Summary
Post-learning sleep contributes to memory consolidation. Yet, it remains contentious whether sleep affords opportunities to modify or update emotional memories, particularly when people would prefer to forget those memories. Here, we attempted to update memories during sleep using spoken positive words paired with cues to recent memories of aversive events. Affective updating using positive words during human non-rapid eye movement (NREM) sleep, compared with using neutral words instead, reduced negative affective judgments in post-sleep tests, suggesting that the recalled events were perceived as less aversive. Electroencephalogram (EEG) analyses showed that positive words modulated theta and spindle/sigma activity; specifically, to the extent that theta power was larger for the positive word than for the following memory cue, participants judged the memory cues less negatively. Moreover, to the extent that sigma power was larger for the positive word than for the following memory cue, participants forgot more episodic details about the aversive event. Notably, when the onset of individual positive words coincided with the up-phase of slow oscillations (a state characterized by increased cortical excitability during NREM sleep), affective updating was more successful. In sum, we altered the affective content of memories via the strategic pairing of positive words and memory cues during sleep, linked with EEG theta power increases and the slow oscillation up-phase. These findings suggest novel possibilities for modifying unwanted memories during sleep, which would not require people to consciously confront memories that they prefer to avoid.
Keywords: memory editing, targeted memory reactivation, sleep learning, theta power, sleep spindle, slow oscillation
eTOC blurb
Tao et al. show that via pairing positive words with aversive memory cues during NREM sleep, people’s affective responses toward cues become less negative, i.e., affective updating. Enhanced theta power elicited by positive words, and the optimal coupling between positive words and slow oscillations up-phases, drive successful affective updating.
Introduction
Sleep sculpts our emotional memories via offline consolidation 1–4. But can memories be updated and modified during sleep? Unwanted memories—such as for traumatic, painful, or shameful experiences—can be particularly debilitating for cognitive functioning and mental well-being 5,6. However, controlling unwanted memories can be daunting, given the challenge of top-down cognitive control abilities 7,8. Moreover, people often wish to avoid thinking of such unwanted memories, thereby precluding direct confrontation and control. It would therefore be desirable to modify unwanted memories without direct confrontation and associated cognitive effort. Here, we examined the novel hypothesis that unwanted memories can be updated during sleep, bypassing the challenge of confronting a negative memory.
An established paradigm to manipulate memory processing during sleep is known as targeted memory reactivation (TMR) 9–11. The TMR method entails the unobtrusive re-presentation of memory cues during post-learning sleep, using a sound or an odor that was paired with prior learning during wakefulness. In this way, cued memories are reactivated, and consolidation moves forward. TMR effects are manifested by differential retrieval for reactivated compared to non-reactivated memory items in post-sleep tests. TMR can influence many types of memory, including spatial memory, motor memory, emotional memory, linguistic memory, and others 9,11–17. Notably, researchers also adapted TMR to modify fearful or emotional memories during sleep 18–26, but the results to date are mixed: TMR weakened, strengthened, or had null effects on emotional memories. This evidence thus does not constitute convincing support for the idea that these TMR methods can effectively update unwanted memories.
Instead of provoking reactivation by presenting a stimulus linked to emotional information from the pre-sleep learning phase, some investigators have used more complex sleep learning or TMR paradigms. Sleep-learning paradigms typically involved attempts to form new associations accomplished via odor-odor, odor-tone, or even word-word stimulus pairing, during sleep 27–29. Beyond forming new associations, researchers have also aimed to promote forgetting during sleep. In a TMR study, Simon and colleagues (2018) first trained tones to be associated with efforts to forget, and then played those forgetting-associated tones in conjunction with memory cues during sleep, which induced forgetting of episodic memories. Episodic forgetting was also shown with a related procedure in which only forgetting-associated tones were played during sleep after directed forgetting was attempted prior to sleep 30.
Another variation on the TMR paradigm, yet to be explored, is to attempt to update a memory by combining a memory cue with a stimulus of opposite valence, i.e., counterconditioning. In a counterconditioning paradigm, a previously conditioned stimulus is paired with a new stimulus of opposite valence, with the aim of modifying evaluations or maladaptive behavior 31–33. For example, when repeatedly paired with a positive or an appetite stimulus, an original fearful memory could be updated to be less negative, while fearful responses gradually diminished via this counterconditioning procedure 31–33. This waking-state counterconditioning research, combined with sleep learning and TMR, raises a novel possibility that through pairing positive emotional stimuli with memory cues during sleep, people may update the affect tone of previously learned aversive memories.
Here, we designed a sleep-based memory-updating procedure to test the extent to which we can update unwanted memories via pairing positive words with memory cues. The procedure consists of three sessions (Figure 1): pre-sleep learning, updating during sleep, and post-sleep test. Prior to sleep, participants learned cue-target pairings involving initially neutral pseudowords and aversive emotional pictures. We also included memory tests following learning to obtain pre-sleep baseline measures. During post-learning NREM sleep, we unobtrusively played spoken updating words (positive or neutral) as unconditioned stimuli, each followed by a pseudoword memory cues. We presented updating words (positive vs. neutral) immediately before the memory cues to ensure that effects were produced by affective counterconditioning instead of memory disruption that might result if words were played following cues (see 17, wherein presenting a spoken word immediately after a memory cue abolished TMR benefits compared to a cue-only condition). To assess behavioral effects of sleep-based updating, we measured participants’ affective judgments and accuracy of unwanted memories after sleep. We hypothesized that by repeatedly pairing positive words with memory cues during NREM sleep, participants’ negative affective responses toward cues would be weakened in the post-sleep tests.
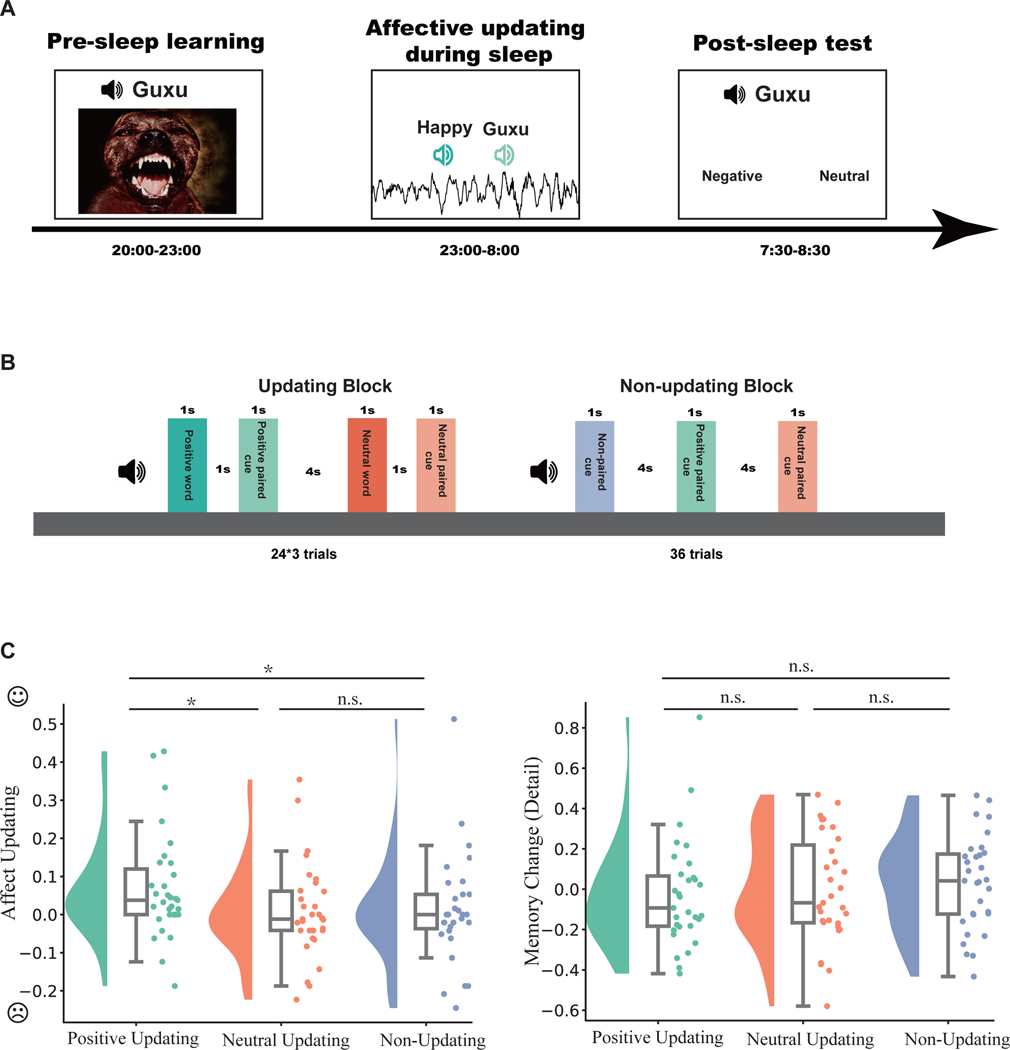
Figure 1: Experiment procedure and affective updating effect.
(A)The general procedure of experiment. (B) Affective updating procedure during sleep (for details, see STAR Methods). (C) Behavioral outcomes of affective updating (left) and memory change of detail (right). Note that the affective updating effect remained significant when using mean ± 2.5 median absolute deviation (MAD) to exclude statistical outliers (p = 0.011). *: p < 0.05.
We further hypothesized that if unwanted memories can be updated, stimulus-elicited brain activity during NREM sleep would be critical for updating to emerge and may be observable in EEG measures. Existing studies showed that 4–8 Hz theta activity was related to memory consolidation during sleep and wakefulness 17,34–37. Some TMR and sleep learning studies further pinpointed the role of theta in emotional processing during NREM sleep, such as in distinguishing between emotional vs. neutral prosodies 38; encoding of emotional stimulus pairings 27,34,39; and reactivation of emotional memory 24,27,34,38–40. We thus hypothesized that the valence of the updating word could modulate theta power during NREM sleep, which could then drive successful affective updating.
