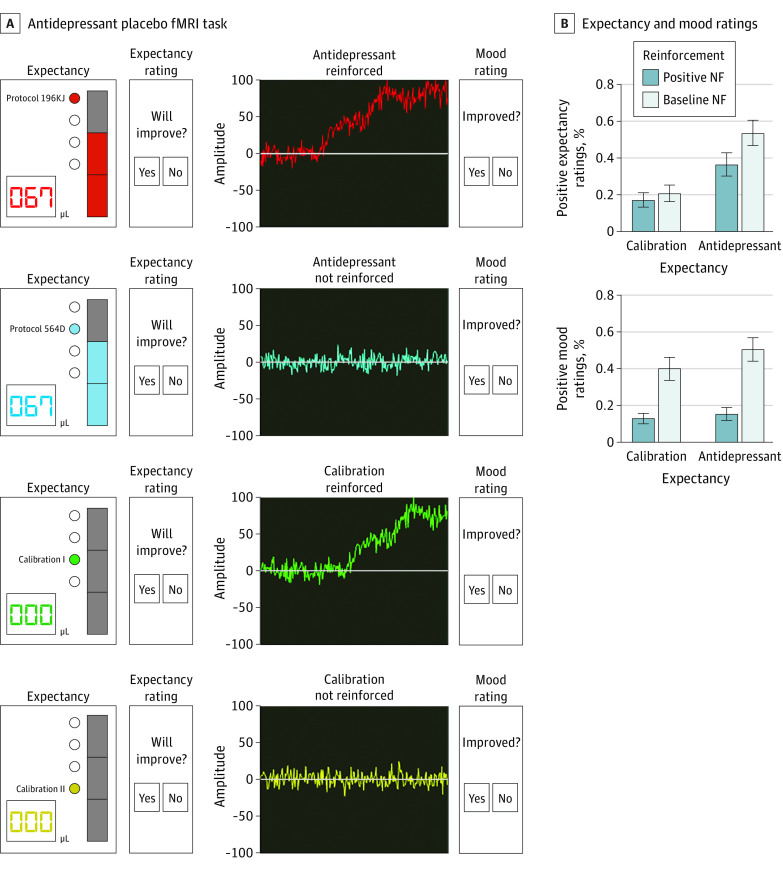
This cross-sectional study investigates the neurobehavioral mechanisms associated with antidepressant placebo effects over time using a reinforcement learning framework.
Key Points
Question
What makes antidepressant placebo effects persist over time?
Findings
Results of this cross-sectional study of 60 patients with major depressive disorder suggest that, on a timescale of minutes, antidepressant placebo effects are maintained by positive feedback loops and reinforcement learning between expectancies and mood improvement. During learning, representations of placebos and their perceived effects are enhanced in primary and secondary sensory cortices; latent learned placebo expectancies are encoded in the salience network.
Meaning
Results suggest that far from being inert, antidepressant placebos set off positive feedback loops between expectancies and learning, resulting in mood improvement.
Abstract
Importance
Despite high antidepressant placebo response rates, the mechanisms underlying the persistence of antidepressant placebo effects are still poorly understood.
Objective
To investigate the neurobehavioral mechanisms underlying the evolution of antidepressant placebo effects using a reinforcement learning (RL) framework.
Design, Setting, and Participants
In this acute within-patient cross-sectional study of antidepressant placebos, patients aged 18 to 55 years not receiving medication for major depressive disorder (MDD) were recruited at the University of Pittsburgh between February 21, 2017, to March 1, 2021.
Interventions
The antidepressant placebo functional magnetic resonance imaging task manipulates placebo-associated expectancies using visually cued fast-acting antidepressant infusions and controls their reinforcement with sham visual neurofeedback while assessing expected and experienced mood improvement.
Main Outcomes and Measures
The trial-by-trial evolution of expectancies and mood was examined using multilevel modeling and RL, relating model-predicted signals to spatiotemporal dynamics of blood oxygenation level–dependent (BOLD) response.
Results
A bayesian RL model comparison in 60 individuals (mean [SE] age, 24.5 [0.8] years; 51 females [85%]) with MDD revealed that antidepressant placebo trial-wise expectancies were updated by composite learning signals multiplexing sensory evidence (neurofeedback) and trial-wise mood (bayesian omnibus risk <0.001; exceedance probability = 97%). Placebo expectancy, neurofeedback manipulations, and composite learning signals modulated the visual cortex and dorsal attention network (threshold-free cluster enhancement [TFCE] = 1 − P >.95). As participants anticipated antidepressant infusions, learned placebo expectancies modulated the salience network (SN, TFCE = 1 – P >.95), positively scaling with depression severity.
Conclusions and Relevance
Results of this cross-sectional study suggest that on a timescale of minutes, antidepressant placebo effects were maintained by positive feedback loops between expectancies and mood improvement. During learning, representations of placebos and their perceived effects were enhanced in primary and secondary sensory cortices. Latent learned placebo expectancies were encoded in the SN.
Introduction
Antidepressant placebos have a profound effect on mood. Empirical studies have begun to investigate the neurobehavioral mechanisms of this effect. However, they have yet to elucidate how antidepressant placebo expectancies evolve and why their effect persists over weeks or months.1,2 Prior placebo analgesia studies have induced placebo effects by (1) setting expectancies using verbal instructions and/or (2) Pavlovian conditioning, through the pairing of an inactive placebo stimulus with an unconditioned stimulus (pain, the psychoactive drug). More recently, reinforcement learning (RL)3,4,5 has provided a computational account of these phenomena wherein expectancy learning starts with a prior (communicated by cues or words) and depends not merely on the contiguity between the conditioned (placebo cues) and unconditioned (biologically active) stimuli but on prediction errors (PEs), or the mismatch between what it is expected (expected value) and what it is experienced. Although RL models have started to help with understanding interindividual variability in placebo analgesic effects, these effects have not been generalized to other clinical conditions where placebo effects are also prevalent, such as depression.
In standard RL, expectancies not confirmed by experience are extinguished,3,4,5,6 as it would be expected for placebo expectancies in the absence of true physiological effects. However, studies of placebo analgesia using RL and bayesian learning models find the opposite. Schenk and colleagues7 recently suggested that placebo analgesia may result from impaired extinction learning caused by prefrontal downregulation of PEs, paralleled by negative coupling between the anterior PFC and the VS. Additionally, Jepma and colleagues8 have demonstrated that placebo effects arise from confirmation biases, where expectancies are selectively reinforced only when new experience confirms prior placebo/nocebo expectancies, and new evidence to the contrary is discounted. In their study, greater expectation updating in response to pain was associated with greater pain-anticipatory brain activity in somatosensory areas, the salience network (SN; ie, anterior midcingulate cortex, midinsula, dorsal striatum), the periaqueductal gray, pons, and the inferior cerebellar vermis. Similarly, Grahl et al9 have related placebo effects to interindividual variability in expectancy precision.
