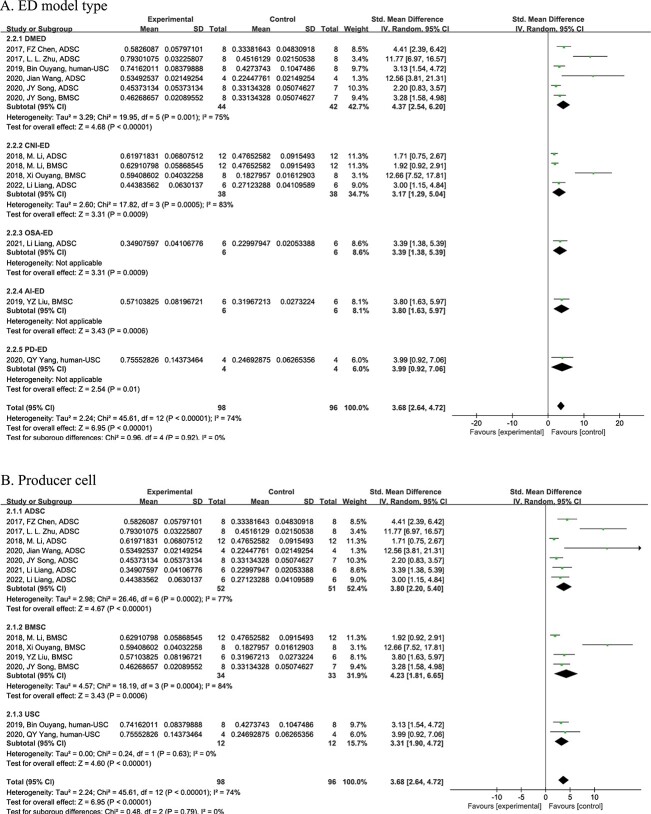
The pooled analysis showed that stem cell–derived exosome therapy ameliorated ICP/MAP significantly (n = 194; standardized mean difference, 3.68; 95% CI, 2.64-4.72; Z = 6.95, P < .01; χ2 = 45.61, I2 = 74%), which hints at the improvement of erectile function (Figure 2A).
Figure 2.
Forest plot for the ICP/MAP changes among different subgroups: (A) ED model type and (B) producer cell. ED, erectile dysfunction; ICP/MAP, intracavernous pressure/mean artery pressure.
Subgroup analysis of ICP/MAP was conducted on the basis of 2 factors: ED model and producer cell. First, the analysis showed that in different ED model types, an increase of ICP/MAP occurred after administration of exosomes as compared with respective controls (diabetic mellitus, P < .01; cavernous nerve injury, P < .01; obstructive sleep apnea, P < .01; artery injury, P < .01; PD, P < .01). However, it showed no statistically significant difference in growth among different ED models (χ2 = 0.96, P = .92) (Figure 2A). Second, exosomes generated by different stem cells could all enhance ICP/MAP vs various controls (ADSCs, P < .01; BMSCs, P < .01; human urine, P < .01). However, no statistically significant difference was observed in producer cell types (χ2 = 0.48, P = .79) (Figure 2B).
Structural changes
To investigate the underlying mechanism of exosome therapy for ED, structural changes were analyzed. Stem cell–derived exosomes restored SM/collagen (n = 144, P < .01), CD31 (n = 44, P < .001), α-SMA (n = 82, P < .006), nNOS (n = 82, P = .03), and eNOS (n = 58, P < .001) damaged by ED, which indicated the amelioration of endothelium and smooth muscle content of cavernosum. Furthermore, the decreases of TGF-β1 (n = 38, P = .003) and caspase 3 (n = 32, P < .001) were observed in the analysis, which meant that exosomes might improve cavernosum structures by inhibiting fibrosis and apoptosis (Table 3).
Table 3.