In addition to theta power, spindle activity within the sigma band (12–16 Hz) and slow-wave activity within delta band (0.5–4 Hz) are instrumental for sleep-mediated memory reactivation and consolidation 2,41–46. Spindle and spindle-related sigma power have also been associated with auditory processing and emotional memory reactivation during NREM sleep 16,17,47,48. Here, given that we presented pairs of stimuli during sleep, we were interested in whether sigma power elicited by the positive words and the memory cues would influence post-sleep memory. In addition, the cortical slow oscillation (SO, 0.5–2 Hz), a defining neural oscillation of deep sleep, encompasses a down-phase and an up-phase that reflect neural hyperpolarization and depolarization, respectively. The SO up-phase is thought to comprise a transient period suitable for high-level cognitive processing and long-distance cross-regions communication 49–51. Indeed, when memory cues or auditory stimulation were applied during the SO up-phase, stronger memory benefits or sleep learning effects emerged 29,52,53. Here, we focused on the contingency between SO phase and onset of spoken words and memory cues, and examined whether such contingencies influenced post-sleep affective updating.
Results
Pairing positive words and memory cues updated affective judgment
To answer our primary research question on sleep-based affective updating, we quantified changes in affective judgments from pre- to post-sleep, i.e., affective updating. We calculated the neutral response ratio by dividing the number of neutral responses by the number of total trials in each of the three conditions (positive vs. neutral vs. non-updating). At the pre-sleep learning session, we confirmed that pairing of pseudowords and aversive images successfully induced negative judgments toward pseudowords (hereafter referred as cues): Participants were more likely to judge the cues as negative than neutral: t(30) = −14.43, p < 0.001, dz = 2.59. Moreover, there were no significant between-condition differences in neutral response ratio during the pre-sleep learning session (mean ± S.E., positive updating: 0.41 ± 0.048; neutral updating: 0.46 ± 0.047; non-updating: 0.38 ± 0.045; F(2,60) = 2.21, p = 0.118).
To quantify the affective updating effect, we computed affective change scores as pre-sleep neutral response ratio subtracted from post-sleep neutral response ratio, with higher scores indicating higher neutral responses. Submitting affective change scores to a one-way analysis of variance (ANOVA, positive vs. neutral vs. non-updating) revealed a significant effect: F(2,60) = 4.23, p = 0.019, ηG2 = 0.043, ηp2 = 0.124. Post-hoc tests showed that positive updating during sleep significantly increased affective change scores than neutral updating (mean ± S.E., positive updating: 0.07± 0.02; neutral updating: 0.01± 0.02; t(30) = 2.36, pcorrected = 0.038, dz = 0.42) and than non-updating (0.01± 0.03; t(30) = 2.41, pcorrected = 0.038, dz = 0.43). The difference between neutral updating and non-updating conditions was not significant (t(30) = −0.10, p = 0.921).
We next examined reaction times (RT) in the affective judgment task. A 3 (positive vs. neutral vs. non-updating) * 2 (negative vs. neutral response) repeated measures ANOVA was conducted on pre- to post-sleep RT changes. There were no significant differences for condition, valence, or their interaction (ps > 0.190). The same analyses on subjective valence and arousal ratings did not reveal significant main or interaction effects (ps > 0.620).
We next sought to explore whether our procedure produced changes in the recall of negative memories. We coded verbally recalled memory with three indices: identification, gist, and detail (STAR Methods). We quantified identification and gist changes via dividing pre-sleep correct responses by post-sleep correct responses. Memory detail scores were Z-normalized within participants to control for between-participant variances in verbal descriptions (Zhuang et al., 2022). Then, memory detail change scores were calculated by subtracting pre-sleep from post-sleep memory detail scores. We did not find significant differences among the three conditions (identification: F(2,60) = 0.17, p = 0.840; gist: F(2,60) = 0.75, p = 0.479; detail: F(2,60) = 0.29, p = 0.752).
Words elicited significant ERPs during NREM sleep
To examine how the sleeping brain responded to updating words, we first calculated brain potentials elicited by updating words across all electrodes. We conducted one-tailed spatial-temporal permutation tests to compare event-related potentials (ERPs) against zero across all channels and time points to identify the peak responses. The results revealed dynamic changes in the brain’s responses to updating words along the time course (pclusters < 0.045; Figure 2). In particular, in the frontal-central regions, two ERP peaks were identified following each of the updating word and memory cue. These findings indicated that the sleeping brain retains some capacity for processing external auditory stimuli such as spoken words (for whole brain analyses, Figure S1).
Figure 2: Word-elicited ERPs during NREM sleep (first: updating word; second: memory cue).
Upper panel: butterfly plot of ERPs to spoken words collapsing across positive and neutral updating conditions. Graded area denotes the global field power (GFP). Middle panel: significant time windows (colored areas) across frontal-central electrodes when comparing ERPs against zero. Bottom panel: grand averaged ERPs across frontal-central electrodes (F1/2, Fz, FC1/2, FCz, C1/2, Cz). See also Figure S1.
Regarding ERPs differences between the positive updating and neutral updating conditions, a two-tailed spatial-temporal permutation test was performed to assess the between-condition differences across all channels and time series. The results revealed no significant differences in ERPs between positive and neutral updating conditions (pclusters > 0.566).
Words elicited delta-theta-alpha and alpha-sigma-beta power during NREM sleep
To investigate word-elicited EEG activity, we performed time-frequency analyses on EEG epochs followed by averaging across conditions, participants, and pre-selected frontal-central channels (F1/2, Fz, FC1/2, FCz, C1/2, Cz; Figure 3A). These electrodes were selected based on previous sleep learning 29 and TMR studies 16. Via a cluster-based, two-tailed one-sample permutation test (1000 randomization and a statistical threshold of 0.05) against zero across time points and frequency bands, we identified one significant cluster, including an earlier delta-theta-alpha cluster (1–12 Hz) and a later alpha-sigma-beta cluster (9–25 Hz) induced by updating words or memory cues (pcluster = 0.001; Figure 3B). For EEG responses elicited by the updating words (positive vs. neutral), we examined the delta-theta cluster during 0.37 s to 1.29 s and the sigma-beta cluster during 0.54 s to 2.08 s (Figure 3C and 3D). For EEG responses elicited by the memory cues, we examined the delta-theta cluster during 2.30 s to 3.69 s and the sigma-beta cluster during 2.34 s to 3.90 s (Figure 3C and 3D). These clusters were generally consistent with previous TMR and sleep learning studies 16,29. We then used these identified clusters as regions of interest (ROIs) in the following analyses.
Figure 3: Word-elicited, time-frequency-resolved EEG activity during NREM sleep.
(A) Time-frequency results averaging across all trials and participants over frontal-central electrodes (F1/2, Fz, FC1/2, FCz, C1/2, Cz). (B) A t-values map from a cluster-based permutation test across frequency bands and time points, representing how updating words and memory cues modulated EEG activity during NREM sleep. Time-frequency plots for (C) positive and for (D) neutral updating conditions, with solid and dash lines highlighting significant clusters derived from (B) within the 4–8 Hz theta and the 12–16 Hz sigma bands, together with their scalp distributions. (E) Theta and (F) sigma power extracted from the significant clusters (highlighted in solid lines) were significantly different between positive and neutral words. *: pcorrected < 0.05. Theta and sigma differences remained significant after excluding statistical outliers (mean ± 2.5 MAD, theta: p = 0.013, sigma: p < 0.001).
Updating words modulated theta and sigma/spindle activity
We first examined the EEG power elicited by positive and neutral words within the earlier 0.37 s to 1.29 s delta-theta cluster (1–8 Hz). To delineate the frequency-specific effect, we separately focused on delta (1–4 Hz) and theta (4–8 Hz). A paired sample t-test showed that positive words elicited significantly stronger theta power (Figure 3E; mean ± S.E., positive word: 3.07 ± 0.40; neutral word: 2.06 ± 0.39; theta: t(29) = 2.47, pcorrected = 0.030, dz = 0.45) than neutral words, while no significant effect was observed in the delta band (delta: t(29) = 1.50, pcorrected = 0.432).
We next examined the later 0.54 s to 2.08 s sigma-beta (12–25 Hz) clusters, focusing on sigma (12–16 Hz) and beta (16–25 Hz) bands separately. Paired sample t-tests showed that positive words elicited significantly greater sigma power than neutral words (sigma: mean ± S.E., positive word: 2.91 ± 0.50; neutral word: 1.70 ± 0.49, t(29) = 3.28, pcorrected = .005, dz = 0.60; Figure 3F). However, this effect was not observed in the beta band (t(29) = 1.65, p =.110). Complementing time-frequency EEG analyses, we found similar results when examining discrete 12–16 Hz fast spindle events and spindle probability: positive words elicited significantly higher fast spindle probability than neutral words within similar time windows as in the sigma power analyses (Figure S2B). Together, results from time-frequency EEG and fast spindle analyses showed that the positive and neutral updating word differentially modulated theta and sigma/spindle activity change during NREM sleep.
Updating words and memory cues jointly modulated theta and sigma activity
We next examined how updating valence and position modulated the dynamics of theta and sigma power. We conducted 2 (updating valence: positive vs. neutral) * 2 (position: first updating words vs. second memory cues) repeated measures ANOVAs on theta and sigma power, separately. Theta (4–8 Hz) and sigma (12–16 Hz) power were extracted within their corresponding significant clusters (Figure 3C and D).