Although antidepressant placebo effects have yet to be explained in terms of RL, recent studies have advanced an account of mood as a higher-order statistic of reinforcement.10,11 Encoding of reinforcement may drive the evolution of mood on a timescale of days,11 and mood can bias how people perceive and learn from rewards.10,11 Although these studies used secondary rewards, while antidepressant expectancies concern mood itself, feedback between learning and mood may play a role in the emergence and maintenance of antidepressant placebo effects. For example, when a patient with high antidepressant placebo expectancies feels even a transient mood improvement, they may encode their experience as more positive, confirming that the placebo is indeed working. Placebo expectancies may influence learning indirectly through mood, where mood fluctuations serve to update expectancies.
We present an experimental study of the association between mood dynamics and antidepressant placebo effects. We investigated neural encoding of placebo expectancies and learning signals. Our antidepressant placebo functional magnetic resonance imaging (fMRI) task12,13 manipulates antidepressant expectancies using purported fast-acting antidepressant infusions and presents ostensible physiological evidence of mood improvement via sham neurofeedback. Using both frequentist multilevel models and bayesian RL model selection, we tested 2 nonmutually exclusive hypotheses: (1) the biased learning hypothesis, which postulates preferential expectancy learning from positive neurofeedback after placebo infusions; and (2) the mood self-reinforcement hypothesis, which postulates that better mood immediately after placebo administration can increment learning of antidepressant placebo expectancies. We derived predicted expectancies and learning signals from the best-fitting RL model to examine their neural encoding. Based on previous placebo analgesia studies,8 we hypothesized that learned antidepressant expectancies would be encoded in the SN, thought to represent expectations of the state of the environment as well as the internal state.14
Methods
Participants, Study Design, and Clinical Assessments
In this cross-sectional study, participants diagnosed with major depressive disorder (MDD) from February 21, 2017, to March 1, 2021, through the University of Pittsburgh’s recruitment website Pitt+Me were recruited.15 Enrolled participants were unmedicated, right-handed, fluent in English, and provided written and signed informed consent with authorized deception, approved by the University of Pittsburgh institutional review board. Participants self-identified with the following race and ethnic groups: African/Black, Asian, Caucasian/White, Hispanic or Latino, and other (ie, American Indian or Alaska Native and Native Hawaiian or other Pacific Islander) or multiracial. Detailed inclusion and exclusion criteria and consenting procedures are described in the eMethods in Supplement 1. Participants completed the physician-administered Hamilton Depression Rating Scale (HDRS)—the primary depression scale used to confirm eligibility at screening—the Montgomery-Åsberg Depression Rating Scale (MADRS),16 and the self-reported Quick Inventory of Depressive Symptomatology.17 This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
Antidepressant Placebo fMRI Task
Participants were deceived about the purpose of the study. The deceptive narrative (eMethods in Supplement 1) constituted an experimental manipulation aimed at investigating the brain effects of a fast-acting antidepressant compared with a conventional antidepressant (expectancy condition or prior expectancies) while recording participants’ brain activity and providing neurofeedback (reinforcement condition). Participants were told that, after each infusion, neurofeedback of positive signal would present with acute mood improvement, whereas baseline neurofeedback signal was unlikely to be followed by mood improvement13 (Figure 1).
Figure 1. Antidepressant Placebo Functional Magnetic Resonance Imaging (fMRI) Task and Behavioral Results.
The antidepressant placebo fMRI task features 2 components of the placebo effect: expectancies (prior expectancies) and their reinforcement, each followed by an expectancy and mood rating cue, respectively. The expectancy condition involves an antidepressant-infusion cue and a no-infusion cue, described as periods of equipment calibration. During the antidepressant infusion cues (4 seconds), a bar is filled at four 1-second periods representing 0%, 33%, 66%, and 100% of the dose administered. During the calibration no-infusion cue (4 seconds) the bar remains empty. For the reinforcement condition (10 seconds), sham neurofeedback acts as a secondary reinforcer of the antidepressant effects. In the high-reinforcement condition, sham neurofeedback is positive 75% of the trials (vs 25% baseline). Participants rate their expected and actual change in mood (yes/no) in response to each infusion/neurofeedback (NF) signal, respectively, by using a keypad and their index fingers. Interstimulus interval duration was randomly sampled from an exponential distribution bounded between 0.33 and 2 seconds. Intertrial intervals were sampled from the uniform distribution with bounds of 4 to 6 seconds. A, Each of the 4 runs included 32 trials for a total task duration of approximately 45 minutes. B, Antidepressant placebo fMRI task model-free behavioral results: the expectancy and reinforcement task conditions significantly predict expectancy and mood ratings during the antidepressant placebo fMRI task.
As previously described,12,13 immediately before the scan, a nurse placed an MRI-compatible intravenous line in the participant’s forearm to administer the antidepressant placebo infusions while participants watched a fragment of the video to be displayed inside of the scanner. During the scanning session, an MRI-compatible pump, controlled from the outside scanning room by pushing the “go” trigger, delivered intravenous saline. The infusion was started manually at a given flow rate and volume, at the beginning of each run, immediately before completing the antidepressant placebo fMRI task (Figure 1).
Statistical Analysis
Analysis of Behavioral Data
We estimated multilevel logistic regression models predicting participants’ evolving expectancies and mood ratings using R, version 4.1.2,18 the R Studio lme419 package. These models estimated the fixed effects of 2 orthogonal experimental conditions (expectancy condition [antidepressant placebo infusion vs calibration] and reinforcement condition [high vs low reinforcement]), and their interaction (eMethods in Supplement 1). All P values were 2-sided, and a P value < .05 was considered statistically significant. Bonferroni correction was applied when needed.
RL Models
To characterize the evolution of participants’ placebo expectancies or learned expectancies, we compared 5 alternative RL models predicting their expectancy ratings. Model parameters were estimated using a hierarchical bayesian procedure based on the Variational Bayes Approach toolbox implemented in MATLAB, version R2021b (MathWorks). Learning models were adapted from a Q-learning rule and Softmax choice function.
Basic Learning Model—Q-Learning Rule and Softmax Function
In the basic learning model (model 1), learned placebo expectancies were updated every time the antidepressant infusion or calibration cue was presented and an outcome (positive or baseline neurofeedback) was observed, as follows:
Qt + 1(s) = Qt(s) + αδt,
where Qt(s) is the learned placebo expectancy of improvement s at trial t, α is a learning rate, and δ is the difference between the actual and expected outcome (PE):
δ = rt − Qt(s),
where, rt is the actual reward outcome (positive or baseline sham neurofeedback).
Of note, our reinforcement manipulation—sham neurofeedback—differs from conventional classical conditioning, as it did not involve a primary reinforcer (eg, sadness induction). Similar secondary reinforcers have been previously used in the field8 and are closely aligned with sensory preconditioning paradigms.20
To account for noise and possible bias in responses, we used a Softmax choice rule with bias (K) and stochasticity/temperature (β) as free parameters. This basic learning model was compared against competing models that would account for individual differences in placebo responses:
Biased learning hypothesis. To account for the possible different learning rates depending on whether participants are updating expectancies for the placebo (αplac) or calibration cue (αcal) (model 2a, placebo learning model) or outcome (positive [αpos_nf] vs baseline neurofeedback [αbase_nf]; model 2b, feedback learning model), we tested 2 alternative models with asymmetrical learning rates.
