Key Points
Question
What is the contemporary prevalence and incidence of alopecia areata (AA), alopecia totalis, and alopecia universalis in the US?
Findings
In this cohort study of a nationwide US employer-sponsored insurance population, AA prevalence was 0.199% to 0.222%, and AA incidence was 91.46 to 92.90 cases per 100 000 patient-years from 2016 to 2019. Rates of AA and alopecia totalis and/or universalis were higher for female vs male individuals, adults vs children and adolescents, and in the Northeast vs other regions.
Meaning
The study results suggest that as the awareness of AA grows and novel therapeutics enter the marketplace, the present estimates could inform future analyses of the burden of disease.
Abstract
Importance
Alopecia areata (AA) is characterized by nonscarring hair loss of the scalp, face, and/or body. Alopecia totalis (AT) and alopecia universalis (AU) involve complete loss of the scalp and body hair, respectively. The epidemiology of AA in the US remains unclear, having previously been extrapolated from older studies that were limited to specific geographic areas or clinical settings, or from self-reported data.
Objective
To estimate the annual prevalence and incidence of AA and AT and/or AU (AT/AU) in the US.
Design, Setting, and Participants
This retrospective, population-based cohort study was conducted from January 2016 to December 2019 and included enrollees in the IBM MarketScan Commercial Claims and Encounters and Medicare Supplemental databases and their dependents, with plan enrollment during each study calendar year and the year prior.
Exposures
Prevalent cases were identified by 1 or more claims for AA or AT/AU (International Statistical Classification of Diseases, Tenth Revision, Clinical Modification [ICD-10-CM] codes L63.x, L63.0, L63.1) during each year of interest or the year prior. Incident cases were identified by 1 or more claims for AA or AT/AU during a specific year and no diagnosis the year prior.
Main Outcomes and Measures
Annual incidence and prevalence rates were calculated and stratified by age, sex, and region. National employer-sponsored insurance population estimates were obtained using population-based weights.
Results
Among eligible patients (2016: n = 18 368 [mean (SD) age, 40.6 (17.9) years; 12 295 women (66.9%)]; 2017: n = 14 372 [mean (SD) age, 39.6 (17.7) years; 9195 women (64.0%)]; 2018: n = 14 231 [mean (SD) age, 38.9 (17.3) years; 8998 women (63.2%)]; 2019: n = 13 455 [mean (SD) age, 39.1 (17.4) years; 8322 women (61.9%)]), AA prevalence increased from 0.199% (95% CI, 0.198%-0.200%) in 2016 to 0.222% (95% CI, 0.221%-0.223%) in 2019. Roughly 5% to 10% of prevalent and incident cases of AA were AT/AU. The prevalence of AT/AU increased from 0.012% (95% CI, 0.012%-0.013%) to 0.019% (95% CI, 0.018%-0.019%) from 2016 to 2019. Incidence of AA per 100 000 person-years ranged from 87.39 (95% CI, 86.84-87.96) in 2017 to 92.90 (95% CI, 92.35-93.45) in 2019. Incidence of AT/AU ranged from 7.09 (95% CI, 6.94-7.25) in 2017 to 8.92 (95% CI, 8.75-9.09) in 2016. Prevalence and incidence of AA and AT/AU were higher among female vs male individuals, adults vs children and adolescents, and in the Northeast vs other regions.
Conclusions and Relevance
The results of this cohort study suggest that these recent AA prevalence and incidence estimates could help improve current understanding of the disease burden. Further research is warranted to elucidate subpopulation differences and trends in AA in the broader US population.
This cohort study examines the annual prevalence and incidence of alopecia areata and alopecia totalis/alopecia universalis in the US.
Introduction
Alopecia areata (AA) is an autoimmune disease characterized by nonscarring hair loss involving the scalp, face, and/or body. The most common forms of AA include small patches of hair loss, complete loss of scalp hair (ie, alopecia totalis [AT]), or complete loss of scalp, facial, and body hair (ie, alopecia universalis [AU]).1 While AA affects both sexes and all age groups, the first onset reportedly occurs by age 40 years in more than 80% of patients and by age 20 years in 40%.2 Although many patients with AA recover within the first year,3,4,5,6,7 an estimated 4.5% to 36.1% of patients may ultimately progress to develop AT and/or AU (AT/AU).3,5,7 Living with AA can be associated with reduced quality of life, social functioning, and psychological well-being,2,8,9,10,11 with substantial costs to patients and health care systems.12,13,14,15
Despite the high burden of disease experienced by patients with AA, to our knowledge, evidence regarding the epidemiology of AA and AT/AU in the US remains limited. Existing estimates of the prevalence and incidence of AA have generally been extrapolated from older studies that were regionally based or conducted in specific clinical settings.5,16,17 The overall prevalence of AA was first estimated at 0.1% to 0.2% in the US based on the 1971 to 1974 First National Health and Nutrition Examination Survey.17 The lifetime incidence risk of AA in the US was estimated at 1.7% and 2.1% by the Rochester Epidemiology Project based on data from 1975 to 1989 and 1990 to 2009, respectively.5,16 However, these incidence rates (IRs) may not be representative of the general US population, as the data were restricted to Olmstead County, Minnesota, and only captured patients who were assessed and received their diagnosis in a clinical setting, primarily by dermatologists. Patients with AA are commonly encountered in clinical settings, comprising about 0.6% to 2.0% of new cases in dermatology clinics in the UK and US.18,19,20 Similarly, hospital-based studies worldwide have estimated the incidence risk of AA to be between 0.57% to 3.8%.2
To ascertain the epidemiology of AA in the broader community, a recent study conducted in 2020 reported the prevalence of AA and AT/AU in a large representative sample of the general US population using a 2017 online cross-sectional survey and clinician adjudication.21 Although the self-reported (0.04%) and clinician-adjudicated prevalence estimates for AT/AU were similar (0.04%), a considerable difference was observed regarding the clinician-adjudicated (0.21%) and self-reported (1.14%) prevalence of AA overall. In light of this evidence, further population-based studies using alternative approaches, such as insurance claims–based analyses, could help to develop annualized estimates of real-world prevalence and incidence5,16,17,21,22 and evaluate differences among subgroups of interest.2,22,23 Accordingly, the present study aimed to provide recent estimates of the annual and point prevalence and incidence of AA and AT/AU by sex, age group, and geographic region using a nationally weighted population from a large, nationwide US employer-sponsored insurance (ESI) claims database.