Analyses of structural changes.
| Biomarker | No. | SMD | 95% CI | Z | P value | χ 2 | I 2 ,% |
|---|---|---|---|---|---|---|---|
| SM/collagen | 144 | 3.71 | 3.10, 4.32 | 11.92 | <.001 | 12.91 | 38 |
| CD31 | 44 | 5.32 | 3.86, 6.78 | 7.14 | <.001 | 2.73 | 27 |
| α-SMA | 82 | 3.57 | 1.01, 6.14 | 2.73 | .006 | 36.3 | 86 |
| eNOS | 58 | 3.27 | 2.39, 4.15 | 7.32 | <.001 | 1.6 | 0 |
| nNOS | 82 | 2.12 | 0.22, 4.03 | 2.19 | .03 | 41.75 | 88 |
| TGF-β1 | 38 | −4.3 | −7.17, −1.43 | 2.94 | .003 | 8.48 | 76 |
| caspase 3 | 32 | −4.42 | −5.86, −2.99 | 6.04 | <.001 | 0.56 | 0 |
Abbreviations: α-SMA, alpha smooth muscle actin; CD31, cluster of differentiation 31; eNOS, endothelial nitric oxide synthase; nNOS, neuronal nitric oxide synthase; SM/collagen, ratio of smooth muscle to collagen; SMD, standardized mean difference; TGF-β1, transforming growth factor β1.
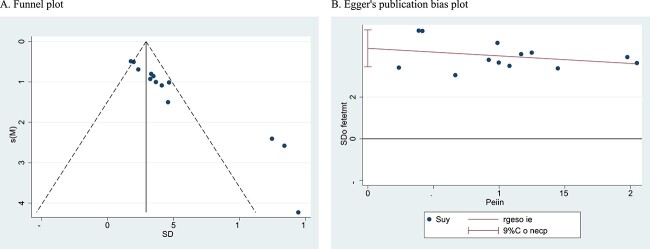
Bias assessment
The funnel plot appeared to be asymmetrical, which indicated that there was publication bias in the ICP/MAP analysis. Furthermore, the Egger test was used to detect publication bias, and its P value (t = 10.77, P < .05) showed bias from small study effects (Figure 3).
Figure 3.
Publication bias test of ICP/MAP: (A) funnel plot and (B) Egger publication bias plot. ICP/MAP, intracavernous pressure/mean artery pressure.
Discussion
A total of 11 published preclinical studies were included in our analysis. Overall, our analysis suggests that stem cell–derived exosomes could ameliorate ED and structural changes in various types of ED models.
Penile erection is a series of vascular events closely related to the endothelium and smooth muscle cells of the corpus cavernosum, which histologically form the basic structure of sinusoids. When the smooth muscle is contracted, the blood inflows through the cavernous artery restrictively, but it outflows through the subtunical venular plexus freely, resulting in a flaccid state of the penis.35 Upon sexual stimulation, nonadrenergic noncholinergic nerve fibers release nitric oxide (NO), which activates guanylyl cyclase to increase the concentration of cGMP (cyclic guanosine monophosphate). Furthermore, acetylcholine released from parasympathetic cholinergic nerve fibers causes activation of adenylyl cyclase, increasing the concentration of cAMP (cyclic adenosine monophosphate). High levels of cGMP and cAMP decrease intracellular Ca2+ levels and lead to smooth muscle cell relaxation, followed by a normal erection. If any of these processes are interrupted, ED may happen. For example, cavernous nerve injury causes downregulation in the nerve signaling of the corpora cavernosa, which reduces the NO level in smooth muscle, increases apoptosis in the smooth muscle and endothelium of blood vessels, and upregulates fibrogenetic cytokines to form collagenization of the smooth muscle. These functional and structural changes lead to veno-occlusive dysfunction.36–38 Hypoxia can cause a decrease in prostaglandin E1 levels of the corpora cavernosa, which commonly inhibits profibrotic cytokines such as TGF-β1.39,40 These profibrotic cytokines enhance collagen deposition, decrease the smooth muscle content, reduce the elasticity of the penis, and impair the ability of the cavernosa to compress the subtunical veins, causing veno-occlusive dysfunction.36 As reported, the mechanisms of diabetic ED observed in rat models may include elevated glycation end products and oxygen-free radical levels, which impaired synthesis of nNOS and decreased cGMP-dependent kinase 1.41,42 In a word, ED is a multifactorial condition with a complex neurovascular process, which is strongly associated with the loss and dysfunction of the corporal endothelium and smooth muscle.