Mood self-reinforcement hypothesis. To test our mood self-reinforcing hypothesis where changes in mood provide reinforcement, we implemented a model where the reward was augmented when mood was rated as improved (ut = r + moodt; model 3, mood learning model).
Biased learning and self-reinforcement hypothesis. Finally, we tested the possibility that biased learning and self-reinforcement contributed to antidepressant expectancies. This model included 2 learning rates, depending on whether participants are updating expectancies for the placebo (αplac) or calibration cue (αcal), in addition to the augmented reward (ut = r + moodt).
Model Comparison and Selection
The basic learning model and its variations were then compared against a null model that assumed no learning:
Qt + 1(s) = Qt(s).
Individual model evidence values were fed to the mbb-vb-toolbox to run a bayesian model comparison.21 Additional details are described in eMethods and eFigure 1 in Supplement 1.
MRI Data Acquisition, Preprocessing, Task-Based and RL-Based MRI Analysis
Acquired images were preprocessed using functions in the following software packages: NiPy, version 0.5.0,22,23 AFNI, version 21.3.17,24 BrainWavelet Toolbox, version 2.0,25 and the FMRIB Software Library, version 6.0 (FSL).26 We have previously detailed this preprocessing pipeline elsewhere27 and in eMethods in Supplement 1. Subject-level and group-level analysis are described in the eMethods and eFigure 2 in Supplement 1.
Results
Model-Free Behavioral Analyses
In this study, a total of 60 individuals (mean [SE] age, 24.5 [0.8] years; 51 females [85%]; 9 males [15%]) with major depressive disorder (MDD) were recruited. Participants identified with the following race and ethnicity categories: 14 African/Black (23%), 10 Asian (16%), 31 Caucasian/White (95%), 3 Hispanic or Latino (5%), and 10 other or multiracial (8%). Credibility assessments are reported in the eResults and eFigure 3 in Supplement 1. As in our previous study,13 participants’ expectancy ratings were higher in the high-expectancy condition (expecting an antidepressant infusion vs calibration, estimate [SE] = 1.01 [0.08]; χ2 = 139.67; P < .001), especially for the highly reinforced antidepressant cue, as reflected by a positive antidepressant × reinforcement interaction (estimate [SE] = 0.46 [ 0.12]; χ2 = 14.47; P < .001) (eTable 1 in Supplement 1). Mood ratings were higher following reinforcement (estimate [SE] = 1.42 [0.08]; χ2 = 273.64; P < .001), and the positive effect of neurofeedback on reported mood was enhanced after the antidepressant drug infusions, as indicated by a positive expectancy × reinforcement interaction (estimate [SE] = 0.27 [0.12]; χ2 = 4.23; P = .03) (Figure 2A and eTable 1 in Supplement 1), suggesting that sensory evidence of an antidepressant effect selectively improves mood in the antidepressant condition. Indeed, replacing the expectancy condition by expectancy ratings in the model predicting mood ratings improved model fit (Akaike information criterion difference favoring model with expectancy ratings = 6486 for 146.5n of observations), suggesting that trial-varying expectancy ratings predict mood ratings beyond the expectancy manipulation effects (eTable 1 in Supplement 1).
Figure 2. Model-Free Neuroimaging Results.
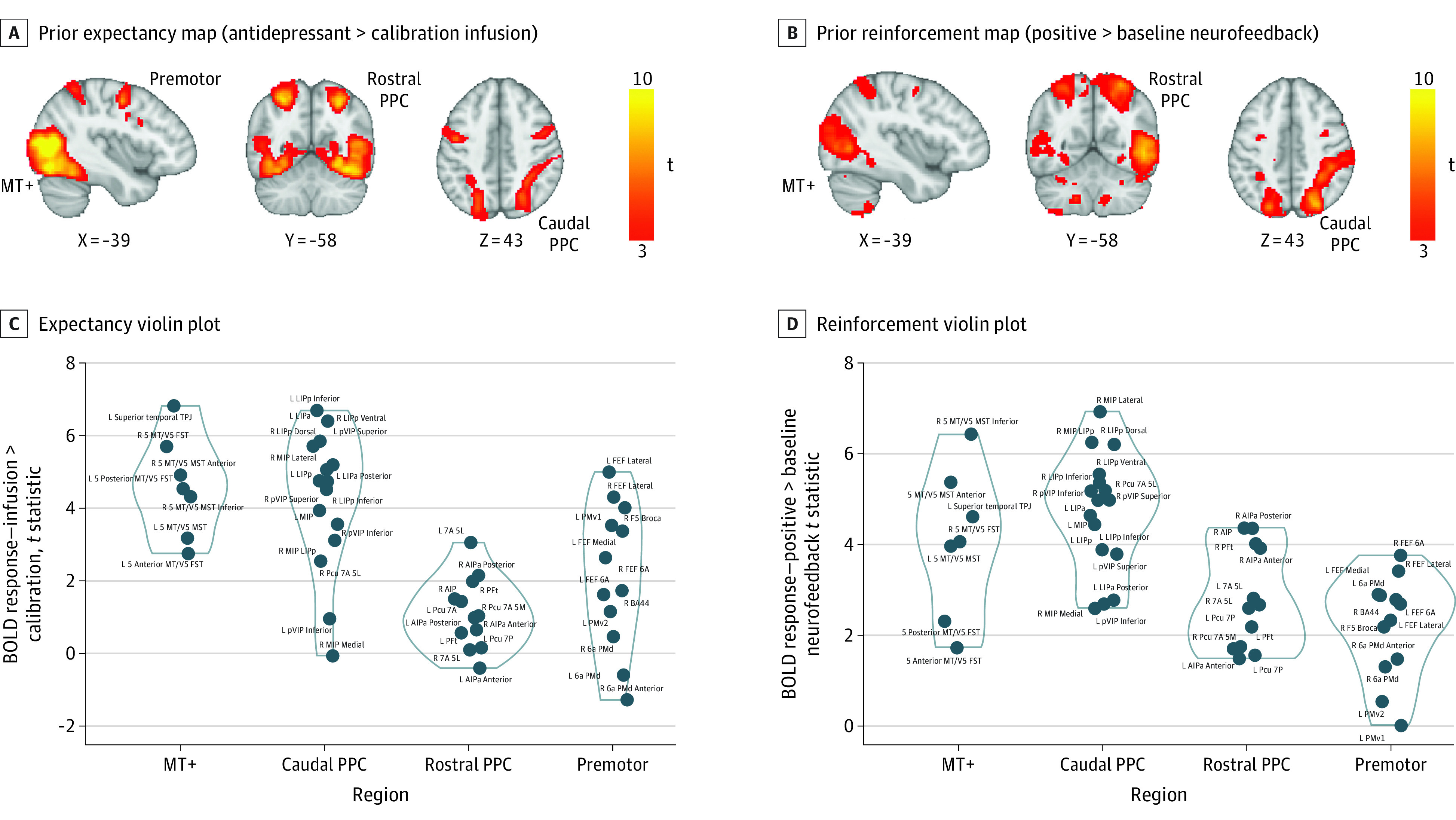
A, The prior placebo expectancy manipulation elicited increased blood oxygenation level–dependent (BOLD) responses in the visual and temporal cortex and the dorsal attention network, including the middle temporal visual area (MT+), the dorsal and rostral posterior parietal cortex (PPC), and the premotor cortex. B, The reinforcement map elicited similar activation of the visual cortex and the dorsal stream. Responses extended to the thalamus and the striatum. Violin plots of t statistics extracted from the expectancy (C) and reinforcement conditions t maps using Schaefer-based dorsal attention network nodes (D). a indicates anterior; AIPa, anterior intraparietal area; BA44, Brodmann area 44; FEF, frontal eye field; FST, superior temporal area; L, lateral; LIP, lateral intraparietal area; MIP, medial intraparietal area; MST, medial superior temporal; p, posterior; Pcu, precuneus; PFt, parietal area Ft; PMd, dorsal premotor cortex; PMv1,2, ventral premotor cortex (subregions 1 and 2); R, rostral; t, t-stat; TPJ, temporoparietal junction; V5, area MT (visual brain); VIP, ventral intraparietal area.