Regarding theta, we found a significant main effect of updating valence: F(1,29) = 4.62, p = 0.040, ηG2 = 0.023, ηp2 = 0.138, with positive updating eliciting greater theta power change than neutral updating (mean ± S.E., positive updating: 3.14 ± 0.36 vs. neutral updating: 2.48 ± 0.35; Figure 4A). We also found the second memory cue tended to elicit a larger theta power change (3.05 ± 0.35) than the preceding updating word (2.56 ± 0.34), although this effect did not reach significance (F(1,29) = 4.14, p = 0.051, ηG2 = 0.013, ηp2 = 0.125). The interaction was not significant (F(1,29) = 1.66, p = 0.208).
Figure 4: Updating words (circles) and memory cues (triangles) modulated theta (A) and sigma (B) power.
Theta and sigma power were derived from their corresponding significant clusters highlighted in Figures 3C and 3D. (C) Theta power differences between positive words and cues positively predicted affective updating. (D) Sigma power differences between positive words and cues negatively predicted memory changes in details. *: p < 0.05, **: p < 0.01. See also Figure S2 and S3.
Regarding sigma, the same ANOVA did not find main effects for either updating valence (F(1,29) = 2.66, p = 0.114) or position (F(1,29) = 0.45, p = 0.507; Figure 4B). However, we found a significant interaction effect (F(1,29) = 11.38, p = 0.002, ηG2 = 0.024, ηp2 = 0.282, Figure 4B). Post-hoc comparisons revealed that in the neutral updating condition, the neutral word elicited a lower sigma power increase than its paired cue (neutral word: 1.70 ± 0.49; cue: 2.69 ± 0.50; t(29) = −2.90, pcorrected = .017, dz = −0.53); whereas in the positive updating condition, the positive word elicited a slightly higher sigma power increase than the memory cue (positive word: 2.91 ± 0.50; cue: 2.31 ± 0.37; t(29) = 1.52, pcorrected = 0.139). We observed a similar interaction with the 12–16 Hz fast spindle probability (Figure S2D).
Theta power difference between positive words and memory cues predicted affective updating
We next sought to ask whether the theta and sigma EEG power changes have any effect on affective updating. To quantify the affective updating effect at a neural level, we subtracted power induced by memory cues from the power induced by updating words. This subtraction thus removed auditory-related neural activity that was not specific to emotion or to cue processing. A higher value would indicate stronger neural processing of updating words than its preceding memory cues, which possibly drives more effective affective updating.
Using this neural metric and the behavioral affective change scores, we examined the relationship between theta and sigma power difference with the affective updating, respectively. We found that during positive updating, theta power differences positively predicted post-sleep affective updating (r(30) = 0.55, p = 0.002; Figure 4C), whereas sigma power differences did not show such a prediction (r(30) = 0.16, p = 0.408). During neutral updating, no significant correlations were found (theta: r(30) = −0.19, p = 0.304; sigma: r(30) = 0.09, p = 0.654).
To verify that the prediction effect was driven by the theta power differences between the updating word and memory cue rather than by the power elicited by either updating word or memory cue, we repeated the analyses using partial correlation, controlling the theta power elicited by individual positive words and cues. The results remained significant (theta: r(30) = 0.56, p = 0.002), confirming that the theta power difference between the positive words and the following cues significantly predicted overnight affective updating.
Sigma power difference between positive words and memory cues predicted memory change
Spindle-related sigma power has been linked to memory processing during sleep. In TMR studies, post-cue sigma power positively predicted memory change, whereas pre-cue sigma showed the opposite predictions 41,54,55. We thus asked whether sigma power differences between the updating words and memory cues predicted memory changes after sleep. We found that sigma power differences negatively predicted memory change in all three measures (identification, gist, and details), yet the relationship was only significant for memory details in the positive updating condition (r(30) = −0.47, p = 0.009; Figure 4D, for identification and gist, r(30)s > −0.03, ps> 0.080). Partial correlation confirmed that in this positive updating condition, only the sigma power differences—rather than the sigma power induced by individual words—predicted memory change in details (r(30) = −0.49, p = 0.009). Together, these results showed that during updating, stronger sigma power elicited by the positive words relative to memory cues resulted in more forgetting of negative memories following sleep.
We examined delta, beta, earlier and later alpha power in affective updating (Figure S3), given their roles in memory and semantic processing during sleep54,56. We also performed analyses to rule out the potential influence of alpha-related arousal (see STAR Methods). Furthermore, spindle analyses showed that the later 8–12 Hz alpha power changes may reflect the activity of slow spindles (Figure S2AC).
Successful affective updating depends on positive word onset within an SO up-phase
Recent sleep learning and TMR studies suggest that the temporal coupling between SO phases and auditory stimulation is instrumental for successful sleep learning and TMR benefits 29,52,57. An intriguing possibility is that the affective updating effect would similarly depend on stimulus-SO coupling. To examine this hypothesis, we categorized individual trials into negative-change and negative-stay sub-conditions based on affective judgment changes (STAR Methods). By definition, negative-change trials were trials showing successful affective updating, i.e., cues previously judged to be negative were later judged to be neutral.
We included negative-change trials across all participants and extracted the instantaneous SO phase when individual updating words and memory cues were played, and we repeated this step for negative-stay trials. Complementing this item-level analysis, we examined the coupling between word onset and SO phase at a participant level, wherein we averaged instantaneous SO phase when updating words and cues were played for negative-change and for negative-stay trials separately within each participant.
The item- and participant-level analyses yielded consistent results: In the positive updating condition, negative-change trials were associated with a significant non-uniform distribution of positive word onsets, and the averaged preferred phase coupled to SO up-phases (trial-level: Z(681) = 7.46, p < 0.001, mvl = 0.105, coupling phase: −0.09°; participant-level: Z(26) = 6.18, p = 0.002, Rayleigh test; mvl = 0.49; coupling phase: −18.51°; see Figure 5A). A similar result was found for memory cues: trial-level: Z(681) = 4.87, p = 0.008, Rayleigh test; mvl = 0.10; coupling phase: −4.91°; participant-level: Z(26) = 2.98, p = 0.049, Rayleigh test; mvl = 0.34; coupling phase: −6.54°, circular mean.
Figure 5: The coupling between SO up-phases and positive word onset drove affective updating.
(AB) The distribution of updating words and cues onsets relative to SO phases. Upper panel: shaded areas in the radar plot indicated the trial numbers of word onset phase distribution from negative-change and negative-stay in positive (A) and neutral (B) updating conditions, using all trials from all participants. The colored circles represent the averaged preferred phase from negative-change and negative-stay sub-conditions, for each participant. Arrows indicated the mean vector length across all participants. Bottom panel: grand-averaged ERPs from negative-change and negative-stay trials in positive (A) and neutral (B) updating, with a 2 Hz low-pass filter applied. (C) Results from the linear mixed model, using the number of individual updating words (left panel) and memory cues (right panel) during SO up-phases to predict affective updating. The coupling results remained significant after excluding outliers (Figure S4). Shaded area indicates 95% CI. **: p < 0.01 ***: p < 0.001.
In contrast, for negative-stay trials, the onset of positive words and memory cues were randomly distributed (positive words: trial-level, Z(681) = 1.75, p = 0.174; participant-level, Z(26) = 1.59; p = 0.205; cues: trial-level, Z(681) = 1.34, p = 0.263; participant-level, Z(26) = 0.11, p = 0.895, Rayleigh tests).
The phase effect was specific to SO events, as the same analyses focusing on 2–4 Hz delta activity did not yield significant effects (Zs < 2.30, ps > 0.100). Repeating the same analysis in the neutral updating condition did not find significant clustering in the negative-change trials (Figure 5B; trial-level: neutral words: Z(412) = 0.57, p = 0.567; cues: Z(412) = 0.61, p = 0.541; participant-level: neutral words, Z(25) = 0.45, p = 0.643; cues, Z(25) = 0.38, p = 0.691) or in the negative-stay trials (trial-level: neutral words: Z(412) = 1.20, p = 0.30; cues: Z(412) = 0.37, p = 0.690; participant-level: neutral words: Z(25) = 0.36, p = 0.698; neutral paired cue: Z(25) = 1.07, p = 0.345).
Next, we conducted an inverted analysis to further demonstrate that the coupling between positive words and SO up-phases drove successful affective updating. We counted how many times each individual positive/neutral word were delivered during an SO up-phase, and used the linear mixed model (LMM) to examine whether this number, the updating valence, and their interaction would predict affective updating (STAR Methods). While neither the number of updating words in SO up-phase (F(1, 29.44) = 0.37, p = 0.550) nor updating valence (F(1, 686.47) = 0.07, p = 0.785) was significant, we found a significant interaction (F(1, 689.21) = 4.25, p = 0.040). That is, the prediction power of affective updating was significantly higher when using the number of up-phase positive words than when using the number of up-phase neutral words (b = 0.025, S.E. = 0.012, t(688) = 2.06, p = 0.040; Figure 5C), suggesting that the number of individual positive words played during SO up-phases drove successful affective updating.
When we used the number of individual memory cues delivered during SO up-phases in the LMM, neither main nor interaction effects were significant (Fs<2.20, ps>0.140). Taken together, these SO phase analyses indicated that the coupling between the onset of positive words and SO up-phases drove successful affective updating.
Discussion
Can negative memories be strategically updated to become less negative during sleep? We demonstrated that our procedures for presenting positive words and memory cues during NREM sleep did indeed produce “affective updating”—participants’ affective judgments about these memories became less negative. In addition to this behavioral effect, we found that EEG theta and sigma power were both implicated in affective updating as well. Notably, at both the item- and participant-level, the timing of positive words relative to the slow oscillation (SO) up-phase contributed to successful affective updating. By demonstrating sleep-based affective updating in association with neural correlates of this updating, the present study provides new knowledge to guide future possibilities for editing unwanted memories.