Methods
Data Source
This study used data from the IBM MarketScan Commercial and Medicare Supplemental databases from January 1, 2015, to December 31, 2019. These databases comprise more than 130 different insurance providers and third-party administrators in the US for more than 25 million patients annually. The data represent the demographic information and medical claims of insured active employees, dependents, early retirees, and consolidated omnibus budget reconciliation act beneficiaries from more than 40 national employers, as well as Medicare-eligible retirees with employer-provided Medicare Supplemental plans. Enrollment history and claims for medical (clinician and institutional) and outpatient pharmacy services are included, and inpatient service records are available at the claim and summarized stay levels. Given that data are deidentified and comply with the patient confidentiality requirements of the Health Insurance Portability and Accountability Act of 1996, this study was exempt from ethics review.
Study Design and Patient Selection
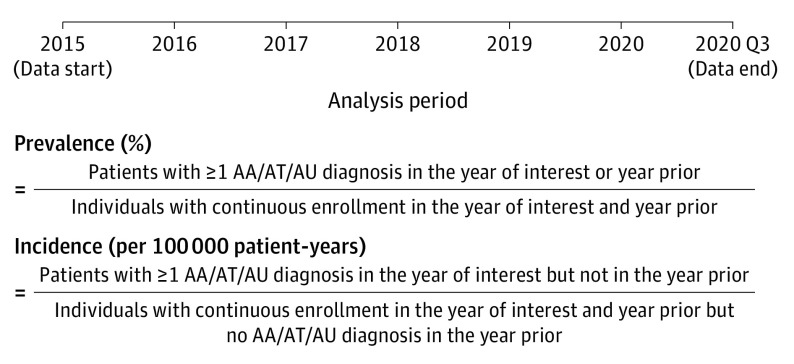
A retrospective, longitudinal cohort study design was used to estimate the annual prevalence rates (PRs) and IRs for AA and AT/AU during the analysis period spanning from 2016 to 2019 (Figure 1; eMethods and eTable 1 in Supplement 1). The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.24 The study population included all commercially insured US individuals with AA and AT/AU who were identified using the International Statistical Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) code L63.x. Diagnoses of AT/AU were imputed forward within each patient based on the presence of at least 1 claim with a diagnosis of AT or AU during the data period available after the first AT/AU diagnosis. Specifically, if a patient had at least 1 diagnosis for AT or AU, all other AA diagnoses for that patient occurring after the first AT or AU diagnosis were considered to be AT/AU, which was consistent with previously published approaches.25
Figure 1. Study Design.
AA indicates alopecia areata; AT, alopecia totalis; AU, alopecia universalis.
Measures and Outcomes
Prevalence and Incidence
For annual prevalence and incidence estimation, all patients had to be continuously enrolled in a health insurance plan for the entire year of analysis and the year prior. If a patient was born during the year of analysis or the year prior, continuous enrollment was required from birth. If the first AA or AT/AU diagnosis occurred after the year of analysis, then the patient was not counted as either a prevalent or incident case for the year of analysis. The number of individuals in the population considered for each annual prevalence estimate (the denominator of the estimate) included all enrollees with continuous health insurance plan coverage from January 1 or birth (whichever came later) to December 31 during the year of analysis and year prior. The number of patients with AA or AT/AU in a given year of analysis, respectively (the numerator of the estimate), was calculated based on the number of patients with 1 or more inpatient (IP) or outpatient (OP) diagnosis codes for AA or AT/AU during the calendar year of analysis or year prior. Individuals in the at-risk population for each annual incidence estimate (the denominator of the estimate) included those with no diagnosis code for AA or AT/AU during the year prior, which served as the washout period. Among the at-risk population, the number of incident patients in a given year of analysis (the numerator of the estimate) included those with 1 or more IP or OP claims with a diagnosis code for AA or AT/AU during the year of analysis but not the year prior. In addition to the annualized estimates described previously, point prevalence was calculated as the number of patients who had at least 1 IP or OP claim with a diagnosis for AA or AT/AU during the 12 months before July 1 of the year of analysis divided by the number of patients who were continuously enrolled during that period.
The crude annual PR for AA and AT/AU was estimated as the number of individuals with AA or AT/AU, respectively, divided by the total number of patients covered in the year of analysis and year prior. Nationally weighted annual PRs were obtained using weights calculated based on data from the American Community Survey, which contains information on census division, age, sex, and policy holder status.26 Crude annual IRs for AA and AT/AU were estimated based on the number of individuals with newly diagnosed AA or AT/AU, respectively, divided by the total number of individuals at risk. Nationally weighted annual IRs were obtained using the same method as described previously.