We further examined moderating effects of depression severity. As detailed in eTable 1 in Supplement 1, the effect sizes of the expectancy and reinforcement manipulations on expectancy and mood ratings were lower in individuals with more severe depression.
Model-Free Analyses of Blood Oxygenation Level–Dependent Responses: Association of Prior Expectancies and Reinforcement
Whole-brain analysis revealed positive responses to placebo expectancy (expecting an antidepressant infusion > calibration) in the visual and temporal cortex and the dorsal attention network (DAN), including the middle temporal visual area (MT+), dorsal and caudal posterior parietal cortex, and right premotor cortex, extending into the middle/inferior frontal gyrus (Figure 2A and C and Table). No effects were observed for the opposite contrast. High (>low) reinforcement elicited similar activation of the visual and temporal cortex (MT+) and the caudal and rostral posterior parietal cortex (PPC). Brainstem responses extended into the thalamus and the striatum (Figure 2B and D and Table). No effects were observed for the opposite contrast.
Table. Task-Based and Reinforcement Learning–Based Neuroimaging Results.
| Cluster size (voxels) | Hemisphere | Regions | Montreal Neurological Institute coordinates | |||
|---|---|---|---|---|---|---|
| t a | x | y | z | |||
| Expectancy (antidepressant > calibration) | ||||||
| 21594 | Bilateral | Visual cortex and DAN (MT+, rostral, and caudal PPC) | 12.3 | 21 | 23 | 34 |
| 524 | Right | Premotor | 7.44 | 57 | 55 | 55 |
| 419 | Left | Premotor | 8.04 | 22 | 54 | 55 |
| 77 | Left | Rostral PCC | 3.44 | 39 | 36 | 59 |
| Reinforcement (positive > baseline neurofeedback) | ||||||
| 27332 | Bilateral | Visual cortex and DAN (MT+, rostral, and caudal PPC) | 11.2 | 47 | 16 | 42 |
| 427 | Left | Premotor | 5.92 | 48 | 58 | 58 |
| 220 | Left | Thalamus | 4.87 | 50 | 44 | 36 |
| 35 | Left | Striatum | 3.9 | 34 | 61 | 32 |
| 47 | Right | Striatum | 3.96 | 45 | 62 | 33 |
| 151 | Right | Caudate | 3.9 | 49 | 57 | 43 |
| Learned value | ||||||
| 11486 | Left | Salience network: cingulate cortex, operculoinsular cortex | 9.84 | 15 | 59 | 48 |
| 2195 | Right | Visual cortex | 7.06 | 48 | 20 | 29 |
| 1896 | Left | MT+ | 7.22 | 21 | 28 | 27 |
| Prediction error | ||||||
| 16105 | Bilateral | Visual cortex and DAN (MT+, rostral, and caudal PPC) | 12.8 | 45 | 17 | 38 |
| 428 | Right | Premotor | 5.93 | 53 | 55 | 57 |
Abbreviations: DAN, dorsal attention network; MT+, middle temporal visual area; PPC, posterior parietal cortex.
Threshold-free cluster enhancement correction.
To understand the relevance of these brain responses to behavioral antidepressant placebo effects, we entered mean coefficients from clusters responsive to expectancy and reinforcement cues into separate linear mixed-methods models predicting participants’ ratings along with the task conditions (eTable 2 in Supplement 1). Greater blood oxygenation level–dependent (BOLD) responses in the MT+ and the premotor cortex during the expectancy condition were associated with a greater effect of the task conditions on expectancy ratings, as reflected by the positive 2-way interaction. Greater BOLD responses in the MT+ and the rostral PPC during the high reinforcement condition were associated with higher mood ratings (eTable 2 in Supplement 1). Expectancy and reinforcement brain responses were not correlated with depression severity (eTable 3 in Supplement 1).
RL Behavioral Modeling
To understand factors controlling the evolution of placebo expectancies, we considered 5 alternative models (Figure 1C). Bayesian model comparison indicated that the model postulating placebo-biased learning and reinforcement by mood dominated all alternatives, after correction for bayesian omnibus risk (bayesian omnibus risk <0.001; exceedance probability = 97%). Model log evidence, or the degree to which expectancy ratings were consistent with RL, was positively correlated with MADRS scores (Pearson r = 0.31; P = .01), after Bonferroni correction (adjusted P = .05; 3 regions × scales P = .02). Model predicted expectancies and simulation-based model recovery are reported in eFigure 4 and eTable 5 in Supplement 1.
Model-Based Analyses of BOLD
To understand the neural dynamics underlying this learning process, we examined trial-level placebo expectancies and PEs from the mood learning model as predictors in a whole-brain analysis. Higher learned expectancies resulting from the RL models and aligned to the expectancy rating event elicited responses throughout the SN, including the dorsal anterior cingulate cortex (dACC) and the operculoinsular cortex (Table and Figure 3A and C). Composite PEs combining both sensory evidence (neurofeedback) and mood recruited the visual cortex and the DAN, including the MT+, dorsal and caudal PPC, and the premotor cortex (Table and Figure 3B and D).
Figure 3. Reinforcement Learning (RL) Model-Based Neuroimaging Results.