Clinically, refractory male ED shows resistance to drug therapy. Facing this obstacle, stem cell therapy is recognized as a promising novel method in ED treatment, and considerable studies have proved its feasibility in animal models and clinical trials,43,44 which may be attributed to their capability of self-renewal, proliferation, and multipotential differentiation. Moreover, the regenerative properties have been established in tissue engineering and regenerative medicine research.45,46
Some studies recently considered that the beneficial effects of transplanted stem cells could not be merely explained by engraftment or differentiation into specific cells.47 Scientists have paid more attention to the paracrine secretion of stem cells, including chemoattractant molecules, bioactive factors, and extracellular vesicles.48,49 Exosomes are 50- to 100-nm membrane-bound extracellular vesicles, in which content varies depending on the original cells and the activation status, including noncoding small RNAs, mRNAs, proteins, and lipids.50 Exosomes have been proved to serve multiple physiologic and pathologic functions via regulating intercellular communication.51 Lai et al52 reported that exosomes derived from mesenchymal stem cells (MSCs) exerted a protective effect on cardiac tissue following myocardial infarction. Zhang et al53 demonstrated that MSC-derived exosomes effectively promoted functional recovery in rats after traumatic brain injury by facilitating endogenous angiogenesis and neurogenesis. When compared with stem cell therapy, exosomes have many advantages, such as greater stability and ease of storage and management, preclusion of the risk of tumor formation, and a lower likelihood of an immune rejection.19,26
Similar to stem cell therapy for ED, our analysis showed that stem cell–derived exosomes increased SM/collagen and the expression of α-SMA, CD31, nNOS, and eNOS damaged in ED. CD31 can be considered a biomarker of endothelium contents,54 while α-SMA and SM/collagen indicated the smooth muscle contents in the corpus cavernosum of rats. This hints that exosomes could improve the tissue structure of the corpus cavernosum to ameliorate erectile function. Moreover, our study suggested the downregulated expression level of TGF-β1 and caspase 3. As a kind of profibrotic cytokine, TGF-β1 was recognized as a key factor related to the formation and development of corporal fibrosis as in PD.55 Kim et al reported that the activation of TGF-β1 signaling initiated collagen accumulation and deposition.56 The antifibrotic effect of exosomes has been demonstrated by some studies in different diseases, such as liver and lung fibrosis.57,58 The downregulation of TGF-β1 in our analysis may hint that the exosomes can also have an antifibrotic effect on ED. Activation of caspases was recognized as the biochemical marker for apoptosis, which is widely used in apoptotic signals examination.59 Vasculogenic ED induced by artery injury was characterized as the ischemic and hypoxic state of the corpus cavernosum, which may increase the release of reactive oxygen species, leading to cell apoptosis.60,61 It is reported that oxidative stress in penile ischemia is an important factor in ED progress.60 In our analysis, the administration of stem cell–derived exosomes decreased the expression level of caspase 3 and TGF-β1, which indicated that exosomes possess the ability to inhibit fibrosis and apoptosis, ensuring the functional endothelium and smooth muscle contents in the corpus cavernosum. The NO/cGMP signaling pathway was important to regulate penile erection, and downregulation of this pathway contributed to ED.62 NO produced by eNOS in cavernous endothelial cells and nNOS in cavernous nerves induce erection by increasing the cGMP content in the smooth muscle cells of the corpus cavernosum.35,63 Song et al reported that exosomes derived from smooth muscle cells regulated the NO/cGMP pathway to ameliorate ED.32 The levels of eNOS and nNOS significantly increased after exosome therapy in our analysis, which indicated that stem cell–derived exosomes might make functional changes in the corpus cavernosum via the NO/cGMP signaling pathway.32 The outcome was consistent with the change of ICP/MAP.