Previous research showed that the sleeping brain responds to external stimuli with a preserved information-processing capacity. Specifically, during NREM sleep, theta power elicited by emotional words could indicate affective information processing 34,38, and theta power elicited by memory cues represents reactivation of emotional memories 24,40. We thus postulated that theta differences between updating words and memory cues could reflect the strength of modulation of affective processing on subsequent memory reactivation. Accordingly, we quantified this modulation effect by calculating theta power differences between updating words and memory cues. Our results indicated that the larger the theta power elicited by positive words than by memory cues, the greater affective updating. Moreover, this effect was specific to the positive updating condition, and there was no such relationship in the neutral updating condition. Thus, successful affective updating may depend on theta activity for affective encoding, for memory reactivation during sleep, or for both.
Sigma EEG power was also implicated in affective updating. Intriguingly, we found that positive words elicited stronger sigma power and more spindles than neutral words, whereas such differences were weaker or even reversed for memory cues. Moreover, the temporal trajectory of spindle probability was consistent with the spindle refractory hypothesis, whereby spindles are segregated by brief refractory periods such that a second spindle is relatively likely 3–6 s after a first spindle 41. Regarding sigma power and memory reprocessing, previous studies showed that pre-cue sigma negatively predicted post-cue sigma power as well as the TMR-induced memory change 41,54,55. Here, given that the two stimuli were played consecutively, sigma induced by updating words could function to decrease the likelihood of spindles following the subsequent memory cues. Accordingly, stronger sigma power elicited by the positive word relative to the memory cue may decrease cue-triggered memory reactivation, thereby leading to episodic forgetting.
Whereas sigma power differences predicted forgetting, memory cue presentations in the non-updating blocks appeared not to influence emotional memory. Importantly, our study combined memory cues with preceding positive or neutral words, which could lead to results different from those of typical TMR studies. In a highly relevant study, Schreiner and colleagues (2015) found that presenting auditory stimuli shortly after memory cues abolished both reactivation-related neural activity and the TMR benefits. It is possible that even when updating words were played before the memory cues, an interfering effect nevertheless occurred, diminishing the TMR benefits from memory cues alone. Another possibility is that updating and non-updating blocks may interactively influence memory reactivation. Unfortunately, we cannot disentangle separate effects that may have resulted from the updating blocks and the non-updating blocks.
Stimulation synchronized with the SO up-phase has been shown to be conducive for successful encoding and memory reactivation during sleep 29,52,64. Indeed, the SO up-phase represents a unique period associated with cortical excitability and neural plasticity that may be essential for information processing during sleep 49,50. Corroborating this hypothesis about the SO up-phase, our results found that at both the item- and participant-level, successful affective updating depended on coincidence between word onset and SO up-phase. Scrutinizing the coupling results suggested that the onset of positive words, but not memory cues, drove affective updating. These results corroborate a recent study that showed that successful learning occurred when the second word of word pairings was delivered at the SO peak 29. Whereas in that study participants learned novel semantic associations, our paradigm involved pairing positive words and aversive memory cues, or counterconditioning 31,32. Extending this research, our study showed that optimal processing of the positive stimuli, as indicated by higher theta power and precise coupling with the SO up-phase, was crucial for affective updating.
What are the possible mechanisms supporting affective updating during sleep? Employing intracranial EEG (iEEG), a recent TMR study found that emotional memory reactivation was accompanied by spindle and SOs in orbitofrontal cortex, and by enhanced theta connectivity between the hippocampus and orbitofrontal cortex 40,58. These results are consistent with the systems consolidation account that emphasizes a critical role of hippocampal-neocortex interactions in memory consolidation 44. Given that affective updating is most effective when updating words were coupled with SO up-phases, a period characterized by long-range cross-region communication 51, it is possible that affective updating during NREM sleep would also engage distributed brain regions implicated in emotional and memory processing (e.g., hippocampus, amygdala, and orbitofrontal cortex). Future research could adopt iEEG or other neuroimaging techniques to investigate whether similar cross-regional communication during pairing contributes to affective updating.
Broadly speaking, our study is related to the “sleep to forget, sleep to remember” (SFSR) hypothesis, which proposes that sleep preserves memory contents while attenuating their affective tone 4. Beyond spontaneous memory and emotional processing during sleep, our findings indicated that the memory and emotional responses could be modified via re-playing memory cues in conjunction with positive words. The SFSR hypothesis proposes an important role for REM sleep in attenuating emotional responses 23,59. Extending our study that focused on NREM sleep, future research could focus on REM sleep to provide additional information about the physiological mechanisms of affective updating.
Limitations shall be noted. First, previous sleep and TMR studies have often shown long-term effects on emotional responses and memory 42,60,61. Given that we did not include a delayed test, whether sleep-based affective updating can be long-lasting warrants future investigation. Second, affective updating was evident in the affective judgment task, which captured fast, spontaneous affective responses. However, subjective emotional ratings did not show any updating effects. Previous sleep learning and TMR effects were evident in indirect measures such as nasal airflow, response speed, and forced choices 27–29,39,62,63. Future research should clarify the extent to which affective updating is evident in different behavioral tasks. Lastly, one fruitful direction to consider is how to boost the magnitude of the affective updating effect. Our findings suggest two routes. First, closed-loop stimulation that delivers updating word at the up-phase of SOs may enhance the affective updating effect 52,64. Second, employing more potent positive stimuli, such as pleasant odors, may elicit stronger theta activity and enhance affective updating. In addition, using pleasant odors as unconditioned stimuli could help capture affective learning effect during sleep, and has been associated with subsequent behavior changes 27,39.
During sleep, the brain continues to process sensory stimuli despite ostensible disconnection from the external world 65. Harnessing the power of the sleeping brain, we showed that affective responses to memory cues could be changed via pairing positive words with these cues during NREM sleep. The present study could help the development of novel paradigms to update or modify unwelcome memories, and pinpoints possible neural mechanisms supporting effective updating. An important question that remains to be tackled in future research will be how to help people better manage unwanted memories they have acquired outside the laboratory, such as from actual traumatic experiences.
STAR★METHODS
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources should be directed to and will be fulfilled by the Lead Contact, Xiaoqing Hu (xiaoqinghu@hku.hk).
Materials availability
This study did not generate new, unique reagents or materials.
Data and code availability
Pre-processed data have been deposited at the open science framework (OSF: https://osf.io/pqcta/).
All original code has been deposited at the open science framework (OSF: https://osf.io/pqcta/).
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Forty-six native Chinese speakers participated in the study. Participants reported regular sleep-wake cycles, did not take any medications that impairs sleep or mood, and had no history or current diagnoses of neurological or psychiatric illnesses. Participants were compensated with a monetary incentive (~ 30 USD) for their participation. Six participants were excluded because they reported hearing the words while sleeping, four participants were excluded because they had fewer than 48 updating trials (i.e., one updating block), and five participants dropped out of the experiment before sleep. One participant’s sleep EEG data were not saved due to equipment breakdown. The final analyses included 31 valid participants in the behavioral analysis (gender: nine male, 22 female; age: mean ± S.D., 21 ± 2) and 30 valid participants in the EEG analysis (with at least 48 updating trials, mean ± S.E., positive: 191 ± 10; neutral: 188 ± 10; t(29) = 1.70, p = 0.100). The study was approved by the Human Research Ethics Committee of the University of Hong Kong. All participants provided written consent prior to participation.
Thirty-six two-syllable pseudowords were created by randomly pairing two neutral characters. We then selected 12 positive words (valence: mean ± S.D., 7.00 ± 1.28; rating obtained from the nine-point Likert-scale, with 1 being extremely negative to 9 being extremely positive) and 12 neutral words (valence: mean ± S.D., 5.29 ± 1.58) from the Chinese Affective Word System 66. Vocalization of the pseudowords, positive words, and neutral words were generated via text-to-speech of iFLYTEK (word duration, mean ± S.D., 0.76 ± 0.10 s), with the positive words rated more positive than the neutral words (t(30) = 18.38, p < 0.001; positive words: mean ± SD., 7.59 ± 0.78; neutral words: 5.45 ± 0.39). For emotional pictures, we selected 36 negative pictures (mean ± S.D., valence: 3.14 ± 0.53; arousal: 4.43 ± 1.22; ratings obtained from the nine-point Likert-scale, with 1 being extremely calm to 9 being extremely excited). These pictures are from three categories: Animal, Baby, Scene, with each category containing 12 pictures (sources: International Affective Picture System, IAPS 67 ; Nencki Affective Picture System, NAPS 68; and from the internet).
METHOD DETAILS
Experimental procedure
Participants completed the following three sessions: 1) pre-sleep learning in which they learned cue-target pairings to acquire negative emotional memories; 2) sleep-based affective updating in which spoken word-cue pairings were unobtrusively played during their NREM sleep; and 3) post-sleep tests in which they were tested on affect responses and memory promoted by cues.
In the pre-sleep learning session (~ 20:30), participants completed the following tasks in order: 1) affective rating of negative pictures; 2) cue-target learning involving pseudowords as cues and negative pictures as targets; 3) baseline affective judgment task; 4) baseline pseudowords memory cue affective rating; 5) positive and neutral words affective rating; and 6) baseline cued recall. Participants went to sleep (~ 23:00) after completing these tasks.
Picture/cue/word rating task
Participants first rated each of the 36 negative emotional pictures on valence (1 being extremely negative to 9 being extremely positive on the Likert scale) and arousal (1 being extremely calm to 9 being extremely excited on the Likert scale). Each trial started with an 0.8-s fixation, followed by pictures being presented on the center of the screen until participants gave responses using a computer mouse. Pictures from all three categories (Animal, Baby, and Scene) were randomly presented. Additionally, participants completed a cue affective rating task in which they rated the valence and arousal of memory cues, in the same way as in the picture rating task.