Annual PRs were expressed as the percentage of prevalent patients in a given year of analysis, and annual IRs were expressed as patients with a new diagnosis per 100 000 person-years (PYs). The 95% CIs were calculated using the Wilson binomial interval approach for weighted annual PRs27 and the Poisson exact approach for weighted annual IRs.
Patient Characteristics
Patient characteristics were summarized among newly diagnosed cases for each calendar year of analysis from 2016 to 2019. Demographic and clinical characteristics were measured at the earliest incident AA diagnosis date in the year of interest. Presence of comorbidities was evaluated using data from the full calendar year. Patient characteristics among annual incident cases were described using means and standard deviations for continuous variables and frequencies and proportions for categorical variables.
Statistical Analysis
In addition to the descriptive analyses described previously, a trends analysis was conducted to estimate temporal changes in annual PR among the overall study sample and stratified by sex (female and male), geographic region (Northeast, Midwest, South, and West), and age group. Specifically, the annual PR during each subsequent calendar year was compared with the annual PR in 2016 using weighted logistic regression models, and changes in annual IRs from 2016 onward were estimated using weighted Poisson regression models. Significance was assessed at an α level of P < .05. All statistical analyses were performed using SAS, version 9.4 (SAS Institute), and R, version 3.6 (R Foundation).
Results
Annual Prevalence and Incidence in the Overall Population
In the overall study population weighted to the US ESI population, the annual prevalence of AA increased slightly from 2016 to 2019. Prevalence rates of AA increased slightly from 0.199% in 2016 to 0.222% in 2019. The annual prevalence rates of AT/AU were 0.012% in 2016 and appeared to increase to 0.019% by 2019, respectively. In the overall study population, the annual incidence of AA per 100 000 PYs was 91.46 in 2016, 87.39 in 2017, 91.32 in 2018 (P = .73), and 92.90 in 2019. The annual incidence of AT/AU per 100 000 PYs ranged between 7.09 in 2017 and 8.92 in 2016.
Annual Prevalence and Incidence by Subgroup
Prevalence rates of AA were higher among female (95% CI, 0.252%–0.271%) vs male individuals (0.145%–0.171%) regardless of age, although this sex difference was more pronounced among adults than in children and adolescents (Figure 2). Prevalence was also higher among adults (range, 0.220%-0.245%) vs children and adolescents (range, 0.120%-0.135%) and in the Northeast (range, 0.273%-0.305%) vs other regions (range, 0.155%-0.222%). Among children and adolescents (Figure 2A), the annual prevalence of AA was highest among adolescents aged 12 