In our meta-analysis, only 2 studies used exosomes generated from human urine–derived stem cells33,34; the others used exosomes derived from ADSCs or BMSCs. In our analysis, 3 kinds of exosomes showed no difference in the therapeutic efficacy. Although exosomes can be generated by most cells, the exosomes derived from MSCs were used in most research to treat ED. MSCs can be isolated from several tissues, such as bone marrow, adipose tissue, Wharton jelly tissue, umbilical cord blood, and neonatal teeth.64 Among them, adipose-derived stem cells and bone marrow–derived stem cells were exploited the most. Noncoding RNAs, such as miRNA, snoRNA, and tRNA, enriched in the exosomes produced by stem cells, may exert important biological functions by conveying properties of parental cells. For example, tRNAs accounted for >50% of the total small RNAs in the exosomes derived from ADSCs, as opposed to merely 23% to 25% in BMSC-derived exosomes.65 Interestingly, some specific tRNAs were more abundant in exosomes than the source cells. It has been proved that miRNAs were the major content of cellular small RNA in MSCs, and the discrepancy might suggest preferential sorting and release.50,65 Moreover, even exosomes originating from the same parental cells might exhibit heterogeneity of content.66–68 The subcellular origin and cell activation status were responsible for the molecular heterogeneity of exosomes.69,70 Due to the limitation of exosome isolation methods, bulk isolates rather than pure exosome population isolates were used in a majority of studies upon evaluation of their therapeutic efficacy.50 Exosomes isolated from urine also contained substantial noncoding small RNAs, such as tRNA and rRNA, while the exact functions need further study. The research on exosome-mediated communication mostly focused on well-known RNA species, such as miRNAs and mRNAs, for the sake of detection sensitivity and specificity of exosome contents.50 Zhu et al30 found that ADSC-derived exosomes contained some microRNAs with proangiogenic (miR-126, miR-130a, and miR-132) and antifibrotic (miR-let7b and miR-let7c) functions. Simultaneously, proteins on the membrane of and in the vesicles get involved in inter- and intracellular signaling mediation. Wang et al28 used the transmembrane serine protease corin in ADSC-derived exosomes to improve ED in diabetic rats and suggested that it may play a role through the ANP/NO/cGMP signaling pathway.
To the best of our knowledge, this is the first meta-analysis providing comprehensive insights into the effects of stem cell–derived exosomes on ED in rats. The value of a systematic review of experimental animal studies has been steadily understood.71,72 The consistent results of exosome therapy efficacy across various ED models in our study could provide reassurance that human beings might also respond in the same way.
There are still several limitations in this study. A high degree of heterogeneity remains in ICP/MAP outcome after subgroup analysis. This may be attributed to the methodological heterogeneity of the studies. Specifically, the exosome types, extraction methods, and animal models used in studies were quite different. Given the limited number of included studies, a persuasive subgroup analysis cannot be performed. Moreover, different software (eg, SPSS, GraphPad Prism, and Stata) was applied across the studies, which may cause high statistical heterogeneity.
The Egger test shows publication bias in the analysis, which may be attributed to the fact that articles with negative conclusions are less likely to be published and retrieved. However, we tried our best to retrieve animal intervention studies on exosome treatment for ED, including preprint databases such as bioRxiv and medRxiv, but failed to find more relevant research, perhaps because exosome therapy for ED is a relatively new topic. Despite the existence of publication bias, our analysis still shows the therapeutic effects of exosomes on ED.
Conclusion
This meta-analysis reveals the therapeutic effects of stem cell–derived exosomes on ED rat models. Exosome administration may improve erectile function by activating the NO/cGMP signaling pathway, ameliorating endothelium, and inhibiting the fibrosis and apoptosis of cavernosum. Stem cell–derived exosomes have great potential to afford a novel cell-free therapy for ED. However, further studies are needed to identify the functional components of exosomes, and clinical trials may be worthwhile to demonstrate the actual effects on the human body.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81871157).
Conflicts of interest: The authors report no conflicts of interest.
Contributor Information
Yunpei Zhu, Department of Urology, Zhongda Hospital, Southeast University, Nanjing, 210009, China; Institute of Urology, Medical College, Southeast University, Nanjing, 210009, China.
Tiancheng Jiang, Department of Urology, Zhongda Hospital, Southeast University, Nanjing, 210009, China; Institute of Urology, Medical College, Southeast University, Nanjing, 210009, China.
Chi Yao, Department of Urology, Zhongda Hospital, Southeast University, Nanjing, 210009, China; Institute of Urology, Medical College, Southeast University, Nanjing, 210009, China.
Jiawei Zhang, Department of Urology, Zhongda Hospital, Southeast University, Nanjing, 210009, China; Institute of Urology, Medical College, Southeast University, Nanjing, 210009, China.