Encoding task
During cue-target learning, participants memorized 36 word-picture pairings involving pseudowords and negative pictures via four rounds of viewing and recognition-feedback. In the viewing phase, each trial started with a fixation (jittered 0.8–1.2 s), followed by two-syllable aurally presented pseudowords (~ 1 s). After a 1-s blank screen, a pseudoword-picture pairing was presented for 1.5 s on the center of the screen, while the spoken pseudoword was played again. After participants viewed all 36 pseudowords-picture pairings, they took a one-minute break, followed by a test-feedback phase. Here, participants were visually and aurally presented with the pseudoword (~ 1 s), together with three pictures being presented on the screen. Pseudowords were randomly assigned to be paired with each picture across participants. Participants were prompted to identify the correct picture that was paired with the spoken pseudoword from the previous viewing session. Note that all pictures in this test-feedback phase were randomly chosen from the earlier viewing session, preventing participants from relying on familiarity to make a correct judgment. Distractors were randomly selected from all viewed pictures for each trial, and were randomly shuffled for each participant. Upon participants’ choice, a “correct” or “incorrect” feedback was provided regardless of accuracy, followed by the presentation of the correct pseudoword-picture pairing for 1.5 s. Participants were instructed to remember the picture in the cue-target pairing in as much detail as possible because there would be another memory recall task in later tests. Participants were presented with their recognition accuracies at the end of each test-feedback phase. This viewing and recognition-feedback round repeated for four times.
Affective judgement task
In the affective judgment task, each trial started with an 0.8–1.2 s fixation, followed by the cues being played aurally and visually on the center of the screen for ~ 1 s. Participants made a speedy negative or neutral affective judgment toward the cue, using the left vs. right keys within 1.5 s. Participants completed this task before and after sleep to measure the affective updating effect.
Cued recall task
In the cued recall tasks, each cue started with a fixation (0.8–1.2 s), followed by the cue word being aurally presented for ~ 1 s. Participants were asked to verbally describe the paired pictures in as much detail as possible within 15 s. The inter-trial interval was set to be 3 s. Participants completed this cued recall task before and after sleep to measure memory changes. Two independent raters blind to experimental conditions rated identification, detail, and gist from the cued recall task according to previous similar studies on the verbal recall of emotional scenes 69. For inconsistent ratings between the two raters, a third rater was involved to reconcile the discrepancy. Identification refers to whether the verbal description included sufficient details to correctly identify the unique picture, with correct identification given a score of 1 (vs. incorrect identification, with a score of 0). Detail refers to the number of meaningful information segments describing the picture, and was scored by counting the number of correct details. Gist refers to whether the verbal description included the key theme of the picture, and was scored by counting the number of correctly identified gist items 69.
Participants woke up at around 07:00 the next morning and completed the following task in order: 1) affective judgment, 2) cue affective rating, and 3) cued recall.
Sleep-based affective updating
We randomly selected two out of three categories of negative memories and their associated memory cues to be paired with positive (one category, 12 cues) or neutral (one category, 12 cues) words during sleep. The remaining one category (12 cues) was assigned to the non-updating condition, i.e., they were not paired with any words during sleep. One of the categories was randomly selected and paired with positive words, while another category was paired with negative words. Picture categories assigned to positive updating, neutral updating, and non-updating conditions were counterbalanced across participants. Linear mixed model (Affective updating ~ 1 + updating_valence * picture_category + (1 | subject)) revealed that picture category did not influence the significance of affective updating results (updating valence: F(1, 708.35) = 6.80, p = 0.009; picture category: F(2, 531.33) = 1.77, p = 0.171; picture category by updating valence interaction: F(2, 90.72) = 0.84, p = 0.435).
Participants went to bed around 23:00. Well-trained experimenters started playing the spoken words when participants entered slow-wave sleep for at least five minutes. Each updating trial started with a spoken positive or neutral word (~ 1 s), followed by a spoken pseudoword memory cue (~ 1 s) with an inter-stimulus-interval (ISI) of 1 s. The inter-trial-interval (ITI) was 4 s. Each updating block contained 24 trials that were randomly presented, with 12 positive words + cue trials and 12 neutral words + cue trials. We presented positive or neutral words before the memory cues to ensure that the effects are due to affective conditioning rather than to memory disruption, which might occur if words were played immediately following memory cues. This latter possibility was observed by Schreiner and colleagues (2015), who reported that presenting a spoken word immediately after a memory cue disrupted the cue-elicited EEG activity, and abolished TMR benefits compared to a cue-only condition. Stimulation was paused for one minute between blocks. After every three updating blocks, a non-updating block was administered. In the non-updating block, all 36 cues (12 positive updating cues, 12 neutral updating cues, and 12 non-updating cues) would be played randomly without any paired words. The ITI was 4 s. Each round included three updating blocks and one non-updating block. We hypothesized that examining cue-elicited EEGs in the non-updating blocks may capture the online change of positive- vs. neutral- vs. non-updating memory cues. Stimulation was paused if participants entered REM or N1 sleep, or showed arousal or wakefulness (e.g., burst of EMGs, alpha activity, etc.). The experimenter would end the procedure when 1) seven rounds (i.e., 21 updating blocks and seven non-updating blocks) were completed or 2) at 02:00 in the morning, whichever came first. The next morning (~ 07:00), participants were woken up and given 15 minutes to recover from sleep inertia.
All experimental tasks were implemented with PsychoPy 3.0 70. During sleep, all aurally presented stimuli were played via a loudspeaker (~ 47-dB sound pressure level) mounted one meter above the bed, with white noise being played throughout the night.
EEG recording and preprocessing
Sleep EEG was recorded using a 64-channel EEG cap connected to an eego amplifier (ANT neuro), with electrodes arranged according to the International 10–20 system. F3/F4, C3/C4, P3/P4, and O1/O2 were selected for online sleep monitoring, with the electrode CPz as the online reference. One EOG channel was placed below the left eye to monitor eye movements. Two additional bipolar EMG electrodes were placed on the chin to record EMG. On-line EEG data were bandpass filtered from 0.5 Hz to 40 Hz for monitoring purposes, with a 500 Hz sampling rate.
We used MNE-Python for offline EEG pre-processing 71. First, EEG data were down-sampled to 200 Hz. Second, raw EEG data were filtered with a bandpass of 0.5–40 Hz. Third, bad channels were visually identified and marked. Fourth, data were re-referenced to the average of all non-marked electrodes after removing the M1 and M2. Fifth, for trials in the updating blocks, continuous EEG data were segmented into short (−1.5 s to 5.5 s) and long (−15 s to 15 s) epochs relative to the onset of the spoken word. We used the short (−1.5 s to 5.5 s) 7000 ms epochs in stimulus-locked event-related potentials (ERPs) and time-frequency analyses; and the long (−15 s to 15 s) 30 s epochs in stimulus-locked sleep event detection analyses on a trial basis. For trials in the non-updating blocks, continuous EEG data were segmented into (−1.5 s to 3.5 s) 5 s epochs relative to the onset of memory cues. Lastly, artifacts were visually inspected and deleted, followed by bad channel interpolation.
Behavioral analysis
For behavioral data, we focused on affective changes from pre- to post-sleep affective responses. Specifically, for the affective judgment task, we calculated the neutral response ratio (0–1: 0 means all responses were negative; 1 means all responses were neutral). We next subtracted the pre-sleep from the post-sleep neutral response ratio, with this affective change score ranging from −1 to 1. A positive affective change score would indicate more neutral (or fewer negative) judgments from pre- to post-sleep, whereas a negative affective change score would indicate fewer neutral (or more negative) judgments from pre- to post-sleep. A zero affective change score would indicate no changes of affective response from pre- to post-sleep. For the affective rating task, we similarly calculated affective rating changes by subtracting pre-sleep baseline valence/arousal ratings from post-sleep ratings. A higher valence/arousal change score would indicate more positive/arousal changes from pre- to post-sleep.
We also measured memory changes from pre- to post-sleep cued recall tasks. Memory change scores were calculated by subtracting pre-sleep baseline memory scores from post-sleep memory scores, with higher change scores indicating larger memory retention.
ERPs and time-frequency analyses
For ERPs, artifact-free short epochs were averaged and baseline corrected (updating trial: −1 s to 0 s; non-updating trial: −1 s to 0 s). For time-frequency analysis, a continuous wavelet transformation with variance cycles (three cycles at 1 Hz in length, increasing linearly with frequency to 15 cycles at 30 Hz) was implemented on updating trial epochs (−1.5 s to 5.5 s) and non-updating trial epochs (−1.5 s to 3.5 s) to obtain power for the frequency range from 1 Hz to 30 Hz, in steps of 0.5 Hz and 5 ms. Epochs were cropped to eliminate edge artifacts (updating trial: −1 s to 5 s; non-updating trial: −1 s to 3 s) after time-frequency transformation. Subsequently, averaged spectral power was normalized (Z-scored) using a (−1 s to −0.2 s) baseline for the updating trial and for the non-updating trial, separately. The time-frequency analysis aimed to identify the brain’s response to auditory stimuli during sleep. Based on the overall auditory-stimulus-induced EEG response against zero, we next analyzed the EEG responses to positive and neutral words. We reported time-frequency and ERP results from the updating trials to investigate the neural mechanisms of affective updating. Results are reported in the main text and in Supplement Figure S1 and S3.
For non-updating blocks that only involved memory cues, we first ran a two-tailed cluster-based permutation test within 1000 randomization across time points and frequency bands at nine interested channels (F1/2, Fz, FC1/2, FCz, C1/2, Cz). Three positive clusters were identified across the delta(1–4Hz), theta(5–9Hz), sigma(12–16Hz), and beta(16–30Hz) bands (pclusters< 0.005). Power values within each band in the identified cluster were extracted from positive paired, neutral paired, and non-paired cues, and were then submitted to positive vs. neutral vs. non-updating ANOVA separately. However, we did not find differences between these conditions (ps > 0.200, Figure S5).