to 17 years (range, 0.149%-0.165%) and lowest among those aged 0 to 5 years (range, 0.067%-0.072%). Among adults (Figure 2B), the highest annual prevalence of AA was observed among individuals aged 18 to 44 years (range, 0.254%-0.278%) and the lowest among those65 years or older (range, 0.150%-0.167%). Similar results were observed for the annual prevalence of AT/AU among age-related subgroups of children and adolescents and adults (Figure 2, C and D).
Figure 2. Annual Prevalence of Alopecia Areata (AA) and Alopecia Totalis (AT)/Alopecia Universalis (AU) by Sex and Age Group From 2016 to 2019.
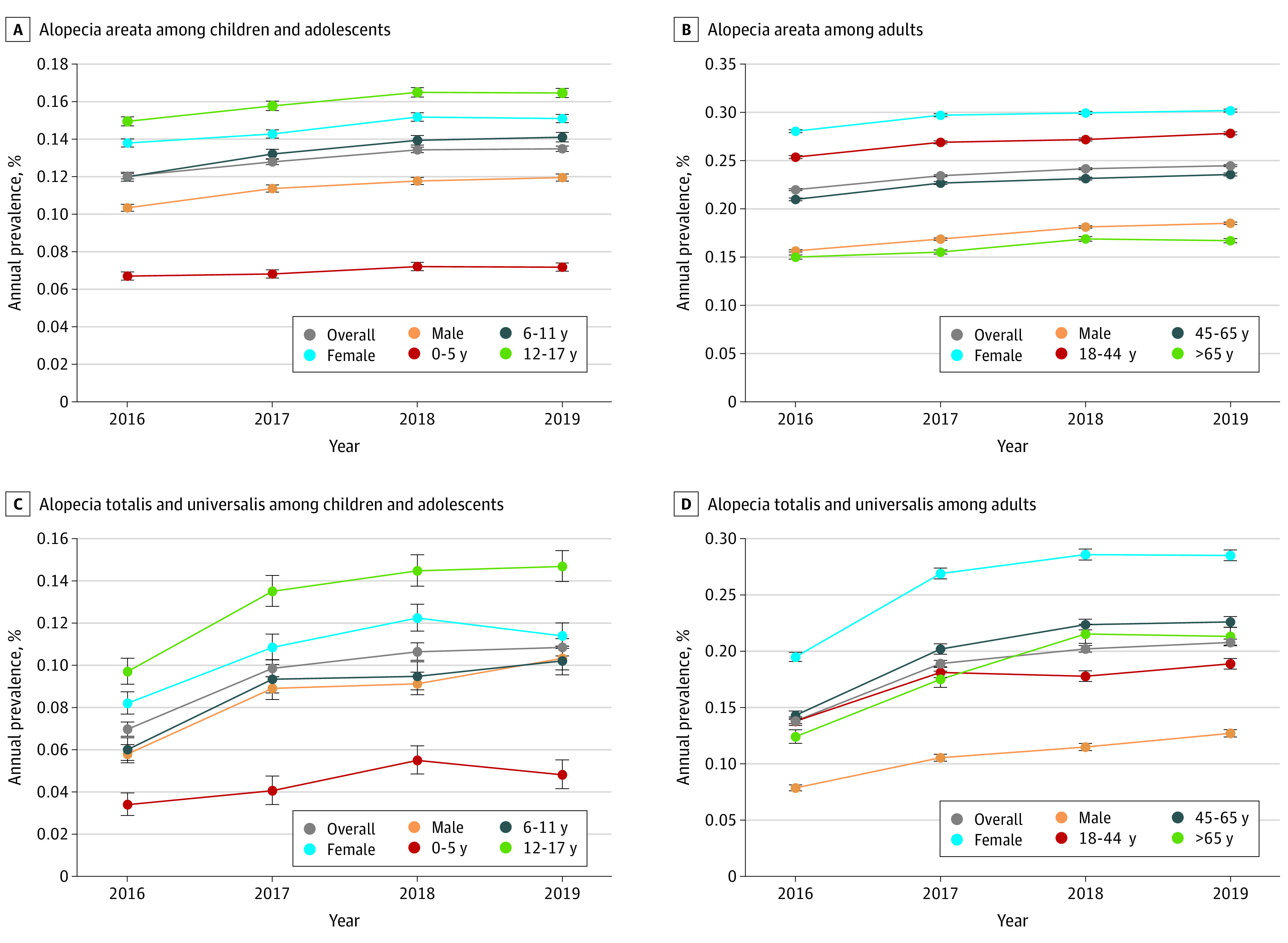
Individual weights in each year (2016-2019) were assigned using data from the American Community Survey based on census division, age, sex, and policy holder status. Annual prevalence was calculated as the number of patients who had at least 1 inpatient or outpatient claim with a diagnosis for AT/AU during the year of analysis or the year prior divided by the number of patients who were continuously enrolled during that period. Binomial exact intervals are shown. The annual prevalence rate during each subsequent calendar year was compared with the annual prevalence rate in 2016 using weighted logistic regression models, with prevalent case status as the dependent variable (cases were coded as 1, noncases were coded as 0) and calendar year indicators included as covariates.
The subgroup analysis of AA incidence yielded a pattern consistent with AA prevalence. Specifically, rates were higher among female (range, 108.53-118.56) vs male individuals (range, 63.68-72.09), adults (range, 95.45-102.63) vs children (range, 56.08-60.05), and in the Northeast (range, 116.95-124.21) vs other regions (range, 67.16-97.12). Likewise, the annual incidence of AA per 100 000 PYs in children and adolescents was highest among individuals aged 12 to 17 years (range, 65.83-63.29; Figure 3A) while the incidence among adults was highest in those aged 18 to 44 years (range, 117.06–119.14; Figure 3B). The incidence of AT/AU by age-related subgroups of children and adolescents and adults followed a similar pattern to that of AA (Figure 3, C and D). A similar sex-related pattern was observed for the annual incidence of AA and AT/AU (Figure 3, A and D), with higher rates among female individuals.
Figure 3. Incidence Rate per 1000 Person-Years of Alopecia Areata (AA) by Sex and Age Group From 2016 to 2019.
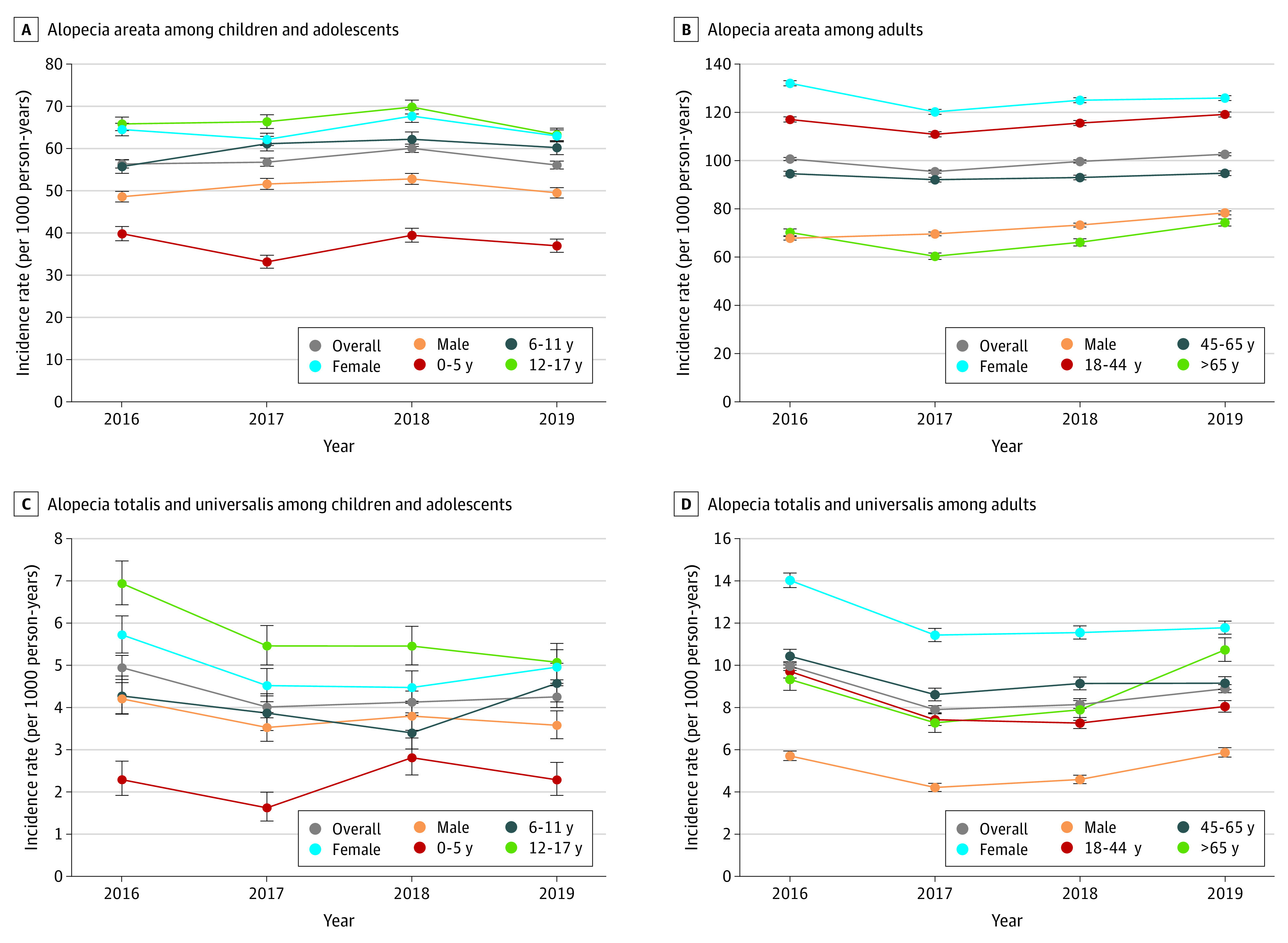