Chao Sun, Department of Urology, Zhongda Hospital, Southeast University, Nanjing, 210009, China; Institute of Urology, Medical College, Southeast University, Nanjing, 210009, China.
Shuqiu Chen, Department of Urology, Zhongda Hospital, Southeast University, Nanjing, 210009, China; Institute of Urology, Medical College, Southeast University, Nanjing, 210009, China.
Ming Chen, Department of Urology, Zhongda Hospital, Southeast University, Nanjing, 210009, China; Institute of Urology, Medical College, Southeast University, Nanjing, 210009, China; Zhongda Hospital Lishui Branch, Southeast University, Nanjing, 210009, China.
References
- 1. Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol. 1994;151(1):54–61. [DOI] [PubMed] [Google Scholar]
- 2. Brookes ST, Link CL, Donovan JL, McKinlay JB. Relationship between lower urinary tract symptoms and erectile dysfunction: results from the Boston Area Community Health Survey. J Urol. 2008;179(1):250–255. [DOI] [PubMed] [Google Scholar]
- 3. Gu X, Thakker PU, Matz EL, et al. Dynamic changes in erectile function and histological architecture after Intracorporal injection of human placental stem cells in a pelvic neurovascular injury rat model. J Sex Med. 2020;17(3):400–411. [DOI] [PubMed] [Google Scholar]
- 4. Shamloul R, Ghanem H. Erectile dysfunction. Lancet. 2013;381(9861):153–165. [DOI] [PubMed] [Google Scholar]
- 5. Malavige LS, Levy JC. Erectile dysfunction in diabetes mellitus. J Sex Med. 2009;6(5):1232–1247. [DOI] [PubMed] [Google Scholar]
- 6. Cui K, Tang Z, Li CC, et al. Lipoxin A4 improves erectile dysfunction in rats with type I diabetes by inhibiting oxidative stress and corporal fibrosis. Asian J Androl. 2018;20(2):166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kaynar M, Gomes ALQ, Sokolakis I, Gul M. Tip of the iceberg: erectile dysfunction and COVID-19. Int J Impot Res. 2022;34(2):152–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Quinlan DM, Nelson RJ, Partin AW, Mostwin JL, Walsh PC. The rat as a model for the study of penile erection. J Urol. 1989;141(3, pt 1):656–661. [DOI] [PubMed] [Google Scholar]
- 9. Yuan J, Lin H, Li P, et al. Molecular mechanisms of vacuum therapy in penile rehabilitation: a novel animal study. Eur Urol. 2010;58(5):773–780. [DOI] [PubMed] [Google Scholar]
- 10. Yao C, Zhang X, Yu Z, Jing J, Sun C, Chen M. Effects of stem cell therapy on diabetic mellitus erectile dysfunction: a systematic review and meta-analysis. J Sex Med. 2022;19(1):21–36. [DOI] [PubMed] [Google Scholar]
- 11. Liu G, Sun X, Bian J, et al. Correction of diabetic erectile dysfunction with adipose derived stem cells modified with the vascular endothelial growth factor gene in a rodent diabetic model. PLoS One. 2013;8(8):e72790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goldstein I, Lue TF, Padma-Nathan H, Rosen RC, Steers WD, Wicker PA. Oral sildenafil in the treatment of erectile dysfunction: Sildenafil Study Group. N Engl J Med. 1998;338(20):1397–1404. [DOI] [PubMed] [Google Scholar]
- 13. Cui H, Liu B, Song Z, et al. Efficacy and safety of long-term tadalafil 5 mg once daily combined with sildenafil 50 mg as needed at the early stage of treatment for patients with erectile dysfunction. Andrologia. 2015;47(1):20–24. [DOI] [PubMed] [Google Scholar]
- 14. Chung E, Wang J. A state-of-art review of low intensity extracorporeal shock wave therapy and lithotripter machines for the treatment of erectile dysfunction. Expert Rev Med Devices. 2017;14(12):929–934. [DOI] [PubMed] [Google Scholar]
- 15. Ghahhari J, De Nunzio C, Lombardo R, et al. Shockwave therapy for erectile dysfunction: which gives the best results? A retrospective national, multi-institutional comparative study of different shockwave technologies. Surg Technol Int. 2022;19(40):213–218. [DOI] [PubMed] [Google Scholar]