Arousal analyses
Because our stimuli-induced time-frequency significant cluster including two parts of alpha activity (earlier alpha within delta-theta-alpha cluster, later alpha within alpha-sigma-beta cluster), we examined arousal-related brain activity to rule out the possibility that the results were partially driven by conscious perception of stimuli or different levels of (micro)arousals between the two updating conditions. According to a recent study that examined arousal during TMR72, we quantified cueing-related arousal by measuring the absolute EEG power spectral difference between pre-updating words (−2–0s) and post-updating words (0–2s). An arousal index was calculated based on the mean of abs(1-postword power/preword power) in steps of 0.5Hz from 0.5Hz to 20Hz. We calculated the power using morlet wavelet time-frequency transformation (tfr_morlet, Python-MNE 1.2, 3 cycles in length at 0.5 Hz, increasing linearly with frequency to 10 cycles at 20 Hz), without baseline normalization. An advantage of this calculation is that “These parameters were chosen to cover the range of frequencies in which arousal-related activity appears, while providing sufficient frequency and time resolution to compute the sleep disruption index” (direct quotation72, page 5). Using this method to quantify arousal, we did not find significant differences of arousal between the positive and neutral words (t(29) = −1.67, p = 0.105), i.e., arousal did not differ between conditions.
Sleep staging analysis
We conducted sleep stage scoring based on a machine learning algorithm Yet Another Spindle Algorithm (YASA73, which was checked by an experienced sleep researcher. EEG data were first re-referenced to FPz per YASA recommendations. The C4 (or C3 if C4 was marked as a bad channel), EOG, and EMG channels were used to feed the algorithm. Before statistics on sleep staging could be calculated, artifacts had to be identified. Table S1 provides information on sleep stages.
Slow oscillations and spindle detection
We extracted slow oscillations (SOs) and sleep spindles implemented in YASA 73. SOs were detected at Fz based on previous research 74,75. EEG data were first bandpass filtered (0.5–2 Hz) using an FIR filter with a transition band of 0.2 Hz. Second, after zero-crossings were detected, events were selected based on duration (0.5 s to 2 s) and amplitude (75 percentile) criteria. Individual SOs were detected on each trial from the (−15 s to 15 s) 30 s long epochs, with the detection results retained in the (−1.5 s to 5.5 s) 7 s epochs.
Fast spindles were detected at Cz 16 using the root mean square (RMS). EEGs were first down-sampled to 100 Hz, followed by bandpass filtered between 12 Hz and 16 Hz. Second, the RMS was calculated at every sample point with a sliding window of 0.3 s at a step of 0.1 s. Spindles thresholds were determined by the mean of RMS plus 1.5 SDs of the signals. The 10% lowest and 10% highest values were removed before computing the SD of RMS. If a sample exceeds this threshold, it would be tagged as a potential spindle. Next, for neighboring potential spindles, they were merged together if the between-spindles intervals were shorter than 0.5 s. Spindle events were counted only if they met the 0.5 s to 2 s duration criterion. Spindles were detected on each (−15 s to 15 s) 30 s long epoch, with the detection results retained in the (−1.5 s to 5.5 s) 7 s epochs. We repeated the same spindle detection method to extract 8–12 Hz slow spindle events at Cz. Spindle probability was then calculated at each time point of the trial according to previous research 16. Spindle results are presented in Figure S2.
Word-SO phase coupling analysis
To investigate how temporal coupling between the word onset and SO phase influences affective updating, we conducted an item-level analysis focusing on the SO phase when playing positive updating stimuli. We divided the positive updating trials into negative-change versus negative-stay trials based on pre- to post-sleep affective judgment changes. We defined trials as negative-change when the affective judgments changed from pre-sleep negative to post-sleep neutral, indicating successful affective updating. We defined trials as negative-stay when both pre- and post-sleep affective judgments were negative, i.e., no affective updating. Participants were excluded from this analysis if they did not have negative-change trials. In the positive updating condition, 26 participants were retained. To examine whether the effect was specifically due to positive updating, we repeated this analysis with neutral updating trials, with 25 participants retained.
We next examined the word-SO phase clustering of negative-change and negative-stay trials in positive and neutral updating conditions, separately. The SOs were identified using the method described above. Given that the updating trials occurred during NREM sleep, we used trials during which at least two SOs occurred during the (−1.5 s to 5.5 s) 7s epochs for subsequent phase analysis. Given that the negative-stay sub-conditions contained more trials than negative-change sub-conditions, we matched the trial numbers by their temporal proximity. Specifically, for each negative-change trial, we retained its closest negative-stay trial to match the number and to control the temporal distance. Next, we extracted the instantaneous phase of the onset of spoken words and memory cues using a Hilbert transform. We examined the coupling between word/cue onset and SO phases using the Rayleigh test.
To further validate the robustness of the word-SO coupling effect, we conducted an inverted analysis. First, upon detection of SOs in each updating trial, we assigned the trial to two sub-conditions: 1) updating words up-phase vs. down-phase and 2) memory cues up-phase vs. down-phase, according to whether their onsets were located between the mid crossing of an SO and its end (up-phase) or between the start of an SO and its mid crossing (down-phase). We counted the number of individual updating word and memory cues in each sub-condition and conducted a linear mixed model (LMM) to explore how the number of individual word/cues in these conditions influenced affective updating. We first focused on the number of individual updating words delivered during up-phases, using the formula described below:
Affective updating ~ 1 + updating_words_up-phase * condition + (1 + updating_words_up-phase | participant).
“updating_words_up-phase” was a continuous variable, denoting the number of individual updating words delivered during the SO up-phase. “condition” was a categorical variable (positive vs. neutral updating).
Next, we focused on the number of individual memory cue delivered during the SO up-phase. The formula was as follows:
Affective updating ~ 1 + memory_cue_up-phase * condition + (1 + memory_cue_up-phase | participant).
“memory_cue_up-phase” was a continuous variable, denoting the number of individual memory cues delivered at the SO up-phase.
QUANTIFICATION AND STATISTICAL ANALYSIS
Jamovi was used to test the statistical significance. For behavioral data, repeated ANOVA was conducted on affect updating and memory changes to test the difference of positive, neutral, and non-updating condition. For ERPs analysis, we used a cluster-based two-tailed, one-sample spatial-temporal permutation test (Python-MNE 1.1,
“spatio_temporal_cluster_1samp_test”) with randomization of 1000 and a statistical threshold of 0.05 against zero. For time-frequency analysis, we used a cluster-based two-tailed, one-sample permutation test (Python-MNE 1.1, “permutation_cluster_1samp_test”) with 1000 randomizations and a statistical threshold of 0.05 against zero.
For extracted EEG power analysis, repeated measure ANOVAs were conducted on the power data within each band to test the effect of updating word and position. Post-hoc analyses and multiple comparison were FDR corrected. An alpha level of 0.05 was used for statistical significance across manuscript. For effect size, given our within-subject designs, we reported both ηG2 and ηp2 accompanying repeated measure analysis of variances (ANOVAs, Lakens et al., 2013). Reporting ηp2 allows direct comparisons between effect sizes from previous similar research in TMR and sleep learning. For paired sample t-test, we reported Cohen’s dz as effect sizes 76. Pearson correlation was used to quantify the relationship between the EEG power difference and behavioral outcomes. Partial correlation was used to further control the possible confounding factors. We used the conservative mean ± 2.5 median absolute deviation (MAD) to detect outliers77.
For phase analysis, the Rayleigh Z test was used to test the uniformity of phase distribution in each updating condition. Specifically, the Rayleigh test examines non-uniformity of event distributions, with a significant result indicating that the events are preferably clustered toward certain phase angles and thus followed a non-uniform distribution. In addition, in lmm analysis, we used Satterthwaite’s method (“ANOVA” function in the package “lmerTest” 78) to test significance levels for fixed and random effects. Post-hoc analyses were conducted using the “emtrends” functions in the package “emmeans” to test interaction effects.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Software and Algorithms | ||
| Python 3.7 | https://www.python.org/ | RRID:SCR_008394 |
| Python package “MNE-Python” (v 1.1.1) |
https://mne.tools/stable/index.html | RRID:SCR_005972 |
| Python package “Pingouin” (v 0.5.2) |
https://pingouin-stats.org/api.html | RRID:SCR_022261 |
| Python package “Numpy” (v 1.19.2) |
https://numpy.org/ | RRID:SCR_008633 |
| Python package “YASA” (v 0.6.2) |
https://raphaelvallat.com/yasa/build/html/index.html | N/a |
| Python package “pandas” (v 1.1.3) |
https://pandas.pydata.org/ | RRID:SCR_018214 |
| Python package “seaborn” (v 0.11.2) |
https://seaborn.pydata.org/index.html | RRID:SCR_018132 |
| R Studio 022.02.2 Build 485 | https://www.rstudio.com/ | RRID: SCR_000432 |
| R (4.2.0) | https://www.r-project.org/ | RRID:SCR_001905 |
| R package “lme4” (v1.1–29) |
https://github.com/lme4/lme4/ | RRID:SCR_015654 |
| R package “lmerTest” (v3.1–3) |
https://github.com/runehaubo/lmerTestR | RRID:SCR_015656 |
| Jamovi (v 2.3.13 ) | https://www.jamovi.org/ | RRID:SCR_016142 |
Highlights:
Pairing positive words with aversive memory cues during NREM sleep updates affect
EEG theta power predicts sleep-based affective updating
Updating is best when positive words occurred during slow oscillation up-phases
Acknowledgements:
This research was supported by the National Natural Science Foundation of China (No. 31922089, No.32171056), Ministry of Science and Technology of China (2022ZD0214100), and General Research Fund (No. 17601318) of Hong Kong Research Grants Council to X.H., and Grants NSF BCS-2048681 and NIH/NINDS R01 NS112942 to K.A.P.