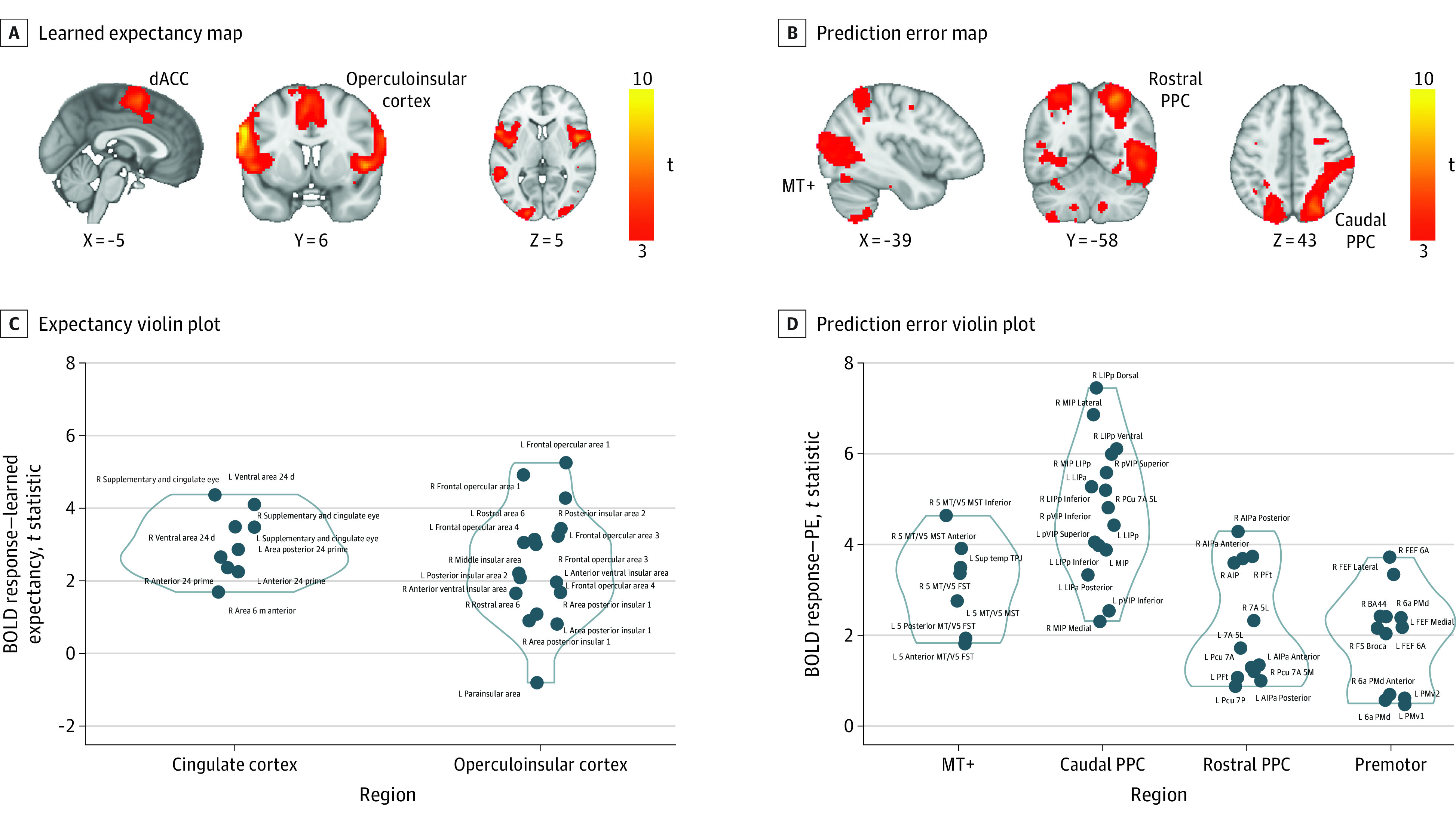
A, The voxelwise neuroimaging RL model revealed that high learned expected values were associated with increased blood oxygenation level–dependent (BOLD) responses extensively throughout the salience network (SN). B, Prediction error (PE) signals were associated with increased BOLD responses in the visual cortex and dorsal attention network (DAN). Violin plots of t statistics extracted from the learned expectancy (C) and PE (D) signal t maps using Schaefer-based SN and DAN nodes. a indicates anterior; AIPa, anterior intraparietal area; BA44, Brodmann area 44; dACC, dorsal anterior cingulate; FEF, frontal eye field; FST, superior temporal area; L, lateral; LIP, lateral intraparietal area; MIP, medial intraparietal area; MST, medial superior temporal; MT+, middle temporal visual area; p, posterior; Pcu, precuneus; PFt, parietal area Ft; PMd, dorsal premotor cortex; PMv1,2, ventral premotor cortex (subregions 1 and 2); PPC, posterior parietal cortex; R, rostral; t, t-stat; TPJ, temporoparietal junction; VIP, ventral intraparietal area.
To examine the behavioral relevance of these SN responses, we tested whether brain responses identified in RL model-based analysis moderated the effect of task conditions on participants’ reported placebo expectancies. SN responses to learned expectancies positively interacted with the high-reinforcement condition predicting expectancy ratings (dACC: estimate [SE] = 5.05 [2.07]; χ2 = 5.94; P = .02) (eTable 4 in Supplement 1). DAN responses to prediction errors positively interacted with the task condition predicting expectancy ratings, as reflected by a 3-way interaction (MT+: estimate [SE] = 12.86 [4.91]; χ2 = 6.86; P = .002; PPC caudal: estimate [SE] = 21.75 [3.96]; χ2 = 30.23; P < .001; PPC rostral: 20.30; estimate [SE] = 20.33 [4.29]; P < .001; PPC premotor: 13.96; estimate [SE] = 17.75 [3.73]; P = .001) (eTable 4 in Supplement 1). Expectancy responses in the SN (dACC) from the learned expectancy map, but not DAN responses from the PE map, scaled positively depression severity (HDRS: r = 0.4, P = .001; adjusted P = 0.05; 6 regions × scales P = .008).
Discussion
In this study, we aimed to understand whether the persistence of antidepressant placebo effects can be explained by (1) biased learning from sensory evidence of efficacy and/or (2) self-reinforcing positive feedback between expectancies of antidepressant effects and mood. Consistent with both hypotheses, multilevel analyses of behavior and RL cognitive modeling revealed learning dynamics wherein high prior expectancies and purported sensory evidence of antidepressant effects led to self-reported mood improvement. The resulting mood improvement was combined with sensory evidence of antidepressant effects, forming learning signals that updated subjective expectancies of the antidepressant effect. Visual displays of placebo infusion cues (expectancy manipulation) and sham neurofeedback supporting its physiological effects (reinforcement manipulation) recruited early visual areas and the DAN. Reinforcement with sham neurofeedback elicited additional striatothalamic responses. DAN responses estimated by model-free analyses positively moderated task manipulation effects on expectancy and mood responses. Latent model-predicted placebo expectancies were encoded in the SN, scaling positively with behavioral dynamics and depression severity. Learning signals were further encoded in visual areas and the DAN but not in striatum (Figure 4).
Figure 4. Conceptual Summary of Results.