- 16. Hatzimouratidis K, Hatzichristou DG. A comparative review of the options for treatment of erectile dysfunction: which treatment for which patient? Drugs. 2005;65(12):1621–1650. [DOI] [PubMed] [Google Scholar]
- 17. Kourembanas S. Exosomes: vehicles of intercellular signaling, biomarkers, and vectors of cell therapy. Annu Rev Physiol. 2015;77:13–27. [DOI] [PubMed] [Google Scholar]
- 18. Hong P, Yang H, Wu Y, Li K, Tang Z. The functions and clinical application potential of exosomes derived from adipose mesenchymal stem cells: a comprehensive review. Stem Cell Res Ther. 2019;10(1):242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ouyang X, Han X, Chen Z, Fang J, Huang X, Wei H. MSC-derived exosomes ameliorate erectile dysfunction by alleviation of corpus cavernosum smooth muscle apoptosis in a rat model of cavernous nerve injury. Stem Cell Res Ther. 2018;9(1):246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li M, Lei H, Xu Y, et al. Exosomes derived from mesenchymal stem cells exert therapeutic effect in a rat model of cavernous nerves injury. Andrology. 2018;6(6):927–935. [DOI] [PubMed] [Google Scholar]
- 21. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Percie du Sert N, Hurst V, Ahluwalia A, et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. PLoS Biol. 2020;18(7):e3000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Weyne E, Ilg MM, Cakir OO, et al. European Society for Sexual Medicine consensus statement on the use of the cavernous nerve injury rodent model to study postradical prostatectomy erectile dysfunction. Sex Med. 2020;8(3):327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. JAMA. 2006;295(6):676–680. [DOI] [PubMed] [Google Scholar]
- 26. Chen F, Zhang H, Wang Z, et al. Adipose-derived stem cell–derived exosomes ameliorate erectile dysfunction in a rat model of type 2 diabetes. J Sex Med. 2017;14(9):1084–1094. [DOI] [PubMed] [Google Scholar]
- 27. Liang L, Zheng D, Lu C, et al. Exosomes derived from miR-301a-3p-overexpressing adipose-derived mesenchymal stem cells reverse hypoxia-induced erectile dysfunction in rat models. Stem Cell Res Ther. 2021;12(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang J, Mi Y, Wu S, et al. Exosomes from adipose-derived stem cells protect against high glucose-induced erectile dysfunction by delivery of corin in a streptozotocin-induced diabetic rat model. Regen Ther. 2020;14:227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liang L, Shen Y, Dong Z, Gu X. Photoacoustic image-guided corpus cavernosum intratunical injection of adipose stem cell–derived exosomes loaded polydopamine thermosensitive hydrogel for erectile dysfunction treatment. Bioact Mater. 2022;9:147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhu LL, Huang X, Yu W, Chen H, Chen Y, Dai YT. Transplantation of adipose tissue-derived stem cell–derived exosomes ameliorates erectile function in diabetic rats. Andrologia. 2018;50(2):e12871. [DOI] [PubMed] [Google Scholar]
- 31. Liu Y, Zhao S, Luo L, et al. Mesenchymal stem cell–derived exosomes ameliorate erection by reducing oxidative stress damage of corpus cavernosum in a rat model of artery injury. J Cell Mol Med. 2019;23(11):7462–7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Song J, Sun T, Tang Z, et al. Exosomes derived from smooth muscle cells ameliorate diabetes-induced erectile dysfunction by inhibiting fibrosis and modulating the NO/cGMP pathway. J Cell Mol Med. 2020;24(22):13289–13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yang Q, Chen W, Han D, et al. Intratunical injection of human urine-derived stem cells derived exosomes prevents fibrosis and improves erectile function in a rat model of Peyronie's disease. Andrologia. 2020;52(11):e13831. [DOI] [PubMed] [Google Scholar]