Footnotes
Declaration of Interests:
The authors declare no competing interests.
Inclusion and Diversity
We support inclusive, diverse, and equitable conduct of research.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Goldstein AN, and Walker MP (2014). The role of sleep in emotional brain function. Annu. Rev. Clin. Psychol 10, 679–708. 10.1146/annurev-clinpsy-032813-153716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rasch B, and Born J (2013). About sleep’s role in memory. Physiol. Rev 93, 681–766. 10.1152/physrev.00032.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Talamini LM, and Juan E (2020). Sleep as a window to treat affective disorders. Curr. Opin. Behav. Sci 33, 99–108. 10.1016/j.cobeha.2020.02.002. [DOI] [Google Scholar]
- 4.Walker MP, and van der Helm E (2009). Overnight therapy? The role of sleep in emotional brain processing. Psychol. Bull 135, 731–748. 10.1037/a0016570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunsmoor JE, Cisler JM, Fonzo GA, Creech SK, and Nemeroff CB (2022). Laboratory models of post-traumatic stress disorder: The elusive bridge to translation. Neuron, 1–23. 10.1016/j.neuron.2022.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pitman RK., Rasmusson AM., Koenen KC., Shin LM., Orr SP., Gilbertson MW., Milad MR., and Liberzon I. (2012). Biological studies of post-traumatic stress disorder. Nat. Rev. Neurosci 13, 769–787. 10.1038/nrn3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson MC, and Hulbert JC (2021). Active forgetting: Adaptation of memory by prefrontal control. Annu. Rev. Psychol 72, 1–36. 10.1146/annurev-psych-072720-094140. [DOI] [PubMed] [Google Scholar]
- 8.Hu X, Bergström ZM, Gagnepain P, and Anderson MC (2017). Suppressing unwanted memories reduces their unintended influences. Curr. Dir. Psychol. Sci 26, 197–206. 10.1177/0963721417689881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paller KA, Creery JD, and Schechtman E (2021). Memory and sleep: How sleep cognition can change the waking mind for the better. Annu. Rev. Psychol 72, 123–130. 10.1146/annurevpsych-010419-050815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rasch B, Büchel C, Gais S, and Born J (2007). Odor cues during slow-wave sleep prompt declarative memory consolidation. Science 315, 1426–1429. 10.1126/science.1138581. [DOI] [PubMed] [Google Scholar]
- 11.Rudoy JD, Voss JL, Westerberg CE, and Paller KA (2009). Strengthening individual memories by reactivating them during sleep. Science 326, 1079. 10.1126/science.1179013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antony JW, Gobel EW, O’Hare JK, Reber PJ, and Paller KA (2012). Cued memory reactivation during sleep influences skill learning. Nat. Neurosci 15, 1114–1116. 10.1038/nn.3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cellini N, and Capuozzo A (2018). Shaping memory consolidation via targeted memory reactivation during sleep. Ann. N. Y. Acad. Sci 1426, 52–71. 10.1111/nyas.13855. [DOI] [PubMed] [Google Scholar]
- 14.Hu X, Cheng LY, Chiu MH, and Paller KA (2020). Promoting memory consolidation during sleep: A meta-analysis of targeted memory reactivation. Psychol. Bull 146, 218–244. 10.1037/bul0000223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oudiette D, and Paller KA (2013). Upgrading the sleeping brain with targeted memory reactivation. Trends Cogn. Sci 17, 142–149. 10.1016/j.tics.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Schechtman E, Antony JW, Lampe A, Wilson BJ, Norman KA, and Paller KA (2021). Multiple memories can be simultaneously reactivated during sleep as effectively as a single memory. Commun. Biol 4, 25. 10.1038/s42003-020-01512-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schreiner T, Lehmann M, and Rasch B (2015). Auditory feedback blocks memory benefits of cueing during sleep. Nat. Commun 6. 10.1038/ncomms9729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ai S-Z, Chen J, Liu J-F, He J, Xue Y-X, Bao Y-P, Han F, Tang X-D, Lu L, and Shi J (2015). Exposure to extinction-associated contextual tone during slow-wave sleep and wakefulness differentially modulates fear expression. Neurobiol. Learn. Mem 123, 159–167. 10.1016/j.nlm.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Ashton JE, Cairney SA, and Gaskell MG (2018). No effect of targeted memory reactivation during slow-wave sleep on emotional recognition memory. J. Sleep Res 27, 129–137. 10.1111/jsr.12542. [DOI] [PubMed] [Google Scholar]
- 20.Cairney SA, Durrant SJ, Hulleman J, and Lewis PA (2014). Targeted memory reactivation during slow wave sleep facilitates emotional memory consolidation. Sleep 37, 701–707. 10.5665/sleep.3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hauner KK, Howard JD, Zelano C, and Gottfried JA (2013). Stimulus-specific enhancement of fear extinction during slow-wave sleep. Nat. Neurosci 16, 1553–1555. 10.1038/nn.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He J., Sun H-Q., Li S-X., Zhang W-H., Shi J., Ai S-Z., Li Y., Li X-J., Tang X-D., and Lu L. (2015). Effect of conditioned stimulus exposure during slow wave sleep on fear memory extinction in humans. Sleep 38, 423–431. 10.5665/sleep.4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hutchison IC, Pezzoli S, Tsimpanouli M-E, Abdellahi MEA, Pobric G, Hulleman J, and Lewis PA (2021). Targeted memory reactivation in REM but not SWS selectively reduces arousal responses. Commun. Biol 4, 404. 10.1038/s42003-021-01854-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lehmann M, Schreiner T, Seifritz E, and Rasch B (2016). Emotional arousal modulates oscillatory correlates of targeted memory reactivation during NREM, but not REM sleep. Sci. Rep 6, 39229. 10.1038/srep39229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pereira SIR, Tsimpanouli M-E, Hutchison I, Schneider J, Anderson IM, McFarquhar M, Elliott R, and Lewis PA (2022). Cueing emotional memories during slow wave sleep modulates next-day activity in the orbitofrontal cortex and the amygdala. NeuroImage 253, 119120. 10.1016/j.neuroimage.2022.119120. [DOI] [PubMed] [Google Scholar]
- 26.van der Heijden AC, van den Heuvel OA, van der Werf YD, Talamini LM, and van Marle HJF (2022). Sleep as a window to target traumatic memories. Neurosci. Biobehav. Rev, 104765. 10.1016/j.neubiorev.2022.104765. [DOI] [PubMed] [Google Scholar]
- 27.Arzi A, Shedlesky L, Ben-Shaul M, Nasser K, Oksenberg A, Hairston IS, and Sobel N (2012). Humans can learn new information during sleep. Nat. Neurosci 15, 1460–1465. 10.1038/nn.3193. [DOI] [PubMed] [Google Scholar]
- 28.Koroma M, Elbaz M, Léger D, and Kouider S (2022). Learning new vocabulary implicitly during sleep transfers with cross-modal generalization into wakefulness. Front. Neurosci 16, 801666. 10.3389/fnins.2022.801666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Züst MA, Ruch S, Wiest R, and Henke K (2019). Implicit vocabulary learning during sleep is bound to slow-wave peaks. Curr. Biol 29, 541–553.e7. 10.1016/j.cub.2018.12.038. [DOI] [PubMed] [Google Scholar]
- 30.Schechtman E, Witkowski S, Lampe A, Wilson BJ, and Paller KA (2020). Targeted memory reactivation during sleep boosts intentional forgetting of spatial locations. Sci. Rep 10, 1–9. 10.1038/s41598-020-59019-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu X, Gawronski B, and Balas R (2017). Propositional versus dual-process accounts of evaluative conditioning: II. The effectiveness of counter-conditioning and counter-instructions in changing implicit and explicit evaluations. Soc. Psychol. Personal. Sci 8, 858–866. 10.1177/1948550617691094. [DOI] [PubMed] [Google Scholar]
- 32.Keller NE, Hennings AC, and Dunsmoor JE (2020). Behavioral and neural processes in counterconditioning: Past and future directions. Behav. Res. Ther 125, 103532. 10.1016/j.brat.2019.103532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Gucht D, Baeyens F, Vansteenwegen D, Hermans D, and Beckers T (2010). Counterconditioning reduces cue-induced craving and actual cue-elicited consumption. Emotion 10, 688–695. 10.1037/a0019463. [DOI] [PubMed] [Google Scholar]
- 34.Canales-Johnson A, Merlo E, Bekinschtein TA, and Arzi A (2020). Neural dynamics of associative learning during human sleep. Cereb. Cortex, 30, 1708–1715. 10.1093/cercor/bhz197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Himmer L, Bürger Z, Fresz L, Maschke J, Wagner L, Brodt S, Braun C, Schönauer M, and Gais S (2021). Localizing spontaneous memory reprocessing during human sleep (Neuroscience) 10.1101ce/2021.11.29.470230. [DOI] [Google Scholar]
- 36.Schreiner T., Doeller CF., Jensen O., Rasch B., and Staudigl T. (2018). Theta phase-coordinated memory reactivation reoccurs in a slow-oscillatory rhythm during NREM sleep. Cell Rep. 25, 296–301. 10.1016/j.celrep.2018.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Staudigl T, and Hanslmayr S (2013). Theta oscillations at encoding mediate the context-dependent nature of human episodic memory. Curr. Biol 23, 1101–1106. 10.1016/j.cub.2013.04.074. [DOI] [PubMed] [Google Scholar]
- 38.Blume C, del Giudice R, Lechinger J, Wislowska M, Heib DPJ, Hoedlmoser K, and Schabus M (2017). Preferential processing of emotionally and self-relevant stimuli persists in unconscious N2 sleep. Brain Lang. 167, 72–82. 10.1016/j.bandl.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 39.Arzi A, Holtzman Y, Samnon P, Eshel N, Harel E, and Sobel N (2014). Olfactory aversive conditioning during sleep reduces cigarette-smoking behavior. J. Neurosci 34, 15382–15393. 10.1523/JNEUROSCI.2291-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Legendre G, Bayer L, Seeck M, Spinelli L, Schwartz S, and Sterpenich V (2022). Reinstatement of emotional associations during human sleep: an intracranial EEG study (Neuroscience) 10.1101/2022.06.24.497499. [DOI] [Google Scholar]