Individual weights in each year (2016-2019) were assigned using data from the American Community Survey based on census division, age, sex, and policy holder status. Incidence rate was calculated as the number of patients who had at least 1 inpatient or outpatient claim with a diagnosis for AA during the year of analysis but not the year prior divided by the total patient-years of continuous enrollment during that period. Wilson score intervals are shown. Changes in annual incidence rates from 2016 onward were estimated using weighted Poisson regression models with a log link function, with incident case counts as the dependent variable, the log of the population at risk included as an offset term, and binary indicators for calendar year included as covariates.
Patient Characteristics
Overall, patient characteristics of incident cases were similar during the analysis period (Table28). On average, patients received a diagnosis of AA at the mean (SD) age of between 38.9 (17.3) (2018) to 40.6 (17.9) years (2016). Approximately 8322 (61.9%) (2019) to 12 295 (66.9%) (2016) of the patients were female, and between 5485 (38.5%) (2018) and 7979 patients (43.4%) (2016) lived in the South. Between 1063 (7.5%) and 1694 patients (9.2%) had AT/AU subtype, and between 7536 (53.0%) (2018) and 7941 patients (55.3%) (2017) with AA or AT/AU first received their diagnosis from a dermatologist. Mean Charlson Comorbidity Index score values and rates of other comorbidities were also relatively similar across years.
Table. Patient Characteristics of the Incidence Cohort From 2016 to 2019.
| Characteristic | No. (%) | |||
|---|---|---|---|---|
| 2016 | 2017 | 2018 | 2019 | |
| No. of patients | 18 368 | 14 372 | 14 231 | 13 455 |
| Demographic and clinical | ||||
| Age, mean (SD), y | 40.6 (17.9) | 39.6 (17.7) | 38.9 (17.3) | 39.1 (17.4) |
| Female | 12 295 (66.9) | 9195 (64.0) | 8998 (63.2) | 8322 (61.9) |
| Male | 6073 (33.1) | 5177 (36.0) | 5374 (36.8) | 5133 (38.1) |
| Geographic region | ||||
| Northeast | 4455 (24.3) | 3522 (24.5) | 4027 (28.3) | 3857 (28.7) |
| Midwest | 3034 (16.5) | 2376 (16.5) | 2437 (17.1) | 2371 (17.6) |
| South | 7979 (43.4) | 6101 (42.5) | 5485 (38.5) | 5262 (39.1) |
| West | 2900 (15.8) | 2373 (16.5) | 2282 (16.0) | 1965 (14.6) |
| Insurance type | ||||
| Managed carea | 14 334 (78.0) | 10 774 (75.0) | 10 813 (76.0) | 10 169 (75.6) |
| Consumer drivenb | 3227 (17.6) | 2930 (20.4) | 2834 (19.9) | 2818 (20.9) |
| Comprehensive | 710 (3.9) | 550 (3.8) | 466 (3.3) | 373 (2.8) |
| Type of AA | ||||
| AT/AU | 1694 (9.2) | 1114 (7.8) | 1063 (7.5) | 1069 (7.9) |
| Non–AT/AU | 16 674 (90.8) | 13 258 (92.2) | 13 168 (92.5) | 12 386 (92.1) |
| First diagnosis by dermatologist | 9916 (54.0) | 7941 (55.3) | 7536 (53.0) | 7242 (53.8) |
| Comorbidities | ||||
| Any atopic comorbiditiesc | 3886 (21.2) | 3127 (21.8) | 3226 (22.7) | 2930 (21.8) |
| Any autoimmune comorbiditiesd | 2948 (16.0) | 2203 (15.3) | 2230 (15.7) | 2092 (15.5) |
| Charlson Comorbidity Index score, mean (SD)28 | 0.4 (0.9) | 0.3 (0.9) | 0.4 (0.9) | 0.3 (0.9) |
Abbreviations: AA, alopecia areata; AT, alopecia totalis; AU, alopecia universalis.