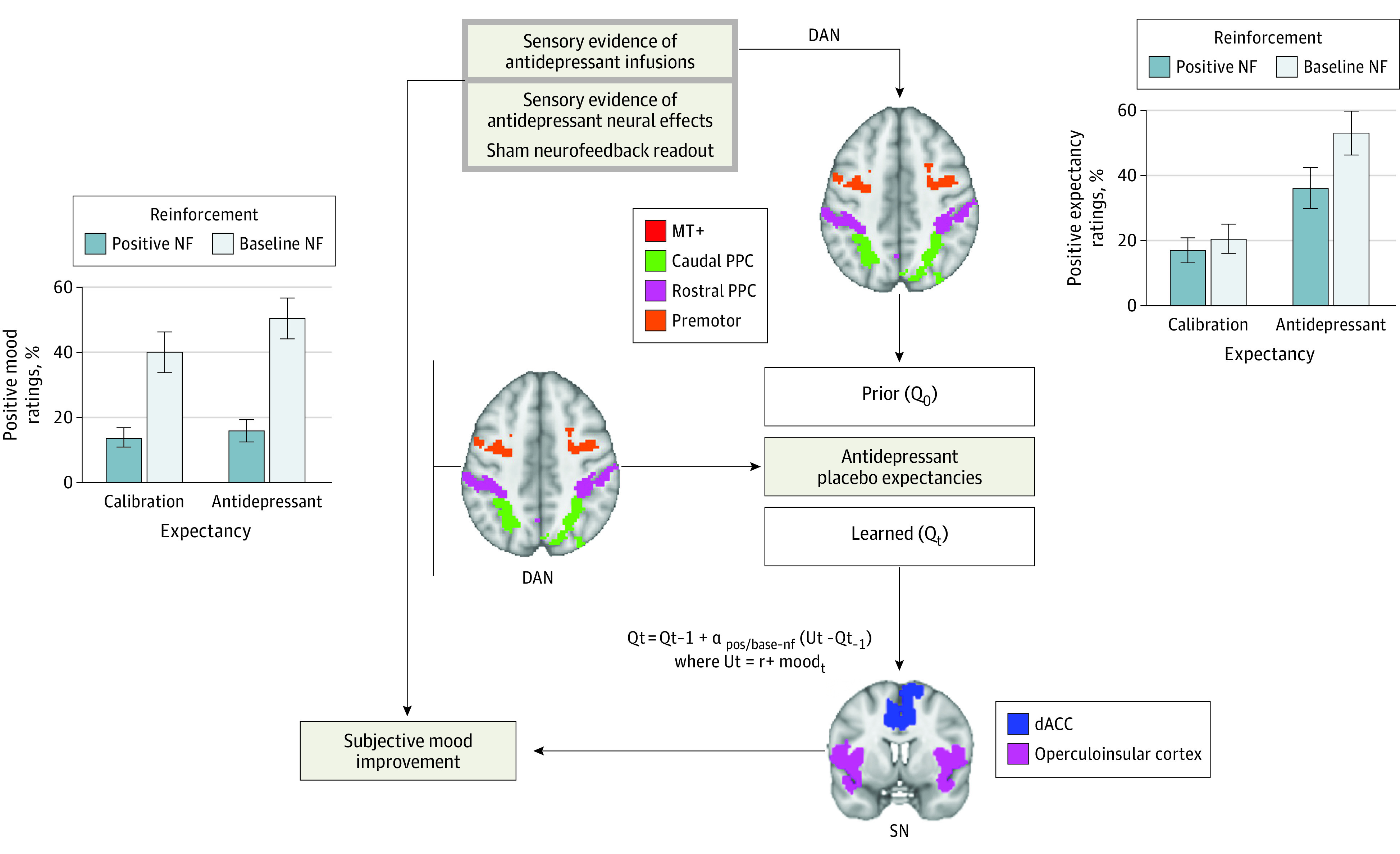
Multilevel analyses of behavior and reinforcement learning cognitive modeling revealed learning dynamics wherein high prior expectancies and sensory evidence of antidepressant effects led to self-reported mood improvement, which, combined with sensory evidence of response, provided learning signals that update patients’ future expectancies of the antidepressant effect. dACC indicates dorsal anterior cingulate; DAN, dorsal attention network; NF, neurofeedback.
First, we replicate previous findings12,13 of antidepressant placebo effects emerging from trial-by-trial manipulations of expectancies (fast-acting antidepressant) and their reinforcement by sham evidence of neural response. Again, expectancy and mood ratings were even more positive when the placebo infusion cue was reinforced with positive sham neurofeedback, suggesting that people’s learning about whether an antidepressant worked was biased by placebo expectancies, a conclusion supported by our computational modeling. Further, RL modeling supported biased learning from placebo cues (infusions), with learning signals that combined sensory evidence of antidepressant effects (sham neurofeedback) and momentary mood. Our computational modeling revealed a positive feedback pattern where momentary improvements in mood, produced in part by sham neurofeedback evidence of an antidepressant’s rapid effects on the brain and in part by prior placebo expectancies, elevated people’s subsequent placebo expectancies. This effect resembles positive feedback dynamics between learning from monetary rewards and mood.10 Our results are largely consistent with findings of Jepma and colleagues,8 who demonstrated that placebo analgesia effects are explained by a combination of expectancy-based pain modulation and a confirmation bias in learning. Taken together, this evidence suggests that persistent placebo effects might be explained by positive feedback loops between prior expectancies and experiences that are resistant to corrective experience.
As in our previous studies, prior expectancies and their reinforcement with sham neurofeedback recruited early visual areas and the DAN, which is consistent with the idea that the DAN maintains a dynamic map of stimuli predicting the antidepressant placebo response. The posterior parietal node integrates visual information from temporo-occipital areas, such as MT+, with parietal somatosensory information into multimodal maps of salient attention and action targets,28,29,30 which are signaled to premotor regions. The DAN has been proposed to encode the focus of attention integrating bottom-up and top-down influences,31 the intention to move,32 expected value,33,34 salience of stimuli,35 expected information gain,36 and environment states relevant to learning from reinforcement.37,38,39 Consistently, our data suggest a dynamic integration of visual and latent affective information in the DAN.
Learned placebo expectancies, on the other hand, were encoded in the SN, a large-scale network anchored on the anterior insula and dACC. The SN subserves context-specific stimulus selection and allocates attention and cognitive resources to adapt behavior to the current context. The anterior insula receives convergent multimodal sensory inputs, eg, auditory and visual.40 In addition to external sensory information, the insula receives interoceptive and autonomic signals important for assigning value to events41,42 and may thus support the emergence and persistence of placebo effects by multiplexing external cues with internal responses. dACC is a higher-order cognitive area involved in error monitoring, learning, and particularly tracking of volatility of reinforcement as well as internal statistics.43,44 dACC neurons represent reinforcement statistics on axes such as good or bad and expected or unexpected.45,46 dACC evaluates information about past actions and expected environmental states against observed outcomes14 to update expectations about the outcomes of decisions or actions.46,47 Consistently, our data suggest that the SN contributes to the emergence and persistence of antidepressant placebo effects by multiplexing external sham neurofeedback cues and mood states.
Finally, our results support previous findings stemming from the placebo analgesia field; however, we provide additional evidence suggesting that they might be specific to depressive symptoms. Depression itself appears to impact constituent processes of the AD placebo effect. As we reported earlier,16 individuals with severe depression are less sensitive to verbally communicated placebo expectancies. At the same time, they display more prominent mood-related learning dynamics, as demonstrated by better behavioral fit of the mood RL model and better neural fit of learned expectancy signals to dACC activity.48
Limitations
Our study has several limitations. First, our neurofeedback manipulation is a secondary reinforcer of antidepressant efficacy, albeit a behaviorally effective one, and future studies need to examine how primary reinforcers shape the antidepressant placebo effect. Second, because of the similarities between competing models, we are unable to generate a sufficiently divergent prediction about the neural activity to support a decisive neural bases comparison. Finally, our findings reveal learning dynamics on a timescale of minutes, and whether they generalize to timescales of weeks remains to be tested in longitudinal studies.