- 41.Antony JW, Piloto L, Wang M, Pacheco P, Norman KA, and Paller KA (2018). Sleep spindle refractoriness segregates periods of memory reactivation. Curr. Biol 28, 1736–1743.e4. 10.1016/j.cub.2018.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cairney SA, Guttesen A. á.Váli, El Marj N, and Staresina BP (2018). Memory consolidation is linked to spindle-mediated information processing during sleep. Curr. Biol 28, 948–954.e4. 10.1016/j.cub.2018.01.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diekelmann S, and Born J (2010). The memory function of sleep. Nat. Rev. Neurosci 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 44.Klinzing JG, Niethard N, and Born J (2019). Mechanisms of systems memory consolidation during sleep. Nat. Neurosci 22. 10.1038/s41593-019-0467-3. [DOI] [PubMed] [Google Scholar]
- 45.Petzka M, Chatburn A, Charest I, Balanos GM, and Staresina BP (2022). Sleep spindles track cortical learning patterns for memory consolidation. Curr. Biol 32, 2349–2356.e4. 10.1016/j.cub.2022.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schreiner T, Petzka M, Staudigl T, and Staresina BP (2021). Endogenous memory reactivation during sleep in humans is clocked by slow oscillation-spindle complexes. Nat. Commun 12, 3112. 10.1038/s41467-021-23520-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andrillon T, Poulsen AT, Hansen LK, L É Ger D, and Kouider S (2016). Neural markers of responsiveness to the environment in human sleep. J. Neurosci 36, 6583–6596. 10.1523/JNEUROSCI.0902-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Andrillon T, and Kouider S (2020). The vigilant sleeper: neural mechanisms of sensory (de)coupling during sleep. Curr. Opin. Physiol 15, 47–59. 10.1016/j.cophys.2019.12.002. [DOI] [Google Scholar]
- 49.Schabus M, Dang-Vu TT, Heib DPJ, Boly M, Desseilles M, Vandewalle G, Schmidt C, Albouy G, Darsaud A, Gais S, et al. (2012). The fate of incoming stimuli during NREM sleep is determined by spindles and the phase of the slow oscillation. Front. Neurol 3. 10.3389/fneur.2012.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Destexhe A, Hughes SW, Rudolph M, and Crunelli V (2007). Are corticothalamic ‘up’ states fragments of wakefulness? Trends Neurosci. 30, 334–342. 10.1016/j.tins.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Niknazar H., Malerba P., and Mednick SC. (2022). Slow oscillations promote long-range effective communication: The key for memory consolidation in a broken-down network. Proc. Natl. Acad. Sci 119, e2122515119. 10.1073/pnas.2122515119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Göldi M, van Poppel EAM, Rasch B, and Schreiner T (2019). Increased neuronal signatures of targeted memory reactivation during slow-wave up states. Sci. Rep 9, 1–10. 10.1038/s41598-019-39178-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ngo HVV, Martinetz T, Born J, and Mölle M (2013). Auditory closed-loop stimulation of the sleep slow oscillation enhances memory. Neuron 78, 545–553. 10.1016/j.neuron.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 54.Antony JW, Cheng LY, Brooks PP, Paller KA, and Norman KA (2018). Neurobiology of learning and memory competitive learning modulates memory consolidation during sleep. Neurobiol. Learn. Mem 155, 216–230. 10.1016/j.nlm.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 55.Wang B, Antony JW, Lurie S, Brooks PP, Paller KA, and Norman KA (2019). Targeted memory reactivation during sleep elicits neural signals related to learning content. J. Neurosci 39, 6728–6736. 10.1523/JNEUROSCI.2798-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wislowska M, Klimesch W, Jensen O, Blume C, and Schabus M (2022). Sleep-specific processing of auditory stimuli is reflected by alpha and sigma oscillations. J. Neurosci, JN-RM-1889–21. 10.1523/JNEUROSCI.1889-21.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Batterink LJ, Creery JD, and Paller KA (2016). Phase of spontaneous slow oscillations during sleep influences memory-related processing of auditory cues. J. Neurosci 36, 1401–1409. 10.1523/JNEUROSCI.3175-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Creery JD, Brang DJ, Arndt JD, Bassard A, Towle VL, Tao JX, Wu S, Rose S, Warnke PC, Issa NP, et al. (2022). Electrophysiological markers of memory consolidation in the human brain when memories are reactivated during sleep. Proc. Natl. Acad. Sci 119, e2123430119. 10.1073/pnas.2123430119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wassing R, Lakbila-Kamal O, Ramautar JR, Stoffers D, Schalkwijk F, and Van Someren EJW (2019). Restless REM sleep impedes overnight amygdala adaptation. Curr. Biol 29, 2351–2358.e4. 10.1016/j.cub.2019.06.034. [DOI] [PubMed] [Google Scholar]
- 60.Rakowska M, Abdellahi MEA, Bagrowska P, Navarrete M, and Lewis PA (2021). Long term effects of cueing procedural memory reactivation during NREM sleep. NeuroImage 244, 118573. 10.1016/j.neuroimage.2021.118573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zeng S, Lin X, Wang J, and Hu X (2021). Sleep’s short-term memory preservation and long-term affect depotentiation effect in emotional memory consolidation: behavioral and EEG evidence. Sleep 44, zsab155. 10.1093/sleep/zsab155. [DOI] [PubMed] [Google Scholar]
- 62.Cairney SA, Durrant SJ, Hulleman J, and Lewis PA (2014). Targeted memory reactivation during slow wave sleep facilitates emotional memory consolidation. Sleep 37, 701–707. 10.5665/sleep.3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hu X, Antony JW, Creery JD, Vargas IM, Bodenhausen GV, and Paller KA (2015). Unlearning implicit social biases during sleep. Science 348, 1013–1015. 10.1126/science.aaa3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ngo H-VV, and Staresina BP (2022). Shaping overnight consolidation via slow-oscillation closed-loop targeted memory reactivation. Proc. Natl. Acad. Sci 119, e2123428119. 10.1073/pnas.2123428119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Andrillon T, and Kouider S (2020). The vigilant sleeper: neural mechanisms of sensory (de)coupling during sleep. Curr. Opin. Physiol 15, 47–59. 10.1016/j.cophys.2019.12.002. [DOI] [Google Scholar]
- 66.Wang Y, Zhou R, and Luo Y (2008). The plot Establishment and Evaluation of Chinese Affective Words System. Chin. Ment. Heal. J 22, 608–612. [Google Scholar]
- 67.Bradley MM., and Lang PJ. (2017). International affective picture system. In Encyclopedia of Personality and Individual Differences (Springer International Publishing; ), pp. 1–4. 10.1007/9783-319-28099-8_42-1. [DOI] [Google Scholar]
- 68.Marchewka A, Żurawski Ł, Jednoróg K, and Grabowska A (2014). The nencki affective picture system (NAPS): Introduction to a novel, standardized, wide-range, high-quality, realistic picture database. Behav. Res. Methods 46, 596–610. 10.3758/s13428-013-0379-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Catarino A, Küpper CS, Werner-Seidler A, Dalgleish T, and Anderson MC (2015). Failing to forget: Inhibitory-control deficits compromise memory suppression in posttraumatic stress disorder. Psychol. Sci 26, 604–616. 10.1177/0956797615569889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peirce JW (2007). PsychoPy-psychophysics software in Python. J. Neurosci. Methods 162, 8–13. 10.1016/j.jneumeth.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gramfort A, Luessi M, Larson E, Engemann DA, Strohmeier D, Brodbeck C, Goj R, Jas M, Brooks T, Parkkonen L, et al. (2013). MEG and EEG data analysis with MNE-Python. Front. Neurosci 7, 1–13. 10.3389/fnins.2013.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Whitmore NW, Bassard AM, and Paller KA (2022). Targeted memory reactivation of face-name learning depends on ample and undisturbed slow-wave sleep. Npj Sci. Learn 7, 1–6. 10.1038/s41539-021-00119-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vallat R, and Walker MP (2021). An open-source, high-performance tool for automated sleep staging. eLife 10, e70092. 10.7554/eLife.70092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Helfrich RF, Mander BA, Jagust WJ, Knight RT, and Walker MP (2017). Old brains come uncoupled in sleep: Slow wave-spindle synchrony, brain atrophy, and forgetting. Neuron, 1–10. 10.1016/j.neuron.2017.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mölle M, Marshall L, Gais S, and Born J (2002). Grouping of spindle activity during slow oscillations in human non-rapid eye movement sleep. J. Neurosci 22, 10941–10947. 10.1523/JNEUROSCI.22-24-10941.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lakens D (2013). Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front. Psychol 4. 10.3389/fpsyg.2013.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Leys C, Ley C, Klein O, Bernard P, and Licata L (2013). Detecting outliers: Do not use standard deviation around the mean, use absolute deviation around the median. J. Exp. Soc. Psychol 49, 764–766. 10.1016/j.jesp.2013.03.013. [DOI] [Google Scholar]
- 78.Kuznetsova A, Brockhoff PB, and Christensen RHB (2017). lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw 82. 10.18637/jss.v082.i13. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Pre-processed data have been deposited at the open science framework (OSF: https://osf.io/pqcta/).
All original code has been deposited at the open science framework (OSF: https://osf.io/pqcta/).
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.