Composite of health maintenance organization, preferred clinician organization, point of service, and exclusive clinician organization plans.
Composite of consumer-driven health plans and high-deductible health plans.
Composite of allergic rhinitis, asthma, atopic dermatitis, celiac disease, chronic urticaria, and conjunctivitis.
Composite of ankylosing spondylitis, Crohn disease, diabetes mellitus, Hashimoto disease, systematic lupus erythematosus, psoriasis, rheumatoid arthritis, Sjögren syndrome, ulcerative colitis, and vitiligo.
Discussion
In a large, nationally representative population of commercially insured patients in the US, the number of prevalent and incident AA and AT/AU cases appeared to increase slightly from 2016 to 2019 in the US. Overall, the prevalence and incidence of AA and AT/AU were higher among female vs male individuals, adults vs children and adolescents, and in the Northeast vs other regions. An estimated 7.5% to 9.2% of prevalent and incident cases of AA were AT/AU. The prevalence and incidence of AA appeared to increase in adolescence (ie, age 12-17 years) and reach a peak in young adulthood (ie, age 18-44 years), with higher rates observed among female individuals regardless of age group.
Alopecia Areata Prevalence Rates
The present study found similar AA PRs (0.199%-0.222% from 2016-2019) to the clinician-adjudicated prevalence rate (0.210% in 2017) reported by a recent survey study,21 which is consistent with earlier estimates from the First National Health and Nutrition Examination Survey (0.1%-0.2% from 1971-1974).17 The self-reported rate of AA in the survey study (1.14% in 2017),21 which accounts for those patients not seeking medical care, suggests that the true prevalence of AA in the community might be higher than reported from medical claims.21,22,29 In the present study, AT/AU PRs (0.012%-0.019% from 2016-2019) were found to be lower than the rate reported in the survey study (0.040% in 2017, respectively).21 Since the current study estimated prevalence in a population of patients seeking care when no approved treatments were available, these rates may be understating the true AT/AU prevalence, particularly in light of prior research that found AT/AU codes may be underused by clinicians.25 With the recent approval of baricitinib by the US Food and Drug Administration as a treatment for AA and other treatments currently in development, more patients with AA may seek treatment and thus get a clinical diagnosis from health care clinicians, which would plausibly increase the incidence and prevalence rates of AA based on claims data. However, this trend may be contingent on the overall safety of the new treatments, as well as their coverage by payers, which is critical to patient access.
The annual incidence of AA in the present study (91.46-92.90 cases per 100 000 PYs from 2016-2019) appeared higher than previous estimates from the Rochester Epidemiology Project (20.2 and 20.9 per 100 000 PYs from 1975-1989 and 1990-2009, respectively).5,16 Further, the IRs of AA and AT/AU in the present study decreased from 2016 to 2017 and then increased slightly from 2017 to 2019. However, the reasons for this trend remain uncertain, and its statistical significance may be a function of the sample size or other random statistical variations present in the data.
A pronounced female preponderance of AA and AT/AU was observed in this study. While it is likely to be multifactorial, this trend could be partially associated with sex differences in health care–seeking behavior. Most new AA consultations in dermatology clinics are women,30 who may experience a greater social and emotional effect of AA than men.30,31 Among more granular age-based subgroups, the present findings are generally aligned with previous reports of the age distribution for AA incidence,32 with younger to middle aged patients (eg, age 30-39 years) representing the largest age group seeking health care for AA.33 Additionally, the present study findings underscore the high rates of AA and AT/AU among children and adolescents in the 12-year to 17-year age range compared with younger children. Apart from the trends toward higher adult and female preponderance in this study, individuals in the Northeast region were found to have higher rates of AA and AT/AU that may reflect, among other factors, regional differences in the density of dermatologists and individual health care–seeking behavior.34
Children and Adolescents
Compared with our results among children and adolescents, similar temporal and subgroup trends were observed in a recent US hospital-based study using electronic health records data. This latter study found that AA prevalence among pediatric patients (mean age, 9 years) had doubled during the last decade (overall prevalence of 0.11% from 2009-2020),35 which was consistent with the slight increase in AA rates over time in the present study. Also consistent with the present study, pediatric AA IRs were higher among children who were older and female; in addition, the latter study identified Asian and Hispanic children as high-risk subgroups. The annual AA PRs among children aged 6 to 11 years in the present study (0.12%-0.14%) were higher than the rates in this hospital-based study (0.04%-0.08%); IRs were also higher in the present study.35
Limitations
The present study had limitations. First, coding inaccuracies or data omissions may have been associated with misidentification of patients with AA and AT/AU. Moreover, we could not discern AA rates by degree of scalp hair loss (outside of AT/AU) as this information was unavailable in claims data. Second, the generalizability of the study findings beyond patients with commercial or Medicare supplemental insurance may be limited.36 Although the current sample was weighted to the US ESI population, we could not control for all potential differences in patient characteristics between our study sample and the general US population. Furthermore, since the present data are limited to patients actively utilizing health care resources for AA, the calculated IRs and PRs might be underestimates. Due to a lack of race and ethnicity information in the claims database, we were not able to calculate incidence and prevalence rates for racial and ethnic subgroups. Finally, despite the 12-month washout period used for identifying incident cases, some of these patients may have received their diagnoses before the data period and were prevalent cases. However, a sensitivity analysis requiring at least 2 claims with AA diagnoses revealed lower incidence and prevalence rates (eTable 2 in Supplement 1) in agreement with prior literature that AA is transient in some cases.37
Conclusions
Evidence regarding the epidemiology of AA and AT/AU in the US remains limited, despite a known clinical, economic, and humanistic burden of disease.2,8,9,10,11,12,13,14,15 Using recent data from a large, nationally representative US ESI population, this population-based cohort study found that AA prevalence was within the range of clinician-adjudicated estimates for the general US population,21 while AA incidence was higher than previous estimates obtained in US clinical settings.5,16 A slight increase in the number of existing and newly diagnosed AA and AT/AU cases from 2016 to 2019 could indicate a growing burden of disease or may reflect an increasing proportion of patients seeking care, with a more pronounced burden among female individuals, adults, and residents of the Northeast. Taken together, this study highlights contemporary trends in the annual prevalence and incidence of AA and AT/AU over time among a large, real-world US population, which may help to inform future analyses of the burden of disease.