- 34. Ouyang B, Xie Y, Zhang C, et al. Extracellular vesicles from human urine-derived stem cells ameliorate erectile dysfunction in a diabetic rat model by delivering proangiogenic microRNA. Sex Med. 2019;7(2):241–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yafi FA, Jenkins L, Albersen M, et al. Erectile dysfunction. Nat Rev Dis Primers. 2016;2:16003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ferrini MG, Kovanecz I, Sanchez S, Umeh C, Rajfer J, Gonzalez-Cadavid NF. Fibrosis and loss of smooth muscle in the corpora cavernosa precede corporal veno-occlusive dysfunction (CVOD) induced by experimental cavernosal nerve damage in the rat. J Sex Med. 2009;6(2):415–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Leungwattanakij S, Bivalacqua TJ, Usta MF, et al. Cavernous neurotomy causes hypoxia and fibrosis in rat corpus cavernosum. J Androl. 2003;24(2):239–245. [DOI] [PubMed] [Google Scholar]
- 38. Mulhall JP, Muller A, Donohue JF, et al. The functional and structural consequences of cavernous nerve injury are ameliorated by sildenafil citrate. J Sex Med. 2008;5(5):1126–1136. [DOI] [PubMed] [Google Scholar]
- 39. Moreland RB. Is there a role of hypoxemia in penile fibrosis: a viewpoint presented to the Society for the Study of Impotence. Int J Impot Res. 1998;10(2):113–120. [DOI] [PubMed] [Google Scholar]
- 40. Moreland RB, Traish A, McMillin MA, Smith B, Goldstein I, Saenz de Tejada I. Investigative urology: PGE sub 1 suppresses the induction of collagen synthesis by transforming growth factor-beta sub 1 in human corpus cavernosum smooth muscle. J Urol. 1995;153(3):826–834. [PubMed] [Google Scholar]
- 41. Moore CR, Wang R. Pathophysiology and treatment of diabetic erectile dysfunction. Asian J Androl. 2006;8(6):675–684. [DOI] [PubMed] [Google Scholar]
- 42. Musicki B, Burnett AL. Endothelial dysfunction in diabetic erectile dysfunction. Int J Impot Res. 2007;19(2):129–138. [DOI] [PubMed] [Google Scholar]
- 43. Matz EL, Terlecki R, Zhang Y, Jackson J, Atala A. Stem cell therapy for erectile dysfunction. Sex Med Rev. 2019;7(2):321–328. [DOI] [PubMed] [Google Scholar]
- 44. Yao C, Zhang X, Yu Z, Jing J, Sun C, Chen M. Effects of stem cell therapy on diabetic mellitus erectile dysfunction: a systematic review and meta-analysis. J Sex Med. 2022;19(1):21–36. [DOI] [PubMed] [Google Scholar]
- 45. Christou I, Mallis P, Michalopoulos E, et al. Evaluation of peripheral blood and cord blood platelet lysates in isolation and expansion of multipotent mesenchymal stromal cells. Bioengineering (Basel). 2018;5(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells: the International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. [DOI] [PubMed] [Google Scholar]
- 47. Bjorge IM, Kim SY, Mano JF, Kalionis B, Chrzanowski W. Extracellular vesicles, exosomes and shedding vesicles in regenerative medicine—a new paradigm for tissue repair. Biomater Sci. 2017;6(1):60–78. [DOI] [PubMed] [Google Scholar]
- 48. da Silva ML, Caplan AI, Nardi NB. In search of the in vivo identity of mesenchymal stem cells. Stem Cells. 2008;26(9):2287–2299. [DOI] [PubMed] [Google Scholar]
- 49. Han C, Sun X, Liu L, et al. Exosomes and their therapeutic potentials of stem cells. Stem Cells Int. 2016;2016:7653489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ferguson SW, Nguyen J. Exosomes as therapeutics: the implications of molecular composition and exosomal heterogeneity. J Control Release. 2016;228:179–190. [DOI] [PubMed] [Google Scholar]
- 51. Li SP, Lin ZX, Jiang XY, Yu XY. Exosomal cargo-loading and synthetic exosome-mimics as potential therapeutic tools. Acta Pharmacol Sin. 2018;39(4):542–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lai RC, Arslan F, Lee MM, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4(3):214–222. [DOI] [PubMed] [Google Scholar]
- 53. Zhang Y, Chopp M, Meng Y, et al. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J Neurosurg. 2015;122(4):856–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. DeLisser HM, Newman PJ, Albelda SM. Molecular and functional aspects of PECAM-1/CD31. Immunol Today. 1994;15(10):490–495. [DOI] [PubMed] [Google Scholar]