Conclusions
In this cross-sectional study, to the extent that our laboratory observations on a timescale of minutes generalize to the clinic, results suggest that antidepressant placebo effects persist in part due to the positive feedback between placebo expectancies and mood. The results also raise the question about the role of learning and expectancy-mood feedback loops in the effects of active antidepressants. Beyond elucidating learning mechanism implicated in antidepressant placebo effects, our findings may inform research on neurofeedback.49 Indeed, similar reinforcement loops might be involved in the persistence of real neurofeedback effects, promoting the learning of self-regulation strategies, and enhancing long-term responses.
eMethods
eFigure 1. Antidepressant Placebo fMRI Task Reinforcement Learning Model-Based Behavioral Results
eFigure 2. Dorsal Attention Network (DAN) and Salience Network (SN) Regions of Interest (ROI)
eResults
eFigure 3. Credibility Questionnaires’ Histograms
eTable 1. Mixed-Effects Models for the Prediction of Expectancy and Mood Ratings and Their Modulation by Depression Severity
eTable 2. Mixed-Effects Models Examining Manipulation Effects on Expectancy and Mood Ratings and Their Moderation by Model-Free Neural Responses During the Antidepressant Placebo fMRI Task
eTable 3. Correlation Between Prior Expectancy (A) and Reinforcement (B) DAN BOLD Responses and Depression Severity
eTable 4. Mixed-Effects Models Examining Manipulation Effects on Expectancy and Mood Ratings and Their Moderation by RL-Based Neural Responses During the Antidepressant Placebo fMRI Task
eFigure 4. Top: Model-Predicted Expectancies Across 4 Key Models. Bottom Left: Our Mood Model Makes a Strong Prediction That if Mood Improved at a Preceding Presentation of the Same Stimulus (Trial t – k), the Expectancy is Heightened at the Current Presentation of This Stimulus k Trials Later at Trial t. Bottom Center: Average Expectancy Ratings by Stimulus Type (Learning Curves). Bottom Right: Average Expectancy Ratings by Reinforcement (Baseline vs Positive Neurofeedback)
eTable 5. Simulation-Based Model Recovery: the Confusion Matrix
Data Sharing Statement
References
- 1.Moseley JB, O’Malley K, Petersen NJ, et al. A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med. 2002;347(2):81-88. doi: 10.1056/NEJMoa013259 [DOI] [PubMed] [Google Scholar]
- 2.Holtzheimer PE, Husain MM, Lisanby SH, et al. Subcallosal cingulate deep brain stimulation for treatment-resistant depression: a multisite, randomised, sham-controlled trial. Lancet Psychiatry. 2017;4(11):839-849. doi: 10.1016/S2215-0366(17)30371-1 [DOI] [PubMed] [Google Scholar]
- 3.Bush RR, Mosteller F. A mathematical model for simple learning. Psychol Rev. 1951;58(5):313-323. doi: 10.1037/h0054388 [DOI] [PubMed] [Google Scholar]
- 4.Sutton RS, Barto AG. Reinforcement Learning: An Introduction. MIT Press; 1998. [Google Scholar]
- 5.Rescorla R, Wagner A. A theory of Pavlovian conditioning: the effectiveness of reinforcement and non-reinforcement. In: Black AH, Prokasy WF, eds. Classical Conditioning II: Current Research and Theory. Appleton-Century-Crofts; 1972:64-99. [Google Scholar]
- 6.Delamater AR, Westbrook RF. Psychological and neural mechanisms of experimental extinction: a selective review. Neurobiol Learn Mem. 2014;108:38-51. doi: 10.1016/j.nlm.2013.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schenk LA, Sprenger C, Onat S, Colloca L, Büchel C. Suppression of striatal prediction errors by the prefrontal cortex in placebo hypoalgesia. J Neurosci. 2017;37(40):9715-9723. doi: 10.1523/JNEUROSCI.1101-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jepma M, Koban L, van Doorn J, Jones M, Wager TD. Behavioural and neural evidence for self-reinforcing expectancy effects on pain. Nat Hum Behav. 2018;2(11):838-855. doi: 10.1038/s41562-018-0455-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grahl A, Onat S, Büchel C. The periaqueductal gray and bayesian integration in placebo analgesia. Elife. 2018;7:e32930. doi: 10.7554/eLife.32930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eldar E, Rutledge RB, Dolan RJ, Niv Y. Mood as representation of momentum. Trends Cogn Sci. 2016;20(1):15-24. doi: 10.1016/j.tics.2015.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eldar E, Niv Y. Interaction between emotional state and learning underlies mood instability. Nat Commun. 2015;6:6149. doi: 10.1038/ncomms7149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peciña M, Heffernan J, Wilson J, Zubieta JK, Dombrovski AY. Prefrontal expectancy and reinforcement-driven antidepressant placebo effects. Transl Psychiatry. 2018;8(1):222. doi: 10.1038/s41398-018-0263-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peciña M, Chen J, Lyew T, Karp JF, Dombrovski AY. μ Opioid antagonist naltrexone partially abolishes the antidepressant placebo effect and reduces orbitofrontal cortex encoding of reinforcement. Biol Psychiatry Cogn Neurosci Neuroimaging. 2021;6(10):1002-1012. doi: 10.1016/j.bpsc.2021.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silvetti M, Seurinck R, Verguts T. Value and prediction error in medial frontal cortex: integrating the single-unit and systems levels of analysis. Front Hum Neurosci. 2011;5:75. doi: 10.3389/fnhum.2011.00075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pitt Plus Me . Home page. Accessed February 21, 2017. https://pittplusme.org/
- 16.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382-389. doi: 10.1192/bjp.134.4.382 [DOI] [PubMed] [Google Scholar]
- 17.Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med. 1996;26(3):477-486. doi: 10.1017/S0033291700035558 [DOI] [PubMed] [Google Scholar]
- 18.Posit (formerly RStudio) . Home page. Accessed August 29, 2022. https://www.rstudio.com/
- 19.Bates D, Maechler M, Bolker B, et al. lme4: Linear mixed-effects models using “eigen” and S4. Published online July 8, 2022. Accessed August 29, 2022. https://CRAN.R-project.org/package=lme4
- 20.White K, Davey GC. Sensory preconditioning and UCS inflation in human “fear” conditioning. Behav Res Ther. 1989;27(2):161-166. doi: 10.1016/0005-7967(89)90074-0 [DOI] [PubMed] [Google Scholar]
- 21.Daunizeau J, Adam V, Rigoux L. VBA: a probabilistic treatment of nonlinear models for neurobiological and behavioural data. PLoS Comput Biol. 2014;10(1):e1003441. doi: 10.1371/journal.pcbi.1003441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorgolewski K, Burns CD, Madison C, et al. Nipype: a flexible, lightweight and extensible neuroimaging data processing framework in python. Front Neuroinform. 2011;5:13. doi: 10.3389/fninf.2011.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rigoux L, Stephan KE, Friston KJ, Daunizeau J. bayesian model selection for group studies—revisited. Neuroimage. 2014;84:971-985. doi: 10.1016/j.neuroimage.2013.08.065 [DOI] [PubMed] [Google Scholar]
- 24.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162-173. doi: 10.1006/cbmr.1996.0014 [DOI] [PubMed] [Google Scholar]