eMethods. Flow of information through the different phases of the review
eTable 1. Point prevalence rates of AA from 2016-2019
eTable 2. Summary of prevalence and incidence rates of AA and AT/AU during 2016-2019—sensitivity analysis
Data sharing statement
References
- 1.McMichael AJ. The genetic epidemiology and autoimmune pathogenesis of alopecia areata. J Eur Acad Dermatol Venereol. 1997;9(1):36-43. doi: 10.1111/j.1468-3083.1997.tb00225.x [DOI] [Google Scholar]
- 2.Villasante Fricke AC, Miteva M. Epidemiology and burden of alopecia areata: a systematic review. Clin Cosmet Investig Dermatol. 2015;8:397-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gip L, Lodin A, Molin L. Alopecia areata: a follow-up investigation of outpatient material. Acta Derm Venereol. 1969;49(2):180-188. [PubMed] [Google Scholar]
- 4.Walker SA, Rothman S. A statistical study and consideration of endocrine influences. J Invest Dermatol. 1950;14(6):403-413. doi: 10.1038/jid.1950.52 [DOI] [PubMed] [Google Scholar]
- 5.Safavi KH, Muller SA, Suman VJ, Moshell AN, Melton LJ III. Incidence of alopecia areata in Olmsted County, Minnesota, 1975 through 1989. Mayo Clin Proc. 1995;70(7):628-633. doi: 10.4065/70.7.628 [DOI] [PubMed] [Google Scholar]
- 6.Pratt CH, King LE Jr, Messenger AG, Christiano AM, Sundberg JP. Alopecia areata. Nat Rev Dis Primers. 2017;3:17011. doi: 10.1038/nrdp.2017.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tosti A, Bellavista S, Iorizzo M. Alopecia areata: a long term follow-up study of 191 patients. J Am Acad Dermatol. 2006;55(3):438-441. doi: 10.1016/j.jaad.2006.05.008 [DOI] [PubMed] [Google Scholar]
- 8.Shi Q, Duvic M, Osei JS, et al. Health-related quality of life (HRQoL) in alopecia areata patients-a secondary analysis of the National Alopecia Areata Registry Data. J Investig Dermatol Symp Proc. 2013;16(1):S49-S50. doi: 10.1038/jidsymp.2013.18 [DOI] [PubMed] [Google Scholar]
- 9.Liu LY, King BA, Craiglow BG. Alopecia areata is associated with impaired health-related quality of life: a survey of affected adults and children and their families. J Am Acad Dermatol. 2018;79(3):556-558.e1. doi: 10.1016/j.jaad.2018.01.048 [DOI] [PubMed] [Google Scholar]
- 10.Rencz F, Gulácsi L, Péntek M, Wikonkál N, Baji P, Brodszky V. Alopecia areata and health-related quality of life: a systematic review and meta-analysis. Br J Dermatol. 2016;175(3):561-571. doi: 10.1111/bjd.14497 [DOI] [PubMed] [Google Scholar]
- 11.Mesinkovska N, King B, Mirmirani P, Ko J, Cassella J. Burden of illness in alopecia areata: a cross-sectional online survey study. J Investig Dermatol Symp Proc. 2020;20(1):S62-S68. doi: 10.1016/j.jisp.2020.05.007 [DOI] [PubMed] [Google Scholar]
- 12.Senna M, Ko J, Tosti A, et al. Alopecia areata treatment patterns, healthcare resource utilization, and comorbidities in the US population using insurance claims. Adv Ther. 2021;38(9):4646-4658. doi: 10.1007/s12325-021-01845-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li SJ, Mostaghimi A, Tkachenko E, Huang KP. Association of out-of-pocket health care costs and financial burden for patients with alopecia areata. JAMA Dermatol. 2019;155(4):493-494. doi: 10.1001/jamadermatol.2018.5218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xenakis J, Meche A, Smith T, Gruben D, Sikirica V. PSY29 economic burden of alopecia areata in a US managed care population. Value Health. 2019;22:S379. doi: 10.1016/j.jval.2019.04.1853 [DOI] [Google Scholar]
- 15.Mostaghimi A, Gandhi K, Done N, et al. All-cause health care resource utilization and costs among adults with alopecia areata: a retrospective claims database study in the United States. J Manag Care Spec Pharm. 2022;28(4):426-434. doi: 10.18553/jmcp.2022.28.4.426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mirzoyev SA, Schrum AG, Davis MDP, Torgerson RR. Lifetime incidence risk of alopecia areata estimated at 2.1% by Rochester Epidemiology Project, 1990-2009. J Invest Dermatol. 2014;134(4):1141-1142. doi: 10.1038/jid.2013.464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Safavi K. Prevalence of alopecia areata in the first National Health and Nutrition Examination Survey. Arch Dermatol. 1992;128(5):702. doi: 10.1001/archderm.1992.01680150136027 [DOI] [PubMed] [Google Scholar]
- 18.Wasserman D, Guzman-Sanchez DA, Scott K, McMichael A. Alopecia areata. Int J Dermatol. 2007;46(2):121-131. doi: 10.1111/j.1365-4632.2007.03193.x [DOI] [PubMed] [Google Scholar]