- 55. Gonzalez-Cadavid NF. Mechanisms of penile fibrosis. J Sex Med. 2009;6(suppl 3):353–362. [DOI] [PubMed] [Google Scholar]
- 56. Kim KK, Sheppard D, Chapman HA. TGF-beta1 signaling and tissue fibrosis. Cold Spring Harb Perspect Biol. 2018;10(4):a022293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Li T, Yan Y, Wang B, et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. 2013;22(6):845–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kuse N, Kamio K, Azuma A, et al. Exosome-derived microRNA-22 ameliorates pulmonary fibrosis by regulating fibroblast-to-myofibroblast differentiation in vitro and in vivo. J Nippon Med Sch. 2020;87(3):118–128. [DOI] [PubMed] [Google Scholar]
- 59. Choudhary GS, Al-Harbi S, Almasan A. Caspase-3 activation is a critical determinant of genotoxic stress-induced apoptosis. Methods Mol Biol. 2015;1219:1–9. [DOI] [PubMed] [Google Scholar]
- 60. Azadzoi KM, Golabek T, Radisavljevic ZM, Yalla SV, Siroky MB. Oxidative stress and neurodegeneration in penile ischaemia. BJU Int. 2010;105(3):404–410. [DOI] [PubMed] [Google Scholar]
- 61. Azadzoi KM, Schulman RN, Aviram M, Siroky MB. Oxidative stress in arteriogenic erectile dysfunction: prophylactic role of antioxidants. J Urol. 2005;174(1):386–393. [DOI] [PubMed] [Google Scholar]
- 62. Angulo J, Gonzalez-Corrochano R, Cuevas P, et al. Diabetes exacerbates the functional deficiency of NO/cGMP pathway associated with erectile dysfunction in human corpus cavernosum and penile arteries. J Sex Med. 2010;7(2, pt 1):758–768. [DOI] [PubMed] [Google Scholar]
- 63. Chiou WF, Liu HK, Juan CW. Abnormal protein expression in the corpus cavernosum impairs erectile function in type 2 diabetes. BJU Int. 2010;105(5):674–680. [DOI] [PubMed] [Google Scholar]
- 64. Protogerou V, Michalopoulos E, Mallis P, et al. Administration of adipose derived mesenchymal stem cells and platelet lysate in erectile dysfunction: a single center pilot study. Bioengineering (Basel). 2019;6(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Baglio SR, Rooijers K, Koppers-Lalic D, et al. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res Ther. 2015;6:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Smith ZJ, Lee C, Rojalin T, et al. Single exosome study reveals subpopulations distributed among cell lines with variability related to membrane content. J Extracell Vesicles. 2015;4:28533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Oksvold MP, Kullmann A, Forfang L, et al. Expression of B-cell surface antigens in subpopulations of exosomes released from B-cell lymphoma cells. Clin Ther. 2014;36(6):847, e1–862. [DOI] [PubMed] [Google Scholar]
- 68. Laulagnier K, Vincent-Schneider H, Hamdi S, Subra C, Lankar D, Record M. Characterization of exosome subpopulations from RBL-2H3 cells using fluorescent lipids. Blood Cells Mol Dis. 2005;35(2):116–121. [DOI] [PubMed] [Google Scholar]
- 69. Tauro BJ, Greening DW, Mathias RA, Mathivanan S, Ji H, Simpson RJ. Two distinct populations of exosomes are released from LIM1863 colon carcinoma cell-derived organoids. Mol Cell Proteomics. 2013;12(3):587–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Roucourt B, Meeussen S, Bao J, Zimmermann P, David G. Heparanase activates the syndecan-syntenin-ALIX exosome pathway. Cell Res. 2015;25(4):412–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sandercock P, Roberts I. Systematic reviews of animal experiments. Lancet. 2002;360(9333):586. [DOI] [PubMed] [Google Scholar]
- 72. Hooijmans CR, Rovers M, de Vries RB, Leenaars M, Ritskes-Hoitinga M. An initiative to facilitate well-informed decision-making in laboratory animal research: report of the first international symposium on systematic reviews in laboratory animal science. Lab Anim. 2012;46(4):356–357. [DOI] [PubMed] [Google Scholar]