- 25.Patel AX, Kundu P, Rubinov M, et al. A wavelet method for modeling and despiking motion artifacts from resting-state fMRI time series. Neuroimage. 2014;95(100):287-304. doi: 10.1016/j.neuroimage.2014.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62(2):782-790. doi: 10.1016/j.neuroimage.2011.09.015 [DOI] [PubMed] [Google Scholar]
- 27.Vanyukov PM, Hallquist MN, Delgado M, Szanto K, Dombrovski AY. Neurocomputational mechanisms of adaptive learning in social exchanges. Cogn Affect Behav Neurosci. 2019;19(4):985-997. doi: 10.3758/s13415-019-00697-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cisek P. Cortical mechanisms of action selection: the affordance competition hypothesis. Philos Trans R Soc Lond B Biol Sci. 2007;362(1485):1585-1599. doi: 10.1098/rstb.2007.2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freedman DJ, Ibos G. An integrative framework for sensory, motor, and cognitive functions of the posterior parietal cortex. Neuron. 2018;97(6):1219-1234. doi: 10.1016/j.neuron.2018.01.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sereno MI, Huang RS. Multisensory maps in parietal cortex. Curr Opin Neurobiol. 2014;24(1):39-46. doi: 10.1016/j.conb.2013.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colby CL, Goldberg ME. Space and attention in parietal cortex. Annu Rev Neurosci. 1999;22(1):319-349. doi: 10.1146/annurev.neuro.22.1.319 [DOI] [PubMed] [Google Scholar]
- 32.Snyder LH, Batista AP, Andersen RA. Intention-related activity in the posterior parietal cortex: a review. Vision Res. 2000;40(10-12):1433-1441. [DOI] [PubMed] [Google Scholar]
- 33.Polanía R, Moisa M, Opitz A, Grueschow M, Ruff CC. The precision of value-based choices depends causally on frontoparietal phase coupling. Nat Commun. 2015;6(1):8090. doi: 10.1038/ncomms9090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Platt ML, Glimcher PW. Neural correlates of decision variables in parietal cortex. Nature. 1999;400(6741):233-238. doi: 10.1038/22268 [DOI] [PubMed] [Google Scholar]
- 35.Leathers ML, Olson CR. In monkeys making value-based decisions, LIP neurons encode cue salience and not action value. Science. 2012;338(6103):132-135. doi: 10.1126/science.1226405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horan M, Daddaoua N, Gottlieb J. Parietal neurons encode information sampling based on decision uncertainty. Nat Neurosci. 2019;22(8):1327-1335. doi: 10.1038/s41593-019-0440-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leong YC, Radulescu A, Daniel R, DeWoskin V, Niv Y. Dynamic interaction between reinforcement learning and attention in multidimensional environments. Neuron. 2017;93(2):451-463. doi: 10.1016/j.neuron.2016.12.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Niv Y, Daniel R, Geana A, et al. Reinforcement learning in multidimensional environments relies on attention mechanisms. J Neurosci. 2015;35(21):8145-8157. doi: 10.1523/JNEUROSCI.2978-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gläscher J, Daw N, Dayan P, O’Doherty JP. States versus rewards: dissociable neural prediction error signals underlying model-based and model-free reinforcement learning. Neuron. 2010;66(4):585-595. doi: 10.1016/j.neuron.2010.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nieuwenhuys R. The insular cortex: a review. Prog Brain Res. 2012;195:123-163. doi: 10.1016/B978-0-444-53860-4.00007-6 [DOI] [PubMed] [Google Scholar]
- 41.Sterzer P, Kleinschmidt A. Anterior insula activations in perceptual paradigms: often observed but barely understood. Brain Struct Funct. 2010;214(5-6):611-622. doi: 10.1007/s00429-010-0252-2 [DOI] [PubMed] [Google Scholar]
- 42.Eckert MA, Menon V, Walczak A, et al. At the heart of the ventral attention system: the right anterior insula. Hum Brain Mapp. 2009;30(8):2530-2541. doi: 10.1002/hbm.20688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silvetti M, Alexander W, Verguts T, Brown JW. From conflict management to reward-based decision-making—actors and critics in primate medial frontal cortex. Neurosci Biobehav Rev. 2014;46(Pt 1):44-57. doi: 10.1016/j.neubiorev.2013.11.003 [DOI] [PubMed] [Google Scholar]
- 44.Alexander WH, Brown JW. Medial prefrontal cortex as an action-outcome predictor. Nat Neurosci. 2011;14(10):1338-1344. doi: 10.1038/nn.2921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Philiastides MG, Biele G, Vavatzanidis N, Kazzer P, Heekeren HR. Temporal dynamics of prediction error processing during reward-based decision-making. Neuroimage. 2010;53(1):221-232. doi: 10.1016/j.neuroimage.2010.05.052 [DOI] [PubMed] [Google Scholar]
- 46.Hayden BY, Heilbronner SR, Pearson JM, Platt ML. Surprise signals in anterior cingulate cortex: neuronal encoding of unsigned reward prediction errors driving adjustment in behavior. J Neurosci. 2011;31(11):4178-4187. doi: 10.1523/JNEUROSCI.4652-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cai X, Padoa-Schioppa C. Neuronal encoding of subjective value in dorsal and ventral anterior cingulate cortex. J Neurosci. 2012;32(11):3791-3808. doi: 10.1523/JNEUROSCI.3864-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jones BDM, Razza LB, Weissman CR, et al. Magnitude of the placebo response across treatment modalities used for treatment-resistant depression in adults: a systematic review and meta-analysis. JAMA Netw Open. 2021;4(9):e2125531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sitaram R, Ros T, Stoeckel L, et al. Closed-loop brain training: the science of neurofeedback. Nat Rev Neurosci. 2017;18(2):86-100. doi: 10.1038/nrn.2016.164 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
eFigure 1. Antidepressant Placebo fMRI Task Reinforcement Learning Model-Based Behavioral Results
eFigure 2. Dorsal Attention Network (DAN) and Salience Network (SN) Regions of Interest (ROI)
eResults
eFigure 3. Credibility Questionnaires’ Histograms
eTable 1. Mixed-Effects Models for the Prediction of Expectancy and Mood Ratings and Their Modulation by Depression Severity
eTable 2. Mixed-Effects Models Examining Manipulation Effects on Expectancy and Mood Ratings and Their Moderation by Model-Free Neural Responses During the Antidepressant Placebo fMRI Task
eTable 3. Correlation Between Prior Expectancy (A) and Reinforcement (B) DAN BOLD Responses and Depression Severity
eTable 4. Mixed-Effects Models Examining Manipulation Effects on Expectancy and Mood Ratings and Their Moderation by RL-Based Neural Responses During the Antidepressant Placebo fMRI Task
eFigure 4. Top: Model-Predicted Expectancies Across 4 Key Models. Bottom Left: Our Mood Model Makes a Strong Prediction That if Mood Improved at a Preceding Presentation of the Same Stimulus (Trial t – k), the Expectancy is Heightened at the Current Presentation of This Stimulus k Trials Later at Trial t. Bottom Center: Average Expectancy Ratings by Stimulus Type (Learning Curves). Bottom Right: Average Expectancy Ratings by Reinforcement (Baseline vs Positive Neurofeedback)
eTable 5. Simulation-Based Model Recovery: the Confusion Matrix
Data Sharing Statement