- 19.Caldwell CC, Saikaly SK, Dellavalle RP, Solomon JA. Prevalence of pediatric alopecia areata among 572,617 dermatology patients. J Am Acad Dermatol. 2017;77(5):980-981. doi: 10.1016/j.jaad.2017.06.035 [DOI] [PubMed] [Google Scholar]
- 20.Dawber D, De Berker D, Wojnarowska F. Disorders of hair: alopecia areata. In: Champion RH, ed. Rook/Wilkinson/Ebling Textbook of Dermatology. Wiley-Blackwell;1998:2919-2927. [Google Scholar]
- 21.Benigno M, Anastassopoulos KP, Mostaghimi A, et al. A large cross-sectional survey study of the prevalence of alopecia areata in the United States. Clin Cosmet Investig Dermatol. 2020;13:259-266. doi: 10.2147/CCID.S245649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee HH, Gwillim E, Patel KR, et al. Epidemiology of alopecia areata, ophiasis, totalis, and universalis: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82(3):675-682. doi: 10.1016/j.jaad.2019.08.032 [DOI] [PubMed] [Google Scholar]
- 23.Strazzulla LC, Wang EHC, Avila L, et al. Alopecia areata: disease characteristics, clinical evaluation, and new perspectives on pathogenesis. J Am Acad Dermatol. 2018;78(1):1-12. doi: 10.1016/j.jaad.2017.04.1141 [DOI] [PubMed] [Google Scholar]
- 24.Vandenbroucke JP, von Elm E, Altman DG, et al. ; STROBE Initiative . Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Ann Intern Med. 2007;147(8):W163-94. doi: 10.7326/0003-4819-147-8-200710160-00010-w1 [DOI] [PubMed] [Google Scholar]
- 25.Lavian J, Li SJ, Lee EY, et al. Validation of case identification for alopecia areata using international classification of diseases coding. Int J Trichology. 2020;12(5):234-237. doi: 10.4103/ijt.ijt_67_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.US Census Bureau . Data from 2009-2011 American Community Survey 3-year public use microdata samples. Accessed January 15, 2021. https://www.census.gov/programs-surveys/acs/microdata.html
- 27.Franco C, Little RJA, Louis TA, Slud EV. Comparative study of confidence intervals for proportions in complex sample surveys. J Surv Stat Methodol. 2019;7(3):334-364. doi: 10.1093/jssam/smy019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676-682. doi: 10.1093/aje/kwq433 [DOI] [PubMed] [Google Scholar]
- 29.Li DG, Huang KP, Xia FD, et al. Development and pilot-testing of the Alopecia Areata Assessment Tool (ALTO). PLoS One. 2018;13(6):e0196517. doi: 10.1371/journal.pone.0196517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marks DH, Penzi LR, Ibler E, et al. The medical and psychosocial associations of alopecia: recognizing hair loss as more than a cosmetic concern. Am J Clin Dermatol. 2019;20(2):195-200. doi: 10.1007/s40257-018-0405-2 [DOI] [PubMed] [Google Scholar]
- 31.Katoulis AC, Christodoulou C, Liakou AI, et al. Quality of life and psychosocial impact of scarring and non-scarring alopecia in women. J Dtsch Dermatol Ges. 2015;13(2):137-142. doi: 10.1111/ddg.12548 [DOI] [PubMed] [Google Scholar]
- 32.Muller SA, Winkelmann RK. Alopecia areata: an evaluation of 736 patients. Arch Dermatol. 1963;88:290-297. doi: 10.1001/archderm.1963.01590210048007 [DOI] [PubMed] [Google Scholar]
- 33.McMichael AJ, Pearce DJ, Wasserman D, et al. Alopecia in the United States: outpatient utilization and common prescribing patterns. J Am Acad Dermatol. 2007;57(2)(suppl):S49-S51. doi: 10.1016/j.jaad.2006.02.045 [DOI] [PubMed] [Google Scholar]
- 34.Yoo JY, Rigel DS. Trends in dermatology: geographic density of US dermatologists. Arch Dermatol. 2010;146(7):779. doi: 10.1001/archdermatol.2010.127 [DOI] [PubMed] [Google Scholar]
- 35.McKenzie PL, Maltenfort M, Bruckner AL, et al. Evaluation of the prevalence and incidence of pediatric alopecia areata using electronic health record data. JAMA Dermatol. 2022;158(5):547-551. doi: 10.1001/jamadermatol.2022.0351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kulaylat AS, Schaefer EW, Messaris E, Hollenbeak CS. Truven Health Analytics MarketScan databases for clinical research in colon and rectal surgery. Clin Colon Rectal Surg. 2019;32(1):54-60. doi: 10.1055/s-0038-1673354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pratt CH, King LE Jr, Messenger AG, Christiano AM, Sundberg JP. Alopecia areata. Nat Rev Dis Primers. 2017;3(1):17011. doi: 10.1038/nrdp.2017.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Flow of information through the different phases of the review
eTable 1. Point prevalence rates of AA from 2016-2019
eTable 2. Summary of prevalence and incidence rates of AA and AT/AU during 2016-2019—sensitivity analysis
Data sharing statement